Abstract
Objective
Sarcopenia is characterized by a loss of muscle mass and strength, which leads to frailty and mortality. Rheumatoid arthritis (RA) is considered to be a cause of sarcopenia. The present study assessed the effectiveness of biological disease-modifying antirheumatic drugs (bDMARDs) on sarcopenia.
Methods
This was a prospective cohort study including 48 patients [11 men, 37 women; 67.5 (57.0-74.8) years old] with RA who started bDMARDs in Niigata Rheumatic Center. We monitored the physical ability, nutritional status and body composition at the baseline, 6 months and 12 months. The physical activity was measured by the Health Assessment Questionnaire (HAQ) and 10-m walking test (10MWT). The nutritional status was assessed by the controlling nutrition status (CONUT) score.
Results
Among the 48 patients who started bDMARDs, 21 were classified as having sarcopenia. The physical activity and nutritional status were significantly ameliorated after 12 months of bDMARDs. The body composition analysis showed a significant increase in the body weight but no significant increase in the skeletal muscle mass index. The proportion of patients diagnosed with sarcopenia decreased significantly after 12 months of bDMARDs (43.8% vs. 27.1%, p=0.039). Among the 21 patients who were diagnosed with sarcopenia when starting bDMARDs, the skeletal muscle index was significantly increased after 12 months of bDMARDs. [5.22 (4.76-5.43) kg/m2 vs. 5.44 (4.84-5.77), p=0.039].
Conclusion
Biologics may be useful in the treatment of sarcopenia through mechanisms such as improving the disease activity, physical activity and nutritional status.
Keywords: biological disease-modifying antirheumatic drugs (bDMARDs), sarcopenia, muscle loss, nutritional status, rheumatoid arthritis
Introduction
Sarcopenia is characterized by a loss of muscle mass and strength, which leads to a reduced physical ability, worsened quality of life (QoL), frailty and mortality (1). The term sarcopenia was first established to assess age-related situations, but the development of sarcopenia is not exclusive to older people. Inflammatory activity, nutritional deficits and an impaired physical activity are reported to be the three main pathogenic mechanisms underlying the development of sarcopenia (2,3).
Rheumatoid arthritis (RA) is a systemic inflammatory disease characterized by chronic, symmetric, and erosive synovitis and is one of the diseases causing secondary sarcopenia (3). Systemic inflammatory process and an impaired physical activity are the characteristic features of RA. In addition, malnutrition is more common in patients with RA than in healthy individuals, being reported to occur in 24.7-26.0% of RA patients (4,5). RA patients therefore are at particular risk of developing sarcopenia.
Muscle loss is regarded as a consequence of the catabolic process induced by chronic inflammatory diseases and has been particularly attributed to proinflammatory cytokines, such as tumor necrosis factor-α (TNF-α), as well as physical inactivity (6). In this context, treatments that reduce inflammation may be able to counteract the development of sarcopenia. Biological disease-modifying antirheumatic drugs (bDMARDs), which target inflammatory cytokines and strongly reduce the disease activity in RA, may thus be good candidates for treating sarcopenia.
The present study assessed the efficacy of bDMARDs on sarcopenia in RA patients.
Materials and Methods
Study population
This was a prospective cohort study. Fifty consecutive patients with RA who started bDMARDs in Niigata Rheumatic Center from April 2018 to October 2018 were enrolled. The decision to start and selection of bDMARDs was made by the attending physicians. Two patients who changed hospitals within the first six months were excluded. All patients included in this study met the 1987 American College of Rheumatology criteria (ACR) or the 2010 ACR/European League Against Rheumatism criteria for a diagnosis of RA.
Assessments
The following categories of measurements were obtained at baseline from the patients' medical records: demographic information (age, sex) and clinical information concerning RA [disease duration, comorbidities, Steinbrocker's classification (Stage) (7), treatment of RA (dose of methotrexate (MTX) and prednisolone (PSL)]. We also monitored the RA disease activity, physical ability, body composition and nutritional status at baseline, 6 months and 12 months. The disease activity was measured by the disease activity score-28 joint count based on erythrocyte sedimentation rate (DAS28-ESR) and clinical disease activity index (CDAI). The physical activity was measured by the Health Assessment Questionnaire (HAQ), 10-m walking test (10MWT) (8), timed up and go (TUG) test (9) and disabilities of the arm, shoulder and hand (DASH) (10). The body composition, including the fat mass and muscle mass, was measured by a bioelectrical impedance analysis (inBody 720Ⓡ; Biospace, Seoul, Korea). The appendicular skeletal muscle mass (ASM) was calculated as the sum of the skeletal muscle mass in the arms and legs. The skeletal muscle index (SMI) was calculated by the sum of the arm and leg skeletal muscle mass in kilograms divided by height in meters squared. The nutritional status was measured by the serum albumin (Alb) level, controlling nutrition status (CONUT) score (11) and prognostic nutritional index (PNI) (12). The CONUT score is a screening tool for determining the nutritional status using daily laboratory information; it is calculated from the serum Alb concentration, total cholesterol level and total lymphocyte count (TLC) (11). The PNI is another screening tool for determining the nutritional status and is calculated by the Alb level and TLC [PNI=(10×Alb)+(0.005×TLC)] (12). The mental status was assessed by Beck's depression inventory second edition (BDI-II) (13). The diagnosis of sarcopenia was made according to the diagnostic algorithm of the Asian working group on sarcopenia in older people (AWGSOP), excluding the criteria concerning older age (14).
