Abstract
Human urothelial carcinoma (UCC) and non-Hodgkin lymphoma are considered environmental cancers in people, but less is known about environment risk for UCC and lymphoma in dogs. The objective of this study was to determine whether dogs with these cancers, compared to unaffected control dogs, live in counties with higher tap water contaminants or higher levels of air pollution as measured by the Environmental Protection Agency (EPA) and by National Air Toxics Assessment chemical exposure risk estimates. Dogs with available home addresses from two previously published case-control populations were included: 66 dogs with UCC and 70 unaffected controls; and 56 boxer dogs with lymphoma and 84 unaffected boxer controls. Tap water total trihalomethanes, which are water disinfection by-products, were more than 3-fold higher in UCC case counties of residence compared to controls (P < 0.0001), and a higher proportion of dogs with UCC lived in counties exceeding EPA ozone limits (41.8%) compared to controls (13.6% P = 0.0008). More boxers with lymphoma lived in counties exceeding EPA ozone limits (52.1%) compared to controls (29.0%; P = 0.018), with higher exposure risk estimates for airborne 1,3 butadiene and formaldehyde (P = 0.004–0.005). These data support the hypothesis that tap water contaminants and airborne environmental pollutants contribute to the risk of both urothelial carcinoma and lymphoma in dogs. If these findings reflect causal relationships, then it is possible that tap water filtration units and more effective air pollution controls could decrease the overall incidence of these cancers in dogs.
Keywords: Environmental risk, Ozone, Total trihalomethanes, Transitional cell carcinoma, Urothelial cell carcinoma
Introduction
Bladder cancer (urothelial cell carcinoma; UCC) and lymphoma in dogs are typically fatal cancers despite chemotherapy and other treatment interventions.1,2 Canine UCC closely resembles the aggressive muscle-invasive form of human UCC3 and canine lymphoma resembles human Non-Hodgkin lymphoma (NHL).4 Human UCC and NHL are considered environmental cancers in people, but less is known about environment risk for UCC and lymphoma in dogs.
Smoking is a major risk factor for UCC in humans, followed by certain occupational chemical exposures,5,6 environmental air pollutants,7 and drinking water contaminants such as arsenic, nitrates, and total trihalomethanes.8–12 Similarly, risk for non-Hodgkin lymphoma in humans has been associated with occupational exposure to many chemicals, including arsenic, chromium, nitrates, chlorinated hydrocarbons, industrial solvents, and pesticides.13–17 Non-occupational risk factors for NHL include exposure to benzene, industrial air pollution, household insecticide use, and nitrates and pesticides in drinking water.17–22
By comparison, UCC in dogs has not been associated with second-hand smoke23,24 but has been associated with household insecticide and herbicide use24,25 and living in areas of higher industrial activity.26 Lymphoma in dogs has also been associated with household use of lawn pesticides and herbicides27–30 and with proximity to industrial areas.31 We recently found an association between lymphoma in the high-risk boxer breed and living within 2 miles of chemical suppliers or crematoria, or within 10 miles of a nuclear power plant.32 Based on these previous findings, we hypothesized that UCC and lymphoma in dogs would track to residence in areas with higher concentrations of specific airborne pollutants and water contaminants.
The specific aim of this study was to determine whether dogs with UCC or lymphoma, compared to older unaffected control dogs, live in counties with higher tap water concentrations of arsenic, nitrates or total trihalomethanes as reported by water utilities, and higher levels of air pollution, as measured by ozone and particulate matter concentrations, and by National Air Toxics Assessment (NATA) chemical exposure risk estimates.
Methods
Study population
Dogs from two previous case-control studies were included in the study population. Dogs with UCC of the bladder or urethra were recruited nationally as part of gene-environment risk study. Cases of UCC were recruited from the University of Wisconsin-Madison, Purdue University, Colorado State University, the University of Georgia, Texas A&M University, private veterinary oncologists, and primary care practices across the country.24 Control dogs were recruited from the University of Wisconsin-Madison, local dog events, and national outreach through breed associations, kennel clubs, and the National Canine Cancer Foundation. Control dogs were selected to match UCC cases for breed and sex-neuter status, and must have reached the median age of onset for canine UCC (≥ 8 years for Scottish terriers and ≥ 11 years for other breeds),3 with no history of systemic cancer and no unresolved lower urinary tract signs.24 Owners completed a household environmental questionnaire, and cases and controls for the UCC study were enrolled from November 2014 to February 2017. Sixty-six of 69 dogs with UCC and 70 of 72 control dogs had unique and complete home addresses for inclusion in the current study (Table 1).
Table 1:
Demographic data for two canine case-control populations analyzed by county of residence for exposure to airborne and drinking water pollutants. UCC – urothelial carcinoma
| Dog with UCC | Unaffected UCC controls | Boxers with lymphoma | Unaffected boxer controls | |
|---|---|---|---|---|
| Original recruited population (n) | 69 | 72 | 63 | 89 |
| Dogs with full addresses for current study (n) | 66 | 70 | 56 | 84 |
| Commonly represented breeds (n ≥ 3) | Mixed 20 Beagle 6 Shetl sheepdog 6 Westie 6 Dachshund 6 Scottish terr 5 Border collie 3 Min Schnauz 3 |
Mixed 22 Beagle 7 Shetl sheepdog 4 Westie 6 Dachshund 6 Scottish terr 6 Border collie 3 Min Schnauz 3 |
Boxers 56 | Boxers 84 |
| Sex and neuter status | FS 42 FI 2 MN 20 MI 2 |
FS 46 FI 1 MN 21 MI 2 |
FS 26 FI 0 MN 27 MI 3 |
FS 46 FI 0 MN 35 MI 3 |
| Median age (range) | 11 years (4–16) | 12 years (8–16) | 8 years (3.5–15) | 10.5 years (10–15) |
| Number of states represented | 19 | 18 | 17 | 27 |
| Number of counties represented | 42 | 41 | 41 | 78 |
| Predominant neighborhood type | Suburban (62.1%) | Suburban (58.6%) | Suburban (66.1%) | Suburban (53.6%) |
| Predominant drinking water source | Municipal (90.9%) Well (6.1%) Other* (3.0%) |
Municipal (92.9%) Well (5.7%) Other (1.4%) |
Municipal (78.6%) Well (10.7%) Other (10.7%) |
Municipal (70.2%) Well (22.6%) Other (7.2%) |
Bottled, filtered, or pond water as reported by the dogs’ owners.
Boxer dogs with lymphoma and older unaffected control boxer dogs were recruited nationally as part of a second gene-environment risk study.32 Boxer lymphoma cases were recruited from the University of Wisconsin-Madison, Colorado State University, the University of Georgia, and private veterinary oncology practices across the country, including Seattle Veterinary Specialists, BluePearl Pet Hospital in Franklin TN, the Wisconsin Veterinary Referral Center, and the nationwide VCA network of specialty oncologists. Controls were clinically unaffected boxer dogs ≥10 years of age, which is the median age of onset for low grade T-cell lymphoma in boxers,33 and were recruited by local and national outreach to boxer breed and rescue organizations. Boxers with systemic disease were included in the control group only if an underlying non-neoplastic etiology had been established and clinical signs were stable. Owners completed a household environmental questionnaire, and boxers were enrolled June 2016 to December 2019 for the lymphoma study. Fifty-six of 63 boxer cases and 84 of 89 boxer controls had available and unique home addresses for inclusion in the current study (Table 1).
Each dog’s home address at the time of enrollment was mapped to individual counties in the United States. Several databases were used to characterize exposure to water and airborne pollution within each county of residence. To correspond with previous dates of enrollment, county data were recorded, as available, for the 5-year period from 2013–2017 for the UCC population and for 2015–2019 for the lymphoma population.
