Abstract
Severe acute respiratory coronavirus 2 (SARS-CoV-2) infection in the young and healthy usually results in an asymptomatic or mild viral syndrome, possibly through an erythropoietin (EPO)-dependent, protective evolutionary landscape. In the old and in the presence of co-morbidities, however, a potentially lethal coronavirus disease 2019 (COVID-19) cytokine storm, through unrestrained renin-angiotensin aldosterone system (RAAS) hyperactivity, has been described. Multifunctional microRNA-155 (miR-155) elevation in malaria, dengue virus (DENV), the thalassemias, and SARS-CoV-1/2, plays critical antiviral and cardiovascular roles through its targeted translational repression of over 140 genes. In the present review, we propose a plausible miR-155-dependent mechanism whereby the translational repression of AGRT1, Arginase-2 and Ets-1, reshapes RAAS towards Angiotensin II (Ang II) type 2 (AT2R)-mediated balanced, tolerable, and SARS-CoV-2-protective cardiovascular phenotypes. In addition, it enhances EPO secretion and endothelial nitric oxide synthase activation and substrate availability, and negates proinflammatory Ang II effects. Disrupted miR-155 repression of AT1R + 1166C-allele, significantly associated with adverse cardiovascular and COVID-19 outcomes, manifests its decisive role in RAAS modulation. BACH1 and SOCS1 repression creates an anti-inflammatory and cytoprotective milieu, robustly inducing antiviral interferons. MiR-155 dysregulation in the elderly, and in comorbidities, allows unimpeded RAAS hyperactivity to progress towards a particularly aggressive COVID-19 course. Elevated miR-155 in thalassemia plausibly engenders a favorable cardiovascular profile and protection against malaria, DENV, and SARS-CoV-2. MiR-155 modulating pharmaceutical approaches could offer novel therapeutic options in COVID-19.
Keywords: MicroRNA-155, Severe acute respiratory coronavirus 2, Renin-angiotensin aldosterone system, Angiotensin-converting enzyme 1, Angiotensin-converting enzyme 2, Erythropoietin
Introduction
Severe acute respiratory coronavirus 2 (SARS-CoV-2) infection causing coronavirus disease 2019 (COVID-19) emerged in the markets of Wuhan, People’s Republic of China in late 2019, and has since had the world in its grip in an unprecedented pandemic, challenging human health and economies [1]. COVID-19 demonstrates a highly variable and unpredictable course; asymptomatic or subclinical in some, inexplicably culminating into a catastrophic hyperinflammation and rapidly progressing to a potentially lethal cytokine storm in others, demanding sophisticated resources from strained health care systems [2]. Co-morbidities associated with chronic inflammatory states such as old age, smoking, hypertension, obesity, diabetes mellitus (DM), and cardiovascular disease (CVD), along with male gender, perilously predispose towards such unfortunate progression [2, 3]. Certain host attributes predict a severe course and impending lethality while various genetic determinants, environmental elements, geography, lifestyle behaviors, and early age, may engender SARS-CoV-2 protection [4, 5].
Upon host invasion, SARS-CoV-2 spike protein (S) interaction with angiotensin-converting enzyme (ACE)2 downregulates ACE2 expression in endothelial cells (EC) and impairs endothelial nitric oxide (NO) synthase (eNOS) activity and downstream NO bioavailability [6, 7]. ACE2 is an important peptide of the renin angiotensin aldosterone system (RAAS), with a crucial role in counterbalancing activation of ACE1 in the vascular endothelium of the lungs and kidneys by cleaving circulating proinflammatory Ang II to vaso-protective Ang 1–7 and promoting eNOS activation [8, 9]. Overwhelmingly generated via eNOS in ECs, NO production is fundamental in maintaining normal endothelial function and defense against insults, injuries, and inflammation [10, 11]. Bioavailable NO potently inhibits leukocyte adhesion and displays significant antithrombotic, antiproliferative, antioxidative, immunoregulatory and microbicidal properties [10, 11]. Thus, through the latent suppression of endothelial expression of ACE2 and eNOS/NO, SARS-CoV-2 renders ACE2’s function in maintaining homeostatic endothelial biology void, and may promote a state of RAAS hyperactivity with elevated angiotensin (Ang II) and aldosterone (ALD) levels and impaired NO bioavailability, all in unison, contributing to the endotheliitis, vascular leakage, and resultant organ injuries observed in COVID-19 [6–9, 12–18]. In the young, however, despite the fact that this ACE1/ACE2 imbalance would be additionally potentiated by lower nasal ACE2 expression, and plausibly also by the serendipitous presence of certain RAAS activating single nucleotide polymorphisms (SNP), this enhanced RAAS overstimulation is apparently well tolerated and in addition renders SARS-CoV-2 infection mild or asymptomatic [17, 19–27].
How do we reconcile these observations? First, why, and how does this SARS-CoV-2-induced pro-inflammatory RAAS state in children and young adults become beneficial for the host? Second, how is the principal ACE1/Ang II/Ang II type 1 receptor (AT1R) axis, that mediates this RAAS hyperactivity, controlled and prevented from deluging into an uninhibited state of a cataclysmic inflammatory response, and progressing to a potentially lethal cytokine storm [8, 28, 29]? Finally, why is this faculty lost in the presence of old age and co-morbidities [3, 29]?
We have put forward an evolutionary congruent, mechanistical explanation accounting for the interaction between host and SARS-CoV-2, that involves an early age, erythropoietin (EPO)-dependent, protective evolutionary landscape [2, 4, 5, 30]. Such an ancestral, protective EPO evolutionary landscape provides the host with a fitness advantage, forming constraints against pathogen adaptation and invasion [31, 32]. The source of this highly significant, age-dependent, anemia-independent EPO elevation observed during the first 13 years of life, highest in the youngest but declining during a child’s development, is unknown. It could be, reasonably, attributed to the significantly higher, age- and genotype-related ACE1 activities in serum and lower nasal ACE2 expression, physiologically found in newborns, healthy children, and teenagers but not in adults [24–26, 33–37]. These early age, physiological states, in certain individuals also serendipitously enhanced by RAAS activating SNPs, elevate ACE1 and would appear to promote a tolerable RAAS hyperactivity, that through elevated Ang II and ALD, both known master regulators of EPO secretion, can plausibly account for the elevated EPO levels seen in the young [31–35]. All molecules in this early age protective evolutionary landscape, involving RAAS-EPO-eNOS interactions, are under significant genetic control aiming to support, augment, and extend EPO elevation and eNOS activity upon pathogen insult, as witnessed by the thalassemias, and protective RAAS and eNOS single nucleotide polymorphisms (SNPs) in malaria and Dengue virus (DENV) infection [27, 31, 32, 38–40]. The resulting elevated EPO levels and the consequently enhanced EPO-eNOS/NO pathway responsiveness, are associated with a better outcome in children with cerebral malaria and may reasonably also promote an early age protection against SARS-CoV-2; indeed, children below the age of 5, when EPO response is maximal, generally experience asymptomatic or mild SARS-CoV-2 infections [4, 30–32, 41–45]. EPO’s non-erythropoietic, extensive tissue protective action is mediated through its immunological effects[30], and enhancement of eNOS/NO pathway activity and subsequently increased vaso-protective NO generation and bioavailability [46–49]. EPO and eNOS together are known to abrogate the NACHT, LRR, and PYD domains-containing protein (NLRP) 3 inflammasome, centrally involved in the development of SARS-CoV-2 endotheliitis, as well as effectively inhibit SARS-CoV-2 early replication and cell entry [16, 44, 47–52]. It is thus, evident, that the host intends, with this physiological, and genetically imprinted, RAAS hyperactivity, to ensure adequate EPO levels to support this EPO-mediated, age-dependent protective evolutionary landscape, plausibly explaining the first question regarding the uncomplicated and rare SARS-CoV-2 infections in the young [30]. While this physiological RAAS hyperactivity, enhanced by RAAS molecule SNPs, engenders beneficial evolutionary effects in the form of protection against malaria and SARS-CoV-2 at an early age, SNP effects can be pleiotropic, and may turn into a detrimental disadvantage in older individuals, as suggested by the malaria-hypertension hypothesis and their association with severe adult COVID-19 outcomes [28, 53–58]. This disadvantage presumably occurs through the loss of eNOS/NO protection and the unopposed action of Ang II via the AT1R, a central player in the RAAS that defines the biological efficacy of Ang II and mediates its vasoconstrictive and pro-inflammatory actions [8]. It is, consequently, imperative to answer the second and third questions, in order to understand how the host controls the resulting RAAS overactivity and diverts Ang II away from the AT1R, and why old age and co-morbidities impact on this ability.
The elusive regulator of the Ang II/AT1R axis within a RAAS hyperactive state
Receptor kinetic studies show that despite Ang II having similar affinities for both its receptors, AT2R stimulation will come into play only at unusually high circulating levels of Ang II, much higher than those needed for AT1R agonism [59]. Moreover, plasma Ang II rather than tissue Ang II is the agonist of AT2R, while the reverse applies to AT1R [59]. Consequently, since AT2R will only engage at high plasma Ang II concentrations and when most AT1R are occupied, the second question of how the Ang II/AT1R signaling is held under control, remains unanswered [59]. There is abundant information, expertly reviewed by Dhangadamajhi and Singh [28], that plasma Ang II in malaria, apart from being conducive to EPO secretion, also possesses immunomodulating properties and direct anti-plasmodial activity, able to inactivate up to 88% of plasmodial sporozoites [28, 60]. Ang II also seems to preserve blood–brain barrier (BBB) integrity, presumably by binding to AT2R [28]. Inhibition of Ang II/AT1R signaling, using pharmacological AT1R block (angiotensin receptor blockers (ARBs) or stimulation of Ang II/AT2R signaling, also appears to confer a survival benefit in an experimental model of CM [28]. Furthermore, studies on a human model of endogenous AT1R antagonism, in patients with Bartter's/Gitelman's syndrome (BS/GS), show that the elevated Ang II and ALD do not result in adverse cardiovascular phenotypes but instead, unopposed AT2R signaling may lie behind increased NO-bioavailability, increased NO-mediated vasodilation, normotension, elevated heme oxygenase 1 (HO-1) with increased plasma antioxidant power, along with elevated expression of EPO [33, 61]. This latter observation is of particularly interest considering that Ang II mediates its EPO secretion regulation through AT1R signaling [33–35, 62]! Evidently, Ang II–induced EPO production persists even when AT1R signaling is disrupted. Presumably, additional mechanisms preserving EPO formation come to play, such as hemodynamic effects and tissue oxygenation of EPO-producing cells, ALD via the mineralocorticoid receptor (MR), direct Ang II and ALD effects on hypoxia-inducible factor (HIF)-1α that induce EPO gene transcription, and compensatory Ang II activation of the AT2R [2, 33, 34, 61–64]. It is, thus, obvious that, RAAS regulation of EPO involves a summation of interconnecting mechanisms, and not exclusively Ang II/AT1R signaling. Enthrallingly, in BS/GS individuals, the endogenous, overactive RAAS environment, elevated Ang II, and ALD, created through AT1R downstream signal disruption, with the simultaneous absence of hypertension and vascular remodeling, apparently also renders them resistant against SARS-CoV-2 infections/COVID-19 [4, 65].
Based on the above intriguing evidence, one must seek what mechanisms the host excogitates that confer endogenous regulation of the AT1R: is it through downregulation of receptor expression, blunting of Ang II/AT1R interaction, dampening of direct Ang II pro-inflammatory effects, or a combination of all of the above? Recently, non-coding RNAs (ncRNA) have been associated with host cell antiviral defense mechanisms, including coronaviruses [66]. One type of such ncRNAs are microRNAs (miRNA), small (18–25 nucleotide long), non-coding, one-stranded RNA molecules, that can target and silence around 60% of all human genes through translational repression [67]. MiRNAs bind to the 3′ untranslated region (3′UTR) of a specific target mRNA, enhancing messenger RNA (mRNA) degradation and inhibiting protein translation, thereby repressing (silencing) gene expression [68]. Viral infections may force the host to elicit hitherto unknown, but evolutionary predetermined, defense programs through miRNA-induced alterations of its immune response [69]. Since a particular miRNA may target one or many different mRNAs while one mRNA may bind many miRNAs, the host can at the same time control diverse aspects of antiviral systemic and immune responses, all in a concerted effort to modulate feedback and control inflammation [67–70]. Moreover, the role of certain miRNAs in the regulation of endothelial function with respect to the RAAS and NO bioavailability, as well as their influence on cytokine and interferon modulation, highlights their potential involvement in the pathogenesis of SARS-CoV-2 and COVID-19 [71, 72].