Statistical analyses
Data are expressed as the median (interquartile range). Differences between each group were compared using a nonparametric Wilcoxon's rank sum test for continuous variables and Fisher's exact test for categorical variables. The comparison of the findings before and after using bDMARDs was performed by Wilcoxon's signed rank test for continuous variables and McNemar's test for categorial variables. All of the statistical analyses were performed using the SPSS software program (ver. 28; SPSS, Chicago, USA). p values of <0.05 were considered to indicate statistical significance.
Ethical considerations
The examinations and treatments were performed within the context of routine care. We obtained written consent from all patients before enrollment in this study. The publication of this study was approved by the ethics committee of Niigata Rheumatic Center (approval number: 2017-017). The study was conducted in accordance with the Declaration of Helsinki.
Results
Characteristics of patients enrolled in this study
Forty-eight patients were enrolled in the study. The characteristics of the patients at the time of starting bDMARDs are summarized in Table 1 (left side). The median age was 67.5 (57.0-74.8) years old, 77.1% of the patients were women, and the median disease duration of RA was 3.0 (1.0-11.8) years. The disease activity measured by the DAS28-ESR was 4.6 (4.0-5.7). MTX was used in 33 (68.8%) patients, and PSL was used in 26 (54.2%) patients. The bDMARDs initiated were adalimumab in 10 (20.8%), certolizumab pegol in 9 (18.8%), abatacept in 9 (18.8%), golimumab in 7 (14.6%), tocilizumab in 5 (10.4%), infliximab in 5 (10.4%) and etanercept in 3 (6.3%) patients.
Table 1.
The Characteristics of the Patients at the Time of Starting bDMARDs.
| All patients n=48 |
Patients with sarcopenia n=21 |
Patients without sarcopenia n=27 |
p | |
|---|---|---|---|---|
| Age (years) | 67.5 (57.0-74.8) | 70.0 (59.0-81.0) | 66.0 (56.0-70.0) | 0.163 |
| Sex, male/female | 11/37 | 2/19 | 9/18 | 0.083 |
| Cormobidities | ||||
| Lung disease, n (%) | 4 (8.3%) | 3 (14.3%) | 1 (3.7%) | 0.306 |
| Chronic kidney disease, n (%) | 9 (18.8%) | 4 (19.0%) | 5 (18.5%) | 1 |
| Diabetes mellitus, n (%) | 7 (14.6%) | 5 (23.8%) | 2 (7.4%) | 0.215 |
| Clinical information of RA | ||||
| Disease duration of RA (years) | 3.0 (1.0-11.8) | 3.1 (1.0-17.5) | 3.0 (1.0-11.2) | 0.584 |
| Steinbricker stage (I/II/III/IV) | 15/14/13/6 | 6/4/7/4 | 9/10/6/2 | |
| DAS28-ESR | 4.6 (4.0-5.7) | 5.2 (4.8-5.4) | 4.4 (3.7-5.1) | 0.057 |
| CDAI | 17.4 (11.7-24.1) | 22.0 (12.6-28.1) | 14.0 (9.8-23.3) | 0.114 |
| Serum CRP (mg/dL) | 0.8 (0.2-3.0) | 1.0 (0.2-5.9) | 0.8 (0.5-1.0) | 0.86 |
| ESR (mm/h) | 30.5 (14.0-74.3) | 28.0 (13.5-71.5) | 33.0 (14.0-79.0) | 0.819 |
| Physical and mental activity | ||||
| HAQ-DI | 0.8 (0.3-1.5) | 1.8 (0.7-2.6) | 0.5 (0.3-1.0) | 0.002* |
| Grip strength | 15.0 (8.3-21.5) | 7.7 (5.0-15.0) | 20.0 (11.6-24.7) | <0.0001* |
| 10MWT (m/s) | 1.6 (0.9-2.0) | 1.1 (0.9-1.8) | 1.7 (1.4-2.0) | 0.063 |
| TUG | 7.8 (6.8-12.6) | 10.3 (7.3-14.8) | 7.3 (1.5-2.0) | 0.017* |
| DASH | 31.9 (19.8-52.5) | 53.4 (28.0-73.8) | 23.3 (17.5-35.0) | 0.001* |
| BDI-II | 14.0 (9.0-19.0) | 13.5 (9.3-17.8) | 15.0 (7.0-19.0) | 0.804 |
| Nutritional status | ||||
| Serum Alb level (g/dL) | 3.9 (3.5-4.2) | 3.9 (3.3-4.2) | 3.9 (3.7-4.2) | 0.364 |
| CONUT score | 2.0 (1.0-3.0) | 2.0 (1.0-5.0) | 1.0 (0.0-3.0) | 0.053 |
| PNI | 45.4 (40.6-50.2) | 44.4 (37.3-49.4) | 45.9 (43.0-50.6) | 0.201 |
