ABSTRACT
The objective of our study was to assess the effect of human umbilical cord blood (HUCB) mesenchymal stem cells (MSCs) transplantation on schistosomal hepatic fibrosis in mice. The study animals were divided into three groups. Group I is a control group, where the mice were infected with Schistosoma mansoni cercariae and remained untreated. The mice of the other two groups were infected and treated with either praziquantel (Group II) or HUCB-MSCs (Group III). Liver function tests, as well as histopathological evaluation of liver fibrosis using hematoxylin and eosin and Masson's trichrome stains, were performed. Additionally, an immunohistochemical study was carried out using anti-glial fibrillary acidic protein (GFAP) in hepatic stellate cells. Compared to the control group, the treated (praziquantel and MSCs) groups showed a substantial improvement, with a significant difference regarding the histopathological evaluation of liver fibrosis in the MSCs-treated group. In conclusion, MSCs could be a promising and efficient cell therapy for liver fibrosis.
KEYWORDS: Liver fibrosis, mesenchymal stem cells, praziquantel, schistosomiasis, glial fibrillary acidic protein
Introduction
Liver fibrosis is considered a serious public health problem. It represents an initial distortion of the hepatic architecture with an extensive accumulation of collagen and extracellular matrix proteins. Possible mechanisms include hepatocyte ischemia, apoptosis with inflammatory cell recruitment, endothelial cell dysfunction, and finally, activation of hepatic stellate cells, which are the effector cells involved in liver fibrosis induction [1]. Liver fibrosis may progress to cirrhosis when the initial injury continues to persist [2].
The progression of liver fibrosis may be influenced by several factors, including viruses, parasites, drugs, alcohol, metabolic disorders, and autoimmune disorders [3]. Schistosoma mansoni infection remains one of the main causes of liver fibrosis, especially in developing countries. It is also considered one of the best experimental models of hepatic fibrosis, reflecting the human disease profile [4]. In experimental animals, S. mansoni eggs are swept into the portal circulation, causing severe periportal hepatic fibrosis similar to the pipe-stem fibrosis found in the hepatosplenic form of the disease in humans [5].
GPraziquantel (PZQ) is the drug of choice for the treatment of schistosomiasis. It demonstrated high efficacy in infected patients while also dramatically reducing the incidence of schistosomiasis when used in endemic areas as a mass drug administration [6]. However, several reports have demonstrated resistance to PZQ [7–9]. Additionally, it has an insignificant effect on the already developed hepatic fibrosis and its complications, such as bleeding varices, liver cell failure, and liver cirrhosis, which may develop when concurrent infection with viral hepatitis occurs [10].
Currently, regardless of the cause, liver transplantation is considered the only curative treatment modality for hepatic fibrosis. Nevertheless, it is unavailable for every patient, and hence, it is important to explore new therapeutic approaches for hepatic fibrosis [11].
Hepatocytes have a substantial capacity to regenerate. However, their proliferative capacity may be insufficient for liver injury improvement even with a share of the bipotent liver progenitor cells that can differentiate into hepatocytes and biliary epithelial cells [12]. Hepatocyte transplantation has been raised as an alternative treatment option to liver transplantation. Even though hepatocyte transplantation is safe, several limitations have emerged due to cell culture difficulties and vulnerability to infection [13].
As an alternative to hepatocytes, several studies have suggested stem cell transplantation as a treatment that may trigger a considerable improvement in liver function [14,15]. Mesenchymal stem cells (MSCs) are highly proliferating, adherent fibroblastic cells that can differentiate into diverse cell types, including hepatocytes. They have been suggested as valuable candidates for the treatment of liver fibrosis [16]. However, the exact mechanisms remain unclear.
Human umbilical cord blood (HUCB) is a rich source of MSCs. It is preferred over other sources as it is from a non-fetal origin, available for collection after delivery, easy to obtain, and remains viable after long-term cryopreservation [17].
This study aimed to investigate the therapeutic potential of HUCB-MSCs in an experimental hepatic schistosomiasis mouse model based on their presumptive capacity to improve inflammation, fibrosis, and liver regeneration.
Methods
Animals and parasites
A total of 60 parasite-free female Swiss albino mice, 4–5 weeks old and weighing 20–25 grams, were obtained from the Biological Unit of Theodor-Bilharz Research Institute, Giza, Egypt. They were maintained in rooms with controlled temperature (22 ± 2 °C), humidity (55% ± 10%), and with continuous air renovation. The animals were housed in a 12 h light/12 h dark cycle (6 AM – 6 PM) and fed rodent diet and water ad libitum, according to institutional and national guidelines.
Infected Biomphalaria alexandrina snails were obtained from the Theodor-Bilharz Research Institute. After cercarial shedding, the female mice were infected by subcutaneous injection with 50 ± 10 cercariae/mouse in the thigh [18]. The infection was confirmed 6 weeks post-injection by parasitological examination of mice feces. Only mice passing viable eggs in their stools were used in the experiment.
Experimental design
Infected mice were randomly allocated into three groups (20 animals each). Group I is a control group, where the mice were infected with Schistosoma mansoni cercariae and remained untreated. The mice of the other two groups were infected and treated ten weeks post-infection with either praziquantel (Group II) or HUCB-MSCs (Group III). The mice of each group were kept separately in their cages and maintained on the same laboratory diet under similar conditions. Twelve weeks after the primary infection, the mice of all groups were sacrificed, their livers and blood samples were subjected to histopathological assessment of liver fibrosis and liver function tests, respectively.
Treatment regimen
Praziquantel
It was available as Epiquantel 600 mg film-coated tablets (EIPICO, Egypt). It was given to S. mansoni-infected mice orally at a dose of 500 mg/kg/dose [19] ten weeks post-infection.
Mesenchymal stem cells
MSCs were administered intravenously via the tail vein to S. mansoni-infected mice at a dose of 1 × 106 MSCs/mouse ten weeks after primary infection [20].
HUCB-derived MSCs preparation and culture
The MSCs used in this study were originally isolated from donated HUCB after full-term cesarean deliveries and consent was taken from the mothers according to the method described by Reddy et al. [21]. Briefly, HUCB was collected from the umbilical cord vein using a 20-mL sterile syringe. The collected blood was emptied into a sterile Falcon tube (50 mL) containing 5000 IU of heparin. All the cord blood samples were processed within 3 hours after delivery. Mononuclear cells were separated from the donated HUCB using Ficoll-Hypaque (p = 1.077 g/L) (Biochrom AG, Germany). The total number of the nucleated cells was determined with a hemocytometer, and the viability was assessed by trypan blue (0.04%) exclusion dye. To obtain MSCs, mononuclear cells were suspended in Dulbecco’s Modified Eagle’s Medium (Lonza, Switzerland) containing 100 U/mL penicillin, 100 mg/mL streptomycin, and 15% fetal bovine serum with 2 mM L-glutamine, 1 mM sodium pyruvate (Sigma-Aldrich, Germany), and 0.25 μg/mL amphotericin (Neon Healthcare Ltd., UK). Seeding the cells into the culture flasks was done at a density of 1 × 106 cells/cm2. After one week, the suspended cells were removed, and adherent cells were then cultured. Cells were maintained in a humidified atmosphere with 5% carbon dioxide at 37 °C. The culture medium was changed every 3–4 days. Cells were detached when reaching 50–60% confluence with 0.25% trypsin-EDTA.
Analysis of MSCs by flow cytometry
Cultured cells were identified by MSCs-related surface markers using flow cytometry as described in previous research [22,23]. Briefly, the cells were harvested and washed several times using phosphate-buffered saline. Labeling the cells was done by adding the following antibodies: FITC-conjugated anti-CD45, PE-conjugated anti-CD34, PE-conjugated anti-CD90, and PE-conjugated anti-CD105, for 20 minutes at room temperature, and then washed. The nonspecific mouse IgG (PE Mouse IgG1 and FITC Mouse IgG1) served as an isotype control to assess background fluorescence intensity. All reagents were purchased from eBio-science, USA. Cells were analyzed using the four-color flow cytometry Becton Dickinson (BD) FACS Calibur instrument (BD, San Diego, California, USA), with data analysis using BD Cell Quest Pro software (BD, version 3, verify software House Topsham, ME, USA).
