ABSTRACT
Objective:
To compare the effects of a home-based pulmonary rehabilitation (PR) program with and without telecoaching on health-related outcomes in COVID-19 survivors.
Methods:
A total of 42 COVID-19 patients who completed medical treatment were randomly divided into two groups: the study (telecoaching) group (n = 21) and the control (no telecoaching) group (n = 21). Both groups participated in an 8-week home-based PR program including education, breathing exercises, strength training, and regular walking. The study group received phone calls from a physiotherapist once a week. Both groups of patients were assessed before and after the program by means of the following: pulmonary function tests; the modified Medical Research Council dyspnea scale; the six-minute walk test; extremity muscle strength measurement; the Saint George’s Respiratory Questionnaire (to assess disease-related quality of life); the Medical Outcomes Study 36-item Short-Form Health Survey (SF-36, to assess overall quality of life); and the Hospital Anxiety and Depression Scale.
Results:
In both groups, there were significant improvements in the following: FVC; the six-minute walk distance; right and left deltoid muscle strength; Saint George’s Respiratory Questionnaire activity domain, impact domain, and total scores; and SF-36 social functioning, role-physical, role-emotional, and bodily pain domain scores (p < 0.05). Decreases in daily-life dyspnea, exertional dyspnea, and exertional fatigue were significant in the study group (p < 0.05), and the improvement in SF-36 social functioning domain scores was greater in the study group (p < 0.05).
Conclusions:
A home-based PR program with telecoaching increases social functioning and decreases daily-life dyspnea, exertional dyspnea, and exertional fatigue in COVID-19 survivors in comparison with a home-based PR program without telecoaching.
Keywords: COVID-19, Exercise, Telerehabilitation, Dyspnea, Fatigue
RESUMO
Objetivo:
Comparar os efeitos de um programa de reabilitação pulmonar (RP) domiciliar com e sem coaching por telefone (telecoaching) nos desfechos relacionados à saúde em sobreviventes da COVID-19.
Métodos:
Um total de 42 pacientes com COVID-19 que completaram o tratamento médico foram aleatoriamente divididos em dois grupos: o grupo com telecoaching (grupo de estudo; n = 21) e o grupo sem telecoaching (grupo controle; n = 21). Ambos os grupos participaram de um programa de RP domiciliar que teve 8 semanas de duração e incluiu educação, exercícios respiratórios, treinamento de força e caminhada regular. O grupo de estudo recebeu telefonemas de um fisioterapeuta uma vez por semana. Ambos os grupos foram avaliados antes e depois do programa por meio de testes de função pulmonar, escala modificada de dispneia do Medical Research Council, teste de caminhada de seis minutos, mensuração da força muscular dos membros superiores e inferiores, Saint George’s Respiratory Questionnaire (para avaliar a qualidade de vida relacionada à doença), Medical Outcomes Study 36-item Short-Form Health Survey (SF-36, para avaliar a qualidade de vida global) e Hospital Anxiety and Depression Scale.
Resultados:
Em ambos os grupos, houve melhoria significativa da CVF; da distância percorrida no teste de caminhada de seis minutos; da força dos músculos deltoides direito e esquerdo; da pontuação obtida nos domínios “atividade” e “impacto” do Saint George’s Respiratory Questionnaire, bem como da pontuação total no questionário; e da pontuação obtida nos domínios “aspectos sociais”, “função física”, “função emocional” e “dor corporal” do SF-36 (p < 0,05). A redução da dispneia na vida diária, da dispneia aos esforços e da fadiga aos esforços foi significativa no grupo de estudo (p < 0,05), e a melhoria da pontuação obtida no domínio “aspectos sociais” do SF-36 foi maior no grupo de estudo (p < 0,05).
Conclusões:
Um programa de RP domiciliar com telecoaching melhora os aspectos sociais e diminui a dispneia na vida diária, a dispneia aos esforços e a fadiga aos esforços em sobreviventes da COVID-19 em comparação com um programa de RP domiciliar sem telecoaching.
Descritores: COVID-19, Exercício físico, Telerreabilitação, Dispneia, Fadiga
INTRODUCTION
COVID-19, a highly contagious respiratory disease, has spread rapidly worldwide, posing a devastating threat to health, economy, and lifestyle. 1 , 2 COVID-19 predominantly affects the respiratory system. Although most patients are asymptomatic, the disease can show clinical progress ranging from upper respiratory tract symptoms to life-threatening severe pneumonia. 3 Flu-like symptoms such as fever, dyspnea, fatigue, cough, expectoration, sore throat, and headache are common in infected individuals. 4 Patients with COVID-19, whether hospitalized or not, continue to have multiple symptoms, particularly dyspnea and fatigue, even approximately 3 months after the onset of symptoms. This indicates the presence of a “post-COVID-19 syndrome” and highlights the unmet health care needs of patients with mild or severe COVID-19. 5
Long-term treatment of critically ill patients in the ICU or inpatient ward, bed rest, continuous quarantine, and social distancing lead patients to be inactive for extended periods, 6 resulting in a decrease in muscle mass and strength; changes in muscle fibers; remodeling of muscle tissue; fatigue; and inflammation. 7 The unpredictability of the disease state, the uncertainty of effective treatment methods, and being afflicted with a deadly disease cause stress. Anxiety, depression, and stress disorder are quite common and severe in patients hospitalized for COVID-19. 8
In patients with COVID-19, pulmonary rehabilitation (PR) facilitates patient follow-up, strengthens health management, and helps patients recover and return to society more quickly and safely. 9 Telehealth practices are the most appropriate method because of the risk of transmission and because they allow social distancing during the COVID-19 pandemic. 10 Pulmonary telerehabilitation has been shown to be as beneficial as conventional PR in patients with chronic respiratory diseases such as COPD, interstitial lung disease, and bronchiectasis, with no safety issues. 11 Studies have described different methods of delivering PR, such as over the phone, through mobile phone applications, through videoconferencing, and through websites. 11 Although home-based programs and telerehabilitation applications are commonly used in the management of COVID-19, there is inadequate information on the best strategy for PR. The objective of the present study was to evaluate whether adding telecoaching to a home-based PR program would have any impact on the effectiveness of the program. To that end, we compared the effects of PR with and without telecoaching on dyspnea, exercise capacity, peripheral muscle strength, quality of life, and psychological symptoms in COVID-19 survivors.
