Abstract
Background
The impact of chronic kidney disease (CKD) on adverse cardiovascular outcomes after percutaneous coronary intervention in patients with diabetes mellitus (DM) is still unclear. This study aimed to systematically assess evidence on this topic.
Methods
The PubMed, Embase, and CENTRAL databases were searched for studies comparing mortality, myocardial infarction (MI), or revascularization outcomes between patients with DM with and without CKD.
Results
In 11 studies, the presence of CKD was associated with significantly increased risk of early all-cause mortality (risk ratio [RR], 3.45; 95% CI, 3.07–3.87; I2 = 0%; P < .001), late all-cause mortality (RR, 2.78; 95% CI, 1.92–4.02; I2 = 83%; P < .001), cardiac mortality (RR, 2.90; 95% CI, 1.99–4.22; I2 = 29%; P < .001), and MI (RR, 1.40; 95% CI, 1.06–1.85; I2 = 13%; P = .02) compared with no CKD. There was no difference in the risk of any revascularization between those with and without CKD. Analysis of adjusted hazard ratios (HRs) indicated significantly increased risk of mortality (HR, 2.64; 95% CI, 1.91–3.64; I2 = 0%; P < .001) in the CKD group but only a nonsignificant tendency of increased MI (HR, 1.59; 95% CI, 0.99–2.54; I2 = 0%; P = .05) and revascularization (HR, 1.24; 95% CI, 0.94–1.63; I2 = 2%; P = .12) in the CKD group.
Conclusion
The presence of CKD in patients with DM significantly increases the risk of mortality and MI. However, CKD had no impact on revascularization rates.
Keywords: Renal failure, kidney disease, mortality, diabetes mellitus, coronary artery disease
Introduction
Percutaneous coronary intervention (PCI) is a common treatment modality for patients with coronary artery disease (CAD). Over the past 2 decades, improvements in procedure devices, stent design, and allied technology combined with judicious patient selection and optimal antiplatelet therapy have made PCI a safe procedure with excellent clinical outcomes.1,2 However, as with any interventional procedure, many associated comorbidities can influence post-PCI patient survival.3
Diabetes mellitus (DM) has been identified as an important risk factor for adverse clinical outcomes after PCI.4,5 Indeed, a recent meta-analysis by Zhou et al6 with data from 139,774 participants has demonstrated that the presence of DM as a comorbidity results in a 2.57-times-higher risk of in-hospital mortality and a 1.38-times-higher risk of major adverse cardiac events after PCI. Such increased risk of mortality has been maintained despite contemporary antiplatelet therapy and large-scale adoption of drug-eluting stents (DES).7,8 Along with such adverse outcomes, DM as a disease can lead to several other organ dysfunctions. Of note, chronic kidney disease (CKD) is a common complication seen with DM, with studies indicating a 3-times-greater risk of renal dysfunction in patients with DM than that in the general population.9
The relationship between CKD and CAD has been well explored in the literature.10 Chronic kidney disease is not only an independent predictor of cardiovascular disease but also a significant comorbidity that can lead to adverse outcomes after cardiac revascularization procedures.10–12 Parikh et al13 demonstrated that the presence of CKD results in increased mortality after PCI and that the rate of adverse outcomes increases with the severity of CKD. Because DM is the cause of CKD in approximately 40% of patients,14 understanding how the presence of CKD affects outcomes for patients with DM who undergo PCI is important. Although there have been studies assessing the role of CKD in PCI outcomes in patients with DM,15,16 to the authors' knowledge, no systematic review or meta-analysis has been attempted to pool evidence on this subject. To bridge this gap in the literature, the current review aimed to perform a systematic literature search for studies assessing the impact of CKD on cardiovascular outcomes in patients with DM undergoing PCI and to pool data for a meta-analysis.
Patients and Methods
This review is based on the Preferred Reporting Items for Systematic Reviews and Meta-Analyses statement17 and the Cochrane Handbook for Systematic Reviews of Intervention.18 However, the study protocol was not pre-registered.
Literature Search
The online PubMed, Embase, and CENTRAL databases were examined for articles of interest to the review that were published between January 1, 2000, and March 1, 2021. No language restriction was applied. The search was carried out in duplicate by 2 reviewers working independently. Terms used for the search strategy were “chronic kidney disease,” “renal disease,” “renal dysfunction,” “dialysis,” “diabetes mellitus,” “percutaneous coronary intervention,” “angioplasty,” “PCI,” and “PCA.” Details of the various combinations used are presented in Supplementary Table I. The initial set of articles was examined using the titles and abstracts to recognize studies requiring full-text analysis. The full texts of selected articles were then extracted for final assessment. These were examined by the 2 reviewers separately based on the inclusion and exclusion criteria. Disagreements were resolved by discussion. The references of the included studies were also searched for any missed articles.
Inclusion and Exclusion Criteria
The Population, Intervention, Comparison, Outcome, and Study design guide was used to assess studies for eligibility. The following criteria were used: population, patients with DM undergoing PCI; intervention, CKD; comparison, no CKD; outcomes of mortality, myocardial infarction (MI), or revascularization rates; and study design, all types.
Studies were included irrespective of the sample size and follow-up duration. Exclusion criteria was as follows: (1) Studies on patients with DM not presenting separate outcome data for CKD and no CKD; (2) studies comparing outcomes of patients with CKD but not reporting separate data for those with DM; (3) studies on all revascularization strategies and not presenting separate data for PCI; (4) studies not describing any outcomes of interest; and (5) review articles, case reports, and unpublished data. If 2 or more studies used the same database or reported overlapping data, the study with the largest sample size was included in the review.
