Abstract
Background
Management of Scedosporium/Lomentospora prolificans infections remains challenging. We described predisposing factors, clinical manifestations, and outcomes of these rare mold infections, including predictors of early (1-month) and late (18-month) all-cause mortality and treatment failure.
Methods
We conducted a retrospective Australian-based observational study of proven/probable Scedosporium/L prolificans infections from 2005 to 2021. Data on patient comorbidities, predisposing factors, clinical manifestations, treatment, and outcomes up to 18 months were collected. Treatment responses and death causality were adjudicated. Subgroup analyses, multivariable Cox regression, and logistic regression were performed.
Results
Of 61 infection episodes, 37 (60.7%) were attributable to L prolificans. Forty-five of 61 (73.8%) were proven invasive fungal diseases (IFDs), and 29 of 61 (47.5%) were disseminated. Prolonged neutropenia and receipt of immunosuppressant agents were documented in 27 of 61 (44.3%) and 49 of 61 (80.3%) episodes, respectively. Voriconazole/terbinafine was administered in 30 of 31 (96.8%) L prolificans infections, and voriconazole alone was prescribed for 15 of 24 (62.5%) Scedosporium spp infections. Adjunctive surgery was performed in 27 of 61 (44.3%) episodes. Median time to death post–IFD diagnosis was 9.0 days, and only 22 of 61 (36.1%) attained treatment success at 18 months. Those who survived beyond 28 days of antifungal therapy were less immunosuppressed with fewer disseminated infections (both P < .001). Disseminated infection and hematopoietic stem cell transplant were associated with increased early and late mortality rates. Adjunctive surgery was associated with lower early and late mortality rates by 84.0% and 72.0%, respectively, and decreased odds of 1-month treatment failure by 87.0%.
Conclusions
Outcomes associated with Scedosporium/L prolificans infections is poor, particularly with L prolificans infections or in the highly immunosuppressed population.
Keywords: lomentosporiosis, outcomes, scedosporiosis, survivors, treatment response
Higher rates of mortality and treatment failure were observed in Lomentospora prolificans infections or in the highly immunosuppressed patients with hematological malignancy or hematopoietic stem cell transplant. Adjunctive surgery was associated with lower mortality and decreased odds of 1-month treatment failure.
Difficult-to-treat Scedosporium spp and Lomentospora prolificans infections mostly affect patients with hematological malignancy (HM), hematopoietic stem cell transplant (HSCT), or solid organ transplant (SOT). Prognosis is poor as these fungi have innate resistance to many, if not all, common antifungal agents [1, 2]. In addition to increased mortality (63.3%), Scedosporium/L prolificans infections are associated with prolonged hospital stay and excess cost [3].
The advent of novel antifungal agents, with improved in vitro and in vivo activity against Scedosporium spp and L prolificans, offers promise [4–7]; however, optimal use of these novel agents remains to be determined. Understanding the infection characteristics of patients who survive beyond 28 days of antifungal therapy can assist patient management. However, none of the few large observational studies [8–12] have evaluated treatment responses of Scedosporium/L prolificans infections beyond 1 year. Conducting a randomized controlled trial to establish evidence for treating Scedosporium/L prolificans infections remains challenging given the low case numbers and heterogeneity of patient populations. This study characterized the predisposing factors, clinical manifestations, and outcomes of Scedosporium/L prolificans infections, particularly in those who survived beyond 28 days of antifungal therapy. The predictors of early and late all-cause mortality and of treatment failure at 1 month following antifungal therapy were determined.
METHODS
A retrospective, 6-center, observational study of proven/probable Scedosporium/L prolificans infections diagnosed between January 2005 and April 2021 was conducted in Australia. Episodes of infection were identified from pathology (microbiology and histopathology) information systems at each hospital. Fungi were identified using standard morphological and phenotypic methods, and species were assigned by matrix-assisted laser desorption/ionization–time of flight mass spectrometry and/or sequencing of the fungal internal transcribed spacer regions [13]. Clinical data were retrieved from medical and pharmacy records.
Each infection episode was reviewed by an expert panel of 3 infectious diseases physicians for study inclusion. Episodes with incomplete medical and pharmacy records where follow-up data were not available, episodes considered to be colonized, or involving patients who had participated in the open-label phase 2b olorofim study (NCT03583164) were excluded. Data collected included patient demographics, underlying conditions, site of infection, causative fungus (Scedosporium spp, L prolificans), fungal copathogens, predisposing factors in the past 30–90 days, antifungal treatment, adjunctive therapies, survival status, and overall treatment response at the time-points of 1, 3, 6, 9, 12, 15, and 18 months. Follow-up was for 18 months or until death or loss to follow-up. Ethics approval was obtained for all hospitals (HREC/63020/PMCC).
Definitions
Definitions for proven/probable invasive fungal disease (IFD) were described according to the updated European Organisation for Research and Treatment of Cancer/Mycoses Study Group Education and Research Consortium (EORTC/MSGERC) [14], with modification for immunocompetent patients where host-specific criteria were not required for classification of probable IFD [15]. For cardiothoracic transplant recipients, proven/probable IFD was assigned according to International Society of Heart and Lung Transplantation definitions [16]. Infection was disseminated if blood cultures were positive or where ≥2 noncontiguous sites were involved. Day of IFD diagnosis was the day of collection of the clinical specimen yielding Scedosporium/L prolificans. The expert panel adjudicated cause of death [17] and antifungal treatment response up to 18 months according to the EORTC/MSGERC categories [18] (Supplementary Table 1).
Statistical Analyses
All analyses were performed using SPSS 28.0 software (IBM Corporation). Analysis was undertaken for all episodes of infection and then stratified into the following subgroups for analyses: (1) causative pathogen (Scedosporium spp, L prolificans); (2) survival beyond 28 days of antifungal therapy (survivor, nonsurvivor); and (3) immunosuppression level (high [eg, HM/HSCT], moderate [eg, SOT, immunosuppressed for reasons other than HM/HSCT and SOT], or low/no [eg, minor/no medical condition]). A χ2 or Fisher exact test was used to compare categorical variables and Wilcoxon rank-sum or Kruskal-Wallis test for continuous variables. Cumulative survival rate post–IFD diagnosis was estimated using the Kaplan-Meier method. Heat maps were generated using Microsoft Word 2016 software to depict overall treatment response of each infection episode. Univariable and multivariable Cox proportional hazard regression analyses were performed for early (1-month) and late (18-month) mortality. The predictors of 1-month treatment failure were determined using multivariate logistic regression. The significance level was set at P < .05.
