Abstract
Background
A SARS-CoV-2+Flu A/B+RSV Combo Rapid test may be more relevant than Rapid Antigen Diagnostic (RAD) tests targeting only SARS-CoV-2 since we are facing a concurrent circulation of these viruses during the winter season.
Objectives
To assess the clinical performance of a SARS-CoV-2+Flu A/B+RSV Combo test in comparison to a multiplex RT-qPCR.
Study Design
Residual nasopharyngeal swabs issued from 178 patients were included. All patients, adults and children, were symptomatic and presented at the emergency department with flu-like symptoms. Characterization of the infectious viral agent was done by RT-qPCR. The viral load was expressed as cycle threshold (Ct). Samples were then tested using the multiplex RAD test Fluorecare®ฏ SARS-CoV-2 & Influenza A/B & RSV Antigen Combo Test. Data analysis was carried out using descriptive statistics.
Results
The sensitivity of the test varies according to the virus, with the highest sensitivity observed for Influenza A (80.8.% [95%CI: 67.2 - 94.4]) and the lowest sensitivity observed for RSV (41.5% [95%CI: 26.2 – 56.8]). Higher sensitivities were observed for samples with high viral loads (Ct < 20) and decrease with low viral loads. The specificity for SARS-CoV-2, RSV and Influenza A and B was >95%.
Conclusions
The Fluorecare® combo antigenic presents satisfying performance in real-life clinical setting for Influenza A and B in samples with high viral load. This could be useful to allow a rapid (self-)isolation as the transmissibility of these viruses increase with the viral load. According to our results, its use to rule-out SARS-CoV-2 and RSV infection is not sufficient.
Keywords: Antigen, SARS-CoV-2, Influenza, RSV, Multiplex, Combo, RAD
1. Introduction
The RNA viruses SARS-CoV-2, influenza A (Flu A), influenza B (Flu B) and the respiratory syncytial virus (RSV) are the most threatening viruses causing acute lower respiratory infections with overlapping clinical manifestations [1]. The early and rapid diagnosis and differentiation between these viruses is essential for clinical management, infection control, and epidemiological surveillance [2]. In parallel to the widely used Reverse Transcriptase – quantitative Polymerase Chain Reaction (RT-qPCR), rapid antigen detection (RAD) tests were developed to allow faster isolation of patients and lower virus spreading [3]. Unlike RT-qPCR, RAD tests do not require laboratory equipment or trained personnel and therefore offer unique advantages from a public health perspective, especially for remote and resource-limited areas, medical emergencies or mass testing purposes [4]. In previous studies, we reported that these RAD tests presented good performance to detect SARS-CoV-2 in patients with high viral loads (Ct < 25) with sensitivities varying from 91 to 98% [5,6]. Nevertheless, a SARS-CoV-2 + Flu A/B + RSV Combo Rapid test may be more relevant since we are facing a concurrent circulation of these viruses during the winter season.
2. Objectives
This study assesses the clinical performance of a SARS-CoV-2 + Flu A/B + RSV Combo test in comparison to a multiplex RT-qPCR.
3. Study design
Residual nasopharyngeal swabs issued from 178 patients who presented between December 25, 2022, and January 6, 2023, at the Clinique Saint-Pierre (Ottignies, Belgium) were included. All patients were symptomatic and presented at the emergency department with flu-like symptoms. Samples included were issued from both adults and children. Nasopharyngeal swabs were collected in Vacuette® Virus Stabilization Tubes (Greiner Bio-One GmbH, Kremsmünster, Austria). Following routine analyses, samples were fully anonymized and frozen at −20°C. Following thawing, RT-qPCR was done with the Allplex® SARS-CoV-2/FluA/FluB/RSV kit (Seegene, Arrow Diagnostics, Seoul, Korea). This method identifies SARS-CoV-2 RNA (by targeting three viral genes: N, S and RdRP), Influenza A, Influenza B and RSV RNA and serves as reference in the current study. The viral load was expressed as a cycle threshold (Ct). Samples with Ct values < 35 were selected for the present study. Samples with higher Ct were not included, as these could be false positive results or residual DNA, with less or no risk of transmission. Following RT-qPCR, samples were tested using the multiplex antigenic test Fluorecare®ฏ SARS-CoV-2 & Influenza A/B & RSV Antigen Combo Test (Microprofit Biotech, Shenzhen, China). Briefly, two drops of the viral transport medium were delivered in the 3 different wells of the device (SARS-CoV-2, Flu A/B and RSV). After 20 min, samples for which both the control and test lines were present were considered positive, while samples for which only the control line was present were considered negative, as objectivated by two independent blinded operators.