Serum liver function parameters
The liver function parameters, including albumin (g/dL), alanine aminotransferase (ALT) (U/L), and aspartate aminotransferase (AST) (U/L), were measured in the serum according to the manufacturer’s instructions using the Dimension automated chemistry analyzer (Siemens, Germany).
Histopathological and immunohistochemical studies
Serial sections (4-µm-thick) of formalin-fixed, paraffin-embedded liver biopsies were stained with hematoxylin and eosin for routine histopathological examination. Masson’s trichrome stain has been used for a better demonstration of fibrous tissue deposition in liver biopsies around the granulomas and periportal areas.
The granuloma diameter was measured at the central laboratory of the Microbiology Department, Faculty of Medicine, Tanta, Egypt, using a Leica Qwin 500 Image Analyzer (LEICA Imaging Systems Ltd, Cambridge, England), which consists of a Leica DM-LB microscope with a JVC color video camera attached to the computer system, Leica Q 500IW. The mean diameter was calculated, tabulated, and statistically analyzed.
Concerning immunohistochemical staining of hepatic stellate cells (HSCs) for glial fibrillary acidic protein (GFAP), liver sections (of 5-μm thickness) were left to dry for 30 minutes at 37 °C. Sections were then deparaffinized, and antigen retrieval was performed in the Dako PT link unit using high and low PH EnVision FLEX Target Retrieval Solutions (reaching 97 °C for 20 min). Immunostaining was accomplished using the Dako Autostainer Link 48. Briefly, a peroxidase blocking reagent was applied, followed by incubation with primary antibodies for 30 minutes. Subsequently, horseradish peroxidase polymer was applied for 20 minutes, and diaminobenzidine was applied as a chromogen. The slides were then counterstained with hematoxylin. The primary antibody used in this study was GFAP, a monoclonal antibody (clone number, Ab-4, MS-688, 1: 100; Labvision, California, USA).
Parenchymal HSCs were identified by morphological and anatomical criteria as being perisinusoidally located, stellate-shaped cells found in the parenchymal lobules or nodules. Estimation of the number of anti-GFAP immunoreactive HSCs was performed independently by two researchers. The number of positive HSCs and negative HSCs for GFAP was separately counted under a light microscope at x200 magnification. Only the cells that displayed nuclei in the section were considered. For each slide, at least 7–10 microscopic fields were randomly chosen. The percentage of positive HSCs to the total number of HSCs was calculated in each slide. The average percentage of activated HSCs (positively stained) was then calculated for each group. Semi-quantitative grading of HSCs immunostained by GFAP was determined as follows: 0: no staining or less than 3% stained; I: 3–33% positive staining; II: 34–66% positive staining; and III: more than 66% positive staining [24].
Statistical analysis
The collected data were organized, tabulated, and statistically analyzed using SPSS software, version 23.0. (IBM Corp., Armonk, N.Y., USA). Differences between the studied groups were tested using the unpaired t-test for numerical data and the chi-square test (χ2) or Fisher’s exact test for nominal data. The degree of fibrosis was compared between groups using the Mann-Whitney U test. P-values of less than 0.05 were considered significant.
Results
Generation and morphological examination of MSCs
The mesenchymal cells started to be generated in the culture media as a fibroblast-like cell colony approximately 4 to 7 days after the first seeding of mononuclear cells. The cell count ranged from 3 × 107 to 5 × 107 cells/mL, and the viability range was 92–95%. By the end of the two weeks, the cultured cells attained the specific features of MSCs as spindle-shaped cells attached to the surface with high proliferation capacity (Figure 1).
Figure 1.
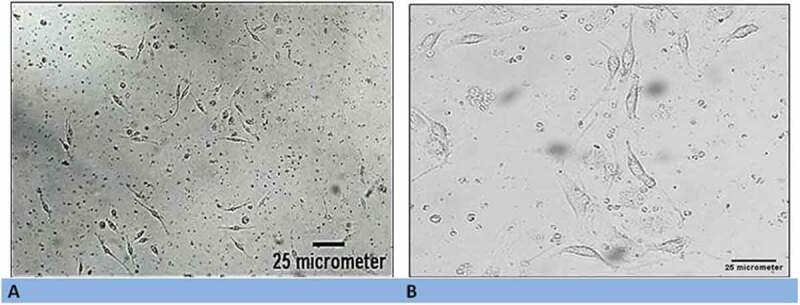
(a) Morphological characteristics of generated mesenchymal stem cells (MSC) using inverted light microscopy (x100). (b) High magnification illustrating the spindle-fibroblast-like shape of MSCs (x200).
Immunophenotyping of human MSCs
Surface antigens were considered positive if more than 20% of cells were positive. The cultured MSCs were positive for CD90 (56.5 ± 5.9 %) and CD105 (69.7 ± 3.7 %) but negative for hematopoietic markers including CD34 and CD45 (Figure 2).
Figure 2.
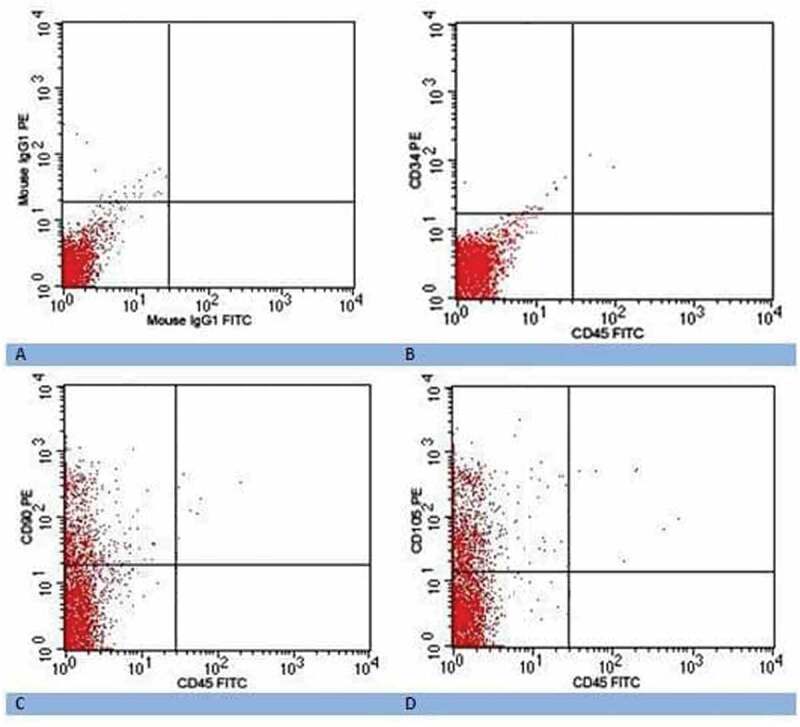
Flow cytometry dot plot showing (a) isotype negative control; (b) negative expression of CD34 and CD45 of MSCs after two weeks; (c) positive expression of CD90 of MSCs after two weeks; and (d) positive expression of CD105 of MSCs after two weeks.
Serum liver function parameters
The liver function parameters, including serum ALT, AST, and albumin, were significantly improved with p1 ˂ 0.01, p1 ˂ 0.001, p1 ˂ 0.01, respectively, in treated group II and p1 ˂ 0.001, p1 ˂ 0.001, and p1 ˂ 0.01, respectively, in treated group III compared to the infected non-treated control group I. No statistically significant difference in liver function parameters was found between treated groups II and III (P2 ˃ 0.05) (Table 1, Figure 3).
Table 1.
