PURPOSE:
Accelerated by the COVID-19 pandemic, the virtual platform has become a prominent medium to deliver mind-body therapies, but the extent to which patients engage in virtual mind-body programming remains unclear. This study aims to assess oncology patient engagement in a virtual mind-body program.
METHODS:
We surveyed oncology patients enrolled in a live-streamed (synchronous) virtual mind-body program in May 2021. Patients self-reported engagement by weekly attendance. We applied multivariate regression to identify associations of engagement with sociodemographic and clinical factors. As an exploratory analysis, we used machine learning to partition engagement subgroups to determine preferential interest in prerecorded (asynchronous) mind-body therapy videos.
RESULTS:
Among 148 patients surveyed (response rate: 21.4%), majority were female (94.5%), White (83.1%), age 65 years or older (64.9%), retired (64.2%), and in survivorship (61.8%). Patient engagement ranged from 1 to 13 classes/week (mean [standard deviation]: 4.23 [2.56]) and was higher for female (β, .82; 95% CI, 0.01 to 1.62), non-White (β, .63; 95% CI, 0.13 to 1.13), and retired patients (β, .50; 95% CI, 0.12 to 0.88). The partition model identified three engagement subgroups: employed (low engagers), retired White (intermediate engagers), and retired non-White (high engagers). Particularly, low engagers had preferential interest in meditation videos (odds ratio, 2.85; 95% CI, 1.24 to 6.54), and both low and high engagers had preferential interest in Tai Chi videos (odds ratio, 2.26; 95% CI, 1.06 to 4.82).
CONCLUSION:
In this cross-sectional study among oncology patients, engagement in virtual mind-body programming was higher for female, non-White, and retired patients. Our findings suggest the need for both synchronous and asynchronous mind-body programming to meet the diverse needs of oncology patients.
INTRODUCTION
Patient engagement has increasingly been recognized as an integral component of cancer care delivery and science.1-3 In recent decades, a growing body of evidence demonstrates that patients who actively engage in health-promoting activities—such as exercising regularly—experience improved symptom control, quality of life, and survival.4-7 However, numerous factors influence patient engagement, in that patients have unique backgrounds, capabilities, interests, or goals that dictate to what extent they are able to engage in their health; thus, promoting patient engagement represents a complex care delivery and research challenge.1,2 The ongoing COVID-19 pandemic further magnifies this challenge as the crisis forces patients to postpone, modify, or remotely engage in health care services and health routines.8,9
Telehealth, and specifically virtual visits, has been widely adopted during the COVID-19 pandemic for patients to communicate and engage with oncology providers.10,11 Telehealth encompasses various ways that patients may remotely interact with their providers, which include synchronous (real-time) interactions (ie, video applications or phone calls) or asynchronous (sequential) interactions (ie, messaging).12 Remarkably, telehealth has proven to be satisfactory and sometimes preferred by patients and providers, citing benefits of convenience and effectiveness.11,13,14 Despite this trend, telehealth remains an understudied model of delivering care across the cancer control continuum, fueling a national research agenda to call for accelerated advancement in the science of telehealth-delivered cancer care.15
Telehealth has also become a medium for patients to engage in health-promoting routines, giving rise to a $1.5 trillion US dollars (USD) virtual wellness industry.16-18 Health- and wellness-oriented virtual platforms can offer various forms of modalities, ranging from more physical-based (ie, fitness and core strength) to more classical mind-body modalities (ie, yoga and meditation) and even creative art interventions (ie, dance and music therapies).19-22 Herein, we will refer to the various virtual health- and wellness-oriented interventions as virtual mind-body therapies or programming. Since the COVID-19 pandemic, a major commercial virtual mind-body platform found a significant increase in the utilization of live-streamed (synchronous) classes from 7% to 85% and prerecorded (asynchronous) videos from 17% to 73%.16 In oncology, physical exercise and mind-body interventions have demonstrated benefits in improving patients' quality of life and symptom burden; however, little is known about oncology patient engagement in virtual mind-body programming.23-30 Early evaluations of virtual mind-body programming at our cancer center demonstrated feasibility with high satisfaction and qualitative benefits to help patients stay physically active, cope with stressors, and foster social relationships.31,32 Further understanding of oncology patient engagement in virtual mind-body programming will help advance virtual care delivery across the cancer control continuum.
We aimed to assess oncology patient engagement in a live-streamed (synchronous) virtual mind-body program and to identify factors that influence engagement. As an exploratory analysis, we then applied machine learning to build a partition model of engagement and hypothesized that this model would allow us to identify distinct patient subgroups with preferential interests in prerecorded (asynchronous) virtual mind-body programming.
METHODS
Study Design
In May 2021, we conducted a cross-sectional survey to assess patient engagement in virtual mind-body programming at the Memorial Sloan Kettering Cancer Center (MSK) as part of the program evaluation and identified patient interest in prerecorded videos of mind-body therapies. We distributed a one-time survey through Qualtrics to all virtual mind-body programming participants. Surveyed participants were all patients with cancer who received care directly or indirectly (ie, second opinion) from MSK. Participants could access survey link via the MSK patient portal system. Patients' surrogates (ie, family or caregivers) may help fill out the surveys on patient behalf. The surveys were collected as is with the assumption that the responses would reflect that of the intended participants. Collected information included patient-reported sociodemographic (age, sex, race, and employment status) and clinical data (phase of cancer care). Completion of the survey was voluntary and optional. All data were deidentified for patient privacy and use to generate analysis. MSK's Institutional Review Board approved the study.
