Abstract
OBJECTIVES:
Arterial diastolic blood pressure (DBP) greater than 25 mm Hg in infants and greater than 30 mm Hg in children greater than 1 year old during cardiopulmonary resuscitation (CPR) was associated with survival to hospital discharge in one prospective study. We sought to validate these potential hemodynamic targets in a larger multicenter cohort.
DESIGN:
Prospective observational study.
SETTING:
Eighteen PICUs in the ICU-RESUScitation prospective trial from October 2016 to March 2020.
PATIENTS:
Children less than or equal to 18 years old with CPR greater than 30 seconds and invasive blood pressure (BP) monitoring during CPR.
INTERVENTIONS:
None.
MEASUREMENTS AND MAIN RESULTS:
Invasive BP waveform data and Utstein-style CPR data were collected, including prearrest patient characteristics, intra-arrest interventions, and outcomes. Primary outcome was survival to hospital discharge, and secondary outcomes were return of spontaneous circulation (ROSC) and survival to hospital discharge with favorable neurologic outcome. Multivariable Poisson regression models with robust error estimates evaluated the association of DBP greater than 25 mm Hg in infants and greater than 30 mm Hg in older children with these outcomes. Among 1,129 children with inhospital cardiac arrests, 413 had evaluable DBP data. Overall, 85.5% of the patients attained thresholds of mean DBP greater than or equal to 25 mm Hg in infants and greater than or equal to 30 mm Hg in older children. Initial return of circulation occurred in 91.5% and 25% by placement on extracorporeal membrane oxygenator. Survival to hospital discharge occurred in 58.6%, and survival with favorable neurologic outcome in 55.4% (i.e., 94.6% of survivors had favorable neurologic outcomes). Mean DBP greater than 25 mm Hg for infants and greater than 30 mm Hg for older children was significantly associated with survival to discharge (adjusted relative risk [aRR], 1.32; 1.01–1.74; p = 0.03) and ROSC (aRR, 1.49; 1.12–1.97; p = 0.002) but did not reach significance for survival to hospital discharge with favorable neurologic outcome (aRR, 1.30; 0.98–1.72; p = 0.051).
CONCLUSIONS:
These validation data demonstrate that achieving mean DBP during CPR greater than 25 mm Hg for infants and greater than 30 mm Hg for older children is associated with higher rates of survival to hospital discharge, providing potential targets for DBP during CPR.
Keywords: cardiopulmonary resuscitation, heart arrest, hemodynamics, outcomes, pediatric
Cardiopulmonary resuscitation (CPR) is provided for greater than 15,000 children with inhospital cardiac arrests (IHCAs) in the United States annually (1). Effectiveness of CPR depends on attaining adequate myocardial blood flow as a result of sufficient coronary perfusion pressure and, thus, arterial diastolic blood pressure (DBP) (2–8). A previous prospective observational multicenter study of 164 children with inhospital CPR and invasive hemodynamic monitoring (Pediatric Intensive Care quality of CPR study [PICqCPR]) established that mean DBP greater than or equal to 25 mmHg in infants and greater than or equal to 30 mm Hg in children greater than or equal to 1 year old during the first 10 minutes of CPR was associated with greater likelihood of survival to hospital discharge and survival with favorable neurologic outcome (4). In addition, no patients survived when mean event-level DBP was less than 16 mm Hg. Those findings have not been validated.
We prospectively gathered invasive blood pressure (BP) data on a much larger population of children enrolled in a prospective interventional trial (ICU-RESUScitation [ICU-RESUS]; NCT02837497) (9). Our goal was to validate the PICqCPR-derived association of DBP with survival to hospital discharge.
MATERIALS AND METHODS
All patients included were enrolled in the ICU-RESUS interventional trial across 18 pediatric ICUs evaluating effects of rigorous CPR training and structured postcardiac arrest debriefing on outcome following IHCA between October 2016 and March 2021 (NCT02837497) (9). The training and debriefing emphasized attention to intra-arrest physiologic targets with special focus on DBP during CPR for patients with invasive BP monitoring. The University of Utah Institutional Review Board (IRB) served as the central IRB and approved the ICU-RESUS study protocol with waiver of informed consent (protocol IRB_00093320) on July 18, 2016. Procedures were followed in accordance with the ethical standards of the central IRB and with the Helsinki Declaration of 1975.
Inclusion criteria were: 1) index CPR event among children greater than or equal to 37 weeks’ gestation and less than or equal to 18 years with chest compressions (CCs) for greater than or equal to 30 seconds in an ICU and 2) invasive BP monitoring before and during CPR. Patients were excluded from ICU-RESUS, if prior to IHCA, they: 1) had documented lack of commitment to aggressive ICU therapies, 2) were brain dead, or 3) had an out-of-hospital cardiac arrest associated with current hospitalization. Exclusion criteria for ICU-RESUS DBP validation were: 1) inability to determine DBP (e.g., lack of arterial waveform resulting from line interruption for blood draw or truncation of BP waveform obscuring DBP) or 2) inability to determine when CPR started and stopped. Data from the intervention training transition period of ICU-RESUS trial were not excluded.
Cardiac arrest and CPR variables were collected by research coordinators per IHCA guidelines (10–12). Baseline Pediatric Cerebral Performance Category (PCPC), Functional Status Scale (FSS), and prearrest Pediatric Risk of Mortality-III (PRISM-III) scores were determined, as previously described (4, 13, 14).
Outcomes
As in the original PICqCPR derivation study, the primary outcome was survival to hospital discharge, and secondary outcomes were survival to hospital discharge with favorable neurological outcome, defined as PCPC 1–3 or no worse than prearrest baseline, and return of spontaneous circulation (ROSC) greater than 20 minutes (4). Substantive new functional morbidity was defined as increase in FSS score greater than or equal to 3 (14). Outcome data were collected by research coordinators and site investigators who were blind to invasive BP analyses. A sensitivity analysis examined the alternative favorable neurologic outcome definition of PCPC 1–2 or no worse than prearrest baseline (4, 12).