Hepatic biochemical parameter levels among the studied experimental animal groups.
| ALT(U/L) | AST(U/L) | Albumin (g/dL) | |
|---|---|---|---|
| Normal control group | 38.25 ± 3.576 | 40.125 ± 3.399 | 3.225 ± 0.409 |
| Infected control group (group I) | 140 ± 52.372 | 214.875 ± 52.305 | 2.175 ± 0.227 |
| P | ˂0.001*** | ˂0.001 *** | ˂0. 01** |
| PZQ treated group (group II) | 78.875 ± 20.911 | 108.75 ± 30.269 | 3.262 ± 0.507 |
| P | ˂ 0.05* | ˂0. 01** | ˃0.05 ns |
| P1 | ˂0. 01** | ˂0.001*** | ˂0. 01** |
| P2 | ˃0.05 ns | ˃0.05 ns | ˃0.05 ns |
| MSCs treated group (group III) | 62.75 ± 15.908 | 76.625 ± 26.715 | 3.213 ± 0.611 |
| P | ˃0.05 ns | ˃0.05 ns | ˃0.05 ns |
| P1 | ˂0.001*** | ˂0.001*** | ˂0.01** |
P indicates significance vs. non-infected control group.
P1 indicates significance vs. infected control group.
P2 indicates significance of PZQ treated group vs. MSCs treated group.
ns: non-significant, * P values ˂ 0.05, ** P values ˂ 0.01, and *** P values ˂ 0.001
Figure 3.
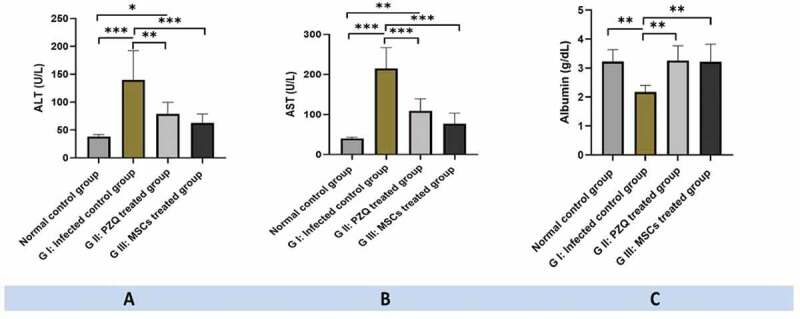
Hepatic biochemical parameter levels among the studied experimental animal groups: (a) serum ALT levels, (b) serum AST levels, and (c) serum albumin levels; showing significantly improved in treated group II and III compared to the infected non-treated control group I. ns: non-significant, * P values ˂ 0.05, ** P values ˂ 0.01, and *** P values ˂ 0.001.
Histopathological hepatic findings
Assessment of hepatic histopathological alterations in experimental animals was carried out using both hematoxylin and eosin and Masson’s trichrome stains. The results of the two stains showed the same pattern. The infected, untreated control group (group I) showed multiple granulomas that were large and diffuse, reaching a diameter of 294.87 ± 12.41 µm. The inflammatory cellular infiltrate was severe, with disruption of tissue architecture as well as cytoplasmic vacuolation and degeneration of hepatocytes. Granulomas were highlighted by concentric fibrosis with many fibroblasts encircling the entrapped eggs. Granulomas were also encircled by a cuff of aggregated lymphocytes, epithelioid cells, eosinophils, and collagenous fibers. Disorganization of hepatic cords and lobular structure was observed owing to the presence of numerous granulomas. In addition, the hepatic sinusoids were dilated and apparently contained more Kupffer cells (Figures 4, 5).
Figure 4.
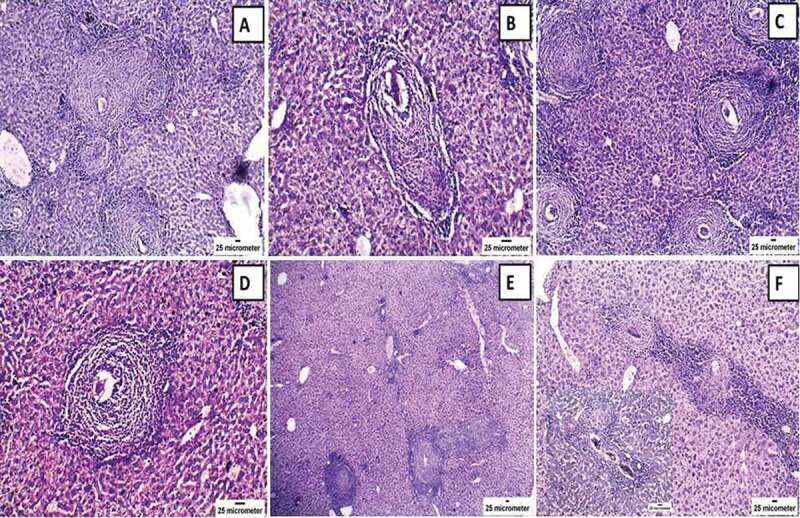
Histopathological changes of hematoxylin and eosin-stained liver of mice infected with Schistosoma mansoni twelve weeks post-infection (control: a & b; treated with praziquantel: c & d; and treated with mesenchymal stem cells: e & f) showing (a) multiple granulomas around Schistosoma mansoni ova and dilatation of portal vein branches with disruption of liver architecture (x100); (b) granuloma around Schistosoma mansoni ova with inflammatory cells mainly lymphocytes, plasma cells, and eosinophils with surrounding concentric fibrosis (x200); (c) & (e): reduction in the number of granulomas; and (d) & (f): decrease the inflammatory cellular infiltrate which is moderate in (d) (x200) and mild in (f) (x100) with insit higher magnification (x 200).
Figure 5.
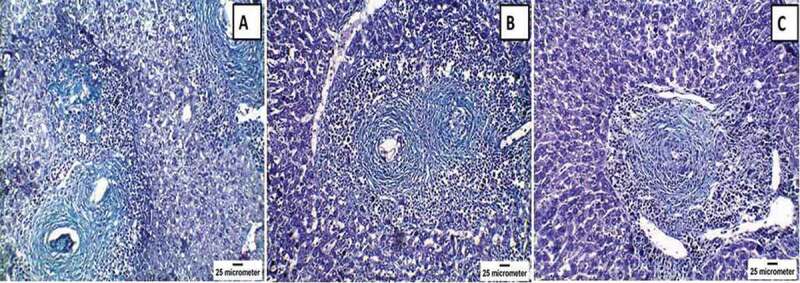
Histopathological changes of Masson’s trichrome (MT)-stained livers of mice infected with Schistosoma mansoni twelve weeks post-infection showing (a) the control infected group with positive staining in the concentric fibrous layers surrounding the granuloma and in the wall of hepatic sinusoids (x200); (b) praziquantel-treated group with moderate positive staining (x200); and (c) mesenchymal stem cells-treated group with mild positive staining (x200). Note the reduction in the number and size of granulomas in both treated groups in comparison to the infected, untreated group.
On the contrary, the PZQ-treated group (group II) showed a reduction in the number and size of the hepatic granulomas (226.89 ± 8.74 µm) with moderate inflammatory cellular infiltrate, while the MSCs-treated group (group III) demonstrated the best results with a marked reduction in the size (187.31 ± 7.90 µm) and the number of hepatic granulomas with mild inflammatory cellular infiltrate (Figures 4, 5). Statistically significant differences in granuloma size were reported among the infected control group and the other treated groups (P= 0.001) (Table 2, Figure 6).
Table 2.
Hepatic granuloma diameter among the studied experimental animal groups.
| G I: Infected control group | G II: PZQ treated group | G III: MSCs treated group | |||
|---|---|---|---|---|---|
| Range | 268.94–320.25 µm | 210.58–244.23 µm | 170.25–197.48 µm | ||
| Mean ± SD | 294.87 ± 12.41 µm | 226.89 ± 8.74 µm | 187.31 ± 7.90 µm | ||
| f. test | |||||
| P. value | |||||
| G I & G II | G I & G III | 60.845 | G II & G III | ||
| 0.001** | 0.001** | 0.001** | 0.001** | ||
** P values ˂ 0.01
Figure 6.