Data Extraction and Quality Assessment
Two reviewers extracted data independently using a prepared sheet. Data were extracted regarding author name; publication year; study type and location; stent type; sample size; patient demographic details; prior MI, hypertension, or stroke; dyslipidemia; 3-vessel disease; mean left ventricular ejection fraction; insulin use; follow-up period; and study outcomes. All extracted data were cross-checked for any errors with the parent article. The outcomes of interest for the meta-analysis were mortality (all-cause and cardiac), MI, and revascularization rates between patients with DM with and without CKD. The impact of the severity of CKD on these outcomes was also assessed.
The quality of included studies was examined by risk using a bias assessment tool for nonrandomized studies.19 Two reviewers examined each study for selection of participants, confounding variables, intervention measurements, blinding of outcome assessment, incomplete outcome data, and selective outcome reporting. Disagreements were resolved by discussion.
Statistical Analysis
Review Manager (RevMan, version 5.3; Cochrane) was used for the meta-analysis. Because of the intrinsic heterogeneity among the studies, the random-effects model was chosen for the meta-analysis. First, data on the absolute number of events of interest (mortality, MI, and revascularization) were extracted. These data were then pooled to calculate risk ratios (RRs) with 95% CIs. A separate analysis was conducted for early mortality (in-hospital and 30-day) and late mortality (until the end of the follow-up period). All-cause and cardiac mortality rates were analyzed separately. Second, multivariable-adjusted hazard ratios (HRs) of the outcome data were extracted from included studies. These data were then pooled using the generic inverse variance function of the software to calculate the pooled effect size. Few studies also reported HRs of a common composite outcome. The composite outcome was defined as combined mortality, MI, and revascularization rates. These data were also pooled in a meta-analysis. Heterogeneity was calculated using the I2 statistic. I2 values of 25% to 50% indicated low heterogeneity, 50% to 75% indicated medium heterogeneity, and greater than 75% indicated substantial heterogeneity. Funnel plots were used to assess publication bias. A P value of <.05 was considered statistically significant.
Results
Search Results and Study Details
A total of 1,145 unique records were identified after the literature search (Fig. 1). On exclusion of 1,110 articles after title and abstract screening, 35 studies with their full texts were analyzed. An additional 24 articles were excluded with reasons. Finally, a total of 11 studies were included in the systematic review and meta-analysis.15,16,20–28 The interreviewer agreement for study selection was high (κ = 0.9).
Fig. 1.
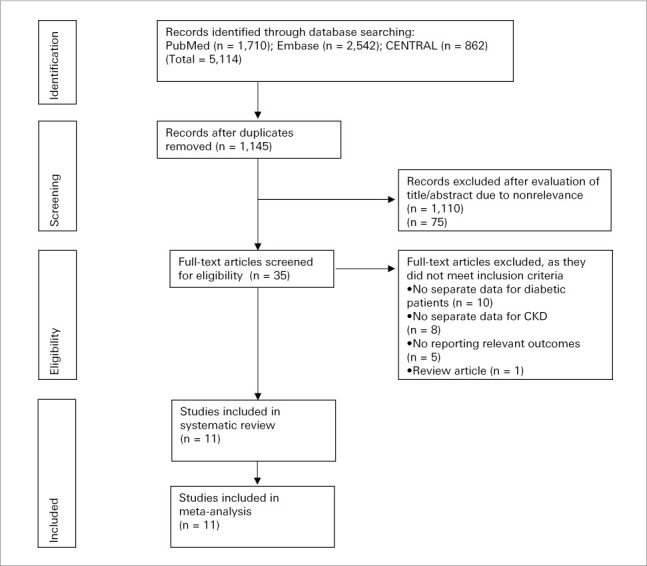
Study flow chart.
The characteristics of included studies are presented in Table I.15,16,20–28 One study28 was a post hoc analysis of 3 multicentric randomized controlled trials (RCTs), and all others were retrospective observational studies. All studies defined CKD as less than 60 mL/min/1.73 m2 of estimated glomerular filtration rate. Two studies15,25 did not report the type of stent used in the PCI. Three studies16,23,26 were exclusively on DES, 2 studies20,21 were on bare-metal stents (BMS), and the remaining studies used a combination of DES and BMS. Only 1 study23 performed propensity-score matching of the study groups. Liosis et al15 reported separate data for patients with ST-segment elevation and non–ST-segment elevation MI, which were pooled separately for the meta-analysis. Two studies15,22 reported only early outcomes; the follow-up of remaining studies varied from 1 to 6.8 years. The risk of bias assessment is depicted in Supplementary Table II.15,16,20–28
TABLE I.
