Abstract
Triplet regimens containing immunomodulatory drugs and proteasome inhibitors (PIs) have improved outcomes and extended survival for patients with relapsed/refractory multiple myeloma (RRMM). We evaluated updated health-related quality of life (HRQoL) findings from the phase 2 ELOQUENT-3 clinical trial (NCT02654132) after 4 years of treatment with elotuzumab plus pomalidomide and dexamethasone (EPd) and assessed the impact of the addition of elotuzumab on patients’ HRQoL. HRQoL was assessed as an exploratory endpoint using the MD Anderson Symptom Inventory for Multiple Myeloma (MDASI-MM), which evaluates symptom severity, symptom interference, and HRQoL, and the 3-level EQ-5D, a patient-reported measure of health utility and general health. Statistical analyses included descriptive responder, longitudinal mixed-model, and time-to-first-deterioration (TTD) analyses using prespecified minimally important differences and responder definitions. Of 117 randomized patients, 106 (EPd, n = 55; pomalidomide and dexamethasone [Pd], n = 51) were eligible for inclusion in HRQoL analyses. Completion rates at almost all on-treatment visits were ≥80%. The proportion of patients treated with EPd who improved or maintained stable HRQoL until cycle 13 ranged from 82% to 96% for MDASI-MM total symptom score and 64% to 85% for MDASI-MM symptom interference. Across measurements, there were no clinically meaningful differences in changes from baseline between treatment arms, and TTD was not significantly different for EPd versus Pd. In conclusion, HRQoL was not impacted by the addition of elotuzumab to Pd and did not significantly deteriorate in patients with RRMM previously treated with lenalidomide and a PI in ELOQUENT-3.
INTRODUCTION
Multiple myeloma (MM) accounts for approximately 1.8% of all cancer diagnoses and 2.1% of all cancer deaths in the United States,1 with an estimated 34,920 cases diagnosed in 2021.2 Worldwide, the estimated number of new cases of MM in 2020 was 176,404.3 Patients with MM often experience poor health-related quality of life (HRQoL), which may substantially deteriorate through multiple treatment lines.4,5 In particular, fatigue and bone pain are 2 of the most common symptoms reported by patients with MM.6 Triplet regimens, specifically those containing immunomodulatory drugs and proteasome inhibitors (PIs), have improved outcomes and extended survival for patients with relapsed/refractory multiple myeloma (RRMM), and abrogate MM-induced symptoms more quickly and efficiently than doublet regimens.7,8 However, the extended use of multiagent treatments has the potential to increase treatment-related side effects.9
In the randomized, phase 2 ELOQUENT-3 trial (NCT02654132), the efficacy and safety of elotuzumab, a humanized immunoglobulin G1 monoclonal antibody, in combination with pomalidomide and dexamethasone (EPd), versus pomalidomide and dexamethasone alone (Pd) were assessed in patients with RRMM previously treated with lenalidomide and a PI. Patients were randomized in a 1:1 ratio to receive either EPd or Pd, stratified by the number of lines of prior therapy (2–3 versus >4) and International Staging System stage at study entry (I–II versus III). At the time of the database lock in November 2018, patients treated with EPd had a 46% reduction in the risk of death versus Pd, without increasing toxicity or affecting HRQoL through 18.3 months of follow-up.10,11 Subsequent clinical data based on a minimum follow-up period of 45 months (database lock: January 2021) demonstrated that median (95% confidence interval [CI]) overall survival was significantly improved with EPd (29.8 [22.9-45.7] months) versus Pd (17.4 [13.8-27.7] months), with a hazard ratio of 0.59 (95% CI, 0.37-0.93; P = 0.022).12,13
To inform treatment-related decision-making, it is important to measure how effective therapies can preserve HRQoL without a negative impact. The objective of this study was to evaluate HRQoL findings from ELOQUENT-3 after 4 years of EPd therapy (data cutoff, January 11, 2021) and assess whether the addition of elotuzumab to Pd impacts patients’ HRQoL. At the 4-year database lock, patients in the EPd group had received a median (range) of 9.0 (1–53) treatment cycles while those in the Pd group received 5.0 (1–50).13 Preliminary HRQoL findings from the 4-year database lock were presented at the American Society of Hematology annual meeting 2021.14 Here, we present more extensive data across all patient-reported outcome (PRO) subscales administered in the study (MD Anderson Symptom Inventory for Multiple Myeloma [MDASI-MM]15 and the 3-level EuroQol 5 dimension [EQ-5D-3L]).
MATERIALS AND METHODS
Study design and HRQoL measures
The ELOQUENT-3 study design has previously been reported.10 HRQoL was assessed as an exploratory endpoint using the MDASI-MM14 and EQ-5D-3L16 at baseline, start of every 28-day treatment cycle, end of treatment, and during follow-up every 3 months.
