INTRODUCTION
Adrenal incidentalomas are adrenal masses greater than 1 cm in size that are detected on cross-sectional imaging performed for an unrelated indication.1 These lesions are common, estimated to be present in 4% of patients on imaging series, and up to 10% of the elderly population.2,3 Most of these lesions are benign nonfunctioning adrenocortical adenomas, with a minority being hormonally active or malignant.
The evaluation and management of adrenal incidentalomas is a multidisciplinary process, relying on the expertise of family physicians, urologists, endocrinologists, and radiologists. In 2011, the Canadian Urological Association (CUA) released a guideline on the management of the incidentally discovered adrenal mass.4 Since then, there have been several clinical practice guidelines published by various endocrine, radiological, and surgical societies.5–8 A review by Maas et al compared these guidelines and found many points of discrepancy and controversy.9 Furthermore, in a letter to the editor from April 2021, McInnes et al suggested an important revision to the current CUA guideline.10
The purpose of this guideline is to provide an updated approach to the diagnosis, management, and followup of adrenal incidentalomas, with a special focus on the areas of discrepancy/controversy existing among the published guidelines from other associations.
METHODS
This guideline was developed by a working group comprised of urologists, endocrinologists, and radiologists across Canada. The working group met virtually on multiple occasions to discuss the priorities for the guideline and to review the manuscript and recommendations. The recommendations and the evidence used to inform each recommendation were reviewed and agreed upon by the working group. When required, consensus was reached by discussion among group members. The target audience of this guideline is healthcare providers who manage patients with adrenal incidentalomas (e.g., family physicians, endocrinologists, internists, urologists, endocrine surgeons, etc.), as well as patients with adrenal incidentalomas.
The Grading of Recommendations Assessment, Development and Evaluation (GRADE ) framework was used as a methodological basis for this guideline. Our evidence synthesis was completed using PubMed, Medline, and Cochrane Library databases.
The first step was defining clinical questions. A list of 12 clinical questions were compiled and are displayed in Table 1. Next, a systematic literature search was conducted to address each question. For questions where there was a recent high-quality guideline analysis and recommendation, focus was given towards any subsequent peer-reviewed publications and adapting the recommendation to a Canadian context. Special attention was paid to areas of controversy/discrepancy between the currently published guidelines.5–8 For each recommendation, the strength of recommendation was reported as weak or strong, and the quality of evidence was evaluated as low, moderate, or high. A summary of all recommendations is displayed in Table 2.
Table 1.
Clinical questions regarding the workup, management, and surveillance of adrenal incidentalomas addressed in the guideline
|
Table 2.
Summary of recommendations
| Recommendation | Strength of recommendation | Quality of evidence | |
| 1 | Workup for an adrenal incidentaloma should include a focused history and physical examination aimed at identifying signs/symptoms of adrenal hormone excess, adrenal malignancy, and/or extra-adrenal malignancy. | Clinical principle | |
| 2 | There should be a low threshold for a multidisciplinary review by endocrinologists, surgeons, and radiologists when the imaging is not consistent with a benign lesion, there is evidence of hormone hypersecretion, the tumor has grown significantly during followup imaging, or adrenal surgery is being considered. | Clinical principle | |
| 3 | Patients found to have an indeterminate incidental adrenal mass should undergo a non-contrast CT as first-line imaging to distinguish benign lesions from those that require further radiological investigation. | Strong | Moderate |
| 4 | Patients who continue to have an indeterminate adrenal mass on non-contrast CT should undergo second-line imaging with either washout CT or chemical-shift MRI. | Weak | Moderate |
| 5 | Adrenal mass biopsy should not be performed routinely for the workup of an adrenal incidentaloma. | Strong | Moderate |
| 6.1 | All patients with adrenal incidentalomas should be screened for autonomous cortisol secretion. | Weak | Moderate |
| 6.2 | 1 mg dexamethasone suppression testing is the preferred screening test for identifying autonomous cortisol secretion when clinically appropriate. | Strong | Moderate |
| 7.1 | Patients with adrenal incidentalomas and hypertension and/or hypokalemia should be screened for primary aldosteronism with an aldosterone-to-renin ratio. | Strong | Moderate |
| 7.2 | Adrenal vein sampling is recommended prior to offering adrenalectomy in patients with primary aldosteronism. | Strong | Moderate |
| 8.1 | We suggest against screening for pheochromocytoma in patients who have unequivocal adrenocortical adenomas confirmed on unenhanced CT (<10 HU) and no signs or symptoms of adrenergic excess. | Weak | Low |
| 8.2 | Patients with adrenal incidentalomas that display ≥10 HU on non-contrast CT or who have signs/symptoms of catecholamine excess should be screened for pheochromocytoma with plasma or 24-hour urinary metanephrines. | Strong | Moderate |
| 9 | In cases of suspected adrenocortical carcinoma and/or when clinical signs of virilization are present, serum testing of excess androgen testing should be performed. | Clinical principle | |
| 10.1 | Patients with unilateral cortisol-secreting adrenal masses and clinically apparent Cushing’s syndrome should undergo unilateral adrenalectomy of the affected adrenal gland. Minimally invasive surgery should be performed when feasible for these procedures. | Clinical principle | |
| 10.2 | Younger patients with mild autonomous cortisol secretion who have progressive metabolic comorbidities attributable to cortisol excess can be considered for adrenalectomy after shared decision-making. Patients not managed surgically should undergo annual clinical screening for new or worsening associated comorbidities. | Weak | Low |
| 11 | Adrenalectomy should be performed for patients with unilateral aldosterone-secreting adrenal masses and pheochromocytomas. Minimally invasive surgery should be performed when feasible for these procedures. | Clinical principle | |
| 12.1 | Minimally invasive adrenalectomy can be offered to patients with suspected adrenocortical carcinomas that can be safely resected without rupturing the tumor capsule. | Weak | Low |
| 12.2 | Open adrenalectomy should be considered for patients with larger adrenocortical carcinomas or those presenting with locally advanced tumors, lymph node metastases, or tumor thrombus in the renal vein/inferior vena cava. | Strong | Low |
| 13 | Patients with benign non-functioning adenomas <4 cm, myelolipomas, and other small masses containing macroscopic fat detected on the initial workup for an adrenal incidentaloma do not require further followup imaging or functional testing. | Strong | Moderate |
| 14.1 | Patients with non-functioning adrenal lesions that are radiologically benign (<10 HU) but >4 cm should undergo repeat imaging in 6–12 months | Weak | Low |
| 14.2 | Adrenalectomy should be considered for patients with adrenal incidentalomas growing >5 mm/year after repeating a functional workup. | Weak | Low |
| 14.3 | No further imaging followup or functional testing is required for patients with adrenal lesions that grow <3 mm/year on followup imaging. | Weak | Low |
| 15 | Shared decision-making between patients and their clinicians should be used for the management of indeterminate non-functioning adrenal lesions. Management options include repeat imaging in 3–6 months vs. surgical resection. | Clinical principle | |
CT: computed tomography.
DEFINITION OF ADRENAL INCIDENTALOMA
An adrenal incidentaloma is an adrenal mass detected on cross-sectional imaging performed for an unrelated indication. The imaging test could not have been ordered to evaluate symptoms of adrenal hormone excess or a suspected adrenal mass. Adrenal masses identified on imaging studies performed for tumor staging in patients with a known cancer are also not considered adrenal incidentalomas. Generally, these masses are found during the workup of signs/symptoms not felt to be related to the adrenal glands, such as abdominal or back pain. Adrenal incidentalomas must also be equal to or greater than 1 cm in size.1
WORKUP OF AN INCIDENTALLY DETECTED ADRENAL MASS
The differential diagnosis for an adrenal incidentaloma is broad. These masses can be broken down into three categories: benign non-functioning, benign hyperfunctioning, and malignant lesions. A full breakdown of potential etiologies of adrenal masses and their estimated prevalence is presented in Table 3.1–4 The most common lesion is a benign non-functioning adrenal adenoma. These are estimated to make up 75% of adrenal incidentalomas. Other potential benign adrenal masses include myelolipomas, cysts, lymphangiomas, and ganglioneuromas.
Table 3.
