Abstract
Background
Latent cytomegalovirus (CMV) infection is immunomodulatory and could affect mRNA vaccine responsiveness. We sought to determine the association of CMV serostatus and prior severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) infection with antibody (Ab) titers after primary and booster BNT162b2 mRNA vaccinations in healthcare workers (HCWs) and nursing home (NH) residents.
Methods
Nursing home residents (N = 143) and HCWs (N = 107) were vaccinated and serological responses monitored by serum neutralization activity against Wuhan and Omicron (BA.1) strain spike proteins, and by bead-multiplex immunoglobulin G immunoassay to Wuhan spike protein and its receptor-binding domain (RBD). Cytomegalovirus serology and levels of inflammatory biomarkers were also measured.
Results
Severe acute respiratory syndrome coronavirus 2-naive CMV seropositive (CMV+) HCWs had significantly reduced Wuhan-neutralizing Ab (P = .013), anti-spike (P = .017), and anti-RBD (P = .011) responses 2 weeks after primary vaccination series compared with responses among CMV seronegative (CMV−) HCWs, adjusting for age, sex, and race. Among NH residents without prior SARS-CoV-2 infection, Wuhan-neutralizing Ab titers were similar 2 weeks after primary series but were reduced 6 months later (P = .012) between CMV+ and CMV− subjects. Wuhan-neutralizing Ab titers from CMV+ NH residents who had prior SARS-CoV-2 infection consistently trended lower than titers from SARS-CoV-2 experienced CMV− donors. These impaired Ab responses in CMV+ versus CMV− individuals were not observed after booster vaccination or with prior SARS-CoV-2 infection.
Conclusions
Latent CMV infection adversely affects vaccine-induced responsiveness to SARS-CoV-2 spike protein, a neoantigen not previously encountered, in both HCWs and NH residents. Multiple antigenic challenges may be required for optimal mRNA vaccine immunogenicity in CMV+ adults.
Keywords: antibodies, COVID-19, cytomegalovirus, SARS-CoV-2, vaccination
SARS-CoV-2-naive CMV-seropositive healthcare workers had reduced vaccine responsiveness to primary vaccination. SARS-CoV-2 naive CMV-seropositive nursing home residents had lower neutralizing antibody titers 6 months after primary vaccination. Latent CMV may predispose people to reduced mRNA vaccine responsiveness.
Coronavirus disease 2019 (COVID-19) is an ongoing, worldwide pandemic caused by severe acute respiratory disease coronavirus 2 (SARS-CoV-2) infection. Because advanced age and multiple comorbidities increase susceptibility to more severe COVID-19 morbidity and mortality, nursing home (NH) residents represent an especially vulnerable population [1]. Emerging evidence suggests that latent herpesvirus infections may predispose people to worse COVID-19 outcomes [2]. For example, individuals who are β-herpesvirus cytomegalovirus (CMV) and α-herpesvirus herpes simplex virus-1 seropositive have disproportionate overrepresentation among patients hospitalized with COVID-19 compared to those with mild disease [3]. In addition, reactivation of the γ-herpesvirus Epstein-Barr virus (EBV) during acute SARS-CoV-2 infection has recently been shown to be associated with worse disease as well as with the onset, persistence, and severity of long-term sequelae [4–8].
On the other hand, evidence from humans and mouse models suggests that latent CMV infection may improve T-cell and antibody (Ab) responses to antigenic challenge. This includes responses to vaccines and infectious agents, possibly via elevated myeloid cell activation and increased soluble interferon levels observed during CMV infection [9, 10]. However, older individuals who have often harbored infection for a longer time may have lost such improvement or even have suppressed responses [9, 11, 12].
One success of the COVID-19 pandemic response has been the unprecedented rapid development and rollout of highly efficacious vaccines. The mRNA-based vaccines have shown remarkable efficacy at preventing severe disease, even from highly evolved SARS-CoV-2 variants, such as the Delta and Omicron variants [13]. However, vaccine-induced total and neutralizing Ab titers wane, particularly in NH populations, 35%–69% of whom have undetectable Wuhan strain neutralizing Ab titers by 6 months after vaccination, suggesting vaccine-mediated protection may lessen over time [14]. This is particularly notable for neutralizing titers to the Omicron variant, which do not readily develop in either healthcare workers (HCWs) or NH residents until after a boost [15]. More importantly, not all factors that govern the generation, peak, and durability of Ab responses after COVID-19 vaccination have been elucidated.
In this report, we assessed the association of latent CMV and EBV infections with Ab responses (neutralizing, anti-spike, anti-receptor binding domain [RBD]) to the BNT162b2 mRNA vaccine in ambulatory HCWs and NH residents before and after the initial vaccination series and boost. For each population, we analyzed the vaccine responses among those who had prior SARS-CoV-2 infection (SARS-CoV-2-naive), in whom vaccine-elicited SARS-CoV-2 spike protein would be a neoantigen never previously encountered, separately from the vaccine responses among those who had prior SARS-CoV-2 infection (SARS-CoV-2-experienced), in whom the pre-existing anti-SARS-CoV-2 immunity could affect vaccine immunogenicity. Our goals were to determine whether latent CMV and/or EBV infections affected vaccine-elicited Ab responses, whether this was associated with age, and whether prior SARS-CoV-2 infection further mediated the response.
METHODS
Study Design and Participants
We collected blood and froze the serum for later analysis from a total of 107 HCWs (aged 26–78, median 48 years) and 143 NH residents (aged 48–99, median 76 years) in northeast Ohio (Table 1). Nursing home residents were administered the BNT162b2 mRNA vaccine (Pfizer/BioNTech) from December 2020 to February 2021 at 4 NHs and vaccinated concurrently with HCWs, by a second dose 3 weeks later during the emergency use authorization period, and then a booster dose with the same vaccine 7 to 10 months after primary series. The mean time from second dose to booster was 273 days, and >80% of the population was vaccinated within 10 days of this mean interval. The minimum interval was 208 days and the maximum interval was 335 days. We did not acquire samples from all participants at every time point, nor did we perform every assay on every sample. We sampled both HCWs and NH residents (1) before they received their first BNT162b2 mRNA vaccine (NNH = 143; NHCW = 74) immunization and (2) again 2 weeks after the second dose (NNH = 138; NHCW = 107), (3) 6 months after the second dose (NNH = 123; NHCW = 93), and (4) before (NNH = 86; NHCW = 54) and (5) 2 weeks after (NNH = 77; NHCW = 47) a third dose (boost). All participants received 2 doses of vaccine in their primary series regardless of prior SARS-CoV-2 infection. Participants received their third dose approximately 9 months after completion of the initial immunization series. We deemed participants to have had prior SARS-CoV-2 infection (“SARS-CoV-2-experienced”) if they had a diagnostic polymerase chain reaction or antigen test that confirmed acute SARS-CoV-2 infection and/or elevated antibody levels to the SARS-CoV-2 spike, RBD, or nucleocapsid before immunization and deemed “SARS-CoV-2-naive” if none of these conditions were met. During this period, laboratory-confirmed SARS-CoV-2 infection in an individual employee or resident triggered contact tracing among HCWs, and within residents' individual NHs, more frequent testing. We removed the few participants who acquired SARS-CoV-2 infection after vaccination (N = 5) from subsequent analyses.
