PURPOSE:
New therapies including oral anticancer agents (OAAs) have improved outcomes for patients with metastatic renal cell carcinoma (mRCC). However, little is known about the quality of end-of-life (EOL) care and systemic therapy use at EOL in patients receiving OAAs or with mRCC.
METHODS:
We retrospectively analyzed EOL care for decedents with mRCC in two parallel cohorts: (1) patients (RCC diagnosed 2004-2015) from the University of North Carolina's Cancer Information and Population Health Resource (CIPHR) and (2) patients (diagnosed 2007-2015) from SEER-Medicare. We assessed hospice use in the last 30 days of life and existing measures of poor-quality EOL care: systemic therapy, hospital admission, intensive care unit admission, and > 1 ED visit in the last 30 days of life; hospice initiation in the last 3 days of life; and in-hospital death. Associations between OAA use, patient and provider characteristics, and EOL care were examined using multivariable logistic regression.
RESULTS:
We identified 410 decedents in the CIPHR cohort (53.4% received OAA) and 1,508 in SEER-Medicare (43.5% received OAA). Prior OAA use was associated with increased systemic therapy in the last 30 days of life in both cohorts (CIPHR: 26.5% v 11.0%; P < .001; SEER-Medicare: 23.4% v 11.7%; P < .001), increased in-hospital death in CIPHR, and increased hospice in the last 30 days in SEER-Medicare. Older patients were less likely to receive systemic therapy or be admitted in the last 30 days or die in hospital.
CONCLUSION:
Patients with mRCC who received OAAs and younger patients experienced more aggressive EOL care, suggesting opportunities to optimize high-quality EOL care in these groups.
INTRODUCTION
Kidney cancer, of which approximately 85% of cases are renal cell carcinoma (RCC), will result in an estimated 13,920 deaths in 2022 in the United Sates.1 With the introduction of new therapies, including antiangiogenic and targeted oral anticancer agents (OAAs), immune checkpoint inhibitors (ICIs), and combinations of these agents, overall survival for metastatic RCC (mRCC) has gradually increased2; however, most patients ultimately die from the disease, and thus, end-of-life (EOL) care is a critical component of quality oncologic care for patients with mRCC, particularly in the era of increasing OAA use.
Quality indicators of EOL care in general populations of patients with cancer have historically focused on omission of hospice and aggressive interventions near EOL.3,4 Commonly accepted measures of low-quality EOL care in patients with cancer include the following: late or no hospice; receipt of chemotherapy at EOL; death in an acute care setting; and > 1 emergency department (ED) visit, hospitalization, or intensive care unit (ICU) admission in the last 30 days of life.3-6
Studies of EOL care in oncology have historically included patients with multiple types of cancers receiving traditional intravenous cytotoxic chemotherapy and have not included patients receiving OAAs. Beginning with the approval of sorafenib in 2005, widespread incorporation of targeted OAAs into RCC treatment transformed how systemic therapy for advanced disease was delivered; thus, a population of patients with mRCC represents a unique opportunity to evaluate how OAAs, now commonly used in the treatment of multiple cancer types, may influence EOL care and may resolve potentially modifiable targets for improving quality of cancer care at EOL. In addition, EOL care may be influenced by other demographic characteristics, which vary between registries. Therefore, using two distinct cohorts of patients with mRCC—a national population-based registry-linked Medicare claims data set and a unique, cancer registry-linked multipayer claims data set from North Carolina—we sought to identify prevalence of and factors associated with poor-quality EOL care with the above measures in the mRCC population.
METHODS
Study Populations
We conducted a retrospective study of decedent patients with mRCC in two separate cohorts that we analyzed in parallel: a cohort of patients age 18 years and older drawn from the University of North Carolina Cancer Information Population Health Resource (CIPHR) and a cohort of patients age 66 years and older sourced from the SEER-Medicare database. CIPHR links the North Carolina Central Cancer Registry data to administrative claims data from private health insurance, Medicare, and Medicaid plans,7 whereas SEER-Medicare links data from the SEER registries covering approximately 30% of the US population to administrative claims data from fee-for-service Medicare only.8 Appendix Figure A1 (online only) illustrates how patients were selected. Eligible patients included those identified in cancer registry data from each cohort diagnosed with stage I-IV RCC (January 1, 2004, to December 31, 2015, for CIPHR and January 1, 2007, to December 31, 2015, for SEER-Medicare; Appendix Table A1, online only). Index date was defined as the RCC diagnosis date from the registry for patients with stage IV disease or the date of the first of two metastatic diagnosis claims for patients diagnosed as stage I-III. Patients were included if they survived ≥ 90 days after the index date and died within the follow-up period (through December 31, 2016). Patients were required to have continuous insurance and prescription medication coverage from their index date until death.
EOL Care Quality Outcomes
Using information drawn from the linked insurance claims, we assessed established measures of poor-quality EOL care: receipt of systemic therapy, hospital admission, ICU admission, and > 1 ED visit within the last 30 days of life; in-hospital death; and hospice initiation within the last 3 days of life.6 Receipt of systemic therapy included both intravenous systemic anticancer therapies and OAAs. Codes used to assess receipt of intravenous and oral chemotherapies are given in Appendix Table A2 (online only). As a high-quality EOL care indicator, we examined hospice use in the last 30 days of life.