Differential diagnosis of adrenal incidentalomas and estimated prevalence
| Type | Range (%) |
|---|---|
|
| |
| Benign non-functional | |
| Non-functioning adenoma | 71–84 |
| Ganglioneuroma | 0–8 |
| Myelolipoma | 7–15 |
| Cysts | 4–22 |
|
| |
| Benign functioning | |
| Cortisol secreting adenoma | 1–30 |
| Aldosterone secreting adenoma | 2–7 |
| Pheochromocytoma | 1.5–14 |
|
| |
| Malignant | |
| Adrenocortical carcinoma | 1.2–12 |
| Metastases | 0–21 |
| Pheochromocytoma | 1.5–14 |
|
| |
| Data from references | 1–4. |
Hyperfunctioning adrenal lesions include cortisol-secreting adenomas (5.3% of all adrenal incidentalomas), aldosterone-secreting adenomas (1%), or catecholamine-secreting pheochromocytomas (5.1%).5 Finally, adrenal incidentalomas could represent malignant lesions, such as adrenocortical carcinoma (ACC) (4.7%) or metastases (2.5%).5 A systematic review found that approximately 20% of all adrenal incidentalomas were potential surgical lesions.3,6
History and physical examination
When an adrenal incidentaloma is detected, a careful evaluation must be carried out to evaluate for any clinical signs or symptoms of a hyperfunctioning lesion or underlying malignancy. The general approach to the clinical history and physical exam for the patient with an incidentally detected adrenal mass is displayed in Table 4.
Table 4.
Elements of a focused history and physical examination are tailored towards detecting possible etiologies for an adrenal incidentaloma
| Condition | History | Physical exam |
|---|---|---|
| Hypercortisolism (Cushing’s s yndrome) | Weight gain, central obesity, easy bruising, severe hypertension, diabetes, proximal muscle weakness, fatigue, depression, sleep disturbances, menstrual irregularities and virilization (in females), or fragility fractures | Hypertension, central obesity, supraclavicular fat accumulation, a dorsocervical fat pad, facial plethora, thinned skin, purple and wide (>1 cm) striae, acne, ecchymoses, hirsutism, and proximal muscle weakness or wasting |
| Aldosteronism | Hypertension, hypokalemia, muscle cramping and weakness, headaches, intermittent or periodic paralysis | Hypertension, fluid retention |
| Pheochromocytoma | Headaches, anxiety attacks, sweating, palpitations, or family history of von Hippel-Lindau disease, multiple endocrine neoplasia type 2, familial paraganglioma syndrome, or neurofibromatosis type 1 | Severe hypertension, tachycardia, arrhythmias, congestive heart failure, excessive sweating, anxiety, and pallor |
| Adrenocortical c arcinoma | Flank pain, vague abdominal discomfort, hypercortisolism, virilization, feminization or aldosteronism | Weight loss, hirsutism, gynecomastia, signs of hypercortisolism |
| Metastasis | Personal and family history of malignant lesions, weight loss, unexplained fevers, lack of adherence to an age-appropriate cancer screening program, and smoking history | Lymphadenopathy, lung mass, breast mass, renal mass or skin lesion suspicious for melanoma, as well as other cancer-specific findings |
■ RECOMMENDATION 1
Workup for an adrenal incidentaloma should include a focused history and physical examination aimed at identifying signs/symptoms of adrenal hormone excess, adrenal malignancy, and/or extra-adrenal malignancy (Clinical principle).
■ RECOMMENDATION 2
There should be a low threshold for a multidisciplinary review by endocrinologists, surgeons, and radiologists when the imaging is not consistent with a benign lesion, there is evidence of hormone hypersecretion, the tumor has grown significantly during followup imaging, or adrenal surgery is being considered (Clinical principle).
Radiological evaluation
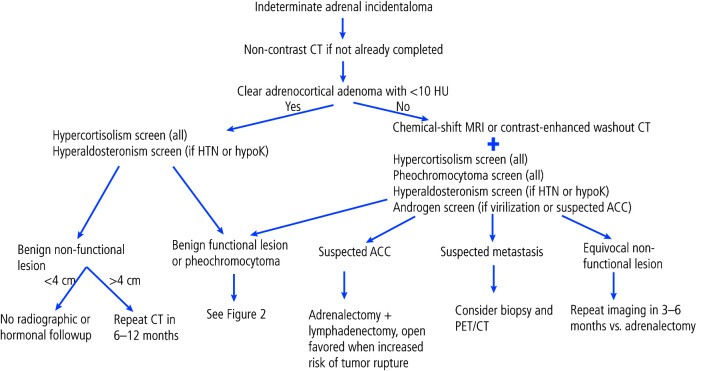
An algorithm for the use of imaging tests in the workup of adrenal incidentalomas is presented in Figure 1. Computed tomography (CT) and magnetic resonance imaging (MR I) are the primary imaging modalities performed to evaluate adrenal incidentalomas.
Figure 1.
Algorithm for the workup of an adrenal incidentaloma. ACC: adrenocortical carcinoma; CT: computed tomography; HTN: hypertension; hypoK: hypokalemia; MRI: magnetic resonance imaging; PET: positron emission tomography.
Figure 2.
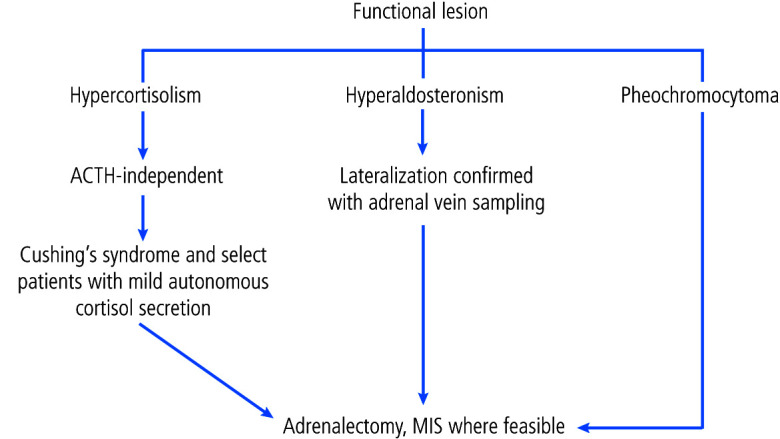
Management of a functional adrenal lesion. ACTH: adrenocorticotropic hormone MIS: minimally invasive surgery.
The first step in characterization of an adrenal mass is to determine if it is benign or malignant. The most validated initial imaging test to characterize adrenal masses is non-contrast CT. A mass that is homogeneous, well-circumscribed, and measures <10 Hounsfield Units (HU) in attenuation can be confidently diagnosed as benign, overwhelmingly representing lipid-rich adrenal cortical adenomas. In a retrospective review of 216 patients who underwent adrenalectomy, 143/143 (100%) patients who had benign features on CT had benign final pathology.11 Similarly, in another retrospective review of 2219 adrenal incidentalomas that were either surgically removed or monitored for at least a year, the risk of finding an ACC was 0% if the HU were <10 on initial imaging (0.5% when HU=10–20 and 6.3% when HU >20).12
In masses that show large areas of macroscopic fat (isoattenuating to retroperitoneal fat and measuring <-10 to -15 HU) a diagnosis of benign myelolipoma can be made.13 Masses with small amounts of macroscopic fat have historically represented a diagnostic dilemma, since macroscopic fat has been found in adrenal cortical carcinoma.14,15 More recent data indicate that many adrenal masses with small amounts of macroscopic fat can be benign adrenal cortical adenomas with myelolipomatous degeneration.16 These can be diagnosed confidently when serial imaging shows a typical adrenal adenoma that later develops areas of macroscopic fat with or without calcification.16 Thus, the presence of small amounts of macroscopic fat in larger, heterogeneous masses should not be considered a diagnostically benign feature.
At a threshold of <10 HU, the sensitivity and specificity of non-contrast CT for benign adenomas is 71% and 98%, respectively.17 Dinnes et al tested this threshold in a systematic review with meta-analysis and found that lesions on non-contrast CT with HU <10 were only identified in those with benign disease.14 It should be noted that approximately 30% of benign adenomas have an attenuation value of >10 HU and are considered lipid-poor, overlapping in density with malignant lesions and pheochromocytomas. Thus, adrenal masses with HU >10 are considered indeterminate.17–19
■ RECOMMENDATION 3
Patients found to have an incidental adrenal mass should undergo a non-contrast CT as first-line imaging to distinguish benign lesions from those that require further radiological investigation (Strong recommendation, moderate-quality evidence).
Masses that do not fit the radiological criteria for lipid-rich adenoma or myelolipoma outlined above can be further evaluated with either a contrast-enhanced washout CT or chemical-shift MR I.