Table 1.
Participant Characteristics
| Characteristics | HCW | NH | P Valuea |
|---|---|---|---|
| N | 107 | 143 | … |
| Age (years), median (IQR) | 48 (29–56) | 76 (70–87) | <.001 |
| Female, N (%) | 56 (52.3) | 54 (37.8) | .028 |
| Race | … | … | … |
| African American | 9 (8.4) | 16 (11.2) | .672 |
| Asian | 5 (4.7) | 1 (0.7) | .087 |
| Hispanic | 4 (3.7) | 1 (0.7) | .167 |
| White | 89 (83.2) | 125 (87.4) | .367 |
| CMV+, N (%) | 54 (50.5) | 89 (62.2) | .071 |
| EBV+, N (%) | 98 (91.6) | 137 (95.8) | .186 |
| SARS-CoV-2-experienced, N (%) | 39 (36.4) | 59 (41.3) | .513 |
| IL-6 (pg/mL), median (IQR) | 3.01 (2.10–4.31) | 6.42 (4.57–9.86) | <.001 |
| CRP (ng/mL), median (IQR) | 4617 (1785–11 182) | 10 779 (3777–28 184) | <.001 |
| sTNFR-I (pg/mL), median (IQR) | 1307 (1122–1588) | 2463 (1982–3410) | <.001 |
| sTNFR-II (pg/mL), median (IQR) | 2257 (1931–2746) | 4305 (3404–5716) | <.001 |
Abbreviations: CMV, cytomegalovirus; CMV+, seropositive; CRP, C-reactive protein; EBV, Epstein-Barr virus; HCW, healthcare workers; IL-6, interleukin 6; IQR, interquartile range; NH, nursing home residents; SARS-CoV-2, severe acute respiratory syndrome coronavirus 2; sTNFR, soluble tumor necrosis factor receptor.
P values less than .05 are considered statistically significant and are depicted in bold font. Categorical variables were analyzed using Fisher's exact test; continuous variables were analyzed using nonparametric Mann-Whitney U test.
Anti-Spike and Anti-Receptor-Binding Domain Assay
We assessed immune responses to BNT162b2 by measuring immunoglobulin G (IgG) to spike protein and its RBD by bead-multiplex immunoassay targeting the Wuhan strain, as we have previously described [14–16]. In short, stabilized full-length Wuhan spike protein (amino acid [aa] 16–1230, with furin site mutated) and RBD (aa 319–541) were conjugated to magnetic microbeads (Luminex, Austin, TX). Antigen-specific IgG was detected in serum/plasma using phycoerythrin-conjugated donkey F(ab)2 antihuman IgG, with Fcγ (Jackson ImmunoResearch, West Grove, PA) added. Using the Magpix assay system (Bio-Rad, Hercules, CA), the mean fluorescent index was recorded. To provide an internal standard and control for plate-to-plate variation, a pool of convalescent plasma was generated from people with known prior SARS-CoV-2 infection and was run on each plate as a standard curve of half-log dilutions starting at a 1:100 dilution. A secondary standard from the Frederick National Laboratory calibrated to the World Health Organization standard 20/136 was used to quantify antibodies to the spike protein in binding Ab units (BAU) per milliliter.
Severe Acute Respiratory Syndrome Coronavirus 2 Pseudovirus Neutralization Assay
The neutralization activity of participant sera against lentiviral particles pseudotyped with Wuhan and Omicron (BA.1) strain spike proteins was assessed as described previously [15, 17, 18]. In short, neutralization assays were performed using a Fluent 780 liquid handler (Tecan, Männedorf, Switzerland) in 384-well plates (Grenier Bio-One, Monroe, NC). Three-fold serial dilutions ranging from 1:12 to 1:8748 were performed and added to 50–250 infectious units of pseudovirus for 1 hour. We calculated the pNT50 values by taking the inverse of the 50% inhibitory concentration value for all samples with a pseudovirus neutralization value of 80% or higher at the highest concentration of serum. The lower limit of detection of this assay is 1:12 dilution.
Cytomegalovirus and Epstein-Barr Virus Serologies and Serum Cytokine Measurements
Serum anti-CMV and anti-EBV IgG serologies were measured by standard enzyme-linked immunosorbent assays following manufacturer's instructions (both kits from Abcam, Cambridge, UK). Serum levels of interleukin (IL)-6, C-reactive protein (CRP), soluble tumor necrosis factor (TNF) receptor (sTNFR)-I, and sTNFR-II were measured by ELLA assay (ProteinSimple, San Jose, CA), per manufacturer's instructions.
Statistical Analyses
For analyses of the association of CMV serostatus on vaccine-induced antibody titers, log-transformed assay values (ie, neutralizing Ab, anti-spike IgG, anti-RBD IgG titers) were compared by least squares multiple linear regression analysis, with adjustment for age, sex, and race. Comparisons of Ab titers between NH and HCW groups, and all comparisons of soluble cytokine levels, were performed without adjustment by nonparametric Mann-Whitney U test. All categorical demographic value differences between groups were analyzed using Fisher's Exact test (eg, sex, race, and CMV and EBV serostatus). All analyses were performed in Prism version 9.5.0 (GraphPad, San Diego, CA), and P values less than .05 were considered statistically significant. Due to the low number of subjects who were EBV seronegative, we did not perform detailed analyses of the association of EBV serostatus on vaccine-induced antibody titers.
Patient Consent Statement
This study was approved by the WCG institutional review board (IRB), and reliant review accepted by Case Western Reserve University local IRB. The WCG IRB approved use of verbal consent and assent for subjects, and documentation of informed consent and assent was obtained from all subjects or their legally authorized representatives.