Data analysis was carried out using descriptive statistics. Sensitivity and specificity were calculated with RT-qPCR results as the reference. The comparison between RT-qPCR viral load (Ct) and the antigenic test result was done using an unpaired t-test, which was computed to assess the difference between groups. One-way ANOVA was performed to compare mean Ct values between each virus. The significance threshold was set at p < 0.05. Data analysis was performed using GraphPad Prism®ฏ software (version 9.0.0, California, USA).
4. Results
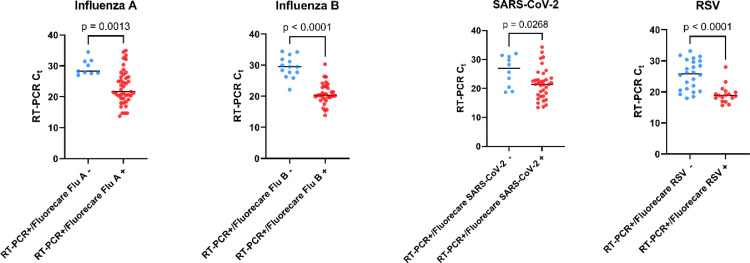
In the entire cohort, the sensitivity of the test varies according to the virus, with the highest sensitivity observed for Influenza A (80.8.% [95%CI: 67.2 – 94.4]) and the lowest sensitivity observed for RSV (41.5% [95%CI: 26.2 – 56.8]). As expected, higher sensitivities were observed for samples with high viral loads (Ct < 20) and decrease with low viral loads (Table 1 ). Samples with RAD negative tests showed lower viral load (higher Ct) compared to RAD positive tests (Fig. 1 ). The specificity for SARS-CoV-2, RSV and Influenza A and B was 100% for SARS-CoV-2 and RSV and 96.0% and 96.9% for influenza A and B, respectively (Table 1). Among the 5 false positive results for Influenza A, one sample showed positive SARS-CoV-2 RT-qPCR (Ct = 13.66), 3 samples were positive for RSV RT-qPCR (Ct = 18.06, 31.80 and 33.15) and one was positive for both SARS-CoV-2 and RSV (Ct = 17.80 and 20.52, respectively). Among the 4 false positive cases for Influenza B, all were positive for Influenza A with RT-qPCR (Ct = 18.00, 19.12, 23.31 and 31.74) (Table 1). Of these, only two showed a positive RAD test for Influenza A. All SARS-CoV-2 samples were expected to be Omicron sublineage according to the current epidemiological situation in Belgium.
Table 1.
Sensitivity and specificity for each virus detected with the Fluorecare® combo antigenic test across various RT-qPCR Ct value ranges. .
| No. of positive patients with RAD test (Sensitivity%) (Pos. / total) | ||||
|---|---|---|---|---|
| Ct (gene N) | Influenza A | Influenza B | SARS-CoV-2 | RSV |
| < 20 | 12/12 (100%) | 12/12 (100%) | 13/15 (86.7%) | 13/17 (76.5%) |
| < 25 | 28/29 (96.5%) | 29/31 (93.5%) | 27/31 (87.1%) | 16/26 (61.5%) |
| < 30 | 37/42 (88.1%) | 30/41 (73.2%) | 31/38 (81.6%) | 17/36 (47.2%) |
| 20 – 25 | 16/17 (94.1%) | 17/19 (89.5%) | 14/16 (87.5%) | 3/9 (33.3%) |
| 25 – 30 | 8/13 (61.5%) | 1/10 (10.0%) | 4/7 (57.1%) | 1/10 (10%) |
| 30 – 35 | 6/10 (60%) | 1/6 (16.6%) | 4/7 (57.1%) | 0/5 (0%) |
|
Total (< 35) [95%CI] |
42/52 (80.8%) [67.2 – 94.4] |
31/47 (65.9%) [51.6 – 80.2] |
35/45 (77.8%) [63.2 – 92.4] |
17/41 (41.5%) [26.2 – 56.8] |
|
Mean Ct [95%CI] |
24.26 [22.68 – 25.84] |
23.40 [21.87–24.93] |
22.58 [20.91–24.24] |
22.88 [21.28–24.48] |
|
No. Of Negative Patients with RAD test (Specificity%) (Neg. / total) | ||||
| Neg. with RT-qPCR [95%CI] |
121/126 (96.0%) [87.3 – 100] |
127/131 (96.9%) [88.33 – 100] |
133/133 (100%) [91.5 – 100] |
135/135 (100%) [91.6 – 100] |
Abbreviations: CI, Confidence Interval; Ct, cycle thresholds; RAD, Rapid Antigen Diagnostic; RSV, Respiratory Syncytial Virus; RT-qPCR, Reverse Transcriptase quantitative Polymerase Chain Reaction.
