Abstract
Background
Patients with chronic kidney disease are at high risk for coronavirus disease 2019. Little is known about immune response to severe acute respiratory syndrome coronavirus 2 vaccination in patients on peritoneal dialysis (PD).
Method
We prospectively enrolled 306 PD patients receiving two doses of vaccines (ChAdOx1-S: 283, mRNA-1273: 23) from July 2021 at a medical center. Humeral and cellular immune responses were assessed by anti-spike IgG concentration and blood T cell interferon-γ production 30 days after vaccination. Antibody ≥0.8 U/mL and interferon-γ ≥ 100 mIU/mL were defined as positive. Antibody was also measured in 604 non-dialysis volunteers (ChAdOx1-S: 244, mRNA-1273: 360) for comparison.
Result
PD patients had less adverse events after vaccinations than volunteers. After the first dose of vaccine, the median antibody concentrations were 8.5 U/mL and 50.4 U/mL in ChAdOx1-S group and mRNA-1273 group of PD patients, and 66.6 U/mL and 195.3 U/mL in ChAdOx1-S group and mRNA-1273 group of volunteers, respectively. And after the second dose of vaccine, the median antibody concentrations were 344.8 U/mL and 9941.0 U/mL in ChAdOx1-S group and mRNA-1273 group of PD patients, and 620.3 U/mL and 3845.0 U/mL in ChAdOx1-S group and mRNA-1273 group of volunteers, respectively. The median IFN-γ concentration was 182.8 mIU/mL in ChAdOx1-S group, which was substantially lower than the median concentration 476.8 mIU/mL in mRNA-1273 group of PD patients.
Conclusions
Both vaccines were safe and resulted in comparable antibody seroconversion in PD patients when compared with volunteers. However, mRNA-1273 vaccine induced significantly higher antibody and T cell response than ChAdOx1-S in PD patients. Booster doses are recommended for PD patients after two doses of ChAdOx1-S vaccination.
Keywords: Humeral immunity, Peritoneal dialysis, SARS-CoV-2, T cell immunity
Introduction
The world continues to be affected profoundly by the global coronavirus disease 2019 (COVID-19) pandemic since the first patient was diagnosed in December 2019.1 Until early October 2022, the severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) has infected more than 600 million people and caused more than 6 million deaths worldwide.1 Although diffuse alveolar damage and acute respiratory failure were the main clinical features, acute kidney injury was found in 5.1% of admitted COVID-19 patients and associated with high in-hospital mortality.2 Moreover, patients with chronic kidney disease (CKD) are at high risk for COVID-19.3, 4, 5, 6 In the OpenSAFELY database that covers 40% of all patients in England, the analysis also demonstrated an increased risk for COVID-19 infection and mortality in patients receiving dialysis therapy.6 A retrospective cohort study showed that 5.5% of patients receiving maintenance hemodialysis (HD) developed COVID-19 and 24.9% of these infected patients died in outpatient clinics in United States.4 A prospective, multicentre, region-wide registry study demonstrated that SARS-coV-2 infection rates were 5.3% and 0.6% in HD patients and general population in Belgium, respectively.7 Infection led to mortality rates 29.6% and 15.3% in HD patients and general population, respectively. A more recent retrospective study on the data of Renal Management Information System (REMIS) at Centers for Medicare and Medicaid Services demonstrated that peritoneal dialysis (PD) was associated with lower adjusted relative rates of COVID-19 hospitalization than HD during epidemiologic weeks of 2020.8 Similar findings showed that incidence of symptomatic COVID-19 in PD patients was close to that of the general population in Wuhan, China.9 In the general population, safety and efficacy of SARS-CoV-2 vaccines have been proved.10, 11, 12 In addition to personal protective measures (masks, social distancing and hand hygiene etc.), vaccination may be the only intervention to protect dialysis patients against COVID-19. However, there are few data available on the efficacy of SARS-CoV-2 vaccine in dialysis patients.13, 14, 15, 16, 17, 18, 19, 20, 21, 22
Although evidence has shown reduced rate of seroconversion after vaccinations in patients receiving maintenance dialysis, vaccinations remain an important component of preventative care against the infection of hepatitis B, influenza, and pneumococcus.23 Recent studies have shown that the level of anti-spike IgG was positive in 90% or more of dialysis patients following two doses of mRNA vaccines BNT 162b2 (BioNTech and Pfizer) or mRNA-1273 (Moderna) but it was significantly lower than health controls.13, 14, 15, 16, 17 A delayed humoral response to SARS-CoV-2 vaccination might happen.16 Of 21 HD patients who did not achieve protective antibody titers 3 weeks after their second dose of BNT 162b2 vaccination, 5 developed protective antibody titers 10 weeks after the second dose.16 Despite concerns about suboptimal antibody response, the risk for SARS-CoV-2 infection and severe outcome could be substantially reduced in dialysis patients receiving mRNA vaccines.21 , 22 Regarding attenuated adenovirus vaccine, the antibody response to ChAdOx1-S (AstraZeneca) was comparable between HD patients and health controls.24 But studies comparing the humoral response in dialysis patients to another attenuated adenovirus vaccine Ad26.COV2.S (Johnson & Johnson) or mRNA vaccines (mRNA-1273 or BNT126b2) showed markedly fewer patients vaccinated with Ad26.COV2.S had detectable or adequate antibody response.18, 19, 20 However, in a large real-world cohort of HD patients, no difference was detected in peri-COVID-19 hospitalizations and deaths among patients receiving BNT126b2 versus Ad26. C)V2.S over the first 6 months postvaccination, despite an inconsistent antibody response to the latter.25 Although PD was associated with lower rate of SARS-CoV-2 infection and hospitalization than HD, the immune response, especially T cell responses to SARS-CoV-2 vaccines in PD patients has not been studied.8 , 9 , 26, 27, 28, 29, 30, 31, 32
In Taiwan, ChAdOx1-S was the first vaccine provided by the government to dialysis patients in hospital from epidemiologic week24 of 2021.33 Then mRNA-1273 was provided in community later.33 Taking advantage of more than 400 PD patients regularly treated in our hospital, here we studied and reported the antibody response to SARS-CoV-2 vaccination in PD patients.