RESULTS
Overall Analysis
Sixty-seven episodes of proven/probable of Scedosporium/L prolificans infections were identified in 65 patients. Five episodes were in the open-label phase 2b olorofim study and data for 1 were incomplete; hence, 61 episodes (60 patients) were evaluable (Table 1), of which 57 (93.4%) were managed as inpatients. The median patient age was 59.0 years and 63.9% of episodes were in males. Common underlying conditions were HM (62.3%), HSCT (27.9%), and diabetes mellitus (26.2%). Receipt of immunosuppressive therapies (80.3%) and prolonged neutropenia (44.3%) were the main predisposing factors. Forty-five of 61 (73.8%) episodes were proven IFD, of which 29 (47.5%) were disseminated. Lomentospora prolificans accounted for 60.7% of episodes (n = 37). Of the 24 episodes of Scedosporium spp infections, 20 were due to Scedosporium apiospermum complex and the remainder were Scedosporium aurantiacum.
Table 1.
Clinical and Infection Characteristics of All Episodes of Infections (N = 61)
| Variable | Overall (N = 61)a |
Stratified by Causative Fungal Pathogen | Stratified by Survival Status Beyond 28 d of Antifungal Therapy | ||||
|---|---|---|---|---|---|---|---|
| Lomentospora prolificans (n = 37) |
Scedosporium sppb (n = 24) |
P Valuec | Survivor (n = 34) | Nonsurvivor (n = 27) | P Valued | ||
| Age, y, median (range) | 59.0 (21.0–91.0) | 59.0 (21.0–75.0) | 60.0 (23.0–91.0) | .154 | 59.5 (23.0–91.0) | 59.0 (21.0–75.0) | .299 |
| Male sex | 39 (63.9) | 27 (73.0) | 12 (50.0) | .068 | 19 (55.9) | 20 (74.1) | .142 |
| Primary diagnosise,f | |||||||
| HM/disorderg | 38 (62.3) | 30 (81.1) | 8 (33.3) | <.001 | 12 (35.3) | 26 (96.3) | <.001 |
| Leukemia | 26 (42.6) | 22 (59.5) | 4 (16.7) | .001 | 8 (23.5) | 18 (66.7) | <.001 |
| HSCT | 17 (27.9) | 13 (35.1) | 4 (16.7) | .116 | 5 (14.7) | 12 (44.4) | .010 |
| Allogeneic | 14 (23.0) | 11 (29.7) | 3 (12.5) | .118 | 4 (11.8) | 10 (37.0) | .020 |
| Autologous | 3 (4.9) | 2 (5.4) | 1 (4.2) | 1.000 | 1 (2.9) | 2 (7.4) | .579 |
| SOT | 9 (14.8) | 3 (8.1) | 6 (25.0) | .136 | 8 (23.5) | 1 (3.7) | .036 |
| Renal transplant | 5 (8.2) | 0 | 5 (20.8) | .007 | 5 (14.7) | 0 | .060 |
| Cardiothoracic transplanth | 4 (6.6) | 3 (8.1) | 1 (4.2) | 1.000 | 3 (8.8) | 1 (3.7) | .623 |
| Immunosuppressedi | 2 (3.3) | 0 | 2 (8.3) | .151 | 2 (5.9) | 0 | .498 |
| Other malignancy | 1 (1.6) | 1 (2.7) | 0 | 1.000 | 1 (2.9) | 0 | 1.000 |
| Other comorbiditiese,j | |||||||
| Diabetes | 16 (26.2) | 7 (18.9) | 9 (37.5) | .107 | 11 (32.4) | 5 (18.5) | .222 |
| Chronic renal disease | 8 (13.1) | 1 (2.7) | 7 (29.2) | .005 | 7 (20.6) | 1 (3.7) | .066 |
| Chronic lung disease | 4 (6.6) | 3 (8.1) | 1 (4.2) | 1.000 | 2 (5.9) | 2 (7.4) | 1.000 |
| Chronic liver disease | 2 (3.3) | 0 | 2 (8.3) | .151 | 2 (5.9) | 0 | .498 |
| Predisposing factorse,k | |||||||
| Baseline neutropenial | 30 (49.2) | 26 (70.3) | 4 (16.7) | <.001 | 5 (14.7) | 25 (92.6) | <.001 |
| Duration of neutropenia, d, median (range)l | 23.5 (1.0–174.0) | 24.0 (1.0–174.0) | 18.5 (13.0–43.0) | .659 | 20.0 (13.0–43.0) | 24.0 (1.0–174.0) | .957 |
| Prolonged neutropeniam | 27 (44.3) | 23 (62.2) | 4 (16.7) | <.001 | 5 (14.7) | 22 (81.5) | <.001 |
| Trauma/open injury | 2 (3.3) | 0 | 2 (8.3) | .151 | 2 (5.9) | 0 | .498 |
| Surgery | 9 (14.8) | 5 (13.5) | 4 (16.7) | .729 | 6 (17.6) | 3 (11.1) | .718 |
| Receipt of immunosuppressive agentse,k | 49 (80.3) | 34 (91.9) | 15 (62.5) | .008 | 22 (64.7) | 27 (100.0) | <.001 |
| Prolonged corticosteroid usen | 14 (23.0) | 8 (21.6) | 6 (25.0) | 1.000 | 5 (14.7) | 9 (33.3) | .251 |
| Chemotherapy | 26 (42.6) | 22 (59.5) | 4 (16.7) | .001 | 7 (20.6) | 19 (70.4) | <.001 |
| Calcineurin inhibitors | 20 (32.8) | 12 (32.4) | 8 (33.3) | .942 | 10 (29.4) | 10 (37.0) | .529 |
| Monoclonal antibodies | 6 (9.8) | 2 (5.4) | 4 (16.7) | .200 | 4 (11.8) | 2 (7.4) | .685 |
| Protein kinase inhibitors | 3 (4.9) | 3 (8.1) | 0 | .272 | 1 (2.9) | 2 (7.4) | .579 |
| Site of infectione,o | |||||||
| Disseminated infection | 29 (47.5) | 27 (73.0) | 2 (8.3) | <.001 | 6 (17.6) | 23 (85.2) | <.001 |
| Blood | 23 (37.7) | 23 (62.2) | 0 | <.001 | 1 (2.9) | 22 (81.5) | <.001 |
| CNS | 13 (21.3) | 11 (29.7) | 2 (8.3) | .046 | 3 (8.8) | 10 (37.0) | .008 |
| Lung | 38 (62.3) | 29 (78.4) | 9 (37.5) | .001 | 14 (41.2) | 24 (88.9) | <.001 |
| Skin/soft tissue infections | 14 (23.0) | 5 (13.5) | 9 (37.5) | .030 | 10 (29.4) | 4 (14.8) | .178 |
| Other proven/probable IFDe,p | 7 (11.5) | 6 (16.2) | 1 (4.2) | .229 | 2 (5.9) | 5 (18.5) | .224 |
Data are presented as No. (%) unless otherwise indicated.
