Abstract
Leprosy (Hansen’s disease) is caused by infection with bacilli of the Mycobacterium leprae complex. It is considered an exotic and rare diagnosis in Missouri. Past leprosy patients diagnosed locally have typically acquired it in areas of the world where leprosy is endemic. However, a recent case in a native Missourian that appears to be locally acquired suggests that leprosy may now be endemic in Missouri, possibly due to the expanded range of its zoonotic vector, the nine-banded armadillo. Health care providers in Missouri should be aware of how leprosy manifests and suspected cases referred to centers such as ours for evaluation and early institution of appropriate treatment.
Introduction
Leprosy (Hansen’s disease) is caused by infection of susceptible individuals with acid-fast bacilli (AFB) of the Mycobacterium leprae complex (M. leprae and M. lepromatosis). These rod-shaped AFB were first described by Gerhard Hansen in 1873.1 M. leprae are slow growing organisms that replicate preferentially in macrophages, endothelial cells, and Schwann cells. They are obligate intracellular organisms and do not grow in artificial media cultures. Their ideal growth occurs between 27–33°C. M. lepromatosis is more recently described as an etiologic agent, though its clinical course may be indistinguishable from infection caused by M. leprae.2
Humans are the primary vector for M. leprae. In the Americas the nine-banded armadillo (Dasypus novemcinctus) is recognized as a zoonotic reservoir for the bacteria.3 Like humans, armadillos can develop the full clinical presentation of leprosy including extensive peripheral nerve involvement.4
Importantly more recent work has detected M. leprae in additional species, including chimpanzees,5 and it is possible additional species capable of infection by M. leprae may be identified. M. lepromatosis has been detected in red squirrels in the UK.6, 7 The mechanism of transmission of M. leprae has been somewhat obscure historically due to variability in host susceptibility to infection and a very long potential incubation period. Respiratory secretions are likely the most common route of transmission. Skin contact and vertical transmission are also possible infection mechanisms. The incubation period is quite broad, with estimates ranging from three to five years for tuberculoid leprosy up to 9 to 12 years for lepromatous leprosy. Longer incubation periods have been described. Importantly, most individuals (up to 90% of the population) appear immune or resistant to M. leprae such that most who are exposed do not acquire the infection.
Risk Factors
Notable risk factors for the acquisition of leprosy include close exposure to individuals with lepromatous/multibacillary leprosy, exposure to armadillos,8 immunosuppression/immunodeficiency, and genetic predisposition (which is poorly understood). A recent review noted that the World Health Organization (WHO) program targeting elimination of leprosy is based solely around eliminating human to human transmission and does not account for the potential of spread via zoonotic vectors. Recent publications suggesting a role for armadillos in zoonotic transmission of leprosy in the U.S.3 and the notable increasing range of armadillos in the U.S. over the past several decades due to human induced environmental change9 (Figure 1) suggest that zoonotic vectors cannot be ignored in addressing the spread of leprosy. Traditionally, in Missouri, leprosy has presented in patients who have immigrated from or spent significant time in endemic areas (such as those who have served in the military). However, recent cases of suspected autochthonous transmission in Missouri suggest zoonotic transmission is already occurring in our state (Dyer JA manuscript submitted). Missouri physicians must now consider the possibility of leprosy occurring in local patients with no notable history of foreign travel.
Figure 1.
A roadkill nine-banded armadillo photographed on Stadium boulevard outside of Faurot Field, University of Missouri, Columbia, Mo.
Clinical Features
There are two main classification systems for the clinical presentation of leprosy that guide management and treatment: 1) the Ridley-Jopling classification combines both clinical features, histopathology, and the bacteriologic index (BI); and 2) the WHO classification system is based on the BI or the number of skin lesions. These systems attempt to account for the wide variability in the clinical manifestations of M. leprae infection. The bacteriologic index (BI) is the density of bacilli in a slit-skin smear (SSS) examination (if SSS is not available then number of skin lesions is used as a surrogate). In the WHO classification system patients are classified as paucibacillary leprosy (PB) if they have very few (one to five) skin lesions and multibacillary (MB) if they have more than five skin lesions. A positive BI classifies patients as MB. The Ridley-Jopling system categorizes leprosy patients along a spectrum between tuberculoid leprosy (TT) and lepromatous leprosy (LL). The term “polar” is used to describe patients with all the classical features of TT or LL, indicating that the patient is at the polar end of one side of the clinical spectrum. This system accounts for the clinical variability by including borderline designations for those patients that do not exhibit all the “polar” features of either TT or LL.2 The individual patient’s cell mediated immune response plays a great role in where along this spectrum individual patients fall.
