Abstract
Significance:
The skin is the crucial first-line barrier against foreign pathogens. Compromise of this barrier presents in the context of inflammatory skin conditions and in chronic wounds. Skin conditions arising from dysfunctional inflammatory pathways severely compromise the quality of life of patients and have a high economic impact on the U.S. health care system. The development of a thorough understanding of the mechanisms that can disrupt skin inflammation is imperative to successfully modulate this inflammation with therapies.
Recent Advances:
Many advances in the understanding of skin inflammation have occurred during the past decade, including the development of multiple new pharmaceuticals. Mechanical force application has been greatly advanced clinically. Bioscaffolds also promote healing, while reducing scarring.
Critical Issues:
Various skin inflammatory conditions provide a framework for analysis of our understanding of the phases of successful wound healing. The large burden of chronic wounds on our society continues to focus attention on the chronic inflammatory state induced in many of these skin conditions.
Future Directions:
Better preclinical models of disease states such as chronic wounds, coupled with enhanced diagnostic abilities of human skin, will allow a better understanding of the mechanism of action. This will lead to improved treatments with biologics and other modalities such as the strategic application of mechanical forces and scaffolds, which ultimately results in better outcomes for our patients.
Keywords: wound healing, inflammation, negative pressure wound therapy, diabetic wound, chronic wound, tissue regeneration

Dennis P. Orgill, MD

Adriana C. Panayi, MD
SCOPE
The skin serves as the crucial first-line barrier of the immune system to foreign pathogens. The different layers of the epidermis provide, in tandem, a physical barrier to water, foreign substances, and other microorganisms. Compromise of this barrier presents in the context of chronic wounds such as diabetic foot ulcers, venous stasis ulcers, and pressure ulcers. These pathologies are caused by hypoperfusion associated with diabetes, incompetent venous valves, and prolonged pressure, including sitting and bed rest.
When left untreated, these chronic wounds can develop infection and eventually lead to sepsis or amputation. Since chronic wounds often occur under chronic inflammatory conditions, the development of a thorough understanding of the conditions and drugs that alter skin inflammation is crucial.
TRANSLATIONAL RELEVANCE
As inflammation in skin conditions has become better understood, so too have the biological, pharmacological, and mechanical treatment options for chronic wounds. Mechanical force application, through vacuum assisted closure (VAC), for example, can stimulate the wound-healing process. Biodegradable scaffolds used on wounds can reduce scarring and wound contraction, while promoting re-epithelialization.1 Wound treatments such as the delivery of exosomes (exos) and other cell-derived therapies show promise and/or have been demonstrated to accelerate wound healing and help treat various skin pathologies.
One limitation of this review is that some studies cited derive their data from rodent experimentation. Although wound-healing physiology is not homologous across rodents and humans, based on extensive prior research we can support the fact that the wound-healing processes are similar enough to make general conclusions. We hope that this review will contribute to a better understanding of the inflammatory processes involved in skin pathology and wound healing to inspire the development of more effective treatments.
CLINICAL RELEVANCE
This review outlines several inflammatory skin conditions arising from diverse inflammatory pathways, including psoriasis, hidradenitis suppurativa, contact dermatitis, toxic epidermal necrolysis (TEN), rosacea, and pyoderma gangrenosum. These inflammatory skin diseases are of high prevalence, placing a total cost in the United States' health care system in the range of billions of dollars (Table 1). Discussion of these conditions provides a framework for analysis of the human response to skin injury, the pathogenic inflammatory response to wound healing, and the failure to heal chronic wounds.
Table 1.
Economic cost and prevalence of inflammatory skin conditions
| Disease | Annual U.S. Cost (2021 USD) | Prevalence, % |
|---|---|---|
| Psoriasis182,183 | $128 billion | 2 |
| Hidradenitis suppurativa184,185 | $2800 per patient | 2 |
| Contact dermatitis37,186 | $1.8 billion | 20 |
| Pyoderma gangrenosum43,187 | $9.5 million | 0.006 |
| Diabetic foot ulcer188,189 | $21 billion | 13 |
| Venous stasis ulcer188,190 | $1.7 billion | 2 |
| Pressure ulcer188,191 | $25 billion | 7 |
| TEN192 | $56000 per patient | 1 |
| Rosacea186,193 | $243 million | 5 |
TEN, toxic epidermal necrolysis.
DISCUSSION
Inflammation
Physiological inflammation
The normal inflammatory response to injury can be divided into two types: acute and chronic. Acute inflammation is an immediate response to injury characterized by innate immune mechanisms and it occurs in three stages. The first stage of acute inflammation classically results in skin redness, warmth, swelling, and pain—the Pillars rubor, calor, tumor, and dolor, as described by Celsus 2,000 years ago. This first stage of inflammation is initiated by sensory nociceptors densely distributed over the skin layers that on injury secrete neuropeptides to induce a local inflammatory response involving vasodilation and protein extravasation.2
Almost all skin cells express neuropeptide G-protein coupled receptors, which on activation trigger the secretion of further neuropeptides and neurotrophins. This exchange between skin cells and nerve cells acts as a positive bidirectional feedback loop that amplifies the inflammatory response.3–6
In the dermis, neuropeptides released from local nerve fibers induce mast cell degranulation and pro-inflammatory histamine and prostaglandin release.7,8 Histamine induces vasodilation and increased vascular permeability. This allows components of the innate immune system to reach the skin tissue and contributes to redness, warmth, and swelling.8 At this point, with help from the complement system, the regenerative stage of inflammation takes place with help from the complement system to facilitate further mast cell degranulation and neutrophil margination, adhesion, and chemotaxis.9–11
Increased levels of neuropeptides promoted the expression of intercellular adhesion molecules (ICAM) and vascular endothelial cell adhesion molecules (VCAM),12 as well as the release of vascular endothelial growth factor (VEGF) from mast cells and other immune cells.13,14 VEGF induces local angiogenesis. Neutrophils marginate near the site of inflammation and adhere to the endothelial wall via the interactions of ICAM and VCAM, before undergoing chemotaxis toward interleukin (IL)-8, C5a, and LTB4, released at the site of injury.
At the site of injury, neutrophils perform phagocytosis of microbes such as Staphylococcus aureus in the skin. Afterward, the neutrophils undergo apoptosis and the third stage of acute inflammation ensues. Macrophages then arrive at the site of injury and perform actions similar to those of neutrophils. Meanwhile, neuropeptides such as neuropeptide substance P (SP) and calcitonin gene related protein enhance the migration and antigen presentation of Langerhans cells, which promotes allergic sensitization.15–17 This favors activation of the humoral immune system and chronic inflammatory response over acute inflammation associated with the cell-mediated immune system.18
In the epidermis, neuropeptide SP released from local nerve fibers stimulates keratinocytes to secrete proinflammatory cytokines.19–22 In addition, both SP and other pro-inflammatory compounds such as interferon-gamma (IFN-γ) activate local fibroblasts to secrete SP, which further amplifies the inflammatory process in acute skin inflammation via a positive feedback loop.23,24 Overexpression of SP and IFN-γ in the dermis has been shown to contribute to fibrosis in chronic skin inflammation such as in atopic dermatitis and psoriasis.3,24
Chronic inflammation is characterized by lymphocytes in the tissue and more adaptive immune responses. Antigen-presenting cells of the skin present antigens on class II major histocompatibility complex (MHC) molecules to CD4+ helper T cells. After co-stimulation, CD4+ T helper 1 (Th1) cells further aid in chronic inflammation. Th1 cells release IFN-γ to induce B cell switching to IgG production for opsonization. Th1 cells additionally release IL-2 to activate CD8+ cytotoxic cells T. The Th2 subtype releases IL-4 and IL-5 to promote B cell switching to IgE production to promote eosinophil recruitment. These actions further help clear pathogens from the site of infection and remove infected tissue.
It is commonly accepted that inflammation is an active process mediated by proinflammatory mediators, whereas the downregulation of such mediators passively promotes tissue homeostasis and inflammation resolution. Serhan recently demonstrated that inflammation resolution can also present as an active process promoted by novel specialized pro-resolving mediators, and the disruption of this process may lead to chronic inflammation.25,26 This is a paradigm shift in inflammation, one that may offer new opportunities for the treatment of inflammatory pathologies in the future.
Pathological inflammation
Pathological inflammation arises when physiological inflammation is interrupted. When macrophages fail to remove the inflammatory substance or pathogen, granulomas may form in which macrophages quarantine the microenvironment, preventing it from exerting more severe effects on the body.27 Many factors and pathways contribute to a chronic inflammatory state in the skin that compromises this physical barrier. The following sections provide an overview of several inflammatory skin conditions ranging from one of the most common skin diseases, rosacea, to one of the most serious, TEN (Fig. 1). These conditions will serve as a framework for current and promising treatments of chronic skin conditions.
Figure 1.
Histological components of healthy skin and key inflammatory skin conditions. Healthy skin consists of a layer of epidermis covering a layer of dermis. The epidermis itself is composed of several different layers, from superficial to deep: stratum corneum, stratum lucidum, stratum granulosum, stratum spinosum, and stratum basale. The dermis is an extracellular matrix predominantly comprising collagens, containing fibroblasts and the neurovascular network. In psoriasis, the stratum corneum becomes abnormal and is characterized by parakeratosis (retention of nuclei) and hyperkeratosis (thickening), manifesting clinically as scale formation. The epidermis is thickened, the rete ridges become elongated, and the vascular network becomes dilated. A lymphocytic infiltrate can be seen within the dermis, with a predominance of T cells and Langerhans cells. Munro's microabscesses, collections of polymorphonuclear leucocytes, can be seen within the stratum corneum. Hidradenitis suppurativa is characterized initially by keratin plug formation, subsequent follicular occlusion, and dilatation, followed by accumulation of cellular debris and cyst formation. This can result in extensive immune cell infiltration, including T cells, B cells, macrophages, and neutrophils. The follicle may eventually rupture and can lead to sinus tract formation. Contact dermatitis is triggered by exposure to contact allergens called haptens that induce a T cell-mediated inflammation. Epidermal edema leads to acanthosis (thickening of the epidermis) with hyperkeratosis and parakeratosis. The inflammatory infiltrate predominately comprises Langerhans cells and extensive cytokine release. In pyoderma gangrenosum, an initial intradermal abscess of a characteristic neutrophilic nature can be followed by epidermal ulceration and superficial dermal necrosis. Surrounding the ulcer is peripheral erythema, and the ulcer site has an undermining border and tenderness. Rosacea has been associated with physical triggers such as heat and certain foods, leading to increased activity of cathelicidin in its active form LL-37. Response is mediated by TRPA1, TRPV1, and TRPV4 channels leading to changes in neurovasculature that manifest as erythema and flushing. TEN is characterized by detachment of skin and mucosa, with an implication of CD8+ T cells that produce Granulysin, resulting in keratinocyte death. TEN, toxic epidermal necrolysis.
Psoriasis
Psoriasis is characterized by excessive growth of the epidermis from aberrant keratinocyte proliferation. Histologically, psoriasis presents as acanthosis (thickening of the stratum spinosum) with parakeratosis (retained nuclei in the stratum corneum). Keratinocytes in patients with psoriasis proliferate faster with shorter differentiation times compared with healthy skin.
Disease initiation and disease progression follow closely related, but different, immune mechanisms. After injury, keratinocytes release LL-37—a cationic antimicrobial peptide—which binds to self DNA or RNA fragments found in damaged skin cells. The resulting LL-37-nucleic acid complex activates plasmacytoid dendritic cells, causing them to release type I IFN.28 These IFN go on to activate myeloid dermal dendritic cells, which migrate to draining lymph nodes to present novel antigens to naive T cells, producing T cells with autoimmune activity.29
After T cell activation, a pro-inflammatory positive feedback IL cycle is created in the psoriatic skin. Dendritic cells and IL-23 further encourage type 17 helper cells (Th17) and cytotoxic (Tc17) functions. IL-23 has been demonstrated to maintain psoriasis-like skin inflammation in murine models.30 Th17 and other specialized T cells serve as sources of IL-17A, IL-17F, and IL-22, which induce keratinocytes to recruit neutrophils, T cells, and LL-37, sustaining the pro-inflammatory loop in psoriasis.31,32 IL-22 impairs keratinocyte differentiation, leading to the keratinocyte hyperplasia observed in psoriasis patients.33
Hidradenitis suppurativa
Hidradenitis suppurativa, or acne inversa, is an inflammatory disease of apocrine gland-bearing skin situated at the axilla, inframammary folds, and groin. Although the main cause of hidradenitis suppurativa is still under investigation, several factors that are inflammatory, environmental, or genetic in nature have been implicated.
