Abstract
Extracellular vesicles (EVs) are nano-sized lipid-bilayer encapsulated vesicles produced by the cells. These EVs are released into the surrounding space by almost all cell types. The EVs help in intercellular communication via their payloads which contain various proteins, lipids, and nucleic acids generated from the donor cells and allow for synergistic responses in surrounding cells. In recent years, EVs have been increasingly important in treating infectious diseases, including respiratory tract infections, urinary tract infections, wound infections, sepsis, and intestinal infections. Studies have confirmed the therapeutic value of mesenchymal stem cell-derived EVs (MSC-EVs) for treating infectious diseases to eliminate the pathogen, modulate the resistance, and restore tissue damage in infectious diseases. This can be achieved by producing antimicrobial substances, inhibiting pathogen multiplication, and activating macrophage phagocytic activity. Pathogen compounds can be diffused by inserting them into EVs produced and secreted by host cells or by secreting them as microbial cells producing EVs carrying signalling molecules and DNA shielding infected pathogens from immune attack. EVs play a key role in infectious pathogenesis and hold great promise for developing innovative treatments. In this review, we discuss the role of MSC-EVs in treating various infectious diseases.
Keywords: Mesenchymal stem cells, Extracellular vesicles, Wound infections, Exosomes, Stem cell therapy
Background
Infectious diseases can cause a variety of health outcomes, ranging from mild illness to significant morbidity and mortality. Infectious diseases are a longstanding and severe public health issue that affects people all over the world. New infectious diseases are arising, and existing ones that were supposed to be under control are restoring strength. The failure of commonly used therapeutic approaches and an increase in the number of severe infectious disease outbreaks have increased the need for alternative therapeutic ways to address infections. Furthermore, it is essential to secure worldwide public health security as the world is approaching an era of extensive antibiotic resistance and the increasing COVID-19 epidemic [1]. Anti-infectious drug development represents a significant advance in the fight against infectious diseases. However, the efficiency of existing anti-infective drugs is fading, as anti-infective agents constantly exert selective pressure on mutations in drug target genes [2]. The effectiveness of current antibiotic treatment is gravely threatened by antibiotic resistance. Developing a unique therapy that does not make resistance worse is crucial. EVs from mesenchymal stem cells (MSCs) are used as an exploratory and promising tool for treating infectious diseases.
EVs are produced by almost all cell types, such as mesenchymal stromal cells, endothelial cells, neurons, B and T cells, dendritic cells, platelets, Schwann cells, and intestinal epithelial cells [3, 4]. These are also found in body fluids like milk, saliva, urine, synovial, and cerebrospinal fluids [5]. Furthermore, the administration route for EVs is quite flexible. EVs can be supplied as intravenous injections. Other delivery methods include direct tissue injections, intraperitoneal injections, and subcutaneous interventions (most of which have been used in studies on tissue repair). Furthermore, EVs are also implanted on scaffolds, and the intratracheal route of administration has been utilised for effective lung diffusion [6].
MSCs are the multipotent population of stromal cells having a wide range of biological functions like multilineage differentiation, anti-inflammatory, immunosuppression, and neuroprotection [7]. MSCs play a vital role in tissue regeneration and homeostasis, making them a promising therapeutic alternative for various diseases [8]. Recent research suggests MSC’s therapeutic effects are due to the released substances. MSCs have a strong paracrine function which is also the key to their therapeutic efficacy. The paracrine effect of MSCs is induced by the release of cytokines, growth factors, and exosomes [9, 10]. The importance of EVs comes from their ability to convey information to target cells, altering the behaviour of the recipient cell transporting protein and nucleic acid payloads such as messenger RNA (mRNA) and micro RNA (miRNA) from one cell to another in a highly selective manner [11]. These EVs are thought to be stable and capable of modulating cellular response in target cells. To enter target cells, EVs use particular receptors. Once recipient cells take up EVs, their biomolecules can control various activities, including gene expression, crucial enzymatic activities, signalling cascades and other mechanisms [12]. MSC-EVs are liable to become an innovative and more effective cell-free therapeutic approach for infectious diseases.
Classification of extracellular vesicles
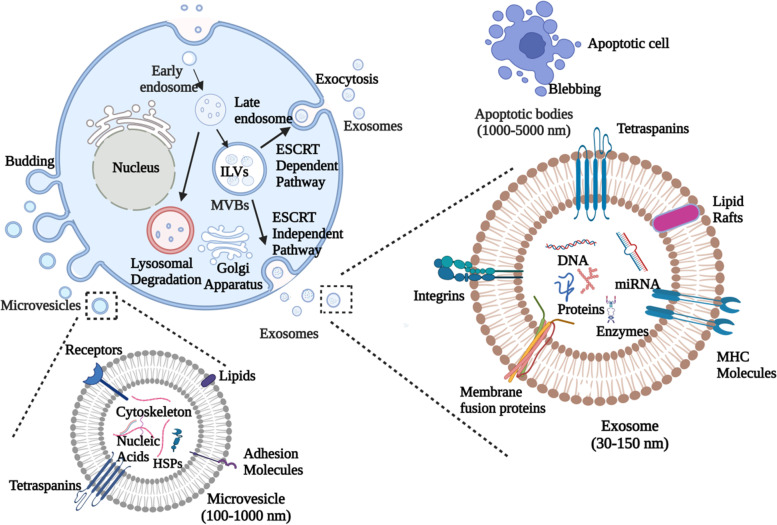
Exosomes, microvesicles, and apoptotic bodies are the three EV subtypes characterised based on their size and biogenesis [13, 14] (Table 1). In all three EV subtypes, a lipid bilayer membrane covers a specific payload of biomolecules, such as proteins, RNA, or cellular waste. Exosomes are EVs with a 30–150 nm diameter generated from multivesicular endosome pathways [4, 28]. Exocytosis of multivesicular bodies (MVBs), the key intermediates in endolysosomal transport, releases exosomes both constitutively and upon stimulation [29]. Ceramide is essential for exosome secretion through the local synthesis of its metabolite sphingosine-1-phosphate (S1P) [30]. Several endosomal sorting complexes required for transport (ESCRT) proteins like Alix, Hrs, and TSG101 have been implicated in lysosome function and exosome release [31–33]. Exosomes can transport mRNA, miRNA, oncogenic receptors, and HIV particles horizontally [34–36].
Table 1.
Classification of EVs
| Conventional classification | As per ISEV guidelines | |||
|---|---|---|---|---|
| Parameters | Exosomes | Microvesicles | Apoptotic bodies | |
| Size | 30–150 nm | 0.1–1 μm | 1–5 μm |
Small EVs/sEVs (< 200 nm diameter) Large EVs/lEVs (> 200 nm diameter) |
| Density | 1.13–1.19 g/ml | Not well defined | 1.16–1.28 g/ml |
Low-density EVs (1.1 to 1.2 g/mL) Medium-density EVs (1.16 g/mL) High-density EVs (1.24–1.28 g/mL) |
| Morphology | Cup shaped | Heterogeneous | Heterogeneous | |
| Origin | Endosomal | Plasma Membrane | Apoptotic cell | |
| Composition | Lipids, proteins, miRNA, mRNA | Lipids, proteins, miRNA, mRNA | Lipids, proteins, DNA, miRNA, mRNA |
CD63+ stained EVs CD81+ stained EVs Annexin V stained EVs |
| Lipids | Cholesterol, ceramide, low phosphatidyl serine exposure, Lyobisphosphatidic acid, Sphingomyelin, | Cholesterol, high phosphatidyl serine exposure | High phosphatidyl serine exposure | |
| Proteins | CD9, CD63, CD81, Annexins, HSPs, Alix, TSG 101, Clathrin, Caveolin, Integrins | CD40, selectins, integrins, flotillins, metalloproteinases | Histones, C3b, thrombospondins | |
| Cells of origin/biogenesis conditions |
Hypoxic EVs Podocyte EVs Apoptotic bodies Large oncosomes |
|||
| References | [15–19] | [16, 18, 20, 21] | [10, 22, 23] | [24–27] |
Microvesicles (MVs) are phospholipid bilayer-encased structures having 100–1000 nm diameter with their size overlapping that of bacteria [13, 37]. The plasma membrane’s controlled release generates them via budding or lipid rafts [38]. The molecular makeup of MVs is still completely unknown, but matrix metalloproteinases (MMPs), glycoproteins, and integrins appear to be abundant in MVs depending on the cell type [39, 40]. MVs, also known as oncosomes, are released by cancer cells. Large oncosomes can be observed in size from 1 to 10 μm [41]. Oncosomes may stimulate organotropic metastatic spread by modulating their target cells’ metabolic and genetic capacities, conferring proteolytic activity and stimulating incursion [42–44]. Apoptotic bodies are the most heterogeneous category of EVs with a wide range of morphology and are generated during apoptosis [45, 46]. They have a diameter of 1–5 μm in the range of platelet size [47]. Apoptosis assures that aged, injured, infected or abnormal cells are selectively removed from normal tissue. Apoptosis is the coordinated disintegration of a cell with cellular debris packed into apoptotic bodies.
The use of the terms described above for the classification of EVs is discouraged due to the significant redundancy among different categories of EVs and the insufficient consensus on precise surface markers. As a result, the International Society for Extracellular Vesicles (ISEV) released the current recommendations in 2018. ISEV recommends the term “extracellular vesicles” as a broad term for nano-sized particles that are normally produced by the cells and are bordered by a lipid bilayer. The current guidelines of ISEV classify the EVs on the basis of (i) size into small EVs (sEVs: diameter of < 200 nm) and medium/large EVs (m/lEVs: diameter > 200 nm) or density with low-density EVs (1.1 to 1.2 g/mL), medium-density EVs (1.16 g/mL), and high-density EVs (1.24–1.28 g/mL); (ii) biological makeup (CD63+ or CD81+ stained EVs, Annexin V stained EVs);and (iii) exosome and microvesicles descriptions should be replaced with explanations of specific biogenesis conditions or cells of origin such as hypoxic EVs, podocyte EVs, apoptotic bodies and large oncosomes [24–27].
Differential ultracentrifugation, filtration, density gradients and immunoaffinity-based isolation procedures are some of the known isolation techniques for EVs [15]. “Ultracentrifugation-linked immunoprecipitation method” is currently the best approach for EV isolation [16, 17]. Furthermore, the different ways for their identification include transmission electron microscope (TEM), nanoparticle tracking analysis (NTA), Western blotting, and recently developed atomic force microscope-infrared spectroscopy (AFM-IR) [18, 19, 24].
The traditional technique for EV isolation makes use of centrifugation to separate particles based on their buoyant density. Following ultracentrifugation, the prepared EV is filtered, and the separated microparticles are chosen based on their size using microfiltration employing filters with different pore sizes. Notably, washing and microfiltration are further EV purification steps that not only improve the EVs’ purity but also reduce their amount. The procedures that combine microfiltration with ultracentrifugation successfully and selectively separate different fractions of microvesicles and exosomes. However, an effective, quick, nearly loss-free and very reproducible approach that enables the isolation of EVs is gel filtration chromatography. The limited yield and relatively high cost of the chromatographic sorbents used in gel chromatography are its drawbacks. The acquired exosomal fraction is also diluted and may need to be concentrated for some applications that follow later. PEG precipitation allows for the simultaneous processing of several samples. This method is most appealing for clinical research because it is easy, quick and affordable, does not distort EVs and needs no extra equipment for isolation, but the sample may be contaminated by various substances like other proteins on a regular basis. To address the issue of protein contamination in the EV fraction, a novel technique for EV isolation utilising a two-phase system with PEG and dextran is provided. In comparison with ultracentrifugation and other kits available, the PEG-dextran solution greatly increases the efficiency of EV isolation, giving the EVs a size and morphology that is similar to that achieved by ultracentrifugation and maintaining the integrity of lipid membranes [20]. There is yet another technique that can be accomplished using affinity-based EV capture using Abs that are specific for a part of the exosome cargo. The clearest illustration of this strategy was provided by Melo and colleagues, who isolated pancreatic cancer-derived exosomes from plasma of a patient and investigated them as prognostic biomarkers of the course and outcome of the disease using immunological capture using Abs-specific for glypican-1 [21]. A better way for isolating EVs should be easy to use, affordable and not require specialised or expensive equipment. It should also be quick and enable isolating EVs from a large sample size. A top objective right now is to develop such EV isolation techniques in order to use them in various clinical and experimental studies.
