Abstract
Strain CHR63 is a salt-sensitive mutant of the moderately halophilic wild-type strain Halomonas elongata DSM 3043 that is affected in the ectoine synthase gene (ectC). This strain accumulates large amounts of Nγ-acetyldiaminobutyrate (NADA), the precursor of ectoine (D. Cánovas, C. Vargas, F. Iglesias-Guerra, L. N. Csonka, D. Rhodes, A. Ventosa, and J. J. Nieto, J. Biol. Chem. 272:25794–25801, 1997). Hydroxyectoine, ectoine, and glucosylglycerate were also identified by nuclear magnetic resonance (NMR) as cytoplasmic organic solutes in this mutant. Accumulation of NADA, hydroxyectoine, and ectoine was osmoregulated, whereas the levels of glucosylglycerate decreased at higher salinities. The effect of the growth stage on the accumulation of solutes was also investigated. NADA was purified from strain CHR63 and was shown to protect the thermolabile enzyme rabbit muscle lactate dehydrogenase against thermal inactivation. The stabilizing effect of NADA was greater than the stabilizing effect of ectoine or potassium diaminobutyrate. A 1H NMR analysis of the solutes accumulated by the wild-type strain and mutants CHR62 (ectA::Tn1732) and CHR63 (ectC::Tn1732) indicated that H. elongata can synthesize hydroxyectoine by two different pathways—directly from ectoine or via an alternative pathway that converts NADA into hydroxyectoine without the involvement of ectoine.
The strategy developed by halophilic and halotolerant bacteria to cope with the osmotic stress imposed by a hypersaline environment involves accumulation of low-molecular-weight organic compounds that are called compatible solutes (2). These compounds can be classified into different categories, such as polyols and heterosides, sugars, amino acids and their derivatives, N-acetylated diamino acids, betaines and tethines, and ectoines (ectoine and hydroxyectoine). Recently, there has been increased interest in compatible solutes in biotechnology, since it has been shown that these compounds are able to protect enzymes and whole cells against stresses, such as the stresses caused by salt, heating, freezing, and desiccation (8, 11).
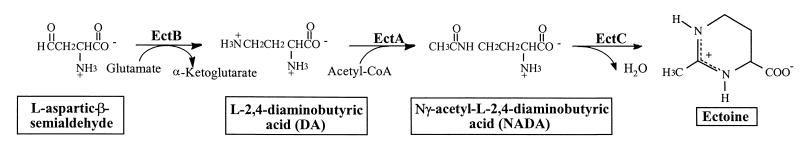
The moderately halophilic bacterium Halomonas elongata exhibits one of the widest ranges of salt tolerance known; this organism is able to grow in the presence of NaCl concentrations ranging from ∼0.1 to ∼4 M in complex medium (26–28). Osmoadaptation is achieved by accumulation of glycine betaine, which is taken up from the medium or synthesized from choline (4, 5), and by synthesis of ectoine and hydroxyectoine (6). The biosynthetic pathway for ectoine in this bacterium has been elucidated at the biochemical and genetic levels (3, 6, 13, 18) (Fig. 1). Ectoine synthesis occurs in three steps. First, aspartate semialdehyde, an intermediate in amino acid metabolism, is converted into diaminobutyrate (DA), which is subsequently acetylated to Nγ-acetyldiaminobutyrate (NADA). Cyclic condensation of this compound leads to formation of ectoine (18, 19). Recently, Cánovas et al. isolated (6) and characterized (3) the genetic region involved in ectoine synthesis in H. elongata DSM 3043. This region comprises the following three genes: ectA, which encodes the DA acetyltransferase; ectB, which encodes the DA transaminase; and ectC, which encodes the ectoine synthase (3). In contrast to the well-characterized ectoine synthesis pathway, the biosynthetic route leading to hydroxyectoine has not been established yet, and the genes involved are not known. Although there is some evidence that hydroxyectoine could be synthesized directly from ectoine (6, 13), this hypothesis lacks firm support.
FIG. 1.
Ectoine biosynthetic pathway. See reference 19. The genes for the three enzymes involved in ectoine synthesis, DA acetyltransferase, l-DA transaminase, and ectoine synthase, are designated ectA, ectB, and ectC, respectively (3). CoA, coenzyme A.
In a recent study, Cánovas et al. (6) demonstrated that NADA, the immediate precursor of ectoine, plays an osmoprotective role in H. elongata. In fact, the salt-sensitive mutant CHR63 (ectC::Tn1732), which accumulated NADA, could tolerate higher levels of salinity than the levels tolerated by mutant CHR62 (ectA::Tn1732), which accumulated DA; this indicated that NADA functions as a compatible solute in mutant CHR63. This was the first indication that NADA, which was discovered as a component of Euphorbia pulcherrima latex (16), could play a role in osmotic adaptation. In this study, NADA was purified from mutant strain CHR63 and shown to stabilize the model enzyme rabbit muscle lactate dehydrogenase (LDH) against inactivation by heat. Moreover, accumulation of solutes was quantified as a function of the NaCl concentration in the medium and the growth phase. Evidence which supports the hypothesis that NADA is a branch point in the synthesis of the compatible solutes ectoine and hydroxyectoine is also presented in this paper.