Adrenal washout CT (contrast-enhanced) has been considered the mainstay for indeterminate adrenal mass evaluation for many years. Adrenal adenomas characteristically take up contrast rapidly and have a rapid loss of contrast or “washout.” Conversely, malignant lesions typically display a slower washout of contrast. These parameters are exploited in contrast-enhanced washout CTs, which quantify the amount of “washout” by measuring lesion attenuation at specific time points during the CT: before injection of contrast medium (HU.pre), at 70 seconds following injection of contrast medium (HU.peak), and then at 15 minutes after contrast injection (HU.15min). From this, the absolute (=100×[HU.peak– HU.15 min]/ [HU.peak–HU.pre]) and relative (=100×[HU.peak–HU.15min]/HU.peak) contrast enhancement washout can be calculated. A relative washout >40% and an absolute washout >60% support the diagnosis of a benign mass.19–23
Recently, contrast-enhanced washout CT has come under scrutiny due to newer data evaluating its test properties.21–23 Limitations of the adrenal washout CT test relate to both false-positive and false-negative rates. It is now widely known that roughly 1/3 of pheochromocytomas may washout in the characteristic range of an adenoma.24,25 Although pheochromocytomas are usually heterogeneous and show higher CT peak attenuation compared to adenoma, there is significant overlap between groups, preventing a confident imaging diagnosis.26 On the contrary, approximately 1/3 of adrenal adenomas do not washout in the adenoma range.27 Moreover, malignant masses can also washout in the adenoma range, which can result in adrenal cortical carcinoma or hypervascular metastases being mistaken for an adenoma on a CT washout.24,28 Clinicians who use washout CTs for characterization of indeterminate adrenal masses should be aware of these above-stated limitations.
A second option for imaging an adrenal mass that is indeterminate on a non-contrast CT scan is chemical-shift MR I. Chemical-shift MR I exploits the different frequency of protons in water and fat and is used to detect microscopic fat.29 Chemical-shift MR I is highly sensitive for microscopic fat and can detect microscopic fat in adrenal adenomas that measure >10 HU on a non-contrast CT and would otherwise be considered lipid-poor.30 Chemical-shift MR I is most useful for adrenal masses that measure 10–30 HU on a non-contrast CT.30 When microscopic fat is identified as a homogeneous signal intensity drop on chemical-shift MR I, these features are diagnostic of lipid-rich adrenal adenoma. Heterogeneous signal intensity drop is a more controversial imaging finding since minute amounts of microscopic fat have been identified in pheochromocytoma, adrenal cortical carcinoma, and some fat-containing metastases.29
A recent systematic review and meta-analysis assessing the test properties of washout CT scans vs. chemical-shift MR I at evaluating indeterminate adrenal lesions was unable to determine superiority of one second-line imaging modality over another. This was largely due to the low number and poor quality of eligible studies.5 Evidence for both washout CT and MR I are weak and there are no head-to-head comparisons for use as a second-line imaging test. The primary advantages of MR I compared to washout CT are the absence of radiation exposure and the lack of iodinated contrast media, making MR I appealing in both young patients (less than 40 years of age), pregnant patients, and those with renal insufficiency. Cost and access, however, are important limitations of MR I in Canada at the present time.
■ RECOMMENDATION 4
Patients who have an indeterminate adrenal mass on non-contrast CT should undergo second-line imaging with either washout CT or chemical-shift MRI (Weak recommendation, moderate-quality evidence).
Role of biopsy
Adrenal mass biopsy is rarely indicated in the workup of an incidental adrenal lesion. Biopsy may be considered when the diagnosis of metastatic disease from an extra-adrenal malignancy would be of value. While biopsy can differentiate metastasis from lipid-poor adenomas and pheochromocytomas, it cannot differentiate an adenoma from an ACC. A 2016 systematic review found that adrenal mass biopsy was associated with a low risk of complication (2.5%) and good diagnostic performance (sensitivity of 87%, specificity 100%).5 Biopsy of suspected ACC should not be routinely performed due to potential risk of tumor seeding the needle tract.31 Prior to biopsy, it is crucial to ensure that a pheochromocytoma has been excluded.
■ RECOMMENDATION 5
Adrenal mass biopsy should not be performed routinely for the workup of an adrenal incidentaloma (Strong recommendation, moderate-quality evidence).
Laboratory evaluation
Adrenal lesions can be hormonally active and secrete cortisol, aldosterone, catecholamines, sex hormones, or steroid precursors. The optimal tests for each of these and their interpretation is outlined in Table 5.
Table 5.
Functional workup for adrenal incidentalomas
| Hormone excess | Population | Tests | Interpretation | Ancillary testing |
|---|---|---|---|---|
| Cortisol | All AIs | 1 mg dexamethasone suppression test: 1 mg taken at 11 pm, serum cortisol measured at 8 am |
|
|
| Aldosterone | Hypertension/Hypokalemia | Aldosterone-to-renin ratio | 20 ng/dL per ng/mL/hr has excellent sensitivity and specificity (>90%) for confirming hyperaldosteronism (labs may have their own calibrated reference cutoffs) | Adrenal vein sampling for lateralization, saline suppression, and saltloading with 24-hr urine aldosterone measurement |
| Catecholamines | HU ≥10 or HU not available |
|
>2X upper limit of normal | N/A |
| Androgens | Suspected ACC or virilization | DHEAS, testosterone | Higher levels suggest greater burden of disease | 17β-estradiol, 17-OH progesterone, androstenedione, 17-OH pregnenolone, 11-deoxycorticosterone, progesterone, and estradiol |
ACC: adrenocortical carcinoma; ACTH: adrenocorticotropic hormone; AI: adrenal incidentaloma; DHEAS: dehydroepiandrosterone sulfate; N/A: not applicable.
The most widely accepted screening test to identify cortisol excess is the 1 milligram (mg) overnight dexamethasone suppression test, although there have been no head-to-head comparisons between alternative tests to assess their diagnostic performance. For the 1 mg overnight dexamethasone suppression test, the patient is given a prescription for 1 mg of dexamethasone to be taken at 11 pm, and the serum cortisol level is measured the following morning at 8 am. Even though cortisol secretion by adrenal adenomas is likely a continuous rather than categorical variable, it is clinically useful to have thresholds. There is evidence to support using a result of ≤50 nmol/L (≤1.8 μg/dL) as sufficient to exclude autonomous cortisol excess (sensitivity >95%). Similarly, cortisol levels >138 nmol/L (>5.0 μg/dL) are in keeping with autonomous cortisol secretions and levels between 51 and 138 nmol/L (1.9–5.0 μg/dL) can be considered equivocal.32–34
There are a few important factors to consider when interpreting the results of a 1 mg overnight dexamethasone suppression test. First, it is important to note that dexamethasone is metabolized through the CYP3A4 enzyme, and its levels can, therefore, be increased or decreased by several interacting medications. Second, estrogens increase cortisol-binding globulin, resulting in a 50% false-positive rate on the 1 mg dexamethasone suppression test in women taking oral contraceptives. Third, patients with critical illness, depression, or shift workers may have a blunted circadian rhythm of cortisol secretion. In these instances, an alternative biochemical test, such as a 24-hour urinary-free cortisol or midnight salivary cortisol, may be considered (Table 5). If hypercortisolism is confirmed on the 1 mg dexamethasone suppression test, referral to endocrinology for additional biochemical tests is warranted. This generally includes confirmatory testing and confirmation of adrenocorticotropic hormone (ACTH)-independent cortisol secretion.
The panel supports screening for cortisol excess in all patients with adrenal incidentalomas given that imaging remains imperfect, with many lipid-poor adenomas overlapping with malignant adrenal masses and pheochromocytomas as discussed above, as well as there likely being a subset of patients with autonomous cortisol secretion but no overt Cushing’s syndrome who could benefit from surgical resection (discussed below).
■ RECOMMENDATION 6.1
All patients with adrenal incidentalomas should be screened for autonomous cortisol secretion (Weak recommendation, moderate-quality evidence).
■ RECOMMENDATION 6.2
1 mg dexamethasone suppression testing is the preferred screening test for identifying autonomous cortisol secretion when clinically appropriate (Strong recommendation, moderate-quality evidence).