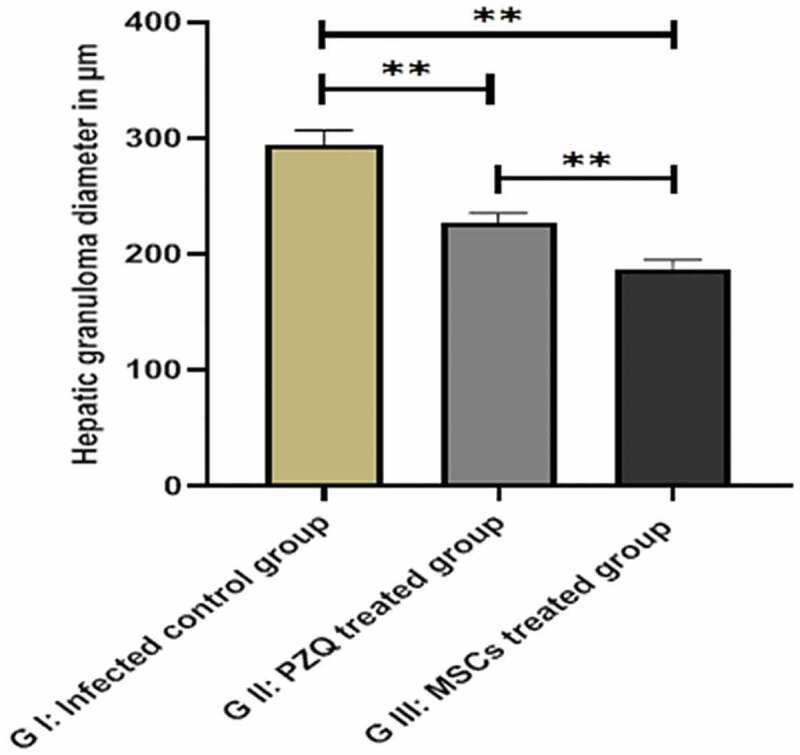
Hepatic granuloma diameter in the studied groups showing a reduction in the hepatic granuloma size in group II and the mesenchymal stem cell-treated group III than the control group I. ns: non-significant, * P values ˂ 0.05, ** P values ˂ 0.01, and *** P values ˂ 0.001.
Immunohistochemical results
Microscopic examination of GFAP immunohistochemically-stained slides revealed that immunostained HSCs were intensely present in the sinus walls as well as in the perivenular areas of the livers of the untreated, infected mice (group I), and 65% of the GFAP-immunostained HSCs were grade III. In contrast, the PZQ-treated group (group II) showed a lower number of GFAP-immunostained HSCs in sinusoidal walls and perivenular areas as 40% of the GFAP-immunostained HSCs were grade II, whereas the MSCs-treated group (group III) had a significantly lower number of GFAP-immunostained HSCs than groups I and II (P= 0.001, P= 0.006), respectively. In this group, 50% of positively stained HSCs were grade I and 25% were negatively stained grade 0, denoting a marked improvement in hepatic fibrosis (Table 3, Figure 7).
Table 3.
Immunohistochemical expression of GFAP of hepatic stellate cells (HSCs) in the liver of the studied experimental animal groups.
| Group (N = 20) | GFAP |
χ2 | P | |||||
|---|---|---|---|---|---|---|---|---|
| 0 | I | II | III | |||||
| G I: Infected control group (%) | 0 | 10 | 25 | 65 | 27.583 | 0.001** | ||
| G II: PZQ treated group (%) | 0 | 25 | 40 | 35 | ||||
| G III: MSCs treated group (%) | 25 | 50 | 20 | 5 | ||||
| G I & G II | G I & G III | G II & G III | ||||||
| 0.151 | 0.001** | 0.006** | ||||||
** P values ˂ 0.01
Semi-quantitative grading of HSCs immunostained by GFAP was determined as follows: 0: no staining or less than 3% stained; I: 3–33% positive staining; II: 34–66% positive staining; and III: more than 66% positive staining.
Figure 7.
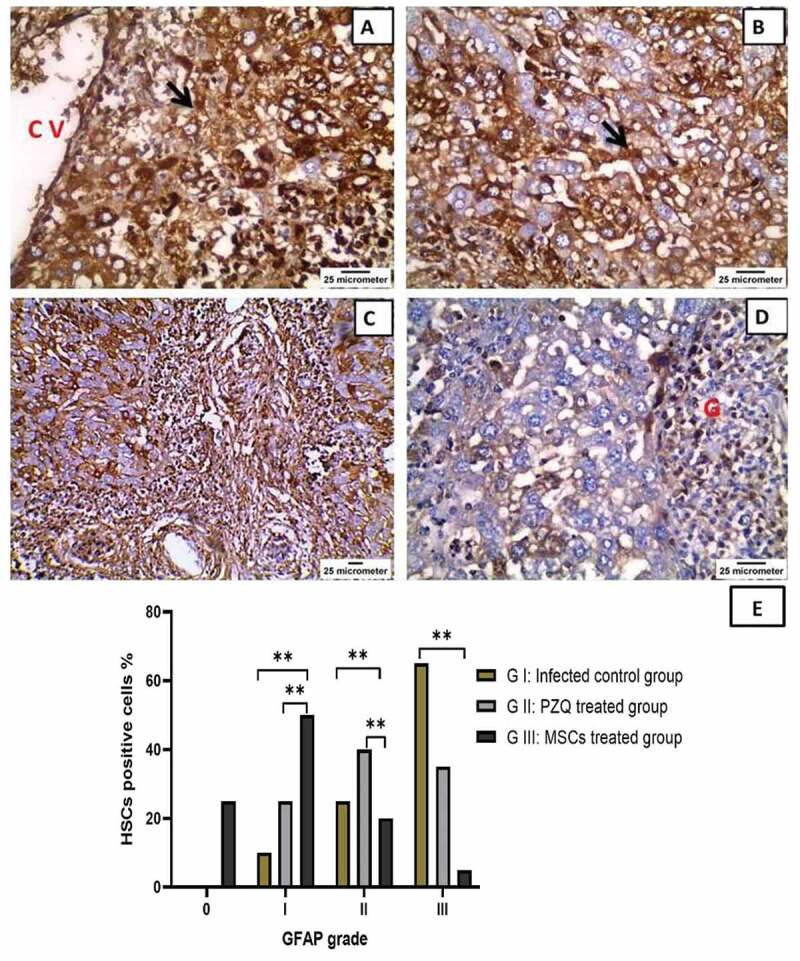
Immunohistochemical staining of glial fibrillary acidic protein (GFAP) in the livers of mice infected with Schistosoma mansoni twelve weeks post-infection showing (a) & (b) the infected, untreated group with intensely immunostained hepatic stellate cells (HSCs) present in the walls of sinuses (black arrow) as well as in the perivenular areas (x400) CV = central vein; (c) praziquantel-treated group with moderate GFAP expression in HSCs in the sinusoidal wall (x200); (d) mesenchymal stem cells-treated group with mild GFAP expression (x400) G = granuloma; and (e) The percentage HSCs positive for GFAP expression in the liver of the studied experimental animal groups. ns: non-significant, * P values ˂ 0.05, ** P values ˂ 0.01, and *** P values ˂ 0.001.
Discussion
This study aimed to investigate the efficacy of HUCB-MSCs in ameliorating S. mansoni-induced liver injury. The antifibrotic and anti-inflammatory properties of HUCB-MSCs were assessed in experimental animals, with the following main findings: (1) administration of 1 × 106 HUCB-MSCs/mouse via the tail vein improved liver functions (AST, ALT, and albumin) (2) a decrease in the size and number of hepatic granulomas with improvement in the liver’s histopathological appearance as evidenced by a decrease in the inflammatory cellular infiltrate and (3) a decrease in the number of activated HSCs immunohistochemically stained with anti-GFAP.
Schistosomal hepatic fibrosis remains a common type of liver fibrosis, particularly in developing countries. It may cause several complications, including portal hypertension, hepatocellular failure with ascites, hepatic encephalopathy, and bleeding tendency. In most cases, concurrent infection with viral hepatitis B and C occurs, ending in liver cirrhosis [2,25]. The patient’s progression to hepatic failure or cirrhosis with irreversible liver dysfunction indicates the need for hepatic replacement procedures such as liver transplantation. Nonetheless, the increased incidence of liver disease, the decreased availability of organ donors, the complications of immunosuppressive therapy, and the poor outcomes in patients on supportive treatment regimens pave the way for developing new treatment modalities such as stem cell therapies for liver regeneration [11].