Abbreviations: CNS, central nervous system; HM, hematological malignancy; HSCT, hematopoietic stem cell transplant; IFD, invasive fungal disease; SOT, solid organ transplant.
One patient had 3 episodes of infection, in which the second episode of infection was excluded due to incomplete records.
Scedosporium apiospermum complex (n = 20) and Scedosporium aurantiacum (n = 4).
P value denotes comparison analyses (Pearson χ2 or Fisher exact test or Wilcoxon rank-sum test) between the subgroups of causative fungal pathogen.
P value denotes comparison analyses (Pearson χ2 or Fisher exact test or Wilcoxon rank-sum test) between the subgroups of survivors and nonsurvivors.
Some may have >1 encounter; not mutually exclusive.
No known host or risk factors for IFD (n = 11).
Other HM/disorders included lymphoma, multiple myeloma, aplastic anemia, and myelodysplastic syndrome (n = 3 each). No significant P values for all comparison analyses.
Cardiothoracic transplant included lung (n = 2), heart (n = 1), and heart-lung (n = 1) transplant. No significant P values for all comparison analyses.
Other immunosuppressed included microscopic polyarteritis and antiphospholipid syndrome (n = 1 each).
Diabetes or chronic renal/lung/liver disease as comorbidities.
Thirty days prior to IFD diagnosis except for the receipt of immunosuppressive agents (90 days prior) and systemic corticosteroids (60 days prior).
Missing data: n = 1.
Defined as absolute neutrophil count <500 cells/µL for >10 days.
Defined as the use of ≥0.3 mg/kg corticosteroids for ≥3 weeks in the past 60 days); missing data: n = 4.
Other sites of infection (eg, heart, kidneys, gastrointestinal, liver, spleen, spine, bone, joint, wound, eye, ear, nasal/sinus, vessels, catheter/prosthetic materials, glands) have relatively small sample size (n < 7) and no significant differences were found between the subgroups stratified by causative fungal pathogen or survival status beyond 28 days of antifungal therapy (P > .05). Sites of infection (eg, kidneys, bone) had small sample size (n < 7), but significant differences were found between the subgroups of survivors and nonsurvivors (P < .05).
Aspergillosis (n = 4), candidemia (n = 3), or mucormycosis (n = 1).
Only 55 episodes of infection received targeted antifungals as 6 deaths occurred prior to the IFD diagnosis. Adjunctive surgery was performed in 27 of 61 episodes (44.3%), with debridement the most common 17 of 61 episodes (27.9%) (Supplementary Table 2). Deaths occurred in 29 episodes at 1-month follow-up; 26 were due to Scedosporium/L prolificans infections (89.7%), translating into an IFD-attributable mortality of 42.6%. Median time to death post–IFD diagnosis was 9.0 (range, 0.0–433.0) days. Treatment success at 18 months was 36.1% (22 episodes).
Subgroup Analyses
Causative Pathogen
Infection episodes due to Scedosporium spp were more common in patients with renal transplant (P = .007) and chronic renal disease (P = .005), whereas L prolificans infection was more common in patients with leukemia (P = .001) (Table 1). Prolonged neutropenia (P < .001), receipt of concomitant immunosuppressant therapy (P = .008), and chemotherapy (P = .001) were associated with L prolificans infections, as were disseminated infections (P < .001), involving blood (P < .001), lung (P = .001), and the central nervous system (CNS) (P = .046) (Table 1). Gastrointestinal tract, adrenal/thyroid, heart, kidney, liver, and spleen involvement only occurred in disseminated L prolificans infections while 2 disseminated Scedosporium spp infections involved the CNS, lung, spine, skin/soft tissue, and large vessels (eg, aortic graft). Scedosporium spp were more likely to affect skin/soft tissue (P = .030; Table 1). For L prolificans infections, voriconazole/terbinafine combination therapy was administered in 30 of 31 episodes (96.8%), while voriconazole monotherapy was prescribed in 15 of 24 (62.5%) Scedosporium spp infections. The median duration of antifungal treatment was shorter for L prolificans, compared with Scedosporium spp infections (median, 10.5 [range, 1.0–585.0] days vs 156.0 [range, 8.0–585.0] days; P < .001) as the majority of those with L prolificans infections were dead at 1 month (n = 26/37 [70.3%]). Adjunctive surgery was more common in Scedosporium spp infections (18/24 [75.0%] vs 9/37 [24.3%]; P < .001).
