Abstract
Background and Aims:
Type 3 innate lymphoid cells (ILC3s) are essential for host defense against infection and tissue homeostasis. However, their role in the development of HCC has not been adequately confirmed. In this study, we investigated the immunomodulatory role of short‐chain fatty acids (SCFAs) derived from intestinal microbiota in ILC3 regulation.
Approach and Results:
We report that Lactobacillus reuteri was markedly reduced in the gut microbiota of mice with HCC, accompanied by decreased SCFA levels, especially acetate. Additionally, transplantation of fecal bacteria from wild‐type mice or L. reuteri could promote an anticancer effect, elevate acetate levels, and reduce IL‐17A secretion in mice with HCC. Mechanistically, acetate reduced the production of IL‐17A in hepatic ILC3s by inhibiting histone deacetylase activity, increasing the acetylation of SRY (sex‐determining region Y)‐box transcription factor 13 (Sox13) at site K30, and decreasing expression of Sox13. Moreover, the combination of acetate with programmed death 1/programmed death ligand 1 blockade significantly enhanced antitumor immunity. Consistently, tumor‐infiltrating ILC3s correlated with negative prognosis in patients with HCC, which could be functionally mediated by acetate.
Conclusions:
These findings suggested that modifying bacteria, changing SCFAs, reducing IL‐17A‐producing ILC3 infiltration, and combining with immune checkpoint inhibitors will contribute to the clinical treatment of HCC.
INTRODUCTION
Accounting for up to 90% of all primary liver malignancies, HCC is a highly aggressive cancer type and the fourth most common cause of cancer‐related deaths worldwide,1 which develops together with fibrosis, cirrhosis, chronic hepatitis, and stromal activation, on the one hand,2 and stimulates antitumor immune response through an inflammatory microenvironment, on the other hand.3 Various tumor‐infiltrating immune cells are functionally altered in the tumor microenvironment (TME), being associated with tumor growth, metastasis, and ultimately prognosis of HCC. Therefore, identifying the various infiltrating immune cells and exploring their role in the TME will be of value for the treatment of HCC.
Recently, innate lymphoid cells, including type 1, type 2 (ILC2), and type 3 (ILC3) subsets, have increasingly been considered key moderators of tissue homeostasis and inflammation by releasing cytokines.4 IL‐22 production is restricted to the natural cytotoxicity‐triggering receptor–positive (NCR+) ILC3 subset, while NCR−ILC3s predominantly produce IL‐17A. IL‐17A was regulated by inhibitor of DNA binding 2 (ID2), SRY (sex‐determining region Y)‐box transcription factor 13 (Sox13), transcription factor 1, and so on.5 In human non‐small cell lung cancer, a substantial number of infiltrating natural killer cell p44‐related protein–positive (NKp44+) ILC3s have been described in tumor‐associated tertiary lymphoid structures as contributing to antitumor immunity.6 However, activation of NKp44+ ILC3s within tumor may not always be desirable. In colorectal cancer, ILC3‐derived IL‐22 induces abnormal epithelial cell proliferation.7 However, the role of ILC3s in liver cancer is still unclear.
Gut microbial dysbiosis has been demonstrated to be the crucial determinant and player in liver diseases, such as NAFLD, lung cancer, and liver cancer. Among the intestinal bacterial metabolites,3 short‐chain fatty acids (SCFAs) have raised concern because of their role in promoting the differentiation of regulatory T (Treg) cells and maintaining intestinal homeostasis.8 Consisting of acetate, propionate, and butyrate, SCFAs are the final products of dietary fiber fermentation through anaerobic bacteria from the phyla Firmicutes and Bacteroidetes, among others.9 Emerging evidence suggests that microbiota‐derived acetate can influence the host immune response and especially exert a range of health‐promoting functions.10 Propionate and butyrate can protect inflammation in the gut through their direct impact on Treg cells.8 It has been demonstrated that butyrate has a remarkable series of antineoplastic properties in addition to its anti‐inflammatory property in the gut: It maintains mucosal integrity and curbs inflammation and carcinogenesis through affecting immunity, epigenetic modulation, and gene expression as the energy source preferred by colonocytes. Produced through the bacterial fermentation of dietary fiber in the colon, SCFAs engage “metabolite‐sensing” G protein–coupled receptors (GPCRs).11 Moreover, it has been recently reported that GPCR 43 (GPR43) can regulate the function of ILC3s as a major receptor for SCFAs,12 while its role in modulating HCC‐derived ILC3s is still unclear.
Here, we found that the Lactobacillus reuteri of HCC mice was significantly down‐regulated, accompanied by a drastic increase in production of IL‐17A in ILC3s. The transplantation of fecal bacteria and acetate administration delayed the growth of tumor and enhanced the efficacy of programmed death 1 (PD‐1) treatment. Mechanistically, acetate inhibited IL‐17A production of ILC3s through the inhibitory activity of histone deacetylase (HDAC) and induction of Sox13 acetylation rather than through GPR43. Besides, it was shown that acetate can attenuate the production of IL‐17A by ILC3s in patients with HCC. To sum up, this study further reveals that acetate plays an immunomodulatory role in modulating ILC3 function.
PATIENTS AND METHODS
Patient samples
All patients received surgical treatment in Jiangsu Province Hospital. The local ethics committee approved the study (EC206/09). All participants were recruited in Jiangsu Province Hospital from September 2019 to October 2020. The individuals were informed about this study and gave consent before specimen collection. Written informed consent was obtained from all participants. The clinical and pathological features of the involved patients in this study were reviewed by a pathologist specializing in HCC. Detailed clinical and pathological information on the patients are presented in Table S1.
Fecal microbiota transplantation and SCFA treatment
The mice were treated with antibiotics for 4 days before stool transplantation experiments. Then, stool samples were mixed with saline solution (20 mg/ml), vortexed, and centrifuged to collect the supernatant. A total of 100 µl of the fecal suspension was administered to mice by oral gavage. The L. reuteri group was orally gavaged with pure L. reuteri (Guangdong Microbial Culture Center 1.614) at a dose of 2 × 108 colony‐forming units/0.2 ml suspended in sterile anaerobic PBS twice per week. Gavage of the same dose of heat‐killed L. reuteri was used as a control. For the SCFA administration groups, wild‐type (WT) mice were treated for 2 weeks with sodium acetate (150 mM), sodium propionate (150 mM), or sodium butyrate (100 mM) dissolved in their autoclaved drinking water and filtered‐sterilized. WT control mice received sodium chloride (150 mM). HCC mice were treated for 2 weeks with sodium acetate (150 mM), sodium propionate (150 mM), or sodium chloride (150 mM) as a control in the drinking water and filtered‐sterilized. Drinking water solutions were freshly prepared and changed every 5 days.
Details of methods used in this study are described in the Supporting Materials and Methods.
RESULTS
Profiles of the gut microbiota and SCFAs in the HCC mouse model
Bacterial DNA was extracted from the feces of 10 mice (six WT and four HCC; establishment of the HCC mouse model shown in Figure S1), and taxonomic profiling was performed through 16S ribosomal RNA gene sequencing. It was found that the two groups were not significantly different in observed species, Chao1 richness, Simpson’s indices, or Shannon diversity at the level of operational taxonomic units (OTUs). However, the gut microbiota was found to have lower alpha‐diversity in mice with HCC than in WT mice, indicating an individual structure with higher homogeneity among HCC individuals (Figure S2A). In addition, it can be noticed from a clustered heatmap demonstrating the Bray‐Curtis dissimilarity between individual samples at the OTU level that the variation in gut microbial communities had no clear distinctive profile between mice within different groups (Figure S2B).
