Abstract
Vitamin C is used in modern medicine supplements for treatment of various disorders associated with oxidative stress, inflammation and immune dysregulation. In this review article, experimental and clinical results regarding the effects of vitamin C on respiratory immunologic, and allergic diseases are reviewed. Various databases and appropriate keywords are used to search the effect of vitamin C on respiratory diseases until the end of May 2022. Books, theses and articles were included. These studies assessed the effects of vitamin C on respiratory disorders including asthma, chronic obstructive pulmonary disease (COPD), lung infection and lung cancer. Vitamin C showed relaxant effect on tracheal smooth muscle via various mechanisms. The preventive effects of vitamin C were mediated by antioxidant, immunomodulatory and anti-inflammatory mechanisms in the experimental animal models of different respiratory diseases. Some clinical studies also indicated the effect of vitamin C on lung cancer and lung infections. Therefore, vitamin C could be used a preventive and/or relieving therapy in respiratory diseases.
Keywords: Vitamin C, Ascorbic acid, Allergy, Asthma, Lung diseases
Introduction
Respiratory, allergic and immunological diseases including chronic obstructive pulmonary disease (COPD), asthma and lung fibrosis and lung infection (caused by bacteria and viruses such as SARS-CoV-2) are serious health problems that can cause significant morbidity and mortality worldwide (Labaki and Han 2020). Since most of these diseases are incurable, current drug therapies focus on controlling symptoms and limiting disease progression by reducing lung inflammation and airway obstruction (Rabe and Schmidt 2001). However, due to various side effects as well as lack of full therapeutic efficacy of the existing drugs, new pharmacological approaches for the treatment of respiratory, allergic and immunological diseases are being sought (Saadat et al. 2021).
Vitamin C is a small, water-soluble antioxidant molecule derived from glucose and is found mainly in fruits and vegetables, especially citrus fruits (Zhitkovich 2020). Most animals synthesize vitamin C in the liver or kidneys, but due to the lack of L-gulonolactone oxidase (GULO), the final enzyme in the biosynthetic process, this vitamin has become an essential nutritional requirement for humans (Kaźmierczak-Barańska et al. 2020). Vitamin C exists in two forms in plasma: (1) the reduced form, ascorbic acid (AA), which is transported into the cells by sodium-vitamin C co‐transporters (SVCTs), and (2) the oxidized form, dehydroascorbic acid (DHA), which is transported into the cells by glucose transporters (GLUTs) (Fig. 1), (Roa et al. 2020). High levels of vitamin C exist in the lungs (Shanklin and O'dell 1966), and its levels in alveolar type II and macrophages are 30 times more than plasma (Castranova et al. 1983). From a functional point of view, vitamin C is involved in many biological processes. Vitamin C prevents oxidative damage to biomolecules by directly scavenging free radicals through donating electrons to these radicals and by indirectly scavenging free radicals through reactivating other free-radical scavengers such as alpha-tocopherol (vitamin E) and glutathione (GSH), as well as inhibiting free-radical-producing enzymes including nicotinamide adenine dinucleotide phosphate (NADPH) and oxidase xanthine oxidase (XO) (Marik 2018; Padayatty et al. 2003). Vitamin C also acts as a cofactor for more than sixty enzymes that catalyze important reactions, including the synthesis of collagen, carnitine, serotonin and norepinephrine, and the regulation of hypoxia-induced transcription factor (HIF) (Ang et al. 2018). In addition, this vitamin decreases the levels of inflammatory mediators by inhibiting the activity of nuclear factor-kappa B (NF-κB) and prevents the entry of immune cells into the microcirculation by inhibiting the expression of intracellular adhesion molecules (Du et al. 2022; Shakoor et al. 2021). Moreover, vitamin C improves microcirculatory blood flow, inhibits apoptosis and augments the bacterial defense (Lavillegrand et al. 2022; Oudemans-van Straaten et al. 2014). In line with these extensive biological activities, the beneficial effects of vitamin C in the context of various diseases have also been reported. For example, in sepsis, vitamin C has been shown to facilitate the production of cortisol, vasopressin, and catecholamines, and protects the endothelial barrier by inhibiting protein phosphatase type 2A (PP2A) (Chang et al. 2020; Teng et al. 2018). Vitamin C was shown to decrease smoking-induced myocardial damage, ameliorate atherosclerosis, and protect the immune system from oxidative stress caused by infection (Das et al. 2012; Wintergerst et al. 2006; Woo et al. 2021). Moreover, vitamin C has been found to increase cell differentiation from somatic cells to induced pluripotent stem cells (iPSCs), which is an important feature in various regenerative processes following acute or chronic diseases (Esteban et al. 2010). In addition, promising findings have been published about the effects of vitamin C on respiratory, allergic and immunological diseases; importantly, abundant presence of vitamin C has been shown in both intracellular and extracellular fluid of the lungs (Maritz 1996). In this regard, treatment with vitamin C was shown to improve influenza and upper respiratory infections (Colunga Biancatelli et al. 2020; van Driel et al. 2019). Vitamin C supplementation has also been indicated to improve antioxidant status and reduced lung function due to smoking and/or COPD (Park et al. 2016a, b; Yieh et al. 2018). In addition, the effect of fruits, vegetables and grains containing vitamin C on COPD was shown (Pirabbasi et al. 2016). However, inconsistent results on the effects of vitamin C supplementation on COPD patients are available (Wu et al. 2007). The effect of vitamin C on COVID-19 was also reported (Hoang et al. 2020; Liu et al. 2020). The safety of high-dose intravenous vitamin C was also shown (Barazzoni et al. 2020; Diyya and Thomas 2022; Karimian et al. 2022; Padayatty et al. 2010).
Fig. 1.
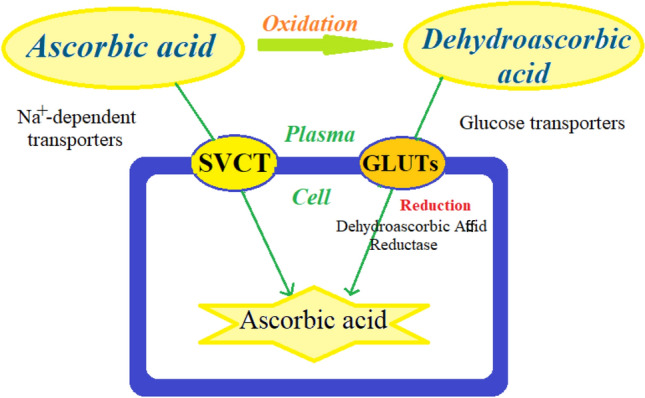
Transmembrane transporters of ascorbic acid and dehydroascorbic acid: sodium-dependent vitamin C transporters (SVCTs) are specific for ascorbic acid, and glucose transporters (GLUTs) are specific for dehydroascorbic acid
Therefore, the present review focuses on experimental and clinical evidence supporting the effects of vitamin C on respiratory, allergic and immunological diseases and the mechanisms involved. The possible effective dose of vitamin C for the treatment of each type of respiratory disorders, as well as its bioavailability and toxicity are also provided.
Main text
Experimental and clinical studies on the effects of vitamin C on respiratory, allergic and immunological diseases, published up to May 2022, in different databases including Google Scholar, PubMed, and Web of Science, using relevant keywords such as “vitamin C”, “ascorbic acid”, “asthma”, “chronic obstructive pulmonary disease”, “lung fibrosis” and “lung cancer” were searched. The reviewed articles were available in full text, and written in English. The titles and abstracts of the studies were screened by four reviewers separately to select relevant articles and disagreements were resolved through consensus. Two reviewers extracted data from the included studies separately by double-checking by the same reviewers. Title, authors, publication year, study design, control groups, model of injury, sample size, in vivo and in vitro intervention, intervention duration, outcome, conclusions, keywords, and sex and age of animals or human from each article were extracted. Two reviewers also evaluated the methodological quality and validity of each included study independently and duplicate references were removed. Letters to editors, abstracts, case-report, and case-series articles were excluded. In total, among the initial 161 articles, 139 papers (33 review articles, 10 book chapters, and 96 original articles) were included in this review and 22 articles were duplicates. The study flowchart is shown in Chart 1.
Chart 1.

Flowchart of the process for selecting studies for this review
The possible bronchodilatory effect
Tracheal smooth muscle relaxation effect, experimental results
To treat obstructive respiratory diseases, the relieving drugs which are compounds that dilate the bronchi and relieve airway obstruction are used (Saadat et al. 2021). The relaxant effect of a compound on the smooth muscle of the trachea indicates its bronchodilatory effect in obstructive respiratory diseases. In this regard, the relaxant effect of vitamin C on tracheal smooth muscle (TSM) along with its underlying mechanisms has been demonstrated in several experimental studies.
Dose-dependent relaxant effect of vitamin C on the smooth muscle of guinea pig trachea, contracted by submaximal concentrations of histamine (1 µg/mL), was reported. In this study, propranolol reduced the relaxant effect of vitamin C on TSM, suggesting that this effect was mediated in part by β-adrenergic receptors stimulation (Zuskin et al. 1973). Relatively high concentrations of vitamin C caused dose-dependent relaxation in TSM pre-contracted by histamine (10–5 M), carbachol (10–6 M), and potassium chloride (KCl) (80 mM). The decrease in the relaxant effect of vitamin C on TSM in the presence of indomethacin (10–5 M) indicated the partial contribution of inhibition of cyclooxygenase pathway in this effect (E. Sipahi & Z. S. Ercan, 1997).
Vitamin C administered for 1 week induced a relaxant effect in BALB/c mice exposed to methacholine aerosol (25 mg/mL) (Jeong et al. 2010). The relaxant effect of vitamin C administered for 5 days on airway resistance was shown in cockroach extract-sensitized BALB/c mice after acute exposure to different doses of methacholine (4, 8, 12, 16 and 20 mg/mL) (Bansal et al. 2014).
The anti-bronchoconstriction effect of sodium ascorbate (200 mg/kg) on airway resistance was shown in guinea pigs after acute exposure to histamine (5 µg/kg) which showed the relaxant effect of sodium ascorbate in TSM in the presence of histamine. Therefore, sodium ascorbate can decline bronchoconstriction caused by histamine in guinea pigs (Dawson et al. 1967).
Scorbutic (50 mg/guinea pig/day) for 3 and 4 weeks induced a relaxant effect in guinea pigs exposed to histamine aerosol. The results showed that AA deficiency causes airway hyperresponsiveness to histamine in guinea pigs (Mohsenin et al. 1988). In addition, in several similar studies, vitamin C decreased bronchoconstriction caused by histamine in guinea pigs (Hemilä 2014; Sipahi and Ercan 1997; Zuskin et al. 1973).
The bronchodilatory effect, clinical results
In three trials, treatment with vitamin C (0.5 to 2 g/day) improved the post-exercise decrease in forced vital volume in one second (FEV1) volume. After short-term heavy physical stress, also treatment with vitamin C, improved the incidence of respiratory symptoms (Hemilä 1996; Hemilä and Chalker 2013).
Common diet and AA supplementation (1500 mg/day) were administered for one-week in a randomized, placebo-controlled double-blind cross-over trial in two groups. The results indicated that vitamin C reduced the post-exercise increase in the urinary markers of bronchoconstrictors prostaglandin D2 (PGD2) and cysteinyl LTs and decrease the NO level in exercise-induced bronchoconstriction (Tecklenburg et al. 2007). The relaxant effects of vitamin C on TSM and its bronchodilatory effects are summarized in Table 1.
Table 1.
