ABSTRACT
Objective:
Transbronchial lung cryobiopsy (TBCB) has developed rapidly and has become one of the research hotspots of lung biopsy technology. The present study sought to evaluate the efficacy of TBCB guided by radial-probe EBUS (RP-EBUS) and a guide sheath (GS) without fluoroscopy for peripheral pulmonary lesions.
Methods:
In this retrospective study, McNemar’s test was used in order to compare TBCB and transbronchial forceps biopsy (TBFB) in terms of diagnostic performance. A multivariate logistic regression model was designed to explore the association between predictive variables and the diagnostic yield of TBCB.
Results:
A total of 168 patients underwent GS-guided RP-EBUS. Of those, 157 had lesions that were visible and 11 had lesions that were not. Of those 157 patients, 24 were excluded because of missing data or an unclear final diagnosis. Therefore, 133 patients underwent RP-EBUS-GS-guided TBFB and TBCB. The pooled diagnostic yield of RP-EBUS-GS-guided TBCB without fluoroscopy was 71.5% (103/144). In 133 patients, the diagnostic yield of TBCB was significantly higher than that of TBFB (77.4% vs. 59.4%; p < 0.05). Multivariate analysis indicated that lesion size and site were independently associated with the diagnostic yield of TBCB (OR = 2.8, p = 0.03 and OR = 4.1, p = 0.01, respectively), although cryoprobe size was not. There was no significant difference between the 1.1-mm cryoprobe and the 1.9-mm cryoprobe in terms of diagnostic performance (78.4% vs. 76.8%; p > 0.05).
Conclusions:
GS-guided RP-EBUS is regarded as a practical option for guiding cryobiopsy, although it may not be able to replace fluoroscopy. Peripheral pulmonary lesions not located in the upper lobes or larger than 30 mm are significantly associated with a higher diagnostic yield of cryobiopsy.
Keywords: Bronchoscopy, Lung neoplasms, Biopsy
RESUMO
Objetivo:
A criobiópsia transbrônquica (CBTB) desenvolveu-se rapidamente e tornou-se um dos focos de pesquisa de tecnologia de biópsia pulmonar. O presente estudo buscou avaliar a eficácia da CBTB guiada por EBUS radial com bainha guia sem fluoroscopia no diagnóstico de lesões pulmonares periféricas.
Métodos:
Neste estudo retrospectivo, o teste de McNemar foi usado para comparar a CBTB e a biópsia transbrônquica com pinça (BTB) quanto ao desempenho diagnóstico. Um modelo de regressão logística multivariada foi criado para explorar a relação entre variáveis preditivas e o rendimento diagnóstico da CBTB.
Resultados:
Um total de 168 pacientes foram submetidos a EBUS radial com bainha guia. Destes, 157 apresentavam lesões que puderam ser visualizadas e 11 apresentavam lesões que não puderam ser visualizadas. Dos 157 pacientes, 24 foram excluídos em virtude de dados incompletos ou diagnóstico final incerto. Portanto, 133 pacientes foram submetidos a BTB e CBTB guiadas por EBUS radial com bainha guia. O rendimento diagnóstico combinado da CBTB guiada por EBUS radial com bainha guia foi de 71,5%. O rendimento diagnóstico da CBTB foi significativamente maior que o da BTB (77,4% vs. 59,4%; p < 0,05). A análise multivariada indicou que o tamanho e o local da lesão apresentaram relação independente com o rendimento diagnóstico da CBTB (OR = 2,8, p = 0,03 e OR = 4,1, p = 0,01, respectivamente); o tamanho da criossonda, por sua vez, não apresentou relação com o rendimento diagnóstico da CBTB. Não houve diferença significativa entre a criossonda de 1,1 mm e a de 1,9 mm no que tange ao desempenho diagnóstico (78,4% vs. 76,8%; p > 0,05).
Conclusões:
EBUS radial com bainha guia é uma opção prática para guiar a criobiópsia, embora talvez não possa substituir a fluoroscopia. Lesões pulmonares periféricas que não estejam nos lobos superiores ou que tenham mais de 30 mm apresentam relação significativa com maior rendimento diagnóstico da criobiópsia.
