Abstract
Materials of different allogeneic or xenogeneic or autologous origins are widely used as soft-tissue fillers or structural scaffolds in the field of cosmetic surgery, while complications including prosthesis infection, donor site deformity and filler embolization have always been difficult problems for plastic surgeons. The application of novel biomaterials may bring in hopeful solutions for these problems. Recently, some advanced biomaterials, such as regenerative biomaterials can effectively promote the repair of defective tissues, which have been proven to have good therapeutic as well as cosmetic effects in cosmetic surgery. Therefore, biomaterials with active compounds have drawn significant attention for the tissue regeneration of reconstructive and esthetic treatment. Some of these applications have achieved better clinical outcomes than traditional biological materials. This review summarized recent progress and clinical applications of advanced biomaterials in cosmetic surgery.
Keywords: applications, biomaterials, cosmetic, soft tissue, scaffolds, regenerative mechanism
Graphical Abstract
Introduction
Over the past few decades, the need for cosmetic surgery from the general population has increased dramatically as appearances have become more and more important in the modern society [1]. Rising demand for cosmetic surgery has also been witnessed worldwide [2]. Cosmetic surgeries are primarily elective procedures aiming to improve appearance. Extra cautions should be taken to minimize risks due to the esthetic rather than therapeutic purpose of the treatment. Risk of infection and long-term complications, including prosthesis deformation, displacement and bone absorption, have been reported in rhinoplasty, breast augmentation and additional plastic surgeries [3]. Autogaft such as autologous-fat and rib cartilage, are often scarce and may lead to donor-site complications [4]. All these clinical problems mainly come from the implanted biomaterials or the implants itself.
Recently, advanced biomaterials have been rapidly developed. Advanced biomaterials require their ability to promote tissue regeneration, without additional damage to donor site, risks of prosthetic rejection and long-term infection. For example, injectable biomaterial is a classical biomaterial to provide sustainable and biocompatible effects for facial esthetic injections [5, 6]. The number of repeated injections required is greatly reduced due to the promotion of tissue regeneration, while also reducing the risk of injection-related complications, including the most severe embolism. Vascular embolism, considered as the most serious complication of soft tissue filler injections, may occur less frequently, by promoting tissue regeneration to reduce the number of repeat injections. Ideally, advanced biomaterial would provide an instant filling effect and promote tissue regeneration to provide a long-term filling effect while the biomaterial would degrade over time. However, there has been a mismatch between the ideal filling effect provided by the biomaterial and the filling effect provided by the regenerated tissue for most biomaterials.
There are various advanced biomaterials for plastic surgery. Some have been well verified in clinical practice. Some long-term effects need further study. This review introduced various cosmetic biomaterials that have been successfully applied in clinical practice. Recent progress and clinical applications including injectable advanced biomaterials, additional components in biomaterials for promoting tissues regeneration were summarized [7, 8]. The challenges and future perspective were also discussed. However, translating advanced biomaterial to clinical application is facing great challenges [9]. The interaction between biomaterials and host tissues, the biocompatibility, safety and biodegradability of implanted biomaterials are important issues that need long-term in-depth study.
Advanced biomaterials for plastic surgery
From the perspective of clinical application, this review more focused on some advanced biomaterials with specific biofunctions, which may help tissue regeneration, biodegradable etc. right now, conventional biomaterials includes hyaluronic acid (HA), poly-l-lactic acid (PLLA), calcium hydroxylapatite (CaHA), bioceramics etc. have been well applied in clinical applications (Table 1).
Table 1.
Overview of advanced biomaterials in plastic surgery
| Clinical administration | Biomaterial | Material type | Clinical usage | Clinical degradation | Properties |
|---|---|---|---|---|---|
| Injection | HA |
|
Esthetic filler |
|
Promotes the generation of Type I and Type III Collagen |
| CaHA | Synthetic biomaterial | Esthetic filler |
|
Stimulates collagen generation | |
| PLLA [10] | Synthetic biomaterial | Esthetic filler |
|
Promotes the generation of Type I and Type III Collagen | |
| Collagen [11] | Natural biomaterial | Esthetic filler |
|
Replaces collagen environment lost with age | |
| Implant | ADM | Semisynthetic biomaterial | Support Silicone Breast Implants | Permanent | Scaffold for host cells to repopulate and revascularize |
| Bioceramics | Synthetic biomaterial | Bone defect reconstruction | Permanent | Promotes osteogenesis and osteoinductivity |
HA, hyaluronic acid; PRP, platelet-rich plasma; PLLA, poly-l-lactic acid; CaHA, calcium hydroxyapatite; ADM, acellular dermla matrices.
Injectable advanced biomaterials
In the facial area, aging is characterized by the volume loss of soft tissue, especially the atrophy of the skin, due to the shrinkage and redistribution of adipose tissue and the reduction of collagen produced by fibroblasts. Therefore, preventing the loss of subcutaneous fat or the reduction of dermal thickness represents an emerging strategy to combat aging of the face. The use of soft tissue fillers is one of the most common techniques to achieve facial rejuvenation. It increases the volume of soft tissues, flattened wrinkles and filled-up superficial defects immediately. However, side effects are not uncommon with filler injections, including pain, redness, hemorrhage, hematoma, erythema, allergic reactions, vascular events, infection, edema and late-onset adverse events [12].
In recent years, facial anti-aging strategy has developed from volume restoration to pursuing the biostimulatory effects of fillers, that is, the process of promoting the orderly production of cells and tissues by the body through regenerative component and restoring the function of the body tissues, which is the core of the modern anti-aging concept. An ideal filler material is one that stimulates collagen production and promotes extracellular matrix remodeling. Many of the injectable regenerative biomaterials already exist in human tissues, offering good biocompatibility. Biodegradable soft tissue fillers are usually resorbed by the body in 3–24 months [13]. To reduce the risk of complication and improve the acceptability of esthetic injection, it is vital to increase the sustainability of regenerative biomaterial or even make the esthetic effect permanent through regeneration of natural human tissue.
Hyaluronic acid
HA is a natural component in connective tissue extracellular matrix. Due to its water-absorbing and hydrophilic characteristics, small mass can occupy large volume with the effect of tissue expansion, at the same time withstand certain pressure, which makes it a filling material that can be applied for different signs of aging by replacing the lost volume [14]. HA has the advantages of being highly versatile and mucoadhesive. Therefore, HA can be used for carrying active factors and drugs [14].
Classification of HA
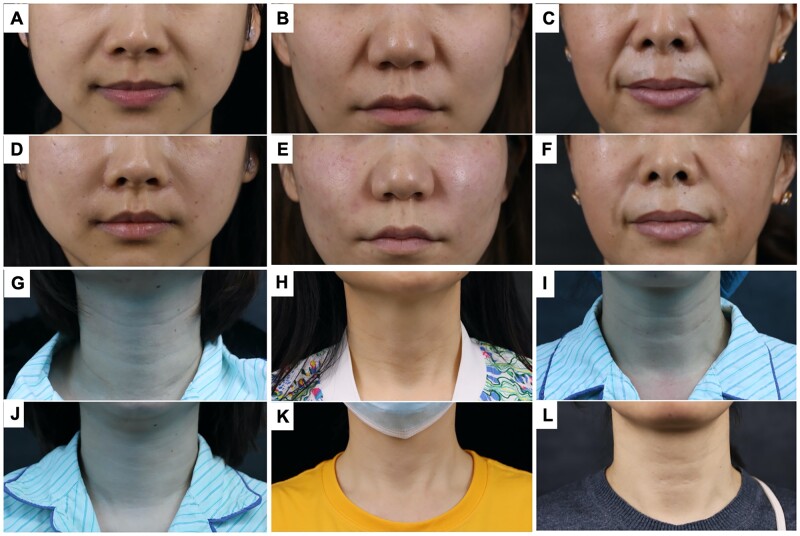
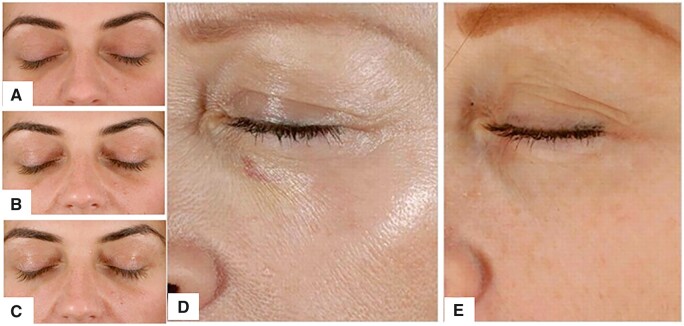
In its native form, HA is known as high molecular weight HA, HA with high molecular weight (HMWHA) usually combines with large amount of water, which can then be decomposed to low molecular weight HA (LMWHA) [14]. HMWHA exhibits anti-inflammatory and immunosuppressive properties, while LMWHA exhibits proinflammatory properties [15]. HMWHA can prevent cells from undergoing apoptosis and modulate cell receptors including CD44, which plays a significant role in promoting cell migration and wound healing [16–18]. Both HMWHA and LMWHA have demonstrated strong antioxidant capability [19, 20]. The level of HA cross-linking also affects the cosmetic effect. HA with higher levels of cross-linking provides more robust structural support through promoting type I collagen synthesis [21], while HA with a lower level of cross-linking provides a more elastic and conforming effect due to hydration. Lannitti et al. [22] combined cross-linked HA and non-cross-linked HA in the rejuvenation of the skin and achieved satisfactory outcomes. The cross-linked HA is firstly injected to provide structural support, and then non-cross-linked HA is injected to facilitate a more natural appearance similar to the borderline skin (Fig. 1).