Virtual Mind-Body Programming
The Integrative Medicine Service (IMS) at MSK implemented a live-streamed, virtual mind-body program on April 1, 2020. The program offers 7-day-a-week virtual mind-body group therapy programming or classes through the Zoom videoconference platform. Classes included instructions that ranged from movement-based modalities (fitness, Tai Chi, yoga, or dance) to meditative therapies (meditation or music therapy). A qualified IMS clinician who had specialty license or training and employed by MSK (eg, fitness instructor, meditation instructor, and music therapist) led each class, which ranged from 30 to 45 minutes and occurred 1 to 4 times per week, totaling 20 classes per week. The classes were interactive, where participants could communicate with their providers and received feedback. There was no participation cap per class; however, class sizes typically range between 10 for music therapy and 150 for fitness classes. Participants could engage in any classes with the option to renew class access under a 1-month ($25 USD), 3-month ($60 USD), or 6-month membership ($120 USD). We informed patients about the class schedule and registration details through the institution's patient portal. Prerecorded mind-body videos were not offered at the time of the study. Classes did not allow for audio or video recording to protect privacy.
Measures
The primary outcome was patient engagement measured by self-reported weekly attendance. We asked patients, “how many virtual mind-body therapy classes have you attended in the last 7 days?” and provided options from 0 to 20 classes. The secondary outcome was self-reported patient interest in prerecorded virtual mind-body therapy classes. We asked patients, “if we provided pre-recorded on-demand videos for members, how long would you like the videos to be?” Response options were 15, 30, 45, or 60 minutes, grouped as interested or not interested for all six categories of virtual mind-body therapy classes (fitness, Tai Chi, yoga, meditation, dance, or music therapy). We assumed that those who responded with a desired length of video time were interested and those who responded not interested were not interested.
We also collected patient demographic and clinical information. Covariates were dichotomized to ease interpretation, including age (younger than 65 v 65 years or older), sex (female v male, not including nonbinary), race (White v non-White, including Black/African American, Asian, Native Hawaiian or Pacific Islander, and more than one race), employment status (employed, including full-time or part-time v retired, including retired or unemployed), and phase of cancer care (active treatment, including newly diagnosed or receiving treatment v post-treatment survivorship). Of note, demographics between the retired and unemployed were similar in our study although comparison was limited because of the modest sample size. Given that both the unemployed and retired patients were not actively employed, we designated the group as retired to emphasize the majority representation (> 90%) of the sample.
Statistical Analysis
We analyzed associations of covariates against the primary outcome (patient engagement) using univariate and multivariate regression analyses. Multivariate models included all covariates that showed a univariate association of P < .10. We then built a partition model of patient engagement using recursive partitioning—a supervised machine learning algorithm—by including the significant multivariate covariates.33-35 The model divided subgroups according to the relationship between covariates and patient engagement by minimizing their sum of squared errors on the basis of logworth statistic, creating a decision tree.36 From the partition model, we defined subgroups with different levels of engagement (ie, low, intermediate, or high engagers). Furthermore, we identified subgroups with substantial and preferential interests in prerecorded virtual mind-body programming. Substantial interest in prerecorded videos was a priori defined as interest > 70%, whereas preferential interest was defined as statistically significant association in engagement subgroups and video categories. Statistical significance was set at P < .05, and analyses were conducted using JMP PRO (version 14.0).37
RESULTS
Baseline Characteristics
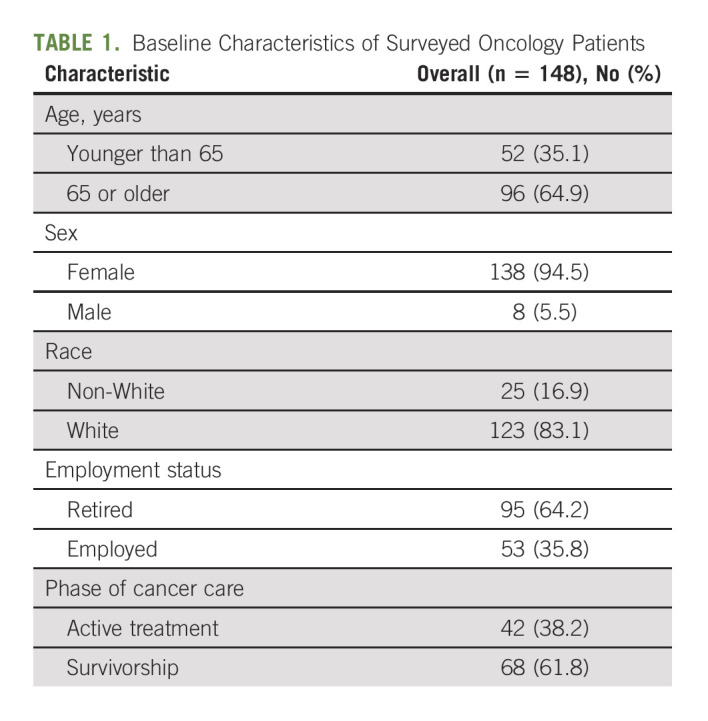
In May 2021, a total of 691 oncology patients participated in virtual mind-body programming, of which 148 (21.4%) completed the one-time survey. Surveyed patients were majority female (94.5%), White (83.1%), age 65 years or older (64.9%), retired (64.2%), and in survivorship (61.8%; Table 1).