Prior Use of an OAA
OAA use during the period from the patient's metastatic diagnosis until 30 days before death was identified using prescription drug files and pharmacy claims by reviewing generic and brand names and national drug codes for sorafenib (approved 2005), sunitinib (2006), pazopanib (2009), everolimus (2009), and axitinib (2012).
Patient- and Provider-Level Variables
We examined other potential factors expected to influence EOL care, including age at mRCC diagnosis, race/ethnicity (non-Hispanic White v Others), sex, residence in a rural or urban area,9 insurance status (CIPHR: private v Medicare only v any Medicaid; SEER-Medicare: Medicare only v dual enrollment in Medicare and Medicaid), number of comorbid conditions in the 12 months pre-mRCC diagnosis (Appendix Table A3, online only), Faurot Frailty score,10 stage at diagnosis (stage IV v < IV), percentage of Medicare beneficiaries in the hospital referral region who died in-hospital the year of metastatic diagnosis,11 and US geographic region (for SEER-Medicare only).12 Sex and race were categorized on the basis of abstracted data from medical records included in the cancer registries.13 Because of small cell sizes, race and ethnicity were grouped into two categories—non-Hispanic White and Others, which included Asian/Pacific Islander, Hispanic Black, Hispanic Others, Hispanic White, non‐Hispanic American Indian, non-Hispanic Black, non‐Hispanic Others, unknown ethnicity Black, and unknown ethnicity White.13 Patients were assigned a modal provider, the physician identified most frequently on claims with a diagnosis code of RCC or metastatic cancer between 2 months before and 3 months after the index date. The rural practice location for the modal provider was assessed using Rural-Urban Commuting Area codes for the provider's zip code.9
Statistical Analysis
Descriptive statistics for each cohort were calculated using means and medians for continuous variables and frequencies and percentages for binary and categorical variables. The distributions of EOL care outcomes were stratified by OAA use; unadjusted group differences were tested using the chi-square test. We estimated odds ratios (ORs) and corresponding 95% confidence limits (CL) using multivariable logistic regression models for the associations between OAA use, patient characteristics, and the EOL quality outcomes. Multicollinearity of covariates was assessed using a variance inflation factor threshold of 5, where no covariates exceeded this threshold.
RESULTS
Study Population
The study samples comprised 410 and 1,508 patients with the median age of 69 and 75 years for the CIPHR and SEER-Medicare populations, respectively (Table 1). Compared with the SEER-Medicare cohort, patients in CIPHR were less often female (34% in CIPHR; 43% in SEER-Medicare) and less frequently lived in an urban area (62%; 80%). Patients in CIPHR were less frequently diagnosed with de novo metastatic disease and had fewer comorbid conditions. In addition, 53% and 44% of decedents received an OAA in CIPHR and SEER-Medicare cohorts, respectively. Among patients who did not receive an OAA, 70% and 67% did not receive any systemic therapy in CIPHR and SEER-Medicare, respectively. Patients who did not receive OAAs but received another systemic therapy received targeted therapy/immunotherapy (49% SEER-Medicare; 47% CIPHR), traditional cytotoxic chemotherapy (44% SEER-Medicare; 35% CIPHR), and cytokine therapy (< 5% in SEER-Medicare).
TABLE 1.
Descriptive Characteristics of the CIPHR and SEER-Medicare Cohorts
Relationship Between Prior OAA Use and EOL Quality Indicators
Overall, 19.3% of decedents in CIPHR and 16.8% in SEER-Medicare received systemic therapy in the 30 days before death (Table 2). Patients who previously received OAAs more frequently received any systemic therapy in the 30 days before death compared with patients who had not previously received OAAs in both CIPHR (26.5% v 11.0%; P < .001) and SEER-Medicare (23.4% v 11.7%; P < .001). Among patients who received OAAs previously, 16% and 14% received an OAA in the 30 days before death in CIPHR and SEER-Medicare, respectively. The overall proportion of decedents with any hospice use in the last 30 days of life was 48.5% in CIPHR and 42.2% in SEER-Medicare. Prior OAA use was associated with any hospice in the 30 days before death in SEER-Medicare (47.5% v 40.6%; P = .007). In SEER-Medicare, hospice initiation in the 3 days before death was higher among prior OAA users compared with nonusers (13.7% v 10.3%; P = .042). In CIPHR, prior OAA use was associated with ICU admission in the 30 days before death (19.6% v 12.0%; P = .037) and with > 1 ED visit in the 30 days before death (16.9% v 6.8%; P = .002). The proportion of patients who died in hospital was similar in both cohorts (21%); however, the difference between prior OAA use and nonuse was only statistically significant in the CIPHR cohort (24.7% v 15.7%; P = .025).
TABLE 2.