RESULTS
Demographics and Seroprevalence of Cytomegalovirus and Epstein-Barr Virus Among Healthcare Workers and Nursing Home Residents
As shown in Table 1, the overall seroprevalence of CMV was 50.5% among HCWs and 62.2% among NH residents (P = .071), and the seroprevalence of EBV was uniformly high—91.6% among HCWs and 95.8% among NH residents (P = .186). A greater proportion of NH residents than HCWs were male (P = .028), due to the recruitment of NH residents from the largely male population at a Veterans' nursing home. For HCWs, CMV seronegative (CMV−) and seropositive (CMV+) persons were similarly aged (48 vs 49.5 years, respectively), had a similar sex distribution (54.7% vs 50% female sex, respectively), similar proportion of EBV seropositivity (86.8% vs 96.3%, respectively), and similar frequencies of prior SARS-CoV-2 infection (35.8% vs 37%, respectively) (Table 2). A greater proportion of CMV− HCWs were White compared with CMV+ HCWs (92.5% vs 74.1%, respectively; P = .018).
Table 2.
Participant Characteristics by CMV Serostatus
| Characteristics | HCW | NH | ||||
|---|---|---|---|---|---|---|
| CMV− | CMV+ | P Valuea | CMV− | CMV+ | P Value | |
| N | 53 | 54 | … | 54 | 89 | … |
| Age (years), median (IQR) | 48 (38.5–56.5) | 49.5 (38.8–56.3) | .402 | 72.5 (67.0–81.3) | 79.0 (71.3–89.0) | .003 |
| Female, N (%) | 29 (54.7) | 27 (50.0) | .700 | 15 (27.8) | 39 (43.8) | .075 |
| Race | ||||||
| African American | 2 (3.8) | 7 (13.0) | .161 | 2 (3.7) | 14 (15.7) | .030 |
| Asian | 1 (1.9) | 4 (7.4) | .363 | 0 (0.0) | 1 (1.1) | >.999 |
| Hispanic | 1 (1.9) | 3 (5.6) | .618 | 0 (0.0) | 1 (1.1) | >.999 |
| White | 49 (92.5) | 40 (74.1) | .018 | 52 (96.3) | 73 (82.0) | .017 |
| EBV+, N (%) | 46 (86.8) | 52 (96.3) | .093 | 51 (94.4) | 86 (96.6) | .673 |
| SARS-CoV-2-experienced, N (%) | 19 (35.8) | 20 (37.0) | >.999 | 16 (29.6) | 43 (48.3) | .036 |
| IL-6 (pg/mL), median (IQR) | 2.85 (1.82–4.26) | 3.23 (2.46–4.61) | .256 | 6.30 (4.41–10.63) | 6.81 (4.97–9.71) | .641 |
| CRP (ng/mL), median (IQR) | 3404 (879–7557) | 5251 (2833–15 492) | .076 | 13 948 (6395–30 600) | 8018 (2520–23 356) | .038 |
| sTNFR-I (pg/mL), median (IQR) | 1268 (1114–1528) | 1373 (1141–1662) | .431 | 2485 (1982–3468) | 2441 (1975–3388) | .580 |
| sTNFR-II (pg/mL), median (IQR) | 2190 (1920–2677) | 2534 (1944–2857) | .347 | 4153 (3335–5504) | 4305 (3450–5755) | .838 |
Abbreviations: CMV, cytomegalovirus; CMV−, CMV seronegative; CMV+, seropositive; CRP, C-reactive protein; EBV, Epstein-Barr virus; HCW, healthcare workers; IL-6, interleukin 6; IQR, interquartile range; NH, nursing home residents; SARS-CoV-2, severe acute respiratory syndrome coronavirus 2; sTNFR, soluble tumor necrosis factor receptor.
P values less than .05 are considered statistically significant and are depicted in bold font. Categorical variables were analyzed using Fisher's exact test; continuous variables were analyzed using nonparametric Mann-Whitney U test.
As shown in Table 2, among NH residents, CMV− donors were significantly younger than CMV+ donors (72.5 vs 79 years, respectively; P = .003). Female donors tended to be enriched within CMV+ donors (43.8% vs 27.8% among CMV− donors; P = .076). The proportion of EBV seropositivity was similar between CMV− and CMV+ donors (94.4% vs 96.6%, respectively), and a lower proportion of CMV− donors were SARS-CoV-2-experienced than the proportion among CMV+ donors (29.6% vs 48.3%, respectively; P = .036). As observed with the HCWs, a greater proportion of CMV− NH residents were White compared with CMV+ NH residents (96.3% vs 82%, respectively; P = .017).
There were no statistically significant differences in any of the demographic or serological indices measured when HCWs and NH residents were stratified by their EBV serostatus (Supplementary Table 1). As shown in Supplementary Table 2, when stratified by their SARS-CoV-2 infection history, a lower proportion of SARS-CoV-2-naive HCWs were female than SARS-CoV-2-experienced HCWs (44.1% vs 66.7%, respectively; P = .028), and a lower proportion of SARS-CoV-2-naive NH residents were CMV+ than SARS-CoV-2-experienced NH residents (54.8% vs 72.9%, respectively; P = .036). All other comparisons were statistically similar among the groups.
Effect of Cytomegalovirus Serostatus on Antibody Responses
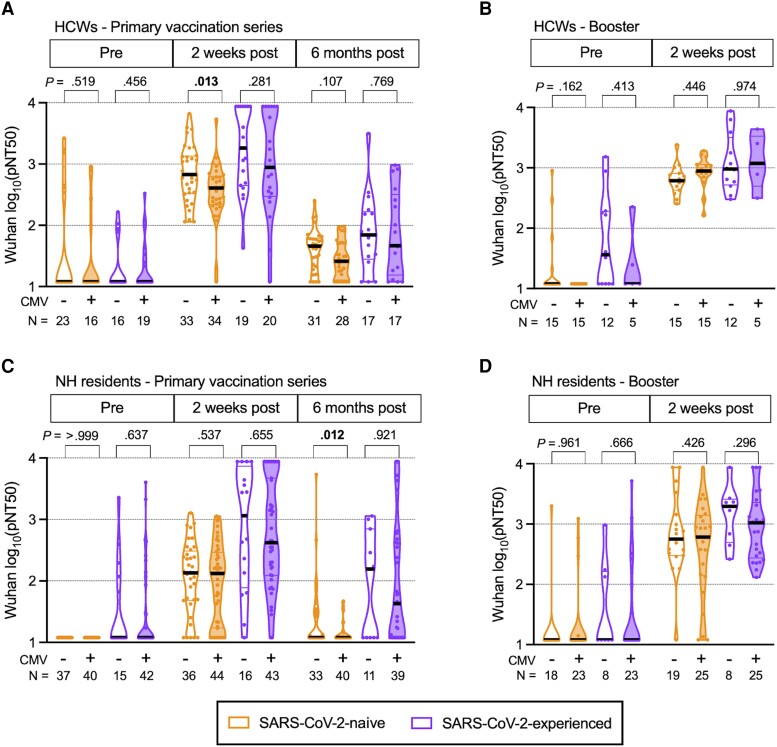
To measure differences in Ab responses between CMV− and CMV+ donors (Supplementary Table 3), we performed multivariable least squares linear regression adjusting for the potential confounders age, sex, and race. After adjustment, we found that among COVID-19-naive HCWs receiving their primary vaccine series, the neutralizing Ab titers to the Wuhan strain for CMV+ donors were significantly reduced at 2 weeks after vaccination (P = .013) compared with CMV− donors (Figure 1A). Neutralizing Ab titers were similar among SARS-CoV-2-experienced donors at 2 weeks after completing the primary series. No significant differences remained in neutralizing Ab titers between CMV+ and CMV− HCWs 0–14 days before their booster (which generally occurred 7–10 months after the primary series). There was also no difference in neutralizing Ab titers between CMV+ and CMV− HCWs 2 weeks after the booster dose, regardless of SARS-CoV-2 infection history (Figure 1B).