The MDASI-MM measures symptom severity of disease and treatment and impact on daily-life function, and comprises 2 domains: total symptom severity (13 core and 7 MM-specific symptom severity items) and symptom interference (3 activity and 3 affective interference items).15 Pain, fatigue, and bone pain were key symptoms additionally analyzed at the item level. All scores range from 0 to 10; higher scores indicate worse HRQoL. Clinically meaningful changes were evaluated in terms of group-level minimally important differences (MIDs) and within-person responder definitions based on standard error of the mean for domains17 and one-half standard deviation (SD) for individual items.18
EQ-5D-3L is a patient-reported measure of health utility and general health, and comprises 5 dimensions: mobility, self-care, usual activities, pain/discomfort, and anxiety/depression; scores are converted into a single summary-utility index ranging from −0.59 to 1 (0 = death; 1 = perfect health) based on United Kingdom empirically derived weights.16 A visual analog scale (VAS) evaluates self-reported health from 0 to 100 (higher scores = better health status). Clinically meaningful score changes were interpreted using MIDs/responder definitions of 0.08 for the United Kingdom utility index and 7 for the VAS.19
Statistical analyses
All randomized patients with baseline and ≥1 post-baseline MDASI-MM/EQ-5D-3L assessment were eligible for inclusion in the HRQoL analysis population used for descriptive responder and longitudinal mixed-model analyses; time-to-first-deterioration (TTD) analyses were based on all randomized patients. Available data and completion rates for HRQoL questionnaires were calculated using the number of randomized and expected patients, respectively, at each time point.
Descriptive responder analysis
The proportion of improved/stable/deteriorated patients’ HRQoL compared with baseline at cycles 2–13 was presented based on prespecified responder definitions for each PRO score.
Longitudinal mixed-model analysis
Longitudinal mixed models for repeated measures (MMRM) analysis generated estimates of least squares (LS) mean change from baseline and LS mean treatment differences. MMRM models included all data up to and including cycle 13 (last on-treatment visit with ≥10 patients completing PROs in each treatment arm). Change from baseline PRO score was included as the dependent variable; covariates included baseline score and stratification factors. Treatment group, visit week, and treatment group × visit week interaction were treated as fixed effects, with visit week as a repeated measure. Models were run using an unstructured covariance matrix.
TTD analysis
The TTD analysis evaluated whether elotuzumab addition to Pd resulted in faster HRQoL deterioration rates. TTD was defined as time to first change from baseline that reached the responder definition threshold for deterioration (ie, MDASI-MM score increase, EQ-5D-3L score decrease) and was calculated using all on-treatment visit data. Patients without a PRO-deterioration event were censored at the date of the last PRO assessment. Hazard ratios were estimated from stratified Cox regression models. Median TTD was estimated using unstratified Kaplan–Meier methodology. Statistical significance presented is at the 0.05 level, with no multiplicity adjustment.
RESULTS
Analysis population and completion rates
Of the 117 randomized patients in the ELOQUENT-3 intent-to-treat population, 106 patients (EPd, n = 55; Pd, n = 51) were eligible for inclusion in the HRQoL analyses. Baseline demographic and clinical characteristics of the HRQoL population were similar for the EPd and Pd arms and were representative of the intent-to-treat study population (Table 1).
Table 1.
Demographic and Clinical Characteristics at Baseline
| Characteristic | Intent-to-treat Population | HRQoL Analysis Population | ||
|---|---|---|---|---|
| EPd (n = 60) | Pd (n = 57) | EPd (n = 55) | Pd (n = 51) | |
| Age at baseline, y | ||||
| Mean (SD) | 66.2 (9.9) | 65.5 (9.9) | 66.9 (9.3) | 65.5 (10.3) |
| Sex, n (%) | ||||
| Female | 28 (46.7) | 22 (38.6) | 26 (47.3) | 21 (41.2) |
| Male | 32 (53.3) | 35 (61.4) | 29 (52.7) | 30 (58.8) |
| Race, n (%) | ||||
| Asian | 15 (25.0) | 9 (15.8) | 15 (27.3) | 8 (15.7) |
| White | 45 (75.0) | 45 (78.9) | 40 (72.7) | 41 (80.4) |
| Black or African American | - | 1 (1.8) | - | - |
| Other | - | 2 (3.5) | - | 2 (3.9) |
| Region, n (%) | ||||
| North America | 3 (5.0) | 7 (12.3) | 3 (5.5) | 6 (11.8) |
| Europe | 44 (73.3) | 43 (75.4) | 39 (70.9) | 39 (76.5) |
| Japan | 13 (21.7) | 7 (12.3) | 13 (23.6) | 6 (11.8) |
| ECOG PS at baseline, n (%) | ||||
| 0 | 28 (46.7) | 23 (40.4) | 26 (47.3) | 21 (41.2) |
| 1 | 28 (46.7) | 26 (45.6) | 25 (45.5) | 23 (45.1) |
| 2 | 4 (6.7) | 8 (14.0) | 4 (7.3) | 7 (13.7) |
| Prior therapy lines, n (%) | ||||
| 2–3 | 37 (61.7) | 35 (61.4) | 34 (61.8) | 32 (62.7) |
| ≥4 | 23 (38.3) | 22 (38.6) | 21 (38.2) | 19 (37.3) |
| ISS stage, n (%) | ||||
| I–II | 52 (86.7) | 51 (89.5) | 48 (87.3) | 45 (88.2) |
| III | 8 (13.3) | 6 (10.5) | 7 (12.7) | 6 (11.8) |
ECOG PS = Eastern Cooperative Oncology Group performance status; EPd = elotuzumab + pomalidomide + dexamethasone; HRQoL = health-related quality of life; ISS = International Staging System; Pd = pomalidomide + dexamethasone; SD = standard deviation.
