Abstract
Mucosal surfaces are distinctive sites exposed to environmental, dietary, and microbial antigens. Particularly in the gut, the host continuously actively adapts via complex interactions between the microbiota and dietary compounds and immune and other tissue cells. Regulatory T cells (Tregs) are critical for tuning the intestinal immune response to self- and non-self-antigens in the intestine. Its importance in intestinal homeostasis is illustrated by the onset of overt inflammation caused by deficiency in Treg generation, function, or stability in the gut. A substantial imbalance in Tregs has been observed in intestinal tissue during pathogenic conditions, when a tightly regulated and equilibrated system becomes dysregulated and leads to unimpeded and chronic immune responses. In this chapter, we compile and critically discuss the current knowledge on the key factors that promote Treg-mediated tolerance in the gut, such as those involved in intestinal Treg differentiation, specificity and suppressive function, and their immunophenotype during health and disease. We also discuss the current state of knowledge on Treg dysregulation in human intestine during pathological states such as inflammatory bowel disease (IBD), necrotizing enterocolitis (NEC), graft-versus-host disease (GVHD), and colorectal cancer (CRC), and how that knowledge is guiding development of Treg-targeted therapies to treat or prevent intestinal disorders.
Keywords: Treg, Immune tolerance, Inflammation, Microbiota, Immunotherapy
9.1. Introduction to Intestinal Tregs
Intestine is a unique site that is constantly exposed to wide array of luminal microbial and dietary antigens. Consequently, while safeguarding the host against the harmful pathogens, the intestinal mucosal immune system has to remain resilient and tolerant to the innocuous food antigens and commensal microbes to be able to endure the immunological insults without getting unduly provoked. The intestine employs many defense mechanisms to maintain its immunosuppressive tone, such as physical barrier (mucus and epithelial layers) or the production of secretory IgA. Tregs are another instrumental players in keeping a check on the activation of local T cells in the intestine (Powrie and Mason 1990; Powrie et al. 1993; Gambineri et al. 2003; Josefowicz et al. 2012).
To better understand the roles of Tregs in the gut, it is important to place it in the context of the intestinal tissue architecture. Histologically, along its entire length, intestine is made up of four layers: mucosa (further consists of the epithelial layer, lamina propria, and muscularis mucosae), submucosa, muscularis propria, and serosa. Lamina propria (LP) compartment consists of a variety of immune cells such as plasma cells, macrophages, dendritic cells, mast cells, and B and T lymphocytes, including Tregs, the subject of this overview. Small intestine (SI) comprises of duodenum, jejunum, and ileum and is characterized by the highly absorptive surface created by finger-like projections called the villi, further enlarged by cellular microvilli located at the apical, lumenfacing membrane of the enterocytes. The small intestinal epithelium consists of invagination forming the crypts of Lieberkuhn. At the base of the crypts reside the intestinal stem cells, which give rise to transient proliferative cells that differentiate and mature into Paneth cells, goblet cells, enteroendocrine cells, and tuft cells as they travel up through the transition zone, to ultimately shed into the lumen at the apex of the villi. Peyer’s patches are organized lymphoid structures unique to the small intestine. They harbor immune cells and are separated from the lumen by a follicle-associated epithelium which contains specialized cells called microfold cells (M cells) capable of sampling the antigens directly from the lumen and delivering it to antigen-presenting cells juxtapositioned at their basolateral side (Fig. 9.1a). The large intestine consists of cecum, colon, and rectum and harbors the bulk of the commensals. Its major function is to absorb water and vitamins while converting undigested food into feces. The most notable histological differences are lack of villi, Paneth cells (except for their metaplastic occurrence in chronic inflammatory bowel diseases), and Peyer’s patches (Fig. 9.1b).
Fig. 9.1.
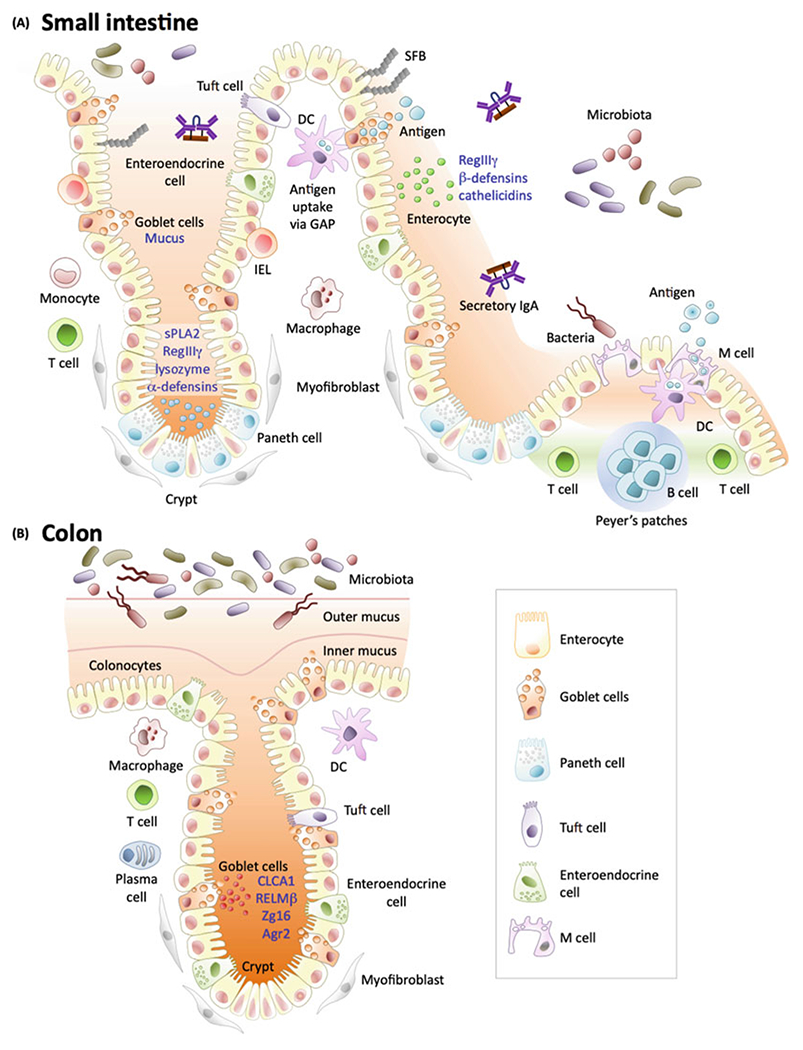
Features of the small intestinal and colonic mucosa. (a) Small intestinal epithelium. Enterocyte-derived antimicrobial peptides: RegIIIγ, β-defensins, cathelicidin. Paneth cell-derived antimicrobial peptides [lysozyme, α-defensins, secretory phospholipase 2 (sPLA2)]. M cells and goblet cells facilitate luminal antigen transfer to the underlying dendritic cells. (b) Colonic epithelium. Goblet cells produce two-layered mucus layer through Muc2 mucin secretion along with other proteins contributing to the colonic epithelial barrier such as anterior gradient protein 2 homolog (Agr2), small lectin-like protein ZG16 (zymogen granulae protein 16), secreted goblet cell-derived protein Clca1 (chloride channel regulator, calciumactivated-1), or Other abbreviations: DC dendritic cell, IEL intraepithelial lymphocyte. (Figure adapted from Allaire et al. with permission) (Allaire et al. 2018)
Most of the information available regarding the development of intestinal Tregs is based on research on mice. In other organs, Tregs comprise approximately 10% of total CD4+ T-cell population However, in the gut, they represent a much higher fraction with >30% of CD4+ T cells in the colonic lamina propria (cLP) and about 20% in the small intestinal lamina propria (siLP) (Weiss et al. 2012; Round and Mazmanian 2010; Atarashi et al. 2011; Geuking et al. 2011; Stefka et al. 2014). The presence of higher proportion of Tregs in the colon, the site of the highest microbial biomass, intuitively suggests that development and maturation of intestinal Tregs is strongly influenced by microbial antigens. Indeed, germ-free (GF) mice and long-term broad spectrum antibiotic-treated specific pathogen-free (SPF) mice have a dramatic reduction in their colonic Tregs compared to their respective controls (Atarashi et al. 2011; Geuking et al. 2011; Weiss et al. 2012). However, the comparable numbers of Tregs present in the siLP of GF and SPF mice indicate that in this intestinal segment, bacterial antigens contribute to the Treg population to a lesser degree (Kim et al. 2016). The same study showed that once the weaned GF mice were switched to antigen-free diet, siLP (but not cLP) Tregs declined, thus revealing a significant role of dietary antigens in regulation of small intestinal Treg population (Kim et al. 2016).
Human CD4+ Tregs are characterized by expression of the transcription factor forkhead box P3 protein (FOXP3, scurfin), high surface expression of CD25, and low or no expression of CD127. Tregs express high levels of CD25 (the IL-2 receptor α-chain) due to their high dependence on interleukin 2 (IL-2) for their development (Zheng et al. 2007; Davidson et al. 2007) and maintenance and peripheral homeostasis (D’Cruz and Klein 2005; Fontenot et al. 2005). The groundbreaking study led by Sagakuchi demonstrated that depletion of CD25+ subpopulation of CD4+ T cells resulted in the manifestation of multiorgan autoimmunity, including intestinal inflammation (Sakaguchi et al. 1995). A few years later, Fiona Powrie and colleagues successfully developed the CD4+CD45RBhi T-cell transfer model of IBD. The consequent discovery that the symptoms of this model could be suppressed via the co-transfer of CD4+CD25+ T cells, was a huge leap in the field (Mottet et al. 2003). Later, FoxP3 was discovered to be a vital transcription factor for the induction and maintenance of CD4+CD25+ Tregs. In “scurfy” mice, an X-linked recessive mutation led to scaly skin, lymphoproliferation, hypergammaglobulinemia, lymphadenomegaly, anemia, runting, and early death (Godfrey et al. 1991). In 2001, seminal genetic studies in the “scurfy” mice led to the discovery of Foxp3 gene (Brunkow et al. 2001). Other contemporary studies revealed that human patients with IPEX syndrome (immunodysregulation polyendocrinopathy enteropathy X-linked syndrome) harbored mutations in Foxp3 gene and its 3′UTR region (Bennett et al. 2001; Wildin et al. 2001). Conspicuously, the FoxP3-deficient mice and patients with IPEX syndrome present with gastrointestinal manifestations in the form of autoimmune enteropathy (Powrie et al. 1993; Gambineri et al. 2003), thus highlighting the role of Tregs in the maintenance of intestinal homeostasis.
While CD4+ Tregs are most widely studied in health and disease, CD8+ Tregs were identified in 1970 by Gershon and Kondo, long before the characterization of CD4+ Tregs (Gershon and Kondo 1970). CD8+ Tregs are a heterogenous population that are not very well characterized and their role in the maintenance of intestinal homeostasis is poorly understood. In this chapter, we will primarily focus on CD4+ Tregs, while the reader is directed to published review articles on CD8+ Tregs and original articles therein (Flippe et al. 2019; Yu et al. 2018; Tsai et al. 2011; Liu et al. 2014; Zhang et al. 2019).
9.2. Intestinal Tregs Subsets
Perhaps as an adaptation to the highly specialized and dynamic intestinal environment, Treg population is characterized by a significant heterogeneity. The current knowledge about the different Treg subsets is mostly based on mice as just like any other biomedical research, studying Treg biology in humans is very challenging. While Foxp3 is considered to be the signature marker associated with murine Tregs, it is not so straight-forward in human Tregs. Moreover, unlike in mice, where a FoxP3 reporter mouse lines are available, it is a technical challenge to isolate human Tregs based on FoxP3 which is expressed intracellularly, which precludes isolation of live cells for further functional studies. Even though the presence of Foxp3 is indispensable for the development and function of human Tregs, it is not necessarily synonymous with immunosuppressive functions. There have been many instances when expression of FoxP3 was shown in activated human T cells that lacked the regulatory activity (Allan et al. 2007; Morgan et al. 2005; Wang et al. 2007; Gavin et al. 2006; Tran et al. 2007). Human Tregs are also known to express cytotoxic T lymphocyte-associated antigen 4 (CTLA4) and glucocorticoid-induced TNF receptor (GITR), but just like CD25, they are not very specific to Tregs and can be expressed by effector T cells (Teff) (Read et al. 2000; Takahashi et al. 2000; McHugh et al. 2002). The CD127 and CD27 markers have been proposed to increase the specificity of Treg identification. CD127 expression is much lower in CD25+Foxp3+ Tregs than in Teff (Seddiki et al. 2006), and this marker is useful to identify Tregs in non-inflammatory conditions. Therefore, most of the human studies are conducted on CD4+CD25brightCD127lo cells isolated from peripheral blood.
In the murine gut, based on transcription factor and surface molecule expression, CD4+ Tregs can be subdivided into the following subsets: GATA3+Helios+(Nrp1+), retinoic acid receptor-related orphan receptor γt (RORγt)-expressing RORγt+Helios− cells, and RORγt− Nrp1−(Helios−) Tregs.
9.2.1. Thymic vs. Peripheral Tregs
Based on their origin, Tregs are classified into thymic Tregs (tTregs) which develop in the thymus and into peripheral Treg which are induced from naïve T cells in the periphery (pTreg). Murine tTreg differentiation into Foxp3+ Treg is a two-step process: First, future Tregs recognize MHCII-presented self-antigen on thymic epithelial cells via a high-affinity T-cell receptor (TCR) (Stritesky et al. 2012; Xing and Hogquist 2012). In the second step, γc cytokines (mainly IL2) and CD28 co-stimulation lead to an induction of FoxP3 expression (Xing and Hogquist 2012; Fontenot et al. 2005; Salomon et al. 2000; Hori et al. 2003; Tai et al. 2005). In contrast, future pTregs exit the thymus as naive CD4+ T cells and differentiate into Foxp3+ Treg cells in the periphery, upon subsequent recognition of their cognate non self-antigen (Shevach and Thornton 2014; Chen et al. 2003; Yadav et al. 2013) and in the presence of a permissive cytokine microenvironment (Zheng et al. 2002, 2004). The role of cytokines and other factors in the development of Tregs is discussed in Sect. 9.4 of this chapter. One of the big challenges that remains in the Treg field is the paucity of markers (especially cell surface proteins) to distinguish the pTregs from the tTregs. So far, Helios and Neuropilin 1 (Nrp1) have shown to be the two most promising markers expressed by tTregs (Thornton et al. 2010; Yadav et al. 2012; Weiss et al. 2012). However, both of these markers are not exclusive to tTregs. The finding that inflammatory milieu upregulates the expression of both Helios and Nrp1, further complicates their utilization as markers (Gottschalk et al. 2012). While Helios is present in 70% of Tregs in peripheral lymphoid tissues (Thornton et al. 2010), the presence of Nrp1 in humans is still questionable. In humans, both of these markers have not proven to be very reliable. A fraction of human tTregs does not express Helios (Himmel et al. 2013), and Nrp1 expression can be induced in activated humans Teff (Milpied et al. 2009). Intestinal Tregs are thought to have a dual origin as they could either arise in thymus or develop in the periphery, in response to local microbiota and food antigens. However, a vast majority of Tregs present in the small intestine and colon are Helios−Nrp1− (Ohnmacht et al. 2015). In fact, it is the frequency of FoxP3+Helios−Nrp1− Tregs which is reduced in the colon of GF and antibiotic-treated SPF mice (Atarashi et al. 2011; Geuking et al. 2011; Weiss et al. 2012).
Development of FoxP3+ pTregs and tTregs is also regulated by Foxp3 intronic enhancers, termed conserved noncoding sequence 1, 2, and 3(CNS1-3). While CNS3 is instrumental in the initial induction of FoxP3 in tTregs, CNS1 harbors a TGFβ-NFAT response element and a binding site for retinoic acid receptor (RAR) and retinoic X receptor (RXR) heterodimer, which is activated by retinoic acid (Zheng et al. 2010; Xu et al. 2010; Tone et al. 2008). CNS1-deficient mice lack pTregs, while their tTregs differentiation remains unaffected (Zheng et al. 2010). Another study reported that CNS1 deficiency in colonic TCR-transgenic T cells only affected initial, but not late, pTreg cell selection (Nutsch et al. 2016). Nonetheless, CNS1-deficient mice display a dysbiotic microbiota with exacerbated Th2 type pathologies in the lung and colon (Josefowicz et al. 2012; Campbell et al. 2018). pTregs have also been shown to suppress Th1-type responses to food antigens in the gut (Kim et al. 2016). Cumulatively, these studies highlight the key role of pTregs in maintaining tolerance toward luminal antigens.
While pTregs remain the predominating Treg population in colon, it has been suggested that tTregs and pTregs supplement the function of each other, partly by expanding their TCR diversity (Haribhai et al. 2011). Moreover, in the absence of pTregs, tTregs can migrate to intestinal LP and expand locally in response to the cross-reactive microbial antigens (Cebula et al. 2013). Using single-cell and high-throughput sequencing on TCRmini mice with “limited but diversified TCR,” this study demonstrated that TCR repertoires of colonic tTregs and pTregs have significant overlap (Cebula et al. 2013). A study with mice with MHCII expression restricted to the cortical thymic epithelial cells and thus cannot provide peripheral MHCII-TCR interaction necessary for the induction of pTregs demonstrated that majority of intestinal Tregs were of thymic origin (Korn et al. 2014). The study revealed that there exists a unique “Treg niche” in the intestine, where tTregs migrate during early life and are maintained without the need for TCR stimulation. However, broad spectrum antibiotic treatment abrogated LP tTregs, suggesting that continuous microbial stimuli might be vital for the maintenance of Tregs in the intestinal compartment (Korn et al. 2014). In summary, both pTregs and tTregs are likely to contribute to the maintenance of intestinal homeostasis, although it is challenging to determine which of the two populations plays a major role.
9.2.2. GATA3+Helios+(Nrp1+) Tregs
GATA-binding protein 3 (GATA3), the canonical Th2-specific transcription factor, is expressed by up to 65% of intestinal Tregs and can be induced in CD4+Foxp3+ Tregs by TCR engagement (Wohlfert et al. 2011). In the same report, human Tregs (CD4+CD25brightCD127lo) isolated from peripheral blood of healthy donors were also able to dramatically upregulate GATA3 expression upon TCR engagement (Wohlfert et al. 2011). However, this subset has not yet been demonstrated in the human gut. GATA3 has been shown to be important for immunosuppressive function and accumulation of Tregs to the extent that GATA3-deficient Tregs were unable to suppress naïve T-cell transfer-induced colitis in mice (Wang et al. 2011). In the mouse gut, GATA3+Helios+ tTregs have been also found to express IL33 receptor ST2 and amphiregulin (Schiering et al. 2014). IL33 is produced by intestinal epithelial cells under inflammatory conditions. In fact, high levels of IL33 have been reported in inflamed lesions of inflammatory bowel disease patients (Kobori et al. 2010; Pastorelli et al. 2010; Seidelin et al. 2010). In the same line of thought, in steady state, ST2+ FoxP3+Tregs showed increased production of IL10 and TGFβ and displayed preferential accumulation in the intestinal LP due to high expression of gut-homing receptors CCR9 and α4β7 (Siede et al. 2016). Furthermore, ST2+ Tregs display an activated phenotype and express high levels of OX40 (Schiering et al. 2014), a secondary co-stimulatory immune checkpoint molecule which is also important for the accumulation of Tregs in colon and for Treg-mediated suppression of naïve T-cell transfer-induced colitis (Griseri et al. 2010). It is thus plausible that GATA3+ tTregs might be home to the sites of damage in the gut to curb excessive inflammation and mediate tissue repair. However, this hypothesis still needs to be verified.
9.2.3. RORγt+Helios− pTregs
Another Treg subset that is abundantly located in the gut expresses the Th17 master transcription factor, retinoic acid receptor-related orphan receptor γt (RORγt). The absence of Helios (Sefik et al. 2015; Ohnmacht et al. 2015) and Nrp1 (Yang et al. 2016) on most of the RORγt+ Tregs suggests their peripheral origin. Owing to their similar expression of RORγt, the RORγt+Foxp3+ Tregs were once considered to be precursors for unstable Tregs that could differentiate into Th17 cells (Zhou et al. 2008; Lochner et al. 2008). However, it was later revealed that, in steady state, RORγt+Helios− FoxP3+ Tregs comprise about 65% and –35% of total Treg population in the colon and small intestine, respectively (Sefik et al. 2015; Ohnmacht et al. 2015; Yang et al. 2016). Comparable frequencies of Rorγt+ Tregs were detected by staining cells from healthy or inflamed (Crohn’s) colon biopsies (Sefik et al. 2015) and among healthy human PBMCs (Duhen et al. 2012), confirming the presence of this subset in humans. Interestingly, there were higher number of circulating IL-17-producing RORγt+Foxp3+CD4+ lymphocytes in IBD patients, and the suppressive ability of these Tregs were reduced by approximately 60% in patients with IBD compared with healthy controls (Ueno et al. 2013).
The plasticity between Th17 cells and RORγt+ Tregs is not very well understood. Development of Th17 cells is dependent on IL6, IL1β, and IL23. However, while IL6 and downstream STAT3 signaling are important for the development of intestinal RORγt+ Tregs, IL1β and IL23 have not been shown to play a role under homeostatic conditions (Sefik et al. 2015; Xu et al. 2010; Yang et al. 2016). Making use of the transcriptomic and epigenetic profiling, Yang et al. (2016) revealed that RORγt+ Foxp3+T cells display higher similarity to Tregs than to Th17 cells and that they represent a lineage-stable population with potent suppressive activity. Using fixed TCRβ transgenic mice, it was shown that TCR repertoire of RORγt+ Foxp3+ Tregs is largely unique compared with other colonic T-cell subsets (Solomon and Hsieh 2016). However, they also observed a subset of Foxp3+RORγt+ TCRs that are shared with Th17 cells. The same study suggested that the development of mucosal RORγt+ Foxp3+T cells is dependent on CX3CR1+ antigen-presenting cells and can be derived from naïve CD4+ T cells in the periphery via a RORγt− Treg intermediate before co-expressing RORγt. Furthermore, RORγt+ Foxp3+Treg subset is readily lost in GF mice or upon antibiotic treatment, signifying the indispensable role of microbiota in their maintenance (Ohnmacht et al. 2015; Sefik et al. 2015). Interestingly, the differentiation of RORγt+ Tregs is also dependent upon constant antigen exposure, as mice deficient in MHCII have decreased frequency of this subset (Ohnmacht et al. 2015). The role of microbiota in the development of RORγt+ Tregs would be further discussed later in this chapter. Additionally, c-Maf, a transcription factor which has already been known to be a positive regulator of Th17 cells, has also been found to be highly expressed by RORγt+ Foxp3+Tregs (Xu et al. 2018). Not only has c-Maf been found to be required for the development of RORγt+ Tregs, but is also important for their suppressive ability, as ablation of c-Maf in Tregs leads to spontaneous inflammation in mice that is not observed in mice with RORγt-deficient Tregs (Sefik et al. 2015; Xu et al. 2018; Yang et al. 2016).
9.2.4. RORγt− Nrp1− (Helios−) pTregs
Another subset of intestinal Tregs are the CD4+Foxp3+RORγt− T cells. They are believed to be of peripheral origin due to absence of Helios or Nrp1. These cells represent about 50% of SI LP Tregs and 15% of colonic Tregs (Kim et al. 2016). Since these Tregs are more frequently present in SI, they are induced by dietary antigens, so much, so that they are not affected by the absence of microbiota but disappear in mice fed “antigen-free” diet (Kim et al. 2016). In fact, the absence of this Treg subset leads to increased susceptibility to food allergy in mice (Kim et al. 2016).
9.2.5. Effector Tregs and IL10+ Tregs
In humans, based on the expression levels of Foxp3 and protein tyrosine phosphatase isoform A (CD45RA), human peripheral blood Foxp3 +CD4+ T cells can be classified into Foxp3loCD45RA+ naive Tregs which after antigenic stimulation differentiate into Foxp3hiCD45RA− Tregs that represent activated, antigen primed, terminally differentiated and highly suppressive effector Tregs or eTregs. In contrast, Foxp3loCD45RA− cells do not possess suppressive activity and can even secrete pro-inflammatory cytokines (Miyara et al. 2009). To understand these cells better, some studies have been carried out with mouse CD44hiCD62LlowKLRG1+FOXP3+ cells, thought to represent the murine counterpart of the human eTregs (Liston and Gray 2014). The CD44hi effector Tregs are most abundant in the colonic LP and depend on constant TCR engagement and subsequent IRF4 expression for their maintenance (Levine et al. 2014). On the other hand, TCR and IRF4 are dispensable for the development of CD44loCD62Lhi naive Tregs as tamoxifen-induced Treg-specific deletion of TCRα or IRF4 does not adversely affect their numbers (Levine et al. 2014). Transcription factors, IRF4 and Blimp-1, jointly regulate the differentiation, function, and homeostasis of eTregs (Levine et al. 2014; Cretney et al. 2011).
Another important Treg subset described in humans is the FOXP3− type 1 (Tr1) cells that develop exclusively in periphery and secrete copious amounts of IL10 and TGFβ1, intermediate levels of IFNγ and no IL4 (Levings and Roncarolo 2005; Roncarolo et al. 2018). In fact, IL10-producing Tr1 cells were originally identified and isolated from patients who have severe combined immunodeficiency (SCID) and had undergone successful HLA-mismatched bone marrow transplantation. In these studies, antigen-specific Tr1 cells were generated in vitro from both mice and human naïve CD4+ T cells (Bacchetta et al. 1994; Groux et al. 1997). The in vitro generated murine Tr1 cells were able to prevent establishment of colitis in SCID mice after CD4+CD45RBhi adoptive T-cell transfer in IL10- and TGFβ-mediated fashion (Groux et al. 1997). Hence, Tr1 cells appear to significantly contribute to maintaining the intestinal tolerance.
9.3. Antigen Specificity of Intestinal Tregs
One of major challenges in the field has been to understand the antigen specificity of intestinal Tregs. For example, are pTregs generated against self-antigens or antigens derived from commensal bacteria? Although some earlier studies have shown that commensal bacteria are not necessary for colonic Treg generation (Min et al. 2007; Round and Mazmanian 2010), generation of Tregs in response to foreign antigens has been established with TCR transgenic models of oral tolerance (Curotto de Lafaille et al. 2008; Sun et al. 2007). Earlier in this chapter, we have described studies with germ-free and antibiotic-treated mice that clearly showed the importance of the gut commensals in driving colonic Treg numbers. Some human gut commensals such as Clostridium clusters IV and XIVa (Atarashi et al. 2011) and Bacteroides fragilis (Round and Mazmanian 2010) have been found to increase the frequency or function of colonic Tregs via protease-resistant capsular polysaccharide (PSA) and a TLR2-dependent mechanism (Round et al. 2011). PSA also induced expression of Foxp3 along with CD39 in naïve human CD4 T cells in vitro while promoting IL-10 secretion (Telesford et al. 2015).
To address TCR specificity, labs developed TCR transgenic mouse lines that respond to antigens derived from commensal bacteria, e.g., flagellin (Cong et al. 2009). However, this approach does not allow for the comparison of the normal in vivo frequency of those TCRs in the Tregs with the effector T cells. The study by Lathrop et al. (2011) analyzed the colonic TCR repertoire and concluded that TCR usage in the colon is very different from that in any other organ and that there was very little overlap in TCRs in Tregs and the effector/memory T cells. This study confirmed that the colonic Treg population is strongly shaped and fine-tuned by the local antigenic milieu and that the effector T cells and Tregs are not necessarily activated by the same antigens. Furthermore, it could be safely inferred that colonic Tregs recognize and suppress the immune response evoked against the antigens from commensal bacteria. In fact, “Limited mice,” which express only a limited TCR repertoire, display shrunken Helios− Tregs and eventually develop loss of tolerance to the gut microbiota and spontaneous development of Th17-driven colitis (Nishio et al. 2015).
9.4. Key Factors Required for the Development and Maintenance of Tregs in the Gut
The intestinal Tregs are very unique and highly specialized due to their role in preventing inflammatory responses to commensal flora, diet, and other innocuous antigens. There are many different mechanisms that are engaged in the induction and maintenance of Tregs in the intestine. While most of these intricate pathways have been elucidated in mice, some attempts have been made to validate them in humans.
9.4.1. Host Factors: Cytokines
As already described in the Introduction to Intestinal Tregs, development of Treg precursors in the thymus requires not only the strong TCR stimulation but also co-stimulation through CD28 and a presence of common-γ-chain cytokines such as IL2 and IL15 (Chinen et al. 2016; Setoguchi et al. 2005). The transcription factor STAT5, which is activated downstream of the IL2 receptor α (CD25), binds to CNS2 in the Foxp3 gene and thus stimulates its expression. In fact, many mouse models mimicking IL2 deficiency have demonstrated IL2 to be a key cytokine for the development and the maintenance of tTregs in the periphery (Papiernik et al. 1998; Almeida et al. 2002; Malek et al. 2002; Setoguchi et al. 2005). Accordingly, CNS2-deficient Tregs are sensitive to low levels of IL2 and eventually lose FoxP3 expression in response to strong TCR activation or pro-inflammatory cytokines, a typical intestinal milieu (Feng et al. 2014; Li et al. 2014). On the other hand, Treg pool can be increased following treatment with agonistic complexes of IL2 or antibody to IL2 (Oldenhove et al. 2009; Wohlfert et al. 2011).
The role of IL2 is central to the development of Tregs as IL2-deficient mice have been known to develop colitis (Sadlack et al. 1993). In general, IL2 potently stimulates T-cell growth and population expansion. However, the function of IL2 signaling for Treg differentiation and maintenance of immune system homeostasis appears to overcome its effects on the effector T cells, since mice deficient in IL-2 or components of the IL-2 receptor, IL-2Ra (CD25) or IL-2Rb (CD122), suffer from an aggressive lymphoproliferative, autoimmune syndrome. Similarly, patients with IL-2Ra deficiency develop immunodeficiency and early onset IBD live-disease (Sharfe et al. 1997). It has been reported that while CD25 activation by IL2 is required for FoxP3+ pTreg cell differentiation, survival, and expansion, it remains dispensable for the induction and differentiation of FoxP3+ tTreg cells (D’Cruz and Klein 2005; Fontenot et al. 2005). However, a later report showed that IL2 seems to be critical for the maintenance of intestinal GATA3+ tTregs, as this subset is severely reduced in the small intestine of IL2−/− mice (Wohlfert et al. 2011). Furthermore, blocking IL2 in a mouse model of oral tolerance to ovalbumin impaired the conversion of adoptively transferred naive OT-II CD4+ T cells into pTreg cells in the mesenteric lymph nodes (MLNs) and Peyer’s Patches (PP) (Edwards et al. 2016). However, the role of IL2 in the induction of RORγt+ and RORγt− pTregs has not been evaluated.
TGFβ gives an initial survival advantage to the Treg precursors in the thymus, though it fails to promote Foxp3 expression and is therefore not important for the induction of tTregs (Li et al. 2006; Marie et al. 2005, 2006; Ouyang et al. 2010; Tone et al. 2008; Konkel and Chen 2011). On the other hand, TGFβ has an unambiguously essential role in the differentiation of pTregs in the intestine, where it is present in copious amounts (Chen et al. 2003; Konkel and Chen 2011). TGFβ1-deficient mice develop lethal multi-organ lymphoproliferative disease, which mainly affects the gut. Notably, this pathology has considerable similarity with the one observed in the Foxp3-deficient mice (Kulkarni et al. 1993; Shull et al. 1992). TGFβ1-activated Smad3 binds to Foxp3 intronic enhancer CNS1 (Josefowicz et al. 2012; Tone et al. 2008; Xu et al. 2010). However, mice with an ablation of the Smad3-binding site in CNS1 did not result in reduction in the frequency of intestinal Tregs (Schlenner et al. 2012). Similarly, the frequency of colonic Tregs is not compromised in mice with the deletion of the cytokine receptor TGFβR1 specifically in Tregs (Konkel and Chen 2011). However, specific Treg subsets were not investigated in that study, and it is possible that while pTregs contracted in the absence of TGFβ signaling, the tTregs expanded to compensate. On the other hand, colitis in mice with TGF-βRII-deficient DCs is accompanied by reduced frequencies of mucosal and MLN Foxp3+ Tregs (Jamwal et al. 2020; Ramalingam et al. 2012).
