Abstract
Background Current practice guidelines for patients with thoracic aortic aneurysms (TAAs) recommend 6 to 12-month intervals for surveillance imaging based on growth estimates of 0.10 to 0.42 cm/y gleaned from limited studies which included patients with thoracoabdominal aneurysms, known acute or chronic aortic dissection, and other syndromic and nonsyndromic high-risk conditions (TAA-HRC) associated with high-risk for adverse aortic events and death. Our objective was to determine TAA growth and event-free survival rates for patients with aortic root or midascending diameters <5.0 cm, and without thoracoabdominal aneurysms, acute or chronic aortic dissection or higher risk syndromic or nonsyndromic conditions (TAA-NoHRC).
Methods A retrospective review of patient records and imaging studies were done. Aortic diameter measurements were all performed by the lead author.
Results For 197 TAA-NoHRC found incidentally during chest imaging, with 616 chest imaging studies over 868 patient-years, the mean aortic root and midascending aortic growth rates were 0.018 and 0.022 cm/y, respectively. The growth rate was significantly lower for aneurysms initially measured at <4.5 cm versus ≥ 4.5 cm at both the aortic root (0.011 vs. 0.068 cm/y) and midascending aorta (0.013 vs. 0.043 cm/y). Survival free from adverse aortic events (dissection, rupture, and surgery) or death at 5 years was 99.5%.
Conclusion Adult TAA-NoHRC patients with initial aortic root and/or ascending aortic diameters <5.0 cm, and particularly <4.5 cm, have very low aortic growth, and adverse event rates which may permit longer intervals between surveillance imaging, up to 3 to 5 years, after initial (6–12 months) stability is documented.
Keywords: thoracic aortic aneurysm, aortic dissection, annual chest imaging, aortic diameter growth rate, practice guidelines
Introduction
Thoracic aortic aneurysms (TAAs) are usually asymptomatic until catastrophic aortic dissection and/or rupture (AoDR) occurs, often in the setting of uncontrolled hypertension. 1 Aortic diameter, measured on chest imaging studies, is a risk determinant for AoDR and the metric used for establishing thresholds at which surgical repair is recommended. 1 Reports of TAA diameter increases of 0.10 to 0.42 cm/y for a variety of adult patient cohorts led to the 2010 American College of Cardiology/American Heart Association (ACC/AHA) Thoracic Aortic Disease (TAD) guideline recommendation that biannual or annual chest imaging should be performed for patients with TAAs to detect aortic growth with a corresponding higher risk of AoDR. 1 2 3 4 5 6 Whether biannual or annual chest imaging is necessary for all TAA patients under surveillance has been questioned particularly given the cumulative radiation exposure risk associated with repeated CT imaging, which provides the best aortic images. 7 8 9
For the 2010 ACC/AHA TAD guideline, there were 118 total recommendations, 75 (64%) of which were the level of evidence C and 42 (36%) were the level of evidence B. For recommendations on imaging for detection or surveillance of TAD, 10 were level C and 3 were level B. The limited evidence base for most topics was a deficiency recognized by the writing committee, and studies to improve the evidence base were encouraged. 1
Thoracic aortic dissection clinics (TADC) are noted in many academic and pediatric medical centers. 10 Bethesda North Hospital is a 362-bed suburban community hospital in southwest Ohio performing 600 to 700 adult cardiac surgical procedures annually. A structured TADC was established in July 2015 utilizing current guidelines as the basis for patient evaluation, management, and education. Previously published adverse event rate estimates which included patients with high-risk syndromic (Marfan or Marfan-like disorders) and nonsyndromic familial conditions, thoraco-abdominal aneurysms, and known acute or chronic aortic dissection associated with high-risk for adverse aortic events and death (TAA-HRC) were used as a basis for aortic imaging surveillance scheduling and patient education. 1
The purposes of this study are to determine aortic diameter growth and adverse event rates for patients with <5.0 cm TAA, without apparent high-risk conditions (TAA-NoHRC) followed in a suburban community hospital setting compared with previous publications, and whether the recommended 6- to 12-month interval for surveillance imaging is appropriate for this subgroup.
Materials and Methods
TADC Structure and Process
Guideline recommendations were utilized wherever possible. 1 2 3 A detailed patient and family history was elicited for possible TAA, AoDR, and/or sudden death without causal verification or postmortem exam, syndromes (Marfan, Turner, Loeys–Dietz, and Ehlers–Danlos vascular type), and history of significant chest trauma or possible inflammatory vasculitides. Where information was unclear or not immediately available, patients and family members were asked to seek additional documentation which was then added to their records. When a positive or suggestive family history of TAD or AoDR emerged, genetic testing and counseling were recommended.
A history of fluoroquinolone use or recorded prescriptions was sought. 11 12 Fluoroquinolones were added to each patient's medication contraindication/allergy list. All blood pressure (BPs) were obtained utilizing the guideline-recommended procedure. 13 Patients were examined for features consistent with high-risk syndromes, primarily Marfan syndrome. Patients who were identified with high-risk syndromic or nonsyndromic (family history of surgery for TAD and/or AoDR) conditions were offered specific surgical consultation.
Prior imaging studies with a possible view of the thoracic aorta were sought. All chest imaging studies for patients as well as first-degree relatives, if available, and with the permission of the individual, were viewed by the lead author. Aortic diameter was obtained from CT or MR images using Merge Picture Archiving and Communication System calipers placed at the outer edge of the aortic wall positioned perpendicular to the axis of flow ( Figs. 1 2 3 4 5 ). Side-by-side comparisons assured that measurements were taken at the same anatomic level and orientation ( Fig. 6 ). The aortic height index was calculated using the method described by Zafar et al. 14
Fig. 1.