Biogenesis of extracellular vesicles
Multiple processes may control the biogenesis and release of EVs from the cell. MVs are formed in areas where there is a significant membrane blebbing [22, 23]. Phospholipase D is activated by ADP-ribosylation factor 6 (ARF6), which is accompanied by an extracellular signal-regulated kinase (ERK). The ERK is trafficked to the plasma membrane, subsequently phosphorylating and activating myosin light chain kinase (MLCK). Finally, MLCK phosphorylates the myosin light chain, which causes the MVs to be released [48]. The ESCRT produces MVs and MVBs. ESCRT is divided into four categories (ESCRT 0, I, II, III) and comprises other proteins like Alix, VPS4, and TSG101. The endosomal membrane is being searched for ubiquitinated transmembrane proteins by the ESCRT 0 complex. The membrane is deformed by ESCRT I and ESCRT II to generate buds with sorted payloads. The ESCRT III causes vesicle scission [49, 50]. The blebbing of the plasma membrane is influenced by various factors that affect membrane deformability and folding, including the lipid content and peripheral cytoskeleton organisation, which modify membrane deformability. MVs have been reported to be abundant in the lipids like phosphatidylserine, lysophosphatidylcholines, sphingomyelins and acylcarnitines [48, 51]. Cholesterol, a significant plasma membrane lipid, is expected to play a key role in MVs formation, and the formation of MVs is reduced when it is depleted [52]. Ceramide that facilitates membrane bending has also been found to control the generation of MVs [53]. The bleb must be squeezed-free from the cell once it has been loaded with payloads by a process mediated by acto-myosin contraction. The downstream kinases like Rho-associated coiled-coil containing kinases (ROCK) and extracellular signal-regulated kinases (ERK) have been shown to increase MV formation when RhoA activity is high [54].
Exosomes are formed by the inward budding of the plasma membrane to produce early endosomes [55]. The early endosomal membrane partially invaginates and buds into adjacent lumina with intracellular content to form intraluminal vesicles (ILVs) [56, 57]. Reports suggest that many ILVs are found in late endosomal structures, known as multivesicular bodies (MVBs). These MVBs can adhere to lysosomes for breakdown or bind to the plasma membrane, which releases exosomes into the extracellular space [58] (Fig. 1). MVBs which are rich in cholesterol are assigned to secretion. In contrast, those who are poor in cholesterol are assigned to lysosome breakdown [59]. The formation of ESCRT is required for exosome formation and secretion [60]. The syndecan-syntenin-ALIX pathway allows ESCRT to generate MVBs. Exosome production is aided by syndecan heparan sulphate proteoglycans which interact with syntenin-1 and the ESCRT’s accessory component Alix [61]. Alix, a characteristic exosomal protein linked to multiple ESCRT proteins (TSG101 and CHMP4), has been reported to play a role in the budding and abscission of the endosomal membrane and the selection of exosomal content via interaction with syndecan [62]. There are, however, ESCRT independent mechanisms for the formation of ILVs, such as tetraspanin complex oligomerisation, ceramide production catalysing sphingomyelinase pathway or ILV budding mediated by phospholipase D2 and ARF6 [63–66]. Different mechanisms for producing MVBs and ILVs depend on the cargo sorted within the respective vesicles [67].
Fig. 1.
Biogenesis of extracellular vesicles. Exosomes are generated by budding of early endosomes into intraluminal vesicles (ILVs) that are released upon fusion of multivesicular bodies (MVBs) with plasma membrane. MVBs usually have one of two destinies either lysosomal degradation or their release on fusion with plasma membrane, allowing their contents to be released into the extracellular environment. This release can be either ESCRT dependent or ESCRT independent. Microvesicles are generated by outward budding of the plasma membrane into the extracellular region. Apoptotic bodies are formed by the blebbing of plasma membrane from apoptotic cells
Apoptotic bodies are the products of apoptosis-programmed cell death. Several changes occur in an apoptotic cell, starting with nuclear chromatin condensation followed by membrane blebbing and protrusion formation and finally breakdown of the cellular content into discrete membrane-contained vesicles known as apoptotic bodies [68]. Macrophages phagocytise and locally remove most apoptotic bodies during normal development [69]. This removal is mediated by the interactions between phagocyte recognition receptors and specific changes in the constitution of the membrane of apoptotic cells [70]. During the production of apoptotic bodies, phosphatidylserines are translocated to the outer leaflet of the lipid bilayer. Annexin V, T-cell immunoglobulin mucin 4 (TIM4), or milk fat globule-EGF factor 8, recognised by phagocytes, bind to these translocated phosphatidylserines [71, 72]. The oxidation of surface molecules is another well-studied membrane change which helps create binding sites for thrombospondin (TSP) or the complement protein C3b. The receptors, in turn, recognise TSP and C3b on phagocytes [73].
MSC-EVs in infectious diseases
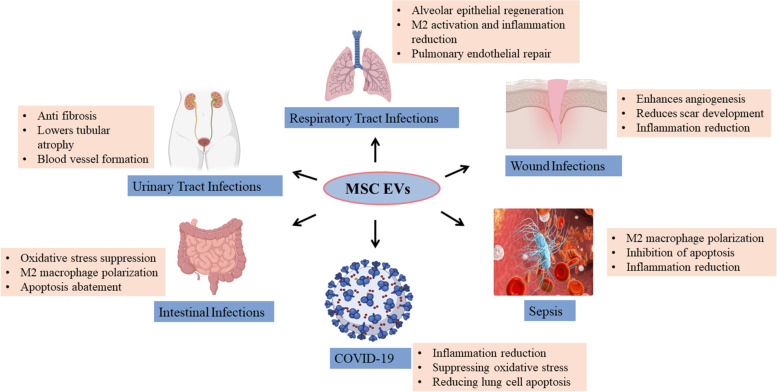
MSC-EVs have several advantages over MSCs in terms of efficacy and safety that they are efficiently circulating and can penetrate biological barriers such as the blood-brain barrier, reduced carcinogenesis, and stable characteristics [74, 75]. MSC have been shown to have regenerative as well as immunomodulatory effect. However, immune rejection can occur in engrafted MSCs, or the target tissue milieu may be unsuitable for them to operate normally. On the other hand, EVs cannot grow or differentiate inside the human body, posing fewer safety problems and ethical and legal issues. The lack of MSC-EVs’ ability to self-replicate lowers the possibility of tumours as well. Furthermore, EVs can maintain high activity levels at low temperatures [76, 77]. MSC-EVs exhibit antibacterial, immunomodulatory, antiapoptotic and antifibrotic properties and can heal injured tissue [78–80]. In comparison with MSCs, MSC-EVs maintain MSC-like biological functions while being more stable and less prone to tumorigenesis, making them an attractive choice for treating different infections [81]. EVs derived from MSCs are used in gene and cell-based treatments as a tailored delivery mechanism for foreign biological and chemical substances [79, 82]. EVs from MSCs are gaining popularity as they minimise several risks associated with MSC-based treatment (Fig. 2).
Fig. 2.
Potential therapeutic role of MSC-EVs in infectious diseases
Respiratory tract infection
The role of EVs in lung diseases has previously been investigated using EVs extracted from bronchial-alveolar lavage fluid (BALF) and lung-derived cells [83, 84]. In several respiratory disorders, EVs have been discovered to play a role. Acute lung injury (ALI) and acute respiratory distress syndrome (ARDS) have been the most thoroughly researched diseases [85]. (ARDS)/(ALI) is a heterogeneous condition marked by extensive endothelial and epithelial damage and a robust inflammatory response [86]. MSC-based therapy has shown promise in pre-clinical models of ALI/ARDS due to its immunomodulation and tissue healing abilities [87]. Since MSCs have low differentiation and engraftment efficacy and a significant tumorigenicity risk, researchers have proposed MSC-EVs as a new cell-free treatment for ALI/ARDS [88]. The significant benefits of MSC-EVs on ALI/ARDS include inflammation reduction, alveolar epithelial regeneration, and improved pulmonary endothelial repair [89].
In a study on bronchopulmonary dysplasia (BPD), including hyperoxia exposure in rat models, intratracheally (IT) administered EVs secreted by MSCs were shown to have an early therapeutic impact. These findings support that IT-administered EVs could prevent/treat BPD by improving defective alveolarisation and pulmonary artery remodelling over time. Anti-inflammatory and proliferative processes may be involved in M2 macrophage polarisation [90, 91]. EVs, specifically MVs, are expected to assist in the recruitment of M1 macrophages to injured epithelial cells, suggesting their role in the beginning and maintaining systemic inflammation in the lung epithelium in case of acute lung injury/inflammation [92, 93]. Intratracheal instillation of MSC-derived EVs decreased extracellular pulmonary water, decreased pulmonary congestion, and reduced pulmonary protein porosity in E. coli endotoxin-induced lung damage [94]. In addition, these MVs lowered macrophage inflammatory protein-2 and neutrophil influx levels in BAL fluid. In an ex vivo perfused human lung, intravenous treatment of MSC MVs dramatically enhanced the rate of alveolar rate clearance, decreased lung protein porosity, and numerically reduced the number of CFU bacteria and inflammation in the wounded alveolus following severe E.coli pneumonia [95]. Inflammatory cytokines, invading leukocytes and the degree of pulmonary congestion, were all reduced due to prophylactic therapy with MSC EVs in rats suffering from traumatic lung injury [96].
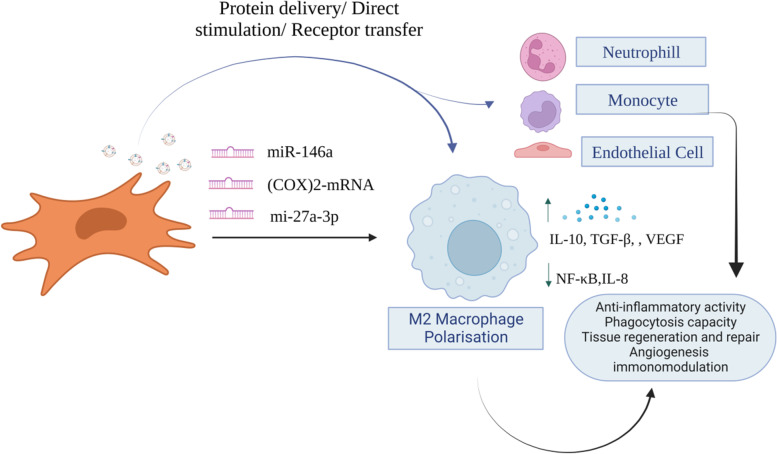
MSC-EVs miRNA, protein, mRNA and mitochondria are important in influencing immune responses and healing ALI lung damage. MSC-EVs have been shown to improve ALI by transferring miR-27a-3p to alveolar macrophages, which inhibits NF-κB transcription and induces M2 activation [97]. MSCs primed with IL-1β produce EVs expressing miR-146a, which generates an M2 macrophage phenotype [98]. These MSC EV-modified alveolar macrophages exhibited increased anti-inflammatory cytokine IL-10 production while decreasing inflammatory cytokines TNF-α and IL-8 release and enhanced phagocytic activity against bacteria [99, 100] (Fig. 3). MSC-EVs reduced lipopolysaccharide (LPS)-induced lung damage by upregulating angiopoietin-1 mRNA [101]. miR-27a-3p was transported from MSC-EVs to alveolar macrophages, regulating macrophage polarisation and reducing acute lung injury [97]. MSC-EVs increased the energy metabolism of acceptor macrophages and decreased silica-induced lung inflammation by transferring mitochondria from MSCs to macrophages [102]. MSC-EVs produce cyclooxygenase (COX)-2 mRNA, an enzyme that triggers the production of prostaglandin E2 [85]. Prostaglandin E2 helps switch phenotypes from pro-inflammatory M1 to anti-inflammatory M2 producing high levels of IL-10 [103, 104]. In pigs with influenza virus-induced ALI, EVs generated from bone marrow MSCs displayed anti-inflammatory and anti-influenza properties. EVs generated from porcine BM-MSCs displayed mesenchymal markers, reduced influenza virus multiplication in vitro and in vivo, and mitigated influenza virus-induced ALI [105]. MSC-EVs communicate with immune cells, causing the synthesis of TGF-β and T-regulatory cells (Tregs) [106]. In influenza virus-infected mice, Tregs increase viral elimination and healing [107, 108].
Fig. 3.
MSC-EVs can produce different kinds of miRNAs causing M2 macrophage polarisation that in turn results in increased cytokine IL-10, VEGF and TGF-β and decreased NF-κB and IL-8 expression. Furthermore, the EVs are internalised into endothelial cells, monocytes, neutrophils and macrophages. As a result of which there is less pro-inflammatory cytokine production, improved phagocytic activity and increased tissue regeneration
COVID-19
The lining of the lung alveoli is composed of a single layer of alveolar type 1 (AT1) and type 2 (AT2) cells. On lung surfaces, ACE2 receptors have been discovered, predominantly on AT2 cells and resident alveolar macrophages [109] SARS-CoV-2 binds with ACE2 receptors expressed on AT2 cells, which are the target cells of SARS-CoV-2. Alveolar cells also express the transmembrane serine protease 2 (TMPRSS2) enzyme, which is important in priming the S (spike) protein of SARS-CoV-2, which in turn facilitates infection of alveolar cells. This increases the production of pro-inflammatory cytokines and chemokines, attracting an increasing number of inflammatory macrophages and circulating immune cells into the sick alveoli, resulting in a cytokine storm (a systemic over-inflammatory condition) [110]. Moreover, cytokine storms influence AT1 and AT2 cells, lowering surfactant production; this increases the alveolar surface tension and collapse and lowers gaseous exchange, ultimately leading to ARDS [111]. ACE2 SARS-CoV-2 receptors generated on MSC-derived EVs decrease the viral infection by competitively inhibiting SARS-CoV-2 binding to alveolar cells, mainly AT2 cells [112] (Fig. 4). A study conducted by Sengupta et al. revealed that inflammatory markers and absolute neutrophilic levels were dramatically decreased. However, total lymphocyte and CD8+ counts significantly increased after 5 days of EV injection in 24 COVID-19 severe pneumonia patients treated with azithromycin and hydroxychloroquine [113].