Figure 1.
HA used in treatment of nasolabial folds (A–C: pretreatment; D–F: post-treatment) and neck wrinkles (G–I: pretreatment; J–L: post-treatment).
Regenerative ability
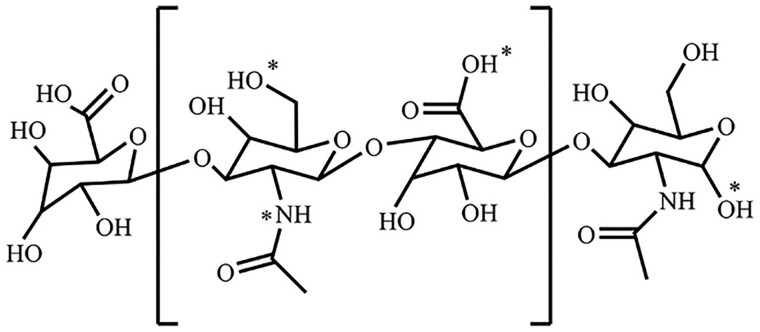
As an injectable biomaterial, HA is commonly used in esthetic surgery. In addition to improving skin hydration and its antioxidant potential [14], HA promotes skin cell regeneration and stimulates dermal fibroblasts to produce collagen [16–18]. Its neocollagenesis mechanism is mainly to promote the formation of new collagen through mechanical stretching and changes in the surrounding structure, thereby inducing the formation of cascades of collagen [14]. Furthermore, HA has a good modification site, so it can be modified to tailor its properties for soft tissue regeneration [14] (Fig. 2).
Figure 2.
The modification sites (*) of HA [23].
Ke et al. [24] analyzed the underlying inflammation induced by fillers on human skin explants. Cutaneous microdialysis techniques are used to measure levels of interleukin 8 (IL-8), tumor necrosis factor α (TNF-α) and histamine. Injections of fillers have been shown to cause changes of local mechanical stress. This mechanical stress further causes changes in the biochemical, metabolic and secretory patterns of surrounding cells. Wang et al. [25] compared forearm skin biopsies in individuals injected with HA or isotonic sodium chloride. Fibroblasts around the injection site have a pronounced elongated mechanically stretched appearance, and immunohistochemistry shows high levels of Type I procollagen. One month after injection, an increase in collagen production was observed and remained elevated for at least 3 months. Scarano et al. [6] reported the effect of LMWHA mixed with amino acid on the rejuvenation of the facial skin. The result showed regeneration of Type III reticular collagen and satisfactory clinical outcomes.
Complications
Early adverse effects include erythema, ecchymosis, hematoma, oedema, infection, anaphylaxis, vascular infarction, soft tissue necrosis, improper placement and distant spread. Late reactions include infection, nodule and granuloma formation, abscess and HA displacement [26]. Vascular infarction and skin necrosis is rare, but potentially extremely devastating complication. It is the result of untreated vascular damage and can be caused by blockage of arteries or veins. Possible causes are direct damage to the vessel wall, careless injection of injectable fillers into the vessel, or direct compression of fillers on the vessels, resulting in lumen obstruction. Injection-related edema is another possible mechanism that impairs blood flow by applying an external force to the walls of blood vessels. Initial signs and symptoms of impaired blood flow include pain, pallor, discoloration and slow capillary refill. If the artery is occluded, immediate and severe pain occurs, while venous occlusion usually presents with a delayed reticular violet appearance. Due to the biodegradability of the product, the use of hyaluronidase can correct adverse reactions at the moment of injection or after injection [27].
Calcium hydroxylapatite
CaHA is a kind of natural mineral existing in the bones which does not require allergy test [13, 28]. CaHA with different morphological structures and particle sizes has different biochemical properties, and the products currently used for cosmetic injections are usually in the form of semi-solid gel. Radiesse® (Merz Pharma GmbH & Co. KGaA, Frankfurt, Germany) contains uniform 25–45 μm CaHA microspheres suspended in a sodium carboxymethylcellulose (CMC) hydrogel matrix. CMC is a gel carrier that has excellent viscosity and elasticity, so it can fill wrinkles immediately after injection. CMC is gradually absorbed by the body after a few weeks of treatment (usually within 8 weeks) [29, 30].
Radiesse has a higher elastic modulus and viscosity compared to HA fillers, providing stronger supporting effect for the injected area. CaHA is FDA-approved for direct injection into the subcutaneous and deep subcutaneous tissues. After injection into the target location, the volume deficit is initially corrected by the gel components and as the gel is gradually absorbed, CaHA microspheres come into contact with the host tissue, activate fibroblasts and promote the regeneration of new tissue, including collagen, proteoglycan and elastin, and other fibrous tissue, which became the main supporting structure 2–3 months after injection [29]. The duration of the lasting effect of CaHA has been reported to range from 12 to 18 months [29, 30].
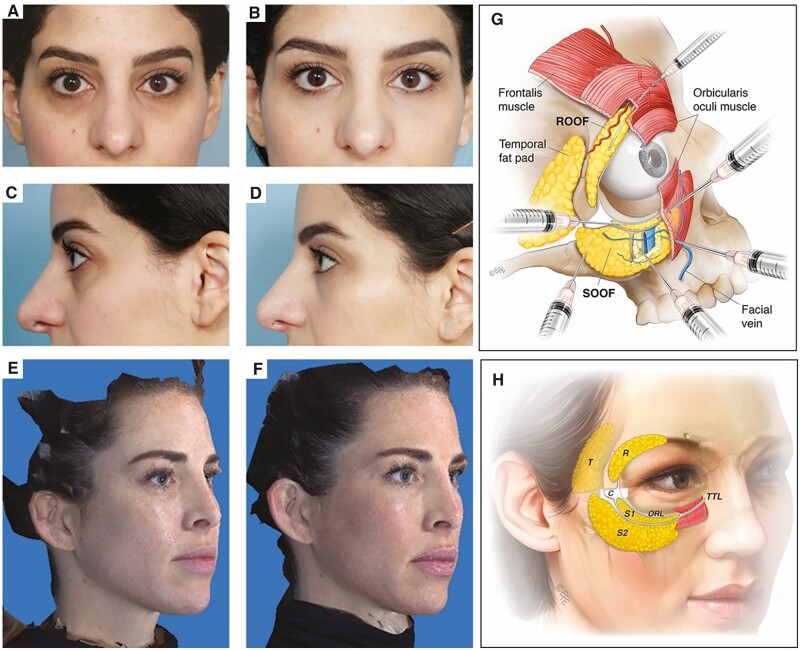
Since CaHA has strong supportive features and is not easily displaced, it is widely used in the treatment of subcutaneous tissue or deep dermal atrophy, including zygomatic area, sub-zygomatic area, suborbital area and nose filling, corner lines, marionette lines, chin lines, mandibular fovea etc. Beer et al. [5] reported an 88% satisfaction rate at 6 months and recommended it as a viable option to treat mid-face volume loss, but long-term results were not reported by this study. In a study with 12-month follow-up, CaHA was found to be significantly superior to nonanimal-stabilized HA (NASHA) [31] (Fig. 3).
