To the Editor,
Development of new-onset oral autoimmune and immune-mediated diseases (e.g., pemphigus vulgaris, oral lichen planus, and bullous pemphigoid (BP)) has been reported after the coronavirus disease 2019 (COVID-19) vaccination [1–3]. Mucous membrane pemphigoid (MMP) is a rare autoimmune bullous disease that commonly affects the oral mucous membrane and conjunctiva. Herein, we present a case of oral MMP following the COVID-19 vaccination with mRNA vaccine (BNT162b2, Pfizer/BioNTech).
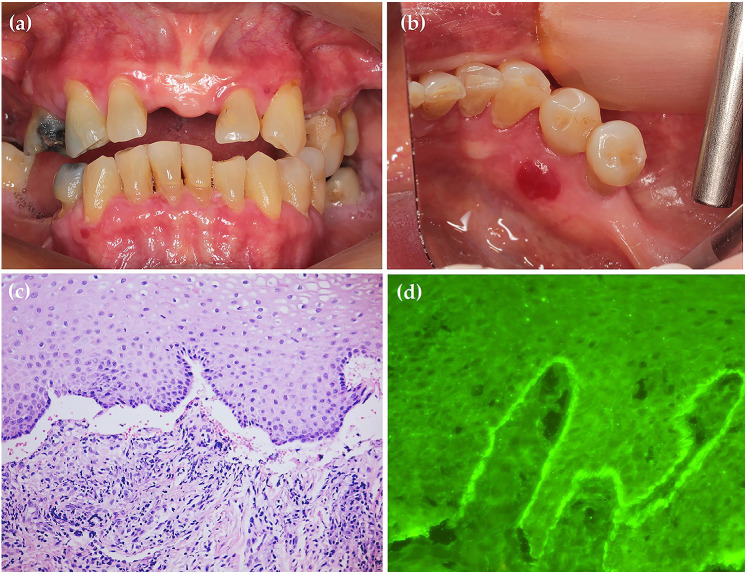
A 74-year-old Thai woman was referred to the Oral Medicine Clinic, Faculty of Dentistry, Mahidol University. The patient reported a two-month history of a burning sensation accompanied by the development of blood-filled vesicles in the oral cavity, which developed three weeks after she had received the first dose of the BNT162b2 vaccine. Upon examination, extraoral areas were unremarkable. Intraoral examination revealed generalized erythematous and erosive areas at the gingiva. A blood-filled vesicle was observed at the lingual gingiva of lower right premolar (Fig. 1). The patient had no significant past medical history and was not taking any medications. Incisional biopsy was performed at the perilesional area of the upper labial gingiva for histopathological examination and direct immunofluorescence (DIF). Histopathologically, the specimen showed subepithelial separation with mild chronic inflammatory cell infiltration in the underlying connective tissue. The DIF findings revealed IgG and C3 positivity at the basement membrane in linear pattern. Given the histopathological and DIF results, the diagnosis of MMP was established. The patient was prescribed oral doxycycline 100 mg twice daily for two weeks. Additionally, high potency topical steroid, 0.1% fluocinolone in orabase, was also prescribed. Ophthalmologic examination by an ophthalmologist showed no evidence of cicatrizing conjunctivitis. After two weeks, her symptom markedly improved. The patient has been followed up for two months and only some mild erythematous areas at the gingiva could be detected. 0.1% fluocinolone in orabase was also continuously prescribed to control the lesion. From this occurrence, the patient decided not to receive the subsequent dose of BNT162b2 vaccine.
Fig. 1.