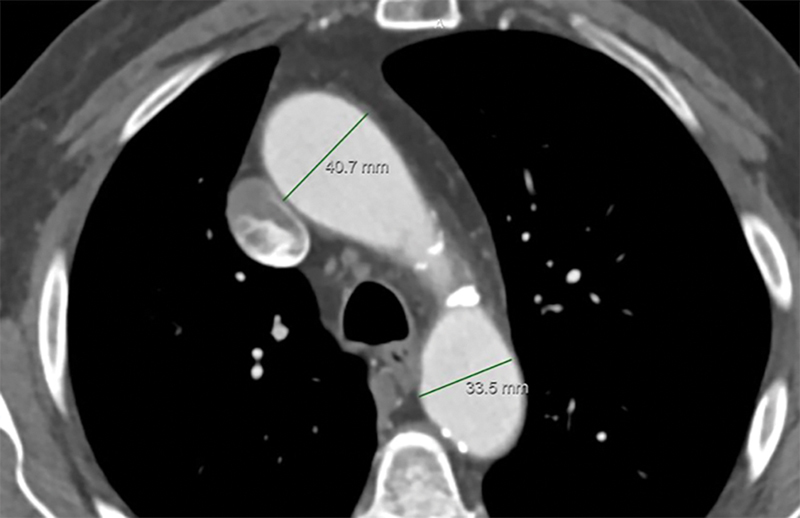
Aortic diameter measurement caliper positioning for: short-axis views of distal-ascending and proximal-descending aorta.
Fig. 2.
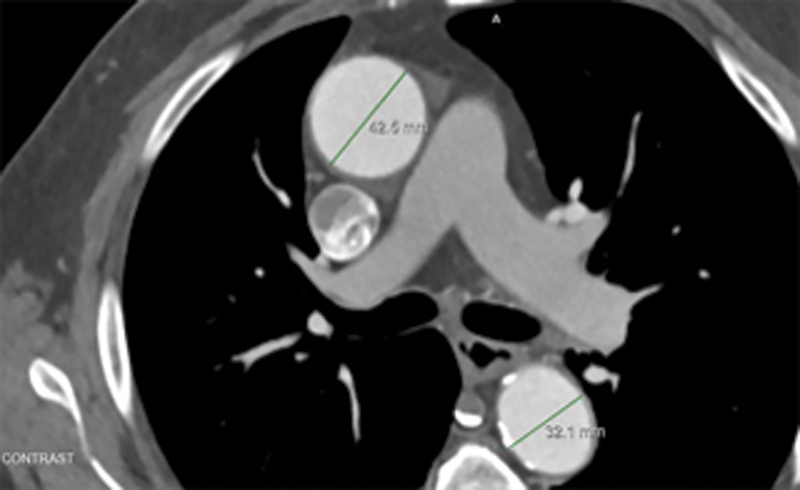
Aortic diameter measurement caliper positioning for: short axis view of midascending and middescending aorta.
Fig. 3.
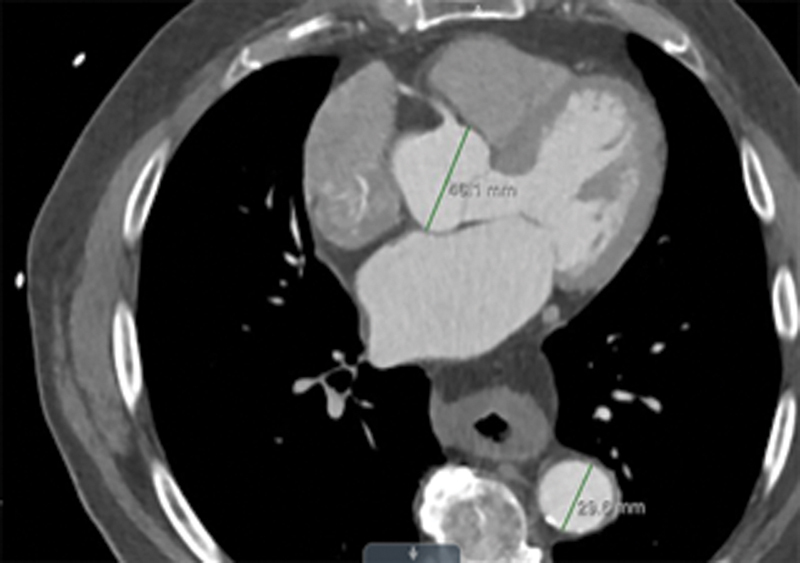
Aortic diameter measurement caliper positioning for: short axis view of aortic root and distal-descending aorta.
Fig. 4.
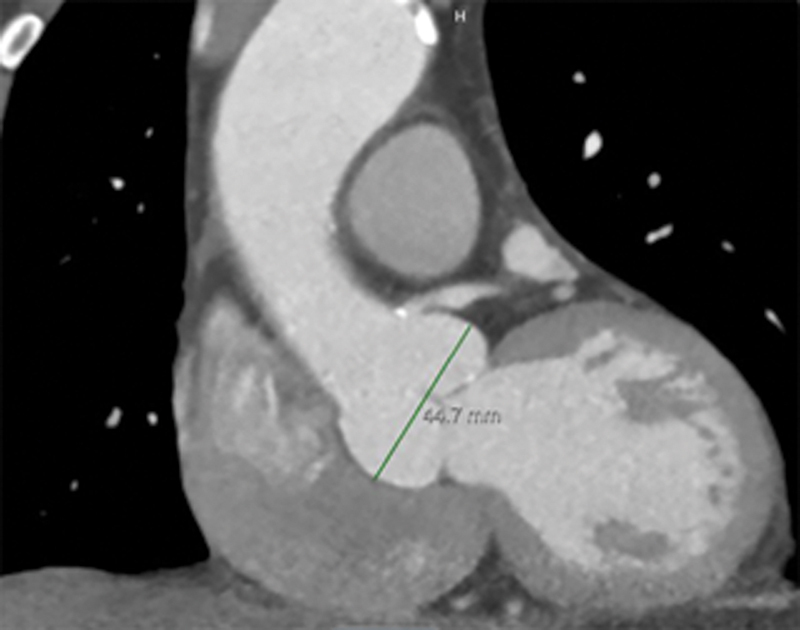
Coronal view of sinus of Valsalva.