With the exception of cycle 14, MDASI-MM and EQ-5D-3L questionnaire completion rates (out of the expected population) were 80% or higher for all on-treatment visits where ≥10 patients remained on study in the EPd arm (Suppl. Table S1). HRQoL was examined between arms up to cycle 13 due to the low number of patients in the Pd arm (<10 patients) after this time point. At cycle 13, 40.0% and 17.5% of the total number of patients randomized to EPd and Pd, respectively, completed the MDASI-MM; available data rates for the EQ-5D-3L were slightly higher (Suppl. Table S1).
Descriptive responder analysis
Mean baseline HRQoL scores were similar between EPd and Pd arms, with the exception of affective interference on the MDASI-MM, which showed greater symptom interference in the EPd arm (EPd: 2.3, Pd: 1.5; Table 2). Table 2 presents the mean baseline scores for the MDASI-MM (domains, subscales, and individual items for the key symptoms of pain, fatigue, and bone pain) and EQ-5D-3L (utility index and VAS).
Table 2.
MDASI-MM and EQ-5D-3L Mean (SD) Baseline Scores in HRQoL Population, by Domain/Symptom
| Domain/Symptom | EPd | Pd |
|---|---|---|
| MDASI-MM a | n = 49 | n = 42 |
| Total symptom severity | 1.5 (1.4) | 1.6 (1.4) |
| Core symptom severity | 1.7 (1.5) | 1.7 (1.5) |
| Module symptom severity | 1.2 (1.3) | 1.4 (1.4) |
| Item 1: Pain | 2.6 (2.5) | 2.7 (2.7) |
| Item 2: Fatigue | 2.8 (2.5) | 3.0 (2.7) |
| Item 20: Bone pain | 2.3 (2.7) | 2.9 (3.0) |
| Symptom interference | 2.5 (2.7) | 2.1 (2.0) |
| Activity interference | 2.6 (2.8) | 2.8 (2.7) |
| Affective interference | 2.3 (2.7) | 1.5 (1.6) |
| EQ-5D-3L | n = 55 | n = 51 |
| Utility indexb | 0.698 (0.283) | 0.677 (0.291) |
| VASc | 65.6 (18.6) | 69.2 (20.9) |
Score ranges from 0 to 10, with a higher score indicating greater symptom severity/interference.
Score ranges from 1 (full health) to 0 (death), with negative scores (to −0.59) reflecting health states valued as worse than death.
Score ranges from 0 to 100, with a higher score indicating a better health status.
EPd = elotuzumab + pomalidomide + dexamethasone; EQ-5D-3L = 3-level EuroQol 5 dimension; HRQoL = health-related quality of life; MDASI-MM = MD Anderson Symptom Inventory for Multiple Myeloma; Pd = pomalidomide + dexamethasone; SD = standard deviation; VAS = visual analog scale.