The possible effects of vitamin C on tracheal smooth muscle (TSM) and its preventive effects on asthma
| Study design | Dose | Admin rout | Effect | References | ||
|---|---|---|---|---|---|---|
| TSM | Exp | Contracted guinea pigs | 20 to 30 µg/mL | – | Relaxing activity on TSM | Zuskin et al. (1973) |
| Contracted guinea pigs TSM | 10–4–10–2 M | – | (Sipahi and Ercan (1997) | |||
| OVA-sensitized guinea pigs | 0.25 g/L | Drinking water | Reduced the responsiveness of TSM | Boskabady and Ziaei (2003) | ||
| TSM of mice | 3 to 5 mg | – | Relaxing activity on TSM | Bansal et al. (2014) | ||
| Contracted guinea pigs | 200 mg/kg | – | Dawson et al. (1967) | |||
| 50 mg | – | [40] | ||||
| BD | Clin | Bronchoconstriction | 0.5 to 2 g/day | Decreased FEV1, improved respiratory symptoms incidence | Hemilä (1996); Hemilä and Chalker (2013) | |
| 1500 mg/day | Increased urinary markers of bronchoconstrictors PGD2 and cysteinyl LTs | Tecklenburg et al. (2007) | ||||
| Asthma | Exp | OVA-sensitized mice | 130 mg/kg | Gavage |
Modulated Th1/Th2 balance Decreased the BALF eosinophilic infiltration |
Chang et al. (2009) |
| Cockroach extract-sensitized BALB/c mice | 308.33 mg/kg | NAS |
Reduced total and differential WBC, EPO, ROS, IL-4, IL-5 Decreased IgE, IgG1, NF-κB p65 protein in lung tissue Increased IL-10 levels and GPx activity in lung tissue |
Bansal et al. (2014) | ||
| OVA-sensitized guinea pigs | 3–5 mg/kg | Gavage | Reduced the number of inflammatory cells in the BALF | Jeong et al. (2010) | ||
| 400 mg/kg | Increased the levels of cGMP | Haines et al. (2011) | ||||
| DNFB-sensitized mice | 200 mg/kg | Lowered inflammatory cell infiltration, increased lung tissue cAMP and cGMP | Haines et al. (2011) | |||
| 0.625 and 5 mg/kg | I.P | Enhanced delayed hypersensitivity, modulated Th1/Th2 balance toward the Th1 | Noh et al. (2005) | |||
| Mouse model of asthma | 130 mg/kg | Gavage | Reduced total and differential WBC count, peribronchial inflammatory cells infiltration, phosphorylated NF-κB and MDA levels | Kianian et al. (2019a) | ||
| Clin | Asthmatic children | 1000 mg/daily | Oral | Improved neutrophil chemotaxis | Anderson and Theron (1983) | |
| 5 g/d for 2 weeks | Reduction incidence and severity of common cold-induced asthma | Hemilä (2013) | ||||
Admin administration, NAS Intranasal, I.P: Intraperitoneally, OVA ovalbumin, TSM tracheal smooth muscle, DNFB hapten 2,4-dinitro-1-fluorobenzene, BD bronchodilator, OVA ovalbumin, cAMP cyclic adenosine monophosphate, cGMP cyclic guanosine monophosphate, IL-13 Interleukin-13. EPO eosinophil peroxidase, ROS reactive oxygen species, GPx glutathione peroxidase, WBC total white blood cell, NF-κB nuclear factor-kappa B, MDA malondialdehyde, FEV1 forced expiratory volume in 1 s, Exp. experimental, Clin. clinical
Preventive effects on asthma
Experimental results
Asthma is one of the most common chronic respiratory diseases worldwide, which places a significant burden on patients and health care systems (Kianian et al. 2020a). The main feature of the pathophysiology of asthma is lung inflammation, oxidative stress and immunological reactions (Kianian et al. 2019a, b; Kianian et al. 2022). Oxidative stress responses to air pollutant exposure trigger inflammation of the lungs by inducing a variety of pro-inflammatory mediators, increasing airway responsiveness, and increasing mucin secretion (Cho and Moon 2010). Persistent lung inflammation, possibly caused by immune responses to repeated inhalation of environmental allergens such as pollen and house dust mites, leads to airway remodeling (e.g., smooth muscle hypertrophy, epithelial mucus metaplasia, and increased deposition of sub-epithelial matrix glycoproteins) (Locksley 2010).
Due to this complex pathophysiology, it can be concluded that a multi-potential drug is necessary for effective treatment of patients with asthma. In this regard, the therapeutic effects of vitamin C on asthma have been evaluated in several studies based on its anti-inflammatory, antioxidant and immunomodulatory effects. The effect of vitamin C administered for 16 days on circulating cytokine levels in 2,4-dinitro-1-fluorobenzene (DNFB)-sensitized BALB/c mice was shown by decreased interleukin (IL)-4 but increased IL-2, tumor necrosis factor-α (TNF-α) and interferon-γ (IFN-γ) levels, indicating an immune response shift to T helper 1 (Th1) (K. Noh et al. 2005a, b).
Vitamin C administered for 5 weeks in a murine model of allergic asthma, increased eosinophilic infiltration into bronchoalveolar lavage fluid (BALF) in ovalbumin (OVA)-sensitized BALB/c mice which modulated Th1/T helper 2 (Th2) balance toward the Th1 pole during the Th2-skewed allergic airway inflammation (Chang et al. 2009). Treatment of allergic airway inflammation in a mouse model of asthma with vitamin C (3–5 mg intraperitoneally (i.p,) for a week) reduced the number of inflammatory cells in the BALF and infiltration of perivascular and peribronchiolar inflammatory cells (Jeong et al. 2010).
In a guinea pig model of asthma, inhalation of OVA caused a significant increase in the number of eosinophils, neutrophils, and macrophages in the BALF. Treatment with 400 mg/kg oral vitamin C prevented the accumulation of these inflammatory cells in the BALF and increased level of cyclic guanosine monophosphate (cGMP), the second messenger in airway smooth muscle cells, indicating that vitamin C can relax the airways and thus reduce the symptoms of asthma (Haines et al. 2011).
Treatment of allergic asthma in cockroach extract-sensitized BALB/c mice with vitamin C for 5 days reduced total and differential total white blood cell (WBC) count, eosinophil peroxidase (EPO) activity and the levels of intracellular reactive oxygen species (ROS), 8-isoprostanese, IL-4 and IL-5 in the BALF, IgE and IgG1 in the serum and NF-κB p65 protein in the lung tissue while increased IL-10 in the BALF and glutathione peroxidase (GPx) activity in the lung tissue. Hematoxylin and eosin (H&E) staining of the lung tissues also showed a high score of inflammation in asthmatic mice, which was significantly reduced by vitamin C treatment (Bansal et al. 2014). Administration of vitamin C for 30 days reduced the responsiveness of tracheal smooth muscle to methacholine and OVA-sensitized guinea pigs. In addition, incubation of TSM with vitamin C caused further reduction in tracheal response to methacholine in the sensitized group (Boskabady and Ziaei 2003).
The effect of vitamin C treatment on lung inflammation and oxidative stress was shown in a mouse model of allergic asthma. The results indicated that 24-day administration of vitamin C significantly reduced WBC count, percentage of neutrophils and eosinophils, infiltration of peribronchial inflammatory cells, expression of phosphorylated nuclear factor-kappa B (NF-κB) and malondialdehyde (MDA) levels (Kianian et al. 2019a). Treatment of OVA-sensitized BALB/c mice with vitamin C for 24 days improved BALF IL-13 and serum IgE levels, as well as goblet hyperplasia and sub-epithelial fibrosis (Kianian et al. 2020a).
Clinical results
In a randomized controlled trial, treatment with vitamin C (1000 mg/daily. i.v.) to asthmatic children, improved neutrophil chemotaxis compared to standard anti-asthma treatment alone (Anderson & Theron, 1983). In addition, in randomized, placebo-controlled, double-blind parallel studies patients with early asthma were treated with 1 g/day vitamin C, 450 mg/day magnesium chelate or placebo for 16 weeks. The findings indicated that treatment with vitamin C or magnesium adds no clinical benefit to current standard therapy of asthma in primary care patients (Fogarty et al. 2003, 2006).
In a meta-analysis including 3 controlled clinical trials (involving 79 children and adults), the efficacy of AA as single or multiple oral doses for different lengths of time (1 g/day for 14 weeks, 2 g/day at two time points, and 5 g/day for 2 weeks), significant reduction of the incidence and severity of common cold, moderate and severe asthma and airway responsiveness to histamine were shown (Hemilä 2013). The preventive effect of vitamin C on asthma is summarized in Table 1.
Preventive effects on COPD
Experimental results
In a guinea pig model of COPD, intramuscular injection of cigarette smoke (CS)-derived p-benzoquinone for 56 days induced significant accumulation of p-benzoquinone in the lung parenchyma and increased airspace size; however, oral vitamin C administration (Ghosh et al. 2015) reduced the formation of protein carbonyls and 8-Oxo-7,8-Dihydro-2′-Deoxyguanosine (8-oxodG) in the lung parenchyma, as well as the inflammatory cells (e.g., macrophages) infiltration and up-regulation of NF-κB, p-IkB-α, matrix metalloproteinase (MMP)-9 TNF-α, IL-8, and MMP-12 in the lungs, indicating a reduction in oxidative stress and inflammation, respectively. Moreover, vitamin C administration reduced collagen deposition in the bronchioles as well as the presence of cytochrome c, Bcl-2-associated X protein (Bax)/B-cell leukemia/lymphoma 2 (BCL-2) ratio, p53, caspase 3, caspase 8 and poly-ADP-ribose polymerase (PARP) activity and deoxyribonucleic acid (DNA) fragmentation in the lungs, indicating decreased airway remodeling and apoptosis (Ghosh et al. 2015).
Treatment of CS-induced emphysema in mice with minimal (0.0375 g/L) and physiologically sufficient vitamin C, reduced oxidative stress, enhanced collagen synthesis and improved vascular endothelial growth factor (VEGF) (K. Koike et al. 2014a, b). Pretreatment with vitamin C in an animal model of tobacco smoke-induced emphysematous injury in guinea pigs significantly suppressed pro-inflammatory protein expressions such as Rtp801, NF-κB, and iNOS as well as MMP-9. The results of this study proved the preventive effect of vitamin c (but no curative effect) against CS-induced lung injury (Gupta et al. 2016).
Vitamin C administration did not decrease the lung cytochrome P4501i9A1 gene expression induced by CS exposure in osteogenic disorder model in Shionogi rats (Ueta et al. 2001). In an in vitro study, vitamin C prevented CS-induced NF-κB activation in alveolar epithelial A549 cells (Das et al. 2013). In another study, vitamin C administration improved emphysematous lung damage induced by CS and p-benzosemiquinone in guinea pigs (Banerjee et al. 2008). Vitamin C administration also protects guinea pigs lung tissue against CS-induced oxidative damage (Panda et al. 2000).
Clinical results
The effects of the N-acetylcysteine (NAC) (600 mg), vitamin C (500 mg), a combination of vitamin C + NAC, and placebo, once daily on the antioxidant status of COPD patients (n = 79) were studied. The results indicated that antioxidant supplementation improved nutritional status and vitamin C was effective in improving antioxidant status (Pirabbasi et al. 2016). The preventive effect of vitamin C (48.50–141.63 mg/kg/day) has been studied in COPD patients. The results showed protective effect of vitamin C against COPD independent of smoking history in the general population (Park et al. 2016a, b).
In a randomized and placebo-controlled study, 35 patients with stable COPD were treated with 200 and 400 mg/day vitamin E or 250 mg/day vitamin C. The results showed that vitamin E and C supplementation for 12 weeks significantly improved the resistance of DNA in WBC against oxidative challenge. The DNA damage induced by H2O2 in the treatment groups with two doses of vitamin E and one dose of vitamin C was significantly reduced compared to the placebo group) p = 0.028 and p = 0.013 for two doses of vitamin E but p = 0.0041 for vitamin C). However, more studies are needed to examine the effect of vitamin C on slowing the decline of lung function in patients with COPD (Wu et al. 2007). Table 2 summarizes the effect of vitamin C on COPD.
Table 2.
The effect of vitamin C on COPD and lung fibrosis
| Study design | Dose | Admin rout | Effects | References | ||
|---|---|---|---|---|---|---|
| COPD | Exp | Guinea Pigs model | 30 mg/kg | Gavage | Decreased airway remodeling, apoptosis and inflammation Improved oxidative stress | Ghosh et al. (2015) |
| 10 mg/kg | Suppressed pro-inflammatory protein expressions such as Rtp801, NF-κB, and iNOS as well as, MMP-9 | Gupta et al. (2016) | ||||
| 15 mg |
Prevented protein damage Reduced inflammation and apoptosis |
Banerjee et al. (2008) | ||||
| 15 mg/day | Reversed protein damage and lipid peroxidation | Panda et al. (2000) | ||||
| Mice model | 0.0375 and 1.5 g/L | – |
Diminished oxidative stress Increased collagen synthesis, improved VEGF expression |
Koike et al. (2014a, b) | ||
| CS exposure in osteogenic disorder rat | 40 mg/kg | – | Do not affect the lung cytochrome P4501A1 gene expression | Ueta et al. (2001) | ||
| CS-induced NF-κB activation in A549 cells | 1 mg/day | – | Decreased NF-κB activation | Das et al. (2013) | ||
| Sepsis-induced acute lung injury in mice | 200 mg/kg | – |
Inhibited inflammation Enhanced epithelial barrier function and alveolar fluid clearance |
Fisher et al. (2012a, b) | ||
| Clin | COPD patients | 500 mg | – | Improved nutritional and antioxidant status, Improve of lung function | Pirabbasi et al. (2016) | |
| 250 mg/day | Improve of lung function | Wu et al. (2007) | ||||
| L.F | Pulmonary endothelial barrier dysfunction. Induced by LPS (in vitro) | 250, 500, 100 μM | Intranasally |
Reversed p53 and phosphorylated cofilin down-regulation Decreased RhoA activation Lowered myosin light-chain phosphorylation |
Barabutis et al. (2017) | |
| LPS-induced lung injury in rat | 250 mg/kg/day | Gavage | Attenuated the inflammatory response and lowered fibrosis in lung tissue | Mohamed et al. (2019) | ||
| Hexavalent chromium-induced fibrosis in rats | 75 mg/kg | Improved fibrotic markers such as fibroblasts and myofibroblasts proliferation and collagen production | Hemmati et al. (2008) | |||
Admin administration, L.F Lung fibrosis, Rtp801 known as Redd1, and encoded by Ddit4, NF-κB Nuclear factor-kappa, B, iNOS Inducible nitric oxide synthase, MMP-9 Matrix metallopeptidase 9, VEGF Vascular endothelial growth factor, LPS Lipopolysaccharide, Exp. experimental, Clin. clinical
Lipopolysaccharide (LPS)-induced lung injury and lung fibrosis
Vitamin C showed therapeutic effects of on sepsis, LPS-induced lung injury and lung fibrosis in some studies. Vitamin C supplementation improved sepsis-induced acute lung injury (ALI) in C57BL/6 mice through inhibition of inflammation, enhancing epithelial barrier function and increasing the clearance of alveolar fluid (Fisher et al. 2012a, b). Administration of a combination of vitamin C and hydrocortisone in an in vitro study improved pulmonary endothelial barrier dysfunction induced by LPS in human lung microvascular endothelial cells. In addition, this combination reversed p53 and phosphorylated cofilin down-regulation and decreased RhoA activation and myosin light-chain phosphorylation (Barabutis et al. 2017). Treatment of LPS-administered rats with vitamin C decreased inflammatory cell infiltration and lung fibrosis and lowered collagen contents and thickening of the alveolar walls (Mohamed et al. 2019). Administration of vitamin C reduced lung fibrotic damage induced by hexavalent chromium in rats through improving fibrotic markers such as fibroblasts and myofibroblasts proliferation as well as collagen production (Hemmati et al. 2008). The effects of vitamin C on LPS-induced lung injury and lung fibrosis are summarized in Table 2.
