Abstract
Background
Diclofenac is a widely used analgesic, anti-inflammatory, antipyretic drug. In several case reports, its use was associated with the occurrence of Kounis syndrome. The aim of this review was to investigate and summarize published cases of Kounis syndrome suspected to be associated with the use of diclofenac.
Methods
Electronic searches were conducted in PubMed/MEDLINE, Scopus, Web of Science, Google Scholar, and the Serbian Citation Index.
Results
Twenty publications describing the 20 patients who met inclusion criteria were included in the systematic review. Specified patient ages ranged from 34 to 81 years. Eighteen (90.0%) patients were male. Five patients (25.0%) reported a previous reaction to diclofenac. Reported time from the used dose of diclofenac to onset of the first reaction symptoms ranged from immediately to 5 hours. Diclofenac caused both type I and type II Kounis syndrome, with the presence of various cardiovascular, gastrointestinal, dermatologic, and respiratory signs and symptoms. Most patients experienced hypotension (n = 15 [75.0%]) and chest pain (n = 12 [60.0%]). The most frequently reported finding on electrocardiogram was ST-segment elevations (n = 17 [85.0%]). Coronary angiogram showed normal coronary vessels in 9 patients (45.0%), with some pathologic findings in 8 patients (40.0%).
Conclusion
Clinicians should be aware that Kounis syndrome may be an adverse effect of diclofenac. Prompt recognition and withdrawal of the drug, with treatment of both allergic and cardiac symptoms simultaneously, is important.
Keywords: Diclofenac, Kounis syndrome, acute coronary syndrome, anaphylaxis
Introduction
In 1991, Kounis and Zavras1 described the “syndrome of allergic angina” and “allergic myocardial infarction,” currently known as Kounis syndrome (KS). Kounis syndrome is the concurrence of acute coronary syndrome (ACS) (including coronary spasm, acute myocardial infarction [AMI], and stent thrombosis) with conditions associated with mast cell and platelet activation in the setting of allergic, hypersensitivity, anaphylactic, or anaphylactoid reactions.2,3
Three variants of KS have been described.3,4 Type I occurs in patients with normal or nearly normal coronary arteries who have no predisposing factors for coronary artery disease and in whom the acute release of inflammatory mediators can lead to either coronary artery spasm without increase of cardiac enzymes or coronary artery spasm progressing to AMI.3,4 Type II refers to patients with culprit but quiescent preexisting atheromatous disease in whom the acute release of inflammatory mediators can induce either coronary artery spasm with normal cardiac enzymes or coronary artery spasm with plaque erosion or rupture manifesting as AMI.3,4 Type III includes patients with drug-eluting stent thrombosis with the presence of mast cells and eosinophils revealed with Giemsa and hematoxylin-eosin staining.3,4 Kounis syndrome is not rare, but it is not well recognized, which leads to underdiagnosis and undertreatment.5 To date, resources on KS are limited, and most of the information comes from clinical case reports and small case series.6
Kounis syndrome is caused by inflammatory mediators such as histamine, platelet activating factor, arachidonic acid products, neutral proteases, and a variety of cytokines and chemokines released during the allergic activation process.3
Diclofenac: Indications, Mechanism of Action, and Adverse Effects
Diclofenac is a phenylacetic acid–derivative nonsteroidal anti-inflammatory drug (NSAID).7 These drugs act by inhibiting cyclooxygenase 1 (COX-1) and COX-2, consequently reducing the synthesis of prostaglandins, prostacyclins, and thromboxanes; in this way, they achieve anti-inflammatory, analgesic, and antipyretic effects.8 Diclofenac, like other NSAIDs, is used in the treatment and management of acute and chronic pain associated with inflammatory conditions9 as well as for the prevention and treatment of postoperative pain.10 Diclofenac preparations pair the drug with a salt, such as sodium, potassium, or epolamine, which (depending on the formulation) can be administered through various routes (eg, oral, intramuscular [IM], intravenous [IV], transdermal, rectal).9
The most common adverse effects of diclofenac are gastrointestinal (GI) problems (erosive gastritis, gastric or duodenal ulcerations, hemorrhage, or perforations), kidney impairment, hypertension, MI, heart failure, stroke, impaired liver function, and increased transaminase levels.9 As with other NSAIDs, allergic reactions, including anaphylactic and anaphylactoid reactions, can occur in rare cases with diclofenac, even without earlier exposure to the drug.11 Hypersensitivity reactions can also progress to KS.11 These NSAID hypersensitivity reactions can be compartmentalized into pharmacologic (secondary to COX-1 inhibition) or a specific (likely immunoglobulin E [IgE]–mediated effect).12 All NSAIDs inhibit COX-1 and thus favor the lipoxygenase pathway of arachidonic acid metabolism, resulting in increased cysteine leukotriene synthesis release of inflammatory mediators, including histamine and tryptase, from mast cells, and eosinophils.13
Diclofenac is 1 of the most prescribed NSAIDs worldwide.14 The goal of this systematic review was to investigate and summarize information about the characteristics of published cases of KS suspected to be associated with the use of diclofenac to provide a useful perspective for better recognition and management of this possible adverse effect of diclofenac.
Patients and Methods
Our systematic review was registered in the International Prospective Register of Systematic Reviews (PROS-PERO) under registration No. CRD42021277053.
Two authors (A.V.P. and M.N.M.) searched the following electronic databases independently from the beginning of indexing to September 15, 2021, with no language or date restriction: PubMed/MEDLINE, Scopus, Web of Science, Google Scholar, and Serbian Citation Index (SCIndeks). Table I shows a detailed search strategy for each database. Case reports or case series that included detailed clinical descriptions of the patients diagnosed with KS (vasospastic allergic angina, allergic MI, or stent thrombosis with occluding thrombus infiltrated by eosinophils or mast cells) caused or suspected to be caused by diclofenac and that had available full text were included in the systematic review. Conference abstracts were included if they contained sufficient data for analysis and quality assessment. At a minimum, the following information had to be available for each patient to include a publication in the review: age group, sex, identification of suspected drug, manifestations of the reaction, clinical course, treatment, and outcome. Case reports or case series were excluded if they (1) did not include a detailed clinical description of each patient (ie, without all previously mentioned minimum required information), (2) reported on patients who received diclofenac with other drugs for whom the exact cause of KS could not be determined or in whom diclofenac was excluded as a possible cause, and (3) reported other cardiac or hypersensitivity adverse effects associated with the use of diclofenac. The reference lists of the retrieved articles were also searched for additional relevant publications.
TABLE I.
Detailed Database Search Strategy
| Database | Search strategy |
|---|---|
| PubMed/MEDLINE | (“diclofenac”[MeSH Terms] OR “diclofenac”[All Fields]) AND (“kounis syndrome”[MeSH Terms] OR (“kounis”[All Fields] AND “syndrome”[All Fields]) OR “kounis syndrome”[All Fields] OR (“kounis syndrome”[MeSH Terms] OR (“kounis”[All Fields] AND “syndrome”[All Fields]) OR “kounis syndrome”[All Fields] OR (“allergic”[All Fields] AND “angina”[All Fields] AND “syndrome”[All Fields]) OR “allergic angina syndrome”[All Fields]) OR (“kounis syndrome”[MeSH Terms] OR (“kounis”[All Fields] AND “syndrome”[All Fields]) OR “kounis syndrome”[All Fields] OR (“allergic”[All Fields] AND “acute”[All Fields] AND “coronary”[All Fields] AND “syndrome”[All Fields]) OR “allergic acute coronary syndrome”[All Fields]) OR (“kounis syndrome”[MeSH Terms] OR (“kounis”[All Fields] AND “syndrome”[All Fields]) OR “kounis syndrome”[All Fields] OR (“allergic”[All Fields] AND “myocardial”[All Fields] AND “infarction”[All Fields]) OR “allergic myocardial infarction”[All Fields])) |
| Web of Science | All databases: TS=(diclofenac AND ((Kounis Syndrome) OR (Allergic Angina Syndrome) OR (Allergic Acute Coronary Syndrome) OR (Allergic Myocardial Infarction))) |
| Scopus | TITLE-ABS-KEY ( diclofenac AND ( ( kounis AND syndrome ) OR ( allergic AND angina AND syndrome ) OR ( allergic AND acute AND coronary AND syndrome ) OR ( allergic AND myocardial AND infarction) ) ) |
| SCIndeks | (ARTAK: diclofenac AND ((Kounis Syndrome) OR (Allergic Angina Syndrome) OR (Allergic Acute Coronary Syndrome) OR (Allergic Myocardial Infarction))) |
| Google Scholar | diclofenac AND ((Kounis Syndrome) OR (Allergic Angina Syndrome) OR (Allergic Acute Coronary Syndrome) OR (Allergic Myocardial Infarction)) |
SCIndeks, Serbian Citation Index.