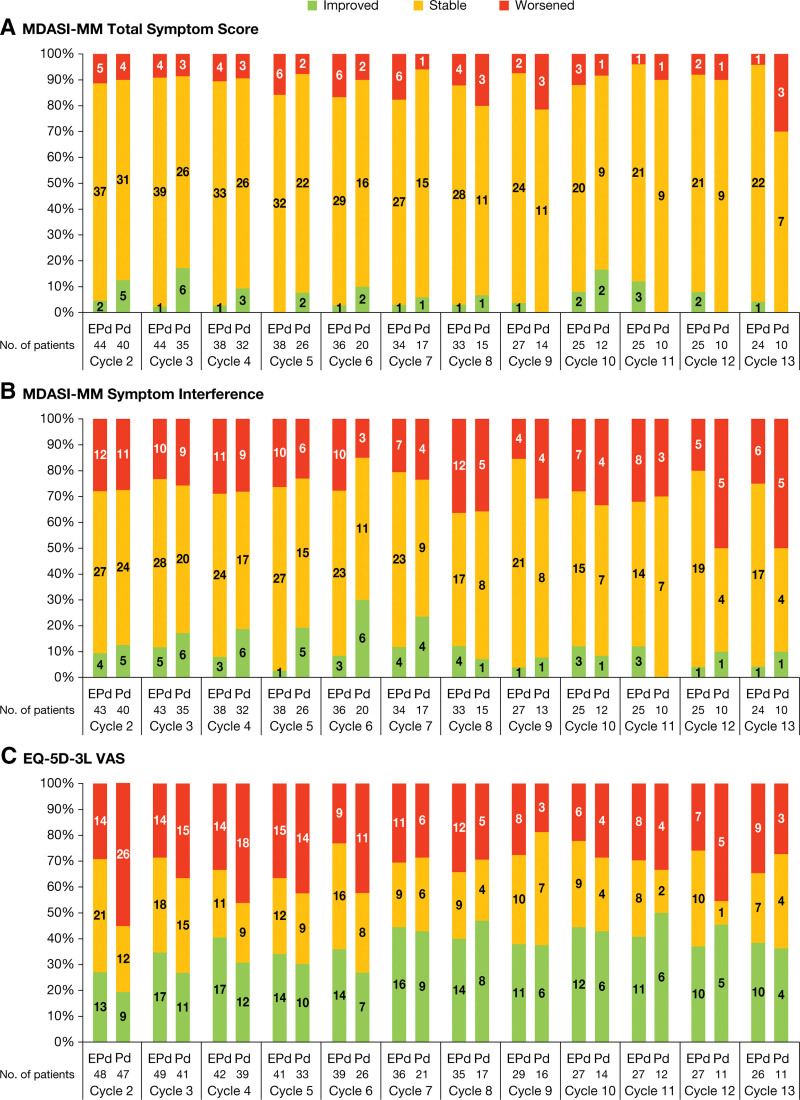
For responder analyses, the proportion of patients in the EPd arm who either improved or maintained stable HRQoL from baseline until cycle 13 ranged from 82% to 96% for MDASI-MM total symptom severity score, and 64% to 85% for MDASI-MM symptom interference (Figure 1A and B). The proportion of patients in the Pd arm who improved or maintained stable HRQoL from baseline was similar to EPd up until cycle 12 for total symptom severity score and cycle 11 for symptom interference, after which a greater proportion of the few remaining patients in the Pd arm worsened. For the EQ-5D-3L VAS, there was a greater proportion of patients at cycle 2 who deteriorated on Pd (55.3%) compared with EPd (29.2%); however, differences between treatment arms at later cycles were less noticeable, with the proportion of patients who improved or maintained stable HRQoL ranging from 63% to 78% for EPd and from 54% to 81% for Pd (Figure 1C). The conclusions for the other scales were consistent with those reported here.
Figure 1.
Proportion improved/stable/worsened patients for (A) MDASI-MM total symptom score, (B) MDASI-MM symptom interference, and (C) EQ-5D-3L VAS at each on-treatment cycle. (A) Responder definition was −0.4 for improvement and +0.4 for worsening HRQoL; (B) Responder definition was −0.7 for improvement and +0.7 for worsening HRQoL; (C) Responder definition was +7 for improvement and −7 for worsening HRQoL. For all graphs (A–C), response calculated relative to baseline; number of patients presented within bars. EPd = elotuzumab + pomalidomide + dexamethasone; EQ-5D-3L = 3-level EuroQol 5 dimension; HRQoL = health-related quality of life; MDASI-MM = MD Anderson Symptom Inventory for Multiple Myeloma; Pd = pomalidomide + dexamethasone; VAS = visual analog scale.
Longitudinal mixed-model analysis
Comparing LS mean treatment differences against the MIDs for the respective scales indicated that there were no overall clinically meaningful differences between the EPd and Pd arms for MDASI-MM (including domains, subscales, and individual items for the key symptoms of pain, fatigue, and bone pain) or EQ-5D-3L (utility index and VAS) (Table 3).
Table 3.
