Abstract
A 19-year-old Japanese man was hospitalized for cardiogenic shock 28 days after receiving a second dose of the coronavirus disease 2019 (COVID-19) mRNA-1273 vaccine. He had had a high fever for three days with vomiting and abdominal pain before arriving at our hospital. The patient visited a local hospital and was diagnosed with heart failure and acute appendicitis. An endomyocardial biopsy specimen showed myocarditis. Thereafter, Impella CP left ventricular assist device implantation and venoarterial peripheral extracorporeal membranous oxygenation were initiated immediately along with inotropic support and steroid pulse therapy. Given these findings, he was finally diagnosed with multiple inflammatory syndrome and fulminant myocarditis.
Keywords: biopsy, cytokine, inflammation
Introduction
Several severe acute respiratory coronavirus 2 (SARS-CoV-2) vaccines have proven safe and efficacious in the prevention of its infection (1-3). Although adverse events following immunization (AEFI), including myocarditis and appendicitis, after SARS-CoV-2 vaccination have been reported, they are rare (4-8). Fulminant myocarditis has also been reported in a few histologically confirmed cases (9).
Multisystem inflammatory syndrome in children (MIS-C) associated with SARS-CoV-2 is rare but leads to a serious and life-threatening hyperinflammatory state 4-6 weeks after SARS-CoV-2 infection in children and adolescents (<21 years old) (10,11). A similar condition has also been reported as a rare complication of SARS-CoV-2 in adults (MIS-A) (11). Recently, MIS-C/A (MIS-C and MIS-A) was reported as a SARS-CoV-2 vaccine AEFI (11-15).
We herein report a 19-year-old patient who had MIS-C with fulminant myocarditis and acute appendicitis 28 days after receiving the second dose of a coronavirus disease 2019 (COVID-19) mRNA vaccine.
Case Report
A 19-year-old Japanese man was admitted to our hospital with heart failure and cardiogenic shock. He had received the second dose of the COVID-19 mRNA-1273 vaccine 28 days earlier. He had a high fever for three days. After experiencing abdominal pain, vomiting, and appetite loss, he visited a local hospital and was diagnosed with acute appendicitis after computed tomography (CT) revealed a swollen appendix (Fig. 1). The patient was transferred to our hospital because of severe cardiac dysfunction. He had no medical or family history and had no history of smoking or alcohol consumption.
Figure 1.
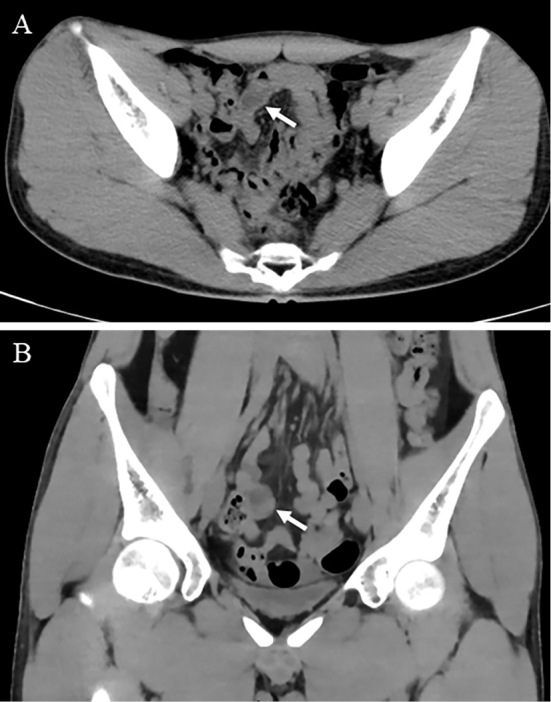
Abdominal computed tomography revealed a swollen appendix (A: transverse view, B: coronal view)
A physical examination on admission revealed the following findings: a blood pressure of 79/50 mmHg; a regular pulse rate of 140 beats per minute; body temperature of 38.6°C; a body mass index of 16.3 kg/m2; and no abnormal findings except for tenderness of the periumbilical region and a 2-finger-breadth palpable liver.
Data of laboratory parameters were as follows: white blood cell count, 12,200/mm3 with lymphopenia (9.4%); C-reactive protein (CRP), 10.27 mg/dL; D-dimer, 1.6 μg/mL; high-sensitivity troponin T, 3.67 ng/mL; creatinine kinase (CK), 476 IU/L; and N-terminal pro-brain natriuretic peptide (NT-proBNP), 24,112 pg/mL. In addition to these findings, we noted liver and renal dysfunction with negative results for COVID-19 antibodies, a real-time reverse transcription polymerase chain reaction assay (RT-PCR), antigens, and routine pathogen tests (Table 1).
