Abstract
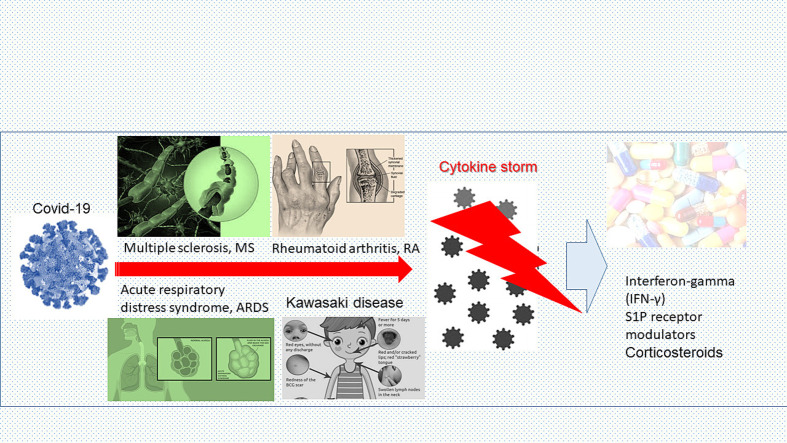
SARS-CoV-2 virus has attracted a lot of attention globally due to the autoimmune and inflammatory processes that were observed during the development of Covid-19 disease. Excessive activation of immune response and triggering of autoantibodies synthesis as well as an excessive synthesis of inflammatory cytokines and the onset of cytokine storm has a vital role in the disease outcome and the occurring autoimmune complications. This scenario is reminiscent of infiltration of lymphocytes and monocytes in specific organs and the increased production of autoantibodies and chemoattractants noted in other inflammatory and autoimmune diseases. The main goal of this study is to investigate the complex inflammatory processes that occur in Covid-19 disease and to find similarities with other inflammatory diseases such as multiple sclerosis (MS), acute respiratory distress syndrome (ARDS), rheumatoid arthritis (RA) and Kawasaki syndrome to advance existing diagnostic and therapeutic protocols. The therapy with Interferon-gamma (IFN-γ) and the use of S1P receptor modulators showed promising results. However, there are many unknowns about these mechanisms and possible novel therapies. Therefore, the inflammation and autoimmunity triggered by Covid-19 should be further investigated to improve existing diagnostic procedures and therapeutic protocols for Covid-19.
Keywords: Autoimmune diseases, Covid-19, Inflammatory reaction, Therapeutic protocols, Immunological reaction, Cytokine storm, B-cell tolerance and T-cell tolerance
Graphical abstract
1. Introduction
The immune system represents a wide palette of different cell types that create complex networks in their interplay, which participate in the body's defence against various pathogens, including bacteria, viruses, tumour cells and other foreign substances. When the immune system mistakenly starts attacking its own tissues and starts creating antibodies against its cells, autoimmune diseases occur. Some autoimmune diseases lead to the damage of only one organ, such as autoimmune orchitis, while other autoimmune diseases can affect the whole organism, such as rheumatoid arthritis. However, the exact cause of autoimmune diseases can often not be determined [1]. In most cases, the appearance of the disease is triggered by many different factors and their combined effects. Among them are viruses, which make this a very interesting phenomenon for observation during the pandemic caused by SARS-CoV-2 virus. Interestingly, disturbances of the immune system have often been reported during or after coronavirus attacks on an organism, which further causes the onset of autoimmune phenomena [2].
Research results presented in the most famous journals and articles after the beginning of this new dangerous pandemic confirmed the connection that exists between many autoimmune diseases and Covid-19 disease. It was shown that Covid-19 induces the activation of different immune cells and increases the synthesis of pro-inflammatory cytokines as well as autoantibodies. The cytokine molecules such as IL-6 and TNF- α can affect endothelial and immune cells in different organs in the body such as the testis, kidney, liver and lungs. Consequently, this leads to the development of more severe forms of Covid-19 as well as the onset of life-threatening complications, such as ARDS or encephalitis [3]. These inflammatory and autoimmune disorders triggered by the Covid-19 virus can be seen in a wide range from only causing the synthesis of autoantibodies to the development of autoimmune diseases. Therefore, the wide palette of specific, general symptoms and signs of the Covid-19 pandemic caused by the changes in immune system including antibody production and the autoimmune characteristics occuring in some patients with Covid-19 infection are particularly interesting for researchers and clinicans [4]. Furthermore, it is highlighted that the impact of coronavirus infection can be manifested systemically in already existing autoimmune diseases such as arthritis or it can have newly diagnosed organ-specific manifestations such as haematological or neurological manifestations [5].
In this context, systematic reviews are being conducted to analyze current data on emerging autoimmune diseases, which are noted during or after the infection with the Covid-19 virus [6], [7]. The search strategy consisted of the research of relevant publications in the indexed databases PubMed and Scopus. Additionally, the related citations and references used in the evaluated articles were also considered for inclusion. Publications were searched by the use of the following terms and keywords: “Coronavirus”, “COVID-19”, “Hypercytokinemia”, “Renin-angiotensin system”, “Immune response, specific”, “Immune response, non-specific”, “Molecular mimicry”, “Immune tolerance”, “Autoantibodies”, “Rheumatoid arthritis”, “Vasculitis”, “Antiphospholipid Syndrome”, “Systemic Vasculitis”, “Orchitis”, “Interstitial lung disease”, “Multisystem inflammatory syndrome, children”, “Multisystem inflammatory syndrome, adults”, “Lupus Erythematosus” and “Systemic”. Publications were excluded if they did not review or research the relationship between autoimmunity and Covid-19. The goals of this review are to analyze the different impacts of the coronavirus on specific examples of autoimmune diseases, but also to give insight into novel pharmacological treatments that can be used in their treatment such as the therapy with Interferon-gamma (IFN-γ) or the sphingosine-1-phosphate (S1P) receptor modulators. Reviews like this can be a very helpful addition to healthcare professionals in daily work and set a good basis for further research and clinical trials.
1.1. Interplay between Covid-19 infection and host's immune system
One of the common characteristics between the infection caused by the coronavirus and other viruses is that they all have the ability to activate different types of immune responses. The first barrier that stands in the way of pathogen organisms including viruses is called the innate immune response. In some cases, the reaction of the host's immune system is not proportional to the threat itself that consequently leads to the development of a very dangerous hyperinflammatory state, which is characterized by an increased level of cytokines that enhance inflammation. This dangerous phenomenon is marked as a cytokine storm and it was mostly recorded in patients who have progressed to a more severe level of Covid-19 infection [8]. Nevertheless, this type of cytokine storm is different from the cytokine storm caused by other viruses [9]. After the SARS-CoV-2 virus enters the host's cell, the cytokine storm that occurs does not cause activation of all types of cytokines [10]. Interestingly, when it comes to the infection with Covid-19, the main attribute of the cytokine storm is a pathological release of a certain group of cytokines in the serum of an infected person and this applies to interleukin IL-2 and IL-7 [11].
Such a high concentration of cytokines leads to the increase of the factor that describes granulocyte-macrophage colonies (GM-CSF) but it also activates the granulocyte colony factor (G-CSF). Additionally, the activation of gamma-induced protein 10 (IP10) by interferons was also observed. The increased presence of cytokines caused by this infection activates the macrophage inflammatory protein 1a (MIP1a) that is an important chemoattractant of monocytes. In many cases where cytokine storm developed, two parameters, interferon-gamma (IFN-Ɣ) and factor α of tumour necrosis (TNF-α) were elevated. In patients with a severe clinical presentation, an increased concentration of chemokines was observed. Interestingly, it almost seems that it is specifically aiming to destabilize the type I response mediated with interferons [8]. The main thing that characterizes the defence against this infection is the increase in levels of interferons, which represents important molecules in the chain of activation of the innate immune response. A prolonged immune response was also observed with Covid-19, as well as it was observed with MERS (Middle East Respiratory Syndrome) and SARS (Severe Acute Respiratory Syndrome). This immune response indicates that it is a virus-induced mechanism that facilitates the breakthrough of a virus in cells of an attacked host [12].
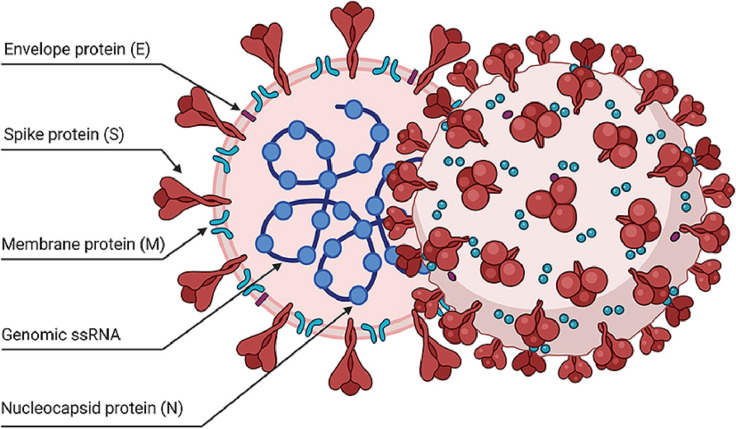
This sequence of events within the immune system enhances the penetration of the virus into the epithelial cells of the lungs, which eventually results in a strong inflammatory response, mostly caused by the activation of a large number of cells, which are a part of non-specific immunity. Fig. 1 shows the structure of the coronavirus that has also been used as a base to start the process of development of vaccines against this virus. The coronavirus has a very specific strategy for achieving its harmful actions, in contrast to similar viruses that attack respiratory organs, such as the virus that causes influenza, marked as type A (IVA). In diseases caused by other viruses, almost a complete reduction of IFNs or chemokines at the time of infection was noted. Unlike them, SARS-CoV-2 has only a weakened IFN immune response, although it shows completely unexpected elevation of different chemokines. This specific compartmentalization that is noted with innate immune response in Covid-19 infection may be the main weapon that this virus uses for an invasion of the respiratory system and to enable viral replication. The consequent increase in a viral load in the affected cells of the epithelium of the host can be the cause of tissue injury and could result in an additional increase in the release of chemokines, as well as in the enhancement of the action of other circulating immune cells. A non-specific immune response would completely and unexpectedly interfere with virus shedding and support further virus replication [8].
Fig. 1.
The graphic displays the structure of SARS-CoV-2 virus particles and its most important structural parts [13].
Optimal specific responses of host's immunity, which can be either mediated by T cell or humoral are characteristic of both asymptomatic patients as well as mildly symptomatic patients. In contrast, patients with severe early-stage Covid-19 expressed prolonged humoral-mediated or/and T cell-mediated protective responses of immunity. It should be noted that the surviving patients had a strong and lasting specific immune response after Covid-19 infection. Additionally, in cases with more expressed and progressive symptoms of Covid-19 infection, a rise in the activation of CD4+ T cells and T helper 7 (Th7) cells was observed, which is a confirmation of the existing increased concentration of cytokines in their organisms [14]. On the contrary, this phenomenon does not occur in patients who are asymptomatic or have a mild clinical picture. It is also interesting that the granulocyte-macrophage colony-stimulating factor (GM-CSF) is highly increased only in more serious cases of infection with SARS CoV-2 and it is not elevated in mildly symptomatic cases. This was not observed when the cause of the infection was the influenza virus [6].
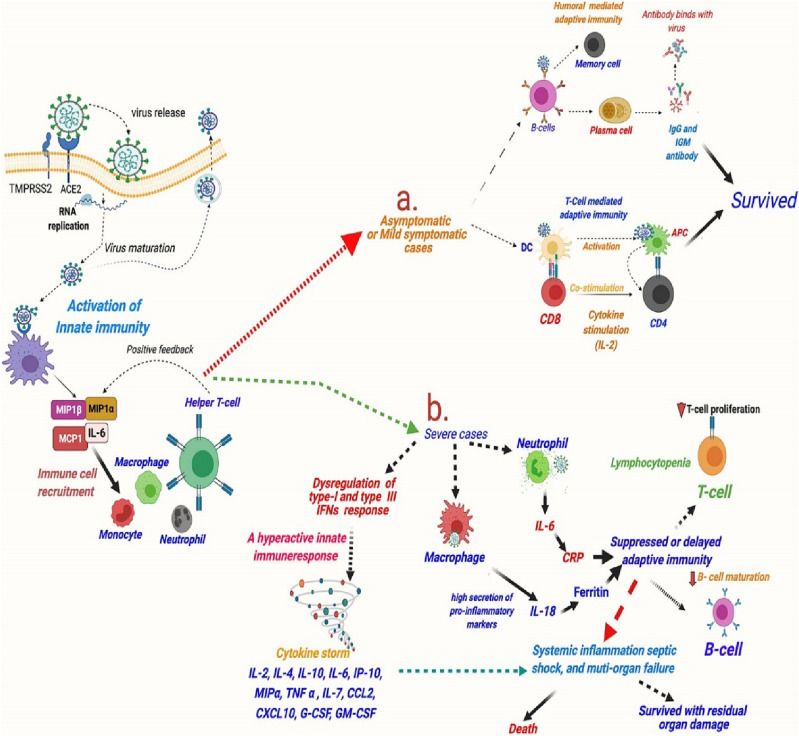
Fig. 2 shows the immune response in asymptomatic patients with mild clinical manifestation and patients who have a more severe clinical picture during or after the infection with Covid-19 [8]. One common feature that is noted, whether the patient had a mild or severe case of Covid-19 is lymphocytopenia, which is especially affecting T cells and a special group of T cells, extremely important for the antiviral activity, called CD8 T cells. Lymphocytopenia is interdependent with the severity of symptoms and a very low number of T cells can serve as a predictor of a poor outcome for the patient. However, the molecular factors responsible for these processes and the activation of the process of killing T cells in infection with this virus are currently unclear [15]. Other common features that are noted in patients with Covid-19 infection, especially in more severe cases, are the onset of the cytokine storm due to the increased synthesis of pro-inflammatory cytokines and the release of interleukins as well as the activation of coagulation pathways and increased development of thrombotic complications [15]. Another mechanism caused by the virus in infected persons that leads to the death of T cells and includes decreased numbers of SARS-CoV-2 entry receptors [16]. In recent studies, it has been proven that SARS-CoV-2 induces lesions and atrophy of lymphoid tissue in human organisms such as the lymph nodes and spleen, consequently lowering the production and effects of T cells [17].
Fig. 2.
The activation of innate and adaptive immune response and cytokine release in asymptomatic, mild clinical and severe clinical manifestation [8].