BP Waveform Analysis
BP waveform data from institutional BedMaster (Excel Medical, Jupiter, FL), IntelliVue Information Center iX (Philips, Andover, MA), or locally developed waveform acquisition systems were downloaded, deidentified, and transmitted to the University of Utah Data Coordinating Center (DCC). Waveforms were reconstructed at Children’s Hospital of Philadelphia, using custom code (MATLAB; The Mathworks, Natick, MA). Coauthors R.A.B., R.W.M., K.G., R.M.S. reviewed reconstructed waveforms and manually annotated: 1) CC starts and stops, 2) nonanalyzable data due to inadequate waveform signal, and 3) nonsustained ROSC. DBP was determined at mid-diastole. Three sites unable to obtain fully electronic waveform data transmitted deidentified waveform data obtained by printing from the local institution’s central monitoring system or by acquiring digital screenshots, which were manually digitized (PlotDigitizer; Version 2.0; Department of Physics, University of South Alabama, Mobile, AL) for analyses in the same manner as fully electronic files.
Means for hemodynamic and CPR quality data, including DBP, systolic blood pressure (SBP), CC rate, and CC fraction, were calculated for each 30-second epoch. Mean DBP and SBP for each patient were defined as average BP over the first 10 minutes of CPR. For patients with less than 10 minutes of CPR BP data, mean BPs were determined for minutes of CPR provided.
Sample Size/Power Analysis
The projected sample size of ICU-RESUS trial was 1,540 patients, of whom 616 patients (40%) were expected to have invasive BP monitoring (4). Based on PICqCPR data, we estimated that 60% of patients would attain the DBP thresholds, 47% of whom would survive to hospital discharge versus 31% survival among patients with lower DBPs (4). Thus, we estimated 85% power to detect significantly higher survival rate for patients attaining DBP thresholds versus patients who do not with a two-sided p value of <0.05.
Statistical Analysis
Patient and event characteristics and outcomes were summarized using frequencies and percentages or medians and quartiles. Associations with survival outcomes were examined using Fisher exact test for categorical variables and the Wilcoxon rank-sum test for ordinal variables.
Multivariable Poisson regression models with robust error estimates were used to evaluate the association of DBP thresholds with survival to hospital discharge, survival to hospital discharge with favorable neurologic outcome, and ROSC (15). Covariates were selected a priori based on established and postulated associations with IHCA outcomes: age category (<1 yr old vs ≥1 yr old), initial cardiac rhythm, location of CPR (CICU vs PICU), and clinical study site (4). Using multivariable Poisson regression models, we evaluated exploratory associations of other potential DBP thresholds during CPR with survival outcomes, including mean DBP greater than or equal to 15/greater than or equal to 20, greater than or equal to 20/greater than or equal to 25, greater than or equal to 30/greater than or equal to 35, and greater than or equal to 35/greater than or equal to 40 mm Hg in infants and older children, respectively.
RESULTS
Among 1,129 children with index CPR events, 574 had invasive BP monitoring when CPR commenced (eFig. 1, http://links.lww.com/CCM/H240). Waveforms were not evaluable in 161 patients: 17 with inability to determine when CPR started or stopped, 47 with inability to determine DBP, and 97 with no waveform transmitted to the DCC. Data from 413 patients were evaluated.
Salient patient characteristics include the following: 64% were infants, 70% had congenital heart disease, 83% had pre-event respiratory insufficiency, 81% had pre-event hypotension, and 50% were postoperative cardiac surgical patients (Table 1). Only one of the patients had CPR for less than 60 seconds. Unadjusted rates of survival to hospital discharge were greater among patients who had normal prearrest PCPC scores, cardiac surgery, and less severe prearrest severity of illness as reflected by PRISM scores, prearrest hypotension, and vasoactive inotrope scores (Table 1).
TABLE 1.
Patient Characteristics
| Survival to hospital discharge |
||||
|---|---|---|---|---|
| Patient Characteristic | Overall (n = 413) |
No (n = 171) |
Yes (n = 242) |
p a |
|
| ||||
| Preexisting conditions | ||||
| Respiratory insufficiency | 343 (83.1%) | 141 (82.5%) | 202 (83.5%) | 0.791b |
| Hypotension | 333 (80.6%) | 149 (87.1%) | 184 (76.0%) | 0.005b |
| Congestive heart failure | 52 (12.6%) | 29 (17.0%) | 23 (9.5%) | 0.034b |
| Pneumonia | 42 (10.2%) | 20 (11.7%) | 22 (9.1%) | 0.412b |
| Sepsis | 60 (14.5%) | 43 (25.1%) | 17 (7.0%) | < 0.001b |
| Renal insufficiency | 52 (12.6%) | 36 (21.1%) | 16 (6.6%) | < 0.001b |
| Malignancy | 18 (4.4%) | 14 (8.2%) | 4 (1.7%) | 0.002b |
| Congenital heart disease | 290 (70.2%) | 111 (64.9%) | 179 (74.0%) | 0.050b |
| Trauma | 6 (1.5%) | 3 (1.8%) | 3 (1.2%) | 0.695b |
| Illness category | ||||
| Medical cardiac | 94 (22.8%) | 45 (26.3%) | 49 (20.2%) | 0.011b |
| Medical noncardiac | 92 (22.3%) | 45 (26.3%) | 47 (19.4%) | |
| Surgical cardiac | 208 (50.4%) | 70 (40.9%) | 138 (57.0%) | |
| Surgical noncardiac or trauma | 19 (4.6%) | 11 (6.4%) | 8 (3.3%) | |
| Baseline Pediatric Cerebral Performance Category scorec | ||||
| 1–Normal | 275 (66.6%) | 103 (60.2%) | 172 (71.1%) | 0.012d |
| 2–Mild disability | 83 (20.1%) | 39 (22.8%) | 44 (18.2%) | |
| 3–Moderate disability | 29 (7.0%) | 11 (6.4%) | 18 (7.4%) | |
| 4–Severe disability | 23 (5.6%) | 16 (9.4%) | 7 (2.9%) | |
| 5–Coma/vegetative state | 3 (0.7%) | 2 (1.2%) | 1 (0.4%) | |
| Baseline Functional Status Scalec | 6.0 [6.0,8.0] | 6.0 [6.0,9.0] | 6.0 [6.0,8.0] | 0.010d |
| Pediatric Risk of Mortalitye | 7.0 [3.0,12.0] | 8.0 [4.0,14.0] | 6.0 [1.0,11.0] | < 0.001d |
| Vasoactive inotropic scoref | 4.5 [0.0,10.0] | 6.0 [1.0,17.0] | 3.1 [0.0,8.5] | < 0.001d |
p for comparison of survivors to hospital discharge versus those not surviving.
Fisher exact test.