The inflammatory mechanism of hidradenitis suppurativa has been associated with increased synthesis and dysregulation of pro-inflammatory miRNA in skin leions.34 Follicle inflammation causes production of cytokeratin 16, which promotes follicular hyperkeratinization and dilatation.35 These processes activate the immune system in apocrine glands. After rupture of the follicle, molecules are exposed to a new dermal immune environment that induces further immune reactions via neutrophils, monocytes, and Th17 cytokines. This inflammation is maintained by repeated neutrophil infiltration via lipocalin-2.36
Contact dermatitis
Contact dermatitis or allergic contact dermatitis is a delayed-type hypersensitivity reaction to environmental stimuli. Common environmental stimuli that trigger contact dermatitis include nickel, fragrances, chromium, and p-phenylenediamine.37 The pathogenesis of allergic contact dermatitis begins with the innate immune system. The triggering irritant penetrates and forms a hapten with self-protein in the skin.38 Studies have indicated that the hapten triggers toll-like receptors (TLR), TLR-2 and TLR-4, by introducing them to breakdown products of hyaluronic acid found in the skin.39
In addition to the TLR-mediated mechanism of immune system activation, haptens can induce formation of the inflammasome complex, which includes NOD-like receptors. The downstream effects of this mechanism result in the release of inflammatory cytokines such as IL-1β.40 These cytokines trigger the migration of dendritic cells to the microenvironment.
The arrival of dendritic cells marks the next phase of allergic contact dermatitis characterized by an adaptive immune response. Dendritic cells along with Langerhans cells present the allergen of interest on their MHC to naive T cells at their T cell receptors in draining lymph nodes. Sensitized effector T cells then migrate to the skin on further allergen exposure with the aid of homing receptors induced by dendritic cells.41
Cytotoxic T cells induce a cytotoxic inflammatory response to the antigen, creating the response seen on patient presentation. Deficiencies in human Treg cells may further exacerbate contact dermatitis, as patients with this condition are unable to desensitize the T cell response to allergens.42 Skin inflammation in contact dermatitis utilizes both innate and adaptive immune responses that may present opportunities for target therapies.
Pyoderma gangrenosum
Pyoderma gangrenosum is an inflammatory disease of a neutrophilic cause with average onset in the mid-40s to -50s and commonly manifested in the lower legs.43 Although its etiology is still unknown, investigations have characterized pyoderma gangrenosum-affected skin as having abundant IL-8, matrix metalloproteinase (MMP)-9, MMP-10, IL-17, myeloperoxidase, and TNF-α with the absence of MMP-1 and MMP-26.44,45
Further studies have found decreased Treg/Th17 ratios in pyoderma gangrenosum-affected skin.46 In the blood of patients affected with pyoderma gangrenosum, CCR5 and CCR6 have been found to be elevated whereas CCR4 is downregulated.47 These findings suggest a dysfunction of neutrophil migration and/or function; however, the exact mechanisms of action are still unclear.
Toxic epidermal necrolysis
The previously mentioned inflammatory dermatoses explain conditions that arise from aberrant physiological processes or contact exposures to foreign substances; however, adverse skin reactions can occur from drugs that are nontopical in nature. TEN is a skin reaction that is a more severe form of Stevens-Johnson Syndrome (SJS), involving more than 30% of the skin. Clinical presentations of SJS/TEN are characterized by detachment of skin and mucosa. Recent studies of the pathogenesis of TEN have implicated CD8+ T cells in TEN.
Granulysin produced by these cells are believed to be responsible for the keratinocyte death seen in this disease.48 Although its pathogenesis remains to be explored, the triggers of TEN include medications such as lamotrigine, carbamazepine, sulfonamides, allopurinol, and nevirapine. Genetic factors further predispose to these drug-induced causes of TEN. HLA-B*1502 in those of Asian ancestry carry higher risks of experiencing SJS/TEN from carbamazepine than others.49 The genetic variation causes immune cells to react strongly against these drugs to induce TEN.
Rosacea
Other noncontact causes of skin inflammation with environmental triggers sometimes result in a less severe course of progression. Rosacea is a long-term skin condition commonly affecting the face. Signs of rosacea include flushing, erythematous papules/pustules, and telangiectasia. The condition can be triggered by temperature extremes, exercise, stress, anxiety, caffeine, and spicy foods. Although triggers have been characterized, the understanding of the pathogenesis of rosacea is currently underway with several associations made with regard to its development.
For example, Demodex mites have been found to have a higher prevalence in rosacea lesions; however, treatments aimed at eradicating colonies showed no improvement of rosacea symptoms.50 In terms of immunologic etiologies, patients with rosacea have also demonstrated increased activity of cathelicidin in its active form LL-37.51 The physical triggers such as heat and certain foods are mediated by TRPA1, TRPV1, and TRPV4 channels, leading to changes in neurovasculature that manifest as erythema and flushing seen in rosacea patients.52,53
Wounds
Acute wound healing
Wound repair is a highly dynamic process that is regulated by complex interactions of extracellular matrix (ECM) molecules, soluble mediators, various resident cells, and infiltrating leukocyte subtypes. Normal wound repair proceeds through four overlapping sequential steps, as described in Figure 2: hemostasis, inflammation, proliferation, and finally maturation/remodeling.54–57
Figure 2.
Phases of wound healing. There are four classic phases of wound healing: hemostasis, inflammation, proliferation, and resolution, with inflammation further separated into two sub-phases: early and late. During the first few hours of healing, the endothelial cells release anti-thrombotic agents to induce platelet aggregation. Factor X induces cleavage of fibrinogen to fibrin, which then cross-links and binds to the platelet aggregate to form a thrombus. The thrombus stops the bleeding and provides the preliminary matrix for healing. Histamine release from mast cells triggers a neutrophilic influx and the onset of inflammation. Through a series of immune responses, debris is removed to prevent infection. Inflammation can be further subdivided into early and late processes, distinguished primarily by their macrophage population. Early inflammation is characterized by a neutrophilic infiltrate and M1 macrophage population, and late inflammation comprising mainly M2 macrophages. During proliferation, an eschar (scab) covers the surface of the wound whereas keratinocytes migrate across the surface for wound closure. At the same time, fibroblasts promote the replacement of the initial fibrin clot with granulation tissue. This stage is also characterized by angiogenesis. The final stage is resolution, during which scar tissue is formed. This typically starts after 3 weeks and can last for longer than 2 years depending on the wound. The deposited matrix undergoes a fibroblast-based remodeling process, blood vessels regress, myofibroblasts are activated, and wound contraction ensues.
In response to an injury, the hemostasis phase of wound healing begins. This step spans seconds to hours and is initiated by endothelial ECM collagen exposure within the wound environment. Metalloproteinases in the wound site degrade endothelial collagen, which activates platelet activation and eventually forms fibrin clots.54–57 The inflammation phase of the wound-healing process can take hours to days to complete, when degraded collagen fragments and local hypoxia cause nearby immune cells such as macrophages to migrate to the wound site.54,58–60
During early inflammation, M1 macrophages activated by IFN-γ promote pro-inflammatory activity.61 During late inflammation, M2 macrophages secrete anti-inflammatory TGF-β1, IL-10, and VEGF to promote lymphatic vessel growth and skin antigen clearance.62 The proliferation step of wound healing can span days to weeks and involves the activation of profibrotic activity by fibroblasts and keratinocytes.54,63
The final step of wound healing occurs when fibroblasts remodel the ECM by realigning and depositing collagen fiber such that collagen is matured into complex structures whose amount and organization determines the tensile strength of healed skin.54–57 Collagen remodeling can occur for months after wound closure, and the tensile strength of repaired tissue can reach up to 85% of normal tissue if the wound healing process occurs without any perturbations.54
Imbalances in enzyme activity during any stage can contribute to wound chronicity. For example, higher than normal levels of macrophages that release pro-inflammatory cytokines or lower levels of reducing enzymes such as Glutathione can promote wound chronicity by either producing or inhibiting the removal of reactive oxygen species (ROS), which consequently degrade the extracellular cell matrix.55–57,62
Wound infection
Biofilm creation is one way in which pathogens impair the skin's wound healing response. S. aureus, one of the most prevalent pathogens of chronic wounds, impairs the wound-healing process by secreting a hyperglycosylated matrix that, when complexed with other bacteria, forms a biofilm that lowers the expression of type I collagen in wounds, impairing granulation tissue formation.64 In addition to impairing type I collagen formation, S. aureus upregulates the synthesis of MMP-2, creating a collagenolytic environment in the skin that prevents biomechanical wound closure.64
The secretion of a biofilm further promotes the progression of a chronic wound state. Pathogens can sometimes trigger lesions in inflammatory skin diseases listed in previous sections. Psoriasis can be exacerbated by the presence of S. aureus, Candida albicans, or Malassezia furfur.65–67 In hidradenitis suppurativa, sweat glands can become infected with bacteria. Early stages of the disease are associated with anaerobic bacteria colonization, whereas later stages are associated with facultative anaerobic and aerobic bacteria. TEN is rarely caused by Mycoplasma pneumoniae infection. Lesions in rosacea are suspected to be caused by an immune response to the bacteria Bacillus oleronius found in Demodex mites.68
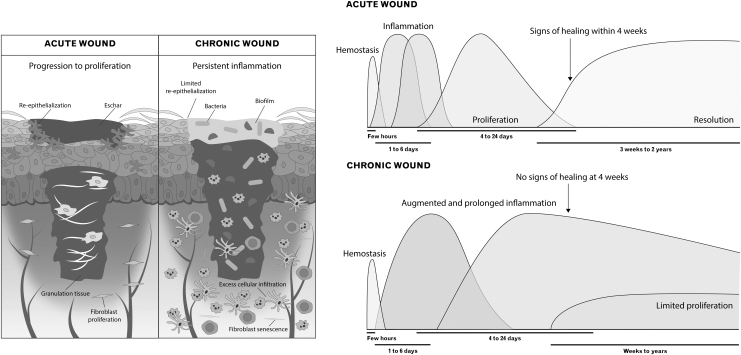
Chronic wounds
Unlike acute wounds, chronic wounds do not progress through the normal four-phase wound-healing process. Instead, they are usually characterized by a longer inflammatory stage, and consequently increased amounts of proinflammatory cytokines, ROS, and proteases (Fig. 3).69–72
Figure 3.
Chronic and acute wounds. Acute wounds typically progress through four phases of healing, with inflammation having two sub-phases, and are characterized by early re-epithelization. Signs of healing tend to occur within 4 weeks. In chronic wounds, hyperproliferation of inflammatory cells, particularly macrophages and neutrophils, results in an augmented and prolonged inflammatory phase. Further, angiogenesis is over-induced, resulting in numerous, but immature and friable microvessels. This establishes a microenvironment that is prone to bacterial infection and biofilm formation. Taken together, these factors limit and delay the proliferative phase and can lead to a lack of resolution even 2 years after wounding.