Laboratory parameters of patients with SARS-CoV-2 infection indicate that increased levels of cytokines may be one of the main reasons standing behind the death of T cells. It was also confirmed that levels of TNF, IL-, IL-8 and IL-10 were higher and associated with lower T-cell counts in severe Covid-19. A review of the available information leads to the conclusion that improved replication of this virus when compared to other viruses such as the influenza virus or other viruses that attack the respiratory system is an important factor that contributes to the increasing risk of mortality and greater virulence of SARS-CoV 2 infection. Replication also contributes to a greater burden and dysregulation of the specific and non-specific immune response. This could also be seen that controlling replication and reducing it by applying appropriate therapy such as antiviral therapy or managing the dysregulated immune response of the host cell by using immunomodulatory therapy is the key to adequate therapy of infection with Covid-19 [8].
1.2. Pathogenesis of Covid-19
1.2.1. Invasion of host cells
Covid-19 virus has the ability to enter into host cells that is one of the most important components of its infectivity and pathogenesis. Entry into the host cell is a major component of interest for the development of adequate, fast human intervention plans that could also boost immunity in the host organism. The first step in the process of invading host cell is binding to a cell receptor. After binding to the receptor that is placed on the attacked cell surface, the virus enters the endosomes. Afterwards, the viral and lysosomal membranes join. The spike protein (S protein) that is expressed on the surface of virus is the main component that participates in the entry of the coronavirus. The viral spike protein is in the form of a trimer. Namely, it has three heads marked as S1 whose role is to connect with the receptor expressed on the host cell. S1 heads are located on top of the membranous S2 stalk [18]. Since the entry of the coronavirus into the host cell is one of the important components of Covid-19 pathogenic potential, this entry mechanism has been thoroughly investigated.
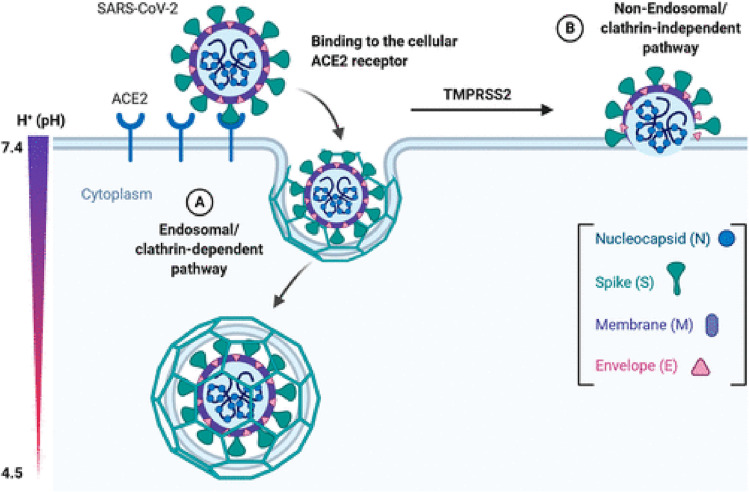
SARS-CoV subunit, marked as subunit S1 on the surface of this virus, binds to its specific receptor and the further role of this completely formed receptor is to recognize the enzyme that converts angiotensin 2 (ACE2) as its receptor. It is a lock that needs to be opened so that the virus enters the targeted cell. The S2 subunit has the function of joining two membranes - the host cell's membrane and the membrane of the virus. To finally fuse the membranes, the activation of spikes at interface of S1 and S2 subunits on the virus is needed. Afterwards, S1 dissociates and S2 has a major structural change in its form. Proteases produced by the SARS-CoV virus additionally activate primarily proteases placed on the surface of the cell (TMPRSS2) and lysosomal proteases cathepsins, which are the priming spike proteins of the virus. This enables the process of degradation of the two membranes and then allows their fusion into one new membrane through a complex process of endocytosis [19] that is shown in Fig. 3 .
Fig. 3.
The entry of SARS-CoV-2 virus in the cell by the interaction of S1 and S2 proteins with receptor-binding domain for angiotensin-converting enzyme 2 [20].
The main cause that leads to symptoms such as fever, headache and respiratory symptoms is the multiplication and release of this virus in the upper respiratory organs. It has been experimentally proven that the virus also causes temporary damage to the olfactory epithelium that results in a temporary dysfunction of the sense of smell. The temporary loss of taste can be explained similarly [21]. The position of ACE 2 receptor in certain tissues explains why Covid-19 virus is targeting more aggressively some tissues over others. If ACE 2 receptor is located on the epithelium of various organs such as endothelial cells of lungs, heart, kidneys, blood vessels and intestines that could explain the enhanced manifestations of various symptoms connected with that organ. Therefore, the placement of ACE 2 receptors is precisely what is underlying cardiovascular complications and gastrointestinal symptoms that occur when human organisms is attacked by this virus. Frequent damage was also observed at post-mortem pathological examinations and the effected organs were mostly the heart, liver and kidneys. This confirms that the virus can directly effect the function of many different organs not only the respiratory tract [22].
Recently, novel routes of Covid-19 entry and invasion of the host cells are being researched such as the interaction of virus with transmembrane protein called cluster of differentiation 147 (CD147). It is proposed that CD147 has an important role in the development of SARS-CoV-2 infection by promoting entry of the virus through endocytosis process. In addition, lower expression or blocking of CD147 was associated with inhibition of SARS-CoV-2 infection. Therefore, the beneficial effects of drugs that interfere with CD147, such as azithromycin are being investigated. However, additional studies are needed to evaluate the dynamic between the effects of CD147 and Covid-19 replication as well as the possible therapy protocols that include these drugs [23].
Another proposed route for the entry of SARS-CoV-2 virus into the host cell is mediated by neuropilin-1 (NRP-1). Neuropilin-1 is a signalling protein expressed on the cell surface that was proven to have the ability to enhance SARS- CoV-2 invasion and dissemination in vitro. The proposed mechanism is the spread of virus through olfactory epithelium into the central nervous system, leading to the development of neurological manifestations seen in patients with Covid-19. It is thought that NRP-1 has an important effect in the cytokine storm often seen in severe cases of Covid-19, possibly by being an immune checkpoint for the memory of T cells. The increased serum levels of NRP-1 have been linked with oxidative stress, inflammation as well as an increased risk of the development of pulmonary thrombosis in these patients. However, there are still many unknowns about the possible role of NRP-1 in the use of antiviral therapy in clinical practice and further studies are needed [24].
A recent study [25] also researched the interaction between the sphingosine-1-phosphate receptor (S1PR) and SARS-CoV-2 virus. It is proposed that the sphingosine-1-phosphate (S1P) has an important role in stabilizing the membrane of endothelial cells. Consequently, improving the maintenance of vascular homeostasis and enhancing the protection against the effects of SARS-CoV-2 virus, especially in central nervous system. The S1P receptor modulators are being researched for their use in the treatment of Covid-19 such as the drug called fingolimod (FTY720). Yet, there are still many unknowns about the possible effects of this therapy and further studies should aim to investigate the effects of S1P analogues on the interaction between the host and SARS-CoV-2.
1.2.2. Initiation of specific immune response
A specific or adaptive immune response is an important factor in organism's defence against viral infections. Specific immune response to the presence of virus is based on the activation of CD4+ T cells, enabling greater manufacture of antibodies that are specific for this virus, through T-dependent activation of B cells. CD8+ T cells are activated. They are cytotoxic and eliminate virus-infected cells. Additionally, increased values of D-dimer and inflammatory cytokines were expressed in patients with more expressed symptoms and signs while the values of T cells and B cells were reduced, which indicates that the immune system cannot adequately regulate the acute phase of infection [26]. A vital role in the pathogenesis of this disease is played by the capacity of coronavirus cell to infect immune system and its different parts. For example, lymphocyte infection has a crucial role in the virally induced pathogenesis of SARS-CoV-2 [27].
1.3. Hypercytokinemia and organ damage
Local secretion of cytokines is considered one of the key pathological correlations responsible for clinical manifestation of ARDS in patients suffering from Covid-19. The results of many studies confirm that cytopathic changes are the main pathological instances in lungs [28]. Therefore, it can be said that severe lung injury occurs in patients with excessive immune response and infection with SARS-CoV-2. Furthermore, excessive activation of immune response as well as an excessive synthesis of inflammatory cytokines and chemical mediators, called cytokine storm has a vital role in the disease outcome. This is one of the most significant factors effecting the severity of Covid-19. It is considered that the severe clinical picture or fatal outcome in patients with Covid-19 disease is connected with excessive secretion of cytokines as well as with severe lymphopenia and thrombosis, but with large infiltration of mononuclear cells in many different organs [29].
The mechanism of cytokines storm is based on the invasion of host cells by SARS-CoV-2. During entry, the spike protein binds to ACE 2 receptor. After entering epithelial cells, the viral cell causes an immune response. A weak interferon response is the cause of excessive cytokines production. Proinflammatory immune responses by Th1 cells, CD14+ and CD16+ monocytes are interfered with by receptors placed on the membrane and downstream signalling pathways. Consequently, the infiltration of macrophages and neutrophils into the lung tissue occurs, resulting in an enormous release of cytokines [35]. It could be seen that infected patients with SARS-CoV-2 have weakened acquired immunity and an uncontrolled innate response to this infection and as a result of such condition, a cytokine storm occurs [11]. Even though the characteristic of Covid-19 disease are mostly respiratory problems, it can damage other systems in the organism and belonging organs such as the heart, liver or kidneys. In a study conducted in Wuhan [28], quite few patients with Covid-19 permanently lost kidney function. The coronavirus binds to the cell via the ACE2 receptor that can be found in tissues of various organs as previously explained. Excessive accumulation of inflammatory cells can lead to damage to the epithelial cells of organs. For these reasons, some patients with this disease require specialized care, sometimes even in intensive care units. Additionally, many studies noted that cytokine storm is one of the most important links in life-threatening spiral that could occur in patients with SARS-CoV-2 infection [28].
1.3.1. Heart damage
Numerous diagnostic procedures discovered that patients tend to develop heart diseases during or after Covid-19. They often have irregular heart rhythms called arrhythmias that is mostly caused by the imbalance of electrolytes, such as hyperkalemia or occurring fever in these patients. Additionally, these damages to the heart muscle can even appear as side effects of drugs used in Covid-19 treatment or the interaction of those drugs. Furthermore, ischemic damage of the heart muscle is noted, mostly because of the hypoxic niche in which heart muscle cells live, because of the onset of pericarditis and myocarditis in infected patients. However, there is a great danger of thrombotic events development, such as pulmonary embolism and deep vein thrombosis, especially in patients with preexisting cardiovascular diseases [30]. The damage to cardiovascular system has also occurred in some patients who did not have heart disease previous to Covid-19 disease but developed it afterwards and the main cause is most likely a cytokine storm [31]. Therefore, it is fundamental to investigate the application of cytokines inhibitor therapy in the hope that these studies will confirm the desired results and widen the therapy options for this disease [28].
1.3.2. Acute respiratory distress syndrome
The lungs are the organ that is most often exposed to the attack of SARS-CoV-2 virus [32]. Pneumonia is a characteristic phenomenon that starts as a result of an infection caused by different types of pathogen microorganisms and it can progress to the appearance of acute respiratory distress syndrome (ARDS) [33]. This can be confirmed on computed tomography by the appearance of a bilateral vitreous appearance. The pathophysiology of ARDS that develops as a consequence of Covid-19 is similar to ARDS that is caused by the actions of other pathogen viruses. Recent studies have noted that neutrophil extracellular traps (NETs), which is a complex network of mitochondrial or nuclear DNA strings and histones have an important role in Covid-19 pathogenesis and the onset of lung complications. Namely, their main role is to capture the pathogens and help their elimination from the host organism. However, in SARS-CoV-2 infection, the formation of NETs and their over-activation is associated with the onset of acute respiratory distress syndrome (ARDS) because of NETs-IL-1β loop and increased synthesis of IL-1β. NETs enhance the development of cytokine storm (CS) in patients with severe Covid-19 as well as the activation of pro-thrombotic cascade [34].
Additionally, the cytokines storm syndrome is a mechanism that leads to accelerated lung injury, mostly caused by the activation of interleukins and activation of coagulation pathways, in which the main role belongs to the thrombin. The main role of thrombin is in complex cascade that results in the creation of a blood clot and prevention of bleeding. This important aspect increases inflammation through receptors activated by proteinases (PAR), especially proteinase 1 that can activate platelets, monocytes and other inflammatory cells [28]. For this reason, it is believed that treatment with PAR 1 antagonists will be very effective in alleviating lung damage caused by a cytokine storm, but additional studies need to be conducted to confirm these drugs as a part of a standard treatment protocol for Covid-19.
1.3.3. Kidney damage
Acute kidney injury is noted in patients with Covid-19 disease, especially in patients who develop more serious symptoms and who are being treated in the intensive care units. It could also be a factor that largely enhances patient's fatal outcome. One of the reasons is cytokine storm that can trigger an inflammatory response, leading to hyperperfusion damage of tubules inside the kidney. The hyperperfusion of the kidney tubules leads to weakening of the function of heart muscle and the onset of cardiorenal syndrome type 1, which is a medical condition described by the simultaneous development of rapid heart failure and acute kidney injury [35]. Direct injury of the host's kidney cells induced by SARS-CoV-2 infection is considered to be an important mechanism that enhances kidney damage because of the activation of ACE 2. It starts a deadly cascade of damaging kidney cells, such as podocytes or cells which form the Bowman's capsule, leading to proteinuria and acute kidney damage [28].
1.3.4. Liver damage
Previously, it was thought that the infection with SARS-CoV-2 virus directly damage the liver. It was thought that it is caused by the onset of viral hepatitis, accompanied by increased levels of bilirubin and aminotransferase in infected patients. However, recent studies show that it is not characteristic that liver injury develops during Covid-19 disease. There is a possibility that the elevated liver enzymes are probably not originating from the liver alone and it is of additional concern that myositis may be the cause of this increase or that the therapy that is being used for this disease might be an underlying initiator of liver damage [28]. On the contrary, substances and cytokines produced in the course of a Covid-19 infection enhance the production of specific proteins in the liver that are responsible for the protection of an organism against pathogens. The increased levels of these proteins could lead to blood clot formation that further leads to the clogging of blood vessels [36]. Consequently, the arrival of oxygen to other organs is prevented that can lead to their failure, the progression of the disease and even death. Nonetheless, new protein modification mechanisms called QTY codes are invented. Structurally, these proteins act similarly to antibodies. They can be injected into the body that could be one of the methods used to treat cytokine storm because they bind to excess cytokines and can slow done or completely inhibit extreme cytokine release [28].