Fig. 5.
Coronal view of sinotubular junction.
Fig. 6.
Example of side-by-side views for comparative measurements.
During visits, aortic images were reviewed with the patient noting the current maximum diameter and potential threshold for recommending surgical repair. Patient-specific information and recommendations were provided to the patient and family and were forwarded to primary care providers (PCPs) and other providers. Patients were instructed to record their BP two to three times per day for 1 to 2 weeks with a scheduled follow-up with their PCP.
Repeat aortic imaging studies were requested at 6 to 12 months after the referral study then annually followed by a clinic visit. Patients for whom results were not forwarded to our office or who did not return at the recommended interval were contacted to determine if any adverse events had occurred and to encourage follow-up.
Study Methods
Patients included in the study group were evaluated and followed in the TADC by the lead author beginning in July 2015. Referral sources included PCPs, emergency department, and specialty practices as well as patient and family self-referral.
A retrospective review of patient data was performed. Structured clinic notes facilitated data acquisition. Imaging studies were all reviewed and aortic diameters were measured by the lead author. The aortic diameter growth rate was calculated by dividing the difference of the diameters by the interval between the first and last aortic examinations.
Of an initial group of 214 patients with initial TAA diameters of 4.0 to 4.9 cm who had not undergone prior thoracic aortic surgery, 17 patients did not have two imaging studies for comparison leaving 197 patients as our study group.
Statistical Methods
Data were analyzed utilizing the SPSS software. One-way analysis of variance (ANOVA), independent samples t -tests and paired samples t -tests, and survival analyses were performed regarding blood pressure measurements, smoker/nonsmoker history and current, aortic diameter, aortic diameter height index, and aortic diameter BSA index at initial contact, and event rates. These tests were also used to compare aortic root diameter by patients to years of follow-up and for subgroup analysis. Nonparametric tests were used in place of ANOVA and t -tests when necessary. Numeric data are presented as mean values ± 95% confidence intervals.
The study was granted a waiver by the TriHealth IRB (21-053).
Results
Patient characteristics are noted in Table 1 . Indications for the initial chest imaging study were varied ( Table 2 ) with lung cancer screening or coronary artery calcium score accounting for 34%, while 3.0% were obtained because of a positive family history of TAD and/or AoDR. None of the patients were determined to have symptoms due to their TAA.
Table 1. Patient characteristics.
| Variable | Value |
|---|---|
| Total number of patients | 197 |
| Sex : | |
| Male, n (%) | 135 (68.5) |
| Female, n (%) | 62 (31.5) |
| Race : | |
| Caucasian, n (%) | 184 (93.4) |
| African American, n (%) | 6 (3.0) |
| Asian, n (%) | 4 (2.0) |
| Hispanic, n (%) | 2 (1.0) |
| Other, n (%) | 1 (1.0) |
| Age at diagnosis, years, mean (± 95% CI) | 62.4 (±1.46) |
| Age at last follow up, years, mean (± 95% CI) | 77.96 (±1.76) |
| Initial height, cm, mean (± 95% CI) | 174.70 (±1.42) |
| Initial weight, kg, mean (± 95% CI) | 93.21 (±2.93) |
| Initial BMI, mean (± 95% CI) | 30.50 (±0.87) |
| History of hypertension, n (%) | 155 (78.7) |
| Initial BP, mm Hg, systolic, mean (± 95% CI) | 126.9 (±1.64) |
| Initial BP, mm Hg, diastolic, mean (± 95% CI) | 76.03 (±1.3) |
| BP initial category : | |
| Normal, n (%) | 59 (29.9) |
| Elevated, n (%) | 65 (33.0) |
| Class I, n (%) | 58 (29.4) |
| Class II, n (%) | 15 (7.6) |
| History of diabetes : | |
| None, n (%) | 158 (80.2) |
| Oral agents, n (%) | 34 (17.3) |
| Insulin dependent, n (%) | 5 (2.5) |
| History of tobacco use: pack years : | |
| None, n (%) | 100 (50.8) |
| >0–19, n (%) | 47 (23.9) |
| ≥20, n (%) | 50 (25.4) |
| Current smoker, n (%) | 31 (15.7) |
| Smoker, quit years, mean (± 95% CI) | 24.3 (± 4.1) |
| Family history of thoracic aortic aneurysm, dissection or rupture, or unknown cause of death : | |
| Mother, n (%) | 4 (2.0) |
| Father, n (%) | 8 (4.1) |
| Siblings, n (%) | 21 (10.7) |
| Children, n (%) | 5 (2.5) |
| Genetic testing performed : | |
| Variant of unknown significance, n (%) | 19 (9.6) |
| Variant of known significance, n (%) | 0 (0.0) |
Abbreviations: BMI, body mass index; BP, blood pressure; CI, confidence interval.
