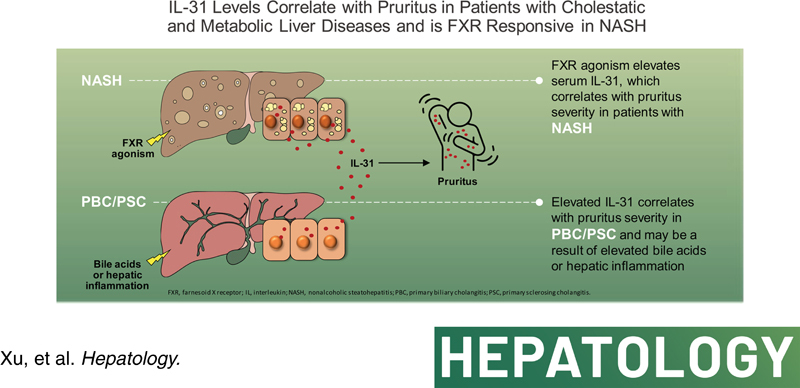
Background and Aims:
Pruritus is associated with multiple liver diseases, particularly those with cholestasis, but the mechanism remains incompletely understood. Our aim was to evaluate serum IL‐31 as a putative biomarker of pruritus in clinical trials of an farnesoid X receptor (FXR) agonist, cilofexor, in patients with NASH, primary sclerosing cholangitis (PSC), and primary biliary cholangitis (PBC).
Approach and Results:
Serum IL‐31 was measured in clinical studies of cilofexor in NASH, PSC, and PBC. In patients with PSC or PBC, baseline IL‐31 was elevated compared to patients with NASH and healthy volunteers (HVs). IL‐31 correlated with serum bile acids among patients with NASH, PBC, and PSC. Baseline IL‐31 levels in PSC and PBC were positively correlated with Visual Analog Scale for pruritus and 5‐D itch scores. In patients with NASH, cilofexor dose‐dependently increased IL‐31 from Week (W)1 to W24. In patients with NASH receiving cilofexor 100 mg, IL‐31 was higher in those with Grade 2–3 pruritus adverse events (AEs) than those with Grade 0–1 pruritus AEs. IL‐31 weakly correlated with C4 at baseline in patients with NASH, and among those receiving cilofexor 100 mg, changes in IL‐31 and C4 from baseline to W24 were negatively correlated. IL‐31 messenger RNA (mRNA) was elevated in hepatocytes from patients with PSC and NASH compared to HVs. In a humanized liver murine model, obeticholic acid increased IL‐31 mRNA expression in human hepatocytes and serum levels of human IL‐31.
Conclusions:
IL‐31 levels correlate with pruritus in patients with cholestatic disease and NASH, with FXR agonist therapy resulting in higher serum levels in the latter group. IL‐31 appears to derive in part from increased hepatocyte expression. These findings have therapeutic implications for patients with liver disease and pruritus.
INTRODUCTION
Pruritus is a poorly understood and debilitating feature of cholestatic liver diseases including primary sclerosing cholangitis (PSC) and primary biliary cholangitis (PBC). The pathobiology of cholestasis‐associated pruritus remains incompletely understood, but elevated serum bile acid (BA) levels may play a prominent role.1 Interestingly, although farnesoid X receptor (FXR) agonists effectively reduce BA levels in patients with PBC and PSC, increases in pruritus have been reported in patients treated with these therapies.2–5 These observations suggest that other potential pruritogens (e.g., autotaxin, individual BA species, others)6–8 may contribute both to the etiology of pruritus associated with cholestasis and FXR agonist therapy.
FXR is an intracellular ligand‐activated nuclear receptor expressed in intestinal epithelial cells and hepatocytes that functions as an enterohepatic regulator of BA homeostasis.9 Activation of FXR by small‐molecule agonists, or its natural ligand BA, in intestinal epithelial cells results in elevated expression and secretion of fibroblast growth factor (FGF)‐19 into the portal circulation.9,10 FGF‐19 binds to its cognate receptor, FGF receptor (FGFR) 4/β‐Klotho receptor complex, on hepatocytes and transcriptionally represses CYP7A1, the rate‐limiting enzyme for BA synthesis via a kinase mediated signaling cascade.10 Changes in circulating FGF‐19, or BA precursors like C4, are biomarkers for FXR activity. BA‐derived steroidal FXR agonists such as obeticholic acid (OCA) or fully synthetic nonsteroidal FXR agonists are approved therapies for PBC or under clinical evaluation in patients with NASH and PSC, respectively.11–16
Although pruritus is not typically associated with NASH, it is a common adverse event associated with FXR agonist therapy.11,13,14,16 In the phase 3 trial of OCA in NASH, 51% of patients treated with OCA 25 mg reported pruritus and 9% discontinued the study due to treatment‐related pruritus.16 Nonsteroidal small‐molecule FXR agonists have also been implicated in inducing pruritus,13,14,17 suggest that pruritus is a class effect of FXR agonism. However, the pruritogen associated with FXR agonist treatment has not been identified. As described above, FXR agonists regulate the BA synthesis pathway by either directly activating hepatocyte FXR or through the FGF‐19–FGFR 4/β‐Klotho receptor axis. Given that patients with NASH treated with the engineered variant of FGF‐19, aldafermin (previously NGM‐282), have not reported pruritus,18 we hypothesized that the pruritogen associated with FXR agonists may involve direct FXR agonism that is independent of FGF‐19 signaling.
IL‐31 is a known pruritogen and an established drug target in atopic dermatitis.19,20 IL‐31 and its receptor heterodimers, IL‐31 receptor A (IL‐31RA) and oncostatin M β, mediate itch signaling through sensory neurons in the dorsal root ganglion.21,22 Overexpression of IL‐31 in rodents or injection of IL‐31 in dogs, monkeys, or humans induces scratching behavior in animals and chronic itch sensation in humans.19,23–25 Nemolizumab, an anti–IL‐31RA antibody, significantly reduces pruritus severity and the sensation of itch in patients with atopic dermatitis.26,27 The link between IL‐31 and pruritus in patients with liver disease is not well described. In a study of patients with PBC, serum IL‐31 levels were positively correlated with platelet count and negatively correlated with the Fibrosis‐4 index.28 Pregnant women with intrahepatic cholestasis and pruritus have higher serum IL‐31 levels compared to control patients.29 However, the correlation of IL‐31 with cholestatic itch and the underlying mechanisms associated with FXR agonist therapy remain unclear.
The objective of this study was to evaluate serum IL‐31 as a putative biomarker of pruritus in clinical trials of an FXR agonist in patients with NASH, PBC, and PSC. This study investigated IL‐31 and associations between changes in serum IL‐31 and pruritus.