Characteristics of Included Studies
| Study | Location | Study type | Stent type(s) | Groups | Sample size, No. | Mean age, y | Male, No. (%) | Prior MI, No. (%) | DL, No. (%) | Prior stroke, No. (%) | HTN, No. (%) | 3-Vessel disease, No. (%) | Mean LVEF, No. (%) | Insulin users, No. (%) | Follow-up |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Nikolsky, et al20 (2004) | USA | Retrospective | BMS | CKD | 529 | 67 | 248 (46.8) | 301 (56.9) | 327 (61.8) | 99 (18.7) | NR | NR | 222 (42) | 290 (54.8) | 1 y |
| No-CKD | 1,046 | 62.4 | 689 (65.9) | 538 (51.4) | 688 (65.8) | 123 (11.8) | NR | NR | 481 (46) | 441 (42.2) | |||||
| Goto, et al21 (2008) | Japan | Retrospective | BMS | CKD | 55 | 66.7 | 34 (62) | 5 (8.9) | 18 (32) | NR | 35 (63) | NR | 28 (51.5) | 5 (9.1) | 6.8 y |
| No-CKD | 165 | 63.9 | 119 (72) | 10 (6.1) | 76 (46) | NR | 109 (66) | NR | 87 (53) | 9 (5.5) | |||||
| Hung, et al22 (2013) | Taiwan | Retrospective | BMS, DES | CKD | 63 | 70.8 | 47 (74.6) | NR | NR | NR | 51 (81) | 7 (11.1) | NR | NR | 30 d |
| No-CKD | 85 | 57.4 | 75 (88.2) | NR | NR | NR | 58 (68.2) | 5 (5.9) | NR | NR | |||||
| Choi, et al23 (2016) | Republic of Korea | Retrospective | DES | CKD | 502 | 68.8 | 319 (63.5) | 135 (26.9) | 153 (30.5) | 53 (10.6) | 422 (84.1) | NR | NR | 25 (5) | 2 y |
| No-CKD | 502 | 68.8 | 325 (64.7) | 136 (27.1) | 147 (29.3) | 53 (10.6) | 416(82.9) | NR | NR | 30 (6) | |||||
| Lima, et al24 (2016) | Brazil | Retrospective | BMS, DES | CKD | 40 | 68 | 21 (52.5) | NR | NR | NR | 31 (77.5) | 24 (60) | 23 (57) | NR | 5.4 y |
| No-CKD | 72 | 58 | 43 (59.7) | NR | NR | NR | 56 (77.8) | 33 (45.8) | 41 (57) | NR | |||||
| Vichova, et al25 (2016) | Czech Republic | Retrospective | NR | CKD | 94 | 72 | 42 (44.7) | 18 (19.1) | NR | 16 (17) | 77 (81.9) | 48 (51) | NR | NR | 1 y |
| No-CKD | 123 | 64.1 | 98 (79.9) | 26 (21.1) | NR | 14 (11.5) | 99 (80.5) | 67 (54.6) | NR | NR | |||||
| Kim, et al26 (2017) | Republic of Korea | Retrospective | DES | CKD | 338 | 71.9 | 169 (50) | 35 (10.4) | 76 (22.5) | 29 (8.7) | 263 (77.7) | 149 (44.1) | NR | 42 (12.4) | 1 y |
| No-CKD | 549 | 63.3 | 388 (70.7) | 59 (10.7) | 129 (23.5) | 38 (7) | 349 (63.6) | 159 (29) | NR | 25 (4.6) | |||||
| Lin, et al27 (2017) | Taiwan | Retrospective | BMS, DES | CKD | 311 | 68.6 | 191 (61.4) | NR | 133 (42.8) | 9 (28) | 220 (70.7) | 93 (29.9) | 171 (55) | NR | 1 y |
| No-CKD | 254 | 59.6 | 194 (76.4) | NR | 139 (54.7) | 12 (4.7) | 151 (59.4) | 59 (23.2) | 150 (59) | NR | |||||
| Farkouh, et al28 (2019) | Multicentric | Post hoc analysis of RCTs | BMS, DES | CKD | 426 | NR | NR | NR | NR | NR | NR | NR | NR | NR | 4.5 y |
| No-CKD | 1,652 | NR | NR | NR | NR | NR | NR | NR | NR | NR | |||||
| Liosis, et al15 (2019) | Germany | Retrospective | NR | a) STEMI CKD | 1,341 | 74.2 | 837 (62.4) | NR | 1,031 (76.9) | 194 (14.5) | 1,262 (94.1) | 712 (53.1) | NR | NR | In-hospital only |
| No-CKD | 3,605 | 66.7 | 2,433 (67.5) | NR | 2,473 (68.6) | 187 (5.2) | 3,108 (86.2) | 1334 (37) | NR | NR | |||||
| b) NSTEMI CKD | 4,534 | 75.3 | 2,965 (65.4) | NR | 3,500 (77.2) | 676 (14.9) | 4,312 (95.1) | 2,707 (59.7) | NR | NR | |||||
| No-CKD | 8,642 | 71 | 5,643 (65.3) | NR | 6,144 (71.1) | 5,704 (66) | 7,752 (89.7) | 3,975 (46) | NR | NR | |||||
| Watanabe, et al16 (2020) | Japan | Retrospective | DES | CKD | 219 | 72 | 160 (73) | 90 (41) | 171 (78) | 21 (9.6) | 193 (88) | 127 (58) | 116 (53) | 53 (24) | 5.1 y |
| No-CKD | 293 | 69 | 231 (79) | 94 (32) | 202 (69) | 15 (5.1) | 237 (81) | 147 (50) | 170 (58) | 38 (13) |
BMS, bare-metal stent; CKD, chronic kidney disease; DES, drug eluting stent; DL, dyslipidemia; HTN, hypertension; LVEF, left ventricular ejection fraction; MI, myocardial infarction; NSTEMI, non–ST-segment elevation MI; NR, not reported; RCT, randomized controlled trial; STEMI, ST-segment elevation MI.
Meta-Analysis of the Absolute Number of Events
Pooled analysis on all-cause mortality indicated that the presence of CKD was associated with significantly increased risk of early (RR, 3.45; 95% CI, 3.07–3.87; I2 = 0%; P < .001) as well as late (RR, 2.78; 95% CI, 1.92–4.02; I2 = 83%; P < .001) mortality compared with patients without CKD (Fig. 2). There was no evidence of publication bias in a funnel plot (Fig. 3). The risk of cardiac mortality was also significantly higher in patients with CKD than in those without CKD (RR, 2.90; 95% CI, 1.99–4.22; I2 = 29%; P < .01) (Fig. 4A). The presence of CKD also significantly increased the risk of MI in patients with DM (RR, 1.40; 95% CI, 1.06–1.85; I2 = 13%; P = .02) (Fig. 4B). However, there was no difference in the risk of any revascularization between CKD and non-CKD groups (RR, 0.96; 95% CI, 0.82–1.13; I2 = 25%; P = .65) (Fig. 4C).
Fig. 2.