TGFβ1 is secreted as an inactive precursor molecule combined with latency-associated peptide (LAP) and needs to be modified by integrins, such as αvβ8 and αvβ6 (Travis et al. 2007). Importantly, the expression of integrin β8 is highly upregulated in intestinal CD103+ DCs (Paidassi et al. 2011), and consequently CD103+ DCs deficient in αvβ8 have a compromised capacity for Treg induction in the MLNs (Worthington et al. 2011). Additionally, LAP could be cleaved by pro-protein convertase enzymes, such as Furin. Notably, Furin-deficient Tregs exhibit defective regulatory function in the naïve T-cell transfer-induced colitis (Pesu et al. 2008). Furthermore, in a mouse model of oral tolerance, a complex of GARP (glycoprotein A repetitions predominant) and latent TGFβ1 on Tregs has been found to be a major source of TGFβ1 needed for the induction of pTreg cells in the MLNs and PPs (Edwards et al. 2016). Overall, IL2 and TGFβ1 seem to be central in Treg homeostasis, so much, so that naïve T cells after in vitro stimulation in the presence of TGFβ and IL2 are induced to differentiate into inducible Tregs (iTregs) (Zheng et al. 2002, 2004).
Although the plasticity of Tregs under inflammatory pressure is well recognized, there are lingering controversies, mainly stemming from varying definitions, protocols, and starting cell populations. One aspect of Treg plasticity is related to reversible FoxP3 expression. The in vitro generated iTregs are generally recognized to have unstable expression of Foxp3 via a mechanism involving histone demethylation at the CNS2 element, resulting in quick reversion to Foxp3− effector T cells (Kanamori et al. 2016). Another aspect of Treg plasticity is the described gain in IL17 production. IL6 is thought to contribute to the latter, although different Treg lineages seem to respond differently. nTregs can be induced to become Th17-like when exposed to IL6 in a TGFβ-dependent manner, but iTregs were resistant to this conversion under the same protocol (Zheng et al. 2008). Xu et al. also showed that CD4+CD25+FoxP3+ mouse splenocytes (constituting primarily nTregs) stimulated with IL6 turned into Th17 cells, even without the presence of exogenous TGFβ (Xu et al. 2007). Similarly, nTregs sorted from mouse thymus or spleens were converted to Th17 cells with CD3/IL6 stimulation, a conversion inhibited by atRA (Zhou et al. 2010). Human peripheral blood CD4+CD25hiCD127loFoxp3+ Tregs stimulated with high-salt medium lose their immunosuppressive function via gaining IFNγ expression, though only a very minor increase of IL17 expression was observed in this cell population. In a more recent mouse study, Luo et al. showed that high-salt diet in vivo and elevated NaCl concentration in vitro did not negatively affected pTregs or iTregs, respectively, but impaired the immunosuppressive function of tTregs and reduced FoxP3 expression (Luo et al. 2019). Thus, none of the Treg population should be considered as entirely stable or terminally differentiated. Their function and phenotype can be affected differently by various stimuli dependent on their lineage, tissue, and/or pathophysiological milieu.
Recently, IL33 has also been deemed instrumental in the induction of Tregs in the gut. IL33 is passively released by stromal or epithelial cells during cell necrosis or tissue damage, defining its role as an alarmin that alerts the immune system during trauma (Pichery et al. 2012). As mentioned earlier, intestinal GATA3+ tTregs, but not RORγt+ pTregs, have high expression of the IL33 receptor ST2. Likewise, in vivo administration of IL33 increases the number of both splenic Tregs and colonic RORγt− Tregs, but not RORγt+ pTregs (Schiering et al. 2014; Sefik et al. 2015). Overall, IL33 seems to positively regulate tTregs but not pTregs in vivo. However, IL33 in combination with TGFβ1 has also been successfully able to induce the differentiation of iTregs (Schiering et al. 2014).
9.4.2. Host Factors: TNFRSF—NF-κB Axis
Survival at a barrier site such as intestine requires adaptation to the changing environmental milieu. Due to continuous exposure to microorganisms, Treg cells display an activated phenotype characterized by the core tumor necrosis factor receptor superfamily (TNFRSF) signature including expression of GITR, OX40, and TNFR2 (Miragaia et al. 2019; Vasanthakumar et al. 2017). The stimulation of TNFRSF members further activates the NF-κB signaling pathway and provides vital survival signals for Tregs (Miragaia et al. 2019; Salomon et al. 2018; Vasanthakumar et al. 2017). OX40 is important for survival, accumulation, and function of Treg cells in the colon (Griseri et al. 2010). Activation of RelA, a NFκB transcription factor, is associated with the adaptation of both tTregs and pTregs in the colon (Vasanthakumar et al. 2017). Overall, the TNFRSF—NF-κB axis may be a central signaling component in Treg maintenance in the gut.
9.4.3. Environmental Factors: Commensal Microbiota
The intestine, especially the colon, harbors abundant bacteria reaching 1012 cells per gram of intestinal content. Bacteria remain in a complex relationship with the host immune system, and among their many functions, they profoundly affect mucosal T cells, including their differentiation into different subsets of effector or regulatory cells (Larmonier et al. 2015). As mentioned in the previous sections, the pTregs are induced, maintained, and “fine-tuned” directly by the commensal bacteria or by the metabolites produced by bacteria. While both RORγt+ and RORγt− pTregs are affected by general presence or absence of microbiota, apparently, only the RORγt+ subset seems to be affected by qualitative changes in the colonic microbiome (Kim et al. 2016; Ohnmacht et al. 2015; Sefik et al. 2015; Ye et al. 2017). Some bacterial genera including Clostridium, Bacteroides, Bifidobacterium, Lactobacillus, and Helicobacter have been shown to promote pTregs in colon (Geuking et al. 2011; Atarashi et al. 2011, 2013; Chai et al. 2017; Kullberg et al. 2002; Sefik et al. 2015). Most of clostridia are gut commensals, with a few exceptions, such as Clostridium perfringens, Clostridium difficile, and Clostridium tetani, which are pathogenic and toxigenic. Oral administration of GF mice with 46 strains of commensal Clostridia showed strong promotion of differentiation, proliferation, and recruitment of Helios−RORγt+ Tregs in the colon (Atarashi et al. 2011). Similar experiments executed with 17 strains of Clostridia isolated from a healthy human adult also demonstrated a strong capacity to induce RORγt+Helios− pTreg cells rather than GATA3+ tTregs in the colon of mice and rats (Atarashi et al. 2013). Clostridia-induced increase in frequency of colonic Tregs is also accompanied by an increase in the expression of IL10, CTLA4, and ICOS on pTregs (Atarashi et al. 2011, 2013) and increased intestinal production of sIgA which coats commensals and reduces T-cell activation by microbial antigens, such as flagellin (Cong et al. 2009). Faecalibacterium prausnitzii (cluster IV) is another member of the class of Clostridia and one of the most abundant residents in healthy humans (Godefroy et al. 2018; Sarrabayrouse et al. 2014). F. prausnitzii promotes the accumulation of Tr1 cells (IL10-producing CD4+CD8αα+ T cells) in the colon and the blood of healthy humans (Sarrabayrouse et al. 2014). Interestingly, relative abundance of F. prausnitzii is decreased in IBD patients (Machiels et al. 2014; Sokol et al. 2008, 2009). F. prausnitzii is capable of inducing IL10 secretion by healthy human DCs (Alameddine et al. 2019) which may also assist with reducing the overall inflammatory tone in the gut.
Apart from the Clostridia, another resident member of the human gut microbiome capable of impacting Treg homeostasis is Bacteroides fragilis. B. fragilis enhances the accumulation of IL10-producing Foxp3+ Tregs in the colon (Round et al. 2011). Monoassociations of mice with other Bacteroides spp., e.g. B. caccae, B. thetaiotaomicron, B. uniformis, B. finegoldii, or B. ovatus, have been shown to induce the accumulation of FOXP3+ cells, particularly Nrp1−RORγt+ pTreg cells in the mouse colon (Faith et al. 2014; Sefik et al. 2015). De novo generation of Tregs, with the capacity to suppress intestinal inflammation, was also observed in GF mice colonized with Bifidobacterium bifidum (Verma et al. 2018). Clostridia, B. fragilis, and B. bifidum have also been shown to increase the expression of CTLA4 on Tregs (Atarashi et al. 2011, 2013; Round et al. 2011; Verma et al. 2018). While it is not yet established if Clostridium and Bacteroides species function in a TCR-dependent manner, a pathobiont Helicobacter hepaticus has been shown to induce antigen-specific RORγt+ Tregs in the colon (Chai et al. 2017; Xu et al. 2018).
Microbes also stimulate the regulatory arm of the mucosal immune system via their components. For example, B. fragilis releases outer membrane vesicles (OMVs) containing polysaccharide A (PSA), the most abundant capsular polysaccharide expressed by B. fragilis. OMVs are taken up by cLP DCs and further elicit the conversion of CD4+ T cells into IL10-producing Foxp3+ Tregs via TLR2 (Round et al. 2011). Similarly, cell surface β-glucan/galactan (CSGG) polysaccharides from B. bifidum are important effector components able to induce Tregs via a DC-dependent and partially TLR2-dependent mechanism (Verma et al. 2018).
9.4.4. Environmental Factors: Dietary and Microbial Metabolites
The specific mechanism by which symbionts stimulate the accumulation of Tregs is not fully understood. It is believed that Clostridia spp. can function synergistically to stimulate IECs leading to Treg induction (Atarashi et al. 2013). One proposed mechanism of action is the cooperative production of short-chain fatty acids (SCFAs), such as butyrate, propionate, and acetate, through fermentation of dietary fiber by the commensals including Clostridium and Bacteroides spp. in the colon (Arpaia et al. 2013; Atarashi et al. 2013; Furusawa et al. 2013; Smith et al. 2013). Colonic SCFAs are absorbed by passive diffusion and by active H+-coupled transport via MCT1 (SLC16A1) and MCT4 (SLC16A3), two electroneutral monocarboxylate transporters, or by electrogenic Na+-coupled monocarboxylate transporters SMCT1 (SLC5A8) and SMCT2 (SLC5A12). Oral administration of butyrate, propionate, and acetate either individually or in combination led to an increase in the number of colonic Treg cells in SPF mice (Smith et al. 2013). Although in monoassociation studies, Sefik et al. could not find a correlation between luminal SCFA concentrations and the frequencies of RORγt+ pTregs (Sefik et al. 2015), this study did not take the combinatorial effects of SCFA producers into account. Upon reaching the colonic LP, SCFAs affect DCs and T cells directly using two mechanisms: via inhibition of histone deacetylases (HDAC) and via signaling through certain G protein–coupled receptors. Butyrate is known to participate in Treg differentiation by facilitating histone H3 acetylation in the promoter region and CNS1 and 3 of Foxp3 gene (Furusawa et al. 2013). Recognition of butyrate by G protein–coupled receptors such as GPR43and GPR15 expressed by colonic Treg cells and by GPR109A expressed by DCs and macrophages (Arpaia et al. 2013; Atarashi et al. 2013; Furusawa et al. 2013; Singh et al. 2014; Smith et al. 2013) also promotes Treg differentiation. Interestingly, SCFA treatment increased Helios+ Treg numbers in GF mice, indicating that SCFAs also promote the expansion of the tTregs present in the colonic LP (Smith et al. 2013).
Aryl hydrocarbon receptor (AhR) is a nuclear sensor expressed by Tregs that helps them sense and react to compounds that act as AhR ligands. Intestinal pTregs have higher expression of AhR than Tregs in any other tissue (Ye et al. 2017). Dietary tryptophan, an essential amino acid, is metabolized by IDO (indoleamine 2,3-dioxygenase) and TDO (tryptophan 2,3-dioxygenase) expressed in IECs and DCs, into several AhR ligands including kynurenine. Kynurenine enhances Foxp3+ Treg generation in vitro in the presence of TGFβ1 (Mezrich et al. 2010). Gut microbes like Lactobacillus spp. can also metabolize tryptophan into many AhR ligands (Gao et al. 2018). AhR is not only important for the induction of Tregs, but it also contributes to their regulatory function. Ahr-expressing Tregs showed enhanced in vivo suppressive activity compared with Ahr-deficient Tregs in a T-cell transfer model of colitis (Ye et al. 2017).
Another dietary metabolite that drives the Treg development in intestine is the all-trans retinoic acid (atRA), a bioactive form of vitamin A. Dietary vitamin A, specifically retinol, is absorbed by the intestinal epithelial cells via passive diffusion. Although epithelial cells are capable of synthesizing atRA from dietary vitamin A (D’Ambrosio et al. 2011), a process regulated by commensal bacteria (Grizotte-Lake et al. 2018), the vast majority of studies to date focused on dendritic cells, macrophages, and eosinophils as sources of atRA for iTreg induction. Aldehyde dehydrogenases ALDH1A1, ALDH1A2, and ALDH1A3 are the rate limiting enzymes in the synthesis of atRA (Bazewicz et al. 2019). Of those three, CD103+DCs express primarily the ALDH1A2 isoform (Coombes et al. 2007). CD103+ DCs are chiefly equipped to promote Tregs in the gut by the activation of latent TGFβ1 via integrin αVβ8. Thus, CD103+ DC-derived RA, along with TGFβ, converts naive FoxP3−CD4+ T cells into FoxP3+ Tregs in both human and mouse (Coombes et al. 2007; Kang et al. 2007; Mucida et al. 2007; Sun et al. 2007). These Tregs also display an enhanced upregulation of the mucosal tissue-homing receptors C-C chemokine receptor type 9 (CCR9) and integrin β7 along with being potent suppressors of effector T cells in a colitis model (Coombes et al. 2007; Kang et al. 2007; Mucida et al. 2007; Sun et al. 2007). These RA-induced human FoxP3+ cells were also chemotactically responsive to CCR9 ligand CCL25, a chemokine specifically expressed by intestinal epithelial cells (Kang et al. 2007). Furthermore, CNS1 non-coding element in the FoxP3 gene, which is required for the generation of pTregs, contains binding sites for the heterodimer of retinoic acid receptor (RAR) and retinoid X receptor (RXR). Upon binding of RA to RAR and RXR, CNS1 undergoes histone acetylation leading to the prolonged and stable expression of FoxP3 in Tregs (Xu et al. 2010). atRA was also shown to promote iTreg development and maintenance via an ERK1/2-dependent mechanism and increased histone methylation and acetylation within the CNS2 element of Foxp3 gene locus (Lu et al. 2011).
Additionally, it is also reported that RA is able to suppress the IL6-induced conversion of Foxp3+ Tregs into inflammatory Th17 cells, a mechanism likely mediated by the retinoic acid receptor α (Elias et al. 2008). Based on experiments with mice fed with a vitamin A–deficient diet or treated with an inhibitor of the RA receptor, vitamin A seems to affect the development of RORγt+ pTregs cells and not Helios+ tTreg cells in the gut (Ohnmacht et al. 2015). While RORγt+ Tregs were restored upon feeding RA, it did not “rescue” the defective generation of RORγt− Tregs (Kim et al. 2016). Notably, an RA-reactive human Treg cell subset expressing natural killer cell receptor CD161 and RORγt has been described recently (Povoleri et al. 2018).
While vitamin A plays the most prominent role in the development of Tregs, there are other vitamins that have been reported to affect this lymphocyte population. Dietary vitamin D3 is metabolized to 1,25-dihyroxyvitamin D3 which binds to the nuclear vitamin D receptor element (VDRE) within the +1714 to +2554 nt non-coding CNS region of the human Foxp gene (Kang et al. 2012). FR4, the receptor for vitamin B9 (folic acid), is highly expressed by Tregs (Yamaguchi et al. 2007) and is known to promote survival of colonic Tregs by upregulating the anti-apoptotic factor BCL2 (Kinoshita et al. 2012). Consistent with this observation, mice fed with a vitamin B9–deficient diet displayed a marked reduction in colonic pTregs and a higher susceptibility to intestinal inflammation (Kinoshita et al. 2012).
9.5. Suppressive Function Mediated by Treg in the Gut
Tregs can exert suppressive function towards a variety of immune cells, such as NK, CD8+, and CD4+ T lymphocytes and antigen-presenting cells (APC). Inhibition of T conventional cells can occur indirectly, i.e., when inhibition involves influencing the activation status of APC, or directly, i.e., in absence of APC. Tregs can suppress intestinal immune responses to maintain homeostasis and exert their functions through various mechanisms, mostly determined on the basis of in vitro assays. The best-known suppressive mechanisms to be discussed here are (1) cytokine-mediated inhibition, (2) expression of inhibitory surface molecules, and (3) cytolysis of target cells. Species-specific mechanisms may complicate data interpretation, and findings identified in murine models may not correspond to those found in human Tregs.
9.5.1. Secretion of Inhibitory Cytokines
FoxP3+ Tregs and Tr1 cells produce high levels of IL10, which can target several cell types in the gut, among them the effector immune cells, to inhibit their expansion and to promote immunosuppression. IL10 also acts in an autocrine manner as a proliferative factor for Tregs, although its requirement for FoxP3+ Tregs and Tr1 maintenance in vivo is unlikely, since both Treg subsets develop normally in the absence of IL10 or IL10R (Maynard et al. 2007; Chaudhry et al. 2011). Even though IL10 can be secreted by many cells of the innate and adaptive immune systems, the importance of IL10 originating in Tregs for the gut homeostasis is illustrated by the development of exacerbated intestinal inflammation when FoxP3+ Tregs lacked the ability to produce IL10, likely due to their inability to suppress pathogenic Th17 cell responses (Asseman et al. 1999; Chaudhry et al. 2011). The high concentration of IL10 produced by mucosal Tr1 enables the suppression of IL1β and TNFα released from human myeloid cells (Cook et al. 2019). In the gut homeostasis, IL10 gene has been identified as susceptibility locus in ulcerative colitis (UC) (Franke et al. 2008) and mutations on IL10RA and IL10RB receptors result in severe and early onset IBD (Glocker et al. 2009; Moran et al. 2013). In the murine colon, RORγ+ FoxP3+ Tregs have been reported to be the main producers of IL10 (Ohnmacht et al. 2015).
The involvement of Treg-derived TGFβ in immune tolerance, inhibition of acute inflammation, and promotion of wound-healing process renders it a pivotal cytokine in the immune regulation in the gut. Soluble and membrane bound TGFβ are produced in high amounts by Tr1 and FoxP3+ Tregs in the gut (Powrie et al. 1996; Levings et al. 2002). TGFβ also contributes to the differentiation of Tregs, converting naïve CD4+ T cells into FoxP3+ Tregs (Chen et al. 2003; Zheng et al. 2002), but other mediators are required for Tregs to fully differentiate since the induction of FoxP3 expression by TGFβ alone does not necessarily confer immunosuppressive abilities to Tregs (Tran et al. 2007). Production of TGFβ by Treg is, however, necessary to prevent intestinal inflammation (Powrie et al. 1996) as much as expression of TGFbRII by the T conventional cells (Gorelik and Flavell 2000), indicating the essential role of Treg-derived TGFβ in controlling pathogenic T-cell response intestinal inflammation.
Tr1 expanded in vitro, but not FoxP3+ Tregs, can also secrete IL22 to promote intestinal barrier function (Cook et al. 2019). Even though IL35 has been described as an important immunoregulatory cytokine secreted by murine Tregs (Collison et al. 2007), human FoxP3+ Tregs in resting or activated state appear not to express IL35 (Bardel et al. 2008).
9.5.2. Expression of Cell Surface Inhibitors of Inflammation
Human Tregs express cell surface molecules that enable the suppression of immune function in target cells via a contact-dependent (e.g., CTLA4, LAG3, and PD1) or contact-independent manner (e.g., IL2R and CD39). The contact-dependent suppression involves binding of the inhibitory receptors expressed on the Treg surface to stimulatory and co-stimulatory receptors expressed on the surface of target cells, ultimately suppressing immunostimulatory intracellular cascades in target cells. The successful antigen presentation by APC requires the antigen-TCR binding in the context of major histocompatibility complex II (MHCII), in coordination with the triggering of CD28 receptors on T cell by co-stimulatory receptors from the B7 family on APC. B7.1 (CD80) and B7.2 (CD86) can bind to a variety of molecule members of the CD28 family: CD28 and ICOS act as positive regulators of T-cell function while CTLA4 and PD1 act as inhibitors. B7.1 and B7.2 are also expressed on Teff cells and can be targeted for inhibition through contact-dependent mechanisms.
9.5.2.1. Contact-Dependent Inhibition
Cytotoxic T-lymphocyte-associated protein 4 (CTLA4, CD152) is highly expressed on FoxP3+ Treg and Tr1 cells relative to the conventional CD4+ T cells. It is a key regulatory molecule, since null or Treg-specific knockout of CTLA4 result in autoimmune disease (Takahashi et al. 2000; Wing et al. 2008). In vitro suppression of Teff cell proliferation by Tregs (in presence of APC) was shown to be dependent on CTLA4 in murine and human cells (Tang et al. 2004; Birebent et al. 2004) and was mediated by CD80/CD86 downregulation on APC (Cederbom et al. 2000). However, production of inflammatory cytokines does not seem to be affected by Tregs’ CTLA4. Expression of lymphocyte activation gene 3 (LAG3) in human Tregs also contributes to their suppressive function (Wang et al. 2018). LAG3 is a CD4 homolog expressed on FoxP3+ and Tr1 T cells and interacts directly with MHCII complex acting as a decoy receptor to inhibit its interaction with the TCR, downregulate cell proliferation and secretion of inflammatory cytokines IFNγ, IL2, and TNFα by APC and B cells (Hannier et al. 1998). Deficiency in LAG3 renders Tregs inefficient to inhibit T-cell proliferation and unable to prevent chronic intestinal inflammation (Huang et al. 2004). PD1 (CD279) is also expressed on both Treg subsets and binds to PD-L1 (B7-H1), PD-L2 (B7-DC), and B7.1. PD1 signaling inhibits TCR-signaling-induced cell cycle progression and TCR expression on CD8+ T cells (Karwacz et al. 2011), and blockade of the PD1/PD-L1 pathway can abrogate Treg-mediated immunoregulation (Kitazawa et al. 2007).
9.5.2.2. Contact-Independent Inhibition
In humans, suppressive Tregs express high levels of CD25. CD25 is a subunit of the high-affinity heterotrimeric IL2 receptor and is also transiently expressed on activated T cells. CD25 expression is a part of the suppressive mechanism of Tregs, supposedly through the scavenging of IL2 and reduction of its effects on T cells (Barthlott et al. 2005). Though the proximity between cells is necessary for inhibition mediated by CD25, contact is not required. Exogenous IL2 abrogates Treg-mediated suppression of Teff in human and mice (Oberle et al. 2007; Pandiyan et al. 2007). Recent reports link the IL2 scavenging by Tregs to induction of apoptosis on Teff cells (Pandiyan et al. 2007), but further studies are required to prove the existence of such regulatory mechanism in humans.
Expression of CD39 on the surface of FoxP3+ Tregs also provides an important immunoregulatory mechanism. Catalysis of ATP to AMP by CD39 (ecto-nucleoside triphosphate diphosphohydrolase 1, E-NTPDase1), and its subsequent hydrolysis into adenosine by CD73 (ecto-5′-nucleotidase, NT5E) regulates the extracellular levels of ATP and adenosine and their effects on immune cells (Su et al. 2019). Since human Tregs do not express high levels of CD73 (in contrast to their murine counterparts), the AMP conversion to adenosine in humans is thought to result from the expression of CD73 on neighboring cells (Dwyer et al. 2010). ATP binding to purinergic receptors such as P2X7 and P2Y2 expressed on the surface of immune cells induces the secretion of inflammatory cytokines and a DC-mediated priming of intestinal Teff cells toward the Th17 phenotype (Pandolfi et al. 2016; Atarashi et al. 2008). On the other hand, activation of A2A receptors by adenosine induces immune anergy and promotes intestinal homeostasis (Ye and Rajendran 2009). Fifty percent of the human Tregs express CD39 (Borsellino et al. 2007), and this population is highly suppressive through the generation of adenosine (Mandapathil et al. 2010). CD39 polymorphisms in humans are associated with IBD susceptibility (Friedman et al. 2009). ATP/adenosine balance by CD39+ Tregs is of relevance to the intestinal inflammation, where high concentrations of ATP produced by the microbiota reach the LP immune cells as a consequence of intestinal epithelial barrier injury (Atarashi et al. 2008).
Inducible co-stimulator (ICOS) has a dual and balancing role to sustain T-cell activation and effector functions and to promote Treg differentiation and suppression. ICOS expression on human Tregs is potentially involved in contact-dependent and -independent immunosuppression in peripheral tissues. High expression of ICOS on natural FoxP3+ Tregs present in thymus, PBMC, and peripheral tissues endows Tregs with the capacity to produce high levels of IL10 and TGFβ to suppress DC and T-cell function, respectively, whereas ICOS−FoxP3+ Tregs play a suppressive function mainly through the secretion of TGFβ1 (Ito et al. 2008). ICOS contact-dependent CD4+ T suppression is mediated by the upregulation of CTLA4 expression on human Tregs (Zheng et al. 2013). Some of the findings in the literature support the role of the microenvironment in the differentiation of ICOS+ Tregs. These cells are the main suppressive subset in human melanoma (Strauss et al. 2008). In the murine gut, colonic LP ICOS+ Tregs have a strong immunosuppressive profile (Nakanishi et al. 2018) and are stimulated locally by human microbiota (Atarashi et al. 2013).
9.5.3. Cytolysis
A less studied mechanism of Treg-mediated immune suppression in human is the secretion of cytolytic enzymes and killing of target cells. Human CD25+ Tregs generated in vitro from CD4+ T cells stimulated with antibodies to CD3/CD46 and with IL2, express granzyme B and induce cell death in autologous cells in a perforin/granzyme-dependent manner (Grossman et al. 2004a). Although CD25high Tregs from human peripheral blood activated in vitro did not express granzyme B, they expressed granzyme A and presented a cytolytic activity toward various autologous immune cells in a perforin-dependent but FasL-independent fashion (Grossman et al. 2004b). The target cells tested included activated CD4+ and CD8+ T cells, CD14+ monocytes, and dendritic cells. However, iTreg-mediated suppression of B cells seems to be independent of cytolysis (Xu et al. 2016). Two studies reported the presence of FoxP3+ Tregs expressing granzyme B in the colon of patients with colorectal cancer (Sun et al. 2020) and in the lungs of RSV-infected mice (Loebbermann et al. 2012). Very few to none of these cells could be found in lymphoid organs or circulating Tregs, suggesting that granzyme B expression is acquired locally by Tregs. These findings, summarized in Fig. 9.2, indicate the participation of perforin/granzyme-dependent cytotoxicity in Treg-mediated suppression in the mucosal surfaces.
Fig. 9.2.
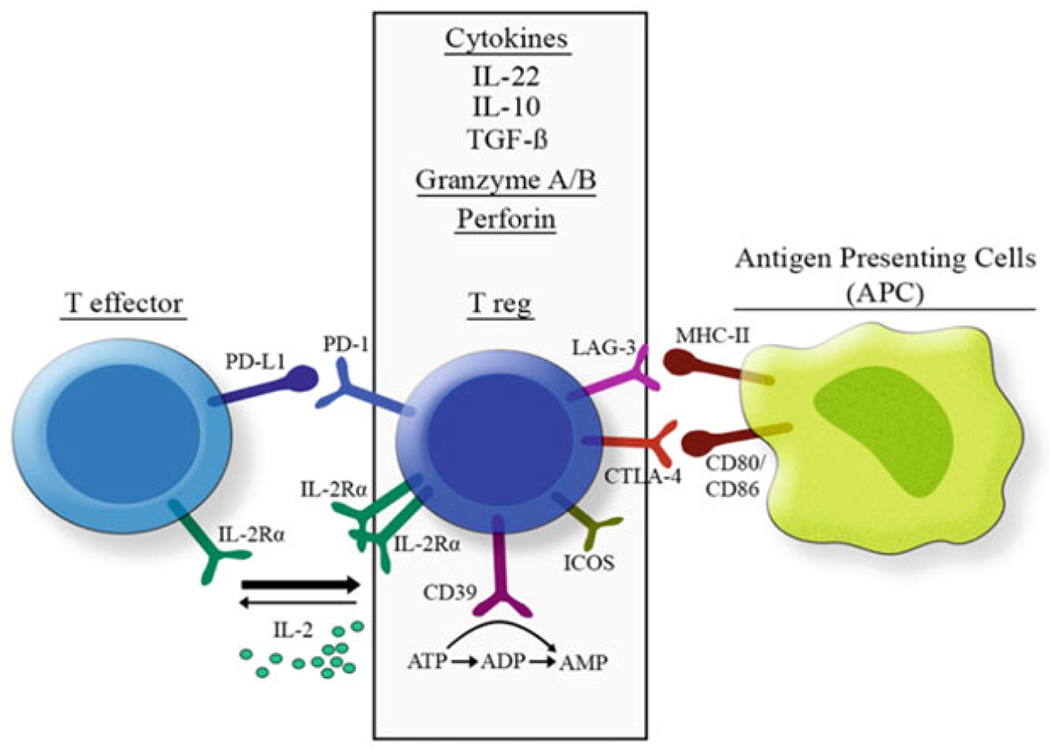
Mechanisms of immunosuppression in human regulatory T cells. Tregs suppress inflammatory cells through secretion of anti-inflammatory cytokines, expression of inhibitory surface molecules and cytolysis of target cells. Secretion of IL22, IL10 and TGFβ, as well as cytolytic Granzyme A/B and Perforin leads to suppression of effector functions of a variety of immune cells. ATP hydrolysis by CD39 also limits the level of such inflammatory signaling molecule in extracellular medium. Expression of LAG3 and CTLA4 constitute negative regulators of APC activation, through MHC competition and inhibition of CD80/86 signaling respectively. High expression of IL2Rα scavenges the extracellular IL2 available for Teff cells, while PD-1 expression inhibits TCR expression and signaling in effector T cells. ICOS induces secretion of TGFβ1 and CTLA4 expression
9.6. Treg During Intestinal Diseases
Tregs are central regulators of the intestinal immune response and inflammation in the gut. Through the described mechanisms, Tregs suppress immune activation and control the intensity of inflammatory responses in the gut. A breakdown of these tolerogenic mechanisms is usually observed under pathological conditions involving exacerbated inflammation in the intestinal mucosa, e.g., in inflammatory bowel disease, celiac disease, or necrotizing enterocolitis (NEC). On the other hand, the function of Tregs can be detrimental under certain circumstances in which the immune response is necessary to eliminate infectious agents or oncogenically transformed cells. In this topic, we will focus on intestinal diseases where a dysfunction of regulatory T cells has been implicated.
9.6.1. Inflammatory Bowel Disease
IBD is a group of chronic inflammatory diseases resulting from exacerbated immune response in the gut mucosa. There is a consensus that the inappropriate immune response driven against commensal intestinal microbes is the key to IBD etiology and is affected by both the environmental factors and genetic susceptibility of the host. Ulcerative colitis (UC) and Crohn’s disease (CD) are the two major types of IBD, distinguished on the basis of histological and endoscopic findings, distribution, and clinical complications (Molodecky et al. 2012). Several lines of evidence support the role of T cells in IBD; however, the adaptive immune profile during CD and UC differs. CD is noted for the involvement of Th1-related cytokines TNFα, IFNγ, and IL12, whereas UC is driven by Th2 and Th9 cytokines such as IL5, IL13, and IL9 (Muzes et al. 2012). Th17-related cytokines, IL17A, and IL23 are increased in intestinal tissue in both UC and CD (Nemeth et al. 2017) and contribute to the disease pathogenesis in these patients.
