Abstract
Background
Penicillin allergy is a commonly listed medication allergy despite rare overall incidence. Many patients erroneously have this label, which has personal, health, and societal costs. Penicillin allergy delabelling requires an oral challenge, which can be a rate limiting step in the de-labeling process; this is even more relevant with the reduction of in-person visits during the COVID-19 pandemic.
Objective
To identify the utility and broader applicability of using a virtually supported platform, initially adopted given COVID-19 restrictions, to expedite penicillin oral provocation challenge and penicillin de-labeling in patients at low to moderate risk of immediate hypersensitivity reaction and based on shared decision making.
Methods
Patients in Vancouver catchment area were referred for penicillin allergy and virtually assessed by the consulting allergist between July 2020 and April 2021. Those deemed appropriate for oral challenge based on the allergist consultant were offered the option of a virtual oral provocation challenge to oral amoxicillin in a subsequent virtual visit. Patients who agreed and were consented underwent a virtually supervised oral amoxicillin challenge during the second virtual visit. Findings are summarized in this case series.
Results
Twenty-three patients, both adult and pediatric, ranging from no to significant co-morbidities were consented and underwent the virtual challenge. One hundred percent of patients were successful with no reaction after an hour post virtual oral provocation challenge with amoxicillin.
Conclusion
Virtual medicine is likely to remain in the allergist’s practice. Virtually supported penicillin allergy delabelling, based on shared decision making and risk stratification, presents another pathway for penicillin allergy delabelling.
Keywords: Drug allergy, Telemedicine, Penicillin allergy, Antimicrobial stewardship, COVID-19
Introduction
Penicillin allergy is the most listed medication allergy in the general population [1]. Five to 15% of patients in developed countries carry a penicillin allergy label; however, anaphylaxis is exceedingly rare, occurring in 0.001–0.0005% of patients [1]. A penicillin allergy label leads to use of alternate antimicrobial agents with more side effects, and to increased rates of multidrug resistant and hospital acquired infections [1]. Erroneous labels of penicillin allergy can prolong hospitalizations and lead to higher readmission rates and higher patient care costs in both inpatient and outpatient setting [2].
Given the personal and societal costs, allergy and immunology organizations [3, 4] recommend proactive penicillin allergy de-labelling. However, even before the COVID-19 pandemic, access to penicillin allergy testing was a rate limiting step in the de-labelling process, particularly in Canada. The gold standard for de-labelling is an oral challenge (OC) with the suspected drug [3], with a growing body of evidence supporting direct OC (without skin testing), if the clinical history is appropriate [3, 5–7]. Faced with similarly urgent need for allergy assessment for food allergy and food introduction in the setting of the COVID-19 pandemic, a Canada wide collaboration established a risk stratification model allowing virtually supervised allergenic food introduction to infants at risk of food allergy [8]. Given the success of the at-risk food introduction model and the ongoing COVID pandemic and restrictions at the time, we identified a need and opportunity to evaluate changes in clinical practice that could offer more remote options for allergist intervention, starting with penicillin de-labelling. Penicillin allergy, risk stratification, and OC are well studied with validated tools. If our practice adaptations using these tools were successful, they could become a permanent option in the event of remote access and could outlive the pandemic. The allergists involved in these virtually supported penicillin allergy de-labelling practices thus performed risk stratification (based on a combination of personalized risk/benefit assessment and a published Canadian consensus statement [3]) and virtually supervised low to intermediate risk amoxicillin oral challenges, to establish an alternate pathway for penicillin allergy de-labelling. The collective findings were reviewed leading to the current submission. The objective of this study is to summarize our experience as well as to identify the utility and broader applicability of using a virtually supported platform, initially adopted given COVID-19 restrictions, to expedite penicillin oral provocation challenge and penicillin de-labeling in patients at low to moderate risk of immediate hypersensitivity reaction and based on shared decision making.