The beauty: MicroRNA-155 target gene repertoire
Minimally detected under normal physiological conditions and mainly expressed in the thymus and spleen, miR-155 is an ancient, evolutionarily well-conserved miRNA, with distinct expression profiles and multifunctionality [70, 73, 74]. MiR-155 targets over 140 genes involved in numerous physiological and pathological processes including hematopoietic lineage differentiation, immunity, inflammation, cancer, CVD, DM, and particularly viral infections [70, 73–77]. MiR-155, is a key modulator of both innate and adaptive immune responses, with critical roles in viral and parasitic infections mounting ancestral mammalian host defense mechanisms against pathogen challenge [70, 73, 74]. MiR-155’s target genes that associate with host defense against malaria, dengue virus (DENV), influenza A, and SARS-CoV-2 infection with COVID-19 are, BTB and CNC homology 1, basic leucine zipper transcription factor 1 (BACH1), suppressor of cytokine signaling 1 (SOCS1), HIF-1α, Arginase-2 (ARG2), E26 Transformation-specific Sequence 1 (ETS-1) factor, and AGTR1 that encodes AT1R (Table 1) [73, 78–82].
Table 1.
Direct targets of microRNA-155 relevant to SARS-CoV-2
| Gene symbol | Full gene name | Action | References |
|---|---|---|---|
| AGTR1 | Angiotensin II type 1 receptor gene | Repressed expression mediates an endogenous AT1R antagonism | [70, 82] |
| ARG2 | Arginase-2 | Repression prevents l-arginine depletion, aids dendritic cell maturation, and averts lung pathologies | [78] |
| BACH1 | BTB and CNC homology 1, basic leucine zipper transcription factor 1 | Repressing BACH1 leads to anti-inflammatory, cytoprotective, antioxidant effects through HO-1, and to induction of antiviral interferon (IFN) | [73, 81] |
| ETS-1 | E26 Transformation-specific Sequence-1 | Repression negates some of Ang II effects involving gene regulation of inflammation, angiogenesis, and vascular remodeling | [70, 82, 83] |
| HIF-1a | Ηypoxia-inducible factor 1α | Promotes HIF-1α recovery and induces EPO gene transcription | [64] |
| SOCS1 | Suppressor of cytokine signaling 1 |
Repression of canonical negative regulation of type I IFN signaling leads to enhanced type I IFN-mediated antiviral response Enhances JAK2/Y343/STAT5 axis: a crucial mediator of EPO-mediated protection against ischemic injury |
[84, 85] |
Several studies have confirmed the prominent position of miR-155 in the regulation of inflammatory responses and RAAS/Ang II/AT1R effects in CVD [70, 82]. Most intriguingly, AT1R-mRNA is an authentic miR-155 target as are Arg2 and Ets-1 [70, 82]. As a repressor of AGTR1, ARG2, and ETS-1 expression, miR-155 has the potential to answer the second question we posed earlier on how the host reconciles a pathogen-induced overtly hyperactive RAAS state with a protective CV phenotype in SARS-CoV-2. Furthermore, repression or modulation of additional gene targets in Table 1 will induce and/or potentiate EPO’s favorable immunological, anti-inflammatory and cytoprotective evolutionary landscape (vide infra: hemoglobin E (HbE)/β-thalassemia) to fight off pathogen replication, imminent invasion, and lower the burden of infection.
The beast: the taming of SARS-CoV-2
In a young and/or previously healthy host, without evidence of comorbidities and/or pharmacological RAAS interventions, our proposed mechanistic pathway commences with the induction of miR-155 upon an impending pathogen invasion such as malaria, DENV, influenza A, or SARS-CoV-1/2. Elevated in-vitro and in-vivo miR-155 levels have been reported in all the above conditions [70, 73, 86–89]. Hadighi et al. found significantly elevated host miRNAs including miR-155 in patients infected with P. vivax [86]. SARS-CoV-1/2 reportedly induce a tenfold upregulation of the miR-155 host gene (MIR155HG) and trigger a 3–16-fold increase of miR-155 in-vitro [88]. SARS-CoV-2 appears to induce host innate immunity earlier and twice as effectively as CoV-1, including miR-155 [88]. In clinical SARS-CoV-2 infection, upregulated miR-155 levels have been reported to date, in all but two studies (Table 2) [90–99]. Elevated miR-155 levels could differentiate between different degrees of COVID-19 severity [90–99]. The contradictory findings in the two studies could be due to differences in sampling timing and elevated BMI and advanced age, factors known to be associated with blunted miR-155 expression [98–101].
Table 2.
In-vitro and clinical studies investigating miR-155 levels in COVID-19
| Authors | Type of study | Result |
|---|---|---|
| Wyler et al. [88] | In-vitro: SARS-CoV-2- infected Calu-3 cells | Ten-fold upregulation of the miR-155 host gene (MIR155HG) and a 3–16-fold increase of miR-155 |
| Haroun et al. [90] | Clinical study | Increased miR-155 expression level in COVID-19 patients vs. controls, in severe vs. moderate COVID-19 patients, and in non-survival vs. survival COVID-19 patients |
| Abbasi-Kolli et al. [91] | Clinical study | Significantly increased miR-155-5p levels in the acute phase of COVID-19 vs. a healthy control group |
| Garg et al. [92] | Clinical study | Significantly increased miR-155 levels in COVID‐19 patients vs. healthy controls. MiR-155 levels could distinguish between COVID‐19 and Influenza‐acute respiratory distress syndrome (ARDS) groups |
| Donyavi et al. [93] | Clinical study | Significantly upregulated miR-155-5p expression level in the COVID-19 group vs. controls. Significant inverse correlation between miR-155-5p and SARS-CoV-2 N-gene and RdRp-gene |
| Gedikbasi et al. [94] | Clinical study |
Significantly upregulated miR-155-5p levels in COVID-19 patients and associated with disease severity SOCS1 expression robustly and negatively correlated with miR-155 |
| Eyileten et al. [95] | Clinical study | MiR-155-5p expression levels differed between healthy individuals and COVID-19 patients and showed increasing trend at day-7 and day-21 after admission |
| Li et al. [96] | Clinical study | Markedly elevated miR-155 in mild/moderate COVID-19 disease vs. severe/critical disease and negative controls |
| Gaytán-Pacheco et al. [97] | Clinical study | Significant upregulation of miR-155 in severe COVID-19 patients versus negative controls |
| Giannella et al. [98] | Clinical study | Significantly downregulated miR-155 levels in severe vs. mild COVID-19, in ICU vs. non-ICU. Predicted increased risk of COVID-19-related sequelae and/or death |
| Kassif-Lerner et al. [99] | Clinical study | 2.5-fold and fivefold less circulating miR-155 in mild and severe COVID-19 disease, respectively, vs. healthy people |
COVID-19 coronavirus disease 2019, ICU intensive care unit, miR-155, MicroRNA-155, SARS-CoV-2 severe acute respiratory coronavirus 2
When SARS-CoV-2 binds to ACE2, its cognate receptor, the SARS-CoV-2 spike 1 protein (S)-ACE2 complex is internalized through AT1R-dependent endocytosis (Figs. 1, 2) and ACE2 is subsequently downregulated [102]. An immediate ACE1/ACE2 imbalance ensues, that strongly engages host humoral and tissue RAAS, leading to enhanced Ang II and ALD formation, while the protective arm of the RAAS is rendered void [2, 4]. As AT1R’s level of expression, defines the biological efficacy of Ang II and thus the degree of RAAS hyperactivity, miR-155’s robust AGTR1/AT1R-mRNA repression will reduce AT1R expression and membrane presence and blunt Ang II action through AT1R [70, 82, 103]. Persisting high plasmatic Ang II concentrations will now be diverted and increasingly engage the AT2R, increasing protective Ang II/AT2R signaling and eNOS/NO pathway activation [8]. AT2R and eNOS/NO are fundamentally involved in NLRP3 regulation, and robustly suppress NLRP3 activation and inflammatory cytokine release and pyroptosis, subsequently alleviating cardiopulmonary and cerebrovascular injury, cardiac remodeling, and inflammation (Fig. 2) [59, 73, 104–106]. In the absence of old age and comorbidities in SARS-CoV-2, miR-155’s purposeful overexpression appears to induce a hyperactive, albeit protective RAAS state, very similar to the one seen with ARBs in CV disease and in BS/GS [2, 61, 73, 104]. Pharmacological RAAS inhibition (RAASi) effects in SARS-CoV-2 have been actively debated [107]. MiR-155 levels are reportedly decreased in coronary artery disease (CAD) compared to healthy subjects and concurrent ARB or ACE inhibitor (ACEi) treatment induced further reduction in miR-155 levels versus no ARB/ACEi [108, 109]. It is thus plausible that, despite a valuable AT1R blockade, the observed miR-155 reduction by ARB/ACEi deprives the host of other miR-155-induced beneficial, antiviral, immunological and cytoprotective effects, or that RAASi is not as potent or efficient as miR-155-induced AT1R downregulation, to promote cardioprotection during COVID-19 (Table 1) [70]. Furthermore, ACEi treatment, known to inhibit EPO secretion through Ang II reduction, may negate EPO’s protective effects [34, 110, 111]. Moreover, while miR-155 levels in BG/GS patients have not been reported, it is worthwhile noting that miR-155 functions as a negative regulator of Ras homolog gene family, member A (RhoA) signaling, reportedly downregulated in BG/GS [112, 113].
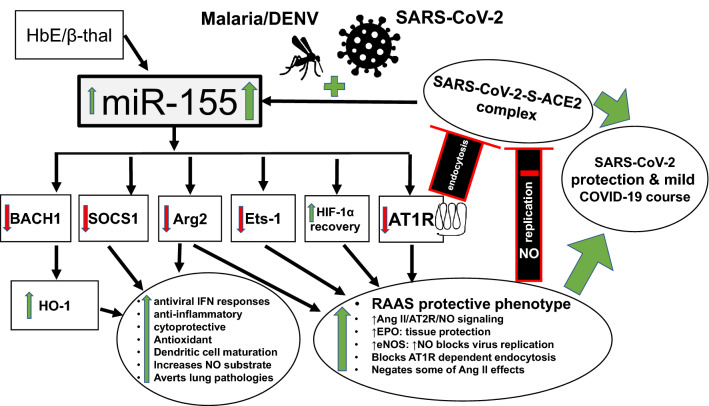
Fig. 1.