Initially, the eligibility of retrieved publications was screened based on the title and abstract by 2 authors (A.V.P., M.N.M.) independently. Where it was not possible to assess whether the publication fully corresponded to the research topic based on the title and information provided in the abstract, the full text of the publication was retrieved and analyzed. Publications were included in the systematic review if all authors agreed that eligibility criteria had been met. Disagreements between individual judgments were resolved by consensus.
The following data were extracted for each described case by 2 authors independently (A.V.P., M.N.M.): demographics (age, sex); country of study; patient medical history; drug dosage; indication; route of diclofenac administration; salt form of diclofenac; concomitant medications; diagnostic investigations (electrocardiography, echocardiography, coronary angiography, myocardial perfusion scintigraphy, laboratory analyses, etc); time to onset of the first symptoms (including symptoms of allergic, hypersensitivity, anaphylactic, or anaphylactoid reaction); setting in which the reaction occurred; KS variant (ie, type I, II, or III); clinical manifestations; complications; treatment; length of stay in hospital; outcome; and information about causality assessment by the authors, if reported (eg, Naranjo score). Another author (N.D.F.) collated the 2 tables and produced the final extraction table.
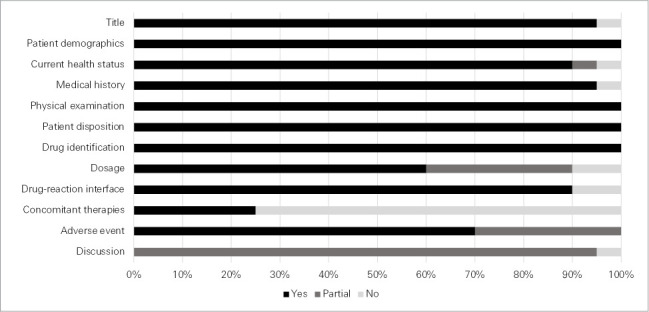
To evaluate the quality of the included cases, the “Guidelines for submitting adverse events reports for publication,” endorsed by the International Society for Pharmacoepidemiology and the International Society of Pharmacovigilance, were used.15 The authors evaluated the items that the guidelines reported as required information: title (consistency of the title with the content of the report), patient information (demographics, current health status, medical history, physical examination, patient disposition), suspected drug information (identification, dosage, drug-reaction interface, concomitant therapies), description of the adverse event, and discussion.15 All items were rated as present (yes), partially present (partial), or absent (no), with descriptive statistics (median, range, proportions), narrative summation, and tabulation of the extracted data.
Results
Figure 1 shows the results of the literature search. Twenty publications (19 case reports2,16–33 and 1 conference abstract34) describing a total of 20 patients who satisfied inclusion criteria were included in the systematic review. The quality assessment of the identified cases is shown in Figure 2. Most of the cases had all required information regarding title (n = 19 [95.0%]), patient demographics (n = 20 [100.0%]), current health status (n = 18 [90.0%]), medical history (n = 19 [95.0%]), physical examination (n = 20 [100.0%]), patient disposition (n = 20 [100.0%]), drug identification (n = 20 [100.0%]), drug-reaction interface (n = 18 [90.0%]), dosage (n = 12 [60.0%]), and adverse events (n = 14 [70.0%]). Only 5 cases (25.0%) had reported an assessment of potential contribution of concomitant therapies. Discussion was rated as partial in the majority of cases (n = 19 [95.0%]), mostly because they did not have specific discussion of the adverse event in relation to product labeling.
Fig. 1.

Chart shows the selection of publications.
SCIndeks, Serbian Citation Index.
Fig. 2.
Quality assessment of the identified cases.
Table II summarizes the basic characteristics of included cases, whereas Table III provides an overview of each included case. Specified patient ages ranged from 34 to 81 years; for 1 patient, only age group was reported (middle age). The majority of the patients were male (n = 18 [90.0%]). Five patients (25.0%) had a history of previous reaction to diclofenac (anaphylaxis,23 hypersensitivity,22 allergic reaction,31 ST-segment elevation MI,27 or chest pain with ischemic ST-segment changes26). One patient (5.0%) had a history of an aspirin-induced asthma attack that required intubation and admission to an intensive care unit.28 A history of diagnosed coronary artery disease and hypertension was reported in 5 patients (25.0%) each, whereas asthma and diabetes were reported in 3 (15.0%) and 2 patients (10.0%), respectively. Many of the cases occurred in Turkey (n = 9 [45.0%]).
TABLE II.
Summary of the Basic Characteristics of Included Cases (N=20)
| Characteristic | Value |
|---|---|
| Age, median (range), y (n=19) | 60.0 (34.0–81.0) |
| Sex, No. (%) | |
| Female | 2 (10.0) |
| Male | 18 (90.0) |
| Country, No. (%) | |
| Austria | 1 (5.0) |
| Egypt | 1 (5.0) |
| India | 2 (10.0) |
| Japan | 1 (5.0) |
| Malaysia | 1 (5.0) |
| Portugal | 1 (5.0) |
| Saudi Arabia | 1 (5.0) |
| Serbia | 1 (5.0) |
| Spain | 1 (5.0) |
| The Netherlands | 1 (5.0) |
| Turkey | 9 (45.0) |
| Diclofenac dosage, No. (%), mg | |
| 12.5 | 1 (5.0) |
| 50 | 4 (20.0) |
| 75 | 4 (20.0) |
| 100 | 3 (15.0) |
| Not reported | 8 (40.0) |
| Diclofenac salt, No. (%) | |
| Sodium | 10 (50.0) |
| Potassium | 4 (20.0) |
| Not reported | 6 (30.0) |
| Route of administration, No. (%) | |
| IM | 9 (45.0) |
| Oral | 7 (35.0) |
| Rectal | 2 (10.0) |
| IV | 1 (5.0) |
| First time after oral, second time after IV | 1 (5.0) |
| Concomitant medications, No. (%) | |
| Yes | 5 (25.0) |
| No | 2 (10.0) |
| Not specified | 13 (65.0) |
| Setting in which reaction occurred, No. (%) | |
| Outpatient | 13 (65.0) |
| Inpatient | 6 (30.0) |
| First time outpatient, second time inpatient | 1 (5.0) |
| Outcome, No. (%) | |
| Survived | 20 (100.0) |
| Died | 0 (0.0) |
IM, intramuscular; IV, intravenous.
TABLE III.
Overview of the Included Cases
| No. | Study | Age, y; sex | Medical history | Diclofenac salt, dose, administration route, indication | Concomitant medications | KS type | Onset | Summary of treatment | LOS | Outcome |
|---|---|---|---|---|---|---|---|---|---|---|
| 1 | Mori et al26 (1997) | 69; M | 7 wk before admission, chest pain with ischemic ST-segment changes after diclofenac sodium (25 mg) suppository; no history of allergic factors or major CAD risk factors | Sodium, 12.5 mg, rectal, fever | NS | NR | 5 min | Nitroglycerin and atropine sulfate IV; intracoronary isosorbide dinitrate after ergonovine provocation test; later diltiazem, long-acting nitrate, and nicorandil | NR | Survived |
| 2 | Blanco et al17 (2003) | 57; M | No history of coronary or vascular risk factors, previous allergies, or drug use | NR, 100 mg, rectal, acute lower back pain | No | NR | 4 h | Methylprednisolone, rt-PA, parenteral nitroglycerin infusion | NR | Survived |
| 3 | Gluvic et al22 (2007) | 66; M | History of diclofenac sodium hypersensitivity, excessive smoking habit | Sodium, NR, IM, pain in left toe from foot injury | NS | NR | Several minutes | CPR (airway intubation, oxygen therapy, establishing IV access, precordial thump, crystalloid administration), oxygen, methylprednisolone, theophylline, IV nitrates (nitroglycerin) | NR | Survived |
| 4 | de Groot et al20 (2009) | 48; M | No history of allergies, eczema, or asthma; surgery for a herniated disc; father and grandfather had died of CV event; smoker; used diclofenac several years earlier without problems | NR, 50 mg, oral, back pain | No | NR | Within minutes | Initially IV fluid replacement, then adrenaline and clemastine IV; IV administration of fluids continued | NR | Survived |
| 5 | Cakar et al19 (2011) | 74; F | No history of allergy at first administration | First time: potassium, NR, oral, NR Second time: potassium, NR, IV, upper respiratory tract infection symptoms |
NS | First time: II Second time: I and II |
First time: 30 min Second time: NR |
First time: IV antihistaminic and prednisolone, saline and dopamine infusions, successful coronary angioplasty with implantation of sequential 3.0- × 16-mm and 3.0- × 8-mm bare-metal stents Second time: IV antihistaminic and prednisolone |
NR | Survived |
| 6 | Granitz et al34 (2011) | 60; M | Planned knee arthroscopy | NR, NR, IV, used during induction of anesthesia | NS | II | Immediately after | Resuscitation, clopidogrel | NR | Survived |
TABLE III.