Mesenchymal stem cell (MSC) therapy has recently emerged as an effective approach to treat a variety of diseases, including liver fibrosis [26]. MSCs are multipotent stem cells located in bone marrow (BM) stroma as well as other organs such as the liver, cord blood, adipose tissue, and the placenta [27]. The majority of human MSCs, regardless of source, express CD105, CD73, and CD90, but not CD34, CD14, CD45, CD11b, CD79α, CD19, or HLA class II. When these cells were isolated by plastic adherence and expanded ex vivo, they were found to differentiate into cell types of mesodermal origin, such as chondrocytes, adipocytes, and osteocytes [28].
MSCs have been shown to have plasticity beyond their traditional mesodermal lineage. They have been induced to generate both ectodermal (neurons) and endodermal (hepatocytes) tissues [29]. In addition, their ease and reproducibility of isolation, high expansion potential, capacity for useful modification using molecular biological engineering techniques, paracrine effects, immune-modulatory properties, migratory behavior, and ethical considerations make them promising candidates for tissue repair and regeneration [30].
Although BM has been identified as the most common source of MSCs, BM aspiration is an invasive and risky technique, particularly in hepatic patients due to their bleeding tendency, susceptibility to infection, and poor general condition. Furthermore, it has been demonstrated that the differentiating potential and number of BM-derived MSCs decrease with age [31].
HUCB is regarded as a crucial source of MSCs. It also has many advantages over other sources, such as vast abundance, ease of acquisition, availability for collection after fetus delivery, low risk of virus transmission, and the ability to be cryopreserved for long periods of time. Furthermore, HUCB-MSCs were found to have a less pronounced immune response as well as unique immunosuppressive properties that allow them to evade host rejection, making them a useful tool for cell therapy and experimental studies [32,33].
The present study demonstrated that HUCB-MSC treatment alleviated liver fibrosis and improved liver functions when compared to the untreated control group. Serum ALT and AST levels usually rise in cases of schistosomiasis, while low levels of liver-associated serum albumin secretions signify a liver function decompensation stage in schistosomiasis infections [34]. Our results reported a statistically significant decrease in the serum levels of ALT and AST as well as an increase in the serum albumin level in group III.
This is consistent with the results of Abou-Zied et al. [35] who reported that serum ALT was a good predictor for the development of liver fibrosis in the S. mansoni-infected mice and that treatment with CB-MSCs improved its level and the levels of serum albumin concentrations. Kamel et al. [33] also showed that the transplantation of HUCB-MSCs in two different experimental models of hepatic fibrosis (schistosomal hepatic fibrosis and chemically induced-fibrosis with carbon tetrachloride) resulted in significantly improved liver functions, engraftment of the fibrotic livers with newly formed hepatocytes with markedly reduced hepatic fibrosis, and a significantly decreased fibrotic index.
Hepatic fibrosis is the wound healing response of the liver to chronic injury. Following repeated injury, the liver undergoes tissue remodeling, resulting in fibrosis, in order to maintain organ integrity. It is characterized by excessive accumulation of extracellular matrix (ECM) proteins, as well as the formation of scar tissue enclosing the site of injury [36,37]. It is mostly a dynamic reversible process, rather than a static condition that can be reversed after the insult has been resolved [38]. Even after the cause has been eliminated, chronic liver injury is associated with a decline in reversibility potential [39].
During the fibrogenic process, several mediators collaborate in an orchestrated fashion. Fibrogenic cells that produce ECM proteins are activated, along with an unbalanced decrease in ECM-removing metalloproteinases (MMPs) [40]. Hepatic stellate cells (HSCs) are one of the most important types of fibrogenic cells. Following liver injury, these cells undergo a complex transformation or activation process that transforms them from a quiescent vitamin A-storing cell to an activated myofibroblast-like cell. Transforming growth factor (TGF-β1) and platelet-derived growth factor are two of the main drivers of HSC activation [41].
Activated HSCs alter the pattern of their gene expression, resulting in a dramatic increase in the synthesis and deposition of ECM proteins such as collagen. At the same time, they increase their proliferation rate, effectively increasing the number of fibrogenic cells in the liver. Furthermore, their activation is associated with an increase in cell contractility, which worsens portal hypertension [42].
The mechanisms by which MSCs exert their anti-fibrotic effects remain unknown. Stem cells were found to be involved in the breakdown of ECM through the expression of MMPs and hepatocyte growth factor (HGF), both of which increase the expression and activity of proteases. Fibrosis can be alleviated by degrading ECM proteins [43,44].
Other studies revealed that MSCs have an inhibitory effect on quiescent HSCs, preventing them from activating into myofibroblasts. This was due to their ability to suppress TGF-β1 expression. Interestingly, they were found to not only prevent HSC activation but also induce apoptosis when they are activated, possibly via HGF [17,37,45].
In addition to the hepatic fibrogenic cells, other cells are implicated in the fibrogenic response. When hepatocyte damage begins with activation of Kupffer cells and subsequent cytokine release, inflammatory cells are recruited to the liver [46]. Activated Kupffer cells, along with the recruitment of inflammatory cells to the liver, have been suggested to stimulate the transformation of HSCs into myofibroblasts, which may occur via Galectin-3 protein production, facilitating fibrosis progression [47–49]. Consistent with this theory, our findings showed that in group III, liver pathology was significantly improved, with a significant reduction in granuloma size and inflammatory cellular infiltrate. This reduction and improvement in liver pathology was statistically significant in group III compared to group II. The decrease in the influx of inflammatory cells may have contributed to the inhibition of HSC activation.
Other authors have also demonstrated the anti-inflammatory effects of MSC therapy [50,51]. Furthermore, MSCs were found to be able to inhibit the chemotactic properties of B cells, resulting in a reduction in inflammation [52,53]. Similarly, T cells produce a large number of cytokines that are involved in fibrosis progression or amelioration. Interleukin 4 (IL-4) and IL-13 are potent inducers of collagen production and fibroblast proliferation [54], while IL-10 has been associated with fibrosis regression [55].
Many researchers have provided explanations for the improvement of inflammation in hepatic tissues reported in our results. Abou Rayia et al. [5] reported that MSC therapy increased the expression of the immunoregulatory cytokine IL-10. Moreover, Lo Sicco et al. [56] demonstrated that stem cells can produce extracellular vesicles with potent anti-inflammatory properties, capable of polarizing macrophages to the regulatory M2 type and downregulating pro-inflammatory cytokines such as IL-6. Intriguingly, MSCs were found to suppress the secretion of Th1 pro-inflammatory cytokines such as tumor necrosis factor-alpha and interferon-gamma and increase the secretion of Th2 immunomodulatory cytokines [57–59].
According to our results, the amelioration detected in liver pathology could also be attributed to a significant reduction in the number of GFAP-immunostained HSCs in group III more than in the other groups. Glial fibrillary acidic protein is a member of the intermediate filaments that maintain the astroglia cell’s mechanical strength and structure. Nonetheless, hepatic expression of GFAP has been observed as a more useful marker for the early activation of HSCs [34]. In schistosomiasis, GFAP-positive cells were reported to predominate in the liver. Moreover, their percentage was correlated with the fibrosis stage [43,60]. Hence, the reduction in their percentage found in our study explains the hepatic fibrosis regression.
The capacity of stem cells to treat a hepatic injury is not only reliant on their paracrine secretions and ability to reduce inflammation and fibrosis, but also on their ability to differentiate into hepatocyte-like cells that express albumin and alpha-fetoprotein as reported by other researchers [17,61,62]. Hence, stem cells may aid liver repair by replacing damaged hepatocytes with normal-functioning cells.
