Abstract
Background and Objective
Nearly one-third of persons with epilepsy will continue having seizures despite trialing multiple antiseizure medications. Epilepsy surgery may be beneficial in these cases, and evaluation at a comprehensive epilepsy center is recommended. Numerous palliative and potentially curative approaches exist, and types of surgery performed may be influenced by center characteristics. This article describes epilepsy center characteristics associated with epilepsy surgery access and volumes in the United States.
Methods
We analyzed National Association of Epilepsy Centers 2019 annual report and supplemental survey data obtained with responses from 206 adult epilepsy center directors and 136 pediatric epilepsy center directors in the United States. Surgical treatment volumes were compiled with center characteristics, including US Census region. We used multivariable modeling with zero-inflated Poisson regression models to present ORs and incidence rate ratios of receiving a given surgery type based on center characteristics.
Results
The response rate was 100% with individual element missingness less than 4% across 352 observations undergoing univariate analysis. Multivariable models included 319 complete observations. Significant regional differences were present. The rates of laser interstitial thermal therapy (LITT) were lower at centers in the Midwest (incidence rate ratio [IRR] 0.74, 95% CI 0.59–0.92; p = 0.006) and Northeast (IRR 0.77, 95% CI 0.61–0.96; p = 0.022) compared with those in the South. Conversely, responsive neurostimulation implantation rates were higher in the Midwest (IRR 1.45, 95% CI 1.1–1.91; p = 0.008) and West (IRR 1.91, 95% CI 1.49–2.44; p < 0.001) compared with the South. Center accreditation level, institution type, demographics, and resources were also associated with variations in access and rates of potentially curative and palliative surgical interventions.
Discussion
Epilepsy surgery procedure volumes are influenced by US epilepsy center region and other characteristics. These variations may affect access to specific surgical treatments for persons with drug resistant epilepsy across the United States.
Epilepsy affects nearly 3.5 million people in the United States.1 Nearly 30% of people with epilepsy have drug resistant epilepsy (DRE), defined by refractory seizures despite appropriate treatment with 2 or more antiseizure medications.2,3 DRE is associated with increased morbidity and mortality, decreased quality of life, and increased health care utilization.4,5
Epilepsy surgery is an effective treatment for carefully selected persons with epilepsy. Resections for focal-onset seizures are superior to medical management alone and may be curative for carefully selected candidates.6-8 Others may benefit from palliative surgery such as neuromodulation and corpus callosotomy, which may reduce the frequency, duration, or severity of seizures.9-12
Surgical options for epilepsy have increased in number and complexity over time.13 Some etiologies and syndromes have multiple potential options. For instance, epilepsy secondary to mesial temporal sclerosis may be approached using anterior temporal lobectomy with amygdalohippocampectomy,6 stereotactic laser interstitial thermal therapy (LITT),14 or neuromodulatory therapy.15 Seizures due to Lennox-Gastaut syndrome (LGS) may be palliated with corpus callosotomy or vagus nerve stimulator (VNS) insertion.16 Clinical trials directly comparing various surgical approaches have not occurred. Choice of surgical procedure is often based on factors such as physician or patient preference and available resources.
Most epilepsy surgeries in the United States occur at an epilepsy center accredited by the National Association of Epilepsy Centers (NAEC). The NAEC collects data from its approximately 260 accredited epilepsy centers on the size and scope of epilepsy monitoring units, personnel, diagnostic testing, surgeries, and other services. Level 3 or level 4 centers are accredited based on center resources, with level 4 centers serving as regional or national referral sites with comprehensive diagnostic and surgical treatment capabilities.17 We hypothesized center characteristics influence surgical treatment practices. We designed and disseminated a supplemental survey to gather more information regarding testing and treatment practices pertaining to epilepsy surgery. We previously reported NAEC member center characteristics associated with presurgical test utilization.18 In this study, we describe center attributes associated with reported utilization of specific surgical techniques.
Methods
We analyzed merged data obtained from the 2019 annual report13 and a supplemental survey on epilepsy surgery practices in the year 2019 from all level 3 and level 4 NAEC epilepsy centers. Data were submitted by center directors, assessed for quality by comparing both to prior years and other centers, and discordant data were reviewed with the member center. The supplemental survey was sent separately to both the adult and pediatric center directors for combined adult/pediatric centers, for a total of 352 center directors surveyed. To combine data from the annual survey and the supplemental survey, surgery volume data from combined adult/pediatric center annual surveys were divided based on age category (younger than 18 years vs 18 years or older) and linked to the supplemental survey from that demographic center director (“pediatric combined” or “adult combined”). All reported data reflect pre–COVID-19 pandemic practices.
Statistical Analysis
Data from responses were described using frequency (percentage of nonmissing totals) for categorical variables and median (interquartile range) for continuous variables. Missing values of treatments were set to zero if meeting certain conditions. If all procedure volumes were missing from a given center, no imputation was performed. If only some procedure volumes were missing, those left blank were recorded as zero.