Overview of the Included Cases
| No. | Study | Age, y; sex | Medical history | Diclofenac salt, dose, administration route, indication | Concomitant medications | KS type | Onset | Summary of treatment | LOS | Outcome |
|---|---|---|---|---|---|---|---|---|---|---|
| 7 | Cagliyan et al18 (2013) | 49; M | CV risk factors (diabetes mellitus, hyperlipidemia, tobacco use), appendectomy before 9 y | Sodium, NR, IM, sore throat, and generalized pain | NS | II | 5 min | Defibrillation, CPR, intracoronary nitroglycerin (no benefit), placement of a floppy guidewire in the LAD (grade 2–3 TIMI flow achieved), successful PPCI (direct stenting of the vessel) | 5 d | Survived |
| 8 | Rodrigues et al2 (2013) | 62; M | Obesity; HTN; history of atopy, with frequent allergic conjunctivitis and rhinitis; food allergies (nut); bronchial asthma | NR, 75 mg, oral, shoulder pain | NS | I | 10 min | Metoclopramide IV, morphine and aspirin IV, dopamine infusion, hydrocortisone, ranitidine IV | 24 h after ICU admission | Survived |
| 9 | Tiwari et al32 (2013) | 64; M | Admitted for surgery after fracture; not a known smoker or alcoholic; no history of allergy, bronchial asthma, or previous surgeries; denied any previous episode of CAD | Sodium, 50 mg, IM, severe pain | NS | I | 10 min | Adrenaline, chlorpheniramine IV, methylprednisolone, IV fluids | NR | Survived |
| 10 | Şahinkuş et al30 (2016) | 34; M | Smoker; no history of allergy or CAD | NR, NR, IM, NR | NS | NR | NR | Acetylsalicylic acid and clopidogrel peroral; discharged with medical therapy (desloratadine, isosorbid-5-mononitrate) | 3 d | Survived |
| 11 | Akboğa et al16 (2017) | 51; M | Former smoker, no history of any allergic disease | Sodium, 100 mg, oral, knee pain | NS | I | 45 min | Pheniramine, dexamethasone, enoxaparin, aspirin | NR | Survived |
| 12 | Gunes et al23 (2017) | 67; M | CAD, HTN, kidney failure, previous anaphylaxis after IM diclofenac potassium almost 1 y previously | Potassium, NR, oral, arthralgia | NS | II | NR | IM pheniramine and dexamethasone, IV infusion of 0.9% NaCl. The occlusion was opened through repeated balloon dilatation, but stenting of the lesion site could not be achieved because of ectasia of the artery | 2d | Survived |
TABLE III.
Overview of the Included Cases
| No. | Study | Age, y; sex | Medical history | Diclofenac salt, dose, administration route, indication | Concomitant medications | KS type | Onset | Summary of treatment | LOS | Outcome |
|---|---|---|---|---|---|---|---|---|---|---|
| 13 | Kerai et al25 (2017) | 47; M | Scheduled for the left parotid gland excision under general anesthesia; no history of asthma or drug or food allergy; no underlying cardiac disease | Sodium, 75 mg, IM, pain management (administered after parotid gland was excised and surgical closure started) | Midazolam, fentanyl, propofol, vecuronium, sevoflurane in an oxygen-nitrous mixture | I | 10 min | IV fluids, sevoflurane was switched off, IV hydrocortisone, chlorpheniramine, ranitidine, dopamine infusion | NR | Survived |
| 14 | İbrahim et al24 (2018) | 54; M | CAD, HTN; no history of allergy to any drug | Sodium, 75 mg, IM, myalgia (severe back pain) | NS | II | Immediately after | IM adrenaline and pheniramine, methylprednisolone, IV fluids, vasopressors; emergency CV percutaneous intervention not deemed necessary; medically treated | 36 h | Survived |
| 15 | Sarıoğlu et al31 (2018) | 64; M | History of allergic reaction to diclofenac potassium; sacroiliitis; no prior CAD; family history of CAD, diabetes, HTN, smoking, hyperlipidemia, and asthma | Potassium, 50 mg, oral, headache | Prednisolone | I | About 1 h | Nasal oxygen, IV isotonic saline, hydrocortisone, SC adrenaline | 4 d | Survived |
| 16 | Rajh et al28 (2019) | 69; M | Type 2 diabetes, bronchial asthma, HTN, GERD, overweight, osteoarthritis, CAD, CABG 6 y before, aspirin-induced asthma attack 20 y before | NR, NR, IM, shoulder pain (osteoarthritis) | Albuterol, budesonide, formoterol | NR | 10 min | Albuterol and ipratropium nebulization, IV methylprednisolone, magnesium sulfate | 1 d | Survived |
| 17 | Yıldırım et al33 (2019) | 81; M | NR | Sodium, 100 mg, oral, knee pain | NS | I | 30 min | IV antihistaminic, prednisolone` | 2 d | Survived |
| 18 | Elsayed21 (2020) | 44; M | Reported no history of cardiac, thyroid, or other relevant diseases | Potassium, 50 mg, oral, recurrent kidney pain | NS | I | 1 h | Oxygen, IM adrenaline, IV hydrocortisone, IV chlorpheniramine, IV 0.9% normal saline, IV Ringer solution; on discharge: IV hydrocortisone, oral chlorpheniramine for 3 d | No (3 h in outpatient clinic) | Survived |
TABLE III.
Overview of the Included Cases
| No. | Study | Age, y; sex | Medical history | Diclofenac salt, dose, administration route, indication | Concomitant medications | KS type | Onset | Summary of treatment | LOS | Outcome |
|---|---|---|---|---|---|---|---|---|---|---|
| 20 | Rui et al29 (2021) | 55; M | CAD, for which stents were inserted into the LAD and RCA; active smoker; no known allergies; no history of atopy | Sodium, 75 mg, IM, lower back pain | Compliant to ACS treatment (drugs not specified) | II | Shortly after | IM adrenaline, IV hydrocortisone, IV chlorphenamine, fluid therapy, oral aspirin, oral clopidogrel, IV streptokinase; consequently had his regular medical therapy continued | Few days | Survived |
ACS, acute coronary syndrome; CABG, coronary artery bypass graft; CAD, coronary artery disease; CPR, cardiopulmonary resuscitation; CV, cardiovascular; F, female; GERD, gastroesophageal reflux disease; HTN, hypertension; ICU, intensive care unit; IM, intramuscular; IV, intravenous; KS, Kounis syndrome; LAD, left anterior descending coronary artery; LOS, length of stay; M, male; NR, not reported; NS, not specified whether the patient received any concomitant medications; PPCI, primary percutaneous coronary intervention; RCA, right coronary artery; rt-PA, recombinant tissue plasminogen activator; SC, subcutaneous; STEMI, ST-segment elevation myocardial infarction; TIMI, Thrombolysis In Myocardial Infarction.
The most frequently used diclofenac dosages that were specified were 50 and 75 mg in 4 cases (20.0%) each. Route of administration was IM in many cases (n = 9 [45.0%]); use of diclofenac with sodium salt was reported in half the patient group. The most frequently reported indication for diclofenac use was pain management (n = 17 [85.0%]). Information about concomitantly used medications is provided in Table III. For most patients, the authors did not specify whether they received concomitant medications (n = 13 [65.0%]).