The results of our study are consistent with earlier studies, with some differences regarding the type and route of cell injection, besides the mode of hepatic function assessment [5,17]. Several clinical trials have also started using different types of cells as autologous BM-derived mononuclear cells [63–65], autologous peripheral blood monocytes [65], or MSCs [66–68]. Also, different modes of administration were tried. Infusion of the cells was performed either via a peripheral vein [66,68], the hepatic artery [63,64,67], or the portal vein [69].
In contrast to our findings, Carvalho et al. [70] reported that BM mesenchymal stromal cells did not reduce fibrosis or improve liver functions in a rat model of severe chronic liver injury. In this model, the authors used both carbon tetrachloride and an alcoholic liquid diet (at a high dose and for a long administration period) to induce fibrosis. Thus, this discrepancy may be due to the discrepancy in the liver fibrosis models used. The severe chronic liver insult used by these authors may have rendered stem cells unable to ameliorate hepatic fibrosis.
Finally, the use of HUCB-MSCs in cell therapy is very promising. However, there are some limitations, such as getting enough cells through repeated culture, determining the optimal dose for treating various stages of liver fibrosis, and maintaining optimal culture conditions to avoid infection.
Conclusion
From our experimental study, MSCs can alleviate liver fibrosis and may serve as a versatile, readily available, novel cell type for the treatment of hepatic fibrosis due to their antifibrotic and anti-inflammatory properties. They may also be used as an alternative to liver transplantation if it is unavailable.
Acknowledgments
Special thanks to Tanta University that provided the complete fund for the present study.
Correction Statement
This article has been republished with minor changes. These changes do not impact the academic content of the article.
Funding Statement
This research was funded by the Tanta University Research Project Unit.
Data availability
The authors confirm that the data supporting the findings of this study are available within the article.
Disclosure statement
No potential conflict of interest was reported by the author(s).
Future recommendation
Further mechanisms underlying MSC-mediated hepatic repair will be studied in the future. In addition, the synergistic effect of the combined use of PZQ and MSCs in schistosomiasis should be elucidated. Indeed, further clinical studies are expeditiously recommended to establish the optimal dose, frequency, and route of administration of this treatment in order to achieve the best results, as well as to identify any potential limitations that may preclude the application of this novel therapy in humans.
Ethics approval
This study was approved by the Ethics Committee of the Faculty of Medicine, Tanta University, Tanta, Egypt. All institutional and national guidelines for the care and use of laboratory animals were followed. All procedures followed were in accordance with the Helsinki Declaration of 1975.
References
- [1].Ghatak S, Biswas A, Dhali GK, et al. Oxidative stress and hepatic stellate cell activation are key events in arsenic induced liver fibrosis in mice. Toxicol Appl Pharmacol. 2011. Feb 15;251(1):59–69. PubMed PMID: 21134390; PubMed Central PMCID: PMCPMC3745774. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [2].Perez HR, Stoeckle JH.. Stuttering: clinical and research update. Can Fam Physician. 2016. Jun;62(6):479–484. PubMed PMID: 27303004; PubMed Central PMCID: PMCPMC4907555. [PMC free article] [PubMed] [Google Scholar]
- [3].Eom YW, Shim KY, Baik SK. Mesenchymal stem cell therapy for liver fibrosis. Korean J Intern Med. 2015Sep;30(5):580–589. PubMed PMID: 26354051; PubMed Central PMCID: PMCPMC4578027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [4].Oliveira SA, Souza BS, Guimaraes-Ferreira CA, et al. Therapy with bone marrow cells reduces liver alterations in mice chronically infected by Schistosoma mansoni. World J Gastroenterol. 2008. Oct 14;14(38):5842–5850. PubMed PMID: 18855983; PubMed Central PMCID: PMCPMC2751894. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [5].Abou Rayia DM, Elmarhoumy SM, Ismail HH, et al. The outcomes of bone marrow stromal cell therapy in schistosomal hepatic fibrosis: an experimental study. J Egypt Soc Parasitol. 2017;47(3):633–642. [Google Scholar]
- [6].Xiao SH, Mei JY, Jiao PY. Schistosoma japonicum-infected hamsters (Mesocricetus auratus) used as a model in experimental chemotherapy with praziquantel, artemether, and OZ compounds. Parasitol Res. 2011Feb;108(2):431–437. PubMed PMID: 20922422. [DOI] [PubMed] [Google Scholar]
- [7].Abou-El-Naga IF. Schistosoma mansoni sarco/endoplasmic reticulum Ca(2+) ATPases (SERCA): role in reduced sensitivity to praziquantel. J Bioenerg Biomembr. 2020Oct;52(5):397–408. PubMed PMID: 32557343; eng. [DOI] [PubMed] [Google Scholar]
- [8].Sanchez MC, Cupit PM, Bu L, et al. Transcriptomic analysis of reduced sensitivity to praziquantel in Schistosoma mansoni. Mol Biochem Parasitol. 2019. Mar;228:6–15. PubMed PMID: 30658180; PubMed Central PMCID: PMCPMC6372308. eng [DOI] [PMC free article] [PubMed] [Google Scholar]
- [9].Botros SS, Bennett JL. Praziquantel resistance. Expert Opin Drug Discov. 2007Oct;2(s1):S35–40. PubMed PMID: 23489031; eng. [DOI] [PubMed] [Google Scholar]
- [10].Appleton CC, Mbaye A. Praziquantel–quality, dosages and markers of resistance. PubMed PMID: 11685889. Trends Parasitol. 2001. Aug;17(8):356–357. [DOI] [PubMed] [Google Scholar]
- [11].Guo Y, Chen B, Chen LJ, et al. Current status and future prospects of mesenchymal stem cell therapy for liver fibrosis. J Zhejiang Univ Sci B. 2016Nov;1711:831–841.PubMed PMID: 27819130; PubMed Central PMCID: PMCPMC5120225 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [12].Papp V, Rokusz A, Dezso K, et al. Expansion of hepatic stem cell compartment boosts liver regeneration. Stem Cells Dev. 2014. Jan 1;23(1):56–65. PubMed PMID: 23952741; PubMed Central PMCID: PMCPMC3870598. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [13].Puppi J, Strom SC, Hughes RD, et al. Improving the techniques for human hepatocyte transplantation: report from a consensus meeting in London. Cell Transplant. 2012;21(1):1–10. PubMed PMID: 21457616. [DOI] [PubMed] [Google Scholar]
- [14].Kharaziha P, Hellstrom PM, Noorinayer B, et al. Improvement of liver function in liver cirrhosis patients after autologous mesenchymal stem cell injection: a phase I-II clinical trial. Eur J Gastroenterol Hepatol. 2009Oct;2110:1199–1205.PubMed PMID: 19455046 [DOI] [PubMed] [Google Scholar]
- [15].Levicar N, Pai M, Habib NA, et al. Long-term clinical results of autologous infusion of mobilized adult bone marrow derived CD34+ cells in patients with chronic liver disease. Cell Prolif. 2008. Feb;41 Suppl 1:115–125. PubMed PMID: 18181952; PubMed Central PMCID: PMCPMC6496663 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [16].Tocci A, Forte L. Mesenchymal stem cell: use and perspectives. Hematol J. 2003;4(2):92–96. PubMed PMID: 12750726. [DOI] [PubMed] [Google Scholar]