For patients with hypertension and/or hypokalemia in whom hyperaldosteronism is suspected, the preferred initial test is the aldosterone/renin ratio (ARR ).35 This test is best done in the morning once the patient has been out of bed for two hours and has been seated for 5–15 minutes. Ideally, patients should be potassium-replete and mineralocorticoid receptor (MR ) antagonists should be withdrawn for at least four weeks before ARR testing. Stopping angiotensin-converting enzyme inhibitors (ACEI) and angiotensin II receptor blockers (ARB ) prior to this testing is not required, as the effect on the ARR is minimal and can be interpreted in light of these confounding factors.35,36
β-adrenergic blockers and central agonists, however, can lead to false-positive results by increasing plasma aldosterone and decreasing renin levels. Ideally, these medications would be halted at least two weeks prior to testing. In most cases, verapamil, hydralazine, prazosin, and doxazosin generally do not have a significant effect on ARR and can be continued or substituted for interfering medications.37
An ARR >20 ng/dL per ng/mL/hr has excellent sensitivity and specificity (>90%) for the diagnosis of hyperaldosteronism.35–38 In addition, suppressed renin levels (i.e., <0.6 ng/mL/hr) are a strong independent predictor of the autonomous aldosterone secretion seen in cases of hyperaldosteronism.39 Saline suppression and salt-loading with 24-hour urine aldosterone measurement are confirmatory tests that can be considered. A comprehensive review of the diagnosis and management of hyperaldosteronism is outside the scope of this guideline, and we direct readers to clinical practice guidelines and excellent expert reviews on this topic.40,41
Furthermore, for most patients, it is paramount to confirm lateralization of aldosterone hypersecretion to the side of the adrenal lesion with adrenal vein sampling (AVS). In a systematic review comprised of 950 patients treated between 1977 and 2009, the authors found that a CT/MR I lesion was discordant with AVS results in 40% of patients. Relying only on CT/MR I results would have led to inappropriate adrenalectomy in 15% of patients (where AVS showed a bilateral problem, better treated with medical therapy), inappropriate exclusion from adrenalectomy in 19% of patients (where AVS showed unilateral secretion), and wrong-sided adrenalectomy in 4% of patients (where AVS showed aldosterone hypersecretion on the contralateral side of the adrenal mass).42,43 The concordance between imaging and AVS appears better in younger patients (age less than 40), but omission of this test in this age group remains controversial.44
■ RECOMMENDATION 7.1
Patients with adrenal incidentalomas and hypertension and/or hypokalemia should be screened for primary aldosteronism with an ARR (Strong recommendation, moderate-quality evidence).
■ RECOMMENDATION 7.2
Adrenal vein sampling is recommended prior to offering adrenalectomy in patients with primary aldosteronism (Strong recommendation, moderate-quality evidence).
Screening for pheochromocytoma is primarily done by measuring plasma-free metanephrines or 24-hour urinary fractionated metanephrines, depending on center-specific testing availability. Plasma normetanephrine levels >2.2 nmol/L or metanephrine levels >1.2 nmol/L are highly specific for cathecholamine hypersecretion.45 A 24-hour urinary metanephrine level two times greater than the upper limit of normal is similarly highly sensitive and specific.46
Traditionally, it has been recommended that all patients with adrenal incidentalomas be tested for pheochromocytomas. Recent evidence from observational studies suggests that biochemical testing for pheochromocytoma is unnecessary in adrenal incidentalomas with unenhanced attenuation of <10 HU (adrenal adenomas).47–49 In the largest of these trials, 99.5% (374/376) of patients with pheochromocytomas had unenhanced attenuation of >10 HU upon retrospective review. The remaining two patients’ masses were exactly 10 HU, with none displaying <10 HU.
Considering this emerging evidence, and the fact that biochemical testing for pheochromocytoma can be cumbersome, time-consuming, and frequently falsely positive, the panel felt it could be omitted in cases when unenhanced CT is clearly in keeping with an adrenocortical adenoma (HU <10).
■ RECOMMENDATION 8.1
Screening for pheochromocytoma can be omitted in patients who have unequivocal adrenocortical adenomas confirmed on unenhanced CT (HU <10) and no signs or symptoms of adrenergic excess (Weak recommendation, low-quality evidence).
■ RECOMMENDATION 8.2
Patients with adrenal incidentalomas that display ≥10 HU on non-contrast CT or who have signs/symptoms of catecholamine excess should be screened for pheochromocytoma with plasma or 24-hour urinary metanephrines (Strong recommendation, moderate-quality evidence).
Adrenocortical carcinoma is responsible for more than half of androgen hypersecretion, which can be confirmed by testing serum levels of dehydroepiandrosterone (DHEA-S), testosterone, 17B-estradiol, 17-OH progesterone, androstenedione, 17-OH pregnenolone, 11-deoxycorticosterone, progesterone, and estradiol.50
■ RECOMMENDATION 9
In cases of suspected ACC and/or when clinical signs of virilization are present, serum testing for excess androgen should be performed (Clinical principle).
MANAGEMENT OF ADRENAL INCIDENTALOMAS
Cortisol-secreting adrenal lesions
It is well-accepted that patients with unilateral cortisol-secreting adrenal lesions and clinical signs/symptoms of Cushing’s syndrome should undergo surgical resection of the hypersecreting adrenal gland;5,51 however, the optimal management of patients with cortisol-secreting adrenal lesions without symptoms of Cushing’s syndrome is less clear. These patients, historically referred to as having subclinical Cushing’s syndrome, are now labelled to have mild autonomous cortisol secretion (MACS). A recent systematic review, comprised of generally low-quality observational studies, showed an association between failed cortisol suppression on 1 mg dexamethasone suppression testing and type 2 diabetes, hypertension, cardiovascular events, vertebral fractures, and mortality.5 Importantly, it also revealed that across three cohort studies with median followups ranging from 3–7.5 years, no patients with failed cortical suppression progressed to develop overt Cushing’s syndrome. Based on this, the panel felt that subclinical Cushing’s should be regarded as having a low risk of progression to overt Cushing’s but can still contribute to medical comorbidity.
To understand the impact of surgery compared to conservative management in patients with MACS, a systematic review consisting of one randomized control trial (RCT) and three observational studies was conducted.5 Despite the RCT, the quality of the evidence was downgraded to low-quality given problems with confounding, bias, imprecision, and indirectness. None of the studies included in the meta-analysis looked at the impact of surgery on vertebral fractures, cardiovascular events, or mortality. The review showed that without surgery, no patients improved with respect to diabetes, hypertension, or dyslipidemia. With surgery, however, improvements were seen in the rates of diabetes and the severity of hypertension and dyslipidemia.5 Based on this data, the panel felt that adrenalectomy could be an option for select patients with MACS, particularly those who are young or have progressive metabolic comorbidities attributable to cortisol excess.
■ RECOMMENDATION 10.1
Patients with unilateral cortisol-secreting adrenal masses and clinically apparent Cushing’s syndrome should undergo unilateral adrenalectomy of the affected adrenal gland. Minimally invasive surgery (MIS) should be performed when feasible (Clinical principle).
■ RECOMMENDATION 10.2
Younger patients with mild autonomous cortisol secretion who have progressive metabolic comorbidities attributable to cortisol excess can be considered for adrenalectomy after shared decision-making. Patients not managed surgically should undergo annual clinical screening for new or worsening associated comorbidities (Weak recommendation, low-quality evidence).
Aldosterone-secreting adenomas and pheochromocytomas
It is also well-accepted that patients with confirmed pheochromocytomas or unilateral aldosterone-producing adrenal adenomas should undergo surgical resection.34,52 The perioperative considerations for removal of a functional adrenal lesion are beyond the scope of this guideline. Specific followup recommendations for pheochromocytomas can be found in a recently published CUA best practice report.53 Following resection of aldosterone-secreting adenomas, postoperative imaging is not necessary and postoperative hormonal workup is only required in the short-term to confirm resolution of hyperfunction. Lack of biochemical cure should raise concern for bilateral disease, recurrence of aldosterone-secreting carcinoma (rare), or removal of the non-hypersecreting adrenal gland if surgery was not guided by AVS.
■ RECOMMENDATION 11
Adrenalectomy should be performed for patients with unilateral aldosterone-secreting adrenal masses and pheochromocytomas. MIS should be performed when feasible (Clinical principle).
Adrenocortical carcinomas
In patients with suspected ACC, resection is recommended.54 A systematic review of the literature looked at the impact of laparoscopic vs. open approach for such cases.5 This systematic review included observational studies of very low quality and had important differences in prognostic factors, such as tumor size and stage. Authors did not detect any difference in completeness of resection, recurrence-free status, and overall survival between laparoscopic and open approaches to adrenalectomy. None of the included studies measured patients’ postoperative pain scores or patient satisfaction, which tend to be improved with laparoscopic vs. open adrenalectomy. There did appear to be increased major postoperative complications with open rather than laparoscopic procedures.5 Other studies have found minimally invasive adrenalectomy for ACC to be associated with higher rates of peritoneal dissemination.55,56 The European Society of Endocrinology set a cutoff of 6 cm for opting for open rather than laparoscopic adrenalectomy, but this was not based on high-quality evidence from clinical studies.5 Similarly, a multidisciplinary expert panel from the University of Southern California strongly recommended an open approach when treating masses >5 cm.9
Based on the above, the panel did not feel it could confidently provide a size cutoff at which point it becomes unsafe to perform minimally invasive adrenalectomy for suspected ACC. It acknowledges that MIS adrenalectomy likely has benefits with decreased morbidity and improved recovery, provided resection can be done without rupture of the tumor capsule, which represents a major risk factor for recurrence.