Methods
This case series describes the use of virtually supported platforms that allowed for remote and virtual delabelling of penicillin allergy using a one step amoxicillin OC. With the limitations of the pandemic, starting in July 2020, patients in the Vancouver and surrounding catchment area who were referred for penicillin allergy were assessed virtually by independent allergists practicing in the community and tertiary health care centres in Vancouver, British Columbia. Given adaptation necessitated by the pandemic restrictions, the option for an amoxicillin OC was offered to all patients and families that were deemed suitable based on (1) the initial review of their reactions, (2) cardiovascular reserve and ability to tolerate anaphylaxis, and (3) urgency of penicillin allergy delabelling. The option for virtual challenge was offered on a case-by-case basis depending on the inflow of relevant referrals from the community. Those individuals who were willing to accept the risk were included in the current communication. There was no pre-specified timeline for this clinical endeavour. The following baseline data was collected: patients’ age, sex, comorbidities, inciting medication, index reaction, risk of reaction, rationale for virtually supervised challenge, and subsequent outcome (Table 1).
Table 1.
Demographic of patient who underwent virtual challenge
| Age | Sex | Comorbidities | Inciting medication | Index reaction | Risk of reactiona | Rationale for virtually supervised challenge based on patient–physician shared decision making | Reactions—immediate or delayedb |
|---|---|---|---|---|---|---|---|
| 3 | M | Multiple food allergies | Amoxicillin | Generalized urticaria | Intermediate | Convenience–distance | None |
| 3 | F | Amoxicillin | Maculopapular rash | Intermediate | Convenience–distance | None | |
| 4 | M | Cystic fibrosis | Amoxicillin clavulanic acid | Maculopapular rash | Intermediate | Convenience–distance | None |
| 4 | M | Asthma, atopic dermatitis | Amoxicillin | Maculopapular rash | Intermediate | Convenience–distance | None |
| 6 | F | Recurrent urinary tract infections, allergic rhinoconjuctivitis | Amoxicillin | Generalized urticaria | Intermediate | Convenience–distance | None |
| 6 | F | Atopic dermatitis | Amoxicillin | Maculopapular rash | Intermediate | Convenience–distance | None |
| 6 | F | Amoxicillin clavulanic acid | Urticaria | Intermediate | Convenience–distance | None | |
| 8 | F | Amoxicillin | Generalized urticaria and facial angioedema | Intermediate | Convenience–distance | None | |
| 8 | M | NSAID allergy | Amoxicillin | Urticaria and angioedema | Intermediate | Convenience–distance | None |
| 8 | M | Cystic fibrosis | Amoxicillin | Urticaria | Intermediate | Convenience | None |
| 11 | F | Amoxicillin | Generalized urticaria | Intermediate | Convenience | None | |
| 12 | M | Food allergy (peanut), asthma, allergic rhinoconjunctivitis | Penicillin | Maculopapular rash | Intermediate | Convenience, less missed school days | None |
| 12 | F | Amoxicillin | Generalized urticaria | Intermediate | Convenience | None | |
| 12 | M | Amoxicillin | Maculopapular rash | Intermediate | Convenience | None | |
| 14 | M | Allergic rhinitis, food allergy, asthma | Amoxicillin | Urticaria | Intermediate | Convenience | None |
| 19 | F | Asthma | Amoxicillin | Generalized urticaria | Intermediate | Convenience, less missed school days | None |
| 37 | M | Relapsing Hodgkin lymphoma, thyroiditis, previous stem cell transplant | Amoxicillin | Rash | Intermediate | Reduce hospital/infectious risk exposure | None |
| 54 | F | Interstitial lung disease on oxygen (end stage) | Amoxicillin | Localized rash on arm | Intermediate | Reduce hospital/infectious risk exposure in patient-high risk for severe COVID19 outcomes | None |
| 65 | F | Myelodysplastic syndrome, dyslipidemia, hypertension, diabetes | Piperacillin tazobactam | Petechial macules/thin papules coalescing into larger purpuric patches on her upper and lower extremities and including trunk, patient also thrombocytopenic | Intermediate | Reduce hospital/infectious risk exposure in immunosuppressed patient | None |
| 66 | F | Multiple myeloma awaiting stem cell transplant, hypertension, diabetes | Amoxicillin | Local rash to chest | Intermediate | None | |
