Abstract
Background
Carpal tunnel syndrome (CTS) is a compression neuropathy of the median nerve causing pain and numbness and tingling typically in the thumb, index and middle finger. It sometimes results in muscle wasting, diminished sensitivity and loss of dexterity. Splinting the wrist (with or without the hand) using an orthosis is usually offered to people with mild‐to‐moderate findings, but its effectiveness remains unclear.
Objectives
To assess the effects (benefits and harms) of splinting for people with CTS.
Search methods
On 12 December 2021, we searched the Cochrane Neuromuscular Specialised Register, CENTRAL, MEDLINE, Embase, AMED, CINAHL, ClinicalTrials.gov, and WHO ICTRP with no limitations. We checked the reference lists of included studies and relevant systematic reviews for studies.
Selection criteria
Randomised trials were included if the effect of splinting could be isolated from other treatment modalities. The comparisons included splinting versus no active treatment (or placebo), splinting versus another disease‐modifying non‐surgical treatment, and comparisons of different splint‐wearing regimens. We excluded studies comparing splinting with surgery or one splint design with another. We excluded participants if they had previously undergone surgical release.
Data collection and analysis
Review authors independently selected trials for inclusion, extracted data, assessed study risk of bias and the certainty in the body of evidence for primary outcomes using the GRADE approach, according to standard Cochrane methodology.
Main results
We included 29 trials randomising 1937 adults with CTS. The trials ranged from 21 to 234 participants, with mean ages between 42 and 60 years. The mean duration of CTS symptoms was seven weeks to five years. Eight studies with 523 hands compared splinting with no active intervention (no treatment, sham‐kinesiology tape or sham‐laser); 20 studies compared splinting (or splinting delivered along with another non‐surgical intervention) with another non‐surgical intervention; and three studies compared different splinting regimens (e.g. night‐time only versus full time).
Trials were generally at high risk of bias for one or more domains, including lack of blinding (all included studies) and lack of information about randomisation or allocation concealment in 23 studies.
For the primary comparison, splinting compared to no active treatment, splinting may provide little or no benefits in symptoms in the short term (< 3 months). The mean Boston Carpal Tunnel Questionnaire (BCTQ) Symptom Severity Scale (SSS) (scale 1 to 5, higher is worse; minimal clinically important difference (MCID) 1 point) was 0.37 points better with splint (95% confidence interval (CI) 0.82 better to 0.08 worse; 6 studies, 306 participants; low‐certainty evidence) compared with no active treatment. Removing studies with high or unclear risk of bias due to lack of randomisation or allocation concealment supported our conclusion of no important effect (mean difference (MD) 0.01 points worse with splint; 95% CI 0.20 better to 0.22 worse; 3 studies, 124 participants). In the long term (> 3 months), we are uncertain about the effect of splinting on symptoms (mean BCTQ SSS 0.64 better with splinting; 95% CI 1.2 better to 0.08 better; 2 studies, 144 participants; very low‐certainty evidence).
Splinting probably does not improve hand function in the short term and may not improve hand function in the long term. In the short term, the mean BCTQ Functional Status Scale (FSS) (1 to 5, higher is worse; MCID 0.7 points) was 0.24 points better (95% CI 0.44 better to 0.03 better; 6 studies, 306 participants; moderate‐certainty evidence) with splinting compared with no active treatment. In the long term, the mean BCTQ FSS was 0.25 points better (95% CI 0.68 better to 0.18 worse; 1 study, 34 participants; low‐certainty evidence) with splinting compared with no active treatment.
Night‐time splinting may result in a higher rate of overall improvement in the short term (risk ratio (RR) 3.86, 95% CI 2.29 to 6.51; 1 study, 80 participants; number needed to treat for an additional beneficial outcome (NNTB) 2, 95% CI 2 to 2; low‐certainty evidence).
We are uncertain if splinting decreases referral to surgery, RR 0.47 (95% CI 0.14 to 1.58; 3 studies, 243 participants; very low‐certainty evidence).
None of the trials reported health‐related quality of life.
Low‐certainty evidence from one study suggests that splinting may have a higher rate of adverse events, which were transient, but the 95% CIs included no effect. Seven of 40 participants (18%) reported adverse effects in the splinting group and 0 of 40 participants (0%) in the no active treatment group (RR 15.0, 95% CI 0.89 to 254.13; 1 study, 80 participants).
There was low‐ to moderate‐certainty evidence for the other comparisons: splinting may not provide additional benefits in symptoms or hand function when given together with corticosteroid injection (moderate‐certainty evidence) or with rehabilitation (low‐certainty evidence); nor when compared with corticosteroid (injection or oral; low certainty), exercises (low certainty), kinesiology taping (low certainty), rigid taping (low certainty), platelet‐rich plasma (moderate certainty), or extracorporeal shock wave treatment (moderate certainty). Splinting for 12 weeks may not be better than six weeks, but six months of splinting may be better than six weeks of splinting in improving symptoms and function (low‐certainty evidence).
Authors' conclusions
There is insufficient evidence to conclude whether splinting benefits people with CTS. Limited evidence does not exclude small improvements in CTS symptoms and hand function, but they may not be clinically important, and the clinical relevance of small differences with splinting is unclear. Low‐certainty evidence suggests that people may have a greater chance of experiencing overall improvement with night‐time splints than no treatment. As splinting is a relatively inexpensive intervention with no plausible long‐term harms, small effects could justify its use, particularly when patients are not interested in having surgery or injections.
It is unclear if a splint is optimally worn full time or at night‐time only and whether long‐term use is better than short‐term use, but low‐certainty evidence suggests that the benefits may manifest in the long term.
Plain language summary
Splinting for carpal tunnel syndrome
Review question
This Cochrane review aimed to compare the benefits and harms of wrist splints with no treatment or other types of treatment for people with carpal tunnel syndrome (CTS).
Background
CTS is a condition where one of the two main nerves in the wrist is compressed. This can lead to pain in the hand and wrist as well as numbness and tingling in the thumb, index and middle finger. Severe compression may result in wasting of hand muscles and loss of dexterity of the hand. CTS is more common in women and in people over 50 years of age.
Many people undergo surgery to treat CTS, though usually non‐surgical treatments, such as splinting, corticosteroid injections (a drug that reduces inflammation) or exercises are offered first. Splinting involves immobilisation of the wrist in a neutral (straight) position, usually leaving the fingers and thumb free to move.
Study characteristics
We collected and analysed all relevant studies to answer our review question and found 29 studies that assessed the safety and benefit of splinting for people with CTS. The average ages of participants were between 42 and 60 years, the number of participants was 1937, and 81% were women. Most had mild‐to‐moderate symptoms.
Key results
When worn for fewer than three months, splinting may not improve CTS symptoms and probably does not improve hand function compared with no intervention. However, people who used a night‐time splint tended to report that overall they felt improvement compared with those that did not use a splint.
In the longer term (more than 3 months), we are still uncertain of the benefits of splinting due to few studies and inconsistent findings across similar studies. We cannot say for certain if splinting provides meaningful improvements in symptoms or function.
We are also uncertain if splinting reduces the need for surgery because only three studies reported this outcome. Splinting may cause temporary side effects such as difficulty in falling asleep or transient tingling after removal of the splint; none of the trials reported any serious side effects. None of the studies reported whether splints improved quality of life.
Some studies assessed if splinting improves outcomes when delivered alongside other treatments. The results suggested that splinting may make little or no difference to outcomes when given together with corticosteroid injection or with various types of rehabilitation.
Splinting was compared with other types of treatments. Splinting does not appear to improve outcomes compared with corticosteroid (injection or oral), exercises, kinesiology taping (stretchy tape), rigid taping, and probably does not improve outcomes compared with platelet‐rich plasma (concentrate of plasma and platelet derived from blood) or extracorporeal shock wave treatment (pulses of high energy sound).
Some studies compared different splint‐wearing regimens. One study found that six months of splinting may improve symptoms and function compared with six weeks of splinting. Another study found that full‐time splinting may not improve outcomes compared to night‐time splinting.
Author's conclusions
Currently, there is limited evidence supporting the use of wrist splints to treat CTS as there are few studies and their findings are inconsistent. While it appears that splinting may not make symptoms worse or result in side effects, splinting may provide little or no benefit for CTS symptoms and hand function, especially in the short term (less than 3 months). One study suggests that night‐time splinting may increase the chance of overall improvement compared with no treatment. Benefits of splinting may occur after months of use, but we need well‐designed research studies to establish how effective splinting is, and to identify the best way to use splints (night‐time or full‐time use; long‐term or short‐term use).
Splinting is relatively inexpensive and has no known long‐term side effects. Therefore, even small benefits may justify its use in people who are not interested in invasive interventions such as surgery.
Certainty of evidence
People in the studies were aware of their treatment. This knowledge can produce more favourable assessments of benefit than when people are unaware of treatment ('blinded'). In the few studies that examined the same treatments and outcomes, findings were inconsistent.
The evidence is up‐to‐date to December 2021.
Summary of findings
Summary of findings 1. SPLINT compared to NO ACTIVE TREATMENT for carpal tunnel syndrome.
| SPLINT compared to NO ACTIVE TREATMENT for carpal tunnel syndrome | ||||||
| Patient or population: carpal tunnel syndrome Setting: outpatient clinics in Italy, Thailand and Turkey; hospital clinic in Australia; education and research hospital in Turkey; auto assembly plant in the USA Intervention: SPLINT Comparison: NO ACTIVE TREATMENT | ||||||
| Outcomes | Anticipated absolute effects* (95% CI) | Relative effect (95% CI) | № of participants (studies) | Certainty of the evidence (GRADE) | Comments | |
| Risk with NO ACTIVE TREATMENT | Risk with SPLINT | |||||
| CTS symptoms (Boston CTS questionnaire) ‐ short‐term improvement: < 3 months Scale: 1 to 5, higher is worse | The mean CTS symptoms severity was 2.37 points | MD 0.37 points better (0.82 better to 0.08 worse) | ‐ | 306 (6 RCTs) | ⊕⊕⊝⊝ Lowa,b | Splint may not improve CTS symptoms in the short term. Absolute difference 9.25% better (20.5% better to 2% worse) with splintc |
| CTS symptoms (Boston CTS questionnaire) ‐ long‐term improvement: > 3 months Scale: 1 to 5, higher is worse | The mean CTS symptoms severity was 2.48 points | MD 0.64 points better (1.2 better to 0.08 better) | ‐ | 144 (2 RCTs) | ⊕⊝⊝⊝ Very lowa,b,d | We are uncertain if splint improves CTS symptoms in the long term. Absolute difference 16% better (30% better to 2% better) with splint |
| Function (Boston CTS questionnaire) ‐ long‐term improvement: > 3 months Scale: 1 to 5, higher is worse | The mean function was 1.77 points | MD 0.25 points better (0.68 better to 0.18 worse) | ‐ | 34 (1 RCT) | ⊕⊕⊝⊝ Lowa,d | Splint may not improve hand function in the long term. Absolute difference 6.25% better (17% better to 4.5% worse) with splint |
| Overall improvement (improved/not improved or worsened) ‐ short‐term improvement: < 3 months | Study population | RR 3.86 (2.29 to 6.51) | 80 (1 RCT) | ⊕⊕⊝⊝ Lowa,d | More people may report overall improvement in the short term with a splint than without a splint. Absolute risk difference 75% better (61% better to 89% better ) with splint. NNTB 2 (95% CI 2 to 2) | |
| 250 per 1000 | 965 per 1000 (573 to 1000) | |||||
| Health‐related quality of life ‐ long‐term improvement: > 3 months | No studies reported this outcome. | ‐ | (0 RCTs) | ‐ | Not estimable. We are uncertain about the effect. | |
| Adverse effects | Study population | RR 15.00 (0.89 to 254.13) | 80 (1 RCT) | ⊕⊕⊝⊝ Lowa,d | Splint may increase risk of transient adverse effects. Absolute risk difference 17% worse (5% worse to 30% worse) with splint | |
| Not calculable from the study data. 0/40 (0%) | Not calculable from the study data. 7/40 (18%) | |||||
| Referral for surgery | Study population | RR 0.47 (0.14 to 1.58) | 243 (3 RCTs) | ⊕⊝⊝⊝ Very lowa,e | We are uncertain if splint can reduce referral for surgery. Absolute risk difference 4% better (11% better to 3% worse) with splint | |
| 79 per 1000 | 37 per 1000 (11 to 125) | |||||
| *The risk in the intervention group (and its 95% CI) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). CI: confidence interval; MD: mean difference; RCT: randomised controlled trial; RR: risk ratio; CTS: carpal tunnel syndrome | ||||||
| GRADE Working Group grades of evidence High certainty: we are very confident that the true effect lies close to that of the estimate of the effect Moderate certainty: we are moderately confident in the effect estimate: The true effect is likely to be close to the estimate of the effect, but there is a possibility that it is substantially different Low certainty: our confidence in the effect estimate is limited: The true effect may be substantially different from the estimate of the effect Very low certainty: we have very little confidence in the effect estimate: The true effect is likely to be substantially different from the estimate of effect | ||||||
aDowngraded one level for high risk of bias in the included studies (lack of blinding) bDowngraded one level for inconsistency (the estimates were not consistent between the studies) cAbsolute risk difference calculated as risk in control group ‐ risk in the splinting group dDowngraded for imprecision (the 95% did not exclude clinically relevant effects) eDowngraded twice for very serious imprecision (95% CIs included substantial effect in both directions)
Background
Description of the condition
Carpal tunnel syndrome (CTS) refers to a condition where the median nerve function is compromised because of compression in the carpal tunnel. Symptoms of CTS include pain in the wrist and hand which can spread to the arm and paraesthesiae (numbness or tingling) in the thumb, index, middle and radial half of the ring finger (Atroshi 1999). Advanced CTS can result in loss of sensitivity in the thumb, index and middle finger, thenar muscle weakness and atrophy and subsequent loss of dexterity (Keir 2005). Suspected risk factors for CTS include diabetes, obesity, menopause, arthritis, hypothyroidism, smoking, and pregnancy (Padua 2016).
The course of CTS is not predictable: some people progress from intermittent paraesthesia to more constant paraesthesia, and eventual thenar atrophy, others experience intermittent exacerbation of sensory symptoms over many years, while others experience spontaneous (and lasting) remission (Braun 1989). There is no reliable data on the number of people who experience spontaneous remission, as such information is often based on assessment using nerve conduction studies, which have been found to correlate weakly with clinical outcomes (Hardoim 2009; Padua 1999; Resende 2003).
The reported prevalence and incidence of CTS has varied across studies depending on the diagnostic criteria used. Results of a Swedish study suggest that the prevalence of CTS in the general population is 3.8% for clinically diagnosed cases and 2.7% for electrophysiologically confirmed cases (Atroshi 1999), and as high as 7.8% in the U.S. working population (Dale 2013). Incidence was 1.7/1000 person years in Finland, and it is associated with age and sex (Pourmemari 2018). People aged less than 25 years accounted for 2.4% of people presenting to Australian general practices with the condition between 2000 and 2009, compared to people aged 45 to 64 years who accounted for 45.5% of these cases (Charles 2009). CTS is reported to affect more women than men (Padua 2016): 67% of CTS encounters at Australian general practices were in women (Charles 2009), and women in their fourth and fifth decades were four times more likely to suffer from CTS compared to men (Atroshi 1999). CTS has been reported to occur more frequently in some professions, where there is frequent grasping, forceful grasping and flexed wrist postures, or exposure to vibration from hand‐held tools (Palmar 2007).
Description of the intervention
Treatment options for CTS are either surgical or non‐surgical. Carpal tunnel release (CTR) has been reported as the most common surgery in the United States, with more than 400,000 CTRs performed annually, with an estimated total cost to the healthcare system of $2 billion (Concannon 2000; Huisstede 2010). Surgical treatment is usually offered to those with advanced CTS, who have constant symptoms, severe sensory disturbance, or thenar motor weakness. Non‐surgical treatments are recommended as an initial treatment for those who have symptoms without evidence of denervation, cannot undergo surgery, or have intermittent symptoms of mild‐to‐moderate CTS. Non‐surgical treatment for CTS includes various interventions such as wrist splinting (with or without the hand included), taping the wrist (e.g. kinesiology taping), injections (including corticosteroid or platelet‐rich plasma (PRP)) into the carpal canal, exercises, yoga, therapeutic ultrasound, laser, acupuncture, activity or ergonomic modification, oral medication, and vitamins (Dong 2020; Geler Kulcu 2016; Muller 2004; O'Connor 2012; Ostergaard 2020).
Splinting generally immobilises the wrist joint by using an external orthosis. The splint usually leaves the fingers and thumb free to move, but some designs may include the fingers. The wrist is generally positioned in a neutral position in the splint; although, the precise angle has yet to be determined, between less than 20 degrees extension and closer to zero degrees has been found to be optimal (Burke 1994). This splint may be worn either at night‐time only or during both the night and day. A thermoplastic splint may be custom fitted to the person with CTS by an occupational therapist, physiotherapist, or hand therapist. Sometimes, a softer, adjustable splint may be fitted; these splints can either be custom‐made or purchased off the shelf. Some splints may permit a restricted range of motion (Wang 2017).
How the intervention might work
In people with CTS, the wrist is usually splinted in a neutral position (i.e. with a straight wrist). When the wrist is in a neutral position, the pressure on the median nerve as it passes through the carpal tunnel is at its lowest. When the wrist is flexed or extended, the pressure increases (Gelberman 1984). As many people sleep with wrists in a flexed position, splinting at night maintains the wrist in the optimal position to reduce pressure within the carpal tunnel. Some have considered whether splinting the hand, in addition to the wrist, provides an additional benefit, as flexion of the fingers may further increase pressure in the carpal tunnel, through movement of the lumbricals, a group of small finger muscles, into the carpal tunnel (Manente 2001).
Why it is important to do this review
CTS creates significant impairment in terms of pain and functional use of the hand. Work days missed due to CTS result in financial loss to both the individual and society. Workers with a CTS diagnosis missed a median of 28 days of work to recuperate (U.S. Bureau of Labor Statistics 2016). For individuals suffering from symptomatic CTS, the direct and indirect costs can average USD 40,000 per year for life (Gabrielli 2020).
Following the publication of the previous versions of this review (O'Connor 2003; Page 2012b), which could not draw firm conclusions on the effect of splinting, the evidence base for all non‐surgical interventions for CTS has grown. Splinting is a common first‐line intervention for those with less severe symptoms or for those who do not wish to pursue more invasive treatment options, yet its efficacy is still unclear (Page 2012b). Cochrane systematic reviews of local corticosteroid injections (Marshall 2007), surgical versus non‐surgical treatment (Verdugo 2008), different surgical treatment options (Scholten 2007), therapeutic ultrasound (Page 2013), ergonomic interventions (O'Connor 2012), acupuncture (Choi 2018), low‐level laser therapy (Rankin 2017), exercise and mobilisation interventions (Page 2012a) for CTS already exist, and up‐to‐date Cochrane systematic reviews of other non‐surgical interventions for CTS (e.g. splinting, oral drugs) are required. Given the personal and financial impact of CTS, there is a need to ascertain the efficacy of splinting for the treatment of CTS.
Objectives
To assess the effects (benefits and harms) of splinting for people with carpal tunnel syndrome.
Methods
Criteria for considering studies for this review
Types of studies
All published and unpublished randomised controlled trials (RCTs) and quasi‐RCTs were eligible for inclusion regardless of publication status and whether they contained outcomes of interest or not. We did not use any language or publication date restrictions or limit the setting of the trials.
Types of participants
All study participants had a diagnosis of carpal tunnel syndrome (CTS), as defined by the authors of each study. We excluded studies that included participants who had previous surgery for CTS.
Types of interventions
We included all splinting interventions, including static (immobilisation), dynamic (allowing a limited range of motion within the splint), or splints aimed at stretching the transverse carpal ligament.
Comparators included no treatment, placebo and other non‐surgical interventions. We also included studies comparing different splinting regimens (i.e. different time periods, or night versus day versus full time).
We excluded the following.
Studies comparing splinting with other non‐surgical treatments that did not have plausible biological mechanism of action to modify the disease or interventions that could be considered as symptom‐modifying interventions. These included, for example, acupuncture, electroacupuncture, yoga, or topical flax seed oil, interferential current, transcutaneous electrical nerve stimulation and phonophoresis;
Studies in which the effect of splinting could not be isolated from the other treatment modalities. That is, splinting was delivered alongside another active treatment and the control group did not receive the same active co‐intervention;
Studies comparing splinting with surgical treatment (as these are reviewed elsewhere, Verdugo 2008);
Studies comparing various splint designs since these comparisons do not inform stakeholders if splints can provide benefits in people with CTS. Moreover, these comparison yield treatment estimates between a specific type of splints and these estimates are likely not applicable. However, these comparisons may be included in future updates if splinting is found to be efficacious.
Types of outcome measures
The outcomes reported in this review have been modified from the original review (O'Connor 2003) and its most recent update (Page 2012b); see Differences between protocol and review. For this update, we used CTS symptoms (continuous outcome) as the primary outcome, since global improvement is infrequently used and the Boston Carpal Tunnel Questionnaire (BCTQ) is a validated responsive measure used in most studies (Leite 2006; Multanen 2020). We prioritised the BCTQ Symptoms Severity Scale for symptoms and used pain (Visual Analogue Scale (VAS) or Numeric Rating Scale (NRS)) as a secondary source of data if the BCTQ was not measured or reported (5 of 11, or 45% items in the BCTQ Symptom Severity Scale are measuring pain).
Furthermore, we did not consider the electrodiagnostic outcomes (e.g. sensory or motor nerve conduction velocity) in this update, as their clinical relevance is unclear (Schrijver 2005; see Differences between protocol and review).
We planned to prioritise one‐year follow‐up for long‐term outcomes, but since no studies reported multiple long‐term time points, we did not have to choose between various time points.
Primary outcomes
-
CTS symptoms (prioritising BCTQ Symptom Severity Scale) at:
Short term (up to 3 months prioritising the time closest to 3 months); and
Long term (over 3 months).
Secondary outcomes
-
Function (CTS‐specific or hand‐specific patient‐reported outcome measure) at:
Short term (up to 3 months, prioritising the time closest to 3 months); and
Long term (over 3 months).
-
Overall improvement of symptoms (dichotomised from global scale (e.g. Likert) or binary outcome categorising participants as improved) at:
Short term (up to 3 months, prioritising the time closest to 3 months); and
Long term (over 3 months).
-
Health‐related quality of life at:
Short term (up to 3 months, prioritising the time closest to 3 months); and
Long term (over 3 months).
Adverse effects at the final time point of the study.
Referral for surgery (number of participants who were referred to surgery or operated, at the final time point of the study).
Search methods for identification of studies
Electronic searches
On 11 December 2020 and 12 December 2021, the Cochrane Neuromuscular Information Specialist searched the following databases for this version of the review:
the Cochrane Neuromuscular Specialised Register via the Cochrane Register of Studies (CRS‐Web) (until Search Date; Appendix 1)
the Cochrane Central Register of Controlled Trials (CENTRAL) via the Cochrane Register of Studies (CRS‐Web) (until Search Date; Appendix 2)
MEDLINE (1946 to 10 December 2021; Appendix 3)
Embase (1974 to Week 49 2021; Appendix 4)
AMED (1985 to December 2021; Appendix 5)
CINAHL Plus (1937 to 12 December 2021; Appendix 6)
ClinicalTrials.Gov (until Search Date; Appendix 7)
WHO ICTRP (until Search Date; Appendix 8)
There was no limitation to date of publication, language, publication status or document type.
Searching other resources
We browsed the reference lists of all included trials and relevant reviews for further relevant studies.
Data collection and analysis
The review authors followed the recommended strategies for data collection and analysis as documented in the Cochrane Handbook for Systematic Reviews of Interventions (Higgins 2021).
Selection of studies
Review authors (TK, VL, SP) working in pairs independently selected trials for possible inclusion based on the review inclusion criteria (study is an RCT or quasi‐RCT; study investigates splinting versus other non‐surgical treatment, no treatment, or placebo, or different splinting regimens for CTS). We then retrieved trials that were potentially eligible for full‐text evaluation to determine whether they met the inclusion criteria. The authors resolved any disagreement via discussion. We also searched PubMed for relevant errata or retraction statements for the included studies, and collated several references related to the same study.
Data extraction and management
Review authors (TK, VL, SP, MP, NMW, DOC) working in pairs independently extracted data from each study using a standardised data extraction form. Authors resolved any discrepancies by discussion. We pilot‐tested the data extraction form and modified it accordingly before use. We recorded the following details.
Participant details (number of participants randomised and analysed, sex, age, duration of symptoms).
Inclusion and exclusion criteria, as well as CTS diagnostic criteria.
Types of interventions used and details of the comparator.
Outcomes, including the type and timing of measures used.
Source of funding and investigators' conflicts of interest.
One review author (VL) compiled all data and entered the data into RevMan Web.
Assessment of risk of bias in included studies
Review authors (TK, VL, SP, MP, NMW, DOC) working in pairs independently assessed the risk of bias of the included studies using The Cochrane Collaboration's risk of bias tool, as described in the Cochrane Handbook for Systematic Reviews of Interventions (Higgins 2011). We assessed the following domains for risk of bias based on information extracted from the reports of the included studies.
Random sequence generation.
Allocation concealment.
Blinding of participants and personnel.
Blinding of outcome assessment.
Incomplete outcome data (defined separately for data measured at 3 months or less, and after 3 months).
Selective reporting.
Other sources of bias. (e.g. inappropriate unit of analysis).
The review authors rated each domain as being at 'low risk of bias', 'unclear risk of bias' or 'high risk of bias'. We resolved any discrepancies through discussion.
Measures of treatment effect
We used Cochrane Review Manager (RevMan) software to perform data analyses (Review Manager 2020). We expressed results as the risk ratio (RR) and 95% confidence interval (CI) for dichotomous outcomes and mean difference (MD) with 95% CI for continuous outcomes when the same measurement tool was used to measure the same outcome across all studies in the meta‐analysis.
When studies used different measurement instruments for the same outcome domain, we used a standardised mean difference (SMD) as a summary measure. We then back‐transformed the SMD to the typical outcome measure (multiplying the SMD and its 95% CI by a typical among‐person standard deviation (SD) (e.g. the SD of the control group at baseline from the most representative trial)). When the outcome measures had a different direction in a meta‐analysis (e.g. higher versus lower is better), we reversed the values so that the direction was the same in all studies.
For dichotomous outcomes, we calculated the number needed to treat for an additional beneficial outcome (NNTB) or number needed to treat for an additional harmful outcome (NNTH) as 1/risk difference when the analysis showed benefit or harm for splinting. NNTB are reported in whole numbers, rounded up.
We set statistical significance at P < 0.05 for all outcomes.
Unit of analysis issues
We sought information about the unit of randomisation used (i.e. wrists or participants, where participants with bilateral CTS received the same intervention for both wrists). In studies that randomised wrists, we sought information about whether each participant's wrist was allocated to different treatments, or whether there was no constraint that each participant's wrist be allocated to different treatments. We preferred participant‐level data whenever it was available. If the authors had randomised wrists and did not report results at the participant level and had not adjusted the analyses for clustering, we considered this a possible source of bias in the 'other bias' domain.
In case of multi‐arm studies, we compared the splinting arm with other eligible arms in separate analyses, avoiding double‐counting the same participants in the total number of the analysed participants.
Dealing with missing data
The review authors sought relevant missing information about the study design or results from the study investigators, where possible. We noted in the Characteristics of included studies tables when authors were contacted for additional data. When SDs of the mean were not reported, we calculated them based on the standard error of the mean, 95% CIs of the mean, reported P values, interquartile range, or range. When the SD was calculated using other measures, we noted this in the Characteristics of included studies table in the notes section.
Assessment of heterogeneity
We assessed clinical diversity by determining whether the characteristics of participants, interventions, outcome measures and timing of outcome measurement were similar across studies. Statistical heterogeneity was assessed by visual inspection of the forest plots and using the Chi2 statistic and the I2 test (Higgins 2002). We interpreted the I2 statistic using the following as an approximate guide (Deeks 2021).
0% to 40% might not be important heterogeneity.
30% to 60% may represent moderate heterogeneity.
50% to 90% may represent substantial heterogeneity.
75% to 100% may represent considerable heterogeneity.
Assessment of reporting biases
To assess publication bias, we intended to generate funnel plots if the review included at least 10 studies examining the same treatment comparison (Page 2021). To assess outcome reporting bias, we searched protocols of trials on the clinical trials register (clinicaltrials.gov), and at the International Clinical Trials Registry Platform of the World Health Organization (apps.who.int/trialssearch), to compare with the corresponding published RCTs (Dwan 2008; Dwan 2011). When the study was not registered, or we could not identify a published protocol, we deemed the study to be at unclear risk of selective reporting bias.
Data synthesis
We defined the following review questions based on the protocol in the previous version and based on the identified comparisons as follows.
Splint versus no active intervention
Splint versus corticosteroid injection
Splint versus oral steroid
Splint plus corticosteroid injection versus corticosteroid injection
Splint versus exercise
Stretching splint versus stretching exercises
Splint versus kinesiology taping
Splint versus rigid tape
Splint versus platelet‐rich plasma (PRP)
Splint versus extracorporeal shockwave therapy (ESWT)
Dynamic splint plus rehabilitation versus rehabilitation
Splint for six weeks versus splint for 12 weeks
Splint for six weeks versus splint for six months
Night‐time splinting versus full‐time splinting
We pooled the results of studies with similar characteristics (participants, interventions, outcome measures and timing of outcome measurement) in a random‐effects meta‐analysis (inverse variance method, DerSimonian‐Laird between‐study variance estimator, Wald‐type method for calculating the 95% CI of the summary effect) for each comparison to provide effect estimates for each outcome that was measured and reported. The primary analysis included all eligible studies. Where we could not pool data, we presented the results as reported by the authors narratively.
We used minimal clinically important difference (MCID) values to assess the clinical importance of differences in patient‐reported outcomes. A wide range of values have been reported as the MCID for the BCTQ Symptom Severity Scale (0.16 to 1.45) and Functional Status Scale (0.47 to 1.6) (De Kleermaeker 2018). We considered a one‐point difference as MCID for the BCTQ Symptom Severity Scale and 0.7 points for the Functional Status Scale (Kim 2013). Furthermore, we used 0.074 points difference as MCID for the EQ‐5D (Walters 2005).
Subgroup analysis and investigation of heterogeneity
We performed no subgroup analyses in this update, as data were not available, but we planned to do subgroup analyses regarding the primary outcome according to the severity of CTS symptoms and sex as per the previous review protocol.
Severity of CTS symptoms: early (E), intermediate (I) and advanced (A) symptoms (Szabo 1992)
Sex: male and female
Sensitivity analysis
To assess the robustness of our findings, we planned sensitivity analyses for studies with low risk of bias in all domains versus those with high or unclear; and low risk of selection bias versus high or unclear risk for the primary comparison (splint versus no active treatment). Since all studies were at high risk of detection and performance bias, we only conducted the latter sensitivity analysis regarding the primary outcome (CTS symptoms).
Summary of findings and assessment of the certainty of the evidence
We presented all outcomes for the primary comparison (splint compared to no active treatment) in the Table 1 and in the additional table (Table 2). We included one effect estimate for each of our primary and secondary outcomes (see Types of outcome measures) and included an overall grading of the evidence related to each of the main outcomes, using the GRADE approach (Schünemann 2017). For binary outcomes, we presented the assumed control group risk and relative risk in the splinting group. We also calculated and noted the absolute risk difference between the intervention and control group, as calculated in GRADEpro GDT (GRADEpro GDT 2021), expressed as a percentage. For continuous outcomes, we reported the weighted mean value for the control group (i.e. mean1 * sample size1 + mean2 * sample size2 + meanx * sample sizex/sum of sample sizes). The absolute difference is expressed as the mean difference (MD) with 95% confidence intervals. We also calculated the relative difference (relative to the scale of the measurement instrument; i.e. MD divided by the scale of the measure and expressed as a percentage).
1. SPLINT compared to NO ACTIVE TREATMENT for carpal tunnel syndrome ‐ outcomes that are not presented in the summary of findings table.
| SPLINT compared to NO ACTIVE TREATMENT for carpal tunnel syndrome ‐ outcomes that are not presented in the summary of findings table | ||||||
| Patient or population: carpal tunnel syndrome Setting: outpatient clinics in Italy, Thailand and Turkey; hospital clinic in Australia; education and research hospital in Turkey Intervention: SPLINT Comparison: NO ACTIVE TREATMENT | ||||||
| Outcomes | Anticipated absolute effects* (95% CI) | Relative effect (95% CI) | № of participants (studies) | Certainty of the evidence (GRADE) | Comments | |
| Risk with NO ACTIVE TREATMENT | Risk with SPLINT | |||||
| Functional status (Boston CTS questionnaire) ‐ short‐term improvement: < 3 months. Scale: 1 to 5, higher is worse | The mean function was 1.97 points | MD 0.24 points better (0.44 better to 0.03 better) | ‐ | 306 (6 RCTs) | ⊕⊕⊕⊝ Moderatea | Splint probably does not improve hand function in the short term. Absolute difference 6% better (11% better to 0.75% better) with splint |
| Overall improvement ‐ long‐term improvement: > 3 months | No studies reported this outcome | ‐ | (0 RCTs) | ‐ | Not estimable. We are uncertain about the effect. | |
| Health‐related quality of life ‐ short‐term improvement: < 3 months | No studies reported this outcome | ‐ | (0 RCTs) | ‐ | Not estimable. We are uncertain about the effect. | |
| *The risk in the intervention group (and its 95% CI) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). CI: confidence interval; MD: mean difference; RCT: randomised controlled trial; RR: risk ratio; CTS: carpal tunnel syndrome | ||||||
| GRADE Working Group grades of evidence High certainty: we are very confident that the true effect lies close to that of the estimate of the effect Moderate certainty: we are moderately confident in the effect estimate: The true effect is likely to be close to the estimate of the effect, but there is a possibility that it is substantially different Low certainty: our confidence in the effect estimate is limited: The true effect may be substantially different from the estimate of the effect Very low certainty: we have very little confidence in the effect estimate: The true effect is likely to be substantially different from the estimate of effect | ||||||
aDowngraded for high risk of bias in the included studies (lack of blinding)
Two review authors (TK, VL) assessed the certainty of the evidence as 'high', 'moderate', 'low', or 'very low' using the five GRADE considerations (study limitations, consistency of effect, imprecision, indirectness and publication bias) to assess the body of evidence. We used GRADEpro software to prepare the Summary of findings table (GRADEpro GDT 2021). We reported decisions to downgrade the certainty of evidence in the footnotes of the Summary of findings table and in the 'Results' section for each outcome. For comparisons and outcomes that were not included in the Summary of Findings table, the certainty of evidence was reported in the results section.
Results
Description of studies
See Characteristics of included studies; Characteristics of excluded studies.
Results of the search
Eight of the 19 trials included in the previous Cochrane Review met the inclusion criteria for this updated review due to the restriction in scope from the original review (De Entrambasaguas 2006, Madjdinasab 2008; Manente 2001; Mishra 2006; Premoselli 2006; Sevim 2004; Walker 2000; Werner 2005). One study awaiting classification in the previous Cochrane Review is now included in the updated review (Taspinar 2007).
The search was updated on 12 December 2021, and we identified 1643 new records, assessed 81 potentially eligible full texts and finally included 20 new studies (Akturk 2018; Chesterton 2018; De Moraes 2021; Eraslan 2014; Gatheridge 2020; Geler Kulcu 2016; Hall 2013; Jaladat 2017; Kocaoglu 2017; Oncu 2014; Rioja Toro 2012; Sanaee 2017; Schmid 2012; So 2018; Ulucakoy 2020; Wang 2017; Willis 2016; Wu 2017; Yazdanpanah 2012) (Figure 1).
1.
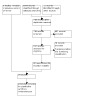
1 ‐ reasons for exclusion: in 5 studies the effect of splinting cannot be isolated from that of the other intervention delivered alongside it; in 29 studies splint applied to each study group; 7 studies compare treatment methods which we defined as not relevant for this review; 5 studies were not a randomised trial
Six studies are currently awaiting assessment for the following reasons:
It is unclear if the study is a randomised controlled trial (RCT) (Bhuva 2019; Riasi 2015);
Results are not yet published in a format that allows for risk of bias assessment and data extraction (Baklaci 2015; Soon 2015);
Two clinical trials from the World Health Organization (WHO) International Clinical Trials Registry Platform (ICTRP) search portal were marked as completed (IRCT2014020416485N1; ISRCTN22916517), but we found no published articles.
We identified seven ongoing studies that seemed to meet our inclusion criteria (Atroshi 2019; and six clinical trials from World Health Organization (WHO) International Clinical Trials Registry Platform (ICTRP) search portal and ClinicalTrials.gov: IRCT20120716010297N5; IRCT20200219046552N1; JPRN‐UMIN000017952; NCT04017390; NCT04515966; NCT04993703).
A flow diagram of the study selection process is presented in Figure 1.
Included studies
Twenty‐nine RCTs, published between and 2000 and 2021, were included in this review.
Participants
The 29 included studies comprised 1937 randomised participants. Some participants had bilateral carpal tunnel syndrome (CTS) and, thus, the studies included 2362 wrists. Three‐hundred‐and‐thirty (19%) participants were men, 1372 (81%) were women, and 235 randomised participants (from 9 studies) did not report the sex distribution (De Entrambasaguas 2006; Gatheridge 2020; Geler Kulcu 2016; Hall 2013; Manente 2001; Sanaee 2017; Schmid 2012; Sevim 2004; Werner 2005).
The trials size varied from 21 to 234 participants. Participants' mean age ranged between 42 and 60 years; one study (Yazdanpanah 2012) did not report the age of participants. The mean duration of CTS symptoms varied from seven weeks to five years in 16 studies; 13 studies did not report the mean duration of symptoms (Chesterton 2018; De Moraes 2021; Eraslan 2014; Jaladat 2017; Madjdinasab 2008; Manente 2001; Oncu 2014; Premoselli 2006; Rioja Toro 2012; Walker 2000; Werner 2005; Willis 2016; Yazdanpanah 2012). Most trialists excluded people with diabetes or rheumatoid arthritis, except for seven studies that included participants with these health conditions (Chesterton 2018; Hall 2013; Taspinar 2007; Ulucakoy 2020; Walker 2000; Werner 2005; Wu 2017). Most of the studies reported having pregnancy as exclusion criteria (Boonhong 2017; Chesterton 2018; Gatheridge 2020; Geler Kulcu 2016; Hall 2013; Jaladat 2017; Madjdinasab 2008; Manente 2001; Mishra 2006; Oncu 2014; Sanaee 2017; Schmid 2012; Sevim 2004; So 2018; Taspinar 2007; Ulucakoy 2020; Wang 2017; Werner 2005; Willis 2016; Wu 2017). One study specifically focused on pregnant women as their population of interest (Yazdanpanah 2012).
Participants in the included studies started with moderate impairment measured by the Boston Carpal Tunnel Questionnaire (BCTQ) Symptom Severity Scale (1 to 5, higher is worse) or Functional Status Scale (1 to 5, higher is worse). The mean symptom severity score at baseline was 2.79 (range from 1.66 to 3.65, data available n = 1689, 23 studies), and the mean functional status score was 2.37 (range from 1.25 to 4.05, data available n = 1577, 22 studies).
Interventions
Splint wear regimen and duration
Treatments varied in duration, type of splint and splint‐wearing regimen. The duration of splint use ranged from one week of nocturnal use (Schmid 2012), to one year of nocturnal use (Sevim 2004), with about half of studies (15 of 29) prescribing a regimen of between two and six weeks. The most common regimen was nocturnal wear (23 of 29 studies, in 8 of which also daytime wear was recommended whenever possible). One study specifically compared night‐time use with full‐time use (Walker 2000). One study did not report how the splint was worn (Kocaoglu 2017), and in two studies the duration of splint use was unclear (Rioja Toro 2012; Wu 2017).
Fifteen studies reported that they monitored compliance/adherence with splint use (Boonhong 2017; Chesterton 2018; Gatheridge 2020; Hall 2013; Manente 2001; Mishra 2006; Premoselli 2006; Sanaee 2017; Schmid 2012; Sevim 2004; So 2018; Walker 2000; Wang 2017; Werner 2005; Willis 2016). The reported compliance/adherence to splint use mainly varied from full to partial (we presented specific information for each study in the Notes section of Characteristics of included studies). Some studies excluded participants who did not comply with splint use from follow‐up (Premoselli 2006; Sanaee 2017). One study formed the control group from the subset of participants who did not comply with the splint regimen (Sevim 2004), but we combined data from the splinting group and this control group to perform an intention‐to‐treat analysis.
Types of splints
Splints were both custom‐made and commercially available. All splints involved wrist support at angles of 'neutral' to 20° of wrist extension. Most splints did not describe joint involvement other than the wrist, except for the MANU hand brace developed by Manente 2001 (fingers 2 to 5 were splinted), and in some cases the MCP joints were also splinted in a 'neutral' position. In one study, instead of immobilising the wrist, the 'Dynasplint' applied pressure across the base of the hand in order to stretch the transverse carpal ligament (Willis 2016). One study used a "limited dynamic wrist splint" that allowed the wrist to move between 15º of flexion and extension without radial or ulnar deviation of the wrist (Jaladat 2017).
Co‐interventions
Ten studies measured the effect of splints delivered with some other non‐surgical intervention: education (Hall 2013), ergonomic education (Boonhong 2017; Werner 2005), an exercise programme (Akturk 2018), a physical therapy programme (consisting of heat application‐ultrasound‐transcutaneous electrical nerve stimulation (TENS) and strengthening exercises) (Eraslan 2014), usual rehabilitation including activity or ergonomic modifications, nerve and tendon gliding exercises, massage, carpal bones and nerve mobilisations, stretches of the upper extremity and flexor retinaculum (Jaladat 2017), tendon and nerve gliding exercises (Geler Kulcu 2016; Oncu 2014), corticosteroid injection (Wang 2017), NSAID, B1 and B6, paraffin bath, ultrasound underwater and grip exercise (Sanaee 2017).
Outcomes
Primary outcome: CTS symptoms
We extracted symptom severity scores of the BCTQ (scale 1 to 5, higher is worse; Levine 1993), whenever possible. In one study (Sevim 2004), symptoms were measured by the Neurological Symptom Score (scale 0 to 3, higher is worse). Symptom severity was measured in 25 studies (Akturk 2018; Boonhong 2017; Chesterton 2018; De Moraes 2021; Eraslan 2014; Gatheridge 2020; Geler Kulcu 2016; Hall 2013; Jaladat 2017; Kocaoglu 2017; Manente 2001; Mishra 2006; Oncu 2014; Premoselli 2006; Rioja Toro 2012; Sanaee 2017; Schmid 2012; Sevim 2004; So 2018; Taspinar 2007; Ulucakoy 2020; Walker 2000; Wang 2017; Werner 2005; Willis 2016; Wu 2017). However, two of the studies did not report the scores and did not respond to queries (Willis 2016; Rioja Toro 2012).
Secondary outcomes
Secondary outcomes were function; overall improvement; health‐related quality of life score (HRQoL); adverse effects; and referral for surgery.
Function
The most commonly assessed secondary outcome was the BCTQ functional status score (scale 1 to 5, higher is worse), measured in 23 studies (Akturk 2018; Boonhong 2017; Chesterton 2018; De Moraes 2021; Eraslan 2014; Gatheridge 2020; Geler Kulcu 2016; Hall 2013; Jaladat 2017; Kocaoglu 2017; Manente 2001; Mishra 2006; Oncu 2014; Premoselli 2006; Rioja Toro 2012; Sanaee 2017; Schmid 2012; So 2018; Taspinar 2007; Ulucakoy 2020; Walker 2000; Wang 2017; Wu 2017).
Overall improvement
Overall improvement, using any measure where participants indicate the overall/global intensity of their complaints compared with baseline, was reported in three studies (De Moraes 2021; Manente 2001; Wang 2017). So 2018 reported satisfaction score on a five‐point scale.
HRQoL
Two studies reported HRQoL (Chesterton 2018 by EQ‐5D‐5L and Taspinar 2007 by Health Assessment Questionnaire).
Adverse events
Adverse effects of splint and other non‐surgical interventions for CTS were measured and reported in nine studies (Boonhong 2017; Chesterton 2018; De Entrambasaguas 2006; De Moraes 2021; Manente 2001; Mishra 2006; Sevim 2004; Taspinar 2007; Wu 2017).
Referral for surgery
Referral for surgery was measured in nine studies (Chesterton 2018; De Moraes 2021; Gatheridge 2020; Hall 2013; Manente 2001; Premoselli 2006; Sanaee 2017; Werner 2005; Willis 2016). However, this was incompletely reported in Hall 2013.
Twenty‐eight studies measured outcomes at short‐term follow‐up (up to 3 months after treatment). Eight studies measured outcomes at long‐term follow‐up (more than 3 months after treatment) (Chesterton 2018; De Moraes 2021; Premoselli 2006; Sanaee 2017; Sevim 2004; Werner 2005; Willis 2016; Wu 2017).
Unit of analysis
The unit of analysis was the wrist in 11 studies (Akturk 2018; De Entrambasaguas 2006; Gatheridge 2020; Geler Kulcu 2016; Mishra 2006; Oncu 2014; Rioja Toro 2012; Sanaee 2017; Taspinar 2007; Walker 2000; Yazdanpanah 2012), and some or all participants in these studies had bilateral CTS.
In seven of these studies (Gatheridge 2020; Geler Kulcu 2016; Mishra 2006; Oncu 2014; Sanaee 2017; Walker 2000; Yazdanpanah 2012), randomisation occurred at the level of participants, and the same intervention was delivered to both wrists.
In Rioja Toro 2012, the authors performed double randomisation at the wrist level (firstly to laser/placebo laser groups and then the same participants to splint/no splint groups), and each participant's wrists could be allocated to the same or different treatments.
It was unclear in three studies whether participants were randomised at the level of the person or the wrist or if people with bilateral CTS received the same or different interventions for each wrist (Akturk 2018; De Entrambasaguas 2006; Taspinar 2007).
The unit of analysis was the participant in 18 studies (Boonhong 2017; Chesterton 2018; De Moraes 2021; Eraslan 2014; Hall 2013; Jaladat 2017; Kocaoglu 2017; Madjdinasab 2008; Manente 2001; Premoselli 2006; Schmid 2012; Sevim 2004; So 2018; Ulucakoy 2020; Wang 2017; Werner 2005; Willis 2016; Wu 2017), even if some or all participants in these studies had bilateral CTS.
In 11 of these studies, only one side was assessed at follow‐up for people with bilateral CTS (Boonhong 2017; Chesterton 2018; De Moraes 2021; Hall 2013; Manente 2001; Premoselli 2006; Schmid 2012; Sevim 2004; So 2018; Wang 2017; Werner 2005). Chesterton 2018 also permitted treatment of the non‐study hand using the research clinical protocol.
In three studies, some included participants had bilateral CTS; however, no clear information was provided with respect to how the trial investigators dealt with and accounted for bilateral CTS in their study design and analysis (outcomes were analysed at the participant level) (Eraslan 2014; Madjdinasab 2008; Ulucakoy 2020).
It was unclear in three studies whether any participant had bilateral CTS and how the study dealt with bilateral CTS if such was present (outcomes were analysed at the participant level) (Jaladat 2017; Kocaoglu 2017; Willis 2016).
Wu 2017 included only people with unilateral CTS in the study and analysis.
Funding
Eight studies reported receiving financial support through various sources (Boonhong 2017; Chesterton 2018; Manente 2001; Schmid 2012; Walker 2000; Werner 2005; Willis 2016; Wu 2017). Three studies declared that no financial support was received (De Moraes 2021; Oncu 2014; Ulucakoy 2020). Eighteen studies did not report information related to the funding (Akturk 2018; De Entrambasaguas 2006; Eraslan 2014; Gatheridge 2020; Geler Kulcu 2016; Hall 2013; Jaladat 2017; Kocaoglu 2017; Madjdinasab 2008; Mishra 2006; Premoselli 2006; Rioja Toro 2012; Sanaee 2017; Sevim 2004; So 2018; Taspinar 2007; Wang 2017; Yazdanpanah 2012).
Excluded studies
In total, we excluded 72 studies after review of the full publication. Reasons for exclusion of studies are given in the 'Characteristics of excluded studies' table. The most common reasons for exclusion were:
Both groups followed a similar splinting regimen, thus the effect of splinting could not be assessed.
The effect of splinting could not be isolated from that of the other concomitant treatment(s) delivered alongside (i.e. splint was delivered with another treatment that was not applied in the control group).
The study compared treatment methods not relevant for this review (see Types of studies): splint and nerve‐ and tendon‐gliding exercises versus gabapentin and nerve‐ and tendon‐gliding exercises; splint versus surgery; splint versus yoga; splint versus acupuncture; splint versus interferential current; splint versus transcutaneous electrical nerve stimulation; splint versus ultrasound and transcutaneous electrical stimulation; splint versus flax seed oil topical gel; splint versus phonophoresis; splint versus phonophoresis with corticosteroid; splint versus phonophoresis with NSAID.
Risk of bias in included studies
For details of risk of bias in the included studies, see the 'Characteristics of included studies' tables and Figure 2.
2.

