Abstract
We studied the metabolism of polycyclic aromatic hydrocarbons (PAHs) by using white rot fungi previously identified as organisms that metabolize polychlorinated biphenyls. Bran flakes medium, which has been shown to support production of high levels of laccase and manganese peroxidase, was used as the growth medium. Ten fungi grown for 5 days in this medium in the presence of anthracene, pyrene, or phenanthrene, each at a concentration of 5 μg/ml could metabolize these PAHs. We studied the oxidation of 10 PAHs by using laccase purified from Coriolopsis gallica. The reaction mixtures contained 20 μM PAH, 15% acetonitrile in 60 mM phosphate buffer (pH 6), 1 mM 2,2′-azinobis-(3-ethylbenzthiazoline-6-sulfonate) (ABTS), and 5 U of laccase. Laccase exhibited 91% of its maximum activity in the absence of acetonitrile. The following seven PAHs were oxidized by laccase: benzo[a]pyrene, 9-methylanthracene, 2-methylanthracene, anthracene, biphenylene, acenaphthene, and phenanthrene. There was no clear relationship between the ionization potential of the substrate and the first-order rate constant (k) for substrate loss in vitro in the presence of ABTS. The effects of mediating substrates were examined further by using anthracene as the substrate. Hydroxybenzotriazole (HBT) (1 mM) supported approximately one-half the anthracene oxidation rate (k = 2.4 h−1) that ABTS (1 mM) supported (k = 5.2 h−1), but 1 mM HBT plus 1 mM ABTS increased the oxidation rate ninefold compared with the oxidation rate in the presence of ABTS, to 45 h−1. Laccase purified from Pleurotus ostreatus had an activity similar to that of C. gallica laccase with HBT alone, with ABTS alone, and with 1 mM HBT plus 1 mM ABTS. Mass spectra of products obtained from oxidation of anthracene and acenaphthene revealed that the dione derivatives of these compounds were present.
Polycyclic aromatic hydrocarbons (PAHs) are pollutants that are found in most terrestrial environments (8, 9). Many fungi can metabolize PAHs by using either a cytochrome P-450 monooxygenase system (2, 22) or, in the case of white rot fungi, lignin peroxidase or related enzymes (22). In organisms that use the cytochrome P-450 system, the trans-dihydrodiol product cannot be used as an energy source, although further metabolism may occur. However, in white rot fungi, such as Phanerochaete chrysosporium, and in Trametes versicolor, mineralization of some PAHs occurs, indicating that complete breakdown of PAHs occurs (21, 22). Fewer examples of in vitro oxidation of PAHs by culture supernatants and purified enzymes have been described, although oxidation of anthracene and pyrene by lignin peroxidase and manganese peroxidase from P. chrysosporium (7, 11, 24) and oxidation of many PAHs by the laccases of T. versicolor (5, 10, 13, 17) have been reported. Manganese peroxidases from Phanerochaete species can also be involved in PAH metabolism (3, 4), and recently a manganese peroxidase preparation from Nematoloma frowardii has been implicated in direct mineralization of aliphatic and aromatic compounds, including pyrene (12).
White rot fungi also can metabolize specific polychlorinated biphenyl (PCB) congeners (1). In the study of Beaudette et al. (1) a number of previously uncharacterized fungi were screened for PCB metabolism and mineralization. Under the conditions used, significant levels of PCB metabolism (87 to 93%) and mineralization (4.7 to 11.1%) were observed in strains of T. versicolor and Bjerkandera adusta but not in P. chrysosporium. Our objectives in this study were (i) to determine if previously uncharacterized fungal strains could metabolize selected PAHs in vivo and (ii) to study PAH metabolism by using partially purified enzymes in vitro. Our long-term goals are to chemically modify PAH-metabolizing enzymes in order to enhance their activities in organic solvents (23–25) and to identify fungal strains that can produce high levels of lignin-degrading enzymes and enzymes with enhanced stability to pH and temperature or with high levels of activity in organic solvents.
MATERIALS AND METHODS
Chemicals.