PATIENTS AND METHODS
Patient populations for clinical serum samples
Human serum samples obtained from five clinical studies of cilofexor were used in this analysis. Samples were taken from healthy volunteers (HVs; n = 60, baseline) who participated in a phase 1 study (NCT02654002)30 and in phase 2 placebo‐controlled studies of patients with noncirrhotic NASH (n = 140; NCT02854605),11 cirrhotic NASH (n = 20; baseline samples from cohorts 7 and 8 in NCT02781584), noncirrhotic PSC (n = 52; NCT02943460),12 and noncirrhotic PBC (n = 71; NCT02943447) (Figure S1). All the subjects provided appropriate consent for participating in the clinical study, and the study was conducted in accordance with the ethics and/or institutional review committees.
Commercially acquired liver biopsy samples for histology study
Liver biopsy samples used for messenger RNA (mRNA) in situ hybridization (ISH) experiments were acquired commercially from HVs (TriStar Technology Group) and patients with histologically confirmed NASH (BioIVT) and PSC (Discovery of Life Sciences). Demographic information for these commercially acquired samples is provided in Table S1.
Assays for serum cytokines
Serum IL‐31 and IL‐4 were detected by Quanterix Simoa HD‐1 analyzer, and single molecule array platforms were performed by Myriad RBM. Capture antibody conjugated paramagnetic beads were incubated with standards, samples, or controls and biotinylated detection antibodies. The beads were then washed and incubated with streptavidin‐ß‐galactosidase. After the final wash, the beads were loaded into the Simoa Disc with enzyme substrate, resorufin ß‐galactopyranoside. The concentration of IL‐31 or IL‐4 in each sample is interpolated from a standard curve. The detection limit for IL‐31 using the Quanterix platform was 0.06–0.089 pg/ml. The coefficient of variation for the same samples tested in different batches was 12% (interquartile range [IQR], 5, 25) with Spearman correlation of 0.98 (p < 0.001). Additional information on other commercially available enzyme‐linked immunosorbent assay kits for detecting IL‐31 and the Quanterix Simoa assay, including its sensitivity and specificity for samples in these experiments, is provided in the Supporting Materials.
IL‐31 mRNA expression and ISH
Hepatic IL‐31 (Thermo Fisher, assay ID: Hs01098710_m1) quantification was analyzed using a droplet digital polymerase chain reaction (ddPCR) assay in a QX200 ddPCR System (Bio‐Rad). This was a duplex assay with a housekeeping TATA‐box binding protein (TBP; Thermo Fisher, assay ID: Hs99999910_m1). The droplets were generated in a QX200 Droplet Generator (Bio‐Rad) according to the manufacturer's instructions. The relative IL‐31 expression was normalized to the TBP expression in the same well. IL‐31 ISH was performed by a standard RNA‐scope platform described previously using a commercial vendor (ACDBio).31 The IL‐31 ISH utilized Fast Red Dye to visualize the positive signal, and all slides were counterstained with hematoxylin for nuclear staining. The nuclei were detected by VisioPharm AI Nuclear Detection deep learning algorithm (Vision PHARM Hoersholm Denmark) at the whole slide level. IL‐31 positive nuclei were counted for each sample to calculate the percentage of IL‐31 positive nuclei (Figure S8).
Chimeric mouse with humanized liver
PXB mice with stable human hepatocyte engraftment in uPA severe combined immunodeficiency background were obtained from PhoenixBio. The human hepatocyte engraftment was assessed by the proportion of human albumin in the serum (>90%). PXB mice were administered OCA (10 mg/kg, BID) for 18 days, and livers and plasma were collected 2 h after the last dose for measurement of IL‐31 mRNA and protein.
Statistical analysis
Wilcoxon rank‐sum test was used to assess differences between groups in continuous variables, including serum IL‐31 levels according to the severity of pruritus (moderate to severe [Grade 2–3] vs. no or minor [Grade 0–1]) and treatment group (cilofexor 100 mg, 30 mg, vs. placebo). Associations between serum IL‐31, BAs, and autotaxin levels and a Visual Analog Scale (VAS) for pruritus and 5‐D itch scores were assessed using Spearman correlations (ρ). Information on imputation of missing biomarker data can be found in the Supporting Material.
RESULTS
Increased serum IL‐31 levels in patients with liver disease
IL‐31 levels were measured in baseline serum samples of HVs and patients with cirrhotic NASH and noncirrhotic NASH, PBC, and PSC who participated in clinical trials of cilofexor. Compared to HVs, serum IL‐31 levels were elevated in all groups of patients with liver disease. In NASH, median (IQR) serum IL‐31 levels were higher among patients with cirrhosis (n = 20) than among noncirrhotic patients (n = 52) (0.3 pg/ml vs. 0.09 pg/ml, p < 0.001; Figure 1A). (Figure 1A). Noncirrhotic patients with PBC and PSC had higher levels of IL‐31 compared to patients with cirrhotic and noncirrhotic NASH (PBC 1.7 pg/ml [0.8, 5.5]) and PSC (2.7 pg/ml [0.8, 5.9], both p < 0.001 versus cirrhotic and noncirrhotic NASH). Serum IL‐31 levels were significantly correlated with serum BA levels (ρ = 0.53, p < 0.001) in the overall NASH, PBC, and PSC population (Figure 1B) but not with other biomarkers of liver injury and fibrosis such as alanine aminotransferase, aspartate aminotransferase, or Enhanced Liver Fibrosis score (data not shown), suggesting that increased serum IL‐31 in patients with liver disease is predominantly related to the degree of cholestasis.
FIGURE 1.
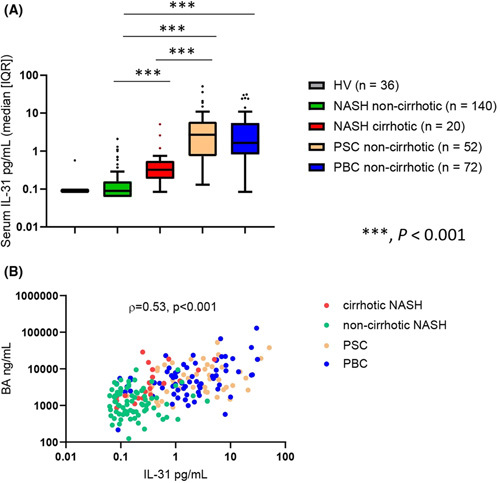
Serum IL‐31 levels in HVs and patients with NASH, PBC, or PSC. (A) Patients with noncirrhotic PBC and PSC had higher levels of serum IL‐31 than HVs or patients with NASH. (B) Serum IL‐31 levels were significantly correlated with BA levels among the patients with NASH, PBC and PSC. BA, bile acid; HV, healthy volunteer; IQR, interquartile range; PBC, primary biliary cholangitis; PSC, primary sclerosing cholangitis. ***p < 0.001.