TABLE 1.
Baseline Characteristics of Surveyed Oncology Patients
Patient Engagement
Patient engagement ranged from 1 to 13 classes/week (mean [standard deviation; SD]: 4.23 [2.56] classes), with the majority taking three to five classes (25th and 75th quartile; Appendix Fig A1, online only). In univariate analysis, female patients were associated with higher engagement than male patients (mean [SD]: 4.33 [2.29] v 2.88 [0.83] classes, P = .001). Retired patients were also associated with higher engagement compared with employed patients (4.56 [2.47] v 3.64 [1.68] classes, P = .008). In multivariate analysis, higher patient engagement (constant: 3.82 classes) was associated with female (beta coefficient [β], .82; 95% CI, 0.01 to 1.62), non-White (β, .63; 95% CI, 0.13 to 1.13), and retirement status (β, .50; 95% CI, 0.12 to 0.88; Table 2).
TABLE 2.
Association of Patient Engagement With Baseline Characteristics
Partition Model
The partition model of patient engagement among all 148 surveyed patients (mean [SD]: 4.22 [2.22] classes) was illustrated (Fig 1A). The model first split patients into subgroups by employment status between retired (n = 95; 4.56 [2.47] classes) and employed patients (n = 53; 3.64 [1.68] classes). Splitting was based on the recursive partitioning, a supervised machine learning algorithm, by minimizing the sum of squared errors. The partition model then split retired patients into subgroups by race between retired non-White (n = 12; 5.83 [4.00] classes) and retired White patients (n = 83; 4.37 [2.14] classes). Overall, the model identified three engagement subgroups: employed (low engagers), retired White (intermediate engagers), and retired non-White (high engagers).
FIG 1.
Partition model of oncology patient engagement and interest in virtual mind-body programming. OR, odds ratio; SD, standard deviation.
Patient Interest
More than half of all patients were interested in prerecorded video classes in fitness (89%), Tai Chi (71%), yoga (69%), dance (68%), meditation (66%), and music therapy (52%; Appendix Fig A2A, online only). Interests in prerecorded videos distributed by patient engagement subgroups are displayed in Appendix Fig A2B-Fig A2G. In particular, employed patients (low engagers) were associated with a higher interest in prerecorded meditation videos than retired patients (intermediate and high engagers; odds ratios [ORs], 2.85; 95% CI, 1.24 to 6.54); employed patients (low engagers) and retired non-White patients (high engagers) were also associated with a higher combined interest in prerecorded Tai Chi videos than retired White patients (intermediate engagers; OR, 2.26; 95% CI, 1.06 to 4.82; Appendix Table A1, online only). Of note, a combined interest in prerecorded meditation videos was higher for the low/high engagers than intermediate engagers (OR, 2.38; 95% CI, 1.12 to 5.06), but the difference was driven by the substantial interest (> 70% interest) in prerecorded meditation videos from low engagers rather than high engagers. Preferential and substantial interests in prerecorded videos by patient engagement subgroups are illustrated in Fig 1B.
DISCUSSION
Patient engagement in health-promoting activities leads to better health outcomes. In this study, we found that sex, race, and employment status influence oncology patient engagement in virtual mind-body programming, favoring female, non-White, and retired patients. We applied a machine learning algorithm to partition three subgroups of patients with differential engagement, including low engagers (employed patients), intermediate engagers (retired White patients), and high engagers (retired non-White patients). Our model identified that low engagers had a preferential interest in meditation videos, and both low and high engagers had a preferential interest in Tai Chi videos. Understanding patient engagement and interest in virtual care is timely and essential for supporting oncology patients' treatment and recovery.
Fueled by the COVID-19 pandemic, the accelerated adoption of telehealth has spurred innovation in health care delivery; although the surge of telehealth adoption has dropped, utilization of virtual health services remains 38 times the pre–COVID-19 era, raising critical questions as to how to leverage this digital shift to promote patient engagement in virtual care.38 To bridge care access during the pandemic, research on telehealth has since been focused on system-level improvement to scale digital infrastructure, from streamlining care delivery processes to enhancing telehealth platform.39-41 However, emerging studies have suggested that telehealth requires personalization—far from a one-size-fits-all model—in that virtual care benefits different patients in different settings at different times.42 For instance, UCLA Health, an academic health care system, has piloted a telehealth triage protocol, with the goal of ensure that virtual care delivery will be appropriate to address patient's unique concerns.42 Similarly, a Korean-based telemedicine program has designed a manual to tailor virtual mind-body interventions to specific patients' symptoms and needs.43 Building on the existing literature, our findings underscore the need to better integrate a sex preferred, racially sensitive, and employment-friendly virtual mind-body therapy experience.