Fig. 4.
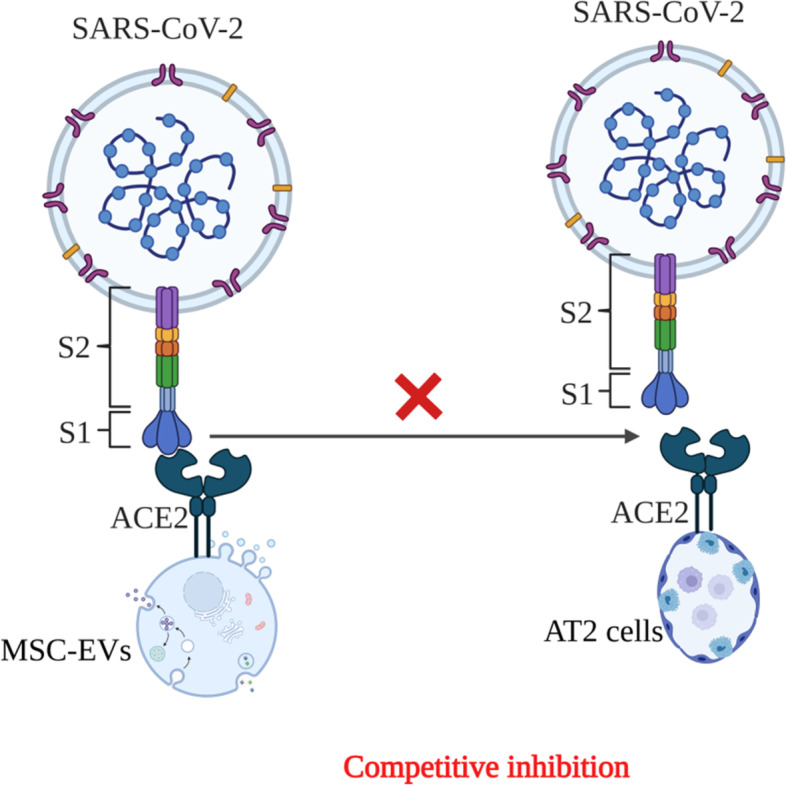
MSC-EVs expressing ACE2 receptor competitively binds to SARS-CoV-2 and prevents the binding of SARS-CoV-2 to AT2 cells
A study by Akbari et al. demonstrated that intravenous injection of MSCs and MSC-EVs showed enormous potential in treatment for COVID-19 patients, with no adverse events during the treatment and follow-up period [114]. MSC-derived EVs exert their therapeutic function in COVID-19 by delivering protective and anti-inflammatory RNAs and proteins to damaged or activated cells in lung tissues. MSC-EVs carry a cargo which consists of different types of microRNAs. It has been reported that miR-124-3p has been involved in suppressing oxidative stress and inflammatory cytokines by binding to its receptor P2X ligand-gated ion channel 7 (P2X7). Another miR-21-5p has been associated with reducing lung cell apoptosis through inhibition of PTEN, and PDCD4-like wise miR-146a participates in transforming macrophages from pro-inflammatory to anti-inflammatory states by suppressing the NF-κB signalling pathway [115]. Hao et al. reported that miR-145 from MSC-EVs increases the phagocytic property of macrophages to enhance the clearance of pathogens at the site of infection [116]. Additionally, MSC-derived EVs increase mitochondrial activity to boost energy production in alveolar cells and increase their repairing capability in the injured lung. A study by Pacienza et al. reported that MSC-EVs reduce lung inflammation by lowering the recruitment of neutrophils and preventing macrophage polarisation via down-regulating macrophage inflammatory protein-2 levels [117].
A non-randomised clinical trial carried out in 2020 evaluated the safety and efficacy of MSC exosomes as treatment for COVID-19. Patients received a single dose of exosomes (ExoFlo™) intravenously. ExoFlo™ showed safety and efficacy with a survival rate of 83%. The ExoFlo™ treatment showed safety, help to restore oxygenation, downregulate cytokine storm and reconstitute immunity, and can be a promising therapy for treatment for COVID-19. Another double-blind, randomised controlled trial (RCT) clinical trial on “Efficacy and Safety of EXOSOME-MSC Therapy to Reduce Hyper-inflammation In Moderate COVID-19 Patients (EXOMSC-COV19)” is currently going on. The EXOSOME-MSC is being tested as adjuvant, as a complementary treatment to standard COVID-19 drugs. It will be injected to participants intravenously twice, in day 1 and day 7 of 14 days of study participation.
Urinary tract infections
The range of infection-related renal disorders is extensive. Acute kidney damage (AKI), glomerulonephritis, tubulointerstitial nephritis, pyelonephritis, and hydronephrosis are all caused by infections through diverse pathways. AKI is among the most common manifestations, which can arise either spontaneously or in the context of earlier chronic kidney disease (CKD). AKI is a rapidly deteriorating renal functioning characterised by elevated blood urea nitrogen and plasma creatinine levels and/or decreased urinary output over hours to days [118, 119]. Most of the patients recovering from AKI have permanent renal impairment and can develop CKD [120]. MSC-EVs stimulate tissue repair by preventing pathophysiological stresses in CKD by addressing fibrosis, lowering tubular atrophy, and swelling and boosting the formation of blood vessels [121, 122]. Furthermore, it has been demonstrated that IGF-1 and IGF-1 receptor mRNA is directly secreted by MSC-EVs into renal tubular epithelial cells (TECs) along with IGF-1 receptors to stimulate kidney healing in AKI [123]. This is thought to happen due to IGF-1-induced stimulation of the Akt signalling pathway [124]. Inhibiting Sema3A significantly activated the Akt and ERK pathways, resulting in cell proliferation and resistance to AKI [125]. MSC-EVs carry other growth factors besides IGF-1 to protect the milieu of affected cells. FGF-2 has an antifibrotic impact, and knocking down FGF-2 stops tissue regeneration and promotes a fibrotic reaction [126]. ADMSC-EVs were reported to have antifibrotic characteristics resulting in reduced renal fibrosis [127, 128].
Wound infections
Wound healing has a complicated mechanism consisting of overlapping processes like haemostasis, inflammation, proliferation, and remodelling [129]. This necessitates intercellular interaction between resident and immune cells through extracellular matrix [130]. EV from MSCs has been found to possess cytokines, chemokines, chemokine receptors, and other immune cell chemoattractant molecules. As a result, the EV may stimulate a more robust defence against infections in injured tissues [131, 132]. Human umbilical vein endothelial cells (HUVEC) were treated with ADMSC-EVs in vitro. Endothelial cells swallowed the EVs, enhancing proliferation, migration, and angiogenesis [133]. These processes were also enhanced by human umbilical cord MSC.
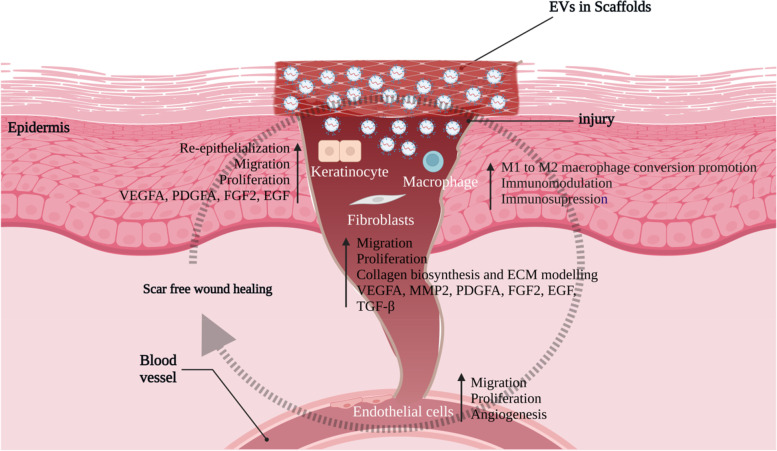
Furthermore, they enhanced angiogenic capacity in a rodent model of second-degree skin burns [134]. MSC-EVs can reduce the deposition of fibroblast collagen, transdifferentiation of fibroblasts to myofibroblasts, and excessive development of scars [135]. MSC-derived EVs assist the healing of diabetic foot ulcers (DFUs) by delivering bioactive molecules. In addition, as a vehicle for non-bioactive compounds such as antibiotics, it can limit bacterial activity and speed up wound healing in bacteria-associated DFUs [136]. DFUs and wounds can be treated effectively with optimised MSC-derived exosomes. Exosomes from MSCs pretreated with salidroside exhibited regeneration of diabetic wounds [137]. According to prior research, ADMSC exosomes integrated with a hydrogel exhibit healing, antimicrobial, and exosome releasing properties [138] (Fig. 5). Exosomes produced from TSG-6-enhanced MSCs inhibited the development of scars by lowering inflammation and preventing collagen deposition [139].
Fig. 5.
Wound healing is enhanced by the local application of scaffolds carrying MSC-EVs. These MSC-EVs enhance the effects of different cell types that are involved in wound healing. These cells include fibroblasts, keratinocytes, endothelial cells and immune cells
Intestinal infections
Intestinal tract homeostasis is crucial owing to the direct exposure to the digestive residue, foreign antigens and millions of pathogens. The intestinal mucosal barrier plays a pivotal role in detecting, clearing the microbial debris, and maintaining a peaceful coexistence. The intestinal defence system consists of the intestinal epithelial cells (IECs), mucus layer and other innate immune system cells.
EVs participate in vascular and epithelial barrier function in wound healing and inflamed intestines. Inflammatory bowel disease (IBD) is a group of persistent, emerging inflammatory diseases of the intestine that are marked by recurring abdominal discomfort, prolonged diarrhoea, and bloody faeces with mucus and pus. There has been a sharp increase in the abundance of Bacteroidetes and Proteobacteria, primarily E.coli in IBD [140]. The severity of colitis was reduced after intravenous administration of bone marrow MSC-EVs, as demonstrated by a reduction in the disease activity index (DAI) and colon damage. The study provided evidence for BMSC-EV’s prospective therapeutic benefits in TNBS-induced colitis, such as inflammatory regulation, oxidative stress suppression, and apoptosis abatement [141]. IBD affects around 3.5 million people worldwide, with its aetiology and pathogenesis still unclear [142]. Several studies of IBD patients report dysbiosis of the gut microbiota with abnormal metabolism of IECs due to a fluctuating abundance of cargo in EVs and MVs [143]. In an active IBD intestinal microenvironment, an increase in the recruitment of innate immune cells such as dendritic cells, neutrophils, monocytes, macrophages, and T cells occurs. As a result, EVs are tightly associated with macrophages in IBD. Additionally, EVs have been reported to have immunosuppressive effects, such as suppress the DCs activation to induce immune tolerance and contributing in Treg activation [144], which will eventually secrete exosomes targeting pro-apoptotic caspase-12 alleviating IBD in mice [145]. In IBD, IECs secrete epithelial cell adhesion molecule-dependent EVs with high TGF-β1 levels, thus maintaining the intestinal tract immune balance and decreasing the IBD severity [146].
Administration of EVs derived from umbilical cord-MSCs has alleviated colitis in mice [147]. In a recent study, human adipose mesenchymal stem cell-derived exosomes (hADSC-Exo) stimulate the proliferation and regeneration of Lgr5-ISCs and epithelial cells and ameliorate TNF-α induced inflammatory damaged mice colon organoids. hADSC-Exos are a potential treatment for IBD as they protect intestine integrity and activate the intestine epithelial cells [148]. Another study reported the MSC-EV-based alleviation of colitis by inducing suppression of colon macrophages. Treatment with MSC-EVs significantly reduced activation of IL-7 and iNOS-signalling pathways in colon macrophages, ultimately resulting in attenuated production of IL-1β, IL-6, TNF-α, and increased secretion of IL-10, leading to colitis alleviation [147]. To examine the effects and possible mechanism of EVs derived from bone marrow MSCs in ulcerative colitis (UC) treatment, an in vitro model of LPS-treated macrophages, and an in vivo dextran sulphate sodium (DSS)-induced mouse model were established to impersonate UC. EVs promote proliferation and dampen the inflammatory response in LPS-induced macrophages. In the in vivo model, administered EV ameliorated the UC symptoms by ameliorating colon mucosa damage and severity, disease activity index, and weight loss while increasing colon length. EVs from bone marrow MSCs attenuated ulcerative colitis by endorsing M2 macrophage polarisation [149]. EVs derived from human placental MSCs have also been utilised to treat colitis, which markedly reduced intestinal inflammation and oxidative stress by dampening the activity of myeloperoxidase (MPO) and reactive oxygen species (ROS) [150] (Fig. 6).