Methodological quality summary: review authors' judgements about each methodological quality item for each included study.
Allocation
Eleven studies reported a method of random sequence generation that we deemed adequate, and we rated them as at low risk of bias (Akturk 2018; Boonhong 2017; Chesterton 2018; Geler Kulcu 2016; Hall 2013; Mishra 2006; Oncu 2014; So 2018; Ulucakoy 2020; Wang 2017; Wu 2017). In four studies (Eraslan 2014; Premoselli 2006; Walker 2000; Werner 2005), the method of sequence generation was a type of alternation (i.e. non‐random), so we rated these studies at high risk of bias. Fourteen studies did not report enough information regarding the method of random sequence generation, therefore, we rated the risk of bias for this domain as unclear (De Entrambasaguas 2006; De Moraes 2021; Gatheridge 2020; Jaladat 2017; Kocaoglu 2017; Madjdinasab 2008; Manente 2001; Rioja Toro 2012; Sanaee 2017; Schmid 2012; Sevim 2004; Taspinar 2007; Willis 2016; Yazdanpanah 2012).
Nine studies described an adequate type of allocation concealment (Boonhong 2017; Chesterton 2018; De Moraes 2021; Gatheridge 2020; Geler Kulcu 2016; Oncu 2014; Schmid 2012; So 2018; Wang 2017). In four studies (Eraslan 2014; Premoselli 2006; Walker 2000; Werner 2005), the method of allocation was a type of alternation (i.e. non‐random), and therefore allocation was not concealed (high risk). Sixteen studies did not report enough information regarding the method of allocation concealment. Therefore, we rated the risk of bias for this domain as unclear (Akturk 2018; De Entrambasaguas 2006; Hall 2013; Jaladat 2017; Kocaoglu 2017; Madjdinasab 2008; Manente 2001; Mishra 2006; Rioja Toro 2012; Sanaee 2017; Sevim 2004; Taspinar 2007; Ulucakoy 2020; Willis 2016; Wu 2017; Yazdanpanah 2012).
Blinding
All participants were aware of the allocation. Although 12 studies reported blinding of assessors or clinicians (Akturk 2018; Boonhong 2017; De Entrambasaguas 2006; Geler Kulcu 2016; Oncu 2014; Premoselli 2006; Sanaee 2017; Schmid 2012; Sevim 2004; Wang 2017; Werner 2005; Wu 2017), we rated blinding of participants (performance bias) at high risk of bias in all 29 studies because all outcomes considered for this review were either self‐reported or could be influenced by the participant knowing the allocation. Participants must have known which group they belonged to from the differences between splinting and control interventions, and no study reported using a placebo splint.
Incomplete outcome data
We rated outcome data collected at three months or less at low risk of bias in 20 studies (Akturk 2018; Boonhong 2017; Chesterton 2018; De Moraes 2021; Eraslan 2014; Gatheridge 2020; Geler Kulcu 2016; Jaladat 2017; Manente 2001; Mishra 2006; Oncu 2014; Sanaee 2017; Schmid 2012; So 2018; Taspinar 2007; Ulucakoy 2020; Walker 2000; Wang 2017; Willis 2016; Wu 2017). In these 20 studies there was either a) no missing data or b) the amount of and reasons for missing data were similar across groups.
In four studies, we rated attrition bias as unclear because the trial authors reported insufficient information on dropouts or reasons for missing data (Kocaoglu 2017; Madjdinasab 2008; Premoselli 2006; Rioja Toro 2012). In five studies, we rated attrition bias as high because of either a) high or imbalanced loss to follow‐up (De Entrambasaguas 2006; Hall 2013; Werner 2005; Yazdanpanah 2012) or b) change of study protocol (in Sevim 2004 a control group was formed from the participants who did not adhere to the study protocol).
We rated six studies reporting outcome data at the long term at low risk of bias (Chesterton 2018; De Moraes 2021; Oncu 2014; Sanaee 2017; Willis 2016; Wu 2017) because there were either a) no missing data or b) the amount and reasons for missing data were similar across groups. We rated one trial as being at unclear risk because the reporting of information on dropouts or reasons for missing data was insufficient (Rioja Toro 2012). We rated one study as being at high risk of bias because of large attrition, which varied between reported outcomes (Werner 2005).
Selective reporting
In 19 studies, we rated the risk of bias from selective reporting as unclear, because, while all the outcomes specified in the 'Methods' section of the trial publication were reported, no study had a published protocol or trial registry entry, or studies did not report some outcomes as predefined (it was unclear in these instances if such reporting was because of the nature of findings) (Akturk 2018; Boonhong 2017; Chesterton 2018; De Entrambasaguas 2006; Eraslan 2014; Gatheridge 2020; Geler Kulcu 2016; Hall 2013; Kocaoglu 2017; Madjdinasab 2008; Oncu 2014; Rioja Toro 2012; Sanaee 2017; Sevim 2004; Taspinar 2007; Ulucakoy 2020; Werner 2005; Wu 2017; Yazdanpanah 2012). In nine studies, we rated the risk of selective reporting as being at low risk of bias, because all the outcomes specified were reported and the study protocol or registry record was available (De Moraes 2021; Jaladat 2017; Manente 2001; Mishra 2006; Premoselli 2006; Schmid 2012; So 2018; Walker 2000; Wang 2017). In one study, we rated the risk of selective reporting as being at high risk of bias, due to partial reporting of prespecified outcomes (Willis 2016).
Other potential sources of bias
We judged three studies as being at high risk of other potential sources of bias because of either a potential conflict of interest or unit of analysis issues (Manente 2001; Oncu 2014; Willis 2016).
Effects of interventions
See: Table 1
1. Splint versus no active treatment
Eight studies compared splinting with no active treatment (Boonhong 2017; Geler Kulcu 2016; Hall 2013; Manente 2001; Oncu 2014; Premoselli 2006; Rioja Toro 2012; Werner 2005). Four of the studies instructed the participants to wear the splints only at night‐time (Manente 2001; Premoselli 2006; Oncu 2014; Werner 2005), while three studies prescribed full‐time splinting (Boonhong 2017; Geler Kulcu 2016; Hall 2013). One study did not report sufficient information to be included in the meta‐analysis (Rioja Toro 2012).
Primary outcome: CTS symptoms
For short‐term follow‐up, we found low‐certainty evidence (downgraded once for risk of bias and once for inconsistency) from six studies indicating that splinting may not improve CTS symptoms compared with no active treatment (measured with the BCTQ Symptom Severity Scale from 1 to 5 points, higher is worse, minimal clinically important difference (MCID) value = 1 point). The heterogeneity may be explained by the risk of selection bias; studies at high risk found an effect, while studies at low risk did not. The mean symptom severity score was 2.37 with no active treatment and 0.37 points better with splint (95% confidence interval (CI) 0.82 better to 0.08 worse; 6 studies, 306 participants, I2 = 93%; Analysis 1.1; Table 1). Geler Kulcu 2016 and Manente 2001 reported data at four weeks; Hall 2013 reported data at eight weeks; Boonhong 2017 and Premoselli 2006 reported data at three months; and Oncu 2014 reported data at 25 days, two months and three months, from which we used the three‐month data.
1.1. Analysis.

Comparison 1: SPLINT VERSUS NO ACTIVE TREATMENT, Outcome 1: CTS symptoms (BCTQ)
For long‐term follow‐up, we downgraded the certainty of evidence to very low (once for risk of bias, once for inconsistency, and once for imprecision as the 95% CI overlapped with the MCID value). Thus, we are uncertain about the effect of splinting after three months. The mean symptom severity score was 2.48 with no active treatment and 0.64 points better with the splint (95% CI 1.2 better to 0.08 better, 2 studies, 144 participants, I2 = 83%; Analysis 1.1; Table 1). Premoselli 2006 reported data at six months, but Werner 2005 at 12 months.
Sensitivity analysis
After removal of studies with an unclear or high risk of selection bias, the effect moved towards splinting having a null effect. At short‐term follow‐up, the mean difference (MD) in the BCTQ symptom severity score between splint and no active treatment group was 0.01 points worse (95% CI 0.20 better to 0.22 worse, 3 studies, 124 participants, I2 = 1%). At long‐term follow‐up, there were no studies with a low risk of selection bias.
We could not study the effect of blinding in a sensitivity analysis because we did not identify any studies that had blinded the participants.
Secondary outcomes
1) Function
For short‐term follow‐up, we found moderate‐certainty evidence (downgraded once for risk of bias) from six trials that splinting probably does not provide a clinically meaningful improvement in hand function (measured by the BCTQ Functional Status Scale from 1 to 5 points, higher is worse, MCID value = 0.7 points) compared with no active treatment. The mean functional status score was 1.97 with no active treatment and 0.24 points better with splint (95% CI 0.44 better to 0.03 better, 6 studies, 306 participants, I2 = 66%; Analysis 1.2; Table 1). Geler Kulcu 2016 and Manente 2001 recorded data at four weeks; Hall 2013 recorded data at eight weeks; Boonhong 2017, Premoselli 2006 and Oncu 2014 recorded data at three months.
1.2. Analysis.

Comparison 1: SPLINT VERSUS NO ACTIVE TREATMENT, Outcome 2: Function (BCTQ)
At long‐term follow‐up, we graded the certainty of evidence as low (downgraded once for risk of bias and once for imprecision as only 1 study with a low number of participants, n = 34, contributed to the analysis and the 95% CIs touched the MCID value). The mean functional status score was 1.77 with no active treatment and 0.25 points better with the splint (95% CI 0.68 better to 0.18 worse, 1 study, 34 participants; Analysis 1.2; Table 1).
2) Overall improvement
One study reported overall improvement (measured by the Global Impression Change Questionnaire, rated in four categories from 'moderate' or 'much' improvement to 'worsening') at short‐term follow‐up (Manente 2001). Since the other studies did not measure this outcome, we could not assess the inconsistency. Manente 2001 also measured the BCTQ scores and was one of the studies reporting the benefit of splinting in the BCTQ Symptom Severity Scale analysis that had high heterogeneity (Analysis 1.1).
We rated the evidence for overall improvement as low certainty (downgraded once for risk of bias and once for imprecision, because only one study with 80 participants contributed to the analysis). The evidence indicates that night‐time splinting may result in higher rates of overall improvement compared with no active treatment. In the splinting group, 40 of 40 participants (100%) improved versus 10 of 40 participants (25%) in the no active treatment group, corresponding to a risk ratio (RR) of 3.86 (95% CI 2.29 to 6.51, 1 study, 80 participants) (Analysis 1.3; Table 1). This equates to a number needed to treat for an additional beneficial outcome (NNTB) of 2 (95% CI 2 to 2).
1.3. Analysis.

Comparison 1: SPLINT VERSUS NO ACTIVE TREATMENT, Outcome 3: Overall improvement
This outcome was not reported at long‐term follow‐up.
3) Health‐related quality of life
None of the studies in this comparison reported this outcome.
4) Adverse effects
Two studies reported this outcome (Boonhong 2017; Manente 2001). Participants who were prescribed a splint reported higher rate of adverse effects (difficulty in falling asleep, n = 3, and transient paraesthesias after removal of the splint, n = 4); however, the 95% CI overlap suggested that there could be no difference between groups. The certainty of evidence was downgraded to low (once for risk of bias and once for imprecision due to low number of events and overlapping 95% CIs suggesting there could be no effect).
Boonhong 2017 reported no serious adverse effects in either group and that "some minor adverse outcomes of splint treatment including itching and feelings of discomfort were reported" but did not report the exact numbers. Thus, this trial did not contribute to analyses.
In Manente 2001, adverse effects were reported by seven of 40 participants (17.5%) in the splinting group and by 0 of 40 participants (0%) in the no active treatment group corresponding to an RR of 15.0 (95% CI 0.89 to 254.13, 1 study, 80 participants) (Analysis 1.4; Table 1).
1.4. Analysis.

Comparison 1: SPLINT VERSUS NO ACTIVE TREATMENT, Outcome 4: Adverse effects
5) Referral for surgery
Three studies reported this outcome (Manente 2001; Premoselli 2006; Werner 2005). We downgraded the certainty of evidence to very low (once for risk of bias, and twice for very serious imprecision, as 95% CIs include substantial effect in both directions). In the splinting group, 4 of 129 participants (3%) were referred to surgery compared to 9 of 114 participants (8%) in the no active treatment group, corresponding to an RR of 0.47 (95% CI 0.14 to 1.58, 3 studies, 243 participants, I2 = 0%) (Analysis 1.5; Table 1). Hall 2013 reported that 19 of 30 participants (63%) had decided not to pursue surgical intervention after the CTS conservative treatment programme (which implies that 11 of 30 (37%) participants had opted for surgery). However, the outcome was not reported for the control group.
1.5. Analysis.

Comparison 1: SPLINT VERSUS NO ACTIVE TREATMENT, Outcome 5: Referral for surgery
2. Splint versus corticosteroid injection
Eight studies were included in this comparison (Chesterton 2018; De Entrambasaguas 2006; De Moraes 2021; Kocaoglu 2017; Sevim 2004; So 2018; Taspinar 2007; Yazdanpanah 2012), of which one study did not report sufficient data to be included in the meta‐analysis (Yazdanpanah 2012).
Primary outcome: CTS symptoms
Moderate‐certainty evidence (downgraded once for risk of bias) indicated that corticosteroids may provide a small but clinically unimportant benefit compared with splinting at short‐term follow‐up. Although the MD favoured corticosteroid injection, the 95% CIs excluded clinically meaningful benefit for injection. The mean symptom severity score (measured by the BCTQ, scale from 1 to 5, higher is worse, MCID value = 1 point) was 1.88 with corticosteroids and 0.28 points worse (95% CI 0.04 worse to 0.51 worse; 5 studies, 459 participants, I2 = 63%) with splints (Analysis 2.1 shows standardised mean difference (SMD) due to various measures at long term).
2.1. Analysis.

Comparison 2: SPLINT VERSUS CORTICOSTEROID INJECTION, Outcome 1: CTS symptoms
At long‐term follow‐up, the certainty of evidence was downgraded to low (due to the risk of bias and unexplained inconsistency), indicating that there may not be clinically important benefit between splint and corticosteroid injection. The SMD was 0.09 (95% CI ‐0.66 to 0.83, 3 studies, 437 participants, I2 = 93%) (Analysis 2.1). This translates to 0.06 points worse (95% CI 0.42 better to 0.52 worse) symptom severity score in the BCTQ Symptom Severity Scale with splinting compared with corticosteroid injection (using standard deviation (SD) of 0.63 at baseline from Chesterton 2018).
Secondary outcomes
1) Function
Moderate‐certainty evidence (downgraded once for risk of bias) indicates that splinting probably provides little or no benefits compared with corticosteroid injection at short‐term follow‐up. The mean functional status score measured by the BCTQ Functional Status Scale (scale from 1 to 5, higher is worse, MCID value = 0.7 points) was 1.76 for those who received a corticosteroid injection, and 0.16 points worse (95% CI 0.04 better to 0.36 worse, 5 studies, 459 participants, I2 = 44%) for those who were prescribed a splint (Analysis 2.2).
2.2. Analysis.

Comparison 2: SPLINT VERSUS CORTICOSTEROID INJECTION, Outcome 2: Function (BCTQ)
At long‐term follow‐up, the evidence was downgraded to very low (once for risk of bias, once for unexplained inconsistency and once for imprecision as the 95% CI overlapped with the MCID value). The mean functional status score was 1.91 for corticosteroid injection, and 0.33 points worse (95% CI 0.40 better to 1.06 worse, 2 studies, 329 participants, I2 = 89%) for those who were prescribed a splint (Analysis 2.2).
2) Overall improvement
De Moraes 2021 measured remission of nocturnal paraesthesias, and we used these data. Moderate‐certainty evidence (downgraded once for risk of bias) indicates that corticosteroid injection probably results in a higher rate of remission from nocturnal paraesthesias both at short‐term and long‐term follow‐up.
At short‐term follow‐up, 19 of 47 participants (40%) in the splinting group and 37 of 52 participants (71%) in the corticosteroid group had improved, corresponding to an RR of 0.57 (95% CI 0.39 to 0.84, 1 study, 99 participants; Analysis 2.3).
2.3. Analysis.

Comparison 2: SPLINT VERSUS CORTICOSTEROID INJECTION, Outcome 3: Overall improvement
At long‐term follow‐up, 13 of 45 participants (29%) in the splinting group and 40 of 50 participants (80%) in the corticosteroid group had improved, corresponding to an RR of 0.36 (95% CI 0.22 to 0.58, 1 study, 95 participants; Analysis 2.3).
So 2018 reported median satisfaction score (0 to 5, higher is better). Median satisfaction was 3 (range 1‐5) in the splinting group and 5 in the corticosteroid injection group.
3) Health‐related quality of life
Chesterton 2018 measured this outcome by EQ‐5D‐5L (scale from 0 to 1, higher is better) and Taspinar 2007 by Health Assessment Questionnaire (scale from 0 to 3, higher is worse). For short‐term follow‐up, we rated the certainty of evidence as low (downgraded once for risk of bias and once for imprecision as the 95% CIs overlapped with the MCID value of 0.074 points). The SMD was ‐0.25 (95% CI ‐0.77 to 0.27, 2 studies, 270 participants, I2 = 57%) favouring corticosteroid. This translates to 0.05 points worse in EQ‐5D‐5L (95% CI 0.15 worse to 0.05 better, MCID 0.074 points) for those prescribed a splint compared with corticosteroid injection (using an SD of 0.2 from Chesterton 2018) (Analysis 2.4).
2.4. Analysis.

Comparison 2: SPLINT VERSUS CORTICOSTEROID INJECTION, Outcome 4: Health‐related quality of life
At the long term, moderate‐certainty evidence (downgraded once due to risk of bias) indicates that splinting probably does not improve health‐related quality of life compared with corticosteroid injection. The mean EQ‐5D‐5L score was 0.82 in the corticosteroid group and 0.01 points better (95% CI 0.04 worse to 0.05 better, 1 study, 234 participants) for those prescribed a splint (Analysis 2.4 shows SMD due to several measures at the short term).
4) Adverse effects
Six studies reported adverse effects (Chesterton 2018; De Entrambasaguas 2006; De Moraes 2021; Sevim 2004; So 2018; Taspinar 2007). Reported adverse effects in the corticosteroid injection group were: skin changes (n = 4), hot flushes (n = 17), and short‐lasting or long‐lasting (over 3 days) pain (n = 53) (Chesterton 2018), vasovagal syncope (n = 1) (De Entrambasaguas 2006), short‐lasting pain (n = 2) or small haematoma (n = 1) (Sevim 2004), short‐lasting pain after the injection (n = 3) (So 2018), and increase in blood glucose level that required increasing the dose of oral antidiabetic drugs (n = 1) (Taspinar 2007). Adverse effects reported in the splinting group were discomfort (n = 11) (Chesterton 2018; So 2018) and allergic reaction on the skin (n = 1) (Sevim 2004).
We downgraded the evidence to very low (once for risk of bias, once for imprecision, and once for inconsistency).
Chesterton 2018 reported 74 of 116 participants (64%) having adverse effects in the corticosteroid injection group versus 7 of 118 participants (6%) having adverse effects in the splinting group.
De Entrambasaguas 2006 reported 1 of 24 participants (4%) having adverse effects in the corticosteroid injection group versus 0 of 26 participants (0%) having adverse effects in the splinting group.
De Moraes 2021 reported zero adverse effects in both groups.
Sevim 2004 reported 3 of 60 participants (5%) having adverse effects in the corticosteroid injection group versus 1 of 60 participants (2%) having adverse effects in the splinting group.
So 2018 reported 3 of 25 participants (12%) having adverse effects in the corticosteroid injection group versus 4 of 25 participants (16%) having adverse effects in the splinting group.
Taspinar 2007 reported 1 of 18 participants (5.5%) having adverse effects in the corticosteroid injection group versus 0 of 18 participants (0%) having adverse effects in the splinting group.
The pooled RR was 0.32 (95% CI 0.08 to 1.26, 6 studies, 590 participants, I² = 67%) (Analysis 2.5).
2.5. Analysis.

Comparison 2: SPLINT VERSUS CORTICOSTEROID INJECTION, Outcome 5: Adverse effects
5) Referral for surgery
Chesterton 2018 reported this outcome at six weeks and at six months follow‐up (in the analysis we included only the results from the 6 months follow‐up) and De Moraes 2021 reported at six months.
The certainty of evidence was rated as very low (downgraded once for risk of bias and twice for serious imprecision). At six months, 15 of 166 participants (9%) in the splinting group were referred to surgery compared to 25 of 168 (15%) in the corticosteroid group, corresponding to an RR of 0.60 (95% CI 0.33 to 1.09, 2 studies, 334 participants, I² = 0%) (Analysis 2.6).
2.6. Analysis.

Comparison 2: SPLINT VERSUS CORTICOSTEROID INJECTION, Outcome 6: Referral for surgery
3. Splint versus oral steroid
One study (Mishra 2006) with 76 participants provided data for this comparison at one month and three months follow‐up. We used the data from three months follow‐up for short‐term analysis. One study (Madjdinasab 2008) reported only electrophysiological outcomes and thus was not included in the meta‐analysis.
Primary outcome: CTS symptoms
For short‐term follow‐up, low‐certainty evidence (downgraded once for risk of bias and once for imprecision, as only one study with a low number of randomised participants, n = 76, contributed to the analysis) suggests that splinting and oral steroid may provide comparable benefits for improving symptoms. At short‐term follow‐up, the mean symptom severity score (measured by the BCTQ Symptom Severity Scale from 1 to 5 points, higher is worse, MCID value = 1 point) was 2.18 for those prescribed an oral steroid and 0.25 points worse (95% CI 0.03 better to 0.53 worse, 1 study, 71 participants) for those prescribed a splint (Analysis 3.1).
3.1. Analysis.

Comparison 3: SPLINT VERSUS ORAL STEROID, Outcome 1: CTS symptoms (BCTQ)
The only study in this comparison did not report this outcome at the long term.
Secondary outcomes
1) Function
For short‐term follow‐up, low‐certainty evidence (downgraded once for risk of bias and once for imprecision, as only 1 study with a low number of randomised participants, n = 76, contributed to the analysis) indicated that hand function (measured by the BCTQ Functional Status Scale from 1 to 5, higher is worse, MCID = 0.7 points) may not differ between splinting and oral steroid use. The mean functional status score was 1.45 for those prescribed an oral steroid and 0.12 points worse (95% CI 0.06 better to 0.3 worse, 1 study, 71 participants) for those prescribed a splint (Analysis 3.2).
3.2. Analysis.

Comparison 3: SPLINT VERSUS ORAL STEROID, Outcome 2: Function (BCTQ)
The only study in this comparison did not report this outcome at the long term.
2) Overall improvement
The only study in this comparison did not report this outcome.
3) Health‐related quality of life
The only study in this comparison did not report this outcome.
4) Adverse effects
Mishra 2006 reported 2 of 36 participants (6%) having adverse effects in the splinting group (discomfort and swelling of the hand) and 0 of 35 participants (0%) having adverse effects in the oral steroid group. This corresponds to an RR of 4.86 (95% CI 0.24 to 97.86, 1 study, 71 participants). The evidence was downgraded to very low (once for risk of bias and twice for very serious imprecision) indicating that we are uncertain about the risk for adverse effects between these two treatments (Analysis 3.3).
3.3. Analysis.

Comparison 3: SPLINT VERSUS ORAL STEROID, Outcome 3: Adverse effects
5) Referral for surgery
The only study in this comparison did not report this outcome.
4. Splint plus corticosteroid injection versus corticosteroid injection
One study with 52 participants provided data for this comparison at six weeks and 12 weeks follow‐up (Wang 2017). We used the data from 12 weeks follow‐up for short‐term analysis.
Primary outcome: CTS symptoms
For short‐term follow‐up, we graded the evidence as being of low certainty (downgraded once for risk of bias and once for imprecision, as only one study with a low number of participants, n = 52, contributed to the analysis). This evidence indicates that splinting given together with corticosteroid injection may not provide benefits in symptoms compared with corticosteroid injection alone. The mean symptom severity score (measured by the BCTQ Symptom Severity Scale from 1 to 5 points, higher is worse, MCID value = 1 point) was 1.49 with corticosteroid injection alone and 0.17 points better (95% CI 0.43 better to 0.09 worse, 1 study, 52 participants) with corticosteroid injection plus splint (Analysis 4.1).
4.1. Analysis.

Comparison 4: SPLINT PLUS CORTICOSTEROID INJECTION VERSUS CORTICOSTEROID INJECTION, Outcome 1: CTS symptoms (BCTQ)
The only study in this comparison did not report this outcome at the long term.
Secondary outcomes
1) Function
Low‐certainty evidence (downgraded once for risk of bias and once for imprecision as only one study with a low number of participants, n = 52, contributed to the analysis) indicated that splinting given together with corticosteroid injection may not provide benefits in function compared with corticosteroid injection alone at short‐term follow‐up. The mean functional status score (measured by the BCTQ Functional Status Scale from 1 to 5 points, higher is worse, MCID value = 0.7 points) was 1.32 with corticosteroid alone and 0.05 points better (95% CI 0.28 better to 0.18 worse, 1 study, 52 participants) with corticosteroid injection plus splint (Analysis 4.2).
4.2. Analysis.

Comparison 4: SPLINT PLUS CORTICOSTEROID INJECTION VERSUS CORTICOSTEROID INJECTION, Outcome 2: Function (BCTQ)
The only study in this comparison did not report this outcome at the long term.
2) Overall improvement
Low‐certainty evidence (downgraded once for risk of bias and once for imprecision) indicated that splinting given together with corticosteroid injection may not result in higher rates of overall improvement compared with corticosteroid alone at short‐term follow‐up. In the splint plus corticosteroid injection group, 20 of 26 participants (77%) reported 'complete recovery' or 'much improved' (measured by a 6‐point Likert‐type scale) compared with 15 of 26 participants (58%) in the corticosteroid injection group alone. This corresponds to an RR of 1.33 (95% CI 0.90 to 1.97, 1 study, 52 participants) (Analysis 4.3).
4.3. Analysis.

Comparison 4: SPLINT PLUS CORTICOSTEROID INJECTION VERSUS CORTICOSTEROID INJECTION, Outcome 3: Overall improvement
The only study in this comparison did not report this outcome at the long term.
3) Health‐related quality of life
The only study in this comparison did not measure this outcome.
4) Adverse effects
The only study in this comparison did not measure this outcome.
5) Referral for surgery
The only study in this comparison did not measure this outcome.
5. Splint versus exercise
One study (Schmid 2012) with 20 participants compared splinting with nerve and tendon gliding exercises at one week.
Primary outcome: CTS symptoms
Low‐certainty evidence (downgraded once for risk of bias and once for imprecision as only one study with a low number of participants, n = 20, contributed to the analysis) indicates that the effect of splinting and nerve and tendon gliding exercises may not differ at short‐term follow‐up. The mean symptom severity score (measured by the BCTQ Symptom Severity Scale from 1 to 5 points, higher is worse, MCID value = 1 point) was 1.73 with exercises and 0.12 points worse (95% CI 0.38 better to 0.62 worse, 1 study, 20 participants) with splinting (Analysis 5.1).
5.1. Analysis.

Comparison 5: SPLINT VERSUS EXERCISE, Outcome 1: CTS symptoms (BCTQ)
The only study in this comparison did not measure this outcome at the long term.
Secondary outcomes
1) Function
Low‐certainty evidence (downgraded once for risk of bias and once for imprecision as only 1 study with a low number of participants, n = 20, contributed to the analysis) indicates that function may not differ at the short term regardless of whether you are prescribed a splint or nerve and tendon gliding exercises. The mean functional status score (measured by the BCTQ Functional Status Score from 1 to 5 points, higher is worse, MCID value = 0.7 points) was 1.15 for those who were prescribed nerve and tendon gliding exercises, but was 0.30 points worse (95% CI 0.11 better to 0.71 worse, 1 study, 20 participants) for those who were prescribed a splint (Analysis 5.2).
5.2. Analysis.

Comparison 5: SPLINT VERSUS EXERCISE, Outcome 2: Function (BCTQ)
The only study in this comparison did not measure this outcome at the long term.
2) Overall improvement
The only study in this comparison did not measure this outcome.
3) Health‐related quality of life
The only study in this comparison did not measure this outcome.
4) Adverse effects
Schmid 2012 reported 0 of 10 participants (0%) having adverse effects in the splinting group and 0 of 10 participants (0%) having adverse effects in the nerve and tendon gliding exercise group. Thus, we could not estimate the risk (very low‐certainty evidence).
5) Referral for surgery
The only study in this comparison did not measure this outcome.
6. Stretching splint versus stretching exercises
One study with 50 participants compared Dynasplint (providing a low load transversal ligament stretching) with stretching exercises (Willis 2016).
Primary outcome: CTS symptoms
Willis 2016 reported symptom severity scores using an atypical scoring version of the BCTQ Symptom Severity Scale at short‐term follow‐up. According to the paper, the stretching splint (Dynasplint) group participants improved from 45.5 to 32.4 (P < 0.001) points, and in the stretching exercises group, the symptoms increased from 44.3 to 46.0 at two months.
The only study in this comparison did not measure symptoms at the long term.
Secondary outcomes
1) Function
The only study in this comparison did not measure this outcome.
2) Overall improvement
The only study in this comparison did not measure this outcome.
3) Health‐related quality of life
The only study in this comparison did not measure this outcome.
4) Adverse effects
The only study in this comparison did not measure this outcome.
5) Referral for surgery
Low‐certainty evidence (downgraded once for risk of bias and once for imprecision due to a low number of events) indicated that a stretching splint may decrease referral to surgery at one year. Willis 2016 reported that 7 of 25 participants (28%) had surgery in the stretching splint group and 16 of 25 participants (64%) in the stretching exercises group, corresponding to an RR of 0.44 (95% CI 0.22 to 0.88, 1 study, 50 participants) (Analysis 6.1).
6.1. Analysis.

Comparison 6: STRETCHING SPLINT VERSUS STRETCHING EXERCISES, Outcome 1: Referral for surgery
7. Splint versus kinesiology taping
Primary outcome: CTS symptoms
Four studies) provided data for this outcome (Akturk 2018; Geler Kulcu 2016; Kocaoglu 2017; Oncu 2014). Akturk 2018 reported data at six weeks; Geler Kulcu 2016 reported data at four weeks; Kocaoglu 2017 reported data at three weeks; Oncu 2014 reported data at 25 days, two months and three months, from which we used the data from 25 days as it was the closest time point to the other studies in this comparison.
For short‐term follow‐up, low‐certainty evidence (downgraded once for risk of bias, once for inconsistency and once for imprecision as the 95% CI overlapped with the MCID value) indicated that splinting may not improve symptoms compared with kinesiology taping. The 95% CI did not exclude a clinically important benefit for kinesiology taping up to three months after starting treatment. The mean symptom severity score (measured by the BCTQ Symptom Severity Scale from 1 to 5 points, higher is worse, MCID value = 1 point) was 2.05 with kinesiology taping and 0.49 points worse (95% CI 0.05 better to 1.03 points worse, 4 studies, 168 participants, I2 = 78%) with a splint (Analysis 7.1).
7.1. Analysis.

Comparison 7: SPLINT VERSUS KINESIOLOGY TAPING, Outcome 1: CTS symptoms (BCTQ)
None of the studies in this comparison reported this outcome at the long term.
Secondary outcomes
1) Function
Four studies (Akturk 2018; Geler Kulcu 2016; Kocaoglu 2017; Oncu 2014) provided data for this outcome. Akturk 2018 reported data at six weeks; Geler Kulcu 2016 reported data at four weeks; Kocaoglu 2017 reported data at three weeks; Oncu 2014 reported data at three months.
Low‐certainty evidence (downgraded once for risk of bias, once for inconsistency and once for imprecision as the 95% CI overlapped with the MCID value) indicated that splinting and kinesiology taping may have comparable effects on hand function at short‐term follow‐up. The 95% CI did not exclude clinically important benefit for kinesiology taping. The mean functional status score (measured by the BCTQ Functional Status Scale from 1 to 5 points, higher is worse, MCID value = 0.7 points) was 1.72 for those prescribed kinesiology tape and 0.11 points worse (95% CI 0.54 better to 0.75 worse, 4 studies, 168 participants, I2 = 79%) for those prescribed a splint (Analysis 7.2).
7.2. Analysis.

Comparison 7: SPLINT VERSUS KINESIOLOGY TAPING, Outcome 2: Function (BCTQ)
None of the studies in this comparison reported this outcome at the long term.
2) Overall improvement
None of the studies in this comparison reported this outcome.
3) Health‐related quality of life
None of the studies in this comparison reported this outcome.
4) Adverse effects
None of the studies in this comparison reported this outcome.
5) Referral for surgery
None of the studies in this comparison reported this outcome.
8. Splint versus rigid tape
One study (Eraslan 2014) with 30 participants provided data for this comparison at three weeks.
Primary outcome: CTS symptoms
For short‐term follow‐up, low‐certainty evidence (downgraded once for risk of bias and once for imprecision) suggests that rigid taping may slightly improve symptoms compared to night‐time splinting but the 95% CI are consistent with both clinically important and unimportant benefit. The mean symptom severity score (measured by the BCTQ Symptom Severity Scale from 1 to 5 points, higher is worse, MCID value = 1 point) was 1.48 with rigid tape and 1.05 points worse (95% CI 0.58 worse to 1.52 worse, 1 study, 30 participants) with a splint (Analysis 8.1).
8.1. Analysis.

Comparison 8: SPLINT VERSUS RIGID TAPE, Outcome 1: CTS symptoms (BCTQ)
The only study in this comparison did not measure this outcome at the long term.
Secondary outcomes
1) Function
Low‐certainty evidence (downgraded once for risk of bias and once for imprecision) suggests that hand function may be better after rigid taping compared with splinting at short‐term follow‐up. The 95% CIs excluded benefit for splinting. The mean functional status score (measured by the BCTQ Functional Status Scale from 1 to 5 points, higher is worse, MCID value = 0.7 points) was 1.71 for those prescribed rigid taping and 0.87 points worse (95% CI 0.48 worse to 1.26 worse, 1 study, 30 participants) for those prescribed a splint (Analysis 8.2).
8.2. Analysis.

Comparison 8: SPLINT VERSUS RIGID TAPE, Outcome 2: Function (BCTQ)
The only study in this comparison did not measure this outcome at the long term.
2) Overall improvement
The only study in this comparison did not measure this outcome.
3) Health‐related quality of life
The only study in this comparison did not measure this outcome.
4) Adverse effects
The only study in this comparison did not measure this outcome.
5) Referral for surgery
The only study in this comparison did not measure this outcome.
9. Splint versus platelet‐rich plasma (PRP)
One study (Wu 2017) with 60 participants provided data for this comparison at one, three, and six months follow‐up. We used the data from three months follow‐up for short‐term analysis.
Primary outcome: CTS symptoms
Low‐certainty evidence (downgraded once for risk of bias and once for imprecision as only one study with a low number of participants, n = 60, contributed to the analysis) suggests the symptom improvement between splinting and PRP may be clinically unimportant both at the short term and long term.
At short‐term follow‐up, the mean symptom severity score (measured by the BCTQ Symptom Severity Scale from 1 to 5 points, higher is worse, MCID value = 1 point) was 1.43 for those who received PRP and 0.21 points worse (95% CI 0.01 worse to 0.41 worse, 1 study, 60 participants) for those prescribed a splint (Analysis 9.1).
9.1. Analysis.

Comparison 9: SPLINT VERSUS PLATELET RICH PLASMA (PRP), Outcome 1: CTS symptoms (BCTQ)
At long‐term follow‐up, the mean symptom severity score was 1.29 for those who received PRP and 0.18 points worse (95% CI 0.01 worse to 0.35 worse, 1 study, 60 participants) for those prescribed a splint (Analysis 9.1).
Secondary outcomes
1) Function
Low‐certainty evidence (downgraded once for risk of bias and once for imprecision as only one study with a low number of participants, n = 60, contributed to the analysis) suggests the difference between splinting and PRP is probably clinically unimportant in function both at the short term and long term.
At short‐term follow‐up, the mean functional status score (measured by the BCTQ Functional Status Scale from 1 to 5 points, higher is worse, MCID value = 0.7 points) was 1.35 with PRP and 0.35 points worse (95% CI 0.16 worse to 0.54 worse, 1 study, 60 participants) with a splint (Analysis 9.2).
9.2. Analysis.

Comparison 9: SPLINT VERSUS PLATELET RICH PLASMA (PRP), Outcome 2: Function (BCTQ)
At long‐term follow‐up, the mean functional status score was 1.30 with PRP and 0.32 points worse (95% CI 0.12 worse to 0.52 worse, 1 study, 60 participants) with a splint (Analysis 9.2).
2) Overall improvement
The only study in this comparison did not measure this outcome.
3) Health‐related quality of life
The only study in this comparison did not measure this outcome.
4) Adverse effects
Wu 2017 reported 0 of 30 participants (0%) having adverse effects in the splinting group and 0 of 30 participants (0%) having adverse effects in the PRP group. Thus, we could not estimate the risk (very low‐certainty evidence).
5) Referral for surgery
The only study in this comparison did not measure this outcome.
10. Splint versus extracorporeal shock wave therapy (ESWT)
One study (Ulucakoy 2020) with 83 participants provided data for this comparison. Results were measured at one month and three months follow‐up. We used the data from three months follow‐up for short‐term analysis.
Primary outcome: CTS symptoms
Based upon moderate‐certainty evidence (downgraded once for risk of bias), splinting probably does not improve symptoms compared with ESWT at short‐term follow‐up. The mean symptom severity score (measured by the BCTQ Symptom Severity Scale from 1 to 5 points, higher is worse, MCID value = 1 point) was 1.8 for those receiving ESWT and 0.1 points better (95% CI 0.40 better to 0.20 worse, 1 study, 83 participants) for those prescribed a splint (Analysis 10.1).
10.1. Analysis.

Comparison 10: SPLINT VERSUS EXTRACORPOREAL SHOCK WAVE THERAPY (ESWT), Outcome 1: CTS symptoms (BCTQ)
The only study in this comparison did not measure this outcome in the long term.
Secondary outcomes
1) Function
Moderate‐certainty evidence (downgraded once for risk of bias) indicates that splinting probably does not improve function compared with ESWT at short‐term follow‐up. The mean functional status score (measured by the BCTQ Functional Status Scale from 1 to 5 points, higher is worse, MCID value = 0.7 points) was 1.9 with ESWT and 0.1 points better (95% CI 0.44 better to 0.24 worse, 1 study, 83 participants) for those prescribed a splint (Analysis 10.2).
10.2. Analysis.

