Abstract
Near-infrared diffuse correlation spectroscopy (NIR-DCS) is an optical imaging technique for measuring relative changes in skeletal muscle microvascular perfusion (i.e., fold change above baseline) during reactive hyperemia testing and exercise and is reported as a blood flow index (BFI). Although it is generally accepted that changes in BFI are primarily driven by changes in muscle perfusion, it is well known that large, hyperthermia-induced changes in cutaneous blood flow can uncouple this relationship. What remains unknown, is how much of an impact that changes in cutaneous perfusion have on NIR-DCS BFI and estimates of skeletal muscle perfusion under thermoneutral conditions, where changes in cutaneous blood flow are assumed to be relatively low. We therefore used epinephrine iontophoresis to pharmacologically block changes in cutaneous perfusion throughout a battery of experimental procedures. The data show that 1) epinephrine iontophoresis attenuates changes in cutaneous perfusion for up to 4-h posttreatment, even in the face of significant neural and local stimuli, 2) under thermoneutral conditions, cutaneous perfusion does not significantly impact NIR-DCS BFI during reactive hyperemia testing or moderate-intensity exercise, and 3) during passive whole body heat stress, when cutaneous vasodilation is pronounced, epinephrine iontophoresis preserves NIR-DCS measures of skeletal muscle BFI during moderate-intensity exercise. Collectively, these data suggest that cutaneous perfusion is unlikely to have a major impact on NIR-DCS estimates of skeletal muscle BFI under thermoneutral conditions, but that epinephrine iontophoresis can be used to abolish cutaneous contamination of the NIR-DCS BFI signal during studies where skin blood flow may be elevated but skeletal muscle perfusion is of specific interest.
Keywords: blood flow index, epinephrine, exercise, iontophoresis, near-infrared diffuse correlation
INTRODUCTION
Near-infrared diffuse correlation spectroscopy (NIR-DCS) is an increasingly popular optical imaging technique that permits noninvasive assessment of skeletal muscle oxygenation and perfusion at the level of the microvasculature (1–4). NIR-DCS is relatively low cost, has no clinical contraindications, and can be performed under a dynamic range of clinical and experimental settings. A potential weakness of NIR-DCS, however, is that measures of perfusion reflect changes in blood flow across all tissues within the region of insonation, including the cutaneous or subcutaneous adipose tissue layers. We, and others, have indeed shown that NIR-DCS is susceptible to large changes in cutaneous blood flow brought on by thermoregulation (1, 5). Less is known, however, regarding the impact of skin blood flow on NIR-DCS estimates of skeletal muscle blood flow under thermoneutral conditions, when changes in skin blood flow are typically less dramatic.
In cerebral tissue, the hard surface of the cranial bones provides a unique opportunity to reduce the impact of skin blood flow on cerebral perfusion by simply applying enough pressure to the probe to abolish skin blood flow (6). However, this technique is not applicable to skeletal muscle, because any added pressure exerted on the probe is translated not just to the skin, but also to the underlying muscle tissue (7). In contrast, time-domain NIR-DCS (8–10), in combination or in isolation with longer near-infrared wavelengths (11), shows great promise for separating deep versus superficial tissue perfusion, but these approaches are not yet widely accessible, nor fully developed.
Alternatively, skin blood flow can be controlled pharmacologically. For example, epinephrine (via activation of postsynaptic adrenergic receptors) is a powerful vasoconstrictor in the skin and is routinely used to minimize bleeding during surgical procedures (12, 13). Under these circumstances, epinephrine is typically injected subcutaneously. However, iontophoresis can also be used to noninvasively and efficiently deliver epinephrine over a large surface area to cause prolonged vasoconstriction of the skin (14–19).
Accordingly, the purpose of the present investigation was to investigate the relative impact that changes in skin blood flow have on NIR-DCS estimations of skeletal muscle blood flow index (BFI) during experiments performed under thermoneutral conditions by using epinephrine iontophoresis to pharmacologically attenuate changes in skin perfusion. Given that skin blood flow may be contributing to the NIR-DCS BFI signal at rest, and that changes in BFI during provocation are expressed as a fold change above baseline, we hypothesized that attenuating changes in skin blood flow via epinephrine iontophoresis may increase the fold change in BFI during reactive hyperemia and handgrip exercise. However, given that our previous work shows that skin blood flow does not affect frequency domain, multidistance NIRS measures of tissue oxygenation (1), we predicted that epinephrine iontophoresis would have no impact on NIRS-based measures of skeletal muscle oxygenation.
METHODS
Ethical Statement
All experimental procedures were approved by the Institutional Review Board of the University of Texas at Arlington (Pro2021-0454) and conformed to the standards set forth by the Declaration of Helsinki, apart from registration in a database. All participants gave written informed consent before any experimental procedures.
Subjects
Twenty healthy young adults (6 females) were recruited from the Dallas-Fort Worth, Texas community using flyers and word of mouth and were screened via phone call. Four subjects completed Part 1, 15 subjects completed Part 2 (one of which also completed Part 1), and five subjects completed Part 3 (one of which completed Part 1 and two of which completed Part 2). Subjects were excluded if they reported a history of cardiovascular, pulmonary, metabolic, or musculoskeletal disease. Female subjects were also excluded if they were pregnant or planning to become pregnant (confirmed by urine pregnancy test before any procedures).
Experimental Approaches
This study was broken into three distinct parts that each addressed a unique component of epinephrine iontophoresis and its impact on NIR-DCS estimations of muscle blood flow. The experimental protocol for each part is described under Experimental Procedures, followed by more detailed descriptions of instrument setup and data acquisition/processing procedures. All testing procedures were performed in a temperature (∼22°C) and ambient light-controlled room and subjects arrived at the laboratory in a fasted state, having abstained from alcohol, caffeine, and vigorous exercise for the 24 h preceding the visit.
Part 1: Pharmacokinetics of epinephrine iontophoresis.
To inform subsequent protocols regarding the effectiveness and time course for cutaneous vasoconstriction, epinephrine iontophoresis was performed in four healthy young adults (1 female) on three discrete positions of the forearm at time points corresponding to 4, 2, and 0 h before the experimental procedures began. A fourth spot was left untreated and served as the control site, and the order of the spots was randomized between subjects. After the final epinephrine-iontophoresis treatment was completed, laser-Doppler probes were secured over each of the four spots to measure changes in skin blood flow during 1) postocclusion reperfusion (i.e., reactive hyperemia), 2) whole body heating to +1°C in body core-temperature, and 3) local heating of the skin surface immediately surrounding the laser-Doppler probes to measure peak skin blood flow (see Fig. 1, A and B). The pharmacokinetics of epinephrine iontophoresis were then determined by quantifying the difference in the magnitude of increase in skin blood flow at the three epinephrine-iontophoresis sites relative to the untreated control site during each of the provocations.
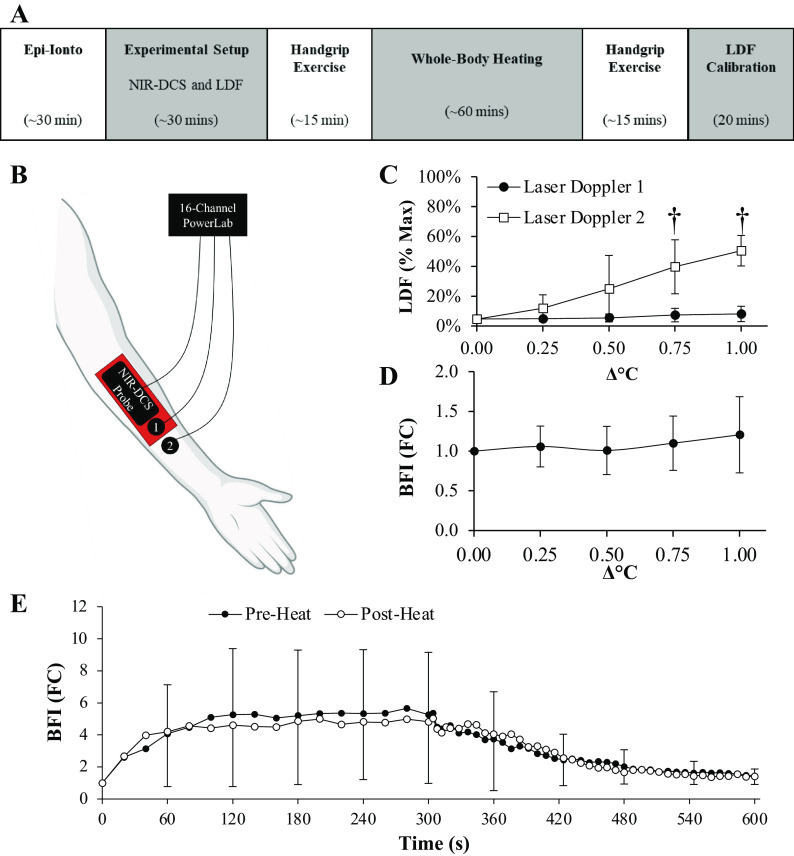
Figure 1.
Epinephrine iontophoresis attenuates peak measures of cutaneous reperfusion. The protocol outline and experimental setup for Part 1 are illustrated in A and B. After 5-min of cuff occlusion, peak measures of cutaneous blood flow measured via laser Doppler flowmetry were attenuated by at least 50% at all three of the treatment sites (C and D). Epinephrine iontophoresis also abolished hyperthermia-induced increases in cutaneous blood flow at the 0 h and 2 h pretreatment sites during whole body heating (E). Cutaneous blood flow was also attenuated at the 4-h pretreatment site, but the effects tended to wear off after +0.50°C. Notably, however, the whole body heating procedures ended ∼5 h after epinephrine iontophoresis had been completed at the 4-h pretreatment site, suggesting that epinephrine iontophoresis does in fact attenuate changes in skin blood flow for up to 4 h before the effects begin to wear off. Data are presented as means ± SD. Laser Doppler flowmetry (LDF) values are normalized as a percentage of the max perfusion (%-Max) observed at the Control site following the local heating procedure. n = 4 for all sites and are presented as means ± SD. However, errors bars are excluded for the 0-, 2-, and 4-h pretreatment lines in C to maintain visual clarity. Figure was created with BioRender.com.