Tap water concentrations of arsenic (in ppb), nitrate (ppm) and total trihalomethanes (ppb) were retrieved from the Environmental Working Group (EWG) database (https://www.ewg.org/tapwater/), which compiles water monitoring data provided annually by public utilities from state and national environmental regulatory agencies; water data were only available through 2017. Concentrations were averaged over the periods of observation (2013–2017 for the UCC population and 2015–2017 for the lymphoma population). Counties were also flagged as exceeding health-based standards for water concentrations of these chemicals if averages were over Environmental Protection Agency (EPA) established compliance limits, which is 10 ppb for arsenic, 10 mg/L (ppm) for nitrate, and 80 ppb for TTHMs. In addition, because dogs were reported by their owners to drink water from different sources, water exposure data were re-analyzed for the sub-group of dogs in each case-control study whose drinking water was primarily (> 50%) from municipal tap water, as opposed to well water, bottled water, filtered water, or other sources.24,32
For measurements of air pollution, we used data for ozone concentrations (in ppb) and airborne fine particulate matter (< 2.5 microns in diameter; PM2.5; in ug/m3) available from the EPA by U.S. county (www.epa.gov). We averaged measurements over 2013–2017 for the UCC population and over 2015–2019 for the lymphoma population and identified counties that had average levels higher than the EPA National Ambient Air Quality Standard limits over the 5-year period (≥ 70 ppb for ozone and ≥ 12 ug/m3 for PM2.5).
In addition, we used data from the EPA’s National Air Toxics Assessment (NATA) program, which were available for the year 2014. These data provide a snapshot of outdoor air quality based on single year measurements of specific chemical emissions by county in the U.S. and are expressed as modeled cancer risk per million people attributable to chronic airborne exposure to these individual chemicals across a lifetime. The NATA database includes 78 airborne chemicals. We edited the list to include only chemicals that have been associated epidemiologically or experimentally with UCC or NHL in people or other animals, based on searches of published studies (PubMed.gov). For individual chemical risk analyses, we further eliminated chemicals for which the cancer risk estimates were very low across U.S. counties (< 0.01/million).
Statistical analyses
For the UCC population, which was recruited using pair matching for breed and sex, average 5-year county of residence-based airborne ozone or PM2.5 concentrations, 5-year tap water arsenic, nitrates, or TTHMs by county of residence, and individual chemical cancer risk estimates from the NATA database were compared between cases and controls using Wilcoxon matched pairs signed rank tests. For the boxer population, which was not pair matched, average 5-year county of residence-based airborne ozone or PM2.5 concentrations, available 3-year tap water arsenic and nitrates by county of residence, and individual chemical cancer risk estimates from the NATA database were compared between cases and controls using Mann Whitney U tests. The proportion of counties of residence with levels ever exceeding EPA guidelines over the observation periods were compared between cases and controls using Fisher’s exact tests, with P < 0.05. Odds ratios with 95% confidence intervals (CI) were used to estimate the increased risk of living in a county with air and water concentrations exceeding EPA limits among cases compared to controls. Given that group sizes were relatively small and cases and controls were comparable in age, sex, and sex (Table 1), which are primary risk factors for canine bladder34 and lymphoma,35,36 adjusted analyses were not conducted. A P < 0.05 considered significant for all analyses except for the NATA data, for which P was adjusted for multiple comparisons using the Bonferroni method (P = 0.05 / number of comparisons).
Results
Dogs with urothelial carcinoma
County of residence data were available for 66 of 69 dogs with UCC of the bladder or urethra and 70 of 72 unaffected controls of comparable sex and breed ≥ 11 years old.24 At the time of original enrollment, dogs with UCC resided in 42 individual counties across 19 states, and control dogs resided in 41 counties across 18 states (Table 1).
Complete data for TTHMs in tap water over the observation period were available for 88.1% of UCC case counties and 82.9% of UCC control counties (Supplemental Table S1). Total trihalomethanes in tap water were more than 3-fold higher in UCC case counties (median 30.3 ppb, range 0.0 to 52.1) compared to UCC control counties (8.0 ppb, range 1.2 to 52.7; P < 0.0001; Figure 1). None exceeded the EPA threshold of 80 ppb. Measured arsenic in tap water was not significantly different between UCC (median 0.011 ppb, range 0.00–5.18) and control counties (0.090 ppb, range 0.00 to 4.02; P = 0.78). No counties exceeded the EPA threshold of 10 ppb arsenic during the observation period of 2013–2017. Similarly, measured nitrate in tap water did not differ significantly between UCC cases and controls (median 0.55 ppm for UCC counties, range 0.0 to 3.6, and median 0.80 ppm for control counties, range 0.0 to 5.9; P = 0.17). No counties exceeded the EPA threshold of 10 ppm for nitrates.
Figure 1:
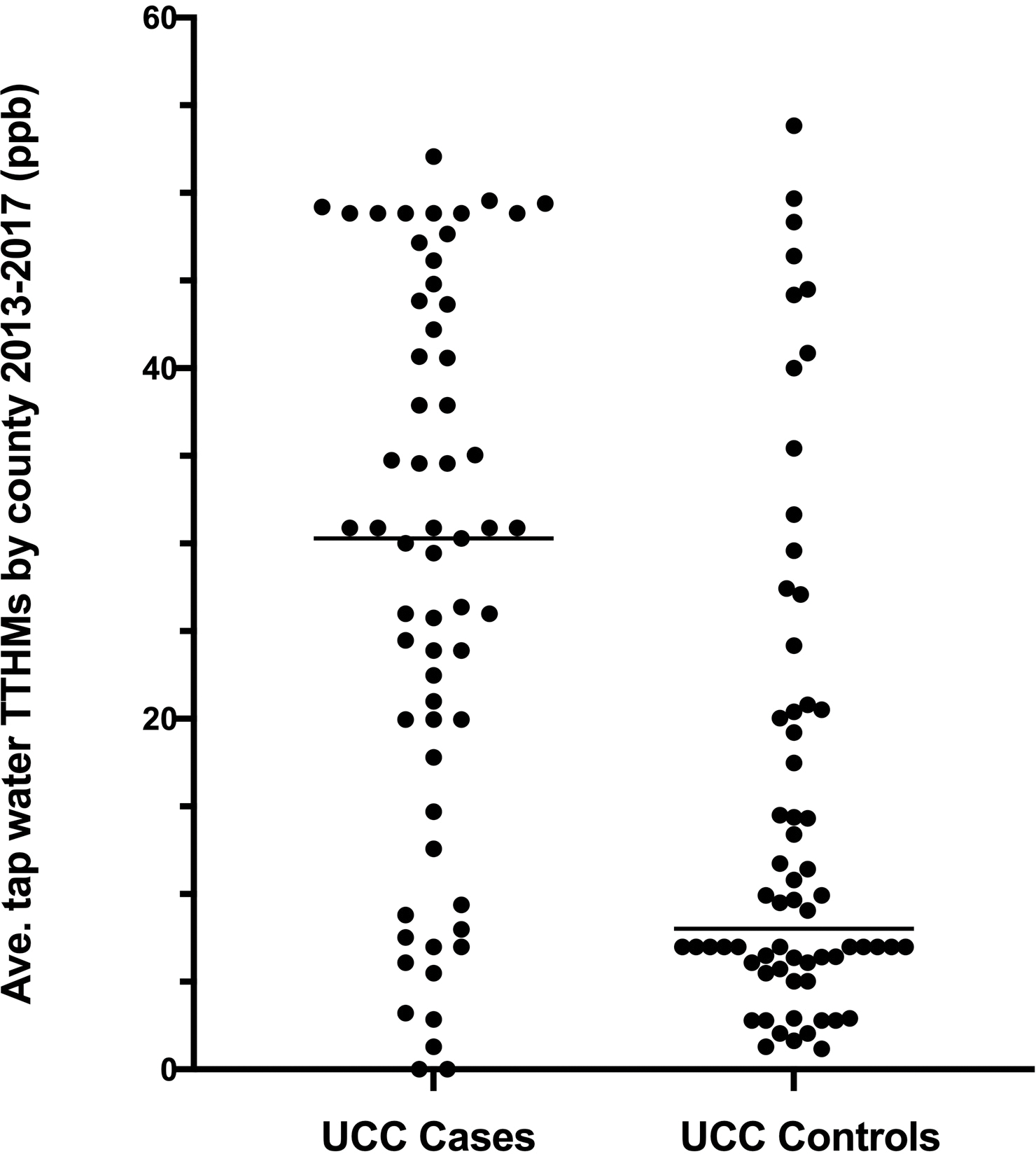
Average tap water total trihalomethane (TTHM) concentrations from public utility data (2013–2017) for counties of residence of dogs diagnosed with bladder cancer (UCC) and unaffected sex- and breed-matched older dogs. Data are shown for paired matches only. P < 0.0001 between groups.