Our partition model offers a novel approach to understanding the influential interactions of patient engagement in virtual mind-body programming. Machine learning approaches have previously been applied to model complex data and demonstrated use to identify heterogeneity in patient engagement.44 Heterogeneous patient engagement subtypes with internet-based cognitive behavioral therapy were associated with different patterns of patient behavior and different levels of improvement in depression and anxiety.44 Our model partitioned patient engagement subgroups by sociocultural factors and predicted preferential interest in mind-body therapies. Such a model may help inform engagement strategies, benchmark expectations, and tailor therapies. For instance, we would define appropriate outreach efforts and therapies to match preferential interest for particular subgroups. Refinement of the model requires more robust data sets and future research to assess its impact on clinical and patient outcomes.
Consequently, our findings require careful interpretation. First, the mechanisms by which sex, race, and employment influence patient engagement are difficult to discern. Previous literature has suggested that gender differences in perceived benefits and purpose of mind-body therapies contribute to greater female engagement.45,46 Racial differences in backgrounds, knowledge, and beliefs are thought to contribute to varying prevalence and types of mind-body therapy use and the likelihood of using telehealth visits.47-50 Employment or income loss after a cancer diagnosis has been described to negatively correlate with patients' treatment adherence; particularly, live-streamed interventions may not be feasible for individuals with conflicting work schedules.51-59 Owing to differences in study designs and data sources, the influential mechanism of specific factors in the setting of virtual mind-body therapies remains unclear and needs future research.
Second, it remains in question as to what signifies meaningful patient engagement in virtual mind-body programming. Defining meaningful engagement, however, is a challenge in and of itself as engagement is a continuum influenced not only by contextual constraints but also by patient goals and needs.60 Although some patients may benefit from sustained, frequent engagement, a single engagement for other patients might already be an accomplishment.60 Consequently, although engagement in virtual mind-body programming has generally reported to be quite high in the research setting, this high level of engagement is unlikely representative in real life, recognizing that engagement varies depending on patients' unique circumstances.61-69 In general, the American Cancer Society recommends that cancer survivors engage in up to at least 150 minutes of moderate-intensity or 75 minutes of vigorous-intensity exercise each week.70 This recommendation is supported by numerous epidemiologic studies, demonstrating the benefits of exercise in reducing cancer risk and all-cause and cancer-specific mortalities. On the contrary, recommendation on engagement in mind-body therapy is less well-established; limited literature has suggested that an interval of 10-20 minutes per day is feasible.71 Future research is needed to refine these recommendations in the virtual setting.
Third, how interest in prerecorded mind-body therapy videos translates to actual patient engagement remains unknown although behavioral health studies suggest that patient interest plays a vital role in influencing engagement.72,73 Unlike live-streamed classes, prerecorded videos are accessible anytime, giving patients the flexibility to engage at a time of their convenience. Videos also allow playback, so patients can choose to replay classes tailored to their specific needs. Some patients may perceive prerecorded classes as more private and prefer this approach to engage over group sessions. In addition, prerecorded content may be a cost-effective, sustainable way to deliver a broad selection of content. As our previous research suggests that live-streamed classes help create a sense of structure and community, future research will be of interest to understand how to structure prerecorded content to best engage patients.32
This study has limitations. The survey response rate is modest and potentially introduces nonresponse or self-selection bias. Given the multifactorial influences of patient engagement, our covariates cannot possibly represent all relevant factors (ie, patient's geographical locations, socioeconomic status, and other clinical factors). The analyzed variables are dichotomized to ease computation, but statistical power is expectantly compromised. Subject to recall or social desirability bias, self-reported patient engagement can be strengthened with actual participation data. However, we did not collect individual attendance given privacy concern and operational limitation in maintaining a manual record. Since weekly attendance, rather than daily class attendance, was reported, correlative insights into specific engagement of each class were limited. The significance of patient engagement in the virtual program remained unclear without a comparative in-person control. Finally, surveyed patients were from a single, cancer-specialized, academic institution; thus, the surveyed population limits generalizability and may not speak to the unique needs of patients in other settings.
In conclusion, patient engagement is essential to achieving optimal health. In this cross-sectional study, we found that female, non-White, and retired patients had higher engagement in live-streamed (synchronous), virtual mind-body programming. Our exploratory partition model presents a novel approach to understanding patient engagement that may be helpful to inform engagement strategy, benchmark expectations, and tailor therapies; however, future research is needed to validate the model. Finally, we found substantial and preferential interests in prerecorded (asynchronous) mind-body therapy videos, suggesting that prerecorded videos may present an attractive, alternative medium for engaging patients in mind-body therapy. As we reimagine digital health in the post–COVID-19 era, our findings support that telehealth is far from a one-size-fits-all model and that we ought to move toward personalization of virtual care.