Severe acute respiratory coronavirus 2 (SARS-CoV-2) but also hemoglobin E (HbE)/β-thalassemia (β-thal), malaria and dengue virus (DENV) robustly increase miRNA-155 (miR-155) levels that, through translational repression of target genes, will lead to SARS-CoV-2 protection and/or asymptomatic or mild coronavirus disease 2019 (COVID-19) course. Repression of angiotensin II type 1 receptor (AT1R), arginase 2 (Arg2) and E26 Transformation-specific Sequence-1 (Ets-1) leads to a protective renin-angiotensin aldosterone system (RAAS) phenotype with erythropoietin (EPO) and endothelial nitric oxide (NO) synthase (eNOS) increase. Repression of BTB and CNC homology 1, basic leucine zipper transcription factor 1 (BACH1), suppressor of cytokine signaling 1 (SOCS1), and promotion of hypoxia-inducible factor 1α (HIF1α) recovery will enhance heme oxygenase (HO)-1 levels and induce anti-inflammatory and cytoprotective programs along with antiviral interferon (IFN) responses. Red colors and signs decrease or inhibit. Green colors and signs increase or stimulate/promote
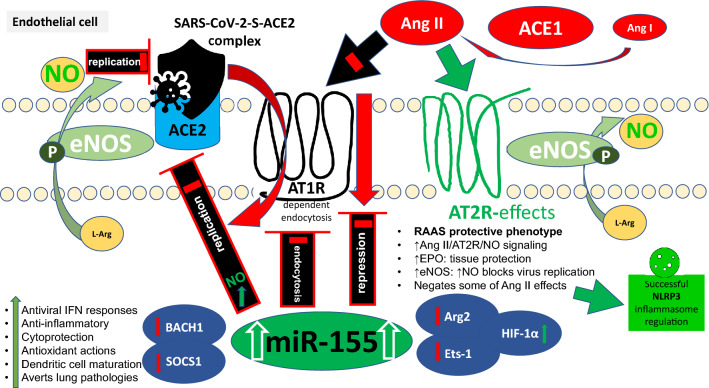
Fig. 2.
MiRNA-155 (miR-155)-induced angiotensin (Ang) II type 1 receptor (AT1R) downregulation and reduced membrane expression will inhibit Ang II pro-inflammatory and vasoconstrictive effects, impede severe acute respiratory coronavirus 2 (SARS-CoV-2) AT1R-dependent endocytosis, dissociate endothelial nitric oxide (NO) synthase (eNOS) from AT1R, and enhance its activity and NO bioavailability, consequently blocking virus replication, and cell entry. Moreover, elevated plasmatic Ang II will increasingly engage the AT2R and induce eNOS/NO-mediated vasculoprotective cellular pathways, resulting in successful regulation of NACHT, LRR, and PYD domains-containing protein (NLRP) 3 inflammasome. Repression of arginase 2 (Arg2) and E26 Transformation-specific Sequence-1 (Ets-1) will improve eNOS substrate availability and negate Ang II-induced endothelial and vascular inflammation, respectively, while hypoxia-inducible factor 1α (HIF1α) recovery will further enhance Ang II-mediated erythropoietin (EPO) secretion. BTB and CNC homology 1, basic leucine zipper transcription factor 1 (BACH1) and suppressor of cytokine signaling 1 (SOCS1) repression will induce robust anti-inflammatory, antioxidant, cytoprotective and interferon (IFN)-mediated antiviral programs
Given the AT1R-mediated signaling in EPO-producing renal cells one would expect that EPO should be reduced when miR-155 is elevated [33–35, 62]. However, Ang II stimulation of HIF-1α expression via AT2R-mediated posttranscriptional mechanism and miR-155’s actions on HIF-1α degradation can induce EPO formation and bypass the hurdle of AT1R repression (Table 1) [61, 64, 114]. The elevated EPO will be available to exert its tissue protective, antiapoptotic, anti-oxidative, and NLRP3 inflammasome abrogating, anti-inflammatory effects via the tissue protective receptor (TPR) that engages eNOS and increases NO bioavailability (Fig. 2) [4, 16, 50, 115, 116]. Interestingly, miR-155 also controls the Janus Kinase (JAK)2/Y343/STAT5 signaling axis required for EPO-mediated protection against renal ischemic injury (Table 1) [85]. Furthermore, increased eNOS activity promoted via unopposed AT2R signaling when AT1R is downregulated (similarly to an ARB block), and through eNOS-AT1R dissociation due to reduced AT1R membrane availability, will ultimately, further increase NO-bioavailability (Fig. 2) [73, 117, 118]. Increased NO bioavailability may halt SARS-CoV-2 infection at an early stage by inhibiting i) palmitoylation and fusion of the SARS-CoV-1/2 spike (S) protein to ACE2, and ii) early production of viral RNA, processes critical in controlling membrane fusion and virion infectivity (Fig. 2) [44]. As SARS-CoV-2-S-ACE2 complex is internalized through an AT1R-dependent endocytosis, reduced AT1R membrane presence through miR-155-induced AT1R repression could theoretically directly inhibit SARS-CoV-2 cell entry (Fig. 2) [102].
Furthermore, another direct target for miR-155, ARG2, constitutively expressed and also inducible in endothelial and kidney cells, is a critical regulator of L-arginine metabolism and NO synthesis, implicated in the development of endothelial dysfunction, CV disease, and diabetic nephropathy [78, 119, 120]. When repressed, Arg2 prevents the depletion of L-arginine, the obligate substrate of eNOS, leading to improved substrate availability and additional increases in NO-production and NO-bioavailability, further aiding the above-mentioned cardio- and renoprotective and antiviral actions (Table 1, Figs. 1, 2) [78, 119]. L-arginine is crucial in promoting dendritic cell maturation and their ability to drive T cell proliferation further improving antiviral responses [78, 79]. Low l-arginine levels impair T cell proliferation and IFN-γ production through reduced expression of the CD3ζ chain, a crucial part of the T-cell antigen receptor complex [121]. Moreover, Arg2 is essential for interleukin (IL)-10/miR-155 axis-induced metabolic reprogramming of inflammatory macrophages, including IL-1β secretion, deciding the fate of a cell’s inflammatory status [80]. Finally, deranged control of Arg2 repression by miR-155 is a potential parameter contributing to the pathogenesis of lung diseases, an observation pertinent to COVID-19 lung pathology[78].
Evincing miR-155’s decisive role in RAAS modulation
The link between miR-155 and its repression of the AT1R is particularly enthralling. The AT1R 1166A/C is a mirSNP (SNP disrupting microRNA targets) as it occurs in the AT1R 3′-UTR [70]. MiR-155 binding is, thus, disrupted in the + 1166C-allele harboring the SNP, as the target for its seed sequence binding to it is absent, rendering the AGTR1 gene with the + 1166C-allele unresponsive, and consequently only the + 1166A-allele expression can be downregulated [70, 103]. This observation biochemically accounts for the increased frequency of hypertension, CV, and metabolic disease associated with the + 1166C polymorphism, due to increased AT1R expression, additionally worsened by Ang II/AT1R downregulation of eNOS phosphorylation and potentially unfavorable eNOS polymorphisms [73, 122, 123]. Captivatingly, and maybe not unexpectedly, in carriers of AT1R + 1166C-allele, the severity of COVID-19 and oxygen dependency was higher compared to the A allele carriers[53]. This observation provides remarkable in-vivo evidence evincing that the impact of miR-155 on RAAS significantly influences COVID-19 course. In addition, reduced membrane expression of AT1R may aid in dampening persistent pro-inflammatory Ang II effects mediated through functional AT1R-autoantibodies (AT1-AA), that may arise through uncontrolled NLRP3-mediated pyroptosis [12, 124–126]. AT1-AAs significantly correlate with IL-6 levels and are implicated in the pathogenesis of systolic blood pressure, pre-eclampsia, and COVID-19 [127, 128].
Finally, Ets-1, acts as a transcriptional mediator of Ang II-induced endothelial and vascular inflammation, angiogenesis, and remodeling[83]. Ets-1 downstream targets include cyclin-dependent kinase inhibitor p21CIP (promoting hypertrophy in vascular smooth muscle cells and dysfunction and cell death in endothelial cells), plasminogen activator inhibitor–1 (PAI-1: critical determinant of the fibrinolytic system and contributing to the development of perivascular fibrosis), vascular cell adhesion molecule 1 (VCAM-1: cell adhesion molecule induced in inflammation), Fms Related Receptor Tyrosine Kinase 1 (FLT-1: a receptor for vascular endothelial growth factor involved in angio- and vasculogenesis), and monocyte chemoattractant protein–1 (MCP-1: mediates inflammatory response in hypertensive vascular disease)[83]. MiR-155 with two target sites in the 3’-UTR of ETS-1, robustly represses it, and its markedly upregulated downstream effectors, thus potently dampening Ang II’s direct pro-inflammatory cardiovascular effects (Table 1, Fig. 1) [82].
Too much of a good thing: excessive miR-155 levels
A vasoplegic state resembling profound RAAS inhibition (even in the absence of ACEi/ARBs), has been reported by certain research groups in sepsis and COVID-19 [129, 130]. MiR-155 elevation is part of an early-stage human septicemic response, peaking at 12 h and decreasing at 48 h, kinetics similar to its induction in SARS-CoV-2 infected human cell lines [74, 88]. However, contradictory miR-155 levels in sepsis underline the need of understanding how miR-155 is implicated in uncontrolled septic inflammatory responses. Elevated levels have been associated with severe condition, poor prognosis, and non-survival, while low levels are implicated in reduced survival in young (< 65 years) critically ill patients [74, 131]. Moreover, persistent miR-155 elevation could lead to decreased AT1R expression with excessive AT1R signaling impairment, contributing to sepsis-induced acute kidney injury [129]. Furthermore, protractedly elevated miR-155 levels may lead to impaired Ang II vascular reactivity due to synergism between AT1R downregulation and Ets-1 repression and could account for COVID-19-induced vasodilatory shock that may improve with Ang II infusion [132–134]. Low miR-155 levels on the other hand, as in the old and/or in comorbidities, could explain a RAAS hyperactive state with cytokine storm [76, 100, 101, 135]. It is to date unclear how miR-155 finetunes this delicate balance in sepsis, but age and comorbidities appear of paramount importance [74, 131]. Clearly more studies are needed to understand its molecular underpinnings in order to reduce excessive inflammation and alleviate tissue and organ damage through tissue and/or systemic miR-155-modulating pharmacological interventions.
MiR-155 engenders SARS-CoV-2 protection in hemoglobin E (HbE)/β-thalassemia carrier state
In our initial review on the HbE/β-thalassemia trait conferring resistance against SARS-CoV-2 infection, subsequently supported in independent reports, we proposed miR-155 as the mediator for this protection [5, 136–138]. Supporting an antiviral effect for the HbE/β-thalassemia trait, akin to its anti-malarial effect, red blood cell precursors in Thai carriers of HbE/β-thalassemia trait were significantly less susceptible to DENV infection[5, 139]. MiR-155 is elevated in HbE/β-thalassemia, enhancing monocyte erythrophagocytic activity, while its exogenous overexpression appears to limit DENV replication in-vitro [81, 140–142]. MiR-155 targets and downregulates BACH1, a sensor of heme levels and a strong repressor of the anti-inflammatory, cytoprotective, and antioxidant protein HO-1, ultimately leading to erythrophagocytosis and induction of antiviral interferon (IFN) responses (Table 1, Fig. 1) [70, 73, 81, 141, 143]. HO-1 is known to exhibit antiviral activity against human immunodeficiency and hepatitis B, C viruses[143]. The anti-DENV effects of HO-1 are exerted through its enzymatic product, biliverdin, an inhibitor of DENV proteases (NS2B/NS3), and DENV protease-suppressed antiviral IFN response is thereby rescued [70, 81, 143]. In experimental models of severe malaria and DENV, HO-1 was shown to control resistance and susceptibility to cerebral malaria and malaria-associated acute lung injury while its pharmacological induction with cobalt protoporphyrin (CoPPIX) reduced experimental cerebral malaria incidence, and demonstrated significant delay in DENV disease onset and mortality, along with lower cerebral DENV load [143, 144]. HO-1’s cytoprotective, anti-inflammatory, and antiviral properties may aid in SARS-CoV-2 protection as its upregulation by the SARS-CoV-2 S protein has been documented in-vitro and some repurposed drugs reportedly protective against COVID-19, increase HO-1 [143, 145, 146].