Comparison 10: SPLINT VERSUS EXTRACORPOREAL SHOCK WAVE THERAPY (ESWT), Outcome 2: Function (BCTQ)
The only study in this comparison did not measure this outcome at the long term.
2) Overall improvement
The only study in this comparison did not measure this outcome.
3) Health‐related quality of life
The only study in this comparison did not measure this outcome.
4) Adverse effects
The only study in this comparison did not measure this outcome.
5) Referral for surgery
The only study in this comparison did not measure this outcome.
11. Dynamic splint plus rehabilitation versus rehabilitation
One study with 24 participants compared a dynamic splint (which allowed a limited range of motion from 15 degrees of flexion to 15 degrees of extension) plus rehabilitation (activity, ergonomic modifications, nerve and tendon gliding exercises, massage, carpal bones and nerve mobilisations, stretches of upper extremity and flexor retinaculum) with rehabilitation alone at six weeks (Jaladat 2017).
Primary outcome: CTS symptoms
Low‐certainty evidence (downgraded once for risk of bias and once for imprecision as only one study with a low number of participants, n = 24 contributed to the analysis) indicates that dynamic splinting given together with rehabilitation may not improve symptoms compared with rehabilitation alone at short‐term follow‐up.
The mean symptom severity score (measured by the BCTQ Symptom Severity Scale from 1 to 5 points, higher is worse, MCID value = 1 point) was 2.25 with rehabilitation and 0.01 points worse (95% CI 0.61 better to 0.63 worse, 1 study, 24 participants) with splinting and rehabilitation (Analysis 11.1).
11.1. Analysis.

Comparison 11: DYNAMIC SPLINT PLUS REHABILITATION VERSUS REHABILITATION, Outcome 1: CTS symptoms (BCTQ)
The only study in this comparison did not measure this outcome at the long term.
Secondary outcomes
1) Function
Low‐certainty evidence (downgraded once for risk of bias and once for imprecision as only 1 one study with a low number of participants, n = 24, contributed to the analysis) indicates that dynamic splinting given together with rehabilitation may not improve function compared with rehabilitation alone. The mean functional status score (measured by the BCTQ Functional Status Scale from 1 to 5 points, higher is worse, MCID value = 0.7 points) was 1.9 with rehabilitation and 0.08 points better (95% CI 0.67 better to 0.51 worse, 1 study, 24 participants) with splinting and rehabilitation (Analysis 11.2).
11.2. Analysis.

Comparison 11: DYNAMIC SPLINT PLUS REHABILITATION VERSUS REHABILITATION, Outcome 2: Function (BCTQ)
The only study in this comparison did not measure this outcome at long‐term follow‐up.
2) Overall improvement
The only study in this comparison did not measure this outcome.
3) Health‐related quality of life
The only study in this comparison did not measure this outcome.
4) Adverse effects
The only study in this comparison did not measure this outcome.
5) Referral to surgery
The only study in this comparison did not measure this outcome.
12. Splint six weeks versus splint 12 weeks
One study with 40 participants compared wearing a neutral wrist splint for six weeks to 12 weeks (Gatheridge 2020). The splint was worn at night primarily, but participants could wear the splint during the day (in addition to night‐time) for activities that provoked symptoms.
Primary outcome: CTS symptoms
Low‐certainty evidence (downgraded once for risk of bias and once for imprecision as only 1 study with a low number of participants, n = 37, contributed to the analysis) indicates that an additional six weeks splinting (6 versus 12 weeks) may not improve symptoms at short‐term follow‐up. At 12 weeks, the mean symptom severity score (measured by the BCTQ Symptom Severity Scale from 1 to 5 points, higher is worse, MCID value = 1 point) was 2.17 after 12 weeks of splinting and 0.18 points better (95% CI 0.62 better to 0.26 worse) with six weeks of splinting (Analysis 12.1).
12.1. Analysis.

Comparison 12: SPLINT SIX WEEKS VERSUS SPLINT 12 WEEKS, Outcome 1: CTS symptoms (BCTQ)
The only study in this comparison did not measure this outcome at the long term.
Secondary outcomes
1) Function
Low‐certainty evidence (downgraded once for risk of bias and once for imprecision as only 1 study with a low number of participants, n = 37, contributed to the analysis) indicates that an additional six weeks of splinting (6 versus 12 weeks) may not improve function beyond that achieved at six weeks total. At 12 weeks post‐commencement of splinting, the BCTQ functional status score (measured by the BCTQ Functional Status Scale from 1 to 5 points, higher is worse, MCID value = 0.7 points) was 1.6 after 12 weeks of splinting and 0.05 points worse (95% CI 0.39 better to 0.49 worse) with six weeks splinting (Analysis 12.2).
12.2. Analysis.

Comparison 12: SPLINT SIX WEEKS VERSUS SPLINT 12 WEEKS, Outcome 2: Function (BCTQ)
The only study in this comparison did not measure this outcome at the long term.
2) Overall improvement
The only study in this comparison did not report this outcome.
3) Health‐related quality of life
The only study in this comparison did not report this outcome.
4) Adverse effects
The only study in this comparison did not report this outcome.
5) Referral for surgery
Gatheridge 2020 reported this outcome at the long term, over three years. We downgraded the certainty of evidence to very low (once for risk of bias and twice for very serious imprecision as the 95% CIs included substantial effect in both directions). In the short‐term splinting group, 2 of 17 participants (12%) had been referred to surgery compared to 4 of 20 participants (20%) in the long‐term splinting group corresponding to an RR of 0.59 (95% CI 0.12 to 2.83, 1 study, 37 participants; Analysis 12.3).
12.3. Analysis.

Comparison 12: SPLINT SIX WEEKS VERSUS SPLINT 12 WEEKS, Outcome 3: Referral for surgery
13. Splint six weeks versus splint six months
One study compared night‐time splinting for six weeks with night‐time splinting for six months (Sanaee 2017). Both groups also received usual care (non‐steroidal anti‐inflammatory drugs (NSAIDs), (naproxen 500 mg three times a day) for 10 days, vitamin B1 and B6 tabs for 6 weeks, physiotherapy (paraffin bath with controlled temperature, ultrasound underwater and grip exercises)). Outcomes were measured at six weeks and six months, but as both groups received exactly the same intervention for the first six weeks, we did not include the six weeks data in the analysis.
Primary outcome: CTS symptoms
At long‐term follow‐up, low‐certainty evidence (downgraded once for risk of bias and once for imprecision as the 95% CI overlapped with the MCID value) indicates that six months of splinting may improve symptoms compared with six weeks of splinting. At six months, the mean symptom severity score (measured by the BCTQ Symptom Severity Scale from 1 to 5 points, higher is worse, MCID value = 1 point) was 2 with six months splinting and 1.3 points worse (95% CI 0.81 worse to 1.79 worse, 1 study, 156 participants) with six weeks of splinting (Analysis 13.1).
13.1. Analysis.

Comparison 13: SPLINT SIX WEEKS VERSUS SPLINT SIX MONTHS, Outcome 1: CTS symptoms (BCTQ)
Secondary outcomes
1) Function
At long‐term follow‐up, moderate‐certainty evidence (downgraded once for risk of bias) indicated that six months of splinting probably improves functional status compared with six weeks of splinting.
At six months, the mean functional status score (measured by the BCTQ Functional Status Scale from 1 to 5 points, higher is worse, MCID value = 0.7 points) was 1.8 with six months splinting and 2.3 points worse (95% CI 1.44 worse to 3.16 worse, 1 study, 156 participants) with six weeks of splinting (Analysis 13.2).
13.2. Analysis.

Comparison 13: SPLINT SIX WEEKS VERSUS SPLINT SIX MONTHS, Outcome 2: Function (BCTQ)
2) Overall improvement
The only study in this comparison did not report this outcome.
3) Health‐related quality of life
The only study in this comparison did not report this outcome.
4) Adverse effects
The only study in this comparison did not report this outcome.
5) Referral for surgery
In the group that was prescribed a splint for six weeks, 4 of 59 participants (7%) had surgery versus 0 of 59 participants (0%) in the group that wore the splint for six months. This corresponds to an RR of 9.00 (95% CI 0.50 to 163.53, 1 study, 118 participants) (Analysis 13.3). We downgraded the certainty of evidence to very low (once for risk of bias and twice for very serious imprecision as the 95% CI included substantial effects in both directions).
13.3. Analysis.

Comparison 13: SPLINT SIX WEEKS VERSUS SPLINT SIX MONTHS, Outcome 3: Referral for surgery
14. Night‐time splinting versus full‐time splinting
One quasi‐randomised trial with 24 participants compared night‐time splinting versus full‐time splinting for six weeks, at six weeks follow‐up (Walker 2000).
Primary outcome: CTS symptoms
Low‐certainty evidence (downgraded once for risk of bias and once for imprecision as only 1 study with a low number of participants, n = 26, contributed to the analysis) indicates that symptoms after six weeks night‐time splinting and full‐time splinting may not differ.
The mean symptom severity score (measured by the BCTQ Symptom Severity Scale from 1 to 5 points, higher is worse, MCID value = 1 point) was 2.3 with night‐time splints and 0.21 points better (95% CI 0.83 better to 0.41 worse, 1 study, 24 participants) with full‐time splinting (Analysis 14.1).
14.1. Analysis.

Comparison 14: NIGHT‐TIME SPLINTING VERSUS FULL‐TIME SPLINTING, Outcome 1: CTS symptoms (BCTQ)
The only study in this comparison did not report this outcome at the long term.
Secondary outcomes
1) Function
Very low‐certainty evidence (downgraded once for risk of bias and twice for imprecision; 1 study with 26 randomised participants and the 95% CI overlapping the MCID) indicates that we are uncertain about the effect on function after six weeks night‐time splinting and full‐time splinting.
At six weeks, the mean functional status score (measured by the BCTQ Functional Status Scale from 1 to 5 points, higher is worse, MCID value = 0.7 points) was 2.14 with night‐time splinting and 0.21 points better (95% CI 0.87 better to 0.45 worse, 1 study, 24 participants) with full‐time splinting (Analysis 14.2).
14.2. Analysis.