METHODS
Study setting and participants
This was a randomized controlled study conducted between February of 2021 and July of 2021. The study was approved by the Research Ethics Committee of the Dr. Suat Seren Chest Diseases and Thoracic Surgery Training and Research Hospital (Protocol no. E-49109414-604.02) and registered at https://clinicaltrials.gov (NCT04791072). All participating patients gave written informed consent. The inclusion criteria were as follows: having been diagnosed with COVID-19; having stayed in the ICU or ward for more than 10 days with or without the need for invasive mechanical ventilation (IMV); having received noninvasive mechanical ventilation (NIMV); having received high-flow oxygen therapy; and having completed medical treatment. COVID-19 patients who received outpatient pharmacological treatment before hospitalization, those who experienced dyspnea for the first time ever because of the disease, and those in whom dyspnea continued despite treatment were also included. 12
An effort was made to include post-acute COVID-19 patients (i.e., those with persistent symptoms at 4 weeks after the onset of symptoms). 13 Patients who were past the post-acute phase, those who had orthopedic problems, those who were receiving treatment for active cancer, and those who declined to participate were excluded from the study. In addition, patients with cardiac and thromboembolic complications were excluded from the study because of their ongoing medical treatment and the potentially harmful effects of exercise. 14
The G*Power software, version 3.1.9.7 (Heinrich Heine University, Düsseldorf, Germany), was used in order to determine the required sample size for the study. It was calculated that at least 21 participants were needed in each group to obtain a power of 80%, with an effect size of 0.80 and a type I error of 0.05, to identify intergroup differences. 15
Procedure
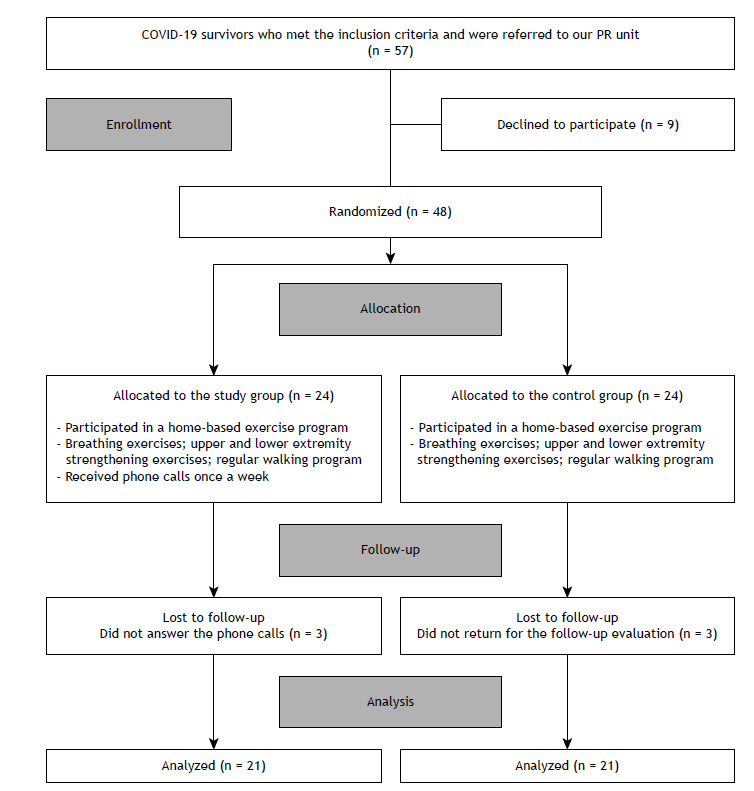
The flow chart of the study is shown in Figure 1. The patients referred to our PR unit were divided into two groups (the study [telecoaching] group and the control [no telecoaching] group) by means of a randomization program. 16 Breathing exercises, as well as exercises focusing on lower and upper extremity strengthening, were prescribed to both groups by a physiotherapist as part of a home-based PR program. All patients were asked to keep an exercise diary to evaluate their compliance with the program. Although the control group was not contacted, the study group was contacted every Monday by a physiotherapist to collect information about their adherence to the program; a motivational speech was made, and the importance of the program was emphasized. During the phone calls, the physiotherapist talked to the patients about the problems that they experienced when doing the exercises and suggested solutions. One researcher, who was blinded to which group the patients were in, entered the data and performed all statistical analyses.
Figure 1. Flow chart of the study. PR: pulmonary rehabilitation.
Outcomes
A detailed anamnesis was obtained from the patients, and their demographic and clinical characteristics were recorded. The Charlson Comorbidity Index was calculated for all patients before SARS-CoV-2 infection. 17 Patients were asked whether they had received mechanical ventilation or high-flow oxygen therapy during hospitalization. The respiratory system was evaluated by a pulmonologist, and the cardiovascular system was evaluated by a cardiologist. Chest X-ray and CT findings were reported by a radiologist at our hospital. Functional assessment was performed in person (in our PR unit) for both groups before and after PR.
The primary outcome of this study was the six-minute walk distance (6MWD). Secondary outcomes included the following: respiratory function; upper and lower extremity muscle strength; perception of dyspnea; quality of life; and psychological symptoms (anxiety and depression).
Exercise capacity
Exercise capacity was determined by the six-minute walk test. Patients were asked to walk as far as possible along a 30-m straight corridor for a period of 6 min. 18
Upper and lower extremity muscle strength
Upper and lower extremity muscle strength was tested against the resistance of the assessing physiotherapist, being graded from 0 (no contraction) to 5 (normal strength). The movement pattern required for each measurement was explained to the patient and demonstrated. The patient was asked to perform the movement in the entire range against gravity. If the patient was able to complete the movement through the full available range against gravity, the joint was placed at the appropriate angle, and resistance was applied gradually. Appropriate feedback and encouragement were given during measurement to promote greater effort. Measurements were made on each patient, first on the right side and then on the left side.
The biceps and deltoid muscles were evaluated for upper extremity muscle strength, and the quadriceps muscle was evaluated for lower extremity muscle strength. 19
Respiratory function
A body plethysmograph (Zan 500; nSpire Health, Inc., Longmont, CO, USA) was used in order to evaluate respiratory function. The following parameters were assessed and recorded: FEV1, FVC, and FEV1/FVC.