The role of lymphadenectomy is not clearly established. The National Comprehensive Cancer Network guidelines recommend concurrent lymphadenectomy when performing an adrenalectomy.57 In a retrospective review of 386 patients with stage I–III ACC who underwent a lymphadenectomy, the authors found that median survival was incrementally worse for patients with more positive nodes, and that lymphadenectomy may be associated with improved survival in cN1 patients. If feasible, performing a lymphadenectomy at the time of ACC resection should be considered, as it can at least provide important prognostic information.58
Lastly, adrenalectomy can be considered in select cases of metastatic ACC when complete resection of the primary tumor and all metastases is feasible at the time of primary diagnosis.54 Management of metastatic ACC is beyond the scope of this guideline.
■ RECOMMENDATION 12.1
Minimally invasive adrenalectomy can be offered to patients with suspected ACC that can be safely resected without rupturing the tumor capsule (Weak recommendation, low-quality evidence).
■ RECOMMENDATION 12.2
Open adrenalectomy should be considered for patients with larger ACC or those presenting with locally advanced tumors, lymph node metastases, or tumor thrombus in the renal vein/inferior vena cava (Strong recommendation, low-quality evidence).
Benign non-functioning lesions
Previous recommendations from the CUA suggested repeating imaging at 12 months from diagnosis for benign non-functioning adrenal lesions and repeating a hormonal workup annually for four years.4
In over 2300 patients who had initial characteristic radiological features of an adenoma, no patients were found to develop an adrenal malignancy.1,59 Similarly, in a study of 973 consecutive patients with an incidental adrenal mass and no history of cancer, no malignant lesions were identified.2 The sole case of malignancy identified in an adrenal lesion, which was referenced in the previous CUA guideline, occurred in a patient with known renal cell carcinoma, and hence the adrenal lesion was not truly an adrenal incidentaloma. In light of the demonstrated safety, as well as the burden of the number of followup imaging tests that would be required given the high prevalence of adrenal incidentalomas and the potential cost to the healthcare system, surveillance imaging is no longer recommended for patients with a characteristic adrenal adenoma (<10 HU on non-contrast CT).
A patient’s risk of developing clinically relevant hormonal excess when the initial workup is in keeping with a non-functioning lesion is also low. A systematic review of over 2000 patients examining the natural history of apparently benign non-functioning adrenal incidentalomas found that the risk of developing clinically apparent Cushing’s, an aldosterone-producing adenoma, or a pheochromocytoma were 0.3%, 0–2%, and 0–2%, respectively.1,5,6, Based on these numbers, over 95% of patients will be screened annually unnecessarily. Although a hormonal workup is non-invasive and has minimal procedural risks, the tests do come with a risk of false-positive results, which could lead to further unnecessary testing, intervention, and harm. Furthermore, there is no evidence that hormonal testing is superior to routine clinical assessment to identify clinically significant hormone excess.
Cawood et al calculated a baseline hormonal workup for an adrenal incidentaloma to cost $120 USD in 2018.59 Extrapolating this to the large number of incident cases detected per year would represent a significant cost burden, especially in a publicly funded healthcare system such as Canada’s. Performing an annual targeted history and physical in these patients, and reserving repeat hormonal testing for those suspected to have developed clinically significant hormone excess is likely to be an efficacious strategy that will decrease the chances of false-positive results and will be cost-effective. For these reasons, repeat hormonal workups are also no longer recommended unless there are new clinical signs and/or symptoms of hormonal excess.
■ RECOMMENDATION 13
Patients with benign non-functioning adenomas <4 cm, myelolipomas, and other small masses containing macroscopic fat detected on the initial workup for an adrenal incidentaloma do not require further followup imaging or functional testing (Strong recommendation, moderate-quality evidence).
An exception to this rule has traditionally been patients with incidentalomas >4 cm. These patients would undergo resection out of concerns for possible malignancy, even if the imaging had characteristically benign features. This was based on retrospective studies showing that most surgically resected pheochromocytomas and ACCs were >4 cm at time of diagnosis.60,61 There is very little data on followup of benign-appearing, large adrenal incidentalomas to guide these decisions. Azoury et al, however, do report that regardless of size, when an adrenal mass is interpreted as benign on CT, there is 100% concordance with benign final pathology.11
Corwin et al studied the growth rate of smaller adrenal masses. In their retrospective review, they compared the growth rate of adrenal adenomas (105) vs. malignant adrenal nodules (26).62 The mean nodule size at baseline was 18.4 mm (range 9–38 mm) for the adenoma group and 29.8 mm (range 0–117 mm) for the malignant group. Their results showed that approximately one-third of radiologically proven adrenal adenomas grew, all at a rate <3 mm/ year. All malignant adrenal nodules grew, and all at a rate >5 mm/year. A growth rate of 3 mm/year distinguished adenomas from malignant nodules with a sensitivity of 100% (95% confidence interval [CI] 86.8–100%) and a specificity of 100% (95% CI 96.6–100%).62
The European Endocrine Society recommends using the RE CIST 1.1 criteria of an increase >20% with an absolute increase of at least 5 mm in diameter to define significant growth.63 It is important to note that the RE CIST 1.1 criteria have not been formally validated in adrenal tumors.
Ultimately, in the setting of a benign-appearing adrenal mass >4 cm, the panel felt that followup imaging after 6–12 months can be considered. Lack of growth of a mass over this period makes a malignancy highly unlikely. Conversely, significant growth of a lesion can be a clue to underlying malignancy and prompt the need for surgical excision. For lesions that grow less than this threshold, re-imaging in 6–12 months can be considered.
■ RECOMMENDATION 14.1
Patients with non-functioning adrenal lesions that are radiologically benign (<10 HU) but >4 cm should undergo repeat imaging in 6–12 months (Weak recommendation, low-quality evidence).
■ RECOMMENDATION 14.2
Adrenalectomy should be considered for patients with adrenal incidentalomas growing >5 mm/year after repeating a functional workup (Weak recommendation, low-quality evidence).
■ RECOMMENDATION 14.3
No further imaging followup or functional testing is required for patients with adrenal lesions that grow <3 mm/year on followup imaging (Weak recommendation, low-quality evidence).
Indeterminate non-functioning lesions
Despite first- and second-line imaging, some adrenal incidentalomas may still remain indeterminate. There is no data to guide the best treatment approach in this clinical scenario.
■ RECOMMENDATION 15
Shared decision-making between patients and their clinicians should be used for the management of indeterminate non-functioning adrenal lesions. Management options include repeat imaging in 3–6 months vs. surgical resection (Clinical principle).
SPECIAL POPULATIONS
Bilateral adrenal incidentalomas
In the setting of bilateral adrenal incidentalomas, each lesion should be separately characterized in the same fashion as a unilateral adrenal incidentaloma, and the same indications for surgery/followup should be followed. Additional considerations include measuring serum 17-hydroxyprogesterone to exclude congenital adrenal hyperplasia, and assessing for adrenal insufficiency in suspected cases of bilateral infiltrative disease, metastases, or hemorrhage.5,64,65 Given that bilateral adrenalectomy is associated with higher morbidity than a unilateral adrenalectomy (such as dependence on lifelong adrenal replacement therapy and risk of adrenal crisis), consideration should be given to adrenal-sparing surgery when appropriate.
Young/pregnant/elderly patients
The prevalence of adrenal incidentalomas increases with age. Adrenal incidentalomas are felt to be rare in childhood and adolescence, have a prevalence of approximately 4% in adults, and 10% in individuals over 70 years of age.66–68 Although there is no strong data to support it, it is generally believed that adrenal lesions in young adults, children, and pregnant patients are more likely to be malignant and, therefore, an evaluation should be expedited. In these individuals, radiation safety is an important consideration and low-dose CT, or chemical-shift MR I may be preferred first-line imaging tests.
A small incidentaloma in the elderly patient is less likely to be malignant and, therefore, when there is no clear sign or suspicion of malignancy, the planned workup and management should be adjusted based on the performance status of the individual and potential clinical gains.