| 70 | F | Locally advanced thymic tumour, hypertension, diabetes | Penicillin | Urticaria | Intermediate | Reduce hospital/infectious risk exposure in patient-high risk for severe COVID19 outcomes | None |
| 70 | F | Diffuse large B-cell lymphoma awaiting bone marrow transplant | Penicillin | Unknown | Intermediate | Convenience | None |
| 71 | M | Interstitial lung disease (end stage) awaiting transplant, emphysema, hypothyroidism, hypertension, dyslipidemia | Penicillin | Local rash | Intermediate | Reduce hospital/infectious risk exposure in patient-high risk for severe COVID19 outcomes | None |
All patients underwent a one-step oral challenge with 250–500 mg of oral amoxicillin (or weight-based dose if pediatric) at their physician’s discretion. Majority of patients lived over 2 h from the centre and/or had co-morbidities putting them at high risk of severe COVID, thus wishing for limited healthcare interaction
aRisk of reaction for proposed oral challenge determined as per the algorithm recommended in reference 3
bReactions—immediate or delayed after the oral challenge took place
The initial consult was conducted on a secure platform which included a video and audio component as this would allow the physician to obtain a baseline appreciation of patient health status within the limitations of virtual visits. During the initial consultation, history was obtained to determine the characteristics of the index reaction, risk of true allergy and risk of adverse reaction to a provocation challenge, according to the Canadian Society of Allergy and Clinical Immunology (CSACI) position statement on beta lactam allergy [3]. The algorithm in the CSACI Position Statement [3] stratifies patients according to risk of future reaction to beta lactam antibiotics and provides guidance on beta-lactam introduction or oral provocation challenge if patients are at low to intermediate risk of reaction. Based on this algorithm, patients that were deemed appropriate for an amoxicillin OC were stratified to the intermediate risk category that still met the criteria to proceed to direct OC, which corresponds to a score of 0–2 (very low to low risk) on the PEN-FAST clinical decision rule. The option to undergo a virtual challenge along with the risks and benefits of the challenge was presented to all patients deemed to be at low to intermediate risk of reaction as per the CSACI beta-lactam allergy risk stratification algorithm [3]. Twenty-three patients accepted the risk and provided verbal informed consent. Features of anaphylaxis, including when to seek medical attention, were discussed. Patients were explicitly told they were able to reconsider at any time including the date of the challenge. All patients who accepted the risk of a virtual challenge between the period of July 2020 to April 2021 are included in the current communication.
Patients who were eligible and consented to proceed with a virtual amoxicillin OC were prescribed amoxicillin for their challenge. Prescriptions for the appropriate dose was called or faxed to their community pharmacy by the attending physician and patients secured the amoxicillin prescription prior to the day of their OC. On the day of the challenge, patients met with their supervising physician on a secure video platform, and a review of their health status was performed to ensure safety. Verbal consent was reviewed, and a single step oral challenge was then carried out, while the physician was on standby via video for 60 min for immediate assistance and guidance in case of a reaction. Patients were required to have a capable friend, family member, or guardian available in their vicinity to function as a caretaker, and counselling provided on when, how, and where to seek immediate medical attention in the event of an adverse reaction requiring immediate care. Given the index history for each patient, a risk of anaphylaxis was felt to be low enough such that no new epinephrine prescriptions were provided for the purpose of the amoxicillin OC alone. Three pediatric patients, ages 3, 12, and 14 had previously diagnosed food allergies and epinephrine auto-injector prescriptions. Caregivers of pediatric patients were in direct contact with the attending physician.