Comparison 14: NIGHT‐TIME SPLINTING VERSUS FULL‐TIME SPLINTING, Outcome 2: Function (BCTQ)
The only study in this comparison did not report this outcome at the long term.
2) Overall improvement
The only study in this comparison did not report this outcome.
3) Health‐related quality of life
The only study in this comparison did not report this outcome.
4) Adverse effects
The only study in this comparison did not report this outcome.
5) Referral for surgery
The only study in this comparison did not report this outcome.
Discussion
Summary of main results
The objective of this review was to assess the effects (benefits and harms) of splinting for people with carpal tunnel syndrome (CTS). We considered the results of 29 trials in a total of 1937 participants. Eight studies examined our primary comparison of splinting versus no treatment, 20 compared splinting with another non‐surgical intervention (or splinting and a co‐intervention compared to the same co‐intervention). Three studies compared different splinting regimens. Most of the participants were female (81 %). Participants had an average age ranging from 42 to 60 years.
Ten studies measured the effect of splint when delivered with some other non‐surgical intervention: an exercise programme (Akturk 2018); a physical therapy programme (consisting of heat‐application‐ultrasound‐TENS) and strengthening exercises (Eraslan 2014); usual rehabilitation including activity or ergonomic modifications, nerve‐ and tendon‐gliding exercises, massage, carpal bones and nerve mobilisations, stretches of upper extremity and flexor retinaculum (Jaladat 2017); tendon and nerve‐gliding exercises (Geler Kulcu 2016; Oncu 2014); corticosteroid injection (Wang 2017); nonsteroidal antiinflammatory drugs (NSAIDs), B1 and B6, paraffin bath, ultrasound underwater and grip exercise (Sanaee 2017); education (Hall 2013); and ergonomic education (Boonhong 2017; Werner 2005).
Splint versus no active treatment
For the primary outcome (symptoms), we found low‐certainty evidence indicating that splinting may provide little or no benefit at short‐term follow‐up compared with no active treatment. The effect estimates excluded clinically relevant benefits, but there is no consensus on the ideal Minimal Clinically Important Difference (MCID) value in this specific context, and the chosen MCID value can affect interpretation (see Overall completeness and applicability of evidence). Regarding long‐term follow‐up (over 3 months), we are uncertain whether splinting provides benefit for CTS symptoms (Table 1).
Lack of blinding and heterogeneity in the treatment effects across the studies were the main limitations in the studies comparing splinting versus no active treatment at short‐term follow‐up. We explored heterogeneity by removing studies at high or unclear risk of selection bias. This left three small studies (i.e. Boonhong 2017; Geler Kulcu 2016; Oncu 2014) with low heterogeneity and moved the effect towards no effect, suggesting that the benefits observed in Manente 2001 and Premoselli 2006 may relate to shortcomings in the randomisation process.
On the other hand, the two studies reporting benefit for splint used splint only at night‐time (Manente 2001; Premoselli 2006), and one study using night‐time splint reported no benefit (Oncu 2014). The other trialists instructed participants to wear splints full time. Thus, inconsistency could also be partially explained by night‐time splinting having a different effect compared with full‐time use. However, one small study comparing night‐time splinting with full‐time splinting did not support this hypothesis (Walker 2000). Thus, it remains unclear if night‐time splinting could improve symptoms, and if those benefits are present only at night‐time or also at daytime, and if they are large enough to be important for people with CTS.
For secondary outcomes, moderate‐certainty evidence suggested that splint probably does not improve hand function in the short term and may not improve hand function in the long term. Low‐certainty evidence based upon one study suggested a benefit for splinting (RR of 3.9) in overall improvement (those reporting 'moderately' or 'much improved') (Manente 2001). This benefit was found in the study, which also reported benefits for CTS symptoms, unlike most studies. In this study, the first author reported owning the patent for the splint being tested. Therefore, we consider the evidence regarding benefits in overall improvement limited until confirmed in other studies.
None of the studies reported general health‐related quality of life (HRQoL) in the primary comparison and the estimates were very imprecise for referral to surgery. We are thus uncertain about the effect of splinting for these outcomes.
Splinting may cause transient adverse effects (difficulty falling asleep and paraesthesia after removal). These adverse effects are generally minor and are not likely to persist after use is discontinued.
Other comparisons
The other comparisons supported the findings from the primary comparison. Splinting may not provide benefits in CTS symptoms or hand function when given together with corticosteroid injection (moderate‐certainty evidence) or with rehabilitation (low‐certainty evidence) nor when compared with corticosteroid (injection or oral; low certainty), exercises (low certainty), kinesiology taping (low certainty), rigid taping (low certainty), and probably does not improve outcomes compared with platelet‐rich plasma (moderate certainty), or extracorporeal shock wave therapy (moderate certainty).
Splinting versus corticosteroid injection
Corticosteroid injections (when compared to splinting) appeared to provide a small, but clinically unimportant benefit in the short term with respect to symptom resolution (but not in improving function). However, this effect vanished at long‐term follow‐up, which is consistent with findings that the effect of corticosteroid injection is likely transient (Huisstede 2010). However, the results were inconsistent at long‐term analysis. One study found that overall improvement (remission from nocturnal paraesthesias) was better with corticosteroid injection both at short‐ and long‐term follow‐up. With respect to health‐related quality of life (HRQoL), corticosteroid injections and splint appeared to be comparable (low certainty), but the confidence intervals (CIs) did not exclude benefit for corticosteroid in the short term. Regarding harms, we did not find statistically significant differences in the reporting of adverse effects or in referral to surgery between the two treatments.
Comparison of splint wearing regimens
Regarding different splint‐wearing regimens, one study found that six months of splinting may improve symptoms and function (low‐certainty evidence) compared with six weeks splinting, suggesting that the benefits of splinting may manifest late (Sanaee 2017). Although the mean difference was at a clinically‐relevant level, the 95% CI overlapped with the MCID value, and it is thus unclear if the effect is clinically relevant. When six weeks of splint use was compared with 12‐week use, there was no evidence of benefit for a longer splinting period in symptoms or function (other outcomes were not measured).
Overall completeness and applicability of evidence
Participants in the studies had a typical age and sex distribution for people with CTS and the results are likely to be applicable to people with mild‐to‐moderate stages of the condition (Atroshi 1999; Concannon 2000; Jablecki 2002; Szabo 1994). Most studies excluded people with severe CTS and since the effect of splinting seems small at best, biological plausibility does not support the hypothesis that splinting would benefit people with more severe symptoms, established axonal loss and subsequent permanent degenerative changes in the median nerve. However, no studies assessed effects in this population, probably because severe CTS is commonly considered an indication for surgery.
Some studies in the splinting versus no active treatment comparison provided education as a co‐intervention (Boonhong 2017; Hall 2013; Werner 2005), or instruction for home‐based exercises along with splinting and in the control group (Geler Kulcu 2016; Oncu 2014). Although education may improve coping and could impact the reported values, we deemed that these co‐interventions were unlikely to bias the comparison as they were offered to participants in both groups. Furthermore, we can presume education to be present in normal clinical practice, including in studies that did not report it explicitly.
Regarding comparisons of splinting with other active non‐surgical treatment modalities, since splinting has not shown efficacy compared with no splint, these studies provide little guidance whether splinting should be used or not. For this reason we excluded comparisons of specific splint designs. The observed changes in these studies may occur due to nonspecific effects and the between‐group differences can be due to harms of the comparator. Although we excluded different splint designs from this update, they may be included in future updates if splinting shows clinically relevant efficacy. The major problem with these comparisons is the applicability of the evidence due to specificity of commercial or individually‐tailored splints,
We could not identify an optimal splint‐wearing regimen based on evidence from studies comparing different regimens. There was limited evidence from one study that the benefit may manifest late (Sanaee 2017), but no studies that compared splinting and no active treatment followed participants for more than six months in the primary analysis and, thus, it is still unclear if splinting can improve symptoms or other outcomes or decrease the need for surgery in long‐term follow‐up.
Another potentially important aspect of CTS that the trials did not specifically study was relief of night‐time symptoms. In early CTS, people may suffer night‐time symptoms almost exclusively. None of the studies specifically measured night‐time symptom relief. Night‐time symptoms comprise only 4/11 (36% weight) items in the BCTQ symptom severity score and patient‐important benefits (e.g. better sleep and consequent improved daytime quality of life) may not be evident when BCTQ is used as the outcome measure for 'symptoms'.
We used CTS symptoms (a continuous outcome) as the primary outcome because very few studies measured global improvement (which was used in the previous update of this review as the primary outcome; Page 2012b) and we could not identify evidence suggesting that global improvement would be a clinically more relevant outcome. We excluded neurophysiological outcomes. Although they may be considered objective measures of improvement, their clinical relevance is unclear; if the possible benefit in conduction velocity does not translate to an improvement in symptoms, function, or health‐related quality of life, it is not likely to be meaningful to people with CTS.
We did not include comparisons to surgery, as they are covered in another review (Verdugo 2008). We also excluded studies that compared splinting with a non‐surgical intervention aimed at symptom modification. These interventions include electroacupuncture; yoga; gabapentin; flax seed oil; interferential current; or phonophoresis.
Quality of the evidence
None of the included studies was at low risk of bias. The main study limitation was lack of blinding (high risk of performance and detection bias). We identified one ongoing study using soft bandage as a placebo control (Atroshi 2019). Presumably soft bandage does not exert an effect, but nevertheless the participants were not blinded, and participant preconceptions can modify perceived improvement. It may not be feasible to create a placebo splint that achieves adequate blinding and does not exert any effect. Although meta‐epidemiological studies indicate that a lack of blinding of participants or study personnel can affect estimates of effect (Savovic 2018), recent evidence suggests that this effect may be small (Moustgaard 2020). As bias from lack of blinding is likely to be context‐dependent, it is unclear how much it can bias the estimates. We hypothesise that if splinting does not show a relevant effect in non‐blinded trials, we should assume that blinded trials will find an even smaller effect.
We downgraded the evidence in the primary outcome for splint compared to no active treatment due to inconsistency. While two studies with high or unclear risk of selection bias reported statistically significant benefits for splinting, the studies at low risk of selection bias found a virtually null effect.
For function, the evidence was moderate (downgraded for risk of bias), the estimates excluding the MCID value. For overall improvement, we downgraded the certainty of evidence to low due to risk of bias and imprecision, as there was only one small study with 80 participants. Regarding harms, we downgraded the certainty of evidence for risk of bias (lack of blinding) and imprecision (the 95% CI included no effect).
Most secondary comparisons included one small unblinded study. Therefore, the estimates were often imprecise (moderate to low certainty of evidence, depending on the comparison and outcome).
Heterogeneity in the reported MCID values adds another layer of uncertainty to interpretation of the results. We used a MCID of 1 point (on a 1 to 5 scale) for BCTQ symptom severity score and 0.7 for BCTQ functional status score (Kim 2013), both from the mid‐range of reported values (De Kleermaeker 2018). MCID values have often been defined in surgical studies, and may not apply directly to splinting. A MCID of 1 point is a relatively high value when translated to a standardised mean difference (SMD) (corresponds with large effect; SMD of 1.25 to 2). Although the chosen MCID value did not affect the estimates, a better understanding of what constitutes a meaningful change in people who use a splint would affect interpretation of results. For example, if we assume a MCID in the symptom scale that is 10% of the scale (0.4 points; corresponding with a medium to large effect, SMD of 0.6 to 1), our conclusions would be that splinting may provide clinically important benefits in carpal tunnel symptoms. However, inconsistency, imprecision and bias would still decrease our certainty, and more rigorous studies are needed.
Conflicts of interest were rarely reported: only two studies reported conflicts of interest that could affect the findings: one due to a patent on the splint used in the trial (Manente 2001), and the other because two authors had been employed by the manufacturer of the tested stretching splint (Willis 2016).
Potential biases in the review process
We conducted searches according to Cochrane methodological standards and did not restrict searches by language or date. It is unlikely that we missed large‐scale rigorous trials. However, based on clinical trial registries, we identified six potentially eligible studies that lacked the needed information or were not published, which are awaiting classification (Baklaci 2015; Bhuva 2019; IRCT2014020416485N1; ISRCTN22916517; Riasi 2015; Soon 2015). Inclusion of these trials could affect the estimates in this review.
Another study was included, but the authors did not report sufficient data to contribute to the analyses (Rioja Toro 2012).
Agreements and disagreements with other studies or reviews
The findings of this review are generally consistent with those of other systematic reviews of non‐surgical interventions for CTS, which conclude that splinting may be more effective than no treatment, but is not more or less effective than other non‐surgical interventions (Ashworth 2010; Gerritsen 2002b; Goodyear‐Smith 2004; Huisstede 2010; Muller 2004; Ono 2010; Piazzini 2007).
Compared to the previous version of this review, we included 20 new studies, and we could pool more data in comparisons of splint versus no active intervention and splint versus corticosteroid. The findings largely agree with the previous version of the review regarding uncertainty of benefits, but we improved certainty regarding symptoms and function in the main analysis (increasing the certainty of evidence from very low to low certainty).
Authors' conclusions
Implications for practice.
There is insufficient evidence to conclude whether wrist splinting provides clinically meaningful benefit for people with carpal tunnel syndrome (CTS). Current limited evidence does not exclude small improvements in CTS symptoms and hand function, but they may not be clinically important. However, the clinical relevance of small differences is still unclear and further research may change this conclusion. People may have greater chance of experiencing overall improvement when they use night‐time splints compared with no splints.
Since splinting is a relatively inexpensive intervention with no plausible long‐term harms, small effects (smaller than the MCID value) could justify use, particularly when people have mild‐to‐moderate CTS symptoms and are not interested in having surgery or injections.
It is still unclear if splint should be used full‐time or at night‐time only and whether long‐term use is better than short‐term use for best response. However, low‐certainty evidence suggests that the benefits may manifest at long‐term follow‐up (after three months) and therefore longer treatment periods can be tried if the person with CTS does not develop adverse effects or worsening of symptoms.
Implications for research.
As long as the efficacy of splinting is unclear, comparing splinting with other treatment modalities (often of ambiguous efficacy) may not improve our understanding of the role of splinting in the treatment of CTS. There is an apparent need for a large rigorous trial comparing splinting with no splinting or placebo and only one of the identified ongoing trials will address this research question (Atroshi 2019). Besides measuring improvement in symptoms and function, the trial authors should measure overall/global improvement, generic health‐related quality of life, and record adverse effects and referral to surgery.
The trials should preferably have a long follow‐up (and continue the intervention for 6 to 12 months or longer) to assess if any effects endure or manifest late. It may also be wise to further explore the optimal splinting strategy in terms of full‐time versus night‐time‐only splinting – people with predominantly night‐time symptoms may be a subgroup worth assessing separately, measuring specifically night‐time symptom relief. Baseline symptom severity is a potential effect modifier and future studies could try to assess if there are subgroups of people that benefit more from splinting.
If participants with bilateral CTS are included in trials, trialists should use statistical methods which take the dependency between wrists into account, and report which statistical methods they used to achieve this. Lastly, MCID values in people undergoing splinting warrants further assessment, as this value can greatly impact the interpretation of the evidence.
What's new
| Date | Event | Description |
|---|---|---|
| 27 February 2023 | New search has been performed | Based on an updated search, 20 new studies were included in the review (Akturk 2018; Boonhong 2017; Chesterton 2018; De Moraes 2021; Eraslan 2014; Gatheridge 2020; Geler Kulcu 2016; Hall 2013; Jaladat 2017; Kocaoglu 2017; Oncu 2014; Rioja Toro 2012; Sanaee 2017; Schmid 2012; So 2018; Ulucakoy 2020; Wang 2017; Willis 2016; Wu 2017; Yazdanpanah 2012); eight studies, included in the previous version of this review, remain included (De Entrambasaguas 2006; Madjdinasab 2008; Manente 2001; Mishra 2006; Premoselli 2006; Sevim 2004; Walker 2000; Werner 2005); one study, which was awaiting classification in the previous version of this review, is now included (Taspinar 2007). |
| 27 February 2023 | New citation required and conclusions have changed | Data, which were not pooled in meta‐analysis in the previous version of this review, are now combined (i.e. Manente 2001 and Premoselli 2006 at short‐term analysis). The previous version of this review did not include any data from Sevim 2004 in analysis, but we have included them. We excluded the following comparisons (included in the previous version of this review):
Changes to outcomes:
|
History
Review first published: Issue 7, 2012
Acknowledgements
We thank Ruth Brassington, Angela Gunn, and Farhad Shokraneh from the Cochrane Neuromuscular Disease Group for their assistance in database searches strategy, and ongoing support for this review. We thank Joona Juurakko for his help in data extraction.
We thank the peer reviewers for their helpful comments.
Catrin Tudur Smith, University of Liverpool
Janet Wale, independent patient advocate
Pietro Caliandro Dipartimento di Scienze dell’Invecchiamento, Neurologiche, Ortopediche e della Testa‐Collo, Fondazione Policlinico Universitario A. Gemelli IRCCS, Rome, Italy
Professor Richard Hughes, Emeritus Professor of Neurology, King's College London
Appendices
Appendix 1. Cochrane Neuromuscular Specialised Register via CRS‐Web search strategy
1 MeSH DESCRIPTOR Carpal Tunnel Syndrome AND INREGISTER 373
2 "carpal tunnel" AND INREGISTER 595
3 ("nerve entrapment" or "nerve compression" or "entrapment neuropathy" or "entrapment neuropathies") and carpal AND INREGISTER 43
4 #1 or #2 or #3 595
5 MeSH DESCRIPTOR Splints AND INREGISTER 74
6 MeSH DESCRIPTOR Braces AND INREGISTER 13
7 splint* or brace* or "wrist support*" AND INREGISTER 185
8 #5 or #6 or #7 185
9 #4 and #8 146
Appendix 2. Cochrane Central Register of Controlled Trials (CENTRAL) via CRS‐Web search strategy
1 MeSH DESCRIPTOR Carpal Tunnel Syndrome AND CENTRAL:TARGET 756
2 "carpal tunnel" AND CENTRAL:TARGET 1694
3 ("nerve entrapment" or "nerve compression" or "entrapment neuropathy" or "entrapment neuropathies") and carpal AND CENTRAL:TARGET 127
4 #1 or #2 or #3 1694
5 MeSH DESCRIPTOR Splints AND CENTRAL:TARGET 465
6 MeSH DESCRIPTOR Braces AND CENTRAL:TARGET 437
7 splint* or brace* or "wrist support*" AND CENTRAL:TARGET 4811
8 #5 or #6 or #7 4811
9 #4 and #8 321
10 INREGISTER 7877
11 #9 NOT #10 179
Appendix 3. MEDLINE (OvidSP) search strategy
Database: Ovid MEDLINE(R) ALL <1946 to December 10, 2021>
1 (randomized controlled trial or controlled clinical trial).pt. or (randomi?ed or placebo or randomly or trial or groups).ab. or drug therapy.fs. (5214596)
2 exp animals/ not humans.sh. (4923984)
3 1 not 2 (4539025)
4 Carpal Tunnel Syndrome.tw. or Carpal Tunnel Syndrome/ or ((nerve entrapment or nerve compression or entrapment neuropath*) and carpal).mp. (11693)
5 SPLINTS/ or BRACES/ or (SPLINT* or BRACE* or WRIST SUPPORT*).tw. (30013)
6 3 and 4 and 5 (208)
7 limit 6 to ed=20201211‐20211231 (6)
8 limit 6 to dt=20201211‐20211231 (7)
9 7 or 8 (13)
Appendix 4. Embase (OvidSP) search strategy
Database: Embase <1974 to 2021 Week 49>
1 Randomized controlled trial/ or Controlled clinical study/ or randomization/ or intermethod comparison/ or double blind procedure/ or human experiment/ or (random$ or placebo or (open adj label) or ((double or single or doubly or singly) adj (blind or blinded or blindly)) or parallel group$1 or crossover or cross over or ((assign$ or match or matched or allocation) adj5 (alternate or group$1 or intervention$1 or patient$1 or subject$1 or participant$1)) or assigned or allocated or (controlled adj7 (study or design or trial)) or volunteer or volunteers).ti,ab. or (compare or compared or comparison or trial).ti. or ((evaluated or evaluate or evaluating or assessed or assess) and (compare or compared or comparing or comparison)).ab. (5590987)
2 limit 1 to (conference abstracts or embase) (4778818)
3 carpal tunnel syndrome/ or carpal tunnel syndrome.tw. or ((nerve entrapment or nerve compression or entrapment neuropath*) and carpal).mp. (17327)
4 splint/ or brace/ or (splint* or brace* or wrist support*).tw. (34265)
5 2 and 3 and 4 (252)
6 limit 5 to em=202050‐202149 (18)
Appendix 5. AMED (OvidSP) search strategy
Database: AMED (Allied and Complementary Medicine) <1985 to December 2021>
1 Randomized controlled trials/ or Random allocation/ or Double blind method/ or Single‐Blind Method/ or exp Clinical Trials/ or (clin* adj25 trial*).tw. or ((singl* or doubl* or treb* or trip*) adj25 (blind* or mask* or dummy)).tw. or placebos/ or placebo*.tw. or random*.tw. or research design/ or Prospective Studies/ or meta analysis/ or (meta?analys* or systematic review*).tw. or control*.tw. or (multicenter or multicentre).tw. or ((study or studies or design*) adj25 (factorial or prospective or intervention or crossover or cross‐over or quasi‐experiment*)).tw. (67795)
2 carpal tunnel syndrome/ or carpal tunnel syndrome.tw. or ((nerve entrapment or nerve compression or entrapment neuropath*) and carpal).mp. (592)
3 splints/ or braces/ or (splint* or brace* or wrist support*).tw. (1526)
4 1 and 2 and 3 (40)
5 limit 4 to yr="2020 ‐Current" (6)
Appendix 6. CINAHL (EBSCOhost) search strategy
Sunday, December 12, 2021 9:02:00 PM
S8 S6 AND S7 1
S7 EM 20180601‐ Limiters ‐ Exclude MEDLINE records 241,618
S6 S3 and S4 and S5 121
S5 (MH "Splints") OR splint* or brace* or "wrist support*" 12,412
S4 (MH "Carpal Tunnel Syndrome") OR "carpal tunnel syndrome" OR ("nerve entrapment" and carpal) OR ("nerve compression" and carpal) OR ("entrapment neuropath*" and carpal) 3,854
S3 S1 NOT S2 876,076
S2 (MH Animals+ OR MH (Animal Studies) OR TI (Animal Model*)) NOT MH (Human) 199,936
S1 MH ("Randomized Controlled Trials" OR "Double‐Blind Studies" OR "Single‐Blind Studies" OR "Random Assignment" OR "Pretest‐Posttest Design" OR "Cluster Sample" OR "Placebos" OR "Crossover Design" OR "Comparative Studies") OR TI (Randomised OR Randomized OR Trial) OR AB (Random* OR (Control W5 Group) OR (Cluster W3 RCT)) OR (MH ("Sample Size") AND AB (Assigned OR Allocated OR Control)) OR PT (Randomized Controlled Trial) 919,968
Appendix 7. ClinicalTrials.Gov search strategy
Advanced Search
Condition or disease: Carpal Tunnel Syndrome
Study type: Interventional Studies (Clinical Trials)
Intervention/treatment: Splint* OR Brace* OR Wrist Support*
3 Studies found
Appendix 8. WHO ICTRP search strategy
Advanced Search
Carpal Tunnel Syndrome in the Condition
Splint* OR Brace* OR Wrist Support* in the Intervention
Recruitment status is ALL
77 records for 77 trials found
Data and analyses
Comparison 1. SPLINT VERSUS NO ACTIVE TREATMENT.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1.1 CTS symptoms (BCTQ) | 7 | Mean Difference (IV, Random, 95% CI) | Subtotals only | |
| 1.1.1 Short‐term improvement: 3 months or less | 6 | 306 | Mean Difference (IV, Random, 95% CI) | ‐0.37 [‐0.82, 0.08] |
| 1.1.2 Long‐term improvement: > 3months | 2 | 144 | Mean Difference (IV, Random, 95% CI) | ‐0.64 [‐1.20, ‐0.08] |
| 1.2 Function (BCTQ) | 6 | Mean Difference (IV, Random, 95% CI) | Subtotals only | |
| 1.2.1 Short‐term improvement: 3 months or less | 6 | 306 | Mean Difference (IV, Random, 95% CI) | ‐0.24 [‐0.44, ‐0.03] |
| 1.2.2 Long‐term improvement: > 3 months | 1 | 34 | Mean Difference (IV, Random, 95% CI) | ‐0.25 [‐0.68, 0.18] |
| 1.3 Overall improvement | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| 1.3.1 Short‐term improvement: 3 months or less | 1 | 80 | Risk Ratio (M‐H, Fixed, 95% CI) | 3.86 [2.29, 6.51] |
| 1.4 Adverse effects | 1 | Risk Ratio (M‐H, Random, 95% CI) | Subtotals only | |
| 1.4.1 Adverse effects | 1 | 80 | Risk Ratio (M‐H, Random, 95% CI) | 15.00 [0.89, 254.13] |
| 1.5 Referral for surgery | 3 | 243 | Risk Ratio (M‐H, Random, 95% CI) | 0.47 [0.14, 1.58] |
Comparison 2. SPLINT VERSUS CORTICOSTEROID INJECTION.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 2.1 CTS symptoms | 6 | Std. Mean Difference (IV, Random, 95% CI) | Subtotals only | |
| 2.1.1 Short‐term improvement: 3 months or less | 5 | 459 | Std. Mean Difference (IV, Random, 95% CI) | 0.38 [0.08, 0.68] |
| 2.1.2 Long‐term improvement: over 3 months | 3 | 437 | Std. Mean Difference (IV, Random, 95% CI) | 0.09 [‐0.66, 0.83] |
| 2.2 Function (BCTQ) | 5 | Mean Difference (IV, Random, 95% CI) | Subtotals only | |
| 2.2.1 Short‐term improvement: 3 months or less | 5 | 459 | Mean Difference (IV, Random, 95% CI) | 0.16 [‐0.04, 0.36] |
| 2.2.2 Long‐term improvement: over 3 months | 2 | 329 | Mean Difference (IV, Random, 95% CI) | 0.33 [‐0.40, 1.06] |
| 2.3 Overall improvement | 1 | Risk Ratio (M‐H, Random, 95% CI) | Subtotals only | |
| 2.3.1 Short‐term improvement: 3 months or less | 1 | 99 | Risk Ratio (M‐H, Random, 95% CI) | 0.57 [0.39, 0.84] |
| 2.3.2 Long‐term improvement: over 3 months | 1 | 95 | Risk Ratio (M‐H, Random, 95% CI) | 0.36 [0.22, 0.58] |
| 2.4 Health‐related quality of life | 2 | Std. Mean Difference (IV, Random, 95% CI) | Subtotals only | |
| 2.4.1 Short‐term improvement: 3 months or less | 2 | 270 | Std. Mean Difference (IV, Random, 95% CI) | ‐0.25 [‐0.77, 0.27] |
| 2.4.2 Long‐term improvement: over 3 months | 1 | 234 | Std. Mean Difference (IV, Random, 95% CI) | 0.04 [‐0.21, 0.30] |
| 2.5 Adverse effects | 6 | Risk Ratio (M‐H, Random, 95% CI) | Subtotals only | |
| 2.5.1 Adverse effects | 6 | 590 | Risk Ratio (M‐H, Random, 95% CI) | 0.32 [0.08, 1.26] |
| 2.6 Referral for surgery | 2 | 334 | Risk Ratio (M‐H, Random, 95% CI) | 0.60 [0.33, 1.09] |
Comparison 3. SPLINT VERSUS ORAL STEROID.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 3.1 CTS symptoms (BCTQ) | 1 | Mean Difference (IV, Random, 95% CI) | Subtotals only | |
| 3.1.1 Short‐term improvement: 3 months or less | 1 | 71 | Mean Difference (IV, Random, 95% CI) | 0.25 [‐0.03, 0.53] |
| 3.2 Function (BCTQ) | 1 | Mean Difference (IV, Random, 95% CI) | Subtotals only | |
| 3.2.1 Short‐term improvement: 3 months or less | 1 | 71 | Mean Difference (IV, Random, 95% CI) | 0.12 [‐0.06, 0.30] |
| 3.3 Adverse effects | 1 | Risk Ratio (M‐H, Random, 95% CI) | Subtotals only | |
| 3.3.1 Adverse effects | 1 | 71 | Risk Ratio (M‐H, Random, 95% CI) | 4.86 [0.24, 97.86] |
Comparison 4. SPLINT PLUS CORTICOSTEROID INJECTION VERSUS CORTICOSTEROID INJECTION.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 4.1 CTS symptoms (BCTQ) | 1 | Mean Difference (IV, Random, 95% CI) | Subtotals only | |
| 4.1.1 Short‐term improvement: 3 months or less | 1 | 52 | Mean Difference (IV, Random, 95% CI) | ‐0.17 [‐0.43, 0.09] |
| 4.2 Function (BCTQ) | 1 | Mean Difference (IV, Random, 95% CI) | Subtotals only | |
| 4.2.1 Short‐term improvement: 3 months or less | 1 | 52 | Mean Difference (IV, Random, 95% CI) | ‐0.05 [‐0.28, 0.18] |
| 4.3 Overall improvement | 1 | Risk Ratio (M‐H, Random, 95% CI) | Subtotals only | |
| 4.3.1 Short‐term improvement: 3 months or less | 1 | 52 | Risk Ratio (M‐H, Random, 95% CI) | 1.33 [0.90, 1.97] |
Comparison 5. SPLINT VERSUS EXERCISE.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 5.1 CTS symptoms (BCTQ) | 1 | Mean Difference (IV, Random, 95% CI) | Subtotals only | |
| 5.1.1 Short‐term improvement: 3 months or less | 1 | 20 | Mean Difference (IV, Random, 95% CI) | 0.12 [‐0.38, 0.62] |
| 5.2 Function (BCTQ) | 1 | Mean Difference (IV, Random, 95% CI) | Subtotals only | |
| 5.2.1 Short‐term improvement: 3 months or less | 1 | 20 | Mean Difference (IV, Random, 95% CI) | 0.30 [‐0.11, 0.71] |
Comparison 6. STRETCHING SPLINT VERSUS STRETCHING EXERCISES.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 6.1 Referral for surgery | 1 | 50 | Risk Ratio (M‐H, Random, 95% CI) | 0.44 [0.22, 0.88] |
Comparison 7. SPLINT VERSUS KINESIOLOGY TAPING.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 7.1 CTS symptoms (BCTQ) | 4 | Mean Difference (IV, Random, 95% CI) | Subtotals only | |
| 7.1.1 Short‐term improvement: 3 months or less | 4 | 168 | Mean Difference (IV, Random, 95% CI) | 0.49 [‐0.05, 1.03] |
| 7.2 Function (BCTQ) | 4 | Mean Difference (IV, Random, 95% CI) | Subtotals only | |
| 7.2.1 Short‐term improvement: 3 months or less | 4 | 168 | Mean Difference (IV, Random, 95% CI) | 0.11 [‐0.54, 0.75] |
Comparison 8. SPLINT VERSUS RIGID TAPE.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 8.1 CTS symptoms (BCTQ) | 1 | Mean Difference (IV, Random, 95% CI) | Subtotals only | |
| 8.1.1 Short‐term improvement: 3 months or less | 1 | 30 | Mean Difference (IV, Random, 95% CI) | 1.05 [0.58, 1.52] |
| 8.2 Function (BCTQ) | 1 | Mean Difference (IV, Random, 95% CI) | Subtotals only | |
| 8.2.1 Short‐term improvement: 3 months or less | 1 | 30 | Mean Difference (IV, Random, 95% CI) | 0.87 [0.48, 1.26] |
Comparison 9. SPLINT VERSUS PLATELET RICH PLASMA (PRP).
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 9.1 CTS symptoms (BCTQ) | 1 | Mean Difference (IV, Random, 95% CI) | Subtotals only | |
| 9.1.1 Short‐term improvement: 3 months or less | 1 | 60 | Mean Difference (IV, Random, 95% CI) | 0.21 [0.01, 0.41] |
| 9.1.2 Long‐term improvement: > 3 months | 1 | 60 | Mean Difference (IV, Random, 95% CI) | 0.18 [0.01, 0.35] |
| 9.2 Function (BCTQ) | 1 | Mean Difference (IV, Random, 95% CI) | Subtotals only | |
| 9.2.1 Short‐term improvement: 3 months or less | 1 | 60 | Mean Difference (IV, Random, 95% CI) | 0.35 [0.16, 0.54] |
| 9.2.2 Long‐term improvement: > 3 months | 1 | 60 | Mean Difference (IV, Random, 95% CI) | 0.32 [0.12, 0.52] |
Comparison 10. SPLINT VERSUS EXTRACORPOREAL SHOCK WAVE THERAPY (ESWT).
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 10.1 CTS symptoms (BCTQ) | 1 | Mean Difference (IV, Random, 95% CI) | Subtotals only | |
| 10.1.1 Short‐term improvement: 3 months or less | 1 | 83 | Mean Difference (IV, Random, 95% CI) | ‐0.10 [‐0.40, 0.20] |
| 10.2 Function (BCTQ) | 1 | Mean Difference (IV, Random, 95% CI) | Subtotals only | |
| 10.2.1 Short‐term improvement: 3 months or less | 1 | 83 | Mean Difference (IV, Random, 95% CI) | ‐0.10 [‐0.44, 0.24] |
Comparison 11. DYNAMIC SPLINT PLUS REHABILITATION VERSUS REHABILITATION.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 11.1 CTS symptoms (BCTQ) | 1 | Mean Difference (IV, Random, 95% CI) | Subtotals only | |
| 11.1.1 Short‐term improvement: 3 months or less | 1 | 24 | Mean Difference (IV, Random, 95% CI) | 0.01 [‐0.61, 0.63] |
| 11.2 Function (BCTQ) | 1 | Mean Difference (IV, Random, 95% CI) | Subtotals only | |
| 11.2.1 Short‐term improvement: 3 months or less | 1 | 24 | Mean Difference (IV, Random, 95% CI) | ‐0.08 [‐0.67, 0.51] |
Comparison 12. SPLINT SIX WEEKS VERSUS SPLINT 12 WEEKS.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 12.1 CTS symptoms (BCTQ) | 1 | Mean Difference (IV, Random, 95% CI) | Subtotals only | |
| 12.1.1 Short‐term improvement: 3 months or less | 1 | 37 | Mean Difference (IV, Random, 95% CI) | ‐0.18 [‐0.62, 0.26] |
| 12.2 Function (BCTQ) | 1 | Mean Difference (IV, Random, 95% CI) | Subtotals only | |
| 12.2.1 Short‐term improvement: 3 months or less | 1 | 37 | Mean Difference (IV, Random, 95% CI) | 0.05 [‐0.39, 0.49] |
| 12.3 Referral for surgery | 1 | Risk Ratio (M‐H, Random, 95% CI) | Subtotals only | |
| 12.3.1 Long‐term improvement: over 3 months | 1 | 37 | Risk Ratio (M‐H, Random, 95% CI) | 0.59 [0.12, 2.83] |
Comparison 13. SPLINT SIX WEEKS VERSUS SPLINT SIX MONTHS.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 13.1 CTS symptoms (BCTQ) | 1 | Mean Difference (IV, Random, 95% CI) | Subtotals only | |
| 13.1.1 Long‐term improvement: > 3 months | 1 | 156 | Mean Difference (IV, Random, 95% CI) | 1.30 [0.81, 1.79] |
| 13.2 Function (BCTQ) | 1 | Mean Difference (IV, Random, 95% CI) | Subtotals only | |
| 13.2.1 Long‐term improvement: > 3 months | 1 | 156 | Mean Difference (IV, Random, 95% CI) | 2.30 [1.44, 3.16] |
| 13.3 Referral for surgery | 1 | 118 | Risk Ratio (M‐H, Random, 95% CI) | 9.00 [0.50, 163.53] |
Comparison 14. NIGHT‐TIME SPLINTING VERSUS FULL‐TIME SPLINTING.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 14.1 CTS symptoms (BCTQ) | 1 | Mean Difference (IV, Random, 95% CI) | Subtotals only | |
| 14.1.1 Short‐term improvement: 3 months or less | 1 | 24 | Mean Difference (IV, Random, 95% CI) | ‐0.21 [‐0.83, 0.41] |
| 14.2 Function (BCTQ) | 1 | Mean Difference (IV, Random, 95% CI) | Subtotals only | |
| 14.2.1 At the end of 6 weeks treatment | 1 | 24 | Mean Difference (IV, Random, 95% CI) | ‐0.21 [‐0.87, 0.45] |
Characteristics of studies
Characteristics of included studies [ordered by study ID]
Akturk 2018.
| Study characteristics | ||
| Methods | Study design: 2‐arm assessor‐blind RCT Setting: hospital physical therapy clinic in Turkey |
|
| Participants |
Details of sampling frame: Total n eligible = not reported Total n excluded pre‐randomisation = not reported Total n randomised = 58 hands (44 participants) Total n available for follow‐up = 58 hands Total n analysed = 58 hands Intervention group 1 (splint + exercise) n = 30 hands Intervention group 2 (kinesiology tape + exercise) n = 28 hands Gender distribution: Total: 6 males; 38 females Intervention group 1 (splint + exercise): 1 male hand; 29 female hands Intervention group 2 (kinesiology tape + exercise): 6 male hands; 22 female hands Mean ± SD age: Intervention group 1 (splint + exercise): 48.2 ± 9.2 Intervention group 2 (kinesiology tape + exercise): 49.2 ± 11.7 Total: aged 20–65 years Mean ± SD duration of CTS symptoms: Intervention group 1 (splint + exercise): 7.13 ± 1.96 weeks Intervention group 2 (kinesiology tape + exercise): 7.6 ± 2.5 weeks Inclusion criteria:
Exclusion criteria:
CTS diagnostic criteria (case definition): People diagnosed with mild‐to‐moderate CTS by electroneuromyography (ENMG) were included in the study CTS severity: Mild‐to‐moderate idiopathic CTS |
|
| Interventions | Group 1: treated with splinting. Flexion, extension, and deviation of the wrist were not allowed in the splinted group but pronation and supination were. Neutral position, volar‐assisted splints were applied and the participants were advised to wear them during the night‐time and also at daytime whenever possible for 35 days. Participant comfort when wearing splint was checked on a weekly basis. Group 2: kinesiology tape was applied using the neural and inhibition techniques recommended for CTS. Alcohol‐drenched cotton was used to clean the skin of oils and moisture with the wrist at 30° extension, the forearm in supination and the elbow in extension. For the neural technique, two 2.5 cm‐wide strips were prepared by measuring the distance from the participant’s first metacarpal joint to medial epicondyle. The 1st band for the median nerve was taped from 2nd and 3rd metacarpophalangeal joints to the medial epicondyle with medium stretch (60%) through the nerve trace. The 2nd strip was applied to the lateral epicondyle from the 4th and 5th metacarpophalangeal joints by applying the inhibition technique. A 3rd strip 4 cm wide was cut and applied to the volar face of the hand with full stretch. Taping was performed by the same physiotherapist twice a week for 5 weeks, 10 times in total. The tape was removed after 48 hours and reapplied after 24 hours of rest due to skin irritation. During this time, the participant was informed that he or she should not engage in activities that could cause excessive sweating and should not be exposed to water. Both groups: All participants were advised to report any discomfort when using the splint or kinesiology tape. The exercises for the tendons and nerve‐shifting were written down and the participants were told to carry them out every day for 35 days. Theexercise programme was given to all groups in the form of a home schedule and they were also given demonstrations. If symptoms were provoked during exercise, it was recommended to continue the exercise regimen using a smaller range of motion. Exercise compliance was monitored with a diary and checked on a weekly basis. |
|
| Outcomes | Outcomes evaluated before treatment and 6 weeks after treatment
|
|
| Funding | Not reported | |
| COI | Not reported | |
| Notes | BCTQ results reported as 10 to 50 in the table. Scale converted to 1 to 5 by dividing by 10. No information on whether the trial allowed participants to use other medication such as analgesic drugs or NSAIDs Exercise compliance was monitored with a diary and checked on a weekly basis, but nothing reported regarding the compliance with splint or kinesiology tape use. The results of compliance not provided |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote: "Randomization was made by drawing lots". |
| Allocation concealment (selection bias) | Unclear risk | Quote: "Randomization was made by drawing lots". Comment: No information regarding the method of allocation was reported. |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Comment: blinding of participants not reported, but due to the nature of the interventions (splint versus taping), it is likely that participants were aware which treatment they were allocated to. Blinded observer is not adequate blinding regarding participant‐reported outcome (BCTQ). Quote: "An observer who was blinded to the treatment method filled in an evaluation form both before treatment and in the sixth week following treatment." |
| Blinding of outcome assessment (detection bias) All outcomes | High risk | Quote: "An observer who was blinded to the treatment method filled in an evaluation form both before treatment and in the sixth week following treatment." Comment: Since participants themselves assessed participant‐reported outcomes and they were likely to be aware of their allocated treatment in this study, we rated the risk as high. |
| Incomplete outcome data (attrition bias) 3 months or less | Low risk | Comment: Not reported, probably no loss to follow‐up |
| Selective reporting (reporting bias) | Unclear risk | Comment: All the outcomes specified in the methods reported, but no study protocol available, therefore, the risk was unclear. |
| Other bias | Unclear risk | Comment: 1) Trial reported BCTQ on a scale of 10 to 50, although normal range is from 1 to 5. As the values reported similarly in each treatment group, we transformed to values to 1 to 5, as this likely does not cause bias. 2) 14 participants had bilateral CTS. Clustering not controlled in the analyses; not clear if these 14 participants were distributed evenly 3) Gender distribution not balanced at baseline (29/30 women versus 22/28 women) |
Boonhong 2017.
| Study characteristics | ||
| Methods | Study design: 3‐month randomised, single‐blind, controlled trial Setting: outpatient unit and electrodiagnostic laboratory of the Department of Rehabilitation Medicine, King Chulalongkorn Memorial Hospital, Bangkok, Thailand |
|
| Participants |
Details of sampling frame: Total n assessed for eligibility = 128 patients Total n excluded pre‐randomisation = 74 patients Total n randomised = 54 participants (hands) Total n available for follow‐up = 54 participants (hands) Total n analysed = 54 participants (hands) Intervention group 1 (splint) n = 28 participants (hands) Intervention group 2 (control) n = 26 participants (hands) Gender distribution: Intervention group 1 (splint): 1 male; 27 female Intervention group 2 (control): 3 male; 23 female Mean ± SD age: Intervention group 1 (splint): 53 ± 12.4 years Intervention group 2 (control): 53.6 ± 12.2 years Mean ± SD duration of CTS symptoms: Intervention group 1 (splint): 6.7 ± 6.6 months Intervention group 2 (control): 6.1 ± 6.6 months Inclusion criteria:
Exclusion criteria:
CTS diagnostic criteria (case definition): Electrodiagnostic tests of sensory and motor nerve conduction were performed at baseline to confirm the diagnosis of mild to moderate CTS (to exclude severe cases of CTS (CMAP amplitude less than 5.0 mV) and other neurological conditions). CTS severity: Mild‐to‐moderate CTS |
|
| Interventions | Group 1 ‐ splinting group: a commercially available adjustable wrist splint that was properly fitted and that immobilised the wrist in the neutral position. Participants were advised to wear the splint as often as possible during the daytime as well as night‐time (during the 3‐month duration of the study) to achieve the maximum effectiveness. Group 2 ‐ control group: received only condition‐related patient instructions and education pamphlet Both groups: Participants were given instruction regarding the nature of CTS and advised to avoid full extension and flexion of the wrist, reduce heavy work activities, and avoid repetitive movements. An education pamphlet was given to participants to learn more by themselves. |
|
| Outcomes | Outcomes were measured at baseline and at 3 months after the start of treatment
|
|
| Funding | The authors gratefully acknowledge the Ratchadapiseksompoj Fund of the Faculty of Medicine, Chulalongkorn University for financial support. Grant number is RA 53/54(2). | |
| COI | The authors had no conflict of interest to declare. | |
| Notes | The article did not report the SD for duration of CTS symptoms but we calculated it from the P value. The article reported the BCTQ symptom severity and functional status as median (IQL 25, IQL 75). There was no explanation for IQL 25 and IQL 75 and we assumed they meant 25th percentile and meant 75th percentile, and calculated IQR and SD based on this assumption. The calculation gave reasonable SD values and the study did not get particularly high or low weight in the analysis. Participants were not allowed other medication, such as analgesic drugs or NSAIDs. All participants in the splinting group reported being able to use their splint during both daytime and night‐time. Mean duration of splint wear was 6.2 ± 2.5 hours/day (min = 2 hours, max = 11 hours) and 8.0 ± 2.0 hours/day (min = 4 hours, max = 11 hours) for daytime and night‐time, respectively. |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote: "The randomized sequence was computer‐generated with results sealed in opaque, tamper‐proof, numbered envelopes." |
| Allocation concealment (selection bias) | Low risk | Quote: "The randomized sequence was computer‐generated with results sealed in opaque, tamper‐proof, numbered envelopes." Comment: No further elaboration of whether the concealment was ensured, but likely that it was |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Quote: "A well‐trained assistant who was blinded to the patient treatments and was not involved in patients’ treatment, explained the questionnaire to the participants without any guide and let them answer independently." Comment: However, splinting and no treatment/education are obviously different from each other, therefore unlikely that the participants were blinded |
| Blinding of outcome assessment (detection bias) All outcomes | High risk | Quote: "All electrodiagnostic studies were conducted by an examiner who was blinded to the patient treatment groups." Comment: However, since participants themselves assessed participant‐reported outcomes and they were likely to be aware of their allocated treatment in this study, we rated the risk as high. |
| Incomplete outcome data (attrition bias) 3 months or less | Low risk | Comment: All participants were accounted for; dropouts and attrition did not happen. |
| Selective reporting (reporting bias) | Unclear risk | Quote: "The protocol for this study has been approved by the Institutional Review Board of the Faculty of Medicine, Chulalongkorn University, Bangkok, Thailand." Comment: All the outcomes specified in the methods reported, but study protocol was not available for reading, therefore the risk of bias was unclear. |
| Other bias | Low risk | Comment: No other risk of bias detected |
Chesterton 2018.
| Study characteristics | ||
| Methods | Study design: pragmatic, 2‐arm, parallel‐group, open‐label, RCT Setting: 25 primary and community musculoskeletal clinics and services in England |
|
| Participants |
Details of sampling frame: Total n eligible = 405 (750 assessed for eligibility) Total n excluded pre‐randomisation = 516 Total n randomised = 234 participants (hands) Intervention group (splint) n = 118 participants (hands) Intervention group (steroid injection) n = 116 participants (hands) Post‐intervention follow‐up at 6 weeks: Total n available for follow‐up (6 weeks) = 217 participants (hands) Total n analysed (6 weeks) = 234 participants (hands) Intervention group (splint) n = 109 participants (hands) Intervention group (steroid injection) n = 108 participants (hands) Post‐intervention follow‐up at 6 months: Total n available for follow‐up (6 months) = 192 participants (hands) Total n analysed (6 months) = 234 participants (hands) Intervention group (splint) n = 96 participants (hands) Intervention group (steroid injection) n = 96 participants (hands) Gender distribution: Intervention group 1 (splint): 37 males; 81 females Intervention group 2 (steroid injection): 43 male; 73 females Mean ± SD age: Intervention group 1 (splint): 52.2 ± 14.9 (median 50.00, IQR 40.75–64.25) Intervention group 2 (steroid injection): 52.6 ± 17 (median 53.50, IQR 39.25–65.00) Duration of CTS symptoms (number of participants): Splint < 3 months: 17 Splint 3–6 months: 33 Splint 6 months to 1 year: 27 Splint > 1 year: 39 Splint missing: 2 Steroid injection < 3 months: 19 Steroid injection 3–6 months: 37 Steroid injection 6 months to 1 year: 22 Steroid injection > 1 year: 34 Steroid injection missing: 4 Inclusion criteria: 1. Aged 18 years or older 2. Presented with a new episode of primary idiopathic mild or moderate CTS, which had been present for longer than 6 weeks Exclusion criteria:
CTS diagnostic criteria (case definition): A general practitioner or trained clinician (physiotherapist or occupational therapist) made the clinical diagnosis, standardised on the basis of presenting symptoms, clinical history, and physical tests using criteria developed as part of a consensus survey of general practitioners from the UK Primary Care Rheumatology Society. Mild CTS was defined as intermittent paraesthesia in the distribution of the median nerve, and moderate as constant paraesthesia, and reversible numbness or pain of idiopathic nature. CTS severity: Mild‐to‐moderate CTS |
|
| Interventions | Group 1 ‐ night‐resting splint to be worn for 6 weeks: a Beta Wrist Brace, which immobilised the wrist in a neutral or slightly extended position (20° from neutral). The splint was fitted according to the size of the participant’s hand and arm with standard splints of differing sizes. The treating clinician showed the participants how to fit and remove the wrist splint and gave them two Arthritis Research UK patient leaflets: CTS and splints for arthritis of the hand and wrist. The clinician instructed the participants to do gentle range‐of‐motion exercises when removing the splint to prevent stiffness and reinforced adherence by verbal instruction. Group 2 ‐ corticosteroid injection: received one injection of 20 mg methylprednisolone acetate (as 20 mg of Depo‐Medrone from 40 mg/mL; Pfizer) via a disposable needle (23 G or 25 G) and syringe which was inserted at the wrist between the proximal and distal wrist crease to infiltrate the carpal tunnel. We did not allow injections into the palm of the hand. Participants were treated by the diagnosing clinician who used a sterile no‐touch technique without local anaesthetic. Participants were advised to wait for 30 min following injection and to rest the injected arm for 48 hours They were given 2 Arthritis Research UK patient leaflets for CTS and local corticosteroid injections. Both groups: no other types of therapy in either group were advised during the first 6 weeks, except for simple analgesia either prescribed or bought over the counter (paracetamol and NSAIDs). |
|
| Outcomes | Outcomes evaluated before, 6 weeks and 6 months after treatment; Secondary outcome measures at 6 weeks, 6 months, 12 months, and 24 months
|
|
| Funding | "This paper presents independent research funded by an Arthritis Research UK grant (Grant Number 20105). EMH is a National Institute for Health Research (NIHR) senior investigator. KSD is part‐funded by a Knowledge Mobilisation Research Fellowship (KMRF‐2014‐03‐002) from the NIHR and the NIHR Collaborations for Leadership in Applied Health Research and Care West Midlands. The views expressed in this paper are those of the author(s) and not necessarily those of the National Health Service, the NIHR, or the Department of Health and Social Care." | |
| COI | The authors declared no competing interests. | |
| Notes | Even though there was a dropout of participants at 6 weeks and 6 months, analyses were based on multiple imputed data. Participants with bilateral CTS were permitted treatment for the non‐study hand according to normal clinical protocols in use at the research site. Some participants were diagnosed with hypothyroidism and diabetes. 5/234 had had surgery for CTS. Outcomes were not reported for this subcohort separately, so we could not exclude these participants. As the proportion was low, we did not exclude this study. In the corticosteroid injection group, 3 participants either received an incorrect injection (n = 2) or additionally to the injection wore a night splint (n = 1). In the night splint group, 28 participants either received a corticosteroid injection in addition to the night splint (n = 2), wore the splint on the wrong hand (n = 3), did not wear the splint for at least 4–6 nights per week (n = 4), or did not provide adherence data (n = 19). |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote: "Participants were randomly assigned (1:1) to either treatment group with permutated blocks of sizes two and four, prestratified by research site. Randomisation was completed by the Keele University (Keele, UK) Clinical Trial Unit’s (CTU) online web or telephone randomisation service." |
| Allocation concealment (selection bias) | Low risk | Quote: "Randomisation was completed by the Keele University (Keele, UK) Clinical Trial Unit’s (CTU) online web or telephone randomisation service. The allocation sequence was not available to research team members. We could not mask treating clinicians or patients to treatment allocation, but we concealed the treatment group allocation during the analyses. A letter was sent to the GPs (general practitioners) of all participants informing them of their patient’s participation in the trial and their treatment allocation." |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Quote: "We could not mask treating clinicians or patients to treatment allocation, but we concealed the treatment group allocation during the analyses." |