Part 2: Impact of cutaneous blood flow on NIR-DCS measures of skeletal muscle BFI under normothermic conditions.
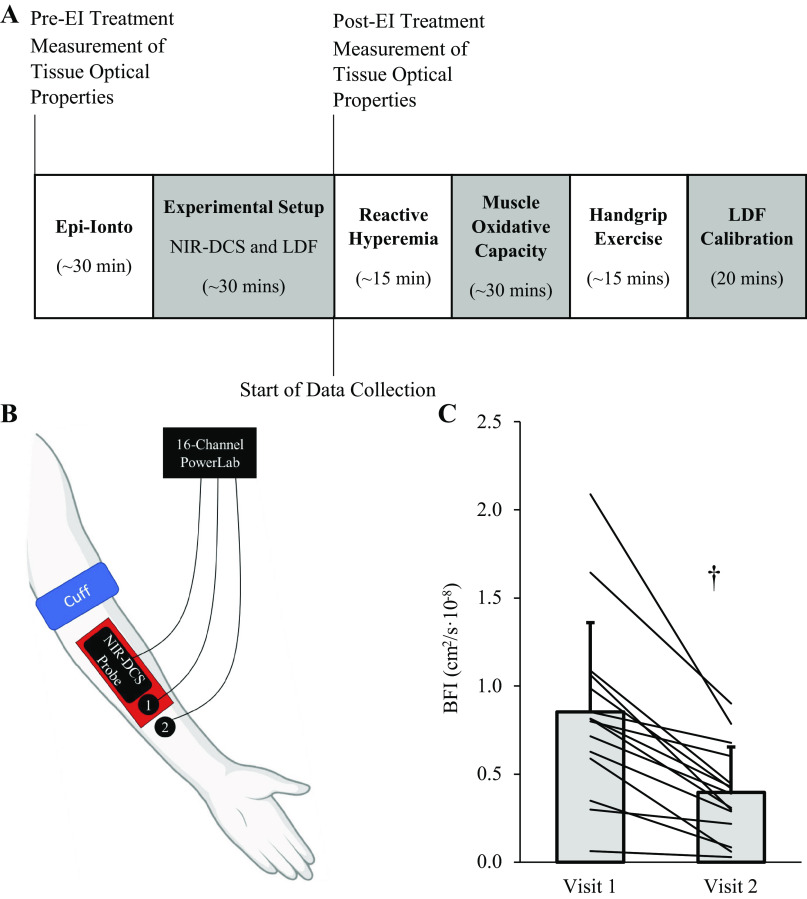
Part 2 examined the impact that epinephrine iontophoresis has on NIR-DCS measures of skeletal muscle BFI throughout a battery of commonly implemented research procedures, including 1) postocclusion reperfusion (i.e., reactive hyperemia), 2) skeletal muscle oxidative capacity testing, and 3) moderate-intensity handgrip exercise. Fifteen healthy young adults (3 females) completed two experimental visits. The experimental protocol for Part 2 is illustrated in Fig. 2A, with the instrument setup illustrated in Fig. 2B. Visit 1 served as the control visit, with epinephrine iontophoresis performed at the start of visit 2 to stimulate cutaneous vasoconstriction and attenuate any changes in skin blood flow during the experimental procedures. In addition, to quantify the impact that epinephrine iontophoresis has on forearm tissue optical properties (i.e., absorption and reduced scattering coefficients, μA and μ′S), we performed 2 min of quiet, resting NIR-DCS acquisition at the start of each visit, as well as immediately after the epinephrine-iontophoresis procedures at visit 2.
Figure 2.
Epinephrine iontorphoresis reduces baseline measures of forearm tissue blood flow index (BFI). The protocol outline and experimental setup for Part 2 are illustrated in A and B. After epinephrine-iontophoresis treatment, absolute measures of tissue BFI (cm2/s·10−8) were significantly lower (C; P ≤ 0.001; †). Data are presented as means ± SD, with each line representing the change in absolute BFI observed from pre- to posttreatment in the 15 subjects of Part 2. Figure was created with BioRender.com.
Part 3: Impact of epinephrine iontophoresis on NIR-DCS estimates of skeletal muscle BFI during handgrip exercise performed before and after whole body heating.
As a final consideration, Part 3 examined if stimulating cutaneous vasoconstriction via epinephrine iontophoresis would preserve NIR-DCS estimates of skeletal muscle blood flow during handgrip exercise performed after whole body heating (1). Five healthy young adults (2 females) performed 5 min of handgrip exercise before and immediately after whole body heating to +1°C in body core temperature. The instrument setup for Part 3 was identical to that described for Part 2.
Experimental Procedures
After informed consent was received at the subject’s first study visit, height and weight were measured on an electrical stadiometer (Professional 500KL, Health-O-Meter, McCook, IL), and seated blood pressure was measured in triplicate using an automated blood pressure cuff (71WX-B Connex Spot Monitor; Welch Allyn Connex, Skaneateles Falls, NY). Skinfold thickness of the left medial forearm was measured in duplicate along three sites (proximal, midpoint, and distal), with mean skinfold thickness taken as the average thickness across all three sites.
Subsequently, subjects laid supine on the examination table with their left arm adducted in a neutral position. For Part 1, laser-Doppler probes were positioned over the three sites treated with epinephrine-iontophoresis, as well as a fourth, untreated control site (Fig. 1B). For Parts 2 and 3, the NIR-DCS probe was positioned over the flexor digitorum profundus, followed distally by two laser-Doppler probes for measurement of cutaneous perfusion (Fig. 2B).
For all procedures, heart rate was measured via three-lead electrocardiography using standard CM5 electrode placement. Beat-by-beat blood pressure was measured in the right hand using finger photoplethysmography (Finometer PRO, Finapres Medical Systems; Arnhem, The Netherlands) that was calibrated to an automated brachial artery blood pressure cuff (71WX-B Connex Spot Monitor; Welch Allyn Connex, Skaneateles Falls, NY). Data acquisition for all measures was synchronized throughout the experimental procedures using a 16-channel data acquisition system (PowerLab 16/35; ADInstruments) and accompanying software (LabChart 8, ADInstruments), and then exported to Excel (Microsoft, Redmond, WA) for postprocessing.
Postocclusive reperfusion.
The postocclusive reperfusion (i.e., reactive hyperemia) protocol consisted of 2 min of quiet resting baseline, 5 min of brachial artery occlusion, and 5 min of reperfusion. Occlusion was achieved by inflating the cuff around the upper arm to 220 mmHg using a Hokanson E10 and AG101 air compressor. Cutaneous and tissue perfusions were measured continuously via laser Doppler flowmetry and NIR-DCS, respectively. All data were downsampled to 1-s averages and exported to Excel (Microsoft, Redmond, WA) for postprocessing of peak laser Doppler flowmetry (LDF), peak BFI (fold change above baseline), mean BFI slope (fold change/s), and time to peak (TTP) BFI, which were calculated as described previously (20).
Skeletal muscle oxidative capacity.
Skeletal muscle oxidative capacity was measured using near-infrared spectroscopy and an intermittent arterial occlusion method (21, 22), as described previously (23). Briefly, subjects performed 2–3 maximum voluntary isometric contractions (MVICs) to measure handgrip strength. After 60 s of resting acquisition, subjects isometrically squeezed a Smedley handgrip dynamometer (Stoelting Corporation, Wood Dale, IL) for ∼10 s at a force equal to 50% MVIC to increase skeletal muscle oxygen consumption (mV̇o2). After 10 s of reperfusion, the cuff around the subject’s upper arm was rapidly inflated/deflated to induce brief periods of ischemia, during which time, mV̇o2 was measured from the slope of the decline in the NIRS-derived [HbO2] signal (i.e., ΔHbO2). Inflation/deflation periods were 5 s on/5 s off for occlusions 1–6, 7 s on/7 s off for occlusions 7–10, 10 s on/15 s off for occlusions 11–14, and 10 s on/20 s off for occlusions 15–18. Subsequently, ΔHbO2 values were plotted over time and fit to a monoexponential equation, of which, the rate constant, kmV̇o2, reflects the skeletal muscle’s oxidative capacity:
| (1) |
where ΔHbO2-FIT is the calculated ΔHbO2 value at time point (t) during recovery, ΔHbO2-Initial is the ΔHbO2 immediately after exercise, and Amp is the amplitude of the response. During the fitting procedure, ΔHbO2-Initial, Amp, and kmVO2 were all left unconstrained.
Handgrip exercise.
The handgrip protocol in the present study was similar to that in our previous work (1). After 2 min of resting baseline, subjects performed 5 min of rhythmic handgrip exercise consisting of intermittent isometric contractions (∼1.7 s on and ∼2.3 s off) at a target intensity of 30% of the subject’s MVIC, followed by 5 min of recovery. Contraction cycles were queued using a prerecorded audio file, while torque generation was continuously displayed on a computer screen. To eliminate motion-induced artifacts in the NIR-DCS BFI signal, data from the handgrip exercise were gated posteriori to only incorporate time periods in which the muscle was not producing torque (24–27) and then averaged in 20-s intervals (i.e., 5 contractions). During recovery, data were averaged in 4-s intervals for the first 20-s and 8-s intervals for the final 280 s.
Whole body heating.
Unless otherwise noted, the experimental procedures for whole body heating in Parts 1 and 3 of the present study were identical to our previous work (1). Briefly, before laying supine on the examination bed, subjects were dressed in a tube-lined water-perfusion suit (BCS-4, Med-Eng Holdings ULC, Ottawa, ON, Canada) attached to a Lauda water heating unit (Alpha A12, LAUDA-Brinkmann, NJ) and homemade water pump. Body core temperature was measured via a sublingual thermistor attached to a Sable TC-2000 Type-T thermocouple meter (Sable Systems International, Las Vegas, NV). At the appropriate time in each protocol, the tube-lined suit was perfused with hot (50°C) water to increase subjects’ body core temperature to +1.0°C. Upon reaching the target temperature, we initiated either the local heating protocol for calibrating the laser-Doppler probes (Part 1) or the postheating 5-min handgrip exercise (Part 3). During whole body heating, data were averaged over 120 s of rest and then in 20-s intervals throughout the rest of the heating process.