Acenaphthene, anthracene, azulene, benzo[aa]pyrene, biphenylene, 2-methylanthracene, 9-methylanthracene, fluoranthene, phenanthrene, pyrene, and 2,5-xylidine were obtained from Aldrich (Oakville, Ontario, Canada). 2,2′-Azino-bis-(3-ethylbenzthiazoline-6-sulfonic acid) (ABTS) and 1-hydroxybenzotriazole (HBT) were obtained from Sigma (St. Louis, Mo.).
Fungi.
B. adusta UAMH 4312, UAMH 7308, and UAMH 8258, Pleurotus ostreatus UAMH 7964 and UAMH 7980, T. versicolor UAMH 8272, Coriolopsis gallica UAMH 8260, and Ganoderma applanatum UAMH 8168 were obtained from the University of Alberta Microfungus Collection and Herbarium, Devonian Botanical Gardens, University of Alberta, Edmonton, Alberta, Canada. P. ostreatus IE-8 was obtained from the Instituto de Ecologia, Xalapa, Veracruz, Mexico, and P. chrysosporium ATCC 24725 was obtained from the American Type Culture Collection, Manassas, Va. The fungi were grown on potato dextrose agar (Difco, Detroit, Mich.) at 28°C for 5 to 7 days before they were stored at 4°C, and they were transferred every 3 months.
Laccase production and purification.
C. gallica inocula were prepared in glucose-malt extract-yeast extract medium (GMY) modified as described by Mester et al. (18). This medium supported excellent growth but relatively low levels of enzyme production (19). GMY contained (per liter) 10 g of glucose, 3.5 g of malt extract (Difco), 2.5 g of yeast extract (Difco), 2.0 g of KH2PO4, and 0.5 g of MgSO4 · 7H2O. Inocula were prepared by homogenizing 1-cm2 portions of surface mycelia from potato dextrose agar plates in 50-ml portions of GMY with an Omnimixer (Sorvall, Norwalk, Conn.) for 10 s. After 3 days of growth in 500-ml shake flasks at 200 rpm and 28°C, the cultures were again homogenized, and 5 to 10% inocula (depending on the density of growth; approximately 2 mg [dry weight]) were used to inoculate production medium. Production medium consisted of 2% (wt/vol) ground cereal bran (Kellogg’s Bran Flakes; Kellogg Company, Battle Creek, Mich.) in 60 mM sodium phosphate buffer (pH 6) (19). The cultures were grown at 28°C for 10 days. Fungal mycelia and residual bran flakes were removed by filtration through cheesecloth prior to centrifugation at 8,000 × g for 20 min. The clarified medium was either precipitated with ammonium sulfate (0 to 80% fraction) (20) and dialyzed or concentrated by ultrafiltration with an Amicon type PM-10 membrane. C. gallica laccase was purified by passage through an anion-exchange column (Whatman type DE-52), followed by gel filtration (Sephadex G-100). The final product represented 40% recovery of the activity in the original culture filtrate; the level of purification was threefold, and the specific activity was 44 enzyme units/mg of protein (as determined by a Bradford protein assay in which serum albumin [Sigma] was used). The purified enzyme produced a single major band on sodium dodecyl sulfate-polyacrylamide gel electrophoresis gels (20) and exhibited no detectable peroxidase activity when o-dianisidine was used as the substrate.
Laccase assay.
Laccase activity was assayed by examining ABTS oxidation (26) in a reaction mixture containing 1 mM ABTS in 0.1 M sodium acetate buffer (pH 4.5) and 5 to 50 μl of enzyme sample. Oxidation was monitored at 436 nm, and 1 U of activity was defined as 1 μmol of ABTS oxidized/min (ɛ436 = 29,300 M−1 cm−1).
PAH transformation studies.