ACKNOWLEDGMENT
We thank all the clinicians and administrative staff who helped with survey development and data collection.
APPENDIX
FIG A1.
Patient engagement in virtual mind-body programming (how many virtual mind-body classes have you attended in the past 7 days?).
FIG A2.
(A) Interest in prerecorded mind-body therapy videos; (B) interest in prerecorded fitness videos; (C) interest in prerecorded music therapy videos; (D) interest in prerecorded dance therapy videos; (E) interest in prerecorded yoga videos; (F) interest in prerecorded meditation videos; (G) interest in prerecorded Tai Chi videos. Engagers categories: H, high; I, intermediate; L, low.
TABLE A1.
Association of the Patient Engagement Group and Interest in Prerecorded Mind-Body Therapy Videos
David G. Pfister
Consulting or Advisory Role: Boehringer Ingelheim, Incyte, MeiraGTx, Nykode Therapeutics
Research Funding: Boehringer Ingelheim (Inst), AstraZeneca (Inst), Exelixis (Inst), Novartis (Inst), MedImmune (Inst), Merck (Inst), Genentech/Roche (Inst), Lilly (Inst), Bayer (Inst), Eisai (Inst), Regeneron (Inst), Atara Biotherapeutics (Inst), MeiraGTx (Inst), Hookipa Pharma (Inst)
Jun J. Mao
Research Funding: Tibet Cheezheng Tibetan Medicine Co, Ltd (Inst)
No other potential conflicts of interest were reported.
PRIOR PRESENTATION
Presented at the 2021 SIO International Conference, Baltimore, MD (oral presentation) on September 26, 2021.
SUPPORT
Supported in part by a National Institutes of Health/National Cancer Institute (NCI) Cancer Center grant (grant No. P30 CA008748) and the Translational and Integrative Medicine Research Fund at the Memorial Sloan Kettering Cancer Center. J.J.M. was supported in part by a National Institutes of Health/National Cancer Institute grant (grant No. R01 CA240417).
AUTHOR CONTRIBUTIONS
Conception and design: Tony K.W. Hung, Eva Pendleton, Jun J. Mao
Financial support: Jun J. Mao
Administrative support: Eva Pendleton, Jun J. Mao
Collection and assembly of data: Tony K.W. Hung, Shelly Latte-Naor, Christina Seluzicki
Data analysis and interpretation: Tony K.W. Hung, Yuelin Li, Gilad J. Kuperman, David G. Pfister
Manuscript writing: All authors
Final approval of manuscript: All authors
Accountable for all aspects of the work: All authors
AUTHORS' DISCLOSURES OF POTENTIAL CONFLICTS OF INTEREST
Assessment of Oncology Patient Engagement and Interest in Virtual Mind-Body Programming: Moving Toward Personalization of Virtual Care
The following represents disclosure information provided by authors of this manuscript. All relationships are considered compensated unless otherwise noted. Relationships are self-held unless noted. I = Immediate Family Member, Inst = My Institution. Relationships may not relate to the subject matter of this manuscript. For more information about ASCO's conflict of interest policy, please refer to www.asco.org/rwc or ascopubs.org/op/authors/author-center.
Open Payments is a public database containing information reported by companies about payments made to US-licensed physicians (Open Payments).
David G. Pfister
Consulting or Advisory Role: Boehringer Ingelheim, Incyte, MeiraGTx, Nykode Therapeutics
Research Funding: Boehringer Ingelheim (Inst), AstraZeneca (Inst), Exelixis (Inst), Novartis (Inst), MedImmune (Inst), Merck (Inst), Genentech/Roche (Inst), Lilly (Inst), Bayer (Inst), Eisai (Inst), Regeneron (Inst), Atara Biotherapeutics (Inst), MeiraGTx (Inst), Hookipa Pharma (Inst)
Jun J. Mao
Research Funding: Tibet Cheezheng Tibetan Medicine Co, Ltd (Inst)
No other potential conflicts of interest were reported.