| Blinding of outcome assessment (detection bias) All outcomes | High risk | Quote: "We could not mask treating clinicians or patients to treatment allocation, but we concealed the treatment group allocation during the analyses." |
| Incomplete outcome data (attrition bias) 3 months or less | Low risk | Comment: All participants were accounted for and reasons for dropouts and attrition were documented (9/118 versus 8/116 missing data at 6 weeks and 20/118 versus 20/118 at 6 months: balanced loss). Authors also did per protocol sensitivity analysis (for 28/118 versus 3/116 participants treatment deviated from protocol). |
| Incomplete outcome data (attrition bias) After 3 months | Low risk | Comment: All participants were accounted for and reasons for dropouts and attrition were documented (9/118 versus 8/116 missing data at 6 weeks and 20/118 versus 20/118 at 6 months: balanced loss). Authors also did per protocol sensitivity analysis (for 28/118 versus 3/116 participants treatment deviated from protocol). |
| Selective reporting (reporting bias) | Unclear risk | Comment: Protocol available. Perceived benefit and satisfaction with treatment seems not to be reported, but since this was a secondary outcome, it was not clear if non‐reporting was related to the nature of the findings. |
| Other bias | Low risk | Comment: No other risk of bias detected |
De Entrambasaguas 2006.
| Study characteristics | ||
| Methods | Study design: Randomised, single‐blind, controlled trial Setting: general university hospital, Spain. Study population included people referred to the clinical neurophysiology service. |
|
| Participants |
Details of sampling frame: Total n eligible = 88 wrists Total n excluded pre‐randomisation = 13 wrists Total n randomised = 75 wrists Intervention group 1 (splint) n = 26 wrists Intervention group 2 (steroid injection) n = 24 wrists Intervention group 3 (phonophoresis) n = 25 wrists Post‐intervention follow‐up: Total n available for follow‐up = 38 participants (52 wrists) Total n analysed = 52 wrists Intervention group 1 (splint) n = 18 wrists Intervention group 2 (steroid injection) n = 18 wrists Intervention group 3 (phonophoresis) n = 16 wrists Gender distribution: Total: 8 male wrists, 44 female wrists (n of participants not reported) Intervention group 1 (splint): 0 male wrists, 18 female wrists Intervention group 2 (steroid injection): 6 male wrists, 12 female wrists Intervention group 3 (phonophoresis): 2 male wrists, 14 female wrists Mean ± SD (range) age: Intervention group 1 (splint): 50 ± 10.7 years Intervention group 2 (steroid injection): 45.4 ± 7.8 years Intervention group 3 (phonophoresis): 58.2 ± 8.1 years Total: 50.7 ± 10.3 (25 to 73) Mean ± SD (range) duration of CTS symptoms: Total: 10.7 ± 20.5 (2–80) months Inclusion criteria: Mild CTS (increase of sensory or mixed latencies of the median nerve, regardless of the amplitude of potentials) or moderate CTS (criteria for mild CTS plus increase of DML of the median nerve) Exclusion criteria:
CTS diagnostic criteria (case definition): Clinical suspicion of CTS was confirmed by means of electromyographic studies (EMG). CTS severity: Mild and moderate CTS |
|
| Interventions | Group 1: splinting – each splint was modelled individually for each hand, and worn for 12 hours daily for four weeks; if uncomfortable, splint was adjusted. Group 2: steroid injection ‐ injection of 40 mg of triamcinolone with 10 mg of lidocaine Group 3: phonophoresis ‐ diclofenac gel was used to administer ultrasound pulses in 10‐minute sessions, 5 days per week, for 4 weeks. | |
| Outcomes | Outcomes assessed at baseline and 1 month after treatment ended:
|
|
| Funding | Not reported | |
| COI | The authors declared that they had no conflict of interest. | |
| Notes | Written in Spanish No treatment had side effects, except 1 participant had vasovagal syncope due to the emotional stress of the injection. As the number of participants (in addition to wrists) was available only for those who completed the study, we used this number when describing results. |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Comment: No information regarding how the random sequence was generated was reported. |
| Allocation concealment (selection bias) | Unclear risk | Comment: No information regarding the method of allocation was reported. |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Quote from translation: "The patients were followed up one month after the end of the given treatment. It consisted of a new clinical evaluation, physical examination and EMG studies, identical to the initial protocol. This evaluation was made single‐blind by the same physician who carried out the first study." Comment: The authors reported that personnel were blinded; however, the participants were not blinded. |
| Blinding of outcome assessment (detection bias) All outcomes | High risk | Quote from translation: "The patients were followed up one month after the end of the given treatment. It consisted of a new clinical evaluation, physical examination and EMG studies, identical to the initial protocol. This evaluation was made single‐blind by the same physician who carried out the first study." Comment: The authors reported that personnel were blinded; however, the participants were not blinded. |
| Incomplete outcome data (attrition bias) 3 months or less | High risk | Comment: A flow chart detailed the number of wrists assigned to each group, plus the number of wrists in each group where participants rejected treatment, did not show up, or were excluded because follow‐up was carried out by physicians not directly involved in the study, or because participants did not follow instructions. Loss to follow‐up and reasons for these losses were not equally balanced across the groups. |
| Selective reporting (reporting bias) | Unclear risk | Comment: According to the translator, all outcomes reported in the Methods section were fully reported in the Results section of the report; however, the study protocol was not available. |
| Other bias | Unclear risk | Not clear how people with bilateral CTS were distributed |
De Moraes 2021.
| Study characteristics | ||
| Methods | Study design: randomised, parallel‐group, single (investigator)‐blinded controlled trial Setting: hand surgery and microsurgery section of Hospital Alvorada, Américas, São Paulo/SP |
|
| Participants |
Details of sampling frame: Total n eligible = not reported Total n excluded pre‐randomisation = not reported Total n randomised = 100 participants (hands) Intervention group 1 (splint) n = 48 participants (hands) Intervention group 2 (steroid injection) n = 52 participants (hands) Post‐intervention follow‐up at 3 months: Total n available for follow‐up (3 months) = 99 participants (hands) Total n analysed (3 months) = 99 participants (hands) Intervention group (splint) n = 47 participants (hands) Intervention group (steroid injection) n = 52 participants (hands) Post‐intervention follow‐up at 6 months: Total n available for follow‐up (6 months) = 95 participants (hands) Total n analysed (6 months) = 95 participants (hands) Intervention group (splint) n = 45 participants (hands) Intervention group (steroid injection) n = 50 participants (hands) Gender: Intervention group 1 (splint): 7 males; 41 females Intervention group 2 (steroid injection): 10 males; 42 females Mean age: Intervention group 1 (splint): 54.4 years (SD not reported) Intervention group 2 (steroid injection): 54.2 years (SD not reported) Duration of CTS symptoms: Inclusion criteria
Exclusion criteria
CTS diagnostic criteria (case definition): A CTS diagnosis was made clinically and supported by electrodiagnostic findings. CTS severity: Moderate to severe CTS |
|
| Interventions | Group 1 ‐ a forearm‐palmar orthosis with the wrist immobilised in a neutral position was used at night while sleeping and removed in the morning. The duration of orthosis use differed because sleeping times were different between individuals. The orthosis was used throughout the study period. Group 2 ‐ corticosteroid injection: 6.43 mg (1 mL) of betamethasone dipropionate, 2.63 mg of betamethasone disodium phosphate, and 0.5 mL of 2% lidocaine (xylocaine), totaling 1.5 mL. After injection, a simple dressing was applied. |
|
| Outcomes | Outcomes evaluated at baseline and after treatment (within the 1st week of the intervention, and 1, 3, and 6 months).
|
|
| Funding | None declared | |
| COI | None declared | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Quote: "The randomization procedure was performed by a person not directly involved in the study." Comment: Random sequence generation, however, not described |
| Allocation concealment (selection bias) | Low risk | Quote: "The allocation of patients was performed using opaque envelopes with consecutive numbers. Envelopes were only opened after verification of inclusion criteria and signing of the informed consent form. The randomization procedure was performed by a person not directly involved in the study." Comment: Adequate allocation concealment |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Quote: "Outcome assessments were performed by blinded researchers." Comment: The participants were not blinded, and outcomes were partially or completely participant‐reported. |
| Blinding of outcome assessment (detection bias) All outcomes | High risk | Quote: "Outcome assessments were performed by blinded researchers." Comment: The participants were not blinded, and outcomes were partially or completely participant‐reported. |
| Incomplete outcome data (attrition bias) 3 months or less | Low risk | Quote: "There was an overall loss to follow‐up of 5 patients (5%). In the orthosis group, 1 patient did not return in the third month of follow‐up, and another 2 were lost in the sixth month (6.25%). In the corticosteroid group, 2 patients (3.8%) did not return in the sixth month assessment." Comment: Small and balanced loss, and reasons reported. Not likely to bias outcomes considerably |
| Incomplete outcome data (attrition bias) After 3 months | Low risk | Small and balanced loss, and reasons reported. Not likely to bias outcomes considerably |
| Selective reporting (reporting bias) | Low risk | Main outcomes defined in the registration were reported. Graham criteria were defined as an outcome in the ClinicalTrials registry but not reported. This likely does not bias the results, as we did not consider those criteria as relevant in this review. |
| Other bias | Low risk | Randomisation and outcome measurement were performed at participant level. No other risk of bias detected |
Eraslan 2014.
| Study characteristics | ||
| Methods |
Study design: 2‐arm quasi‐randomised trial Setting: unclear |
|
| Participants |
Details of sampling frame: Total n eligible = not reported Total n excluded pre‐randomisation = not reported Total n randomised = 30 participants (47 wrists) Total n available for follow‐up = 30 Total n analysed = 30 Intervention group 1 (splint) n = 15 Intervention group 2 rigid taping) = 15 Gender distribution Intervention group 1 (splint): 2 male, 13 females Intervention group 2 (rigid taping): 2 male, 13 females Mean ± SD (range) age: Intervention group 1 (splint): 43.5 ± 13 Intervention group 2 (rigid taping): 48.9 ± 12 Total mean: 46.2 (aged between 21 and 71 years) Mean ± SD (range) duration of symptoms, months Not reported Inclusion criteria: CTS according to the electroneuromyography (ENMG) and American Association of Electrodiagnostic Medicine criteria Exclusion criteria:
CTS diagnostic criteria (case definition): Not reported CTS severity: Not reported Protocol violators: None |
|
| Interventions | Group 1 ‐ splint: On the 6th day of the treatment, participants were recommended night splint without thumb support. While allowing pronation and supination of the wrist, a neutral positioned splint with volar support was used, which would not allow flexion, extension and deviation. The participants were advised to use their splints for an average of 8 hours each night. Group 2 ‐ rigid taping: Rigid taping was applied by stretching the carpometacarpal joint with the anchor tie around the thumb, relaxing the flexor retinaculum, and finally stretching the metacarpophalangeal joint and closing it with the last anchor tie on the thumb. Both groups: physical therapy programme consisting of 21 sessions of hot application‐ultrasound‐TENS. Hot application for 20 minutes, ultrasound for 4 minutes (1.5 W/cm2 for 2 minutes in each hand), and 20 minutes of TENS (continuous mode) were applied once a day, 6 sessions a week. In the first 6 days of treatment, the participants were recommended to restrict excessive hand and wrist activities. On the 6th day of the treatment, both groups were given tendon shifting, median nerve shifting, strengthening exercises for the wrist, strengthening exercises for the intrinsic muscles, especially strengthening and stretching exercises for the thumb (abductor pollicis muscle) and median nerve stretching. |
|
| Outcomes | Outcomes were collected at baseline and at the 21st day of the treatment
|
|
| Funding | Not reported | |
| COI | Not reported | |
| Notes | Article in Turkish, translation used Results from BCTQ not reported in scale 1 to 5. We assumed that total sum was reported and divided symptom score by 11 and functional score by 8 (as per instructions of the scale). |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | High risk | Quote from translation: "Patients were allocated to two treatment groups as night splint and rigid taping. The first patient assigned to a group according to the coin toss method. The following patients were grouped considering the arrival order." Comment: Only the first participant was randomised and the rest were sequentially allocated, which is not true randomisation. |
| Allocation concealment (selection bias) | High risk | Quote from translation: "Patients were allocated to two treatment groups as night splint and rigid taping. The first patient assigned to a group according to the coin toss method. The following patients were grouped considering the arrival order." Comment: No true randomisation and the sequence was not concealed from the investigators. |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Comment: Not reported, but due to the nature of the interventions (splint versus taping), it is likely that participants were aware of their allocated treatment. |
| Incomplete outcome data (attrition bias) 3 months or less | Low risk | Comment: Data given for all participants in the table |
| Selective reporting (reporting bias) | Unclear risk | Comment: No protocol available to confirm the planned outcomes, therefore the risk was unclear. BCTQ reported fully for both groups |
| Other bias | Low risk | Comment: 1) The BCTQ scores were reported in a modified way. The standard method is to divide by the number of questions (scale 1 to 5) but the authors reported the total score (scale probably 19 to 95). However, this did not cause bias because both groups were reported similarly. 2) The level of analysis was the participant, therefore clustering should not affect the analysis. |
Gatheridge 2020.
| Study characteristics | ||
| Methods | Study design: prospective, unblinded, randomised clinical trial with 12‐week follow‐up Setting: United States Air Force Academy |
|
| Participants |
Details of sampling frame: Total n eligible = not reported Total n excluded pre‐randomisation = not reported Total n randomised = 31 (38 hands) Intervention group 1 (splint 6 weeks) = not reported Intervention group 2 (splint 12 weeks) = not reported Post‐intervention follow‐up: Total n available for follow‐up = 30 (37 hands) Total n analysed = 30 (37 hands) Intervention group 1 (splint 6 weeks) = 14 (17 hands) Intervention group 2 (splint 12 weeks) = 16 (20 hands) Gender distribution: Intervention group 1 (splint 6 weeks): 4 males; 10 females Intervention group 2 (splint 12 weeks): 6 male; 10 females Mean ± SD age: Intervention group 1 (splint 6 weeks): 46.8 ± 6.35 (range 34–61) Intervention group 2 (splint 12 weeks): 47 ± 6.35 (range 26–72) Mean ± SD duration of CTS symptoms: Intervention group 1 (splint 6 weeks): 51.2 ± 15.17 (range 12–156) weeks Intervention group 2 (splint 12 weeks): 61.4 ± 15.17 (range 6–520) weeks Inclusion criteria:
Exclusion criteria:
CTS diagnostic criteria (case definition): Electrodiagnostic testing (to confirm having unilateral or bilateral mild or mild to moderate CTS) CTS severity: Mild‐to‐moderate CTS |
|
| Interventions | Group 1: neutral wrist splint for 6 weeks Group 2: neutral wrist splint for 12 weeks All participants were fitted with the Hely & Weber Titan Wrist Lacing Orthosis by manufacturer instructions in a wrist neutral position, where the wrist is in straight alignment with the forearm, limiting pressure on the carpal tunnel space. All participants received the same instruction for wearing the wrist splint, to include wearing the wrist splint every night for the specified duration. Participants were advised that they could wear the splint during the day (in addition to night‐time) for activities that provoked symptoms; however, they were encouraged to attempt nocturnal splinting first. Participants were not given any work or activity restrictions. |
|
| Outcomes | Outcomes were assessed at baseline, 6 and 12 weeks
|
|
| Funding | Not reported | |
| COI | Not reported | |
| Notes | SD value for age and duration of symptoms not reported in the article, but we calculated it from P value. At 6 and 12 weeks, participants were asked about their wrist splint compliance and, in a separate unvalidated survey, if they minded wearing the wrist splint. At 6 weeks, compliance was similar for both groups (P = 0.856). A total of 47% of the cohort reported wearing their wrist splint every night, and 91% reported wearing it for at least 5 to 7 nights. In weeks 6 to 12, 84% of participants in group 2 continued to wear the splint more than 5 nights. The majority of participants in group 1 did not wear the wrist splint (P = 0.004) during this time. 4 participants (5 hands) in group 1 reported that they did continue to wear the wrist splint, 2 wearing the splint frequently (5–7 nights). |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Quote: "Subjects were randomized to wear the wrist splint for either 6 weeks (group A) or 12 weeks (group B) by random block permutation, for which the PI was blinded". Comment: No further description how randomisation was achieved |
| Allocation concealment (selection bias) | Low risk | Quote: "Subjects were randomized to wear the wrist splint for either 6 weeks (group A) or 12 weeks (group B) by random block permutation, for which the PI was blinded. Once the subject was enrolled, the PI and subjects were unblinded to that subjects’ splint use duration." Comment: No further information on allocation concealment |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Quote: "Once the subject was enrolled, the PI [principal investigator] and subjects were unblinded to that subject's splint use duration." |
| Blinding of outcome assessment (detection bias) All outcomes | High risk | Quote: "Once the subject was enrolled, the PI and subjects were unblinded to that subject's splint use duration." |
| Incomplete outcome data (attrition bias) 3 months or less | Low risk | The dropouts documented and reasons provided |
| Selective reporting (reporting bias) | Unclear risk | Comment: All outcomes specified in the methods were reported, but no study protocol was available, therefore the risk of bias is unclear. |
| Other bias | Unclear risk | Comment: 30 participants, 38 hands in the study. Clustering not controlled for. Bilateral condition in 3 versus 4 participants in the groups. Unclear if this could bias the comparison. |
Geler Kulcu 2016.
| Study characteristics | ||
| Methods | Study design: Randomised 3‐arm, sham‐controlled trial Setting: Turkey |
|
| Participants |
Details of sampling frame:
Total n assessed for eligibility = 57
Total n excluded pre‐randomisation = 12
Total n randomised = 45 participants (65 wrists)
Intervention group 1 (splint) n = 15 (21 wrists)
Intervention group 2 (kinesiology tape) n = 15 (22 wrists)
Intervention group 3 (placebo kinesiology tape) n = 15 (22 wrists)
Post‐intervention follow‐up at 4 weeks:
Total n available for follow‐up = 40 participants (60 wrists)
Intervention group 1 (splint) n = 14 (20 wrists)
Intervention group 2 (kinesiology tape) n = 13 (20 wrists)
Intervention group 3 (placebo kinesiology tape) n = 13 (20 wrists)
Gender distribution Intervention group 1 (splint): 1 male; 13 females Intervention group 2 (kinesiology tape): 1 males; 12 females Intervention group 3 (placebo kinesiology tape): 0 males; 13 females Mean ± SD age: Intervention group 1 (splint): 51.3 ± 8.3 (range 40–65) Intervention group 2 (kinesiology tape): 49.8 ±11.5 (range 20–62) Intervention group 3 (placebo kinesiology tape): 48.95 ± 6.0 (range 40–60) Total: 50.02 ± 8.79 (20–65) years Mean ± SD (range) duration of CTS symptoms Not reported. All had CTS < 1 year Inclusion criteria 1. Mild‐to‐moderate CTS according to a nerve conduction study 2. 18 years or older 3. Symptoms for less than 1 year Exclusion criteria
CTS diagnostic criteria (case definition): 1. People diagnosed by EMG as having mild to moderate CTS (according to NCSs) CTS severity: Mild‐to‐moderate CTS |
|
| Interventions | Group 1 ‐ wrist orthosis: Participants were applied with a custom‐made volar thermoplastic wrist orthotic device in a neutral wrist position. The participants were encouraged to use the orthosis night and day, whenever possible, for 4 weeks. Group 2 ‐ kinesiology tape: Tape with a width of 5 cm and a thickness of 0.5 mm was used. Kinesio Tex I Strip was measured from elbow to fingertips and cut. It was folded approximately 2 blocks from the end and cut into 2 triangles on the fold. The 3rd and 4th fingers were slipped through holes and Kinesio Tex was applied on the dorsum of the hand with no tension. The position of elbow extension, wrist extension, and radial deviation was provided, and Kinesio Tex was applied from hand to medial epicondyle with 15% to 25% tension and ended at medial epicondyle with no tension. The second Kinesio Tex I Strip was measured for wrist size and cut. It was applied to the carpal tunnel region with 25% to 35% tension. This technique has been described by Kase and colleagues (Kase 1998). Applied tension to kinesiology tape were performed according to the visible pores on the kinesiology tape. Participants were taped by a doctor certified to apply kinesiology tape. Kinesiology tape was applied at the beginning of the week, to stay on for 5 days, with a 2‐day rest, a total of 4 times. Group 3 ‐ sham kinesiology tape: Tape with a width of 5 cm and a thickness of 0.5 mm was used. Kinesio Tex I Strip was applied without having the proper position and with no tension (in a manner inconsistent with the technique described in Group 1). Kinesiology tape was applied at the beginning of the week, to stay on for 5 days, with a 2‐day rest, a total of 4 times. All groups: All participants received a home exercise programme during the 4 weeks, consisting of tendon‐gliding exercises. To follow up and to improve compliance, each participant was asked to document in a supplied diary what they did, i.e. how many times they did each exercise in a day. The diaries were checked every visit. |
|
| Outcomes | Outcomes were assessed at 4 weeks
|
|
| Funding | Not reported | |
| COI | Not reported | |
| Notes | We assumed by reading the paper that the follow‐up data collection occurred at 4 weeks, but this was not explicitly stated. VAS – data analysis implied that this was a Numerical Rating Scale, not a VAS. Results from BCTQ not reported on a 1 to 5 scale. We assumed that investigators reported the total sum and divided the symptom score by 11 and the functional score by 8 (as per instructions of the scale). The number of participants and hands reported in the main text and Figure 1 were not the same. We used data from Figure 1 as these data appeared to be correct. Adherence to exercise was monitored by documenting what was done in a diary. |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote: "Patients were randomly assigned to one of the three groups using a secure system of opaque closed envelopes numbered 1–3. Wrists of the patients with bilateral CTS were allocated to the same group according to the envelope number that the patient chose. The first group received KT [kinesiology tape], the second group received sham KT, and the third group received an OD [orthotic device], performed by a researcher not involved in the study." Comment: The random sequence was probably adequately generated, as it appeared the participants randomly selected the envelope containing the randomisation allocation. |
| Allocation concealment (selection bias) | Low risk | Quote: "Patients were randomly assigned to one of the three groups using a secure system of opaque closed envelopes numbered 1–3. Wrists of the patients with bilateral CTS were allocated to the same group according to the envelope number that the patient chose. The first group received KT, the second group received sham KT, and the third group received an OD, performed by a researcher not involved in the study." Comment: The allocation sequence appeared to have been adequately concealed prior to assignment of interventions. |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Quote: "The investigator applying the treatments was different from the investigator evaluating the outcome measures; the latter was blind to which series of treatments (experimental KT, placebo KT, or OD) each patient was about to receive or had just received. The patients in Group 1 and Group 2 were blind to the treatments." Comment: The participants in Groups 1 and 2 were blinded to whether they were receiving the experimental or sham intervention. However, the participants in Group 3 were not blinded. Furthermore, due to the nature of the treatments, those providing treatment were unlikely to be blinded to the intervention groups. |
| Blinding of outcome assessment (detection bias) All outcomes | High risk | Quote: "The investigator applying the treatments was different from the investigator evaluating the outcome measures; the latter was blind to which series of treatments (experimental KT, placebo KT, or OD) each patient was about to receive or had just received." Comment: The outcome assessors were likely blinded to the treatment group. However, since participants themselves assessed participant‐reported outcomes and they were likely to be aware of their allocated treatment in this study, we rated the risk as high. |
| Incomplete outcome data (attrition bias) 3 months or less | Low risk | Comment: The number of dropouts at 4‐week follow‐up: 1) kinesiology tape group: 2/15 (13%) 2) placebo kinesiology tape group: 2/15 (13%) 3) orthotic device group 1/15 (7%) Dropouts and protocol violators are clearly documented in the flow diagram and were not included in the analysis. |
| Selective reporting (reporting bias) | Unclear risk | Comment: All outcomes specified in the methods were reported, but no study protocol was available, therefore the risk was unclear. |
| Other bias | Unclear risk | Quote: "Patients with bilateral symptoms were asked to complete two questionnaires, one for each hand separately." Comment: The unit of analysis was hands (with some participants contributing 2 hands to the analysis). Although unlikely, it was unclear whether a unit of analysis error may have occurred in the data analysis. |
Hall 2013.
| Study characteristics | ||
| Methods | Study design: randomised controlled 2‐arm unblinded trial Setting: hospital clinic, Australia |
|
| Participants |
Details of sampling frame Total n eligible = 116 Total n excluded pre‐randomisation = unclear (58 participants were excluded because they did not meet the selection criteria) Total n randomised = 62 participants (hands) Intervention group 1 (splint and education) n = 31 participants (hands) Intervention group 2 (control ‐ no treatment) n = 31 participants (hands) Post‐intervention follow‐up: Total n available for follow‐up = 54 participants (hands) Total n analysed = 54 participants (hands) Intervention group 1 (splint and education) n available for follow‐up = 30 participants (hands) Intervention group 2 (control ‐ no treatment) n available for follow‐up = 24 participants (hands) Gender distribution Intervention group 1 (splint and education): 9 males; 21 females Intervention group 2 (control ‐ no treatment): 5 males; 19 females Mean ± SD age: Intervention group 1 (splint and education): 53.8 ± 5.6 Intervention group 2 (control ‐ no treatment): 54.9 ± 4.7 Mean (range) duration of CTS symptoms: Intervention group 1 (splint and education): 28.3 (26‐132) months Intervention group 2 (control ‐ no treatment): 37.9 (28‐132) months Inclusion criteria
Exclusion criteria: Any medical, cognitive, perceptual, or language deficits that prevented the comprehension of instructions or attendance at appointments CTS diagnostic criteria (case definition): Not reported CTS severity: Not reported Protocol violators: Intervention protocol violators were not included in the analysis. |
|
| Interventions | Group 1 ‐splint and education: 8‐week treatment programme involving a full‐time wrist splint and education sessions conducted by an occupational therapist. The programme included prescription and fitting of a wrist‐support splint and structured education sessions with a focus on self‐management. Over the 8‐week period, each participant received 2 treatment sessions in the 1st week and between week 2‐4 and a 20 min phone call at week 7. The wrist splint positioned the wrist in a neutral wrist position and allowed full finger and thumb motion. One of 4 choices of splint were provided: Otto Bock Manu Basic; Roylan Enlarged Thumb Hole D‐Ring Wrist Brace; Otto Bock Manu Comfort; or custom‐made thermoplastic wrist splint. Education included pathology of CTS, risk identification, and goal setting designed to teach self‐management of CTS symptoms e.g. avoidance of tasks that aggravate symptoms. The night‐time compliance rate for wearing the splint averaged 89%, but compliance dropped to 81% during the daytime. Group 2 ‐ control: participants were assessed and observed but received no intervention during the 8‐week study period. | |
| Outcomes | The outcomes were measured at baseline and at 8 weeks.
|
|
| Funding | Not reported | |
| COI | Not reported | |
| Notes | We decided to include this study in splint versus no active treatment (education about the condition was considered as non‐disease modifying side intervention as in the other analyses where the participants received information about the condition). We did not include the data regarding referral to surgery because of partial reporting: Study reported that 19/30 participants (63%) had decided not to pursue surgical intervention after the CTS conservative treatment programme (which implies that 11/30 participants had still opted for surgery); however, the outcome was not reported for the control group. The night‐time compliance rate for wearing the splint averaged 89%, but compliance dropped to 81% during the daytime. |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote: "Participants who were invited and agreed to participate in the study were randomly assigned to the intervention or control group using a blocking strategy, implemented by the first author, to recruit participants to each study arm at equal rates." Comment: It is likely that sequence generation was adequate. |
| Allocation concealment (selection bias) | Unclear risk | Comment: It is not clear from the publication whether the allocation sequence was adequately concealed until the interventions were assigned. |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Quote: "The two treating occupational therapists, who were not blinded to group allocation, performed all data collection and provided interventions according to the study's assessment and treatment procedure manual." Comment: Due to the nature of the interventions, it is unlikely that participants were blinded. The treating therapists were not blinded to the interventions. |
| Blinding of outcome assessment (detection bias) All outcomes | High risk | Quote: "The two treating occupational therapists, who were not blinded to group allocation, performed all data collection and provided interventions according to the study's assessment and treatment procedure manual." Comment: However, since participants themselves assessed participant‐reported outcomes and they were likely to be aware of their allocated treatment in this study, we rated the risk as high. |
| Incomplete outcome data (attrition bias) 3 months or less | High risk | Quote: "31 were assigned to each group (treatment and control group). Eight participants withdrew, were excluded, or did not complete the study after group allocation (1 from the treatment group and 7 from the control group). The reasons for non‐compliance included vocational requirements that prohibited wearing of splints during work hours and habitual participation in activities such as swimming or cooking that precluded wearing a splint. Discomfort and perceived negative cosmetic effects were also reported as reasons not to wear splint." Comment: All participants were accounted for and reasons for dropouts and attrition were documented, but the loss was not balanced 1/31 (3%) versus 7/31 (23%). |
| Selective reporting (reporting bias) | Unclear risk | Comment: All the outcomes specified in the methods reported, but no study protocol available, therefore the risk was unclear. |
| Other bias | Low risk | Quote: "Participants could have symptoms in one or both hands, but only one hand, with the worst symptoms, was chosen as the study hand". Comment: Only 1 hand for each participant was enrolled in the study, therefore a unit of analysis error was not observed. All outcomes were measured using appropriate reliable and valid methods. |
Jaladat 2017.
| Study characteristics | ||
| Methods | Study design: RCT Setting: Iran |
|
| Participants |
Details of sampling frame: Total n eligible = not reported Total n excluded pre‐randomisation = not reported Total n randomised = 24 Total n available for follow‐up = 24 Total n analysed = 24 Intervention group 1 (splint + routine rehabilitation) n = 12 Intervention group 2 (routine rehabilitation) n = 12 Gender distribution: Intervention group 1 (splint + routine rehabilitation): 0 males, 12 females Intervention group 2 (routine rehabilitation): 0 males, 12 females Mean ± SD age: Intervention group 1 (splint + routine rehabilitation): 47.42 years (SD not reported) Intervention group 2 (routine rehabilitation): 45.76 years (SD not reported) Mean ± SD duration of CTS symptoms: Not reported Inclusion criteria:
Exclusion criteria:
CTS diagnostic criteria (case definition): 1. Classification of NCV findings to a cluster of mild, moderate, and severe CTS 1.1. Median nerve SDL (mild > 3.7; moderate > 4.5; severe > 5.3) 1.2. Median nerve wrist SNCV (mild < 40; moderate < 35; severe < 30) 1.3. Median nerve DML (mild > 4.5; moderate > 5.5; severe > 6.5) 1.4. Median motor response amplitude compared to other hand (mild ‐ ; moderate < 50%; severe ‐ ) 1.5. Median nerve compound latency (mild > 2.4; moderate > 2.8; severe > 3.2) 1.6. Median nerve compound amplitude compared to other hand (mild < 50%; moderate ‐ ; severe ‐ ) CTS severity: Mild‐to‐moderate |
|
| Interventions | Group 1 ‐ splint + routine rehabilitation: treatment for 6 weeks. Splint: thermoplastic customised limited dynamic wrist splint (with a range of motion between 15° flexion & 15° extension), which they had to wear for 6 to 8 hours a day Group 2 ‐ routine rehabilitation: treatment for 6 weeks (including activity/ergonomic modifications, nerve and tendon gliding exercises, massage, carpal bones and nerve mobilisations, stretches of upper extremity and flexor retinaculum) |
|
| Outcomes | Outcomes evaluated before treatment and 6 weeks after treatment
|
|
| Funding | Not reported | |
| COI | Authors reported no conflict of interest. | |
| Notes | Results from BCTQ not reported on a scale of 1 to 5. We assumed that the total sum was reported and investigators divided the symptom score by 11 and the functional score by 8 (as per instructions of the scale). | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Quote: "This interventional study was designed as a randomized controlled trial". "The patients were randomly divided into control and treatment groups". Comment: No further information provided about sequence generation provided |
| Allocation concealment (selection bias) | Unclear risk | Quote: "The study was planned as a prospective, randomized, single‐blind study." Comment: No further information provided about allocation concealment |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Comment: No information about blinding provided, but splinting and routine rehabilitation treatment methods are obviously different from each other, therefore unlikely that the participants were blinded |
| Blinding of outcome assessment (detection bias) All outcomes | High risk | Comment: No information about blinding provided, but splinting and routine rehabilitation treatment methods are obviously different from each other, therefore unlikely that blinding was possible |
| Incomplete outcome data (attrition bias) 3 months or less | Low risk | Comment: No loss to follow‐up reported |
| Selective reporting (reporting bias) | Low risk | Comment: All outcomes defined in the registration (IRCT2015061522753N1) and methods, were reported at 6 weeks. |
| Other bias | Low risk | Comment: In both groups, the participants could have rehabilitation including activity/ergonomic modifications, nerve and tendon gliding exercises, massage, carpal bones and nerve mobilisations, and stretches of the upper extremity and flexor retinaculum. The authors did not measure the use of co‐interventions but no reason to assume that they would differ |
Kocaoglu 2017.
| Study characteristics | ||
| Methods | Study design: parallel‐group, 3‐arm RCT Setting: Turkey |
|
| Participants |
Details of sampling frame: Total n eligible = not reported Total n excluded pre‐randomisation = not reported Total n randomised = 60 Total n available for follow‐up = not reported Total n analysed = not reported Intervention group 1 (splint) n = 20 Intervention group 2 (kinesiology tape) n = 20 Intervention group 3 (Steroid/local anaesthetic injection) n = 20 Gender distribution: Total: 2 males, 58 females Intervention group 1 (splint): not reported Intervention group 2 (kinesiology tape): not reported Intervention group 3 (Steroid/local anaesthetic injection): not reported Mean ± SD age: Total: 48.2 ± 8.9 years Intervention group 3 (splint): not reported Intervention group 1 (kinesiology tape): not reported Intervention group 2 (Steroid/local anaesthetic injection): not reported Mean ± SD duration of CTS symptoms: Total: disease‐duration, 2.8 + 3.5 months Intervention group 3 (splint): not reported Intervention group 1 (kinesiology tape): not reported Intervention group 2 (Steroid/local anaesthetic injection): not reported Inclusion criteria: 1. People with CTS Exclusion criteria: Not reported CTS diagnostic criteria (case definition): Not reported CTS severity: Not reported |
|
| Interventions | Group 1 ‐ splinting for 3 weeks Group 2 ‐ kinesiology taping: performed 3 times at 4‐day intervals Group 3 ‐ a steroid/local anaesthetic injection to carpal tunnel |
|
| Outcomes | The clinical and electrophysiologic studies were performed at baseline and at the 3rd week
|
|
| Funding | Not reported | |
| COI | Not reported | |
| Notes | Number of participants randomised in each group was not reported, but we assumed that the numbers were equal. Study reported as a congress abstract, not much information available |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Quote: "The patients were randomized into three groups receiving either KT performed three‐times by intervals of 4‐day (Group 1); a single S/LA injection to carpal‐tunnel (Group 2); or splinting alone for three‐weeks (Group 3)." Comment: Random sequence generation not described |
| Allocation concealment (selection bias) | Unclear risk | Quote: "The patients were randomized into three groups receiving either KT performed three‐times by intervals of 4‐day (Group 1); a single S/LA injection to carpal‐tunnel (Group 2); or splinting alone for three‐weeks (Group 3)." Comment: Allocation concealment not described |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Comment: The authors did not report any attempt at blinding. Due to the nature of the interventions, it is unlikely that participants were blinded. |
| Blinding of outcome assessment (detection bias) All outcomes | High risk | Comment: Since participants themselves assessed participant‐reported outcomes and they were likely to be aware of their allocated treatment in this study, we rated the risk as high. |
| Incomplete outcome data (attrition bias) 3 months or less | Unclear risk | Comment: loss to follow‐up not described |
| Selective reporting (reporting bias) | Unclear risk | Comment: All the outcomes specified in the methods reported, but no study protocol available, therefore the risk was unclear. |
| Other bias | Unclear risk | Comment: No apparent other sources of bias |
Madjdinasab 2008.
| Study characteristics | ||
| Methods | Study design: RCT Setting: Iran |
|
| Participants |
Details of sampling frame: Total n eligible = not reported Total n excluded pre‐randomisation = not reported Total n randomised = 48 participants Intervention group 1 (splint) n = 24 participants Intervention group 2 (oral steroid) n = 24 participants Gender distribution: Intervention group 1 (splint): 2 males, 22 females Intervention group 2 (oral steroid): 2 males, 22 females Mean ± SD age: Intervention group 1 (splint): 43 years Intervention group 2 (oral steroid): 40 years Total mean: 42.19 (range 21 to 65 years) Mean ± SD duration of CTS symptoms: Not reported Inclusion criteria:
Exclusion criteria:
CTS diagnostic criteria (case definition): Electrophysiological criteria were used for diagnosis of CTS. CTS severity: Not reported |
|
| Interventions | Group 1 ‐ commercially available splint: worn at night and for as long as possible during the day for 6 weeks (wrist splinting in neutral position) Group 2 ‐ oral steroid: Prednisolone 20 mg/day for 2 weeks Both groups were given advice to avoid extreme wrist flexion/extension, excessive hand movement and hand rest. The participants were also asked not to use additional medicines or other methods of treatment during the study period. |
|
| Outcomes | Outcomes assessed at baseline and at the end of 6 weeks treatment
|
|
| Funding | Not reported | |
| COI | Not reported | |
| Notes | No self‐reported outcomes (e.g. symptoms, pain) or function outcomes were reported as being measured in this study. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Quote: "They were randomly divided into two groups. Splint groups (N = 24) used splint for six weeks; and steroid group (N = 24) used oral Prednisolone 20 mg/day for two weeks." Comment: No information reported on how the randomisation sequence was generated |
| Allocation concealment (selection bias) | Unclear risk | Quote: "They were randomly divided into two groups. Splint groups (N = 24) used splint for six weeks; and steroid group (N = 24) used oral Prednisolone 20 mg/day for two weeks." Comment: No information reported on how adequately the randomisation sequence was concealed |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Quote: "This double blind study was carried out in 48 idiopathic CTS patients". Comment: The authors reported that this was a double‐blind study, but did not indicate who specifically was blinded (participants, personnel delivering the treatment, or outcome assessors). Due to the nature of the interventions (splint versus oral steroid), it is likely that participants were aware of their allocated treatment. |
| Blinding of outcome assessment (detection bias) All outcomes | High risk | Quote: "This double blind study was carried out in 48 idiopathic CTS patients". Comment: The authors reported that this was a double‐blind study, but did not indicate who specifically was blinded (participants, personnel delivering the treatment, or outcome assessors). Due to the nature of the interventions (splint versus oral steroid), it is likely that participants were aware of their allocated treatment. |
| Incomplete outcome data (attrition bias) 3 months or less | Unclear risk | Quote: "In splint group three patients and in steroid group two patients did not complete the study and were eliminated." Comment: 21/24 of the splint group and 22/24 of the prednisolone group completed assessments. The reasons for participants not completing the study were not reported, so it is not possible to determine whether the dropouts could have had an impact on the results. |
| Selective reporting (reporting bias) | Unclear risk | Comment: All outcomes reported in the Methods section of the publication were reported in the Results section of the publication. However, the only reported outcomes were electrophysiologic measures. Most other CTS RCTs also measured symptoms and function and without access to a protocol for this study, we could not determine whether those clinical outcomes were measured but not reported in the publication. |
| Other bias | Low risk | Comment: No other sources of bias identified |
Manente 2001.
| Study characteristics | ||
| Methods | Study design: RCT Setting: Italy |
|
| Participants |
Details of sampling frame: Total n eligible = 151 screened Total n excluded pre‐randomisation = not reported Total n randomised = 83 participants (83 wrists) Intervention group 1 (splint) n = 41 participants (41 wrists) Intervention group 2 (no treatment) n = 42 participants (42 wrists) Post‐intervention follow‐up: Total n available for follow‐up = 80 participants (80 wrists) Total n analysed = 80 participants Intervention group 1 (splint) n = 40 participants (40 wrists) Intervention group 2 (no treatment) n = 40 participants (40 wrists) Gender distribution: Intervention group 1 (splint): 4 males, 36 females Intervention group 2 (no treatment): 7 males, 33 females Mean ± SD age: Intervention group 1 (splint): 46.10 ± 12.94 years Intervention group 2 (no treatment): 50.0 ± 12.65 years Mean ± SD duration of CTS symptoms: Not reported Inclusion criteria:
Exclusion criteria:
CTS diagnostic criteria (case definition): On the basis of the electrophysiological results, hands were classified according to the following neurophysiological classification:
|
|
| Interventions | Group 1 ‐ splint: worn at night for 4 weeks (hand brace, called Manu) Group 2 ‐ control (no treatment): participants were asked to wait for an observational period of 4 weeks. All participants were required to agree not to receive other treatments, or change work duties or medications during the study, or otherwise report it. |
|
| Outcomes | Outcome assessed at 2 weeks and at the end of 4 weeks of treatment
|
|
| Funding | This study was supported by a grant from the Italian Ministry for Scientific and Technological Research. | |
| COI | First author is the owner of the patent for the brace, which was pending at the time of publication. | |
| Notes | Compliance and tolerability was assessed by a questionnaire asking how many nights in 4 weeks the participant wore the hand brace: all 28 nights; most (at least 21) nights; half (about 14) of the nights; and some (less than 7) nights, and whether there were adverse effects. Of the 40 participants in the treated group, 38 wore the hand brace for all or most of the nights. |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Quote: "Subjects were randomized into two groups by having them select sealed envelopes containing a group assignment". Comment: Insufficient information provided to determine whether an adequate method was used to generate random sequence |
| Allocation concealment (selection bias) | Unclear risk | Quote: "Subjects were randomized into two groups by having them select sealed envelopes containing a group assignment". Comment: not specified whether envelopes were opaque or sequentially numbered and distributed |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Comment: Participants, personnel, and outcome assessors were not blinded to treatment allocation (confirmed by study authors via personal communication). Assessment of symptoms, functional status, and global impression of change may be biased. |
| Blinding of outcome assessment (detection bias) All outcomes | High risk | Comment: However, since participants themselves assessed participant‐reported outcomes and they were likely to be aware of their allocated treatment in this study, we rated the risk as high. |
| Incomplete outcome data (attrition bias) 3 months or less | Low risk | Comment: Only 1 participant in the treatment group was lost to follow‐up and 2 participants in the control group were excluded after randomisation because they underwent surgery. This is unlikely to have introduced substantial bias in the comparison of outcomes for each group. |
| Selective reporting (reporting bias) | Low risk | Comment: All outcomes stated in the methods section of the publication were reported in the results. |
| Other bias | High risk | Comment: First author is the owner of the patent for the brace, which was pending at the time of publication. |
Mishra 2006.
| Study characteristics | ||
| Methods | Study design: RCT Setting: neurology outpatient department of a tertiary care centre, India |
|
| Participants |
Details of sampling frame: Total n assessed for eligibility = 66 participants (117 hands) Total n excluded pre‐randomisation = 26 participants Total n randomised = 40 participants (71 hands) Total n available for follow‐up = 40 participants (71 hands) Total n analysed = 71 wrists Intervention group 1 (splint) n = 20 participants (36 wrists) Intervention group 2 (oral steroid) n = 20 participants (35 wrists) Gender distribution: Intervention group 1 (splint): 3 males, 17 females Intervention group 2 (oral steroid): 4 males, 16 females Mean ± SD (range) age: Intervention group 1 (splint): 42.91 ± 9.39 (range 23 to 60) years Control group 2 (oral steroid): 41.57 ± 9.26 (range 28 to 60) years Mean ± SD duration of CTS symptoms: Intervention group 1 (splint): 6.40 ± 7.09 months Control group 2 (oral steroid): 6.31 ± 7.50 months Inclusion criteria: Symptoms suggestive of CTS of at least 1‐month duration and electrophysiological evidence of median neuropathy at wrist Exclusion criteria:
CTS diagnostic criteria (case definition): The clinical criteria laid down by the American Academy of Neurology were used for diagnosis of CTS. The electrophysiological criteria used for the diagnosis of CTS included the presence of 2 or more of the following:
CTS severity: Not reported |
|
| Interventions | Group 1 ‐ commercially available carpal tunnel splint: worn in the neutral position at night and as much as possible during the daytime for 4 weeks. In the case of bilateral symptoms, both hands were treated. Participants were also told not use additional medicines or other methods of treatment during the study period. Group 2 ‐ oral steroid: prednisolone 20 mg/day was taken for 2 weeks followed by 10 mg/day for another 2 weeks. Advice to avoid extremes of wrist flexion or extension, excessive hand movement and hand rest was common to both groups. |
|
| Outcomes | Outcomes assessed before treatment and at the end of 4 weeks of treatment and at 8 weeks post‐treatment:
|
|
| Funding | Not reported | |
| COI | Not reported | |
| Notes | Compliance was reported as excellent in steroid group, while 3 participants were not using splint regularly as prescribed (only 5 to 6 days per week instead of most of the time daily as advised) also taking NSAIDs like nimesulide and diclofenac. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote: "Randomization was done using the table of random numbers." Comment: The randomisation sequence was probably adequately generated. |
| Allocation concealment (selection bias) | Unclear risk | Quote: "All patients were randomly allocated to one of the following two groups: 1. Splinting in neutral position. 2. Oral steroid. Randomization was done using the table of random numbers." Comment: Not enough information to determine whether the treatment allocation was adequately concealed until interventions were assigned |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Quote: "A prospective randomised open‐label clinical and electrophysiological study of efficacy of splinting and oral steroids for the treatment of CTS was done." Comment: Participants were probably aware of which intervention they received. |
| Blinding of outcome assessment (detection bias) All outcomes | High risk | Comment: Since participants themselves assessed participant‐reported outcomes and they were likely to be aware of their allocated treatment in this study, we rated the risk as high. |
| Incomplete outcome data (attrition bias) 3 months or less | Low risk | Comment: No withdrawals, dropouts or losses to follow‐up were reported, and the authors indicated in the results tables that data was based on all 71 randomised wrists. |
| Selective reporting (reporting bias) | Low risk | Comment: All of the study's outcomes (prespecified in the Methods section of the study report) were reported in the prespecified way. |
| Other bias | Low risk | Comment: No other sources of bias identified |
Oncu 2014.
| Study characteristics | ||
| Methods | Study design: RCT, blind assessor Setting: physical therapy outpatient clinic, Turkey |
|
| Participants |