In consideration of the relationship between gut bacteria and HCC in HCC mice, the next attempt was to determine whether the gut microbial composition of WT mice was different from that of HCC ones. For this purpose, the general landscape of the gut microbiome in every mouse of both groups was assessed first, revealing the existence of similar communities (Figure S2C). Then, a comparison was made between the abundance of OTUs in HCC and WT mice, which disclosed that each group had rich specific bacterial communities at a variety of taxonomic levels (Figure S3A–E). To further investigate these findings, linear discriminant analysis of effect size was carried out to conduct high‐dimensional class comparisons13 to detect whether WT and HCC mice were significantly different in the predominance of bacterial communities (Figure S2D,E): HCC mice were predominated by Erysipelotrichales and Bifidobacteriales at the order level, while WT ones exhibited a dominance of Lactobacillales and Bacteroidales.
According to partial least squares discriminant analysis (PLS‐DA), samples of WT and HCC mice presented a distinct clustering pattern (Figure 1A). The score of variable importance in projection (VIP) for the gut microbiota indicated the significant contribution of L. reuteri to the separation of groups (Figure 1B). Moreover, HCC mice exhibited a marked reduction in the enrichment of L. reuteri compared with WT ones (mean reads, 7.476% vs. 1.876%, p = 0.004; Figure 1C; Figure S2E). To identify whether specific components of gut microbial metabolites promote antitumor immunity, we performed high‐throughput metabolomics analyses with samples from the colonic contents of HCC or WT mice. Compared with WT mice, about three‐quarters of the detected metabolites were decreased in mice with HCC (Figure 1D). The decreased metabolites involved in many metabolic pathways were critical for host physiology in HCC mice (Figure 1E,F).
FIGURE 1.
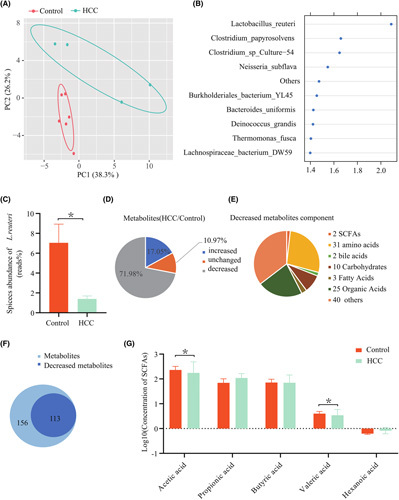
Gut microbial and SCFA alterations in mice with HCC. (A) PLS‐DA score image shows the abundance of species from samples of mice with HCC (green points) and controls (red points). The significant difference between the two groups (*p < 0.05) was evaluated using permutational multivariate analysis of variance with the Bray‐Curtis distance metric. (B) VIP score of PLS‐DA. The discriminative ability of different taxa between the HCC and control groups was ranked by applying VIP scores. The important distinction is the taxon with a VIP score > 1.4. (C) Species abundance of L. reuteri in the HCC and control groups. (D) Metabolomics analysis of gut microbial metabolites in colon contents from mice with or without HCC. (E) Components of the decreased gut microbial metabolite ( >10‐fold) in colon content in HCC mice compared with control mice. (F) Comparison of the decreased gut microbial metabolites in post‐HCC mice ( >10‐fold) and control mice. (G) Levels of portal vein serum SCFAs of acetate, propionate, butyrate, valerate, and caproate were detected in controls (n = 5) and HCC mice (n = 6). The concentration of SCFAs was determined by log10. p values were determined by a two‐tailed Mann‐Whitney U test. PC, principal component
Emerging evidence suggested that gut microbiota is strongly associated with the development, progression, and risk of HCC. Produced in the colon and metabolized by intestinal bacteria–derived enzymes, SCFAs are of critical importance for the maintenance of healthy gut microbiota, innate immunity, as well as balanced lipid and carbohydrate metabolism.14 In this study, a substantial reduction in acetate (mean value of concentration, 113.8 vs. 281.2 μmol/L; p = 0.019) and valerate (mean value of concentration, 2.7 vs. 4.2 μmol/L; p = 0.04) serum was observed in the HCC group compared with the WT one (Figure 1G). A recent study also indicates an increase in the serum concentrations of SCFAs after L. reuteri treatment.15 These data suggest that the gut microbiota of HCC mice suffered a significant down‐regulation of L. reuteri, which in turn exerted an impact on the metabolism of SCFAs in such mice.
Fecal microbiota of WT mice or L. reuteri transplantation delayed tumor growth
To further ascertain the role of the gut microbiota in mice with HCC, stools from WT, HCC, L. reuteri, or Trans‐L. reuteri mice with antibiotic treatment (ABTx) were administered by oral gavage to HCC recipient mice (called Trans‐Control, Trans‐HCC, Trans‐L. reuteri, and Trans‐L. reuteri+ABTx, respectively) (Figure 2A). Notably, oral gavage with L. reuteri or stools from WT mice reduced the number and relative size of liver tumors in HCC mice (Figure 2B–D). All HCC mice developed typical HCC accompanied by fibrosis, as analyzed by the whole liver (Figure 2B) and hematoxylin and eosin (H&E) analyses (Figure 2E). Ki67+ tumor cells were diminished in Trans‐Control and Trans‐L. reuteri mice (Figure 2E). The hepatic injury of HCC mice transplanted with L. reuteri and WT mice stool was milder than that of mice transplanted with HCC mice stool and Trans‐L. reuteri+ABTx, as disclosed by alanine and aspartate aminotransferase (ALT and AST), alkaline phosphatase (ALP), and γ‐glutamyl transpeptidase (γ‐GT) (Figure 2F). These data indicate that the gut microbiota from WT mice, especially L. reuteri, modulated the hepatic injury and delayed the tumor progression of HCC mice to a certain extent. We also tested SCFAs in the portal vein serum, and the results revealed that acetate was dramatically reduced in the Trans‐HCC and Trans‐L. reuteri+ABTx groups in comparison with the Trans‐Control or Trans‐L. reuteri group (Figure 2G).
FIGURE 2.
