Abstract
Hepatocellular carcinoma (HCC) is a common cause of cancer‐related mortality and morbidity worldwide. With the obesity pandemic, NAFLD‐related HCC is contributing to the burden of disease exponentially. Genetic predisposition and clinical risk factors for NAFLD‐related HCC have been identified. Cirrhosis is a well‐known and major risk factor for NAFLD‐related HCC. However, the occurrence of NAFLD‐related HCC in patients without cirrhosis is increasingly recognized and poses a significant challenge regarding cancer surveillance. It is of paramount importance to develop optimal risk stratification scores and models to identify subsets of the population at high risk so they can be enrolled in surveillance programs. In this review, we will discuss the risks and prediction models for NAFLD‐related HCC.
INTRODUCTION
NAFLD is a spectrum of chronic liver disease (CLD) ranging from isolated hepatic steatosis and NASH to cirrhosis and/or hepatocellular carcinoma (HCC). A recent meta‐analysis showed that the global prevalence of NAFLD during 1990–2019 was approximately 30%, with the trend analysis revealing that 37% of adults worldwide may have NAFLD as of 2019.1 HCC is the fourth leading cause of cancer death worldwide and the second‐leading cause of years of life lost to cancer.2 According to the Global Burden of Disease 2015 study, there was an increase of 75% in liver cancer incidence between 1990 and 2015.3 Currently, NAFLD‐related HCC accounts for 1%–38% of the HCC burden in different countries/regions.4 NAFLD‐related HCC likely will increase significantly over time due to the growing obesity epidemic. Studies assessing the temporal trends in HCC attributed to NAFLD have alluded to this fact (Figure 1). Among patients with NAFLD‐associated cirrhotic liver (CL), the estimated annual incidence of HCC complication ranges from 0.5% to 2.6%. Less frequently, NAFLD‐related HCC has also been reported in observational studies in patients with noncirrhotic liver (NCL) at rates between 0.1 and 0.8 per 1000 patient‐years.4–7
FIGURE 1.
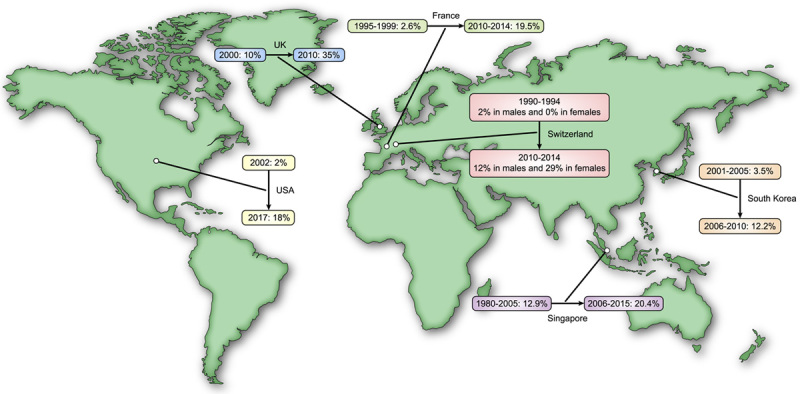
Temporal trends of NAFLD‐related hepatocellular carcinoma (HCC) in different countries.11–16
NAFLD‐related HCC is already becoming the leading cause of HCC among liver transplant candidates in the United States.8–10 This alarming trend and poor prognosis associated with NAFLD‐related HCC highlights the importance of early HCC detection based on risk factors. In this review, we will summarize the risks and prediction models for NAFLD‐related HCC. Future HCC surveillance programs may incorporate risk stratification methods using these prediction models.
SEARCH STRATEGY AND SELECTION CRITERIA
A literature review was performed by searching PUBMED for relevant full‐text articles through April 2003 for risk factors for NAFLD‐related HCC. The authors searched for articles using keywords, “liver cirrhosis,” “non‐alcoholic fatty liver,” “non‐alcoholic steatohepatitis,” “non‐cirrhosis,” “hepatocellular carcinoma,” “risk factors,” and “NAFLD‐related HCC,” in various combinations and with various synonyms. A separate search was conducted for each risk factor identified in the initial search with the particular risk factor in focus and NAFLD‐related HCC as the keywords. Similarly for the HCC risk prediction models, relevant full‐text articles through November 2014 were included with search terms “NAFLD‐related hepatocellular carcinoma,” “non‐alcoholic steatohepatitis,” “risk stratification,” “prediction scores/models,” “cirrhosis,” and “non‐cirrhosis” in various combinations. An additional search of article reference lists was performed to identify further studies. Only English‐language publications were considered. Articles using prediction scores for other CLD‐related HCC without liver steatosis (LS) or NAFLD were excluded.
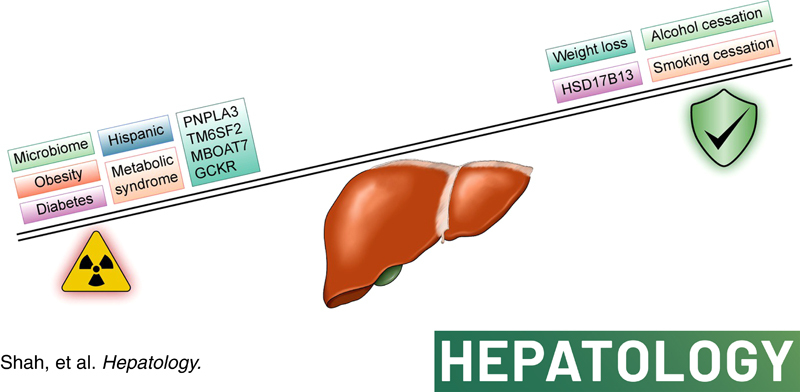
RISK FACTORS FOR NAFLD‐RELATED HCC (Figure 2)
FIGURE 2.
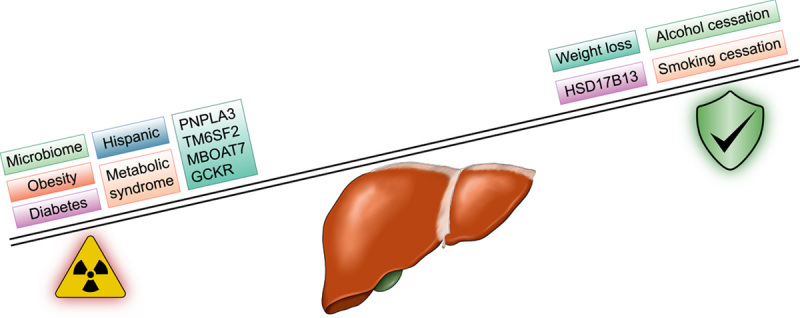
Risk and protective factors for NAFLD‐related hepatocellular carcinoma (HCC). GCKR, glucokinase regulator; HSD17B13, 17‐β hydroxysteroid dehydrogenase 13; MBOAT7, membrane bound O‐acyltransferase domain‐containing 7; PNPLA3; patatin‐like phospholipase domain‐containing 3; TM6SF2, transmembrane 6 superfamily member 2.
Diabetes mellitus
Diabetes mellitus (DM) is a recognized risk factor for HCC regardless of the etiology of CLD. Yang et al. found that among patients with NASH cirrhosis, the presence of DM was associated with a fourfold increase in HCC risk (hazard ratio [HR], 4.2; 95% confidence interval [CI], 1.2–14.2; p = 0.02.17 In a large European cohort of 136,703 patients with NAFLD that included 6425 (4.7%) patients with advanced fibrosis, Alexander et al. showed that DM was the strongest risk factor for the development of HCC.18 Kanwal et al. reported a similarly strong association between DM and HCC; in a US cohort of 271,906 patients with NAFLD where 253 had HCC, DM had the strongest association with HCC (adjusted HR, 2.77; 95% CI, 2.03–3.77).19 Duration of DM was also found to correlate with the development of HCC. Hassan et al. found that those who had DM for 10 years had a twofold increased cancer risk compared to those with the disease for 5 years (odds ratio [OR], 2.2; 95% CI, 1.2–4.8).20 In a UK cohort, Dyson et al. observed more than a 10‐fold increase in NAFLD‐related HCC between 2000 and 2010, and the elderly population with DM or metabolic syndrome (MetS) had the highest HCC‐associated mortality.12
Medications
Studies have evaluated the impact of antidiabetic medications on modifying HCC risk because diabetes is a well‐known risk factor. A recent study by Kramer et al. showed that adequate glycemic control was associated with a 31% lower risk of HCC in patients with NAFLD and DM. They also showed that metformin use was associated with a 20% lower risk of HCC, whereas insulin use in combination with other oral antidiabetics increased HCC risk by 1.6–1.7 fold.21 Similarly, Wang et al. showed a reduced HCC risk with metformin (risk ratio [RR], 0.3) and an increased HCC risk with sulphonylureas or insulin (RR, 4.0).22 Results from two meta‐analyses showed a similar trend with metformin being associated with approximately 50% HCC risk reduction.23,24 Contrary to these findings, a database study that included 18,080 patients with NAFLD NCL who were followed for 6.3 years did not find an association between HCC and metformin use.25
Similarly, statins have also been associated with having anticarcinogenic effects.26 A database study from Taiwan that included 18,080 patients with NAFLD showed an inverse association (OR, 0.29; 95% CI, 0.12–0.68) between statin use and HCC.25 German et al. evaluated such an association in a retrospective case‐control study of 102 patients with NAFLD with 34 HCC cases, and statin was found to be protective against HCC (OR, 0.20; 95% CI, 0.07–0.60).27 A recent retrospective study showed that dose‐dependent statin use was associated with significant HCC risk reduction in NASH cirrhosis.28 Although these studies show favorable findings, a study with an NAFLD cohort of 458 patients with advanced fibrosis showed no such association.29
The uncontrolled and retrospective nature of these studies limits the interpretation of the potential chemopreventive benefits of these medications and as such cannot be routinely recommended exclusively for HCC prevention.
Obesity
Numerous studies identified the association of obesity and HCC risk. Marrero et al. showed that among patients with cirrhosis, obesity has a fourfold increased HCC risk compared to subjects of normal weight (OR, 4.3; 95% CI, 2.1–8.4). Patients with obesity and cirrhosis were 47 times more likely to have HCC compared to persons without liver disease (OR, 47.8; 95% CI, 9.6–74.5).30 Moreover, Ohki et al. found that the presence of visceral fat was an independent risk factor for HCC recurrence after curative treatment (RR, 1.08 per 1 cm2 of visceral fat).31 Age of onset of adiposity also impacts the development of HCC and mortality. Obesity in early adulthood increased the risk of HCC in both men (OR, 2.3; 95% CI, 1.2–4.4) and women (OR, 3.6; 95% CI, 1.5–8.9).32 A prospective cohort study from the American Cancer Society found that cancer mortality in men and women with overweight was 52% and 62% higher compared to subjects with normal weight, respectively.33 These collective findings provided strong evidence that obesity, especially early age onset and presence of visceral fat, impacts HCC development and the associated increase in mortality.