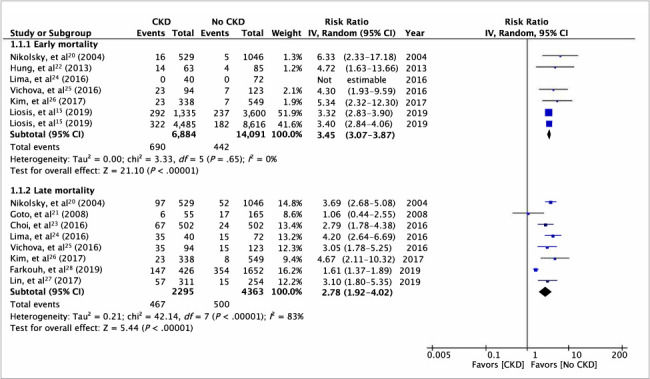
Results of the meta-analysis of early and late all-cause mortality between patients with diabetes mellitus with and without CKD. The total number of patients in Liosis et al15 excludes patients lost to follow-up. P < .05 was considered statistically significant.
CKD, chronic kidney disease; df, degree of freedom; IV, inverse variance.
Fig. 3.
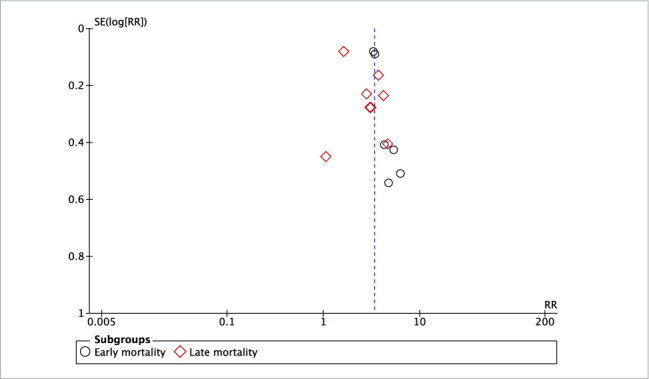
Funnel plot for the meta-analysis of mortality between patients with diabetes mellitus with and without chronic kidney disease.
RR, risk ratio.
Fig. 4.
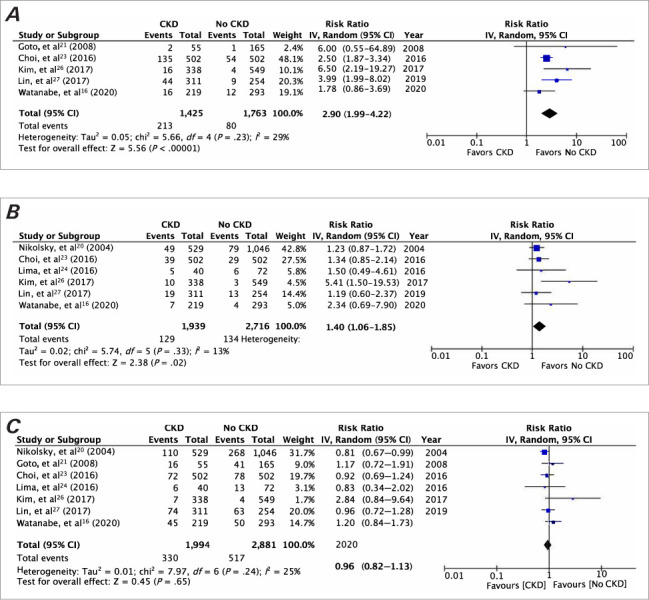
Meta-analysis of A) cardiac mortality, B) myocardial infarction, and C) revascularization between patients with diabetes mellitus with and without CKD. P < .05 was considered statistically significant.
CKD, chronic kidney disease; df, degree of freedom; IV, inverse variance.
Meta-Analysis of Adjusted Outcomes
Only 4 studies reported a multivariable-adjusted comparison of patients with and without CKD, and only 3 studies were available for meta-analysis of the following outcomes. For the composite outcome, meta-analysis indicated significantly increased combined risk of mortality, MI, and revascularization in patients with CKD compared with patients without CKD (HR, 1.70; 95% CI, 1.23–2.34; I2 = 0%; P = .001) (Supplementary Fig. 1). A separate meta-analysis of these 3 outcomes indicated a significantly increased risk of mortality (HR, 2.64; 95% CI, 1.91–3.64; I2 = 0%; P < .001) in the CKD group but only a nonsignificant tendency of increased MI (HR, 1.59; 95% CI, 0.99–2.54; I2 = 0%; P = .05) and revascularization (HR, 1.24; 95% CI, 0.94–1.63; I2 = 2%; P = .12) in the CKD group (Supplementary Fig. 1).
Outcomes Based on Severity of CKD
Because of interstudy heterogeneity and inadequate data reporting, a quantitative analysis on the impact of the severity of CKD on patient outcomes could not be conducted. However, a detailed description of outcomes reported by the studies is presented in Table II.15,16,20,22,23,25,27
TABLE II.
Outcomes of studies based on severity of CKD
| Study | CKD classification based on eGFR (mL/min/1.73 m2) | Outcomes |
|---|---|---|
| Nikolsky, et al20 (2004) | Dialysis | There was significantly higher in-hospital mortality and 1-y mortality in the dialysis group than in the nondialysis group (P < .001). |
| Nondialysis (eGFR not specified) | ||
| Hung, et al22 (2013) | Stage 1: ≥90 | Patients with stage 3 and 4 CKD had significantly higher risk of mortality than did patients with stage 1 and 2 disease (P < .001). |
| Stage 2: 60–89 | ||
| Stage 3: 30–59 | ||
| Stage 4: <30 | ||
| Choi, et al23 (2016) | Moderate: 30–60 | There was a significantly higher risk of cardiac death in both the moderate (P = .02) and severe (P < .001) CKD groups than in patients with normal renal function. |
| Severe: <30 | ||
| Vichova, et al25 (2016) | Moderate: 30–60 | There was significantly higher in-hospital mortality in the severe CKD group than in the moderate CKD group (P = .002). |
| Severe: <30 | ||
| Lin, et al27 (2017) | Stage 1: ≥90 | The advanced-stage CKD group (stage 4 and 5) had significantly higher cardiovascular mortality and all-cause mortality than the early-stage CKD group (both P < .001). There was no difference in these groups in terms of recurrent MI (P = .06) or revascularization (P = .20). |
| Stage 2: 60–89 | ||
| Stage 3: 30–59 | ||
| Stage 4: 15–29 | ||
| Stage 5: <15 | ||
| Liosis, et al15 (2019) | Mild: 45–60 | In-hospital mortality was seen to increase significantly with increase in severity of CKD (P < .001). |
| Moderate: 30–45 | ||
| Severe: <30 | ||
| Watanabe, et al16 (2020) | Mild: 45–60 | The target lesion failure, cardiac mortality, and MI were significantly higher in the severe CKD group than in other groups (all P ≤ .001). There was no difference in the rate of revascularization in the CKD groups (P = .31). |
| Moderate: 30–45 | ||
| Severe: <30 |
CKD, chronic kidney disease; eGFR, estimated glomerular filtration rate; MI, myocardial infarction.