History of extra-adrenal malignancy
Available evidence suggests that in patients with a history of an extra-adrenal malignancy, only 7% of adrenal metastases have <10 HU on CT. Lesions >10 HU, however, are malignant in 70% of patients with a history of extra-adrenal malignancy.5 When characterization of these lesions will alter clinical management, adrenal biopsy and flourine-18 fluorodeoxyglucose positron emission tomography (18F-FDG-PET ) can be useful adjuncts.69,70
18F-FDG-PET is a nuclear medicine imaging test conducted after the intravenous injection of 18F-FDG. Uptake of 18F-FDG occurs in cells with increased energy requirements, such as malignant tumors. These studies are often combined with CT for better anatomic overlay and are most useful in oncology patients who have a large, enlarging, or indeterminate adrenal mass where exclusion of metastasis is warranted.70–73 Although adenomas generally do not exhibit significant FDG uptake on PET imaging, exceptions exist. Accordingly, increased standardized uptake values (SUVs) of an adrenal lesion with otherwise benign imaging characteristics must be interpreted in the context of each clinical scenario.74,75
Surgery or other local therapies can be considered for metastasis to the adrenal gland on a case-by-case basis where potential oncological benefit exists, as determined by a multidisciplinary team.
Partial adrenalectomy
Patients with hereditary syndromes are at increased likelihood of disease in the contralateral gland. Similarly, pheochromocytomas and aldosterone-producing adenomas can also have bilateral involvement. It is also estimated that approximately 1% of the population have adrenal gland function affected by various pathological processes, such as infectious or infiltrative processes. In all these patients, there may be an emerging role for partial adrenalectomy to avoid the need for lifelong adrenal replacement therapy. 76
FUTURE DIRECTIONS
Our current workup of incidental adrenal lesions has sensitivity and specificity limitations in determining whether an incidental adrenal lesion is benign or malignant. As a result, some patients receive unnecessary adrenalectomy, some patients’ care is delayed, and some are subjected to unnecessary followup investigations with associated ionizing radiation. To address this, recent research efforts have focused on the development of urine steroid metabolomics to better risk-stratify patients. Studies have shown that ACC have a distinct pattern of urinary corticosteroid excretion, characterized by an excess of precursor steroid metabolites. Using a combination of mass spectrometry-based urinary steroid metabolite profiling and machine learning-based data analysis, urine steroid metabolomic testing can quantify this.
In a 2020, prospective, multicenter study (EURINE-ACT), urine steroid metabolomics were used in conjunction with tumor diameter and imaging characteristics to characterize adrenal incidentalomas, and this “triple test” strategy had a sensitivity of 82.7% and specificity of 95.7%.77 Interestingly, in this study, the authors also showed that using a cutoff of 20 HU instead of 10 HU increased the specificity of unenhanced CT scans in detecting malignant lesion to 80% from 64%, while sensitivity remained similar. These results are certainly promising and require further validation. Hopefully, these non-invasive tests are able to help further risk-stratify patients in the future, allowing for prompt management of patients with ACC, and avoiding the need for unnecessary followup testing and associated harms in those with benign lesions.
CONCLUSIONS
Incidental adrenal masses are common, and most of these lesions are benign. Nonetheless, identification and timely management of functional and malignant lesions is crucial. This guideline provides a contemporary approach to the appropriate clinical, radiographical, and endocrine assessments required for the evaluation, management, and followup of patients with such lesions.
Footnotes
COMPETING INTERESTS: The authors and reviewers do not report any competing personal or financial interests related to this work.
REFERENCES
- 1.NIH state-of-the-science statement on management of the clinically inapparent adrenal mass (“incidentaloma”) NIH Consens State Sci Statements. 2002;19:1–25. Available at https://consensus.nih.gov/2002/2002AdrenalIncidentalomasos021main.htm. [PubMed] [Google Scholar]
- 2.Song JH, Chaudhry FS, Mayo-Smith WW. The incidental adrenal mass on CT: Prevalence of adrenal disease in 1049 consecutive adrenal masses in patients with no known malignancy. AJR Am J Roentgenol. 2008;190:1163–8. doi: 10.2214/AJR.07.2799. [DOI] [PubMed] [Google Scholar]
- 3.Young WF., JrManagement approaches to adrenal incidentalomas: A view from Rochester, MinnesotaEndocrinol Metab Clin North Am 200029159–85. 10.1016/S0889-8529(05)70122-5 [DOI] [PubMed] [Google Scholar]
- 4.Kapoor A, Morris T, Rebello R. Guidelines for the management of the incidentally discovered adrenal mass. Can Urol Assoc J. 2011;5:241–7. doi: 10.5489/cuaj.657. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Fassnacht M, Arlt W, Bancos I, et al. Management of adrenal incidentalomas: European Society of Endocrinology clinical practice guideline in collaboration with the European Network for the Study of Adrenal Tumors. Eur J Endocrin. 2016175:G1–34. doi: 10.1530/EJE-16-0467. [DOI] [PubMed] [Google Scholar]
- 6.Zeiger MA, Thompson GB, Duh QY, et al. American Association of Clinical Endocrinologists and American Association of Endocrine Surgeons medical guidelines for the management of adrenal incidentalomas. Endocrine Pract. 2009;15:1–20. doi: 10.4158/EP.15.S1.1. [DOI] [PubMed] [Google Scholar]
- 7.Lee JM, Kim MK, Ko SH, et al. Clinical guidelines for the management of adrenal incidentaloma. Endocrin Metab. 2017;32:200–18. doi: 10.3803/EnM.2017.32.2.200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Mayo-Smith WW, Song JH, Boland GL, et al. Management of incidental adrenal masses: A white paper of the ACR Incidental Findings Committee. J Am Coll Radiol. 2017;14:1038–44. doi: 10.1016/j.jacr.2017.05.001. [DOI] [PubMed] [Google Scholar]
- 9.Maas M, Nassiri N, Bhanvadia S, et al. Discrepancies in the recommended management of adrenal incidentalomas by various guidelines. J Urol. 2021;205:52–9. doi: 10.1097/JU.0000000000001342. [DOI] [PubMed] [Google Scholar]
- 10.McInnes M, Schieda N. Revising adrenal incidentalomas followup recommendations in CUA guideline. Can Urol Assoc J. 2021;15:E232. doi: 10.5489/cuaj.7267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Azoury SC, Nagarajan N, Young A, et al. Computed tomography in the management of adrenal tumors. J Computer Assist Tomograph. 2017;41:628–32. doi: 10.1097/RCT.0000000000000578. [DOI] [PubMed] [Google Scholar]
- 12.Kahramangil B, Kose E, Remer EM, et al. A modern assessment of cancer risk in adrenal incidentalomas. Ann Surg. 2020;275:e238–44. doi: 10.1097/SLA.0000000000004048. [DOI] [PubMed] [Google Scholar]
- 13.Young WF., Jr The incidentally discovered adrenal mass. N Engl J Med. 2007;356:601–10. doi: 10.1056/NEJMcp065470. [DOI] [PubMed] [Google Scholar]
- 14.Dinnes J, Bancos I, Ferrante di Ruffano L, et al. Imaging for the diagnosis of malignancy in incidentally discovered adrenal masses — a systematic review and meta-analysis. Eur J Endocrinol. 2016;175:R51–64. doi: 10.1530/EJE-16-0461. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Ranathunga DS, Cherpak LA, Schieda N, et al. Macroscopic fat in adrenocortical carcinoma: A systematic review. Am J Roentgenol. 2020;214:390–4. doi: 10.2214/AJR.19.21851. [DOI] [PubMed] [Google Scholar]