Fungal inocula were grown in GMY at 28°C for 3 days and homogenized as described above. The transformation medium (25 ml of 2% bran flakes in 60 mM phosphate buffer [pH 6] in a 125-ml flask) was inoculated with 5% fungal homogenate, and the culture was grown for 3 days before 1 ml of a solution containing 0.125 mg of PAH/ml of dimethylformamide was added to give a final concentration of 5 μg of PAH/ml. At this concentration dimethylformamide had no observable effect on fungal growth. Growth and PAH transformation were continued for an additional 5 days at 28°C with shaking. Culture growth and metabolism were stopped by adding 25 ml of reduced tetrahydrofuran (refluxed with FeSO4 and then distilled in order to eliminate reactive peroxides) to extract the unreacted PAHs and metabolites. One-milliliter samples were then centrifuged (14,000 × g for 3 min), and 50 μl was used for a high-performance liquid chromatography (HPLC) analysis on a Hypersil BDS-C18 5 μm reverse-phase column (200 by 2.1 mm; Hewlett-Packard, Avondale, Pa.) by using isocratic elution with acetonitrile-water (60:40, vol/vol). The decrease in the amount of PAH in samples was estimated by measuring the peak area of UV absorbance with a Turbachrom workstation computer (Perkin-Elmer, Norwalk, Conn.). Fungi were grown in triplicate flasks, and the experiment was repeated three times over a 1-year period. The results are reported below as means and standard deviations based on the results obtained with nine flasks. In control cultures that were grown for 3 days and then autoclaved before PAHs were added, the levels of recovery were equivalent to the levels obtained when PAH medium alone. Tetrahydrofuran extracted >95% of the added PAH compared to heat-treated control flasks. Even after 10 days of incubation the levels of control PAH recovery were 93% ± 12%. Extraction of the residual PAHs by tetrahydrofuran was very rapid, as no differences in the levels of recovery were observed when data from 1-min, 1-h, and 3-day extractions performed in triplicate were compared.
Laccase-mediated oxidation of PAHs.
Laccase-mediated oxidation of PAHs was determined by incubating a mixture of individual PAHs (20 μM) and ABTS (1 mM) in 15% acetonitrile in 0.1 M acetate buffer (pH 4.5) with 5 U of C. gallica laccase in a 100-μl reaction mixture. Acetonitrile aids in PAH solubilization, and in 15% acetonitrile C. gallica laccase exhibited 91% ± 1% of the activity exhibited in buffer alone. The assay was started by adding enzyme and was terminated by adding acetonitrile to a final concentration of 50%. Boiled enzyme controls exhibited no activity. After centrifugation, 50-μl samples were analyzed by HPLC by using the C18 reverse-phase column described above and isocratic elution with acetonitrile-water (60:40). Peak areas were calculated, and the first-order oxidation reaction rates were plotted by fitting the data to the equation At = A0e−kt.
To obtain enough products for identification by gas chromatography-mass spectrometry (GC-MS), 10-ml reaction mixtures containing 20 μM PAH were treated with laccase (5 U), 1 mM ABTS, and 1 mM HBT for most compounds; the only exception was phenanthrene, for which the total reaction volume was 5 ml. After 18 h the mixtures were acidified and extracted five times with 2 ml of methylene chloride. The extracts were combined, dried over anhydrous sodium sulfate, and concentrated under nitrogen prior to analysis by GC-MS. GC-MS was carried out by using a Hewlett-Packard GC (model 6890) coupled to an MS detector (model 5972). The GC-MS was equipped with a type SPB-20 column (30 m by 0.25 mm; Supelco); the temperature program started with 90°C for 2 min, and then the temperature was increased to 290°C at a rate of 8°C/min and kept at 290°C for 10 min. The chemical structures of the biocatalytic oxidation products were determined by comparing their mass spectra with the mass spectra of standards.
RESULTS
In vivo fungal metabolism of anthracene, pyrene, and phenanthrene.