Table 1.
Laboratory Data.
| WBC | 12,200 | /μL | RF | <5.0 | IU/mL (<15) | |||
| Seg | 69.9 | % | Anti-nuclear antibody | <80 | ||||
| Lymph | 9.4 | % | Anti-dsDNA antibody | <10 | IU/mL (<12.0) | |||
| Mono | 19.5 | % | Anti-ssDNA antibody | <10 | IU/mL (<25.0) | |||
| RBC | 4.89×104 | /μL | CH50 | 30.5 | /mL (30-46) | |||
| Hb | 14.2 | g/dL | MPO-ANCA | <1.0 | U/mL (<3.5) | |||
| Hct | 40.9 | % | PR3-ANCA | <1.0 | U/mL (<3.5) | |||
| Plt | 163×103 | /μL | Anti-SS-A antibody | <1.0 | U/mL (<10.0) | |||
| PT-INR | 1.22 | Anti-SS-B antibody | <1.0 | U/mL (<10.0) | ||||
| APTT | 28.5 | s | NTproBNP | 24,112 | pg/mL | |||
| D-dimer | 1.6 | μg/mL | CRP | 10.27 | mg/dL | |||
| T-Bil | 0.7 | mg/dL | SARS-CoV-2-Ab | <0.1 | COI (<0.1) | |||
| AST | 51 | IU/L | SARS-CoV2-PCR | (-) | ||||
| ALT | 43 | IU/L | SARS-CoV2-Ag | (-) | ||||
| ALP | 36 | IU/L | Procalcitonin | 0.156 | ng/mL (<0.046) | |||
| LDH | 347 | IU/L | β-D glucan | 5.0 | pg/mL (<20) | |||
| γ-GTP | 33 | IU/L | Influenza antigen | (-) | ||||
| CK | 476 | IU/L | Urinary antigen of Legionella | (-) | ||||
| CKMB | 17 | IU/L | Legionella nucleic acid (sputum) | (-) | ||||
| hs-TnT | 3.67 | ng/mL | Mycoplasma nucleic acid (sputum) | (-) | ||||
| Na | 135 | mEq/L | HSV IgG | 12.5 | (<2.0) | |||
| K | 4.0 | mEq/L | HSV IgM | 0.29 | (<0.80) | |||
| Cl | 97 | mEq/L | HSV DNA PCR | (-) | ||||
| Ca | 8.7 | mg/dL | Chlamydia pneumoniae IgG | 27 | (<30) | |||
| BUN | 23 | mg/dL | Chlamydia pneumoniae IgA | 5 | (<8.0) | |||
| Cre | 1.07 | mg/dL | CMV antibody IgG | 12.9 | (<2.0) | |||
| TP | 6.1 | g/dL | CMV antibody IgM | 0.04 | (<0.80) | |||
| Alb | 3.3 | g/dL | CMV nucleic acid | (-) | ||||
| UA | 6.3 | mg/dL | Urinary antigen of Strept. pneumoniae | (-) | ||||
| TG | 64 | mg/dL | EBV VCA IgG | 40 | (<1.0) | |||
| LDL-C | 49 | mg/dL | EBV VCA IgM | <10 | (<10) | |||
| HDL-C | 39 | mg/dL | ||||||
| FPG | 130 | mg/dL | EBV EBNA IgG | 2.6 | (<1.0) | |||
| HbA1c | 5.3 | % | T-SPOT. TB | (-) |
WBC: white blood cell count, RBC: red blood cell count, Hb: hemoglobin, Hct: hematocrit, Plt: platelet count, PT-INR: prothrombin time-international normalized ratio, APTT: activated partial thromboplastin time, T-bil: total bilirubin , AST: aspartate aminotransferase, ALT: alanine aminotransferase, ALP: alkaline phosphatase, LDH: lactate dehydrogenase, γ-GTP: γ-glutamyl transpeptidase, CK: creatine kinase, hs-TnT: high sensitive-troponin T, BUN: blood urea nitrogen, Cre: creatinine, TP: total protein, Alb: albumin, UA: uric acid, TG: triglyceride, LDL-C: low-density lipoprotein cholesterol, HDL-C: high-density lipoprotein cholesterol, FPG: fasting plasma glucose, HbA1c: hemoglobin A1c, NT-pro BNP: N terminal-pro brain natriuretic peptide, CRP: C-reactive protein, SARS-CoV-2-Ab: SARS-CoV-2-antibody, SARS-CoV2-PCR: SARS-CoV2-polymerase chain reaction, SARS-CoV2-Ag: SARS-CoV2-antigen, MPO-ANCA: myeloperoxydase-antineutrophil cytoplasmic antibody, PR3-ANCA: proteinase 3-antineutrophil cytoplasmic antibody, urinary antigen of Strept. pneumoniae: urinary antigen of Streptococcus pneumoniae, CMV: cytomegalovirus, EBV VCA: Epstein-Barr virus virus capsid antigen