Baseline Pediatric Cerebral Performance Category and Functional Status Scale represent subject status prior to the event leading to hospitalization.
Wilcoxon rank-sum test.
Pediatric Risk of Mortality was evaluated 2–6 hr prior to the event.
Vasoactive inotropic score was evaluated 2 hr prior to the event.
CPR event features include the following: 68% occurred in CICUs, 8% had initial shockable rhythms, immediate cause of arrest was hypotension in 71% and respiratory decompensation in 44%, and epinephrine was provided for 86% (Table 2). Unadjusted rates of survival to discharge were superior among patients in CICUs and among patients with shockable rhythm, shorter CPR duration, and less exposure to epinephrine, calcium, or bicarbonate. Unadjusted rates of survival to discharge were lower among patients with prearrest hypotension and with prearrest interventions of vasoactive infusions, invasive mechanical ventilation, and central venous catheters (Table 2).
TABLE 2.
Event Characteristics
| Event Characteristic | Survival to hospital discharge |
|||
|---|---|---|---|---|
| Overall (n = 413) |
No (n = 171) |
Yes (n = 242) |
p a | |
|
| ||||
| Location of CPR event | ||||
| PICU | 134 (32.4%) | 68 (39.8%) | 66 (27.3%) | 0.010b |
| Cardiac ICU | 279 (67.6%) | 103 (60.2%) | 176 (72.7%) | |
| Interventions in place prior to event | ||||
| Central venous catheter | 357 (86.4%) | 156 (91.2%) | 201 (83.1%) | 0.019b |
| Vasoactive infusion | 301 (72.9%) | 144 (84.2%) | 157 (64.9%) | < 0.001b |
| Invasive mechanical ventilation | 343 (83.1%) | 157 (91.8%) | 186 (76.9%) | < 0.001b |
| Noninvasive ventilation | 37 (9.0%) | 11 (6.4%) | 26 (10.7%) | 0.162b |
| Immediate cause(s) of event | ||||
| Arrhythmia | 68 (16.5%) | 24 (14.0%) | 44 (18.2%) | 0.284b |
| Cyanosis without respiratory decompensation | 17 (4.1%) | 6 (3.5%) | 11 (4.5%) | 0.802b |
| Hypotension | 291 (70.5%) | 137 (80.1%) | 154 (63.6%) | < 0.001b |
| Respiratory decompensation | 183 (44.3%) | 63 (36.8%) | 120 (49.6%) | 0.012b |
| CPR timec | ||||
| Weekday | 232 (56.2%) | 96 (56.1%) | 136 (56.2%) | 0.770b |
| Weeknight | 83 (20.1%) | 32 (18.7%) | 51 (21.1%) | |
| Weekend | 98 (23.7%) | 43 (25.1%) | 55 (22.7%) | |
| First documented rhythm at time of CPR | ||||
| Pulseless electrical activity/asystole | 160 (38.7%) | 75 (43.9%) | 85 (35.1%) | 0.068b |
| Ventricular fibrillation/tachycardia | 34 (8.2%) | 9 (5.3%) | 25 (10.3%) | |
| Bradycardia with poor perfusion | 219 (53.0%) | 87 (50.9%) | 132 (54.5%) | |
| Duration of CPR (minutes) | 7 (3–23) | 19 (4–44) | 5 (2–12) | < 0.0014 |
| Duration of CPR (min) | ||||
| < 6 | 182 (44.1%) | 50 (29.2%) | 132 (54.5%) | < 0.001b |
| 6–15 | 86 (20.8%) | 28 (16.4%) | 58 (24.0%) | |
| 16–35 | 65 (15.7%) | 34 (19.9%) | 31 (12.8%) | |
| > 35 | 80 (19.4%) | 59 (34.5%) | 21 (8.7%) | |
| Epinephrine during CPR | 357 (86.4%) | 159 (93.0%) | 198 (81.8%) | 0.001b |
| Minutes to first dose | 1 (0–2) | 1 (0–2) | 1 (0–2) | 0.616d |
| Number of doses | 2 (1–5) | 4 (2–8) | 2 (1–3) | < 0.001d |
| Average interval between dosese | 4.5 (3.3–7.4) | 4.5 (3.3–7.5) | 4.5 (3.1–7.0) | 0.765d |
| Calcium | 194 (47.0%) | 101 (59.1%) | 93 (38.4%) | < 0.001b |
| Sodium bicarbonate | 217 (52.5%) | 118 (69.0%) | 99 (40.9%) | < 0.001b |
CPR = cardiopulmonary resuscitation.
p for comparison of survivors to hospital discharge vs those not surviving.
Fisher exact test.
Weekday is between 7 am and 11 pm Monday to Friday; weeknight is after 11 pm Monday to Thursday; weekend is 11 pm Friday to 7 am Monday.
Wilcoxon rank-sum test.
Average interval between epinephrine doses is only calculated on subjects with at least two doses of epinephrine.
Hemodynamic measures and outcomes are summarized in Tables 3 and 4. Overall, 85.5% of the patients attained thresholds of mean DBP greater than or equal to 25 mm Hg in infants and greater than or equal to 30 mmHg in older children. Initial return of circulation occurred in 91.5%: 25% by placement on extracorporeal membrane oxygenator (ECMO). Survival to hospital discharge occurred in 58.6% and survival with favorable neurologic outcome in 55.4% (i.e., 94.6% of survivors had favorable neurologic outcomes).
TABLE 3.
Hemodynamic Measures During Cardiopulmonary Resuscitation
| Hemodynamic/CPR Quality Metric | Survival to hospital discharge |
|||
|---|---|---|---|---|
| Overall (n = 413) |
No (n = 171) |
Yes (n = 242) |
p a | |
|
| ||||
| Pediatric Intensive Care Quality of CPR thresholds | ||||
| Diastolic blood pressure (mm Hg) ≥25 for infants or ≥30 for children | 353 (85.5%) | 139 (81.3%) | 214 (88.4%) | 0.048b |
| Systolic blood pressure (mm Hg) ≥60 for infants or ≥80 for children | 279 (68.4%) | 108 (63.9%) | 171 (71.5%) | 0.106b |
| Diastolic blood pressure (mm Hg) | ||||
| Age ≤1 yr | 36 (30–46) | 32 (27–43) | 38 (30–47) | 0.006c |
| Age >1 yr | 43 (35–56) | 42 (34–57) | 44 (36–55) | 0.505c |
| Systolic blood pressure (mm Hg) | ||||
| Age ≤1 yr | 74 (56–92) | 70 (54–86) | 79 (58–92) | 0.098c |
| Age >1 yr | 95 (73–120) | 98 (66–123) | 93 (78–114) | 0.764c |
| Average pulse pressure (mm Hg) | 38 (26–56) | 38 (26–61) | 38 (26–54) | 0.548c |
| Average chest compression rate (per minute) | 121 (112–130) | 121 (112–128) | 121 (112–132) | 0.429c |
| Average chest compression fraction | 0.97 (0.92–1.00) | 0.96 (0.92–0.99) | 0.97 (0.92–1.00) | 0.170c |
CPR = cardiopulmonary resuscitation.
p for comparison of survivors to hospital discharge vs those not surviving.