Cilofexor increased serum IL‐31 in patients with NASH and correlated with pruritus
In the 24‐week phase 2 study of cilofexor in noncirrhotic NASH (NCT02854605), 8 of 56 (14%), 1 of 56 (2%), and 0 of 28 patients receiving cilofexor 100 mg, cilofexor 30 mg, or placebo, respectively, had Grade 2–3 pruritus (Table 1). Several potential pruritogens were explored in the study, including changes in serum BA concentration and composition, autotaxin, and IL‐31 levels. Baseline BA levels were not different between patients with Grade 2–3 pruritus compared to patients without pruritus at Weeks 1 or 4 (data not shown), and BA composition11 or autotaxin levels were not associated with cilofexor treatment (Figure S2). Cilofexor increased median serum IL‐31 in a dose‐dependent manner, with cilofexor 100 mg resulting in significantly higher serum IL‐31 levels compared to placebo as early as the first week (Figure 2A, p < 0.001, cilofexor 100 mg vs. placebo for all time points postbaseline). At Week (W)24, the cilofexor 100 mg group had significantly higher median IL‐31 levels (0.26 pg/ml [IQR, 0.13, 0.82] compared to the cilofexor 30 mg (0.15 pg/ml [0.062, 0.27], p = 0.003) and placebo groups (0.062 [0.062, 0.12], p < 0.001) (Table 2).
TABLE 1. Summary of pruritusa in a noncirrhotic NASH Phase 2 study.
| CILO 100 mg (n = 56) | CILO 30 mg (n = 56) | PBO (n = 28) | |
|---|---|---|---|
| Grade 1, n (%) | 8 (14) | 10 (18) | 5 (18) |
| Grade 2, n (%) | 7 (13) | 1 (2) | 0 |
| Grade 3, n (%) | 1 (2) | 0 | 0 |
| Any Grade, n (%) | 16 (29) | 11 (20) | 5 (18) |
Abbreviations: CILO, cilofexor; PBO, placebo.
Pruritus and pruritus generalized in the adverse event list.
FIGURE 2.
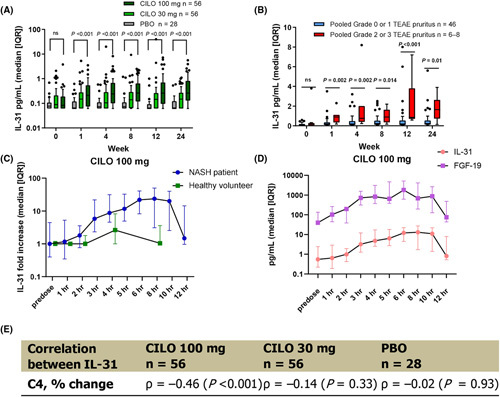
Serum IL‐31 levels in patients with NASH administered cilofexor. (A) CILO increased serum IL‐31 in a dose‐dependent manner. (B) Serum IL‐31 levels were significantly higher in patients with NASH receiving CILO 100 mg who had severe pruritus (Grades 2–3) compared to patients with none or mild pruritus (Grades 0–1). (C) Patients with NASH had greater increase of serum IL‐31 levels than healthy volunteers (HVs) after cilofexor administration. (D) The increase of serum IL‐31 had a temporal correlation with serum FGF‐19 changes in both HVs and patients with NASH. (E) Changes of serum IL‐31 and C4 from baseline to Week (W)24 in the cilofexor 100 mg group were significantly negatively correlated. CILO, cilofexor; FGF, fibroblast growth factor; IQR, interquartile range; ns, not significant; PBO, placebo; TEAE, treatment‐emergent adverse event.
TABLE 2. Serum IL‐31 levels in noncirrhotic NASH Phase 2 study.
| CILO 100 mg (n = 56) | CILO 30 mg (n = 56) | PBO (n = 28) | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Visit | n a | Median (IQR) | n a | Median (IQR) | n a | Median (IQR) | Trend p value | p value 100 mg vs. PBO | p value 30 mg vs. PBO | p value 100 mg vs. 30 mg |
| Baseline | 54 | 0.083 (0.062, 0.190) | 56 | 0.09 (0.062, 0.210) | 28 | 0.077 (0.062, 0.105) | 0.50 | 0.360 | 0.390 | 0.96 |
| Week 1 | 54 | 0.21 (0.076, 0.54) | 56 | 0.15 (0.062, 0.280) | 27 | 0.062 (0.062, 0.12) | <0.001 | <0.001 | 0.004 | 0.055 |
| Week 4 | 53 | 0.24 (0.11, 0.670) | 54 | 0.105 (0.062, 0.280) | 27 | 0.074 (0.062, 0.110) | <0.001 | <0.001 | 0.051 | 0.008 |
| Week 8 | 54 | 0.26 (0.12, 0.620) | 53 | 0.14 (0.067, 0.290) | 27 | 0.074 (0.062, 0.110) | <0.001 | <0.001 | 0.037 | 0.002 |
| Week 12 | 53 | 0.26 (0.097, 0.70) | 52 | 0.145 (0.062, 0.245) | 25 | 0.062 (0.062, 0.086) | <0.001 | <0.001 | 0.011 | 0.003 |
| Week 24 | 52 | 0.26 (0.13, 0.815) | 51 | 0.15 (0.062, 0.270) | 24 | 0.062 (0.062, 0.115) | <0.001 | <0.001 | 0.006 | 0.003 |
Abbreviations: CILO, cilofexor; IQR, interquartile range; PBO, placebo.
Patient numbers decrease over time either because patients discontinued the study or missed the sample collection period.
Among 56 patients with NASH in the cilofexor 100 mg group, 8 (14%) reported Grade 2 or 3 pruritus. At W24, serum IL‐31 levels in these patients were 7‐fold higher (1.7 pg/ml [0.92, 2.2]) than among patients with Grade 0–1 pruritus in this treatment group (0.24 pg/ml [0.12, 0.53], p = 0.010) (Table 3). Patients with Grade 2–3 pruritus had significantly higher serum IL‐31 serum levels than those with Grade 0–1 pruritus at all postbaseline time points and as early as the first week: W1 (5.1‐fold increase, p = 0.002), W4 (2.9‐fold increase, p = 0.005), W8 (3.8‐fold increase, p = 0.014), W12 (4.5‐fold increase, p < 0.001), and W24 (7‐fold increase, p = 0.01) (Figure 2B, Table 3). These data indicate that cilofexor dose‐dependently increased IL‐31 levels in patients with NASH, with this increase associated with higher grades of pruritus.
TABLE 3. Serum IL‐31 levels in patients with noncirrhotic NASH with pruritus treated with cilofexor 100 mg.
| Max Grade 0–1 pruritus (n = 48) | Max Grade 2–3 pruritus (n = 8) | ||||
|---|---|---|---|---|---|
| Visit | n a | Median (IQR) | n a | Median (IQR) | Wilcoxon p value |
| Baseline | 46 | 0.078 (0.062, 0.19) | 8 | 0.11 (0.062, 0.23) | 0.662 |
| Week 1 | 46 | 0.18 (0.071, 0.35) | 8 | 0.90 (0.37, 1.1) | 0.002 |
| Week 4 | 46 | 0.19 (0.084, 0.46) | 7 | 0.75 (0.4, 2) | 0.005 |
| Week 8 | 46 | 0.24 (0.097, 0.5) | 8 | 0.89 (0.475, 1.55) | 0.014 |
| Week 12 | 46 | 0.19 (0.076, 0.52) | 7 | 0.85 (0.7, 3.8) | <0.001 |
| Week 24 | 46 | 0.24 (0.12, 0.53) | 6 | 1.7 (0.92, 2.2) | 0.010 |
Abbreviation: IQR, interquartile range.