Furthermore, SOCS1, a canonical negative regulator of type I IFN signaling, is targeted by miR-155 in macrophages, and SOCS1 knockdown mediates the enhancing effect of miR-155 on type I IFN-mediated antiviral response (Table 1, Fig. 1) [84]. MiR-155’s central role in host defense in a model of coronavirus-induced neurological disease underscores its importance in enhancing antiviral T cell responses including IFN-γ secretion, cytolytic activity, and homing to the central nervous system (CNS) in response to viral infection [147]. Aggravated disease course, increased morbidity/mortality, and an inability to control viral replication within the CNS was reported in miR-155-knockout (KO) mice[147]. Induction of ectopic upregulation of miR-155 in the liver of mice using hepatotropic adeno-associated virus 8 (AAV8) vectors achieved complete protection against infectious parasite challenge through direct suppression of SOCS1 [148]. MiR-155 mediated downregulation of AT1R (vide supra) in antigen-specific CD8 + T cells could affect a variety of downstream functions ushering the host towards a malaria protective phenotype, highlighting miR-155’s fundamental role in Plasmodium liver infection in-vivo [148–150]. As previously mentioned, Hadighi et al. found significantly elevated host miRNAs, including miR-155, in patients infected with P. vivax [86].
Elevated miR-155 levels with repression of relevant target genes could convincingly account for the favorable inflammatory profile (Ets-1 repression with lower PAI-1 levels), better lipidemic and metabolic profile (SOCS1), better ambulatory blood pressure control (lower AT1R expression), and EPO-mediated renoprotection against ischemic injury through the SOCS1/JAK2/Y343/STAT5 axis, altogether leading to overall better CV health, repeatedly reported in HbE/β-thalassemia carrier state [151–153]. An advantageous basal health condition in thalassemia carriers at the time of the initiation of SARS-CoV-2 infection, may potentially lead to a more favorable COVID-19 outcome [138]. MiR-155 can thus eloquently engender HbE/β-thalassemia’s protective effects in malaria, DENV, and SARS-CoV-2 (Fig. 1) [4, 5, 81, 85, 86, 89, 139, 141, 142, 148–150, 154].
Conclusions and future perspectives
As a multifunctional miRNA, miR-155 critically modulates innate, humoral, and cellular immune responses during viral infections [155]. In the current review, we propose that the elevated miR-155 levels in SARS-CoV-2 infection appear, anticipatorily and purposefully, to prepare the host for a SARS-CoV-2-S-ACE2-induced RAAS hyperactivity [88, 90–99]. At a young age, and in the absence of comorbidities or pharmacological interventions, a judiciously initiated and choreographed miR-155 circuitry is expected to promote immediate, early, and late protection against SARS-CoV-2 and its complications[2, 4, 70, 73, 85, 156]. MiR-155-mediated translational repression of AGTR1, ARG2 and ETS-1 (Table 1), purposefully tames this SARS-CoV-2-induced RAAS hyperactivity into a balanced, tolerable, and defensive RAAS state, that through AT2R, promotes a protective EPO evolutionary landscape and NLRP3 inflammasome regulation (Fig. 1, 2) [30, 106]. MiR-155 engendered AT1R downregulation and reduced membrane availability coaxes a RAAS cardioprotective state [73], avails increased eNOS/NO pathway activation [47, 157], the latter further potentiated by Arg2 repression [80, 119, 158], leading to increased NO-bioavailability and impaired AT1R-mediated endocytosis, SARS-CoV-2 replication, and cell entry (Fig. 2) [44, 102]. Furthermore, Ets-1 repression negates proinflammatory Ang II effects [83]. Disrupted miR-155 repression of the AT1R + 1166C-allele, associated with adverse CV and COVID-19 outcomes, biochemically manifests this miR’s decisive role in RAAS modulation [53, 122, 123]. Finally, BACH1 and SOCS1 repression enhances host antiviral responses to fight off pathogen invasion, simultaneously creating an anti-inflammatory, cytoprotective, antioxidant milieu, through HO-1 increase, that robustly lowers inflammatory burden (Fig. 1) [73, 81, 84, 85]. In situations when miR-155 homeostasis is compromised (T2DM, sarcopenia, obesity, smoking, aging, male gender, CVD and renal disease, or pharmacological interventions), unimpeded RAAS stimulation and inappropriately low EPO levels, with subsequent EPO/eNOS-NO protection override, may allow NLRP3 dysregulation, and progress towards a particularly aggressive COVID-19 course with aberrant immune response and immunopathological consequences [2, 4, 63, 76, 77, 100, 101, 109, 135, 159, 160]. Genetic variants of the molecules in the RAAS and of eNOS may additionally and differentially impact on SARS-CoV-2 protection [30].
MiR-155 convincingly integrates disparate evidence in SARS-CoV-2 infection and COVID-19 course and appears as a valuable diagnostic marker and prognostic tool [90–93]. Further studies on miR-155, and other miRNAs and their genetic polymorphisms, will clarify discrepancies in their differentially expressed miRNA profiles and improve our understanding of their pathophysiology. Tissue specific studies and characterization of miR-155 temporal expression trajectory, are particularly important. MiR-155 modulation approaches could offer innovative prevention and treatment strategies, but specific and directed tissue, rather than global modulation, might offer superior therapeutic advantages [87, 135]. ARDS in influenza A responded better with lung alveolar type II cell miR-155 inhibition rather than global inhibition that also involved miR-155 from inflammatory leukocytes that invaded the lung at a later stage [87]. In the present clinical management of COVID-19, careful combination of MR-antagonists (aldactone, eplerenone, finerenone) to avoid hyperkalemia, with a calcium channel blocker may prove effective in the elderly [135, 161]. MR inhibition will rescue and restore the profoundly low basal serum miR-155 levels in the aging vasculature and block two sequential steps involving miR-155 targets, Cav1.2 (L-type calcium channel (LTCC) subunit) and AT1R that contribute to hypertension [135]. On the other hand, ARB or ACEi treatment further reduces miR-155 levels versus no ARB/ACEi [108]. Moreover, adding metformin in selected patient groups (obesity and T2DM) might confer additional benefits since metformin therapy prior to admission in patients with COVID-19 and pre-existing T2DM is associated with a significant reduction of in-hospital mortality [162]. Metformin has also been reported to improve high fat-induced inflammation in vascular endothelium through increased expression of miR-155 levels [163].
In the coevolutionary virus-host arms race, viral miRNAs have possibly evolved to exploit pre-existing host gene regulatory pathways, in yet unknown ways, to provide a viral replicative advantage [164]. Kaposi's-sarcoma-associated herpes virus, Marek’s disease virus, and Ebola virus, all encode miR-155 analogs, while Epstein Barr virus can even induce host miR-155 [73, 88]. It is thus, conceivable, that viral miR-155 induction is an invasive viral strategy in SARS-CoV-2 infection [73, 88]. Numerous miRNAs are part of the vast and intricately coordinated processes, interactively affecting multiple regulatory pathways [68]. We undoubtedly acknowledge that additional miRNAs are involved in the etiopathology of SARS-CoV-2. However, our translational approach on miR-155 offers an understanding of this multifunctional miRNA’s permeative effects in health and disease and highlights the need for circulating miRNA profiling to identify clusters and signatures, that will aid in patient stratification and treatment.
Author contributions
KIP conceived and conceptualized the pathophysiology, designed the review, drafted the initial manuscript, and reviewed and revised the manuscript. AP performed the literature search, extracted vital information, contributed to the synthesis of the review, and reviewed and revised the manuscript. T-CA coordinated and supervised literature search, made substantial and direct intellectual contributions and critically reviewed the manuscript for important intellectual content. All authors approved the submitted final manuscript and agree to be accountable for all aspects of the work.
Funding
No financial support has been received in any form.
Data availability
All data analysed during this narrative review are included in this published article.
Declarations
Conflict of interest
The authors have no relevant financial or non-financial interests to disclose.
Ethics approval
Not applicable.
Informed consent
Not applicable.
Footnotes
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Contributor Information
K. I. Papadopoulos, Email: kostas@thaistemlife.co.th
A. Papadopoulou, Email: Alexandra.Papadopoulou3@gmail.com
T. C. Aw, Email: tarchoon@gmail.com
References
- 1.Maxmen A. Wuhan market was epicentre of pandemic's start, studies suggest. Nature. 2022;603(7899):15–16. doi: 10.1038/d41586-022-00584-8. [DOI] [PubMed] [Google Scholar]
- 2.Papadopoulos KI, Sutheesophon W, Aw T-C. The influence of renin angiotensin aldosterone system (RAAS), endothelial nitric oxide synthase (eNOS) and erythropoietin (EPO) on COVID-19 complications. Chem Biol Interact. 2022;354:109834. 10.1016/j.cbi.2022.109834. [DOI] [PMC free article] [PubMed]
- 3.Sudhakar M, Winfred SB, Meiyazhagan G, Venkatachalam DP. Mechanisms contributing to adverse outcomes of COVID-19 in obesity. Mol Cell Biochem. 2022;477(4):1155–1193. doi: 10.1007/s11010-022-04356-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Papadopoulos KI, Sutheesophon W, Manipalviratn S, Aw TC. Age and genotype dependent erythropoietin protection in COVID-19. World J Stem Cells. 2021;13(10):1513–1529. doi: 10.4252/wjsc.v13.i10.1513. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Papadopoulos KI, Sutheesophon W, Manipalviratn S, Aw TC. A Southeast Asian Perspective on the COVID-19 pandemic: hemoglobin E (HbE)-trait confers resistance against COVID-19. Med Sci Monit Basic Res. 2021;27:e929207. 10.12659/MSMBR.929207. [DOI] [PMC free article] [PubMed]
- 6.Varga Z, Flammer AJ, Steiger P, Haberecker M, Andermatt R, Zinkernagel AS, et al. Endothelial cell infection and endotheliitis in COVID-19. Lancet. 2020;395(10234):1417–1418. doi: 10.1016/s0140-6736(20)30937-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Lei Y, Zhang J, Schiavon CR, He M, Chen L, Shen H, et al. SARS-CoV-2 spike protein impairs endothelial function via downregulation of ACE 2. Circ Res. 2021;128(9):1323–1326. doi: 10.1161/CIRCRESAHA.121.318902. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Benigni A, Cassis P, Remuzzi G. Angiotensin II revisited: new roles in inflammation, immunology and aging. EMBO Mol Med. 2010;2(7):247–257. doi: 10.1002/emmm.201000080. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Sampaio WO, Souza dos Santos RA, Faria-Silva R, da Mata Machado LT, Schiffrin EL, Touyz RM. Angiotensin-(1–7) through receptor Mas mediates endothelial nitric oxide synthase activation via Akt-dependent pathways. Hypertension. 2007;49(1):185–92. 10.1161/01.HYP.0000251865.35728.2f. [DOI] [PubMed]