Chronic wounds have higher levels of leukocyte infiltration due to a heightened inflammatory state, which is often characterized by a variety of pathological changes such as an increased presence of macrophages and lymphocytes at the wound site.73 Higher leukocyte infiltration ultimately prolongs oxidative stress and exacerbates tissue damage by increasing local ROS production.54 Elevated ROS results in impaired wound healing by increasing cell apoptosis, senescence lipid peroxidation, protein modification, and DNA damage.73
In fact, chronic wounds have been successfully induced in diabetic mice by administering an antioxidant enzyme inhibitor in the wound environment, which blocks antioxidant enzymes from converting ROS to nontoxic molecules.54
Oxidative stress contributes to macrophage differentiation and polarization, which ultimately prolongs the inflammation stage of wound healing. Specifically, oxidative stress results in the downregulation of the genes responsible for hematopoietic stem cells differentiation to monocytes/macrophages, and it reduces macrophage infiltration, driving polarization toward M1 macrophages.73–75 Higher M1 macrophage phenotypes can lead to an overproduction of the inflammatory cytokine TNF-α, which in abnormally high levels induces chronicity by the overproduction of ROS and degradation of the ECM via the activation of metalloproteases.75–79
Overall, ROS plays a key role in chronic inflammation by promoting leukocyte adhesion and chemotaxis of proinflammatory factors into the wound, while inhibiting keratinocyte migration and re-epithelization.80,81
A prolonged inflammatory stage in chronic wounds is also characterized by a longer period of angiogenesis. During normal wound healing, newly formed blood vessels are highly disorganized and poorly perfused during the inflammation stage, but a switch from proangiogenic to antiangiogenic factors during the proliferation and maturation phase leads to angiomaturation and apoptosis of immature vessels with restoration of a vascular environment.73,82 Blood vessels in chronic wounds are prone to leakage and damage because they remain immature and poorly perfused, characterized by a lack of surrounding pericyte vascular smooth muscle cells.73,83
Further, chronic wounds experience epidermal hyperproliferation of cell types such as keratinocytes, which clinically leads to overgrowth of the wound margin and raised borders.73,84 Such pocket-like wounds are more difficult to clean and more prone to infection, contributing to wound chronicity.54,85
Higher levels of bacterial colonies and biofilm production, specifically by species such as S. aureus and coagulase negative staphylococci, also contribute to wound chronicity by exacerbating leukocyte infiltration and subsequent ROS damage.86,87 Further, although the production of type III collagen by fibroblasts is a characteristic of the late inflammation phase of normal wound healing, type III is commonly overproduced in chronic wounds.54,73
Modulation of inflammation
Wound care clinicians have succeeded in treating several conditions by modulating the inflammatory response to skin irritation and trauma. Although the cascade of initiating and mediating factors of inflammation has not been fully elucidated, it is understood that the disruption of intact skin initiates an inflammatory response that helps to regenerate or repair the disturbed tissue.88 If the stress is transient and the system in good health, growth factors and chemokines produced by macrophages in the skin stimulate granulation tissue formation and re-epithelialization and the tissue can return to homeostasis relatively quickly.
Under sustained insult, this inflammatory response can become maladaptive and contribute to pathological wound healing. However, when elements of the physiologic adaptive response are missing, as in some disease states, this process can be interrupted.88 Clinicians have attempted to influence wound healing by modulating systemic and localized inflammatory processes.
Corticosteroids
Corticoids are one of the most widely used treatments for skin inflammation. The downstream effects of corticosteroids are varied but physiologically aligned, consisting of anti-inflammatory and immunosuppressive responses. Corticoids most commonly act by binding to intracellular glucocorticoid receptors (GR) that then either bind directly to other transcription factors or to glucocorticoid response elements (GRE) located in the DNA.
For example, by binding directly to NF-κb and AP-1 and inhibiting their activity, GR prevents both factors from activating DNA transcription of pro-inflammatory cytokines. In addition, GR can bind to certain GREs that promote the expression of mitogen-activated protein kinase (MAPK) phosphatase 1, which inhibits the pro-inflammatory MAPK pathway.89 Corticoids also bind to certain GREs that suppress the expression of phospholipase A2, which decreases the synthesis of pro-inflammatory molecules such as prostaglandins and leukotrienes.90
More generally, corticoids exert effects on various cells of the immune system. Corticoids induce apoptosis in T lymphocytes, decreasing local inflammatory responses.91,92 Corticoids also affect macrophages by reducing their migratory functions and causing them to decrease secretions of collagenase, elastase, and plasminogen activators.93,94
Corticosteroids have proven useful in treating many skin conditions. In psoriasis, topical corticosteroids can lower relapse and induce remission of disease for up to 6 months of treatment.95 Anti-inflammatory properties of corticosteroids also reduce local inflammations in hidradenitis suppurativa.96 For pyoderma gangrenosum, topical corticosteroids are first-line for small lesions with slow progression whereas larger, more rapidly progressive lesions are treated with systemic corticosteroids.97 In TEN, corticosteroids are an ineffective monotherapy, but they have beneficial results in combination with intravenous immunoglobulin therapy.98
Although acute treatment with corticosteroids has pro-healing anti-inflammatory effects, chronic steroid therapy can lead to delayed wound healing and a higher incidence of local infection and dehiscence.99 In one study involving lagomorphs, vitamin A administration has been utilized to reverse some of the harmful effects by promoting epithelialization and collagen synthesis.100
Antibiotics
Chronic inflammatory environments compromise the body's natural skin barrier to commensal bacteria. Pathogenic infiltration of normal skin microbiota can precipitate in the formation of ulcers and abscesses. Thus, antibiotics are often prescribed to prevent or treat infections occurring from these inflammatory skin conditions. However, in many skin conditions, antibiotics serve the dual role of combating infection as well as decreasing inflammation. Studies have found that macrolide antibiotic treatment successfully reduced plaque psoriasis severity and area in patients.101
Proposed mechanisms of actions for the efficacy of macrolides in psoriasis include the inhibition of pro-inflammatory cytokines and compounds that promote neutrophil chemotaxis.
Antibiotics are also often used in hidradenitis suppurativa depending on the severity of disease. Early hidradenitis is treated with topical antibiotics, with clindamycin being the most effective.102 Additional antibiotics used in inflammatory skin conditions include tetracyclines, which have been suggested for treatment in hidradenitis suppurativa103 and pyoderma gangrenosum.104 Doxycycline, a type of tetracycline, additionally shows great benefits in treating rosacea when used in a modified release form in combination with metronidazole or in combination with ivermectin.105,106
Biologics
Biologics are complex molecules derived from natural sources and are a rapidly expanding class of drugs used for inflammatory skin conditions. Monoclonal antibodies have shown great efficacy in treating inflammatory dermatoses. Adalimumab, a fully human monoclonal antibody against TNF-α, has been studied for use in many skin conditions, including psoriasis,107 hidradenitis suppurativa,108 and pyoderma gangrenosum.109 Adverse effects of these anti-TNF-α biologics are similar to those of immunosuppressants since TNF-α stimulates the immune response.110
A range of FDA-approved biologics used in the treatment of inflammatory dermatoses and common adverse reactions are outlined in Table 2. Additional information on biologics currently under FDA review is included in Supplementary Table S1. Until biosimilars become more widely available, costs of these biologic therapies will limit their use in the United States.
Table 2.
Available FDA-approved biologics used in the treatment of hidradenitis suppurativa, psoriasis, pyoderma gangrenosum, toxic epidermal necrolysis, rosacea, and allergic contact dermatitis
| Disease | Target | Drug Name | ClinicalTrials.gov Identifier | Phase 4 Recruitment Status | Adverse Reactions |
|---|---|---|---|---|---|
| HS | TNF-α | Adalimumab | NCT02808975 | Complete | Infection, URI, Antibody development, Injection site reaction, Positive ANA titer, Headache, Skin rash, Increased creatine phosphokinase |
| PP | IL-17A | Secukinumab | NCT02798211 | Complete | Infection, URI |
| IL-17A | Ixekizumab | NCT03151551 | Complete | Infection, URI, Antibody development, Injection site reaction, Neutropenia | |
| IL-17 receptor A | Brodalumab | NCT03403036 | Complete | Infection, URI | |
| IL-12/IL-23 | Ustekinumab | NCT01511315 | Complete | Infection, URI, Antibody development | |
| IL-23 | Tildrakizumab | NCT04271540 | Recruiting | Infection, URI | |
| IL-23 | Risankizumab | NCT04340076 | Recruiting | Infection, URI, Antibody development | |
| IL-23 | Guselkumab | NCT03573323 | Completed | Infection, URI | |
| TNF-α | Adalimumab | NCT01265823 | Complete | Infection, URI, Antibody development, Injection site reaction, Positive ANA titer, Headache, Skin rash, Increased creatine phosphokinase | |
| TNF-α | Etanercept | NCT00967538 | Complete | Infection, URI, Antibody development, Injection site reaction, Positive ANA titer, Skin rash, Diarrhea | |
| TNF-α | Infliximab | NCT00779675 | Complete | Infection, URI, Antibody development, Positive ANA titer, double-stranded DNA antibody development, Infusion related reaction, Headache, Nausea, Abdominal pain, Abscess, Increased serum alanine aminotransferase | |
| TNF-α | Certolizumab pegol | NCT02326298 | Complete | Infection, URI, Antibody development, Nausea | |
| PG | No FDA approved biologics | ||||
| TEN | No FDA approved biologics | ||||
| RSA | No FDA approved biologics | ||||
| ACD | No FDA approved biologics | ||||
Listed for each drug is the drug's target, identifier number, recruitment status as of October 2021, and common adverse reactions with incidences indicated in the most recent FDA label.
ACD, allergic contact dermatitis; ANA, antinuclear antibody; HS, hidradenitis suppurativa; IL, interleukin; PG, pyoderma gangrenosum; PP, plaque psoriasis; RSA, rosacea; TNF-α, tumour necrosis factor alpha; URI, upper respiratory infection.
Immunosuppressants
Immunosuppressants target immune cells that are responsible for releasing host immune cell secreted biological response modifiers. These drugs serve as additional treatment where corticosteroids may be contraindicated due to toxicity. In the realm of inflammatory modulation by immunosuppressants, methotrexate stands out as one of the most prominent immunosuppressants with anti-inflammatory properties. Methotrexate is one immunosuppressant that functions as a folic acid analogue that inhibits dihydrofolate reductase, which, in turn, impairs the production of thymidine, purines, and amino acids.
This effect culminates in decreased cytokine production in immune cells.111 Specifically, methotrexate treatments promote accumulation of 5-aminoimidazole-4-carboxamide ribonucleotide (AICAR) and release of adenosine.112 Adenosine interacts with receptors inhibiting the release of inflammatory TNF-α, IL-6, and IL-8 from monocytes.112
Mechanical forces
The discovery of reduced scarring from incisions made parallel to natural bands of tension in skin (Langer lines) first brought attention to the effects of forces on wound inflammation and healing.113 Within the first 2 weeks of injury, the epidermis may close but the wound healing process underneath the surface is not complete. If the wound continues to endure mechanical stress even from natural skin tension, inflammation may persist and ultimately lead to pathologic scarring114 such as hypertrophic and keloid scars.115,116 The prevention of scar formation remains a difficult task, with clinicians currently using taping strategies, adjunctive chemotherapies ,and cryosurgery to further control the inflammatory process.117–120
Mechanical forces stimulate mechanoreceptors to induce biochemical signaling that is involved in the life cycle, proliferation, and migration cellular processes in a process known as mechanotransduction.121–124 Examples include mechano-regulated differentiation of human monocytes and their expression of proteolytic MMPs.125 One clinical implication of mechanical forces include skin stretch with tissue-expanders, which can be utilized to increase the size of donor site tissue for the reconstruction of compromised tissue elsewhere on the patient's own integument.126,127
The mechanisms that mechanical forces transduce biochemical signals have yet to be fully elucidated and findings depend on the model studied, type of mechanical stress induced, and tissue scaffolding selected.128 However, mechanical forces generally alter dermal inflammation through downstream effects on cytokines, including IL-1α, IL-1β, IL-6, and TNF-α.129,130 Skin stretch increases the quantity of local M2 macrophages, which promotes tissue proliferation and vascularization.131
However, Aragona et al. found that inflammation is not a key driver of skin-stretch induced tissue expansion. Using microarray analysis, they found that many of the genes upregulated by stretch expansion are also upregulated in wound healing, and that by knocking out the cytoskeleton remodeling genes Diaph3 and Myh9, they inhibited normal downstream effects of stretch on nuclear translocation of YAP1 and megakaryoblastic leukemia/myocardin-like 1 (MKL1), which led to barrier defects and complete inhibition of proliferation in response to stretch.132 A further study of these pathways may unlock more potential applications of skin stretch in wound care.