Multiple human genome-wide association studies (GWAS) have identified a remarkable proportion of single-nucleotide polymorphisms (SNPs) in genes that affect T-cell function in patients with IBD. Among them, polymorphisms in genes linked to the IL10 signaling pathway are associated with severe early onset of IBD (Farh et al. 2015; Glocker et al. 2009). IL10 signaling is essential to maintain homeostasis and mucosal barrier function and to the control of effector and suppressor cells. As described earlier in this chapter, Tr1 and FoxP3+ Tregs are the two major producers of IL10 in the gut. The pivotal role of FoxP3+ Tregs in maintaining intestinal homeostasis is exemplified by the presence of severe intestinal inflammation in patients with FoxP3 mutations, who suffer with global failure in Treg development (Bennett et al. 2001). Interestingly, patients with IBD do not have a quantitative deficiency in frequency of FoxP3+ Tregs in the gut. On the contrary, an increase in this population has been observed in the mucosa and regional lymph nodes of CD and UC patients (Reikvam et al. 2011; Yu et al. 2007). Ex vivo, CD4+CD25+ cells isolated from UC patients showed suppressive activity, and the authors suggested that this activity may either be insufficient or impaired in vivo, in the face of overwhelming mucosal inflammation (Yu et al. 2007).
Helios+ and Helios− FoxP3+ cells in the mucosa of IBD patients were found to express similar levels of immunoinhibitory receptors CD25, CD39, CTLA4, TIGIT, and PD-1 compared to control patients (Lord et al. 2015). Analyses of circulating Tregs in IBD can be controversial, and the frequency of FoxP3+ Tregs and Tr1 cells has been found either increased (Vitale et al. 2019) or decreased (Khalili et al. 2018). In murine adoptive naïve T-cell transfer model of colitis, a reliable and representative model of IBD (te Velde et al. 2007), the administration of a T-cell population containing IL10-producing Tregs, protected from intestinal inflammation (Asseman et al. 1999). CTLA4 was found to be mutated in mucosal immune cells in early onset CD (Zeissig et al. 2015). Impaired dimerization and binding of the mutated CTLA4 to CD80 could explain the loss of Treg regulatory mechanisms in IBD. Chao et al. (2018) showed that lack of CTLA4 in CD4+ T cells in mice impairs differentiation of follicular Tregs and triggers the accumulation of autoantibodies in intestinal epithelial cells. This defect exacerbated the immune response to MCMV (mouse cytomegalovirus), resulting in acute intestinal injury and death.
Another mechanism driving impaired immunosuppression in IBD may be related to FoxP3+ Treg survival in the inflamed intestine as a consequence of the hyperinflammatory environment. It has been reported that Tregs from the inflamed tissue of IBD patients show evidence of enhanced apoptosis, which can be reversed by anti-TFNα treatment (Veltkamp et al. 2011). In line with the evidence of altered Treg survival during IBD, polymorphisms in genes encoding the IL2RA and IL2RB subunits, indispensable for Treg survival in peripheral tissues, were identified in patients with CD and UC (Bouzid et al. 2013). ST2/IL33 axis has also been shown to enhance Treg stability and/or survival. Under homeostasis, IL33 binds to suppressor of tumorigenicity 2 (ST2) highly expressed on colonic Tregs, stimulates FoxP3 and GATA3 expression, and promotes Treg function through enhancing TGFβ1-mediated differentiation, also an inducer of survival pathway in T cells (Schiering et al. 2014). IL33 signaling in DCs may indirectly contribute to this process, by stimulating DC production of IL2 (Matta et al. 2014). Increased levels of sST2, an extracellular ST2 decoy receptor for IL33, in the mucosa and serum of IBD patients is positively associated with the disease severity, suggesting that this axis maybe be disrupted during chronic inflammation in patients with IBD (Boga et al. 2016). In sepsis-surviving patient, IL33 contributes to long-term immunosuppression by expanding the Tregs (Nascimento et al. 2017). However, in UC patients, increased IL33 mRNA expression was reversed in remission induced with infliximab, a TNFα inhibitor, which could be interpreted that IL33 may have a dual role in IBD patients (Gundersen et al. 2016; Chen et al. 2020; Pastorelli et al. 2013).
In addition to a reduced survival observed in Tregs from patients with IBD, alterations in Treg function and phenotype have been reported. Zhou et al. showed that FoxP3+ Tregs have reduced FoxP3 expression and acquire inflammatory phenotype when exposed to an inflammatory milieu (Zhou et al. 2009). Loss of FoxP3 interaction with the epigenetic enzyme Enhancer of Zeste Homolog 2 (EZH2) observed in mucosal Tregs of IBD patients may result from Treg exposure to IL6 (Bamidele et al. 2019) and may lead to an impaired repression of inflammatory genes, normally targeted by FoxP3/EZH2 complex in CD4+ T cells (Sarmento et al. 2017). Sanctuary et al. (2019) described a mechanism for TNFα-induced loss of Treg immunosuppressive function. According to the authors, TNFα induces miR-106a microRNA in both humans and mice, which through NFκB promoter binding suppresses post-transcriptional regulation of IL10 release by Tregs.
In homeostatic conditions, intestinal FoxP3+ Tregs express RORγT. While this population is highly immunosuppressive, it may also display considerable plasticity and gain the ability to produce IL17A in inflammation associated with CD and UC. IL17A producing Tregs from IBD patients showed defective suppressive function, and differences in cytokine requirements for that transition were observed in patient peripheral blood cells from CD and UC (Ueno et al. 2013). In CD patients, IL1β/TGFβ/IL23 are more effective in inducing IL17A+ FoxP3+ Tregs, while cells from UC patients required IL21 and TGFβ (Ueno et al. 2013). Patients with IBD also demonstrated increased Treg/Th1 crossover populations in T-bet+FoxP3+ Treg (Li et al. 2017), but the pathways involved in acquisition of T-bet in human intestinal FoxP3+ Tregs and their role during IBD have not been investigated. To complicate matters, another study showed that despite producing IL17, IL17+CD161+ Tregs enriched in the CD mucosa remained highly suppressive and were enriched for wound healing genes (e.g., PDGFA and CFS2), including soluble mediators (e.g., IL17A and IL22) known for accelerating epithelial barrier healing (Povoleri et al. 2018).
The mucosal inflammatory milieu of IBD may also impair the responsiveness of the effector cells to Treg-mediated suppression. Inflammatory cytokines and inducers of NFκb in T lymphocytes, such as TNFα, have anti-apoptotic effects in these cells (Dudley et al. 1999) and could lead to pathogenic T-cell resistance to mechanisms of cell death induced by Tregs during IBD. Such resistance could promote the persistence and perpetuation of pathogenic CD4+ T cells in the gut tissue. Mechanisms of resistance to TGFβ have also been identified in the LP effector cells during IBD. High levels of Smad7 produced by mucosal T lymphocytes in patients with IBD, likely as a consequence of increased TNFα, IL1β, and IFNγ expression (Ulloa et al. 1999), turn pathogenic Teff cells insensitive to regulation by TGFβ (Laudisi et al. 2016), despite the increased levels of TGFβ in IBD tissues (Babyatsky et al. 1996).
Most of the knowledge on Treg dysfunction in IBD comes from studies on FoxP3+ Treg subset, and the characteristics and function of Tr1 in patients with IBD have yet to be further investigated. A recent report has identified IFNγ+ IL10-producing Tr1 as CCR5+PD-1+ cells in the human intestine but did not indicate altered frequencies of these population in CD or UC. However, Tr1 showed reduced IL10 expression when isolated from the IBD mucosa, a decrease that could be reproduced by treatment with of IL-23 and IL1β (Alfen et al. 2018).
9.6.2. Celiac Disease
Celiac disease is a T-cell-mediated disease triggered by ingestion of gliadin, the main component of the gluten, in genetically predisposed individuals. Gliadin-derived peptides activate T helper (Th) cells to produce Th1 and Th17 signature cytokines IFNγ (Nilsen et al. 1998) and IL17A (Fernandez et al. 2011) that contribute to the expansion and maintenance of the inflammatory response in the gut of individuals with celiac disease. A break of tolerance to dietary gliadin, and the known role of Tregs in immune tolerance to dietary components, prompted several groups to investigate the Treg population patients with celiac disease. The findings offered some parallels and similarities with IBD. Like in IBD, the numbers of FoxP3+ Tregs are increased in the intestinal mucosa of celiac patients with active disease (Cianci et al. 2012), and its local expansion after gliadin stimulation (Zanzi et al. 2011) suggested that it is an insufficient attempt at reigning in the Teff cells during this disease. Celiac intestinal mucosa also harbors Tr1 cells, able to produce IL10 and TGFβ and to suppress proliferation of pathogenic T cells in vitro (Gianfrani et al. 2006). In fact, upregulation of these two immunosuppressive cytokines by Tregs was observed along with the exacerbated proinflammatory immune response found in celiac disease (Lahat et al. 1999). In vitro studies support the regulatory role of IL10 in the mucosal T cells from celiac patients by inhibiting IFNγ production by gliadin-specific Teff cells (Salvati et al. 2005).
The negative regulation of the immune response exerted by Tregs is not enough to control the ongoing immune response in celiac patients. Circulating FoxP3+ Tregs from children with celiac disease show high expression of CD101 and CD129 (Akesson et al. 2015) and 80% of the circulating FoxP3+ Tregs from celiac patients express high levels of CD39 (Cook et al. 2017), all indicators of their suppressor activity. FoxP3+ Tregs from the intestine of celiac patients showed no impaired function in vitro (Zanzi et al. 2011), but Cook et al. showed that circulating gluten-specific FoxP3+CD39+ Tregs from celiac disease patients can have reduced suppressive function in response to a polyclonal stimulus (Cook et al. 2017). These results suggest that the intestinal and the inflammatory environment established during the course of the disease may locally impair crucial regulatory mechanisms afforded by Tregs. The overexpression of intestinal IL15 in celiac disease could contribute to this phenomenon. IL15 impairs TGFβ signaling in biopsies from active celiac disease (Benahmed et al. 2007), dampens Treg suppressive activity (Zanzi et al. 2011), and makes T effector cells refractory to the regulatory effects of Tregs through the activation of phosphatidylinositol 3-kinase (PI3K) pathway (Ben Ahmed et al. 2009). Additionally, microbiome-derived metabolites have shown to modulate alternative splicing and the expression of FoxP3 isoforms in intestinal biopsies from patients with active celiac disease, where increased expression of FoxP3 shorter D2 isoform over full length has been observed (Serena et al. 2017). The alternatively spliced isoform FoxP3 D2, thought to be induced by butyrate produced by bacteria expanding during the active celiac disease in presence of IFNγ, is less effective in downregulating the Th17 differentiation. In this scenario, the switch to FoxP3 isoform D2 in patients with celiac disease could promote local Th17 immune response (Serena et al. 2017). Therefore, the microenvironment in the intestinal tissue of patients with celiac disease might orchestrate the differentiation and/or functionality of Tregs, thus restricting their activity.
9.6.3. Necrotizing Enterocolitis
Necrotizing enterocolitis (NEC) is an immunopathology that most commonly affects infants born prematurely. The disease is characterized by dysregulated inflammatory host responses to luminal bacteria with increased levels of inflammatory mediators, such as TNFα and plateletactivating factor (PAF) in the small intestinal tissue and serum (Caplan et al. 1990). The inflammatory injury in NEC causes a variable spectrum of damage to the intestinal tract, from mucosal injury to full-thickness necrosis. Multiple factors are involved in the precipitation of NEC, including formula feeding, ischemic/hypoxic injury, and abnormal bacterial colonization.
The population of FoxP3+ Tregs develop early during gestation. They are detected as early as 23 weeks of gestational age in large and small bowel. Treg numbers are stable during the later phases of development and do not change with increasing intrauterine development or postnatal age (Weitkamp et al. 2009). The immaturity of the immune system in preterm babies is thought to be a critical pathogenic factor in NEC. Several studies have found important correlations between preterm birth and growth restriction with Treg function in the intestinal tissue. The FoxP3+ Treg frequency in the circulating blood of preterm infants inversely correlated with gestational age at birth, indicating a contraction in this population after birth in preterm children (Qazi et al. 2020). Treg suppressive index, which reflects the expression level of FoxP3, is also decreased in preterm infants (Mukhopadhyay et al. 2014). Their homing markers may also be affected in preterm children (Qazi et al. 2020) and children with NEC (Weitkamp et al. 2013). In infants with growth restrictions, Mukhopadhyay et al. found a markedly reduced proportion of circulating Tregs in the cord blood (Mukhopadhyay et al. 2014). In this vulnerable group of infants, circulating FoxP3+ Tregs also showed a reduced expression of the gut homing integrin α4β7, which could also be an indicator of reduced capacity of Tregs to reach the intestinal mucosa (Qazi et al. 2020). Therefore, lower frequency, impaired homing, and/or impaired function of Tregs observed in preterm children may be implicated, or at least contribute to the pathogenesis of NEC.
In NEC, the inflammatory microenvironment may contribute to the impairment of Treg differentiation and expansion, which could perpetuate the inflammation. Flow cytometry analysis has shown lower frequency of FoxP3+ Treg cells in ileum (Weitkamp et al. 2013) and peripheral blood mononuclear cells of infants with NEC (Pang et al. 2018a). The frequency of intestinal FoxP3+ Tregs was recovered in tissue from healed postoperative NEC (Weitkamp et al. 2013, suggesting that Tregs from children with NEC have no intrinsic failure in expansion and accumulation in intestinal tissue. Studies of Tregs in infants with NEC need to be interpreted with caution due the diversity of markers used to identify these cells in different investigations. Detecting FoxP3 expression in Tregs is a useful tool to characterize the frequency of Tregs in the inflamed tissue, but it requires cell fixation and does not permit downstream functional analysis. Because of that, isolation of human Tregs for in vitro assays is usually based on high expression of IL2Rα (CD25). Human CD4+CD25+/hi Tregs from NEC infants showed reduced expression of CTLA4, LAG3, and Helios, reduced capability to suppress CD4 Teff proliferation in vitro, and remarkably failed to suppress IL17A expression in these cells (Pang et al. 2018a). An imbalance caused by an increased ratio of Th17/Treg cells in the LP was shown to contribute to excessive inflammatory response in NEC (Egan et al. 2016), and the restoration of this balance after Treg induction by retinoic acid administration decreased NEC severity (Nino et al. 2017). IL6, which inhibits TGFβ-induced Treg differentiation and favors Th17 lineage, was found to be increased in the small intestine affected by NEC and could participate in the contraction of Tregs and in perpetuating the disease (Weitkamp et al. 2013). Indeed, IL6 neutralization restored FoxP3+ Tregs and ameliorated experimental NEC in mice (Ma et al. 2019). Monocytes in NEC patients were suggested to play a role in Th17/Treg imbalance by promoting the differentiation of RORγt-expressing Th17 cells via production of IL6 (Pang et al. 2018b). The plasticity of Treg phenotype in hyperinflammatory environment, as we discussed earlier in the chapter, could also contribute to Treg dysfunction in NEC patients. IL17+ Tregs were increased in peripheral blood of NEC patients, and this population could be promoted by in vitro Treg exposure to IL6 (Ma et al. 2019).
9.6.4. Graft-Versus-Host Disease (GVHD)
GVHD is a syndrome commonly associated with allogeneic hematopoietic stem cell transplantation (HCT), caused by the recognition of the host as foreign by the immune cells that remain in the donated tissue (graft). The naïve donor T cells migrate to target organs such as the colon, small bowel, liver, and skin where they cause tissue damage (Wysocki et al. 2005). These tissues are potentially attractive sites for the donor T cells due the release of danger-associated molecular patterns (DAMPs), the presence and epithelial translocation of pathogen-associated molecular patterns (PAMPs), and inflammatory cytokines induced during irradiation and/or chemotherapy. Host’s activated APC activate donor T cells, leading to their proliferation and differentiation toward Th1 and Th17 cells, which cause inflammation and damage at mucosal tissues, including the gut. In human allogeneic transplant, localization of Foxp3+ Tregs in the gut has shown negative correlation with the severity of intestinal inflammation in GVHD (Rieger et al. 2006). Increased frequencies of Tregs with gut-homing phenotype (α4β7+) is associated with reduced gut GVHD in patients subjected to neutrophil engraftment (Engelhardt et al. 2011, 2012), indicating that Tregs inhibit immune responses at mucosal sites during GVHD. However, the involvement of Tregs in homeostasis during this syndrome may differ at different sites of the gastrointestinal tract, since no correlation between Treg frequency and the histological or clinical severity of gastrointestinal GVHD was observed in gastric antral biopsies in allogeneic hematopoietic stem cell transplantation (allo-HCT)recipients (Lord et al. 2011). Both tTreg and pTreg cells are important to mediate immunosuppression in the intestine during GVHD, and their transfer prior or along with the donor T cells has shown to diminish the incidence of GVHD and improved survival (Edinger et al. 2003). The literature about the use of Treg induction or infusion as means of GVHD prevention will be discussed in the next section of this chapter.
9.6.5. Tregs in Colorectal Cancer
Cancers are not just masses of transformed cells, but are complex aggregates with non-transformed cells, cytokines, chemokines, growth factors, and inflammatory and matrix remodeling enzymes. Colorectal cancer (CRC) remains one of the leading causes of death worldwide. Besides genetic predisposition and environmental factors, IBD-associated inflammation has been linked to CRC in an “inflammation-dysplasia-carcinoma sequence” (Zisman and Rubin 2008). The tumor microenvironment is thus a result of complex host-tumor interactions on multiple molecular and cellular levels. Immune cells contribute to this microenvironment with the primary goal to efficiently recognize and destroy neoplastic cells in a process known as tumor-immunosurveillance (Dunn et al. 2004). Tumor infiltrating lymphocytes (TILs) like CD8+ cytotoxic T lymphocytes (CTLs) are the major key players in tumor-immunosurveillance. Infiltration of CD8+ cells in solid tumors including colorectal carcinoma has been correlated with favorable prognosis (Ohtani 2007). In the inflamed colon, Tregs accumulate locally in an effort to limit the inflammation and mitigate the related tissue damage (Holmen et al. 2006). Furthermore, it has been demonstrated that CRC could secrete CCL5 which recruits Tregs to tumors through CCL5/CCR5 signaling (Chang et al. 2012). In fact, CCR5hiFoxp3+ Tregs are more suppressive than CCR5loFoxP3+ Tregs in human CRC (Ward et al. 2015). Tumors can also secrete angiogenic factors such as vascular endothelial growth factor (VEGF) that help recruit Tregs to tumors. Blocking VEGF pathways inhibited the recruitment of FoxP3+ Tregs in a mouse model of CRC and impeded the proliferation of human Tregs in vitro (Terme et al. 2013). The high frequency of Tregs might be important for controlling cancer-driving inflammation, but at the same time, they may promote tumor progression by obstructing specific immune responses and contributing to escape of tumor cells from the immune surveillance.
As of now, the state of the field seems to be divided regarding the role of Tregs in the outcome of CRC. Most of the TILs in CRC patients are CD3+ and using CD3+/FoxP3+ T-cell ratio as an appropriate prognostic marker in CRC, a low intraepithelial CD3+/FOXP3+ T-cell ratio has been associated with shorter patient survival (Sinicrope et al. 2009). Another study used intra-tumoral CD8+ T-cell/FoxP3+ cell ratio as a predictive marker for overall survival and found FoxP3+ Tregs to be associated with lymph node metastases (Suzuki et al. 2010). Similarly, a preliminary study showed that peripheral blood derived CD4+CD25+ T-cell lines derived from CRC patient and inhibited the proliferation of autologous HLA-A1 restricted CTLs via TGFβ, thus allowing for tumor cells to escape the immunosurveillance (Somasundaram et al. 2002). Moreover, patients with less abundant Foxp3 Tregs displayed improved T-cell responses (Galon et al. 2006).
There have been reports of antigen-specific CD3+ T-cell responses in CRC, but spontaneous tumor clearance is a very rare occurrence (Campi et al. 2003; Nagorsen et al. 2005; Sinicrope et al. 2009). Apparently, Treg suppression in CRC is tumor-associated antigen (TAA) specific and not systemic (Betts et al. 2012; Clarke et al. 2006). Another detailed analysis using various CRC-specific TAAs such as carcinoembryonic antigen (CEA) peptides, telomerase, Her-2/neu, and MUC-1 showed that they can lead to Treg activation (Bonertz et al. 2009), which may explain the ineffective immune response against the tumor. Moreover, depleting Tregs in PBMCs from CRC patients markedly enhanced the IFNγ and TNFα production in T cells, which were stimulated with a CEA peptide (Yaqub et al. 2008). TAA-specific Tr1 cells have also been identified using a p53 peptide (Bueter et al. 2006). Highly suppressive TIM3-expressing FoxP3+Helios+ Tregs have also been demonstrated at different stages of CRC (Toor et al. 2019). Tumor-infiltrating highly suppressive Tregs that express interleukin-1 receptor 2 (IL1R2), PD-1 Ligand 1, PD-1 Ligand 2, TNFRSF9, CCR1and CCR8 have also been reported (De Simone et al. 2016; Fujimoto et al. 2018). Interestingly, high expression in Treg cell signature genes within whole tumor transcriptome, such as LAYN (a transmembrane protein with homology to c-type lectin), and of MAGEH1 (a member of the melanoma antigen gene family) or CCR8, correlated with poor prognosis (De Simone et al. 2016). Another study with CRC patients showed increased levels of Tr1 markers LAG3 and CD49b, as well as IL17 which were inversely associated with patient survival (Chen and Chen 2014). TAA-specific Tregs suppress APC and T-cell function via IL-10 and NK cell function by producing TGFβ, thus incapacitating both adaptive and innate immunity against cancer (Zou 2006). Additionally, tumor-associated Tregs have been shown to express very high levels of CD39 and inhibit the in vitro T-cell transendothelial migration via adenosine synthesis (Sundstrom et al. 2016).
The field is not universally in agreement about the role of Tregs in CRC progression, as the picture becomes more nuanced. Some studies showed favorable outcomes associated with high infiltration with FoxP3+ Tregs at different stages of CRC (Frey et al. 2010; Lee et al. 2018; Salama et al. 2009). It is also worth noting that effector Tregs (eTregs) are very frequently found in CRC patients. In an interesting study, Saito and colleagues revealed the heterogeneity of FoxP3 expression in tumor infiltrating Tregs (Saito et al. 2016). The authors showed that CRC tumors with favorable outcomes were actually more infiltrated with Foxp3loCD45RA+ non-Tregs and upregulated inflammatory genes like Il12a, Il12b, Tgfb1, and Tnf. On the other hand, CRC with higher infiltration of FOXP3hiCD45RA− eTregs resulted in poor prognosis and lower disease-free survival (Saito et al. 2016). Complementing this study, Lin et al. demonstrated that eTregs, but not Foxp3loCD45RA+ non-Tregs, were associated with late-stage CRC (Lin et al. 2013). Another study showed that FoxP3+ eTregs that express Blimp1, a critical regulator of CD4 T cell exhaustion, have been identified in human CRC and were associated with long-term disease-free survival (Ward-Hartstonge et al. 2017). FoxP3+ Tregs might also be involved in regulating the immune response directed against high-level microsatellite instability (MSI) CRC at the primary tumor site. Significantly elevated FoxP3+ Tregs were associated with CRC patients with known MSI status (Michel et al. 2008).
The discrepancies in the role of Tregs may partly be explained by Treg plasticity. Inflammation in the gut and tumor microenvironment drive certain Tregs to display plasticity and an acquisition of a pro-inflammatory phenotype. RORγt+FOXP3+ Tregs with pathogenic properties have been identified in CRC patients and were enriched in patients with late-stage cancer (Blatner et al. 2012). Interestingly, RORγt+FOXP3+ Tregs in CRC patients produced both IL10 and IL17 (Blatner et al. 2012), indicating that the balance between protective and pathogenic Tregs could potentially influence the disease outcome. Murine Tregs have also been shown to lose FoxP3 expression and gain the ability to produce IL17, in response to an inflammatory bacterial insult in the gut (Yurchenko et al. 2012). Populations of IL17-producing Tregs have been identified in mouse polyps and human CRC (Blatner et al. 2010). Interestingly, this Treg reprogramming does not represent a classical Th17 conversion as these IL17-producing Tregs did not lose their suppressive function (Blatner et al. 2010). It has been suggested that these Tregs might be transitional and have reserved the ability to differentiate into FoxP3+ Tregs or TH17 cells depending on the local milieu of tumor microenvironment (Du et al. 2014). In a mouse model of CRC, TGFβ and prostaglandin E2 mediated the differentiation of Th17 cells into suppressive IL17+ and IL17−FoxP3+ Tregs (Downs-Canner et al. 2017). Indeed, IL17-producing FoxP3+ Tregs have been identified in human CRC (Girardin et al. 2013; Kryczek et al. 2011) with the ability to suppress the proliferation of CD8+ TILs stimulated with CRC antigens (Ma and Dong 2011).
Besides CD4+Foxp3+ Tregs, there have been some unconventional Tregs that have been identified in the context of CRC. Populations of FoxP3+ and FoxP3− T cells that express latency-associated protein (LAP) have been identified in CRC (Mahalingam et al. 2014; Scurr et al. 2014; Zhong et al. 2017). The tumor-infiltrating LAP+FOXP3− cells had much greater suppressive capacity than LAP−FoxP3+ Tregs isolated from the peripheral blood (Scurr et al. 2014) and were associated with poor prognosis. CD8+FoxP3+ Tregs have also been identified in CRC tissues (Taylor et al. 2016). Another study demonstrated tumor infiltrating CD8+CD25+FoxP3+ cells to be able to express CTLA4 and GITR and suppress CD4+ T-cell proliferation and IFNγ production ex vivo (Chaput et al. 2009).
9.7. Treg-Related Therapeutic Approaches
Based on the observed impairment of Treg immunosuppressive abilities in patients with inflammatory disorders of the gut, therapeutic approaches aiming to correct such deficiencies may have the potential to protect the gut tissue from excessive inflammation and successfully restore intestinal homeostasis. Studies on Treg immunotherapy with infusion of autologous Treg or mesenchymal stem cells (MSCs), or enhancement of Treg numbers and function via administration of antibodies, cytokines, or dietary interventions, have shown preliminary efficacy. In this section, we discuss the available evidence for Treg-targeted therapies to modulate intestinal disorders.
9.7.1. Treg Infusion
Purity, homing ability, antigen-specificity, and survival of Treg cells all need to be carefully considered during the design of therapies involving Treg infusion. Varying in vitro protocols are used to obtain sufficient numbers of Tregs to be used in immunotherapy. The expansion of polyclonal FoxP3+ Treg isolated from peripheral blood and the differentiation of Tr1 from human CD4+ T in the presence of IL10 and allogenic monocytes are the most common methods employed to obtain Tregs. The use of specific markers to properly identify and isolate Treg is critical to prevent contamination with other cells. Also, to appropriately reach the desired site of action, infused Tregs should express homing receptor(s) that guide their migration to the gut where the cells can act directly at the inflamed site or to the draining lymph nodes to modulate T-cell priming. Engineering or selecting the right antigen specificity of the infused Tregs can influence the expansion of these cells in the gut, where TCR engagement by microbial and/or dietary antigens would promote Treg expansion, retention, and function after transfer.
T cells expanded from the population of blood CD25+CD127lowCD45RA+ (natural) Tregs may represent a promising T-cell transfer therapy approach in CD, as they do not convert to Th17, home to the small intestine and suppress the activation of immune cells in the intestinal tissue of CD patients (Canavan et al. 2016). Phase I and II clinical trials to test the safety and efficacy of Treg immunotherapy using cells obtained with this protocol are ongoing (NCT03185000). The upregulation of α4β7 and CCR9, gut homing molecules, and the induction of a transcriptional profile consistent with gut-homing can be enhanced by addition of atRA, usually produced by CD103+ cells in the gut, during in vitro expansion of human pediatric thymic and adult blood Tregs (Hoeppli et al. 2019). Additionally, the expansion of Tregs from patients with CD in the presence of RAR568, specific agonist for RA receptor A was even more efficient than atRA in inducing α4β7 (Goldberg et al. 2019). New approaches aim to generate antigen-specific Tregs, which would decrease the risk of a non-specific inhibition, allow the use of lower numbers of Tregs and increase the chance of Treg expansion in the gut. As proof of principle, in a phase I/IIa clinical study in 20 patients with refractory CD, a reduction of 40% in Crohn’s Disease Activity Index (CDAI) was observed after treatment with single-injection of Tr1 ovalbumin-specific Tregs in response to ovalbumin ingestion (Desreumaux et al. 2012).
In patients with GVHD, tTreg infusion was shown to be well tolerated with no toxicities reported and improved clinical outcomes if injected alone or in conjunction with other anti-GVHD therapies (Duggleby et al. 2018). More recently, a study in mice showed that treatment with selective inhibitor of bromodomain and extra-terminal (BET) proteins, which reduce pro-inflammatory cytokine production, can be successfully combined with Treg expansion therapy for the treatment of GVHD (Copsel et al. 2018).
The field of NEC is perhaps less advanced both in the available knowledge of Treg function and their potential use as preventative therapy. One study has reported that administration of Tregs in a mouse model of NEC has attenuated the severity of NEC by limiting the immune response, also indicating the potential of Tregs in controlling human NEC (Dingle et al. 2013). Additionally, in very low birth weight (VLBW) neonates fed with lactoferrin (LF), the major protein in mammalian milk, prevention of NEC has correlated with increased levels of circulating FoxP3+ Tregs (Akin et al. 2014).
9.7.2. Promoting the Number and Function of Endogenous Tregs
Indirect interventions to affect the gut Treg population represent viable approaches aimed to maximize Treg immunosuppression in vivo. While majority of the available data comes from preclinical models, some therapies have found their way into ongoing clinical trials and are discussed in this section.
9.7.2.1. Targeting Cytokines and Inflammatory Pathways
Treatments to improve Treg population based on the administration of cytokines known to promote their survival and expansion or inhibition of pro-inflammatory cytokines known to promote Treg dysfunction/cell death have been explored in several studies.
Administration of low doses of IL2 preferentially activates Treg cells due to their abundant expression of CD25, avoiding its unspecific action on other immune cells expressing lower levels of CD25. Low dose of IL2 promotes proliferation, thymic export, and resistance to apoptosis among FoxP3+ Tregs (Matsuoka et al. 2013). In a phase I study, patients with active chronic GVHD (cGVHD) refractory to glucocorticoid treatment treated daily with low-dose IL2, augmented Tregs and demonstrated clinical improvement (Koreth et al. 2011). In a phase II trial, 61% of the patients had a clinical response at multiple cGVHD sites, including gastrointestinal tract. During 2 years of extended IL2 therapy, both the clinical response and Treg cell immune response persisted (Koreth et al. 2016). Currently, a clinical trial phase II is recruiting patients to study the induction of Tregs by low-dose IL2 in CD and UC (NCT01988506). Other soluble factor beside IL2 can be used in therapies aimed at Treg expansion. In a mouse model of cGVHD, granulocyte-macrophage colony-stimulating factor (GM-CSF) therapy increased the number CD4+CD8− DCs, expanded Tregs, and protected against the development of skin GVHD (Hotta et al. 2019). The potential of GM-CSF therapy for the recovery of intestinal homeostasis is yet to be addressed in future studies.