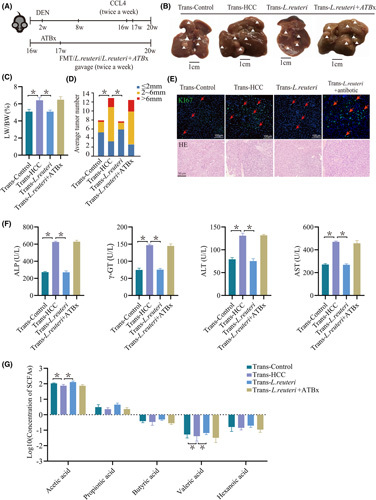
Healthy control fecal microbiota or L. reuteri transplantation inhibited tumor growth in the HCC mouse model. (A) Schematic plot of the HCC model induced by diethylnitrosamine‐CCl4. Diethylnitrosamine (25 mg/kg) was injected i.p. into 14‐day‐old mice, followed by another injection after 6 weeks with CCl4 (2 mL/kg) twice a week for 12 weeks. Sixteen‐week‐old mice were transplanted with HCC, control fecal microbiota, L. reuteri, or L. reuteri+ABTx. (B) Macrograph of livers in male HCC mice kept with Trans‐Control (left) and with Trans‐HCC (second from left), and with oral administration of L. reuteri (right). HCCs are represented by arrowheads. (C,D) The liver weight to body weight ratio was analyzed, and the average number of liver tumors and the relative size distribution were divided into ≤ 2 mm, 2–6 mm, and >6 mm (p < 0.01). (E) Representative images and statistical analysis of H&E and Ki67 staining of liver sections in various mice, as indicated (n = 5). (F) Hepatic function indexes: ALP, AST, ALT, γ‐GT. (G) Levels of portal vein serum SCFAs of acetate, propionate, butyrate, valerate, and caproate were detected in Trans‐Control (n = 5), Trans‐HCC (n = 5), Trans‐L. reuteri, and Trans‐L. reuteri+ABTx (n = 5). p values were determined by the two‐tailed Mann‐Whitney U test. DEN, diethylnitrosamine; FMT, fecal microbiota transplantation; LW/BW, liver weight to body weight ratio
L. Reuteri mediated an antitumor effect through inhibiting IL‐17A‐ producing ILC3 function
Gut microbiota and its metabolites are likely to affect oncogenesis, tumor progression, and response to cancer therapy through their influence on the immune system.16 In addition to SCFAs, hepatic immune cells were screened. In liver samples, Il17a mRNA experienced a reduction after oral gavage with L. reuteri and stools from WT mice compared with the Trans‐HCC group (mean, 1.423 or 0.548 vs. 5.366, p = 0.0286; Figure 3A). As analyzed by immunofluorescence and H&E analysis, a decrease in IL‐17A+ tumor cells was observed in Trans‐Control and Trans‐L. reuteri mice (Figure 3B). Consistent with the previous data, serum IL‐17A levels were reduced in Trans‐Control and Trans‐L. reuteri mice compared with Trans‐HCC mice (Figure 3C). To further identify IL‐17A‐producing cells, ILC3s from the liver were detected using flow cytometry. The Trans‐Control and Trans‐L. reuteri mice exhibited a notably lower percentage of IL‐17A+ cells in hepatic ILC3s (cluster of differentiation 45–positive [CD45+] lineage− retinoic acid–related orphan receptor γt–positive [RORγt+]) compared with the Trans‐HCC group (mean, 4.33% or 4.55% vs. 7.85%, p = 0.029; Figure 3D; Figure S4A). IL‐17A is produced by ILC3 cells and other types of cells like T helper 17 (Th17) cells and T‐cell receptor γδ T cells.17 However, no substantial difference in Th17 and γδ T cells was observed among three groups of recipient mice (Figure S4B), but IL‐17A protein levels in the serum were significantly diminished in Trans‐Control and Trans‐L. reuteri mice (Figure 3C). However, we detected only very slight changes, if any, in the frequency of the lymph node and splenic IL‐17A+ cell subsets; provision of L. reuteri or normal stools to HCC animals resulted in a robust decrease in IL‐17A expression in hepatic ILC3 cells (Figure 3D) but not thymic or splenic IL‐17A‐secreting cells (Figure S5A–C). Additionally, ILC3s were not involved in production of IL‐10 or IL‐13 (Figure S4C). Cumulatively, these findings demonstrate that the gut microbiota of WT mice, especially L. reuteri, can reduce the level of IL‐17A produced by hepatic ILC3s in recipient mice.
FIGURE 3.
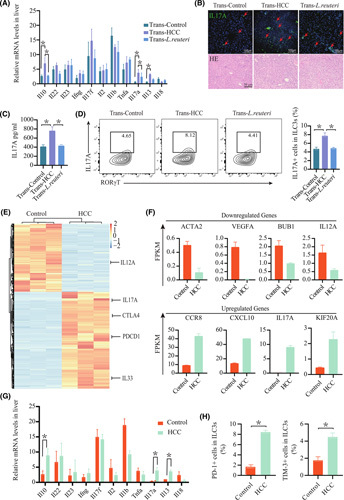
Molecular characterization of ILC3 cells in the liver by RNA‐seq. (A) Measurement of Il22, Ifng, Il10, Il13, Il17a, Il17f, Il18, Il1b, Il2, Il23, and Tnfa mRNA expression levels in livers by real‐time quantitative PCR (n = 5; *p < 0.05). (B) Representative images and statistical analysis of H&E and IL‐17‐A immunofluorescence staining of liver sections in various mice, as indicated (n = 5). (C) IL‐17A levels in the serum of Trans‐Control, Trans‐HCC, and Trans‐L. reuteri mice (n = 5). (D) Flow‐cytometric analysis of IL‐17A+ cells in CD45+ lineage−RORγt+ ILC3s from the liver. Similar results were observed in three independent experiments. Percentage of IL‐17A cells in ILC3s from the liver (n ≥ 4). (E) Hierarchical clustering of all genes. (F) Representative expression map of differentially expressed genes including metabolism, tumor promotion, and cytokine secretion in ILC3 cells between tumor and control tissues (p < 0.01, by negative binomial generalized linear model). (G) Measurement of Il22, Ifng, Il10, Il13, Il17a, Il17f, Il18, Il1b, Il2, Il23, and Tnfa mRNA expression levels in livers by real‐time quantitative PCR (n ≥ 3; *p < 0.05). (H) Percentage of PD‐1 or TIM‐3 cells in ILC3s from the liver (n ≥ 4). BUB1, budding uninhibited by benzimidazoles 1; CCR8, chemokine (C‐C motif) receptor 8; CTLA4, cytotoxic T lymphocyte antigen 4; FPKM, fragments per kilobase per million; PDCD1, programmed cell death 1
ILC3 cells were further sorted from the tumor liver tissues of three mice with HCC and normal liver tissues of three WT mice by RNA sequencing (RNA‐seq) to determine their global differential expression gene. RNA‐seq analysis showed that ILC3 cells derived from tumors had >6000 differentially expressed genes in comparison with those from normal liver tissues, indicating an abnormal gene expression profile of ILC3 cells in the HCC microenvironment (Figure 3E). The next aim was to explore the change of biological pathways in tumor ILC3 cells because of those up‐regulated and down‐regulated genes. Up‐regulated genes including chemokine (C‐C motif) receptor 8 and chemokine (C‐X‐C motif) receptor 10 (CXCL10), were abundant in the pathways involved in cytokine–cytokine receptor interaction, while significantly down‐regulated genes such as actin alpha 2 (ACTA2), VEGFA, and IL‐12A in tumor ILC3 cells were rich in the pathways of proteoglycans in cancer, consistent with the findings that tumor ILC3 cells suffered a severe impairment in effector function (Figure 3F; Figure S6A).
HCC development is fostered by genes abnormally up‐regulated in tumor‐associated ILC3 cells, such as IL‐17A, CXCL10, and PD‐1. Especially, IL‐17A may directly act on endothelial cells to stimulate tumor growth through angiogenesis18,19 or up‐regulate the permeability of adhesion molecules and endothelial cells, thereby promoting the metastasis of secondary sites.20 IL‐17A is the most up‐regulated gene in ILC3 cells derived from tumors, in line with the previous result of cytokine secretion. HCC mice showed a drastic increase in Il17A mRNA among these cytokine mRNAs compared with the control one (Figure 3G). Likewise, a significant increase in tumor‐derived ILC3 cells with expression of PD‐1 and T‐cell Ig mucin family member 3 (TIM‐3) was observed compared with normal liver mice (Figure 3H; Figure S6B). Similar to the previous RNA‐seq results, compared with Trans‐HCC, marked down‐regulation of PD‐1 (mean, 8.5% vs. 4.7%, p = 0.0019; 8.5% vs. 4.8%, p = 0.0013) and TIM‐3 (mean, 6.2% vs. 3.7%, p = 0.012; 6.2% vs. 3.6%, p = 0.01) on ILC3 cells was detected in Trans‐Control and Trans‐L. reuteri mice (Figure S6C). Hence, these results suggested that ILC3 cells accelerated HCC progression in the HCC microenvironment.