Material and methods
Study design and cohorts
In this prospective single-center cohort study, we enrolled 306 PD patients agreeing to receive two doses of ChAdOx1-S vaccines 12 weeks apart, two doses of mRNA-1273 vaccines 4 weeks apart from July 12 2021 according to the guidelines of Taiwan Center for Disease Control (CDC).33 Totally 604 non-dialysis/non-immunosuppressive volunteers receiving two doses of ChAdOx1-S vaccines or two doses of mRNA-1273 were also studied. All participants were instructed to record any adverse reactions. The blood was collected before vaccination, 28 days after first and second doses for analysis. Antibodies to the nucleotide protein were measured before enrollment, after the first or the second vaccination to exclude COVID-19 infection. The primary outcome was serum anti-spike IgG concentration and blood T cell production of interferon-γ (IFN-γ) after vaccination. Secondary outcome included systemic reactions after vaccination, such as fever, injection site tenderness, general malaise and myalgia. The definition of severe adverse event was Guillain-Barré syndrome, immune thrombocytopenia, myocarditis, pericarditis, angioedema, or anaphylaxis.
The study was approved by the Ethics Committee of National Taiwan University Hospital (IRB No.202106106RINB). Written informed consents were from all participants.
Laboratory tests for anti-SARS-CoV-2 spike IgG
Anti-spike IgG in serum was determined by Elecsys® Anti-SARS-CoV-2 S Immunoassay (Roche Diagnostics, Mannheim, Germany). Antibody concentration ≥0.8 U/mL was defined as positive.
Laboratory tests for T cell response to SARS-CoV-2 spike protein
T cell production of IFN-γ in whole blood was quantified by Interferon-Gamma-Release Assay (IGRA) (EUROIMMUN Medical Laboratory Diagnostics, Lübeck, Germany) after stimulation by SARS-CoV-2 spike protein.34 , 35 IFN-γ concentration above that of the higher calibrator (2500 mIU/mL) was considered as equal to 2500 mIU/mL. IFN-γ concentration ≥100 mIU/mL was defined as positive.36
Statistical analysis
Data were expressed as mean (95% confidence interval), median (range) or number (percentage). Independent t test or Mann–Whitney test was used to compare continuous data between groups. Chi-square or Fisher's exact test was used to compare categorical data shown as count and percentage between groups. General linear model was used to predict the risk factors of anti-spike IgG and IFN-γ after the second dose of vaccine. Statistical analyses were performed using SAS software, version 9.4 (SAS Institute Inc). P < 0.05 was considered statistically significant.
Results
Baseline characteristics of PD patients
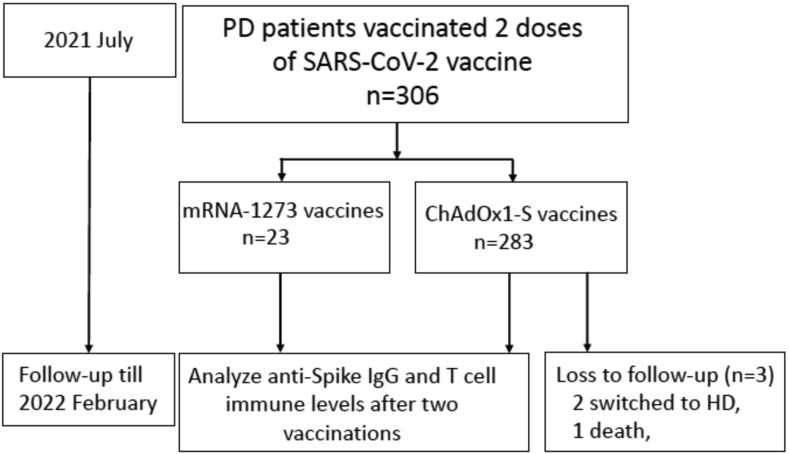
Totally 306 PD patients were prospectively enrolled to received two doses of SARS-CoV-2 vaccines from July 2021 and followed until February 2022 (Fig. 1 ). Antibodies against the nucleotide protein were negative in all participants. In PD patients, 283 and 23 received two doses of ChAdOx1-S and two doses of mRNA-1273 vaccines, respectively. Baseline characteristics of all PD patients were showed in Table 1 . No difference in sex, age, comorbidities and laboratory test results was noted between PD patients receiving ChAdOx1-S and mRNA-1273 vaccines, but the average dialysis vintage was longer in mRNA-1273 group (Table 1). In the follow-up period, one patient died and 2 patients switched to HD due to PD peritonitis in ChAdOx1-S group after completing analysis of immune response.
Figure 1.
Flow diagram of all participants. Abbreviations: HD, hemodialysis; IgG, immunoglobulin; PD, peritoneal dialysis.
Table 1.
Baseline characteristics of PD patients.
| All (n = 306) | ChAdOx1-S (n = 283) | mRNA-1273 (n = 23) | P value | |
|---|---|---|---|---|
| Male (n (%)) | 161 (52.6) | 152 (53.7) | 9 (39.1) | 0.198 |
| Age (years, mean (95% C.I.)) | 55.6 (54.1–57.1) | 55.6 (54.0–57.1) | 55.0 (49.6–60.4) | 0.848 |
| Dialysis vintage (months, mean (95% C.I.)) | 51.9 (46.4–57.3) | 50.2 (44.7–55.7) | 72.9 (47.3–98.5) | 0.030 |
| Comorbidities (n (%)) | ||||
| Diabetes mellitus | 97 (31.7) | 92 (32.5) | 3 (13.0) | 0.061 |
| Cardiovascular disease | 75 (24.5) | 69 (24.4) | 5 (21.7) | >0.1 |
| Previous cancer historya | 22 (7.2) | 21 (7.4) | 1 (4.4) | >0.1 |
| Previous kidney transplantation | 10 (3.3) | 8 (2.8) | 2 (8.7) | 0.168 |
| Laboratory test (mean (95% C.I.)) | ||||
| Albumin (mg/dL) | 3.85 (3.81–3.90) | 3.85 (3.81–3.90) | 3.84 (3.61–4.08) | 0.902 |
| Hemoglobin (g/dL) | 11.0 (10.8–11.1) | 10.9 (10.8–11.1) | 11.1 (10.6–11.6) | 0.495 |
| Leukocyte (1000/μL) | 7.48 (7.19–7.77) | 7.47 (7.16–7.77) | 7.62 (6.53–8.72) | 0.775 |
Abbreviations: C.I., confidence interval.