| Body composition | ||||
| BMI | 21.5 (19.8-24.3) | 20.7 (18.9-22.6) | 22.9 (20.5-26.7) | 0.007* |
| Total body weight (kg) | 52.2 (46.8-61.0) | 48.7 (43.7-52.5) | 58.1 (51.2-70.1) | <0.0001* |
| Total fat mass (kg) | 15.8 (11.9-20.7) | 13.8 (11.3-18.0) | 16.6 (13.0-22.7) | 0.057 |
| Total muscle mass (kg) | 33.7 (30.9-38.9) | 31.0 (29.0-33.0) | 38.5 (33.8-44.7) | <0.0001* |
| ASM (kg) | 13.7 (11.9-16.5) | 12.0 (10.6-13.3) | 16.3 (13.7-19.5) | <0.0001* |
| SMI (kg/m2) | 5.67 (5.09-6.74) | 5.22 (4.76-5.43) | 6.38 (5.70-7.25) | <0.0001* |
| Treatment of RA | ||||
| ADA 10 (20.8%), CZP 9 (18.8%), | ADA 3 (14.3%), CZP 6 (28.6%), | ADA 7 (25.9%), CZP 3 (11.1%), | ||
| bDMARDs usage, n (%) | ABT 9 (18.8%), GLM 7 (14.6%), | ABT 5 (23.8%), GLM 3 (14.3%), | ABT 4 (14.8%), GLM 4 (14.8%), | |
| TCZ 5 (10.4%), IFX 5 (10.4%), ETN 3 (6.3%) TCZ 1 (4.8%), IFX 2 (9.5%), ETN 1 (4.8%) TCZ 4 (14.8%), IFX 3 (11.1%), ETN 2 (7.4%) |
||||
| MTX usage, n (%) | 33 (68.8%) | 13 (61.9%) | 20 (74.0%) | 0.531 |
| MTX dosage among users (mg/week) | 10.0 (6.0-11.0) | 8.0 (6.0-10.0) | 10.0 (6.0-12.0) | 0.235 |
| PSL usage, n (%) | 26 (54.2%) | 13 (61.9%) | 13 (48.1%) | 0.393 |
| PSL dosage among users (mg/day) | 4.5 (2.9-5.0) | 5.0 (2.5-6.3) | 4.5 (2.9-5.0) | 0.84 |
| Cumulative dose of PSL (mg) | 70.0 (0-2,986) | 130 (0-6,646) | 0 (0-2,943) | 0.597 |
RA: rheumatoid arthritis, DAS28-ESR: disease activity score 28 joint count erythrocyte sedimentation rate, CDAI: clinical disease activity index, ESR: erythrocyte sedimentation rate, HAQ-DI: health assessment questionnaire disability index, TUG: timed up and go test, 10MWT: 10-m walking test, DASH: disabilities of the arm, shoulder and hand, BDI-II: Beck depression inventory second edition, CONUT: controlling nutritional status, PNI: prognostic nutritional index, BMI: body mass index, ASM: appendicular skeletal muscle mass, SMI: skeletal muscle index, bDMARDs: biological disease-modifying antirheumatic drugs, ADA: adalimumab, CZP: certolizumab pegol, ABT: abatacept, GLM: golimumab, TCZ: tocilizumab, IFX: infliximab, ETN: etanercept, MTX: methotrexate, PSL: prednisolone
At the time of starting bDMARDs, 21 patients (43.8%) met the AGWSOP diagnostic criteria for sarcopenia except for the item concerning older age. We then compared the characteristics of the sarcopenia group with those of the non-sarcopenia group (Table 1, right side). In addition to the items contained in the AGWSOP criteria (grip strength, 10MWT, limb muscle mass), the results for the HAQ, TUG and DASH were significantly worse in the sarcopenia group than in the non-sarcopenia group, and the body mass index (BMI) was significantly lower in the sarcopenic group than in the non-sarcopenia group.
Effects of 6 and 12 months of bDMARDs
The changes in the parameters before and after 6 and 12 months of bDMARDs therapy are shown in Table 2. The disease activity measured by the DAS28-ESR and CDAI was significantly ameliorated after 12 months of bDMARDs. Furthermore, after 12 months of bDMARDs, all parameters regarding the physical and mental activity we measured were significantly improved, including the HAQ, grip strength, 10MWT and TUG. The nutritional status as measured by the serum Alb level, CONUT score and PNI was also ameliorated. Regarding the body composition, the total body weight and total fat mass were significantly increased after 12 months of bDMARDs therapy. However, no significant increase was observed in the total muscle mass or ASM.