The reported time from the dose of diclofenac used to onset of the first symptoms of the reaction ranged from immediately to 5 hours. Most reactions occurred in the outpatient setting (n = 13 [65.0%]). The diagnosis was based on clinical presentation and use of relevant diagnostic investigations. Patients generally had clinical symptoms and signs associated with allergic, hypersensitivity, or anaphylactic reactions (only 1 patient with recurrent KS had no sign of systemic allergic reactions, except coronary spasm27) accompanied by cardiac manifestations. In total, 15 (75.0%) patients experienced hypotension, and 12 (60.0%) had chest pain. Tachycardia and atrioventricular block were seen in 8 (40.0%) and 3 (15.0%) patients, respectively. Cardiac arrest, ventricular fibrillation, and cardiogenic shock complicated the clinical course in 3 (15.0%), 2 (10.0%), and 1 (5.0%) patients, respectively. Premature ventricular contractions, palpitations, raised jugular venous pressure, and apparent pathologic venous pulsations were sporadically reported (n = 1 [5.0%]). Nausea and vomiting were reported in 4 patients (20.0%). Dermatologic manifestations, including rash, erythema, and urticaria, were reported in 14 patients (70.0%). Itching (pruritus) was reported in 8 patients (40.0%), and edema (facial, mucous membrane, lips) was reported in 3 patients (15.0%). Sweating was described in 4 patients (20.0%), and eye lacrimation was seen in 1 patient (5.0%). Loss of consciousness, syncope, or collapse was reported in 8 patients (40.0%). Respiratory signs and symptoms, such as tachypnea, dyspnea, wheezing, sense of suffocation, irregular spontaneous respirations with cyanosis, and prolonged expiratory time, were described in 6 patients (30.0%). One patient (5.0%) experienced partial respiratory acidosis and hematuria; another patient (5.0%) developed pulmonary edema.
Detailed results of relevant cardiovascular diagnostic investigations for each patient are provided in Table IV. Electrocardiography, coronary angiography, echocardiography, and myocardial perfusion scintigraphy were performed in 20 (100.0%), 17 (85.0%), 12 (60.0%), and 1 (5.0%) patients, respectively. The most frequently reported finding on electrocardiograms were ST-segment elevations (n = 17 [85.0%]). Decreased heart wall motion (hypokinesia) was reported in 5 patients (25.0%), and an ejection fraction of less than 40% was reported in 3 patients (15.0%). Coronary angiogram showed normal coronary vessels in 9 patients (45.0%), with pathologic findings seen in 8 patients (40.0%). Dipyridamole myocardial perfusion scintigraphy showed no ischemic tissue of myocardium in 1 patient (5.0%).19 Elevated cardiac enzyme levels were reported in 14 patients (70.0%). Eosinophilia was reported in 3 patients (15.0%). Elevated tryptase was reported in 2 patients (10.0%), whereas 2 patients (10.0%) had normal tryptase levels measured several hours after presentation. Elevated IgE levels were reported in 5 patients (25.0%), whereas 2 patients (10.0%) had normal IgE levels measured several hours after presentation. Hyperglycemia and hyperlipidemia were reported in 3 (15.0%) and 2 (10.0%) patients, respectively. Elevated white blood cell counts (neutrophilia) were reported in 4 patients (20.0%). The activities of protein C, protein S, antithrombin III, and activated protein C were within normal limits; antiphospholipid antibodies and factor V Leiden mutation were negative in 1 patient (5.0%).
TABLE IV.
Results of Relevant Cardiovascular Diagnostic Investigations
| No. | Study | ECG | Echocardiogram | Coronary angiogram | Cardiac enzymes | Diagnosis (KS type), as reported by the authors |
|---|---|---|---|---|---|---|
| 1 | Mori et al26 (1997) | Significant STEs in leads II, III, aVF, V5, and V6, with 2:1 AV block | Normal | Normal coronary trees; ergonovine provocation test showed severe spasm at middle portion of RCA and LCA accompanied by chest pain and ischemic ST changes promptly resolved by 2.5 mg intracoronary isosorbide dinitrate | NR | Vasospastic angina associated with anaphylactic reaction |
| 2 | Blanco et al17 (2003) | Elevation of the ST segment of leads V1–V4 indicative of anterior acute MI | Anterior hypokinesia with global EF of 49% | Normal, with no atherosclerotic organic lesions | Elevated total CK and troponin T | Anterior acute MI associated with anaphylactic reaction |
| 3 | Gluvic et al22 (2007) | Up to 5-mm STE in inferior and entire precordial leads | No motility disorders of heart muscle (EF, 53%; trace mitral and tricuspid regurgitation) | Not performed (refused by patient) | Elevated CK, normal troponin I | ACS associated with type I hypersensitivity reaction |
| 4 | de Groot et al20 (2009) | STEs in leads II, III, and aVF; acute inferolateral MI diagnosed | NR | No significant coronary stenosis | Elevated troponin T, whereas CK, and CK-MB remained normal | STEMI associated with anaphylaxis |
| 5 | Cakar et al19 (2011) | First time: 1-mm STE in inferior derivations, reciprocal ST-segment depression up to 4 mm in entire precordial leads and third-degree AV block Second time: same as previously |
NR | First time: 2 sequential 70% RCA lesions and noncritical lesion in LAD | Normal | ACS associated with anaphylaxis (first time: II; second time: I and II) |
| 6 | Granitz et al34 (2011) | Transient STEs over inferior posterior wall | NR | Stenosing CAD could be ruled out angiographically, but ubiquitous plaques found | Elevated troponin, without CK deflection | ACS associated with anaphylactic reaction (II) |
| 7 | Cagliyan et al18 (2013) | Emergent ECG compatible with acute anterior MI; fourth-hour control ECG showed resolution of STE | NR | LAD occluded just distal to the first diagonal branch | Marked elevation | Anterior acute MI triggered by allergic reaction (II) |
| 8 | Rodrigues et al2 (2013) | STE in the inferior leads | No motility disorders of heart wall muscle or other abnormalities | No lesions on coronary vessels or contractility abnormalities | Elevated troponin I and CK-MB | ACS associated with anaphylaxis (I) |
| 9 | Tiwari et al32 (2013) | STE in leads II, III, and aVF; STEMI of inferior wall diagnosed | NR | Normal coronary vessels | Elevated troponin T, normal CK, and CK-MB | STEMI associated with anaphylaxis (I) |
TABLE IV.
Results of Relevant Cardiovascular Diagnostic Investigations
| No. | Study | ECG | Echocardiogram | Coronary angiogram | Cardiac enzymes | Diagnosis (KS type), as reported by the authors |
|---|---|---|---|---|---|---|
| 10 | Şahinkuş et al30 (2016) | STE in leads D2, D3, aVF, V4–V6; reciprocal ST-segment depression in leads aVL, V1, V2; diagnosed with acute inferolateral wall MI | EF, 60%; left ventricle wall motion normal | No pathological changes, such as plaque rupture, thrombus, or dissection | Elevated troponin I | STEMI secondary to allergic reaction |
| 11 | Akboğa et al16 (2017) | 2- to 3-mm STE in D2, D3, and aVF leads; reciprocal changes in other leads | Normal | Normal coronary arteries without obstruction | Normal | ACS associated withanaphylaxis (I) |
| 12 | Gunes et al23 (2017) | First ECG: slight STE in inferior leads (II, III, aVF) accompanied by slight ST-segment depression and negative T waves in leads V2–V5; Second ECG: prominent STE in inferior leads (II, III, aVF) accompanied by prominent ST-segment depression and biphasic T waves in leads V2–V5 |
Left ventricular EF, 25% | Total (100%) occlusion of RCA by thrombus material | NR | ACS associated withanaphylaxis (II) |
| 13 | Kerai et al25 (2017) | ST depression and T-wave inversion in leads II and III; VF from V2–V5 suggestive of inferior and lateral wall MI | NR | Normal coronary vasculature | Elevated CK-MB and troponin I | Acute MI associated with anaphylaxis (I) |
| 14 | İbrahim et al24 (2018) | Normal ECG—no changes | EF, 25%; global hypokinesia of left ventricle | Not performed—emergency cardiovascular percutaneous intervention not necessary | Elevated troponin and CK-MB | ACS associated withanaphylaxis (II) |
| 15 | Sarıoğlu et al31 (2018) | Sinus tachycardia and STE in leads D1 and aVL, with reciprocal ST depressions in leads D3 and aVF | Lateral and anterolateral wall hypokinesis, with EF of 35%; no valve abnormality | Normal coronary arteries without significant lesions | Elevated troponin I, normal CK-MB | ACS associated with anaphylactic reaction (I) |
| 16 | Rajh et al28 (2019) | STEs in leads III and aVF, with changes in leads V4, V5, V6, I, and II | NR | No new occlusions, confirmation of preexisting 3-vessel disease (100% occluded LAD, 20%–30% of LCA, and 70% of RCA); graft study demonstrated patency through left internal mammary artery to left anterior ascending artery and saphenous vein graft to diagonal arteries | Elevated CK, normal troponin T | STEMI associated with allergic reaction |
TABLE IV.