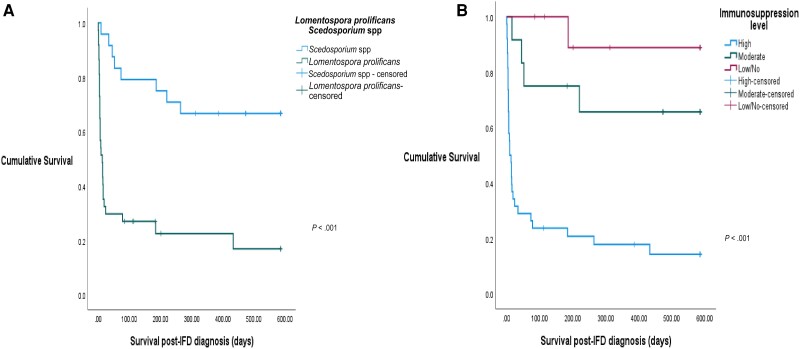
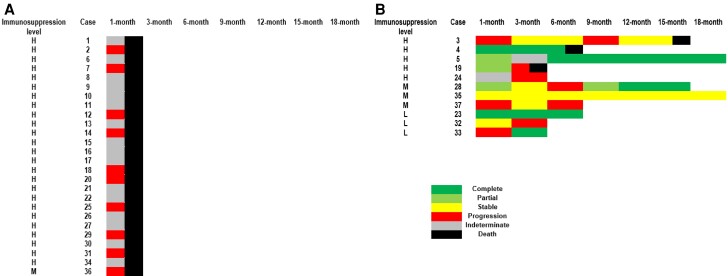
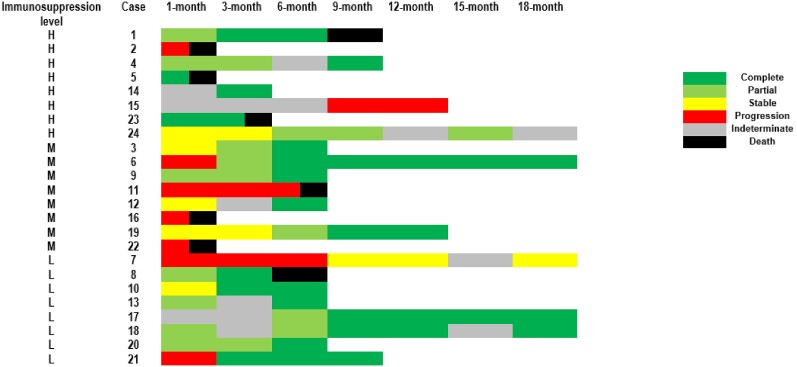
A lower survival rate was noted in L prolificans infections (Figure 1A) where the median time to death post–IFD diagnosis was 6.0 (range, 0.0–433.0) days, shorter than those with Scedosporium spp infections (median, 62.5 [range, 9.0–264.0] days; P < .001). Only 5 L prolificans infections (n = 5/37 [13.5%]) were treated successfully at 1 month and 18 months. At 18-month follow-up, 2 had stable L prolificans infections, 14 had progression, and 16 had indeterminate responses; of these, 29 died and 3 were lost to follow-up (Figure 2Aand 2B). More Scedosporium spp infections (n = 9/24 [37.5%]) were deemed to have treatment success at 1 month. At 18-month follow-up, 17 attained treatment success (n = 17/24 [70.8%]), 1 had stable Scedosporium spp infection, 5 had progression, and 1 had indeterminate response; of these, 8 died and 1 was lost to follow-up (Figure 3).
Figure 1.
Survival curves stratified by causative fungal pathogen (A) and immunosuppression level (B). Abbreviation: IFD, invasive fungal disease.
Figure 2.
Heat maps depicting overall treatment response up to 18-month follow-up after initiation of antifungal treatment for the nonsurvivor subgroup with Lomentospora prolificans infection (n = 26; A) and the survivor subgroup with L prolificans infection (n = 11; B). Cases 24, 32, and 37 were lost to follow-up. A cutoff of ±1.5 months around each follow-up time point was used to allow for the fact that follow-up visits may not be conducted at exactly each time point given the retrospective study design. Immunosuppression level: high (H; eg, hematological malignancy [HM], hematopoietic stem cell transplant [HSCT]); moderate (M; eg, solid organ transplant [SOT], immunosuppressed for reasons other than HM/HSCT and SOT); or low/no (L; eg, minor/no medical condition).
Figure 3.
Heat maps depicting overall treatment response up to 18-month follow-up after initiation of antifungal treatment for those with Scedosporium spp infections (n = 24). Case 15 was lost to follow-up. A cutoff of ±1.5 months around each follow-up time point was used to allow for the fact that follow-up visits may not be conducted at exactly each time point given the retrospective study design. Only case 2 did not survive beyond 28 days of antifungal therapy. Immunosuppression level: high (H; eg, hematological malignancy [HM], hematopoietic stem cell transplant [HSCT]); moderate (M; eg, solid organ transplant [SOT], immunosuppressed for reasons other than HM/HSCT and SOT); or low/no (L; eg, minor/no medical condition).
Survival Following 28 Days of Antifungal Therapy
Compared with episodes involving nonsurvivors, survivors were less immunosuppressed, with lower proportions of HSCT (P = .010), HM, prolonged neutropenia, and chemotherapy (all P < .001) (Table 1). There were more Scedosporium spp infections among survivors (n = 23/34 [67.6%]) compared with nonsurvivors (n = 1/27 [3.7%]) (P < .001). A smaller proportion of survivors had disseminated infections (P < .001; Table 1). Antifungal treatment duration (median, 166.0 [range, 32.0–585.0] days vs 5.0 [range, 1.0–18.0] days) and time to death post–IFD diagnosis (median, 131.0 [range, 34.0–433.0] days vs 6.0 [range, 0.0–24.0] days) were longer among survivors than nonsurvivors (both P < .001). A higher proportion of the survivors (n = 25/34 [73.5%]) received adjunctive surgery compared with nonsurvivors (n = 2/27 [7.4%]) (P < .001). All nonsurvivors failed treatment, whereas 58.8% (n = 20/34) of the survivors had treatment failure at 1-month follow-up (P < .001; Figures 2 and 3).
Degree of Immunosuppression
Compared with those not considered highly immunosuppressed (Supplementary Table 3), prolonged neutropenia and immunosuppressive treatments were more common in infection episodes affecting highly immunosuppressed patients (both P < .001). Most infections in the highly immunosuppressed subgroup were disseminated (P < .001), with L prolificans more frequently isolated (n = 30/38 [78.9%]) (P < .001). The duration of antifungal treatment was shorter (median, 10.5 [range, 1.0–585.0] days) in the highly immunosuppressed subgroup (P < .001), and the survival rate was reduced (P < .001) (Figure 1B), with a median time to death post–IFD diagnosis of 7.0 (range, 0.0–433.0) days. Up to 82% of the highly immunosuppressed subgroup failed treatment by 1-month follow-up (n = 31/38) (Figures 2 and 3). All of those with low/no immunosuppression received adjunctive surgery.