- 10.Zhao Y, Vanhoutte PM, Leung SWS. Vascular nitric oxide: beyond eNOS. J Pharmacol Sci. 2015;129(2):83–94. doi: 10.1016/j.jphs.2015.09.002. [DOI] [PubMed] [Google Scholar]
- 11.Förstermann U, Münzel T. Endothelial nitric oxide synthase in vascular disease: from marvel to menace. Circulation. 2006;113(13):1708–1714. doi: 10.1161/circulationaha.105.602532. [DOI] [PubMed] [Google Scholar]
- 12.Ratajczak MZ, Bujko K, Ciechanowicz A, Sielatycka K, Cymer M, Marlicz W, et al. SARS-CoV-2 entry receptor ACE2 is expressed on very small CD45(-) precursors of hematopoietic and endothelial cells and in response to virus spike protein activates the Nlrp3 Inflammasome. Stem Cell Rev Rep. 2021;17(1):266–277. doi: 10.1007/s12015-020-10010-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Teuwen LA, Geldhof V, Pasut A, Carmeliet P. COVID-19: the vasculature unleashed. Nat Rev Immunol. 2020;20(7):389–391. doi: 10.1038/s41577-020-0343-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Camargo RL, Bombassaro B, Monfort-Pires M, Mansour E, Palma AC, Ribeiro LC et al. Plasma angiotensin II is increased in critical coronavirus disease 2019. Front Cardiovasc Med. 2022;9:847809. 10.3389/fcvm.2022.847809. [DOI] [PMC free article] [PubMed]
- 15.Carpenter RM, Young MK, Petri WAO, Lyons GR, Gilchrist C, Carey RM et al. Repressed Ang 1–7 in COVID-19 is inversely associated with inflammation and coagulation. mSphere. 2022:e0022022. 10.1128/msphere.00220-22. [DOI] [PMC free article] [PubMed]
- 16.Sogawa Y, Nagasu H, Itano S, Kidokoro K, Taniguchi S, Takahashi M et al. The eNOS-NO pathway attenuates kidney dysfunction via suppression of inflammasome activation in aldosterone-induced renal injury model mice. PLoS One. 2018;13(10):e0203823. 10.1371/journal.pone.0203823 [DOI] [PMC free article] [PubMed]
- 17.Villard O, Morquin D, Molinari N, Raingeard I, Nagot N, Cristol JP et al. The plasmatic aldosterone and C-reactive protein levels, and the severity of covid-19: the Dyhor-19 Study. J Clin Med. 2020;9(7). 10.3390/jcm9072315. [DOI] [PMC free article] [PubMed]
- 18.Bruder-Nascimento T, Ferreira NS, Zanotto CZ, Ramalho F, Pequeno IO, Olivon VC, et al. NLRP3 inflammasome mediates aldosterone-induced vascular damage. Circulation. 2016;134(23):1866–1880. doi: 10.1161/circulationaha.116.024369. [DOI] [PubMed] [Google Scholar]
- 19.Jackson CB, Farzan M, Chen B, Choe H. Mechanisms of SARS-CoV-2 entry into cells. Nat Rev Mol Cell Biol. 2021 doi: 10.1038/s41580-021-00418-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Verdecchia P, Cavallini C, Spanevello A, Angeli F. The pivotal link between ACE2 deficiency and SARS-CoV-2 infection. Eur J Intern Med. 2020;76:14–20. doi: 10.1016/j.ejim.2020.04.037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Angeli F, Zappa M, Reboldi G, Trapasso M, Cavallini C, Spanevello A, et al. The pivotal link between ACE2 deficiency and SARS-CoV-2 infection: 1 year later. Eur J Intern Med. 2021;93:28–34. doi: 10.1016/j.ejim.2021.09.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Wu Z, Hu R, Zhang C, Ren W, Yu A, Zhou X. Elevation of plasma angiotensin II level is a potential pathogenesis for the critically ill COVID-19 patients. Crit Care. 2020;24(1):290. doi: 10.1186/s13054-020-03015-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Osman IO, Melenotte C, Brouqui P, Million M, Lagier JC, Parola P et al. Expression of ACE2, Soluble ACE2, Angiotensin I, Angiotensin II and Angiotensin-(1–7) Is modulated in COVID-19 patients. Front Immunol. 2021;12:625732. 10.3389/fimmu.2021.625732. [DOI] [PMC free article] [PubMed]
- 24.Bunyavanich S, Do A, Vicencio A. Nasal gene expression of angiotensin-converting enzyme 2 in children and adults. JAMA. 2020;323(23):2427–2429. doi: 10.1001/jama.2020.8707. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Heinonen S, Helve O, Andersson S, Janér C, Süvari L, Kaskinen A. Nasal expression of SARS-CoV-2 entry receptors in newborns. Arch Dis Child Fetal Neonatal Ed. 2022;107(1):95–97. doi: 10.1136/archdischild-2020-321334. [DOI] [PubMed] [Google Scholar]
- 26.Hasan MR, Ahmad MN, Dargham SR, Zayed H, Al Hashemi A, Ngwabi N et al. Nasopharyngeal expression of angiotensin-converting enzyme 2 and transmembrane serine protease 2 in children within SARS-CoV-2-infected family clusters. Microbiol Spectr. 2021;9(3):e0078321. 10.1128/Spectrum.00783-21. [DOI] [PMC free article] [PubMed]
- 27.Dhangadamajhi G, Mohapatra BN, Kar SK, Ranjit M. Gene polymorphisms in angiotensin I converting enzyme (ACE I/D) and angiotensin II converting enzyme (ACE2 C–>T) protect against cerebral malaria in Indian adults. Infect Genet Evol. 2010;10(2):337–341. doi: 10.1016/j.meegid.2010.01.009. [DOI] [PubMed] [Google Scholar]
- 28.Dhangadamajhi G, Singh S. Malaria link of hypertension: a hidden syndicate of angiotensin II, bradykinin and sphingosine 1-phosphate. Hum Cell. 2021;34(3):734–744. doi: 10.1007/s13577-021-00513-3. [DOI] [PubMed] [Google Scholar]
- 29.Augustine R, S A, Nayeem A, Salam SA, Augustine P, Dan P et al. Increased complications of COVID-19 in people with cardiovascular disease: role of the renin-angiotensin-aldosterone system (RAAS) dysregulation. Chem Biol Interact. 2021;351:109738. 10.1016/j.cbi.2021.109738. [DOI] [PMC free article] [PubMed]
- 30.Papadopoulos KI, Papadopoulou A, Aw T-C. A protective erythropoietin evolutionary landscape, NLRP3 inflammasome regulation, and multisystem inflammatory syndrome in children. Hum Cell. 2022 doi: 10.1007/s13577-022-00819-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.O'Donnell A, Premawardhena A, Arambepola M, Allen SJ, Peto TE, Fisher CA, et al. Age-related changes in adaptation to severe anemia in childhood in developing countries. Proc Natl Acad Sci USA. 2007;104(22):9440–9444. doi: 10.1073/pnas.0703424104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Abugri J, Tetteh JK, Oseni LA, Mensah-Brown HE, Delimini RK, Obuobi DO, et al. Age-related pattern and monocyte-acquired haemozoin associated production of erythropoietin in children with severe malarial anaemia in Ghana. BMC Res Notes. 2014;7:551. doi: 10.1186/1756-0500-7-551. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Yasuoka Y, Izumi Y, Nagai T, Fukuyama T, Nakayama Y, Inoue H, et al. Fludrocortisone stimulates erythropoietin production in the intercalated cells of the collecting ducts. Biochem Biophys Res Commun. 2018;503(4):3121–3127. doi: 10.1016/j.bbrc.2018.08.102. [DOI] [PubMed] [Google Scholar]
- 34.Yasuoka Y, Izumi Y, Fukuyama T, Inoue H, Oshima T, Yamazaki T, et al. Effects of Angiotensin II on erythropoietin production in the kidney and liver. Molecules. 2021;26(17):5399. doi: 10.3390/molecules26175399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Kim YC, Mungunsukh O, Day RM. Erythropoietin regulation by angiotensin II. Vitam Horm. 2017;105:57–77. doi: 10.1016/bs.vh.2017.02.001. [DOI] [PubMed] [Google Scholar]
- 36.Lopez-Sublet M, Caratti di Lanzacco L, Danser AHJ, Lambert M, Elourimi G, Persu A. Focus on increased serum angiotensin-converting enzyme level: from granulomatous diseases to genetic mutations. Clin Biochem. 2018;59:1–8. 10.1016/j.clinbiochem.2018.06.010. [DOI] [PubMed]
- 37.Cambien F, Alhenc-Gelas F, Herbeth B, Andre JL, Rakotovao R, Gonzales MF, et al. Familial resemblance of plasma angiotensin-converting enzyme level: the Nancy Study. Am J Hum Genet. 1988;43(5):774–780. [PMC free article] [PubMed] [Google Scholar]
- 38.Dhangadamajhi G, Mohapatra BN, Kar SK, Ranjit M. Endothelial nitric oxide synthase gene polymorphisms and Plasmodium falciparum infection in Indian adults. Infect Immun. 2009;77(7):2943–2947. doi: 10.1128/IAI.00083-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Dhangadamajhi G, Mohapatra BN, Kar SK, Ranjit MR. A new allele (eNOS4e) in the intron 4 (VNTR) of eNOS gene in malaria infected individuals of the population of Orissa (an eastern Indian state) Nitric Oxide. 2010;22(1):58–59. doi: 10.1016/j.niox.2009.11.010. [DOI] [PubMed] [Google Scholar]
- 40.Dos Santos ACM, de Moura EL, da Silva DM, Araujo Moura AW, Ferreira JM, Lira Neto AB, et al. Association of polymorphisms in serotonin and nitric oxide genes with clinical outcome of dengue in Brazilian northeast population. Acta Trop. 2019;190:144–148. doi: 10.1016/j.actatropica.2018.11.015. [DOI] [PubMed] [Google Scholar]
- 41.Dufort EM, Koumans EH, Chow EJ, Rosenthal EM, Muse A, Rowlands J, et al. Multisystem inflammatory syndrome in children in New York State. N Engl J Med. 2020;383(4):347–358. doi: 10.1056/NEJMoa2021756. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Casals-Pascual C, Idro R, Picot S, Roberts DJ, Newton CR. Can erythropoietin be used to prevent brain damage in cerebral malaria? Trends Parasitol. 2009;25(1):30–36. doi: 10.1016/j.pt.2008.10.002. [DOI] [PubMed] [Google Scholar]
- 43.Casals-Pascual C, Idro R, Gicheru N, Gwer S, Kitsao B, Gitau E, et al. High levels of erythropoietin are associated with protection against neurological sequelae in African children with cerebral malaria. Proc Natl Acad Sci USA. 2008;105(7):2634–2639. doi: 10.1073/pnas.0709715105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Akaberi D, Krambrich J, Ling J, Luni C, Hedenstierna G, Jarhult JD et al. Mitigation of the replication of SARS-CoV-2 by nitric oxide in vitro. Redox Biol. 2020;37:101734. 10.1016/j.redox.2020.101734. [DOI] [PMC free article] [PubMed]
- 45.Guan SP, Seet RCS, Kennedy BK. Does eNOS derived nitric oxide protect the young from severe COVID-19 complications? Ageing Res Rev. 2020;64:101201. 10.1016/j.arr.2020.101201. [DOI] [PMC free article] [PubMed]
- 46.Alnaeeli M, Wang L, Piknova B, Rogers H, Li X, Noguchi CT. Erythropoietin in brain development and beyond. Anat Res Int. 2012;2012:953264. 10.1155/2012/953264. [DOI] [PMC free article] [PubMed]