Recent studies have highlighted the role of bariatric surgery and its oncologic benefits on HCC incidence. Rustgi et al. used a retrospective administrative database to analyze 98,090 patients with NAFLD and severe obesity, of whom 33,435 (34.1%) underwent bariatric surgery. In addition to reduced risk of obesity‐related cancers, HCC risk was also found to be lowered, with an adjusted HR of 0.48 (95% CI, 0.24–0.89).34 Similar findings were reciprocated by Ramai et al. in a retrospective study that included 19,514,750 patients (18,423,546 controls and 1,091,204 patients with bariatric surgery). The study showed a pooled unadjusted OR of 0.40 (95% CI, 0.28–0.57) for HCC incidence, favoring bariatric surgery compared with no surgery; however, the analysis was limited by high heterogeneity (I 2 = 79%).35 The beneficial outcomes with bariatric surgery could be related to the reduction of the inflammatory state, as studies have shown a progressive reduction in hepatic fibrosis and NASH with bariatric surgery.36 In summary, clinical studies evaluating bariatric surgery suggest its use beyond weight loss strategy in patients at particularly high risk; however, with the increasing global NAFLD burden, it might not be feasible to expand the indication on a population level.
Given the worldwide obesity pandemic, it is of utmost importance to risk stratify obesity for HCC development. In a case‐control study involving 518 HCC cases and 1036 frequency‐matched controls, Nasereldin et al. assessed the association of HCC risk and obesity based on individuals underlying metabolic dysfunction (i.e., dyslipidemia, hypertension, and diabetes). Authors did not find any association between HCC risk and being overweight or obese in participants without any metabolic abnormalities. However, among the participants with metabolic dysfunction, being overweight (OR, 1.89; 95% CI, 1.31–2.72) or obese (OR, 1.50; 95% CI, 1.07–2.09) was associated with higher HCC risk.37
It is reasonable to assume that HCC risk might be higher in noncirrhotic individuals with NASH compared to individuals with obesity without NASH. However, it is very premature to draw such conclusions at this time given lack of prospective studies in a diverse NAFLD population to establish these associations.
MetS
MetS is a constellation of conditions that includes obesity, impaired glucose tolerance, dyslipidemia, and hypertension. Besides its association with increased risk of cardiovascular disease, MetS is also associated with the development of HCC. In a large European cohort study comprising 578,700 subjects where 155 had HCC, Borena et al. found that MetS had an RR of 1.46 (1.16–1.84) for HCC.38 Similarly, Welzel et al. analyzed the Surveillance, Epidemiology, and End Results (SEER‐Medicare data) from 1993 to 2005 and found that MetS was significantly associated with increased HCC risk (OR, 2.13; 95% CI, 1.96–2.31; p < 0.0001).39
Mild‐moderate alcohol drinking
The deleterious effects of continuous and excessive ethanol intake on the liver are well established; however, there is uncertainty regarding the effects of mild to moderate ethanol consumption. Studies to date provided inconsistent evidence. Chang et al. assessed the relationship between mild‐moderate drinking and worsening of noninvasive fibrosis scores in 58,927 Korean adults with NAFLD and initially low fibrosis scores for a median of 4.9 years. In total, 5303 (9%) subjects had progression of FIB‐4 from low to intermediate or high scores. Those with moderate drinking were more likely to have increased fibrosis compared to nondrinkers with HR 1.29 (95% CI, 1.23).40 Excessive alcohol consumption predisposes for liver cancer.41 There are limited data on the association of mild to moderate ethanol intake and HCC risk in NAFLD. A study by Ascha et al. found that even mild drinking habit increased risk of carcinogenesis in a NASH‐associated cirrhosis cohort with HR 3.8 (95% CI, 1.6–8.9; p = 0.002). A limitation of the study was that only patients with decompensated liver disease were included.42 A multivariate analysis of a recent study that included patients with biopsy‐proven NAFLD with a spectrum of liver fibrosis severity showed that mild alcohol intake of <20 g/day increased the HCC risk, especially among those with advanced F3–4 fibrosis (p = 0.04; RR, 4.83).43
Smoking
Smoking, in general, has been associated with liver cancer. The 2014 US Surgeon General’s report found that current and former smoking was associated with a 70% and 40% increased risk of liver cancer, respectively.44 Similarly, in a meta‐analysis of 81 studies by Abdel‐Rahman et al., the pooled ORs for HCC development were 1.55 (95% CI, 1.46–1.65) in current smokers and 1.39 (95% CI, 1.26–1.52) in former smokers.45 There are no specific data on the effect of smoking in NAFLD‐related HCC risk currently.
Gut microbiome and bile acids
Increased gut permeability and altered microbiome composition are associated with NAFLD and its disease severity.46 Preclinical models have suggested the contributing role of gut microbiota in hepatocarcinogenesis.47 One of the first culture‐based studies from Poland prospectively analyzed the gut microbial profile of 15 patients with HCC and 15 non‐HCC patients and found that HCC was associated with significantly increased fecal counts of Escherichia coli (p = 0.025).48 Another study by Ponziani et al. on a NASH cohort demonstrated a reduction in Akkermansia and Bifidobacterium species among patients with HCC compared to those with cirrhosis without HCC.49 These bacterial species have also been studied in animal models, and their reduced abundance was found to be correlated with increased hepatic inflammation, which in turn can promote hepatocarcinogenesis.50
The gut microbiome has been shown to affect the diversity and pool of bile acids.51 Gut microbiome has been implicated in modulating the farnesoid X receptor (FXR), a bile acid‐activated nuclear receptor. There is evidence that FXR prevents liver injury and carcinogenesis and modulates fibrosis.52,53
Ethnicity and Genetics
Genome‐wide association studies have uncovered variations in genes such as patatin‐like phospholipase domain‐containing protein 3 (PNPLA3), transmembrane 6 superfamily member 2 (TM6SF2), membrane bound O‐acyltransferase domain‐containing 7 (MBOAT7), glucokinase regulator (GCKR), and 17‐β hydroxysteroid dehydrogenase 13 (HSD17B13) that can influence the natural history of NAFLD. Worldwide, variations in genetic and metabolic makeup that contribute to NAFLD have been identified (Figure 3). In Japanese populations, various studies have shown association of genetic polymorphism with NAFLD including PNPLA3 (rs738409‐GG genotype), microsomal triglyceride transfer protein [‐493(G/T)], adiponectin (+45GG), angiotensinogen (rs 7079), and angiotensin II (rs3772622, rs3772633, rs2276736, rs3772630, rs3772627).54–58 A different set of genetic makeup has been associated with NAFLD in Asian Indians, including apolipoprotein C3 (rs651821 C‐482T, T‐455C) and peroxisome proliferator‐activated receptor γ (Pro12Ala).59,60 On the European side, heterogeneity also exists in the genetic composition associated the with NAFLD. For instance, Italian cohort studies have identified adiponectin (+45T/G), ectoenzyme nucleotide pyrophosphate phosphodiesterase 1 (ENPP1) (Lys121Gln), and IL28B rs12979860 (cc).61–63 Similarly, in a United Kingdom and Italian cohort, ENPP1 121Gln and insulin receptor substrate‐1 972Arg polymorphisms were found to be associated with severity in patients with NAFLD.62 In a Mexican cohort, lysophospholipase‐like 1, protein phosphatase 1 regulatory subunit 3B, and GCKR were found to be associated with NAFLD.64
FIGURE 3.
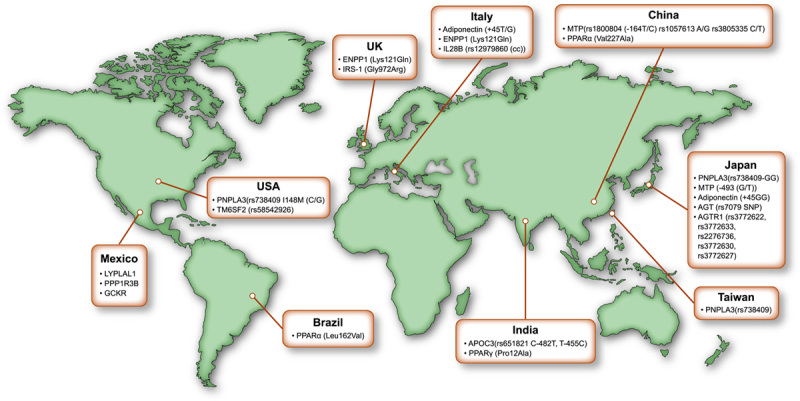
Global genetic variation contributing to NAFLD. AGT, angiotensinogen; AGTR1, angiotensin II; APOC3, apolipoprotein C3; ENPP1, ectoenzyme nucleotide pyrophosphate phosphodiesterase 1; IRS, insulin receptor substrate; MTP, microsomal triglyceride transfer protein; PNPLA3, patatin‐like phospholipase domain‐containing protein 3; PPAR, peroxisome proliferator‐activated receptor.57–67
There are significant racial and ethnic disparities in the prevalence of NAFLD in the US, with the highest prevalence in Hispanic populations and lowest in Black populations.65 Hispanic populations are noted to have the highest rate of MetS, intraperitoneal and hepatic fat content, and NAFLD‐related HCC.66–68 A study by Kallwitz et al. showed that Hispanics with American ancestry had increased risk for NAFLD whereas those with African and European ancestry had inverse relation with NAFLD.69
More recently, there is an increased awareness of the genetic predispositions of NAFLD and HCC. PNPLA3 has very strong association with NAFLD.70–72 In a case‐control study, Hassan et al. investigated the impact of PNPLA3 genetic variation (rs738409: C>G) on HCC risk between 257 histologically confirmed HCC (60.7% cirrhotic) and 494 healthy controls and found that the GG genotype was found to a have higher risk of HCC for subjects than CC or CG genotypes (OR, 3.21; 95% CI, 1.7–6.4).73 Similarly, a study by Liu et al. looked at the PNPLA3 rs738409 genotype frequencies between 100 subjects with NAFLD‐related HCC and 275 controls with histologically proven NAFLD. Their study showed that after adjusting for age, sex, DM, body mass index (BMI), and presence of cirrhosis, carriage of each copy of the rs738409 minor (G) allele conferred an additive risk for HCC (adjusted OR 2.26 [95% CI, 1.23–4.14]; p = 0.0082), with GG homozygotes exhibiting a fivefold [1.47–17.29], p = 0.01 increased risk over CC. This risk effect was even more pronounced when GG homozygotes were compared with the general population of the UK CC homozygotes (OR, 12.19; 95% CI, 6.89–21.58; p < 0.0001).71
A meta‐analysis of 24 studies with 9915 patients looking at the effect of PNPLA3 on fibrosis progression and HCC occurrence showed that PNPLA3 was associated with a higher risk of HCC in patients with cirrhosis (OR, 1.4; 95% CI, 1.12–1.75). This risk was even higher in patients with NASH or alcohol‐related cirrhosis (OR, 1.67; 95% CI, 1.27–2.21) in a subgroup analysis but not with other etiologies of cirrhosis.74 In multivariate models after adjusting for confounding factors in a cohort of 1020 patients with HCC, 2484 healthy subjects, and 2021 patients with CLDs, Yang et al. showed that both the PNPLA3 and TM6SF2 polymorphism were associated with the development of HCC (OR, 1.67 and 1.45, respectively).75
In a Japanese cohort of 902 patients with histologically proven NAFLD, including 58 NASH‐HCC cases, a significant association of PNPLA3 was observed between NASH‐HCC and controls (OR, 3.37; 95% CI, 2.21–5.14; p = 1.8 × 10‐8).76 Another retrospective study by Seko et al. included a Japanese cohort of 238 patients with biopsy‐proven NAFLD. Over a follow‐up of 6.1 years, 10 patients (4.2%) developed NASH‐HCC, and PNPLA3 genotype GG was found to be an independent risk factor for HCC with HR 6.36; p = 0.019.77
PATTERNS AND RISKS OF NAFLD‐RELATED HCC (Figure 4)
FIGURE 4.
