Abstract
Molecular typing has been used previously to identify and trace dissemination of pathogenic and spoilage bacteria associated with food processing. Amplified fragment length polymorphism (AFLP) is a novel DNA fingerprinting technique which is considered highly reproducible and has high discriminatory power. This technique was used to fingerprint 88 Pseudomonas fluorescens and Pseudomonas putida strains that were previously isolated from plate counts of carcasses at six processing stages and various equipment surfaces and environmental sources of a poultry abattoir. Clustering of the AFLP patterns revealed a high level of diversity among the strains. Six clusters (clusters I through VI) were delineated at an arbitrary Dice coefficient level of 0.65; clusters III (31 strains) and IV (28 strains) were the largest clusters. More than one-half (52.3%) of the strains obtained from carcass samples, which may have represented the resident carcass population, grouped together in cluster III. By contrast, 43.2% of the strains from most of the equipment surfaces and environmental sources grouped together in cluster IV. In most cases, the clusters in which carcass strains from processing stages grouped corresponded to the clusters in which strains from the associated equipment surfaces and/or environmental sources were found. This provided evidence that there was cross-contamination between carcasses and the abattoir environment at the DNA level. The AFLP data also showed that strains were being disseminated from the beginning to the end of the poultry processing operation, since many strains associated with carcasses at the packaging stage were members of the same clusters as strains obtained from carcasses after the defeathering stage.
Pseudomonas spp. (specifically, Pseudomonas fluorescens, Pseudomonas putida, and Pseudomonas fragi) are considered the principal spoilage microorganisms of refrigerated poultry (4, 12, 32). The higher the level of these and other psychrotrophic spoilage bacteria on carcasses at the end of processing, the shorter the shelf life of the refrigerated product. Spoilage by Pseudomonas spp. results in a variety of off odors and flavors in chicken meat due to the proteolytic and lipolytic activities of the microorganisms (38). These bacteria enter the poultry abattoir on the feathers and feet of the live birds, as well as in water and ice supplies (4, 27). Most spoilage bacteria found on incoming live birds are destroyed during the scalding stage, which is the initial stage of processing and at which the temperatures are between 50 and 63°C, but recontamination occurs during subsequent processing stages due to the ability of the bacteria to multiply on all wet surfaces (17, 18, 26). Pseudomonas spp. have been observed to multiply on soiled poultry processing equipment surfaces, such as conveyor belts, knives, wire mesh gloves, and immersion chiller tanks (22). Certain exopolymer-producing strains of P. fragi have also been reported to act as primary colonizers for biofilm formation by the psychrotrophic pathogen Listeria monocytogenes (33). Thus, it is important to identify points of cross-contamination by Pseudomonas spp. and associated spoilage strains in poultry abattoirs in order to implement steps to control or eliminate these organisms.
DNA-based techniques have proven to be invaluable in identifying and characterizing bacteria associated with food (25, 29, 35). Methods such as plasmid profiling, PCR, randomly amplified polymorphic DNA analysis, and ribotyping have been used to identify and trace the dissemination of pathogenic and spoilage bacteria in different parts of the food chain (3, 6, 13–15, 28, 37). The amplified fragment length polymorphism (AFLP) technique is a novel PCR-based DNA fingerprinting technique which was originally developed for plant breeding purposes (39). AFLP has recently been used in the genotyping of different bacterial species and strains, such as Acinetobacter spp. (24), Aeromonas spp. (21), Xanthomonas spp. (9, 23), Helicobacter pylori (19), Pseudomonas syringae (11), and Vibrio vulnificus (2). The AFLP technique is based on the principles of restriction fragment length polymorphism analysis and PCR amplification. AFLP involves ligation of adaptor molecules to restriction enzyme fragments, which subsequently serve as primer binding sites for PCR amplification (23, 39). The amplification process is selective since the AFLP primers contain one or more nucleotides on their 3′ ends; thus, only a subset of the restriction fragments are amplified (23).
In this study, AFLP was used to DNA fingerprint potential spoilage Pseudomonas strains obtained from various sources in a poultry abattoir in an attempt to determine possible sites of cross-contamination. To our knowledge, there have been no previous reports of the use of DNA fingerprinting to trace Pseudomonas spp. and related spoilage bacteria through poultry processing operations.
MATERIALS AND METHODS
Bacterial strains and culture conditions.