MATERIALS AND METHODS
Strains and growth conditions.
H. elongata DSM 3043 (wild type) (27), CHR61 (a spontaneous rifampin-resistant mutant of the wild-type strain), CHR62 (ectA::Tn1732), and CHR63 (ectC::Tn1732) have been described previously (3, 6). H. elongata strains were grown in defined M63 medium containing 20 mM glucose as the sole carbon source (7). The pH of the medium was adjusted to 7.2 with KOH. Liquid cultures were incubated at 37°C in an orbital shaker at 200 rpm. Cell growth was monitored by measuring the optical density at 600 nm (OD600).
To study the effect of salinity on the accumulation of organic solutes, cultures were grown in media containing 0.5 to 1.5 M NaCl until they reached the late exponential phase (OD600, 1.0 to 1.2). The effect of the growth phase on the accumulation of solutes was examined by growing cells in medium containing 1.0 M NaCl. For the experiments performed with mutant CHR62 that were designed to investigate the ectoine-hydroxyectoine interconversion, media were supplemented with 1 mM NADA, 1 mM ectoine, or 1 mM hydroxyectoine. Cells were harvested by centrifugation (8,000 × g, 30°C, 10 min).
Cell protein contents.
The protein contents of the cells were determined by the Bradford assay (1) after cell lysis with 1 M NaOH (100°C, 10 min) and neutralization with 1 M HCl.
Extraction and determination of intracellular solutes by NMR spectroscopy.
Cell pellets obtained from cultures of H. elongata were extracted twice with boiling 80% ethanol by the method of Reed et al. (21), modified as previously described by Martins and Santos (17). Freeze-dried extracts were dissolved in D2O for nuclear magnetic resonance (NMR) analysis. Solutes were quantified by 1H NMR by using formate as an internal concentration standard. For quantification purposes, spectra were acquired with a 6-μs pulse width (corresponding to a 60° flip angle) and a repetition delay of 65 s. 1H NMR spectra were recorded at 300.14 MHz with a Bruker model AMX300 spectrometer equipped with a 5-mm-diameter broad-band inverse probe head. 13C NMR spectra were obtained with a Bruker model DRX500 spectrometer at 125.77 MHz.
Purification of NADA.
An ethanol-soluble extract of an H. elongata CHR63 culture grown in M63 medium supplemented with 0.75 M NaCl was applied onto an activated Dowex 50W-X8 cation-exchange resin column (16 by 2.8 cm). Elution was performed with a linear gradient of perchloric acid (0 to 2 M) at a flow rate of 2.5 ml min−1. Aliquots of each fraction were neutralized with KOH and were analyzed for the presence of amino acid derivatives by using the ninhydrin test. Fractions containing amino acid derivatives were freeze-dried and analyzed by 1H NMR spectroscopy. The fractions that eluted between 1.5 and 1.7 M perchloric acid contained hydroxyectoine and NADA, and the fractions that eluted between 1.7 and 2 M perchloric acid contained pure NADA. Samples containing pure NADA were pooled, and salts were removed by passage through an ion retardation column (type AG11 A8; 45 by 2.5 cm). After lyophilization, ultrapure water was added to obtain a 0.6 M NADA solution. This solution was stored at −20°C until it was used. No cations (Na+ or K+) were detected by plasma emission spectroscopy performed with a Jobin Yvon spectrometer (model JY24).
Purification of glucosylglycerate.
An ethanol-soluble extract of an H. elongata CHR63 culture grown in M63 medium containing 1.0 M NaCl was applied to an activated Dowex 50W-X8 resin (H+ form) column and eluted with distilled water. The eluted fractions were analyzed for carbohydrate by the method of Dubois et al. (9). The fractions containing carbohydrates were pooled, neutralized with 1 M KOH, lyophilized, and dissolved in D2O; these fractions were used for 1H NMR analysis.
Measurements of enzyme activity.
Rabbit muscle LDH, which was obtained from the supplier as a suspension in ammonium sulfate, was applied to a type PD-10 column and eluted with 10 mM potassium phosphate buffer (pH 7.6). LDH activity was assayed by monitoring oxidation of NADH on the basis of measurements of absorbance at 340 nm, as determined with a spectrophotometer (model DW-2; Olis) equipped with a thermostat-equipped cuvette holder at 30°C. The standard reaction mixture contained 80 mM Tris-HCl (pH 7.6), 1.6 mM pyruvate (sodium salt), 0.2 mM NADH, and 0.25 μg of enzyme in a total volume of 1 ml (25). The reaction was initiated by adding pyruvate.
