Keywords: enteric glia, enteric nervous system, interstitial cells of Cajal, myenteric plexus, vagus nerve
Abstract
Of all the organ systems in the body, the gastrointestinal tract is the most complicated in terms of the numbers of structures involved, each with different functions, and the numbers and types of signaling molecules utilized. The digestion of food and absorption of nutrients, electrolytes, and water occurs in a hostile luminal environment that contains a large and diverse microbiota. At the core of regulatory control of the digestive and defensive functions of the gastrointestinal tract is the enteric nervous system (ENS), a complex system of neurons and glia in the gut wall. In this review, we discuss 1) the intrinsic neural control of gut functions involved in digestion and 2) how the ENS interacts with the immune system, gut microbiota, and epithelium to maintain mucosal defense and barrier function. We highlight developments that have revolutionized our understanding of the physiology and pathophysiology of enteric neural control. These include a new understanding of the molecular architecture of the ENS, the organization and function of enteric motor circuits, and the roles of enteric glia. We explore the transduction of luminal stimuli by enteroendocrine cells, the regulation of intestinal barrier function by enteric neurons and glia, local immune control by the ENS, and the role of the gut microbiota in regulating the structure and function of the ENS. Multifunctional enteric neurons work together with enteric glial cells, macrophages, interstitial cells, and enteroendocrine cells integrating an array of signals to initiate outputs that are precisely regulated in space and time to control digestion and intestinal homeostasis.
CLINICAL HIGHLIGHTS.
How gastrointestinal homeostasis is controlled is fundamental to understanding the etiology of gastrointestinal diseases. This review addresses how the digestive and defensive functions of the gastrointestinal tract are regulated by the enteric nervous system. Enteric neurons function in circuits together with enteric glial cells, macrophages, interstitial cells, and enteroendocrine cells to initiate outputs (e.g., motility, secretion) that are precisely regulated in space and time to control intestinal homeostasis. A deep understanding of how enteric neural control is regulated will guide future investigations into pathophysiological conditions and gastrointestinal diseases.
1. INTRODUCTION AND SCOPE OF THE REVIEW
The dual roles of digestion and defense place an enormous burden on the gastrointestinal (GI) tract. The gut must supply the energy, nutrients, vitamins, fluid, and electrolytes required for survival and effective reproduction and, at the same time, protection against hostile microorganisms, toxins, and environmental contaminants ingested along with food and water. Defensive mechanisms are also required to protect against digestion itself; acid, digestive enzymes, food antigens, and bile acids are all potentially harmful. Another important stress faced by the gut is the mechanical stress (stretch and strain) associated with the movements of the gut. These two principal functions of the gut occur simultaneously and are seamlessly regulated and integrated. The dynamic interplay between defense and digestion occurs in the context of the continual turnover of the GI epithelium and the maintenance of an optimal luminal environment to support the gut microbiome: the vast numbers of commensal microbes that live in the gut lumen (1–3).
Digestion is organized in distinct phases that involve the sight, smell, and initial taste of the food (and, for humans, the intricacies of the preparation of the meal). These sensory stimuli are integrated in the central nervous system (CNS) and initiate the cephalic phase of digestion. After an oral phase of digestion, food enters the esophagus and stomach, activating the gastric phase of digestion, which is then followed sequentially by the intestinal and colonic phases of digestion. Given the complexity of digestion, the relative dimensions of the gut, and the time it takes to process meals, organizing and regulating digestion and defense is a task of considerable magnitude: a task worthy of a “brain.” In fact, vertebrates utilize two “brains” to regulate the gut: the CNS (brain and spinal cord) and the enteric nervous system (ENS), the system of nerves within the wall of the GI tract.
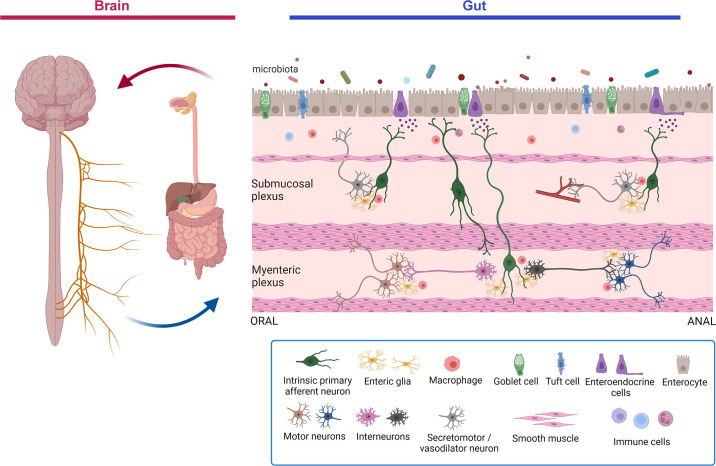
The majority of the regulatory control of the GI tract occurs locally and is directed by the “brain in the gut,” the ENS (4–6). The cephalic phase of digestion is initiated by the CNS, which interacts directly with the ENS to initiate digestive functions in the wall of the gut. Bidirectional communication between the ENS and the CNS, the gut-brain axis, integrates digestive and defensive functions of the gut with those of other organs to maintain homeostasis (7–10) (FIGURE 1).
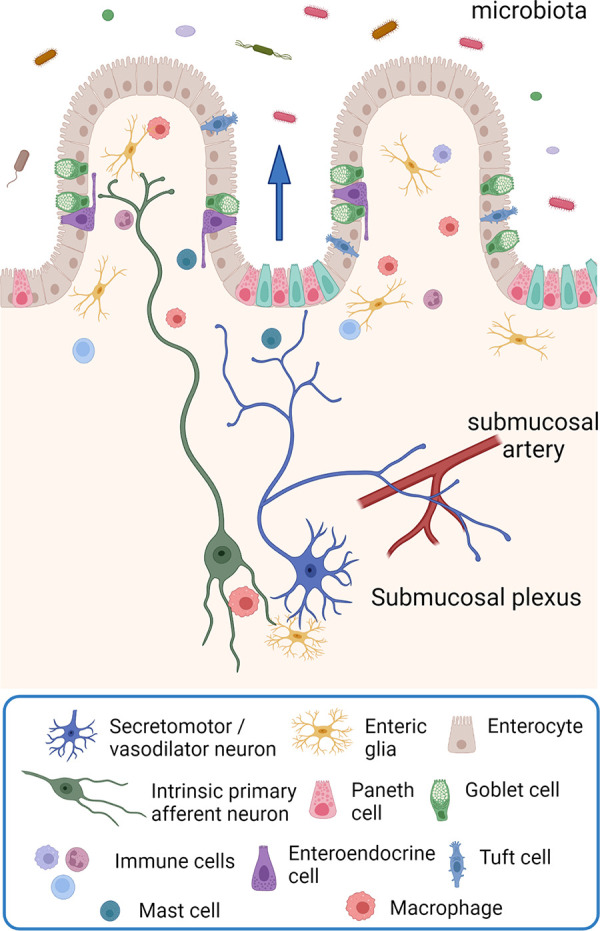
Figure 1.
Schematic overview of the gut-brain-microbiota axis. The gut-brain axis is a bidirectional communication axis that influences the digestive and defensive functions of the gut to regulate homeostasis. The gut microbiota communicates with epithelial cells, including enteroendocrine, tuft cells, and immune cells in the lamina propria that in turn communicate with enteric nerves, enteric glia, and the terminals of extrinsic primary afferent nerves (not shown) to modulate reflex control of gut function. Autonomic reflexes are initiated via vagal and spinal primary afferent pathways, as well as viscerofugal enteric neurons, and central neural circuitry of the brain and spinal cord is mobilized. Top-down control of the gastrointestinal tract occurs via autonomic efferent pathways. Alterations in gut motility and secretion can modulate the microbial environment in the gut. Modifications in the nature of these bidirectional interactions in response to perturbations such as stress or infections can alter the behavior of this system, sometimes manifesting as disorders of brain-gut interactions such as irritable bowel syndrome. Image created with BioRender.com, with permission.
The ENS has evolved over millennia. Seen in cnidarians (i.e., hydra), invertebrates (e.g., mollusks, insects, and worms), and all vertebrate species, the ENS evolved before the CNS (11). This is possibly one of the evolutionary steps that allowed the mammalian CNS to emerge without the need to contain the vast numbers of neurons and glia required to control digestion and defense. The ENS therefore gives the CNS the “freedom to think,” since the local regulation of digestive functions occurs autonomously and generally in the absence of conscious sensation. As suggested by Furness and Stebbing (11), the ENS should be considered the “first brain” and the CNS the “second brain,” not the other way round (12)!
The topic of the ENS is immense, and doing justice to it fully would take a book, as has been the case in the past (4, 5, 12). In this review, we build on these findings by providing an overview of the general structure of the enteric nervous system, including a discussion of whether a blood-ENS barrier exists. We then consider the cellular and molecular architecture of the ENS, including all the cell types found in, or associated with, enteric ganglia. The review aims to show how these diverse cell types are integrated to regulate GI physiology. After this we discuss the functional organization of the enteric nervous system and its relation to the autonomic nervous system. We place into context the recently discovered features of mammalian enteric neural control, with reference to historical milestones in the development of the field.
Although we touch on older literature, we highlight the many new developments in the field and use them to illustrate how our understanding of the physiology and pathophysiology of enteric neural control has been revolutionized in recent years. Notably, we describe the molecular architecture of the ENS based on the use of single-cell RNA technologies, local immune control by the ENS, regulation of intestinal barrier function by enteric neurons and glia, organization and function of enteric motor circuits, plasticity of the ENS, extrinsic autonomic neural control, the role of the gut microbiome in regulating the structure and function of the ENS, and luminal signaling mechanisms involving enteroendocrine cells.
We fully consider the role of the ENS in host defense, focusing on how immune cells are integrated into the functional circuitry of the ENS, highlighting some particularly novel aspects of the neuroimmunophysiology of the GI tract. We also draw readers’ attention to excellent recent reviews on this topic (13–23). An area of emerging importance is ENS-CNS communication in the development and expression of neurological disorders. These findings, which have been recently reviewed (7, 24–26), point to a remarkable role for the ENS and gut-brain axis in mediating aspects of diseases thought until recently to be purely CNS disorders. Similarly, enteric reflexes and motor control have been recently reviewed, and we direct readers to these excellent articles (27–32). Finally, we do not discuss enteric neural development, as this subject has been covered extensively, and we direct interested readers to these articles (33–43).
In a final section, we look to the future and consider the impact of the application of modern molecular, genetic, and neuroscientific techniques on our understanding of ENS physiology, to fill critical knowledge gaps remaining in the field.
2. GENERAL STRUCTURE OF THE ENTERIC NERVOUS SYSTEM
In the intestines of vertebrates, the ENS is characterized by the presence of neurons and glia organized in ganglia arranged as two or more distinct enteric plexuses and their interconnecting neural pathways (4, 5). The ganglionated plexuses provide local nervous control of the tissues and cells adjacent to the ganglia, including smooth muscle, blood vessels, glands, and immune cells and tissues. The myenteric plexus (formerly, Auerbach’s plexus) lies between the longitudinal and circular muscle layers, whereas the submucosal plexus is found within the connective tissues of the submucosa (FIGURE 2). The submucosal plexus consists of a single layer of rather small ganglia in common laboratory species (i.e., mouse, rat, and guinea pig), but in larger mammals and humans it consists of two layers of ganglia, called the inner and outer submucosal plexus [formerly Meissner’s plexus and Henle’s/Schabadasch’s plexus (the exact attribution is uncertain), respectively] (4, 44–46). There are extensive connections among ganglia along the length of the gut, including projections between myenteric and submucosal plexuses. The magnitude of the ENS is unmatched in the peripheral nervous system: it has been estimated that the human ENS contains ∼200 million neurons, with three to five times as many enteric glial cells (4, 5, 47). Given its size, complexity, and ability to function autonomously, the analogy to the brain, mentioned above, seems warranted and, as we will see, goes further than simply the number of neurons.
Figure 2.
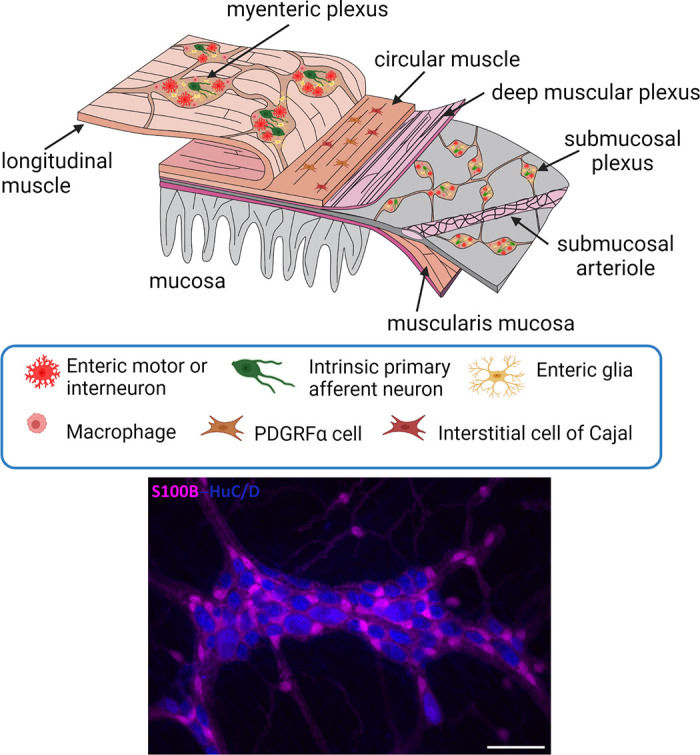
Top: the general organization of the enteric nervous system. The myenteric plexus lies between the longitudinal and circular muscle layers. The submucosal plexus lies in the submucosa and in larger animals (not shown) consists of an outer and an inner component. Nerve fibers connect the ganglia and form nerve plexuses that innervate the longitudinal muscle, circular muscle, muscularis mucosa, and mucosa. There are also enteric nerves innervating arteries in the submucosa, as well as the gut-associated lymphoid tissues. The mucosa is densely innervated. Enteric glia are found associated with enteric nerves throughout the wall of the gut. Bottom: organization of enteric neurons (labeled with ant-HuC/D, blue) and enteric glia (labeled with anti-S100B, pink) in the myenteric plexus of the mouse ileum. Note that enteric glia outnumber enteric neurons and the presence of extraganglionic enteric glial cells. Scale bar, 100 µm. See glossary for abbreviations. Unpublished photomicrograph provided by C. M. MacNaughton.
The ENS extends the length of the GI tract, from the esophagus to the anus, as a continuous uninterrupted network of ganglia and nerve bundles (4, 5). It is organized into two (or three) ganglionated plexuses, but this arrangement is only fully elaborated in the small and large intestines. In the esophagus and stomach, the vast majority of neurons are found in the myenteric plexus. In larger mammals, a very sparse submucosal plexus exists, in these regions consisting of only a few ganglia or isolated neurons. In typical laboratory species there are few if any submucosal ganglia in the esophagus and stomach. The functional significance of the lack of the submucosal plexus in the esophagus and stomach remains to be fully determined, but it may be because these organs receive an extensive vagal efferent innervation that serves to regulate secretion, which occurs predominantly during the cephalic and gastric phases of digestion.
The arrangement of the ENS facilitates its functions. The role of the myenteric plexus is to control GI motility by regulating contractility in the longitudinal and circular muscles (48–50). The submucosal plexus (or inner submucosal plexus in larger animals) controls secretory functions, absorption, and blood flow (51, 52). In larger animals, the outer submucosal plexus provides additional control of the circular and longitudinal muscles (53–55). In addition, there are scattered enteric neurons in the base of the mucosa of larger animals and humans that presumably control mucosal function. Considering the crucial defensive functions of the gut, the contributions of the two plexuses are actually not well defined, but presumably there are important contributions from both plexuses. The enteric plexuses are extensively interconnected to allow for the coordinated regulation of multiple gut functions, e.g., secretion and motility in a given region (4, 5).
The accessory organs of the digestive system also contain ganglionated plexuses that share properties of parasympathetic and enteric ganglia. There are ganglia in the gallbladder, sphincter of Oddi, and pancreas (56–58). These ganglia are in continuity with the ENS of the duodenum (59, 60) and are important for reflex regulation of secretion, as well as motility in the biliary system. Most evidence points to the fact that these ganglia act more like autonomic (parasympathetic) ganglia than enteric ganglia, which have a greater integrative role based on local inputs. Together, the accessory organs of the digestive system play an integral role in the duodenal phase of digestion.
2.1. Is There a Blood-Enteric Nervous System Barrier?
At an ultrastructural level, the ganglia of the ENS exhibit a dense neuropil devoid of connective tissue or blood vessels that resembles the CNS (4, 61). Given this “brainlike” structure, it is of relevance to consider whether there is a blood-enteric ganglia barrier, analogous to the blood-brain barrier of the CNS. In enteric ganglia, neurons are only partially surrounded by glial cells, and enteric axons are not wrapped in glial sheaths, as even unmyelinated axons are in the periphery. Thus, superficially, it appears that there is not a blood-ganglion barrier, allowing ready access to circulating molecules or drugs. This was supported by the work of Jacobs (62) and later by Mawe and colleagues (63), who showed high-molecular-weight tracers in myenteric ganglia of the rat ileum and enteric ganglia of the guinea pig gallbladder, respectively, after intravenous administration. In contrast, Gershon and Bursztajn (64) showed that albumin and horseradish peroxidase could not penetrate the capsule of the myenteric ganglia of the mouse jejunum after intravenous injection. These data were recently confirmed and extended by Dora et al. (65), who used confocal and electron microscopy to characterize a blood-myenteric plexus barrier in the proximal colon of mice. They demonstrated a blood-myenteric plexus barrier, consisting of the extracellular matrix proteins agrin and collagen-4 and enteric glial end feet, that is reminiscent of the blood-brain barrier (65). These authors also found that the blood-myenteric plexus barrier was disrupted when the colon was inflamed, and this led to an infiltration of macrophages into the myenteric plexus. Further studies are needed to expand these interesting findings and to examine whether there is a similar barrier in the submucosal plexus, but the answer to the question, Is there a blood-enteric barrier? appears to be yes. It will be informative to determine which molecules are excluded and which penetrate this barrier and under what circumstances the blood-myenteric plexus barrier is disrupted, as this will likely explain some aspects of GI pathophysiology that we have yet to fully understand. The existence of these extracellular matrix proteins in the ENS suggests the possibility of perineuronal nets. Perineuronal nets are extracellular matrix structures that are important for synaptic stabilization and plasticity, especially during development, and are involved in functional recovery after lesions and play key roles in interactions between nerve processes and astrocytes in the CNS (66–68). Extracellular matrix proteins, beyond those noted above, are found in the ENS (e.g., Refs. 69, 70). Interestingly, one of these, tenascin-x, was recently shown to be expressed in mouse and human myenteric neurons and found to be important in the regulation of gastric emptying in the mouse (69, 71). Recent transcriptomic data reveal that some of the key perineuronal net molecules are also expressed in the ENS, e.g., chondroitin sulfate proteoglycans, tenascin, and versican (72). Collectively, these findings support the possibility that perineuronal nets contribute to synaptic integrity and plasticity in the ENS.
3. CELLULAR AND MOLECULAR ARCHITECTURE OF THE ENTERIC NERVOUS SYSTEM
Ever since the earliest anatomical and physiological studies of the ENS (4), it has been clear that it is a complex neural network, consisting of a heterogeneous mixture of neurons and glia, that is involved in the nervous control of digestion through reflex regulation of gut motility and secretion. It was also recognized that, in particular, the myenteric plexus is closely associated with nonneuronal cells that were also arranged in a network. Recent studies have shed new light on the remarkable complexity of the cellular and molecular architecture of the ENS (73–75).
3.1. Enteric Neurons
Enteric neurons were originally classified based on the appearance of the soma and the nature of their dendritic processes and axonal arborizations. Two major subtypes were recognized, which are referred to as Dogiel type I and type II neurons. Dogiel type I neurons appear flattened and exhibit short, club-shaped, lamellar dendrites and a single axon, and Dogiel type II neurons extend multiple axons to other ganglia as well as to the mucosa. A third class of neuron (Dogiel type III neurons) was also observed that had numerous tapering dendrites that ended within the ganglion of origin (4). Subsequent work, especially in the pig and human, has revealed many additional morphologies of enteric neurons in the myenteric and submucosal plexuses. These include subtypes of neurons that are found along the length of the gut [e.g., (Dogiel) type I neurons] but others with more restricted distributions in specific regions of the gut (e.g., type V neurons that are only found in the human upper small intestine) and neurons with filamentous dendrites (76, 77). It is not clear whether the dendritic processes of enteric neurons behave like dendritic spines of the CNS, but classical spines have not been described on enteric neurons. Nevertheless, enteric neurons in animals and humans are richly decorated with synapses, on their soma and dendrites (61, 78–81), and in the guinea pig ENS they function similarly to central synapses in terms of their dynamic properties and neurotransmitter release mechanisms (82). Moreover, synaptic contacts are also observed in the interganglionic fiber bundles of the ENS, suggesting that neuronal activity is regulated both within ganglia and at extraganglionic sites between ganglia and presumably between the submucosal and myenteric plexuses (4, 61, 79, 83). An important observation was made by Gabella in his extensive ultrastructural examination of the myenteric plexus (61). He noted that
“Clusterings of agranular vesicles, thickenings of the axon membrane, and dense projections are frequently observed in small varicosities inside the ganglia; in place of the expected post-synaptic element there is a glial cell body or a glial process without any obvious ‘post-junctional’ specialization.”
He went on to say,
“An extremely puzzling observation is the occurrence of morphological specializations in axons contacting glial cells. Apparently these axons are a specific type of agranular vesicle-containing axon. The number of contacts is rather high and it is very likely that each glial cell has one or more.”
These striking observations were generally overlooked until relatively recently, when functional evidence was provided for direct neuronal-glial communication and circuit-specific enteric glia involved in the control of colonic motility (discussed in more detail in sect. 3.2.2) (84–87). Thus, like the brain (88, 89), there is the possibility of a functionally important tripartite synapse in the ENS. The detailed features of this arrangement that have been elaborated in the CNS remain to be fully understood in the ENS. Another feature in which the neuropil of the ENS resembles that of the CNS is the lack of sheaths surrounding individual unmyelinated axons. In the ENS, unmyelinated axons exist in bundles that are immediately adjacent to one another.
Unlike neurons of the CNS, which are largely protected from mechanical deformation, enteric neurons are regularly subject to distortion, varying in shape with contraction and distension of the musculature of the gut wall even under physiological conditions (90). The extent of the deformation of enteric neurons is unique in the nervous system, but the mechanisms underlying their survival under these conditions remain to be fully elucidated. One controversial theory proposed by Kulkarni et al. (91) is that because of these stresses there is extensive enteric neuronal apoptosis that is balanced by an equal degree of neurogenesis that maintains the overall integrity of the ENS throughout life. Data to support one aspect of these findings have been described by Becker et al. (92), who used an ex vivo organotypic culturing system of the ENS and measured proliferation, finding high levels of ethynyl-labeled deoxyuridine+ (proliferating) neurons in young adult mice that were reduced in older mice. Further evidence in support of these findings comes from De Schepper et al. (93) and Chandrasekharan et al. (94), who demonstrated, using cleaved caspase-3 as a marker, constitutively high levels of neuronal apoptosis in enteric neurons in the myenteric plexus that range from ∼5% to 10% in the human and mouse, respectively, with higher levels in the submucosal plexus of the mouse (93). Using cleaved caspase-1 as a marker, Ye et al. (95) also found that ∼5% of neurons in the myenteric plexus under physiological conditions were undergoing pyroptosis, and this number substantially increased with obesity. In contrast, Gianino et al. (96) found no evidence of neuronal apoptosis based on use of cleaved caspase-3, and neither Bradley et al. (97) nor Joseph et al. (98) were able to detect 5-bromo-2-deoxyuridine, another marker of cell proliferation, in enteric neurons under physiological conditions. In a direct attempt to replicate the findings of Kulkarni et al., Virtanen et al. (99) were unable to do so and concluded that there was no evidence of neuronal proliferation in myenteric neurons in the mouse. In a twist to this story, Iruzubieta et al. (100) found no evidence of proliferation in myenteric neurons in the healthy human colon but instead described it only in interstitial cells of Cajal (ICCs). They went on to propose ICCs as neural stem cells, which will require further exploration. Therefore, while this interesting debate continues and until further studies are undertaken to address this issue more systematically, the question of how the ENS is maintained despite the physiological stresses it undergoes remains to be fully resolved. If there were to be very rapid turnover of the neurons, a big challenge would be to explain how the intricacy of the enteric neural circuits would be maintained given their extensive projections. As we discuss in sect. 6, there are conditions in which plasticity of the ENS has been demonstrated and neuronal proliferation occurs.
3.1.1. Electrophysiological properties of enteric neurons.
Electrophysiological recordings were first made from myenteric neurons in the early 1970s by Hirst, Mayer, North, Wood, and their colleagues (101–104). These investigators discovered unique properties of enteric neurons and subdivided them into two major classes, those that received fast synaptic input (S neurons) and those with a prolonged afterhyperpolarization (AH neurons). The two classes of neurons had morphological features of Dogiel type I and Dogiel type II neurons, respectively, with type I neurons exhibiting short, club-shaped, and lamellar dendrites and a single axon and type II neurons extending multiple axons to other ganglia as well as to the mucosa (105). Subsequently, recordings of enteric neurons made in other species, including humans, have confirmed the two major classes of enteric neurons (106–110).
The two major morphological classes of enteric neurons have markedly different electrophysiological characteristics that govern the activity of these neurons (4–6). S neurons are relatively depolarized at rest and exhibit actions potentials carried by tetrodotoxin (TTX)-sensitive sodium channels that are followed by a short-duration afterhyperpolarization, allowing them to fire repeatedly if stimulated sufficiently. They receive extensive synaptic inputs that generate fast and slow excitatory postsynaptic potentials (EPSPs), which can readily summate to fire action potentials. AH neurons, on the other hand, are relatively inexcitable, demonstrating little spontaneous activity. Furthermore, AH neurons are characterized by having both an early and a late afterhyperpolarization and action potentials that are only partially blocked by TTX, consisting of currents carried by voltage-gated sodium and calcium channels. The firing frequency of AH neurons is limited by the late afterhyperpolarization, but as we discuss below this is subject to regulation in many different conditions, contributing to plasticity of the ENS circuits (sect. 6). With the use of sharp electrode recording techniques, AH neurons are reported to receive few if any fast EPSPs (103, 104, 111). However, given the right conditions they do in fact receive fast synaptic inputs (112, 113). AH neurons also have prominent slow EPSPs. Slow EPSPs in AH neurons can be elicited by a variety of neurotransmitters and immune/inflammatory mediators, allowing for repetitive firing by suppression of the late afterhyperpolarization (111, 114). The ionic mechanisms of action potential generation in the ENS are well understood in the guinea pig, but less so in other species. We direct interested readers to many previous reviews on this topic (5, 114, 115). Recently, simultaneous patch-clamp and calcium imaging was performed on primary cultures of myenteric neurons from the mouse, using a line of mice with a genetically encoded calcium reporter (116). Here, both major classes of enteric neurons were once again identified, and calcium transients were found to be elicited by single action potentials. Interestingly, the responses to nicotinic acetylcholine receptor stimulation were found to distinguish AH and S neurons (116).
On the basis of their electrophysiological properties and projections, Dogiel type II neurons were classified as intrinsic primary afferent (intrinsic sensory) neurons and Dogiel type I motor and interneurons (29, 111, 117). However, the general concept of linking specific functional roles to individual classes of enteric neuron based on morphological or electrophysiological properties is no longer tenable. Although projection to a specific target (e.g., mucosa, circular muscle, longitudinal muscle) confers potential functions linked to that target, it appears that all classes of enteric neuron are multifunctional in nature. For example, in the myenteric plexus, Dogiel type I neurons, traditionally considered interneurons or motor neurons, are also mechanosensory (118–122) and Dogiel type II neurons are more likely to be integrators of neuronal function since they receive fast and slow synaptic inputs from enteric neurons and extrinsic spinal primary afferents (111–114, 123) and have been shown to be immuno-motor neurons directly regulating barrier function, as we discuss in sects. 3.1.5 and 5.4.
3.1.2. Organization of enteric neuronal circuitry.
Bayliss and Starling in their seminal 1899 paper describing the “movements and innervation of the small intestine” (124) concluded that
“The peristaltic contractions are true coordinated reflexes, started by mechanical stimulation of the intestine, and carried out by the local nervous mechanism (Auerbach’s plexus). They are independent of the connections of the gut with the central nervous system. […] The production of the true peristaltic wave is dependent on the unvarying response of the intestinal nervous mechanism to local stimulation, the law of the intestine. This law is as follows:—Local stimulation of the gut produces excitation above and inhibition below the excited spot. These effects are dependent on the activity of the local nervous mechanism.”
Eighteen years after Bayliss and Starling published their groundbreaking work, Trendelenburg (1917) extended their observations by conducting the first studies of isolated guinea pig small intestine (125). He demonstrated in an organ bath that he could elicit a reflex response by increasing intraluminal pressure and that this reflex would persist for hours if the preparation was maintained in a healthy state. Trendelenburg used the term “peristaltic reflex,” which remains in current use to describe the propulsive motor activity in the intestines, and he correctly attributed this to actions of the nervous system. Trendelenburg credited Carl Lüderitz for the peristaltic reflex, calling it “the Lüderitz–Bayliss–Starling reflex” (125). It has now become clear that, in addition to Bayliss and Starling, Lüderitz made important contributions to the discovery of smooth muscle responses after distension of the intestine, and in addition to the peristaltic pattern of motility, he demonstrated the importance of intrinsic nerves and the role of longitudinal muscle in this response (126).
The traditional model of “the law of the intestine” has served as a template for conceptualizing and understanding the organization of propulsive reflex circuitry of the ENS, despite evidence that this “law” is not rigidly obeyed in the organization of ENS circuitry controlling motility. For example, Spencer et al. (127) simultaneously recorded longitudinal and circular muscle from the guinea pig ileum while distending the segment and found that synchronous contractions occurred in both the longitudinal and circular muscle orally and anally to the stimulus. These contractions were blocked by the sodium channel blocker TTX, revealing them to be neurally mediated. These authors showed that distal transit was facilitated by apamin-sensitive inhibitory neurotransmission that modulated the amplitude of the cholinergic contraction anal to the stimulus (127).
Thus, the commonly accepted view of the ENS is that it is organized into discrete neuronal circuits that are coordinated to reflexly regulate gut function. With an apparently random collection of neurons organized in ganglia of varying sizes, unraveling this organization presented a considerable challenge. Overcoming this challenge involved gaining the combined knowledge of the physiological, morphological, and neurochemical properties of the many subpopulations of enteric neurons, as well as their unique projection patterns. This ultimately required the application of microsurgical lesioning, retrograde tracing technologies, intracellular labeling, and immunohistochemistry applied to discrete regions of the gut (5, 29, 117, 128–130). As a result of the tenacity of those undertaking these studies, the characterization of most of the major functional classes of enteric neurons in the mammalian GI tract was largely accomplished. These data have allowed the development of a coherent view of the various subpopulations of enteric neurons that contribute to the regulation of digestive functions. The same cannot be said for the organization of the nerve circuits underlying defensive functions of the gut, which still require complete elucidation. As we discuss in sect. 3.1.4, molecular characterization of enteric neurons requires a reevaluation of the categories of enteric neurons and how they relate to the functional organization of the ENS. The current organizational scheme dominates the way the ENS is viewed and is found throughout the literature (e.g., see Refs. 29, 32, 72, 131–134).
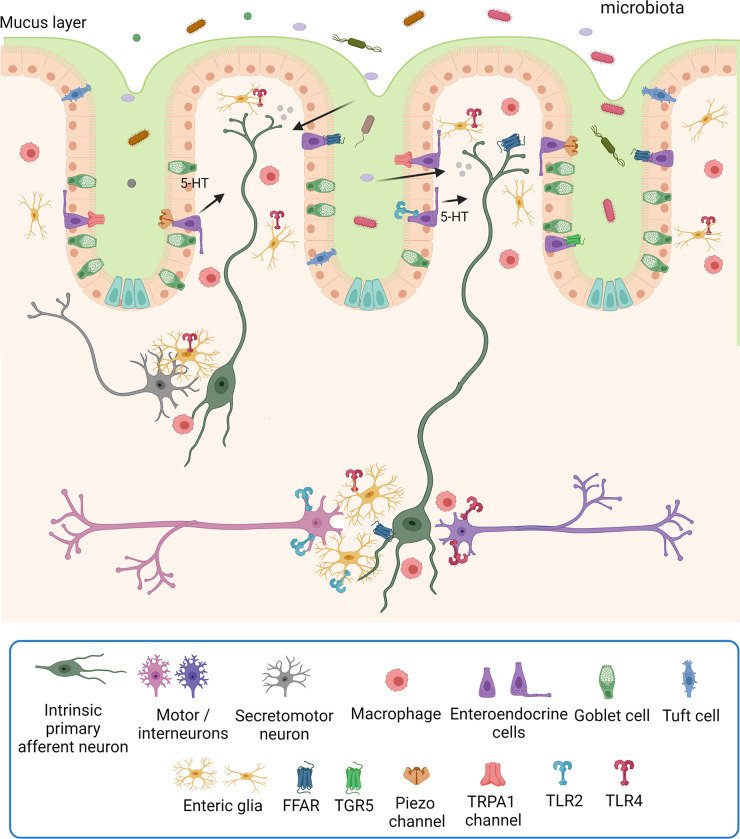
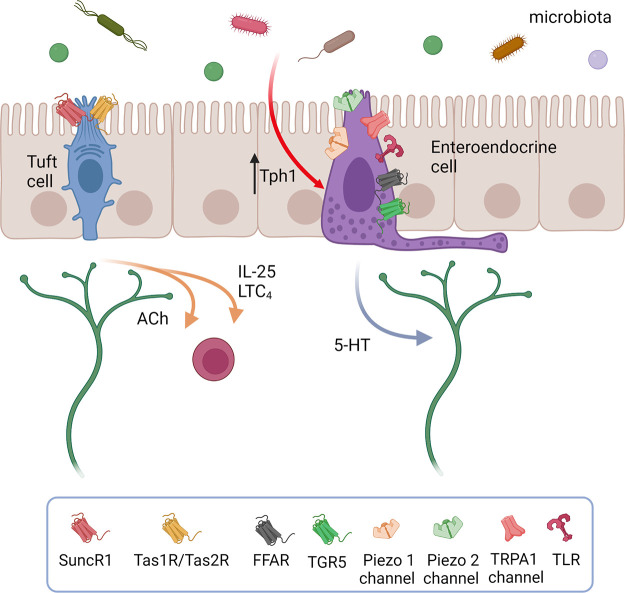
The basic circuits of reflex neural control elicited from the gut lumen consist of 1) intrinsic primary afferent (intrinsic sensory) neurons that project to the mucosa, where they are activated by an epithelial sensor, since enteric nerves do not project into the epithelium, but lie beneath it; 2) motor, vasomotor, and/or secretomotor neurons; and 3) interneurons (FIGURES 3 AND 4). The best-understood epithelial sensors are enterochromaffin cells that primarily release serotonin (5-hydroxytryptamine, 5-HT) in response to mechanical or chemical stimuli. We discuss the transduction of luminal stimuli further in sect. 5.1. This apparently simple reflex arrangement as illustrated in FIGURES 3 AND 4 serves to define the elements that make up the nerve circuits in the intestines but belies the complexity of enteric neural control.
Figure 3.
The organization of submucosal secretomotor and vasodilator reflexes. Chemical or mechanical stimulation of the mucosa activates enteroendocrine cells, which, in turn, excite submucosal primary afferent neurons that then activate secretomotor and/or vasodilator neurons to stimulate fluid secretion (indicted by the arrow) and/or vasodilatation. Fluid secretion may be amplified by nerve-mediated mast cell activation (13, 135) or modulated by enteric glia (136). Extrinsic primary sensory neurons may also directly evoke a secretory response via axon reflexes (not shown), but there does not appear to be efferent preganglionic input to submucosal ganglia. There are also long secretomotor and vasodilator reflexes involving the myenteric plexus (not shown) (137, 138). Image created with BioRender.com, with permission.
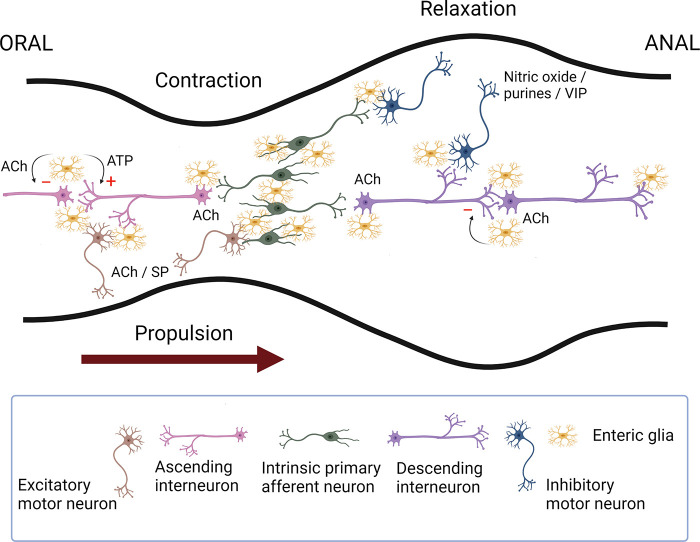
Figure 4.
Schematic representation of the enteric neural circuitry underlying the neural control of the circular muscle (the main driving force for propulsion). Intrinsic primary afferent neurons synapse with ascending and descending interneurons that run along the length of the intestine, which then activate excitatory (orally) and inhibitory (anally) motor neurons. Upon a chemical or mechanical luminal stimulus, intrinsic primary afferent neurons cause the activation of ascending interneurons that synapse with excitatory motor neurons evoking an oral contraction, whereas the activation of descending interneurons leads to the excitation of inhibitory motor neurons eliciting a relaxation anally. This polarized reflex circuitry allows for a pressure gradient to be developed that propels the contents anally. The primary transmitters involved include acetylcholine (ACh) for all of the pathways except for inhibitory motor neurons that use nitric oxide and purine nucleotides as their primary neurotransmitters. Substance P (SP) or a related tachykinin provides an important adjunct to the excitatory innervation of smooth muscle; likewise, vasoactive intestinal peptide (VIP) is an adjunct to the inhibitory innervation of smooth muscle. Enteric glia also play an active role in regulating intestinal motility. Neuronal recruitment in the descending circuitry is subject to glial inhibitory control in a muscarinic M3-dependent manner. Enteric glial purinergic signaling preferentially stimulates neuronal responses within the ascending circuitry, which may also be under inhibitory regulation (see Ref. 85 for details). Image created with BioRender.com, with permission.
It is clear that intrinsic primary afferent neurons have a sensory function (111, 114, 139, 140); however, the terms “intrinsic primary afferent” and “intrinsic sensory” are potentially misleading, as it has become apparent that these are multifunctional neurons in enteric circuits, as proposed by Wood (6) and discussed above. These neurons respond to luminal chemical and mechanical stimulation and initiate reflex activity in submucosal and myenteric neural circuits (111, 114, 139, 140). Initiating the activity of enteric neural circuits by circumferential stretch, as occurs when a bolus or fecal pellet is in the intestines, reveals a more complex situation, at least in the guinea pig. Spencer, Smith, and their colleagues (120, 141, 142) made simultaneous recordings from myenteric neurons and adjacent circular muscle in flat-sheet preparations. They showed that AH neurons were silent in preparations where muscle tone and contraction were abolished, despite the ongoing excitatory and inhibitory junction potentials that were recorded in the circular muscle. Therefore, these AH neurons could not be generating the ongoing junction potential activity in the muscle. When they recorded from excitatory and inhibitory motor neurons they found they exhibited short periodic bursts of fast EPSPs that preceded the excitatory and inhibitory junction potentials, suggesting that these were generated by mechanosensitive interneurons, and this was consistent with recordings made from these cells under various conditions (120, 141, 142). Extending these observations, Dickson et al. (143) demonstrated that mechanosensitive myenteric neurons also respond to longitudinal stretch, and they characterized these as nitric oxide-expressing descending interneurons. In addition to these studies, Mazzuoli and Schemann (144) identified a population of rapidly adapting mechanosensitive neurons that were activated by mechanical stimuli mimicking deformation of the myenteric plexus during contractile activity of the gut. They extended these findings to the mouse small and large intestine, where they constituted 22% and 15% of myenteric neurons in the ileal and colonic myenteric plexus, respectively (119), and to the corpus of the guinea pig stomach, where many classes of mechanosensitive neuron were described (145).
Enteric reflex circuits located solely within the submucosal plexus appear to be monosynaptic, since submucosal interneurons have not been described, whereas those involving the myenteric plexus are generally polysynaptic (32, 51). Submucosal reflexes control blood flow and secretion in the intestines of laboratory animals (52, 137, 146) but not in the esophagus or stomach, since these organs possess few, if any, submucosal ganglia. In larger mammals and humans, the submucosal plexus is also involved in motility, via projections to the circular muscle layer, although the organization of this reflex circuitry is not well understood. A population of submucosal neurons are also mechanosensitive (147).
Control of the enteric reflexes by the myenteric plexus is more complex where there is clear polarity to the circuitry (FIGURE 4). Intrinsic sensory neurons of the myenteric plexus have processes that reach into the lamina propria underlying the epithelial layer. Furthermore, they synapse on classes of interneurons that have ascending and descending projections within the myenteric plexus or that project to the submucosal plexus. Interneurons, in turn, synapse with 1) excitatory and inhibitory motor neurons that regulate circular and longitudinal smooth muscle contractility, 2) vasomotor neurons, 3) secretomotor neurons, 4) motor neurons that regulate enteroendocrine cell immune and defensive functions, and 5) viscerofugal neurons that are involved in intestino-intestinal reflexes discussed below. Motor reflexes can be elicited by stretch or distension of the gut that does not involve mechanosensory elements in the mucosa (120, 141, 142). In this case, mechanosensitive (Dogiel type I) myenteric neurons initiate the reflex (118, 120, 141, 142).
Some interesting features that relate to the organization of enteric reflexes and specific functional subclasses of neurons emerged from studies that examined the projection patterns of enteric neurons (29, 117, 128–130). One feature is that longitudinal muscle motor neurons project only a short distance either orally or anally from their cell bodies, suggesting that regulation along the length of the gut occurs in a segmentally restricted manner. The role of the longitudinal muscle in propulsive motility is important, and whether it contracts in or out of synchrony with the circular muscle was unclear. Detailed recordings made in the guinea pig ileum (127) and distal colon (148–150) and in the human esophagus (151) reveal that at least in these regions of the gut there is strong synchrony between the contractions and the coordinated activity of the neuronal pathways to the two muscle layers. In contrast, circular muscle motor neurons fall into two distinct classes, with short and long projections both orally and anally, that provide the basis for contraction or relaxation of longer segments of bowel behind or in front of a bolus, respectively.
Another feature is that in general there are more types of descending interneuron than ascending, they project over longer distances, and their neurochemistry is more complex, suggestive of distinct functional roles for the different subclasses of interneuron. In the guinea pig small intestine, there is only a single class of ascending interneuron, whereas in the colon there are three classes, which reflects the regional organization of enteric reflexes along the length of the bowel (117, 130).
Intrinsic primary afferent neurons project circumferentially only a short distance from the cell body, so they are activated locally (111, 114). Moreover, several lines of evidence indicate that intrinsic primary afferent neurons are arranged in interconnected, self-reinforcing networks that sustain activity once it is initiated (111, 114). These polymodal multifunctional neurons are particularly interesting because their state of excitability is governed/regulated by the environmental conditions within the gut wall, e.g., in states of inflammation or immune activation their activity is increased, and by the presence or absence of gut microbiota and their products. In addition, they are involved in local immune regulation, as we discuss further in sects. 3.1.5 and 5.4.
Moving beyond traditional approaches, Lasrado et al. (152) used single-cell transcriptomics and mosaic mutagenesis to discover that the ENS of the mouse small intestine is composed of overlapping clonal units founded by postmigratory neural crest-derived progenitors. The size of the clonal units depends on the properties of the founder progenitors, interactions with descendants of lineally unrelated neighboring units, and the physical growth changes of the gut during development. Although all the clones contribute to the myenteric plexus, only a subset contribute to the submucosal plexus, and these are organized radially in serosal-mucosal columns. When electrically stimulated, the clonally related enteric neuronal populations exhibited a greater degree of synchronous activity than unrelated neurons. Both excitatory and inhibitory neurons were identified within each of these clones, suggesting that these distinct neuronal populations may function synchronously under some conditions (152). Examples of synchronized firing of neuronal assemblies have been demonstrated for stretch-activated neuronal pathways to the longitudinal and circular muscle in the guinea pig distal colon (141, 148) and mouse large intestine (153–155). Together these data reveal a new way of considering the organization of the ENS that lends itself to functional integration of the ENS circuits that control motility, and potentially other gut functions.
Wood (6) has proposed that the ENS functions as a series of central pattern generators, modified by sensory inputs, rather than in traditional reflex arcs. Central pattern generators are groups of neurons that fire rhythmically in the absence of sensory input. In the CNS, they are important for the control of rhythmic activities such as locomotion and respiration (156, 157). The movements of the gut are often rhythmic in nature; therefore, it is not unreasonable to consider pattern generators as a potential mechanism to explain spontaneous motor functions that demonstrate such rhythmicity (see Ref. 158 for detailed discussion of pattern generators in motility). Direct observation of rhythmic firing in the ENS underlying rhythmic electrical depolarizations in smooth muscle of the mouse colon was made by Spencer et al. (153), which is consistent with this idea. However, these recordings were not made in the complete absence of sensory input. Spencer and Smith (120), however, recorded spontaneous bursting activity in S neurons of the guinea pig distal colon in the absence of sensory inputs, and, similarly, Wood (159) made extracellular recordings of bursting neurons from the cat small intestine in a high-magnesium bathing solution that would block all synaptic inputs to these cells. Although these examples can be criticized (for example, pinning out of the preparations could activate stretch-sensitive neurons), spontaneous activity in the ENS is readily observed by calcium imaging in primary cultures of enteric neurons (160) and spontaneous synaptic activity is detected in undissected preparations of the mouse colon (161), although the origins of this activity remain to be determined. Together these suggest that there needs to be reconsideration of the manner in which enteric neural circuitry is organized, to include both traditional reflex arcs and nontraditional organizational principles (at least for the control of motility) that include synchronized assemblies of neurons that are potentially controlled by pattern generators.
The assemblies of neurons that comprise the ENS are organized in circuits that control the functions of the gut in a region-specific manner. Li et al. (162) determined, using calcium imaging, viral vector tracing, and immunohistochemistry, that the complexity of the motor patterns of the proximal colon is far greater than that of the distal colon, directly reflecting the additional repertoire of motor patterns observed in the proximal colon where the luminal contents contain more water. These authors were also able to determine that the polarity and nature of cholinergic neurotransmission varied in a regionally specific manner. Whether the organizational principles identified in the colon apply to the small intestine remains to be determined. But assuming that to be the case, these data are consistent with the concept that form follows function; the roles of each gut region are regulated by a network of neurons whose complexity is tuned to the needs dictated by the specific functional roles that need to be fulfilled.
How the luminal contents of the gut elicit the reflexes that promote both digestive and host defense remains the subject of much ongoing research that has been recently reviewed (22, 27–32). The initiation of digestive reflexes relies on the transduction of chemical and mechanical events by cells located in the epithelium (chemical and mechanical) and enteric neurons (mechanical), as we discuss in sect. 5.1. Consistent with the suggestions made above that simple reflexes are not solely involved in the regulation of gut function upon the presence of luminal contents, Chambers et al. (163) proposed that two rhythm generators regulate segmentation in the gut in the presence of luminal nutrients. Segmentation is the motor pattern of the fed state in the small intestine that promotes the digestion of food and absorption of nutrients (164, 165). One of these rhythm generators drives contractions within the bursts of segmenting motor activity and the other the occurrence of bursts, which depends on feedback to the intrinsic sensory neuron circular muscle contraction (163). They demonstrated that both 5-HT and cholecystokinin (CCK), released from enteroendocrine cells of the epithelium, initiate this motor pattern (164). However, segmentation can occur after blocking neuronal activity with TTX (166). Under these conditions Huizinga et al. (166) revealed that the segmentation motor pattern emerges when the amplitude of the dominant pacemaker in the gut, in this case the mechanical slow wave generated by ICCs associated with the myenteric plexus, is modulated by the phase of an induced lower-frequency rhythmic transient depolarization, generated by ICC associated with the deep muscular plexus. When these patterns of smooth muscle activity are merged, they result in the waxing and waning of the amplitude of the slow wave and a pattern of segmentation motor activity. Of course, physiologically neural activity is present and, in all likelihood, works in concert with the ICC pacemaker activities to generate segmentation in the small intestine. We discuss the role of ICCs in enteric neural control further in sect. 3.3.1.
To date, however, the enteric neural circuits that control the defensive functions of the gut are not as clearly defined, though considerable progress has been made (see Ref. 22). The elucidation of the defensive enteric neural circuits is an exciting and emerging area of research that is propelling the field forward.
The recognition of the multifunctional nature of all classes of enteric neurons suggests that the time has come to reconsider a simple model of reflex arc circuits of the ENS that is commonly used to describe motor reflexes in the gut (as shown in FIGURE 4) as a more complex one in which multifunctional neurons integrate the local environmental conditions of the gut (luminal, immune, and microbial) to produce coordinated outputs that are both digestive and defensive in nature and involve both pattern generators and reflex mechanisms. This new model will need to involve not only neurons but also enteric glia, interstitial cells, and macrophages as additional vital elements in the enteric neural circuits, as we discuss in sects. 3.2, 3.3, and 3.4. In sum, over the last 10 years our view of how the ENS functions has been greatly expanded, and with the inclusion of transcriptomic data (see sect. 3.1.4) it will evolve further in the next few years.
3.1.3. Chemical neuroanatomy of the ENS.
Functional, biochemical, histochemical, and immunohistochemical studies have defined the conserved primary neurotransmitters that characterize the major classes of neurons in the myenteric plexus of the mammalian ENS. In the adult, it is generally accepted that most myenteric neurons are either cholinergic or nitrergic (167, 168). Some neurons appear to be both cholinergic and nitrergic, and there is a population of cells that do not express the rate-limiting enzymes for the production of either ACh (cholineacetyltransferase, ChAT) or nitric oxide (nitric oxide synthase, NOS). Some studies, however, report higher proportions of myenteric neurons that express neither NOS or ChAT (169, 170) and that some enteric neurons also express enteric glial markers (at least in adolescent mice) (169), challenging the accepted paradigms of chemical neuroanatomy and highlighting the need for care when examining and categorizing enteric neuronal populations. Unlike myenteric neurons, submucosal neurons rarely express NOS. But again, there are two main populations: cholinergic neurons and vasoactive intestinal peptide (VIP)-expressing, noncholinergic neurons (TABLES 1 AND 2).
Table 1.
Characterization of enteric neurons that innervate the upper gastrointestinal tract
| Region | Type of Neuron | Location of Cell Bodies | Primary Transmitter(s) | Cotransmitters | Neurochemical Coding | References |
|---|---|---|---|---|---|---|
| Esophagus | Excitatory smooth and striated muscle motor neuron | Myenteric plexus | ACh | Tachykinin, enkephalin | ChAT/tachykinin | (171–173) |
| Inhibitory smooth and striated muscle motor neuron | Myenteric plexus | Nitric oxide | VIP, ATP, NPY, galanin | NOS/VIP | (171–173) | |
| Interneuron | Myenteric plexus | ACh, nitric oxide | Enkephalin | Enkephalin* | (173) | |
| Esophofugal neuron† | Myenteric plexus | Nitric oxide | VIP | NOS/VIP | (174, 175) | |
| Esophofugal neuron† | Myenteric plexus | ACh | Calretinin | ACh/calretinin | (175) | |
| Stomach | Excitatory muscle motor neuron | Myenteric plexus | ACh | Tachykinin, GRP, enkephalin | ChAT/tachykinin, ChAT/enkephalin | (129, 176–179) |
| Inhibitory muscle motor neuron | Myenteric plexus | Nitric oxide | VIP, ATP, NPY, GRP, enkephalin | NOS/VIP, NOS/NPY | (129, 176–179) | |
| Ascending interneuron | Myenteric plexus | ACh | Calbindin | ChAT/calbindin | (180) | |
| Descending interneuron | Myenteric plexus | ACh, nitric oxide | Calbindin, 5-HT | ChAT/calbindin/±5-HT‡, NOS/calbindin‡ | (180) | |
| Vasodilator neuron | Myenteric plexus, submucosal plexus | VIP | VIP | (129) | ||
| Myenteric plexus | ACh | Calretinin | ACh/calretinin | (129) | ||
| Motor neurons to parietal cells | Myenteric plexus | ACh | ChAT/GRP/VIP | (129) | ||
| Motor neurons to gastrin cells | Myenteric plexus | GRP | VIP | GRP | (129) | |
| Motor neurons to chief cells | Myenteric plexus | GRP | GRP/VIP | (129) |
The chemical coding and projection pattern of enteric neurons were largely established in the guinea pig GI tract. Increasingly, data are becoming available from mouse and human. The patterns of innervation described in this table are not necessarily correct for every species but are broadly reflective of neurochemically coded, polarized projection patterns of enteric innervation. 5-HT, serotonin; ACh, acetylcholine; ATP, adenosine triphosphate; ChAT, cholineacetyltransferase; GRP, gastrin-releasing peptide; NOS, nitric oxide synthase; NPY, neuropeptide Y; VIP, vasoactive intestinal peptide. *Based on their location. †Based on retrograde labeling these neurons project to the trachea. ‡ChAT/calbindin/±5-HT have long projections; NOS/calbindin have short projections.
Table 2.