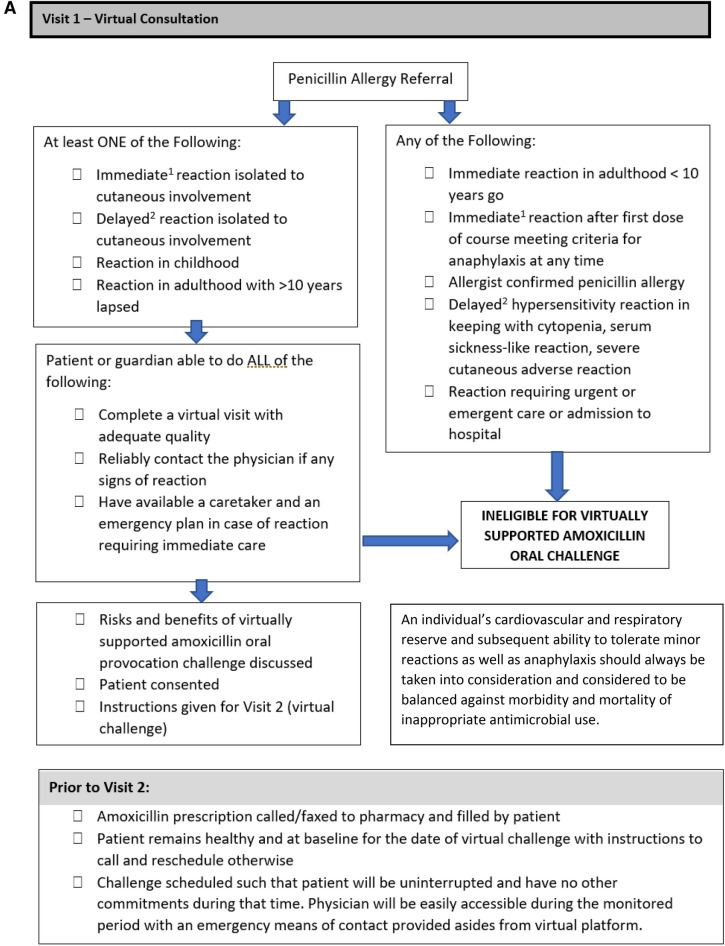
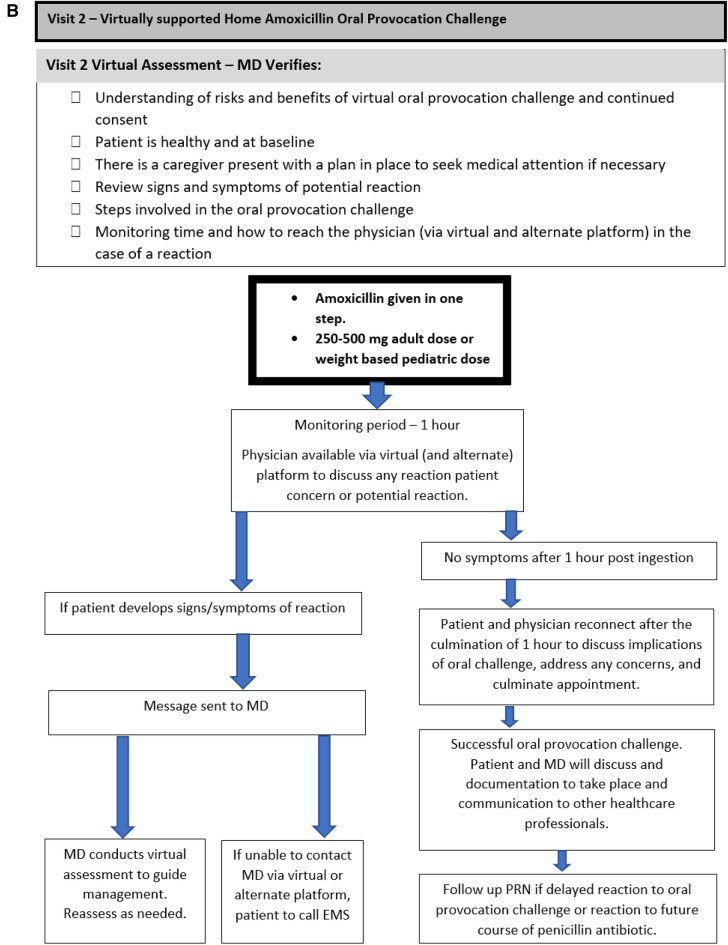
Two virtual visits were required for the virtual challenge to take place, with the initial virtual visit functioning as consultation and assessment of eligibility for the virtual challenge, and the second virtual visit functioning as the visit where the remote OC took place with the algorithm summarized in Fig. 1. Risks, benefits, and consent to proceed with the virtual amoxicillin OC was discussed during both encounters.
Fig. 1.