Separate regression models were built for each of the surgical treatments as dependent variables, which included temporal lobectomy, extratemporal resection, hemispherotomy/ectomy, LITT, corpus callosotomy, VNS implantation, and responsive neurostimulation (RNS) implantation. Deep brain stimulation cases were excluded because of lack of reliable reporting. Potential model independent variables included organization accreditation level (level 3 vs 4), center director demographic (pediatric vs adult patients), institution type (academic, private practice, or teaching affiliate), US geographic region (South, Midwest, Northeast, or West), number of epileptologists with 2 or more years of fellowship training, percent of resections performed with electrocorticography (ECOG; by 10% incremental increase), availability of image-guided robotics (yes vs no), availability of magnetoencephalography (MEG; yes vs no), availability of positron emission tomography (PET; yes vs no), and availability of single-photon emission computed tomography (SPECT; yes vs no). Variables that were highly imbalanced were not used in the multivariable models, and this most commonly was the distribution of treatment by the organization accreditation level.
Many centers reported performing none of any given treatment type (count = 0). Therefore, we used zero-inflated (ZIF) Poisson regression models18 for these highly skewed count data. These multivariable models had 2 components: one for modeling the count of treatments with Poisson regression and another for modeling excess zeros in the data. There are 2 sources of zeros in a ZIF Poisson model: excess zeros that come from the binary component and zeros that come from the count component.
Estimates from the first component (ZIF) of the ZIF Poisson model are presented as ORs. The original binary model captures the probability of no treatment. For ease of interpretation, we present inverted ORs to interpret odds of performing any of a given procedure. An OR greater than 1 indicates increased odds of performing the procedure; an OR less than 1 indicates decreased odds of performing that procedure. Estimates from the second component (Poisson regression) are presented as incidence rate ratios (IRRs). An IRR greater than 1 indicates a factor by which the treatment rate for a given surgery is higher than the rate for the reference category; an IRR less than 1 indicates the factor by which the rate is decreased compared with the reference category.
All statistical analyses were performed in R version 4.0 (R Core Team, Vienna, Austria) with reproducible programing in R Markdown. p values ≤ 0.05 were considered statistically significant. ZIF Poisson models were constructed with the zeroinfl function from R package pscl.19 Backward stepwise model selection based on the Akaike information criterion (AIC) was performed using the stepAIC function from R package MASS.20
Standard Protocol Approvals, Registrations, and Patient Consents
The ethical standards committee at Nationwide Children's Hospital determined this study exempt from institutional review board approval.
Data Availability
Qualified researchers may request data using the NAEC policy governing the release of member center data (naec-epilepsy.org/wp-content/uploads/NAECBoardPoliciesforDataAccess.pdf).
Results
Exploratory Characteristics
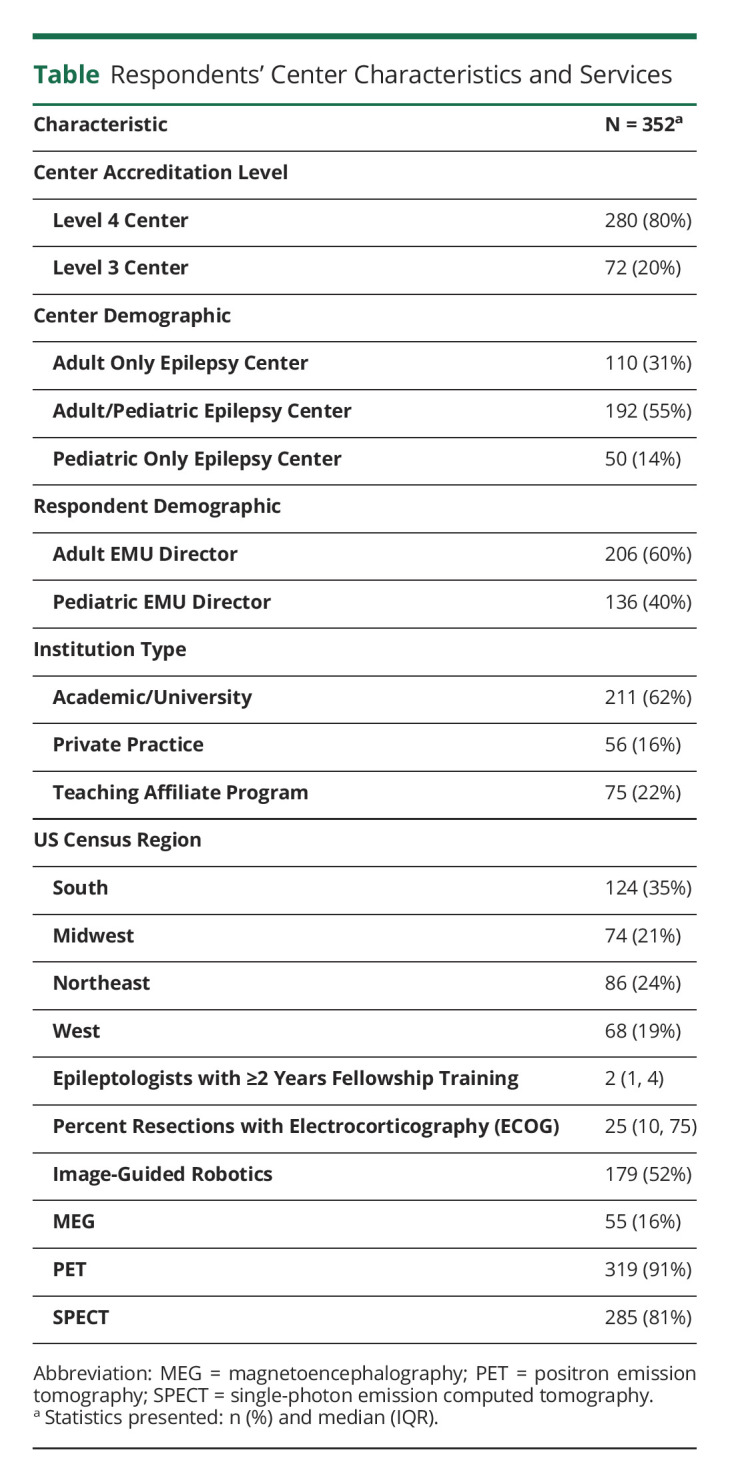
The overall response rate was 100%, although some fields were not completed across all respondents. A total of 352 observations were included. Level 4 EMU centers accounted for 280 (80%) of the observations, and 211 (62%) were characterized as academic/university type institutions. The respondents were 206 (60%) adult EMU directors and 136 (40%) pediatric EMU directors. Epilepsy center demographics and characteristics are summarized in Table. Degree of missingness for all variables was less than 4%. Only statistically significant outcomes are described.