Cancer-free during study.
The adverse events after vaccinations in PD patients
No serious adverse events were reported by PD patients after vaccinations. The most frequently reported adverse events in PD patients included injection site tenderness (49.1%), generalized malaise (44.8%), fever (37.4%) in ChAdOx1-S group, and myalgia (17.4%), fever (17.4%), generalized malaise (17.4%) in mRNA-1273 group (Table 2 ). In PD patients, ChAdOx1-S group only had more generalized malaise than mRNA-1273 group (P < 0.001) In the same study period, adverse events in 604 volunteers were recorded (Table 2). The most frequently reported adverse events in volunteers included myalgia (67.3%), generalized malaise (64.6%), headache (45.1%) in ChAdOx1-S group, and injection site tenderness (47.7%), generalized malaise (41.7%), fever (33.0%) in mRNA-1273 group. In volunteers, ChAdOx1-S group suffered from less injection site tenderness but more myalgia, generalized, headache than mRNA-1273 group (P = 0.004, <0.001, <0.001, <0.001, respectively). PD patients had more injection site tenderness but less myalgia, generalized malaise and headache than volunteers after ChAdOx1-S vaccination (P = 0.003, <0.001, <0.001, <0.001, respectively). Moreover, PD patients had less myalgia, generalized malaise and headache than volunteers after mRNA-1273 vaccination (P = 0.03, <0.001, = 0.002, respectively). Overall, PD patients had less adverse events including myalgia, generalized malaise and headache after vaccination than volunteers (P < 0.001, <0.001, <0.001, respectively).
Table 2.
Adverse events in PD patients and volunteers.
| Adverse Events | PD patients |
Volunteers |
aP | bP | cP | dP | eP | ||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| ChAdOx1-S (n = 283) | mRNA-1273 (n = 23) | ChAdOx1-S (n = 244) | mRNA-1273 (n = 360) | ||||||||||
| Fever | 106 | (37.4) | 4 | (17.3) | 101 | (39.23) | 151 | (33.0) | 0.05 | 0.09 | 0.65 | 0.11 | 0.84 |
| Injection site. tenderness | 139 | (49.1) | 8 | (34.7) | 94 | (36.6) | 218 | (47.7) | 0.18 | 0.004 | 0.003 | 0.22 | 0.20 |
| Myalgia | 76 | (26.8) | 4 | (17.3) | 173 | (67.3) | 179 | (32.6) | 0.32 | <0.001 | <0.001 | 0.03 | <0.001 |
| Generalized. malaise | 127 | (44.8) | 1 | (4.4) | 166 | (64.6) | 229 | (41.7) | <0.001 | <0.001 | <0.001 | <0.001 | <0.001 |
| Headache | 31 | (10.9) | 0 | (0) | 116 | (45.1) | 133 | (24.2) | 0.09 | <0.001 | <0.001 | 0.002 | <0.001 |
Data were shown as n (%).
P value between ChAdOx1-S group and mRNA-1273 group in PD patients.
P value between ChAdOx1-S group and mRNA-1273 group in volunteers.
P value between PD patients and volunteers receiving ChAdOx1-S vaccine.
P value between PD patients and volunteers receiving mRNA-1273 vaccine.
P value between all PD patients and all volunteers.
Lower serum anti-spike IgG concentrations in PD patients after the first dose of vaccine
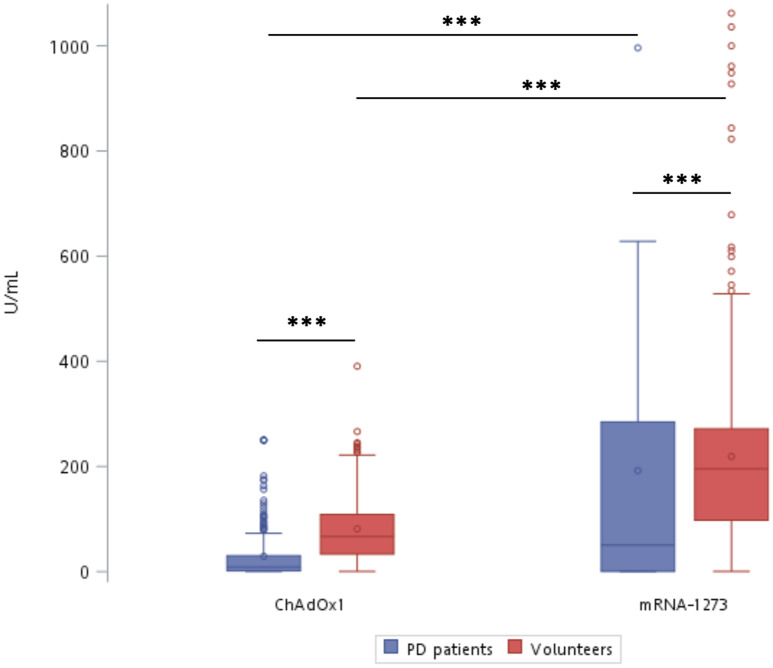
After the first dose of vaccine, positive rates for antibody detection were 80.5% and 73.3% in ChAdOx1-S group and mRNA-1273 group of PD patients, respectively (P = 0.71). The positive rates for antibody detection were 98.7% and 99.7% in ChAdOx1-S group and mRNA-1273 group of volunteers, higher than ChAdOx1-S group and mRNA-1273 group of PD patients, respectively (P < 0.001 for both ChAdOx1-S group and mRNA-1273 groups). The median antibody levels were 8.5 U/mL and 50.4 U/mL in ChAdOx1-S and mRNA-1273 group of PD patients, and 66.6 U/mL and 195.3 U/mL in ChAdOx1-S and mRNA-1273 group of volunteers, respectively (Fig. 2 ). Serum antibody concentrations induced by ChAdOx1-S vaccine were lower than those induced by mRNA-1273 vaccine in both PD patients and volunteers (Fig. 2). Moreover, serum antibody concentrations were lower in PD patients than those in volunteers receiving corresponding vaccine (Fig. 2).
Figure 2.