SCFAs inhibit the function of ILC3s for IL‐17A production in vitro
IL‐23 has been implicated in the activation and induction of ILC3s to produce IL‐17A.21 To confirm whether SCFAs monitor ILC3 function, the effects of acetate, propionate, and butyrate on the production of type 3 cytokine by IL‐23‐activated ILC3s were evaluated. Liver‐derived ILC3s were sorted and treated with SCFAs in the presence of IL‐23. These results showed that acetate dramatically reduced the percentage of IL‐17A‐producing ILC3 cells in a dose‐dependent manner. And acetate exhibited the strongest effect on inhibiting IL‐17A production compared with butyrate and propionate (Figure 4A,B; Figure S7A).
FIGURE 4.
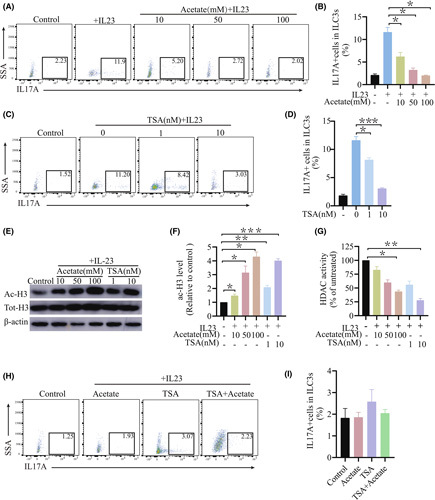
SCFAs inhibited production of IL‐17A by ILC3s in vitro. (A) Representative flow diagram showing intracellular expression of IL‐17A in ILC3s after 48 h of treatment with acetate at different concentrations. (B) Total IL‐17A+ ILC3s after 48 h of treatment assessed in (A) (n ≥ 4). (C,D) ILC3s from the livers of control mice were treated with 40 ng/ml IL‐23 in the presence or absence of acetate or TSA at the indicated concentrations. Representative western blot and relative expression of acetyl‐histone H3 levels after 6 h of treatment. Normalization of data against the total H3. (E) HDAC activity in ILC3 nuclear extracts after 6 h of treatment. (F,G) Flow‐cytometric analysis of the percentage of IL‐17A cells in ILC3s from livers after 48 h of treatment with TSA at different concentrations. Total IL‐17A+ ILC3s after 48 h of treatment in (C) (n ≥ 4). (H) ILC3s from the liver were treated with 50 mmol/L acetate, 40 ng/ml IL‐23, or 10 nmol/L TSA for 48 h. Total IL‐17A+ ILC3s after 48 h of treatment (n ≥ 4). (I) Total IL‐17A+ ILC3s after 48 h of treatment in E (n ≥ 4). ac‐H3, acetyl‐histone H3; SSA, side scattering analysis
GPR43 transcript of colonic ILC3s is an SCFA‐sensing GPCR that plays an immunomodulatory role in the modulation of inflammation and intestinal homeostasis.22 The colonic ILC3 population and its function can be selectively promoted by GPR43 agonism.11 Loss of GPR43 in ILC3s can reduce production of ILC3‐derived IL‐17A and colonic ILC3 in situ proliferation.12 Thus, GPR43 expression in ILC3s was significantly up‐regulated through IL‐23 treatment in comparison with control values, while acetate cotreatment exerted no effect on its expression levels (Figure S7B). IL‐23‐activated ILC3s were treated with the GPR43 agonist 4‐CMTB, and flow‐cytometric analysis of IL‐17A confirmed the influence of GPR43 activation on ILC3 function. Unlike acetate, 4‐CMTB treatment failed to reduce the level of IL‐23‐induced IL‐17A (Figure S7C). More importantly, acetate cotreatment with 4‐CMTB resulted in synergistic inhibition, suggesting that the production of IL‐17A in ILC3s regulated by acetate was independent of the GPR43‐mediated pathway (Figure S7D). In general, the findings suggest that ILC3 function is not affected by GPR43 activation.
To study the influence of HDAC inhibition on ILC3 function, IL‐23‐activated ILC3s were treated with enhanced efficacy of trichostatin A (TSA) for the assessment of its impact on ILC3 production. Similar to acetate, TSA significantly decreased the production of IL‐17A at the protein level in a dose‐dependent manner, indicating that impaired inhibition of ILC3 function by HDAC (Figure 4F,H).
To ascertain whether acetate‐mediated inhibition depends on the activity of HDAC inhibition, ILC3s were treated with acetate or TSA along with IL‐23. In dendritic cells, acetate acts on HDAC targets with TSA by means of cotreatment tactics.23 It is worth noting that no cooperative or additive effects were observed in the cotreatment of TSA and acetate. Consequently, acetate treatment cannot further strengthen TSA‐mediated inhibition (Figure 4G,I). The results confirmed the presupposition that acetate inhibits ILC3 function by inhibiting HDAC activity.
We asked how acetate affects the activity of IL‐17A production in ILC3s. RNA‐seq data of ILC3s showed that Sox13, a transcription factor that has been shown to regulate IL‐17A production, was up‐regulated in mice with HCC (Figure 5A; Figure S6D). ILC3s treated with acetate showed a significant decrease of Sox13 mRNA levels (Figure 5B). Moreover, upon acetate treatment, the acetylation of Sox13 in 293T cell transfected with Sox13 plasmid was observed by immunoprecipitation (IP) (Figure 5C). We noticed that Sox13 expression at the total protein level remained unchanged, while the Sox13 mRNA level was actually decreased after acetate treatment (Figure 5B,C). This discrepancy may be involved in complicating posttranscriptional and translational regulation processes.24 To determine candidate acetylation sites of Sox13, the acetylation modification site of Sox13 was predicted using the following website: http://csspalm.biocuckoo.org/. The result suggested two potential acetylation sites, K29 and K30, which were highly conserved among species (Figure 5D). The sites were then detected by IP analysis of His‐tagged Sox13 from acetate‐treated 293T cells, which implied that the lysine residue K30 was the main acetylation site on Sox13 (Figure 5E). To verify this, His‐tagged Sox13 mutants in which the lysine residues were changed to arginine by site‐directed mutagenesis were transfected into 293T cells. Upon acetate treatment, compared with WT Sox13, Sox13‐K30R showed markedly reduced acetylation and enhanced IL‐17A expression (Figure 5E,F). Next, the transcription factor Sox13, which potentially binds to the promoter region of IL‐17A, was predicted by the JASPAR database, revealing striking enrichment of motifs within protein‐binding sites (Figure 5G). Then, chromatin IP assays were used to determine whether Sox13 promotes the transcription of IL‐17A. DNA bound to Sox13 was pulled down and further PCR‐amplified with primers specific for the two binding domains of the IL‐17A promoter, which confirmed that Sox13 binding to IL‐17A was severely impaired by acetate (Figure 5H). For functional analysis of the binding sites, we constructed luciferase reporter plasmids containing the WT IL‐17A promoter Sox13‐binding motifs and mutant ones; luciferase expression activity demonstrated that the inserted WT Sox13‐binding domains were activated in 293T cells (Figure 5I). This indicates that Sox13 is sufficient to mediate the acetate effect on IL‐17A production in ILC3s.
FIGURE 5.