Figure 1.
Wuhan strain neutralization titers in primary series and booster series with BNT162b2 mRNA vaccination in healthcare workers (HCW) and nursing home (NH) residents, with and without prior severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) infection. Violin plots show Wuhan (vaccine) strain pseudovirus neutralization (pNT50) values for (A) HCWs during primary series, (B) HCWs during booster series, (C) NH residents during primary series, and (D) NH residents during booster series. The upper limit of detection of the assay is 1:8748 and the lower limit of detection is 1:12. Participants who were SARS-CoV-2 naive (SARS-CoV-2-naive) are shown in orange, participants with prior SARS-CoV-2 infection (SARS-CoV-2-experienced) are shown in purple, open plots are from cytomegalovirus (CMV) seronegative (CMV−) donors, and filled plots are from CMV seropositive (CMV+) donors. N values indicate the number of participants measured in each group. P values are between CMV− and CMV+, determined by least squares multiple linear regression, adjusted for age, sex, and race. P values less than .05 are considered statistically significant and are depicted in bold font.
Similar to our previous reports [14, 16], SARS-CoV-2-naive NH residents had substantially reduced Wuhan strain neutralizing Ab titers compared with HCWs before vaccination and 2 weeks and 6 months after vaccination, regardless of their CMV serostatus (Supplementary Table 4). We did not observe these differences when comparing SARS-CoV-2-experienced NH residents or HCWs. Cytomegalovirus seropositivity was associated with significantly reduced Wuhan strain neutralizing Ab titers 6 months after the primary vaccine in SARS-CoV-2-naive NH residents (P = .012) (Figure 1C). No other differences in Wuhan-neutralizing Ab titers after the primary series or booster were seen according to CMV serostatus among SARS-CoV-2-naive NH residents. Of note, neutralizing Ab titers trended lower among CMV+ SARS-CoV-2-experienced NH residents at all postvaccine timepoints, but these differences were not statistically significant (Figure 1C and D).
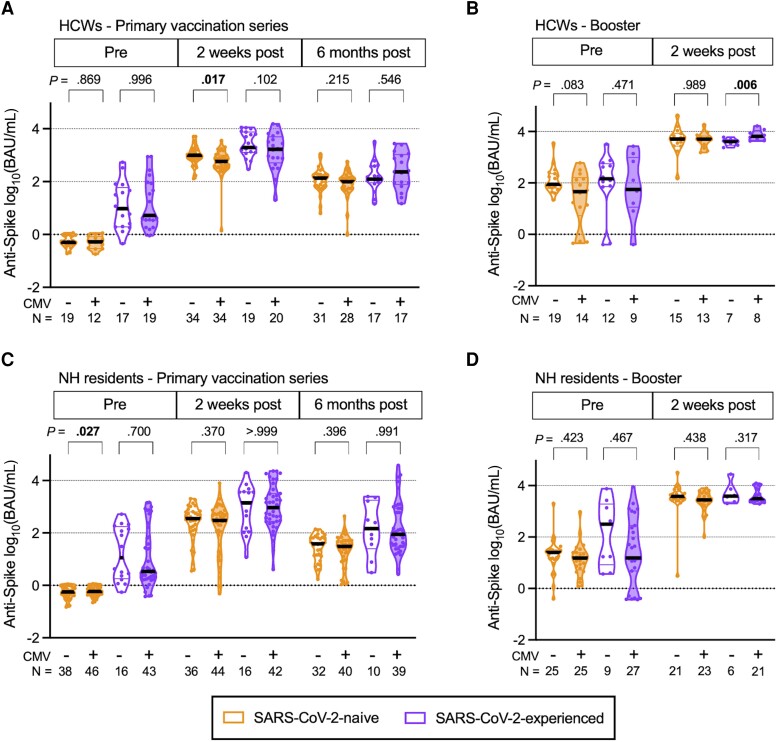
We next measured the titers of anti-spike Abs, expressed as binding Ab units (BAU) per milliliter and anti-RBD titers, in AU (Supplementary Table 3). As we observed with Wuhan-neutralizing Ab titers, we found a significant reduction in anti-spike Ab titers (P = .017) (Figure 2A) and anti-RBD titers (P = .011) (Supplementary Figure 1A) among CMV+ SARS-CoV-2-naive HCWs compared to titers among CMV− HCWs at 2 weeks after primary vaccination and in anti-RBD titers prebooster (P = .042) (Supplementary Figure 1B), after adjustment for age, sex, and race. It is interesting to note that we found a small but significant increase in anti-spike Ab titers among SARS-CoV-2-experienced CMV+ HCWs compared with CMV− donors (P = .006) (Figure 2B). As with the Wuhan-neutralizing titers, we did not observe any differences in anti-spike or anti-RBD Ab titers between CMV+ and CMV− donors after the primary series (Figure 2C, Supplementary Figure 1C) or booster (Figure 2D, Supplementary Figure 1D) among NH residents, regardless of SARS-CoV-2 infection history.
Figure 2.