MMRMs Analysis of Change From Baseline in PRO Scales (HRQoL Analysis Population)
| Item/Domain | EPd: N, LS Mean (95% CI) | Pd: N, LS Mean (95% CI) | Treatment Difference LS Mean (95% CI)a | Treatment Difference P Value |
MID |
|---|---|---|---|---|---|
| MDASI-MMb | |||||
| Total symptom severity | 48, 0.5 (0.1–0.9) | 40, 0.4 (−0.1 to 0.9) | 0.1 (−0.5 to 0.6) | 0.811 | 0.4 |
| Core symptom severity | 48, 0.4 (−0.1 to 0.8) | 40, 0.3 (−0.2 to 0.9) | 0.1 (−0.5 to 0.6) | 0.846 | 0.5 |
| Module symptom severity | 47, 0.5 (0.1–0.9) | 40, 0.4 (−0.1 to 0.9) | 0.1 (−0.4 to 0.6) | 0.597 | 0.7 |
| Item 1: Pain | 48, 0.2 (−0.6 to 0.9) | 40, 0.2 (−0.7 to 1.1) | −0.1 (−1.1 to 0.9) | 0.884 | 1.3 |
| Item 2: Fatigue | 48, 0.9 (0.1–1.7) | 40, 0.9 (−0.1 to 1.8) | 0.0 (−1.0 to 1.0) | 0.987 | 1.3 |
| Item 20: Bone pain | 47, 0.1 (−0.8 to 0.9) | 40, −0.2 (−1.1 to 0.8) | 0.2 (−0.9 to 1.3) | 0.695 | 1.5 |
| Symptom interference | 47, 0.5 (−0.2 to 1.2) | 40, 0.8 (0.0–1.5) | −0.3 (−1.1 to 0.5) | 0.462 | 0.7 |
| Activity interference | 47, 0.6 (−0.2 to 1.3) | 40, 0.8 (−0.1 to 1.6) | −0.2 (−1.0 to 0.7) | 0.708 | 0.8 |
| Affective interference | 47, 0.2 (−0.4 to 0.9) | 40, 0.6 (−0.2 to 1.3) | −0.4 (−1.2 to 0.4) | 0.344 | 0.8 |
| EQ-5D-3Lc | |||||
| Utility index | 54, −0.005 (−0.073 to 0.063) | 50, −0.053 (−0.130 to 0.023) | 0.048 (−0.032 to 0.129) | 0.237 | 0.08 |
| VAS | 52, 1.4 (−3.6 to 6.4) | 48, −3.3 (−8.7 to 2.2) | 4.7 (−0.9 to 10.2) | 0.098 | 7 |
Includes all data up to cycle 13 (where both treatment arms had ≥10 patients).
A treatment difference that exceeds the MID indicates a clinically meaningful difference between treatment arms.
Score ranges from 0 to 10, with a higher score indicating greater symptom severity/interference. A positive LS mean change from baseline indicates worsening and a positive LS mean treatment difference indicates greater symptom burden/interference for EPd.
Utility index score ranges from 1 (full health) to 0 (death), with negative scores (to −0.59) reflecting health states valued as worse than death. VAS score ranges from 0 to 100. A positive LS mean change from baseline indicates improvement and a positive LS mean treatment difference indicates better HRQoL for EPd.
CI = confidence interval; EPd = elotuzumab + pomalidomide + dexamethasone; EQ-5D-3L = 3-level EuroQol 5 dimension; HRQoL = health-related quality of life; LS = least squares; MDASI-MM = MD Anderson Symptom Inventory for Multiple Myeloma; MID = minimally important difference; MMRM = mixed models for repeated measures; Pd = pomalidomide + dexamethasone; PRO = patient-reported outcome; VAS = visual analog scale.
TTD analysis
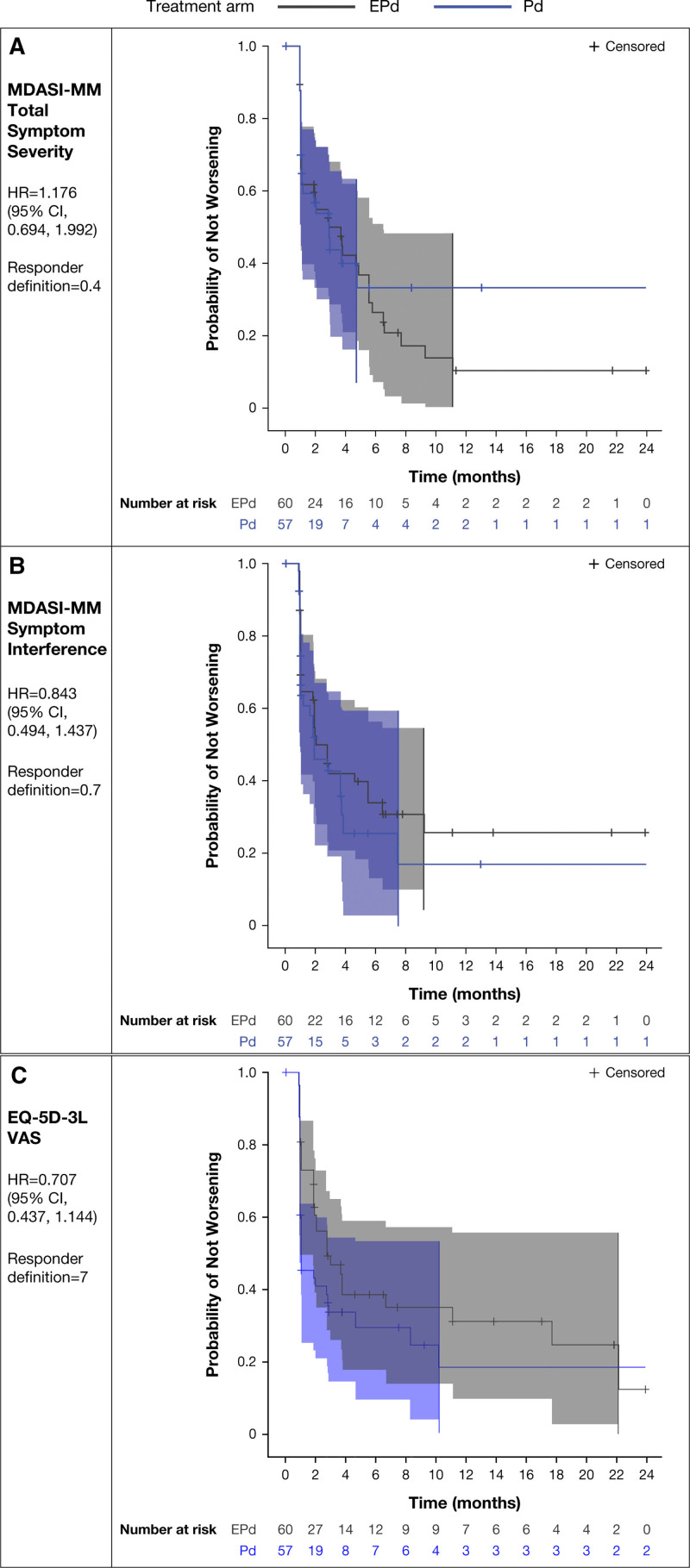