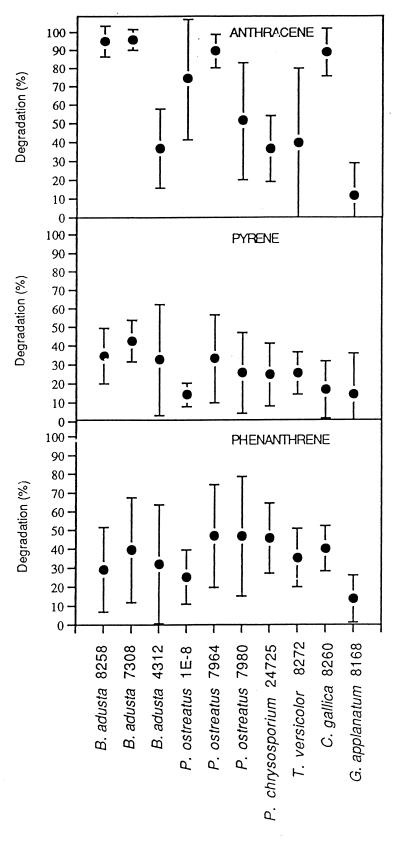
In preliminary experiments, the abilities of 20 fungal isolates to metabolize PAHs were compared. These isolates included three isolates of B. adusta, seven isolates of P. ostreatus, six isolates of P. chrysosporium, and single isolates of C. gallica, T. versicolor, Flammulina velutipes, and G. applanatum. Ten of these strains metabolized less than 15% of the PAH added and were not investigated further. The abilities of the other 10 fungal strains to metabolize three PAHs were examined following 5 days of growth in 2% bran flakes medium containing 5 μg of PAH per ml (Fig. 1). The data obtained were generally consistent; there was relatively narrow variation in individual three-flask experiments but broader variation when the data from three experiments were examined together. The consistency of the three-flask experiment was increased by the high efficiency of extraction of PAH by tetrahydrofuran, which was >90% in the control (autoclaved mycelium). For most strains, anthracene, the PAH with the lowest ionization potential, was the most readily metabolized substrate, while phenanthrene and pyrene were the least readily metabolized substrates.
FIG. 1.
In vivo metabolism of anthracene, pyrene, and phenanthrene by white rot fungi.
In vitro oxidation of PAHs by C. gallica laccase.
We examined the ability of C. gallica laccase to metabolize various PAHs (Table 1). The reactions were carried out in the presence of 15% acetonitrile; under these conditions the laccase retained about 90% of its activity in buffer alone, but the substrate was not saturating and a first-order reaction was evident (Fig. 2). Of the 10 potential substrates tested, 7 were significantly metabolized by laccase in the presence of the mediating substrate ABTS and slowly metabolized in its absence. Two PAHs, pyrene and fluoranthene, were not metabolized, and azulene autooxidized in the presence of ABTS without enzyme. There was no obvious relationship between the ionization potential of a PAH and its rate constant for oxidation.
TABLE 1.
Rates of reaction of C. gallica laccase with PAHs
| PAH | Wavelengtha (nm) | Ionization potential (eV)b | Rate constant (h−1)c |
|---|---|---|---|
| Benzo[a]pyrene | 295 | 7.12 | 83 |
| 9-Methylanthracene | 253 | 7.23 | 240 |
| 2-Methylanthracene | 247 | 7.42 | 4.9 |
| Azulene | 270 | 7.43 | NEOd |
| Anthracene | 225 | 7.55 | 5.2 |
| Biphenylene | 246 | 7.58 | 3.8 |
| Acenaphthene | 226 | 7.7 | 10 |
| Pyrene | 236 | 7.72 | NRe |
| Fluoranthene | 235 | 7.76 | NR |
| Phenanthrene | 250 | 8.03 | 0.8 |
HPLC detection wavelength.
Data from reference 15.
First-order rate constant for substrate loss.
NEO, nonenzymatic oxidation.
NR, no reaction.
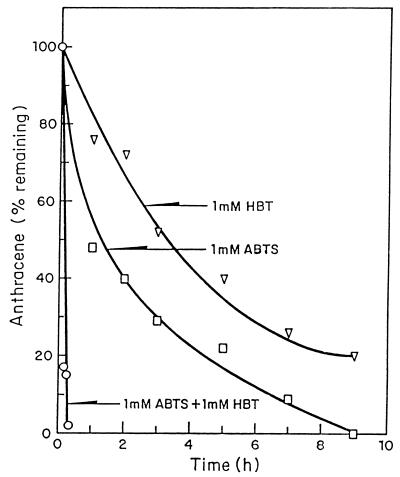
FIG. 2.