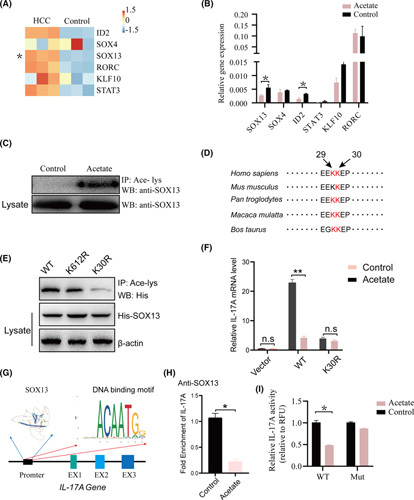
Acetate‐modulated production of IL‐17A in ILC3s through enhancing acetylation of Sox13. (A) ILC3 cells from WT and HCC livers were sorted. RNA‐seq was performed. Expression of ID2, SOX4, SOX13, RORC, KLF10, and STAT3 is shown in a heatmap. Sox13 was significantly up‐regulated in HCC mice. (B) Measurement of ID2, SOX4, SOX13, RORC, KLF10, and STAT3 mRNA expression levels in livers by real‐time quantitative PCR (n ≥ 3; *p < 0.05). (C) Acetylation of Sox13 in 293T cells with Sox13 overexpression in the presence of acetate. The antibodies used for IP and western blotting were antiacetylated lysine and anti‐Sox13, respectively. (D) Alignment of the Sox13 amino acid sequence from various species. Red highlight indicates the conserved K29 and K30. (E,F) Acetylation of Sox13 mutants expressed in 293T cells. The K29R and K30R and the Lys29 and Lys30 residues were replaced by Arg. (F) After being transfected with vector control, WT Sox13, or Sox13‐K30R, the IL‐17A mRNA level in 293T cells was detected upon acetate treatment. (G) The representative Sox13 DNA binding motif was predicted using the following website: https://jaspar.genereg.net. (H) The binding of Sox13 to the binding domain within an IL‐17A promoter was analyzed using chromatin IP and real‐time quantitative PCR in 293T cells transfected with His‐flagged Sox13 plasmid and IL‐17A promoter plasmid following treatment with acetate. (I) The 293T cells transfected with WT Sox13 binding domain or mutant Sox13 binding domain reporter vectors were treated with or without acetate 2 days posttransfection. Sox13 activity was assessed by luciferase. EX, exon; KLF10, Kruppel like factor 10; MUT, mutant; RORC, ROR complex; STAT3, signal transducer and activator of transcription 3; WB, western blotting
Combination PD‐1 monoclonal antibody therapy with SCFA administration enhanced the antitumor effect in mice with HCC
To confirm the role of acetate in anticancer therapy, 8‐week‐old mice were exposed to SCFAs through the drinking water to evaluate the physiological significance. Another group of mice received acetate in the drinking water, followed by PD‐1 therapy (Figure 6A). In this study, significant down‐regulation of PD‐1 on ILC3 cells was detected in acetate and acetate+PD‐1 compared with HCC mice. Likewise, ILC3 cell expression of TIM‐3 was also significantly decreased in acetate and acetate+PD‐1 mice (Figure S9). We did not observe any change in the frequency of PD‐1+ ILC3s or TIM‐3+ ILC3s in mice that received butyrate or propionate in the drinking water (Figure S9). These data suggested that acetate administration combined with PD‐1 therapy, but not butyrate or propionate administration, could delay tumor growth (Figures S6B–D and S8B–E).
FIGURE 6.
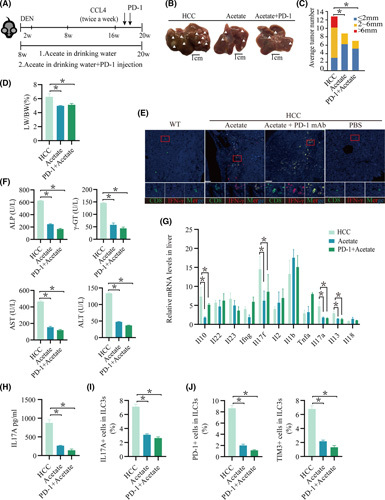
The combination of ICIs with SCFA administration facilitates the immune response in mice with HCC. (A) Schematic plot of the HCC model induced by diethylnitrosamine‐CCl4. PD‐1 (20 mg/kg) was injected twice per week during the last 3 weeks of CCl4 treatment. Mice were given drinking water containing acetate from 16 weeks of age for 20 weeks. IL‐17A levels in the supernatant of in vitro–cultured ILC3s treated with acetate (n ≥ 4/group). (B) Macrograph of livers in HCC mice kept with HCC (left), with acetate (second from left), and with PD‐1+acetate (right). Arrowheads indicate HCCs. (C,D) The liver weight to body weight ratio was analyzed, and the average number of liver tumors and the relative size distribution were divided into ≤ 2 mm, 2–6 mm, and >6 mm (p < 0.01). (E) Expression of CD8 and interferon‐γ in tumor tissue was determined by immunohistochemistry. (F) Hepatic function indexes: ALP, AST, ALT, γ‐GT. (G) Measurement of Il22, Ifng, Il10, Il13, Il17a, Il17f, Il18, Il1b, Il2, Il23, and Tnfa mRNA expression levels in livers by real‐time quantitative PCR (n ≥ 3; *p < 0.05). (H) IL‐17A levels in the serum of acetate, PD‐1+acetate, and HCC mice (n ≥ 3 mice/group). (I) Percentage of IL‐17A cells in ILC3s from the liver (n ≥ 4). (J) Percentage of PD‐1 or TIM‐3 cells in ILC3s from the liver (n ≥ 4). DEN, diethylnitrosamine; LW/BW, liver weight to body weight ratio; mAb, monoclonal antibody
To further verify the efficacy of immunosuppressive agents, we used multiplex immunofluorescence staining and found that more cytotoxic T lymphocyte (CTL) infiltration was observed in the combined group (Figure 6E). The hepatic injury was milder than that in other groups (Figure 6F; Figure S8D). The mice with acetate administration and PD‐1 treatment showed a decrease in Il17A mRNA among these cytokine mRNAs compared with the others (Figure 6G; Figure S8F). Similarly, the serum IL‐17A levels and the number of IL‐17A+ cells in ILC3s in the liver of mice administered acetate combined with PD‐1 treatment were decreased (Figure 6G–I; Figure S8G–I). Propionate and butyrate administration had no influence on the levels of serum IL‐17A, the percentage of IL‐17A+ ILC3s in the liver, and tumor growth (Figure S8G–I). These data suggest that acetate administration, but not propionate or butyrate, can improve liver injury and inhibit tumor growth in the HCC model. Consistent with this finding, it was observed in the HCC model that the addition of PD‐1 and acetate as a combined treatment produced greater antitumor effects than those acetates alone mediated as a single modality (Figure 6A–J). This shows that the combination of acetate with immune checkpoint inhibitors (ICIs) played an important role in antitumor effects.
ILC3 infiltration correlated with unfavorable outcomes in HCC
Based on the ILC3 cell gene list (KIT, CXCL8, IL4I1, IL1R1, MAF BZIP transcription factor F [MAFF], RUNX family transcription factor 3 [RUNX3]) in tumors, patients were divided into ILC3‐high and ILC3‐low groups using the mean value of each gene as the cutoff, to ascertain the relationship between ILC3 cell infiltration and survival. HCC survival data from The Cancer Genome Atlas indicated that patients with higher expression of ILC3 had a significant association with reduced survival (p = 0.0026; HR, 1.7; Figure 7A). Furthermore, high expression Sox13 in HCC was associated with poor prognosis (p = 0.027; HR, 1.63; Figure 7B). SCFAs in the portal vein serum were tested in patients with HCC. Clinical specimens were divided into an acetate‐high group and an acetate‐low group based on the content of acetate. Consistent with mice, significant down‐regulation of acetate was detected in HCC compared with healthy donors (Figure 7C). Multiplex immunofluorescence staining was used to describe tumor immune infiltrates in patients with HCC, and a greater density of ILC3s (CD45+CD3–RORγt+) in tumors was found in the acetate‐high group (mean, 1 vs. 3 cells/core; p = 0.0001; Figure 7D). In our study, we found that the IL‐17‐secreting ability of ILC3 cells was significantly inhibited from acetate‐high patients compared with acetate‐low patients, but this was not related to propionate (Figure 7D–F). Next, to investigate whether acetate regulates IL‐17A secretion, ILC3s from peripheral blood were sorted, cultured, and treated with acetate. Secretion of IL‐17A protein, Sox13, and Il17A mRNA levels in cultured ILC3s was significantly decreased in the presence of acetate (Figure 7I,J). Moreover, the percentage of peripheral blood IL‐17A+ ILC3s was reduced in patients with high acetate levels in the hepatic portal vein serum compared to those with low acetate levels (Figure 7G,H). Collectively, the high density of tumor‐infiltrated ILC3 cells manifested poor clinical outcomes in patients with HCC, according to the analysis of all patients.