Patient numbers decrease over time either because patients discontinued the study or missed the sample collection period.
Cilofexor induced higher serum IL‐31 in patients with NASH compared to HVs
In the phase 1 study (NCT02654002), HVs were treated with cilofexor 30, 100, or 300 mg, and serum IL‐31 or FGF‐19 levels were assessed in predose and postdose (1–8 h) samples. A similar intensive sampling study was also performed in the study of cilofexor in noncirrhotic NASH. Cilofexor had similar target engagement in HVs and patients with NASH, as demonstrated by similarly increasing FGF‐19 levels with 100 mg of cilofexor in HVs and patients with NASH (peak concentrations of 591.6 pg/ml and 829.7 pg/ml at 4 h postdose, respectively) (Figure S3A). However, the same dose of cilofexor 100 mg led to higher serum levels of IL‐31 in patients with noncirrhotic NASH compared to HVs. In HVs, serum IL‐31 achieved a peak concentration of 0.19 pg/ml (0.1, 0.94), representing a 2.6‐fold increase from predose levels (Figure S3B). In contrast, the peak serum concentration of IL‐31 in patients with noncirrhotic NASH was 13 pg/ml, a 23.6‐fold increase from predose (Figure 2C). Thus, induction of IL‐31 secondary to cilofexor was more pronounced in patients with NASH than in HVs.
Increased serum IL‐31 in NASH is correlated with pharmacodynamic markers of FXR agonism
FGF‐19 and C4 are established pharmacodynamic biomarkers of FXR agonism and associated with BA synthesis. In serially collected serum samples from patients with NASH, 100 mg cilofexor increased both IL‐31 and FGF‐19 to peak concentrations at 4–6 h postdose (Figure 2D). At W24, C4 levels were significantly lower in both the cilofexor 30 mg and 100 mg groups compared to placebo, as previously reported.11 Although serum IL‐31 was weakly correlated with C4 at baseline (ρ = ‐0.25, p = 0.068), changes in IL‐31 and C4 from baseline to W24 in the cilofexor 100 mg group were significantly negatively correlated (Figure 2E, ρ = −0.46, p < 0.001). The correlations between changes in serum IL‐31 and pharmacodynamic markers of FXR agonism provide further evidence of an on‐target effect of cilofexor.
Cilofexor did not alter serum IL‐31 levels in patients with PSC or PBC
Serum IL‐31 levels were assessed in two phase 2, placebo‐controlled studies of cilofexor in PBC and PSC at baseline, W1, W4, and W12. In contrast to the increased serum IL‐31 levels observed in patients with NASH (Figure 2A), cilofexor did not alter serum IL‐31 in patients with PSC (Figure 3A). Cilofexor did not exacerbate pruritus in patients with PSC with a numerically lower proportion of patients having Grade 2–3 pruritus compared to patients administered placebo.12 Cilofexor also did not alter the VAS for pruritus or the 5‐D Itch score in patients with PSC or PBC from W1 to W12 compared to placebo (Figure S4). Associations between serum IL‐31 and these patient‐reported outcomes were analyzed in patients with PSC and PBC at baseline. Serum IL‐31 levels were significantly correlated with 5‐D Itch (ρ = 0.42, p = 0.003) and VAS for pruritus (ρ = 0.44, p = 0.002) in patients with PSC (Figure 3B,C). Serum autotaxin levels at baseline were also significantly correlated with VAS and 5‐D Itch scores in patients with PSC (Table S2), confirming previous reports that autotaxin is associated with cholestatic itch.7
FIGURE 3.
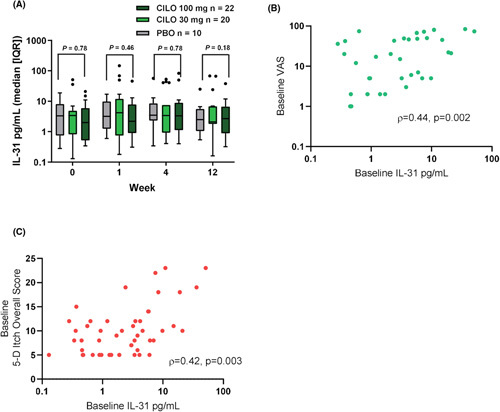
Serum IL‐31 levels in patients with PSC. (A) Administration of CILO did not alter serum IL‐31 levels in patients with PSC over 12 weeks. (B) Spearman correlation of VAS and serum IL‐31 levels at baseline. (C) Spearman correlation of 5D Itch overall score and serum IL‐31 levels at baseline. CILO, cilofexor; IQR, interquartile range; PBO, placebo; PSC, primary sclerosing cholangitis; VAS, Visual Analog Scale.
In patients with PBC, treatment with cilofexor did not affect serum IL‐31 levels in samples collected at W1, W4, and W12 compared to patients receiving placebo (Figure 4A). At baseline, serum IL‐31 was correlated with VAS (ρ = 0.39, p < 0.001) and 5‐D Itch scores (ρ = 0.48, p < 0.001) (Figure 4B,C). In serially collected serum samples collected postdose, 100 mg cilofexor mildly increased serum IL‐31 levels from a median of 3 pg/ml at predose to 7.4 pg/ml (2.5‐fold increase) at 5 h postdose (Figure 4D).
FIGURE 4.
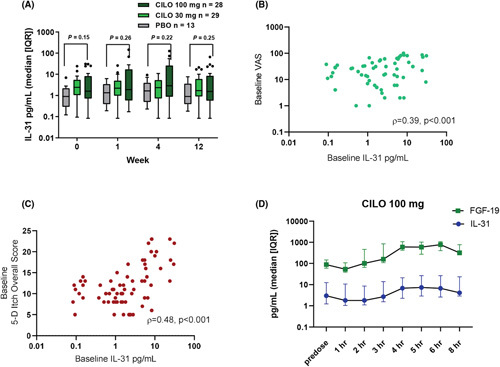
Serum IL‐31 levels in patients with PBC. (A) In patients with PBC, treatment with CILO did not change serum IL‐31 levels over 12 weeks. (B) Spearman correlation of VAS and serum IL‐31 levels at baseline. (C) Spearman correlation of 5D Itch overall score and serum IL‐31 levels at baseline. (D) 100 mg CILO mildly increased serum IL‐31 levels within 12 h postdose period. CILO, cilofexor; FGF, fibroblast growth factor; IQR, interquartile range; PBC, primary biliary cholangitis; PBO, placebo; VAS, Visual Analog Scale.