AMG714, a neutralizing anti-IL15 monoclonal antibody, in a clinical phase IIa study in patients with refractory coeliac disease was safe, and improved the clinical symptoms even though it did not prevent mucosal injury after gluten challenge, particularly diarrhea in patients who underwent gluten challenge (Lahdeaho et al. 2019; Cellier et al. 2019). Although not directly studied in clinical trials, IL15 blockade would be expected to restore TGFβ signaling in the LP immune cells and promote the local Treg population.
Restoring TGFβ sensitivity has also been an active area of research and pharmacologic developments. The oral administration of SMAD7 antisense oligonucleotide, GED-0301 (Morgensen; Celgene), was shown safe and effective in double-blind, placebo-controlled trials (Monteleone et al. 2015, 2016; Feagan et al. 2018), although a more recent clinical trial with CD patients failed to show a benefit over placebo (Sands et al. 2019).
Several studies in mice had shown that therapy that promotes IL33 signaling in FoxP3+ Tregs with either rIL33 or sST2-blocking antibody showed the potential to be effective in models of exacerbated intestinal inflammation. The response to such treatments may be contextual, as the intestinal inflammation could be ameliorated if rIL33 was administered during chronic phases of intestinal inflammation (Grobeta et al. 2012), but was exacerbated in acute settings (Oboki et al. 2010). The latter may be attributed to the role of IL33 as alarmin, instigating a pro-inflammatory response in the gut. On the other hand, in chronic stages of the intestinal inflammation, rIL33 could promote tissue homeostasis and healing. In a model of CD, the protection promoted by rIL-33 was mediated by the induction of FoxP3+ Treg expansion and CD103+ DCs in the gut (Duan et al. 2012). The same way, ST2+ Treg expansion during rIL33 pre-conditioning therapy or blocking sST2 during peri-transplant period, decreased GVHD severity and mortality (Matta et al. 2016) (Zhang et al. 2015). One study in patients with celiac disease showed even higher levels of IL33 and sST2 in serum and mucosal samples of celiac patients than in patients with Crohn’s disease, which suggests that the blocking sST2 therapy approach could also have a favorable outcome in celiac disease patients (Lopez-Casado et al. 2017).
Inhibition of inflammatory pathways may also improve Treg survival in the gut. For example, the anti-TNFα therapy has shown to reverse FoxP3+ Treg apoptosis in IBD patients (Li et al. 2015). Blocking antibody against OX40L-OX40, promoted Treg responses, such as increased IL10-producing CD4+ T cells and the frequency of CD25+Foxp3+CD127loCTLA4+CCR4+ Tregs, reduced T-cell infiltration in various sites of inflammation including the gut, and reduced the severity of the GVHD in immunodeficient mice transplanted with human peripheral blood mononuclear cells (Tripathi et al. 2019).
9.7.2.2. Mesenchymal Stem Cell (MSC) Infusion
Mesenchymal Stem Cells (MSC) are multipotent non-hematopoietic cells present in different tissues, which due to their immunomodulatory characteristics are considered as new therapeutic agents in the cell-based therapy of IBD, NEC, and GVHD. Different tissues were used as the source of MSCs in research studies, including bone marrow, amniotic fluid, umbilical cord, and placenta, where these cells are typically identified based on the expression of markers CD73, CD90, and CD105, and lack of CD34, CD45, CD11b, CD14, CD19, or CD79a (Drucker et al. 2018; Chen et al. 2013). Recently published studies emphasized the crucial importance of MSC for the attenuation of colonic inflammation mainly through the promotion of M2 macrophage lineage (Giri et al. 2020), induction of intestinal epithelial repair (Gong et al. 2016), and the expansion of colonic Treg population (Vicente-Suarez et al. 2015). The effects of MSCs on macrophage-mediated mucosal repair involves polarization of IL10+ tissue macrophages via CCL2 and CXCL12 (Giri et al. 2020). In Treg generation, MSCs can either act directly by delivering sphingosine 1-phosphate (S1P) to CD4+ T cells and promoting FoxP3-expressing and IL10-producing Tregs (Li et al. 2019). Indirectly, MSCs can promote a regulatory phenotype in DCs, associated with high production of IDO1 (Zhang et al. 2018), suppression of expansion of inflammatory Th1 and Th17 cells, and favoring of conversion to CD4+CD25+FoxP3+ Tregs (Harrell et al. 2018; Zhang et al. 2017).
Cell-free MSC-based therapy have also been postulated as potentially safer. Conditioned medium containing regulatory soluble mediators and exosomes secreted by MSCs mimicked the ability of the MSCs to increase total number of IL10- and TGFβ-producing Tregs in injured tissues and peripheral lymph organs of mice with autoimmune and chronic inflammatory diseases, including IBD (Heidari et al. 2018).
Thus far, MSC infusion in humans has been reported to be safe over long-term use. The efficacy and diversity of the MSC-based therapy protocols being tested in human intestinal disorders have been extensively revised elsewhere (Gregoire et al. 2017; Moheb-Alian et al. 2016; Gao et al. 2016). The improvement in the intestinal disease outcome after MSC therapy is related to improvement in mucosal frequency of FoxP3+ Tregs. Allogeneic bone marrow-derived mesenchymal stromal cell (bmMSC) therapy in perianal Crohn’ s disease fistulas resulted in closure at week 24 and remained closed after 4 years (Barnhoorn et al. 2020). Ciccocioppo et al. showed an increased percentage of circulating and mucosal Tregs in CD patients after MSC therapy (Ciccocioppo et al. 2011). The use of MSC as adjuvant therapy combined with conventional pharmacological approaches has also been considered. The combination of MSC with anti-cytokine therapy to treat CD patients with perianal lesions showed significant improvement in comparison to the control group receiving cell therapy only (Knyazev et al. 2018). Neutralizing TNFα and IFNγ during MSC-based therapy may increase their efficacy by removing the ability of these two cytokines to impair the pro-repair effects of MSCs (Hu et al. 2019) and increase Treg generation/viability in the inflamed intestine (Visperas et al. 2014) (Veltkamp et al. 2011).
Several trials in safety and efficacy of MSC therapy are ongoing in both IBD and GHVD (clinicaltrial.gov). The results of this novel therapeutic approach and in-depth information of how its findings relate to Treg improvement in the intestinal lamina propria are awaited by the scientific community.
9.7.2.3. Induction of Tregs by Microbiota
In humans and mice, deviation in gut microbial composition from homeostatic condition, referred to as dysbiosis, is associated with intestinal immune disorders, including NEC (Denning and Prince 2018), celiac disease (Girbovan et al. 2017), and IBD (Ni et al. 2017). In patients with GVHD, the conditioning regimen and medications given to patients undergoing allo-HCT reduce the microbial diversity and disturb the homeostatic crosstalk between the microbiome and the host immune system (Hidaka et al. 2018). However, the direct causal relationship between dysbiosis and human intestinal disorders is not straightforward. While it may be possible that dysbiosis arises as a consequence of the inflammatory milieu in the gut, it is also well established that transfer of dysbiosis community can increase susceptibility to and severity of experimental colitis (Harrison et al. 2018; Gerassy-Vainberg et al. 2018; Garrett et al. 2007). Despite that, research into fecal microbiome transplant (FMT) or next-generation probiotics and preclinical studies with mouse models of intestinal inflammation show promise.
In humans, FMT from healthy individuals to subjects with active CD have successfully increased intestinal microbial diversity and mucosal CD4+CD25+CD127low Tregs (Vaughn et al. 2016). However, clinical studies of FMT in active IBD, especially CD, brought mixed results, with notable cases of disease exacerbation attributed to the introduction of novel antigens to an already overreactive immune system and/or increasing bacterial load (Sarrabayrouse et al. 2020; Browne and Kelly 2017).
Administration of selected bacterial species or micro-communities derived from the human gut microbiota offers a promising therapeutic alternative to FMT for the prevention and/or treatment of dysregulated intestinal immune responses. Bacterial species from strains clusters IV, XIVa, and XVIII of Clostridia (Narushima et al. 2014), Bacteroides fragilis (Round and Mazmanian 2010) and Akkermansia muciniphila (Zhai et al. 2019) have been reported to promote Tregs in murine colon, as was described in more detail in Sect. 9.4.3. Short-chain fatty acids (SCFAs) such as butyrate and propionate produced by Clostridia through fermentation of mainly undigested dietary carbohydrates are the main mediators of Treg induction by this bacterial population. This aspect of Treg regulation was also discussed in more detail in Sect. 9.4.3. With the notable exception of NEC, where the relative abundance of Clostridia clusters IV, XIVa is increased as compared to healthy infants (Brower-Sinning et al. 2014), these bacterial groups are generally decreased in the intestine under inflammatory conditions such as IBD and celiac disease (Narushima et al. 2014; Sanchez et al. 2010). Their reduction may contribute to intestinal immune dysregulation, and replenishment may offer a therapeutic strategy.
Clostridium F. prausnitzii can induce an immunoregulatory phenotype in human dendritic cells, characterized by the expression of IL10, IL27, CD39, IDO1, and PDL-1 and inhibition of inflammatory mediator production in response to TLR4 stimulation, and ultimately promotes colonic Treg development (Alameddine et al. 2019).
B. fragilis colonization in mice was found to reduce colitis and inflammation-associated colon cancer in mice in a mechanism dependent on the production on PSA and TLR2 signaling (Lee et al. 2018), and promoted circulating Tregs, alleviating intestinal inflammation (Tan et al. 2019). PSA produced by B. fragilis-derived PSA (but no other TRL2 ligands) stimulated FoxP3+ Tregs while suppressing intestinal Th17 generation. IL17 suppression by PSA is a critical mechanism by which B. fragilis associates with its host and promotes mucosal colonic niche and immune tolerance (Round et al. 2011). The first study in B. fragilis PSA induction of human Tregs showed that PSA presentation by total APC induced human peripheral CD4+ T differentiation into IL10-producing Tr1 cells (Kreisman and Cobb 2011). Later, Telesford et al. showed that PSA promoted frequency and function in human IL10-producing CD39+Foxp3+ Tregs in the presence of DCs through HLA-DR, CD86, CD40, and PD-L1 and enhanced Treg suppressive function (Telesford et al. 2015). Therefore, the presence and specific phenotype of the APC is required to prime CD4+ T cells with PSA to promote differentiation into one of the two Treg subtypes. IBD patients have a significantly lower relative abundance of B. fragilis actively producing PSA, thus not just overall decrease of B. fragilis but also a reduction in PSA production by this species may contribute to the breakdown of the intestinal homeostasis in IBD patients (Blandford et al. 2019).
Akkermansia muciniphila, a mucin-degrading and abundant member of a healthy microbiota, is reduced in IBD (Lopez-Siles et al. 2018; Earley et al. 2019). It is primarily localized in the transverse and descending colon, where its abundance depends on the mucus thickness and is affected by the immune status of the host (Van den Abbeele et al. 2010). A. muciniphila administration in mice modulated the intestinal inflammation and ameliorated acute colitis (Bian et al. 2019). Its beneficial effect in chronic colitis was associated with an induction of Treg differentiation (Zhai et al. 2019). The role of A. muciniphila in preventing intestinal disorders has to be addressed with caution because in contrast to the findings in colitis, expansion of A. muciniphila in mice with GVHD was associated with loss of the colonic mucus layer and compromised intestinal barrier function (Shono et al. 2016).
There is an accumulating evidence that restoration of intestinal flora by classical probiotic bacteria also generates FoxP3 Tregs in the gut, making the probiotic consumption an interesting approach to induce this cell population in patients with intestinal disorders. Commonly probiotic mixture contains Bifidobacterium spp., Lactobacillus spp., and Streptococcus thermophilus. The beneficial impact of probiotics on intestinal homeostasis has been shown in many studies (Abraham and Quigley 2017; Cristofori et al. 2018) (Patel and Underwood 2018; Andermann et al. 2016). For instance, L. fermentum, L. acidophilus, and L. casei Lbs2 protect from experimental colitis, through the induction of Treg responses (Jang et al. 2019; Park et al. 2018; Thakur et al. 2016). Treg promotion by Lactobacilli is usually mediated by induction of a regulatory phenotype in the intestinal DCs (Mikulic et al. 2017). Furthermore, Bifidobacteria and S. thermophilus stimulated significant concentrations of TGFβ in human PBMC (Donkor et al. 2012). Oral consumption of Bifidobacterium infantis induces FoxP3 expression and enhances IL10 and TGFβ production in the mouse colon (Zhou et al. 2019). Although limited clinical trials suggest a promising efficacy of classical probiotic strains and their combinations in ulcerative colitis, there remains a lot of skepticism in the IBD field regarding the efficacy of such approaches in Crohn’s disease (Guandalini and Sansotta 2019).
9.7.2.4. Dietary Vitamins
Vitamins are organic dietary compounds with significant impact on the gut mucosal immune function. As we described in more detail in Sect. 9.4.4., vitamins A and D provide important signaling cues for the generation and survival of intestinal Foxp3+ Tregs (Huang et al. 2018; Fakhoury et al. 2020). Retinoic acid (RA), product of vit A degradation by RALDH2 expressed by CD103+ DCs in the intestine, has a large spectrum of modulatory effects on Tregs. Such pivotal role of vitamin A metabolites on intestinal Foxp3+ Tregs and homeostasis is illustrated by the findings that vitamin A deficiency exacerbates experimental colitis (Reifen et al. 2002) and supplementation of vitamin A results in decreased inflammatory markers in NEC patients (Xiao et al. 2018). Therefore, administration of vitamin A/retinoic acid in clinical patients with outcomes related to intestinal inflammation may stimulate FoxP3+ Tregs. A clinical trial on the efficacy of high doses of vitamin A to prevent gastrointestinal GVHD out of the Ohio State University Comprehensive Cancer Center is in early stages (NCT03719092), and the outcome of this therapeutic approach may inform other intestinal disorders going forward.
Along with vitamin A, vitamin D is another potent immunomodulator that affects T cells directly and indirectly (Cantorna et al. 2015, 2019). We have already discussed the effects of vitamin D on Treg biology earlier in this chapter. In IBD patients, VDR polymorphisms have been associated with higher disease risk and increased morbidity (Eloranta et al. 2011). Vitamin D deficiency is commonly observed in IBD patients, and association between vitamin D status and the extent of inflammation has been reported by several studies (Jorgensen et al. 2013; Gubatan et al. 2019; Fletcher et al. 2019; Burrelli Scotti et al. 2019; Law et al. 2019). In a double-blind randomized controlled trial, where 90 mild-to-moderate UC patients were administered one single muscular injection of vitamin D (7.5 mg) and reduced serum concentrations of TNFα, IFNγ, and IL12p70 levels, but it did not affect serum IL4 and IL10 levels. However, no other clinical parameters were evaluated or included in the report (Sharifi et al. 2019). In a murine model of GVHD, vitamin D promoted FoxP3+ Treg generation and slowed the disease progression (Ni et al. 2019). A severe vitamin D deficiency has also been documented in patients with celiac disease (Sulimani 2019) and maternal vitamin D deficiency has been proposed to be a new risk factor for NEC in preterm infants (Cetinkaya et al. 2017). Importantly, vitamin D has pleiotropic effects on the cells of the innate and adaptive immune system and affects intestinal barrier function and gut microbial ecology, thus any beneficial effects of this vitamin/hormone cannot be solely attributed to promotion of Tregs and their immunosuppressive functions.
Finally, dietary plant sterols capable of reducing intestinal inflammation in mice also promote Treg numbers. These compounds are consumed in habitual diets derived from vegetable oils, grain products, nuts, seeds, fruits, and vegetables (te Velde et al. 2015). Fiber-enriched diets were associated with a higher colonic in situ production of total SCFA including butyrate, which promotes the gut Tregs (Rodriguez-Cabezas et al. 2002). Dietary interventions and application of prebiotics that enhance butyrate production, such as 2′-fucosyllactose, may help induce remission in UC without involving additional immune suppression (phase IV clinical trial NCT02345733) or may contribute to the maintenance of remission in pediatric and young adult IBD patients undergoing anti-cytokine therapy (NCT03847467). Collectively, the available data suggests somewhat instinctively that the supplementation with vitamins (or correction of deficiencies) and intake of a balanced diet rich in fruits, grains, and vegetables should be a part of the comprehensive therapy of intestinal inflammatory disorders, and a part of the associated mechanism may be through promotion of intestinal Tregs and immunoregulatory environment in the gut.
9.8. Conclusions
Intestinal Tregs is a complex and heterogenous population of immunosuppressive cells whose development and function are affected (positively and negatively) by an array of factors, both host-intrinsic and extrinsic. They play crucial roles in maintaining the low mucosal inflammatory tone in the gut under continuous antigenic challenge, contribute to the maintenance of intestinal barrier function, keep the immune response to pathogens in check, and promote the resolution of inflammation and mucosal restitution. As such, they represent attractive target of research and therapeutic developments. Although much is known already about their phenotypic and functional features in mice, translating these results to humans has been challenging. A better understanding of human Tregs at mucosal sites, especially in the gut, will help guide better therapeutic options, primary or adjuvant, to treat chronic inflammatory conditions of the gut.
Acknowledgment
We thank Dr. Claudio Bernardazzi M. Azevedo for his assistance with the preparation of figures.
Contributor Information
Vanessa R. Figliuolo da Paz, Department of Pediatrics, University of Arizona, Tucson, AZ, USA.
Deepa R. Jamwal, Department of Pediatrics, University of Arizona, Tucson, AZ, USA
Pawel R. Kiela, Department of Pediatrics, University of Arizona, Tucson, AZ, USA; Department of Immunobiology, University of Arizona, Tucson, AZ, USA
References
- Abraham BP, Quigley EMM (2017) Probiotics in inflammatory bowel disease. Gastroenterol Clin N Am 46(4):769–782. 10.1016/j.gtc.2017.08.003 [DOI] [PubMed] [Google Scholar]
- Akesson K, Tompa A, Ryden A, Faresjo M (2015) Low expression of CD39(+)/CD45RA(+) on regulatory T cells (Treg ) cells in type 1 diabetic children in contrast to high expression of CD101(+)/CD129(+) on Treg cells in children with coeliac disease. Clin Exp Immunol 180(1):70–82. 10.nn/cei.12559 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Akin IM, Atasay B, Dogu F, Okulu E, Arsan S, Karatas HD, Ikinciogullari A, Turmen T (2014) Oral lactoferrin to prevent nosocomial sepsis and necrotizing enterocolitis of premature neonates and effect on T-regulatory cells. Am J Perinatol 31(12):1111–1120. 10.1055/s-0034-1371704 [DOI] [PubMed] [Google Scholar]
- Alameddine J, Godefroy E, Papargyris L, Sarrabayrouse G, Tabiasco J, Bridonneau C, Yazdanbakhsh K, Sokol H, Altare F, Jotereau F (2019) Faecalibacterium prausnitzii skews human DC to prime IL10-producing T cells through TLR2/6/JNK signaling and IL-10, IL-27, CD39, and IDO-1 induction. Front Immunol 10:143. 10.3389/fimmu.2019.00143 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alfen JS, Larghi P, Facciotti F, Gagliani N, Bosotti R, Paroni M, Maglie S, Gruarin P, Vasco CM, Ranzani V, Frusteri C, Iseppon A, Moro M, Crosti MC, Gatti S, Pagani M, Caprioli F, Abrignani S, Flavell RA, Geginat J (2018) Intestinal IFN-gamma-producing type 1 regulatory T cells coexpress CCR5 and programmed cell death protein 1 and downregulate IL-10 in the inflamed guts of patients with inflammatory bowel disease. J Allergy Clin Immunol 142(5):1537–1547.e1538. 10.1016/j.jaci.2017.12.984 [DOI] [PubMed] [Google Scholar]
- Allaire JM, Crowley SM, Law HT, Chang SY, Ko HJ, Vallance BA (2018) The intestinal epithelium: central coordinator of mucosal immunity. Trends Immunol 39(9):677–696. 10.1016/j.it.2018.04.002 [DOI] [PubMed] [Google Scholar]
- Allan SE, Crome SQ, Crellin NK, Passerini L, Steiner TS, Bacchetta R, Roncarolo MG, Levings MK (2007) Activation-induced FOXP3 in human T effector cells does not suppress proliferation or cytokine production. Int Immunol 19(4):345–354. 10.1093/intimm/dxm014 [DOI] [PubMed] [Google Scholar]
- Almeida AR, Legrand N, Papiernik M, Freitas AA (2002) Homeostasis of peripheral CD4+ T cells: IL-2R alpha and IL-2 shape a population of regulatory cells that controls CD4+ T cell numbers. J Immunol 169(9):4850–4860. 10.4049/jimmunol.169.9.4850 [DOI] [PubMed] [Google Scholar]
- Andermann TM, Rezvani A, Bhatt AS (2016) Microbiota manipulation with prebiotics and probiotics in patients undergoing stem cell transplantation. Curr Hematol Malig Rep 11(1):19–28. 10.1007/s11899-016-0302-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arpaia N, Campbell C, Fan X, Dikiy S, van der Veeken J, deRoos P, Liu H, Cross JR, Pfeffer K, Coffer PJ, Rudensky AY (2013) Metabolites produced by commensal bacteria promote peripheral regulatory T-cell generation. Nature 504(7480):451–455. 10.1038/nature12726 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Asseman C, Mauze S, Leach MW, Coffman RL, Powrie F (1999) An essential role for interleukin 10 in the function of regulatory T cells that inhibit intestinal inflammation. J Exp Med 190(7):995–1004. 10.1084/jem.190.7.995 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Atarashi K, Nishimura J, Shima T, Umesaki Y, Yamamoto M, Onoue M, Yagita H, Ishii N, Evans R, Honda K, Takeda K (2008) ATP drives lamina propria T(H)17 cell differentiation. Nature 455(7214):808–812. 10.1038/nature07240 [DOI] [PubMed] [Google Scholar]
- Atarashi K, Tanoue T, Shima T, Imaoka A, Kuwahara T, Momose Y, Cheng G, Yamasaki S, Saito T, Ohba Y, Taniguchi T, Takeda K, Hori S, Ivanov II, Umesaki Y, Itoh K, Honda K (2011) Induction of colonic regulatory T cells by indigenous Clostridium species. Science 331(6015):337–341. 10.1126/science.1198469 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Atarashi K, Tanoue T, Oshima K, Suda W, Nagano Y, Nishikawa H, Fukuda S, Saito T, Narushima S, Hase K, Kim S, Fritz JV, Wilmes P, Ueha S, Matsushima K, Ohno H, Olle B, Sakaguchi S, Taniguchi T, Morita H, Hattori M, Honda K (2013) Treg induction by a rationally selected mixture of Clostridia strains from the human microbiota. Nature 500(7461):232–236. 10.1038/nature12331 [DOI] [PubMed] [Google Scholar]
- Babyatsky MW, Rossiter G, Podolsky DK (1996) Expression of transforming growth factors alpha and beta in colonic mucosa in inflammatory bowel disease. Gastroenterology 110(4):975–984. 10.1053/gast.1996.v110.pm8613031 [DOI] [PubMed] [Google Scholar]
- Bacchetta R, Bigler M, Touraine JL, Parkman R, Tovo PA, Abrams J, de Waal Malefyt R, de Vries JE, Roncarolo MG (1994) High levels of interleukin 10 production in vivo are associated with tolerance in SCID patients transplanted with HLA mismatched hematopoietic stem cells. J Exp Med 179(2):493–502. 10.1084/jem.179.2.493 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bamidele AO, Svingen PA, Sagstetter MR, Sarmento OF, Gonzalez M, Braga Neto MB, Kugathasan S, Lomberk G, Urrutia RA, Faubion WA Jr (2019) Disruption of FOXP3-EZH2 interaction represents a pathobiological mechanism in intestinal inflammation. Cell Mol Gastroenterol Hepatol 7(1):55–71. 10.1016/j.jcmgh.2018.08.009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bardel E, Larousserie F, Charlot-Rabiega P, Coulomb-L’Hermine A, Devergne O (2008) Human CD4+ CD25+ Foxp3+ regulatory T cells do not constitutively express IL-35. J Immunol 181(10):6898–6905. 10.4049/jimmunol.181.10.6898 [DOI] [PubMed] [Google Scholar]
- Barnhoorn MC, Wasser M, Roelofs H, Maljaars PWJ, Molendijk I, Bonsing BA, Oosten LEM, Dijkstra G, van der Woude CJ, Roelen DL, Zwaginga JJ, Verspaget HW, Fibbe WE, Hommes DW, Peeters K, van der Meulen-de Jong AE (2020) Long-term evaluation of allogeneic bone marrow-derived mesenchymal stromal cell therapy for Crohn’s disease perianal fistulas. J Crohns Colitis 14(1):64–70. 10.1093/ecco-jcc/jjz116 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barthlott T, Moncrieffe H, Veldhoen M, Atkins CJ, Christensen J, O’Garra A, Stockinger B (2005) CD25+ CD4+ T cells compete with naive CD4+ T cells for IL-2 and exploit it for the induction of IL-10 production. Int Immunol 17(3):279–288. 10.1093/intimm/dxh207 [DOI] [PubMed] [Google Scholar]
- Bazewicz CG, Dinavahi SS, Schell TD, Robertson GP (2019) Aldehyde dehydrogenase in regulatory T-cell development, immunity and cancer. Immunology 156(1):47–55. 10.11n/imm.13016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ben Ahmed M, Belhadj Hmida N, Moes N, Buyse S, Abdeladhim M, Louzir H, Cerf-Bensussan N (2009) IL-15 renders conventional lymphocytes resistant to suppressive functions of regulatory T cells through activation of the phosphatidylinositol 3-kinase pathway. J Immunol 182(11):6763–6770. 10.4049/jimmunol.0801792 [DOI] [PubMed] [Google Scholar]
- Benahmed M, Meresse B, Arnulf B, Barbe U, Mention JJ, Verkarre V, Allez M, Cellier C, Hermine O, Cerf-Bensussan N (2007) Inhibition of TGF-beta signaling by IL-15: a new role for IL-15 in the loss of immune homeostasis in celiac disease. Gastroenterology 132(3):994–1008. 10.1053/j.gastro.2006.12.025 [DOI] [PubMed] [Google Scholar]
- Bennett CL, Christie J, Ramsdell F, Brunkow ME, Ferguson PJ, Whitesell L, Kelly TE, Saulsbury FT, Chance PF, Ochs HD (2001) The immune dysregulation, polyendocrinopathy, enteropathy, X-linked syndrome (IPEX) is caused by mutations of FOXP3. Nat Genet 27(1):20–21. 10.1038/83713 [DOI] [PubMed] [Google Scholar]
- Betts G, Jones E, Junaid S, El-Shanawany T, Scurr M, Mizen P, Kumar M, Jones S, Rees B, Williams G, Gallimore A, Godkin A (2012) Suppression of tumour-specific CD4(+) T cells by regulatory T cells is associated with progression of human colorectal cancer. Gut 61(8):1163–1171. 10.1136/gutjnl-2011-300970 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bian X, Wu W, Yang L, Lv L, Wang Q, Li Y, Ye J, Fang D, Wu J, Jiang X, Shi D, Li L (2019) Administration of Akkermansia muciniphila ameliorates dex-tran sulfate sodium-induced ulcerative colitis in mice. Front Microbiol 10:2259. 10.3389/fmicb.2019.02259 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Birebent B, Lorho R, Lechartier H, de Guibert S, Alizadeh M, Vu N, Beauplet A, Robillard N, Semana G (2004) Suppressive properties ofhuman CD4+CD25 + regulatory T cells are dependent on CTLA-4 expression. Eur J Immunol 34(12):3485–3496. 10.1002/eji.200324632 [DOI] [PubMed] [Google Scholar]