Negative pressure wound therapy
Negative pressure wound therapy (NPWT) is a mechanical technology used by clinicians to promote healing in slow-healing cutaneous wounds by optimizing local wound conditions.133 Despite uncertain clinical efficacy, the use of NPWT has become prominent in the treatment of acute and chronic wounds since its introduction in 1997.134 For example, NPWT has been identified as a safe and valuable component of care for hidradenitis suppurativa and pyoderma gangrenosum when added to standard of care surgical intervention and immunosuppression.135,136
Falling under the umbrella of NPWT, VAC involves filling a wound with a porous material and attaching a drainage vacuum port to induce negative pressure.133 The NPWT promotes healing through a number of mechanisms, including inflammation modulation, removal of harmful inflammatory byproducts, wound approximation, blood and lymphatic vessel growth stimulation, bacterial burden reduction, and exudate removal (Fig. 4).137–140
Figure 4.
Effects of NPWT. NPWT exerts both macrodeformational and microdeformational forces, both of which are believed to induce angiogenesis and lymphangiogenesis. The mechanisms are multifactorial, but they include increased release of growth factors, edema-induced changes in hydrostatic compression and osmotic tension, and leucocyte elimination during exudate removal.132 In a recent murine experiment from our group (unpublished data), we showed that NPWT is able to stimulate lymphangiogenesis in diabetic wounds, leading to an overall promotion of healing. NPWT, negative pressure wound therapy.
In diabetic foot ulcers treated with NPWT, the inflammatory mediators IL-1β, TNF-α, and MMP-1 are downregulated whereas VEGF, TGF-β1, and TIMP metallopeptidase inhibitor 1 are upregulated.141 In 2019, Wang et al. found that IL-6, TNF-α, and inducible nitric oxide synthase were downregulated after NPWT via the MAPK-JNK pathway.142 More recently, Song et al. showed that NPWT reduced levels of proinflammatory cytokines and suppressed autophagy and overall macrophage inflammation to promote wound healing in diabetic mice.143
In addition to the effects on inflammation, NPWT stimulates wound healing by reducing collagen turnover in the remodeling stage. Mous et al. found that the levels of MMP-9 enzyme and proenzyme, a collagenase believed to be partly responsible for poor wound closure, were significantly lower in wounds treated with NPWT.144 Although there is a lack of preclinical basic science research that could elucidate the mechanisms of NPWT, its rapid adoption and prominent usage today in multimodal treatment of chronic wounds lends to the importance of further study.
Clinically available scaffolds
Clinically available biological scaffolds (bioscaffolds) provide surgeons with tools to reconstruct a wide array of structural defects. Bioscaffolds are dynamic structures that modulate the inflammation of chronic wounds to facilitate repair or regeneration of stromal tissues. Bioscaffolds are able to accomplish this because they possess physicochemical and structural properties that allow for both their passive and active interactions with host tissue cells.
They comprise a variety of bioactive components, such as adhesion ligands, that assist in establishing topography and promote correct cell deposition and alignment, and biological cues such as growth factors, nucleic acids, and cytokines that interact with the local ECM and promote tissue proliferation.145,146
On implantation, multiple inflammation pathways are activated. Skin injury initiates an acute inflammatory response, causing secondary damage to the surrounding tissue that can hinder the integration of host cells into the scaffold.147,148 Implantation can also intensify the skin's inflammatory response by triggering a foreign body reaction. For example, naturally derived templates may contain impurities and synthetic polymers may contain pro-inflammatory byproducts, all of which act as “non-self” signals that increase local inflammation.149–152
The transition from an inflammatory M1 wound phenotype to an anti-inflammatory M2 phenotype is correlated with an increase in type 2 (Th2) cytokine secretion by CD4+ T (Th) cells, which ultimately leads to inflammation resolution.153,154 A quicker resolution of inflammation increases the chance of implant acceptance and is ideally achieved within 2 weeks.
Bioscaffolds can modulate the inflammatory response and promote regenerative healing through a multifactorial process. Prior research has shown that scaffolds made of immune-inert biomaterials or hydrophilic and neutral materials can promote healing through a decrease in dendritic cell maturation, monocyte/macrophage differentiation, leukocyte migration, and pro-inflammatory cytokine production.143,155–158 Further, implantation of acellular scaffolds without chemical crosslinking and serum-coating can reduce the inflammatory response (Fig. 5).159,160
Figure 5.
Wound-healing effects of scaffold application. The effects of scaffolds are multiple and varied but can be broadly classified under three main groups: surface adaptation, immune modulation, and structural modification. Superficially, scaffolds can help maintain a moist environment, promoting healing while inhibiting bacterial contamination and biofilm formation. At the immune level, scaffolds have been shown to be able to modify the macrophage population, to reduce cytokine production, and to increase leucocyte migration. Finally, structurally, scaffolds can promote re-epithelization, enhance granulation tissue formation, and improve angiogenesis. The three categories illustrated aim at providing an overview of the effects of scaffold application. Undoubtedly, the response to implanted material is complex and depends on multiple factors beyond the physico-chemical, biomechanical, and immunogenic properties of the scaffold.
Scaffolds with aligned fiber topography have been shown to reduce capsule formation whereas microporosity enables cell fusion and infiltration of the scaffold, promoting tissue granulation and angiogenesis, and reducing protein adsorption, monocyte adhesion, and pro-inflammatory cytokines.161–163
Examples of biological scaffolds used to treat nonhealing wounds that are readily available on the market include synthetic (NovoSorb™ Biodegradable Temporising Matrix [BTM]), semisynthetic (Integra®), and acellular (Alloderm®) matrices. The BTM consists of a synthetic polyurethane polymerized matrix that eliminates any risk of cross-species residual antigenicity, reduces the chance of infection, and shows similar wound-healing effects compared with the Integra semisynthetic scaffold.164,165
In a recently developed murine model of chronic wound healing, diabetic wounds treated with Integra Dermal Regeneration Template, a collagen-glycosaminoglycan (collagen-GAG) bioactive scaffold, exhibited a lower level of inflammation and a higher level of microvasculature maturation in comparison to untreated chronic wounds.54
Wounds displayed faster wound healing due to enhanced cellular proliferation, collagen deposition, keratinocyte migration, and microvessel maturation. The Integra collagen-GAG matrix provided a favorable environment for keratinocyte survival via a decreased chronic inflammatory state and with migration occurring at the wound border and micrograft edges toward the center of the wound.54
Acellular dermal matrices such as Alloderm act as 3D scaffolds that are infiltrated by host cells, including fibroblasts and endothelial cells, while resisting adhesion formation. They can degrade over time and are replaced by host ECM to promote healing.166,167
Clinically available cell-based therapies
Many already established cell-based bioengineered skin products exist on the U.S. market that promote wound healing via a variety of processes, one of which includes the modification of cellular and molecular inflammatory signals. Examples include epithelial autografts (Epicel® and ReCell®), cellular dermal substitutes (Dermagraft®), and bilayered skin substitutes (Apligraf®). Epicel acts as an autologous skin product that involves sheets of keratinocytes attached to a supportive petrolatum gauze backing that is removed 1 week after direct treatment of difficult-to-heal skin burns.168,169
This skin product is mainly used for patients who have extensive burns and lack enough donor skin for engraftment. ReCell is another autologous treatment commonly known as “Spray on Skin” that utilizes donor skin to produce a spray that once applied can regenerate a new outer layer of skin.170 The major benefit of ReCell is that a large wound area can be treated with a relatively small amount of donor skin. Apligraf is a bi-layered living-skin construct that involves a dermis and well-differentiated epidermis.171
This skin construct has been valuable for treating chronic wounds such as diabetic foot ulcers and has been shown to decrease wound-healing time. Dermagraft is a dermal substitute that involves cryopreserved human neonatal fibroblasts attached to a bioabsorbable polyglactin mesh matrix.172 This dermal substitute has been used to accelerate chronic wound healing, such as full-thickness foot ulcers, by promoting re-epithelialization via activation of the patient's own keratinocytes.
New advances in tissue processing have also come from placenta-derived constructs. The ECM sheets derived from human placenta are rich in growth factors and promote full-thickness wound healing by providing a microenvironment favorable to both angiogenesis and the growth and differentiation of cells.173 Further, acellular dermal matrices can also act as a scaffold to promote re-epithelialization and neoangiogenesis of the chronic wound bed. Multiple studies and reviews of chronic wounds have demonstrated improved healing times and wound area reductions.174–176
In recent years, exosomes have garnered attention and shown promise as a potential treatment option for skin conditions involving chronic inflammation. Exosomes are small vesicles containing bioactive molecules such as lipids, proteins, mRNAs, and microRNAs that fuse with target cells to affect cellular functions.177,178
For example, a recent study combined a decellularized small intestinal submucosa (SIS), mesoporous bioactive glass (MBG), and bone marrow derived mesenchymal stem cell (BMSC) exosomes to produce a hydrogel scaffold dressing (SIS/MBG@Exos), which allows for sustained release of bioactive BMSC exosomes. The scaffold promoted proliferation, migration, and angiogenesis, and it accelerated diabetic wound healing.179
Other possible exosome treatments include mesenchymal stem cell-derived exos containing pioglitazone, which activates the PI3K/AKT/eNOS pathway and decreases oxidative stress.162 Further, exosomes derived from adipose-derived stem cells containing nuclear factor-erythroid factor 2-related factor 2 (Nrf2) protein promote the Nrf2/Keap1 pathway in rodent chronic wounds, decreasing oxidative stress.180
In another study, exos carrying human beta-defensins, a group of antimicrobial peptides with pro- and anti-inflammatory functions that are usually released by resident cells of the epidermis to fight exogeneous pathogens, were used as a treatment of chronic wounds.181 Although not widely used clinically, exos demonstrate promise and deserve further attention.
SUMMARY
Skin inflammation can be characterized as a deviation from the normal physiological pathways of wound healing. It is important to recognize that any treatment used to resolve skin inflammation is an attempt to reorchestrate events at the molecular level, so it is essential to take into account the physiological underpinnings of all relevant processes. In comparison to acute wound healing, which progresses through a series of well-characterized phases, chronic wounds can stall at any phase of the wound-healing process and commonly exhibit a longer and altered inflammatory phase, leading to a variety of high-cost skin conditions such as psoriasis, hidradenitis, contact dermatitis, rosacea, TEN, and pyoderma gangrenosum.
Clinical modulation of chronic inflammatory states may involve therapeutics such as the administration of corticoids, biologics, antibiotics, mechanical forces, bioscaffolds, skin-based products, and exosomes. The goal of such treatments is to provide inflammation resolution, which can result in decreased scarring and increased scaffold engrafting.
TAKE HOME MESSAGES
The normal physiology of wound healing involves a concerted inflammatory response by different types of cells involved in cell-mediated immunity.
The altered healing of chronic wounds can be characterized by dysfunction in regulating the inflammatory phase of wound healing.
Inflammatory skin conditions are of significant prevalence and high cost to the U.S. health care system.
By modulating the cellular pathways underpinning chronic inflammation, quicker resolution and wound healing can be achieved.
Targeting mechanotransduction pathways associated with inflammation such as the administration of VAC, NPWT, and skin stretch can lead to anti-inflammatory effects.
Application of bioscaffolds and anti-inflammatory agents such as skin-cell products and bioactive compounds are established treatments for chronic wounds.
