Abstract
Multipotent mesenchymal stromal cells (MSCs) have been described as bone marrow stromal cells, which can form cartilage, bone or hematopoietic supportive stroma. In 2006, the International Society for Cell Therapy (ISCT) established a set of minimal characteristics to define MSCs. According to their criteria, these cells must express CD73, CD90 and CD105 surface markers; however, it is now known they do not represent true stemness epitopes. The objective of the present work was to determine the surface markers for human MSCs associated with skeletal tissue reported in the literature (1994–2021). To this end, we performed a scoping review for hMSCs in axial and appendicular skeleton. Our findings determined the most widely used markers were CD105 (82.9%), CD90 (75.0%) and CD73 (52.0%) for studies performed in vitro as proposed by the ISCT, followed by CD44 (42.1%), CD166 (30.9%), CD29 (27.6%), STRO-1 (17.7%), CD146 (15.1%) and CD271 (7.9%) in bone marrow and cartilage. On the other hand, only 4% of the articles evaluated in situ cell surface markers. Even though most studies use the ISCT criteria, most publications in adult tissues don't evaluate the characteristics that establish a stem cell (self-renewal and differentiation), which will be necessary to distinguish between a stem cell and progenitor populations. Collectively, MSCs require further understanding of their characteristics if they are intended for clinical use.
Keywords: Mesenchymal stem cell (MSCs), Cell surface marker, Bone, Cartilage, Bone marrow
1. Introduction
The bone marrow (BM) contains cells with self-renewal and differentiation capacity, known as multipotent mesenchymal stromal cells (MSCs) related to the skeletal system, which can differentiate into skeletal lineages (cartilage, bone, hematopoietic support stroma, adipocytes and fibroblasts). These cells represent a population destined to sustain skeletal tissue throughout the life of an individual [1]. Worldwide there has been an increasing need for skeletal tissue regeneration (bone or cartilage), due to an augmentation in trauma, cancer, bone and joint disorders, as well as ageing, associated with a decline in the quality of life. Hence, therapies have been proposed using stem cells, where MSCs would be an ideal source; however, their true nature and location for isolation has not been yet determined, because MSCs reside in different skeletal anatomical sites. Therefore, the aim of this work was to perform a scoping review regarding MSCs’ cell surface markers to establish the status reported in the literature.
The concept of post-natal mesenchymal stem cells originated with Friedenstein in 1966 from studies isolating BM stroma [2]. In 1988 Owen and Friedenstein described these BM stromal cells were made up of a heterogeneous population possibly containing stem and progenitor cells with the capacity to differentiate into diverse tissues including bone [3]. Following, Arnold Caplan in 1991 coined the term mesenchymal stem cells (MSCs) to describe a limited population of cells responsible for bone and cartilage formation in the embryo and its repair and turnover in adult tissues [4]. However, the term “mesenchymal” is not appropriate since it refers to a histological description of a cell with migratory capacity in the embryo, which can derive from mesoderm, neural crest or ectoderm [5]. Skeletal tissue has different embryological origins, such as somites, which give rise to the axial skeleton (vertebrae), lateral plate mesoderm forming appendicular bones and neural crest cells developing into the visceral cranium. Therefore, there is not a unique origin to form the skeleton and the term MSCs would be incorrect [5].
Since the year 2000 until now, publications associated with the term “MSCs” have starkly increased. Additionally, the term MSCs has extended into non-skeletal tissues, such as umbilical cord [6], adipose tissue [7] and placenta [8]. Moreover, lack of scientific rigor in defining postnatal skeletal stem cells has been identified [5]. In 2006 the International Society for Cell Therapy (ISCT) in an effort to establish a general consensus regarding multipotent mesenchymal stromal cells proposed a series of characteristics to define MSCs. These criteria included: adherence to plastic under standard culture conditions, expression of cell surface markers CD105, CD90 and CD73; absence of hematopoietic cell surface markers CD45, CD34, CD14, CD11b, CD79a or CD19 and HLA-DR, and last the capacity to differentiate in vitro into osteoblasts, adipocytes and chondrocytes, as determined by staining techniques [9]. Despite the fact that over 15 years have passed the same criteria are still used, even though they don't validate a true skeletal stem cell [10]. In 2018 Chan et al. reported “true skeletal stem cells” from fetal and adult tissues with the following cell surface markers: PDPN+, CD146-, CD73+, CD164+ with the capacity of self-renewal and multipotency generating bone, cartilage and stroma progenitors [11]. However, the scientific community has not echoed Chan's work.