Results of Relevant Cardiovascular Diagnostic Investigations
| No. | Study | ECG | Echocardiogram | Coronary angiogram | Cardiac enzymes | Diagnosis (KS type), as reported by the authors |
|---|---|---|---|---|---|---|
| 17 | Yıldırım et al33 (2019) | 2- to 3-mm STE in leads D2–D3 and aVF; reciprocal changes in other leads | NR | Normal coronary arteries without obstruction | NR | MI (I) |
| 18 | Elsayed21 (2020) | Sinus tachycardia (130/min), with a few univocal PVCs and ST-segment depression in the anterior (I, aVL, and V6) and inferior (II and aVF) leads | No detected abnormalities; EF, 66% | NR | NR | ACS associated with anaphylaxis (I) |
| 19 | Özdemir et al27 (2020) | Prominent STE in leads II, III, aVF, V5, and V6; ST-segment depression in leads aVL and V1–V3, with AV complete block | EF, 60%; mild hypokinesia in midsegment of inferior wall of left ventricle | Dominant LCX with nonsignificant plaque; LAD with normal appearance; RCA was nondominant without a fixed stenosis | Elevated troponin I | Recurrent allergic STEMI with no sign of systemic allergic reactions, except coronary spasm (between I and II) |
| 20 | Rui et al29 (2021) | Significant STEs over V2–V4, with reciprocal ST-segment depressions over leads II, III, and aVF (anteroseptal STE with reciprocal changes) | Anterior wall hypokinesia | No acute culprit lesions in the LAD; mild in-stent restenosis in the LAD; no remnants to suggest a recent thrombus or plaque rupture | Elevated CK and troponin T | STEMI secondary to anaphylactic reaction(II) |
ACS, acute coronary syndrome; AV, atrioventricular; CAD, coronary artery disease; CK, creatine kinase; CK-MB, creatine kinase myocardial band; ECG, electrocardiogram; EF, ejection fraction; KS, Kounis syndrome; LAD, left anterior descending coronary artery; LCA, left coronary artery; LCX, left circumflex coronary artery; MI, myocardial infarction; NR, not reported; PVC, premature ventricular contraction; RCA, right coronary artery; STE, ST-segment elevation; STEMI, ST-segment elevation myocardial infarction; VF, ventricular fibrillation.
The authors diagnosed KS type I and type II in 7 (35.0%) and 5 (25.0%) patients, respectively. The clinical situation for 1 patient (5.0%) was described to be between types I and II (the patient had coronary disease with elevated troponin I levels but no severe stenosis, plaque erosion, or rupture).27 In another case (n = 1 [5.0%]),19 the authors described a 74-year-old female patient who experienced an anaphylactic reaction after taking oral diclofenac potassium and type II KS (ST-segment elevations in inferior derivations resulting from coronary artery spasm and underlying coronary artery disease); 2 months later, after receiving IV diclofenac potassium, she felt chest pain, and her electrocardiograms showed the same findings as those observed during the previous application. Coronary angiography was not repeated because myocardial perfusion imaging showed no coronary ischemia; therefore, the authors concluded that this case is an example of both type I (at the second application because no coronary ischemia occurred after coronary stenting) and type II KS at the same time.19 The authors did not report KS type for 6 cases (30.0%).
Causality assessment findings using Naranjo score were reported in just 1 case.21 The author calculated that the Naranjo score was +10, indicating a definite relationship between the adverse drug reaction and the culprit drug: oral diclofenac potassium.21 Positive rechallenge of signs and symptoms associated with KS after diclofenac administration was described in 3 patients (15.0%)—1 each in Mori et al,26 Cakar et al,19 and Özdemir et al.27
Most patients (n = 19 [95.0%]) required hospitalization for treatment, although 1 patient (5.0%) was able to go home without issues 3 hours after treatment in an outpatient clinic.21 Four patients (20.0%) were treated in an intensive care unit, and 3 patients (15.0%) were treated in a cardiac care unit. The specified total length of hospital stay for these 7 patients ranged from 1 to 5 days (median, 2 days). During management of the reaction, diclofenac was discontinued in all patients after they experienced first symptoms. The summary of treatment for each patient is provided in Table III. Corticosteroids (n = 14 [70.0%]), histamine receptor 1 (H1) antihistamines (n = 11 [55.0%]), and IV fluids (n = 11 [55.0%]) were the most frequently prescribed treatments, followed by adrenaline (n = 6 [30.0%]), antiplatelet drugs (n = 6 [30.0%]), nitrates (n = 5 [25.0%]), vasopressors (n = 4 [20.0%]), bronchodilators (n = 3 [15.0%]), oxygen (n = 3 [15.0%]), the anticoagulant enoxaparin (n = 2 [10.0%]), morphine (n = 2 [10.0%]), the H2 antihistamine ranitidine (n = 2 [10.0%]), atropine (n = 2 [10.0%]), the calcium channel blocker diltiazem (n = 2 [10.0%]), statins (n = 1 [5.0%]), nicorandil (n = 1 [5.0%]), ramipril (n = 1 [5.0%]), magnesium sulfate (n = 1 [5.0%]), and metoclopramide (n = 1 [5.0%]). Reperfusion therapy was performed in 4 patients (20.0%): 2 (10.0%) patients received fibrinolytics, and the other 2 (10.0%) underwent stent placement. In 1 patient (5.0%), an occlusion was opened by repeated balloon dilatation, but a stent could not be placed at the lesion site because of ectasia of the artery. Resuscitation had to be performed in 3 patients (15.0%), and 1 patient (5.0%) required defibrillation. All patients survived.
Discussion
This review observed the clinical relevance of diclofenac preparations as a cause of KS. This life-threatening adverse effect occurred with therapeutic doses of diclofenac and was reported significantly more frequently in men than in women. Most patients included in this review did not have a history of cardiovascular disease or allergic reaction to diclofenac. The drug caused both type I and type II KS, with various cardiovascular, GI, dermatologic, and respiratory signs and symptoms. Although there were no fatal outcomes, severe cases with complications such as cardiac arrest, ventricular fibrillation, cardiogenic shock, and pulmonary edema were reported.
Etiology and Epidemiology of Kounis Syndrome
Many triggers of KS have been identified to date, such as different kinds of food, insect bites, various medications, environmental exposures, and several health conditions.3 Among medications, antibiotics and NSAIDs are the most common triggers.35,36 Kounis syndrome can occur in all age categories, but almost 70% of all reported cases to date have been in people between 40 and 70 years of age,35 which is in accordance with the results of this review. What is particularly interesting among the results presented here, however, is that diclofenac-induced KS occurred much more frequently in men than in women (90% vs 10%). Similarly, results of a systematic review conducted by Ridella et al37 showed that 76% of patients with KS following β-lactam antibiotic use were men. Awareness and knowledge of KS are particularly high among physicians in southern Europe4; that finding was confirmed in the present study, with almost half of all reported cases of diclofenac-induced KS coming from Turkey. It is difficult to determine the precise prevalence and incidence of KS.4 Many cases certainly remain unrecognized, misdiagnosed, or unreported by clinicians.6,38 Although overall the number of identified cases of KS is low, it has grown exponentially over the past few years,39 reflecting increasing awareness among physicians.40
Pathophysiology
Figure 3 provides an overview of the proposed pathophysiology of KS. Central in the pathogenesis is mast cell degranulation and the release of inflammatory mediators. Mast cell degranulation can occur either as a result of antigen binding to IgE antibodies on the mast cell surface or as a consequence of complement system activation.41 Many mediators are released from mast cell granules, including histamine, platelet-activating factor, chemokines, cytokines, arachidonic acid products (eg, leukotrienes, thromboxane), and neutral proteases (eg, tryptase, chymase, cathepsin D).42 These inflammatory mediators cause anaphylactic symptoms in patients with KS. Histamine induces coronary vasoconstriction, decreases diastolic blood pressure, and activates platelets.3,35 Leukotrienes, chymase, and cathepsin D also have powerful vasoconstrictive effects.43,44 Thromboxane stimulates platelet aggregation,45 and tryptase caused fibrin degradation and thus contributes to the destabilization of thrombi.46 Neutral proteases through metalloproteinase activation can cause plaque rupture or erosion.47 Among medications, NSAIDs are frequently involved in allergic reactions, affecting 20% to 25% of all patients evaluated in allergy units.48–50 These drugs may cause hypersensitive reactions by 2 different mechanisms. The first is mediated by drug-specific IgE antibodies when a patient is hypersensitive to 1 specific chemical group of NSAIDs but tolerant of NSAIDs that are not structurally similar.51,52 The second mechanism is a consequence of the pharmacologic action of this group of drugs because they inhibit cyclooxygenase and stimulate the lipoxygenase pathway of arachidonic acid metabolism, increasing leukotriene production.53 In this case, there is cross-reactive hypersensitivity toward NSAIDs from different chemical groups.50 Anaphylactic reaction to diclofenac can appear in patients who have never been exposed to this drug before.54 Severe allergic reactions are more common after IM and IV administration of diclofenac, although cases have been reported after oral, rectal, and subcutaneous administration, as well.54 This finding is in accordance with the results in the current study, with almost half of reported cases of KS occurring after the IM administration of diclofenac, although this side effect also occurred after oral, rectal, and IV administration.