Effects of the mediating substrates HBT and ABTS on anthracene metabolism by C. gallica laccase.
The mass spectrum of the oxidation product obtained from anthracene matched the mass spectrum of anthraquinone, and (M+) 208 m/z, 180 m/z, 162 m/z, and 75 m/z ions were the major ions. We did not quantify the conversion of anthracene to anthraquinone, but an HPLC analysis showed that anthraquinone was by far the most abundant metabolite, although traces of a second unknown product were detected. Two oxidized products were obtained from acenaphthene, and the mass spectra of these compounds indicated that one of them was the dione with (M+) 182 m/z, 154 m/z, and 126 m/z as the major ions. The other product was a monohydroxydione derivative with (M+) 198 m/z, 164 m/z, and 126 m/z as the major ions. Although phenanthrene was clearly oxidized, we could not definitely identify the oxidation product.
Effect of mediating substrate concentration on anthracene oxidation.
We investigated the effects of two mediating substrates, HBT and ABTS, on anthracene oxidation. When 5 U of laccase was used, 20 μM anthracene was totally oxidized in 10 min in the presence of 1 mM ABTS plus 1 mM HBT (Fig. 2). When only 1 mM ABTS was the mediating substrate, all of the anthracene was oxidized in 9 h, and when only 1 mM HBT was the mediating substrate, 80% of the anthracene was oxidized in 9 h.
Comparison of anthracene oxidation by laccases from C. gallica and P. ostreatus.
We compared the activities of two laccase preparations, one obtained from C. gallica and one obtained P. ostreatus (17), by using 20 μM anthracene and different combinations of the two mediating substrates HBT and ABTS. In the presence of 1 mM HBT the reaction rate for C. gallica laccase was 0.08 nmol · h−1 · U−1, and the reaction rate for P. ostreatus laccase was 0.09 nmol · h−1 · U−1. When 1 mM ABTS was used, the corresponding rates were 0.16 and 0.17 nmol · h−1 · U−1, respectively. However, when both mediating substrates were used (each at a final concentration of 1 mM), the anthracene metabolism rates increased by factors of about 10, to 1.43 nmol · h−1 · U−1 for C. gallica laccase and to 2.09 nmol · h−1 · U−1 for the P. ostreatus enzyme. Thus, the activities of the two enzyme preparations were essentially identical, but in both cases the two mediating substrates stimulated activity not in an additive fashion but in a synergistic fashion.
DISCUSSION
In vivo PAH metabolism.
We wanted to identify new sources of enzymes that can be used for metabolism of PAHs and hypothesized that fungi capable of metabolizing PCBs (1) might also exhibit activity against PAHs. Our results confirmed this hypothesis because we found (Fig. 1) that about one-half the white rot fungi examined could metabolize anthracene, pyrene, and phenanthrene, although we do not know the mechanism used. While there was significant variation in our data, the trends are clear. In most cases the relative rates of metabolism were highest for the compound with the lowest ionization potential, anthracene, while the values for phenanthrene and pyrene, which have higher ionization potentials, were lower. Triplicate flasks in individual experiments gave more reproducible data than the variations shown by the error bars in Fig. 1, which indicate the standard deviations for the nine flasks of the three separate experiments performed. Although approximately equivalent amounts of biomass were present in each inoculum, the 10 cultures grew at different rates, and the 5-day incubation with PAHs introduced another potential variable in the three experiments. The levels of recovery of PAHs from killed mycelia were 93% ± 12% for up to 10 days after PAHs were added. Given that PAHs are hydrophobic and, like PCBs (1), may nonspecifically bind to glassware and biomass, part of the PAH loss may have been due to absorption. However, the reproducibility of the control extraction results and the results obtained with triplicate experimental flasks compared to the differences between the results of repeated experiments indicated that nonspecific PAH binding was a minor component of the total loss of PAH.