Table 2. Indication for initial chest imaging study.
| Variable | Value |
|---|---|
| Lung cancer screening, n (%) | 36 (18.3) |
| Coronary artery calcium score, n (%) | 31 (15.7) |
| Shortness of breath, acute or chronic, n (%) | 25 (12.7) |
| Chest X-ray abnormality, n (%) | 21 (10.7) |
| Echocardiogram abnormality, n (%) | 20 (10.2) |
| Cancer staging, n (%) | 14 (7.1) |
| Trauma, n (%) | 8 (4.1) |
| Abdominal imaging abnormality, n (%) | 7 (3.6) |
| Family history of TAD, n (%) | 6 (3.0) |
| Neck mass, n (%) | 2 (1.0) |
| AAA evaluation, planned repair, n (%) | 2 (1.0) |
| Pre-Afib ablation evaluation, n (%) | 1 (0.5) |
| Other, n (%) | 3 (1.5) |
Abbreviations: AAA, abdominal aortic aneurysm; TAD, thoracic aortic disease.
History of HTN was noted for 78% overall. While the diagnosis or HTN class was not established in the TADC, we noted TADC-recorded BPs with 33.0% elevated (systolic 120–129 and diastolic <80 mmHg), 29.4% stage 1 (systolic 130-139 or diastolic 80-89 mmHg), and 7.6% stage 2 (systolic >140 or diastolic >90 mmHg) ranges.
Antihypertensive agents included a beta blocker for 70.3%, angiotensin-receptor blocker for 31.0%, and calcium channel blocker for 32.9%. Other antihypertensive agents were used by 62.5% with many taking more than one agent. Of all patients listed as having HTN, 3.9% were taking no antihypertensive agents, due to adverse side effects or medication intolerance.
Echocardiogram findings are noted in Table 3 . Aortic valve morphology per echocardiography reports was trileaflet for 72.6%, bileaflet for 4.6%, and indeterminate for 22.8%.
Table 3. Echocardiogram findings.
| Variable | Value |
|---|---|
| LVEF : | |
| ≥55 %, n (%) | 177 (89.8) |
| <55 %, n (%) | 7 (3.6) |
| Indeterminate, n (%) | 13 (6.6) |
| Aortic root diameter, internal, cm, mean (± 95% CI) | 3.58 (±0.07) |
| Aortic valve morphology : | |
| Trileaflet, n (%) | 143 (72.6) |
| Bileaflet, n (%) | 9 (4.6) |
| Indeterminate, n (%) | 45 (22.8) |
Abbreviations: CI, confidence interval; LVEF, left ventricular ejection fraction.
Follow-up duration from the first available imaging study ranged from <1 to 20 years, with a mean of 2.89 ± 0.3 years, and a total of 868 patient-years. Follow-up by medical record review was 100% complete for this cohort as of April 2021.
Aortic diameters for each level are noted in Table 4 . Differences between the initial and last imaging studies were significant for all levels except at the sinotubular junction.
Table 4. Aortic diameter measurements (cm).
| Location | Initial diameter | Initial 95% CI | Last diameter | Last 95% CI | p -Value |
|---|---|---|---|---|---|
| Root, short axis | 3.892 | (3.822, 3.962) | 3.906 | (3.836, 3.976) | 0.00 a |
| Sinus of valsalva | 3.954 | (3.884, 4.024) | 3.978 | (3.908, 4.048) | 0.00 a |
| Sinotubular junction | 3.387 | (3.333, 3.441) | 3.405 | (3.350, 3.459) | NS |
| Midascending | 4.293 | (4.223, 4.363) | 4.303 | (4.233, 4.373) | 0.00 a |
| Distal ascending | 3.676 | (3.619, 3.734) | 3.681 | (3.626, 3.736) | 0.00 a |
| Arch | 3.133 | (1.986, 4.281) | 3.167 | (1.941, 4.392) | 0.00 a |
| Proximal descending | 2.939 | (2.879, 3.000) | 2.956 | (2.895, 3.017) | 0.00 a |
| Middescending | 2.687 | (2.640, 2.734) | 2.705 | (2.657, 2.754) | 0.00 a |
| Distal descending | 2.591 | (2.547, 2.636) | 2.600 | (2.556, 2.644) | 0.00 a |
| Diaphragmatic hiatus | 2.509 | (2.463, 2.555) | 2.515 | (2.468, 2.563) | 0.00 a |
Abbreviations: CI, confidence interval; NS, nonsignificant.
Significance at the 0.01 level
Aortic growth rates are listed in Table 5 . The mean growth rate was 0.018 and 0.022 cm/year for aortic root and midascending aorta, respectively. The growth rate was significantly greater for aneurysms initially measured at 4.5 to 5.0 cm versus < 4.5 cm at both aortic root (0.068 vs. 0.011 cm/year) and midascending aorta (0.043 vs. 0.013 cm/y) levels. Aortic growth stratified by BP levels was not statistically different. Potential risk factors including the presence of bileaflet aortic valve, or aortic diameter indexed to height, did not significantly contribute to greater aortic growth.
Table 5. Mean aortic diameter growth rates (cm/year).
| Location | Cohort | 95% CI | <4.5 | 95% CI | ≥4.5 | 95% CI |
|---|---|---|---|---|---|---|
| Root, short axis | 0.018 | (0.003, 0.033) | 0.011 | (−0.003, 0.026) | 0.068 | (0.008, 0.129) |
| Sinus of valsalva | 0.021 | (0.001, 0.040) | 0.024 | (−0.011, 0.050) | −0.011 | (−0.091, 0.070) |
| Sinotubular junction | 0.005 | (−0.012, 0.022) | 0.005 | (−0.012, 0.022) | 0.000 | (0.000, 0.000) |
| Midascending | 0.022 | (−0.001, 0.044) | 0.013 | (−0.019, 0.044) | 0.043 | (0.022, 0.064) |
| Distal ascending | 0.019 | (0.006, 0.032) | 0.017 | (0.001, 0.032) | 0.408 | (−0.641, 1.458) |
| Proximal descending | 0.016 | (−0.003, 0.035) | 0.016 | (−0.003, 0.035) | N/A | N/A |
| Middescending | 0.017 | (0.008, 0.026) | 0.017 | (0.008, 0.026) | N/A | N/A |
| Distal descending | 0.016 | (0.005, 0.028) | 0.016 | (0.005, 0.028) | N/A | N/A |
| Diaphragmatic hiatus | 0.006 | (−0.009, 0.021) | 0.006 | (−0.009, 0.021) | N/A | N/A |
Abbreviations: CI, confidence interval; N/A, not available.