Dyspnea
The modified Medical Research Council (mMRC) dyspnea scale, 20 consisting of five grades, was used in order to determine the severity of dyspnea, with 0 being the best grade and 4 being the worst grade. The modified Borg scale, 21 with a scoring system ranging from 0 to 10, was used in order to evaluate dyspnea and fatigue on exertion, with 0 being the best score and 10 being the worst score.
Psychological symptoms
The Hospital Anxiety and Depression Scale, consisting of 14 questions, was used in order to determine the psychological status of patients. A score of 0-7 indicated a normal status; a score of 8-11 indicated a borderline status; and a score > 11 indicated anxiety or depression. 22
Quality of life
The Saint George’s Respiratory Questionnaire (SGRQ) was used in order to determine disease-specific quality of life. High scores indicated worsening of the disease and an increase in symptoms. 23 The Medical Outcomes Study 36-item Short-Form Health Survey (SF-36) was used in order to measure the overall quality of life. A higher score translated to a better quality of life. 24
Home-based PR program
Our home-based PR program was explained to all patients in detail by a physiotherapist in our PR unit. All patients were asked to keep an exercise diary. The home-based program included breathing exercises, strength training, and a regular walking program. Table 1 summarizes the program. Bronchial hygiene techniques were taught to the patients in need by means of pulmonary auscultation. Breathing exercises included pursed-lip breathing, diaphragmatic breathing, and thoracic expansion exercises. Strengthening exercises were performed with free weights for the biceps and deltoid muscles (in the upper extremity) and the quadriceps and gastrocnemius muscles (in the lower extremity). In addition, for the lower extremities, squat and calf-raise exercises were given, with each exercise being repeated 8-10 times per set, 1-2 sets per day, 5-7 days per week. All strengthening exercises were started without weight. During the program, half a kilogram of weight was added to every 6 periods of exercise on the basis of a modified Borg scale score of ≤ 3. All patients were given an exercise booklet. A 2-min break was given between exercises for resting. Patients participated in a walking exercise program for a total of 20-30 min in an outdoor environment without a slope or in an indoor environment with good ventilation and a slope. The exercise was adjusted according to the target HR and the modified Borg scale score. 25 , 26 The exercise duration was increased when dyspnea and fatigue perception scores were ≤ 3 on the modified Borg scale. The patients were instructed to keep their oxygen saturation above 90% and their HR below 124 bpm in order to perform the exercise within safe limits and avoid cardiopulmonary complications. 26
Table 1. Home-based pulmonary rehabilitation program for COVID-19 survivors.
| Breathing exercises | • Techniques: diaphragmatic breathing, pursed-lip breathing, thoracic expansion exercises • Frequency: 8-10 repetitions per set, 1-2 sets per day, 5-7 days per week |
| Strength training | • Intensity: perceived fatigue of ≤ 3 on the modified Borg scale • Frequency: 8-10 repetitions per set, 1-2 sets per day, 5-7 days per week • Progression: Weights were increased when perceived dyspnea and fatigue were ≤ 3 on the modified Borg scale. |
| Walking program | • Intensity: Fatigue was targeted at ≤ 3 on the modified Borg scale. • Frequency: 3-5 days per week, 20-30 min per day • Progression: Walking speed and increased duration targeting a score of ≤ 3 on the modified Borg scale for perceived dyspnea and fatigue. |
The patients were reevaluated 8 weeks after completing the home-based exercise program, and telecoaching was added to the study group. Respiratory function, perception of dyspnea, psychological symptoms, and quality of life were evaluated by the same pulmonologist, and exercise capacity, muscle strength, and compliance with the program were evaluated by the same physiotherapist. The exercise diary kept by the patients was used in order to evaluate their compliance with the program.
Statistical analysis
All statistical analyses were performed with the IBM SPSS Statistics software package, version 20.0 (IBM Corporation, Armonk, NY, USA). The normality of the data was evaluated by the Shapiro-Wilk test and histograms. Descriptive statistics were reported as mean ± standard deviation, median (interquartile range), or proportion. Results were reported by comparing post-intervention and baseline values. The independent sample t-test or the Mann-Whitney U test was used in order to compare baseline characteristics, and two-way ANOVA with Bonferroni correction was used in order to compare variables before and after treatment in each group. A value of p < 0.05 was considered statistically significant.
RESULTS
Of the 57 patients who met the inclusion criteria, 9 declined to participate in the study. The remaining 48 patients were randomized into study (telecoaching) and control (no telecoaching) groups. Of those 48 patients, 3 (all of whom were in the study group) did not answer the phone calls. A total of 3 controls were unable to return to our PR unit for the follow-up evaluation: 2 moved to another city, and 1 had a vertigo attack. Therefore, 6 patients (3 from each of the two groups) were excluded from the analysis. A total of 42 COVID-19 patients therefore completed the study. Of those, 21 were in the study group and 21 were in the control group (Figure 1). The baseline values for those who withdrew from the study were similar to those for those who remained in the study (p > 0.05).
The rate of compliance with the exercise program was 90% in the study group and 70% in the control group. After receiving feedback from study and control group patients at the end of the program, we determined that they did their breathing exercises fully but failed to comply with the walking program.
The demographic and physical characteristics of the patients in the study and control groups were similar (p > 0.05), the exception being that the control group comprised older patients (p = 0.009). The number of patients with bilateral radiological findings was higher in the study group (p = 0.048). In addition, 2 of the patients in the study group had nodules, and 1 had pleurisy, which was not present in the control group. The number of patients receiving home oxygen therapy was higher in the control group (p = 0.044). The hospital and ICU length of stay, the number of patients who received IMV or NIMV, and the number of patients who received high-flow oxygen therapy were similar in both groups (p > 0.05; Table 2).
