Abstract
Recent studies have reported that autoantibodies against glial fibrillary acidic protein (GFAP), a major cytoskeletal protein expressed in astrocytes, can lead to GFAP astrocytopathy, an autoimmune central nervous system inflammatory disease. We herein report the unique case of a 59-year-old Japanese woman with GFAP astrocytopathy who presented with characteristic symptoms, including signs of meningeal irritation, cerebellar ataxia, and bladder/rectal dysfunction, in the absence of specific findings on initial brain magnetic resonance imaging (MRI). The patient exhibited new abnormal changes mainly in the brainstem on follow-up MRI, illustrating the need to recognize that MRI abnormalities may appear later in GFAP astrocytopathy.
Keywords: glial fibrillary acidic protein, astrocytopathy, magnetic resonance imaging, brainstem
Introduction
Glial fibrillary acidic protein (GFAP) astrocytopathy, first reported in 2016, is an autoimmune inflammatory disease of the central nervous system (CNS) that shows a good response to corticosteroid treatment (1). The clinical manifestations of GFAP astrocytopathy include meningoencephalitis-like symptoms, such as fever and headache, as well as involuntary movement, cerebellar ataxia, exaggerated tendon reflexes and bladder-rectal dysfunction. Antibodies against GFAP, an intermediate filament protein present in the cytoskeleton of astrocytes, are detected in the cerebrospinal fluid (CSF) of affected patients (1).
The pathogenesis underlying GFAP astrocytopathy remains unclear because of the lack of a sufficient number of reported cases. The characteristic magnetic resonance imaging (MRI) abnormality is the vertical contrast effect around the lateral ventricles, which is found in approximately half of patients (1-3).
We herein report the unique case of a patient with GFAP astrocytopathy who exhibited delayed MRI changes despite normal findings on initial brain MRI.
Case Report
A 59-year-old Japanese woman with a history of nonrecurrent breast cancer developed headache and general malaise. She developed a high fever (39.6°C) on day 6, tremor in the bilateral upper limbs on day 7, and sursumvergence lasting several minutes following impaired consciousness for more than 10 hours on day 8. On day 10, she was unable to stand and complained of hallucinations, including the presence of red and blue threads in her visual field and chime-like sounds; therefore, she visited the emergency room of our hospital. Meningoencephalitis was suspected, and she was transferred to our department on day 11.
On admission, her body temperature was 38.9°C, and the Glasgow Come Scale was E4V4M6 (mild disorientation). Overt signs of meningeal irritation were not present. However, she had a partial defect in her right visual field, saccadic eye movements with mild limitation of abduction in the left eye, resting and postural tremor in the bilateral upper limbs, myoclonus in the left upper limb, left-dominant cerebellar ataxia, hyperreflexia in the bilateral upper limbs, decreased tendon reflexes in the bilateral lower limbs, reduced vibratory sensation of the left lower limb, and urinary retention requiring bladder catheter placement, and constipation.
Laboratory tests showed signs of inflammation [white blood cell count, 1.92×104/μL; C-reactive protein, 1.48 mg/dL; interleukin-6 (IL-6), 26.5 pg/mL]. Blood β-D-glucan and antigens for Cryptococcus, Aspergillus, and Candida were negative. A CSF examination revealed pleocytosis (126 cells/μL; 123 mononuclear cells/μL and 3 polynuclear cells/μL) with increased protein (126 mg/dL) and IL-6 (263 pg/mL), normal CSF glucose (70 mg/dL), and high adenosine deaminase (ADA, 11 U/L). Oligoclonal bands were negative, but myelin basic protein was positive. Polymerase chain reaction was negative for herpes simplex virus, varicella zoster virus, and mycobacterium tuberculosis in the CSF. The patient was negative for autoantibodies against myelin oligodendrocyte glycoprotein, N-methyl-D-aspartate receptor (NMDAR), leucine-rich glioma-inactivated 1 protein, contactin-associated protein-like 2, and α-3-hydroxy-5-methyl-4-isoxazole propionic acid. Initial brain MRI on day 12 showed only a slightly hyperintense region in the left basal ganglia on T2-weighted and fluid-attenuated inversion recovery (FLAIR) images (Ingenia 1.5 T, TE: 100 ms, TR: 4,121 ms, Thick: 5.0 mm). Chest and abdominal computed tomography showed no mass lesions.