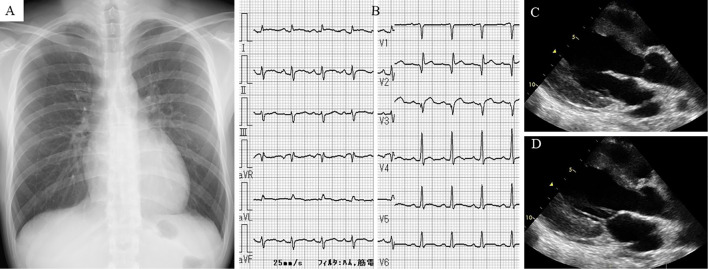
Chest radiography showed normal findings, with a cardiothoracic ratio of 49.7% (Fig. 2A). Electrocardiography (ECG) revealed sinus tachycardia; abnormal Q waves in leads V1-3; and slight ST-segment elevation in leads I, II, aVF, and V2-6 (Fig. 2B). Echocardiography revealed diffuse left ventricular hypokinesis [left ventricular ejection fraction (LVEF), 34%], thickened interventricular septal wall (12 mm), and LV posterior wall (12 mm) with normal LV dimensions and pericardial effusion (Fig. 2C, D). Coronary angiography revealed no remarkable findings.
Figure 2.
Chest radiography showed normal findings with a cardiothoracic ratio of 49.7% (A). Electrocardiography revealed sinus tachycardia, abnormal Q waves in leads V1-3, and slight ST-segment elevation in leads I, II, aVF, and V2-6 (B). Transthoracic echocardiography showing left ventricular hypokinesis with mild pericardial effusion (C: end-diastolic phase of parasternal long-axis view, D: end-systolic phase of the parasternal long-axis view).
He received tracheal intubation because of respiratory alkalosis (pH, 7.503; PCO2, 26.1 mmHg; PO2, 90.1 mmHg; HCO3-, 20.3 mEq/L; base excess, -1.0 mEq/L); and an increased lactic acid level (2.2 mmol/L) with nasal oxygen inhalation (3 L/min).
Cardiac catheterization on admission revealed normal coronary arteries. The results of a pressure study were as follows: pulmonary capillary wedge pressure (a wave/v wave/mean), 31/27/26 mmHg; pulmonary artery pressure (systolic/diastolic/mean), 35/26/31 mmHg; right ventricular pressure (systolic/diastolic/end-diastolic pressure), 38/9/12 mmHg; right atrial pressure (a wave/v wave/mean), 15/11/11 mmHg; left ventricular pressure (systolic/diastolic/end-diastolic pressure), 84/19/28 mmHg; cardiac output, 3.01 L/min; cardiac index, 1.85 L/min/m2.
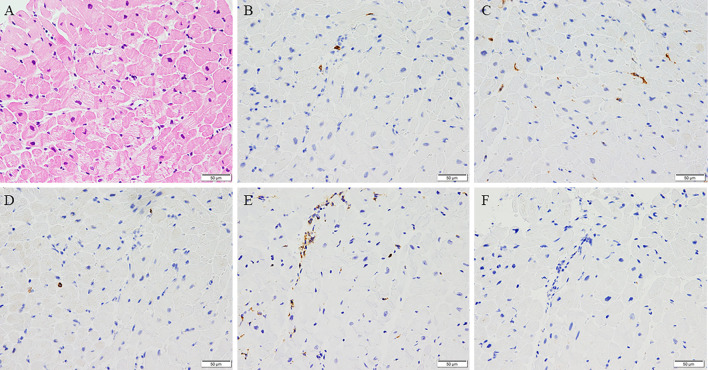
Implantation of an Impella CP left ventricular assist device and veno-arterial peripheral extracorporeal membrane oxygenation (VA ECMO) were immediately initiated along with inotropic support [noradrenalin (0.05 μg/kg/min)]. He also received methylprednisolone (1 g/day for 3 days) and immunoglobulin (5 g/day for 3 days), along with azithromycin (500 mg/day for 3 days). An endomyocardial biopsy specimen showed mild myocyte damage and cell infiltration (Fig. 3A) with T cells (Fig. 3B) [both CD4+ (Fig. 3C) and CD8+ cells (Fig. 3D)], an increased number of macrophages (Fig. 3E), and a decreased number of B cells (Fig. 3F). Based on these findings, he was diagnosed with fulminant myocarditis.