Fisher exact test.
Wilcoxon rank-sum test.
TABLE 4.
Outcomes in ICU-Resuscitation cohort and Pediatric Intensive Care Quality of Cardiopulmonary Resuscitation Cohort Cohorts
| Outcome | ICU-Resuscitation Cohort (n = 413) | Pediatric Intensive Care Quality of Cardiopulmonary Resuscitation Cohort (n = 164) |
|---|---|---|
|
| ||
| Return of circulation | 378 (91.5%) | 148 (90.2%) |
| Return of spontaneous circulation ≥20 min | 274 (66.3%) | 112 (68.3%) |
| Transitioned to extracorporeal membrane oxygenation | 104 (25.2%) | 36 (22.0%) |
| Hospital discharge outcomes | ||
| Survival | 242 (58.6%) | 77 (47.0%) |
| Survival with favorable neurologic outcomea | 229 (55.4%) | 70 (42.7%) |
| Survival to hospital discharge with PCPC of 1, 2, or no worse than baseline | 205 (49.6%) | 62 (37.8%) |
| PCPC | ||
| 1–Normal | 107 (25.9%) | 24 (14.6%) |
| 2–Mild disability | 78 (18.9%) | 27 (16.5%) |
| 3–Moderate disability | 37 (9.0%) | 17 (10.4%) |
| 4–Severe disability | 19 (4.6%) | 8 (4.9%) |
| 5–Coma/vegetative state | 1 (0.2%) | 1 (0.6%) |
| 6–Death | 171 (41.4%) | 87 (53.0%) |
| FSS in survivors | 8.0 (7.0–11.0) | 9.0 (8.0–12.0) |
| FSS change from baseline in survivors | 1.0 (0.0–3.0) | 0.0 (0.0–3.0) |
| New morbidity at hospital dischargeb | 76 (18.4%) | 22 (13.4%) |
FSS = Functional Status Score, PCPC = Pediatric Cerebral Performance Category.
Favorable neurologic outcome was defined as no more than moderate disability or no worsening from baseline PCPC. Baseline PCPC represents subject status prior to the event leading to hospitalization.
New morbidity among survivors was defined as a worsening from baseline FSS by 3 points or more.
Adjusting for age, initial rhythm, location (PICU or CICU), and institution, attaining the DBP thresholds was significantly associated with survival to discharge (adjusted relative risk [aRR], 1.32; 1.01–1.74; p = 0.03) and ROSC (aRR, 1.49; 1.12–1.97; p = 0.002) but did not reach significance for survival to hospital discharge with favorable neurologic outcome (aRR, 1.30; 0.98–1.72; p = 0.051) (Fig. 1). Association of DBP threshold attainment with the alternative favorable neurologic outcome definition was similar (aRR, 1.34; 0.97–1.85; p = 0.055) (eTables 1–3, http://links.lww.com/CCM/H240)).
Figure 1.
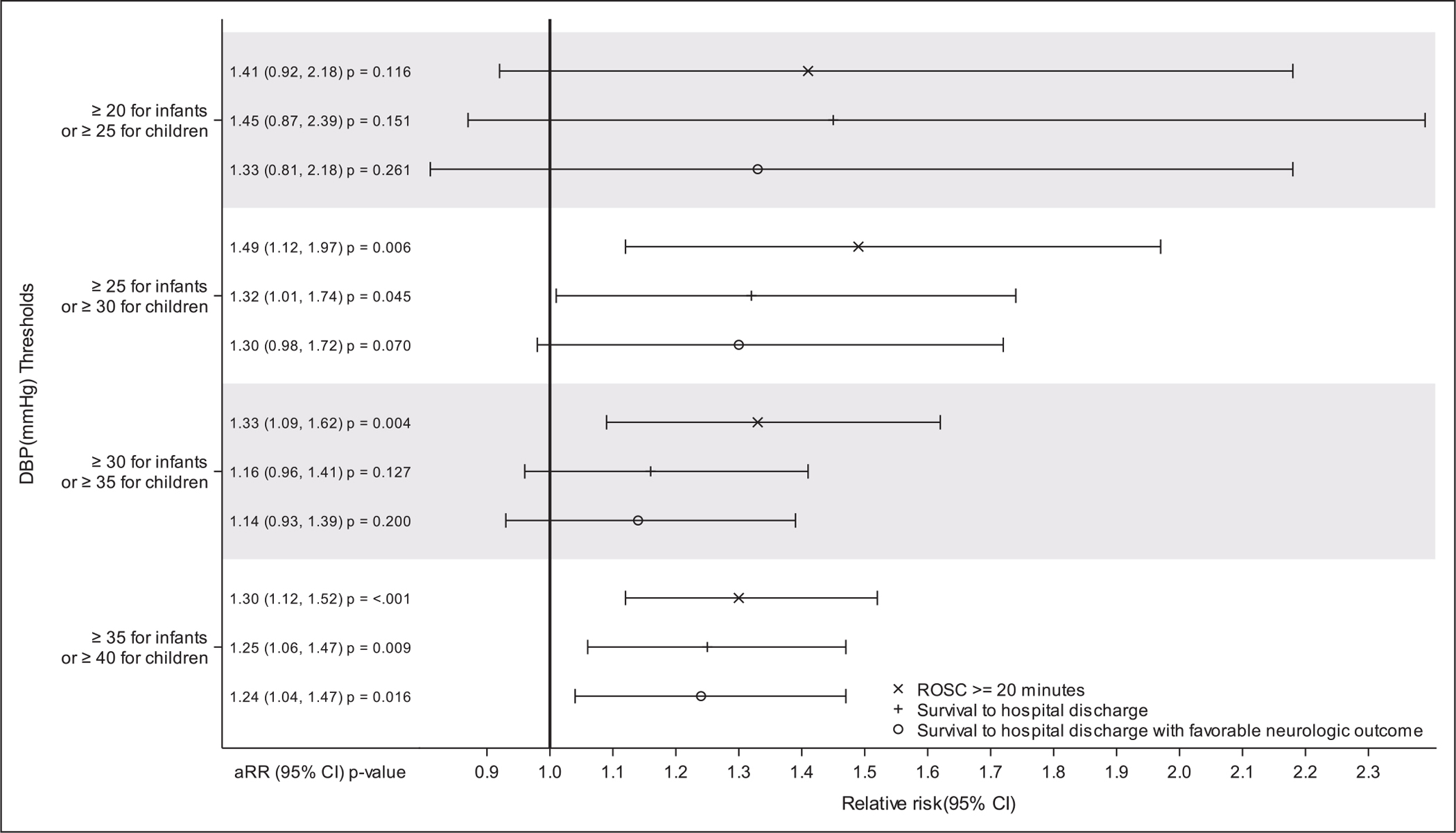
Forest plot of multivariable models of the association of average diastolic blood pressures over the first 10 minutes of cardiopulmonary resuscitation with survival outcomes. Multivariable models adjusted for age category (<1 yr, ≥1 yr), first documented rhythm, location of cardiopulmonary resuscitation, and study site. Favorable neurologic outcome was defined as no more than moderate disability or no worsening from baseline Pediatric Cerebral Performance Category (PCPC). Baseline PCPC represents subject status prior to the event leading to hospitalization. aRR = adjusted relative risk, ROSC = return of spontaneous circulation.