There were no statistically significant differences in TTD in HRQoL between the EPd and Pd arms. Hazard ratios (95% CI) for MDASI-MM total symptom severity score, MDASI-MM symptom interference, and EQ-5D-3L VAS were 1.176 (0.694–1.992), 0.843 (0.494–1.437), and 0.707 (0.437–1.144), respectively. Kaplan–Meier curves, displaying the proportion of patients who have not experienced a deterioration in PRO score over time, showed overlapping confidence bands, indicating no statistically significant differences in TTD between the EPd and Pd arms (Figure 2). The conclusions for the other scales were consistent with those reported here.
Figure 2.
Time to first deterioration Kaplan–Meier plots (95% confidence bands) for (A) MDASI-MM total symptom severity, (B) MDASI-MM symptom interference, and (C) EQ-5D-3L VAS (continued on next page). CI = confidence interval; EPd = elotuzumab + pomalidomide + dexamethasone; EQ-5D-3L = 3-level EuroQol 5 dimension; HR = hazard ratio; MDASI-MM = MD Anderson Symptom Inventory for Multiple Myeloma; Pd = pomalidomide + dexamethasone; VAS = visual analog scale.
DISCUSSION
In patients with RRMM previously treated with lenalidomide and a PI, combining elotuzumab with Pd did not lead to any additional deterioration of HRQoL. The proportion of patients who improved or maintained stable HRQoL until cycle 13 was similar between treatment arms. Across measurements, there were no clinically meaningful differences in LS means between treatment arms, and TTD in disease- or treatment-related symptoms was not significantly different for EPd versus Pd after up to 4 years of therapy. These findings are consistent with HRQoL analyses conducted on the prior database lock.11 For example, the trend for delayed TTD in EQ-5D-3L VAS observed on this updated 4-year database lock (hazard ratio, 0.71 [95% CI, 0.44–1.14]) is very consistent with the hazard ratio based on the prior database lock with a minimum follow-up of 18.3 months (0.70 [0.43–1.14]).11
The preservation of patient-reported HRQoL with the addition of elotuzumab to Pd is consistent with the previously published clinician-reported adverse event data for ELOQUENT-3. The proportion of patients who experienced adverse events that led to treatment discontinuation (EPd: 18.3%, Pd: 23.6%) was relatively similar between the 2 treatment arms.12,13 Instances of bone pain and fatigue-related adverse events were both low, with ≤20% patients in each treatment arm experiencing any grade of each of these adverse events based on clinician reports.12,13
ELOQUENT-3 was an international study for which the MDASI-MM and EQ-5D-3L questionnaires were translated into the local language for each country where available. Since a Polish-language translation was not available specifically for the MDASI-MM, no MDASI-MM data were collected from the 16 patients enrolled at sites in Poland (7 and 9 patients randomized to EPd and Pd, respectively). These patients were not included in the calculation of the expected number of subjects for the MDASI-MM, but resulted in lower available data rates for the MDASI-MM compared with the EQ-5D-3L.