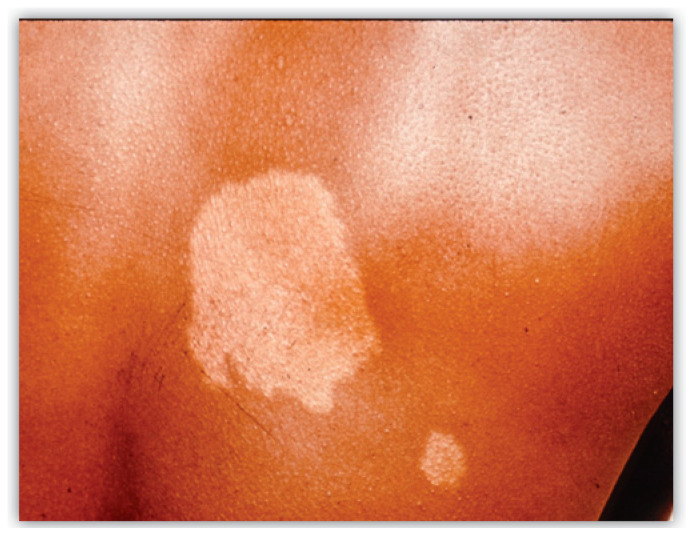
The most common clinical presentation of leprosy is hypopigmented or erythematous patches that exhibit associated loss of sensation (Figure 2). Neural involvement occurs very early in the process and assessing for altered sensation can expedite the diagnosis. Clinical findings are determined by the type of immune response mounted by the patient. TT patients have a vigorous cell mediated immune response (Th1) which impairs proliferation of the bacilli. TT patients have a less severe disease course, often having only a few (or single) hypopigmented well-defined skin lesions (macules or patches/plaques) that show decreased or absent sensation and often loss of hair. TT skin lesions tend to appear erythematous in fair skinned patients and hypopigmented in patients with darker skin types. Borderline patients are unstable in their immune response. Many patients eventually fall into this clinical category.11
Figure 2.
Typical clinical findings of tuberculoid leprosy. Hypopigmented patches with loss of sensation. (Photo courtesy of the University ofMissouri collection)
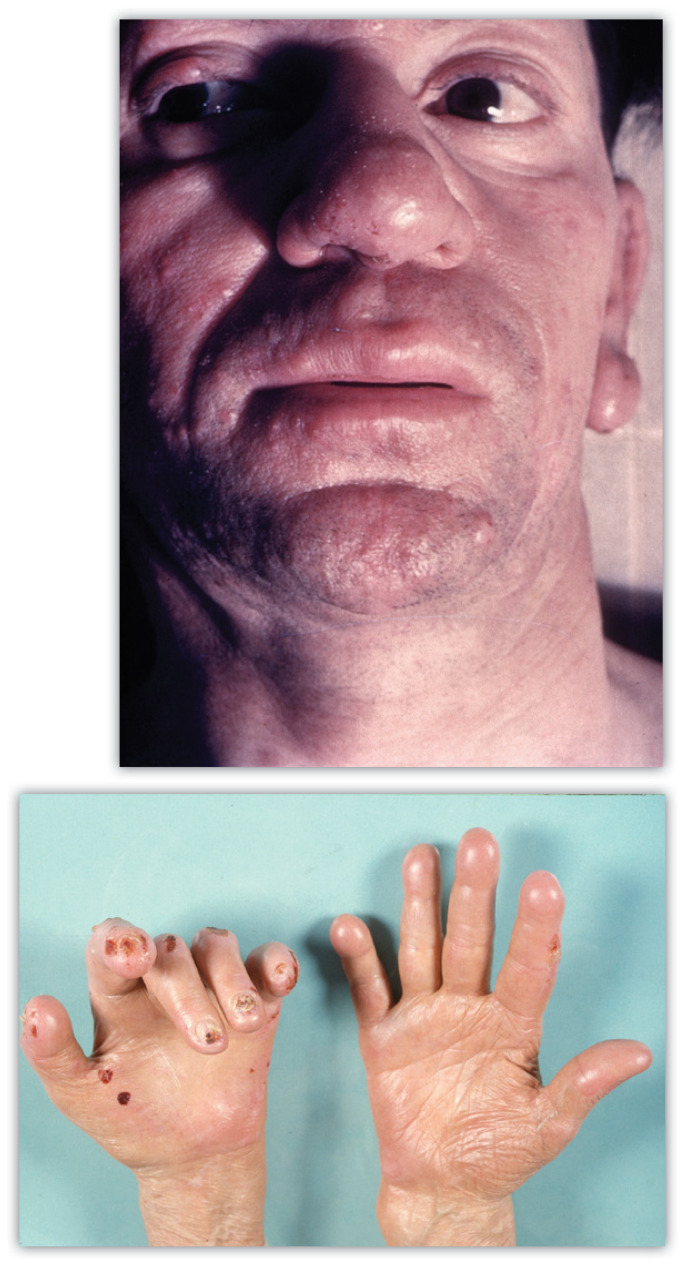
LL patients seem to lack a true cell mediated immune response, allowing the leprosy bacilli to spread widely. Patients may manifest with widespread anesthetic erythematous to brawny indurated papules and plaques. These may be diffuse or focally localized over certain areas of the body. Some scaling may be noted in more involved areas. With time skin infiltration leads to the development of the classic “leonine facies” (Figure 3a). Decreased sweating, hair loss, and anesthesia of involved areas is typically noted. Involved digits and limbs may demonstrate ulceration and distal resorption due to ongoing chronic injury (Figure 3b). Multiple additional organs can be involved, including the adrenal, kidney, testes, and others. Testicular atrophy can lead to gynecomastia in affected males.