Table.
Respondents' Center Characteristics and Services
Measuring Treatment Differences
Outcomes were described for ORs and IRRs for both potentially curative and palliative surgical procedures. ORs were quantified to capture the probability of performing at least one procedure of a given type at a respondent's center with a specified characteristic, holding all other variables constant in the model. IRR quantified the expected rate of treatment, or volume, from a respondent's center holding all other variables constant in the model.
Potentially Curative Surgeries
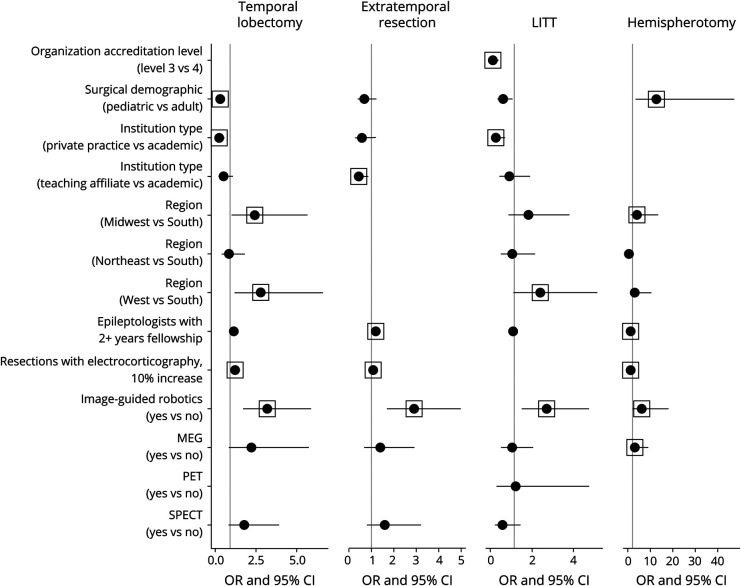
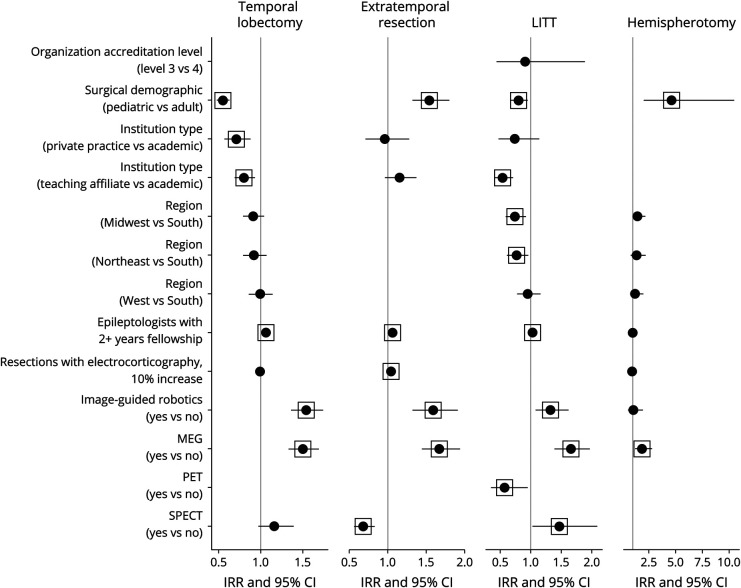
Potentially curative surgeries included temporal lobectomy, extratemporal resection, LITT, and hemispherotomy/ectomy. The odds of performing potentially curative surgeries were higher at centers with image-guided robotics (Figure 1), and treatment rates were higher at centers with MEG (Figure 2). Treatment rates were higher for each potentially curative procedure except hemispherotomy/ectomy at centers with a greater number of epileptologists with at least 2 years of fellowship training.