Table 2.
The Changes in the Parameters before and after 6 and 12 Months of bDMARDs.
| n=48 | Baseline | 6 months after bDMARDs | 12 months after bDMARDs | p (before vs. 12 months) |
|---|---|---|---|---|
| Disease activity of RA | ||||
| DAS28-ESR | 4.6 (4.0-5.7) | 2.6 (1.8-3.5) | 2.6 (1.7-3.5) | <0.001* |
| CDAI | 17.4 (11.7-24.1) | 7.0 (1.7-11.0) | 7.6 (1.3-11.0) | <0.001* |
| Serum CRP (mg/dL) | 0.8 (0.2-3.0) | 0.06 (0.02-0.2) | 0.08 (0.02-0.2) | <0.001* |
| ESR (mm/mL) | 30.5 (14.0-74.3) | 12.0 (7.0-19.0) | 10.0 (6.0-24.8) | <0.001* |
| Physical and mental activity | ||||
| HAQ-DI | 0.8 (0.3-1.5) | 0.3 (0.0-1.1) | 0.3 (0.0-1.1) | <0.001* |
| Grip strength (kg) | 15.0 (8.3-21.5) | 19.5 (12.7-27.9) | 19.0 (10.8-28.3) | <0.001* |
| 10MWT (m/s) | 1.6 (0.9-2.0) | 1.7 (1.3-2.0) | 1.8 (1.3-2.0) | 0.005* |
| TUG | 7.8 (6.8-12.6) | 7.1 (6.3-8.9) | 7.1 (6.3-8.7) | 0.011* |
| DASH | 31.9 (19.8-52.5) | 18.3 (6.7-36.7) | 16.0 (7.9-32.1) | <0.001* |
| BDI-II | 14.0 (9.0-19.0) | 9.0 (6.7-36.7) | 7.0 (1.0-16.0) | 0.003* |
| Nutritional status | ||||
| Serum Alb level (g/dL) | 3.9 (3.5-4.2) | 4.2 (3.9-4.4) | 4.2 (3.9-4.4) | <0.001* |
| CONUT score | 2.0 (1.0-3.0) | 1.0 (0.0-2.0) | 1.0 (0.0-2.0) | <0.001* |
| PNI | 45.4 (40.6-50.2) | 50.1 (47.3-53.2) | 50.0 (46.9-54.0) | <0.001* |
| Body composition | ||||
| BMI | 21.5 (19.8-24.3) | 22.2 (20.1-24.6) | 22.1 (20.1-24.8) | 0.003* |
| Total body weight (kg) | 52.2 (46.8-61.0) | 53.1 (47.7-61.4) | 53.5 (47.8-62.1) | 0.004* |
| Total fat mass (kg) | 15.8 (11.9-20.7) | 17.2 (12.5-21.0) | 16.7 (12.7-21.9) | 0.002* |
| Total muscle mass (kg) | 33.7 (30.9-38.9) | 34.2 (31.8-39.0) | 33.9 (31.3-39.6) | 0.156 |
| ASM (kg) | 13.7 (11.9-16.5) | 13.8 (12.5-16.4) | 13.8 (12.6-16.9) | 0.061 |
| SMI (kg/m2) | 5.67 (5.09-6.74) | 5.69 (5.29-6.41) | 5.77 (5.27-6.44) | 0.063 |
| Treatment of RA | ||||
| MTX usage, n (%) | 33 (68.8%) | 31 (64.6%) | 27 (56.3%) | 0.292 |
| MTX dosage among users (mg/week) | 10.0 (6.0-11.0) | 8.0 (6.0-10.0) | 8.0 (6.0-10.0) | 0.063 |
| PSL usage, n (%) | 26 (54.2%) | 26 (54.2%) | 21 (43.8%) | 0.414 |
| PSL dosage among users (mg/day) | 4.5 (2.9-5.0) | 2.8 (2.0-5.0) | 2.5 (1.3-4.0) | 0.004* |
RA: rheumatoid arthritis, DAS28-ESR: disease activity score 28 joint count erythrocyte sedimentation rate, CDAI: clinical disease activity index, ESR: erythrocyte sedimentation rate, HAQ-DI: health assessment questionnaire disability index, TUG: timed up and go test, 10MWT: 10-m walking test, DASH: disabilities of the arm, shoulder and hand, BDI-II: Beck depression inventory second edition, CONUT: controlling nutritional status, PNI: prognostic nutritional index, BMI: body mass index, ASM: appendicular skeletal muscle mass, SMI: skeletal muscle index, bDMARDs: biological disease-modifying antirheumatic drugs, MTX: methotrexate, PSL: prednisolone
When comparing the 21 patients who were diagnosed with sarcopenia at the time of starting bDMARDs, the ASM [12.0 (10.6-13.3) vs. 12.9 (10.3-13.8) kg, p=0.042] and SMI [5.22 (4.76-5.43) vs. 5.44 (4.84-5.77), p=0.039] were also significantly increased after 12 months of bDMARDs therapy (Table 3).