Figure 3.
Cell infiltration in the myocardium (A, Hematoxylin and Eosin staining) with CD3+ cells (B), both CD4+ cells (C), CD8+ cells (D), more CD68+ cells (E), and fewer CD20+ cells (F) (×200).
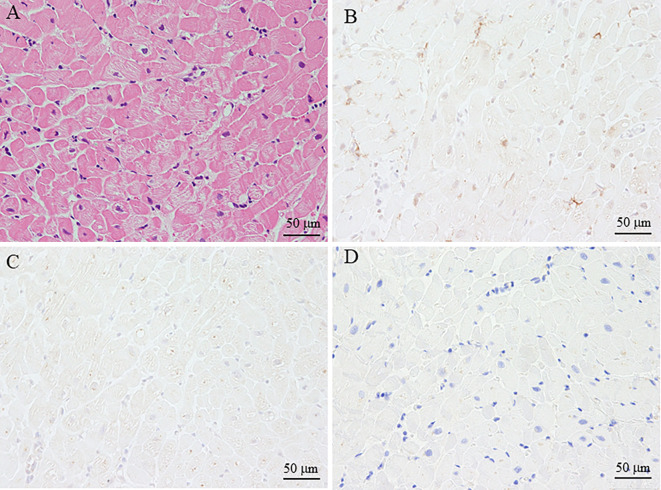
We also performed immunostaining for the myocardium biopsy specimen using antibodies against angiotensin-converting enzyme 2 (ACE2), SARS-CoV-2 spike S protein, and C4d to evaluate the relationship between myocarditis and COVID-19 vaccination. The myocytes were negative for these antibodies (Fig. 4).
Figure 4.
Immunostaining of the myocardium biopsy sample using antibodies for ACE2, SARS-CoV-2 (COVID-19) spike protein, and C4d. In the myocardium biopsy sample with myocarditis (A, Hematoxylin and Eosin staining, ×200), ACE2 (B, ×200), SARS-CoV-2 (COVID-19) spike protein (C, ×200), and C4d (D) were negative in myocytes. SARS-CoV-2: severe acute respiratory syndrome coronavirus 2, COVID-19: coronavirus disease 2019, ACE2: angiotensin-converting enzyme 2
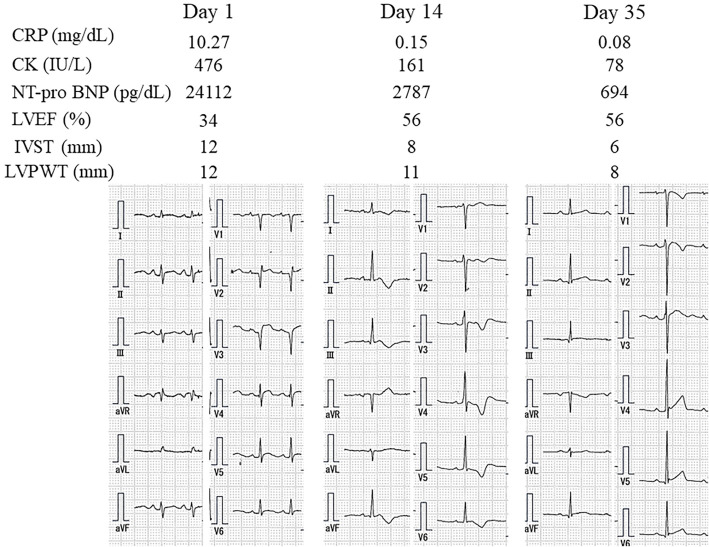
His cardiac function gradually improved, and he was weaned from VA ECMO and Impella CP eight days and nine days after admission, respectively. CT revealed no swelling of the appendix seven days after admission. After treatment for heart failure with furosemide (40 mg/day), enalapril (1.25 mg/day), and carvedilol (2.5 mg/day), his condition gradually improved. Two weeks after admission, his cardiac function had recovered to an almost normal systolic function with an LVEF of 56%, normal LV wall thickness on echocardiography, and normal CK levels. The patient experienced liver dysfunction due to enalapril (1.25 mg/day), which was changed to losartan (12.5 mg/day). The patient was discharged with a serum NT-proBNP level of 694 pg/mL and normal ECG findings (Fig. 5) 5 weeks after admission and was prescribed losartan (12.5 mg/day) and carvedilol (5 mg/day).