Adjusted associations of alternative DBP thresholds with survival outcomes are shown in Figure 1 and eTable 4 (http://links.lww.com/CCM/H240). Mean DBP greater than or equal to 15/greater than or equal to 20 mm Hg and mean DBP greater than or equal to 20/greater than or equal to 25 mm Hg were not significantly associated with any outcome. Mean DBP greater than or equal to 30/greater than or equal to 35 mm Hg was significantly associated with ROSC (aRR, 1.33; 1.09–1.62; p = 0.002), but not survival to hospital discharge or survival with favorable neurologic outcome. Mean DBP greater than or equal to 35/greater than or equal to 40 mm Hg was significantly associated with ROSC (aRR 1.30; 1.12–1.52; p < 0.001), survival to hospital discharge (aRR, 1.25; 1.06–1.47; p = 0.008), and survival with favorable neurologic outcome (aRR, 1.24; 1.04–1.47; p = 0.014). Only 147 of 242 (61%) patients with survival to hospital discharge and only 138/229 (60%) with favorable neurologic outcomes attained greater than or equal to 35/greater than or equal to 40 mm Hg threshold compared with 214 of 242 (88%) with survival to discharge and 202 of 229 (88%) with favorable neurologic outcomes who attained the greater than or equal to 25/greater than or equal to 30 mm Hg threshold (eTable 4, http://links.lww.com/CCM/H240).
Only two patients attained ROSC and survived to hospital discharge with mean DBP less than 16 mm Hg. Both were infants: one with mean DBP 7 mm Hg and one with mean DBP 15 mm Hg. All other infants and children who survived to hospital discharge had mean DBP greater than 16 mm Hg. One older child attained ROSC and survived to discharge with mean DBP 17 mm Hg.
DISCUSSION
American Heart Association CPR guidelines recommend using physiologic parameters such as BP to monitor and optimize CPR quality (16, 17). PICqCPR was the first clinical investigation to demonstrate associations of DBP during CPR with the important outcomes of survival to hospital discharge and survival with favorable neurologic outcome (4). Those findings are consistent with foundational large animal CPR studies that established survival depends on attaining adequate DBP and coronary perfusion pressure during CPR to drive sufficient myocardial blood flow and that BP-directed CPR can improve outcomes (2–8, 18). Two adult studies of prolonged out-of-hospital cardiac arrests showed that invasive BP after Emergency Department arrival is associated with ROSC, but only one of 150 patients survived to hospital discharge (19, 20). The ICU-RESUS validation data support American Heart Association guidelines for using arterial BP to monitor and perhaps optimize CPR.
These ICU-RESUS data from 413 patients with IHCA validate previous PICqCPR findings from 164 patients that established the association of DBP thresholds during early minutes of CPR with survival outcomes. In this much larger data set, when mean DBP was greater than or equal to 25 mm Hg in infants and greater than or equal to 30 mm Hg in older children during the first 10 minutes of CPR, patients were 49% more likely to attain ROSC and 32% more likely to survive to hospital discharge compared with patients not attaining these DBP thresholds. Alternative DBP thresholds 5 and 10 mm Hg lower or higher were not significantly associated with greater likelihood of higher rates of survival outcomes than the DBP thresholds derived from PICqCPR data. For pediatric IHCAs with invasive BP monitoring, these data provide support for targeting DBP greater than or equal to 25 mm Hg in infants and greater than or equal to 30 mm Hg in older children during CPR. In addition, only two patients survived to hospital discharge with DBP less than 16 mm Hg in either the original PICqCPR derivation study or the present validation study, and both were infants, suggesting the particular importance of attaining DBP greater than 15 mm Hg.
Prearrest patient characteristics and arrest event characteristics of the ICU-RESUS and PICqCPR patients were similar except ICU-RESUS patients had lower frequency of sepsis (15% vs 27%) and higher frequency of normal pre-arrest neurologic status (67% vs 47%) (eTables 5 and 6, http://links.lww.com/CCM/H240). Yet, ICU-RESUS patients were more likely to attain: 1) higher SBPs during CPR, suggesting more forceful CCs, and 2) threshold DBPs associated with superior outcomes (86% vs 62%) (eTable 7, http://links.lww.com/CCM/H240). Higher SBPs and DBPs presumably resulted from cumulative training and experience at ICU-RESUS sites, as the intervention highlighted intra-arrest physiologic targets with special focus on DBP during CPR and nearly all ICU-RESUS sites participated in PICqCPR (4, 9).