Predictors of Mortality and Treatment Failure
Disseminated infection and HSCT were associated with 6.87 times (hazard ratio [HR], 6.87 [95% confidence interval {CI}, 2.63–17.96]) and 2.17 times (HR, 2.17 [95% CI, 1.01–4.66]) the rate of 1-month mortality adjusting for adjunctive surgery (Table 2). Receipt of adjunctive surgery was associated with an 84.0% reduction (HR, 0.16 [95% CI, .05–.48]) in the rate of 1-month mortality following Scedosporium/L prolificans infections, adjusting for disseminated infection and HSCT. The same predictors were observed for 18-month mortality when adjusted for causative pathogen (Table 2). Receipt of adjunctive surgery was associated with an 87.0% reduction (odds ratio, 0.13 [95% CI, .02–.77]) in the odds of treatment failure by 1-month follow-up, adjusting for disseminated infection, HM, and causative pathogen (Supplementary Table 4).
Table 2.
Predictors of 1-Month (Early) and 18-Month (Late) All-Cause Mortality Following Diagnosis of Invasive Scedosporium and Lomentospora prolificans Infections
| Characteristic | 1-mo (Early) | 18-mo (Late) | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Univariable Analysis | Multivariable Analysis | Univariable Analysis | Multivariable Analysis | |||||||||
| HR | (95% CI) | P Value | HR | (95% CI) | P Value | HR | (95% CI) | P Value | HR | (95% CI) | P Value | |
| Age | 0.98 | (.96–1.00) | .117 | … | … | 0.99 | (.97–1.01) | .339 | … | … | ||
| Male sex | 1.51 | (.69–3.32) | .306 | … | … | 1.50 | (.75–3.00) | .247 | … | … | ||
| Year of infectiona | 0.76 | (.31–1.86) | .546 | … | … | 0.86 | (.38–1.96) | .716 | … | … | ||
| Underlying condition/risk factors | ||||||||||||
| HM | 14.11 | (3.33–59.73) | <.001 | … | … | 8.02 | (3.09–20.81) | <.001 | … | … | ||
| HSCT | 0.56 | (.39–.81) | .002 | 2.17 | (1.01–4.66) | .046 | 2.85 | (1.47–5.53) | .002 | 2.03 | (1.01–4.10) | .047 |
| SOT | 0.31 | (.07–1.31) | .111 | … | … | 0.34 | (.11–1.12) | .077 | … | … | ||
| Prolonged neutropenia | 10.15 | (4.05–25.43) | <.001 | … | … | 8.39 | (3.99–17.63) | <.001 | … | … | ||
| Trauma | 0.05 | (.00–128.65) | .448 | … | … | 0.05 | (.00–29.07) | .348 | … | … | ||
| Surgery | 0.55 | (.17–1.81) | .321 | … | … | 0.37 | (.12–1.22) | .103 | … | … | ||
| Prolonged corticosteroid use | 1.11 | (.53–2.36) | .780 | … | … | 1.39 | (.67–2.88) | .383 | … | … | ||
| Cancer chemotherapy | 5.08 | (2.29–11.27) | <.001 | … | … | 4.57 | (2.31–9.06) | <.001 | … | … | ||
| Calcineurin inhibitors | 1.30 | (.62–2.76) | .490 | … | … | 1.02 | (.51–2.02) | .965 | … | … | ||
| Monoclonal antibodies | 1.16 | (.35–3.83) | .810 | … | … | 1.26 | (.44–3.55) | .668 | … | … | ||
| Protein kinase inhibitors | 1.91 | (.45–8.04) | .378 | … | … | 1.44 | (.35–6.00) | .616 | … | … | ||
| Site of infection/coinfection | ||||||||||||
| Disseminated infection | 8.38 | (3.36–20.90) | <.001 | 6.87 | (2.63–17.96) | <.001 | 6.52 | (3.14–13.56) | <.001 | 5.11 | (2.13–12.29) | <.001 |
| Blood | 18.27 | (7.22–46.19) | <.001 | … | … | 17.80 | (7.54–41.99) | <.001 | … | … | ||
| CNS | 2.68 | (1.24–5.80) | .012 | … | … | 2.44 | (1.20–5.00) | .014 | … | … | ||
| Lung | 8.26 | (2.49–27.42) | <.001 | … | … | 4.56 | (1.99–10.45) | <.001 | … | … | ||
| Other proven/probable IFD | 2.17 | (.83–5.69) | .116 | … | … | 2.05 | (.85–4.92) | .109 | … | … | ||
| Receipt of treatment | ||||||||||||
| Combination antifungal therapy | 1.44 | (.63–3.25) | .386 | … | … | 1.26 | (.63–2.51) | .518 | … | … | ||
| Adjunctive surgery | 0.11 | (.04–.31) | <.001 | 0.16 | (.05–.48) | .001 | 0.20 | (.09–.42) | <.001 | 0.28 | (.12–.67) | .004 |
| Causative fungal pathogen | ||||||||||||
| Lomentospora prolificans | 9.59 | (2.88–31.93) | <.001 | … | … | 5.15 | (2.30–11.53) | <.001 | 1.90 | (.70–5.15) | .208 | |
| Scedosporium spp | 0.10 | (.03–.35) | <.001 | … | … | 0.19 | (.09–.44) | <.001 | … | … | ||
Cox regression model: One variable from each category/domain (ie, underlying condition/risk factor, site of infection, receipt of treatment, causative fungal pathogen) in the univariable analysis was chosen based on either the narrower CI or clinical grounds. The number of variables included in each adjusted model was based on a minimum of 10 outcomes per explanatory variable to avoid overfitting.
Abbreviations: CI, confidence interval; CNS, central nervous system; HM, hematological malignancy; HR, hazard ratio; HSCT, hematopoietic stem cell transplant; IFD, invasive fungal disease; SOT, solid organ transplant.
Category of year of infection (ie, 2005–2010 vs 2011–2021).