Table 2. Comparison of sociodemographic and clinical characteristics between study and control group patients.a .
| Variable | Group | p | |
|---|---|---|---|
| Study | Control | ||
| (n = 21) | (n = 21) | ||
| Male | 13 (68.4) | 15 (78.9) | 0.714* |
| Age, years | 57.67 ± 8.42 | 63.67 ± 7.90 | 0.009† |
| BMI, kg/m2 | 29.98 ± 6.37 | 28.75 ± 3.51 | 0.352† |
| Smoking status Current smoker Former smoker Never smoker |
1 (5.3) 8 (42.1) 10 (52.6) |
0 (0.0) 13 (68.4) 6 (31.6) |
0.203* |
| Smoking history, pack-years | 36.33 ± 21.73 | 37.50 ± 23.40 | 0.913† |
| Presence of comorbidity | 14 (73.7) | 13 (68.4) | 0.721* |
| CCI | 0 [0-1] | 1 [0-1] | 0.488 |
| Radiological finding Bilateral Unilateral Pleural effusion Ground-glass opacity Nodule |
14 (73.6) 8 (42.1) 1 (5.2) 14 (73.6) 2(10.5) |
8 (42.1) 11 (57.8) 0 (0.0) 16 (84.2) 0 (0.0) |
0.048 0.330 - 0.633 - |
| Hospital LOS, days | 12 [5-15] | 11 [8-14] | 0.975‡ |
| ICU LOS, days | 0 [0-11] | 2.5 [0-9] | 0.638‡ |
| NIMV | 6 (31.6) | 9 (47.4) | 0.319* |
| IMV | 2 (10.5) | 3 (15.8) | 0.631* |
| HFOT | 3 (15.8) | 5 (26.3) | 0.473* |
| HOT | 9 (47.4) | 15 (78.9) | 0.044* |
CCI: Charlson Comorbidity Index; LOS: length of stay; NIMV: noninvasive mechanical ventilation; IMV: invasive mechanical ventilation; HFOT: high-flow oxygen therapy; and HOT: home oxygen therapy. aValues expressed as n (%), mean ± SD, or median [IQR]. *Pearson’s chi-square test. †Independent sample t-test. ‡Mann-Whitney U test.
Table 3 provides a between-group comparison of outcome measures before and after the home-based PR program. In the study and control groups, there was a significant increase in the following: FVC (in % of predicted); 6MWD; right and left deltoid muscle strength; SGRQ activity domain, impact domain, and total scores; and SF-36 social functioning, role-physical, role-emotional, and bodily pain domain scores (p < 0.05). Daily-life dyspnea (as measured by the mMRC scale), exertional dyspnea and fatigue (as measured by the modified Borg scale), lower extremity muscle strength, and SF-36 role-physical and bodily pain domain scores decreased significantly, although only in the study group (p < 0.05). Figure 2 shows the changes in the 6MWD, mMRC scale scores, exertional dyspnea, and exertional fatigue before and after PR in the study and control groups.
Table 3. Comparison of pre- and post-rehabilitation outcome measures.a .
| Variable | Study group (n = 21) |
Control group (n = 21) |
F | p‡ | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Before PR | After PR | ∆ | F | p† | Before PR | After PR | ∆ | F | p† | |||
| PFT FEV1 (% predicted) FVC (% predicted) FEV1/FVC |
85.05 ± 4.44 83.94 ± 3.76 83.47 ± 3.92 |
89.63 ± 4.96 90.94 ± 4.11 86.76 ± 4.05 |
4.57 ± 2.41 7.01 ± 2.72 3.28 ± 2.73 |
3.586 6.619 1.445 |
0.066 0.014 0.237 |
80.00 ± 4.44 77.47 ± 3.76 86.15 ± 3.92 |
84.68 ± 4.96 83.57 ± 4.11 86.84 ± 4.05 |
4.68 ± 2.41 6.10 ± 2.72 0.68 ± 2.73 |
3.753 5.035 0.063 |
0.061 0.031 0.804 |
0.603 1.476 0.234 |
0.443 0.232 0.632 |
| mMRC scale | 2.63 ± 0.33 | 1.21 ± 0.300 | 1.42 ± 0.24 | 33.38 | < 0.001 | 2.31 ± 0.33 | 1.84 ± 0.30 | 0.474 ± 0.24 | 3.710 | 0.062 | 0.143 | 0.708 |
| Six-minute walk test 6MWD ∆Dyspnea (mBORG) ∆Leg fatigue (mBORG) |
378.1 ± 28.9 2.368 ± 0.38 2.158 ± 0.27 |
440.9 ± 25.8 1.368 ± 0.31 1.263 ± 0.29 |
62.8 ± 15.1 1.00 ± 0.31 0.89 ± 0.30 |
17.222 10.09 8.862 |
< 0.001 0.003 0.005 |
325.1 ± 28.9 2.051 ± 0.38 1.789 ± 0.27 |
381.7 ± 25.8 2.158 ± 0.31 1.787 ± 0.29 |
56.6 ± 15.1 0.10 ± 0.31 0.02 ± 0.01 |
14.013 0.112 0.001 |
0.001 0.740 0.998 |
1.671 0.336 0.934 |
0.204 0.565 0.340 |
| Muscle strength Right biceps Left biceps Right deltoid Left deltoid Right quadriceps Left quadriceps |