Anti-spike antibody (Ab) titers in primary series and booster series with BNT162b2 mRNA vaccination in healthcare workers (HCW) and nursing home (NH) residents, with and without prior severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) infection. Violin plots show anti-spike Ab values depicted in the binding arbitrary units/milliliter (BAU/mL) based on the World Health Organization standard for (A) HCWs during primary series, (B) HCWs during booster series, (C) NH residents during primary series, and (D) NH residents during booster series. The cutoff for a positive anti-spike response over prepandemic controls is 3.8 BAU/mL. Participants who were SARS-CoV-2 naive (SARS-CoV-2-naive) are shown in orange, participants with prior SARS-CoV-2 infection (SARS-CoV-2-experienced) are shown in purple, open plots are from cytomegalovirus (CMV) seronegative (CMV−) donors, and filled plots are from CMV seropositive (CMV+) donors. N values indicate the number of participants measured in each group. P values are between CMV− and CMV+, determined by least squares multiple linear regression, adjusted for age, sex, and race. P values less than .05 are considered statistically significant and are depicted in bold font.
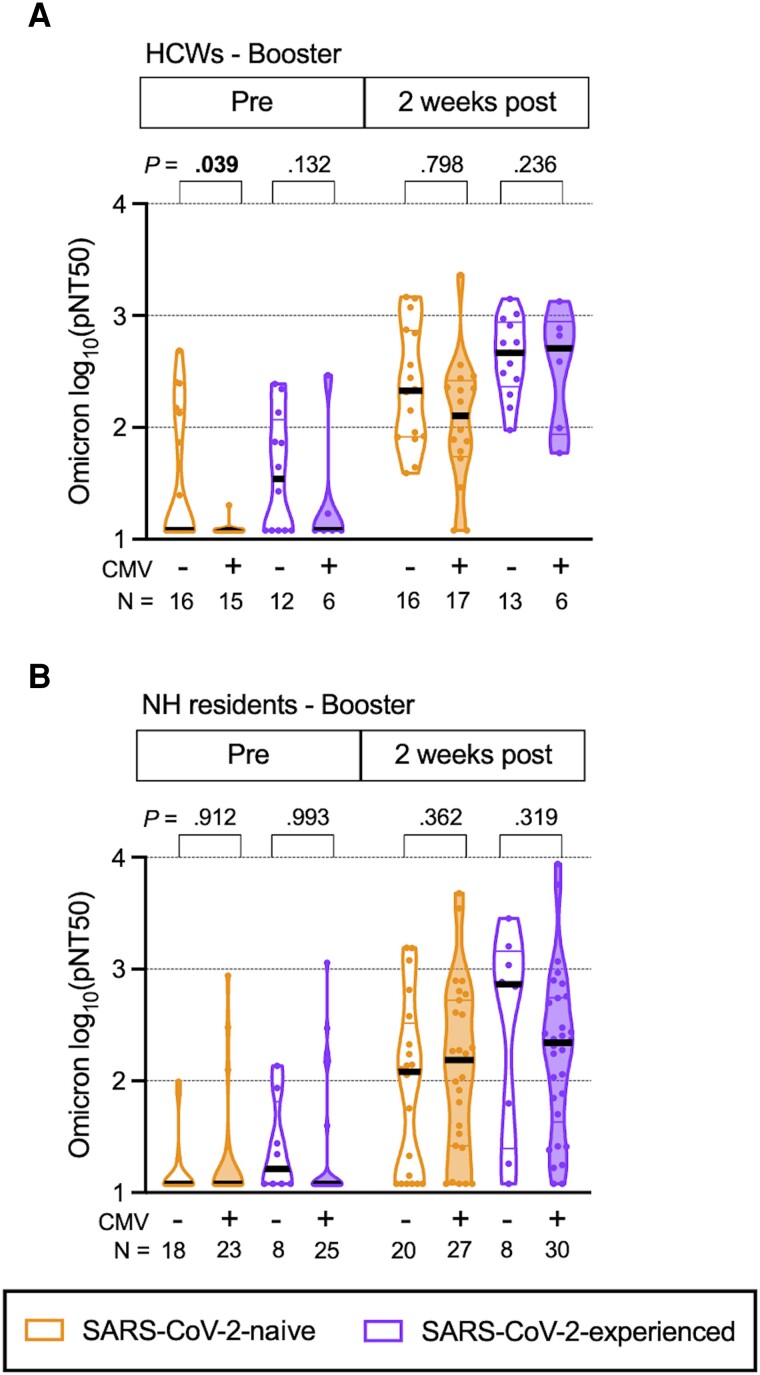
Because neutralizing Ab titers to the early Omicron subvariant BA.1 do not readily develop among HCWs or NH residents until after a booster dose [15], we tested whether CMV infection was associated with impaired neutralizing Ab titers to the Omicron (BA.1) strain in HCW and NH residents during the booster series. Although CMV+ SARS-CoV-2-naive HCWs had reduced Omicron (BA.1)-neutralizing Ab titers _before their booster dose (P = .039) (Figure 3A), we found no other statistically significant differences in Omicron (BA.1)-neutralizing Ab titers between CMV+ and CMV− donors, whether they were HCWs or NH residents, regardless of SARS-CoV-2 infection history.
Figure 3.
Omicron (BA.1) strain neutralization titers over time in booster series with BNT162b2 mRNA vaccination in healthcare workers (HCW) and nursing home (NH) residents, with and without prior severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) infection. Violin plots show Omicron (BA.1) strain pseudovirus neutralization (pNT50) values during the booster series for (A) HCWs and (B) NH residents. The upper limit of detection of the assay is 1:8748 and the lower limit of detection is 1:12. Participants who were SARS-CoV-2 naive (SARS-CoV-2-naive) are shown in orange, participants with prior SARS-CoV-2 infection (SARS-CoV-2-experienced) are shown in purple, open plots are from cytomegalovirus (CMV) seronegative (CMV−) donors, and filled plots are from CMV seropositive (CMV+) donors. N values indicate the number of participants measured in each group. P values are between CMV− and CMV+, determined by least squares multiple linear regression, adjusted for age, sex, and race. P values less than .05 are considered statistically significant and are depicted in bold font.
Prevaccination Levels of Soluble Inflammatory Mediators
We next considered whether CMV infection-associated inflammation at the time of immunization could have contributed to a differential vaccine efficacy in our cohort. Using available prevaccination samples, we measured 4 factors that, although not unique to CMV, have a demonstrated association with CMV infection: IL-6, CRP, sTNFR-I, and sTNFR-II [19–23]. Although we found that NH residents had significantly elevated levels of all 4 analytes compared with HCWs (Table 1), the only difference we observed among NH residents was that CMV− NH residents had significantly higher CRP levels than CMV+ NH residents (Table 2). We found no differences among HCWs whether we stratified by CMV or EBV serostatus, or prior SARS-CoV-2 experience (Table 2, Supplementary Tables 1 and 2). We also found no correlations among all HCWs or NH residents between any of the analytes and neutralizing Ab titers 2 weeks after the primary series (data not shown).