Lower serum levels of anti-spike antibody after the first dose of ChAdOx1-S and mRNA-1273 vaccines in PD patients and volunteers. Box and Whisker plots showed the data of 25th–75th percentile, and the data of 10th–90th percentile, respectively. Horizontal bar in Box represented the average. ∗P < 0.05 and ∗∗∗P < 0.001 by Mann–Whitney test.
Comparable serum anti-spike IgG concentrations in PD patients and volunteers after the second dose of vaccine
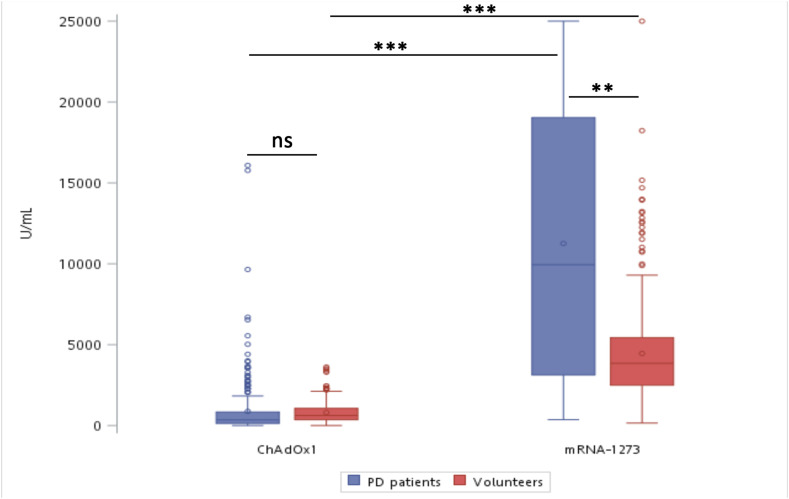
After the second dose of vaccine, positive rates for antibody detection increased to 98.2% and 100.0% in ChAdOx1-S and mRNA-1273 group of PD patients (P = 1), 100.0% and 100.0% in ChAdOx1-S and mRNA-1273 group of volunteers, respectively. The positive rates between PD patients and volunteers were not different after ChAdOx1-S vaccination, either (P = 0.16). The median antibody concentrations were 344.8 U/mL and 9941.0U/mL in ChAdOx1-S group and mRNA-1273 group of PD patients, and 620.3 U/mL and 3845.0 U/mL in ChAdOx1-S group and mRNA-1273 group of volunteers, respectively (Fig. 3 ). Although serum antibody concentrations were not different between PD patients and volunteers after the second dose of ChAdOx1-S vaccine, mRNA-1273 vaccine induced higher serum antibody concentrations than ChAdOx1-S vaccine in both PD patients and volunteers (Fig. 3). Interestingly, serum antibody concentrations in PD patients were higher than those in volunteers after the second dose of mRNA-1273 vaccine (Fig. 3).
Figure 3.
Comparable serum levels of anti-spike antibody after the second dose of ChAdOx1-S and mRNA-1273 vaccines in PD patients and volunteers. Box and Whisker plots showed the data of 25th–75th percentile, and the data of 10th–90th percentile, respectively. Horizontal bar in Box represented the average. ∗∗P < 0.01 and ∗∗∗P < 0.001 by Mann–Whitney test. ns. Non-significant.
Lower blood T cell response to spike protein in ChAdOx1-S group of PD patients
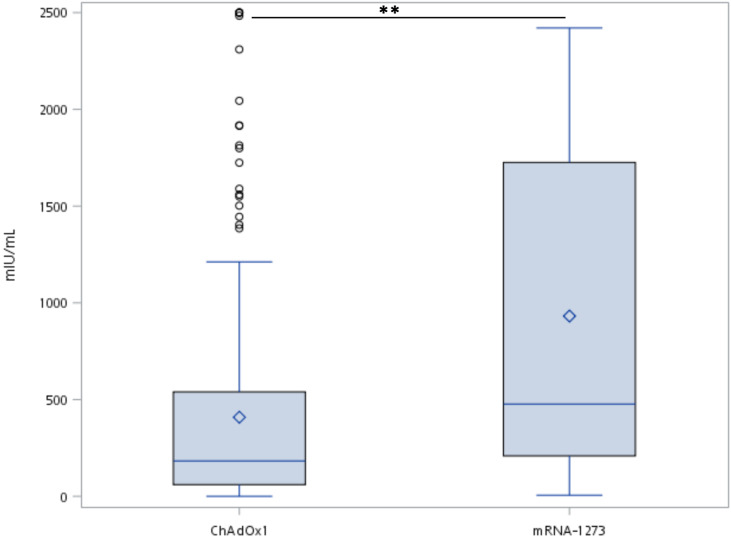
The positive rates for blood T cell IFN-γ production in response to spike protein stimulation was 92.5% and 100% in ChAdOx1-S group and mRNA-1273 group, respectively (P = 0.61). The median IFN-γ concentration was 182.8 mIU/mL in ChAdOx1-S group, which was substantially lower than the median concentration 476.8 mIU/mL in mRNA-1273 group (Fig. 4 ).
Figure 4.
Higher blood T cell response to spike protein in mRNA-1273 group of PD patients. Blood T cell response was identified by interferon-gamma release assays. Box and Whisker plots showed the data of 25th–75th percentile, and the data of 10th–90th percentile, respectively. Horizontal bar in Box represented the average. ∗∗P < 0.01 by Mann–Whitney test.
Factors associated with serum anti-spike IgG and blood T cell IFN-γ production in PD patients after SARS-CoV-2 vaccination
The association of baseline characteristics and comorbidities with serum antibody concentration and blood T cell IFN-γ production were analyzed in PD patients (Table 3 ). We observed longer PD vintage was substantially associated with higher antibody and IFN-γ concentrations after ChAdOx1-S vaccination in PD patients (Table 3). Cancer history was positively associated with antibody concentration, not IFN-γ concentration after ChAdOx1-S vaccination (Table 3). Old age was negatively associated with IFN-γ concentration, not antibody concentration after mRNA-1273 vaccination (Table 3).
Table 3.