The 88 Pseudomonas strains used in this study were obtained from a previous microbiological survey performed in a poultry abattoir to determine bacterial numbers on carcasses at different stages of processing, on equipment surfaces, and in the environment (17). Presumptive Pseudomonas colonies were randomly selected from duplicate cetrimide-fucidin-cephaloridine (Oxoid, Basingstoke, United Kingdom)-supplemented Pseudomonas agar base (Oxoid) plates containing between 30 and 300 colonies (20). The isolates were purified on plate count agar (Biolab, Midrand, South Africa) by incubation at 25°C for 24 h and were tentatively confirmed to be isolates of Pseudomonas spp. by checking for production of cytochrome oxidase and the ability to utilize glucose in the oxidation-fermentation test (16, 20). The isolates were identified to the species level by the Analytical Profile Index procedure by using the API 20NE system (bioMérieux, Marcy l’Etoile, France). All of the strains were stored at −70°C in tryptone soya broth (Oxoid) supplemented with 15% (vol/vol) glycerol.
In this study, we used only representatives of the previously described potential spoilage organisms P. fluorescens (80 strains) and P. putida (8 strains). A total of 44 of these strains were obtained from the neck skin of carcasses at six processing stages, and 44 strains were obtained from various equipment surfaces and environmental sources closely associated with these processing stages (see Table 1).
TABLE 1.
Numbers and sources of Pseudomonas strains in clusters I through VI, as determined by AFLP fingerprint analysis
| Source | Processing stage or designation | No. of strains analyzed | No. of strains
|
||||||
|---|---|---|---|---|---|---|---|---|---|
| Cluster I | Cluster II | Cluster III | Cluster IV | Cluster V | Cluster VI | Ungrouped | |||
| Carcass samples | |||||||||
| After defeathering | A | 5 | 1 | 3 | 1 | ||||
| Before evisceration | B | 8 | 5 | 2 | 1 | ||||
| After evisceration | C | 8 | 2 | 5 | 1 | ||||
| After spray washing | D | 7 | 2 | 5 | |||||
| After immersion chilling | E | 8 | 5 | 2 | 1 | ||||
| After packaging | F | 8 | 1 | 3 | 3 | 1 | |||
| Samples from equipment surfaces and environ-mental samples | |||||||||
| Rubber fingers at exit of defeathering machine | RF | 6 | 3 | 3 | |||||
| Plastic curtain at exit of defeathering machine | DC | 6 | 2 | 1 | 2 | 1 | |||
| Stainless steel chute leading into evisceration area | SE | 6 | 5 | 1 | |||||
| Stainless steel evisceration equipment | EE | 3 | 2 | 1 | |||||
| Stainless steel chute leading into immersion chiller | SS | 5 | 5 | ||||||
| Immersion chiller outlet water | SCB | 7 | 1 | 4 | 2 | ||||
| Whole-carcass packaging conveyor belt | CON | 1 | 1 | ||||||
| Stainless steel whole-carcass packaging equipment | PE | 6 | 4 | 2 | |||||
| Air of the defeathering area | DEF | 1 | 1 | ||||||
| Air of the packaging area | PACK | 3 | 1 | 1 | 1 | ||||
| Total | 88 | 4 | 10 | 31 | 28 | 10 | 3 | 2 | |
DNA isolation.
Bacterial strains were grown in tryptone soya broth at 25°C for 18 h. Genomic DNA was extracted from 5-ml cultures by the cetyltrimethylammonium bromide method of Bickley et al. (7). To obtain very pure DNA, two phenol extractions were included in the DNA isolation procedure. DNA pellets were resuspended in 20 to 40 μl of TE buffer (10 mM Tris-HCl, 1 mM EDTA; pH 8.0) and treated with RNase at 37°C for 30 min. DNA concentrations were estimated by agarose gel electrophoresis with diluted samples of λ DNA (Boehringer GmbH, Mannheim, Germany).
AFLP reaction.
An AFLP ligation and preselective amplification kit (Perkin-Elmer, Foster City, Calif.) was used; this kit provided the EcoRI and MseI restriction enzyme adapters, PCR primers, and PCR mixture for the AFLP reactions. The procedure used was essentially the procedure described by the manufacturer. We prepared a restriction-ligation mixture containing 500 ng of genomic DNA, 1 μl of 10× T4 DNA ligase buffer, 1 μl of 0.5 M nuclease-free NaCl (BDH, Dorset, England), 0.5 μl of a 10-mg/ml bovine serum albumin (New England Biolabs, Beverly, Mass.) solution, 1 μl (each) of the double-stranded EcoRI and MseI adapters (Perkin-Elmer), and 1.6 μl of an enzyme mixture consisting of 1 U of T4 DNA ligase (Boehringer), 5 U of EcoRI (Boehringer), and 1 U of TruI (Boehringer), which is an isoschizomer of MseI. The restriction-ligation mixture was incubated at 37°C for 3 h, and then it was diluted with 0.1 M TE buffer (20 mM Tris-HCl, 0.1 mM EDTA; pH 8.0) to a final volume of 200 μl. DNA amplification was performed in a 20-μl (total volume) mixture consisting of 4 μl of the diluted restriction-ligation mixture, 1 μl of EcoRI and MseI preselective amplification primer pairs (Perkin-Elmer), and 15 μl of AFLP core mixture (Perkin-Elmer). The EcoRI and MseI primers each had a single selective nucleotide on the 3′ end (A and C, respectively). PCR amplifications were performed with a Perkin-Elmer model 2400 thermocycler by using the following protocol: an initial step consisting of 72°C for 2 min, 20 cycles consisting of 94°C for 1 s, 56°C for 30 s, and 72°C for 2 min, and a final extension step consisting of 72°C for 7 min. The PCR products were stored at −20°C until electrophoresis was performed.