Characterization of enteric neurons that innervate the lower gastrointestinal tract
| Region | Type of Neuron | Location of Cell Bodies | Primary Transmitter(s) | Cotransmitters | Neurochemical Coding | References |
|---|---|---|---|---|---|---|
| Small and large intestines | Excitatory muscle motor neuron | Myenteric plexus | ACh | Tachykinin, enkephalin, GABA, calretinin | ChAT/tachykinin, ChAT/Calretinin | (5, 117, 181) |
| Inhibitory muscle motor neuron | Myenteric plexus | Nitric oxide | VIP, ATP, enkephalin, NPY, GABA | NOS/VIP | (5, 117, 181, 182) | |
| Ascending interneuron | Myenteric plexus | ACh | Calretinin, enkephalin, tachykinin, Calbindin | ChAT/Calretinin | (5, 117, 181, 183) | |
| Descending interneuron | Myenteric plexus | ACh | 5-HT, somatostatin | ChAT/5-HT, ChAT/somatostatin* | (5, 117, 181) | |
| Descending interneuron | Myenteric plexus | ACh | VIP, NPY, nitric oxide | ChAT/VIP, NOS/VIP | (5, 117, 181) | |
| Intrinsic primary afferent neuron | Myenteric plexus; submucosal plexus | ACh, CGRP | CGRP,† neuromedin U, calretinin, calbindin,‡ tachykinin | CGRP/neuromedin U | (5, 117, 181) | |
| Intestinofugal neuron | Myenteric plexus | ACh | VIP, GRP, CART, CCK | (5, 117, 181) | ||
| Cholinergic secretomotor neurons | Myenteric plexus; submucosal plexus | ACh | NPY | ChAT/NPY | (5, 117, 181, 184) | |
| Noncholinergic secretomotor/vasodilator neurons | Submucosal plexus | VIP | Galanin, CART, dynorphin | VIP/GAL | (5, 117, 181, 184) | |
| Vasodilator neurons | Submucosal plexus | ChAT | Calretinin | ChAT/calretinin | (5, 117, 181, 184) |
The chemical coding and projection pattern of enteric neurons were largely established in the guinea pig GI tract. Increasingly, data are becoming available from mouse and human. The patterns of innervation described in this table are not necessarily correct for every species but are broadly reflective of neurochemically coded, polarized projection patterns of enteric innervation. 5-HT, serotonin; ACh, acetylcholine; ATP, adenosine triphosphate; CART, cocaine and amphetamine-regulated transcript; CGRP, calcitonin gene-related peptide; ChAT, cholineacetyltransferase; GABA, gamma aminobutyric acid; GAL, galanin; GRP, gastrin-releasing peptide; NOS, nitric oxide synthase; NPY, neuropeptide Y; VIP, vasoactive intestinal peptide. *This class of interneuron is not found in the guinea pig colon. †CGRP varicosities from intrinsic primary afferents form “baskets” around calretinin and NOS neurons in the mouse colon (185). ‡Calbindin-immunoreactive varicosities from intrinsic primary afferents form “baskets” around calretinin and calbindin neurons in the guinea pig colon (186, 187).
In both the submucosal and myenteric plexuses, these major neuronal populations can be further subdivided into various subsets based on their expression of other markers (mostly neuropeptides and calcium binding proteins). These neurochemically coded subsets have been functionally identified based on the use of lesions and retrograde tracing of their projections to specific targets in the gut wall (29). The proportions of each neurochemically coded subset of neurons vary somewhat along the length of the gut in a region-specific manner. There are also interspecies differences in the neurochemical coding patterns, but all mammals that have been examined in detail have the same main populations and all the major functional subtypes of neurons. In total ∼15 classes of functionally defined, neurochemically coded enteric neurons have been identified in the intestines (TABLES 1 AND 2), with a smaller number in the stomach and esophagus.
There are species differences in the neurochemical coding of enteric neurons, as well as regional variation in the proportions of the neuronal subtypes along the length of the gut (5, 76, 128–130, 171, 188–190). However, there are some general points that have emerged that link the neurochemical code of a given neuron to its functional role in the ENS in the defined region of gut. For example, substance P or a related tachykinin is generally in excitatory motor neurons, VIP is an important secretomotor transmitter, and calcitonin gene-related peptide (CGRP) is found in intrinsic primary afferent neurons. Neurochemical coding can be applied to the polarity of projections. A polarized pattern of innervation of the circular muscle has been demonstrated for the stomach and intestines, with descending pathways being primarily nitrergic whereas ascending pathways are primarily cholinergic. Similarly, for many regions of the gut, ascending pathways to the mucosa are primarily cholinergic whereas descending pathways are primarily noncholinergic. We draw the readers’ attention to excellent reviews on this subject (5, 76, 128–130, 171, 188–190).
3.1.4. Molecular characterization of enteric neurons.
In recent years, the application of single-cell sequencing technology to the ENS has revolutionized our understanding of the molecular architecture, developmental programs, and the biology of enteric neurons and glia. More than a dozen studies have used a variety of single-cell RNA platforms to sequence the entire transcriptome of human and mouse myenteric neurons from the small and large intestines (191). Our understanding of submucosal plexus neurons and glia is nascent, in part because of the technical difficulty of isolating them and their relatively low numbers compared with cells in the myenteric plexus. These hypothesis-generating studies have produced vast data sets that will take a substantial effort to fully reconcile with our existing knowledge of the ENS, and indeed with each other, since no two studies have used the same approaches (e.g., single nucleus vs. single cell, different transgenic mice, different sorting parameters), all of which have specific strengths and limitations. There are also issues with comparing transcriptomic studies to the existing literature because of limitations of antibody staining, sample sizes, and species differences (e.g., a lot of older work was done in the guinea pig, whereas most studies now use the mouse or human) (191). Nevertheless, some striking commonalities between the studies have emerged that offer exciting opportunities. For example, the Nmu gene, which encodes the peptide neuromedin U (NMU), was recognized to be a marker gene of a population of murine intrinsic primary afferent neurons by Zeisel et al. (192) (ENT9, small intestine), Morarach et al. (134) (ENC 6, small intestine), May-Zhang et al. (193) (cluster 5, small and large intestine) and Drokhlyansky et al. (72) (PSN 1, small intestine) (TABLE 3) (72, 134, 192, 193, 194). It should be noted that Drokhlyansky et al. (72) identified a second population of intrinsic primary afferent neurons in the large intestine (PSN 2) that also highly expressed Nmu, but these neurons coexpressed the same genes that Morarach et al. (134) and May-Zhang et al. (193) identified in their studies that were expressed in Nmu neurons (Nog, Pcdh10, and Cbln2), which suggests that these are potentially the same cell populations. Of course, in these data sets there are inconsistencies that need to be resolved, and this will happen as the data are more closely analyzed, especially as much is available on websites (e.g., https://singlecell.broadinstitute.org/single_cell). Moreover, with a greater level of confidence in the assignment of cell populations, it is now possible to develop hypothesis-driven experiments that explore the functional significance and roles of these molecularly characterized neurons, whose receptors, ion channels, transcription factors, adhesion molecules, etc. are all defined (transcriptionally, at least).
Table 3.
Subtypes of myenteric neurons of the intestines
| Type of Neuron | Shape | Primary Neurotransmitter(s) | Additional Transmitters and/or Neurochemical Markers | E.physiological Classification (guinea pig, mouse) | Potential Subtype Designation from Single-Cell RNA Studies |
|---|---|---|---|---|---|
| Excitatory muscle motor neuron | Dogiel type I/small simple neurons | Acetylcholine, tachykinin | Enkephalin, GABA, Calretinin | S cell | ENT4–6 (192) |
| PEMN (72) | |||||
| ENC1–4 (134) | |||||
| Cluster 0, 3 (193) | |||||
| Chat 1–3 (194) | |||||
| Inhibitory muscle motor neuron | Dogiel type I | Nitric oxide | VIP, ATP, NPY | S cell | ENT2–3 (192) |
| PIMN (72) | |||||
| ENC8, 9 (134) | |||||
| Cluster 1, 2 (193) | |||||
| Nos 1, 2 (194) | |||||
| Ascending interneuron | Dogiel type I | Acetylcholine | Tachykinin, calretinin, enkephalin | S cell | ENT6, 7 (192) |
| PIN (72) | |||||
| ENC4, 12 (134) | |||||
| Cluster 3s (193) | |||||
| Chat 4 (194) | |||||
| Descending interneuron | Dogiel type I/filamentous neurons | Acetylcholine, ATP | 5-HT, somatostatin | S cell | PIN (72) |
| ENC5, 12 (134) | |||||
| Cluster 6s, 9 (193) | |||||
| Chat 4 (194) | |||||
| Descending interneuron | Dogiel type I | Nitric oxide | VIP | S cell | ENT1 (192) |
| PIN/PSVN (72) | |||||
| ENC10 (134) | |||||
| Cluster 10 (193) | |||||
| Nos. 1, 2 (194) | |||||
| Intrinsic primary afferent neuron | Dogiel type II | Acetylcholine, CGRP | Neuromedin U, advillin, calbindin, calretinin, IB4 | AH cell | ENT9 (192) |
| PSN1 (72) | |||||
| ENC6 (134) | |||||
| Cluster 5, 6 (193) | |||||
| Calcb (194) | |||||
| Intestinofugal neurons | Dogiel type I | Acetylcholine | VIP, GRP, CCK, CART | S cell | ENT8 (192) |
| PSN3 (72) | |||||
| ENC7 (134) | |||||
| Cluster 7s (193) |
In Drokhlyansky et al. (72), there are multiple classes of neuron type within given clusters. 5-HT and somatostatin are two separate groups in the single-cell RNA (scRNA) data set of Morarach et al. (134). 5-HT is a subgroup within the heterogeneous ENC12, whereas somatostatin interneurons correspond to ENC5. See glossary for abbreviations.
There are some other interesting early findings that have emerged from these exciting studies. First, there is regional enrichment of various genes in identified subsets of neurons along the length of the gut, likely reflective of differences in the specific functional circuits (193). Second, human enteric neuron subsets share considerable overlap with core transcriptional programs in the mouse (72, 193, 194). This adds further support to an evolutionary conservation of function between species and the relevance of studies conducted in rodent models. Third, these data sets strongly support the concept that enteric neurons are involved in bidirectional neuroimmune interactions in the GI tract, e.g., via the release and response to cytokines (72, 134, 192–194). Fourth, despite its established expression, the rate-limiting enzyme for serotonin, tryptophan hydroxylase 2 (TPH2), was not detected in the mouse intestine in these studies (though it was found in the human ENS) (72, 134, 192). Exactly why this should be the case remains to be determined, but it may be due to the low abundance of enteric serotonergic neurons (∼1–2% of total myenteric neurons) (181, 195). In the future, extending these approaches to models of disease, and using additional tools to examine epigenomic modifications, offers the chance to make enormous gains in our understanding of the physiology of the ENS.
Moving beyond healthy animals, transcriptional studies have now also been performed in mouse models of inflammatory bowel disease (IBD) and human transcriptomic data sets of samples from patients with ulcerative colitis (196). With the use of the murine dextran sodium sulfate (DSS), T-cell transfer, and oxazolone models of colitis, it was shown that there is an induction of gene transcription of several neuronal (e.g., Nos1, Bdnf, and Htr2b) and glial (e.g., S100b, Gfap, and Cdh19) marker genes that increased as the degree of colitis increased in these models. Similar findings of gene upregulation were made in human IBD data sets (196). Moreover, in the mouse models of colitis, as the severity of inflammation resolved there was a rapid downregulation of gene expression. These changes were accompanied by immunohistochemical evidence for increased innervation density of the colonic mucosa. Together, they reveal the dynamic nature of transcriptional regulation of enteric neuronal and glial genes in gut inflammation and the potential pathways that are activated (or repressed) under these conditions. In the future, studies like this will shed new light on the impact of disease on the ENS and allow for the identification of molecular pathways involved in, for example, neuro-immune, neuro-epithelial, and neuro-muscular interactions that are modified in disease. These too will greatly increase our understanding of the pathophysiology of the ENS.
3.1.5. Microbial regulation of enteric neuronal gene transcription and function.
It has been established that the transcriptome and function of the developing and adult ENS are modified by the presence of the gut microbiota (TABLE 4) (197–228). Muller et al. (201) performed transcriptomic profiling of enteric neurons along the length of the gut, comparing germ-free mice to those raised under specific pathogen-free (SPF) conditions. In the duodenum, there were no transcripts upregulated by the presence of a gut microbiota, in contrast to the ileum and colon, where many genes were specifically upregulated. Interestingly, microbiota-dependent transcripts included Nmu, which is found in intrinsic primary afferent neurons that project to the intestinal mucosa. Intriguingly, quantification of the numbers of neurons in the myenteric plexus of germ-free versus SPF mice revealed a significant reduction in numbers in the duodenum and ileum but not in the colon, which seems somewhat counterintuitive and not readily explicable to this point. They also measured activity of enteric neurons based on the expression of phosphorylated cAMP response element-binding protein (pCREB). They found less activity in neurons in the ileum myenteric plexus of germ-free mice compared with SPF mice, suggesting that enteric neurons may be hypoexcitable in the absence of a normal gut microbiota (201). A potential molecular mechanism for this observation was provided by Obata and colleagues (204).
Table 4.
The effects of microbiota (bacteria, fungi, and viruses) on the enteric nervous system
| Model | Effects on the ENS and Intestinal Motility | References |
|---|---|---|
|
Germ-free (GF) mice
| ||
| GF mice studied at postnatal day 3 | ↓ myenteric neuronal and nerve fiber density and ↑ proportion of myenteric nitrergic neurons in the jejunum and ileum, ↓ frequency and amplitude of muscle contractions | (197) |
| Colonization of adult GF mice | ↑ neuronal proliferation involving mucosal and neuronal 5-HT and activation of 5-HT4 receptors, ↑ intestinal transit rates | (198) |
| Colonization of adult GF mice | Initial colonization and homeostasis of mucosal enteric glial cells are regulated by the indigenous gut microbiota. | (199) |
| Colonization of adult GF mice | Restoration of intrinsic and extrinsic nerve functions toward normal levels | (200) |
| GF mice | The number of CART-expressing neurons, which project to prevertebral ganglia, was increased by twofold in mice with a gut microbiota. Microbiota depletion resulted in loss of these neurons and impaired glucose regulation. | (201) |
| GF mice colonized with B. thetaiotaomicron | Colonization of GF mice with B. thetaiotaomicron restored gut motility and increased neuronal and glial density. | (202) |
| GF, specific pathogen-free, and conventionalized-GF mice studied at 8–12 wk | Decreased AH neuron excitability in GF mice | (203) |
| Analysis of differential gene expression and motility in GF vs colonized GF mice | Microbial breakdown of tryptophan creates metabolites that can promote expression and activation of the aryl hydrocarbon receptor, which is expressed by neurons and is linked to increased peristaltic activity. | (204) |
| High-fat diet in GF vs. conventionally raised mice | High-fat diet reduced duodenal nitrergic neuron density in conventionally raised but not GF mice. | (205) |
| Antibiotic treatment | ||
| Vancomycin treatment in neonatal mice | ↓ myenteric neuronal and nerve fiber density, ↑ proportion of myenteric cholinergic neurons and ↓ in nitrergic neurons, ↑ frequency and amplitude of colonic migrating motor complexes | (206) |
| Vancomycin treatment in neonatal mice studied 6 wk later | Treatment had sexually dimorphic effects on neurochemistry and neuronal activity. Myenteric and submucosal neuronal excitability caused by synaptic activation was differentially altered between males and females. Only male mice showed perturbed myenteric neurochemistry, and only females showed slower GI transit. | (207) |
| Vancomycin treatment in adolescent mice | ↓in myenteric and submucosal neurons and ↓ in colonic migrating motor complexes. | (208) |
| Ampicillin treatment in adult mice | ↓colonic motility, fewer myenteric nitrergic neurons, reduced colonic neurogenesis that was reversible | (209) |
| Broad-spectrum antibiotic treatment in juvenile mice | Altered glial network, loss of myenteric plexus neurons, altered cholinergic, tachykininergic, and nitrergic neurotransmission associated with reduced number of NOS neurons, decreased motility | (210) |
| Broad-spectrum antibiotic treatment in adult mice | ↓ enteric neurons in the ileum and proximal colon in both myenteric and submucosal plexuses, ↓ ileal myenteric glia. Recovery of microbiota restored intestinal function and stimulated neurogenesis and gliogenesis. | (211) |
| Effects of (probiotic) bacteria and fungi | ||
| Lactobacillus rhamnosus GG in GF mice | ↑expression of neuronal choline acetyl transferase, enhanced motility | (212) |
| Bacteroides thetaiotaomicron in GF mice | ↑ neuronal density, enhanced motility | (202) |
| Pediococcus acidilactici treatment in piglets | ↑ in galanin- and CGRP-immunoreactive neurons in the ileal submucosal plexus and ↑ in enteric glial cells in the inner and outer submucosal plexuses | (212, 213) |
| Bacteroides fragilis added to epithelium while recording neuronal activity | ↑excitability of AH neurons. The action is mediated by the capsular exopolysaccharide of B. fragilis, polysaccharide A. | (214) |
| L. rhamnosus added to the epithelium while recording vagal afferent activity | ↑in vagal afferent activity that appears to involve a nicotinic synapse between enteric neurons and vagal afferents | (215) |
| Ex vivo myenteric plexus preparations exposed to B. longum | ↓ excitability of AH neurons due to opening of potassium channels and closing of hyperpolarization-activated cation channels | (216) |
| Treatment of pigs with the probiotic yeast S. boulardii | No change in neuronal numbers but a decrease in the number of neurons with calbindin immunoreactivity. No changes were detected for other neurochemical markers. | (217) |
| Treatment of Herpes simplex virus type 1 (HSV-1)-infected mice with S. boulardii | S. boulardii treatment ameliorated HSV-1-induced dysmotility and changes in neurons and glia, assessed by immunohistochemistry. | (218) |
| Viral infection of the ENS | ||
| Macaques with simian immunodeficiency virus | Myenteric ganglionitis that was more severe in the small than large intestine. Despite the presence of inflammatory cells in the ganglia, the ENS was not a target of the infection. | (219) |
| HSV-1 infection in rat | Latent infection of myenteric ganglia | (220) |
| HSV-1 infection in mice | Oral inoculation with HSV-1 led to viral spread throughout the myenteric and submucosal plexuses. | (221) |
| HSV-1 infection in mice | A replication-defective strain of HSV-1 caused ganglionitis and dysmotility, demonstrating that replication of the virus was not required for GI disruption. | (222) |
| Varicella zoster virus in isolated guinea pig enteric ganglia | Latent infections of enteric neurons are observed with no preference for primary afferent neurons. Lytic infections of neurons occur when the enteric neurons are cocultured with nonneuronal Varicella zoster virus-infected cells. | (223) |
| Evaluation of ENS in resected bowels of children with history of Varicella zoster virus infection | Varicella zoster virus was detected in enteric neurons of children with a history of varicella. | (224) |
| Varicella zoster virus in achalasia | Varicella zoster virus is detected in myenteric neurons of individuals with achalasia. | (225, 226) |
| JC virus in chronic intestinal pseudoobstruction | JC virus infection occurs in the myenteric plexus in a high proportion of patients with chronic intestinal pseudoobstruction. JC virus was detected in enteric glial cells. | (227) |
| Evaluation of postmortem tissues in SARS-CoV-2 (COVID-19) cases | Widespread presence of SARS-CoV-2 nucleocapsid protein in neurons of the myenteric plexus. | (228) |
See glossary for abbreviations.
Obata and colleagues (204) discovered that the aryl hydrocarbon receptor (AHR), a transcription factor whose activity is controlled by a variety of dietary and microbial metabolites, is a critical regulatory element that integrates information from the luminal environment to produce a physiological output of intestinal neural circuits to maintain intestinal homeostasis. To demonstrate this, they compared the transcriptional profiles from enteric neurons in the colon of germ-free and SPF mice and examined them to determine which genes were specifically upregulated by the presence of the gut microbiota. Among the most highly expressed genes found after microbial colonization was Ahr, the gene encoding the AHR. The expression of Ahr correlated with microbial abundance along the length of the gut, being relatively low in the proximal bowel and increasing distally. The major target of AHR signaling was shown to be Cyp1a1, a cytochrome P-450 gene, and Kcnj12, an inwardly rectifying potassium channel gene that regulates neuronal excitability. They extended these studies to examine propulsive motility and showed that knockout of Ahr in enteric neurons led to reduced GI transit, similar to the slowing of motility in mice treated with antibiotics to deplete the majority of gut bacteria (204). Activation of the AHR in enteric neurons of antibiotic-treated mice with a dietary AHR ligand rescued the phenotype, directly demonstrating the role of this receptor in promoting propulsive motility along the GI tract.
The regulation of gut functions is also influenced by microbial signaling to enteric neurons via toll-like receptors (TLRs). Toll-like receptors 1, 2, 3, 4, 7, and 13 are expressed on enteric neurons (72, 192, 229). Despite the very high levels of expression of TLR3, a receptor for double-stranded RNA, in many classes of enteric neuron, its function in the ENS has not been established. In fact, our understanding of the role of the TLRs is limited to TLR2 and TLR4 (209, 230–234) (FIGURE 5). Anitha and colleagues (230) demonstrated that TLR4-deficient mice had delayed intestinal transit due to reduced nitrergic relaxation associated with the loss of NOS neurons. They noted the similarities with germ-free mice, which also have delayed intestinal transit and loss of NOS neurons, and showed that TLR4 promotes neuronal survival (230). Toll-like receptor 4 signaling in the ENS shapes inhibitory neurotransmission via both nitrergic and purinergic neurotransmission (233) and modulates the effects of morphine on intestinal transit (234). Toll-like receptor 2 is important in maintaining the integrity of the ENS by promoting neurogenesis though glial cell line-derived growth factor family ligands (see also sect. 6.3) (209, 231, 232) (FIGURE 5). Further discussion of the microbial factors regulating gut function via receptors and ion channels on enteroendocrine cells is found in sect. 5.1.
Figure 5.
Microbiota influence the activity of many cell types in the gut wall, including enteroendocrine cells, tuft cells, immune cells, enteric glia, and enteric neurons, via many different mechanisms including via the production of short-chain fatty acids that activate free fatty acid receptors (FFARs) (235, 236) and bile acids that activate the TGR5 receptor (237), mechanosensitive Piezo1 channels (238), transient receptor potential (TRP)A1 channels (239) and toll-like receptors (TLRs) 2 and 4 (230, 231, 240, 241); see sects. 5.1, 6.2, and 6.5 and FIGURE 9. In addition, the gut microbiota is essential for the maintenance of mucosal enteric glial cells (199), which are key players in mucosal homeostasis (242). Microbial products can directly influence the activity of intrinsic primary afferent neurons (203, 214, 215, 243, 244). See glossary for other abbreviations. Image created with BioRender.com, with permission.
Together these studies provide insights into ways in which the gut microbiota can shape the phenotypes of enteric neurons, the integrity of the ENS, and functions of the neural circuitry. These are just examples of the effects of the gut microbiota, and we are just scratching the surface of revealing and understanding these interactions. The addition of probiotic bacteria and fungi impacts the structure and function of the ENS as we outline in TABLE 4. The ENS is also subject to viral infection, and this too alters the properties of both enteric neurons and glia, leading to functional alterations in enteric neural control in humans and animals (TABLE 4 and recent reviews in Refs. 24, 245–247). This vast and ever-growing topic has been the subject of recent reviews that outline in more detail many of the studies identified in TABLE 4 (7, 9, 21, 43, 248–252). This is an area of intense interest, and future studies are likely to reveal the roles of specific bacterial and fungal populations on the ENS, the roles of the TLRs and microbial receptors like the nucleotide-binding and oligomerization domain receptors that are widely expressed in enteric neurons (74), and how specific neural circuits are differentially regulated in health and in pathophysiological states. Collectively, these future studies will offer new and interesting insights into the neural control of intestinal homeostasis.
3.1.6. Enteric neurons as elements in the immunoregulatory control of gut homeostasis.
Neurons of the myenteric and submucosal plexuses are intimately linked to the immune regulation of intestinal homeostasis in addition to being positioned to respond to microbial mediators (FIGURE 6). This topic has been extensively reviewed, and interested readers are directed to excellent recent articles on this topic (20–22, 74, 253–257).
Figure 6.
A schematic illustration of the interactions between the gut immune and enteric nervous systems in the intestinal mucosa. Bidirectional communication between enteric nerves and immune cells regulates the local environment of the gut. Some of the cellular mediators of neuroimmune signaling in the gut are illustrated. There is extensive multidirectional signaling between intestinal epithelial cells (enterocytes, goblet cells, tuft cells, and enteroendocrine cells), enteric nerves, and enteric glia. Enteric nerves receive and integrate information from enteroendocrine cells, gut microbiota, and enteric glia; they also communicate with enteric glia, epithelia, the microbiota, and various populations of immune cells. Tuft cells and goblet cells are key mediators of epithelial-immune signaling, and tuft cells also signal to enteric nerves. Details of the specific molecular signaling mechanisms are described in the text. A cryptopatch is an aggregate of lymphoid cells found in the intestinal lamina propria. 5-HT, serotonin; ACh, acetylcholine; CGRP, calcitonin gene-related peptide; GFL, glial cell line-derived neurotrophic factor family ligand; IL, interleukin; ILC2, type 2 innate lymphoid cell; ILC3, type 3 innate lymphoid cell; NMU, neuromedin U; SP, substance P; VIP, vasoactive intestinal peptide. Image created with BioRender.com, with permission.
Here we will use three recent studies to illustrate how the ENS regulates aspects of epithelial immunity. Of particular note is that enteric neurons make and release cytokines as well as classical neurotransmitters. One of the key cytokines involved in the control of intestinal barrier function is interleukin (IL)-18 (258, 259). Recently, Jarret et al. (259) discovered that IL-18 produced by enteric neurons plays a key role in combating infection by the pathogen Salmonella typhimurium. They described a population of nitrergic myenteric neurons that make IL-18, as well as another group of myenteric neurons that were not nitrergic but were apparently not cholinergic. Mice that had IL-18 selectively knocked out in enteric neurons, but not those with IL-18 knocked out in immune or epithelial cells, were susceptible to S. typhimurium infection. They demonstrated that neuronal IL-18 is required for the production of antimicrobial peptides by goblet cells in the colon (FIGURE 7). Interestingly, they showed that in the distal colon IL-18 signaling occurs directly with goblet cells but, in the proximal colon this signaling is indirect, suggesting the involvement of other cell types in this signaling cascade. Given the role of nitrergic myenteric neurons in the control of motility, they investigated whether IL-18 was involved in intestinal and colonic transit and found that ablating IL-18 from enteric neurons had no effect on these parameters of motility (259). The recent single-cell sequencing study by Drokhlyansky et al. confirms IL-18 gene expression in myenteric neurons of the human and mouse small and large intestine (72) but reveals that the highest level of gene expression in the colon is in intrinsic primary afferent neurons that coexpress neuromedin U (72). This is interesting, as these neurons project to the intestinal mucosa (260) whereas there is only a sparse projection of NOS neurons to the mucosa (261), with most NOS projections within the ENS (181, 262). Unraveling of the exact circuitry of this important homeostatic system is needed to fully reconcile these findings. Nevertheless, this interesting study revealed that enteric neurons can produce an immunoregulatory cytokine necessary to prevent pathogenic bacterial infection through interactions with goblet cells (FIGURE 6).
Figure 7.
Enteric neural regulation of goblet cell secretion is a key element of host defense. Goblet cell secretion of antimicrobial peptides and mucus is differentially regulated by acetylcholine (mediated by mAChR1 muscarinic receptors) and IL-18. Acetylcholine also regulates the passage of antigen through goblet cells by activation of mAChR3 or mAChR4 muscarinic receptors. See text for details. Image created with BioRender.com, with permission.
Goblet cell mucus secretion is known to be regulated by both cholinergic and noncholinergic neural pathways (263, 264), so it appears that mucus and antimicrobial peptides are differentially regulated. This is also the case for the regulation of mucus secretion and the endocytotic uptake and transcytosis of antigen (265), an important component of epithelial immunity. Gustafsson et al. (265) demonstrated that ACh regulates the passage of antigen through goblet cells by activation of mAChR3 or mAChR4 muscarinic receptors whereas the secretion of mucus is mediated by mAChR1 muscarinic receptors. Thus, goblet cells can differentially regulate the mucus barrier and the delivery of luminal antigens to the immune system (FIGURE 7).
As outlined above, there is an important relationship between the gut microbiota and the ENS. A key immune cell population that are also regulated by commensal microbiota are regulatory T cells (266). Yan et al. (261) examined the interaction between regulatory T cells, the gut microbiota, and the ENS and discovered a regulatory circuit in which microbial signals determine neuronal density and activation, which via the release of IL-6 modulates the generation of a regulatory T-cell population important for immune tolerance. Here it was shown that enteric nerves expressing NOS and CGRP are in close apposition to regulatory T cells in the colonic mucosa. In vitro, they found that myenteric neurons inhibited the differentiation of regulatory T cells, which was shown to be due to IL-6. When mice were monocolonized with bacteria known to induce T cells in the wall of the gut, there was an apparent reduction in neuronal density in the myenteric plexus, a reduction in nerve fibers projecting to the mucosa, and downregulation of a number of neurotransmitter transcripts including Nos1, Vip, Calcb, and Tac1, which encode NOS, VIP, CGRP-β, and substance P, respectively. These neuronal populations include those that make IL-6. When IL-6 was knocked out from enteric neurons, regulatory T cells increased in the gut wall. In this study, Yan et al. established a connection between gut microbes, signaling in the ENS, and differentiation of regulatory T cells centering around IL-6.
Together, these interesting studies illustrate the sophistication and complexity of immunoregulatory signaling in the GI tract and how the maintenance of intestinal homeostasis is due to integration of neuronal, immune, and microbial signaling using conserved molecules (e.g., cytokines) that can be produced in a cell-autonomous fashion according to the circuits involved. These studies build on past work demonstrating important functional interactions between enteric nerves and cells and organs of the innate and adaptative immune systems in the wall of the gut (reviewed in Refs. 20–22, 74, 253–257). Elaboration of these defensive circuits is an important future direction for the field.
3.2. Enteric Glia
Enteric glia represent a large, structurally and molecularly diverse population of nonneuronal cells that are distributed in all layers and along the length of the GI tract, in close association with the ENS and nerve fibers that innervate the gut (84, 242, 267, 268). Major populations of enteric glia are found in the myenteric and submucosal plexuses and in the lamina propria of the mucosa. Enteric glia were largely ignored from earlier accounts of the integrated physiology of the ENS, but through the use of molecular genetics and imaging technologies many remarkable functions for these fascinating cells have been discovered in the last 10–15 years. The functions of enteric glia and their diverse roles in health and disease have been recently reviewed (41, 242, 268, 269). Here we consider the integrative physiological roles of enteric glia in the light of some significant recent findings.
As noted in sect. 3.1 and recently described in more detail, enteric glia are well positioned to respond to neuronal activity by virtue of the synaptic and nonsynaptic specializations on their cell membranes (61, 83, 270). They influence motility, secretion, and barrier function, and it has become clear that neuro-glial communication is bidirectional, at least in the myenteric plexus (242). The close association between neurons and glia prompted Boesmans et al. (271) to investigate the spatial aspects of enteric neuron-glia communication within myenteric ganglia. They developed a highly innovative method to stimulate single enteric neurons by photolysis of a caged compound that rapidly elevated intracellular calcium levels while monitoring the activity of neighboring enteric glial cells that expressed a genetically encoded calcium indicator (271). They demonstrated that enteric neurons employ pannexins to signal to enteric glial cells in a paracrine fashion through the release of a purinergic transmitter, a finding that confirmed prior observations (84, 272, 273). Furthermore, they extended this to show that this signaling occurs within spatially restricted neuron-glia units. In light of these important findings, we should probably consider myenteric neural circuits in terms of neuro-glial units rather than in terms of the function of separate cell types, in much the same way as smooth muscle and interstitial cells function as a syncytium (274). We elaborate further on this idea below.
3.2.1. Enteric glial cell subtypes.
Single-cell transcriptomic analyses of the human and mouse intestines have revealed distinct subpopulations of enteric glia in the myenteric plexus that vary between the regions of the gut examined and between human and mouse (72, 192–194). For technical reasons, and because of the different approaches used, there are also some differences between these studies. It should also be noted that glia in other regions of the gut, such as the submucosal plexus and mucosa, have not yet been characterized at the transcriptional level, so our understanding of the molecular diversity of enteric glia is still rudimentary.
Drokhlyansky et al. (72) identified three subsets of enteric glia in the mouse colon, which is similar to work of Wright et al. (194), who identified four subsets. Wright et al. (194) revealed many interesting features of colonic enteric glia in the mouse, including the expression of specific classes of ion channels [e.g., transient receptor potential channel M member 3 (Trpm3), sodium voltage-gated channel alpha subunit 7 (Scn7a)], neurotransmitter receptors [e.g., ionotropic glutamate receptor genes (e.g., Grid2, Grik2)], a variety of signaling pathways, and presynaptic protein genes. Similar findings were also made in the mouse colon by Drokhlyansky et al. (72), indicating that they are robust. Interestingly, comparing mouse to human colon, orthologs of many of the genes found in the mouse (e.g., TRPM3, SCNA7, GRIK2) are also highly expressed in the colonic myenteric plexus, in one or more of the six glial subtypes that have been defined in the human colon (72). Since many of these genes and their gene products have not yet been explored functionally, these studies have revealed many new avenues of future investigation.
In the mouse small intestine, Zeisel et al. (192) identified seven subpopulations of enteric glia in the myenteric plexus of young animals, one of which was actively proliferating based on its expression of DNA topoisomerase II alpha gene (Top2a). Although Drokhlyansky et al. (72) identified only two populations of enteric glia in their analysis of the adult mouse small intestinal myenteric plexus, one of these (Glia 2) was distinguished by a large proportion of cells expressing high levels of Top2a. Interestingly, the equivalent population of enteric glia in the colon (Glia 2) also expressed high levels of Top2a, but in a much smaller population of cells (72), though this may be due to factors related to the liberation of cells from the different tissues. This observation suggests that glial homeostasis is dynamic and differs along the length of the bowel in the mouse. The factors that regulate baseline enteric glial proliferation in adult animals remain to be elucidated. Glial homeostasis in the mouse gut is in part regulated by the microbiome (199, 211), which also varies along the length of the bowel. Hence it is tempting to speculate that microbial factors might contribute to these findings, especially in light of the finding that the short-chain fatty acid butyrate, which is produced by the gut microbiota, inhibits proliferation of enteric glia in vivo (275). A recent study revealed that the dependence of mucosal glia on the gut microbiota was not the same in the human gut, which appears to develop independent of the microbiota (276). Glial proliferation is also regulated by estrogen receptor β (277), but whether this is involved physiologically has not been studied.
Enteric infection, inflammation, and chemical injury to the myenteric plexus all stimulate enteric glial proliferation, and it has been proposed that proliferating enteric glia in adult mice contribute to enteric neurogenesis by transdifferentiation (41, 97, 98, 278, 279). This response may involve the actively proliferating glial subtype, but further studies are needed to determine if this is the case. However, other classes of enteric glia may also serve as progenitor cells. Middelhoff et al. (280) described a population of enteric glia expressing doublecortin-like kinase 1 (Dclk1), which have the molecular characteristics of progenitor cells (coexpression of the genes for p75 neurotrophin receptor and nestin). There is a marked expansion of these Dclk1-positive cells in response to elevated levels of 5-HT in the gut and in response to whole body radiation injury, which causes increased 5-HT release and a marked increase in 5-HT enterochromaffin cell numbers (the major source of 5-HT in the gut) (280). These data suggest that proliferation of glia and glial progenitor cells are key determinants of ENS homeostasis in health and disease, and that the microbiota, enterochromaffin cells/5-HT are critical factors in signaling to enteric glia to maintain the integrity of the ENS.
3.2.2. Structural and functional relationships between enteric glia and other cells in the GI tract.
Bidirectional communication between enteric glia and neurons occurs in the myenteric plexus (242), but how neuron-glial communication shapes the activity of polarized synaptic circuits was not known until recently, when Gulbransen and colleagues (85) shed light on this topic using calcium imaging combined with chemogenetics. They demonstrated that distinct sets of enteric glia were recruited by ascending, descending, and circumferentially activated neural pathways and that glial activation has differential reciprocal effects that are specific to each neural pathway (FIGURE 4). Moreover, they found that enteric glia were functionally heterogeneous and interacted in a cell- and network-specific manner, depending on the polarity of the neural projection patterns (85). These interesting observations show that neurons in the myenteric plexus regulate neural activity in a circuit-specific manner, analogous to astrocytes in the CNS. Thus, taken together with data on the spatial relationships of neurons and enteric glia (271), at least in the myenteric plexus, it is clear that enteric glia function as a physiological unit with enteric neurons in neuro-glial units. Recently, Seguella et al. (281) demonstrated regional heterogeneity between myenteric glial cells of the duodenum and colon of the mouse. Using calcium imaging, they showed that enteric glia in the duodenum and colon show differential responses to adenosine diphosphate and CCK both within and between these regions of the gut. Moreover, these differences translated into differential control of contractility. These data strongly suggest that there are region-specific glial mechanisms that regulate digestive and presumably other gut functions, and they underscore the potential sophistication of neuro-glial units in the control of neural circuits.
The relationships that glia have with other cells in the gut wall extend beyond the neuro-glial unit to include enteroendocrine cells in the mucosa (282), epithelial cells in the crypts (283), and immune cells in the submucosa (284) and myenteric plexus (285, 286). In some cases, a spatially defined unit has been demonstrated, e.g., with enteroendocrine cells (282), T cells and macrophages (285–287), but in others the association is functional. For example, in a recent study Stakenborg et al. (287) demonstrated that inflammation of the muscularis externa of the small intestine caused by manipulation following laparotomy caused the upregulation of C-C chemokine family ligand (CCL)2 (monocyte chemoattractant protein-1) and colony-stimulating factor (CSF)-1 by enteric glia that promote the recruitment of monocytes to the muscularis and their differentiation into pro-resolving macrophages leading to tissue recovery. This example illustrates how enteric glia in the myenteric plexus act as cellular integrators that help protect the plexus from injury. Once a more complete picture of the molecular characteristics of the different populations of enteric glial cells emerges, functionally identified, molecularly characterized subsets of enteric glia are likely to be found to play a variety of key signaling functions in the GI tract, including the promotion of intestinal homeostasis and barrier function, as we discuss below.
3.2.3. Enteric glia and the maintenance of intestinal homeostasis.
An early report that generated considerable momentum in the field was the observation that ablating enteric glia caused a fulminant jejuno-ileitis in mice (288). These findings suggested that enteric glia play an essential role in maintaining the integrity of the bowel and implied that their loss or dysfunction may contribute to inflammatory bowel diseases or other inflammatory disorders of the gut. Although the essential nature of enteric glia for the maintenance of epithelial integrity has been challenged (289), recent findings have revealed that there are two redundant subpopulations of mucosal enteric glia that express glial-fibrillary acid protein (GFAP) and proteolipid protein (PLP)-1 that can compensate for each other to a large extent, but when both are ablated there is a complete collapse of epithelial integrity (283). The GFAP enteric glial cell population is the source of Wingless and Int-1 (Wnt) ligands, which are essential for regeneration of the intestinal epithelium by activating Leucine-rich repeat-containing G protein-coupled receptor 5 intestinal stem cells. Consistent with these findings, mice with the GFAP subpopulation ablated have a more severe phenotype in response to DSS, with significant impairments in epithelial regeneration and defective mucosal healing (283).
Extending the idea that enteric glia are important in maintaining intestinal epithelial integrity, Kovler et al. (241) showed that in a mouse model of necrotizing enterocolitis GFAP+ enteric glia were lost as the disease progressed. Necrotizing enterocolitis is a severe intestinal inflammatory condition of premature infants, characterized by a breakdown in intestinal barrier function, and similar findings were made in samples from human infants with necrotizing enterocolitis. They then showed that mice with selective ablation of either GFAP-, PLP-1-, or Sox10-expressing glia [Sox10 is expressed in all populations of enteric glia (72, 192)] had more severe intestinal inflammation. When TLR4 was selectively ablated in enteric glia, mice retained their glial populations and the expression of disease was greatly attenuated. They demonstrated that brain-derived neurotrophic factor (BDNF) was the molecular mediator of these effects and that BDNF administered to the glial ablated mice restored gut function and reduced intestinal inflammation (241). Together, these findings indicate that enteric glia regulate intestinal homeostasis by controlling intestinal barrier function and epithelial regeneration through the release of molecular mediators including Wnt ligands and BDNF.
The factors that regulate enteric glial homeostasis in the mucosa are not yet fully understood, but a remarkable study by Kabouridis et al. (199) revealed that the enteric glia in the lamina propria are dynamically regulated by the presence of the gut microbiota. In germ-free mice or mice treated as adults with high doses of antibiotics, they provided evidence with genetic lineage tracing that in adult mice the mucosal enteric glial cell network is continuously renewed by cells originating in the enteric plexuses. Using a genetic reporter, Vicentini et al. (211) found that PLP-1-expressing enteric glia of the intestinal mucosa are not subject to this microbial regulation, but as noted above this is only one subpopulation of mucosal enteric glial cells. The glial populations in the myenteric plexus, but curiously not the submucosal plexus (211), may also be subject to some degree of regulation by the gut microbiota (198, 211).
Enteric glia regulate barrier function, but when barrier function is compromised enteric glia may become activated and display the characteristics of reactive gliosis, with consequences in both the gut and brain. Reactive gliosis is a highly complex continuum of dynamic states whereby enteric glia respond to a perturbation through changes in morphology and/or the expression of proinflammatory and other molecular markers (see Ref. 242 for a full discussion). Seguella et al. (290) showed that feeding mice a high-fat diet that impairs duodenal barrier function led to activation of enteric glia. Associated with that, they demonstrated alterations in synaptic processes in the myenteric plexus and dendritic spines in the hippocampus, which correlated with a reduction in BDNF at both sites. These CNS changes correlated with increased levels of anxiety and depression, which were dependent on the activated enteric glia since they were abolished in animals treated with fluorocitrate, a glial metabolic inhibitor (290). This intriguing study illustrates how enteric glia are a key regulatory element of the gut-brain axis.
3.2.4. Enteric glia and the regulation of intestinal immunity.
Another important feature of enteric glia is that they express a variety of immune-related signaling molecules such as major histocompatibility class II and various cytokines (242). Given their positions in the wall of the gut and their rich immunological signaling capacity, enteric glia are poised to interact with the immune system, serving to integrate a variety of signals to maintain homeostasis. Thus, enteric glia respond to infection and bacterial products and signal to various immune cell types in the wall of the gut. For example, when activated, enteric glia interact with mast cells to regulate colonic barrier function by regulating mast cell degranulation (291). They interact with macrophages to regulate visceral sensitivity (see below) (285, 292) and with T and B cells via major histocompatibility class II signaling, which activates an autophagy pathway in enteric glia (286). Enteric glia release glial-derived neurotrophic factor family ligands that bind to the protein tyrosine kinase receptor Ret on type 3 innate lymphoid cells that release IL-22 to regulate barrier function and inflammation (284).
In a recent study Progatzky et al. (293) investigated infection with the intestinal roundworm Heligmosomoides polygyrus. H. polygyrus infection led to reactive gliosis and a glia-specific gene signature indicative of response to interferon gamma (IFNγ), a gene signature they also observed in tissues from patients with ulcerative colitis, indicating that it is a conserved response pathway in these cells. They then demonstrated that IFNγ-mediated activation of enteric glia in the external muscle layers of the gut regulates the immune response and repair of tissue damage, as well as intestinal motility. They showed that the upregulation of the chemokine CXCL10 in enteric glia in response to IFNγ signaling is required for a regulated inflammatory response and resolution of the pathology (293). Collectively, these studies illustrate the complex ways in which enteric glia promote tissue integrity and homeostasis by interacting with the host immune system to regulate a variety of gut functions including epithelial permeability, motility, and visceral sensitivity.
3.3. Interstitial Cells
Another set of cells that play important roles in motility and neurotransmission in the GI tract are interstitial cells (294). One class of interstitial cells, which are involved in these activities, is called interstitial cells of Cajal (ICCs), named for Santiago Ramón y Cajal, who first described them. A second class of cells was discovered more recently and has been termed platelet-derived growth factor receptor (PDGFR)α cells. These cells are sometimes referred to as telocytes (295–297); however, telocytes are characterized by long processes and are mobile, whereas the PDGFRα cells in the gut lack long processes and appear to be fixed in their positions at the interface of nerve fibers and smooth muscle. The term has, however, been adopted to refer to a population of PDGFRα cells that form a pericryptal sheath that express the winged helix transcription factor Foxl1 and are involved in the maintenance of the intestinal stem cell niche (298, 299).
Interstitial cells of Cajal and PDGFRα cells lie in close contact with smooth muscle cells (294), and ICCs and PDGFRα cells form gap junctions with smooth muscle cells (300–302). Electrical coupling allows changes in the excitability of the interstitial cells to affect the excitability of smooth muscle cells, which turns out to be an extremely important aspect of the regulation of motility in the GI tract. Collectively, the syncytium consisting of smooth muscle cells, ICCs, and PDGFR-α cells has been referred to as the SIP syncytium because these cells are electrically coupled by gap junctions and they appear to function as a unit (303).
3.3.1. Interstitial cells of Cajal.
ICCs express the receptor tyrosine kinase c-Kit, which has served as a critical tool for identifying and isolating these cells, and they also express ANO1 Ca2+-activated Cl− channels, which are important for their functions, as described below. Single-cell RNA sequencing of ICCs, sorted by their expression of c-Kit and ANO1, has demonstrated that these cells also express caveolin 1 and Protein Kinase cGMP-Dependent 1 (304). This is consistent with the fact that two features of these cells are that they are rich in calveoli (301) and they are sensitive to nitric oxide (305).
ICCs are divided into subtypes based on their locations, including ICC-MY cells (MY for myenteric), which form a network in the layer of the myenteric plexus, and ICC-IM and ICC-DMP cells (IM for intramuscular; DMP for deep muscular plexus), which are located within bundles of smooth muscle and lie near nerve fibers (294, 306). ICC-MY cells serve as the pacemakers for the phasic, rhythmic electrical and contractile activity that is exhibited by GI smooth muscle (294, 303). Spontaneous, rhythmic depolarizations of ICC-MY cells are generated by ANO1 Ca2+-activated Cl− channels, which are activated by Ca2+ released from intracellular stores (307, 308). Since these cells lack voltage-activated Ca2+ channels, they exhibit rhythmic slow wave depolarizations without action potential spikes. As these slow waves spread via gap junctions into the smooth muscle, the depolarizations activate voltage-activated L-type Ca2+ channels, resulting in spikes on the slow waves in smooth muscle that supply the Ca2+ that drives rhythmic contractions. Modulation of the frequency and amplitude of the slow waves by motor neuron inputs influences the strength of muscle tension in a given region at a given time.
It is now widely accepted that interstitial cells are important mediators of neuromuscular transmission in the GI tract. As described above, excitatory neuromuscular transmission is primarily mediated by ACh, which acts on muscarinic receptors to elicit transient depolarizations called excitatory junction potentials. Inhibitory neuromuscular transmission is primarily mediated by purines and nitric oxide, which cause rapid and prolonged hyperpolarizations referred to as fast and slow inhibitory junction potentials (IJPs).
ICC-IM cells are important mediators of excitatory neuromuscular transmission as well as the nitrergic component of inhibitory transmission (305). Acetylcholine released from the terminals of excitatory motor neurons activates M3 muscarinic receptors, leading to release of Ca2+ from intracellular stores and activation of ANO1 channels (309–311). This results in a transient depolarization that spreads through the smooth muscle as an excitatory junction potential. Nitric oxide released onto ICC-IM cells causes a prolonged hyperpolarization that spreads into the muscle as a slow IJP. Within the ICC-IM cell, nitric oxide binds to soluble guanylate cyclase, which produces cyclic GMP, and this leads to a membrane hyperpolarization by activating large-conductance potassium channels (312).
Interstitial cells of Cajal are integral components of the intrinsic motor circuitry of the GI tract that generates motor patterns. For example, activation of AH neurons increases ICC activity and as a result pacemaker activity of the ICC (313). It was proposed that this interaction contributes to the shift of intestinal activity from an interdigestive state reflected by peristalsis to a digestive state involving a segmentation motility pattern. Similar responses are not observed when S cells are stimulated. Furthermore, neuronally released nitric oxide, acting via deep muscular plexus ICCs, is required for distension-induced clustering of myogenic contractions (314). A recent study by Koh and colleagues (315) provides further, compelling evidence for a critical role of nitrergic neuron-ICC interactions in the generation of propulsive motor activity. They demonstrated that the initiation of colonic migrating motor complexes is not activated by cholinergic, excitatory input but rather nitric oxide, acting via ICCs, sets up the colonic migrating motor complex by providing a critical inhibitory response that is then followed by increased Ca2+ transients in colonic ICC that activate ANO1 Ca2+-activated Cl− channels, causing rebound excitation in the colonic smooth muscle (315).
3.3.2. Platelet-derived growth factor receptor α cells.
Platelet-derived growth factor receptor (PDGFR)α cells are a distinct population of interstitial cells that have unique transcriptional signatures and consist of two subpopulations that express PDGFRα at high and low levels (316). Inhibitory motor neurons release purinergic transmitters onto PDGFRα cells, resulting in the fast component of the IJP, and there is strong evidence that β-nicotinamide adenine dinucleotide is a major mediator of these signals (317). Purines activate P2Y1 receptors on the PDGFRα cells, and this leads to a sequence of events including activation of Gq signal transduction pathways and small-conductance Ca2+-activated K+ (SK3) channels (317, 318). This results in a transient hyperpolarization that spreads into the smooth muscle as a fast IJP. In addition, evoked IJPs mediated by PDGFRα cells contribute to tonic inhibition of intestinal smooth muscle, although it is not clear whether this involves spontaneous input to these cells from inhibitory motor neurons or spontaneous activity of the PDGFRα cells themselves.
3.4. Muscularis Macrophages
Under baseline conditions, macrophages are the most abundant immune cells in the GI tract (319). The major populations of macrophages residing in the lamina propria and the muscularis layers of the gut include monocyte-derived mature macrophages and monocyte-derived inflammatory macrophages (319, 320), as well as the recently described self-maintaining macrophages (93). The latter are found in all layers of the gut but are concentrated in the regions of the ENS and submucosal blood vessels. If these cells are selectively depleted, neuronal apoptosis occurs and blood vessel morphology is disrupted (93), illustrating their fundamental importance. A recent single-cell transcriptomic analysis of human colonic macrophages extended our understanding of these major subpopulations (321). Domanska et al. (321) demonstrated that lamina propria macrophages can be further divided into those that have upregulated proinflammatory gene clusters and others that have antigen presentation and phagocytic roles based on the gene profiles. Muscularis macrophages can be subdivided into a population that has genes involved in immune activation and angiogenesis that are upregulated and one that is involved in neuronal homeostasis, with functions such as synaptic pruning (321). The full molecular characterization of macrophages will allow for future hypothesis-driven studies that explore the molecular mechanisms of their roles in the ENS.
Within the muscularis of both human and mouse, macrophages are found in close association with the myenteric plexus, smooth muscle cells, interstitial cells of Cajal, and PDGFRα cells (322). Both extraganglionic and intraganglionic macrophages are found in the myenteric plexus, with intraganglionic macrophages lying in contact with enteric neurons and glia and potentially serving as the microglial cells of the ENS (65, 323, 324). If there is a blood-ENS barrier as outlined in sect. 2.1, this raises the question of how blood-derived monocytes get access to the myenteric plexus. The mechanisms remain to be determined, but of note, CCL2 has been reported in the human myenteric plexus (325) and, as we have discussed, it can be upregulated in the mouse ENS in inflammatory conditions (287), suggesting that there is a signal to attract monocytes to the plexus.
Muscularis macrophages make cell-to-cell contacts with PDGFRα and smooth muscle but apparently not with ICCs or intramuscular nerve fibers (322). Extraganglionic macrophages do not have cell contacts with enteric neurons. Macrophages display a diverse and complex morphology with extensive branches, spines, and numerous filopodia observed in fixed tissues, indicative of a highly motile phenotype (324). In addition, they are in close contact with the varicosities of extrinsic sympathetic nerves (324).
Lamina propria macrophages preferentially express a proinflammatory phenotype, consistent with a defensive role, whereas muscularis macrophages have a tissue-reparative or protective phenotype (320). Interesting studies from Mucida’s group revealed that sympathetic stimulation via β2-adrenergic receptors triggers polarization of muscularis macrophages. When mice were infected with an attenuated strain of Salmonella typhimurium these macrophages further enhanced tissue-protective programs, through reflex activation of sympathetic inputs to the gut (320).
3.4.1. Macrophage functions.
The functions of macrophages in relation to the ENS have been revealed over recent years, and it is clear that they are multifunctional cells in the ENS (319, 326). They have a number of interesting regulatory roles that we discuss in detail below, but it is important to note that they have phagocytic functions in the ENS and rapidly clear neuronal debris when enteric neurons die (91, 93).
Under physiological conditions, muscularis macrophages can influence the contractility of smooth muscle and motility along the length of the gut, as well as the integrity of the ENS, via the secretion of bone morphogenic protein-2 (327). In a reciprocal fashion, enteric neurons release the growth factor CSF-1, which is important for macrophage homeostasis (327). Interestingly, this mutual arrangement is modulated by the gut microbiota. In animals depleted of normal bacterial numbers by antibiotics, motility is slowed and there is a loss of enteric neurons. This appears to be due to loss of CSF-1 release in the ENS leading to loss of bone morphogenic protein-2 because of reduced macrophage numbers and a breakdown in the normal reciprocal regulatory state (327). Although enteric neurons release CSF-1, the primary source of CSF-1 in the ENS appears to be enteric glia (285). This suggests an interesting, but complex, tripartite relationship between neurons, glia, and macrophages, where neuronal-glial cross talk tunes the signaling cascades required for ENS homeostasis conferred at least in part by the closely associated macrophages. When self-maintaining macrophages are depleted, neuronal activity, contractility, and transit are also all reduced (93). In addition, neuronally mediated epithelial ion transport is reduced, revealing interactions between submucosal neurons and macrophages (93).
Another interesting function of muscularis macrophages is that they appear to mediate the anti-inflammatory effects of vagal stimulation in the gut. The vagal anti-inflammatory pathway is a key mechanism by which the stimulation of the vagus nerve, and activation of α7 nicotinic acetylcholine receptors (α7nAChRs), modulates the immune system, reducing inflammation (328, 329). Boeckxstaens and colleagues (330) showed that in the intestine muscularis macrophages are found near cholinergic nerve fibers arising from enteric neurons that are innervated by the vagus nerve. The expression of α7nAChR on intestinal muscularis macrophages, but not on nonimmune cells, is essential for the protective effects of the vagus nerve, and α7nAChR-activated Jak2-STAT3 pathways limit proinflammatory cytokine production in muscularis macrophages (330, 331), presumably polarizing them to an alternatively activated reparative phenotype, although this has yet to be directly demonstrated.
Although muscularis and self-maintaining macrophages may be involved in the physiological control of motility and are important in maintaining the integrity of the ENS, when polarized toward an inflammatory phenotype they lead to a breakdown in the integrity of the ENS and loss of normal function. For example, in DSS colitis, there is disruption of the blood-myenteric plexus barrier in the colon, allowing the penetration of 4-kDa fluorescein isothiocyanate (FITC)-dextran and an infiltration of immune cells (323). If macrophages are depleted with clodronate liposomes the blood-myenteric barrier remains intact, indicating that in a proinflammatory setting macrophage activation is deleterious to the integrity of the ENS.