DISCUSSION
Cytomegalovirus, a highly prevalent pathogen, has infected approximately half the adults in the United States, and approximately 100% of adults elsewhere [24, 25]. Although primary CMV infection is usually mild or asymptomatic, the virus is retained in a latent state and is never fully cleared from the host. Cytomegalovirus reactivation from latency can be a major problem in settings of immune compromise, such as in transplant recipients or in those with untreated human immunodeficiency virus infection. Even in the absence of overt reactivation, carriage of CMV is associated with inflammation and numerous complications and comorbidities, suggesting that long-term effects of CMV can lead to poor health outcomes. Indeed, in older persons, having CMV is a major component of the immune risk phenotype, which is associated with reduced longevity [26]. However, latent CMV infection may also protect the host from subsequent pathogen challenge, as has been observed in mouse models of bacterial and viral infection, but this protection has been shown to wane over time [9, 10].
How CMV infection affects vaccine immunogenicity is unknown. One report suggests that inflammatory signals caused by latent CMV infection may activate the immune system, and thereby improve antibody titers with influenza vaccination [9], but this effect may be temporary. Other studies have shown conflicting results, with CMV being associated with impaired influenza vaccine responses in both older and younger adults [27–33], possibly via reduced B-cell activity [31], or no having no effect [34, 35], and all of these may be confounded by various levels of pre-existing immunity to seasonal influenza strains in the vaccine recipients [36]. Few studies to date have examined CMV and SARS-CoV-2 vaccine immunogenicity, none of which have found a substantial association of CMV serostatus with vaccine-elicited antibody responses in either younger or older adults [37–39].
In this report, we investigated the association of latent CMV infection with Pfizer/BioNTech BNT162b2 mRNA vaccine immunogenicity in NH residents and HCWs who either had or did not have prior SARS-CoV-2 infection. We measured vaccine responses in 3 ways and found lower Ab titers in the serum of CMV+ HCWs than in serum from CMV− HCWs 2 weeks after the primary vaccination series, even after adjustment for age, sex, and race. The difference disappeared after the booster dose and was absent among HCWs who had prior SARS-CoV-2 experience. Thus, in this population of adult HCWs, latent CMV infection is associated with a diminished response to SARS-CoV-2 spike protein, primarily when presented a neoantigen having only been encountered in the context of the vaccine. In SARS-CoV-2-experienced HCWs, who had previously been infected with SARS-CoV-2 and for whom spike is not a neoantigen, CMV infection did not appear to greatly affect vaccine-enhanced spike-specific recall responses. Indeed, for SARS-CoV-2-experienced HCWs who have encountered spike at least 3 times (prior infection, primary vaccination, boost), we actually observed a small but significant increase in anti-spike Ab titers in CMV+ donors. Unexpectedly, however, NH residents—who have elevated levels of systemic inflammatory mediators compared with HCWs before vaccination—did not exhibit CMV-associated reduced Ab titers 2 weeks after either the primary vaccination series or the booster series but did have a small but significant reduction in neutralizing Ab titers 6 months after primary vaccination. These results suggest that despite their overall elevated inflammatory profile compared with HCWs, CMV carriage in older individuals does not seem to substantially impair mRNA vaccine immunogenicity.
Epstein-Barr virus is another very common herpesvirus associated with comorbidities, including recent mechanistic evidence of a role for EBV in the development of multiple sclerosis [40, 41]. Epstein-Barr virus establishes latent infection in B cells, making it plausible that latent EBV infection could affect vaccine-induced Ab titers. Because so few of our donors were seronegative for EBV (NNH = 6; NHCW = 9), and because we saw no substantial demographic differences between EBV− and EBV+ donors (Supplementary Table 1), we did not further assess the effect of EBV on Ab titers after vaccination.
This report is not without limitations. First, we had a relatively small sample size for each group. This hinders our power to test the association of EBV serostatus with vaccine efficacy because of the high seroprevalence in the study populations. Second, not all subjects are represented at each time point, limiting our ability to make longitudinal assessments of vaccine activity. Third, our NH residents are disproportionately male due to the recruitment from the largely male population at the Veterans' nursing home. Although our analyses were controlled for age, sex, and race, these discrepancies limit the generalizability of our findings. Fourth, SARS-CoV-2-experienced NH residents may have been asymmetrically culled by lethal infection, because prevaccine a substantial proportion of these vulnerable individuals died from infection. This survivor effect might be an explanation for the significantly increased proportion of CMV seropositivity among SARS-CoV-2-experienced NH residents. Fifth, we only measured surrogate immunologic readouts of protection and did not track rates of postvaccination breakthrough infections. Finally, we did not assess T-cell immunity in these assays. Given the profound effect of latent CMV infection on T-cell responses [42, 43], it will be of interest to measure the association of CMV serostatus with vaccine-induced T-cell responses in these donors and in other well characterized vaccine cohorts.
CONCLUSIONS
Our results demonstrate that latent CMV infection has a detrimental effect on BNT162b2 mRNA vaccine responsiveness in a well characterized cohort of HCWs when spike protein is a neoantigen. This may leave CMV+ adult vaccine recipients more susceptible to SARS-CoV-2 breakthrough infections after the primary series than CMV− vaccinees. Because CMV seroprevalence tends to increase with age, our findings also may identify a contributor to the lower Ab titers seen in older adult populations in other studies, compared with titers in younger individuals. It is notable that CMV+ study participants did mount robust Ab responses to a third vaccination, including neutralizing Ab titers to Omicron (BA.1), offering support for primary and booster vaccinations for everyone, including those with latent CMV infection.
Supplementary Material
Acknowledgments
We thank Michael Chicchelly, Brian Clagett, Dominic Dorazio, and Lenore Carias for excellent technical assistance and Brigid Wilson for statistical and data support.
Author contributions. MLF, OAO, JB, and DHC had full access to all the data in the study and take responsibility for the integrity of the data and the accuracy of the data analyses. MLF, MML, and DHC contributed to concept and design. MLF, OAO, DM, MP, MLS, and JB contributed to acquisition, analysis, or interpretation of data. MLF, MML, and DHC contributed to drafting the manuscript. All authors contributed to critical revision of the manuscript for important intellectual content. MLF and JB contributed to statistical analysis. ABB, CLK, MML, and DHC obtained funding. ABB, CLK, SG, and DHC contributed to administrative, technical, or material support. ABB, CLK, SG, MML, and DHC supervised the work.