Descritores: Broncoscopia, Neoplasias pulmonares, Biópsia
INTRODUCTION
In recent years, because of the widespread use of chest CT in physical examination, more cases of peripheral pulmonary lesions (PPLs) have been found. 1 PPLs can often go undiagnosed by liquid biopsy, sputum cultures, and sputum smears, a lung biopsy therefore being necessary, especially in cases of suspected malignancy. Since the 1990s, interventional pulmonology has developed rapidly. 2 Most PPLs can be diagnosed by nonsurgical biopsy, including transbronchial forceps biopsy (TBFB), CT-guided transthoracic needle biopsy, and transbronchial lung cryobiopsy (TBCB). 3 , 4
The cryoprobe is a novel biopsy tool that allows tissue to be obtained in a 360° manner laterally from the tip of the probe. In recent years, it has been increasingly used in lung biopsy. One meta-analysis demonstrated that the pooled diagnostic yield of TBCB was as high as 77% (95% CI, 71-84%). 5 Previous studies have focused on the 1.9-mm cryoprobe, with patients undergoing fluoroscopy-guided TBCB or TBCB without the assistance of a guide sheath (GS). 5 - 7 In addition, some single-center studies have analyzed the factors that may affect the efficacy of cryobiopsy. However, controversy remains as to whether the size of the cryoprobe is an independent factor. 3 , 4 , 8 In particular, there is a lack of studies comparing the new 1.1-mm cryoprobe and the traditional 1.9-mm cryoprobe in terms of diagnostic efficiency. Therefore, the objective of our study was to evaluate the performance of TBCB guided by radial-probe EBUS (RP-EBUS) with a GS (RP-EBUS-GS-guided TBCB) without fluoroscopy for the diagnosis of PPLs.
METHODS
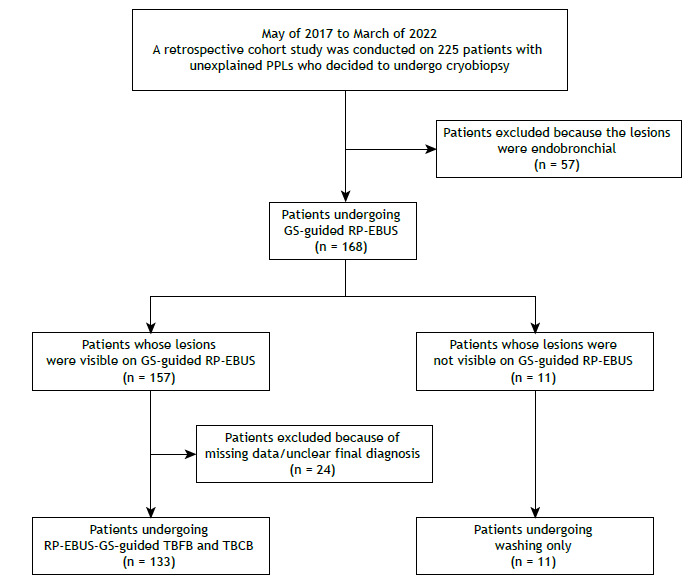
This was a retrospective observational study conducted between May of 2017 and March of 2022 in the Department of Pulmonary and Critical Care Medicine of the Affiliated Hospital of Medical School, Ningbo University, located in Ningbo, China. A total of 225 patients who had unexplained PPLs and who had originally planned to undergo cryobiopsy were enrolled in the study. Patients presenting with endobronchial lesions were excluded, as were those for whom lesion-related data were missing and those in whom diagnosis was uncertain. Therefore, 133 patients undergoing RP-EBUS-GS-guided TBFB and TBCB were analyzed in this study. Figure 1 shows a flow chart of the patient selection process. All procedures were performed in accordance with the 2019 Chinese expert consensus on the standardized procedure and technique of transbronchial cryobiopsy. 9 This study was approved by the Research Ethics Committee of the Affiliated Hospital of Ningbo University (Protocol no. XJS20210612), and written informed consent was obtained from all participants. Clinical information regarding patient characteristics was based on patient medical records.
Figure 1. Flow chart of the study. PPLs: peripheral pulmonary lesions; GS-guided RP-EBUS: radial-probe EBUS performed with a guide sheath; TBFB: transbronchial forceps biopsy; and TBCB: transbronchial lung cryobiopsy.
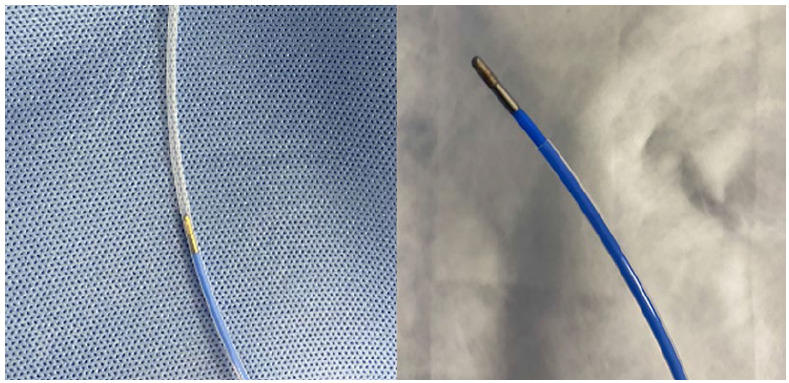
In this study, a standard bronchoscope (BF-1TQ290/BF-1T260; Olympus Corporation, Tokyo, Japan; distal end outer diameter, 5.9 mm; working channel, 3.0/2.8 mm), a radial probe (UM-S20-20R; Olympus Corporation; outer diameter, 1.7 mm), a GS (K-203/201; Olympus Corporation; outer diameter, 2.55/1.95 mm), and a 1.9-mm cryoprobe (AS Medizintechnik GmbH, Tuttlingen, Germany) were used in combination. A thin bronchoscope (BF-P260F/BF-F260/BF-260; Olympus Corporation; distal end outer diameter, 4.0/5.5/4.9 mm; working channel, 2.0 mm) was used in conjunction with a radial probe (UM-S20-17S; Olympus Corporation; outer diameter, 1.4 mm), a GS (K-201; Olympus Corporation; outer diameter, 1.95 mm), and a 1.1-mm cryoprobe (AS Medizintechnik GmbH). Because the K-203 GS was 2 cm longer than the 1.9-mm cryoprobe, we trimmed the distal end of the GS so that the tip of the probe was at least 1 cm out of the GS (Figure 2). The K-201 GS required no modification to be used with the 1.1-mm cryoprobe.