Generalized erythematous and erosive areas at the gingiva (a). A blood-filled vesicle 7 × 6 × 2 mm. in diameter at the lower right lingual gingiva (b). The specimen illustrated subepithelial separation with mild chronic inflammatory cell infiltration in the underlying connective tissue; original magnification ×200 (c). The DIF findings demonstrated IgG and C3 positivity at the basement membrane in linear pattern; original magnification ×400 (d)
MMP is a rare subepithelial blistering disorder characterized by linear deposition of autoantibodies (e.g., BP180, BP230, laminin 332, and α6β4 integrin) along the basement membrane zone [4]. The cause of MMP is unclear; however, vaccine-induced MMP was reported in diphtheria-tetanus vaccination [5]. Furthermore, some pieces of evidence suggested that the COVID-19 vaccine may trigger or flare pemphigus and pemphigoid diseases, especially BP [6, 7]. BP, a common pemphigoid disease, was also reported to develop after administration with several vaccines including Diphtheria-Tetanus-Pertussis, polio, Bacillus Calmette–Guérin, tetanus, herpes zoster, and COVID-19 [3, 5]. Although the possible role of COVID-19 vaccine-induced MMP might be difficult to prove, post-vaccination autoimmunity may be induced by either antigen-specific molecular mimicry or nonspecific bystander activation [5]. Therefore, this report underlies the fact that oral autoimmune and immune-mediated diseases, including MMP, might be able to develop after the COVID-19 vaccine administration.
To date, the COVID-19 pandemic remains an important issue globally. Large-scale vaccination is a cornerstone of a COVID-19 prevention strategy. Since many people have received multiple doses or boosters of the COVID-19 vaccine, oral health practitioners should recognize the possibility of a new-onset oral MMP development after the COVID-19 vaccination.
Acknowledgements
The authors would like to thank all staffs of the Department of Oral Medicine and Periodontology, Faculty of Dentistry, Mahidol University, and laboratory staffs of the Department of Medical Technology, Institute of Dermatology, for their kind support.
Author Contributions
DR: Conceptualization, resources, investigation, and writing—original draft preparation. NR: Writing—Review & Editing. RJ: Resources.
Funding
None.
Data Availability
The data presented in this study are available on request from the corresponding author. The data are not publicly available because of ethical restrictions.
Code Availability
Not applicable
Declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Ethical Approval
For this type of study formal consent is not required.
Informed Consent
Informed consent was obtained from the patient.
Consent for Publication
Consent for publication was obtained from the patient.
Footnotes
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Thongprasom K, Pengpis N, Phattarataratip E, Samaranayake L. Oral pemphigus after COVID-19 vaccination. Oral Dis. 2022;28(Suppl 2):2597–2598. doi: 10.1111/odi.14034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Kaomongkolgit R, Sawangarun W. Oral lichen planus following mRNA COVID-19 vaccination. Oral Dis. 2022;28(Suppl 2):2622–2623. doi: 10.1111/odi.14182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Hali F, Sr., Araqi L, Jr, Marnissi F, Meftah A, Chiheb S. Autoimmune bullous dermatosis following COVID-19 vaccination: a Series of five cases. Cureus. 2022;14(3):e23127. doi: 10.7759/cureus.23127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Xu HH, Werth VP, Parisi E, Sollecito TP. Mucous membrane pemphigoid. Dent Clin North Am. 2013;57(4):611–630. doi: 10.1016/j.cden.2013.07.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Sezin T, Egozi E, Hillou W, Avitan-Hersh E, Bergman R. Anti-laminin-332 mucous membrane pemphigoid developing after a diphtheria tetanus vaccination. JAMA Dermatol. 2013;149(7):858–862. doi: 10.1001/jamadermatol.2013.741. [DOI] [PubMed] [Google Scholar]
- 6.Damiani G, Pacifico A, Pelloni F, Iorizzo M. The first dose of COVID-19 vaccine may trigger pemphigus and bullous pemphigoid flares: is the second dose therefore contraindicated? J Eur Acad Dermatol Venereol. 2021;35(10):e645–e7. doi: 10.1111/jdv.17472. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Bardazzi F, Carpanese MA, Abbenante D, Filippi F, Sacchelli L, Loi C. New-onset bullous pemphigoid and flare of pre-existing bullous pemphigoid after the third dose of the COVID-19 vaccine. Dermatol Ther. 2022;35(7):e15555. doi: 10.1111/dth.15555. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The data presented in this study are available on request from the corresponding author. The data are not publicly available because of ethical restrictions.
Not applicable