Figure 3.
Frontal and lateral views before (A, C) and 2 weeks after CaHA injection (B, D). photographs showing right oblique views (E) before and 3 months after (F) injection of CaHA. The anatomical map (G) shows a safe ‘c-angle’ volume augmentation technique relative to the local anatomy [31].
Complications of Radiesse included cellulitis, tissue necrosis and nodule formation. More serious but uncommon complications include recurrence of shingles, arterial embolism leading to infarction, temporary blindness and oculomotor palsy. Among them, the most worrying complications are vascular damage and tissue necrosis. The interglabellar region is supplied by trochlear artery, resulting in the highest risk of tissue necrosis after filler injection. Nodules may appear when Radiesse is injected into more superficial areas such as the lip mucosa, tear trough and neck lines [32]. Unlike nodules of hyaluronidase-soluble HA-based fillers, CAHA-based filler nodules do not have an established dissolution method and can last for years. Recent case studies have shown that sodium thiosulfate (STS; 250 mg/ml) may be effective in dissolving CAHA nodules [33]. STS is a calcium chelator most commonly used for keratosis, dermatitis or calcifications. One hypothesis is that STS chelates with calcium to form a compound that can be removed through the lymphatic system. Another hypothesis is that it increases the solubility of calcium, thereby reducing the precipitation of calcium in tissues. Since the specific mechanism of STS is still unclear, cautions should be taken by applying conservative dosages. One possible regimen is to inject a small amount of STS into the nodule site (0.1–0.3 ml per palpable nodule) and repeat the injections as necessary at several visits. It is also important to note that it should not be mixed with lidocaine, as this may reduce efficacy.
Poly-L-lactic acid
PLLA was first used as a facial filler in 2004 for lipotrophic HIV patients. After that PLLA is more widely used to increase soft tissue volume.
Studies have shown that PLLA is a degradable regenerative biomaterial that promotes the synthesis of collagen in the injected area [34–36]. PLLA particles are between 40 and 63 μm in diameter. This particle size avoids phagocytosis by dermal macrophages and prevents passage through the capillary wall, but is small enough to be easily injected with a needle as fine as 26 gauge. After injection, PLLA filler maintains a certain lactate concentration by continuously releasing lactic acid to promote the host’s own collagen synthesis [34–36]. In rodents, infiltration of lymphocytes and activation of fibroblasts can be observed within extracellular matrix surrounding the microspheres; this is often expected in foreign body reactions [37, 38]. A histological analysis of nasolabial tissue at 12 and 30 months after injection of PLLA showed aggregation of collagen fibers and giant cells [34]. In a clinical study conducted by Stein et al. [35], PLLA was injected in the upper arm of 21 patients. After the injection, they found substantial Type III and Type I collagen and upregulated gene expression for their transcripts. At 9 months after the injection, the PLLA was not found under the microscope and was considered completely degraded [35].
In a study including 106 patients, 99.1% of satisfaction was achieved 2 years after injection in the upper face, middle face and lower face regions [39]. Palm et al. [40] retrospectively reviewed 130 patients who received PLLA, 75% of the patients rated the treatment effect as good to excellent. It is reported that the effect of PLLA may last as long as 3 years [41]. However, concerns have been raised regarding nodule and papule formation in the early use of PLLA [42], specific consideration of injection technique, the timing of injection sessions and injection volume should be taken.
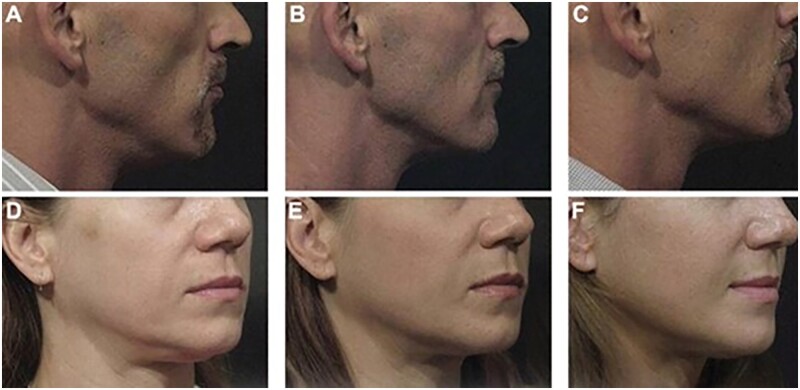
A 48-year-old man presented with facial lipoatrophy (Fig. 4A). The reduction of facial fat magnified bulges caused by muscles and bones. He was injected with three vials of PLLA at a time, three times every 6 weeks (9 vials in total) (Fig. 4B). Figure 4C shows the effect 3 months after the last treatment. Another 42-year-old woman came for aging treatment (Fig. 4D). She was injected with two vials of PLLA at a time and three injections every 4 weeks (six vials in total). Figure 4E shows the effect during treatment, and Fig. 4F was 1 year after last treatment (Fig. 4F) [43].
Figure 4.
PLLA used in enhanced facial contour [43].
Hexsel et al. [44] describe a new technique that enhances skin and subcutaneous tissue support by injecting small amounts of collagen stimulators into the cheekbones and preauricular area. It gently pulls up the skin, increases the firmness of the skin and delays the normal process of aging facial sagging.
In addition to its classic application in facial area, PLLA is currently used for volume increase, body contouring, sagging skin, scarring and fine line expansion in areas beyond the face such as neck and chest, buttocks, abdomen, arms, thighs, knees and hands. A recent survey reported that hip augmentation (42.4%) is the second most common use of PLLA in the USA, after HIV lipoatrophy (46.8%), which is enough to demonstrate the importance of PLLA in the field of body therapy [45].
Short-term adverse effects of PLLA injection include pain, edema, bleeding, ecchymosis, overcorrection, embolism and localized cellulitis. These side effects usually occur within a few days after the injection and heal on their own within 1–2 weeks [41]. Delayed and persistent nodule is a major complication of PLLA, with an incidence ranging from 5% to more than 40%, but nodule formation can be avoided by proper dilution, placing the product at a deeper anatomical level, minimizing volume in each injection site, and active post-treatment massage (at least five times a day for 5 min for more than 5 days). A retrospective study of more than 100 patients with PLLA injections for cosmetic needs has found that nodules were most likely to appear on the hands (12.5%) and cheeks (7.2%), so cautions should be taken when treating these areas [46]. Avoid injecting PLLA into the lips, as nodules are most likely to form. Treatment options for late-onset subcutaneous nodules include topical steroid injections, systemic steroids, systemic antibiotics, intense pulsed light, 5-fluorouracil, allopurinol and surgical excision.
Collagen is a fibrillar protein that naturally exists in many tissues of the human body. It serves a connective role in tissues of the skin, joint and bone [47]. In humans, collagen production decreases with aging, which causes facial wrinkles with a thin dermal layer and flat rete ridges [48]. The common natural source of collagen comes from animals, including bovine and porcine. There are as many as 26 types of collagen characterized by different amino acid sequences and different kinds of cross-linkage [47]. One study reported porcine Type I collagen achieved satisfactory correction of wrinkles in up to 12-month follow-up, comparable to that of bovine collagen [48]. Recent studies have reported that cross-linked collagen may have the ability to prolong the cosmetic effect and show comparable efficacy to that of HA [49, 50]. Delivery methods have also been changing to minimize trauma and discomfort during injection. Sun et al. [51] has successfully developed a polyvinylpyrrolidone microneedles system to deliver Type I collagen into human skin.