Figure 1. Forest Plot for OR (Zero-Inflated Component) of Potentially Curative Treatment Models.
Several independent variables were excluded by the model selection process using the AIC criteria, and some were manually removed because of imbalance between categories (see Supplementary Tables, links.lww.com/WNL/C466). Box indicates a statistically significant value (p ≤ 0.05). Abbreviation: AIC = Akaike information criterion.
Figure 2. Forest Plot for Incidence Rate Ratio (Count Component) of Potentially Curative Treatment Models.
Several independent variables were excluded by the model selection process using the AIC criteria, and some were manually removed because of imbalance between categories (see eTables 1–4, links.lww.com/WNL/C466). Box indicates a statistically significant value (p ≤ 0.05). Abbreviation: AIC = Akaike information criterion.
Temporal Lobectomy
Respondents from 208 (79%) level 4 centers and 9 (14%) level 3 centers reported performing at least 1 temporal lobectomy. Characteristics associated with higher odds (OR; 95% CI; p value) included location in the Midwest region (vs South; 2.43; 1.04–5.66; 0.039), West region (vs South; 2.8; 1.19–6.62; 0.019), and greater utilization of intraoperative ECOG (1.23; 1.12–1.36; <0.001). Odds were lower when reported by pediatric center directors (0.31; 0.16–0.59; <0.001) and those in private practice (vs academic; 0.25; 0.11–0.57; 0.001). The treatment rate (IRR; 95% CI; p value) was also lower when reported by pediatric center directors (0.55; 0.48–0.63; <0.001) and those in private practice (vs academic; 0.71; 0.57–0.88; 0.002).
Extratemporal Resection
At least 1 extratemporal resection was reported by 174 directors, including 169 (64%) at level 4 centers and 5 (7.7%) at level 3 centers. Odds were higher with greater utilization of intraoperative ECOG (1.09; 1.01–1.18; 0.019) and lower for directors at teaching affiliate centers (vs academic; 0.44; 0.23–0.86; 0.016). The treatment rate was higher when reported by pediatric center directors (1.54; 1.32–1.8; <0.001) and those with greater utilization of intraoperative ECOG (1.04; 1.02–1.07; <0.001). Availability of SPECT was associated with lower rates (0.68; 0.56–0.83; <0.001).
LITT
131 respondents reported performing at least 1 LITT, including 126 (48%) at level 4 centers and 5 (7.7%) at level 3 centers. Odds were greater in the West region (vs South; 2.4; 1.12–5.13; 0.024), and they were lower at level 3 (vs level 4; 0.12; 0.04–0.39; <0.001) and private practice (vs academic; 0.26; 0.09–0.7; 0.008) centers. The treatment rate was higher at centers with SPECT (1.47; 1.03–2.09; 0.035). The treatment rate was lower at centers with PET (0.57; 0.35–0.95; 0.031), teaching affiliate centers (vs academic; 0.54; 0.41–0.71; <0.001), when reported by pediatric center director respondents (0.8; 0.68–0.95; 0.012), or at centers in the Midwest (0.74; 0.59–0.92; 0.006) and Northeast (0.77; 0.61–0.96; 0.022) compared with centers in the South.
Hemispherotomy/Ectomy
At least 1 hemispherotomy/ectomy was reported by directors at 63 level 4 centers, most of whom (57%) were at pediatric-only centers. No level 3 centers performed this procedure. Odds were higher at centers in the Midwest region (vs South; 3.96; 1.17–13.43; 0.027) and those reporting greater utilization of intraoperative ECOG (1.26; 1.09–1.46; 0.001). Odds (12.71; 3.4–47.49; <0.001) and treatment rates (4.62; 2.04–10.47; <0.001) were higher when reported by pediatric center directors.