Fig. 3.

An overview of Kounis syndrome pathophysiology.
AMI, acute myocardial infarction; PLT, platelet; GP IIb/IIIa R, glycoprotein IIb/IIIa receptor.
Symptoms
Kounis syndrome can manifest with various symptoms, but the main clinical symptoms and signs are always associated with subclinical, clinical, acute, or chronic allergic reactions accompanied by cardiac symptomatology.3 In 80% of cases, the symptoms appear within 1 hour after exposure to the trigger.35 This study found that the reported time from the use of diclofenac to the onset of symptoms ranged from immediately to 5 hours; however, Ridella et al37 showed that the time between β-lactam antibiotic administration and occurrence of symptoms in patients with KS is shorter, ranging from immediate to 2.5 hours. Except for 1 patient whose only symptom was coronary spasm,27 all patients included in this review had symptoms and signs associated with allergic, hypersensitivity, or anaphylactic reactions. The most common cardiac symptoms were chest pain (60%) and hypotension (75%); dermatologic, respiratory, and GI symptoms were reported in 70%, 30%, and 20% of patients, respectively. There were no significant differences in the prevalence of symptoms among patients in whom the trigger for KS was β-lactam antibiotics, where chest pain was reported in 65% of patients; hypotension in approximately 71% of patients; and dermatologic, respiratory, and GI symptoms in approximately 77%, 41%, and 12% of patients, respectively.37
Diagnosis
The diagnosis of KS is based on reliable anamnestic data, clinical symptoms, and signs that emphasize multisystem involvement as well as on laboratory, electrocardiographic, echocardiographic, and angiographic evidence.3,35,39 When KS is suspected, it is necessary first to determine whether the patient has a history of allergic reactions. It has been shown that 25% of patients have a known history of allergy, mostly to the trigger.35 The results of this study are in accordance with these findings: 5 patients (25.0%) with diclofenac-induced KS had had a previous reaction to the culprit drug, and 1 patient had a history of an aspirin-induced asthma attack and obviously had cross-reactive hypersensitivity to diclofenac. Regarding laboratory findings, measuring serum tryptase, histamine, cardiac enzyme, and cardiac troponin levels may be helpful.3,35 Histamine has a half-life of 8 minutes,53,55 however, so blood samples should be collected promptly after symptom onset.56 Tryptase has a half-life of approximately 90 minutes,57 so testing for tryptase may be more useful than for histamine. The role of IgE levels in diagnosis remains unclear,55 although elevated IgE levels were reported in 20% of patients with diclofenac-induced KS and in approximately 24% of patients in whom β-lactam antibiotics were the trigger.37 Cardiospecific enzymes, such as troponin I and T, creatine kinase, and creatine kinase myocardial band, are important indicators of myocardial injury associated with the allergic insult.3,35,58 Abdelghany et al35 found that approximately 60% of all patients with KS had elevated troponin levels, and elevated levels of various cardiac enzymes were observed in 70% of patients in this systematic review. Electrocardiography is also important in the diagnosis of KS.6 In patients with diclofenac-induced KS, the most frequently reported finding was ST-segment elevation (85% of patients). An even higher proportion of electrocardiogram findings indicating ST elevation (95%) was observed in patients with KS resulting from the use of β-lactam antibiotics.37 Transthoracic echocardiography may be useful for the differential diagnostic exclusion of pericarditis or aortic dissection as potential causes of chest pain,6 and cardiac catheterization may show coronary vasospasm or stenosis.35
Abdelghany et al35 showed that type I is the most common variant of KS, accounting for approximately 73% of cases, followed by the type II and type III variants, whose frequencies are approximately 22% and 5%, respectively. Type I was also the most common variant in KS cases resulting from β-lactam antibiotics (70.5%); the remaining 29.5% of cases were categorized as type II.37 In contrast, this study found that among patients with diclofenac-induced KS, the difference in the frequency of the type I variant was not as drastically pronounced (35% in type I vs 25% in type II). It is important to emphasize, however, that in more than one-third of cases, the authors did not state or were unsure of the type of KS.
Treatment
No precise guidelines exist for the management of KS.35 In patients with type II KS, it is necessary to treat both the allergic and the cardiac symptoms simultaneously; in patients with the type I variant, treatment of the allergic event alone can abolish symptoms.3 For relief of allergic symptoms, hydrocortisone 1 to 2 mg/kg/day and H1 and H2 antihistamines such as diphenhydramine 1 to 2 mg/kg and ranitidine 1 mg/kg are considered adequate.3,6 Because of increased vascular permeability during anaphylaxis, up to 50% of intravascular fluid volume may be transferred into the extravascular space within 10 minutes, so fluid replacement is advised.6 Fluid replacement should be performed with caution in patients with left ventricular dysfunction, however, because of the increased risk of pulmonary edema.6 Supplemental oxygen should be administered to all patients.6 For treatment of coronary vasospasm, calcium channel blockers are recommended, whereas nitrates could be used in patients with normal blood pressure.3
Note that use of adrenaline in this setting is controversial: although adrenaline is life-saving in anaphylaxis, it may worsen vasospasm in patients with KS because it acts on α-adrenergic receptors.3 In addition, adrenaline preparations contain sulfite as a preservative and antioxidant, which can cause allergic and anaphylactoid reactions.59,60 Finally, adrenaline could be ineffective in patients with KS who previously used β-blockers.3,6
Fentanyl and its derivatives are the analgesics of choice for the treatment of chest pain in patients with KS.3,6 Other opioids, such as morphine, codeine, and meperidine, should be avoided because they may worsen allergic reaction. Similarly, IV acetaminophen should be avoided because of the risk of hypotension.3,36 In the type III variant, clinicians should follow the most recent guidelines for the treatment of ACS.3,35
Prognosis
Generally, ACS within KS has a better prognosis and lower mortality rate than for conventional types of ACS.3 Complete resolution of contractile abnormalities and full recovery are usually seen.6,35 Abdelghany et al35 showed that the death rate in patients with KS is 2.9%, but patients hospitalized for KS experience higher all-cause in-hospital mortality, prolonged hospital length of stay, higher hospitalization charges, and more frequent transfers to other facilities than patients hospitalized for non-KS allergy, hypersensitivity, or anaphylactic reactions.61
Limitations
This systematic review has the following shortcomings: (1) it analyzed a relatively small number of reported cases with diclofenac-induced KS; (2) the completeness of the included cases varied, and important information was missing or incompletely presented (eg, dose of administered diclofenac, duration of treatment before symptom onset); (3) data on the type of KS were missing in several case reports; (4) in the vast majority of the case reports analyzed, assessment of the potential contribution of concomitant therapies was missing; and (5) even if cases contained all the essential information, it was often not possible to establish definitive conclusions on causality.15 Despite these limitations, the results of this review could help clinicians in various specialties better recognize and manage diclofenac-induced KS.
Conclusion
Diclofenac administered at therapeutic doses, regardless of route of administration, may cause KS, even in patients without a known history of hypersensitivity and allergic reactions. Clinicians should be aware that KS may be an adverse effect of diclofenac: prompt recognition and withdrawal of the culprit drug, with treatment of both allergic and cardiac symptoms simultaneously, are of the utmost importance.
Abbreviations and Acronyms
- ACS
acute coronary syndrome
- AMI
acute myocardial infarction
- COX-1
cyclooxygenase 1
- GI
gastrointestinal
- IgE
immunoglobulin E
- IM
intramuscular
- IV
intravenous
- KS
Kounis syndrome
- MI
myocardial infarction
- NSAID
nonsteroidal anti-inflammatory drug
- SCIndeks
Serbian Citation Index
Funding Statement
Funding/Support: This study was partially supported by grant No. 175007 from the Serbian Ministry of Education, Science and Technological Development.
Footnotes
Conflict of Interest Disclosures: No potential conflict of interest was reported by the authors.