Lung cancer
Both experimental and clinical studies indicated therapeutic effects of vitamin C on lung cancer.
Experimental results
The expression and distribution of vitamin C transporters in tumor cells and the route of administration of vitamin C (oral or intravenous) are factors influencing the effects of vitamin C on cancer progression. In addition, the relationship between cancer and vitamin C is related to its pro-oxidant, gene expression regulating and antioxidant properties (Vissers and Das 2018). Combined α-tocopherol and vitamin C supplementation prevented lung squamous metaplasia induced by CS in ferrets via inhibition of cyclin D1 expression and restoring retinoic acid levels in the lung. Vitamin C administration also prevented excessive proliferation in human lung epithelial cells (A549) (Kim et al. 2012).
Clinical results
In a meta-analysis of 18 articles, 21 studies including 8938 cases of lung cancer were evaluated. The results showed that consumption of vitamin C may have a protective effect against lung cancer. In fact, there is a linear dose–response relationship between the level of vitamin C intake and lung cancer risk. The risk of lung cancer was reduced by 7% for every 100 mg/day increase in vitamin C intake (Luo et al. 2014). The relationship between supplemental with multivitamins, vitamin C, vitamin E and folate with incidence of lung cancer was examined in a total of 521 cases of lung cancer patients. The results showed that supplemental vitamin E was associated with a slight increase in the risk of lung cancer, while multivitamins, vitamin C, and folate supplementation were not associated with a decrease in the risk of lung cancer. Therefore, the use of these supplements is suggested to patients to prevent lung cancer (Slatore et al. 2008). The effects of vitamin C on lung cancer are summarized in Table 3.
Table 3.
The effect of vitamin C on lung cancer and lung infection
| Study design | Dose | Admin rout | Effect | References | ||
|---|---|---|---|---|---|---|
| LC | Exp | A549 | 210 mg/day | Inhibited cyclin D1 expression and restoring retinoic acid levels in the lung | Kim et al. (2012a, b) | |
| Clin | – | 75 g | Improved CEA | González et al. (2016) | ||
| 100 mg/day | Decreased risk of lung cancer | Luo et al. (2014) | ||||
| – | Not associated with a decrease in the risk of lung cancer | Slatore et al. (2008) | ||||
| L-In | Exp | Pigs model | – | Antibacterial effect | [56] | |
| – |
Increased FVC and FEV1% Therapeutic effect of high-dose vitamin C on lung infection |
Yi et al. (2019) | ||||
| 800 mg/kg | Antibacterial effect in the high-dose vitamin C | (Jensen et al. 2012) | ||||
| Rhesus monkeys’ model | – | Reduced susceptibility of lung of rhesus monkeys to infections by bacteria and other microorganisms | (Sabin 1939) | |||
| Clin | Pneumonia | – | Not associated with improvement of the 28-day mortality and prognosis | Lee et al. (2021a, b) | ||
| – | Not support with vitamin C in the prevention and treatment | Hui et al. (2022) | ||||
| COVID-19 | 50 mg/kg/day | Did not use less mechanical ventilation | Kumari et al. (2020) | |||
| PTB | – | Decreased serum levels of ascorbic acid in PTB and pneumonia in PTB, but not in pneumonia | Bakaev and Duntau (2004) | |||
| Sepsis | 50, 200 mg/kg/day, 24 h | Positively impact the extent of multiple organ failure, biomarkers of inflammation and endothelial injury | Syed et al. (2014) | |||
| ARI | 200 mg | Decreased respiratory symptom score | Hunt et al. (1994) | |||
| COVID-19 | 60 mg/kg/ | Decrease inflammation, duration of mechanical ventilation, vasopressor use without affecting mortality | Mahmoodpoor et al. (2021) | |||
| 1 g/day | Shortened the duration of colds in adults | Hemilä (1999) | ||||
Admin administration, LC lung cancer, Exp. experimental, Clin. clinical, i.v. intravenous, L-In lung infection, FVC forced vital capacity, FEV1 first second of forced expiration, ARI acute respiratory infections, PTB pulmonary tuberculosis, CEA carcinoembryonic antigen
Lung infection
The vitamin C effects on lung infection were shown in both experimental and clinical studies. Vitamin C is synthesized by most mammals in their liver. Guinea pig, which is a rare species with the absence of this capacity, is regarded as an appropriate empirical model for investigations on the impacts of this vitamin. The intake of this vitamin influences the guinea pigs’ susceptibility to infections induced by bacteria and microorganisms (Goldschmidt et al. 1988). Five pneumonia cases were seen in rhesus monkeys in a group of 25 monkeys that had vitamin C deficiency in their diet. However, 21 control monkeys administered vitamin C did not show any cases of pneumonia, which suggests the possibility of the influence of primate vitamin C intake on pneumonia susceptibility (Sabin 1939).
Vitamin C treatment improved lung infection in a rat model of bronchial asthma. The control group and case group received a high amount of vitamin C, respectively (82 rats in each group). The results showed that lung function was not significantly different between the two groups before treatment. However, after treatment, case group showed significantly higher levels of FEV1, FEV1%, and FEV1/FVC compared to the control group. The results indicated a therapeutic impact of high doses of vitamin C in pulmonary infection in rats with bronchial asthma, which may be of clinical value in preventing the lung infection (Yi et al. 2019).
Treatment of male C57BL/6 mice with vitamin C (200 mg/kg, i.p.) or dehydroascorbic acid (200 mg/kg) protected mice against the harmful outcomes of sepsis by various mechanisms, such as the pro-inflammatory response attenuation, epithelial barrier function increment, inhibition of coagulation abnormalities related to sepsis, and alveolar fluid clearance enhancement. In animals with lung injury induced by sepsis, administration of vitamin C causes improving bronchoalveolar epithelial barrier function, attenuating neutrophil sequestration, and increasing alveolar fluid clearance, which are all crucial factors for normal functioning of lung (Fisher et al. 2012).
Vitamin C showed an anti-infection effect on the growth of Pseudomonas aeruginosa. The present research studied 56 guinea pigs, and 36 pigs for establishing a chronic P. aeruginosa lung infection. The findings proved that the plasma antioxidant capacity induced by the infection was more significant in the vitamin C-deficient diet, which implies the therapeutic effect of consumption of high-dose antioxidants during lung infection (Jensen et al. 2012). Therefore, vitamin C could also affect lower respiratory tract infections.
Clinical studies
Treatment of pneumonia patients (35 cases) with a single-dose vitamin C for 28 days indicated that there was no association between the vitamin C treatment alone and prognosis and mortality improvement in individuals with respiratory failure and severe viral pneumonia (Lee et al. 2021a, b). The preventive effect of vitamin C has been studied in patients with COVID-19 and pneumonia. According to the results, treatment with only high oral or i.v. doses of vitamin C in these patients exerts a therapeutic effect via different biological pathways (Hui et al. 2022).
In a randomized, double-blind clinical trial, COVID-19 patients in Pakistan with respiratory symptoms were allocated into two groups. One group received vitamin C along with the standard therapy, and the other group received standard therapy only. The results proved that in 150 patients with severe COVID-19, cases that received vitamin C showed the elimination of symptoms earlier; however, the application of mechanical ventilation was not less in them (Kumari et al. 2020).
A clinical trial measured the dehydroascorbic, ascorbic, and diketogulonic acid (DKG) levels in the serum of pneumonia and pulmonary tuberculosis (PTB) patients. The results demonstrated the reduction of the AA serum levels in pneumonia and PTB, and there was a reduction in the dehydroascorbic acid level in PTB, while it was not noted in pneumonia (Bakaev and Duntau 2004). In a randomized, double-blind clinical trial, 24 individuals with severe sepsis were allocated to 3 groups for receiving a placebo and 2 doses of vitamin C. The results demonstrated that vitamin C positively affected the level of multiple organ failure and inflammation biomarkers and endothelial injury (Syed et al. 2014). In a similar clinical study, 57 elderly patients with acute respiratory infections were randomly treated with placebo or vitamin C for 2 and 4 weeks. Respiratory symptom score showed a reduction in the vitamin C-treated group in comparison with placebo group (Hunt et al. 1994). Pneumonia patients (18 cases) were allocated to placebo or intervention groups receiving standard treatment plus i.v. vitamin C for 96 h. As shown, it was safe to intravenously administer a moderately high level of vitamin C to severe pneumonia patients, with the possibility of reducing the mechanical ventilation period, vasopressor use, and inflammation without considerably affecting mortality (Mahmoodpoor et al. 2021).
The placebo-controlled trials studies showed that the vitamin C administration (1 g/day) reduced the duration of adult colds averagely by 6% and by 17% in children and ≥ 2 g/day vitamin C decreased the period of colds in children by 6% and in adults by 21% (Hemilä 1999). In a randomized controlled trial, 308 adults with COVID-19 transferred to the ICU; patients were randomized to two groups for receiving high dose (200 mg/kg) and low dose (50 mg/kg) of vitamin C. The results indicated that high-dose vitamin C suppressed cytokine storms caused by COVID-19, improved pulmonary function and reduced the risk of ARDS in patients with COVID-19 (Liu et al. 2020). Vitamin C impacts on lung infections are summarized in Table 3.
The effect of vitamin C on other lung disorders
In other lung disorders also, the effects of vitamin C were demonstrated. In an in vitro study, vitamin C showed protective effects against hyperoxia-induced epithelial disruption. The results of this study showed that these protective effects are achieved by modulating the zona occludens-1 (ZO-1) and reducing pro-inflammatory cytokines level (Al‐Shmgani et al. 2013). The results of another study showed that the combination of vitamin C and E can reduce the destructive effects of hyperoxia on bronchial epithelium cells by reducing oxidative damage and improving the antioxidant system (Al-Shmgani et al. 2012). In an in vivo study, pretreatment with vitamin C and vitamin E significantly improved hyperoxia-induced down-regulation of cough reflex and oxidative damage in guinea pigs (Brozmanova et al. 2006).
Combined vitamin C and vitamin E administration ameliorated lung damage induced by acute swimming in rats. This study showed that the protective effects of this combination are mediated through antioxidant activity (Al-Hashem 2012). In addition, vitamin C administration protects lung tissue damage against ARDS induced by oleic acid trough improving histopathological markers such as hyperemia in vessels and thickening in bronchoalveolar septum. In addition, vitamin C supplementation reduced cytokine production, inflammatory cell infiltrations and oxidative biomarkers in the lung tissue (Erol et al. 2019). Vitamin C and NAC administration improved lung tissue injury induced by bile duct-ligation (BDL) in rats. This study indicated that treatment with NAC plus vitamin C decreased total bilirubin value, interstitial edema, focal metaplasia's of alveolar lining cells, and severely damaged pulmonary architecture (Ozturk et al. 2008). Vitamin C administration (200 mg/kg/day) improved benzene inhalation-induced lung injury in rats through modulating metalloproteinase-1 mRNA expression (Sourour et al. 2012). In addition, treatment with vitamin C significantly mitigated lung injury induced by zinc oxide nanoparticles inhalation in rats. Protective effect of vitamin C was mediated through suppressing acute oxidative stress, lactate dehydrogenase (LDH) activity and IL-6 concentration. In addition, vitamin C improved cytokine-induced neutrophil chemoattractant (CINC)-1, CINC-3, and HO-1 genes expression (Fukui et al. 2015). Vitamin C administration (100 µM) inhibited acrolein-induced cytotoxicity in cultured human bronchial epithelial cells via suppression of apoptosis and oxidant production (Nardini et al. 2002). Vitamin C (5–30 mM) protected human bronchial epithelial cells (16HBE cell) against PM2.5-induced oxidative damage. This study showed that vitamin C could suppress ROS generation in cells and improve mitochondrial function as well as inflammation status (Jin et al. 2016).