Association of baseline characteristics and comorbidities with anti-spike IgG and IFN-γ concentrations in PD patients after the second dose of vaccine.
| Anti-spike IgG |
IFN-γ |
|||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| ChAdOx1-S |
mRNA-1273 |
ChAdOx1-S |
mRNA-1273 |
|||||||||
| Coef. | (95% C.I.) | P | Coef. | (95% C.I.) | P | Coef. | (95% C.I.) | P | Coef. | (95% C.I.) | P | |
| Age (year) | −0.5 | (-15.8-14.7) | 0.94 | 209.6 | (-95.6-514.9) | 0.16 | −0.6 | (-5.7–4.5) | 0.81 | −36.0 | (-70.9–1.0) | 0.04 |
| Gender (female) | 217.9 | (-188.6-624.4) | 0.29 | 4446.0 | (-3297.7-12189.7) | 0.24 | −102.7 | (-235.4-30.1) | 0.12 | 274.4 | (-697-1245.8) | 0.55 |
| Diabetes mellitus | −215.3 | (-646.9-216.3) | 0.32 | −1352.3 | (-12932.4-10227.8) | 0.81 | −52.1 | (-192.5-90.4) | 0.47 | −660.5 | (-2087.2-8766.1) | 0.33 |
| Cardiovascular. disease | 90.5 | (-382.3-563.4) | 0.70 | 1279.6 | (-8171-10730.1) | 0.78 | 18.4 | (-141.7-178.5) | 0.82 | −206.8 | (-1327.9-914.4) | 0.69 |
| Previous cancer history | 858.0 | (89.8–1626.3) | 0.02 | −6777.2 | (-25679.4-12124.9) | 0.46 | −66.5 | (-324.9-192) | 0.61 | – | – | – |
| Previous kidney. transplantation | 160.8 | (-1065.1-1385.3) | 0.79 | 3332.1 | (-10445.4-17109.7) | 0.62 | −100.4 | (-480.1-279.3) | 0.60 | 284.1 | (-1182.9-1751.2) | 0.68 |
| Dialysis vintage (month) | 6.2 | (2.0–10.5) | 0.004 | −11.6 | (-79.0-55.7) | 0.72 | 2.0 | (0.6–3.5) | 0.006 | −0.8 | (-10.6-9.1) | 0.87 |
Abbreviations: IFN-γ, interferon-gamma.
The association of adverse events with antibody and IFN-γ concentrations were analyzed in PD patients, too (Table 4 ). We observed that fever and myalgia were substantially positively associated with higher IFN-γ concentrations after mRNA-1273 vaccination (Table 4). However, the antibody and IFN-γ concentrations were not associated with any studied adverse events after ChAdOx1-S vaccination (Table 4).
Table 4.
Association of adverse events with anti-spike IgG and IFN-γ concentration after the second dose of vaccine.
| Anti-spike IgG |
IFN-γ |
|||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| ChAdOx1-S |
mRNA-1273 |
ChAdOx1-S |
mRNA-1273 |
|||||||||
| Coef. | (95% C.I.) | P | Coef. | (95% C.I.) | P | Coef. | (95% C.I.) | P | Coef. | (95% C.I.) | P | |
| Fever | −175.0 | (−594–244.1) | 0.41 | 2963.8 | (−7251.6–13179.2) | 0.55 | 56.1 | (−79.5–191.7) | 0.41 | 1667.4 | (860.8–2474.1) | <0.001 |
| Injection site tenderness | 395.2 | (−8.4–798.8) | 0.054 | −4090.3 | (−12077.3–3896.6) | 0.29 | 16.5 | (−116.4–149.5) | 0.80 | −242.0 | (−1240.8–756.7) | 0.61 |
| Myalgia | −339.3 | (−795.8–117.1) | 0.14 | 5268.3 | (−4754.02–15290.7) | 0.28 | −38.6 | (−185.2–108.1) | 0.60 | 1508.2 | (604.5–2412) | 0.003 |
| General malaise | −399.7 | (−805.4–5.9) | 0.05 | −1032.5 | (−20177.4–18112.4) | 0.91 | −43.7 | (−177.2–89.6) | 0.51 | 1346.3 | (−516.9–3209.5) | 0.14 |
| Headache | −298.3 | (−947.6–351) | 0.36 | – | – | 77.1 | (−125.5–279.6) | 0.45 | – | – | – | |
Discussion
Here we report five important findings for SARS-CoV-2 vaccination in PD patients: (1) A delayed anti-spike IgG response to both ChAdOx1-S and mRNA-1273 vaccinations happened in PD patients; (2) Anti-spike IgG concentrations after two doses of both ChAdOx1-S vaccination were comparable in PD patients and non-dialysis volunteers; (3) Blood T cell IFN-γ production after spike protein stimulation in mRNA-1273 group was higher than ChAdOx1-S group of PD patients; (4) Longer PD vintage was associated with higher anti-spike IgG and IFN-γ concentrations in ChAdOx1-S group of PD patients; and (5) Adverse events were less reported in PD patients than volunteers.
Our data endorsed SARS-CoV-2 vaccination in PD patients based on the safety and immune response although a delayed anti-spike IgG response to vaccination was noted when compared to volunteers. No serious adverse event was reported in our PD patients and the adverse events were less in PD patients than volunteers. Our data showed the positive rates for anti-spike IgG detection increased substantially to almost 100% in both ChAdOx1-S group and mRNA-1273 groups of PD patients after the second dose of vaccine, and the serum concentrations of anti-spike IgG were comparable in ChAdOx1-S group of both PD patients and volunteers. Interestingly, our data showed that serum anti-spike IgG concentrations in PD patients were higher than volunteers after the second dose of mRNA-1273 vaccine. Our findings endorsed the safety and efficacy of SARS-coV-2 vaccination in PD patients after two doses of vaccines, a result different from previous studies showing lower seroconversion rate of vaccination and lower anti-spike IgG concentrations in dialysis patients (more patients received HD therapy) than general population.13, 14, 15, 16, 17 , 23 Interestingly, Duarte et al. demonstrated that PD patients developed higher anti-spike IgG concentrations than HD patients after SARS-CoV-2 vaccination (most patients received mRNA vaccines including mRNA-1273 and BNT162b2).27 Moreover, Speer et al. demonstrated that PD patients after BNT162b2 vaccination had lower concentrations of anti-spike IgG and neutralizing antibody than healthy controls, but higher than HD patients.29 But the other studies did not demonstrate different anti-spike IgG concentrations between PD and HD patients.30 , 31
HD and immunosuppressive therapy were negative predictors of seroconversion after SARS-coV-2 vaccination in HD patients compared with healthy control.37 Furthermore, younger age and better dialysis quality were independent characteristics associated with a higher anti-spike IgG concentrations after vaccination.37 Compared with volunteers, our study showed non-inferior and higher anti-spike IgG concentrations in PD patients after two doses of ChAdOx1-S and mRNA-1273 vaccines, respectively. One of possible reasons was our PD patients in ChAdOx1-S group received two doses of ChAdOx1-S vaccines 12 weeks apart, not 28 days, which might increase their immune responses.38 The other one reason was that baseline characteristics of our non-dialysis/non-immunosuppressive volunteers were not recorded in detail, which might have effect on the immune response to SARS-CoV-2 vaccination.