End-of-Life Quality Indicators in CIPHR and SEER-Medicare Decedent Cohorts, overall and Stratified by OAA Usea
Inpatient Admission Within the 30 Days Before Death
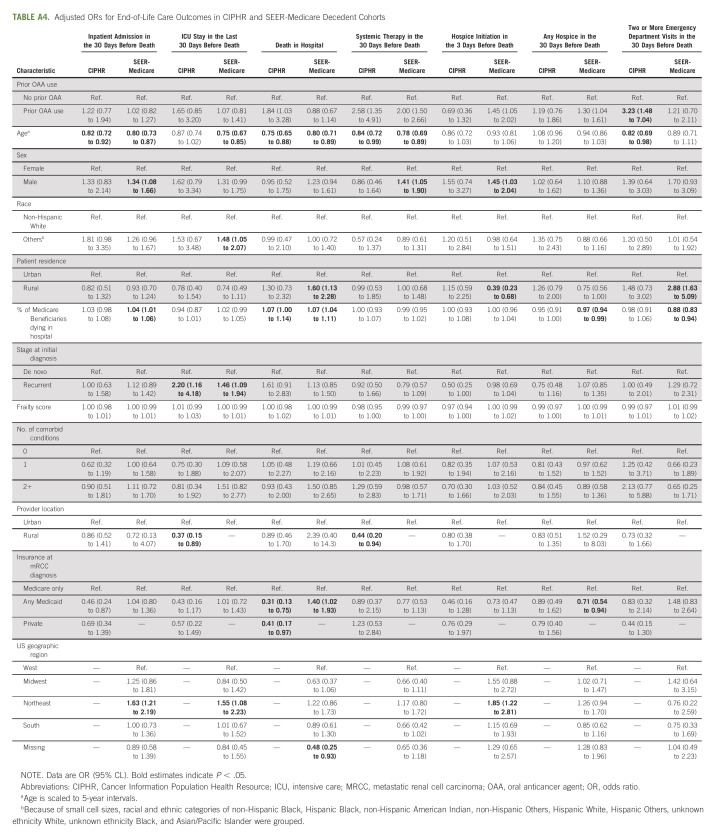
Prior OAA use (v no prior OAA use) was not associated with inpatient admission in the 30 days before death in both cohorts. Older patients were less likely to be admitted in the 30 days before death in both cohorts (CIPHR: OR = 0.82 [95% CL, 0.72 to 0.92]; SEER-Medicare: OR = 0.80 [95% CL: 0.73 to 0.87]; Fig 1). In SEER-Medicare, patients were more likely to be admitted in the 30 days before death if they were male (v female; OR, 1.34; 95% CL, 1.08 to 1.66) or living in the Northeast United States (v west; OR, 1.63; 95% CL, 1.21 to 2.19; Fig 1 and Appendix Table A4, online only).
FIG 1.
Adjusted ORs for end-of-life care outcomes in (A) CIPHR and (B) SEER-Medicare decedent cohorts. Results are shown for prior OAA use (v no prior OAA use), agea, sex (male v female), raceb (Others v non-Hispanic White), patient residence (rural v urban), and insurance at mRCC diagnosis (any Medicaid v Medicare only). Full results are presented in Appendix Table A4. aAge is scaled to 5-year intervals. bBecause of small cell sizes, racial and ethnic categories of non-Hispanic Black, Hispanic Black, non‐Hispanic American Indian, non‐Hispanic Others, Hispanic White, Hispanic Others, unknown ethnicity White, Unknown ethnicity Black, and Asian/Pacific Islander were grouped. CIPHR, Cancer Information Population Health Resource; ICU, intensive care; mRCC, metastatic renal cell carcinoma; OAA, oral anticancer agent; OR, odds ratio; RCC, renal cell carcinoma.
ICU Admission Within the 30 Days Before Death
Prior OAA (v no prior OAA use) use was not associated with ICU admission in the 30 days before death in both cohorts. Patients were more likely to be admitted to an ICU in the 30 days before death if they were diagnosed with recurrent mRCC (v de novo mRCC) in both cohorts (CIPHR: OR = 2.20 [95% CL, 1.16 to 4.18]; SEER-Medicare: OR = 1.46 [95% CL, 1.09 to 1.94]). In SEER-Medicare, patients were less likely to be admitted to an ICU in the 30 days before death if they were older (OR, 0.75; 95% CL, 0.67 to 0.85) but more likely to be admitted to an ICU in the 30 days before death if they were not non-Hispanic White (v non-Hispanic White; OR, 1.48; 95% CL, 1.05 to 2.07) or lived in the Northeast United States (v west; OR, 1.55; 95% CL, 1.08 to 2.23).
Death in Hospital
Patients in CIPHR were more likely to die in hospital if they previously received an OAA (v no prior OAA; OR, 1.84; 95% CL, 1.03 to 3.28; Fig 1). In both cohorts, older patients were less likely to die in hospital (CIPHR: OR = 0.75 [95% CL, 0.65 to 0.88]; SEER-Medicare: OR = 0.80 [95% CL, 0.71 to 0.89]). In SEER-Medicare, patients with dual Medicaid insurance (compared with Medicare only) were more likely to die in hospital (OR, 1.40; 95% CL, 1.02 to 1.93) as were patients living in a rural location (v urban; OR, 1.60; 95% CL, 1.13 to 2.28).