Table 3.
The Changes in the Parameters before and after 6 and 12 Months of bDMARDs Therapy in the 21 Patients Who Were Diagnosed with Sarcopenia at the Time of Starting bDMARDs.
| n=21 | baseline | 6 months after bDMARDs | 12 months after bDMARDs | p (before vs. 12 months) |
|---|---|---|---|---|
| Body composition | ||||
| BMI | 20.7 (18.9-22.6) | 20.5 (19.5-22.6) | 20.9 (19.5-22.8) | 0.04* |
| Total body weight (kg) | 48.7 (43.7-52.5) | 48.8 (44.4-54.3) | 49.7 (44.1-55.0) | 0.044* |
| Total fat mass (kg) | 13.8 (11.3-18.0) | 15.4 (11.6-18.2) | 14.6 (12.3-18.6) | 0.009* |
| Total muscle mass (kg) | 31.0 (29.0-33.0) | 31.9 (28.0-33.6) | 32.1 (28.2-34.1) | 0.161 |
| ASM (kg) | 12.0 (10.6-13.3) | 12.9 (10.2-13.8) | 12.9 (10.3-13.8) | 0.042* |
| SMI (kg/m2) | 5.22 (4.76-5.43) | 5.41 (4.80-5.60) | 5.44 (4.84-5.77) | 0.039* |
BMI: body mass index, ASM: appendicular skeletal muscle mass, SMI: skeletal muscle index, bDMARDs: biological disease-modifying antirheumatic drugs
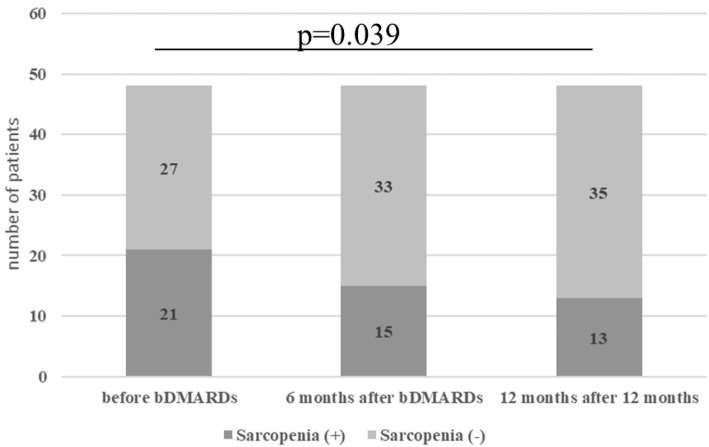
Figure shows the number of patients diagnosed with sarcopenia. The proportion of patients diagnosed with sarcopenia significantly decreased after 12 months of bDMARDs therapy (from 43.8% to 27.1%, p=0.039). Among the 21 patients who had been diagnosed with sarcopenia when starting bDMARDs, 11 still had sarcopenia, but 10 had escaped their sarcopenic state. Two patients who were not sarcopenic before bDMARDs became sarcopenic.
Figure.
The proportion of patients diagnosed with sarcopenia decreased significantly after 12 months of treatment with bDMARDs.
We compared the parameters between the 11 patients still with sarcopenia and the 10 patients without sarcopenia (Table 4). The age [77.0 (67.0-82.0) vs. 59.5 (29.3-73.8) years old, p=0.041] was significantly older and the disease duration of RA [9.0 (3.0-41.0) vs. 1.5 (1.0-5.1) years, p=0.029] significantly longer in the patients who still had sarcopenia than in those without it. The patients who were non-sarcopenic after 12 months had greater improvement in HAQ and BDI-II than the patients who still had sarcopenia.
Table 4.
The Comparison of the Parameters between the 11 Patients Still with Sarcopenia and the 10 Patients without Sarcopenia.