Proinflammatory macrophage polarization is also involved in the delayed gastric emptying that occurs in diabetes. In a series of studies (332–336), Farrugia and colleagues have elegantly demonstrated that in diabetes classically activated macrophages release inflammatory mediators that reduce the numbers of ICCs and myenteric neurons, leading to delayed gastric emptying. Moreover, they showed that maintenance of normal ICC networks depends on the ability of cytoprotective macrophages to suppress the damaging effects of proinflammatory cytokines released from activated macrophages polarized by the increased levels of oxidative stress that occur in diabetes (332–336). A similar shift in polarization of macrophages occurs in aging and is related to inflammation-induced ENS degeneration and to changes in the composition of the gut microbiota (92). Macrophage polarization in intestinal inflammation not only has local effects in the gut but also affects the extrinsic innervation. For example, Grubišić and colleagues (285) found that visceral hypersensitivity caused by intestinal inflammation was mediated by enteric glial release of CSF-1 via connexin 43 signaling and the activation of muscularis macrophages.
During some enteric infections, there is a loss of enteric neurons and associated disruptions to GI motility. Investigating how the ENS integrity is maintained, Mucida’s group have made some striking observations. They showed that infection of mice with attenuated S. typhimurium, Toxoplasma gondii, and Trypanosoma cruzi leads to a sustained loss of neurons in the submucosal and myenteric plexuses of the ileum and colon (337), despite evidence for ongoing adult neurogenesis (91). However, a subsequent infection with the same or a different pathogen produced no additional loss of neurons. If the gut microbiota was normalized, neuronal numbers recovered, indicating that there is a role for the gut microbial factors in stimulating the recovery of enteric neurons. The loss of enteric neurons involved NOD-like receptor family pyrin domain containing 6 inflammasome and caspase 11-dependent mechanisms. In contrast to intestinal inflammation that seems to cause an indiscriminate loss of neurons (338), infection leads to the loss of VGlut2-positive intrinsic primary afferent neurons [VGlut2 neurons are a subset of intrinsic primary afferents (72, 134, 192)] but not NOS- or somatostatin-containing neurons (337). Other subsets of neurons were not assessed in this study. When muscularis macrophages are depleted before infection there is a greater loss of neurons, indicating that in bacterial infection muscularis macrophages appear to limit excessive damage.
Sympathetic projections from prevertebral ganglia are responsible for mediating the activation and polarization of protective muscularis macrophages, and the loss of neurons is prevented by pharmacological activation of β2 receptors during infection (337). Exactly how sympathetic pathways are activated in vivo remains to be determined. The effects of multiple infections on neuronal loss and the impact of helminth infections compared with bacterial infections have also been investigated (339). After helminth or bacterial infection (via distinct immunological mechanisms) muscularis macrophages are activated to prevent neuronal loss during subsequent challenge with an unrelated pathogen. Thus, it appears that muscularis macrophages trigger a state of tolerance aimed to preserve the integrity of the ENS and gut function in case of subsequent reinfection (339).
Together, these findings reveal a remarkable complexity and sophistication of enteric neural regulation of sensory, motor, and secretory function of the gut involving distinct populations of macrophages interacting with intrinsic and extrinsic nerves and nonneuronal elements (enteric glia, PDFGRα cells, and interstitial cells of Cajal) of the ENS, modulated by the gut microbiota. When these new insights are applied to the human gut we will gain a more comprehensive understanding of the complex neuro-immune interactions that exist under both healthy and pathological conditions, as well as during the process of recovery from infections and inflammation. This knowledge will hopefully be leveraged to develop novel treatment strategies for GI motility disorders.
3.5. Similarities and Dissimilarities Between the Human ENS and Commonly Used Laboratory Species
Extensive morphological, immunohistochemical, and functional studies have been conducted in the human, pig, mouse, rat, and guinea pig ENS. Both similarities and differences can be observed regionally along the gut and within the same region of gut between different species. These variations lead to functional differences that reflect species-specific physiology, but, unfortunately, they complicate the translation of laboratory data to the clinic.
At a gross structural level, the most obvious differences between larger species, such as human and pig, and the rodent ENS is the presence of a single layer of submucosal ganglia in the rodent intestine versus two distinct layers in the human and pig (29). The distinction probably reflects the need for distributed local control of the circular muscle by the outer submucosal plexus in species with a thicker muscle layer. The inner submucosal plexus of humans and pigs controls secretory and vascular functions, like that of the submucosal plexus of rodents. As might be expected, there are notable differences in the neuronal subtypes (46, 76, 340, 341), projection patterns (342, 343), and neurochemical coding (342, 344–349) between the neurons in the two submucosal plexuses.
Within each plexus, there are markedly different neuronal densities across species between the mouse, guinea pig, and human, and, interestingly, the total numbers of enteric neurons mirror the numbers of neurons in the spinal cord of these species (47). In rodents the number of myenteric neurons is roughly double that of submucosal neurons, but in humans it is ∼35% larger, presumably because some of the functions of the outer submucosal plexus are controlled by the myenteric plexus in rodents (47). There is also a higher density of enteric glia in the human intestine compared to that in the guinea pig. The medians of the glial cell-to-neuron ratio ranged from 1.3 to 1.9 and from 5.9 to 7.0 in the human inner and outer submucosal plexus and myenteric plexus, respectively. In the guinea pig, this ratio is 0.8–1.0 in submucosal plexus and 1.7 in myenteric plexus (350). Given the recent recognition of the diversity of functional roles of enteric glia, as we have discussed above, these data suggest that enteric glia in larger animals have a particularly important position in the regulation of enteric neural control.
Marked differences exist in the general complexity of the morphology of human (and porcine) enteric neurons compared with that of rodents, as briefly discussed above. These include larger neurons and a greater number of morphological cell types in larger species. However, all of the major neurochemical subtypes of neurons (cholinergic, nitrergic, VIPergic, etc.) are found in all monogastric species studied to date. Despite the differences in neuronal density and morphology, the major functional classes of neurons (S and AH neurons) are found in all species where electrophysiological recordings have been obtained, including the human colonic myenteric plexus (106, 109). However, the proportion of AH neurons appears to be far lower in the human colon than in the rat, mouse, and guinea pig colon (107, 108, 351–353), and there are some other electrophysiological differences, but the sample sizes of the human studies are too small to determine how significant they are (106, 109).
Based on direct comparative functional studies, neurons of the human ENS show differences and similarities to those of rodents. For example, Schemann’s group found that protease-activated receptor (PAR)1 is the major functional neuronal protease receptor in human enteric neurons, whereas in guinea pigs it is PAR2 and PAR4 (354–356). Similarly, Benko et al. (357) found that neurogenic cholinergic contractions of human small intestine were unaffected by morphine, whereas longitudinal contractions of the guinea pig ileum were concentration-dependently suppressed by it, suggesting that presynaptic opioid receptor expression and/or function differs between humans and guinea pigs. In contrast, Reed et al. (358) demonstrated that stress hormones increased descending inhibition through purinergic and nitrergic mechanisms in both the mouse and human colon and Fornai et al. (359) showed that modulation of cholinergic excitatory neurotransmission in the colon by cyclooxygenase inhibitors was comparable between mouse and human. Likewise, Schemann et al. (360) found that a human mast cell mediator cocktail excites similar proportions of submucosal neurons in the human and guinea pig. Taken together, it is apparent that although there may be specific mechanistic differences in the control of distinct functions between humans and animals, most gut functions are well conserved, and so are the neural control mechanisms that regulate them.
Single-cell RNA transcriptomic studies have revealed several parallels between the mouse and human ENS but also marked differences that further illustrate why translation from the bench to the bedside is often difficult (72, 134, 193, 194, 361). Nevertheless, human and mouse share many fundamental transcriptional programs and congruence in the major populations of enteric neurons, though our understanding of the transcriptional programs in enteric glia is less advanced. An example of a conserved marker of neuronal subtypes between mouse and human is the gene encoding neuromedin U (Nmu/NMU). In the mouse ENS, Nmu is found exclusively in intrinsic primary afferent neurons of the myenteric plexus in the small (and probably) large intestine (72, 134). In the human, NMU is found in a similar subset of small intestinal myenteric neurons but a smaller proportion of colonic neurons (72, 193, 194), although the range of coexpressed genes in these populations varies somewhat between species and between different studies. Another significant similarity between mouse and human is the regional expression of the Cckar/CCKAR in a subpopulation of duodenal myenteric neurons (193). These detailed molecular studies are at an early stage, and it will be exciting to see to what extent we can use these data to further resolve the physiology of the ENS in the future.
Finally, it should be noted that within commonly used rodent models of the ENS there are also both marked similarities and notable significant differences between mouse, rat, and guinea pig. These shared and disparate features need consideration when generalizing findings that have only been made in a single species. For example, in the guinea pig small intestine, calbindin and calretinin are expressed in nonoverlapping populations of intrinsic primary afferent and excitatory longitudinal muscle motor neurons/ascending interneurons, respectively (117). In the mouse calretinin and calbindin overlap in a population of intrinsic primary afferent neurons, and calretinin is also found in excitatory circular muscle motor neurons as well as the other two populations found in the guinea pig (181, 362). There are also different numbers of neuronal classes present in the mouse small (and large) intestine compared with the guinea pig ileum (181).
4. FUNCTIONAL ORGANIZATION OF THE ENTERIC NERVOUS SYSTEM AND ITS RELATION TO THE AUTONOMIC NERVOUS SYSTEM
The ENS is classified as the third division of the autonomic nervous system (363), with the parasympathetic and the sympathetic divisions being the first and second. In developing this classification, Langley stated at the time, “This classification is, I think, still advisable for the central connexion of the enteric nerve cells is still uncertain, and evidence has been obtained that they have automatic and reflex functions which other peripheral nerve cells do not possess.”
In the intestines, although the ENS can function autonomously, physiological functions of the GI tract are normally influenced by reciprocal connections between the ENS and the CNS (FIGURE 1). Together these make up essential elements of the “brain-gut axis” that functions to integrate activity between the gut and the brain (8, 10, 364). Enteric neurons are not traditional postganglionic autonomic neurons. For example, based on tracing studies, most myenteric ganglia in the intestines do not receive vagal preganglionic projections, and in the ganglia that do most neurons do not receive direct vagal input (365). Furthermore, vagal efferent projections to submucosal ganglia have not yet been observed. Therefore, enteric neurons of the intestines receive most of their synaptic signals from other enteric neurons, and they integrate activity from local circuits in the wall of the gut with preganglionic inputs from the sympathetic and parasympathetic nervous systems. Indeed, the ENS is the major target of these autonomic nerves, which for the most part do not directly innervate the structures of the gut wall, except for sympathetic innervation to blood vessels and sphincters (4, 5, 366, 367).
Autonomic control of the GI tract is, however, important for normal GI function (FIGURE 8). Vagal efferent signals are vital for normal esophageal and gastric motor and secretory function and as the mediator of the cephalic phase of digestion (51) as well as for host defense via the vagal anti-inflammatory pathways discussed above (328, 329). Lumbosacral parasympathetic inputs to the distal colon are important for colonic motility and defecation. In both cases, these inputs are via the ENS, not directly to smooth muscle or glands.
Figure 8.
A schematic illustration of the autonomic and primary afferent innervation of the gastrointestinal (GI) tract. The GI tract receives a parasympathetic preganglionic innervation from the vagus and pelvic nerves and a rich sympathetic efferent innervation from postganglionic neurons located in the abdominal prevertebral ganglia (celiac, superior mesenteric, and inferior mesenteric ganglia). Viscerofugal enteric neurons provide input from the small and large intestines to the sympathetic ganglia and from the esophagus to the trachea. Primary afferent neurons carry signals from the gut to the central nervous system. Vagal afferents have their cell bodies in the nodose ganglia, and spinal primary afferent nerves run with the sympathetic and pelvic splanchnic nerves and have their cell bodies in dorsal root ganglia. It should be noted that sympathetic and parasympathetic efferent fibers innervate the enteric nervous system and not the smooth muscle or mucosal tissues of the GI tract, with the exception of a direct sympathetic innervation of submucosal arteries and sphincteric smooth muscle. Adapted in part from Refs 21, 29. Image created with BioRender.com, with permission.
Sympathetic inputs to the gut tonically regulate secretion and motility (366). The action on secretion is via a direct postsynaptic input to enteric secretomotor neurons, whereas the actions on motility are indirect, via inhibitory presynaptic inputs that limit excitation of enteric neurons (51). There appears to be tonic sympathetic input to the distal colon that limits the degree of excitation of the myenteric plexus (368), and there is a degree of sympathetic tone to sphincteric smooth muscle. Reflex activation of sympathetic nerves inhibits motility. In a recent study, Parker et al. (369) examined the association of noradrenergic varicosities with the types of enteric neurons in the human colonic myenteric plexus. They found that sympathetic axons preferentially targeted cholinergic excitatory neurons compared with nitrergic neurons, indicating that the inhibitory modulation of human colonic motility by the sympathetic nervous system is probably via the reduction in excitatory transmitter release. Generalized sympathetic activity occurs via spinal reflexes in response to stimulation of the gut or through other mechanisms, e.g., painful stimuli. Another class of sympathetic reflexes termed intestino-intestinal reflexes involve the abdominal prevertebral ganglia, bypassing the CNS. Intestino-intestinal reflexes are initiated by viscerofugal enteric neurons (see below) that activate sympathetic postganglionic neurons in the prevertebral ganglia providing feedback from distal regions of the gut to regulate (slow) propulsion in more proximal regions of the gut. As we will see below, sympathetic nerves are also important in neuroimmune interactions in the gut.
Recent studies using optogenetics have demonstrated that sympathetic neurons differentially regulate the activities of neurons in the proximal versus the distal colon to promote distinct changes in motility patterns in these regions (370). Sympathetic inputs to the myenteric plexus have differential effects on the enteric neural circuitry. In the proximal colon, myenteric neuron responses to ascending input were significantly decreased when sympathetic nerves were stimulated, but responses to descending input were not affected. In contrast, in the distal colon myenteric neuron responses to descending inputs were significantly reduced after sympathetic stimulation, but ascending inputs were not affected (370). This illustrates that output from sympathetic prevertebral ganglia to the ENS is highly organized and results in region-specific modulation of motor patterns. Moreover, these results suggest that, physiologically, the sympathetic innervation is organized in a proximal to distal manner to prevent the anterograde movement of fecal contents in the distal colon and reduce the likelihood of reflex-initiated propulsive contractions in the proximal colon. This study illustrates the power of combining physiological approaches with the use of fluorescence imaging and optogenetics, providing significant novel insights into the organization and function of the neural control of the GI tract.
Sympathetic inputs to the gut not only regulate motility and digestive functions of the gut but also have an impact on defensive function. A recent study used the power of optogenetics to reveal that stimulation of sympathetic nerves innervating the mouse colon regulates the degree of colitis after DSS treatment by reducing immune cell trafficking to the colon by downregulating the expression of the cell adhesion molecule mucosal vascular addressin cell adhesion molecule 1 (371). This study provided direct mechanistic evidence that sympathetic activation can regulate immune cell abundance in the gut wall. Previous studies have shown that activation of the sympathetic nervous system by stress, which is well recognized to reduce intestinal barrier function, enhances bacterial proliferation and virulence mechanisms (256, 372). Neural-immune-microbial interactions in the gut, including interkingdom signaling mechanisms, are increasingly recognized to regulate host-pathogen interactions as well as intestinal homeostasis. Thus, both sympathetic and vagal innervation of the gut wall regulate local intestinal inflammation, barrier function, and host defense.
4.1. Primary Afferent Innervation of the GI Tract
Autonomic inputs to the GI tract can be initiated centrally, as occurs in the cephalic phase of digestion, or via reflex activation from the gut itself. The GI tract is richly innervated by primary afferent nerves that run together with the vagal and spinal innervation of the gut and provide the afferent arm of autonomic reflexes (FIGURE 8).
Vagal afferent neurons innervating the gut have their cell bodies in the nodose ganglia and terminals in the ENS, smooth muscle, and mucosa. Elegant anterograde tracing studies by Powley and colleagues have revealed specialized vagal afferent terminals in the myenteric plexus termed intraganglionic laminar endings (IGLEs) (708). These mechanosensitive terminal structures are found throughout the length of the GI tract and consist of flattened plates of lamelliform terminal puncta in contact with myenteric ganglia. Generally regarded as tension receptors, IGLEs also respond to neurotransmitters/enteroendocrine peptides such as CCK and therefore can integrate mechanosensory neural signals with local or circulating chemical signals.
The vagal afferent innervation of smooth muscle occurs through widely arborizing intramuscular arrays, with long neurites that run in parallel to the muscle fibers and in close association with ICCs. They are found mostly in the stomach, with few in the intestines. Based on their position, it is suggested these intramuscular arrays are stretch sensors, responding to gastric filling (373).
The final class of vagal afferents are mucosal arborizations. These appear as free nerve endings in the mucosa throughout the GI tract and seem to be polymodal in nature. The molecular identity of every class of vagal afferent was recently revealed by single-cell RNA analysis (374). The diversity of neuronal subtypes reveals a chemo- and mechanosensory system with the capacity to respond to a wide variety of chemicals including enteroendocrine peptides, neurotransmitters, nutrients, inflammatory and microbial mediators, as well as mechanical stimuli through mechanosensitive ion channels (374). Future studies that attribute a functional role and target of innervation to every molecularly defined class of neuron will be of immense value. It is important to also highlight the remarkable plasticity of nodose ganglion neurons in response to nutritional status. Nodose ganglion cells alter their expression of transmitters [e.g., cocaine- and amphetamine-regulated transcript (CART), CCK, melanin concentrating hormone] and receptors (e.g., cannabinoid CB1, leptin) in response to food deprivation, feeding, and obesity (375). Thus, this system is highly dynamic and adapted to integrate a variety of peripheral signals to maintain metabolic homeostasis and to mediate digestive reflexes, particularly those involving the organs of the proximal GI tract.
Spinal afferents have their cell bodies in dorsal root ganglia, and like vagal afferents they have arborizations in the myenteric plexus, circular muscle, submucosa, and mucosal layers of the gut (376) and like vagal afferents have been characterized by detailed transcriptomic analyses. There is substantial molecular heterogeneity of these neurons, differences between neurons at different spinal levels (thoracic, lumbar, and sacral), some significant sex differences, as well as the discovery of many novel genes whose roles remain to be determined (377–380). In pioneering studies unbiased single-cell RNA sequencing was performed on retrogradely labeled colonic primary afferent neurons from both thoracolumbar and lumbosacral regions of the mouse (377). Here seven subtypes of colonic primary afferent neurons were described that encompassed the known modalities of colonic neuronal sensitivity. This study offered novel insights into the discrete neuronal populations activated by specific enteroendocrine/neurotransmitter ligands (e.g., 5-HT), bacterial signaling molecules (e.g., TLR4 and TLR5), and mechano- and chemosensory stimulation (377).
An important role for spinal primary afferents innervating the GI tract is mediating host defense (21, 257, 381). They accomplish this through axon reflexes that cause vasodilatation, increasing local blood flow (382), and by regulating immune function and inflammation (21, 257, 381). Recently, Lai et al. (383) showed that spinal primary afferents regulate the density of microfold (M) cells in Peyer’s patches of the ileum via the release of CGRP. This is accompanied by a change in the composition of the gut microbiota and in particular the levels of segmentous filamentous bacteria. By suppressing the density of M cells, spinal afferents regulate segmentous filamentous bacteria and thereby limit entry of S. typhimurium. The spinal afferents directly sense S. typhimurium and release CGRP that enhances intestinal barrier function. It remains unresolved how CGRP regulates M cell homeostasis or how the primary afferents sense the presence of S. typhimurium (383). These findings illustrate another example of interkingdom signaling and serve to show that the extrinsic innervation of the gut, as well as enteric nerves (see sect. 5.4), plays key roles in host defense and the maintenance of intestinal homeostasis.
There are important functional differences between vagal and spinal afferents. Stimulation of spinal afferents evokes the sensation of pain, and they are importantly involved in defense mechanisms (257, 384–386); vagal afferents are also involved in defense (e.g., vomiting), but the sensations evoked by vagal stimulation are not painful (385). Both spinal and vagal afferents are involved in the homeostatic control of gut function, with vagal afferents strongly involved in the generation of interoceptive sensations, including mood (7, 8). Vagal afferents are also important for initiating digestive reflexes as well as influencing hunger and satiety (387, 388) and the reward value of food (389). The autonomic reflexes evoked by stimulating these two populations of afferents reflect their roles in the maintenance of homeostasis, e.g., regulation of motility, secretion, blood flow, and defense. For example, stimulation of low-threshold vagal afferents in the esophagus by ingested food results in proximal gastric relaxation and gastric accommodation of a meal, whereas nociceptive stimulation of the intestine activates spinal afferents that result in the inhibition of gastric motility, limiting gastric emptying.
4.2. Viscerofugal Enteric Neurons and Their Role in Autonomic Control
In addition to vagal and spinal afferent projections from the gut to the CNS, five populations of enteric neurons have been described that project away from the gut (FIGURE 8). In the esophagus, there are two populations of myenteric neurons that project to the trachea (174, 175). A population of myenteric inhibitory neurons containing NOS and VIP projects to the trachealis muscle and mediates relaxation (174). Second, a small group of myenteric cholinergic neurons coexpressing ChAT and calretinin provide an excitatory input to tracheal ganglia (175). These inputs could explain why esophageal reflux, which leads to activation of these esophago-tracheal projection neurons, is linked to changes in airway reactivity. There are a few scattered enteric neurons in the stomach and duodenum that project to the dorsal motor nucleus of the vagus in the brain stem (365), the main source of vagal efferent neurons to the gut, but the function of these unusual projections remains to be established. A population of neurons in the colon and rectum project directly to the spinal cord and may be involved in nociceptive processing from the distal gut (390, 391).
Finally, there are enteric neurons that project from the myenteric plexus at all levels of the gut to abdominal prevertebral ganglia (392–394). The majority of these “intestinofugal” neurons are found in the colon and distal ileum and were originally described by Szurszewski and colleagues as providing the afferent limb of intestino-intestinal reflexes (395, 396). This is a population of mechanosensitive cholinergic myenteric neurons that also express VIP, CCK, gastrin-releasing peptide (GRP), and CART (29). In the guinea pig, they have been shown to have lamellar expansions in the myenteric plexus (394), consistent with their mechanosensory function. In a novel preparation, Hibberd et al. (397) recorded extracellularly from intestinofugal neurons in rectal nerve trunks of the mouse colon and demonstrated that they fired in 2-Hz bursts that aligned with the 2-Hz smooth muscle voltage oscillations of the colonic motor complex. The burst firing pattern emerged from the assemblies of intestinofugal neurons each making a partial contribution to the net firing pattern, illustrating a completely novel form of enteric neuronal synchronization. This firing pattern leads to reflex sympathetic activation of neurons of the inferior mesenteric ganglion (397).
Exciting recent work has shed new light on the function of intestinofugal neurons. Muller et al. (201) demonstrated that CART+ neurons in the ileum and colon are regulated by the intestinal microbiota. In germ-free mice there are substantively fewer cells expressing CART, and after reconstitution of the gut flora CART expression reaches normal levels. This loss of CART expression is due to a selective loss of these neurons in the ileum but not the colon, where there is no loss of neurons per se in germ-free mice. These data suggest that neuronal gene transcription is modulated by the gut microbiota in the colon but that loss of CART gene-expressing neurons occurred in the ileum. Many of these CART+ neurons project from the gut to the celiac-superior mesenteric ganglia, and they are therefore intestinofugal neurons (201). Selectively exciting these neurons with excitatory designer receptor exclusively activated by designer drugs (DREADDs) leads to reductions in food intake, elevated levels of blood glucose, and a reduction in insulin levels with no change in plasma glucagon. Careful tracing of the circuit leading to these metabolic effects revealed that the CART+ neurons project to the celiac-superior mesenteric ganglia and activate a synaptic pathway involving sympathetic activation of the liver and pancreas. These remarkable findings leave many questions unanswered about intestinofugal neurons. For example, how do they sense the presence or absence of the gut microbiota since they do not project within the gut beyond the myenteric plexus? What triggers the firing of these neurons under physiological conditions? Are the mechanosensitive properties of intestinofugal neurons linked to their roles in the control of blood glucose levels, and why would distally located mechanosensitive enteric neurons be involved in glucoregulation? Answers to these questions will open new vistas in enteric neurobiology.
A follow-up study by Zhang et al. (398) built on these findings and demonstrated a remarkable interorgan neural circuit that utilizes intestinofugal neurons as the afferent limb of the ileal brake. The ileal brake is a reflex feedback mechanism by which nutrients in the ileum trigger the slowing of the proximal gut to allow additional time for digestion and absorption (399). Previously, intestinofugal neurons have been proposed as a potential mediator of this reflex in response to luminal fat (400), though this was never directly proven. Zhang et al. used optogenetics and chemogenetics to directly stimulate intestinal L cells, which release glucagon-like peptide (GLP)-1, and showed that this initiated gastric distension and a reduction in food intake. They demonstrated that GLP-1 receptor was present on a population of ileal neurons and that activation of these neurons initiated the reflex-mediated gastric accommodation. Activation of these receptors evoked expression in neurons of c-Fos in the celiac ganglion. Using focal viral transfection of the celiac ganglion, they demonstrated that the GLP-1 receptor-expressing neurons of the ileum are intestinofugal neurons and that these are both necessary and sufficient to activate the ileal brake mechanism. Furthermore, they also showed that gastric NOS-inhibitory motor neurons were responsible for slowing gastric emptying and causing the gastric distension that is responsible for the reduction of food intake (398). This was very surprising, since it would have been expected that activation of sympathetic neurons innervating the stomach would lead to presynaptic inhibition of excitatory cholinergic neurons (presumably via adrenergic receptors) rather than a direct activation of gastric inhibitory motor neurons (129). This raises new questions about the details of the sympathetic innervation of the stomach that require elucidation. Another interesting observation in this paper is that the gastric distension caused by GLP-1 activation of intestinofugal neurons is signaled via spinal and not vagal afferent pathways (398). The spinal afferents then activated the lateral reticular formation and the parasubthalamic nucleus of the lateral hypothalamus to cause appetite suppression. It appears in this case that the levels of gastric distension evoked a nociceptive response leading to the production of an aversive response to food intake (based on the orofacial features produced by stimulation). Nevertheless, this finding necessitates a reevaluation of the roles of vagal and spinal primary afferent neurons in the physiological regulation of gut function, since generally it was believed that vagal and not spinal afferents were responsible for feedback regulation to gastric distension (401).
5. INNERVATION OF THE GASTROINTESTINAL MUCOSA
Most of the digestive and defensive functions of the gut occur in the mucosa. A dense network of nerve fibers innervates the mucosa of the GI tract (4, 5). In the small intestine there are discrete plexuses in the crypt region and villi that are differentially chemically coded (5), probably reflecting functional differences along the crypt-villus axis. In the stomach and large intestine, which lack villi, the innervation is more generalized but serves similar functions. The mucosal innervation derives from neurons in the submucosal and myenteric plexuses, as well as extrinsic spinal and vagal primary afferent nerves and sympathetic postganglionic nerve fibers. Recently, Smith-Edwards and colleagues (370) used optogenetic approaches to stimulate the sympathetic input to the colonic epithelium. They made the interesting observation that sympathetic stimulation evoked increases in intracellular calcium in crypt epithelial cells in the proximal colon via α2-adrenoceptors but had no effect in the distal colon, which was shown to have the capacity to respond to norepinephrine (370). The functional consequences of this epithelial activation by the sympathetic innervation in the proximal colon remain to be understood. There is also a dense network of enteric glia in the mucosa, including glial cell bodies (136, 242, 267), as discussed above. The enteric innervation of the mucosa serves many physiological roles including the regulation of mucosal growth and cell proliferation, control of secretion, and the maintenance of epithelial barrier function (16, 22, 51, 136, 402), as we discuss further in sect. 5.3. Furthermore, recent transcriptomic studies have revealed enormous potential for interactions of the ENS with the diversity of cells in the intestinal epithelium (72). These exciting novel studies open up a vast array of new possibilities to examine the neuro-glial-epithelial unit (136) as a node of functional integration in the GI tract and will be the subject of many future investigations.
5.1. Enteroendocrine Cells and the Transduction of Luminal Stimuli
A notable feature of the enteric innervation of the mucosa is that enteric nerves do not penetrate the epithelial lining of the GI tract. Therefore, to receive information about the luminal capacity or contents, enteric nerves and glia rely on signals transduced by “luminal sensors,” specialized epithelial cells that have the ability to respond to mechanical or chemical stimuli and release a variety of peptides and the amines 5-HT and melatonin via granular exocytosis (403–414). Most of these cells are enteroendocrine cells, including 5-HT-containing enterochromaffin cells, the largest subpopulation of enteroendocrine cells and the most thoroughly studied population of epithelial sensory cells (7, 415–417). Recent studies using three-dimensional (3-D) electron microscopy and confocal imaging have demonstrated an intimate “synaptic” relationship between enteroendocrine cells and nerves and glia (282, 406, 407, 418), providing an anatomical substrate for activation of enteric and extrinsic reflexes, and glial signaling (FIGURE 9).
Figure 9.
Enterochromaffin cells and tuft cells are regulated by the microbiota. Tuft cells respond to microbial signals via succinate (SuncR1) and taste (Tas1R/Tas2R) receptors and release acetylcholine (ACh) and immune mediators [IL-25 and leukotriene C4 (LTC4)] to regulate neuroimmune signaling in the lamina propria. The gut microbiota can modulate the release of serotonin (5-HT) by influencing the expression of tryptophan hydroxylase 1 (Tph1), the rate-limiting enzyme for the production of 5-HT. Enterochromaffin cells respond to microbial signals via Piezo1 channels, transient receptor potential (TRP)A1 channels, and toll-like receptors (TLR) 2 and 4. They also respond to short-chain fatty acids via free fatty acid receptors (FFARs) and bile acids via TGR5 (GPBAR1) receptors. These cells are also mechanosensitive (via Piezo2 channels). In all cases, 5-HT that is released acts on a variety of 5-HT receptors on enteric and extrinsic primary afferent nerves to regulate motility, secretion, and defensive responses. See text for details. Image created with BioRender.com, with permission.
This arrangement has the potential for pathophysiological “transmission” of misfolded proteins found in enteroendocrine cells to the ENS and CNS (420). Specifically, it has been shown that both prion protein and α-synuclein are present in enteroendocrine cells and that the potential exists for this to be a route of transmission from the gut to the brain via the ENS and/or vagus nerve (419–421). Although direct proof that this occurs in humans remains to be shown, these findings are very intriguing, particularly in light of GI manifestations of many neurodegenerative diseases (7, 24–26).
Recently, a new approach to investigate epithelial signaling to the ENS was introduced that used optogenetic activation of the epithelium combined with calcium imaging of myenteric neurons (422). Using a villin promotor to direct the expression of channel rhodopsin to the colonic epithelium and measuring intracellular calcium as a proxy for activity in enteric neurons, Najjar and colleagues (422) demonstrated that stimulation of epithelial cells resulted in activation of what appears to be a population of intrinsic primary afferent neurons of the myenteric plexus. Stimulation of the distal, but interestingly not the proximal, colon caused local contractions and increased the rate of colonic migrating motor complexes. The motor effects were blocked by TTX and hexamethonium, consistent with them being activated by intrinsic primary afferent neurons (which are cholinergic). Epithelium-evoked contractions in the distal colon were also reduced by both ATP and 5-HT receptor antagonists, consistent with an enteroendocrine transduction mechanism (422). This study illustrates the potential for using modern molecular genetic approaches to study the extent to which the different populations of enteroendocrine cells signal to enteric neurons (and glia) by direct synaptic mechanisms and/or by paracrine mechanisms of volume transmission following release of their mediators into the lamina propria.
Mechanical and chemical stimulation of the gut by the passage of a bolus, pressure due to distension or microbial mediators, nutrients, toxins, or irritants activates the mechano- and/or chemosensory systems of the intestinal epithelium to initiate digestive and defensive reflexes (409, 413, 423–426). Tremendous advances in our understanding of epithelial sensing have been made in the last few years, based on the use of single-cell RNA technologies, optogenetics, and transgenics.
Single-cell RNA studies of enteroendocrine cell populations in human and mouse have revealed an exquisite diversity of cell types with the capacity to respond to a wide variety of signals originating luminally (e.g., diet, microbes) and from the local cellular environment (e.g., immune, paracrine, and neural) (427–430). In particular, they express an incredible array of G protein-coupled receptors (427, 430), ideally positioning them as sentinels for the integration of local signaling with bodily homeostasis (415). Because of the nature of their outputs, paracrine, humoral, and neural, these secretory cells are capable of regulating homeostasis over a range of timescales and both locally and at a distance from the gut.
Enterochromaffin cells serve as environmental sensors integrating microbial and dietary signals with the release of 5-HT and the initiation of motor reflexes. Yano et al. (431) showed that 5-HT biosynthesis via tryptophan hydroxylase 1 is regulated by the presence of the commensal microbiota in the colonic epithelium, specifically by indigenous spore-forming bacteria (FIGURE 9). This effect is not due to the induction of regulatory T cells, since it was maintained in Rag1 knockout mice and is mediated by metabolites produced by these bacteria (431). Following from this, Sugisawa et al. (238) revealed one mechanism by which enterochromaffin cells sense bacteria. They showed that the cation channel Piezo1 acts as a sensor for single-stranded RNA and linked this to the production of 5-HT by enterochromaffin cells of the small and large intestine. Interestingly, deletion of Piezo1 from epithelial cells conferred resistance to DSS-induced colitis (238), indicating that the release of 5-HT in the mucosa by mucosal irritants, which leads to intestinal inflammation via disruption of autophagy (432, 433), is regulated, at least in part, via this ion channel. In addition to the Piezo1 channel, enterochromaffin cells are enriched in transient receptor potential ankyrin A1 (TRPA1) channels and the olfactory receptor Olfr558 (418). These were shown by Bellono et al. (418) to be irritant and microbial metabolite receptors, respectively. Furthermore, they also demonstrated that enterochromaffin cells could be activated by catecholamines, released from sympathetic nerves, via α2a-adrenergic receptors, indicating a modulatory role of the autonomic nervous system and further capacity for integration by enterochromaffin cells (418).
Microbial catabolites of dietary tryptophan are also sensed by enterochromaffin cells, leading to 5-HT release and activation of cholinergic nerves. Using in vivo real-time measurements of enteroendocrine cells and enteric nervous system activity in zebrafish, Ye et al. (434) discovered that the bacteria Edwardsiella tarda activates enteroendocrine cells (including enterochromaffin cells) through the TRPA1 receptor. Activation of this receptor maintains microbial homeostasis by regulating intestinal motility. Finally, host-derived immune signals released after parasitic infection are also integrated by enterochromaffin cells to maintain the intestinal homeostasis. Chen et al. (239) showed that the alarmin cytokine IL-33 released from the epithelium upon parasitic infection with Trichuris muris promoted worm expulsion by the release of 5-HT from enterochromaffin cells. This effect also required elevated intracellular calcium levels and the activation of TRPA1 channels (FIGURE 9).
Not only do enterochromaffin cells monitor the luminal environment as chemosensors, they are also mechanosensors of the gut and form a large subpopulation of mechanosensory enteroendocrine cells that express the Piezo2 ion channel (408, 435) (FIGURE 9). About 80% of enterochromaffin cells express Piezo2, and the vast majority of Piezo2-expressing cells are enterochromaffin cells. Beyder and colleagues (435) showed that Piezo2 enteroendocrine cells respond to tactile stimuli and can detect even small luminal forces in the small and large intestines. Mechanistically, activation of Piezo2 channels by force leads to the opening of a rapidly activating cationic receptor current. This current initiates a sustained increase in intracellular calcium involving calcium-induced calcium release mechanisms activated through intracellular store receptors, ryanodine receptors, and inositol (1,4,5)-trisphosphate (IP3) calcium store receptors. Activation of these receptors then leads to the release of 5-HT (436). As with their chemosensory outputs, mechanosensory activation of these cells regulates intestinal transit and gastric emptying (408, 435, 437).
Together these studies reveal that enterochromaffin cells are a key node of integration of dietary, microbial, immune, and neural signaling; they are also mechanosensory, responding to the forces generated by the luminal contents of the gut. 5-HT release is a common output for the initiation of reflexes that activate the brain-gut axis locally (via the ENS) and centrally (via activation of vagal and spinal primary afferent nerves) (409, 418, 434).
It should be noted that attempting to gain a complete understanding of the physiological roles of 5-HT in the gut and the actions of gut-derived 5-HT outside the gut is not for the faint of heart. As summarized in TABLE 5, at least seven different types of 5-HT receptor are expressed in the GI tract, and they are expressed on enteric neurons, extrinsic primary afferents, interstitial cells, smooth muscle cells, enteroendocrine cells, enterocytes, goblet cells, and immune cells, and some of these receptors exhibit constitutive activity.
Table 5.
Subtypes of 5-HT receptor in the GI tract
| Receptor | Cell Type | Gene Transcripts: Human Large Intestine* | Gene Transcripts: Mouse Small Intestine* | Gene Transcripts: Mouse Large Intestine* | Gene Transcripts: Mouse Colonic-Projecting Dorsal Root Ganglion Neurons or Nodose Ganglion Neurons† | Physiological Action in the GI Tract | References for Functional Actions |
|---|---|---|---|---|---|---|---|
| 5-HT1 | Myenteric neuron | HTR1E (excitatory and inhibitory motor neurons) | Htr1b (sensory neurons, secretomotor/vasomotor neurons, inhibitory motor neurons), Htr1f (excitatory motor neurons) | Htr1b (sensory neurons), Htr1f (excitatory motor neurons) | Presynaptic inhibition, neuronal hyperpolarization, decreased ACh release | (438–440) | |
| Spinal afferent | Htr1a, Htr1b, Htr1d, Htr1f | Function not determined | |||||
| Epithelium | Htr1b, Htr1d, Htr1f | Promotes colorectal cancer stem cell self-renewal and tumorigenesis. | (441) | ||||
| 5-HT2 | Myenteric neuron | HTR2A, HTR2B, HTR2C (excitatory and inhibitory motor neurons, secretomotor/vasomotor neurons, sensory neurons) | Htr2a, Htr2b, Htr2c (interneurons, secretomotor/vasomotor neurons, excitatory motor neurons, sensory neurons) | Htr2a, Htr2b, Htr2c (Excitatory and Inhibitory motor neurons, Sensory neurons) | Excitatory actions in human colon | (442) | |
| Submucosal neuron | Secretomotor; promotes epithelial proliferation via 5-HT2A receptors on submucosal neurons. | (443, 444) | |||||
| ICC | Promotes proliferation. | (445, 446) | |||||
| Smooth muscle | Contraction | (442) | |||||
| Epithelium | Secretion | (443) | |||||
| Spinal afferent | Htr2a, Htr2b, Htr2c | Function not determined | |||||
| Immune cells | Recruitment of various immune cell populations, anti- inflammatory macrophage polarization | (447) | |||||
| 5-HT3 | Myenteric neuron | HTR3A (sensory neurons), HTR3B (sensory neurons, excitatory motor neurons) | Htr3a, Htr3b (sensory neurons) | Htr3a, Htr3b (sensory neurons, secretomotor/vasomotor neurons) | Rapid depolarization | (448–450) | |
| Submucosal neuron | Rapid depolarization | (451, 452) | |||||
| Vagal afferent | Htr3a, HTr3b | Activation | (374, 453–455) | ||||
| Spinal afferent | Htr3a, Htr3b | Activation | (454, 456) | ||||
| Epithelium | Secretion | (457, 458) | |||||
| Immune cells | Activation; anti-inflammatory actions | (447, 459) | |||||
| 5-HT4 | Myenteric neuron | HTR4 (excitatory motor neurons, interneurons) | Htr4 (excitatory motor neurons, sensory neurons, interneurons) | Htr4 (excitatory motor neurons, sensory neurons, interneurons, secretomotor/vasomotor neurons) | Presynaptic facilitation | (438–440, 460–463) | |
| Enteric glia | Htr4 | Htr4 | Function not determined | ||||
| Submucosal neuron | Presynaptic facilitation | (461, 462, 464, 465) | |||||
| Smooth muscle | Htr4 | Relaxation | (461, 466–469) | ||||
| Spinal afferent | Htr4 | Function not determined | |||||
| Enteroendocrine cell | Htr4 | Htr4 | 5-HT release | (470, 471) | |||
| Enterocyte | Htr4 | Cl− and bicarbonate secretion, proliferation, migration | (470, 472–474) | ||||
| Goblet cell | Htr4 | Mucus secretion | (470, 475, 476) | ||||
| Immune cells | Htr4 | Modulates the differentiation of dendritic cells from human monocytes. | (447, 477) | ||||
| 5-HT5 | Myenteric neuron | Htr5a, Htr5b (low expression in sensory neurons, interneurons) | Htr5a, Htr5b (low expression in motor neurons, sensory neurons, interneurons) | Function not determined | |||
| Spinal afferent | Htr5a, Htr5b | Function not determined | |||||
| 5-HT6 | Myenteric neuron | Htr6 (low expression in inhibitory motor neurons, sensory neurons) | Htr6 (low expression in inhibitory motor neurons, sensory neurons) | Function not determined | |||
| Spinal afferent | Htr6 | Function not determined | |||||
| 5-HT7 | Myenteric neuron | HTR7 (excitatory motor neurons) | Htr7 (low expression in sensory neurons, interneurons) | Htr7 (low expression in excitatory motor neurons, sensory neurons, interneurons) | Slow depolarization | (478, 479) | |
| Submucosal neuron | Function not determined | (479) | |||||
| Smooth muscle | Accommodation of circular muscle | (479) | |||||
| Immune cells | Promotes anti- inflammatory macrophage polarization; modulates the differentiation of dendritic cells from human monocytes; activation of dendritic cells. | (477, 480) | |||||
| Spinal afferent | Htr7 | Function not determined |
Another 5-HT receptor that has been described in the enteric nervous system is the 5-HT1P receptor. Features of this receptor include the activation of prolonged depolarizations when it is activated by administration of exogenous 5-HT and mediation of slow EPSPs (481). Furthermore, this receptor has a high affinity for [3H]-5-HT (481, 482), which was a criterion for the 5-HT1 class of 5-HT receptors before molecular characterization. The 5-HT1P receptor is rather enigmatic because efforts to clone it have so far failed. There have been suggestions that the 5-HT1P receptor might actually be the 5-HT4 receptor or the 5-HT7 receptor, but it appears to have distinct pharmacological properties (483). The existence of a distinct 5-HT1P receptor was supported recently by a study showing that a receptor with properties of the 5-HT1P receptor, but not 5-HT7, mediates prolonged excitation of human submucosal neurons (484). Taken together, there is ample evidence for the 5-HT1P to exist as a receptor based on classical binding and pharmacological studies using intracellular electrophysiology and calcium imaging techniques, and if this receptor does indeed exist it mediates prolonged excitation of enteric neurons. However, until molecular validation of this receptor is provided, the existence of the 5-HT1P entity as a distinct 5-HT receptor will remain in question.
The exact circumstances by which 5-HT release regulates propulsive motility have been subject to considerable debate, but there is now abundant evidence demonstrating that 5-HT is not necessary for the activation of propulsive colonic motility (reviewed in Ref. 485) but there is also strong evidence indicating that 5-HT released from enterochromaffin cells can activate motor responses, and this is supported by the clinical use of 5-HT4 agonists to treat constipation and 5-HT3 antagonists to treat diarrhea (486, 487). Release of 5-HT from enterochromaffin cells also activates secretory and vasodilatory reflexes involving the submucosal plexus (488, 489). Other potential actions of 5-HT in the gut include promoting neuronal regeneration and proliferation (198, 490–494), proliferation of interstitial cells of Cajal and epithelial cells (445, 446, 474), and both pro- and anti-inflammatory actions (432, 474, 495–497). Furthermore, gut-derived 5-HT is a negative regulator of bone density (498) and may contribute to bone loss in inflammatory bowel disease (499).
Other populations of enteroendocrine cells are important “taste cells” of the gut as well as being nutrient and metabolite chemosensors (413, 423–426). Much is known about how individual cells respond to components of the luminal environment and transduce signals. How they integrate their outputs to regulate digestive and defensive reflexes remains to be fully elucidated. However, remarkable progress has been made lately, as discussed above for intestinal L cells (sect. 4.2). Specifically, the use of molecular genetic and optogenetic approaches allows for cell-specific activation of individual components and the elucidation of the enteric neural pathways that integrate luminally triggered digestive and defensive reflexes.
5.2. Tuft Cells and Their Relationship to the Enteric Nervous System
A discrete chemosensory cell population in the intestines are tuft cells (410, 411, 427, 500). Tuft cells are characterized by their long microvillus tuft, a well-defined tubulovesicular system, and the expression of Dclk1 and the transcription factor POU class 2 homeobox 3. They express a variety of taste receptors, ion channels, G protein-coupled receptors, and the transduction apparatus involved in luminal sensing (FIGURE 9). These cells are particularly interesting, as they communicate with local nerves via the release of ACh, with immune cells via the release of IL-25, and likely with both via the release of a variety of eicosanoids (410, 500–502). Tuft cells play a key role in activating type 2 mucosal immune responses to rid the gut of helminth parasites as well as regulating epithelial cell proliferation after mucosal injury (501, 503–505). Tuft cells are near enteric nerves, notably those expressing CGRP (506). However, the extent to which these cells are involved in neural or glial communication in the gut remains to be determined.
5.3. Enteric Neural Control of Mucosal Growth and Proliferation, Secretion, and Epithelial Barrier Function
The intestinal epithelium is a selectively permeable barrier that facilitates the paracellular and transcellular movement of nutrients, water, and electrolytes (507–510). Intestinal epithelial cells have a rapid rate of turnover (2–4 days), and elaborate mechanisms ensure the continuous restitution of any breach of integrity under physiological conditions (511). The monolayer of polarized epithelial cells that forms a continuous interface along the gut is not completely impermeable since it is essential for a degree of antigen leakage to occur for immune education and the establishment of oral tolerance. However, it must be sufficiently tight to inhibit the unwanted passage of luminal toxins, antigens, pathogens, and commensal bacteria (507–509). Tight junctions regulate paracellular transport across the intestinal barrier (512, 513). The protein complexes that form tight junctions are located between adjacent epithelial cells and consist of the transmembrane proteins occludin, claudin, junctional adhesion molecule, and tricellulin (514–518). The intracellular domains of the transmembrane proteins are anchored to the actin cytoskeleton by scaffold proteins, including zonula occludens 1 (ZO-1) (514). The regulation of tight junctions is a dynamic process that controls the paracellular permeability of electrolytes, nutrients, and water (507, 512). Transcellular permeability occurs via membrane transporters, ion channels, pumps, and vesicular mechanisms (507, 510).
Intestinal barrier function is maintained by the physical barriers of the epithelium, and the tight junctions between them, as well as an epithelial surface barrier consisting of mucus, antimicrobial peptides (i.e., defensins), trefoil factors, immunoglobulin A, water, and electrolytes that are secreted by epithelial cells (519–523). There is increasing evidence that the ENS is involved in the control of epithelial barrier function, as well as the regulation of mucosal growth and proliferation.
5.3.1. Enteric neural regulation of epithelial cell proliferation.
A vital part of epithelial barrier function is the constant renewal of the epithelium by proliferation of stem cells. The ENS plays a role in the regulation of epithelial proliferation and differentiation. We have already discussed the role of enteric glia in the maintenance of epithelial homeostasis (sect. 3.2.3). Enteric nerves also play a role in these processes.
It has been known for many years that vagotomy alters the morphology of the gastric mucosa (524), illustrating that the enteric innervation has either direct or indirect influences on the stem cell niche to regulate epithelial cell proliferation. Interestingly, ablation of the myenteric plexus with the detergent benzalkonium chloride markedly alters the morphology of the mucosa in the intrinsically denervated segment (525–527). When the myenteric plexus of the ileum is ablated, there is marked crypt cell proliferation altering the mucosal architecture to produce long fingerlike villi with an altered distribution of enteroendocrine cells (527, 528). The mediators of these effects remain to be determined, though VIP has been implicated in the regulation of epithelial cell and goblet cell proliferation (529, 530). Enteric nerves are also involved in the epithelial proliferative response to the enteroendocrine peptide GLP-2 that is released from L cells (531). The role of VIP in this response was examined in VIP knockout mice (532). Here, it was shown that GLP-2 significantly increased crypt cell proliferation and small bowel growth to comparable levels in animals with and without the VIP gene. These data suggest that other enteric neurotransmitters must also be involved in regulating epithelial cell proliferation, although which ones remains uncertain. Using a Transwell culture system, Puzan et al. (533) found that enteric neurons and glia cocultured with an epithelial cell monolayer improved the health of the epithelium by enhancing the organization of the tight junctions, thereby reducing epithelial permeability. ENS cocultures also led to enhanced differentiation of epithelial cells, increasing the proportion of enteroendocrine cells. Although the mediators of this response were not directly studied, it was notable that there was enhanced cytokine production (IL-10, transforming growth factor-β1, and macrophage inflammatory protein-2) when enteric neural cultures were added to the epithelial monolayers (533). Since enteric nerves and glia can synthesize and secrete cytokines, these may be produced by enteric neurons or enteric glia in response to the epithelial cues and may play a role in the enhanced epithelial barrier function.
One potential neuronal mediator of epithelial proliferation is 5-HT. Mucosal growth and proliferation of mucosal cells is greater in mice that lack the serotonin reuptake transporter SERT or wild-type mice given selective serotonin reuptake inhibitors (SSRIs) (444). Surprisingly, mucosal growth was also enhanced in mice lacking Tph1 that were given SSRI, an effect that was not seen in mice lacking Tph2, suggesting that the myenteric neurons mediate the proliferative effect of 5-HT on the mucosa. Since 5-HT expressing nerves do not innervate the mucosa, the effects were found to be indirect. The effect of 5-HT was mediated by 5-HT2A receptors on submucosal cholinergic neurons, which innervate the epithelium (444).
Serotonin can also influence epithelial proliferation via activation of the 5-HT4 receptor (474). When colonic epithelial 5-HT4 receptors are activated by enema administration of an agonist, there is an increase in the number of epithelial cells immunoreactive for the proliferation marker Ki67. Furthermore, proliferation is decreased by administration of a 5-HT4 antagonist, indicating that 5-HT4 receptor activation is involved in epithelial barrier homeostasis.
The cholinergic innervation of the stem cell niche mediates proliferation via M1 muscarinic receptors (534) and in the colon via M3 muscarinic receptors (535). In the mouse stomach, the two major sources of ACh are enteric nerves and Dclk1+ tuft cells (536). Cholinergic stimulation of gastric organoids with carbachol induces nerve growth factor expression in an M3-dependent manner. Nerve growth factor overexpression in the gastric epithelium expands the population of enteric nerves and promotes carcinogenesis (536). Ablation of Dclk1+ cells or blockade of nerve growth factor signaling inhibits epithelial proliferation and tumorigenesis.
In the colon, enteric serotonergic neurons also contribute to tumorigenesis by acting on colorectal cancer stem cells. Zhu et al. (441) found compelling evidence for the expression of Tph2 in the mouse ENS and documented a critical role of these neurons in the control of mucosal growth in the context of the gut microbiota. They showed that 5-HT produced by enteric neurons promotes stemness and tumorigenesis via subtypes of the 5-HT1 receptor (HTR1B, HTR1D, and HTR1F) in colorectal cancer stem cells. Mechanistically, the colorectal cancer-enriched microbiota metabolite isovalerate initiates Tph2 expression, leading to 5-HT production. 5-HT signaling is correlated with the severity of colorectal cancer, and blocking it inhibits the self-renewal of colorectal cancer stem cells and tumors. Together these studies reveal instances where the ENS contributes to pathophysiological conditions in the gut (536). The role of the ENS in the development and progression of GI cancers has recently been extensively reviewed (537).
5.3.2. Role of enteric nerves in the control of epithelial permeability.
Direct evidence for the role of enteric nerves in the regulation of intestinal permeability under physiological conditions is limited. Overman et al. (538) showed that the addition of TTX to preparations of the porcine ileum reduced mucosal to serosal flux of a 4-kDa macromolecular marker that passed through epithelial tight junctions. These data suggest that there is a tonic control of epithelial permeability by enteric nerves. Interestingly, TTX reduced mucosal mast cell degranulation, so it is not clear whether the neural effect on permeability is directly or indirectly mediated. These findings are similar to those obtained by Cameron and Perdue (539) in mouse jejunum. They showed that TTX reduced the baseline flux of horseradish peroxidase, another macromolecular permeability marker that is transcellularly transported. They obtained similar results with the muscarinic cholinergic antagonist atropine, suggesting a cholinergic regulation of transcellular transport. Consistent with this finding, the cholinergic agonist bethanechol stimulated an increase in the flux of horseradish peroxidase through activation of M3 receptors (539). A recently published study further examined the role of the ENS on the acute regulation of intestinal permeability in response to luminal nutrients (540). Here segments of jejunum and ileum from mice were mounted in Ussing chambers and permeability to 4-kDa FITC-dextran was recorded after mucosal stimulation with either nutrients or 5% Intralipid. Mucosal lipopolysaccharide (LPS) was also studied. Enteric neurons were inhibited with TTX or activated with veratridine. Neither TTX nor veratridine altered baseline epithelial permeability. Intralipid acutely reduced permeability to FITC-dextran, whereas, as expected, LPS increased it. TTX pretreatment abolished the effect of Intralipid and exacerbated the LPS-induced increase in permeability. This study showed that neither neuronal or enteric glial activity is required for the maintenance of baseline intestinal permeability; however, neuronal activity is essential for the acute regulation of intestinal permeability in response to luminal lipids and LPS. These findings reveal the sophistication of the neural regulation of this vital element of host defense.
Other studies that have examined the role of the ENS have looked at the effects of stress, since it is well known that stress increases intestinal permeability. Gareau et al. (541) demonstrated that horseradish peroxidase flux was greater in young rats subjected to maternal separation stress than in nonstressed control animals, and they provided strong evidence that the stress mediator corticotropin-releasing factor (CRF) acted via cholinergic nerves to regulate intestinal permeability. This enhanced permeability was reduced to below control levels with atropine and further reduced by the nicotinic receptor antagonist hexamethonium. These data support observations demonstrating a role for cholinergic and adrenergic nerves and mast cells in regulating CRF-induced enhanced permeability in the rat colon (542, 543). CRF-induced c-Fos activation in cholinergic neurons of the myenteric plexus supports these observations (544, 545). However, at least when injected intraperitoneally, CRF only seems to activate colonic myenteric, but not submucosal, neurons and not those in the small intestine or stomach (544). In other studies, Saunders et al. (546) also found a muscarinic cholinergic enhancement of permeability in stressed rat jejunum in which nicotinic receptor stimulation was not involved. Further evidence for a role of cholinergic nerves in the control of epithelial permeability comes from the investigation of bile acid enhancement of colonic permeability. Here, both nicotinic and muscarinic receptors were identified as contributing to the serosal to luminal flux of mannitol and urea in response to ligand activation of the G protein-coupled bile acid receptor GPBAR1 (aka TGR5) (547–549).
5.3.2.1. role of vasoactive intestinal peptide in the regulation of epithelial permeability and barrier function.