Figure 2. Modified guide sheath.
All procedures were performed by expert bronchoscopists. For each lesion, cytological samples were obtained by brushing, whereas histological samples were obtained by TBFB and TBCB. Rapid on-site cytopathological evaluation was performed as routinely described. The bronchoscope was advanced to the target bronchus by following the route designed prior to performing the procedure. The radial probe in the GS was inserted into the working channel of the bronchoscope and advanced without fluoroscopic guidance. After detection of a low-echo area, the position of the ultrasound probe was adjusted until the maximum image area was reached. The probe was then removed, whereas the GS was kept in place for subsequent sampling. If PPLs were invisible on RP-EBUS, TBCB and TBFB were not performed, and only local washing was performed.
First, small biopsy forceps were inserted into the GS for sample collection. To ensure tissue volume, five samples were collected from each lesion. 10 For TBCB, after PPLs were located by means of RP-EBUS, the cryoprobe was inserted into the GS and activated with carbon dioxide to a pressure of 50 bar for 4 s. 9 Then, tissues at the tip of the cryoprobe were removed together with the bronchoscope. Another bronchoscope was immediately inserted in order to assess the degree of airway bleeding upon removal of the first bronchoscope. If no effective samples were obtained, the freezing time was increased by 2 s for rebiopsy. To reduce the risk of bleeding and pneumothorax, TBCB was performed no more than three times per site. A chest X-ray was performed to look for evidence of pneumothorax and other complications after each procedure.
Bronchoscopy results were categorized as diagnostic or nondiagnostic. Specimens showing clear malignant features were considered true positives. For a benign diagnosis, if the pathological or microbiological diagnosis of the sample was consistent with the final clinical diagnosis, the procedure was considered diagnostic. Any lesions without pathological findings or suspicious findings were considered negative cases. All nondiagnostic biopsy samples underwent another diagnostic test (repeat bronchoscopy, CT-guided biopsy, surgery, or a follow-up CT scan six months later) in order to confirm the clinical diagnosis. In the analysis of pooled diagnostic yield, the lesions that were not visible were categorized as undiagnosed by histological biopsy.
Descriptive statistics are presented as frequency, proportion, and mean ± standard deviation. McNemar’s test was used in order to analyze the diagnostic yield of TBCB and TBFB samples. Univariate and multivariate logistic regression models were used in order to analyze factors affecting the diagnostic yield of TBCB. The level of statistical significance was set at p < 0.05. All statistical analyses were performed with the IBM SPSS Statistics software package, version 26 (IBM Corporation, Armonk, NY, USA).
RESULTS
A retrospective cohort study was conducted on 225 patients who had unexplained PPLs and who had originally planned to undergo cryobiopsy. Lesions were excluded if they were endobronchial (in 57 patients). Thus, 168 patients underwent GS-guided RP-EBUS. Of those, 157 had lesions that were visible on RP-EBUS and 11 had lesions that were not visible on RP-EBUS. Of the 157 patients whose lesions were visible on RP-EBUS, 24 were excluded because of missing data or an unclear final diagnosis. Therefore, 133 patients (86 males and 47 females) undergoing RP-EBUS-GS-guided TBFB and TBCB were enrolled in the univariate and multivariate logistic regression analyses. The mean age was 59.1 ± 13.2 years. Approximately half of the patients had nodules, with the other half having lesions ≥ 30 mm in size. Most of the lesions were not located in the upper lobes (in 102 patients; 76.7%), and only 31 patients (23.3%) had lesions that were located in the upper lobes. Of the 133 patients enrolled in the analyses, 99 (74.4%) were enrolled as cases of concentric probe orientation (toward the center), with only 34 (25.6%) being enrolled as cases of eccentric and adjacent orientation (toward the side). A 1.9-mm cryoprobe was used in 82 (61.7%) of the study participants, and a 1.1-mm cryoprobe was used in 51 (38.3%). The characteristics of the patients and target lesions are described in Table 1.