4.737 ± 0.12 4.737 ± 0.12 4.422 ± 0.15 4.474 ± 0.14 4.737 ± 0.09 4.737 ± 0.09 |
4.947 ± 0.03 4.947 ± 0.03 4.895 ± 0.07 4.895 ± 0.11 5.00 ± 0.03 5.00 ± 0.00 |
0.211 ± 0.12 0.211 ± 0.12 0.474 ± 0.12 0.421 ± 0.11 0.263 ± 0.08 0.105 ± 0.13 |
3.097 3.064 13.75 14.58 8.654 7.627 |
0.087 0.089 0.001 0.001 0.006 0.009 |
4.572 ± 0.12 4.522 ± 0.12 4.474 ± 0.15 4.368 ± 0.14 4.842 ± 0.09 4.842 ± 0.09 |
5.00 ± 0.037 5.00 ± 0.037 4.842 ± 0.07 4.632 ± 0.11 4.947 ± 0.03 5.00 ± 0.00 |
0.421 ± 0.12 0.474 ± 0.12 0.368 ± 0.120 0.263 ± 0.11 0.105 ± 0.08 0.001 ± 0.01 |
12.387 15.51 8.321 5.696 1.385 2.746 |
0.001 < 0.001 0.007 0.001 0.247 0.106 |
0.298 0.664 0.062 0.250 0.610 0.610 |
0.589 0.421 0.805 0.620 0.440 0.440 |
| SGRQ Symptoms Activity Impact Total |
40.78 ± 6.19 62.56 ± 4.85 41.46 ± 5.69 47.77 ± 4.97 |
35.25 ± 5.23 49.06 ± 6.32 30.05 ± 5.77 36.67 ± 5.45 |
−5.52 ± 3.61 −13.49 ± 4.70 −11.41 ± 3.42 −11.09 ± 2.87 |
2.340 8.244 11.07 14.94 |
0.135 0.007 0.002 <0.001 |
36.55 ± 6.19 65.19 ± 4.85 47.24 ± 5.69 50.91 ± 4.97 |
31.38 ± 5.23 52.95 ± 6.32 33.3 ± 5.77 39.03 ± 5.45 |
−5.202 ± 3.61 −12.24 ± 4.70 −13.86 ± 3.42 −11.88 ± 2.87 |
2.070 6.783 16.34 17.14 |
0.159 0.013 < 0.001 < 0.001 |
0.229 0.148 0.516 0.200 |
0.635 0.703 0.477 0.657 |
| SF-36 Physical functioning Social functioning Role-physical Role-emotional General health Mental health Bodily pain Vitality |
52.36 ± 6.25 32.23 ± 6.22 15.78 ± 6.90 24.36 ± 7.12 57.89 ± 5.53 63.57 ± 4.72 48.94 ± 7.32 50.00 ± 4.98 |
66.84 ± 6.97 68.42 ± 6.10 50.00 ± 8.84 50.63 ± 8.57 62.63 ± 5.81 69.21 ± 5.76 78.15 ± 5.22 62.63 ± 5.76 |
14.47 ± 6.08 36.18 ± 6.13 34.21 ± 9.11 26.26 ± 8.78 4.737 ± 4.34 5.631 ± 3.63 29.21 ± 6.03 12.63 ± 4.72 |
5.653 34.79 14.08 8.937 1.187 2.395 23.45 7.134 |
0.023 < 0.001 0.001 0.005 0.283 0.130 < 0.001 0.011 |
52.89 ± 6.25 44.07 ± 6.22 22.36 ± 6.90 27.94 ± 7.21 53.94 ± 5.53 58.10 ± 4.72 59.47 ± 7.32 47.10 ± 4.98 |
59.47 ± 6.95 57.23 ± 6.10 43.42 ± 8.84 47.10 ± 8.57 57.36 ± 5.81 64.0 ± 5.76 73.50 ± 5.22 53.15 ± 5.76 |
6.579 ± 6.08 13.15 ± 6.13 21.05 ± 9.11 19.15 ± 8.78 3.421 ± 4.34 5.895 ± 0.11 14.02 ± 6.03 6.05 ± 4.72 |
1.168 4.601 5.333 4.755 0.619 2.624 5.408 1.638 |
0.287 0.039 0.027 0.036 0.436 0.068 0.026 0.209 |
0.004 1.812 0.454 0.123 0.254 0.672 1.033 0.169 |
0.953 0.187 0.505 0.728 0.617 0.418 0.316 0.684 |
| HADS Anxiety Depression |
6.263 ± 1.04 5.895 ± 0.98 |
5.105 ± 1.10 6.00 ± 1.10 |
1.15 ± 0.070 0.10 ± 0.58 |
2.263 0.033 |
0.141 0.857 |
7.158 ± 1.04 7.260 ± 0.09 |
7.737 ± 1.10 7.158 ± 1.10 |
0.57 ± 0.45 0.10 ± 0.58 |
0.566 0.033 |
0.457 0.857 |
0.370 0.968 |
0.547 0.332 |
PR: pulmonary rehabilitation; PFT: pulmonary function testing; mMRC: modified Medical Research Council; 6MWD: six-minute walk distance; mBORG: modified Borg scale; SGRQ: Saint George’s Respiratory Questionnaire; SF-36: Medical Outcomes Study 36-item Short-Form Health Survey; and HADS: Hospital Anxiety and Depression Scale. aData presented as mean ± standard error of the mean. Repeated-measures ANOVA. †Within-group differences. ‡Comparison of baseline values in the groups.
Figure 2. Changes in the six-minute walk distance (6MWD), modified Medical Research Council (mMRC) scale scores, exertional dyspnea, and exertional fatigue before and after pulmonary rehabilitation (PR) in the study (telecoaching) and control (no telecoaching) groups.
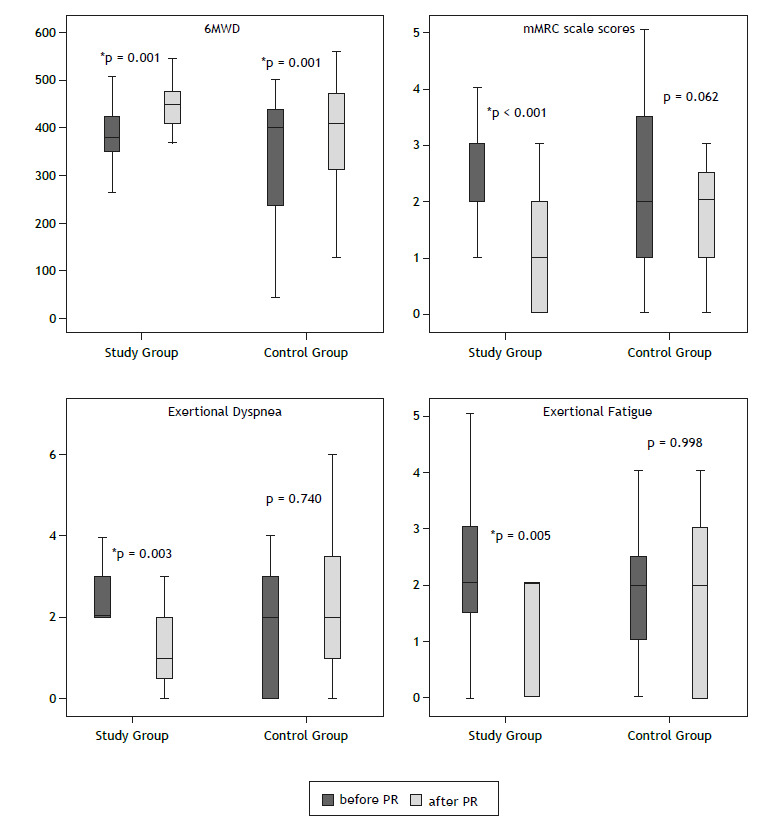
Right and left biceps muscle strength increased, although only in the control group (p < 0.05). No significant changes were observed in FEV1 (in % of predicted), FEV1/FVC, anxiety scores, or depression scores (p > 0.05; Table 3).