Supplementary Material
Abbreviations and Acronyms
- ACD
allergic contact dermatitis
- AICAR
5-aminoimidazole-4-carboxamide ribonucleotide
- ANA
antinuclear antibody
- AP-1
activator protein 1
- BMSC
bone marrow derived mesenchymal stem cell
- BTM
Biodegradable Temporising Matrix
- C3, C4, C5
complement protein
- CD4+, CD8+
cluster of differentiation
- ECM
extracellular matrix
- exos
exosomes
- GAG
glycosaminoglycan
- GR
glucocorticoid receptors
- GRE
glucocorticoid response element
- HS
hidradenitis suppurativa
- ICAM
intercellular adhesion molecules
- IFN-γ
interferon-gamma
- IL
interleukin
- JNK
c-Jun NH(2)-terminal kinases
- LL-37
cathelicidin antimicrobial peptide (active form)
- LTB4
leukotriene B4
- MAPK
mitogen-activated protein kinase
- MBG
mesoporous bioactive glass
- MHC
major histocompatibility complex
- MMP
matrix metalloproteinase
- NF-κb
nuclear factor kappa-light-chain-enhancer of activated B cells
- NPWT
negative pressure wound therapy
- Nrf2
nuclear factor-erythroid factor 2-related factor 2
- PG
pyoderma gangrenosum
- PP
plaque psoriasis
- ROS
reactive oxygen species
- RSA
rosacea
- SIS
small intestinal submucosa
- SJS
Stevens-Johnson Syndrome
- SP
substance P
- TEN
toxic epidermal necrolysis
- TGF-β1
transforming growth factor beta 1
- Th1
type 1 helper T cell
- Th2
type 2 helper T cell
- TLR
toll-like receptors
- TNF-α
tumour necrosis factor alpha
- URI
upper respiratory infection
- VAC
vacuum assisted closure
- VCAM
vascular endothelial cell adhesion molecules
- VEGF
vascular endothelial growth factor
AUTHORs' CONTRIBUTIONS
D.P.O. and A.C.P. planned, conceptualized, and orchestrated the writing; D.Y.M., B.N., and O.D. wrote the article; D.P.O. and A.C.P. verified the accuracy of key statements; A.C.P. and D.Y.M. made the figures and video; M.W., D.P.O., and A.C.P. critically edited the article; and the article was reviewed by all the authors.
ACKNOWLEDGMENTS AND FUNDING SOURCES
None declared. This work was funded by The Gillian Reny Stepping Strong Center for Trauma Innovation and is in part supported by NIH T35 HL110843 fellowship to Brian Ng.
AUTHOR DISCLOSURE AND GHOSTWRITING
The authors declare no competing financial interests.
ABOUT THE AUTHORS
Dany Y. Matar is a researcher at Brigham and Women's Hospital and an undergraduate student at Washington University in St. Louis studying Biochemistry and Molecular Biology.
Brian Ng, BA, is a medical student at Saint Louis University School of Medicine. He is a researcher at Brigham and Women's Hospital and a 2021 Summer Research Fellow in the Harvard-Longwood Short-Term Research Training in Vascular Surgery (NIH-T35) Program.
Oliver Darwish, BA, is a medical student at California Northstate University College of Medicine. He is a research collaborator at Brigham and Women's Hospital and a previous 2020 Summer Research Fellow in the Harvard-Longwood Short-Term Research Training in Vascular Surgery (NIH-T35) program.
Mengfan Wu, MD, PhD, is a postdoctoral research fellow in the Division of Plastic Surgery, Brigham and Women's Hospital, Harvard Medical School and a plastic surgeon in the Department of Plastic Surgery, Peking University, P.R. China.
Dennis P. Orgill, MD, PhD, directs the Wound Healing and Tissue Engineering Laboratory at Brigham and Women's Hospital, where he is a Plastic Surgeon. He is Professor of Surgery at Harvard Medical School.
Adriana C. Panayi, MD, is a Principal Investigator at Brigham and Women's Hospital and an instructor at Harvard Medical School. Dr. Panayi's research areas are wound healing, with a focus on chronic wounds.
SUPPLEMENTARY MATERIAL
REFERENCES
- 1. Soller EC, Tzeranis DS, Miu K, So PTC, Yannas IV. Common features of optimal collagen scaffolds that disrupt wound contraction and enhance regeneration both in peripheral nerves and in skin. Biomaterials 2012;33:4783–4791. [DOI] [PubMed] [Google Scholar]
- 2. Jans R, Sartor M, Jadot M, Poumay Y. Calcium entry into keratinocytes induces exocytosis of lysosomes. Arch Dermatol Res 2004;296:30–41. [DOI] [PubMed] [Google Scholar]
- 3. Liezmann C, Klapp B, Peters EM. Stress, atopy and allergy. Dermatoendocrinol 2011;3:37–40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Botchkarev VA, Yaar M, Peters EM, et al. Neurotrophins in skin biology and pathology. J Invest Dermatol 2006;126:1719–1727. [DOI] [PubMed] [Google Scholar]
- 5. Roosterman D, Goerge T, Schneider SW, Bunnett NW, Steinhoff M. Neuronal control of skin function: the skin as a neuroimmunoendocrine organ. Physiol Rev 2006;86:1309–1379. [DOI] [PubMed] [Google Scholar]
- 6. Cevikbas F, Steinhoff A, Homey B, Steinhoff M. Neuroimmune interactions in allergic skin diseases. Curr Opin Allergy Clin Immunol 2007;7:365–373. [DOI] [PubMed] [Google Scholar]
- 7. Wallengren J. Vasoactive peptides in the skin. J Investig Dermatol Symp Proc 1997;2:49–55. [DOI] [PubMed] [Google Scholar]
- 8. Yoshikai Y. Roles of prostaglandins and leukotrienes in acute inflammation caused by bacterial infection. Curr Opin Infect Dis 2001;14:257–263. [DOI] [PubMed] [Google Scholar]
- 9. Walport MJ. Complement. First of two parts. N Engl J Med 2001;344:1058–1066. [DOI] [PubMed] [Google Scholar]
- 10. El-Lati SG, Dahinden CA, Church MK. Complement peptides C3a- and C5a-induced mediator release from dissociated human skin mast cells. J Invest Dermatol 1994;102:803–806. [DOI] [PubMed] [Google Scholar]
- 11. Goldman AS, Prabhakar BS. Immunology Overview. Medical Microbiology Galveston: University of Texas Medical Branch at Galveston, 1996. [PubMed] [Google Scholar]
- 12. Lindsey KQ, Caughman SW, Olerud JE, Bunnett NW, Armstrong CA, Ansel JC. Neural regulation of endothelial cell-mediated inflammation. J Investig Dermatology Symp Proc 2000;5:74–78. [DOI] [PubMed] [Google Scholar]
- 13. Brain SD, Williams TJ. Interactions between the tachykinins and calcitonin gene-related peptide lead to the modulation of oedema formation and blood flow in rat skin. Br J Pharmacol 1989;97:77–82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Saria A. Substance P in sensory nerve fibres contributes to the development of oedema in the rat hind paw after thermal injury. Br J Pharmacol 1984;82:217–222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Nakano Y. Stress-induced modulation of skin immune function: two types of antigen-presenting cells in the epidermis are differentially regulated by chronic stress. Br J Dermatol 2004;151:50–64. [DOI] [PubMed] [Google Scholar]
- 16. Beresford L, Orange O, Bell EB, Miyan JA. Nerve fibres are required to evoke a contact sensitivity response in mice. Immunology 2004;111:118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Joachim RA, Handjiski B, Blois SM, Hagen E, Paus R, Arck PC. Stress-induced neurogenic inflammation in murine skin skews dendritic cells towards maturation and migration. Am J Pathol 2008;173:1379–1388. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Ding W, Stohl L, Wagner JA, Granstein RD. Calcitonin gene-related peptide biases Langerhans cells towards Th2-type immunity. J Immunol 2008;181:6020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Park YM, Kim CW. The effects of substance P and vasoactive intestinal peptide on interleukin-6 synthesis in cultured human keratinocytes. J Dermatol Sci 1999;22:17–23. [DOI] [PubMed] [Google Scholar]
- 20. Song I-S, Bunnett NW, Olerud JE, et al. Substance P induction of murine keratinocyte PAM 212 interleukin 1 production is mediated by the neurokinin 2 receptor (NK-2R). Exp Dermatol 2000;9:42–52. [DOI] [PubMed] [Google Scholar]
- 21. Burbach GJ, Kim KH, Zivony AS, et al. The neurosensory tachykinins substance P and neurokinin A directly induce keratinocyte nerve growth factor. J Invest Dermatol 2001;117:1075–1082. [DOI] [PubMed] [Google Scholar]
- 22. Dallos A, Kiss M, Polyánka H, Dobozy A, Kemény L, Husz S. Effects of the neuropeptides substance P, calcitonin gene-related peptide, vasoactive intestinal polypeptide and galanin on the production of nerve growth factor and inflammatory cytokines in cultured human keratinocytes. Neuropeptides 2006;40:251–263. [DOI] [PubMed] [Google Scholar]
- 23. Liu J-Y, Hu J-H, Zhu Q-G, Li F-Q, Sun H-J. Substance P receptor expression in human skin keratinocytes and fibroblasts. Br J Dermatol 2006;155:657–662. [DOI] [PubMed] [Google Scholar]
- 24. Bae S-J, Matsunaga Y, Takenaka M, et al. Substance P induced preprotachykinin-A mRNA, neutral endopeptidase mRNA and substance P in cultured normal fibroblasts. Int Arch Allergy Immunol 2002;127:316–321. [DOI] [PubMed] [Google Scholar]
- 25. Serhan CN. Novel pro-resolving lipid mediators in inflammation are leads for resolution physiology. Nature 2014;510:92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Serhan CN, Savill J. Resolution of inflammation: the beginning programs the end. Nat Immunol 2005;6:1191–1197. [DOI] [PubMed] [Google Scholar]
- 27. Kumar V, Abul A, Aster J. Robbins & Cotran Pathologic Basis of Disease, 10th ed. Amsterdam, Netherlands: Elsevier-Health Sciences Division, 2020. [Google Scholar]
- 28. Lande R, Gregorio J, Facchinetti V, et al. Plasmacytoid dendritic cells sense self-DNA coupled with antimicrobial peptide. Nature 2007;449:564–569. [DOI] [PubMed] [Google Scholar]
- 29. Hänsel A, Günther C, Ingwersen J, et al. Human slan (6-sulfo LacNAc) dendritic cells are inflammatory dermal dendritic cells in psoriasis and drive strong Th17/Th1 T-cell responses. J Allergy Clin. Immunol 2011;127:787–794.e9. [DOI] [PubMed] [Google Scholar]
- 30. van der Fits L, Mourits S, Voerman JS, et al. Imiquimod-induced psoriasis-like skin inflammation in mice is mediated via the IL-23/IL-17 axis. J Immunol 2009;182:5836–5845. [DOI] [PubMed] [Google Scholar]
- 31. Pantelyushin S, Haak S, Ingold B, et al. Rorγt + innate lymphocytes and γδ T cells initiate psoriasiform plaque formation in mice. J Clin Invest 2012;122:2252–2256. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Jovanovic DV, Di Battista JA, Martel-Pelletier J, et al. IL-17 stimulates the production and expression of proinflammatory cytokines, IL-beta and TNF-alpha, by human macrophages. J Immunol 1998;160:3513–3521. [PubMed] [Google Scholar]
- 33. Wolk K, Witte E, Warszawska K, et al. The Th17 cytokine IL-22 induces IL-20 production in keratinocytes: a novel immunological cascade with potential relevance in psoriasis. Eur J Immunol 2009;39:3570–3581. [DOI] [PubMed] [Google Scholar]
- 34. Hessam S, Sand M, Skrygan M, Gambichler T, Bechara FG. Inflammation induced changes in the expression levels of components of the microRNA maturation machinery Drosha, Dicer, Drosha co-factor DGRC8 and Exportin-5 in inflammatory lesions of hidradenitis suppurativa patients. J Dermatol Sci 2016;82:166–174. [DOI] [PubMed] [Google Scholar]
- 35. Janse IC, Blok JL, Diercks GFH, Horváth B, Jonkman MF. Hidradenitis suppurativa: a disease of infundibular epidermis rather than pilosebaceous units? Br J Dermatol 2017;176:1659–1661. [DOI] [PubMed] [Google Scholar]