Filling the tear trough with collagen is a safe and minimally invasive cosmetic procedure. According to a clinical trial [52], a total of 10 female patients were treated with collagen material for tear trough deformity. All patients had excellent clinical outcomes. No swelling or lumps were observed after treatment. All patients resumed normal work and social activities immediately after treatment. At the 3-month follow-up, patient satisfaction was high (Fig. 5).
Figure 5.
(A–C) Pretreatment and post-treatment view of a 31-year-old woman. (D, E) Pretreatment and post-treatment view of a 55-year-old woman [52].
Implanted advanced biomaterials and 3D printing technology
Traditionally, autologous grafts and synthetic permanent implants have been used to repair structural defects or as an augmentation to achieve esthetic effects. However, both autologous grafts and synthetic permanent implants face many challenges. For the autologous graft, the challenges include additional operation conducted at the donor-site, which might lead to donor-site deficit and even further complications. For instance, thoracic deformity can be found in microtia children treated with autologous rib cartilage [53]. Additionally, autologous graft can be insufficient to achieve ideal surgical outcome [54]. For the synthetic permanent implant, challenges include risk of infection, which is always present as long as the implant is within the body. Also, due to the lack of integration with surrounding tissue, the long-term effect may be compromised. Pseudocapsule formation and bone resorption under stress have been reported in breast augmentation, mandible augmentation and rhinoplasty using silicone [3].
For any regenerative biomaterial, the scaffold is considered the basic structure that provides appropriate physical environment for tissue regeneration [55–57]. Various substances can be integrated or added into the scaffold to induce and accelerate the intended biological process (angiogenesis, fibrosis, osteogenesis etc.). The regenerative biomaterial scaffold may be used as a supplement instead of traditional material in plastic and cosmetic surgery. For example, bioceramics including HA, TCP and bioactive glasses have been selected for repairing bone defect. One of the advantages of ceramics constructs is that the ion-rich microenvironment promotes cell proliferation by close cell–cell interaction [58]. It provides structural support for bone tissue and has the potential to interact with the surrounding tissue as well. Recent progress has been made in constructing nano scaled biomimetic scaffolds, including ion-functionalized scaffolds, decellularized extracellular matrix scaffolds [59]. Some of them provide strong structural support as well as physical and bioactive properties, which promote tissue regeneration [60]. The osteogenesis and osteoinductivity can be further enhanced when other materials such as polymers are incorporated. In polymers, polyetherketoneketone has shown promising results in mechanical support and osteointegration in recent studies [61–63]. Hydroxyapatite combined with osteogenesis induction materials shows promising outcome in treating bone defects. Hydroxyapatite provides structural support for about 1-2 years before degradation while the osteogenesis process gradually fills the defected area.
The complexity of structure and function in the craniofacial areas makes reconstructing challenging. Extensive research has been conducted on the use of regenerative biomaterials and 3D printing technologies in the reconstruction of bone and cartilage defects in specific region [64]. Three-dimensional (3D) printing has the capability to remodel the regenerative biomaterial into an ideal shape based on personalized clinical needs. Porosity, elasticity, surface morphology, and other physical properties can be modified by integrating different material and printing techniques. A recent study has also shown the possibility of printing cells into a construct [65]. Inzana et al. [66] printed calcium phosphate constructure while incorporating a collagen coating, making the implant osteoconductive, biodegradable, and with a stable 3D structure. Another study using inkjet technique integrated other osteoconductive materials, including BMP-2, into micro-porous scaffolds and observed tissue formation [67]. Integrating 3D printing and regenerative biomaterial may provide a personalized, stable and self-regenerative biomaterial with added pro-regeneration ingredients.
Few attempts have been made in the clinical application of 3D printed regenerative biomaterial to reconstruct craniofacial defects. Brie et al. [68] used HA and resin as material to produce 3D-printed implants and treated 8 patients with craniofacial defects. In 12-month follow-up, no major complications were observed, and the cosmetic effect were considered satisfactory. Staffa et al. [69] developed fully biodegradable implant using high porosity HA, which achieved satisfactory esthetic result with no prosthesis fragmentation note in 12–79 months follow up. Hikita et al. [70] used α-TCP instead of HA or β-TCP as the printing material since there is no need for acid-based binders or polymer solution with α-TCP, therefore, it may provide better biodegradability.
Additional components for promoting tissues regeneration
On the basis of ensuring biocompatibility and safety, advanced biomaterials can be compounded with some other additional components. These additional components can generate some synergistic effects to promote tissue repair and regeneration. Key components including growth factors, platelet rich plasma (PRP), stem cells, extracellular vesicles (EVs) and growth factors, which can be integrated into biomaterials for further enhanced biofunctions.
Platelet rich plasma
Activated platelet release a variety of growth factors and cytokines, which mediate inflammation, angiogenesis and synthesis of ECM. Platelet-derived growth factors and fibroblast growth factors have had positive influence on inflammation response, granulation tissue formation and remodeling process. Vascular endothelial growth factor derived from PRP have been proved shown an important role in promoting the formation of skin capillaries, and it is conducive to skin repair and regeneration [71]. One study combined PRP and grafting biomaterial, and it showed superior osteogenesis compared with graft alone [72]. As endogenous signaling molecules, growth factors play a crucial role in regulating tissue regeneration.
In esthetic dermatology, collagen stimulation is the main purpose for using PRP. Studies have shown improved skin color and texture with PRP injection through intradermal or subdermal injection. In 5 months after three injections of PRP into the wrinkles of the face, significant improvement was found regarding general appearance, skin firmness and correction of wrinkles [73]. When combined with micron-needling, PRP effectively improved the appearance of acne scars [74]. PRP should have a concentration of platelets four to seven times above the physiologic concentration. However, the relationship between PRP concentration and its efficacy is not fully investigated [75–78].
Stem cell
Studies have shown that biomaterial combined stem cell therapy could promote muscle and bone regeneration [79]. Stem cells have the potential to differentiate into many esthetic related tissues. Stem cells are conducive to the differentiation of fibroblasts and endothelial cells, which is good for promoting muscle tissue regeneration. Biomaterials may regulate the microenvironment of the stem cell and promote proliferation and differentiation [80]. Biomaterial provides the surrounding environment to support specific cellular functions. Previous studies reported polymeric materials, including Collagen I, supported myogenesis [81]. The proteins, such as fibrin would promote myogenesis too. Some positive results have been observed in animal models of muscle trauma [82, 83].
Extracellular vesicles
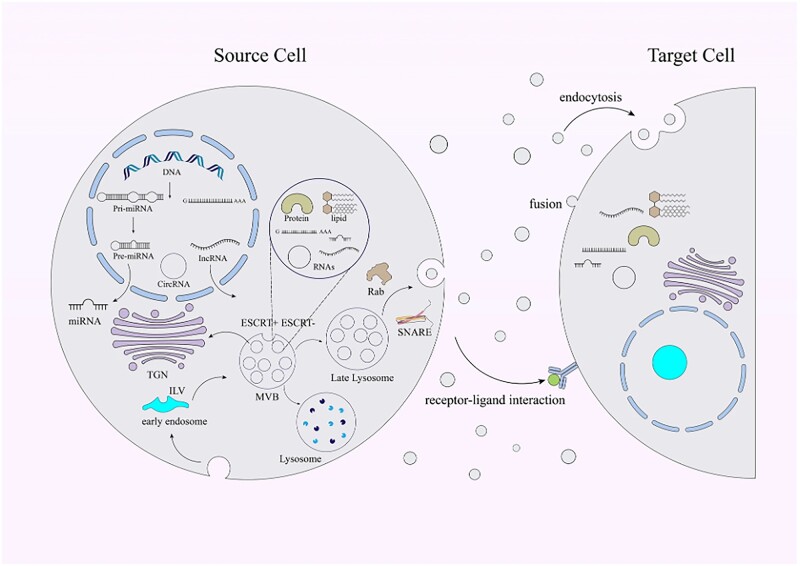
EVs have attracted considerable attention over the past few years. EVs are small particles of nanoscale vesicles with lipid membranes secreted by cells, which are shed by almost all types of cells in vitro and in vivo. The classifications of EVs mainly include apoptotic bodies, microvesicles and exosomes. They are consisted of phospholipid bilayers incorporating several different surface and membrane proteins. Furthermore, EVs can carry microRNA, proteins and resistance genes. EVs are cellular information disseminators which can specifically transfer biological information between donor and recipient cells. Therefore, EVs can be delivered by some biomaterials to treat diseases.