Fig. 6.
MSC-EVs have the ability to deliver antioxidant enzymes like superoxide dismutase (SOD), glutathione S-transferase (GST) and catalase (CAT) directly into the target cells. These anti-oxidant enzymes effectively reduce oxidative stress and inflammation in intestines
Sepsis
Sepsis is a severe organ dysfunction due to a deregulated host response to infection. Presently, sepsis incidence is approximately 270 cases per 100,000 persons per year, with an approximate 26% mortality rate [151]. Sepsis usually involves an imbalance of the anti- and pro-inflammatory components of the immune system, characterised by immune system hyperactivation followed by an aggravated anti-inflammatory state, ultimately leading to immunosuppression [152]. It is accompanied by coagulation and haemodynamic alterations and cellular injuries leading to the development of multiple organ dysfunction (MOD). EVs are released from cells upon activation and apoptosis and will express membrane epitopes which are specific to their parental cells. In sepsis models of mice and patients, high numbers of EVs in the blood are reported compared to healthy individuals by flow cytometry and NTA techniques [153]. All immune cells, endothelial cells, RBCs, and platelets are reported as sources of EVs in the blood in both disease and healthy states. Septic EVs contribute to the spread of inflammation and exert pro- and anti-inflammatory characteristics. A massive cytokine storm characterises early-phase sepsis in the circulation wherein EVs are identified as carriers of chemokines, cytokines, and growth factors [154, 155]. During systemic inflammatory conditions, released EVs contain DAMPs, including heat shock proteins (HSPs), histones, and high-motility group box-1 (HMGB1) [156, 157]. C-reactive protein (CRP), an acute phase protein, is another pro-inflammatory protein in septic EVs. Hepatocytes primarily secrete CRP in response to systemic inflammatory conditions and tissue damage. Septic EVs have also been reported to contain differentially expressed miRNAs associated with inflammatory pathways. EVs can influence clinical outcomes as well as predict survival. However, beneficial and detrimental roles are associated with sepsis depending on the cellular source and the disease phase during which the EVs are being studied.
In animal models, MSC-EVs have been proven to improve the outcome of sepsis. miRNAs from MSC-EVs vigorously exert their role in sepsis. miR-21 in MSC-EV was upregulated in IL-1β-stimulated MSCs inducing M2 polarisation of macrophages in vitro and in vivo sepsis [158]. miRNA-146a was also upregulated in MSC-EVs primed with IL-1β, which effectively induces M2 polarisation by altering TRAF6, IRF5 and IRAK1 signalling [98]. These studies support the notion of pretreating MSCs with pro-inflammatory cytokines, eventually augmenting the immunomodulatory function of MSCs.
MSC-Exo therapy may complement as adjuvant therapy in sepsis-induced acute kidney, liver, and cardiovascular system injuries. The capability of MSC-Exos has been validated to rescue the renal function in sepsis [159]. Kidney morphology was found to be more intact after MSC-Exo intervention in caecal ligation and puncture (CLP)-induced sepsis mice. A reduced inflammatory cell infiltration, decreased kidney interstitial oedema, and higher integrity of brush borders were observed in HE staining kidney tissues [160]. Renal function was also restored, which was confirmed by a blood test. MSC-Exos have been reported to show hepatic protection in acute liver injury demonstrated by improved hepatic function indicators, a lower degree of hepatocellular necrosis and inflammation [161]. The therapeutic effects of MSC-Exos lie in innate immune system modulation. MSC-Exos participate in hepatocyte haemostasis maintenance by inhibiting cell apoptosis. BMSC-Exos reportedly reduce apoptosis of hepatocytes by increasing levels of autophagy marker proteins, an increasing number of autophagosomes and microtubule-associated protein 1A/1B-light chain 3, Beclin-1 [162]. MSC-Exos are reported to suppress inflammation and maintain calcium homeostasis under septic conditions. Wang et al. (2015) injected MSC-Exos intravenously in septic mice. It resulted in overall mice survival by inhibiting cardiomyocytes’ death and attenuating excess inflammation via miR-233. MSC-Exos represent an efficacious cell-free therapeutic modality for sepsis treatment depending on its anti-inflammatory and anti-apoptotic activities [163].
Pharmacokinetics of EVs
It is essential to understand the pharmacokinetics of EVs in order to deliver therapeutic molecules to precise sites in the body. The duration during which large concentrations of EVs are kept in the bloodstream after intravenous injection is significant. EVs generally concentrate in the organs of reticuloendothelial system (RES), also called as mononuclear phagocyte system (MPS) [164]. The best way to enhance EV pharmacokinetics is to prevent MPS accumulation. The MPS captures nearly all of the EVs when they are systemically delivered. The quick removal of EVs from the bloodstream is caused by MPS macrophages. Additionally, since macrophages have phosphatidyl serine (PS) binding receptor molecules, the negatively charged surface of EVs, which is derived from the similarly negatively charged PS, is what drives the majority of macrophage uptake [165, 166].
The pharmacokinetics of EVs is also greatly influenced by their source. The reason behind this is that every EV has a unique makeup. EVs from various cell types demonstrated somewhat varied biodistribution. The pharmacokinetics is greatly impacted by the mode of administration. Intravenous injection of EVs causes fast removal from blood and build-up in MPS-related tissues, as was previously stated. The novel method for delivering drugs into the brain is through intranasal administration. Oral administration, which can use EVs obtained from food, is a promising approach as it is minimally intrusive, yet it is still unknown if EVs can enter the bloodstream by intestinal absorption while retaining their integrity [167, 168].
Another useful strategy for avoiding MPS capture may be PEGylation. PEG is a hydrophilic polymer that, when coupled to nanoparticles, forms chains that cover the exterior of these nanoparticles. The contact between nanoparticles and proteins or cells can be diminished, and the half-life can be extended owing to the steric hindrance influence of PEG chains. Such methods can be used to enhance the pharmacokinetics of EVs [164]. Matsumoto et al. revealed that injecting a liposome containing PS or phosphatidylglycerol prior to systemic administration increased EVs in blood circulation [166]. These methods are useful for enhancing the pharmacokinetics of EVs and enabling tailored distribution to particular cells or organs.
Toxicity and tumorigenicity tests
MSC-EVs have already undergone numerous preclinical and clinical trials. MSCEV tolerance has been documented in a large number of studies; however, security-related concerns have received far less attention. However, a study was conducted on the toxicity of MSC-EVs. Hyun et al. performed toxicity assays like in vitro photosensitisation, local lymph node assay, acute oral toxicity, and eye and skin irritation. Their results suggested that the MSCEVs are risk-free and have no side effects. The ability of MSC-EVs to shield cells from UV irradiation damage was demonstrated [169]. EVs produced from wild-type and modified HEK293T cells (non-MSC-EVs) did not cause any toxicity or a significant immunological reaction in immune-competent C57BL/6 mice [170]. To examine the safety of hucMSC exosomes in vivo, rats with acute myocardial infarction were injected intravenously with the exosomes. In addition to protecting against weight reduction, hucMSC exosomes had no negative impacts on liver or kidney function [171]. In vitro toxicological evaluations were used to determine the safety of EVs derived from MSCs or bovine milk. Neither the alkaline comet assay nor the micronucleus assay revealed any genotoxic effects from either MSC-EVs or bovine milk-derived EVs [172].
Exosomes from colorectal cancer stem cells increased the lifespan of bone marrow-derived neutrophils and caused them to have a protumoural character. Interleukin-1 expression was increased by tumour exosomal triphosphate RNAs via a pattern recognition-NF-κB signalling axis to maintain survival of neutrophils [173]. Exosomes derived from melanoma that carry oncogenic molecular reprogramming cause naive MSCs to turn into melanoma-like cells that overexpress programmed cell death protein 1(mMSCPD-1+). In vivo, oncogenic factors are expressed, and tumour growth is induced by exosomes and mMSCPD-1+ cells [174]. A study by Gu et al. revealed that MSC-EVs enhanced the proliferation and metastatic properties of gastric cancer cells ex vivo. The epithelial-mesenchymal transition was reported to be induced by MSC-EVs, which improved the migration and proliferation of HGC 27 cells. The tumorigenicity of gastric cancer cells was also increased by MSC-EVs [175].
Conclusion
MSC-EVs have a potential role in therapeutic regimens for infectious diseases, including intestinal infections, respiratory infections, and sepsis in recent years. The therapeutic mechanisms include immunomodulation, direct antimicrobial effects, and tissue repair. MSC-EVs reportedly exert their effect through the transfer of mRNAs, proteins, and miRNAs. EVs secreted by MSCs have emerged as a cell-free alternative to MSC-based therapies owing to their low risk of immunogenicity, tumorigenicity, and higher safety. Even though EV-based therapies are more attractive in terms of safety, specific concerns should be addressed for their clinical use. There is a wide variation and a lack of standardisation procedures regarding MSC expansion or EV purification methods. Protocol standardisation and potency tests are crucial for clinical grade exosomes or EV preparations. However, further investigations are required to fully elucidate the immunomodulation mechanism and the EV cargo responsible for the cellular level alterations specific to clinical conditions. Furthermore, toxicity profiling, which may include evaluations of immunological responses, need to be carried out on all EVs preparations.
Acknowledgements
The diagrams were made in biorender.com.
Code availability
Not applicable
Abbreviations
- EVs
Extracellular vesicles
- MSCs
Mesenchymal stem cells
- MSC-EVs
Mesenchymal stem cell-derived extracellular vesicles
- mRNA
Messenger RNA
- miRNA
Micro RNA
- MVBs
Multivesicular bodies
- S1P
Sphingosine-1-phosphate
- ESCRT
Endosomal sorting complex required for transport
- Alix
ALG-2-interacting protein X
- HRS
Hepatocyte growth factor-regulated tyrosine kinase substrate
- TSG-101
Tumor susceptibility gene 101
- HIV
Human immunodeficiency virus
- MVs
Microvesicles
- MMPs
Matrix metalloproteinases
- ISEV
International Society for Extracellular Vesicles
- sEVs
Small EV
- m/lEVs
Medium/large EVs
- TEM
Transmission electron microscope
- NTA
Nanoparticle tracking analysis
- AFM-IR
Atomic force microscope-infrared spectroscopy
- ARF6
ADP-ribosylation factor 6
- ERK
Extracellular signal regulated kinase
- MLCK
Myosin light chain kinase
- VPS4
Vacuolar protein sorting-associated protein
- ROCK
Rho-associated coiled-coil containing kinases
- ILVs
Intraluminal vesicles
- CHMP4
Charged multivesicular body protein
- TIM4
T-cell immunoglobulin mucin 4
- EGF
Epithelial growth factor
- TSP
Thrombospondin
- MHC
Major histocompatibility complex
- BALF
Bronchial-alveolar lavage fluid
- ALI
Acute lung injury
- ARDS
Acute respiratory distress syndrome
- BPD
Bronchopulmonary dysplasia
- IT
Intratracheally
- LPS
Lipopolysaccharide
- COX
Cyclooxygenase
- TGF-β
Transforming growth factor
- Tregs
T-regulatory cells
- AT1
Alveolar type 1 cell
- AT2
Alveolar type 2 cell
- ACE2 receptor
Angiotensin-converting enzyme 2 receptor
- TMPRSS2
Transmembrane serine protease 2
- P2X7
P2X ligand-gated ion channel 7
- AKI
Acute kidney injury
- CKD
Chronic kidney disease
- IGF
Insulin-like growth factor
- TECs
Tubular epithelial cells
- ADMSCs
Adipose-derived mesenchymal stem cells
- HUVEC
Human umbilical vein endothelial cells
- DFUs
Diabetic foot ulcers
- IECs
Intestinal epithelial cells
- GI
Gastrointestinal
- MOD
Multiple organ dysfunction
- DAMPs
Damage-associated molecular patterns
- HSPs
Heat shock proteins
- HMGB1
Histones and high-motility group box-1
- CRP
C-reactive protein
- TRAF6
Tumour necrosis factor receptor (TNFR)-associated factor 6
- IRF
Interferon regulatory factor
- IRAK1
Interleukin-1 receptor-associated kinase 1
- TLR
Toll-like receptors
- HAP
Hemagglutinin protease
- IBD
Inflammatory bowel disease
- DCs
Dendritic cells
- hADSC-Exo
Human adipose mesenchymal stem cell-derived exosomes
- UC
Ulcerative colitis
- DSS
Dextran sulphate sodium
- MPO
Myeloperoxidase
- TNBS
2,4,6-Trinitrobenzene sulfonic acid
- ROS
Reactive oxygen species
- SOD
Superoxide dismutase
- CAT
Catalase
- GST
Glutathione-s-transferase
- CLP
Cecal ligation and puncture
- RES
Reticuloendothelial system
- MPS
Mononuclear phagocyte system
Authors’ contributions
TM and AS wrote and edited the manuscript. SMA and PAS conceived and finalised the review. SS NF, LAD, SI, MBG, and JN helped in the writing and collection of information. The authors read and approved the final manuscript.