Pretreatment with vitamin C in a rat model of lung ischemia/reperfusion (I/R) significantly decreased lung tissue MDA levels, intense interstitial leukocyte infiltration and plasma leukocyte sequestration (Kim et al. 2012a, b). In another study in reperfusion injury in a lung auto-transplantation model in sheep, vitamin C pretreatment significantly lowered arterio-alveolar oxygen difference (AaDO2), pulmonary vascular resistance (PVR), work of breathing (WOB) and the plasma level of polymorphonuclear neutrophils (PMNs) (Demertzis et al. 2000).
Intravenous injection of vitamin C and continuous infusion in combination with vitamin E in a pig model of lung I/R, improved oxygenation and also decreased pulmonary inflammation (Wagner et al. 2002). Oral vitamin C therapy in an I/R induced lung injury model in Sprague–Dawley rats significantly attenuated microvascular leakage and neutrophil infiltration (Kearns et al. 1999). The effects of vitamin C on other lung disorders are summarized in Table 4.
Table 4.
The effect of vitamin C on other lung disorders
| Exp model | Dose | Effect | References | ||
|---|---|---|---|---|---|
| OFILI | Oleic acid-induced lung injury in rat | 100 mg/kg | Gavage | Reduced cytokine production, inflammatory cell infiltrations and oxidative biomarkers in lung tissue | Erol et al. (2019) |
| BDL-induced lung injury in rat | 200 mg/kg | Decreased total bilirubin value, interstitial edema, focal metaplasia’s of alveolar lining cells | Ozturk et al. (2008) | ||
| Benzene inhalation-induced lung injury in rats | 200 mg/kg | Decreased interstitial edema, focal metaplasia's of alveolar lining cells | Sourour et al. (2012) | ||
| ZON inhalation-induced lung injury in rats | 1% aqueous solution | Modulated metalloproteinase-1 mRNA expression | Sourour et al. (2012) | ||
| Hyperoxia-induced epithelial disruption | 10 (−6) or 10 (−7) M |
Modulated the ZO-1 levels Reduced pro-inflammatory cytokine |
Al‐Shmgani et al. (2013) | ||
| Hyperoxia-induced bronchial epithelium cells injury | – | – |
Reduced oxidative damage Improved the antioxidant system |
Al-Shmgani et al. (2012) | |
| Lung damage induced by acute swimming in the rats | – | – | Improved antioxidant system | Al-Hashem (2012) | |
| Acrolein-induced cytotoxicity in CHBEC | – | – | Suppressed the apoptosis and oxidant production | Nardini et al. (2002) | |
| PM2.5-induced oxidative damage in 16HBE cell γ | 5–30 mM | – | Suppressed ROS generation, improved mitochondrial function and inflammation | Jin et al. (2016) | |
| O L. D | Lung I/R in rats | 100 mg/kg | Gavage | Decreased lung tissue MDA levels, interstitial leukocyte infiltration and blood leukocyte sequestration | Kim et al. (2012a, b) |
| 3.3 gr | Attenuated microvascular leakage and neutrophil infiltration | Kearns et al. (1999) | |||
| Lung I/R in sheep | 1 g/kg | Lowered AaDO2, PVR, WOB and PMN count | Demertzis et al. (2000) | ||
| Lung I/R in pigs | 1 mg and 125 mg/h |
Improved oxygenation Decreased pulmonary inflammation |
Wagner et al. (2002a, b) | ||
Admin administration, OFILI other factors induced lung injury, O L. D other lung disorders, I/R ischemia–reperfusion, LDH lactate dehydrogenase, IL-6 interleukin-6, CINC-1 cytokine-induced neutrophil chemoattractant, HO-1 heme oxygenase-1, ROS reactive oxygen species, BDL bile duct-ligation, Exp experimental, ZON zinc oxide nanoparticles, CHBEC cultured human bronchial epithelial cells, PVR pulmonary vascular resistance, AaDO2 arterio-alveolar oxygen difference
Allergic disorders
Experimental results
The effect of vitamin C on allergic disorders was indicated in several studies. In a study of 18 guinea pigs, administration of Na-ascorbate (the mineral salt of AA which is more bioavailable than any other form of vitamin C), suppressed severe anaphylactic shock. In addition, administration of vitamin C for 4 days before and 1 day after the passive transfer of rabbit decreased anti-HGG antibody (human gamma globulin) and mortality (11%) compared to the control group (40%). Therefore, it can be concluded that AA has a protective role in preventing anaphylaxis (Pavlović and Fraser 1988).
In an in vitro study on HaCaT human keratinocyte cell line, the proliferation of HaCaT and primary human keratinocytes induced by house dust mite (HDM) was significantly suppressed by vitamin C and aptamin. In addition, T cell migration, and thymus and activation-regulated chemokine (TARC) production were reduced. Therefore, the results showed the potential effect of aptamin (a modified form of vitamin C that consists aptamers, DNA fragments) on inflammatory lesions such as atopic dermatitis (AD) (D. Lee et al. 2021a, b).
Clinical results
In 40 allergic rhinitis patients, the effect of vitamin C on clinical manifestations was investigated. This trial was a prospective controlled study with an experimental group (vitamin C group, 20 subjects) and placebo group (20 subjects). Administration of vitamin C and a sugar pill orally as a placebo was done for 1½ years, and the plasma levels of AA and its effect on the signs and symptoms of rhinitis were recorded. The results showed that administration of vitamin C improved the symptoms of allergic rhinitis such as sneezing, lacrimation, itching and malaise. After treatment with vitamin C, plasma AA level was also increased (Tongtako et al. 2018). In another study conducted on 27 patients, the effect of vitamin C supplementation on the symptoms of rhinitis was investigated. Administration of vitamin C for 8 weeks (2000 mg/day) along with exercise including walking and/or running on a treadmill with heart rate reserve of 65–70% for 30 min per session, 3 times a week for 8 weeks, caused a significant reduction in nasal congestion, sneezing, nasal itching, runny nose, nasal blood flow and MDA levels (Tongtako et al. 2018). Following acute oral administration of vitamin C on two consecutive days, in a double-blind cross-over, placebo-controlled design in patients with allergic rhinitis, the bronchial responsiveness index (PC15FEV1) increased significantly 1 h after vitamin C treatment (Bucca et al. 1990).
In 71 patients with respiratory or skin allergic disorders, the effect of adjunctive treatment of intravenous vitamin C on specific and non-specific symptoms of the disease (fatigue, sleep disorders, depression and lack of mental concentration) was evaluated. Administering i.v. vitamin C reduced allergy-related symptoms (Vollbracht et al. 2018). The effects of vitamin C on allergic disorders are summarized in Table 5.
Table 5.
The effect of vitamin C on allergic and immunologic disorders
| Dose | Admin rout | Effect | References | |||
|---|---|---|---|---|---|---|
| Al. D | Exp | Guinea pigs, anaphylaxis | 281 mg/day, Na-ascorbate | Gavage | Suppressed severe anaphylactic shock, decreased mortality | Pavlović and Fraser (1988) |
| Clin | Allergic rhinitis | 1 g/day | I.v. | Improved the symptoms of allergic rhinitis | Munjal (2020) | |
| 2000 mg/day, 8 weeks | Decreased nasal congestion, sneezing, nasal itching, runny nose, NBF and MDA levels | Tongtako et al. (2018) | ||||
| 2 g, 2 days | Increased PC15FEV1 | Bucca et al. (1990) | ||||
| 7.5 g/50 mL | Decreased allergy-related symptoms | Vollbracht et al. (2018) | ||||
| Im. D | Exp | HaCaT human keratinocyte cell line | – | Gavage | Decreased T cell migration and TARC production | Lee et al. (2021a, b) |
| LPS in guinea pigs | 0, 25, or 250 mg | Reduced mRNA levels of cytokines | Fraser et al. (1980) | |||
| Guinea pigs | 4 g/kg for 14 days | Improved T lymphocyte function in cell-mediated cytotoxicity | Anthony et al. (1979) | |||
| Mouse model | 5 mg/day | Increased levels of TNF-α and IFN-γ and decreased level of IL-4 | Noh et al. (2005) | |||
| Mice model | 10 μg/mL | Up-regulated IL-17 through Jmjd2 histone demethylase enzymes | Song et al. (2017) | |||
| Cell line U937 | – | Inhibited apoptotic pathways in monocytes | Perez-Cruz et al. (2003) | |||
| OVA-sensitized BALB/c mice | 130 mg/kg | Improved BALF IL-13 and serum IgE levels | Kianian et al. (2020a, b) |
Admin administration, Al. D. allergic disorders, Im. D. immunologic disorders, Exp. experimental, Clin. clinical, i.v. intravenous, PC15FEV1 15 percent decrease in forced expiratory volume, MDA Malondialdehyde, NBF Nasal blood flow, TARC Thymus and activation-regulated chemokine, IL-4 interleukin-4, IgE immunoglobulin E, IL-17 interleukin-17, IL-13 interleukin-13, TNF-α tumor necrosis factor alpha, IFN-γ interferon gamma, Ref references, Exp experimental
Immunologic disorders
The effects of vitamin C on allergic disorders were also investigated in several studies. Treatment with of Na-ascorbate in LPS-treated guinea pigs reduced mRNA levels of cytokines, IFN-γ and TNF-α (Fraser et al. 1980). The anti-inflammatory effect of vitamin C in a guinea pig model of cell-mediated cytotoxicity and humoral immune response was evaluated by administration of daily gavages of vitamin C for 14 days. The results indicated that AA deficiency may cause of T lymphocyte function impairment in cell-mediated cytotoxicity or changed the number or function of another cell type (Anthony et al. 1979). In a mouse model of T cell activation, treatment with vitamin C on 2,4-dinitro-1-fluorobenzene (DNFB)-induced delayed-type hypersensitivity response, increased levels of TNF-α and IFN-γ but decreased IL-4 level (Noh et al. 2005).
Vitamin C in wild-type (WT) mice model, up-regulated IL-17 by Jmjd2 histone demethylase enzymes and the related H3K9 histone modifications (Song et al. 2017). It was shown that intracellular accumulation of vitamin C, in monocytes in cell line U937, inhibits apoptotic pathways. In addition, vitamin C may regulate distinct genes expression in macrophages, which are induced by receptor FAS via NF-κB-light-chain-enhancer of activated B cells activation (Perez-Cruz et al. 2003).The effects of vitamin C on immunologic disorders are summarized in Table 5.
Possible effective dose of vitamin C on various lung disorders
Experimental results
Table 6 summarizes the various doses of vitamin C used in experimental models of various respiratory disorders. Animal models of asthma were treated with vitamin C at the minimum, maximum and average dose of 0.625, 400 and 130 mg/kg, respectively, and at the minimum, maximum and average total dose of 0.03 mg, 4.16 and about 38.8 g, respectively.
Table 6.
