Abstract
Objectives
Stereotactic radiosurgery (SRS) is part of the multimodality treatment for patients with cancer. The objective of this study is to determine factors which influence overall survival (OS) of Filipino patients who underwent SRS for metastatic tumors of the spine.
Methods
This is a retrospective analysis of a cohort of Filipino patients treated with spine SRS for metastatic tumors in a single institution. Putative predictors were determined by the institution’s spine SRS team and described in the cohort. A Cox proportional hazards regression model was utilized to construct a model based on the predictors determined by the institution’s spine SRS team.
Results
A total of 51 consecutive patients with 68 spine metastases were treated with SRS at our institution. The median OS was 13.1 months (95% CI of 7.1 to 19.1). On multivariate analysis, significant predictors that are associated with OS were visceral tumor origin (adjusted HR: 3.08, 95% CI of 1.24 to 7.64, p = 0.015) and cardiovascular disease (adjusted HR: 2.50, 95% CI of 1.04 to 5.94, p = 0.039) with dose and number of fractions as co-variates [Model Wald χ2 (5, N = 51) = 11.11 (p = 0.049)].
Conclusions
The presence of visceral tumor origins and cardiovascular disease are independent factors that are associated with lower overall survival in Filipino patients with spine metastasis treated with SRS.
Keywords: Stereotactic radiosurgery, spine metastasis, cardiovascular disease, visceral tumor origin, overall survival
INTRODUCTION
Spine metastasis occurs in more than 40% of disseminated cancers and this occurs mostly in the bony components. The incidence of spine metastasis is expected to rise due to improved therapy of cancer that prolongs patient survival [1], hence the potential challenges to its management should be realized. The most common histologic origins are the breast, prostate, and lung cancer [2], while visceral tumor origins include the lungs, pleura, liver and the rest of the gastrointestinal tract. Studies on survival after spine metastasis have shown through recursive partitioning analysis that Karnofsky Performance Status Scale (KPS), control of primary tumor, and visceral metastases are predictors for survival [3]. The regional or local management of spine metastasis include surgery and radiotherapy [4]. Surgery is mainly indicated for obtaining tissue samples, spine stabilization, and immediate decompression. However, surgery may not be applicable for patients with poor medical conditions, limited life expectancies, and extensive spinal involvement. Spine Stereotactic Radiosurgery (SRS) may be offered to this subset of patients because if can be used safely and effectively [5,6].
Our institution is the only institution in the Philippines to offer spine SRS. The patients undergoing this procedure have a median Overall Survival (OS) of 16 months and a progression-free survival (PFS) of 13 months [7]. To date, no studies have been performed to determine factors that influence OS in Filipinos with spine metastasis treated with SRS. This study aims to determine these significant predictors to help in risk estimation and in improvement of management protocols and patient care.
MATERIALS AND METHODS
Study population
We studied 51 consecutive patients treated with SRS for spine metastasis (average age was 59.7 ± 13.9 years and 22 were women and 29 were men). Treatment with spine SRS was initiated only after approval of the institution’s spine SRS team which was composed of Neurosurgeons, Neuro-Oncologists, Radiation Oncologists, and Medical Physicists. The patients should have the following criteria to be treated with SRS: 1) spine metastasis by histopathology and imaging using Magnetic Resonance Imaging (MRI), Computed Tomography (CT), or Positron Emission Tomography (PET); 2) localized spine metastasis at 1 to 3 sites with a maximum involvement of 2 contiguous vertebral bodies; 3) numerical rating scale for pain > 5/10; 4) mild to moderate neurologic signs (e.g. radiculopathy, dermatomal sensory change, manual motor strength < 4/5 (problems with ambulation for the lower extremity and arm-raising and finger function for the upper extremity)); 5) paraspinal mass with at least a 3 mm gap from the spinal cord; and 6) paraspinal mass with the spine metastatic tumor that is less than 5 cm in its largest diameter. Pediatric patients below 18 years of age and pregnancy during the time of treatment were not treated with spine SRS.
Radiosurgery technique
These patients underwent either a single- or multiple- fraction SRS treatment procedure. For single fraction treatment, median doses for the planned target volumes were usually 16 Gy [13 to 18 Gy]. For 3 to 5 fraction treatment procedures, median doses were usually 3 fractions of 9 Gy each (27 Gy total) or 5 fractions of 7 Gy each (35 Gy total). The decision for fractionation and dose was based on the tumor volume, proximity to the spinal cord and other organs-at-risk, and previous radiotherapy, congruent with Zeng et al. [8]. The number of isocenters/spinal levels treated for a patient ranged from one to three. All treatment plans were reviewed retrospectively.