Epinephrine iontophoresis.
Consistent with previous work (14–19), we performed epinephrine iontophoresis for 20 min using a 1:1,000 solution of epinephrine and a current density of 0.15–0.18 mA/cm2, which was below the recommended 0.5 mA/cm2 safety limit (28, 29). First, subjects were screened for electrically implanted medical devices and asked to remove all jewelry. A three-dimensional (3-D) printed plastic plate was then secured over the region of interest using athletic tape. This plate served to keep the epinephrine solution within the region of interest, as any leaking of epinephrine away from this area would increase the effective “treatment area,” thereby decreasing the electrical current density and reducing the potential effectiveness of the epinephrine-iontophoresis treatment. The gauze pads were then positioned over the treatment area and soaked in the 1:1,000 solution of epinephrine (PAR Pharmaceuticals).
Next, homemade copper electrodes were secured over the treatment and electrical dissipation sites. For Part 1, each of the three treatment sites was covered by a 3 × 3 cm (9 cm2) electrode. For Parts 2 and 3 of the study, iontophoresis was performed on two spots: a 3 × 3 cm (9 cm2) area for the treated laser Doppler site and a 6 × 3 cm (17 cm2) area for the NIR-DCS site. The dissipation electrode was liberally covered in electrode gel (Signa Gel, Parker Laboratories Inc., NJ) and then positioned on the upper arm. Notably, the dissipation electrode was always at least twice the size of the active electrode to minimize subject discomfort.
Subsequently, the electrodes were connected to an ActivaDose-II iontophoresis unit (ActivaTek, Inc.), with the anode (+) attached to the treatment electrode and the cathode (−) attached to the dissipation electrode. The electrical current (in mA) was set to produce a current density of 0.15–0.18 mA/cm2 conducting through the treatment electrode, and iontophoresis was conducted for 20 min, after which time the electrodes were removed and the skin was gently cleaned and inspected for signs of electrical burns.
Near-infrared DCS.
Forearm heme content (hemoglobin + myoglobin; μM), oxygenation (i.e., StO2%; oxy-heme/total heme), and blood flow index (BFI; cm2/s·10−8) were measured continuously using a MetaOx frequency-domain near-infrared diffuse correlation spectrometer (ISS, Inc., Champaign, IL). Briefly, tissue optical properties [i.e., absorption (μA) and reduced scattering (μ′S) coefficients] were calculated via frequency-domain multidistance (FDMD) near-infrared spectroscopy (NIRS). The derived μA and μ′S were then incorporated into the modified Beer–Lambert law to derive absolute concentrations of oxygenated and deoxygenated hemoglobin + myoglobin within the tissue’s region of insonation (30–32). In addition, relative measures of forearm tissue microvascular perfusion were derived via diffuse correlation spectroscopy (DCS). Briefly, DCS can be used to estimate relative (i.e., normalized to a resting baseline) changes in tissue perfusion by measuring fluctuations in photon intensity (i.e., photon counts) from small laser speckles, and fitting the decay of the temporal autocorrelation of these speckle fluctuations to a diffusion equation (33, 34). Although muscle and other motion artifacts affect DCS measures of tissue perfusion, under resting conditions, the decay of the speckle light-intensity temporal autocorrelation is primarily driven by the movement of red blood cells within the tissue’s region of insonation (35–37).
All NIR-DCS measurements were collected at a sampling frequency of 5 Hz. A single NIR-DCS probe that contained multiple source-detector pairs for NIRS and DCS measures was positioned longitudinally over the flexor digitorum profundus and secured in place using self-adhering wrap. The near-infrared component of the probe consisted of eight laser diodes operating at wavelengths of 670, 690, 700, 730, 750, 785, 808, and 830 nm, and contained four laser diodes separated from a single detector by distances of 2.0, 2.5, 3.5, and 4.5 cm. However, although optical measures for the 670-nm wavelength were collected and logged by the MetaOx, this wavelength was not used to calculate tissue optical properties or heme-contents during the experiments because previous pilot work in our laboratory had shown that during prolonged forearm ischemia (i.e., such as the 5 min of cuff-induced ischemia used in the present investigation), there is substantial signal loss at the 670 nm wavelength, which affects estimations of tissue oxy- and deoxy-heme contents at the end of ischemia. Alternatively, the DCS component of the probe consisted of four photon-counting detectors located 2.4 cm away from a long coherence laser operating at a wavelength of 850 nm. In accordance with our recent work (33), the MetaOx was programmed to calculate NIR-DCS measures of BFI using a 3-s rolling average of the μA and μ′S. Throughout all experimental procedures, tissue BFI was normalized to rest and reported as a fold change above baseline. For measures of forearm perfusion during handgrip exercise, NIR-DCS measures of BFI were gated to periods in which the muscle was not producing force to avoid motion artifacts.
Laser Doppler flowmetry.
Changes in cutaneous blood flow were measured using laser Doppler flowmetry (LDF) via two multifiber integrating probes (VP7a/T; Moor Instruments, Wilmington, DE) positioned distally to the NIR-DCS probe. Both probes were housed in a local heating unit (PF-450; Perimed Instruments, Stockholm, Sweden) set to thermoneutrality (33°C) at the start of experimentation. Consistent with our approach for NIR-DCS measures of forearm BFI, all LDF measures of cutaneous perfusion during exercise were gated to periods in which the muscle was not producing force to avoid motion artifacts. After completion of all experimental procedures, the local heating units were set to 44°C to derive site-specific measures of peak cutaneous blood flow. All measures of cutaneous blood flow were normalized to the peak flow observed at the end of local heating and expressed as a percent maximum. However, the vasoconstrictive effects of epinephrine iontophoresis were so potent that it diminished the vasodilatory effects of local heating as long as 3- to 4-h posttreatment. Thus, in contrast to our previous work (1), comparisons of peak cutaneous blood flow across treated versus untreated sites were not directly comparable. As such, we made two important methodological adjustments: 1) the local heating procedures were only performed for 20 min, and 2) when an LDF site was treated with epinephrine iontophoresis, the perfusion units measured from that site during experimentation (i.e., reactive hyperemia, handgrip exercise, whole body heating, etc.) were normalized to the peak perfusion units measured at the untreated control site.
Statistical analyses.
All statistical analyses were conducted using SPSS v28.0 (IBM Statistics, Armonk, NY). Data are presented as means ± SD, with P values <0.05 considered statistically significant, and effect size () reported where appropriate. Before any statistical comparisons were conducted, data were tested for normal distribution using the Shapiro–Wilk test. Normally distributed data were analyzed by paired tests or repeated-measures ANOVAs. If significant main or interaction effects were observed, post hoc comparisons were analyzed using the estimated marginal means or paired t tests. For non-normally distributed data, paired t tests were replaced by the Wilcoxon signed-rank test and repeated-measures ANOVA’s were replaced by Friedman’s test. In the event of a significant Friedman’s test, post hoc comparisons were analyzed using individual Wilcoxon signed rank tests.
RESULTS
Participant characteristics for each part of the study are described in Table 1. Overall, epinephrine iontophoresis was well tolerated across the 80+ treatment sites. One subject reported a small cut on their arm a few days after testing near the position of a treatment site from Part 1. A second subject exhibited small dark spots beneath the treatment area during Part 2 that was potentially consistent with a first-degree burn. Data collection was terminated for this subject and the spots began dissipating within 24 h, with no additional adverse effects reported thereafter. Strategies for minimizing subject discomfort and adverse events are explained in discussion.
Table 1.
Participant characteristics
| Measure | Part 1 | Part 2 | Part 3 |
|---|---|---|---|
| n | 4 (1 F) | 15 (3 F) | 5 (2 F) |
| Age | 26.5 ± 4.9 | 23.6 ± 5.9 | 28.1 ± 7.2 |
| Height, cm | 175.9 ± 7.8 | 171.9 ± 8.3 | 168.2 ± 9.1 |
| Weight, kg | 71.2 ± 11.4 | 71.6 ± 18.0 | 68.6 ± 18.2 |
| Body fat, % | 22.3 ± 5.8 | 27.5 ± 9.7 | 29.6 ± 7.5 |
| Handgrip strength, kg | – | 38.1 ± 7.8 | 35.2 ± 10.6 |
| Half skinfold thickness, mm | – | – | – |
| Mean | – | 3.35 ± 1.42 | 3.58 ± 1.05 |
A total of 20 healthy young adults participated in the present study. One of the subjects who completed Part 1 also completed Part 2, and another completed Part 3. Two of the subjects from Part 2 also completed Part 3. Data are presented as means ± SD.
Part 1: Pharmacokinetics of Epinephrine Iontophoresis
Table 2 describes the average time (in minutes) between the end of each epinephrine-iontophoresis treatment, and the start of reactive hyperemia or completion of whole body and local heating. On average, the time separating the end of epinephrine iontophoresis and the start of experimental procedures 2 h and 4 h pretesting sites were both within 5 min of our goal start times.
Table 2.
Time separating the end of epinephrine-iontophoresis treatment and the start/end of experimental procedures for Part 1
| Measure | 0-H Pre, Min | 2-H Pre, Min | 4-H Pre, Min |
|---|---|---|---|
| Start of 5-min occlusion test | 20.5 ± 9.7 | 123.8 ± 4.0 | 243.3 ± 3.4 |
| Start of WBH test | 35.8 ± 7.9 | 139.0 ± 2.6 | 258.5 ± 1.3 |
| End of WBH test | 78.0 ± 10.5 | 181.3 ± 5.1 | 300.8 ± 4.6 |
| End of LDF calibration | 101.5 ± 9.7 | 204.8 ± 3.4 | 324.3 ± 3.2 |
Times are given in minutes as means ± SD. LDF, laser Doppler flowmetry; pre, pretesting; WBH, whole body heating.