The method previously described for determining prolyl hydroxylase activity was used to detect putative NADA hydroxylase activity in crude cell extracts of H. elongata CHR63 (24). To do this, cells were grown in M63 medium supplemented with 1.5 M NaCl and harvested during the exponential growth phase; the cell pellet was suspended in a saline solution (1.5 M NaCl) containing the protease inhibitor phenylmethylsulfonyl fluoride at a concentration of 1 mM, 1 mM EDTA, 1 mM MgCl2, and 2 mg of DNase ml−1. After the cells were broken by passage through a French press, the resulting extract was centrifuged (15,000 × g, 30 min, 4°C) to remove the cell debris. The supernatant solution was dialyzed overnight against 20 mM phosphate buffer (pH 7.2).
Thermal stability assays.
The thermal stability assay mixtures contained 50 μg of LDH ml−1 with or without one of the following solutes: KCl, DA, NADA, ectoine, and hydroxyectoine. All of the solutes were used at a final concentration of 0.5 M. Mixtures were placed in Eppendorf tubes and incubated at 50 or 55°C in a water bath, and aliquots were withdrawn at different times. The aliquots were cooled in an ice bath and immediately assayed for enzyme activity. Results are presented below as percentages of activity compared with the activities of aliquots kept at room temperature.
Chemicals.
Type II rabbit muscle LDH (E.C. 1.1.1.27) was obtained from Sigma. Dowex AG 50W-X8 and the ion retardation resin AG 11 A8 were purchased from Bio-Rad Laboratories (Richmond, Calif.). The PD-10 column was obtained from Pharmacia Fine Chemicals (Uppsala, Sweden). DA was obtained from Sigma. Ectoine and hydroxyectoine were generously provided by Bitop GmbH.
RESULTS
Identification of the organic solutes that accumulated in H. elongata mutant strain CHR63.
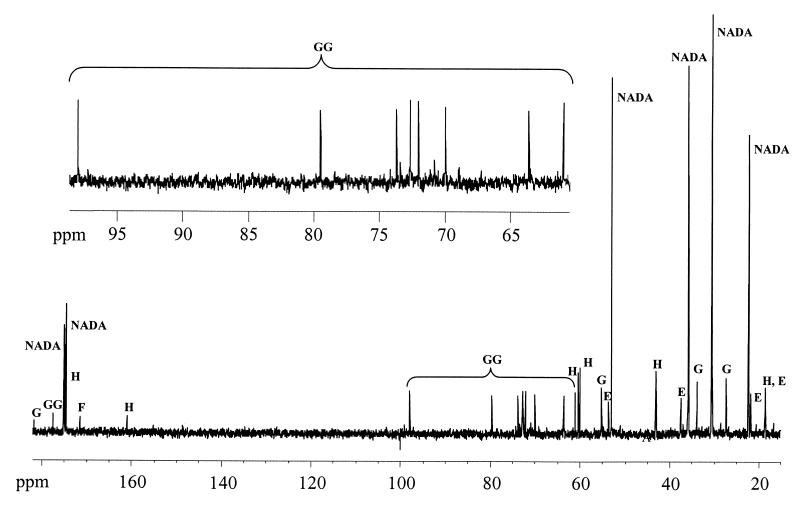
The 13C NMR spectra of ethanol extracts of the mutant CHR63 contained several sets of resonances that were assigned to NADA, hydroxyectoine, ectoine, and glucosylglycerate (Fig. 2). Small amounts of glutamate, lactate, trehalose, and alanine were detected in the 1H NMR spectra of the same extracts (data not shown). Resonances were assigned by comparison with previously published chemical shift values (6, 12, 23, 29). In most cases assignments were confirmed by spiking samples with the pure compounds. The only exception was glucosylglycerate; assignment of this organic solute was confirmed by partially purifying an ethanol extract and performing 1H and 13C NMR analyses. The 13C NMR spectrum contained only nine major carbon resonances (at 61.0, 63.6, 70.0, 72.0, 72.7, 73.7, 79.3, 97.9, and 177.5 ppm), whose chemical shifts agreed with the chemical shifts previously obtained for this compound in Methanohalophilus sp. and the blue-green alga Agmenellum quadruplicatum (14, 22).
FIG. 2.
13C NMR spectrum of the crude extract of H. elongata mutant strain CHR63 grown in M63 medium supplemented with 1.5 M NaCl. The major solutes were NADA, hydroxyectoine (H), ectoine (E), glutamate (G), and glucosylglycerate (GG). Formate (F) was added to the sample as an internal concentration standard.