Funding
No funding was available
Availability of data and materials
Not applicable
Declarations
Ethics approval and consent to participate
None
Consent for publication
Not applicable
Competing interests
The authors declare that they have no competing interests.
Footnotes
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Contributor Information
Tasaduq Manzoor, Email: tasaduqmanzoor88@gmail.com.
Afnan Saleem, Email: afnankhan1082@gmail.com.
Nida Farooq, Email: farooquinida.as@gmail.com.
Lateef Ahmad Dar, Email: darlateef116@gmail.com.
Junaid Nazir, Email: junaidnaxir@gmail.com.
Sahar Saleem, Email: saleem.sehar@gmail.com.
Sameena Ismail, Email: sameena17@gmail.com.
Mudasir Bashir Gugjoo, Email: mbgugjoo@gmail.com.
Parvaiz A. Shiekh, Email: sparvaiz@cbme.iitd.ac.in
Syed Mudasir Ahmad, Email: mudasirbio@gmail.com.
References
- 1.Van Puyvelde S, Deborggraeve S, Jacobs J. Why the antibiotic resistance crisis requires a One Health approach. Lancet Infect Dis. 2018;18:132–134. doi: 10.1016/S1473-3099(17)30704-1. [DOI] [PubMed] [Google Scholar]
- 2.Holmes AH, Moore LSP, Sundsfjord A, Steinbakk M, Regmi S, Karkey A, et al. Understanding the mechanisms and drivers of antimicrobial resistance. Lancet. 2016;387:176–187. doi: 10.1016/S0140-6736(15)00473-0. [DOI] [PubMed] [Google Scholar]
- 3.Raposo G, Stoorvogel W. Extracellular vesicles: exosomes, microvesicles, and friends. J Cell Biol. 2013;200:373–383. doi: 10.1083/jcb.201211138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Yáñez-Mó M, Siljander PR-M, Andreu Z, Bedina Zavec A, Borràs FE, Buzas EI, et al. Biological properties of extracellular vesicles and their physiological functions. J Extracell Vesicles. 2015;4:27066. doi: 10.3402/jev.v4.27066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Kalra H, Drummen GPC, Mathivanan S. Focus on extracellular vesicles: introducing the next small big thing. Int J Mol Sci. 2016;17:170. doi: 10.3390/ijms17020170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Tieu A, Lalu MM, Slobodian M, Gnyra C, Fergusson DA, Montroy J, et al. An analysis of mesenchymal stem cell-derived extracellular vesicles for preclinical use. ACS Nano. 2020;14:9728–9743. doi: 10.1021/acsnano.0c01363. [DOI] [PubMed] [Google Scholar]
- 7.Parekkadan B, Milwid JM. Mesenchymal stem cells as therapeutics. Annu Rev Biomed Eng. 2010;12:87. doi: 10.1146/annurev-bioeng-070909-105309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Yudintceva NM, Bogolyubova IO, Muraviov AN, Sheykhov MG, Vinogradova TI, Sokolovich EG, et al. Application of the allogenic mesenchymal stem cells in the therapy of the bladder tuberculosis. J Tissue Eng Regen Med. 2018;12:e1580–e1593. doi: 10.1002/term.2583. [DOI] [PubMed] [Google Scholar]
- 9.Paliwal S, Chaudhuri R, Agrawal A, Mohanty S. Regenerative abilities of mesenchymal stem cells through mitochondrial transfer. J Biomed Sci. 2018;25:1–12. doi: 10.1186/s12929-018-0429-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Liang Y, Duan L, Lu J, Xia J. Engineering exosomes for targeted drug delivery. Theranostics. 2021;11:3183. doi: 10.7150/thno.52570. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Yin K, Wang S, Zhao RC. Exosomes from mesenchymal stem/stromal cells: a new therapeutic paradigm. Biomark Res. 2019;7:1–8. doi: 10.1186/s40364-019-0159-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Nguyen TH, Duong CM, Nguyen X-H, Than UTT. Mesenchymal stem cell-derived extracellular vesicles for osteoarthritis treatment: extracellular matrix protection, chondrocyte and osteocyte physiology, pain and inflammation management. Cells. 2021;10:2887. doi: 10.3390/cells10112887. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.György B, Szabó TG, Pásztói M, Pál Z, Misják P, Aradi B, et al. Membrane vesicles, current state-of-the-art: emerging role of extracellular vesicles. Cell Mol Life Sci. 2011;68:2667–2688. doi: 10.1007/s00018-011-0689-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Merchant ML, Rood IM, Deegens JKJ, Klein JB. Isolation and characterization of urinary extracellular vesicles: implications for biomarker discovery. Nat Rev Nephrol. 2017;13:731–749. doi: 10.1038/nrneph.2017.148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Zhang Y, Bi J, Huang J, Tang Y, Du S, Li P. Exosome: a review of its classification, isolation techniques, storage, diagnostic and targeted therapy applications. Int J Nanomed. 2020;15:6917. doi: 10.2147/IJN.S264498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Tengattini S. Chromatographic approaches for purification and analytical characterization of extracellular vesicles: recent advancements. Chromatographia. 2019;82:415–424. doi: 10.1007/s10337-018-3637-7. [DOI] [Google Scholar]
- 17.Melling GE, Carollo E, Conlon R, Simpson JC, Carter DRF. The challenges and possibilities of extracellular vesicles as therapeutic vehicles. Eur J Pharm Biopharm. 2019;144:50–56. doi: 10.1016/j.ejpb.2019.08.009. [DOI] [PubMed] [Google Scholar]
- 18.Tkach M, Théry C. Communication by extracellular vesicles: where we are and where we need to go. Cell. 2016;164:1226–1232. doi: 10.1016/j.cell.2016.01.043. [DOI] [PubMed] [Google Scholar]
- 19.Kim SY, Khanal D, Kalionis B, Chrzanowski W. High-fidelity probing of the structure and heterogeneity of extracellular vesicles by resonance-enhanced atomic force microscopy infrared spectroscopy. Nat Protoc. 2019;14:576–593. doi: 10.1038/s41596-018-0109-3. [DOI] [PubMed] [Google Scholar]
- 20.Konoshenko MY, Lekchnov EA, Vlassov AV, Laktionov PP. Isolation of extracellular vesicles: general methodologies and latest trends. Biomed Res Int. 2018;1-27. [DOI] [PMC free article] [PubMed]
- 21.Melo SA, Luecke LB, Kahlert C, Fernandez AF, Gammon ST, Kaye J, et al. Glypican-1 identifies cancer exosomes and detects early pancreatic cancer. Nature. 2015;523:177–182. doi: 10.1038/nature14581. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Di Vizio D, Morello M, Dudley AC, Schow PW, Adam RM, Morley S, et al. Large oncosomes in human prostate cancer tissues and in the circulation of mice with metastatic disease. Am J Pathol. 2012;181:1573–1584. doi: 10.1016/j.ajpath.2012.07.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.D’Souza-Schorey C, Clancy JW. Tumor-derived microvesicles: shedding light on novel microenvironment modulators and prospective cancer biomarkers. Genes Dev. 2012;26:1287–1299. doi: 10.1101/gad.192351.112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Théry C, Witwer KW, Aikawa E, Alcaraz MJ, Anderson JD, Andriantsitohaina R, et al. Minimal information for studies of extracellular vesicles 2018 (MISEV2018): a position statement of the International Society for Extracellular Vesicles and update of the MISEV2014 guidelines. J Extracell Vesicles. 2018;7:1535750. doi: 10.1080/20013078.2018.1535750. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Lötvall J, Hill AF, Hochberg F, Buzás EI, Di Vizio D, Gardiner C, et al. Minimal experimental requirements for definition of extracellular vesicles and their functions: a position statement from the International Society for Extracellular Vesicles. J. Extracell Vesicles. 2014;3(1):26913. [DOI] [PMC free article] [PubMed]
- 26.Witwer KW, Buzás EI, Bemis LT, Bora A, Lässer C, Lötvall J, et al. Standardization of sample collection, isolation and analysis methods in extracellular vesicle research. J Extracell Vesicles. 2013;2:20360. doi: 10.3402/jev.v2i0.20360. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Soekmadji C, Hill AF, Wauben MH, Buzás EI, Di Vizio D, Gardiner C, et al. Towards mechanisms and standardization in extracellular vesicle and extracellular RNA studies: results of a worldwide survey. J Extracell Vesicles. 2018;7:1535745. doi: 10.1080/20013078.2018.1535745. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Gurunathan S, Kang M-H, Jeyaraj M, Qasim M, Kim J-H. Review of the isolation, characterization, biological function, and multifarious therapeutic approaches of exosomes. Cells. 2019;8:307. doi: 10.3390/cells8040307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Théry C, Ostrowski M, Segura E. Membrane vesicles as conveyors of immune responses. Nat Rev Immunol. 2009;9:581–593. doi: 10.1038/nri2567. [DOI] [PubMed] [Google Scholar]
- 30.Kajimoto T, Okada T, Miya S, Zhang L, Nakamura S. Ongoing activation of sphingosine 1-phosphate receptors mediates maturation of exosomal multivesicular endosomes. Nat Commun. 2013;4:1–13. doi: 10.1038/ncomms3712. [DOI] [PubMed] [Google Scholar]
- 31.Eitan E, Suire C, Zhang S, Mattson MP. Impact of lysosome status on extracellular vesicle content and release. Ageing Res Rev. 2016;32:65–74. doi: 10.1016/j.arr.2016.05.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Rusten TE, Simonsen A. ESCRT functions in autophagy and associated disease. Cell Cycle. 2008;7:1166–1172. doi: 10.4161/cc.7.9.5784. [DOI] [PubMed] [Google Scholar]
- 33.Colombo M, Raposo G, Théry C. Biogenesis, secretion, and intercellular interactions of exosomes and other extracellular vesicles. Annu Rev Cell Dev Biol. 2014;30:255–289. doi: 10.1146/annurev-cellbio-101512-122326. [DOI] [PubMed] [Google Scholar]
- 34.Ding G, Zhou L, Qian Y, Fu M, Chen J, Chen J, et al. Pancreatic cancer-derived exosomes transfer miRNAs to dendritic cells and inhibit RFXAP expression via miR-212-3p. Oncotarget. 2015;6:29877. doi: 10.18632/oncotarget.4924. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Saleem SN, Abdel-Mageed AB. Tumor-derived exosomes in oncogenic reprogramming and cancer progression. Cell Mol Life Sci. 2015;72:1–10. doi: 10.1007/s00018-014-1710-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Izquierdo-Useros N, Naranjo-Gómez M, Archer J, Hatch SC, Erkizia I, Blanco J, et al. Capture and transfer of HIV-1 particles by mature dendritic cells converges with the exosome-dissemination pathway. Blood. 2009;113:2732–2741. doi: 10.1182/blood-2008-05-158642. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.György B, Módos K, Pállinger E, Pálóczi K, Pásztói M, Misják P, et al. Detection and isolation of cell-derived microparticles are compromised by protein complexes resulting from shared biophysical parameters. Blood. 2011;117:e39–e48. doi: 10.1182/blood-2010-09-307595. [DOI] [PubMed] [Google Scholar]
- 38.Feng R, Ullah M, Chen K, Ali Q, Lin Y, Sun Z. Stem cell-derived extracellular vesicles mitigate ageing-associated arterial stiffness and hypertension. J Extracell Vesicles. 2020;9:1783869. doi: 10.1080/20013078.2020.1783869. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.de Lizarrondo SM, Roncal C, Calvayrac O, Rodríguez C, Varo N, Purroy A, et al. Synergistic effect of thrombin and CD40 ligand on endothelial matrix metalloproteinase-10 expression and microparticle generation in vitro and in vivo. Arterioscler Thromb Vasc Biol. 2012;32:1477–1487. doi: 10.1161/ATVBAHA.112.248773. [DOI] [PubMed] [Google Scholar]
- 40.Mezouar S, Darbousset R, Dignat-George F, Panicot-Dubois L, Dubois C. Inhibition of platelet activation prevents the P-selectin and integrin-dependent accumulation of cancer cell microparticles and reduces tumor growth and metastasis in vivo. Int J Cancer. 2015;136:462–475. doi: 10.1002/ijc.28997. [DOI] [PubMed] [Google Scholar]
- 41.Meehan B, Rak J, Di Vizio D. Oncosomes–large and small: what are they, where they came from? J Extracell Vesicles. 2016;5:33109. doi: 10.3402/jev.v5.33109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Ciardiello C, Leone A, Lanuti P, Roca MS, Moccia T, Minciacchi VR, et al. Large oncosomes overexpressing integrin alpha-V promote prostate cancer adhesion and invasion via AKT activation. J Exp Clin Cancer Res. 2019;38:1–16. doi: 10.1186/s13046-019-1317-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Cufaro MC, Pieragostino D, Lanuti P, Rossi C, Cicalini I, Federici L, et al. Extracellular vesicles and their potential use in monitoring cancer progression and therapy: the contribution of proteomics. J Oncol. 2019;1639854. [DOI] [PMC free article] [PubMed]