REFERENCES
- 1.Klein WMP, O’Connell ME, Bloch MH, et al. : Behavioral research in cancer prevention and control: Emerging challenges and opportunities. J Natl Cancer Inst 114:179-186, 2022 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Carman KL, Dardess P, Maurer M, et al. : Patient and family engagement: A framework for understanding the elements and developing interventions and policies. Health Aff 32:223-231, 2013 [DOI] [PubMed] [Google Scholar]
- 3.Deverka PA, Bangs R, Kreizenbeck K, et al. : A new framework for patient engagement in cancer clinical trials cooperative group studies. J Natl Cancer Inst 110:553-559, 2018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Lee K, Tripathy D, Demark-Wahnefried W, et al. : Effect of aerobic and resistance exercise intervention on cardiovascular Disease risk in women with early-stage breast cancer: A randomized clinical trial. JAMA Oncol 5:710-714, 2019 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Mustian KM, Alfano CM, Heckler C, et al. : Comparison of pharmaceutical, psychological, and exercise treatments for cancer-related fatigue: A meta-analysis. JAMA Oncol 3:961-968, 2017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Knols R, Aaronson NK, Uebelhart D, et al. : Physical exercise in cancer patients during and after medical treatment: A systematic review of randomized and controlled clinical trials. J Clin Oncol 23:3830-3842, 2005 [DOI] [PubMed] [Google Scholar]
- 7.Cannioto RA, Hutson A, Dighe S, et al. : Physical activity before, during, and after chemotherapy for high-risk breast cancer: Relationships with survival. J Natl Cancer Inst 113:54-63, 2021 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Schrag D, Hershman DL, Basch E: Oncology practice during the COVID-19 pandemic. JAMA 323:2005-2006, 2020 [DOI] [PubMed] [Google Scholar]
- 9.Moore S, Leung B, Bates A, Ho C: Social isolation: Impact on treatment and survival in patients with advanced cancer. J Clin Oncol 36, 2018. (suppl 34; abstr 156) [Google Scholar]
- 10.Arem H, Moses J, Cisneros C, et al. : Cancer provider and survivor experiences with telehealth during the COVID-19 pandemic. JCO Oncol Pract 18:e452-e461, 2022 [DOI] [PubMed] [Google Scholar]
- 11.Manz C, Baxter NN, duPont NC, et al. : Patterns of telehealth utilization during the COVID-19 pandemic and preferences for post-pandemic telehealth use: A national survey of oncology clinicians. J Clin Oncol 39, 2021. (suppl 15; abstr 1580) [Google Scholar]
- 12.Sirintrapun SJ, Lopez AM: Telemedicine in cancer care. Am Soc Clin Oncol Ed book 38:540-545, 2018 [DOI] [PubMed] [Google Scholar]
- 13.Neeman E, Kumar D, Lyon L, et al. : Attitudes and perceptions of multidisciplinary cancer care clinicians toward telehealth and secure messages. JAMA Netw Open 4:e2133877, 2021 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Wehrle CJ, Lee SW, Devarakonda AK, Arora TK: Patient and physician attitudes toward telemedicine in cancer clinics following the COVID-19 pandemic. JCO Clin Cancer Inform 5:394-400, 2021 [DOI] [PubMed] [Google Scholar]
- 15.National Institutes of Health : Centers on Telehealth Research for Cancer-Related Care (P50 Clinical Trial Required). RFA-CA-21-029.2021, 2021. https://grants.nih.gov/grants/guide/rfa-files/RFA-CA-21-029.html
- 16.Cording J: How COVID-19 Is Transforming the Fitness Industry. Forbes, 2020. https://www.forbes.com/sites/jesscording/2020/07/13/covid-19-transforming-fitness-industry/?sh=720638630a74 [Google Scholar]
- 17.Wasil AR, Gillespie S, Patel R, et al. : Reassessing evidence-based content in popular smartphone apps for depression and anxiety: Developing and applying user-adjusted analyses. J Consult Clin Psychol 88:983-993, 2020 [DOI] [PubMed] [Google Scholar]
- 18.Callaghan S, Lösch M, Pione A, Teichner W: Feeling Good: The Future of the $1.5 Trillion Wellness Market. McKinsey & Company, 2021. https://www.mckinsey.com/industries/consumer-packaged-goods/our-insights/feeling-good-the-future-of-the-1-5-trillion-wellness-market [Google Scholar]
- 19.MindBody, Inc : https://www.mindbodyonline.com
- 20.Cancer Treatment Centers of America : BurnAlong. https://www.cancercenter.com/our-services/virtual-wellness-and-exercise-classes [Google Scholar]
- 21.Yoga4Cancer. https://y4c.com/classes-for-survivors/ [Google Scholar]
- 22.Glo. https://www.glo.com [Google Scholar]
- 23.Duncan M, Moschopoulou E, Herrington E, et al. : Review of systematic reviews of non-pharmacological interventions to improve quality of life in cancer survivors. BMJ Open 7:e015860, 2017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Hökkä M, Kaakinen P, Pölkki T: A systematic review: Non-pharmacological interventions in treating pain in patients with advanced cancer. J Adv Nurs 70:1954-1969, 2014 [DOI] [PubMed] [Google Scholar]
- 25.So WKW, Law BMH, Chan DNS, et al. : The effect of nonpharmacological interventions on managing symptom clusters among cancer patients: A systematic review. Cancer Nurs 43:E304-E327, 2020 [DOI] [PubMed] [Google Scholar]
- 26.Juvet LK, Thune I, Elvsaas I, et al. : The effect of exercise on fatigue and physical functioning in breast cancer patients during and after treatment and at 6 months follow-up: A meta-analysis. Breast 33:166-177, 2017 [DOI] [PubMed] [Google Scholar]