Details of sampling frame: Total n eligible = not reported Total n excluded pre‐randomisation = not reported Total n randomised = 40 participants (60 wrists) Total n available for follow‐up = 40 participants (60 wrists) Total n analysed = 40 participants (60 wrists) Intervention group 1 (splint + exercise) n = 15 wrists Intervention group 2 (kinesiology tape + exercise) n = 15 wrists Intervention group 3 (kinesiology tape + splint + exercise) n = 15 wrists Intervention group 4 (exercise) n = 15 wrists Gender distribution: Intervention group 1 (splint + exercise): not reported Intervention group 2 (kinesiology tape + exercise): not reported Intervention group 3 (kinesiology tape + splint + exercise): not reported Intervention group 4 (exercise): not reported Total: 40 females, 0 males Mean ± SD (range) age: Intervention group 1 (splint + exercise): not reported Intervention group 2 (kinesiology tape + exercise): not reported Intervention group 3 (kinesiology tape + splint + exercise): not reported Intervention group 4 (exercise): not reported Total: 48.97+10.66 Mean ± SD (range) duration of CTS symptoms: Intervention group 1 (splint + exercise): not reported Intervention group 2 (kinesiology tape + exercise): not reported Intervention group 3 (kinesio tape + splint + exercise): not reported Intervention group 4 (exercise): not reported Inclusion criteria:
Exclusion criteria:
CTS diagnostic criteria (case definition):
CTS severity: Mild‐to‐moderate |
|
| Interventions | Group 1 ‐splint and exercise (tendon and nerve gliding exercises for 25 days): Participants received a splint in a neutral position with a volar support which allowed wrist pronation and supination while extension and deviation were restricted. Participants were recommended to use the splint at night for 25 days. Group 2 ‐kinesiology taping and exercise (tendon and nerve gliding exercises for 25 days): kinesiology taping was performed using neural technique and ligament technique/space correction techniques. which are recommended for CTS. Group 3 ‐ kinesiology taping (see above) and splint (see above) and exercise (tendon and nerve gliding exercises for 25 days) Group 4 ‐ exercise only: (tendon and nerve gliding exercises for 25 days) |
|
| Outcomes | Outcomes assessed at 25 days post‐randomisation, and 2 and 3 months follow‐up
|
|
| Funding | The authors declared that this study has received no financial support. | |
| COI | No conflict of interest was declared by the authors. | |
| Notes | Article in Turkish, translation used BCTQ results reported as mean from 1 to 5 (higher is worse) in the article, table 1 and 2 Results for BCTQ functional status score provided for 3 months only (not for 25 days and 2 months) The authors reported that 10 of 40 participants had bilateral CTS, however the total number of wrists in the study were 60 (not 50). We used 40 as the number of participants (60 wrists) in the analysis. |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote from translation: "Four different treatment alternatives were created via computer by a statistical expert and the same person enumerated from high to low in an order and put them into envelopes. 40 patients were assigned to 4 groups according to the sequential (ranking) randomization method. The person who performs the therapy opened the envelope and carried out the treatment method from the envelope regarding the number of the patient." |
| Allocation concealment (selection bias) | Low risk | Quote from translation: "Four different treatment alternatives were created via computer by a statistical expert and the same person enumerated from high to low in an order and put them into envelopes. 40 patients were assigned to 4 groups according to the sequential (ranking) randomization method. The person who performs the therapy opened the envelope and carried out the treatment method from the envelope regarding the number of the patient." |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Comment: Study was single (observer)‐blind. Given the nature of the assigned interventions, participants were not blind. |
| Blinding of outcome assessment (detection bias) All outcomes | High risk | Quote from translation: "One and same blind observer filled the assessment forms of all patients included in the study before, at 25th day (end of the study) and 2 and 3 months after the study. All patients were evaluated by ENMG by the same and blind (for the treatment) observer before and 3 months after the study in the same ENGM laboratory." Comment: Since participants themselves assessed participant‐reported outcomes and they were likely to be aware of their allocated treatment in this study, we rated the risk as high. |
| Incomplete outcome data (attrition bias) 3 months or less | Low risk | Comment: No dropouts reported, and data reported as being based on all wrists that were randomised (n = 60) |
| Incomplete outcome data (attrition bias) After 3 months | Low risk | Comment: No dropouts reported, and data reported as being based on all wrists that were randomised (n = 60) |
| Selective reporting (reporting bias) | Unclear risk | Comment: No protocol available, and outcome data not reported for all outcomes at all time points, but unclear if this was related to the nature of the findings |
| Other bias | High risk | Quote: "The same treatment method from a single envelope was implemented for both hands of cases having bilateral CTS (10 of 40 cases) not to influence the study results." Comment: There were 40 participants, with 60 wrists affected. Analysis was based on the number of wrists, and there was no attempt to adjust the analysis for the correlation between wrists. |
Premoselli 2006.
| Study characteristics | ||
| Methods | Study design: quasi‐RCT Setting: outpatient clinic, Italy |
|
| Participants |
Details of sampling frame: Total n eligible = not reported Total n excluded pre‐randomisation = not reported Total n randomised = 50 participants (50 wrists) randomised Intervention group 1 (splint) n = 25 participants (wrists) Intervention group 2 (no treatment) n = 25 participants (wrists) Post‐intervention follow‐up at 3 months: Total n available for follow‐up = 49 participants (wrists) Total n analysed = 48 participants (wrists) Intervention group 1 (splint) n = 24 participants (wrists) Intervention group 2 (no treatment) n = 24 participants (wrists) Post‐intervention follow‐up at 6 months: Total n available for follow‐up = 41 participants (wrists) Total n analysed = 34 participants (wrists) Intervention group 1 (splint) n = 18 participants (wrists) Intervention group 2 (no treatment) n = 16 participants (wrists) Gender distribution: Intervention group 1 (splint): 2 males, 23 females Intervention group 2 (no treatment): 3 males, 22 females Mean ± SD age: Intervention group: 53.1 ± 13.3 yrs Control group: 46.5 ± 13.8 yrs Mean ± SD duration of CTS symptoms: Not reported Inclusion criteria:
Exclusion criteria:
CTS diagnostic criteria (case definition): Electrodiagnostic evaluation CTS severity: Mild CTS |
|
| Interventions | Group 1 ‐ neutral custom‐moulded thermoplastic resin wrist splints: worn at night‐time only, for a minimum of 6 hours per night, for 6 months Group 2 ‐ no intervention: participants simply monitored |
|
| Outcomes | Outcomes assessed at baseline, at 3 months, and at the end of 6 months of treatment:
|
|
| Funding | Not reported | |
| COI | Not reported | |
| Notes | At the 6‐month follow‐up visit, 1/25 case group participants dropped out from the study because of surgical treatment, and 5/25 control group dropouts underwent surgical treatment; 2/25 case group participants dropped out because they failed to comply adequately with wearing the splint. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | High risk | Quote: "The randomisation protocol was based on the last visit booking number (even or odd)." Comment: The trial authors used a non‐random component in the sequence generation process. |
| Allocation concealment (selection bias) | High risk | Quote: "The randomisation protocol was based on the last visit booking number (even or odd)." Comment: The trials authors did not adequately conceal the treatment allocation until interventions were assigned. |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Comment: Due to the nature of the interventions, it is likely that participants were aware of which treatment they received (night‐time splint or no intervention). |
| Blinding of outcome assessment (detection bias) All outcomes | High risk | Quote: "The examiner was blinded to treatment status (control or treatment)." Comment: Since participants themselves assessed participant‐reported outcomes and they were likely to be aware of their allocated treatment in this study, we rated the risk as high. |
| Incomplete outcome data (attrition bias) 3 months or less | Unclear risk | Quote: "Fifty patients (50 hands) were enrolled, of which 36 completed the study at 6 months." Quote: "At the three‐month follow‐up visit, 24/25 case patients and 24/25 control patients were evaluated." Quote: "At the six‐month follow‐up visit, 18 case group subjects and 16 control group subjects were evaluated." Comment: The numbers in these 3 quotes do not add up. In the abstract, it says that 36 participants were available at 6 months follow‐up, but in the text, it says that 34 (18 + 16) participants were available at 6 months follow‐up. Therefore, it is not clear how many participants were lost to follow‐up and the reasons for these losses. Furthermore, 7 (3 versus 4) participants at long‐term follow‐up were excluded due to not adhering to the treatment, which may cause bias, but loss seems to be balanced between the groups. |
| Selective reporting (reporting bias) | Low risk | Comment: All outcomes stated in the methods section of the publication were reported as prespecified. |
| Other bias | Low risk | Comment: No other sources of bias identified |
Rioja Toro 2012.
| Study characteristics | ||
| Methods | Study design: a prospective, blinded, randomised, placebo‐controlled study Setting: Traumatology and Rehabilitation Service, Spain |
|
| Participants |
Details of sampling frame: Total n eligible = not reported Total n excluded pre‐randomisation = not reported Total n randomised = 49 (98 hands) Total n available for follow‐up = unclear Total n analysed = 86 hands Intervention group 1 (splint & real laser) n = 22 hands Intervention group 2 (splint & placebo laser) n = 23 hands Intervention group 3 (real laser) n = 24 hands Intervention group 4 (placebo laser) n = 17 hands Gender distribution: Total: 3 males; 46 females Intervention group 1 (splint & real laser): not reported Intervention group 2 (splint & placebo laser): not reported Intervention group 3 (real laser): not reported Intervention group 4 (placebo laser): not reported Mean ± SD (range) age: Total: 49 ± 11.1 Intervention group 1 (splint & real laser): not reported Intervention group 2 (splint & placebo laser): not reported Intervention group 3 (real laser): not reported Intervention group 4 (placebo laser): not reported Mean ± SD (range) duration of CTS symptoms: Not reported Inclusion criteria:
Exclusion criteria:
CTS diagnostic criteria (case definition): 1. The diagnostic criteria are those recommended by the American Academy of Emergency Medicine: 1.1. Sensory latency difference (degree of CTS: mild < 0.5 ms; moderate 0.5‐0.8 ms; severe > 0.8 ms or not evoked) 1.2. Spontaneous activity at rest (degree of CTS: mild ‐ not; moderate ‐ not; severe ‐ yes (sometimes)) 1.3. Voluntary activity (degree of CTS: mild ‐ normal; moderate ‐ normal; severe ‐ neurogenic pattern) 1.4. DML (degree of CTS: mild ‐ normal; moderate ‐ normal or slight increase; severe ‐ augmented) 1.5. Motor potential synchronisation (degree of CTS: mild ‐ yes; moderate ‐ yes; severe ‐ desynchronised or decreased in amplitude, or both) 1.6. Motor driving speed (degree of CTS: mild ‐ normal; moderate ‐ normal; severe ‐ diminished) CTS severity: Mild‐to‐moderate CTS |
|
| Interventions | Group 1 ‐splint during night & laser treatment Group 2 ‐ splint during night & placebo Group 3 ‐ laser treatment Group 4 ‐ control (placebo laser) For participants wearing splint: wrist orthosis with palmar metal strap was used (night‐time), without encompassing the metacarpophalangeal joints and in a neutral wrist position (0° position). In all participants treated with real laser, a total energy dose of 945 J was used in an area of 4 x 4 cm2 (59 J/cm2) and of 5 J of total dose in the same area of 4 x 4 cm2 (0.3 J/cm2) in the treatments with placebo laser. The rhythm of the sessions has been 5 per week for 4 weeks. In all of them, a careful cleaning of the skin was carried out prior to each treatment session, to avoid losses due to reflection. All participants had either not started treatment with NSAIDs, or had stopped at least 1 month before. None were undergoing local treatments with iontophoresis, ultrasounds, etc., or they had abandoned it more than a month ago. |
|
| Outcomes | Outcomes evaluated before treatment and 1 and 3 months after treatment
|
|
| Funding | Not reported | |
| COI | The authors declared that they had no conflict of interest. | |
| Notes | Article in Spanish, translation used The number of participants and hands reported in the main text and table 6 seems to be not the same ‐ we used data from table 6, assuming that the table 6 reported hands. |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Quote: "The patients who passed the selection of units A and B, went to unit C (physiotherapy) where, randomly, one wrist was treated with Lv and the other with Lp (total 49 hands with Lv and 49 hands with Lp). Also, 27 patients were randomly indicated to use a wrist orthosis with palmar metal strap (night use), without encompassing the metacarpophalangeal joints and in a neutral wrist position (0°position); in 15 of them the orthosis was put on the most affected hand, and in 12 on both hands (total 39 hands with orthosis)." Comment: Not clear how the randomisation was done |
| Allocation concealment (selection bias) | Unclear risk | Comment: No information regarding the method of allocation was reported. |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Comment: Blinding of real laser versus placebo laser probably possible, however, it is unlikely that blinding for orthosis was possible. |
| Blinding of outcome assessment (detection bias) All outcomes | High risk | Comment: Due to the nature of the interventions, it is likely that participants were aware of which treatment they were allocated to, therefore we rated the risk as high. |
| Incomplete outcome data (attrition bias) 3 months or less | Unclear risk | Comment: No flowchart and not completely clear if all participants were followed up or if the authors only reported data for those that participated in the follow‐up visits. |
| Incomplete outcome data (attrition bias) After 3 months | Unclear risk | Comment: Numbers of participants not reported |
| Selective reporting (reporting bias) | Unclear risk | Comment: The outcomes were reported incompletely (only as P values from statistical analysis). |
| Other bias | Unclear risk | Comment: 49 participants had bilateral CTS (98 hands). The unit of analysis (hand or participant) was unclear, as the tables reported data for 86 participants. |
Sanaee 2017.
| Study characteristics | ||
| Methods | Study design: 2‐arm RCT Setting: outpatient clinics of physical medicine and rehabilitation of Shiraz University of Medical Sciences |
|
| Participants |
Details of sampling frame: Total n assessed for eligibility = 140 Total n excluded pre‐randomisation = 22 Total n randomised = 118 Intervention group 1 (short‐term splinting) n = 59 participants (94 hands) Intervention group 2 (long‐term splinting) n = 59 participants (94 hands) Post‐intervention follow‐up: Total n available for follow‐up = 94 participants (156 hands) Total n analysed = 94 participants (156 hands) Intervention group 1 (short‐term splinting) n = 80 hands Intervention group 2 (long‐term splinting) n = 76 hands Gender distribution: Intervention group 1 (short‐term splinting): not reported Intervention group 2 (long‐term splinting): not reported Mean ± SD age: Intervention group 1 (short‐term splinting): 47.4 ± na Intervention group 2 (long‐term splinting): 45 ± na Mean ± SD duration of CTS symptoms (months): Intervention group 1 (short‐term splinting): 7.8 ± na Intervention group 2 (long‐term splinting): 7.9 ± na Inclusion criteria:
Exclusion criteria:
CTS diagnostic criteria (case definition): Not reported (included only severe as defined in the inclusion criteria) CTS severity: Severe CTS |
|
| Interventions | Group 1: NSAID (naproxen 500 mg 3 times a day) for 10 days, vitamin B1 and B6 tabs for 6 weeks, physiotherapy (paraffin bath with controlled temperature, ultrasound under water and grip exercise), and Dr.K.H. splint (a wrist splint keeping the wrist in 5º of dorsiflexion), for 6 weeks Group 2: the group received the same medical and physical therapy as the first group, splint 23 hours/day for the first 6 weeks, continuing use just at night till 6 months. Both groups also received recommendation for activity modification. |
|
| Outcomes | Outcomes were assessed at baseline and at 6 weeks and 6 months.
|
|
| Funding | Not reported | |
| COI | Not reported | |
| Notes | Of all the 118 participants entering the study, 4 participants (3%) left the study going through the surgery. SD for outcomes were not provided in the article, but we calculated them from P value. The authors reported that 5 participants in the short‐term group and 15 participants in the long‐term group did not comply with the treatment (these participants were regarded as study dropouts). |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Quote: "After filling in the informed consent, the subjects entered our study and randomly divided into two groups. We first randomized the patients with bilateral and then patients with unilateral severe CTS so that each group contains the same number of bilateral and unilateral involvement." Comment: Random sequence generation was not described. |
| Allocation concealment (selection bias) | Unclear risk | Comment: Allocation concealment was not described. |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Comment: Blinding of participants not reported, but due to the nature of the interventions, it is likely that participants were aware of which treatment they were allocated to. |
| Blinding of outcome assessment (detection bias) All outcomes | High risk | Quote: "Both groups went under the evaluation of symptoms and function with CTSAQ (Boston questionnaire) and electrodiagnostical study by the same blind physiatrist at six weeks, and six months after the beginning of the study." Comment: Since participants themselves assessed participant‐reported outcomes and they were likely to be aware of their allocated treatment in this study, we rated the risk as high. |
| Incomplete outcome data (attrition bias) 3 months or less | Low risk | Comment: Follow‐up data available for 80/94 (85%) in group 1 and 76/94 (81%) in group 2. The loss and reasons were comparable; differences were so small that they are unlikely to bias the results. |
| Incomplete outcome data (attrition bias) After 3 months | Low risk | Comment: Follow‐up data available for 80/94 (85%) in group 1 and 76/94 (81%) in group 2. The loss and reasons were comparable; differences were so small that they are unlikely to bias the results. |
| Selective reporting (reporting bias) | Unclear risk | Comment: No protocol available to check. All outcomes described in the methods were reported. Adverse events not reported; unclear if measured |
| Other bias | Unclear risk | Comment: Clustering not controlled (hand was the subject). The number of participants with bilateral disease was balanced. Unclear if this could cause bias. Normal distribution not tested, T‐test used; variances not given in the results |
Schmid 2012.
| Study characteristics | ||
| Methods | Study design: randomised, single‐centre, assessor‐blinded, controlled pilot study Setting: Princess Alexandra Hospital Australia (people awaiting electrodiagnostic testing) |
|
| Participants |
Details of sampling frame: Total n assessed for eligibility = 111 Total n excluded pre‐randomisation = 90 Total n randomised = 21 Total n available for follow‐up = 21 Post‐intervention follow‐up: Total n analysed = 20 Intervention group 1 (splinting) n = 10 Intervention group 2 (exercise) n = 10 Gender distribution: Intervention group 1 (splinting): 7 males; 3 females Intervention group 2 (exercise): 5 males; 5 females Mean ± SD age: Group 1: 57.9 ± 16.3 Group 2: 49.9 ± 12.5 Mean ± SD duration of symptoms (months): Group 1: 62.8 ± 56.1 Group 2: 54.6 ± 47.6 Inclusion criteria: 1. Meeting clinical and electrodiagnostic criteria for mild or moderate CTS Exclusion criteria:
CTS diagnostic criteria (case definition): Clinical findings and electrodiagnostic mild or moderate grade findings CTS severity: Mild or moderate CTS |
|
| Interventions | Group 1 ‐ splinting: Participants received a prefabricated wrist splint (Access Health, Blackburn, Australia) that they wore at night for 1 week. All participants, irrespective of their treatment allocation, were encouraged to continue with their normal daily activities. Group 2 ‐ exercises: 1‐week home programme of nerve and tendon gliding exercises. The programme was instructed by a physiotherapist specialised in musculoskeletal management. For the tendon gliding exercises, the hand positions described by Wehbe and colleagues (Wehbe 1985) were adopted in 4 separate exercises. The nerve gliding exercises were based on recent biomechanical insights. Rather than progressively elongating the median, nerve exercises were selected which maximise nerve excursion while minimising an increase in nerve strain. The exercises were preceded by one warming‐up exercise that included forward and backward rolling of the shoulder girdle. Ten repetitions of each exercise were performed per session. One session took approximately 2 min to complete. Participants were asked to complete 10 sessions per day. On the day of the 1‐week follow‐up assessment, no exercises were performed in order to evaluate the prolonged rather than immediate effects of exercise. Participants were instructed that exercises should not provoke any symptoms. If symptoms were provoked, it was recommended to continue the exercise regimen using a smaller range of motion. Each participant was contacted by phone after the 1st and 3rd day to ensure that the prescribed exercise regimen did not cause any discomfort. Exercise compliance was monitored with a diary. |
|
| Outcomes | Outcomes were collected after first 10‐minute session of treatment and 1 week after recruitment.
|
|
| Funding | The study was funded through the Health Practitioner Research Scheme from Queensland Health, Australia and Project grant 511161 from the National Health and Medical Research Council (NHMRC) of Australia. | |
| COI | All authors declared no conflict of interest. | |
| Notes | Total BCTQ reported in the article, but results of Symptom Severity Scale and Functional Status Scale were sent to us by the author. Pain, numbness and BCTQ only collected/reported at baseline and 1 week (not after the 1st treatment). Direction of VAS not explicitly declared: “visual analogue scales (VAS) were completed for current level of pain and numbness (ranging from no pain/numbness to worst ever pain/numbness)”. All participants received the treatment as allocated and adhered to the prescribed exercise programme and splinting regimen. |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Quote: "Patients who met clinical and electrodiagnostic criteria for mild or moderate CTS (S‐Table 1) were randomly allocated to receive either night splinting (n = 10) or nerve and tendon gliding exercises (n = 10; Fig. 1). Allocation was stratified for CTS severity based on electrodiagnostic test results." Comment: "The method of sequence generation not declared |
| Allocation concealment (selection bias) | Low risk | Quote: "Concealed random allocation was performed by an independent investigator using sealed envelopes." |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Comment: Blinding of participants not reported, but due to the nature of the interventions, it is likely that participants were aware of which treatment they were allocated to. |
| Blinding of outcome assessment (detection bias) All outcomes | High risk | Quote: "All MRI [magnetic resonance imaging] scans were coded and an investigator blinded to the group allocation took all measurements". Comment: Since participants themselves assessed participant‐reported outcomes and they were likely to be aware of their allocated treatment in this study, we rated the risk as high. |
| Incomplete outcome data (attrition bias) 3 months or less | Low risk | Quote: "21 patients were recruited from a list of patients awaiting electrodiagnostic testing at a neurology department of a public hospital. One patient discontinued the study after the first appointment due to time constraints." |
| Selective reporting (reporting bias) | Low risk | Comment: All outcomes which were planned in methods were reported. Considering the aims of this pilot study, it seems improbable that some outcomes were left unreported. Protocol could not be found in ICTRP or Clinicaltrials.gov. |
| Other bias | Low risk | Comment: No other sources of bias identified |
Sevim 2004.
| Study characteristics | ||
| Methods | Study design: RCT Setting: orthopaedic outpatient clinics of Mersin University Hospital, Turkey |
|
| Participants |
Details of sampling frame: Total n eligible = not reported Total n excluded pre‐randomisation = not reported Total n randomised = 120 participants (120 wrists) Intervention group 1 (proximal injection group) n = 30 wrists Intervention group 2 (distal injection group) n = 30 wrists Intervention group 3 (splint group) n = 60 wrists Post‐intervention follow‐up: Total n available for follow‐up = 108 participants (108 wrists) Total n analysed = 108 participants (108 wrists) Intervention group 1 (proximal injection group) n = 28 wrists Intervention group 2 (distal injection group) n = 29 wrists Intervention group 3 (splint group) n = 28 wrists Intervention group 4 ("control" group) n = 23 wrists Gender distribution (reported for participants available for follow‐up analysis (n = 108)): Total: 16 males, 92 females Intervention group 1 (proximal injection group): 1 male, 27 females Intervention group 2 (distal injection group): 5 male, 24 females Intervention group 3 (splint group): 6 males, 22 females Intervention group 4 ("control" group): 4 males, 19 females Mean ± SD (range) age (reported for participants available for follow‐up analysis (n = 108)): Total sample: 46.27 ± 10.24 yrs (range 23 to 71 years) Intervention group 1 (proximal injection group): 43.89 ± 10.54 years (range not reported) Intervention group 2 (distal injection group): 45.45 ± 11.60 years (range not reported) Intervention group 3 (splint group): 49.71 ± 9.75 years (range not reported) Intervention group 4 ("control" group): 46.00 ± 7.90 years (range not reported) Mean ± SD (range) duration of CTS symptoms: Total sample: range 5 months to 30 years (mean ± SD not reported) Inclusion criteria:
Exclusion criteria:
CTS severity: Mild‐to‐moderate |
|
| Interventions | Group 1 ‐ proximal steroid injection containing 3 mg betamethasone disodium phosphate and 3 mg betamethasone acetate suspension (Celestone Chronodose), mixed with 0.5 cc of a lidocaine HCl solution (Aritmal ampul 2%, 5 cc). The injection site was the volar side of the forearm 4 cm proximal to the wrist crease between the tendons of the radial flexor muscle; the long palmar muscle and the needle was inserted with an angle of 10º to 20º before injection of the solution. All the participants were injected once. Group 2 ‐ distal steroid injection containing 3 mg betamethasone disodium phosphate and 3 mg betamethasone acetate suspension (Celestone Chronodose), mixed with 0.5 cc of a lidocaine HCl solution (Aritmal ampul 2%, 5 cc). The needle was inserted at the anterior wrist flexion crease just near to ulnar side of the palmaris longus tendon and angulated 45º distally as well as 45º radially. All the participants were injected once. Group 3 ‐splinting was performed by placing a standard lightweight wrist splint with a metal strip extending across the wrist to the midpalm region. The splint was bent so the wrist would be in neutral position (0° to 5° extended). The participants were instructed to wear the splints every night until the 1‐year follow‐up (average 11 months, range 9 to 14), and to mark each night that they had worn the splints on a calendar. Group 4 ‐ control group formed by the subset of participants who were randomised to the splint group but who did not comply with wearing the splint 6 to 7 days per week during the 1‐year treatment period (average 11 months, range 9 to 14), and instead wore the splint less than 1 night per week. |
|
| Outcomes | Outcomes assessed at baseline and at the end of 12 months treatment (average of 11 months after the start of treatment, range 9 to 14 months)
|
|
| Funding | Not reported | |
| COI | Not reported | |
| Notes | Of the 60 participants instructed to wear their splints every night, 28 (46.6%) used the splints for an average of 6 to 7 days per week. Control group was formed by the subset of participants who were randomised to the splint group but who did not comply with wearing the splint 6 to 7 days per week during the treatment period, and instead wore the splint less than one night per week. We combined data from the splinting and control group (as per ITT principle) and used these combined data in the analyses. |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Quote: "Patients were randomly assigned to one of the 3 groups: splint group (60 patients), distal injection group (30 patients) and proximal injection group (30 patients)." Comment: Not enough information to determine the adequacy of the randomisation sequence generation |
| Allocation concealment (selection bias) | Unclear risk | Quote: "Patients were randomly assigned to one of the 3 groups: splint group (60 patients), distal injection group (30 patients) and proximal injection group (30 patients)." Comment: Not enough information to determine whether the allocation sequence was adequately concealed until interventions were assigned |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Comment: Due to the nature of the interventions, participants and personnel were aware of treatment allocations. |
| Blinding of outcome assessment (detection bias) All outcomes | High risk | Quote: "Two authors (HK and MA), blinded to the electrophysiologic findings and treatment methods of the patients throughout the study, assessed the patients using a structured questionnaire regarding possible symptoms of carpal tunnel syndrome." Quote: "Electrophysiological examinations were performed on the chosen hand of each patient before and after the treatment, by the same author (SS) who was blinded to treatment methods and historical data throughout the study." Comment: Outcome assessors were probably blind to treatment allocation. However, since participants themselves assessed participant‐reported outcomes and they were likely to be aware of their allocated treatment in this study, we rated the risk as high. |
| Incomplete outcome data (attrition bias) 3 months or less | High risk | Quote: "At the end of 11 months (range, 9 to 14 months), contact with one patient from the proximal injection group and one from the distal injection group were lost for follow‐up. Another patient from the proximal injection group refused the electrophysiologic follow‐up examination. These 3 patients were dropped from the final analysis. Of the 60 participants in the splint group, 9 wore the splints on average 1‐5 nights per week and were excluded. Twenty‐three from this group wore the splints less than 1 night per week and were considered to form a control group. The remaining 28 patients wore the splints 6‐7 nights per week and they were taken as the properly used splint group. Thus, follow‐up evaluation was performed on 28 patients from the proximal injection group, 29 from the distal injection group, 28 from the splint group and 23 from the control group. These 108 participants were re‐evaluated by the same methods used at baseline and by the same physicians." Comment: Withdrawals and reasons for these were clearly reported. Participants who did not adhere to the splint protocol were entered into a 'control' group. We combined these participants into the splinting group (as per ITT principle). However, 9 participants were excluded due to not adhering to the treatment and this may have caused bias. |
| Selective reporting (reporting bias) | Unclear risk | Quote: "The Ethics Committee of Mersin Medical Faculty approved the study protocol." Data for the neurological symptom score (patient self‐reported outcome) provided, however, the trial authors did not indicate what the possible total NSS was. The protocol was not available for reading. |
| Other bias | Low risk | Comment: No other sources of bias identified |
So 2018.
| Study characteristics | ||
| Methods | Study design: prospective, randomised, parallel‐group clinical trial Setting: medical clinic of a local hospital (Kwong Wah Hopital), Hong Kong, China. |
|
| Participants |
Details of sampling frame: Total n eligible = not reported Total n excluded pre‐randomisation = not reported Total n randomised = 50 Total n available for follow‐up = 50 Total n analysed = 50 Intervention group 1 (splint) n = 25 Intervention group 2 (steroid) n = 25 Gender distribution: Intervention group 1 (splint): 22 males, 3 females Intervention group 2 (steroid): 21 males, 4 females Mean ± SD age: Intervention group 1 (splint): 57.28 ± 9.75 Intervention group 2 (steroid): 57.32 ± 9.12 Median ± SD duration of CTS symptoms: Intervention group 1 (splint): 104 weeks (range 39–1040) Intervention group 2 (steroid): 78 weeks (range 12–1040) Inclusion criteria:
Exclusion criteria:
CTS diagnostic criteria (case definition):
CTS severity: Mild, moderate and severe NCV abnormality |
|
| Interventions | Group 1 ‐ After randomisation, the hands of the participants in the splinting group were splinted in a neutral position with a standard cotton–polyester splint. Participants were instructed to use the splints during night‐time for 1 month. Group 2 ‐ the local injection of steroid was performed by the same investigator after the randomisation. Using a sterile technique, 20 mg methylprednisolone acetate premixed with lidocaine was injected using a 25‐guage 9 5/8” needle. The needle was inserted medially to the palmaris longus tendon at the distal palmar crease in the wrist at an angle of 45° to the forearm. The steroid was injected at approximately 1 cm below the skin. The needle was repositioned if there was any resistance to injection, or any pain or paraesthesia in the median nerve territory. |
|
| Outcomes | Outcomes were assessed at baseline and at 4 weeks follow‐up.
|
|
| Funding | Not reported | |
| COI | The authors reported no conflict of interest. | |
| Notes | The article reported the mean change on the BCTQ Symptom Severity Scale and Functional Status Scale, but the trial author provided end point scores (for Symptom Severity Scale and Functional Status Scale). Participants were encouraged to mark each night they had worn the splints on a calendar to ensure compliance, but results for adherence were not reported. |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote: "They were then allocated to one of the two treatment arms according to the randomization procedure using sequentially numbered opaque sealed envelopes (SNOSE). Two sets of [an] equal number of sealed opaque envelopes containing a sheet of paper marked Steroid Injection or Splinting were shuffled very thoroughly. The envelopes were then marked on the front with a unique number sequentially starting from one. Patients were thus randomly assigned to one of the two treatment arms according to what was marked in these envelopes." |
| Allocation concealment (selection bias) | Low risk | Quote: "They were then allocated to one of the two treatment arms according to the randomization procedure using sequentially numbered opaque sealed envelopes (SNOSE). Two sets of [an] equal number of sealed opaque envelopes containing a sheet of paper marked Steroid Injection or Splinting were shuffled very thoroughly. The envelopes were then marked on the front with a unique number sequentially starting from one. Patients were thus randomly assigned to one of the two treatment arms according to what was marked in these envelopes." |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Quote: "However, the open label design of the study means the potential ascertainment bias introduced by unblinding is not excluded." Comment: Blinding of participants not attempted |
| Incomplete outcome data (attrition bias) 3 months or less | Low risk | Comment: Authors did not report any dropouts. Text did not explicitly give numbers in the follow‐up but the numbers in the table implied that there were no dropouts. |
| Selective reporting (reporting bias) | Low risk | Comment: All prespecified outcomes reported. Protocol available |
| Other bias | Low risk | Comment: Hand used as a unit. There was no apparent source of bias. |
Taspinar 2007.
| Study characteristics | ||
| Methods | Study design: single‐blind, randomised, prospectively planned study Setting: Şişli Etfal Training and Research Hospital, İstanbul, Turkey |
|
| Participants |
Details of sampling frame: Total n eligible = 66 Total n excluded pre‐randomisation = 31 Total n randomised = 35 (54 hands) Total n available for follow‐up = 54 Total n analysed = 54 hands Intervention group 1 (splint) n = 18 hands Intervention group 2 (corticosteroid injection) n = 18 hands Intervention group 3 (physiotherapy) n = 18 hands Gender distribution: Intervention group 1 (splint): not reported Intervention group 2 (corticosteroid injection): not reported Intervention group 3 (physiotherapy): not reported Total: 0 males, 35 females Mean ± SD age: Intervention group 1 (splint): 55.36 ± 7.63 Intervention group 2 (corticosteroid injection): 51.53 ± 9.64 Intervention group 3 (physiotherapy): 53.86 ± 11.52 Total mean: 53.20 ± 9.34 Mean ± SD duration of CTS symptoms: Intervention group 1 (splint): 4.61 ± 3.39 years Intervention group 2 (corticosteroid injection): 2.3 ± 2.07 years Intervention group 3 (physiotherapy): 2.87 ± 2.34 years Inclusion criteria:
Exclusion criteria:
CTS diagnostic criteria (case definition):
CTS severity: Mild and moderate EMG findings |
|
| Interventions | Group 1 ‐ splint: wrist splint with volar support and neutral position made of thermoplastic material, splinting at night for 3 months Group 2 ‐steroid injection: 1 mL Diprospan (5 mg betamethasone dipropionate + 2 mg betamethasone sodium phosphate) Group 3 ‐physical therapy: ultrasound (US) and TENS were applied for a total of 10 sessions, 5 days a week. Continuous US was applied to the volar face of the wrist and the palm at a dose of 1 watt/cm2 at 1 MHz for 5 minutes. Conventional TENS (slightly above the sensory threshold), negative electrode was placed in the middle of the forearm; positive electrode was placed in the palm and applied for 20 minutes. |
|
| Outcomes | Outcomes evaluated before treatment and 3 months after
|
|
| Funding | Not reported | |
| COI | Not reported | |
| Notes | Written in Turkish; translation used Authors reported that in only one of their participants no steroid‐related side effects were observed, however, no clear information regarding side effects in splinting or steroid injection group was reported. |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Quote: "Patients included in the study were sequentially randomized into three groups of 18 hands each." Comment: No information about randomisation method provided. The study was planned as a single‐blind, randomised, prospective study. |
| Allocation concealment (selection bias) | Unclear risk | Comment: No information about allocation concealment provided. The study was planned as a prospective, randomised, single‐blind study. |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Comment: No information about blinding process provided, but because splinting and steroid injection were obviously different from each other, it is unlikely that the participants were blinded. |
| Blinding of outcome assessment (detection bias) All outcomes | High risk | Comment: Since participants themselves assessed participant‐reported outcomes and they were likely to be aware of their allocated treatment in this study, we rated the risk as high. |
| Incomplete outcome data (attrition bias) 3 months or less | Low risk | Comment: no dropouts reported and likely all participants had follow‐up data but no flow chart provided |
| Selective reporting (reporting bias) | Unclear risk | Comment: All the outcomes specified in the methods reported, but no study protocol available, therefore unclear |
| Other bias | Unclear risk | Quote: "A total of 54 hands of 35 patients included in the study were sequentially randomized into three groups of 18 hands each." Comment: Not clear how many participants in each group and no information how the participants with bilateral disease were distributed |
Ulucakoy 2020.
| Study characteristics | ||
| Methods | Study design: single‐centre, double‐blind, prospective, randomised, placebo‐controlled study Setting: Physical Medicine and Rehabilitation outpatient clinic of Ankara Numune Training and Research Hospital, Ankara, Turkey |
|
| Participants |
Details of sampling frame: Total n assessed for eligibility = 323 Total n excluded pre‐randomisation = 134 Total n randomised = 189 (295 wrists) Intervention group 1 (splint) n = 47 Intervention group 2 (splint + radial extracorporeal shock wave therapy (rESWT)) n = 47 Intervention group 3 (rESWT) n = 45 Intervention group 4 (splint + placebo rESWT) n = 50 Post‐intervention follow‐up at 1 month: Total n available for follow‐up = 174 (270 wrists) Total n analysed = 174 (270 wrists) Intervention group 1 (splint) n = 42 (67 wrists) Intervention group 2 (splint + rESWT) n = 45 (66 wrists) Intervention group 3 (rESWT) n = 43 (62 wrists) Intervention group 4 (splint + placebo rESWT) n = 44 (75 wrists) Post‐intervention follow‐up at 3 months: Total n available for follow‐up = 168 (259 wrists) Total n analysed = 168 (259 wrists) Intervention group 1 (splint) n = 42 (67 wrists) Intervention group 2 (splint + rESWT) n = 42 (60 wrists) Intervention group 3 (rESWT) n = 41 (58 wrists) Intervention group 4 (splint + placebo rESWT) n = 43 (74 wrists) Gender distribution: Intervention group 1 (splint): 7 males, 40 females Intervention group 2 (splint + rESWT): 8 males, 39 females Intervention group 3 (rESWT): 4 males, 41 females Intervention group 4 (splint + placebo rESWT): 3 males, 47 females Mean ± SD age: Intervention group 1 (splint): 48.1 ± 10.1 Intervention group 2 (splint + rESWT): 48.4 ± 10.1 Intervention group 3 (rESWT): 50 ± 8.6 Intervention group 4 (splint + placebo rESWT): 48.5 ± 9.8 Total mean: 48.8 ± 9.5 Mean ± SD duration of CTS symptoms (months): Intervention group 1 (splint): 22.2 ± 26.9 Intervention group 2 (splint + rESWT): 33.7 ± 38.1 Intervention group 3 (rESWT): 23.5 ± 27.3 Intervention group 4 (splint + placebo rESWT): 24.8 ± 31.5 Inclusion criteria: 1. Diagnosed with mild‐to‐moderate CTS Exclusion criteria:
CTS diagnostic criteria (case definition): Electrodiagnostic studies CTS severity: Mild‐to‐moderate CTS |
|
| Interventions | Group 1: splint Group 2: splint + rESWT Group 3: rESWT Group 4: splint + placebo rESWT In splint groups, a wrist splint with suitable size was advised to be used every night and as much as possible during the day for 3 months. In rESWT groups, the forearm and fingers of the participant were placed on the table with the palm facing up, and median nerve was found with musculoskeletal ultrasonography (US) (LOGIQ® GE Healthcare Ultrasound, Korea). rESWT was performed with the Vibrolith ESWT device (Elmed Medical Systems, Orlando, FL, USA) and the probe was located perpendicularly on the median nerve. The treatment area included the proximal carpal tunnel at the level of the pisiform bone that was shown by transverse US image. The rESWT was applied with 1000 shots, 0.05 mJ/mm2 intensity of energy and frequency of 5 Hz. The rESWT was administered consecutively for 3 weeks, once a week. |
|
| Outcomes | Outcomes evaluated at baseline (pretreatment) and at 1 and 3 months after treatment.
|
|
| Funding | The authors received no financial support for the research and/or authorship of this article. | |
| COI | The authors declared no conflicts of interest with respect to the authorship and/or publication of this article. | |
| Notes | Outcomes evaluated at baseline (pretreatment) and at 1 and 3 months after treatment, but in analysis, results at 3 months used. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote: "A total of 189 patients (295 wrists) were randomized to four groups by an independent researcher using [a] stratified randomization method. In this randomization, the researcher specified stratification according to the factors (age, sex, and CTS severity) which may affect the outcomes of intervention. The patients were, then, assigned to intervention groups using a computer‐generated randomization of study numbers." |
| Allocation concealment (selection bias) | Unclear risk | Quote: "..were randomized to four groups by an independent researcher using [a] stratified randomization method." Comment: No further explanation provided on whether the sequence was concealed |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Quote: "All interventions were carried out by a single physician who was blinded to the outcome measurements and randomization. Only the sound was heard without energy for placebo rESWT in Group 4 patients." Comment: No information provided about participant blinding, but splinting and ESWT were obviously different from each other, therefore unlikely that the participants were blinded. |
| Blinding of outcome assessment (detection bias) All outcomes | High risk | Quote: "All interventions were carried out by a single physician who was blinded to the outcome measurements and randomization. Only the sound was heard without energy for placebo rESWT in Group 4 patients." Comment: No information about whether participant blinding was provided, but splinting and ESWT were obviously different from each other, therefore unlikely that the participants were blinded. |
| Incomplete outcome data (attrition bias) 3 months or less | Low risk | Comment: All participants were accounted for and reasons for dropout and attrition were documented. Loss to follow‐up was 5/47 and 2/45 at one month and 5/47 and 4/45 at 3 months in splint and ESWT groups, respectively. Small and quite balanced loss, not likely to bias the outcomes considerably |
| Selective reporting (reporting bias) | Unclear risk | Quote: "The study protocol was approved by the Ankara Numune Training and Research Hospital Ethics Committee (No. 829/2016)." Comment: All the outcomes specified in the methods reported, but study protocol was not available for reading, therefore it was unclear. |
| Other bias | Low risk | Comment: Unit of analysis seemed to be participant, bilateral involvement dispersed evenly between the groups. |
Walker 2000.
| Study characteristics | ||
| Methods | Study design: quasi‐randomised controlled trial (prospective, unblinded, randomised clinical trial) Setting: Veterans Administration Medical Center, outpatient clinic |
|
| Participants |
Details of sampling frame: Total n eligible = not reported Total n excluded pre‐randomisation = not reported Total n randomised = 21 (30 wrists) Total n available for follow‐up = 17 (24 wrists) Total n analysed = 17 (24 wrists) Intervention group 1 (full‐time splint) n = 11 wrists (completed the study) Intervention group 2 (night‐time splint) n =13 wrists (completed the study) Gender distribution: Total: 20 males; 1 female (randomised) Intervention group 1 (full‐time splint): 7 males; 0 female (completed the study) Intervention group 2 (night‐time splint): 9 males; 1 female (completed the study) Mean ± SD age: Intervention group 1 (full‐time splint): 59.8 ± 9 yrs Intervention group 2 (night‐time splint): 60.7 ± 13 yrs Total mean: 60.0 ± 11.2 Mean ± SD duration of CTS symptoms: not reported Inclusion criteria:
Exclusion criteria: Not reported CTS diagnostic criteria (case definition): Electrodiagnostic studies CTS severity: Mild, moderate and severe CTS |
|
| Interventions | Group 1: full‐time wear of wrist splint for 6 weeks Group 2: night‐time only wear of wrist splint for 6 weeks Both groups used custom‐made, thermoplastic, lightweight, low‐profile, neutral‐positioned wrist splint. No work or activity restrictions were given to the participants. |
|
| Outcomes | Outcome assessed at the end of six weeks of treatment:
|
|
| Funding | The authors reported that no commercial party having a direct financial interest in the results of the research supporting this article had or would confer a benefit upon the authors or upon any organisation with which the authors were associated. | |
| COI | Not reported | |
| Notes | Strict adherence to specific splint‐wearing instructions was reported in 46% of hands, and partial compliance was reported by the remainder. Complete or nearly complete night‐time wear of splints was reported by 85% of the night‐only group, and by 100% of the full‐time group. Complete to near complete daytime wear was reported by only 27% of hands in the full‐time group, with the remainder reporting partial compliance. Despite instructions for night wear only, 23% of hands in the night‐only group reported limited daytime use of splints. One participant in the full‐time wear group reported very poor daytime wear compliance because he felt the splints interfered with his job performance. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | High risk | Quote: "The randomization protocol was based on the last digit of the subject's Social Security number." Comment: Allocation sequence was not truly random. |
| Allocation concealment (selection bias) | High risk | Comment: The last digit of the participant's social security number was used, therefore, allocation was not concealed. |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Comment: The authors described the trial as "unblinded". Participants were not blinded to splint‐wearing. Self‐administered questionnaires for the Symptom Severity Scale, Functional Status Scale, and splint‐wearing compliance for the last 2 weeks of the trial may have been influenced by the participant's knowledge of their own splint‐wearing behaviour. |
| Blinding of outcome assessment (detection bias) All outcomes | High risk | Comment: Since the outcome assessors for participant‐reported outcomes were participants themselves and since it is likely that participants were aware of which treatment they were allocated to, we rated the risk is rated as high. |
| Incomplete outcome data (attrition bias) 3 months or less | Low risk | Quote: "Subjects were informed that steroid injections and surgery were also treatment options for CTS, and participation in this study did not prohibit them from seeking additional treatment, but they would be dropped from the study if they did so". Comment: One participant from each group was excluded because they had surgery or steroid injections. Losses from each group were balanced (2 participants from each group) and unlikely to be a source of bias. |
| Selective reporting (reporting bias) | Low risk | Comment: All measures appeared to be reported as described in the protocol of the trial publication. |
| Other bias | Low risk | Quote: "..subjects with bilateral involvement always received the same instructions for both hands...measures were taken for each hand". Comment: Participants were allocated to treatment groups (not hands), therefore, those with bilateral involvement each contributed two hands to the analysis. The number of bilateral cases were similar in both treatment groups, so a unit of analysis error is unlikely to have occurred. |
Wang 2017.
| Study characteristics | ||
| Methods | Study design: prospective, single‐blind, RCT Setting: Taipei Veterans General Hospital, tertiary care centre |
|
| Participants |
Details of sampling frame: Total n assessed for eligibility = 72 Total n excluded pre‐randomisation = 20 Total n randomised = 52 (93 hands) Total n available for follow‐up = 52 Total n analysed = 52 Intervention group 1 (steroid injection + splinting) n = 26 Intervention group 2 (steroid injection) n = 26 Gender distribution: Intervention group 1 (steroid injection + splinting): 6 males, 20 females Intervention group 2 (steroid injection): 5 males, 21 females Mean ± SD age: Intervention group 1 (steroid injection + splinting): 54.34 ± 9.86 Intervention group 2 (steroid injection): 55.76 ± 8.56 Mean ± SD duration of CTS symptoms: Intervention group 1 (steroid injection + splinting): 3‐6 months, n = 6; 6‐12 months, n = 6; 1‐2 years, n = 6; > 2 years, n = 8 Intervention group 2 (steroid injection): 3‐6 months, n = 5; 6‐12 months, n = 7; 1‐2 years, n = 5; > 2 years, n = 9 Inclusion criteria:
To confirm the diagnosis of CTS, motor and sensory nerve conduction studies were performed as follows. The electrophysiological tests were considered supportive of CTS when the interval between the median and ulnar sensory peak latencies exceeded 0.5 ms. Exclusion criteria:
CTS diagnostic criteria (case definition): The electrophysiological tests were considered supportive of CTS when the interval between the median and ulnar sensory peak latencies exceeded 0.5 ms. CTS severity: Not reported Protocol violators: 2 participants (7.6%) wore the splints 1 to 5 nights/week |
|