The results of the two-way ANOVA comparing the study and control groups showed that the improvements in daily-life dyspnea, exertional dyspnea, exertional fatigue, and SF-36 social functioning domain scores were significantly greater in the study group (p < 0.05; Table 4).
Table 4. Group effect, time effect, and group-time effect on the outcome variables.
| Variable | Group effect | Time effect | Group-time effecta | Partial h2 | Statistical power | |||
|---|---|---|---|---|---|---|---|---|
| F | p | F | p | F | p | |||
| PFT FEV1 (% predicted) FVC (% predicted) FEV1/FVC |
2.257 1.745 0.068 |
0.142 0.195 0.796 |
7.339 11.60 1.055 |
0.010 0.002 0.311 |
0.001 0.054 0.453 |
0.976 0.817 0.505 |
0.001 0.001 0.012 |
0.050 0.056 0.100 |
| mMRC scale | 0.143 | 0.708 | 17.05 | < 0.001 | 7.420 | 0.010 | 0.171 | 0.755 |
| Six-minute walk test 6MWD Dyspnea (mBORG) Leg fatigue (mBORG) |
2.257 0.281 0.055 |
0.142 0.599 0.816 |
31.152 4.039 4.431 |
< 0.001 0.052 0.042 |
0.083 6.163 4.431 |
0.775 0.018 0.042 |
0.002 0.146 0.110 |
0.059 0.676 0.535 |
| Muscle strength Right biceps Left biceps Right deltoid Left deltoid Right quadriceps Left quadriceps |
0.298 0.664 0.001 1.208 0.107 0.610 |
0.589 0.421 0.997 0.279 0.745 0.440 |
13.93 16.18 21.73 19.25 8.486 9.763 |
0.001 < 0.001 < 0.001 < 0.001 0.006 0.004 |
1.544 2.394 0.340 1.025 1.558 0.610 |
0.221 0.131 0.564 0.318 0.220 0.440 |
0.041 0.062 0.009 0.028 0.041 0.017 |
0.228 0.325 0.088 0.167 0.229 0.118 |
| SGRQ Symptoms Activity Impact Total |
0.274 0.203 0.347 0.150 |
0.604 0.655 0.559 0.701 |
4.406 14.99 27.16 32.05 |
0.043 < 0.001 < 0.001 < 0.001 |
0.004 0.036 0.255 0.038 |
0.949 0.851 0.616 0.847 |
0.001 0.001 0.007 0.001 |
0.050 0.054 0.078 0.054 |
| SF-36 Physical functioning Social functioning Role-physical Role-emotional General health Mental health Bodily pain Vitality |
0.170 0.002 0.001 0.001 0.386 0.584 0.137 0.816 |
0.683 0.966 0.998 0.998 0.538 0.450 0.713 0.372 |
5.980 32.34 18.37 13.36 1.761 5.016 25.69 7.805 |
0.019 < 0.001 < 0.001 0.001 0.193 0.031 < 0.001 0.008 |
0.841 7.045 1.042 0.327 0.046 0.003 3.169 0.968 |
0.365 0.012 0.314 0.571 0.832 0.960 0.083 0.332 |
0.023 0.164 0.028 0.009 0.001 0.001 0.081 0.026 |
0.145 0.733 0.168 0.086 0.055 0.050 0.410 0.160 |
| HADS Anxiety Depression |
1.549 0.792 |
0.221 0.379 |
0.283 0.001 |
0.598 0.998 |
2.546 0.065 |
0.119 0.800 |
0.066 0.002 |
0.342 0.057 |
PFT: pulmonary function testing; mMRC: modified Medical Research Council; 6MWD: six-minute walk distance; mBORG: modified Borg scale; SGRQ: Saint George’s Respiratory Questionnaire; SF-36: Medical Outcomes Study 36-item Short-Form Health Survey; and HADS: Hospital Anxiety and Depression Scale. aGroup-time effect is also known as comparison of ∆ values. Repeated-measures ANOVA.
DISCUSSION
In the present study, COVID-19 survivors participated in an 8-week home-based exercise program, with telecoaching being added to the study group. We found that the 6MWD, FVC, and quality of life significantly improved in both groups. Upper and lower extremity muscle strength significantly increased in the telecoaching group, whereas, in the control group, only upper extremity muscle strength increased. Daily-life dyspnea, exertional dyspnea, and exertional fatigue decreased significantly, although only in the telecoaching group. When the two groups were compared in terms of the benefits of the program, the improvement in daily-life dyspnea, exertional dyspnea, exertional fatigue, and social functioning was significantly greater in the telecoaching group than in the control group. No side effects were observed in either group during the program.
Given the elevated risk of hospital spread, rehabilitation should be provided through telemedicine, with minimal contact. This can be provided to COVID-19 patients through remote consultation (telecoaching) or online training (telerehabilitation). In this study, telecoaching was preferred because of the easy and widespread use of mobile phones.
In our study, ground-glass opacity was the most common radiographic finding. Bilateral findings were more common in the study group than in the control group (73% vs. 42%). In our study, there was no difference between the two groups in terms of the length of stay in the ward/ICU or the use of IMV and NIMV. Although the two groups were similar in terms of the number of patients receiving high-flow oxygen therapy, the number of patients receiving home oxygen therapy was higher in the control group. It is known that oxygen therapy has positive effects on functional recovery and quality of life. 27 Therefore, the improvement in functional parameters and quality of life despite a lower rate of compliance with the program in the control group might be due to the high number of patients receiving oxygen therapy in this group.