- Blandford LE, Johnston EL, Sanderson JD, Wade WG, Lax AJ (2019) Promoter orientation of the immunomodulatory Bacteroides fragilis capsular polysaccharide A (PSA) is off in individuals with inflammatory bowel disease (IBD). Gut Microbes 10(5):569–577. 10.1080/19490976.2018.1560755 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blatner NR, Bonertz A, Beckhove P, Cheon EC, Krantz SB, Strouch M, Weitz J, Koch M, Halverson AL, Bentrem DJ, Khazaie K (2010) In colorectal cancer mast cells contribute to systemic regulatory T-cell dysfunction. Proc Natl Acad Sci USA 107(14):6430–6435. 10.1073/pnas.0913683107 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blatner NR, Mulcahy MF, Dennis KL, Scholtens D, Bentrem DJ, Phillips JD, Ham S, Sandall BP, Khan MW, Mahvi DM, Halverson AL, Stryker SJ, Boller AM, Singal A, Sneed RK, Sarraj B, Ansari MJ, Oft M, Iwakura Y, Zhou L, Bonertz A, Beckhove P, Gounari F, Khazaie K (2012) Expression of RORgammat marks a pathogenic regulatory T cell subset in human colon cancer. Sci Transl Med 4(164):164ra159. 10.1126/scitranslmed.3004566 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boga S, Alkim H, Koksal AR, Ozagari AA, Bayram M, Tekin Neijmann S, Sen I, Alkim C (2016) Serum ST2 in inflammatory bowel disease: a potential biomarker for disease activity. J Investig Med 64(5):1016–1024. 10.1136/jim-2016-000062 [DOI] [PubMed] [Google Scholar]
- Bonertz A, Weitz J, Pietsch DH, Rahbari NN, Schlude C, Ge Y, Juenger S, Vlodavsky I, Khazaie K, Jaeger D, Reissfelder C, Antolovic D, Aigner M, Koch M, Beckhove P (2009) Antigen-specific Tregs control T cell responses against a limited repertoire of tumor antigens in patients with colorectal carcinoma. J Clin Invest 119(11):3311–3321. 10.1172/JCI39608 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Borsellino G, Kleinewietfeld M, Di Mitri D, Sternjak A, Diamantini A, Giometto R, Hopner S, Centonze D, Bernardi G, Dell’Acqua ML, Rossini PM, Battistini L, Rotzschke O, Falk K (2007) Expression of ectonucleotidase CD39 by Foxp3+ Treg cells: hydrolysis of extracellular ATP and immune suppression. Blood 110(4):1225–1232. 10.1182/blood-2006-12-064527 [DOI] [PubMed] [Google Scholar]
- Bouzid D, Amouri A, Fourati H, Marques I, Abida O, Tahri N, Goncalves CP, Masmoudi H (2013) Polymorphisms in the IL2RA and IL2RB genes in inflammatory bowel disease risk. Genet Test Mol Biomarkers 17(11):833–839. 10.1089/gtmb.2013.0291 [DOI] [PubMed] [Google Scholar]
- Brower-Sinning R, Zhong D, Good M, Firek B, Baker R, Sodhi CP, Hackam DJ, Morowitz MJ (2014) Mucosa-associated bacterial diversity in necrotizing enterocolitis. PLoS One 9(9):e105046. 10.1371/journal.pone.0105046 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Browne AS, Kelly CR (2017) Fecal transplant in inflammatory bowel disease. Gastroenterol Clin N Am 46(4):825–837. 10.1016/j.gtc.2017.08.005 [DOI] [PubMed] [Google Scholar]
- Brunkow ME, Jeffery EW, Hjerrild KA, Paeper B, Clark LB, Yasayko SA, Wilkinson JE, Galas D, Ziegler SF, Ramsdell F (2001) Disruption of a new forkhead/winged-helix protein, scurfin, results in the fatal lymphoproliferative disorder of the scurfy mouse. Nat Genet 27(1):68–73. 10.1038/83784 [DOI] [PubMed] [Google Scholar]
- Bueter M, Gasser M, Schramm N, Lebedeva T, Tocco G, Gerstlauer C, Grimm M, Nichiporuk E, Thalheimer A, Thiede A, Meyer D, Benichou G, Waaga-Gasser AM (2006) T-cell response to p53 tumor-associated antigen in patients with colorectal carcinoma. Int J Oncol 28(2):431–438 [PubMed] [Google Scholar]
- Burrelli Scotti G, Afferri MT, De Carolis A, Vaiarello V, Fassino V, Ferrone F, Minisola S, Nieddu L, Vernia P (2019) Factors affecting vitamin D deficiency in active inflammatory bowel diseases. Dig Liver Dis 51(5):657–662. 10.1016/j.dld.2018.11.036 [DOI] [PubMed] [Google Scholar]
- Campbell C, Dikiy S, Bhattarai SK, Chinen T, Matheis F, Calafiore M, Hoyos B, Hanash A, Mucida D, Bucci V, Rudensky AY (2018) Extrathymically generated regulatory T cells establish a niche for intestinal borderdwelling Bacteria and affect physiologic metabolite balance. Immunity 48(6):1245–1257. e1249. 10.1016/j.immuni.2018.04.013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Campi G, Crosti M, Consogno G, Facchinetti V, Conti-Fine BM, Longhi R, Casorati G, Dellabona P, Protti MP (2003) CD4(+) T cells from healthy subjects and colon cancer patients recognize a carcinoembryonic antigen-specific immunodominant epitope. Cancer Res 63(23):8481–8486 [PubMed] [Google Scholar]
- Canavan JB, Scotta C, Vossenkamper A, Goldberg R, Elder MJ, Shoval I, Marks E, Stolarczyk E, Lo JW, Powell N, Fazekasova H, Irving PM, Sanderson JD, Howard JK, Yagel S, Afzali B, MacDonald TT, Hernandez-Fuentes MP, Shpigel NY, Lombardi G, Lord GM (2016) Developing in vitro expanded CD45RA+ regulatory T cells as an adoptive cell therapy for Crohn’s disease. Gut 65(4):584–594. 10.1136/gutjnl-2014-306919 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cantorna MT, Snyder L, Lin YD, Yang L (2015) Vitamin D and 1,25(OH)2D regulation of T cells. Nutrients 7(4):3011–3021. 10.3390/nu7043011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cantorna MT, Snyder L, Arora J (2019) Vitamin A and vitamin D regulate the microbial complexity, barrier function, and the mucosal immune responses to ensure intestinal homeostasis. Crit Rev Biochem Mol Biol 54(2):184–192. 10.1080/10409238.2019.1611734 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Caplan MS, Sun XM, Hseuh W, Hageman JR (1990) Role of platelet activating factor and tumor necrosis factor-alpha in neonatal necrotizing enterocolitis. J Pediatr 116(6):960–964. 10.1016/s0022-3476(05)80661-4 [DOI] [PubMed] [Google Scholar]
- Cebula A, Seweryn M, Rempala GA, Pabla SS, McIndoe RA, Denning TL, Bry L, Kraj P, Kisielow P, Ignatowicz L (2013) Thymus-derived regulatory T cells contribute to tolerance to commensal microbiota. Nature 497(7448):258–262. 10.1038/nature12079 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cederbom L, Hall H, Ivars F (2000) CD4+CD25+ regulatory T cells down-regulate co-stimulatory molecules on antigen-presenting cells. Eur J Immunol 30(6):1538–1543. [DOI] [PubMed] [Google Scholar]
- Cellier C, Bouma G, van Gils T, Khater S, Malamut G, Crespo L, Collin P, Green PHR, Crowe SE, Tsuji W, Butz E, Cerf-Bensussan N, Macintyre E, Parnes JR, Leon F, Hermine O, Mulder CJ, Investigators R-ISG (2019) Safety and efficacy of AMG 714 in patients with type 2 refractory coeliac disease: a phase 2a, randomised, double-blind, placebo-controlled, parallel-group study. Lancet Gastroenterol Hepatol 4(12):960–970. 10.1016/S2468-1253(19)30265-1 [DOI] [PubMed] [Google Scholar]
- Cetinkaya M, Erener-Ercan T, Kalayci-Oral T, Babayigit A, Cebeci B, Semerci SY, Buyukkale G (2017) Maternal/neonatal vitamin D deficiency: a new risk factor for necrotizing enterocolitis in preterm infants? J Perinatol 37(6):673–678. 10.1038/jp.2017.18 [DOI] [PubMed] [Google Scholar]
- Chai JN, Peng Y, Rengarajan S, Solomon BD, Ai TL, Shen Z, Perry JSA, Knoop KA, Tanoue T, Narushima S, Honda K, Elson CO, Newberry RD, Stappenbeck TS, Kau AL, Peterson DA, Fox JG, Hsieh CS (2017) Helicobacter species are potent drivers of colonic T cell responses in homeostasis and inflammation. Sci Immunol 2(13):eaal5068. 10.1126/sciimmunol.aal5068 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chang LY, Lin YC, Mahalingam J, Huang CT, Chen TW, Kang CW, Peng HM, Chu YY, Chiang JM, Dutta A, Day YJ, Chen TC, Yeh CT, Lin CY (2012) Tumor-derived chemokine CCL5 enhances TGF-beta-mediated killing of CD8(+) T cells in colon cancer by T-regulatory cells. Cancer Res 72(5):1092–1102. 10.1158/0008-5472.CAN-11-2493 [DOI] [PubMed] [Google Scholar]
- Chao G, Li X, Ji Y, Zhu Y, Li N, Zhang N, Feng Z, Niu M (2018) CTLA-4 regulates T follicular regulatory cell differentiation and participates in intestinal damage caused by spontaneous autoimmunity. Biochem Biophys Res Commun 505(3):865–871. 10.1016/j.bbrc.2018.09.182 [DOI] [PubMed] [Google Scholar]
- Chaput N, Louafi S, Bardier A, Charlotte F, Vaillant JC, Menegaux F, Rosenzwajg M, Lemoine F, Klatzmann D, Taieb J (2009) Identification of CD8 +CD25+Foxp3+ suppressive T cells in colorectal cancer tissue. Gut 58(4):520–529. 10.1136/gut.2008.158824 [DOI] [PubMed] [Google Scholar]
- Chaudhry A, Samstein RM, Treuting P, Liang Y, Pils MC, Heinrich JM, Jack RS, Wunderlich FT, Burning JC, Muller W, Rudensky AY (2011) Interleukin-10 signaling in regulatory T cells is required for suppression of Th17 cell-mediated inflammation. Immunity 34(4):566–578. 10.1016/j.immuni.2011.03.018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen J, Chen Z (2014) The effect of immune microenvironment on the progression and prognosis of colorectal cancer. Med Oncol 31(8):82. 10.1007/s12032-014-0082-9 [DOI] [PubMed] [Google Scholar]
- Chen W, Jin W, Hardegen N, Lei KJ, Li L, Marinos N, McGrady G, Wahl SM (2003) Conversion of peripheral CD4+CD25-naive T cells to CD4+CD25+ regulatory T cells by TGF-beta induction of transcription factor Foxp3. J Exp Med 198(12):1875–1886. 10.1084/jem.20030152 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen M, Su W, Lin X, Guo Z, Wang J, Zhang Q, Brand D, Ryffel B, Huang J, Liu Z, He X, Le AD, Zheng SG (2013) Adoptive transfer of human gingiva-derived mesenchymal stem cells ameliorates collagen-induced arthritis via suppression of Th1 and Th17 cells and enhancement of regulatory T cell differentiation. Arthritis Rheum 65(5):1181–1193. 10.1002/art.37894 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen J, He Y, Tu L, Duan L (2020) Dual immune functions of IL-33 in inflammatory bowel disease. Histol Histopathol 35(2):137–146. 10.14670/HH-18-149 [DOI] [PubMed] [Google Scholar]
- Chinen T, Kannan AK, Levine AG, Fan X, Klein U, Zheng Y, Gasteiger G, Feng Y, Fontenot JD, Rudensky AY (2016) An essential role for the IL-2 receptor in Treg cell function. Nat Immunol 17(11):1322–1333. 10.1038/ni.3540 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cianci R, Cammarota G, Frisullo G, Pagliari D, Ianiro G, Martini M, Frosali S, Plantone D, Damato V, Casciano F, Landolfi R, Paola Batocchi A, Pandolfi F (2012) Tissue-infiltrating lymphocytes analysis reveals large modifications of the duodenal “immunological niche” in coeliac disease after gluten-free diet. Clin Transl Gastroenterol 3:e28. 10.1038/ctg.2012.22 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ciccocioppo R, Bernardo ME, Sgarella A, Maccario R, Avanzini MA, Ubezio C, Minelli A, Alvisi C, Vanoli A, Calliada F, Dionigi P, Perotti C, Locatelli F, Corazza GR (2011) Autologous bone marrow-derived mesenchymal stromal cells in the treatment of fistulising Crohn’s disease. Gut 60(6):788–798. 10.1136/gut.2010.214841 [DOI] [PubMed] [Google Scholar]
- Clarke SL, Betts GJ, Plant A, Wright KL, El-Shanawany TM, Harrop R, Torkington J, Rees BI, Williams GT, Gallimore AM, Godkin AJ (2006) CD4+CD25 +FOXP3+ regulatory T cells suppress anti-tumor immune responses in patients with colorectal cancer. PLoS One 1:e129. 10.1371/journal.pone.0000129 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Collison LW, Workman CJ, Kuo TT, Boyd K, Wang Y, Vignali KM, Cross R, Sehy D, Blumberg RS, Vignali DA (2007) The inhibitory cytokine IL-35 contributes to regulatory T-cell function. Nature 450(7169):566–569. 10.1038/nature06306 [DOI] [PubMed] [Google Scholar]
- Cong Y, Feng T, Fujihashi K, Schoeb TR, Elson CO (2009) A dominant, coordinated T regulatory cell-IgA response to the intestinal microbiota. Proc Natl Acad Sci U S A 106(46):19256–19261. 10.1073/pnas.0812681106 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cook L, Munier CML, Seddiki N, van Bockel D, Ontiveros N, Hardy MY, Gillies JK, Levings MK, Reid HH, Petersen J, Rossjohn J, Anderson RP, Zaunders JJ, Tye-Din JA, Kelleher AD (2017) Circulating gluten-specific FOXP3(+)CD39(+) regulatory T cells have impaired suppressive function in patients with celiac disease. J Allergy Clin Immunol 140(6):1592–1603.e1598. 10.1016/j.jaci.2017.02.015 [DOI] [PubMed] [Google Scholar]
- Cook L, Stahl M, Han X, Nazli A, MacDonald KN, Wong MQ, Tsai K, Dizzell S, Jacobson K, Bressler B, Kaushic C, Vallance BA, Steiner TS, Levings MK (2019) Suppressive and gut-reparative functions of human type 1 T regulatory cells. Gastroenterology 157(6):1584–1598. 10.1053/j.gastro.2019.09.002 [DOI] [PubMed] [Google Scholar]
- Coombes JL, Siddiqui KR, Arancibia-Carcamo CV, Hall J, Sun CM, Belkaid Y, Powrie F (2007) A functionally specialized population of mucosal CD103+ DCs induces Foxp3+ regulatory T cells via a TGF-beta and retinoic acid-dependent mechanism. J Exp Med 204(8):1757–1764. 10.1084/jem.20070590 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Copsel SN, Lightbourn CO, Barreras H, Lohse I, Wolf D, Bader CS, Manov J, Kale BJ, Shah D, Brothers SP, Perez VL, Komanduri KV, Wahlestedt C, Levy RB (2018) BET bromodomain inhibitors which permit Treg function enable a combinatorial strategy to suppress GVHD in pre-clinical allogeneic HSCT. Front Immunol 9:3104. 10.3389/fimmu.2018.03104 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cretney E, Xin A, Shi W, Minnich M, Masson F, Miasari M, Belz GT, Smyth GK, Busslinger M, Nutt SL, Kallies A (2011) The transcription factors Blimp-1 and IRF4 jointly control the differentiation and function of effector regulatory T cells. Nat Immunol 12(4):304–311. 10.1038/ni.2006 [DOI] [PubMed] [Google Scholar]
- Cristofori F, Indrio F, Miniello VL, De Angelis M, Francavilla R (2018) Probiotics in celiac disease. Nutrients 10(12):1824. 10.3390/nu10121824 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Curotto de Lafaille MA, Kutchukhidze N, Shen S, Ding Y, Yee H, Lafaille JJ (2008) Adaptive Foxp3+ regulatory T cell-dependent and -independent control of allergic inflammation. Immunity 29(1):114–126. 10.1016/j.immuni.2008.05.010 [DOI] [PubMed] [Google Scholar]
- D’Ambrosio DN, Clugston RD, Blaner WS (2011) Vitamin a metabolism: an update. Nutrients 3(1):63–103. 10.3390/nu3010063 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davidson TS, DiPaolo RJ, Andersson J, Shevach EM (2007) Cutting edge: IL-2 is essential for TGF-beta-mediated induction of Foxp3+ T regulatory cells. J Immunol 178(7):4022–4026. 10.4049/jimmunol.178.7.4022 [DOI] [PubMed] [Google Scholar]
- D’Cruz LM, Klein L (2005) Development and function of agonist-induced CD25+Foxp3+ regulatory T cells in the absence of interleukin 2 signaling. Nat Immunol 6(11):1152–1159. 10.1038/ni1264 [DOI] [PubMed] [Google Scholar]
- De Simone M, Arrigoni A, Rossetti G, Gruarin P, Ranzani V, Politano C, Bonnal RJP, Provasi E, Sarnicola ML, Panzeri I, Moro M, Crosti M, Mazzara S, Vaira V, Bosari S, Palleschi A, Santambrogio L, Bovo G, Zucchini N, Totis M, Gianotti L, Cesana G, Perego RA, Maroni N, Pisani Ceretti A, Opocher E, De Francesco R, Geginat J, Stunnenberg HG, Abrignani S, Pagani M (2016) Transcriptional landscape of human tissue lymphocytes unveils uniqueness of tumor-infiltrating T regulatory cells. Immunity 45(5):1135–1147. 10.1016/j.immuni.2016.10.021 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Denning NL, Prince JM (2018) Neonatal intestinal dysbiosis in necrotizing enterocolitis. Mol Med 24(1):4. 10.1186/s10020-018-0002-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Desreumaux P, Foussat A, Allez M, Beaugerie L, Hebuterne X, Bouhnik Y, Nachury M, Brun V, Bastian H, Belmonte N, Ticchioni M, Duchange A, Morel-Mandrino P, Neveu V, Clerget-Chossat N, Forte M, Colombel JF (2012) Safety and efficacy of antigen-specific regulatory T-cell therapy for patients with refractory Crohn’s disease. Gastroenterology 143(5):1207–1217. e1202. 10.1053/j.gastro.2012.07.116 [DOI] [PubMed] [Google Scholar]
- Dingle BM, Liu Y, Fatheree NY, Min J, Rhoads JM, Tran DQ (2013) FoxP3(+) regulatory T cells attenuate experimental necrotizing enterocolitis. PLoS One 8(12):e82963. 10.1371/journal.pone.0082963 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Donkor ON, Ravikumar M, Proudfoot O, Day SL, Apostolopoulos V, Paukovics G, Vasiljevic T, Nutt SL, Gill H (2012) Cytokine profile and induction of T helper type 17 and regulatory T cells by human peripheral mononuclear cells after microbial exposure. Clin Exp Immunol 167(2):282–295. 10.1111/j.1365-2249.2011.04496.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- Downs-Canner S, Berkey S, Delgoffe GM, Edwards RP, Curiel T, Odunsi K, Bartlett DL, Obermajer N (2017) Suppressive IL-17A(+)Foxp3(+) and ex-Th17 IL-17A (neg)Foxp3(+) Treg cells are a source of tumour-associated Treg cells. Nat Commun 8:14649. 10.1038/ncomms14649 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Drucker NA, McCulloh CJ, Li B, Pierro A, Besner GE, Markel TA (2018) Stem cell therapy in necrotizing enterocolitis: current state and future directions. Semin Pediatr Surg 27(1):57–64. 10.1053/j.sempedsurg.2017.11.011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Du R, Zhao H, Yan F, Li H (2014) IL-17+Foxp3+ T cells: an intermediate differentiation stage between Th17 cells and regulatory T cells. J Leukoc Biol 96(1):39–48. 10.1189/jlb.1RU0114-010RR [DOI] [PubMed] [Google Scholar]
- Duan L, Chen J, Zhang H, Yang H, Zhu P, Xiong A, Xia Q, Zheng F, Tan Z, Gong F, Fang M (2012) Interleukin-33 ameliorates experimental colitis through promoting Th2/Foxp3(+) regulatory T-cell responses in mice. Mol Med 18:753–761. 10.2119/molmed.2011.00428 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dudley E, Hornung F, Zheng L, Scherer D, Ballard D, Lenardo M (1999) NF-kappaB regulates Fas/APO-1/CD95- and TCR- mediated apoptosis of T lymphocytes. Eur J Immunol 29(3):878–886. [DOI] [PubMed] [Google Scholar]
- Duggleby R, Danby RD, Madrigal JA, Saudemont A (2018) Clinical grade regulatory CD4(+) T cells (Tregs): moving toward cellular-based immunomodulatory therapies. Front Immunol 9:252. 10.3389/fimmu.2018.00252 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Duhen T, Duhen R, Lanzavecchia A, Sallusto F, Campbell DJ (2012) Functionally distinct subsets of human FOXP3+ Treg cells that phenotypically mirror effector Th cells. Blood 119(19):4430–4440. 10.1182/blood-2011-11-392324 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dunn GP, Old LJ, Schreiber RD (2004) The immunobiology of cancer immunosurveillance and immunoediting. Immunity 21(2):137–148. 10.1016/j.immuni.2004.07.017 [DOI] [PubMed] [Google Scholar]
- Dwyer KM, Hanidziar D, Putheti P, Hill PA, Pommey S, McRae JL, Winterhalter A, Doherty G, Deaglio S, Koulmanda M, Gao W, Robson SC, Strom TB (2010) Expression of CD39 by human peripheral blood CD4+ CD25+ T cells denotes a regulatory memory phenotype. Am J Transplant 10(11):2410–2420. 10.1111/j.1600-6143.2010.03291.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- Earley H, Lennon G, Balfe A, Coffey JC, Winter DC, O’Connell PR (2019) The abundance of Akkermansia muciniphila and its relationship with sulphated colonic mucins in health and ulcerative colitis. Sci Rep 9(1):15683. 10.1038/s41598-019-51878-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Edinger M, Hoffmann P, Ermann J, Drago K, Fathman CG, Strober S, Negrin RS (2003) CD4+CD25+ regulatory T cells preserve graft-versus-tumor activity while inhibiting graft-versus-host disease after bone marrow transplantation. Nat Med 9(9):1144–1150. 10.1038/nm915 [DOI] [PubMed] [Google Scholar]
- Edwards JP, Hand TW, Morais da Fonseca D, Glass DD, Belkaid Y, Shevach EM (2016) The GARP/latent TGF-beta1 complex on Treg cells modulates the induction of peripherally derived Treg cells during oral tolerance. Eur J Immunol 46(6):1480–1489. 10.1002/eji.201546204 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Egan CE, Sodhi CP, Good M, Lin J, Jia H, Yamaguchi Y, Lu P, Ma C, Branca MF, Weyandt S, Fulton WB, Nino DF, Prindle T Jr, Ozolek JA, Hackam DJ (2016) Toll-like receptor 4-mediated lymphocyte influx induces neonatal necrotizing enterocolitis. J Clin Invest 126(2):495–508. 10.1172/JCI83356 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Elias KM, Laurence A, Davidson TS, Stephens G, Kanno Y, Shevach EM, O’Shea JJ (2008) Retinoic acid inhibits Th17 polarization and enhances FoxP3 expression through a Stat-3/Stat-5 independent signaling pathway. Blood 111(3):1013–1020. 10.1182/blood-2007-06-096438 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eloranta JJ, Wenger C, Mwinyi J, Hiller C, Gubler C, Vavricka SR, Fried M, Kullak-Ublick GA, Swiss IBDCSG (2011) Association of a common vitamin D-binding protein polymorphism with inflammatory bowel disease. Pharmacogenet Genomics 21(9):559–564. 10.1097/FPC.0b013e328348f70c [DOI] [PubMed] [Google Scholar]
- Engelhardt BG, Jagasia M, Savani BN, Bratcher NL, Greer JP, Jiang A, Kassim AA, Lu P, Schuening F, Yoder SM, Rock MT, Crowe JEJ (2011) Regulatory T cell expression of CLA or alpha(4)beta(7) and skin or gut acute GVHD outcomes. Bone Marrow Transplant 46(3):436–442. 10.1038/bmt.2010.127 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Engelhardt BG, Sengsayadeth SM, Jagasia M, Savani BN, Kassim AA, Lu P, Shyr Y, Yoder SM, Rock MT, Crowe JE Jr (2012) Tissue-specific regulatory T cells: biomarker for acute graft-vs-host disease and survival. Exp Hematol 40(12):974–982. e971. 10.1016/j.exphem.2012.08.002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Faith JJ, Ahern PP, Ridaura VK, Cheng J, Gordon JI (2014) Identifying gut microbe-host phenotype relationships using combinatorial communities in gnotobiotic mice. Sci Transl Med 6(220):220ra211. 10.1126/scitranslmed.3008051 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fakhoury HMA, Kvietys PR, AlKattan W, Anouti FA, Elahi MA, Karras SN, Grant WB (2020) Vitamin D and intestinal homeostasis: barrier, microbiota, and immune modulation. J Steroid Biochem Mol Biol 200:105663. 10.1016/j.jsbmb.2020.105663 [DOI] [PubMed] [Google Scholar]
- Farh KK, Marson A, Zhu J, Kleinewietfeld M, Housley WJ, Beik S, Shoresh N, Whitton H, Ryan RJ, Shishkin AA, Hatan M, Carrasco-Alfonso MJ, Mayer D, Luckey CJ, Patsopoulos NA, De Jager PL, Kuchroo VK, Epstein CB, Daly MJ, Hafler DA, Bernstein BE (2015) Genetic and epigenetic fine mapping of causal autoimmune disease variants. Nature 518(7539):337–343. 10.1038/nature13835 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Feagan BG, Sands BE, Rossiter G, Li X, Usiskin K, Zhan X, Colombel JF (2018) Effects of Mongersen (GED-0301) on endoscopic and clinical outcomes in patients with active Crohn’s disease. Gastroenterology 154(1):61–64.e66. 10.1053/j.gastro.2017.08.035 [DOI] [PubMed] [Google Scholar]
- Feng Y, Arvey A, Chinen T, van der Veeken J, Gasteiger G, Rudensky AY (2014) Control of the inheritance of regulatory T cell identity by a cis element in the Foxp3 locus. Cell 158(4):749–763. 10.1016/j.cell.2014.07.031 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fernandez S, Molina IJ, Romero P, Gonzalez R, Pena J, Sanchez F, Reynoso FR, Perez-Navero JL, Estevez O, Ortega C, Santamaria M (2011) Characterization of gliadin-specific Th17 cells from the mucosa of celiac disease patients. Am J Gastroenterol 106(3):528–538. 10.1038/ajg.2010.465 [DOI] [PubMed] [Google Scholar]
- Fletcher J, Cooper SC, Ghosh S, Hewison M (2019) The role of vitamin D in inflammatory bowel disease: mechanism to management. Nutrients 11(5):1019. 10.3390/nu11051019 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Flippe L, Bezie S, Anegon I, Guillonneau C (2019) Future prospects for CD8(+) regulatory T cells in immune tolerance. Immunol Rev 292(1):209–224. 10.1111/imr.12812 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fontenot JD, Rasmussen JP, Gavin MA, Rudensky AY (2005) A function for interleukin 2 in Foxp3-expressing regulatory T cells. Nat Immunol 6(11):1142–1151. 10.1038/ni1263 [DOI] [PubMed] [Google Scholar]
- Franke A, Balschun T, Karlsen TH, Sventoraityte J, Nikolaus S, Mayr G, Domingues FS, Albrecht M, Nothnagel M, Ellinghaus D, Sina C, Onnie CM, Weersma RK, Stokkers PC, Wijmenga C, Gazouli M, Strachan D, McArdle WL, Vermeire S, Rutgeerts P, Rosenstiel P, Krawczak M, Vatn MH, INSTEN Study Group, Mathew CG, Schreiber S (2008) Sequence variants in IL10, ARPC2 and multiple other loci contribute to ulcerative colitis susceptibility. Nat Genet 40(11):1319–1323. 10.1038/ng.221 [DOI] [PubMed] [Google Scholar]
- Frey DM, Droeser RA, Viehl CT, Zlobec I, Lugli A, Zingg U, Oertli D, Kettelhack C, Terracciano L, Tornillo L (2010) High frequency of tumor-infiltrating FOXP3(+) regulatory T cells predicts improved survival in mismatch repair-proficient colorectal cancer patients. Int J Cancer 126(11):2635–2643. 10.1002/ijc.24989 [DOI] [PubMed] [Google Scholar]
- Friedman DJ, Kunzli BM, YI AR, Sevigny J, Berberat PO, Enjyoji K, Csizmadia E, Friess H, Robson SC (2009) From the cover: CD39 deletion exacerbates experimental murine colitis and human polymorphisms increase susceptibility to inflammatory bowel disease. Proc Natl Acad Sci U S A 106(39):16788–16793. 10.1073/pnas.0902869106 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fujimoto H, Saito Y, Ohuchida K, Kawakami E, Fujiki S, Watanabe T, Ono R, Kaneko A, Takagi S, Najima Y, Hijikata A, Cui L, Ueki T, Oda Y, Hori S, Ohara O, Nakamura M, Saito T, Ishikawa F (2018) Deregulated mucosal immune surveillance through gut-associated regulatory T cells and PD-1(+) T cells in human colorectal cancer. J Immunol 200(9):3291–3303. 10.4049/jimmunol.1701222 [DOI] [PubMed] [Google Scholar]
- Furusawa Y, Obata Y, Fukuda S, Endo TA, Nakato G, Takahashi D, Nakanishi Y, Uetake C, Kato K, Kato T, Takahashi M, Fukuda NN, Murakami S, Miyauchi E, Hino S, Atarashi K, Onawa S, Fujimura Y, Lockett T, Clarke JM, Topping DL, Tomita M, Hori S, Ohara O, Morita T, Koseki H, Kikuchi J, Honda K, Hase K, Ohno H (2013) Commensal microbe-derived butyrate induces the differentiation of colonic regulatory T cells. Nature 504(7480):446–450. 10.1038/nature12721 [DOI] [PubMed] [Google Scholar]
- Galon J, Costes A, Sanchez-Cabo F, Kirilovsky A, Mlecnik B, Lagorce-Pages C, Tosolini M, Camus M, Berger A, Wind P, Zinzindohoue F, Bruneval P, Cugnenc PH, Trajanoski Z, Fridman WH, Pages F (2006) Type, density, and location of immune cells within human colorectal tumors predict clinical outcome. Science 313(5795):1960–1964. 10.1126/science.1129139 [DOI] [PubMed] [Google Scholar]
- Gambineri E, Torgerson TR, Ochs HD (2003) Immune dysregulation, polyendocrinopathy, enteropathy, and X-linked inheritance (IPEX), a syndrome of systemic autoimmunity caused by mutations of FOXP3, a critical regulator of T-cell homeostasis. Curr Opin Rheumatol 15(4):430–435. 10.1097/00002281-200307000-00010 [DOI] [PubMed] [Google Scholar]
- Gao F, Chiu SM, Motan DA, Zhang Z, Chen L, Ji HL, Tse HF, Fu QL, Lian Q (2016) Mesenchymal stem cells and immunomodulation: current status and future prospects. Cell Death Dis 7:e2062. 10.1038/cddis.2015.327 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gao J, Xu K, Liu H, Liu G, Bai M, Peng C, Li T, Yin Y (2018) Impact of the gut microbiota on intestinal immunity mediated by tryptophan metabolism. Front Cell Infect Microbiol 8:13. 10.3389/fcimb.2018.00013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Garrett WS, Lord GM, Punit S, Lugo-Villarino G, Mazmanian SK, Ito S, Glickman JN, Glimcher LH (2007) Communicable ulcerative colitis induced by T-bet deficiency in the innate immune system. Cell 131(1):33–45. 10.1016/jxell.2007.08.017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gavin MA, Torgerson TR, Houston E, DeRoos P, Ho WY, Stray-Pedersen A, Ocheltree EL, Greenberg PD, Ochs HD, Rudensky AY (2006) Single-cell analysis of normal and FOXP3-mutant human T cells: FOXP3 expression without regulatory T cell development. Proc Natl Acad Sci U S A 103(17):6659–6664. 10.1073/pnas.0509484103 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gerassy-Vainberg S, Blatt A, Danin-Poleg Y, Gershovich K, Sabo E, Nevelsky A, Daniel S, Dahan A, Ziv O, Dheer R, Abreu MT, Koren O, Kashi Y, Chowers Y (2018) Radiation induces proinflammatory dysbiosis: transmission of inflammatory susceptibility by host cytokine induction. Gut 67(1):97–107. 10.1136/gutjnl-2017-313789 [DOI] [PubMed] [Google Scholar]
- Gershon RK, Kondo K (1970) Cell interactions in the induction of tolerance: the role of thymic lymphocytes. Immunology 18(5):723–737 [PMC free article] [PubMed] [Google Scholar]