Fig. 1.
Representation of positive and negative results on the Fluorecare® combo antigenic test as a function of the Ct value in RT-qPCR.
Abbreviations: Ct, cycle thresholds; RSV, Respiratory Syncytial Virus; RT-PCR, Reverse Transcriptase quantitative Polymerase Chain Reaction.
According to our results, this device presents good performance for Influenza A, B and SARS-CoV-2 in patients presenting high viral loads (Ct < 25). Clinical performance was more limited for low viral loads (Ct > 25) and were insufficient for RSV. Similar to our results, Franck et al. reported sensitivity below 50% for three distinct RSV RAD tests [7], while Reina et al. noted also significantly lower Ct values (≈19) in samples positive for RSV RAD test [8]. On the other hand, concordant results were observed by Moesker et al. with the BinaxNow Influenza AB® and BinaxNow RSV® [9], which highlighted 30% of false positive Influenza RAD tests, as caused by RSV. As reported by several authors, sensitivity of RSV RAD tests seems higher in pediatric context, due to higher viral loads in this population. Therefore, one limitation of this study is that it includes a heterogeneous population. However, we are confident in our results since our mean RSV Ct value is very close to those reported in studies investigating solely pediatric samples [8].
Compared to previous SARS-CoV-2 RAD tests, the Fluorecare® combo antigenic test seems less sensitive, although ideally, the comparison should be made on the same selection of samples [5,6]. False negative samples predominantly displayed high Ct values which reflects the lack of sensitivity of these RAD techniques.
5. Conclusions
To conclude, the Fluorecare® combo antigenic does not reach WHO's minimum performance requirement of 80% sensitivity for SARS-CoV-2 in our study population. For influenza A and B its performance is limited to samples with high viral load (i.e. Ct values below 25). For RSV, whatever the Ct value, its performance is insufficient and the use of this RAD to rule-out RSV infection should be avoided. Additional studies are needed to assess performance in asymptomatic individuals but we do not expect better performance than in the present cohort based on our previous experience with SARS-CoV-2 RAD tests [6]. In addition, external validation of such RAD tests utilizing an EQA proficiency panel would be important [10]. Such rapid test could nevertheless be useful to allow a rapid (self-) isolation as the transmissibility of these viruses increase with the viral load [11]. However, while its use in the clinical setting is certainly limited due to the easier access RT-qPCR multiplexing platforms, its ambulatory deployment should be accompanied by a statement of its current limited performance in samples with expected low viral load. These samples may turn falsely negative with the current device and in case of any doubt, have to be confirmed by RT-qPCR.
Declaration of Competing Interest
The authors have no relevant conflicts of interest to disclose in relation to this article.