Vasoactive intestinal peptide is an important secretomotor transmitter extensively expressed throughout the intestinal mucosa (550). The secretomotor innervation is largely from a subset of submucosal neurons but in some regions such as the stomach may also come as a direct projection from the myenteric plexus (184, 551). Using a human coculture model, Neunlist et al. (552) showed that electrical field stimulation of submucosal neurons elicits a reduction in flux of paracellular permeability markers across a monolayer of epithelial cells. This effect is blocked by a VIP receptor antagonist and mimicked by application of VIP directly to the monolayer. This effect was determined to be due to an increased expression of the tight junction scaffolding protein ZO-1. This observation was further explored by Conlin et al. (553) in a model of colitis. They found that VIP could prevent the increase in mannitol flux observed in animals infected with the bacterium Citrobacter rodentium (553). In studies of isolated, gut pathogen-infected epithelial monolayers, VIP reduced the redistribution of tight junction proteins by preventing an increase in the myosin light chain kinase expression and the phosphorylation of myosin light chain (553). Building on these observations Wu et al. (554) used VIP knockout mice to evaluate the role of VIP in chemically induced colitis. VIP knockout mice display distorted colonic crypts, defects in epithelial cell proliferation and migration, increased apoptosis, and altered permeability. Chemically induced colitis was more severe in VIP knockout mice than in wild-type animals. Treatment with VIP rescued the phenotype, protecting knockout mice against DSS colitis. These data suggest that VIP plays a vital role in the development and maintenance of colonic epithelial barrier integrity and promotes epithelial repair and homeostasis during colitis. The role of VIP has also been assessed in low-birth-weight neonatal piglets fed various diets (555). In these animals, VIP reduced epithelial permeability in the jejunum but had no effect in the ileum, and its effects were not observed when animals were on a high-protein diet.
In contrast to the findings that VIP reduces intestinal permeability, a study in rat and human tissues found that acute, stress-induced increase in bacterial uptake in rat ileal mucosa was prevented by injection of a VIP receptor antagonist (or a mast cell stabilizer) (556). Consistent with these results, VIP stimulated permeability and increased bacterial translocation (556). In this study, VIP receptors were found to be localized on mast cells, supporting the functional observations. Similar findings were made in the human ileum but interestingly not the human colon (556). It may be that species differences account for these data, but it is clear that further studies are required to completely ascertain the role of VIP in different regions of the gut under both physiological and pathophysiological conditions. VIP also regulates aspects of intestinal immunity and microbiota homeostasis, which we discuss in sect. 5.4.
5.3.3. Enteric neural control of secretion.
Fluid secretion is an important component of epithelial function (507, 522). For example, acid is secreted in the stomach for digestion but also to sterilize food, and this is controlled by both the intrinsic and extrinsic innervation of the gut. Specialized glands in the duodenal submucosa (Brünner’s glands) are able to sustain high levels of bicarbonate secretion to protect it from gastric acid and pepsin (557). Similarly, along the intestine bursts of secretion, regulated largely by enteric cholinergic and VIP-expressing nerves, protect the mucosa from physical damage in response to mechanical stimulation, and the fluid itself serves to dilute potential cytotoxins (522, 558).
The regulation of secretion is largely accomplished by neurons in the submucosal or inner submucosal plexus of larger animals and humans, as noted above. The two major subtypes of secretomotor neurons are distinguished by their expression of ACh or VIP: cholinergic or noncholinergic secretomotor neurons (52, 184, 559, 560). It should be noted that many of these neurons also regulate vasodilatation, which occurs in parallel to fluid secretion (52, 146). Secretomotor reflex circuits are generally localized to the site of the stimulus, i.e., are short local reflexes, (e.g., see Ref. 561). However, there is also evidence for secretomotor reflex pathways that involve neurons in the myenteric plexus (138, 562). Secretomotor reflexes are stimulated by mucosal distortion/distension elicited from the lumen of the gut (563–566). They can also be evoked by luminal nutrients (567, 568) and toxins (569, 570).
In the stomach, acid secretion is controlled by vagal cholinergic and enteric neuronal pathways (involving both ACh and GRP) that regulate an initial cephalic phase of acid secretion, followed by a sustained secretion of acid during the gastric phase for digestive and antimicrobial functions (571). Acid stimulates blood flow required to sustain secretion (which requires considerable energy) and provides a mechanism for clearance of toxic metabolites. The local vasodilatation is regulated by the release of CGRP from primary afferent nerves by activation of TRPV1 receptors (by protons) and by nitric oxide from the endothelium (382, 572–574). CGRP is also a negative-feedback signal for acid secretion. It accomplishes this by releasing somatostatin from D cells of the gastric epithelium (571). However, this protective mechanism can be commandeered under pathophysiological conditions. One mechanism used by Helicobacter pylori hijacks these physiological gastroprotective mechanisms. Zaki et al. (575) demonstrated that H. pylori activates capsaicin-sensitive primary afferents to release CGRP, which by releasing somatostatin inhibits histamine secretion from enterochromaffin-like cells and so reduces acid secretion, allowing bacteria to survive in the stomach.
In the small intestine, bicarbonate secretion from Brünner’s glands in the duodenum is important for the neutralization of gastric acid. Cholinergic and noncholinergic (VIP) enteric nerves regulate bicarbonate secretion (576, 577), and Brünner’s glands are richly innervated by VIP and other nerves (578, 579). However, Moore et al. (557, 580), using a novel in vitro model in which the Brünner’s glands were directly visualized, concluded that, in the guinea pig, Brünner’s glands are innervated by cholinergic vagal efferent fibers but apparently not by submucosal secretomotor nerves. Nevertheless, it is clear that there is a functional enteric innervation, the details of which still require complete elucidation.
Epithelial ion transport in the small and large intestine is regulated by the enteric neural pathways discussed above. Interestingly, in addition to direct actions on the epithelium, there is considerable evidence that mucosal mast cells are targets of the enteric innervation and contribute to electrogenic ion transport and fluid secretion that accompanies it (510, 581–583). In a recent study, Buhner et al. (584) used calcium imaging of nerve-mast cell signaling in the human intestine and found that roughly one-third of the mast cells in a given field of view responded with a sharp rise in intracellular calcium in response to stimulation of submucosal neurons. The mast cell response was abolished by TTX and reduced by antagonists of VIP and CGRP but interestingly not substance P, which activates mast cells in the mouse small intestine (585). Also in this study, the authors showed that only a few submucosal neurons responded to mast cell degranulation, which releases a multitude of mast cell mediators that can be neuroactive (584). In contrast, in the guinea pig small intestine degranulation of mast cells readily excites submucosal neurons and reduces their noradrenergic inhibitory inputs (586), Although these studies reveal additional interesting species differences, they also highlight the conserved nature of nerve-mast cell communication in the intestine. Nerve-mast cell relationships in the control of barrier function are important in pathophysiological conditions, including stress and food allergy, and may involve both submucosal and myenteric neural pathways (587, 588).
5.3.3.1. mucus, trefoil factors, defensins, and secretory iga.
The secretion of mucus from goblet cells that are distributed along the length of the gut serves a vital role as a lubricant for the passage of luminal contents and for protection against bacteria and toxins (519, 589, 590). Mucins and trefoil factors secreted by goblet cells enhance barrier function and promote epithelial restitution and repair. In the small intestine, Paneth cells provide an additional level of host defense through the secretion of antimicrobial peptides called defensins (521, 591, 592). The secretion of polymeric IgA from the epithelium to the gut lumen also contributes an immunological barrier (593). The degree to which each of these secretory components is under neural control varies, but few studies have investigated them in a systematic manner. In all cases, cholinergic control is exercised through muscarinic receptor mechanisms, and where it has been examined VIP also seems to be prosecretory (594–598). These data are consistent with the known pattern of innervation of the mucosa from submucosal neurons. The vagus plays an important role in the tonic secretion of gastric mucus (599) but does not seem to have such a role in the jejunum (263). Sympathetic mechanisms or stress increases the secretion of IgA and mucin (594, 595, 598), although it has been reported that norepinephrine inhibits the secretion of IgA (600). Hoffman et al. (470) investigated the distribution of 5-HT4 receptors along the GI tract and found they were present on goblet cells (and enterocytes). When 5-HT4 agonists were added to the gut lumen they caused cavitation of goblet cells, an indicator of mucin secretion. These observations extend earlier work in which 5-HT4 receptor mechanisms were shown to be involved in cholera toxin-induced mucin secretion (476). Together, these data support the concept that local activation of mucosal nerves and enterochromaffin cells serve as important defense mechanisms in response to potentially injurious luminal factors.
5.4. Neuroimmune Interactions in the Regulation of Mucosal Immunity and Homeostasis
The gut-associated lymphoid tissues and diffuse immune systems of the GI tract receive extensive innervation (601–606). Nerves in the wall of the gut respond to threats as an alarm system and serve as effectors contributing to a reinforcement of barrier function (22, 23). Much of the focus has been on the role of the extrinsic nerves. Vagal afferents are activated by local immune and inflammatory stimuli, as well as enteroendocrine mediators, and initiate vagal reflexes including the vagal anti-inflammatory reflex (607–609). Spinal afferents express a variety of receptors including TRPV1 and PAR2 receptors, allowing them to respond to inflammatory mediators and noxious chemical mediators and respond with the release of peptides such as substance P and CGRP that locally regulate barrier and immune function and blood flow (21, 381, 382, 610, 611). This gives rise to neurogenic inflammation, an acute, protective response to injury involving resident mast cells and other leukocytes.
Nevertheless, the ENS plays a significant role in the regulation of immune function as it relates to barrier functions of the gut. This topic has been reviewed recently (13, 16, 22, 23, 255, 256), so we highlight the role of the ENS with some recent examples of studies that reveal novel mechanisms of integrated host defense by which enteric neurotransmitters regulate barrier function. As is the case for other elements of host defense, there are few systematic studies that have examined the role of endogenously released enteric neurotransmitters on specific immune functions in the gut. In part this was because it is hard to selectively activate enteric neurons physiologically in vivo or in ex vivo preparations. However, with the advent of molecular genetic approaches (85, 422, 612, 613), these studies are now far more feasible and, in the future, likely to be undertaken.
As discussed above, secretomotor neurons expressing VIP serve as an important regulator of ion transport and have a key role in the control of barrier function. Three recent, somewhat controversial, discoveries have shed light on interesting mechanisms by which VIP promotes defense against enteric pathogens and inflammatory injury by modulating the activity of group 3 innate lymphoid cells (ILC3 cells) (614–616). Seillet et al. (616) showed that the circadian pattern of production of the cytoprotective cytokine IL-22 in ILC3 cells in the mouse small intestine can be modulated by food intake, independent of the gut microbiome; elevated levels of IL-22 in ILC3 are synchronized with increased food intake in the dark phase of the circadian cycle. To investigate the mechanism of this regulatory control, the authors performed single-cell RNA sequencing of the ILC3 cells and noted the expression of VPAC2, one of the receptors for VIP in these cells, and they found that ILC3s in cryptopatches and isolated lymphoid follicles were in close association with VIP-immunoreactive nerves and the expression of VIP in submucosal neurons was regulated by feeding (616). VIP was shown to regulate IL-22 production in vitro and in vivo, and this was regulated by VPAC2. Finally, they showed that VPAC2 knockout mice treated with DSS had more severe intestinal inflammation. Together, these data reveal an enteric neuronal VIP-VPAC2 ILC3 signaling pathway that is important for the dynamic control of IL-22 production in the gut and that increased protection of the epithelial barrier is linked to feeding, when the luminal content of the gut is greatest.
An orchestrated homeostatic neuroimmune response was also examined by Talbot et al. (615), who found virtually the opposite results: that the control of IL-22 by VPAC2 receptors was reduced by their activation and that activation of VIP neurons by chemogenetics led to greater bacterial translocation and reduced survival of mice infected with the enteropathogen Citrobacter rodentium. However, like Seillet et al., they found that feeding regulated VIP expression but that it was associated with reduced IL-22 production. At this point it is unclear why these studies have such disparate results.
The third study examining the role of VIP in neuroimmune regulation in the gut was conducted by Yu et al. (614). In this study, these authors showed that VIP promoted ILC3 recruitment to the intestine through the other VIP receptor, VPAC1, that was independent of the gut microbiota or adaptive immunity. They demonstrated that mice deficient in VIP or VPAC1 have reduced numbers of intestinal ILC3s and correspondingly impaired production of IL-22, which, as Seillet et al. observed, rendered them more susceptible to intestinal inflammation caused by C. rodentium (614). These findings suggest that VIP regulates the recruitment of intestinal ILC3s and formation of postnatal intestinal lymphoid tissues that protect the gut against enteric pathogens like C. rodentium (614). Why this study found VPAC1 and not VPAC2 signaling mechanisms also remains to be determined. The state-of-the-art approaches used in these three studies pave the way for further examination of the role of not only VIP but other enteric transmitters in the neuroimmune control of epithelial barrier function, and they also highlight the need for additional work in this area. Moreover, as discussed in sect. 3.2.4 ILC3 cells are also regulated by enteric glia (284), so development of an integrated model that explains the neuroimmune regulation of barrier function needs to consider all the elements of the ENS.
Vasoactive intestinal peptide is not only involved in the regulation of mucosal immunity; it also regulates microbiota homeostasis. By combining transcriptomics, chemogenetics, and the coculture of enteric neuron-intestinal organoids, Lei et al. (617) showed that VIP-expressing neurons regulate fucosylation (fut2 expression) of intestinal epithelial cells via VPAC1 (VIPR1) through activation of the Erk1/2-c-Fos pathways. They demonstrated that perturbation of enteric neurons leads to gut dysbiosis with an imbalance between beneficial Bifidobacterium and the opportunistic pathogen Enterococcus faecalis. This resulted in increased susceptibility to alcohol-associated liver disease (617). This study illustrates another, previously unknown, mechanism by which the ENS regulates intestinal homeostasis.
Intrinsic primary afferent neurons of the ENS are a subpopulation of cholinergic neurons characterized by the expression of NMU, as discussed in sect. 3.1.2. These neurons, in addition to having a sensory function, also serve in immune regulation in the intestine, for which they are well positioned as they send projections to the lamina propria. The close association of enteric nerves with innate immune cells in the intestinal mucosa led Klose et al. (618) and Cardoso et al. (619) to investigate communication between enteric nerves and group 2 innate lymphoid cells (ILC2 cells). ILC2 cells produce type 2 cytokines, which are important in host defense against intestinal helminths, such as ringworms and roundworms, and promote tissue repair (620). They found that the most differentially expressed gene for neurotransmitter ligands was Nmu1, the receptor for NMU, and that this was selectively expressed in ILC2 cells (618, 619). Neuromedin U nerves were in close proximity to ILC2 cells, and NMU stimulated the production of type 2 cytokines. They then investigated the role of NMU in immunity to nematode parasites (roundworms) and showed that it enhanced the immune response to the worms and promoted their clearance. Cardoso et al. (619) also revealed that the intrinsic primary afferents responded to the worm products and alarmin (IL-33) released by the worm through MYD88-dependent activation of Nmu gene expression. Tsou et al. (621) recently extended these observations, showing that that NMU stimulates colonic ILC2s to produce amphiregulin. Amphiregulin is a member of the epidermal growth factor family and is important for the control of barrier function and promoting immunity to infection. Interestingly, they showed that ILC2s were the critical source of amphiregulin that could not be compensated for by other sources, including CD4+ T cells, illustrating that these neuroimmune interactions have nonredundant protective functions (621). Together, these data reveal a previously unknown relationship between the ENS and innate immune cells that coordinates a response to accelerate the expulsion of enteric pathogens.
Although NMU activates ILC2 cells, the factors regulating their activation remained less well understood. Two groups discovered that another peptide expressed by intrinsic primary afferent neurons, CGRP, was a negative regulator of ILC2 cells (622, 623). Here it was shown that CGRP differentially modulates type 2 cytokine production via the CGRP receptor when ILC2 cells are stimulated by NMU and IL-33, reducing IL-13 production but promoting the production of IL-5. This results in the stimulation of eosinophilia (to rid the body of the parasite) while limiting fibrosis which is stimulated by IL-13 (622, 623). It remains to be determined whether there is a differential release of the two peptides (CGRP and NMU) that allows for effective regulation of ILC2 cells or whether there are other mechanisms that effectively allow for the spatial and/or temporal discrimination of these signals by ILC2 cells.
These examples reveal the intricacy and sophistication of enteric neuroimmune interactions, and although we are learning more about the complexities of host defense, there is much further work required to fully elucidate this complex aspect of enteric physiology. One of the big challenges that remains is to understand the regulatory functions of the ENS that restore homeostasis when immune interactions lead to a breakdown in host defense.
6. NEUROPLASTICITY IN THE ENTERIC NERVOUS SYSTEM
One of the most remarkable features of even simple nervous systems is their inherent plasticity, that is, their ability to respond and adapt to a variety of conditions, to “learn” and “remember,” and to respond to a changing environment to optimize neural connectivity and behavior. Of course, plastic changes can also be maladaptive, leading to instabilities in neural networks and pathophysiological states. In the ENS, we see examples of adaptive changes that maintain the integrity of the ENS after injury. However, other forms of neuroplasticity appear to compromise normal function and may explain the symptoms of the GI disorders in which they occur.
The ENS in humans and animals demonstrates remarkable functional and structural plasticity under a wide variety of conditions in health and disease. This includes changes to the properties of enteric glia and neurons and changes to the expression of neurotransmitters in different subsets of enteric neurons. Under physiological conditions, there are alterations to transcriptional activity in enteric neurons due to circadian rhythms and with aging (72, 624). There is neurochemical and phenotypic plasticity in response to diet (625–629) and after treatment with probiotics (213, 216). These changes include loss of neurons in response to a high-fat diet, with NOS neurons being particularly affected, as well as changes to receptor sensitivity (e.g., enhanced nicotinic responsivity in mice fed a high-fat diet) and other functional alterations to ENS signaling (625–629). Enteric neurons of the guinea pig display enhanced excitability following repetitive action potential generation, and mouse myenteric neurons display enhanced activity following intracellular depolarization (630–632). Guinea pig myenteric and submucosal neurons have altered synaptic inputs (S neurons) and become hyperexcitable (AH neurons) in intestinal inflammation (451, 633–641).
When subjected to a physical or chemical lesion, the ENS can respond and reinnervate a denervated segment of gut. For example, after a complete transection and reanastomosis of the guinea pig ileum, descending axons of myenteric VIP, GRP, and somatostatin neurons were able to regrow across the anastomosis within ∼2 mo after the lesion, which was also the time it took for a functional recovery of the migrating myoelectric complex (642).
Neurogenesis is also a feature of ENS plasticity. Chemical lesion of a segment of mouse colon with the detergent benzalkonium chloride led to complete ablation of myenteric ganglia. Within 7 days nerve fibers were observed, and within ∼2 mo nerves extensively reinnervated the region that was ablated. Only occasional neurons were observed in the ablated region of the gut, but their presence suggested the potential for adult enteric neurogenesis (643). More recent studies have revealed adult enteric neurogenesis in the mouse ileum after treatment with benzalkonium chloride (278) and in the colon in mouse models of colitis (279, 493). Neurogenesis in adult animals has also been observed under physiological conditions in the mouse intestine after treatment with a 5-HT4 receptor agonist (490) and in the mouse ileum (644) and in rat and guinea pig rectum (494, 645) after an anastomosis and treatment with a 5-HT4 receptor agonist. It is also observed in mice following microbial recolonization after treatment of adult animals with antibiotics or in germ-free mice treated with TLR2 agonists (209, 211).
The known molecular mediators of neuroplasticity in the ENS include changes in cAMP signaling (646), adenosine (647), prostaglandins (648–650), cytokines (651), protease-activated receptors (356, 652, 653), mast cell mediators (652, 654, 655), and neurotrophic factors (656–658). Recent studies have also shown that the microbial products and/or alterations in the composition of the gut microbiota give rise to marked changes in neuronal activity and the composition of neuronal subsets in the ENS (198, 200–204, 211, 214, 243, 244, 337).
Plasticity is not restricted to enteric neurons; enteric glia also exhibit plasticity with alterations in glial populations in response to changes in the composition of the gut microbiota (199, 211), diet (290, 659), reactive gliosis in response to inflammation (242, 660), and changes in enteric glial function observed in early-life adversity (661). There is some evidence to suggest that there may be sex-dependent differences in the ENS (289, 662), but currently few studies have examined this in detail.
In the CNS, learning and memory are regarded as one of the most important forms of neuroplasticity. Schemann and colleagues (663) recently reviewed this topic in the context of the ENS, asking: How smart is the gut? Although they identified many open questions and fascinating areas for future study, based on the current literature, including most of the examples given above and others, they concluded that the ENS displays evidence of learning and memory, conditioning, long-term potentiation, and adaptation (663). These findings further support the concept of the “brain in the gut” and illustrate the capacity of the ENS to provide the complex integrated outputs needed to control the motor, secretory, vasomotor, and defensive functions of the gut under an incredibly wide variety of conditions.
6.1. Neuroplasticity in AH Neurons
The best-studied examples of neuroplasticity in defined enteric neuronal populations are related to the properties of AH neurons, intrinsic primary afferent neurons of the myenteric and submucosal plexuses. Because AH neurons project to the lamina propria of the intestine, they are directly exposed to luminal signaling molecules in the gut and are susceptible to pathophysiological changes that are centered in the gut mucosa, such as intestinal inflammation (FIGURE 10).
Figure 10.
The elements of secretomotor and peristaltic reflex circuits in the colon and the impact of colitis at specific sites of this circuitry. Chemical or mechanical stimuli applied to the mucosa results in the release of serotonin (5-HT) from enterochromaffin cells in the epithelium. This in turn activates intrinsic primary afferent neurons that send projections into the submucosal and myenteric plexuses. These activate secretomotor reflexes in the submucosal plexus and the 2 limbs of the peristaltic reflex in the myenteric plexus. Oral to the stimulus interneurons synapse with and activate excitatory motor neurons to trigger smooth muscle contractions, whereas anal to the stimulus interneurons synapse with and activate inhibitory motor neurons to trigger relaxation of smooth muscle. Colitis is characterized by increased 5-HT availability in the lamina propria, increased excitability of intrinsic primary afferent neurons, facilitation of interneuronal synaptic activity, decreased purinergic neuromuscular transmission, loss of myenteric neurons, and reactive gliosis in myenteric enteric glia. Colitis is also associated with altered patterns of gene expression in the enteric nervous system (196). Collectively, these plastic changes in the enteric neural circuitry alter secretomotor and motor control in the gut during active periods of colitis, and many of them persist after the resolution of colitis. See text for details. Adapted in part from Ref. 664. Image created with BioRender.com, with permission.
By virtue of the complement of receptors and ion channels that regulate their excitability (111, 114, 115), AH neurons are capable of markedly altered states of excitability. For example, after prolonged periods of low-frequency (1 Hz) synaptic activation, AH neurons in myenteric ganglia of the guinea pig ileum exhibit a slowly developing, sustained increase in excitability associated with depolarization and increased input resistance. This increased excitability lasts for many hours after stimulation and is due to phosphorylation of the calcium-activated potassium channels that are responsible for the late AH, which normally limits the excitability of these neurons (631, 632). This is an example of long-term potentiation in the ENS. In the brain, long-term potentiation depends in part on the release of activity-dependent neurotransmitters in a retrograde manner from postsynaptic neurons that act on presynaptic terminals to provide feedback regulation of synaptic input to these cells (665). It remains to be determined whether retrograde transmission is a feature of AH neurons, as this has not been examined. However, Hons et al. (630) demonstrated a novel form of metaplasticity in the mouse myenteric plexus through the balance of endocannabinoid and purinergic signaling at enteric synapses. A purinergic signal is involved in synaptic regulation by increasing transmitter release in an activity-dependent manner, a feature revealed in the absence of cannabinoid (CB)1 receptors. CB1 receptors modulate cholinergic transmission in myenteric neurons through presynaptic mechanisms: activity-independent inhibition of synapses and activity-dependent inhibition of neurotransmitter release (630).
The excitability of AH neurons is enhanced by slow excitatory synaptic inputs. Slow EPSPs in AH neurons can be generated by several different neurotransmitters (e.g., substance P, 5-HT, VIP, CGRP) and enteroendocrine cell transmitters (e.g., 5-HT, CCK) that act via adenylyl cyclase-protein kinase A or phospholipase C-diacylglycerol lipase-protein kinase C pathways. Slow EPSPs can also be elicited by the paracrine release of immune mediators such as histamine, cysteinyl leukotrienes, serine proteases, IL-1β, and IL-6, as shown by Wood and colleagues, that also activate these intracellular signaling cascades (651, 653, 666, 667). Thus, AH cells are poised to integrate environmental, immune, and neural inputs, and their outputs alter the gain in enteric reflex circuitry.
In addition to regulating the synaptic properties of AH cells, their membrane properties are also subject to modulation. Studies of inflammation-induced changes to enteric neurons have consistently demonstrated alterations in the electrical properties of AH neurons. Whether in the myenteric (633–636, 639, 649, 668) or submucosal (451, 586, 634, 654, 655) plexus, jejunum (668), ileum (586, 639), or colon (451, 633–636, 649, 654, 655) or via different inflammatory mechanisms [Trichinella spiralis-induced enteritis, trinitrobenzene sulfonic acid (TNBS) colitis or β-lactoglobulin-sensitized guinea pigs exposed to the milk protein], AH neuron excitability is consistently enhanced.
The mechanisms responsible for inflammation-induced AH neuron hyperexcitability vary depending on the location and type of inflammatory response or the inputs these cells receive. The most consistent feature of inflammation-induced neuroplasticity in AH neurons is a reduction in the magnitude of the long afterhyperpolarization that limits the excitability of these cells. In T. spiralis enteritis, the AH neurons exhibit a lower input resistance and their membrane potential is depolarized (668). These features, along with the suppressed late AH, indicate that a reduction in the activity of the intermediate-conductance Ca2+-activated K+ channel (IKCa) is involved in the increase in excitability in this condition. In TNBS colitis, a major contributor to the hyperexcitability in AH neurons is an increase in a hyperpolarization-activated cation conductance (Ih, due to hyperpolarization-activated cyclic nucleotide-gated channels), with no change in the IKCa (633). The impact of these changes in excitability in colitis is considerable. The heighted excitability of these cells leads to the generation of spontaneous action potentials in ∼50% of the neurons in the inflamed colon (633). This leads to the activation of ascending and descending signals arising from multiple regions in an inflamed segment of bowel simultaneously, which leads to a form of intestinal fibrillation (664), and a failure of normal propulsive motility. The inflammatory mediators responsible for the increased AH excitability include prostaglandins derived from cyclooxygenase 2, prostaglandin E2, mast cell tryptase, and PAR2 activation (356, 635, 648, 649, 652).
There is strong evidence indicating that the inflammation-induced changes in AH neuron excitability can result in disrupted propulsive motor activity (638, 649). In the normal colon, the velocity of propulsive motility is significantly decreased when IKCa channels are inhibited, resulting in an increase in AH neuron excitability (638). The rate of colonic motility is significantly higher in TNBS-inflamed colons from animals in which cyclooxygenase 2 is inhibited to prevent the inflammation-induced hyperexcitability of AH neurons (649). Consistent with this, propulsive motility is also improved in TNBS-inflamed colons after application of hyperpolarization-activated cyclic nucleotide-gated channel blockers to inhibit the Ih in AH neurons, thus dampening their excitability (638) (FIGURE 10).
Taken together, these findings underscore the delicate balance of enteric neural circuits and the central role that AH neurons play in the regulation of network activity by virtue of their anatomical arrangement in the ENS and their ability to integrate a variety of signals. Interestingly, the inflammation-induced changes in AH neuron excitability and motility persist beyond recovery of inflammation (634, 635), indicating that plasticity of these neurons could be a contributing factor in functional GI disorders.
6.2. The Influence of the Gut Microbiome on the Neuronal Excitability of AH Neurons
In addition to being regulated by enteroendocrine peptides and 5-HT, immune/inflammatory mechanisms, and neurotransmitters, the excitability of AH neurons can also be influenced by the gut microbiome and microbial mediators (FIGURE 5). In a series of studies, Kunze and colleagues revealed that IKCa channels are the molecular target of microbial regulation of AH neuron excitability in both rats and mice (200, 203, 214, 216, 243, 244, 669). Recordings of AH cell excitability made from jejunal myenteric neurons in germ-free mice revealed that they were relatively unexcitable and had a prolonged late AH (203). Addition of the IKCa channel blocker TRAM-34 to specific pathogen-free mice resulted in a depolarized resting membrane potential and reduction in the amplitude of the slow AH, effects that were almost absent in germ-free mice (200). These data reveal that the function of IKCa channels is regulated by the presence of gut microbes. To investigate the regulation of AH neuronal excitability further, a variety of different microbes and their mediators were examined. The probiotic bacteria Lactobacillus reuteri, Lactobacillus rhamnosus (JB-1), and Bacteroides fragilis selectively increased the excitability of AH neurons through a decreased late AH caused by a reduction in IKCa channel expression, leading to alterations in motility (214, 243, 669). In contrast, B. longum conditioned medium reduced the excitability of AH neurons compared with unconditioned medium (216). Mao et al. (214) examined the mechanisms by which L. rhamnosus (JB-1) and B. fragilis caused their effects, using a compartmentalized organ bath. They found that when action potential generation was blocked in the epithelial compartment where the bacteria were added, there was no increase in the excitability of AH neurons. These data suggest that luminal bacteria interact with cells in the epithelium (presumably enteroendocrine cells) that then regulate the activity of the AH neurons.
6.3. Regeneration and Neurogenesis in the Adult Enteric Nervous System
Since the ENS is essential for life, having the ability to regenerate when injured would seem to be an important evolutionary adaptation. Thus, when it was recognized that the ENS could make functional reconnections after a physical or chemical lesion (642, 643), this suggested the presence of progenitor/stem cells that could repopulate a portion of the damaged neural network. Subsequent investigations to understand the nature of adult enteric nervous system neurogenesis have revealed that enteric glia have a neurogenic potential and are able to generate new neurons in response to injury and inflammation (278, 279, 493, 670). This response appears to be due to the transdifferentiation of enteric glia (493), rather than by mitosis and cell division, since there is little or no incorporation of the thymidine analog bromodeoxyuridine into adult enteric neurons after injury (98, 99). The glial populations that provide the reserve capacity of the adult ENS have been characterized and include Dclk1-positive glial cells and those that express Sox2 and nestin (91, 280, 670, 671).
6.4. Role of 5-HT in Adult Enteric Neurogenesis
The molecular mediators and mechanisms of adult enteric neurogenesis are becoming better understood, though a full appreciation based on systematic studies is currently lacking, especially regarding the extent of neurogenesis (or not) in the submucosal plexus. Serotonin acting via 5-HT4 receptors appears to be a key signaling molecule that promotes neurogenesis in the adult ENS. Gershon and colleagues (490) were the first to demonstrate that administration of a 5-HT4 agonist stimulated neurogenesis in the myenteric plexus. Evidence that 5-HT4 agonists would promote neurogenesis that led to a functional recovery following surgical reanastomosis of the ileum and rectum substantiated the significance of these findings (494, 644, 645, 672–674). The intracellular mechanism of 5-HT4 signaling to promote neurogenesis and axon growth at an anastomosis involves the protein tyrosine kinase receptor Ret (675), the critical regulator of ENS development (676), but whether this is more widely the case is not known.
Evidence to support a physiological role of 5-HT in promoting enteric neurogenesis comes from the genetic deletion of SERT, the use of mice that express a hyperfunctional form of SERT, and mice that lack Tph2; all have abnormal numbers of enteric neurons (491, 677, 678). SERT knockout mice (and animals chronically treated with an SSRI) have a hyperplastic ENS, whereas mice expressing the hyperfunctional form of SERT have a hypoplastic ENS, as do mice that lack Tph2. Mice that express a decreased functional form of TPH2 also have a hypoplastic ENS, which can be rescued by a long-acting 5-hydroxytryptophan, which suggests that in adult mice 5-HT is active in promoting enteric neurogenesis (678). Taken together, these observations support the role of endogenous 5-HT as a physiological regulator of enteric neurogenesis. The studies discussed above also show that enteric neurons are the source of the 5-HT. Enterochromaffin cells are not the source, because the deletion of Tph1 does not mimic the deficiency of neurons seen in mice lacking Tph2 (677). Specific classes of enteric neuron, moreover, depend on neuronal serotonin for their development/survival, whereas other types of enteric neuron do not (677). Interestingly, however, Kawahara et al. (645) compared a 5-HT4 receptor agonist with an SSRI (which presumably raised endogenous levels of 5-HT by reducing its reuptake) and found that the SSRI treatment was ineffective at promoting regeneration of nerve fibers across an anastomosis or generating neural stem cells. Therefore, the conditions and extent to which endogenous 5-HT is a physiological regulator of enteric neurogenesis remain to be fully determined.
In adult germ-free mice the ENS is not fully developed, and intestinal transit is delayed relative to conventionally raised, SPF mice (198, 203, 228). The addition of a normal microbiota modifies the neuroanatomy of the ENS and increases neurogenesis, leading to the maturation of the ENS and the normalization of intestinal transit (198). De Vadder et al. (198) colonized germ-free Tph1−/− mice that lack mucosal 5-HT and found that neurogenesis in the ENS of these mice was significantly attenuated. In addition, they demonstrated that treatment of germ-free mice with a 5-HT4 receptor antagonist at the time of recolonization resulted in a decrease in the number of myenteric neurons and the number of new neurons that express nestin. They also noted that the 5-HT innervation of the myenteric plexus was reduced in germ-free mice but restored upon recolonization. Consistent with these findings, treatment of germ-free mice with a 5-HT4 receptor agonist was associated with faster intestinal transit and an increased number of enteric neurons (198). Together, these data suggest that colonization by the gut microbiota stimulates neuronal and mucosal 5-HT release and that 5-HT4 receptor activation contributes in a significant way to differentiation and maturation of enteric neurons.
In complementary findings, Belkind-Gerson et al. (493) showed that colitis induces adult enteric neurogenesis through a 5-HT4-dependent mechanism. After induction of DSS colitis in normal mice, they observed an increase in neurogenesis and the number of myenteric neurons. These effects were markedly attenuated by treatment with a 5-HT4 receptor antagonist. Interestingly, they also observed marked glial proliferation in the myenteric plexus of mice with colitis, and this too was attenuated by the 5-HT4 receptor antagonist (493). The source of 5-HT was not determined in this study.
Taken together, these studies highlight the importance of 5-HT in the integrity of the ENS, but they also reveal gaps in our understanding of the actual sources of 5-HT that mediate these effects and triggers for its release. Whether adult neurogenesis is regulated by other enteroendocrine amines or peptides or by neurotransmitters is not known, but it seems unlikely that this is the sole intercellular system for this purpose.
6.5. The Gut Microbiome and Neurogenesis in the Adult Enteric Nervous System
As noted above, the microbiota is important in regulating the numbers of enteric neurons and glia in the gut wall (198, 201, 211, 230). In adult mice treated with antibiotics to deplete the vast majority of intestinal bacteria, there is a loss of enteric neurons from the myenteric and submucosal plexuses (201, 211, 230). Nitrergic neurons are particularly vulnerable to the loss of microbial signaling (211, 230). The mechanism of enteric neurodegeneration after ablation of the gut microbiota by antibiotic treatment was discovered by Muller et al. (201). They showed that in antibiotic-treated mice death of enteric neurons was mediated by an inflammasome pathway involving caspase 11 and NOD-like receptor family pyrin domain containing 6 as key molecular mediators (201). Interestingly, feeding of mice with a high-fat Western diet, which causes marked gut microbial dysbiosis, led to dysmotility and loss of enteric neurons via neuronal pyroptosis mediated by caspase 11 (95). Mice with a genetic knockout of caspase 11 were protected from the effects of a Western diet. The effects of the Western diet on neurodegeneration were recapitulated in vitro by the TLR4 ligand LPS and the saturated fatty acid palmitate. Binding of LPS to TLR4 leads to the activation of nuclear factor kappa B signaling and the transcription of caspase 11, which leads to inflammasome activation and neuronal death (95). However, it remains unclear whether LPS gains access to the myenteric plexus in vivo. How the loss of LPS that occurs after bacterial depletion leads to inflammasome activation remains to be determined.
Importantly, when the microbiota are allowed to naturally recolonize the gut after antibiotic treatment the numbers of neurons recover to control levels in the ileum and colon, suggesting that the presence of the gut microbiota stimulates neurogenesis (201, 211). When examining microbe-related neurogenesis, Vicentini et al. (211) found evidence for Sox2-expressing neurons in the myenteric plexus but interestingly not in the submucosal plexus. Sox 2 has been shown previously to be a marker of enteric neurogenesis (279, 493, 671). These data therefore suggest that different mechanisms of neurogenesis occur in the different plexuses in the wall of the gut. The microbial mediators of this response were also investigated, and they found that short-chain fatty acids could rescue neuronal numbers in both enteric plexuses in the ileum and colon of antibiotic-treated mice. A potential mechanism is via the 5-HT4 receptor. Since short-chain fatty acids increase tryptophan hydroxylase 1 expression and levels of 5-HT in the gut (431, 679), and the 5-HT4 receptor has been implicated in neurogenesis (see above), it is plausible to hypothesize a link between the two.
In addition to 5-HT effects, TLR activation could contribute to the influence of microbes on ENS integrity. The TLR4 ligand LPS was found to be important for neuronal survival, as others have shown (230), but it could not rescue the neuronal loss after antibiotic treatment (211). A recent report has described the effects of TLR2 in rescuing neuronal loss after antibiotic treatment by administering lipoteichoic acid, a TLR2 agonist (209). These findings complement other work showing that TLR2 regulates the integrity of the ENS via glial cell line-derived growth factor-Ret signaling pathways (231). Thus, a balance of microbial factors is required for the integrity of the ENS: TLR4 signaling is important for neuronal survival, whereas TLR2 activation may be required for neurogenesis. Additional studies are needed to delineate the interplay between TLR2 and TLR4, as well as other microbial-derived ligands and their contributions to adult neurogenesis. The importance of these mechanisms of adult neurogenesis is underscored by studies showing that infection-induced neuronal loss can be reversed after reconstitution of the normal gut microbiota after antibiotic treatment (337). For further in-depth accounts of this topic, readers are directed to recent reviews (7, 9, 43, 250, 253).
7. FUTURE DIRECTIONS AND PERSPECTIVES
The gut differs from all other organs of the body by having an extensive intrinsic nervous system, the ENS. The assemblies of neurons that comprise the ENS are organized in circuits that control the functions of the gut in a region-specific manner and do so utilizing pattern generators modulated by sensory input as well as by reflex mechanisms. We now know that these neurons do not function independently. The basic neural circuits receive strong bidirectional influences from enteric glial cells, muscularis macrophages, and enteroendocrine cells. These cells respond to the plethora of digestive, microbial, and immune signals that are integral to the physiological roles of the gut: digestion and host defense. Moreover, the ENS utilizes two populations of interstitial cells to mediate its effects on smooth muscle.
The ENS is bidirectionally linked to the CNS via the autonomic and primary afferent innervation of the GI tract, forming essential neural components of the microbiota-gut-brain axis. Through this, the brain has indirect influence over gut functions and, to an extent not previously appreciated, the gut has a dramatic effect on the function of the brain. This is exemplified by the recognition that many neurological disorders are now considered to be disorders of the microbiota-gut-brain axis, including Parkinson’s disease, amyotrophic lateral sclerosis, multiple sclerosis, and Alzheimer’s disease (7, 24–26). Moreover, it has become clear that mood disorders are influenced by the microbiota-gut-brain axis (7). The ENS is not a relay of autonomic efferent outflow between the CNS and the effector tissues of the gut or a set of simple reflex pathways: multifunctional enteric neurons integrate both intrinsic and extrinsic neural and nonneural inputs to initiate their outputs that precisely control the functions of the gut in space and over time.
As we have outlined, the intrinsic primary afferent neurons of the ENS are especially well positioned to integrate luminal signals to initiate appropriate defensive and digestive reflexes because of their mucosal projections. As we learn more about these cells from single-cell sequencing studies, and through the use of molecular genetics that allow them to be selectively activated or inhibited, their functions will become even better understood. To this point we know very little about the intrinsic primary afferent neurons located in the submucosal plexus compared with what is known about those located in the myenteric plexus. Although we can expect many similarities in terms of the expression of marker genes and their products, we might expect the submucosal neurons to have unique molecular signatures aligned to their functional roles in the regulation of secretomotor and/or vasomotor reflexes, in regulation of the epithelium, or in host defense.
The vast area of the gut is protected by an equally extensive immune system whose roles are intimately linked to that of the ENS through reciprocal interactions that on one hand help direct immune responses to ensure the viability of the gut when it is threatened and on the other protect the ENS and help maintain its integrity. The ENS and immune systems are located beneath the epithelium, which contains rich and diverse populations of enteroendocrine and tuft cells. These cells are the sensory transducers of luminal signals, but their function is modulated by neural, paracrine, and immune signaling inputs that ultimately shape the secretory output of these cells.
The last 10–15 years have seen our understanding of the integrated actions of the ENS increase exponentially. Two factors have driven this accelerated pace of discovery. First, the recognition that the gut microbiome is an essential determinant of neural, immune, and endocrine homeostasis: It is interesting to note that reasonably influential reviews of the ENS from ∼10 years ago that recognized the interactions between the cellular elements of the ENS in the control of intestinal homeostasis did not mention the gut microbiome (29, 136, 267, 329). Only a few pioneering groups recognized its importance then (680–682), but nowadays it is widely recognized that the function of the ENS is closely tied to the presence of the gut microbiota. There remain many things that we do not know about microbial-enteric neural interactions in the gut. For example, much of the focus on the microbiome has been on understanding the role of bacteria, but fungi are also important. Fungal colonization promotes major shifts in bacterial microbiome ecology and has an independent effect on innate and adaptive immune systems (683), but their role in the regulation of the ENS remains poorly understood. Furthermore, we know little about specific bacterial species and the way they interact with the ENS, and many of the vast array of microbial metabolites have been poorly studied to this point. Studies making use of gnotobiotic mice and mice colonized with a stable microbiota of a defined composition will further advance our understanding of the role of gut bacteria in the regulation of the ENS.
Second, advances in technologies that have been applied to studies of the ENS, notably molecular genetics, imaging, and transgenics, have revolutionized our understanding of enteric neural circuitry. As we have reviewed, application of these technologies has provided investigators the unparalleled opportunity to perform deterministic analyses of the factors involved in enteric neural control. As these technologies are integrated into studies of ENS function we will learn even more about the functional circuitries of the ENS. For example, the first use of optogenetic actuators in studies of the ENS in 2017 by Stamp and colleagues (684) paved the way for recent advances that have shed new light (pun intended!) on the enteric and extrinsic control of colonic motility and the synaptic circuitry of the colon (370, 422, 685–688), small intestinal motility (613), the regulation of peristalsis (689), the regulation of colitis (371), and microbial regulation of enteric neural control (434). Similarly, genetically encoded calcium indicators and chemogenetic tools (DREADDs) have led to remarkable new insights into the regulation of enteric neural and glial circuitry (85, 690). These new tools, combined with the use of engineered viruses for the noninvasive delivery of defined genes to make cell-specific reporters, knockouts, optogenetic actuators, etc., will further revolutionize our understanding of the role of the ENS in shaping the gut environment (612, 691–693). We are poised to be able to take the single-cell RNA data on the composition of the ENS to make transgenic mice with neuronal or glial subtype-specific gene deletions and to use mouse transgenics to an even greater extent to reveal the functions of specific neuronal populations in the circuitry of the ENS. Major advances in imaging technology are also poised to offer new insights into the ENS circuitry. The application of clearing and tissue visualization combined with new microscopy modalities such as light-sheet microscopy to the ENS will allow for three-dimensional imaging without the need for sectioning or dissection. These approaches are beginning to be employed (694–699) and with broader application will allow for better understanding of the spatial interactions between cell types of the ENS and tissues and cells of the GI tract.
Important advances in biology have been made using model organisms. With the advances discussed above, mammalian model organisms, notably the mouse, remain at the forefront. However, there is great value to be gained by using the strengths of model organisms such as the zebrafish and Drosophila melanogaster. These are yielding very interesting findings and offer unique advantages by virtue of their biology (434, 700–703). One finding that is particularly intriguing is that in Drosophila the plasticity of enteric neurons was found to be important for reproductive success, because hormones functionally remodeled these neurons to allow for greater capacity for food intake (704). In mammals, we know relatively little about how the maternal ENS is regulated in pregnancy and lactation. In fact, despite the well-recognized sex differences in the expression of GI diseases that involve the ENS (705), limited advances have been made in understanding sex differences in the enteric neural control of homeostasis. However, more attention is being paid to the role of sex in studies of the ENS, and as shown by Rao and colleagues (289, 706), sex hormones play an important role in shaping the control of GI motility and sex differences are important features of enteric glial control of motility. Further work in this area will add considerably to a better understanding of the role of the ENS in males and females.
In conclusion, this review has discussed selected examples of the historical and current advances in the enteric neural control of gut homeostasis. The ENS is truly an integrative nervous system. In the last 5 years there have been remarkable new findings that have redefined the molecular architecture of the ENS, local immune control by the ENS, regulation of intestinal barrier function by enteric neurons and glia, organization and function of enteric motor circuits, neuroplasticity of the ENS, extrinsic autonomic neural control, the role of the gut microbiome in regulating the structure and function of the ENS, and luminal signaling mechanisms involving enteroendocrine cells. The ENS is often likened to a brain in the gut, and, as we have seen, it has many of the features of the brain, the most important of which includes an incredible degree of plasticity. To control the functions of the gut, the ENS is endowed with hierarchically organized “programs” (6, 707), which are constantly adapting to the environmental challenges imposed on the gut. Although our understanding of the neural circuitry of digestion is quite well developed, the neural circuits of mucosal defense are less well understood. With the rapid adoption of new advanced technologies, we anticipate that many new findings will emerge. An area of important future research will be to apply the new knowledge of the neural control of the gut outlined in this review to disease states. Our understanding of the pathophysiology of the ENS is advancing, but further efforts are required to define molecular targets in the ENS for the treatment of GI diseases.
GRANTS
Original work in the authors’ laboratories is supported by grants from the Canadian Institutes of Health Research (FDN148380 to K.A.S.) and the National Institutes of Health (NOA R21AT011203 and DK113800 to G.M.M).
DISCLOSURES
K.A.S. has provided scientific advice and assistance to Arena Pharmaceuticals and GW Pharmaceuticals, has served on a speaker bureau for Abbvie, and has received research support from Takeda Pharmaceuticals and Abalone, Inc. G.M.M. has received research support from Takeda Pharmaceuticals and is on the Scientific Advisory Board of Dignify Therapeutics.
AUTHOR CONTRIBUTIONS
K.A.S. and G.M.M. developed the concept of this review, reviewed the literature, wrote the drafts, edited and revised the manuscript, and approved the final manuscript for submission.
ACKNOWLEDGMENTS
We are grateful to Drs. Jaime Belkind-Gerson, Robert Heuckeroth, Ulrika Marklund, Kenton Sanders, and Estelle Spear for valuable advice and discussion. We thank Catherine MacNaughton (Sharkey lab) for the micrograph in FIGURE 2 and for editorial assistance, Sandra Sharkey for drawing FIGURE 2, TOP, and members of the Sharkey and Mawe laboratories for editorial comments.