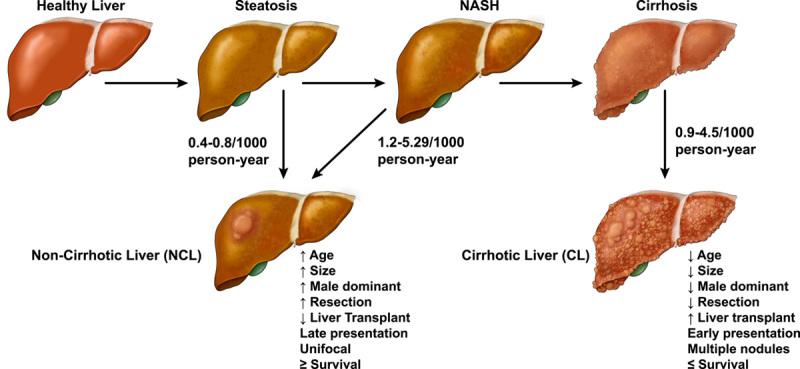
Natural history of NAFLD‐related hepatocellular carcinoma (HCC). Data for reported incidences from references.7,80,89,90 Reported HCC incidence in simple steatosis and NASH in the figure are based on studies that utilized histological diagnosis as inclusion criteria. Given the limited number of studies, these rates might not be a robust reflection of actual incidence.
The rates of progression of NAFLD to HCC varied according to different fibrosis stages.78,79 A recent meta‐analysis showed that the incidence of HCC increased from 0.4/1000 (95% CI, 0.29–0.66) in isolated steatosis to 5.29/1000 (95% CI, 0.75–37.5) in established NASH, which is an increase over 10‐fold.80 Similarly, in a Swedish study, the absolute rates of HCC per 1000 patients/year with isolated steatosis, NASH without fibrosis, early fibrosis, and cirrhosis progressively increased from 0.8, 1.2, 2.3, to 6.2, respectively.7 Studies have attempted to determine the relative importance of histological parameters such as steatosis, inflammation, and fibrosis in contributing HCC development. In a Veterans Affairs (VA) study, patients with MetS were found to have more than a fivefold risk of developing HCC in the absence of cirrhosis compared with patients with hepatitis‐related HCC.81 Angulo et al. found that fibrosis was an independent risk of HCC regardless of the presence of steatosis and inflammation in NAFLD.82
There are differential patterns and risks of NAFLD‐related HCC in NCL and CL as summarized in Table 1 and Figure 4. In a prospective Japanese cohort of 935 patients with NAFLD, Tobari et al. identified that NCL HCC were more likely to be associated with older age, male sex, and large tumor size compared to those with CL HCC. There were, however, no differences in tumor histology or differentiation between the two groups. Those with NCL HCC had significantly higher 5‐ and 10‐year survival rates of 68.6% NCL versus 47.8% CL and 57.3% NCL versus 12.9% CL, respectively. That was most likely due to a lower recurrence rate and preserved liver function in NCL HCC.83 In a Swedish cohort of 225 patients with NAFLD‐related HCC, 83 with NCL HCC were compared with 142 with CL HCC.84 The NCL HCC cohort was significantly older, had a low prevalence of type 2 diabetes mellitus (T2DM), had larger tumors, and more frequently had tumor resection but liver transplantation (LT) was less frequent. There was no significant difference in survival; the median survival was 16 months in this cohort.84 In a retrospective analysis in the US, Mohamad et al. reported similar findings among 36 patients with NCL HCC and NAFLD and 47 patients with CL HCC and NAFLD. Patients with NCL HCC were older, less likely to have diabetes, presented with a single nodule and large tumor size, and were more likely to undergo tumor resection than LT.85 Leung et al. studied the characteristics of HCC in an Australian cohort of 8 NCL HCC with 46 CL HCC and found that patients with NCL had larger tumor size at diagnosis and failed to meet Milan criteria for LT, whereas no significant difference was seen in age, T2DM, or HCC differentiation.86 These HCC risk predispositions between patients with NAFLD NCL and patients with CL NAFLD were further evaluated in a recent international retrospective study.87 Among 470 with NCL HCC and 770 with CL HCC, Chen et al. reported that the NCL HCC cohort had more male patients, had less frequent diabetes, had higher rates of unifocal cancer, and were more likely to have tumor resection than LT. No survival difference was seen.87 The study results of Kodama et al. reinforced the observations that in patients without advanced stage 3–4 fibrosis, their tumor size tended to be significantly larger but HCC recurrence was lower after curative treatment.88
TABLE 1. Studies comparing NAFLD‐related HCC in patients who are noncirrhotic versus patients who are cirrhotic.
| Country, study period | No. of patients with HCC | Survival/mortality rate | Recurrence rate (no. of patients) (rate) | (Noncirrhotic NAFLD vs. cirrhotic NAFLD) |
|---|---|---|---|---|
| Japan, 1991–201883 | 48 vs. 71 | 10‐year survival 57.3% vs. 12.9% (p < 0.01) | (34 HCC vs. 49) 5‐year 40.9% vs. 85% (p < 0.01) | >Male sex, >age, >light drinker, >tumor size, >dyslipidemia, <T2D, =HCC histology and differentiation |
| Sweden, 2004–201784 | 83 vs. 142 | Mortality rate 71% vs. 69% (aHR, 0.93; 95% CI, 0.58–1.51; p = 0.78) | >Age, =male sex, <T2D, =HCC differentiation, >tumor size, <LT, >resection | |
| USA, 2003–201285 | 36 vs. 47 | Mortality rate (aHR, 1.5; 95% CI, 0.57–4; p = 0.4) | 21 HCC vs. 37 86% vs. 14% (p < 0.001) | >Age, =male sex, <T2D, =HCC differentiation, >tumor size, >single nodule, <LT, >resection, |
| Australia, 2000–201286 | 8 vs. 46 | Male sex not assessed, =age, =T2D, =HCC differentiation, >tumor size larger, =median no of HCC, >failed the Milan criteria for LT, >resection | ||
| USA and East/Southeast Asia, 2005–201787 | 470 vs. 770 | Survival (HR, 1.14; 95% CI, 0.94–1.37) | >Male sex, =age, <T2D, HCC differentiation not assessed, >tumor size larger in noncirrhotic p < 0.001, >unifocal cancer, >resection, <LT |
Note: >significantly higher/larger, <significantly lower/smaller, =no significant difference.
Abbreviations: aHR, adjusted hazard ratio; CI, confidence interval; HCC, hepatocellular carcinoma; HR, hazard ratio; LT, liver transplantation; T2D, type 2 diabetes mellitus.
In summary, NAFLD‐related HCC among NCL usually presents in older male individuals with a larger unifocal tumor and are more likely to undergo resection than LT compared to NCL HCC. There were conflicting findings on mortality; some studies found no difference, whereas others reported that NCL HCC had better survival over CL HCC. This could be secondary to overall preserved liver function and lower tumor recurrence of patients with NCL HCC.66
RISK PREDICTION MODELS FOR NAFLD‐RELATED HCC IN PATIENTS WITH CIRRHOSIS
There are established guidelines for HCC surveillance in all patients with cirrhosis regardless of the underlying etiology. Because HCC risk is not uniform across all patients with cirrhosis, many prediction models were developed to better risk stratify the patients (Table 2). One of the first such models is the ADRESS‐HCC risk model that was applied to estimate the 1‐year probability of HCC among 34,932 patients with cirrhosis with various etiologies. Six baseline clinical variables age, diabetes, race, etiology of cirrhosis, sex, and severity of liver dysfunction were found to be independently associated with HCC and were used to develop the ADRESS prediction model. After rigorous internal and external validation, the model was found to have a moderate ability to separate patients with cirrhosis who will or will not develop HCC based on a concordance index (c‐index) of 0.7.91 Knowing the disease‐specific incidence of HCC is very important to guide the frequency of HCC surveillance. Sharma et al. developed a score known as the Toronto HCC Risk Index (THRI). Their cohort consisted of 2079 patients with cirrhosis with different etiologies and 226 (10.8%) developed HCC. Their model was able to separate patients into risk groups based on scores. The 10‐year cumulative HCC incidence was predicted to be 3%, 10%, and 32% with scores <120, 120–240, and >240, respectively.92 Morat et al. further validated the THRI score in a weighted multivariate model. There were 752 patients with cirrhosis who had ALD (n = 529), chronic hepatitis C virus (HCV) infection (n = 145), and NAFLD (n = 78). Among them, 85 patients (11%) developed HCC. They concluded that an individualized model is more useful for the prediction of HCC occurrence in patients with cirrhosis.93 Ioannou et al. developed models estimating HCC risk in patients from a VA cohort of 7068 patients with cirrhosis and NAFLD. The mean annualized HCC incidence was 1.56%. The final model included seven predictors: age, sex, diabetes, BMI, platelet count, serum albumin, and aspartate aminotransferase/alanine aminotransferase (ALT) ratio. The models exhibited a very good area under the receiver operating characteristic curve (AUROC) of 0.75 for NAFLD‐cirrhosis.94 In an Italian study consisted of a long follow‐up of 471 consecutive patients with NAFLD‐related cirrhosis, Grimaudo et al. confirmed that the combined values of PNPLA3 genotypes, liver function tests, and portal hypertension status were able to stratify the HCC risk.95 More recently, Lambrecht et al. developed an APAC score based on age, soluble platelet‐derived growth factor receptor beta, α‐fetoprotein (AFP), and creatinine. The score was evaluated in a cohort of 267 patients with cirrhosis with various etiologies; among them, 122 had HCC. The APAC score was able to predict HCC more accurately than the GALAD score (area under curve [AUC] 0.95 vs. 0.90, p = 0.003). In a subanalysis of NAFLD‐related cirrhosis, the APAC score performed equally well with an AUC of 0.95. The results suggest that the diagnostic accuracy of the APAC score was independent of the etiology of the underlying disease.96
TABLE 2. Risk prediction models for NAFLD‐related HCC in patients with cirrhosis.