A limitation of the MMRM methodology implemented for this analysis is that it assumes data are missing at random and, as such, the inability of patients to respond to PRO assessments can result in a bias in favor of responders. To evaluate the impact of missing data in ELOQUENT-3, mean scores were plotted by the time of last assessment. The resultant plots (not shown) demonstrated no clear patterns in PRO scores related to drop-out time of assessment, suggesting the reasonableness of the assumption of data missing at random in longitudinal analysis modeling. However, this study was not powered to detect differences in PRO endpoints, and any treatment differences observed should be interpreted with caution, given the small number of patients completing PRO assessments in the Pd treatment arm by cycle 13. The lack of blinding in ELOQUENT-3 is an additional limitation.
It is also important to note that all the analyses presented here are based on PRO data collected on-treatment, during which time the patient completion rate of PROs was high. Therefore, although data availability at survival follow-up visits is limited (completion rates <50%), further exploration of PRO analyses using survival follow-up data may be warranted. Collection of HRQoL data beyond disease progression is challenging, potentially due to reduced patient engagement post-progression. Time-to-event analyses presented here are limited to TTD. Time to confirmed deterioration, defined as a deterioration event where the subsequent assessment after the first deterioration also reaches the predefined deterioration threshold, would additionally be informative to explore.
Finally, although the MID values of 7 and 0.08 are commonly used in the literature to define clinically meaningful changes in EQ-5D-3L VAS and utility index scores, respectively, it is important to note that these thresholds were determined from a study involving patients across 11 different types of cancer, not including MM.19 Therefore, further research is recommended to confirm the appropriateness of those thresholds in an MM population.
In conclusion, HRQoL was not impacted by the addition of elotuzumab to Pd and did not significantly deteriorate in patients with RRMM previously treated with lenalidomide and a PI in ELOQUENT-3 after up to 4 years of EPd therapy. These patient-reported HRQoL findings complement existing follow-up data, which show that patients who were treated with EPd experienced clinically meaningful improvements in survival without impacting HRQoL,10–12 further supporting the clinical value of EPd in adult patients with RRMM who have received ≥2 prior therapies including lenalidomide and a PI.
AUTHOR CONTRIBUTIONS
AP, JSM, KW, MAD, MC, and ME were involved in conceptualization/design and data acquisition. CY, FT, JL-B, JT, and MG did the analysis. AP, CY, FT, JL-B, JSM, JT, KW, MAD, MC, ME, and MG participated in data interpretation.
DISCLOSURES
KW reports consultancy for AbbVie, Adaptive Biotechnologies, and Oncopeptides; consultancy, research funding, and honoraria from Amgen, BMS, Celgene, and Sanofi; consultancy and honoraria from GSK; research funding and honoraria from Janssen; and honoraria from Karyopharm, Novartis, Pfizer, Roche, and Takeda. MAD reports honoraria from advisory boards from Amgen, BMS, Celgene, Janssen, and Takeda. JSM reports consultancy fees and sits on advisory boards for AbbVie, Amgen, BMS, Celgene, GSK, Janssen, Karyopharm, MSD, Novartis, Regeneron, Roche, Sanofi, SecuraBio, and Takeda. AP reports consultancy for Amgen, Celgene/BMS, GSK, Karyopharm, and Oncopeptides. ME reports consultancy fees and honoraria from Amgen, BMS, GSK, and Sanofi; research funding and honoraria from Janssen; and honoraria from Takeda. FT, CY, and MG report employment with Adelphi Values, a consultancy firm that received payment from BMS for the execution of this study. JL-B reports equity and employment with BMS. JT reports consultancy for BMS. MC reports honoraria from AbbVie, Adaptive Biotechnologies, Amgen, GSK, and Takeda; and consultancy and honoraria from Celgene/BMS, Janssen, and Sanofi.
SOURCES OF FUNDING
The study was supported by Bristol Myers Squibb and AbbVie Biotherapeutics. David Yao (employed by BMS at the time of this study) supported data interpretation of these analyses. The authors received editorial support in the preparation of this manuscript from Jared Saunders, PhD, of Excerpta Medica, funded by Bristol Myers Squibb.
Supplementary Material
Footnotes
Data sharing statement: BMS policy on data sharing may be found at https://www.bms.com/researchers-and-partners/independent-research/data-sharing-request-process.html.
Supplemental digital content is available for this article.