Factors
Variables considered by the institution’s spine SRS team in the analysis of factors associated with OS included the following: KPS < 80, Cardiovascular Disease (CVD), Diabetes Mellitus (DM), primary tumor control, chemotherapy, extraspinal metastasis, previous extraspinal Radiation Therapy (RT), history of spine surgery, vertebral compression fracture, pain, motor deficit, sensory deficit, visceral origin, gross tumor volume (GTV), dose, number of fractions, and the interaction term of dose*fractions.
The patient’s medical history (medical, oncologic, and spine), initial KPS and Eastern Cooperative Oncology Group (ECOG) Performance Status score, tumor characteristics (primary control and visceral origin), and symptoms (pain and motor/sensory deficits were collected from medical records written by the treating physicians before spine SRS. Treatment data were collected from the institution’s spine SRS database. Overall survival data were collected from the database and hospital/clinic medical records. The patients who were alive during follow-up were censored during analysis. Strict patient confidentiality was maintained throughout in all stages of the study. Only the investigators were allowed to gather patient data and analyze patient records.
Sample size and missing data
The values for effect size, accrual time, and accrual patterns were taken from Mariano et al. to compute for the sample size requirement of this study [3]. A two-sided Log-Rank test with an overall sample size of 50 subjects achieved a 90.3% power at an alpha set at 0.05, with a hazard ratio (HR) of 1.7. The study was expected to last for 8 time periods of which subject accrual was expected to occur in the first time period. No subjects were expected to drop out. Complete case analysis was used if there were any missing data.
Statistical analysis
Categorical variables were presented as counts (percentages) and continuous variables were described using measures of central tendency depending on the normality of data using the Shapiro-Wilk test. Kaplan-Meier survival analysis was used to estimate OS. Cox proportional hazards regression analysis was performed to assess the relationship between each variable and OS. Variables in the final model were selected according to a stepwise method and those deemed to have clinical importance by the authors were included. To determine the independent factors affecting overall survival, hazard ratios were evaluated after adjustment for other factors. All tests were two-sided and p values were considered significant if they were less than 0.05. Analysis was performed using SPSS version 26.0 statistical software.
RESULTS
Patient characteristics
The clinical data for these 51 patients with spine metastasis were summarized in Table 1. Fifty-one consecutive patients with spine metastasis (22 female and 29 male ranging from 23 to 85 years [59.7 ± 13.8 years]) underwent spine SRS at our institution. The median ECOG Performance Status grade was 2. The median KPS score was 80 [50 – 90]. Many of patients with co-morbidities had CVD (35.3%) and DM (15.7%) (Table 1). The predominant histological diagnoses were lung (21.6%) and prostate (13.7%), in accordance with the study by Lun et al. [9]. Twenty-one patients did not have extraspinal metastasis. The bone (31.4%) and brain (11.8%) were the most common sites of extraspinal metastasis. Seventeen patients (33.3%) were given extraspinal RT. The most common sites of RT prior to SRS were bone (29.4%) and head and neck (17.6%). Twenty-one (41.2%) patients received chemotherapy (Table 1).
Table 1.
Patient demographics, disease characteristics, and treatment findings
| Parameter | Value |
|---|---|
| Age (years) | 59.7 ± 13.9 |
| Sex | |
| Female | 22 (43.1%) |
| Male | 29 (56.9%) |
| ECOG | 2.00 [IQR 1-3] |
| KPS | 80 [IQR 50-90] |
| Medical history | |
| Cardiovascular disease | 18 (35.3%) |
| Diabetes mellitus | 8 (15.7%) |
| Oncologic history | |
| Primary tumor control | 21 (41.2%) |
| Chemotherapy | 21 (41.2%) |
| Radiotherapy | 17 (33.3%) |
| Spine history | |
| Surgery | 16 (31.4%) |
| VCF | 14 (27.5%) |
| Symptoms | |
| Pain | 31 (60.8%) |
| Motor deficit | 14 (27.5%) |
| Sensory deficit | 6 (11.8%) |
| Visceral origin | 28 (54.9%) |
| GTV (mL) | 43.1 [IQR 14.0-80.7] |
| Dose (Gy) | 16 [IQR 15-24] |
| Fractions (no.) | 1 [IQR 1-3] |
Spine SRS treatment
The objective of treatment was palliation in 14 (27.5%) patients. The number of isocenters treated in a patient ranged from one to three. Majority of patients (74.5%) had one isocenter, twelve (23.5%) had two isocenters, and one (2.0%) had three isocenters. A total of 68 spine metastases were treated in our sample. The most common isocenters were located in the thoracic (55.9%) and lumbar (22.1%). According to Spine Instability Neoplastic Score (SINS) classification [10] on location, most spine metastases were either junctional (39.7%) or semi-rigid (38.2%). The most common histological diagnoses were small cell lung carcinoma (19.1%), prostate adenocarcinoma (13.2%) and breast carcinoma (11.8%). Sixty (88.2%) of the lesions were deemed sensitive to radiation.