1.3.5. Central nervous system
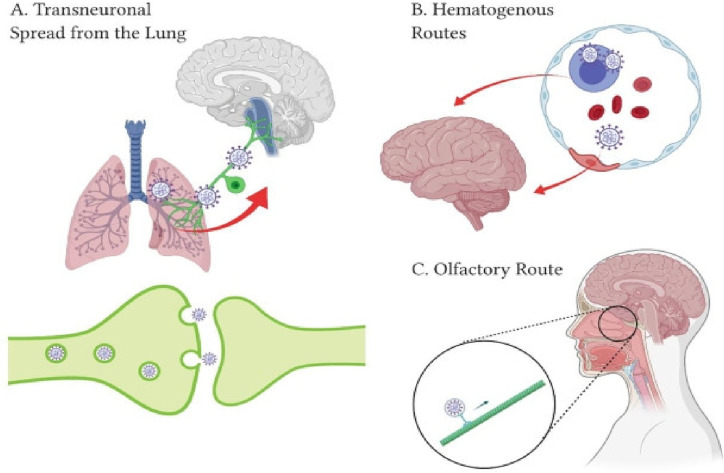
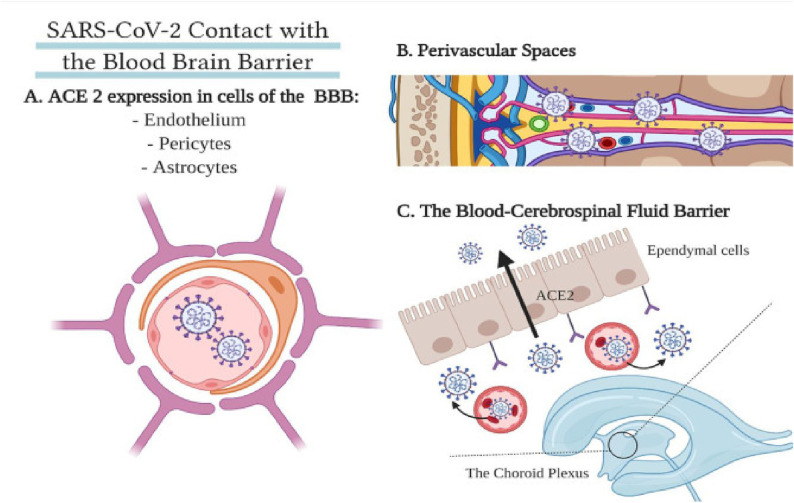
Neurological manifestations such as headache, ataxia and memory impairment are present in a large number of Covid-19 cases, which is an indication that this infection has an important neurological impact. Interestingly, it was noted that these manifestations mostly occurred in patients with more severe clinical picture. Neurological symptoms can occur as a consequence of the damage found in skeletal muscles because of ACE receptors involvement and elevated pro-inflammatory cytokines [12]. Many different mechanisms are proposed as the key to deciphering the involvement of CNS such as spread of virus via synapses through endocytosis and vesicles filled with SARS-CoV-2 travelling through axons. It is well-known that this virus attacks immune system cells, therefore, it is possible that it infiltrates and uses them as a carrier through brain-blood barrier [38].
Furthermore, different studies confirmed that oxidative stress and elevation of inflammatory cytokines can cause neuroinflammation and polyneuropathy [37]. Guillain-Barre syndrome can occur between 5 and 10 days after the first symptoms appear [38]. The multisystem inflammatory syndrome occurs mostly in pediatric population with features that reminiscence to Kawasaki disease. This can also occur in adults, however, rarely noted [39]. More recent studies have shown that glibenclamide has the potential to be a protective agent that decreases the process of neuroinflammation in brain. Glibenclamide is a drug used as a therapy option for the treatment of diabetes mellitus type 2. It can inhibit the adenosine triphosphate (ATP) channels in pancreatic cells in type 1 sulfonylurea receptors (SUR-1). However, it has strong anti-inflammatory properties. Glibenclamide inhibits the function of nod-like receptor pyrin 3 (NLRP3) that is induced by SARS-CoV-2 invasion. This reduces the damage of brain-blood barrier caused by oxidative stress. Therefore, glibenclamide inhibits the activation of NLRP3 inflammasome, lowers oxidative stress as well as the over-activation of microglial cells in the brain tissue and release of pro-inflammatory cytokines [40]. Consequently, the onset of cytokine storm is less likely to occur and the process of neuroinflammation is reduced in patients with neurological manifestations of Covid-19 [41].
1.4. Importance of renin-angiotensin system
Pneumonia caused by Covid-19 virus is atypical pneumonia with uncharacteristic pathophysiological phenomena underlying its development [32]. Interestingly, SARS-CoV-2 utilizes the ACE2 as an entrance to the host cell and thereby induces an effect on the renin-angiotensin system (RAS) [42]. The most important role of RAS is the control of blood pressure. In recent studies, numerous pathological processes conditioned by the classical RAS pathway, in which angiotensin binds to AT1 and AT2 receptors have been studied and described. The protective RAS pathway in which angiotensin connects with MAS receptors and causes dilatation of the blood vessels and has anti-inflammatory effects, may be overlooked and compromised in Covid-19. This could cause excessive stimulation of the classical pathway that can lead to the damage of endothelial cells, respiratory and cardiovascular damage, hypercoagulation, inflammatory processes and resistance to insulin.
The hypothesis of excessive activation of RAS caused by Covid-19 infection can be used to understand many of the previously mentioned clinical and epidemiological findings that are characteristics of Covid-19 disease [43]. This is especially important in early days of this infection and in sensitive patients with existing risk-factors. In sensitive individuals, this is quite likely to have the largest impact on the early development of the disease in combination with increased cytokine release. This mechanism is very prevalent when talking about the severe form of Covid-19 infection, but it is not unique. The most important role that this hypothesis has is in the prevention of the disease worsening. The use of certain drugs, such as AT2R agonists could enable the host's protective response. Additionally, some studies suggested that the use of fenofibrate that is an agonist of peroxisome proliferator-activated receptor alpha (PPAR-α) has beneficial effects in the treatment of Covid-19. This drug expresses strong anti-inflammatory anti-thrombotic properties. The proposed mechanism is the inactivation of spike protein that SARS-CoV-2 virus uses as an entry point to bind the ACE2 receptor. Consequently, the initial invasion of SARS-CoV-2 virus into the host cell is disabled [44]. Research on this topic is of great importance and has been greatly intensified in the previous period. Therefore, the results of these studies show that therapeutic approach is recommended for application based on these hypotheses [45].
1.5. Risk factors for more severe Covid-19 disease
Identifying high-risk populations for progression from mild Covid-19 infection to severe and critical illness is crucial for the strategies used for combating Covid-19. A large number of risk factors have been identified as being related to the progression of this disease and its severity [46]. Some of the most important are age, male sex, co-morbidities such as increased body mass index (BMI) and blood pressure, cardiovascular diseases (CVDs), heart, liver and kidney damage, previously developed pulmonary diseases and some other chronic illnesses. Some consequences of Covid-19 disease also include coagulation imbalance, acute kidney injury and thromboembolism [47]. Potential complications and progression of the Covid-19 disease are monitored through laboratory parameters, including the monitoring of interleukin levels, highly sensitive positive inflammation reactant called C-reactive protein (CRP), lymphocytes, platelet count, D-dimer [48], SpO2 (oxygen saturation), peripheral capillary oxygen saturation and ferritin [49]. A report from Japan's Covid-19 registry noted that patients who had co-morbidities such as kidney dysfunction, liver disease, diabetes, hypertension, obesity and older age tended to develop a more severe clinical picture than patients without such comorbidities. Additionally, patients who have previously been diagnosed with lung diseases developed critical illnesses with a high probability of death, especially in the case of chronic obstructive pulmonary disease (COPD). The course of the Covid-19 disease in lungs is usually monitored by chest CT (computed tomography) scan [49].
1.5.1. Initiation of non-specific immune response
The decisive role in the protection and ultimate barrier against pathogens (such as viruses) is non-specific or innate immune response. Type I interferon (IFN) activation is first response of the host's immune system to viral infection. Its activation is the main factor that controls viral multiplication and it elicits an adequate adaptive immune response. However, viruses use different mechanisms to suppress the antiviral immune response [50]. First of all, the process of suppressing type I interferon is activated by viruses. This complex process is characteristic of coronavirus with expressed pathogenic abilities, SARS-CoV and MERS-CoV is connected with disease's course and outcomes. One of the studies [51] compared the level of interferon in a patient infected with MERS-CoV with different levels of disease severity. A significantly lower level of type I interferon was observed in patients with a severe clinical picture, especially in population of patients for whom this disease ended fatally, in comparison to the levels of type I IFN in patients who recovered or had a mild clinical manifestation . IFN production is conditioned by the activation of IFN-regulatory factor type III (IRF3) that is inhibited after SARS-CoV infection. Inhibition of type III interferon regulatory factors was also observed after SARS-CoV infection. Viral proteins ORF4a, ORF4b and ORF5 inactivate the nuclear translocation of IRF type 3 that effects the expression of IFN. In addition, MERS-CoV 4a accessory protein stops IFN production through a direct interplay with RNA. It is envisioned that SARS-CoV-2 uses similar mechanisms as other types of coronaviruses such as SARS-CoV and MERS-CoV to alter the type I IFN response because the genetic similarity between them is high [51].
However, several sequence analysis studies [51] have shown certain differences in SARS-CoV-2 that make it more susceptible to type I interferon than these other types of coronavirus. SARS-CoV-1 has a similar rate of replication as SARS-CoV-2. However, it is much more sensitive to type I IFN treatment and a significant reduction in virus replication is observed after type I IFN treatment that could represent one of the potential therapeutic protocols for Covid-19 disease. Unfortunately, prolongation of type I IFN response disrupts the early control of virus and thus induces an influx of inflammatory cells such as neutrophils, monocytes and macrophages that onsets a dangerous cascade formation of pro-inflammatory cytokines [52]. In some cases, this is the threshold for the triggering of a cytokine storm [53]. Cytokine levels are especially elevated in more severe forms of disease caused by infection. The number of total lymphocytes and neutrophils in blood changes with the patient's condition, therefore, those with a severe form have very low values of lymphocytes and high neutrophils unlike those with mild symptoms [54].
2. Immunological tolerance and autoimmunity
Immune tolerance is known as the state in which immune system dose does not react to its own antigens or to a specific antigen that can cause an immune response in the body. Immune tolerance is broadly categorized as central and peripheral tolerance with different mechanisms that provide and maintain this extremely important state of self-tolerance [55]. This classification is based on the location of the initial induction of this process. If it is induced in the thymus and bone marrow then such tolerance is called central tolerance and if the condition is induced in other tissues and lymph nodes then it is called peripheral tolerance. The mechanisms by which these two forms of tolerance develop are different, but the result is almost identical [56]. The human immune system is constantly evolving, trying to protect the host as efficiently as possible from different pathogens. It consists of innate and acquired immunity. An innate or non-specific mechanism exists even before contact with a pathogen occurs and represents a key part of the first line of defence against pathogens through numerous mechanisms. Another part of the immune system is acquired or specific. This defence barrier works through the activation of receptors that are distributed among lymphocytes. The wide range of these receptors provides an adequate response to most pathogens [55].
Immune tolerance is of fundamental importance for normal physiology. Through central tolerance, the immune system learns how to distinguish a foreign antigen from the hosts and it destroys lymphocytes with a very important unwanted characteristic of autoreactivity. If these lymphocytes survive, eventually they lead to the onset of different diseases with autoimmune characteristics. On the other hand, the main role of peripheral tolerance is to prevent an excessive immune response to foreign bodies after lymphocytes, both T and B, leave thymus, mature and enter the lymph nodes and other tissues. Deficiencies of both types of immunity, peripheral and central tolerance are the ones to blame for the autoimmune diseases onset and progression [56]. Understanding the mechanism behind the process of tolerance is of key importance. Understanding the lack of tolerance and how it leads to the development of the disease could later be used in their therapy. This would help to replace the current long-term drug treatment regimes with highly effective short-term treatments [57]. Therefore, the basic role of immune tolerance is to prevent an excessive immune response and to ensure that it regulates the response to foreign bodies adequately without harming the host itself [56].
2.1. T lymphocyte tolerance
The basic role of the T cells is to recognize part of the pathogen, through the surface proteins that are a part od the major histocompatibility complex (MHC) group of molecules that are placed in the host cells [58]. As such, they can cause serious injuries to healthy tissue if they are not aiming at the right target, which means they will not react to foreign antigens but their own. B-lymphocyte tolerance is in some way conditioned by T lymphocyte tolerance that gives it additional importance in this entire process. Based on this, it can be concluded that the eventual failure of T-cell tolerance can cause large number of disorders including different autoimmune diseases [59]. After the formation of T-cell receptor and emergence of progenitor T-cell in thymus, the mechanism of T tolerance is triggered. However, the mechanism of central toleration alone is not sufficient because not all antigens, which T cells need to recognize correctly reach the thymus. Through special mechanisms, central T cells are provided with additional help in the form of peripheral T cell effects and tolerance [60].
2.1.1. Central T cell tolerance
Central T cell tolerance has been mostly noticed in thymus because it develops during the stay of T lymphocytes in the thymus. At the beginning, all precursors of T cells have the same genome and then different receptors arise and their rearrangement occurs. After the recombination, there is a rearrangement of T-cell receptor (TCR) genes [61]. T-cell genes contain a large number of parts that need to be physically recombined, consequently leading to the insertion of new bases. As a result, the diversity of these genes and receptors is increased that leads to the formation of complementarity-determining regions (CDR). Such combinations in genome lead to the creation of receptors for T-lymphocytes, however the synthesis of antibodies against different types of foreign antigens. Therefore, a strong host response to rapidly evolving pathogens is developed. T-cells are self-regulatory that means they could alone recognize the antigen that is present in the host and is considered to be the foreign antigen [62]. During the maturation of T-cells in thymus, they are still sensitive to the host antigens [63]. The development of central tolerance largely depends on the encounter of lymphocytes with autoantigen. This means they can develop central tolerance only in lymphatic organs such as thymus [64].
The thymus has one important specificity – it is the source of antigens that are originating from certain organs. The mechanism of central T-cell tolerance is based on the recognition of MCH molecules during the development of T-cells through selection processes that are of fundamental importance. There are two types of selection that T-cells go through, namely positive and negative selection [64]. Positive selection occurs in the thymus cortex. Thymic epithelial cells that are rich in MCH molecules on the surface, take part in this process. Those cells that do not recognize MCH peptide do not bind to this protein and die. This selection mechanism tells that T-cells recognize the antigen but only in MCH. This is essential because the main role of T-cells is to recognize pathogenic cells. The selection process determines whether a T-cell will become a CD4+ or a CD8+ cell. All thymocytes are double positive before the positive selection process and during this process, they are converted to either positive or negative. Whether they turn positive or negative depends on the cell's ability to recognize MCH [64].