Electrophoresis and detection of PCR products.
The amplified fragments were separated electrophoretically in a denaturing 4% polyacrylamide sequencing gel with 8 M ultrapure urea (ICN Biochemicals Inc., Aurora, Ohio). The short glass plate was treated with Bind Silane (Promega, Madison, Wis.) in order to chemically cross-link the gel to the glass plate. Equal volumes of loading buffer (95% formamide, 20 mM EDTA, 0.05% bromophenol blue, 0.05% xylene cyanol) and amplified product (3 μl) were loaded into each lane. Prior to loading of the gel, samples were heated at 90°C for 3 min and then snap-cooled on ice to prevent DNA secondary structures from reannealing. Electrophoresis was performed with a model S2 sequencing gel apparatus (Gibco, BRL Life Technologies, Gaithersburg, Md.) at 55 W with 1× Tris-borate-EDTA (TBE) as the running buffer in the upper compartment and 1× TBE supplemented with 0.5 M sodium acetate in the lower compartment to prevent gel “frowning” (1). After electrophoresis, AFLP fingerprints were detected by a modification of the silver staining method of Bassam et al. (5). The gel was fixed in 12% acetic acid while it was still attached to the glass plate, stained with a solution containing 1 g of silver nitrate (BDH, Poole, England) per liter and 2.5 ml of 37% formaldehyde (BDH) per liter, and developed with a solution containing 30 g of sodium carbonate (Merck, Darmstadt, Germany) per liter, 2.5 ml of 37% formaldehyde per liter, and 500 μl of a 10-mg/ml sodium thiosulfate (BDH) solution per liter. The developing reaction was stopped by adding 12% acetic acid. The gels were allowed to air dry overnight before the results were recorded.
AFLP analysis.
The gels were scanned with a Hewlett-Packard ScanJet IIcx scanner and were stored as tagged image format files. The AFLP patterns were normalized, and similarity levels were calculated by using the PC Windows software package GelManager, version 1.5 (BioSystematica, Devon, United Kingdom). So that we could compare and normalize fingerprints between gels with GelManager, molecular weight marker X (Boehringer) and the 1-kb Plus ladder (Gibco) were included at five-lane intervals on every gel as standards. The levels of similarity between AFLP fingerprints were expressed as Dice coefficients (SD), which were calculated by determining the ratio of twice the number of bands shared by two patterns to the total number of bands in both patterns (8, 34). The position tolerance for band matching was set at 0.01 in. (8), where the standards electrophoresed at five-lane intervals on every gel exhibited levels of similarity of 100%. Strains were clustered by using the unweighted pair group method with arithmetic averages (UPGMA) (8, 34).
RESULTS
A representative silver-stained AFLP gel is shown in Fig. 1. Clustering of the AFLP data by UPGMA resulted in the dendrogram shown in Fig. 2. Six clusters (clusters I through VI) were delineated at an arbitrary SD level of 0.65, and two strains remained ungrouped. The numbers and sources of the Pseudomonas strains in the clusters are summarized in Table 1. The largest clusters were clusters III and IV, which contained 31 and 28 strains, respectively. Of the 31 strains in cluster III, 23 (74.2%) were obtained from carcass (product) samples taken at the six processing stages samples (stages A to F) (Table 1); thus, 52.3% of all of the strains isolated from carcasses were members of this cluster. The eight remaining strains in cluster III were obtained from nonproduct sources; five of these strains were obtained from a stainless steel chute onto which carcasses dropped after hock cutting in order to enter the evisceration area, one strain was obtained from a plastic curtain at the exit of the defeathering machine against which carcasses brushed, one strain was obtained from the immersion chiller water, and one strain was obtained from air of the packaging area (sources SE, DC, SCB, and PACK, respectively) (Fig. 2 and Table 1). A number of cluster III strains obtained from different sources exhibited high levels of similarity. For instance, strains 200 and 206, which originated from carcasses before and after evisceration, respectively, were 93% similar, and their AFLP fingerprints differed by only three bands. Also, strains 193 and 314, which were isolated from carcasses before the evisceration processing stage and the air of the packaging area, respectively, exhibited a level of similarity of 92%. In addition, the AFLP pattern of strain 231, which was obtained from immersion-chilled carcasses, differed from the AFLP pattern of strain 261 by only two bands, and strain 231 was 96% similar to strain 261, which was recovered from the chute leading into the evisceration area (Fig. 2).