- 27.Bourke L, Smith D, Steed L, et al. : Exercise for men with prostate cancer: A systematic review and meta-analysis. Eur Urol 69:693-703, 2016 [DOI] [PubMed] [Google Scholar]
- 28.Cramer H, Lauche R, Klose P, et al. : Yoga for improving health-related quality of life, mental health and cancer-related symptoms in women diagnosed with breast cancer. Cochrane Database Syst Rev 1:CD010802, 2017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Hall DL, Luberto CM, Philpotts LL, et al. : Mind-body interventions for fear of cancer recurrence: A systematic review and meta-analysis. Psychooncology 27:2546-2558, 2018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Lyman GH, Greenlee H, Bohlke K, et al. : Integrative therapies during and after breast cancer treatment: ASCO endorsement of the SIO clinical practice guideline. J Clin Oncol 36:2647-2655, 2018 [DOI] [PubMed] [Google Scholar]
- 31.Trevino KM, Raghunathan N, Latte-Naor S, et al. : Rapid deployment of virtual mind-body interventions during the COVID-19 outbreak: Feasibility, acceptability, and implications for future care. Support Care Cancer 29:543-546, 2021 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Emard N, Lynch KA, Liou KT, et al. : Virtual mind-body programming for patients with cancer during COVID-19: A qualitative study. JMIR Cancer 7:e27384, 2021 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Kass GV: An exploratory technique for investigating large quantities of categorical data. Appl Stat 29:119-127, 1980 [Google Scholar]
- 34.Hawkins DM, Kass GV: Automatic interaction detection, in Hawkins DM. (ed): Topics in Applied Multivariate Analysis. Cambridge, United Kingdom, Cambridge University Press, 1982, pp 267-300 [Google Scholar]
- 35.Strobl C, Malley J, Tutz G: An introduction to recursive partitioning: Rationale, application, and characteristics of classification and regression trees, bagging, and random forests. Psychol Methods 14:323-348, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Sall J: Monte Carlo Calibration of Distributions of Partition Statistics. Cary, NC, SAS Institute, 2002. https://www.jmp.com/content/dam/jmp/documents/en/white-papers/montecarlocal.pdf [Google Scholar]
- 37.JMP®. Version 14. Cary, NC. SAS Institute, 1989–2021 [Google Scholar]
- 38.Bestsennyy O, Gilbert G, Harris A, Rost J: Telehealth: A Quarter-Trillion-Dollar Post-COVID-19 Reality? McKinsey & Company, 2021. https://www.mckinsey.com/industries/healthcare-systems-and-services/our-insights/telehealth-a-quarter-trillion-dollar-post-covid-19-reality [Google Scholar]
- 39.Royce TJ, Sanoff HK, Rewari A: Telemedicine for cancer care in the time of COVID-19. JAMA Oncol 6:1698-1699, 2020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Lopez AM, Lam K, Thota R: Barriers and facilitators to telemedicine: Can you hear me now? Am Soc Clin Oncol Ed Book 41:25-36, 2021 [DOI] [PubMed] [Google Scholar]
- 41.Artandi M, Thomas S, Shah NR, Srinivasan M: Rapid system transformation to more than 75% primary care video visits within three weeks at Stanford: Response to public safety crisis during a pandemic. NEJM Catalyst, 2020. https://catalyst.nejm.org/doi/full/10.1056/CAT.20.0100 [Google Scholar]
- 42.Croymans D, Hurst I, Han M: Telehealth: The right care, at the right time, via the right medium. NEJM Catalyst, 2020. https://catalyst.nejm.org/doi/full/10.1056/CAT.20.0564 [Google Scholar]
- 43.Kwon CY, Kwak HY, Kim JW: Using mind-body modalities via telemedicine during the COVID-19 crisis: Cases in the Republic of Korea. Int J Environ Res Public Health 17:4477, 2020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Chien I, Enrique A, Palacios J, et al. : A machine learning approach to understanding patterns of engagement with internet-delivered mental health interventions. JAMA Netw Open 3:e2010791, 2020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Upchurch DM, Johnson PJ: Gender differences in prevalence, patterns, purposes, and perceived benefits of meditation practices in the United States. J Womens Health 28:135-142, 2019 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Craft BB, Carroll HA, Lustyk MKB: Gender differences in exercise habits and quality of life reports: Assessing the moderating effects of reasons for exercise. Int J Lib Arts Soc Sci 2:65-76, 2014 [PMC free article] [PubMed] [Google Scholar]
- 47.Arthur KN, Belliard JC, Hardin SB, et al. : Reasons to use and disclose use of complementary medicine use—An insight from cancer patients. Cancer Clin Oncol 2:81-92, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Pierce RP, Stevermer JJ: Disparities in use of telehealth at the onset of the COVID-19 public health emergency. J Telemed Telecare 29:3-9, 2023 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Waseem N, Boulanger M, Yanek LR, Feliciano JL: Disparities in telemedicine success and their association with adverse outcomes in patients with thoracic cancer during the COVID-19 pandemic. JAMA Netw Open 5:e2220543, 2022 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Fischer SH, Ray KN, Mehrotra A, et al. : Prevalence and characteristics of telehealth utilization in the United States. JAMA Netw Open 3:e2022302, 2020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Banegas MP, Guy GP Jr, de Moor JS, et al. : For working-age cancer survivors, medical debt and bankruptcy create financial hardships. Health Aff 35:54-61, 2016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Howlader N, Noone AM, Krapcho M, et al. : SEER Cancer Statistics Review, 1975-2016. Bethesda, MD, National Cancer Institute, 2019 [Google Scholar]