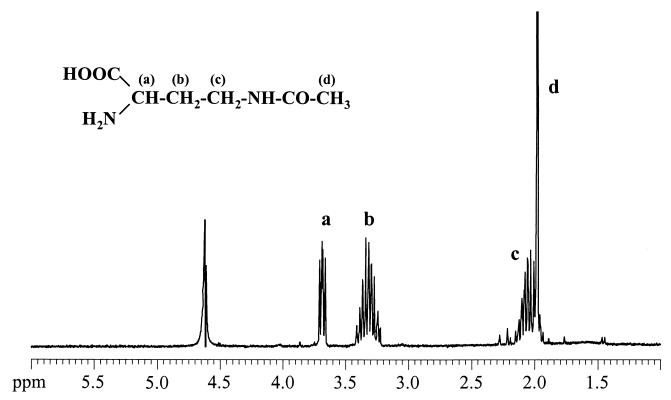
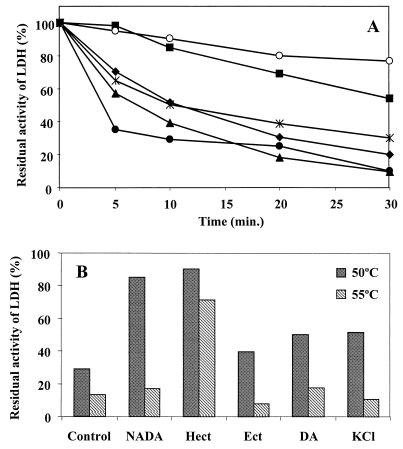
Effect of NADA as an enzyme thermostabilizer.
Since it had been shown that NADA could function as a compatible solute in H. elongata (6), we decided to investigate whether NADA could also act as an enzyme stabilizer. This compound was purified from mutant CHR63. The 1H NMR spectrum of the pure compound is shown in Fig. 3. The ability of NADA to stabilize rabbit muscle LDH was compared with the abilities of ectoine, hydroxyectoine, and DA, the immediate precursor of NADA in members of the genus Halomonas (Fig. 4A). At 50°C, NADA had a significant thermostabilizing effect on LDH; this effect was surpassed only by the effect of hydroxyectoine. However, when the enzyme was incubated at a higher temperature, 55°C, hydroxyectoine was the only solute that significantly protected the enzyme against thermal inactivation, and NADA was a poor stabilizer (Fig. 4B). The potassium salt of DA had a weak stabilizing effect at 50°C, comparable to the effect of KCl.
FIG. 3.
1H NMR (300-MHz) spectrum of NADA after purification from H. elongata CHR63.
FIG. 4.
Effects of NADA (■), ectoine (Ect) (▴), hydroxyectoine (Hect) (○), potassium DA (✠), and potassium chloride (⧫) on thermal inactivation of rabbit muscle LDH. (A) The enzyme was incubated at 50°C in the absence (●) or in the presence of one of the solutes, and samples were withdrawn at different times. (B) The enzyme was incubated at 50 or 55°C for 10 min in the presence or in the absence of one of the solutes. The activity of the enzyme was immediately assayed. Values are expressed as percentages of activity compared to the activity of the enzyme incubated at room temperature. All of the results are averages of the values from at least two independent experiments. The standard deviation was equal to or less than 10%.
Accumulation of organic solutes by mutant CHR63 as a function of salinity and growth phase.
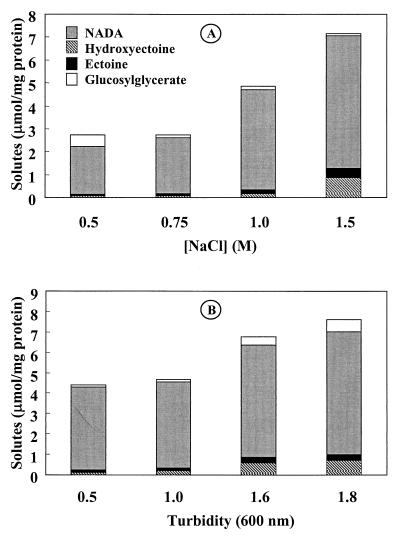
In addition to the major compound NADA, which was previously detected in mutant CHR63, we identified ectoine, hydroxyectoine, and glucosylglycerate as significant members of the intracellular pool of solutes in this organism. We investigated the accumulation of solutes as a function of the salt concentration in the medium and the growth phase (Fig. 5). NADA was by far the predominant solute and accumulated at all of the salinities and growth stages examined. H. elongata CHR63 grows optimally in the presence of NaCl concentrations ranging from 0.75 to 1.0 M (6). The concentration of the total pool of organic solutes was maintained at approximately 2.8 μmol mg of protein−1 at NaCl concentrations up to 0.75 M. At the highest NaCl concentration used (1.5 M), the concentration of the total solute pool increased to 7.2 μmol mg of protein−1, and NADA accounted for 80% of the total pool. However, hydroxyectoine (12%) and ectoine (6%) also made relevant contributions. At NaCl concentrations of 0.75 to 1.5 M, the levels of NADA, ectoine, and hydroxyectoine increased 2.4-, 4.7-, and 10-fold, respectively, showing that accumulation of these solutes is osmoregulated in strain CHR63. In contrast, glucosylglycerate did not function as a compatible solute, since it accumulated preferentially at low levels of salinity. Low levels of glutamate, trehalose, alanine, and lactate were also detected, but they were not accurately quantified; the combined contents of these minor solutes did not exceed 10% of the total solute pool (Fig. 5A).