The higher rate of attaining PICqCPR-derived DBP thresholds may have led to improved outcomes compared with the PICqCPR patients. ICU-RESUS patients more commonly survived to hospital discharge (59% vs 47%), survived with favorable neurologic outcomes (55% vs 43%), and survived with PCPC 1 or 2 (45% vs 31%) (Table 4). Ironically, these impressive DBPs during CPR and remarkable outcomes throughout both control and intervention periods of ICU-RESUS may have precluded the interventional trial from demonstrating improved outcomes, as the intervention was intended to improve outcomes by improving CPR performance and hemodynamics (4).
Attaining these DBP thresholds was associated with survival to hospital discharge in both cohorts, yet it was associated with survival to hospital discharge with favorable neurologic outcome only in PICqCPR derivation patients. In contrast, maintaining DBP above those thresholds was only associated with ROSC in ICU-RESUS validation patients. Reasons for these apparent discrepancies are not clear, but the Forest Plot of outcomes and the consistent direction of associations across outcomes suggest that the statistical differences in relative rates of these outcomes with adequate DBP in the two cohorts may be due to variability around the aRR ratios and/or sample size (Fig. 1). We also suspect that ceiling effects may be a confounder, as some patients may have diseases and/or pathobiological processes that preclude ROSC, survival, and/or survival with favorable neurologic outcomes despite excellent DBP during CPR.
Establishing and validating the association of hemodynamic thresholds with clinically important outcomes are a necessary step toward the potentially transformative novel approach of hemodynamic-directed CPR when invasive BP monitoring is available. For example, when DBP is below a target threshold, rescuers can attempt to increase DBP by ensuring adequate CC rate and force/depth, avoiding interruptions, and encouraging adequate venous return by allowing full chest recoil. When the DBP remains lower than the hemodynamic thresholds despite appropriate basic life support and adequate vasopressor support, resuscitating teams should consider potentially reversible causes of low DBP during CPR, such as hypoxemia, hypercarbic acidosis, pulmonary thromboembolism, tension pneumothorax, cardiac tamponade, toxins, and hypovolemia. When DBP is above hemodynamic thresholds without resultant ROSC, resuscitating teams can focus on other issues, such as adequate oxygenation and ventilation, hypoglycemia, hyperkalemia, toxins, or myocardial pathology that may preclude ROSC (e.g., postsurgical cardiomyopathy or myocarditis, each perhaps needing emergent ECMO).
As an exploratory aim, we evaluated alternative DBP thresholds: greater than or equal to 15/greater than or equal to 20, greater than or equal to 20/greater than or equal to 25, greater than or equal to 30/greater than or equal to 35, and greater than or equal to 35/greater than or equal to 40 mm Hg in infants and older children, respectively (Fig. 1). In PICqCPR patients, DBP greater than or equal to 20/greater than or equal to 25 mm Hg in infants and older children, respectively, was associated with survival to hospital discharge and survival with favorable neurologic outcome, yet these findings were not confirmed in the ICU-RESUS validation cohort (4). Conversely, DBP greater than or equal to 35/greater than or equal to 40 mm Hg, respectively, was associated with ROSC, survival to hospital discharge, and survival with favorable neurologic outcome in the ICU-RESUS cohort, yet these thresholds were not demonstrated to be associated with survival outcomes in any previous study, and intermediate thresholds of DBP greater than or equal to 30/greater than or equal to 35 mm Hg were not associated with survival outcomes. In addition, aRRs of survival outcomes in ICU-RESUS patients were not higher with DBP greater than or equal to 35/greater than or equal to 40 mm Hg versus DBP greater than or equal to 25/greater than or equal to 30 mm Hg. Furthermore, only 61% of patients who survived to discharge and 60% who survived with favorable neurologic outcome attained DBP greater than or equal to 35/greater than or equal to 40 mm Hg versus 88% of patients with each outcome attained DBP greater than or equal to 25/greater than or equal to 30 mm Hg. Although alternative thresholds warrant further investigation, available data provide strongest support for association of DBP greater than or equal to 25/greater than or equal to 30 mm Hg with superior outcomes.
Generalizability of these findings should be cautiously interpreted due to inherent limitations. First, the observational design precludes attribution of causality. However, numerous prospective randomized interventional animal studies have shown that BP-targeted CPR results in superior outcomes compared with one-size-fits-all CPR with standard depth and fixed epinephrine dosing (5, 6, 18, 21, 22). This validation study supports potential patient-centered hemodynamic targets during CPR for infants and children with invasive hemodynamic monitoring. Second, ICU-RESUS sites are all large academic North American pediatric ICUs, and patient mix and quality of care before and after cardiac arrests may differ from other institutions. Third, CPR was outstanding with median CC fractions of 0.97, 86% attainment of DBP thresholds, and outcomes superior to previous large-scale reports (4, 23–27). Fourth, survival rates from CPR depend on many other factors in addition to DBP during CPR, including underlying diseases and pathobiology, causes of the arrest, comorbidities, and pre-arrest and postarrest care (16, 17, 28–30). Fifth, DBPs were measured post hoc from waveforms transmitted to blinded investigators, whereas providers may not be able to determine DBP as precisely on bedside monitors during CPR. This same argument pertains to real-time use of many ICU bedside measurements, yet providers use such data to guide patient care every day. Sixth, the study population of 415 patients was smaller than the expected 616 patients primarily because waveform data could not be evaluated in 161 patients. Seventh, 49% of ICU-RESUS patients did not have invasive BP monitoring at time of arrest, yet 51% did have invasive BP monitoring and thus could potentially benefit from BP-directed CPR.
CONCLUSIONS
This multicenter prospective observational study validates PICqCPR-derived findings that attaining mean DBP greater than or equal to 25 mm Hg during CPR in infants less than 1 year old and greater than or equal to 30 mm Hg in older children is associated with higher likelihood of survival to hospital discharge. These data support targeting DBP greater than or equal to 25 mm Hg during CPR in infants and greater than or equal to 30 mm Hg in older children.
Supplementary Material
KEY POINTS.
Question:
Can findings from a single derivation study establishing that mean DBP greater than 25 mm Hg for infants and greater than 30 mm Hg for older children during CPR is associated with survival to hospital discharge be validated?
Findings:
In this much larger multicenter prospective observational study, when mean DBP during CPR is greater than or equal to 25 mm Hg in infants and greater than or equal to 30 mm Hg in older children, patients were 49% more likely to attain ROSC and 32% more likely to survive to hospital discharge compared with patients not attaining these DBP thresholds.
Meaning:
These data support targeting DBP greater than or equal to 25 mm Hg in infants and greater than or equal to 30 mm Hg in older children during CPR.