Henceforth, after decades the biological definition of true MSCs remains a topic of discussion. Despite the lack of knowledge, an interest still prevails regarding this cell population for therapeutic purposes. Moreover, in vitro cell culture can modify protein expression properties [1]. To date it is not clear whether cell epitopes can ascertain MSCs; hence, the objective of this work was to identify from the literature which cell surface markers have been used to characterize hMSCs related to the skeletal system, since tissue of origin has been used indiscriminately. Additionally, this review assessed if markers were determined from in vitro studies or established by immunohistochemistry (IHC) from tissues in situ.
2. Materials and methods
PRISMA guidelines were used to perform a scoping review to identify in the literature cell surface markers that have been utilized to identify possible MSCs in vitro and in situ based on indexed publications in Medline (PubMed), Scopus and Web of Science databases. The following search equation was used “skeletal stem cells, mesenchymal stem cells, cell surface markers, in vitro, in vivo and in situ”, which should be included in the title, abstract or keywords (Table 1). Year of publication was not restricted, where the last date of search was July 26, 2022.
Table 1.
Terms and keywords selected for each evaluated variable. A search in Medline (PubMed), Scopus and Web of Science was performed using equations for all three variables.
| Variable | Keywords | Equation |
|---|---|---|
| Skeletal stem cells | “Skeletal stem cells” “Mesenchymal stem cells” ” Stromal stem cells” “Mesenchymal Stromal stem cells” |
“Skeletal stem cells” OR “Mesenchymal stem cells“ OR “stromal stem cells” OR “Mesenchymal Stromal stem cells” |
| AND | ||
| Cell Surface marker | “Surface markers” Biomarkers “clusters of differentiation” Identification |
“Surface markers” OR Biomarkers OR “clusters of differentiation” OR Identification |
| AND | ||
| in situ and in vitro | “in vivo” “in vitro” “Cell culture” “in situ” |
“in vivo” OR “in vitro” OR “Cell culture” OR culture OR “in situ” |
| NOT | ||
| Equine OR Bovine OR Rhesus OR Rat OR Mice OR Rodent OR Rabbit OR Porcine OR Canine OR Ovine OR Cryconservation OR Adipose OR “Umbilical Cord” OR Placenta OR “Dental pulp” OR Neuron OR Hepatitis OR Arterio* OR Skin OR Murine OR Mucosa OR Pancreatic* OR animal. | ||
For this study, no review protocol was required since this work was not associated with a clinical trial.
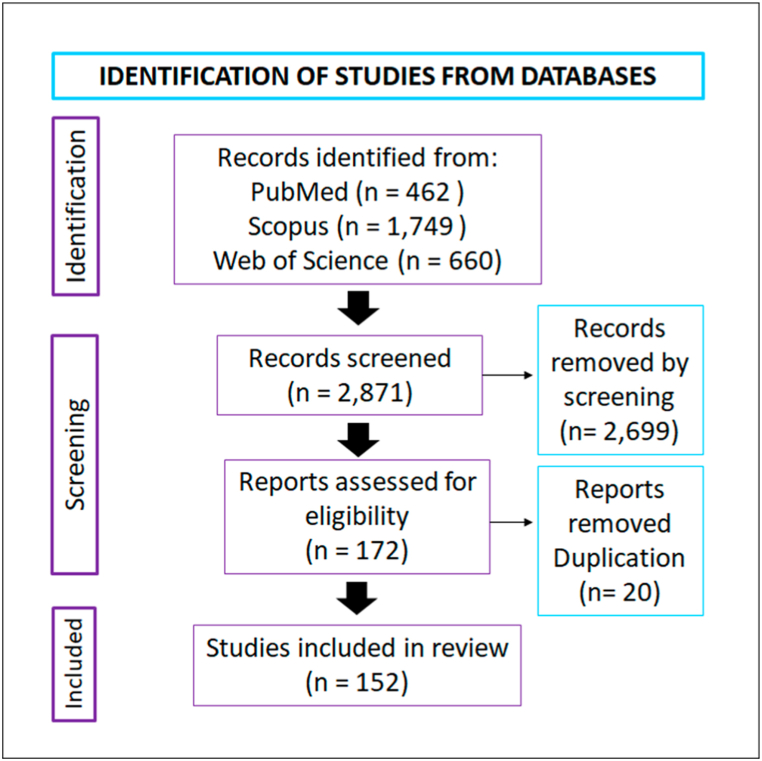
A total of 462 articles was obtained in PubMed (the search was refined by including only humans and NOT taking into account the following: Hematopoietic* OR peripheral blood OR menstrual blood OR cardio* OR “salivary glands” OR synovial OR papilla OR heart* OR denta* OR muscle OR Cornea* OR synovi* OR “Schwann cell” OR vascular OR brain OR odont* OR neural OR liver OR hepatic OR aorta OR amniotic OR muscle OR adipo* OR uro* OR Menstrual OR Ovari* OR cord OR endometr* OR periodontal* OR ligament OR tendon OR synovi* OR cardio* OR diabetes OR jelly OR lung OR colo* OR spleen or kidney). In contrast, initially 2716 articles were found in Scopus; however, after applying the filter “human” and “humans” from the listing “Refine results” a total of 1749 articles were attained. Last, in Web of Science 660 publications were identified for a total of 2871 articles. With this total an excel table was created with information containing author, year of publication and journal assigning a number to each retrieved article. Eighteen observers revised the 2871 articles. After this first selection a total of 172 articles were selected based on inclusion and exclusion criteria. Exclusion criteria included: animals (2.4%), visceral skeleton (0.7%), synovial tissue (0.5%), commercial stem cells also considering TERT MSCs (3.7%), other tissues other than bone and cartilage (25.2%), articles not in English (0.2%), non-research articles (25.8%) and non-related (35.6%). Following, only three observers performed a second round of revision where duplicated articles were excluded, for a final total of 152 articles where 94.8% of the initial 2871 articles were omitted (Fig. 1).