The effective dose of vitamin C on various disorders in experimental studies
| Exp. model | Animal | Dose/BW | Admin duration | Admin rout | Total dose | References |
|---|---|---|---|---|---|---|
| Asthma | Mice | 308 mg/kg | 5 days | I.n. | 6.6 mg | Bansal et al. (2014) |
| 0.625 and 5 mg/kg | 3 days | Gavage | 1.875–15 mg | Noh et al. (2005) | ||
| 130 mg/kg | 5 weeks | Gavage | 91 mg | Chang et al. (2009) | ||
| 130 mg/kg | 53 days | 138 mg | Kianian et al. (2019a) | |||
| 200 mg/kg | 26 days | 104 mg | Kianian et al. (2019b) | |||
| GP | 3–5 mg/kg | 6 days | 5.4 and 6 mg | Jeong et al. (2010) | ||
| 400 mg/kg | 26 days | 3.12 g | Haines et al. (2011) | |||
| COPD | Mice | 40 and 1500 mg/kg | 2 months | 48 mg and 1.8 g | Kengo Koike et al. (2014a, b) | |
| Rats | 40 mg/kg | 25 days | 235 mg | Ueta et al. (2001) | ||
| GP | 30 mg/kg | 8 weeks | 588 g | Ghosh et al. (2015) | ||
| 10 mg/kg | 28 days | 98 mg | Gupta et al. (2016) | |||
| 15 mg/day | 21 days | 315 mg | Banerjee et al. (2008) | |||
| Lung fibrosis | Rats | 75 mg/kg | 3 weeks | Gavage | 307 mg | Hemmati et al. (2008) |
| L-In | Rats | 250 mg/kg | 30 days | 1.65 g | Mohamed et al. (2019) | |
| GP | 800 mg/kg | 2 months | 14.5 g | Jensen et al. (2012) | ||
| OFILI | Rats | 100 mg/kg | 3 weeks | 525 mg | Pavlović and Fraser (1988) | |
| 200 mg/kg | 3 weeks | 945 mg | Öztürk et al. (2008) | |||
| 200 mg/kg | 4 weeks | 924 mg | Mongi et al. (2011) | |||
| 100 mg/kg | 6 weeks | 840 mg | Kim (2012) | |||
| 3.3 g/day | 5 days | 16.5 g | Kearns et al. (1999) | |||
| GP | 125 mg/h and 1 g | – | I.v. | 1.125 g | Wagner et al. (2002) | |
| 0.25 or 250 mg | 28 days | 7 mg and 7 g | Fraser et al. (1980) | |||
| 4 g/kg | 14 days | – | 21 g | Anthony et al. (1979) | ||
| 281 mg/day | SD | I.p. | 281 mg | Pavlović and Fraser (1988) | ||
| Mice | 5 mg/day | 2 weeks | I.p. | 70 mg | Noh et al. (2005) | |
| 130 mg/kg | 8 weeks | I.p. | 145 mg | Kianian et al. (2020b) | ||
| 200 mg/kg | 4 weeks | I.p. | 112 mg | Sourour et al. (2012) |
Admin administration, COPD chronic obstructive pulmonary disease, OFILI other factors induced lung injury, L-In lung infection, GP guinea pigs, i.p intraperitoneal, Ref references, Exp experimental
Animal models of COPD were treated with vitamin C at the minimum, maximum and average dose of 10, 1500 and 272 mg/kg, respectively, and at the minimum, maximum and average total dose of 48 mg, 1.8 g and about 452 mg, respectively. In animal models of lung infection, vitamin C was administered at the minimum, maximum and average dose of 250, 800 and 525 mg/kg, respectively, and at the minimum, maximum and average total dose of 1.65, 14.5 and about 8 g, respectively. Animal models of another lung injury were treated with vitamin C at the minimum, maximum and average dose of 0.6 mg/kg, 4000 mg/kg and 6.7 g/kg, respectively, and at the minimum, maximum and average total dose of 7 mg, 21 g and 4 g, respectively.
Clinical results
Table 7 shows that in clinical studies, asthmatic patients were treated with vitamin C at the minimum, maximum and average total dose of 180 g, 48 g and 109 g, respectively. In patients with COPD, vitamin C was administered by oral rout at 250 mg/day for 12 weeks (total administered dose 21 g) (Wu et al. 2007) and at dose 500 mg/day for 6 months (total administered dose 90 g) (Pirabbasi et al. 2016).
Table 7.
The effective dose of vitamin C on various disorders in clinical studies
| Clinical condition | Dose | Admin rout | Admin duration | Total dose | References |
|---|---|---|---|---|---|
| Bronchoconstriction | 1500 mg/day | Oral | 1 week | 455 g | Riordan et al. (2000) |
| Asthma | 1 g/day | I.V | 6 months | 180 g | Anderson and Theron (1983) |
| 1 g/day | Oral | 16 weeks | 112 g | Fogarty et al. (2003; Andrew Fogarty et al. (2006) | |
| 1 g/day | Oral | 14 weeks | 98 g | Anah et al. (1980) | |
| COPD | 250 mg/day | Oral | 12 weeks | 21 g | Wu et al. (2007) |
| 500 mg/day | Oral | 6 months | 90 g | Pirabbasi et al. (2016) | |
| LC | 65 g/day | I.V | 1 week | 455 g | Bansal et al. (2014) |
| 150 g | I.V | 1 day | 150 g | González et al. (2016) | |
| 100 mg/day | Oral | – | – | Bansal et al. (2014); Kumari et al. (2020) | |
| L-In | 50 mg/kg | I.V | 28 days | 105 g | Bansal et al. (2014); Kumari et al. (2020) |
| 60 mg/kg | I.V | 4 days | 18 g | Bansal et al. (2014); Mahmoodpoor et al. (2021) | |
| 50 or 200 mg/kg | I.V | 4 days | 15 and 60 g | Syed et al. (2014) | |
| 6 g/day | I.V | 4 days | 24 g | Lee et al. (2021a, b) | |
| 200 mg/kg | Oral | 2 weeks | 2.8 g | Hunt et al. (1994) | |
| 2 g/day | Oral | 2 days | 4 g | Bucca et al. (1990) | |
| 2000 mg/day | Oral | 8 weeks | 112 g | Tongtako et al. (2018) | |
| 7.5 g/day | Oral | 10–14 weeks | 525 and 735 g | Vollbracht et al. (2018) |
Admin administration, COPD chronic obstructive pulmonary disease, L-In lung infection, Ref references, I.V intravenous, LC lung cancer
Patients with lung infection were also treated with vitamin C at the minimum, maximum and average dose of 2.6, 200 and 73.7 mg/kg, respectively, and at the minimum, maximum and average total dose of 2.8 g, 105 g and 37.4 g, respectively. Vitamin C was administered in patients with lung infection at the minimum, maximum and average dose of 2.6, 200 and 73.7 mg/kg, respectively, and at the minimum, maximum and average total dose of 2.8, 105 and 37.4 g, respectively.
Bioavailability, registered drug forms and toxicity of vitamin C
The vitamin C bioavailability can be affected by various factors, such as pH, the interaction of other organic compounds, the interaction between vitamin C and minerals, the presence of oxygen, the type of processing, and lifestyle. Nevertheless, as shown by previous research works, this can be prevented by retaining or enhancing the bioavailability and bioactivity of vitamin C. Encapsulation, the formation of emulsions, and using antioxidants are among the actions done for improving the bioactivity and bioavailability of vitamin C (Vissers et al. 2013).
There are various formulations for synthetic vitamin C, but some types showed better bioavailability (Nyyssönen et al. 1997) including AA preparations available as capsules, tablet, or powders, or as formulations with slow release. It is not clear if there is any form that has higher absorbability than others (Yung et al. 1982). These forms are different in terms of product stability in storage since exposure to heat, light, or air readily oxidizes ascorbate. Therefore, it is suggested to keep them in tablets or capsules instead of powder in long time storage conditions. Ascorbate in the form of liquid is generally more unsteady and heating in the commercial preparation of fruit juices can destroy it. For ensuring the products’ consistency, synthetic ascorbate is generally added to them before sale (Vissers et al. 2013).
Since in clinical practice, vitamin C is used in higher bolus amounts compared to the vitamin C available in food, it is attempted to formulate them in such a way that the free acid effect is mitigated and more prolonged and slower uptake is ensured (Doseděl et al. 2021). AA mineral salts, mainly calcium ascorbate or sodium, are neutral (Carr and Frei 1999). In “Ester C”, calcium ascorbate is mostly combined with some metabolites, and it is different from esterified ascorbate, which comprises mixes of lipids and vitamin C, leading to increased bioavailability (Carr and Frei 1999). Nevertheless, there is not sufficient in vivo data determining the relative efficacy of these products in comparison to AA, either from a food source or a purified form.
In terms of the toxicity, low toxicity is generally exhibited by vitamin C. The LD50 is accepted as 11,900 mg (11.9 g) per kg in rats. Zero deaths from toxicity of vitamin C have been reported by the American Association of Poison Control Centers (Litovitz et al. 1999).
A single dose of 5–10 g vitamin C (oral form) causes abdominal bloating with pain and/or transient osmotic diarrhea. However, it is considered that even such a high dosage is safe. Nevertheless, these adverse reactions can be reduced by its intake with food (Fukushima and Yamazaki 2010; Johnston and Cox 2001; Levine et al. 1999; World Health Organization 1999).
It should be noted that most patients can tolerate even very high doses of intravenous vitamin C (between 1 and 200 g) (Padayatty et al. 2010). According to a prospective cohort research, the oral intake of vitamin C above 1 g elevated the stone formation risk by 41% (Taylor et al. 2004). Thus, doctors should not recommend vitamin C at doses above 1 g for daily usage on a routine basis (Levine et al. 1999).
Conclusions
Reviewed studies indicated the effect of vitamin C on various lung disorders including asthma, COPD, lung fibrosis, lung cancer, lung infections as well as allergic and immunologic disorders with anti-inflammatory, antioxidant and immunomodulatory mechanisms, in both experimental and clinical studies.
Various experimental studies showed the relaxant effect of vitamin C on TSM mediated by various mechanisms including β2-adrenoceptors stimulatory, inhibitory effect on muscarinic and histamine H1 receptors, phosphodiesterase enzyme-like and calcium channel blocking mechanisms. These findings suggest the possible bronchodilatory effects of the vitamin C in obstructive respiratory diseases but further clinical trials should be performed to examine this effect in different obstructive pulmonary disorders.
The preventive effect of vitamin C was demonstrated in different respiratory diseases, including asthma, COPD, lung fibrosis, lung cancer, and other respiratory disorders.
Treatment with the vitamin C affects different lung cancer in vitro, in vivo and in clinical studies through affecting cell viability and other molecular mechanisms.
In experimental studies, the effects of vitamin C on lung infections caused by various viruses and batteries were reported. Clinical studies also demonstrate that timely administration of high dose of vitamin C improves the outcome of COVID-19 infection.
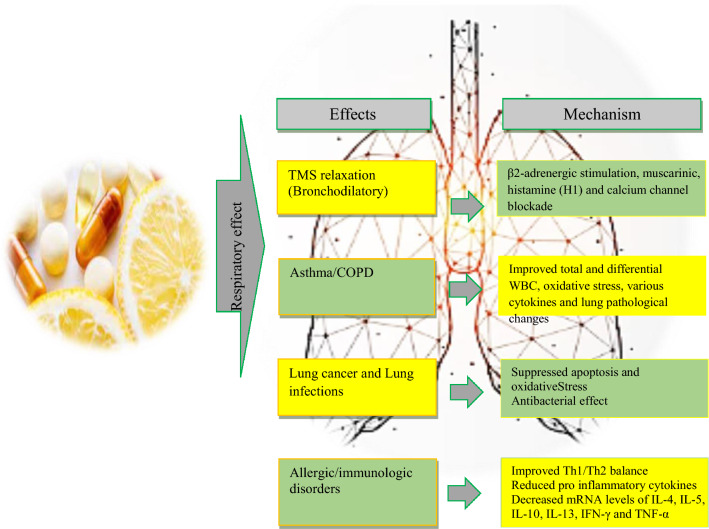
The preventive or prophylactic effects of the vitamin C on various allergic and immunologic disorders were also indicated. The possible cellular and molecular mechanisms of the preventive effects of vitamin C on various respiratory disorders were shown to be due to its anti-inflammatory, immunomodulatory and antioxidant properties in different studies. Anti-inflammatory effects of the vitamin C on lung inflammation were reported by reduction of total and WBC in BALF and blood of asthma and COPD and others respiratory disorders. Vitamin C also improved the levels of inflammatory mediators such as 8-isoprostanese, vascular endothelial growth factor, pro-inflammatory protein expressions such as Rtp801, NF-κB, and iNOS as well as MMP-9 and MMP-12 gene expression in the BALF and lung tissues. Regarding the antioxidant effects of vitamin C, it declined free radicals. Serum and BALF level of oxidants markers such as MDA and erythropoietin were reduced but antioxidants including catalase and glutathione peroxidase were increased in asthmatic animals by treatment with vitamin C. In addition, vitamin C suppressed ROS generation in cells and improved mitochondrial function as well as inflammation status. Immune-modulatory effects of vitamin C were indicated in animal models by decreasing serum and BALF levels of IL-6, IL-1β, TNFα, IL-4 and IL-5 but enhanced IFN-γ and Treg cells. Treatment with vitamin C also shifted Th1/Th2 balance toward the Th1 pole. In addition, vitamin C down-regulated gene expression of pro-inflammatory cytokines such as TNFα and IL-1 that is dependent on ROS by inhibiting NF-kB transcription. The levels of IL-4 and IL-5 in the BALF, IgE and IgG1 levels in the serum and NF-κB p65 protein levels in the lung tissue were improved by treatment with vitamin C. Lung pathological changes in various respiratory diseases and tracheal responsiveness to different stimuli, mainly methacholine, were improved due to vitamin C therapy which are suggested to be due to anti-inflammatory, antioxidant and immunomodulatory properties of this agent. Figures 2 and 3 show various effects of vitamin C on respiratory system and some of its possible mechanisms.
Fig. 2.

Possible mechanisms of the effect’s vitamin C on respiratory disorders. IL-6 Interleukin-6, IL-1β Interleukin-1β, IFN-γ Interferon-γ
Fig. 3.