When the subgroup of dogs who drank predominantly municipal tap water were analyzed separately, TTHMs by county of residence were still significantly higher for UCC cases (30.9 ppb, range 0.0 to 49.6; n = 26) compared to controls (7.0 ppb, range 1.6 to 46.4; n = 27; P = 0.0013). Median tap water arsenic (0.000 versus 0.178 ppb; P = 0.05; n = 17 per group) and nitrates (0.35 versus 0.85 ppm; P = 0.08; n = 25 per group) were still not significantly higher in UCC cases versus controls. We did lose sample size when matched cases and controls were not both drinking predominantly municipal water or one of the pair had missing county data.
Complete airborne ozone data from 2013–2017 were available for 70.7% of UCC case counties and 65.9% of UCC control counties (Supplemental Table S1). Averaged 5-year concentrations of airborne ozone were modestly but significantly higher in counties where dogs with UCC resided (median 69 ppb, range 58–80 ppb; n = 53) compared to counties where unaffected dogs resided (66 ppb, range 55–85 ppb; n = 54; P = 0.011; Figure 2A). A higher proportion of dogs with UCC lived in counties exceeding EPA ozone limits of 70 ppb (41.8%; 23 of 55) compared to control dogs (13.6%; 8 of 59; P = 0.0008), with an odds ratio for excessive ozone exposure of 4.58 (95% CI, 1.88–11.24) in dogs with bladder cancer.
Figure 2:
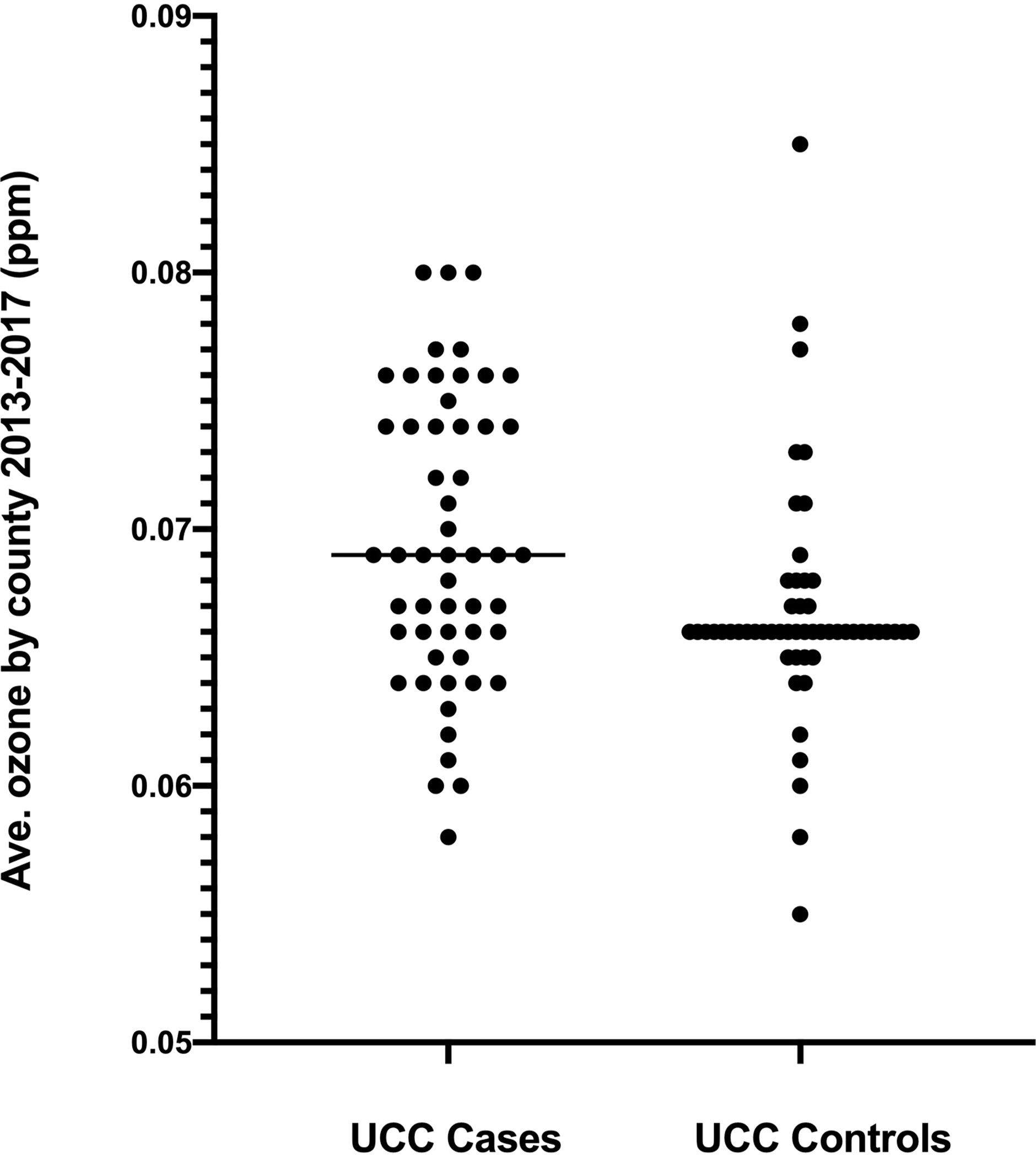
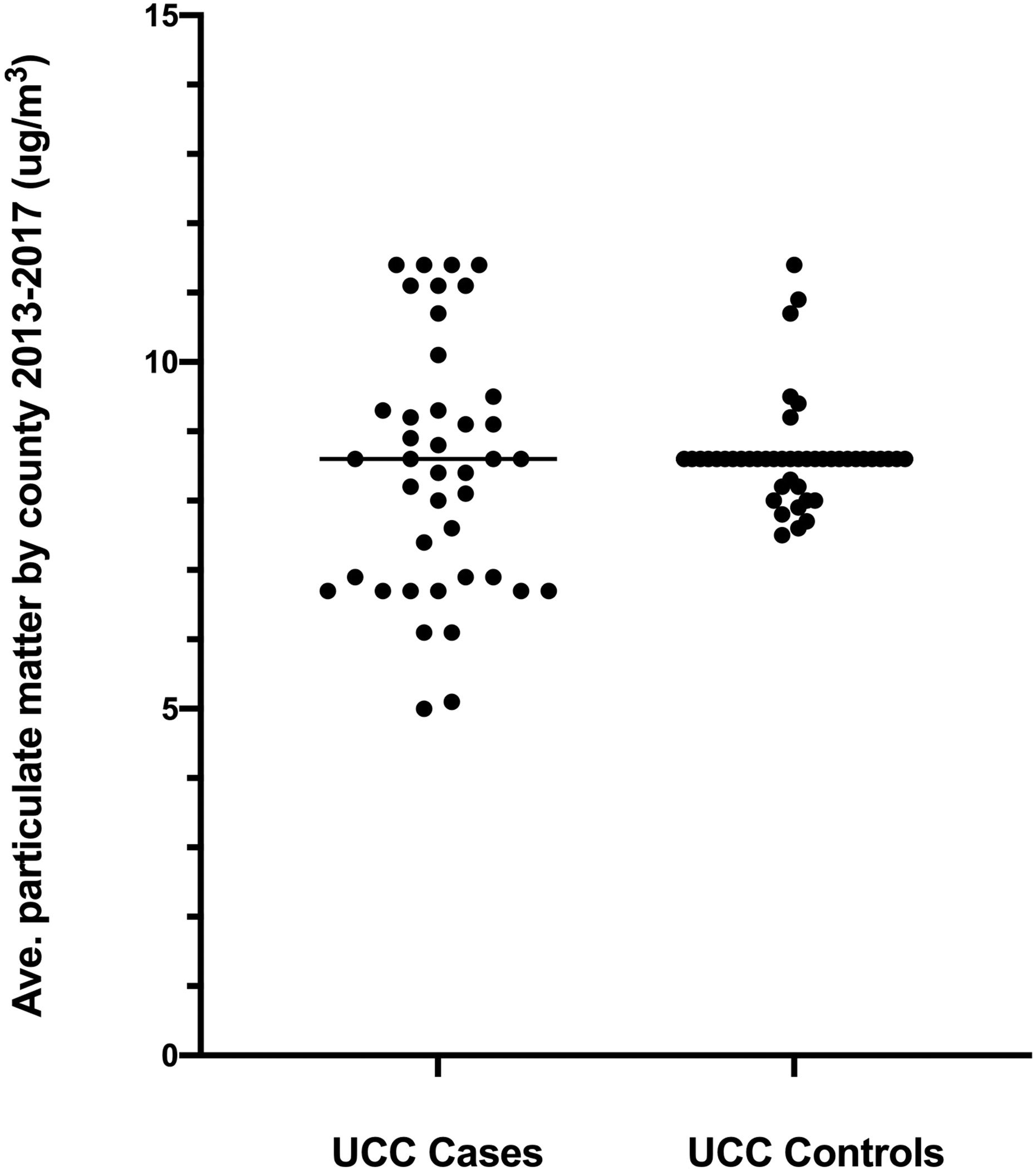
Panel A. Average ground level ozone concentrations based on EPA data from 2013–2017 for counties of residence of dogs diagnosed with bladder cancer (UCC) and unaffected sex- and breed matched older dogs. P = 0.011 between groups. Panel B. Airborne particulate matter < 2.5 microns (PM2.5) from 2013–2017 in the same two populations. P = 0.71 between groups. Data are shown for paired matches only.