Figure 5.
Time course of electrocardiography, echocardiographic data, and laboratory data. CRP: C-reactive protein, CK: creatinine kinase, NT-proBNP: N-terminal pro-brain natriuretic peptide, LVEF: left ventricular ejection fraction, IVST: interventricular septal thickness, LVPW: left ventricular posterior wall thickness
Tests performed for infections showed negative results for the following: two sets of blood cultures taken before administration of antibiotics; PCR for COVID-19; IgM antibody for cytomegalovirus and Epstein-Barr virus-viral capsid antigen; influenza A and B kits; and viral antibodies (paired serum samples) against adenovirus, Coxsackie virus (A16, A7, B1, B2, B3, B4, B5, and B6), echovirus (3, 6, 7, 11, and 12), and parainfluenza virus (1, 2, and 3). Immunology screening results for antinuclear antigen (ANA) and antineutrophil cytoplasmic antibodies (ANCA) were normal (Table 1). Given these results, he was finally diagnosed with fulminant myocarditis and acute appendicitis related to the COVID-19 vaccine. In addition, this patient also met the level 1 diagnostic criteria (definitive case) of vaccine-induced MIS-C according to the Brighton Collaboration Network definition (11). The criteria are as follows: age <21 years old; a fever for 3 days; ≥2 clinical features of multiple organ involvement [gastrointestinal (abdominal pain, vomiting), and shock/hypotension]; laboratory evidence of inflammation (elevated CRP levels), ≥2 measures of disease activity (elevated NT-proBNP and troponin, lymphopenia, low LVEF on echocardiography, and ECG changes consistent with myocarditis), and SARS-CoV-2 vaccination.
Discussion
We encountered a patient with MIS-C with fulminant myocarditis and acute appendicitis 28 days after receiving the second dose of a COVID-19 mRNA vaccine. He recovered after treatment with steroids and antibiotics, in addition to Impella CP implantation and VA ECMO support.
There have been no reports of patients with myocarditis and appendicitis at the same time after COVID-19 mRNA vaccination, although COVID-19 mRNA vaccination is associated with an elevated risk of both myocarditis and appendicitis (8).
We searched for previous reports on MIS and the COVID-19 vaccine and identified 67. Patients with evidence of COVID-19 infection were excluded from the study. Finally, we reviewed 19 cases of myocarditis and evaluated the vaccine type, duration between vaccination and the first sign of symptoms, presence of hypotension/shock, and treatments including medication and VA ECMO, in addition to those in our case (Table 2) (13,14,16-26). The vaccine dose and duration between vaccination and the MIS-C/A onset varied among the reports, indicating that the occurrence of MIS-C/A after COVID-19 mRNA vaccination is heterogeneous, and the underlying mechanisms may differ among cases.
Table 2.
Multisysten Inflammatory Syndrome with Myocarditis after COVID-19 Vaccination in the Previous Reports and Our Report.