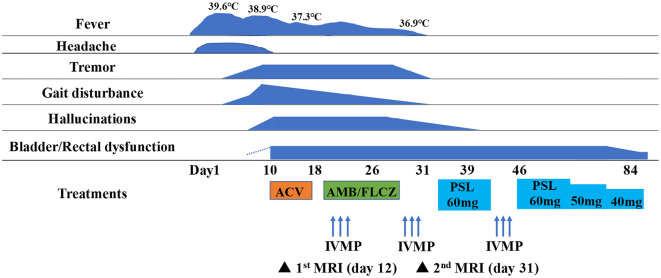
Fig. 1 summarizes the clinical course of the case. The patient was initially treated with acyclovir (500 mg i.v. bid) for viral meningoencephalitis. However, CSF examination on day 25 revealed sustained pleocytosis (81 cells/μL, all mononuclear) and a decrease in the glucose level (45 mg/dL) with a blood glucose level of 158 mg/dL. Therefore, the patient was administered amphotericin B and fluconazole for possible fungal infection. Autoimmune-mediated encephalitis could not be denied; therefore, other treatments were stopped, and 3 courses of high-dose intravenous methylprednisolone followed by oral prednisolone (60 mg/day) were started. The dose of prednisolone was reduced by 10 mg every 2 to 3 weeks.
Figure 1.
Clinical course of the patient. At the beginning of the disease, she developed signs of meningeal irritation, after which various symptoms such as tremor, gait disturbance and hallucinations appeared. Based on the CSF findings, she was initially treated for viral meningoencephalitis, but the symptoms were not improved. Autoimmune encephalitis was taken into consideration and steroid treatment was administered, resulting in the improvement of her symptoms. ACV: acyclovir, AMB/FLCZ: amphotericin B/fluconazole, IVMP: intravenous methylprednisolone, PSL: prednisolone
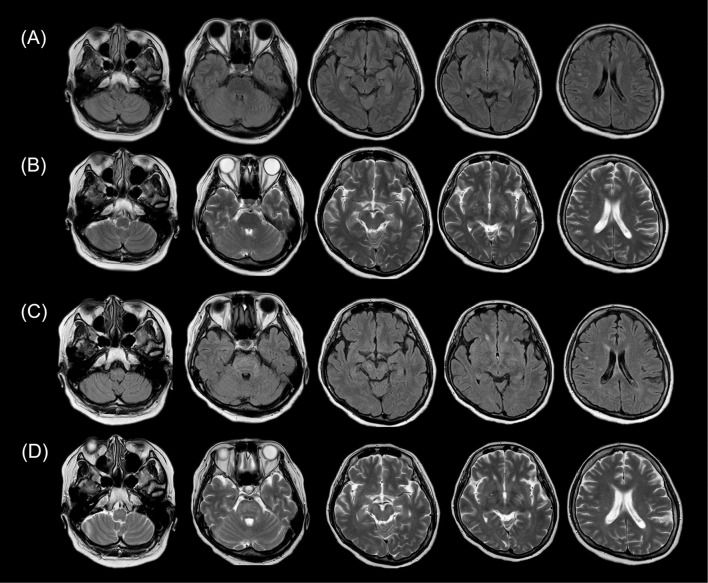
The patient's symptoms, such as her headache, fever, tremor, gait disturbance and hallucinations, were significantly improved following the steroid treatment, except for bladder and rectal dysfunction. However, follow-up brain MRI on day 31 showed new hyperintense lesions in the midbrain, middle cerebellar peduncle, pons, and medulla on T2-weighted and FLAIR images (Ingenia 1.5 T, TE: 100 ms, TR: 3,803 ms, Thick: 3.0 mm) (Fig. 2). Contrast enhancement was not observed. Spinal cord MRI on day 38 showed no obvious abnormalities.
Figure 2.
T2-weighted and fluid-attenuated inversion recovery (FLAIR) brain magnetic resonance imaging (MRI) on days 12 and 31. FLAIR (A) and T2-weighted (B) brain MRI on day 12 showing slightly hyperintense lesion in the left basal ganglia. There were no obvious changes elsewhere. Follow-up brain MRI on day 31 showing new hyperintense lesions in the midbrain, middle cerebellar peduncle, pons, and medulla on FLAIR (C) and T2-weighted images (D).
The patient was transferred to a rehabilitation hospital on day 84 with a possible diagnosis of GFAP astrocytopathy based on the favorable steroid response and characteristic findings, including signs of meningeal irritation, cerebellar ataxia, involuntary movement, bladder and rectal dysfunction as well as the increased ADA level and persistent pleocytosis in the CSF. The patient showed anti-GFAP antibodies on cell- and tissue-based assays, confirming the diagnosis of GFAP astrocytopathy.
At the last follow-up at seven months after initial admission, the patient did not have any neurological sequelae.
Discussion
Patients with GFAP astrocytopathy typically exhibit meningoencephalitis-like symptoms, such as involuntary movement, cerebellar ataxia, hyperreflexia and bladder and rectal dysfunction; some patients also develop spinal cord lesions, papilledema or hyponatremia (3). Other characteristics of GFAP astrocytopathy include long-term mononuclear cell-predominant pleocytosis, high ADA levels and anti-GFAP antibody positivity in the CSF (3).