Complete data for airborne particulate matter < 2.5 microns (PM2.5) from 2013–2017 were only available for 39% of UCC case counties and 31.7% of UCC control counties. Recorded values for PM2.5 did not differ in resident counties between UCC cases (median 8.6 ug/m3, range 5.0–11.4 ug/m3; n = 41) and controls (median 8.6 ug/m3, range 7.5–11.4 ug/m3; n = 45; P = 0.71; Figure 2B). PM2.5 concentrations reached or exceeded the EPA limit of 12 ug/m3 in 21.4% of UCC counties (9 of 42), but only 8.2% of control counties (4 of 49); however, this did not reach significance (P = 0.13), nor did the odds of excess PM2.5 exposure between UCC cases and controls (OR 3.07, 95% CI 0.83–9.52; P = 0.13).
When National Air Toxics Assessment data were reviewed, 6 chemicals were identified to have possible associations with UCC in the literature; one chemical, tetrachloroethylene,37 was censored due to low overall exposure risk estimates, leaving 5 airborne chemicals for individual risk assessment for UCC: acrylonitrile,38 arsenic,39 benzene,40 chromium VI,41 and cadmium.42 Chemical exposure cancer risk estimates were available for these 5 chemicals in all but one county of residence in the UCC case-control population. However, none of these 5 estimated chemical exposures were associated with UCC risk in dogs in this population (Table 2).
Table 2:
Chemical exposure risk by county of residence, modeled as lifetime attributed cancer risk per million people, from the National Air Toxics Assessment (NATA) database. Data are shown for county of residence for dogs with urothelial carcinoma (UCC) and matched unaffected control dogs enrolled from 2014–2017. NATA data were available for 2014 only. Data are presented as mean values with observed ranges. Aggregate risk was calculated as the sum of individual risk across all chemicals for which there were available data.
| Airborne chemical | Attributed human cancer risk in residence counties for dogs with UCC | Attributed human cancer risk in residence counties for control dogs | P value (adjusted threshold = 0.008) |
|---|---|---|---|
| Acrylonitrile | 0.018 (0.000–0.045) | 0.020 (0.001–0.198) | 0.05 |
| Airborne arsenic | 0.120 (0.038–0.483) | 0.115 (0.046–0.322) | 0.71 |
| Benzene | 2.86 (0.74–6.61) | 2.97 (1.94–4.78) | 0.35 |
| Chromium VI | 0.141 (0.012–0.763) | 0.168 (0.019–0.817) | 0.28 |
| Cadmium | 0.013 (0.003–0.101) | 0.013 (0.006–0.259) | 0.27 |
| Aggregate risk | 3.22 (0.90–7.08) | 3.29 (2.17–5.27) | 0.46 |
Boxer dogs with lymphoma
United States county of residence data were available for 56 of 63 boxers with lymphoma and 84 of 89 unaffected control boxers ≥ 10 years old from a previous case-control study.32 At the time of original enrollment, boxers with lymphoma resided in 41 different counties across 17 states, and unaffected control boxers resided in 78 different counties across 27 states (Table 1).
Complete data for airborne ozone were available from 2015–2019 for 80.4% of lymphoma case counties and 71.8% of lymphoma control counties (Supplemental Table S1). Average airborne ozone concentrations were significantly higher for residence counties of boxers with lymphoma (70 ppb, range 55–82 ppb; n = 48) compared to those of control boxers (66 ppb, range 55–111 ppb; n = 62; P = 0.006; Figure 3A). Twenty-five of 48 boxers with lymphoma (52.1%) lived in counties with average ozone ≥ 70 ppb, which was significantly higher than that for control boxers (18 of 62; 29.0%; P = 0.018). The odds ratio for excess ozone concentrations in counties of residence for lymphoma cases versus controls was 2.66 (95% CI, 1.21–5.65; P = 0.018).
Figure 3:
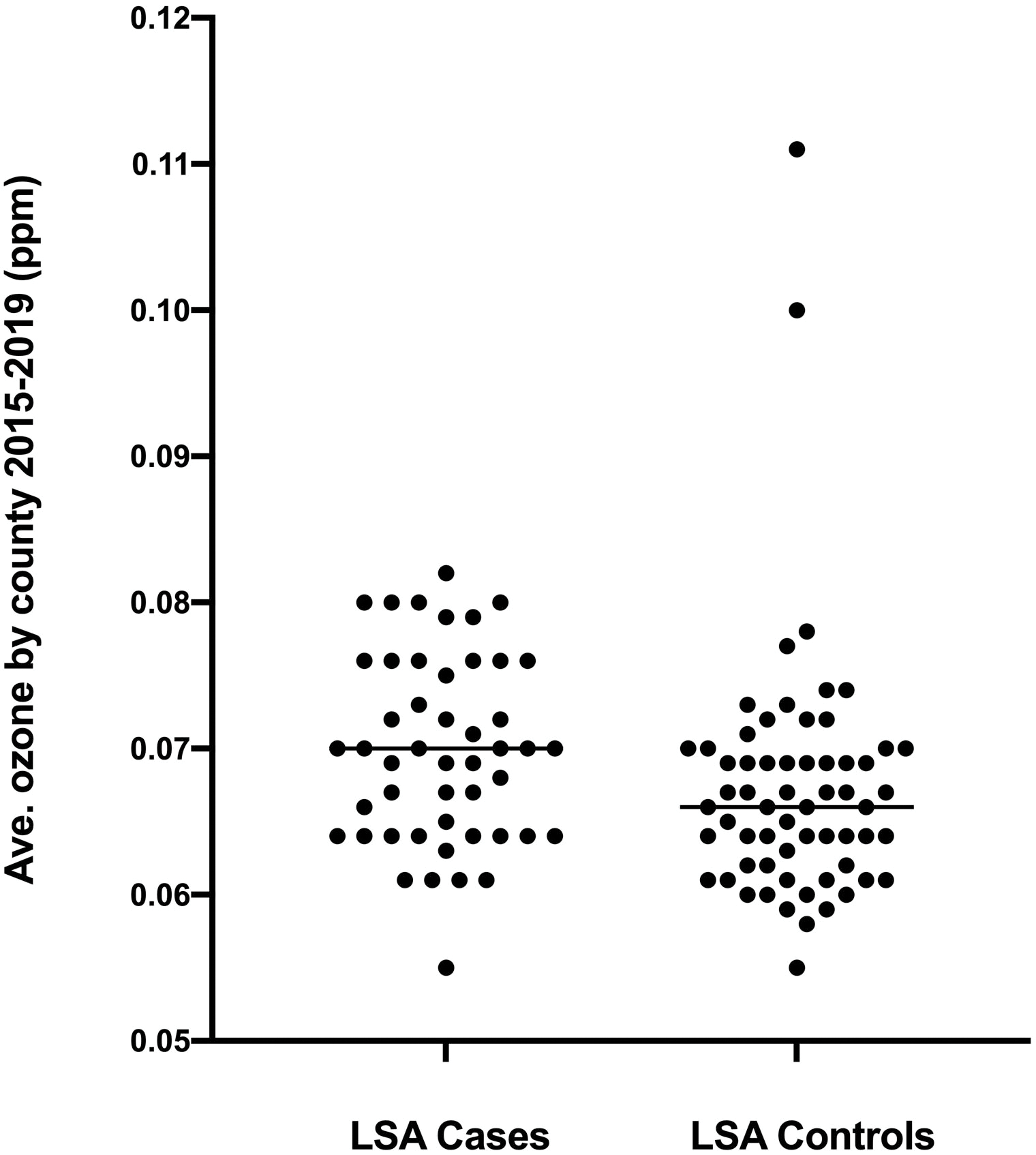