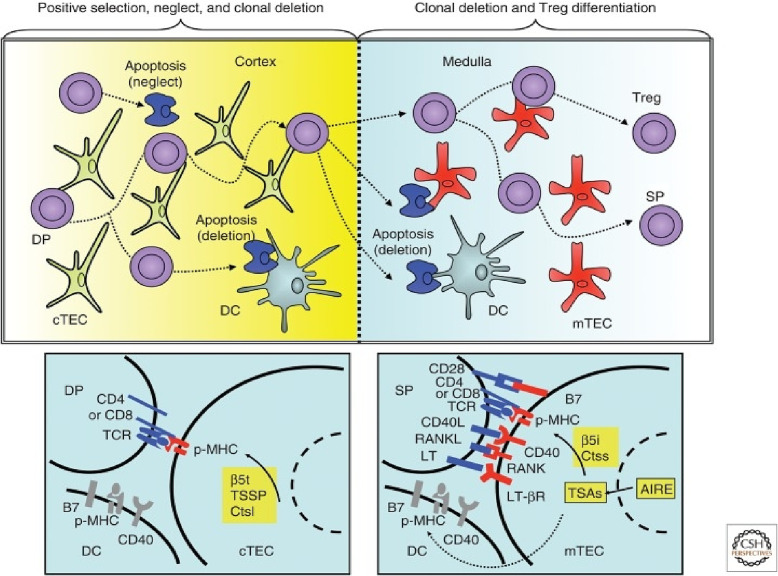
Negative selection takes place in the thymic medullary epithelial cells (mTECs) and dendritic cells. The interaction of an antigen-presenting cell and an immature lymphocyte potentiates the deletion of clones of T lymphocytes that have the ability to attack their own cells. This process is largely conditioned by the expression of ectopic antigens specific to tissues. However, the process in thymus cannot destroy every self-regulatory T-cell. Thus, for example, T-cells that bind to a protein may be found elsewhere in the body or T-cells may be at a different stage of development [60]. Fig. 4 shows the different types of cells that create a central immune tolerance. To prevent the development of autoimmunity through the development of effector cells, the organism developed a mechanism of peripheral tolerance. This was necessary because some self-reactive lymphocytes can escape the mechanism of central tolerance.
Fig. 4.
Different types of cells are included in the development of central immune tolerance through positive selection, neglect, clonal deletion and Treg differentiation [60].
2.1.2. Peripheral T cell tolerance
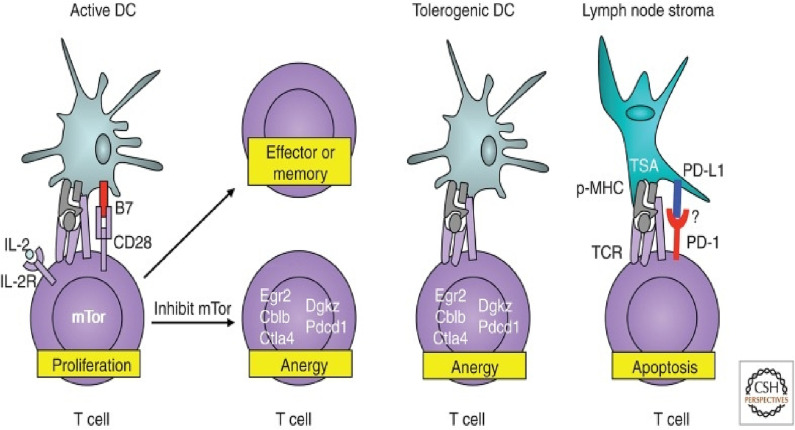
Peripheral tolerance occurs after the exit of T-cells from thymus. The main goal of all complex processes that in the end develop peripheral tolerance is to prevent T-cells that have escaped central tolerance from causing autoimmune disease. Although central tolerance mechanisms are very efficient, they cannot eliminate all self-reactive lymphocytes [65]. The reason for this is that not all autoantigens are expressed at the primary site of lymphocyte development that is the thymus. Peripheral tolerance leads to the inactivation of effector T-cells. This can happen by clonal deletion or changing to regulatory T-cells (Treg). Regulatory T-cells are created during the development of T-cells and their main role is to suppress effector cells in the periphery. Some of the mechanisms for removing self-reactive T-cells in the periphery are clonal deletion and conversion to Tregs, suppression and induced anarchy as shown in Fig. 5 . The main cause of adaptive immune system activation is the activation of dendritic cells. Dendritic cells could also cause tolerance to CD4+ and CD8+. Immature dendritic cells bind antigens from the periphery and present them to T cells that are inactive. If the cell recognizes the antigen, its transformation into Treg will occur. As dendritic cells mature, they lose these abilities. In addition, other cells can cause similar effect [56]. Suppression of Treg effector T-cells is also an important mechanism in protection against autoimmunity. Treg cells are generated in the periphery or the thymus. Through different mechanisms, Tregs can protect the organism from autoimmunity [60].
Fig. 5.
Representation of the development of peripheral tolerance of T cells and the processes of anergy and apoptosis [60].
2.2. B lymphocyte tolerance
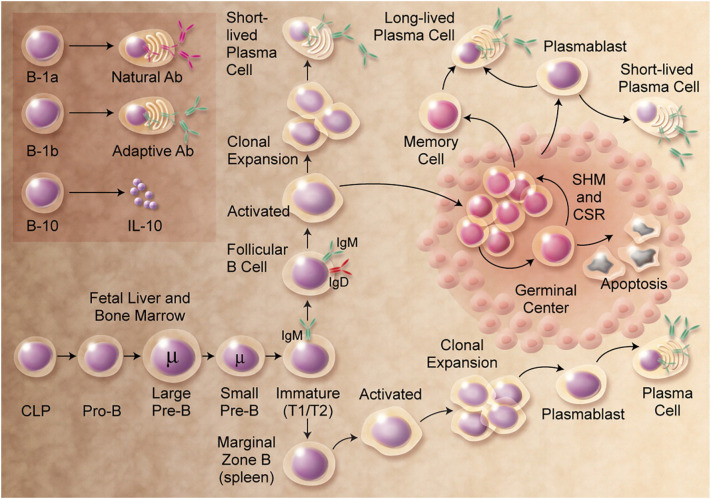
B cells participate in host defence through effects of antibodies that are created as the protective mechanism after microorganisms' presence is detected. The development of B cells is shown in Fig. 6 . The lack of B cells in some patients leads to the inability to produce antibodies and a high tendency to infection [66]. Specifically, each B cell owns a unique antigen receptor (BCR) [67]. After cell identifies an antigen from the side that is on the membrane, the reactive B cells start a mechanism in which the cells rapidly multiply to increase their number and then secrete their specific antibody in the form of immunoglobulins: IgM, IgD, IgG, IgA or IgE. Activated B cells can improve antigen specificity and affinity, together with CD4+ T follicular helper cells (TFH) and other cell types [68]. The basic role of antibodies is to provide an adequate response to a large number of infections. However, there are cases when antibodies can be pathogenic causing the development of a large number of autoimmune diseases, such as type I diabetes, rheumatoid arthritis and others [69].
Fig. 6.
Detail mechanism underlying the development of B cells, starting from the common lymphoid precursor (CLP) to the mature B cell that synthesis antibodies [70].
2.2.1. Central B cell tolerance
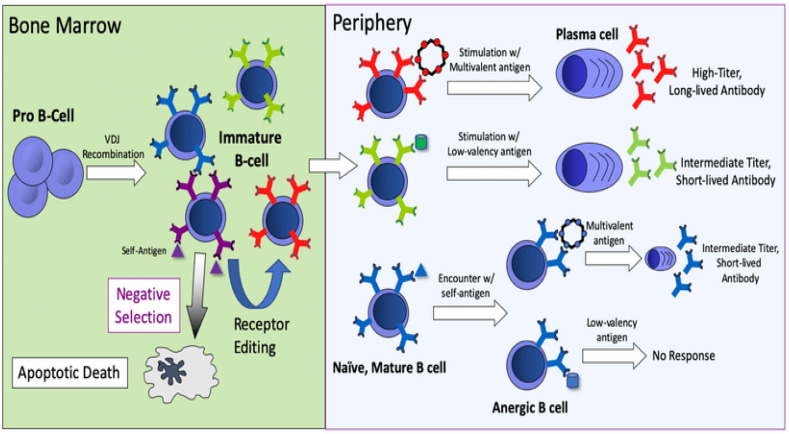
Antigen identification by immature B-cells in bone marrow is of crucial importance for the development of central tolerance for these cells. By the use of this mechanism, the produced cells will not recognize internal, host antigens but they can recognize pathogens, i.e. foreign antigens. Therefore, this can be concluded that the central point of B-cell tolerance is in bone marrow (BM) [63]. In the process of negative selection, immature B-cells have reduced reactivity and at that stage, they recognize only the molecules that are in the bone marrow. Upon exposure to an antigen, the B-cell loses its receptor and that process is called clonal deletion [71]. B-cells that have surface IgM are eliminated in the process of clonal deletion. This kind of cell death is also called programmed death or apoptosis. However, through various research, it was concluded that by genetic rearrangement only reactive B-cells can be saved and receptors can be replaced. Immature B-cells, also called low-valent cells do not die but they lose the ability to express IgM. Something similar happens with developed tolerance to autoantibodies and drugs [63]. For this reason, such B-cells go to the periphery, express IgD and cannot respond to antigens. They are called anergic cells. A foreign antigen can only be recognized by B-cells that have not encountered the antigen in the bone marrow and that possess both IgM and IgD receptors. Defective tolerance of central B cells has been observed in many patients with autoimmune diseases such as type 1 diabetes, rheumatoid arthritis and others [71].
2.2.2. Peripheral B cell tolerance
Central B-tolerance and the deletion of autoreactive B cells is sometimes not fully completed and self-reactive B cells migrate to the periphery. To avoid the migration of self-reactive B-cells and their dangerous, unwanted effects, many mechanisms related to peripheral tolerance have been developed. Peripheral tolerance occurs when B-cells encounter an antigen in the periphery. Further, if B-cells meet with an antigen on the periphery, these could be anergic. Such anergic cells cannot ask for help from T cells, so their lifespan is shortened [72]. The main influence on an adequate B cell response is the presence of T-cell that is specific for a certain antigen. The production of autoantibodies is precisely organized by the presence of T-cells [73]. Although many different mechanisms that are controlling self-reactive B cells are known, in the case of autoimmune diseases, central and peripheral mechanisms of tolerance fail [73]. Many studies have analyzed the pathways that control the reaction of B cells and in the case of autoimmune diseases, these simply do not work [73]. Such data on mechanisms that fail and lead to a loss of central and peripheral tolerance could be of great importance because they provide information on how these autoimmune diseases are triggered. They can also be used for therapeutic purposes. Fig. 7 shows the mechanism of B cell central and peripheral tolerance.
Fig. 7.
Central B cell tolerance occurring in the bone marrow and peripheral B cell tolerance [74].
2.3. Potential molecular mechanisms of autoimmunity in Covid-19
With the emergence of an infection caused by SARS-CoV-2, research about the connection between infections and autoimmune diseases was conducted. The results of a large number of studies show a connection between infections and autoimmunity [75]. That connection is mostly represented by processes such as molecular mimicry, hyperstimulation and dysregulation of the immune system.
2.3.1. Molecular mimicry
Viruses use different ways to infect the host, often using the host's biochemical pathways. One of the primary ways of infecting the host is molecular mimicry of proteins. This mechanism has been long known and the phenomenon of Covid pandemic caused by SARS-CoV-2 intensified these studies. Viruses often use the host's machinery to more effectively evade the immune system's response, but primarily they are used for the cycle of replication. The viral proteins are similar to host proteins because of this process. It is thought that viruses have managed to imitate the host proteins. This is supported by the fact that viral material of corona virus was found in other organs as well, such as kidneys, brain, heart and liver in addition to the lungs [6]. Through a detailed study of Covid-19 disease, it was concluded that there is a possibility of molecular mimicry. In an other study [76] the sharing of peptides, mostly hexapeptides and heptapeptides between SARS-CoV-2 spike glycoproteins and human proteins was confirmed.
Interestingly, a large number of these proteins are closely related to pulmonary, cardiac, vascular, coagulation and immune disorders. Other studies [6] have shown associations and matches between SARS-CoV-2 and human proteins, including pulmonary surfactant, brainstem neuronal proteins, chaperones, heat shock proteins, ankyrin, an odorant receptor and many other proteins associated with parts of the respiratory system as well as some which contribute to the onset of different autoimmune diseases. One of them is polymerase 9 (PARP9) that contributes to the development of diseases in the lower respiratory organs. It is important to state that monoclonal antibodies that are aimed against SARS-CoV-2 spike protein and nucleoprotein can create a cross-reaction with a large number of proteins in the tissues such as nuclear antigens, isolable nuclear antigen, mitochondria, thyroglobulin and many other proteins. Therefore, the main aim of the mechanism of molecular mimicry is to facilitate infection, through imitating host proteins. As is the case with other viruses, SARS-CoV-2 also often encodes host proteins precisely to ease the infection. This is also one of the characteristics of other corona viruses such as MERS-CoV [33].
2.3.2. Autoantibodies
Stimulation of immune system is a complex process that has many benefits for human organism, but if its activation escapes control mechanisms, many serious consequences can occur. On one hand, the immune system and the pathways that regulates are necessary for defence against various exogenous substances and potentially infectious substances. On the other hand, hyperstimulation of the immune response can trigger autoimmune disorders. Many factors can contribute to autoimmune diseases developing in a patient and some of the most influential ones are genetic factors, hormones, a weakened immune response as well as individual environmental factors. The results of numerous studies confirm that people infected with Covid-19 develop a greater number of autoantibodies. For the vast majority of antibodies, it is unknown whether they were created after the patient's recovery from the infection or whether they existed before the infection as well as whether they will effect the possible onset of disease with autoimmune characteristics in the future [77].
The first line of defence against viral infections is represented by type I interferons (IFN). Autoantibodies that neutralize type I IFN have long been known. Such results have been reported in patients with viral infections, but also in some patients with chronic systemic diseases. Until the emergence of SARS-CoV-2 virus, these antibodies were considered to be clinically negligible. The synthesis of these autoantibodies can start in childhood, but they are characteristically found in patients with autoimmune polyendocrine syndrome type-1 (APS-1), found in patients with hypomorphic RAG1 or RAG2 mutations as well as many other genetic mutations. From these results and the significant function of type I IFN in the immune response, it could be assumed that innate errors in antiviral immune response in type I IFN may be a possible basis for the development of severe and even life-threatening Covid-19 [77].
The results also indicate in patients with autoantibodies that neutralize IFN-α2 show that an enviable number of patients who developed a severe clinical picture and life-threatening Covid-19 possessed these neutralizing autoantibodies. This should also be mentioned that none of these neutralizing autoantibodies was detected in asymptomatic patients or patients with a mild clinical picture. The factors that influenced the probability of finding autoantibodies are gender (over 90 % in men) and age of the patient [77]. These results were subsequently confirmed by many publications around the world. Patients with APS-1 belong to the group of high-risk patients with an increased risk of developing severe disease during Covid-19 infection [78]. Considering such evidence, it can be concluded that neutralizing autoantibodies to type I IFN is one of the important factors in the development of complications during Covid-19 infection. This could also be assumed that these autoantibodies help other viral diseases especially in the elderly people [77], [78].