| Age (years) | Sex | Type of vaccine | Vaccine dose | Days from vaccination to onset | Hypotension/ shock | Treatment | VA-ECMO | Outcome | Ref. | |
|---|---|---|---|---|---|---|---|---|---|---|
| 1 | 12-15, 3 cases 18-20, 3 cases |
M, 3 | BNT162b | 1st | NA | 2 cases | IVIG,4 cases steroid, 4 cases |
- | Recovered | 13 |
| 2 | BNT162b | 2nd | 21 days | - | Recovered | 13 | ||||
| 3 | BNT162b | 2nd | 14 days | - | Recovered | 13 | ||||
| 4 | BNT162b | 2nd | 84 days | - | Recovered | 13 | ||||
| 5 | BNT162b | 2nd | 5 day | - | Recovered | 13 | ||||
| 6 | BNT162b | 2nd | 0 days | - | Recovered | 13 | ||||
| 7 | 12 | M | mRNA-1273 | 2nd | 35 days | + | IVIG | - | Recovered | 16 |
| 8 | 12 | M | BNT162b | 2nd | 2 days | - | - | - | Recovered | 17 |
| 9 | 14 | F | BNT162b | 2nd | 60 days | + | IVIG+steroid | - | Recovered | 18 |
| 10 | 16 | M | BNT162b | 1st | 12 days | - | IVIG+steroid | - | Recovered | 19 |
| 11 | 17 | F | BNT162b | 1st | 7 days | - | IVIG+steroid | - | Recovered | 20 |
| 12 | 19 | M | mRNA-1273 | 2nd | 28 days | + | IVIG+steroid | + | Recovered | Our |
| 13 | Late teens | F | BNT162b | 2nd | 20 days | - | IVIG+steroid | - | Recovered | 21 |
| 14 | 22 | F | ChAdOX1 | 1st | 10 days | + | IVIG+steroid+tocilizmab | - | Recovered | 22 |
| 15 | 24 | M | BNT162b | 2nd | 14 days | - | Steroid | - | Recovered | 23 |
| 16 | 27 | M | BNT162b | 2nd | 2 days | + | IVIG+steroid+anakinra | + | Dead | 24 |
| 17 | 34 | F | BNT162b | 1st | 9 days | + | IVIG+steroid+anakinra | + | Recovered | 24 |
| 18 | 44 | F | BNT162b | NA | 2 days | + | Steroid | - | Recovered | 14 |
| 19 | 46 | M | Ad26.COV2.S | NA | 12 days | - | Steroid | - | Recovered | 25 |
| 20 | 67 | M | ChAdOX1 | 1st | 6 days | - | Steroid | - | Recovered | 26 |
F: female, IVIG: human immunoglobulin, M: male, NA: not applicable, Ref.: reference, VA-ECMO: veno-arterial peripheral extracorporeal membrane oxygenation
Of the 20 patients, including our own, 9 had hypotension/shock, and 3 [case 12 (our case), 16, and 17] were treated with VA ECMO in addition to other medical treatments (Tab 2). A myocardial biopsy was performed in only 2 of the 20 patients: in our patient (case 12) and case 17. Case 17 underwent a myocardial biopsy on day 13 of hospital admission that showed cardiomyocytes with focal cytoplasmic vacuolization and rare interstitial lymphocytic infiltration, suggesting healing myocarditis. Our myocardial biopsy showed cell infiltration (more macrophages than T and B cells) without ACE2, spike protein, or C4d expression in myocytes, which differed from our previously reported case of fulminant myocarditis after COVID-19 vaccination without MIS (27). These findings suggest that the pathophysiology of myocarditis following COVID-19 vaccination may be multifactorial.
Several reports have shown histopathological features of MIS following SARS-Cov-2 infection (28-32). Cardiac injury in MIS following viral infection may occur either because of direct cardiac invasion of the virus or inflammatory response by cytokine release (cytokine storm) (29). The presence of more macrophages and less T cell infiltration (29) or mild T cell (31) without substantial myocyte necrosis suggested an inflammatory response by cytokine release but not direct cardiac damage due to viral invasion. As these histopathological features were similar to those of our patient, myocarditis in MIS following COVID-19 vaccination may be due to hyperinflammation induced by cytokine release, although the precise mechanism that leads to hyperinflammation in MIS is unknown.
There have been several case reports of MIS-C associated with SARS-CoV-2 infection and acute appendicitis (33-37). These previous findings suggested that it may be difficult to make the differential diagnosis between acute appendicitis and MIS-C, as some patients may have both acute appendicitis and MIS-C, and others may have appendix inflammation (mimicking appendicitis) as gastrointestinal involvement due to MIS-C. However, there have been no reports of MIS-C after COVID-19 mRNA vaccination along with acute appendicitis. Our patient was clinically diagnosed with acute appendicitis based on his symptoms, laboratory data, and CT findings, and he had no appendectomy because his condition was ameliorated with treatment including steroid pulse therapy for fulminant myocarditis in addition to antibiotics. We should therefore consider MIS-C in patients with multisystem organ involvement, including suspected acute appendicitis, after COVID-19 mRNA vaccination because the treatment may differ among patients with acute appendicitis and MIS-C.