Metabolism of PAHs by whole fungal cultures has been demonstrated previously (7, 22). At least two mechanisms are involved; one uses the cytochrome P-450 system (2, 22), and the other uses the soluble extracellular enzymes of lignin catabolism, including lignin peroxidase (7, 11), manganese peroxidase (3, 4), and laccase (5, 10, 13, 17). There were no studies involving many fungi and several PAHs until this study.
In vitro PAH metabolism.
Both laccase and manganese peroxidase are known to metabolize individual or small groups of PAHs (3–5, 10, 12, 13, 17). We examined the in vitro metabolism of PAHs by the laccase from C. gallica. When purified laccase was used, 22% of 20 μM anthracene was metabolized in 15% acetonitrile–acetate buffer (pH 4.5) over a 6-h period in the absence of mediating substrates. Addition of 1 mM ABTS as a mediating substrate increased this value to 60% in 6 h. Of the 10 PAHs tested, 7 were oxidized by C. gallica laccase; these compounds included phenanthrene, which has a sufficiently high ionization potential (8.03 eV) that this result was unexpected (14), but not pyrene or fluoranthene, which have lower ionization potentials. Phenanthrene degradation by P. ostreatus has been ascribed to a cytochrome P-450 monooxygenase system (2), while Collins et al. (10) were not able to demonstrate oxidation of phenanthrene by T. versicolor laccase and attributed this result to the relatively high ionization potential. C. gallica laccase catalyzed low-level but consistent phenanthrene degradation in this study. 9-Methylanthracene was the substrate that was most rapidly oxidized (Table 1) and was completely oxidized in the presence of ABTS within 10 min.
Laccases and mediating substrates.
The accelerative ability of the laccase-mediating substrates ABTS and HBT appears to act differently in different systems. ABTS was about twice as efficient a mediating substrate as HBT for oxidizing anthracene (Fig. 2). The two mediating substrates, each at a concentration of 1 mM, increased the oxidation rate about 10-fold compared with the rate obtained with ABTS alone. Other groups of workers have reported quite different data. Using veratryl alcohol as a model lignin compound, Bourbonnais and Paice (6) showed that at a concentration of 1 mM, HBT was about twice as effective as ABTS at stimulating veratryl alcohol oxidation when T. versicolor laccase was used and that the two mediating substrates, each at a concentration of 0.5 mM, were as effective as HBT at a concentration of 1 mM. However Johannes et al. (13), using T. versicolor laccase to oxidize 84 μM anthracene, found that ABTS at a concentration of 1 mM was about seven times more effective than HBT after 72 h and that 0.1 mM ABTS was about 10 times more effective than HBT. Clearly, the stimulatory effects of these mediating substrates depend very much on the system being analyzed. We also compared reaction rates for oxidation of anthracene by using laccase purified from C. gallica and laccase purified from P. ostreatus and found that the rates were very similar. These data suggest that under the conditions which we used other laccase preparations would act similarly and that the differences described above were due to experimental conditions. At the PAH concentration (20 μM) used in our in vitro studies, a mediating substrate concentration of 1 mM resulted in a complete loss of anthracene within 10 min. Reducing the mediating substrate concentration 10-fold reduced the reaction rate 50% over the same period.
In this study we examined 20 white rot fungal strains originally selected on the basis of potential PCB metabolism (1) and found that 10 of them can metabolize at least three PAHs. Most of these fungi produce extracellular oxidative enzymes involved in lignin degradation when they are grown in a 2% cereal bran medium (19). These enzymes are primarily laccases and manganese peroxidases. Some of the fungi produce only laccase, some produce only manganese peroxidase, and some produce both enzymes (19). Purified laccase from C. gallica, a fungus not previously described as an organism that can metabolize PAHs, can oxidize benzo[a]pyrene, 9-methylanthracene, 2-methylanthracene, anthracene, biphenylene, acenaphthene, and phenanthrene but not pyrene or fluoranthene. The first-order rate constants for these reactions are not clearly related to the ionization potentials of the PAHs. C. gallica laccase was relatively stable in aqueous 15% acetonitrile solutions, suggesting that it may be suitable for proposed studies on activity in various solvents. The ability of this enzyme to oxidize anthracene was enhanced by the mediating substrates ABTS and HBT separately, and together ABTS and HBT acted in a synergistic manner. Mediating substrates, such as ABTS and HBT, extend the substrate range of laccases to nonphenolic subunits of lignin (6). When oxidized by laccase, HBT forms a nitroxy radical, a potent electrophile; oxy radicals are known to cooxidize PAHs (5). The synergy among ABTS, HBT, and anthracene oxidation is a novel observation, and its mechanism is unknown. Perhaps the interaction of the two oxidized species in some way activates one compound to become a more potent oxidant than when it is oxidized by laccase directly or protects laccase activity from inactivation due to interaction with the mediating substrate free radical (16). Similar anthracene oxidation properties were exhibited by the P. ostreatus laccase, suggesting that these properties may be general properties of laccases under the conditions which we used.