REFERENCES
- 1.Padala SA, Barsouk A, Barsouk A, et al. Epidemiology, staging, and management of multiple myeloma. Med Sci (Basel). 2021;9:3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.American Cancer Society. Key statistics about multiple myeloma. 2022. Available at: https://www.cancer.org/cancer/multiple-myeloma/about/key-statistics.html. Accessed April 4, 2002.
- 3.International Agency for Research on Cancer, World Health Organization. Cancer Today: Estimated number of new cases in 2020, worldwide, both sexes, all ages (excl. NMSC). 2020. Available at: https://gco.iarc.fr/today/online-analysis-table?v=2020&mode=cancer&mode_population=continents&population=900&populations=900&key=asr&sex=0&cancer=39&type=0&statistic=5&prevalence=0&population_group=0&ages_group%5B%5D=0&ages_group%5B%5D=17&group_cancer=1&include_nmsc=0&include_nmsc_other=1. Accessed May 9, 2022.
- 4.Jordan K, Proskorovsky I, Lewis P, et al. Effect of general symptom level, specific adverse events, treatment patterns, and patient characteristics on health-related quality of life in patients with multiple myeloma: results of a European, multicenter cohort study. Support Care Cancer. 2014;22:417–426. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Engelhardt M, Ihorst G, Singh M, et al. Real-world evaluation of health-related quality of life in patients with multiple myeloma from Germany. Clin Lymphoma Myeloma Leuk. 2021;21:e160–e175. [DOI] [PubMed] [Google Scholar]
- 6.Kyle RA, Gertz MA, Witzig TE, et al. Review of 1027 patients with newly diagnosed multiple myeloma. Mayo Clin Proc. 2003;78:21–33. [DOI] [PubMed] [Google Scholar]
- 7.Rajkumar SV, Kumar S. Multiple myeloma: diagnosis and treatment. Mayo Clin Proc. 2016;91:101–119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Nijhof IS, van de Donk N, Zweegman S, et al. Current and new therapeutic strategies for relapsed and refractory multiple myeloma: an update. Drugs. 2018;78:19–37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Chakraborty R, Majhail NS. Treatment and disease-related complications in multiple myeloma: implications for survivorship. Am J Hematol. 2020;95:672–690. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Dimopoulos MA, Dytfeld D, Grosicki S, et al. Elotuzumab plus pomalidomide and dexamethasone for multiple myeloma. N Engl J Med. 2018;379:1811–1822. [DOI] [PubMed] [Google Scholar]
- 11.Weisel K, Paner A, Engelhardt M, et al. Impact of elotuzumab plus pomalidomide and dexamethasone on health-related quality of life in patients with relapsed/refractory multiple myeloma enrolled in the ELOQUENT-3 study. Blood. 2019;134(Suppl 1):3480. [Google Scholar]
- 12.Dimopoulos MA, Dytfeld D, Grosicki S, et al. P-193: Elotuzumab plus pomalidomide/dexamethasone for relapsed/refractory multiple myeloma: final overall survival from the phase 2 ELOQUENT-3 trial. Clin Lymphoma Myeloma Leuk. 2021;21(Suppl 2):S143–S144. [Google Scholar]
- 13.Dimopoulos MA, Dytfeld D, Grosicki S, et al. Elotuzumab plus pomalidomide and dexamethasone for relapsed/refractory multiple myeloma: final overall survival analysis from the randomized phase II ELOQUENT-3 trial. J Clin Oncol. 2023;41:568–578. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Weisel K, Dimopoulos MA, San-Miguel J, et al. Impact of elotuzumab plus pomalidomide/dexamethasone on health-related quality of life for patients with relapsed/refractory multiple myeloma (RRMM): final data from the phase 2 ELOQUENT-3 trial. Blood. 2021;138(Suppl 1):1662. [Google Scholar]
- 15.Jones D, Vichaya EG, Wang XS, et al. Validation of the M. D. Anderson Symptom Inventory multiple myeloma module. J Hematol Oncol. 2013;6:13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Dolan P. Modeling valuations for EuroQol health states. Med Care. 1997;35:1095–1108. [DOI] [PubMed] [Google Scholar]
- 17.Wyrwich KW, Tierney WM, Wolinsky FD. Further evidence supporting an SEM-based criterion for identifying meaningful intra-individual changes in health-related quality of life. J Clin Epidemiol. 1999;52:861–873. [DOI] [PubMed] [Google Scholar]
- 18.Norman GR, Sloan JA, Wyrwich KW. Interpretation of changes in health-related quality of life: the remarkable universality of half a standard deviation. Med Care. 2003;41:582–592. [DOI] [PubMed] [Google Scholar]
- 19.Pickard AS, Neary MP, Cella D. Estimation of minimally important differences in EQ-5D utility and VAS scores in cancer. Health Qual Life Outcomes. 2007;5:70. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.