GLOSSARY
- 5-HT
Serotonin
- α7nAChR
α7 Nicotinic acetylcholine receptors
- ACh
Acetylcholine
- AH
Afterhyperpolarization
- AHR
Aryl hydrocarbon receptor
- BDNF
Brain-derived neurotrophic factor
- CART
Cocaine- and amphetamine-related transcript
- CCK
Cholecystokinin
- CGRP
Calcitonin gene-related peptide
- ChAT
Cholineacetyltransferase
- CCL2
C-C chemokine family ligand 2
- CNS
Central nervous system
- CRF
Corticotropin-releasing factor
- CSF-1
Colony-stimulating factor-1
- Dclk1
Doublecortin-like kinase 1
- DREADDs
Designer receptors exclusively activated by designer drugs
- DSS
Dextran sodium sulfate
- ENS
Enteric nervous system
- EPSP
Excitatory postsynaptic potential
- GABA
Gamma aminobutyric acid
- GAL
Galanin
- GF
Germ free
- GFAP
Glial fibrillary acidic protein
- GFL
Glial cell line-derived neurotrophic factor family ligand
- GI
Gastrointestinal
- GLP-1
Glucagon-like peptide-1
- GRP
Gastrin-releasing peptide
- HSV-1
Herpes simplex virus type 1
- IBD
Inflammatory bowel disease
- ICC
Interstitial cell of Cajal
- ICC-DMP
Deep muscular interstitial cell of Cajal
- ICC-IM
Intramuscular interstitial cell of Cajal
- IFNγ
Interferon gamma
- IGLE
Intraganglionic laminar ending
- Ih
Hyperpolarization-activated cation conductance
- IJP
Inhibitory junction potential
- IKCa
Intermediate-conductance Ca2+-activated K+ channel
- IL
Interleukin
- ILC
Innate lymphoid cell
- LPS
Lipopolysaccharide
- NMU
Neuromedin U
- NOS
Nitric oxide synthase
- NPY
Neuropeptide Y
- PAR
Protease-activated receptor
- pCREB
Phosphorylated cAMP response element-binding protein
- PDGFRα
Platelet-derived growth factor receptor-α
- PLP-1
Proteolipid protein-1
- S
Synaptic
- SARS-CoV-2
Severe acute respiratory syndrome coronavirus 2
- SERT
Selective serotonin reuptake transporter
- SP
Substance P
- SPF
Specific pathogen free
- SSRI
Selective serotonin reuptake inhibitor
- TLR
Toll-like receptor
- TNBS
Trinitrobenzene sulfonic acid
- Top2a
DNA topoisomerase II alpha
- TPH
Tryptophan hydroxylase
- TRP
Transient receptor potential
- TRPA1
Transient receptor potential ankyrin A1
- TRPM3
Transient receptor potential channel M member 3
- TTX
Tetrodotoxin
- VIP
Vasoactive intestinal peptide
- Wnt
Wingless and Int-1
REFERENCES
- 1. Bäckhed F, Ley RE, Sonnenburg JL, Peterson DA, Gordon JI. Host-bacterial mutualism in the human intestine. Science 307: 1915–1920, 2005. doi: 10.1126/science.1104816. [DOI] [PubMed] [Google Scholar]
- 2. Lozupone CA, Stombaugh JI, Gordon JI, Jansson JK, Knight R. Diversity, stability and resilience of the human gut microbiota. Nature 489: 220–230, 2012. doi: 10.1038/nature11550. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Sommer F, Bäckhed F. The gut microbiota—masters of host development and physiology. Nat Rev Microbiol 11: 227–238, 2013. doi: 10.1038/nrmicro2974. [DOI] [PubMed] [Google Scholar]
- 4. Furness JB, Costa M. The Enteric Nervous System. Edinburgh: Churchill Livingstone, 1987. [Google Scholar]
- 5. Furness JB. The Enteric Nervous System. Oxford, UK: Blackwell, 2006. [Google Scholar]
- 6. Wood JD. Enteric nervous system: reflexes, pattern generators and motility. Curr Opin Gastroenterol 24: 149–158, 2008. doi: 10.1097/MOG.0b013e3282f56125. [DOI] [PubMed] [Google Scholar]
- 7. Margolis KG, Cryan JF, Mayer EA. The microbiota-gut-brain axis: from motility to mood. Gastroenterology 160: 1486–1501, 2021. doi: 10.1053/j.gastro.2020.10.066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Martin CR, Osadchiy V, Kalani A, Mayer EA. The brain-gut-microbiome axis. Cell Mol Gastroenterol Hepatol 6: 133–148, 2018. doi: 10.1016/j.jcmgh.2018.04.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Cryan JF, O’Riordan KJ, Cowan CS, Sandhu KV, Bastiaanssen TF, Boehme M, et al. The microbiota-gut-brain axis. Physiol Rev 99: 1877–2013, 2019. doi: 10.1152/physrev.00018.2018. [DOI] [PubMed] [Google Scholar]
- 10. Rhee SH, Pothoulakis C, Mayer EA. Principles and clinical implications of the brain-gut-enteric microbiota axis. Nat Rev Gastroenterol Hepatol 6: 306–314, 2009. doi: 10.1038/nrgastro.2009.35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Furness JB, Stebbing MJ. The first brain: species comparisons and evolutionary implications for the enteric and central nervous systems. Neurogastroenterol Motil 30: e13234, 2018. doi: 10.1111/nmo.13234. [DOI] [PubMed] [Google Scholar]
- 12. Gershon MD. The Second Brain. New York: Harper Collins, 1998. [Google Scholar]
- 13. Sharkey KA, Beck PL, McKay DM. Neuroimmunophysiology of the gut: advances and emerging concepts focusing on the epithelium. Nat Rev Gastroenterol Hepatol 15: 765–784, 2018. doi: 10.1038/s41575-018-0051-4. [DOI] [PubMed] [Google Scholar]
- 14. Veiga-Fernandes H, Pachnis V. Neuroimmune regulation during intestinal development and homeostasis. Nat Immunol 18: 116–122, 2017. doi: 10.1038/ni.3634. [DOI] [PubMed] [Google Scholar]
- 15. Veiga-Fernandes H, Mucida D. Neuro-immune interactions at barrier surfaces. Cell 165: 801–811, 2016. doi: 10.1016/j.cell.2016.04.041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Fornai M, van den Wijngaard RM, Antonioli L, Pellegrini C, Blandizzi C, de Jonge WJ. Neuronal regulation of intestinal immune functions in health and disease. Neurogastroenterol Motil 30: e13406, 2018. doi: 10.1111/nmo.13406. [DOI] [PubMed] [Google Scholar]
- 17. Powell N, Walker MM, Talley NJ. The mucosal immune system: master regulator of bidirectional gut-brain communications. Nat Rev Gastroenterol Hepatol 14: 143–159, 2017. doi: 10.1038/nrgastro.2016.191. [DOI] [PubMed] [Google Scholar]
- 18. Lai NY, Mills K, Chiu IM. Sensory neuron regulation of gastrointestinal inflammation and bacterial host defence. J Intern Med 282: 5–23, 2017. doi: 10.1111/joim.12591. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Holland AM, Bon-Frauches AC, Keszthelyi D, Melotte V, Boesmans W. The enteric nervous system in gastrointestinal disease etiology. Cell Mol Life Sci 78: 4713–4733, 2021. doi: 10.1007/s00018-021-03812-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Wang H, Foong JP, Harris NL, Bornstein JC. Enteric neuroimmune interactions coordinate intestinal responses in health and disease. Mucosal Immunol 15: 27–39, 2022. doi: 10.1038/s41385-021-00443-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Jacobson A, Yang D, Vella M, Chiu IM. The intestinal neuro-immune axis: crosstalk between neurons, immune cells, and microbes. Mucosal Immunol 14: 555–565, 2021. doi: 10.1038/s41385-020-00368-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Populin L, Stebbing MJ, Furness JB. Neuronal regulation of the gut immune system and neuromodulation for treating inflammatory bowel disease. FASEB Bioadv 3: 953–966, 2021. doi: 10.1096/fba.2021-00070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Schneider KM, Kim J, Bahnsen K, Heuckeroth RO, Thaiss CA. Environmental perception and control of gastrointestinal immunity by the enteric nervous system. Trends Mol Med 28: 989–1005, 2022. doi: 10.1016/j.molmed.2022.09.005. [DOI] [PubMed] [Google Scholar]
- 24. Rao M, Gershon MD. The bowel and beyond: the enteric nervous system in neurological disorders. Nat Rev Gastroenterol Hepatol 13: 517–528, 2016. doi: 10.1038/nrgastro.2016.107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Travagli RA, Browning KN, Camilleri M. Parkinson disease and the gut: new insights into pathogenesis and clinical relevance. Nat Rev Gastroenterol Hepatol 17: 673–685, 2020. doi: 10.1038/s41575-020-0339-z. [DOI] [PubMed] [Google Scholar]
- 26. Singh A, Dawson TM, Kulkarni S. Neurodegenerative disorders and gut-brain interactions. J Clin Invest 131: e143775, 2021. doi: 10.1172/JCI143775. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Spencer NJ, Hu H. Enteric nervous system: sensory transduction, neural circuits and gastrointestinal motility. Nat Rev Gastroenterol Hepatol 17: 338–351, 2020. doi: 10.1038/s41575-020-0271-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Costa M, Spencer NJ, Brookes SJ. The role of enteric inhibitory neurons in intestinal motility. Auton Neurosci 235: 102854, 2021. doi: 10.1016/j.autneu.2021.102854. [DOI] [PubMed] [Google Scholar]
- 29. Furness JB. The enteric nervous system and neurogastroenterology. Nat Rev Gastroenterol Hepatol 9: 286–294, 2012. doi: 10.1038/nrgastro.2012.32. [DOI] [PubMed] [Google Scholar]
- 30. Huizinga JD, Hussain A, Chen JH. Interstitial cells of Cajal and human colon motility in health and disease. Am J Physiol Gastrointest Liver Physiol 321: G552–G575, 2021. doi: 10.1152/ajpgi.00264.2021. [DOI] [PubMed] [Google Scholar]
- 31. Wood JD. Motor behavior of mouse large intestine: a minireview. Neurogastroenterol Motil 33: e14206, 2021. doi: 10.1111/nmo.14206. [DOI] [PubMed] [Google Scholar]
- 32. Fung C, Vanden Berghe P. Functional circuits and signal processing in the enteric nervous system. Cell Mol Life Sci 77: 4505–4522, 2020. doi: 10.1007/s00018-020-03543-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Rao M, Gershon MD. Enteric nervous system development: what could possibly go wrong? Nat Rev Neurosci 19: 552–565, 2018. doi: 10.1038/s41583-018-0041-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Hao MM, Foong JP, Bornstein JC, Li ZL, Vanden Berghe P, Boesmans W. Enteric nervous system assembly: functional integration within the developing gut. Dev Biol 417: 168–181, 2016. doi: 10.1016/j.ydbio.2016.05.030. [DOI] [PubMed] [Google Scholar]
- 35. Foong JP. Postnatal development of the mouse enteric nervous system. Adv Exp Med Biol 891: 135–143, 2016. doi: 10.1007/978-3-319-27592-5_13. [DOI] [PubMed] [Google Scholar]
- 36. Bondurand N, Southard-Smith EM. Mouse models of Hirschsprung disease and other developmental disorders of the enteric nervous system: old and new players. Dev Biol 417: 139–157, 2016. doi: 10.1016/j.ydbio.2016.06.042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Young HM, Stamp LA, McKeown SJ. ENS development research since 1983: great strides but many remaining challenges. Adv Exp Med Biol 891: 53–62, 2016. doi: 10.1007/978-3-319-27592-5_6. [DOI] [PubMed] [Google Scholar]
- 38. Lake JI, Heuckeroth RO. Enteric nervous system development: migration, differentiation, and disease. Am J Physiol Gastrointest Liver Physiol 305: G1–G24, 2013. doi: 10.1152/ajpgi.00452.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Sasselli V, Pachnis V, Burns AJ. The enteric nervous system. Dev Biol 366: 64–73, 2012. doi: 10.1016/j.ydbio.2012.01.012. [DOI] [PubMed] [Google Scholar]
- 40. Nagy N, Goldstein AM. Enteric nervous system development: a crest cell’s journey from neural tube to colon. Semin Cell Dev Biol 66: 94–106, 2017. doi: 10.1016/j.semcdb.2017.01.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Boesmans W, Nash A, Tasnády KR, Yang W, Stamp LA, Hao MM. Development, diversity, and neurogenic capacity of enteric glia. Front Cell Dev Biol 9: 775102, 2021. doi: 10.3389/fcell.2021.775102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Kang YN, Fung C, Vanden Berghe P. Gut innervation and enteric nervous system development: a spatial, temporal and molecular tour de force. Development 148: dev18253, 2021. doi: 10.1242/dev.182543. [DOI] [PubMed] [Google Scholar]
- 43. Joly A, Leulier F, De Vadder F. Microbial modulation of the development and physiology of the enteric nervous system. Trends Microbiol 29: 686–699, 2021. doi: 10.1016/j.tim.2020.11.007. [DOI] [PubMed] [Google Scholar]
- 44. Christensen J, Rick GA. Intrinsic nerves in the mammalian colon: confirmation of a plexus at the circular muscle-submucosal interface. J Auton Nerv Syst 21: 223–231, 1987. doi: 10.1016/0165-1838(87)90025-7. [DOI] [PubMed] [Google Scholar]
- 45. Fang S, Wu R, Christensen J. Intramucosal nerve cells in human small intestine. J Auton Nerv Syst 44: 129–136, 1993. doi: 10.1016/0165-1838(93)90025-p. [DOI] [PubMed] [Google Scholar]
- 46. Brehmer A, Rupprecht H, Neuhuber W. Two submucosal nerve plexus in human intestines. Histochem Cell Biol 133: 149–161, 2010. doi: 10.1007/s00418-009-0657-2. [DOI] [PubMed] [Google Scholar]
- 47. Michel K, Kuch B, Dengler S, Demir IE, Zeller F, Schemann M. How big is the little brain in the gut? Neuronal numbers in the enteric nervous system of mice, Guinea pig, and human. Neurogastroenterol Motil 34: e14440, 2022. doi: 10.1111/nmo.14440. [DOI] [PubMed] [Google Scholar]
- 48. Sanders KM, Hwang SJ, Ward SM. Neuroeffector apparatus in gastrointestinal smooth muscle organs. J Physiol 588: 4621–4639, 2010. doi: 10.1113/jphysiol.2010.196030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Spencer NJ, Dinning PG, Brookes SJ, Costa M. Insights into the mechanisms underlying colonic motor patterns. J Physiol 594: 4099–4116, 2016. doi: 10.1113/JP271919. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Smith TK, Koh SD. A model of the enteric neural circuitry underlying the generation of rhythmic motor patterns in the colon: the role of serotonin. Am J Physiol Gastrointest Liver Physiol 312: G1–G14, 2017. doi: 10.1152/ajpgi.00337.2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Furness JB, Callaghan BP, Rivera LR, Cho HJ. The enteric nervous system and gastrointestinal innervation: integrated local and central control. Adv Exp Med Biol 817: 39–71, 2014. doi: 10.1007/978-1-4939-0897-4_3. [DOI] [PubMed] [Google Scholar]
- 52. Vanner S, MacNaughton WK. Submucosal secretomotor and vasodilator reflexes. Neurogastroenterol Motil 16, Suppl 1: 39–43, 2004. doi: 10.1111/j.1743-3150.2004.00473.x. [DOI] [PubMed] [Google Scholar]
- 53. Hens J, Gajda M, Scheuermann DW, Adriaensen D, Timmermans JP. The longitudinal smooth muscle layer of the pig small intestine is innervated by both myenteric and submucous neurons. Histochem Cell Biol 117: 481–492, 2002. doi: 10.1007/s00418-002-0406-2. [DOI] [PubMed] [Google Scholar]
- 54. Porter AJ, Wattchow DA, Brookes SJ, Costa M. Projections of nitric oxide synthase and vasoactive intestinal polypeptide-reactive submucosal neurons in the human colon. J Gastroenterol Hepatol 14: 1180–1187, 1999. doi: 10.1046/j.1440-1746.1999.02026.x. [DOI] [PubMed] [Google Scholar]
- 55. Furness JB, Lloyd KC, Sternini C, Walsh JH. Projections of substance P, vasoactive intestinal peptide and tyrosine hydroxylase immunoreactive nerve fibres in the canine intestine, with special reference to the innervation of the circular muscle. Arch Histol Cytol 53: 129–140, 1990. doi: 10.1679/aohc.53.129. [DOI] [PubMed] [Google Scholar]
- 56. Woods CM, Mawe GM, Toouli J, Saccone GT. The sphincter of Oddi: understanding its control and function. Neurogastroenterol Motil 17: 31–40, 2005. doi: 10.1111/j.1365-2982.2005.00658.x. [DOI] [PubMed] [Google Scholar]
- 57. Balemba OB, Salter MJ, Mawe GM. Innervation of the extrahepatic biliary tract. Anat Rec A Discov Mol Cell Evol Biol 280: 836–847, 2004. doi: 10.1002/ar.a.20089. [DOI] [PubMed] [Google Scholar]
- 58. Kirchgessner AL, Gershon MD. Innervation of the pancreas by neurons in the gut. J Neurosci 10: 1626–1642, 1990. doi: 10.1523/JNEUROSCI.10-05-01626.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59. Kennedy AL, Mawe GM. Duodenal sensory neurons project to sphincter of Oddi ganglia in guinea pig. J Neurosci 18: 8065–8073, 1998. doi: 10.1523/JNEUROSCI.18-19-08065.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60. Mawe GM, Kennedy AL. Duodenal neurons provide nicotinic fast synaptic input to sphincter of Oddi neurons in guinea pig. Am J Physiol Gastrointest Liver Physiol 277: G226–234, 1999. doi: 10.1152/ajpgi.1999.277.1.G226. [DOI] [PubMed] [Google Scholar]
- 61. Gabella G. Fine structure of the myenteric plexus in the guinea-pig ileum. J Anat 111: 69–97, 1972. [PMC free article] [PubMed] [Google Scholar]
- 62. Jacobs JM. Penetration of systemically injected horseradish peroxidase into ganglia and nerves of the autonomic nervous system. J Neurocytol 6: 607–618, 1977. doi: 10.1007/BF01205222. [DOI] [PubMed] [Google Scholar]
- 63. Mawe GM, Talmage EK, Cornbrooks EB, Gokin AP, Zhang L, Jennings LJ. Innervation of the gallbladder: structure, neurochemical coding, and physiological properties of guinea pig gallbladder ganglia. Microsc Res Tech 39: 1–13, 1997. doi:. [DOI] [PubMed] [Google Scholar]
- 64. Gershon MD, Bursztajn S. Properties of the enteric nervous system: limitation of access of intravascular macromolecules to the myenteric plexus and muscularis externa. J Comp Neurol 180: 467–488, 1978. doi: 10.1002/cne.901800305. [DOI] [PubMed] [Google Scholar]
- 65. Dora D, Ferenczi S, Stavely R, Toth VE, Varga ZV, Kovacs T, Bodi I, Hotta R, Kovacs KJ, Goldstein AM, Nagy N. Evidence of a myenteric plexus barrier and its macrophage-dependent degradation during murine colitis: implications in enteric neuroinflammation. Cell Mol Gastroenterol Hepatol 12: 1617–1641, 2021. doi: 10.1016/j.jcmgh.2021.07.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66. Fawcett JW, Oohashi T, Pizzorusso T. The roles of perineuronal nets and the perinodal extracellular matrix in neuronal function. Nat Rev Neurosci 20: 451–465, 2019. doi: 10.1038/s41583-019-0196-3. [DOI] [PubMed] [Google Scholar]
- 67. Reichelt AC, Hare DJ, Bussey TJ, Saksida LM. Perineuronal nets: plasticity, protection, and therapeutic potential. Trends Neurosci 42: 458–470, 2019. doi: 10.1016/j.tins.2019.04.003. [DOI] [PubMed] [Google Scholar]
- 68. Song I, Dityatev A. Crosstalk between glia, extracellular matrix and neurons. Brain Res Bull 136: 101–108, 2018. doi: 10.1016/j.brainresbull.2017.03.003. [DOI] [PubMed] [Google Scholar]
- 69. Aktar R, Peiris M, Fikree A, Cibert-Goton V, Walmsley M, Tough IR, Watanabe P, Araujo EJ, Mohammed SD, Delalande JM, Bulmer DC, Scott SM, Cox HM, Voermans NC, Aziz Q, Blackshaw LA. The extracellular matrix glycoprotein tenascin-X regulates peripheral sensory and motor neurones. J Physiol 596: 4237–4251, 2018. doi: 10.1113/JP276300. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70. Bannerman PG, Mirsky R, Jessen KR, Timpl R, Duance VC. Light microscopic immunolocalization of laminin, type IV collagen, nidogen, heparan sulphate proteoglycan and fibronectin in the enteric nervous system of rat and guinea pig. J Neurocytol 15: 733–743, 1986. doi: 10.1007/BF01625191. [DOI] [PubMed] [Google Scholar]
- 71. Aktar R, Peiris M, Fikree A, Eaton S, Kritas S, Kentish SJ, Araujo EJ, Bacarin C, Page AJ, Voermans NC, Aziz Q, Blackshaw LA. A novel role for the extracellular matrix glycoprotein-Tenascin-X in gastric function. J Physiol 597: 1503–1515, 2019. doi: 10.1113/JP277195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72. Drokhlyansky E, Smillie CS, Van Wittenberghe N, Ericsson M, Griffin GK, Eraslan G, Dionne D, Cuoco MS, Goder-Reiser MN, Sharova T, Kuksenko O, Aguirre AJ, Boland GM, Graham D, Rozenblatt-Rosen O, Xavier RJ, Regev A. The human and mouse enteric nervous system at single-cell resolution. Cell 182: 1606–1622.e23, 2020. doi: 10.1016/j.cell.2020.08.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73. Marklund U. Diversity, development and immunoregulation of enteric neurons. Nat Rev Gastroenterol Hepatol 19: 85–86, 2022. doi: 10.1038/s41575-021-00553-y. [DOI] [PubMed] [Google Scholar]
- 74. Jakob MO, Kofoed-Branzk M, Deshpande D, Murugan S, Klose CS. An integrated view on neuronal subsets in the peripheral nervous system and their role in immunoregulation. Front Immunol 12: 679055, 2021. doi: 10.3389/fimmu.2021.679055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75. Guyer RA, Mueller JL, Goldstein AM. Applications of single-cell sequencing technology to the enteric nervous system. Biomolecules 12: 452, 2022. doi: 10.3390/biom12030452. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76. Brehmer A. Classification of human enteric neurons. Histochem Cell Biol 156: 95–108, 2021. doi: 10.1007/s00418-021-02002-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77. Brehmer A, Schrodl F, Neuhuber W. Morphological classifications of enteric neurons–100 years after Dogiel. Anat Embryol (Berl) 200: 125–135, 1999. doi: 10.1007/s004290050267. [DOI] [PubMed] [Google Scholar]
- 78. Pompolo S, Furness JB. Ultrastructure and synaptic relationships of calbindin-reactive, Dogiel type II neurons, in myenteric ganglia of guinea-pig small intestine. J Neurocytol 17: 771–782, 1988. doi: 10.1007/BF01216705. [DOI] [PubMed] [Google Scholar]
- 79. Gabella G. On the ultrastructure of the enteric nerve ganglia. Scand J Gastroenterol Suppl 71: 15–25, 1982. [PubMed] [Google Scholar]
- 80. Böttner M, Zorenkov D, Hellwig I, Barrenschee M, Harde J, Fricke T, Deuschl G, Egberts JH, Becker T, Fritscher-Ravens A, Arlt A, Wedel T. Expression pattern and localization of alpha-synuclein in the human enteric nervous system. Neurobiol Dis 48: 474–480, 2012. doi: 10.1016/j.nbd.2012.07.018. [DOI] [PubMed] [Google Scholar]
- 81. Böttner M, Fricke T, Müller M, Barrenschee M, Deuschl G, Schneider SA, Egberts JH, Becker T, Fritscher-Ravens A, Ellrichmann M, Schulz-Schaeffer WJ, Wedel T. Alpha-synuclein is associated with the synaptic vesicle apparatus in the human and rat enteric nervous system. Brain Res 1614: 51–59, 2015. doi: 10.1016/j.brainres.2015.04.015. [DOI] [PubMed] [Google Scholar]
- 82. Vanden Berghe P, Klingauf J. Spatial organization and dynamic properties of neurotransmitter release sites in the enteric nervous system. Neuroscience 145: 88–99, 2007. doi: 10.1016/j.neuroscience.2006.11.048. [DOI] [PubMed] [Google Scholar]
- 83. Gabella G. Ultrastructure of the nerve plexuses of the mammalian intestine: the enteric glial cells. Neuroscience 6: 425–436, 1981. doi: 10.1016/0306-4522(81)90135-4. [DOI] [PubMed] [Google Scholar]
- 84. Gulbransen BD, Sharkey KA. Purinergic neuron-to-glia signaling in the enteric nervous system. Gastroenterology 136: 1349–1358, 2009. doi: 10.1053/j.gastro.2008.12.058. [DOI] [PubMed] [Google Scholar]
- 85. Ahmadzai MM, Seguella L, Gulbransen BD. Circuit-specific enteric glia regulate intestinal motor neurocircuits. Proc Natl Acad Sci USA 118: e2025938118, 2021. doi: 10.1073/pnas.2025938118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86. Boesmans W, Cirillo C, Van den Abbeel V, Van den Haute C, Depoortere I, Tack J, Vanden Berghe P. Neurotransmitters involved in fast excitatory neurotransmission directly activate enteric glial cells. Neurogastroenterol Motil 25: e151–e160, 2013. doi: 10.1111/nmo.12065. [DOI] [PubMed] [Google Scholar]
- 87. Broadhead MJ, Bayguinov PO, Okamoto T, Heredia DJ, Smith TK. Ca2+ transients in myenteric glial cells during the colonic migrating motor complex in the isolated murine large intestine. J Physiol 590: 335–350, 2012. doi: 10.1113/jphysiol.2011.219519. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88. Lyon KA, Allen NJ. From synapses to circuits, astrocytes regulate behavior. Front Neural Circuits 15: 786293, 2021. doi: 10.3389/fncir.2021.786293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89. Durkee CA, Araque A. Diversity and specificity of astrocyte-neuron communication. Neuroscience 396: 73–78, 2019. doi: 10.1016/j.neuroscience.2018.11.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90. Gabella G, Trigg P. Size of neurons and glial cells in the enteric ganglia of mice, guinea-pigs, rabbits and sheep. J Neurocytol 13: 49–71, 1984. doi: 10.1007/BF01148318. [DOI] [PubMed] [Google Scholar]
- 91. Kulkarni S, Micci MA, Leser J, Shin C, Tang SC, Fu YY, Liu L, Li Q, Saha M, Li C, Enikolopov G, Becker L, Rakhilin N, Anderson M, Shen X, Dong X, Butte MJ, Song H, Southard-Smith EM, Kapur RP, Bogunovic M, Pasricha PJ. Adult enteric nervous system in health is maintained by a dynamic balance between neuronal apoptosis and neurogenesis. Proc Natl Acad Sci USA 114: E3709–E3718, 2017. doi: 10.1073/pnas.1619406114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92. Becker L, Nguyen L, Gill J, Kulkarni S, Pasricha PJ, Habtezion A. Age-dependent shift in macrophage polarisation causes inflammation-mediated degeneration of enteric nervous system. Gut 67: 827–836, 2018. doi: 10.1136/gutjnl-2016-312940. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93. De Schepper S, Verheijden S, Aguilera-Lizarraga J, Viola MF, Boesmans W, Stakenborg N, Voytyuk I, Schmidt I, Boeckx B, Dierckx D, Casterlé I, Baekelandt V, Gonzalez Dominguez E, Mack M, Depoortere I, De Strooper B, Sprangers B, Himmelreich U, Soenen S, Guilliams M, Vanden Berghe P, Jones E, Lambrechts D, Boeckxstaens G. Self-maintaining gut macrophages are essential for intestinal homeostasis. Cell 175: 400–415.e13, 2018. doi: 10.1016/j.cell.2018.07.048. [DOI] [PubMed] [Google Scholar]
- 94. Chandrasekharan B, Anitha M, Blatt R, Shahnavaz N, Kooby D, Staley C, Mwangi S, Jones DP, Sitaraman SV, Srinivasan S. Colonic motor dysfunction in human diabetes is associated with enteric neuronal loss and increased oxidative stress. Neurogastroenterol Motil 23: 131–138.e26, 2011. doi: 10.1111/j.1365-2982.2010.01611.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95. Ye L, Li G, Goebel A, Raju AV, Kong F, Lv Y, Li K, Zhu Y, Raja S, He P, Li F, Mwangi SM, Hu W, Srinivasan S. Caspase-11-mediated enteric neuronal pyroptosis underlies Western diet-induced colonic dysmotility. J Clin Invest 130: 3621–3636, 2020. doi: 10.1172/JCI130176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96. Gianino S, Grider JR, Cresswell J, Enomoto H, Heuckeroth RO. GDNF availability determines enteric neuron number by controlling precursor proliferation. Development 130: 2187–2198, 2003. doi: 10.1242/dev.00433. [DOI] [PubMed] [Google Scholar]
- 97. Bradley JS Jr, Parr EJ, Sharkey KA. Effects of inflammation on cell proliferation in the myenteric plexus of the guinea-pig ileum. Cell Tissue Res 289: 455–461, 1997. doi: 10.1007/s004410050891. [DOI] [PubMed] [Google Scholar]
- 98. Joseph NM, He S, Quintana E, Kim YG, Núñez G, Morrison SJ. Enteric glia are multipotent in culture but primarily form glia in the adult rodent gut. J Clin Invest 121: 3398–3411, 2011. doi: 10.1172/JCI58186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99. Virtanen H, Garton DR, Andressoo JO. Myenteric neurons do not replicate in small intestine under normal, physiological conditions in adult mouse. Cell Mol Gastroenterol Hepatol 14: 27–34, 2022. doi: 10.1016/j.jcmgh.2022.04.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100. Iruzubieta P, Cantarero I, Monzón M, Lahoz M, Junquera C. Supporting evidence of human enteric nervous system adult neurogenesis: presence of primary cilia and adult neurogenesis markers. Cell Mol Neurobiol 42: 473–481, 2022. doi: 10.1007/s10571-020-01017-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101. Wood JD, Mayer CJ. Patterned discharge of six different neurons in a single enteric ganglion. Pflugers Arch 338: 247–256, 1973. doi: 10.1007/BF00587390. [DOI] [PubMed] [Google Scholar]
- 102. Wood JD. Electrical discharge of single enteric neurons of guinea pig small intestine. Am J Physiol 225: 1107–1113, 1973. doi: 10.1152/ajplegacy.1973.225.5.1107. [DOI] [PubMed] [Google Scholar]
- 103. Hirst GD, Holman ME, Spence I. Two types of neurones in the myenteric plexus of duodenum in the guinea-pig. J Physiol 236: 303–326, 1974. doi: 10.1113/jphysiol.1974.sp010436. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104. Nishi S, North RA. Intracellular recording from the myenteric plexus of the guinea-pig ileum. J Physiol 231: 471–491, 1973. doi: 10.1113/jphysiol.1973.sp010244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105. Furness JB, Costa M. Types of nerves in the enteric nervous system. Neuroscience 5: 1–20, 1980. doi: 10.1016/0306-4522(80)90067-6. [DOI] [PubMed] [Google Scholar]
- 106. Brookes SJ, Ewart WR, Wingate DL. Intracellular recordings from myenteric neurones in the human colon. J Physiol 390: 305–318, 1987. doi: 10.1113/jphysiol.1987.sp016702. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107. Browning KN, Lees GM. Myenteric neurons of the rat descending colon: electrophysiological and correlated morphological properties. Neuroscience 73: 1029–1047, 1996. doi: 10.1016/0306-4522(96)00118-2. [DOI] [PubMed] [Google Scholar]
- 108. Nurgali K, Stebbing MJ, Furness JB. Correlation of electrophysiological and morphological characteristics of enteric neurons in the mouse colon. J Comp Neurol 468: 112–124, 2004. doi: 10.1002/cne.10948. [DOI] [PubMed] [Google Scholar]
- 109. Carbone SE, Jovanovska V, Nurgali K, Brookes SJ. Human enteric neurons: morphological, electrophysiological, and neurochemical identification. Neurogastroenterol Motil 26: 1812–1816, 2014. doi: 10.1111/nmo.12453. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110. Cornelissen W, De Laet A, Kroese AB, Van Bogaert PP, Scheuermann DW, Timmermans JP. Electrophysiological features of morphological Dogiel type II neurons in the myenteric plexus of pig small intestine. J Neurophysiol 84: 102–111, 2000. doi: 10.1152/jn.2000.84.1.102. [DOI] [PubMed] [Google Scholar]
- 111. Furness JB, Kunze WA, Bertrand PP, Clerc N, Bornstein JC. Intrinsic primary afferent neurons of the intestine. Prog Neurobiol 54: 1–18, 1998. doi: 10.1016/s0301-0082(97)00051-8. [DOI] [PubMed] [Google Scholar]
- 112. Grafe PG, Wood JD, Mayer CJ. Fast excitatory postsynaptic potentials in AH (Type 2) neurons of guinea pig myenteric plexus. Brain Res 163: 349–352, 1979. doi: 10.1016/0006-8993(79)90365-2. [DOI] [PubMed] [Google Scholar]
- 113. Hibberd TJ, Travis L, Wiklendt L, Costa M, Brookes SJ, Hu H, Keating DJ, Spencer NJ. Synaptic activation of putative sensory neurons by hexamethonium-sensitive nerve pathways in mouse colon. Am J Physiol Gastrointest Liver Physiol 314: G53–G64, 2018. doi: 10.1152/ajpgi.00234.2017. [DOI] [PubMed] [Google Scholar]
- 114. Furness JB, Jones C, Nurgali K, Clerc N. Intrinsic primary afferent neurons and nerve circuits within the intestine. Prog Neurobiol 72: 143–164, 2004. doi: 10.1016/j.pneurobio.2003.12.004. [DOI] [PubMed] [Google Scholar]
- 115. Wood JD, Kirchgessner A. Slow excitatory metabotropic signal transmission in the enteric nervous system. Neurogastroenterol Motil 16, Suppl 1: 71–80, 2004. doi: 10.1111/j.1743-3150.2004.00479.x. [DOI] [PubMed] [Google Scholar]
- 116. Li Z, Boesmans W, Kazwiny Y, Hao MM, Vanden Berghe P. Simultaneous whole-cell patch-clamp and calcium imaging on myenteric neurons. Am J Physiol Gastrointest Liver Physiol 323: G341–G347, 2022. doi: 10.1152/ajpgi.00162.2022. [DOI] [PubMed] [Google Scholar]
- 117. Brookes SJ. Classes of enteric nerve cells in the guinea-pig small intestine. Anat Rec 262: 58–70, 2001. doi:. [DOI] [PubMed] [Google Scholar]
- 118. Mazzuoli-Weber G, Schemann M. Mechanosensitivity in the enteric nervous system. Front Cell Neurosci 9: 408, 2015. doi: 10.3389/fncel.2015.00408. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119. Mazzuoli G, Schemann M. Mechanosensitive enteric neurons in the myenteric plexus of the mouse intestine. PLoS One 7: e39887, 2012. doi: 10.1371/journal.pone.0039887. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120. Spencer NJ, Smith TK. Mechanosensory S-neurons rather than AH-neurons appear to generate a rhythmic motor pattern in guinea-pig distal colon. J Physiol 558: 577–596, 2004. doi: 10.1113/jphysiol.2004.063586. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121. Hibberd TJ, Zagorodnyuk VP, Spencer NJ, Brookes SJ. Identification and mechanosensitivity of viscerofugal neurons. Neuroscience 225: 118–129, 2012. doi: 10.1016/j.neuroscience.2012.08.040. [DOI] [PubMed] [Google Scholar]
- 122. Hibberd TJ, Spencer NJ, Zagorodnyuk VP, Chen BN, Brookes SJ. Targeted electrophysiological analysis of viscerofugal neurons in the myenteric plexus of guinea-pig colon. Neuroscience 275: 272–284, 2014. doi: 10.1016/j.neuroscience.2014.04.066. [DOI] [PubMed] [Google Scholar]
- 123. Wang GD, Wang XY, Liu S, Qu M, Xia Y, Needleman BJ, Mikami DJ, Wood JD. Innervation of enteric mast cells by primary spinal afferents in guinea pig and human small intestine. Am J Physiol Gastrointest Liver Physiol 307: G719–G731, 2014. doi: 10.1152/ajpgi.00125.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124. Bayliss WM, Starling EH. The movements and innervation of the small intestine. J Physiol 24: 99–143, 1899. doi: 10.1113/jphysiol.1899.sp000752. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125. Trendelenburg P. Physiological and pharmacological investigations of small intestinal peristalsis. Translation of the article “Physiologische und pharmakologische Versuche uber die Dünndarmperistaltik”, Arch. Exp. Pathol. Pharmakol. 81, 55-129, 1917. Naunyn Schmiedebergs Arch Pharmacol 373: 101–133, 2006. doi: 10.1007/s00210-006-0052-7. [DOI] [PubMed] [Google Scholar]
- 126. Schemann M, Mai G, Costa M, Enck P. Translating the seminal findings of Carl Luderitz: a description in English of his extraordinary studies of gastrointestinal motility accompanied by a historical view of peristalsis. Neurogastroenterol Motil 33: e13995, 2021. doi: 10.1111/nmo.13995. [DOI] [PubMed] [Google Scholar]
- 127. Spencer N, Walsh M, Smith TK. Does the guinea-pig ileum obey the ‘law of the intestine’? J Physiol 517: 889–898, 1999. doi: 10.1111/j.1469-7793.1999.0889s.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128. Schemann M, Reiche D, Michel K. Enteric pathways in the stomach. Anat Rec 262: 47–57, 2001. doi:. [DOI] [PubMed] [Google Scholar]
- 129. Furness JB, Di Natale M, Hunne B, Oparija-Rogenmozere L, Ward SM, Sasse KC, Powley TL, Stebbing MJ, Jaffey D, Fothergill LJ. The identification of neuronal control pathways supplying effector tissues in the stomach. Cell Tissue Res 382: 433–445, 2020. doi: 10.1007/s00441-020-03294-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130. Costa M, Brookes SH. Architecture of enteric neural circuits involved in intestinal motility. Eur Rev Med Pharmacol Sci 12: 3–19, 2008. [PubMed] [Google Scholar]
- 131. Costa M, Brookes SJ, Hennig GW. Anatomy and physiology of the enteric nervous system. Gut 47, Suppl 4: iv15–iv19, 2000. doi: 10.1136/gut.47.suppl_4.iv15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132. Gershon MD, Margolis KG. The gut, its microbiome, and the brain: connections and communications. J Clin Invest 131: e143768, 2021. doi: 10.1172/JCI143768. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133. Mawe GM, Sharkey KA. The intrinsic reflex circuitry of the inflamed colon. Adv Exp Med Biol 891: 153–157, 2016. doi: 10.1007/978-3-319-27592-5_15. [DOI] [PubMed] [Google Scholar]
- 134. Morarach K, Mikhailova A, Knoflach V, Memic F, Kumar R, Li W, Ernfors P, Marklund U. Diversification of molecularly defined myenteric neuron classes revealed by single-cell RNA sequencing. Nat Neurosci 24: 34–46, 2021. doi: 10.1038/s41593-020-00736-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135. Perdue MH, McKay DM. Integrative immunophysiology in the intestinal mucosa. Am J Physiol Gastrointest Liver Physiol 267: G151–G165, 1994. doi: 10.1152/ajpgi.1994.267.2.G151. [DOI] [PubMed] [Google Scholar]
- 136. Neunlist M, Van Landeghem L, Mahé MM, Derkinderen P, Des Varannes SB, Rolli-Derkinderen M. The digestive neuronal-glial-epithelial unit: a new actor in gut health and disease. Nat Rev Gastroenterol Hepatol 10: 90–100, 2013. doi: 10.1038/nrgastro.2012.221. [DOI] [PubMed] [Google Scholar]
- 137. Reed DE, Vanner SJ. Long vasodilator reflexes projecting through the myenteric plexus in guinea-pig ileum. J Physiol 553: 911–924, 2003. doi: 10.1113/jphysiol.2003.053140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138. Reed DE, Vanner S. Mucosal stimulation activates secretomotor neurons via long myenteric pathways in guinea pig ileum. Am J Physiol Gastrointest Liver Physiol 292: G608–G614, 2007. doi: 10.1152/ajpgi.00364.2006. [DOI] [PubMed] [Google Scholar]
- 139. Kunze WA, Furness JB, Bertrand PP, Bornstein JC. Intracellular recording from myenteric neurons of the guinea-pig ileum that respond to stretch. J Physiol 506: 827–842, 1998. doi: 10.1111/j.1469-7793.1998.827bv.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140. Furness JB, Kunze WA, Clerc N. Nutrient tasting and signaling mechanisms in the gut. II. The intestine as a sensory organ: neural, endocrine, and immune responses. Am J Physiol Gastrointest Liver Physiol 277: G922–G928, 1999. doi: 10.1152/ajpgi.1999.277.5.G922. [DOI] [PubMed] [Google Scholar]
- 141. Spencer NJ, Hennig GW, Smith TK. A rhythmic motor pattern activated by circumferential stretch in guinea-pig distal colon. J Physiol 545: 629–648, 2002. doi: 10.1113/jphysiol.2002.028647. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142. Spencer NJ, Dickson EJ, Hennig GW, Smith TK. Sensory elements within the circular muscle are essential for mechanotransduction of ongoing peristaltic reflex activity in guinea-pig distal colon. J Physiol 576: 519–531, 2006. doi: 10.1113/jphysiol.2006.109561. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143. Dickson EJ, Spencer NJ, Hennig GW, Bayguinov PO, Ren J, Heredia DJ, Smith TK. An enteric occult reflex underlies accommodation and slow transit in the distal large bowel. Gastroenterology 132: 1912–1924, 2007. doi: 10.1053/j.gastro.2007.02.047. [DOI] [PubMed] [Google Scholar]
- 144. Mazzuoli G, Schemann M. Multifunctional rapidly adapting mechanosensitive enteric neurons (RAMEN) in the myenteric plexus of the guinea pig ileum. J Physiol 587: 4681–4694, 2009. doi: 10.1113/jphysiol.2009.177105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 145. Mazzuoli-Weber G, Schemann M. Mechanosensitive enteric neurons in the guinea pig gastric corpus. Front Cell Neurosci 9: 430, 2015. doi: 10.3389/fncel.2015.00430. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 146. Vanner S, Surprenant A. Neural reflexes controlling intestinal microcirculation. Am J Physiol Gasrointest Liver Physiol 271: G223–G230, 1996. doi: 10.1152/ajpgi.1996.271.2.G223. [DOI] [PubMed] [Google Scholar]
- 147. Filzmayer AK, Elfers K, Michel K, Buhner S, Zeller F, Demir IE, Theisen J, Schemann M, Mazzuoli-Weber G. Compression and stretch sensitive submucosal neurons of the porcine and human colon. Sci Rep 10: 13791, 2020. doi: 10.1038/s41598-020-70216-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 148. Spencer NJ, Hennig GW, Smith TK. Stretch-activated neuronal pathways to longitudinal and circular muscle in guinea pig distal colon. Am J Physiol Gastrointest Liver Physiol 284: G231–G241, 2003. doi: 10.1152/ajpgi.00291.2002. [DOI] [PubMed] [Google Scholar]
- 149. Spencer NJ, Hennig GW, Smith TK. Spatial and temporal coordination of junction potentials in circular muscle of guinea-pig distal colon. J Physiol 535: 565–578, 2001. doi: 10.1111/j.1469-7793.2001.00565.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 150. Spencer NJ, Smith TK. Simultaneous intracellular recordings from longitudinal and circular muscle during the peristaltic reflex in guinea-pig distal colon. J Physiol 533: 787–799, 2001. doi: 10.1111/j.1469-7793.2001.00787.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 151. Mittal RK, Padda B, Bhalla V, Bhargava V, Liu J. Synchrony between circular and longitudinal muscle contractions during peristalsis in normal subjects. Am J Physiol Gastrointest Liver Physiol 290: G431–G438, 2006. doi: 10.1152/ajpgi.00237.2005. [DOI] [PubMed] [Google Scholar]
- 152. Lasrado R, Boesmans W, Kleinjung J, Pin C, Bell D, Bhaw L, McCallum S, Zong H, Luo L, Clevers H, Vanden Berghe P, Pachnis V. Lineage-dependent spatial and functional organization of the mammalian enteric nervous system. Science 356: 722–726, 2017. doi: 10.1126/science.aam7511. [DOI] [PubMed] [Google Scholar]
- 153. Spencer NJ, Hibberd TJ, Travis L, Wiklendt L, Costa M, Hu H, Brookes SJ, Wattchow DA, Dinning PG, Keating DJ, Sorensen J. Identification of a rhythmic firing pattern in the enteric nervous system that generates rhythmic electrical activity in smooth muscle. J Neurosci 38: 5507–5522, 2018. doi: 10.1523/JNEUROSCI.3489-17.2018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 154. Spencer NJ, Hennig GW, Dickson E, Smith TK. Synchronization of enteric neuronal firing during the murine colonic MMC. J Physiol 564: 829–847, 2005. doi: 10.1113/jphysiol.2005.083600. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 155. Spencer NJ, Travis L, Wiklendt L, Costa M, Hibberd TJ, Brookes SJ, Dinning P, Hu H, Wattchow DA, Sorensen J. Long range synchronization within the enteric nervous system underlies propulsion along the large intestine in mice. Commun Biol 4: 955, 2021. doi: 10.1038/s42003-021-02485-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 156. Del Negro CA, Funk GD, Feldman JL. Breathing matters. Nat Rev Neurosci 19: 351–367, 2018. doi: 10.1038/s41583-018-0003-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 157. Grillner S. Biological pattern generation: the cellular and computational logic of networks in motion. Neuron 52: 751–766, 2006. doi: 10.1016/j.neuron.2006.11.008. [DOI] [PubMed] [Google Scholar]
- 158. Huizinga JD, Lammers WJ. Gut peristalsis is governed by a multitude of cooperating mechanisms. Am J Physiol Gastrointest Liver Physiol 296: G1–G8, 2009. doi: 10.1152/ajpgi.90380.2008. [DOI] [PubMed] [Google Scholar]
- 159. Wood JD. Effects of elevated magnesium on discharge of myenteric neurons of cat small bowel. Am J Physiol 229: 657–662, 1975. doi: 10.1152/ajplegacy.1975.229.3.657. [DOI] [PubMed] [Google Scholar]
- 160. Boesmans W, Ameloot K, van den Abbeel V, Tack J, Vanden Berghe P. Cannabinoid receptor 1 signalling dampens activity and mitochondrial transport in networks of enteric neurones. Neurogastroenterol Motil 21: 958–e977, 2009. doi: 10.1111/j.1365-2982.2009.01300.x. [DOI] [PubMed] [Google Scholar]
- 161. Margiotta JF, Smith-Edwards KM, Nestor-Kalinoski A, Davis BM, Albers KM, Howard MJ. Synaptic components, function and modulation characterized by GCaMP6f Ca2+ imaging in mouse cholinergic myenteric ganglion neurons. Front Physiol 12: 652714, 2021. doi: 10.3389/fphys.2021.652714. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 162. Li Z, Hao MM, Van den Haute C, Baekelandt V, Boesmans W, Vanden Berghe P. Regional complexity in enteric neuron wiring reflects diversity of motility patterns in the mouse large intestine. Elife 8: e42914, 2019. doi: 10.7554/eLife.42914. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 163. Chambers JD, Bornstein JC, Thomas EA. Multiple neural oscillators and muscle feedback are required for the intestinal fed state motor program. PLoS One 6: e19597, 2011. doi: 10.1371/journal.pone.0019597. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 164. Ellis M, Chambers JD, Gwynne RM, Bornstein JC. Serotonin and cholecystokinin mediate nutrient-induced segmentation in guinea pig small intestine. Am J Physiol Gastrointest Liver Physiol 304: G749–G761, 2013. doi: 10.1152/ajpgi.00358.2012. [DOI] [PubMed] [Google Scholar]
- 165. Gwynne RM, Bornstein JC. Mechanisms underlying nutrient-induced segmentation in isolated guinea pig small intestine. Am J Physiol Gastrointest Liver Physiol 292: G1162–G1172, 2007. doi: 10.1152/ajpgi.00441.2006. [DOI] [PubMed] [Google Scholar]
- 166. Huizinga JD, Chen JH, Zhu YF, Pawelka A, McGinn RJ, Bardakjian BL, Parsons SP, Kunze WA, Wu RY, Bercik P, Khoshdel A, Chen S, Yin S, Zhang Q, Yu Y, Gao Q, Li K, Hu X, Zarate N, Collins P, Pistilli M, Ma J, Zhang R, Chen D. The origin of segmentation motor activity in the intestine. Nat Commun 5: 3326, 2014. doi: 10.1038/ncomms4326. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 167. Hao MM, Bornstein JC, Young HM. Development of myenteric cholinergic neurons in ChAT-Cre;R26R-YFP mice. J Comp Neurol 521: 3358–3370, 2013. doi: 10.1002/cne.23354. [DOI] [PubMed] [Google Scholar]
- 168. Erickson CS, Lee SJ, Barlow-Anacker AJ, Druckenbrod NR, Epstein ML, Gosain A. Appearance of cholinergic myenteric neurons during enteric nervous system development: comparison of different ChAT fluorescent mouse reporter lines. Neurogastroenterol Motil 26: 874–884, 2014. doi: 10.1111/nmo.12343. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 169. Parathan P, Wang Y, Leembruggen AJ, Bornstein JC, Foong JP. The enteric nervous system undergoes significant chemical and synaptic maturation during adolescence in mice. Dev Biol 458: 75–87, 2020. doi: 10.1016/j.ydbio.2019.10.011. [DOI] [PubMed] [Google Scholar]
- 170. Avetisyan M, Wang H, Schill EM, Bery S, Grider JR, Hassell JA, Stappenbeck T, Heuckeroth RO. Hepatocyte growth factor and MET support mouse enteric nervous system development, the peristaltic response, and intestinal epithelial proliferation in response to injury. J Neurosci 35: 11543–11558, 2015. doi: 10.1523/JNEUROSCI.5267-14.2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 171. Neuhuber WL, Wörl J. Enteric co-innervation of striated muscle in the esophagus: still enigmatic? Histochem Cell Biol 146: 721–735, 2016. doi: 10.1007/s00418-016-1500-1. [DOI] [PubMed] [Google Scholar]
- 172. Neuhuber WL, Raab M, Berthoud HR, Wörl J. Innervation of the mammalian esophagus. Adv Anat Embryol Cell Biol 185: 1–73, 2006. [PubMed] [Google Scholar]
- 173. Kuramoto H, Yoshimura R, Sakamoto H, Kadowaki M. Regional variations in the number distribution of intrinsic myenteric neurons and coinnervated motor endplates on the striated muscles in the rat esophagus. Auton Neurosci 219: 25–32, 2019. doi: 10.1016/j.autneu.2019.03.004. [DOI] [PubMed] [Google Scholar]
- 174. Fischer A, Canning BJ, Undem BJ, Kummer W. Evidence for an esophageal origin of VIP-IR and NO synthase-IR nerves innervating the guinea pig trachealis: a retrograde neuronal tracing and immunohistochemical analysis. J Comp Neurol 394: 326–334, 1998. doi:. [DOI] [PubMed] [Google Scholar]
- 175. Mazzone SB, McGovern AE. Innervation of tracheal parasympathetic ganglia by esophageal cholinergic neurons: evidence from anatomic and functional studies in guinea pigs. Am J Physiol Lung Cell Mol Physiol 298: L404–L416, 2010. doi: 10.1152/ajplung.00166.2009. [DOI] [PubMed] [Google Scholar]
- 176. Michel K, Reiche D, Schemann M. Projections and neurochemical coding of motor neurones to the circular and longitudinal muscle of the guinea pig gastric corpus. Pflugers Arch 440: 393–408, 2000. doi: 10.1007/s004240000299. [DOI] [PubMed] [Google Scholar]
- 177. Pfannkuche H, Firzlaff U, Sann H, Reiche D, Schemann M. Neurochemical coding and projection patterns of gastrin-releasing peptide-immunoreactive myenteric neurone subpopulations in the guinea-pig gastric fundus. J Chem Neuroanat 19: 93–104, 2000. doi: 10.1016/S0891-0618(00)00057-0. [DOI] [PubMed] [Google Scholar]
- 178. Pfannkuche H, Reiche D, Sann H, Schemann M. Different subpopulations of cholinergic and nitrergic myenteric neurones project to mucosa and circular muscle of the guinea-pig gastric fundus. Cell Tissue Res 292: 463–475, 1998. doi: 10.1007/s004410051075. [DOI] [PubMed] [Google Scholar]
- 179. Pfannkuche H, Reiche D, Firzlaff U, Sann H, Schemann M. Enkephalin-immunoreactive subpopulations in the myenteric plexus of the guinea-pig fundus project primarily to the muscle and not to the mucosa. Cell Tissue Res 294: 45–55, 1998. doi: 10.1007/s004410051155. [DOI] [PubMed] [Google Scholar]
- 180. Reiche D, Michel K, Pfannkuche H, Schemann M. Projections and neurochemistry of interneurones in the myenteric plexus of the guinea-pig gastric corpus. Neurosci Lett 295: 109–112, 2000. doi: 10.1016/s0304-3940(00)01617-7. [DOI] [PubMed] [Google Scholar]
- 181. Sang Q, Young HM. Chemical coding of neurons in the myenteric plexus and external muscle of the small and large intestine of the mouse. Cell Tissue Res 284: 39–53, 1996. doi: 10.1007/s004410050565. [DOI] [PubMed] [Google Scholar]
- 182. Humenick A, Chen BN, Lauder CIW, Wattchow DA, Zagorodnyuk VP, Dinning PG, Spencer NJ, Costa M, Brookes SJ. Characterization of projections of longitudinal muscle motor neurons in human colon. Neurogastroenterol Motil 31: e13685, 2019. doi: 10.1111/nmo.13685. [DOI] [PubMed] [Google Scholar]
- 183. Humenick A, Chen BN, Wattchow DA, Zagorodnyuk VP, Dinning PG, Spencer NJ, Costa M, Brookes SJ. Characterization of putative interneurons in the myenteric plexus of human colon. Neurogastroenterol Motil 33: e13964, 2021. doi: 10.1111/nmo.13964. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 184. Bornstein JC, Furness JB. Correlated electrophysiological and histochemical studies of submucous neurons and their contribution to understanding enteric neural circuits. J Auton Nerv Syst 25: 1–13, 1988. doi: 10.1016/0165-1838(88)90002-1. [DOI] [PubMed] [Google Scholar]
- 185. Smolilo DJ, Hibberd TJ, Costa M, Wattchow DA, De Fontgalland D, Spencer NJ. Intrinsic sensory neurons provide direct input to motor neurons and interneurons in mouse distal colon via varicose baskets. J Comp Neurol 528: 2033–2043, 2020. doi: 10.1002/cne.24872. [DOI] [PubMed] [Google Scholar]
- 186. Smolilo DJ, Costa M, Hibberd TJ, Wattchow DA, Spencer NJ. Morphological evidence for novel enteric neuronal circuitry in guinea pig distal colon. J Comp Neurol 526: 1662–1672, 2018. doi: 10.1002/cne.24436. [DOI] [PubMed] [Google Scholar]
- 187. Smolilo DJ, Costa M, Hibberd TJ, Brookes SJ, Wattchow DA, Spencer NJ. Distribution, projections, and association with calbindin baskets of motor neurons, interneurons, and sensory neurons in guinea-pig distal colon. J Comp Neurol 527: 1140–1158, 2019. doi: 10.1002/cne.24594. [DOI] [PubMed] [Google Scholar]
- 188. Furness JB, Young HM, Pompolo S, Bornstein JC, Kunze WA, McConalogue K. Plurichemical transmission and chemical coding of neurons in the digestive tract. Gastroenterology 108: 554–563, 1995. doi: 10.1016/0016-5085(95)90086-1. [DOI] [PubMed] [Google Scholar]
- 189. Neunlist M, Reiche D, Michel K, Pfannkuche H, Hoppe S, Schemann M. The enteric nervous system: region and target specific projections and neurochemical codes. Eur J Morphol 37: 233–240, 1999. doi: 10.1076/ejom.37.4.233.4720. [DOI] [PubMed] [Google Scholar]
- 190. Timmermans JP, Adriaensen D, Cornelissen W, Scheuermann DW. Structural organization and neuropeptide distribution in the mammalian enteric nervous system, with special attention to those components involved in mucosal reflexes. Comp Biochem Physiol A Physiol 118: 331–340, 1997. doi: 10.1016/s0300-9629(96)00314-3. [DOI] [PubMed] [Google Scholar]