3. Systemic autoimmune manifestations associated with Covid-19
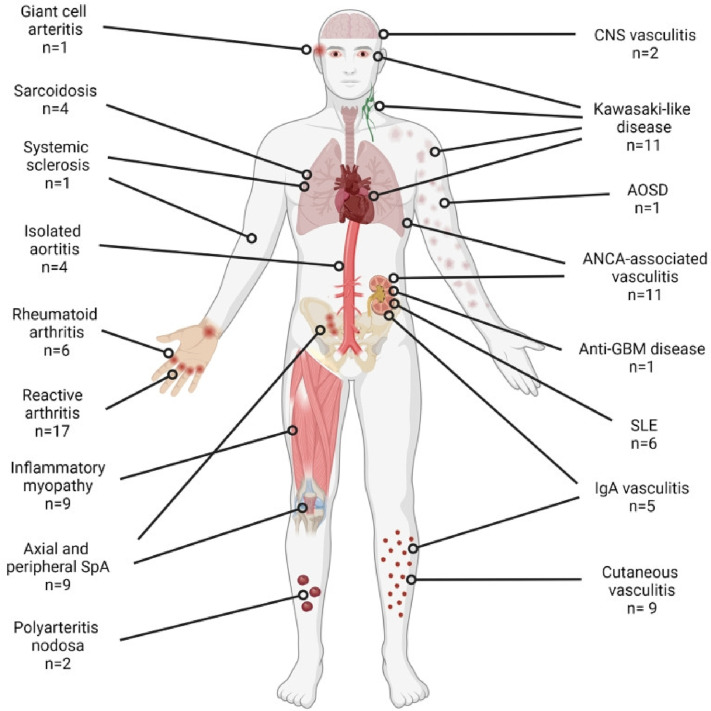
Viral infections such as Covid-19 are closely related to many autoimmune diseases. Some of these are rheumatoid arthritis (RA), systemic lupus erythematosus (SLE), vasculitis, a multisystem inflammatory syndrome in children and adults (MIS-C and MIS-A), antiphospholipid syndrome (APS) as well as many other autoimmune diseases [79]. Fig. 8 shows organic systems that could be effected by Covid-19.
Fig. 8.
The number of cases noted and organs affected by the new-onset rheumatic autoimmune diseases reported during or after Covid-19 [79].
3.1. Systemic lupus erythematosus
Systemic lupus erythematosus (SLE) is one of the systemic manifestations associated with Covid-19 infection. Several studies [79], [80] have described an association between SLE and Covid-19 infection. The mechanism behind this process is still unclear, but it is thought that these patients had an increased activation of B cells and also an increase in interferon release. Additionally, there is a possibility that patients already had developed SLE but the symptoms were not expressed yet or were not properly diagnosed and Covid-19 only accelerated the onset of more severe symptoms. Further studies are needed to clarify this phenomenon. Acute kidney injury was the main complication that was noted after the onset of SLE. The thrombocytopenia was presented to a greater extent than leucopenia and a lesser extent, pericardial effusion. Patients with Covid-19, who developed SLE were treated with corticosteroids, namely dexamethasone or prednisone and to a lesser extent with chloroquine. In addition, plasmapheresis and intravenous immunoglobulin were used to treat some patients [79].
3.2. Rheumatoid arthritis
Rheumatoid arthritis is a disease that is part of a wide spectrum of diseases that have autoimmunity as their main characteristic, in this case mostly associated with inflammation of the mucous membrane lining the joint. The cause of this disease is still unknown, but many different factors were associated such as nutrition, infections, enzyme, hormone imbalances, stress and other forms of emotional trauma. They can either trigger or significantly worsen the disease. However, it is interesting that excessive activation of cytokines in RA is similar to that noted in cases infected with SARS-CoV-2 virus [80]. Many different studies suggested that rheumatoid arthritis leads to an increased chance of acquiring infection with SARS-CoV-2 due to immunologic dysfunction and the use of potent immunomodulatory medications [36]. There is evidence that Covid-19 infections can affect the musculoskeletal system and even trigger the onset of rheumatoid arthritis.
Some studies [79] suggested that Covid-19 infection often presents itself with joint pain and this symptom appears during the period of infection but during the recovery period. The main characteristic is arthralgia (pain in joints) that often occurs with myalgia (pain in muscles) [81]. In addition, the data showed that arthralgia and myalgia were present in patients with this infection in a high percentage (over 40 %) [79]. The most likely mechanism is immuno-inflammation that could lead to the start of inflammatory changes in joints, called arthritis in patients with Covid-19 infection. Interestingly, Covid-19 and rheumatoid arthritis share two potentially similar mechanisms in their development. The SARS-CoV-2 virus uses an ACE receptor on the cell to attack and infiltrate them. This leads to increased activation of these receptors and production of angiotensin II, consequently leading to increased production of vascular growth factors (VEGF).
This cascade is eventually responsible for the entry and infiltration of inflammatory cells in healthy tissues. This could be seen in RA as well as a rise in the number of aberrant macrophage clusters in alveoli in Covid-19 and synovial liquid in RA [36]. In conclusion, respiratory infection with SARS-CoV-2 is associated with the development and progression of rheumatoid arthritis. Rheumatoid arthritis can affect the course of Covid-19 disease because these patients often have comorbidities in a much higher percentage than individuals without rheumatoid arthritis such as asthma, diabetes and many others [81]. Therefore, additional follow-up is needed in patients with both rheumatoid arthritides and are suffering from Covid-19. However, in the group of patients with predisposition for RA development because Covid-19 infection could be a trigger for its onset.
3.3. Vasculitis
Vasculitis is a clinical-pathological process characterized by inflammation and necrosis (death) of blood vessels throughout whole organism or in a specific organ. The consequences of these pathological events usually compromise the lumen of blood vessels - arteries, arterioles, capillaries and venules. The disease is most likely caused by several different factors. It is believed that some hereditary factors, infection or other factors from the external environment can have a significant effect on the onset of the disease [82]. Additionally, people suffering from vasculitis have a hyperactive immune system. Normally, the human body fights against various infections (viruses and bacterias). In patients with vasculitis, the immune system attacks its own blood vessels causing chronic inflammation [83]. The mechanism of vasculitis development can be compared to the mechanism of the onset of infection with Covid-19 because both develop as a consequence of hyperactivation of the immune response.
It is thought that this virus starts a process of pyroptosis. The term pyroptosis is used to describe endothelial and innate cells of the immune system after being damaged by the virus or some other toxic agent, release cellular components that enhance inflammation and oxidative stress. This could be proven by a noted increase in the levels of molecules produced in these damaging processes such as low-density lipoproteins, heat-shock proteins, proteases and phospholipids that have been oxidized. For this reason, it is considered that vasculitis can often accompany a virus infection and that could develop as a more serious consequence of Covid-19. Furthermore, this is confirmed by a study that investigated the interplay between vasculitis and Covid-19 [84]. A maculopapular purple rash was observed in a large number of patients but papular and erythematous rashes were common. Such manifestations indicate a clear connection between these two diseases. Corticosteroids were mostly administered to patients as well as intravenous immunoglobulins [38].
Leukocytoclastic (LCV) forms are the most common forms of vasculitis detected in patients infected by SARS-CoV-2. The presence of immunoglobulin A vasculitis (IgAV) and Kawasaki disease were slightly less expressed in adult patients than in pediatric patients [84]. IgA vasculitis (IgAV) is mostly detected in pediatric patients but it was also noted in adult patients. This type of vasculitis is a systemic vasculitis that effects small blood vessels and is characterized by non-thrombocytopenic palpable purpura, joint pain and abdominal pain. For the treatment of IgAV, no special therapy is used other than supportive therapy because it resolves on its own. Leukocytoclastic vasculitis is very similar to IgA vasculitis. This is systemic, usually effects small blood vessels and mainly occurs in adults [85]. Kawasaki disease is a type of vasculitis that occurs predominantly in children up to five years of age. The symptoms associated with Kawasaki disease are rash, fever and swelling of the extremities. It could cause heart problems if it is not properly treated. The manifestations of all types of vasculitis are related and similar to the manifestations that are characteristic of Covid-19 that speaks in the favour of their connection [79].
3.4. Antiphospholipid syndrome
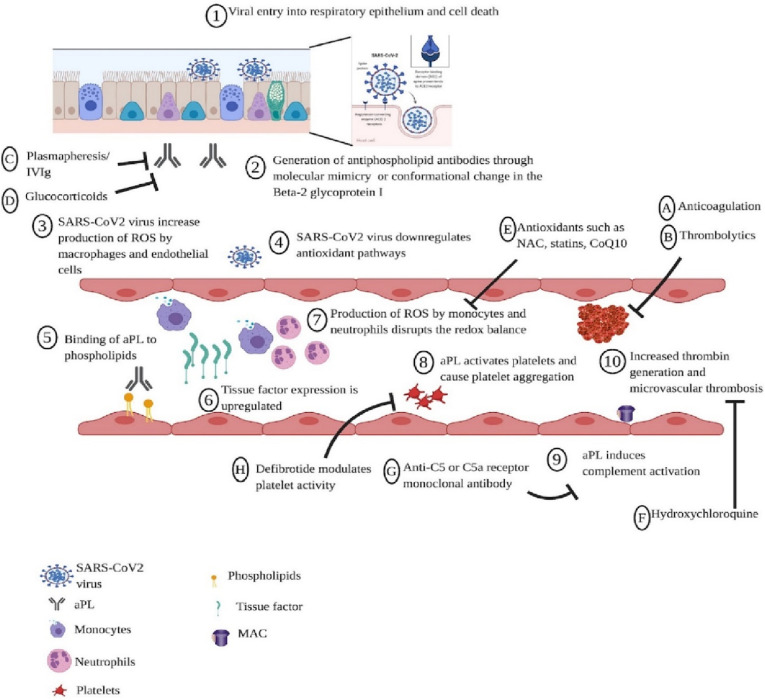
Antiphospholipid syndrome (APS) is a part of the complex group of autoimmune diseases, which are characterized by the production of specific antibodies that attack healthy host cells. This antibody is called antiphospholipid antibody (aPL). The common complications that the action of these antibodies can cause are complex and dangerous such as the formation of blood clots and low levels of platelets (thrombocytopenia) [86]. This is noted that patients have APS do not manifest often clinical symptoms and positive antibodies cannot be detected. Interestingly, a person can be positive for the antibody test (aPL) without ever developing APS and without any clinical symptoms [87]. Catastrophic antiphospholipid syndrome (CAPS) is a more serious and dangerous type of this autoimmune condition with a severe manifestation of APS. This CAPS syndrome develops in a small number of patients (around 1 %) or even less patients with already developed APS. CAPS has a very high mortality rate of around 50 % that is the reason this is called catastrophic [88]. Fig. 9 shows the mechanisms of APS pathophysiology.
Fig. 9.
Proposed mechanisms of APS pathophysiology and potential therapeutic targets for coronavirus disease [87].
In CAPS, blood clots form in a short time, usually within 24 h. This causes serious dysfunction and injury of lungs and leads to neurological disfunction due to brain injury [89]. The international CAPS registry has published key data for diagnosing this syndrome and therapeutic guidelines for the treatment of effected patients [90]. In the review of registry data of cases with developed CAPS, it was noted that a large number of cases (almost half) had a viral infection previous to the onset of this disease [91]. The Covid-19 infection is not only causing damage to the lungs but also to other organs at large extent [92]. Based on the analysis of swabs from people with SARS-CoV-2 virus, the infection that can cause inflammation and damage small vessels of the mucosa was noted. This is caused by the formation of small blood clots, called microthrombi. This microthrombi can easily cause the appearance of APS or even in the worst case CAPS [87].
Furthermore, in many registered cases of Covid-19 a frequent occurrence of deep vein thrombosis, pulmonary embolism and microthrombi formation has occurred [93]. The frequency of these occurrences is significantly higher than expected in hospitalized patients with more severe form of the disease. Although it was previously demonstrated through experiments on animals that aPL are thrombogenic. A recent research shows that there must be a factor that will initiate the entire process of thrombosis. SARS-CoV-2 can cause damage to the endothelium that is manifested by dysregulation of redox balance due to the increased amount of reactive oxygen molecules produced by macrophages. Viruses, such as the coronavirus, reduce the effects of antioxidant pathways by binding to ACE2 receptor, thereby inhibiting nitric oxide pathways, especially nitric oxide synthesis. That dangerous inhibition of antioxidant pathways can cause a series of cascade reactions such as the activation of cascade coagulation and the formation of a thrombus [87].
3.5. Multisystem inflammatory syndrome in children
A large number of accompanying disorders caused by this disease were reported in Covid-19 pandemic, such as a multisystem inflammatory syndrome. This syndrome was mostly observed in pediatric population. The mechanism of this disorder is most likely based on the development of hyperinflammatory shock but further studies need to research this phenomenon [94]. Interestingly, all children with developed MIS-C had confirmed SARS-CoV-2 infection and MIS symptoms appeared approximately 2 weeks after being infected with the coronavirus that indicates the cause of MIS development is the aggressive immune reaction. It was shown that the dysfunction of endothelial cells is the main issue because increased levels of C5b-9 (a weapon of the complement system used for damaging cell membranes) and autoantibodies that attacked endothelial cells were detected [95].
The clinical manifestations that occurred in reported patients with this multisystem inflammatory syndrome are very similar to the symptoms that were present in Kawasaki disease, which is common in infants and young children. The most common symptoms observed in patients with multi-inflammatory syndrome were fever, rash, gastrointestinal problems with stomach pain and elevated heart damage parameters. The presence of this syndrome in children was confirmed in many later publications, with a high prevalence (above 30 %). Therefore, additional studies should identify the exact cause and underlying risk factors for MIS onset in pediatric patients and the potential prevention of its development [95].
3.6. Multisystem inflammatory syndrome in adults
In addition to the disease occurring in children (MIS-C), MIS has been identified in adults effected with SARS-CoV-2 virus. The identification of multi-inflammatory syndrome in adults (MIS-A) is an important problem. The reason for this is the appearance of many other hyperinflammatory throughout the body that accompany the infection of Covid-19. This kind of clinical dilemma is very difficult to solve. Additionally, distinguishing MIS-A from other diseases such as biphasic acute Covid-19 is often complex because there is an overlap both in their manifestations and in diagnosis. It is further complicated by the fact that MIS-A has not been investigated enough in Covid-19 infection and its temporal relationship with the infection is not known [41]. The most commonly used therapy for MIS-A is the use of corticosteroids, immunoglobulins and agonists of IL-1 receptor, anakinra that had a good effect on the treatment of children with MIS-C. Acute myocarditis has often been described in cases with MIS-A and it is treated with corticosteroids [95]. A better description of MIS-A is of key importance because the treatment may differ from some other accompanying diseases as well as from severe Covid-19 [41].