- [17].Jung KH, Shin HP, Lee S, et al. Effect of human umbilical cord blood-derived mesenchymal stem cells in a cirrhotic rat model. Liver Int. 2009Jul;296:898–909.PubMed PMID: 19422480; eng [DOI] [PubMed] [Google Scholar]
- [18].Elkhafif N, Voss B, Hammam O, et al. Homing of transplanted bone marrow cells in livers of Schistosoma mansoni-infected mice. APMIS. 2010Apr;1184:277–287.PubMed PMID: 20402673 [DOI] [PubMed] [Google Scholar]
- [19].Ismail HI, Farrag AK, Abo-Raya AA. Pygeum africanum as an inhibitor for fibrogenesis: does it have a role in experimental hepatic schistosomal granuloma. Egyp J Med Microbiol. 2001;10:533–542. [Google Scholar]
- [20].Cho KA, Ju SY, Cho SJ, et al. Mesenchymal stem cells showed the highest potential for the regeneration of injured liver tissue compared with other subpopulations of the bone marrow. Cell Biol Int. 2009Jul;337:772–777.PubMed PMID: 19427913 [DOI] [PubMed] [Google Scholar]
- [21].Reddy NP, Vemuri MC, Pallu R. Isolation of stem cells from human umbilical cord blood. Methods Mol Biol. 2007;407:149–163. PubMed PMID: 18453255; eng. [DOI] [PubMed] [Google Scholar]
- [22].Khalifa YH, Mourad GM, Stephanos WM, et al. Bone marrow-derived mesenchymal stem cell potential regression of dysplasia associating experimental liver fibrosis in albino rats. Biomed Res Int. 2019;2019:5376165. PubMed PMID: 31781620; PubMed Central PMCID: PMCPMC6874956. eng. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [23].Starc N, Ingo D, Conforti A, et al. Biological and functional characterization of bone marrow-derived mesenchymal stromal cells from patients affected by primary immunodeficiency. Sci Rep. 2017. Aug 15;7(1):8153. PubMed PMID: 28811575; PubMed Central PMCID: PMCPMC5557950. eng. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [24].Carotti S, Morini S, Corradini SG, et al. Glial fibrillary acidic protein as an early marker of hepatic stellate cell activation in chronic and posttransplant recurrent hepatitis C. Liver Transplant. 2008Jun;146:806–814.PubMed PMID: 18508359; eng [DOI] [PubMed] [Google Scholar]
- [25].Darwish MA, Faris R, Darwish N, et al. Hepatitis c and cirrhotic liver disease in the Nile delta of Egypt: a community-based study. Am J Trop Med Hyg. 2001. Mar-Apr;643–4:147–153. PubMed PMID: 11442209. [DOI] [PubMed] [Google Scholar]
- [26].Moodley Y, Atienza D, Manuelpillai U, et al. Human umbilical cord mesenchymal stem cells reduce fibrosis of bleomycin-induced lung injury. Am J Pathol. 2009Jul;1751:303–313.PubMed PMID: 19497992; PubMed Central PMCID: PMCPMC2708816 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [27].Kucia M, Reca R, Jala VR, et al. Bone marrow as a home of heterogenous populations of nonhematopoietic stem cells. Leukemia. 2005Jul;197:1118–1127.PubMed PMID: 15902288; eng [DOI] [PubMed] [Google Scholar]
- [28].Dominici M, Le Blanc K, Mueller I, et al. Minimal criteria for defining multipotent mesenchymal stromal cells. The International Society for Cellular Therapy Position Statement Cytotherapy. 2006;8(4):315–317. PubMed PMID: 16923606; eng. [DOI] [PubMed] [Google Scholar]
- [29].Lee OK, Kuo TK, Chen WM, et al. Isolation of multipotent mesenchymal stem cells from umbilical cord blood. Blood. 2004. Mar 1;103(5):1669–1675. PubMed PMID: 14576065; eng. [DOI] [PubMed] [Google Scholar]
- [30].Brooke G, Cook M, Blair C, et al. Therapeutic applications of mesenchymal stromal cells. Semin Cell Dev Biol. 2007Dec;186:846–858.PubMed PMID: 18024097; eng [DOI] [PubMed] [Google Scholar]
- [31].D’Ippolito G, Schiller PC, Ricordi C, et al. Age-related osteogenic potential of mesenchymal stromal stem cells from human vertebral bone marrow. J Bone Miner Res. 1999Jul;147:1115–1122.PubMed PMID: 10404011; eng [DOI] [PubMed] [Google Scholar]
- [32].Coulson-Thomas VJ, Gesteira TF, Hascall V, et al. Umbilical cord mesenchymal stem cells suppress host rejection: the role of the glycocalyx. J Biol Chem. 2014. Aug 22;289(34):23465–23481. PubMed PMID: 24986866; PubMed Central PMCID: PMCPMC4156039. eng. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [33].Kamel MM, Baz HGE, Demerdash Z, et al. Cord blood-derived mesenchymal stem cells with hepatogenic differentiation potential ameliorate chronic liver affection in experimental models. Adv Clin Exp Med. 2018Oct;2710:1329–1339.PubMed PMID: 30048056; eng [DOI] [PubMed] [Google Scholar]
- [34].Hassan MM, Hwang LY, Hatten CJ, et al. Risk factors for hepatocellular carcinoma: synergism of alcohol with viral hepatitis and diabetes mellitus. Hepatology. 2002Nov;365:1206–1213.PubMed PMID: 12395331 [DOI] [PubMed] [Google Scholar]
- [35].Abou-Zied AM, Soliman RH, Hefila SM, et al. Biochemical and Parasitological Studies on the Effect of hUCB-Selected CD34(+) Progenitor/Stem Cells in Mice Infected with Schistosoma mansoni. Int J Stem Cells. 2014Nov;72:98–107.PubMed PMID: 25473447; PubMed Central PMCID: PMCPMC4249909. eng [DOI] [PMC free article] [PubMed] [Google Scholar]
- [36].Poynard T, Lebray P, Ingiliz P, et al. Prevalence of liver fibrosis and risk factors in a general population using non-invasive biomarkers (FibroTest). BMC Gastroenterol. 2010. Apr 22;10:40. PubMed PMID: 20412588; PubMed Central PMCID: PMCPMC2864202. eng. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [37].Zhao DC, Lei JX, Chen R, et al. Bone marrow-derived mesenchymal stem cells protect against experimental liver fibrosis in rats. World J Gastroenterol. 2005. Jun 14;11(22):3431–3440. PubMed PMID: 15948250; PubMed Central PMCID: PMCPMC4315999. eng. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [38].Schuppan D, Afdhal NH. Liver cirrhosis. Lancet. 2008. Mar 8;371(9615):838–851. PubMed PMID: 18328931; PubMed Central PMCID: PMCPMC2271178. eng. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [39].Fagone P, Mangano K, Pesce A, et al. Emerging therapeutic targets for the treatment of hepatic fibrosis. Drug Discov Today. 2016Feb;212:369–375.PubMed PMID: 26523773; eng [DOI] [PubMed] [Google Scholar]
- [40].Fagone P, Mangano K, Mammana S, et al. Identification of novel targets for the diagnosis and treatment of liver fibrosis. Int J Mol Med. 2015Sep;363:747–752.PubMed PMID: 26135677; eng [DOI] [PubMed] [Google Scholar]
- [41].Eng FJ, Friedman SL, Fibrogenesis I. New insights into hepatic stellate cell activation: the simple becomes complex. Am J Physiol Gastrointest Liver Physiol. 2000Jul;279(1):G7–g11. PubMed PMID: 10898741; eng. [DOI] [PubMed] [Google Scholar]
- [42].Tsukada S, Parsons CJ, Rippe RA. Mechanisms of liver fibrosis. Clin Chim Acta. 2006Feb;364(1–2):33–60. PubMed PMID: 16139830; eng. [DOI] [PubMed] [Google Scholar]
- [43].Chang YJ, Liu JW, Lin PC, et al. Mesenchymal stem cells facilitate recovery from chemically induced liver damage and decrease liver fibrosis. Life Sci. 2009. Sep 23;85(13–14):517–525. PubMed PMID: 19686763; eng. [DOI] [PubMed] [Google Scholar]
- [44].Ozaki I, Zhao G, Mizuta T, et al. Hepatocyte growth factor induces collagenase (matrix metalloproteinase-1) via the transcription factor Ets-1 in human hepatic stellate cell line. J Hepatol. 2002Feb;362:169–178.PubMed PMID: 11830328; eng [DOI] [PubMed] [Google Scholar]