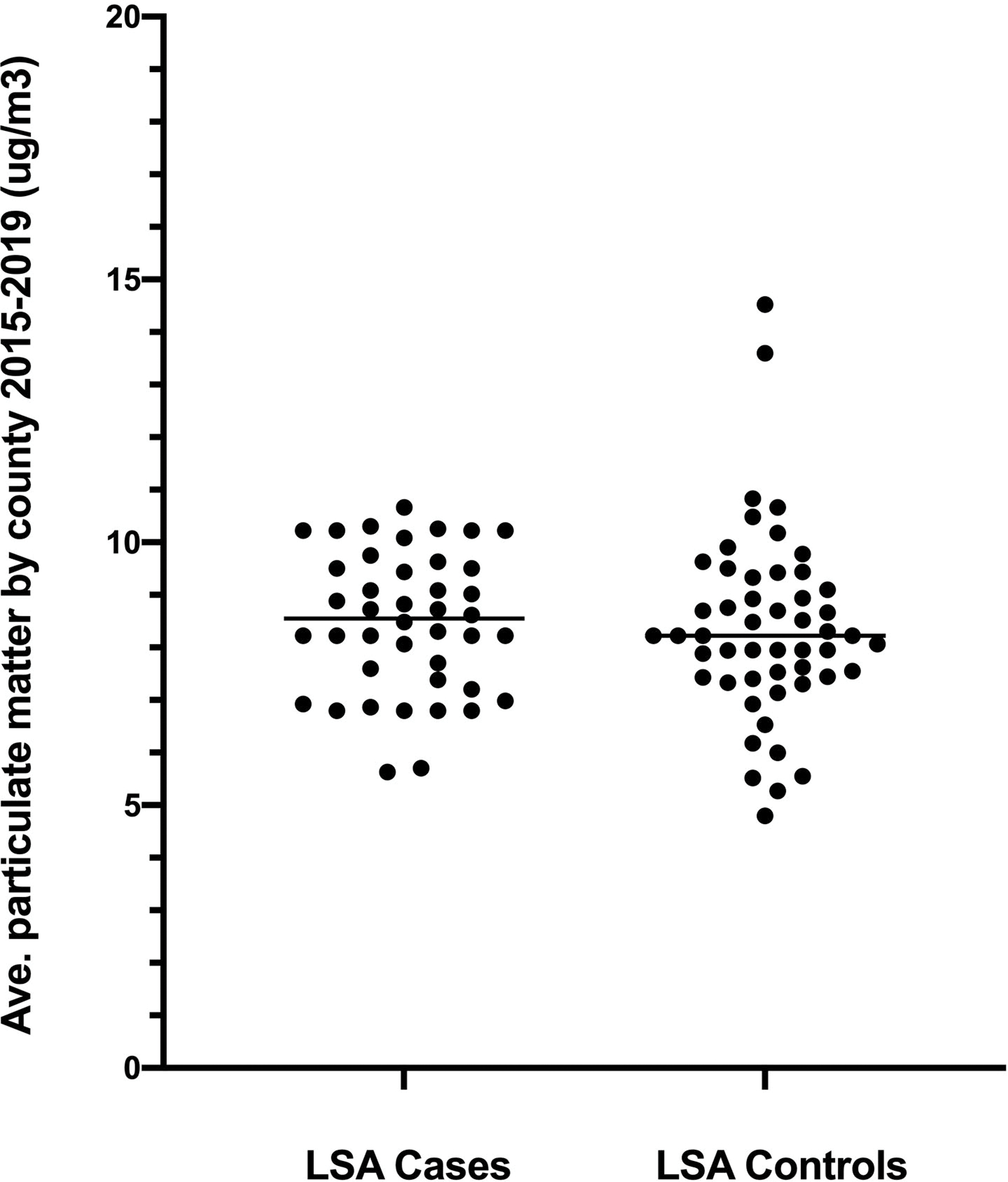
Panel A. Average ground level ozone concentrations based on EPA data from 2015–2019 for counties of residence of boxer dogs with lymphoma and unaffected sex-matched older boxers. P = 0.006 between groups. Panel B. Airborne particulate matter < 2.5 microns (PM2.5) from 2015–2019 in the same two populations. P = 0.40 between groups.
Complete data for airborne PM2.5 were only available for 65.8% of lymphoma case counties and 42.3% of lymphoma control counties from 2015–2019. From the available data, average PM2.5 concentrations within county of residence did not differ between lymphoma cases (median PM2.5 8.6 ug/m3, range 5.6–10.7 ug/m3; n = 42) and controls (median 8.2 ug/m3, range 4.8–14.5 ug/m3; n = 51; P = 0.40; Figure 3B). There was also no significant difference in the proportion of counties that ever exceeded 12 ug/m3 of particulate matter during the observation period of 2015–2019 (15.9% versus 11.8%, respectively; P = 0.57), and the odds of excess PM2.5 exposure was not different between lymphoma cases and controls (OR 1.42, 95% CI 0.47–4.87; P = 0.57).
Complete tap water arsenic data were available for 60.9% of lymphoma case counties and 50% of lymphoma control counties. From available data, boxer dogs with lymphoma did not show a difference in county tap water arsenic concentrations (median 0.000 ppb, range 0.00–2.26; n = 39) compared to control boxers (0.000 ppb, range 0.000 to 2.24, n = 54; P = 0.39), and none exceeded the EPA threshold for arsenic. When only boxers who drank predominantly municipal tap water were analyzed (44 cases and 59 controls), there was still no significant difference between groups for tap water arsenic (0.000 vs. 0.001 ppb, respectively; P = 0.09)
Tap water nitrate data were recorded for 85.3% of lymphoma case counties and 67.9% of lymphoma control counties. We did not detect a difference in tap water nitrate between lymphoma cases (median 0.28 ppm, range 0.00–5.75; n = 53) and controls (0.39 ppm, range 0.00–5.75; P = 0.14). No counties exceeded the EPA threshold for nitrates. When the subgroup of boxers who drank predominantly municipal tap water were analyzed, there was still no significant difference between cases and controls for tap water nitrates (0.0276 vs. 0.403 ppm, respectively; P = 0.24) Total trihalomethanes were not compared in the boxer population because there was no support for an association between TTHMs and lymphoma in the literature.
Of 78 chemicals in the NATA database, we identified 15 with plausible associations with human NHL or lymphoma in animal models; six of these chemicals were not included due to low cancer exposure risk estimates in the county databases (2,4-toluene diisocyanate, coke oven emissions, hexachlorobenzene, methylene chloride, tetrachloroethylene, and vinyl chloride), leaving 9 chemicals with reported associations with lymphoma for risk assessment: 1,3 butadiene,43 benzene,44 chromium VI,45 cadmium,46 carbon tetrachloride,47 ethylene oxide,48 formaldehyde,49 tetrachloroethylene,37 and polycyclic aromatic hydrocarbons50 (Table 3). Cancer risk estimates were available in the NATA database for all but one county in the lymphoma case-control population. Boxer dogs with lymphoma were more likely to live in a county with significantly higher airborne exposure risk estimates for 1,3 butadiene (about 27% higher) and formaldehyde (about 39% higher), but lower exposure to carbon tetrachloride (by a slight 1.5%), with significantly higher aggregate airborne chemical risk estimates overall (by about 28%) for dogs with lymphoma compared to controls (P = 0.0005; Table 3).