Systemic Therapy Within the 30 Days Before Death
Compared with patients who had not previously received an OAA, patients who previously received an OAA were more likely to receive systemic therapy in the last 30 days of life in both cohorts (CIPHR: OR = 2.58 [95% CL, 1.35 to 4.91]; SEER-Medicare: OR = 2.00 [95% CL, 1.50 to 2.66]; Fig 1). Older patients less often received systemic therapy in the 30 days before death in both cohorts (CIPHR: OR = 0.84 [95% CL, 0.72 to 0.99]; SEER-Medicare: OR = 0.78 [95% CL, 0.69 to 0.89]).
Hospice Initiation Within the 3 Days Before Death
In SEER-Medicare, patients were more likely to initiate hospice in the last 3 days of life if they had previously received an OAA (OR, 1.45; 95% CL, 1.05 to 2.02), were male (v female; OR, 1.45; 95% CL, 1.03 to 2.04), or lived in the northeast (v west; OR, 1.85; 95% CL, 1.22 to 2.81). In SEER-Medicare, patients were less likely to initiate hospice in the last 3 days of life if they lived in a rural location (v urban; OR, 0.39; 95% CL, 0.23 to 0.68).
More Than One ED Visit Within the 30 Days Before Death
In CIPHR, patients were more likely to have > 1 ED visit in the 30 days before death if they had previously received an OAA (OR, 3.23; 95% CL, 1.48 to 7.04) and less likely if they were older (OR, 0.82; 95% CL, 0.69 to 0.98). In SEER-Medicare, patients were more likely to have > 1 ED visit in the 30 days before death if they lived in a rural location (OR, 2.88; 95% CL, 1.63 to 5.09).
Any Hospice Within the 30 Days Before Death
To identify variables associated with hospice use at EOL, a measure of high-quality care, we examined any hospice use in the 30 days before death (Fig 1). In SEER-Medicare, prior OAA use was associated with increased hospice use in the 30 days before death (OR, 1.30; 95% CL, 1.04 to 1.61). Compared with patients with Medicare alone, patients with dual Medicaid and Medicare had lower odds of hospice use in the 30 days before death (OR, 0.71; 95% CL, 0.54 to 0.94) in SEER-Medicare.
DISCUSSION
We report the first description, to our knowledge, of EOL care in patients with mRCC and in patients receiving OAAs as part of routine oncologic care. Our data, drawn from two complementary cohorts, illuminate real-world patterns of EOL care and factors associated with these care components during the era of OAAs. Prior OAA use was associated with increased likelihood of receiving systemic therapy in the last 30 days of life in both cohorts. Prior OAA use was also associated with increased likelihood of hospice use in the last 30 days of life. Younger patients experienced more aggressive EOL care with higher likelihood of inpatient death, inpatient admission, and systemic therapy use in the last 30 days of life in both cohorts. In SEER-Medicare, patients who were dual-enrolled in Medicare and Medicaid, a traditionally vulnerable population, had more aggressive EOL care including higher likelihood of in-hospital death and lower likelihood of any hospice use in the last 30 days of life. Other factors associated with low-quality EOL care included non-White race, male sex, rural location, northeast region, and recurrent metastatic disease.
In the time between metastatic diagnosis and 30 days before death, 53% of patients in CIPHR and 43% of patients in SEER-Medicare received an OAA. In the last 30 days of life, 27% and 23% of these patients in CIPHR and SEER-Medicare, respectively, continued to receive systemic therapy, some of whom continued OAA use. The use of systemic therapy near EOL has been shown not to improve quality of life, while subjecting patients to unnecessary toxicities.14 Studies characterizing the negative impact of systemic therapy near EOL have largely included patients receiving traditional cytotoxic chemotherapy, before widespread use of newer targeted OAAs and ICIs. In one study using 2012-2013 Massachusetts private insurance claims, OAA use sharply declined during the last 30 days of life and even more so at 14 days before EOL in multiple cancer types, but they did not examine how OAA use may affect other aspects of EOL care.15
One potential explanation for the increased likelihood of hospice enrollment for patients on OAAs is that these patients may be more likely to see a medical oncologist,16,17 and in previous studies, patients with advanced cancer cared for by medical oncologists were more likely to enroll in hospice.18 Alternatively, concurrent increases in OAA use and national increases in hospice enrollment over the past two decades may account for the increased likelihood of hospice enrollment for patients on OAAs.19 However, rates of OAA use for patients with mRCC increased significantly from 2007 to 2015 in SEER-Medicare, but in CIPHR, OAA use rates varied with no consistent trend over this time period.20,21
Previous studies of patients with multiple cancer types have demonstrated similar trends to our study with younger patients and dual-eligible beneficiaries receiving more aggressive EOL care.22-24 We did not see differences in EOL care metrics among racial or ethnic groups except for increased risk of ICU admission in the last 30 days of life for non-White patients in SEER-Medicare. Although it is encouraging not to see differences in EOL care quality by race or ethnicity, we exercise caution when interpreting these findings, given the small number of non-White patients and conflicting findings in other treatment settings and cancer types.22,25-27