Cilofexor did not activate T helper 2 cells in patients with NASH and PSC
We wanted to explore the cellular source of IL‐31 in patients with liver disease. IL‐31 is expressed and secreted by activated T helper 2 (Th2) cells, and Th2 cells contribute to elevated serum IL‐31 in patients with atopic dermatitis.19,32 IL‐4 is a type II immune cytokine that increases with Th2 response.33,34 In patients with NASH, cilofexor 100 mg did not increase serum IL‐4 concentrations, whereas cilofexor 30 mg caused a numerical decrease in IL‐4 levels that returned to baseline by W24 (Figure S5A). The changes in serum IL‐31 in patients with NASH treated with cilofexor were not significantly correlated with changes in serum IL‐4 levels. Patients with PSC experienced a decrease in median IL‐4 levels as soon as W1 that normalized to near (but less than) baseline levels by W12 (Figure S5B). Thus, cilofexor did not alter serum IL‐4 levels in patients with NASH or PSC, suggesting that the increased serum IL‐31 in patients with NASH treated with cilofexor is unlikely due to Th2 cell activation.
IL‐31 expression is elevated in hepatocytes from patients with NASH and PSC
The correlation between serum IL‐31 and C4/FGF‐19 in patients with NASH treated with cilofexor suggested a direct effect of cilofexor on the primary FXR‐expressing cells, hepatocytes, and/or intestinal epithelial cells. We investigated IL‐31 expression by ISH in commercially procured samples of ileum and liver from HVs and patients with NASH and PSC and found IL‐31–positive (IL‐31+) cells only present in the liver (Figure S7). In NASH and PSC, both nonparenchymal cells and hepatocytes stained positively for IL‐31 in liver biopsies, whereas IL‐31+ staining in liver samples from healthy donors was nearly absent (Figure 5A). The number of IL‐31+ cells were elevated in the liver biopsy samples from patients with NASH (0.74%) and PSC (0.85%) compared to HVs (0.18%) (Figure 5D). The IL‐31+ hepatocytes were located in both periductal and central venous areas, without a clear zonal distribution.
FIGURE 5.
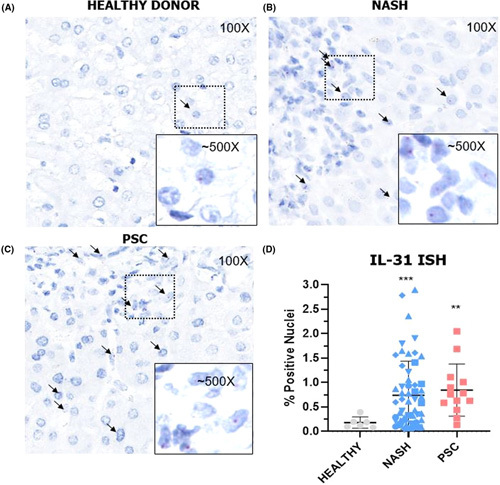
IL‐31 expression in livers from patients with NASH or primary biliary cholangitis (PBC). IL‐31+ cells are found in in liver samples from healthy volunteers (HVs) (A), patients with NASH (B), or patients with PSC (C). (D) The number of IL‐31+ cells were elevated in liver biopsy in patients with NASH and PSC compared to HVs. ISH, in situ hybridization; FXR, farnesoid X receptor; IQR, interquartile range; PSC, primary sclerosing cholangitis. **p < 0.01, ***p < 0.001.
OCA elevated IL‐31 expression and secretion from human hepatocytes in PXB mouse
We next evaluated the direct effect of the FXR agonist OCA (10 mg/kg once daily vs. vehicle for 18 days) on human hepatocytes in chimeric PXB mice. The Simoa assay was validated to detect human (not murine) IL‐31 (Figure S6). OCA increased IL‐31 hepatic mRNA expression (7.8‐fold higher than vehicle, p < 0.001) (Figure 6A). The chimeric mice treated with OCA also had higher circulating levels of human IL‐31 (1.1 pg/ml [0.25, 2.7]) than the vehicle group (0.085 pg/ml [0.045, 0.13], p = 0.005) (Figure 6B). Hepatic IL‐31 mRNA correlated with serum IL‐31 levels in PXB mice treated with OCA (ρ = 0.61, p < 0.001) (Figure 6C). The FXR agonist, OCA, therefore directly increased hepatocyte IL‐31 gene expression and circulating human IL‐31 in the humanized liver murine model.
FIGURE 6.
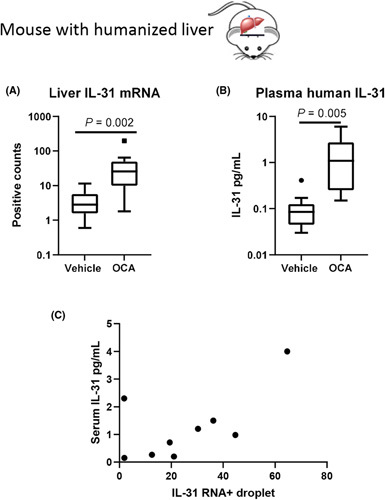
Elevated hepatic IL‐31 expression and plasma IL‐31 in PXB mice treated by obeticholic acid (OCA). Chimeric mice treated with OCA had higher levels of IL‐31 than vehicle controls in liver messenger RNA (mRNA) (A) and circulating (B). (C) The liver IL‐31 mRNA and circulating IL‐31 protein was significantly correlated.
DISCUSSION
Pruritus, a poorly understood and debilitating feature of cholestatic liver disease, is also commonly associated with treatment with OCA2,3,15 and other nonsteroidal FXR agonists.5,11,13,14 IL‐31 is a known pruritogen whose blockade significantly reduces pruritus in patients with atopic dermatitis.26,27 The present study demonstrated that circulating levels of IL‐31 correlate with pharmacodynamic markers of FXR agonism in recent clinical trials of the FXR agonist cilofexor. In a clinical trial evaluating the nonsteroidal FXR agonist cilofexor as a treatment for NASH, the highest serum IL‐31 levels were observed in patients who reported the most severe pruritus. Experimentally, the steroidal FXR agonist OCA directly increased circulating human IL‐31 concentrations and hepatic IL‐31 expression in a humanized liver murine model. Therefore, the present study provides a potential explanation for the somewhat perplexing observation of why both steroidal and nonsteroidal FXR agonists may cause pruritus despite concomitant reductions in levels of BAs.2,5,11,13–15,35
To the best of our knowledge, the cellular source of circulating IL‐31 in liver diseases had not been previously reported. Dillon et al. first established IL‐31 as a direct pruritogen experimentally and identified its highest expression in activated T helper cells.19 In our study, serum IL‐31 had a weak association with serum IL‐4, but significantly correlated with circulating BA levels. This association suggested that the liver may represent a source of IL‐31. Indeed, we observed that circulating IL‐31 levels were elevated in patients with NASH, PSC, and PBC versus healthy controls with the highest levels found in the cholestatic disorders (Figure 1A). ISH demonstrated nuclear localization of IL‐31 RNA mostly in hepatocytes and in some nonparenchymal cells in NASH and PSC livers but not in healthy controls. Experimental FXR agonism with OCA in the humanized liver murine model demonstrated a significant increase in IL‐31 gene expression and nearly a 10‐fold increase in circulating human IL‐31 protein. These findings suggest that FXR agonism in the liver is the primary determinant of IL‐31 given the species differences in FXR found in this murine model where only the hepatocytes are human in origin. Other recent studies also point to an increase in circulating IL‐31 with the development of experimental NASH.36 Together these data provide evidence that the liver may represent a significant source of circulating IL‐31 in clinical liver diseases.