Table 3:
Chemical exposure risk by county of residence, modeled as lifetime attributed cancer risk per million people, from the National Air Toxics Assessment (NATA) database. Data are shown for county of residence for boxer dogs with lymphoma and unaffected control boxer dogs enrolled from 2016–2019. NATA data were available for 2014 only and are presented as mean values with observed ranges across each dog population. Aggregate risk was calculated as the sum of individual risk across all chemicals for which there were data.
| Airborne chemical | Attributed human cancer risk in residence counties for dogs with lymphoma | Attributed human cancer risk in residence counties for unaffected control dogs | P value (adjusted threshold 0.005) |
|---|---|---|---|
| 1,3 butadiene | 0.873 (0.213–2.86) | 0.690 (0.089–2.80) | P = 0.004 |
| Benzene | 2.88 (1.68–6.61) | 2.95 (0.65–6.61) | P = 0.19 |
| Chromium VI | 0.170 (0.045–1.40) | 0.178 (0.013–2.03) | P = 0.87 |
| Cadmium | 0.0167 (0.0046–0.2129) | 0.0193 (0.0031–0.2588) | P = 0.15 |
| Carbon tetrachloride | 3.19 (2.62–3.30) | 3.24 (2.64–3.30) | P = 0.001 |
| Ethylene oxide | 0.625 (0.009–20.1) | 0.379 (0.000–17.5) | P = 0.45 |
| Formaldehyde | 18.24 (8.28–29.24) | 13.17 (7.33–26.99) | P = 0.005 |
| Trichloroethylene | 0.0468 (0.0016–0.3664) | 0.0529 (0.0031–0.2766) | P = 0.41 |
| Polycyclic aromatic hydrocarbons | 0.104 (0.058–0.773) | 0.100 (0.041–0.472) | P = 0.24 |
| Aggregate risk | 29.32 (14.01–43.19) | 22.93 (10.90–42.22) | P = 0.0005 |
Discussion
Urothelial carcinoma and lymphoma are commonly diagnosed cancers in dogs that cause significant morbidity, do not respond fully to current treatment regimens, and typically end in death or euthanasia.1,2 Similar cancers in humans have been associated epidemiologically with environmental chemical exposures.12,17 We hypothesized that the same would be true for dogs, and we utilized publicly available water utility and EPA air pollution data by U.S. county of residence to test this hypothesis in two case-control populations of dogs.
We found that dogs with UCC were more likely to live in counties with higher measured tap water concentrations of TTHMs. Total trihalomethanes are reactive byproducts of water disinfection, especially chlorination, which are mutagenic51 and have been linked to bladder cancer in people.9,10 In contrast to our findings, a previous study in dogs did not find an association between bladder cancer and estimated trihalomethane exposure.52 This previous study also used municipal water data, but controls were not breed-matched to UCC cases. The study did consider the dog’s current and previous two residences and used averaged water utility data over the dog’s lifespan, where available, which is an advantage over our study. We only recorded current addresses and did not have information on how long the dogs had lived in their current homes. If the associations between UCC and drinking water TTHMs in previous human studies and in our study are indicative of a causal relationship, it is possible that consistent use of water filtration systems that are designed to remove TTHMs from tap water could decrease the incidence of bladder cancer attributable to exposure to these disinfection byproducts.
We also found that dogs with UCC lived in areas with significantly higher airborne levels of ozone compared to unaffected older controls, and that nearly 42% of dogs with UCC, compared to about 14% of control dogs, lived in countries with average ozone concentrations higher than EPA recommendations. In people, ambient ozone exposure is a risk factor for mortality from bladder cancer.53 Ozone is a harmful air pollutant at ground level and is a major component of smog. Ozone (O3) is generated from oxidative reactions between nitrogen and volatile organic compounds in the air.54 While ozone is not directly carcinogenic, it serves as a marker of poor air quality (epa.gov). Therefore, the association between UCC and ozone concentrations in our study may be a surrogate for exposures to volatile organic compounds or other pollutants that were not measured directly in the EPA database.
Boxer dogs with lymphoma, compared to unaffected older boxers, also came from counties with significantly higher airborne levels of ozone, with 52% of lymphoma cases living in areas that exceed EPA recommendations compared to only 29% of unaffected control boxers (P = 0.018). While ozone itself is not directly associated with NHL in humans, precursor airborne volatile organic compounds have been.17,48,54 This is consistent with our findings from the NATA database that dogs with lymphoma lived in counties with higher exposure risk estimates from the volatile organic compounds 1,3 butadiene and formaldehyde. The chemical 1,3 butadiene is found in automobile exhaust and pollution from rubber, chemical, and petroleum manufacturing. Formaldehyde is present in “off-gassing” from adhesives in wood products such as paneling and medium-density fiberboard, and in emissions from automobiles, kerosene heaters, and gas stoves. Both of these chemicals are classified as human carcinogens by the World Health Organization, and exposure to 1,3 butadiene54 and formaldehyde48 are reported risk factors for NHL in people.
We did not detect associations between average airborne concentrations of PM2.5 by county of residence and either UCC or lymphoma in dogs. These fine inhalable particles arise from vehicular emissions, smokestacks, roadway paving, construction, power plants, and wildfires, and may consist of a wide range of chemicals including sulfate, nitrate, ammonium, black carbon, volatile organics, and heavy metals.55 Higher PM2.5 concentrations were associated with both UCC and NHL in a longitudinal study of more than 300,000 people;56 however, even in this large population, these associations were no longer significant when adjusted for multiple comparisons over many tumor types. Furthermore, a Spanish case-control study on bladder cancer risk did not find an association between PM2.5 and UCC.57 In contrast, NHL has been significantly associated with higher PM2.5 concentrations, particularly black carbon and volatile organics.58,59 These subsets of PM2.5 were not available in the EPA database for our study. In addition, EPA data were relatively sparse for PM2.5 concentrations, so our negative findings may have been due in part to missing EPA data and inadequate power. For example, for proportions of dogs living in counties exceeding EPA limits for PM2.5 (UCC cases 21.4%, controls 8.2%), we would have needed 113 dogs in each group to show this difference, if real, to be significant.
We did not find associations between average tap water arsenic concentrations by county and UCC or lymphoma in dogs, either in the whole population or in the subgroups of dogs with municipal tap water as their predominant drinking source. This is in contrast to demonstrated associations between drinking water arsenic and human UCC in regions such as Taiwan, Argentina, and Chile.60 None of the U.S. counties in our study approached the high drinking water arsenic levels (50–150 ppb) reported in these non-U.S. studies. Arsenic levels, however, can exceed EPA guidelines in some well water in the U.S, which would not be captured by municipal tap water data. Between 6 and 23% of dogs in each population were drinking predominantly well water according to previous owner questionnaires, and we did not have access to chemical concentration data for individual wells for either study. Therefore, our data cannot rule out an association between arsenic exposure and either UCC or lymphoma in dogs, and direct measurements in individual dogs are indicated.
We did not find associations between tap water nitrates and either UCC or lymphoma in dogs. Nitrate can react with other chemicals in water to generate reactive N-nitroso compounds that are carcinogenic, and drinking water nitrate has been linked to bladder cancer11,61 and NHL18,20 in people. Dogs’ consumption of chemicals in tap water can also vary based on home filtration systems or provision of bottled water as an alternative drinking water source. While few owners in our previous case-control studies reported using bottled water for their dogs,24,32 most were unsure about what water filtration systems, if any, were in their homes, so we were unable to account for this variable. Follow-up studies are indicated to measure urinary nitrates in dogs with UCC and lymphoma.
Our study has important limitations. While we had home addresses for dogs at the time of diagnosis or matched enrollment, we did not have full residence histories throughout each dog’s life. Our study groups were relatively small, which did not allow adjusted analyses for potential covariates. Data were not available from some counties for certain chemicals, which may have limited power to detect differences for water arsenic and nitrates or specific NATA chemical risks. For example, for the small observed differences in NATA risk from airborne PAHs (boxer lymphoma cases, 0.1877, control boxers 0.1210) we would have needed 127 boxer dogs in each group to show this difference, if real, to be significant. In addition, our cases and controls were not matched for age, since we deliberately chose our unaffected control groups to be at or over the median age of onset of UCC 24 or T cell lymphoma. 32 This could have created some unintended structural bias for the current analyses.
Even with county level data, individual airborne exposures can vary widely based on proximity to nearby factories, wind patterns, and time spent outdoors. Indoor air pollution, such as PM2.5 from frying food, wood stoves, or fireplaces, are not captured by the EPA data. Further, data from NATA were only available for 2014, and data are reported as a modeled cancer risk based on lifetime human exposures rather than actual concentrations, so the application of these data to dogs is an extrapolation that may not be valid. For water utility and EPA data, we averaged exposures over 5 years, starting with one year prior to the first case diagnosed in each population, but it is not clear what the best time frame is for assessing these exposures relative to UCC and lymphoma risk in dogs. Finally, our cases and controls were not recruited using the exact same methodologies; most, but not all, UCC and lymphoma cases were recruited through an oncologist or primary care veterinarian, while most unaffected control dogs were recruited directly through outreach to owners. This difference could have led to structural bias in the two study populations that influenced our results. Because environment was one of the testable variables in the original studies, we wanted to avoid drawing controls from the same hospital populations as the cases. Case and control populations was drawn from 17 to 27 states, so we were able to include dogs from a wide geographic range.