FIGURE 7.
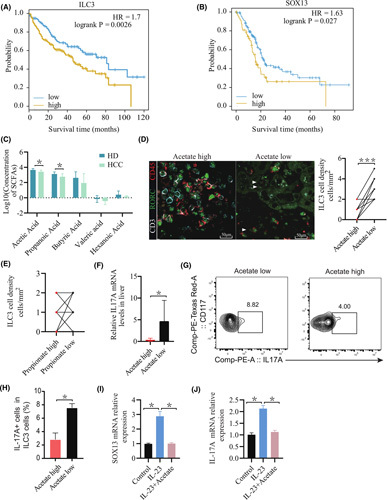
Acetate negatively correlates with hepatic ILC3 infiltration in individuals with HCC. (A) Kaplan‐Meier curves of OS based on ILC3 cell frequencies in The Cancer Genome Atlas ILC3 cell gene list (KIT, CXCL8, IL4I1, IL1R1, MAFF, RUNX3). (B) Kaplan‐Meier curves of OS based on Sox13 expression in HCC in The Cancer Genome Atlas. (C) Levels of portal vein serum SCFAs of acetate, propionate, butyrate, valerate, and caproate were detected in individuals with HCC (n = 8) and healthy donors (n = 15). p values were determined by the two‐tailed Mann‐Whitney U test. (D,E) Representative graphs of ILC3 cell distribution in tumor tissues. The method was used to define the subset as CD45+CD3‐RORγt+ cells. Compared with the acetate‐high group and the acetate‐low group (D) or compared with the propionate‐high group and the propionate‐low group (E), the absolute number of ILC3 cells was significantly reduced in the acetate‐high group (p < 0.001; n = 10). (F) Measurement of IL‐17A mRNA expression levels in livers by real‐time quantitative PCR in individuals in the acetate‐high group (n = 3) and the acetate‐low group (n = 4) (*p < 0.05). (G,H) Human ILC3s were sorted from peripheral blood mononuclear cells of patients with HCC. (G) The percentage of peripheral blood IL‐17A+ cells in ILC3 cells was determined by flow‐cytometric analysis. (H) Representative graphs of IL‐17A+ILC3 cells in patients with high levels of serum acetate and those with low acetate levels (n ≥ 3) (*p < 0.05). (I,J) Human ILC3s were sorted from healthy donor peripheral blood mononuclear cells and cultured with IL‐2 (10 ng/ml) and IL‐7 (10 ng/ml) in the presence or absence of acetate or IL‐23 (100 ng/ml). IL‐17A and Sox13 mRNA expression levels of ILC3s after 2 days of treatment (n ≥ 3) (*p < 0.05). HD, healthy donors; RORC, ROR complex
Activation of HSCs is an important factor that leads to liver fibrosis, which will eventually promote the development of HCC. Therefore, we further investigated the role of IL‐17A in the activation and senescence of HSCs and, thus, in the promotion of cirrhosis and development of HCC. First, the clinical specimens were divided into an IL‐17A‐high group and an IL‐17A‐low group based on the degree of IL‐17A+ in patients with HCC. To confirm tissue cirrhosis, we used immunohistochemical and Sirius red staining, and higher HSC activation (α‐smooth muscle actin [α‐SMA] and IL‐1β were used as markers) was observed in the IL‐17A‐high group (Figure S10A,B). RNA was extracted from the tissues to detect differences in the HSC activation‐related genes Acta2, collagen type I alpha 1 chain, Tgfb1, and tissue inhibitor of metalloproteinase 1. The IL‐17A‐high group had higher expression levels of these genes than the IL‐17A‐low group, which indicated a higher level of fibrosis (Figure S10D). To study the influence of ILC3 cells on HSC activation and fibrosis, we cocultured LX‐2 cells with ILC3s obtained from peripheral blood of patients with HCC and observed a significant increase in α‐SMA and collagen I, while coculture in the presence of acetate or α‐IL‐17A antibody led to a drastic decrease (Figure S10C). Taken together, the IL‐17A‐producing ILC3 cells contribute to HSC activation and fibrosis
DISCUSSION
The first single‐agent ICI to be approved in HCC was the anti‐PD‐1 nivolumab, demonstrating that the objective response rate and median overall survival (OS) were only 15% and 1 years, respectively.25 The definite but limited clinical benefit of single‐agent anti‐PD‐1 treatment gave fresh impetus to the search for strategies to optimize ICI treatment. In this study, we found that the reduction of gut‐derived L. reuteri and its acetate metabolite was involved in the development of HCC, accompanied by increased IL‐17A‐producing ILC3s. Further functional studies indicated that acetate inhibited IL‐17A production of HCC‐derived ILC3s in the manner of the hepatoenteric axis. This function of acetate is involved in HDAC inhibitor activity and the acetylation of Sox13 in ILC3s. Additionally, we reported that the combination of acetate with PD‐1/programmed death ligand 1 (PD‐L1) blockade enhanced anticancer immunity and improved therapeutic efficacy in the HCC mice model. Hence, we suggested that modifying the gut microbiota by altering acetate metabolism may be of value for the treatment of HCC.
Emerging studies with both mice and human patients have suggested that the gut microbiota plays a pivotal role in antitumor efficacy by shaping host immunity.3 In this study, a marked reduction in the enrichment of L. reuteri was observed in the HCC mice model compared with WT mice. Moreover, HCC mice that received transplantation of L. reuteri exhibited a significant antitumor effect. L. reuteri is a symbiotic bacterium in the digestive tract of many vertebrate hosts.26 Besides, a study found that a mouse model of pancreatic ductal adenocarcinoma that received gemcitabine in combination with L. reuteri GMNL‐89 had lower levels of ALT and AST.27 Previous studies have proven that the heterolactic L. reuteri uses a variety of monosaccharides and disaccharides to produce lactate together with ethanol and/or acetate in a 6‐phosphogluconate/phosphoketolase pathway–dependent manner,28 consistent with our results that L. reuteri was related to acetate metabolism in mice with HCC.
Recent studies have shown that gut microbiota and metabolites participated in modulating the functional state of immune cells.3 Our findings revealed that the IL‐17A‐producing ILC3 population was significantly reduced in the HCC mice model which received transplantation of L. reuteri or acetate administration. The role of ILC3s as a newly identified population in tumor progression remains controversial. Our results revealed that hepatic ILC3 was a poor prognosis factor in patients with HCC. Consistent with our findings, another recent study demonstrated that NCR− ILC3s promote HCC development in the IL‐23/IL‐17 axis–dependent pathway.29 These results indicated that considerable heterogeneity exists in ILC3s and their location in organs, and this may influence the overall effect on antitumor immunity and outcome. IL‐17A production could be the most important aspect of the ILC3 immunosuppressive behavior.29 Though IL‐17A was also produced by Th17 and γδ T cells, the number of γδ T and Th17 cells remained unchanged in hepatoma mice. A recent study indicated that IL‐17A drove TGF‐β‐dependent liver fibrosis,30 which is consistent with our results that tumor‐derived ILC3s promoted HSC activation and fibrosis in an IL‐17A‐dependent manner. In addition, the association of tumor‐infiltrating ILC3s with less acetate in patients with HCC has been observed. Therefore, we proposed that acetate participated in regulating the function of ILC3s for IL‐17A production to postpone progression of HCC.