- 47.Teng R, Calvert JW, Sibmooh N, Piknova B, Suzuki N, Sun J, et al. Acute erythropoietin cardioprotection is mediated by endothelial response. Basic Res Cardiol. 2011;106(3):343–354. doi: 10.1007/s00395-011-0158-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Suresh S, Rajvanshi PK, Noguchi CT. The many facets of erythropoietin physiologic and metabolic response. Front Physiol. 2019;10:1534. doi: 10.3389/fphys.2019.01534. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Keswani SC, Bosch-Marcé M, Reed N, Fischer A, Semenza GL, Höke A. Nitric oxide prevents axonal degeneration by inducing HIF-1-dependent expression of erythropoietin. Proc Natl Acad Sci USA. 2011;108(12):4986–4990. doi: 10.1073/pnas.1019591108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Cao F, Tian X, Li Z, Lv Y, Han J, Zhuang R, et al. Suppression of NLRP3 inflammasome by erythropoietin via the EPOR/JAK2/STAT3 pathway contributes to attenuation of acute lung injury in mice. Front Pharmacol. 2020;11:306. doi: 10.3389/fphar.2020.00306. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Liu F, Wen Y, Kang J, Wei C, Wang M, Zheng Z, et al. Regulation of TLR4 expression mediates the attenuating effect of erythropoietin on inflammation and myocardial fibrosis in rat heart. Int J Mol Med. 2018;42(3):1436–1444. doi: 10.3892/ijmm.2018.3707. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Khan AI, Coldewey SM, Patel NS, Rogazzo M, Collino M, Yaqoob MM, et al. Erythropoietin attenuates cardiac dysfunction in experimental sepsis in mice via activation of the β-common receptor. Dis Model Mech. 2013;6(4):1021–1030. doi: 10.1242/dmm.011908. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Izmailova O, Shlykova O, Vatsenko A, Ivashchenko D, Dudchenko M, Koval T et al. Allele C (rs5186) of at1r is associated with the severity of COVID-19 in the Ukrainian population. Infect Genet Evol. 2022;98:105227. 10.1016/j.meegid.2022.105227. [DOI] [PMC free article] [PubMed]
- 54.Yamamoto N, Ariumi Y, Nishida N, Yamamoto R, Bauer G, Gojobori T et al. SARS-CoV-2 infections and COVID-19 mortalities strongly correlate with ACE1 I/D genotype. Gene. 2020;758:144944. 10.1016/j.gene.2020.144944. [DOI] [PMC free article] [PubMed]
- 55.Feng S, Song F, Guo W, Tan J, Zhang X, Qiao F, et al. Potential genes associated with COVID-19 and comorbidity. Int J Med Sci. 2022;19(2):402–415. doi: 10.7150/ijms.67815. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Tiwari A, De A, Pande V, Sinha A. Interlinking antecedent malaria and hypertension through angiotensin II in India. Front Cardiovasc Med. 2021;8. 10.3389/fcvm.2021.729525. [DOI] [PMC free article] [PubMed]
- 57.Gallego-Delgado J, Rodriguez A. Malaria and hypertension. Another co-evolutionary adaptation? Front Cell Infect Microbiol. 2014;4:121. 10.3389/fcimb.2014.00121. [DOI] [PMC free article] [PubMed]
- 58.Gallego-Delgado J, Walther T, Rodriguez A. The high blood pressure-malaria protection hypothesis. Circ Res. 2016;119(10):1071–1075. doi: 10.1161/circresaha.116.309602. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Schalekamp MA, Danser AH. How does the angiotensin II type 1 receptor 'trump' the type 2 receptor in blood pressure control? J Hypertens. 2013;31(4):705–712. doi: 10.1097/HJH.0b013e32835d6d11. [DOI] [PubMed] [Google Scholar]
- 60.Torres MD, Silva AF, Alves FL, Capurro ML, Miranda A, Cordeiro RM, et al. Evidences for the action mechanism of angiotensin II and its analogs on Plasmodium sporozoite membranes. J Pept Sci. 2016;22(3):132–142. doi: 10.1002/psc.2849. [DOI] [PubMed] [Google Scholar]
- 61.Calò LA, Davis PA, Maiolino G, Pagnin E, Ravarotto V, Naso E, et al. Assessing the relationship of angiotensin II Type 1 receptors with erythropoietin in a human model of endogenous angiotensin II Type 1 receptor antagonism. Cardiorenal Med. 2015;6(1):16–24. doi: 10.1159/000439183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Vlahakos DV, Marathias KP, Madias NE. The role of the renin-angiotensin system in the regulation of erythropoiesis. Am J Kidney Dis. 2010;56(3):558–565. doi: 10.1053/j.ajkd.2009.12.042. [DOI] [PubMed] [Google Scholar]
- 63.Marathias KP, Lambadiari VA, Markakis KP, Vlahakos VD, Bacharaki D, Raptis AE, et al. Competing effects of renin angiotensin system blockade and sodium-glucose cotransporter-2 inhibitors on erythropoietin secretion in diabetes. Am J Nephrol. 2020;51(5):349–356. doi: 10.1159/000507272. [DOI] [PubMed] [Google Scholar]
- 64.Sun CY, Zhang XP, Liu F, Wang W. Orchestration of lincRNA-p21 and miR-155 in modulating the adaptive dynamics of HIF-1α. Front Genet. 2020;11:871. doi: 10.3389/fgene.2020.00871. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Calo LA, Rigato M, Sgarabotto L, Gianesello L, Bertoldi G, Ravarotto V et al. ACE2 and SARS-CoV-2 infection risk: insights from patients with two rare genetic tubulopathies, Gitelman's and Bartter's Syndromes. Front Med (Lausanne). 2021;8:647319. 10.3389/fmed.2021.647319. [DOI] [PMC free article] [PubMed]
- 66.Chow JT, Salmena L. Prediction and analysis of SARS-CoV-2-targeting MicroRNA in human lung epithelium. Genes (Basel). 2020;11(9). 10.3390/genes11091002. [DOI] [PMC free article] [PubMed]
- 67.Papadopoulos KI, Wattanaarsakit P, Prasongchean W, Narain R. 10—gene therapies in clinical trials. In: Narain R, editor. Polymers and Nanomaterials for gene therapy. Woodhead Publishing; 2016. p. 231–56.
- 68.Kozomara A, Birgaoanu M, Griffiths-Jones S. miRBase: from microRNA sequences to function. Nucl Acids Res. 2018;47(D1):D155–D162. doi: 10.1093/nar/gky1141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Bautista-Becerril B, Pérez-Dimas G, Sommerhalder-Nava PC, Hanono A, Martínez-Cisneros JA, Zarate-Maldonado B et al. miRNAs, from evolutionary junk to possible prognostic markers and therapeutic targets in COVID-19. Viruses. 2021;14(1). 10.3390/v14010041. [DOI] [PMC free article] [PubMed]
- 70.Elton TS, Selemon H, Elton SM, Parinandi NL. Regulation of the MIR155 host gene in physiological and pathological processes. Gene. 2013;532(1):1–12. doi: 10.1016/j.gene.2012.12.009. [DOI] [PubMed] [Google Scholar]
- 71.Nemecz M, Alexandru N, Tanko G, Georgescu A. Role of MicroRNA in endothelial dysfunction and hypertension. Curr Hypertens Rep. 2016;18(12):87. doi: 10.1007/s11906-016-0696-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Kohanbash G, Okada H. MicroRNAs and STAT interplay. Semin Cancer Biol. 2012;22(1):70–75. doi: 10.1016/j.semcancer.2011.12.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Faraoni I, Antonetti FR, Cardone J, Bonmassar E. miR-155 gene: A typical multifunctional microRNA. Biochimica et Biophysica Acta (BBA)—Molecular Basis of Disease. 2009;1792(6):497–505. 10.1016/j.bbadis.2009.02.013. [DOI] [PubMed]
- 74.Chen M, Wang F, Xia H, Yao S. MicroRNA-155: regulation of immune cells in sepsis. Mediators Inflamm. 2021;2021:8874854. doi: 10.1155/2021/8874854. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Mashima R. Physiological roles of miR-155. Immunology. 2015;145(3):323–333. doi: 10.1111/imm.12468. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Jankauskas SS, Gambardella J, Sardu C, Lombardi A, Santulli G. Functional role of miR-155 in the pathogenesis of diabetes mellitus and its complications. Noncoding RNA. 2021;7(3). 10.3390/ncrna7030039. [DOI] [PMC free article] [PubMed]
- 77.Huang S, Xiang C, Song Y. Identification of the shared gene signatures and pathways between sarcopenia and type 2 diabetes mellitus. PLoS One. 2022;17(3):e0265221. 10.1371/journal.pone.0265221. [DOI] [PMC free article] [PubMed]
- 78.Dunand-Sauthier I, Irla M, Carnesecchi S, Seguín-Estévez Q, Vejnar CE, Zdobnov EM, et al. Repression of arginase-2 expression in dendritic cells by microRNA-155 is critical for promoting T cell proliferation. J Immunol. 2014;193(4):1690–1700. doi: 10.4049/jimmunol.1301913. [DOI] [PubMed] [Google Scholar]
- 79.Martí i Líndez AA, Dunand-Sauthier I, Conti M, Gobet F, Núñez N, Hannich JT et al. Mitochondrial arginase-2 is a cell‑autonomous regulator of CD8+ T cell function and antitumor efficacy. JCI Insight. 2019;4(24). 10.1172/jci.insight.132975. [DOI] [PMC free article] [PubMed]
- 80.Dowling JK, Afzal R, Gearing LJ, Cervantes-Silva MP, Annett S, Davis GM, et al. Mitochondrial arginase-2 is essential for IL-10 metabolic reprogramming of inflammatory macrophages. Nat Commun. 2021;12(1):1460. doi: 10.1038/s41467-021-21617-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Wong RR, Abd-Aziz N, Affendi S, Poh CL. Role of microRNAs in antiviral responses to dengue infection. J Biomed Sci. 2020;27(1):4. doi: 10.1186/s12929-019-0614-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Zhu N, Zhang D, Chen S, Liu X, Lin L, Huang X, et al. Endothelial enriched microRNAs regulate angiotensin II-induced endothelial inflammation and migration. Atherosclerosis. 2011;215(2):286–293. doi: 10.1016/j.atherosclerosis.2010.12.024. [DOI] [PubMed] [Google Scholar]
- 83.Zhan Y, Brown C, Maynard E, Anshelevich A, Ni W, Ho IC, et al. Ets-1 is a critical regulator of Ang II-mediated vascular inflammation and remodeling. J Clin Invest. 2005;115(9):2508–2516. doi: 10.1172/jci24403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Wang P, Hou J, Lin L, Wang C, Liu X, Li D, et al. Inducible microRNA-155 feedback promotes type I IFN signaling in antiviral innate immunity by targeting suppressor of cytokine signaling 1. J Immunol. 2010;185(10):6226–6233. doi: 10.4049/jimmunol.1000491. [DOI] [PubMed] [Google Scholar]
- 85.Breggia AC, Wojchowski DM, Himmelfarb J. JAK2/Y343/STAT5 signaling axis is required for erythropoietin-mediated protection against ischemic injury in primary renal tubular epithelial cells. Am J Physiol Renal Physiol. 2008;295(6):F1689–F1695. doi: 10.1152/ajprenal.90333.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Hadighi R, Heidari A, Fallah P, Keshavarz H, Tavakoli Z, Sholeh M et al. Key plasma microRNAs variations in patients with Plasmodium vivax malaria in Iran. Heliyon. 2022;8(3):e09018. 10.1016/j.heliyon.2022.e09018. [DOI] [PMC free article] [PubMed]
- 87.Woods PS, Doolittle LM, Rosas LE, Nana-Sinkam SP, Tili E, Davis IC. Increased expression of microRNA-155-5p by alveolar type II cells contributes to development of lethal ARDS in H1N1 influenza A virus-infected mice. Virology. 2020;545:40–52. doi: 10.1016/j.virol.2020.03.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Wyler E, Mosbauer K, Franke V, Diag A, Gottula LT, Arsie R et al. Transcriptomic profiling of SARS-CoV-2 infected human cell lines identifies HSP90 as target for COVID-19 therapy. iScience. 2021;24(3):102151. 10.1016/j.isci.2021.102151. [DOI] [PMC free article] [PubMed]
- 89.Su Y-C, Huang Y-F, Wu Y-W, Chen H-F, Wu Y-H, Hsu C-C, et al. MicroRNA-155 inhibits dengue virus replication by inducing heme oxygenase-1-mediated antiviral interferon responses. FASEB J. 2020;34(6):7283–7294. doi: 10.1096/fj.201902878R. [DOI] [PubMed] [Google Scholar]