This study provides a valuable view of real-world patterns in EOL care for patients with mRCC, many receiving OAAs as part of routine treatment, but is subject to the limitations that are part of retrospective analyses of registry and claims databases. CIPHR represents a novel cohort of patients with mRCC from multiple payers. However, the data are limited to patients who were diagnosed and received care in North Carolina and may not reflect patterns of care in other regions. SEER-Medicare, while encompassing patients across the United States, is limited to older patients and may not be generalizable to patients under age 66 years. In addition, there are limitations to the use of SEER-Medicare data to identify patients with localized RCC with later progression to mRCC, so it is possible that some of these patients were excluded from our study cohort.28 Our study included patients treated between 2004 and 2016, which encompassed the approval times of most OAAs used in RCC; however, frontline management of mRCC has subsequently changed to include ICIs alone and in combination with OAAs, which might have influenced more recent EOL patterns of care. Because ICIs are commonly combined with OAAs, we believe that the implications of this study will remain largely unchanged by these developments but still warrant future studies. We defined OAA use at EOL as having a prescription drug claim within 30 days of death, as has been performed in previous studies evaluating OAA use near EOL.15,27 This methodology does not capture whether patients took the prescribed OAA and may incorrectly identify patients who stop taking an OAA despite having filled the prescription. Finally, decisions around EOL care are highly personal and complicated, incorporating unique patient preferences and physician guidance, which are not captured in these data. Although higher-quality EOL care is generally characterized as less aggressive care, specifics of EOL care are personal decisions and not captured in a retrospective study.
Despite these limitations, to our knowledge, our study is the first to describe patterns of EOL care for patients with mRCC in the OAA era. Patients receiving OAAs, common in contemporary oncology treatment, remained on therapy near the EOL more frequently; however, they were also more likely to enroll in hospice care. For clinicians, these data suggest that OAA receipt in clinically declining patients may be a marker of aggressive EOL care, presenting an opportunity to optimize EOL care through interventions such as early palliative care engagement. Better understanding of the unique factors influencing physician and patient decision making around EOL care, particularly for patients receiving OAAs, warrants continuous evaluation to optimize EOL care.
ACKNOWLEDGMENT
The authors acknowledge the efforts of the National Cancer Institute; the Office of Research, Development, and Information, CMS; Information Management Services (IMS), Inc; and the Surveillance, Epidemiology, and End Results (SEER) Program tumor registries in the creation of the SEER-Medicare database.
APPENDIX
FIG A1.
Cohort selection diagrams for (A) CIPHR and (B) SEER-Medicare. aRequired continuous enrollment in parts A and B for Medicare patients. bMetastatic index date was the first metastatic claim date for both stages I-III and earliest metastatic claim date for stage IV. cRequired continuous enrollment in parts A, B, and D for Medicare patients. CIPHR, Cancer Information and Population Health Resource.
TABLE A1.
Codes to Identify Renal Cell Carcinoma and Metastatic Cancer

TABLE A2.
Codes and Generic Drug Names Used to Identify the Receipt of Intravenous and Oral Chemotherapies

TABLE A3.
Coding Definitions for Patient Comorbid Conditions

TABLE A4.
Adjusted ORs for End-of-Life Care Outcomes in CIPHR and SEER-Medicare Decedent Cohorts
Hannah E. Dzimitrowicz
Research Funding: Pfizer (Inst)
Lauren E. Wilson
Research Funding: AstraZeneca (Inst)
Lisa P. Spees
Research Funding: AstraZeneca/Merck
Christopher D. Baggett
Employment: Rho
Melissa A. Greiner
Research Funding: Novartis (Inst), Philips Healthcare (Inst), iRhythm Technologies (Inst)
Deborah R. Kaye
Consulting or Advisory Role: Janssen
Research Funding: Blue Cross Blue Shield of Michigan
Tian Zhang
Leadership: Capio Biosciences, Archimmune Therapeutics
Stock and Other Ownership Interests: Capio Biosciences, Archimmune Therapeutics, Nanorobotics
Honoraria: MJH Life Sciences, Pacific Genuity, Aptitude Health, Curio Science, PeerView, Clinical Care Options
Consulting or Advisory Role: Janssen, Exelixis, Pfizer, Bristol Myers Squibb, Seattle Genetics, Dendreon, Calithera Biosciences, QED Therapeutics, Eisai, Aravive, Bayer, Lilly, AVEO, Merck, Sanofi/Aventis
Speakers' Bureau: Genomic Health, Sanofi/Aventis