Experimental and clinical effects of vitamin C on respiratory and allergic disorders
The minimum, maximum and average effective doses of vitamin C for the treatment of different respiratory diseases both in experimental and clinical were also provided. The findings described in this article suggest a promising therapeutic effect of this agent on various respiratory diseases including lung insult induced by COVID-19. The major limitation regarding the effect of vitamin C on lung disorders is limited clinical studies done in this field. In addition, the exact doses of vitamin C for the treatment of each disorder should be determined in further studies. Therefore, more clinical trials are needed to examine the effects of vitamin C on respiratory disorders. In addition, to systematically assess the results of previous research, meta-analyses should be done before the production of vitamin C-based drugs for clinical use.
Acknowledgements
We would like to thank the authors of all papers which are included in the present review article.
Abbreviations
- AA
Ascorbic acid
- AD
Atopic dermatitis
- BALF
Broncho-alveolar lavage fluid
- cAMP
Cyclic adenosine monophosphate
- cGMP
Cyclic guanosine monophosphate
- COPD
Chronic obstructive pulmonary disease
- DHA
Dehydroascorbic acid
- EPO
Eosinophil peroxidase
- GLUTs
Glucose transporters
- GSH
Glutathione
- GULO
L-Gulonolactone oxidase
- HDM
House dust mite
- H&E
Hematoxylin and eosin
- HGG
Human gamma globulin
- IFN-γ
Interferon-γ
- IgE
Immunoglobulin E
- IL
Interleukin
- iNOS
Inducible nitric oxide synthase
- iPSCs
Induced pluripotent stem cells
- KCl
Potassium chloride
- MDA
Malondialdehyde
- MMP-9
Matrix metallopeptidase 9
- NBF
Nasal blood flow
- NF-κB
Nuclear factor-kappa B
- OVA
Ovalbumin
- PP2A
Protein phosphatase type 2A
- ROS
Reactive oxygen species
- SVCTs
Sodium-vitamin C co‐transporters
- TARC
Thymus and activation-regulated chemokine
- TNF-α
Tumor necrosis factor-α
- WBC
White blood cell
- XO
Xanthine oxidase
- DNFB
2,4-Dinitro-1-fluorobenzene
Author contributions
All the authors contributed to the study conception and design. MHEG, FK, SB, SB, NM, and MB prepared the first draft of the manuscript and helped in the revision of the final version of the manuscript. M.H.B designed the study, critically edited and revised the manuscript. All the authors read and approved the final manuscript.
Funding
There are no funding sources.
Data availability
Not applicable.
Declarations
Conflict of interest
The authors declare that there are not competing interest in this article.
Ethical approval
Not applicable.
Consent to participate
Not applicable.
Consent for publication
Not applicable.
Footnotes
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- Al-Hashem FH. Potential roles for vitamins E and C in combination in modulating exhaustive swimming and high altitude-associated lung injury in rats. Saudi Med J. 2012;33(4):367–374. [PubMed] [Google Scholar]
- Al-Shmgani HS, Moate RM, Sneyd JR, Macnaughton PD, Moody AJ. Hyperoxia-induced ciliary loss and oxidative damage in an in vitro bovine model: the protective role of antioxidant vitamins E and C. Biochem Biophys Res Commun. 2012;429(3–4):191–196. doi: 10.1016/j.bbrc.2012.10.113. [DOI] [PubMed] [Google Scholar]
- Al-Shmgani HS, Moate RM, Macnaughton PD, Sneyd JR, Moody AJ. Effects of hyperoxia on the permeability of 16 HBE 14o–cell monolayers–the protective role of antioxidant vitamins E and C. FEBS J. 2013;280(18):4512–4521. doi: 10.1111/febs.12413. [DOI] [PubMed] [Google Scholar]
- Anah C, Jarike L, Baig H. High dose ascorbic acid in Nigerian asthmatics. Trop Geogr Med. 1980;32(2):132–137. [PubMed] [Google Scholar]
- Anderson R, Hay I, van Wyk HA, Theron A. Ascorbic acid in bronchial asthma. S Afr Med J. 1983;63(17):649–652. [PubMed] [Google Scholar]
- Ang A, Pullar JM, Currie MJ, Vissers MCM. Vitamin C and immune cell function in inflammation and cancer. Biochem Soc Trans. 2018;46(5):1147–1159. doi: 10.1042/bst20180169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Anthony LE, Kurahara CG, Taylor KB. Cell-mediated cytotoxicity and humoral immune response in ascorbic acid-deficient guinea pigs. Am J Clin Nutr. 1979;32(8):1691–1698. doi: 10.1093/ajcn/32.8.1691. [DOI] [PubMed] [Google Scholar]
- Bakaev V, Duntau A. Ascorbic acid in blood serum of patients with pulmonary tuberculosis and pneumonia. Int J Tuberc Lung Dis. 2004;8(2):263–266. [PubMed] [Google Scholar]
- Banerjee S, Chattopadhyay R, Ghosh A, Koley H, Panda K, Roy S, Chatterjee IB. Cellular and molecular mechanisms of cigarette smoke-induced lung damage and prevention by vitamin C. J Inflamm (lond) 2008;5:21. doi: 10.1186/1476-9255-5-21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bansal P, Saw S, Govindaraj D, Arora N. Intranasal administration of a combination of choline chloride, vitamin C, and selenium attenuates the allergic effect in a mouse model of airway disease. Free Radical Biol Med. 2014;73:358–365. doi: 10.1016/j.freeradbiomed.2014.05.018. [DOI] [PubMed] [Google Scholar]
- Barabutis N, Khangoora V, Marik PE, Catravas JD. Hydrocortisone and ascorbic acid synergistically prevent and repair lipopolysaccharide-induced pulmonary endothelial barrier dysfunction. Chest. 2017;152(5):954–962. doi: 10.1016/j.chest.2017.07.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barazzoni R, Bischoff SC, Breda J, Wickramasinghe K, Krznaric Z, Nitzan D, Singer P. ESPEN expert statements and practical guidance for nutritional management of individuals with SARS-CoV-2 infection. Elsevier; 2020. pp. 1631–1638. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boskabady MH, Ziaei T. Effect of ascorbic acid on airway responsiveness in ovalbumin sensitized guinea pigs. Respirology. 2003;8(4):473–478. doi: 10.1046/j.1440-1843.2003.00511.x. [DOI] [PubMed] [Google Scholar]
- Brozmanova M, Plevkova J, Bartos V, Plank L, Javorka M, Tatar M. The interaction of dietary antioxidant vitamins and oxidative stress on cough reflex in guinea-pigs after long term oxygen therapy. J Physiol Pharmacol. 2006;57:45–54. [PubMed] [Google Scholar]
- Bucca C, Rolla G, Oliva A, Farina J. Effect of vitamin C on histamine bronchial responsiveness of patients with allergic rhinitis. Ann Allergy. 1990;65(4):311–314. [PubMed] [Google Scholar]
- Carr AC, Frei B. Toward a new recommended dietary allowance for vitamin C based on antioxidant and health effects in humans. Am J Clin Nutr. 1999;69(6):1086–1107. doi: 10.1093/ajcn/69.6.1086. [DOI] [PubMed] [Google Scholar]
- Castranova V, Wright J, Colby H, Miles PR. Ascorbate uptake by isolated rat alveolar macrophages and type II cells. J Appl Physiol. 1983;54(1):208–214. doi: 10.1152/jappl.1983.54.1.208. [DOI] [PubMed] [Google Scholar]
- Chang HH, Chen CS, Lin JY. High dose vitamin C supplementation increases the Th1/Th2 cytokine secretion ratio, but decreases eosinophilic infiltration in bronchoalveolar lavage fluid of ovalbumin-sensitized and challenged mice. J Agric Food Chem. 2009;57(21):10471–10476. doi: 10.1021/jf902403p. [DOI] [PubMed] [Google Scholar]
- Chang P, Liao Y, Guan J, Guo Y, Zhao M, Hu J, Liu Z. Combined treatment with hydrocortisone, vitamin c, and thiamine for sepsis and septic shock: a randomized controlled trial. Chest. 2020;158(1):174–182. doi: 10.1016/j.chest.2020.02.065. [DOI] [PubMed] [Google Scholar]
- Cho YS, Moon H-B. The role of oxidative stress in the pathogenesis of asthma. Allergy, Asthma Immunol Res. 2010;2(3):183–187. doi: 10.4168/aair.2010.2.3.183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Colunga Biancatelli RML, Berrill M, Marik PE. The antiviral properties of vitamin C. Expert Rev Anti Infect Ther. 2020;18(2):99–101. doi: 10.1080/14787210.2020.1706483. [DOI] [PubMed] [Google Scholar]
- Das A, Dey N, Ghosh A, Das S, Chattopadhyay DJ, Chatterjee IB. Molecular and cellular mechanisms of cigarette smoke-induced myocardial injury: prevention by vitamin C. PLoS ONE. 2012;7(9):e44151. doi: 10.1371/journal.pone.0044151. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- Das B, Maity PC, Sil AK. Vitamin C forestalls cigarette smoke induced NF-kappaB activation in alveolar epithelial cells. Toxicol Lett. 2013;220(1):76–81. doi: 10.1016/j.toxlet.2013.04.009. [DOI] [PubMed] [Google Scholar]
- Dawson W, Hemsworth B, Stockham M. Actions of sodium ascorbate on smooth muscle. Br J Pharmacol Chemother. 1967;31(2):269. doi: 10.1111/j.1476-5381.1967.tb01997.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Demertzis S, Scherer M, Langer F, Dwenger A, Hausen B, Schafers HJ. Ascorbic acid for amelioration of reperfusion injury in a lung autotransplantation model in sheep. Ann Thorac Surg. 2000;70(5):1684–1689. doi: 10.1016/s0003-4975(00)01846-4. [DOI] [PubMed] [Google Scholar]
- Diyya SM, Thomas NV. Multiple micronutrient supplementation: as a supportive therapy in the treatment of COVID-19. BioMed Res Int. 2022;2022:1–7. doi: 10.1155/2022/3323825. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Doseděl M, Jirkovský E, Macáková K, Krčmová LK, Javorská L, Pourová J, Mladěnka P. Vitamin C—sources, physiological role, kinetics, deficiency, use, toxicity, and determination. Nutrients. 2021;13(2):615. doi: 10.3390/nu13020615. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Du YT, Long Y, Tang W, Liu XF, Dai F, Zhou B. Prooxidative inhibition against NF-κB-mediated inflammation by pharmacological vitamin C. Free Radic Biol Med. 2022;180:85–94. doi: 10.1016/j.freeradbiomed.2022.01.007. [DOI] [PubMed] [Google Scholar]
- Erol N, Saglam L, Saglam YS, Erol HS, Altun S, Aktas MS, Halici MB. The protection potential of antioxidant vitamins against acute respiratory distress syndrome: a rat trial. Inflammation. 2019;42(5):1585–1594. doi: 10.1007/s10753-019-01020-2. [DOI] [PubMed] [Google Scholar]
- Esteban MA, Wang T, Qin B, Yang J, Qin D, Cai J, Pei D. Vitamin C enhances the generation of mouse and human induced pluripotent stem cells. Cell Stem Cell. 2010;6(1):71–79. doi: 10.1016/j.stem.2009.12.001. [DOI] [PubMed] [Google Scholar]
- Fisher BJ, Kraskauskas D, Martin EJ, Farkas D, Wegelin JA, Brophy D, Natarajan R. Mechanisms of attenuation of abdominal sepsis induced acute lung injury by ascorbic acid. Am J Physiol Lung Cell Mol Physiol. 2012;303(1):L20–32. doi: 10.1152/ajplung.00300.2011. [DOI] [PubMed] [Google Scholar]
- Fogarty A, Lewis S, Scrivener S, Antoniak M, Pacey S, Pringle M, Britton J. Oral magnesium and vitamin C supplements in asthma: a parallel group randomized placebo-controlled trial. Clin Exp Allergy. 2003;33(10):1355–1359. doi: 10.1046/j.1365-2222.2003.01777.x. [DOI] [PubMed] [Google Scholar]
- Fogarty A, Lewis SA, Scrivener SL, Antoniak M, Pacey S, Pringle M, Britton J. Corticosteroid sparing effects of vitamin C and magnesium in asthma: a randomised trial. Respir Med. 2006;100(1):174–179. doi: 10.1016/j.rmed.2005.03.038. [DOI] [PubMed] [Google Scholar]
- Fraser RC, Pavlović S, Kurahara CG, Murata A, Peterson NS, Taylor KB, Feigen GA. The effect of variations in vitamin C intake on the cellular immune response of guinea pigs. Am J Clin Nutr. 1980;33(4):839–847. doi: 10.1093/ajcn/33.4.839. [DOI] [PubMed] [Google Scholar]