Cellular immune response is important for the protective adaptive immunity but it was less assessed in most of the previous studies for SARS-CoV-2 vaccination in dialysis patients.37 , 39 The ROMANOV study reported HD patients had lower humeral and cellular immune responses after two doses of BNT162b2 vaccines than healthy controls.37 Broseta et al. reported that only 62% of HD patients 3 weeks after two doses of mRNA-1273 or BNT162b2 vaccines had a positive blood T cell response to SARS-CoV-2 peptide pools.39 In our study, we found that two doses of vaccines induced positive T cell response in 92.9% of ChAdOx1-S group and 100.0% of mRNA-1273 group in PD patients. This may be because their samples were collected 3 weeks after vaccinations earlier than our analysis. Unfortunately, we could not compare the T cell response between PD patients and volunteers in our study because different method was used in volunteers.
In our PD patients after ChAdOx1-S vaccination, PD vintage, not age, was positively associated with higher concentrations of anti-spike IgG and IFN-γ. We thought patients with longer PD vintage should have superior generalized health status for better technique survival and response to vaccination. Evidence has shown lower seroconversion rate or delayed immune response in patients with cancer after SARS-CoV-2 vaccination,40 but our data showed that previous history of cancer was associated with higher anti-spike IgG concentrations in our PD patients after ChAdOx1-S vaccination. The reason for higher anti-spike IgG concentrations in our PD patients with previous history of cancer was not clear. We thought patients could survive from cancer and became cancer-free should have better generalized health status for response to vaccination.
There are few studies of SARS-CoV-2 vaccination in PD patients. As we know, our study was the first prospective study of SARS-CoV-2 vaccination in more than 300 PD patients. However, this study was limited by a single-center study and non-random allocation of vaccine type. Moreover, this study had only 23 PD patients receiving two doses of mRNA-1273 vaccines, which was too small a number of patients to make a solid conclusion though old age was negatively associated with IFN-γ levels in mRNA-1273 group. One recent study demonstrated a negative correlation with a drop-off age over 80 years between T cell response and age.41
Although no neutralizing antibody was measured in our participants, a previous study proved that anti-spike IgG detected by Elecsys® Anti-SARS-CoV-2 S Immunoassay can predict SARS-CoV-2 neutralizing antibodies.42 Moreover, Takei et al. also reported a linear correlation between Elecsys® anti-spike IgG assay and the neutralization assay (r = 0.7253, r2 = 0.5261) in 146 serum samples from 59 patients with COVID-19.43
In conclusion, almost 100% of PD patients had positive seroconversion and developed comparable humeral immune response with less adverse events after two doses of SARS-CoV-2 vaccination when compared with volunteers. Although more than 90% of PD patients also developed positive T cell response after two doses of SARS-CoV-2 vaccination, mRNA-1273 vaccine induced significantly higher antibody and T cell response than ChAdOx1-S vaccine in PD patients. Booster doses are recommended for PD patients after two doses of ChAdOx1-S vaccination.
Author contributions
Z-YY, C-FL, Y-TC, P-RH, Y-MC and S-LL designed the studies. Z-YY, S-YY, S-IC, M-JL, C-MK and Y-TH assisted in the experiments. Z-YY and T-SL assisted in the statistical analysis. Y-TC and S-LL provided the funding for the study and drafted the manuscript.
Financial support
YTC is supported by National Science and Technology Council (NSTC) (111-2314-B-002-182, 111-2218-E-002-042-), National Taiwan University Hospital (MM022-2) and Mrs. Hsiu-Chin Lee Kidney Research Foundation. SLL is supported by NSTC (108-2314-B-002-078-MY3, 110-2314-B-002-208 and 111-2314-B-002-037), NTUH (110-S4837, 111-S0014, 111-TMU022), NTUH and National Taiwan University College of Medicine (NSCCMOH-131-43, 145-72), NTU (NTU-CC-110L893304, 111L892704) and Taiwan Health Foundation.
Data statement
Raw data are available from the study investigators on request.
Declaration of competing interest
The authors declare no conflict of interests.
Acknowledgement
We thank Yan-Hua Su (National Taiwan University) and all PD nurses in National Taiwan University Hospital for their experiment supports in this study.
References
- 1.World Health Organization (WHO) WHO coronavirus (COVID-19) dashboard https://covid19.who.int/, Accessed 10 October 2022.