3.7. Other systemic autoimmune diseases
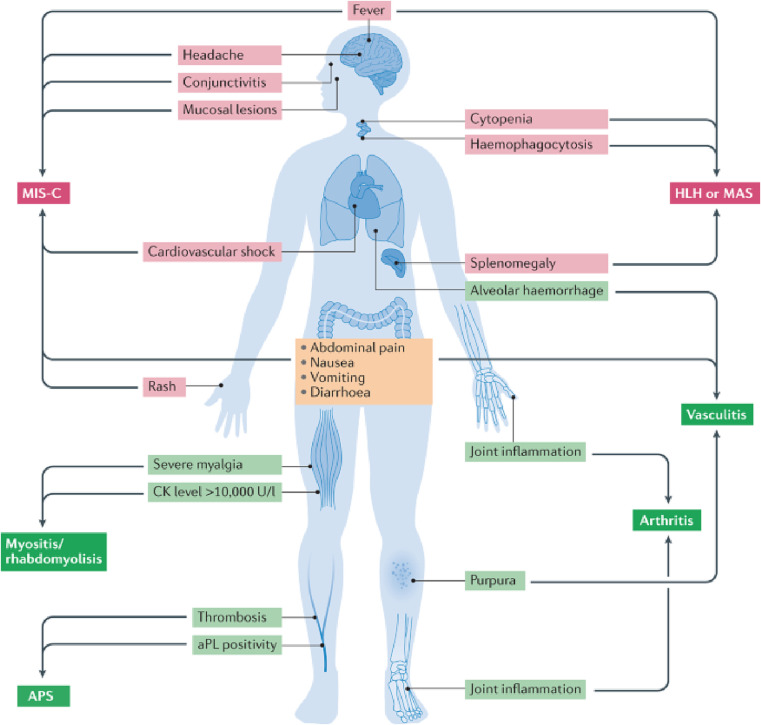
Covid-19 disease is accompanied by numerous disorders both organ-specific and systemic that is shown in Fig. 10 . A large number of publications [79] evidenced the occurrence of a wide range of systemic diseases that were triggered by Covid-19 infection. The most frequently reported disorders were systemic lupus erythematosus (SLE) and myositis of proximal limbs but also paraspinal myositis [96]. Both types of myositis were diagnosed on magnetic resonance imaging (MRI) as well as by the recorded elevation of creatine kinase (CK) level that is a marker of muscle damage. The biggest problem in the studies that followed these cases is incomplete diagnostics, such as an absence of muscle biopsy or electromyography. Cases of ankylosing spondylitis have also been reported, causing back or joint pain in people with polymyalgia rheumatica, pain occurred in the muscles but it was resolved with the use of adequate therapy (hydroxychloroquine) [5].
Fig. 10.
Presentation of the most common symptoms and signs accompanying systemic immune disease in patients with Covid-19 [5].
In addition, novel studies have shown the association between the onset of diabetes mellitus type 1 and Covid-19 infection. Previously, it was proven that viral infections such as enterovirus infection can increase the production of antibodies that attack pancreatic beta cells that are responsible for insulin production. This is an underlying mechanism of onset of diabetes mellitus type 1. The SARS-CoV-2 could enhance the production of autoantibodies against antigens expressed in the pancreatic beta cell and induce their damage. In addition, it was recently discovered that ACE2 receptor is expressed in pancreatic endocrine cells responsible for insulin production. This receptor is the main entry point that SARS-CoV-2 virus uses to invade host cells. Therefore, SARS CoV-2 could directly attack human pancreatic cells and lead to their apoptosis. However, the data that would support these hypotheses are still insufficient and further studies should investigate the interplay between Covid-19 and diabetes mellitus type 1 as well as possible therapies that the patients would benefit from [97].
The latest studies found a link between autoimmune diseases of the thyroid gland such as hyperthyroidism with Covid-19 infection. It was also shown that SARS-CoV-2 can induce thyrotoxicosis and autoimmune thyroiditis. The proposed mechanism behind this is the use of ACE2 receptor and transmembrane protease serine 2 (TMPRSS2) as the main complex that induces the SARS-CoV-2 invasion of the host cell. These receptors are expressed in both the thyroid gland and lungs. Therefore, patients infected with Covid-19 are at increased risk of induction or relapse of autoimmune hyperthyroidism. The cytokine storm that is induced in severe cases of SARS-CoV-2 infection and the released proinflammatory cytokines have a thyrotoxic potential. This is often seen in older patients that could increase the mortality in Covid-19. In addition, systemic inflammation and the over-activation of immune cells, especially the lymphocytes, resemble the processes noted in thyroid diseases that is enhanced by the synthesis of autoantibodies in immune cells. However, further studies need to investigate these proposed mechanisms as well as therapeutic approaches that can improve outcomes of Covid-19 in patients with or without preexisting disease of thyroid gland [98], [99].
4. Organ-specific autoimmune manifestations associated with Covid-19
After the beginning of Covid-19 pandemic, various organ damages have been reported. The most commonly effected organs are the lungs, heart, liver and kidneys, but their manifestations could be presented differently. Mechanisms of organ damage caused by the infection of Covid-19 are numerous. Cytokine storm is thought to be among the leading causes of organ damage that is influenced by many other factors through many different mechanisms such as inflammation, ischemia and hypoxia. However, these mechanisms have not been completely researched such as in the case of the injury of the myocardium in the heart of person who have developed a multi-inflammatory syndrome (MIS). Among the most common unusual side effects of Covid-19 were haematological, neurological, skin and ocular manifestations [100].
4.1. Haematological manifestations
Haematological manifestations often accompany viral infections and Covid-19 is not an exemption. For example, lymphopenia was detected in numerous viral infections associated with a severe clinical picture or poor outcomes [100]. Interestingly, haematological manifestation that was often seen in patients with this infection was lymphopenia as well as leukopenia and thrombocytopenia, these are characteristic of this infection [100], [101]. Lymphopenia and leukopenia were particularly noticeable in patients with a severe clinical picture. Lymphopenia was often seen in critically ill patients, especially if they developed ARDS [100]. Therefore, lymphopenia is a significant sign of disease's severity. Cytopenia has been rarely reported in people infected with Covid-19 and it was mostly detected in asymptomatic patients.
Thrombocytopenia is frequently seen in people with Covid-19. Interestingly, a close association of thrombocytopenia with poor outcomes in patients with Covid-19 was noted. This is additionally confirmed by research data that showed a linear association between the deaths caused by Covid-19 and the registered number of platelets in the blood. Possible mechanisms should yet be identified but it is thought that the main role belongs to the insufficiency of platelets production caused by cytokine storm. It is possible that the destruction and damage of the platelets is happening on the periphery because of a direct attack on host's platelets as well as an increase in autoantibodies production [102]. In addition, it was reported that thrombocytopenia was present in severe forms of the disease. Consequently, thrombocytopenia can be used as a biomarker for the detection of organ damage and the level of platelets could be used as a predictor of the disease prognosis because of having an important role in its course [100].
Interestingly, other autoimmune haematological manifestations of Covid-19 have been detected such as immune thrombocytopenic purpura (ITP). This phenomenon has been observed in a large number of patients over 50 years, usually 14 days after the initial infection. Serological testing and detection of IgG antibodies presence may indicate a delayed immune response. Autoimmune hemolytic anemia (AIHA) was also detected in elderly [103]. The symptoms characteristic of AIHA is asthenia and jaundice that are manifested during the first seven days of disease's course. Additionally, in severe Covid-19 disease, a significant rise of the D-dimer values was noted in patients. This points out that the activation of a dangerous coagulation cascade is often seen in people with Covid-19. The increased value of D-dimer was noted mostly at advanced stages of this disease and it was significantly lower in the group of patients with moderate or mild disease, which indicates that this parameter is related to the course of disease. Therefore, it should be monitored to detect hypercoagulation on time and prevent its full onset [5].
4.2. Skin manifestations
Different presentations of skin symptoms and signs can be used as clues to determine the severity of infection in viral diseases including Covid-19. The skin manifestations caused by Covid-19 in adult patients that are mostly recorded are morbilliform rash, urticarial lesions, vesicles, pseudo-chills and vaso-occlusive lesions [104]. The maculopapular lesions are often seen as a localized skin manifestation accompanying this infection. Additionally, skin lesions can develop as a side effect of medications. By reviewing the results of different studies, it was determined that almost half of the skin lesions that occur in Covid-19 are maculopapular lesions [105]. Age is a risk factor for onset of these lesions, also found in younger people. These lesions are most often located on the trunk of the body [100].
Furthermore, an increased incidence of sores, also called pernio has been noticed in patients with Covid-19. They occur on certain parts of the body, mostly feet and hands, these are characterized by inflammation of the skin. These lesions are characterized by a change in the skin colour to red or blue and swelling of the extremities. A popular term used to describe infected patients with these lesions is “Covid on the toes”. Medical literature supports the association of these lesions with the infection of Covid-19 or with some autoimmune diseases although these lesions are mostly of unknown causes [100]. Additionally, the vesicular rash was recorded in some of the patients. It manifests itself as lesions that resemble bags that are filled with liquid and are located under the epithelial layer. A common name used for such lesions is blisters. The size of these lesions often does not exceed 1 cm and rarely appear individually but rather in groups.
Risk factors that lead to this phenomenon are infection by viruses and bacteria, drugs, heatness and contact dermatitis. They rarely occur in Covid-19 disease and they are most often localized on the extremities and occur in middle-aged people [100]. However, the main problem is that these lesions could also occur as a side effect of drugs and Covid-19- infected patients are usually treated with many different drugs. Therefore, it is not advised to use maculopapular lesions and any other skin lesions as the diagnostic characteristic of infection with this virus. Drugs that can potentially lead to the appearance of these sores are mostly from the group of antiretroviral drugs such as ritonavir and lopinavir as well as intravenous immunoglobulin (IVIG) that are often used in the treatment of Covid-19 patients [106].
4.3. Ocular manifestations
The presence of the previously detected coronaviruses has been noted in tear secretions, cornea and conjunctival cells. This indicates that these types of coronavirus could cause conjunctivitis, especially due to a lack of treatment and low level of prevention. Interestingly, ocular manifestations are often registered in Covid-19 and research shows that one out of ten patients with this disease had some type of ocular manifestation [107]. Conjunctivitis is the most commonly reported ophthalmic complication confirmed in patients suffering from Covid-19 [108]. This virus often causes a more severe form of conjunctivitis with pronounced cilia-conjunctival hyperemia. The time of this ocular manifestation is not precisely defined but the most likely time of appearance of conjunctivitis is between 5 and 20 days after the initial detection of symptoms. The mostly used eye drops to treat these viral conjunctivitides are ribavirin eye drops, although further studies need to be conducted to confirm their safety. In recent research, case series of oculomotor paralysis (OMP) and retinopathy have also been described [107].
A review of the medical literature also found that medicines used in the therapy protocols for Covid-19 can cause ocular changes such as cataracts and ocular hypertension. This was often noted in more severe clinical cases. In addition to conjunctivitis that was the most common ocular manifestation (Over 80 %), dry eye, redness, itching, eye pain and discharge were noted [107]. The most common symptoms of conjunctivitis can be redness and itching but it could also cause fever. Conjunctivitis commonly occurs in adults and less in children. Importantly, this conjunctivitis has a high risk of transmission for people who treat infected patients, therefore additional safety measures should be applied when treating a patient with conjunctivitis and Covid-19 [109].
4.4. Neurological manifestations
Several reports confirmed that neurological manifestations are highly prevalent in patients suffering from Covid-19. The most common neurological symptoms accompanying the Covid-19 infection are anosmia, ageusia, myalgia, vertigo, headache, encephalitis and Guillain-Barre syndrome [110], [111]. Anosmia and ageusia were often detected in patients infected with this virus. More severe complications in the neurological sphere such as Guillain-Barre syndrome or stroke occurred very rarely. It was proposed that the transmission of a virus from the respiratory system to the brain can occur because of a “cytokine storm” and the injuries of the brain-blood barrier as a consequence of their effects [40]. Interestingly, virus proteins and increased markers of inflammation, such as C-reactive protein (CRP), fibrinogen and D-dimer were detected in the cerebrospinal fluid of patients that were diagnosed with encephalitis. Additionally, it was shown that different antibodies appear after Covid-19 infection and some of them affect the neurological system such as antibodies against anti-myelin oligodendrocyte glycoprotein (MOG) and antibodies against hypocretin receptors.
Hypocretin is a neuropeptide that regulates important brain functions such as sleep, appetite, and wakefulness but some extracerebral processes such as heart contractility [103]. Another pathophysiological mechanism is shown in Fig. 11 . The earlier explained mechanism of the binding of coronavirus to the ACE2 is a key factor that enables the entrance of the coronavirus into host cells. ACE2 receptors are expressed in different cell types in the brain such as astrocytes, cells that line the endothelium of blood vessels and pericytes. ACE2 can be expressed in blood vessels and the choroidal epithelium in choroid plexus of the lateral ventricles, possibly allowing the penetration of SARS-CoV-2 virus into the cerebrospinal fluid (CSF). Fig. 12 shows this interaction of coronavirus with the ACE2 receptor. Although the expression of ACE2 was also found in a larger number of different types of cells in brain's tissues, these are still less expressed in the brain than in peripheral tissues. Therefore, it is hypothesized that direct infection of the brain by SARS-CoV-2 virus is also possible but more research is required to precisely decipher Covid-19 virus affinity towards the brain and possible prevention and therapy of neurological manifestations of this disease [111].
Fig. 11.
One of the possible neuropathological mechanisms of SARS-CoV-2 [111].
Fig. 12.
Touchpoints of SARS-CoV-2 and blood-brain barrier [111].
4.5. Interstitial lung disease
The system that is often affected by the SARS-CoV-2 virus is respiratory system especially the lungs. Interestingly, pneumonia that has SARS-CoV-2 as the main cause of infection could cause secondary pulmonary fibrosis. Secondary pulmonary fibrosis can potentially prolong the respiratory symptoms caused by this infection that is called interstitial lung disease (ILD) [112]. Interstitial lung disease can significantly increase the risk of critical illness or mortality [113]. The therapy used to treat patients with IDL is mainly based on corticosteroids [114]. Among the diseases that have the predisposition to cause secondary lung infections such as interstitial lung disease is diabetes mellitus [115]. The patients diagnosed with diabetes mellitus more often develop asthma, COPD, pneumonia and pulmonary fibrosis [116]. The processes behind this phenomenon have not been fully elucidated yet, so further research is necessary although it is thought that autoantibodies could play an important role. The therapeutic strategy for IDL is not established and immunosuppressants are thought to be useful in the treatment of ILD [113]. Additionally, novel studies have shown that pirfenidone (PFN), the anti-fibrotic agent has beneficial effects on the pulmonary fibrosis that occurs after the Covid-19 infection. PFN can lower the activation of inflammatory cells and decrease the release of pro-inflammatory cytokines such as transforming growth factor beta (TGF-β1). Consequently, the proliferation of fibroblasts and the synthesis of extracellular matrix, especially collagen is decreased. However, the use of PFN in the treatment of pulmonary complications after Covid-19 should be researched in future studies [41].