Notwithstanding the difference in outcomes and classifications used by studies, the majority of studies reported an increased risk of adverse outcomes with increased severity of CKD.
Discussion
Diabetes mellitus is known to be one of the strongest predictors of poor outcomes after PCI.4,5 Not only do data from observational studies demonstrate the adverse effect of DM on PCI,6 but evidence from high-quality RCTs has also confirmed the same. In a subset analysis of the ACUITY trial,29 patients with DM had significantly higher early mortality, ischemia events, and major bleeding episodes than in those without DM. An important reason for this difference is that patients with DM tend to have more severe and diffuse CAD than do those without DM.30 In addition to higher baseline disease activity, DM also leads to increased platelet reactivity, poor response to antiplatelet therapy, and elevated inflammatory markers owing to the state of chronic inflammation.31–33 These pathophysiological factors are also important contributors to unfavorable outcomes following PCI in patients with DM. Because DM also leads to worsening of renal function14 and CKD in itself is associated with worse outcomes after PCI,13 it is important to clarify the role of renal disease in outcomes for patients with DM. In this context, the systematic review and meta-analysis are important as the first to evaluate the subject in question. In the analysis combining data from 20,975 patients, CKD was found to increase the risk of early all-cause mortality by 3.45 times in patients with DM who underwent PCI. Similarly, a 2.78-times-increased risk of late all-cause mortality and a 2.90-times-increased risk of cardiac mortality with combined CKD and DM were noted compared with DM alone. Furthermore, CKD increased the risk of MI by 40% in patients with DM who underwent PCI but did not affect revascularization rates. It is important to note that these values were obtained by pooling data mostly from unmatched studies with significant differences in baseline characteristics between the study groups. Therefore, there is a high possibility that multiple confounding preoperative, intraoperative, and postoperative variables could have skewed the outcomes of this analysis. In the absence of evidence from RCTs, multivariable-adjusted regression analysis or use of propensity-score matching has been recommended to negate the effect of confounding factors.34 However, baseline matching of the cohort was performed only by Choi et al,23 and adjusted HRs of outcomes were not universally reported. Despite this limitation, the increased risk of all-cause mortality with combined CKD and DM was seen in all studies examined except Goto et al.21 The limited sample size of that study could have contributed to the nonsignificant outcome. Furthermore, the meta-analysis of adjusted HRs was in concurrence with the results of the absolute number of events, indicating that presence of CKD does have a role in increasing mortality and MI rates in patients with DM.
Adverse outcomes resulting from CKD in PCI have been attributed to several possible reasons. The presence of CKD and CKD-associated bone mineral disorders is known to cause vascular calcifications, which increase the complexity of PCI.35 Research indicates poorer outcomes after PCI in patients with calcified lesions.36 Also, common pathophysiological processes in DM and CKD, such as alteration of cellular calcium homeostasis, chronic inflammation with cardiac cell apoptosis, myocardial alterations leading to fibrosis, and concentric remodeling, can lead to a significant reduction in left ventricular function, which in turn leads to increased mortality and risk of heart failure.25,37,38 The role of reactive oxygen species (ROS) in influencing PCI outcomes has recently been recognized.39 The presence of ROS leads to endothelial dysfunction, neointimal hyperplasia, and vascular smooth muscle cell hypertrophy, which can affect PCI outcomes. Hyperglycemia and overt DM are associated with increased ROS, and their role has also been identified in CKD.40,41 The combined oxidative damage induced by the 2 diseases could lead to worse outcomes after PCI. In addition, the combination of CKD and DM is associated with increased incidence of hypertension, dyslipidemia, and multivessel disease compared with DM alone.27 All of these factors are also known to contribute to worse outcomes after PCI.42,43 Angiolillo et al44 demonstrated that CKD reduces the activity of antiplatelet drugs and further increases platelet reactivity in patients with DM. Poor response to antiplatelet drugs is an important cause of adverse events after PCI.44 It is also plausible that as the severity of CKD increases, the impact of these pathophysiological processes expands proportionally, leading to further deterioration of outcomes. Research on patients with CKD has indicated only worse outcomes after PCI with increasing severity of disease.13 However, because of limited data, a quantitative assessment of this association in the cohort of patients with DM was not able to be made.
Although this study demonstrates that CKD is an important cause of adverse PCI outcomes in patients with DM, research suggests that poor outcomes in patients with DM may be related to the presence of CKD alone, and non-CKD DM may have limited contribution. In 1 of the included studies, Liosis et al15 demonstrated that in-hospital mortality is increased in patients with DM only in the subset of those with CKD and not in those with DM alone. Similarly, Lin et al27 reported a comparable risk of all-cause mortality between patients without CKD or DM and patients with DM alone (3.9% vs 5.9%, respectively) but a significantly higher risk of mortality (18.3%) in patients with CKD and DM who underwent PCI. These findings, along with the results of this study, have important clinical implications, as they demonstrate that patients with DM and CKD are a high-risk subset who require close monitoring and optimal perioperative and postoperative management. These findings may also be helpful when discussing the prognosis of PCI with this cohort of patients.