- 36. Wolk K, Wenzel J, Tsaousi A, et al. Lipocalin-2 is expressed by activated granulocytes and keratinocytes in affected skin and reflects disease activity in acne inversa/hidradenitis suppurativa. Br J Dermatol 2017;177:1385–1393. [DOI] [PubMed] [Google Scholar]
- 37. Thyssen JP, Linneberg A, Menné T, Johansen JD. The epidemiology of contact allergy in the general population—prevalence and main findings. Contact Dermatitis 2007;57:287–299. [DOI] [PubMed] [Google Scholar]
- 38. Aiba S, Terunuma A, Manome H, Tagami H. Dendritic cells differently respond to haptens and irritants by their production of cytokines and expression of co-stimulatory molecules. Eur J Immunol 1997;27:3031–3038. [DOI] [PubMed] [Google Scholar]
- 39. Jiang D, Liang J, Fan J, et al. Regulation of lung injury and repair by Toll-like receptors and hyaluronan. Nat Med 2005;11:1173–1179. [DOI] [PubMed] [Google Scholar]
- 40. Watanabe H, Gaide O, Pétrilli V, et al. Activation of the IL-1beta-processing inflammasome is involved in contact hypersensitivity. J Invest Dermatol 2007;127:1956–1963. [DOI] [PubMed] [Google Scholar]
- 41. Edele F, Molenaar R, Gütle D, et al. Cutting edge: instructive role of peripheral tissue cells in the imprinting of T cell homing receptor patterns. J Immunol 2008;181:3745–3749. [DOI] [PubMed] [Google Scholar]
- 42. Dubois B, Chapat L, Goubier A, Papiernik M, Nicolas JF, Kaiserlian D. Innate CD4+CD25+ regulatory T cells are required for oral tolerance and inhibition of CD8+ T cells mediating skin inflammation. Blood 2003;102:3295–3301. [DOI] [PubMed] [Google Scholar]
- 43. Xu A, Balgobind A, Strunk A, Garg A, Alloo A. Prevalence estimates for pyoderma gangrenosum in the United States: an age- and sex-adjusted population analysis. J Am Acad Dermatol 2020;83:425–429. [DOI] [PubMed] [Google Scholar]
- 44. Oka M, Berking C, Nesbit M, et al. Interleukin-8 overexpression is present in pyoderma gangrenosum ulcers and leads to ulcer formation in human skin xenografts. Lab Investig 2011;80:595–604. [DOI] [PubMed] [Google Scholar]
- 45. Bister V, Mäkitalo L, Jeskanen L, Saarialho-Kere U. Expression of MMP-9, MMP-10 and TNF-alpha and lack of epithelial MMP-1 and MMP-26 characterize pyoderma gangrenosum. J Cutan Pathol 2000;34:889–898. [DOI] [PubMed] [Google Scholar]
- 46. Caproni M, Antiga E, Volpi W, et al. The Treg/Th17 cell ratio is reduced in the skin lesions of patients with pyoderma gangrenosum. Br J Dermatol 2015;173:275–278. [DOI] [PubMed] [Google Scholar]
- 47. Quaglino P, Fava P, Caproni M, et al. Phenotypical characterization of circulating cell subsets in pyoderma gangrenosum patients: the experience of the Italian immuno-pathology group. J Eur Acad Dermatology Venereol 2007;30:655–658. [DOI] [PubMed] [Google Scholar]
- 48. Chung WH, Hung SI, Yang JY, et al. Granulysin is a key mediator for disseminated keratinocyte death in Stevens-Johnson syndrome and toxic epidermal necrolysis. Nat Med 2008;14:1343–1350. [DOI] [PubMed] [Google Scholar]
- 49. Chung WH, Hung SI, Hong HS, et al. Medical genetics: a marker for Stevens-Johnson syndrome. Nature 2008;428:486. [DOI] [PubMed] [Google Scholar]
- 50. Murillo N, Aubert J, Raoult D. Microbiota of Demodex mites from rosacea patients and controls. Microb Pathog 2004;71–72:37–40. [DOI] [PubMed] [Google Scholar]
- 51. Kim JY:Kim YJ, Lim BJ, Sohn HJ, Shin D, Oh SH. Increased expression of cathelicidin by direct activation of protease-activated receptor 2: possible implications on the pathogenesis of rosacea. Yonsei Med J 2014;55:1648–1655. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Sulk M, Seeliger S, Aubert J, et al. Distribution and expression of non-neuronal transient receptor potential (TRPV) ion channels in rosacea. J Invest Dermatol 2012;132:1253–1262. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. Steinhoff M, Buddenkotte J, Aubert J, et al. Clinical, cellular, and molecular aspects in the pathophysiology of rosacea. J Investig Dermatol Symp Proc 2011;15:2–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54. Panayi AC, Endo Y, Karvar M, et al. Low mortality oxidative stress murine chronic wound model. BMJ Open Diabetes Res Care 2020;8:e001221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55. Eming SA, Martin P, Tomic-Canic M. Wound repair and regeneration: mechanisms, signaling, and translation. Sci Transl Med 2014;6:265sr6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56. Sun BK, Siprashvili Z, Khavari PA. Advances in skin grafting and treatment of cutaneous wounds. Science 2014;346:941–945. [DOI] [PubMed] [Google Scholar]
- 57. Gurtner GC, Werner S, Barrandon Y, Longaker MT. Wound repair and regeneration. Nature 2008;453:314–321. [DOI] [PubMed] [Google Scholar]
- 58. Tandara AA, Mustoe TA. Oxygen in wound healing–more than a nutrient. World J Surg 2004;28:294–300. [DOI] [PubMed] [Google Scholar]
- 59. Turabelidze A, Dipietro LA. Inflammation and wound healing. Endod Top 2012;24:26–38. [Google Scholar]
- 60. Koh TJ, DiPietro LA. Inflammation and wound healing: the role of the macrophage. Expert Rev Mol Med 2011;13:e23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61. Ley K. M1 means kill; M2 means heal. J Immunol 2017;199:2191–2193. [DOI] [PubMed] [Google Scholar]
- 62. Kataru RP, Jung K, Jang C, et al. Critical role of CD11b+ macrophages and VEGF in inflammatory lymphangiogenesis, antigen clearance, and inflammation resolution. Blood 2009;113:5650–5659. [DOI] [PubMed] [Google Scholar]
- 63. Caley MP, Martins VL, O'Toole EA. Metalloproteinases and wound healing. Adv Wound Care (New Rochelle) 2015;4:225–234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64. Roy S, Santra S, Das A, et al. Staphylococcus aureus biofilm infection compromises wound healing by causing deficiencies in granulation tissue collagen. Ann Surg 2020;271:1174–1185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65. Leung DYM, Travers JB, Giorno R, et al. Evidence for a streptococcal superantigen-driven process in acute guttate psoriasis. J Clin Invest 1995;96:2106–2112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66. Waldman A, Gilhar A, Duek L, Berdicevsky I. Incidence of Candida in psoriasis—a study on the fungal flora of psoriatic patients. Mycoses 2001;44:77–81. [DOI] [PubMed] [Google Scholar]
- 67. Baroni A, Paoletti I, Ruocco E, Agozzino M, Tufano MA, Donnarumma G. Possible role of Malassezia furfur in psoriasis: modulation of TGF-beta1, integrin, and HSP70 expression in human keratinocytes and in the skin of psoriasis-affected patients. J Cutan Pathol 2004;31:35–42. [DOI] [PubMed] [Google Scholar]
- 68. Lacey N, Delaney S, Kavanagh K, Powell FC. Mite-related bacterial antigens stimulate inflammatory cells in rosacea. Br J Dermatol 2007;157:474–481. [DOI] [PubMed] [Google Scholar]
- 69. Loots MAM, Kenter SB, Au FL, et al. Fibroblasts derived from chronic diabetic ulcers differ in their response to stimulation with EGF, IGF-I, bFGF and PDGF-AB compared to controls. Eur J Cell Biol 2002;81:153–160. [DOI] [PubMed] [Google Scholar]
- 70. Kanter JE, Kramer F, Barnhart S, et al. Diabetes promotes an inflammatory macrophage phenotype and atherosclerosis through acyl-CoA synthetase 1. Proc Natl Acad Sci U S A 2012;109:E715–E724. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71. Sen CK, Roy S. Redox signals in wound healing. Biochim Biophys Acta 2008;1780:1348–1361. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72. Enoch S, Grey JE, Harding KG. Recent advances and emerging treatments. BMJ 2006;332:962–965. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73. Schafer M, Werner S. Oxidative stress in normal and impaired wound repair. Pharmacol Res 2008;58:165–171. [DOI] [PubMed] [Google Scholar]
- 74. Zhang W, Chen L, Xiong Y, et al. Antioxidant therapy and antioxidant-related bionanomaterials in diabetic wound healing. Front Bioeng Biotechnol 2021;9:707479. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75. Khanna S, Biswas S, Shang Y, et al. Macrophage dysfunction impairs resolution of inflammation in the wounds of diabetic mice. PLoS One 2010;5:e9539. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76. Liu R, Bal HS, Desta T, Behl Y, Graves DT. Tumor necrosis factor-α mediates diabetes-enhanced apoptosis of matrix-producing cells and impairs diabetic healing. Am J Pathol 2006;168:757–764. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77. Han YP, Tuan TL, Wu H, Hughes M, Garner WL. TNF-alpha stimulates activation of pro-MMP2 in human skin through NF-(kappa)B mediated induction of MT1-MMP. J Cell Sci 2001;114:131–139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78. Kasiewicz LN, Whitehead KA. Lipid nanoparticles silence tumor necrosis factor α to improve wound healing in diabetic mice. Bioeng Transl Med 2019;4:75–82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79. Xu F, Zhang C, Graves DT. Abnormal cell responses and role of TNF-α in impaired diabetic wound healing. Biomed Res Int 2013;2013:754802. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80. Kishimoto TK, Jutila MA, Berg EL, Butcher EC. Neutrophil Mac-1 and MEL-14 adhesion proteins inversely regulated by chemotactic factors. Science 1989;245:1238–1241. [DOI] [PubMed] [Google Scholar]
- 81. Ponugoti B, Xu F, Zhang C, Tian C, Pacios S, Graves DT. FOXO1 promotes wound healing through the up-regulation of TGF-β1 and prevention of oxidative stress. J Cell Biol 2013;203:327–343. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82. Greenhalgh DG. The role of apoptosis in wound healing. Int J Biochem Cell Biol 1998;30:1019–1030. [DOI] [PubMed] [Google Scholar]
- 83. Gerhardt H, Betsholtz C. Endothelial-pericyte interactions in angiogenesis. Cell Tissue Res 2003;314:15–23. [DOI] [PubMed] [Google Scholar]
- 84. Usui ML, Mansbridge JN, Carter WG, Fujita M, Olerud JE. Keratinocyte migration, proliferation, and differentiation in chronic ulcers from patients with diabetes and normal wounds. J Histochem Cytochem 2008;56:687–696. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85. Manuela B, Milad K, Anna-Lena S, Julian-Dario R, Ewa Klara S. Acute and chronic wound fluid inversely influence wound healing in an in-vitro 3D wound model. J Tissue Repair Regen 2017;1:1–11. [Google Scholar]
- 86. James GA, Swogger E, Wolcott R, et al. Biofilms in chronic wounds. Wound Repair Regen 2008;16:37–44. [DOI] [PubMed] [Google Scholar]
- 87. Gjødsbøl K, Christensen JJ, Karlsmark T, Jørgensen B, Klein BM, Krogfelt KA. Multiple bacterial species reside in chronic wounds: a longitudinal study. Int Wound J 2006;3:225–231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88. Medzhitov R. Origin and physiological roles of inflammation. Nature 2008;454:428–435. [DOI] [PubMed] [Google Scholar]
- 89. Vandevyver S, Dejager L, Van Bogaert T, et al. Glucocorticoid receptor dimerization induces MKP1 to protect against TNF-induced inflammation. J Clin Invest 2012;122:2130–2140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90. Newton R, Kuitert LM, Slater DM, Adcock IM, Barnes PJ. Cytokine induction of cytosolic phospholipase A2 and cyclooxygenase-2 mRNA is suppressed by glucocorticoids in human epithelial cells. Life Sci 1996;60:67–78. [DOI] [PubMed] [Google Scholar]