One of the advantages of EVs is that they exhibit low immunogenicity when used autologously. Therefore, EVs potentially have limited side effects. The size and lipid membrane composition of EVs allow them to fuse with target cells while avoiding degradation easily. In addition, EVs avoid inherent toxicity compared to synthetic nanoparticles (Fig. 6).
Figure 6.
Schematic illustration of the biogenesis, compositions and also release of the EVs [84].
Growth factor
Tissue regeneration aims to achieve functional recovery after injury by creating an environment that enables self-repair. In order to achieve tissue regeneration, it is necessary to introduce exogenous cells, biomaterial scaffolds and bioactive molecules into the tissue. Growth factors play important roles in directing regeneration pathways among these active ingredients. Growth factors belong to a new class of polypeptide hormones. These polypeptides can stimulate DNA synthesis and mitosis in cells. Growth factors have been isolated from platelets, submaxillary gland, pituitary gland, brain and cultured cells in vitro.
Exogenous growth factors are now widely used in trauma and wound healing treatment, such as epidermal growth factor and fibroblast growth factor, which are the main kinds of growth factors applied. Usually, biomaterials such as hydrogels are used as carriers, along with growth factors to be injected subcutaneously or into muscles, which can promote tissue repair. The deficiency of growth factors has been proven to be associated with delayed wound healing [85], which makes applying growth factors a potential treatment option. However, the effect of the therapeutic application of growth factors is limited because of the low stability and limited absorption. It is difficult to send enough active and stable growth factors to the designated area and maintain a specific concentration in the area. Delivery systems based on biomaterials may help us overcome this difficulty [86]. PLGA, alginate microspheres, HA and collagen have been used as the drug delivery systems either separately or combined [87–90]. Recently, developments have been reported in creating a porous topology of natural ECM using micro nanofibers electrospinning technique [91, 92].
Challenges and future perspective
On the premise of safety and minimizing complications, maximizing the effect of treatment is the unremitting pursuit for plastic surgery. Take ear reconstruction as an example, microtia is a congenital malformation of the external ear, which results in abnormal appearance and loss of function. Autologous rib cartilage remained the primary option for microtia reconstruction. However, scarcity of the cartilage, the influence of thoracic growth and the skill required for a surgeon to carve the complex 3D structure of the external ear remained some problems [4]. The utilization of regenerative biomaterial in this field solved the problem of source scarcity. More artificial synthetic biomaterials, instead of being taken from patients themselves, will be an important direction of development in cosmetic surgery field. In an ideal scenario, there is a dynamic balance between the beauty and the safe. Security, minimally invasive and permanent repair are the best choices. However, at present, how to accurately control compatibility, biodegradation, retention time and other aspects remains to be explored.
On the other hand, some challenges still need to be concerned. Some traditional biomaterials, such as CaHA, PMMA and PLLA for cosmetic injection, there are still many clinical cases of failure reported. Some permanent or semi-permanent biomaterials take years, or even decades, to degrade in vivo. Which may cause multiple complications after subcutaneous injection cases, including subcutaneous lesions, erythema, granuloma etc. The improvement of the biocompatibility, biosafety and biodegradability of these materials is a great challenge. In addition, different countries have different approval and access thresholds. How to effectively evaluate the safety of regulatory cosmetic surgery biomaterials is also another major challenge. These problems should not be ignored.
Conclusions
Although faced with various challenges, regenerative biomaterial provides promising treatments in the field of plastic surgery. Additional investigations should be focused on translating the research achievements to clinical practice in some of these promising regenerative biomaterials while ensuring biocompatibility and safety. The quality of ideal regenerative biomaterial includes: (i) lasting effect, (ii) safety, (iii) degradable, (iv) regenerative, (v) easy to remodel and (vi) dynamic balance between degradation and regeneration. Therefore, the regenerative biomaterial is gradually replaced by the regenerative tissue while sustaining satisfactory surgical effect throughout the treatment.
Contributor Information
Hairui Li, Department of Plastic Reconstructive and Aesthetic Surgery, West China Tianfu Hospital, Sichuan University, Chengdu 610213, China; Department of Burn and Plastic Surgery, West China School of Medicine, West China Hospital, Sichuan University, Chengdu 610041, China.
Xiujuan Xu, National Engineering Research Center for Biomaterials, Sichuan University, Chengdu 610064, China; College of Biomedical Engineering, Sichuan University, Chengdu 610064, China.
Lina Wu, National Engineering Research Center for Biomaterials, Sichuan University, Chengdu 610064, China; College of Biomedical Engineering, Sichuan University, Chengdu 610064, China.
Xi Chen, Department of Orthopedic Surgery, West China Hospital, West China School of Medicine, Sichuan University, Chengdu 610041, China.
Haris Akhter, Department of Plastic and Reconstructive Surgery, University of Nebraska Medical Center, Nebraska 68588, USA.
Yixi Wang, Department of Burn and Plastic Surgery, West China School of Medicine, West China Hospital, Sichuan University, Chengdu 610041, China.
Ping Song, National Engineering Research Center for Biomaterials, Sichuan University, Chengdu 610064, China; College of Biomedical Engineering, Sichuan University, Chengdu 610064, China.
Xiaoxia Liao, Department of Burn and Plastic Surgery, West China School of Medicine, West China Hospital, Sichuan University, Chengdu 610041, China.
Zhenyu Zhang, Department of Burn and Plastic Surgery, West China School of Medicine, West China Hospital, Sichuan University, Chengdu 610041, China; Department of Plastic Reconstructive and Aesthetic Surgery, West China Tianfu Hospital, Sichuan University, Chengdu 610213, China.
Zhengyong Li, Department of Burn and Plastic Surgery, West China School of Medicine, West China Hospital, Sichuan University, Chengdu 610041, China; Department of Plastic Reconstructive and Aesthetic Surgery, West China Tianfu Hospital, Sichuan University, Chengdu 610213, China.
Changchun Zhou, National Engineering Research Center for Biomaterials, Sichuan University, Chengdu 610064, China; College of Biomedical Engineering, Sichuan University, Chengdu 610064, China.
Ying Cen, Department of Burn and Plastic Surgery, West China School of Medicine, West China Hospital, Sichuan University, Chengdu 610041, China.
Hua Ai, National Engineering Research Center for Biomaterials, Sichuan University, Chengdu 610064, China; College of Biomedical Engineering, Sichuan University, Chengdu 610064, China.
Xingdong Zhang, National Engineering Research Center for Biomaterials, Sichuan University, Chengdu 610064, China; College of Biomedical Engineering, Sichuan University, Chengdu 610064, China.
Funding
This work was partially supported by the National Natural Science Foundation of China (81171731). 135 project for disciplines of excellence, West China Hospital Sichuan University (Nos ZYGD21001, ZYJC21026, ZYJC21077, ZYPY20003, ZYPY20004). Project of Chengdu Science and Technology Bureau (2021-YF05-01619-SN, 2021-RC05-00022-CG). Sichuan Science and Technology Program (2022YFG0066, 2022NSFSC0717). Science and Technology Project of Tibet Autonomous Region (XZ202202YD0013C).
Conflicts of interest statement. None declared.