4.6. Orchitis
The human testes are also at risk of becoming collateral damage of Covid-19 infection. The most common manifestation is orchitis, although it is not an often-seen consequence of this disease [117]. The mechanism that helps this virus to avoid blood-testis barrier is most likely connected with the presence of ACE2 in the testis. However, this receptor is not expressed enough on the cell surface in the testes to enable the virus entrance into cells. Therefore, it is thought that TMPRSS2 has an important role. It is a protease that is responsible for the priming of the spike protein of this virus and it helps ACE2 to open the door of the host cell for its entry [118]. The inflammatory response that is triggered by the production of molecules marked as cytokines such as IL-6 and TNF- α effects the testes that could change and disrupt the barrier between testis and blood. Additionally, the increased body temperature in infections such as Covid-19 could directly damage testicular cells and lead to leukocyte infiltration [119]. The number of studies investigated the effects of SARS-CoV-2 virus on the male reproductive system is insufficient. Because the consequences of inflammation in these organs are serious such as sterility or impaired spermatogenesis, further studies are needed to properly address the diagnostic and therapeutic protocols for this disease.
5. Conclusion
Covid-19 infection is a trigger for various types of autoimmune diseases that manifest either as a systemic disease or as an organ-specific one. Excessive activation of immune response and triggering of autoantibodies synthesis as well as an excessive synthesis of inflammatory cytokines and onset of cytokine storm have a vital role in the disease outcome. These processes are important factors in onset of autoimmune complications of Covid-19 such as arthritis, vasculitis, antiphospholipid syndrome and multisystem inflammatory syndrome. Yet, many unknowns need to be deciphered about Covid-19 autoimmune manifestations. The precise knowledge of complex mechanisms behind the inflammatory and autoimmune reactions could impose novel therapeutic options for their treatment, especially for the use of immunosuppressants and different receptor modulators in limiting the body's immune response. Corticosteroids are known for reducing suppressive action of the immune response that has an impact on mortality reduction. Additionally, the therapy with Interferon-gamma (IFN-γ) showed promising results as well as the use of S1P receptor modulators, such as the drug called fingolimod. However, there are still many unknowns about the use of these novel therapies. Therefore, the inflammation and autoimmunity triggered by Covid-19 should be further investigated to improve existing diagnostic procedures and therapeutic protocols for Covid-19.
CRediT authorship contribution statement
Emina Karahmet Sher: Data Curation, Investigation, Formal Analysis, Methodology, Software, Supervision, Resources, Writing-Original Draft. Adnan Ćosović, Amina Džidić-Krivić, Esma Karahmet Farhat: Conceptualization, Visualization, Writing-Original Draft, Resources, Validation, Supervision. Emma Pinjić: Visualization, Software, Formal Analysis, Resources. Farooq Sher: Software, Resources, Writing-Original Draft, Administration, Funding Acquisition, Submission, Reviewing and Editing.
Declaration of competing interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Acknowledgement
The authors are grateful for the financial support from the International Society of Engineering Science and Technology (ISEST), UK.
References
- 1.Hysa E., et al. Immune system activation in polymyalgia rheumatica: which balance between autoinflammation and autoimmunity? A systematic review. Autoimmun. Rev. 2022;21(2) doi: 10.1016/j.autrev.2021.102995. [DOI] [PubMed] [Google Scholar]
- 2.Galeotti C., Bayry J. Autoimmune and inflammatory diseases following COVID-19. Nat. Rev. Rheumatol. 2020;16(8):413–414. doi: 10.1038/s41584-020-0448-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Gupta M., Weaver D.F. COVID-19 as a trigger of brain autoimmunity. ACS Chem. Neurosci. 2021;12(14):2558–2561. doi: 10.1021/acschemneuro.1c00403. [DOI] [PubMed] [Google Scholar]
- 4.Knight J.S., et al. The intersection of COVID-19 and autoimmunity. J. Clin. Invest. 2021;131(24) doi: 10.1172/JCI154886. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Ramos-Casals M., Brito-Zerón P., Mariette X. Systemic and organ-specific immune-related manifestations of COVID-19. Nat. Rev. Rheumatol. 2021;17(6):315–332. doi: 10.1038/s41584-021-00608-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Tang K.-T., Hsu B.-C., Chen D.-Y. Autoimmune and rheumatic manifestations associated with COVID-19 in adults: an updated systematic review. Front. Immunol. 2021;12 doi: 10.3389/fimmu.2021.645013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Ehrenfeld M., et al. Covid-19 and autoimmunity. Autoimmun. Rev. 2020;19(8) doi: 10.1016/j.autrev.2020.102597. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Kumar A., et al. COVID-19 mechanisms in the human body—what we know so far. Front. Immunol. 2021;12 doi: 10.3389/fimmu.2021.693938. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Mobasheri L., et al. SARS-CoV-2 triggering autoimmune diseases. Cytokine. 2022;154 doi: 10.1016/j.cyto.2022.155873. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Nile S.H., et al. COVID-19: pathogenesis, cytokine storm and therapeutic potential of interferons. Cytokine Growth Factor Rev. 2020;53:66–70. doi: 10.1016/j.cytogfr.2020.05.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Hu B., Huang S., Yin L. The cytokine storm and COVID-19. J. Med. Virol. 2021;93(1):250–256. doi: 10.1002/jmv.26232. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Ziegler C.G.K. Harvard University; 2021. Deconstructing Cell Intrinsic Immunity and Host-Pathogen Interactions Using Single-Cell Genomics. [Google Scholar]
- 13.Pizzato M. SARS-CoV-2 and the host cell: a tale of interactions. Front. Virol. 2022:1. [Google Scholar]
- 14.Jose R.J., Manuel A. COVID-19 cytokine storm: the interplay between inflammation and coagulation. Lancet Respir. Med. 2020;8(6):e46–e47. doi: 10.1016/S2213-2600(20)30216-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Carissimo G., et al. Whole blood immunophenotyping uncovers immature neutrophil-to-VD2 T-cell ratio as an early marker for severe COVID-19. Nat. Commun. 2020;11(1):1–12. doi: 10.1038/s41467-020-19080-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Belaid B., et al. T cell counts and IL-6 concentration in blood of north african COVID-19 patients are two independent prognostic factors for severe disease and death. J. Leukoc. Biol. 2022;111(1):269–281. doi: 10.1002/JLB.4COVA1020-703R. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Sattler A., et al. SARS–CoV-2–specific T cell responses and correlations with COVID-19 patient predisposition. J. Clin. Invest. 2020;130(12):6477–6489. doi: 10.1172/JCI140965. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Shang J. Cell Entry Mechanisms of SARS-CoV-2. 117(21) 2020. pp. 11727–11734. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Prasansuklab A., et al. Anti-COVID-19 drug candidates: a review on potential biological activities of natural products in the management of new coronavirus infection. J. Tradit. Complement. Med. 2021;11(2):144–157. doi: 10.1016/j.jtcme.2020.12.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Xiu S., et al. Inhibitors of SARS-CoV-2 entry: current and future opportunities. J. Med. Chem. 2020;63(21):12256–12274. doi: 10.1021/acs.jmedchem.0c00502. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Doty R.L. Olfactory dysfunction in COVID-19: pathology and long-term implications for brain health. Trends Mol. Med. 2022;28(9) doi: 10.1016/j.molmed.2022.06.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Chen Z., et al. SARS-CoV-2 cell entry and targeted antiviral development. Acta Pharm. Sin. B. 2021;11(12):3879–3888. doi: 10.1016/j.apsb.2021.05.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Behl T., et al. CD147-spike protein interaction in COVID-19: get the ball rolling with a novel receptor and therapeutic target. Sci. Total Environ. 2022;808 doi: 10.1016/j.scitotenv.2021.152072. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Mayi B.S. 17(1) 2021. The Role of Neuropilin-1 in COVID-19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Pan Y., et al. Role of the SphK-S1P-S1PRs pathway in invasion of the nervous system by SARS-CoV-2 infection. Clin. Exp. Pharmacol. Physiol. 2021;48(5):637–650. doi: 10.1111/1440-1681.13483. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Zhang E., Ma Z., Lu M. Contribution of T-and B-cell intrinsic toll-like receptors to the adaptive immune response in viral infectious diseases. Cell. Mol. Life Sci. 2022;79(11):1–15. doi: 10.1007/s00018-022-04582-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Pawliczak R. The immune response to SARS-CoV-2. Focus on severe COVID-19 pathogenesis. Alergol. Pol. Pol. J. Allergol. 2020;7(3):146–152. [Google Scholar]
- 28.Mustafa M.I., et al. Cytokine storm in COVID-19 patients, its impact on organs and potential treatment by QTY code-designed detergent-free chemokine receptors. Mediat. Inflamm. 2020;2020 doi: 10.1155/2020/8198963. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Coperchini F., et al. The cytokine storm in COVID-19: an overview of the involvement of the chemokine/chemokine-receptor system. Cytokine Growth Factor Rev. 2020;53:25–32. doi: 10.1016/j.cytogfr.2020.05.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Zaki N., Alashwal H., Ibrahim S. Association of hypertension, diabetes, stroke, cancer, kidney disease, and high-cholesterol with COVID-19 disease severity and fatality: a systematic review. Diabetes Metab. Syndr. Clin. Res. Rev. 2020;14(5):1133–1142. doi: 10.1016/j.dsx.2020.07.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Petrey A.C., et al. Cytokine release syndrome in COVID-19: innate immune, vascular, and platelet pathogenic factors differ in severity of disease and sex. J. Leukoc. Biol. 2021;109(1):55–66. doi: 10.1002/JLB.3COVA0820-410RRR. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Prnjavorac B., et al. Chest x-ray resolution after SARS-CoV-2 infection. Med. Glas. 2021;18(2) doi: 10.17392/1391-21. [DOI] [PubMed] [Google Scholar]
- 33.Grasselli G., et al. Pathophysiology of COVID-19-associated acute respiratory distress syndrome: a multicentre prospective observational study. Lancet Respir. Med. 2020;8(12):1201–1208. doi: 10.1016/S2213-2600(20)30370-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Al-Kuraishy H.M., et al. Neutrophil extracellular traps (NETs) and Covid-19: a new frontiers for therapeutic modality. Int. Immunopharmacol. 2022;104 doi: 10.1016/j.intimp.2021.108516. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Ahmadian E., et al. Covid-19 and kidney injury: pathophysiology and molecular mechanisms. Rev. Med. Virol. 2021;31(3) doi: 10.1002/rmv.2176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Wu J., et al. Liver diseases in COVID-19: etiology, treatment and prognosis. World J. Gastroenterol. 2020;26(19):2286. doi: 10.3748/wjg.v26.i19.2286. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Karahmet E., et al. Clinical use of an analysis of oxidative stress and IL-6 as the promoters of diabetic polyneuropathy. Med. Glas. 2021;18(1):12–17. doi: 10.17392/1279-21. [DOI] [PubMed] [Google Scholar]
- 38.Emina K., et al. IDF21-0423 Michigan neuropathy screening for assessing diabetes in participants and correlation to the immune response. J. Diabetes Res. Clin. Pract. 2022:186. [Google Scholar]
- 39.McIntosh K., Hirsch M.S., Bloom A. In: COVID-19: Clinical Features. UpToDate. Post T.W., editor. UpToDate; Waltham, MA: 2021. [Google Scholar]
- 40.Batiha G.E. Targeting of Neuroinflammation by Glibenclamide in Covid-19: Old Weapon From Arsenal. 2022. pp. 1–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Al-Kuraishy H.M. Pirfenidone and Post-Covid-19 Pulmonary Fibrosis: Invoked Again for Realistic Goals. 30(6) 2022. pp. 2017–2026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Ni W., et al. Role of angiotensin-converting enzyme 2 (ACE2) in COVID-19. Crit. Care. 2020;24(1):1–10. doi: 10.1186/s13054-020-03120-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Cenko E., et al. Cardiovascular disease and COVID-19: a consensus paper from the ESC working group on Coronary Pathophysiology & Microcirculation, ESC working group on thrombosis and the Association for Acute CardioVascular care (ACVC), in collaboration with the european heart rhythm association (EHRA) Cardiovasc. Res. 2021;117(14):2705–2729. doi: 10.1093/cvr/cvab298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Alkhayyat S.S., et al. Fenofibrate for COVID-19 and related complications as an approach to improve treatment outcomes: the missed key for holy grail. Inflamm. Res. 2022;71(10–11):1159–1167. doi: 10.1007/s00011-022-01615-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Wiese O., Allwood B., Zemlin A. COVID-19 and the renin-angiotensin system (RAS): a spark that sets the forest alight? Med. Hypotheses. 2020;144 doi: 10.1016/j.mehy.2020.110231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Zhang J. Risk factors for disease severity, unimprovement, and mortality in COVID-19 patients in Wuhan, China. Clin. Microbiol. Infect. 2020;26(6):767–772. doi: 10.1016/j.cmi.2020.04.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Tian J., et al. Clinical characteristics and risk factors associated with COVID-19 disease severity in patients with cancer in Wuhan, China: a multicentre, retrospective, cohort study. Lancet Oncol. 2020;21(7):893–903. doi: 10.1016/S1470-2045(20)30309-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Al-Kuraishy H.M., et al. Calprotectin: the link between acute lung injury and gastrointestinal injury in Covid-19: ban or boon. Curr. Protein Pept. Sci. 2022;23(5):310–320. doi: 10.2174/1389203723666220610124303. [DOI] [PubMed] [Google Scholar]
- 49.Kutsuna S. Clinical manifestations of coronavirus disease 2019. JMA J. 2021;4(2):76–80. doi: 10.31662/jmaj.2021-0013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Chumakov K. Old vaccines for new infections: exploiting innate immunity to control COVID-19 and prevent future pandemics. Proc. Natl. Acad. Sci. 2021;118(21) doi: 10.1073/pnas.2101718118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Al-Kuraishy H.M., et al. Testosterone in COVID-19: an adversary bane or comrade boon. Frontiers in cellular and infection. Microbiology. 2021:832. doi: 10.3389/fcimb.2021.666987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Pearce L., Davidson S.M., Yellon D.M. The cytokine storm of COVID-19: a spotlight on prevention and protection. Expert Opin. Ther. Targets. 2020;24(8):723–730. doi: 10.1080/14728222.2020.1783243. [DOI] [PubMed] [Google Scholar]