- 90.Haroun RA, Osman WH, Amin RE, Hassan AK, Abo-Shanab WS, Eessa AM. Circulating plasma miR-155 is a potential biomarker for the detection of SARS-CoV-2 infection. Pathology. 2022;54(1):104–110. doi: 10.1016/j.pathol.2021.09.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Abbasi-Kolli M, Nahand JS, Kiani SJ, Khanaliha K, Khatami A, Taghizadieh M et al. The expression patterns of MALAT-1, NEAT-1, THRIL, and miR-155–5p in the acute to the post-acute phase of COVID-19 disease. Br J Infect Dis. 2022:102354. 10.1016/j.bjid.2022.102354. [DOI] [PMC free article] [PubMed]
- 92.Garg A, Seeliger B, Derda AA, Xiao K, Gietz A, Scherf K, et al. Circulating cardiovascular microRNAs in critically ill COVID-19 patients. Eur J Heart Fail. 2021;23(3):468–475. doi: 10.1002/ejhf.2096. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Donyavi T, Bokharaei-Salim F, Baghi HB, Khanaliha K, Alaei Janat-Makan M, Karimi B et al. Acute and post-acute phase of COVID-19: analyzing expression patterns of miRNA-29a-3p, 146a-3p, 155–5p, and let-7b-3p in PBMC. Int Immunopharmacol. 2021;97:107641. 10.1016/j.intimp.2021.107641. [DOI] [PMC free article] [PubMed]
- 94.Gedikbasi A, Adas G, Isiksacan N, Kart Yasar K, Canbolat Unlu E, Yilmaz R et al. The effect of host miRNAs on prognosis in COVID-19: miRNA-155 may promote severity via targeting suppressor of cytokine signaling 1 (SOCS1) Gene. Genes (Basel). 2022;13(7). 10.3390/genes13071146. [DOI] [PMC free article] [PubMed]
- 95.Eyileten C, Wicik Z, Simões SN, Martins-Jr DC, Klos K, Wlodarczyk W, et al. Thrombosis-related circulating miR-16-5p is associated with disease severity in patients hospitalised for COVID-19. RNA Biol. 2022;19(1):963–979. doi: 10.1080/15476286.2022.2100629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Li S, Duan X, Li Y, Li M, Gao Y, Li T, et al. Differentially expressed immune response genes in COVID-19 patients based on disease severity. Aging (Albany NY) 2021;13(7):9265–9276. doi: 10.18632/aging.202877. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Gaytán-Pacheco N, Ibáñez-Salazar A, Herrera-Van Oostdam AS, Oropeza-Valdez JJ, Magaña-Aquino M, Adrián López J et al. miR-146a, miR-221, and miR-155 are involved in inflammatory immune response in severe COVID-19 patients. Diagnostics (Basel). 2022;13(1). 10.3390/diagnostics13010133. [DOI] [PMC free article] [PubMed]
- 98.Giannella A, Riccetti S, Sinigaglia A, Piubelli C, Razzaboni E, Di Battista P et al. Circulating microRNA signatures associated with disease severity and outcome in COVID-19 patients. Front Immunol. 2022;13:968991. 10.3389/fimmu.2022.968991. [DOI] [PMC free article] [PubMed]
- 99.Kassif-Lerner R, Zloto K, Rubin N, Asraf K, Doolman R, Paret G, et al. miR-155: a potential biomarker for predicting mortality in COVID-19 patients. J Person Med. 2022;12(2):324. doi: 10.3390/jpm12020324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Vonhögen IGC, Mohseni Z, Winkens B, Xiao K, Thum T, Calore M, et al. Circulating miR-216a as a biomarker of metabolic alterations and obesity in women. Noncoding RNA Res. 2020;5(3):144–152. doi: 10.1016/j.ncrna.2020.08.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Noren Hooten N, Abdelmohsen K, Gorospe M, Ejiogu N, Zonderman AB, Evans MK. microRNA expression patterns reveal differential expression of target genes with age. PLoS One. 2010;5(5):e10724. 10.1371/journal.pone.0010724. [DOI] [PMC free article] [PubMed]
- 102.Ogunlade BO, Lazartigues E, Filipeanu CM. Angiotensin Type 1 receptor-dependent internalization of SARS-CoV-2 by angiotensin-converting enzyme 2. Hypertension. 2021;77(4):e42–e43. doi: 10.1161/HYPERTENSIONAHA.120.16795. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Sethupathy P, Borel C, Gagnebin M, Grant GR, Deutsch S, Elton TS, et al. Human microRNA-155 on chromosome 21 differentially interacts with its polymorphic target in the AGTR1 3' untranslated region: a mechanism for functional single-nucleotide polymorphisms related to phenotypes. Am J Hum Genet. 2007;81(2):405–413. doi: 10.1086/519979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Pacurari M, Tchounwou PB. Role of MicroRNAs in renin-angiotensin-aldosterone system-mediated cardiovascular inflammation and remodeling. Int J Inflam. 2015;2015:101527. 10.1155/2015/101527. [DOI] [PMC free article] [PubMed]
- 105.Valle Raleigh J, Mauro AG, Devarakonda T, Marchetti C, He J, Kim E, et al. Reperfusion therapy with recombinant human relaxin-2 (Serelaxin) attenuates myocardial infarct size and NLRP3 inflammasome following ischemia/reperfusion injury via eNOS-dependent mechanism. Cardiovasc Res. 2017;113(6):609–619. doi: 10.1093/cvr/cvw246. [DOI] [PubMed] [Google Scholar]
- 106.Tapia Cáceres F, Gaspari TA, Hossain MA, Samuel CS. Relaxin inhibits the cardiac myofibroblast NLRP3 inflammasome as part of its anti-fibrotic actions via the angiotensin Type 2 and ATP (P2X7) receptors. Int J Mol Sci. 2022;23(13). 10.3390/ijms23137074. [DOI] [PMC free article] [PubMed]
- 107.Kow CS, Ramachandram DS, Hasan SS. Effects of angiotensin II receptor blockers on the risk of mortality in patients with COVID-19: an updated systematic review and meta-analysis of randomized trials. Am J Hypertens. 2022;35(8):763–764. doi: 10.1093/ajh/hpac070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Weber M, Baker MB, Patel RS, Quyyumi AA, Bao G, Searles CD. MicroRNA expression profile in CAD patients and the impact of ACEI/ARB. Cardiol Res Pract. 2011;2011:532915. 10.4061/2011/532915. [DOI] [PMC free article] [PubMed]
- 109.Fichtlscherer S, Rosa SD, Fox H, Schwietz T, Fischer A, Liebetrau C, et al. Circulating MicroRNAs in patients with coronary artery disease. Circ Res. 2010;107(5):677–684. doi: 10.1161/CIRCRESAHA.109.215566. [DOI] [PubMed] [Google Scholar]
- 110.Kamper AL, Nielsen OJ. Effect of enalapril on haemoglobin and serum erythropoietin in patients with chronic nephropathy. Scand J Clin Lab Invest. 1990;50(6):611–618. doi: 10.3109/00365519009089178. [DOI] [PubMed] [Google Scholar]
- 111.Pratt MC, Lewis-Barned NJ, Walker RJ, Bailey RR, Shand BI, Livesey J. Effect of angiotensin converting enzyme inhibitors on erythropoietin concentrations in healthy volunteers. Br J Clin Pharmacol. 1992;34(4):363–365. doi: 10.1111/j.1365-2125.1992.tb05644.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Bijkerk R, de Bruin RG, van Solingen C, Duijs JM, Kobayashi K, van der Veer EP, et al. MicroRNA-155 functions as a negative regulator of RhoA signaling in TGF-β-induced endothelial to mesenchymal transition. Microrna. 2012;1(1):2–10. doi: 10.2174/2211536611201010002. [DOI] [PubMed] [Google Scholar]
- 113.Calò LA. Vascular tone control in humans: insights from studies in Bartter's/Gitelman's syndromes. Kidney Int. 2006;69(6):963–966. doi: 10.1038/sj.ki.5000253. [DOI] [PubMed] [Google Scholar]
- 114.Wolf G, Schroeder R, Stahl RA. Angiotensin II induces hypoxia-inducible factor-1 alpha in PC 12 cells through a posttranscriptional mechanism: role of AT2 receptors. Am J Nephrol. 2004;24(4):415–421. doi: 10.1159/000080086. [DOI] [PubMed] [Google Scholar]
- 115.Su KH, Shyue SK, Kou YR, Ching LC, Chiang AN, Yu YB, et al. beta Common receptor integrates the erythropoietin signaling in activation of endothelial nitric oxide synthase. J Cell Physiol. 2011;226(12):3330–3339. doi: 10.1002/jcp.22678. [DOI] [PubMed] [Google Scholar]
- 116.Heinisch O, Zeyen T, Goldmann T, Prinz M, Huber M, Jung J, et al. Erythropoietin abrogates post-ischemic activation of the NLRP3, NLRC4, and AIM2 inflammasomes in microglia/macrophages in a TAK1-dependent manner. Transl Stroke Res. 2022;13(3):462–482. doi: 10.1007/s12975-021-00948-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Sun H-X, Zeng D-Y, Li R-T, Pang R-P, Yang H, Hu Y-L, et al. Essential role of MicroRNA-155 in regulating endothelium-dependent vasorelaxation by targeting endothelial nitric oxide synthase. Hypertension. 2012;60(6):1407–1414. doi: 10.1161/HYPERTENSIONAHA.112.197301. [DOI] [PubMed] [Google Scholar]
- 118.Su KH, Tsai JY, Kou YR, Chiang AN, Hsiao SH, Wu YL, et al. Valsartan regulates the interaction of angiotensin II type 1 receptor and endothelial nitric oxide synthase via Src/PI3K/Akt signalling. Cardiovasc Res. 2009;82(3):468–475. doi: 10.1093/cvr/cvp091. [DOI] [PubMed] [Google Scholar]
- 119.Durante W, Johnson FK, Johnson RA. Arginase: a critical regulator of nitric oxide synthesis and vascular function. Clin Exp Pharmacol Physiol. 2007;34(9):906–911. doi: 10.1111/j.1440-1681.2007.04638.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Morris SM, Jr, Gao T, Cooper TK, Kepka-Lenhart D, Awad AS. Arginase-2 mediates diabetic renal injury. Diabetes. 2011;60(11):3015–3022. doi: 10.2337/db11-0901. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Rodríguez PC, Ochoa AC. Arginine regulation by myeloid derived suppressor cells and tolerance in cancer: mechanisms and therapeutic perspectives. Immunol Rev. 2008;222:180–191. doi: 10.1111/j.1600-065X.2008.00608.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Ceolotto G, Papparella I, Bortoluzzi A, Strapazzon G, Ragazzo F, Bratti P, et al. Interplay between miR-155, AT1R A1166C polymorphism, and AT1R expression in young untreated hypertensives. Am J Hypertens. 2011;24(2):241–246. doi: 10.1038/ajh.2010.211. [DOI] [PubMed] [Google Scholar]
- 123.Stanković A, Kolaković A, Živković M, Djurić T, Bundalo M, Končar I, et al. Angiotensin receptor type 1 polymorphism A1166C is associated with altered AT1R and miR-155 expression in carotid plaque tissue and development of hypoechoic carotid plaques. Atherosclerosis. 2016;248:132–139. doi: 10.1016/j.atherosclerosis.2016.02.032. [DOI] [PubMed] [Google Scholar]
- 124.Espitia-Corredor JA, Boza P, Espinoza-Pérez C, Lillo JM, Rimassa-Taré C, Machuca V, et al. Angiotensin II triggers NLRP3 inflammasome activation by a Ca(2+) signaling-dependent pathway in rat cardiac fibroblast Ang-II by a Ca(2+)-dependent mechanism triggers NLRP3 inflammasome in CF. Inflammation. 2022 doi: 10.1007/s10753-022-01707-z. [DOI] [PubMed] [Google Scholar]
- 125.Ciechanowicz AK, Lay WX, Prado Paulino J, Suchocki E, Leszczak S, Leszczak C et al. Angiotensin 1–7 stimulates proliferation of lung bronchoalveolar progenitors-implications for SARS-CoV-2 infection. Cells. 2022;11(13). 10.3390/cells11132102. [DOI] [PMC free article] [PubMed]
- 126.Cau SB, Bruder-Nascimento A, Silva MB, Ramalho FNZ, Mestriner F, Alves-Lopes R et al. Angiotensin-II activates vascular inflammasome and induces vascular damage. Vascul Pharmacol. 2021;139:106881. 10.1016/j.vph.2021.106881. [DOI] [PMC free article] [PubMed]
- 127.Papadopoulos KI, Manipalviratn S, Aw TC. Further observations on pregnancy complications and COVID-19 infection. JAMA Pediatr. 2021 doi: 10.1001/jamapediatrics.2021.2613. [DOI] [PubMed] [Google Scholar]
- 128.Wallukat G, Hohberger B, Wenzel K, Furst J, Schulze-Rothe S, Wallukat A et al. Functional autoantibodies against G-protein coupled receptors in patients with persistent Long-COVID-19 symptoms. J Transl Autoimmun. 2021;4:100100. 10.1016/j.jtauto.2021.100100. [DOI] [PMC free article] [PubMed]