The complex causal mechanisms that contribute to pruritus led us to investigate other postulated pruritogens in the present study. Studies of BA and its receptor, Mas‐related GPR family member X4 (MRGPRX4), and their relation to pruritus are under investigation. Deoxycholic acid (DCA), an unconjugated secondary BA, is among the most potent ligands for MRGPRX4.37–39 However, despite reductions in total BA levels with cilofexor treatment in both NASH and PSC, relative levels of DCA, as well as the overall BA composition, did not change.12,17 Experimentally, the addition of OCA to the PXB humanized mice did not alter the hepatic expression of MRGPRX4 (data not shown). However, this finding does not preclude a role for this receptor in the dorsal root ganglion in mediating cholestatic itch. Autotaxin, a secreted enzyme that generates lysophosphatidic acid, is elevated in patients with PBC with cholestatic itch.7 Our study confirmed significant correlations between VAS and 5‐D Itch scores with baseline serum autotaxin levels in patients with PBC and PSC (Table S2). However, our studies of cilofexor in NASH and PSC (Figure S2) and a previous study of OCA in patients with PBC,2 indicated that FXR agonism does not alter serum autotaxin levels. These findings suggest that the pruritus associated with FXR agonists may be distinct from the pruritus that originates from excess autotaxin activity.
The notion that multiple molecular mediators may differentially contribute to a complex symptom such as pruritus is suggested by the discrepant findings in this study in which patients with NASH demonstrated induction of IL‐31 with cilofexor, whereas patients with PBC and PSC showed no increase. In patients with PSC and PBC, baseline IL‐31 levels correlated with pruritus, but levels of IL‐31 were six to nine‐fold higher in patients with PBC/PSC versus cirrhotic NASH at baseline. In the Phase 2 NASH study, cilofexor led to a 13‐fold increase in IL‐31 levels that were associated with the development of pruritus. These cilofexor‐induced levels, however, were still below the levels seen in cholestatic liver disease at baseline prior to cilofexor treatment. In contrast, cilofexor did not increase IL‐31 in both cholestatic diseases, suggesting a potential “ceiling” effect of this cytokine. Determining the relative contribution of IL‐31 in mediating pruritus in these various liver diseases will likely require targeted therapies that interfere with IL‐31 directly.
Identification of IL‐31 as a causative pruritogen in other liver diseases, most notably cholestatic disorders, could provide clinical benefit to patients suffering from intractable pruritus. The ileal BA transporter (IBAT) inhibitor, linerixibat, reduced cholestatic itch in patients with PBC with moderate‐to‐severe pruritus40; another IBAT inhibitor, maralixibat, reduced serum BA levels and pruritus in patients with Alagille syndrome, an inherited cholestatic disease often characterized by severe pruritus.41 Determining whether IL‐31 levels decline in response to successful antipruritic interventions such as BA binding resins or IBAT inhibitors would provide further support for the role of IL‐31 in mediating cholestatic pruritus. Therapeutics directly targeting IL‐31 such as nemolizumab (an anti–IL‐31RA antibody) and abrocitinib (a Janus kinase inhibitor, targeting IL‐31 and other inflammatory pathways) have demonstrated efficacy in mitigating pruritus associated with atopic dermatitis.26,27,42 In addition to their ability to ameliorate pruritus in patients with atopic dermatitis, these molecules also have a known safety profile.26,27,42 Future randomized trials using clinically characterized anti–IL‐31 blocking agents would provide a critical test of IL‐31’s role as a cytokine in mediating the pruritus associated with cholestatic diseases.
In conclusion, clinical use of the FXR agonist cilofexor is associated with elevated serum IL‐31 and pruritus severity in NASH, whereas baseline levels of IL‐31 in PSC and PBC were associated with pruritus. FXR agonism in a humanized hepatic murine model directly induces IL‐31, thereby demonstrating that the liver can be a source of this putative pruritogen in response to FXR agonists. These findings have therapeutic implications for the pruritus associated with FXR agonists and warrant exploration in other etiologies, especially in those patients with cholestatic disorders.
Acknowledgments
AUTHOR CONTRIBUTIONS
Jun Xu, Andrew N. Billin, Chuhan Chung and Robert P. Myers, developed the manuscript. Ya Wang and Matt Peach analyzed the data. Matt Peach, Jen-Chieh Chuang, Julie Lin, Wen-Wei Tsai, Sangeetha Mahadevan, Wesley Minto, Lauri Diehl, and Ruchi Gupta provided data in clinical or preclinical study. Michael Trauner, Keyur Patel, Mazen Noureddin, Kris V. Kowdley, Aliya Gulamhusein and Christopher L. Bowlus monitored the clinical study. All the authors reviewed and edited the manuscript.
FUNDING INFORMATION
Funding for this project was provided by Gilead Sciences, Inc. Funding for this analysis was provided by Gilead Sciences, Inc. Medical writing support for the initial draft was provided by Gregory Suess, PhD, of AlphaScientia, LLC.
CONFLICT OF INTEREST
Andrew N. Billin owns stock in and is employed by Gilead. Christopher L. Bowlus consults for and received grants from Cymabay, GSK, and Mirum. He received grants from Intercept, Takeda, BMS, BiomX, Gilead, Novartis, and Pliant. Chuhan Chung owns stock in and is employed by Inipharm. He owns stock in Gilead. Jen-Chieh Chuang owns stock in and is employed by TLC Inc. Jun Xu owns stock in and is employed by Gilead. Keyur Patel consults for Gilead. He advises Intercept. Kris V. Kowdley consults for, is on the speakers’ bureau for, and received grants from Gilead and Intercept. He consults for and received grants from Hightide, Mirum, NGM, and 89 Bio. He consults for Madrigal, PTG, and Genfit. He is on the speakers’ bureau for AbbVie. He received grants from GSK, Pliant, Hanmi, Viking, and Pfizer. Lauri Diehl owns stock in and is employed by Gilead. Michael Trauner consults for, is on the speakers’ bureau for, and received grants from Falk, Intercept, Gilead, and MSD. He consults for and received grants from Alberio. He consults for BiomX, Boehringer Ingelheim, Genfit, Janssen, Novartis, Shire, Phenex, and Regulus. He received grants from Cymabay, Takeda, Alnylam, Ultragenyx, and AbbVie. He is the coinventor of patents on the medical use of 24-norursodesoxycholic acid. Matt Peach owns stock in and is employed by Gilead. Mazen Noureddin owns stock in Anaetos, Chrownwell, Ciema, Rivus Pharma, and Viking. He advises and received grants from Gilead, Pfizer, and Madrigal. He advises 89BIO, Altimmune, cohBar, Cytodyn, Intercept, Novo Nordisk, Blade, EchoSens, Fractyl, NorthSea, Perspecturm, Terns, Siemens, and Roche. He received grants from Allergan, BMS, Galmed, Galectin, Genfit, Shire, Zydus, Conatus, Enanta, and Novartis. Mina Khoshdeli owns stock in and is employed by Gilead. Robert P. Myers owns stock in and is employed by The Liver Company. He receives stock from Gilead. Ryan S. Huss owns stock in and is employed by The Liver Company. He receives stock from Gilead. Wiley Minto owns stock in and is employed by Gilead.