Acknowledgments
None
References
- 1.Cilloniz C., Luna C.M., Hurtado J.C., Marcos M.A., Torres A. Respiratory viruses: their importance and lessons learned from COVID-19. Eur. Respir. Rev. 2022;31(166) doi: 10.1183/16000617.0051-2022. Dec 31PubMed PMID: 36261158. Pubmed Central PMCID: PMC9724808. Epub 20221019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Gradisteanu Pircalabioru G., Iliescu F.S., Mihaescu G., Cucu A.I., Ionescu O.N., Popescu M., et al. Advances in the rapid diagnostic of viral respiratory tract infections. Front. Cell. Infect. Microbiol. 2022;12 doi: 10.3389/fcimb.2022.807253. PubMed PMID: 35252028. Pubmed Central PMCID: PMC8895598. Epub 20220210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Toptan T., Eckermann L., Pfeiffer A.E., Hoehl S., Ciesek S., Drosten C., et al. Evaluation of a SARS-CoV-2 rapid antigen test: potential to help reduce community spread? J. Clin. Virol. 2021 Feb;135 doi: 10.1016/j.jcv.2020.104713. PubMed PMID: 33352470. Pubmed Central PMCID: PMC7832367. Epub 20201205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Lee J.J., Verbakel J.Y., Goyder C.R., Ananthakumar T., Tan P.S., Turner P.J., et al. The clinical utility of point-of-care tests for influenza in ambulatory care: a systematic review and meta-analysis. Clin. Infect. Dis. 2019;69(1):24–33. doi: 10.1093/cid/ciy837. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Favresse J., Gillot C., Oliveira M., Cadrobbi J., Elsen M., Eucher C., et al. Head-to-head comparison of rapid and automated antigen detection tests for the diagnosis of SARS-CoV-2 infection. J. Clin. Med. 2021;10(2) doi: 10.3390/jcm10020265. Jan 13PubMed PMID: 33450853. Pubmed Central PMCID: PMC7828347. Epub 20210113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Bayart J.L., Degosserie J., Favresse J., Gillot C., Didembourg M., Djokoto H.P., et al. Analytical sensitivity of six SARS-CoV-2 rapid antigen tests for omicron versus delta variant. Viruses. 2022;14(4) doi: 10.3390/v14040654. Mar 22PubMed PMID: 35458384. Pubmed Central PMCID: PMC9031584. Epub 20220322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Franck K.T., Schneider U.V., Ma C.M.G., Knudsen D., Lisby G. Evaluation of immuview RSV antigen test (SSI siagnostica) and BinaxNOW RSV card (alere) for rapid detection of respiratory syncytial virus in retrospectively and prospectively collected respiratory samples. J. Med. Virol. 2020 doi: 10.1002/jmv.26369. Jul 29PubMed PMID: 32725889. Epub 20200729. [DOI] [PubMed] [Google Scholar]
- 8.Reina J., Morales C., Busquets M., Norte C. Usefulness of Ct value in acute respiratory infections caused by respiratory syncytial virus A and B and influenza virus A (H1N1)pdm09, A (H3N2) and B. Enferm. Infecc. Microbiol. Clin. (English ed) 2018;36(6):332–335. doi: 10.1016/j.eimc.2017.04.008. Jun-JulPubMed PMID: 28601216. Epub 20170607. Utilidad del valor Ct en las infecciones respiratorias agudas causadas por el virus respiratorio sincitial A y B y los virus gripales A (H1N1)pdm09, A (H3N2) y B. [DOI] [PubMed] [Google Scholar]
- 9.Moesker F.M., van Kampen J.J.A., Aron G., Schutten M., van de Vijver D., Koopmans M.P.G., et al. Diagnostic performance of influenza viruses and RSV rapid antigen detection tests in children in tertiary care. J. Clin. Virol. 2016;79:12–17. doi: 10.1016/j.jcv.2016.03.022. JunPubMed PMID: 27045454. Pubmed Central PMCID: PMC7185377. Epub 20160325. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Donoso Mantke O., Corman V.M., Taddei F., McCulloch E., Niemeyer D., Grumiro L., et al. Importance of external quality assessment for SARS-CoV-2 antigen detection during the COVID-19 pandemic. J. Clin. Virol. 2022;154 doi: 10.1016/j.jcv.2022.105222. SepPubMed PMID: 35797940. Pubmed Central PMCID: PMC9235289. Epub 20220627. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Klompas M., Milton D.K., Rhee C., Baker M.A., Leekha S. Current insights into respiratory virus transmission and potential implications for infection control programs : a narrative review. Ann. Intern. Med. 2021;174(12):1710–1718. doi: 10.7326/M21-2780. DecPubMed PMID: 34748374. Epub 20211109. [DOI] [PubMed] [Google Scholar]