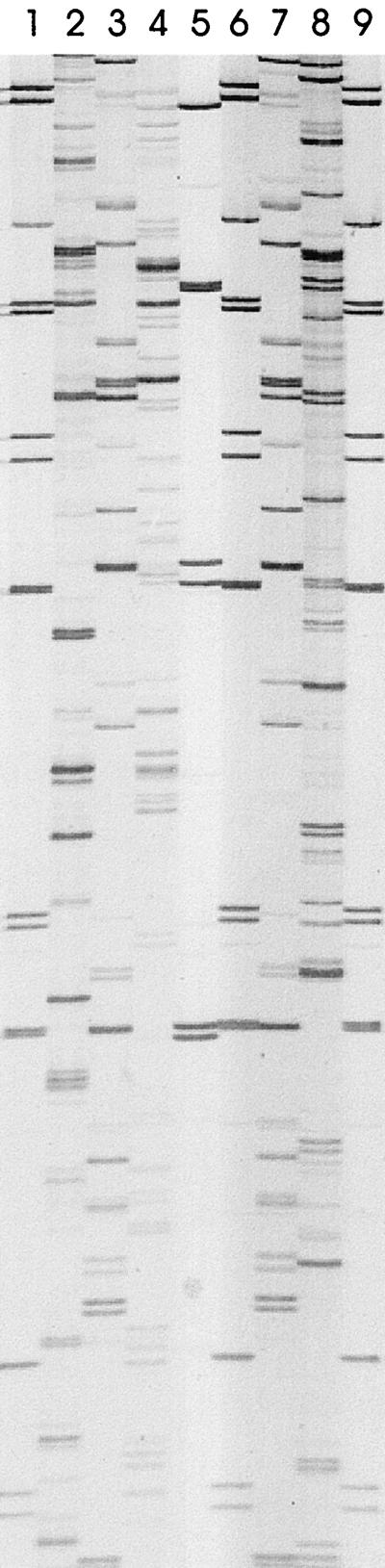
FIG. 1.
AFLP patterns of five Pseudomonas strains isolated from carcasses after the immersion chilling processing stage (stage E) (Table 1). Lanes 1, 6, and 9, molecular weight marker X; lane 2, strain 223; lane 3, strain 224; lane 4, strain 226; lane 5, 1-kb Plus ladder; lane 7, strain 227; lane 8, strain 228.
FIG. 2.
Dendrogram based on AFLP fingerprints of 88 P. fluorescens and P. putida strains obtained from carcasses (44 strains) and equipment surfaces and environmental sources (44 strains) associated with poultry processing. For a description of strain sources see Table 1. The dendrogram was constructed by using UPGMA. Levels of similarity between AFLP fingerprints were calculated by using SD. Clusters were delineated at an arbitrary SD level of 0.65.
In contrast to the cluster III strains, 19 of the 28 strains (67.9%) in cluster IV were obtained from the equipment surfaces and environmental sources sampled, excluding the chute leading into the evisceration area, the whole-carcass packaging conveyor belt, and the air of the defeathering area (Fig. 2 and Table 1). Thus, 43.2% of all of the strains obtained from nonproduct sources were members of cluster IV. This cluster also contained strains obtained from carcass samples taken from four of the six processing stages (that is, processing stages A, C, E, and F) (Fig. 2 and Table 1).
Like cluster IV, clusters II and V, each of which contained 10 strains, consisted mainly of strains obtained from the equipment surfaces and environmental sources sampled (six and seven strains, respectively) (Fig. 2 and Table 1). These sources included the two equipment surfaces associated with the defeathering machine (sources RF and DC) (Table 1), the chute leading into the evisceration area, the immersion chiller water, and the whole-carcass packaging equipment (Table 1). The remaining two clusters, clusters I and VI, were the smallest clusters and contained four and three strains, respectively (Fig. 2 and Table 1).