Table 1. Characteristics of the patients and target lesions.a .
| Characteristic | Result |
|---|---|
| Sample, N | 133 |
| Patient characteristics | |
| Age, yearsb | 59.1 ± 13.2 |
| Sex | |
| Male | 86 (64.7) |
| Female | 47 (35.3) |
| Target lesion characteristics | |
| Lesion size, mm | |
| <30 | 60 (45.1) |
| ≥30 | 73 (54.9) |
| Lesion site | |
| Upper lobe | 31 (23.3) |
| Other | 102 (76.7) |
| Probe orientation | |
| Toward the side | 34 (25.6) |
| Toward the center | 99 (74.4) |
| Cryoprobe size | |
| 1.9 mm | 82 (61.7) |
| 1.1 mm | 51 (38.3) |
| Final diagnosis | |
| Benign lesion | 93 (69.9) |
| Malignant lesion | 40 (30.1) |
Data expressed as n (%), except where otherwise indicated. bData expressed as mean ± SD.
In this study, the pooled diagnostic yield of RP-EBUS-GS-guided TBCB without fluoroscopy was 71.5% (103/144). For the 133 patients who underwent RP-EBUS-GS-guided TBFB and TBCB, the diagnostic yield of TBCB was as high as 77.4%, which was significantly higher than that of TBFB (59.4%; p < 0.05). In order to show the performance of TBCB and TBFB more clearly, the lesions were divided into ten groups on the basis of five different factors (lesion size, lesion site, final diagnosis, probe orientation, and cryoprobe size), which are shown in Table 2. The data show that the diagnostic yield of TBCB was higher than that of TBFB in all groups. However, there was no statistically significant difference when the lesions were ≥ 30 mm in size and malignant.
Table 2. Comparison of diagnostic yield between transbronchial forceps biopsy and transbronchial lung cryobiopsy.a .
| Variable | Group | p* | |
|---|---|---|---|
| TBFB | TBCB | ||
| All | 59.4 (79/133) | 77.4 (103/133) | <0.01 |
| Lesion size, mm | |||
| < 30 | 43.3 (26/60) | 68.3 (41/60) | <0.01 |
| ≥ 30 | 72.6 (53/73) | 84.9 (62/73) | 0.09 |
| Lesion site | |||
| Upper lobe | 51.6 (16/31) | 61.3 (19/31) | 0.51 |
| Other | 61.8 (63/102) | 82.4 (84/102) | <0.01 |
| Probe orientation | |||
| Toward the side | 50.0 (17/34) | 70.6 (24/34) | 0.04 |
| Toward the center | 62.6 (62/99) | 79.8 (79/99) | <0.01 |
| Final diagnosis | |||
| Benign lesion | 63.4 (59/93) | 80.6 (75/93) | <0.01 |
| Malignant lesion | 50.0 (20/40) | 70.0 (28/40) | 0.057 |
| Cryoprobe size | |||
| 1.9 mm | 61 (50/82) | 76.8 (63/82) | 0.01 |
| 1.1 mm | 56.8 (29/51) | 78.4 (40/51) | 0.02 |
TBFB: transbronchial forceps biopsy; and TBCB: transbronchial lung cryobiopsy. aData presented as % (n/N). *McNemar’s test.
Table 3 shows five factors that may affect the diagnostic yield of TBCB. Univariate and multivariate analyses indicated that lesion size and lesion site were independently associated with the diagnostic yield of TBCB (OR = 2.8, p = 0.03 and OR = 4.1, p = 0.01, respectively). However, there was no significant difference between the 1.1-mm cryoprobe and the 1.9-mm cryoprobe in terms of diagnostic performance (78.4% vs. 76.8%; p > 0.05).
Table 3. Multivariate logistic regression model for the diagnostic yield of transbronchial lung cryobiopsy.a .
| Category | Group | Diagnostic yield | Univariate analysis | Multivariate analysis | ||||
|---|---|---|---|---|---|---|---|---|
| C2 | p | B | OR (95% CI) | p | ||||
| Lesion size, mm | < 30 | 68.3 (41/60) | 5.2 | 0.04 | 0 | 1 | 0.03 | |
| ≥ 30 | 84.9 (62/73) | 1.1 | 2.8 (1.1-6.9) | |||||
| Lesion site | Upper lobe | 61.3 (19/31) | 6 | 0.02 | 0 | 1 | 0.01 | |
| Other | 82.4 (84/102) | 1.4 | 4.1 (1.5-11.3) | |||||
| Probe orientation | Toward the side | 70.6 (24/34) | 1.2 | 0.34 | 0 | 1 | 0.56 | |
| Toward the center | 79.8 (79/99) | 0.3 | 1.3 (0.5-3.6) | |||||
| Final diagnosis | Benign lesion | 80.6 (75/93) | 1.8 | 0.26 | 0 | 1 | 0.12 | |
| Malignant lesion | 70.0 (28/40) | −0.7 | 0.5 (0.2-1.2) | |||||
| Cryoprobe size | 1.9 mm | 76.8 (63/82) | 0.05 | 0.84 | 0 | 1 | 0.22 | |
| 1.1 mm | 78.4 (40/51) | 0.6 | 1.9 (0.7-5.0) | |||||
Data presented as % (n/N).