Survival free from aortic dissection or rupture, aortic surgery, or death for any reason was 99.5% at 5 years ( Fig. 7 ). There were no adverse aortic events or surgery performed for any of these patients. None of these patients reached the 5.5 cm diameter or the 0.5 cm/y growth threshold for recommending surgery during the period of follow-up. The only death was due to a radiographically confirmed intracerebral hemorrhage with a significant neurologic deficit leading to an extended hospitalization and subsequent hospice stay.
Fig. 7.
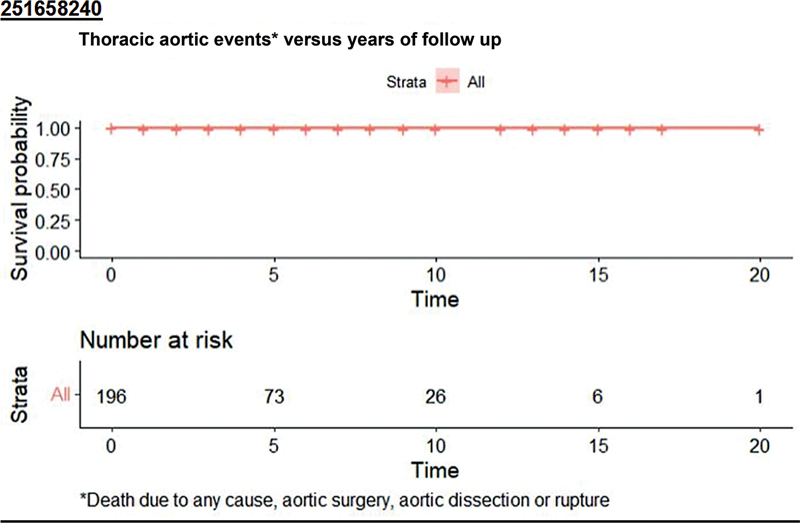
Freedom from adverse aortic events or death versus time of follow-up.
Fig. 8 shows the longitudinal patient-specific diameter measurements for this cohort at three levels.
Fig. 8.
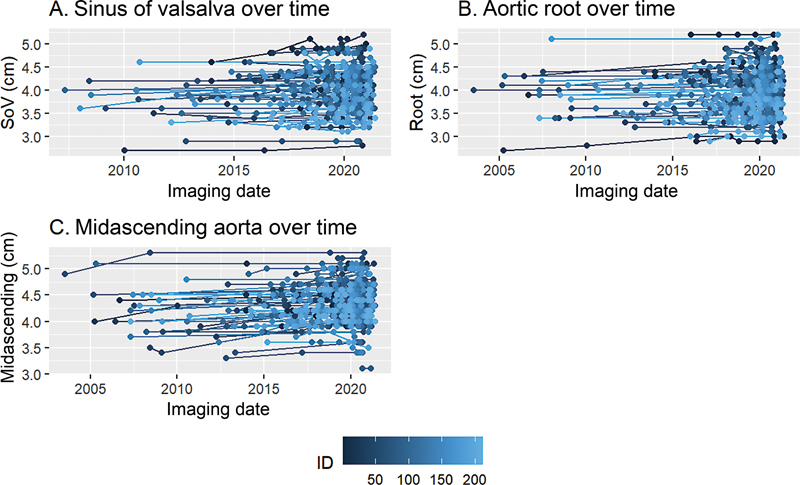
Longitudinal patient-specific aortic diameter measurements. (A) Sinus of valsalva, coronal view, cm. (B) Aortic root, short axis view, cm. (C) Midascending aorta, short axis view, cm.
Of 166 initial chest imaging studies where the aortic root and/or midascending aortic diameter was ≥4.0 cm, an aneurysm was not noted on the imaging report for 51 (31%).
Family history was positive for TAD, AoDR, or unknown cause of death for 38 patients including 21 (10.7%) siblings, 12 (6.1%) parents, and 5 (2.5%) children of patients. Genetic testing and counseling were completed for 19 patients with no variants of known significance associated with aortopathy detected. None of the 197 patients exhibited the history or phenotypic features of syndromic (Marfan or Marfan-like) aortic disease or other HRCs associated with TAD or AoDR.
TAAs were initially discovered as an incidental finding for virtually all of our patients without a known family history of TAD. Subsequent imaging demonstrated previously undiagnosed nonaortic pathology for 48 patients, mostly benign, but including one lung cancer, one renal cancer, and one with multiple pulmonary emboli.
Discussion
In this retrospective review of adult TAA-NoHRC patients, mostly referred because of an incidental finding of TAA on chest imaging performed for other reasons, with baseline aortic root and/or ascending aortic diameters <5.0 cm, followed in a suburban community hospital TADC, both the aortic diameter growth and adverse event rates were very low.