A flow chart and checklist of a virtually supported penicillin oral challenge. A Visit 1: virtual consultation and preparation. B Visit 2: virtually supported home amoxicillin oral provocation challenge. (1) Immediate reaction is defined as Type I hypersensitivity reaction that is IgE mediated and occurs within 2 h of the first dose of medication and lasts < 24 h. (2) Delayed hypersensitivity reaction are defined as Type II–IV reactions that typically take > 24 h to develop and raise concern for end organ involvement (cytopenias, renal/hepatic dysfunction, serum sickness), or severe cutaneous adverse reactions (skin desquamation, purpura, mucosal lesions, SJS/TEN, DRESS, AGEP)
(Figure adapted from Mack et al. [8]. JACI: In Practice)
After successful completion of the amoxicillin OC, patients were counselled on the low risk of delayed reactions and when to seek urgent medical care if required. They were provided with instructions to contact the attending physician in the event of any delayed reaction. No formal follow up was scheduled at the end of the OC if patients were otherwise well, with follow up planned on an as needed basis in the event of delayed reactions to the amoxicillin OC or in the context of a course of amoxicillin taken for a subsequent infection.
Results
Twenty-three patients completed the virtually supervised challenge. Demographic information and reaction characteristics are outlined in Table 1. Clinical history revealed the majority of the patients experienced isolated cutaneous symptoms, including urticaria, non-urticarial exanthem, and mild angioedema. The majority of patients had also experienced mild, delayed cutaneous reactions, occurring > 24 h from onset of treatment. Finally, over half the patients undergoing the amoxicillin OC had their index reaction prior to age 18 and had at least 1 year pass since the reaction. A detailed description of reaction characteristics is outlined in Table 2. In the patients who were offered virtual amoxicillin OC, all previous penicillin reactions were self-limited and resolved within 2–4 days of cessation of antibiotic use with or without the use of antihistamines for symptom relief. Epinephrine was not required for any of the index reactions.
Table 2.
Reaction history and characteristics of patients who underwent the virtually supervised oral amoxicillin challenge
| Reaction history and characteristics | Proportion of patients |
|---|---|
| Symptoms concerning for cytopenia, serum sickness-like reactions, severe cutaneous adverse reactions | None |
| Timing to reaction from first dose | |
| • Immediate (< 6 h) | 1 (4.3%) |
| • Delayed (> 24 h) | 16 (69.6) |
| • Unknown | 6 (26.1%) |
| Cutaneous involvement onlya | 19 (83%) |
| • Generalized urticaria | 7 (30.4%) |
| • Non-urticarial exanthem | 11(47.8%) |
| • Angioedema | 1 (4.3%) |
| • Remote, unknown | 1 (4.3%) |
| Index reaction prior to age 18 | 15 (65.2%) |
| Time elapsed since index reaction | |
| • > 1 year | 21 (91.3%) |
| • < 1 year | 2 (8.7%) |
aWith no history of severe cutaneous adverse reactions
Of the 23 patients challenged, no patients experienced a reaction during the observation period of the virtually supervised challenge. No delayed reactions have been reported to date, either as a consequence of the challenge, or with treatment with a penicillin antibiotic. A minimum of 7 months has elapsed since the last virtual amoxicillin OC reported in this paper.
Discussion
This diverse group of patients included adults with significant medical comorbidities at baseline and would almost certainly require antibiotics in the near future, including patients awaiting bone marrow and solid organ transplants. Although some of these patients were deemed to have limited cardiovascular reserve, the urgency of delabelling the antibiotic allergy took precedence, based on shared decision making with the allergist. Despite the variable age and health status, with appropriate patient selection, there was a 100% success rate in re-introduction of amoxicillin through a virtually supervised OC.
With the limitations of the COVID-19 pandemic, modifications of ambulatory allergy/immunology services were needed to appropriately triage patients and while we are currently seeing a return to in-person visits, we must learn from our past experiences. Shaker et al. proposed a patient prioritization schematic recommending that except for certain patient populations within the specialty, such as those with primary immunodeficiency, there is limited need for face-to-face visits [9]. Our study is the first demonstration that virtually supported penicillin allergy de-labeling is a reasonable method of health care delivery, including in different age groups, and in the presence of co-morbidities as illustrated in our small sampling. When carried out by an experienced professional, who conducted risk stratification of (1) penicillin anaphylaxis and (2) ability to withstand anaphylaxis, and then mutually agreed on delabelling in appropriately selected patients, our virtually supported penicillin de-labelling has led to positive results, de-labelling patients in a timely and cost effective manner during the pandemic.