One study including 225 patients with inflammatory CNS disease reported that the frequency of anti-GFAP antibody positivity was 6.2%, which was similar to that of anti-NMDAR antibody positivity (5.8%) (3), suggesting that GFAP astrocytopathy is not an extremely rare autoimmune inflammatory disease of the CNS. A well-known typical MRI feature of GFAP astrocytopathy is radial contrast enhancement around the lateral ventricles (1-3). Brain biopsies from patients with GFAP astrocytopathy exhibit marked inflammatory response in the perivascular legions, indicating gadolinium leakage from blood vessels (4). Importantly, these typical MRI features were not present in the current patient, who did not exhibit obvious abnormalities in the initial brain MRI obtained early during the clinical presentation but developed abnormal brainstem lesions on follow-up MRI. Regardless, her symptoms improved with steroid treatment.
This is the unique case of a patient with GFAP astrocytopathy in whom the emergence of MRI abnormalities was delayed. One study reported the delayed detection of abnormalities in deep gray matter and brainstem on brain MRI in pediatric patients with acute disseminated encephalomyelitis (5), and the authors concluded that these MRI findings might herald a prolonged clinical course and lack of response to glucocorticoid therapy. However, in the current case the MRI abnormalities appeared later while the patient was exhibiting improvement in clinical symptoms. Similarly, one case study reported adult patients with acute disseminated encephalomyelitis in whom the emergence of MRI abnormalities accompanied an improvement in clinical symptoms (6).
The current case is notable for several unique clinical symptoms, including sursumvergence and visual and auditory hallucinations. Oculogyric crisis has been reported in a patient with GFAP astrocytopathy (7); however, we considered that the sursumvergence was due to epileptic seizure rather than oculogyric crisis because of the prolonged alteration in consciousness. No study to date reported hallucinations in patients with GFAP astrocytopathy. We speculate the midbrain as the source of the hallucinations, due to the presence of color accompanied by auditory hallucinations (8) and the patient's awareness that the hallucinations were unnatural.
In conclusion, this unique case of GFAP astrocytopathy with delayed appearance of brain MRI abnormalities suggests that repeated brain MRI might have clinical value in terms of the elucidation of the lesion site in patients presenting with characteristic clinical symptoms of GFAP astrocytopathy in the absence of abnormal findings on initial MRI.
The authors state that they have no Conflict of Interest (COI).
Acknowledgments
We would like to acknowledge Dr. Keiko Tanaka, a senior lecturer in the Department of Animal Model Development, Brain Research Institute of Niigata University for her cooperation in measuring anti-NMDAR, anti-leucine-rich glioma-inactivated 1 protein, anticontactin-associated protein-like 2, anti-α-3-hydroxy-5-methyl-4-isoxazole propionic acid antibodies.
References
- 1. Fang B, McKeon A, Hinson SR, et al. Autoimmune glial fibrillary acidic protein astrocytopathy: a novel meningoencephalomyelitis. JAMA Neurol 73: 1297-1307, 2016. [DOI] [PubMed] [Google Scholar]
- 2. Flanagan EP, Hinson SR, Lennon VA, et al. Glial fibrillary acidic protein immunoglobulin G as biomarker of autoimmune astrocytopathy: analysis of 102 patients. Ann Neurol 81: 298-309, 2017. [DOI] [PubMed] [Google Scholar]
- 3. Kimura A, Takekoshi A, Yoshikura N, Hayashi Y, Shimohata T. Clinical characteristics of autoimmune GFAP astrocytopathy. J Neuroimmunol 332: 91-98, 2019. [DOI] [PubMed] [Google Scholar]
- 4. Long Y, Liang J, Xu H, et al. Autoimmune glial fibrillary acidic protein astrocytopathy in Chinese patients: a retrospective study. Eur J Neurol 25: 477-483, 2018. [DOI] [PubMed] [Google Scholar]
- 5. Khurana DS, Melvin JJ, Kothare SV, et al. Acute disseminated encephalomyelitis in children: discordant neurologic and neuroimaging abnormalities and response to plasmapheresis. Pediatrics 116: 431-436, 2005. [DOI] [PubMed] [Google Scholar]
- 6. Honkaniemi J, Dastidar P, Kähärä V, Haapasalo H. Delayed MR imaging changes in acute disseminated encephalomyelitis. AJNR Am J Neuroradiol 22: 1117-1124, 2001. [PMC free article] [PubMed] [Google Scholar]
- 7. Equiza J, Rodríguez-Antigüedad J, Campo-Caballero D, et al. Autoimmune GFAP astrocytopathy presenting with remarkable CNS hyperexcitability and oculogyric crises. J Neuroimmunol 359: 577695, 2021. [DOI] [PubMed] [Google Scholar]
- 8. Manford M, Andermann F. Complex visual hallucinations. Clinical and neurobiological insights. Brain 121: 1819-1840, 1998. [DOI] [PubMed] [Google Scholar]