Limitations
The limitations of this study need to be specified. Foremost, the number of studies included in this meta-analysis was not very large. Furthermore, not all studies reported data on all outcomes, which diminished the power of individual analysis. Second, as discussed earlier, the results should be interpreted with caution, as several potential confounding factors could have influenced study outcomes. The majority of studies were retrospective in nature, with a large tendency of selection bias. Meta-analysis of only adjusted outcomes from all studies would have provided the best available evidence; however, these data were not widely reported. Also, a subgroup analysis based on the severity of CKD could not be conducted; only a qualitative analysis could be carried out. Third, the included studies were conducted over a long period spanning almost 2 decades. To include only recent evidence, only studies published in 2000 or later were searched; however, there have been several changes in PCI technology and cardiovascular drugs during this period that could have influenced outcomes. Fourth, an important difference across studies was in the different types of stents used. Although earlier studies used only BMS, more recent ones used only DES. Because of limited data, the impact of stent types on study outcomes could not be assessed. Last, the way in which antidiabetic therapy may affect outcomes in patients with CKD who undergo PCI could not be assessed. In recent years, studies have shown that sodium-glucose cotransporter-2 inhibitors used in the management of DM have cardioprotective and renoprotective functions. These drugs are known to significantly reduce the risk of worsening renal function and prevent progression to end-stage renal disease.45 In this context, it would be interesting for future studies to explore the association of sodium-glucose cotransporter-2 inhibitors and outcomes in patients with CKD who undergo PCI. There is also a need for future studies to compare outcomes of revascularization vs nonrevascularization (where feasible) in patients with CKD with cardiovascular disease to further enhance current evidence.
Based on the current results, the authors believe that clinicians should take adequate care and prioritize treatment of patients with CKD who require PCI. These patients should also be counseled regarding the increased risk of complications and be provided with highly supervised perioperative care.
Conclusion
Data from mostly retrospective studies indicate that the presence of CKD in patients with DM significantly increases the risk of mortality and MI. However, CKD was found to have no impact on revascularization rates. Further larger-scale studies assessing outcomes of PCI based on CKD severity are needed to supplement current conclusions.
Supplementary Material
Abbreviations and Acronyms
- BMS
bare-metal stent
- CAD
coronary artery disease
- CKD
chronic kidney disease
- DES
drug-eluting stent
- DM
diabetes mellitus
- HR
hazard ratio
- MI
myocardial infarction
- PCI
percutaneous coronary intervention
- ROS
reactive oxygen species
- RR
risk ratio
Funding Statement
Funding/Support: None
Footnotes
Author Contributions: W.J. conceived and designed the study; Y.Z. and S.C. were involved in literature search and data collection; W.J. and Y.Z. analyzed the data; S.L. wrote the paper; and S.L. reviewed and edited the manuscript. All authors read and approved the final manuscript.
Conflict of Interest Disclosures: None
References
- 1.Sawano M, Yamaji K, Kohsaka S et al. Contemporary use and trends in percutaneous coronary intervention in Japan: an outline of the J-PCI registry. Cardiovasc Interv Ther . 2020;35(3):218–226. doi: 10.1007/s12928-020-00669-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Koshy SKG, George LK, Das P. Cost-effectiveness and outcomes with early or same-day discharge after elective percutaneous coronary intervention. Curr Cardiol Rep . 2020;22(6):42. doi: 10.1007/s11886-020-01286-1. [DOI] [PubMed] [Google Scholar]
- 3.Gayed M, Yadak N, Qamhia W, Daralammouri Y, Ohlow MA. Comorbidities and complications in nonagenarians undergoing coronary angiography and intervention. Int Heart J . 2017;58(2):180–184. doi: 10.1536/ihj.16-083. [DOI] [PubMed] [Google Scholar]
- 4.Lin MJ, Chang YJ, Chen CY, Huang CC, Chuang TY, Wu HP. Influence of hypercholesterolemia and diabetes on long-term outcome in patients with stable coronary artery disease receiving percutaneous coronary intervention. Medicine (Baltimore) . 2019;98(34):e16927. doi: 10.1097/MD.0000000000016927. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Zhu Y, Meng S, Chen M et al. Long-term prognosis of chronic total occlusion treated by successful percutaneous coronary intervention in patients with or without diabetes mellitus: a systematic review and meta-analysis. Cardiovasc Diabetol . 2021;20(1):29. doi: 10.1186/s12933-021-01223-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Zhou X, Zhang C, Feng J, Ouyang S, Niu P, Dai Z. In-hospital, short-term and long-term adverse clinical outcomes observed in patients with type 2 diabetes mellitus vs non-diabetes mellitus following percutaneous coronary intervention. Medicine (Baltimore) . 2019;98(8):e14669. doi: 10.1097/MD.0000000000014669. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Pi SH, Rhee TM, Lee JM et al. Outcomes in patients with diabetes mellitus according to insulin treatment after percutaneous coronary intervention in the second-generation drug-eluting stent era. Am J Cardiol . 2018;121(12):1505–1511. doi: 10.1016/j.amjcard.2018.02.034. [DOI] [PubMed] [Google Scholar]