In our study, most of the cases of bleeding were categorized as grade 0 or 1; that is, bleeding that can stop on its own or that requires suction to clear. Only a few of the patients who underwent TBCB with a 1.9-mm cryoprobe had grade 2 bleeding requiring wedging, cold saline solution lavage, and regional instillation of epinephrine (5 mL; 1:10,000), 9 without the need for further intervention (bronchial blocker or surgical intervention). Pneumothorax occurred in only 4 patients and was managed by closed thoracic drainage. Of the 4 cases of pneumothorax, 3 were caused by the 1.9-mm cryoprobe and 1 was caused by the 1.1-mm cryoprobe.
DISCUSSION
Various techniques such as robot technology, electromagnetic navigation, virtual bronchoscopy, and RP-EBUS have been used in order to diagnose PPLs, and these techniques have improved diagnostic efficiency. 11 - 13 Nevertheless, the application of these technologies plays a role in guiding the bronchoscope to locate the lesion. In some cases, even if the lesion can be located, it is still difficult to obtain effective pathological samples because the volume and quality of the samples are determined by biopsy methods, such as TBFB, TBCB, and needle aspiration. 14 Therefore, there is a need to improve biopsy techniques in order to address the diagnostic dilemma of PPLs.
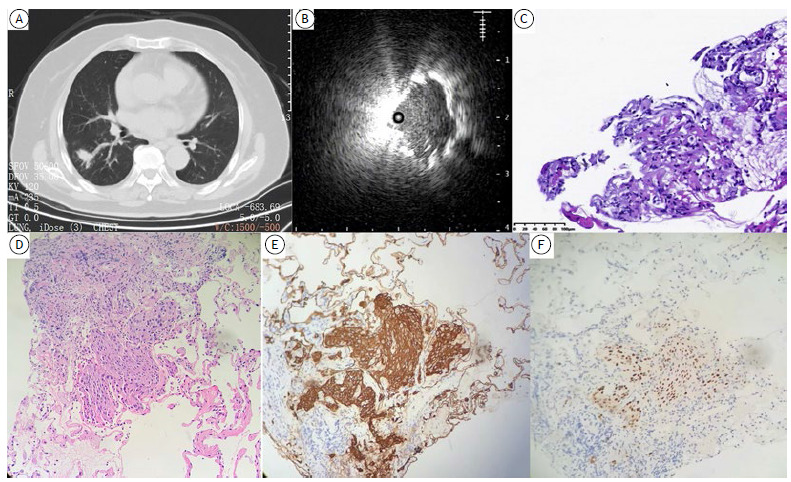
Cryobiopsy is a novel biopsy tool. For eccentrically and adjacently oriented lesions, our results show that the diagnostic yield of TBCB is significantly higher than that of TBFB, a finding that is consistent with the literature. 15 In the present study, the diagnostic yield of TBCB was higher than that of TBFB in all groups. It is well recognized that cryobiopsy samples are preferred for molecular testing and immunohistochemical analysis, being superior in terms of tissue volume when compared with forceps biopsy samples. 5 , 16 In one patient in the present study, the sample obtained by TBFB was limited, without any evidence of malignancy. However, the sample obtained by TBCB showed suspicious tumor cell clumps. Therefore, TBCB samples were used for further immunohistochemical analysis to confirm the diagnosis. Various staining methods and biomarker analyses led us to conclude that the patient had poorly differentiated lung squamous cell carcinoma (Figure 3). Therefore, it is evident that the higher quality of cryobiopsy samples allows morphological diagnosis and biomarker analysis, especially for malignancies with minimal histological heterogeneity. Cryobiopsy avoids repeat procedures and contributes to precision medicine against lung cancer.
Figure 3. A patient with poorly differentiated lung squamous cell carcinoma underwent transbronchial forceps biopsy (TBFB) and transbronchial lung cryobiopsy (TBCB) guided by radial-probe EBUS and a guide sheath. In A, solitary peripheral pulmonary nodule (of 2.0 cm in size) in the dorsal segment of the right lower lobe. In B, radial-probe EBUS showing an eccentric lesion, with no surrounding vessels. In C, TBFB showing no features of suspected malignancy (H&E; magnification, ×200). In D, TBCB showing suspicious tumor cell clumps (H&E; magnification, ×200). In E, immunohistochemistry showing strong and diffuse positivity for cytokeratins. In F, immunohistochemistry showing strong and diffuse positivity for p40.
The GS has been reported to play an important role in ensuring accurate and consistent cryobiopsy. 14 , 17 , 18 However, in most centers, cryobiopsy is performed without the assistance of a GS. In this study, a 1.9-mm cryoprobe was housed within a K-203 GS for sampling. Because the GS was 2 cm longer than the 1.9-mm cryoprobe (which was 1,050 mm long), the GS had to be modified in order to be used for cryobiopsy in our study. Herath et al. trimmed the GS by 3 cm from the distal end to enable contact with the lesion. 17 The 1.1-mm cryoprobe used in the present study was compatible with the K-201 GS (outer diameter, 1.95 mm). In order to make the procedure smoother, all instruments should be selected and measured preoperatively, including the radial probe, the cryoprobe, and the GS.