- 44.Minciacchi VR, You S, Spinelli C, Morley S, Zandian M, Aspuria P-J, et al. Large oncosomes contain distinct protein cargo and represent a separate functional class of tumor-derived extracellular vesicles. Oncotarget. 2015;6:11327. doi: 10.18632/oncotarget.3598. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Simpson RJ, Mathivanan S. Extracellular microvesicles: the need for internationally recognised nomenclature and stringent purification criteria. OMICS Group.; 2012. [Google Scholar]
- 46.Taylor RC, Cullen SP, Martin SJ. Apoptosis: controlled demolition at the cellular level. Nat Rev Mol cell Biol. 2008;9:231–241. doi: 10.1038/nrm2312. [DOI] [PubMed] [Google Scholar]
- 47.Barros FM, Carneiro F, Machado JC, Melo SA. Exosomes and immune response in cancer: friends or foes? Front Immunol. 2018;9:730. doi: 10.3389/fimmu.2018.00730. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Muralidharan-Chari V, Clancy JW, Sedgwick A, D’Souza-Schorey C. Microvesicles: mediators of extracellular communication during cancer progression. J Cell Sci. 2010;123:1603–1611. doi: 10.1242/jcs.064386. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Hanson PI, Cashikar A. Multivesicular body morphogenesis. Annu Rev Cell Dev Biol. 2012;28:337–362. doi: 10.1146/annurev-cellbio-092910-154152. [DOI] [PubMed] [Google Scholar]
- 50.Juan T, Fürthauer M. Biogenesis and function of ESCRT-dependent extracellular vesicles. Semin Cell Dev Biol. 2018;74:66–77. [DOI] [PubMed]
- 51.Haraszti RA, Didiot M-C, Sapp E, Leszyk J, Shaffer SA, Rockwell HE, et al. High-resolution proteomic and lipidomic analysis of exosomes and microvesicles from different cell sources. J Extracell Vesicles. 2016;5:32570. doi: 10.3402/jev.v5.32570. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Del Conde I, Shrimpton CN, Thiagarajan P, López JA. Tissue-factor–bearing microvesicles arise from lipid rafts and fuse with activated platelets to initiate coagulation. Blood. 2005;106:1604–1611. doi: 10.1182/blood-2004-03-1095. [DOI] [PubMed] [Google Scholar]
- 53.Budnik V, Ruiz-Cañada C, Wendler F. Extracellular vesicles round off communication in the nervous system. Nat Rev Neurosci. 2016;17:160–172. doi: 10.1038/nrn.2015.29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Sedgwick AE, Clancy JW, Olivia Balmert M, D’Souza-Schorey C. Extracellular microvesicles and invadopodia mediate non-overlapping modes of tumor cell invasion. Sci Rep. 2015;5:1–14. doi: 10.1038/srep14748. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.McAndrews KM, Kalluri R. Mechanisms associated with biogenesis of exosomes in cancer. Mol Cancer. 2019;18:1–11. doi: 10.1186/s12943-019-0963-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Huotari J, Helenius A. In: Endosome maturation. John EJ, editor. Chichester: Wiley; 2011. pp. 3481–3500. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Minciacchi VR, Freeman MR, Di Vizio D. Extracellular vesicles in cancer: exosomes, microvesicles and the emerging role of large oncosomes. Semin Cell Dev Biol. 2015;40:41–51. [DOI] [PMC free article] [PubMed]
- 58.Williams RL, Urbé S. The emerging shape of the ESCRT machinery. Nat Rev Mol cell Biol. 2007;8:355–368. doi: 10.1038/nrm2162. [DOI] [PubMed] [Google Scholar]
- 59.Möbius W, Ohno-Iwashita Y, Donselaar EG, Oorschot VM, Shimada Y, Fujimoto T, Heijnen HF, Geuze HJ, Slot JW. Immunoelectron microscopic localization of cholesterol using biotinylated and non-cytolytic perfringolysin O. J Histochem Cytochem. 2002;50(1):43–55. [DOI] [PubMed]
- 60.Kalluri R, LeBleu VS. The biology, function, and biomedical applications of exosomes. Science (80- ). American Association for the. Advance Sci. 2020;367:eaau6977. doi: 10.1126/science.aau6977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Roucourt B, Meeussen S, Bao J, Zimmermann P, David G. Heparanase activates the syndecan-syntenin-ALIX exosome pathway. Cell Res. 2015;25:412–428. doi: 10.1038/cr.2015.29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Villarroya-Beltri C, Baixauli F, Gutiérrez-Vázquez C, Sánchez-Madrid F, Mittelbrunn M. Sorting it out: regulation of exosome loading. Semin Cancer Biol. 2014;28:3–13. [DOI] [PMC free article] [PubMed]
- 63.Stuffers S, Sem Wegner C, Stenmark H, Brech A. Multivesicular endosome biogenesis in the absence of ESCRTs. Traffic. 2009;10:925–937. doi: 10.1111/j.1600-0854.2009.00920.x. [DOI] [PubMed] [Google Scholar]
- 64.Van Niel G, Charrin S, Simoes S, Romao M, Rochin L, Saftig P, et al. The tetraspanin CD63 regulates ESCRT-independent and-dependent endosomal sorting during melanogenesis. Dev Cell. 2011;21:708–721. doi: 10.1016/j.devcel.2011.08.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Trajkovic K, Hsu C, Chiantia S, Rajendran L, Wenzel D, Wieland F, et al. Ceramide triggers budding of exosome vesicles into multivesicular endosomes. Science (80- ). American Association for the. Advance Sci. 2008;319:1244–1247. doi: 10.1126/science.1153124. [DOI] [PubMed] [Google Scholar]
- 66.Ghossoub R, Lembo F, Rubio A, Gaillard CB, Bouchet J, Vitale N, et al. Syntenin-ALIX exosome biogenesis and budding into multivesicular bodies are controlled by ARF6 and PLD2. Nat Commun. 2014;5:1–12. doi: 10.1038/ncomms4477. [DOI] [PubMed] [Google Scholar]
- 67.Carayon K, Chaoui K, Ronzier E, Lazar I, Bertrand-Michel J, Roques V, et al. Proteolipidic composition of exosomes changes during reticulocyte maturation. J Biol Chem. 2011;286:34426–34439. doi: 10.1074/jbc.M111.257444. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Atkin-Smith GK, Tixeira R, Paone S, Mathivanan S, Collins C, Liem M, et al. A novel mechanism of generating extracellular vesicles during apoptosis via a beads-on-a-string membrane structure. Nat Commun. 2015;6:1–10. doi: 10.1038/ncomms8439. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Erwig LP, Henson PM. Clearance of apoptotic cells by phagocytes. Cell Death Differ. 2008;15:243–250. doi: 10.1038/sj.cdd.4402184. [DOI] [PubMed] [Google Scholar]
- 70.Vandivier RW, Ogden CA, Fadok VA, Hoffmann PR, Brown KK, Botto M, et al. Role of surfactant proteins A, D, and C1q in the clearance of apoptotic cells in vivo and in vitro: calreticulin and CD91 as a common collectin receptor complex. J Immunol. 2002;169:3978–3986. doi: 10.4049/jimmunol.169.7.3978. [DOI] [PubMed] [Google Scholar]
- 71.Akers JC, Gonda D, Kim R, Carter BS, Chen CC. Biogenesis of extracellular vesicles (EV): exosomes, microvesicles, retrovirus-like vesicles, and apoptotic bodies. J Neurooncol. 2013;113:1–11. doi: 10.1007/s11060-013-1084-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Martínez MC, Freyssinet J-M. Deciphering the plasma membrane hallmarks of apoptotic cells: phosphatidylserine transverse redistribution and calcium entry. BMC Cell Biol. 2001;2:1–11. doi: 10.1186/1471-2121-2-20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Savill J. Recognition and phagocytosis of cells undergoing apoptosis. Br Med Bull. 1997;53:491–508. doi: 10.1093/oxfordjournals.bmb.a011626. [DOI] [PubMed] [Google Scholar]
- 74.Saint-Pol J, Gosselet F, Duban-Deweer S, Pottiez G, Karamanos Y. Targeting and crossing the blood-brain barrier with extracellular vesicles. Cells. 2020;9:851. doi: 10.3390/cells9040851. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Becker A, Thakur BK, Weiss JM, Kim HS, Peinado H, Lyden D. Extracellular vesicles in cancer: cell-to-cell mediators of metastasis. Cancer Cell. 2016;30:836–848. doi: 10.1016/j.ccell.2016.10.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Wen S, Dooner M, Cheng Y, Papa E, Del Tatto M, Pereira M, et al. Mesenchymal stromal cell-derived extracellular vesicles rescue radiation damage to murine marrow hematopoietic cells. Leukemia. 2016;30:2221–2231. doi: 10.1038/leu.2016.107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Mendt M, Kamerkar S, Sugimoto H, McAndrews KM, Wu C-C, Gagea M, et al. Generation and testing of clinical-grade exosomes for pancreatic cancer. JCI Insight. 2018;3(8):e99263. [DOI] [PMC free article] [PubMed]
- 78.Bruno S, Grange C, Collino F, Deregibus MC, Cantaluppi V, Biancone L, et al. Microvesicles derived from mesenchymal stem cells enhance survival in a lethal model of acute kidney injury. PLoS One. 2012;7:e33115. doi: 10.1371/journal.pone.0033115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Yan Y, Jiang W, Tan Y, Zou S, Zhang H, Mao F, et al. hucMSC exosome-derived GPX1 is required for the recovery of hepatic oxidant injury. Mol Ther. 2017;25:465–479. doi: 10.1016/j.ymthe.2016.11.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Blazquez R, Sanchez-Margallo FM, de la Rosa O, Dalemans W, Álvarez V, Tarazona R, et al. Immunomodulatory potential of human adipose mesenchymal stem cells derived exosomes on in vitro stimulated T cells. Front Immunol. 2014;5:1–9. doi: 10.3389/fimmu.2014.00556. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Thirabanjasak D, Tantiwongse K, Thorner PS. Angiomyeloproliferative lesions following autologous stem cell therapy. J Am Soc Nephrol. 2010;21:1218–1222. doi: 10.1681/ASN.2009111156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Lee C, Mitsialis SA, Aslam M, Vitali SH, Vergadi E, Konstantinou G, et al. Exosomes mediate the cytoprotective action of mesenchymal stromal cells on hypoxia-induced pulmonary hypertension. Circulation. 2012;126:2601–2611. doi: 10.1161/CIRCULATIONAHA.112.114173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Carnino JM, Lee H, Jin Y. Isolation and characterization of extracellular vesicles from Broncho-alveolar lavage fluid: a review and comparison of different methods. Respir Res. 2019;20:1–11. doi: 10.1186/s12931-019-1210-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Lee H, Zhang D, Laskin DL, Jin Y. Functional evidence of pulmonary extracellular vesicles in infectious and noninfectious lung inflammation. J Immunol. 2018;201:1500–1509. doi: 10.4049/jimmunol.1800264. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Monsel A, Zhu Y, Gennai S, Hao Q, Hu S, Rouby J-J, et al. Therapeutic effects of human mesenchymal stem cell–derived microvesicles in severe pneumonia in mice. Am J Respir Crit Care Med. 2015;192:324–336. doi: 10.1164/rccm.201410-1765OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Wilson KP, Black J-AF, Thomson JA, Kim EE, Griffith JP, Navia MA, et al. Structure and mechanism of interleukin-lβ converting enzyme. Nature. 1994;370:270. doi: 10.1038/370270a0. [DOI] [PubMed] [Google Scholar]
- 87.Laffey JG, Matthay MA. Fifty years of research in ARDS. Cell-based therapy for acute respiratory distress syndrome. Biology and potential therapeutic value. Am J Respir Crit Care Med. 2017;196:266–273. doi: 10.1164/rccm.201701-0107CP. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Eggenhofer E, Luk F, Dahlke MH, Hoogduijn MJ. The life and fate of mesenchymal stem cells. Front Immunol. 2014;5:148. doi: 10.3389/fimmu.2014.00148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Shah TG, Predescu D, Predescu S. Mesenchymal stem cells-derived extracellular vesicles in acute respiratory distress syndrome: a review of current literature and potential future treatment options. Clin Transl Med. 2019;8:1–6. doi: 10.1186/s40169-019-0242-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Porzionato A, Zaramella P, Dedja A, Guidolin D, Bonadies L, Macchi V, et al. Intratracheal administration of mesenchymal stem cell-derived extracellular vesicles reduces lung injuries in a chronic rat model of bronchopulmonary dysplasia. Am J Physiol Cell Mol Physiol. 2021;320:L688–L704. doi: 10.1152/ajplung.00148.2020. [DOI] [PubMed] [Google Scholar]