FIG. 5.
Accumulation of solutes in H. elongata CHR63. (A) Cells were grown in M63 medium supplemented with different salt concentrations and samples were withdrawn in the late exponential phase. (B) Cells were grown in M63 medium supplemented with 1.0 M NaCl, and samples were taken at different growth stages. Turbidity (OD600) values of 0.5 and 1.0 correspond to the early and late exponential phases of growth, respectively, and values of 1.6 and 1.8 correspond to the stationary phase. The solutes were identified and quantified by 1H NMR by using ethanol cell extracts. The values are the means of values from at least two independent determinations, and the individual values did not differ by more than 20%.
The levels of solutes were clearly affected by the growth phase (Fig. 5B). There were no differences in the concentrations of the cytoplasmic pools of compounds in the early (OD600, 0.5) and late (OD600, 1) exponential phases of growth. However, during the stationary phase (OD600, 1.6 to 1.8), the concentrations of all solutes increased considerably. It is noteworthy that a threefold increase in the levels of hydroxyectoine was detected during the stationary phase of growth (Fig. 5B).
Evidence that NADA is a branch point for the synthesis of ectoine and hydroxyectoine.
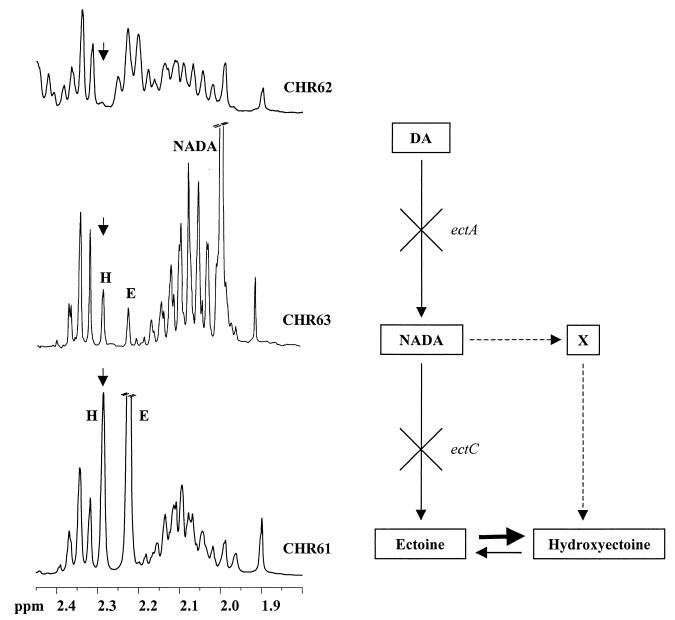
The observation that in H. elongata mutant CHR63 the hydroxyectoine/ectoine ratio was considerably higher than the ratio in the wild-type strain (6; this study) led us to the hypothesis that NADA could be a direct precursor for the synthesis of hydroxyectoine via an alternative pathway different from the pathway previously proposed (in which ectoine is used as a precursor) (6, 13). In order to test this hypothesis, two mutant strains, CHR62 (ectA::Tn1732) and CHR63 (ectC::Tn1732), and the wild-type strain of H. elongata were cultivated in M63 medium containing 0.65 M NaCl, harvested at an OD600 of 0.9, and examined for the presence of ectoine, hydroxyectoine, and NADA. Mutant CHR62, which is affected in the DA acetyltransferase gene (ectA), did not synthesize ectoine or hydroxyectoine (Fig. 6), whereas mutant CHR63, which is affected in the ectoine synthase gene (ectC), and the wild-type strain synthesized hydroxyectoine and ectoine with relative proportions of 1.3:1 and 0.4:1, respectively.
FIG. 6.