DATA AVAILABILITY STATEMENT
Anonymized individual patient data will be shared upon request for research purposes dependent upon the nature of the request, the merit of the proposed research, the availability of the data, and its intended use. More information on data transparency from Gilead Sciences, Inc., can be found at https://www.gileadclin icaltrials.com/transparency-policy/.
Footnotes
Abbreviations: BA, bile acid; CILO, cilofexor; FGF, fibroblast growth factor; FXR, farnesoid X receptor; HV, healthy volunteer; IBAT, ileal bile acid transporter; IL‐31RA, IL‐31 receptor A; IQR, interquartile range; ISH, in situ hybridization; mRNA, messenger RNA; OCA, obeticholic acid; PBC, primary biliary cholangitis; PSC, primary sclerosing cholangitis; VAS, Visual Analog Scale; W, Week.
Funding informationGilead Sciences
Supplemental Digital Content is available for this article. Direct URL citations appear in the printed text and are provided in the HTML and PDF versions of this article on the journal’s website, www.hepjournal.com.
Contributor Information
Jun Xu, Email: jun.xu@gilead.com.
Andrew N. Billin, Email: andrew.billin@gilead.com.
REFERENCES
- 1.Beuers U, Kremer AE, Bolier R, Elferink RP. Pruritus in cholestasis: facts and fiction. Hepatology. 2014;60:399–407. [DOI] [PubMed] [Google Scholar]
- 2.Nevens F, Andreone P, Mazzella G, Strasser SI, Bowlus C, Invernizzi P, et al. A placebo‐controlled trial of obeticholic acid in primary biliary cholangitis. N Engl J Med. 2016;375:631–43. [DOI] [PubMed] [Google Scholar]
- 3.Hirschfield GM, Mason A, Luketic V, Lindor K, Gordon SC, Mayo M, et al. Efficacy of obeticholic acid in patients with primary biliary cirrhosis and inadequate response to ursodeoxycholic acid. Gastroenterology. 2015;148:751–61.e8. [DOI] [PubMed] [Google Scholar]
- 4.Kowdley KV, Vuppalanchi R, Levy C, Floreani A, Andreone P, LaRusso NF, et al. A randomized, placebo‐controlled, phase II study of obeticholic acid for primary sclerosing cholangitis. J Hepatol. 2020;73:94–101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Kowdley KV, Minuk GY, Pagadala MR, Gulamhusein A, Swain MG, Neff GW, et al. The nonsteroidal farnesoid X receptor (FXR) agonist cilofexor improves liver biochemistry in patients with primary biliary cholangitis (PBC): a phase 2, randomized, placebo‐controlled trial. Hepatology. 2019;70(S1):abstract 45. [Google Scholar]
- 6.Kremer AE, van Dijk R, Leckie P, Schaap FG, Kuiper EM, Mettang T, et al. Serum autotaxin is increased in pruritus of cholestasis, but not of other origin, and responds to therapeutic interventions. Hepatology. 2012;56:1391–400. [DOI] [PubMed] [Google Scholar]
- 7.Kremer AE, Martens JJ, Kulik W, Ruëff F, Kuiper EM, van Buuren HR, et al. Lysophosphatidic acid is a potential mediator of cholestatic pruritus. Gastroenterology. 2010;139:1008–18, 1018.e1. [DOI] [PubMed] [Google Scholar]
- 8.Meixiong J, Vasavda C, Snyder SH, Dong X. MRGPRX4 is a G protein‐coupled receptor activated by bile acids that may contribute to cholestatic pruritus. Proc Natl Acad Sci U S A. 2019;116:10525–30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Chiang JY. Bile acids: regulation of synthesis. J Lipid Res. 2009;50:1955–66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Holt JA, Luo G, Billin AN, Bisi J, McNeill YY, Kozarsky KF, et al. Definition of a novel growth factor‐dependent signal cascade for the suppression of bile acid biosynthesis. Genes Dev. 2003;17:1581–91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Patel K, Harrison SA, Elkhashab M, Trotter JF, Herring R, Rojter SE, et al. Cilofexor, a nonsteroidal FXR agonist, in patients with noncirrhotic NASH: a phase 2 randomized controlled trial. Hepatology. 2020;72:58–71. [DOI] [PubMed] [Google Scholar]
- 12.Trauner M, Gulamhusein A, Hameed B, Caldwell S, Shiffman ML, Landis C, et al. The nonsteroidal farnesoid X receptor agonist cilofexor (GS‐9674) improves markers of cholestasis and liver injury in patients with primary sclerosing cholangitis. Hepatology. 2019;70:788–801. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Lucas KJ, Lopez P, Lawitz EJ, Sheikh A, Aizenberg D, Hsia S, et al. Safety and efficacy of tropifexor in patients with fibrotic nonalcoholic steatohepatitis: 48‐week results from part C of the phase 2 flight‐FXR study. Hepatology. 2020;72(S1):abstract 139. [Google Scholar]
- 14.Ratziu V, Rinella ME, Neuschwander‐Tetri BA, Lawitz E, Denham D, Kayali Z, et al. EDP‐305 in patients with NASH: a phase II double‐blind placebo‐controlled dose‐ranging study. J Hepatol. 2021;76:506–17. [DOI] [PubMed] [Google Scholar]
- 15.Neuschwander‐Tetri BA, Loomba R, Sanyal AJ, Lavine JE, Van Natta ML, Abdelmalek MF, et al. Farnesoid X nuclear receptor ligand obeticholic acid for non‐cirrhotic, non‐alcoholic steatohepatitis (FLINT): a multicentre, randomised, placebo‐controlled trial. Lancet. 2015;385:956–65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Younossi ZM, Ratziu V, Loomba R, Rinella M, Anstee QM, Goodman Z, et al. Obeticholic acid for the treatment of non‐alcoholic steatohepatitis: interim analysis from a multicentre, randomised, placebo‐controlled phase 3 trial. Lancet. 2019;394:2184–96. [DOI] [PubMed] [Google Scholar]
- 17.Patel K, Harrison SA, Elkashab M, Trotter JF, Herring R, Rojter S, et al. Cilofexor, a nonsteroidal FXR agonist, in non‐cirrhotic patients with nonalcoholic steatohepatitis: a phase 2 randomized controlled trial. Hepatology. 2020;72:58–71. [DOI] [PubMed] [Google Scholar]
- 18.Harrison SA, Rossi SJ, Paredes AH, Trotter JF, Bashir MR, Guy CD, et al. NGM282 improves liver fibrosis and histology in 12 weeks in patients with nonalcoholic steatohepatitis. Hepatology. 2020;71:1198–212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Dillon SR, Sprecher C, Hammond A, Bilsborough J, Rosenfeld‐Franklin M, Presnell SR, et al. Interleukin 31, a cytokine produced by activated T cells, induces dermatitis in mice. Nat Immunol. 2004;5:752–60. [DOI] [PubMed] [Google Scholar]