Palliative Surgeries
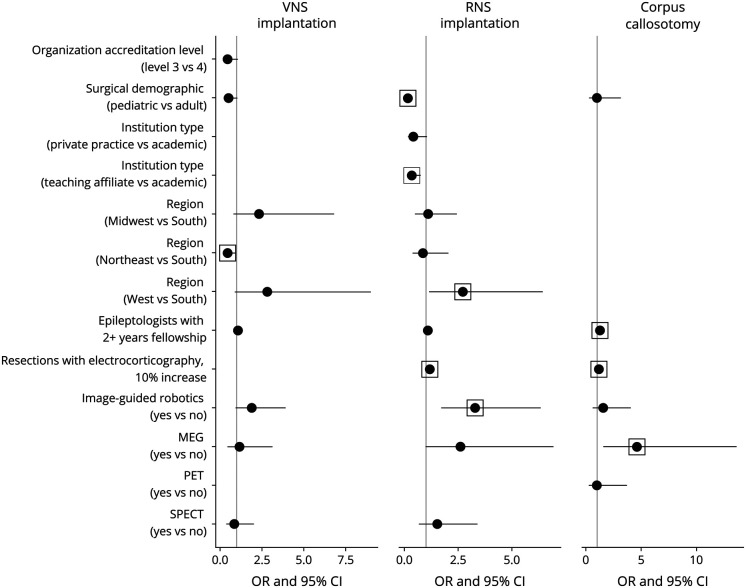
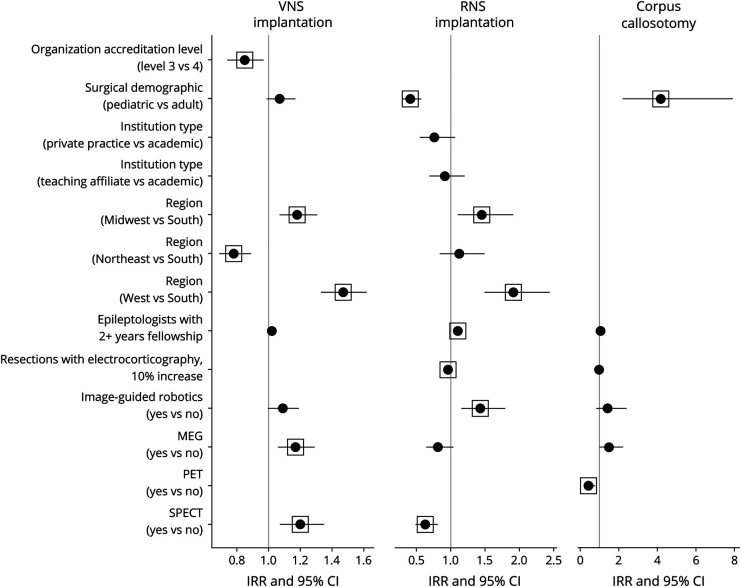
Palliative surgeries studied included VNS implantation, RNS implantation, and corpus callosotomy. Implantation rates of VNS and RNS were higher at centers with a greater number of epileptologists with at least 2 years of fellowship training and centers with access to image-guided robotics (Figures 3 and 4).
Figure 3. Forest Plot for OR (Zero-Inflated Component) of Palliative Treatment Models.
Several independent variables were excluded by the model selection process using the AIC criteria, and some were manually removed because of imbalance between categories (see eTables 1–4, links.lww.com/WNL/C466). Box indicates a statistically significant value (p ≤ 0.05). Abbreviation: AIC = Akaike information criterion.
Figure 4. Forest Plot for Incidence Rate Ratio (Count Component) of Palliative Treatment Models.
Several independent variables were excluded by the model selection process using the AIC criteria, and some were manually removed because of imbalance between categories (see eTables 1–4, links.lww.com/WNL/C466). Box indicates a statistically significant value (p ≤ 0.05). Abbreviation: AIC = Akaike information criterion.
VNS Implantation
276 center directors reported performing at least 1 VNS implantation at their site. Odds were lower in the Northeast (vs South; 0.45; 0.21–0.96; 0.039). The treatment rate was higher at centers with access to MEG (1.17; 1.06–1.29; 0.002) and SPECT (1.2; 1.07–1.35; 0.002) or those in the Midwest (1.18; 1.07–1.31; 0.001) and West (1.47; 1.33–1.62; <0.001) compared with the South. The treatment rate was lower at level 3 centers (0.85; 0.74–0.97; 0.013) and at those in the Northeast (vs South; 0.78; 0.69–0.89; <0.001).
RNS Implantation
At least 1 RNS implantation was reported by 134 center directors, including 131 (50%) at level 4 centers and 3 (4.6%) at level 3 centers. Odds were greater in the West region (vs South; 2.72; 1.15–6.45; 0.023) and those reporting a greater percentage of surgeries performed with ECOG (1.18; 1.07–1.31; 0.001). Odds were lower when reported by pediatric center directors (0.16; 0.07–0.34; <0.001) and those at teaching affiliate centers (vs academic; 0.34; 0.15–0.75; 0.008). The treatment rate was higher in the Midwest (1.45; 1.1–1.91; 0.008) and West (1.91; 1.49–2.44; <0.001) compared with the South. The treatment rate was lower at centers with greater utilization of intraoperative ECOG (0.96; 0.93–0.98; 0.003), with access to SPECT (0.63; 0.49–0.81; <0.001) and when reported by pediatric center directors (0.41; 0.29–0.57; <0.001).
Corpus Callosotomy
Respondents at 71 centers reported performing at least 1 corpus callosotomy, with 99% at level 4 academic (83%) or teaching affiliate (15%) centers. Higher odds were associated with greater utilization of intraoperative ECOG at the center (1.16; 1.02–1.32; 0.021) and access to MEG (4.6; 1.55–13.6; 0.006). Treatment rates were higher when reported by pediatric directors (4.17; 2.21–7.89; <0.001) and lower in centers with access to PET (0.43; 0.24–0.78; 0.005).
Discussion
We evaluated the association of epilepsy center characteristics on surgical treatments used for DRE to better understand epilepsy management in the United States. Epilepsy surgery is the best option for curing or palliating unrelenting seizures for persons with DRE, yet it remains underutilized.21-24 We previously reported variations in presurgical testing linked to center characteristics.18 This study identified additional epilepsy center characteristics associated with variations in access and volume of specific procedures in the United States.