Epinephrine iontophoresis attenuated the change in skin blood flow during reactive hyperemia (Fig. 1, C and D), whole body heat stress (Fig. 1E), and local heating for up to 4-h posttreatment. For reactive hyperemia, a significant main effect of treatment was observed for peak measures of postocclusive reperfusion (Fig. 1, C and D; P = 0.040; = 0.789). All three treatment sites were attenuated by at least 50% compared with the control site, with the 0-h pretreatment site being significantly lower than both the control (P = 0.007) and 2-h pretreatment site (P = 0.028).
For whole body heat stress, a significant main effect of treatment was found for cutaneous perfusion (%max) during whole body heating (Fig. 1E; P ≤ 0.001; = 0.950), with the control site exhibiting greater cutaneous perfusion than all three treatment sites (P ≤ 0.003, all). We also observed a significant site × temperature interaction effect (P = 0.015; = 0.807), with the control site exhibiting greater perfusion than the 4-h pretreatment site at baseline (P = 0.036) and all three treatments sites at +0.25, +0.50, and +0.75°C (P ≤ 0.01, all). At +1.00°C, the control site also exhibited greater perfusion than the 0- and 2-h pretreatment sites (P ≤ 0.001, all) but not the 4-h pretreatment site (P = 0.061).
To assess the impact that epinephrine iontophoresis had on the maximal capacity for skin vasodilation and perfusion (measured via LDF perfusion units—PU), we performed 20 min of local heating at the end of every experiment. A significant main effect of treatment was observed for maximal PU (main effect P = 0.003; = 0.781), with the 0- and 2-h pretreatment sites being lower than the control site (P ≤ 0.012), and the 0-h pretreatment site also being lower than the 2-h pretreatment site (P ≤ 0.001).
Part 2: Impact of Cutaneous Blood Flow on NIR-DCS BFI under Normothermic Conditions
Consistent with Part 1, epinephrine iontophoresis significantly attenuated cutaneous perfusion measured via laser Doppler flowmetry following the local heating procedures (Site × Time interaction effect P ≤ 0.001), with the epinephrine-iontophoresis-treated site (LDF-1 at visit 2; 148.4 ± 56.3 PU) being ∼40% lower than all untreated sites: LDF-1, visit 1 (256.3 ± 47.8 PU); LDF-2, visit 1 (252.5 ± 62.5 PU, P ≤ 0.001), and LDF-2, visit 2 (254.6 ± 60.7 PU, P ≤ 0.001). Notably, baseline NIR-DCS BFI (cm2/s·10−8) was lower following epinephrine-iontophoresis treatment at visit 2 compared with visit 1 in all subjects (Fig. 2C, 0.395 ± 0.26 vs. 0.852 ± 0.51 cm2/s·10−8; P ≤ 0.001), with the average reduction being ∼54%. Epinephrine iontophoresis did not significantly impact the NIRS-derived tissue scattering coefficient (µS; P > 0.05 for all wavelengths), but the tissue absorption coefficient (µA) was significantly greater for all wavelengths (P < 0.05; Table 3) except for 808 nM (P = 0.068), indicating an increase in photon absorption by the forearm tissue posttreatment.
Table 3.
Changes in tissue absorption (µA) and scattering (µS) coefficients after epinephrine-iontophoresis treatment
| Wavelength, nm | µA, cm−1 |
µS, cm−1 |
|||
|---|---|---|---|---|---|
| n | Pre- | Post- | Pre- | Post- | |
| 670 | 16 | 0.169 ± 0.05 | 0.180 ± 0.05† | 7.48 ± 3.8 | 7.13 ± 3.9 |
| 690 | 16 | 0.153 ± 0.04 | 0.163 ± 0.04† | 7.27 ± 3.7 | 6.95 ± 3.8 |
| 700 | 16 | 0.140 ± 0.03 | 0.148 ± 0.03† | 6.85 ± 3.6 | 6.56 ± 3.7 |
| 730 | 16 | 0.137 ± 0.03 | 0.144 ± 0.03† | 6.39 ± 3.4 | 6.10 ± 3.5 |
| 750 | 16 | 0.163 ± 0.04 | 0.173 ± 0.04† | 6.31 ± 3.5 | 6.02 ± 3.5 |
| 785 | 16 | 0.162 ± 0.04 | 0.169 ± 0.04† | 5.90 ± 3.2 | 5.63 ± 3.2 |
| 808 | 11 | 0.174 ± 0.05 | 0.181 ± 0.05 | 6.04 ± 3.7 | 5.68 ± 3.7 |
| 830 | 16 | 0.185 ± 0.04 | 0.193 ± 0.04† | 5.42 ± 3.1 | 5.20 ± 3.1 |
Technical difficulties prevented data collection from the 808 wavelength in 5 of the subjects. n = 16 for all other wavelengths (fifteen subjects from Part 2 and one subject from Part 3). Data are presented as means ± SD. nm, nanometer; µA, absorption coefficient; µS, reduced scattering coefficient; pre- and post- refer to before and after epinephrine-iontophoresis treatment.
Postocclusive reperfusion.
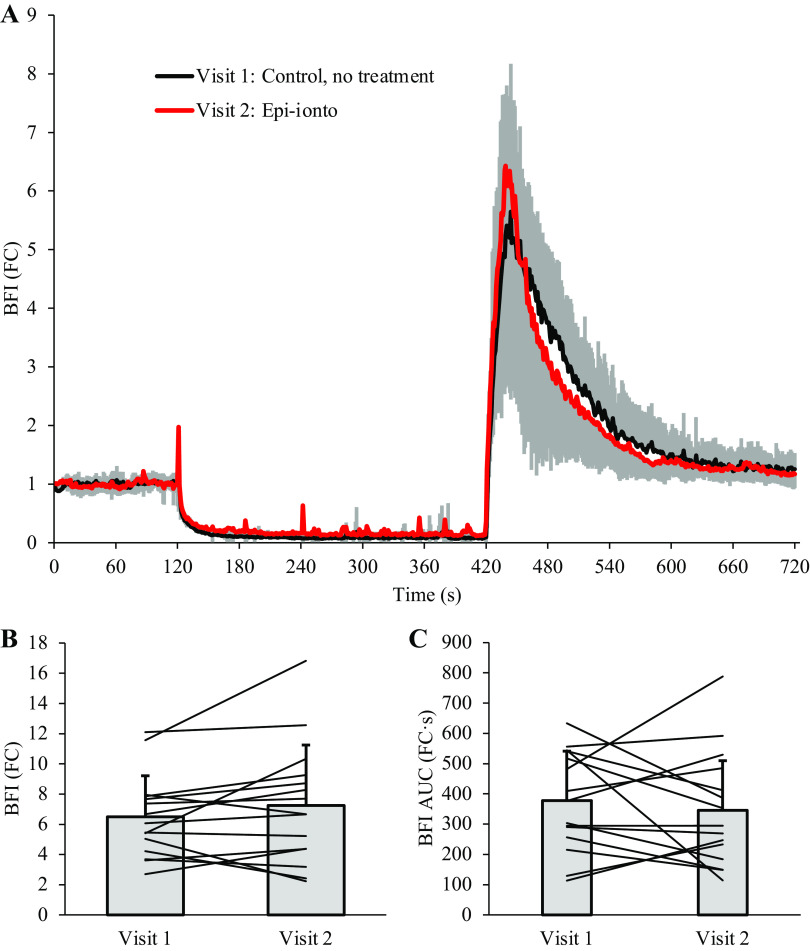
Table 4 describes measures of deoxygenation and reperfusion during the 5-min cuff occlusion protocol. There were no differences observed between visits 1 and 2 for StO2% at baseline (P = 0.739) or the end of cuff occlusion (P = 0.186), nor the StO2 area under the curve during ischemia (P = 0.416), which indicates that the ischemic stimulus to vasodilate was comparable across visits. Consistent with Part 1, epinephrine iontophoresis significantly attenuated peak measures of skin perfusion beneath the treated LDF probe after 5 min of cuff occlusion at visit 2 (Visit × Site interaction effect P ≤ 0.001), with peak perfusion from LDF-1 at visit 2 being significantly lower than all untreated sites: versus LDF-1, visit 1 (P ≤ 0.001); versus LDF-2, visit 1 (P ≤ 0.001), and versus LDF-2, visit 2 (P ≤ 0.001). We did not observe a significant effect of visit for NIR-DCS meaures of forearm tissue reperfusion (Fig. 3), including peak BFI (P = 0.195), BFI slope (P = 0.422), and time-to-peak BFI (P = 0.088). BFI area under the curve [AUC; fold-change (FC) above baseline] during recovery was also similar across Visits over the first 30 s of reperfusion (Table 4; P = 0.069), the middle 31–180 s (P = 0.204), and the final 180 s of recovery (P = 0.554).
Table 4.
Measures of cutaneous and forearm microvascular perfusion after the 5-min cuff occlusion protocol
| Measure | n | Visit 1 | Visit 2 |
|---|---|---|---|
| StO2%-baseline, % | 15 | 71.6 ± 3.7 | 71.9 ± 3.7 |
| StO2%-end occlusion, % | 15 | 45.9 ± 8.0 | 47.3 ± 6.3 |
| StO2%-AUC, %·s | 15 | 4,113.6 ± 1,021.5 | 3,969.4 ± 1,143.5 |
| Peak LDF-1, %-Max | 15 | 40.8 ± 11.1 | 16.6 ± 7.5† |
| Peak LDF-2, %-Max | 15 | 41.8 ± 11.8 | 41.4 ± 9.4 |
| Peak BFI, fold-change | 15 | 6.49 ± 2.7 | 7.25 ± 4.0 |
| BFI slope, fold-change/s | 13 | 0.230 ± 0.125 | 0.258 ± 0.154 |
| TTP BFI, s | 13 | 22.5 ± 4.2 | 20.0 ± 4.4 |
Data are reported as means ± SD. n = 13 for blood flow index (BFI) slope and time to peak (TTP) BFI due to poor data quality in two subjects. AUC, area under the curve; LDF-1 and -2, laser Doppler positions 1 (treated) and 2 (untreated); StO2%, tissue oxygen saturation index.