- 91. Hakem R, Hakem A, Duncan GS, et al. Differential requirement for caspase 9 in apoptotic pathways in vivo. Cell 1998;94:339–352. [DOI] [PubMed] [Google Scholar]
- 92. Yoshida H, Kong YY, Yoshida R, et al. Apaf1 is required for mitochondrial pathways of apoptosis and brain development. Cell 1998;94:739–750. [DOI] [PubMed] [Google Scholar]
- 93. Desgeorges T, Caratti G, Mounier R, Tuckermann J, Chazaud B. Glucocorticoids shape macrophage phenotype for tissue repair. Front Immunol 2019;10:1591. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94. Werb Z. Biochemical actions of glucocorticoids on macrophages in culture: specific inhibition of elastase, collagenase, and plasminogen activator secretion and effects on other metabolic functions. J Exp Med 1978;147:1695–1712. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95. Papp KA, Dhadwal G, Gooderham M, et al. Emerging paradigm shift toward proactive topical treatment of psoriasis: a narrative review. Dermatol Ther 2021;34:e15104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96. Ballard K, Shuman VL. Hidradenitis suppurativa In: StatPearls [Internet]. Treasure Island, FL: StatPearls Publishing, 2022. [PubMed] [Google Scholar]
- 97. Ahronowitz I, Harp J, Shinkai K. Etiology and management of pyoderma gangrenosum: a comprehensive review. Am J Clin Dermatol 2012;13:191–211. [DOI] [PubMed] [Google Scholar]
- 98. Tsai TY, Huang IH, Chao YC, et al. Treating toxic epidermal necrolysis with systemic immunomodulating therapies: a systematic review and network meta-analysis. J Am Acad Dermatol 2021;84:390–397. [DOI] [PubMed] [Google Scholar]
- 99. Wang AS, Armstrong EJ, Armstrong AW. Corticosteroids and wound healing: clinical considerations in the perioperative period. Am J Surg 2013;206:410–417. [DOI] [PubMed] [Google Scholar]
- 100. Phillips JD, Kim CS, Fonkalsrud EW, Zeng H, Dindar H. Effects of chronic corticosteroids and vitamin a on the healing of intestinal anastomoses. Am J Surg 1992;163:71–77. [DOI] [PubMed] [Google Scholar]
- 101. Polat M, Lenk N, Yalcin B, et al. Efficacy of erythromycin for psoriasis vulgaris. Clin Exp Dermatol 2007;32:295–297. [DOI] [PubMed] [Google Scholar]
- 102. Clemmensen OJ. Topical treatment of hidradenitis suppurativa with clindamycin. Int J Dermatol 1983;22:325–328. [DOI] [PubMed] [Google Scholar]
- 103. Zouboulis CC, Desai N, Emtestam L, et al. European S1 guideline for the treatment of hidradenitis suppurativa/acne inversa. J Eur Acad Dermatol Venereol 2015;29:619–644. [DOI] [PubMed] [Google Scholar]
- 104. Mrcp JB-J, Tan SV, Graham-Brown RAC, Pembroke AC. The successful use of minocycline in pyoderma gangrenosum—a report of seven cases and review of the literature. J Dermatolog Treat 1989;1:23–25. [Google Scholar]
- 105. Schaller M, Kemény L, Havlickova B, et al. A randomized phase 3b/4 study to evaluate concomitant use of topical ivermectin 1% cream and doxycycline 40-mg modified-release capsules, versus topical ivermectin 1% cream and placebo in the treatment of severe rosacea. J Am Acad Dermatol 2020;82:336–343. [DOI] [PubMed] [Google Scholar]
- 106. Fowler JF Jr. Combined effect of anti-inflammatory dose doxycycline (40-mg doxycycline, USP monohydrate controlled-release capsules) and metronidazole topical gel 1% in the treatment of rosacea. J Drugs Dermatol 2007;6:641–645. [PubMed] [Google Scholar]
- 107. Reich K, Nestle FO, Papp K, et al. Infliximab induction and maintenance therapy for moderate-to-severe psoriasis: a phase III, multicentre, double-blind trial. Lancet 2005;366:1367–1374. [DOI] [PubMed] [Google Scholar]
- 108. Lu J-W, Huang Y-W, Chen T-L. Efficacy and safety of adalimumab in hidradenitis suppurativa. Medicine (Baltimore) 2021;100:e26190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109. Brooklyn TN, Dunnill MG, Shetty A, et al. Infliximab for the treatment of pyoderma gangrenosum: a randomised, double blind, placebo controlled trial. Gut 2006;55:505–509. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110. Alikhan A, Sayed C, Alavi A, et al. North American clinical management guidelines for hidradenitis suppurativa: a publication from the United States and Canadian Hidradenitis Suppurativa Foundations: Part I: diagnosis, evaluation, and the use of complementary and procedural management. J Am Acad Dermatol 2019;81:76–90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111. Haroon N, Srivastava R, Misra R, Aggarwal A. A novel predictor of clinical response to methotrexate in patients with rheumatoid arthritis: a pilot study of in vitro T cell cytokine suppression. J Rheumatol 2008;35:975–978. [PubMed] [Google Scholar]
- 112. Cronstein BN, Naime D, Ostad E. The antiinflammatory mechanism of methotrexate. Increased adenosine release at inflamed sites diminishes leukocyte accumulation in an in vivo model of inflammation. J Clin Invest 1993;92:2675–2682. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113. Meyer M, McGrouther DA. A study relating wound tension to scar morphology in the pre-sternal scar using Langers technique. Br J Plast Surg 1991;44:291–294. [DOI] [PubMed] [Google Scholar]
- 114. Ogawa R. Keloid and hypertrophic scars are the result of chronic inflammation in the reticular dermis. Int J Mol Sci 2017;18:606. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115. Ogawa R, Okai K, Tokumura F, et al. The relationship between skin stretching/contraction and pathologic scarring: the important role of mechanical forces in keloid generation. Wound Repair Regen 2012;20:149–157. [DOI] [PubMed] [Google Scholar]
- 116. Akaishi S, Akimoto M, Ogawa R, Hyakusoku H. The relationship between keloid growth pattern and stretching tension: visual analysis using the finite element method. Ann Plast Surg 2008;60:445–451. [DOI] [PubMed] [Google Scholar]
- 117. Daya M. Abnormal scar modulation with the use of micropore tape. Eur J Plast Surg 2011;34:45–51. [Google Scholar]
- 118. Reiffel RS. Prevention of hypertrophic scars by long-term paper tape application. Plast Reconstr Surg 1995;96:1715–1718. [DOI] [PubMed] [Google Scholar]
- 119. Shah VV, Aldahan AS, Mlacker S, Alsaidan M, Samarkandy S, Nouri K. 5-Fluorouracil in the treatment of keloids and hypertrophic scars: a comprehensive review of the literature. Dermatol Ther 2016;6:169–183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120. Awad SM, Ismail SA, Sayed DS, Refaiy AE, Makboul R. Suppression of transforming growth factor-beta1 expression in keloids after cryosurgery. Cryobiology 2017;75:151–153. [DOI] [PubMed] [Google Scholar]
- 121. Huang S, Chen CS, Ingber DE. Control of cyclin D1, p27(Kip1), and cell cycle progression in human capillary endothelial cells by cell shape and cytoskeletal tension. Mol Biol Cell 1998;9:3179–3193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122. Pathak A, Kumar S. Independent regulation of tumor cell migration by matrix stiffness and confinement. Proc Natl Acad Sci U S A 2012;109:10334–10339. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123. Huang C, Holfeld J, Schaden W, Orgill D, Ogawa R. Mechanotherapy: revisiting physical therapy and recruiting mechanobiology for a new era in medicine. Trends Mol Med 2013;19:555–564. [DOI] [PubMed] [Google Scholar]
- 124. Fu S, Panayi A, Fan J, et al. Mechanotransduction in wound healing: from the cellular and molecular level to the clinic. Adv Skin Wound Care 2021;34:67–74. [DOI] [PubMed] [Google Scholar]
- 125. Yang JH, Sakamoto H, Xu EC, Lee RT. Biomechanical regulation of human monocyte/macrophage molecular function. Am J Pathol 2000;156:1797–804. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126. Gibson T, Kenedi RM. Biomechanical properties of skin. Surg Clin North Am 1967;47:279–294. [DOI] [PubMed] [Google Scholar]
- 127. Mayer HF, Loustau HD. Capsular grafts and flaps in immediate prosthetic breast reconstruction. Aesthetic Plast Surg 2014;38:129–138. [DOI] [PubMed] [Google Scholar]
- 128. Maruyama K, Nemoto E, Yamada S. Mechanical regulation of macrophage function—cyclic tensile force inhibits NLRP3 inflammasome-dependent IL-1β secretion in murine macrophages. Inflamm Regen 2019;39:3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129. Chen W, Fu X, Sun X, Sun T, Zhao Z, Sheng Z. Analysis of differentially expressed genes in keloids and normal skin with cDNA microarray1. J Surg Res 2003;113:208–216. [DOI] [PubMed] [Google Scholar]
- 130. Messadi DV, Doung HS, Zhang Q, et al. Activation of NFkappaB signal pathways in keloid fibroblasts. Arch Dermatol Res 2004;296:125–133. [DOI] [PubMed] [Google Scholar]
- 131. Ding J, Lei L, Liu S, et al. Macrophages are necessary for skin regeneration during tissue expansion. J Transl Med 2019;17:36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132. Aragona M, Sifrim A, Malfait M, et al. Mechanisms of stretch-mediated skin expansion at single-cell resolution. Nature 2020;584:268–273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133. Panayi AC, Leavitt T, Orgill DP. Evidence based review of negative pressure wound therapy. World J Dermatol 2017;6:1–16. [Google Scholar]
- 134. Peinemann F, Labeit A. Negative pressure wound therapy: a systematic review of randomized controlled trials from 2000 to 2017. J Evid Based Med 2019;12:125–132. [DOI] [PubMed] [Google Scholar]
- 135. Taylor EM, Hamaguchi R, Kramer KM, Kimball AB, Orgill DP. Plastic surgical management of hidradenitis suppurativa. Plast Reconstr Surg 2021;147:479–491. [DOI] [PubMed] [Google Scholar]
- 136. Eisendle K, Thuile T, Deluca J, Pichler M. Surgical treatment of pyoderma gangrenosum with negative pressure wound therapy and skin grafting, including xenografts: personal experience and comprehensive review on 161 cases. Adv Wound Care 2020;9:405–425. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137. Frear CC, Zang T, Griffin BR, et al. The modulation of the burn wound environment by negative pressure wound therapy: insights from the proteome. Wound Repair Regen 2021;29:288–297. [DOI] [PubMed] [Google Scholar]
- 138. Orgill DP, Manders EK, Sumpio BE, et al. The mechanisms of action of vacuum assisted closure: more to learn. Surgery 2009;146:40–51. [DOI] [PubMed] [Google Scholar]
- 139. Labanaris AP, Polykandriotis E, Horch RE. The effect of vacuum-assisted closure on lymph vessels in chronic wounds. J Plast Reconstr Aesthet Surg 2009;62:1068–1075. [DOI] [PubMed] [Google Scholar]
- 140. Ludolph I, Fried FW, Kneppe K, Arkudas A, Schmitz M, Horch RE. Negative pressure wound treatment with computer-controlled irrigation/instillation decreases bacterial load in contaminated wounds and facilitates wound closure. Int Wound J 2018;15:978–984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141. Karam RA, Rezk NA, Abdel Rahman TM, Al Saeed M. Effect of negative pressure wound therapy on molecular markers in diabetic foot ulcers. Gene 2018;667:56–61. [DOI] [PubMed] [Google Scholar]
- 142. Wang T, Li X, Fan L, et al. Negative pressure wound therapy promoted wound healing by suppressing inflammation via down-regulating MAPK-JNK signaling pathway in diabetic foot patients. Diabetes Res Clin Pract 2019;150:81–89. [DOI] [PubMed] [Google Scholar]