Emerging evidence has indicated that SCFAs regulate the function of immune cells through HDAC inhibition and GPR41/43‐dependent metabolism regulation.11 In general, acetylation of histones by histone acetyltransferases enhances chromatin accessibility and facilitates gene transcription. HDACs reverse the process by driving a return to a silent state typical of more condensed chromatin.31 A recent study has shown that ILC2‐driven airway hyperreactivity and airway inflammation can be significantly ameliorated by systemic and local administration of butyrate.32 In another study, SCFAs promote the production of IL‐22 in CD4+ T cells and ILCs through HDAC inhibition and GPR41, rather than GPR43 or GPR109a.31 Here, we found that acetate can reduce the level of IL‐17A induced by ILC3s. Mechanistically, the acetate reduced production of IL‐17A in ILC3s is independent of GPR41/GPR43 but dependent on direct HDAC inhibitor activity, subsequently increasing the acetylation of Sox13.
Studies in recent years have shown that the outcome of cancer immunotherapy can be enhanced by the influence of the gut microbiome and its metabolite on the immune system.33 Compared with germ‐free mice or mice with interfering microbiota, mice with healthy gut microbiota usually respond more favorably to anti‐PD‐1 treatment.34] In this study, we further corroborated these observations that ILC3 cells in liver cancer can also express immune checkpoint molecules, which may result in immune escape of liver cancer. Notably, the combined blockade of PD‐1/PD‐L1 with acetate administration could enhance immunity against HCC by reducing the number of ILC3s and promoting CTL infiltration into tumors in vivo. These findings suggest that the combination of PD‐1/PD‐L1 blockade with acetate administration has better efficacy than monotherapy, suggesting the potential value of this strategy.
Although our studies provide evidence that L. reuteri–derived acetate decreases ILC3 production of IL‐17A through HDAC inhibition, providing a function of SCFAs in the regulation of the antitumor immune response in HCC, there are several limitations. Due to the lack of sequence analysis for bacterial screening for patient stools, this finding may not fully reflect the in vivo conditions of patients with HCC. Furthermore, the correlations between L. reuteri and IL‐17A+ ILC3 cells in patients with HCC are unclear. Therefore, further research is needed to study the composition and characteristics of human gut microbiota in HCC. In addition to SCFAs, L. reuteri may produce other metabolites involved in functional changes of the immune response and tumor development, which will be further explored in subsequent studies.
In conclusion, our study has demonstrated that the gut microbial metabolite acetate can modulate ILC3 function through Sox13‐dependent signaling and that the combination of PD‐1/PD‐L1 blockade with acetate administration enhances antitumor immunity in an HCC mouse model, suggesting that gut microbial metabolites can promote anticancer immunity to sufficiently improve immunotherapy efficacy; therefore, manipulation of gut microbial metabolites could be of great value for HCC therapy.
Supplementary Material
Acknowledgments
AUTHOR CONTRIBUTIONS
Yun Chen supervised, designed, and funded the study. Chupeng Hu, Bingqing Xu, and Wenhua You performed experiments and analyzed the 16s RNA sequencing data. Jinying Lu, Deyuan Kong, and Yu Jin performed experiments, interpreted the data and prepared the figures. Xiaodong Wang, Wen‐Hua Wan, Xiaoxin Mu, and Dongju Feng contributed biopsy samples and pathology analysis. Chupeng Hu and Bingqing Xu prepared the initial draft. Yun Chen, Xiaodong Wang, Wen‐Hua Wan, and Hua Sun reviewed and finalized the paper. All authors read and approved the manuscript.
ACKNOWLEDGMENT
We thank Prof. Xiaohuan Guo from Tsinghua University for providing professional advice and technical support. Supported by project grants from the National Natural Science Foundation of China (82071767, 82102876).
CONFLICT OF INTEREST
The author declares that there is no conflict of interest.
DATA AVAILABILITY STATEMENT
The details of data sharing are provided in the Supporting information. Further information and requests for resources and reagents should be directed to and will be fulfilled by the corresponding authors: Yun Chen, chenyun@njmu.edu.cn.
ETHICS STATEMENT
Written informed consent was obtained from all participants who received surgical treatment in Jiangsu Province Hospital. The specimen collection in our study from patients with Hepatocellular Carcinoma was approved by the local ethical committee (approval no.: EC206/09). All experiments were performed in accordance with relevant guidelines and regulations.
Footnotes
Abbreviations: ACTA2, actin alpha 2; ALP, alkaline phosphatase; ALT, alanine aminotransferase; AST, aspartate aminotransferase; ABTx, antibiotic treatment; CD, cluster of differentiation; CXCL8/10, chemokine (C‐X‐C motif) ligand 8/10; γ‐GT, γ‐glutamyl transpeptidase; GPCR, G protein–coupled receptor; GPR43, GPCR 43; HDAC, histone deacetylase; H&E, hematoxylin and eosin; ICI, immune checkpoint inhibitor; ID2, inhibitor of DNA binding 2; ILC3, type 3 innate lymphoid cell; IP, immunoprecipitation; NCR, natural cytotoxicity‐triggering receptor; OS, overall survival; OTU, operational taxonomic unit; PD‐1, programmed death 1; PD‐L1, programmed death ligand 1; PLS‐DA, partial least squares discriminant analysis; RNA‐seq, RNA sequencing; RORγt, retinoic acid–related orphan receptor γt; SCFA, short chain fatty acid; Sox13, SRY (sex‐determining region Y)‐box transcription factor 13; Th17, T helper 17; TIM‐3, T‐cell Ig mucin family member 3; TSA, trichostatin A; VIP, variable importance in projection; WT, wild type.
Chupeng Hu, Bingqing Xu, Xiaodong Wang, and Wen-Hua Wan contributed equally to this work.
Funding information Supported by project grants from the National Natural Science Foundation of China (82071767, 82102876)
Supplemental Digital Content is available for this article. Direct URL citations appear in the printed text and are provided in the HTML and PDF versions of this article on the journal's website, www.hepjournal.com.