Overall conclusions
These data support the hypothesis that environmental pollutants in the air and tap water may contribute to the risk of both urothelial carcinoma and lymphoma in dogs. In particular, we identified exposure to higher tap water concentrations of water disinfection byproducts (TTHMs) and higher airborne concentrations of ozone, which may act as a surrogate for volatile organic compounds (VOCs), as risk factors for bladder cancer in dogs. We found higher airborne exposures to ozone, 1,3 butadiene and formaldehyde as risk factors for lymphoma, at least in the boxer breed. Water filtration systems can be used to decrease TTHM exposure in drinking water, but avoiding airborne pollutants is more difficult. Indoor air pollution can be decreased by improving ventilation, reducing frying as a cooking method, choosing paints and construction materials that are low in VOCs, and minimizing the use of woodstoves or open fireplaces. If our findings are indicative of a causal relationship between outdoor air pollution and UCC or lymphoma in dogs, then it is possible that more effective air pollution control could decrease the overall incidence of these cancers in dogs.
Supplementary Material
Acknowledgments:
We thank Kristen Malecki, PhD, MPH, Department of Population Health Sciences, University of Wisconsin-Madison, for helpful advice on statistical analyses.
References
- 1.Burgess KE, DeRegis CJ. Urologic oncology. Vet Clin North Am Small Anim Pract. 2019;49(2):311–323. [DOI] [PubMed] [Google Scholar]
- 2.Zandvliet M Canine lymphoma: a review. Vet Q. 2016;36(2):76–104. [DOI] [PubMed] [Google Scholar]
- 3.Knapp DW, Glickman NW, Denicola DB, Bonney PL, Lin TL, Glickman LT. Naturally-occurring canine transitional cell carcinoma of the urinary bladder A relevant model of human invasive bladder cancer. Urol Oncol. 2000;5(2):47–59. [DOI] [PubMed] [Google Scholar]
- 4.Richards KL, Suter SE. Man’s best friend: what can pet dogs teach us about non-Hodgkin’s lymphoma? Immunol Rev. 2015;263(1):173–191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Koutros S, Lynch CF, Ma X, et al. Heterocyclic aromatic amine pesticide use and human cancer risk: results from the U.S. Agricultural Health Study. Int J Cancer 2009;124(5):1206–1212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Letasiova S, Medve’ova A, Sovcikova A, et al. Bladder cancer, a review of the environmental risk factors. Environ Health. 2012;11 Suppl 1:S11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Liu CC, Tsai SS, Chiu HF, Wu TN, Chen CC, Yang CY. Ambient exposure to criteria air pollutants and risk of death from bladder cancer in Taiwan. Inhal Toxicol. 2009;21(1):48–54. [DOI] [PubMed] [Google Scholar]
- 8.Smith AH, Goycolea M, Haque R, Biggs ML. Marked increase in bladder and lung cancer mortality in a region of Northern Chile due to arsenic in drinking water. Am J Epidemiol. 1998;147(7):660–669. [DOI] [PubMed] [Google Scholar]
- 9.Villanueva CM, Cantor KP, Grimalt JO, et al. Bladder cancer and exposure to water disinfection by-products through ingestion, bathing, showering, and swimming in pools. Am J Epidemiol. 2007;165(2):148–156. [DOI] [PubMed] [Google Scholar]
- 10.Bove GE, Jr., Rogerson PA, Vena JE. Case-control study of the effects of trihalomethanes on urinary bladder cancer risk. Arch Environ Occup Health. 2007;62(1):39–47. [DOI] [PubMed] [Google Scholar]
- 11.Jones RR, Weyer PJ, DellaValle CT, et al. Nitrate from drinking water and diet and bladder cancer among postmenopausal women in iowa. Environ Health Perspect. 2016;124(11):1751–1758. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Cumberbatch MGK, Jubber I, Black PC, et al. Epidemiology of bladder cancer: A systematic review and contemporary update of risk factors in 2018. Eur Urol. 2018;74(6):784–795. [DOI] [PubMed] [Google Scholar]
- 13.Fritschi L, Benke G, Hughes AM, et al. Occupational exposure to pesticides and risk of non-Hodgkin’s lymphoma. Am J Epidemiol. 2005;162(9):849–857. [DOI] [PubMed] [Google Scholar]
- 14.Seidler A, Mohner M, Berger J, et al. Solvent exposure and malignant lymphoma: a population-based case-control study in Germany. J Occup Med Toxicol. 2007;2:2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Richardson DB, Terschuren C, Hoffmann W. Occupational risk factors for non-Hodgkin’s lymphoma: a population-based case-control study in Northern Germany. Am J Ind Med. 2008;51(4):258–268. [DOI] [PubMed] [Google Scholar]
- 16.Orsi L, Delabre L, Monnereau A, et al. Occupational exposure to pesticides and lymphoid neoplasms among men: results of a French case-control study. Occupational and environmental medicine. 2009;66(5):291–298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Moubadder L, McCullough LE, Flowers CR, Koff JL. Linking Environmental Exposures to Molecular Pathogenesis in Non-Hodgkin Lymphoma Subtypes. Cancer Epidemiol Biomarkers Prev. 2020;29(10):1844–1855. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Ward MH, Mark SD, Cantor KP, Weisenburger DD, Correa-Villasenor A, Zahm SH. Drinking water nitrate and the risk of non-Hodgkin’s lymphoma. Epidemiology. 1996;7(5):465–471. [PubMed] [Google Scholar]
- 19.O’Connor SR, Farmer PB, Lauder I. Benzene and non-Hodgkin’s lymphoma. J Pathol. 1999;189(4):448–453. [DOI] [PubMed] [Google Scholar]
- 20.Gulis G, Czompolyova M, Cerhan JR. An ecologic study of nitrate in municipal drinking water and cancer incidence in Trnava District, Slovakia. Environ Res. 2002;88(3):182–187. [DOI] [PubMed] [Google Scholar]
- 21.Rhoades MG, Meza JL, Beseler CL, et al. Atrazine and nitrate in public drinking water supplies and non-hodgkin lymphoma in nebraska, USA. Environ Health Insights. 2013;7:15–27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Ortega-Garcia JA, Lopez-Hernandez FA, Carceles-Alvarez A, Fuster-Soler JL, Sotomayor DI, Ramis R. Childhood cancer in small geographical areas and proximity to air-polluting industries. Environ Res. 2017;156:63–73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Glickman LT, Schofer FS, McKee LJ, Reif JS, Goldschmidt MH. Epidemiologic study of insecticide exposures, obesity, and risk of bladder cancer in household dogs. J Toxicol Environ Health. 1989;28(4):407–414. [DOI] [PubMed] [Google Scholar]
- 24.Luethcke KR, Ekena J, Chun R, Trepanier LA. Glutathione S-transferase theta genotypes and environmental exposures in the risk of canine transitional cell carcinoma. J Vet Intern Med. 2019;33(3):1414–1422. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Glickman LT, Raghavan M, Knapp DW, Bonney PL, Dawson MH. Herbicide exposure and the risk of transitional cell carcinoma of the urinary bladder in Scottish Terriers. J Am Vet Med Assoc. 2004;224(8):1290–1297. [DOI] [PubMed] [Google Scholar]
- 26.Hayes HM, Jr., Hoover R, Tarone RE. Bladder cancer in pet dogs: a sentinel for environmental cancer? Am J Epidemiol. 1981;114(2):229–233. [DOI] [PubMed] [Google Scholar]
- 27.Hayes HM, Tarone RE, Cantor KP, Jessen CR, McCurnin DM, Richardson RC. Case-control study of canine malignant lymphoma: positive association with dog owner’s use of 2,4-dichlorophenoxyacetic acid herbicides. J Natl Cancer Inst. 1991;83(17):1226–1231. [DOI] [PubMed] [Google Scholar]
- 28.Hayes HM, Tarone RE, Cantor KP. On the association between canine malignant lymphoma and opportunity for exposure to 2,4-dichlorophenoxyacetic acid. Environ Res. 1995;70(2):119–125. [DOI] [PubMed] [Google Scholar]