Figures 3a and 3b.
Typical clinical findings of lepromatous/ multlbaclllary leprosy.
A-top. Erythematous, indurated granulomatous patches and plaques with facial infiltration and leonine facies.
B-bottom. Taught acral skin with distal digital resorption and ulceration due to loss of sensation (Photo 3a courtesy of the University of Missouri collection)
Ocular
Ocular involvement occurs in over 70% of leprosy patients and assessing its presence and severity is important for patient management. Blindness may result in ~5%. There can be direct damage to the ocular nerves (ophthalmic and/or facial) and/or invasion of the anterior ocular chamber by the bacilli. Lagophthalmos (decreased eyelid closure) is the most common clinical finding, with other findings, such as corneal abrasion or ulceration, related to impaired sensation.
Mucosal Involvement
Nasal mucosal involvement is quite common in LL, likely because it is one of the main transmission sites for the bacillus. Oral mucosal lesions are nonspecific but may eventually ulcerate.2, 11
Diagnosis
The diagnosis of leprosy is based on clinical findings along with results of a SSS or skin biopsy. Importantly, a recent review highlighted that failure to consider leprosy in the differential diagnosis for patients is often the greatest impediment to making the diagnosis. Leprosy would not be a diagnosis typically considered for granulomatous skin eruptions in patients from Missouri, but it now must be considered a possibility, even in patients with a negative travel history. In a patient with a suspicious skin eruption, a thorough history, including all travel, should be taken. Loss of or decrease in sensation in a hypopigmented or erythematous skin patch should raise concern for leprosy.