DISCUSSION
Both Scedosporium spp and L prolificans infections are associated with high morbidity and mortality [9, 10, 19–21]. Including only proven/probable infections, we found substantive differences in the clinical manifestations, patient risk populations, and outcomes for these 2 fungal pathogen groups. While differences in the epidemiology and features of Scedosporium/L prolificans infections are appreciated [9, 10], regional and even institutional-specific data are essential to inform timely management decisions. For the first time, our study provided a longitudinal view of adjudicated treatment response of each infection episode at regular intervals (ie, 1, 3, 6, 9, 12, 15, and 18 months). These data, in addition to those comparing the survivor and nonsurvivor subgroups, are keys to guide optimal use of both current and newer antifungals.
Notably, the predominance of L prolificans over Scedosporium spp infections in our study contrasts with surveys performed elsewhere [10–12]. This is due at least in part to geographical differences in species distribution, which may depend on local environmental (eg, soil pH) or climatic conditions [1, 22, 23], further consolidating the results of an earlier Australian study [24]. Interestingly, organ involvement outside the CNS and lung was reported only in disseminated L prolificans infections. Disseminated scedosporiosis has been described [10, 25], but our study observed more localized and indolent skin/soft tissue infections with Scedosporium spp. While distinct clinical presentations between S apiospermum and Scedosporium boydii were reported in a large French observational study [12], we are unable to confirm this as the different species within the S apiospermum complex were not further differentiated given the retrospective nature of our study. The finding that L prolificans infections are often disseminated with a propensity to affect the lungs and CNS emphasizes the need for urgent investigation for infection elsewhere, as fungemia progresses rapidly [8–12, 26]. Expedited collection of blood cultures/tissue specimens, adoption of molecular techniques [13], and multisystem imaging are essential to timely identify disseminated infections. Siderophores may also have an emerging role as an early diagnostic biomarker in IFD [27], which merits future investigations.
Importantly, we have shown that the clinical manifestations, predisposing factors, treatment modalities, and outcomes of the survivors differed significantly from the nonsurvivors, and these have not been extensively studied before. Appreciation of these characteristics and outcomes of patients who survived beyond 28 days of antifungal therapy provides insights on identifying optimal time of initiation and treatment duration of antifungal agents. The survivor subgroup was less immunosuppressed and had lower proportions of HM/HSCT patients with prolonged neutropenia and receipt of immunosuppressive agents, particularly chemotherapy. This could explain the predominance of Scedosporium spp infections, with relatively lower numbers of disseminated disease with blood, CNS, or lung involvements as well as lower mortality and failure rates observed in the survivors. We also noted that up to 74.0% of the survivors had adjunctive surgery, with a longer median time to death of 131.0 days post–IFD diagnosis reported, consistent with previous observations that adjunctive surgery confers improved survival [9, 11, 19]. Indeed, for the first time, we have demonstrated that adjunctive surgery was associated with a decreased odds of treatment failure at 1-month follow-up.
Our study has provided insights that stratifying patients according to degree of immunosuppression is useful for clinical management and predicting survival. The poor outcomes observed in infections among the most immunosuppressed subgroup (HM/HSCT patients), combined with the fact that L prolificans was the main causative species, highlights the critical need for early detection/diagnosis of these subgroups of infections. We observed greater mortality in the highly immunosuppressed and L prolificans subgroups where HSCT and disseminated infection were predictors of both early and late mortality (Table 2). Disseminated infection with fungemia, CNS involvement, and HM are associated with increased mortality in Scedosporium/L prolificans infections [9, 10, 12, 19–21]. Apart from host immunosuppression, site of infection, and delayed diagnosis, these fatal outcomes are in part due to limited treatment options. Currently available antifungal agents exhibit high minimum inhibitory concentrations against L prolificans in particular [2, 10, 28]. Hence, there is an unmet need for novel antifungal agents that have excellent activity against these fungi.
A key finding of our study is the higher rate of treatment failure in L prolificans infections compared with those caused by Scedosporium spp. The higher proportion of HM/HSCT patients with disseminated infections in the L prolificans subgroup is 1 likely explanation. While Jenks et al [9] reported that treatment failure rates were the highest in L prolificans cases with HM (81.0%) or with disseminated infections (84.0%), they defined those with stable disease as treatment success, in contrast with our approach guided by the EORTC/MSGERC [18]. The EORTC/MSGERC criteria may have limitations in classifying treatment response for certain Scedosporium/L prolificans infections, which tend to be chronic or slowly progressive (eg, osteomyelitis, sinusitis). Recent viewpoints have highlighted that stable disease may signal early control of IFDs awaiting immune recovery in immunocompromised hosts and is a reasonable therapeutic goal in the real-world setting [29]. Combined voriconazole/terbinafine therapy has been associated with improved survival and treatment success for L prolificans infections [8, 9, 28]; however, we did not observe this in our study. The majority of lomentosporiosis cases were treated with voriconazole/terbinafine for a median of only 10.5 days and died by 1 month (Figure 2), questioning the effectiveness of such therapy. Conversely, we noted that most Scedosporium spp infections responded to treatment between 3 and 6 months of follow-up (Figure 3), suggesting that prolonged antifungal therapy of at least 6 months may be warranted. This is pertinent as the recommendation regarding optimal treatment duration for scedosporiosis is not well-established [30].
This study of adult patients has several limitations, including its retrospective design that included a relatively smaller proportion of SOT recipients. As this study encompassed only 6 tertiary hospitals, the findings may not be generalizable elsewhere; however, all of these hospitals delivered care to patients with Scedosporium/L prolificans infections in a similar manner, with broad adherence to national management guidelines [31]. This study may have been underpowered to detect a difference where one might exist given the low case numbers that are commonly seen in studies of rare IFDs. Finally, analysis of Scedosporium spp infections was not further stratified to species level, and thus we were unable to determine if fungal species (eg, S aurantiacum, S boydii) within this group influenced outcomes.
Managing Scedosporium/L prolificans infections remains challenging given their complex clinical setting. We highlight the pressing need for better therapies, particularly for HM/HSCT patients or those with L prolificans infections. Our clinical data support the notion that stable disease may represent a successful response with these infections. In the less immunosuppressed or survivor subgroup, we noted that prolonged therapy of currently available antifungal agents is required, with slow responses and late failures observed. For new antifungal agents yet to be marketed, these granular data regarding differences in outcomes of Scedosporium/L prolificans infections and the impact of immunosuppression provide useful information for comparative effectiveness studies.