Coady et al established an annual risk estimate for aortic dissection, rupture, or death for smaller aneurysms: <4 cm, 8.8%; 4.0 to 4.9 cm, 9.5%; 5.0 to 5.9 cm, 17.8%, and ≥ 6 cm, 27.9%. 5 This cohort included patients who presented with AoDR or who died from TAD during the study interval and patients with chronic dissection or known Marfan syndrome. Zafar et al indexed aortic diameter to height to estimate risk for patients with ascending aortic aneurysms. 14 Aortic diameter to height indices as cm/m of ≤ 2.43, 2.44 to 3.17, 3.21 to 4.06, and ≥ 4.1 were associated with a 4, 7, 12, and 18% average yearly risk of complications, respectively.
Oladokun et al reviewed 11 publications which included 1,383 patients with TAA growth rates of 0.2 to 4.2 mm/y. 15 For ascending and aortic arch aneurysms, the growth rate ranged from 0.2 to 2.8 mm/y. Large aneurysm diameter, distal locations, and the presence of Marfan syndrome and/or bicuspid aortic valve were associated with increased growth. This analysis included patients with thoraco-abdominal TAAs as well as chronic dissection.
For aortic root and/or ascending aortic aneurysms in TAA-NoHRC, Gagne-Loranger et al reported lower growth (0.42 ± 0.82 mm/y) and event rates (99.4% freedom from an acute aortic-related event at 5 years) for 251 adults with root/ascending aortic aneurysm diameter of 40 to 50 mm. 7 Their TADC approach included 24-hour BP monitoring with a target BP < 140/90. In young (<60 years old) active patients, a kinesiology evaluation was performed to assess BP elevation during isometric exercises using standardized protocols in order to provide patient-specific recommendations on activity. Not noted were target BP reached, compliance with recommended activity restrictions. Antihypertensive agents were listed for 81.2% of patients with a beta blocker used by 59.9%.
Geisbüsch et al reported 166 patients without history of Marfan syndrome or surgical repair and with ascending TAAs <5.0 cm with two or more imaging studies. 8 No AODR occurred during a follow-up of 971 patient-years. Three patients had surgical repair because of uncontrolled HTN, not because of increased aortic diameter.
McLarty et al reported 110 TAA-NoHRC patients in a Veterans Administration Medical Center setting. 9 Baseline diameters were 4.45 ± 0.37 cm (range 3.8–5.4 cm), with 94 (86%) involving the ascending aorta. During the 8-year follow-up, aortic diameter increased 0.03 ± 0.24 cm per patient per year. Patients were divided into two groups depending upon whether they did or did not reach a primary adverse end point (aortic diameter increase ≥ 0.5 cm/y, diameter increase to > 5.5 cm, offered surgery for aneurysm, and aneurysm-related death). The 13 (12.7%) who reached the adverse endpoint had higher index aortic diameter (4.81 vs. 4.40 cm) and a higher growth rate per year (0.16 vs. 0.01 cm) compared to the nonendpoint patients. The only variable to achieve significance in the univariate time to event screening was aneurysm index size. Only one “not-at-risk” patient with an index aneurysm size under 4.3 cm reached the endpoint (aneurysm growth ≥ 0.5 cm/year) within 120 days after initial diagnosis.
Kim et al reported 4,654 adults with nonsyndromic TAAs followed by echocardiography. 16 Of 4,526 adults with the initial aortic diameter of 4.0 to 5.0 cm, the probability of AoDR within 5 years based upon initial aortic diameter was 0.1% at 4.0 cm, 0.4% at 4.5 cm, and 1.1% at 5.0 cm. The probability of an adverse event or undergoing surgical repair within 5 years was 1.6% at 4.0 cm, 6.4% at 4.5 cm, and 21.2% at 5.0 cm.
Our findings are more consistent with data extracted from the above studies for aortic root and ascending TAAs in TAA-NoHRC adults. 7 8 9 16
Beta-blockers were taken by 40.6% of our patients with initial BPs in the elevated, stage 1 or stage 2 ranges. Beta-blockers were recommended in the HTN guideline as the preferred antihypertensive agents in patients with HTN and TAD. 13 The 2010 ACC/AHA TAD guideline recommended antihypertensive therapy with a goal of <140/90 mm Hg (without diabetes) or < 130/80 mm Hg (diabetes or chronic renal disease) to reduce the risk of stroke, myocardial infarction, heart failure, and cardiovascular death. 1 Most of the supporting data for these recommendations came from studies of patients with chronic aortic dissection, not from studies of low-risk patients with intact TAAs.
For other cardiovascular risk reduction, the 2010 ACC/AHA TAD guideline recommended lipid profile optimization, smoking cessation, and other atherosclerosis risk-reduction measures for patients with small aneurysms not requiring surgery, as well as for patients who were not surgical or stent graft candidates. 1 These principles were emphasized to all patients as well.
Regarding surveillance imaging frequency, the 2010 ACC/AHA TAD guideline recommended an echocardiogram at the time of diagnosis of Marfan syndrome and 6 months thereafter to determine the rate of enlargement of the aorta. Since echocardiograms do not visualize the entire thoracic aorta, CT and/or MR imaging are usually performed. Additionally, annual imaging was recommended for patients with Marfan syndrome if stability of the aortic diameter was documented at the initial follow-up. If the maximal aortic diameter was ≥ 4.5 cm, or if the aortic diameter shows significant growth from baseline, more frequent imaging was recommended. The 2011 Japanese 3 and 2014 European guidelines 2 recommended annual imaging for all TAAs < 4.5 cm, and every 6-month imaging for TAAs ≥ 4.5 to 5.5 cm, with the latter modified if stability was confirmed by serial imaging.