Almost all patients recovered by treatment with steroids and/or intravenous immune globulin (IVIG); anakinra, an interleukin (IL)-1 receptor antagonist; or tocilizumab, an IL-6 receptor antagonist. However, one patient without hypotension/shock (case 8) recovered without these treatments, and one patient (case 16) with fulminant myocarditis died despite treatment with IVIG, steroids, and anakinra (Table 2). Although there are no prospective studies, immunomodulatory therapy may be effective for myocarditis associated with MIS-C. Further studies are required to determine the optimal treatment for this condition.
In conclusion, myocarditis associated with MIS-C should be considered in patients even after COVID-19 mRNA vaccination for timely and proper treatment, although this is very rare.
The authors state that they have no Conflict of Interest (COI).
References
- 1. Baden LR, El Sahly HM, Essink B, et al. Efficacy and safety of the mRNA-1273 SARS-CoV-2 vaccine. N Engl J Med 384: 403-416, 2021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Thomas SJ, Moreira ED Jr, Kitchin N, et al. Safety and efficacy of the BNT162b2 mRNA COVID-19 vaccine through 6 months. N Engl J Med 385: 1761-1773, 2021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Voysey M, Clemens SAC, Madhi SA, et al. Safety and efficacy of the ChAdOx1 nCoV-19 vaccine (AZD1222) against SARS-CoV-2: an interim analysis of four randomised controlled trials in Brazil, South Africa, and the UK. Lancet 397: 99-111, 2021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Mevorach D, Anis E, Cedar N, et al. Myocarditis after BNT162b2 mRNA vaccine against COVID-19 in Israel. N Engl J Med 385: 2140-2149, 2021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Klein NP, Lewis N, Goddard K, et al. Surveillance for adverse events after COVID-19 mRNA vaccination. JAMA 326: 1390-1399, 2021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Montgomery J, Ryan M, Engler R, et al. Myocarditis following immunization with mRNA COVID-19 vaccines in members of the US military. JAMA Cardiol 6: 1202-1206, 2021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Witberg G, Barda N, Hoss S, et al. Myocarditis after COVID-19 vaccination in a large health care organization. N Engl J Med 385: 2132-2139, 2021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Barda N, Dagan N, Ben-Shlomo Y, et al. Safety of the BNT162b2 mRNA COVID-19 vaccine in a nationwide setting. N Engl J Med 385: 1078-1090, 2021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Verma AK, Lavine KJ, Lin CY. Myocarditis after COVID-19 mRNA vaccination. N Engl J Med 385: 1332-1334, 2021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Feldstein LR, Rose EB, Horwitz SM, et al. Multisystem inflammatory syndrome in U.S. children and adolescents. N Engl J Med 383: 334-346, 2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Vogel TP, Top KA, Karatzios C, et al. Multisystem inflammatory syndrome in children and adults (MIS-C/A): case definition & guidelines for data collection, analysis, and presentation of immunization safety data. Vaccine 39: 3037-3049, 2021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Narvel H, Kaur A, Seo J, Kumar A. Multisystem inflammatory syndrome in adults or hemophagocytic lymphohistiocytosis: a clinical conundrum in fully vaccinated adults with breakthrough COVID-19 infections. Cureus 14: e22123, 2022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Yousaf AR, Cortese MM, Taylor AW, et al. Reported cases of multisystem inflammatory syndrome in children aged 12-20 years in the USA who received a COVID-19 vaccine, December, 2020, through August, 2021: a surveillance investigation. Lancet Child Adolesc Health 6: 303-312, 2022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Nune A, Iyengar KP, Goddard C, Ahmed AE. Multisystem inflammatory syndrome in an adult following the SARS-CoV-2 vaccine (MIS-V). BMJ Case Rep 14: 2021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Patel T, Kelleman M, West Z, et al. Comparison of multisystem inflammatory syndrome in children-related myocarditis, classic viral myocarditis, and COVID-19 vaccine-related myocarditis in children. J Am Heart Assoc 11: e024393, 2022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Abdelgalil AA, Saeedi FA. Multisystem inflammatory syndrome in a 12-year-old boy after mRNA-SARS-CoV-2 vaccination. Pediatr Infect Dis J 41: e93-e94, 2022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Poussaint TY, LaRovere KL, Newburger JW, et al. Multisystem inflammatory-like syndrome in a child following COVID-19 mRNA vaccination. Vaccines (Basel) 