- Geuking MB, Cahenzli J, Lawson MA, Ng DC, Slack E, Hapfelmeier S, McCoy KD, Macpherson AJ (2011) Intestinal bacterial colonization induces mutualistic regulatory T cell responses. Immunity 34(5):794–806. 10.1016/j.immuni.2011.03.021 [DOI] [PubMed] [Google Scholar]
- Gianfrani C, Levings MK, Sartirana C, Mazzarella G, Barba G, Zanzi D, Camarca A, Iaquinto G, Giardullo N, Auricchio S, Troncone R, Roncarolo MG (2006) Gliadin-specific type 1 regulatory T cells from the intestinal mucosa of treated celiac patients inhibit pathogenic T cells. J Immunol 177(6):4178–4186. 10.4049/jimmunol.177.6.4178 [DOI] [PubMed] [Google Scholar]
- Girardin A, McCall J, Black MA, Edwards F, Phillips V, Taylor ES, Reeve AE, Kemp RA (2013) Inflammatory and regulatory T cells contribute to a unique immune microenvironment in tumor tissue of colorectal cancer patients. Int J Cancer 132(8):1842–1850. 10.1002/ijc.27855 [DOI] [PubMed] [Google Scholar]
- Girbovan A, Sur G, Samasca G, Lupan I (2017) Dysbiosis a risk factor for celiac disease. Med Microbiol Immunol 206(2):83–91. 10.1007/s00430-017-0496-z [DOI] [PubMed] [Google Scholar]
- Giri J, Das R, Nylen E, Chinnadurai R, Galipeau J (2020) CCL2 and CXCL12 derived from mesenchymal stromal cells cooperatively polarize IL-10+ tissue macrophages to mitigate gut injury. Cell Rep 30(6):1923–1934. e1924. 10.1016/j.celrep.2020.01.047 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Glocker EO, Kotlarz D, Boztug K, Gertz EM, Schaffer AA, Noyan F, Perro M, Diestelhorst J, Allroth A, Murugan D, Hatscher N, Pfeifer D, Sykora KW, Sauer M, Kreipe H, Lacher M, Nustede R, Woellner C, Baumann U, Salzer U, Koletzko S, Shah N, Segal AW, Sauerbrey A, Buderus S, Snapper SB, Grimbacher B, Klein C (2009) Inflammatory bowel disease and mutations affecting the interleukin-10 receptor. N Engl J Med 361(21):2033–2045. 10.1056/NEJMoa0907206 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Godefroy E, Alameddine J, Montassier E, Mathe J, Desfrancois-Noel J, Marec N, Bossard C, Jarry A, Bridonneau C, Le Roy A, Sarrabayrouse G, Kerdreux E, Bourreille A, Sokol H, Jotereau F, Altare F (2018) Expression of CCR6 and CXCR6 by gut-derived CD4(+)/CD8alpha(+) T-regulatory cells, which are decreased in blood samples from patients with inflammatory bowel diseases. Gastroenterology 155(4):1205–1217. 10.1053/j.gastro.2018.06.078 [DOI] [PubMed] [Google Scholar]
- Godfrey VL, Wilkinson JE, Rinchik EM, Russell LB (1991) Fatal lymphoreticular disease in the scurfy (sf) mouse requires T cells that mature in a sf thymic environment: potential model for thymic education. Proc Natl Acad Sci U S A 88(13):5528–5532. 10.1073/pnas.88.13.5528 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goldberg R, Scotta C, Cooper D, Nissim-Eliraz E, Nir E, Tasker S, Irving PM, Sanderson J, Lavender P, Ibrahim F, Corcoran J, Prevost T, Shpigel NY, Marelli-Berg F, Lombardi G, Lord GM (2019) Correction of defective T-regulatory cells from patients with Crohn’s disease by ex vivo ligation of retinoic acid receptor-alpha. Gastroenterology 156(6):1775–1787. 10.1053/j.gastro.2019.01.025 [DOI] [PubMed] [Google Scholar]
- Gong W, Guo M, Han Z, Wang Y, Yang P, Xu C, Wang Q, Du L, Li Q, Zhao H, Fan F, Liu Q (2016) Mesenchymal stem cells stimulate intestinal stem cells to repair radiation-induced intestinal injury. Cell Death Dis 7(9):e2387. 10.1038/cddis.2016.276 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gorelik L, Flavell RA (2000) Abrogation of TGFbeta signaling in T cells leads to spontaneous T cell differentiation and autoimmune disease. Immunity 12(2):171–181. 10.1016/s1074-7613(00)80170-3 [DOI] [PubMed] [Google Scholar]
- Gottschalk RA, Corse E, Allison JP (2012) Expression of Helios in peripherally induced Foxp3+ regulatory T cells. J Immunol 188(3):976–980. 10.4049/jimmunol.1102964 [DOI] [PubMed] [Google Scholar]
- Gregoire C, Lechanteur C, Briquet A, Baudoux E, Baron F, Louis E, Beguin Y (2017) Review article: mesenchymal stromal cell therapy for inflammatory bowel diseases. Aliment Pharmacol Ther 45(2):205–221. 10.1111/apt.13864 [DOI] [PubMed] [Google Scholar]
- Griseri T, Asquith M, Thompson C, Powrie F (2010) OX40 is required for regulatory T cell-mediated control of colitis. J Exp Med 207(4):699–709. 10.1084/jem.20091618 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grizotte-Lake M, Zhong G, Duncan K, Kirkwood J, Iyer N, Smolenski I, Isoherranen N, Vaishnava S (2018) Commensals suppress intestinal epithelial cell retinoic acid synthesis to regulate interleukin-22 activity and prevent microbial dysbiosis. Immunity 49(6):1103–1115. e1106. 10.1016/j.immuni.2018.11.018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grobeta P, Doser K, Falk W, Obermeier F, Hofmann C (2012) IL-33 attenuates development and perpetuation of chronic intestinal inflammation. Inflamm Bowel Dis 18(10):1900–1909. 10.1002/ibd.22900 [DOI] [PubMed] [Google Scholar]
- Grossman WJ, Verbsky JW, Barchet W, Colonna M, Atkinson JP, Ley TJ (2004a) Human T regulatory cells can use the perforin pathway to cause autologous target cell death. Immunity 21(4):589–601. 10.1016/j.immuni.2004.09.002 [DOI] [PubMed] [Google Scholar]
- Grossman WJ, Verbsky JW, Tollefsen BL, Kemper C, Atkinson JP, Ley TJ (2004b) Differential expression of granzymes A and B in human cytotoxic lymphocyte subsets and T regulatory cells. Blood 104 (9):2840–2848. 10.1182/blood-2004-03-0859 [DOI] [PubMed] [Google Scholar]
- Groux H, O’Garra A, Bigler M, Rouleau M, Antonenko S, de Vries JE, Roncarolo MG (1997) A CD4+ T-cell subset inhibits antigen-specific T-cell responses and prevents colitis. Nature 389(6652):737–742. 10.1038/39614 [DOI] [PubMed] [Google Scholar]
- Guandalini S, Sansotta N (2019) Probiotics in the treatment of inflammatory bowel disease. Adv Exp Med Biol 1125:101–107. 10.1007/5584_2018_319 [DOI] [PubMed] [Google Scholar]
- Gubatan J, Chou ND, Nielsen OH, Moss AC (2019) Systematic review with meta-analysis: association of vitamin D status with clinical outcomes in adult patients with inflammatory bowel disease. Aliment Pharmacol Ther 50(11–12):1146–1158. 10.1111/apt.15506 [DOI] [PubMed] [Google Scholar]
- Gundersen MD, Goll R, Hol J, Olsen T, Rismo R, Sorbye SW, Sundnes O, Haraldsen G, Florholmen J (2016) Loss of interleukin 33 expression in colonic crypts - a potential marker for disease remission in ulcerative colitis. Sci Rep 6:35403. 10.1038/srep35403 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hannier S, Tournier M, Bismuth G, Triebel F (1998) CD3/TCR complex-associated lymphocyte activation gene-3 molecules inhibit CD3/TCR signaling. J Immunol 161(8):4058–4065 [PubMed] [Google Scholar]
- Haribhai D, Williams JB, Jia S, Nickerson D, Schmitt EG, Edwards B, Ziegelbauer J, Yassai M, Li SH, Relland LM, Wise PM, Chen A, Zheng YQ, Simpson PM, Gorski J, Salzman NH, Hessner MJ, Chatila TA, Williams CB (2011) A requisite role for induced regulatory T cells in tolerance based on expanding antigen receptor diversity. Immunity 35(1):109–122. 10.1016/j.immuni.2011.03.029 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harrell CR, Simovic Markovic B, Fellabaum C, Arsenijevic A, Djonov V, Arsenijevic N, Volarevic V (2018) Therapeutic potential of mesenchymal stem cell-derived exosomes in the treatment of eye diseases. Adv Exp Med Biol 1089:47–57. 10.1007/5584_2018_219 [DOI] [PubMed] [Google Scholar]
- Harrison CA, Laubitz D, Ohland CL, Midura-Kiela MT, Patil K, Besselsen DG, Jamwal DR, Jobin C, Ghishan FK, Kiela PR (2018) Microbial dysbiosis associated with impaired intestinal Na(+)/H(+) exchange accelerates and exacerbates colitis in ex-germ free mice. Mucosal Immunol 11(5):1329–1341. 10.1038/s41385-018-0035-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heidari M, Pouya S, Baghaei K, Aghdaei HA, Namaki S, Zali MR, Hashemi SM (2018) The immunomodulatory effects of adipose-derived mesenchymal stem cells and mesenchymal stem cells-conditioned medium in chronic colitis. J Cell Physiol 233(11):8754–8766. 10.1002/jcp.26765 [DOI] [PubMed] [Google Scholar]
- Hidaka D, Hayase E, Shiratori S, Hasegawa Y, Ishio T, Tateno T, Okada K, Goto H, Sugita J, Onozawa M, Nakagawa M, Kahata K, Endo T, Hashimoto D, Teshima T (2018) The association between the incidence of intestinal graft-vs-host disease and antibiotic use after allogeneic hematopoietic stem cell transplantation. Clin Transpl 32(9):e13361. 10.1111/ctr.13361 [DOI] [PubMed] [Google Scholar]
- Himmel ME, MacDonald KG, Garcia RV, Steiner TS, Levings MK (2013) Helios+ and Helios− cells coexist within the natural FOXP3+ T regulatory cell subset in humans. J Immunol 190(5):2001–2008. 10.4049/jimmunol.1201379 [DOI] [PubMed] [Google Scholar]
- Hoeppli RE, MacDonald KN, Leclair P, Fung VCW, Mojibian M, Gillies J, Rahavi SMR, Campbell AIM, Gandhi SK, Pesenacker AM, Reid G, Lim CJ, Levings MK (2019) Tailoring the homing capacity of human Tregs for directed migration to sites of Th1-inflammation or intestinal regions. Am J Transplant 19(1):62–76. 10.1111/ajt.14936 [DOI] [PubMed] [Google Scholar]
- Holmen N, Lundgren A, Lundin S, Bergin AM, Rudin A, Sjovall H, Ohman L (2006) Functional CD4 +CD25high regulatory T cells are enriched in the colonic mucosa of patients with active ulcerative colitis and increase with disease activity. Inflamm Bowel Dis 12(6):447–456. 10.1097/00054725-200606000-00003 [DOI] [PubMed] [Google Scholar]
- Hori S, Nomura T, Sakaguchi S (2003) Control of regulatory T cell development by the transcription factor Foxp3. Science 299(5609):1057–1061. 10.1126/science.1079490 [DOI] [PubMed] [Google Scholar]
- Hotta M, Yoshimura H, Satake A, Tsubokura Y, Ito T, Nomura S (2019) GM-CSF therapy inhibits chronic graft-versus-host disease via expansion of regulatory T cells. Eur J Immunol 49(1):179–191. 10.1002/eji.201847684 [DOI] [PubMed] [Google Scholar]
- Hu S, Yuan J, Xu J, Li X, Zhang G, Ma Q, Zhang B, Hu T, Song G (2019) TNF-alpha and IFN-gamma synergisti-cally inhibit the repairing ability of mesenchymal stem cells on mice colitis and colon cancer. Am J Transl Res 11(9):6207–6220 [PMC free article] [PubMed] [Google Scholar]
- Huang CT, Workman CJ, Flies D, Pan X, Marson AL, Zhou G, Hipkiss EL, Ravi S, Kowalski J, Levitsky HI, Powell JD, Pardoll DM, Drake CG, Vignali DA (2004) Role of LAG-3 in regulatory T cells. Immunity 21(4):503–513. 10.1016/j.immuni.2004.08.010 [DOI] [PubMed] [Google Scholar]
- Huang Z, Liu Y, Qi G, Brand D, Zheng SG (2018) Role of vitamin a in the immune system. J Clin Med 7(9):258. 10.3390/jcm7090258 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ito T, Hanabuchi S, Wang YH, Park WR, Arima K, Bover L, Qin FX, Gilliet M, Liu YJ (2008) Two functional subsets of FOXP3+ regulatory T cells in human thymus and periphery. Immunity 28(6):870–880. 10.1016/j.immuni.2008.03.018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jamwal DR, Marati RV, Harrison CA, Midura-Kiela MT, Figliuolo Paz VR, Besselsen DG, Ghishan FK, Kiela PR (2020) Total CD3 T cells are necessary and sufficient to induce colitis in Immunodeficient mice with dendritic cell-specific deletion of TGFbR2: a novel IBD model to study CD4 and CD8 T-cell interaction. Inflamm Bowel Dis 26(2):229–241. 10.1093/ibd/izz191 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jang YJ, Kim WK, Han DH, Lee K, Ko G (2019) Lactobacillus fermentum species ameliorate dextran sulfate sodium-induced colitis by regulating the immune response and altering gut microbiota. Gut Microbes 10(6):696–711. 10.1080/19490976.2019.1589281 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jorgensen SP, Hvas CL, Agnholt J, Christensen LA, Heickendorff L, Dahlerup JF (2013) Active Crohn’s disease is associated with low vitamin D levels. J Crohns Colitis 7(10):e407–e413. 10.1016/j.crohns.2013.01.012 [DOI] [PubMed] [Google Scholar]
- Josefowicz SZ, Niec RE, Kim HY, Treuting P, Chinen T, Zheng Y, Umetsu DT, Rudensky AY (2012) Extrathymically generated regulatory T cells control mucosal TH2 inflammation. Nature 482 (7385):395–399. 10.1038/nature10772 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kanamori M, Nakatsukasa H, Okada M, Lu Q, Yoshimura A (2016) Induced regulatory T cells: their development, stability, and applications. Trends Immunol 37(11):803–811. 10.1016/j.it.2016.08.012 [DOI] [PubMed] [Google Scholar]
- Kang SG, Lim HW, Andrisani OM, Broxmeyer HE, Kim CH (2007) Vitamin a metabolites induce gut-homing FoxP3+ regulatory T cells. J Immunol 179(6):3724–3733. 10.4049/jimmunol.179.6.3724 [DOI] [PubMed] [Google Scholar]
- Kang SW, Kim SH, Lee N, Lee WW, Hwang KA, Shin MS, Lee SH, Kim WU, Kang I (2012) 1,25-Dihyroxyvitamin D3 promotes FOXP3 expression via binding to vitamin D response elements in its conserved noncoding sequence region. J Immunol 188(11):5276–5282. 10.4049/jimmunol.1101211 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Karwacz K, Bricogne C, MacDonald D, Arce F, Bennett CL, Collins M, Escors D (2011) PD-L1 co-stimulation contributes to ligand-induced T cell receptor down-modulation on CD8+ T cells. EMBO Mol Med 3(10):581–592. 10.1002/emmm.201100165 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Khalili A, Ebrahimpour S, Maleki I, Abediankenari S, Afrouzi MM (2018) CD4+CD25+CD127low FoxP3+ regulatory T cells in Crohn’s disease. Rom J Intern Med 56(3):158–166. 10.2478/rjim-2018-0006 [DOI] [PubMed] [Google Scholar]
- Kim KS, Hong SW, Han D, Yi J, Jung J, Yang BG, Lee JY, Lee M, Surh CD (2016) Dietary antigens limit mucosal immunity by inducing regulatory T cells in the small intestine. Science 351(6275):858–863. 10.1126/science.aac5560 [DOI] [PubMed] [Google Scholar]
- Kinoshita M, Kayama H, Kusu T, Yamaguchi T, Kunisawa J, Kiyono H, Sakaguchi S, Takeda K (2012) Dietary folic acid promotes survival of Foxp3 + regulatory T cells in the colon. J Immunol 189(6):2869–2878. 10.4049/jimmunol.1200420 [DOI] [PubMed] [Google Scholar]
- Kitazawa Y, Fujino M, Wang Q, Kimura H, Azuma M, Kubo M, Abe R, Li XK (2007) Involvement of the programmed death-1/programmed death-1 ligand pathway in CD4+CD25+ regulatory T-cell activity to suppress alloimmune responses. Transplantation 83(6):774–782. 10.1097/01.tp.0000256293.90270.e8 [DOI] [PubMed] [Google Scholar]
- Knyazev OV, Fadeeva NA, Kagramanova AV, Belyakov NI, Orlova NV, Lishchinskaya AA, Konoplyannikov AG, Parfenov AI (2018) Stem cell therapy for perianal Crohn’s disease. Ter Arkh 90(3):60–66. 10.26442/terarkh201890360-66 [DOI] [PubMed] [Google Scholar]
- Kobori A, Yagi Y, Imaeda H, Ban H, Bamba S, Tsujikawa T, Saito Y, Fujiyama Y, Andoh A (2010) Interleukin-33 expression is specifically enhanced in inflamed mucosa of ulcerative colitis. J Gastroenterol 45(10):999–1007. 10.1007/s00535-010-0245-1 [DOI] [PubMed] [Google Scholar]
- Konkel JE, Chen W (2011) Balancing acts: the role of TGF-beta in the mucosal immune system. Trends Mol Med 17(11):668–676. 10.1016/j.molmed.2011.07.002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koreth J, Matsuoka K, Kim HT, McDonough SM, Bindra B, Alyea EP 3rd, Armand P, Cutler C, Ho VT, Treister NS, Bienfang DC, Prasad S, Tzachanis D, Joyce RM, Avigan DE, Antin JH, Ritz J, Soiffer RJ (2011) Interleukin-2 and regulatory T cells in graft-versus-host disease. N Engl J Med 365(22):2055–2066. 10.1056/NEJMoa1108188 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koreth J, Kim HT, Jones KT, Lange PB, Reynolds CG, Chammas MJ, Dusenbury K, Whangbo J, Nikiforow S, Alyea EP III, Armand P, Cutler CS, Ho VT, Chen YB, Avigan D, Blazar BR, Antin JH, Ritz J, Soiffer RJ (2016) Efficacy, durability, and response predictors of low-dose interleukin-2 therapy for chronic graft-versus-host disease. Blood 128(1):130–137. 10.1182/blood-2016-02-702852 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Korn LL, Hubbeling HG, Porrett PM, Yang Q, Barnett LG, Laufer TM (2014) Regulatory T cells occupy an isolated niche in the intestine that is antigen independent. Cell Rep 9(5):1567–1573. 10.1016/j.celrep.2014.11.006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kreisman LS, Cobb BA (2011) Glycoantigens induce human peripheral Tr1 cell differentiation with gut-homing specialization. J Biol Chem 286(11):8810–8818. 10.1074/jbc.M110.206011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kryczek I, Wu K, Zhao E, Wei S, Vatan L, Szeliga W, Huang E, Greenson J, Chang A, Rolinski J, Radwan P, Fang J, Wang G, Zou W (2011) IL-17+ regulatory T cells in the microenvironments of chronic inflammation and cancer. J Immunol 186(7):4388–4395. 10.4049/jimmunol.1003251 [DOI] [PubMed] [Google Scholar]
- Kulkarni AB, Huh CG, Becker D, Geiser A, Lyght M, Flanders KC, Roberts AB, Sporn MB, Ward JM, Karlsson S (1993) Transforming growth factor beta 1 null mutation in mice causes excessive inflammatory response and early death. Proc Natl Acad Sci U S A 90(2):770–774. 10.1073/pnas.90.2770 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kullberg MC, Jankovic D, Gorelick PL, Caspar P, Letterio JJ, Cheever AW, Sher A (2002) Bacteria-triggered CD4(+) T regulatory cells suppress ? Helicobacter hepaticus-induced colitis. J Exp Med 196(4):505–515. 10.1084/jem.20020556 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lahat N, Shapiro S, Karban A, Gerstein R, Kinarty A, Lerner A (1999) Cytokine profile in coeliac disease. Scand J Immunol 49(4):441–446. 10.1046/j.1365-3083.1999.00523.x [DOI] [PubMed] [Google Scholar]
- Lahdeaho ML, Scheinin M, Vuotikka P, Taavela J, Popp A, Laukkarinen J, Koffert J, Koivurova OP, Pesu M, Kivela L, Lovro Z, Keisala J, Isola J, Parnes JR, Leon F, Maki M (2019) Safety and efficacy of AMG 714 in adults with coeliac disease exposed to gluten challenge: a phase 2a, randomised, doubleblind, placebo-controlled study. Lancet Gastroenterol Hepatol 4(12):948–959. 10.1016/S2468-1253(19)30264-X [DOI] [PubMed] [Google Scholar]
- Larmonier CB, Shehab KW, Ghishan FK, Kiela PR (2015) T lymphocyte dynamics in inflammatory bowel diseases: role of the microbiome. Biomed Res Int 2015:504638. 10.1155/2015/504638 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lathrop SK, Bloom SM, Rao SM, Nutsch K, Lio CW, Santacruz N, Peterson DA, Stappenbeck TS, Hsieh CS (2011) Peripheral education of the immune system by colonic commensal microbiota. Nature 478(7368):250–254. 10.1038/nature10434 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Laudisi F, Dinallo V, Di Fusco D, Monteleone G (2016) Smad7 and its potential as therapeutic target in inflammatory bowel diseases. Curr Drug Metab 17(3):303–306. 10.2174/1389200217666151210130103 [DOI] [PubMed] [Google Scholar]
- Law AD, Dutta U, Kochhar R, Vaishnavi C, Kumar S, Noor T, Bhadada S, Singh K (2019) Vitamin D deficiency in adult patients with ulcerative colitis: prevalence and relationship with disease severity, extent, and duration. Indian J Gastroenterol 38(1):6–14. 10.1007/s12664-019-00932-z [DOI] [PubMed] [Google Scholar]
- Lee YK, Mehrabian P, Boyajian S, Wu WL, Selicha J, Vonderfecht S, Mazmanian SK (2018) The protective role of Bacteroides fragilis in a murine model of colitis-associated colorectal cancer. mSphere 3(6):e00587–18. 10.1128/mSphere.00587-18 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Levine AG, Arvey A, Jin W, Rudensky AY (2014) Continuous requirement for the TCR in regulatory T cell function. Nat Immunol 15(11):1070–1078. 10.1038/ni.3004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Levings MK, Roncarolo MG (2005) Phenotypic and functional differences between human CD4+CD25+ and type 1 regulatory T cells. Curr Top Microbiol Immunol 293:303–326. 10.1007/3-540-27702-1_14 [DOI] [PubMed] [Google Scholar]
- Levings MK, Sangregorio R, Sartirana C, Moschin AL, Battaglia M, Orban PC, Roncarolo MG (2002) Human CD25+CD4+ T suppressor cell clones produce transforming growth factor beta, but not interleukin 10, and are distinct from type 1 T regulatory cells. J Exp Med 196(10):1335–1346. 10.1084/jem.20021139 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li MO, Sanjabi S, Flavell RA (2006) Transforming growth factor-beta controls development, homeostasis, and tolerance of T cells by regulatory T cell-dependent and -independent mechanisms. Immunity 25(3):455–471. 10.1016/j.immuni.2006.07.011 [DOI] [PubMed] [Google Scholar]
- Li X, Liang Y, LeBlanc M, Benner C, Zheng Y (2014) Function of a Foxp3 cis-element in protecting regulatory T cell identity. Cell 158(4):734–748. 10.1016/j.cell.2014.07.030 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li Z, Vermeire S, Bullens D, Ferrante M, Van Steen K, Noman M, Rutgeerts P, Ceuppens JL, Van Assche G (2015) Restoration of Foxp3+ regulatory T-cell subsets and Foxp3-type 1 regulatory-like T cells in inflammatory bowel diseases during anti-tumor necrosis factor therapy. Inflamm Bowel Dis 21(10):2418–2428. 10.1097/MIB.0000000000000509 [DOI] [PubMed] [Google Scholar]
- Li J, Ueno A, Iacucci M, Fort Gasia M, Jijon HB, Panaccione R, Kaplan GG, Beck PL, Luider J, Barkema HW, Qian J, Gui X, Ghosh S (2017) Crossover subsets of CD4(+) T lymphocytes in the intestinal lamina propria of patients with Crohn’s disease and ulcerative colitis. Dig Dis Sci 62(9):2357–2368. 10.1007/s10620-017-4596-9 [DOI] [PubMed] [Google Scholar]
- Li Y, Wang F, Guo R, Zhang Y, Chen D, Li X, Tian W, Xie X, Jiang Z (2019) Exosomal sphingosine 1-phosphate secreted by mesenchymal stem cells regulated Treg/Th17 balance in aplastic anemia. IUBMB Life 71(9):1284–1292. 10.1002/iub.2035 [DOI] [PubMed] [Google Scholar]
- Lin YC, Mahalingam J, Chiang JM, Su PJ, Chu YY, Lai HY, Fang JH, Huang CT, Chiu CT, Lin CY (2013) Activated but not resting regulatory T cells accumulated in tumor microenvironment and correlated with tumor progression in patients with colorectal cancer. Int J Cancer 132(6):1341–1350. 10.1002/ijc.27784 [DOI] [PubMed] [Google Scholar]
- Liston A, Gray DH (2014) Homeostatic control of regulatory T cell diversity. Nat Rev Immunol 14(3):154–165. 10.1038/nri3605 [DOI] [PubMed] [Google Scholar]
- Liu Y, Lan Q, Lu L, Chen M, Xia Z, Ma J, Wang J, Fan H, Shen Y, Ryffel B, Brand D, Quismorio F, Liu Z, Horwitz DA, Xu A, Zheng SG (2014) Phenotypic and functional characteristic of a newly identified CD8 + Foxp3- CD103+ regulatory T cells. J Mol Cell Biol 6(1):81–92. 10.1093/jmcb/mjt026 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lochner M, Peduto L, Cherrier M, Sawa S, Langa F, Varona R, Riethmacher D, Si-Tahar M, Di Santo JP, Eberl G (2008) In vivo equilibrium of proinflammatory IL-17+ and regulatory IL-10+ Foxp3+ RORgamma t+ T cells. J Exp Med 205(6):1381–1393. 10.1084/jem.20080034 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Loebbermann J, Thornton H, Durant L, Sparwasser T, Webster KE, Sprent J, Culley FJ, Johansson C, Openshaw PJ (2012) Regulatory T cells expressing granzyme B play a critical role in controlling lung inflammation during acute viral infection. Mucosal Immunol 5(2):161–172. 10.1038/mi.2011.62 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lopez-Casado MA, Lorite P, Palomeque T, Torres MI (2017) Potential role of the IL-33/ST2 axis in celiac disease. Cell Mol Immunol 14(3):285–292. 10.1038/cmi.2015.85 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lopez-Siles M, Enrich-Capo N, Aldeguer X, Sabat-Mir M, Duncan SH, Garcia-Gil LJ, Martinez-Medina M (2018) Alterations in the abundance and co-occurrence of Akkermansia muciniphila and Faecalibacterium prausnitzii in the colonic mucosa of inflammatory bowel disease subjects. Front Cell Infect Microbiol 8:281. 10.3389/fcimb.2018.00281 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lord JD, Hackman RC, Gooley TA, Wood BL, Moklebust AC, Hockenbery DM, Steinbach G, Ziegler SF, McDonald GB (2011) Blood and gastric FOXP3+ T cells are not decreased in human gastric graft-versus-host disease. Biol Blood Marrow Transplant 17(4):486–496. 10.1016/j.bbmt.2010.09.015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lord JD, Shows DM, Chen J, Thirlby RC (2015) Human blood and mucosal regulatory T cells express activation markers and inhibitory receptors in inflammatory bowel disease. PLoS One 10(8):e0136485. 10.1371/journal.pone.0136485 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lu L, Ma J, Li Z, Lan Q, Chen M, Liu Y, Xia Z, Wang J, Han Y, Shi W, Quesniaux V, Ryffel B, Brand D, Li B, Liu Z, Zheng SG (2011) All-trans retinoic acid promotes TGF-beta-induced Tregs via histone modification but not DNA demethylation on Foxp3 gene locus. PLoS One 6(9):e24590. 10.1371/journal.pone.0024590 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Luo Y, Xue Y, Wang J, Dang J, Fang Q, Huang G, Olsen N, Zheng SG (2019) Negligible effect of sodium chloride on the development and function of TGF-beta-induced CD4(+) Foxp3(+) regulatory T cells. Cell Rep 26(7):1869–1879. e1863. 10.1016/j.celrep.2019.01.066 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ma C, Dong X (2011) Colorectal cancer-derived Foxp3(+) IL-17(+) T cells suppress tumour-specific CD8+ T cells. Scand J Immunol 74(1):47–51. 10.1111/j.1365-3083.2011.02539.x [DOI] [PubMed] [Google Scholar]
- Ma F, Li S, Gao X, Zhou J, Zhu X, Wang D, Cai Y, Li F, Yang Q, Gu X, Ge W, Liu H, Xiao X, Hao H (2019) Interleukin-6-mediated CCR9(+) interleukin-17-producing regulatory T cells polarization increases the severity of necrotizing enterocolitis. EBioMedicine 44:71–85. 10.1016/j.ebiom.2019.05.042 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Machiels K, Joossens M, Sabino J, De Preter V, Arijs I, Eeckhaut V, Ballet V, Claes K, Van Immerseel F, Verbeke K, Ferrante M, Verhaegen J, Rutgeerts P, Vermeire S (2014) A decrease of the butyrate-producing species Roseburia hominis and Faecalibacterium prausnitzii defines dysbiosis in patients with ulcerative colitis. Gut 63(8):1275–1283. 10.1136/gutjnl-2013-304833 [DOI] [PubMed] [Google Scholar]
- Mahalingam J, Lin CY, Chiang JM, Su PJ, Chu YY, Lai HY, Fang JH, Huang CT, Lin YC (2014) CD4(+) T cells expressing latency-associated peptide and Foxp3 are an activated subgroup of regulatory T cells enriched in patients with colorectal cancer. PLoS One 9(9):e108554. 10.1371/journal.pone.0108554 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Malek TR, Yu A, Vincek V, Scibelli P, Kong L (2002) CD4 regulatory T cells prevent lethal autoimmunity in IL-2Rbeta-deficient mice. Implications for the nonredundant function of IL-2. Immunity 17(2):167–178. 10.1016/s1074-7613(02)00367-9 [DOI] [PubMed] [Google Scholar]
- Mandapathil M, Hilldorfer B, Szczepanski MJ, Czystowska M, Szajnik M, Ren J, Lang S, Jackson EK, Gorelik E, Whiteside TL (2010) Generation and accumulation of immunosuppressive adenosine by human CD4+CD25highFOXP3+ regulatory T cells. J Biol Chem 285(10):7176–7186. 10.1074/jbc.M109.047423 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marie JC, Letterio JJ, Gavin M, Rudensky AY (2005) TGF-beta1 maintains suppressor function and Foxp3 expression in CD4+CD25+ regulatory T cells. J Exp Med 201(7):1061–1067. 10.1084/jem.20042276 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marie JC, Liggitt D, Rudensky AY (2006) Cellular mechanisms of fatal early-onset autoimmunity in mice with the T cell-specific targeting of transforming growth factor-beta receptor. Immunity 25(3):441–454. 10.1016/j.immuni.2006.07.012 [DOI] [PubMed] [Google Scholar]
- Matsuoka K, Koreth J, Kim HT, Bascug G, McDonough S, Kawano Y, Murase K, Cutler C, Ho VT, Alyea EP, Armand P, Blazar BR, Antin JH, Soiffer RJ, Ritz J (2013) Low-dose interleukin-2 therapy restores regulatory T cell homeostasis in patients with chronic graft-versus-host disease. Sci Transl Med 5(179):179ra143. 10.1126/scitranslmed.3005265 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matta BM, Lott JM, Mathews LR, Liu Q, Rosborough BR, Blazar BR, Turnquist HR (2014) IL-33 is an unconventional Alarmin that stimulates IL-2 secretion by dendritic cells to selectively expand IL-33R/ST2+ regulatory T cells. J Immunol 193(8):4010–4020. 10.4049/jimmunol.1400481 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matta BM, Reichenbach DK, Zhang X, Mathews L, Koehn BH, Dwyer GK, Lott JM, Uhl FM, Pfeifer D, Feser CJ, Smith MJ, Liu Q, Zeiser R, Blazar BR, Turnquist HR (2016) Peri-alloHCT IL-33 administration expands recipient T-regulatory cells that protect mice against acute GVHD. Blood 128(3):427–439. 10.1182/blood-2015-12-684142 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maynard CL, Harrington LE, Janowski KM, Oliver JR, Zindl CL, Rudensky AY, Weaver CT (2007) Regulatory T cells expressing interleukin 10 develop from Foxp3+ and Foxp3- precursor cells in the absence of interleukin 10. Nat Immunol 8(9):931–941. 10.1038/ni1504 [DOI] [PubMed] [Google Scholar]