Research Funding: Janssen (Inst), Pfizer (Inst), Merrimack (Inst), Novartis (Inst), OmniSeq (Inst), Regeneron (Inst), Merck (Inst), Mirati Therapeutics (Inst), Astellas Pharma, Loxo/Lilly (Inst)
Patents, Royalties, Other Intellectual Property: Circulating tumor cell novel capture by c-MET technology (Inst), Prochelators as Targeted Prodrugs for Prostate Cancer (Inst)
Travel, Accommodations, Expenses: Janssen
Daniel George
Leadership: Capio Biosciences
Honoraria: Sanofi, Bayer, Exelixis, EMD Serono, OncLive, Pfizer, UroToday, Acceleron Pharma, American Association for Cancer Research, Axess Oncology, Janssen Oncology, Millennium Medical Publishing
Consulting or Advisory Role: Bayer, Exelixis, Pfizer, Sanofi, Astellas Pharma, Innocrin Pharma, Bristol Myers Squibb, Genentech, Janssen, Merck Sharp & Dohme, Myovant Sciences, AstraZeneca, Michael J. Hennessy Associates, Constellation Pharmaceuticals, Physicians' Education Resource, Propella Therapeutics, RevHealth, xCures
Speakers' Bureau: Sanofi, Bayer, Exelixis
Research Funding: Exelixis (Inst), Janssen Oncology (Inst), Novartis (Inst), Pfizer (Inst), Astellas Pharma (Inst), Bristol Myers Squibb (Inst), Acerta Pharma (Inst), Bayer (Inst), Dendreon (Inst), Innocrin Pharma (Inst), Calithera Biosciences (Inst), Sanofi/Aventis (Inst)
Travel, Accommodations, Expenses: Bayer, Exelixis, Merck, Pfizer, Sanofi, Janssen Oncology, UroToday
Charles D. Scales Jr
Research Funding: Pfizer (Inst), Exelixis (Inst), Merck (Inst), Bristol Myers Squibb-Ono Pharmaceutical (Inst), Astellas Pharma (Inst)
Jessica E. Pritchard
Research Funding: St Jude Medical (Inst), Pfizer (Inst), Boston Scientific (Inst)
Cary P. Gross
Research Funding: Johnson & Johnson (Inst), AstraZeneca (Inst), Genentech
Michaela A. Dinan
Research Funding: AstraZeneca (Inst), Janssen
Stephanie B. Wheeler
Research Funding: Pfizer (Inst)
Travel, Accommodations, Expenses: Pfizer
No other potential conflicts of interest were reported.
DISCLAIMER
The ideas and opinions expressed herein are those of the author(s), and endorsement by the State of California Department of Public Health, the National Cancer Institute, and the Centers for Disease Control and Prevention or their Contractors and Subcontractors is not intended nor should be inferred. The content is solely the responsibility of the authors and does not necessarily represent the official views of the NIH.
PRIOR PRESENTATION
Previously presented at ASCO Genitourinary Cancers Symposium 2022, February 17-19, 2022 in San Francisco, CA.
SUPPORT
Supported by the National Cancer Institute (NCI R01-CA226842-02; PI: M.A.D.). Federal money is financing 100% of the cost. The collection of cancer incidence data used in this study was supported by the California Department of Public Health as part of the statewide cancer reporting program mandated by California Health and Safety Code Section 103885; the National Cancer Institute's Surveillance, Epidemiology and End Results Program under contract No. HHSN261201000140C awarded to the Cancer Prevention Institute of California, contract No. HHSN261201000035C awarded to the University of Southern California, and contract No. HHSN261201000034C awarded to the Public Health Institute; and the Centers for Disease Control and Prevention's National Program of Cancer Registries, under agreement No. U58DP003862-01 awarded to the California Department of Public Health. Database infrastructure was supported through the UNC Clinical and Translational Science Award (UL1TR001111) and the UNC LCCC, University Cancer Research Fund via the State of North Carolina.
AUTHOR CONTRIBUTIONS
Conception and design: Hannah E. Dzimitrowicz, Lauren E. Wilson, Melissa A. Greiner, Daniel George, Charles D. Scales, Jessica E. Pritchard, Cary P. Gross, Michaela A. Dinan, Stephanie B. Wheeler
Financial support: Michaela A. Dinan
Administrative support: Jessica E. Pritchard, Stephanie B. Wheeler
Provision of study materials or patients: Stephanie B. Wheeler
Collection and assembly of data: Hannah E. Dzimitrowicz, Bradford E. Jackson, Melissa A. Greiner, Daniel George, Stephanie B. Wheeler
Data analysis and interpretation: Hannah E. Dzimitrowicz, Lauren E. Wilson, Bradford E. Jackson, Lisa P. Spees, Christopher D. Baggett, Melissa A. Greiner, Deborah R. Kaye, Tian Zhang, Daniel George, Charles D. Scales, Jessica E. Pritchard, Michael S. Leapman, Stephanie B. Wheeler
Manuscript writing: All authors
Final approval of manuscript: All authors
Accountable for all aspects of the work: All authors
AUTHORS' DISCLOSURES OF POTENTIAL CONFLICTS OF INTEREST
End-of-Life Care for Patients with Metastatic Renal Cell Carcinoma in the Era of Oral Anticancer Therapy
The following represents disclosure information provided by authors of this manuscript. All relationships are considered compensated unless otherwise noted. Relationships are self-held unless noted. I = Immediate Family Member, Inst = My Institution. Relationships may not relate to the subject matter of this manuscript. For more information about ASCO's conflict of interest policy, please refer to www.asco.org/rwc or ascopubs.org/op/authors/author-center.