| Patients with sarcopenia n=11 |
Patients without sarcopenia n=10 |
p | ||||
|---|---|---|---|---|---|---|
| Age (years) | 77.0 (67.0-82.0) | 59.5 (29.3-73.8) | 0.041* | |||
| Sex, male/female | 0/11 | 2/8 | 0.083 | |||
| Cormobidities | ||||||
| Lung disease, n (%) | 2 (18.2%) | 1 (10.0%) | 1 | |||
| Chronic kidney disease, n (%) | 2 (18.2%) | 2 (20.0%) | 1 | |||
| Diabetes mellitus, n (%) | 2 (18.2%) | 3 (30.0%) | 0.635 | |||
| Clinical information of RA | ||||||
| Disease duration of RA (years) | 9.0 (3.0-41.0) | 1.5 (1.0-5.1) | 0.029* | |||
| Steinbricker stage (I/II/III/IV) | 2/2/4/3 | 4/2/3/1 | ||||
| Changes of DAS28-ESR from baseline | -2.4 [-4.2- (-1.7)] | -3.0 [-4.0- (-1.5)] | 0.654 | |||
| Changes of serum CRP from baseline (mg/dL) | -0.8 [-4.6- (-0.3)] | -0.2 [-10.4- (-0.04)] | 0.605 | |||
| Physical and mental activity | ||||||
| Changes of HAQ-DI from baseline | -0.8 (-1.0-0.0) | -0.6 (-1.3-0.0) | 0.023* | |||
| Changes of TUG from baseline | -1.1 (-2.7-5.8) | -1.3 [-5.8- (-0.2)] | 0.314 | |||
| Changes of BDI-II | -1.0 (-11.0-3.0) | -5.0 [-8.8- (-0.8)] | 0.034* | |||
| Nutritional status | ||||||
| Changes of CONUT score from baseline | -1.0 [-3.0- (-1.0)] | -1.0 (-3.25-0.0) | 0.863 | |||
| Changes of PNI from baseline | 4.4 [2.9-8.0)] | 7.7 (3.8-13.1) | 0.173 | |||
| Treatment of RA | ||||||
| ADA 3 (27.2%), CZP 1 (9.0%), | ADA 0 (0.0%), CZP 5 (50.0%), | |||||
| bDMARDs usage, n (%) | ABT 2 (18.2%), GLM 1 (9.0%), | ABT 3 (30.0%), GLM 2 (20.0%), | ||||
| TCZ 1 (9.0%), IFX 1 (9.0%), | TCZ 0 (0.0%), IFX 1 (10.0%), | |||||
| ETN 1 (9.0%) | ETN 0 (0.0%) | |||||
| MTX usage, n (%) | 6 (54.5%) | 7 (70.0%) | 0.659 | |||
| PSL usage, n (%) | 6 (54.5%) | 5 (50.0%) | 1 | |||
| Cumulative PSL usage over 12-months (mg) | 62 (0-810) | 539 (0-1,496) | 0.684 |
RA: rheumatoid arthritis, DAS28-ESR: disease activity score 28 joint count erythrocyte sedimentation rate, CONUT: controlling nutritional status, PNI: prognostic nutritional index, bDMARDs: biological disease-modifying antirheumatic drugs, ADA: adalimumab, CZP: certolizumab pegol, ABT: abatacept, GLM: golimumab, TCZ: tocilizumab, IFX: infliximab, ETN: etanercept
Discussion
In this prospective study, we demonstrated the efficacy of bDMARDs on the disease activity, physical and mental activity and nutritional status. Of note, the skeletal muscle mass was increased in the sarcopenic patients, and the number of patients diagnosed with sarcopenia was significantly decreased. However, older patients with a longer disease duration were likely to still have sarcopenia at the end of treatment.
Sarcopenia is a syndrome characterized by the loss of skeletal muscle mass and strength, which leads to adverse outcomes, such as frailty and mortality (2). The European working group on sarcopenia in older people (EWGSOP) developed a practical clinical definition and consensus diagnostic criteria for sarcopenia. The measurement of the muscle function (gait speed and grip strength) and muscle mass is needed to diagnose sarcopenia (2). The AWGSOP determined suitable cut-off values for all measurements in Asian populations (14).
In our study, the prevalence of sarcopenia among RA patients was 43.8%. This result was slightly higher than in previous reports describing sarcopenia in RA patients [37.1% (15) and 39.8% (16)] and was markedly higher than in the general Japanese elderly [8.06-22.1% (17,18)]. In previous reports, multiple factors were reported to be associated with sarcopenia in RA patients, including the age (15,16,19,20), disease duration (15), joint destruction (15,16), serum C-reactive protein (CRP) level (20), malnutrition (15), BMI (20) and presence of disability (19). The patients included in our study tended to be older with a longer disease duration and tended to have higher CRP levels because they failed conventional synthetic DMARD treatment and needed bDMARD treatment. Therefore, our patients seemed to be prone to sarcopenia at the time of starting bDMARDs.
In the present study, the systemic inflammation measured by the ESR, CRP and DAS28-ESR; the physical activity measured by the HAQ, 10MWT, TUG, and DASH; and the nutritional status measured by the serum Alb level, CONUT score and PNI were all ameliorated by bDMARD treatment. In addition, the mental status measured by the BDI-II was also ameliorated. Inflammation, physical activity and the nutritional status are considered to be major factors affecting sarcopenia (2). In this study, the proportion of patients diagnosed with sarcopenia was significantly reduced following bDMARD treatment, probably due to an improvement in the associated factors or by the direct effect of bDMARDs inhibiting inflammatory cytokines, such as TNF-α or IL-6.