References
- 1. Griffiths D, Mullock A.. Cosmetic surgery: regulatory challenges in a global beauty market. Health Care Anal 2018;26:220–34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Babadi H, Fereidooni-Moghadam M, Dashtbozorgi B, Cheraghian B.. Investigating psychosocial causes of the tendency for facial cosmetic surgery. Aesthetic Plast Surg 2018;42:1157–63. [DOI] [PubMed] [Google Scholar]
- 3. Rohrich RJ, Kaplan J, Dayan E.. Silicone implant illness: science versus myth? Plast Reconstr Surg 2019;144:98–109. [DOI] [PubMed] [Google Scholar]
- 4. Magritz R, Siegert R.. Auricular reconstruction: surgical innovations, training methods, and an attempt for a look forward. Facial Plast Surg 2014;30:183–93. [DOI] [PubMed] [Google Scholar]
- 5. Beer K, Yohn M, Cohen JL.. Evaluation of injectable CaHA for the treatment of mid-face volume loss. J Drugs Dermatol 2008;7:359–66. [PubMed] [Google Scholar]
- 6. Scarano A, Sbarbati A, Amore R, Iorio EL, Ferraro G, Marchetti M, Amuso D.. The role of hyaluronic acid and amino acid against the aging of the human skin: a clinical and histological study. J Cosmet Dermatol 2021;20:2296–304. [DOI] [PubMed] [Google Scholar]
- 7. Klopfleisch R, Jung F.. The pathology of the foreign body reaction against biomaterials. J Biomed Mater Res A 2017;105:927–40. [DOI] [PubMed] [Google Scholar]
- 8. Świętek M, Brož A, Tarasiuk J, Wroński S, Tokarz W, Kozieł A, Błażewicz M, Bačáková L.. Carbon nanotube/iron oxide hybrid particles and their PCL-based 3D composites for potential bone regeneration. Mater Sci Eng C Mater Biol Appl 2019;104:109913. [DOI] [PubMed] [Google Scholar]
- 9. Pashuck ET, Stevens MM.. Designing regenerative biomaterial therapies for the clinic. Sci Transl Med 2012;4:160sr4. [DOI] [PubMed] [Google Scholar]
- 10. Liu MH, Beynet DP, Gharavi NM.. Overview of deep dermal fillers. Facial Plast Surg 2019;35:224–9. [DOI] [PubMed] [Google Scholar]
- 11. Liu B, Xu Z, Yu R, Wang J, Wang Z, Harrell CR.. The use of type I and type III injectable human collagen for dermal fill: 10 years of clinical experience in China. Sem Plast Surg 2005;19:241–50. [Google Scholar]
- 12. Ozturk CN, Li Y, Tung R, Parker L, Piliang MP, Zins JE.. Complications following injection of soft-tissue fillers. Aesthet Surg J 2013;33:862–77. [DOI] [PubMed] [Google Scholar]
- 13. Kulichova D, Borovaya A, Ruzicka T, Thomas P, Gauglitz GG.. Understanding the safety and tolerability of facial filling therapeutics. Expert Opin Drug Saf 2014;13:1215–26. [DOI] [PubMed] [Google Scholar]
- 14. Aya KL, Stern R.. Hyaluronan in wound healing: rediscovering a major player. Wound Repair Regen 2014;22:579–93. [DOI] [PubMed] [Google Scholar]
- 15. Litwiniuk M, Krejner A, Speyrer MS, Gauto AR, Grzela T.. Hyaluronic acid in inflammation and tissue regeneration. Wounds 2016;28:78–88. [PubMed] [Google Scholar]
- 16. Lesley J, Hascall VC, Tammi M, Hyman R.. Hyaluronan binding by cell surface CD44. J Biol Chem 2000;275:26967–75. [DOI] [PubMed] [Google Scholar]
- 17. Jiang D, Liang J, Fan J, Yu S, Chen S, Luo Y, Prestwich GD, Mascarenhas MM, Garg HG, Quinn DA, Homer RJ, Goldstein DR, Bucala R, Lee PJ, Medzhitov R, Noble PW.. Regulation of lung injury and repair by toll-like receptors and hyaluronan. Nat Med 2005;11:1173–9. [DOI] [PubMed] [Google Scholar]
- 18. Bourguignon LY, Zhu H, Chu A, Iida N, Zhang L, Hung MC.. Interaction between the adhesion receptor, CD44, and the oncogene product, p185HER2, promotes human ovarian tumor cell activation. J Biol Chem 1997;272:27913–8. [DOI] [PubMed] [Google Scholar]
- 19. Ye J, Zhang H, Wu H, Wang C, Shi X, Xie J, He J, Yang J.. Cytoprotective effect of hyaluronic acid and hydroxypropyl methylcellulose against DNA damage induced by thimerosal in Chang conjunctival cells. Graefes Arch Clin Exp Ophthalmol 2012;250:1459–66. [DOI] [PubMed] [Google Scholar]
- 20. Ke C, Sun L, Qiao D, Wang D, Zeng X.. Antioxidant activity of low molecular weight hyaluronic acid. Food Chem Toxicol 2011;49:2670–5. [DOI] [PubMed] [Google Scholar]
- 21. Quan T, Wang F, Shao Y, Rittié L, Xia W, Orringer JS, Voorhees JJ, Fisher GJ.. Enhancing structural support of the dermal microenvironment activates fibroblasts, endothelial cells, and keratinocytes in aged human skin in vivo. J Invest Dermatol 2013;133:658–67. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Iannitti T, Morales-Medina JC, Coacci A, Palmieri B.. Experimental and clinical efficacy of two hyaluronic acid-based compounds of different cross-linkage and composition in the rejuvenation of the skin. Pharm Res 2016;33:2879–90. [DOI] [PubMed] [Google Scholar]
- 23. Huang G, Chen J.. Preparation and applications of hyaluronic acid and its derivatives. Int J Biol Macromol 2019;125:478–84. [DOI] [PubMed] [Google Scholar]
- 24. Ke C, Wang D, Sun Y, Qiao D, Ye H, Zeng X.. Immunostimulatory and antiangiogenic activities of low molecular weight hyaluronic acid. Food Chem Toxicol 2013;58:401–7. [DOI] [PubMed] [Google Scholar]
- 25. Wang F, Garza LA, Kang S, Varani J, Orringer JS, Fisher GJ, Voorhees JJ.. In vivo stimulation of de novo collagen production caused by cross-linked hyaluronic acid dermal filler injections in photodamaged human skin. Arch Dermatol 2007;143:155–63. [DOI] [PubMed] [Google Scholar]
- 26. Convery C, Davies E, Murray G, Walker L.. Delayed-onset nodules (DONs) and considering their treatment following use of hyaluronic acid (HA) fillers. J Clin Aesthet Dermatol 2021;14:E59–67. [PMC free article] [PubMed] [Google Scholar]
- 27. Aviv U, Haik J, Weiss N, Berl A, Ofir H, Nardini G, Cleary M, Kornhaber R, Harats M.. Treatment algorithm for hyaluronic acid-related complication based on a systematic review of case reports, case series, and clinical experience. Craniomaxillofac Trauma Reconstr 2020;13:313–28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Fallacara A, Manfredini S, Durini E, Vertuani S.. Hyaluronic acid fillers in soft tissue regeneration. Facial Plast Surg 2017;33:87–96. [DOI] [PubMed] [Google Scholar]
- 29. Jacovella PF. Use of calcium hydroxylapatite (Radiesse) for facial augmentation. Clin Interv Aging 2008;3:161–74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Goldie K, Peeters W, Alghoul M, Butterwick K, Casabona G, Chao YYY, Costa J, Eviatar J, Fabi SG, Lupo M, Sattler G, Waldorf H, Yutskovskaya Y, Lorenc P.. Global consensus guidelines for the injection of diluted and hyperdiluted calcium hydroxylapatite for skin tightening. Dermatol Surg 2018;44(Suppl 1):S32–41. [DOI] [PubMed] [Google Scholar]
- 31. Moers-Carpi MM, Tufet JO.. Calcium hydroxylapatite versus nonanimal stabilized hyaluronic acid for the correction of nasolabial folds: a 12-month, multicenter, prospective, randomized, controlled, split-face trial. Dermatol Surg 2008;34:210–5. [DOI] [PubMed] [Google Scholar]