- 2.Cheng Y., Luo R., Wang K., Zhang M., Wang Z., Dong L., et al. Kidney disease is associated with in-hospital death of patients with COVID-19. Kidney Int. 2020;97:829–838. doi: 10.1016/j.kint.2020.03.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Thaunat O., Legeai C., Anglicheau D., Couzi L., Blancho G., Hazzan M., et al. IMPact of the COVID-19 epidemic on the moRTAlity of kidney transplant recipients and candidates in a French Nationwide registry sTudy (IMPORTANT) Kidney Int. 2020;98(6):1568–1577. doi: 10.1016/j.kint.2020.10.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Hsu C.M., Weiner D.E., Aweh G., Miskulin D.C., Manley H.J., Stewart C., et al. COVID-19 among US dialysis patients: risk factors and outcomes from a national dialysis provider. Am J Kidney Dis. 2021;77(5):748–756 e1. doi: 10.1053/j.ajkd.2021.01.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Valeri A.M., Robbins-Juarez S.Y., Stevens J.S., Ahn W., Rao M.K., Radhakrishnan J., et al. Presentation and outcomes of patients with ESKD and COVID-19. J Am Soc Nephrol. 2020;31(7):1409–1415. doi: 10.1681/ASN.2020040470. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Williamson E.J., Walker A.J., Bhaskaran K., Bacon S., Bates C., Morton C.E., et al. Factors associated with COVID-19-related death using OpenSAFELY. Nature. 2020;584(7821):430–436. doi: 10.1038/s41586-020-2521-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.De Meester J., De Bacquer D., Naesens M., Meijers B., Couttenye M.M., De Vriese A.S., et al. Incidence, characteristics, and outcome of COVID-19 in adults on kidney replacement therapy: a regionwide registry study. J Am Soc Nephrol. 2021;32(2):385–396. doi: 10.1681/ASN.2020060875. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Weinhandl E.D., Wetmore J.B., Peng Y., Liu J., Gilbertson D.T., Johansen K.L. Initial effects of COVID-19 on patients with ESKD. J Am Soc Nephrol. 2021;32(6):1444–1453. doi: 10.1681/ASN.2021010009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Jiang H.J., Tang H., Xiong F., Chen W.L., Tian J.B., Sun J., et al. COVID-19 in peritoneal dialysis patients. Clin J Am Soc Nephrol. 2020;16(1):121–123. doi: 10.2215/CJN.07200520. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Baden L.R., El Sahly H.M., Essink B., Kotloff K., Frey S., Novak R., et al. Efficacy and safety of the mRNA-1273 SARS-CoV-2 vaccine. N Engl J Med. 2021;384(5):403–416. doi: 10.1056/NEJMoa2035389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Polack F.P., Thomas S.J., Kitchin N., Absalon J., Gurtman A., Lockhart S., et al. Safety and efficacy of the BNT162b2 mRNA Covid-19 vaccine. N Engl J Med. 2020;383(27):2603–2615. doi: 10.1056/NEJMoa2034577. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Voysey M., Clemens S.A.C., Madhi S.A., Weckx L.Y., Folegatti P.M., Aley P.K., et al. Safety and efficacy of the ChAdOx1 nCoV-19 vaccine (AZD1222) against SARS-CoV-2: an interim analysis of four randomised controlled trials in Brazil, South Africa, and the UK. Lancet. 2021;397(10269):99–111. doi: 10.1016/S0140-6736(20)32661-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Agur T., Ben-Dor N., Goldman S., Lichtenberg S., Herman-Edelstein M., Yahav D., et al. Antibody response to mRNA SARS-CoV-2 vaccine among dialysis patients - a prospectivecohort study. Nephrol Dial Transplant. 2021;36:1347–1349. doi: 10.1093/ndt/gfab155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Grupper A., Sharon N., Finn T., Cohen R., Israel M., Agbaria A., et al. Humoral response to the Pfizer BNT162b2 vaccine in patients undergoing maintenance hemodialysis. Clin J Am Soc Nephrol. 2021;16(7):1037–1042. doi: 10.2215/CJN.03500321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Yanay N.B., Freiman S., Shapira M., Wishahi S., Hamze M., Elhaj M., et al. Experience with SARS-CoV-2 BNT162b2 mRNA vaccine in dialysis patients. Kidney Int. 2021;99(6):1496–1498. doi: 10.1016/j.kint.2021.04.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Simon B., Rubey H., Treipl A., Gromann M., Hemedi B., Zehetmayer S., et al. Haemodialysis patients show a highly diminished antibody response after COVID-19 mRNA vaccination compared with healthy controls. Nephrol Dial Transplant. 2021;36(9):1709–1716. doi: 10.1093/ndt/gfab179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Stumpf J., Siepmann T., Lindner T., Karger C., Schwobel J., Anders L., et al. Humoral and cellular immunity to SARS-CoV-2 vaccination in renal transplant versus dialysis patients: a prospective, multicenter observational study using mRNA-1273 or BNT162b2 mRNA vaccine. Lancet Reg Health Eur. 2021;9:100–178. doi: 10.1016/j.lanepe.2021.100178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Garcia P., Anand S., Han J., Montez-Rath M.E., Sun S., Shang T., et al. COVID-19 vaccine type and humoral immune response in patients receiving dialysis. J Am Soc Nephrol. 2022;33(1):33–37. doi: 10.1681/ASN.2021070936. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Mulhern J.G., Fadia A., Patel R., Ficociello L.H., Willetts J., Dahne-Steuber I.A., et al. Humoral response to mRNA versus an adenovirus vector-based SARS-CoV-2 vaccine in dialysis patients. Clin J Am Soc Nephrol. 2021;16(11):1720–1722. doi: 10.2215/CJN.06450521. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Hsu C.M., Weiner D.E., Aweh G.N., Manley H.J., Ladik V., Frament J., et al. Seroresponse to SARS-CoV-2 vaccines among maintenance dialysis patients. Am J Kidney Dis. 2022;79(2):307–310. doi: 10.1053/j.ajkd.2021.10.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Oliver M.J., Thomas D., Balamchi S., Ip J., Naylor K., Dixon S.N., et al. Vaccine effectiveness against SARS-CoV-2 infection and severe outcomes in the maintenance dialysis population in Ontario, Canada. J Am Soc Nephrol. 2022;33(4):839–849. doi: 10.1681/ASN.2021091262. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Sibbel S., McKeon K., Luo J., Wendt K., Walker A.G., Kelley T., et al. Real-world effectiveness and immunogenicity of BNT162b2 and mRNA-1273 SARS-CoV-2 vaccines in patients on hemodialysis. J Am Soc Nephrol. 2022;33(1):49–57. doi: 10.1681/ASN.2021060778. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Krueger K.M., Ison M.G., Ghossein C. Practical guide to vaccination in all stages of CKD, including patients treated by dialysis or kidney transplantation. Am J Kidney Dis. 2020;75(3):417–425. doi: 10.1053/j.ajkd.2019.06.014. [DOI] [PubMed] [Google Scholar]