| Interventions | Group 1 ‐ steroid injection‐plus‐splinting group: Customised volar thermoplastic wrist splint with the wrist placed in a neutral position. Volar splints were made by an experienced occupational therapist. The treating therapist asked participants to wear the splints while sleeping and also during daytime whenever possible for 12 weeks. Group 2 ‐ steroid injection group: All participants received a single ultrasound‐guided carpal tunnel injection in the affected hand at the beginning of the treatment. Ultrasound‐guided injections were given using a 6‐ to 18‐MHz linear array probe. A single physiatrist performed all injections according to the technique described by Smith and colleagues (Smith 2008). The participant was placed in an upright sitting position, with the hand resting on the table, the forearm supinated, and the wrist placed in slight dorsiflexion. The ultrasound probe was placed transversely at the level of the proximal carpal inlet. After sterile preparation, a 25‐G, 1.5‐in needle was inserted in‐plane immediately superficial and lateral to the ulnar artery and directly above and below the median nerve. The injection fluid contained 1 mL of 10 mg (10 mg/mL) triamcinolone acetonide (Shincort) mixed with 1 mL of 2% lidocaine hydrochloride (Xylocaine). Participants were instructed to avoid analgesic/anti‐inflammatory drugs for the study period. |
|
| Outcomes | Participants were evaluated before the treatment and at 6 and 12 weeks after the onset of treatment.
Compliance with the splint‐wearing at night was recorded. Each participant was given a form to record each night he/she wore the splint. The data were collected at 6 and 12 weeks, and the investigator recorded the total number of nights they wore the splint in the 12‐week study duration. The average nights per week of splint wear for each participant was calculated. |
|
| Funding | Not reported | |
| COI | Not reported | |
| Notes | Of the 26 participants in the steroid injection‐plus‐splinting group, 24 (92.3%) wore the splints 6 to 7 nights/week. The remaining 2 participants (7.6%) wore the splints 1 to 5 nights/week. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote: "Randomization was performed by an independent research assistant who was blinded to treatment and assessment by using a random number generator in blocks of 4 with no stratification." |
| Allocation concealment (selection bias) | Low risk | Quote: "The allocation of participants to treatment condition was concealed in envelopes, which was opened by a research assistant after the patients’ baseline assessment." |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Quote: "Based on the nature of the interventions, it was difficult to blind patients." Comment: No blinding was attempted. |
| Blinding of outcome assessment (detection bias) All outcomes | High risk | Quote: "Assessments were performed by a physiatrist who was blinded to treatment allocation during the assessments. To ensure blinding, at the beginning of each interview the patients were instructed not to divulge his/her group assignment to the assessors and not to bring the splint with them if patients were assigned to the steroid injection‐plus splinting group." Comment: Since participants themselves assessed participant‐reported outcomes and they were not blinded, we rated the risk as high. |
| Incomplete outcome data (attrition bias) 3 months or less | Low risk | Comment: No loss to follow‐up |
| Selective reporting (reporting bias) | Low risk | Comment: All the outcomes reported |
| Other bias | Low risk | Comment: No other sources of bias identified |
Werner 2005.
| Study characteristics | ||
| Methods | Study design: quasi‐RCT Setting: a Midwestern auto assembly plant, USA |
|
| Participants |
Details of sampling frame: Total n assessed for eligibility = 2636 Total n excluded pre‐randomisation = 2475 Total n randomised = 161 (161 wrists) randomised Intervention group 1 (splint + ergonomic education) n = 86 wrists Intervention group 2 (ergonomic education) n = 75 wrists Post‐intervention follow‐up: Total n available for follow‐up = 112 Total n analysed = 112 Intervention group 1 (splint + ergonomic education) n = 63 wrists Intervention group 2 (ergonomic education) n = 49 wrists Gender distribution: Intervention group 1 (splint + ergonomic education): 30 males, 33 females (completed the study) Intervention group 2 (ergonomic education): 25 males, 24 females (completed the study) Mean ± SD (range) age (for those who completed the study): Intervention group 1 (splint + ergonomic education): 44.74 ± 1.02 (25.6 to 59.0) years Intervention group 2 (ergonomic education): 43.77 ± 1.44 (25.5 to 59.2) years Mean ± SD duration of CTS symptoms: Not reported Inclusion criteria:
Exclusion criteria:
CTS diagnostic criteria (case definition): Study examined the efficacy of splints among participants with symptoms consistent with CTS, but not necessarily having the diagnosis established. CTS severity: Not reported |
|
| Interventions | Group 1 ‐ splint + ergonomic education: Customised wrist splints and ergonomic education ‐ participants were fitted with a custom wrist‐hand orthosis that maintained the wrist in a neutral posture, and was worn at night for 6 weeks. Participants received instructions in how to reduce ergonomic stressors in the work and home environments by viewing a 20‐minute video on CTS and ergonomic risk factors. The focus of the video was industrial ergonomics and the prevention of repetitive strain disorders. Group 2 ‐ergonomic education alone: Ergonomic education alone via the same 20‐minute video on CTS and ergonomic risk factors presented to participants in the intervention group |
|
| Outcomes | Outcomes assessed at baseline, and 3, 6, and a mean of 12 months (range 7 to 15 months) follow‐up (after the end of treatment)
|
|
| Funding | Supported in the whole by joint funds from the United Auto Workers (UAW) and General Motors (GM) National Joint Committee on Health and Safety "No commercial party having a direct interest in the results of the research supporting this article has or will confer a benefit on the author(s) or on any organization with which the author(s) is/are associated." |
|
| COI | Not reported | |
| Notes | According to the trial authors, half the participants did not complete the questionnaires at 3‐month and 6‐month follow‐up, so no data from these time points were reported. The authors did not report numbers regarding adherence to splint use. |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | High risk | Quote: "Subjects were randomized to either a treatment or a control group, depending on whether the last digit of their Social Security number was odd or even." Comment: The trial authors used a non‐random component in the sequence generation process. |
| Allocation concealment (selection bias) | High risk | Quote: "Subjects were randomized to either a treatment or a control group, depending on whether the last digit of their Social Security number was odd or even. Subjects were not informed of the sequence for random allocation nor were they told to which group they were assigned until after consenting to participate." Comment: The trials authors did not adequately conceal the treatment allocation until interventions were assigned, as a non‐random process was used. |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Quote: "Subjects were not blinded to their treatment, and the primary outcome measure was a self‐reported symptom severity score." Comment: Participants were not blinded to treatment allocation. |
| Blinding of outcome assessment (detection bias) All outcomes | High risk | Quote: "The nerve conduction data were collected at baseline and at the 12‐month follow‐up. Subjects reported to the medical department to have the testing done during regular work hours, and the person doing the testing was blinded to the treatment assignment." Comment: Since participants themselves assessed participant‐reported outcomes and they were likely to be aware of their allocated treatment in this study, we rated the risk as high. |
| Incomplete outcome data (attrition bias) 3 months or less | High risk | Quote: "Data collection was incomplete at the 3‐ and 6‐month follow‐up periods. Subjects were contacted by a study site coordinator and were reminded to fill out the questionnaire, but about half of the subjects did not complete the 3‐ or 6‐month questionnaires. The trend in outcome measures at 3 and 6 months was similar to the results at 12 months." Comment: Data not complete for all outcomes, with no explanation as to how this may have impacted on the data reported |
| Incomplete outcome data (attrition bias) After 3 months | High risk | Quote: "The 12‐month follow‐up data are presented because they represent a more complete data set...The 12‐month follow‐up was actually a range of follow‐up times, with an average of 12 months and a range of 7 to 15 months." Comment: Data not complete for each outcome, with no explanation as to how this may have impacted on the data reported |
| Selective reporting (reporting bias) | Unclear risk | Comment: The results of participant self‐reported outcomes reported; however, protocol not available |
| Other bias | Low risk | Comment: No other sources of bias identified |
Willis 2016.
| Study characteristics | ||
| Methods | Study design: Unblinded RCT Setting: | |
| Participants |
Details of sampling frame Total n eligible = 60 Total n excluded pre‐randomisation = 10 Total n randomised = 50 Total n available for follow‐up = 50 Total n analysed = 50 Intervention group 1 (splint) n = 25 Intervention group 2 (control) n = 25 Gender distribution Intervention group 1 (splint): 7 males; 18 females Intervention group 2 (control): 3 males; 22 females Mean ± SD (range) age Intervention group 1 (splint): not reported Intervention group 2 (control): not reported Total: 51 ± 12.6 Mean SD (range) duration of symptoms Not reported Inclusion criteria
Exclusion criteria
CTS diagnostic criteria (case definition) Physical examination and a NCS of the median nerve following electrodiagnostic standards established by the American Association for Hand Surgery CTS severity: Not reported |
|
| Interventions | Group 1 ‐ splint: The participants were treated with Dynasplint stretching modality. Participants wore the device for two 30‐minute sessions per day with regular increases in splint tension for 60 days. Each Dynasplint was customised by one technician to fit each participant’s hand length, width, and girth. The first week of Dynasplint use was an accommodation period for the participant, and participants were encouraged to wear the unit twice daily for 15 minutes each session. Time was then increased by 2 to 4 minutes each following session. After the participant comfortably wore the device for two 30‐minute sessions each day for 1 week, instructions were given to increase the tension of the Dynasplint device once every 2 weeks, based on comfort and tolerance. If the new tension setting caused excess joint fatigue or “soreness,” the participant was instructed to reduce the time to 15 minutes, twice daily, and work the time back up to 30 minutes, twice daily. The goal was to wear the modality for 30 minutes, twice a day and increase the tension twice a month, based on comfort and tolerance. All participants were instructed to communicate on compliance through weekly transfer of wearing diary records to the prescribing physician. Group 2 ‐ control: The control participants were only treated with NSAIDs, stretching exercises, and instructions for reducing the potential movement causing the pain. Steroid injections were not given. |
|
| Outcomes | The outcomes were assessed at 2 months (BCTQ score) and at 12 months (rate of surgery).
|
|
| Funding | The author(s) disclosed receipt of the following financial support for the research, authorship, and/or publication of this article: funding to operate Galveston Clinical Research is obtained through book revenues, allowing this to be completely independent research. | |
| COI | The author(s) declared the following potential conflicts of interest with respect to the research, authorship, and/or publication of this article: "Neither author has conflict of interest at this time. Neither author has received any earnings or compensation for this publication; nor will any earnings be awarded in the future. Dr. F.B.W. was employed by Galveston Clinical Research Foundation at the time of this study. He had a previous affiliation with the parent company of Dynasplint Systems, Inc. but the affiliation including all compensation or earnings were completed in 2013. B.F. was previously employed by Dynasplint Systems but her employment was also completed in 2013." | |
| Notes | BCTQ score scale is normally from 1 to 5. The authors have used a modified version "The Levine‐Katz survey used in the study measures symptoms in two categories, totaling 100 points." The trial reported 100% compliance over 60 days and after completion of the controlled trial, treatments over the second phase (12 months) were intentionally unregulated but were tracked. |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Quote: "Randomly assigned to either the experimental or control group". Comment: No further description of the random sequence generation |
| Allocation concealment (selection bias) | Unclear risk | Comment: It was not clear if concealment of allocation was achieved. |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Comment: Participants were not blinded to the allocation. |
| Blinding of outcome assessment (detection bias) All outcomes | High risk | Comment: Because it is likely that participants were aware of which treatment they were allocated to, we rated the risk as high. |
| Incomplete outcome data (attrition bias) 3 months or less | Low risk | Comment: 100% follow‐up rate at 2 months but data incompletely reported |
| Incomplete outcome data (attrition bias) After 3 months | Low risk | Comment: 100% follow‐up data reported (but only for the rate of surgery) |
| Selective reporting (reporting bias) | High risk | Comment: Partial reporting; at long‐term follow‐up, the authors reported only surgery rates, and at short‐term follow‐up no scores. |
| Other bias | High risk | Comment: Corresponding author is an employee of Dynasplint Systems, Inc. |
Wu 2017.
| Study characteristics | ||
| Methods | Study design: prospective, single‐blinded, RCT Setting: Tri‐Service General Hospital, Taiwan |
|
| Participants |
Details of sampling frame: Total n assessed for eligibility = 80 Total n excluded pre‐randomisation = 20 Total n randomised = 60 Total n available for follow‐up = 60 Total n analysed = 60 Intervention group 1 (splint) n = 30 Intervention group 2 (platelet‐rich plasma (PRP)) n = 30 Gender distribution: Intervention group 1 (splint): 5 males; 25 females Intervention group 2 (PRP): 3 males; 27 females Mean ± SD age: Intervention group 1 (splint): 54.27 ± 7.34 Intervention group 2 (PRP): 57.87 ± 8.27 Mean ± SD duration of CTS symptoms (months): Intervention group 1 (splint): 30.70 ± 33.0 Intervention group 2 (PRP): 34.43 ± 31.1 Inclusion criteria: Paraesthesia or dysaesthesia, painful swelling with clumsy weakness of the hand exacerbated by sleep or repetitive use of the wrist, and relieved by shaking the hand with postural change, AND 1 or more of the following:
Participants diagnosed with mild‐to‐moderate unilateral CTS with clinical symptoms for at least 3 months undergoing electrophysiological study and ultrasonography were enrolled. Exclusion criteria:
CTS diagnostic criteria (case definition): The cut‐off points or normal range of the electrophysiological study for CTS in this study were as follows:
Participants with mild and moderate CTS were categorised by the electrophysiological classification of CTS by Padua and colleagues (Padua 1997): mild: only abnormal digit/wrist SNCV with normal DML; moderate: abnormal digit/wrist SNCV and abnormal DML; or severe: absence of SNCV and abnormal DML. CTS severity: Mild‐to‐moderate CTS |
|
| Interventions | Group 1 ‐ received a night splint through the study period: The splint was applied in a neutral position to restrict the wrist as previously described. The controls were instructed to put on the splint overnight for at least 8 hours daily throughout the study period. Group 2 ‐ PRP group: participants were injected with one dose of 3 mL of PRP using ultrasound guidance. Ten mL of blood sample were drawn from the antecubital vein using RegentKit‐THT‐1 (RegenLab SA, Mont‐sur‐Lausanne, Switzerland) followed by centrifugation at 3400 rpm for 15 minutes at room temperature using Regen Lab PRP Centri, yielding 3.5 mL of PRP. The RegentKit‐THT‐1 has sodium citrate solution as an anticoagulant, and autologous thrombin as an activator to advance platelet activation and conversion of fibrinogen to fibrin. For quality tests, 0.5 mL of the PRP sample was sent to the laboratory and 3 mL was used for the ultrasound‐guided injection. The concentration of platelets and leukocytes in the PRP was approximately 2.7 ± 0.4 times and 1.2 ± 0.4 times that in whole blood, respectively. The ultrasound‐guided PRP injection was performed by the same physiatrist, using ultrasonography (MyLab™ 25Gold, Esaote, Genova, Italy). With the palm facing upwards and the wrist slightly extended, the median nerve was identified at the inlet of the proximal carpal tunnel (pisiform level). The ultrasound‐guided injection was conducted using the in‐plane ulnar approach. The ulnar artery was identified using Doppler imaging, and a 25‐gauge needle was passed from the ulnar side of the wrist toward the median nerve. After placing the needle tip on the median nerve, 2 mL of PRP was injected to peel the nerve off the flexor retinaculum via hydrodissection. An additional 1 mL of PRP was delivered to the inferior part of the median nerve and the median nerve was peeled from the underlying subsynovial connective tissue. After this, the entire carpal tunnel was scanned to ensure that the PRP had spread throughout the proximal‐to‐distal area of the carpal tunnel. Both groups: All participants were instructed to refrain from any other management approaches, such as analgesics, steroid injections, or physical therapy, for CTS symptoms from 2 weeks before and throughout the study period, and were requested to report receiving any of these therapies. |
|
| Outcomes | Outcomes were assessed before intervention and at months 1, 3, and 6 after treatment
|
|
| Funding | The study is supported by the Ministry of Science and Technology, Taiwan, Republic of China (grant no. MOST 105‐2314‐B‐016‐046). | |
| COI | The authors declared that they had no competing interests. | |
| Notes | Results from BCTQ not reported in scale 1 to 5. We assumed that the total sum was reported and divided the symptom score by 11 and functional score by 8 (as per instructions of the scale). Authors reported standard error (SE) for age and duration of symptoms, but we calculated SD (SE * SQRT n). |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote: "The enrolled patients were block randomized in a 1:1 ratio into two groups, control and PRP groups, by an independent researcher via computer‐generated randomization of study numbers (Microsoft Excel, Microsoft Inc., Redmond, WA, USA)." |
| Allocation concealment (selection bias) | Unclear risk | Comment: The method of allocation concealment not described |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Comment: Blinding of participants not attempted |
| Blinding of outcome assessment (detection bias) All outcomes | High risk | Quote: "One physiatrist (Dr. Ke) with 5 years’ experience in musculoskeletal ultrasonography and electrophysiological study, who was blinded to the patients’ randomization, performed all the measurements in all patients of both groups before intervention and at months 1, 3, and 6 after treatment." Comment: However, as the participants were likely aware of which treatment they were allocated to, we rated the risk as high. |
| Incomplete outcome data (attrition bias) 3 months or less | Low risk | Comment: No loss to follow‐up |
| Incomplete outcome data (attrition bias) After 3 months | Low risk | Comment: No loss to follow‐up |
| Selective reporting (reporting bias) | Unclear risk | Comment: According to the protocol, outcomes were assessed at 1, 2, 4, 8, 12, 16 and 24 weeks. Results reported at 1, 3 and 6 months. Unclear if this was related to the nature of the findings |
| Other bias | Low risk | Comment: No other sources of bias identified |
Yazdanpanah 2012.
| Study characteristics | ||
| Methods | Study design: RCT Setting: S. Mofateh Clinic, Iran |
|
| Participants |
Details of sampling frame: Total n eligible = 28 Total n excluded pre‐randomisation = not applicable Total n randomised = 28 Intervention group 1 (splint) n = 14 Intervention group 2 (steroid injection) n = 14 Post‐intervention follow‐up: Total n available for follow‐up = 25 Total n analysed = 25 Intervention group 1 (splint) n = 11 Intervention group 2 (steroid injection) n = 14 Gender distribution: Intervention group 1 (splint): 0 males, 14 females Intervention group 2 (steroid injection): 0 males, 14 females Mean ± SD age: Total: range between 26 to 49 years, and among them the range of 28‐31 years had the most frequency Mean ± SD duration of CTS symptoms: Not reported Inclusion criteria:
Exclusion criteria:
CTS diagnostic criteria (case definition):
Severity of CTS was based on neurophysiology findings and guideline values of the Association of the Electrodiagnostic Medicine. |
|
| Interventions | Group 1 ‐ splint: Splint in neutral position during night for 6 weeks (wrist splint made by Iran Odor Company in Iran) Group 2 ‐ steroid injection: triamcinolone (Triamhexal) 40 mg; made by Holzkirchen factory in Germany, was injected without anaesthetic substance and using needle number 23, with 30º angle on radial side of wrist in carpal tunnel. |
|
| Outcomes | Outcomes were evaluated before treatment and 2 months after treatment. Electrophysiologic parameters of median and ulnar nerves were recorded before and 2 months after the steroid injection and wrist splint. The effectiveness of treatment was described in the manner of 1) change of disease stage from severe to moderate or mild or 2) complete recovery. In cases of observing changes from severe to lower stages of the disease in electrodiagnostic studies, treatment was considered successful and otherwise failed. |
|
| Funding | Not reported | |
| COI | Not reported | |
| Notes | Inclusion criteria were: If a pregnant woman had severe CTS, she was entered in the research. Nevertheless, in the results the authors reported that participants had varying severity of CTS. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Quote: "Then, the patients were randomly placed in one of treatments groups including steroid injection and wrist splint treatments." Comment: No further information about the sequence generation provided |
| Allocation concealment (selection bias) | Unclear risk | Comment: No information about allocation concealment provided |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Comment: No information about blinding provided. Splinting and steroid injection were obviously different from each other, therefore, unlikely that the participants were blinded. |
| Blinding of outcome assessment (detection bias) All outcomes | High risk | Comment: No information about blinding provided. Splinting and steroid injection were obviously different from each other, therefore, unlikely that the participants were blinded. |
| Incomplete outcome data (attrition bias) 3 months or less | High risk | Quote: "Three women in splint group refused to continue treatment". Comment: This corresponded to 21% loss in one group and no loss in the other. |
| Selective reporting (reporting bias) | Unclear risk | Comment: No protocol or registration provided. Only electrodiagnostic studies reported and unclear if clinical outcomes measured |
| Other bias | Unclear risk | Comment 1: All participants were pregnant. It was unclear if pregnancy is an effect modifier. Comment 2: Randomisation done at participant level, but unit of analysis was hand. Not clear how the participants with bilateral disease were distributed, also not clear information if all 50 studied hands actually were affected by CTS or not. Quote: "Among the studied women, 5 (20 percent) were suffering in the left hand, 14 (56%) in the right hand and 6 (24%) in both hands. In view of severity of CTS among 50 studied hands, 31 (62%) were suffering from severe CTS, 6 hands (12%) were normal from the beginning. Also among 50 hands, 9 (18%) were in mild CTS and 4 hands (8%) were in moderate stage of CTS." |
ASHT: American Society of Hand Therapists BCTQ: Boston Carpal Tunnel Questionnaire CMAP: compound motor action potential CTS: carpal tunnel syndrome CTSAQ: Carpal Tunnel Syndrome Assessment Questionnaire DML: distal motor latency DN4: Douleur Neuropathique en 4 Questions‐questionnaire DSL: distal sensory latency E(N)MG: electro(neuro)myography EQ‐5D‐5L: EuroQol 5‐dimension 5‐level health status and quality of life measure EuroQol: EuroQol Groupt GP: general practitioner HCI: hydrochloric acid IQR: interquartile range ITT: intention‐to‐treat KT: kinesiotaping Lp: placebo laser Lv: real laser M(N)CV: motor (nerve) conduction velocity MRI: magnetic resonance image na: not available NCS: nerve conduction study NCV: nerve conduction velocity NSAID: nonsteroidal antiinflammatory drug NSS: Neurologic Symptom Score OD: orthotic device PI: principal investigator RCT: randomised controlled trial rESWT: radial extracorporeal shock wave therapy rpm: rounds per minute SD: standard deviation SDL: sensory distal latency SE: standard error SNAP: sensory nerve action potential SNCV: sensory (nerve) conduction velocity SNOSE: sequentially numbered opaque sealed envelopes SQRT: square root TENS: transcutaneous electrical nerve stimulation US: ultrasound VAS: visual analog scale
Characteristics of excluded studies [ordered by study ID]
| Study | Reason for exclusion |
|---|---|
| Akalin 2002 | The same type of splint was applied to each group in this trial. |
| Arinci Incel 2005 | Study compared treatment methods which we defined as not relevant for this review. |
| Asgari 2020 | Splint applied to each group in this trial |
| Bagheri 2011 | Splint applied to each group in this trial |
| Baker 2012 | Splint applied to each group in this trial |
| Bardak 2009 | The effect of splinting could not be isolated from that of the other intervention delivered alongside it. |
| Baysal 2006 | The same type of splint was applied to each group in this trial. |
| Bilgici 2010 | The effect of splinting could not be isolated from that of the other intervention delivered alongside it. |
| Brininger 2007 | Splint applied to each group in this trial |
| Bulut 2015 | Splint applied to each group in this trial |
| Burke 1994 | Splint applied to each group in this trial |
| Bye 2011 | Splint applied to each group in this trial |
| Celiker 2002 | The effect of splinting could not be isolated from that of the other intervention delivered alongside it. |
| Chung 2016 | Splint applied to each group in this trial |
| CTRI/2015/02/005531 | Splint applied to each group in this trial |
| Daniel 2000 | Not a randomised trial |
| Davis 1998 | The same type of splint was applied to each group in this trial. |
| De Angelis 2009 | Splint applied to each group in this trial |
| Dehghani 2012 | Splint applied to each group in this trial |
| Dincer 2009 | Splint applied to each group in this trial |
| DRKS00014585 | Study compared treatment methods which we defined as not relevant for this review. |
| Eftekharsadat 2011 | The same type of splint was applied to each group in this trial. |
| Ekim 2008 | The same type of splint was applied to each group in this trial. |
| Evcik 2007 | The same type of splint was applied to each group in this trial. |
| Farahmand 2021 | Splint applied to both study groups |
| Garfinkel 1998 | Study compared treatment methods which we defined as not relevant for this review. |
| Garland 1964 | Study compared treatment methods which we defined as not relevant for this review. |
| Gerritsen 2002 | Study compares treatment methods which we defined as not relevant for this review |
| Golriz 2016 | Study compared two types of splint. |
| Gurcay 2009 | The same type of splint was applied to each group in this trial. |
| Halac 2015 | Not a randomised trial |
| Heebner 2008 | The same type of splint was applied to each group in this trial. |
| Hojjati 2020 | Splint applied to each of the study groups |
| IRCT2012122811912N1 | The effect of splinting could not be isolated from that of the other intervention delivered alongside it; study compared treatment methods that we defined as not relevant for this review. |
| IRCT20130612013651N4 | Splint applied to each group in this trial |
| IRCT2013092612450N1 | Splint applied to each group in this trial |
| IRCT2014083118991N1 | Splint applied to each group in this trial |
| IRCT201412014641N9 | The effect of splinting could not be isolated from that of the other intervention delivered alongside it. |
| IRCT20190429043421N1 | Splint applied to each group in this trial |
| IRCT20201130049540N1 | Splint seemed to be applied in both interventions. |
| Kamanli 2011 | The same type of splint was applied to each group in this trial. |
| Khosrawi 2012 | Splint applied to each group in this trial |
| Koca 2014 | Study compared treatment methods which we defined as not relevant for this review. |
| Krause 2020 | The effect of splinting could not be isolated from that of the other intervention delivered alongside it. |
| Kumnerddee 2010 | Study compared treatment methods which we defined as not relevant for this review. |
| Lewis 2020 | The effect of splinting could not be isolated from that of the other intervention delivered alongside it. |
| Luong 2017 | Not a randomised controlled trial |
| Mansiz Kaplan 2018 | Splint applied to each group in this trial |
| Mohammadabadi 2018 | Splint applied to each group in this trial |
| NCT04043780 | Splint applied to each group in this trial |
| NCT04416867 | Splint applied to each of the study groups |
| NCT04733209 | The effect of splint could not be isolated from the co‐interventions delivered with it. |
| Pinar 2005 | The same type of splint was applied to each group in this trial. |
| Politis 2015 | Splint applied to each group in this trial |
| Raeissadat 2014 | Splint applied to each group in this trial |
| Raji 2019 | The effect of splinting could not be isolated from that of the other intervention delivered alongside it. |
| Rayegani 2013 | Splint applied to each group in this trial |
| Roitberg 2003 | Not a randomised controlled trial (the article was a commentary) |
| Ruksen 2011 | The same type of splint was applied to each group in this trial. |
| Salehi 2019 | Splint applied to each group in this trial |
| Setayesh 2017 | Study compared treatment methods which we defined as not relevant for this review. |
| Šošić 2020 | Splint applied to each group in this trial |
| Soyupek 2012 | Study compared treatment methods which we defined as not relevant for this review. |
| Storey 2013 | Splint applied to each group in this trial |
| Talebi 2013 | Splint applied to each group in this trial |
| Toopchizadeh 2020 | Splint applied to each group in this trial |
| Ucan 2006 | Study compared treatment methods which we defined as not relevant for this review; splint applied to each group |
| Weintraub 2000 | The effectiveness of a wrist support strap (not a splint) was investigated in this study. |
| Weng 2016 | Not a randomised trial |
| Yao 2019 | Not a randomised trial |
| Zhang 2018 | Study compared treatment methods which we defined as not relevant for this review; the effect of splinting could not be isolated from that of the other intervention delivered alongside it. |
| Zinnuroglu 2010 | Splint applied to each group in this trial |
Characteristics of studies awaiting classification [ordered by study ID]
Baklaci 2015.
| Methods | Study design: Prospective, randomised, placebo‐controlled clinical study |
| Participants |
Details of sampling frame: Total n = 80 participants Group 1 (physical therapy) n = 21 Group 2 (splinting) n = 22 Group 3 (physical therapy + splinting) n = 18 Group 4 (placebo (sham) physical therapy) n = 19 Gender distribution: Total: 78 female, 2 male Duration of CTS symptoms: Not reported Inclusion criteria: People with CTS Exclusion criteria: Not reported |
| Interventions | Group 1: Physical therapy ‐ steroid iontophoresis using dexamethasone as a conductive agent (500 g/mol, 2.5 mA–4 mA), continuous ultrasound at 2.0 W/cm2 (3 MHz, 4 cm2 probe area), paraffin bath for hands was applied to the physical therapy group for 15 sessions. Group 2: Lightweight, neutral positioned wrist splint was applied to the 2nd group for 1 month (24 hours a day). Group 3: Both physical therapy and splinting were applied to the 3rd group in the same way and for the same duration as 1st and 2nd groups. Group 4: Placebo (sham) ultrasound and placebo (sham) iontophoresis without steroid (both without energy emission) were applied to the sham therapy group. |
| Outcomes | Outcomes evaluated at baseline after the treatment and at 1st, 3rd and 6th
|
| Notes | Report available only as a conference abstract |
Bhuva 2019.
| Methods | Study design: unclear (awaiting information from the authors) |
| Participants |
Details of sampling frame: Total n analysed = 26 (30 wrists) Gender distribution: Total: 7 males, 19 females Duration of CTS symptoms: 2 to 5 months Inclusion criteria:
Exclusion criteria:
|
| Interventions | Group 1: Customised splint, ultrasound and exercises (splint to wear every night for 4 weeks) Group 2: Soft tissue mobilisation, ultrasound, exercises |
| Outcomes | Outcomes evaluated at baseline and post‐treatment (after 4 weeks)
|
| Notes | Email sent to the authors but no response. Unclear if randomised trial or not |
IRCT2014020416485N1.
| Methods | Study design: Randomised, not blinded, parallel‐group, placebo‐controlled Setting: Baqiyatallah University of Medical Sciences polyclinic and University of Social Welfare and Rehabilitation Sciences affiliated clinics |
| Participants |
Details of sampling frame: Target sample size: 150 Gender: Both Age: No age limit Duration of CTS symptoms (N of participants): Inclusion criteria: People with mild to moderate CTS with no history of surgical and local steroid injection, any age or gender Exclusion criteria: Severe CTS and severe dysfunction that needs surgical median nerve release History of other therapeutic approaches History of allergy against steroids or triamcinolone Steroid contraindications History of cardiac arrhythmia Diabetes mellitus Hand surgeries in past 3 months Rheumatoid arthritis Cervical radiculopath Thyroid dysfunctions |
| Interventions | Group 1: injection of 80 mg triamcinolone with 1 mL lidocaine 2% by hydrodissection method Group 2: injection of 40 mg triamcinolone with 1 mL distilled water and 1 ml lidocaine 2% by hydrodissection method Group 3: injection of 2 mL distilled water and 1 mL lidocaine 2% by hydrodissection method Group 4: injection of 40 mg triamcinolone without hydrodissection method Group 5: wrist splinting for 6 weeks |
| Outcomes |
|
| Notes | Recruitment complete |
ISRCTN22916517.
| Methods | Study design: Randomised, open cross‐over study Primary study design: interventional Secondary study design: RCT Trial setting: not specified |
| Participants | Participant inclusion criteria: 80 participants in total: 40 with primary CTS and 40 with secondary CTS Participant type: patient Age group: not specified Gender: not specified Target number of participants: 80 Participant exclusion criteria: not provided at time of registration |
| Interventions | Wrist splints versus steroid injection |
| Outcomes | Primary outcome measure: proportion of participants improved at 6 weeks Secondary outcome measures: VAS for pain and tingling, grip strength test, adverse effects of treatment, recurrence or surgery within 12 months |
| Notes | Overall trial status: completed (12 September 2003) Recruitment status: no longer recruiting (12 September 2003) Publication status: results overdue (20 August 2015) |
Riasi 2015.
| Methods | Study design: unclear (awaiting information from the authors) Setting: neurology clinic of Vali Asr Hospital of Birjand |
| Participants |
Details of sampling frame: Total n analysed = 40 participants (wrists) Gender distribution: Total: 10 males, 30 females Age: 32.75 ± 4.33 years (ranging from 20 to 48 years) Duration of CTS symptoms: CTS diagnosis confirmed based on clinical examinations (Phalen’s and Tinel’s sign) and a proximal and distal amplitude difference higher than 50% and delayed distal motor conduction velocity lower than 20 m/s Inclusion criteria:
Exclusion criteria:
|
| Interventions | Group 1: ibuprofen (800 mg twice daily for 4‐6 weeks) with a short wrist splint Group 2: ibuprofen (800 mg twice daily for 4‐6 weeks) |
| Outcomes | Outcomes evaluated at baseline and after 4 to 6 weeks of treatment NCV and EMG examinations |
| Notes | Email sent to the authors, awaiting response. Unclear if randomised trial or not |
Soon 2015.
| Methods | Single‐blinded, randomised clinical trial with a 2 × 2 factorial design Setting: university clinical research centre and a network of clinical practices |
| Participants | Total n = 120 participants Group 1 (splint + multimodal approach comprising of manual mobilisation techniques, education and nerve and tendon gliding exercises (MEX)) n = 30 Group 2 (splint + ultrasound) n = 30 Group 3 (MEX alone) n = 30 Group 4 (ultrasound alone) n = 30 Inclusion criteria: Mild‐to‐moderate CTS based on clinical criteria and electrodiagnostic tests |
| Interventions | Group 1: splint + MEX Group 2: splint + ultrasound Group 3: MEX alone Group 4: ultrasound alone Commercially available hand splint was used to keep the wrist in the neutral position. Participants who received the hand splint were advised to use the splint during sleep. |
| Outcomes | Outcomes evaluated at baseline and at 7, 12 and 52 weeks follow‐up
|
| Notes | Report available only as a conference abstract |
BCTQ: Boston Carpal Tunnel Questionnaire CTS: carpal tunnel syndrome DML: distal motor latency EMG: electromyography GROC: Global Rating of Change MEX: multimodal approach comprising of manual mobilisation techniques, education and nerve and tendon gliding exercises NCV: nerve conduction velocity RCT: randomised controlled trial SF‐36: 36‐Item Short Form Health Survey SNCV: sensory nerve conduction velocity VAS: visual analog scale
Characteristics of ongoing studies [ordered by study ID]
Atroshi 2019.
| Study name | Evaluation of wrist splinting for the treatment of carpal tunnel syndrome |
| Methods | Study design: Prospective, randomised, parallel‐group superiority clinical trial Setting: Department of Orthopedics, Hässleholm‐Kristianstad‐Ystad |
| Participants |
Details of sampling frame: Total n for randomisation = 112 Gender distribution: Age: 25 to 65 years old Duration of CTS symptoms: Inclusion criteria
Exclusion criteria
|
| Interventions | Group 1: rigid wrist splint Group 2: soft wrist bandage (placebo) Splints and soft wrist bandages are going to be used initially for 6 weeks at night and, if possible, during the day |
| Outcomes | Outcomes will be measured at baseline and at 6, 12, 24, and 52 weeks after treatment starts.
|
| Starting date | Recruitment started 4 June 2018 and was expected to conclude by the end of 2020. |
| Contact information | Correspondence: Isam Atroshi, isam.atroshi@med.lu.se 1. Department of Clinical Sciences – Orthopedics, Lund University, SE‐22100 Lund, Sweden 2. Department of Orthopedics Hässleholm‐Kristianstad, Hässleholm Hospital, SE‐28125 Hässleholm, Sweden |
| Notes |
IRCT20120716010297N5.
| Study name | The long‐term effect of low level laser therapy on clinical symptoms and electrophysiologic parameters in patients with carpal tunnel syndrome |
| Methods | Study design: Double‐blind, RCT Setting: outpatient clinics of Isfahan University of Medical Sciences |
| Participants |
Details of sampling frame: Target sample size = 64 Gender: Both Age: From 30 years old to 65 years old Inclusion criteria:
Exclusion criteria:
|
| Interventions | Group 1: low level laser therapy Group 2: sham laser therapy + vitamin B1 (300 mg per day) + static night splinting for wrist for 2 months |
| Outcomes | Outcomes measured at baseline and 6 months after treatment
|
| Starting date | 2014/06/05 |
| Contact information | Shila Haghighat, +98 31 3233 0091, sh‐haghighat@med.mui.ac.ir |
| Notes | Recruitment status: not yet recruiting |
IRCT20200219046552N1.
| Study name | Comparison of local corticosteroid injection and high‐intensity laser outcomes in moderate carpal tunnel syndrome (CTS) |
| Methods | Study design: A concealed, randomised, open‐label, sham‐controlled clinical trial with a parallel‐group design |
| Participants |
Details of sampling frame: Target sample size: 126 Gender: Both Age: No age limit Inclusion criteria:
Exclusion criteria:
|
| Interventions | Group 1: splint + painkillers (meloxicam 15 mg) + vitamin B1 Group 2: 40 mg of methylprednisolone (Depromedrol) in combination with 1% lidocaine Group 3: laser therapy |
| Outcomes | Outcomes evaluated at baseline and 6 months later.
|
| Starting date | 20 April 2020 |
| Contact information | Mozaffar Hosseininezhad, +98 13 3332 2444, hosseininezhadm@gmail.com |
| Notes | Recruitment status: recruiting |
JPRN‐UMIN000017952.
| Study name | Nerve gliding exercise for carpal tunnel syndrome |
| Methods | Study design: Interventional, factorial, randomised, open (no blinding) |
| Participants |
Details of sampling frame: Sample size: 60 Gender: Both Age: 20 to 65 years old Inclusion criteria: Grade 1 (Hamada classification) Exclusion criteria:
etc. |
| Interventions | Group 1: nerve gliding, splinting, rest Group 2: splinting, rest Group 3: rest |
| Outcomes | Improvement of symptoms |
| Starting date | 15 June 2015 |
| Contact information | Yuki Hara, 029‐8533219, yukihara@md.tsukuba.ac.jp |
| Notes | Recruitment status: recruiting |
NCT04017390.
| Study name | The effect of theraworx foam on the cross‐sectional area of the median nerve in patients with CTS |
| Methods | Study design: Randomised, parallel assignment, double (participant, investigator) masked |
| Participants |
Details of sampling frame: Estimated enrolment = 60 participants Age: 18 years and older Inclusion criteria
Exclusion criteria
|
| Interventions | Group 1: Theraworx foam Group 2: placebo foam Group 3: Theraworx foam and night splint Group 4: placebo foam and night‐time splint |
| Outcomes | Outcomes evaluated at baseline and after treatment (2 weeks)
|
| Starting date | Estimated study start date: December 2021 |
| Contact information | 1. John Fowler, 412‐605‐3245, fowlerjr@upmc.edu 2. Karen Wasil, 412‐605‐3221, wasilkf2[at]upmc.edu |
| Notes |
NCT04515966.
| Study name | Randomised controlled trial of ultrasound‐guided steroid injection versus wrist splint in patients with CTS |
| Methods | Study design: Randomised, parallel assignment, open‐label (no masking) |
| Participants |
Details of sampling frame: Estimated enrolment: 70 participants Gender: Both Age: 18 years to 75 years (adult, older adult) Duration of CTS symptoms: Inclusion criteria
Exclusion criteria
|
| Interventions | Group 1: ultrasound‐guided steroid injection Group 2: wrist splint |
| Outcomes | Outcomes evaluated at baseline and after treatment (6 weeks, 12 weeks, 6 months, 1 year).
|
| Starting date | Estimated study start date: 1 October 2020 |
| Contact information | Roy N Morcos, (330)729‐8700, roy_morcos@mercy.com |
| Notes |
NCT04993703.
| Study name | Effect of kinesiotaping and night splinting in patients with carpal tunnel syndrome |
| Methods | Study design: Double‐blind (participant‐, outcomes assessor‐blinded) RCT |
| Participants |
Details of sampling frame: Estimated enrolment: 45 participants Gender: Both Age: Ages eligible for study: 30 years to 60 years Inclusion criteria:
Exclusion criteria:
|
| Interventions | Group 1: kinesiotaping Group 2: night splinting Group 3: control group |
| Outcomes | Outcomes assessed at baseline and 8‐week follow‐up
|
| Starting date | 2021/08/06 |
| Contact information | Leyla Eraslan, +905348488373, leylaeraslan@hacettepe.edu.tr |
| Notes | Recruitment status: recruiting |
BCTQ: Boston Carpal Tunnel Questionnaire CTS: carpal tunnel syndrome DML: distal motor latency EMG: electromyography EQ‐5D: EuroQol 5‐dimension health status and quality of life measure NCV: nerve conduction velocity quickDASH: 11‐item Disabilities of the Arm, Shoulder, and Hand scale RCT: randomised controlled trial VAS: visual analog scale
Differences between protocol and review
In this update, we did not include neurophysiological parameters. We also changed symptoms (continuous outcome) as the primary outcome as only one paper had measured overall improvement (binary outcome). Changes to outcomes were as follows.
The primary outcome was amended from "short‐term overall improvement" (in the previous version of this review) to "CTS symptoms" because the included studies did not measure this outcome in the previous review and a preliminary overview (before data extraction) suggested that this outcome is rarely measured and reported.
We excluded "improvement in neurophysiologic parameters" since they are not patient‐important outcomes.
We added "referral to surgery", because it may be important in clinical decision‐making.
We excluded the following comparisons included in the previous version of this review.
Different splint designs were excluded until splints show efficacy since all identified comparisons tested some specific splint against another specific splint and inference to other splints was limited;
Splint versus interventions that are unlikely to be disease‐modifying or it was impossible to isolate the effect of the splint (splint versus yoga; splint verus acupuncture; splint plus nerve and tendon gliding exercises with gabapentin plus nerve and tendon gliding exercises; splint plus steroid injection versus therapeutic ultrasound; splint plus steroid injection plus nerve and tendon gliding exercises versus nerve and tendon gliding exercises; splint plus steroid injection versus nerve and tendon gliding exercises; splint plus NSAIDs versus local corticosteroid injection).
For the first version of the splints review, the primary outcome (see Page 2012b) was short‐term overall improvement (any measure in which patients indicate the intensity of their complaints compared with baseline) (three months or less; reported as a dichotomous outcome). Secondary outcomes were as follows.
Adverse effects.
Short‐term improvement in CTS symptoms (for example, pain, paraesthesia, nocturnal paraesthesia) (three months or less).
Short‐term improvement in functional ability or health‐related quality of life (three months or less).
Short‐term improvement in neurophysiologic parameters (three months or less).
Long‐term improvement in CTS symptoms (greater than three months).
Long‐term improvement in functional ability or health‐related quality of life (greater than three months).
In the original version of the review, Non‐surgical interventions for carpal tunnel syndrome by O'Connor and colleagues (O'Connor 2003), types of outcome measures were as follows.
The primary outcome measure was improvement in clinical symptoms, such as pain and paraesthesiae, at least three months after the end of treatment.
Secondary outcome measures included:
Improvement in functional status and/or health‐related quality of life parameters at least three months after treatment;
Improvement in objective physical examination measures, such as grip, pinch strength, and sensory perception at least three months after treatment;
Improvement in neurophysiological parameters three months after treatment;
Clinical improvement at less than three months of follow‐up;
Clinical improvement at one year after treatment;
Need for surgical release of the flexor retinaculum during follow‐up.
Contributions of authors
TEEMU KARJALAINEN (TK) was involved in the design of the update; screening of the search results and assessing retrieved full texts, appraising risk of bias; extracting data; performing analyses; interpreting analyses including certainty of evidence; and writing of the review.
VIEDA LUSA (VL) was involved in the screening of the search results and assessing retrieved full texts, appraising the risk of bias; extracting data; performing analyses; interpreting the evidence, including certainty of evidence; and writing of the review.
SUSAN PETERS (SP) screening of the search results and assessing retrieved full texts, appraising risk of bias; extracting data; performing analyses; interpreting the evidence, including certainty of evidence; and writing of the review.
MATTHEW PAGE (MP) was involved in the following stages of the review: appraising the risk of bias of papers; extracting data from papers; editing the review.
NICOLA MASSY‐WESTROPP (NMW) was involved in the following stages of the review: appraising the risk of bias of papers; extracting data from papers; approval of the final review.
DENISE O'CONNOR (DOC) was responsible for: appraising the risk of bias of papers; extracting data from papers; approval of the final review.
Sources of support
Internal sources
No sources of support provided
External sources
-
Teemu Karjalainen, Finland
TK is supported by a Finnish Medical Foundation research grant.
-
Vieda Lusa, Finland
Vieda Lusa is supported by Hospital Nova Foundation (Sairaala Novan säätiö) research grant.
-
Cochrane Neuromuscular, UK
This project was supported by the National Institute for Health and Care Research (NIHR) via Cochrane Infrastructure funding to Cochrane Neuromuscular. The views and opinions expressed therein are those of the authors and do not necessarily reflect those of the Systematic Reviews Programme, NIHR, NHS or the Department of Health. Cochrane Neuromuscular is also supported by the Queen Square Centre for Neuromuscular Diseases.
-
Matthew Page, Australia
Matthew J Page is supported by an Australian Research Council Discovery Early Career Award (DE200101618).
-
Susan Peters, USA
Susan Peters is supported by funding recieved from the National Institute for Occupational Safety and Health (U19 OH008861).
-
Denise O'Connor, Australia
Denise O'Connor is supported by an Australian National Health and Medical Research Council (NHMRC) Public Health Fellowship (#606726).
Declarations of interest
TVK declares no conflicts of interests in this review. As a consultant in hand surgery, he frequently sees and treats people with carpal tunnel syndrome in a public hospital (salaried position). He also receives remuneration for private practice.
VL declares no conflicts of interests in this review.
MJP declares no conflicts of interests in this review.
NMW declares no conflicts of interests in this review. She is an Interplast Australia Volunteer presenter of hand therapy and anatomy webinars and direct hand therapy training and a volunteer research advocacy and education committee member for Arthritis South Australia, volunteer research committee member for Australian Hand Therapy Association.
DOC declares no conflicts of interests in this review. She is an Editor, Cochrane EPOC, Director, Cochrane EPOC Australasian Satellite and Editor, Cochrane Musculoskeletal.
SEP declares no conflicts of interests in this review.
New search for studies and content updated (conclusions changed)
References
References to studies included in this review
Akturk 2018 {published data only}
- Aktürk S, Büyükavcı R, Aslan Ö, Ersoy Y. Comparison of splinting and Kinesio taping in the treatment of carpal tunnel syndrome: a prospective randomized study. Clinical Rheumatology 2018;37(9):2465-9. [DOI] [PubMed] [Google Scholar]
Boonhong 2017 {published data only}
- Boonhong J, Panyasriwanit S. Neutral wrist splint for mild to moderate carpal tunnel syndrome. Chulalongkorn Medical Journal 2017;61(2):151-63. [Google Scholar]
- Panyasriwanit S. Combination of wrist splint and conventional treatment vs conventional treatment alone in carpal tunnel syndrome. Archives of Physical Medicine and Rehabilitation 2013;94(10):e63. [Google Scholar]
- TCTR20160209001. Three-month neutral wrist splint in mild to moderate carpal tunnel syndrome: a randomized controlled trial. who.int/trialsearch/Trial2.aspx?TrialID=TCTR20160209001 (first received 9 February 2016).
Chesterton 2018 {published data only}
- Burton CL, Rathod-Mistry T, Blagojevic-Bucknall M, Chesterton LS, Davenport G, Dziedzic KS, et al. P143 The clinical and cost-effectiveness of corticosteroid injection versus night splints for carpal tunnel syndrome: 24-month follow-up of an open-label, parallel group, randomised controlled trial. Rheumatology 2020;59(Suppl_2):111.138. [Google Scholar]
- Chesterton LS, Blagojevic-Bucknall M, Burton C, Dziedzic KS, Davenport G, Jowett SM, et al. The clinical and cost-effectiveness of corticosteroid injection versus night splints for carpal tunnel syndrome (INSTINCTS trial): an open-label, parallel group, randomised controlled trial. Lancet 2018;392(10156):1423-33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chesterton LS, Dziedzic KS, Van der Windt DA, Davenport G, Myers HL, Rathod T, et al. The clinical and cost effectiveness of steroid injection compared with night splints for carpal tunnel syndrome: the INSTINCTS randomised clinical trial study protocol. BMC Musculoskeletal Disorders 2016;17(1):1-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- EUCTR2013-001435-48-GB. Injections versus splints in carpal tunnel syndrome. clinicaltrialsregister.eu/ctr-search/trial/2013-001435-48/results (first received 3 June 2013).
- ISRCTN09392969. The clinical and cost effectiveness of a steroid injection versus a night splint for carpal tunnel syndrome. who.int/trialsearch/Trial2.aspx?TrialID=ISRCTN09392969 (first received 1 May 2014).
- NCT02038452. Injection versus splinting in carpal tunnel syndrome. clinicaltrials.gov/show/NCT02038452 (first received 16 Jan 2014).
De Entrambasaguas 2006 {published data only}