- 191. Dharshika C, Gulbransen BD. Enteric neuromics: how high-throughput “omics” deepens our understanding of enteric nervous system genetic architecture. Cell Mol Gastroenterol Hepatol 15: 487–504, 2022. doi: 10.1016/j.jcmgh.2022.10.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 192. Zeisel A, Hochgerner H, Lönnerberg P, Johnsson A, Memic F, van der Zwan J, Häring M, Braun E, Borm LE, La Manno G, Codeluppi S, Furlan A, Lee K, Skene N, Harris KD, Hjerling-Leffler J, Arenas E, Ernfors P, Marklund U, Linnarsson S. Molecular architecture of the mouse nervous system. Cell 174: 999–1014.e22, 2018. doi: 10.1016/j.cell.2018.06.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 193. May-Zhang AA, Tycksen E, Southard-Smith AN, Deal KK, Benthal JT, Buehler DP, Adam M, Simmons AJ, Monaghan JR, Matlock BK, Flaherty DK, Potter SS, Lau KS, Southard-Smith EM. Combinatorial transcriptional profiling of mouse and human enteric neurons identifies shared and disparate subtypes in situ. Gastroenterology 160: 755–770.e26, 2021. doi: 10.1053/j.gastro.2020.09.032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 194. Wright CM, Schneider S, Smith-Edwards KM, Mafra F, Leembruggen AJ, Gonzalez MV, Kothakapa DR, Anderson JB, Maguire BA, Gao T, Missall TA, Howard MJ, Bornstein JC, Davis BM, Heuckeroth RO. scRNA-Seq reveals new enteric nervous system roles for GDNF, NRTN, and TBX3. Cell Mol Gastroenterol Hepatol 11: 1548–1592.e1, 2021. doi: 10.1016/j.jcmgh.2020.12.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 195. Okamoto T, Barton MJ, Hennig GW, Birch GC, Grainger N, Corrigan RD, Koh SD, Sanders KM, Smith TK. Extensive projections of myenteric serotonergic neurons suggest they comprise the central processing unit in the colon. Neurogastroenterol Motil 26: 556–570, 2014. doi: 10.1111/nmo.12302. [DOI] [PubMed] [Google Scholar]
- 196. Gonzalez Acera M, Bubeck M, Mascia F, Diemand L, Sturm G, Kühl AA, Atreya R, Lie DC, Neurath MF, Schumann M, Klose CS, Trajanoski Z, Becker C, Patankar JV. Dynamic, transient, and robust increase in the innervation of the inflamed mucosa in inflammatory bowel diseases. Cells 10: 2253, 2021. doi: 10.3390/cells10092253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 197. Collins J, Borojevic R, Verdu EF, Huizinga JD, Ratcliffe EM. Intestinal microbiota influence the early postnatal development of the enteric nervous system. Neurogastroenterol Motil 26: 98–107, 2014. doi: 10.1111/nmo.12236. [DOI] [PubMed] [Google Scholar]
- 198. De Vadder F, Grasset E, Mannerås Holm L, Karsenty G, Macpherson AJ, Olofsson LE, Bäckhed F. Gut microbiota regulates maturation of the adult enteric nervous system via enteric serotonin networks. Proc Natl Acad Sci USA 115: 6458–6463, 2018. doi: 10.1073/pnas.1720017115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 199. Kabouridis PS, Lasrado R, McCallum S, Chng SH, Snippert HJ, Clevers H, Pettersson S, Pachnis V. Microbiota controls the homeostasis of glial cells in the gut lamina propria. Neuron 85: 289–295, 2015. doi: 10.1016/j.neuron.2014.12.037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 200. McVey Neufeld KA, Perez-Burgos A, Mao YK, Bienenstock J, Kunze WA. The gut microbiome restores intrinsic and extrinsic nerve function in germ-free mice accompanied by changes in calbindin. Neurogastroenterol Motil 27: 627–636, 2015. doi: 10.1111/nmo.12534. [DOI] [PubMed] [Google Scholar]
- 201. Muller PA, Matheis F, Schneeberger M, Kerner Z, Jové V, Mucida D. Microbiota-modulated CART+ enteric neurons autonomously regulate blood glucose. Science 370: 314–321, 2020. doi: 10.1126/science.abd6176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 202. Aktar R, Parkar N, Stentz R, Baumard L, Parker A, Goldson A, Brion A, Carding S, Blackshaw A, Peiris M. Human resident gut microbe Bacteroides thetaiotaomicron regulates colonic neuronal innervation and neurogenic function. Gut Microbes 11: 1745–1757, 2020. doi: 10.1080/19490976.2020.1766936. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 203. McVey Neufeld KA, Mao YK, Bienenstock J, Foster JA, Kunze WA. The microbiome is essential for normal gut intrinsic primary afferent neuron excitability in the mouse. Neurogastroenterol Motil 25: 183–e188, 2013. doi: 10.1111/nmo.12049. [DOI] [PubMed] [Google Scholar]
- 204. Obata Y, Castaño A, Boeing S, Bon-Frauches AC, Fung C, Fallesen T, de Agüero MG, Yilmaz B, Lopes R, Huseynova A, Horswell S, Maradana MR, Boesmans W, Vanden Berghe P, Murray AJ, Stockinger B, Macpherson AJ, Pachnis V. Neuronal programming by microbiota regulates intestinal physiology. Nature 578: 284–289, 2020. doi: 10.1038/s41586-020-1975-8. [DOI] [PubMed] [Google Scholar]
- 205. Nyavor Y, Brands CR, May G, Kuther S, Nicholson J, Tiger K, Tesnohlidek A, Yasuda A, Starks K, Litvinenko D, Linden DR, Bhattarai Y, Kashyap PC, Forney LJ, Balemba OB. High-fat diet-induced alterations to gut microbiota and gut-derived lipoteichoic acid contributes to the development of enteric neuropathy. Neurogastroenterol Motil 32: e13838, 2020. doi: 10.1111/nmo.13838. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 206. Hung LY, Boonma P, Unterweger P, Parathan P, Haag A, Luna RA, Bornstein JC, Savidge TC, Foong JP. Neonatal antibiotics disrupt motility and enteric neural circuits in mouse colon. Cell Mol Gastroenterol Hepatol 8: 298–300.e6, 2019. doi: 10.1016/j.jcmgh.2019.04.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 207. Poon SS, Hung LY, Wu Q, Parathan P, Yalcinkaya N, Haag A, Luna RA, Bornstein JC, Savidge TC, Foong JP. Neonatal antibiotics have long term sex-dependent effects on the enteric nervous system. J Physiol 600: 4303–4323, 2022. doi: 10.1113/JP282939. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 208. Hung LY, Parathan P, Boonma P, Wu Q, Wang Y, Haag A, Luna RA, Bornstein JC, Savidge TC, Foong JP. Antibiotic exposure postweaning disrupts the neurochemistry and function of enteric neurons mediating colonic motor activity. Am J Physiol Gastrointest Liver Physiol 318: G1042–G1053, 2020. doi: 10.1152/ajpgi.00088.2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 209. Yarandi SS, Kulkarni S, Saha M, Sylvia KE, Sears CL, Pasricha PJ. Intestinal bacteria maintain adult enteric nervous system and nitrergic neurons via toll-like receptor 2-induced neurogenesis in mice. Gastroenterology 159: 200–213.e8, 2020. doi: 10.1053/j.gastro.2020.03.050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 210. Caputi V, Marsilio I, Filpa V, Cerantola S, Orso G, Bistoletti M, Paccagnella N, De Martin S, Montopoli M, Dall’Acqua S, Crema F, Di Gangi IM, Galuppini F, Lante I, Bogialli S, Rugge M, Debetto P, Giaroni C, Giron MC. Antibiotic-induced dysbiosis of the microbiota impairs gut neuromuscular function in juvenile mice. Br J Pharmacol 174: 3623–3639, 2017. doi: 10.1111/bph.13965. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 211. Vicentini FA, Keenan CM, Wallace LE, Woods C, Cavin JB, Flockton AR, Macklin WB, Belkind-Gerson J, Hirota SA, Sharkey KA. Intestinal microbiota shapes gut physiology and regulates enteric neurons and glia. Microbiome 9: 210, 2021. doi: 10.1186/s40168-021-01165-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 212. Chandrasekharan B, Saeedi BJ, Alam A, Houser M, Srinivasan S, Tansey M, Jones R, Nusrat A, Neish AS. Interactions between commensal bacteria and enteric neurons, via FPR1 induction of ROS, increase gastrointestinal motility in mice. Gastroenterology 157: 179–192.e2, 2019. doi: 10.1053/j.gastro.2019.03.045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 213. di Giancamillo A, Vitari F, Bosi G, Savoini G, Domeneghini C. The chemical code of porcine enteric neurons and the number of enteric glial cells are altered by dietary probiotics. Neurogastroenterol Motil 22: e271–e278, 2010. doi: 10.1111/j.1365-2982.2010.01529.x. [DOI] [PubMed] [Google Scholar]
- 214. Mao YK, Kasper DL, Wang B, Forsythe P, Bienenstock J, Kunze WA. Bacteroides fragilis polysaccharide A is necessary and sufficient for acute activation of intestinal sensory neurons. Nat Commun 4: 1465, 2013. doi: 10.1038/ncomms2478. [DOI] [PubMed] [Google Scholar]
- 215. Perez-Burgos A, Mao YK, Bienenstock J, Kunze WA. The gut-brain axis rewired: adding a functional vagal nicotinic “sensory synapse”. FASEB J 28: 3064–3074, 2014. doi: 10.1096/fj.13-245282. [DOI] [PubMed] [Google Scholar]
- 216. Khoshdel A, Verdu EF, Kunze W, McLean P, Bergonzelli G, Huizinga JD. Bifidobacterium longum NCC3001 inhibits AH neuron excitability. Neurogastroenterol Motil 25: e478–e484, 2013. doi: 10.1111/nmo.12147. [DOI] [PubMed] [Google Scholar]
- 217. Kamm K, Hoppe S, Breves G, Schröder B, Schemann M. Effects of the probiotic yeast Saccharomyces boulardii on the neurochemistry of myenteric neurones in pig jejunum. Neurogastroenterol Motil 16: 53–60, 2004. doi: 10.1046/j.1365-2982.2003.00458.x. [DOI] [PubMed] [Google Scholar]
- 218. Brun P, Scarpa M, Marchiori C, Sarasin G, Caputi V, Porzionato A, Giron MC, Palù G, Castagliuolo I. Saccharomyces boulardii CNCM I-745 supplementation reduces gastrointestinal dysfunction in an animal model of IBS. PLoS One 12: e0181863, 2017. doi: 10.1371/journal.pone.0181863. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 219. Orandle MS, Veazey RS, Lackner AA. Enteric ganglionitis in rhesus macaques infected with simian immunodeficiency virus. J Virol 81: 6265–6275, 2007. doi: 10.1128/JVI.02671-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 220. Brun P, Giron MC, Zoppellaro C, Bin A, Porzionato A, De Caro R, Barbara G, Stanghellini V, Corinaldesi R, Zaninotto G, Palù G, Gaion RM, Tonini M, De Giorgio R, Castagliuolo I. Herpes simplex virus type 1 infection of the rat enteric nervous system evokes small-bowel neuromuscular abnormalities. Gastroenterology 138: 1790–1801, 2010. doi: 10.1053/j.gastro.2010.01.036. [DOI] [PubMed] [Google Scholar]
- 221. Gesser RM, Koo SC. Oral inoculation with herpes simplex virus type 1 infects enteric neuron and mucosal nerve fibers within the gastrointestinal tract in mice. J Virol 70: 4097–4102, 1996. doi: 10.1128/JVI.70.6.4097-4102.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 222. Brun P, Qesari M, Marconi PC, Kotsafti A, Porzionato A, Macchi V, Schwendener RA, Scarpa M, Giron MC, Palù G, Calistri A, Castagliuolo I. Herpes simplex virus type 1 infects enteric neurons and triggers gut dysfunction via macrophage recruitment. Front Cell Infect Microbiol 8: 74, 2018. doi: 10.3389/fcimb.2018.00074. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 223. Chen JJ, Gershon AA, Li ZS, Lungu O, Gershon MD. Latent and lytic infection of isolated guinea pig enteric ganglia by varicella zoster virus. J Med Virol 70, Suppl 1: S71–S78, 2003. doi: 10.1002/jmv.10325. [DOI] [PubMed] [Google Scholar]
- 224. Chen JJ, Gershon AA, Li Z, Cowles RA, Gershon MD. Varicella zoster virus (VZV) infects and establishes latency in enteric neurons. J Neurovirol 17: 578–589, 2011. doi: 10.1007/s13365-011-0070-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 225. Robertson CS, Martin BA, Atkinson M. Varicella-zoster virus DNA in the oesophageal myenteric plexus in achalasia. Gut 34: 299–302, 1993. doi: 10.1136/gut.34.3.299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 226. Naik RD, Vaezi MF, Gershon AA, Higginbotham T, Chen JJ, Flores E, Holzman M, Patel D, Gershon MD. Association of achalasia with active varicella zoster virus infection of the esophagus. Gastroenterology 161: 719–721.e2, 2021. doi: 10.1053/j.gastro.2021.04.057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 227. Selgrad M, De Giorgio R, Fini L, Cogliandro RF, Williams S, Stanghellini V, Barbara G, Tonini M, Corinaldesi R, Genta RM, Domiati-Saad R, Meyer R, Goel A, Boland CR, Ricciardiello L. JC virus infects the enteric glia of patients with chronic idiopathic intestinal pseudo-obstruction. Gut 58: 25–32, 2009. doi: 10.1136/gut.2008.152512. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 228. Gray-Rodriguez S, Jensen MP, Otero-Jimenez M, Hanley B, Swann OC, Ward PA, Salguero FJ, Querido N, Farkas I, Velentza-Almpani E, Weir J, Barclay WS, Carroll MW, Jaunmuktane Z, Brandner S, Pohl U, Allinson K, Thom M, Troakes C, Al-Sarraj S, Sastre M, Gveric D, Gentleman S, Roufosse C, Osborn M, Alegre-Abarrategui J. Multisystem screening reveals SARS-CoV-2 in neurons of the myenteric plexus and in megakaryocytes. J Pathol 257: 198–217, 2022. doi: 10.1002/path.5878. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 229. Barajon I, Serrao G, Arnaboldi F, Opizzi E, Ripamonti G, Balsari A, Rumio C. Toll-like receptors 3, 4, and 7 are expressed in the enteric nervous system and dorsal root ganglia. J Histochem Cytochem 57: 1013–1023, 2009. doi: 10.1369/jhc.2009.953539. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 230. Anitha M, Vijay-Kumar M, Sitaraman SV, Gewirtz AT, Srinivasan S. Gut microbial products regulate murine gastrointestinal motility via Toll-like receptor 4 signaling. Gastroenterology 143: 1006–16.e4, 2012. doi: 10.1053/j.gastro.2012.06.034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 231. Brun P, Giron MC, Qesari M, Porzionato A, Caputi V, Zoppellaro C, Banzato S, Grillo AR, Spagnol L, De Caro R, Pizzuti D, Barbieri V, Rosato A, Sturniolo GC, Martines D, Zaninotto G, Palù G, Castagliuolo I. Toll-like receptor 2 regulates intestinal inflammation by controlling integrity of the enteric nervous system. Gastroenterology 145: 1323–1333, 2013. doi: 10.1053/j.gastro.2013.08.047. [DOI] [PubMed] [Google Scholar]
- 232. Brun P, Gobbo S, Caputi V, Spagnol L, Schirato G, Pasqualin M, Levorato E, Palù G, Giron MC, Castagliuolo I. Toll like receptor-2 regulates production of glial-derived neurotrophic factors in murine intestinal smooth muscle cells. Mol Cell Neurosci 68: 24–35, 2015. doi: 10.1016/j.mcn.2015.03.018. [DOI] [PubMed] [Google Scholar]
- 233. Caputi V, Marsilio I, Cerantola S, Roozfarakh M, Lante I, Galuppini F, Rugge M, Napoli E, Giulivi C, Orso G, Giron MC. Toll-like receptor 4 modulates small intestine neuromuscular function through nitrergic and purinergic pathways. Front Pharmacol 8: 350, 2017. doi: 10.3389/fphar.2017.00350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 234. Beckett EA, Staikopoulos V, Hutchinson MR. Differential effect of morphine on gastrointestinal transit, colonic contractions and nerve-evoked relaxations in Toll-like receptor deficient mice. Sci Rep 8: 5923, 2018. doi: 10.1038/s41598-018-23717-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 235. Nøhr MK, Pedersen MH, Gille A, Egerod KL, Engelstoft MS, Husted AS, Sichlau RM, Grunddal KV, Poulsen SS, Han S, Jones RM, Offermanns S, Schwartz TW. GPR41/FFAR3 and GPR43/FFAR2 as cosensors for short-chain fatty acids in enteroendocrine cells vs FFAR3 in enteric neurons and FFAR2 in enteric leukocytes. Endocrinology 154: 3552–3564, 2013. doi: 10.1210/en.2013-1142. [DOI] [PubMed] [Google Scholar]
- 236. Bertrand PP, Kunze WA, Bornstein JC, Furness JB, Smith ML. Analysis of the responses of myenteric neurons in the small intestine to chemical stimulation of the mucosa. Am J Physiol Gastrointest Liver Physiol 273: G422–G435, 1997. doi: 10.1152/ajpgi.1997.273.2.G422. [DOI] [PubMed] [Google Scholar]
- 237. Lieu T, Jayaweera G, Bunnett NW. GPBA: a GPCR for bile acids and an emerging therapeutic target for disorders of digestion and sensation. Br J Pharmacol 171: 1156–1166, 2014. doi: 10.1111/bph.12426. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 238. Sugisawa E, Takayama Y, Takemura N, Kondo T, Hatakeyama S, Kumagai Y, Sunagawa M, Tominaga M, Maruyama K. RNA sensing by gut Piezo1 is essential for systemic serotonin synthesis. Cell 182: 609–624.e21, 2020. doi: 10.1016/j.cell.2020.06.022. [DOI] [PubMed] [Google Scholar]
- 239. Chen Z, Luo J, Li J, Kim G, Stewart A, Urban JF Jr, Huang Y, Chen S, Wu LG, Chesler A, Trinchieri G, Li W, Wu C. Interleukin-33 promotes serotonin release from enterochromaffin cells for intestinal homeostasis. Immunity 54: 151–163.e6, 2021. doi: 10.1016/j.immuni.2020.10.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 240. Wang H, Kwon YH, Dewan V, Vahedi F, Syed S, Fontes ME, Ashkar AA, Surette MG, Khan WI. TLR2 plays a pivotal role in mediating mucosal serotonin production in the gut. J Immunol 202: 3041–3052, 2019. doi: 10.4049/jimmunol.1801034. [DOI] [PubMed] [Google Scholar]
- 241. Kovler ML, Salazar AJ, Fulton WB, Lu P, Yamaguchi Y, Zhou Q, Sampah M, Ishiyama A, Prindle T Jr, Wang S, Jia H, Wipf P, Sodhi CP, Hackam DJ. Toll-like receptor 4-mediated enteric glia loss is critical for the development of necrotizing enterocolitis. Sci Transl Med 13: eabg3459, 2021. doi: 10.1126/scitranslmed.abg3459. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 242. Seguella L, Gulbransen BD. Enteric glial biology, intercellular signalling and roles in gastrointestinal disease. Nat Rev Gastroenterol Hepatol 18: 571–587, 2021. doi: 10.1038/s41575-021-00423-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 243. Kunze WA, Mao YK, Wang B, Huizinga JD, Ma X, Forsythe P, Bienenstock J. Lactobacillus reuteri enhances excitability of colonic AH neurons by inhibiting calcium-dependent potassium channel opening. J Cell Mol Med 13: 2261–2270, 2009. doi: 10.1111/j.1582-4934.2009.00686.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 244. Al-Nedawi K, Mian MF, Hossain N, Karimi K, Mao YK, Forsythe P, Min KK, Stanisz AM, Kunze WA, Bienenstock J. Gut commensal microvesicles reproduce parent bacterial signals to host immune and enteric nervous systems. FASEB J 29: 684–695, 2015. doi: 10.1096/fj.14-259721. [DOI] [PubMed] [Google Scholar]
- 245. Gershon M, Gershon A. Varicella-zoster virus and the enteric nervous system. J Infect Dis 218: S113–S119, 2018. doi: 10.1093/infdis/jiy407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 246. De Giorgio R, Ricciardiello L, Naponelli V, Selgrad M, Piazzi G, Felicani C, Serra M, Fronzoni L, Antonucci A, Cogliandro RF, Barbara G, Corinaldesi R, Tonini M, Knowles CH, Stanghellini V. Chronic intestinal pseudo-obstruction related to viral infections. Transplant Proc 42: 9–14, 2010. doi: 10.1016/j.transproceed.2009.12.014. [DOI] [PubMed] [Google Scholar]
- 247. Julio-Pieper M, López-Aguilera A, Eyzaguirre-Velásquez J, Olavarría-Ramírez L, Ibacache-Quiroga C, Bravo JA, Cruz G. Gut susceptibility to viral invasion: contributing roles of diet, microbiota and enteric nervous system to mucosal barrier preservation. Int J Mol Sci 22: 4734, 2021. doi: 10.3390/ijms22094734. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 248. Foong JP, Hung LY, Poon S, Savidge TC, Bornstein JC. Early life interaction between the microbiota and the enteric nervous system. Am J Physiol Gastrointest Liver Physiol 319: G541–G548, 2020. doi: 10.1152/ajpgi.00288.2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 249. Heiss CN, Olofsson LE. The role of the gut microbiota in development, function and disorders of the central nervous system and the enteric nervous system. J Neuroendocrinol 31: e12684, 2019. doi: 10.1111/jne.12684. [DOI] [PubMed] [Google Scholar]
- 250. Obata Y, Pachnis V. The effect of microbiota and the immune system on the development and organization of the enteric nervous system. Gastroenterology 151: 836–844, 2016. doi: 10.1053/j.gastro.2016.07.044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 251. Karakan T, Ozkul C, Küpeli Akkol E, Bilici S, Sobarzo-Sánchez E, Capasso R. Gut-brain-microbiota axis: antibiotics and functional gastrointestinal disorders. Nutrients 13: 389, 2021. doi: 10.3390/nu13020389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 252. Fried S, Wemelle E, Cani PD, Knauf C. Interactions between the microbiota and enteric nervous system during gut-brain disorders. Neuropharmacology 197: 108721, 2021. doi: 10.1016/j.neuropharm.2021.108721. [DOI] [PubMed] [Google Scholar]
- 253. Yoo BB, Mazmanian SK. The enteric network: interactions between the immune and nervous systems of the gut. Immunity 46: 910–926, 2017. doi: 10.1016/j.immuni.2017.05.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 254. Kulkarni S, Kurapati S, Bogunovic M. Neuro-innate immune interactions in gut mucosal immunity. Curr Opin Immunol 68: 64–71, 2021. doi: 10.1016/j.coi.2020.09.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 255. Klose CS, Veiga-Fernandes H. Neuroimmune interactions in peripheral tissues. Eur J Immunol 51: 1602–1614, 2021. doi: 10.1002/eji.202048812. [DOI] [PubMed] [Google Scholar]
- 256. Ramirez V, Swain S, Murray K, Reardon C. Neural immune communication in the control of host-bacterial pathogen interactions in the gastrointestinal tract. Infect Immun 88: e00928-19, 2020. doi: 10.1128/IAI.00928-19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 257. Klein Wolterink RG, Wu GS, Chiu IM, Veiga-Fernandes H. Neuroimmune interactions in peripheral organs. Annu Rev Neurosci 45: 339–360, 2022. doi: 10.1146/annurev-neuro-111020-105359. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 258. Nowarski R, Jackson R, Gagliani N, de Zoete MR, Palm NW, Bailis W, Low JS, Harman CC, Graham M, Elinav E, Flavell RA. Epithelial IL-18 equilibrium controls barrier function in colitis. Cell 163: 1444–1456, 2015. doi: 10.1016/j.cell.2015.10.072. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 259. Jarret A, Jackson R, Duizer C, Healy ME, Zhao J, Rone JM, Bielecki P, Sefik E, Roulis M, Rice T, Sivanathan KN, Zhou T, Solis AG, Honcharova-Biletska H, Vélez K, Hartner S, Low JS, Qu R, de Zoete MR, Palm NW, Ring AM, Weber A, Moor AE, Kluger Y, Nowarski R, Flavell RA. Enteric nervous system-derived IL-18 orchestrates mucosal barrier immunity. Cell 180: 813–814, 2020. doi: 10.1016/j.cell.2020.02.004. [DOI] [PubMed] [Google Scholar]
- 260. Furness JB, Pompolo S, Murphy R, Giraud A. Projections of neurons with neuromedin U-like immunoreactivity in the small intestine of the guinea-pig. Cell Tissue Res 257: 415–422, 1989. doi: 10.1007/BF00261844. [DOI] [PubMed] [Google Scholar]
- 261. Yan Y, Ramanan D, Rozenberg M, McGovern K, Rastelli D, Vijaykumar B, Yaghi O, Voisin T, Mosaheb M, Chiu I, Itzkovitz S, Rao M, Mathis D, Benoist C. Interleukin-6 produced by enteric neurons regulates the number and phenotype of microbe-responsive regulatory T cells in the gut. Immunity 54: 499–513.e5, 2021. doi: 10.1016/j.immuni.2021.02.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 262. Sang Q, Williamson S, Young HM. Projections of chemically identified myenteric neurons of the small and large intestine of the mouse. J Anat 190: 209–222, 1997. doi: 10.1046/j.1469-7580.1997.19020209.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 263. Greenwood B, Mantle M. Mucin and protein release in the rabbit jejunum: effects of bethanechol and vagal nerve stimulation. Gastroenterology 103: 496–505, 1992. doi: 10.1016/0016-5085(92)90839-q. [DOI] [PubMed] [Google Scholar]
- 264. Phillips TE, Phillips TH, Neutra MR. Regulation of intestinal goblet cell secretion. IV. Electrical field stimulation in vitro. Am J Physiol Gastrointest Liver Physiol 247: G682–G687, 1984. doi: 10.1152/ajpgi.1984.247.6.G682. [DOI] [PubMed] [Google Scholar]
- 265. Gustafsson JK, Davis JE, Rappai T, McDonald KG, Kulkarni DH, Knoop KA, Hogan SP, Fitzpatrick JA, Lencer WI, Newberry RD. Intestinal goblet cells sample and deliver lumenal antigens by regulated endocytic uptake and transcytosis. Elife 10: e67292, 2021. doi: 10.7554/eLife.67292. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 266. Ansaldo E, Farley TK, Belkaid Y. Control of Immunity by the Microbiota. Annu Rev Immunol 39: 449–479, 2021. doi: 10.1146/annurev-immunol-093019-112348. [DOI] [PubMed] [Google Scholar]
- 267. Gulbransen BD, Sharkey KA. Novel functional roles for enteric glia in the gastrointestinal tract. Nat Rev Gastroenterol Hepatol 9: 625–632, 2012. doi: 10.1038/nrgastro.2012.138. [DOI] [PubMed] [Google Scholar]
- 268. Rosenberg HJ, Rao M. Enteric glia in homeostasis and disease: from fundamental biology to human pathology. iScience 24: 102863, 2021. doi: 10.1016/j.isci.2021.102863. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 269. Schonkeren SL, Thijssen MS, Vaes N, Boesmans W, Melotte V. The emerging role of nerves and glia in colorectal cancer. Cancers (Basel) 13: 152, 2021. doi: 10.3390/cancers13010152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 270. Gabella G. Enteric glia: extent, cohesion, axonal contacts, membrane separations and mitochondria in Auerbach’s ganglia of guinea pigs. Cell Tissue Res 389: 409–426, 2022. doi: 10.1007/s00441-022-03656-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 271. Boesmans W, Hao MM, Fung C, Li Z, Van den Haute C, Tack J, Pachnis V, Vanden Berghe P. Structurally defined signaling in neuro-glia units in the enteric nervous system. Glia 67: 1167–1178, 2019. doi: 10.1002/glia.23596. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 272. Gomes P, Chevalier J, Boesmans W, Roosen L, van den Abbeel V, Neunlist M, Tack J, Vanden Berghe P. ATP-dependent paracrine communication between enteric neurons and glia in a primary cell culture derived from embryonic mice. Neurogastroenterol Motil 21: 870-e62, 2009. doi: 10.1111/j.1365-2982.2009.01302.x. [DOI] [PubMed] [Google Scholar]
- 273. Gulbransen BD, Bashashati M, Hirota SA, Gui X, Roberts JA, MacDonald JA, Muruve DA, McKay DM, Beck PL, Mawe GM, Thompson RJ, Sharkey KA. Activation of neuronal P2X7 receptor-pannexin-1 mediates death of enteric neurons during colitis. Nat Med 18: 600–604, 2012. doi: 10.1038/nm.2679. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 274. Sanders KM, Ward SM, Koh SD. Interstitial cells: regulators of smooth muscle function. Physiol Rev 94: 859–907, 2014. doi: 10.1152/physrev.00037.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 275. Cossais F, Durand T, Chevalier J, Boudaud M, Kermarrec L, Aubert P, Neveu I, Naveilhan P, Neunlist M. Postnatal development of the myenteric glial network and its modulation by butyrate. Am J Physiol Gastrointest Liver Physiol 310: G941–G951, 2016. doi: 10.1152/ajpgi.00232.2015. [DOI] [PubMed] [Google Scholar]
- 276. Inlender T, Nissim-Eliraz E, Stavely R, Hotta R, Goldstein AM, Yagel S, Gutnick MJ, Shpigel NY. Homeostasis of mucosal glial cells in human gut is independent of microbiota. Sci Rep 11: 12796, 2021. doi: 10.1038/s41598-021-92384-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 277. D’Errico F, Goverse G, Dai Y, Wu W, Stakenborg M, Labeeuw E, De Simone V, Verstockt B, Gomez-Pinilla PJ, Warner M, Di Leo A, Matteoli G, Gustafsson JA. Estrogen receptor beta controls proliferation of enteric glia and differentiation of neurons in the myenteric plexus after damage. Proc Natl Acad Sci USA 115: 5798–5803, 2018. doi: 10.1073/pnas.1720267115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 278. Laranjeira C, Sandgren K, Kessaris N, Richardson W, Potocnik A, Vanden Berghe P, Pachnis V. Glial cells in the mouse enteric nervous system can undergo neurogenesis in response to injury. J Clin Invest 121: 3412–3424, 2011. doi: 10.1172/JCI58200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 279. Belkind-Gerson J, Graham HK, Reynolds J, Hotta R, Nagy N, Cheng L, Kamionek M, Shi HN, Aherne CM, Goldstein AM. Colitis promotes neuronal differentiation of Sox2+ and PLP1+ enteric cells. Sci Rep 7: 2525, 2017. doi: 10.1038/s41598-017-02890-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 280. Middelhoff M, Valenti G, Tomassoni L, Ochiai Y, Belin B, Takahashi R, Malagola E, Nienhüser H, Finlayson M, Hayakawa Y, Zamechek LB, Renz BW, Westphalen CB, Quante M, Margolis KG, Sims PA, Laise P, Califano A, Rao M, Gershon MD, Wang TC. Adult enteric Dclk1-positive glial and neuronal cells reveal distinct responses to acute intestinal injury. Am J Physiol Gastrointest Liver Physiol 322: G583–G597, 2022. doi: 10.1152/ajpgi.00244.2021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 281. Seguella L, McClain JL, Esposito G, Gulbransen BD. Functional intraregional and interregional heterogeneity between myenteric glial cells of the colon and duodenum in mice. J Neurosci 42: 8694–8708, 2022. doi: 10.1523/JNEUROSCI.2379-20.2022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 282. Bohórquez DV, Samsa LA, Roholt A, Medicetty S, Chandra R, Liddle RA. An enteroendocrine cell-enteric glia connection revealed by 3D electron microscopy. PLoS One 9: e89881, 2014. doi: 10.1371/journal.pone.0089881. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 283. Baghdadi MB, Ayyaz A, Coquenlorge S, Chu B, Kumar S, Streutker C, Wrana JL, Kim TH. Enteric glial cell heterogeneity regulates intestinal stem cell niches. Cell Stem Cell 29: 86–100.e6, 2022. doi: 10.1016/j.stem.2021.10.004. [DOI] [PubMed] [Google Scholar]
- 284. Ibiza S, García-Cassani B, Ribeiro H, Carvalho T, Almeida L, Marques R, Misic AM, Bartow-McKenney C, Larson DM, Pavan WJ, Eberl G, Grice EA, Veiga-Fernandes H. Glial-cell-derived neuroregulators control type 3 innate lymphoid cells and gut defence. Nature 535: 440–443, 2016. doi: 10.1038/nature18644. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 285. Grubišić V, McClain JL, Fried DE, Grants I, Rajasekhar P, Csizmadia E, Ajijola OA, Watson RE, Poole DP, Robson SC, Christofi FL, Gulbransen BD. Enteric glia modulate macrophage phenotype and visceral sensitivity following inflammation. Cell Rep 32: 108100, 2020. doi: 10.1016/j.celrep.2020.108100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 286. Chow AK, Grubišić V, Gulbransen BD. Enteric glia regulate lymphocyte activation via autophagy-mediated MHC-II expression. Cell Mol Gastroenterol Hepatol 12: 1215–1237, 2021. doi: 10.1016/j.jcmgh.2021.06.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 287. Stakenborg M, Abdurahiman S, De Simone V, Goverse G, Stakenborg N, van Baarle L, Wu Q, Pirottin D, Kim JS, Chappell-Maor L, Pintelon I, Thys S, Pollenus E, Boon L, Van den Steen P, Hao M, Van Ginderachter JA, Boeckxstaens GE, Timmermans JP, Jung S, Marichal T, Ibiza S, Matteoli G. Enteric glial cells favor accumulation of anti-inflammatory macrophages during the resolution of muscularis inflammation. Mucosal Immunol 15: 1296–1308, 2022. doi: 10.1038/s41385-022-00563-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 288. Bush TG, Savidge TC, Freeman TC, Cox HJ, Campbell EA, Mucke L, Johnson MH, Sofroniew MV. Fulminant jejuno-ileitis following ablation of enteric glia in adult transgenic mice. Cell 93: 189–201, 1998. doi: 10.1016/s0092-8674(00)81571-8. [DOI] [PubMed] [Google Scholar]
- 289. Rao M, Rastelli D, Dong L, Chiu S, Setlik W, Gershon MD, Corfas G. Enteric glia regulate gastrointestinal motility but are not required for maintenance of the epithelium in mice. Gastroenterology 153: 1068–1081.e7, 2017. doi: 10.1053/j.gastro.2017.07.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 290. Seguella L, Pesce M, Capuano R, Casano F, Pesce M, Corpetti C, Vincenzi M, Maftei D, Lattanzi R, Del Re A, Sarnelli G, Gulbransen BD, Esposito G. High-fat diet impairs duodenal barrier function and elicits glia-dependent changes along the gut-brain axis that are required for anxiogenic and depressive-like behaviors. J Neuroinflammation 18: 115, 2021. doi: 10.1186/s12974-021-02164-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 291. Meira de-Faria F, Casado-Bedmar M, Lindqvist C, Jones MP, Walter SA, Keita AV. Altered interaction between enteric glial cells and mast cells in the colon of women with irritable bowel syndrome. Neurogastroenterol Motil 33: e14130, 2021. doi: 10.1111/nmo.14130. [DOI] [PubMed] [Google Scholar]
- 292. Lucarini E, Seguella L, Vincenzi M, Parisio C, Micheli L, Toti A, Corpetti C, Del Re A, Squillace S, Maftei D, Lattanzi R, Ghelardini C, Di Cesare Mannelli L, Esposito G. Role of enteric glia as bridging element between gut inflammation and visceral pain consolidation during acute colitis in rats. Biomedicines 9: 1671, 2021. doi: 10.3390/biomedicines9111671. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 293. Progatzky F, Shapiro M, Chng SH, Garcia-Cassani B, Classon CH, Sevgi S, Laddach A, Bon-Frauches AC, Lasrado R, Rahim M, Amaniti EM, Boeing S, Shah K, Entwistle LJ, Suárez-Bonnet A, Wilson MS, Stockinger B, Pachnis V. Regulation of intestinal immunity and tissue repair by enteric glia. Nature 599: 125–130, 2021. doi: 10.1038/s41586-021-04006-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 294. Sanders KM, Kito Y, Hwang SJ, Ward SM. Regulation of gastrointestinal smooth muscle function by interstitial cells. Physiology (Bethesda) 31: 316–326, 2016. doi: 10.1152/physiol.00006.2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 295. Popescu LM, Faussone-Pellegrini MS. TELOCYTES—a case of serendipity: the winding way from interstitial cells of Cajal (ICC), via interstitial Cajal-like cells (ICLC) to TELOCYTES. J Cell Mol Med 14: 729–740, 2010. doi: 10.1111/j.1582-4934.2010.01059.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 296. Vannucchi MG, Traini C, Manetti M, Ibba-Manneschi L, Faussone-Pellegrini MS. Telocytes express PDGFRalpha in the human gastrointestinal tract. J Cell Mol Med 17: 1099–1108, 2013. doi: 10.1111/jcmm.12134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 297. Vannucchi MG. The telocytes: ten years after their introduction in the scientific literature. An update on their morphology, distribution, and potential roles in the gut. Int J Mol Sci 21: 4478, 2020. doi: 10.3390/ijms21124478. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 298. Kaestner KH. The intestinal stem cell niche: a central role for Foxl1-expressing subepithelial telocytes. Cell Mol Gastroenterol Hepatol 8: 111–117, 2019. doi: 10.1016/j.jcmgh.2019.04.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 299. Kurahashi M, Nakano Y, Peri LE, Townsend JB, Ward SM, Sanders KM. A novel population of subepithelial platelet-derived growth factor receptor alpha-positive cells in the mouse and human colon. Am J Physiol Gastrointest Liver Physiol 304: G823–G834, 2013. doi: 10.1152/ajpgi.00001.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 300. Baker SA, Hennig GW, Ward SM, Sanders KM. Temporal sequence of activation of cells involved in purinergic neurotransmission in the colon. J Physiol 593: 1945–1963, 2015. doi: 10.1113/jphysiol.2014.287599. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 301. Horiguchi K, Komuro T. Ultrastructural observations of fibroblast-like cells forming gap junctions in the W/Wnu mouse small intestine. J Auton Nerv Syst 80: 142–147, 2000. doi: 10.1016/S0165-1838(00)00089-8. [DOI] [PubMed] [Google Scholar]
- 302. Komuro T, Seki K, Horiguchi K. Ultrastructural characterization of the interstitial cells of Cajal. Arch Histol Cytol 62: 295–316, 1999. doi: 10.1679/aohc.62.295. [DOI] [PubMed] [Google Scholar]
- 303. Sanders KM, Koh SD, Ro S, Ward SM. Regulation of gastrointestinal motility–insights from smooth muscle biology. Nat Rev Gastroenterol Hepatol 9: 633–645, 2012. doi: 10.1038/nrgastro.2012.168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 304. Foong D, Liyanage L, Zhou J, Zarrouk A, Ho V, O’Connor MD. Single-cell RNA sequencing predicts motility networks in purified human gastric interstitial cells of Cajal. Neurogastroenterol Motil 34: e14303, 2022. doi: 10.1111/nmo.14303. [DOI] [PubMed] [Google Scholar]
- 305. Klein S, Seidler B, Kettenberger A, Sibaev A, Rohn M, Feil R, Allescher HD, Vanderwinden JM, Hofmann F, Schemann M, Rad R, Storr MA, Schmid RM, Schneider G, Saur D. Interstitial cells of Cajal integrate excitatory and inhibitory neurotransmission with intestinal slow-wave activity. Nat Commun 4: 1630, 2013. doi: 10.1038/ncomms2626. [DOI] [PubMed] [Google Scholar]
- 306. Blair PJ, Rhee PL, Sanders KM, Ward SM. The significance of interstitial cells in neurogastroenterology. J Neurogastroenterol Motil 20: 294–317, 2014. doi: 10.5056/jnm14060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 307. Zhu MH, Kim TW, Ro S, Yan W, Ward SM, Koh SD, Sanders KM. A Ca2+-activated Cl- conductance in interstitial cells of Cajal linked to slow wave currents and pacemaker activity. J Physiol 587: 4905–4918, 2009. doi: 10.1113/jphysiol.2009.176206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 308. Gomez-Pinilla PJ, Gibbons SJ, Bardsley MR, Lorincz A, Pozo MJ, Pasricha PJ, Van de Rijn M, West RB, Sarr MG, Kendrick ML, Cima RR, Dozois EJ, Larson DW, Ordog T, Farrugia G. Ano1 is a selective marker of interstitial cells of Cajal in the human and mouse gastrointestinal tract. Am J Physiol Gastrointest Liver Physiol 296: G1370–G1381, 2009. doi: 10.1152/ajpgi.00074.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 309. Drumm BT, Rembetski BE, Huynh K, Nizar A, Baker SA, Sanders KM. Excitatory cholinergic responses in mouse colon intramuscular interstitial cells of Cajal are due to enhanced Ca2+ release via M3 receptor activation. FASEB J 34: 10073–10095, 2020. doi: 10.1096/fj.202000672R. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 310. Sung TS, Hwang SJ, Koh SD, Bayguinov Y, Peri LE, Blair PJ, Webb TI, Pardo DM, Rock JR, Sanders KM, Ward SM. The cells and conductance mediating cholinergic neurotransmission in the murine proximal stomach. J Physiol 596: 1549–1574, 2018. doi: 10.1113/JP275478. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 311. Bayguinov PO, Hennig GW, Smith TK. Ca2+ imaging of activity in ICC-MY during local mucosal reflexes and the colonic migrating motor complex in the murine large intestine. J Physiol 588: 4453–4474, 2010. doi: 10.1113/jphysiol.2010.196824. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 312. Zhu Y, Huizinga JD. Nitric oxide decreases the excitability of interstitial cells of Cajal through activation of the BK channel. J Cell Mol Med 12: 1718–1727, 2008. doi: 10.1111/j.1582-4934.2008.00217.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 313. Zhu YF, Wang XY, Lowie BJ, Parsons S, White L, Kunze W, Pawelka A, Huizinga JD. Enteric sensory neurons communicate with interstitial cells of Cajal to affect pacemaker activity in the small intestine. Pflugers Arch 466: 1467–1475, 2014. doi: 10.1007/s00424-013-1374-1. [DOI] [PubMed] [Google Scholar]
- 314. Parsons SP, Huizinga JD. Nitric oxide is essential for generating the minute rhythm contraction pattern in the small intestine, likely via ICC-DMP. Front Neurosci 14: 592664, 2020. doi: 10.3389/fnins.2020.592664. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 315. Koh SD, Drumm BT, Lu H, Kim HJ, Ryoo SB, Kim HU, Lee JY, Rhee PL, Wang Q, Gould TW, Heredia D, Perrino BA, Hwang SJ, Ward SM, Sanders KM. Propulsive colonic contractions are mediated by inhibition-driven poststimulus responses that originate in interstitial cells of Cajal. Proc Natl Acad Sci USA 119: e2123020119, 2022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 316. Ha SE, Lee MY, Kurahashi M, Wei L, Jorgensen BG, Park C, Park PJ, Redelman D, Sasse KC, Becker LS, Sanders KM, Ro S. Transcriptome analysis of PDGFRalpha+ cells identifies T-type Ca2+ channel CACNA1G as a new pathological marker for PDGFRalpha+ cell hyperplasia. PLoS One 12: e0182265, 2017. doi: 10.1371/journal.pone.0182265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 317. Sanders KM, Mutafova-Yambolieva VN. Neurotransmitters responsible for purinergic motor neurotransmission and regulation of GI motility. Auton Neurosci 234: 102829, 2021. doi: 10.1016/j.autneu.2021.102829. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 318. Kurahashi M, Mutafova-Yambolieva V, Koh SD, Sanders KM. Platelet-derived growth factor receptor-alpha-positive cells and not smooth muscle cells mediate purinergic hyperpolarization in murine colonic muscles. Am J Physiol Cell Physiol 307: C561–C570, 2014. doi: 10.1152/ajpcell.00080.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 319. Yip JL, Balasuriya GK, Spencer SJ, Hill-Yardin EL. The role of intestinal macrophages in gastrointestinal homeostasis: heterogeneity and implications in disease. Cell Mol Gastroenterol Hepatol 12: 1701–1718, 2021. doi: 10.1016/j.jcmgh.2021.08.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 320. Gabanyi I, Muller PA, Feighery L, Oliveira TY, Costa-Pinto FA, Mucida D. Neuro-immune interactions drive tissue programming in intestinal macrophages. Cell 164: 378–391, 2016. doi: 10.1016/j.cell.2015.12.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 321. Domanska D, Majid U, Karlsen VT, Merok MA, Beitnes AR, Yaqub S, Baekkevold ES., Jahnsen FL. Single-cell transcriptomic analysis of human colonic macrophages reveals niche-specific subsets. J Exp Med 219: e20211846, 2022. doi: 10.1084/jem.20211846. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 322. Ji S, Traini C, Mischopoulou M, Gibbons SJ, Ligresti G, Faussone-Pellegrini MS, Sha L, Farrugia G, Vannucchi MG, Cipriani G. Muscularis macrophages establish cell-to-cell contacts with telocytes/PDGFRalpha-positive cells and smooth muscle cells in the human and mouse gastrointestinal tract. Neurogastroenterol Motil 33: e13993, 2021. doi: 10.1111/nmo.13993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 323. Dora D, Arciero E, Hotta R, Barad C, Bhave S, Kovacs T, Balic A, Goldstein AM, Nagy N. Intraganglionic macrophages: a new population of cells in the enteric ganglia. J Anat 233: 401–410, 2018. doi: 10.1111/joa.12863. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 324. Phillips RJ, Powley TL. Macrophages associated with the intrinsic and extrinsic autonomic innervation of the rat gastrointestinal tract. Auton Neurosci 169: 12–27, 2012. doi: 10.1016/j.autneu.2012.02.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 325. Mazzucchelli L, Hauser C, Zgraggen K, Wagner HE, Hess MW, Laissue JA, Mueller C. Differential in situ expression of the genes encoding the chemokines MCP-1 and RANTES in human inflammatory bowel disease. J Pathol 178: 201–206, 1996. doi:. [DOI] [PubMed] [Google Scholar]
- 326. Delfini M, Stakenborg N, Viola MF, Boeckxstaens G. Macrophages in the gut: masters in multitasking. Immunity 55: 1530–1548, 2022. doi: 10.1016/j.immuni.2022.08.005. [DOI] [PubMed] [Google Scholar]
- 327. Muller PA, Koscsó B, Rajani GM, Stevanovic K, Berres ML, Hashimoto D, Mortha A, Leboeuf M, Li XM, Mucida D, Stanley ER, Dahan S, Margolis KG, Gershon MD, Merad M, Bogunovic M. Crosstalk between muscularis macrophages and enteric neurons regulates gastrointestinal motility. Cell 158: 1210, 2014. doi: 10.1016/j.cell.2014.08.002. [DOI] [PubMed] [Google Scholar]
- 328. Tracey KJ. Physiology and immunology of the cholinergic antiinflammatory pathway. J Clin Invest 117: 289–296, 2007. doi: 10.1172/JCI30555. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 329. Matteoli G, Boeckxstaens GE. The vagal innervation of the gut and immune homeostasis. Gut 62: 1214–1222, 2013. doi: 10.1136/gutjnl-2012-302550. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 330. Matteoli G, Gomez-Pinilla PJ, Nemethova A, Di Giovangiulio M, Cailotto C, van Bree SH, Michel K, Tracey KJ, Schemann M, Boesmans W, Vanden Berghe P, Boeckxstaens GE. A distinct vagal anti-inflammatory pathway modulates intestinal muscularis resident macrophages independent of the spleen. Gut 63: 938–948, 2014. doi: 10.1136/gutjnl-2013-304676. [DOI] [PubMed] [Google Scholar]
- 331. de Jonge WJ, van der Zanden EP, The FO, Bijlsma MF, van Westerloo DJ, Bennink RJ, Berthoud HR, Uematsu S, Akira S, van den Wijngaard RM, Boeckxstaens GE. Stimulation of the vagus nerve attenuates macrophage activation by activating the Jak2-STAT3 signaling pathway. Nat Immunol 6: 844–851, 2005. doi: 10.1038/ni1229. [DOI] [PubMed] [Google Scholar]
- 332. Cipriani G, Gibbons SJ, Verhulst PJ, Choi KM, Eisenman ST, Hein SS, Ordog T, Linden DR, Szurszewski JH, Farrugia G. Diabetic Csf1(op/op) mice lacking macrophages are protected against the development of delayed gastric emptying. Cell Mol Gastroenterol Hepatol 2: 40–47, 2016. doi: 10.1016/j.jcmgh.2015.09.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 333. Grover M, Bernard CE, Pasricha PJ, Parkman HP, Gibbons SJ, Tonascia J, Koch KL, McCallum RW, Sarosiek I, Hasler WL, Nguyen LA, Abell TL, Snape WJ, Kendrick ML, Kellogg TA, McKenzie TJ, Hamilton FA, Farrugia G; NIDDK Gastroparesis Clinical Research Consortium (GpCRC). Diabetic and idiopathic gastroparesis is associated with loss of CD206-positive macrophages in the gastric antrum. Neurogastroenterol Motil 29: 10.1111/nmo.13018, 2017. doi: 10.1111/nmo.13018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 334. Eisenman ST, Gibbons SJ, Verhulst PJ, Cipriani G, Saur D, Farrugia G. Tumor necrosis factor alpha derived from classically activated “M1” macrophages reduces interstitial cell of Cajal numbers. Neurogastroenterol Motil 29: e12984, 2017. doi: 10.1111/nmo.12984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 335. Cipriani G, Gibbons SJ, Miller KE, Yang DS, Terhaar ML, Eisenman ST, Ordog T, Linden DR, Gajdos GB, Szurszewski JH, Farrugia G. Change in populations of macrophages promotes development of delayed gastric emptying in mice. Gastroenterology 154: 2122–2136.e12, 2018. doi: 10.1053/j.gastro.2018.02.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 336. Cipriani G, Terhaar ML, Eisenman ST, Ji S, Linden DR, Wright AM, Sha L, Ordog T, Szurszewski JH, Gibbons SJ, Farrugia G. Muscularis propria macrophages alter the proportion of nitrergic but not cholinergic gastric myenteric neurons. Cell Mol Gastroenterol Hepatol 7: 689–691.e4, 2019. doi: 10.1016/j.jcmgh.2019.01.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 337. Matheis F, Muller PA, Graves CL, Gabanyi I, Kerner ZJ, Costa-Borges D, Ahrends T, Rosenstiel P, Mucida D. Adrenergic signaling in muscularis macrophages limits infection-induced neuronal loss. Cell 180: 64–78.e16, 2020. doi: 10.1016/j.cell.2019.12.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 338. Linden DR, Couvrette JM, Ciolino A, McQuoid C, Blaszyk H, Sharkey KA, Mawe GM. Indiscriminate loss of myenteric neurones in the TNBS-inflamed guinea-pig distal colon. Neurogastroenterol Motil 17: 751–760, 2005. doi: 10.1111/j.1365-2982.2005.00703.x. [DOI] [PubMed] [Google Scholar]
- 339. Ahrends T, Aydin B, Matheis F, Classon CH, Marchildon F, Furtado GC, Lira SA, Mucida D. Enteric pathogens induce tissue tolerance and prevent neuronal loss from subsequent infections. Cell 184: 5715–5727.e12, 2021. doi: 10.1016/j.cell.2021.10.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 340. Brehmer A, Stach W, Addicks K. Fine structural distinction between ganglia of the outer and inner submucosal plexus in porcine small intestine. Acta Anat (Basel) 151: 188–193, 1994. doi: 10.1159/000147662. [DOI] [PubMed] [Google Scholar]
- 341. Wedel T, Roblick U, Gleiss J, Schiedeck T, Bruch HP, Kühnel W, Krammer HJ. Organization of the enteric nervous system in the human colon demonstrated by wholemount immunohistochemistry with special reference to the submucous plexus. Ann Anat 181: 327–337, 1999. doi: 10.1016/S0940-9602(99)80122-8. [DOI] [PubMed] [Google Scholar]
- 342. Timmermans JP, Scheuermann DW, Stach W, Adriaensen D, De Groodt-Lasseel MH. Functional morphology of the enteric nervous system with special reference to large mammals. Eur J Morphol 30: 113–122, 1992. [PubMed] [Google Scholar]
- 343. Hens J, Schrödl F, Brehmer A, Adriaensen D, Neuhuber W, Scheuermann DW, Schemann M, Timmermans JP. Mucosal projections of enteric neurons in the porcine small intestine. J Comp Neurol 421: 429–436, 2000. doi:. [DOI] [PubMed] [Google Scholar]
- 344. Balemba OB, Grøndahl ML, Mbassa GK, Semuguruka WD, Hay-Smith A, Skadhauge E, Dantzer V. The organisation of the enteric nervous system in the submucous and mucous layers of the small intestine of the pig studied by VIP and neurofilament protein immunohistochemistry. J Anat 192: 257–267, 1998. doi: 10.1046/j.1469-7580.1998.19220257.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 345. Mazzoni M, Caremoli F, Cabanillas L, de Los Santos J, Million M, Larauche M, Clavenzani P, De Giorgio R, Sternini C. Quantitative analysis of enteric neurons containing choline acetyltransferase and nitric oxide synthase immunoreactivities in the submucosal and myenteric plexuses of the porcine colon. Cell Tissue Res 383: 645–654, 2021. doi: 10.1007/s00441-020-03286-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 346. Timmermans JP, Scheuermann DW, Stach W, Adriaensen D, De Groodt-Lasseel MH, Polak JM. Neuromedin U-immunoreactivity in the nervous system of the small intestine of the pig and its coexistence with substance P and CGRP. Cell Tissue Res 258: 331–337, 1989. doi: 10.1007/BF00239453. [DOI] [PubMed] [Google Scholar]
- 347. Poonyachoti S, Kulkarni-Narla A, Brown DR. Chemical coding of neurons expressing delta- and kappa-opioid receptor and type I vanilloid receptor immunoreactivities in the porcine ileum. Cell Tissue Res 307: 23–33, 2002. doi: 10.1007/s00441-001-0480-0. [DOI] [PubMed] [Google Scholar]
- 348. Wojtkiewicz J, Gonkowski S, Bladowski M, Majewski M. Characterisation of cocaine- and amphetamine- regulated transcript-like immunoreactive (CART-LI) enteric neurons in the porcine small intestine. Acta Vet Hung 60: 371–381, 2012. doi: 10.1556/AVet.2012.032. [DOI] [PubMed] [Google Scholar]
- 349. Oponowicz A, Kozłowska A, Gonkowski S, Godlewski J, Majewski M. Changes in the distribution of cocaine- and amphetamine-regulated transcript-containing neural structures in the human colon affected by the neoplastic process. Int J Mol Sci 19: 414, 2018. doi: 10.3390/ijms19020414. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 350. Hoff S, Zeller F, von Weyhern CW, Wegner M, Schemann M, Michel K, Rühl A. Quantitative assessment of glial cells in the human and guinea pig enteric nervous system with an anti-Sox8/9/10 antibody. J Comp Neurol 509: 356–371, 2008. doi: 10.1002/cne.21769. [DOI] [PubMed] [Google Scholar]
- 351. Tamura K, Wood JD. Electrical and synaptic properties of myenteric plexus neurones in the terminal large intestine of the guinea-pig. J Physiol 415: 275–298, 1989. doi: 10.1113/jphysiol.1989.sp017722. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 352. Nurgali K, Furness JB, Stebbing MJ. Correlation of electrophysiology, shape and synaptic properties of myenteric AH neurons of the guinea pig distal colon. Auton Neurosci 103: 50–64, 2003. doi: 10.1016/s1566-0702(02)00212-6. [DOI] [PubMed] [Google Scholar]