In our study, the patients in the study group were younger than were those in the control group. Although this is not a limitation, given that our study was a randomized controlled study, when we evaluate the effect of age on the results, the respiratory, functional, and psychological values measured before the program were similar between the two groups. The gains might appear to be higher if the baseline physical functions were worse in the older group. Therefore, we do not think that the younger patients in the study group inflated the results. However, compliance with telerehabilitation might be higher in younger patients, and this might explain why the improvement was greater in the study group.
It has been shown that PR increases exercise capacity, increases muscle strength, improves the quality of life, and reduces dyspnea. 3 In a study investigating the effect of telerehabilitation, there was no improvement in the respiratory function of COVID-19 survivors who had a perception of dyspnea of 2-3 on the mMRC scale after discharge. 28 In our study, a significant increase in FVC values was observed in both the study and control groups.
In one study, exercise capacity significantly increased following the telerehabilitation provided to COVID-19 patients after discharge, and dyspnea significantly decreased. 29 Similarly, in our study, there was an increase in the 6MWD and a decrease in the perception of dyspnea in the study group. In the control group, the 6MWD increased, and the perception of dyspnea did not decrease.
In one study, lower extremity muscle strength exercises were included in the telerehabilitation exercise program, and it was observed that muscle strength increased at the end of the program. 9 In our study, both upper and lower extremity muscle strength exercises were given. Although lower and upper extremity muscle strength increased in the study group, only upper extremity muscle strength increased in the control group. The higher increase in lower extremity muscle strength in the study group might be due to greater compliance with the walking program, as well as to peripheral strengthening.
Telerehabilitation provided to COVID-19 survivors improves the quality of life of patients. 30 In our study, there was an improvement in all parameters except for SGRQ symptoms domain scores in both groups. There was an improvement in most SF-36 domains in the study group, whereas, in the control group, fewer SF-36 domains were found to have improved. Anxiety and depression scores did not change in either group. This result suggests that COVID-19 survivors with symptoms of anxiety and depression should seek professional psychological support.
In our study, the rate of adherence to the telerehabilitation program was 90%. As expected, compliance with the program was higher in the telecoaching group. This rate is close to that reported elsewhere (88%). 9 In a study in which only breathing exercises were prescribed to elderly COVID-19 patients, the respiratory function, 6MWD, and quality of life of the patients improved. 31
In our study, the follow-up evaluation revealed that the patients in the control group performed breathing exercises and peripheral muscle strength exercises but failed to walk regularly. The increase in muscle strength in the upper extremities of control group patients might be due to increased use of upper extremities in daily life. The lower extremity muscle strength of control group patients may not have increased because they did not adhere to the walking program.
The relatively small number of patients participating in the study prevented us from making further between-group comparisons. The evaluation of upper and lower extremity muscle strength, the evaluation of health-related and disease-specific quality of life, and the examination of psychological symptoms are some of the strengths of the present study.
In conclusion, a home-based PR program increases exercise capacity, muscle strength, and quality of life in COVID-19 survivors. Adding telecoaching to the program results in more significant improvements in daily-life dyspnea, exertional dyspnea, exertional fatigue, and social functioning. Providing COVID-19 survivors with a home-based exercise program (and telecoaching, if possible) reduces the negative effects of the disease.
Footnotes
Financial support: None.
2 Study carried out at the Dr. Suat Seren Chest Diseases and Thoracic Surgery Training and Research Hospital, Izmir, Turkey.
3(ClinicalTrials.gov identifier: NCT04791072 [https://www.clinicaltrials.gov/])
REFERENCES
- 1.Woods JA, Hutchinson NT, Powers SK, Roberts WO, Gomez-Cabrera MC, Radak Z. The COVID-19 pandemic and physical activity. Sports Med Health Sci. 2020;2(2):55–64. doi: 10.1016/j.smhs.2020.05.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Barker-Davies RM, O'Sullivan O, Senaratne KPP, Baker P, Cranley M, Dharm-Datta S, et al. The Stanford Hall consensus statement for post-COVID-19 rehabilitation. Br J Sports Med. 2020;54(16):949–959. doi: 10.1136/bjsports-2020-102596. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Grigoletto I, Cavalheri V, Lima FF, Ramos EMC. Recovery after COVID-19 The potential role of pulmonary rehabilitation. Braz J Phys Ther. 2020;24(6):463–464. doi: 10.1016/j.bjpt.2020.07.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Carfì A, Bernabei R, Landi F. Gemelli Against COVID-19 Post-Acute Care Study Group Persistent Symptoms in Patients After Acute COVID-19. JAMA. 2020;324(6):603–605. doi: 10.1001/jama.2020.12603. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Goërtz YMJ, Van Herck M, Delbressine JM, Vaes AW, Meys R, Machado FVC, et al. Persistent symptoms 3 months after a SARS-CoV-2 infection the post-COVID-19 syndrome?. ERJ Open. Res. 2020;6(4):00542–02020. doi: 10.1183/23120541.00542-2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Salawu A, Green A, Crooks MG, Brixey N, Ross DH, Sivan M. A Proposal for Multidisciplinary Tele-Rehabilitation in the Assessment and Rehabilitation of COVID-19 Survivors. Int J Environ Res Public Health. 2020;17(13):4890–4890. doi: 10.3390/ijerph17134890. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Sagarra-Romero L, Viñas-Barros A. COVID-19 Short and Long-Term Effects of Hospitalization on Muscular Weakness in the Elderly. Int J Environ Res Public Health. 2020;17(23):8715–8715. doi: 10.3390/ijerph17238715. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Zandifar A, Badrfam R, Yazdani S, Arzaghi SM, Rahimi F, Ghasemi S. Prevalence and severity of depression, anxiety, stress and perceived stress in hospitalized patients with COVID-19. J Diabetes Metab Disord. 2020;19(2):1431–1438. doi: 10.1007/s40200-020-00667-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Vitacca M. Will the. COVID tsunami be able to impose tele-rehabilitation as a system opportunity?. Pulmonology. 2020;26(6):338–339. doi: 10.1016/j.pulmoe.2020.08.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Zhao HM, Xie YX, Wang C. Chinese Association of Rehabilitation MedicineRespiratory Rehabilitation Committee of Chinese Association of Rehabilitation MedicineCardiopulmonary Rehabilitation Group of Chinese Society of Physical Medicine and Rehabilitation Recommendations for respiratory rehabilitation in adults with coronavirus disease 2019. Chin Med J (Engl) 2020;133(13):1595–1602. doi: 10.1097/CM9.0000000000000848. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Cox NS, Dal Corso S, Hansen H, McDonald CF, Hill CJ, Zanaboni P. Telerehabilitation for chronic respiratory disease. Cochrane Database Syst Rev. 2021;1(1):CD013040–CD013040. doi: 10.1002/14651858.CD013040.pub2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.British Thoracic Society . Delivering rehabilitation to patients surviving COVID-19 using an adapted pulmonary rehabilitation approach - BTS guidance. 2020. [Google Scholar]
- 13.Alschuler L, Chiasson AM, Horwitz R, Sternberg E, Crocker R, Weil A. Integrative medicine considerations for convalescence from mild-to-moderate COVID-19 disease. Explore (NY) 2022;18(2):140–148. doi: 10.1016/j.explore.2020.12.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Beddhu S, Bruns FJ, Saul M, Seddon P, Zeidel ML. A simple comorbidity scale predicts clinical outcomes and costs in dialysis patients. Am J Med. 2000;108(8):609–613. doi: 10.1016/S0002-9343(00)00371-5. [DOI] [PubMed] [Google Scholar]
- 15.Faul F, Erdfelder E, Lang AG, Buchner A. G*Power 3 a flexible statistical power analysis program for the social, behavioral, and biomedical sciences. Behav Res Methods. 2007;39(2):175–191. doi: 10.3758/BF03193146. [DOI] [PubMed] [Google Scholar]
- 16.Urbaniak GC, Plous S. Research Randomizer (Version 4.0) [Computer software] http://www.randomizer.org
- 17.Charlson ME, Carrozzino D, Guidi J, Patierno C. Charlson Comorbidity Index A Critical Review of Clinimetric Properties. Psychother Psychosom. 2022;91(1):8–35. doi: 10.1159/000521288. [DOI] [PubMed] [Google Scholar]
- 18.ATS Committee on Proficiency Standards for Clinical Pulmonary Function Laboratories ATS statement guidelines for the six-minute walk test [published correction appears in Am J Respir Crit Care. Med. 2016;193(10):1185–1185. doi: 10.1164/ajrccm.166.1.at1102. [DOI] [PubMed] [Google Scholar]
- 19.Cuthbert SC, Goodheart GJ., Jr On the reliability and validity of manual muscle testing a literature review. Chiropr Osteopat. 2007;15:4–4. doi: 10.1186/1746-1340-15-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Sweer L, Zwillich CW. Dyspnea in the patient with chronic obstructive pulmonary disease Etiology and management. Clin Chest Med. 1990;11(3):417–445. doi: 10.1016/S0272-5231(21)00710-3. [DOI] [PubMed] [Google Scholar]
- 21.Borg GA. Psychophysical bases of perceived exertion. Med Sci Sports Exerc. 1982;14(5):377–381. doi: 10.1249/00005768-198205000-00012. [DOI] [PubMed] [Google Scholar]
- 22.Aydemir O, Guvenir T, Kuey L, Kultur S. Reliability and validity of the Turkish version of hospital anxiety and depression scale [Article in Turkish] Turkish. J Psychiat. 1997;8:280–287. [Google Scholar]
- 23.Polatli M, Yorgancioglu A, Aydemir Ö, Yilmaz Demirci N, Kirkil G, Atis Nayci S. Validity and reliability of Turkish version of St. George's respiratory questionnaire [Article in Turkish]. Tuberk Toraks. 2013;61(2):81–87. doi: 10.5578/tt.5404. [DOI] [PubMed] [Google Scholar]
- 24.Kocyigit H, Aydemir O, Fisek G, Olmez N, Memis A. Validity and reliability of Turkish version of Short form SF-36 [Article in Turkish] Turk Drug. Ther. 1999;12:102–106. [Google Scholar]
- 25.Wang TJ, Chau B, Lui M, Lam GT, Lin N, Humbert S. Physical Medicine and Rehabilitation and Pulmonary Rehabilitation for COVID-19. Am J Phys Med Rehabil. 2020;99(9):769–774. doi: 10.1097/PHM.0000000000001505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Yang LL, Yang T. Pulmonary rehabilitation for patients with coronavirus disease 2019 (COVID-19) Chronic Dis Transl Med. 2020;6(2):79–86. doi: 10.1016/j.cdtm.2020.05.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Eaton T, Lewis C, Young P, Kennedy Y, Garrett JE, Kolbe J. Long-term oxygen therapy improves health-related quality of life. Respir Med. 2004;98(4):285–293. doi: 10.1016/j.rmed.2003.10.008. [DOI] [PubMed] [Google Scholar]
- 28.Li J, Xia W, Zhan C, Liu S, Yin Z, Wang J, et al. Effectiveness of a telerehabilitation program for COVID-19 survivors (TERECO) on exercise capacity, pulmonary function, lower limb muscle strength, and quality of life: a randomized controlled trial. medRxiv; 2021. [DOI] [Google Scholar]
- 29.Paneroni M, Vitacca M, Bernocchi P, Bertacchini L, Scalvini S. Feasibility of tele-rehabilitation in survivors of COVID-19 pneumonia. Pulmonology. 2022;28(2):152–154. doi: 10.1016/j.pulmoe.2021.03.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Tanguay P, Marquis N, Gaboury I, Kairy D, Touchette M, Tousignant M. Telerehabilitation for Post-Hospitalized COVID-19 Patients A Proof-of-Concept Study During a Pandemic. Int J Telerehabil. 2021;13(1):e6383. doi: 10.5195/ijt.2021.6383. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Liu K, Zhang W, Yang Y, Zhang J, Li Y, Chen Y. Respiratory rehabilitation in elderly patients with COVID-19 A randomized controlled study. Complement Ther Clin Pract. 2020;39:101166–101166. doi: 10.1016/j.ctcp.2020.101166. [DOI] [PMC free article] [PubMed] [Google Scholar]