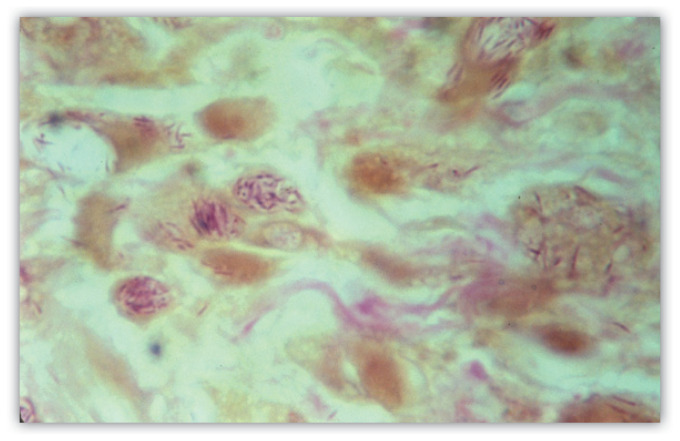
Nerve enlargement and/or muscle weakness in the nerve distribution may also be seen. Only peripheral nerves are involved, commonly the ulnar or peroneal nerve in more advanced cases. Patients may exhibit absent sensation throughout the areas supplied by the affected nerve. Sensation may be assessed using monofilaments identical to those used to screen for diabetic neuropathy (monofilament testing was initially developed to assess and screen leprosy patients and was only later adapted to assess other forms of neuropathy). SSS exam should be performed. While SSS are often negative, in positive cases it is quite helpful. The most active lesions should be targeted although high yield anatomic sites (often cooler areas of the body such as the earlobes) can also be sampled. Instructions for performing a proper SSS exam may be found on the website of the National Hansen’s Disease Center (NHDC: https://www.hrsa.gov/hansens-disease/diagnosis/skin-smears). Confirmatory skin biopsy with polymerase chain reaction (PCR) studies to screen for M. leprae complex DNA are more commonly used. Patients with TT typically exhibit epithelioid granulomas, lymphocytic infiltration around adnexae and nerves, and very few (often absent) AFB on histologic examination. In LL histocyte collections are diffuse and not organized. Histiocytes with foamy cytoplasm (Virchow cells) with large numbers of AFB are typical. Clumps of bacilli are called globi (Figure 4). AFB within peripheral nerves in a biopsy specimen is diagnostic. While skin biopsy is not necessary for diagnosing leprosy it can be a valuable adjunct study in these patients.2, 10, 11
Figure 4.
A positive slit skin smear from a patient with multibacillary leprosy. Acid fast bacilli (AFB) are visible. Groups of organisms (globi) are present.
Polymerase Chain Reaction
Polymerase chain reaction (PCR) is used to support the suspicion of a clinical diagnosis of leprosy. PCR is highly sensitive in patients with a positive BI, however in those with negative BI it can be much more variable. The National Hansens Disease Program (NHDP) performs these free of charge (contact information at end of article).
Additional Diagnostic Procedures
Nerve conduction studies may be utilized to assess the degree of nerve involvement and treatment response. Ultrasound of high yield peripheral nerves has been increasingly utilized in recent decades to objectively measure nerve thickening in suspected cases.11
Differential Diagnosis
The differential diagnosis for leprosy is broad and includes several innocuous conditions (Table 1). Assessing sensation in involved areas in any cutaneous eruption where leprosy is in the differential is critical to minimize delays in diagnosis and treatment of affected patients. However, neural involvement can be subtle, especially in early cases, and skin biopsy can be helpful. In Missouri patients eventually diagnosed with leprosy had been previously diagnosed with pityriasis alba, post-inflammatory hypopigmentation from eczema, granuloma annulare, cutaneous sarcoidosis, and diabetic neuropathy with ulceration among other diagnoses.12 Anesthesia of the involved areas was often a clinical clue.
Table 1.