Open Payments is a public database containing information reported by companies about payments made to US-licensed physicians (Open Payments).
Hannah E. Dzimitrowicz
Research Funding: Pfizer (Inst)
Lauren E. Wilson
Research Funding: AstraZeneca (Inst)
Lisa P. Spees
Research Funding: AstraZeneca/Merck
Christopher D. Baggett
Employment: Rho
Melissa A. Greiner
Research Funding: Novartis (Inst), Philips Healthcare (Inst), iRhythm Technologies (Inst)
Deborah R. Kaye
Consulting or Advisory Role: Janssen
Research Funding: Blue Cross Blue Shield of Michigan
Tian Zhang
Leadership: Capio Biosciences, Archimmune Therapeutics
Stock and Other Ownership Interests: Capio Biosciences, Archimmune Therapeutics, Nanorobotics
Honoraria: MJH Life Sciences, Pacific Genuity, Aptitude Health, Curio Science, PeerView, Clinical Care Options
Consulting or Advisory Role: Janssen, Exelixis, Pfizer, Bristol Myers Squibb, Seattle Genetics, Dendreon, Calithera Biosciences, QED Therapeutics, Eisai, Aravive, Bayer, Lilly, AVEO, Merck, Sanofi/Aventis
Speakers' Bureau: Genomic Health, Sanofi/Aventis
Research Funding: Janssen (Inst), Pfizer (Inst), Merrimack (Inst), Novartis (Inst), OmniSeq (Inst), Regeneron (Inst), Merck (Inst), Mirati Therapeutics (Inst), Astellas Pharma, Loxo/Lilly (Inst)
Patents, Royalties, Other Intellectual Property: Circulating tumor cell novel capture by c-MET technology (Inst), Prochelators as Targeted Prodrugs for Prostate Cancer (Inst)
Travel, Accommodations, Expenses: Janssen
Daniel George
Leadership: Capio Biosciences
Honoraria: Sanofi, Bayer, Exelixis, EMD Serono, OncLive, Pfizer, UroToday, Acceleron Pharma, American Association for Cancer Research, Axess Oncology, Janssen Oncology, Millennium Medical Publishing
Consulting or Advisory Role: Bayer, Exelixis, Pfizer, Sanofi, Astellas Pharma, Innocrin Pharma, Bristol Myers Squibb, Genentech, Janssen, Merck Sharp & Dohme, Myovant Sciences, AstraZeneca, Michael J. Hennessy Associates, Constellation Pharmaceuticals, Physicians' Education Resource, Propella Therapeutics, RevHealth, xCures
Speakers' Bureau: Sanofi, Bayer, Exelixis
Research Funding: Exelixis (Inst), Janssen Oncology (Inst), Novartis (Inst), Pfizer (Inst), Astellas Pharma (Inst), Bristol Myers Squibb (Inst), Acerta Pharma (Inst), Bayer (Inst), Dendreon (Inst), Innocrin Pharma (Inst), Calithera Biosciences (Inst), Sanofi/Aventis (Inst)
Travel, Accommodations, Expenses: Bayer, Exelixis, Merck, Pfizer, Sanofi, Janssen Oncology, UroToday
Charles D. Scales Jr
Research Funding: Pfizer (Inst), Exelixis (Inst), Merck (Inst), Bristol Myers Squibb-Ono Pharmaceutical (Inst), Astellas Pharma (Inst)
Jessica E. Pritchard
Research Funding: St Jude Medical (Inst), Pfizer (Inst), Boston Scientific (Inst)
Cary P. Gross
Research Funding: Johnson & Johnson (Inst), AstraZeneca (Inst), Genentech
Michaela A. Dinan
Research Funding: AstraZeneca (Inst), Janssen
Stephanie B. Wheeler
Research Funding: Pfizer (Inst)
Travel, Accommodations, Expenses: Pfizer
No other potential conflicts of interest were reported.