- Fukui H, Iwahashi H, Endoh S, Nishio K, Yoshida Y, Hagihara Y, Horie M. Ascorbic acid attenuates acute pulmonary oxidative stress and inflammation caused by zinc oxide nanoparticles. J Occup Health. 2015;57(2):118–125. doi: 10.1539/joh.14-0161-OA. [DOI] [PubMed] [Google Scholar]
- Fukushima R, Yamazaki E. Vitamin C requirement in surgical patients. Curr Opin Clin Nutr Metab Care. 2010;13(6):669–676. doi: 10.1097/MCO.0b013e32833e05bc. [DOI] [PubMed] [Google Scholar]
- Ghosh A, Ganguly S, Dey N, Banerjee S, Das A, Chattopadhyay DJ, Chatterjee IB. Causation of cigarette smoke-induced emphysema by p-benzoquinone and its prevention by vitamin C. Am J Respir Cell Mol Biol. 2015;52(3):315–322. doi: 10.1165/rcmb.2013-0545OC. [DOI] [PubMed] [Google Scholar]
- Goldschmidt M, Masin W, Brown L, Wyde P. The effect of ascorbic acid deficiency on leukocyte phagocytosis and killing of actinomyces viscosus. Int J Vit Nutr Res. 1988;58(3):326–334. [PubMed] [Google Scholar]
- González MJ, Berdiel MJ, Miranda-Massari JR, López D, Rodríguez-López JL, Adrover-López PA, Duconge J. High dose intravenous vitamin c treatment in a patient with lung cancer: a case report. Clin Case Rep Rev. 2016;2(6):454–455. doi: 10.15761/CCRR.1000243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gupta I, Ganguly S, Rozanas CR, Stuehr DJ, Panda K. Ascorbate attenuates pulmonary emphysema by inhibiting tobacco smoke and Rtp801-triggered lung protein modification and proteolysis. Proc Natl Acad Sci U S A. 2016;113(29):E4208–4217. doi: 10.1073/pnas.1600056113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haines DD, Varga B, Bak I, Juhasz B, Mahmoud FF, Kalantari H, Tosaki A. Summative interaction between astaxanthin, Ginkgo biloba extract (EGb761) and vitamin C in suppression of respiratory inflammation: a comparison with ibuprofen. Phytother Res. 2011;25(1):128–136. doi: 10.1002/ptr.3160. [DOI] [PubMed] [Google Scholar]
- Hemilä H. Vitamin C and common cold incidence: a review of studies with subjects under heavy physical stress. Int J Sports Med. 1996;17(05):379–383. doi: 10.1055/s-2007-972864. [DOI] [PubMed] [Google Scholar]
- Hemilä H. Vitamin C supplementation and common cold symptoms: factors affecting the magnitude of the benefit. Med Hypotheses. 1999;52(2):171–178. doi: 10.1054/mehy.1997.0639. [DOI] [PubMed] [Google Scholar]
- Hemilä H. Vitamin C and common cold-induced asthma: a systematic review and statistical analysis. Allergy Asthma Clin Immunol. 2013;9(1):1–10. doi: 10.1186/1710-1492-9-46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hemilä H. The effect of vitamin C on bronchoconstriction and respiratory symptoms caused by exercise: a review and statistical analysis. Allergy Asthma Clin Immunol. 2014;10(1):1–11. doi: 10.1186/1710-1492-10-58. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hemilä H, Chalker E. Vitamin C for preventing and treating the common cold. Cochrane Database Syst Rev. 2013 doi: 10.1002/14651858.CD000980.pub4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hemmati AA, Nazari Z, Ranjbari N, Torfi A. Comparison of the preventive effect of vitamin C and E on hexavalent chromium induced pulmonary fibrosis in rat. Inflammopharmacology. 2008;16(4):195–197. doi: 10.1007/s10787-008-7004-4. [DOI] [PubMed] [Google Scholar]
- Hoang BX, Shaw G, Fang W, Han B. Possible application of high-dose vitamin C in the prevention and therapy of coronavirus infection. J Global Antimicrob Resist. 2020;23:256–262. doi: 10.1016/j.jgar.2020.09.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hui L, Nelson E, Lin S, Zhao J. The role of vitamin C in pneumonia and COVID-19 infection in adults with European ancestry: a Mendelian randomisation study. Eur J Clin Nutr. 2022;76(4):588–591. doi: 10.1038/s41430-021-00993-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hunt C, Chakravorty N, Annan G, Habibzadeh N, Schorah C. The clinical effects of vitamin C supplementation in elderly hospitalised patients with acute respiratory infections. Int J Vit Nutr Res. 1994;64(3):212–219. [PubMed] [Google Scholar]
- Jensen PØ, Lykkesfeldt J, Bjarnsholt T, Hougen HP, Høiby N, Ciofu O. Poor antioxidant status exacerbates oxidative stress and inflammatory response to Pseudomonas aeruginosa lung infection in guinea pigs. Basic Clin Pharmacol Toxicol. 2012;110(4):353–358. doi: 10.1111/j.1742-7843.2011.00822.x. [DOI] [PubMed] [Google Scholar]
- Jeong YJ, Kim JH, Kang JS, Lee WJ, Hwang YI. Mega-dose vitamin C attenuated lung inflammation in mouse asthma model. Anat Cell Biol. 2010;43(4):294–302. doi: 10.5115/acb.2010.43.4.294. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jin X, Su R, Li R, Song L, Chen M, Cheng L, Li Z. Amelioration of particulate matter-induced oxidative damage by vitamin c and quercetin in human bronchial epithelial cells. Chemosphere. 2016;144:459–466. doi: 10.1016/j.chemosphere.2015.09.023. [DOI] [PubMed] [Google Scholar]
- Johnston CS, Cox SK. Plasma-saturating intakes of vitamin C confer maximal antioxidant protection to plasma. J Am Coll Nutr. 2001;20(6):623–627. doi: 10.1080/07315724.2001.10719159. [DOI] [PubMed] [Google Scholar]
- Karimian P, Tahami MS, Sayyahfar S, Delavar MA. Association of vitamin D and severity of COVID-19 in children. Eur J Transl Myol. 2022;32(2):10453. doi: 10.4081/ejtm.2022.10453. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaźmierczak-Barańska J, Boguszewska K, Adamus-Grabicka A, Karwowski BT. Two faces of vitamin C-antioxidative and pro-oxidative agent. Nutrients. 2020 doi: 10.3390/nu12051501. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kearns S, Kelly C, Barry M, Abdih H, Condron C, Leahy A, Bouchier-Hayes D. Vitamin C reduces ischaemia-reperfusion-induced acute lung injury. Eur J Vasc Endovasc Surg. 1999;17(6):533–536. doi: 10.1053/ejvs.1999.0833. [DOI] [PubMed] [Google Scholar]
- Kianian F, Kadkhodaee M, Sadeghipour HR, Karimian SM, Seifi B. An overview of high-mobility group box 1, a potent pro-inflammatory cytokine in asthma. J Basic Clin Physiol Pharmacol. 2020 doi: 10.1515/jbcpp-2019-0363. [DOI] [PubMed] [Google Scholar]
- Kianian F, Karimian SM, Kadkhodaee M, Takzaree N, Seifi B, Adeli S, Sadeghipour HR. Combination of ascorbic acid and calcitriol attenuates chronic asthma disease by reductions in oxidative stress and inflammation. Respiratory Physiol Neurobiol. 2019;270:103265. doi: 10.1016/j.resp.2019.103265. [DOI] [PubMed] [Google Scholar]
- Kianian F, Karimian SM, Kadkhodaee M, Takzaree N, Seifi B, Sadeghipour HR. Protective effects of ascorbic acid and calcitriol combination on airway remodelling in ovalbumin-induced chronic asthma. Pharm Biol. 2020;58(1):107–115. doi: 10.1080/13880209.2019.1710218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kianian F, Sadeghipour HR, Karimian SM, Kadkhodaee M, Seifi B. Protective effects of hydrogen sulfide on anxiety in ovalbumin-induced chronic asthma. Physiol Pharmacol. 2019;23(3):208–214. [Google Scholar]
- Kianian F, Seifi B, Kadkhodaee M, Sadeghipour HR, Ranjbaran M. Nephroprotection through modifying the apoptotic tnf-α/erk1/2/bax signaling pathway and oxidative stress by long-term sodium hydrosulfide administration in ovalbumin-induced chronic asthma. Immunol Invest. 2022;51(3):602–618. doi: 10.1080/08820139.2020.1858860. [DOI] [PubMed] [Google Scholar]
- Kim Y, Chongviriyaphan N, Liu C, Russell RM, Wang X-D. Combined α-tocopherol and ascorbic acid protects against smoke-induced lung squamous metaplasia in ferrets. Lung Cancer. 2012;75(1):15–23. doi: 10.1016/j.lungcan.2011.05.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koike K, Ishigami A, Sato Y, Hirai T, Yuan Y, Kobayashi E, Takahashi K. Vitamin C prevents cigarette smoke–induced pulmonary emphysema in mice and provides pulmonary restoration. Am J Respir Cell Mol Biol. 2014;50(2):347–357. doi: 10.1165/rcmb.2013-0121OC. [DOI] [PubMed] [Google Scholar]
- Kumari P, Dembra S, Dembra P, Bhawna F, Gul A, Ali B, Rizwan A. The role of vitamin C as adjuvant therapy in COVID-19. Cureus. 2020 doi: 10.7759/cureus.11779. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Labaki WW, Han MK. Chronic respiratory diseases: a global view. Lancet Respir Med. 2020;8(6):531–533. doi: 10.1016/s2213-2600(20)30157-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lavillegrand JR, Raia L, Urbina T, Hariri G, Gabarre P, Bonny V, Ait-Oufella H. Vitamin C improves microvascular reactivity and peripheral tissue perfusion in septic shock patients. Crit Care. 2022;26(1):25. doi: 10.1186/s13054-022-03891-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee D, Kim Y, Jo H, Go C, Jeong Y, Jang Y, Kang JS. The anti-inflammatory effect of aptamin c on house dust mite extract-induced inflammation in keratinocytes via regulation of il-22 and gdnf production. Antioxidants. 2021;10(6):945. doi: 10.3390/antiox10060945. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee S-I, Lim C-M, Koh Y, Huh J-W, Lee JS, Hong S-B. The effectiveness of vitamin C for patients with severe viral pneumonia in respiratory failure. J Thorac Dis. 2021;13(2):632. doi: 10.21037/jtd-20-1306. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Levine M, Rumsey SC, Daruwala R, Park JB, Wang Y. Criteria and recommendations for vitamin C intake. JAMA. 1999;281(15):1415–1423. doi: 10.1001/jama.281.15.1415. [DOI] [PubMed] [Google Scholar]
- Litovitz TL, Klein-Schwartz W, Caravati EM, Youniss J, Crouch B, Lee S. 1998 annual report of the American Association of Poison Control Centers toxic exposure surveillance system. Am J Emerg Med. 1999;17(5):435–487. doi: 10.1016/S0735-6757(99)90254-1. [DOI] [PubMed] [Google Scholar]
- Liu F, Zhu Y, Zhang J, Li Y, Peng Z. Intravenous high-dose vitamin C for the treatment of severe COVID-19: study protocol for a multicentre randomised controlled trial. BMJ Open. 2020;10(7):e039519. doi: 10.1136/bmjopen-2020-039519. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Locksley RM. Asthma and allergic inflammation. Cell. 2010;140(6):777–783. doi: 10.1016/j.cell.2010.03.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Luo J, Shen L, Zheng D. Association between vitamin C intake and lung cancer: a dose-response meta-analysis. Sci Rep. 2014;4(1):1–7. doi: 10.1038/srep06161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mahmoodpoor A, Shadvar K, Sanaie S, Hadipoor MR, Pourmoghaddam MA, Saghaleini SH. Effect of Vitamin C on mortality of critically ill patients with severe pneumonia in intensive care unit: a preliminary study. BMC Infect Dis. 2021;21(1):1–7. doi: 10.1186/s12879-021-06288-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marik PE. Hydrocortisone, ascorbic acid and thiamine (HAT Therapy) for the treatment of sepsis. Focus on ascorbic acid. Nutrients. 2018 doi: 10.3390/nu10111762. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maritz GS. Ascorbic acid. Protection of lung tissue against damage. Subcell Biochem. 1996;25:265–291. doi: 10.1007/978-1-4613-0325-1_14. [DOI] [PubMed] [Google Scholar]
- Mohamed HA, Elbastawisy YM, Elsaed WM. Attenuation of lipopolysaccharide-induced lung inflammation by ascorbic acid in rats: Histopathological and ultrastructural study. SAGE Open Med. 2019;7:2050312119828260. doi: 10.1177/2050312119828260. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mohsenin V, Tremml P, Rothberg K, Souhrada M, Douglas J. Airway responsiveness and prostaglandin generation in scorbutic guinea pigs. Prostaglandins Leukot Essent Fatty Acids. 1988;33(3):149–155. [PubMed] [Google Scholar]