Differential diagnosis of leprosy subtypes:
| Leprosy subtype | Differential diagnostic considerations |
|---|---|
| Tuberculoid; Indeterminate | Pityriasis alba; post-inflammatory hypopigmentation; vitiligo; segmental vitiligo; tinea versicolor; nummular eczema |
| Borderline tuberculoid | Granuloma annulare; interstitial granulomatous dermatitis; disseminated granuloma annulare; sarcoidosis; erythema annulare centrifugum; tinea corporis |
| Mid-borderline and borderline lepromatous | Necrobiosis lipoidica; granuloma annulare; necrobiotic xanthogranuloma; gummatous syphilis; Sweet’s syndrome; lichen planus; ichthyosis vulgaris; parapsoriasis; mycosis fungoides; mastocytosis; morphea |
| Lepromatous | Alternative cutaneous mycobacterial infections (tuberculosis; M. marinum; M. avium-intracullare…etc); deep fungal infection (sporotrichosis; blastomycosis; chromoblastomycosis; lobomycosis); leishmaniasis; erythema elevatum diutinum; xanthogranulomas; trichoepitheliomas; verrucous lesions. |
| Isolated neural | Peripheral nerve sheath tumor |
Adapted from: Moschella SL, Garcia-Albea V. Differential diagnosis of leprosy. In: Scollard DM, Gillis TP, eds. International Textbook of Leprosy. Available at: https://www.internationaltextbookofleprosy.org/. Accessed August 12, 2022
Immune Reactions
Immune reactions to the M. leprae bacilli are one of the major causes of morbidity and even mortality in leprosy patients. The sudden activation of a more pronounced immune response can lead to significant inflammatory reactions, often involving infected nerves and worsening existing nerve damage. In severe cases it can lead to great morbidity or even death. Development of these immune reactions may prompt patients to seek medical care, often for the first time. Clinicians should be aware of how such reactions can manifest. There are three main types of leprosy reactions, Type 1 reactions, type 2 reactions, and the rare Lucio phenomenon. They are considered medical emergencies though there is a great range in their severity. Early recognition and treatment is mandatory for limiting morbidity.10
Type 1 reactions (T1R) manifest as subacute to acute inflammation of the skin and nerves. They result from a sudden increase in cell-mediated immunity and delayed-type hypersensitivity to antigens from the M. leprae complex bacilli. These reactions can occur at any time, including long after treatment has ceased. They occur in 30–50% of patients. Involved skin areas become red and edematous. Swelling of the limbs and face may occur. T1R are the main cause of nerve damage in leprosy patients and may present with sudden worsening of nerve palsies, paresthesia, foot drop and facial palsy. Diagnosis of these reactions is clinical, and they may be the reason for initial presentation of the patient. Systemic corticosteroids are the mainstay of therapy.
Type 2 reactions (T2R; erythema nodosum leprosum (ENL)) present as recurrent outbreaks of superficial and deep painful erythematous nodules. These may progress to ulceration with purulent discharge (mimicking erythema induratum). It is more common in LL patients. Lesions often occur on the extremities. Histopathology from an early lesion (first 24–72 hours) may show a neutrophilic infiltrate which can help confirm the diagnosis. Systemic symptoms may accompany the outbreaks such as fever, arthralgia, neuritis, malaise and lymphadenitis. ENL episodes are typically one to three weeks in duration. If present for more than six months it is considered chronic ENL. A high BI is a notable risk factor. Mild cases may respond to nonsteroidal anti-inflammatory drugs (NSAIDs) but any nerve involvement should prompt the use of systemic corticosteroids. Thalidomide is a drug of choice for ENL but is hard to obtain and expensive.
Lucio phenomenon is rare in the U.S., occurring in patients with diffuse LL. It is a thrombotic reaction characterized by the development of purpuric and discolored skin lesions that become necrotic and heal with atrophic scars. Histopathology can be helpful in the diagnosis 10,11
Conclusion
Leprosy is considered a rare and exotic diagnosis, something the clinician will see only once or twice in a career. Recently patients with suspected autochthonous acquisition of leprosy in Missouri have been identified. This is not surprising considering the rapid expansion of the range of nine banded armadillos, the classic zoonotic vector for leprosy, due to human induced environmental change. In fact it is to be expected and has been anticipated in our department for the past decade. High clinical suspicion is key to the early diagnosis of leprosy. Recognizing the various clinical presentations of leprosy and assessing clinically suspicious lesions for loss of sensation is within the scope of all providers. At the University of Missouri our Dermatology Department has partnered with the Division of Infectious Disease and Department of Neurology to form the Missouri Hansen’s Disease Resource Center. Clinicians with suspected cases of Hansen’s disease are encouraged to reach out to our faculty for questions, input, teleconsultation, and/or clinical evaluation as part of our ongoing research studies on Hansen’s disease in the state of Missouri. Our Dermatopathology lab can process SSS and skin biopsies and perform all staining necessary for these evaluations. We have several ongoing projects investigating the prevalence of Hansen’s disease and its transmission in our state.