Some studies have shown that the diagnostic performance of TBFB for PPLs is just as good with fluoroscopy as it is without it. 19 , 20 Therefore, it is reasonable to assume that it is possible to forgo fluoroscopy during cryobiopsy. In this study, we demonstrated the utility of RP-EBUS-GS-guided TBCB without fluoroscopy in evaluating PPLs. The pooled yield of 71.5% is at least good, even if it is inferior to that of TBCB with fluoroscopy, which is of 97%. 4 In addition, there are reports that cryobiopsy without fluoroscopy can increase the risk of pneumothorax. 4 , 21 In this study, the incidence of pneumothorax was only 3%, which might be due to the use of a GS in the whole process of cryobiopsy. Therefore, although GS-guided RP-EBUS may not replace fluoroscopy in determining the precise site for cryobiopsy, it is also safe and effective.
Previous single-center studies have reported several factors that can affect the diagnostic yield of cryobiopsy, such as probe orientation, lesion site, and lesion size. 22 , 23 On the basis of previous reports and clinical experience, we discussed five factors that can affect the yield of cryobiopsy. Univariate and multivariate analysis indicated that PPLs that were not located in the upper lobes were significantly associated with a higher diagnostic yield of cryobiopsy (OR = 4.1; 95% CI, 1.5-11.3; p = 0.01). PPLs in the upper lobes are notoriously difficult to be accessed with bronchoscopes. 22 , 24 We also encountered such difficulties. In addition, the size of the lesions was a significant factor affecting the diagnostic yield of cryobiopsy, a finding that is consistent with those of a report providing the largest data series of TBCB. 4
In this study, the diagnostic yield of TBCB performed with a 1.1-mm cryoprobe was not significantly higher than that of TBCB performed with a 1.9-mm cryoprobe (78.4% vs. 76.8%; p = 0.84), a finding that is consistent with those of Lonny et al. 8 Some studies have reported that the 1.1-mm cryoprobe is a dramatic improvement over the 1.9-mm cryoprobe in diagnosing specific lesions (such as ground-glass nodules, lesions located in the upper lobe, and lesions near the pleura), 25 - 27 the diagnostic yield of the 1.1-mm cryoprobe therefore being higher than that of larger probes. Previous studies have suggested that when an ultrathin cryoprobe and a GS are used for cryobiopsy, it is no longer necessary to remove en bloc the bronchoscope from the patient in tissue sampling, and this is beneficial to repeat biopsy and early monitoring of bleeding. 5 , 28 However, the fact that the GS used in conjunction with the ultrathin cryoprobe is also thinner means that the samples might be too big to be taken out. 26 It is necessary to determine the appropriate activation time because the area of the samples increases with increasing activation time. 8 However, in the present study, the number of enrolled patients undergoing TBCB with a 1.1-mm cryoprobe was relatively limited, being insufficient for a more detailed comparison between the 1.1-mm cryoprobe and the 1.9-mm cryoprobe.
Our study has some limitations. First, because of the retrospective design, the study may have a bias on patient and lesion characteristics. Second, the number of enrolled patients undergoing TBCB with a 1.1-mm cryoprobe was small, further studies therefore being needed.
Cryobiopsy is a safe and effective method for diagnosing PPLs. Moreover, GS-guided RP-EBUS is considered a practical option for guiding the procedure, although it may not be able to replace fluoroscopy. PPLs not located in the upper lobes or larger than 30 mm are significantly associated with a higher diagnostic yield of cryobiopsy, and the 1.1-mm and 1.9-mm cryoprobes have similar diagnostic performance.
ACKNOWLEDGMENTS
We thank all of the patients who agreed to participate in the study and all of the researchers who contributed to the study.
Footnotes
1 Study carried out in the Department of Pulmonary and Critical Care Medicine, The Affiliated Hospital of Medical School, Ningbo University, Jiangbei District, Ningbo, Zhejiang Province, China.
Financial support: This study received financial support from the Affiliated Hospital of the Medical School of Ningbo University Youth Talent Cultivation Program (Grant no. FYQM-LC-202003), the Ningbo Social and Scientific Development Fund (Grant no. 2015C50012), the Ningbo Health Youth Technical Key Talents Training Special Project (Grant no. 2020SWSQNGG-05), the Natural Science Foundation of Ningbo (Grant nos. 2018A610271 and 2017A610250), the Ningbo Medical Science and Technology Project (Grant no. 2021Y13), the Medical Health Science and Technology Project of Zhejiang Provincial Health Commission (Grant no. 2016KYB268), and the Zhejiang Provincial Health Science and Technology Plan (Grant no. 2022RC247).