- 16.Guccione J, Soliman M, Zhang M, et al. Imaging characteristics of pathologically proven adrenal adenomas with myelolipomatous degeneration: Correlation with clinical and pathologic features. Br J Radiol. 2022;95:20210555. doi: 10.1259/bjr.20210555. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Boland GW, Lee MJ, Gazelle GS, et al. Characterization of adrenal masses using unenhanced CT: An analysis of the CT literature. Am J Roentgenol. 1998;171:201–4. doi: 10.2214/ajr.171.1.9648789. [DOI] [PubMed] [Google Scholar]
- 18.Caoili EM, Korobkin M, Francis IR, et al. Delayed enhanced CT of lipid-poor adrenal adenomas. Am J Roentgenol. 2000;175:1411–5. doi: 10.2214/ajr.175.5.1751411. [DOI] [PubMed] [Google Scholar]
- 19.Pena CS, Boland GW, Hahn PF, et al. Characterization of indeterminate (lipid-poor) adrenal masses: Use of washout characteristics at contrast-enhanced CT. Radiology. 2000;217:798–802. doi: 10.1148/radiology.217.3.r00dc29798. [DOI] [PubMed] [Google Scholar]
- 20.Zhang HM, Perrier ND, Grubbs EG, et al. CT features and quantification of the characteristics of adrenocortical carcinomas on unenhanced and contrast-enhanced studies. Clin Radiol. 2012;67:38–46. doi: 10.1016/j.crad.2011.03.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Corwin MT, Remer EM. Adrenal washout CT: Point-not useful for characterizing incidentally discovered adrenal nodules. Am J Roentgenol. 2021;216:1166–7. doi: 10.2214/AJR.20.24417. [DOI] [PubMed] [Google Scholar]
- 22.Corwin MT, Badawy M, Caoili EM, et al. Incidental adrenal nodules in patients without known malignancy: Prevalence of malignancy and utility of washout CT for characterization — a multi-institutional study. Am J Roentgenol. 2022;219:804–12. doi: 10.2214/AJR.22.27901. [DOI] [PubMed] [Google Scholar]
- 23.Dunnick NR, Korobkin M. Imaging of adrenal incidentalomas: Current status. Am J Roentgenol. 2002;179:559–68. doi: 10.2214/ajr.179.3.1790559. [DOI] [PubMed] [Google Scholar]
- 24.Szolar DH, Kammerhuber FH. Adrenal adenomas and non-adenomas: Assessment of washout at delayed contrast-enhanced CT. Radiology. 1998;207:369–75. doi: 10.1148/radiology.207.2.9577483. [DOI] [PubMed] [Google Scholar]
- 25.Akbulut S, Erten O, Kahramangil B, et al. A critical analysis of computed tomography washout in lipid-poor adrenal incidentalomas. Ann Surg Oncol. 2021;28:2756–62. doi: 10.1245/s10434-020-09329-1. [DOI] [PubMed] [Google Scholar]
- 26.Schieda N, Siegelman ES. Update on CT and MRI of adrenal nodules. Am J Roentgenol. 2017;208:1206–17. doi: 10.2214/AJR.16.17758. [DOI] [PubMed] [Google Scholar]
- 27.Schieda N, Alrashed A, Flood TA, et al. Comparison of quantitative MRI and CT washout analysis for differentiation of adrenal pheochromocytoma from adrenal adenoma. Am J Roentgenol. 2016;206:1141–8. doi: 10.2214/AJR.15.15318. [DOI] [PubMed] [Google Scholar]
- 28.Choi YA, Kim CK, Park BK, et al. Evaluation of adrenal metastases from renal cell carcinoma and hepatocellular carcinoma: Use of delayed contrast-enhanced CT. Radiology. 2013;266:514–20. doi: 10.1148/radiol.12120110. [DOI] [PubMed] [Google Scholar]
- 29.Schieda N, Al Dandan O, Kielar AZ, et al. Pitfalls of adrenal imaging with chemical shift MRI. Clin Radiol. 2014;69:1186–97. doi: 10.1016/j.crad.2014.06.020. [DOI] [PubMed] [Google Scholar]
- 30.Haider MA, Ghai S, Jhaveri K, et al. Chemical-shift MR imaging of hyperattenuating (>10 HU) adrenal masses: Does it still have a role? Radiology. 2004;231:711–6. doi: 10.1148/radiol.2313030676. [DOI] [PubMed] [Google Scholar]
- 31.Mody MK, Kazerooni EA, Korobkin M. Percutaneous CT-guided biopsy of adrenal masses: Immediate and delayed complications. J Comp Assist Tomograph. 1995;19:434–9. doi: 10.1097/00004728-199505000-00017. [DOI] [PubMed] [Google Scholar]
- 32.Di Dalmazi G, Vicennati V, Rinaldi E, et al. Progressively increased patterns of subclinical cortisol hypersecretion in adrenal incidentalomas differently predict major metabolic and cardiovascular outcomes: A large, cross-sectional study. Eur J Endocrin. 2012;166:669–77. doi: 10.1530/EJE-11-1039. [DOI] [PubMed] [Google Scholar]
- 33.Debono M, Bradburn M, Bull M, et al. Cortisol as a marker for increased mortality in patients with incidental adrenocortical adenomas. J Clin Endocrinol Metab. 2014;99:4462–70. doi: 10.1210/jc.2014-3007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Di Dalmazi G, Vicennati V, Garelli S, et al. Cardiovascular events and mortality in patients with adrenal incidentalomas that are either non-secreting or associated with intermediate phenotype or subclinical Cushing’s syndrome: A 15-year retrospective study. Lancet Diabetes Endocrinol. 2014;2:396–405. doi: 10.1016/S2213-8587(13)70211-0. [DOI] [PubMed] [Google Scholar]
- 35.Funder JW, Carey RM, Fardella C, et al. Case detection, diagnosis, and treatment of patients with primary aldosteronism: An Endocrine Society clinical practice guideline. J Clin Endocrinol Metab. 2008;93:3266–3281. doi: 10.1210/jc.2008-0104. [DOI] [PubMed] [Google Scholar]
- 36.Seifarth C, Trenkel S, Schobel H, et al. Influence of antihypertensive medication on aldosterone and renin concentration in the differential diagnosis of essential hypertension and primary aldosteronism. Clin Endocrinol. 2002;57:457–65. doi: 10.1046/j.1365-2265.2002.01613.x. [DOI] [PubMed] [Google Scholar]
- 37.Jędrusik P, Symonides B, Lewandowski J, et al. The effect of antihypertensive medications on testing for primary aldosteronism. Front Pharmacol. 2021;12:684111. doi: 10.3389/fphar.2021.684111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Stowasser M, Gordon RD. Primary aldosteronism-careful investigation is essential and rewarding. Mol Cell Endocrinol. 2004;217:33–9. doi: 10.1016/j.mce.2003.10.006. [DOI] [PubMed] [Google Scholar]
- 39.Hundemer GL, Baudrand R, Brown JM, et al. Renin phenotypes characterize vascular disease, autonomous aldosteronism, and mineralocorticoid receptor activity. J Clin Endocrinol Metab. 2017;102:1835–43. doi: 10.1210/jc.2016-3867. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Funder JW, Carey RM, Mantero F, et al. The management of primary aldosteronism: Case detection, diagnosis, and treatment: An endocrine society clinical practice guideline. J Clin Endocrinol Metab. 2016;101:1889–916. doi: 10.1210/jc.2015-4061. [DOI] [PubMed] [Google Scholar]
- 41.Vaidya A, Carey RM. Evolution of the primary aldosteronism syndrome: Updating the approach. J Clin Endocrinol Metab. 2020;105:3771–83. doi: 10.1210/clinem/dgaa606. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Tan YY, Ogilvie JB, Triponez F, et al. Selective use of adrenal venous sampling in the lateralization of aldosterone-producing adenomas [with discussion] World J Surg. 2006;30:879–87. doi: 10.1007/s00268-005-0622-8. [DOI] [PubMed] [Google Scholar]
- 43.Kempers MJ, Lenders JW, van Outheusden L, et al. Systematic review: Diagnostic procedures to differentiate unilateral from bilateral adrenal abnormality in primary aldosteronism. Ann Intern Med. 2009;151:329–37. doi: 10.7326/0003-4819-151-5-200909010-00007. [DOI] [PubMed] [Google Scholar]
- 44.Quencer KB, Singh A, Sharma A. Best practices: Indications and procedural controversies of adrenal vein sampling for primary aldosteronism. Am J Roentgenol. 2022;17:1–1. doi: 10.2214/AJR.22.27692. [DOI] [PubMed] [Google Scholar]
- 45.Van Berkel A, Lenders JW, Timmers HJ. Diagnosis of endocrine disease: Biochemical diagnosis of phaeochromocytoma and paraganglioma. Eur J Endocrinol. 2014;170:R109–19. doi: 10.1530/EJE-13-0882. [DOI] [PubMed] [Google Scholar]