| Model | Country | Study design | Output | Variables | Major etiology | Predictive ability | Validation |
|---|---|---|---|---|---|---|---|
| THRI score92 | Canada | Retrospective | 10‐year HCC incidence | Age, sex, etiology, platelets | Steatohepatitis, viral, PBC, AIH | C Statistic | Internal |
| Low risk: 3% | Validation cohort: 0.77 | External | |||||
| Medium risk: 10% | Derivation cohort: 0.77 | ||||||
| High risk: 32% | |||||||
| ADRESS‐HCC91 | USA | Retrospective | 1‐year HCC risk | Age, diabetes, race, etiology, sex, Child‐Pugh score | NASH, HCV, alcohol, HBV, other | C Statistic | Internal |
| Score ≥ 4.67: ≥1.5% per year | Derivation: 0.704 | External | |||||
| Validation: 0.691 | |||||||
| Grimaudo et al.95 | Italy | Prospective longitudinal | aHR | PNPLA3 rs738409, F3–4 fibrosis, liver function, portal hypertension | NAFLD | No | |
| PNPLA3 G variant: 2.68; p = 0.04 | |||||||
| F3–4 fibrosis (Inf p < 0.001) | |||||||
| Morat et al.93 | Belgium | Observational | 10‐year HCC incidence | Weighted scores | NAFLD, HCV, alcohol | Validation of THRI score | |
| Low risk: 9.1% | Age, sex, etiology, platelets | ||||||
| Medium risk: 15.7% | |||||||
| High risk: 29% | |||||||
| Ioannou et al.94 | USA | Retrospective | 5‐year HCC risk | Age, sex, diabetes, BMI, platelet count, serum albumin and AST/√ALT ratio | NAFLD, alcohol | C Statistic 0.75 for HCC in NAFLD‐cirrhosis | Internal |
| Low risk: <5% | |||||||
| Medium risk: 5%–15% | |||||||
| High risk: >15% | |||||||
| Annual HCC risk | |||||||
| Low risk: 0%–1% | |||||||
| Medium risk: >1%–3% | |||||||
| High risk: >3% | |||||||
| APAC score96 | Germany | Observational cohort study | Age, sPDGFRβ, AFP, and creatinine | NAFLD, viral, alcohol | AUROC 0.95 (95% CI, 0.91–0.99) SN: 84.62%, SP: 90.91% for HCC in NAFLD‐cirrhosis | Internal |
Abbreviations: AFP, α‐fetoprotein; aHR, adjusted hazard ratio; AIH, autoimmune hepatitis; AST, aspartate aminotransferase; AUC, area under curve; AUROC, area under the receiver operating characteristic curve; BMI, body mass index; CI, confidence interval; HBV, hepatitis B virus; HCC, hepatocellular carcinoma; HCV, hepatitis C virus; PBC, primary biliary cholangitis; PNPLA3, patatin‐like phospholipase domain‐containing protein 3; SN, sensitivity; SP, specificity; sPDGFRβ, soluble platelet‐derived growth factor receptor beta; THRI, Toronto HCC Risk Index.
RISK PREDICTION MODELS FOR NAFLD‐RELATED HCC IN PATIENTS WITHOUT CIRRHOSIS
Current guidelines recommend HCC surveillance if the annual HCC risk is >1.5%. That was based on the cost‐effective analysis. This recommendation is appropriate for patients with NAFLD and cirrhosis.97 However, up to 30% of patients with NAFLD‐related HCC do not have cirrhosis,98 and HCC risk in NAFLD NCL is approximately 0.1 to 0.8 per 1000 patient‐years.4–7 Given this dilemma, cost‐effective HCC surveillance strategies based on individualized genetic and clinical profiles to identify patients with high‐risk NAFLD without cirrhosis are needed. The polygenic risk score (PRS) has shown encouraging performance in diagnosing HCC among patients with NAFLD NCL. One of the first studies utilizing PRS along with clinical factors was done in an Italian cohort of 765 patients with NAFLD NCL. Donati et al. developed a combined risk score incorporating the clinical (age, sex, obesity, T2DM, advanced fibrosis) and genetic risk factors (PNPLA3 I148M, TM6SF2 E167K, and MBOAT7 rs641738 C>T). The resulting model had a 0.96 ± 0.4 AUROC for detecting HCC cases with 96% sensitivity and 89% specificity. When they only applied the clinical factors to the model, the AUROC (0.93 ± 0.5) remained favorable. Thus, the addition of a polygenic component to their model did not significantly improve the predictive accuracy of clinical factors. This nonsignificance was maintained even in a subgroup analysis of patients without severe fibrosis.99 Another study by Pelusi et al. evaluated the contributions of rare pathogenic variants in addition to known genetic variants to HCC risk in a European cohort of 142 with NAFLD‐HCC, 59 with NAFLD with advanced fibrosis, 50 matched healthy controls, and 404 healthy individuals from the 1000 Genomes database. They were able to detect enrichment of rare pathologic variants in the candidate genes among NAFLD‐HCC cases versus controls (OR, 3.5; 95% CI, 2.2‐inf; p = 1.9 × 10‐6). Their comprehensive PRS, including the rare variants, predicted NAFLD‐HCC with higher diagnostic accuracy (OR, 4.96; 95% CI, 3.29–7.55), and it significantly outperformed the common genetic risk factors, including the PNPLA3 I148M variant alone, or a combination of PNPLA3 I148M and TM6SF2 E167K variant. Also, addition of the PRS to a model based on the classical risk factors (age, sex, presence of type 2 diabetes and of advanced fibrosis) increased the ability to discriminate NAFLD‐HCC (AUC 0.903 vs. 0.89, p = 0.03).100 As noted in previous studies, different genetic polymorphisms had variable effects on HCC risks; HSD17B13, for example, has protective effects, whereas others like PNPLA3 (I148M variant) increase the risk.101,102 PRS with weighted effect of each genetic variant likely would be more predictive. Bianco et al. applied the weighted effect of known genetic variants to compose HCC risk scores for patients with NAFLD with CL and NCL. They developed two scoring systems, namely, PRS‐HFC with four variants (PNPLA3, TM6SF2, MBOAT7, GCKR) and PRS‐5, a modified PRS‐HFC score with adjustment for the rs72613567 HSD17B13 variant. These scores were evaluated in an NAFLD cohort of 2566 patients that included 226 with HCC (Italian and UK cohort). The scores were subsequently validated with a cohort of 427 German patients with NAFLD (16.8% with HCC, n = 72) and 364,048 subjects from general population of UK Biobank cohort that included 202 with HCC. These scores were able to predict HCC equally well in subjects with NAFLD both with and without cirrhosis.103 Similarly, Gellert‐Kristensen et al. showed large populations of 110,761 individuals from the Danish general population and 334,691 individuals from the UK Biobank in whom PNPLA3, TM6SF2, and HSD17B13 variants were assessed and translated into PRS ranging from 0 to 6 risk. This PRS was found to be associated with up to 12‐fold higher risk of cirrhosis and up to 29‐fold higher risk of HCC.104 These results of PRS are promising in identifying patients with increased HCC risk; however, there are limitations. Firstly, most studies used AUROC as a prediction tool in defined patient populations that needs to be carefully validated in the population. Secondly, these scores were derived from individuals of European descent and may not be generalizable to populations of other ethnicities. It is very likely that different PRS need to be developed for patients with various ethnicity‐specific genetic backgrounds.
More recently, risk stratification scores have been developed in predicting NAFLD‐related HCC among those with NCL without using any genetic input as a variable. The GALAD score (based on patient sex; age; and serum levels of AFP, AFP isoform L3, and des‐gamma‐carboxy prothrombin) has been applied in several studies with favorable results. In a German study, GALAD score was evaluated in 356 patients with NAFLD (125 with and 231 without HCC), and it was able to identify patients with various stages of HCC with an AUROC of 0.96. In a subgroup analysis that included only patients with NCL NASH (HCC, n = 30 vs. 182 controls), GALAD achieved an AUROC of 0.98 for detection of HCC with 93.3% sensitivity and 96.1% specificity. This study demonstrated that the GALAD score could predict HCC regardless of the cirrhosis status of patients with similar accuracy.105 Sinn et al. developed and validated a novel risk score for HCC using six independent risk factors including age, sex, ALT, total cholesterol, DM, and smoking. The score was developed in a general population cohort (n = 467,206) in South Korea that excluded patients with viral hepatitis, cirrhosis, and heavy alcohol use. The model was able to stratify patients with 10‐year risk HCC risk ranging from 0.0% (lowest) to 6.16% (highest) with AUROC of 0.83 (95% CI, 0.77–0.88). The validation cohort (n = 91,357) had AUROC 0.92 (95% CI, 0.89–0.95). This study was limited because the diagnosis of NAFLD was not clearly defined; ALT was used as a surrogate for NAFLD.106 In a longitudinal European study, Younes et al. applied a number of established scoring systems; namely, NAFLD‐fibrosis score (NFS), Fibrosis‐4 score (FIB‐4), BARD (BMI, AST/ALT ratio, and diabetes) score, APRI score, and the Hepamet fibrosis score (HFS) to predict HCC in 1173 patients with NAFLD with NCL. These patients were followed for a mean follow‐up period of 81 months, and 17 patients (1.5%) developed HCC. NFS performed significantly better than any other NSS (c‐index 0.901 ± 0.0302; AUC ingrated across time (iAUC) = 0.889 ± 0.048). This was followed by FIB‐4 (c‐index 0.853 ± 0.0516), HFS (c‐index 0.824 ± 0.0578), and BARD (c‐index 0.772 ± 0.0345), in descending order.107 In future studies, some of these clinical scores could be combined with PRS for comprehensive risk stratification of patients with NAFLD (Table 3).