Asymptomatic TAAs are found as an incidental finding on chest imaging performed for a variety of reasons. As the potential safety of reduced radiation exposure protocols has improved, CT screening for coronary artery disease, lung cancer, and other diseases has become increasingly popular. In emergency departments, CT scans are a mainstay of diagnostic tools for a variety of presentations. In a review of 541 patients with a CT scan as part of their trauma evaluation, Treskes et al found one patient with a penetrating thoracic aortic ulcer and one patient with a <5.5cm TAA. 17 Itani et al reported on 6,971 patients with chest CT imaging for lung nodule screening. 18 TAAs were found in 11 (0.16%). Additionally, as we found, there may be significant findings that were underreported. Krueger et al published an “alert” for radiologists which included sinus of Valsalva aneurysms, TAAs, and bicuspid aortic valve on a list of possible incidental cardiovascular findings on thoracic CT imaging. 19
While contrast CT is the preferred imaging technique, there are patients for whom other types of periodic imaging are needed such as noncontrast low-dose lung cancer screening studies. For such studies, precise definition of aortic wall or intraluminal pathology may not be discernable. If aortic diameter growth is in question, more definitive aortic imaging studies may be needed. The potential risk of repeated radiation exposure has been of some concern. 20
Study Limitations
The duration of follow-up was up to 20 years. Mean follow-up was 2.89 ± 0.3 years, and 37% of our patients were followed up to 5 years. Longer duration follow-up may demonstrate higher growth or adverse event rates.
We did not use BPs recorded in the clinic setting to define either the diagnosis or category of HTN. We did not monitor ambulatory or home BPs, which are known to be prognostically better predictors of cardiovascular risk. 21 Definitive diagnosis and management of HTN were deferred to PCPs or HTN specialists. Whether a dedicated TADC approach and/or documented BP control to optimal levels are associated with lower aortic growth or event-free outcomes would require a more extensive study.
Aortic diameter measurements were performed by the lead author. The measurements were not taken in a blinded fashion. Side-by-side comparisons provided consistency of data acquisition from each consecutive study. Where pulsation artifact or indistinct aortic borders did not allow appropriate caliper positioning, values were not recorded. Of the 616 imaging studies reviewed, indistinct aortic borders, pulsation artifacts, or a nonvisualized segment preventing precise diameter measurements were noted at short axis aortic root 72 (11.7%), coronal view sinus of Valsalva 139 (22.6%), coronal view sinotubular junction 145 (23.5%), short axis mid-ascending 9 (1.5%), short axis distal-ascending 21 (3.4%), short axis proximal-descending 22 (2.1%), short axis mid-descending 32 (5.2%), distal-descending 17 (2.8%), diaphragmatic hiatus 31 (5.0%).
Publications cited with low-risk patients showing low aortic growth and adverse aortic event rates may be subject to publication bias. Larger patient cohort studies with greater longitudinal follow-up data are needed to better inform guideline recommendations.
Conclusion
TAAs involving the aortic root and/or ascending aorta measuring <4.5 cm in maximum diameter, in adult patients without syndromic or nonsyndromic HRCs, have very low growth and adverse event rates, lower than suggested by current guidelines. If a first follow-up aortic imaging study at 6 to 12 months does not demonstrate an increase in aortic diameter and if patients follow guideline-based recommendations particularly achieving BP goals, such patients are very unlikely to have an adverse aortic event or rapid growth. For such patients, intervals longer than 1 year, possibly extending to 3 to 5 years, for subsequent surveillance imaging could be considered to reduce cumulative radiation exposure and cost of repeated imaging studies.
Acknowledgments
None.
Funding Statement
Funding This work was funded by Educational Grant from the Bethesda Foundation.
Footnotes
Conflict of Interest The authors declare no conflict of interest related to this article.
References
- 1.American College of Cardiology Foundation/American Heart Association Task Force on Practice Guidelines ; American Association for Thoracic Surgery ; American College of Radiology ; American Stroke Association ; Society of Cardiovascular Anesthesiologists ; Society for Cardiovascular Angiography and Interventions ; Society of Interventional Radiology ; Society of Thoracic Surgeons ; Society for Vascular Medicine . Hiratzka L F, Bakris G L, Beckman J A. 2010 ACCF/AHA/AATS/ACR/ASA/SCA/SCAI/SIR/STS/SVM guidelines for the diagnosis and management of patients with Thoracic Aortic Disease: a report of the American College of Cardiology Foundation/American Heart Association Task Force on Practice Guidelines, American Association for Thoracic Surgery, American College of Radiology, American Stroke Association, Society of Cardiovascular Anesthesiologists, Society for Cardiovascular Angiography and Interventions, Society of Interventional Radiology, Society of Thoracic Surgeons, and Society for Vascular Medicine. Circulation. 2010;121(13):e266–e369. doi: 10.1161/CIR.0b013e3181d4739e. [DOI] [PubMed] [Google Scholar]