- 143. Song H, Xu Y, Chang W, Zhuang J, Wu X. Negative pressure wound therapy promotes wound healing by suppressing macrophage inflammation in diabetic ulcers. Regen Med 2020;15:2341–2349. [DOI] [PubMed] [Google Scholar]
- 144. Mous CM, van Toorenenbergen AW, Heule F, Hop WC, Hovius SER. The role of topical negative pressure in wound repair: expression of biochemical markers in wound fluid during wound healing. Wound Repair Regen 2008;16:488–494. [DOI] [PubMed] [Google Scholar]
- 145. Hersel U, Dahmen C, Kessler H. RGD modified polymers: biomaterials for stimulated cell adhesion and beyond. Biomaterials 2003;24:4385–4415. [DOI] [PubMed] [Google Scholar]
- 146. Drury JL, Mooney DJ. Hydrogels for tissue engineering: scaffold design variables and applications. Biomaterials 2003;24:4337–4351. [DOI] [PubMed] [Google Scholar]
- 147. Pineau I, Lacroix S. Proinflammatory cytokine synthesis in the injured mouse spinal cord: multiphasic expression pattern and identification of the cell types involved. J Comp Neurol 2007;500:267–85. [DOI] [PubMed] [Google Scholar]
- 148. Duffield JS. The inflammatory macrophage: a story of Jekyll and Hyde. Clin Sci (Lond) 2003;104:27–38. [DOI] [PubMed] [Google Scholar]
- 149. Nilsson B, Ekdahl KN, Mollnes TE, Lambris JD. The role of complement in biomaterial-induced inflammation. Mol Immunol 2007;44:82–94. [DOI] [PubMed] [Google Scholar]
- 150. Christenson EM, Dadsetan M, Anderson JM, Hiltner A. Biostability and macrophage-mediated foreign body reaction of silicone-modified polyurethanes. J Biomed Mater Res A 2005;74:141–155. [DOI] [PubMed] [Google Scholar]
- 151. Christenson EM, Dadsetan M, Wiggins M, Anderson JM, Hiltner A. Poly(carbonate urethane) and poly(ether urethane) biodegradation: in vivo studies. J Biomed Mater Res A 2004;69:407–416. [DOI] [PubMed] [Google Scholar]
- 152. Ebert M, Ward B, Anderson J, McVenes R, Stokes K. In vivo biostability of polyether polyurethanes with polyethylene oxide surface-modifying end groups; resistance to biologic oxidation and stress cracking. J Biomed Mater Res A 2005;75:175–184. [DOI] [PubMed] [Google Scholar]
- 153. Mosmann TR, Coffman RL. TH1 and TH2 cells: different patterns of lymphokine secretion lead to different functional properties. Annu Rev Immunol 1989;7:145–173. [DOI] [PubMed] [Google Scholar]
- 154. Mosmann TR, Coffman RL. Heterogeneity of cytokine secretion patterns and functions of helper T cells. Adv Immunol 1989;46:111–147. [DOI] [PubMed] [Google Scholar]
- 155. Higuchi A, Shirano K, Harashima M, et al. Chemically modified polysulfone hollow fibers with vinylpyrrolidone having improved blood compatibility. Biomaterials 2002;23:2659–2666. [DOI] [PubMed] [Google Scholar]
- 156. MacDonald DE, Deo N, Markovic B, Stranick M, Somasundaran P. Adsorption and dissolution behavior of human plasma fibronectin on thermally and chemically modified titanium dioxide particles. Biomaterials 2002;23:1269–1279. [DOI] [PubMed] [Google Scholar]
- 157. Hess H, Vogel V. Molecular shuttles based on motor proteins: active transport in synthetic environments. J Biotechnol 2001;82:67–85. [DOI] [PubMed] [Google Scholar]
- 158. Wang S, Gupta AS, Sagnella S, Barendt PM, Kottke-Marchant K, Marchant RE. Biomimetic fluorocarbon surfactant polymers reduce platelet adhesion on PTFE/ePTFE surfaces. J Biomater Sci Polym Ed 2009;20:619. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 159. Badylak SF, Valentin JE, Ravindra AK, McCabe GP, Stewart-Akers AM. Macrophage phenotype as a determinant of biologic scaffold remodeling. Tissue Eng Part A 2008;14:1835–1842. [DOI] [PubMed] [Google Scholar]
- 160. Acharya AP, Dolgova NV, Clare-Salzler MJ, Keselowsky BG. Adhesive substrate-modulation of adaptive immune responses. Biomaterials 2008;29:4736–4750. [DOI] [PubMed] [Google Scholar]
- 161. Bota PCS, Collie AM, Puolakkainen P, et al. Biomaterial topography alters healing in vivo and monocyte/macrophage activation in vitro. J Biomed Mater Res A 2010;95A:649–657. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 162. Bridges AW, Singh N, Burns KL, Babensee JE, Andrew Lyon L, García AJ. Reduced acute inflammatory responses to microgel conformal coatings. Biomaterials 2008;29:4605–4615. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 163. Zeng Q, Macri L, Prasad A, et al. 6.20 Skin tissue engineering. In: Ducheyne P, ed. Comprehensive Biomaterials II. Amsterdam, Netherlands: Elsevier, 2017:334–382. [Google Scholar]
- 164. Greenwood JE, Schmitt BJ, Wagstaff MJD. Experience with a synthetic bilayer Biodegradable Temporising Matrix in significant burn injury. Burn Open 2018;2:17–34. [Google Scholar]
- 165. Cheshire PA, Herson MR, Cleland H, Akbarzadeh S. Artificial dermal templates: a comparative study of NovoSorb™ Biodegradable Temporising Matrix (BTM) and Integra(®) Dermal Regeneration Template (DRT). Burns 2016;42:1088–1096. [DOI] [PubMed] [Google Scholar]
- 166. Expert working group. Acellular matrices for the treatment of wounds—wounds international. 2011. https://www.woundsinternational.com/resources/details/acellular-matrices-treatment-wounds (last accessed April 29, 2022).
- 167. Nyame TT, Chiang HA, Orgill DP. Clinical applications of skin substitutes. Surg Clin North Am 2014;94:839–850. [DOI] [PubMed] [Google Scholar]
- 168. Wright KA, Nadire KB, Busto P, Tubo R, McPherson JM, Wentworth BM. Alternative delivery of keratinocytes using a polyurethane membrane and the implications for its use in the treatment of full-thickness burn injury. Burns 1998;24:7–17. [DOI] [PubMed] [Google Scholar]
- 169. Huang S, Fu X. Tissue-engineered skin: bottleneck or breakthrough. Int J Burns Trauma 2011;1:1–10. [PMC free article] [PubMed] [Google Scholar]
- 170. Peirce SC, Carolan-Rees G. ReCell® spray-on skin system for treating skin loss, scarring and depigmentation after burn injury: a NICE Medical Technology Guidance. Appl Health Econ Health Policy 2019;17:131–141. [DOI] [PubMed] [Google Scholar]
- 171. Eaglstein WH, Iriondo M, Laszlo K. A composite skin substitute (graftskin) for surgical wounds. A clinical experience. Dermatol Surg 1995;21:839–843. [DOI] [PubMed] [Google Scholar]
- 172. Aggarwala S, Harish V, Roberts S, et al. Treatment of partial thickness burns: a prospective, randomized controlled trial comparing four routinely used burns dressings in an ambulatory care setting. J Burn Care Res 2021;42:934–943. [DOI] [PubMed] [Google Scholar]
- 173. Choi JS, Kim JD, Yoon HS, Cho YW. Full-thickness skin wound healing using human placenta-derived extracellular matrix containing bioactive molecules. Tissue Eng Part A 2013;19:329.-–339. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 174. Zelen CM, Orgill DP, Serena T, et al. A prospective, randomised, controlled, multicentre clinical trial examining healing rates, safety and cost to closure of an acellular reticular allogenic human dermis versus standard of care in the treatment of chronic diabetic foot ulcers. Int Wound J 2017;14:307–315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 175. Walters J, Cazzell S, Pham H, Vayser D, Reyzelman A. Healing rates in a multicenter assessment of a sterile, room temperature, acellular dermal matrix versus conventional care wound management and an active comparator in the treatment of full-thickness diabetic foot ulcers. Eplasty 2016;16:e10–e10. [PMC free article] [PubMed] [Google Scholar]
- 176. Reyzelman A, Crews RT, Moore JC, et al. Clinical effectiveness of an acellular dermal regenerative tissue matrix compared to standard wound management in healing diabetic foot ulcers: a prospective, randomised, multicentre study. Int Wound J 2009;6:196–208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 177. Liu C, Zhang W, Li Y, et al. Microfluidic sonication to assemble exosome membrane-coated nanoparticles for immune evasion-mediated targeting. Nano Lett 2019;19:7836–7844. [DOI] [PubMed] [Google Scholar]
- 178. Luo Z-W, Li FX, Liu YW, et al. Aptamer-functionalized exosomes from bone marrow stromal cells target bone to promote bone regeneration. Nanoscale 2019;11:20884–20892. [DOI] [PubMed] [Google Scholar]
- 179. Hu Y, Wu B, Xiong Y, et al. Cryogenic 3D printed hydrogel scaffolds loading exosomes accelerate diabetic wound healing. Chem Eng J 2021;426:130634. [Google Scholar]
- 180. Li X, Xie X, Lian W, et al. Exosomes from adipose-derived stem cells overexpressing Nrf2 accelerate cutaneous wound healing by promoting vascularization in a diabetic foot ulcer rat model. Exp Mol Med 2018;504:50:1–14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 181. Mi B, Liu J, Liu Y, et al. The designer antimicrobial peptide A-hBD-2 facilitates skin wound healing by stimulating keratinocyte migration and proliferation. Cell Physiol Biochem 2018;51:647–663. [DOI] [PubMed] [Google Scholar]
- 182. Brezinski EA, Dhillon JS, Armstrong AW. Economic burden of psoriasis in the United States. JAMA Dermatol 2015;151:651. [DOI] [PubMed] [Google Scholar]
- 183. Lomholt G. Prevalence of skin diseases in a population: a consensus study from the Faroe Islands. Dan Med Bull 1964;11:1–7. [PubMed] [Google Scholar]
- 184. Marvel J, Vlahiotis A, Sainski-Nguyen A, Willson T, Kimball A. Disease burden and cost of hidradenitis suppurativa: a retrospective examination of US administrative claims data. BMJ Open 2019;9:e030579. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 185. Jemec GBE. Clinical practice. Hidradenitis suppurativa. N Engl J Med 2012;366:158–164. [DOI] [PubMed] [Google Scholar]
- 186. American Academy of Dermatology/Milliman. Burden of Skin Disease 2017. www.aad.org/BSD (last accessed April 29, 2022).
- 187. Narla S, Silverberg JI. The inpatient burden and comorbidities of pyoderma gangrenosum in adults in the United States. Arch Dermatol Res 2021;313:245–253. [DOI] [PubMed] [Google Scholar]
- 188. Nussbaum SR, Carter MJ, Fife CE, et al. An economic evaluation of the impact, cost, and medicare policy implications of chronic nonhealing wounds. Value Health 2018;21:27–32. [DOI] [PubMed] [Google Scholar]
- 189. Zhang P, Lu J, Jing Y, Tang S, Zhu D, Bi Y. Global epidemiology of diabetic foot ulceration: a systematic review and meta-analysis. Ann Med 2017;49:106–116. [DOI] [PubMed] [Google Scholar]
- 190. Xie T, Ye J, Rerkasem K, Mani R. The venous ulcer continues to be a clinical challenge: an update. Burn Trauma 2018;6:8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 191. Whittington K, Patrick M, Roberts JL. A national study of pressure ulcer prevalence and incidence in acute care hospitals. J Wound Ostomy Continence Nurs 2000;27:a107879. [DOI] [PubMed] [Google Scholar]
- 192. Hsu DY, Brieva J, Silverberg NB, Silverberg JI. Morbidity and mortality of Stevens-Johnson syndrome and toxic epidermal necrolysis in United States adults. J Invest Dermatol 2016;136:1387–1397. [DOI] [PubMed] [Google Scholar]
- 193. Okhovat J-P, Armstrong AW. Updates in rosacea: epidemiology, risk factors, and management strategies. Curr Dermatol Rep 2014;3:23–28. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.