DISCUSSION
AFLP fingerprinting of the 88 Pseudomonas isolates, which were primarily P. fluorescens strains, revealed that the strains were very diverse (Fig. 2). The gels were normalized so that at a position tolerance level for band matching of 0.01 in. the standards included on every gel exhibited levels of similarity of 100%. Although some Pseudomonas strains appeared to be 100% similar visually (e.g., strains 224 and 227) (Fig. 1), the levels of similarity assigned by the program were considerably lower, because the analysis became more stringent as the number of bands increased and also because there were slight variations in band width and mobility which could be detected in the same gel. The levels of similarity which we obtained are therefore not absolute, but nevertheless they are useful for determining relationships among members of the population, as shown in Fig. 2. Taking this fact into account does not minimize the finding that there was a great deal of diversity among the Pseudomonas strains found in the processing plant. This was not unexpected as the two Pseudomonas spp. analyzed in this study, especially P. fluorescens, have been shown to be remarkably heterogeneous and can be subdivided into subspecies or biovars, on the basis of detailed phenotypic, chemotaxonomic, and genotypic data (32, 36). The isolates analyzed in this study were not identified to the subspecies level but were identified to the species level with the API 20NE system. The P. fluorescens and P. putida strains identified with this system, however, did not cluster separately at the DNA level (Fig. 2). The difference between the phenotypic and genotypic classifications of these two species was consistent with the findings of Christensen et al. (10), who used 23S ribosomal DNA sequencing and could not clearly distinguish among different P. fluorescens biovars and P. putida biovar B taxa which were phenotypically distinguishable by biochemical tests and intracellular protein profiles. The P. putida strains identified by the API 20NE system in the present study could be representatives of P. putida biovar B and for this reason clustered with P. fluorescens strains at the DNA level. The variability in the results obtained with the API 20NE system and AFLP fingerprinting provides further evidence that reexamination and possible reclassification of these two species are necessary.
The two largest clusters obtained with the AFLP data were clusters III and IV. Cluster III consisted mainly of product strains (74.2% of the cluster III strains) obtained from the six carcass sampling sites, and cluster IV consisted mainly of nonproduct strains (67.9% of the cluster IV strains) obtained from 7 of the 10 equipment surfaces and environmental sources examined (Table 1). Thus, more than one-half (52.3%) of the product strains were members of cluster III, while 43.2% of the nonproduct strains were members of cluster IV (Table 1). On the basis of these results, we speculated that the product strains in cluster III represented the resident population of the carcasses. Likewise, the nonproduct strains in cluster IV probably represented a separate population which was associated with the equipment surfaces and environmental sources in the abattoir. In a similar study, different populations of fluorescent pseudomonads from turkey meat and the surface of a meat mincer used to mince this meat were differentiated by randomly amplified polymorphic DNA profiles and carbon assimilation patterns (28). Similarly, Dodd et al. (13) recovered different populations of Staphylococcus aureus (based on plasmid profiling) from the rubber fingers of a defeathering machine and from freshly slaughtered carcasses in a poultry abattoir. The populations associated with the equipment surfaces in both of these studies were presumed to be endemic to the processing plants. In the present study, the presence of endemic strains on equipment surfaces could not be confirmed conclusively, since all of the strains analyzed were obtained in a single survey.
Clustering of the Pseudomonas AFLP patterns also provided evidence at the DNA level that cross-contamination between the carcasses and the abattoir environment was occurring. The clusters in which carcass strains obtained from each processing stage grouped corresponded, in most cases, to the clusters in which strains obtained from the associated equipment surfaces and/or environmental sources were found. For instance, strains obtained from carcasses that had been defeathered (processing stage A) were members of clusters II through IV (Table 1). The equipment surfaces associated with this stage were the rubber fingers and plastic curtain at the exit of the defeathering machine (sources RF and DC, respectively) (Table 1). The strains obtained from the RF sample grouped in clusters IV and V, and the strains obtained from the DC sample grouped in clusters II through V (Table 1). Based on the data described above, the clusters containing the strains obtained from processing stage A carcasses mirrored the clusters in which all of the strains obtained from the two associated equipment surfaces were found. Rubber fingers are well-known sites of cross-contamination in poultry abattoirs because they are difficult to clean and disinfect; this allows microorganisms to persist in surface channels and cracks (26, 30).
Cross-contamination was also detected at the evisceration stage (processing stage C). Strains obtained from carcasses at this stage grouped in clusters III, IV, and VI, while strains obtained from the chute leading into the evisceration area (source SE) and the evisceration equipment (source EE) were members of clusters III and V and clusters IV and VI, respectively (Table 1). Strains obtained from the immersion-chilled carcasses (processing stage E) were members of clusters III through V, as were the strains obtained from the immersion chiller water (Table 1). Cluster IV contained all five strains that originated from the chute leading into the immersion chiller, four strains that originated from the immersion chiller water, and two strains that originated from immersion-chilled carcasses (Table 1). In previous studies performed in the same abattoir the workers observed significant increases in the Pseudomonas counts for carcasses after this processing stage (17), and based on the results described above this was a result of cross-contamination from the chute and chiller water. Cross-contamination between carcasses and the abattoir environment was also highlighted at processing stage F (packaging). Strains obtained from packaged carcasses were members of clusters I through IV, while strains obtained from the associated equipment surfaces (sources PE and CON) (Table 1) and an environmental source (source PACK) (Table 1) were members of clusters II and IV (source PE strains), cluster I (source CON strains), and clusters III and IV (source PACK strains). Cluster II contained three of the eight strains obtained from packaged carcasses and four of the six strains obtained from the packaging equipment (Table 1). This equipment surface was previously identified as a site of cross-contamination with Serratia spp. by workers who used phenotypic characterization techniques (17).