An erroneous label of penicillin allergy confers significant burden to patients, and to an already stretched health care system. The process of de-labeling penicillin allergy was made even more challenging given access limitations due to the ongoing COVID-19 pandemic. Penicillin de-labelling allows for safer and more broad-spectrum therapeutic options for the outpatient setting. Virtual care can be central in providing services within a risk-stratified context [9]. The need for social distancing in doctor’s offices, to limit exposure to vulnerable individuals, and for rapid evaluation provided rationale for this new model of penicillin allergy assessment.
Limitations of this adaptive practice may include the one hour of observation after a single step amoxicillin challenge, given that many patients had a history of delayed reactions. A recent study by Van Gasse et al. demonstrated negligible value in prolonged drug challenges in the context of delayed or unclear reactions. In their study, only three of 128 patients undergoing a prolonged challenge had reactions. The reactions were mild maculopapular cutaneous reactions, which alone would not be a contraindication to the drug. In our virtual delabelling protocol, patients were advised to notify their attending physician in the event of any reaction thought to be due to the single step OC or due to a course of a penicillin antibiotic after delabelling. This would allow for recognition of a wider patient population and provide real world data for clinical decision making and antimicrobial guidance and would allow patients to have penicillin antibiotics as an available medication in the interim.
Another limitation in the current communication is the small number of patients described. Given the limitations of patient referrals and suitability for a remote challenge, the small number of patients who agreed to undergo virtually supervised challenge do represent a wide spectrum of ages and health statuses and indicates feasibility for larger-scaled studies. We describe our clinical findings as a prospective first step for future studies as well as a pragmatic means of empowering our colleges with similar challenges faced around penicillin de-labelling. There is both pediatric and adult data reporting the safety of amoxicillin OC without skin prick testing [5, 7]. The heterogenous nature of sampling highlights this fact suggesting that as a treatment option, virtual amoxicillin OC and penicillin de-labelling can have a profound impact across the age spectrum. Patient selection and steps taken in this study closely resemble the practice of many allergists across the country. Yet, we would like to caution that the risk of any individual patient reacting increases with increasing frequency of challenges. As such, we stress the importance of a careful discussion and shared decision making around risk and benefits, as no oral challenge, regardless of index reaction history or personal health status is without risk. For clinicians who may want to adopt virtual penicillin de-labelling option to their practice, steps can be taken to mitigate the potential for adverse outcomes, even if not adopted in the current communication. The risk and benefits of acquiring an epinephrine auto-injector solely for the purpose of the challenge is one such reasonable option and a great juncture for shared decision making. Alternatively, we can recruit the help of our community medicine colleagues to arrange more local yet well-equipped environments for patients at high risk of adverse outcome due to limited cardiovascular or respiratory reserve.
Finally, the results obtained are that of two independent practitioners in Vancouver BC and by its very nature cannot at this time be considered to be broadly generalizable but allows for the dissemination of knowledge and experience that may be applicable in specific settings.
Conclusion
Virtual amoxicillin OC in the carefully selected patient may be an approach to management that can be adopted long after the restrictions of the pandemic have been lifted and represents a shift in paradigm for drug allergy testing to allow patients to have access to optimal antimicrobial agents when needed, especially those residing in remote locations where travel presents an added layer of complexity to the assessment.
Acknowledgements
Not applicable.
Abbreviations
- CSACI
Canadian Society of Allergy and Clinical Immunology
- OC
Oral challenge
- SCAR
Severe cutaneous adverse reaction
Author contributions
AG, GS, and SJ were involved in writing the manuscript. RM and SE provided the datasets for the current paper. AG, GS, SJ, RM, SE, and SW were all involved in reviewing, editing, the manuscript. All authors read and approved the final manuscript.
Funding
None.
Declarations
Ethics approval and consent to participate
The University of British Columbia/BC Children’s Hospital Research Ethics Board has reviewed the current project and agrees with the authors that it is quality improvement/evaluation and does not require REB review.
Consent for publication
The datasets generated during the current study are not publicly available as they do contain patient information. Datasets may be available from corresponding authors so long as they do not contain patient identifying information or after appropriate consent is obtained from the patients.