- 29.Takashima-Uebelhoer BB, Barber LG, Zagarins SE, et al. Household chemical exposures and the risk of canine malignant lymphoma, a model for human non-Hodgkin’s lymphoma. Environ Res. 2012;112:171–176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Schofield I, Stevens KB, Pittaway C, et al. Geographic distribution and environmental risk factors of lymphoma in dogs under primary-care in the UK. J Small Anim Pract. 2019;60(12):746–754. [DOI] [PubMed] [Google Scholar]
- 31.Gavazza A, Presciuttini S, Barale R, Lubas G, Gugliucci B. Association between canine malignant lymphoma, living in industrial areas, and use of chemicals by dog owners. J Vet Intern Med. 2001;15(3):190–195. [PubMed] [Google Scholar]
- 32.Craun K, Ekena J, Sacco J, Jiang T, Motsinger-Reif A, Trepanier LA. Genetic and environmental risk for lymphoma in boxer dogs. J Vet Intern Med. 2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Jankowska U, Jagielski D, Czopowicz M, Sapierzynski R. The animal-dependent risk factors in canine T-cell lymphomas. Vet Comp Oncol. 2015. [DOI] [PubMed] [Google Scholar]
- 34.Knapp DW, Ramos-Vara JA, Moore GE, Dhawan D, Bonney PL, Young KE. Urinary bladder cancer in dogs, a naturally occurring model for cancer biology and drug development. ILAR J. 2014;55(1):100–118. [DOI] [PubMed] [Google Scholar]
- 35.Bennett PF, Taylor R, Williamson P. Demographic risk factors for lymphoma in Australian dogs: 6201 cases. J Vet Intern Med. 2018;32(6):2054–2060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Pittaway C, Schofield I, Dobson J, O’Neill DG, Brodbelt DC. Incidence and risk factors for the diagnosis of lymphoma in dogs in UK primary-care practice. J Small Anim Pract. 2019;60(10):581–588. [DOI] [PubMed] [Google Scholar]
- 37.Guyton KZ, Hogan KA, Scott CS, et al. Human health effects of tetrachloroethylene: key findings and scientific issues. Environ Health Perspect. 2014;122(4):325–334. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Koutros S, Lubin JH, Graubard BI, et al. Extended mortality follow-up of a cohort of 25,460 workers exposed to acrylonitrile. Am J Epidemiol. 2019;188(8):1484–1492. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Christoforidou EP, Riza E, Kales SN, et al. Bladder cancer and arsenic through drinking water: a systematic review of epidemiologic evidence. J Environ Sci Health A 2013;48(14):1764–1775. [DOI] [PubMed] [Google Scholar]
- 40.Hadkhale K, Martinsen JI, Weiderpass E, et al. Occupational exposure to solvents and bladder cancer: A population-based case control study in Nordic countries. Int J Cancer. 2017;140(8):1736–1746. [DOI] [PubMed] [Google Scholar]
- 41.Reed O, Jubber I, Griffin J, et al. Occupational bladder cancer: A cross section survey of previous employments, tasks and exposures matched to cancer phenotypes. PLoS One. 2020;15(10):e0239338. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Feki-Tounsi M, Hamza-Chaffai A. Cadmium as a possible cause of bladder cancer: a review of accumulated evidence. Environ Sci Pollut Res Int. 2014;21(18):10561–10573. [DOI] [PubMed] [Google Scholar]
- 43.Melnick RL, Kohn MC. Mechanistic data indicate that 1,3-butadiene is a human carcinogen. Carcinogenesis. 1995;16(2):157–163. [DOI] [PubMed] [Google Scholar]
- 44.Teras LR, Diver WR, Deubler EL, et al. Residential ambient benzene exposure in the United States and subsequent risk of hematologic malignancies. Int J Cancer. 2019;145(10):2647–2660. [DOI] [PubMed] [Google Scholar]
- 45.Briggs NC, Levine RS, Hall HI, Cosby O, Brann EA, Hennekens CH. Occupational risk factors for selected cancers among African American and White men in the United States. Am J Public Health. 2003;93(10):1748–1752. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Kelly RS, Lundh T, Porta M, et al. Blood erythrocyte concentrations of cadmium and lead and the risk of B-cell non-Hodgkin’s lymphoma and multiple myeloma: a nested case-control study. PLoS One. 2013;8(11):e81892. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Callahan CL, Stewart PA, Friesen MC, et al. Case-control investigation of occupational exposure to chlorinated solvents and non-Hodgkin’s lymphoma. Occup Environ Med. 2018;75(6):415–420. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Bulka C, Nastoupil LJ, Koff JL, et al. Relations between residential proximity to epa-designated toxic release sites and diffuse large b-cell lymphoma incidence. South Med J. 2016;109(10):606–614. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Lamplugh A, Harries M, Xiang F, Trinh J, Hecobian A, Montoya LD. Occupational exposure to volatile organic compounds and health risks in Colorado nail salons. Environ Pollut. 2019;249:518–526. [DOI] [PubMed] [Google Scholar]
- 50.DellaValle CT, Deziel NC, Jones RR, et al. Polycyclic aromatic hydrocarbons: determinants of residential carpet dust levels and risk of non-Hodgkin lymphoma. Cancer Causes Control. 2016;27(1):1–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Daiber EJ, DeMarini DM, Ravuri SA, et al. Progressive increase in disinfection byproducts and mutagenicity from source to tap to swimming pool and spa water: Impact of human inputs. Environ Sci Technol. 2016;50(13):6652–6662. [DOI] [PubMed] [Google Scholar]
- 52.Backer LC, Coss AM, Wolkin AF, Flanders WD, Reif JS. Evaluation of associations between lifetime exposure to drinking water disinfection by-products and bladder cancer in dogs. J Am Vet Med Assoc. 2008;232(11):1663–1668. [DOI] [PubMed] [Google Scholar]
- 53.Smith ND, Prasad SM, Patel AR, et al. Bladder cancer mortality in the United States: A geographic and temporal analysis of socioeconomic and environmental factors. J Urol. 2016;195(2):290–296. [DOI] [PubMed] [Google Scholar]
- 54.Simpson IJ, Marrero JE, Batterman S, Meinardi S, Barletta B, Blake DR. Air quality in the Industrial Heartland of Alberta, Canada and potential impacts on human health. Atmos Environ 2013;81:702–709. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Liang CS, Duan FK, He KB, Ma YL. Review on recent progress in observations, source identifications and countermeasures of PM2.5. Environ Int. 2016;86:150–170. [DOI] [PubMed] [Google Scholar]
- 56.Coleman NC, Burnett RT, Higbee JD, et al. Cancer mortality risk, fine particulate air pollution, and smoking in a large, representative cohort of US adults. Cancer Causes Control. 2020;31(8):767–776. [DOI] [PubMed] [Google Scholar]
- 57.Turner MC, Gracia-Lavedan E, Cirac M, et al. Ambient air pollution and incident bladder cancer risk: Updated analysis of the Spanish Bladder Cancer Study. Int J Cancer. 2019;145(4):894–900. [DOI] [PubMed] [Google Scholar]
- 58.Hvidtfeldt UA, Erdmann F, Urhoj SK, et al. Residential Exposure to PM2.5 Components and Risk of Childhood Non-Hodgkin Lymphoma in Denmark: A Nationwide Register-Based Case-Control Study. Int J Environ Res Public Health. 2020;17(23). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Taj T, Poulsen AH, Ketzel M, et al. Long-term exposure to PM2.5 and its constituents and risk of Non-Hodgkin lymphoma in Denmark: A population-based case-control study. Environ Res. 2020;188:109762. [DOI] [PubMed] [Google Scholar]
- 60.Saint-Jacques N, Parker L, Brown P, Dummer TJ. Arsenic in drinking water and urinary tract cancers: a systematic review of 30 years of epidemiological evidence. Environ Health. 2014;13:44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Weyer PJ, Cerhan JR, Kross BC, et al. Municipal drinking water nitrate level and cancer risk in older women: the Iowa Women’s Health Study. Epidemiology. 2001;12(3):327–338. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