The average GTV was 34.98 mL [0.6 – 192.3]. Thirty-nine (76.5%) underwent single fraction treatment, hence bringing down the average dose to 16 Gy [8 – 35]. Single-fraction and five-fraction SRS were used to treat 52 (76.5%) and 10 (14.7%) lesions respectively. Average GTV for single-fraction and five-fraction SRS were 30.8 [0.6 – 192.3] and 66.2 [19.2 – 157.7] respectively. Average doses for single-fraction and five-fraction SRS were 16 Gy [8 – 24] and 27.5 Gy [22.5 – 35.0] respectively.
Overall survival
The follow-up period ranged from 1 to 69 months. The median OS of patients who underwent spine SRS was 13.1 months (95% CI of 7.1 to 19.1) (Figure 1). Thirty (58.8%) patients died during the study period. The most common causes of death were acute respiratory failure (9/30), septic shock (6/30), and primary tumor progression in nine (30.0%), six (20.0%), and five (16.7%) patients, respectively.
Figure 1.
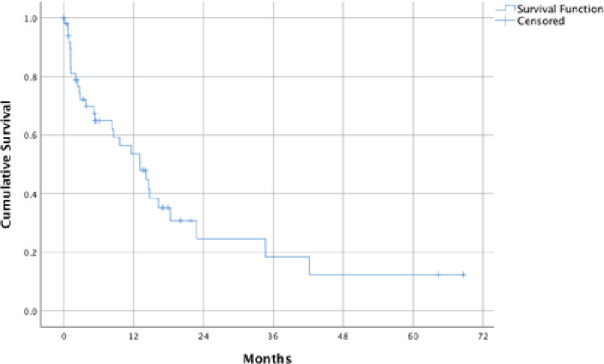
OS from time of SRS.
On univariate analysis (Table 2), significant predictors were visceral tumor origin (HR: 2.477, 95% CI of 1.108 to 5.539, p = 0.027) and dose (HR: 1.001, 95% CI of 1.000 to 1.001, p = 0.040). On multivariate analysis (Table 3), a model (Wald χ2 (5, N = 51) = 11.11 (p = 0.049)) was created where visceral tumor origin (HR: 3.077, 95% CI of 1.239 to 7.643, p = 0.015) and cardiovascular disease (HR: 2.495, 95% CI of 1.048 to 5.938, p = 0.039) were independently associated with OS. Other covariates included in the model were dose (p = 0.188), number of fractions (p = 0.978), and the interaction term, dose*fractions (p = 0.702). Survival functions adjusted for covariate means were created to aid risk stratification for patients in Figures 2 and 3.
Table 2.
Factors influencing survival (univariate analysis)
| Parameter | HR | 95% CI | p Value |
|---|---|---|---|
| KPS < 80 | 0.715 | 0.348-1.472 | 0.363 |
| CVD | 0.730 | 0.352-1.512 | 0.396 |
| DM | 1.686 | 0.713-3.986 | 0.234 |
| Primary tumor control | 0.682 | 0.321-1.448 | 0.319 |
| Chemotherapy | 1.674 | 0.810-3.458 | 0.164 |
| Extraspinal metastasis | 1.467 | 0.691-3.115 | 0.319 |
| Previous extraspinal RT | 0.813 | 0.354-1.864 | 0.624 |
| Spine surgery | 0.931 | 0.439-1.972 | 0.852 |
| VCF | 1.048 | 0.446-2.459 | 0.915 |
| Pain | 1.688 | 0.787-3.620 | 0.179 |
| Motor deficit | 1.409 | 0.680-2.919 | 0.179 |
| Sensory deficit | 0.809 | 0.243-2.696 | 0.730 |
| Visceral origin | 2.477 | 1.108-5.539 | 0.027 |
| GTV (mL) | 1.003 | 0.996-1.010 | 0.475 |
| Dose (Gy) | 1.001 | 1.000-1.001 | 0.040 |
| Fractions (no.) | 1.153 | 0.918-1.449 | 0.222 |
| Dose*Fractions | 1.000 | 1.000-1.000 | 0.129 |
Table 3.