- 129.Leisman DE, Fernandes TD, Bijol V, Abraham MN, Lehman JR, Taylor MD, et al. Impaired angiotensin II type 1 receptor signaling contributes to sepsis-induced acute kidney injury. Kidney Int. 2021;99(1):148–160. doi: 10.1016/j.kint.2020.07.047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Akin S, Schriek P, van Nieuwkoop C, Neuman RI, Meynaar I, van Helden EJ, et al. A low aldosterone/renin ratio and high soluble ACE2 associate with COVID-19 severity. J Hypertens. 2022;40(3):606–614. doi: 10.1097/hjh.0000000000003054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Tacke F, Spehlmann ME, Vucur M, Benz F, Luedde M, Cardenas DV, et al. miR-155 predicts long-term mortality in critically ill patients younger than 65 years. Mediators Inflamm. 2019;2019:6714080. doi: 10.1155/2019/6714080. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Vasques-Nóvoa F, Laundos TL, Cerqueira RJ, Quina-Rodrigues C, Soares-dos-Reis R, Baganha F, et al. MicroRNA-155 amplifies nitric Oxide/cGMP signaling and impairs vascular angiotensin II reactivity in septic shock. Crit Care Med. 2018;46(9):e945–e954. doi: 10.1097/ccm.0000000000003296. [DOI] [PubMed] [Google Scholar]
- 133.Leisman DE, Mastroianni F, Fisler G, Shah S, Hasan Z, Narasimhan M et al. Physiologic response to angiotensin II treatment for coronavirus disease 2019-induced vasodilatory shock: a retrospective matched cohort study. Crit Care Explor. 2020;2(10):e0230. 10.1097/cce.0000000000000230. [DOI] [PMC free article] [PubMed]
- 134.Zangrillo A, Landoni G, Beretta L, Morselli F, Serpa Neto A, Bellomo R. Angiotensin II infusion in COVID-19-associated vasodilatory shock: a case series. Crit Care. 2020;24(1):227. doi: 10.1186/s13054-020-02928-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.DuPont JJ, McCurley A, Davel AP, McCarthy J, Bender SB, Hong K et al. Vascular mineralocorticoid receptor regulates microRNA-155 to promote vasoconstriction and rising blood pressure with aging. JCI Insight. 2016;1(14):e88942. 10.1172/jci.insight.88942. [DOI] [PMC free article] [PubMed]
- 136.Atmakusuma TD. COVID-19 in patients with transfusion dependent thalassemia in Indonesia: characteristics of the disease and patients, and comparison between epidemiological data for COVID-19 and thalassemia in Indonesia and South-East Asia. Hematol Rep. 2022;13(4):9379. doi: 10.4081/hr.2021.9379. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137.Haghpanah S, Hosseini-Bensenjan M, Sayadi M, Karimi M. Incidence rate of COVID-19 infection in hemoglobinopathies: a systematic review and meta-analysis. Hemoglobin. 2021:1–9. 10.1080/03630269.2021.1927751. [DOI] [PubMed]
- 138.El-Battrawy I, Longo F, Núñez Gil IJ, Abumayyaleh M, Gianesin B, Estrada V et al. Thalassaemia is paradoxically associated with a reduced risk of in-hospital complications and mortality in COVID-19: data from an international registry. J Cell Mol Med. 10.1111/jcmm.17026. [DOI] [PMC free article] [PubMed]
- 139.Sornjai W, Khungwanmaythawee K, Svasti S, Fucharoen S, Wintachai P, Yoksan S, et al. Dengue virus infection of erythroid precursor cells is modulated by both thalassemia trait status and virus adaptation. Virology. 2014;471–473:61–71. doi: 10.1016/j.virol.2014.10.004. [DOI] [PubMed] [Google Scholar]
- 140.Leecharoenkiat K, Tanaka Y, Harada Y, Chaichompoo P, Sarakul O, Abe Y, et al. Plasma microRNA-451 as a novel hemolytic marker for β0-thalassemia/HbE disease. Mol Med Rep. 2017;15(5):2495–2502. doi: 10.3892/mmr.2017.6326. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141.Srinoun K, Nopparatana C, Wongchanchailert M, Fucharoen S. MiR-155 enhances phagocytic activity of β-thalassemia/HbE monocytes via targeting of BACH1. Int J Hematol. 2017;106(5):638–647. doi: 10.1007/s12185-017-2291-4. [DOI] [PubMed] [Google Scholar]
- 142.Das SS, Das S, Byram PK, Rahaman M, Dolai TK, Chatterjee A et al. MicroRNA expression patterns in HbE/β-thalassemia patients: the passwords to unlock fetal hemoglobin expression in β-hemoglobinopathies. Blood Cells Mol Dis. 2021;87:102523. 10.1016/j.bcmd.2020.102523. [DOI] [PubMed]
- 143.Toro A, Ruiz MS, Lage-Vickers S, Sanchis P, Sabater A, Pascual G, et al. A journey into the clinical relevance of heme oxygenase 1 for human inflammatory disease and viral clearance: why does it matter on the COVID-19 scene? Antioxidants. 2022;11(2):276. doi: 10.3390/antiox11020276. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144.Pena AC, Pamplona A. Heme oxygenase-1, carbon monoxide, and malaria—the interplay of chemistry and biology. Coordinat Chem Rev. 2022;453:214285. 10.1016/j.ccr.2021.214285.
- 145.Singh RD, Barry MA, Croatt AJ, Ackerman AW, Grande JP, Diaz RM et al. The spike protein of SARS-CoV-2 induces heme oxygenase-1: Pathophysiologic implications. Biochim Biophys Acta Mol Basis Dis. 2022;1868(3):166322. 10.1016/j.bbadis.2021.166322. [DOI] [PMC free article] [PubMed]
- 146.Hooper PL. COVID-19 and heme oxygenase: novel insight into the disease and potential therapies. Cell Stress Chaperones. 2020;25(5):707–710. doi: 10.1007/s12192-020-01126-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 147.Dickey LL, Worne CL, Glover JL, Lane TE, O'Connell RM. MicroRNA-155 enhances T cell trafficking and antiviral effector function in a model of coronavirus-induced neurologic disease. J Neuroinflammation. 2016;13(1):240. doi: 10.1186/s12974-016-0699-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 148.Hentzschel F, Hammerschmidt-Kamper C, Börner K, Heiss K, Knapp B, Sattler JM, et al. AAV8-mediated in vivo overexpression of miR-155 enhances the protective capacity of genetically attenuated malarial parasites. Mol Ther. 2014;22(12):2130–2141. doi: 10.1038/mt.2014.172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 149.Silva-Filho JL, Caruso-Neves C, Pinheiro AAS. Angiotensin II type-1 receptor (AT1R) regulates expansion, differentiation, and functional capacity of antigen-specific CD8+ T cells. Sci Rep. 2016;6(1):35997. doi: 10.1038/srep35997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 150.Silva-Filho JL, Caruso-Neves C, Pinheiro AAS. Targeting angiotensin II Type-1 receptor (AT1R) inhibits the harmful phenotype of plasmodium-specific CD8+ T cells during blood-stage malaria. Front Cell Infect Microbiol. 2017;7. 10.3389/fcimb.2017.00042. [DOI] [PMC free article] [PubMed]
- 151.Triantafyllou AI, Vyssoulis GP, Karpanou EA, Karkalousos PL, Triantafyllou EA, Aessopos A, et al. Impact of β-thalassemia trait carrier state on cardiovascular risk factors and metabolic profile in patients with newly diagnosed hypertension. J Hum Hypertens. 2014;28(5):328–332. doi: 10.1038/jhh.2013.102. [DOI] [PubMed] [Google Scholar]
- 152.Triantafyllou AI, Farmakis DT, Lampropoulos KM, Karkalousos PL, Triantafyllou EA, Papingiotis G, et al. Impact of β-thalassemia trait carrier state on inflammatory status in patients with newly diagnosed hypertension. J Cardiovasc Med (Hagerstown) 2019;20(5):284–289. doi: 10.2459/jcm.0000000000000787. [DOI] [PubMed] [Google Scholar]
- 153.Lin X, Qin Y, Jia J, Lin T, Lin X, Chen L et al. MiR-155 enhances insulin sensitivity by coordinated regulation of multiple genes in mice. PLoS Genet. 2016;12(10):e1006308. 10.1371/journal.pgen.1006308. [DOI] [PMC free article] [PubMed]
- 154.Tseng CK, Lin CK, Wu YH, Chen YH, Chen WC, Young KC, et al. Human heme oxygenase 1 is a potential host cell factor against dengue virus replication. Sci Rep. 2016;6:32176. doi: 10.1038/srep32176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 155.Jafarzadeh A, Naseri A, Shojaie L, Nemati M, Jafarzadeh S, Bannazadeh Baghi H et al. MicroRNA-155 and antiviral immune responses. Int Immunopharmacol. 2021;101:108188. 10.1016/j.intimp.2021.108188. [DOI] [PubMed]
- 156.Seifi BKM, Ranjbaran M, Bakhshi E. Nephroprotection through the Akt/eNOS pathway by centrally administered erythropoietin in a rat model of fixed-volume hemorrhage. Life Sci. 2018;193:180–185. doi: 10.1016/j.lfs.2017.11.003. [DOI] [PubMed] [Google Scholar]
- 157.Beleslin-Čokić BB, Cokić VP, Wang L, Piknova B, Teng R, Schechter AN, et al. Erythropoietin and hypoxia increase erythropoietin receptor and nitric oxide levels in lung microvascular endothelial cells. Cytokine. 2011;54(2):129–135. doi: 10.1016/j.cyto.2011.01.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 158.De Santi C, Nally FK, Afzal R, Duffy CP, Fitzsimons S, Annett SL, et al. Enhancing arginase 2 expression using target site blockers as a strategy to modulate macrophage phenotype. Mol Ther Nucl Acids. 2022;29:643–655. doi: 10.1016/j.omtn.2022.08.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 159.Fujita Y, Doi Y, Hamano T, Hatazaki M, Umayahara Y, Isaka Y, et al. Low erythropoietin levels predict faster renal function decline in diabetic patients with anemia: a prospective cohort study. Sci Rep. 2019;9(1):14871. doi: 10.1038/s41598-019-51207-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 160.Symeonidis A, Kouraklis-Symeonidis A, Psiroyiannis A, Leotsinidis M, Kyriazopoulou V, Vassilakos P, et al. Inappropriately low erythropoietin response for the degree of anemia in patients with noninsulin-dependent diabetes mellitus. Ann Hematol. 2006;85(2):79–85. doi: 10.1007/s00277-005-1102-9. [DOI] [PubMed] [Google Scholar]
- 161.Pitt B, Agarwal R, Anker SD, Ruilope LM, Rossing P, Ahlers C et al. Association of finerenone use with reduction in treatment-emergent pneumonia and COVID-19 adverse events among patients with type 2 diabetes and chronic kidney disease: a FIDELITY pooled secondary analysis. JAMA Netw Open. 2022;5(10):e2236123. 10.1001/jamanetworkopen.2022.36123. [DOI] [PMC free article] [PubMed]
- 162.Ma Z, Patel N, Vemparala P, Krishnamurthy M. Metformin is associated with favorable outcomes in patients with COVID-19 and type 2 diabetes mellitus. Sci Rep. 2022;12(1):5553. doi: 10.1038/s41598-022-09639-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 163.Gou L, Liu G, Ma R, Regmi A, Zeng T, Zheng J, et al. High fat-induced inflammation in vascular endothelium can be improved by Abelmoschus esculentus and metformin via increasing the expressions of miR-146a and miR-155. Nutr Metab (Lond) 2020;17:35. doi: 10.1186/s12986-020-00459-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 164.Carl JW, Jr., Trgovcich J, Hannenhalli S. Widespread evidence of viral miRNAs targeting host pathways. BMC Bioinform. 2013;14 Suppl 2(Suppl 2):S3. 10.1186/1471-2105-14-s2-s3. [DOI] [PMC free article] [PubMed]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
All data analysed during this narrative review are included in this published article.