- McHugh RS, Whitters MJ, Piccirillo CA, Young DA, Shevach EM, Collins M, Byrne MC (2002) CD4(+) CD25(+) immunoregulatory T cells: gene expression analysis reveals a functional role for the glucocorticoid-induced TNF receptor. Immunity 16(2):311–323. 10.1016/s1074-7613(02)00280-7 [DOI] [PubMed] [Google Scholar]
- Mezrich JD, Fechner JH, Zhang X, Johnson BP, Burlingham WJ, Bradfield CA (2010) An interaction between kynurenine and the aryl hydrocarbon receptor can generate regulatory T cells. J Immunol 185(6):3190–3198. 10.4049/jimmunol.0903670 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Michel S, Benner A, Tariverdian M, Wentzensen N, Hoefler P, Pommerencke T, Grabe N, von Knebel Doeberitz M, Kloor M (2008) High density of FOXP3-positive T cells infiltrating colorectal cancers with microsatellite instability. Br J Cancer 99(11):1867–1873. 10.1038/sj.bjc.6604756 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mikulic J, Longet S, Favre L, Benyacoub J, Corthesy B (2017) Secretory IgA in complex with Lactobacillus rhamnosus potentiates mucosal dendritic cell-mediated Treg cell differentiation via TLR regulatory proteins, RALDH2 and secretion of IL-10 and TGF-beta. Cell Mol Immunol 14(6):546–556. 10.1038/cmi.2015.110 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Milpied P, Renand A, Bruneau J, Mendes-da-Cruz DA, Jacquelin S, Asnafi V, Rubio MT, MacIntyre E, Lepelletier Y, Hermine O (2009) Neuropilin-1 is not a marker of human Foxp3+ Treg. Eur J Immunol 39(6):1466–1471. 10.1002/eji.200839040 [DOI] [PubMed] [Google Scholar]
- Min B, Thornton A, Caucheteux SM, Younes SA, Oh K, Hu-Li J, Paul WE (2007) Gut flora antigens are not important in the maintenance of regulatory T cell heterogeneity and homeostasis. Eur J Immunol 37(7):1916–1923. 10.1002/eji.200737236 [DOI] [PubMed] [Google Scholar]
- Miragaia RJ, Gomes T, Chomka A, Jardine L, Riedel A, Hegazy AN, Whibley N, Tucci A, Chen X, Lindeman I, Emerton G, Krausgruber T, Shields J, Haniffa M, Powrie F, Teichmann SA (2019) Single-cell transcriptomics of regulatory T cells reveals trajectories of tissue adaptation. Immunity 50(2):493–504. e497. 10.1016/j.immuni.2019.01.001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miyara M, Yoshioka Y, Kitoh A, Shima T, Wing K, Niwa A, Parizot C, Taflin C, Heike T, Valeyre D, Mathian A, Nakahata T, Yamaguchi T, Nomura T, Ono M, Amoura Z, Gorochov G, Sakaguchi S (2009) Functional delineation and differentiation dynamics of human CD4+ T cells expressing the FoxP3 transcription factor. Immunity 30(6):899–911. 10.1016/j.immuni.2009.03.019 [DOI] [PubMed] [Google Scholar]
- Moheb-Alian A, Forouzesh F, Rostami-Nejad M, Rostami K (2016) Mesenchymal stem cells as potential therapeutic approaches in celiac disease. Gastroenterol Hepatol Bed Bench 9(suppl 1):S1–S7 [PMC free article] [PubMed] [Google Scholar]
- Molodecky NA, Soon IS, Rabi DM, Ghali WA, Ferris M, Chernoff G, Benchimol EI, Panaccione R, Ghosh S, Barkema HW, Kaplan GG (2012) Increasing incidence and prevalence of the inflammatory bowel diseases with time, based on systematic review. Gastroenterology 142(1):46–54.e42, quiz e30. 10.1053/j.gastro.2011.10.001 [DOI] [PubMed] [Google Scholar]
- Monteleone G, Neurath MF, Ardizzone S, Di Sabatino A, Fantini MC, Castiglione F, Scribano ML, Armuzzi A, Caprioli F, Sturniolo GC, Rogai F, Vecchi M, Atreya R, Bossa F, Onali S, Fichera M, Corazza GR, Biancone L, Savarino V, Pica R, Orlando A, Pallone F (2015) Mongersen, an oral SMAD7 antisense oligonucleotide, and Crohn’s disease. N Engl J Med 372(12):1104–1113. 10.1056/NEJMoa1407250 [DOI] [PubMed] [Google Scholar]
- Monteleone G, Di Sabatino A, Ardizzone S, Pallone F, Usiskin K, Zhan X, Rossiter G, Neurath MF (2016) Impact of patient characteristics on the clinical efficacy of mongersen (GED-0301), an oral Smad7 antisense oligonucleotide, in active Crohn’s disease. Aliment Pharmacol Ther 43(6):717–724. 10.1111/apt.13526 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moran CJ, Walters TD, Guo CH, Kugathasan S, Klein C, Turner D, Wolters VM, Bandsma RH, Mouzaki M, Zachos M, Langer JC, Cutz E, Benseler SM, Roifman CM, Silverberg MS, Griffiths AM, Snapper SB, Muise AM (2013) IL-10R polymorphisms are associated with very-early-onset ulcerative colitis. Inflamm Bowel Dis 19(1):115–123. 10.1002/ibd.22974 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morgan ME, van Bilsen JH, Bakker AM, Heemskerk B, Schilham MW, Hartgers FC, Elferink BG, van der Zanden L, de Vries RR, Huizinga TW, Ottenhoff TH, Toes RE (2005) Expression of FOXP3 mRNA is not confined to CD4+CD25+ T regulatory cells in humans. Hum Immunol 66(1):13–20. 10.1016/j.humimm.2004.05.016 [DOI] [PubMed] [Google Scholar]
- Mottet C, Uhlig HH, Powrie F (2003) Cutting edge: cure of colitis by CD4+CD25+ regulatory T cells. J Immunol 170(8):3939–3943. 10.4049/jimmunol.170.8.3939 [DOI] [PubMed] [Google Scholar]
- Mucida D, Park Y, Kim G, Turovskaya O, Scott I, Kronenberg M, Cheroutre H (2007) Reciprocal TH17 and regulatory T cell differentiation mediated by retinoic acid. Science 317(5835):256–260. 10.1126/science.1145697 [DOI] [PubMed] [Google Scholar]
- Mukhopadhyay D, Weaver L, Tobin R, Henderson S, Beeram M, Newell-Rogers MK, Perger L (2014) Intrauterine growth restriction and prematurity influence regulatory T cell development in newborns. J Pediatr Surg 49(5):727–732. 10.1016/j.jpedsurg.2014.02.055 [DOI] [PubMed] [Google Scholar]
- Muzes G, Molnar B, Tulassay Z, Sipos F (2012) Changes of the cytokine profile in inflammatory bowel diseases. World J Gastroenterol 18(41):5848–5861. 10.3748/wjg.v18.i41.5848 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nagorsen D, Scheibenbogen C, Letsch A, Germer CT, Buhr HJ, Hegewisch-Becker S, Rivoltini L, Thiel E, Keilholz U (2005) T cell responses against tumor associated antigens and prognosis in colorectal cancer patients. J Transl Med 3(1):3. 10.1186/1479-5876-3-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nakanishi Y, Ikebuchi R, Chtanova T, Kusumoto Y, Okuyama H, Moriya T, Honda T, Kabashima K, Watanabe T, Sakai Y, Tomura M (2018) Regulatory T cells with superior immunosuppressive capacity emigrate from the inflamed colon to draining lymph nodes. Mucosal Immunol 11(2):437–448. 10.1038/mi.2017.64 [DOI] [PubMed] [Google Scholar]
- Narushima S, Sugiura Y, Oshima K, Atarashi K, Hattori M, Suematsu M, Honda K (2014) Characterization of the 17 strains of regulatory T cell-inducing human-derived Clostridia. Gut Microbes 5(3):333–339. 10.4161/gmic.28572 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nascimento DC, Melo PH, Pineros AR, Ferreira RG, Colon DF, Donate PB, Castanheira FV, Gozzi A, Czaikoski PG, Niedbala W, Borges MC, Zamboni DS, Liew FY, Cunha FQ, Alves-Filho JC (2017) IL-33 contributes to sepsis-induced long-term immunosuppression by expanding the regulatory T cell population. Nat Commun 8:14919. 10.1038/ncomms14919 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nemeth ZH, Bogdanovski DA, Barratt-Stopper P, Paglinco SR, Antonioli L, Rolandelli RH (2017) Crohn’s disease and ulcerative colitis show unique cytokine profiles. Cureus 9(4):e1177. 10.7759/cureus.1177 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ni J, Wu GD, Albenberg L, Tomov VT (2017) Gut microbiota and IBD: causation or correlation? Nat Rev Gastroenterol Hepatol 14(10):573–584. 10.1038/nrgastro.2017.88 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ni C, Gan X, Li X, Sun H, Chen Z, Lu H (2019) Vitamin D alleviates acute graft-versus-host disease through promoting the generation of Foxp3(+) T cells. Ann Transl Med 7(23):748. 10.21037/atm.2019.11.102 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nilsen EM, Jahnsen FL, Lundin KE, Johansen FE, Fausa O, Sollid LM, Jahnsen J, Scott H, Brandtzaeg P (1998) Gluten induces an intestinal cytokine response strongly dominated by interferon gamma in patients with celiac disease. Gastroenterology 115(3):551–563. 10.1016/s0016-5085(98)70134-9 [DOI] [PubMed] [Google Scholar]
- Nino DF, Sodhi CP, Egan CE, Zhou Q, Lin J, Lu P, Yamaguchi Y, Jia H, Martin LY, Good M, Fulton WB, Prindle T Jr, Ozolek JA, Hackam DJ (2017) Retinoic acid improves incidence and severity of necrotizing enterocolitis by lymphocyte balance restitution and repopulation of LGR5+ intestinal stem cells. Shock 47(1):22–32. 10.1097/SHK.0000000000000713 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nishio J, Baba M, Atarashi K, Tanoue T, Negishi H, Yanai H, Habu S, Hori S, Honda K, Taniguchi T (2015) Requirement of full TCR repertoire for regulatory T cells to maintain intestinal homeostasis. Proc Natl Acad Sci U S A 112(41):12770–12775. 10.1073/pnas.1516617112 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nutsch K, Chai JN, Ai TL, Russler-Germain E, Feehley T, Nagler CR, Hsieh CS (2016) Rapid and efficient generation of regulatory T cells to commensal antigens in the periphery. Cell Rep 17(1):206–220. 10.1016/j.celrep.2016.08.092 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oberle N, Eberhardt N, Falk CS, Krammer PH, Suri-Payer E (2007) Rapid suppression of cytokine transcription in human CD4+CD25 T cells by CD4+Foxp3+ regulatory T cells: independence of IL-2 consumption, TGF-beta, and various inhibitors of TCR signaling. J Immunol 179(6):3578–3587. 10.4049/jimmunol.179.6.3578 [DOI] [PubMed] [Google Scholar]
- Oboki K, Ohno T, Kajiwara N, Arae K, Morita H, Ishii A, Nambu A, Abe T, Kiyonari H, Matsumoto K, Sudo K, Okumura K, Saito H, Nakae S (2010) IL-33 is a crucial amplifier of innate rather than acquired immunity. Proc Natl Acad Sci U S A 107(43):18581–18586. 10.1073/pnas.1003059107 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ohnmacht C, Park JH, Cording S, Wing JB, Atarashi K, Obata Y, Gaboriau-Routhiau V, Marques R, Dulauroy S, Fedoseeva M, Busslinger M, Cerf-Bensussan N, Boneca IG, Voehringer D, Hase K, Honda K, Sakaguchi S, Eberl G (2015) MUCOSAL IMMUNOLOGY. The microbiota regulates type 2 immunity through RORgammat(+) T cells. Science 349(6251):989–993. 10.1126/science.aac4263 [DOI] [PubMed] [Google Scholar]
- Ohtani H (2007) Focus on TILs: prognostic significance of tumor infiltrating lymphocytes in human colorectal cancer. Cancer Immun 7:4. [PMC free article] [PubMed] [Google Scholar]
- Oldenhove G, Bouladoux N, Wohlfert EA, Hall JA, Chou D, Dos Santos L, O’Brien S, Blank R, Lamb E, Natarajan S, Kastenmayer R, Hunter C, Grigg ME, Belkaid Y (2009) Decrease of Foxp3+ Treg cell number and acquisition of effector cell phenotype during lethal infection. Immunity 31(5):772–786. 10.1016/j.immuni.2009.10.001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ouyang W, Beckett O, Ma Q, Li MO (2010) Transforming growth factor-beta signaling curbs thymic negative selection promoting regulatory T cell development. Immunity 32(5):642–653. 10.1016/j.immuni.2010.04.012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paidassi H, Acharya M, Zhang A, Mukhopadhyay S, Kwon M, Chow C, Stuart LM, Savill J, Lacy-Hulbert A (2011) Preferential expression of integrin alphavbeta8 promotes generation of regulatory T cells by mouse CD103+ dendritic cells. Gastroenterology 141(5):1813–1820. 10.1053/j.gastro.2011.06.076 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pandiyan P, Zheng L, Ishihara S, Reed J, Lenardo MJ (2007) CD4+CD25+Foxp3+ regulatory T cells induce cytokine deprivation-mediated apoptosis of effector CD4+ T cells. Nat Immunol 8(12):1353–1362. 10.1038/ni1536 [DOI] [PubMed] [Google Scholar]
- Pandolfi JB, Ferraro AA, Sananez I, Gancedo MC, Baz P, Billordo LA, Fainboim L, Arruvito L (2016) ATP-induced inflammation drives tissue-resident Th17 cells in metabolically unhealthy obesity. J Immunol 196(8):3287–3296. 10.4049/jimmunol.1502506 [DOI] [PubMed] [Google Scholar]
- Pang Y, Du X, Xu X, Wang M, Li Z (2018a) Impairment of regulatory T cells in patients with neonatal necrotizing enterocolitis. Int Immunopharmacol 63:19–25. 10.1016/j.intimp.2018.07.029 [DOI] [PubMed] [Google Scholar]
- Pang Y, Du X, Xu X, Wang M, Li Z (2018b) Monocyte activation and inflammation can exacerbate Treg/Th17 imbalance in infants with neonatal necrotizing enterocolitis. Int Immunopharmacol 59:354–360. 10.1016/j.intimp.2018.04.026 [DOI] [PubMed] [Google Scholar]
- Papiernik M, de Moraes ML, Pontoux C, Vasseur F, Penit C (1998) Regulatory CD4 T cells: expression of IL-2R alpha chain, resistance to clonal deletion and IL-2 dependency. Int Immunol 10(4):371–378. 10.1093/intimm/10.4.371 [DOI] [PubMed] [Google Scholar]
- Park JS, Choi JW, Jhun J, Kwon JY, Lee BI, Yang CW, Park SH, Cho ML (2018) Lactobacillus acidophilus improves intestinal inflammation in an acute colitis mouse model by regulation of Th17 and Treg cell balance and fibrosis development. J Med Food 21(3):215–224. 10.1089/jmf.2017.3990 [DOI] [PubMed] [Google Scholar]
- Pastorelli L, Garg RR, Hoang SB, Spina L, Mattioli B, Scarpa M, Fiocchi C, Vecchi M, Pizarro TT (2010) Epithelial-derived IL-33 and its receptor ST2 are dysregulated in ulcerative colitis and in experimental Th1/Th2 driven enteritis. Proc Natl Acad Sci U S A 107(17):8017–8022. 10.1073/pnas.0912678107 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pastorelli L, De Salvo C, Vecchi M, Pizarro TT (2013) The role of IL-33 in gut mucosal inflammation. Mediat Inflamm 2013:608187. 10.1155/2013/608187 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Patel RM, Underwood MA (2018) Probiotics and necrotizing enterocolitis. Semin Pediatr Surg 27(1):39–46. 10.1053/j.sempedsurg.2017.11.008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pesu M, Watford WT, Wei L, Xu L, Fuss I, Strober W, Andersson J, Shevach EM, Quezado M, Bouladoux N, Roebroek A, Belkaid Y, Creemers J, O’Shea JJ (2008) T-cell-expressed proprotein convertase furin is essential for maintenance of peripheral immune tolerance. Nature 455(7210):246–250. 10.1038/nature07210 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pichery M, Mirey E, Mercier P, Lefrancais E, Dujardin A, Ortega N, Girard JP (2012) Endogenous IL-33 is highly expressed in mouse epithelial barrier tissues, lymphoid organs, brain, embryos, and inflamed tissues: in situ analysis using a novel Il-33-LacZ gene trap reporter strain. J Immunol 188(7):3488–3495. 10.4049/jimmunol.1101977 [DOI] [PubMed] [Google Scholar]
- Povoleri GAM, Nova-Lamperti E, Scotta C, Fanelli G, Chen YC, Becker PD, Boardman D, Costantini B, Romano M, Pavlidis P, McGregor R, Pantazi E, Chauss D, Sun HW, Shih HY, Cousins DJ, Cooper N, Powell N, Kemper C, Pirooznia M, Laurence A, Kordasti S, Kazemian M, Lombardi G, Afzali B (2018) Human retinoic acid-regulated CD161 (+) regulatory T cells support wound repair in intestinal mucosa. Nat Immunol 19(12):1403–1414. 10.1038/s41590-018-0230-z [DOI] [PMC free article] [PubMed] [Google Scholar]
- Powrie F, Mason D (1990) OX-22high CD4+ T cells induce wasting disease with multiple organ pathology: prevention by the OX-22low subset. J Exp Med 172(6):1701–1708. 10.1084/jem.172.6.1701 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Powrie F, Leach MW, Mauze S, Caddle LB, Coffman RL (1993) Phenotypically distinct subsets of CD4+ T cells induce or protect from chronic intestinal inflammation in C. B-17 scid mice. Int Immunol 5(11):1461–1471. 10.1093/intimm/5.11.1461 [DOI] [PubMed] [Google Scholar]
- Powrie F, Carlino J, Leach MW, Mauze S, Coffman RL (1996) A critical role for transforming growth factor-beta but not interleukin 4 in the suppression of T helper type 1-mediated colitis by CD45RB(low) CD4+ T cells. J Exp Med 183(6):2669–2674. 10.1084/jem.183.6.2669 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Qazi KR, Bach Jensen G, van der Heiden M, Bjorkander S, Holmlund U, Haileselassie Y, Kokkinou E, Marchini G, Jenmalm MC, Abrahamsson T, Sverremark-Ekstrom E (2020) Extremely preterm infants have significant alterations in their conventional T cell compartment during the first weeks of life. J Immunol 204(1):68–77. 10.4049/jimmunol.1900941 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ramalingam R, Larmonier CB, Thurston RD, Midura-Kiela MT, Zheng SG, Ghishan FK, Kiela PR (2012) Dendritic cell-specific disruption of TGF-beta receptor II leads to altered regulatory T cell phenotype and spontaneous multiorgan autoimmunity. J Immunol 189(8):3878–3893. 10.4049/jimmunol.1201029 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Read S, Malmstrom V, Powrie F (2000) Cytotoxic T lymphocyte-associated antigen 4 plays an essential role in the function of CD25(+)CD4(+) regulatory cells that control intestinal inflammation. J Exp Med 192(2):295–302. 10.1084/jem.192.2.295 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reifen R, Nur T, Ghebermeskel K, Zaiger G, Urizky R, Pines M (2002) Vitamin A deficiency exacerbates inflammation in a rat model of colitis through activation of nuclear factor-kappaB and collagen formation. J Nutr 132(9):2743–2747. 10.1093/jn/132.9.2743 [DOI] [PubMed] [Google Scholar]
- Reikvam DH, Perminow G, Lyckander LG, Gran JM, Brandtzaeg P, Vatn M, Carlsen HS (2011) Increase of regulatory T cells in ileal mucosa of untreated pediatric Crohn’s disease patients. Scand J Gastroenterol 46(5):550–560. 10.3109/00365521.2011.551887 [DOI] [PubMed] [Google Scholar]
- Rieger K, Loddenkemper C, Maul J, Fietz T, Wolff D, Terpe H, Steiner B, Berg E, Miehlke S, Bornhauser M, Schneider T, Zeitz M, Stein H, Thiel E, Duchmann R, Uharek L (2006) Mucosal FOXP3+ regulatory T cells are numerically deficient in acute and chronic GvHD. Blood 107(4):1717–1723. 10.1182/blood-2005-06-2529 [DOI] [PubMed] [Google Scholar]
- Rodriguez-Cabezas ME, Galvez J, Lorente MD, Concha A, Camuesco D, Azzouz S, Osuna A, Redondo L, Zarzuelo A (2002) Dietary fiber down-regulates colonic tumor necrosis factor alpha and nitric oxide production in trinitrobenzenesulfonic acid-induced colitic rats. J Nutr 132(11):3263–3271. 10.1093/jn/132.11.3263 [DOI] [PubMed] [Google Scholar]
- Roncarolo MG, Gregori S, Bacchetta R, Battaglia M, Gagliani N (2018) The biology of T regulatory type 1 cells and their therapeutic application in immune-mediated diseases. Immunity 49(6):1004–1019. 10.1016/j.immuni.2018.12.001 [DOI] [PubMed] [Google Scholar]
- Round JL, Mazmanian SK (2010) Inducible Foxp3+ regulatory T-cell development by a commensal bacterium of the intestinal microbiota. Proc Natl Acad Sci U S A 107(27):12204–12209. 10.1073/pnas.0909122107 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Round JL, Lee SM, Li J, Tran G, Jabri B, Chatila TA, Mazmanian SK (2011) The Toll-like receptor 2 pathway establishes colonization by a commensal of the human microbiota. Science 332(6032):974–977. 10.1126/science.1206095 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sadlack B, Merz H, Schorle H, Schimpl A, Feller AC, Horak I (1993) Ulcerative colitis-like disease in mice with a disrupted interleukin-2 gene. Cell 75(2):253–261. 10.1016/0092-8674(93)80067-0 [DOI] [PubMed] [Google Scholar]
- Saito T, Nishikawa H, Wada H, Nagano Y, Sugiyama D, Atarashi K, Maeda Y, Hamaguchi M, Ohkura N, Sato E, Nagase H, Nishimura J, Yamamoto H, Takiguchi S, Tanoue T, Suda W, Morita H, Hattori M, Honda K, Mori M, Doki Y, Sakaguchi S (2016) Two FOXP3(+)CD4(+) T cell subpopulations distinctly control the prognosis of colorectal cancers. Nat Med 22(6):679–684. 10.1038/nm.4086 [DOI] [PubMed] [Google Scholar]
- Sakaguchi S, Sakaguchi N, Asano M, Itoh M, Toda M (1995) Immunologic self-tolerance maintained by activated T cells expressing IL-2 receptor alpha-chains (CD25). Breakdown of a single mechanism of self-tolerance causes various autoimmune diseases. J Immunol 155(3):1151–1164 [PubMed] [Google Scholar]
- Salama P, Phillips M, Grieu F, Morris M, Zeps N, Joseph D, Platell C, Iacopetta B (2009) Tumor-infiltrating FOXP3+ T regulatory cells show strong prognostic significance in colorectal cancer. J Clin Oncol 27(2):186–192. 10.1200/JCO.2008.18.7229 [DOI] [PubMed] [Google Scholar]
- Salomon B, Lenschow DJ, Rhee L, Ashourian N, Singh B, Sharpe A, Bluestone JA (2000) B7/CD28 costimulation is essential for the homeostasis of the CD4+CD25+ immunoregulatory T cells that control autoimmune diabetes. Immunity 12(4):431–440. 10.1016/s1074-7613(00)80195-8 [DOI] [PubMed] [Google Scholar]
- Salomon BL, Leclerc M, Tosello J, Ronin E, Piaggio E, Cohen JL (2018) Tumor necrosis factor alpha and regulatory T cells in oncoimmunology. Front Immunol 9:444. 10.3389/fimmu.2018.00444 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Salvati VM, Mazzarella G, Gianfrani C, Levings MK, Stefanile R, De Giulio B, Iaquinto G, Giardullo N, Auricchio S, Roncarolo MG, Troncone R (2005) Recombinant human interleukin 10 suppresses gliadin dependent T cell activation in ex vivo cultured coeliac intestinal mucosa. Gut 54(1):46–53. 10.1136/gut.2003.023150 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sanchez E, Donat E, Ribes-Koninckx C, Calabuig M, Sanz Y (2010) Intestinal bacteroides species associated with coeliac disease. J Clin Pathol 63(12):1105–1111. 10.1136/jcp.2010.076950 [DOI] [PubMed] [Google Scholar]
- Sanctuary MR, Huang RH, Jones AA, Luck ME, Aherne CM, Jedlicka P, de Zoeten EF, Collins CB (2019) miR-106a deficiency attenuates inflammation in murine IBD models. Mucosal Immunol 12(1):200–211. 10.1038/s41385-018-0091-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sands BE, Feagan BG, Sandborn WJ, Schreiber S, Peyrin-Biroulet L, Colombel JF, Rossiter G, Usiskin K, Ather S, Zhan X, D’Haens G (2019) Mongersen (GED-0301) for active Crohn’s disease: results of a phase 3 study. Am J Gastroenterol 115:738–745. 10.14309/ajg.0000000000000493 [DOI] [PubMed] [Google Scholar]
- Sarmento OF, Svingen PA, Xiong Y, Sun Z, Bamidele AO, Mathison AJ, Smyrk TC, Nair AA, Gonzalez MM, Sagstetter MR, Baheti S, McGovern DP, Friton JJ, Papadakis KA, Gautam G, Xavier RJ, Urrutia RA, Faubion WA (2017) The role of the histone methyltransferase enhancer of Zeste homolog 2 (EZH2) in the Pathobiological mechanisms underlying inflammatory bowel disease (IBD). J Biol Chem 292(2):706–722. 10.1074/jbc.M116.749663 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sarrabayrouse G, Bossard C, Chauvin JM, Jarry A, Meurette G, Quevrain E, Bridonneau C, Preisser L, Asehnoune K, Labarriere N, Altare F, Sokol H, Jotereau F (2014) CD4CD8alphaalpha lymphocytes, a novel human regulatory T cell subset induced by colonic bacteria and deficient in patients with inflammatory bowel disease. PLoS Biol 12(4):e1001833. 10.1371/journal.pbio.1001833 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sarrabayrouse G, Landolfi S, Pozuelo M, Willamil J, Varela E, Clark A, Campos D, Herrera C, Santiago A, Machiels K, Vermeire S, Marti M, Espin E, Manichanh C (2020) Mucosal microbial load in Crohn’s disease: a potential predictor of response to faecal microbiota transplantation. EBioMedicine 51:102611. 10.1016/j.ebiom.2019.102611 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schiering C, Krausgruber T, Chomka A, Frohlich A, Adelmann K, Wohlfert EA, Pott J, Griseri T, Bollrath J, Hegazy AN, Harrison OJ, Owens BMJ, Lohning M, Belkaid Y, Fallon PG, Powrie F (2014) The alarmin IL-33 promotes regulatory T-cell function in the intestine. Nature 513(7519):564–568. 10.1038/nature13577 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schlenner SM, Weigmann B, Ruan Q, Chen Y, von Boehmer H (2012) Smad3 binding to the foxp3 enhancer is dispensable for the development of regulatory T cells with the exception of the gut. J Exp Med 209(9):1529–1535. 10.1084/jem.20112646 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scurr M, Ladell K, Besneux M, Christian A, Hockey T, Smart K, Bridgeman H, Hargest R, Phillips S, Davies M, Price D, Gallimore A, Godkin A (2014) Highly prevalent colorectal cancer-infiltrating LAP(+) Foxp3(−) T cells exhibit more potent immunosuppressive activity than Foxp3(+) regulatory T cells. Mucosal Immunol 7(2):428–439. 10.1038/mi.2013.62 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seddiki N, Santner-Nanan B, Martinson J, Zaunders J, Sasson S, Landay A, Solomon M, Selby W, Alexander SI, Nanan R, Kelleher A, Fazekas de St Groth B (2006) Expression of interleukin (IL)-2 and IL-7 receptors discriminates between human regulatory and activated T cells. J Exp Med 203(7):1693–1700. 10.1084/jem.20060468 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sefik E, Geva-Zatorsky N, Oh S, Konnikova L, Zemmour D, McGuire AM, Burzyn D, Ortiz-Lopez A, Lobera M, Yang J, Ghosh S, Earl A, Snapper SB, Jupp R, Kasper D, Mathis D, Benoist C (2015) MUCOSAL IMMUNOLOGY. Individual intestinal symbionts induce a distinct population of RORgamma(+) regulatory T cells. Science 349(6251):993–997. 10.1126/science.aaa9420 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seidelin JB, Bjerrum JT, Coskun M, Widjaya B, Vainer B, Nielsen OH (2010) IL-33 is upregulated in colonocytes of ulcerative colitis. Immunol Lett 128(1):80–85. 10.1016/j.imlet.2009.11.001 [DOI] [PubMed] [Google Scholar]
- Serena G, Yan S, Camhi S, Patel S, Lima RS, Sapone A, Leonard MM, Mukherjee R, Nath BJ, Lammers KM, Fasano A (2017) Proinflammatory cytokine interferon-gamma and microbiome-derived metabolites dictate epigenetic switch between forkhead box protein 3 isoforms in coeliac disease. Clin Exp Immunol 187(3):490–506. 10.1111/cei.12911 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Setoguchi R, Hori S, Takahashi T, Sakaguchi S (2005) Homeostatic maintenance of natural Foxp3(+) CD25(+) CD4(+) regulatory T cells by interleukin (IL)-2 and induction of autoimmune disease by IL-2 neutralization. J Exp Med 201(5):723–735. 10.1084/jem.20041982 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sharfe N, Dadi HK, Shahar M, Roifman CM (1997) Human immune disorder arising from mutation of the alpha chain of the interleukin-2 receptor. Proc Natl Acad Sci U S A 94(7):3168–3171. 10.1073/pnas.94.7.3168 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sharifi A, Vahedi H, Nedjat S, Rafiei H, Hosseinzadeh-Attar MJ (2019) Effect of single-dose injection of vitamin D on immune cytokines in ulcerative colitis patients: a randomized placebo-controlled trial. APMIS 127(10):681–687. 10.1111/apm.12982 [DOI] [PubMed] [Google Scholar]