- Mongi S, Mahfoud M, Amel B, Kamel J. Protective effects of vitamin C against haematological and biochemical toxicity induced by deltamethrin in male Wistar rats. Ecotoxicol Environ Saf. 2011;74(6):1765–1769. doi: 10.1016/j.ecoenv.2011.04.003. [DOI] [PubMed] [Google Scholar]
- Munjal M, Khurana AS, Bajwa N, Munjal S, Dhawan N, et al. Study of vitamin C therapy in allergic rhinitis. Int J Otorhinolaryngol Head Neck Surg. 2020;6:1951–1955. doi: 10.18203/issn.2454-5929.ijohns20204616. [DOI] [Google Scholar]
- Nardini M, Finkelstein EI, Reddy S, Valacchi G, Traber M, Cross CE, van der Vliet A. Acrolein-induced cytotoxicity in cultured human bronchial epithelial cells. Modulation by alpha-tocopherol and ascorbic acid. Toxicology. 2002;170(3):173–185. doi: 10.1016/s0300-483x(01)00540-6. [DOI] [PubMed] [Google Scholar]
- Noh K, Lim H, Moon S-K, Kang JS, Lee WJ, Lee D, Hwang Y-I. Mega-dose Vitamin C modulates T cell functions in Balb/c mice only when administered during T cell activation. Immunol Lett. 2005;98(1):63–72. doi: 10.1016/j.imlet.2004.10.012. [DOI] [PubMed] [Google Scholar]
- Nyyssönen K, Poulsen H, Hayn M, Agerbo P, Porkkala-Sarataho E, Kaikkonen J, Salonen J. Effect of supplementation of smoking men with plain or slow release ascorbic acid on lipoprotein oxidation. Eur J Clin Nutr. 1997;51(3):154–163. doi: 10.1038/sj.ejcn.1600376. [DOI] [PubMed] [Google Scholar]
- World Health Organization (1999) Scurvy and its prevention and control in major emergencies. World Health Organization (No. WHO/NHD/99.11)
- Oudemans-van Straaten HM, Spoelstra-de Man AM, de Waard MC. Vitamin C revisited. Crit Care. 2014;18(4):460. doi: 10.1186/s13054-014-0460-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ozturk H, Terzi EH, Ozturk H, Kukner A. The effects of N-acetylcysteine and vitamin C on liver and pulmonary tissue damage in rats following bile duct ligation. Saudi Med J. 2008;29(11):1580–1584. [PubMed] [Google Scholar]
- Padayatty SJ, Katz A, Wang Y, Eck P, Kwon O, Lee JH, Levine M. Vitamin C as an antioxidant: evaluation of its role in disease prevention. J Am Coll Nutr. 2003;22(1):18–35. doi: 10.1080/07315724.2003.10719272. [DOI] [PubMed] [Google Scholar]
- Padayatty SJ, Sun AY, Chen Q, Espey MG, Drisko J, Levine M. Vitamin C: intravenous use by complementary and alternative medicine practitioners and adverse effects. PLoS ONE. 2010;5(7):e11414. doi: 10.1371/journal.pone.0011414. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Panda K, Chattopadhyay R, Chattopadhyay DJ, Chatterjee IB. Vitamin C prevents cigarette smoke-induced oxidative damage in vivo. Free Radic Biol Med. 2000;29(2):115–124. doi: 10.1016/s0891-5849(00)00297-5. [DOI] [PubMed] [Google Scholar]
- Park HJ, Byun MK, Kim HJ, Kim JY, Kim Y-I, Yoo K-H, Ahn CM. Dietary vitamin C intake protects against COPD: the Korea National Health and Nutrition Examination Survey in 2012. Int J Chronic Obstr Pulmonary Dis. 2016;11:2721. doi: 10.2147/COPD.S119448. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pavlović S, Fraser R. Effects of different levels of vitamin C intake on the vitamin C concentration in guinea pigs plasma and the effect of vitamin C intake on anaphylaxis. Medecine Interne. 1988;26(3):235–244. [PubMed] [Google Scholar]
- Perez-Cruz I, Carcamo JM, Golde DW. Vitamin C inhibits FAS-induced apoptosis in monocytes and U937 cells. Blood. 2003;102(1):336–343. doi: 10.1182/blood-2002-11-3559. [DOI] [PubMed] [Google Scholar]
- Pirabbasi E, Shahar S, Manaf ZA, Rajab NF, Manap RA. Efficacy of ascorbic acid (vitamin C) and/N-acetylcysteine (NAC) supplementation on nutritional and antioxidant status of male chronic obstructive pulmonary disease (COPD) patients. J Nutr Sci Vitaminol. 2016;62(1):54–61. doi: 10.3177/jnsv.62.54. [DOI] [PubMed] [Google Scholar]
- Rabe KF, Schmidt DT. Pharmacological treatment of asthma today. Eur Respir J Suppl. 2001;34:34s–40s. doi: 10.1183/09031936.01.00252501. [DOI] [PubMed] [Google Scholar]
- Riordan N, Riordan H, Casciari J. Clinical and experimental experiences with intravenous vitamin C. J Orthomol Med. 2000;15(4):201–213. [Google Scholar]
- Roa FJ, Peña E, Gatica M, Escobar-Acuña K, Saavedra P, Maldonado M, Muñoz-Montesino C. Therapeutic use of vitamin C in cancer: physiological considerations. Front Pharmacol. 2020;11:211. doi: 10.3389/fphar.2020.00211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saadat S, Aslani MR, Ghorani V, Keyhanmanesh R, Boskabady MH. The effects of Nigella sativa on respiratory, allergic and immunologic disorders, evidence from experimental and clinical studies, a comprehensive and updated review. Phytother Res. 2021;35(6):2968–2996. doi: 10.1002/ptr.7003. [DOI] [PubMed] [Google Scholar]
- Sabin AB. Vitamin C in relation to experimental poliomyelitis: With incidental observations on certain manifestations in macacus rhesus monkeys on a scorbutic diet. J Exp Med. 1939;69(4):507–516. doi: 10.1084/jem.69.4.507. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shakoor H, Feehan J, Al Dhaheri AS, Ali HI, Platat C, Ismail LC, Stojanovska L. Immune-boosting role of vitamins D, C, E, zinc, selenium and omega-3 fatty acids: could they help against COVID-19? Maturitas. 2021;143:1–9. doi: 10.1016/j.maturitas.2020.08.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shanklin D, O’dell T. Ascorbic acid and the lung. Nature. 1966;210(5043):1329–1331. doi: 10.1038/2101329a0. [DOI] [PubMed] [Google Scholar]
- Sipahi E, Ercan ZS. The mechanism of the relaxing effect of ascorbic acid in guinea pig isolated tracheal muscle. Gen Pharmacol. 1997;28(5):757–760. doi: 10.1016/S0306-3623(96)00277-7. [DOI] [PubMed] [Google Scholar]
- Slatore CG, Littman AJ, Au DH, Satia JA, White E. Long-term use of supplemental multivitamins, vitamin C, vitamin E, and folate does not reduce the risk of lung cancer. Am J Respir Crit Care Med. 2008;177(5):524–530. doi: 10.1164/rccm.200709-1398OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Song MH, Nair VS, Oh KI. Vitamin C enhances the expression of IL17 in a Jmjd2–dependent manner. BMB Rep. 2017;50(1):49. doi: 10.5483/BMBRep.2017.50.1.193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sourour DA, El Margoushy NM, El Nemr GM. Protective effects of vitamin C against benzene-induced lung injury in rats. Med J Cairo Univ. 2012;80(1):545–558. [Google Scholar]
- Syed AA, Knowlson S, Sculthorpe R, Farthing D, DeWilde C, Farthing CA, Gupta S. Phase I safety trial of intravenous ascorbic acid in patients with severe sepsis. J Transl Med. 2014;12(1):1–10. doi: 10.1186/1479-5876-12-32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Taylor EN, Stampfer MJ, Curhan GC. Dietary factors and the risk of incident kidney stones in men: new insights after 14 years of follow-up. J Am Soc Nephrol. 2004;15(12):3225–3232. doi: 10.1097/01.ASN.0000146012.44570.20. [DOI] [PubMed] [Google Scholar]
- Tecklenburg SL, Mickleborough TD, Fly AD, Bai Y, Stager JM. Ascorbic acid supplementation attenuates exercise-induced bronchoconstriction in patients with asthma. Respir Med. 2007;101(8):1770–1778. doi: 10.1016/j.rmed.2007.02.014. [DOI] [PubMed] [Google Scholar]
- Teng J, Pourmand A, Mazer-Amirshahi M. Vitamin C: The next step in sepsis management? J Crit Care. 2018;43:230–234. doi: 10.1016/j.jcrc.2017.09.031. [DOI] [PubMed] [Google Scholar]
- Tongtako W, Klaewsongkram J, Mickleborough TD, Suksom D. Effects of aerobic exercise and vitamin C supplementation on rhinitis symptoms in allergic rhinitis patients. Asian Pac J Allergy Immunol. 2018;36(4):222–231. doi: 10.12932/AP-040417-0066. [DOI] [PubMed] [Google Scholar]
- Ueta E, Suzuki E, Nanba E, Tadokoro Y, Otsuka Y, Kurata T. Regulation of cigarette smoke-induced cytochrome P4501A1 gene expression in osteogenic disorder Shionogi rat liver and in lung by large ascorbic acid dose. Biosci Biotechnol Biochem. 2001;65(11):2548–2551. doi: 10.1271/bbb.65.2548. [DOI] [PubMed] [Google Scholar]
- van Driel ML, Beller EM, Thielemans E, Deckx L, Price-Haywood E, Clark J, De Sutter AIM. Oral vitamin C supplements to prevent and treat acute upper respiratory tract infections. Cochrane Database Syst Rev. 2019 doi: 10.1002/14651858.CD013292. [DOI] [Google Scholar]
- Vissers MC, Das AB. Potential mechanisms of action for vitamin C in cancer: reviewing the evidence. Front Physiol. 2018;9:809. doi: 10.3389/fphys.2018.00809. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vissers MC, Carr AC, Pullar JM, Bozonet SM. The bioavailability of vitamin C from kiwifruit. Adv Food Nutr Res. 2013;68:125–147. doi: 10.1016/B978-0-12-394294-4.00007-9. [DOI] [PubMed] [Google Scholar]
- Vollbracht C, Raithel M, Krick B, Kraft K, Hagel AF. Intravenous vitamin C in the treatment of allergies: an interim subgroup analysis of a long-term observational study. J Int Med Res. 2018;46(9):3640–3655. doi: 10.1177/0300060518777044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wagner FM, Weber AT, Ploetze K, Schubert F, Pfeiffer S, Albrecht S, Schueler S. Do vitamins C and E attenuate the effects of reactive oxygen species during pulmonary reperfusion and thereby prevent injury? Ann Thorac Surg. 2002;74(3):811–818. doi: 10.1016/S0003-4975(02)03666-4. [DOI] [PubMed] [Google Scholar]
- Wintergerst ES, Maggini S, Hornig DH. Immune-enhancing role of vitamin C and zinc and effect on clinical conditions. Ann Nutr Metab. 2006;50(2):85–94. doi: 10.1159/000090495. [DOI] [PubMed] [Google Scholar]
- Woo KS, Yip TWC, Chook P, Koon KV, Leong HC, Feng XH, Kwok TCY. Vitamins B-12 and C supplementation improves arterial reactivity and structure in passive smokers: implication in prevention of smoking-related atherosclerosis. J Nutr Health Aging. 2021;25(2):248–254. doi: 10.1007/s12603-020-1529-7. [DOI] [PubMed] [Google Scholar]
- Wu H, Hsu W, Yeh (2007) Vitamin E and vitamin C supplementation in patients with chronic obstructive pulmonary disease. Int J Vit Nutr Res 77(4):272–279 [DOI] [PubMed]
- Yi C, Ji M, LiJuan C, YouLei Z, XuFen B. Effect of high-dose vitamin C on pulmonary infection in rats with bronchial asthma and its effect on pulmonary function. Chin J Nosocomiol. 2019;29(19):2905–2909. [Google Scholar]
- Yieh L, McEvoy CT, Hoffman SW, Caughey AB, MacDonald KD, Dukhovny D. Cost effectiveness of vitamin c supplementation for pregnant smokers to improve offspring lung function at birth and reduce childhood wheeze/asthma. J Perinatol. 2018;38(7):820–827. doi: 10.1038/s41372-018-0135-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yung S, Mayersohn M, Robinson JB. Ascorbic acid absorption in humans: a comparison among several dosage forms. J Pharm Sci. 1982;71(3):282–285. doi: 10.1002/jps.2600710304. [DOI] [PubMed] [Google Scholar]
- Zhitkovich A. Nuclear and Cytoplasmic Functions of Vitamin C. Chem Res Toxicol. 2020;33(10):2515–2526. doi: 10.1021/acs.chemrestox.0c00348. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zuskin E, Lewis AJ, Bouhuys A. Inhibition of histamine-induced airway constriction by ascorbic acid. J Allergy Clin Immunol. 1973;51(4):218–226. doi: 10.1016/0091-6749(73)90141-3. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Not applicable.