Disclaimer. The funding organizations had no role in the design and conduct of the study; collection, management, analysis, and interpretation of the data; preparation, review, or approval of the manuscript; and decision to submit the manuscript for publication.
Financial support. This work was supported by the National Institute on Drug Abuse Avenir New Innovator Award DP2DA040254, the MGH Transformative Scholars Program, and a Massachusetts Consortium on Pathogenesis Readiness (MassCPR) Grant (to ABB); National Institutes of Health National Institute of Allergy and Infectious Diseases (AI129709-03S1) and the National Cancer Institute (CA260539-01) (to CLK); the Richard J. Fasenmyer Foundation (to MML); US Centers for Disease Control and Prevention (200-2016-91773); and the US Department of Veterans Affairs (BX005507-01; to DHC).
Contributor Information
Michael L Freeman, Division of Infectious Diseases and HIV Medicine, Department of Medicine, Case Western Reserve University, Cleveland, Ohio, USA.
Oladayo A Oyebanji, Division of Infectious Diseases and HIV Medicine, Department of Medicine, Case Western Reserve University, Cleveland, Ohio, USA.
Daniela Moisi, Division of Infectious Diseases and HIV Medicine, Department of Medicine, Case Western Reserve University, Cleveland, Ohio, USA.
Michael Payne, Center for Global Health and Diseases, Department of Pathology, Case Western Reserve University, Cleveland, Ohio, USA.
Maegan L Sheehan, Ragon Institute of MGH, MIT, and Harvard, Cambridge, Massachusetts, USA.
Alejandro B Balazs, Ragon Institute of MGH, MIT, and Harvard, Cambridge, Massachusetts, USA.
Jürgen Bosch, Department of Health Services, Policy, and Practice, Brown University School of Public Health, Providence, Rhode Island, USA.
Christopher L King, Center for Global Health and Diseases, Department of Pathology, Case Western Reserve University, Cleveland, Ohio, USA.
Stefan Gravenstein, Department of Health Services, Policy, and Practice, Brown University School of Public Health, Providence, Rhode Island, USA; Center on Innovation in Long-Term Services and Supports, Providence Veterans Administration Medical Center, Providence, Rhode Island, USA; Division of Geriatrics and Palliative Medicine, Alpert Medical School of Brown University, Providence, Rhode Island, USA.
Michael M Lederman, Division of Infectious Diseases and HIV Medicine, Department of Medicine, Case Western Reserve University, Cleveland, Ohio, USA.
David H Canaday, Division of Infectious Diseases and HIV Medicine, Department of Medicine, Case Western Reserve University, Cleveland, Ohio, USA; Louis Stokes Cleveland Department of Veterans Affairs Medical Center, Geriatric Research Education and Clinical Center, Cleveland, Ohio, USA.
Supplementary Data
Supplementary materials are available at Open Forum Infectious Diseases online. Consisting of data provided by the authors to benefit the reader, the posted materials are not copyedited and are the sole responsibility of the authors, so questions or comments should be addressed to the corresponding author.
References
- 1. Arons MM, Hatfield KM, Reddy SC, et al. Presymptomatic SARS-CoV-2 infections and transmission in a skilled nursing facility. N Engl J Med 2020; 382:2081–90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Alanio C, Verma A, Mathew D, et al. Cytomegalovirus latent infection is associated with an increased risk of COVID-19-related hospitalization. J Infect Dis 2022; 226:463–73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Shrock E, Fujimura E, Kula T, et al. Viral epitope profiling of COVID-19 patients reveals cross-reactivity and correlates of severity. Science 2020; 370:eabd4250. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Paolucci S, Cassaniti I, Novazzi F, et al. EBV DNA increase in COVID-19 patients with impaired lymphocyte subpopulation count. Int J Infect Dis 2021; 104:315–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Im JH, Nahm CH, Je YS, et al. The effect of Epstein-Barr virus viremia on the progression to severe COVID-19. Medicine (Baltimore) 2022; 101:e29027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Zubchenko S, Kril I, Nadizhko O, Matsyura O, Chopyak V. Herpesvirus infections and post-COVID-19 manifestations: a pilot observational study. Rheumatol Int 2022; 42:1523–30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Gold JE, Okyay RA, Licht WE, Hurley DJ. Investigation of long COVID prevalence and its relationship to Epstein-Barr virus reactivation. Pathogens 2021; 10:763. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Su Y, Yuan D, Chen DG, et al. Multiple early factors anticipate post-acute COVID-19 sequelae. Cell 2022; 185:881–95.e20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Furman D, Jojic V, Sharma S, et al. Cytomegalovirus infection enhances the immune response to influenza. Sci Transl Med 2015; 7:281ra243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Barton ES, White DW, Cathelyn JS, et al. Herpesvirus latency confers symbiotic protection from bacterial infection. Nature 2007; 447:326–9. [DOI] [PubMed] [Google Scholar]
- 11. van den Berg SPH, Wong A, Hendriks M, Jacobi RHJ, van Baarle D, van Beek J. Negative effect of age, but not of latent cytomegalovirus infection on the antibody response to a novel influenza vaccine strain in healthy adults. Front Immunol 2018; 9:82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Nicoli F, Clave E, Wanke K, et al. Primary immune responses are negatively impacted by persistent herpesvirus infections in older people: results from an observational study on healthy subjects and a vaccination trial on subjects aged more than 70 years old. EBioMedicine 2022; 76:103852. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Tartof SY, Slezak JM, Puzniak L, et al. Durability of BNT162b2 vaccine against hospital and emergency department admissions due to the omicron and delta variants in a large health system in the USA: a test-negative case-control study. Lancet Respir Med 2022; 10:689–99. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Canaday DH, Carias L, Oyebanji OA, et al. Reduced BNT162b2 messenger RNA vaccine response in severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2)-naive nursing home residents. Clin Infect Dis 2021; 73:2112–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Canaday DH, Oyebanji OA, White E, et al. COVID-19 vaccine booster dose needed to achieve omicron-specific neutralisation in nursing home residents. EBioMedicine 2022; 80:104066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Canaday DH, Oyebanji OA, Keresztesy D, et al. Significant reduction in vaccine-induced antibody levels and neutralization activity among healthcare workers and nursing home residents 6 months following coronavirus disease 2019 BNT162b2 mRNA vaccination. Clin Infect Dis 2022; 75:e884–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Garcia-Beltran WF, Lam EC, St Denis K, et al. Multiple SARS-CoV-2 variants escape neutralization by vaccine-induced humoral immunity. Cell 2021; 184:2523. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Garcia-Beltran WF, St Denis KJ, Hoelzemer A, et al. mRNA-based COVID-19 vaccine boosters induce neutralizing immunity against SARS-CoV-2 omicron variant. Cell 2022; 185:457–66.e4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Blankenberg S, Rupprecht HJ, Bickel C, et al. Cytomegalovirus infection with interleukin-6 response predicts cardiac mortality in patients with coronary artery disease. Circulation 2001; 103:2915–21. [DOI] [PubMed] [Google Scholar]