Supplementary Material
Contributor Information
Chin Fen Neoh, National Centre for Infections in Cancer, Peter MacCallum Cancer Centre, Melbourne, Australia; Department of Infectious Diseases, Peter MacCallum Cancer Centre, Melbourne, Australia; Sir Peter MacCallum Department of Oncology, University of Melbourne, Melbourne, Australia.
Sharon C A Chen, Centre for Infectious Diseases and Microbiology Laboratory Services, New South Wales Health Pathology, Westmead Hospital, Sydney, Australia; Faculty of Health Sciences, Sydney Medical School, University of Sydney, Sydney, Australia.
Amy Crowe, Department of Infectious Diseases, St Vincent's Hospital Melbourne, Melbourne, Australia.
Kate Hamilton, Centre for Infectious Diseases and Microbiology Laboratory Services, New South Wales Health Pathology, Westmead Hospital, Sydney, Australia.
Quoc A Nguyen, Department of Clinical Microbiology and Infectious Diseases, St Vincent's Hospital Sydney, Sydney, Australia; Kolling Institute, Faculty of Medicine and Health, University of Sydney and Northern Sydney Local Health District, Sydney, Australia.
Debbie Marriott, Department of Clinical Microbiology and Infectious Diseases, St Vincent's Hospital Sydney, Sydney, Australia.
Jason A Trubiano, National Centre for Infections in Cancer, Peter MacCallum Cancer Centre, Melbourne, Australia; Sir Peter MacCallum Department of Oncology, University of Melbourne, Melbourne, Australia; Department of Infectious Diseases, Austin Hospital, Melbourne, Australia.
Tim Spelman, Department of Health Services Research, Peter MacCallum Cancer Centre, Melbourne, Australia.
David C M Kong, National Centre for Antimicrobial Stewardship, Peter Doherty Institute for Infections and Immunity, Melbourne, Australia; Centre for Medicine Use and Safety, Monash Institute of Pharmaceutical Sciences, Faculty of Pharmacy and Pharmaceutical Sciences, Monash University, Melbourne, Australia; Pharmacy Department, Grampians Health–Ballarat, Melbourne, Australia; School of Medicine, Deakin University, Geelong, Australia.
Monica A Slavin, National Centre for Infections in Cancer, Peter MacCallum Cancer Centre, Melbourne, Australia; Department of Infectious Diseases, Peter MacCallum Cancer Centre, Melbourne, Australia; Sir Peter MacCallum Department of Oncology, University of Melbourne, Melbourne, Australia.
Supplementary Data
Supplementary materials are available at Open Forum Infectious Diseases online. Consisting of data provided by the authors to benefit the reader, the posted materials are not copyedited and are the sole responsibility of the authors, so questions or comments should be addressed to the corresponding author.
Notes
Author contributions. Study conceptualization and design: C. F. N., S. C.-A. C., D. C. M. K., and M. A. S. Data collection: C. F. N., S. C.-A. C., K. H., and Q. A, N. Adjudication of treatment outcomes: S. C.-A. C., A. C., and M. A. S. Provision of patients: S. C.-A. C., A. C., D. M., J. A. T., and M. A. S. Data analysis: C. F. N. with statistical advice from T. S. Drafted the manuscript: C. F. N. All authors reviewed and agreed to the final version of the manuscript.
Acknowledgments. We thank pathology services at all study sites (Peter MacCallum Pathology, Melbourne Health Shared Pathology Service, Austin Pathology, St Vincent's Pathology [Melbourne], New South Wales Health Pathology, and SydPath [St Vincent's Sydney Pathology]) for their support.
Patient consent. This study was approved by the Peter MacCallum Cancer Centre Human Research Ethics Committee under a national mutual acceptance scheme with a patient waiver of consent given the noninterventional, retrospective nature of the study design (HREC/63020/PMCC).
Disclaimer. The funder was not involved in the study design, data collection, data analysis, or manuscript preparation.
Financial support. This work was supported by F2G Ltd, Manchester, United Kingdom.
References
- 1. Ramirez-Garcia A, Pellon A, Rementeria A, et al. Scedosporium and Lomentospora: an updated overview of underrated opportunists. Med Mycol 2018; 56(Suppl 1):S102–25. [DOI] [PubMed] [Google Scholar]
- 2. Lackner M, de Hoog GS, Verweij PE, et al. Species-specific antifungal susceptibility patterns of Scedosporium and Pseudallescheria species. Antimicrob Agents Chemother 2012; 56:2635–42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Heng SC, Slavin MA, Chen SC, et al. Hospital costs, length of stay and mortality attributable to invasive scedosporiosis in haematology patients. J Antimicrob Chemother 2012; 67:2274–82. [DOI] [PubMed] [Google Scholar]
- 4. Biswas C, Law D, Birch M, et al. In vitro activity of the novel antifungal compound F901318 against Australian Scedosporium and Lomentospora fungi. Med Mycol 2018; 56:1050–4. [DOI] [PubMed] [Google Scholar]
- 5. Seyedmousavi S, Chang YC, Youn JH, et al. In vivo efficacy of olorofim against systemic scedosporiosis and lomentosporiosis. Antimicrob Agents Chemother 2021; 65:e0043421. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Alkhazraji S, Gebremariam T, Alqarihi A, et al. Fosmanogepix (APX001) is effective in the treatment of immunocompromised mice infected with invasive pulmonary scedosporiosis or disseminated fusariosis. Antimicrob Agents Chemother 2020; 64:e01735-19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Rivero-Menendez O, Cuenca-Estrella M, Alastruey-Izquierdo A. In vitro activity of APX001A against rare moulds using EUCAST and CLSI methodologies. J Antimicrob Chemother 2019; 74:1295–9. [DOI] [PubMed] [Google Scholar]
- 8. Jenks JD, Seidel D, Cornely OA, et al. Voriconazole plus terbinafine combination antifungal therapy for invasive Lomentospora prolificans infections: analysis of 41 patients from the FungiScope registry 2008–2019. Clin Microbiol Infect 2020; 26:784.e1–5. [DOI] [PubMed] [Google Scholar]
- 9. Jenks JD, Seidel D, Cornely OA, et al. Clinical characteristics and outcomes of invasive Lomentospora prolificans infections: analysis of patients in the FungiScope registry. Mycoses 2020; 63:437–42. [DOI] [PubMed] [Google Scholar]