A comparison of the clusters to which strains obtained from carcasses at the first and last processing stages belonged showed that the clusters were identical (i.e., clusters II through IV) (Table 1). However, one strain obtained from packaged carcasses was a member of cluster I. Thus, carcasses leaving the abattoir harbored practically the same strains as the strains associated with carcasses after the defeathering stage. Strains were thus disseminated from the beginning to the end of the processing line. Evidence of this phenomenon was also found in a distinct subcluster within cluster II containing strains 235 through 257, which were 72% similar (Fig. 2). This subcluster contained three strains obtained from packaged carcasses, three strains obtained from packaging equipment, one strain obtained from defeathered carcasses, and one strain obtained from the plastic curtain at the exit of the defeathering machine (Fig. 2). Cluster IV also contained a subcluster of strains (strains 249 through 258), which were 75% similar; this finding showed that the rubber fingers and plastic curtain were the sources of a strain obtained from the packaging equipment (Fig. 2). Thus, strains recovered from packaged carcasses and packaging equipment appeared to have originated from carcasses and equipment surfaces associated with defeathering. The source and dissemination of strains associated with the chute leading into the evisceration area were also noted. Strain 260, which was obtained from the chute, could have originated from the plastic curtain since it was 90% similar to strain 255, which was obtained from this equipment surface (cluster III) (Fig. 2). Furthermore, another strain obtained from the chute (strain 261) appeared to be disseminated from this site to carcasses at processing stages D and E. This was observed in the subcluster of cluster III containing strains 220 through 261, which were 85% similar (Fig. 2). Based on these findings, the AFLP technique can be used to determine the origins and spread of specific Pseudomonas strains in a processing environment.
In conclusion, this study highlighted the discriminative power of AFLP for differentiating between potential Pseudomonas spoilage strains associated with poultry carcasses and the abattoir environment. The ability of this technique to identify cross-contamination sites should be helpful in identifying steps which can be used to eliminate or control resident spoilage populations associated with equipment surfaces and the processing environment. Furthermore, the use of AFLP fingerprints in tracing the sources and dissemination of strains may be extended to determine the etiology of food-borne bacterial pathogens associated with poultry processing, such as Escherichia coli O157:H7, Listeria monocytogenes, and Salmonella spp.
ACKNOWLEDGMENT
We are grateful to the Foundation of Research Development for financial support of this study.
REFERENCES
- 1.Aarts H J M, van Lith L A J T, Keijer J. High-resolution genotyping of Salmonella strains by AFLP-fingerprinting. Lett Appl Microbiol. 1998;26:131–135. doi: 10.1046/j.1472-765x.1998.00302.x. [DOI] [PubMed] [Google Scholar]
- 2.Arias C R, Verdonck L, Swings J, Garay E, Aznar R. Intraspecific differentiation of Vibrio vulnificus biotypes by amplified fragment length polymorphism and ribotyping. Appl Environ Microbiol. 1997;63:2600–2606. doi: 10.1128/aem.63.7.2600-2606.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Arimi S M, Ryser E T, Pritchard T J, Donnelly C W. Diversity of Listeria ribotypes recovered from dairy cattle, silage, and dairy processing environments. J Food Prot. 1997;60:811–816. doi: 10.4315/0362-028X-60.7.811. [DOI] [PubMed] [Google Scholar]
- 4.Barnes E M. Microbiological problems of poultry at refrigerator temperatures—a review. J Sci Food Agric. 1976;27:777–782. doi: 10.1002/jsfa.2740270813. [DOI] [PubMed] [Google Scholar]
- 5.Bassam B J, Caetano-Anollés G, Gresshoff P M. Fast and sensitive silver staining of DNA in polyacrylamide gels. Anal Biochem. 1991;196:80–83. doi: 10.1016/0003-2697(91)90120-i. [DOI] [PubMed] [Google Scholar]