1H NMR (300-MHz) spectra of ethanol extracts of H. elongata salt-sensitive mutant strains CHR62 (ectA::Tn1732) and CHR63 (ectC::Tn1732) and of CHR61 (wild-type strain) grown in M63 medium supplemented with 0.65 M NaCl. The arrows indicate the position of the hydroxyectoine resonance. Abbreviations: H, hydroxyectoine; E, ectoine. The biosynthetic pathway for ectoines from DA is also shown. The solid and dashed lines indicate established and proposed steps, respectively. The letter X represents an intermediate in hydroxyectoine biosynthesis that has not been identified so far.
To assess the occurrence of direct interconversion between hydroxyectoine and ectoine, mutant CHR62 was grown in M63 medium supplemented with 1 mM NADA, 1 mM ectoine, or 1 mM hydroxyectoine, and 2.0 M NaCl. Hydroxyectoine and ectoine at a molar ratio of 2:1 were synthesized from NADA. When ectoine was provided, the hydroxyectoine/ectoine ratio was 0.7, and this ratio increased to 7.0 when hydroxyectoine was provided instead of ectoine (results not shown). These results show not only that hydroxyectoine can be synthesized directly from ectoine but also that conversion of ectoine to hydroxyectoine is a reversible reaction, which under the conditions tested seemed to be favored in the direction of hydroxyectoine synthesis.
In summary, in the organisms in which the intracellular concentration of NADA was high, the hydroxyectoine level was significantly higher than the level of ectoine, suggesting that in H. elongata DSM 3043 NADA is the branch point in the biosynthetic pathway to ectoines (Fig. 6).
DISCUSSION
The stabilizing effects of osmolytes, such as glycerol and sugars, on enzymes and whole cells were well-known long before the term compatible solute was introduced by Brown in 1976 (2). More recently, compatible solutes have received considerable attention due to their remarkable protection of enzymes against heat, salt, freezing, and drying. Although general mechanisms for this protection have been postulated, the degree of stabilization achieved seems to depend on the specific enzyme-solute pair under investigation (20; see references 8 and 10 for reviews).
The labile enzyme rabbit muscle LDH has often been used as a model system for enzyme stabilization tests (10, 15, 20). This enzyme is very sensitive to high temperatures and also is rapidly inactivated by freeze-thaw treatment (10). We found that NADA, the precursor of ectoine, protected this enzyme against inactivation by heat at 50°C. This protection was comparable to that of hydroxyectoine and much better than that of ectoine or DA. This was surprising, since NADA can be considered the hydrolyzed linear form of ectoine.
To our knowledge, the stabilization properties of solutes belonging to the N-acetylated diamino acid group (such as Nδ-acetylornithine and Nɛ-acetyllysine) have been studied by using freeze-thaw cycles, but no thermal protection results have been reported. An important feature of this kind of compound is that the acetyl moiety must be attached to the N-terminal position ω to provide complete protection, and α-acetylated isomers are poorer protectors (6, 10). Although NADA very efficiently protected against LDH inactivation at 50°C, it did not have any stabilizing effect at 55°C. In contrast, solutes, such as mannosylglycerate, which are accumulated by thermophilic and hyperthermophilic organisms, and hydroxyectoine are able to provide remarkable protection against inactivation at high temperatures (10, 20).
It was not known until very recently that NADA plays a role in the osmotic adaptation of bacteria (6); here we show that this compound can also be added to the repertory of enzyme stabilizers. However, further studies on the stabilizing effect of NADA on a variety of enzymes subjected to different types of stress must be performed in order to evaluate the ability of NADA as a general enzyme stabilizer. In this respect, H. elongata salt-sensitive mutant CHR63 has great potential in biotechnology as a source of a cocktail of compatible solutes that comprehend primarily NADA and hydroxyectoine, two very efficient enzyme stabilizers.
The data for accumulation of intracellular solutes as a function of the NaCl concentration in the medium strongly suggest that synthesis of NADA, ectoine, and hydroxyectoine is osmoregulated in mutant CHR63. However, this organism is not able to grow in the presence of NaCl concentrations greater than 1.5 M (6), in contrast to the wild-type strain. This suggests that ectoines are probably more efficient osmolytes in vivo than NADA.
Although hydroxyectoine undoubtedly can be used as an enzyme-stabilizing agent (10), the biosynthetic pathway of this compound has not been determined. The results of previous studies performed with H. elongata mutants impaired in the synthesis of ectoine suggested that hydroxyectoine might be synthesized directly by ectoine hydroxylation (6, 13). In fact, interconversion of ectoine and hydroxyectoine was demonstrated by Göller et al. (13) with mutant SAA4, which contains a mutation in the same gene as H. elongata CHR62. These results were confirmed in the present work. In addition, we obtained evidence that there is an alternative route, in which NADA is converted into hydroxyectoine without the involvement of ectoine as an intermediate metabolite. In the pathway proposed here (Fig. 6), hydroxyectoine is synthesized via a two-step pathway involving hydroxylation of NADA to produce 3-hydroxyl-Nγ-acetyldiaminobutyrate, which is subsequently converted to hydroxyectoine by the action of a putative hydroxyectoine synthase. We looked for this activity in extracts of H. elongata CHR63 cells, but we failed to detect it, probably due to instability of the enzyme.