Factors influencing overall survival (multivariate analysis)
| Parameter | Adjusted HR | 95% CI | p Value |
|---|---|---|---|
| Visceral origin | 3.077 | 1.239-7.643 | 0.015 |
| CVD | 2.495 | 1.048-5.938 | 0.039 |
| Dose (Gy) | 1.002 | 0.999-1.004 | 0.188 |
| Fractions (no.) | 1.022 | 0.208-5.035 | 0.978 |
| Dose*Fractions | 1.000 | 0.999-1.001 | 0.702 |
Figure 2.
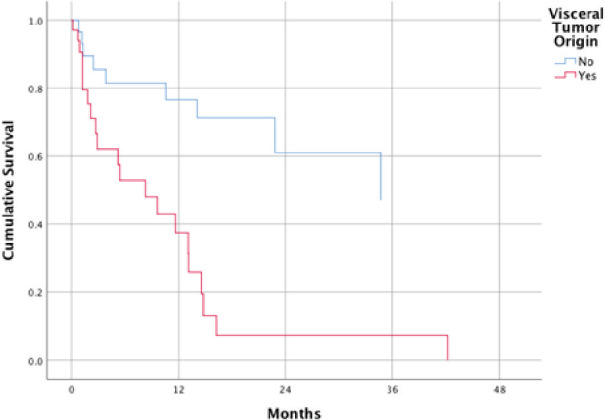
Survival function of patients with and without visceral tumor origins at mean of covariates.
Figure 3.
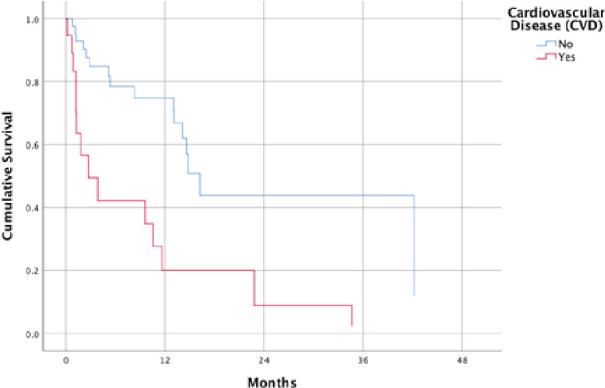
Survival function of patients with and without cardiovascular disease at mean of covariates.
DISCUSSION
Visceral tumor origin was found to be an independent factor influencing OS in our study and this is congruent to results of many studies [1,9,11,12]. The hazard ratio (HR) calculated in our sample is greater compared to other studies. Although this may be due to the small sample size of this study, it should be taken into consideration when advising patients to undergo SRS. Patients who have non-visceral tumor origins have the significant advantage of undergoing SRS over patients who have visceral tumor origins.
It is interesting to note that CVD is a significant predictor of decreased OS in our sample. Though most studies mentioned that majority of patients with spine metastasis are plagued with cardiovascular co-morbidities such as hypertension and coronary artery disease, no study has found an association between CVD and OS. The decreased OS in patients with CVD may have implications in SRS treatment like radiation induced heart disease or aggravation of heart disease as cited by Wang et al. [13]. However, because of our study’s small sample size, it may be better to verify this with a prospective study with a greater study population. For now, it may suffice that thorough cardiopulmonary pre-SRS risk assessment and Cardiology follow-up every 3 months be done on Filipino patients undergoing SRS to mitigate modifiable factors for patients with increased risk for mortality.
Survival functions at means of covariates for both visceral tumor origins and CVD were constructed to aid in risk assessment of patients at specific time points. This may aid in clinical decision making after spine SRS.
CONCLUSION
SRS is a safe and effective treatment for spine metastasis. Filipino patients who are treated with SRS for spine metastasis at our institution have poorer median OS if they have CVD and visceral tumor origins.
ACKNOWLEDGMENTS
Authors’ disclosure of potential conflicts of interest
The authors have nothing to disclose.