TABLE 3. Risk prediction models for NAFLD‐related hepatocellular carcinoma in patients without cirrhosis.
| Model | Study design | Variables | Population | Country | Predictive ability (AUROC, HR) | Validation |
|---|---|---|---|---|---|---|
| Bianco et al.103 | Cross‐sectional | PRS‐HFC: PNPLA3‐TM6SF2‐MBOAT7‐GCKR | NAFLD with NCL | Italy | PRS‐HFC: 0.64 (43% SN, 80% SP) | Yes |
| PRS‐5: PRS‐HFC score adjusted for rs72613567 HSD17B13 variant | UK | PRS‐5: 0.65 (43% SN, 79% SP) | ||||
| Germany | ||||||
| Gellert‐Kristensen et al.104 | Prospective | PRS: PNPLA3+TM6SF2+HSD17B13 | General population | UK | HR 29 (95% CI, 17–51), p < 0.001 | No |
| Denmark | ||||||
| Pelusi et al.100 | Retrospective cohort | PRS: PNPLA3+TM6SF2+MBOAT7+variants | NAFLD with NCL | Italy | 0.9 ± 0.04 (93% SN, 86% SP) | Yes |
| Clinical: Age, sex, obesity, T2DM, severe fibrosis | UK | |||||
| Non‐Finnish Europeans | ||||||
| Donati et al.99 | Retrospective cohort | PRS: PNPLA3, TM6SF2, and MBOAT7 | NAFLD with NCL | Italy | 0.96 ± 0.04 (96% SN, 89% SP) | No |
| Clinical: Age, sex, obesity, T2DM, severe fibrosis | ||||||
| Sinn et al.106 | Retrospective cohort | Age, sex, smoking, DM, total cholesterol, ALT | General population | South Korea | 0.83 (95% CI, 0.77–0.88) | Yes |
| Best et al.105GALAD score | Retrospective cohort | Sex, age, AFP‐L3, AFP, des‐gamma carboxyprothrombin | NAFLD with NCL and CL | Germany | CL 0.93 (93.3% SN and 96.1% SP) | Validation of established NSS |
| NCL 0.98 (85.7% SN and 96.2% SP) | ||||||
| NFS | Longitudinal Study Younes et al.107 | Age, BMI, DM, AST, ALT, platelets, albumin | NAFLD with NCL and CL | UK | 0.901 ± 0.0302a | Validation of established NSS |
| FIB‐4 | Age, AST, ALT, platelets | Italy | 0.853 ± 0.0516 | |||
| APRI | AST, ALT, platelet | Spain | 0.788 ± 0.0362 | |||
| BARD | BMI, AST, ALT, T2DM | 0.772 ± 0.0345 | ||||
| HFS | Age, sex, AST, albumin, HOMA, DM, platelets | 0.824 ± 0.0578) |
Abbreviations: AFP, α‐fetoprotein; AFP‐L3, AFP isoform L3; ALT, alanine aminotransferase; AST, asparatate aminotransferase; AUROC, area under the receiver operating characteristic curve; BARD, BMI, AST/ALT ratio, and diabetes; BMI, body mass index; CI, confidence interval; CL, cirrhotic liver; DM, diabetes mellitus; FIB‐4, fibrosis‐4; GCKR, glucokinase regulator; HFS, Hepamet fibrosis score; HOMA, homeostatic model assessment; HR, hazard ratio; HSD17B13, 17‐β hydroxysteroid dehydrogenase 13; MBOAT7, membrane bound O‐acyltransferase domain‐containing 7; PRS, polygenic risk score; NCL, noncirrhotic liver; PNPLA3, patatin‐like phospholipase domain‐containing protein 3; SN, sensitivity; SP, specificity; T2DM, type 2 diabetes mellitus; TM6SF2, transmembrane 6 superfamily member 2.
Denotes statistically higher c‐indices with respect to the NSS without asterisk for the same comparison.
HCC RISK PREDICTION MODELS WITH LS IN HCV AFTER DIRECT‐ACTING ANTIVIRAL
In the direct‐acting antiviral (DAA) era, the risk of HCC continues to persist among patients with chronic hepatitis C (CHC) despite the high rates of sustained virological response (SVR) especially among those with preexisting cirrhosis. At 1, 2, and 3‐year post‐SVR, the cumulative incidence of HCC was 1.1%, 1.9%, and 2.8%, respectively.108 In a number of studies, NAFLD was believed to contribute to the HCC occurrence in a subset of patients with CHC.109–111 Peleg et al. showed that LS is an independent and strong predictor of all‐cause mortality and HCC among patients with CHC who achieved SVR. In that study, 515 patients with CHC who achieved DAA‐induced SVR were followed for a mean duration of 24 months. In the first model, LS was significantly associated with HCC (HR, 7.51; 95% CI, 3.61–13.36; p < 0.001) even after adjustment to other components of the MetS. In the second model, patients who had both LS and advanced fibrosis were found to have the highest HCC risk and all‐cause mortality (HR, 17.56; 95% CI, 2.37–75.11; p = 0.005), which was followed by the presence of LS without advanced fibrosis (HR, 9.21; 95% CI, 1.11–62.53; p = 0.030).111 Degasperi et al. recently assessed the association between PRS (consisting of PNPLA3, MBOAT7, TM6SF2, GCKR) of LS and HCC in patients with CHC treated with DAAs in an Italian cohort. They followed 509 consecutive patients with cirrhosis and found that during a median follow‐up of 43 (3–57) months, 36 of 452 (8%) patients developed de novo HCC. They showed that PRS score >0.597 (HR, 2.30; p = 0.04) was an independent predictor of de novo HCC. Adding clinical factors (male sex, diabetes, albumin) to the PRS model further improved HCC risk prediction. Combining both genetic and clinical variables, patients with ≥3 risk factors had a significantly higher 4‐year cumulative de novo HCC incidence than those with <3 risk factors (80% vs. 8%). These data strongly suggest that LS promotes HCC.109 More recently, Ji et al. evaluated a prospective cohort of patients with CHC who achieved SVR after DAA (n = 519) and pegylated interferon‐based therapy (n = 817), respectively. After a median post‐SVR follow‐up of 48 months, HCC developed in 54 (4.4%) subjects. They formulated a nomogram to estimate the HCC risk among patients with CHC and SVR. The presence of NAFLD contributed independently to the HCC risks. The nomogram had a c‐index of 0.835 (95% CI, 0.783–0.866).110
FUTURE PERSPECTIVE, RESEARCH DIRECTIONS
The high NAFLD prevalence exponentially contributes to the overall disease burden of CLD. NAFLD‐related HCC in NCL is increasingly recognized and alarming. New strategies are necessary for HCC screening in these patient populations. Routine HCC surveillance of all patients with NAFLD and NCL would severely constrain the healthcare system and is not cost‐effective. It is of paramount importance to develop optimal risk stratification scores and models to identify subsets of the population with high risks so they can be enrolled in surveillance programs (Figure 5). Such HCC prediction models need to be carefully validated in prospective cohorts of diverse populations to confirm their broad applicability. Because patients with NAFLD and NCL are mostly encountered in primary care settings, it would be ideal if the HCC prediction scores can be generated with simple demographic and clinical variables; that would facilitate the timely referral of patients at greatest HCC risk. Allocated resources are needed to develop the HCC surveillance programs. Equally importantly, ongoing research efforts are essential to identify disease modifying factors and treatment options that can prevent NAFLD disease progression and HCC complication.
FIGURE 5.
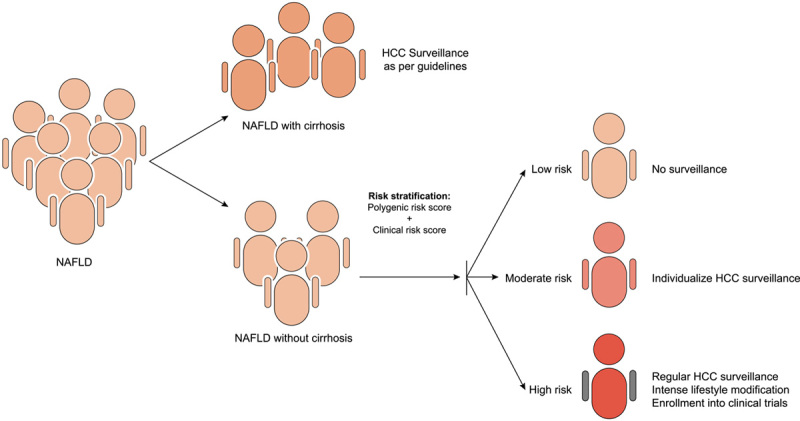
Model for risk stratification in NAFLD‐related hepatocellular carcinoma (HCC).
Acknowledgments
AUTHOR CONTRIBUTIONS
Conceptualization: Pir Ahmad Shah and Stephen A. Harrison. Writing ‐ Original Draft: Pir Ahmad Shah, Rashmee Patil, and Stephen A. Harrison. Writing ‐ Review & Editing: Pir Ahmad Shah, Rashmee Patil, and Stephen A. Harrison. Supervision: Stephen A. Harrison.
CONFLICTS OF INTEREST
Dr. Harrison owns stock in Akero Therapeutics, Galectin Therapeutics, Genfit Corp, Hepion Pharmaceuticals, Metactine, NGM Biopharmaceuticals, Chronwell, Cirius Therapeutics, and HistoIndex. Dr. Harrison consults for and advises Altimmune, Alimentiv, Akero Therapeutics, Axcella Pharmaceuticals, Boston Pharmaceuticals, Echosens North America, Enyo Pharma S.A., Galectin Therapeutics, Genfit Corp, Gilead Sciences, Hepion Pharmaceuticals, Medpace, Metacrine, NGM Biopharmaceuticals, Novartis, Northsea Therapeutics, Cirius Therapeutics, HistoIndex, Hightide Therapeutics, Intercept Pharmaceuticals, Madrigal Pharmaceuticals, Novo Nordisk, PathAI, Perspectum Diagnostics, Poxel, Sagimet Biosciences, Sonic Incytes, Terns, and Viking. Dr. Harrison has received grants from Altimmune, Akero, Axcella Pharmaceuticals, Boehringer Ingelheim, Corcept Therapeutics, Enyo Pharma S.A., Ionis, 89Bio, Galectin, Gilead, Genfit, Hepion Therapeutics, Intercept, Madrigal Pharmaceuticals, Novo Nordisk, Novartis, NGM Biopharmaceuticals, Poxel, Sagimet Biosciences, Viking.
Footnotes
Abbreviations: AFP, α‐fetoprotein; ALT, alanine aminotransferase; AUC, area under curve; AUROC, area under the receiver operating characteristic curve; BMI, body mass index; CHC, chronic hepatitis C; CI, confidence interval; c‐index, concordance index; CL, cirrhotic liver; CLD, chronic liver disease; DM, diabetes mellitus; GCKR, glucokinase regulator; HCC, hepatocellular carcinoma; HCV, hepatitis C virus; HFS, Hepamet fibrosis score; HR, hazard ratio; HSD17B13, 17‐β hydroxysteroid dehydrogenase 13; LS, liver steatosis; LT, liver transplantation; MBOAT7, membrane bound O‐acyltransferase domain‐containing 7; MetS, metabolic syndrome; NCL, noncirrhotic liver; OR, odds ratio; PNPLA3, patatin‐like phospholipase domain‐containing protein 3; PRS, polygenic risk score; RR, risk ratio; SVR, sustained virological response; T2DM, type 2 diabetes mellitus; THRI, Toronto HCC Risk Index; TM6SF2, transmembrane 6 superfamily member 2.