- 91.You J, Zhou O, Liu J, Zou W, Zhang L, Tian D, et al. Human umbilical cord mesenchymal stem cell-derived small extracellular vesicles alleviate lung injury in rat model of bronchopulmonary dysplasia by affecting cell survival and angiogenesis. Stem Cells Dev. 2020;29:1520–1532. doi: 10.1089/scd.2020.0156. [DOI] [PubMed] [Google Scholar]
- 92.Lee H, Zhang D, Zhu Z, Dela Cruz CS, Jin Y. Epithelial cell-derived microvesicles activate macrophages and promote inflammation via microvesicle-containing microRNAs. Sci Rep. 2016;6:1–11. doi: 10.1038/srep35250. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Lee H, Zhang D, Wu J, Otterbein LE, Jin Y. Lung epithelial cell–derived microvesicles regulate macrophage migration via MicroRNA-17/221–induced integrin β1 recycling. J Immunol. 2017;199:1453–1464. doi: 10.4049/jimmunol.1700165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Zhu Y, Feng X, Abbott J, Fang X, Hao Q, Monsel A, et al. Human mesenchymal stem cell microvesicles for treatment of Escherichia coli endotoxin-induced acute lung injury in mice. Stem Cells. 2014;32:116–125. doi: 10.1002/stem.1504. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Park J, Kim S, Lim H, Liu A, Hu S, Lee J, et al. Therapeutic effects of human mesenchymal stem cell microvesicles in an ex vivo perfused human lung injured with severe E. coli pneumonia. Thorax. 2019;74:43–50. doi: 10.1136/thoraxjnl-2018-211576. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Li Q-C, Liang Y, Su Z-B. Prophylactic treatment with MSC-derived exosomes attenuates traumatic acute lung injury in rats. Am J Physiol Cell Mol Physiol. 2019;316:L1107–L1117. doi: 10.1152/ajplung.00391.2018. [DOI] [PubMed] [Google Scholar]
- 97.Wang J, Huang R, Xu Q, Zheng G, Qiu G, Ge M, et al. Mesenchymal stem cell–derived extracellular vesicles alleviate acute lung injury via transfer of miR-27a-3p. Crit Care Med. 2020;48:e599–e610. doi: 10.1097/CCM.0000000000004315. [DOI] [PubMed] [Google Scholar]
- 98.Song Y, Dou H, Li X, Zhao X, Li Y, Liu D, et al. Exosomal miR-146a contributes to the enhanced therapeutic efficacy of interleukin-1β-primed mesenchymal stem cells against sepsis. Stem Cells. 2017;35:1208–1221. doi: 10.1002/stem.2564. [DOI] [PubMed] [Google Scholar]
- 99.Morrison TJ, Jackson MV, Cunningham EK, Kissenpfennig A, McAuley DF, O’Kane CM, et al. Mesenchymal stromal cells modulate macrophages in clinically relevant lung injury models by extracellular vesicle mitochondrial transfer. Am J Respir Crit Care Med. 2017;196:1275–1286. doi: 10.1164/rccm.201701-0170OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Ionescu L, Byrne RN, van Haaften T, Vadivel A, Alphonse RS, Rey-Parra GJ, et al. Stem cell conditioned medium improves acute lung injury in mice: in vivo evidence for stem cell paracrine action. Am J Physiol Cell Mol Physiol. 2012;303:L967–L977. doi: 10.1152/ajplung.00144.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Tang X-D, Shi L, Monsel A, Li X-Y, Zhu H-L, Zhu Y-G, et al. Mesenchymal stem cell microvesicles attenuate acute lung injury in mice partly mediated by Ang-1 mRNA. Stem Cells. 2017;35:1849–1859. doi: 10.1002/stem.2619. [DOI] [PubMed] [Google Scholar]
- 102.Phinney DG, Di Giuseppe M, Njah J, Sala E, Shiva S, St Croix CM, et al. Mesenchymal stem cells use extracellular vesicles to outsource mitophagy and shuttle microRNAs. Nat Commun. 2015;6:1–15. doi: 10.1038/ncomms9472. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Németh K, Leelahavanichkul A, Yuen PST, Mayer B, Parmelee A, Robey PG, et al. Bone marrow stromal cells attenuate sepsis via prostaglandin E2–dependent reprogramming of host macrophages to increase their interleukin-10 production. Nat Med. 2009;15:42–49. doi: 10.1038/nm.1905. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Islam MN, Das SR, Emin MT, Wei M, Sun L, Westphalen K, et al. Mitochondrial transfer from bone-marrow–derived stromal cells to pulmonary alveoli protects against acute lung injury. Nat Med. 2012;18:759–765. doi: 10.1038/nm.2736. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Khatri M, Richardson LA, Meulia T. Mesenchymal stem cell-derived extracellular vesicles attenuate influenza virus-induced acute lung injury in a pig model. Stem Cell Res Ther. 2018;9:1–13. doi: 10.1186/s13287-018-0774-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Chen W, Huang Y, Han J, Yu L, Li Y, Lu Z, et al. Immunomodulatory effects of mesenchymal stromal cells-derived exosome. Immunol Res. 2016;64:831–840. doi: 10.1007/s12026-016-8798-6. [DOI] [PubMed] [Google Scholar]
- 107.Moser EK, Hufford MM, Braciale TJ. Late engagement of CD86 after influenza virus clearance promotes recovery in a FoxP3+ regulatory T cell dependent manner. PLoS Pathog. 2014;10:e1004315. doi: 10.1371/journal.ppat.1004315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Oliphant S, Lines JL, Hollifield ML, Garvy BA. Regulatory T cells are critical for clearing influenza A virus in neonatal mice. Viral Immunol. 2015;28:580–589. doi: 10.1089/vim.2015.0039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Li Y, Zhou W, Yang L, You R. Physiological and pathological regulation of ACE2, the SARS-CoV-2 receptor. Pharmacol Res. Elsevier. 2020;157:104833. doi: 10.1016/j.phrs.2020.104833. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Chatterjee S. Understanding the nature of variations in structural sequences coding for coronavirus spike, envelope, membrane and nucleocapsid proteins of SARS-CoV-2. Envel Membr Nucleocapsid Proteins SARS-CoV-2. 2020.
- 111.Xia X, Yuan P, Liu Y, Wang Y, Cao W, Zheng JC. Emerging roles of extracellular vesicles in COVID-19, a double-edged sword? Immunology. 2021;163:416–430. doi: 10.1111/imm.13329. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Wan Y, Shang J, Graham R, Baric RS, Li F. Receptor recognition by the novel coronavirus from Wuhan: an analysis based on decade-long structural studies of SARS coronavirus. J Virol. 2020;94(7):e00127-20. [DOI] [PMC free article] [PubMed]
- 113.Sengupta V, Sengupta S, Lazo A, Woods P, Nolan A, Bremer N. Exosomes derived from bone marrow mesenchymal stem cells as treatment for severe COVID-19. Stem Cells Dev. 2020;29:747–754. doi: 10.1089/scd.2020.0080. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Akbari A, Rezaie J. Potential therapeutic application of mesenchymal stem cell-derived exosomes in SARS-CoV-2 pneumonia. Stem Cell Res Ther. 2020;11:1–10. doi: 10.1186/s13287-020-01866-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Xia X, Wang Y, Huang Y, Zhang H, Lu H, Zheng JC. Exosomal miRNAs in central nervous system diseases: biomarkers, pathological mediators, protective factors and therapeutic agents. Prog Neurobiol. 2019;183:101694. doi: 10.1016/j.pneurobio.2019.101694. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Hao Q, Gudapati V, Monsel A, Park JH, Hu S, Kato H, et al. Mesenchymal stem cell–derived extracellular vesicles decrease lung injury in mice. J Immunol. 2019;203:1961–1972. doi: 10.4049/jimmunol.1801534. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Pacienza N, Lee RH, Bae E-H, Kim D, Liu Q, Prockop DJ, et al. In vitro macrophage assay predicts the in vivo anti-inflammatory potential of exosomes from human mesenchymal stromal cells. Mol Ther Clin Dev. 2019;13:67–76. doi: 10.1016/j.omtm.2018.12.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Grange C, Skovronova R, Marabese F, Bussolati B. Stem cell-derived extracellular vesicles and kidney regeneration. Cells. 2019;8:1240. doi: 10.3390/cells8101240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Makris K, Spanou L. Acute kidney injury: definition, pathophysiology and clinical phenotypes. Clin Biochem Rev. 2016;37:85. [PMC free article] [PubMed] [Google Scholar]
- 120.Mehta RL, Cerdá J, Burdmann EA, Tonelli M, García-García G, Jha V, et al. International Society of Nephrology’s 0by25 initiative for acute kidney injury (zero preventable deaths by 2025): a human rights case for nephrology. Lancet. 2015;385:2616–2643. doi: 10.1016/S0140-6736(15)60126-X. [DOI] [PubMed] [Google Scholar]
- 121.Gatti S, Bruno S, Deregibus MC, Sordi A, Cantaluppi V, Tetta C, et al. Microvesicles derived from human adult mesenchymal stem cells protect against ischaemia–reperfusion-induced acute and chronic kidney injury. Nephrol Dial Transplant. 2011;26:1474–1483. doi: 10.1093/ndt/gfr015. [DOI] [PubMed] [Google Scholar]
- 122.Ramírez-Bajo MJ, Martín-Ramírez J, Bruno S, Pasquino C, Banon-Maneus E, Rovira J, et al. Nephroprotective potential of mesenchymal stromal cells and their extracellular vesicles in a murine model of chronic cyclosporine nephrotoxicity. Front cell Dev Biol. 2020;8:296. doi: 10.3389/fcell.2020.00296. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Gao LI, Zhong X, Jin J, Li J, Meng X. Potential targeted therapy and diagnosis based on novel insight into growth factors, receptors, and downstream effectors in acute kidney injury and acute kidney injury-chronic kidney disease progression. Signal Transduct Target Ther; 2020. pp. 1–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Ullah I, Subbarao RB, Rho GJ. Human mesenchymal stem cells-current trends and future prospective. Biosci Rep. 2015;35(2):e00191. [DOI] [PMC free article] [PubMed]
- 125.Zhu G, Pei L, Lin F, Yin H, Li X, He W, et al. Exosomes from human-bone-marrow-derived mesenchymal stem cells protect against renal ischemia/reperfusion injury via transferring miR-199a-3p. J Cell Physiol. 2019;234:23736–23749. doi: 10.1002/jcp.28941. [DOI] [PubMed] [Google Scholar]
- 126.Iwasaki M, Adachi Y, Minamino K, Suzuki Y, Zhang Y, Okigaki M, et al. Mobilization of bone marrow cells by G-CSF rescues mice from cisplatin-induced renal failure, and M-CSF enhances the effects of G-CSF. J Am Soc Nephrol. 2005;16:658–666. doi: 10.1681/ASN.2004010067. [DOI] [PubMed] [Google Scholar]
- 127.Eirin A, Zhu X-Y, Puranik AS, Tang H, McGurren KA, van Wijnen AJ, et al. Mesenchymal stem cell–derived extracellular vesicles attenuate kidney inflammation. Kidney Int. 2017;92:114–124. doi: 10.1016/j.kint.2016.12.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Nagaishi K, Mizue Y, Chikenji T, Otani M, Nakano M, Konari N, et al. Mesenchymal stem cell therapy ameliorates diabetic nephropathy via the paracrine effect of renal trophic factors including exosomes. Sci Rep. 2016;6:1–16. doi: 10.1038/srep34842. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Li J, Chen J, Kirsner R. Pathophysiology of acute wound healing. Clin Dermatol. 2007;25:9–18. doi: 10.1016/j.clindermatol.2006.09.007. [DOI] [PubMed] [Google Scholar]
- 130.Gurtner GC, Werner S, Barrandon Y, Longaker MT. Wound repair and regeneration. Nature. 2008;453:314–321. doi: 10.1038/nature07039. [DOI] [PubMed] [Google Scholar]
- 131.Mardpour S, Hamidieh AA, Taleahmad S, Sharifzad F, Taghikhani A, Baharvand H. Interaction between mesenchymal stromal cell-derived extracellular vesicles and immune cells by distinct protein content. J Cell Physiol. 2019;234:8249–8258. doi: 10.1002/jcp.27669. [DOI] [PubMed] [Google Scholar]
- 132.Casado-Díaz A, Quesada-Gómez JM, Dorado G. Extracellular vesicles derived from mesenchymal stem cells (MSC) in regenerative medicine: applications in skin wound healing. Front Bioeng Biotechnol. 2020;8:146. doi: 10.3389/fbioe.2020.00146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Ren S, Chen J, Duscher D, Liu Y, Guo G, Kang Y, et al. Microvesicles from human adipose stem cells promote wound healing by optimizing cellular functions via AKT and ERK signaling pathways. Stem Cell Res Ther. 2019;10:1–14. doi: 10.1186/s13287-019-1152-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.Zhang B, Wang M, Gong A, Zhang X, Wu X, Zhu Y, et al. HucMSC-exosome mediated-Wnt4 signaling is required for cutaneous wound healing. Stem Cells. 2015;33:2158–2168. doi: 10.1002/stem.1771. [DOI] [PubMed] [Google Scholar]