The observation that hydroxyectoine predominates over ectoine in organisms with high intracellular concentrations of NADA (mutants CHR63 and CHR62 grown in the presence of NADA) suggests that the first enzyme involved in the alternative pathway for synthesis of hydroxyectoine has a lower affinity for the substrate than ectoine synthase has. However, the relative contributions of the two pathways to synthesis of hydroxyectoine in the wild-type strain of H. elongata cannot be deduced from the data presented here. Work is in progress to fully elucidate the pathways for biosynthesis of hydroxyectoine by isolating the genes and characterizing the key enzymes.
ACKNOWLEDGMENTS
We thank Bitop GmbH for kindly providing ectoine and hydroxyectoine.
D. Cánovas and N. Borges contributed equally to this work.
D. Cánovas acknowledges a fellowship from the Spanish Ministerio de Educación y Ciencia. This work was supported by the European Commission BIOTECH Programme Extremophiles as Cell Factories (grant BIO4-CT96-0488), by PRAXIS XXI and FEDER, Portugal (grant PRAXIS/2/2.1/BIO/1109/95), by the Spanish Ministerio de Educación y Cultura (grants PB97-0722 and BIO97-1876-CE), and by the Junta de Andalucía.
REFERENCES
- 1.Bradford M M. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal Biochem. 1976;72:248–254. doi: 10.1016/0003-2697(76)90527-3. [DOI] [PubMed] [Google Scholar]
- 2.Brown A D. Microbial water stress. Bacteriol Rev. 1976;40:803–846. doi: 10.1128/br.40.4.803-846.1976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Cánovas D, Vargas C, Calderón M I, Ventosa A, Nieto J J. Characterization of the genes for the biosynthesis of the compatible solute ectoine in the moderately halophilic bacterium Halomonas elongata DSM 3043. Syst Appl Microbiol. 1998;21:487–497. doi: 10.1016/S0723-2020(98)80060-X. [DOI] [PubMed] [Google Scholar]
- 4.Cánovas D, Vargas C, Csonka L N, Ventosa A, Nieto J J. Osmoprotectants in Halomonas elongata: high-affinity betaine transport system and choline-betaine pathway. J Bacteriol. 1996;178:7221–7226. doi: 10.1128/jb.178.24.7221-7226.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Cánovas D, Vargas C, Csonka L N, Ventosa A, Nieto J J. Synthesis of glycine betaine from exogenous choline in the moderately halophilic bacterium Halomonas elongata. Appl Environ Microbiol. 1998;64:4095–4097. doi: 10.1128/aem.64.10.4095-4097.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Cánovas D, Vargas C, Iglesias-Guerra F, Csonka L N, Rhodes D, Ventosa A, Nieto J J. Isolation and characterization of salt-sensitive mutants of the moderate halophile Halomonas elongata and cloning of the ectoine synthesis genes. J Biol Chem. 1997;272:25794–25801. doi: 10.1074/jbc.272.41.25794. [DOI] [PubMed] [Google Scholar]
- 7.Cohen G N, Rickenberg R H. Concentration specifique reversible des aminoacides chez E. coli. Ann Inst Pasteur (Paris) 1956;91:693–720. [PubMed] [Google Scholar]
- 8.da Costa M S, Santos H, Galinski E A. An overview of the role and diversity of compatible solutes in Bacteria and Archaea. Adv Biochem Engl Biotechnol. 1998;61:117–153. doi: 10.1007/BFb0102291. [DOI] [PubMed] [Google Scholar]
- 9.Dubois M, Gilles K A, Hamilton J K, Rebers P A, Smith F. Colorimetric method for determination of sugars and related substances. Anal Chem. 1956;28:350–356. [Google Scholar]
- 10.Galinski E A. Compatible solutes of halophilic eubacteria: molecular principles, water-solute interaction, stress protection. Experientia. 1993;49:487–496. [Google Scholar]
- 11.Galinski E A. Osmoadaptation in bacteria. In: Poole R K, editor. Advances in microbial physiology. London, United Kingdom: Academic Press; 1995. pp. 273–328. [PubMed] [Google Scholar]
- 12.Galinski E A, Pfeiffer H P, Trüper H G. 1,4,5,6-Tetrahydro-2-methyl-4-pyrimidinecarboxylic acid. A novel cyclic amino acid from halophilic phototrophic bacteria of the genus Ectothiorhodospira. Eur J Biochem. 1985;149:135–139. doi: 10.1111/j.1432-1033.1985.tb08903.x. [DOI] [PubMed] [Google Scholar]