- 2.ESC Committee for Practice Guidelines ; The Task Force for the Diagnosis and Treatment of Aortic Diseases of the European Society of Cardiology (ESC) . Erbel R, Aboyans V, Boileau C. 2014 ESC Guidelines on the diagnosis and treatment of aortic diseases: Document covering acute and chronic aortic diseases of the thoracic and abdominal aorta of the adult. Eur Heart J. 2014;35(41):2873–2926. doi: 10.1093/eurheartj/ehu281. [DOI] [PubMed] [Google Scholar]
- 3.JCS Joint Working Group . Guidelines for diagnosis and treatment of aortic aneurysm and aortic dissection (JCS 2011): digest version. Circ J. 2013;77(03):789–828. doi: 10.1253/circj.cj-66-0057. [DOI] [PubMed] [Google Scholar]
- 4.Cambria R A, Gloviczki P, Stanson A W. Outcome and expansion rate of 57 thoracoabdominal aortic aneurysms managed nonoperatively. Am J Surg. 1995;170(02):213–217. doi: 10.1016/s0002-9610(99)80289-x. [DOI] [PubMed] [Google Scholar]
- 5.Coady M A, Rizzo J A, Hammond G L.What is the appropriate size criterion for resection of thoracic aortic aneurysms? J Thorac Cardiovasc Surg 199711303476–491., discussion 489–491 [DOI] [PubMed] [Google Scholar]
- 6.Davies R R, Goldstein L J, Coady M A. Yearly rupture or dissection rates for thoracic aortic aneurysms: simple prediction based on size. Ann Thorac Surg. 2002;73(01):17–27. doi: 10.1016/s0003-4975(01)03236-2. [DOI] [PubMed] [Google Scholar]
- 7.Gagné-Loranger M, Dumont É, Voisine P, Mohammadi S, Dagenais F. Natural history of 40-50 mm root/ascending aortic aneurysms in the current era of dedicated thoracic aortic clinics. Eur J Cardiothorac Surg. 2016;50(03):562–566. doi: 10.1093/ejcts/ezw123. [DOI] [PubMed] [Google Scholar]
- 8.Geisbüsch S, Stefanovic A, Schray D. A prospective study of growth and rupture risk of small-to-moderate size ascending aortic aneurysms. J Thorac Cardiovasc Surg. 2014;147(01):68–74. doi: 10.1016/j.jtcvs.2013.06.030. [DOI] [PubMed] [Google Scholar]
- 9.McLarty A J, Bishawi M, Yelika S B, Shroyer A L, Romeiser J. Surveillance of moderate-size aneurysms of the thoracic aorta. J Cardiothorac Surg. 2015;10:17. doi: 10.1186/s13019-015-0220-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Bathen T, Krohg-Sørensen K, Lidal I B. Multidisciplinary aortopathy clinics: a systematic scoping review of the literature and evaluation of patient experiences from a newly started clinic in Norway. Am J Med Genet A. 2020;182(11):2552–2569. doi: 10.1002/ajmg.a.61827. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Singh S, Nautiyal A. Aortic dissection and aortic aneurysms associated with fluoroquinolones: a systematic review and meta-analysis. Am J Med. 2017;130(12):1449–1457. doi: 10.1016/j.amjmed.2017.06.029. [DOI] [PubMed] [Google Scholar]
- 12.Frankel W C, Trautner B W, Spiegelman A, Grigoryan L, LeMaire S A. Patients at risk for aortic rupture often exposed to fluoroquinolones during hospitalization. Antimicrob Agents Chemother. 2019;63(02):e01712–e01718. doi: 10.1128/AAC.01712-18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Whelton P K, Carey R M, Aronow W S. 2017 ACC/AHA/AAPA/ABC/ACPM/AGS/APhA/ASH/ASPC/NMA/PCNA guideline for the prevention, detection, evaluation, and management of high blood pressure in adults: a report of the American College of Cardiology/American Heart Association Task Force on Clinical Practice Guidelines. J Am Coll Cardiol. 2018;71(19):e127–e248. doi: 10.1016/j.jacc.2017.11.006. [DOI] [PubMed] [Google Scholar]
- 14.Zafar M A, Li Y, Rizzo J A. Height alone, rather than body surface area, suffices for risk estimation in ascending aortic aneurysm. J Thorac Cardiovasc Surg. 2018;155(05):1938–1950. doi: 10.1016/j.jtcvs.2017.10.140. [DOI] [PubMed] [Google Scholar]
- 15.Oladokun D, Patterson B O, Sobocinski J. Systematic review of the growth rates and influencing factors in thoracic aortic aneurysms. Eur J Vasc Endovasc Surg. 2016;51(05):674–681. doi: 10.1016/j.ejvs.2016.01.017. [DOI] [PubMed] [Google Scholar]
- 16.Kim J B, Spotnitz M, Lindsay M E, MacGillivray T E, Isselbacher E M, Sundt T M., III Risk of aortic dissection in the moderately dilated ascending aorta. J Am Coll Cardiol. 2016;68(11):1209–1219. doi: 10.1016/j.jacc.2016.06.025. [DOI] [PubMed] [Google Scholar]
- 17.REACT-2 study group . Treskes K, Bos S A, Beenen L FM. High rates of clinically relevant incidental findings by total-body CT scanning in trauma patients; results of the REACT-2 trial. Eur Radiol. 2017;27(06):2451–2462. doi: 10.1007/s00330-016-4598-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Itani Y, Watanabe S, Masuda Y. Measurement of aortic diameters and detection of asymptomatic aortic aneurysms in a mass screening program using a mobile helical computed tomography unit. Heart Vessels. 2002;16(02):42–45. doi: 10.1007/s380-002-8315-1. [DOI] [PubMed] [Google Scholar]
- 19.Krueger M, Cronin P, Sayyouh M, Kelly A M. Significant incidental cardiac disease on thoracic CT: what the general radiologist needs to know. Insights Imaging. 2019;10(01):10–26. doi: 10.1186/s13244-019-0693-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Fazel R, Krumholz H M, Wang Y. Exposure to low-dose ionizing radiation from medical imaging procedures. N Engl J Med. 2009;361(09):849–857. doi: 10.1056/NEJMoa0901249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Niiranen T J, Mäki J, Puukka P, Karanko H, Jula A M. Office, home, and ambulatory blood pressures as predictors of cardiovascular risk. Hypertension. 2014;64(02):281–286. doi: 10.1161/HYPERTENSIONAHA.114.03292. [DOI] [PubMed] [Google Scholar]