REFERENCES
- 1.Le MH, Yeo YH, Li X, Li J, Zou B, Wu Y, et al. 2019 global NAFLD prevalence: a systematic review and meta‐analysis. Clin Gastroenterol Hepatol. 2021. 10.1016/j.cgh.2021.12.002 [DOI] [PubMed] [Google Scholar]
- 2.Fitzmaurice C, Abate D, Abbasi N, Abbastabar H, Abd‐Allah F, Abdel‐Rahman O, et al. Global, regional, and national cancer incidence, mortality, years of life lost, years lived with disability, and disability‐adjusted life‐years for 29 cancer groups, 1990 to 2017: a systematic analysis for the global burden of disease study. JAMA Oncol. 2019;5:1749–68. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Akinyemiju T, Abera S, Ahmed M, Alam N, Alemayohu MA, Allen C, et al. The burden of primary liver cancer and underlying etiologies from 1990 to 2015 at the global, regional, and national level: results from the Global Burden of Disease Study 2015. JAMA Oncol. 2017;3:1683–91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Huang DQ, El‐Serag HB, Loomba R. Global epidemiology of NAFLD‐related HCC: trends, predictions, risk factors and prevention. Nat Rev Gastroenterol Hepatol. 2021;18:223–38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Kogiso T, Tokushige K. The current view of nonalcoholic fatty liver disease‐related hepatocellular carcinoma. Cancers. 2021;13:516. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Younossi ZM, Golabi P, de Avila L, Paik JM, Srishord M, Fukui N, et al. The global epidemiology of NAFLD and NASH in patients with type 2 diabetes: a systematic review and meta‐analysis. J Hepatol. 2019;71:793–801. [DOI] [PubMed] [Google Scholar]
- 7.Simon TG, Roelstraete B, Sharma R, Khalili H, Hagström H, Ludvigsson JF. Cancer risk in patients with biopsy‐confirmed nonalcoholic fatty liver disease: a population‐based cohort study. Hepatology. 2021;74(5):2410–23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Wong RJ, Aguilar M, Cheung R, Perumpail RB, Harrison SA, Younossi ZM, et al. Nonalcoholic steatohepatitis is the second leading etiology of liver disease among adults awaiting liver transplantation in the United States. Gastroenterology. 2015;148:547–555. [DOI] [PubMed] [Google Scholar]
- 9.Chalasani N, Younossi Z, Lavine JE, Charlton M, Cusi K, Rinella M, et al. The diagnosis and management of nonalcoholic fatty liver disease: practice guidance from the American Association for the Study of Liver Diseases. Hepatology. 2018;67:328–57. [DOI] [PubMed] [Google Scholar]
- 10.Younossi Z, Stepanova M, Ong JP, Jacobson IM, Bugianesi E, Duseja A, et al. Nonalcoholic steatohepatitis is the fastest growing cause of hepatocellular carcinoma in liver transplant candidates. Clin Gastroenterol Hepatol. 2019;17:748–55.e3. [DOI] [PubMed] [Google Scholar]
- 11.Younossi ZM, Otgonsuren M, Henry L, Venkatesan C, Mishra A, Erario M, et al. Association of nonalcoholic fatty liver disease (NAFLD) with hepatocellular carcinoma (HCC) in the United States from 2004 to 2009. Hepatology. 2015;62:1723–30. [DOI] [PubMed] [Google Scholar]
- 12.Dyson J, Jaques B, Chattopadyhay D, Lochan R, Graham J, Das D, et al. Hepatocellular cancer: the impact of obesity, type 2 diabetes and a multidisciplinary team. J Hepatol. 2014;60:110–17. [DOI] [PubMed] [Google Scholar]
- 13.Pais R, Fartoux L, Goumard C, Scatton O, Wendum D, Rosmorduc O, et al. Temporal trends, clinical patterns and outcomes of NAFLD‐related HCC in patients undergoing liver resection over a 20‐year period. Aliment Pharmacol Ther. 2017;46:856–63. [DOI] [PubMed] [Google Scholar]
- 14.Cho EJ, Kwack MS, Jang ES, You SJ, Lee JH, Kim YJ, et al. Relative etiological role of prior hepatitis B virus infection and nonalcoholic fatty liver disease in the development of non‐B non‐C hepatocellular carcinoma in a hepatitis B‐endemic area. Digestion. 2011;84(Suppl 1):17–22. [DOI] [PubMed] [Google Scholar]
- 15.Liew ZH, Goh GB, Hao Y, Chang PE, Tan CK. Comparison of hepatocellular carcinoma in patients with cryptogenic versus hepatitis B etiology: a study of 1079 cases over 3 decades. Dig Dis Sci. 2019;64:585–90. [DOI] [PubMed] [Google Scholar]
- 16.Myers S, Neyroud‐Caspar I, Spahr L, Gkouvatsos K, Fournier E, Giostra E, et al. NAFLD and MAFLD as emerging causes of HCC: a populational study. JHEP Rep. 2021;3:100231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Yang JD, Ahmed F, Mara KC, Addissie BD, Allen AM, Gores GJ, et al. Diabetes is associated with increased risk of hepatocellular carcinoma in patients with cirrhosis from nonalcoholic fatty liver disease. Hepatology. 2020;71:907–16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Alexander M, Loomis AK, van der Lei J, Duarte‐Salles T, Prieto‐Alhambra D, Ansell D, et al. Risks and clinical predictors of cirrhosis and hepatocellular carcinoma diagnoses in adults with diagnosed NAFLD: real‐world study of 18 million patients in four European cohorts. BMC Med. 2019;17:95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Kanwal F, Kramer JR, Li L, Dai J, Natarajan Y, Yu X, et al. Effect of metabolic traits on the risk of cirrhosis and hepatocellular cancer in nonalcoholic fatty liver disease. Hepatology. 2020;71:808–19. [DOI] [PubMed] [Google Scholar]
- 20.Hassan MM, Curley SA, Li D, Kaseb A, Davila M, Abdalla EK, et al. Association of diabetes duration and diabetes treatment with the risk of hepatocellular carcinoma. Cancer. 2010;116:1938–46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Kramer JR, Natarajan Y, Dai J, Yu X, Li L, El‐Serag HB, et al. Effect of diabetes medications and glycemic control on risk of hepatocellular cancer in patients with nonalcoholic fatty liver disease. Hepatology. 2022;75:1420–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Wang P, Kang D, Cao W, Wang Y, Liu Z. Diabetes mellitus and risk of hepatocellular carcinoma: a systematic review and meta‐analysis. Diabetes Metab Res Rev. 2012;28:109–22. [DOI] [PubMed] [Google Scholar]
- 23.Zhou YY, Zhu GQ, Liu T, Zheng JN, Cheng Z, Zou TT, et al. Systematic review with network meta‐analysis: antidiabetic medication and risk of hepatocellular carcinoma. Sci Rep. 2016;6:33743. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Singh S, Singh PP, Singh AG, Murad MH, Sanchez W. Anti‐diabetic medications and the risk of hepatocellular cancer: a systematic review and meta‐analysis. Am J Gastroenterol. 2013;108:881–91; quiz 892. [DOI] [PubMed] [Google Scholar]
- 25.Lee TY, Wu JC, Yu SH, Lin JT, Wu MS, Wu CY. The occurrence of hepatocellular carcinoma in different risk stratifications of clinically noncirrhotic nonalcoholic fatty liver disease. Int J Cancer. 2017;141:1307–114. [DOI] [PubMed] [Google Scholar]
- 26.Demierre MF, Higgins PDR, Gruber SB, Hawk E, Lippman SM. Statins and cancer prevention. Nat Rev Cancer. 2005;5:930–42. [DOI] [PubMed] [Google Scholar]
- 27.German MN, Lutz MK, Pickhardt PJ, Bruce RJ, Said A. Statin use is protective against hepatocellular carcinoma in patients with nonalcoholic fatty liver disease: a case‐control study. J Clin Gastroenterol. 2020;54:733–40. [DOI] [PubMed] [Google Scholar]
- 28.Pinyopornpanish K, Al‐Yaman W, Butler RS, Carey W, McCullough A, Romero‐Marrero C. Chemopreventive effect of statin on hepatocellular carcinoma in patients with nonalcoholic steatohepatitis cirrhosis. Am J Gastroenterol. 2021;116:2258–69. [DOI] [PubMed] [Google Scholar]
- 29.Vilar‐Gomez E, Calzadilla‐Bertot L, Wai‐Sun Wong V, Castellanos M, Aller‐de la Fuente R, Metwally M, et al. Fibrosis severity as a determinant of cause‐specific mortality in patients with advanced nonalcoholic fatty liver disease: a multi‐national cohort study. Gastroenterology. 2018;155:443–57.e17. [DOI] [PubMed] [Google Scholar]
- 30.Marrero JA, Fontana RJ, Fu S, Conjeevaram HS, Su GL, Lok AS. Alcohol, tobacco and obesity are synergistic risk factors for hepatocellular carcinoma. J Hepatol. 2005;42:218–224. [DOI] [PubMed] [Google Scholar]
- 31.Ohki T, Tateishi R, Shiina S, Goto E, Sato T, Nakagawa H, et al. Visceral fat accumulation is an independent risk factor for hepatocellular carcinoma recurrence after curative treatment in patients with suspected NASH. Gut. 2009;58:839–44. [DOI] [PubMed] [Google Scholar]
- 32.Hassan MM, Abdel‐Wahab R, Kaseb A, Shalaby A, Phan AT, El‐Serag HB, et al. Obesity early in adulthood increases risk but does not affect outcomes of hepatocellular carcinoma. Gastroenterology. 2015;149:119–29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Calle EE, Rodriguez C, Walker‐Thurmond K, Thun MJ. Overweight, obesity, and mortality from cancer in a prospectively studied cohort of US adults. N Engl J Med. 2003;348:1625–38. [DOI] [PubMed] [Google Scholar]
- 34.Rustgi VK, Li Y, Gupta K, Minacapelli CD, Bhurwal A, Catalano C, et al. Bariatric surgery reduces cancer risk in adults with nonalcoholic fatty liver disease and severe obesity. Gastroenterology. 2021;161:171–84.e10. [DOI] [PubMed] [Google Scholar]
- 35.Ramai D, Singh J, Lester J, Khan SR, Chandan S, Tartaglia N, et al. Systematic review with meta‐analysis: bariatric surgery reduces the incidence of hepatocellular carcinoma. Aliment Pharmacol Ther. 2021;53:977–84. [DOI] [PubMed] [Google Scholar]
- 36.Lassailly G, Caiazzo R, Ntandja‐Wandji LC, Gnemmi V, Baud G, Verkindt H, et al. Bariatric surgery provides long‐term resolution of nonalcoholic steatohepatitis and regression of fibrosis. Gastroenterology. 2020;159:1290–301.e5. [DOI] [PubMed] [Google Scholar]