- 46.Perry CG, Sawka AM, Singh R, et al. The diagnostic efficacy of urinary fractionated metanephrines measured by tandem mass spectrometry in detection of pheochromocytoma. Clin Endocrinol. 2007;66:703–8. doi: 10.1111/j.1365-2265.2007.02805.x. [DOI] [PubMed] [Google Scholar]
- 47.Canu L, Van Hemert JA, Kerstens MN, et al. CT characteristics of pheochromocytoma: Relevance for the evaluation of adrenal incidentaloma. J Clin Endocrinol Metab. 2019;104:312–8. doi: 10.1210/jc.2018-01532. [DOI] [PubMed] [Google Scholar]
- 48.Buitenwerf E, Korteweg T, Visser A, et al. Unenhanced CT imaging is highly sensitive to exclude pheochromocytoma: A multicenter study. Eur J Endocrinol. 2018;178:431–7. doi: 10.1530/EJE-18-0006. [DOI] [PubMed] [Google Scholar]
- 49.Gruber LM, Strajina V, Bancos I, et al. Not all adrenal incidentalomas require biochemical testing to exclude pheochromocytoma: Mayo clinic experience and a meta-analysis. Gland Surg. 2020;9:362. doi: 10.21037/gs.2020.03.04. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Allolio B, Fassnacht M. Clinical review: Adrenocortical carcinoma; clinical update. J Clin Endocrinol Metab. 2006;91:2027–37. doi: 10.1210/jc.2005-2639. [DOI] [PubMed] [Google Scholar]
- 51.Nieman LK, Biller BM, Findling JW, et al. Treatment of Cushing’s syndrome: An Endocrine Society clinical practice guideline. J Clin Endocrinol Metab. 2015;100:2807–31. doi: 10.1210/jc.2015-1818. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Lenders JW, Duh QY, Eisenhofer G, et al. Pheochromocytoma and paraganglioma: An Endocrine Society clinical practice guideline. J Clin Endocrinol Metab. 2014;99:1915–42. doi: 10.1210/jc.2014-1498. [DOI] [PubMed] [Google Scholar]
- 53.Kumar RM, Violette PD, Tran C, et al. Canadian Urological Association best practice report: Long-term surveillance following resection of pheochromocytoma. Can Urol Assoc J. 2019;13:372–6. doi: 10.5489/cuaj.6254. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Fassnacht M, Allolio B. Clinical management of adrenocortical carcinoma. Best Pract Res Clin Endocrinol Metab. 2009;23:273–89. doi: 10.1016/j.beem.2008.10.008. [DOI] [PubMed] [Google Scholar]
- 55.Payabyab EC, Balasubramaniam S, Edgerly M, et al. Adrenocortical cancer: A molecularly complex disease where surgery matters. Clin Cancer Res. 2016;22:4989. doi: 10.1158/1078-0432.CCR-16-1570. [DOI] [PubMed] [Google Scholar]
- 56.Wu K, Liu Z, Liang J, et al. Laparoscopic vs. open adrenalectomy for localized (stage 1/2) adrenocortical carcinoma: Experience at a single, high-volume center. Surgery. 2018;164:1325. doi: 10.1016/j.surg.2018.07.026. [DOI] [PubMed] [Google Scholar]
- 57.Shah MH, Goldner WS, Benson AB, et al. Neuroendocrine and adrenal tumors, version 2.2021, NCCN clinical practice guidelines in oncology. J Natl Compr Canc Netw. 2021;19:839–68. doi: 10.6004/jnccn.2021.0032. [DOI] [PubMed] [Google Scholar]
- 58.Tseng J, DiPeri T, Chen Y, et al. Adrenocortical carcinoma: The value of lymphadenectomy. Ann Surg Oncol. 2022;29:1965–70. doi: 10.1245/s10434-021-11051-5. [DOI] [PubMed] [Google Scholar]
- 59.Cawood TJ, Hunt PJ, O’Shea D, et al. Recommended evaluation of adrenal incidentalomas is costly, has high false-positive rates and confers a risk of fatal cancer that is similar to the risk of the adrenal lesion becoming malignant; time for a rethink? Eur J Endocrinol. 2009;161:513–27. doi: 10.1530/EJE-09-0234. [DOI] [PubMed] [Google Scholar]
- 60.Angeli A, Osella G, Alì A, et al. Adrenal incidentaloma: An overview of clinical and epidemiological data from the National Italian Study Group. Horm Res. 1997;47:279. doi: 10.1159/000185477. [DOI] [PubMed] [Google Scholar]
- 61.Terzolo M, Ali A, Osella G, et al. Prevalence of adrenal carcinoma among incidentally discovered adrenal masses. A retrospective study from 1989–1994. Arch Surg. 1997;132:914–9. doi: 10.1001/archsurg.1997.01430320116020. [DOI] [PubMed] [Google Scholar]
- 62.Corwin MT, Navarro SM, Malik DG, et al. Differences in growth rate on CT of adrenal adenomas and malignant adrenal nodules. Am J Roentgenol. 2019;213:632–6. doi: 10.2214/AJR.19.21342. [DOI] [PubMed] [Google Scholar]
- 63.Eisenhauer EA, Therasse P, Bogaerts J, et al. New response evaluation criteria in solid tumors: Revised RECIST guideline (version 1.1) Eur J Cancer. 2009;45:228–47. doi: 10.1016/j.ejca.2008.10.026. [DOI] [PubMed] [Google Scholar]
- 64.Jaresch S, Kornely E, Kley HK, et al. Adrenal incidentaloma and patients with homozygous or heterozygous congenital adrenal hyperplasia. J Clin Endocrinol Metab. 1992;74:685–89. doi: 10.1210/jcem.74.3.1311000. [DOI] [PubMed] [Google Scholar]
- 65.Del Monte P, Bernasconi D, Bertolazzi L, et al. Increased 17 alpha-hydroxyprogesterone response to ACTH in silent adrenal adenoma: Cause or effect? Clin Endocrinol. 1995;42:273–7. doi: 10.1111/j.1365-2265.1995.tb01875.x. [DOI] [PubMed] [Google Scholar]
- 66.Bovio S, Cataldi A, Reimondo G, et al. Prevalence of adrenal incidentaloma in a contemporary computerized tomography series. J Endocrinol Invest. 2006;29:298–302. doi: 10.1007/BF03344099. [DOI] [PubMed] [Google Scholar]
- 67.Hammarstedt L, Muth A, Wangberg B, et al. Adrenal lesion frequency: A prospective, cross-sectional CT study in a defined region, including systematic re-evaluation. Acta Radiologica. 2010;51:1149–56. doi: 10.3109/02841851.2010.516016. [DOI] [PubMed] [Google Scholar]
- 68.Davenport C, Liew A, Doherty B, et al. The prevalence of adrenal incidentaloma in routine clinical practice. Endocrine. 2011;40:80–3. doi: 10.1007/s12020-011-9445-6. [DOI] [PubMed] [Google Scholar]
- 69.Bancos I, Tamhane S, Shah M, et al. The diagnostic performance of adrenal biopsy: A systematic review and meta-analysis. Eur J Endocrinol. 2016;175:R60–85. doi: 10.1530/EJE-16-0297. [DOI] [PubMed] [Google Scholar]
- 70.Mackie GC, Shulkin BL, Ribeiro RC, et al. Use of [18F] fluorodeoxyglucose positron emission tomography in evaluating locally recurrent and metastatic adrenocortical carcinoma. J Clin Endocrinol Metab. 2006;91:2665–71. doi: 10.1210/jc.2005-2612. [DOI] [PubMed] [Google Scholar]
- 71.Groussin L, Bonardel G, Silvera S, et al. 18F-Fluorodeoxyglucose positron emission tomography for the diagnosis of adrenocortical tumors: A prospective study in 77 operated patients. J Clin Endocrinol Metab. 2009;94:1713–22. doi: 10.1210/jc.2008-2302. [DOI] [PubMed] [Google Scholar]
- 72.Deandreis D, Leboulleux S, Caramella C, et al. FDG-PET in the management of patients with adrenal masses and adrenocortical carcinoma. Hormones Cancer. 2011;2:354–62. doi: 10.1007/s12672-011-0091-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Becherer A, Vierhapper H, Potzi C, et al. FDG-PET in adrenocortical carcinoma. Cancer Biother Radiopharm. 2001;16:289–95. doi: 10.1089/108497801753131363. [DOI] [PubMed] [Google Scholar]
- 74.Canter D, Simhan J, Wu KN, et al. Intensely positron emission tomography-avid benign adrenal adenoma. Urology. 2011;78:1307–8. doi: 10.1016/j.urology.2011.01.049. [DOI] [PubMed] [Google Scholar]
- 75.Boland GW, Dwamena BA, Jagtiani Sangwaiya M, et al. Characterization of adrenal masses by using FDG-PET: A systematic review and meta-analysis of diagnostic test performance. Radiology. 2011;259:117–26. doi: 10.1148/radiol.11100569. [DOI] [PubMed] [Google Scholar]
- 76.Madala A, Daugherty M, Bratslavsky G. Partial adrenalectomy — why should it be considered? Urol Pract. 2015;2:359–66. doi: 10.1016/j.urpr.2015.03.006. [DOI] [PubMed] [Google Scholar]
- 77.Bancos I, Taylor AE, Chortis V, et al. Urine steroid metabolomics for the differential diagnosis of adrenal incidentalomas in the EURINE-ACT study: A prospective test validation study. Lancet Diabetes Endocrinol. 2020;8:773–81. doi: 10.1016/S2213-8587(20)30218-7. [DOI] [PMC free article] [PubMed] [Google Scholar]