The most novel findings relate to variations in specific procedures based on location. US census geographic regions drove differences in both potentially curative and palliative surgery types after correcting for other characteristics. For instance, the odds of performing a hemispherotomy/ectomy were nearly 4 times greater in the Midwest compared with the South. Location also influenced treatment volumes, as evidenced by centers in the West having a rate of RNS implantation almost 2 times greater than those in the South. Potential causes of geographic influences on epilepsy surgery utilization may include patient sociodemographics, payer practices, or the influence of training institution and relative proximity of eventual practice location.
Previous studies demonstrated other regional disparities in epilepsy care in the United States. The odds of obtaining presurgical neuropsychological testing for DRE are lower in the South compared with the Midwest. This may be due to a strain on resources, as a greater proportion of patients with epilepsy live in the South than in other regions of the United States,25-27 and all regions are undersupplied with neurologists aside from the Northeast.28 Seizure outcomes also vary with location. Persons in the Northeast receive care from epilepsy specialists more often than all other regions,26,27 and they report higher rates of seizure control compared with those in the South.26 The degree to which regional differences in testing and treatment interact, affect outcomes, and drive cost remains uncertain and requires further study.
Some associations were expected and confirmed. Level 4 centers had greater odds and treatment rates for each surgery type because they serve as regional or national referral sites with expertise in specialized neuroimaging, intracranial EEG, and more complex surgical techniques.17 This imbalance led to the exclusion of accreditation level from models for temporal lobectomy, extratemporal resections, corpus callosotomy, RNS implantation, and hemispherotomy/ectomy. Institution type was also excluded from corpus callosotomy and hemispherotomy/ectomy because most occur at academic or teaching affiliate programs. Only 52% of respondents have access to image-guided robotics, and yet, this factor was positively correlated with both greater odds of offering each procedure aside from VNS and corpus callosotomy as well as higher treatment volumes for temporal lobectomy, extratemporal resection, LITT, and RNS (Figures 1-4). Procedures more likely to be performed in children, including hemispherotomy, extratemporal resection, and corpus callosotomy, were associated with a higher OR in epileptologists with at least 2 years of fellowship training and a greater percentage of surgeries performed with ECOG, likely reflective of these procedures being concentrated in level 4 academic centers.
Recent advances in stereoelectroencephalography, LITT, and neuromodulation have expanded potential treatment approaches for DRE. For instance, temporal lobectomy and LITT may be appropriate options for a given patient, and the choice between the 2 options may be affected by center characteristics. The treatment rate for performing LITT was lower at centers with access to PET and in the Midwest and Northeast compared with the South, whereas it was higher at centers with SPECT. These influences were not present in temporal lobectomy treatment rates. Center features may also influence palliative choices, such as corpus callosotomy or VNS, 2 treatment options for LGS.29 Callosotomy had a higher treatment rate when reported by pediatric directors and at centers without PET, whereas VNS implantations were not affected by these characteristics. Additional interactions between center influences on presurgical testing and surgery may further affect access to specific treatments. Studying specific scenarios or patient cohorts across centers may further elucidate drivers for decision making.
Availability of MEG, PET and SPECT was associated with individual procedure access and volume. The odds of performing hemispherotomy/ectomy or corpus callosotomy were greater at centers with access to MEG, and treatment rates for most procedures were also higher at these institutions. By contrast, corpus callosotomy treatment rates were lower at centers with PET compared with those without, likely because of a large number of directors at centers with PET ([199 [75%]) not having performed a corpus callosotomy. Furthermore, access to PET was associated with lower LITT rates, and access to SPECT was associated with higher LITT and RNS rates but lower extratemporal resection rates. Centers with higher LITT rates may rely less on PET, especially in lesional temporal epilepsy. The opposite influences of SPECT on LITT and extratemporal resections may represent alternative approaches to similar patients as LITT becomes more common13 and as efficacy data emerge.30 Clinical trials directly examining LITT and resection are needed.
The results of this study are strengthened by the census survey methodology because of requirement for NAEC accreditation, with a 100% response rate and a low range of missingness. Future iterations of the survey may include required fields to improve data completeness. Findings are limited primarily by data acquisition methods because surgery numbers and other data are manually entered into the survey, rather than obtained from claims data or similar source. This analysis is limited to center characteristics and does not account for population characteristics, such as patient demographics, which would provide further insight on our initial findings. Although NAEC member centers do not provide the entirety of epilepsy care in the United States, they likely represent most of the specialized evaluations and procedures for those with DRE because limited available data suggest low utilization of epilepsy surgery outside NAEC member centers.13 Therefore, our analysis is likely generalizable regarding the current state of surgical treatment for DRE in the United States.
This study identifies effects of epilepsy center characteristics on surgical volumes, which may contribute to disparities in epilepsy surgery access.31 These findings provide a critical foundation to better examine outcomes for persons with DRE. Future work should examine the large-scale effects of referral collaborations, location, and payer mix on access to epilepsy surgery. In addition, comparative study of the impact of variation in presurgical testing and choice of surgery on patient outcomes and cost are urgently needed.
Acknowledgment
The authors would like to acknowledge the contributions of NAEC staff members Ellen Riker, Barbara Small, and Johanna Gray as well as the Medical Directors of NAEC Accredited Epilepsy Centers who submit data annually to NAEC.