ACKNOWLEDGMENTS
The ICU-RESUS Investigator Group: Robert M. Sutton, MD, MSCE, Robert A. Berg, MD, Ron W. Reeder, PhD, Ryan W. Morgan, MD, MTR, Vinay M. Nadkarni, MD, MS, Heather A. Wolfe, MD, MSHP, Tageldin Ahmed, MD, Michael J. Bell, MD, Robert Bishop, MD, Matthew Bochkoris, MD, Candice Burns, MD, Joseph A. Carcillo, MD, Todd C. Carpenter, MD, J. Michael Dean, MD, J. Wesley Diddle, MD, Myke Federman, MD, Richard Fernandez, MD, Ericka L. Fink, MD, MS, Deborah Franzon, MD, Aisha H. Frazier, MD, Stuart H. Friess MD, Kathryn Graham, MLAS, Mark Hall, MD, David Hehir, MD, Christopher M. Horvat, MD, MHA, Leanna L. Huard, MD, William P. Landis, Tensing Maa, MD, Arushi Manga, MD, Patrick S. McQuillen, MD, Kathleen L. Meert, MD, Peter M. Mourani, MD, Maryam Y. Naim, MD, MSCE, Daniel Notterman, MD, Chella A. Palmer, MPH, Murray M. Pollack, MD, Anil Sapru, MD, Carleen Schneiter, MD, Matthew P. Sharron, MD, Ashley Siems, MD, Neeraj Srivastava, MD, Sarah Tabbutt, MD, PhD, Bradley Tilford, MD, Shirley Viteri, MD, David Wessel, MD, Andrew R. Yates, MD, Athena F. Zuppa, MD, MSCE.
Supported, in part, by the following grants: R01HL131544, U01HD049934, UG1HD049981, UG1HD049983, UG1HD050096, UG1HD063108, UG1HD083166, UG1HD083170, and UG1HD083171.
Footnotes
Supplemental digital content is available for this article. Direct URL citations appear in the printed text and are provided in the HTML and PDF versions of this article on the journal’s website (http://journals.lww.com/ccmjournal)
This work was performed at The Children’s Hospital of Philadelphia, University of Utah, Children’s Hospital of Michigan, Children’s National Hospital, Children’s Hospital Colorado, UPMC Children’s Hospital of Pittsburgh, Mattel Children’s Hospital, Nationwide Children’s Hospital, Benioff Children’s Hospital, Alfred I. duPont Hospital for Children, and Washington University School of Medicine.
Drs. Berg, Morgan, Reeder, Bell, Carcillo, Carpenter, Dean, Fink, Hall, McQuillen, Meert, Mourani, Pollack, Sapru, Wessel, Wolfe, Yates, Zuppa, and Sutton received National Institutes of Health (NIH) grant funding to their institution related to this project. Drs. Berg’s and Sutton’s institutions received funding from the National Heart, Lung, and Blood Institute (NHLBI). Drs. Berg’s, Horvat’s, McQuillen’s, Sapru’s, Schneiter’s, and Zuppa’s institutions received funding from the National Institute of Child Health and Human Development (NICHD). Drs. Berg, Morgan, Reeder, Bell, Carcillo, Carpenter, Dean, Fink, Franzon, Frazier, Friess, Hall, Horvat, Manga, McQuillen, Meert, Mourani, Naim, Pollack, Sapru, Schneiter, Wessel, Wolfe, Yates, and Sutton received support for article research from the NIH. Dr. Morgan’s institution received funding from the NHLBI (K23HL148541). Drs. Reeder’s, Bell’s, Carcillo’s, Carpenter’s, Dean’s, Fink’s, Frazier’s, Friess’s, Hall’s, Manga’s, Meert’s, Mourani’s, Nadkarni’s, Naim’s, Pollack’s, Wessel’s, Wolfe’s, and Yates’ institutions received funding from the NIH. Dr. Fink’s institution received funding from the Neurocritical Care Society; she received funding from the Child Neurology Society. Drs. Fink and Hall received funding from the American Board of Pediatrics. Dr. Hall received funding from Abbvie and Kiadis. Dr. Maa’s institution received funding from the Children’s Hospital of Philadelphia, the NHBLI (R01HL131544), and the NICHD (U01HD049934, UG1HD049981, UG1HD049983, UG1HD050096, UG1HD063108, UG1HD083166, UG1HD083170, and UG1HD083171). Dr. Nadkarni’s institution received funding from Zoll Medical, the American Heart Association RQI Partners, and Nihon-Kohden; he disclosed that he is the Society of Critical Care Medicine President elect Citizen. CPR Foundation Board member volunteer, and an International Liaison Committee on Resuscitation board member volunteer. Dr. Notterman received funding from GenoTwin SAB. Dr. Sutton disclosed that he is the Chair of the Pediatric Research Task Force of the American Heart Association’s Get with the Guidelines Resuscitation National Registry and a Pediatric Advanced Life Support author. The remaining authors have disclosed that they do not have any potential conflicts of interest.