REFERENCES
- 1.Kubes P, Jenne C. Immune responses in the liver. Annu Rev Immunol. 2018;36:247–77. [DOI] [PubMed] [Google Scholar]
- 2.Ringelhan M, Pfister D, O'Connor T, Pikarsky E, Heikenwalder M. The immunology of hepatocellular carcinoma. Nat Immunol. 2018;19:222–32. [DOI] [PubMed] [Google Scholar]
- 3.Rooks MG, Garrett WS. Gut microbiota, metabolites and host immunity. Nat Rev Immunol. 2016;16:341–52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Vivier E, Artis D, Colonna M, Diefenbach A, Di Santo JP, Eberl G, et al. Innate lymphoid cells: 10 years on. Cell. 2018;174:1054–66. [DOI] [PubMed] [Google Scholar]
- 5.Malhotra N, Narayan K, Cho O, Sylvia K, Yin C, Melichar H, et al. A network of high‐mobility group box transcription factors programs innate interleukin‐17 production. Immunity. 2013;38:681–93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Carrega P, Loiacono F, Di Carlo E, Scaramuccia A, Mora M, Conte R, et al. NCR+ILC3 concentrate in human lung cancer and associate with intratumoral lymphoid structures. Nat Commun. 2015;6:8280. [DOI] [PubMed] [Google Scholar]
- 7.Kirchberger S, Royston DJ, Boulard O, Thornton E, Franchini F, Szabady RL, et al. Innate lymphoid cells sustain colon cancer through production of interleukin‐22 in a mouse model. J Exp Med. 2013;210:917–31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Furusawa Y, Obata Y, Fukuda S, Endo TA, Nakato G, Takahashi D, et al. Commensal microbe‐derived butyrate induces the differentiation of colonic regulatory T cells. Nature. 2013;504:446–50. [DOI] [PubMed] [Google Scholar]
- 9.Canani RB, Costanzo MD, Leone L, Pedata M, Meli R, Calignano A. Potential beneficial effects of butyrate in intestinal and extraintestinal diseases. World J Gastroenterol. 2011;17:1519–28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Balmer ML, Ma EH, Thompson AJ, Epple R, Unterstab G, Lötscher J, et al. Memory CD8+ T cells balance pro‐ and anti‐inflammatory activity by reprogramming cellular acetate handling at sites of infection. Cell Metab. 2020;32:457–67.e5. [DOI] [PubMed] [Google Scholar]
- 11.Tan JK, McKenzie C, Marino E, Macia L, Mackay CR. Metabolite‐sensing G protein–coupled receptors—facilitators of diet‐related immune regulation. Annu Rev Immunol. 2017;35:371–402. [DOI] [PubMed] [Google Scholar]
- 12.Chun E, Lavoie S, Fonseca‐Pereira D, Bae S, Michaud M, Hoveyda HR, et al. Metabolite‐sensing receptor Ffar2 regulates colonic group 3 innate lymphoid cells and gut immunity. Immunity. 2019;51:871–84.e6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Segata N, Izard J, Waldron L, Gevers D, Miropolsky L, Garrett WS, et al. Metagenomic biomarker discovery and explanation. Genome Biol. 2011;12:R60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Yao Y, Cai X, Fei W, Ye Y, Zhao M, Zheng C. The role of short‐chain fatty acids in immunity, inflammation and metabolism. Crit Rev Food Sci Nutr. 2022;62:1–12. [DOI] [PubMed] [Google Scholar]
- 15.Oh J‐H, Alexander LM, Pan M, Schueler KL, Keller MP, Attie AD, et al. Dietary fructose and microbiota‐derived short‐chain fatty acids promote bacteriophage production in the gut symbiont lactobacillus reuteri. Cell Host Microbe. 2019;25:273–84.e6. [DOI] [PubMed] [Google Scholar]
- 16.Singh RP, Bashir H, Kumar R. Emerging role of microbiota in immunomodulation and cancer immunotherapy. Semin Cancer Biol. 2021;70:37–52. [DOI] [PubMed] [Google Scholar]
- 17.Vitiello GA, Miller G. Targeting the interleukin‐17 immune axis for cancer immunotherapy. J Exp Med. 2020;217(1):e20190456. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Van Hede D, Polese B, Humblet C, Wilharm A, Renoux V, Dortu E, et al. Human papillomavirus oncoproteins induce a reorganization of epithelial‐associated gammadelta T cells promoting tumor formation. Proc Natl Acad Sci U S A. 2017;114:E9056–65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Silva‐Santos B. Promoting angiogenesis within the tumor microenvironment: the secret life of murine lymphoid IL‐17‐producing gammadelta T cells. Eur J Immunol. 2010;40:1873–6. [DOI] [PubMed] [Google Scholar]
- 20.Kulig P, Burkhard S, Mikita‐Geoffroy J, Croxford AL, Hövelmeyer N, Gyülvészi G, et al. IL‐17A‐mediated endothelial breach promotes metastasis formation. Cancer Immunol Res. 2016;4:26–32. [DOI] [PubMed] [Google Scholar]
- 21.Bielecki P, Riesenfeld SJ, Hütter J‐C, Torlai Triglia E, Kowalczyk MS, Ricardo‐Gonzalez RR, et al. Skin‐resident innate lymphoid cells converge on a pathogenic effector state. Nature. 2021;592:128–32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Koh A, De Vadder F, Kovatcheva‐Datchary P, Backhed F. From dietary fiber to host physiology: short‐chain fatty acids as key bacterial metabolites. Cell. 2016;165:1332–45. [DOI] [PubMed] [Google Scholar]
- 23.Arpaia N, Campbell C, Fan X, Dikiy S, van der Veeken J, deRoos P, et al. Metabolites produced by commensal bacteria promote peripheral regulatory T‐cell generation. Nature. 2013;504:451–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Barrett LW, Fletcher S, Wilton SD. Regulation of eukaryotic gene expression by the untranslated gene regions and other non‐coding elements. Cell Mol Life Sci. 2012;69:3613–34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Zhu XD, Sun HC. Emerging agents and regimens for hepatocellular carcinoma. J Hematol Oncol. 2019;12:110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Duar RM, Frese SA, Lin XB, Fernando SC, Burkey TE, Tasseva G, et al. Experimental evaluation of host adaptation of Lactobacillus reuteri to different vertebrate species. Appl Environ Microbiol. 2017;83:e00132‐17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Chen SM, Chieng WW, Huang SW, Hsu LJ, Jan MS. The synergistic tumor growth‐inhibitory effect of probiotic Lactobacillus on transgenic mouse model of pancreatic cancer treated with gemcitabine. Sci Rep. 2020;10:20319. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Mai TT, Tran DQ, Roos S, Rhoads JM, Liu Y. Human breast milk promotes the secretion of potentially beneficial metabolites by probiotic Lactobacillus reuteri DSM 17938. Nutrients. 2019;11(7):1548. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Liu Y, Song Y, Lin D, Lei L, Mei YU, Jin Z, et al. NCR– group 3 innate lymphoid cells orchestrate IL‐23/IL‐17 axis to promote hepatocellular carcinoma development. EBioMedicine. 2019;41:333–44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Fabre T, Molina MF, Soucy G, Goulet JP, Willems B, Villeneuve JP, et al. Type 3 cytokines IL‐17A and IL‐22 drive TGF‐beta‐dependent liver fibrosis. Sci Immunol. 2018;3(28):eear7754. [DOI] [PubMed] [Google Scholar]
- 31.Yang W, Yu T, Huang X, Bilotta AJ, Xu L, Lu Y, et al. Intestinal microbiota–derived short‐chain fatty acids regulation of immune cell IL‐22 production and gut immunity. Nat Commun. 2020;11:4457. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Thio CL, Chi PY, Lai AC, Chang YJ. Regulation of type 2 innate lymphoid cell–dependent airway hyperreactivity by butyrate. J Allergy Clin Immunol. 2018;142:1867–83.e12. [DOI] [PubMed] [Google Scholar]
- 33.Gopalakrishnan V, Spencer CN, Nezi L, Reuben A, Andrews MC, Karpinets TV, et al. Gut microbiome modulates response to anti‐PD‐1 immunotherapy in melanoma patients. Science. 2018;359:97–103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Sivan A, Corrales L, Hubert N, Williams JB, Aquino‐Michaels K, Earley ZM, et al. Commensal Bifidobacterium promotes antitumor immunity and facilitates anti‐PD‐L1 efficacy. Science. 2015;350:1084–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The details of data sharing are provided in the Supporting information. Further information and requests for resources and reagents should be directed to and will be fulfilled by the corresponding authors: Yun Chen, chenyun@njmu.edu.cn.