ACKNOWLEDGMENTS
This work was supported by grant IN 220597 from DGPA-UNAM to (R.V.D.) and by grant A6482 from NSERC (to M.A.P.).
REFERENCES
- 1.Beaudette L E, Davies S, Fedorak P M, Ward O P, Pickard M A. Comparison of biodegradation and mineralization as methods for measuring loss of selected polychlorinated biphenyl congeners in cultures of four white rot fungi. Appl Environ Microbiol. 1998;64:2020–2025. doi: 10.1128/aem.64.6.2020-2025.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Bezalel L, Hadar Y, Cerniglia C E. Enzymatic mechanisms involved in phenanthrene degradation by the white rot fungus Pleurotus ostreatus. Appl Environ Microbiol. 1997;63:2495–2501. doi: 10.1128/aem.63.7.2495-2501.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Bogan B, Lamar R T. Polycyclic aromatic hydrocarbon-degrading capabilities of Phanerochaete laevis HHB-1625 and its extracellular ligninolytic enzymes. Appl Environ Microbiol. 1996;62:1597–1603. doi: 10.1128/aem.62.5.1597-1603.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Bogan B, Lamar R T, Hammel K E. Fluorene oxidation in vivo by Phanerochaete chrysosporium and in vitro during manganese peroxidase-dependent lipid peroxidation. Appl Environ Microbiol. 1996;62:1788–1792. doi: 10.1128/aem.62.5.1788-1792.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Bohmer S, Messner K, Srebotnik E. Oxidation of phenanthrene by a fungal laccase in the presence of 1-hydroxybenzotriazole and unsaturated lipids. Biochem Biophys Res Commun. 1998;244:233–238. doi: 10.1006/bbrc.1998.8228. [DOI] [PubMed] [Google Scholar]
- 6.Bourbonnais R, Paice M G. Oxidation of non-phenolic substrates. An expanded role for laccase in lignin biodegradation. FEBS Lett. 1990;267:99–102. doi: 10.1016/0014-5793(90)80298-w. [DOI] [PubMed] [Google Scholar]
- 7.Bumpus J A. Biodegradation of polycyclic aromatic hydrocarbons by Phanerochaete chrysosporium. Appl Environ Microbiol. 1989;55:154–158. doi: 10.1128/aem.55.1.154-158.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Cerniglia C. Biodegradation of polycyclic aromatic hydrocarbons. Curr Opin Biotechnol. 1993;4:331–338. [Google Scholar]
- 9.Cerniglia C. Fungal metabolism of polycylic aromatic hydrocarbons: past, present and future applications in bioremediation. J Ind Microbiol Biotechnol. 1997;19:324–333. doi: 10.1038/sj.jim.2900459. [DOI] [PubMed] [Google Scholar]
- 10.Collins P J, Kotterman M J J, Field J A, Dobson A D W. Oxidation of anthracene and benzo[a]pyrene by laccases from Trametes versicolor. Appl Environ Microbiol. 1996;62:4563–4567. doi: 10.1128/aem.62.12.4563-4567.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Hammel K E, Green B, Gai W Z. Ring fission of anthracene by a eukaryote. Proc Natl Acad Sci USA. 1991;88:10605–10608. doi: 10.1073/pnas.88.23.10605. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Hofrichter M, Scheibner K, Schneegass I, Fritsche W. Enzymatic combustion of aromatic and aliphatic compounds by manganese peroxidase from Nematoloma frowardii. Appl Environ Microbiol. 1998;64:399–404. doi: 10.1128/aem.64.2.399-404.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Johannes C, Majcherczyk A, Huttermann A. Degradation of anthracene by laccase of Trametes versicolor in the presence of different mediating substrate compounds. Appl Microbiol Biotechnol. 1996;46:313–317. doi: 10.1007/s002530050823. [DOI] [PubMed] [Google Scholar]