- [45].Zhang B, Inagaki M, Jiang B, et al. Effects of bone marrow and hepatocyte transplantation on liver injury. J Surg Res. 2009Nov;1571:71–80.PubMed PMID: 19345373; eng [DOI] [PubMed] [Google Scholar]
- [46].Muddu AK, Guha IN, Elsharkawy AM, et al. Resolving fibrosis in the diseased liver: translating the scientific promise to the clinic. Int J Biochem Cell Biol. 2007;39(4):695–714. PubMed PMID: 17110155; eng. [DOI] [PubMed] [Google Scholar]
- [47].Li LC, Li J, Gao J. Functions of galectin-3 and its role in fibrotic diseases. J Pharmacol Exp Ther. 2014Nov;351(2):336–343. PubMed PMID: 25194021; eng. [DOI] [PubMed] [Google Scholar]
- [48].Pellicoro A, Ramachandran P, Iredale JP, et al. Liver fibrosis and repair: immune regulation of wound healing in a solid organ. Nat Rev Immunol. 2014Mar;143:181–194.PubMed PMID: 24566915; eng [DOI] [PubMed] [Google Scholar]
- [49].Imamura M, Ogawa T, Sasaguri Y, et al. Suppression of macrophage infiltration inhibits activation of hepatic stellate cells and liver fibrogenesis in rats. Gastroenterology. 2005Jan;1281:138–146.PubMed PMID: 15633130; eng [DOI] [PubMed] [Google Scholar]
- [50].Zhang Z, Wang FS. Stem cell therapies for liver failure and cirrhosis. J Hepatol. 2013Jul;59(1):183–185. PubMed PMID: 23353868; eng. [DOI] [PubMed] [Google Scholar]
- [51].Li J, Li M, Niu B, et al. Therapeutic potential of stem cell in liver regeneration. Front Med. 2011Mar;51:26–32.PubMed PMID: 21681671; eng [DOI] [PubMed] [Google Scholar]
- [52].Liu J, Liu Q, Chen X. The Immunomodulatory Effects of Mesenchymal Stem Cells on Regulatory B Cells. Front Immunol. 2020;11:1843. PubMed PMID: 32922398; PubMed Central PMCID: PMCPMC7456948. eng. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [53].Franquesa M, Hoogduijn MJ, Bestard O, et al. Immunomodulatory effect of mesenchymal stem cells on B cells. Front Immunol. 2012;3:212. PubMed PMID: 22833744; PubMed Central PMCID: PMCPMC3400888. eng. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [54].Chiaramonte MG, Donaldson DD, Cheever AW, et al. An IL-13 inhibitor blocks the development of hepatic fibrosis during a T-helper type 2-dominated inflammatory response. J Clin Invest. 1999Sep;1046:777–785.PubMed PMID: 10491413; PubMed Central PMCID: PMCPMC408441. eng [DOI] [PMC free article] [PubMed] [Google Scholar]
- [55].Safadi R, Ohta M, Alvarez CE, et al. Immune stimulation of hepatic fibrogenesis by CD8 cells and attenuation by transgenic interleukin-10 from hepatocytes. Gastroenterology. 2004Sep;1273:870–882.PubMed PMID: 15362042; eng [DOI] [PubMed] [Google Scholar]
- [56].Lo Sicco C, Reverberi D, Balbi C, et al. Mesenchymal stem cell-derived extracellular vesicles as mediators of anti-inflammatory effects: endorsement of macrophage polarization. Stem Cells Transl Med. 2017Mar;63:1018–1028.PubMed PMID: 28186708; PubMed Central PMCID: PMCPMC5442783. eng [DOI] [PMC free article] [PubMed] [Google Scholar]
- [57].Zhu M, Hua T, Ouyang T, et al. Applications of mesenchymal stem cells in liver fibrosis: novel strategies, mechanisms, and clinical practice. Stem Cells Int. 2021;2021:6546780. PubMed PMID: 34434239; PubMed Central PMCID: PMCPMC8380491. eng. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [58].Parekkadan B, van Poll D, Megeed Z, et al. Immunomodulation of activated hepatic stellate cells by mesenchymal stem cells. Biochem Biophys Res Commun. 2007. Nov 16;363(2):247–252. PubMed PMID: 17869217; PubMed Central PMCID: PMCPMC2096777. eng. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [59].Hu C, Zhao L, Zhang L, et al. Mesenchymal stem cell-based cell-free strategies: safe and effective treatments for liver injury. Stem Cell Res Ther. 2020. Sep 3;11(1):377. PubMed PMID: 32883343; PubMed Central PMCID: PMCPMC7469278. eng. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [60].Chang D, Ramalho LN, Ramalho FS, et al. Hepatic stellate cells in human schistosomiasis mansoni: a comparative immunohistochemical study with liver cirrhosis. Acta Trop. 2006Mar;973:318–323.PubMed PMID: 16473318; eng [DOI] [PubMed] [Google Scholar]
- [61].Eissa M, Elarabany N, Hyder A. In vitro efficacy of liver microenvironment in bone marrow mesenchymal stem cell differentiation. Vitro Cell Dev Biol Anim. 2020Apr;56(4):341–348. PubMed PMID: 32270392; eng. [DOI] [PubMed] [Google Scholar]
- [62].Zhou X, Cui L, Zhou X, et al. Induction of hepatocyte-like cells from human umbilical cord-derived mesenchymal stem cells by defined microRNAs. J Cell Mol Med. 2017May;215:881–893.PubMed PMID: 27874233; PubMed Central PMCID: PMCPMC5387126. eng [DOI] [PMC free article] [PubMed] [Google Scholar]
- [63].Lyra AC, Soares MB, da Silva LF, et al. Infusion of autologous bone marrow mononuclear cells through hepatic artery results in a short-term improvement of liver function in patients with chronic liver disease: a pilot randomized controlled study. Eur J Gastroenterol Hepatol. 2010Jan;221:33–42.PubMed PMID: 19654548 [DOI] [PubMed] [Google Scholar]
- [64].Spahr L, Chalandon Y, Terraz S, et al. Autologous bone marrow mononuclear cell transplantation in patients with decompensated alcoholic liver disease: a randomized controlled trial. PLoS One. 2013;8(1):e53719. PubMed PMID: 23341981; PubMed Central PMCID: PMCPMC3544843. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [65].Yannaki E, Anagnostopoulos A, Kapetanos D, et al. Lasting amelioration in the clinical course of decompensated alcoholic cirrhosis with boost infusions of mobilized peripheral blood stem cells. Exp Hematol. 2006Nov;3411:1583–1587.PubMed PMID: 17046578 [DOI] [PubMed] [Google Scholar]
- [66].El-Ansary M, Abdel-Aziz I, Mogawer S, et al. Phase II trial: undifferentiated versus differentiated autologous mesenchymal stem cells transplantation in Egyptian patients with HCV induced liver cirrhosis. Stem Cell Rev Rep. 2012Sep;83:972–981.PubMed PMID: 21989829 [DOI] [PubMed] [Google Scholar]
- [67].Peng L, Xie DY, Lin BL, et al. Autologous bone marrow mesenchymal stem cell transplantation in liver failure patients caused by hepatitis B: short-term and long-term outcomes. Hepatology. 2011. Sep 2;54(3):820–828. PubMed PMID: 21608000. [DOI] [PubMed] [Google Scholar]
- [68].Mohamadnejad M, Alimoghaddam K, Bagheri M, et al. Randomized placebo-controlled trial of mesenchymal stem cell transplantation in decompensated cirrhosis. Liver Int. 2013Nov;3310:1490–1496.PubMed PMID: 23763455 [DOI] [PubMed] [Google Scholar]
- [69].Salama H, Zekri AR, Bahnassy AA, et al. Autologous CD34+ and CD133+ stem cells transplantation in patients with end stage liver disease. World J Gastroenterol. 2010. Nov 14;16(42):5297–5305. PubMed PMID: 21072892; PubMed Central PMCID: PMCPMC2980678. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [70].Carvalho AB, Quintanilha LF, Dias JV. Bone marrow multipotent mesenchymal stromal cells do not reduce fibrosis or improve function in a rat model of severe chronic liver injury. Stem cells. 2008. ;26(5): 1307–1314. doi: 10.1634/stemcells.2007-0941. PubMed PMID: 18308943; eng. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The authors confirm that the data supporting the findings of this study are available within the article.