- De Entrambasaguas M, Mañez I, Girona G, Lopez-Santoveña F, Poyatos YJ. Steroid injection, wrist splinting and phonophoresis in carpal tunnel syndrome [Infiltracion de esteroides, ferula de muneca fonoforesis en el syndrome del tunel carpiano]. Rehabilitacion 2006;40(4):193-200. [Google Scholar]
De Moraes 2021 {published data only}
- De Moraes VY, Queiroz J, Raduan-Neto J, Fernandes M, Okamura A, Belloti JC. Nonsurgical treatment for symptomatic carpal tunnel syndrome: a randomized clinical trial comparing local corticosteroid injection versus night orthosis. Journal of Hand Surgery 2021;46(4):P295-300.e1. [CLINICALTRIALS: NCT03196817] [DOI] [PubMed] [Google Scholar]
- NCT03196817. Non-surgical treatment of carpal tunnel syndrome: night splint versus local corticosteroid infiltration. clinicaltrials.gov/show/NCT03196817 (first received 23 June 2017).
Eraslan 2014 {published data only}
- Eraslan L, Yuce D, Kermalli AM, Baltaci G. Comparison of short-term effects of rigid tape and night splint on pain and function in patient with carpal tunnel syndrome: a randomized clinical trial. Türk Fizyoterapi ve Rebilitasyon Dergisi 2014;25(1):8-15. [Google Scholar]
Gatheridge 2020 {published data only}
- Gatheridge MA, Sholty EA, Inman A, Pattillo M, Mindrup F, Sanderson DL. Splinting in carpal tunnel syndrome: the optimal duration. Military Medicine 2020;185(11-12):e2049-54. [DOI] [PubMed] [Google Scholar]
Geler Kulcu 2016 {published data only}
- Geler Kulcu D, Bursali C, Aktas I, Bozkurt Alp S, Unlu Ozkan F, Akpinar P. Kinesiotaping as an alternative treatment method for carpal tunnel syndrome. Turkish Journal of Medical Sciences 2016;46(4):1042-9. [DOI] [PubMed] [Google Scholar]
Hall 2013 {published data only}
- Hall B, Lee HC, Fitzgerald H, Byrne B, Barton A, Lee AH. Investigating the effectiveness of full-time wrist splinting and education in the treatment of carpal tunnel syndrome: a randomized controlled trial. American Journal of Occupational Therapy 2013;67(4):448-59. [DOI] [PubMed] [Google Scholar]
Jaladat 2017 {published data only}
- IRCT2015061522753N1. Effects of wrist limited dynamic splint on carpal tunnel syndrome. who.int/trialsearch/Trial2.aspx?TrialID=IRCT2015061522753N1 (first received 22 Feb 2015).
- Jaladat SM, Abdolvahab M, Moghadam BA, Ashraf A, Jalili M, Baghestani A. The effectiveness of limited dynamic wrist splints on the symptoms, function, and strength of women with carpal tunnel syndrome: a controlled trial study. Journal of Rehabilitation Sciences and Research 2017;1(4):1-5. [Google Scholar]
Kocaoglu 2017 {published data only}
- Kocaoglu S, Esmer A, Okumus M, Ozturk E, Kaygisiz F, Demir G. The kinesio taping, local anesthetic/steroid injection and splinting in the treatment of patients with carpal tunnel syndrome: a clinical and electrophysiological study. Osteoporosis International 2017;28(Suppl 1):S447. [Google Scholar]
Madjdinasab 2008 {published data only}
- Madjdinasab N, Zadeh NS, Assarzadegan F, Ali AM, Pipelzadeh M. Efficacy comparison of splint and oral steroid therapy in nerve conduction velocity and latency median nerve in carpal tunnel syndrome. Pakistan Journal of Medical Sciences 2008;24(5):725-8. [EMBASE: 2008533657] [Google Scholar]
Manente 2001 {published data only}
- Manente G, Torrieri F, Di Blasio F, Staniscia T, Romano F, Uncini A. An innovative hand brace for carpal tunnel syndrome: a randomized controlled trial. Muscle & Nerve 2001;24(8):1020-5. [PMID: ] [DOI] [PubMed] [Google Scholar]
Mishra 2006 {published data only}
- Mishra S, Prabhakar S, Lal V, Modi M, Das CP, Khurana D. Efficacy of splinting and oral steroids in the treatment of carpal tunnel syndrome: a prospective randomized clinical and electrophysiological study. Neurology India 2006;54(3):286-90. [DOI] [PubMed] [Google Scholar]
Oncu 2014 {published data only}
- Oncu J, Iliser R, Koymen Yilmaz F, Kuran B. Efficacy of kinesiotaping on symptoms, hand functions, and hand grip strength in carpal tunnel syndrome: a single-blind and randomized controlled study. Turkiye Fiziksel Tip ve Rehabilitasyon Dergisi 2014;60(Suppl 1):S43-51. [Google Scholar]
Premoselli 2006 {published data only}
- Premoselli S, Sioli P, Grossi A, Cerri C. Neutral wrist splinting in carpal tunnel syndrome: a 3- and 6-month clinical and neurophysiologic follow-up evaluation of night-only splint therapy. Europa Medicophysica 2006;42(2):121-6. [PMID: ] [PubMed] [Google Scholar]
Rioja Toro 2012 {published data only}
- Rioja Toro J, Estévez Poy PJ, Martínez Pardo F. A prospective, randomized and placebo controlled study of the efficacy of treatment with laser, with or without splinting of wrist in idiopathic carpal tunnel syndrome. Rehabilitacion 2012;46(2):92-102. [Google Scholar]
Sanaee 2017 {published data only}
- IRCT2013091814704N1. Effectiveness of long term using of splinting in severe CTS. who.int/trialsearch/Trial2.aspx?TrialID=IRCT2013091814704N1 (first received 17 May 2015).
- Sanaee S, Roshanzamir S, Homayounee K. Efficacy of long-term splinting in the treatment of severe carpal tunnel syndrome. Journal of Musculoskeletal Research 2017;20(02):1750013. [Google Scholar]
Schmid 2012 {published data only}
- ACTRN12611000690954. The effect of splinting and exercises on swelling of the nerve in patients with carpal tunnel syndrome. anzctr.org.au/ACTRN12611000690954.aspx (first received 7 July 2011).
- Schmid AB, Elliott JM, Strudwick MW, Little M, Coppieters MW. Effect of splinting and exercise on intraneural edema of the median nerve in carpal tunnel syndrome - an MRI study to reveal therapeutic mechanisms. Journal of Orthopaedic Research 2012;30(8):1343-50. [DOI] [PubMed] [Google Scholar]
Sevim 2004 {published data only}
- Sevim S, Dogu O, Camdeviren H, Kaleagasi H, Aral M, Arslan E, et al. Long-term effectiveness of steroid injections and splinting in mild and moderate carpal tunnel syndrome. Neurological Sciences 2004;25(2):48-52. [PMID: ] [DOI] [PubMed] [Google Scholar]
So 2018 {published data only}
- NCT02140632. Efficacy study of local steroid injection and wrist splinting for carpal tunnel syndrome. clinicaltrials.gov/show/NCT02140632 (first received 16 May 2014).
- So H, Chung V, Cheng J, Yip ML. Local steroid injection versus wrist splinting for carpal tunnel syndrome: a randomized clinical trial. Annals of the Rheumatic Diseases 2017;76(Suppl 2):998–9. [DOI] [PubMed] [Google Scholar]
- So H, Chung V. Local steroid injection versus wrist splinting for carpal tunnel syndrome: a randomized clinical trial and a qualitative exploration. Annals of the Rheumatic Diseases 2015;74:1218. [DOI] [PubMed] [Google Scholar]
- So H, Chung VCH, Cheng JCK, Yip RML. Local steroid injection versus wrist splinting for carpal tunnel syndrome: a randomized clinical trial. International Journal of Rheumatic Diseases 2018;21(1):102–7. [DOI] [PubMed] [Google Scholar]
Taspinar 2007 {published data only}
- Taşpınar Ş, Şahin F, Erçalık C, Kuran B, Barkut K, Çelik M, et al. Comparison of the efficacy of corticosteroid injection, night splint, and physiotherapy in diabetic carpal tunnel syndrome [Diyabetik karpal tünel sendromunda kortikosteroid enjeksiyonu, gece ateli ve fizik tedavinin etkinliğinin karşılaştırılması]. Turkish Journal of Physical Medicine and Rehabilitation 2007;53(2):54-60. [Google Scholar]
Ulucakoy 2020 {published data only}
- Ulucaköy RK, Yurdakul FG, Bodur H. Extracorporeal shock wave therapy as a conservative treatment option for carpal tunnel syndrome: a double-blind, prospective, randomized, placebo-controlled study. Turkish Journal of Physical Medicine and Rehabilitation 2020;66(4):388–97. [DOI] [PMC free article] [PubMed] [Google Scholar]
Walker 2000 {published data only}
- Walker WC, Metzler M, Cifu DX, Swartz Z. Neutral wrist splinting in carpal tunnel syndrome: a comparison of night-only versus full-time wear instructions. Archives of Physical Medicine and Rehabilitation 2000;81(4):424-9. [PMID: ] [DOI] [PubMed] [Google Scholar]
Wang 2017 {published data only}
- NCT02708693. Efficacy of combined ultrasound guided steroid injection and splinting in patients with carpal tunnel syndrome. clinicaltrials.gov/show/NCT02708693 (first received 15 March 2016).
- Wang JC, Liao KK, Lin KP, Chou CL, Yang TF, Huang YF, et al. Efficacy of combined ultrasound-guided steroid injection and splinting in patients with carpal tunnel syndrome: a randomized controlled trial. Archives of Physical Medicine and Rehabilitation 2017;98(5):947-56. [DOI] [PubMed] [Google Scholar]
Werner 2005 {published data only}
- Werner RA, Franzblau A, Gell N. Randomized controlled trial of nocturnal splinting for active workers with symptoms of carpal tunnel syndrome. Archives of Physical Medicine and Rehabilitation 2005;86(1):1-7. [PMID: ] [DOI] [PubMed] [Google Scholar]
Willis 2016 {published data only}
- NCT00558116. Treatment of carpal tunnel syndrome with dynamic splinting. clinicaltrials.gov/show/NCT00558116 (first received 14 Nov 2007).
- Willis FB, Fowler B. Longitudinal outcomes following a randomized controlled trial of dynamic splint stretching for carpal tunnel syndrome. Hand 2016;11(3):290-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
Wu 2017 {published data only}
- NCT02539186. The effect of platelet rich plasma for carpal tunnel syndrome. clinicaltrials.gov/show/NCT02539186 (first received 2 Sep 2015).
- Wu YT, Ho TY, Chou YC, Ke MJ, Li TY, Huang GS, et al. Six-month efficacy of platelet-rich plasma for carpal tunnel syndrome: a prospective randomized, single-blind controlled trial. Scientific Reports 2017;7(1):94. [DOI] [PMC free article] [PubMed] [Google Scholar]
Yazdanpanah 2012 {published data only}
- IRCT2012110210799N3. Efficacy of steroid injection and wrist splint in carpal tunnel syndrome. who.int/trialsearch/Trial2.aspx?TrialID=IRCT2012110210799N3 (first received 12 Feb 2013).
- Yazdanpanah P, Aramesh ST, Malekzadeh JM, Rahimipour SH. Comparison efficacy of triamcinolone and wrist splint in severe carpal tunnel syndrome in pregnancy. Gynecology & Obstetrics 2012;2(5):134. [Google Scholar]
References to studies excluded from this review
Akalin 2002 {published data only}
- Akalin E, El O, Peker O, Senocak O, Tamci S, Gülbahar S, et al. Treatment of carpal tunnel syndrome with nerve and tendon gliding exercises. American Journal of Physical Medicine & Rehabilitation 2002;81(2):108-13. [PMID: ] [DOI] [PubMed] [Google Scholar]
Arinci Incel 2005 {published data only}
- Arinci Incel N, Sezgin M, Aksit C, Sahin G. Effect of gabapentin in carpal tunnel syndrome: a controlled clinical trial [Karpal tunnel sendromunda gabapentin tedavisinin etkinligi: karsilastirmali klinik calisma]. Journal of Rheumatology and Medical Rehabilitation 2005;16(4):224-30. [EMBASE: 2007022406] [Google Scholar]
Asgari 2020 {published data only}
- Asgari MR, Mosaviinejad SS, Ebrahimian A, Aminianfar A, Ghorbani R, Babamohamadi H. The effects of acupressure on the symptoms severity and function status and electrodiagnostic findings in patients with carpal tunnel syndrome. Complementary Therapies in Medicine 2020;51:102420. [DOI] [PubMed] [Google Scholar]
- IRCT201609178717N4. The effect of acupressure in the treatment of carpal tunnel syndrome. who.int/trialsearch/Trial2.aspx?TrialID=IRCT201609178717N4 (first received 24 Oct 2016).
Bagheri 2011 {published data only}
- Bagheri A, Reisi M, Vahab Kashani R. Comparison of therapeutic effects of dorsal wrist splint with cockup splint in carpal tunnel syndrome based on median sensory nerve conduction measurements. Journal of Gorgan University of Medical Sciences 2011;13(3(39)):42-7. [Google Scholar]
- IRCT138903224157N1. Effect of splinting for carpal tunnel syndrome. who.int/trialsearch/Trial2.aspx?TrialID=IRCT138903224157N1 (first received 5 Mar 2009).
Baker 2012 {published data only}
- Baker NA, Moehling K, Rubinstein E, Gustafson N, Baratz M. Lumbrical splinting and stretching versus standard treatment on grip, pinch, and dexterity in people with carpal tunnel syndrome. Arthritis and Rheumatism 2012;64:S1025-6. [Google Scholar]
- Baker NA, Moehling KK, Rubinstein EN, Wollstein R, Gustafson NP, Baratz M. The comparative effectiveness of combined lumbrical muscle splints and stretches on symptoms and function in carpal tunnel syndrome. Archives of Physical Medicine and Rehabilitation 2012;93(1):1-10. [DOI] [PubMed] [Google Scholar]
- NCT00803257. Effect of lumbrical stretching on carpal tunnel syndrome. clinicaltrials.gov/show/NCT00803257 (first received 5 Dec 2008).
Bardak 2009 {published data only}
- Bardak AN, Alp M, Erhan B, Paker N, Kaya B, Onal AE. Evaluation of the clinical efficacy of conservative treatment in the management of carpal tunnel syndrome. Advances in Therapy 2009;26(1):107-16. [PMID: ] [DOI] [PubMed] [Google Scholar]
Baysal 2006 {published data only}
- Baysal O, Altay Z, Ozcan C, Ertem K, Yologlu S, Kayhan A. Comparison of three conservative treatment protocols in carpal tunnel syndrome. International Journal of Clinical Practice 2006;60(7):820-8. [PMID: ] [DOI] [PubMed] [Google Scholar]
Bilgici 2010 {published data only}
- Biligici A, Ulusoy H, Canturk F. The comparison of ultrasound treatment and local steroid injection plus splinting in the carpal tunnel syndrome: a randomized controlled trial. Bratislavske Lekarske Listy 2010;111(12):659-65. [PMID: ] [PubMed] [Google Scholar]
Brininger 2007 {published data only}
- Brininger TL, Rogers JC, Holm MB, Baker NA, Li ZM, Goitz RJ. Efficacy of a fabricated customized splint and tendon and nerve gliding exercises for the treatment of carpal tunnel syndrome: a randomized controlled trial. Archives of Physical Medicine and Rehabilitation 2007;88(11):1429-35. [PMID: ] [DOI] [PubMed] [Google Scholar]
- NCT00336622. Efficacy of wrist/hand splints and tendon/nerve gliding exercises for carpal tunnel syndrome: a RCT. clinicaltrials.gov/show/NCT00336622 (first received 14 June 2006).
Bulut 2015 {published data only}
- Bulut GT, Caglar NS, Aytekin E, Ozgonenel L, Tutun S, Demir SE. Comparison of static wrist splint with static wrist and metacarpophalangeal splint in carpal tunnel syndrome. Journal of Back and Musculoskeletal Rehabilitation 2015;28(4):761-7. [DOI] [PubMed] [Google Scholar]
Burke 1994 {published data only}
- Burke DT, Burke MM, Stewart GW, Cambre A. Splinting for carpal tunnel syndrome: in search of the optimal angle. Archives of Physical Medicine and Rehabilitation 1994;75(11):1241-4. [PMID: ] [DOI] [PubMed] [Google Scholar]
Bye 2011 {published data only}
- Bye R, Hajiaqai B, Frorough B. Comparison between efficacy of manu splint and cock-up splint in carpal tunnel syndrome treatment. Journal of Babul University Medical Sciences 2011;13(1):51-7. [Google Scholar]
Celiker 2002 {published data only}
- Celiker R, Arslan S, Inanici F. Corticosteroid injection vs. nonsteroidal antiinflammatory drug and splinting in carpal tunnel syndrome. American Journal of Physical Medicine and Rehabilitation 2002;81(3):182-6. [PMID: ] [DOI] [PubMed] [Google Scholar]
Chung 2016 {published data only}
- Chung VC, Ho RS, Liu S, Chong MK, Leung AW, Yip BH, et al. Electroacupuncture and splinting versus splinting alone to treat carpal tunnel syndrome: a randomized controlled trial. Canadian Medical Association Journal 2016;188(12):867-75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chung VC, Wong SY, Kung K, Zee CY, Leung WN, Chong KC, et al. Electroacupuncture and wrist splinting for carpal tunnel syndrome: a randomised trial. Hong Kong Medical Journal 2017;23 (3 Suppl 2):28-31. [PubMed] [Google Scholar]
CTRI/2015/02/005531 {published data only}
- CTRI/2015/02/005531. Device study to evaluate the effect of CarpaStretch in treating carpal tunnel syndrome (a medical condition which causes pain, numbness and tingling in the wrist and fingers due to compression of median nerve in the wrist). who.int/trialsearch/Trial2.aspx?TrialID=CTRI/2015/02/005531 (first received 11 Feb 2015).
Daniel 2000 {published data only}
- Daniel ES, Paul S. A comparison study of the volar wrist cock-up splint and ulnar gutter splint in carpal tunnel syndrome. Occupational Therapy in Health Care 2000;12(4):79-93. [DOI] [PubMed] [Google Scholar]
Davis 1998 {published data only}
- Davis PT, Hulbert JR, Kassak KM, Meyer JJ. Comparative efficacy of conservative medical and chiropractic treatments for carpal tunnel syndrome: a randomized clinical trial. Journal of Manipulative and Physiological Therapeutics 1998;21(5):317-26. [PMID: ] [PubMed] [Google Scholar]
De Angelis 2009 {published data only}
- De Angelis MV, Pierfelice F, Di Giovanni P, Staniscia T, Uncini A. Efficacy of a soft hand brace and a wrist splint for carpal tunnel syndrome: a randomized controlled study. Acta Neurologica Scandinavica 2009;119(1):68-74. [PMID: ] [DOI] [PubMed] [Google Scholar]
Dehghani 2012 {published data only}
- Dehghani M, Sohrabi F, Teimouri M . The effects of topical corticosteroid injection on carpal tunnel syndrome. Journal of Isfahan Medical School 2012;29:2521-6. [Google Scholar]
Dincer 2009 {published data only}
- Dincer U, Cakar E, Kiralp MZ, Kilac H, Dursun H. The effectiveness of conservative treatments of carpal tunnel syndrome: splinting, ultrasound, and low-level laser therapies. Photomedicine and Laser Surgery 2009;27(1):119-25. [DOI] [PubMed] [Google Scholar]
DRKS00014585 {published data only}
- DRKS00014585. Therapy of carpal tunnel syndrom. who.int/trialsearch/Trial2.aspx?TrialID=DRKS00014585 (first received 26 June 2018).
Eftekharsadat 2011 {published data only}
- Eftekharsadat B, Shakouri SK, Shimia M, Rahbar M, Ghojazadeh M, Rashidi MR, et al. Effect of E. laciniata (L) ointment on mild and moderate carpal tunnel syndrome: a double-blind, randomized clinical trial. Phytotherapy Research 2011;25(2):290-5. [PMID: ] [DOI] [PubMed] [Google Scholar]
Ekim 2008 {published data only}
- Ekim A, Colak E. Ultrasound treatment in carpal tunnel syndrome: a placebo controlled study. Turkiye Fiziksel Tip ve Rehabilitasyon Dergisi 2008;54(3):96-101. [Google Scholar]
Evcik 2007 {published data only}
- Evcik D, Kavuncu V, Cakir T, Subasi V, Yaman M. Laser therapy in the treatment of carpal tunnel syndrome: a randomized controlled trial. Photomedicine and Laser Surgery 2007;25(1):34-9. [PMID: ] [DOI] [PubMed] [Google Scholar]
Farahmand 2021 {published data only}
- Farahmand B, Pourhosaingholi E, Bagheri A. Investigating the effects of volar wrist cock-up splint and dorsal lock wrist hand orthosis in reducing signs of carpal tunnel syndrome. Medical Journal of the Islamic Republic of Iran 2021;35:53. [DOI] [PMC free article] [PubMed] [Google Scholar]
Garfinkel 1998 {published data only}
- Garfinkel MS, Singhal A, Katz WA, Allan DA, Reshetar R, Schumacher HR Jr. Yoga-based intervention for carpal tunnel syndrome: a randomized trial. JAMA 1998;280(18):1601-3. [PMID: ] [DOI] [PubMed] [Google Scholar]
Garland 1964 {published data only}
- Garland H, Taverne D, Clark JMP. Surgical treatment for the carpal tunnel syndrome. Lancet 1964;1:1129-30. [DOI] [PubMed] [Google Scholar]
Gerritsen 2002 {published data only}
- Gerritsen AA, De Vet HC, Scholten RJ, Bertelsmann FW, De Krom MC, Bouter LM. Splinting vs surgery in the treatment of carpal tunnel syndrome: a randomized controlled trial. JAMA 2002;288(10):1245-51. [DOI] [PubMed] [Google Scholar]
- ISRCTN18853827. Splinting or surgery for carpal tunnel syndrome. www.isrctn.com/ISRCTN18853827 (first received 11 Feb 2002).
Golriz 2016 {published data only}
- Golriz B, Ahmadi Bani M, Arazpour M, Bahramizadeh M, Curran S, Madani SP. Comparison of the efficacy of a neutral wrist splint and a wrist splint incorporating a lumbrical unit for the treatment of patients with carpal tunnel syndrome. Prosthetics and Orthotics International 2016;40(5):617-23. [DOI] [PubMed] [Google Scholar]
Gurcay 2009 {published data only}
- Gurcay E, Unlu E, Gurcay AG, Tuncay R, Cakci A. Evaluation of the effect of local corticosteroid injection and anti-inflammatory medication in carpal tunnel syndrome. Scottish Medical Journal 2009;54(1):4-6. [PMID: ] [DOI] [PubMed] [Google Scholar]
Halac 2015 {published data only}
- Halac G, Demir S, Yucel H, Niftaliyev E, Kocaman G, Duruyen H, et al. Splinting is effective for night-only symptomatic carpal tunnel syndrome patients. Journal of Physical Therapy Science 2015;27(4):993-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
Heebner 2008 {published data only}
- Heebner ML, Roddey TS. The effects of neural mobilization in addition to standard care in persons with carpal tunnel syndrome from a community hospital. Journal of Hand Therapy 2008;21(3):229-41. [DOI] [PubMed] [Google Scholar]
Hojjati 2020 {published data only}
- Hojjati F, Afjei MH, Ebrahimi Takamjani I, Rayegani SM, Sarrafzadeh J, Raeissadat SA, et al. The effect of high-power and low-power lasers on symptoms and the nerve conduction study in patients with carpal tunnel syndrome. A prospective randomized single-blind clinical trial. Journal of Lasers in Medical Sciences 2020;11(Suppl 1):S73-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
IRCT2012122811912N1 {published data only}
- IRCT2012122811912N1. Comparing the effect of acupuncture and medical treatment on clinical symptoms and ElectroMyoGraphy/Nerve Conduction Velocity (EMG/NCV) changes in patients with CTS. who.int/trialsearch/Trial2.aspx?TrialID=IRCT2012122811912N1 (first received 6 Sep 2015).
IRCT20130612013651N4 {published data only}
- IRCT20130612013651N4. Evaluation of the effect of interventions near and far to the carpal tunnel using dry needle method and routine physiotherapy. who.int/trialsearch/Trial2.aspx?TrialID=IRCT20130612013651N4 (first received 23 July 2020).
IRCT2013092612450N1 {published data only}
- IRCT2013092612450N1. Tendon/nerve gliding exercises in carpal tunnel syndrome. who.int/trialsearch/Trial2.aspx?TrialID=IRCT2013092612450N1 (first received 17 Mar 2014).
IRCT2014083118991N1 {published data only}
- IRCT2014083118991N1. The effectiveness of extracorporeal shock wave therapy and wrist splint for management of carpal tunnel syndrome. who.int/trialsearch/Trial2.aspx?TrialID=IRCT2014083118991N1 (first received 16 Oct 2014).
IRCT201412014641N9 {published data only}
- IRCT201412014641N9. Comparison of two therapeutic methods in patients with mild to moderate carpal tunnel syndrome. who.int/trialsearch/Trial2.aspx?TrialID=IRCT201412014641N9 (first received 10 Jan 2015).
IRCT20190429043421N1 {published data only}
- IRCT20190429043421N1. Effect of Fatih massage on carpal tunnel syndrome. who.int/trialsearch/Trial2.aspx?TrialID=IRCT20190429043421N1 (first received 22 July 2019).
IRCT20201130049540N1 {published data only}
- IRCT20201130049540N1. Evaluation of the effect of thermal therapy by Kay method (a kind of local Dagh darmani in Traditional Iranian Medicine) on reducing the sensory and functional symptoms of mild and moderate idiopathic carpal tunnel syndrome. en.irct.ir/trial/52653 (first received 16 Jan 2021).
Kamanli 2011 {published data only}
- Kamanli A, Bezgincan M, Kaya A. Comparison of local steroid injection into carpal tunnel via proximal and distal approach in patients with carpal tunnel syndrome. Bratislavské Lekárske Listy 2011;112(6):337-41. [PubMed] [Google Scholar]
Khosrawi 2012 {published data only}
- IRCT201108174242N2. Acupuncture in treatment of carpal tunnel syndrome. en.irct.ir/trial/4501 (first received 24 Sep 2011).
- Khosrawi S, Moghtaderi A, Haghighat S. Acupuncture in treatment of carpal tunnel syndrome: a randomized controlled trial study. Journal of Research in Medical Sciences 2012;17(1):1-7. [PMC free article] [PubMed] [Google Scholar]
Koca 2014 {published data only}
- Koca I, Boyaci A, Tutoglu A, Ucar M, Kocaturk O. Assessment of the effectiveness of interferential current therapy and TENS in the management of carpal tunnel syndrome: a randomized controlled study. Rheumatology International 2014;34(12):1639-45. [DOI] [PubMed] [Google Scholar]
Krause 2020 {published data only}
- Krause D, Roll SC, Javaherian-Dysinger H, Daher N. Comparative efficacy of the dorsal application of Kinesio tape and splinting for carpal tunnel syndrome: a randomized controlled trial. Journal of Hand Therapy 2021;34(3):351-61. [DOI] [PubMed] [Google Scholar]
- NCT03360344. Non-surgical intervention for carpal tunnel syndrome. clinicaltrials.gov/ct2/show/NCT03360344 (first received 4 Dec 2017).
Kumnerddee 2010 {published data only}
- Kumnerddee W, Kaewtong A. Efficacy of acupuncture versus night splinting for carpal tunnel syndrome: a randomized clinical trial. Journal of the Medical Association of Thailand 2010;93(12):1463-9. [PubMed] [Google Scholar]
Lewis 2020 {published data only}
- ACTRN12613001095752. The effect of a hand therapy preoperative screening and intervention clinic on patient and hospital outcomes: a randomised control trial. anzctr.org.au/ACTRN12613001095752.aspx (first received 30 Sep 2013).
- Lewis KJ, Coppieters MW, Ross L, Hughes I, Vicenzino B, Schmid AB. Group education, night splinting and home exercises reduce conversion to surgery for carpal tunnel syndrome: a multicentre randomised trial. Journal of Physiotherapy 2020;66(2):97-104. [DOI] [PubMed] [Google Scholar]
- Lewis KJ, Coppieters MW, Vicenzino B, Hughes I, Ross L, Schmid AB. Occupational therapists, physiotherapists and orthopaedic surgeons agree on the decision for carpal tunnel surgery. International Journal of Health Policy and Management 2022;11(7):1001-8. [DOI: 10.34172/ijhpm.2020.227] [PMID: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lewis KJ, Ross L, Coppieters MW, Vicenzino B, Schmid AB. Education, night splinting and exercise versus usual care on recovery and conversion to surgery for people awaiting carpal tunnel surgery: a protocol for a randomised controlled trial. BMJ Open 2016;6(9):e012053. [DOI] [PMC free article] [PubMed] [Google Scholar]
Luong 2017 {published data only}
- Luong P, King F, Li ZM, Dickason M, Diamond M, Son J. Pilot clinical study of a novel unobtrusive carpal tunnel tissue manipulation device in reducing symptoms of carpal tunnel syndrome. Arthritis and Rheumatology 2017;69(Suppl 10):2953-4. [Google Scholar]
Mansiz Kaplan 2018 {published data only}
- Mansiz Kaplan B, Akyuz G, Kokar S, Yagci I. Comparison of the effectiveness of orthotic intervention,kinesiotaping, and paraffin treatments in patients with carpal tunnel syndrome: a single-blind and randomized controlled study. Journal of Hand Therapy 2019;32(3):297-304. [DOI] [PubMed] [Google Scholar]
Mohammadabadi 2018 {published data only}
- IRCT20180511039611N1. Study of the effect of wrist mobilization on carpal tunnel syndrome. who.int/trialsearch/Trial2.aspx?TrialID=IRCT20180511039611N1 (first received 4 Aug 2018).
- Mohammadabadi HD, Azizi S, Dadarkhah A, Soltani ZR. Evaluation of effectiveness of the wrist mobilization compared with local corticosteroid injection in treatment of moderate carpal tunnel syndrome. Annals of Military and Health Sciences Research 2018;16(2):e83335. [Google Scholar]
NCT04043780 {published data only}
- NCT04043780. Clinical validation of a decompression prototype splint for patients with carpal tunnel syndrome. clinicaltrials.gov/show/NCT04043780 (first received 2 Aug 2019).
NCT04416867 {published data only}
- NCT04416867. Radial extracorporeal shock wave therapy in the treatment of carpal tunnel syndrome. clinicaltrials.gov/ct2/show/NCT04416867 (first received 4 June 2020).
NCT04733209 {published data only}
- NCT04733209. Mobilization with movement in carpal tunnel syndrome. clinicaltrials.gov/ct2/show/NCT04733209 (first received 1 Feb 2021).
Pinar 2005 {published data only}
- Pinar L, Enhos A, Ada S, Güngör N. Can we use nerve gliding exercises in women with carpal tunnel syndrome? Advances in Therapy 2005;22(5):467-75. [PMID: ] [DOI] [PubMed] [Google Scholar]
Politis 2015 {published data only}
- Politis M. A customizable, modular brace for carpal tunnel syndrome. Journal of Injury, Function and Rehabilitation 2015;7(9 Suppl 1):S158-9. [Google Scholar]
Raeissadat 2014 {published data only}
- Raeissadat SA, Rayegani SM, Rezaei S, Sedighipour L, Bahrami MH, Eliaspour D, et al. The effect of polarized polychromatic noncoherent light (bioptron) therapy on patients with carpal tunnel syndrome. Journal of Lasers in Medical Science 2014;5(1):39-46. [PMC free article] [PubMed] [Google Scholar]
Raji 2019 {published data only}
- IRCT20100313003551N9. The effects of exercise therapy on functional status in people with carpal tunnel syndrome. who.int/trialsearch/Trial2.aspx?TrialID=IRCT20100313003551N9 (first received 10 Mar 2018).
- Raji P, Deyabadi S, Bagheri H, Heidarimoghadam R, Jalili M, Mohammadi Y. The effect of exercise therapy on functional status of patients with carpal tunnel syndrome (original study in Persian). Journal of Medical Council of Iran 2019;37(1):28-32. [Google Scholar]
Rayegani 2013 {published data only}
- Rayegani SM, Bahrami MH, Eliaspour D, Raeissadat SA, Shafi Tabar Samakoosh M, Sedihgipour L, et al. The effects of low intensity laser on clinical and electrophysiological parameters of carpal tunnel syndrome. Journal of Lasers in Medical Science 2013;4(4):182-9. [PMC free article] [PubMed] [Google Scholar]
Roitberg 2003 {published data only}
- Roitberg B. A randomized trial of splinting vs. surgery for carpal tunnel syndrome. Surgical Neurology 2003;59(1):5-6. [DOI] [PubMed] [Google Scholar]
Ruksen 2011 {published data only}
- Ruksen S, Oz B, Olmez N, Memis A. Comparison of clinical effectiveness of corticosteroid phonophoresis and local steroid injection treatment in carpal tunnel syndrome. Turkiye Fiziksel Tip ve Rehabilitasyon Dergisi 2011;57(3):119-23. [Google Scholar]
Salehi 2019 {published data only}
- Salehi S, Hesami O, Esfehani MP, Khosravi S, Rashed A, Haghighatzadeh M, et al. The effectiveness of exercise therapy and dry needling on wrist range of motion, pinch and grip force in carpal tunnel syndrome: a randomized clinical trial. Asian Journal of Sports Medicine 2019;10:e83927. [Google Scholar]
Setayesh 2017 {published data only}
- IRCT2014112120023N1. Comparison of therapeutic effects of linseed oil topical gel, with wrist splint in carpal tunnel syndrome. who.int/trialsearch/Trial2.aspx?TrialID=IRCT2014112120023N1 (first received 12 May 2015).
- Setayesh M, Sadeghifar AR, Nakhaee N, Kamalinejad M, Rezaeizadeh H. A topical gel from flax seed oil compared with hand splint in carpal tunnel syndrome: a randomized clinical trial. Journal of Evidence-Based Complementary & Alternative Medicine 2017;22(3):462-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
Šošić 2020 {published data only}
- Šošić L, Bojnec V, Lonzarić D, Jesenšek Papež B. An advanced stage of carpal tunnel syndrome - is night-time splinting still effective? International Journal of Occupational Medicine and Environmental Health 2020;33(6):771-80. [DOI] [PubMed] [Google Scholar]
Soyupek 2012 {published data only}
- Soyupek F, Yesildag A, Kutluhan S, Askin A, Ozden A, Uslusoy GA, et al. Determining the effectiveness of various treatment modalities in carpal tunnel syndrome by ultrasonography and comparing ultrasonographic findings with other outcomes. Rheumatology International 2012;32(10):3229-34. [DOI] [PubMed] [Google Scholar]
Storey 2013 {published data only}
- ISRCTN26618585. Prospective clinical trial comparing C-TracTM splint with conventional resting splint for treatment of carpal tunnel syndrome. who.int/trialsearch/Trial2.aspx?TrialID=ISRCTN26618585 (first received 22 June 2006).
- Storey P, Armstrong D, Dear H, Bradley M, Burke F. Pilot randomised controlled trial comparing C-Trac splints with Beta wrist braces for the management of carpal tunnel syndrome. Hand Therapy 2013;18(2):35-41. [Google Scholar]
Talebi 2013 {published data only}
- IRCT138903094052N1. The effect of physiotherapy on symptoms of nerve entrapment syndrome. who.int/trialsearch/Trial2.aspx?TrialID=IRCT138903094052N1 (first received 18 Oct 2008).
- Talebi GA, Eteraf Oskouei MA, Shakouri SK. The effect of neuromobilization on clinical findings in patients with carpal tunnel syndrome. Journal of Rehabilitation 2013;13(3):74-83. [Google Scholar]
Toopchizadeh 2020 {published data only}
- Toopchizadeh V, Karimnia S, Sadat BE, Jahanjoo F, Pezeshki MZ. Effects of forearm myofascial trigger point dry needling on pain and function of patients with carpal tunnel syndrome. Crescent Journal of Medical and Biological Sciences 2020;7(3):362–7. [Google Scholar]
Ucan 2006 {published data only}
- Ucan H, Yagci I, Yilmaz L, Yagnurku F, Keskin D, Bodur H. Comparison of splinting, splinting plus local steroid injection and open carpal tunnel release outcomes in idiopathic carpal tunnel syndrome. Rheumatology International 2006;27:45-51. [DOI] [PubMed] [Google Scholar]
- Yağci I, Uçan H, Yilmaz L, Yağmurlu F, Keskin D, Bodur H. Comparison of splinting, splinting plus local steroid injection and surgery in carpal tunnel syndrome treatment. Turkish Journal of Physical Medicine and Rehabilitation 2006;52(2):55-60. [Google Scholar]
Weintraub 2000 {published data only}
- Weintraub MI, Cole SP. Neuromagnetic treatment of pain in refractory carpal tunnel syndrome: an electrophysiological and placebo analysis. Journal of Back and Musculoskeletal Rehabilitation 2000;15(2-3):77-81. [DOI] [PubMed] [Google Scholar]
Weng 2016 {published data only}
- Weng C, Dong H, Chu H, Lu Z. Clinical and electrophysiological evaluation of neutral wrist nocturnal splinting in patients with carpal tunnel syndrome. Journal of Physical Therapy Science 2016;28(8):2274-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
Yao 2019 {published data only}
- Yao Y, Grandy E, Jenkins L, Hou J, Evans PJ, Seitz WH Jr, et al. Changes of median nerve conduction, cross-sectional area and mobility by radioulnar wrist compression intervention in patients with carpal tunnel syndrome. Journal of Orthopaedic Translation 2019;18:13-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
Zhang 2018 {published data only}
- Zhang K, Yang Y, Xu S, Shou Y, Jiang H, Zhang B. A case control study on the treatment of carpal tunnel syndrome with needle Dao. China Journal of Orthopaedics and Traumatology 2018;31(6):497-9. [DOI] [PubMed] [Google Scholar]
Zinnuroglu 2010 {published data only}
- Zinnuroglu M, Baspinar M, Beyazova M. Carpal lock and the volar supporting orthosis in mild and moderate carpal tunnel syndrome. American Journal of Physical Medicine and Rehabilitation 2010;89(9):759-64. [PMID: ] [DOI] [PubMed] [Google Scholar]
References to studies awaiting assessment
Baklaci 2015 {published data only}
- Baklaci K. Long-term effectiveness of physical therapy modalities in carpal tunnel syndrome. Internal Medicine Journal 2015;45(Suppl 2):12. [Google Scholar]
Bhuva 2019 {published data only}
- Bhuva VD, Jagtap V. Effect of customized splint and soft tissue mobilization exercises in carpal tunnel syndrome. Indian Journal of Physiotherapy & Occupational Therapy 2019;13(3):121-6. [Google Scholar]
IRCT2014020416485N1 {published data only}
- IRCT2014020416485N1. Therapeutic effect of ultrasound-guided local steroid injection with hydro-dissection in carpal tunnel syndrome. who.int/trialsearch/Trial2.aspx?TrialID=IRCT2014020416485N1 (first received 4 May 2014).
ISRCTN22916517 {published data only}
- ISRCTN22916517. Comparison of wrist splints and steroid injection for carpal tunnel syndrome. who.int/trialsearch/Trial2.aspx?TrialID=ISRCTN22916517 (first received 12 September 2003).
Riasi 2015 {published data only}
- Riasi H, Rajabpour Sanati A, Salehi F, Salehian H, Ghaemi K. Analyzing the therapeutic effects of short wrist splint in patients with carpal tunnel syndrome (CTS) under ibuprofen treatment from an EMG-NCV perspective. Journal of Medicine and Life 2015;8(Special Issue 4):154-8. [PMC free article] [PubMed] [Google Scholar]
Soon 2015 {published data only}
- ACTRN12613000140752. A randomised clinical trial of physiotherapy, therapeutic ultrasound and hand splinting for carpal tunnel syndrome. who.int/trialsearch/Trial2.aspx?TrialID=ACTRN12613000140752 (first received 6 February 2013).
- Soon B, Vicenzino B, Plumbe L, Coppieters M. Randomised clinical trial on efficacy of combining hand splinting with physiotherapy or ultrasound treatment for patients with carpal tunnel syndrome. Physiotherapy (United Kingdom) 2015;101:eS1422-eS3. [Google Scholar]
References to ongoing studies
Atroshi 2019 {published data only}
- Atroshi I, Tadjerbashi K, McCabe SJ, Ranstam J. Treatment of carpal tunnel syndrome with wrist splinting: study protocol for a randomized placebo-controlled trial. Trials 2019;20(1):531. [DOI: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
- ISRCTN81836603. Evaluation of wrist splinting for the treatment of carpal tunnel syndrome. who.int/trialsearch/Trial2.aspx?TrialID=ISRCTN81836603 (first received 2 May 2018).
IRCT20120716010297N5 {published data only}
- IRCT20120716010297N5. The long-term effect of low level laser therapy on carpal tunnel syndrome. who.int/trialsearch/Trial2.aspx?TrialID=IRCT20120716010297N5 (first received 20 June 2018).
IRCT20200219046552N1 {published data only}
- IRCT20200219046552N1. Comparison of local corticosteroid injection and high-intensity laser outcomes in moderate carpal tunnel syndrome (CTS). who.int/trialsearch/Trial2.aspx?TrialID=IRCT20200219046552N1 (first received 29 March 2020).
JPRN‐UMIN000017952 {published data only}
- JPRN-UMIN000017952. Nerve gliding exercise for carpal tunnel syndrome. who.int/trialsearch/Trial2.aspx?TrialID=JPRN-UMIN000017952 (first received 1 July 2015).
NCT04017390 {published data only}
- NCT04017390. The effect of theraworx foam in carpal tunnel syndrome. clinicaltrials.gov/show/NCT04017390 (first received 12 July 2019).
NCT04515966 {published data only}
- NCT04515966. A comparison of ultrasound-guided steroid injection with wrist splint in carpal tunnel syndrome. clinicaltrials.gov/show/NCT04515966 (first received 17 August 2020).
NCT04993703 {published data only}
- NCT04993703. Effect of kinesiotaping and night splinting in patients with carpal tunnel syndrome. clinicaltrials.gov/ct2/show/NCT04993703 (first received 6 August 2021).
Additional references
Ashworth 2010
- Ashworth N. Carpal tunnel syndrome. Clinical Evidence 2010;2010:1114. [PMID: ] [PMC free article] [PubMed] [Google Scholar]
Atroshi 1999
- Atroshi I, Gummesson C, Johnsson R, Ornstein E, Ranstam J, Rosén I. Prevalence of carpal tunnel syndrome in a general population. JAMA 1999;282(2):153-8. [PMID: ] [DOI] [PubMed] [Google Scholar]
Braun 1989
- Braun RM, Davidson K, Doehr S. Provocative testing in the diagnosis of dynamic carpal tunnel syndrome. Journal of Hand Surgery (American Volume) 1989;14(2 Pt 1):195-7. [PMID: ] [DOI] [PubMed] [Google Scholar]
Charles 2009
- Charles J, Fahridin S, Britt H. Carpal tunnel syndrome. Australian Family Physician 2009;38(9):665. [PMID: ] [PubMed] [Google Scholar]
Choi 2018
- Choi GH, Wieland LS, Lee H, Sim H, Lee MS, Shin BC. Acupuncture and related interventions for the treatment of symptoms associated with carpal tunnel syndrome. Cochrane Database of Systematic Reviews 2018, Issue 12. Art. No: CD011215. [DOI: 10.1002/14651858.CD011215.pub2] [DOI] [PMC free article] [PubMed] [Google Scholar]
Concannon 2000
- Concannon MJ, Brownfield ML, Puckett CL. The incidence of recurrence after endoscopic carpal tunnel release. Plastic and Reconstructive Surgery 2000;105(5):1662-5. [PMID: ] [DOI] [PubMed] [Google Scholar]
Dale 2013
- Dale AM, Harris-Adamson C, Rempel D, Gerr F, Hegmann K, Silverstein B, et al. Prevalence and incidence of carpal tunnel syndrome in US working populations: pooled analysis of six prospective studies. Scandinavian Journal of Work, Environment, and Health 2013;39(5):495-505. [DOI: 10.5271/sjweh.3351] [PMID: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
Deeks 2021
- Deeks JJ, Higgins JP, Altman DG (editors). Chapter 10: Analysing data and undertaking meta-analyses. In: Higgins JP, Thomas J, Chandler J, Cumpston M, Li T, Page MJ, et al, editor(s). Cochrane Handbook for Systematic Reviews of Interventions version 6.2 (updated February 2021). Cochrane, 2021. Available from www.training.cochrane.org/handbook.
De Kleermaeker 2018
- De Kleermaeker FG, Boogaarts HD, Meulstee J, Verhagen WI. Minimal clinically important difference for the Boston Carpal Tunnel Questionnaire: new insights and review of literature. Journal of Hand Surgery, European Volume 2018;44(3):283-9. [DOI] [PubMed] [Google Scholar]
Dong 2020
- Dong C, Sun Y, Qi Y, Zhu Y, Wei H, Wu D, et al. Effect of platelet-rich plasma injection on mild or moderate carpal tunnel syndrome: an updated systematic review and meta-analysis of randomized controlled trials. BioMed Research International 2020;2020:5089378. [DOI: 10.1155/2020/5089378] [DOI] [PMC free article] [PubMed] [Google Scholar]
Dwan 2008
- Dwan K, Altman DG, Arnaiz JA, Bloom J, Chan AW, Cronin E, et al. Systematic review of the empirical evidence of study publication bias and outcome reporting bias. PLOS One 2008;3(8):e3081. [PMID: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
Dwan 2011
- Dwan K, Altman DG, Cresswell L, Blundell M, Gamble CL, Williamson PR. Comparison of protocols and registry entries to published reports for randomised controlled trials. Cochrane Database of Systematic Reviews 2011, Issue 1. Art. No: MR000031. [DOI: 10.1002/14651858.MR000031.pub2] [DOI] [PMC free article] [PubMed] [Google Scholar]
Gabrielli 2020
- Gabrielli AS, Lesiak AC, Fowler JR. The direct and indirect costs to society of carpal tunnel release. Hand 2020;15(2):NP26-30. [DOI: 10.117/1558944718810855] [DOI] [PMC free article] [PubMed] [Google Scholar]
Gelberman 1984
- Gelberman RH, Szabo RM, Mortenson MM. Carpal tunnel pressures and wrist position in patients with Colles fractures. Journal of Trauma, Injury, Infection and Critical Care 1984;24(8):747-9. [PMID: ] [DOI] [PubMed] [Google Scholar]
Gerritsen 2002b
- Gerritsen AA, De Krom MC, Struijs MA, Scholten RJ, De Vet HC, Bouter LM. Conservative treatment options for carpal tunnel syndrome: a systematic review of randomised controlled trials. Journal of Neurology 2002;249(3):272-80. [PMID: ] [DOI] [PubMed] [Google Scholar]
Goodyear‐Smith 2004
- Goodyear-Smith F, Arroll B. What can family physicians offer patients with carpal tunnel syndrome other than surgery? A systematic review of nonsurgical management. Annals of Family Medicine 2004;2(3):267-73. [PMID: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
GRADEpro GDT 2021 [Computer program]
- GRADEpro Guideline Development Tool. McMaster University and Evidence Prime, 2021. Available from gradepro.org.
Graham 2006
- Graham B, Regehr G, Naglie G, Wright JG. Development and validation of diagnostic criteria for carpal tunnel syndrome. Journal of Hand Surgery 2006;31(6):919e924. [PubMed] [Google Scholar]
Hardoim 2009
- Hardoim DG, De Oliveira GB, Kouyoumdjian JA. Carpal tunnel syndrome: long-term nerve conduction studies in 261 hands. Arquivos de Neuro-Psiquiatria 2009;67(1):69-73. [DOI] [PubMed] [Google Scholar]
Higgins 2002
- Higgins JP, Thompson SG. Quantifying heterogeneity in meta-analysis. Statistics in Medicine 2002;21(11):1539-58. [DOI] [PubMed] [Google Scholar]
Higgins 2011
- Higgins JP, Altman DG, Sterne JA. Chapter 8: Assessing risk of bias in included studies. In: Higgins JP, Green S, editor(s). Cochrane Handbook for Systematic Reviews of Interventions Version 5.1.0 (updated March 2011). The Cochrane Collaboration, 2011. Available from training.cochrane.org/handbook/archive/v5.1.
Higgins 2021
- Higgins JP, Thomas J, Chandler J, Cumpston M, Li T, Page MJ, et al (editors). Cochrane Handbook for Systematic Reviews of Interventions version 6.2 (updated February 2021). Cochrane, 2021. Available from www.training.cochrane.org/handbook.
Huisstede 2010
- Huisstede BM, Hoogvliet P, Randsdorp MS, Glerum S, Van Middelkoop M, Koes BW. Carpal tunnel syndrome. Part 1: Effectiveness of nonsurgical treatments - a systematic review. Archives of Physical Medicine and Rehabilitation 2010;91(7):981-1004. [PMID: ] [DOI] [PubMed] [Google Scholar]
Jablecki 2002
- Jablecki CK, Andary MT, Floeter MK, Miller RG, Quartly CA, Vennix MJ, et al, American Association of Electrodiagnostic Medicine, American Academy of Neurology, American Academy of Physical Medicine and Rehabilitation. Practice parameter: electrodiagnostic studies in carpal tunnel syndrome. Report of the American Association of Electrodiagnostic Medicine, American Academy of Neurology, and the American Academy of Physical Medicine and Rehabilitation. Neurology 2002;58(11):1589-92. [PMID: ] [DOI] [PubMed] [Google Scholar]
Kase 1998
- Kase K, Hashimoto T, Okane T. Kinesio Perfect Taping Manual: Amazing Taping Therapy to Eliminate Pain and Muscle Disorders. Albuquerque, NM, USA : Kinesio Taping Association, 1998. [Google Scholar]
Keir 2005
- Keir P, Rempel D. Pathomechanics of peripheral nerve loading. Journal of Hand Therapy 2005;18(2):259-69. [PMID: ] [DOI] [PubMed] [Google Scholar]
Kim 2013
- Kim JK, Jeon SH. Minimal clinically important differences in the Carpal Tunnel Questionnaire after carpal tunnel release. Journal of Hand Surgery. European Volume 2013;38(1):75-9. [DOI] [PubMed] [Google Scholar]
Leite 2006
- Leite JC, Jerosch-Herold C, Song F. A systematic review of the psychometric properties of the Boston Carpal Tunnel Questionnaire. BMC Musculoskeletal Disorders 2006;7:78. [DOI] [PMC free article] [PubMed] [Google Scholar]
Levine 1993
- Levine DW, Simmons BP, Koris MJ, Daltroy LH, Hohl GG, Fossel AH, et al. A self-administered questionnaire for the assessment of severity of symptoms and functional status in carpal tunnel syndrome. Journal of Bone and Joint Surgery. American Volume 1993;75(11):1585-92. [DOI] [PubMed] [Google Scholar]
Marshall 2007
- Marshall SC, Tardif G, Ashworth NL. Local corticosteroid injection for carpal tunnel syndrome. Cochrane Database of Systematic Reviews 2007, Issue 2. Art. No: CD001554. [DOI: 10.1002/14651858.CD001554.pub2] [DOI] [PubMed] [Google Scholar]
Moustgaard 2020
- Moustgaard H, Clayton GL, Jones HE, Boutron I, Jørgensen L, Laursen DRT, et al. Impact of blinding on estimated treatment effects in randomised clinical trials: meta-epidemiological study. BMJ 2020;368:l6802. [DOI] [PMC free article] [PubMed] [Google Scholar]
Muller 2004
- Muller M, Tsui D, Schnurr R, Biddulph-Deisroth, Hard J, MacDermid J. Effectiveness of hand therapy interventions in primary management of carpal tunnel syndrome: a systematic review. Journal of Hand Therapy 2004;17(2):210-28. [PMID: ] [DOI] [PubMed] [Google Scholar]
Multanen 2020
- Multanen J, Ylinen J, Karjalainen T, Ikonen J, Häkkinen A, Repo JP. Structural validity of the Boston Carpal Tunnel Questionnaire and its short version, the 6-Item CTS symptoms scale: a Rasch analysis one year after surgery. BMC Musculoskeletal Disorders 2020;21(1):609. [DOI] [PMC free article] [PubMed] [Google Scholar]
O'Connor 2012
- O’Connor D, Page MJ, Marshall SC, Massy-Westropp N. Ergonomic positioning or equipment for treating carpal tunnel syndrome. Cochrane Database of Systematic Reviews 2012, Issue 1. Art. No: CD009600. [DOI: 10.1002/14651858.CD009600] [DOI] [PMC free article] [PubMed] [Google Scholar]
Ono 2010
- Ono S, Clapham PJ, Chung KC. Optimal management of carpal tunnel syndrome. International Journal of General Medicine 2010;3:255-61. [PMID: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
Ostergaard 2020
- Ostergaard PJ, Meyer MA, Earp BE. Non-operative treatment of carpal tunnel syndrome. Current Reviews in Musculoskeletal Medicine 2020;13(2):141-7. [PMID: 10.1007/s12178-020-09616-0] [DOI] [PMC free article] [PubMed] [Google Scholar]
Padua 1997
- Padua L, LoMonaco M, Gregori B, Valente EM, Padua R, Tonali P. Neurophysiological classification and sensitivity in 500 carpal tunnel syndrome hands. Acta Neurologica Scandinavica 1997;96(4):211–7. [DOI: 10.1111/j.1600-0404.1997.tb00271.x] [DOI] [PubMed] [Google Scholar]
Padua 1999
- Padua L, Padua R, Lo Monaco M, Aprile I, Tonali P. Multiperspective assessment of carpal tunnel syndrome: a multicenter study. Italian CTS Study Group. Neurology 1999;53(8):1654-9. [DOI] [PubMed] [Google Scholar]
Padua 2016
- Padua L, Coraci D, Erra C, Pazzaglia C, Paolasso I, Loreti C, et al. Carpal tunnel syndrome: clinical features, diagnosis, and management. Lancet Neurology 2016;15(12):1273-84. [DOI: 10.1016/S1474-4422(16)30231-9] [PMID: ] [DOI] [PubMed] [Google Scholar]
Page 2012a
- Page MJ, O'Connor D, Pitt V, Massy‐Westropp N. Exercise and mobilisation interventions for carpal tunnel syndrome. Cochrane Database of Systematic Reviews 2012, Issue 6. Art. No: CD009899. [DOI: 10.1002/14651858.CD009899] [DOI] [PubMed] [Google Scholar]
Page 2013
- Page MJ, O'Connor D, Pitt V, Massy-Westropp N. Therapeutic ultrasound for carpal tunnel syndrome. Cochrane Database of Systematic Reviews 2013, Issue 3. Art. No: CD009601. [DOI: 10.1002/14651858.CD009601.pub2] [DOI] [PMC free article] [PubMed] [Google Scholar]
Page 2021
- Page MJ, Higgins JP, Sterne JA. Chapter 13: Assessing risk of bias due to missing results in a synthesis. In:Higgins JPT, Thomas J, Chandler J, Cumpston M, Li T, Page MJ, et al, editor(s). Cochrane Handbook for Systematic Reviews of Interventions version 6.2 (updated February 2021). Cochrane, 2021. Available from www.training.cochrane.org/handbook.
Palmar 2007
- Palmer K, Harris C, Coggon D. Carpal tunnel syndrome and its relation to occupation: a systematic literature review. Occupational Medicine 2007;57(1):57-66. [DOI] [PubMed] [Google Scholar]
Piazzini 2007
- Piazzini DB, Aprile I, Ferrara PE, Bertolini C, Tonali P, Maggi L, et al. A systematic review of conservative treatment of carpal tunnel syndrome. Clinical Rehabilitation 2007;21(4):299-314. [DOI] [PubMed] [Google Scholar]
Pourmemari 2018
- Pourmemari MH, Heliövaara M, Viikari-Juntura E, Shiri R. Carpal tunnel release: lifetime prevalence, annual incidence, and risk factors. Muscle & Nerve 2018;58(4):497-502. [DOI] [PubMed] [Google Scholar]
Rankin 2017
- Rankin IA, Sargean H, Rehman H, Selvan Gurusamy K. Low-level laser therapy for carpal tunnel syndrome. Cochrane Database of Systematic Reviews 2017, Issue 8. Art. No: CD012765. [DOI: 10.1002/14651858.CD012765] [DOI] [PMC free article] [PubMed] [Google Scholar]
Resende 2003
- Resende LA, Tahara A, Fonseca RG, Sardenberg T. The natural history of carpal tunnel syndrome: a study of 20 hands evaluated 4 to 9 years after initial diagnosis. Electromyography and Clinical Neurophysiology 2003;43(5):301-4. [PubMed] [Google Scholar]
Review Manager 2020 [Computer program]
- Review Manager. Version 5.4. The Cochrane Collaboration, 2020.
Savovic 2018
- Savovic J, Turner RM, Mawdsley D, Jones HE, Beynon R, Higgins JP, et al. Association between risk-of-bias assessments and results of randomized trials in Cochrane reviews: the ROBES meta-epidemiologic study. American Journal of Epidemiology 2018;187(5):1113-22. [DOI] [PMC free article] [PubMed] [Google Scholar]
Scholten 2007
- Scholten RJ, Van der Molen AM, Uitdehaag BM, Bouter LM, De Vet HC. Surgical treatment options for carpal tunnel syndrome. Cochrane Database of Systematic Reviews 2007, Issue 4. Art. No: CD003905. [DOI: 10.1002/14651858.CD003905.pub3] [DOI] [PMC free article] [PubMed] [Google Scholar]
Schrijver 2005
- Schrijver HM, Gerritsen AA, Strijers RL, Uitdehaag BM, Scholten RJ, De Vet Henrica CW, et al. Correlating nerve conduction studies and clinical outcome measures on carpal tunnel syndrome: lessons from a randomized controlled trial. Journal of Clinical Neurophysiology 2005;22(3):216-21. [PubMed] [Google Scholar]
Schünemann 2017
- Schünemann HJ, Oxman AD, Vist GE, Higgins JP, Deeks JJ, Glasziou P, et al, Cochrane Applicability and Recommendations Methods Group. Chapter 12: Interpreting results and drawing conclusions. In: Higgins JPT, Green S, editor(s). Cochrane Handbook for Systematic Reviews of Interventions Version 5.1.0 (updated June 2017). The Cochrane Collaboration, 2017. Available from: training.cochrane.org/handbook/archive/v5.1.
Smith 2008
- Smith J, Wisniewski SJ, Finnoff JT, Payne JM. Sonographically guided carpal tunnel injections: the ulnar approach. Journal of Ultrasound in Medicine 2008;27(10):1485-90. [DOI] [PubMed] [Google Scholar]
Szabo 1992
- Szabo RM, Madison M. Carpal tunnel syndrome. Orthopedic Clinics of North America 1992;23(1):103-9. [MEDLINE: ] [PubMed] [Google Scholar]
Szabo 1994
- Szabo RM, Steinberg DR. Nerve entrapment syndromes in the wrist. Journal of the American Academy of Orthopedic Surgeons 1994;2(2):115-23. [DOI] [PubMed] [Google Scholar]
U.S. Bureau of Labor Statistics 2016
- US Bureau of Labor Statistics. Nonfatal occupational injuries and illnesses requiring days away from work. www.bls.gov/news.release/pdf/osh2.pdf (accessed 1 September 2022).
Verdugo 2008
- Verdugo RJ, Salinas RS, Castillo J, Cea JG. Surgical versus non-surgical treatment for carpal tunnel syndrome. Cochrane Database of Systematic Reviews 2008, Issue 4. Art. No: CD001552. [DOI: 10.1002/14651858.CD001552.pub2] [DOI] [PMC free article] [PubMed] [Google Scholar]
Walters 2005
- Walters SJ, Brazier JE. Comparison of the minimally important difference for two health state utility measures: EQ-5D and SF-6D. Quality of Life Research 2005;6:1523-32. [DOI: 10.1007/s11136-004-7713-0] [DOI] [PubMed] [Google Scholar]
Wehbe 1985
- Wehbe MA, Hunter JM. Flexor tendon gliding in the hand. Part II. Differential gliding. Journal of Hand Surgery 1985;10(4):575–9. [DOI] [PubMed] [Google Scholar]
Williams 1992
- Williams TM, Mackinnon SE, Novak CB, McCabe S, Kelly L. Verification of the pressure provocative test in carpal tunnel syndrome. Annals of Plastic Surgery 1992;29(1):8-11. [DOI: 10.1097/00000637-199207000-00003] [DOI] [PubMed] [Google Scholar]
References to other published versions of this review
O'Connor 2003
- O'Connor D, Marshall S, Massy-Westropp N. Non-surgical treatment (other than steroid injection) for carpal tunnel syndrome. Cochrane Database of Systematic Reviews 2003, Issue 1. Art. No: CD003219. [DOI: 10.1002/14651858.CD003219] [DOI] [PMC free article] [PubMed] [Google Scholar]
Page 2012b
- Page MJ, Massy-Westropp N, O'Connor D, Pitt V. Splinting for carpal tunnel syndrome. Cochrane Database of Systematic Reviews 2012, Issue 7. Art. No: CD010003. [DOI: 10.1002/14651858.CD010003] [DOI] [PMC free article] [PubMed] [Google Scholar]