REFERENCES
- 1.Holmberg MJ, Ross CE, Fitzmaurice GM, et al. : Annual incidence of adult and pediatric in-hospital cardiac arrest in the United States. Circ Cardiovasc Qual Outcomes 2019; 12:e005580. [PMC free article] [PubMed] [Google Scholar]
- 2.Sanders AB, Ewy GA, Taft TV: Prognostic and therapeutic importance of the aortic diastolic pressure in resuscitation from cardiac arrest. Crit Care Med 1984; 12:871–873 [DOI] [PubMed] [Google Scholar]
- 3.Kern KB, Ewy GA, Voorhees WD, et al. : Myocardial perfusion pressure: A predictor of 24-hour survival during prolonged cardiac arrest in dogs. Resuscitation 1988; 16:241–250 [DOI] [PubMed] [Google Scholar]
- 4.Berg RA, Sutton RM, Reeder RW, et al. : Association between diastolic blood pressure during pediatric in-hospital cardiopulmonary resuscitation and survival. Circulation 2018; 137:1784–1795 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Lautz AJ, Morgan RW, Karlsson M, et al. : Hemodynamic-directed cardiopulmonary resuscitation improves neurologic outcomes and mitochondrial function in the heart and brain. Crit Care Med 2019; 47:e241–e249 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Sutton RM, Friess SH, Naim MY, et al. : Patient-centric blood pressure-targeted cardiopulmonary resuscitation improves survival from cardiac arrest. Am J Respir Crit Care Med 2014; 190:1255–1262 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Morgan RW, French B, Kilbaugh TJ, et al. : A quantitative comparison of physiologic indicators of cardiopulmonary resuscitation quality: Diastolic blood pressure versus end-tidal carbon dioxide. Resuscitation 2016; 104:6–11 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Pearson JW, Redding JS: Peripheral vascular tone on cardiac resuscitation. Anesth Analg 1965; 44:746–752 [PubMed] [Google Scholar]
- 9.Sutton RM, Wolfe HA, Reeder RW, et al. : Effect of physiologic point-of-care cardiopulmonary resuscitation training on survival with favorable neurologic outcome in cardiac arrest in pediatric ICUs: A randomized clinical trial. JAMA 2022; 327:934–945 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Nolan JP, Berg RA, Andersen LW, et al. : Cardiac arrest and cardiopulmonary resuscitation outcome reports: Update of the Utstein resuscitation registry template for in-hospital cardiac arrest: A consensus report from a task force of the international liaison committee on resuscitation (American Heart Association, European Resuscitation Council, Australian and New Zealand Council on Resuscitation, Heart and Stroke Foundation of Canada, InterAmerican Heart Foundation, Resuscitation Council of Southern Africa, Resuscitation Council of Asia). Circulation. 2019; 140:e746–e757 [DOI] [PubMed] [Google Scholar]
- 11.Jacobs I, Nadkarni V, Bahr J, et al. : Cardiac arrest and cardiopulmonary resuscitation outcome reports: Update and simplification of the Utstein templates for resuscitation registries. A statement for healthcare professionals from a task force of the international liaison committee on resuscitation (American Heart Association, European Resuscitation Council, Australian Resuscitation Council, New Zealand Resuscitation Council, Heart and Stroke Foundation of Canada, InterAmerican Heart Foundation, Resuscitation Council of Southern Africa). Resuscitation 2004; 63:233–249 [DOI] [PubMed] [Google Scholar]
- 12.Topjian AA, Scholefield BR, Pinto NP, et al. : P-COSCA (Pediatric Core Outcome Set for Cardiac Arrest) in children: An advisory statement from the international liaison committee on resuscitation. Resuscitation 2021; 162:351–364 [DOI] [PubMed] [Google Scholar]
- 13.Pollack MM, Holubkov R, Funai T, et al. : Simultaneous prediction of new morbidity, mortality, and survival without new morbidity from pediatric intensive care: A new paradigm for outcomes assessment. Crit Care Med 2015; 43:1699–1709 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Pollack MM, Holubkov R, Glass P, et al. : Functional status scale: New pediatric outcome measure. Pediatrics 2009; 124:e18–e28 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Greenland S: Model-based estimation of relative risks and other epidemiologic measures in studies of common outcomes and in case-control studies. Am J Epidemiol 2004; 160:301–305 [DOI] [PubMed] [Google Scholar]
- 16.Topjian AA, Raymond TT, Atkins D, et al. : Part 4: Pediatric basic and advanced life support: 2020 American Heart Association guidelines for cardiopulmonary resuscitation and emergency cardiovascular care. Circulation 2020; 142(16_Suppl_2):S469–S523 [DOI] [PubMed] [Google Scholar]
- 17.Panchal AR, Bartos JA, Cabanas JG, et al. : Part 3: Adult basic and advanced life support: 2020 American Heart Association guidelines for cardiopulmonary resuscitation and emergency cardiovascular care. Circulation 2020; 142(16_Suppl_2):S366–S468 [DOI] [PubMed] [Google Scholar]
- 18.Morgan RW, Kilbaugh TJ, Shoap W, et al. : A hemodynamic-directed approach to pediatric cardiopulmonary resuscitation (HD-CPR) improves survival. Resuscitation 2017; 111:41–47 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Paradis NA, Martin GB, Rivers EP, et al. : Coronary perfusion pressure and the return of spontaneous circulation in human cardiopulmonary resuscitation. JAMA 1990; 263:1106–1113 [PubMed] [Google Scholar]
- 20.Koyama Y, Matsuyama T, Inoue Y: Association between haemodynamics during cardiopulmonary resuscitation and patient outcomes. Resuscitation 2022; 170:295–302 [DOI] [PubMed] [Google Scholar]
- 21.Morgan RW, Sutton RM, Karlsson M, et al. : Pulmonary vasodilator therapy in shock-associated cardiac arrest. Am J Respir Crit Care Med 2018; 197:905–912 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Naim MY, Sutton RM, Friess SH, et al. : Blood pressure- and coronary perfusion pressure-targeted cardiopulmonary resuscitation improves 24-hour survival from ventricular fibrillation cardiac arrest. Crit Care Med 2016; 44:e1111–e1117 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Holmberg MJ, Wiberg S, Ross CE, et al. : Trends in survival after pediatric in-hospital cardiac arrest in the United States. Circulation 2019; 140:1398–1408 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Wolfe H, Zebuhr C, Topjian AA, et al. : Interdisciplinary ICU cardiac arrest debriefing improves survival outcomes. Crit Care Med 2014; 42:1688–1695 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Berg RA, Nadkarni VM, Clark AE, et al. : Incidence and outcomes of cardiopulmonary resuscitation in PICUs. Crit Care Med 2016; 44:798–808 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Berg RA, Sutton RM, Holubkov R, et al. : Ratio of PICU versus ward cardiopulmonary resuscitation events is increasing. Crit Care Med 2013; 41:2292–2297 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Alten JA, Klugman D, Raymond TT, et al. : Epidemiology and outcomes of cardiac arrest in pediatric cardiac ICUs. Pediatr Crit Care Med 2017; 18:935–943 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Morgan RW, Kirschen MP, Kilbaugh TJ, et al. : Pediatric in-hospital cardiac arrest and cardiopulmonary resuscitation in the United States: A review. JAMA Pediatr 2021; 175:293–302 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Chan PS, Berg RA, Spertus JA, et al. : Risk-standardizing survival for in-hospital cardiac arrest to facilitate hospital comparisons. J Am Coll Cardiol 2013; 62:601–609 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Topjian AA, de Caen A, Wainwright MS, et al. : Pediatric postcardiac arrest care: A scientific statement from the American Heart Association. Circulation 2019; 140:e194–e233 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