†Significantly different from all other measures of peak cutaneous blood flow (P < 0.05).
Figure 3.
Blood flow index (BFI) during postocclusive reperfusion. Epinephrine iontophoresis did not affect peak BFI (fold change above baseline), BFI slope, or time-to-peak BFI after 5 min of cuff occlusion. Although the area under the curve was slightly lower after epinephrine-iontophoresis treatment, the difference was not statistically significant. n = 15, data are presented as means ± SD. FC, fold change.
Skeletal muscle oxidative capacity.
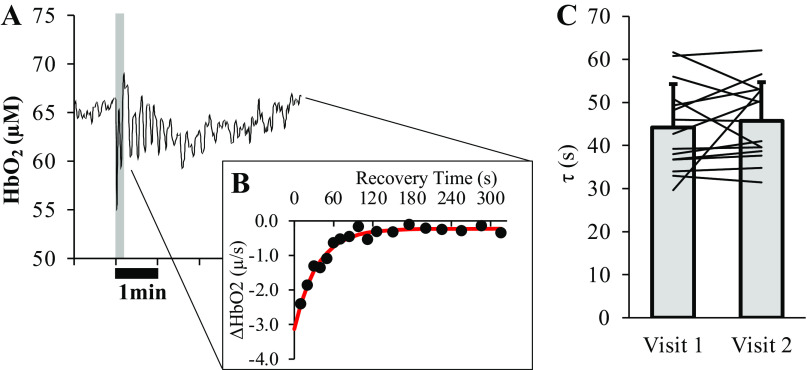
Skeletal muscle oxidative capacity was not significantly different between visits 1 and 2 (Fig. 4, 44.2 ± 10.1 vs. 45.7 ± 8.9 s, P = 0.461).
Figure 4.
Skeletal muscle oxidative capacity. Skeletal muscle oxidative capacity was determined by plotting the slopes of HbO2 observed during repeated arterial occlusions after 10 s of isometric handgrip exercise at 50% of maximum voluntary isometric contraction (MVIC). A representative trace showing changes in HbO2 during baseline, handgrip, and the first few minutes of recovery from one subject is given in A, with the gray-shaded region illustrating the 10-s period of isometric hand-gripping. B, ΔHbO2 values (black circles) from each of the arterial occlusions, which were subsequently fit with a monoexponential function, of which τ represents the skeletal muscle oxidative capacity. No differences were observed in skeletal muscle oxidative capacity (τ, in s) measured across visits (C; P = 0.461). Bar graph in B is presented as means ± SD, along with a line representing the average τ measured in each of the 15 subjects at visits 1 and 2. HbO2, oxyheme content in micromoles (µM), τ, tau of HbO2 recovery.
Handgrip exercise.
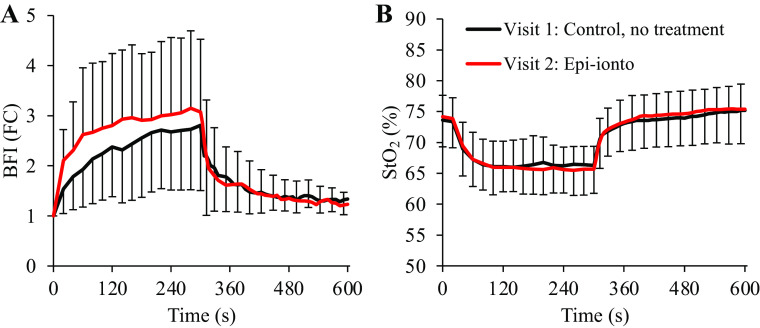
A significant Site × Visit interaction effect was observed for LDF measures of skin blood flow during handgrip exercise (P = 0.002), with LDF-1 being lower than LDF-2 at all time points (P ≤ 0.02) except baseline (P = 0.110). However, no main effect of visit was observed for BFI (fold change above baseline; Fig. 5A; P = 0.075). Similarly, there were no differences in BFI AUC during recovery over the first 30 s (P = 0.820), middle 31–180 s (P = 0.865), or final 180 s (P = 0.281).
Figure 5.
Impact of epinephrine iontophoresis on near-infrared diffuse correlation spectroscopy (NIR-DCS) blood flow index (BFI) and tissue oxygenation during handgrip exercise. Attenuating changes in cutaneous perfusion via epinephrine iontophoresis did not significantly affect NIR-DCS BFI during moderate-intensity handgrip exercise (A). These results suggest that changes in cutaneous blood flow contribute very little to the NIR-DCS BFI signal during moderate-intensity exercise in thermoneutral environments, and that changes in NIR-DCS BFI during exercise are likely dominated by changes in perfusion of the underlying skeletal muscle. Similarly, cutaneous blood flow had little-to-no impact on NIRS-derived measures of forearm tissue oxygenation (B). n = 15 for all data points. Data are presented as means ± SD, with error bars omitted intermittently during the recovery period to avoid illustrative congestion. FC, fold-change.
There were also no differences in any NIRS-based measures of tissue oxygenation during handgrip exercise across visits, including deoxyheme (HHb, main effect of visit P = 0.903), oxyheme (HbO2, main effect of visit P = 0.436), total heme (HbTot, main effect of visit P = 0.733), or StO2% (Fig. 5B; main effect of visit P = 0.709).
Part 3: Impact of Epinephrine Iontophoresis on NIR-DCS BFI during WBH
Figure 6 illustrates the changes in LDF measures of cutaneous blood flow and NIR-DCS measures of tissue BFI during whole body heating to +1.0°C observed during Part 3. A significant Site × Temperature interaction effect was observed for LDF (P ≤ 0.001). No changes in skin blood flow were observed for the Site treated by epinephrine iontophoresis (P > 0.05 for all temperature comparisons), but skin blood flow was significantly elevated beneath the untreated site (LDF-2) after +0.50°C (P ≤ 0.021 for +0°C, +0.25°C, and + 0.50°C vs. +0.75°C and +1.00°C). In contrast, no main effect was observed for changes in NIR-DCS BFI across all temperatures (P = 0.485; = 0.184).
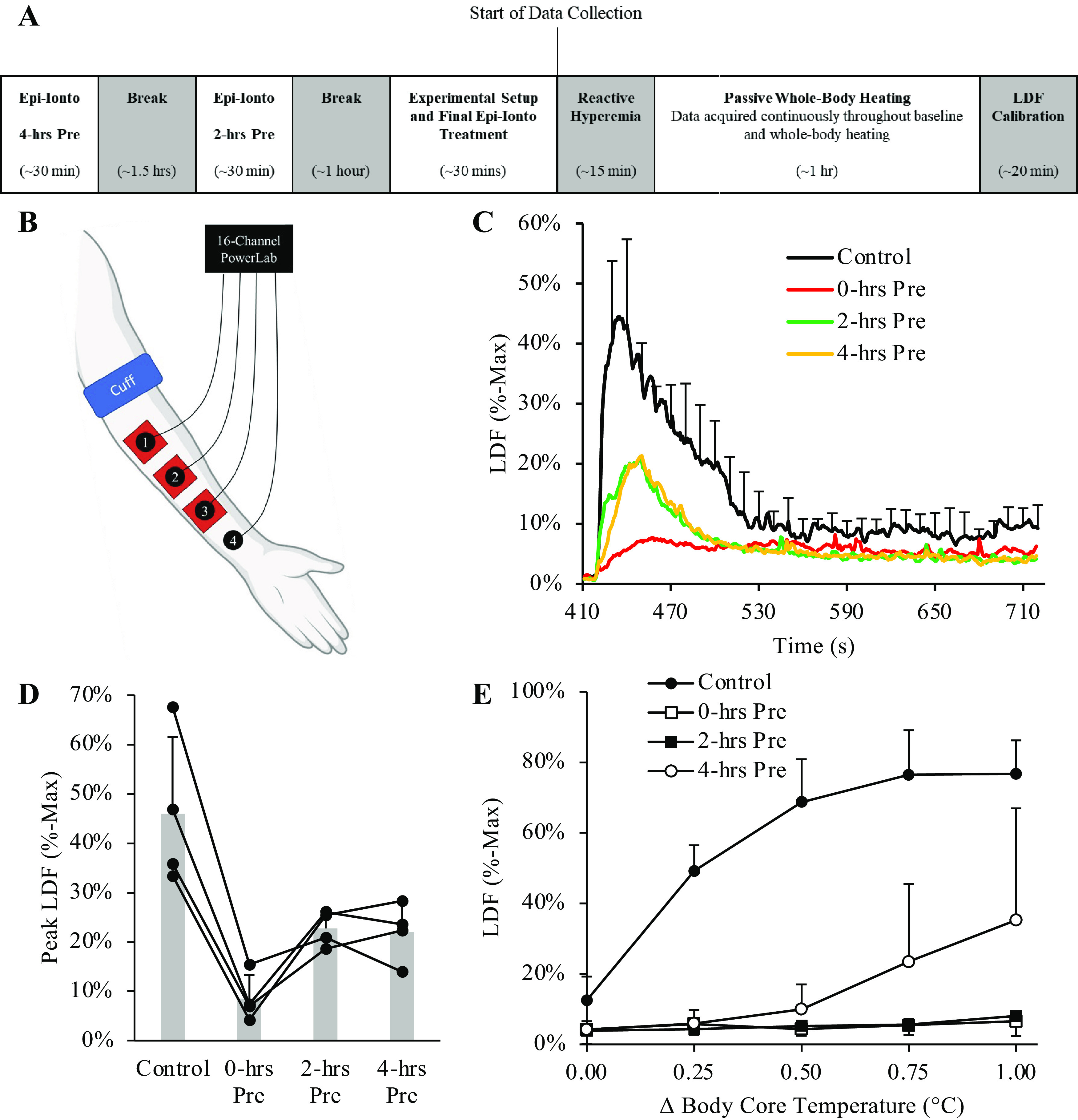
Figure 6.