- 6.Beyer W, Mukendi F M, Kimmig P, Böhm R. Suitability of repetitive-DNA-sequence-based PCR fingerprinting for characterizing epidemic isolates of Salmonella enterica serovar Saintpaul. J Clin Microbiol. 1998;36:1549–1554. doi: 10.1128/jcm.36.6.1549-1554.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Bickley J, Short J K, McDowell D G, Parkes H C. Polymerase chain reaction (PCR) detection of Listeria monocytogenes in diluted milk and reversal of PCR inhibition caused by calcium ions. Lett Appl Microbiol. 1996;22:153–158. doi: 10.1111/j.1472-765x.1996.tb01131.x. [DOI] [PubMed] [Google Scholar]
- 8.BioSystematica. GelManager for Windows version 1.5 user’s manual. Devon, United Kingdom: BioSystematica; 1998. [Google Scholar]
- 9.Bragard C, Singer E, Alizadeh A, Vauterin L, Maraite H, Swings J. Xanthomonas translucens from small grains: diversity and phytopathological relevance. Phytopathology. 1997;87:1111–1117. doi: 10.1094/PHYTO.1997.87.11.1111. [DOI] [PubMed] [Google Scholar]
- 10.Christensen H, Boye M, Poulsen L K, Rasmussen O F. Analysis of fluorescent pseudomonads based on 23S ribosomal DNA sequences. Appl Environ Microbiol. 1994;60:2196–2199. doi: 10.1128/aem.60.6.2196-2199.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Clerc A, Manceau C, Nesme X. Comparison of randomly amplified polymorphic DNA with amplified fragment length polymorphism to assess genetic diversity and genetic relatedness within genospecies III of Pseudomonas syringae. Appl Environ Microbiol. 1998;64:1180–1187. doi: 10.1128/aem.64.4.1180-1187.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Cox N A, Juven B J, Thomson J E, Mercuri A J, Chew V. Spoilage odors in poultry meat produced by pigmented and nonpigmented Pseudomonas. Poult Sci. 1975;54:2001–2006. [Google Scholar]
- 13.Dodd C E R, Chaffey B J, Waites W M. Plasmid profiles as indicators of the source of contamination of Staphylococcus aureus endemic within poultry processing plants. Appl Environ Microbiol. 1998;54:1541–1549. doi: 10.1128/aem.54.6.1541-1549.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Dykes G A, Geornaras I, Papathanasopoulos M A, von Holy A. Plasmid profiles of Listeria species associated with poultry processing. Food Microbiol. 1994;11:519–523. [Google Scholar]
- 15.Farber J M. An introduction to the hows and whys of molecular typing. J Food Prot. 1996;59:1091–1101. doi: 10.4315/0362-028X-59.10.1091. [DOI] [PubMed] [Google Scholar]
- 16.Fischer P L, Jooste P J, Novello J C. Evaluation of rapid plate counting techniques for the enumeration of psychrotrophic bacteria in raw milk. S Afr J Dairy Sci. 1986;18:137–141. [Google Scholar]
- 17.Geornaras I, de Jesus A, van Zyl E, von Holy A. Microbiological survey of a South African poultry processing plant. J Basic Microbiol. 1995;35:73–82. doi: 10.1002/jobm.3620350204. [DOI] [PubMed] [Google Scholar]
- 18.Geornaras I, de Jesus A E, van Zyl E, von Holy A. Bacterial populations of different sample types from carcasses in the dirty area of a South African poultry abattoir. J Food Prot. 1997;60:551–554. doi: 10.4315/0362-028X-60.5.551. [DOI] [PubMed] [Google Scholar]
- 19.Gibson J R, Slater E, Xerry J, Tompkins D S, Owen R J. Use of an amplified fragment length polymorphism technique to fingerprint and differentiate isolates of Helicobacter pylori. J Clin Microbiol. 1998;36:2580–2585. doi: 10.1128/jcm.36.9.2580-2585.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Harrigan W F, McCance M E. Laboratory methods in microbiology. 3rd ed. London, United Kingdom: Academic Press; 1966. [Google Scholar]
- 21.Huys G, Coopman R, Janssen P, Kersters K. High-resolution genotypic analysis of the genus Aeromonas by AFLP fingerprinting. Int J Syst Bacteriol. 1996;46:572–580. doi: 10.1099/00207713-46-2-572. [DOI] [PubMed] [Google Scholar]
- 22.International Commission on Microbiological Specifications for Foods. Microorganisms in foods. 6. Microbial ecology of food commodities. London, United Kingdom: Blackie Academic & Professional; 1998. [Google Scholar]