- 353. Lomax AE, Sharkey KA, Bertrand PP, Low AM, Bornstein JC, Furness JB. Correlation of morphology, electrophysiology and chemistry of neurons in the myenteric plexus of the guinea-pig distal colon. J Auton Nerv Syst 76: 45–61, 1999. doi: 10.1016/S0165-1838(99)00008-9. [DOI] [PubMed] [Google Scholar]
- 354. Kugler EM, Mazzuoli G, Demir IE, Ceyhan GO, Zeller F, Schemann M. Activity of protease-activated receptors in primary cultured human myenteric neurons. Front Neurosci 6: 133, 2012. doi: 10.3389/fnins.2012.00133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 355. Mueller K, Michel K, Krueger D, Demir IE, Ceyhan GO, Zeller F, Kreis ME, Schemann M. Activity of protease-activated receptors in the human submucous plexus. Gastroenterology 141: 2088–2097.e1, 2011. doi: 10.1053/j.gastro.2011.08.034. [DOI] [PubMed] [Google Scholar]
- 356. Linden DR, Manning BP, Bunnett NW, Mawe GM. Agonists of proteinase-activated receptor 2 excite guinea pig ileal myenteric neurons. Eur J Pharmacol 431: 311–314, 2001. doi: 10.1016/s0014-2999(01)01447-9. [DOI] [PubMed] [Google Scholar]
- 357. Benko R, Molnár Z, Nemes D, Dékány A, Kelemen D, Illényi L, Cseke L, Papp A, Varga G, Bartho L. Unexpected insensitivity of the cholinergic motor responses to morphine in the human small intestine. Pharmacology 86: 145–148, 2010. doi: 10.1159/000316637. [DOI] [PubMed] [Google Scholar]
- 358. Reed DE, Zhang Y, Beyak MJ, Lourenssen S, Blennerhassett MG, Paterson WG, Vanner SJ. Stress increases descending inhibition in mouse and human colon. Neurogastroenterol Motil 28: 569–580, 2016. doi: 10.1111/nmo.12755. [DOI] [PubMed] [Google Scholar]
- 359. Fornai M, Blandizzi C, Colucci R, Antonioli L, Bernardini N, Segnani C, Baragatti B, Barogi S, Berti P, Spisni R, Del Tacca M. Role of cyclooxygenases 1 and 2 in the modulation of neuromuscular functions in the distal colon of humans and mice. Gut 54: 608–616, 2005. doi: 10.1136/gut.2004.053322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 360. Schemann M, Michel K, Ceregrzyn M, Zeller F, Seidl S, Bischoff SC. Human mast cell mediator cocktail excites neurons in human and guinea-pig enteric nervous system. Neurogastroenterol Motil 17: 281–289, 2005. doi: 10.1111/j.1365-2982.2004.00591.x. [DOI] [PubMed] [Google Scholar]
- 361. Memic F, Knoflach V, Morarach K, Sadler R, Laranjeira C, Hjerling-Leffler J, Sundström E, Pachnis V, Marklund U. Transcription and signaling regulators in developing neuronal subtypes of mouse and human enteric nervous system. Gastroenterology 154: 624–636, 2018. doi: 10.1053/j.gastro.2017.10.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 362. Furness JB, Robbins HL, Xiao J, Stebbing MJ, Nurgali K. Projections and chemistry of Dogiel type II neurons in the mouse colon. Cell Tissue Res 317: 1–12, 2004. doi: 10.1007/s00441-004-0895-5. [DOI] [PubMed] [Google Scholar]
- 363. Langley JN. The Autonomic Nervous System. Part 1. Cambridge, UK: W. Heffer and Sons, 1921. [Google Scholar]
- 364. Mayer EA, Raybould HE. Role of visceral afferent mechanisms in functional bowel disorders. Gastroenterology 99: 1688–1704, 1990. doi: 10.1016/0016-5085(90)90475-g. [DOI] [PubMed] [Google Scholar]
- 365. Holst MC, Kelly JB, Powley TL. Vagal preganglionic projections to the enteric nervous system characterized with Phaseolus vulgaris-leucoagglutinin. J Comp Neurol 381: 81–100, 1997. doi:. [DOI] [PubMed] [Google Scholar]
- 366. Lomax AE, Sharkey KA, Furness JB. The participation of the sympathetic innervation of the gastrointestinal tract in disease states. Neurogastroenterol Motil 22: 7–18, 2010. doi: 10.1111/j.1365-2982.2009.01381.x. [DOI] [PubMed] [Google Scholar]
- 367. McIntyre AS, Thompson DG. Review article: adrenergic control of motor and secretory function in the gastrointestinal tract. Aliment Pharmacol Ther 6: 125–142, 1992. doi: 10.1111/j.1365-2036.1992.tb00257.x. [DOI] [PubMed] [Google Scholar]
- 368. Yuyama N, Mizuno J, Tsuzuki H, Wada-Takahashi S, Takahashi O, Tamura K. Effects of extrinsic autonomic inputs on expression of c-Fos immunoreactivity in myenteric neurons of the guinea pig distal colon. Brain Res 948: 8–16, 2002. doi: 10.1016/s0006-8993(02)02943-8. [DOI] [PubMed] [Google Scholar]
- 369. Parker DR, Wiklendt L, Humenick A, Chen BN, Sia TC, Wattchow DA, Dinning PG, Brookes SJ. Sympathetic pathways target cholinergic neurons in the human colonic myenteric plexus. Front Neurosci 16: 863662, 2022. doi: 10.3389/fnins.2022.863662. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 370. Smith-Edwards KM, Edwards BS, Wright CM, Schneider S, Meerschaert KA, Ejoh LL, Najjar SA, Howard MJ, Albers KM, Heuckeroth RO, Davis BM. Sympathetic input to multiple cell types in mouse and human colon produces region-specific responses. Gastroenterology 160: 1208–1223.e4, 2021. doi: 10.1053/j.gastro.2020.09.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 371. Schiller M, Azulay-Debby H, Boshnak N, Elyahu Y, Korin B, Ben-Shaanan TL, Koren T, Krot M, Hakim F, Rolls A. Optogenetic activation of local colonic sympathetic innervations attenuates colitis by limiting immune cell extravasation. Immunity 54: 1022–1036.e8, 2021. doi: 10.1016/j.immuni.2021.04.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 372. Hughes DT, Sperandio V. Inter-kingdom signalling: communication between bacteria and their hosts. Nat Rev Microbiol 6: 111–120, 2008. doi: 10.1038/nrmicro1836. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 373. Phillips RJ, Powley TL. Tension and stretch receptors in gastrointestinal smooth muscle: re-evaluating vagal mechanoreceptor electrophysiology. Brain Res Brain Res Rev 34: 1–26, 2000. doi: 10.1016/s0165-0173(00)00036-9. [DOI] [PubMed] [Google Scholar]
- 374. Kupari J, Häring M, Agirre E, Castelo-Branco G, Ernfors P. An atlas of vagal sensory neurons and their molecular specialization. Cell Rep 27: 2508–2523.e4, 2019. doi: 10.1016/j.celrep.2019.04.096. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 375. de Lartigue G. Role of the vagus nerve in the development and treatment of diet-induced obesity. J Physiol 594: 5791–5815, 2016. doi: 10.1113/JP271538. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 376. Spencer NJ, Zagorodnyuk V, Brookes SJ, Hibberd T. Spinal afferent nerve endings in visceral organs: recent advances. Am J Physiol Gastrointest Liver Physiol 311: G1056–G1063, 2016. doi: 10.1152/ajpgi.00319.2016. [DOI] [PubMed] [Google Scholar]
- 377. Hockley JR, Taylor TS, Callejo G, Wilbrey AL, Gutteridge A, Bach K, Winchester WJ, Bulmer DC, McMurray G, Smith ES. Single-cell RNAseq reveals seven classes of colonic sensory neuron. Gut 68: 633–644, 2019. doi: 10.1136/gutjnl-2017-315631. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 378. Smith-Anttila CJ, Mason EA, Wells CA, Aronow BJ, Osborne PB, Keast JR. Identification of a sacral, visceral sensory transcriptome in embryonic and adult mice. eNeuro 7: ENEURO.0397-19.2019, 2020. doi: 10.1523/ENEURO.0397-19.2019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 379. Ray P, Torck A, Quigley L, Wangzhou A, Neiman M, Rao C, Lam T, Kim JY, Kim TH, Zhang MQ, Dussor G, Price TJ. Comparative transcriptome profiling of the human and mouse dorsal root ganglia: an RNA-seq-based resource for pain and sensory neuroscience research. Pain 159: 1325–1345, 2018. doi: 10.1097/j.pain.0000000000001217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 380. Meerschaert KA, Adelman PC, Friedman RL, Albers KM, Koerber HR, Davis BM. Unique molecular characteristics of visceral afferents arising from different levels of the neuraxis: location of afferent somata predicts function and stimulus detection modalities. J Neurosci 40: 7216–7228, 2020. doi: 10.1523/JNEUROSCI.1426-20.2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 381. Chu C, Artis D, Chiu IM. Neuro-immune interactions in the tissues. Immunity 52: 464–474, 2020. doi: 10.1016/j.immuni.2020.02.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 382. Holzer P. Role of visceral afferent neurons in mucosal inflammation and defense. Curr Opin Pharmacol 7: 563–569, 2007. doi: 10.1016/j.coph.2007.09.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 383. Lai NY, Musser MA, Pinho-Ribeiro FA, Baral P, Jacobson A, Ma P, Potts DE, Chen Z, Paik D, Soualhi S, Yan Y, Misra A, Goldstein K, Lagomarsino VN, Nordstrom A, Sivanathan KN, Wallrapp A, Kuchroo VK, Nowarski R, Starnbach MN, Shi H, Surana NK, An D, Wu C, Huh JR, Rao M, Chiu IM. Gut-innervating nociceptor neurons regulate Peyer’s patch microfold cells and SFB levels to mediate salmonella host defense. Cell 180: 33–49.e22, 2020. doi: 10.1016/j.cell.2019.11.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 384. Grundy L, Erickson A, Brierley SM. Visceral pain. Annu Rev Physiol 81: 261–284, 2019. doi: 10.1146/annurev-physiol-020518-114525. [DOI] [PubMed] [Google Scholar]
- 385. Brookes SJ, Spencer NJ, Costa M, Zagorodnyuk VP. Extrinsic primary afferent signalling in the gut. Nat Rev Gastroenterol Hepatol 10: 286–296, 2013. doi: 10.1038/nrgastro.2013.29. [DOI] [PubMed] [Google Scholar]
- 386. Baral P, Udit S, Chiu IM. Pain and immunity: implications for host defence. Nat Rev Immunol 19: 433–447, 2019.doi: 10.1038/s41577-019-0147-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 387. Powley TL. Brain-gut communication: vagovagal reflexes interconnect the two “brains”. Am J Physiol Gastrointest Liver Physiol 321: G576–G587, 2021. doi: 10.1152/ajpgi.00214.2021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 388. Kim DY, Heo G, Kim M, Kim H, Jin JA, Kim HK, Jung S, An M, Ahn BH, Park JH, Park HE, Lee M, Lee JW, Schwartz GJ, Kim SY. A neural circuit mechanism for mechanosensory feedback control of ingestion. Nature 580: 376–380, 2020. doi: 10.1038/s41586-020-2167-2. [DOI] [PubMed] [Google Scholar]
- 389. Han W, Tellez LA, Perkins MH, Perez IO, Qu T, Ferreira J, Ferreira TL, Quinn D, Liu ZW, Gao XB, Kaelberer MM, Bohórquez DV, Shammah-Lagnado SJ, de Lartigue G, de Araujo IE. A neural circuit for gut-induced reward. Cell 175: 887–888, 2018. doi: 10.1016/j.cell.2018.10.018. [DOI] [PubMed] [Google Scholar]
- 390. Doerffler-Melly J, Neuhuber WL. Rectospinal neurons: evidence for a direct projection from the enteric to the central nervous system in the rat. Neurosci Lett 92: 121–125, 1988. doi: 10.1016/0304-3940(88)90046-8. [DOI] [PubMed] [Google Scholar]
- 391. Neuhuber WL, Appelt M, Polak JM, Baier-Kustermann W, Abelli L, Ferri GL. Rectospinal neurons: cell bodies, pathways, immunocytochemistry and ultrastructure. Neuroscience 56: 367–378, 1993. doi: 10.1016/0306-4522(93)90338-g. [DOI] [PubMed] [Google Scholar]
- 392. Messenger JP, Furness JB. Distribution of enteric nerve cells that project to the coeliac ganglion of the guinea-pig. Cell Tissue Res 269: 119–132, 1992. doi: 10.1007/BF00384732. [DOI] [PubMed] [Google Scholar]
- 393. Messenger JP, Furness JB. Distribution of enteric nerve cells projecting to the superior and inferior mesenteric ganglia of the guinea-pig. Cell Tissue Res 271: 333–339, 1993. doi: 10.1007/BF00318620. [DOI] [PubMed] [Google Scholar]
- 394. Sharkey KA, Lomax AE, Bertrand PP, Furness JB. Electrophysiology, shape, and chemistry of neurons that project from guinea pig colon to inferior mesenteric ganglia. Gastroenterology 115: 909–918, 1998. doi: 10.1016/s0016-5085(98)70263-x. [DOI] [PubMed] [Google Scholar]
- 395. Crowcroft PJ, Holman ME, Szurszewski JH. Excitatory input from the distal colon to the inferior mesenteric ganglion in the guinea-pig. J Physiol 219: 443–461, 1971. doi: 10.1113/jphysiol.1971.sp009671. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 396. Szurszewski JH, Ermilov LG, Miller SM. Prevertebral ganglia and intestinofugal afferent neurones. Gut 51: i6–i10, 2002. doi: 10.1136/gut.51.suppl_1.i6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 397. Hibberd TJ, Yew WP, Chen BN, Costa M, Brookes SJ, Spencer NJ. A novel mode of sympathetic reflex activation mediated by the enteric nervous system. eNeuro 7: ENEURO.0187-20.2020, 2020. doi: 10.1523/ENEURO.0187-20.2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 398. Zhang T, Perkins MH, Chang H, Han W, de Araujo IE. An inter-organ neural circuit for appetite suppression. Cell 185: 2478–2494.e28, 2022. doi: 10.1016/j.cell.2022.05.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 399. Maljaars PW, Peters HP, Mela DJ, Masclee AA. Ileal brake: a sensible food target for appetite control. A review. Physiol Behav 95: 271–281, 2008. doi: 10.1016/j.physbeh.2008.07.018. [DOI] [PubMed] [Google Scholar]
- 400. Van Citters GW, Lin HC. Ileal brake: neuropeptidergic control of intestinal transit. Curr Gastroenterol Rep 8: 367–373, 2006. doi: 10.1007/s11894-006-0021-9. [DOI] [PubMed] [Google Scholar]
- 401. Blackshaw LA, Brookes SJ, Grundy D, Schemann M. Sensory transmission in the gastrointestinal tract. Neurogastroenterol Motil 19: 1–19, 2007. doi: 10.1111/j.1365-2982.2006.00871.x. [DOI] [PubMed] [Google Scholar]
- 402. Sharkey KA, Savidge TC. Role of enteric neurotransmission in host defense and protection of the gastrointestinal tract. Auton Neurosci 181: 94–106, 2014. doi: 10.1016/j.autneu.2013.12.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 403. Latorre R, Sternini C, De Giorgio R, Greenwood-Van Meerveld B. Enteroendocrine cells: a review of their role in brain-gut communication. Neurogastroenterol Motil 28: 620–630, 2016. doi: 10.1111/nmo.12754. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 404. Liddle RA. Interactions of gut endocrine cells with epithelium and neurons. Compr Physiol 8: 1019–1030, 2018. doi: 10.1002/cphy.c170044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 405. Linan-Rico A, Ochoa-Cortes F, Beyder A, Soghomonyan S, Zuleta-Alarcon A, Coppola V, Christofi FL. Mechanosensory signaling in enterochromaffin cells and 5-HT release: potential implications for gut inflammation. Front Neurosci 10: 564, 2016. doi: 10.3389/fnins.2016.00564. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 406. Bohórquez DV, Shahid RA, Erdmann A, Kreger AM, Wang Y, Calakos N, Wang F, Liddle RA. Neuroepithelial circuit formed by innervation of sensory enteroendocrine cells. J Clin Invest 125: 782–786, 2015. doi: 10.1172/JCI78361. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 407. Kaelberer MM, Rupprecht LE, Liu WW, Weng P, Bohórquez DV. Neuropod cells: emerging biology of the gut-brain sensory transduction. Annu Rev Neurosci 43: 337–353, 2020. doi: 10.1146/annurev-neuro-091619-022657. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 408. Alcaino C, Knutson KR, Treichel AJ, Yildiz G, Strege PR, Linden DR, Li JH, Leiter AB, Szurszewski JH, Farrugia G, Beyder A. A population of gut epithelial enterochromaffin cells is mechanosensitive and requires Piezo2 to convert force into serotonin release. Proc Natl Acad Sci USA 115: E7632–E7641, 2018. doi: 10.1073/pnas.1804938115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 409. Mercado-Perez A, Beyder A. Gut feelings: mechanosensing in the gastrointestinal tract. Nat Rev Gastroenterol Hepatol 19: 283–296, 2022. doi: 10.1038/s41575-021-00561-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 410. Burman A, Kaji I. Luminal chemosensory cells in the small intestine. Nutrients 13: 3712, 2021. doi: 10.3390/nu13113712. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 411. O’Leary CE, Schneider C, Locksley RM. Tuft cells—systemically dispersed sensory epithelia integrating immune and neural circuitry. Annu Rev Immunol 37: 47–72, 2019. doi: 10.1146/annurev-immunol-042718-041505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 412. Worthington JJ, Reimann F, Gribble FM. Enteroendocrine cells-sensory sentinels of the intestinal environment and orchestrators of mucosal immunity. Mucosal Immunol 11: 3–20, 2018. doi: 10.1038/mi.2017.73. [DOI] [PubMed] [Google Scholar]
- 413. Yang M, Reimann F, Gribble FM. Chemosensing in enteroendocrine cells: mechanisms and therapeutic opportunities. Curr Opin Endocrinol Diabetes Obes 28: 222–231, 2021. doi: 10.1097/MED.0000000000000614. [DOI] [PubMed] [Google Scholar]
- 414. MacEachern SJ, Keenan CM, Papakonstantinou E, Sharkey KA, Patel BA. Alterations in melatonin and 5-HT signalling in the colonic mucosa of mice with dextran-sodium sulfate-induced colitis. Br J Pharmacol 175: 1535–1547, 2018. doi: 10.1111/bph.14163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 415. Spohn SN, Mawe GM. Non-conventional features of peripheral serotonin signalling—the gut and beyond. Nat Rev Gastroenterol Hepatol 14: 412–420, 2017. doi: 10.1038/nrgastro.2017.51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 416. Diwakarla S, Fothergill LJ, Fakhry J, Callaghan B, Furness JB. Heterogeneity of enterochromaffin cells within the gastrointestinal tract. Neurogastroenterol Motil 29: 10.1111/nmo.13101, 2017. doi: 10.1111/nmo.13101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 417. Jones LA, Sun EW, Martin AM, Keating DJ. The ever-changing roles of serotonin. Int J Biochem Cell Biol 125: 105776, 2020. doi: 10.1016/j.biocel.2020.105776. [DOI] [PubMed] [Google Scholar]
- 418. Bellono NW, Bayrer JR, Leitch DB, Castro J, Zhang C, O’Donnell TA, Brierley SM, Ingraham HA, Julius D. Enterochromaffin cells are gut chemosensors that couple to sensory neural pathways. Cell 170: 185–198.e16, 2017. doi: 10.1016/j.cell.2017.05.034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 419. Chandra R, Hiniker A, Kuo YM, Nussbaum RL, Liddle RA. alpha-Synuclein in gut endocrine cells and its implications for Parkinson’s disease. JCI Insight 2: e92295, 2017. doi: 10.1172/jci.insight.92295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 420. Marcos Z, Pffeifer K, Bodegas ME, Sesma MP, Guembe L. Cellular prion protein is expressed in a subset of neuroendocrine cells of the rat gastrointestinal tract. J Histochem Cytochem 52: 1357–1365, 2004. doi: 10.1177/002215540405201012. [DOI] [PubMed] [Google Scholar]
- 421. Ford MJ, Burton LJ, Morris RJ, Hall SM. Selective expression of prion protein in peripheral tissues of the adult mouse. Neuroscience 113: 177–192, 2002. doi: 10.1016/s0306-4522(02)00155-0. [DOI] [PubMed] [Google Scholar]
- 422. Najjar SA, Edwards BS, Albers KM, Davis BM, Smith-Edwards KM. Optogenetic activation of the distal colon epithelium engages enteric nervous system circuits to initiate motility patterns. Am J Physiol Gastrointest Liver Physiol 321: G426–G435, 2021. doi: 10.1152/ajpgi.00026.2021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 423. Psichas A, Reimann F, Gribble FM. Gut chemosensing mechanisms. J Clin Invest 125: 908–917, 2015. doi: 10.1172/JCI76309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 424. Steensels S, Depoortere I. Chemoreceptors in the gut Annu Rev Physiol 80: 117–141, 2018. doi: 10.1146/annurev-physiol-021317-121332. [DOI] [PubMed] [Google Scholar]
- 425. Furness JB, Rivera LR, Cho HJ, Bravo DM, Callaghan B. The gut as a sensory organ. Nat Rev Gastroenterol Hepatol 10: 729–740, 2013. doi: 10.1038/nrgastro.2013.180. [DOI] [PubMed] [Google Scholar]
- 426. Furness JB. Integrated neural and endocrine control of gastrointestinal function. Adv Exp Med Biol 891: 159–173, 2016. doi: 10.1007/978-3-319-27592-5_16. [DOI] [PubMed] [Google Scholar]
- 427. Haber AL, Biton M, Rogel N, Herbst RH, Shekhar K, Smillie C, Burgin G, Delorey TM, Howitt MR, Katz Y, Tirosh I, Beyaz S, Dionne D, Zhang M, Raychowdhury R, Garrett WS, Rozenblatt-Rosen O, Shi HN, Yilmaz O, Xavier RJ, Regev A. A single-cell survey of the small intestinal epithelium. Nature 551: 333–339, 2017. doi: 10.1038/nature24489. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 428. Yan KS, Gevaert O, Zheng GX, Anchang B, Probert CS, Larkin KA, et al. Intestinal enteroendocrine lineage cells possess homeostatic and injury-inducible stem cell activity. Cell Stem Cell 21: 78–90.e6, 2017. doi: 10.1016/j.stem.2017.06.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 429. Gehart H, van Es JH, Hamer K, Beumer J, Kretzschmar K, Dekkers JF, Rios A, Clevers H. Identification of enteroendocrine regulators by real-time single-cell differentiation mapping. Cell 176: 1158–1173.e16, 2019. doi: 10.1016/j.cell.2018.12.029. [DOI] [PubMed] [Google Scholar]
- 430. Beumer J, Puschhof J, Bauzá-Martinez J, Martinez-Silgado A, Elmentaite R, James KR, et al. High-resolution mRNA and secretome atlas of human enteroendocrine cells. Cell 182: 1062–1064, 2020. doi: 10.1016/j.cell.2020.08.005. [DOI] [PubMed] [Google Scholar]
- 431. Yano JM, Yu K, Donaldson GP, Shastri GG, Ann P, Ma L, Nagler CR, Ismagilov RF, Mazmanian SK, Hsiao EY. Indigenous bacteria from the gut microbiota regulate host serotonin biosynthesis. Cell 161: 264–276, 2015. doi: 10.1016/j.cell.2015.02.047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 432. Ghia JE, Li N, Wang H, Collins M, Deng Y, El-Sharkawy RT, Cote F, Mallet J, Khan WI. Serotonin has a key role in pathogenesis of experimental colitis. Gastroenterology 137: 1649–1660, 2009. doi: 10.1053/j.gastro.2009.08.041. [DOI] [PubMed] [Google Scholar]
- 433. Haq S, Wang H, Grondin J, Banskota S, Marshall JK, Khan II, Chauhan U, Côté F, Kwon YH, Philpott D, Brumell JH, Surette M, Steinberg GR, Khan WI. Disruption of autophagy by increased 5-HT alters gut microbiota and enhances susceptibility to experimental colitis and Crohn’s disease. Sci Adv 7: eabi6442, 2021. doi: 10.1126/sciadv.abi6442. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 434. Ye L, Bae M, Cassilly CD, Jabba SV, Thorpe DW, Martin AM, Lu HY, Wang J, Thompson JD, Lickwar CR, Poss KD, Keating DJ, Jordt SE, Clardy J, Liddle RA, Rawls JF. Enteroendocrine cells sense bacterial tryptophan catabolites to activate enteric and vagal neuronal pathways. Cell Host Microbe 29: 179–196.e9, 2021. doi: 10.1016/j.chom.2020.11.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 435. Treichel AJ, Finholm I, Knutson KR, Alcaino C, Whiteman ST, Brown MR, Matveyenko A, Wegner A, Kacmaz H, Mercado-Perez A, Gajdos GB, Ordog T, Grover M, Szurszewski J, Linden DR, Farrugia G, Beyder A. Specialized mechanosensory epithelial cells in mouse gut intrinsic tactile sensitivity. Gastroenterology 162: 535–547.e13, 2022. doi: 10.1053/j.gastro.2021.10.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 436. Knutson KR, Whiteman ST, Alcaino C, Mercado-Perez A, Finholm I, Serlin HK, Bellampalli SS, Linden DR, Farrugia G, Beyder A. Intestinal enteroendocrine cells rely on ryanodine and IP3 calcium store receptors for mechanotransduction. J Physiol 601: 287–305, 2023. doi: 10.1113/JP283383. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 437. Wei L, Singh R, Ha SE, Martin AM, Jones LA, Jin B, Jorgensen BG, Zogg H, Chervo T, Gottfried-Blackmore A, Nguyen L, Habtezion A, Spencer NJ, Keating DJ, Sanders KM, Ro S. Serotonin deficiency is associated with delayed gastric emptying. Gastroenterology 160: 2451–2466.e19, 2021. doi: 10.1053/j.gastro.2021.02.060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 438. Galligan JJ. Electrophysiological studies of 5-hydroxytryptamine receptors on enteric neurons. Behav Brain Res 73: 199–201, 1996. doi: 10.1016/0166-4328(96)00096-4. [DOI] [PubMed] [Google Scholar]
- 439. Pan H, Galligan JJ. Effects of 5-HT1A and 5-HT4 receptor agonists on slow synaptic potentials in enteric neurons. Eur J Pharmacol 278: 67–74, 1995. doi: 10.1016/0014-2999(95)00101-p. [DOI] [PubMed] [Google Scholar]
- 440. Pan H, Galligan JJ. 5-HT1A and 5-HT4 receptors mediate inhibition and facilitation of fast synaptic transmission in enteric neurons. Am J Physiol Gastrointest Liver Physiol 266: G230–G238, 1994. doi: 10.1152/ajpgi.1994.266.2.G230. [DOI] [PubMed] [Google Scholar]
- 441. Zhu P, Lu T, Chen Z, Liu B, Fan D, Li C, Wu J, He L, Zhu X, Du Y, Tian Y, Fan Z. 5-Hydroxytryptamine produced by enteric serotonergic neurons initiates colorectal cancer stem cell self-renewal and tumorigenesis. Neuron 110: 2268–2282.e4, 2022. doi: 10.1016/j.neuron.2022.04.024. [DOI] [PubMed] [Google Scholar]
- 442. Borman RA, Tilford NS, Harmer DW, Day N, Ellis ES, Sheldrick RL, Carey J, Coleman RA, Baxter GS. 5-HT2B receptors play a key role in mediating the excitatory effects of 5-HT in human colon in vitro. Br J Pharmacol 135: 1144–1151, 2002. doi: 10.1038/sj.bjp.0704571. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 443. Johnson PJ, Bornstein JC, Furness JB, Woollard DJ, Orrman-Rossiter SL. Characterization of 5-hydroxytryptamine receptors mediating mucosal secretion in guinea-pig ileum. Br J Pharmacol 111: 1240–1244, 1994. doi: 10.1111/j.1476-5381.1994.tb14878.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 444. Gross ER, Gershon MD, Margolis KG, Gertsberg ZV, Li Z, Cowles RA. Neuronal serotonin regulates growth of the intestinal mucosa in mice. Gastroenterology 143: 408–417 e402, 2012. doi: 10.1053/j.gastro.2012.05.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 445. Wouters MM, Gibbons SJ, Roeder JL, Distad M, Ou Y, Strege PR, Szurszewski JH, Farrugia G. Exogenous serotonin regulates proliferation of interstitial cells of Cajal in mouse jejunum through 5-HT2B receptors. Gastroenterology 133: 897–906, 2007. doi: 10.1053/j.gastro.2007.06.017. [DOI] [PubMed] [Google Scholar]
- 446. Tharayil VS, Wouters MM, Stanich JE, Roeder JL, Lei S, Beyder A, Gomez-Pinilla PJ, Gershon MD, Maroteaux L, Gibbons SJ, Farrugia G. Lack of serotonin 5-HT2B receptor alters proliferation and network volume of interstitial cells of Cajal in vivo. Neurogastroenterol Motil 22: 462–469, 2010. doi: 10.1111/j.1365-2982.2009.01435.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 447. Shajib MS, Khan WI. The role of serotonin and its receptors in activation of immune responses and inflammation. Acta Physiol (Oxf) 213: 561–574, 2015. doi: 10.1111/apha.12430. [DOI] [PubMed] [Google Scholar]
- 448. Zhou X, Galligan JJ. Synaptic activation and properties of 5-hydroxytryptamine(3) receptors in myenteric neurons of guinea pig intestine. J Pharmacol Exp Ther 290: 803–810, 1999. [PubMed] [Google Scholar]
- 449. Tack JF, Janssens J, Vantrappen G, Wood JD. Actions of 5-hydroxytryptamine on myenteric neurons in guinea pig gastric antrum. Am J Physiol Gastrointest Liver Physiol 263: G838–G846, 1992. doi: 10.1152/ajpgi.1992.263.6.G838. [DOI] [PubMed] [Google Scholar]
- 450. Wade PR, Mawe GM, Branchek TA, Gershon MD. Use of stereoisomers of zacopride to analyze actions of 5-hydroxytryptamine on enteric neurons. Am J Physiol Gasrointest Liver Physiol 260: G80–G90, 1991. doi: 10.1152/ajpgi.1991.260.1.G80. [DOI] [PubMed] [Google Scholar]
- 451. Lomax AE, Mawe GM, Sharkey KA. Synaptic facilitation and enhanced neuronal excitability in the submucosal plexus during experimental colitis in guinea-pig. J Physiol 564: 863–875, 2005. doi: 10.1113/jphysiol.2005.084285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 452. Vanner S, Surprenant A. Effects of 5-HT3 receptor antagonists on 5-HT and nicotinic depolarizations in guinea-pig submucosal neurones. Br J Pharmacol 99: 840–844, 1990. doi: 10.1111/j.1476-5381.1990.tb13017.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 453. Hillsley K, Grundy D. Sensitivity to 5-hydroxytryptamine in different afferent subpopulations within mesenteric nerves supplying the rat jejunum. J Physiol 509: 717–727, 1998. doi: 10.1111/j.1469-7793.1998.717bm.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 454. Yu Y, Villalobos-Hernandez EC, Pradhananga S, Baker CC, Keating C, Grundy D, Lomax AE, Reed DE. Deoxycholic acid activates colonic afferent nerves via 5-HT3 receptor-dependent and -independent mechanisms. Am J Physiol Gastrointest Liver Physiol 317: G275–G284, 2019. doi: 10.1152/ajpgi.00016.2019. [DOI] [PubMed] [Google Scholar]
- 455. Hillsley K, Kirkup AJ, Grundy D. Direct and indirect actions of 5-hydroxytryptamine on the discharge of mesenteric afferent fibres innervating the rat jejunum. J Physiol 506: 551–561, 1998. doi: 10.1111/j.1469-7793.1998.551bw.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 456. Hicks GA, Coldwell JR, Schindler M, Ward PA, Jenkins D, Lynn PA, Humphrey PP, Blackshaw LA. Excitation of rat colonic afferent fibres by 5-HT3 receptors. J Physiol 544: 861–869, 2002. doi: 10.1113/jphysiol.2002.025452. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 457. Bhattarai Y, Schmidt BA, Linden DR, Larson ED, Grover M, Beyder A, Farrugia G, Kashyap PC. Human-derived gut microbiota modulates colonic secretion in mice by regulating 5-HT3 receptor expression via acetate production. Am J Physiol Gastrointest Liver Physiol 313: G80–G87, 2017. doi: 10.1152/ajpgi.00448.2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 458. Kapeller J, Houghton LA, Mönnikes H, Walstab J, Möller D, Bönisch H, Burwinkel B, Autschbach F, Funke B, Lasitschka F, Gassler N, Fischer C, Whorwell PJ, Atkinson W, Fell C, Büchner KJ, Schmidtmann M, van der Voort I, Wisser AS, Berg T, Rappold G, Niesler B. First evidence for an association of a functional variant in the microRNA-510 target site of the serotonin receptor-type 3E gene with diarrhea predominant irritable bowel syndrome. Hum Mol Genet 17: 2967–2977, 2008. doi: 10.1093/hmg/ddn195. [DOI] [PubMed] [Google Scholar]
- 459. Kato S. Role of serotonin 5-HT3 receptors in intestinal inflammation. Biol Pharm Bull 36: 1406–1409, 2013. doi: 10.1248/bpb.b13-00363. [DOI] [PubMed] [Google Scholar]
- 460. Han C, Geng Q, Qin J, Li Y, Yu H. Activation of 5-hydroxytryptamine 4 receptor improves colonic barrier function by triggering mucin 2 production in a mouse model of type 1 diabetes. Am J Pathol 192: 876–886, 2022. doi: 10.1016/j.ajpath.2022.03.002. [DOI] [PubMed] [Google Scholar]
- 461. Liu M, Geddis MS, Wen Y, Setlik W, Gershon MD. Expression and function of 5-HT4 receptors in the mouse enteric nervous system. Am J Physiol Gastrointest Liver Physiol 289: G1148–G1163, 2005. doi: 10.1152/ajpgi.00245.2005. [DOI] [PubMed] [Google Scholar]
- 462. Poole DP, Xu B, Koh SL, Hunne B, Coupar IM, Irving HR, Shinjo K, Furness JB. Identification of neurons that express 5-hydroxytryptamine4 receptors in intestine. Cell Tissue Res 325: 413–422, 2006. doi: 10.1007/s00441-006-0181-9. [DOI] [PubMed] [Google Scholar]
- 463. Tonini M, Galligan JJ, North RA. Effects of cisapride on cholinergic neurotransmission and propulsive motility in the guinea pig ileum. Gastroenterology 96: 1257–1264, 1989. doi: 10.1016/s0016-5085(89)80012-5. [DOI] [PubMed] [Google Scholar]
- 464. Makimoto N, Sakurai-Yamashita Y, Furuichi A, Kawakami S, Enjoji A, Kanematsu T, Taniyam K. In vivo assessment of acceleration of motor activity associated with acetylcholine release via 5-hydroxytryptamine4 receptor in dog intestine. Jpn J Pharmacol 90: 28–35, 2002. doi: 10.1254/jjp.90.28. [DOI] [PubMed] [Google Scholar]
- 465. Fang X, Liu S, Wang XY, Gao N, Hu HZ, Wang GD, Cook CH, Needleman BJ, Mikami DJ, Xia Y, Fei GJ, Hicks GA, Wood JD. Neurogastroenterology of tegaserod (HTF 919) in the submucosal division of the guinea-pig and human enteric nervous system. Neurogastroenterol Motil 20: 80–93, 2008. doi: 10.1111/j.1365-2982.2007.00983.x. [DOI] [PubMed] [Google Scholar]
- 466. Tam FS, Hillier K, Bunce KT, Grossman C. Differences in response to 5-HT4 receptor agonists and antagonists of the 5-HT4-like receptor in human colon circular smooth muscle. Br J Pharmacol 115: 172–176, 1995. doi: 10.1111/j.1476-5381.1995.tb16335.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 467. McLean PG, Coupar IM, Molenaar P. A comparative study of functional 5-HT4 receptors in human colon, rat oesophagus and rat ileum. Br J Pharmacol 115: 47–56, 1995. doi: 10.1111/j.1476-5381.1995.tb16318.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 468. Prins NH, Van Haselen JF, Lefebvre RA, Briejer MR, Akkermans LM, Schuurkes JA. Pharmacological characterization of 5-HT4 receptors mediating relaxation of canine isolated rectum circular smooth muscle. Br J Pharmacol 127: 1431–1437, 1999. doi: 10.1038/sj.bjp.0702665. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 469. Prins NH, Shankley NP, Welsh NJ, Briejer MR, Lefebvre RA, Akkermans LM, Schuurkes JA. An improved in vitro bioassay for the study of 5-HT4 receptors in the human isolated large intestinal circular muscle. Br J Pharmacol 129: 1601–1608, 2000. doi: 10.1038/sj.bjp.0703254. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 470. Hoffman JM, Tyler K, MacEachern SJ, Balemba OB, Johnson AC, Brooks EM, Zhao H, Swain GM, Moses PL, Galligan JJ, Sharkey KA, Greenwood-Van Meerveld B, Mawe GM. Activation of colonic mucosal 5-HT4 receptors accelerates propulsive motility and inhibits visceral hypersensitivity. Gastroenterology 142: 844–854.e4, 2012. doi: 10.1053/j.gastro.2011.12.041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 471. Okumura M, Hamada A, Ohsaka F, Tsuruta T, Hira T, Sonoyama K. Expression of serotonin receptor HTR4 in glucagon-like peptide-1-positive enteroendocrine cells of the murine intestine. Pflugers Arch 472: 1521–1532, 2020. doi: 10.1007/s00424-020-02453-7. [DOI] [PubMed] [Google Scholar]
- 472. Bhattarai Y, Williams BB, Battaglioli EJ, Whitaker WR, Till L, Grover M, Linden DR, Akiba Y, Kandimalla KK, Zachos NC, Kaunitz JD, Sonnenburg JL, Fischbach MA, Farrugia G, Kashyap PC. Gut microbiota-produced tryptamine activates an epithelial G-protein-coupled receptor to increase colonic secretion. Cell Host Microbe 23: 775–785.e5, 2018. doi: 10.1016/j.chom.2018.05.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 473. Kaji I, Akiba Y, Said H, Narimatsu K, Kaunitz JD. Luminal 5-HT stimulates colonic bicarbonate secretion in rats. Br J Pharmacol 172: 4655–4670, 2015. doi: 10.1111/bph.13216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 474. Spohn SN, Bianco F, Scott RB, Keenan CM, Linton AA, O’Neill CH, Bonora E, Dicay M, Lavoie B, Wilcox RL, MacNaughton WK, De Giorgio R, Sharkey KA, Mawe GM. Protective actions of epithelial 5-hydroxytryptamine 4 receptors in normal and inflamed colon. Gastroenterology 151: 933–944.e3, 2016. doi: 10.1053/j.gastro.2016.07.032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 475. Bhattarai Y, Jie S, Linden DR, Ghatak S, Mars RA, Williams BB, Pu M, Sonnenburg JL, Fischbach MA, Farrugia G, Sha L, Kashyap PC. Bacterially derived tryptamine increases mucus release by activating a host receptor in a mouse model of inflammatory bowel disease. iScience 23: 101798, 2020. doi: 10.1016/j.isci.2020.101798. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 476. Moore BA, Sharkey KA, Mantle M. Role of 5-HT in cholera toxin-induced mucin secretion in the rat small intestine. Am J Physiol Gastrointest Liver Physiol 270: G1001–G1009, 1996. doi: 10.1152/ajpgi.1996.270.6.G1001. [DOI] [PubMed] [Google Scholar]
- 477. Kim JJ, Bridle BW, Ghia JE, Wang H, Syed SN, Manocha MM, Rengasamy P, Shajib MS, Wan Y, Hedlund PB, Khan WI. Targeted inhibition of serotonin type 7 (5-HT7) receptor function modulates immune responses and reduces the severity of intestinal inflammation. J Immunol 190: 4795–4804, 2013. doi: 10.4049/jimmunol.1201887. [DOI] [PubMed] [Google Scholar]
- 478. Monro RL, Bornstein JC, Bertrand PP. Slow excitatory post-synaptic potentials in myenteric AH neurons of the guinea-pig ileum are reduced by the 5-hydroxytryptamine7 receptor antagonist SB 269970. Neuroscience 134: 975–986, 2005. doi: 10.1016/j.neuroscience.2005.05.006. [DOI] [PubMed] [Google Scholar]
- 479. Tonini M, Vicini R, Cervio E, De Ponti F, De Giorgio R, Barbara G, Stanghellini V, Dellabianca A, Sternini C. 5-HT7 receptors modulate peristalsis and accommodation in the guinea pig ileum. Gastroenterology 129: 1557–1566, 2005. doi: 10.1053/j.gastro.2005.08.005. [DOI] [PubMed] [Google Scholar]
- 480. Idzko M, Panther E, Stratz C, Müller T, Bayer H, Zissel G, Dürk T, Sorichter S, Di Virgilio F, Geissler M, Fiebich B, Herouy Y, Elsner P, Norgauer J, Ferrari D. The serotoninergic receptors of human dendritic cells: identification and coupling to cytokine release. J Immunol 172: 6011–6019, 2004. doi: 10.4049/jimmunol.172.10.6011. [DOI] [PubMed] [Google Scholar]
- 481. Mawe GM, Branchek TA, Gershon MD. Peripheral neural serotonin receptors: identification and characterization with specific antagonists and agonists. Proc Natl Acad Sci USA 83: 9799–9803, 1986. doi: 10.1073/pnas.83.24.9799. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 482. Branchek TA, Mawe GM, Gershon MD. Characterization and localization of a peripheral neural 5-hydroxytryptamine receptor subtype (5-HT1P) with a selective agonist, 3H-5-hydroxyindalpine. J Neurosci 8: 2582–2595, 1988. doi: 10.1523/JNEUROSCI.08-07-02582.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 483. Gershon MD. Review article: roles played by 5-hydroxytryptamine in the physiology of the bowel. Aliment Pharmacol Ther 13, Suppl 2: 15–30, 1999. [PubMed] [Google Scholar]
- 484. Annaházi A, Berger TE, Demir IE, Zeller F, Müller M, Anneser M, Skerra A, Michel K, Schemann M. Metabotropic 5-HT receptor-mediated effects in the human submucous plexus. Neurogastroenterol Motil 34: e14380, 2022. doi: 10.1111/nmo.14380. [DOI] [PubMed] [Google Scholar]
- 485. Spencer NJ, Costa M, Hibberd TJ, Wood JD. Advances in colonic motor complexes in mice. Am J Physiol Gastrointest Liver Physiol 320: G12–G29, 2021. doi: 10.1152/ajpgi.00317.2020. [DOI] [PubMed] [Google Scholar]
- 486. Bharucha AE, Wouters MM, Tack J. Existing and emerging therapies for managing constipation and diarrhea. Curr Opin Pharmacol 37: 158–166, 2017. doi: 10.1016/j.coph.2017.10.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 487. Wadhwa A, Camilleri M, Grover M. New and investigational agents for irritable bowel syndrome. Curr Gastroenterol Rep 17: 46, 2015. doi: 10.1007/s11894-015-0473-x. [DOI] [PubMed] [Google Scholar]
- 488. Mawe GM, Hoffman JM. Serotonin signalling in the gut—functions, dysfunctions and therapeutic targets. Nat Rev Gastroenterol Hepatol 10: 473–486, 2013. doi: 10.1038/nrgastro.2013.105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 489. Hansen MB, Witte AB. The role of serotonin in intestinal luminal sensing and secretion. Acta Physiol (Oxf) 193: 311–323, 2008. doi: 10.1111/j.1748-1716.2008.01870.x. [DOI] [PubMed] [Google Scholar]
- 490. Liu MT, Kuan YH, Wang J, Hen R, Gershon MD. 5-HT4 receptor-mediated neuroprotection and neurogenesis in the enteric nervous system of adult mice. J Neurosci 29: 9683–9699, 2009. doi: 10.1523/JNEUROSCI.1145-09.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 491. Margolis KG, Li Z, Stevanovic K, Saurman V, Israelyan N, Anderson GM, Snyder I, Veenstra-VanderWeele J, Blakely RD, Gershon MD. Serotonin transporter variant drives preventable gastrointestinal abnormalities in development and function. J Clin Invest 126: 2221–2235, 2016. doi: 10.1172/JCI84877. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 492. Bianco F, Bonora E, Natarajan D, Vargiolu M, Thapar N, Torresan F, Giancola F, Boschetti E, Volta U, Bazzoli F, Mazzoni M, Seri M, Clavenzani P, Stanghellini V, Sternini C, De Giorgio R. Prucalopride exerts neuroprotection in human enteric neurons. Am J Physiol Gastrointest Liver Physiol 310: G768–G775, 2016. doi: 10.1152/ajpgi.00036.2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 493. Belkind-Gerson J, Hotta R, Nagy N, Thomas AR, Graham H, Cheng L, Solorzano J, Nguyen D, Kamionek M, Dietrich J, Cherayil BJ, Goldstein AM. Colitis induces enteric neurogenesis through a 5-HT4-dependent mechanism. Inflamm Bowel Dis 21: 870–878, 2015. doi: 10.1097/MIB.0000000000000326. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 494. Matsuyoshi H, Kuniyasu H, Okumura M, Misawa H, Katsui R, Zhang GX, Obata K, Takaki M. A 5-HT4-receptor activation-induced neural plasticity enhances in vivo reconstructs of enteric nerve circuit insult. Neurogastroenterol Motil 22: 806–813, 2010. doi: 10.1111/j.1365-2982.2010.01474.x. [DOI] [PubMed] [Google Scholar]
- 495. Kim JJ, Wang H, Terc JD, Zambrowicz B, Yang QM, Khan WI. Blocking peripheral serotonin synthesis by telotristat etiprate (LX1032/LX1606) reduces severity of both chemical- and infection-induced intestinal inflammation. Am J Physiol Gastrointest Liver Physiol 309: G455–G465, 2015. doi: 10.1152/ajpgi.00299.2014. [DOI] [PubMed] [Google Scholar]
- 496. Margolis KG, Stevanovic K, Li Z, Yang QM, Oravecz T, Zambrowicz B, Jhaver KG, Diacou A, Gershon MD. Pharmacological reduction of mucosal but not neuronal serotonin opposes inflammation in mouse intestine. Gut 63: 928–937, 2014. doi: 10.1136/gutjnl-2013-304901. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 497. Haub S, Ritze Y, Bergheim I, Pabst O, Gershon MD, Bischoff SC. Enhancement of intestinal inflammation in mice lacking interleukin 10 by deletion of the serotonin reuptake transporter. Neurogastroenterol Motil 22: 826–834, 2010. doi: 10.1111/j.1365-2982.2010.01479.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 498. Yadav VK, Balaji S, Suresh PS, Liu XS, Lu X, Li Z, Guo XE, Mann JJ, Balapure AK, Gershon MD, Medhamurthy R, Vidal M, Karsenty G, Ducy P. Pharmacological inhibition of gut-derived serotonin synthesis is a potential bone anabolic treatment for osteoporosis. Nat Med 16: 308–312, 2010. doi: 10.1038/nm.2098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 499. Lavoie B, Roberts JA, Haag MM, Spohn SN, Margolis KG, Sharkey KA, Lian JB, Mawe GM. Gut-derived serotonin contributes to bone deficits in colitis. Pharmacol Res 140: 75–84, 2019. doi: 10.1016/j.phrs.2018.07.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 500. Hendel SK, Kellermann L, Hausmann A, Bindslev N, Jensen KB, Nielsen OH. Tuft cells and their role in intestinal diseases. Front Immunol 13: 822867, 2022. doi: 10.3389/fimmu.2022.822867. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 501. Middelhoff M, Westphalen CB, Hayakawa Y, Yan KS, Gershon MD, Wang TC, Quante M. expressing tuft cells: critical modulators of the intestinal niche? Am J Physiol Gastrointest Liver Physiol 313: G285–G299, 2017. doi: 10.1152/ajpgi.00073.2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 502. Gerbe F, Jay P. Intestinal tuft cells: epithelial sentinels linking luminal cues to the immune system. Mucosal Immunol 9: 1353–1359, 2016. doi: 10.1038/mi.2016.68. [DOI] [PubMed] [Google Scholar]
- 503. Gerbe F, Sidot E, Smyth DJ, Ohmoto M, Matsumoto I, Dardalhon V, Cesses P, Garnier L, Pouzolles M, Brulin B, Bruschi M, Harcus Y, Zimmermann VS, Taylor N, Maizels RM, Jay P. Intestinal epithelial tuft cells initiate type 2 mucosal immunity to helminth parasites. Nature 529: 226–230, 2016. doi: 10.1038/nature16527. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 504. von Moltke J, Ji M, Liang HE, Locksley RM. Tuft-cell-derived IL-25 regulates an intestinal ILC2-epithelial response circuit. Nature 529: 221–225, 2016. doi: 10.1038/nature16161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 505. Howitt MR, Lavoie S, Michaud M, Blum AM, Tran SV, Weinstock JV, Gallini CA, Redding K, Margolskee RF, Osborne LC, Artis D, Garrett WS. Tuft cells, taste-chemosensory cells, orchestrate parasite type 2 immunity in the gut. Science 351: 1329–1333, 2016. doi: 10.1126/science.aaf1648. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 506. Cheng X, Voss U, Ekblad E. Tuft cells: Distribution and connections with nerves and endocrine cells in mouse intestine. Exp Cell Res 369: 105–111, 2018. doi: 10.1016/j.yexcr.2018.05.011. [DOI] [PubMed] [Google Scholar]
- 507. Turner JR. Intestinal mucosal barrier function in health and disease. Nat Rev Immunol 9: 799–809, 2009. doi: 10.1038/nri2653. [DOI] [PubMed] [Google Scholar]
- 508. Turner HL, Turner JR. Good fences make good neighbors: gastrointestinal mucosal structure. Gut Microbes 1: 22–29, 2010. doi: 10.4161/gmic.1.1.11427. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 509. Odenwald MA, Turner JR. The intestinal epithelial barrier: a therapeutic target? Nat Rev Gastroenterol Hepatol 14: 9–21, 2017. doi: 10.1038/nrgastro.2016.169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 510. Keita AV, Söderholm JD. The intestinal barrier and its regulation by neuroimmune factors. Neurogastroenterol Motil 22: 718–733, 2010. doi: 10.1111/j.1365-2982.2010.01498.x. [DOI] [PubMed] [Google Scholar]
- 511. Patankar JV, Becker C. Cell death in the gut epithelium and implications for chronic inflammation. Nat Rev Gastroenterol Hepatol 17: 543–556, 2020. doi: 10.1038/s41575-020-0326-4. [DOI] [PubMed] [Google Scholar]
- 512. Laukoetter MG, Bruewer M, Nusrat A. Regulation of the intestinal epithelial barrier by the apical junctional complex. Curr Opin Gastroenterol 22: 85–89, 2006. doi: 10.1097/01.mog.0000203864.48255.4f. [DOI] [PubMed] [Google Scholar]
- 513. Buckley A, Turner JR. Cell biology of tight junction barrier regulation and mucosal disease. Cold Spring Harb Perspect Biol 10: a029314, 2018. doi: 10.1101/cshperspect.a029314. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 514. Otani T, Furuse M. Tight junction structure and function revisited. Trends Cell Biol 30: 805–817, 2020. doi: 10.1016/j.tcb.2020.08.004. [DOI] [PubMed] [Google Scholar]
- 515. Günzel D, Yu AS. Claudins and the modulation of tight junction permeability. Physiol Rev 93: 525–569, 2013. doi: 10.1152/physrev.00019.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 516. Feldman GJ, Mullin JM, Ryan MP. Occludin: structure, function and regulation. Adv Drug Deliv Rev 57: 883–917, 2005. doi: 10.1016/j.addr.2005.01.009. [DOI] [PubMed] [Google Scholar]
- 517. Martìn-Padura I, Lostaglio S, Schneemann M, Williams L, Romano M, Fruscella P, Panzeri C, Stoppacciaro A, Ruco L, Villa A, Simmons D, Dejana E. Junctional adhesion molecule, a novel member of the immunoglobulin superfamily that distributes at intercellular junctions and modulates monocyte transmigration. J Cell Biol 142: 117–127, 1998. doi: 10.1083/jcb.142.1.117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 518. Ikenouchi J, Furuse M, Furuse K, Sasaki H, Tsukita S, Tsukita S. Tricellulin constitutes a novel barrier at tricellular contacts of epithelial cells. J Cell Biol 171: 939–945, 2005. doi: 10.1083/jcb.200510043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 519. Pelaseyed T, Bergström JH, Gustafsson JK, Ermund A, Birchenough GM, Schütte A, van der Post S, Svensson F, Rodríguez-Piñeiro AM, Nystrom EE, Wising C, Johansson ME, Hansson GC. The mucus and mucins of the goblet cells and enterocytes provide the first defense line of the gastrointestinal tract and interact with the immune system. Immunol Rev 260: 8–20, 2014. doi: 10.1111/imr.12182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 520. Bevins CL, Salzman NH. Paneth cells, antimicrobial peptides and maintenance of intestinal homeostasis. Nat Rev Microbiol 9: 356–368, 2011. doi: 10.1038/nrmicro2546. [DOI] [PubMed] [Google Scholar]
- 521. Clevers HC, Bevins CL. Paneth cells: maestros of the small intestinal crypts. Annu Rev Physiol 75: 289–311, 2013. doi: 10.1146/annurev-physiol-030212-183744. [DOI] [PubMed] [Google Scholar]
- 522. Barrett KE, Keely SJ. Chloride secretion by the intestinal epithelium: molecular basis and regulatory aspects. Annu Rev Physiol 62: 535–572, 2000. doi: 10.1146/annurev.physiol.62.1.535. [DOI] [PubMed] [Google Scholar]
- 523. Keely SJ, Barrett KE. Intestinal secretory mechanisms and diarrhea. Am J Physiol Gastrointest Liver Physiol 322: G405–G420, 2022. doi: 10.1152/ajpgi.00316.2021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 524. Axelson J, Ekelund M, Håkanson R, Sundler F. Gastrin and the vagus interact in the trophic control of the rat oxyntic mucosa. Regul Pept 22: 237–243, 1988. doi: 10.1016/0167-0115(88)90036-5. [DOI] [PubMed] [Google Scholar]
- 525. Hadzijahic N, Renehan WE, Ma CK, Zhang X, Fogel R. Myenteric plexus destruction alters morphology of rat intestine. Gastroenterology 105: 1017–1028, 1993. doi: 10.1016/0016-5085(93)90944-8. [DOI] [PubMed] [Google Scholar]
- 526. Holle GE. Changes in the structure and regeneration mode of the rat small intestinal mucosa following benzalkonium chloride treatment. Gastroenterology 101: 1264–1273, 1991. doi: 10.1016/0016-5085(91)90076-w. [DOI] [PubMed] [Google Scholar]
- 527. Holle GE, Dietl J, Demir I. Influence of the intramural innervation on the morphogenesis of the enteroendocrine cells and the genetic construct involved (review). Int J Mol Med 11: 275–285, 2003. doi: 10.3892/ijmm.11.3.275. [DOI] [PubMed] [Google Scholar]
- 528. Zucoloto S, de Deus DA, Martins AA, Muglia VF, Kajiwara JK, Garcia SB. The relationship between myenteric neuronal denervation, smooth muscle thickening and epithelial cell proliferation in the rat colon. Res Exp Med (Berl) 197: 117–124, 1997. doi: 10.1007/s004330050061. [DOI] [PubMed] [Google Scholar]
- 529. Toumi F, Neunlist M, Cassagnau E, Parois S, Laboisse CL, Galmiche JP, Jarry A. Human submucosal neurones regulate intestinal epithelial cell proliferation: evidence from a novel co-culture model. Neurogastroenterol Motil 15: 239–242, 2003. doi: 10.1046/j.1365-2982.2003.00409.x. [DOI] [PubMed] [Google Scholar]
- 530. Schwerdtfeger LA, Tobet SA. Vasoactive intestinal peptide regulates ileal goblet cell production in mice. Physiol Rep 8: e14363, 2020. doi: 10.14814/phy2.14363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 531. Bjerknes M, Cheng H. Modulation of specific intestinal epithelial progenitors by enteric neurons. Proc Natl Acad Sci USA 98: 12497–12502, 2001. doi: 10.1073/pnas.211278098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 532. Yusta B, Holland D, Waschek JA, Drucker DJ. Intestinotrophic glucagon-like peptide-2 (GLP-2) activates intestinal gene expression and growth factor-dependent pathways independent of the vasoactive intestinal peptide gene in mice. Endocrinology 153: 2623–2632, 2012. doi: 10.1210/en.2012-1069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 533. Puzan M, Hosic S, Ghio C, Koppes A. Enteric nervous system regulation of intestinal stem cell differentiation and epithelial monolayer function. Sci Rep 8: 6313, 2018. doi: 10.1038/s41598-018-24768-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 534. Greig CJ, Armenia SJ, Cowles RA. The M1 muscarinic acetylcholine receptor in the crypt stem cell compartment mediates intestinal mucosal growth. Exp Biol Med (Maywood) 245: 1194–1199, 2020. doi: 10.1177/1535370220938375. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 535. Raufman JP, Samimi R, Shah N, Khurana S, Shant J, Drachenberg C, Xie G, Wess J, Cheng K. Genetic ablation of M3 muscarinic receptors attenuates murine colon epithelial cell proliferation and neoplasia. Cancer Res 68: 3573–3578, 2008. doi: 10.1158/0008-5472.CAN-07-6810. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 536. Hayakawa Y, Sakitani K, Konishi M, Asfaha S, Niikura R, Tomita H, Renz BW, Tailor Y, Macchini M, Middelhoff M, Jiang Z, Tanaka T, Dubeykovskaya ZA, Kim W, Chen X, Urbanska AM, Nagar K, Westphalen CB, Quante M, Lin CS, Gershon MD, Hara A, Zhao CM, Chen D, Worthley DL, Koike K, Wang TC. Nerve growth factor promotes gastric tumorigenesis through aberrant cholinergic signaling. Cancer Cell 31: 21–34, 2017. doi: 10.1016/j.ccell.2016.11.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 537. Vaes N, Idris M, Boesmans W, Alves MM, Melotte V. Nerves in gastrointestinal cancer: from mechanism to modulations. Nat Rev Gastroenterol Hepatol 19: 768–784, 2022. doi: 10.1038/s41575-022-00669-9. [DOI] [PubMed] [Google Scholar]