Footnotes
Author contributions
Conception and design: Ibet Marie Y. Sih, Roy Allan Dominique G. Torcuator, Erickson F. Torio, Maurice V. Bayhon,
Data collection: Jo-Celine M. Leong, Jonna Mae D. Maala, Charlene Mary C. Mercado, Thomas Vincent T. Vergara, Jan Rehino M. Yanto
Data analysis and interpretation: Ma. Socorro S.D. Santos
Manuscript writing: Ibet Marie Y. Sih, Roy Allan Dominique G. Torcuator, Erickson F. Torio, Maurice V. Bayhon
Final approval of manuscript: Carlo G. Barredo, Rhoderick M. Casis, Juan Manuel L. Mariano, Manuel M. Mariano, Roy Allan Dominique G. Torcuator, Miriam Joy C. Calaguas, Angela P. Camacho, Kathleen Jane U. Cortez, Juan Martin J. Magsanoc, Julius Cezar P. Rojales, Thomas Vincent T. Vergara
REFERENCES
- 1.Park HJ, Kim HJ, Won JH, Lee SC, Chang AR. Stereotactic body radiotherapy (SBRT) for spinal metastases: who will benefit the most from SBRT? Technol Cancer Res Treat 2015. Apr;14(2):159-67. doi: . Epub 2014 Nov 21. [DOI] [PubMed] [Google Scholar]
- 2.Maccauro G, Spinelli MS, Mauro S, Perisano C, Graci C, Rosa MA. Physiopathology of spine metastasis Int J Surg Oncol 2011;2011:107969. doi: . Epub 2011 Aug 10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Mariano JM, Torio EF, Santos MS, Sih IM, Torcuator RA. Stereotactic radiosurgery and stereotactic fractionated radiotherapy for metastatic tumors of the spine Neurol Res 2017. Apr;39(4):298-304. doi: . Epub 2017 Mar 7. [DOI] [PubMed] [Google Scholar]
- 4.Ryu SI, Chang SD, Kim DH, Murphy MJ, Le QT, Martin DP, Adler JR. Image-guided hypo-fractionated stereotactic radiosurgery to spinal lesions Neurosurgery 2001. Oct;49(4):838-46. doi: . [DOI] [PubMed] [Google Scholar]
- 5.Azad TD, Esparza R, Chaudhary N, Chang SD. Stereotactic radiosurgery for metastasis to the craniovertebral junction preserves spine stability and offers symptomatic relief J Neurosurg Spine 2016. Feb;24(2):241-247. doi: . SPINE 15190. Epub 2015 Oct 30. [DOI] [PubMed] [Google Scholar]
- 6.Benzil DL, Saboori M, Mogilner AY, Rocchio R, Moorthy CR. Safety and efficacy of stereotactic radiosurgery for tumors of the spine J Neurosurg 2004. Nov;101 Suppl 3:413-8. [PubMed] [Google Scholar]
- 7.Jawad MS, Fahim DK, Gerszten PC, Flickinger JC, Sahgal A, Grills IS, Sheehan J, Kersh R, Shin J, Oh K, Mantel F, Guckenberger M. on behalf of the Elekta Spine Radiosurgery Research Consortium Vertebral compression fractures after stereotactic body radiation therapy: a large, multi-institutional, multinational evaluation J Neurosurg Spine 2016. Jun;24(6):928-36. doi: . SPINE 141261. Epub 2016 Feb 19. [DOI] [PubMed] [Google Scholar]
- 8.Zeng KL, Tseng CL, Soliman H, Weiss Y, Sahgal A, Myrehaug S. Stereotactic body radiotherapy (SBRT) for oligometastatic spine metastases: An overview Front Oncol 2019. May 1;9:337. doi: . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Lun DX, Chen NW, Feng JT, Yang XG, Xu ZW, Li F, Hu YC. Visceral metastasis: A prognostic factor of survival in patients with spinal metastases Orthop Surg 2020. Apr;12(2):552-560. doi: . Epub 2020 Mar 29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Thibault I, Atenafu EG, Chang E, Chao S, Ameen AO, Zhou S, Boehling N, Balagamwala EH, Cunha M, Cho J, Angelov L, Brown PD, Suh J, Rhines LD, Fehlings MG, Sahgal A. Risk of vertebral compression fracture specific to osteolytic renal cell carcinoma spinal metastases after stereotactic body radiotherapy: A multi-institutional study J Radiosurg SBRT 2015;3(4):297-305. [PMC free article] [PubMed] [Google Scholar]
- 11.Hosono N, Ueda T, Tamura D, Aoki Y, Yoshikawa H. Prognostic relevance of clinical symptoms in patients with spinal metastases Clin Orthop Relat Res 2005. Jul;(436):196-201. doi: . [DOI] [PubMed] [Google Scholar]
- 12.Gao ZY, Zhang T, Zhang H, Pang CG, Jiang WX. Prognostic factors for overall survival in patients with spinal metastasis secondary to prostate cancer: a systematic review and meta-analysis BMC Musculoskelet Disord 2020. Jun 17;21(1):388. doi: . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Wang H, Wei J, Zheng Q, Meng L, Xin Y, Yin X, Jiang X. Radiation-induced heart disease: a review of classification, mechanism and prevention Int J Biol Sci 2019. Aug 8;15(10):2128-2138. doi: . [DOI] [PMC free article] [PubMed] [Google Scholar]