REFERENCES
- 1.Spelic D, Hilohi M, Farris K, Eicholtz G, Elee J, Ortego J. The Nationwide Evaluation of X-Ray Trends, Part I More Than 40 Years of Surveying the US Radiology Practice. J Am Coll Radiol. 2016;13(6):713–715. doi: 10.1016/j.jacr.2016.03.013. [DOI] [PubMed] [Google Scholar]
- 2.Bolliger CT, Mathur PN, Beamis JF, Becker HD, Cavaliere S, Colt H. ERS/ATS statement on interventional pulmonology European Respiratory Society/American Thoracic Society. Eur Respir J. 2002;19(2):356–373. doi: 10.1183/09031936.02.00204602. [DOI] [PubMed] [Google Scholar]
- 3.Fu YF, Zhang JH, Wang T, Shi YB. Endobronchial ultrasound-guided versus computed tomography-guided biopsy for peripheral pulmonary lesions A meta-analysis. Clin Respir J. 2021;15(1):3–10. doi: 10.1111/crj.13275. [DOI] [PubMed] [Google Scholar]
- 4.Herth FJ, Mayer M, Thiboutot J, Kapp CM, Sun J, Zhang X. Safety and Performance of Transbronchial Cryobiopsy for Parenchymal Lung Lesions. Chest. 2021;160(4):1512–1519. doi: 10.1016/j.chest.2021.04.063. [DOI] [PubMed] [Google Scholar]
- 5.Sryma PB, Mittal S, Madan NK, Tiwari P, Hadda V, Mohan A, et al. Efficacy of Radial Endobronchial Ultrasound (R-EBUS) guided transbronchial cryobiopsy for peripheral pulmonary lesions (PPL's): A systematic review and meta-analysis. Pulmonology. 2021 doi: 10.1016/j.pulmoe.2020.12.006. [DOI] [PubMed] [Google Scholar]
- 6.Ye J, Zhang R, Ma S, Wang L, Jin W. Endobronchial ultrasound plus fluoroscopy-guided biopsy compared to fluoroscopy-guided transbronchial biopsy for obtaining samples of peripheral pulmonary lesions A systematic review and meta-analysis. Ann Thorac Med. 2017;12(2):114–120. doi: 10.4103/atm.ATM_298_16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Imabayashi T, Uchino J, Yoshimura A, Chihara Y, Tamiya N, Kaneko Y. Safety and Usefulness of Cryobiopsy and Stamp Cytology for the Diagnosis of Peripheral Pulmonary Lesions. Cancers (Basel) 2019;11(3):410–410. doi: 10.3390/cancers11030410. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Yarmus LB, Semaan RW, Arias SA, Feller-Kopman D, Ortiz R, Bösmuller H. A Randomized Controlled Trial of a Novel Sheath Cryoprobe for Bronchoscopic Lung Biopsy in a Porcine Model. Chest. 2016;150(2):329–336. doi: 10.1016/j.chest.2016.01.018. [DOI] [PubMed] [Google Scholar]
- 9.Guo S, Li Q, Jiang J, Luo F, Li Y, Jin F. Chinese expert consensus on the standardized procedure and technique of transbronchial cryobiopsy. J Thorac Dis. 2019;11(12):4909–4917. doi: 10.21037/jtd.2019.12.36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Yamada N, Yamazaki K, Kurimoto N, Asahina H, Kikuchi E, Shinagawa N. Factors related to diagnostic yield of transbronchial biopsy using endobronchial ultrasonography with a guide sheath in small peripheral pulmonary lesions. Chest. 2007;132(2):603–608. doi: 10.1378/chest.07-0637. [DOI] [PubMed] [Google Scholar]
- 11.Gildea TR, Mazzone PJ, Karnak D, Meziane M, Mehta AC. Electromagnetic navigation diagnostic bronchoscopy a prospective study. Am J Respir Crit Care Med. 2006;174(9):982–989. doi: 10.1164/rccm.200603-344OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Ishida T, Asano F, Yamazaki K, Shinagawa N, Oizumi S, Moriya H. Virtual bronchoscopic navigation combined with endobronchial ultrasound to diagnose small peripheral pulmonary lesions a randomised trial. Thorax. 2011;66(12):1072–1077. doi: 10.1136/thx.2010.145490. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Kuo CH, Lin SM, Lee KY, Chung FT, Lo YL, Hsiung TC. Endobronchial ultrasound-guided transbronchial biopsy and brushing a comparative evaluation for the diagnosis of peripheral pulmonary lesions. Eur J Cardiothorac Surg. 2014;45(5):894–898. doi: 10.1093/ejcts/ezt472. [DOI] [PubMed] [Google Scholar]
- 14.Schuhmann M, Bostanci K, Bugalho A, Warth A, Schnabel PA, Herth FJ. Endobronchial ultrasound-guided cryobiopsies in peripheral pulmonary lesions a feasibility study. Eur Respir J. 2014;43(1):233–239. doi: 10.1183/09031936.00011313. [DOI] [PubMed] [Google Scholar]