Several reports have described the effects of bDMARDs on the body composition or sarcopenia. Our study included the largest number of patients among these studies. Metsios et al. followed 20 patients who took a TNF-α inhibitor for 12 weeks (21), Serelis et al. followed 19 women who took a TNF-α inhibitor for 1 year (22), Engvall et al. conducted a randomized control study with 18 patients recruited for infliximab therapy and followed them for 2 years (23). In these reports, the fat mass was increased by the therapy (22,23), but the muscle mass was not changed (21-23). Tournadre et al. followed 21 patients who took tocilizumab for 1 year and found an increase in the muscle mass (24). In our study, the muscle mass did not increase in a total of 48 patients. However, on assessing the data in detail, the muscle mass was found to have significantly increased in the 21 patients who had sarcopenia when starting bDMARDs. Because of the small number of patients, we were unable to assess whether a TNF-α inhibitor or non-TNF-α inhibitor was more effective for increasing the muscle mass. Differences in the disease duration, disease activity or baseline muscle mass might be the reason for the conflicting results.
While the number of patients diagnosed with sarcopenia was reduced in this study, patients with an older age and longer disease duration tended to still have sarcopenia at the end of observation. The earlier introduction of bDMARDs might be needed to prevent and overcome sarcopenia.
Conditions related to sarcopenia include sarcopenic obesity and rheumatoid cachexia. The term sarcopenic obesity is used to refer to sarcopenia concurrent with excess adiposity (25). In addition to the decreased muscle mass, obesity may pose a risk for diabetes, hypertension and cardiovascular disease (26). Furthermore, adipose itself is a potent source of inflammatory cytokines that may contribute to systemic inflammation (27). The term cachexia is used to refer to a wasting disorder that accompanies many chronic diseases and results from an imbalance in the energy requirement and energy uptake, leading to a loss of adipose tissue and skeletal muscle (28). Rheumatoid cachexia is reported to be a predictor of death (29). In both sarcopenic obesity and rheumatoid cachexia, inflammatory cytokines are believed to play a major role (27,29), and the effects of adequate treatment, including biologic agents, may be expected.
There are several limitations in the present study. First, this was a cohort study with a relatively small number of patients in a single center. We were unable to analyze the effect of each bDMARDs because of the small number. Second, we did not recruit a control group, so concluding the specific effects of bDMARDs is difficult. However, while it was difficult to identify any direct or indirect effect of bDMARDs, we did observe a significant improvement in all parameters, including the skeletal muscle mass, and a decreased prevalence of sarcopenia. Third, the evaluation of sarcopenia is complex; for example, an impaired grip strength may also be associated with joint destruction of RA. There is no specific evaluation tool for diagnosing sarcopenia in RA patients. The EWGSOP criteria were validated for the evaluation of age-related sarcopenia, so we need to be cautious when applying them to secondary sarcopenia.
However, to our knowledge, the present study is the first to describe the improvement of sarcopenia by bDMARDs by measuring both the muscle mass and physical function. We suggest that rheumatologists consider the earlier induction of sufficient therapy, like bDMARDs, in order to prevent and ameliorate a sarcopenic state.
The authors state that they have no Conflict of Interest (COI).
References
- 1. Beaudart C, Rizzoli R, Bruyere O, Regomster JY, Biver E. Sarcopenia: burden and challenges for public health. Arch Public Health 72: 45, 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Cruz-Jentoft AJ, Baeyens JP, Bauer JM, et al. ; European Working Group on Sarcopenia in Older People. Sarcopenia: european consensus on definition and diagnosis: report of the European Working Group on Sarcopenia in Older People. Age Ageing 39: 412-423, 2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Santilli V, Bernetti A, Mangone M, Paoloni M. Clinical definition of sarcopenia. Clin Cases Miner Bone Metab 11: 177-180, 2014. [PMC free article] [PubMed] [Google Scholar]
- 4. Fukuda W, Yamazaki T, Akaogi T, et al. Malnutrition and disease progression in patients with rheumatoid arthritis. Mod Rheumatol 15: 104-107, 2005. [DOI] [PubMed] [Google Scholar]
- 5. Helliwell M, Coombes EJ, Moody BJ, Batstone GF, Robertson JC. Nutritional status in patients with rheumatoid arthritis. Ann Rheum Dis 43: 386-390, 1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Rall LC, Roubenoff R. Rheumatoid cachexia: metabolic abnormalities, mechanisms and interventions. Rheumatology (Oxford) 46: 1824-1827, 2007. [DOI] [PubMed] [Google Scholar]