Glossary
- AIC
Akaike information criterion
- DBS
deep brain stimulation
- DRE
drug resistant epilepsy
- ECOG
electrocorticography
- IRRs
incidence rate ratios
- LGS
Lennox-Gastaut syndrome
- LITT
laser interstitial thermal therapy
- MEG
magnetoencephalography
- NAEC
National Association of Epilepsy Centers
- PET
positron emission tomography
- RNS
responsive neurostimulation
- SPECT
single-photon emission computed tomography
- VNS
vagus nerve stimulator
- ZIF
zero-inflated
Appendix 1. Authors
Appendix 2. Coinvestigators

Study Funding
This study was supported by Award Number 45141-0001-0321 from Nationwide Children's Hospital and Award Number 810712-1221-00 from the National Association of Epilepsy Centers.
Disclosure
The authors report no disclosures relevant to the manuscript. Go to Neurology.org/N for full disclosures.
References
- 1.Zack MM, Kobau R. National and state estimates of the numbers of adults and children with active epilepsy - United States, 2015. MMWR Morb Mortal Wkly Rep. 2017;66(31):821-825. doi: 10.15585/mmwr.mm6631a1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Kwan P, Brodie MJ. Early identification of refractory epilepsy. N Engl J Med. 2000;342(5):314-319. doi: 10.1056/nejm200002033420503 [DOI] [PubMed] [Google Scholar]
- 3.Kwan P, Arzimanoglou A, Berg AT, et al. Definition of drug resistant epilepsy: consensus proposal by the ad hoc task force of the ILAE commission on therapeutic strategies: definition of drug resistant epilepsy. Epilepsia. 2009;51(6):1069-1077. doi: 10.1111/j.1528-1167.2009.02397.x [DOI] [PubMed] [Google Scholar]
- 4.Taylor RS, Sander JW, Taylor RJ, Baker GA. Predictors of health-related quality of life and costs in adults with epilepsy: a systematic review: Quality of Life and Costs in Epilepsy. Epilepsia. 2011;52(12):2168-2180. doi: 10.1111/j.1528-1167.2011.03213.x [DOI] [PubMed] [Google Scholar]
- 5.Ostendorf AP, Gedela S. Effect of epilepsy on families, communities, and society. Semin Pediatr Neurol. 2017;24(4):340-347. doi: 10.1016/j.spen.2017.10.007 [DOI] [PubMed] [Google Scholar]
- 6.Wiebe S, Blume WT, Girvin JP, Eliasziw M. A randomized, controlled trial of surgery for temporal-lobe epilepsy. N Engl J Med. 2001;345(5):311-318. doi: 10.1056/nejm200108023450501 [DOI] [PubMed] [Google Scholar]
- 7.Engel J, McDermott MP, Wiebe S, et al. Early surgical therapy for drug-resistant temporal lobe epilepsy: a randomized trial. JAMA. 2012;307(9):922-930. doi: 10.1001/jama.2012.220 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Dwivedi R, Ramanujam B, Chandra PS, et al. Surgery for drug-resistant epilepsy in children. N Engl J Med. 2017;377(17):1639-1647. doi: 10.1056/nejmoa1615335 [DOI] [PubMed] [Google Scholar]
- 9.Morrell MJ, RNS System, Epilepsy Study Group. Responsive cortical stimulation for the treatment of medically intractable partial epilepsy. Neurology. 2011;77(13):1295-1304. doi: 10.1212/wnl.0b013e3182302056.in. [DOI] [PubMed] [Google Scholar]
- 10.Graham D, Tisdall MM, Gill D. Corpus callosotomy outcomes in pediatric patients: a systematic review. Epilepsia. 2016;57(7):1053-1068. doi: 10.1111/epi.13408 [DOI] [PubMed] [Google Scholar]
- 11.Chan AY, Rolston JD, Lee B, Vadera S, Englot DJ. Rates and predictors of seizure outcome after corpus callosotomy for drug-resistant epilepsy: a meta-analysis. J Neurosurg. 2018:1-10. doi: 10.3171/2017.12.JNS172331 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Ryvlin P, Rheims S, Hirsch LJ, Sokolov A, Jehi L. Neuromodulation in epilepsy: state-of-the-art approved therapies. Lancet Neurol. 2021;20(12):1038-1047. doi: 10.1016/s1474-4422(21)00300-8 [DOI] [PubMed] [Google Scholar]
- 13.Ostendorf AP, Ahrens SM, Lado FA, et al. United States epilepsy center characteristics: a data analysis from the National Association of Epilepsy Centers. Neurology. 2022;98(5):e449-e458. doi: 10.1212/WNL.0000000000013130 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Kang JY, Wu C, Tracy J, et al. Laser interstitial thermal therapy for medically intractable mesial temporal lobe epilepsy. Epilepsia. 2016;57(2):325-334. doi: 10.1111/epi.13284 [DOI] [PubMed] [Google Scholar]
- 15.Geller EB, Skarpaas TL, Gross RE, et al. Brain-responsive neurostimulation in patients with medically intractable mesial temporal lobe epilepsy. Epilepsia. 2017;58(6):994-1004. doi: 10.1111/epi.13740 [DOI] [PubMed] [Google Scholar]