- 8.Bundhun PK, Yanamala CM, Huang F. Should a prolonged duration of dual anti-platelet therapy be recommended to patients with diabetes mellitus following percutaneous coronary intervention? A systematic review and meta-analysis of 15 studies. BMC Cardiovasc Disord . 2016;16(1):161. doi: 10.1186/s12872-016-0343-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Shen Y, Cai R, Sun J et al. Diabetes mellitus as a risk factor for incident chronic kidney disease and end-stage renal disease in women compared with men: a systematic review and meta-analysis. Endocrine . 2017;55(1):66–76. doi: 10.1007/s12020-016-1014-6. [DOI] [PubMed] [Google Scholar]
- 10.Gansevoort RT, Correa-Rotter R, Hemmelgarn BR et al. Chronic kidney disease and cardiovascular risk: epidemiology, mechanisms, and prevention. Lancet . 2013;382(9889):339–352. doi: 10.1016/S0140-6736(13)60595-4. [DOI] [PubMed] [Google Scholar]
- 11.Wu P, Luo F, Fang Z. Multivessel coronary revascularization strategies in patients with chronic kidney disease: a meta-analysis. Cardiorenal Med . 2019;9(3):145–159. doi: 10.1159/000494116. [DOI] [PubMed] [Google Scholar]
- 12.Lin MJ, Yang WC, Chen CY et al. Hypertension and chronic kidney disease affect long-term outcomes in patients with stable coronary artery disease receiving percutaneous coronary intervention. Sci Rep . 2018;8(1):17673. doi: 10.1038/s41598-018-35982-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Parikh PB, Jeremias A, Naidu SS et al. Impact of severity of renal dysfunction on determinants of in-hospital mortality among patients undergoing percutaneous coronary intervention. Catheter Cardiovasc Interv . 2012;80(3):352–357. doi: 10.1002/ccd.23394. [DOI] [PubMed] [Google Scholar]
- 14.Mozaffarian D, Benjamin EJ, Go AS et al. Heart disease and stroke statistics-2015 update: a report from the American Heart Association. Circulation . 2015;131(4):e29–322. doi: 10.1161/CIR.0000000000000152. [DOI] [PubMed] [Google Scholar]
- 15.Liosis S, Hochadel M, Darius H et al. Effect of renal insufficiency and diabetes mellitus on in-hospital mortality after acute coronary syndromes treated with primary PCI. Results from the ALKK PCI Registry. Int J Cardiol . 2019;292:43–49. doi: 10.1016/j.ijcard.2019.04.071. [DOI] [PubMed] [Google Scholar]
- 16.Watanabe Y, Mitomo S, Naganuma T et al. Impact of chronic kidney disease in patients with diabetes mellitus after percutaneous coronary intervention for left main distal bifurcation (from the Milan and New–Tokyo (MITO) Registry) Am J Cardiol . 2021;138:33–39. doi: 10.1016/j.amjcard.2020.10.021. [DOI] [PubMed] [Google Scholar]
- 17.Moher D, Liberati A, Tetzlaff J, Altman DG;, PRISMA Group Preferred reporting items for systematic reviews and meta-analyses: the PRISMA Statement. PLoS Med . 2009;6(7):e1000097. doi: 10.1371/journal.pmed.1000097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Higgins J, Thomas J, Chandler J Cochrane Handbook for Systematic Reviews of Interventions Version 6. Cochrane; 2019. [Google Scholar]
- 19.Kim SY, Park JE, Lee YJ et al. Testing a tool for assessing the risk of bias for nonrandomized studies showed moderate reliability and promising validity. J Clin Epidemiol . 2013;66(4):408–414. doi: 10.1016/j.jclinepi.2012.09.016. [DOI] [PubMed] [Google Scholar]
- 20.Nikolsky E, Mehran R, Turcot D et al. Impact of chronic kidney disease on prognosis of patients with diabetes mellitus treated with percutaneous coronary intervention. Am J Cardiol . 2004;94(3):300–305. doi: 10.1016/j.amjcard.2004.04.023. [DOI] [PubMed] [Google Scholar]
- 21.Goto K, Shiode N, Shirota K et al. Impact of impaired renal function and diabetes on long-term prognosis in patients undergoing primary angioplasty for acute coronary syndrome. Intern Med . 2008;47(10):907–913. doi: 10.2169/internalmedicine.47.0932. [DOI] [PubMed] [Google Scholar]
- 22.Hung CC, Huang WC, Chiou KR et al. Chronic kidney disease, but not diabetes, can predict 30-day outcomes in patients with ST-elevation myocardial infarction after primary percutaneous coronary intervention: a single-center experience. Acta Cardiol Sin . 2013;29(5):395–403. [PMC free article] [PubMed] [Google Scholar]
- 23.Choi KH, Yang JH, Kim JH et al. The impact of renal dysfunction on the long term clinical outcomes of diabetic patients undergoing percutaneous coronary intervention in the drug-eluting stent era. PLoS One . 2016;11(1):e0141846. doi: 10.1371/journal.pone.0141846. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Lima EG, Hueb W, Gersh BJ et al. Impact of chronic kidney disease on long-term outcomes in type 2 diabetic patients with coronary artery disease on surgical, angioplasty, or medical treatment. Ann Thorac Surg . 2016;101(5):1735–1744. doi: 10.1016/j.athoracsur.2015.10.036. [DOI] [PubMed] [Google Scholar]
- 25.Vichova T, Knot J, Ulman J, Maly M, Motovska Z. The impact of stage of chronic kidney disease on the outcomes of diabetics with acute myocardial infarction treated with percutaneous coronary intervention. Int Urol Nephrol . 2016;48(7):1137–1143. doi: 10.1007/s11255-016-1260-9. [DOI] [PubMed] [Google Scholar]