- 32. Nipshagen M, Velthuis P, Cook E, Mosmuller D.. Non-inflammatory nodule formation after hyperdiluted calcium hydroxyapatite treatment in the neck area. Dermatol Ther 2020;33:e14272. [DOI] [PubMed] [Google Scholar]
- 33. Rullan P, Olson R, Lee K.. The use of intralesional sodium thiosulfate to dissolve facial nodules from calcium hydroxylapatite. Dermatol Surg 2020;46:1366–8. [DOI] [PubMed] [Google Scholar]
- 34. Vleggaar D, Bauer U.. Facial enhancement and the European experience with sculptra (poly-l-lactic acid). J Drugs Dermatol 2004;3:542–7. [PubMed] [Google Scholar]
- 35. Stein P, Vitavska O, Kind P, Hoppe W, Wieczorek H, Schürer NY.. The biological basis for poly-L-lactic acid-induced augmentation. J Dermatol Sci 2015;78:26–33. [DOI] [PubMed] [Google Scholar]
- 36. Goldberg D, Guana A, Volk A, Daro-Kaftan E.. Single-arm study for the characterization of human tissue response to injectable poly-L-lactic acid. Dermatol Surg 2013;39:915–22. [DOI] [PubMed] [Google Scholar]
- 37. Gogolewski S, Jovanovic M, Perren SM, Dillon JG, Hughes MK.. Tissue response and in vivo degradation of selected polyhydroxyacids: polylactides (PLA), poly(3-hydroxybutyrate) (PHB), and poly(3-hydroxybutyrate-co-3-hydroxyvalerate) (PHB/VA). J Biomed Mater Res 1993;27:1135–48. [DOI] [PubMed] [Google Scholar]
- 38. Anderson JM, Rodriguez A, Chang DT.. Foreign body reaction to biomaterials. Semin Immunol 2008;20:86–100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Schierle CF, Casas LA.. Nonsurgical rejuvenation of the aging face with injectable poly-L-lactic acid for restoration of soft tissue volume. Aesthet Surg J 2011;31:95–109. [DOI] [PubMed] [Google Scholar]
- 40. Palm MD, Woodhall KE, Butterwick KJ, Goldman MP.. Cosmetic use of poly-l-lactic acid: a retrospective study of 130 patients. Dermatol Surg 2010;36:161–70. [DOI] [PubMed] [Google Scholar]
- 41. Funt D, Pavicic T.. Dermal fillers in aesthetics: an overview of adverse events and treatment approaches. Clin Cosmet Investig Dermatol 2013;6:295–316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Bartus C, William Hanke C, Daro-Kaftan E.. A decade of experience with injectable poly-L-lactic acid: a focus on safety. Dermatol Surg 2013;39:698–705. [DOI] [PubMed] [Google Scholar]
- 43. Fitzgerald R, Graivier MH, Kane M, Lorenc ZP, Vleggaar D, Werschler WP, Kenkel JM.. Update on facial aging. Aesthet Surg J 2010;30(Suppl):11S–24S. [DOI] [PubMed] [Google Scholar]
- 44. Hexsel D, Camozzato F, Valente-Bezerra I, Silva A, Siega C.. L-Lift technique using poly-l-lactic acid: a pilot study. Dermatol Surg 2021;47:1087–92. [DOI] [PubMed] [Google Scholar]
- 45. Christen M. Collagen stimulators in body applications: a review focused on poly-L-lactic acid (PLLA). Clin Cosmet Investig Dermatol 2022;15:997–1019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Jeon Y, Koo D, Lee J.. Late onset foreign body reaction due to poly-L-lactic acid facial injections for cosmetic purpose. Ann Dermatol 2020;32:519–22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Avila Rodríguez MI, Rodríguez Barroso LG, Sánchez ML.. Collagen: a review on its sources and potential cosmetic applications. J Cosmet Dermatol 2018;17:20–6. [DOI] [PubMed] [Google Scholar]
- 48. Lee JH, Choi YS, Kim SM, Kim YJ, Rhie JW, Jun YJ.. Efficacy and safety of porcine collagen filler for nasolabial fold correction in Asians: a prospective multicenter, 12 months follow-up study. J Korean Med Sci 2014;29(Suppl 3):S217–21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Narins RS, Brandt FS, Lorenc ZP, Maas CS, Monheit GD, Smith SR.. Twelve-month persistency of a novel ribose-cross-linked collagen dermal filler. Dermatol Surg 2008;34(Suppl 1):S31–9. [DOI] [PubMed] [Google Scholar]
- 50. Monstrey SJ, Pitaru S, Hamdi M, Van Landuyt K, Blondeel P, Shiri J, Goldlust A, Shoshani D.. A two-stage phase I trial of Evolence30 collagen for soft-tissue contour correction. Plast Reconstr Surg 2007;120:303–11. [DOI] [PubMed] [Google Scholar]
- 51. Sun W, Inayathullah M, Manoukian MA, Malkovskiy AV, Manickam S, Marinkovich MP, Lane AT, Tayebi L, Seifalian AM, Rajadas J.. Transdermal delivery of functional collagen via polyvinylpyrrolidone microneedles. Ann Biomed Eng 2015;43:2978–90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Goldberg DJ. Correction of tear trough deformity with novel porcine collagen dermal filler (Dermicol-P35). Aesthet Surg J 2009;29:S9–S11. [DOI] [PubMed] [Google Scholar]
- 53. Nahabedian MY. Acellular dermal matrices in primary breast reconstruction: principles, concepts, and indications. Plast Reconstr Surg 2012;130:44s–53s. [DOI] [PubMed] [Google Scholar]
- 54. Al Sufyani MA, Al Hargan AH, Al Shammari NA, Al Sufyani MA.. Autologous fat transfer for breast augmentation: a review. Dermatol Surg 2016;42:1235–42. [DOI] [PubMed] [Google Scholar]
- 55. Pool SMW, Wolthuizen R, Mouës-Vink CM.. Silicone breast prostheses: a cohort study of complaints, complications, and explanations between 2003 and 2015. J Plast Reconstr Aesthet Surg 2018;71:1563–9. [DOI] [PubMed] [Google Scholar]
- 56. Deva AK, Merten S, Chang L.. Silicone in nasal augmentation rhinoplasty: a decade of clinical experience. Plast Reconstr Surg 1998;102:1230–7. [DOI] [PubMed] [Google Scholar]
- 57. Saleh HA, Lohuis PJ, Vuyk HD.. Bone resorption after alloplastic augmentation of the mandible. Clin Otolaryngol Allied Sci 2002;27:129–32. [DOI] [PubMed] [Google Scholar]
- 58. Lobo SE, Glickman R, da Silva WN, Arinzeh TL, Kerkis I.. Response of stem cells from different origins to biphasic calcium phosphate bioceramics. Cell Tissue Res 2015;361:477–95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59. Jiang S, Wang M, He J.. A review of biomimetic scaffolds for bone regeneration: toward a cell-free strategy. Bioeng Transl Med 2021;6:e10206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60. Zhu S, Zhang X, Chen X, Wang Y, Li S, Qiu G, Qian W.. Nano-Hydroxyapatite scaffold based on recombinant human bone morphogenetic protein 2 and its application in bone defect repair. J Biomed Nanotechnol 2021;17:1330–8. [DOI] [PubMed] [Google Scholar]
- 61. Roskies MG, Fang D, Abdallah MN, Charbonneau AM, Cohen N, Jordan JO, Hier MP, Mlynarek A, Tamimi F, Tran SD.. Three-dimensionally printed polyetherketoneketone scaffolds with mesenchymal stem cells for the reconstruction of critical-sized mandibular defects. Laryngoscope 2017;127:E392–8. [DOI] [PubMed] [Google Scholar]
- 62. Adamzyk C, Kachel P, Hoss M, Gremse F, Modabber A, Hölzle F, Tolba R, Neuss S, Lethaus B.. Bone tissue engineering using polyetherketoneketone scaffolds combined with autologous mesenchymal stem cells in a sheep calvarial defect model. J Craniomaxillofac Surg 2016;44:985–94. [DOI] [PubMed] [Google Scholar]