- 23.Janssen P, Coopman R, Huys G, Swings J, Bleeker M, Vos P, Zabeau M, Kersters K. Evaluation of the DNA fingerprinting method AFLP as a new tool in bacterial taxonomy. Microbiology. 1996;142:1881–1893. doi: 10.1099/13500872-142-7-1881. [DOI] [PubMed] [Google Scholar]
- 24.Koeleman J G M, Stoof J, Biesmans D J, Savelkoul P H M, Vandenbroucke-Grauls C M J E. Comparison of amplified ribosomal DNA restriction analysis, random amplified polymorphic DNA analysis, and amplified fragment length polymorphism fingerprinting for identification of Acinetobacter genomic species and typing of Acinetobacter baumannii. J Clin Microbiol. 1998;36:2522–2529. doi: 10.1128/jcm.36.9.2522-2529.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Matthews K R, Roberson J, Gillespie B E, Luther D A, Oliver S P. Identification and differentiation of coagulase-negative Staphylococcus aureus by polymerase chain reaction. J Food Prot. 1997;60:686–688. doi: 10.4315/0362-028X-60.6.686. [DOI] [PubMed] [Google Scholar]
- 26.Mead G C. Hygiene problems and control of process contamination. In: Mead G C, editor. Processing of poultry. London, United Kingdom: Elsevier Applied Science; 1989. pp. 183–220. [Google Scholar]
- 27.Mead G C, Adams B W, Parry R T. The effectiveness of in-plant chlorination in poultry processing. Br Poult Sci. 1975;16:517–526. doi: 10.1080/00071667508416220. [DOI] [PubMed] [Google Scholar]
- 28.Michiels C W, Schellekens M, Soontjens C C F, Hauben K J A. Molecular and metabolic typing of resident and transient fluorescent pseudomonad flora from a meat mincer. J Food Prot. 1997;60:1515–1519. doi: 10.4315/0362-028X-60.12.1515. [DOI] [PubMed] [Google Scholar]
- 29.Miyamoto T, Tian H Z, Okabe T, Trevanich S, Asoh K, Tomoda S, Honjoh K-I, Hatano S. Application of random amplified polymorphic DNA analysis for detection of Salmonella spp. in foods. J Food Prot. 1998;61:785–791. doi: 10.4315/0362-028x-61.7.785. [DOI] [PubMed] [Google Scholar]
- 30.Notermans S, Dormans J A M A, Mead G C. Contribution of surface attachment to establishment of microorganisms in food processing plants: a review. Biofouling. 1991;5:21–36. [Google Scholar]
- 31.Palleroni N J. Introduction to the family Pseudomonadaceae. In: Balows A, Trüper H G, Dworkin M, Harder W, Schleifer K-H, editors. The prokaryotes. 2nd ed. New York, N.Y: Springer-Verlag; 1992. pp. 3071–3085. [Google Scholar]
- 32.Russell S M, Fletcher D L, Cox N A. Spoilage bacteria of fresh broiler chicken carcasses. Poult Sci. 1996;75:2041–2047. doi: 10.3382/ps.0742041. [DOI] [PubMed] [Google Scholar]
- 33.Sasahara K C, Zottola E A. Biofilm formation by Listeria monocytogenes utilizes a primary colonizing microorganism in flowing systems. J Food Prot. 1993;56:1022–1028. doi: 10.4315/0362-028X-56.12.1022. [DOI] [PubMed] [Google Scholar]
- 34.Sneath P H A, Sokal R R. Numerical taxonomy: the principles and practice of numerical classification. W. H. San Francisco, Calif: Freeman; 1973. [Google Scholar]
- 35.Strom M S. Phenotypic and genetic characterization of a non-hemolytic variant of Listeria monocytogenes from cold-smoked salmon. Food Microbiol. 1998;15:329–337. [Google Scholar]
- 36.Vancanneyt M, Torck U, Dewettinck D, Vaerewijck M, Kersters K. Grouping of pseudomonads by SDS-PAGE of whole-cell proteins. Syst Appl Microbiol. 1996;19:556–568. [Google Scholar]
- 37.van der Vossen J M B M, Hofstra H. DNA based typing, identification and detection systems for food spoilage microorganisms: development and implementation. Int J Food Microbiol. 1996;33:35–49. doi: 10.1016/0168-1605(96)01136-1. [DOI] [PubMed] [Google Scholar]
- 38.Vanderzant C, Ousley T J. Utilization of some carbon and nitrogen sources by Pseudomonas fluorescens. J Food Sci. 1963;28:123–129. [Google Scholar]
- 39.Vos P, Hogers R, Bleeker M, Reijans M, van de Lee T, Hornes M, Frijters A, Pot J, Peleman J, Kuiper M, Zabeau M. AFLP: a new technique for DNA fingerprinting. Nucleic Acids Res. 1995;23:4407–4414. doi: 10.1093/nar/23.21.4407. [DOI] [PMC free article] [PubMed] [Google Scholar]