- 26.Kim SM, Tripathy DR, et al. Impact of chronic kidney disease on clinical outcomes in diabetic patients undergoing percutaneous coronary intervention in the era of newer-generation drug-eluting stents. Korean Circ J . 2017;47(2):222–230. doi: 10.4070/kcj.2016.0312. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Lin MJ, Lee J, Chen CY, Huang CC, Wu HP. Chronic kidney disease and diabetes associated with long-term outcomes in patients receiving percutaneous coronary intervention. BMC Cardiovasc Disord . 2017;17(1):242. doi: 10.1186/s12872-017-0673-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Farkouh ME, Sidhu MS, Brooks MM et al. Impact of chronic kidney disease on outcomes of myocardial revascularization in patients with diabetes. J Am Coll Cardiol . 2019;73(4):400–411. doi: 10.1016/j.jacc.2018.11.044. [DOI] [PubMed] [Google Scholar]
- 29.Feit F, Manoukian SV, Ebrahimi R et al. Safety and efficacy of bivalirudin monotherapy in patients with diabetes mellitus and acute coronary syndromes. a report from the ACUITY (Acute Catheterization and Urgent Intervention Triage Strategy) trial. J Am Coll Cardiol . 2008;51(17):1645–1652. doi: 10.1016/j.jacc.2007.11.081. [DOI] [PubMed] [Google Scholar]
- 30.Bauer T, Möllmann H, Weidinger F et al. Impact of diabetes mellitus status on coronary pathoanatomy and interventional treatment: insights from the Euro heart survey PCI registry. Catheter Cardiovasc Interv . 2011;78(5):702–709. doi: 10.1002/ccd.22939. [DOI] [PubMed] [Google Scholar]
- 31.Angiolillo DJ, Bernardo E, Sabaté M et al. Impact of platelet reactivity on cardiovascular outcomes in patients with type 2 diabetes mellitus and coronary artery disease. J Am Coll Cardiol . 2007;50(16):1541–1547. doi: 10.1016/j.jacc.2007.05.049. [DOI] [PubMed] [Google Scholar]
- 32.Geisler T, Anders N, Paterok M et al. Platelet response to clopidogrel is attenuated in diabetic patients undergoing coronary stent implantation. Diabetes Care . 2007;30(2):372–374. doi: 10.2337/dc06-1625. [DOI] [PubMed] [Google Scholar]
- 33.Winzap P, Davies A, Klingenberg R et al. Diabetes and baseline glucose are associated with inflammation, left ventricular function and short- and long-term outcome in acute coronary syndromes: role of the novel biomarker Cyr 61. Cardiovasc Diabetol . 2019;18(1):142. doi: 10.1186/s12933-019-0946-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Yao X, Lei W, Shi N et al. Impact of initial dialysis modality on the survival of patients with ESRD in eastern China: a propensity-matched study. BMC Nephrol . 2020;21(1):310. doi: 10.1186/s12882-020-01909-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Cano-Megías M, Guisado-Vasco P, Bouarich H et al. Coronary calcification as a predictor of cardiovascular mortality in advanced chronic kidney disease: a prospective long-term follow-up study. BMC Nephrol . 2019;20(1):188. doi: 10.1186/s12882-019-1367-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Kim MC, Ahn Y, Sim DS et al. Impact of calcified bifurcation lesions in patients undergoing percutaneous coronary intervention using drug-eluting stents: results from the COronary BIfurcation Stent (COBIS) II registry. EuroIntervention . 2017;13(3):338–344. doi: 10.4244/EIJ-D-16-00264. [DOI] [PubMed] [Google Scholar]
- 37.Wang Q, Tan K, Xia H, Gao Y. The further negative effect of hyperuricemia on left ventricular structure and function in patients with type 2 diabetes mellitus: a transthoracic 3d speckle tracking imaging study. Metab Syndr Relat Disord . 2019;17(9):436–443. doi: 10.1089/met.2019.0048. [DOI] [PubMed] [Google Scholar]
- 38.Ersbøll M, Valeur N, Hassager C, Søgaard P, Køber L. The association between renal impairment and cardiac structure and function in patients with acute myocardial infarction. Am Heart J . 2014;167(4):506–513. doi: 10.1016/j.ahj.2013.12.029. [DOI] [PubMed] [Google Scholar]
- 39.Gallo G, Pierelli G, Forte M, Coluccia R, Volpe M, Rubattu S. Role of oxidative stress in the process of vascular remodeling following coronary revascularization. Int J Cardiol . 2018;268:27–33. doi: 10.1016/j.ijcard.2018.05.046. [DOI] [PubMed] [Google Scholar]
- 40.Adeshara KA, Diwan AG, Tupe RS. Diabetes and complications: cellular signaling pathways, current understanding and targeted therapies. Curr Drug Targets . 2016;17(11):1309–1328. doi: 10.2174/1389450117666151209124007. [DOI] [PubMed] [Google Scholar]
- 41.Irazabal MV, Torres VE. Reactive oxygen species and redox signaling in chronic kidney disease. Cells . 2020;9(6):1342. doi: 10.3390/cells9061342. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Zhao X, Xu L, Jiang L et al. Real-world outcomes of different treatment strategies in patients with diabetes and three-vessel coronary disease: a mean follow-up 6.3 years study from China. Cardiovasc Diabetol . 2021;20(1):16. doi: 10.1186/s12933-020-01193-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Bundhun PK, Wu ZJ, Chen MH. Impact of modifiable cardiovascular risk factors on mortality after percutaneous coronary intervention: a systematic review and meta-analysis of 100 studies. Medicine (Baltimore) . 2015;94(50):e2313. doi: 10.1097/MD.0000000000002313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Angiolillo DJ, Bernardo E, Capodanno D et al. Impact of chronic kidney disease on platelet function profiles in diabetes mellitus patients with coronary artery disease taking dual antiplatelet therapy. J Am Coll Cardiol . 2010;55(11):1139–1146. doi: 10.1016/j.jacc.2009.10.043. [DOI] [PubMed] [Google Scholar]
- 45.Li N, Lv D, Zhu X et al. Effects of SGLT2 Inhibitors on renal outcomes in patients with chronic kidney disease: a meta-analysis. Front Med (Lausanne) . 2021;1(8):728089. doi: 10.3389/fmed.2021.728089. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