- 37.Nasereldin DS, White LJ, Hodge DO, Roberts LR, Patel T, Antwi SO. Association of metabolic health phenotypes, obesity, and hepatocellular carcinoma risk. Dig Liver Dis. 2021. 10.1016/j.dld.2021.12.002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Borena W, Strohmaier S, Lukanova A, Bjørge T, Lindkvist B, Hallmans G, et al. Metabolic risk factors and primary liver cancer in a prospective study of 578,700 adults. Int J Cancer. 2012;131:193–200. [DOI] [PubMed] [Google Scholar]
- 39.Welzel TM, Graubard BI, Zeuzem S, El‐Serag HB, Davila JA, McGlynn KA. Metabolic syndrome increases the risk of primary liver cancer in the United States: a study in the SEER‐Medicare database. Hepatology. 2011;54:463–71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Chang Y, Cho YK, Kim Y, Sung E, Ahn J, Jung HS, et al. Nonheavy drinking and worsening of noninvasive fibrosis markers in nonalcoholic fatty liver disease: a cohort study. Hepatology. 2019;69:64–75. [DOI] [PubMed] [Google Scholar]
- 41.Bagnardi V, Rota M, Botteri E, Tramacere I, Islami F, Fedirko V, et al. Alcohol consumption and site‐specific cancer risk: a comprehensive dose–response meta‐analysis. Br J Cancer. 2015;112:580–93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Ascha MS, Hanouneh IA, Lopez R, Tamimi TAR, Feldstein AF, Zein NN. The incidence and risk factors of hepatocellular carcinoma in patients with nonalcoholic steatohepatitis. Hepatology. 2010;51:1972–78. [DOI] [PubMed] [Google Scholar]
- 43.Kimura T, Tanaka N, Fujimori N, Sugiura A, Yamazaki T, Joshita S, et al. Mild drinking habit is a risk factor for hepatocarcinogenesis in non‐alcoholic fatty liver disease with advanced fibrosis. World J Gastroenterol. 2018;24:1440–50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Alberg AJ, Shopland DR, Cummings KM. The 2014 Surgeon General's report: commemorating the 50th anniversary of the 1964 report of the Advisory Committee to the US Surgeon General and updating the evidence on the health consequences of cigarette smoking. Am J Epidemiol. 2014;179:403–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Abdel‐Rahman O, Helbling D, Schöb O, Eltobgy M, Mohamed H, Schmidt J, et al. Cigarette smoking as a risk factor for the development of and mortality from hepatocellular carcinoma: an updated systematic review of 81 epidemiological studies. J Evid Based Med. 2017;10:245–54. [DOI] [PubMed] [Google Scholar]
- 46.Boursier J, Mueller O, Barret M, Machado M, Fizanne L, Araujo‐Perez F, et al. The severity of nonalcoholic fatty liver disease is associated with gut dysbiosis and shift in the metabolic function of the gut microbiota. Hepatology. 2016;63:764–775. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Dapito D, Mencin A, Gwak GY, Pradere JP, Jang MK, Mederacke I, et al. Promotion of hepatocellular carcinoma by the intestinal microbiota and TLR4. Cancer Cell. 2012;21:504–16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Grąt M, Wronka KM, Krasnodębski M, Masior Ł, Lewandowski Z, Kosińska I, Grąt K, et al. Profile of gut microbiota associated with the presence of hepatocellular cancer in patients with liver cirrhosis. Transplantation Proc. 2016;48:1687–91. [DOI] [PubMed] [Google Scholar]
- 49.Ponziani FR, Bhoori S, Castelli C, Putignani L, Rivoltini L, Del Chierico F, et al. Hepatocellular carcinoma is associated with gut microbiota profile and inflammation in nonalcoholic fatty liver disease. Hepatology. 2019;69:107–20. [DOI] [PubMed] [Google Scholar]
- 50.Wu W, Lv L, Shi D, Ye J, Fang D, Guo F, et al. Protective effect of Akkermansia muciniphila against immune‐mediated liver injury in a mouse model. Front Microbiol. 2017;8:1804. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Cornberg M, Lok AF, Terrault NA, Zoulim F, Berg T, Brunetto MR, et al. Guidance for design and endpoints of clinical trials in chronic hepatitis B ‐ report from the 2019 EASL‐AASLD HBV Treatment Endpoints Conference. J Hepatol. 2020;72:539–557. [DOI] [PubMed] [Google Scholar]
- 52.Meng Z, Wang Y, Wang L, Jin W, Liu N, Pan H, et al. FXR regulates liver repair after CCl4‐induced toxic injury. Mol Endocrinol. 2010;24:886–97. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Yang F, Huang X, Yi T, Yen Y, Moore DD, Huang W. Spontaneous development of liver tumors in the absence of the bile acid receptor farnesoid X receptor. Can Res. 2007;67:863–7. [DOI] [PubMed] [Google Scholar]
- 54.Ono M, Ochi T, Munekage K, Ogasawara M, Hirose A, Nozaki Y, et al. Angiotensinogen gene haplotype is associated with the prevalence of Japanese non‐alcoholic steatohepatitis. Hepatol Res. 2011;41:1223–9. [DOI] [PubMed] [Google Scholar]
- 55.Yoneda M, Hotta K, Nozaki Y, Endo H, Uchiyama T, Mawatari H, et al. Association between angiotensin II type 1 receptor polymorphisms and the occurrence of nonalcoholic fatty liver disease. Liver Int. 2009;29:1078–85. [DOI] [PubMed] [Google Scholar]
- 56.Kawaguchi T, Sumida Y, Umemura A, Matsuo K, Takahashi M, Takamura T, et al. Genetic polymorphisms of the human PNPLA3 gene are strongly associated with severity of non‐alcoholic fatty liver disease in Japanese. PLoS One. 2012;7:e38322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Tokushige K, Hashimoto E, Noto H, Yatsuji S, Taniai M, Torii N, et al. Influence of adiponectin gene polymorphisms in Japanese patients with non‐alcoholic fatty liver disease. J Gastroenterol. 2009;44:976–82. [DOI] [PubMed] [Google Scholar]
- 58.Namikawa C, Shu‐Ping Z, Vyselaar JR, Nozaki Y, Nemoto Y, Ono M, et al. Polymorphisms of microsomal triglyceride transfer protein gene and manganese superoxide dismutase gene in non‐alcoholic steatohepatitis. J Hepatol. 2004;40:781–6. [DOI] [PubMed] [Google Scholar]
- 59.Petersen KF, Dufour S, Hariri A, Nelson‐Williams C, Foo JN, Zhang XM, et al. Apolipoprotein C3 gene variants in nonalcoholic fatty liver disease. N Engl J Med. 2010;362:1082–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Gupta AC Chaudhory AK, Sukriti, Pande C Sakhuja P Singh Y et al. Peroxisome proliferators‐activated receptor γ2 Pro12Ala variant is associated with body mass index in non‐alcoholic fatty liver disease patients. Hepatol Int. 2011;5:575–80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Musso G, Gambino R, De Michieli F, Durazzo M, Pagano G, Cassader M. Adiponectin gene polymorphisms modulate acute adiponectin response to dietary fat: possible pathogenetic role in NASH. Hepatology. 2008;47:1167–77. [DOI] [PubMed] [Google Scholar]
- 62.Dongiovanni P, Valenti L, Rametta R, Daly AK, Nobili V, Mozzi E, et al. Genetic variants regulating insulin receptor signalling are associated with the severity of liver damage in patients with non‐alcoholic fatty liver disease. Gut. 2010;59:267–73. [DOI] [PubMed] [Google Scholar]
- 63.Petta S, Grimaudo S, Cammà C, Cabibi D, Marco VD, Licata G, et al. IL28B and PNPLA3 polymorphisms affect histological liver damage in patients with non‐alcoholic fatty liver disease. J Hepatol. 2012;56:1356–62. [DOI] [PubMed] [Google Scholar]
- 64.Larrieta‐Carrasco E, Flores YN, Macías‐Kauffer LR, Ramírez‐Palacios P, Quiterio M, Ramírez‐Salazar EG, et al. Genetic variants in COL13A1, ADIPOQ and SAMM50, in addition to the PNPLA3 gene, confer susceptibility to elevated transaminase levels in an admixed Mexican population. Exp Mol Pathol. 2018;104:50–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Rich NE, Hester C, Odewole M, Murphy CC, Parikh ND, Marrero JA, et al. Racial and ethnic differences in presentation and outcomes of hepatocellular carcinoma. Clin Gastroenterol Hepatol. 2019;17:551–9.e1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Kanwal F, Kramer JR, Mapakshi S, Natarajan Y, Chayanupatkul M, Richardson PA, et al. Risk of hepatocellular cancer in patients with non‐alcoholic fatty liver disease. Gastroenterology. 2018;155:1828–37.e2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Ford ES, Giles WH, Dietz WH. Prevalence of the metabolic syndrome among US adults: findings from the third National Health and Nutrition Examination Survey. JAMA. 2002;287:356–9. [DOI] [PubMed] [Google Scholar]
- 68.Weston SR, Leyden W, Murphy R, Bass NM, Bell BP, Manos MM, et al. Racial and ethnic distribution of nonalcoholic fatty liver in persons with newly diagnosed chronic liver disease. Hepatology. 2005;41:372–9. [DOI] [PubMed] [Google Scholar]
- 69.Kallwitz ER, Tayo BO, Kuniholm MH, Cai J, Daviglus M, Cooper RS, et al. American ancestry is a risk factor for suspected nonalcoholic fatty liver disease in Hispanic/Latino adults. Clin Gastroenterol Hepatol. 2019;17:2301–9. [DOI] [PubMed] [Google Scholar]
- 70.Romeo S, Kozlitina J, Xing C, Pertsemlidis A, Cox D, Pennacchio LA, et al. Genetic variation in PNPLA3 confers susceptibility to nonalcoholic fatty liver disease. Nat Genet. 2008;40:1461–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Liu YL, Patman G, Leathart J, Piguet AC, Burt A, Dufour JF, et al. Carriage of the PNPLA3 rs738409 C> G polymorphism confers an increased risk of non‐alcoholic fatty liver disease associated hepatocellular carcinoma. J Hepatol. 2014;61:75–81. [DOI] [PubMed] [Google Scholar]
- 72.Trépo E, Valenti L. Update on NAFLD genetics: from new variants to the clinic. J Hepatol. 2020;72:1196–209. [DOI] [PubMed] [Google Scholar]
- 73.Hassan MM, Kaseb A, Etzel CJ, El‐Serag H, Spitz MR, Chang P, et al. Genetic variation in the PNPLA3 gene and hepatocellular carcinoma in USA: risk and prognosis prediction. Mol Carcinog. 2013;52(S1):139–47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Singal AG, Manjunath H, Yopp AC, Beg MS, Marrero JA, Gopal P, et al. The effect of PNPLA3 on fibrosis progression and development of hepatocellular carcinoma: a meta‐analysis. Am J Gastroenterol. 2014;109:325–34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Yang J, Trépo E, Nahon P, Cao Q, Moreno C, Letouzé E, et al. PNPLA3 and TM6SF2 variants as risk factors of hepatocellular carcinoma across various etiologies and severity of underlying liver diseases. Int J Cancer. 2019;144:533–44. [DOI] [PubMed] [Google Scholar]