- Shevach EM, Thornton AM (2014) tTregs, pTregs, and iTregs: similarities and differences. Immunol Rev 259(1):88–102. 10.1111/imr.12160 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shono Y, Docampo MD, Peled JU, Perobelli SM, Velardi E, Tsai JJ, Slingerland AE, Smith OM, Young LF, Gupta J, Lieberman SR, Jay HV, Ahr KF, Porosnicu Rodriguez KA, Xu K, Calarfiore M, Poeck H, Caballero S, Devlin SM, Rapaport F, Dudakov JA, Hanash AM, Gyurkocza B, Murphy GF, Gomes C, Liu C, Moss EL, Falconer SB, Bhatt AS, Taur Y, Pamer EG, van den Brink MRM, Jenq RR (2016) Increased GVHD-related mortality with broad-spectrum antibiotic use after allogeneic hematopoietic stem cell transplantation in human patients and mice. Sci Transl Med 8(339):339ra371. 10.1126/scitranslmed.aaf2311 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shull MM, Ormsby I, Kier AB, Pawlowski S, Diebold RJ, Yin M, Allen R, Sidman C, Proetzel G, Calvin D et al. (1992) Targeted disruption of the mouse transforming growth factor-beta 1 gene results in multifocal inflammatory disease. Nature 359(6397):693–699. 10.1038/359693a0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Siede J, Frohlich A, Datsi A, Hegazy AN, Varga DV, Holecska V, Saito H, Nakae S, Lohning M (2016) IL-33 receptor-expressing regulatory T cells are highly activated, Th2 biased and suppress CD4 T cell proliferation through IL-10 and TGFbeta release. PLoS One 11(8):e0161507. 10.1371/journal.pone.0161507 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Singh N, Gurav A, Sivaprakasam S, Brady E, Padia R, Shi H, Thangaraju M, Prasad PD, Manicassamy S, Munn DH, Lee JR, Offermanns S, Ganapathy V (2014) Activation of Gpr109a, receptor for niacin and the commensal metabolite butyrate, suppresses colonic inflammation and carcinogenesis. Immunity 40(1):128–139. 10.1016/j.immuni.2013.12.007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sinicrope FA, Rego RL, Ansell SM, Knutson KL, Foster NR, Sargent DJ (2009) Intraepithelial effector (CD3+)/regulatory (FoxP3+) T-cell ratio predicts a clinical outcome of human colon carcinoma. Gastroenterology 137(4):1270–1279. 10.1053/j.gastro.2009.06.053 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith PM, Howitt MR, Panikov N, Michaud M, Gallini CA, Bohlooly YM, Glickman JN, Garrett WS (2013) The microbial metabolites, short-chain fatty acids, regulate colonic Treg cell homeostasis. Science 341(6145):569–573. 10.1126/science.1241165 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sokol H, Pigneur B, Watterlot L, Lakhdari O, Bermudez-Humaran LG, Gratadoux JJ, Blugeon S, Bridonneau C, Furet JP, Corthier G, Grangette C, Vasquez N, Pochart P, Trugnan G, Thomas G, Blottiere HM, Dore J, Marteau P, Seksik P, Langella P (2008) Faecalibacterium prausnitzii is an anti-inflammatory commensal bacterium identified by gut microbiota analysis of Crohn disease patients. Proc Natl Acad Sci U S A 105(43):16731–16736. 10.1073/pnas.0804812105 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sokol H, Seksik P, Furet JP, Firmesse O, Nion-Larmurier I, Beaugerie L, Cosnes J, Corthier G, Marteau P, Dore J (2009) Low counts of Faecalibacterium prausnitzii in colitis microbiota. Inflamm Bowel Dis 15(8):1183–1189. 10.1002/ibd.20903 [DOI] [PubMed] [Google Scholar]
- Solomon BD, Hsieh CS (2016) Antigen-specific development of mucosal Foxp3+RORgammat+ T cells from regulatory T cell precursors. J Immunol 197(9):3512–3519. 10.4049/jimmunol.1601217 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Somasundaram R, Jacob L, Swoboda R, Caputo L, Song H, Basak S, Monos D, Peritt D, Marincola F, Cai D, Birebent B, Bloome E, Kim J, Berencsi K, Mastrangelo M, Herlyn D (2002) Inhibition of cytolytic T lymphocyte proliferation by autologous CD4+/CD25+ regulatory T cells in a colorectal carcinoma patient is mediated by transforming growth factor-beta. Cancer Res 62(18):5267–5272 [PubMed] [Google Scholar]
- Stefka AT, Feehley T, Tripathi P, Qiu J, McCoy K, Mazmanian SK, Tjota MY, Seo GY, Cao S, Theriault BR, Antonopoulos DA, Zhou L, Chang EB, Fu YX, Nagler CR (2014) Commensal bacteria protect against food allergen sensitization. Proc Natl Acad Sci U S A 111(36):13145–13150. 10.1073/pnas.1412008111 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Strauss L, Bergmann C, Szczepanski MJ, Lang S, Kirkwood JM, Whiteside TL (2008) Expression of ICOS on human melanoma-infiltrating CD4 +CD25highFoxp3+ T regulatory cells: implications and impact on tumor-mediated immune suppression. J Immunol 180(5):2967–2980. 10.4049/jimmunol.180.5.2967 [DOI] [PubMed] [Google Scholar]
- Stritesky GL, Jameson SC, Hogquist KA (2012) Selection of self-reactive T cells in the thymus. Annu Rev Immunol 30:95–114. 10.1146/annurev-immunol-020711-075035 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Su W, Chen X, Zhu W, Yu J, Li W, Li Y, Li Z, Olsen N, Liang D, Zheng SG (2019) The cAMP-adenosine feedback loop maintains the suppressive function of regulatory T cells. J Immunol 203(6):1436–1446. 10.4049/jimmunol.1801306 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sulimani RA (2019) Celiac disease and severe vitamin D deficiency: the case for anti-tissue transglutaminase antibody screening. Arch Osteoporos 14(1):30. 10.1007/s11657-018-0554-1 [DOI] [PubMed] [Google Scholar]
- Sun CM, Hall JA, Blank RB, Bouladoux N, Oukka M, Mora JR, Belkaid Y (2007) Small intestine lamina propria dendritic cells promote de novo generation of Foxp3 T reg cells via retinoic acid. J Exp Med 204(8):1775–1785. 10.1084/jem.20070602 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sun B, Liu M, Cui M, Li T (2020) Granzyme B-expressing Treg cells are enriched in colorectal cancer and present the potential to eliminate autologous T conventional cells. Immunol Lett 217:7–14. 10.1016/j.imlet.2019.10.007 [DOI] [PubMed] [Google Scholar]
- Sundstrom P, Stenstad H, Langenes V, Ahlmanner F, Theander L, Ndah TG, Fredin K, Borjesson L, Gustavsson B, Bastid J, Quiding-Jarbrink M (2016) Regulatory T cells from colon cancer patients inhibit effector T-cell migration through an adenosine-dependent mechanism. Cancer Immunol Res 4(3):183–193. 10.1158/2326-6066.CIR-15-0050 [DOI] [PubMed] [Google Scholar]
- Suzuki H, Chikazawa N, Tasaka T, Wada J, Yamasaki A, Kitaura Y, Sozaki M, Tanaka M, Onishi H, Morisaki T, Katano M (2010) Intratumoral CD8(+) T/FOXP3 (+) cell ratio is a predictive marker for survival in patients with colorectal cancer. Cancer Immunol Immunother 59(5):653–661. 10.1007/s00262-009-0781-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tai X, Cowan M, Feigenbaum L, Singer A (2005) CD28 costimulation of developing thymocytes induces Foxp3 expression and regulatory T cell differentiation independently of interleukin 2. Nat Immunol 6(2):152–162. 10.1038/ni1160 [DOI] [PubMed] [Google Scholar]
- Takahashi T, Tagami T, Yamazaki S, Uede T, Shimizu J, Sakaguchi N, Mak TW, Sakaguchi S (2000) Immunologic self-tolerance maintained by CD25(+)CD4(+) regulatory T cells constitutively expressing cytotoxic T lymphocyte-associated antigen 4. J Exp Med 192(2):303–310. 10.1084/jem.192.2.303 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tan H, Zhao J, Zhang H, Zhai Q, Chen W (2019) Novel strains of Bacteroides fragilis and Bacteroides ovatus alleviate the LPS-induced inflammation in mice. Appl Microbiol Biotechnol 103(5):2353–2365. 10.1007/s00253-019-09617-1 [DOI] [PubMed] [Google Scholar]
- Tang Q, Boden EK, Henriksen KJ, Bour-Jordan H, Bi M, Bluestone JA (2004) Distinct roles of CTLA-4 and TGF-beta in CD4+CD25+ regulatory T cell function. Eur J Immunol 34(11):2996–3005. 10.1002/eji.200425143 [DOI] [PubMed] [Google Scholar]
- Taylor ES, McCall JL, Girardin A, Munro FM, Black MA, Kemp RA (2016) Functional impairment of infiltrating T cells in human colorectal cancer. Onco Targets Ther 5(11):e1234573. 10.1080/2162402X.2016.1234573 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Telesford KM, Yan W, Ochoa-Reparaz J, Pant A, Kircher C, Christy MA, Begum-Haque S, Kasper DL, Kasper LH (2015) A commensal symbiotic factor derived from Bacteroides fragilis promotes human CD39(+)Foxp3(+) T cells and Treg function. Gut Microbes 6(4):234–242. 10.1080/19490976.2015.1056973 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Terme M, Pernot S, Marcheteau E, Sandoval F, Benhamouda N, Colussi O, Dubreuil O, Carpentier AF, Tartour E, Taieb J (2013) VEGFA-VEGFR pathway blockade inhibits tumor-induced regulatory T-cell proliferation in colorectal cancer. Cancer Res 73(2):539–549. 10.1158/0008-5472.CAN-12-2325 [DOI] [PubMed] [Google Scholar]
- Thakur BK, Saha P, Banik G, Saha DR, Grover S, Batish VK, Das S (2016) Live and heat-killed probiotic Lactobacillus casei Lbs2 protects from experimental colitis through toll-like receptor 2-dependent induction of T-regulatory response. Int Immunopharmacol 36:39–50. 10.1016/j.intimp.2016.03.033 [DOI] [PubMed] [Google Scholar]
- Thornton AM, Korty PE, Tran DQ, Wohlfert EA, Murray PE, Belkaid Y, Shevach EM (2010) Expression of Helios, an Ikaros transcription factor family member, differentiates thymic-derived from peripherally induced Foxp3+ T regulatory cells. J Immunol 184(7):3433–3441. 10.4049/jimmunol.0904028 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tone Y, Furuuchi K, Kojima Y, Tykocinski ML, Greene MI, Tone M (2008) Smad3 and NFAT cooperate to induce Foxp3 expression through its enhancer. Nat Immunol 9(2):194–202. 10.1038/ni1549 [DOI] [PubMed] [Google Scholar]
- Toor SM, Murshed K, Al-Dhaheri M, Khawar M, Abu Nada M, Elkord E (2019) Immune checkpoints in circulating and tumor-infiltrating CD4(+) T cell subsets in colorectal cancer patients. Front Immunol 10:2936. 10.3389/fimmu.2019.02936 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tran DQ, Ramsey H, Shevach EM (2007) Induction of FOXP3 expression in naive human CD4+FOXP3 T cells by T-cell receptor stimulation is transforming growth factor-beta dependent but does not confer a regulatory phenotype. Blood 110(8):2983–2990. 10.1182/blood-2007-06-094656 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Travis MA, Reizis B, Melton AC, Masteller E, Tang Q, Proctor JM, Wang Y, Bernstein X, Huang X, Reichardt LF, Bluestone JA, Sheppard D (2007) Loss of integrin alpha(v)beta8 on dendritic cells causes autoimmunity and colitis in mice. Nature 449(7160):361–365. 10.1038/nature06110 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tripathi T, Yin W, Xue Y, Zurawski S, Fujita H, Hanabuchi S, Liu YJ, Oh S, Joo H (2019) Central roles of OX40L-OX40 interaction in the induction and progression of human T cell-driven acute graft-versus-host disease. Immunohorizons 3(3):110–120. 10.4049/immunohorizons.1900001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tsai S, Clemente-Casares X, Santamaria P (2011) CD8(+) Tregs in autoimmunity: learning “self”-control from experience. Cell Mol Life Sci 68(23):3781–3795. 10.1007/s00018-011-0738-y [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ueno A, Jijon H, Chan R, Ford K, Hirota C, Kaplan GG, Beck PL, Iacucci M, Fort Gasia M, Barkema HW, Panaccione R, Ghosh S (2013) Increased prevalence of circulating novel IL-17 secreting Foxp3 expressing CD4+ T cells and defective suppressive function of circulating Foxp3+ regulatory cells support plasticity between Th17 and regulatory T cells in inflammatory bowel disease patients. Inflamm Bowel Dis 19(12):2522–2534. 10.1097/MIB.0b013e3182a85709 [DOI] [PubMed] [Google Scholar]
- Ulloa L, Doody J, Massague J (1999) Inhibition of transforming growth factor-beta/SMAD signalling by the interferon-gamma/STAT pathway. Nature 397(6721):710–713. 10.1038/17826 [DOI] [PubMed] [Google Scholar]
- Van den Abbeele P, Grootaert C, Marzorati M, Possemiers S, Verstraete W, Gerard P, Rabot S, Bruneau A, El Aidy S, Derrien M, Zoetendal E, Kleerebezem M, Smidt H, Van de Wiele T (2010) Microbial community development in a dynamic gut model is reproducible, colon region specific, and selective for Bacteroidetes and Clostridium cluster IX. Appl Environ Microbiol 76(15):5237–5246. 10.1128/AEM.00759-10 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vasanthakumar A, Liao Y, Teh P, Pascutti MF, Oja AE, Garnham AL, Gloury R, Tempany JC, Sidwell T, Cuadrado E, Tuijnenburg P, Kuijpers TW, Lalaoui N, Mielke LA, Bryant VL, Hodgkin PD, Silke J, Smyth GK, Nolte MA, Shi W, Kallies A (2017) The TNF receptor superfamily-NF-kappaB Axis is critical to maintain effector regulatory T cells in lymphoid and non-lymphoid tissues. Cell Rep 20(12):2906–2920. 10.1016/jxelrep.2017.08.068 [DOI] [PubMed] [Google Scholar]
- Vaughn BP, Vatanen T, Allegretti JR, Bai A, Xavier RJ, Korzenik J, Gevers D, Ting A, Robson SC, Moss AC (2016) Increased intestinal microbial diversity following fecal microbiota transplant for active Crohn’s disease. Inflamm Bowel Dis 22(9):2182–2190. 10.1097/MIB.0000000000000893 [DOI] [PMC free article] [PubMed] [Google Scholar]
- te Velde AA, de Kort F, Sterrenburg E, Pronk I, ten Kate FJ, Hommes DW, van Deventer SJ (2007) Comparative analysis of colonic gene expression of three experimental colitis models mimicking inflammatory bowel disease. Inflamm Bowel Dis 13(3):325–330. 10.1002/ibd.20079 [DOI] [PubMed] [Google Scholar]
- te Velde AA, Brull F, Heinsbroek SE, Meijer SL, Lutjohann D, Vreugdenhil A, Plat J (2015) Effects of dietary plant sterols and Stanol esters with low- and high-fat diets in chronic and acute models for experimental colitis. Nutrients 7(10):8518–8531. 10.3390/nu7105412 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Veltkamp C, Anstaett M, Wahl K, Moller S, Gangl S, Bachmann O, Hardtke-Wolenski M, Langer F, Stremmel W, Manns MP, Schulze-Osthoff K, Bantel H (2011) Apoptosis of regulatory T lymphocytes is increased in chronic inflammatory bowel disease and reversed by anti-TNFalpha treatment. Gut 60(10):1345–1353. 10.1136/gut.2010.217117 [DOI] [PubMed] [Google Scholar]
- Verma R, Lee C, Jeun EJ, Yi J, Kim KS, Ghosh A, Byun S, Lee CG, Kang HJ, Kim GC, Jun CD, Jan G, Suh CH, Jung JY, Sprent J, Rudra D, De Castro C, Molinaro A, Surh CD, Im SH (2018) Cell surface polysaccharides of Bifidobacterium bifidum induce the generation of Foxp3(+) regulatory T cells. Sci Immunol 3(28):eaat6975. 10.1126/sciimmunol.aat6975 [DOI] [PubMed] [Google Scholar]
- Vicente-Suarez I, Larange A, Reardon C, Matho M, Feau S, Chodaczek G, Park Y, Obata Y, Gold R, Wang-Zhu Y, Lena C, Zajonc DM, Schoenberger SP, Kronenberg M, Cheroutre H (2015) Unique lamina propria stromal cells imprint the functional phenotype of mucosal dendritic cells. Mucosal Immunol 8(1):141–151. 10.1038/mi.2014.51 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Visperas A, Shen B, Min B (2014) gammadelta T cells restrain extrathymic development of Foxp3 +−inducible regulatory T cells via IFN-gamma. Eur J Immunol 44(8):2448–2456. 10.1002/eji.201344331 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vitale A, Strisciuglio C, Vitale S, Santopaolo M, Bruzzese D, Micillo T, Scarpato E, Miele E, Staiano A, Troncone R, Matarese G, Gianfrani C (2019) Increased frequency of regulatory T cells in pediatric inflammatory bowel disease at diagnosis: a compensative role? Pediatr Res 87:853. 10.1038/s41390-019-0662-7 [DOI] [PubMed] [Google Scholar]
- Wang J, Ioan-Facsinay A, van der Voort EI, Huizinga TW, Toes RE (2007) Transient expression of FOXP3 in human activated nonregulatory CD4+ T cells. Eur J Immunol 37(1):129–138. 10.1002/eji.200636435 [DOI] [PubMed] [Google Scholar]
- Wang Y, Su MA, Wan YY (2011) An essential role of the transcription factor GATA-3 for the function of regulatory T cells. Immunity 35(3):337–348. 10.1016/j.immuni.2011.08.012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang J, Ti Y, Wang Y, Guo G, Jiang H, Chang M, Qian H, Zhao J, Sun G (2018) LAG-3 represents a marker of CD4+ T cells with regulatory activity in patients with bone fracture. Immunol Investig 47(5):492–503. 10.1080/08820139.2018.1458107 [DOI] [PubMed] [Google Scholar]
- Ward ST, Li KK, Hepburn E, Weston CJ, Curbishley SM, Reynolds GM, Hejmadi RK, Bicknell R, Eksteen B, Ismail T, Rot A, Adams DH (2015) The effects of CCR5 inhibition on regulatory T-cell recruitment to colorectal cancer. Br J Cancer 112(2):319–328. 10.1038/bjc.2014.572 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ward-Hartstonge KA, McCall JL, McCulloch TR, Kamps AK, Girardin A, Cretney E, Munro FM, Kemp RA (2017) Inclusion of BLIMP-1(+) effector regulatory T cells improves the Immunoscore in a cohort of New Zealand colorectal cancer patients: a pilot study. Cancer Immunol Immunother 66(4):515–522. 10.1007/s00262-016-1951-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weiss JM, Bilate AM, Gobert M, Ding Y, Curotto de Lafaille MA, Parkhurst CN, Xiong H, Dolpady J, Frey AB, Ruocco MG, Yang Y, Floess S, Huehn J, Oh S, Li MO, Niec RE, Rudensky AY, Dustin ML, Littman DR, Lafaille JJ (2012) Neuropilin 1 is expressed on thymus-derived natural regulatory T cells, but not mucosa-generated induced Foxp3+ T reg cells. J Exp Med 209(10):1723–1742, S1721. 10.1084/jem.20120914 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weitkamp JH, Rudzinski E, Koyama T, Correa H, Matta P, Alberty B, Polk DB (2009) Ontogeny of FOXP3(+) regulatory T cells in the postnatal human small intestinal and large intestinal lamina propria. Pediatr Dev Pathol 12(6):443–449. 10.2350/08-09-0533.1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weitkamp JH, Koyama T, Rock MT, Correa H, Goettel JA, Matta P, Oswald-Richter K, Rosen MJ, Engelhardt BG, Moore DJ, Polk DB (2013) Necrotising enterocolitis is characterised by disrupted immune regulation and diminished mucosal regulatory (FOXP3)/effector (CD4, CD8) T cell ratios. Gut 62(1):73–82. 10.1136/gutjnl-2011-301551 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wildin RS, Ramsdell F, Peake J, Faravelli F, Casanova JL, Buist N, Levy-Lahad E, Mazzella M, Goulet O, Perroni L, Bricarelli FD, Byrne G, McEuen M, Proll S, Appleby M, Brunkow ME (2001) X-linked neonatal diabetes mellitus, enteropathy and endocrinopathy syndrome is the human equivalent of mouse scurfy. Nat Genet 27(1):18–20. 10.1038/83707 [DOI] [PubMed] [Google Scholar]
- Wing K, Onishi Y, Prieto-Martin P, Yamaguchi T, Miyara M, Fehervari Z, Nomura T, Sakaguchi S (2008) CTLA-4 control over Foxp3+ regulatory T cell function. Science 322(5899):271–275. 10.1126/science.1160062 [DOI] [PubMed] [Google Scholar]
- Wohlfert EA, Grainger JR, Bouladoux N, Konkel JE, Oldenhove G, Ribeiro CH, Hall JA, Yagi R, Naik S, Bhairavabhotla R, Paul WE, Bosselut R, Wei G, Zhao K, Oukka M, Zhu J, Belkaid Y (2011) GATA3 controls Foxp3(+) regulatory T cell fate during inflammation in mice. J Clin Invest 121(11):4503–4515. 10.1172/JCI57456 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Worthington JJ, Czajkowska BI, Melton AC, Travis MA (2011) Intestinal dendritic cells specialize to activate transforming growth factor-beta and induce Foxp3+ regulatory T cells via integrin alphavbeta8. Gastroenterology 141(5) 1802–1812:1802. 10.1053/j.gastro.2011.06.057 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wysocki CA, Panoskaltsis-Mortari A, Blazar BR, Serody JS (2005) Leukocyte migration and graft-versus-host disease. Blood 105(11):4191–4199. 10.1182/blood-2004-12-4726 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xiao S, Li Q, Hu K, He Y, Ai Q, Hu L, Yu J (2018) Vitamin A and retinoic acid exhibit protective effects on necrotizing enterocolitis by regulating intestinal flora and enhancing the intestinal epithelial barrier. Arch Med Res 49(1):1–9. 10.1016/j.arcmed.2018.04.003 [DOI] [PubMed] [Google Scholar]
- Xing Y, Hogquist KA (2012) T-cell tolerance: central and peripheral. Cold Spring Harb Perspect Biol 4(6):a006957. 10.1101/cshperspect.a006957 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu L, Kitani A, Fuss I, Strober W (2007) Cutting edge: regulatory T cells induce CD4+CD25-Foxp3-T cells or are self-induced to become Th17 cells in the absence of exogenous TGF-beta. J Immunol 178(11):6725–6729. 10.4049/jimmunol.178.11.6725 [DOI] [PubMed] [Google Scholar]
- Xu L, Kitani A, Stuelten C, McGrady G, Fuss I, Strober W (2010) Positive and negative transcriptional regulation of the Foxp3 gene is mediated by access and binding of the Smad3 protein to enhancer I. Immunity 33(3):313–325. 10.1016/j.immuni.2010.09.001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu A, Liu Y, Chen W, Wang J, Xue Y, Huang F, Rong L, Lin J, Liu D, Yan M, Li QZ, Li B, Song J, Olsen N, Zheng SG (2016) TGF-beta-induced regulatory T cells directly suppress B cell responses through a noncytotoxic mechanism. J Immunol 196(9):3631–3641. 10.4049/jimmunol.1501740 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu M, Pokrovskii M, Ding Y, Yi R, Au C, Harrison OJ, Galan C, Belkaid Y, Bonneau R, Littman DR (2018) c-MAF-dependent regulatory T cells mediate immunological tolerance to a gut pathobiont. Nature 554(7692):373–377. 10.1038/nature25500 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yadav M, Louvet C, Davini D, Gardner JM, Martinez-Llordella M, Bailey-Bucktrout S, Anthony BA, Sverdrup FM, Head R, Kuster DJ, Ruminski P, Weiss D, Von Schack D, Bluestone JA (2012) Neuropilin-1 distinguishes natural and inducible regulatory T cells among regulatory T cell subsets in vivo. J Exp Med 209(10):1713–1722, S1711–1719. 10.1084/jem.20120822 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yadav M, Stephan S, Bluestone JA (2013) Peripherally induced tregs - role in immune homeostasis and autoimmunity. Front Immunol 4:232. 10.3389/fimmu.2013.00232 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yamaguchi T, Hirota K, Nagahama K, Ohkawa K, Takahashi T, Nomura T, Sakaguchi S (2007) Control of immune responses by antigen-specific regulatory T cells expressing the folate receptor. Immunity 27(1):145–159. 10.1016/j.immuni.2007.04.017 [DOI] [PubMed] [Google Scholar]
- Yang BH, Hagemann S, Mamareli P, Lauer U, Hoffmann U, Beckstette M, Fohse L, Prinz I, Pezoldt J, Suerbaum S, Sparwasser T, Hamann A, Floess S, Huehn J, Lochner M (2016) Foxp3(+) T cells expressing RORgammat represent a stable regulatory T-cell effector lineage with enhanced suppressive capacity during intestinal inflammation. Mucosal Immunol 9(2):444–457. 10.1038/mi.2015.74 [DOI] [PubMed] [Google Scholar]
- Yaqub S, Henjum K, Mahic M, Jahnsen FL, Aandahl EM, Bjornbeth BA, Tasken K (2008) Regulatory T cells in colorectal cancer patients suppress anti-tumor immune activity in a COX-2 dependent manner. Cancer Immunol Immunother 57(6):813–821. 10.1007/s00262-007-0417-x [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ye JH, Rajendran VM (2009) Adenosine: an immune modulator of inflammatory bowel diseases. World J Gastroenterol 15(36):4491–4498. 10.3748/wjg.15.4491 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ye J, Qiu J, Bostick JW, Ueda A, Schjerven H, Li S, Jobin C, Chen ZE, Zhou L (2017) The aryl hydrocarbon receptor preferentially marks and promotes gut regulatory T cells. Cell Rep 21(8):2277–2290. 10.1016/j.celrep.2017.10.114 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yu QT, Saruta M, Avanesyan A, Fleshner PR, Banham AH, Papadakis KA (2007) Expression and functional characterization of FOXP3+ CD4+ regulatory T cells in ulcerative colitis. Inflamm Bowel Dis 13(2):191–199. 10.1002/ibd.20053 [DOI] [PubMed] [Google Scholar]
- Yu Y, Ma X, Gong R, Zhu J, Wei L, Yao J (2018) Recent advances in CD8(+) regulatory T cell research. Oncol Lett 15(6):8187–8194. 10.3892/ol.2018.8378 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yurchenko E, Shio MT, Huang TC, Da Silva Martins M, Szyf M, Levings MK, Olivier M, Piccirillo CA (2012) Inflammation-driven reprogramming of CD4+ Foxp3+ regulatory T cells into pathogenic Th1/Th17 T effectors is abrogated by mTOR inhibition in vivo. PLoS One 7(4):e35572. 10.1371/journal.pone.0035572 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zanzi D, Stefanile R, Santagata S, Iaffaldano L, Iaquinto G, Giardullo N, Lania G, Vigliano I, Vera AR, Ferrara K, Auricchio S, Troncone R, Mazzarella G (2011) IL-15 interferes with suppressive activity of intestinal regulatory T cells expanded in celiac disease. Am J Gastroenterol 106(7):1308–1317. 10.1038/ajg.2011.80 [DOI] [PubMed] [Google Scholar]
- Zeissig S, Petersen BS, Tomczak M, Melum E, Huc-Claustre E, Dougan SK, Laerdahl JK, Stade B, Forster M, Schreiber S, Weir D, Leichtner AM, Franke A, Blumberg RS (2015) Early-onset Crohn’s disease and autoimmunity associated with a variant in CTLA-4. Gut 64(12):1889–1897. 10.1136/gutjnl-2014-308541 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhai R, Xue X, Zhang L, Yang X, Zhao L, Zhang C (2019) Strain-specific anti-inflammatory properties of two Akkermansia muciniphila strains on chronic colitis in mice. Front Cell Infect Microbiol 9:239. 10.3389/fcimb.2019.00239 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang J, Ramadan AM, Griesenauer B, Li W, Turner MJ, Liu C, Kapur R, Hanenberg H, Blazar BR, Tawara I, Paczesny S (2015) ST2 blockade reduces sST2-producing T cells while maintaining protective mST2-expressing T cells during graft-versus-host disease. Sci Transl Med 7(308):308ra160. 10.1126/scitranslmed.aab0166 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang W, Zhou L, Dang J, Zhang X, Wang J, Chen Y, Liang J, Li D, Ma J, Yuan J, Chen W, Zadeh HH, Olsen N, Zheng SG (2017) Human gingiva-derived mesenchymal stem cells ameliorate Streptozoticin-induced T1DM in mice via suppression of T effector cells and up-regulating Treg subsets. Sci Rep 7(1):15249. 10.1038/s41598-017-14979-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang Q, Fu L, Liang Y, Guo Z, Wang L, Ma C, Wang H (2018) Exosomes originating from MSCs stimulated with TGF-beta and IFN-gamma promote Treg differentiation. J Cell Physiol 233(9):6832–6840. 10.1002/jcp.26436 [DOI] [PubMed] [Google Scholar]
- Zhang X, Ouyang X, Xu Z, Chen J, Huang Q, Liu Y, Xu T, Wang J, Olsen N, Xu A, Zheng SG (2019) CD8+CD103+ iTregs inhibit chronic graft-versus-host disease with lupus nephritis by the increased expression of CD39. Mol Ther 27(11):1963–1973. 10.1016/j.ymthe.2019.07.014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zheng SG, Gray JD, Ohtsuka K, Yamagiwa S, Horwitz DA (2002) Generation ex vivo of TGF-beta-producing regulatory T cells from CD4+CD25-precursors. J Immunol 169(8):4183–4189. 10.4049/jimmunol.169.8.4183 [DOI] [PubMed] [Google Scholar]
- Zheng SG, Wang JH, Gray JD, Soucier H, Horwitz DA (2004) Natural and induced CD4+CD25+ cells educate CD4+CD25− cells to develop suppressive activity: the role of IL-2, TGF-beta, and IL-10. J Immunol 172(9):5213–5221. 10.4049/jimmunol.172.9.5213 [DOI] [PubMed] [Google Scholar]
- Zheng SG, Wang J, Wang P, Gray JD, Horwitz DA (2007) IL-2 is essential for TGF-beta to convert naive CD4+CD25− cells to CD25+Foxp3+ regulatory T cells and for expansion of these cells. J Immunol 178(4):2018–2027. 10.4049/jimmunol.178.4.2018 [DOI] [PubMed] [Google Scholar]
- Zheng SG, Wang J, Horwitz DA (2008) Cutting edge: Foxp3+CD4+CD25+ regulatory T cells induced by IL-2 and TGF-beta are resistant to Th17 conversion by IL-6. J Immunol 180(11):7112–7116. 10.4049/jimmunol.180.n.7112 [DOI] [PubMed] [Google Scholar]
- Zheng Y, Josefowicz S, Chaudhry A, Peng XP, Forbush K, Rudensky AY (2010) Role of conserved non-coding DNA elements in the Foxp3 gene in regulatory T-cell fate. Nature 463(7282):808–812. 10.1038/nature08750 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zheng J, Chan PL, Liu Y, Qin G, Xiang Z, Lam KT, Lewis DB, Lau YL, Tu W (2013) ICOS regulates the generation and function of human CD4+ Treg in a CTLA-4 dependent manner. PLoS One 8(12):e82203. 10.1371/journal.pone.0082203 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhong W, Jiang ZY, Zhang L, Huang JH, Wang SJ, Liao C, Cai B, Chen LS, Zhang S, Guo Y, Cao YF, Gao F (2017) Role of LAP(+)CD4(+) T cells in the tumor microenvironment of colorectal cancer. World J Gastroenterol 23(3):455–463. 10.3748/wjg.v23.i3.455 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhou L, Lopes JE, Chong MM, Ivanov II, Min R, Victora GD, Shen Y, Du J, Rubtsov YP, Rudensky AY, Ziegler SF, Littman DR (2008) TGF-beta-induced Foxp3 inhibits T(H)17 cell differentiation by antagonizing RORgammat function. Nature 453(7192):236–240. 10.1038/nature06878 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhou X, Bailey-Bucktrout SL, Jeker LT, Penaranda C, Martinez-Llordella M, Ashby M, Nakayama M, Rosenthal W, Bluestone JA (2009) Instability of the transcription factor Foxp3 leads to the generation of pathogenic memory T cells in vivo. Nat Immunol 10(9):1000–1007. 10.1038/ni.1774 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhou X, Kong N, Wang J, Fan H, Zou H, Horwitz D, Brand D, Liu Z, Zheng SG (2010) Cutting edge: all-trans retinoic acid sustains the stability and function of natural regulatory T cells in an inflammatory milieu. J Immunol 185(5):2675–2679. 10.4049/jimmunol.1000598 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhou L, Liu D, Xie Y, Yao X, Li Y (2019) Bifidobacterium infantis induces protective colonic PD-L1 and Foxp3 regulatory T cells in an acute murine experimental model of inflammatory bowel disease. Gut Liver 13(4):430–439. 10.5009/gnl18316 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zisman TL, Rubin DT (2008) Colorectal cancer and dysplasia in inflammatory bowel disease. World J Gastroenterol 14(17):2662–2669. 10.3748/wjg.14.2662 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zou W (2006) Regulatory T cells, tumour immunity and immunotherapy. Nat Rev Immunol 6(4):295–307. 10.1038/nri1806 [DOI] [PubMed] [Google Scholar]