- 14.Kersten P J, Kalyanaraman B, Hammel K E, Reinhammar B. Comparison of lignin peroxidase, horseradish peroxidase and laccase in the oxidation of methoxybenzenes. Biochem J. 1990;268:475–480. doi: 10.1042/bj2680475. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Levin R D, Lias S G. Ionization potentials and appearance potential measurements, 1971–1981. U.S. National Standard Reference Data Series No. 71. Washington, D.C: U.S. Bureau of Standards; 1982. [Google Scholar]
- 16.Li K, Xu F, Eriksson K-E L. Comparison of fungal laccases and redox mediators in oxidation of a nonphenolic lignin model compound. Appl Environ Microbiol. 1999;65:2654–2660. doi: 10.1128/aem.65.6.2654-2660.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Majcherczyk A, Johannes C, Huttermann A. Oxidation of polycyclic aromatic hydrocarbons (PAH) by laccase of Trametes versicolor. Enzyme Microb Technol. 1998;22:335–341. [Google Scholar]
- 18.Mester T, Pena M, Field J A. Nutrient regulation of extracellular peroxidases in the white rot fungus Bjerkandera sp. strain BOS55. Appl Microbiol Biotechnol. 1996;44:778–784. [Google Scholar]
- 19.Pickard, M. A., H. Vandertol, R. Roman, and R. Vazquez-Duhalt. High production of ligninolytic enzymes from white rot fungi in cereal bran liquid medium. Can. J. Microbiol., in press.
- 20.Rodriguez E, Pickard M A, Vazquez-Duhalt R. Industrial dye decolorization by laccases from ligninolytic fungi. Curr Microbiol. 1999;38:27–32. doi: 10.1007/pl00006767. [DOI] [PubMed] [Google Scholar]
- 21.Sack U, Heinze T M, Deck J, Cerniglia C E, Martens R, Zadrazil F, Fritsche W. Comparison of phenanthrene and pyrene degradation by different wood-decaying fungi. Appl Environ Microbiol. 1997;63:3919–3925. doi: 10.1128/aem.63.10.3919-3925.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Sutherland J B, Rafii F, Khan A A, Cerniglia C E. Mechanisms of polycyclic aromatic hydrocarbon degradation. In: Young L Y, Cerniglia C E, editors. Microbial transformation and degradation of toxic organic chemicals. New York, N.Y: Wiley-Liss; 1995. [Google Scholar]
- 23.Tinoco R, Vazquez-Duhalt R. Chemical modification of cytochrome c improves the catalytic properties of polycyclic aromatic hydrocarbons. Enzyme Microb Technol. 1998;22:8–12. [Google Scholar]
- 24.Vazquez-Duhalt R, Westlake D W S, Fedorak P M. Lignin peroxidase oxidation of aromatic compounds in systems containing aromatic solvents. Appl Environ Microbiol. 1994;60:459–466. doi: 10.1128/aem.60.2.459-466.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Vazquez-Duhalt R, Westlake D W S, Fedorak P M. Kinetics of chemically modified lignin peroxidase and enzymatic oxidation of aromatic nitrogen-containing compounds. Appl Microbiol Biotechnol. 1995;42:675–681. [Google Scholar]
- 26.Wolfenden B S, Wilson R L. J. Chem. Soc. Perkin Trans. II:805–812. 1982. Radical cations as reference chromogens in studies of one-electron transfer reactions: pulse radiolysis studies of 2,2′-azinobis-(3-ethylbenzthiazoline-6-sulfonate) [Google Scholar]