- 20. Muhlestein JB, Horne BD, Carlquist JF, et al. Cytomegalovirus seropositivity and C-reactive protein have independent and combined predictive value for mortality in patients with angiographically demonstrated coronary artery disease. Circulation 2000; 102:1917–23. [DOI] [PubMed] [Google Scholar]
- 21. Montag C, Wagner J, Gruska I, Hagemeier C. Human cytomegalovirus blocks tumor necrosis factor alpha- and interleukin-1beta-mediated NF-kappaB signaling. J Virol 2006; 80:11686–98. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Freeman ML, Mudd JC, Shive CL, et al. CD8 T-cell expansion and inflammation linked to CMV coinfection in ART-treated HIV infection. Clin Infect Dis 2016; 62:392–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Limaye AP, Stapleton RD, Peng L, et al. Effect of ganciclovir on IL-6 levels among cytomegalovirus-seropositive adults with critical illness: a randomized clinical trial. JAMA 2017; 318:731–40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Bates M, Brantsaeter AB. Human cytomegalovirus (CMV) in Africa: a neglected but important pathogen. J Virus Erad 2016; 2:136–42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Cannon MJ, Schmid DS, Hyde TB. Review of cytomegalovirus seroprevalence and demographic characteristics associated with infection. Rev Med Virol 2010; 20:202–13. [DOI] [PubMed] [Google Scholar]
- 26. Pawelec G, Akbar A, Caruso C, Solana R, Grubeck-Loebenstein B, Wikby A. Human immunosenescence: is it infectious? Immunol Rev 2005; 205:257–68. [DOI] [PubMed] [Google Scholar]
- 27. Trzonkowski P, Mysliwska J, Szmit E, et al. Association between cytomegalovirus infection, enhanced proinflammatory response and low level of anti-hemagglutinins during the anti-influenza vaccination–an impact of immunosenescence. Vaccine 2003; 21:3826–36. [DOI] [PubMed] [Google Scholar]
- 28. Alonso Arias R, Moro-Garcia MA, Echeverria A, Solano-Jaurrieta JJ, Suarez-Garcia FM, Lopez-Larrea C. Intensity of the humoral response to cytomegalovirus is associated with the phenotypic and functional status of the immune system. J Virol 2013; 87:4486–95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Derhovanessian E, Theeten H, Hähnel K, Van Damme P, Cools N, Pawelec G. Cytomegalovirus-associated accumulation of late-differentiated CD4 T-cells correlates with poor humoral response to influenza vaccination. Vaccine 2013; 31:685–90. [DOI] [PubMed] [Google Scholar]
- 30. Derhovanessian E, Maier AB, Hähnel K, McElhaney JE, Slagboom EP, Pawelec G. Latent infection with cytomegalovirus is associated with poor memory CD4 responses to influenza A core proteins in the elderly. J Immunol 2014; 193:3624–31. [DOI] [PubMed] [Google Scholar]
- 31. Frasca D, Diaz A, Romero M, Landin AM, Blomberg BB. Cytomegalovirus (CMV) seropositivity decreases B cell responses to the influenza vaccine. Vaccine 2015; 33:1433–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Turner JE, Campbell JP, Edwards KM, et al. Rudimentary signs of immunosenescence in cytomegalovirus-seropositive healthy young adults. Age (Dordr) 2014; 36:287–97. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Wald A, Selke S, Magaret A, Boeckh M. Impact of human cytomegalovirus (CMV) infection on immune response to pandemic 2009 H1N1 influenza vaccine in healthy adults. J Med Virol 2013; 85:1557–60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. den Elzen WP, Vossen AC, Cools HJ, Westendorp RG, Kroes AC, Gussekloo J. Cytomegalovirus infection and responsiveness to influenza vaccination in elderly residents of long-term care facilities. Vaccine 2011; 29:4869–74. [DOI] [PubMed] [Google Scholar]
- 35. Haq K, Fulop T, Tedder G, et al. Cytomegalovirus seropositivity predicts a decline in the T cell but not the antibody response to influenza in vaccinated older adults independent of type 2 diabetes status. J Gerontol A Biol Sci Med Sci 2017; 72:1163–70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Jackson SE, Redeker A, Arens R, et al. CMV immune evasion and manipulation of the immune system with aging. Geroscience 2017; 39:273–91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Sharpe HR, Provine NM, Bowyer GS, et al. CMV-associated T cell and NK cell terminal differentiation does not affect immunogenicity of ChAdOx1 vaccination. JCI Insight 2022; 7:e154187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Breznik JA, Huynh A, Zhang A, et al. Cytomegalovirus seropositivity in older adults changes the T cell repertoire but does not prevent antibody or cellular responses to SARS-CoV-2 vaccination. J Immunol 2022; 209:1892–905. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Jo N, Hidaka Y, Kikuchi O, et al. Impaired CD4+ T cell response in older adults is associated with reduced immunogenicity and reactogenicity of mRNA COVID-19 vaccination. Nature Aging 2023; 3:82–92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Bjornevik K, Cortese M, Healy BC, et al. Longitudinal analysis reveals high prevalence of Epstein-Barr virus associated with multiple sclerosis. Science 2022; 375:296–301. [DOI] [PubMed] [Google Scholar]
- 41. Lanz TV, Brewer RC, Ho PP, et al. Clonally expanded B cells in multiple sclerosis bind EBV EBNA1 and GlialCAM. Nature 2022; 603:321–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Klenerman P, Oxenius A. T cell responses to cytomegalovirus. Nat Rev Immunol 2016; 16:367–77. [DOI] [PubMed] [Google Scholar]
- 43. van den Berg SPH, Pardieck IN, Lanfermeijer J, et al. The hallmarks of CMV-specific CD8 T-cell differentiation. Med Microbiol Immunol 2019; 208:365–73. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.