- 135.Li Y, Zhang J, Shi J, Liu K, Wang X, Jia Y, et al. Exosomes derived from human adipose mesenchymal stem cells attenuate hypertrophic scar fibrosis by miR-192-5p/IL-17RA/Smad axis. Stem Cell Res Ther. 2021;12:1–16. doi: 10.1186/s13287-021-02568-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.Raghav A, Tripathi P, Mishra BK, Jeong G-B, Banday S, Gautam KA, et al. Mesenchymal stromal cell-derived tailored exosomes treat bacteria-associated diabetes foot ulcers: a customized approach from bench to bed. Front Microbiol. 2021;12:712588. [DOI] [PMC free article] [PubMed]
- 137.Ariyanti AD, Zhang J, Marcelina O, Nugrahaningrum DA, Wang G, Kasim V, et al. Salidroside-pretreated mesenchymal stem cells enhance diabetic wound healing by promoting paracrine function and survival of mesenchymal stem cells under hyperglycemia. Stem Cells Transl Med. 2019;8:404–414. doi: 10.1002/sctm.18-0143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138.Shiekh PA, Singh A, Kumar A. Exosome laden oxygen releasing antioxidant and antibacterial cryogel wound dressing OxOBand alleviate diabetic and infectious wound healing. Biomaterials. 2020;249:120020. doi: 10.1016/j.biomaterials.2020.120020. [DOI] [PubMed] [Google Scholar]
- 139.Jiang L, Zhang Y, Liu T, Wang X, Wang H, Song H, et al. Exosomes derived from TSG-6 modified mesenchymal stromal cells attenuate scar formation during wound healing. Biochimie. 2020;177:40–49. doi: 10.1016/j.biochi.2020.08.003. [DOI] [PubMed] [Google Scholar]
- 140.Knights D, Silverberg MS, Weersma RK, Gevers D, Dijkstra G, Huang H, et al. Complex host genetics influence the microbiome in inflammatory bowel disease. Genome Med. 2014;6:1–11. doi: 10.1186/s13073-014-0107-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141.Yang J, Liu X-X, Fan H, Tang Q, Shou Z-X, Zuo D-M, et al. Extracellular vesicles derived from bone marrow mesenchymal stem cells protect against experimental colitis via attenuating colon inflammation, oxidative stress and apoptosis. PLoS One. 2015;10:e0140551. doi: 10.1371/journal.pone.0140551. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142.Kaplan GG. The global burden of IBD: from 2015 to 2025. Nat Rev Gastroenterol Hepatol. 2015;12:720–727. doi: 10.1038/nrgastro.2015.150. [DOI] [PubMed] [Google Scholar]
- 143.Chang X, Wang S-L, Zhao S-B, Shi Y-H, Pan P, Gu L, et al. Extracellular vesicles with possible roles in gut intestinal tract homeostasis and IBD. Mediators Inflamm. 2020;2020:1945832. [DOI] [PMC free article] [PubMed]
- 144.Ocansey DKW, Zhang L, Wang Y, Yan Y, Qian H, Zhang X, et al. Exosome-mediated effects and applications in inflammatory bowel disease. Biol Rev Camb Philos Soc. 2020;95:1287–1307. doi: 10.1111/brv.12608. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 145.Liao F, Lu X, Dong W. Exosomes derived from T regulatory cells relieve inflammatory bowel disease by transferring miR-195a-3p. IUBMB Life. 2020;72:2591–2600. doi: 10.1002/iub.2385. [DOI] [PubMed] [Google Scholar]
- 146.Jiang L, Shen Y, Guo D, Yang D, Liu J, Fei X, et al. EpCAM-dependent extracellular vesicles from intestinal epithelial cells maintain intestinal tract immune balance. Nat Commun. 2016;7:1–16. doi: 10.1038/ncomms13045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 147.Wu Y, Qiu W, Xu X, Kang J, Wang J, Wen Y, et al. Exosomes derived from human umbilical cord mesenchymal stem cells alleviate inflammatory bowel disease in mice through ubiquitination. Am J Transl Res. 2018;10:2026. [PMC free article] [PubMed] [Google Scholar]
- 148.Yu H, Yang X, Xiao X, Xu M, Yang Y, Xue C, et al. Human adipose mesenchymal stem cell-derived exosomes protect mice from DSS-induced inflammatory bowel disease by promoting intestinal-stem-cell and epithelial regeneration. Aging Dis. 2021;12:1423. doi: 10.14336/AD.2021.0601. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 149.Cao L, Xu H, Wang G, Liu M, Tian D, Yuan Z. Extracellular vesicles derived from bone marrow mesenchymal stem cells attenuate dextran sodium sulfate-induced ulcerative colitis by promoting M2 macrophage polarization. Int Immunopharmacol. 2019;72:264–274. doi: 10.1016/j.intimp.2019.04.020. [DOI] [PubMed] [Google Scholar]
- 150.Duan L, Huang H, Zhao X, Zhou M, Chen S, Wang C, et al. Extracellular vesicles derived from human placental mesenchymal stem cells alleviate experimental colitis in mice by inhibiting inflammation and oxidative stress. Int J Mol Med. 2020;46:1551–1561. doi: 10.3892/ijmm.2020.4679. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 151.Singer M, Deutschman CS, Seymour CW, Shankar-Hari M, Annane D, Bauer M, et al. The third international consensus definitions for sepsis and septic shock (Sepsis-3) Jama. 2016;315:801–810. doi: 10.1001/jama.2016.0287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 152.Gyawali B, Ramakrishna K, Dhamoon AS. Sepsis: the evolution in definition, pathophysiology, and management. London: SAGE open Med. SAGE Publications Sage UK; 2019. p. 2050312119835043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 153.Xu J, Feng Y, Jeyaram A, Jay SM, Zou L, Chao W. Circulating plasma extracellular vesicles from septic mice induce inflammation via microRNA-and TLR7-dependent mechanisms. J Immunol. Am Assoc Immnol. 2018;201:3392–3400. doi: 10.4049/jimmunol.1801008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 154.Chaudhry H, Zhou J, Zhong YIN, Ali MM, McGuire F, Nagarkatti PS, et al. Role of cytokines as a double-edged sword in sepsis. In vivo (Brooklyn) 2013;27:669–684. [PMC free article] [PubMed] [Google Scholar]
- 155.Gao K, Jin J, Huang C, Li J, Luo H, Li L, Huang Y, Jiang Y. Exosomes derived from septic mouse serum modulate immune responses via exosome-associated cytokines. Front Immunol. 2019;10:1560. [DOI] [PMC free article] [PubMed]
- 156.Tulapurkar ME, Ramarathnam A, Hasday JD, Singh IS. Bacterial lipopolysaccharide augments febrile-range hyperthermia-induced heat shock protein 70 expression and extracellular release in human THP1 cells. PLoS One. San Francisco: Public Library of Science; 2015. p. e0118010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 157.Coleman LG, Jr, Maile R, Jones SW, Cairns BA, Crews FT. HMGB1/IL-1β complexes in plasma microvesicles modulate immune responses to burn injury. PLoS One. 2018;13:e0195335. doi: 10.1371/journal.pone.0195335. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 158.Yao M, Cui B, Zhang W, Ma W, Zhao G, Xing L. Exosomal miR-21 secreted by IL-1β-primed-mesenchymal stem cells induces macrophage M2 polarization and ameliorates sepsis. Life Sci. 2021;264:118658. doi: 10.1016/j.lfs.2020.118658. [DOI] [PubMed] [Google Scholar]
- 159.Gang D, Yu CJ, Zhu S, Zhu P, Nasser MI. Application of mesenchymal stem cell-derived exosomes in kidney diseases. Cell Immunol. 2021;364:104358. doi: 10.1016/j.cellimm.2021.104358. [DOI] [PubMed] [Google Scholar]
- 160.Gao F, Zuo B, Wang Y, Li S, Yang J, Sun D. Protective function of exosomes from adipose tissue-derived mesenchymal stem cells in acute kidney injury through SIRT1 pathway. Life Sci. 2020;255:117719. doi: 10.1016/j.lfs.2020.117719. [DOI] [PubMed] [Google Scholar]
- 161.Zhang S, Hou Y, Yang J, Xie D, Jiang L, Hu H, et al. Application of mesenchymal stem cell exosomes and their drug-loading systems in acute liver failure. J Cell Mol Med. Wiley Online. Library. 2020;24:7082–7093. doi: 10.1111/jcmm.15290. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 162.Zhao S, Liu Y, Pu Z. Bone marrow mesenchymal stem cell-derived exosomes attenuate D-GaIN/LPS-induced hepatocyte apoptosis by activating autophagy in vitro. Drug Des Devel Ther. 2019;13:2887. doi: 10.2147/DDDT.S220190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 163.Wang X, Gu H, Qin D, Yang L, Huang W, Essandoh K, et al. Exosomal miR-223 contributes to mesenchymal stem cell-elicited cardioprotection in polymicrobial sepsis. Sci Rep. 2015;5:13721. doi: 10.1038/srep13721. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 164.Immordino ML, Dosio F, Cattel L. Stealth liposomes: review of the basic science, rationale, and clinical applications, existing and potential. Int J Nanomedicine. 2006;1:297. [PMC free article] [PubMed] [Google Scholar]
- 165.Takahashi Y, Nishikawa M, Shinotsuka H, Matsui Y, Ohara S, Imai T, et al. Visualization and in vivo tracking of the exosomes of murine melanoma B16-BL6 cells in mice after intravenous injection. J Biotechnol. 2013;165:77–84. doi: 10.1016/j.jbiotec.2013.03.013. [DOI] [PubMed] [Google Scholar]
- 166.Matsumoto A, Takahashi Y, Nishikawa M, Sano K, Morishita M, Charoenviriyakul C, et al. Role of phosphatidylserine-derived negative surface charges in the recognition and uptake of intravenously injected B16BL6-derived exosomes by macrophages. J Pharm Sci. 2017;106:168–175. doi: 10.1016/j.xphs.2016.07.022. [DOI] [PubMed] [Google Scholar]
- 167.Munagala R, Aqil F, Jeyabalan J, Gupta RC. Bovine milk-derived exosomes for drug delivery. Cancer Lett. 2016;371:48–61. doi: 10.1016/j.canlet.2015.10.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 168.Wang B, Zhuang X, Deng Z-B, Jiang H, Mu J, Wang Q, et al. Targeted drug delivery to intestinal macrophages by bioactive nanovesicles released from grapefruit. Mol Ther. 2014;22:522–534. doi: 10.1038/mt.2013.190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 169.Ha DH, Kim S-D, Lee J, Kwon HH, Park G-H, Yang SH, et al. Toxicological evaluation of exosomes derived from human adipose tissue-derived mesenchymal stem/stromal cells. Regul Toxicol Pharmacol. 2020;115:104686. doi: 10.1016/j.yrtph.2020.104686. [DOI] [PubMed] [Google Scholar]
- 170.Zhu X, Badawi M, Pomeroy S, Sutaria DS, Xie Z, Baek A, et al. Comprehensive toxicity and immunogenicity studies reveal minimal effects in mice following sustained dosing of extracellular vesicles derived from HEK293T cells. J Extracell vesicles. 2017;6:1324730. doi: 10.1080/20013078.2017.1324730. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 171.Sun LI, Xu R, Sun X, Duan Y, Han Y, Zhao Y, et al. Safety evaluation of exosomes derived from human umbilical cord mesenchymal stromal cell. Cytotherapy. 2016;18:413–422. doi: 10.1016/j.jcyt.2015.11.018. [DOI] [PubMed] [Google Scholar]
- 172.Maji S, Yan IK, Parasramka M, Mohankumar S, Matsuda A, Patel T. In vitro toxicology studies of extracellular vesicles. J Appl Toxicol. 2017;37:310–318. doi: 10.1002/jat.3362. [DOI] [PubMed] [Google Scholar]
- 173.Hwang W-L, Lan H-Y, Cheng W-C, Huang S-C, Yang M-H. Tumor stem-like cell-derived exosomal RNAs prime neutrophils for facilitating tumorigenesis of colon cancer. J Hematol Oncol. 2019;12:1–17. doi: 10.1186/s13045-019-0699-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 174.Gyukity-Sebestyén E, Harmati M, Dobra G, Németh IB, Mihály J, Zvara Á, et al. Melanoma-derived exosomes induce PD-1 overexpression and tumor progression via mesenchymal stem cell oncogenic reprogramming. Front Immunol. 2019;10:2459. doi: 10.3389/fimmu.2019.02459. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 175.Gu H, Ji R, Zhang X, Wang M, Zhu W, Qian H, et al. Exosomes derived from human mesenchymal stem cells promote gastric cancer cell growth and migration via the activation of the Akt pathway. Mol Med Rep. 2016;14:3452–3458. doi: 10.3892/mmr.2016.5625. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Not applicable