- 24.Yadav A.K., Gondil V.S., Singla M., Goyal A., Kaushal R., Chauhan M., et al. Humoral response to one and two doses of ChAdOx1-S vaccine in patients on hemodialysis. Clin J Am Soc Nephrol. 2021;16:1875–1876. doi: 10.2215/CJN.10170721. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Brunelli S.M., Sibbel S., Karpinski S., Marlowe G., Walker A.G., Giullian J., et al. Comparative effectiveness of mRNA-based BNT162b2 vaccine versus adenovirus vector-based Ad26.COV2.S vaccine for the prevention of COVID-19 among dialysis patients. J Am Soc Nephrol. 2022;33(4):688–697. doi: 10.1681/ASN.2021101395. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Htay H., Foo M.W.Y., Gan S.S.W., Jayaballa M., Oei E.L., Tan M.S.H., et al. COVID-19 vaccination in peritoneal dialysis patients. Int Urol Nephrol. 2022;29:1–7. doi: 10.1007/s11255-022-03302-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Duarte R., Roldao M., Figueiredo C., Luz I., Ferrer F., Goncalves H., et al. Humoral response to BNT162b2 mRNA COVID-19 vaccine in peritoneal and hemodialysis patients: a comparative study. Ther Apher Dial. 2022;26(4):790–796. doi: 10.1111/1744-9987.13766. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Quiroga B., Soler M.J., Ortiz A., Martinez Vaquera S., Jarava Mantecon C.J., Useche G., et al. Safety and immediate humoral response of COVID-19 vaccines in chronic kidney disease patients: the SENCOVAC study. Nephrol Dial Transplant. 2022;37(10):1868–1878. doi: 10.1093/ndt/gfab313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Speer C., Schaier M., Nusshag C., Tollner M., Buylaert M., Kalble F., et al. Longitudinal humoral responses after COVID-19 vaccination in peritoneal and hemodialysis patients over twelve weeks. Vaccines. 2021;9(10):1130. doi: 10.3390/vaccines9101130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Patecki M., Merscher S., Dumann H., Bernhardt W., Dopfer-Jablonka A., Cossmann A., et al. Similar humoral immune responses in peritoneal dialysis and haemodialysis patients after two doses of the SARS-CoV-2 vaccine BNT162b2. Perit Dial Int. 2022;42(1):100–101. doi: 10.1177/08968608211055631. [DOI] [PubMed] [Google Scholar]
- 31.Nacasch N., Cohen-Hagai K., Benchetrit S., Zitman-Gal T., Einbinder Y., Erez D., et al. Comparison of long-term antibody response to mRNA SARS-CoV-2 vaccine among peritoneal dialysis and hemodialysis patients. Nephrol Dial Transplant. 2022;37(3):602–604. doi: 10.1093/ndt/gfab321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Bensouna I., Caudwell V., Kubab S., Acquaviva S., Pardon A., Vittoz N., et al. SARS-CoV-2 antibody response after a third dose of the BNT162b2 vaccine in patients receiving maintenance hemodialysis or peritoneal dialysis. Am J Kidney Dis. 2022;79(2):185–192 e1. doi: 10.1053/j.ajkd.2021.08.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Taiwan Centers for Diseases Control (T-CDC) COVID-19 (SARS-CoV-2 infection) https://www.cdc.gov.tw/En Accessed 16 April 2022.
- 34.Malipiero G., Moratto A., Infantino M., D'Agaro P., Piscianz E., Manfredi M., et al. Assessment of humoral and cellular immunity induced by the BNT162b2 SARS-CoV-2 vaccine in healthcare workers, elderly people, and immunosuppressed patients with autoimmune disease. Immunol Res. 2021;69(6):576–583. doi: 10.1007/s12026-021-09226-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Fernandez-Gonzalez M., Agullo V., Padilla S., Garcia J.A., Garcia-Abellan J., Botella A., et al. Clinical performance of a standardized SARS-CoV-2 interferon-gamma release assay for simple detection of T-cell responses after infection or vaccination. Clin Infect Dis. 2022;75:e338–e346. doi: 10.1093/cid/ciab1021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Schrezenmeier E., Bergfeld L., Hillus D., Lippert J.D., Weber U., Tober-Lau P., et al. Immunogenicity of COVID-19 tozinameran vaccination in patients on chronic dialysis. Front Immunol. 2021;12:690–698. doi: 10.3389/fimmu.2021.690698. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Espi M., Charmetant X., Barba T., Koppe L., Pelletier C., Kalbacher E., et al. The ROMANOV study found impaired humoral and cellular immune responses to SARS-CoV-2 mRNA vaccine in virus-unexposed patients receiving maintenance hemodialysis. Kidney Int. 2021;100(4):928–936. doi: 10.1016/j.kint.2021.07.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Voysey M., Costa Clemens S.A., Madhi S.A., Weckx L.Y., Folegatti P.M., Aley P.K., et al. Single-dose administration and the influence of the timing of the booster dose on immunogenicity and efficacy of ChAdOx1 nCoV-19 (AZD1222) vaccine: a pooled analysis of four randomised trials. Lancet. 2021;397(10277):881–891. doi: 10.1016/S0140-6736(21)00432-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Broseta J.J., Rodriguez-Espinosa D., Rodriguez N., Mosquera M.D.M., Marcos M.A., Egri N., et al. Humoral and cellular responses to mRNA-1273 and BNT162b2 SARS-CoV-2 vaccines administered to hemodialysis patients. Am J Kidney Dis. 2021;78(4):571–581. doi: 10.1053/j.ajkd.2021.06.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Corti C., Antonarelli G., Scotte F., Spano J.P., Barriere J., Michot J.M., et al. Seroconversion rate after vaccination against COVID-19 in patients with cancer-a systematic review. Ann Oncol. 2022;33(2):158–168. doi: 10.1016/j.annonc.2021.10.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.El Karoui K., De Vriese A.S. COVID-19 in dialysis: clinical impact, immune response, prevention, and treatment. Kidney Int. 2022;101(5):883–894. doi: 10.1016/j.kint.2022.01.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Resman Rus K., Korva M., Knap N., Avsic Zupanc T., Poljak M. Performance of the rapid high-throughput automated electrochemiluminescence immunoassay targeting total antibodies to the SARS-CoV-2 spike protein receptor binding domain in comparison to the neutralization assay. J Clin Virol. 2021;139 doi: 10.1016/j.jcv.2021.104820. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Takei S., Ai T., Yamamoto T., Igawa G., Kanno T., Tobiume M., et al. Performance evaluation of the Roche Elecsys(R) Anti-SARS-CoV-2 immunoassays by comparison with neutralizing antibodies and clinical assessment. PLoS One. 2022;17(9) doi: 10.1371/journal.pone.0274181. [DOI] [PMC free article] [PubMed] [Google Scholar]