- 15.Kho SS, Chan SK, Yong MC, Tie ST. Performance of transbronchial cryobiopsy in eccentrically and adjacently orientated radial endobronchial ultrasound lesions. ERJ Open Res. 2019;5(4):00135–02019. doi: 10.1183/23120541.00135-2019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Haentschel M, Boeckeler M, Bonzheim I, Schimmele F, Spengler W, Stanzel F. Influence of Biopsy Technique on Molecular Genetic Tumor Characterization in Non-Small Cell Lung Cancer-The Prospective, Randomized, Single-Blinded, Multicenter PROFILER Study Protocol. Diagnostics (Basel) 2020;10(7):459–459. doi: 10.3390/diagnostics10070459. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Herath S, Yap E. Novel hybrid cryo-radial method an emerging alternative to CT-guided biopsy in suspected lung cancer. A prospective case series and description of technique. Respirol Case Rep. 2017;6(2):e00287. doi: 10.1002/rcr2.287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Nasu S, Okamoto N, Suzuki H, Shiroyama T, Tanaka A, Samejima Y. Comparison of the Utilities of Cryobiopsy and Forceps Biopsy for Peripheral Lung Cancer. Anticancer Res. 2019;39(10):5683–5688. doi: 10.21873/anticanres.13766. [DOI] [PubMed] [Google Scholar]
- 19.Zheng X, Xie F, Li Y, Chen J, Jiang Y, Sun J. Ultrathin bronchoscope combined with virtual bronchoscopic navigation and endobronchial ultrasound for the diagnosis of peripheral pulmonary lesions with or without fluoroscopy A randomized trial. Thorac Cancer. 2021;12(12):1864–1872. doi: 10.1111/1759-7714.13995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Hong KS, Ahn H, Lee KH, Chung JH, Shin KC, Jin HJ. Radial Probe Endobronchial Ultrasound Using Guide Sheath-Guided Transbronchial Lung Biopsy in Peripheral Pulmonary Lesions without Fluoroscopy. Tuberc Respir Dis (Seoul) 2021;84(4):282–290. doi: 10.4046/trd.2021.0002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Dhooria S, Mehta R, Srinivasan A, Madan K, Sehgal I, Pattabhiraman V. The safety and efficacy of different methods for obtaining transbronchial lung cryobiopsy in diffuse lung diseases. Clin Respir J. 2018;12(4):1711–1720. doi: 10.1111/crj.12734. [DOI] [PubMed] [Google Scholar]
- 22.Matsumoto Y, Nakai T, Tanaka M, Imabayashi T, Tsuchida T, Ohe Y. Diagnostic Outcomes and Safety of Cryobiopsy Added to Conventional Sampling Methods An Observational Study. Chest. 2021;160(5):1890–1901. doi: 10.1016/j.chest.2021.05.015. [DOI] [PubMed] [Google Scholar]
- 23.Kronborg-White S, Sritharan SS, Madsen LB, Folkersen B, Voldby N, Poletti V. Integration of cryobiopsies for interstitial lung disease diagnosis is a valid and safe diagnostic strategy-experiences based on 250 biopsy procedures. J Thorac Dis. 2021;13(3):1455–1465. doi: 10.21037/jtd-20-2431. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Haidong H, Yunye N, Wei Z, Zarogoulidis P, Hohenforst-Schmidt W, Man Y. Multiple guided technologies based on radial probe endobronchial ultrasound for the diagnosis of solitary peripheral pulmonary lesions a single-center study. J Cancer. 2017;8(17):3514–3521. doi: 10.7150/jca.20035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Kho SS, Chai CS, Nyanti LE, Ismail AMB, Tie ST. Combination of 1 1 mm flexible cryoprobe with conventional guide sheath and therapeutic bronchoscope in biopsy of apical upper lobe solitary pulmonary nodule. BMC Pulm Med. 2020;20(1):158–158. doi: 10.1186/s12890-020-01199-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Hetzel J, Linzenbold W, Boesmueller H, Enderle M, Poletti V. Evaluation of Efficacy of a New Cryoprobe for Transbronchial Cryobiopsy A Randomized, Controlled in vivo Animal Study. Respiration. 2020;99(3):248–256. doi: 10.1159/000506017. [DOI] [PubMed] [Google Scholar]
- 27.Jiang S, Liu X, Chen J, Ma H, Xie F, Sun J. A pilot study of the ultrathin cryoprobe in the diagnosis of peripheral pulmonary ground-glass opacity lesions. Transl Lung Cancer Res. 2020;9(5):1963–1973. doi: 10.21037/tlcr-20-957. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Verhoeven RLJ, Vos S, van der Heijden EHFM. Multi-modal tissue sampling in cone beam CT guided navigation bronchoscopy comparative accuracy of different sampling tools and rapid on-site evaluation of cytopathology. J Thorac Dis. 2021;13(7):4396–4406. doi: 10.21037/jtd-21-518. [DOI] [PMC free article] [PubMed] [Google Scholar]