- 10. Seidel D, Meissner A, Lackner M, et al. Prognostic factors in 264 adults with invasive Scedosporium spp. and Lomentospora prolificans infection reported in the literature and FungiScope. Crit Rev Microbiol 2019; 45:1–21. [DOI] [PubMed] [Google Scholar]
- 11. Seidel D, Hassler A, Salmanton-Garcia J, et al. Invasive Scedosporium spp. and Lomentospora prolificans infections in pediatric patients: analysis of 55 cases from FungiScope and the literature. Int J Infect Dis 2020; 92:114–22. [DOI] [PubMed] [Google Scholar]
- 12. Bronnimann D, Garcia-Hermoso D, Dromer F, Lanternier F; French Mycoses Study Group . Scedosporiosis/lomentosporiosis observational study (SOS): clinical significance of Scedosporium species identification. Med Mycol 2021; 59:486–97. [DOI] [PubMed] [Google Scholar]
- 13. Chen SCA, Halliday CL, Hoenigl M, Cornely OA, Meyer W. Scedosporium and Lomentospora infections: contemporary microbiological tools for the diagnosis of invasive disease. J Fungi (Basel) 2021; 7:23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Donnelly JP, Chen SC, Kauffman CA, et al. Revision and update of the consensus definitions of invasive fungal disease from the European Organization for Research and Treatment of Cancer and the Mycoses Study Group Education and Research Consortium. Clin Infect Dis 2020; 71:1367–76. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Slavin M, van Hal S, Sorrell TC, et al. Invasive infections due to filamentous fungi other than Aspergillus: epidemiology and determinants of mortality. Clin Microbiol Infect 2015; 21:490.e1–10. [DOI] [PubMed] [Google Scholar]
- 16. Husain S, Mooney ML, Danziger-Isakov L, et al. A 2010 working formulation for the standardization of definitions of infections in cardiothoracic transplant recipients. J Heart Lung Transplant 2011; 30:361–74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Wingard JR, Ribaud P, Schlamm HT, Herbrecht R. Changes in causes of death over time after treatment for invasive aspergillosis. Cancer 2008; 112:2309–12. [DOI] [PubMed] [Google Scholar]
- 18. Segal BH, Herbrecht R, Stevens DA, et al. Defining responses to therapy and study outcomes in clinical trials of invasive fungal diseases: Mycoses Study Group and European Organization for Research and Treatment of Cancer consensus criteria. Clin Infect Dis 2008; 47:674–83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Husain S, Munoz P, Forrest G, et al. Infections due to Scedosporium apiospermum and Scedosporium prolificans in transplant recipients: clinical characteristics and impact of antifungal agent therapy on outcome. Clin Infect Dis 2005; 40:89–99. [DOI] [PubMed] [Google Scholar]
- 20. Lamaris GA, Chamilos G, Lewis RE, Safdar A, Raad II, Kontoyiannis DP. Scedosporium infection in a tertiary care cancer center: a review of 25 cases from 1989–2006. Clin Infect Dis 2006; 43:1580–4. [DOI] [PubMed] [Google Scholar]
- 21. Alvarez-Uria A, Guinea JV, Escribano P, et al. Invasive Scedosporium and Lomentosora infections in the era of antifungal prophylaxis: a 20-year experience from a single centre in Spain [manuscript published online ahead of print 4 August 2020]. Mycoses 2020. 10.1111/myc.13154 [DOI] [PubMed] [Google Scholar]
- 22. Kaltseis J, Rainer J, De Hoog GS. Ecology of Pseudallescheria and Scedosporium species in human-dominated and natural environments and their distribution in clinical samples. Med Mycol 2009; 47:398–405. [DOI] [PubMed] [Google Scholar]
- 23. Harun A, Gilgado F, Chen SC, Meyer W. Abundance of Pseudallescheria/Scedosporium species in the Australian urban environment suggests a possible source for scedosporiosis including the colonization of airways in cystic fibrosis. Med Mycol 2010; 48:S70–76. [DOI] [PubMed] [Google Scholar]
- 24. Heath CH, Slavin MA, Sorrell TC, et al. Population-based surveillance for scedosporiosis in Australia: epidemiology, disease manifestations and emergence of Scedosporium aurantiacum infection. Clin Microbiol Infect 2009; 15:689–93. [DOI] [PubMed] [Google Scholar]
- 25. Rammaert B, Neoh ZCF, Chen SCA, Kong DCM, Slavin MA. Scedosporium and Lomentospora infections in lung transplant recipients. Curr Fungal Infect Rep 2021; 15:49–66. [Google Scholar]
- 26. Cobo F, Lara-Oya A, Rodriguez-Granger J, Sampedro A, Aliaga-Martinez L, Navarro-Mari JM. Infections caused by Scedosporium/Lomentospora species: clinical and microbiological findings in 21 cases. Med Mycol 2018; 56:917–25. [DOI] [PubMed] [Google Scholar]
- 27. Kriegl L, Havlicek V, Dichtl K, Egger M, Hoenigl M. Siderophores: a potential role as a diagnostic for invasive fungal disease. Curr Opin Infect Dis 2022; 35:485–92. [DOI] [PubMed] [Google Scholar]
- 28. Jenks JD, Reed SL, Seidel D, et al. Rare mould infections caused by Mucorales, Lomentospora prolificans and Fusarium, in San Diego, CA: the role of antifungal combination therapy. Int J Antimicrob Agents 2018; 52:706–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Slavin MA, Chen YC, Cordonnier C, et al. When to change treatment of acute invasive aspergillosis: an expert viewpoint. J Antimicrob Chemother 2021; 77:16–23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Hoenigl M, Salmanton-Garcia J, Walsh TJ, et al. Global guideline for the diagnosis and management of rare mould infections: an initiative of the European Confederation of Medical Mycology in cooperation with the International Society for Human and Animal Mycology and the American Society for Microbiology. Lancet Infect Dis 2021; 21:e246–57. [DOI] [PubMed] [Google Scholar]
- 31. Bupha-Intr O, Butters C, Reynolds G, et al. Consensus guidelines for the diagnosis and management of invasive fungal disease due to moulds other than Aspergillus in the haematology/oncology setting, 2021. Intern Med J 2021; 51(Suppl 7):177–219. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.