10: 43, 2021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. DeJong J, Sainato R, Forouhar M, Robinson D, Kunz A. Multisystem inflammatory syndrome in a previously vaccinated adolescent female with sickle cell disease. Pediatr Infect Dis J 41: e104-e105, 2022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Hugh McGann P, Krim AOA, Green J, Venturas J. Multi inflammatory syndrome in a 16-year-old male following first dose of m-RNA COVID-19 vaccination. Clin Infect Pract 14: 100139, 2022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Jain E, Donowitz JR, Aarons E, Marshall BC, Miller MP. Multisystem inflammatory syndrome in children after SARS-CoV-2 vaccination. Emerg Infect Dis 28: 990-993, 2022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Wangu Z, Swartz H, Doherty M. Multisystem inflammatory syndrome in children (MIS-C) possibly secondary to COVID-19 mRNA vaccination. BMJ Case Rep 15: 2022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Choi YK, Moon JY, Kim J, et al. Postvaccination multisystem inflammatory syndrome in adult with no evidence of prior SARS-CoV-2 infection. Emerg Infect Dis 28: 411-414, 2022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Durucan I, Guner S, Kilickiran Avci B, Unverengil G, Melikoglu M, Ugurlu S. Post COVID-19 vaccınatıon inflammatory syndrome: a case report. Mod Rheumatol Case Rep. Forthcoming. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Abbate A, Gavin J, Madanchi N, et al. Fulminant myocarditis and systemic hyperinflammation temporally associated with BNT162b2 mRNA COVID-19 vaccination in two patients. Int J Cardiol 340: 119-121, 2021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Bova C, Vigna E, Gentile M. Multisystem inflammatory syndrome after Ad26.COV2.S vaccination. IDCases 27: e01411, 2022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Park JW, Yu SN, Chang SH, Ahn YH, Jeon MH. Multisystem inflammatory syndrome in an adult after COVID-19 vaccination: a case report and literature review. J Korean Med Sci 36: e312, 2021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Kawano H, Motokawa T, Kurohama H, et al. Fulminant myocarditis 24 days after coronavirus disease messenger ribonucleic acid vaccination: a case report. Intern Med 61: 2319-2325, 2022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Beaudry JT, Dietrick B, Lammert DB, et al. Fatal SARS-CoV-2 inflammatory syndrome and myocarditis in an adolescent: a case report. Pediatr Infect Dis J 40: e72-e76, 2021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Bemtgen X, Klingel K, Hufnagel M, et al. Case report: lymphohistiocytic myocarditis with severe cardiogenic shock requiring mechanical cardiocirculatory support in multisystem inflammatory syndrome following SARS-CoV-2 infection. Front Cardiovasc Med 8: 716198, 2021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Vannella KM, Oguz C, Stein SR, et al. Evidence of SARS-CoV-2-specific T-cell-mediated myocarditis in a MIS-A case. Front Immunol 12: 779026, 2021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Carrasco-Molina S, Ramos-Ruperto L, Ibáñez-Mendoza P, et al. Cardiac involvement in adult multisystemic inflammatory syndrome related to COVID-19. Two case reports. J Cardiol Cases 26: 24-27, 2022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Aldeghaither S, Qutob R, Assanangkornchai N, et al. Clinical and histopathologic features of myocarditis in multisystem inflammatory syndrome (adult)-associated COVID-19. Crit Care Explor 10: e0630, 2022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Hwang M, Wilson K, Wendt L, et al. The great gut mimicker: a case report of MIS-C and appendicitis clinical presentation overlap in a teenage patient. BMC Pediatr 21: 258, 2021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Hofto ME, Schmit EO, Sharma M, Samuy N. Acute appendicitis associated with multisystem inflammatory syndrome in children. Cureus 13: e15893, 2021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Anderson J, Bhisitkul D, Pham T, Wilson K, Barbera AR. Multisystem inflammatory syndrome presenting as early acute appendicitis. Cureus 13: e20200, 2021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Trevisan M, Amaddeo A, Taddio A, Boscarelli A, Barbi E, Cozzi G. Case report: simil-appendicitis presentation may precede cardiac involvement in MIS-C patient. Front Pediatr 10: 832391, 2022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Martin A, Otto T, Smith T. A case of COVID-19 mimicking acute appendicitis in multi-system inflammatory syndrome. Cureus 13: e15600, 2021. [DOI] [PMC free article] [PubMed] [Google Scholar]