- 16.Rolston JD, Englot DJ, Wang DD, Garcia PA, Chang EF. Corpus callosotomy versus vagus nerve stimulation for atonic seizures and drop attacks: a systematic review. Epilepsy Behav. 2015;51:13-17. doi: 10.1016/j.yebeh.2015.06.001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Labiner DM, Bagic AI, Herman ST, Fountain NB, Walczak TS, Gumnit RJ. Essential services, personnel, and facilities in specialized epilepsy centers-Revised 2010 guidelines: guidelines for Specialized Epilepsy Centers. Epilepsia. 2010;51(11):2322-2333. doi: 10.1111/j.1528-1167.2010.02648.x [DOI] [PubMed] [Google Scholar]
- 18.Ahrens SM, Arredondo KH, Bagic AI, et al. Epilepsy center characteristics and geographic region influence presurgical testing in the United States. Epilepsia. Published online November 1, 2022. doi: 10.1111/epi.17452. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Lambert D. Zero-inflated Poisson regression, with an application to defects in manufacturing. Technometrics. 1992;34:1-14. doi: 10.2307/1269547 [DOI] [Google Scholar]
- 20.Zeileis A, Kleiber C, Jackman S. Regression models for count data in R. J Stat Softw. 2008;27(8):1-25. doi: 10.18637/jss.v027.i08 [DOI] [Google Scholar]
- 21.Venables W, Ripley B. Modern Applied Statistics with S, 4th ed: Springer; 2002. [Google Scholar]
- 22.Schiltz NK, Koroukian SM, Lhatoo SD, Kaiboriboon K. Temporal trends in pre-surgical evaluations and epilepsy surgery in the U.S. from 1998 to 2009. Epilepsy Res. 2013;103(2-3):270-278. doi: 10.1016/j.eplepsyres.2012.07.016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Pestana Knight EM, Schiltz NK, Bakaki PM, Koroukian SM, Lhatoo SD, Kaiboriboon K. Increasing utilization of pediatric epilepsy surgery in the United States between 1997 and 2009. Epilepsia. 2015;56(3):375-381. doi: 10.1111/epi.12912 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Okubo Y, Fallah A, Hayakawa I, Handa A, Nariai H. Trends in hospitalization and readmission for pediatric epilepsy and underutilization of epilepsy surgery in the United States. Seizure. 2020;80:263-269. doi: 10.1016/j.seizure.2020.05.013 [DOI] [PubMed] [Google Scholar]
- 25.Beatty CW, Lockrow JP, Gedela S, Gehred A, Ostendorf AP. The missed value of underutilizing pediatric epilepsy surgery: a systematic review. Semin Pediatr Neurol. 2021;39:100917. doi: 10.1016/j.spen.2021.100917 [DOI] [PubMed] [Google Scholar]
- 26.Pisu M, Kratt P, Faught E, et al. Geographic variation of epilepsy for older Americans: how close to the geographic variation of stroke?. Epilepsia. 2012;53(12):2186-2193. doi: 10.1111/j.1528-1167.2012.03640.x [DOI] [PubMed] [Google Scholar]
- 27.Tian N, Boring M, Kobau R, Zack MM, Croft JB. Active epilepsy and seizure control in adults—United States, 2013 and 2015. MMWR Morb Mortal Wkly Rep. 2018;67(15):437-442. doi: 10.15585/mmwr.mm6715a1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Szaflarski M, Wolfe JD, Tobias JGS, Mohamed I, Szaflarski JP. Poverty, insurance, and region as predictors of epilepsy treatment among US adults. Epilepsy Behav. 2020;107:107050. doi: 10.1016/j.yebeh.2020.107050 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Dall TM, Storm MV, Chakrabarti R, et al. Supply and demand analysis of the current and future US neurology workforce. Neurology. 2013;81(5):470-478. doi: 10.1212/wnl.0b013e318294b1cf [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Ostendorf AP, Ng Y-T. Treatment-resistant Lennox-Gastaut syndrome: therapeutic trends, challenges and future directions. Neuropsychiatr Dis Treat. 2017;13:1131-1140. doi: 10.2147/ndt.s115996 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Barot N, Batra K, Zhang J, et al. Surgical outcomes between temporal, extratemporal epilepsies and hypothalamic hamartoma: systematic review and meta-analysis of MRI-guided laser interstitial thermal therapy for drug-resistant epilepsy. J Neurol Neurosurg Psychiatry. 2022;93(2):133-143. doi: 10.1136/jnnp-2021-326185 [DOI] [PubMed] [Google Scholar]
- 32.Samanta D, Singh R, Gedela S, Scott Perry M, Arya R. Underutilization of epilepsy surgery: Part II: strategies to overcome barriers. Epilepsy Behav. 2021;117:107853. doi: 10.1016/j.yebeh.2021.107853 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Qualified researchers may request data using the NAEC policy governing the release of member center data (naec-epilepsy.org/wp-content/uploads/NAECBoardPoliciesforDataAccess.pdf).