References
- 1.Kounis NG, Zavras GM. Histamine-induced coronary artery spasm: the concept of allergic angina. Br J Clin Pract . 1991;45(2):121–128. [PubMed] [Google Scholar]
- 2.Luís Rodrigues MC, Coelho D, Granja C. Drugs that may provoke Kounis syndrome. Braz J Anesthesiol . 2013;63(5):426–428. doi: 10.1016/j.bjan.2013.04.006. [DOI] [PubMed] [Google Scholar]
- 3.Kounis NG. Kounis syndrome: an update on epidemiology, pathogenesis, diagnosis and therapeutic management. Clin Chem Lab Med . 2016;54(10):1545–1559. doi: 10.1515/cclm-2016-0010. [DOI] [PubMed] [Google Scholar]
- 4.Kounis NG. Coronary hypersensitivity disorder: the Kounis syndrome. Clin Ther . 2013;35(5):563–571. doi: 10.1016/j.clinthera.2013.02.022. [DOI] [PubMed] [Google Scholar]
- 5.Li J, Zheng J, Zhou Y, Liu X, Peng W. Acute coronary syndrome secondary to allergic coronary vasospasm (Kounis syndrome): a case series, follow-up and literature review. BMC Cardiovasc Disord . 2018;18(1):42. doi: 10.1186/s12872-018-0781-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Biteker M. Current understanding of Kounis syndrome. Expert Rev Clin Immunol . 2010;6(5):777–788. doi: 10.1586/eci.10.47. [DOI] [PubMed] [Google Scholar]
- 7.Gan TJ. Diclofenac: an update on its mechanism of action and safety profile. Curr Med Res Opin . 2010;26(7):1715–1731. doi: 10.1185/03007995.2010.486301. [DOI] [PubMed] [Google Scholar]
- 8.Jordan S, White J. Non-steroidal anti-inflammatory drugs: clinical issues. Nurs Stand . 2001;15(23):45–52. doi: 10.7748/ns2001.02.15.23.45.c2986. [DOI] [PubMed] [Google Scholar]
- 9.Alfaro RA, Davis DD. Diclofenac . StatPearls Publishing; Updated September 17, 2021. Accessed January 10, 2023. http://www.ncbi.nlm.nih.gov/books/NBK557879/ [PubMed] [Google Scholar]
- 10.Pal A, Biswas J, Mukhopadhyay P, Sanyal P, Dasgupta S, Das S. Diclofenac is more effective for post-operative analgesia in patients undergoing lower abdominal gynecological surgeries: a comparative study. Anesth Essays Res . 2014;8(2):192–196. doi: 10.4103/0259-1162.134502. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Flynn Pharma Ltd; 2021. AKIS 75mg/ml solution for injection. Summary of product characteristics. Accessed January 10, 2023. https://www.medicines.org.uk/emc/product/9399/smpc%202021. [Google Scholar]
- 12.Modena B, White AA, Woessner KM. Aspirin and nonsteroidal antiinflammatory drugs hypersensitivity and management. Immunol Allergy Clin North Am . 2017;37(4):727–749. doi: 10.1016/j.iac.2017.07.008. [DOI] [PubMed] [Google Scholar]
- 13.Schwartz BG, Daulat S, Kuiper J. The Kounis-Zavras syndrome with the Samter-Beer triad. Proc (Bayl Univ Med Cent) . 2011;24(2):107–109. doi: 10.1080/08998280.2011.11928695. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.McGettigan P, Henry D. Use of non-steroidal anti-inflammatory drugs that elevate cardiovascular risk: an examination of sales and essential medicines lists in low-, middle-, and high-income countries. PLoS Med . 2013;10(2):e1001388. doi: 10.1371/journal.pmed.1001388. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Kelly WN, Arellano FM, Barnes J, et al. International Society for Pharmacoepidemiology. International Society of Pharmacovigilance Guidelines for submitting adverse event reports for publication. Drug Saf . 2007;30(5):367–373. doi: 10.2165/00002018-200730050-00001. [DOI] [PubMed] [Google Scholar]
- 16.Akboğa MK, Akyel A, Aydoğdu S. Acute coronary syndrome due to diclofenac-induced anaphylaxis: type 1 Kounis syndrome. Gazi Med J . 2017;28(1):44–45. [Google Scholar]
- 17.Blanco VMR, Möller I, Catalán F, Casares G. Acute myocardial infarction during a diclofenac-associated anaphylactic reaction. [In Spanish.] Med Clin (Barc) . 2003;121(7):278. doi: 10.1016/s0025-7753(03)75196-3. [DOI] [PubMed] [Google Scholar]
- 18.Cagliyan CE, Balli M, Tekin K, Turkmen S, Tanboga İH. Kounis syndrome triggered by diclofenac sodium injection which leads to myocardial infarction and cardiac arrest. J Cardiol Cases . 2013;8(1):e17–e19. doi: 10.1016/j.jccase.2013.03.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Cakar MA, Gündüz H, Kocayilnit I, Kocayiǧit DF, Vatan MB, Tamer A. Acute coronary syndrome due to diclofenac potassium induced anaphylaxis: two Kounis syndrome variants in the same patient. Anadolu Kardiyol Derg . 2011;11(1):88–89. doi: 10.5152/akd.2011.017. [DOI] [PubMed] [Google Scholar]
- 20.de Groot JWB, Gosselink ATM, Ottervanger JP. Acute ST-segment elevation myocardial infarction associated with diclofenac-induced anaphylaxis: case report. Am J Crit Care . 2009;18(4):388, 386–387. doi: 10.4037/ajcc2009100. [DOI] [PubMed] [Google Scholar]
- 21.Elsayed YMH. Diclofenac potassium-induced anaphylaxis with an allergic acute coronary syndrome and premature ventricular contractions; outpatient clinic management; a case report. Res Int J Cardiol Cardio Med . 2020;1(1):13–16. doi: 10.37179/rijccm.000005. [DOI] [Google Scholar]
- 22.Gluvic ZM, Putniković B, Panic M, Stojkovic A, Rasic-Milutinovic Z, Jankovic-Gavrilovic J. Acute coronary syndrome in diclofenac sodium-induced type I hypersensitivity reaction: Kounis syndrome. Malta Med J . 2007;19(3):36–39. [Google Scholar]
- 23.Gunes H, Sonmez FT, Saritas A, Koksal Y. Kounis syndrome induced by oral intake of diclofenac potassium. Iran J Allergy Asthma Immunol . 2017;16(6):565–568. [PubMed] [Google Scholar]
- 24.İbrahim A, Çolak Ş, Erdoğan MÖ, Afacan MA, Sarıtaş A, Kandiş H. Kounis syndrome as a result of anaphylactic reaction to diclofenac sodium: a case report. J Surg Med . 2018;2(3):337–338. doi: 10.28982/josam.402775. [DOI] [Google Scholar]
- 25.Kerai S, Sehrawat L, Saxena KN, Taneja B. Occurrence of Kounis syndrome under anesthesia. J Anaesthesiol Clin Pharmacol . 2017;33(2):276–277. doi: 10.4103/0970-9185.209737. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Mori E, Ikeda H, Ueno T et al. Vasospastic angina induced by nonsteroidal anti-inflammatory drugs. Clin Cardiol . 1997;20(7):656–658. doi: 10.1002/clc.4960200713. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Özdemir E, Karakaya Z, Karaca M, Topal F, Payza U. Recurrent diclofenac-induced acute myocardial infarction: an interesting case with wandering ST-segment elevation. Hong Kong J Emerg Med . 2020;27(5):304–307. doi: 10.1177/1024907919845526. [DOI] [Google Scholar]
- 28.Rajh F, Raja R, Rajah F, AlAli A. Acute ST-segment elevation myocardial infarction following intramuscular diclofenac: a case of Kounis syndrome. J Emerg Med . 2019;57(1):e5–e8. doi: 10.1016/j.jemermed.2019.03.026. [DOI] [PubMed] [Google Scholar]
- 29.Rui TC, Sani H, Zulkufli NS. A case of acute ST-elevation myocardial infarction (STEMI) secondary to anaphylactic reaction: type 2 Kounis syndrome. Malaysian J Med Health Sci . 2021;17(2):314–316. [Google Scholar]
- 30.Şahinkuş S, Yılmaz S, Yaylacı S, Can Y, Kocayiğit İ, Gündüz H. Kounis syndrome due to diclofenac injection. Alban Med J . 2016;1:63–66. [Google Scholar]
- 31.Sarıoğlu G, Kurtoglu E, Çakmak T, Güngören F, Hidayet Ş. Kounis syndrome due to oral intake of diclofenac potassium: a case report. [In Turkish.] Bozok Tıp Dergisi . 2018;8(3):132–135. doi: 10.16919/bozoktip.383792. [DOI] [Google Scholar]