Near-infrared diffuse correlation spectroscopy (NIR-DCS) measures of blood flow index (BFI) during handgrip exercise performed before and after whole body heating. The experimental protocol is outlined in the top panel (A, unlabeled), with the instrumental setup given in B. Consistent with our prior work (1), whole body heating to +1.0°C increased cutaneous blood flow beneath the untreated laser Doppler flowmetry site (LDF-2; C; †P < 0.05 vs. +0°C, +0.25°C, and + 0.50°C). However, treatment with epinephrine iontophoresis abolished changes in cutaneous blood flow beneath LDF-1 throughout the whole body heating protocol, as well as changes in NIR-DCS BFI (D). Accordingly, NIR-DCS indices of skeletal muscle BFI were similar during handgrip exercise performed before and after whole body heating (E). These data demonstrate that epinephrine iontophoresis not only acutely abolishes changes in cutaneous blood flow but can also be used to preserve NIR-DCS indices of skeletal muscle BFI during studies in which cutaneous blood flow is expected to significantly increase. n = 5 for C and D, and n = 4 for E due to one subject not being able to complete the postheating handgrip exercise. Data are presented as means ± SD. Figure was created with BioRender.com.
For the handgrip exercises performed before and after whole body heating, no main effects of heat were observed for BFI (Fig. 6D; P = 0.757; = 0.037), whereas LDF measures of skin blood flow (P ≤ 0.003) were significantly greater during the postheating handgrip exercise than preheating exercise. A significant Site × Condition was also observed for LDF measures of skin blood flow (P = 0.005), with no differences observed for LDF-1 (epinephrine iontophoresis-treated site) pre- versus postheating (P = 0.150), or when comparing preheating measures of skin blood flow from LDF-1 versus LDF-2 (P = 0.851). In contrast, skin blood flow beneath the untreated site (LDF-2) was significantly higher postheating versus preheating (P = 0.004) and when comparing the untreated versus treated sites postheating (LDF-1 < LDF-2; P = 0.008).
DISCUSSION
The objective of the present study was to examine the impact that cutaneous blood flow has on NIR-DCS measures of BFI during experiments performed under thermoneutral conditions by using epinephrine iontophoresis to attenuate changes in cutaneous perfusion by acutely stimulating skin vasoconstriction. The primary novel findings of the present study are threefold. First, we provide quantitative evidence that epinephrine iontophoresis attenuates changes in cutaneous blood flow for up to 4 h posttreatment, even in the face of significant neural- and local stimuli for cutaneous vasodilation. Second, cutaneous blood flow does not appear to have a significant impact on NIR-DCS measures of skeletal muscle BFI during vascular function testing or moderate-intensity exercise performed in thermoneutral experimental conditions. Third, when cutaneous vasodilation is sufficient to affect NIR-DCS estimations of skeletal muscle BFI (1), epinephrine iontophoresis can acutely, and noninvasively, abolish changes in skin blood flow over large surface areas, which preserves NIR-DCS estimations of skeletal muscle BFI during moderate-intensity exercise. This suggests that epinephrine iontophoresis can be used to attenuate changes in cutaneous perfusion during studies in which skin blood flow may reasonably be expected to increase sufficiently to affect NIR-DCS estimations of skeletal muscle BFI, such as whole body heating or high-intensity exercise with large muscle masses (i.e., knee extension, running, or cycling).
Several studies have demonstrated that epinephrine iontophoresis causes profound vasoconstriction in the skin, resulting in blanching (14–19). In 1969, LaJoie et al. reported that blanching of the skin persisted for up to 5–6 h, but their experiments ended within ∼3 h of epinephrine-iontophoresis treatment, so no quantitative data were available to define how long the vasoconstrictive effects of epinephrine iontophoresis actually persist. Part 1 of the present study addressed this gap by performing epinephrine iontophoresis on three discrete locations of the forearm at timepoints corresponding to 4-, 2-, and 0 h before the start of experimentation. As shown in Fig. 1, laser-Doppler indices of cutaneous blood flow were attenuated at all three locations throughout all testing procedures. Although the effects of epinephrine iontophoresis began to wear off during whole body heating at temperatures above +0.5°C, it is worth noting that these data were collected ∼5 h after treatment had been completed on the 4-h pre-experimentation spot (Table 2). Similarly, we saw no changes in cutaneous blood flow throughout whole body heating beneath the 2- and 0-h pre-experimentation spots. Accordingly, we conclude that epinephrine iontophoresis effectively attenuates changes in cutaneous blood flow for ∼3–4 h posttreatment.
Subsequently, Part 2 directly examined the impact that cutaneous blood flow has on NIR-DCS BFI throughout a battery of experimental procedures that are commonly performed in our laboratory, and others; including 1) postocclusive reactive hyperemia, 2) skeletal muscle oxidative capacity assessment, and 3) moderate-intensity exercise. Previous studies using epinephrine iontophoresis showed that skin blood flow accounts for ∼20%–40% of forearm perfusion at rest under thermoneutral conditions (15, 19). Given that NIR-DCS BFI reflects the “net” perfusion of all tissues within the probe’s region of insonation (∼½ source-detector separation), we hypothesized that attenuating cutaneous perfusion via epinephrine iontophoresis would reduce BFI at rest. We also predicted that if resting BFI was reduced, then the fold change in BFI during physiological provocation would be greater.
As shown in Fig. 2, baseline BFI was indeed lower after epinephrine-iontophoresis treatment in all 15 subjects. However, attenuating changes in cutaneous perfusion did not affect the fold change in BFI during reactive hyperemia (Fig. 3) or moderate-intensity handgrip exercise (Fig. 5). Collectively, these data suggest that changes in cutaneous perfusion exert little-to-no impact on forearm BFI during experiments performed under thermoneutral conditions. Or, put another way, that muscle perfusion dominates the NIR-DCS BFI signal until skin blood flow increases substantially above some “threshold value” (1).
The NIRS-intermittent occlusion method for measuring skeletal muscle oxidative capacity has become increasingly popular over the past decade due to its low cost and clinical feasibility (21, 22). However, previous work has shown that changes in skin blood flow can distort NIRS estimations of muscle oxygenation (38), thereby potentially confounding estimations of skeletal muscle oxidative capacity. The results of the present study demonstrate that cutaneous perfusion has little-to-no effect on NIRS measures of skeletal muscle oxygenation when using a frequency-domain, multidistance NIR spectrometer, and therefore have little-to-no impact on estimations of skeletal muscle oxidative capacity derived from the NIRS-intermittent occlusion method (Fig. 4). Whether this observation holds true for nonspatially resolved spectroscopy approaches, such as the more commonly employed continuous-wave devices, requires further investigation.
Finally, Part 3 of this study investigated whether epinephrine iontophoresis could preserve NIR-DCS BFI kinetics under conditions where cutaneous blood flow increases sufficiently to affect NIR-DCS estimates of skeletal muscle perfusion. For these experiments, subjects performed handgrip exercises before and after whole body heating to +1°C. Consistent with our previous work using Botox (1), and the results from Parts 1 and 3 of the present study, epinephrine iontophoresis abolished changes in cutaneous perfusion throughout whole body heating. Remarkably, NIR-DCS BFI was also unchanged throughout heating, and as a result, BFI kinetics during handgrip exercise was almost identical. As such, we interpret these results as an indication that epinephrine iontophoresis can be used to attenuate (if not completely abolish) changes in cutaneous perfusion during experiments in which skin blood flow may be reasonably expected to increase enough to uncouple changes in NIR-DCS BFI from changes in muscle perfusion. For example, moderate-intensity handgrip exercise would not be expected to challenge whole body thermoregulation. However, heat generation during large muscle-mass exercises, like cycling or knee extension, may stimulate sweating and increases in skin blood flow that could affect NIR-DCS BFI. Quaresima et al. (39) recently investigated changes in skeletal muscle- and subcutaneous tissue perfusions by using a NIR-DCS probe with both long (25 mm) and short (5 mm) source-detector separations. Although the increase in subcutaneous BFI during exercise was ∼77% lower than the increase in BFI from skeletal muscle, these differences were much less pronounced during recovery. Future work will be needed to delineate what effects, if any, that skin blood flow may have on NIR-DCS estimates of skeletal muscle BFI during high-intensity whole body exercises that pose thermoregulatory challenges.
Epinephrine iontophoresis substantially reduced skin blood flow and, in many cases, caused visible blanching of the skin. This observation suggests that cutaneous red blood cell content was reduced following epinephrine-iontophoresis treatment, which should, theoretically, also reduce tissue photon absorption and µA. However, as shown in Table 3, µA was greater following epinephrine-iontophoresis treatment. The explanation for this observation is not readily apparent, but may be related to changes in net photon trajectory through the forearm tissues. Specifically, reducing red blood cell content in the skin may have also reduced photon scattering by the superficial tissues. In effect, this would allow a greater proportion of the incident light emitted by the laser to reach the muscle tissues that exhibit high photon absorption in the 600–900 nm wavelength region. Although the data collected in the present study cannot offer any additional insight into this observation, the impact that epinephrine iontophoresis has on photon movement through cutaneous versus muscle tissues warrants additional investigation by future studies.
Methodological Considerations for Iontophoresis
One of the main challenges of iontophoresis is ensuring subject safety and minimizing discomfort during treatment. Skin irritation and erythema are common adverse events but typically resolve within 3–4 h. During treatment, subjects often reported feeling tingling or pins and needles beneath the electrodes, which subsided within 3–5 min. Similarly, some subjects also reported feeling “hot-spots” beneath the dissipation electrode. This is a sign of high-current density, likely due to the electrode gel being unevenly distributed. In our experience, this was easily resolved by gently moving the dissipation electrode back and forth to redistribute the gel. If the sensation persists, treatment should be stopped immediately, and the skin should be checked for burns before reapplying the electrode gel and resuming treatment. The most significant risk of iontophoresis is burning of the skin. Electrolysis of water beneath metal electrodes leads to oxidation/reduction reactions, which can cause chemical burns due to large alterations in skin pH (29, 40). Thermal burns can also occur if the skin is exposed to high-current density for prolonged periods of time. However, these risks can be minimized by following a few simple rules.