Competing interests
SJ has been on speaker’s bureaus for Aralez, AstraZeneca, Sanofi, and Novartis. TW has provided speaking engagements for Pfizer and Stallergenes-Greer and has been part of an advisory board for Sanofi, Aralez Pharmaceuticals, Leo Pharma and ALK. RM has been on advisory boards for Sanofi and ALK, and has received honoraria from Novartis, Astra Zeneca, CSL Behring, and Pediapharm. SE, AG, and GS have no conflicts of interest.
Footnotes
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Contributor Information
Arian Ghassemian, Email: aghasse3@uwo.ca.
Geetanjalee Sadi, Email: sadig@myumanitoba.ca.
Raymond Mak, Email: raymond.mak@almumni.ubc.ca.
Stephanie Erdle, Email: stephanie.erdle@cw.bc.ca.
Tiffany Wong, Email: tiffany.wong@cw.bc.ca.
Samira Jeimy, Email: samira.jeimy@lhsc.on.ca.
References
- 1.Blumenthal KG, Peter JG, Trubiano JA, Phillips EJ. Antibiotic allergy. Lancet. 2019;393(10167):183–198. doi: 10.1016/S0140-6736(18)32218-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Castells M, Khan DA, Phillips EJ. Penicillin allergy. N Engl J Med. 2019;381(24):2338–2351. doi: 10.1056/NEJMra1807761. [DOI] [PubMed] [Google Scholar]
- 3.Jeimy S, Ben-Shoshan M, Abrams EM, Ellis AK, Connors L, Wong T. Practical guide for evaluation and management of beta-lactam allergy: position statement from the Canadian Society of Allergy and Clinical Immunology. Allergy Asthma Clin Immunol. 2020;16(1):1. doi: 10.1186/s13223-020-00494-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Lang DM, Castells MC, Khan DA, Macy EM, Murphy AW. Penicillin allergy testing should be performed routinely in patients with self-reported penicillin allergy. J Allergy Clin Immunol Pract. 2017;5(2):333–334. doi: 10.1016/j.jaip.2016.12.010. [DOI] [PubMed] [Google Scholar]
- 5.Mill C, Primeau MN, Medoff E, Lejtenyi C, O’Keefe A, Netchiporouk E, Dery A, Ben-Shoshan M. Assessing the diagnostic properties of a graded oral provocation challenge for the diagnosis of immediate and nonimmediate reactions to amoxicillin in children. JAMA Pediatr. 2016;170(6):e160033. doi: 10.1001/jamapediatrics.2016.0033. [DOI] [PubMed] [Google Scholar]
- 6.Trubiano JA, Vogrin S, Chua KY, Bourke J, Yun J, Douglas A, Stone CA, Yu R, Groenendijk L, Holmes NE, Phillips EJ. Development and validation of a penicillin allergy clinical decision rule. JAMA Intern Med. 2020;180(5):745–752. doi: 10.1001/jamainternmed.2020.0403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Tucker MH, Lomas CM, Ramchandar N, Waldram JD. Amoxicillin challenge without penicillin skin testing in evaluation of penicillin allergy in a cohort of Marine recruits. J Allergy Clin Immunol Pract. 2017;5(3):813–815. doi: 10.1016/j.jaip.2017.01.023. [DOI] [PubMed] [Google Scholar]
- 8.Mack DP, Hanna MA, Abrams EM, Wong T, Soller L, Erdle SC, Jeimy S, Protudjer JL, Chan ES. Virtually supported home peanut introduction during COVID-19 for at-risk infants. J Allergy Clin Immunol Pract. 2020;8(8):2780–2783. doi: 10.1016/j.jaip.2020.05.048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Shaker MS, Oppenheimer J, Grayson M, Stukus D, Hartog N, Hsieh EW, Rider N, Dutmer CM, Vander Leek TK, Kim H, Chan ES. COVID-19: pandemic contingency planning for the allergy and immunology clinic. J Allergy Clin Immunol Pract. 2020;8(5):1477–1488. doi: 10.1016/j.jaip.2020.03.012. [DOI] [PMC free article] [PubMed] [Google Scholar]