- 20.Kabashima K, Irie H. Interleukin‐31 as a clinical target for pruritus treatment. Front Med. 2021;8:638325. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Arai I, Tsuji M, Miyagawa K, Takeda H, Akiyama N, Saito S. Repeated administration of IL‐31 upregulates IL‐31 receptor A (IL‐31RA) in dorsal root ganglia and causes severe itch‐associated scratching behaviour in mice. Exp Dermatol. 2015;24:75–8. [DOI] [PubMed] [Google Scholar]
- 22.Tseng PY, Hoon MA. Oncostatin M can sensitize sensory neurons in inflammatory pruritus. Sci Transl Med. 2021;13:eabe3037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Gonzales AJ, Humphrey WR, Messamore JE, Fleck TJ, Fici GJ, Shelly JA, et al. Interleukin‐31: its role in canine pruritus and naturally occurring canine atopic dermatitis. Vet Dermatol. 2013;24:48–53.e11‐2. [DOI] [PubMed] [Google Scholar]
- 24.Oyama S, Kitamura H, Kuramochi T, Higuchi Y, Matsushita H, Suzuki T, et al. Cynomolgus monkey model of interleukin‐31‐induced scratching depicts blockade of human interleukin‐31 receptor A by a humanized monoclonal antibody. Exp Dermatol. 2018;27:14–21. [DOI] [PubMed] [Google Scholar]
- 25.Hawro T, Saluja R, Weller K, Altrichter S, Metz M, Maurer M. Interleukin‐31 does not induce immediate itch in atopic dermatitis patients and healthy controls after skin challenge. Allergy. 2014;69:113–7. [DOI] [PubMed] [Google Scholar]
- 26.Ruzicka T, Hanifin JM, Furue M, Pulka G, Mlynarczyk I, Wollenberg A, et al. Anti‐interleukin‐31 receptor A antibody for atopic dermatitis. N Engl J Med. 2017;376:826–35. [DOI] [PubMed] [Google Scholar]
- 27.Kabashima K, Matsumura T, Komazaki H, Kawashima M. Trial of nemolizumab and topical agents for atopic dermatitis with pruritus. N Engl J Med. 2020;383:141–50. [DOI] [PubMed] [Google Scholar]
- 28.Mu N, Lin F, Jiang Z, Liang Y, Yang Z. Implication of increased serum IL‐31 for primary biliary cholangitis. Immunol Invest. 2021;50:662–70. [DOI] [PubMed] [Google Scholar]
- 29.Basile F, Santamaria A, Mannucci C, Rizzo L, Gangemi S, D'Anna R, et al. Interleukin 31 is involved in intrahepatic cholestasis of pregnancy. J Matern Fetal Neonatal Med. 2017;30:1124–7. [DOI] [PubMed] [Google Scholar]
- 30.Myers RP, Djedjos C, Kirby B, Bilin A, Khan M, Gosink J, et al. A198 pharmacodynamic effects of the oral, non‐steroidal farnesoid X receptor agonist GS‐9674 in healthy volunteers. J Can Assoc Gastroenterol. 2018;1:346. [Google Scholar]
- 31.Wang F, Flanagan J, Su N, Wang LC, Bui S, Nielson A, et al. RNAscope: a novel in situ RNA analysis platform for formalin‐fixed, paraffin‐embedded tissues. J Mol Diagn. 2012;14:22–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Lu J, Wu K, Zeng Q, Xiang Y, Gao L, Huang J. Serum interleukin‐31 level and pruritus in atopic dermatitis: a meta‐analysis. Zhong Nan Da Xue Xue Bao Yi Xue Ban. 2018;43:124–30. [DOI] [PubMed] [Google Scholar]
- 33.Stott B, Lavender P, Lehmann S, Pennino D, Durham S, Schmidt‐Weber CB. Human IL‐31 is induced by IL‐4 and promotes TH2‐driven inflammation. J Allergy Clin Immunol. 2013;132:446–54.e5. [DOI] [PubMed] [Google Scholar]
- 34.Castellani ML, Felaco P, Galzio RJ, Tripodi D, Toniato E, De Lutiis MA, et al. IL‐31 a Th2 cytokine involved in immunity and inflammation. Int J Immunopathol Pharmacol. 2010;23:709–13. [DOI] [PubMed] [Google Scholar]
- 35.Kremoser C. FXR agonists for NASH: how are they different and what difference do they make? J Hepatol. 2021;75:12–5. [DOI] [PubMed] [Google Scholar]
- 36.Ganguly S, Muench GA, Shang L, Rosenthal SB, Rahman G, Wang R, et al. Nonalcoholic steatohepatitis and HCC in a hyperphagic mouse accelerated by Western diet. Cell Mol Gastroenterol Hepatol. 2021;12:891–920. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Stofan M, Guo GL. Bile acids and FXR: novel targets for liver diseases. Front Med. 2020;7:544. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Bartholomew TC, Summerfield JA, Billing BH, Lawson AM, Setchell KD. Bile acid profiles of human serum and skin interstitial fluid and their relationship to pruritus studied by gas chromatography‐mass spectrometry. Clin Sci (Lond). 1982;63:65–73. [DOI] [PubMed] [Google Scholar]
- 39.Kremer AE, Oude Elferink RP, Beuers U. Pathophysiology and current management of pruritus in liver disease. Clin Res Hepatol Gastroenterol. 2011;35:89–97. [DOI] [PubMed] [Google Scholar]
- 40.Takemura K, Takizawa E, Tamori A, Nakamae M, Kubota H, Uchida‐Kobayashi S, et al. Association of serum autotaxin levels with liver fibrosis in patients pretreatment and posttreatment with chronic hepatitis C. J Gastroenterol Hepatol. 2021;36:217–24. [DOI] [PubMed] [Google Scholar]
- 41.Gonzales E, Hardikar W, Stormon M, Baker A, Hierro L, Gliwicz D, et al. Efficacy and safety of maralixibat treatment in patients with Alagille syndrome and cholestatic pruritus (ICONIC): a randomised phase 2 study. Lancet. 2021;398:1581–92. [DOI] [PubMed] [Google Scholar]
- 42.Bieber T, Simpson EL, Silverberg JI, Thaci D, Paul C, Pink AE, et al. Abrocitinib versus placebo or dupilumab for atopic dermatitis. N Engl J Med. 2021;384:1101–12. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Anonymized individual patient data will be shared upon request for research purposes dependent upon the nature of the request, the merit of the proposed research, the availability of the data, and its intended use. More information on data transparency from Gilead Sciences, Inc., can be found at https://www.gileadclin icaltrials.com/transparency-policy/.