First, never allow a metal electrode to come in direct contact with the subject’s skin, as this will quickly lead to a thermal burn. This issue can be easily avoided by using a thick layer of gauze to separate the treatment electrode from the skin and a thick layer of electrode gel beneath the dissipation electrode. Similarly, when constructing homemade electrodes, always use pure copper and never use a copper-aluminum alloy (i.e., a mix of two or more metals). Although copper-aluminum alloy sheets are both cheap and easy to find at local hardware stores, copper and aluminum have different electrical conductance properties. As such, any deterioration of the outer copper shell will expose the underlying aluminum and alter how the electrical current emitted by the iontophoresis device is dissipated across the surface of the electrode and into the skin. This can cause a thermal burn. It is also recommended that the dissipation electrode be at least twice the size of the active electrode to minimize subject discomfort. Finally, liberally soaking the gauze beneath the treatment electrode with epinephrine, will help ensure that the electrical current is dissipated uniformly across the skin. Electrode gel should also be applied liberally beneath the dissipation electrode.
Limitations
A major limitation of the present study is that we did not collect any gold-standard measures of muscle perfusion, such as those obtained by arterial spin-labeling MRI (41). By normalizing BFI values to a standard measure of perfusion, cross-visit and intersubject variability may have been greatly reduced for the variables measured in Part 2 of the present study. Similarly, gold-standard measures of muscle perfusion would help to better inform our conclusions about the effectiveness of epinephrine iontophoresis for attenuating changes in cutaneous blood flow during whole body heating and preserving BFI kinetics during exercise performed during hyperthermia.
Peak measures of skin perfusion measured by laser Doppler flowmetry are often measured following 30+ min of local heating to induce maximal vasodilation beneath the LDF probe. Our decision to only perform local heating for 20 min was based on our previous work (1), and the observation that epinephrine iontophoresis substantially attenuated skin blood flow in Part 1 of the present study. Indeed, the results of Part 1 suggested that we likely would have had to perform local heating for 2+ h to achieve a true “maximal” measure of skin blood flow beneath the epinephrine-iontophoresed tissue, which would have added significant burden to the participants. Moreover, we saw little-to-no differences in peak LDF across the three untreated measurement sites during the reactive hyperemia testing of Part 2 (Table 4). However, because we did not perform local heating long enough to obtain a maximal measure of skin perfusion beneath the epinephrine-iontophoresed tissue, we cannot quantify the absolute magnitude by which it attenuated skin blood flow. Nevertheless, the results of this study demonstrate that epinephrine iontophoresis can be used to eliminate any potentially confounding effects that increased skin blood flow may have on NIR-DCS estimates of skeletal muscle BFI.
Another limitation is that we did not measure the depth that epinephrine penetrated the forearm tissues during iontophoresis, and therefore cannot conclusively determine if epinephrine affected skeletal muscle blood flow. To the best of our knowledge, this has never been directly investigated. Draper et al. reported that iontophoresis can drive lidocaine 5 mm into cutaneous tissue when the lidocaine solution also contains epinephrine (42). It seems unlikely that epinephrine iontophoresis affected skeletal muscle blood flow in the present study though, given that changes in BFI and THb during reactive hyperemia and exercise were not significantly different across visits.
Finally, although the experimental procedures performed in Part 2 are commonly performed by several laboratories that use NIR-DCS to investigate skeletal muscle blood flow (1, 2, 4, 26, 43, 44), the procedures do not, in and of themselves, induce hyperthermia. Thus, the results of the present study are less applicable to whole body exercises, such as cycling or knee extension, where exercise-induced hyperthermia may stimulate increases in cutaneous perfusion to augment sweating. However, the results of Part 3 demonstrate that even if exercise-induced hyperthermia does cause large increases in cutaneous perfusion, epinephrine iontophoresis can be used to abolish changes in skin blood flow beneath the NIR-DCS probe.
Perspectives and Significance
The present study demonstrated that epinephrine iontophoresis attenuates (if not abolishes) changes in cutaneous perfusion for up to 3–4 h posttreatment and eliminates cutaneous impairment of NIR-DCS BFI during hyperthermic handgrip exercise. Together, these data suggest that epinephrine iontophoresis can be used to eliminate any potentially confounding effects that increased skin blood flow may have on NIR-DCS estimates of skeletal muscle BFI. However, for experiments performed in thermoneutral environments, the NIR-DCS BFI signal appears to be almost wholly dominated by changes in the underlying skeletal muscle, with little-to-no contributions from the skin. Thus, epinephrine iontophoresis does not appear to be necessary for NIR-DCS studies of skeletal muscle blood flow when changes in cutaneous perfusion are expected to be small.
DATA AVAILABILITY
Data will be made available upon reasonable request.
GRANTS
This work was supported by the Potratz Family’s generous contributions and the National Institutes for Health Grant R15HL140989 (to M. D. Nelson).
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the authors.
AUTHOR CONTRIBUTIONS
M.F.B., C.A.T., D.W., and M.D.N. conceived and designed research; M.F.B., A.P.-C., A.P.O., and J.M. performed experiments; M.F.B. analyzed data; M.F.B., A.P.-C., A.P.O., J.M., R.M.B., C.A.T., D.W., and M.D.N. interpreted results of experiments; M.F.B. and M.D.N. prepared figures; M.F.B. drafted manuscript; M.F.B., A.P.-C., A.P.O., J.M., R.M.B., C.A.T., D.W., and M.D.N. edited and revised manuscript; M.F.B., A.P.-C., A.P.O., J.M., R.M.B., C.A.T., D.W., and M.D.N. approved final version of manuscript.
ACKNOWLEDGMENTS
We thank the subjects for time, patience, and efforts throughout this study.
REFERENCES
- 1. Bartlett MF, Akins JD, Oneglia AP, Brothers RM, Wilkes D, Nelson MD. Impact of cutaneous blood flow on NIR-DCS measures of skeletal muscle blood flow index. J Appl Physiol (1985) 131: 914–926, 2021. doi: 10.1152/japplphysiol.00337.2021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Ichinose M, Nakabayashi M, Ono Y. Rapid vasodilation within contracted skeletal muscle in humans: new insight from concurrent use of diffuse correlation spectroscopy and Doppler ultrasound. Am J Physiol Heart Circ Physiol 320: H654–H667, 2021. doi: 10.1152/ajpheart.00761.2020. [DOI] [PubMed] [Google Scholar]
- 3. Ichinose M, Nakabayashi M, Ono Y. Sympathoexcitation constrains vasodilation in the human skeletal muscle microvasculature during postocclusive reactive hyperemia. Am J Physiol Heart Circ Physiol 315: H242–H253, 2018. doi: 10.1152/ajpheart.00010.2018. [DOI] [PubMed] [Google Scholar]
- 4. Didier KD, Hammer SM, Alexander AM, Caldwell JT, Sutterfield SL, Smith JR, Ade CJ, Barstow TJ. Microvascular blood flow during vascular occlusion tests assessed by diffuse correlation spectroscopy. Exp Physiol 105: 201–210, 2020. doi: 10.1113/EP087866. [DOI] [PubMed] [Google Scholar]
- 5. Nakabayashi M, Ono Y. Detection of blood flow speed in shallow and deep tissues using diffuse correlation spectroscopy. Adv Biomed Eng 6: 53–58, 2017. doi: 10.1117/1.JBO.25.9.097003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Baker W, Parthasarathy A, Ko T, Busch D, Abramson K, Tzeng S, Mesquita R, Durduran T, Greenberg J, Kung D, Yodh A. Pressure modulation algorithm to separate cerebral hemodynamic signals from extracerebral artifacts. Neurophotonics 2: 035004, 2015. doi: 10.1117/1.NPh.2.3.035004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Wang D, Baker WB, He H, Gao P, Zhu L, Peng Q, Li Z, Li F, Chen T, Feng H. Influence of probe pressure on the pulsatile diffuse correlation spectroscopy blood flow signal on the forearm and forehead regions. Neurophotonics 6: 035013, 2019. doi: 10.1117/1.NPh.6.3.035013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Tamborini D, Stephens K, Wu M, Farzam P, Siegel A, Shatrovoy O, Blackwell M, Boas D, Carp S, Franceschini M. Portable system for time-domain diffuse correlation spectroscopy. IEEE Trans Biomed Eng 66: 3014–3025, 2019. [Erratum in IEEE Trans Biomed Eng 67: 1229, 2020]. doi: 10.1109/TBME.2019.2899762. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Pagliazzi M, Colombo L, Vidal-Rosas EE, Dragojević T, Parfentyeva V, Culver JP, Konugolu Venkata Sekar SK, Di Sieno L, Contini D, Torricelli A, Pifferi A, Dalla Mora A, Durduran T. Time resolved speckle contrast optical spectroscopy at quasi-null source-detector separation for non-invasive measurement of microvascular blood flow. Biomed Opt Express 12: 1499–1511, 2021. doi: 10.1364/BOE.418882. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Samaei S, Sawosz P, Kacprzak M, Pastuszak Ż, Borycki D, Liebert A. Time-domain diffuse correlation spectroscopy (TD-DCS) for noninvasive, depth-dependent blood flow quantification in human tissue in vivo. Sci Rep 11: 1817, 2021. doi: 10.1038/s41598-021-81448-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Carp S, Tamborini D, Mazumder D, Wu K, Robinson M, Stephens K, Shatrovoy O, Lue N, Ozana N, Blackwell M, Franceschini M. Diffuse correlation spectroscopy measurements of blood flow using 1064 nm light. J Biomed Opt 25: 097003, 2020. doi: 10.1117/1.JBO.25.9.097003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. McKee DE, Lalonde DH, Thoma A, Dickson L. Achieving the optimal epinephrine effect in wide awake hand surgery using local anesthesia without a tourniquet. Hand (NY) 10: 613–615, 2015. doi: 10.1007/s11552-015-9759-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Hernandez A, Rosario M, Mendoza-Torres R, Taguba CRM, Garcia A, Battad G. Evaluating clinical outcomes for determining the optimal delay to skin incision under WALANT: a prospective series of 34 patients from a low-resource tertiary setting. Adv Orthop 2020: 9351354, 2020. doi: 10.1155/2020/9351354. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Cooper K, Edholm O, Mottram R. The blood flow in skin and muscle of the human forearm. J Physiol 128: 258–267, 1955. doi: 10.1113/jphysiol.1955.sp005304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Edholm O, Fox R, MacPherson R. The effect of body heating on the circulation in skin and muscle. J Physiol 134: 612–619, 1956. doi: 10.1113/jphysiol.1956.sp005669. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Zelis R, Mason D. Comparison of the reflex reactivity of skin and muscle veins in the human forearm. J Clin Invest 48: 1870–1877, 1969. doi: 10.1172/JCI106153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Zelis R, Mason D, Braunwald E. Partition of blood flow to the cutaneous and muscular beds of the forearm at rest and during leg exercise in normal subjects and in patients with heart failure. Circ Res 24: 799–806, 1969. doi: 10.1161/01.res.24.6.799. [DOI] [PubMed] [Google Scholar]
- 18. LaJoie W, Wong W, Hyman C, Austin E. Cutaneous ischemia from epinephrine ion transfer and counterpressure. J Appl Physiol (1985) 27: 400–402, 1969. doi: 10.1152/jappl.1969.27.3.400. [DOI] [PubMed] [Google Scholar]
- 19. Detry J, Brengelmann G, Rowell L, Wyss C. Skin and muscle components of forearm blood flow in directly heated resting man. J Appl Physiol (1985) 32: 506–511, 1972. doi: 10.1152/jappl.1972.32.4.506. [DOI] [PubMed] [Google Scholar]
- 20. Bartlett MF, Oneglia A, Jaffery MF, Manitowabi-Huebner S, Hueber DM, Nelson MD. Kinetic differences between macro- and microvascular measures of reactive hyperemia. J Appl Physiol (1985) 129: 1183–1192, 2020. doi: 10.1152/japplphysiol.00481.2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Ryan TE, Southern WM, Reynolds MA, McCully KK. A cross-validation of near-infrared spectroscopy measurements of skeletal muscle oxidative capacity with phosphorus magnetic resonance spectroscopy. J Appl Physiol (1985) 115: 1757–1766, 2013. doi: 10.1152/japplphysiol.00835.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Adami A, Rossiter HB. Principles, insights, and potential pitfalls of the noninvasive determination of muscle oxidative capacity by near-infrared spectroscopy. J Appl Physiol (1985) 124: 245–248, 2018. doi: 10.1152/japplphysiol.00445.2017. [DOI] [PubMed] [Google Scholar]
- 23. Chung S, Rosenberry R, Ryan T, Munson M, Dombrowsky T, Park S, Nasirian A, Haykowsky MJ, Nelson MD. Near-infrared spectroscopy detects age-related differences in skeletal muscle oxidative function: promising implications for geroscience. Physiol Rep 6: e13588, 2018. doi: 10.14814/phy2.13588. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Gurley K, Shang Y, Yu G. Noninvasive optical quantification of absolute blood flow, blood oxygenation, and oxygen consumption rate in exercising skeletal muscle. J Biomed Opt 17: 075010, 2012. doi: 10.1117/1.JBO.17.7.075010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Rosenberry R, Tucker WJ, Haykowsky MJ, Trojacek D, Chamseddine HH, Arena-Marshall CA, Zhu Y, Wang J, Kellawan JM, Tian F, Nelson MD. Determinants of skeletal muscle oxygen consumption assessed by near-infrared diffuse correlation spectroscopy during incremental handgrip exercise. J Appl Physiol (1985) 127: 698–706, 2019. doi: 10.1152/japplphysiol.00273.2019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Tucker WJ, Rosenberry R, Trojacek D, Chamseddine H, Arena-Marshall C, Zhu Y, Wang J, Kellawan J, Haykowsky M, Tian F, Nelson M. Studies into the determinants of skeletal muscle oxygen consumption: novel insight from near-infrared diffuse correlation spectroscopy. J Physiol 597: 2887–2901, 2019. doi: 10.1113/JP277580. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Tucker WJ, Rosenberry R, Trojacek D, Sanchez B, Bentley RF, Haykowsky MJ, Tian F, Nelson MD. Near-infrared diffuse correlation spectroscopy tracks changes in oxygen delivery and utilization during exercise with and without isolated arterial compression. Am J Physiol Regul Integr Comp Physiol 318: R81–R88, 2020. doi: 10.1152/ajpregu.00212.2019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Dixit N, Bali V, Baboota S, Ahuja A, Ali J. Iontophoresis—an approach for controlled drug delivery: a review. Curr Drug Deliv 4: 1–10, 2007. doi: 10.2174/1567201810704010001. [DOI] [PubMed] [Google Scholar]
- 29. Roustit M, Blaise S, Cracowski JL. Trials and tribulations of skin iontophoresis in therapeutics. Br J Clin Pharmacol 77: 63–71, 2014. doi: 10.1111/bcp.12128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Hueber DM, Franceschini MA, Ma HY, Zhang Q, Ballesteros JR, Fantini S, Wallace D, Ntziachristos V, Chance B. Non-invasive and quantitative near-infrared haemoglobin spectrometry in the piglet brain during hypoxic stress, using a frequency-domain multidistance instrument. Phys Med Biol 46: 41–62, 2001. doi: 10.1088/0031-9155/46/1/304. [DOI] [PubMed] [Google Scholar]
- 31. Barstow TJ. Understanding near infrared spectroscopy and its application to skeletal muscle research. J Appl Physiol (1985) 126: 1360–1376, 2019. doi: 10.1152/japplphysiol.00166.2018. [DOI] [PubMed] [Google Scholar]
- 32. Gratton E, Fantini S, Franceschini MA, Gratton G, Fabiani M. Measurements of scattering and absorption changes in muscle and brain. Philos Trans R Soc Lond B Biol Sci 352: 727–735, 1997. doi: 10.1098/rstb.1997.0055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Bartlett MF, Jordan SM, Hueber DM, Nelson MD. Impact of changes in tissue optical properties on near-infrared diffuse correlation spectroscopy measures of skeletal muscle blood flow. J Appl Physiol (1985) 130: 1183–1195, 2021. doi: 10.1152/japplphysiol.00857.2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Lin W, Busch D, Goh C, Barsi J, Floyd T. Diffuse correlation spectroscopy analysis implemented on a field programmable gate array. IEEE Access 7: 122503–122512, 2019. doi: 10.1109/access.2019.2938085. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Durduran T, Choe R, Baker WB, Yodh AG. Diffuse optics for tissue monitoring and tomography. Rep Prog Phys 73: 076701, 2010. doi: 10.1088/0034-4885/73/7/076701. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Boas DA, Sakadžić S, Selb J, Farzam P, Franceschini MA, Carp SA. Establishing the diffuse correlation spectroscopy signal relationship with blood flow. Neurophotonics 3: 031412, 2016. doi: 10.1117/1.NPh.3.3.031412. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Boas DA, Yodh AG. Spatially varying dynamical properties of turbid media probed with diffusing temporal light correlation. J Opt Soc Am A 14: 192–215, 1997. doi: 10.1364/JOSAA.14.000192. [DOI] [Google Scholar]
- 38. Tew GA, Ruddock AD, Saxton JM. Skin blood flow differentially affects near-infrared spectroscopy-derived measures of muscle oxygen saturation and blood volume at rest and during dynamic leg exercise. Eur J Appl Physiol 110: 1083–1089, 2010. doi: 10.1007/s00421-010-1596-2. [DOI] [PubMed] [Google Scholar]
- 39. Quaresima V, Farzam P, Anderson P, Farzam PY, Wiese D, Carp SA, Ferrari M, Franceschini MA. Diffuse correlation spectroscopy and frequency-domain near-infrared spectroscopy for measuring microvascular blood flow in dynamically exercising human muscles. J Appl Physiol (1985) 127: 1328–1337, 2019. doi: 10.1152/japplphysiol.00324.2019. [DOI] [PubMed] [Google Scholar]
- 40. Costello CT, Jeske AH. Iontophoresis: applications in transdermal medication delivery. Phys Ther 75: 554–563, 1995. doi: 10.1093/ptj/75.6.554. [DOI] [PubMed] [Google Scholar]
- 41. Yu G, Floyd TF, Durduran T, Zhou C, Wang J, Detre JA, Yodh AG. Validation of diffuse correlation spectroscopy for muscle blood flow with concurrent arterial spin labeled perfusion MRI. Opt Express 15: 1064–1075, 2007. doi: 10.1364/oe.15.001064. [DOI] [PubMed] [Google Scholar]
- 42. Draper DO, Coglianese M, Castel C. Absorption of iontophoresis-driven 2% lidocaine with epinephrine in the tissues at 5 mm below the surface of the skin. J Athl Train 46: 277–281, 2011. doi: 10.4085/1062-6050-46.3.277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Ichinose M, Nakabayashi M, Ono Y. Difference in the integrated effects of sympathetic vasoconstriction and local vasodilation in human skeletal muscle and skin microvasculature. Physiol Rep 7: e14070, 2019. doi: 10.14814/phy2.14070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Hammer SM, Alexander AM, Didier KD, Smith JR, Caldwell JT, Sutterfield SL, Ade CJ, Barstow TJ. The noninvasive simultaneous measurement of tissue oxygenation and microvascular hemodynamics during incremental handgrip exercise. J Appl Physiol (1985) 124: 604–614, 2018. doi: 10.1152/japplphysiol.00815.2017. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Data will be made available upon reasonable request.