- 63. Alqurashi H, Khurshid Z, Syed AUY, Rashid Habib S, Rokaya D, Zafar MS.. Polyetherketoneketone (PEKK): an emerging biomaterial for oral implants and dental prostheses. J Adv Res 2021;28:87–95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64. Tao O, Kort-Mascort J, Lin Y, Pham HM, Charbonneau AM, ElKashty OA, Kinsella JM, Tran SD.. The applications of 3D printing for craniofacial tissue engineering. Micromachines (Basel) 2019;10. doi: 10.3390/mi10070480. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65. Shirazi SF, Gharehkhani S, Mehrali M, Yarmand H, Metselaar HS, Adib Kadri N, Osman NA.. A review on powder-based additive manufacturing for tissue engineering: selective laser sintering and inkjet 3D printing. Sci Technol Adv Mater 2015;16:033502. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66. Inzana JA, Olvera D, Fuller SM, Kelly JP, Graeve OA, Schwarz EM, Kates SL, Awad HA.. 3D printing of composite calcium phosphate and collagen scaffolds for bone regeneration. Biomaterials 2014;35:4026–34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67. Cooper GM, Miller ED, Decesare GE, Usas A, Lensie EL, Bykowski MR, Huard J, Weiss LE, Losee JE, Campbell PG.. Inkjet-based biopatterning of bone morphogenetic protein-2 to spatially control calvarial bone formation. Tissue Eng Part A 2010;16:1749–59. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68. Brie J, Chartier T, Chaput C, Delage C, Pradeau B, Caire F, Boncoeur MP, Moreau JJ.. A new custom made bioceramic implant for the repair of large and complex craniofacial bone defects. J Craniomaxillofac Surg 2013;41:403–7. [DOI] [PubMed] [Google Scholar]
- 69. Staffa G, Nataloni A, Compagnone C, Servadei F.. Custom made cranioplasty prostheses in porous hydroxy-apatite using 3D design techniques: 7 years experience in 25 patients. Acta Neurochir (Wien) 2007;149:161–70; discussion 170. [DOI] [PubMed] [Google Scholar]
- 70. Hikita A, Chung UI, Hoshi K, Takato T.. Bone regenerative medicine in oral and maxillofacial region using a three-dimensional printer. Tissue Eng Part A 2017;23:515–21. [DOI] [PubMed] [Google Scholar]
- 71. Zarei F, Soleimaninejad M.. Role of growth factors and biomaterials in wound healing. Artif Cells Nanomed Biotechnol 2018;46:906–11. [DOI] [PubMed] [Google Scholar]
- 72. Ortega-Mejia H, Estrugo-Devesa A, Saka-Herrán C, Ayuso-Montero R, López-López J, Velasco-Ortega E.. Platelet-rich plasma in maxillary sinus augmentation. Syst Rev Mater (Basel) 2020;13. doi: 10.3390/ma13030622. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73. Leo MS, Kumar AS, Kirit R, Konathan R, Sivamani RK.. Systematic review of the use of platelet-rich plasma in aesthetic dermatology. J Cosmet Dermatol 2015;14:315–23. [DOI] [PubMed] [Google Scholar]
- 74. El-Domyati M, Abdel-Wahab H, Hossam A.. Microneedling combined with platelet-rich plasma or trichloroacetic acid peeling for management of acne scarring: a split-face clinical and histologic comparison. J Cosmet Dermatol 2018;17:73–83. [DOI] [PubMed] [Google Scholar]
- 75. Graziani F, Ivanovski S, Cei S, Ducci F, Tonetti M, Gabriele M.. The in vitro effect of different PRP concentrations on osteoblasts and fibroblasts. Clin Oral Implants Res 2006;17:212–9. [DOI] [PubMed] [Google Scholar]
- 76. Yuksel EP, Sahin G, Aydin F, Senturk N, Turanli AY.. Evaluation of effects of platelet-rich plasma on human facial skin. J Cosmet Laser Ther 2014;16:206–8. [DOI] [PubMed] [Google Scholar]
- 77. Andia I, Abate M.. Platelet-rich plasma: underlying biology and clinical correlates. Regen Med 2013;8:645–58. [DOI] [PubMed] [Google Scholar]
- 78. Kim DH, Je YJ, Kim CD, Lee YH, Seo YJ, Lee JH, Lee Y.. Can platelet-rich plasma be used for skin rejuvenation? Evaluation of effects of platelet-rich plasma on human dermal fibroblast. Ann Dermatol 2011;23:424–31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79. Dunn A, Talovic M, Patel K, Patel A, Marcinczyk M, Garg K.. Biomaterial and stem cell-based strategies for skeletal muscle regeneration. J Orthop Res 2019;37:1246–62. [DOI] [PubMed] [Google Scholar]
- 80. Midgley AC, Wei Y, Li Z, Kong D, Zhao Q.. Nitric-oxide-releasing biomaterial regulation of the stem cell microenvironment in regenerative medicine. Adv Mater 2020;32:e1805818. [DOI] [PubMed] [Google Scholar]
- 81. Pollot BE, Rathbone CR, Wenke JC, Guda T.. Natural polymeric hydrogel evaluation for skeletal muscle tissue engineering. J Biomed Mater Res 2018;106:672–9. [DOI] [PubMed] [Google Scholar]
- 82. Huang YC, Dennis RG, Larkin L, Baar K.. Rapid formation of functional muscle in vitro using fibrin gels. J Appl Physiol (1985) 2005;98:706–13. [DOI] [PubMed] [Google Scholar]
- 83. Page RL, Malcuit C, Vilner L, Vojtic I, Shaw S, Hedblom E, Hu J, Pins GD, Rolle MW, Dominko T.. Restoration of skeletal muscle defects with adult human cells delivered on fibrin microthreads. Tissue Eng Part A 2011;17:2629–40. [DOI] [PubMed] [Google Scholar]
- 84. Bian D, Wu Y, Song G, Azizi R, Zamani A.. The application of mesenchymal stromal cells (MSCs) and their derivative exosome in skin wound healing: a comprehensive review. Stem Cell Res Ther 2022;13:24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85. Falanga V. Chronic wounds: pathophysiologic and experimental considerations. J Invest Dermatol 1993;100:721–5. [DOI] [PubMed] [Google Scholar]
- 86. Park JW, Hwang SR, Yoon IS.. Advanced growth factor delivery systems in wound management and skin regeneration. Molecules 2017;22:1259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87. Dong X, Xu J, Wang W, Luo H, Liang X, Zhang L, Wang H, Wang P, Chang J.. Repair effect of diabetic ulcers with recombinant human epidermal growth factor loaded by sustained-release microspheres. Sci China C Life Sci 2008;51:1039–44. [DOI] [PubMed] [Google Scholar]
- 88. Elçin YM, Dixit V, Gitnick G.. Extensive in vivo angiogenesis following controlled release of human vascular endothelial cell growth factor: implications for tissue engineering and wound healing. Artif Organs 2001;25:558–65. [DOI] [PubMed] [Google Scholar]
- 89. Niiyama H, Kuroyanagi Y.. Development of novel wound dressing composed of hyaluronic acid and collagen sponge containing epidermal growth factor and vitamin C derivative. J Artif Organs 2014;17:81–7. [DOI] [PubMed] [Google Scholar]
- 90. Shimizu N, Ishida D, Yamamoto A, Kuroyanagi M, Kuroyanagi Y.. Development of a functional wound dressing composed of hyaluronic acid spongy sheet containing bioactive components: evaluation of wound healing potential in animal tests. J Biomater Sci Polym Ed 2014;25:1278–91. [DOI] [PubMed] [Google Scholar]
- 91. Jayarama Reddy V, Radhakrishnan S, Ravichandran R, Mukherjee S, Balamurugan R, Sundarrajan S, Ramakrishna S.. Nanofibrous structured biomimetic strategies for skin tissue regeneration. Wound Repair Regen 2013;21:1–16. [DOI] [PubMed] [Google Scholar]
- 92. Jang JH, Castano O, Kim HW.. Electrospun materials as potential platforms for bone tissue engineering. Adv Drug Deliv Rev 2009;61:1065–83. [DOI] [PubMed] [Google Scholar]