- 32.Tiwari AK, Tomar GS, Ganguly CS, Kapoor MC. Kounis syndrome resulting from anaphylaxis to diclofenac. Indian J Anaesth . 2013;57(3):282–284. doi: 10.4103/0019-5049.115614. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Yildirim T, Yıldırım SE. A forgotten cause of myocardial infarction in an octogenarian patient: type 1 Kounis syndrome. Balıkesir Med J . 2019;3(3):165–167. doi: 10.33716/bmedj.599198. [DOI] [Google Scholar]
- 34.Granitz C, Kraus J, Schuler J, Pichler M. An unusual cause of acute coronary syndrome: diclofenac-induced Kounis syndrome. [In German.] Wiener klinische Wochenschrift . 2011;123(17–18):A56. [Google Scholar]
- 35.Abdelghany M, Subedi R, Shah S, Kozman H. Kounis syndrome: a review article on epidemiology, diagnostic findings, management and complications of allergic acute coronary syndrome. Int J Cardiol . 2017;232:1–4. doi: 10.1016/j.ijcard.2017.01.124. [DOI] [PubMed] [Google Scholar]
- 36.Renda F, Landoni G, Trotta F et al. Kounis syndrome: an analysis of spontaneous reports from international pharmacovigilance database. Int J Cardiol . 2016;203:217–220. doi: 10.1016/j.ijcard.2015.10.003. [DOI] [PubMed] [Google Scholar]
- 37.Ridella M, Bagdure S, Nugent K, Cevik C. Kounis syndrome following beta-lactam antibiotic use: review of literature. Inflamm Allergy Drug Targets . 2009;8(1):11–16. doi: 10.2174/187152809787582462. [DOI] [PubMed] [Google Scholar]
- 38.Fourie P. Kounis syndrome: a narrative review. South Afr J Anaesth Analg . 2016;22(2):72–80. doi: 10.1080/22201181.2016.1154309. [DOI] [Google Scholar]
- 39.Di Toro ML, Stub D. Kounis syndrome: a case report and literature review of pre-hospital treatment. Australas J Paramedicine . 2018;15(4):1–7. doi: 10.33151/ajp.15.4.614. [DOI] [Google Scholar]
- 40.Renda F, Marotta E, Landoni G, Belletti A, Cuconato V, Pani L. Kounis syndrome due to antibiotics: a global overview from pharmacovigilance databases. Int J Cardiol . 2016;224:406–411. doi: 10.1016/j.ijcard.2016.09.066. [DOI] [PubMed] [Google Scholar]
- 41.Kounis NG. Kounis syndrome (allergic angina and allergic myocardial infarction): a natural paradigm? Int J Cardiol . 2006;110(1):7–14. doi: 10.1016/j.ijcard.2005.08.007. [DOI] [PubMed] [Google Scholar]
- 42.Gilfillan AM, Tkaczyk C. Integrated signalling pathways for mast-cell activation. Nat Rev Immunol . 2006;6(3):218–230. doi: 10.1038/nri1782. [DOI] [PubMed] [Google Scholar]
- 43.Carl-McGrath S, Gräntzdörffer I, Lendeckel U, Ebert MP, Röcken C. Angiotensin II-generating enzymes, angiotensin-converting enzyme (ACE) and mast cell chymase (CMA1), in gastric inflammation may be regulated by H. pylori and associated cytokines. Pathology . 2009;41(5):419–427. doi: 10.1080/00313020902885037. [DOI] [PubMed] [Google Scholar]
- 44.Riccioni G, Zanasi A, Vitulano N, Mancini B, D'Orazio N. Leukotrienes in atherosclerosis: new target insights and future therapy perspectives. Mediators Inflamm . 2009;2009:737282. doi: 10.1155/2009/737282. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Arshad M, Vijay V, Floyd BC et al. Thromboxane receptor stimulation suppresses guanylate cyclase-mediated relaxation of radial arteries. Ann Thorac Surg . 2006;81(6):2147–2154. doi: 10.1016/j.athoracsur.2006.01.024. [DOI] [PubMed] [Google Scholar]
- 46.Kounis NG, Tsigkas G, Almpanis G, Kouni SN, Kounis GN, Mazarakis A. Anaphylaxis-induced hyperfibrinogenolysis and the risk of Kounis syndrome: the dual action of tryptase. Am J Emerg Med . 2011;29(9):1229–1230. doi: 10.1016/j.ajem.2011.06.002. [DOI] [PubMed] [Google Scholar]
- 47.Johnson JL, Jackson CL, Angelini GD, George SJ. Activation of matrix-degrading metalloproteinases by mast cell proteases in atherosclerotic plaques. Arterioscler Thromb Vasc Biol . 1998;18(11):1707–1715. doi: 10.1161/01.atv.18.11.1707. [DOI] [PubMed] [Google Scholar]
- 48.Faich GA. Adverse-drug-reaction monitoring. N Engl J Med . 1986;314(24):1589–1592. doi: 10.1056/NEJM198606123142427. [DOI] [PubMed] [Google Scholar]
- 49.Doña I, Blanca-López N, Cornejo-García JA et al. Characteristics of subjects experiencing hypersensitivity to non-steroidal anti-inflammatory drugs: patterns of response. Clin Exp Allergy . 2011;41(1):86–95. doi: 10.1111/j.1365-2222.2010.03651.x. [DOI] [PubMed] [Google Scholar]
- 50.Kowalski ML, Makowska JS, Blanca M et al. Hypersensitivity to nonsteroidal anti-inflammatory drugs (NSAIDs)—classification, diagnosis and management: review of the EAACI/ENDA(#) and GA2LEN/HANNA*. Allergy . 2011;66(7):818–829. doi: 10.1111/j.1398-9995.2011.02557.x. [DOI] [PubMed] [Google Scholar]
- 51.Quiralte J, Blanco C, Castillo R, Delgado J, Carrillo T. Intolerance to nonsteroidal antiinflammatory drugs: results of controlled drug challenges in 98 patients. J Allergy Clin Immunol . 1996;98(3):678–685. doi: 10.1016/s0091-6749(96)70102-1. [DOI] [PubMed] [Google Scholar]
- 52.Carmona MJ, Blanca M, Garcia A et al. Intolerance to piroxicam in patients with adverse reactions to nonsteroidal antiinflammatory drugs. J Allergy Clin Immunol . 1992;90(6 pt 1):873–879. doi: 10.1016/0091-6749(92)90459-f. [DOI] [PubMed] [Google Scholar]
- 53.Szczeklik A, Stevenson DD. Aspirin-induced asthma: advances in pathogenesis, diagnosis, and management. J Allergy Clin Immunol . 2003;111(5):913–921. doi: 10.1067/mai.2003.1487. quiz 922. [DOI] [PubMed] [Google Scholar]
- 54.Yiannakopoulou E, editor. Diclofenac Pharmacology Uses and Adverse Effects . Nova Science Publishers; 2019. [Google Scholar]
- 55.Biteker M, Ekşi Duran N, Sungur Biteker F, et al. Allergic myocardial infarction in childhood: Kounis syndrome. Eur J Pediatr . 2010;169(1):27–29. doi: 10.1007/s00431-009-0965-5. [DOI] [PubMed] [Google Scholar]
- 56.Zavras GM, Papadaki PJ, Kokkinis CE et al. Kounis syndrome secondary to allergic reaction following shellfish ingestion. Int J Clin Pract . 2003;57(7):622–624. [PubMed] [Google Scholar]
- 57.Castells MC, Irani AM, Schwartz LB. Evaluation of human peripheral blood leukocytes for mast cell tryptase. J Immunol . 1987;138(7):2184–2189. [PubMed] [Google Scholar]
- 58.Lippi G, Buonocore R, Schirosa F, Cervellin G. Cardiac troponin I is increased in patients admitted to the emergency department with severe allergic reactions. A case-control study. Int J Cardiol . 2015;194:68–69. doi: 10.1016/j.ijcard.2015.05.093. [DOI] [PubMed] [Google Scholar]
- 59.Riggs BS, Harchelroad FP, Jr, Poole C. Allergic reaction to sulfiting agents. Ann Emerg Med . 1986;15(1):77–79. doi: 10.1016/s0196-0644(86)80492-9. [DOI] [PubMed] [Google Scholar]
- 60.Yang WH, Purchase EC. Adverse reactions to sulfites. CMAJ . 1985;133(9):865–867. 880. [PMC free article] [PubMed] [Google Scholar]
- 61.Desai R, Parekh T, Patel U et al. Epidemiology of acute coronary syndrome co-existent with allergic/hypersensitivity/anaphylactic reactions (Kounis syndrome) in the United States: a nationwide inpatient analysis. Int J Cardiol . 2019;292:35–38. doi: 10.1016/j.ijcard.2019.06.002. [DOI] [PubMed] [Google Scholar]