- 7. Steinbrocker O, Traeger CH, Batterman RC. Therapeutic criteria in rheumatoid arthritis. JAMA 140: 659-662, 1949. [DOI] [PubMed] [Google Scholar]
- 8. Bloem BR, Marinus J, Almeida Q, et al. Measurement instruments to assess posture, gait, and balance in Parkinson's disease: critique and recommendations. Mov Disord 31: 1343-1355, 2016. [DOI] [PubMed] [Google Scholar]
- 9. Podsiadlo D, Rishardson S. The timed “up & go”: a test of basic functional mobility for frail elderly persons. J Am Geriatr Soc 39: 142-148, 1991. [DOI] [PubMed] [Google Scholar]
- 10. Gummesson C, Atroshi I, Ekdahll C. The disabilities of the arm, shoulder and hand (DASH) outcome questionnaire: longitudinal construct validity and measuring self-rated health change after surgery. BMC Musculoskelet Disord 4: 11, 2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Ignacio de Ulíbarri J, González-Madroño A, de Villar NG, et al. CONUT: a tool for controlling nutritional status. First validation in a hospital population. Nutr Hosp 20: 38-45, 2005. [PubMed] [Google Scholar]
- 12. Onodera T, Goseki N, Kosaki G. Prognostic nutritional index in gastrointestinal surgery of malnourished cancer patients. Nihon Geka Gakkai Zasshi (J Jpn Surg Soc) 85: 1001-1005, 1984(in Japanese). [PubMed] [Google Scholar]
- 13. Beck AT, Ward CH, Mendelson M, Mock J, Erbaugh J. An inventory for measuring depression. Arch Gen Psychiatry 4: 561-571, 1961. [DOI] [PubMed] [Google Scholar]
- 14. Chen LK, Liu LK, Woo J, et al. Sarcopenia in Asia: consensus report of the Asian Working Group for Sarcopenia. J Am Med Dir Assoc 15: 95-101, 2014. [DOI] [PubMed] [Google Scholar]
- 15. Torii M, Hashimoto M, Hanai A, et al. Prevalence and factors associated with sarcopenia in patients with rheumatoid arthritis. Mod Rheumatol 29: 589-595, 2019. [DOI] [PubMed] [Google Scholar]
- 16. Ngeuleu A, Allali F, Medrare L, Madhi A, Rkain H, Hajjaj-Hassouni N. Sarcopenia in rheumatoid arthritis: prevalence, influence of disease activity and associated factors. Rheumatol Int 37: 1015-1020, 2017. [DOI] [PubMed] [Google Scholar]
- 17. Yamada M, Kimura Y, Ishiyama D, et al. Differential characteristics of skeletal muscle in community-dwelling older adults. J Am Med Dir Assoc 18: 807.e9-807.e16, 2017. [DOI] [PubMed] [Google Scholar]
- 18. Nishiguchi S, Yamada M, Fukutani N, et al. Differential association of frailty with cognitive decline and sarcopenia in community-dwelling older adults. J Am Med Dir Assoc 16: 120-124, 2015. [DOI] [PubMed] [Google Scholar]
- 19. Barone M, Viggiani MT, Anelli MG, et al. Sarcopenia in patients with rheumatic diseases: prevalence and associated risk factors. J Clin Med 7: 504, 2018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Mochizuki T, Yano K, Ikari K, Okazaki K. Sarcopenia-associated factors in Japanese patients with rheumatoid arthritis: a cross-sectional study. Geriatr Gerontol Int 19: 907-912, 2019. [DOI] [PubMed] [Google Scholar]
- 21. Metsios GS, Stavropoulos-Kalinoglou A, Douglas KMJ, et al. Blockade of tumor necrosis factor-α in rheumatoid arthritis: effects on components rheumatoid cachexia. Rheumatol Oxf Engl 46: 1824-1827, 2007. [DOI] [PubMed] [Google Scholar]
- 22. Serelis J, Kontogianni MD, Katsiougiannis S, Bletsa M, Tektonidou MG, Skopouli FN. Effect of anti-TNF treatment on body composition and serum adiponectin levels of women with rheumatoid arthritis. Clin Rheumatol 27: 795-797, 2008. [DOI] [PubMed] [Google Scholar]
- 23. Engvall IL, Tengstrand B, Brismar K, Hafström I. Infliximab therapy increases body fat mass in early rheumatoid arthritis independently of changes in disease activity and levels of leptin and adiponectin: a randomised study over 21 months. Arthritis Res Ther 12: R197, 2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Tournadre A, Pereira B, Dutheil F, et al. Changes in body composition and metabolic profile during interleukin 6 inhibition in rheumatoid arthritis. J Cachexia Sarcopenia Muscle 8: 639-646, 2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Letarouilly JG, Flipo RM, Cortet B, Tournadre A, Paccou J. Body composition in patients with rheumatoid arthritis: a narrative literature review. Ther Adv Musculoskelet Dis 13: 1759720X211015006, 2021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Abbasi F, Brown BW Jr, Lamendola C, McLaughlin T, Reaven GM. Relationship between obesity, insulin resistance, and coronary heart disease risk. J Am Coll Cardiol 4: 937-943, 2002. [DOI] [PubMed] [Google Scholar]
- 27. Hotamisligil GS, Arner P, Caro JF, Atkinson RL, Spiegelman BM. Increased adipose tissue expression of tumor necrosis factor-α in human obesity and insulin resistance. J Clin Invest 95: 2409-2415, 1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Rohm M, Zeigerer A, Machado J, Herzig S. Energy metabolism in cachexia. EMBO Rep 20: e47258, 2019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Walsmith J, Roubenoff R. Cachexia in rheumatoid arthritis. Int J Cardiol 85: 89-99, 2002. [DOI] [PubMed] [Google Scholar]