- 53.Horie S., et al. Emerging pharmacological therapies for ARDS: COVID-19 and beyond. Intensive Care Med. 2020;46(12):2265–2283. doi: 10.1007/s00134-020-06141-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Thorne L.G., et al. SARS-CoV-2 sensing by RIG-I and MDA5 links epithelial infection to macrophage inflammation. EMBO J. 2021;40(15) doi: 10.15252/embj.2021107826. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Netea M.G., et al. Innate and adaptive immune memory: an evolutionary continuum in the host’s response to pathogens. Cell Host Microbe. 2019;25(1):13–26. doi: 10.1016/j.chom.2018.12.006. [DOI] [PubMed] [Google Scholar]
- 56.Cifuentes-Rius A., et al. Inducing immune tolerance with dendritic cell-targeting nanomedicines. Nat. Nanotechnol. 2021;16(1):37–46. doi: 10.1038/s41565-020-00810-2. [DOI] [PubMed] [Google Scholar]
- 57.Abbas A.K., Lichtman A.H., Pillai S. Elsevier Health Sciences; 2021. Cellular and Molecular Immunology E-book. [Google Scholar]
- 58.Pishesha N., Harmand T.J., Ploegh H.L. A guide to antigen processing and presentation. Nat. Rev. Immunol. 2022:1–14. doi: 10.1038/s41577-022-00707-2. [DOI] [PubMed] [Google Scholar]
- 59.d'Izarny-Gargas T., Durrbach A., Zaidan M. Efficacy and tolerance of immune checkpoint inhibitors in transplant patients with cancer: a systematic review. Am. J. Transplant. 2020;20(9):2457–2465. doi: 10.1111/ajt.15811. [DOI] [PubMed] [Google Scholar]
- 60.T. Xing , Cell tolerance: central and peripheral, Cold Spring Harb. Perspect. Biol., (4): a006957. [DOI] [PMC free article] [PubMed]
- 61.Klein L., Robey E.A., Hsieh C.-S. Central CD4+ T cell tolerance: deletion versus regulatory T cell differentiation. Nat. Rev. Immunol. 2019;19(1):7–18. doi: 10.1038/s41577-018-0083-6. [DOI] [PubMed] [Google Scholar]
- 62.Sprent J., Kishimoto H. SC the royal society. Philos. Trans. R. Soc. Lond. Biol. Sci. Ser. B. 2001;356(1409-1412):609–616. doi: 10.1098/rstb.2001.0846. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Meffre E., O'Connor K.C. Impaired B-cell tolerance checkpoints promote the development of autoimmune diseases and pathogenic autoantibodies. Immunol. Rev. 2019;292(1):90–101. doi: 10.1111/imr.12821. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Klein L., et al. Positive and negative selection of the T cell repertoire: what thymocytes see (and don't see) Nat. Rev. Immunol. 2014;14(6):377–391. doi: 10.1038/nri3667. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.ElTanbouly M.A., Noelle R.J. Rethinking peripheral T cell tolerance: checkpoints across a T cell’s journey. Nat. Rev. Immunol. 2021;21(4):257–267. doi: 10.1038/s41577-020-00454-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Peng B., Ming Y., Yang C. Regulatory B cells: the cutting edge of immune tolerance in kidney transplantation. Cell Death Dis. 2018;9(2):1–13. doi: 10.1038/s41419-017-0152-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Wang J., et al. Profiling the origin, dynamics, and function of traction force in B cell activation. Sci. Signal. 2018;11(542) doi: 10.1126/scisignal.aai9192. [DOI] [PubMed] [Google Scholar]
- 68.Tong P., et al. Memory B cell repertoire for recognition of evolving SARS-CoV-2 spike. Cell. 2021;184(19):4969–4980. doi: 10.1016/j.cell.2021.07.025. e15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Nemazee D. Mechanisms of central tolerance for B cells. Nat. Rev. Immunol. 2017;17(5):281–294. doi: 10.1038/nri.2017.19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.LeBien T.W., Tedder T.F. B lymphocytes: how they develop and function. Blood, J. Am. Soc. Hematol. 2008;112(5):1570–1580. doi: 10.1182/blood-2008-02-078071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Damato V., Evoli A., Iorio R. Efficacy and safety of rituximab therapy in neuromyelitis optica spectrum disorders: a systematic review and meta-analysis. JAMA Neurol. 2016;73(11):1342. doi: 10.1001/jamaneurol.2016.1637. 2016. [DOI] [PubMed] [Google Scholar]
- 72.Tan C., et al. Self-reactivity on a spectrum: a sliding scale of peripheral B cell tolerance. Immunol. Rev. 2019;292(1):37–60. doi: 10.1111/imr.12818. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Brooks J.F., et al. Peripheral tolerance checkpoints imposed by ubiquitous antigen expression limit antigen-specific B cell responses under strongly immunogenic conditions. J. Immunol. 2020;205(5):1239–1247. doi: 10.4049/jimmunol.2000377. [DOI] [PubMed] [Google Scholar]
- 74.Chackerian B., Peabody D.S. Factors that govern the induction of long-lived antibody responses. Viruses. 2020;12(1):74. doi: 10.3390/v12010074. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Damoiseaux J., et al. Autoantibodies and SARS-CoV2 infection: the spectrum from association to clinical implication: report of the 15th Dresden symposium on autoantibodies. Autoimmun. Rev. 2022;21(3) doi: 10.1016/j.autrev.2021.103012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Adiguzel Y. Molecular mimicry between SARS-CoV-2 and human proteins. Autoimmun. Rev. 2021;20(4) doi: 10.1016/j.autrev.2021.102791. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Bastard P., et al. Autoantibodies neutralizing type I IFNs are present in~ 4% of uninfected individuals over 70 years old and account for~ 20% of COVID-19 deaths. Sci. Immunol. 2021;6(62) doi: 10.1126/sciimmunol.abl4340. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Bastard P., et al. Vaccine breakthrough hypoxemic COVID-19 pneumonia in patients with auto-abs neutralizing type I IFNs. Sci. Immunol. 2022 doi: 10.1126/sciimmunol.abp8966. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Gracia-Ramos A.E., Martin-Nares E., Hernández-Molina G. New onset of autoimmune diseases following COVID-19 diagnosis. Cells. 2021;10(12):3592. doi: 10.3390/cells10123592. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Rodríguez Y., et al. Autoinflammatory and autoimmune conditions at the crossroad of COVID-19. J. Autoimmun. 2020;114 doi: 10.1016/j.jaut.2020.102506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Dewanjee S., et al. COVID-19 and rheumatoid arthritis crosstalk: emerging association, therapeutic options and challenges. Cells. 2021;10(12):3291. doi: 10.3390/cells10123291. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Blevins B.L., et al. Brain arteriolosclerosis. Acta Neuropathol. 2021;141(1):1–24. doi: 10.1007/s00401-020-02235-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Noris M., Benigni A., Remuzzi G. The case of complement activation in COVID-19 multiorgan impact. Kidney Int. 2020;98(2):314–322. doi: 10.1016/j.kint.2020.05.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Wong K., et al. COVID-19 associated vasculitis: a systematic review of case reports and case series. Ann. Med. Surg. 2022;74 doi: 10.1016/j.amsu.2022.103249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Vinayagam S., Sattu K. SARS-CoV-2 and coagulation disorders in different organs. Life Sci. 2020;260 doi: 10.1016/j.lfs.2020.118431. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Borghi M.O., et al. Anti-phospholipid antibodies in COVID-19 are different from those detectable in the anti-phospholipid syndrome. Front. Immunol. 2020;11 doi: 10.3389/fimmu.2020.584241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Tung M.L., et al. Anti-phospholipid syndrome and COVID-19 thrombosis: connecting the dots. Rheumatol. Adv. Pract. 2021;5(1) doi: 10.1093/rap/rkaa081. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Hollerbach A., et al. Pathogenic lipid-binding antiphospholipid antibodies are associated with severity of COVID-19. J. Thromb. Haemost. 2021;19(9):2335–2347. doi: 10.1111/jth.15455. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Serrano M., et al. COVID-19 and the antiphospholipid syndrome. Autoimmun. Rev. 2022;21 doi: 10.1016/j.autrev.2022.103206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Legault K., et al. McMaster RARE-bestpractices clinical practice guideline on diagnosis and management of the catastrophic antiphospholipid syndrome. J. Thromb. Haemost. 2018;16(8):1656–1664. doi: 10.1111/jth.14192. [DOI] [PubMed] [Google Scholar]
- 91.López-Benjume B., et al. Eculizumab use in catastrophic antiphospholipid syndrome (CAPS): descriptive analysis from the “CAPS registry”. Autoimmun. Rev. 2022;21(4) doi: 10.1016/j.autrev.2022.103055. [DOI] [PubMed] [Google Scholar]
- 92.Pengo V., Denas G. Diagnostics and treatment of thrombotic antiphospholipid syndrome (APS): a personal perspective. Thromb. Res. 2018;169:35–40. doi: 10.1016/j.thromres.2018.07.011. [DOI] [PubMed] [Google Scholar]
- 93.Price L.C. Thrombosis and COVID-19 pneumonia: the clot thickens! Eur. Respiratory Soc. 2020;56 doi: 10.1183/13993003.01608-2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Ahmed M., et al. Multisystem inflammatory syndrome in children: a systematic review. EClinicalMedicine. 2020;26 doi: 10.1016/j.eclinm.2020.100527. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Godfred-Cato S., et al. COVID-19–associated multisystem inflammatory syndrome in children—United States, March–July 2020. Morb. Mortal. Wkly Rep. 2020;69(32):1074. doi: 10.15585/mmwr.mm6932e2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Wang X., et al. COVID-19 and antiphospholipid antibodies: a position statement and management guidance from AntiPhospholipid syndrome Alliance for clinical trials and InternatiOnal networking (APS ACTION) Lupus. 2021;30(14):2276–2285. doi: 10.1177/09612033211062523. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Qeadan F., et al. The associations between COVID-19 diagnosis, type 1 diabetes, and the risk of diabetic ketoacidosis: a nationwide cohort from the US using the cerner real-world data. PLoS One. 2022;17(4) doi: 10.1371/journal.pone.0266809. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Scappaticcio L. Impact of COVID-19 on the Thyroid Gland: An Update. 22(4) 2021. pp. 803–815. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Croce L. The Cytokine Storm and Thyroid Hormone Changes in COVID-19. 44(5) 2021. pp. 891–904. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Mina A., Van Besien K., Platanias L.C. Hematological manifestations of COVID-19. Leuk. Lymphoma. 2020;61(12):2790–2798. doi: 10.1080/10428194.2020.1788017. [DOI] [PubMed] [Google Scholar]
- 101.Terpos E., et al. Hematological findings and complications of COVID-19. Am. J. Hematol. 2020;95(7):834–847. doi: 10.1002/ajh.25829. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Hottz E.D., et al. Platelet activation and platelet-monocyte aggregate formation trigger tissue factor expression in patients with severe COVID-19. Blood. 2020;136(11):1330–1341. doi: 10.1182/blood.2020007252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Jäger U., et al. Diagnosis and treatment of autoimmune hemolytic anemia in adults: recommendations from the first international consensus meeting. Blood Rev. 2020;41 doi: 10.1016/j.blre.2019.100648. [DOI] [PubMed] [Google Scholar]
- 104.Jamshidi P. Skin manifestations in COVID-19 patients: Are they indicators for disease severity? A systematic review. Front. Med. 2021;8:634208. doi: 10.3389/fmed.2021.634208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Singh H., et al. Cutaneous manifestations of COVID-19: a systematic review. Adv. Wound Care. 2021;10(2):51–80. doi: 10.1089/wound.2020.1309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Rizk J.G., et al. Pharmaco-immunomodulatory therapy in COVID-19. Drugs. 2020;80(13):1267–1292. doi: 10.1007/s40265-020-01367-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Nasiri N., et al. Ocular manifestations of COVID-19: a systematic review and meta-analysis. J. Ophthalmic Vision Res. 2021;16(1):103. doi: 10.18502/jovr.v16i1.8256. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Zhong Y., et al. Ocular manifestations in COVID-19 patients: a systematic review and meta-analysis. Travel Med. Infect. Dis. 2021;44 doi: 10.1016/j.tmaid.2021.102191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Hu K. StatPearls [Internet] StatPearls Publishing; 2022. Ophthalmic manifestations of coronavirus (COVID-19) [PubMed] [Google Scholar]
- 110.Pezzini A., Padovani A. Lifting the mask on neurological manifestations of COVID-19. Nat. Rev. Neurol. 2020;16(11):636–644. doi: 10.1038/s41582-020-0398-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Johansson A., et al. Neurological manifestations of COVID-19: a comprehensive literature review and discussion of mechanisms. J. Neuroimmunol. 2021;358 doi: 10.1016/j.jneuroim.2021.577658. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Wong A.W., et al. Practical considerations for the diagnosis and treatment of fibrotic interstitial lung disease during the coronavirus disease 2019 pandemic. Chest. 2020;158(3):1069–1078. doi: 10.1016/j.chest.2020.04.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Uemasu K., et al. Post-COVID-19 interstitial lung disease presenting with profound hypoxemia: report of three cases demonstrating a good response to high-dose corticosteroid therapy. J. Infect. Chemother. 2022;28(2):321–325. doi: 10.1016/j.jiac.2021.11.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Myall K.J., et al. Persistent post–COVID-19 interstitial lung disease. An observational study of corticosteroid treatment. Ann. Am. Thorac. Soc. 2021;18(5):799–806. doi: 10.1513/AnnalsATS.202008-1002OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Raghu G., et al. Diagnosis of idiopathic pulmonary fibrosis. An official ATS/ERS/JRS/ALAT clinical practice guideline. Am. J. Respir. Crit. Care Med. 2018;198(5):e44–e68. doi: 10.1164/rccm.201807-1255ST. [DOI] [PubMed] [Google Scholar]
- 116.Ehrlich S.F., et al. Patients diagnosed with diabetes are at increased risk for asthma, chronic obstructive pulmonary disease, pulmonary fibrosis, and pneumonia but not lung cancer. Diabetes Care. 2010;33(1):55–60. doi: 10.2337/dc09-0880. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Sengupta P., Leisegang K., Agarwal A. The impact of COVID-19 on the male reproductive tract and fertility: a systematic review. Arab. J. Urol. 2021;19(3):423–436. doi: 10.1080/2090598X.2021.1955554. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Sheikhzadeh Hesari F., Hosseinzadeh S.S., Sardroud M.A.Asl Monadi. Review of COVID-19 and male genital tract. Andrologia. 2021;53(1) doi: 10.1111/and.13914. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Tian Y., Zhou L.-Q. Evaluating the impact of COVID-19 on male reproduction. Reproduction. 2021;161(2):R37–R44. doi: 10.1530/REP-20-0523. [DOI] [PubMed] [Google Scholar]