- 538. Overman EL, Rivier JE, Moeser AJ. CRF induces intestinal epithelial barrier injury via the release of mast cell proteases and TNF-alpha. PLoS One 7: e39935, 2012. doi: 10.1371/journal.pone.0039935. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 539. Cameron HL, Perdue MH. Muscarinic acetylcholine receptor activation increases transcellular transport of macromolecules across mouse and human intestinal epithelium in vitro. Neurogastroenterol Motil 19: 47–56, 2007. doi: 10.1111/j.1365-2982.2006.00845.x. [DOI] [PubMed] [Google Scholar]
- 540. Cavin JB, Cuddihey H, MacNaughton WK, Sharkey KA. Acute regulation of intestinal ion transport and permeability in response to luminal nutrients: the role of the enteric nervous system. Am J Physiol Gastrointest Liver Physiol 318: G254–G264, 2020. doi: 10.1152/ajpgi.00186.2019. [DOI] [PubMed] [Google Scholar]
- 541. Gareau MG, Jury J, Perdue MH. Neonatal maternal separation of rat pups results in abnormal cholinergic regulation of epithelial permeability. Am J Physiol Gastrointest Liver Physiol 293: G198–G203, 2007. doi: 10.1152/ajpgi.00392.2006. [DOI] [PubMed] [Google Scholar]
- 542. Santos J, Saunders PR, Hanssen NP, Yang PC, Yates D, Groot JA, Perdue MH. Corticotropin-releasing hormone mimics stress-induced colonic epithelial pathophysiology in the rat. Am J Physiol Gastrointest Liver Physiol 277: G391–G399, 1999. doi: 10.1152/ajpgi.1999.277.2.G391. [DOI] [PubMed] [Google Scholar]
- 543. Barreau F, Cartier C, Leveque M, Ferrier L, Moriez R, Laroute V, Rosztoczy A, Fioramonti J, Bueno L. Pathways involved in gut mucosal barrier dysfunction induced in adult rats by maternal deprivation: corticotrophin-releasing factor and nerve growth factor interplay. J Physiol 580: 347–356, 2007. doi: 10.1113/jphysiol.2006.120907. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 544. Yuan PQ, Million M, Wu SV, Rivier J, Taché Y. Peripheral corticotropin releasing factor (CRF) and a novel CRF1 receptor agonist, stressin1-A activate CRF1 receptor expressing cholinergic and nitrergic myenteric neurons selectively in the colon of conscious rats. Neurogastroenterol Motil 19: 923–936, 2007. doi: 10.1111/j.1365-2982.2007.00978.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 545. Miampamba M, Maillot C, Million M, Taché Y. Peripheral CRF activates myenteric neurons in the proximal colon through CRF1 receptor in conscious rats. Am J Physiol Gastrointest Liver Physiol 282: G857–G865, 2002. doi: 10.1152/ajpgi.00434.2001. [DOI] [PubMed] [Google Scholar]
- 546. Saunders PR, Hanssen NP, Perdue MH. Cholinergic nerves mediate stress-induced intestinal transport abnormalities in Wistar-Kyoto rats. Am J Physiol Gastrointest Physiol 273: G486–G490, 1997. doi: 10.1152/ajpgi.1997.273.2.G486. [DOI] [PubMed] [Google Scholar]
- 547. Cipriani S, Mencarelli A, Chini MG, Distrutti E, Renga B, Bifulco G, Baldelli F, Donini A, Fiorucci S. The bile acid receptor GPBAR-1 (TGR5) modulates integrity of intestinal barrier and immune response to experimental colitis. PLoS One 6: e25637, 2011. doi: 10.1371/journal.pone.0025637. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 548. Sun Y, Fihn BM, Sjövall H, Jodal M. Enteric neurones modulate the colonic permeability response to luminal bile acids in rat colon in vivo. Gut 53: 362–367, 2004. doi: 10.1136/gut.2003.015867. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 549. Fihn BM, Sjöqvist A, Jodal M. Involvement of enteric nerves in permeability changes due to deoxycholic acid in rat jejunum in vivo. Acta Physiol Scand 178: 241–250, 2003. doi: 10.1046/j.1365-201X.2003.01144.x. [DOI] [PubMed] [Google Scholar]
- 550. Keast JR, Furness JB, Costa M. Distribution of certain peptide-containing nerve fibres and endocrine cells in the gastrointestinal mucosa in five mammalian species. J Comp Neurol 236: 403–422, 1985. doi: 10.1002/cne.902360308. [DOI] [PubMed] [Google Scholar]
- 551. Furness JB, Bornstein JC, Smith TK, Murphy R, Pompolo S. Correlated functional and structural analysis of enteric neural circuits. Arch Histol Cytol 52: 161–166, 1989. doi: 10.1679/aohc.52.Suppl_161. [DOI] [PubMed] [Google Scholar]
- 552. Neunlist M, Toumi F, Oreschkova T, Denis M, Leborgne J, Laboisse CL, Galmiche JP, Jarry A. Human ENS regulates the intestinal epithelial barrier permeability and a tight junction-associated protein ZO-1 via VIPergic pathways. Am J Physiol Gastrointest Liver Physiol 285: G1028–G1036, 2003. doi: 10.1152/ajpgi.00066.2003. [DOI] [PubMed] [Google Scholar]
- 553. Conlin VS, Wu X, Nguyen C, Dai C, Vallance BA, Buchan AM, Boyer L, Jacobson K. Vasoactive intestinal peptide ameliorates intestinal barrier disruption associated with Citrobacter rodentium-induced colitis. Am J Physiol Gastrointest Liver Physiol 297: G735–G750, 2009. doi: 10.1152/ajpgi.90551.2008. [DOI] [PubMed] [Google Scholar]
- 554. Wu X, Conlin VS, Morampudi V, Ryz NR, Nasser Y, Bhinder G, Bergstrom KS, Yu HB, Waterhouse CC, Buchan AM, Popescu OE, Gibson WT, Waschek JA, Vallance BA, Jacobson K. Vasoactive intestinal polypeptide promotes intestinal barrier homeostasis and protection against colitis in mice. PLoS One 10: e0125225, 2015. doi: 10.1371/journal.pone.0125225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 555. Boudry G, Morise A, Seve B, Le Huërou-Luron I. Effect of milk formula protein content on intestinal barrier function in a porcine model of LBW neonates. Pediatr Res 69: 4–9, 2011. doi: 10.1203/PDR.0b013e3181fc9d13. [DOI] [PubMed] [Google Scholar]
- 556. Keita AV, Carlsson AH, Cigéhn M, Ericson AC, McKay DM, Söderholm JD. Vasoactive intestinal polypeptide regulates barrier function via mast cells in human intestinal follicle-associated epithelium and during stress in rats. Neurogastroenterol Motil 25: e406–e417, 2013. doi: 10.1111/nmo.12127. [DOI] [PubMed] [Google Scholar]
- 557. Moore BA, Kim D, Vanner S. Neural pathways regulating Brunner’s gland secretion in guinea pig duodenum in vitro. Am J Physiol Gastrointest Liver Physiol 279: G910–G917, 2000. doi: 10.1152/ajpgi.2000.279.5.G910. [DOI] [PubMed] [Google Scholar]
- 558. Chu S, Schubert ML. Gastric secretion. Curr Opin Gastroenterol 29: 636–641, 2013. doi: 10.1097/MOG.0b013e328365efc7. [DOI] [PubMed] [Google Scholar]
- 559. Foong JP, Tough IR, Cox HM, Bornstein JC. Properties of cholinergic and non-cholinergic submucosal neurons along the mouse colon. J Physiol 592: 777–793, 2014. doi: 10.1113/jphysiol.2013.265686. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 560. Mongardi Fantaguzzi C, Thacker M, Chiocchetti R, Furness JB. Identification of neuron types in the submucosal ganglia of the mouse ileum. Cell Tissue Res 336: 179–189, 2009. doi: 10.1007/s00441-009-0773-2. [DOI] [PubMed] [Google Scholar]
- 561. Neunlist M, Schemann M. Polarised innervation pattern of the mucosa of the guinea pig distal colon. Neurosci Lett 246: 161–164, 1998. doi: 10.1016/s0304-3940(98)00257-2. [DOI] [PubMed] [Google Scholar]
- 562. Jodal M, Holmgren S, Lundgren O, Sjöqvist A. Involvement of the myenteric plexus in the cholera toxin-induced net fluid secretion in the rat small intestine. Gastroenterology 105: 1286–1293, 1993. doi: 10.1016/0016-5085(93)90130-5. [DOI] [PubMed] [Google Scholar]
- 563. Weber E, Neunlist M, Schemann M, Frieling T. Neural components of distension-evoked secretory responses in the guinea-pig distal colon. J Physiol 536: 741–751, 2001. doi: 10.1111/j.1469-7793.2001.00741.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 564. Vermillion DL, Gillespie JP, Cooke AR, Wood JD. Does 5-hydroxytryptamine influence “purinergic” inhibitory neurons in the intestine? Am J Physiol Endocrinol Metab 237: E198–E202, 1979. doi: 10.1152/ajpendo.1979.237.2.E198. [DOI] [PubMed] [Google Scholar]
- 565. Cooke HJ, Xue J, Yu JG, Wunderlich J, Wang YZ, Guzman J, Javed N, Christofi FL. Mechanical stimulation releases nucleotides that activate P2Y1 receptors to trigger neural reflex chloride secretion in guinea pig distal colon. J Comp Neurol 469: 1–15, 2004. doi: 10.1002/cne.10960. [DOI] [PubMed] [Google Scholar]
- 566. Christofi FL, Wunderlich J, Yu JG, Wang YZ, Xue J, Guzman J, Javed N, Cooke H. Mechanically evoked reflex electrogenic chloride secretion in rat distal colon is triggered by endogenous nucleotides acting at P2Y1, P2Y2, and P2Y4 receptors. J Comp Neurol 469: 16–36, 2004. doi: 10.1002/cne.10961. [DOI] [PubMed] [Google Scholar]
- 567. See NA, Bass P. Glucose-induced ion secretion in rat jejunum: a mucosal reflex that requires integration by the myenteric plexus. J Auton Nerv Syst 42: 33–40, 1993. doi: 10.1016/0165-1838(93)90339-v. [DOI] [PubMed] [Google Scholar]
- 568. Diener M, Vujicic Z, Scharrer E. Neuronally mediated anion secretion induced by short-chain fatty acids in the rat distal small intestine. Acta Physiol Scand 157: 33–40, 1996. doi: 10.1046/j.1365-201X.1996.470184000.x. [DOI] [PubMed] [Google Scholar]
- 569. Lundgren O, Jodal M. The enteric nervous system and cholera toxin-induced secretion. Comp Biochem Physiol A Physiol 118: 319–327, 1997. doi: 10.1016/s0300-9629(96)00312-x. [DOI] [PubMed] [Google Scholar]
- 570. Lundgren O, Peregrin AT, Persson K, Kordasti S, Uhnoo I, Svensson L. Role of the enteric nervous system in the fluid and electrolyte secretion of rotavirus diarrhea. Science 287: 491–495, 2000. doi: 10.1126/science.287.5452.491. [DOI] [PubMed] [Google Scholar]
- 571. Schubert ML, Shamburek RD. Control of acid secretion. Gastroenterol Clin N Am 19: 1–25, 1990. doi: 10.1016/S0889-8553(21)00454-4. [DOI] [PubMed] [Google Scholar]
- 572. Schicho R, Schemann M, Pabst MA, Holzer P, Lippe IT. Capsaicin-sensitive extrinsic afferents are involved in acid-induced activation of distinct myenteric neurons in the rat stomach. Neurogastroenterol Motil 15: 33–44, 2003. doi: 10.1046/j.1365-2982.2003.00384.x. [DOI] [PubMed] [Google Scholar]
- 573. Holzer P. Gastroduodenal mucosal defense. Curr Opin Gastroenterol 16: 469–478, 2000. doi: 10.1097/00001574-200011000-00003. [DOI] [PubMed] [Google Scholar]
- 574. Holzer P. Local microcirculatory reflexes and afferent signalling in response to gastric acid challenge. Gut 47, Suppl 4: iv46–iv48, 2000. doi: 10.1136/gut.47.suppl_4.iv46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 575. Zaki M, Coudron PE, McCuen RW, Harrington L, Chu S, Schubert ML. H. pylori acutely inhibits gastric secretion by activating CGRP sensory neurons coupled to stimulation of somatostatin and inhibition of histamine secretion. Am J Physiol Gastrointest Liver Physiol 304: G715–G722, 2013. doi: 10.1152/ajpgi.00187.2012. [DOI] [PubMed] [Google Scholar]
- 576. Fei G, Fang X, Wang GD, Liu S, Wang XY, Xia Y, Wood JD. Neurogenic mucosal bicarbonate secretion in guinea pig duodenum. Br J Pharmacol 168: 880–890, 2013. doi: 10.1111/j.1476-5381.2012.02218.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 577. Hogan DL, Yao B, Steinbach JH, Isenberg JI. The enteric nervous system modulates mammalian duodenal mucosal bicarbonate secretion. Gastroenterology 105: 410–417, 1993. doi: 10.1016/0016-5085(93)90714-n. [DOI] [PubMed] [Google Scholar]
- 578. Ferri GL, Botti P, Biliotti G, Rebecchi L, Bloom SR, Tonelli L, Labò G, Polak JM. VIP-, substance P- and met-enkephalin-immunoreactive innervation of the human gastroduodenal mucosa and Brunner’s glands. Gut 25: 948–952, 1984. doi: 10.1136/gut.25.9.948. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 579. Bosshard A, Chery-Croze S, Cuber JC, Dechelette MA, Berger F, Chayvialle JA. Immunocytochemical study of peptidergic structures in Brunner’s glands. Gastroenterology 97: 1382–1388, 1989. doi: 10.1016/0016-5085(89)90380-6. [DOI] [PubMed] [Google Scholar]
- 580. Moore BA, Morris GP, Vanner S. A novel in vitro model of Brunner’s gland secretion in the guinea pig duodenum. Am J Physiol Gastrointest Liver Physiol 278: G477–G485, 2000. doi: 10.1152/ajpgi.2000.278.3.G477. [DOI] [PubMed] [Google Scholar]
- 581. Buhner S, Schemann M. Mast cell-nerve axis with a focus on the human gut. Biochim Biophys Acta 1822: 85–92, 2012. doi: 10.1016/j.bbadis.2011.06.004. [DOI] [PubMed] [Google Scholar]
- 582. Berin MC, McKay DM, Perdue MH. Immune-epithelial interactions in host defense. Am J Trop Med Hyg 60: 16–25, 1999. doi: 10.4269/ajtmh.1999.60.16. [DOI] [PubMed] [Google Scholar]
- 583. MacNaughton WK, Leach KE, Prud’homme-Lalonde L, Ho W, Sharkey KA. Ionizing radiation reduces neurally evoked electrolyte transport in rat ileum through a mast cell-dependent mechanism. Gastroenterology 106: 324–335, 1994. doi: 10.1016/0016-5085(94)90589-4. [DOI] [PubMed] [Google Scholar]
- 584. Buhner S, Barki N, Greiter W, Giesbertz P, Demir IE, Ceyhan GO, Zeller F, Daniel H, Schemann M. Calcium imaging of nerve-mast cell signaling in the human intestine. Front Physiol 8: 971, 2017. doi: 10.3389/fphys.2017.00971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 585. Wang L, Stanisz AM, Wershil BK, Galli SJ, Perdue MH. Substance P induces ion secretion in mouse small intestine through effects on enteric nerves and mast cells. Am J Physiol Gastrointest Liver Physiol 269: G85–G92, 1995. doi: 10.1152/ajpgi.1995.269.1.G85. [DOI] [PubMed] [Google Scholar]
- 586. Liu S, Hu HZ, Gao N, Gao C, Wang G, Wang X, Peck OC, Kim G, Gao X, Xia Y, Wood JD. Neuroimmune interactions in guinea pig stomach and small intestine. Am J Physiol Gastrointest Liver Physiol 284: G154–G164, 2003. doi: 10.1152/ajpgi.00241.2002. [DOI] [PubMed] [Google Scholar]
- 587. Yashiro T, Ogata H, Zaidi SF, Lee J, Hayashi S, Yamamoto T, Kadowaki M. Pathophysiological roles of neuro-immune interactions between enteric neurons and mucosal mast cells in the gut of food allergy mice. Cells 10: 1586, 2021. doi: 10.3390/cells10071586. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 588. Albert-Bayo M, Paracuellos I, González-Castro AM, Rodríguez-Urrutia A, Rodríguez-Lagunas MJ, Alonso-Cotoner C, Santos J, Vicario M. Intestinal mucosal mast cells: key modulators of barrier function and homeostasis. Cells 8: 135, 2019. doi: 10.3390/cells8020135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 589. McGuckin MA, Lindén SK, Sutton P, Florin TH. Mucin dynamics and enteric pathogens. Nat Rev Microbiol 9: 265–278, 2011. doi: 10.1038/nrmicro2538. [DOI] [PubMed] [Google Scholar]
- 590. Gustafsson JK, Johansson ME. The role of goblet cells and mucus in intestinal homeostasis. Nat Rev Gastroenterol Hepatol 19: 785–803, 2022. doi: 10.1038/s41575-022-00675-x. [DOI] [PubMed] [Google Scholar]
- 591. Salzman NH. Paneth cell defensins and the regulation of the microbiome: detente at mucosal surfaces. Gut Microbes 1: 401–406, 2010. doi: 10.4161/gmic.1.6.14076. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 592. Lueschow SR, McElroy SJ. The paneth cell: the curator and defender of the immature small intestine. Front Immunol 11: 587, 2020. doi: 10.3389/fimmu.2020.00587. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 593. Brandtzaeg P. Gate-keeper function of the intestinal epithelium. Benef Microbes 4: 67–82, 2013. doi: 10.3920/BM2012.0024. [DOI] [PubMed] [Google Scholar]
- 594. Lyte M, Vulchanova L, Brown DR. Stress at the intestinal surface: catecholamines and mucosa-bacteria interactions. Cell Tissue Res 343: 23–32, 2011. doi: 10.1007/s00441-010-1050-0. [DOI] [PubMed] [Google Scholar]
- 595. Schmidt LD, Xie Y, Lyte M, Vulchanova L, Brown DR. Autonomic neurotransmitters modulate immunoglobulin A secretion in porcine colonic mucosa. J Neuroimmunol 185: 20–28, 2007. doi: 10.1016/j.jneuroim.2006.10.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 596. Ogata H, Podolsky DK. Trefoil peptide expression and secretion is regulated by neuropeptides and acetylcholine. Am J Physiol Gastrointest Liver Physiol 273: G348–G354, 1997. doi: 10.1152/ajpgi.1997.273.2.G348. [DOI] [PubMed] [Google Scholar]
- 597. Moro F, Levenez F, Durual S, Plaisancié P, Thim L, Giraud AS, Cuber JC. Secretion of the trefoil factor TFF3 from the isolated vascularly perfused rat colon. Regul Pept 101: 35–41, 2001. doi: 10.1016/S0167-0115(01)00257-9. [DOI] [PubMed] [Google Scholar]
- 598. Castagliuolo I, Lamont JT, Qiu B, Fleming SM, Bhaskar KR, Nikulasson ST, Kornetsky C, Pothoulakis C. Acute stress causes mucin release from rat colon: role of corticotropin releasing factor and mast cells. Am J Physiol Gastrointest Liver Physiol 271: G884–G892, 1996. doi: 10.1152/ajpgi.1996.271.5.G884. [DOI] [PubMed] [Google Scholar]
- 599. Somasundaram K, Ganguly AK. The effect of subdiaphragmatic vagotomy on the gastric mucus barrier in rats. Clin Exp Pharmacol Physiol 14: 735–741, 1987. doi: 10.1111/j.1440-1681.1987.tb01899.x. [DOI] [PubMed] [Google Scholar]
- 600. Schmidt PT, Eriksen L, Loftager M, Rasmussen TN, Holst JJ. Fast acting nervous regulation of immunoglobulin A secretion from isolated perfused porcine ileum. Gut 45: 679–685, 1999. doi: 10.1136/gut.45.5.679. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 601. Straub RH, Wiest R, Strauch UG, Harle P, Scholmerich J. The role of the sympathetic nervous system in intestinal inflammation. Gut 55: 1640–1649, 2006. doi: 10.1136/gut.2006.091322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 602. Lawson VA, Furness JB, Klemm HM, Pontell L, Chan E, Hill AF, Chiocchetti R. The brain to gut pathway: a possible route of prion transmission. Gut 59: 1643–1651, 2010. doi: 10.1136/gut.2010.222620. [DOI] [PubMed] [Google Scholar]
- 603. Chiocchetti R, Mazzuoli G, Albanese V, Mazzoni M, Clavenzani P, Lalatta-Costerbosa G, Lucchi ML, Di Guardo G, Marruchella G, Furness JB. Anatomical evidence for ileal Peyer’s patches innervation by enteric nervous system: a potential route for prion neuroinvasion? Cell Tissue Res 332: 185–194, 2008. doi: 10.1007/s00441-008-0583-y. [DOI] [PubMed] [Google Scholar]
- 604. Crivellato E, Soldano F, Travan L. A light and electron microscopic quantitative analysis of nerve-immune cell contacts in the gut-associated lymphoid tissue of the mouse colon. J Submicrosc Cytol Pathol 34: 55–66, 2002. [PubMed] [Google Scholar]
- 605. Crivellato E, Soldano F, Travan L, Fusaroli P, Mallardi F. Apposition of enteric nerve fibers to plasma cells and immunoblasts in the mouse small bowel. Neurosci Lett 241: 123–126, 1998. doi: 10.1016/s0304-3940(98)00004-4. [DOI] [PubMed] [Google Scholar]
- 606. Felten SY, Felten DL, Bellinger DL, Olschowka JA. Noradrenergic and peptidergic innervation of lymphoid organs. Chem Immunol 52: 25–48, 1992. [PubMed] [Google Scholar]
- 607. Reardon C, Murray K, Lomax AE. Neuroimmune communication in health and disease. Physiol Rev 98: 2287–2316, 2018. doi: 10.1152/physrev.00035.2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 608. Bonaz B. Anti-inflammatory effects of vagal nerve stimulation with a special attention to intestinal barrier dysfunction. Neurogastroenterol Motil 34: e14456, 2022. doi: 10.1111/nmo.14456. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 609. Stakenborg N, Viola MF, Boeckxstaens GE. Intestinal neuro-immune interactions: focus on macrophages, mast cells and innate lymphoid cells. Curr Opin Neurobiol 62: 68–75, 2020. doi: 10.1016/j.conb.2019.11.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 610. Pinho-Ribeiro FA, Verri WA Jr, Chiu IM. Nociceptor sensory neuron-immune interactions in pain and inflammation. Trends Immunol 38: 5–19, 2017. doi: 10.1016/j.it.2016.10.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 611. Pontarollo G, Mann A, Brandão I, Malinarich F, Schöpf M, Reinhardt C. Protease-activated receptor signaling in intestinal permeability regulation. FEBS J 287: 645–658, 2020. doi: 10.1111/febs.15055. [DOI] [PubMed] [Google Scholar]
- 612. Drumm BT, Cobine CA, Baker SA. Insights on gastrointestinal motility through the use of optogenetic sensors and actuators. J Physiol 600: 3031–3052, 2022. doi: 10.1113/JP281930. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 613. Spencer NJ, Travis L, Hibberd T, Kelly N, Feng J, Hu H. Effects of optogenetic activation of the enteric nervous system on gastrointestinal motility in mouse small intestine. Auton Neurosci 229: 102733, 2020. doi: 10.1016/j.autneu.2020.102733. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 614. Yu HB, Yang H, Allaire JM, Ma C, Graef FA, Mortha A, Liang Q, Bosman ES, Reid GS, Waschek JA, Osborne LC, Sokol H, Vallance BA, Jacobson K. Vasoactive intestinal peptide promotes host defense against enteric pathogens by modulating the recruitment of group 3 innate lymphoid cells. Proc Natl Acad Sci USA 118: e2106634118, 2021. doi: 10.1073/pnas.2106634118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 615. Talbot J, Hahn P, Kroehling L, Nguyen H, Li D, Littman DR. Feeding-dependent VIP neuron-ILC3 circuit regulates the intestinal barrier. Nature 579: 575–580, 2020. doi: 10.1038/s41586-020-2039-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 616. Seillet C, Luong K, Tellier J, Jacquelot N, Shen RD, Hickey P, Wimmer VC, Whitehead L, Rogers K, Smyth GK, Garnham AL, Ritchie ME, Belz GT. The neuropeptide VIP confers anticipatory mucosal immunity by regulating ILC3 activity. Nat Immunol 21: 168–177, 2020. doi: 10.1038/s41590-019-0567-y. [DOI] [PubMed] [Google Scholar]
- 617. Lei C, Sun R, Xu G, Tan Y, Feng W, McClain CJ, Deng Z. Enteric VIP-producing neurons maintain gut microbiota homeostasis through regulating epithelium fucosylation. Cell Host Microbe 30: 1417–1434.e8, 2022. doi: 10.1016/j.chom.2022.09.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 618. Klose CS, Mahlakõiv T, Moeller JB, Rankin LC, Flamar AL, Kabata H, Monticelli LA, Moriyama S, Putzel GG, Rakhilin N, Shen X, Kostenis E, König GM, Senda T, Carpenter D, Farber DL, Artis D. The neuropeptide neuromedin U stimulates innate lymphoid cells and type 2 inflammation. Nature 549: 282–286, 2017. doi: 10.1038/nature23676. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 619. Cardoso V, Chesné J, Ribeiro H, García-Cassani B, Carvalho T, Bouchery T, Shah K, Barbosa-Morais NL, Harris N, Veiga-Fernandes H. Neuronal regulation of type 2 innate lymphoid cells via neuromedin U. Nature 549: 277–281, 2017. doi: 10.1038/nature23469. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 620. Bouchery T, Le Gros G, Harris N. ILC2s—Trailblazers in the host response against intestinal helminths. Front Immunol 10: 623, 2019. doi: 10.3389/fimmu.2019.00623. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 621. Tsou AM, Yano H, Parkhurst CN, Mahlakõiv T, Chu C, Zhang W, He Z, Jarick KJ, Zhong C, Putzel GG, Hatazaki M, Consortium J, Lorenz IC, Andrew D, Balderes P, Klose CS, Lira SA, Artis D; JRI IBD Live Cell Bank Consortium. Neuropeptide regulation of non-redundant ILC2 responses at barrier surfaces. Nature 611: 787–793, 2022. doi: 10.1038/s41586-022-05297-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 622. Nagashima H, Mahlakoiv T, Shih HY, Davis FP, Meylan F, Huang Y, Harrison OJ, Yao C, Mikami Y, Urban JF Jr, Caron KM, Belkaid Y, Kanno Y, Artis D, O’Shea JJ. Neuropeptide CGRP limits group 2 innate lymphoid cell responses and constrains type 2 inflammation. Immunity 51: 682–695.e6, 2019. doi: 10.1016/j.immuni.2019.06.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 623. Wallrapp A, Burkett PR, Riesenfeld SJ, Kim SJ, Christian E, Abdulnour RE, Thakore PI, Schnell A, Lambden C, Herbst RH, Khan P, Tsujikawa K, Xavier RJ, Chiu IM, Levy BD, Regev A, Kuchroo VK. Calcitonin gene-related peptide negatively regulates alarmin-driven type 2 innate lymphoid cell responses. Immunity 51: 709–723.e6, 2019. doi: 10.1016/j.immuni.2019.09.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 624. Hoogerwerf WA, Hellmich HL, Cornélissen G, Halberg F, Shahinian VB, Bostwick J, Savidge TC, Cassone VM. Clock gene expression in the murine gastrointestinal tract: endogenous rhythmicity and effects of a feeding regimen. Gastroenterology 133: 1250–1260, 2007. doi: 10.1053/j.gastro.2007.07.009. [DOI] [PubMed] [Google Scholar]
- 625. Stenkamp-Strahm CM, Nyavor YE, Kappmeyer AJ, Horton S, Gericke M, Balemba OB. Prolonged high fat diet ingestion, obesity, and type 2 diabetes symptoms correlate with phenotypic plasticity in myenteric neurons and nerve damage in the mouse duodenum. Cell Tissue Res 361: 411–426, 2015. doi: 10.1007/s00441-015-2132-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 626. Beraldi EJ, Soares A, Borges SC, de Souza AC, Natali MR, Bazotte RB, Buttow NC. High-fat diet promotes neuronal loss in the myenteric plexus of the large intestine in mice. Dig Dis Sci 60: 841–849, 2015. doi: 10.1007/s10620-014-3402-1. [DOI] [PubMed] [Google Scholar]
- 627. Beraldi EJ, Borges SC, de Almeida FLA, Dos Santos A, Saad MJ, Buttow NC. Colonic neuronal loss and delayed motility induced by high-fat diet occur independently of changes in the major groups of microbiota in Swiss mice. Neurogastroenterol Motil 32: e13745, 2020. doi: 10.1111/nmo.13745. [DOI] [PubMed] [Google Scholar]
- 628. Reichardt F, Baudry C, Gruber L, Mazzuoli G, Moriez R, Scherling C, Kollmann P, Daniel H, Kisling S, Haller D, Neunlist M, Schemann M. Properties of myenteric neurones and mucosal functions in the distal colon of diet-induced obese mice. J Physiol 591: 5125–5139, 2013. doi: 10.1113/jphysiol.2013.262733. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 629. Reichardt F, Chassaing B, Nezami BG, Li G, Tabatabavakili S, Mwangi S, Uppal K, Liang B, Vijay-Kumar M, Jones D, Gewirtz AT, Srinivasan S. Western diet induces colonic nitrergic myenteric neuropathy and dysmotility in mice via saturated fatty acid- and lipopolysaccharide-induced TLR4 signalling. J Physiol 595: 1831–1846, 2017. doi: 10.1113/JP273269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 630. Hons IM, Storr MA, Mackie K, Lutz B, Pittman QJ, Mawe GM, Sharkey KA. Plasticity of mouse enteric synapses mediated through endocannabinoid and purinergic signaling. Neurogastroenterol Motil 24: e113–e124, 2012. doi: 10.1111/j.1365-2982.2011.01860.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 631. Clerc N, Furness JB, Kunze WA, Thomas EA, Bertrand PP. Long-term effects of synaptic activation at low frequency on excitability of myenteric AH neurons. Neuroscience 90: 279–289, 1999. doi: 10.1016/S0306-4522(98)00431-X. [DOI] [PubMed] [Google Scholar]
- 632. Nguyen TV, Stebbing MJ, Clerc N, Kawai M, Harvey JR, Furness JB. Evidence for protein kinase involvement in long-term postsynaptic excitation of intrinsic primary afferent neurons in the intestine. Auton Neurosci 115: 1–6, 2004. doi: 10.1016/j.autneu.2004.06.006. [DOI] [PubMed] [Google Scholar]
- 633. Linden DR, Sharkey KA, Mawe GM. Enhanced excitability of myenteric AH neurones in the inflamed guinea-pig distal colon. J Physiol 547: 589–601, 2003. doi: 10.1113/jphysiol.2002.035147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 634. Lomax AE, O’Hara JR, Hyland NP, Mawe GM, Sharkey KA. Persistent alterations to enteric neural signaling in the guinea pig colon following the resolution of colitis. Am J Physiol Gastrointest Liver Physiol 292: G482–G491, 2007. doi: 10.1152/ajpgi.00355.2006. [DOI] [PubMed] [Google Scholar]
- 635. Krauter EM, Strong DS, Brooks EM, Linden DR, Sharkey KA, Mawe GM. Changes in colonic motility and the electrophysiological properties of myenteric neurons persist following recovery from trinitrobenzene sulfonic acid colitis in the guinea pig. Neurogastroenterol Motil 19: 990–1000, 2007. doi: 10.1111/j.1365-2982.2007.00986.x. [DOI] [PubMed] [Google Scholar]
- 636. Krauter EM, Linden DR, Sharkey KA, Mawe GM. Synaptic plasticity in myenteric neurons of the guinea-pig distal colon: presynaptic mechanisms of inflammation-induced synaptic facilitation. J Physiol 581: 787–800, 2007. doi: 10.1113/jphysiol.2007.128082. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 637. Hons IM, Burda JE, Grider JR, Mawe GM, Sharkey KA. Alterations to enteric neural signaling underlie secretory abnormalities of the ileum in experimental colitis in the guinea pig. Am J Physiol Gastrointest Liver Physiol 296: G717–G726, 2009. doi: 10.1152/ajpgi.90472.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 638. Hoffman JM, McKnight ND, Sharkey KA, Mawe GM. The relationship between inflammation-induced neuronal excitability and disrupted motor activity in the guinea pig distal colon. Neurogastroenterol Motil 23: 673-e279, 2011. doi: 10.1111/j.1365-2982.2011.01702.x. [DOI] [PubMed] [Google Scholar]
- 639. Nurgali K, Nguyen TV, Matsuyama H, Thacker M, Robbins HL, Furness JB. Phenotypic changes of morphologically identified guinea-pig myenteric neurons following intestinal inflammation. J Physiol 583: 593–609, 2007. doi: 10.1113/jphysiol.2007.135947. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 640. Nurgali K, Nguyen TV, Thacker M, Pontell L, Furness JB. Slow synaptic transmission in myenteric AH neurons from the inflamed guinea pig ileum. Am J Physiol Gastrointest Liver Physiol 297: G582–G593, 2009. doi: 10.1152/ajpgi.00026.2009. [DOI] [PubMed] [Google Scholar]
- 641. Nurgali K, Qu Z, Hunne B, Thacker M, Pontell L, Furness JB. Morphological and functional changes in guinea-pig neurons projecting to the ileal mucosa at early stages after inflammatory damage. J Physiol 589: 325–339, 2011. doi: 10.1113/jphysiol.2010.197707. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 642. Galligan JJ, Furness JB, Costa M. Migration of the myoelectric complex after interruption of the myenteric plexus: intestinal transection and regeneration of enteric nerves in the guinea pig. Gastroenterology 97: 1135–1146, 1989. doi: 10.1016/0016-5085(89)91683-1. [DOI] [PubMed] [Google Scholar]
- 643. Hanani M, Ledder O, Yutkin V, Abu-Dalu R, Huang TY, Härtig W, Vannucchi MG, Faussone-Pellegrini MS. Regeneration of myenteric plexus in the mouse colon after experimental denervation with benzalkonium chloride. J Comp Neurol 462: 315–327, 2003. doi: 10.1002/cne.10721. [DOI] [PubMed] [Google Scholar]
- 644. Goto K, Kato G, Kawahara I, Luo Y, Obata K, Misawa H, Ishikawa T, Kuniyasu H, Nabekura J, Takaki M. In vivo imaging of enteric neurogenesis in the deep tissue of mouse small intestine. PLoS One 8: e54814, 2013. doi: 10.1371/journal.pone.0054814. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 645. Kawahara I, Kuniyasu H, Matsuyoshi H, Goto K, Obata K, Misawa H, Fujii H, Takaki M. Comparison of effects of a selective 5-HT reuptake inhibitor versus a 5-HT4 receptor agonist on in vivo neurogenesis at the rectal anastomosis in rats. Am J Physiol Gastrointest Liver Physiol 302: G588–G597, 2012. doi: 10.1152/ajpgi.00284.2011. [DOI] [PubMed] [Google Scholar]
- 646. Chen Z, Suntres Z, Palmer J, Guzman J, Javed A, Xue J, Yu JG, Cooke H, Awad H, Hassanain HH, Cardounel AJ, Christofi FL. Cyclic AMP signaling contributes to neural plasticity and hyperexcitability in AH sensory neurons following intestinal Trichinella spiralis-induced inflammation. Int J Parasitol 37: 743–761, 2007. doi: 10.1016/j.ijpara.2006.12.018. [DOI] [PubMed] [Google Scholar]
- 647. Christofi FL, Wood JD. Presynaptic inhibition by adenosine A1 receptors on guinea pig small intestinal myenteric neurons. Gastroenterology 104: 1420–1429, 1993. doi: 10.1016/0016-5085(93)90351-c. [DOI] [PubMed] [Google Scholar]
- 648. Manning BP, Sharkey KA, Mawe GM. Effects of PGE2 in guinea pig colonic myenteric ganglia. Am J Physiol Gastrointest Liver Physiol 283: G1388–G1397, 2002. doi: 10.1152/ajpgi.00141.2002. [DOI] [PubMed] [Google Scholar]
- 649. Linden DR, Sharkey KA, Ho W, Mawe GM. Cyclooxygenase-2 contributes to dysmotility and enhanced excitability of myenteric AH neurones in the inflamed guinea pig distal colon. J Physiol 557: 191–205, 2004. doi: 10.1113/jphysiol.2004.062174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 650. Frieling T, Rupprecht C, Kroese AB, Schemann M. Effects of the inflammatory mediator prostaglandin D2 on submucosal neurons and secretion in guinea pig colon. Am J Physiol Gastrointest Physiol 266: G132–G139, 1994. doi: 10.1152/ajpgi.1994.266.1.G132. [DOI] [PubMed] [Google Scholar]
- 651. Xia Y, Hu HZ, Liu S, Ren J, Zafirov DH, Wood JD. IL-1beta and IL-6 excite neurons and suppress nicotinic and noradrenergic neurotransmission in guinea pig enteric nervous system. J Clin Invest 103: 1309–1316, 1999. doi: 10.1172/JCI5823. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 652. Reed DE, Barajas-Lopez C, Cottrell G, Velazquez-Rocha S, Dery O, Grady EF, Bunnett NW, Vanner SJ. Mast cell tryptase and proteinase-activated receptor 2 induce hyperexcitability of guinea-pig submucosal neurons. J Physiol 547: 531–542, 2003. doi: 10.1113/jphysiol.2002.032011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 653. Gao C, Liu S, Hu HZ, Gao N, Kim GY, Xia Y, Wood JD. Serine proteases excite myenteric neurons through protease-activated receptors in guinea pig small intestine. Gastroenterology 123: 1554–1564, 2002. doi: 10.1053/gast.2002.36581. [DOI] [PubMed] [Google Scholar]
- 654. Frieling T, Cooke HJ, Wood JD. Neuroimmune communication in the submucous plexus of guinea pig colon after sensitization to milk antigen. Am J Physiol Gastrointest Liver Physiol 267: G1087–G1093, 1994. doi: 10.1152/ajpgi.1994.267.6.G1087. [DOI] [PubMed] [Google Scholar]
- 655. Frieling T, Palmer JM, Cooke HJ, Wood JD. Neuroimmune communication in the submucous plexus of guinea pig colon after infection with Trichinella spiralis. Gastroenterology 107: 1602–1609, 1994. doi: 10.1016/0016-5085(94)90798-6. [DOI] [PubMed] [Google Scholar]
- 656. Zhang L, Wang R, Bai T, Xiang X, Qian W, Song J, Hou X. EphrinB2/ephB2-mediated myenteric synaptic plasticity: mechanisms underlying the persistent muscle hypercontractility and pain in postinfectious IBS. FASEB J 33: 13644–13659, 2019. doi: 10.1096/fj.201901192R. [DOI] [PubMed] [Google Scholar]
- 657. Bodin R, Paillé V, Oullier T, Durand T, Aubert P, Le Berre-Scoul C, Hulin P, Neunlist M, Cissé M. The ephrin receptor EphB2 regulates the connectivity and activity of enteric neurons. J Biol Chem 297: 101300, 2021. doi: 10.1016/j.jbc.2021.101300. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 658. Liu S. Neurotrophic factors in enteric physiology and pathophysiology. Neurogastroenterol Motil 30: e13446, 2018. doi: 10.1111/nmo.13446. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 659. Stenkamp-Strahm C, Patterson S, Boren J, Gericke M, Balemba O. High-fat diet and age-dependent effects on enteric glial cell populations of mouse small intestine. Auton Neurosci 177: 199–210, 2013. doi: 10.1016/j.autneu.2013.04.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 660. Pochard C, Coquenlorge S, Freyssinet M, Naveilhan P, Bourreille A, Neunlist M, Rolli-Derkinderen M. The multiple faces of inflammatory enteric glial cells: is Crohn’s disease a gliopathy? Am J Physiol Gastrointest Liver Physiol 315: G1–G11, 2018. doi: 10.1152/ajpgi.00016.2018. [DOI] [PubMed] [Google Scholar]
- 661. McClain JL, Mazzotta EA, Maradiaga N, Duque-Wilckens N, Grants I, Robison AJ, Christofi FL, Moeser AJ, Gulbransen BD. Histamine-dependent interactions between mast cells, glia, and neurons are altered following early-life adversity in mice and humans. Am J Physiol Gastrointest Liver Physiol 319: G655–G668, 2020. doi: 10.1152/ajpgi.00041.2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 662. Makowska K, Gonkowski S. Age and sex-dependent differences in the neurochemical characterization of calcitonin gene-related peptide-like immunoreactive (CGRP-LI) nervous structures in the porcine descending colon. Int J Mol Sci 20: 1024, 2019. doi: 10.3390/ijms20051024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 663. Schemann M, Frieling T, Enck P. To learn, to remember, to forget—How smart is the gut? Acta Physiol (Oxf) 228: e13296, 2020. doi: 10.1111/apha.13296. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 664. Mawe GM. Colitis-induced neuroplasticity disrupts motility in the inflamed and post-inflamed colon. J Clin Invest 125: 949–955, 2015. doi: 10.1172/JCI76306. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 665. Malenka RC, Bear MF. LTP and LTD: an embarrassment of riches. Neuron 44: 5–21, 2004. doi: 10.1016/j.neuron.2004.09.012. [DOI] [PubMed] [Google Scholar]
- 666. Nemeth PR, Ort CA, Wood JD. Intracellular study of effects of histamine on electrical behaviour of myenteric neurones in guinea-pig small intestine. J Physiol 355: 411–425, 1984. doi: 10.1113/jphysiol.1984.sp015427. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 667. Liu S, Hu HZ, Gao C, Gao N, Wang G, Wang X, Gao X, Xia Y, Wood JD. Actions of cysteinyl leukotrienes in the enteric nervous system of guinea-pig stomach and small intestine. Eur J Pharmacol 459: 27–39, 2003. doi: 10.1016/S0014-2999(02)02820-0. [DOI] [PubMed] [Google Scholar]
- 668. Palmer JM, Wong-Riley M, Sharkey KA. Functional alterations in jejunal myenteric neurons during inflammation in nematode-infected guinea pigs. Am J Physiol Gastrointest Liver Physiol 275: G922–G935, 1998. doi: 10.1152/ajpgi.1998.275.5.G922. [DOI] [PubMed] [Google Scholar]
- 669. Wang B, Mao YK, Diorio C, Wang L, Huizinga JD, Bienenstock J, Kunze W. Lactobacillus reuteri ingestion and IKCa channel blockade have similar effects on rat colon motility and myenteric neurones. Neurogastroenterol Motil 22: 98–107, 2010. doi: 10.1111/j.1365-2982.2009.01384.x. [DOI] [PubMed] [Google Scholar]
- 670. Belkind-Gerson J, Carreon-Rodriguez A, Benedict LA, Steiger C, Pieretti A, Nagy N, Dietrich J, Goldstein AM. Nestin-expressing cells in the gut give rise to enteric neurons and glial cells. Neurogastroenterol Motil 25: 61–69.e7, 2013. doi: 10.1111/nmo.12015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 671. Heanue TA, Pachnis V. Prospective identification and isolation of enteric nervous system progenitors using Sox2. Stem Cells 29: 128–140, 2011. doi: 10.1002/stem.557. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 672. Katsui R, Kojima Y, Kuniyasu H, Shimizu J, Koyama F, Fujii H, Nakajima Y, Takaki M. A new possibility for repairing the anal dysfunction by promoting regeneration of the reflex pathways in the enteric nervous system. Am J Physiol Gastrointest Liver Physiol 294: G1084–G1093, 2008. doi: 10.1152/ajpgi.00345.2007. [DOI] [PubMed] [Google Scholar]
- 673. Takaki M, Goto K, Kawahara I, Nabekura J. Activation of 5-HT4 receptors facilitates neurogenesis of injured enteric neurons at an anastomosis in the lower gut. J Smooth Muscle Res 51: 82–94, 2015. doi: 10.1540/jsmr.51.82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 674. Goto K, Kawahara I, Inada H, Misawa H, Kuniyasu H, Nabekura J, Takaki M. Activation of 5-HT4 receptors facilitates neurogenesis from transplanted neural stem cells in the anastomotic ileum. J Physiol Sci 66: 67–76, 2016. doi: 10.1007/s12576-015-0396-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 675. Goto K, Kawahara I, Kuniyasu H, Takaki M. A protein tyrosine kinase receptor, c-RET signaling pathway contributes to the enteric neurogenesis induced by a 5-HT4 receptor agonist at an anastomosis after transection of the gut in rodents. J Physiol Sci 65: 377–383, 2015. doi: 10.1007/s12576-015-0377-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 676. Laranjeira C, Pachnis V. Enteric nervous system development: Recent progress and future challenges. Auton Neurosci 151: 61–69, 2009. doi: 10.1016/j.autneu.2009.09.001. [DOI] [PubMed] [Google Scholar]
- 677. Li Z, Chalazonitis A, Huang YY, Mann JJ, Margolis KG, Yang QM, Kim DO, Coté F, Mallet J, Gershon MD. Essential roles of enteric neuronal serotonin in gastrointestinal motility and the development/survival of enteric dopaminergic neurons. J Neurosci 31: 8998–9009, 2011. doi: 10.1523/JNEUROSCI.6684-10.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 678. Israelyan N, Del Colle A, Li Z, Park Y, Xing A, Jacobsen JP, Luna RA, Jensen DD, Madra M, Saurman V, Rahim R, Latorre R, Law K, Carson W, Bunnett NW, Caron MG, Margolis KG. Effects of serotonin and slow-release 5-hydroxytryptophan on gastrointestinal motility in a mouse model of depression. Gastroenterology 157: 507–521.e4, 2019. doi: 10.1053/j.gastro.2019.04.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 679. Reigstad CS, Salmonson CE, Rainey JF 3rd, Szurszewski JH, Linden DR, Sonnenburg JL, Farrugia G, Kashyap PC. Gut microbes promote colonic serotonin production through an effect of short-chain fatty acids on enterochromaffin cells. FASEB J 29: 1395–1403, 2015. doi: 10.1096/fj.14-259598. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 680. Collins SM, Surette M, Bercik P. The interplay between the intestinal microbiota and the brain. Nat Rev Microbiol 10: 735–742, 2012. doi: 10.1038/nrmicro2876. [DOI] [PubMed] [Google Scholar]
- 681. Bercik P, Collins SM, Verdu EF. Microbes and the gut-brain axis. Neurogastroenterol Motil 24: 405–413, 2012. doi: 10.1111/j.1365-2982.2012.01906.x. [DOI] [PubMed] [Google Scholar]
- 682. Grenham S, Clarke G, Cryan JF., Dinan TG. Brain-gut-microbe communication in health and disease. Front Physiol 2: 94, 2011. doi: 10.3389/fphys.2011.00094. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 683. van Tilburg Bernardes E, Pettersen VK, Gutierrez MW, Laforest-Lapointe I, Jendzjowsky NG, Cavin JB, Vicentini FA, Keenan CM, Ramay HR, Samara J, MacNaughton WK, Wilson RJ, Kelly MM, McCoy KD, Sharkey KA, Arrieta MC. Intestinal fungi are causally implicated in microbiome assembly and immune development in mice. Nat Commun 11: 2577, 2020. doi: 10.1038/s41467-020-16431-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 684. Stamp LA, Gwynne RM, Foong JP, Lomax AE, Hao MM, Kaplan DI, Reid CA, Petrou S, Allen AM, Bornstein JC, Young HM. Optogenetic demonstration of functional innervation of mouse colon by neurons derived from transplanted neural cells. Gastroenterology 152: 1407–1418, 2017. doi: 10.1053/j.gastro.2017.01.005. [DOI] [PubMed] [Google Scholar]
- 685. Hibberd TJ, Feng J, Luo J, Yang P, Samineni VK, Gereau RW, Kelley N, Hu H, Spencer NJ. Optogenetic induction of colonic motility in mice. Gastroenterology 155: 514–528.e6, 2018. doi: 10.1053/j.gastro.2018.05.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 686. Smith-Edwards KM, Najjar SA, Edwards BS, Howard MJ, Albers KM, Davis BM. Extrinsic primary afferent neurons link visceral pain to colon motility through a spinal reflex in mice. Gastroenterology 157: 522–536.e2, 2019. doi: 10.1053/j.gastro.2019.04.034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 687. Gould TW, Swope WA, Heredia DJ, Corrigan RD, Smith TK. Activity within specific enteric neurochemical subtypes is correlated with distinct patterns of gastrointestinal motility in the murine colon. Am J Physiol Gastrointest Liver Physiol 317: G210–G221, 2019. doi: 10.1152/ajpgi.00252.2018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 688. Perez-Medina AL, Galligan JJ. Optogenetic analysis of neuromuscular transmission in the colon of ChAT-ChR2-YFP BAC transgenic mice. Am J Physiol Gastrointest Liver Physiol 317: G569–G579, 2019. doi: 10.1152/ajpgi.00089.2019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 689. Fujii K, Nakajo K, Egashira Y, Yamamoto Y, Kitada K, Taniguchi K, Kawai M, Tomiyama H, Kawakami K, Uchiyama K, Ono F. Gastrointestinal neurons expressing HCN4 regulate retrograde peristalsis. Cell Rep 30: 2879–2888.e3, 2020. doi: 10.1016/j.celrep.2020.02.024. [DOI] [PubMed] [Google Scholar]
- 690. Boesmans W, Hao MM, Vanden Berghe P. Optogenetic and chemogenetic techniques for neurogastroenterology. Nat Rev Gastroenterol Hepatol 15: 21–38, 2018. doi: 10.1038/nrgastro.2017.151. [DOI] [PubMed] [Google Scholar]
- 691. Chan KY, Jang MJ, Yoo BB, Greenbaum A, Ravi N, Wu WL, Sánchez-Guardado L, Lois C, Mazmanian SK, Deverman BE, Gradinaru V. Engineered AAVs for efficient noninvasive gene delivery to the central and peripheral nervous systems. Nat Neurosci 20: 1172–1179, 2017. doi: 10.1038/nn.4593. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 692. Buckinx R, Van Remoortel S, Gijsbers R, Waddington SN, Timmermans JP. Proof-of-concept: neonatal intravenous injection of adeno-associated virus vectors results in successful transduction of myenteric and submucosal neurons in the mouse small and large intestine. Neurogastroenterol Motil 28: 299–305, 2016. doi: 10.1111/nmo.12724. [DOI] [PubMed] [Google Scholar]
- 693. Yoo BB, Griffiths JA, Thuy-Boun PS, Cantu V, Weldon KC, Challis C, Sweredoski MJ, Chan KY, Thron TM, Sharon G, Moradian A, Zhu Q, Shaffer J, Wolan D, Dorrestein PE, Knight R, Gradinaru V, Mazmanian SK. Targeted activation of enteric neurons shapes the gut environment of mice (Preprint). bioRxiv 2021.04.12.439539, 2021. doi: 10.1101/2021.04.12.439539. [DOI]
- 694. Yuan PQ, Bellier JP, Li T, Kwaan MR, Kimura H, Taché Y. Intrinsic cholinergic innervation in the human sigmoid colon revealed using CLARITY, three-dimensional (3D) imaging, and a novel anti-human peripheral choline acetyltransferase (hpChAT) antiserum. Neurogastroenterol Motil 33: e14030, 2021. doi: 10.1111/nmo.14030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 695. Graham KD, López SH, Sengupta R, Shenoy A, Schneider S, Wright CM, Feldman M, Furth E, Valdivieso F, Lemke A, Wilkins BJ, Naji A, Doolin EJ, Howard MJ, Heuckeroth RO. Robust, 3-dimensional visualization of human colon enteric nervous system without tissue sectioning. Gastroenterology 158: 2221–2235.e5, 2020. doi: 10.1053/j.gastro.2020.02.035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 696. Bossolani GDP, Pintelon I, Detrez JD, Buckinx R, Thys S, Zanoni JN, De Vos WH, Timmermans JP. Comparative analysis reveals Ce3D as optimal clearing method for in toto imaging of the mouse intestine. Neurogastroenterol Motil 31: e13560, 2019. doi: 10.1111/nmo.13560. [DOI] [PubMed] [Google Scholar]
- 697. El-Nachef WN, Hu C, Bronner ME. Whole gut imaging allows quantification of all enteric neurons in the adult zebrafish intestine. Neurogastroenterol Motil 34: e14292, 2022. doi: 10.1111/nmo.14292. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 698. Fu YY, Peng SJ, Lin HY, Pasricha PJ, Tang SC. 3-D imaging and illustration of mouse intestinal neurovascular complex. Am J Physiol Gastrointest Liver Physiol 304: G1–G11, 2013. doi: 10.1152/ajpgi.00209.2012. [DOI] [PubMed] [Google Scholar]
- 699. Liu YA, Chung YC, Pan ST, Shen MY, Hou YC, Peng SJ, Pasricha PJ, Tang SC. 3-D imaging, illustration, and quantitation of enteric glial network in transparent human colon mucosa. Neurogastroenterol Motil 25: e324–e338, 2013. doi: 10.1111/nmo.12115. [DOI] [PubMed] [Google Scholar]
- 700. McCallum S, Obata Y, Fourli E, Boeing S, Peddie CJ, Xu Q, Horswell S, Kelsh RN, Collinson L, Wilkinson D, Pin C, Pachnis V, Heanue TA. Enteric glia as a source of neural progenitors in adult zebrafish. Elife 9: e56086, 2020. doi: 10.7554/eLife.56086. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 701. Rolig AS, Mittge EK, Ganz J, Troll JV, Melancon E, Wiles TJ, Alligood K, Stephens WZ, Eisen JS, Guillemin K. The enteric nervous system promotes intestinal health by constraining microbiota composition. PLoS Biol 15: e2000689, 2017. doi: 10.1371/journal.pbio.2000689. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 702. Hamilton MK, Wall ES, Robinson CD, Guillemin K, Eisen JS. Enteric nervous system modulation of luminal pH modifies the microbial environment to promote intestinal health. PLoS Pathog 18: e1009989, 2022. doi: 10.1371/journal.ppat.1009989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 703. El-Nachef WN, Bronner ME. De novo enteric neurogenesis in post-embryonic zebrafish from Schwann cell precursors rather than resident cell types. Development 147: dev186619, 2020. doi: 10.1242/dev.186619. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 704. Hadjieconomou D, King G, Gaspar P, Mineo A, Blackie L, Ameku T, Studd C, de Mendoza A, Diao F, White BH, Brown AE, Plaçais PY, Préat T, Miguel-Aliaga I. Enteric neurons increase maternal food intake during reproduction. Nature 587: 455–459, 2020. doi: 10.1038/s41586-020-2866-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 705. Prusator DK, Chang L. Sex-related differences in GI disorders. Handb Exp Pharmacol 239: 177–192, 2017. doi: 10.1007/164_2016_121. [DOI] [PubMed] [Google Scholar]
- 706. Rastelli D, Robinson A, Lagomarsino VN, Matthews LT, Hassan R, Perez K, Dan W, Yim PD, Mixer M, Prochera A, Shepherd A, Sun L, Hall K, Ballou S, Lembo A, Nee J., Rao M. Diminished androgen levels are linked to irritable bowel syndrome and cause bowel dysfunction in mice. J Clin Invest 132: e150789, 2022. doi: 10.1172/JCI150789. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 707. Wood JD. Enteric neuroimmunophysiology and pathophysiology. Gastroenterology 127: 635–657, 2004. doi: 10.1053/j.gastro.2004.02.017. [DOI] [PubMed] [Google Scholar]
- 708. Powley TL, Phillips RJ. Musings on the wanderer: what's new in our understanding of vago-vagal reflexes? I. Morphology and topography of vagal afferents innervating the GI tract. Am J Physiol Gastrointest Liver Physiol 283: G1217–G1225, 2002. doi: 10.1152/ajpgi.00249.2002. [DOI] [PubMed] [Google Scholar]