- 76.Kawaguchi T, Shima T, Mizuno M, Mitsumoto Y, Umemura A, Kanbara Y, et al. Risk estimation model for nonalcoholic fatty liver disease in the Japanese using multiple genetic markers. PLoS One. 2018;13:e0185490. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Seko Y, Sumida Y, Tanaka S, Mori K, Taketani H, Ishiba H, et al. Development of hepatocellular carcinoma in Japanese patients with biopsy‐proven non‐alcoholic fatty liver disease: association between PNPLA3 genotype and hepatocarcinogenesis/fibrosis progression. Hepatol Res. 2017;47:1083–92. [DOI] [PubMed] [Google Scholar]
- 78.Anstee QM, Reeves HL, Kotsiliti E, Govaere O, Heikenwalder M. From NASH to HCC: current concepts and future challenges. Nat Rev Gastroenterol Hepatol. 2019;16:411–28. [DOI] [PubMed] [Google Scholar]
- 79.Starley BQ, Calcagno CJ, Harrison SA. Nonalcoholic fatty liver disease and hepatocellular carcinoma: a weighty connection. Hepatology. 2010;51:1820–32. [DOI] [PubMed] [Google Scholar]
- 80.Younossi ZM, Koenig AB, Abdelatif D, Fazel Y, Henry L, Wymer M. Global epidemiology of nonalcoholic fatty liver disease—meta‐analytic assessment of prevalence, incidence, and outcomes. Hepatology. 2016;64:73–84. [DOI] [PubMed] [Google Scholar]
- 81.Mittal S, El‐Serag HB, Sada YH, Kanwal F, Duan Z, Temple S, et al. Hepatocellular carcinoma in the absence of cirrhosis in United States veterans is associated with nonalcoholic fatty liver disease. Clin Gastroenterol Hepatol. 2016;14:124–31.e1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Angulo P, Kleiner DE, Dam‐Larsen S, Adams LA, Bjornsson ES, Charatcharoenwitthaya P, et al. Liver fibrosis, but no other histologic features, is associated with long‐term outcomes of patients with nonalcoholic fatty liver disease. Gastroenterology. 2015;149:389–97.e10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Tobari M, Hashimoto E, Taniai M, Kodama K, Kogiso T, Tokushige K, et al. The characteristics and risk factors of hepatocellular carcinoma in nonalcoholic fatty liver disease without cirrhosis. J Gastroenterol Hepatol. 2020;35:862–9. [DOI] [PubMed] [Google Scholar]
- 84.Bengtsson B, Stål P, Wahlin S, Björkström NK, Hagström H. Characteristics and outcome of hepatocellular carcinoma in patients with NAFLD without cirrhosis. Liver Int. 2019;39:1098–108. [DOI] [PubMed] [Google Scholar]
- 85.Mohamad B, Shah V, Onyshchenko M, Elshamy M, Aucejo F, Lopez R, et al. Characterization of hepatocellular carcinoma (HCC) in non‐alcoholic fatty liver disease (NAFLD) patients without cirrhosis. Hepatol Int. 2016;10:632–9. [DOI] [PubMed] [Google Scholar]
- 86.Leung C, Yeoh SW, Patrick D, Ket S, Marion K, Gow P, et al. Characteristics of hepatocellular carcinoma in cirrhotic and non‐cirrhotic non‐alcoholic fatty liver disease. World J Gastroenterol. 2015;21:1189–96. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Chen VL, Yeh ML, Yang JD, Leong J, Huang DQ, Toyoda H, et al. Effects of cirrhosis and diagnosis scenario in metabolic‐associated fatty liver disease‐related hepatocellular carcinoma. Hepatol Commun. 2021;5:122–32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Kodama K, Kawaguchi T, Hyogo H, Nakajima T, Ono M, Seike M, et al. Clinical features of hepatocellular carcinoma in nonalcoholic fatty liver disease patients without advanced fibrosis. J Gastroenterol Hepatol. 2019;34:1626–32. [DOI] [PubMed] [Google Scholar]
- 89.Hsiang JC, Bai WW, Raos Z, Stableforth W, Upton A, Selvaratnam S, et al. Epidemiology, disease burden and outcomes of cirrhosis in a large secondary care hospital in South Auckland, New Zealand. Intern Med J. 2015;45:160–9. [DOI] [PubMed] [Google Scholar]
- 90.Ioannou GN, Green P, Lowy E, Mun EJ, Berry K. Differences in hepatocellular carcinoma risk, predictors and trends over time according to etiology of cirrhosis. PLoS One. 2018;13:e0204412. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Flemming JA, Yang JD, Vittinghoff E, Kim WR, Terrault NA. Risk prediction of hepatocellular carcinoma in patients with cirrhosis: the ADRESS‐HCC risk model. Cancer. 2014;120:3485–93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Sharma SA, Kowgier M, Hansen BE, Brouwer WP, Maan R, Wong D, et al. Toronto HCC Risk Index: a validated scoring system to predict 10‐year risk of HCC in patients with cirrhosis. J Hepatol. 2018;68:92–99. [DOI] [PubMed] [Google Scholar]
- 93.Marot A, Henrion J, Knebel JF, Deltenre P. Individualized prediction of hepatocellular carcinoma occurrence in a large cohort of patients with cirrhosis. J Hepatol. 2018;69:975–6. [DOI] [PubMed] [Google Scholar]
- 94.Ioannou GN, Green P, Kerr KF, Berry K. Models estimating risk of hepatocellular carcinoma in patients with alcohol or NAFLD‐related cirrhosis for risk stratification. J Hepatol. 2019;71:523–33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Grimaudo S, Pipitone RM, Pennisi G, Celsa C, Cammà C, Di Marco V, et al. Association between PNPLA3 rs738409 C>G variant and liver‐related outcomes in patients with nonalcoholic fatty liver disease. Clin Gastroenterol Hepatol. 2020;18:935–44.e3. [DOI] [PubMed] [Google Scholar]
- 96.Lambrecht J, Porsch‐Özçürümez M, Best J, Jost‐Brinkmann F, Roderburg C, Demir M, et al. The APAC score: a novel and highly performant serological tool for early diagnosis of hepatocellular carcinoma in patients with liver cirrhosis. J Clin Med. 2021;10:3392. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Loomba R, Lim JK, Patton H, El‐Serag HB. AGA clinical practice update on screening and surveillance for hepatocellular carcinoma in patients with nonalcoholic fatty liver disease: expert review. Gastroenterology. 2020;158:1822–30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Stine JG, Wentworth BJ, Zimmet A, Rinella ME, Loomba R, Caldwell SH, et al. Systematic review with meta‐analysis: risk of hepatocellular carcinoma in non‐alcoholic steatohepatitis without cirrhosis compared to other liver diseases. Aliment Pharmacol Ther. 2018;48:696–703. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Donati B, Dongiovanni P, Romeo S, Meroni M, McCain M, Miele L, et al. MBOAT7 rs641738 variant and hepatocellular carcinoma in non‐cirrhotic individuals. Sci Rep. 2017;7:4492. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Pelusi S, Baselli G, Pietrelli A, Dongiovanni P, Donati B, McCain MV, et al. Rare pathogenic variants predispose to hepatocellular carcinoma in nonalcoholic fatty liver disease. Sci Rep. 2019;9:3682. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Abul‐Husn NS, Cheng X, Li AH, Xin Y, Schurmann C, Stevis P, et al. A protein‐truncating HSD17B13 variant and protection from chronic liver disease. N Engl J Med. 2018;378:1096–106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Stender S, Loomba R. PNPLA3 genotype and risk of liver and all‐cause mortality. Hepatology. 2020;71:777–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Bianco C, Jamialahmadi O, Pelusi S, Baselli G, Dongiovanni P, Zanoni I, et al. Non‐invasive stratification of hepatocellular carcinoma risk in non‐alcoholic fatty liver using polygenic risk scores. J Hepatol. 2021;74:775–82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Gellert‐Kristensen H, Richardson TG, Davey Smith G, Nordestgaard BG, Tybjærg‐Hansen A, Stender S. Combined effect of PNPLA3, TM6SF2, and HSD17B13 variants on risk of cirrhosis and hepatocellular carcinoma in the general population. Hepatology. 2020;72:845–56. [DOI] [PubMed] [Google Scholar]
- 105.Best J, Bechmann LP, Sowa JP, Sydor S, Dechêne A, Pflanz K, et al. GALAD score detects early hepatocellular carcinoma in an international cohort of patients with nonalcoholic steatohepatitis. Clin Gastroenterol Hepatol. 2020;18:728–35.e4. [DOI] [PubMed] [Google Scholar]
- 106.Sinn DH, Kang D, Cho SJ, Paik SW, Guallar E, Cho J, et al. Risk of hepatocellular carcinoma in individuals without traditional risk factors: development and validation of a novel risk score. Int J Epidemiol. 2020;49:1562–71. [DOI] [PubMed] [Google Scholar]
- 107.Younes R, Caviglia GP, Govaere O, Rosso C, Armandi A, Sanavia T, et al. Long‐term outcomes and predictive ability of non‐invasive scoring systems in patients with non‐alcoholic fatty liver disease. J Hepatol. 2021;75(4):786–94. [DOI] [PubMed] [Google Scholar]
- 108.Terrault NA, Hassanein TI. Management of the patient with SVR. J Hepatol. 2016;65:S120–S129. [DOI] [PubMed] [Google Scholar]
- 109.Degasperi E, Galmozzi E, Pelusi S, D’Ambrosio R, Soffredini R, Borghi M, et al. Hepatic fat—genetic risk score predicts hepatocellular carcinoma in patients with cirrhotic HCV treated with DAAs. Hepatology. 2020;72:1912–23. [DOI] [PubMed] [Google Scholar]
- 110.Ji D, Chen GF, Niu XX, Zhang M, Wang C, Shao Q, et al. Non‐alcoholic fatty liver disease is a risk factor for occurrence of hepatocellular carcinoma after sustained virologic response in chronic hepatitis C patients: a prospective four‐years follow‐up study. Metabolism Open. 2021;10:100090. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Peleg N, Issachar A, Sneh Arbib O, Cohen‐Naftaly M, Harif Y, Oxtrud E, et al. Liver steatosis is a major predictor of poor outcomes in chronic hepatitis C patients with sustained virological response. J Viral Hepatitis. 2019;26:1257–65. [DOI] [PubMed] [Google Scholar]