REFERENCES
- 1.Siegel RL, Miller KD, Fuchs HE, Jemal A: Cancer statistics, 2022. CA Cancer J Clin 72:7-33, 2022 [DOI] [PubMed] [Google Scholar]
- 2.Li P, Wong YN, Armstrong K, et al. : Survival among patients with advanced renal cell carcinoma in the pretargeted versus targeted therapy eras. Cancer Med 5:169-181, 2016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Earle CC, Park ER, Lai B, et al. : Identifying potential indicators of the quality of end-of-life cancer care from administrative data. J Clin Oncol 21:1133-1138, 2003 [DOI] [PubMed] [Google Scholar]
- 4.Earle CC, Neville BA, Landrum MB, et al. : Evaluating claims-based indicators of the intensity of end-of-life cancer care. Int J Qual Health Care 17:505-509, 2005 [DOI] [PubMed] [Google Scholar]
- 5.Palliative Care and End-of-Life Care. https://www.qualityforum.org/topics/palliative_care_and_end-of-life_care.aspx. [Google Scholar]
- 6.Morden NE, Chang CH, Jacobson JO, et al. : End-of-life care for Medicare beneficiaries with cancer is highly intensive overall and varies widely. Health Aff 31:786-796, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Meyer AM, Olshan AF, Green L, et al. : Big data for population-based cancer research: The integrated cancer information and surveillance system. North Carolina Med J 75:265-269, 2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Enewold L, Parsons H, Zhao L, et al. : Updated overview of the SEER-Medicare data: Enhanced content and applications. J Natl Cancer Inst Monogr 2020:3-13, 2020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Rural-Urban Commuting Area Codes. https://www.ers.usda.gov/data-products/rural-urban-commuting-area-codes.aspx [Google Scholar]
- 10.Faurot KR, Jonsson Funk M, Pate V, et al. : Using claims data to predict dependency in activities of daily living as a proxy for frailty. Pharmacoepidemiol Drug Saf 24:59-66, 2015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Dartmouth Atlas of Healthcare. http://dartmouthatlas.org [Google Scholar]
- 12.2010 census regions and divisions of the United States (U.S. Department of Commerce, Economics and statistics Administration, U.S. Census Bureau, Geography Division). https://www.census.gov/geographies/reference-maps/2010/geo/2010-census-regions-and-divisions-of-the-united-states.html
- 13.Interpreting Race and Ethnicity in Cancer Data. United States Cancer Statistics. http://medbox.iiab.me/modules/en-cdc/www.cdc.gov/cancer/npcr/uscs/technical_notes/interpreting/race.htm [Google Scholar]
- 14.Prigerson HG, Bao Y, Shah MA, et al. : Chemotherapy use, performance status, and quality of life at the end of life. JAMA Oncol 1:778-784, 2015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Zerillo JA, Stuver SO, Fraile B, et al. : Understanding oral chemotherapy prescribing patterns at the end of life at a comprehensive cancer center: Analysis of a Massachusetts payer claims database. JCO Oncol Pract 11:372-377, 2015 [DOI] [PubMed] [Google Scholar]
- 16.Spees LP, Wheeler SB, Jackson BE, et al. : Provider- and patient-level predictors of oral anticancer agent initiation and adherence in patients with metastatic renal cell carcinoma. Cancer Med 10:6653-6665, 2021 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Kaye DR, Wilson LE, Greiner MA, et al. : Patient, provider, and hospital factors associated with oral anti-neoplastic agent initiation and adherence in older patients with metastatic renal cell carcinoma. J Geriatr Oncol 13:614-623, 2022 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Obermeyer Z, Powers BW, Makar M, et al. : Physician characteristics strongly predict patient enrollment in hospice. Health Aff 34:993-1000, 2015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Patel MN, Nicolla JM, Friedman FAP, et al. : Hospice use among patients with cancer: Trends, barriers, and future directions. JCO Oncol Pract 16:803-809, 2020 [DOI] [PubMed] [Google Scholar]
- 20.Wilson LE, Spees L, Pritchard J, et al. : Real-world utilization of oral anticancer agents and related costs in older adults with metastatic renal cell carcinoma in the United States. Kidney Cancer 5:115-127, 2021 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Wheeler SB, Spees LP, Jackson BE, et al. : Patterns and predictors of oral anticancer agent use in diverse patients with metastatic renal cell carcinoma. JCO Oncol Pract 17:e1895-e1904, 2021 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Earle CC, Neville BA, Landrum MB, et al. : Trends in the aggressiveness of cancer care near the end of life. J Clin Oncol 22:315-321, 2004 [DOI] [PubMed] [Google Scholar]
- 23.Falchook AD, Dusetzina SB, Tian F, et al. : Aggressive end-of-life care for metastatic cancer patients younger than age 65 years. J Natl Cancer Inst 109:djx028, 2017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Herrel LA, Zhu Z, Ryan AM, et al. : Intensity of end-of-life care for dual-eligible beneficiaries with cancer and the impact of delivery system affiliation. Cancer 127:4628-4635, 2021 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Wadiwala J, Patel M, Li C, et al. : Health care disparities and barriers to palliative care among metastatic renal cell carcinoma patients: An NCDB analysis. J Clin Oncol 39, 2021. (suppl 15; abstr 4545) [Google Scholar]
- 26.Chen Y, Criss SD, Watson TR, et al. : Cost and utilization of lung cancer end-of-life care among racial-ethnic minority groups in the United States. Oncologist 25:e120-e129, 2020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Ferrario A, Xu X, Zhang F, et al. : Intensity of end-of-life care in a cohort of commercially insured women with metastatic breast cancer in the United States. JCO Oncol Pract 17:e194-e203, 2021 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Nordstrom BL, Whyte JL, Stolar M, et al. : Identification of metastatic cancer in claims data: Metastatic cancer in claims. Pharmacoepidemiol Drug Saf 21:21-28, 2012 [DOI] [PubMed] [Google Scholar]