- 53.Fenn KM, Evans SB, McCorkle R, et al. : Impact of financial burden of cancer on survivors' quality of life. JCO Oncol Pract 10:332-338, 2014 [DOI] [PubMed] [Google Scholar]
- 54.Zafar SY, McNeil RB, Thomas CM, et al. : Population-based assessment of cancer survivors' financial burden and quality of life: A prospective cohort study. JCO Oncol Pract 11:145-150, 2015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Dusetzina SB, Winn AN, Abel GA, et al. : Cost sharing and adherence to tyrosine kinase inhibitors for patients with chronic myeloid leukemia. J Clin Oncol 32:306-311, 2014 [DOI] [PubMed] [Google Scholar]
- 56.Neugut AI, Subar M, Wilde ET, et al. : Association between prescription co-payment amount and compliance with adjuvant hormonal therapy in women with early-stage breast cancer. J Clin Oncol 29:2534-2542, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Lathan CS, Cronin A, Tucker-Seeley R, et al. : Association of financial strain with symptom burden and quality of life for patients with lung or colorectal cancer. J Clin Oncol 34:1732-1740, 2016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Holland JC, Andersen B, Breitbart WS, et al. : Distress management. J Natl Compr Canc Netw 11:190-209, 2013 [DOI] [PubMed] [Google Scholar]
- 59.Meeker CR, Geynisman DM, Egleston BL, et al. : Relationships among financial distress, emotional distress, and overall distress in insured patients with cancer. JCO Oncol Pract 12:e755-e764, 2016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Torous J, Michalak EE, O'Brien HL: Digital health and engagement-looking behind the measures and methods. JAMA Netw Open 3:e2010918, 2020 [DOI] [PubMed] [Google Scholar]
- 61.Lester E, DiStefano S, Mace R, et al. : Virtual mind-body treatment for geographically diverse youth with neurofibromatosis: A pilot randomized controlled trial. Gen Hosp Psychiatry 62:72-78, 2020 [DOI] [PubMed] [Google Scholar]
- 62.Luberto CM, Perez GK, Finkelstein-Fox L, et al. : Acceptability of a virtual mind-body intervention for parents of children with autism or learning disabilities. Glob Adv Health Med 10:216495612110478, 2021 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Fell L, Goshe B, Traeger L, et al. : Acceptability of a virtual mind-body group intervention for teen siblings of children with autism spectrum disorder. J Autism Dev Disord 52:5243-5252, 2022 [DOI] [PubMed] [Google Scholar]
- 64.Kubo A, Kurtovich E, McGinnis M, et al. : A randomized controlled trial of mHealth mindfulness intervention for cancer patients and informal cancer caregivers: A feasibility study within an integrated health care delivery system. Integr Cancer Ther 18:153473541985063, 2019 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Rosen KD, Paniagua SM, Kazanis W, et al. : Quality of life among women diagnosed with breast cancer: A randomized waitlist controlled trial of commercially available mobile app-delivered mindfulness training. Psychooncology 27:2023-2030, 2018 [DOI] [PubMed] [Google Scholar]
- 66.Russell L, Ugalde A, Orellana L, et al. : A pilot randomised controlled trial of an online mindfulness-based program for people diagnosed with melanoma. Support Care Cancer 27:2735-2746, 2019 [DOI] [PubMed] [Google Scholar]
- 67.Mikolasek M, Witt CM, Barth J: Adherence to a mindfulness and relaxation self-care app for cancer patients: Mixed-methods feasibility study. JMIR Mhealth Uhealth 6:e11271, 2018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Tkatch R, Musich S, MacLeod S, et al. : A qualitative study to examine older adults' perceptions of health: Keys to aging successfully. Geriatr Nurs 38:485-490, 2017 [DOI] [PubMed] [Google Scholar]
- 69.Riley TD, Roy S, Parascando JA, et al. : Mindfulness-based stress reduction live online during the COVID-19 pandemic: A mixed methods feasibility study. J Integr Complement Med 28:497-506, 2022 [DOI] [PubMed] [Google Scholar]
- 70.Rock CL, Thomson C, Gansler T, et al. : American Cancer Society guideline for diet and physical activity for cancer prevention. CA Cancer J Clin 70:245-271, 2020 [DOI] [PubMed] [Google Scholar]
- 71.Basso JC, McHale A, Ende V, et al. : Brief, daily meditation enhances attention, memory, mood, and emotional regulation in non-experienced meditators. Behav Brain Res 356:208-220, 2019 [DOI] [PubMed] [Google Scholar]
- 72.Eng L, Liu SY, Zhang Q, et al. : Cancer patient interest and perceptions of health behavior change programs. J Clin Oncol 36, 2018. (suppl 7; abstr 98) [Google Scholar]
- 73.Sajjadi NB, Feldman K, Shepard S, et al. : Public interest and behavior change in the United States regarding colorectal cancer following the death of Chadwick Boseman: Infodemiology investigation of internet search trends nationally and in at-risk areas. JMIR Infodemiology 1:e29387, 2021 [DOI] [PMC free article] [PubMed] [Google Scholar]