Resources
Missouri Hansen’s Disease Resource Center: c/o University of Missouri Department of Dermatology; phone: 573-882-8578; fax: 573-884-5947; email: dyerja@health.missouri.edu
National Hansen’s Disease Resource Center: https://www.hrsa.gov/hansens-disease
International Textbook of Leprosy: Free, comprehensive, and up to date online resource: Scollard DM, Gillis TP, eds. International Textbook of Leprosy. Available at: https://www.internationaltext bookofleprosy.org/. Accessed August 12, 2022.
Footnotes
Andrea Gilmore, MD, and James Roller, MD, are in the Department of Dermatology, University of Missouri-Columbia School of Medicine. Jonathan A. Dyer, MD, (above) is the Philip C. Anderson Chair and Professor Departments of Dermatology and Child Health, also at University of Missouri-Columbia, Columbia, Missouri.
Disclosure
None reported.
References
- 1.Hansen GA. On the Etiology of Leprosy, British and Foreign Medico-Chirurgical Review. 1875;55:459–89. [PMC free article] [PubMed] [Google Scholar]
- 2.Maymone M, Venkatesh S, Laughter M, et al. Leprosy: Clinical aspects and diagnostic techniques. J Am Acad Dermatol. 2020;83:1–14. doi: 10.1016/j.jaad.2019.12.080. [DOI] [PubMed] [Google Scholar]
- 3.Truman RW, Singh P, Sharma R, et al. Probable zoonotic leprosy in the southern United States. N Engl J Med. 2011;364:1626–1633. doi: 10.1056/NEJMoa1010536. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Pena MT, Lahiri R, Ebenezer GJ, et al. The Armadillo as a Model for Leprosy Nerve Function Impairment: Preventative and Therapeutic Interventions. Front Med. 2022;9:879097. doi: 10.3389/fmed.2022.879097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Hockings KJ, Mubemba B, Avanzi C, et al. Leprosy in wild chimpanzees. Nature. 2021;598:652–656. doi: 10.1038/s41586-021-03968-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Meredith A, Del Pozo J, Smith S, Milne E, Stevenson K, McLuckie J. Leprosy in red squirrels in Scotland. Vet Rec. 2014;175:285–286. doi: 10.1136/vr.g5680. [DOI] [PubMed] [Google Scholar]
- 7.Avanzi C, Del-Pozo J, Benjak A, et al. Red squirrels in the British Isles are infected with leprosy bacilli. Science. 2016;354:744–747. doi: 10.1126/science.aah3783. [DOI] [PubMed] [Google Scholar]
- 8.da Silva MB, Portela JM, Li W, et al. Evidence of zoonotic leprosy in Para, Brazilian Amazon, and risks associated with human contact or consumption of armadillos PLoS Negl Trop Dis 201812e0006532 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Taulman JF, Robbins L. Range expansion and distributional limits of the nine-banded armadillo in the United States: an update of Taulman & Robbins 1996. Journal of Biogeography. 2014;41:1626–1630. [Google Scholar]
- 10.Maymone M, Venkatesh S, Laughter M, et al. Leprosy: Treatment and management of complications. J Am Acad Dermatol. 2020;83:17–30. doi: 10.1016/j.jaad.2019.10.138. [DOI] [PubMed] [Google Scholar]
- 11.Scollard DM, Gillis TP, editors. [Accessed August 12, 2022];International Textbook of Leprosy. Available at: https://www.internationaltextbookofleprosy.org/ [Google Scholar]
- 12.Westblom TU, Roller JA. Leprosy in Missouri. Mo Med. 1987;84(11):699–701. [PubMed] [Google Scholar]