- 13.Göller K, Ofer A, Galinski E A. Construction and characterization of an NaCl-sensitive mutant of Halomonas elongata impaired in ectoine biosynthesis. FEMS Microbiol Lett. 1998;161:293–300. doi: 10.1111/j.1574-6968.1998.tb12960.x. [DOI] [PubMed] [Google Scholar]
- 14.Kollman V H, Hanner J L, London R E, Adame E G, Walker T E. Photosynthetic preparation and characterization of 13C-labeled carbohydrates in Agmenellum quadruplicatum. Carbohydr Res. 1979;73:193–202. [Google Scholar]
- 15.Lamosa, P., A. Burke, R. Peist, R. Huber, M. Y. Liu, G. Silva, C. Rodrigues-Pousada, J. LeGall, C. Maycock, and H. Santos. Unpublished data. [DOI] [PMC free article] [PubMed]
- 16.Liss I. N-Acetyldiamniobuttersäure, eine neue Aminosäure aus dem Latex von Euphorbia pulcherrima Willd ex Klotzsch. Phytochemistry. 1962;1:87–88. [Google Scholar]
- 17.Martins L O, Santos H. Accumulation of mannosylglycerate and di-myo-inositol-phosphate by Pyrococcus furiosus in response to salinity and temperature. Appl Environ Microbiol. 1995;61:3299–3303. doi: 10.1128/aem.61.9.3299-3303.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Ono H, Sawada K, Khunajakr N, Tao T, Yamamoto M, Hiramoto M, Shinmyo A, Takano M, Murooka Y. Characterization of biosynthetic enzymes for ectoine as a compatible solute in a moderately halophilic eubacterium, Halomonas elongata. J Bacteriol. 1999;181:91–99. doi: 10.1128/jb.181.1.91-99.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Peters P, Galinski E A, Trüper H G. The biosynthesis of ectoine. FEMS Microbiol Lett. 1990;71:157–162. [Google Scholar]
- 20.Ramos A, Raven N D H, Sharp R J, Bartolucci S, Rossi M, Cannio R, Lebbink J, Van der Oost J, de Vos W M, Santos H. Stabilization of enzymes against thermal stress and freeze-drying by mannosylglycerate. Appl Environ Microbiol. 1997;63:4020–4025. doi: 10.1128/aem.63.10.4020-4025.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Reed R H, Borowitzka L J, Mackay M A, Chudek J A, Foster R, Warr S R C, Moore D J, Stewart W D P. Organic solute accumulation and osmotic stress in cyanobacteria. J Gen Microbiol. 1984;130:1–4. [Google Scholar]
- 22.Robertson D E, Lai M C, Gunsalus R P, Roberts M F. Composition, variation, and dynamics of major osmotic solutes in Methanohalophilus strain FDF1. Appl Environ Microbiol. 1992;58:2438–2443. doi: 10.1128/aem.58.8.2438-2443.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Severin J, Wohlfarth A, Galinski E A. The predominant role of recently discovered tetrahydropyrimines for osmoadaptation of halophilic eubacteria. J Gen Microbiol. 1992;138:1629–1638. [Google Scholar]
- 24.Tuderman L, Myllylä R, Kilvirikko K I. Mechanism of the prolyl hydroxylase reaction. Eur J Biochem. 1977;80:341–348. doi: 10.1111/j.1432-1033.1977.tb11888.x. [DOI] [PubMed] [Google Scholar]
- 25.Vassault A. Lactate dehydrogenase. UV-method with pyruvate and NADH. In: Bergmeyer H U, editor. Methods of enzymatic analysis. 3rd ed. III. Weinheim, Germany: VCH; 1987. pp. 118–126. [Google Scholar]
- 26.Ventosa A, Nieto J J, Oren A. Biology of moderately halophilic aerobic bacteria. Microbiol Mol Biol Rev. 1998;62:504–544. doi: 10.1128/mmbr.62.2.504-544.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Vreeland R H, Litchfield C D, Martin E L, Elliot E. Halomonas elongata, a new genus and species of extremely salt-tolerant bacteria. Int J Syst Bacteriol. 1980;30:485–495. [Google Scholar]
- 28.Vreeland R H, Martin E L. Growth characteristics, effects of temperature, and ion specificity of the halotolerant bacterium Halomonas elongata. Can J Microbiol. 1980;26:746–752. [Google Scholar]
- 29.Wohlfarth A, Severin J, Galinski E A. The spectrum of compatible solutes in heterotrophic eubacteria of the family Halomonadaceae. J Gen Microbiol. 1990;136:705–712. [Google Scholar]