Fig. 1.
Article selection. Literature search was performed in three databases Medline (PubMed), Scopus, and Web of Science obtaining a total of 2871 articles. After a revision performed by 18 observers, 152 articles were selected.
3. Results
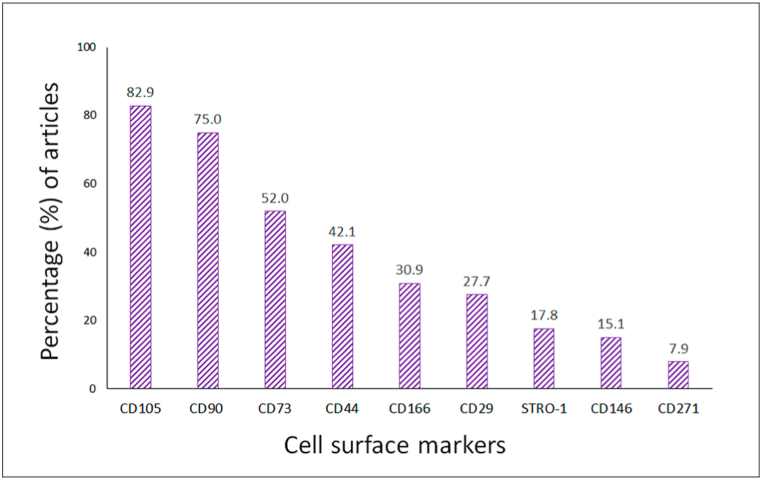
The objective of the present work was to determine MSCs in vitro or in situ cell surface markers associated with skeletal tissue according to what has been reported in the literature for Medline (PubMed), Scopus and Web of Science. Based on what has been published MSCs were classified according to their anatomical location: bone marrow (82.0%) and cartilage (11.3%). Furthermore, for 6.7% of the publications out of the 152 articles did not describe site of isolation. Moreover, for some publications more than one anatomical site was characterized, for example bone marrow and cartilage. Additionally, for the 152 articles evaluated the most frequent cell surface markers were CD105+, CD90+ and CD73+, followed by CD44+, CD166+, CD29+, STRO-1+, CD146+ and CD271+ (Fig. 2).
Fig. 2.
Percentage of articles evaluating cell surface markers in vitro. The most frequently used markers agree with those proposed by ISCT 2006: CD105 (82.9%), CD90 (75.0%), CD73 (52.0%), followed by CD44 (42.1), CD166 (30.9%), CD29 (27.7%), STRO-1 (17.8%), CD146 (15.1%) and CD271 (7.9%).
3.1. Bone marrow cell surface markers
Most studies isolated cells from bone marrow (92.1%) from the following anatomical sites: femur, hip, iliac crest, vertebra and knee. The most frequent cell surface markers from cells cultured in vitro were those proposed by the ISCT (CD105+, CD73+ and CD90+), followed by CD44+, CD166+, CD29+, STRO-1+, CD146+ and CD271+ (Table 2).
Table 2.
Cell Surface markers assessed from in vitro studies in cells isolated from bone marrow in femur, hip, iliac crest, vertebra and knee. Negative cell surface markers were not included, such as CD45, CD34, CD14, CD11b, CD79a, CD19 and HLA-DR.
| Cell Surface marker | Anatomical site | Reference |
|---|---|---|
| CD105, CD90 and CD73 | Femur | [[12], [13], [14], [15], [16], [17], [18], [19], [20], [21], [22], [23], [24], [25], [26], [27], [28]] |
| Iliac crest | [[29], [30], [31], [32], [33], [34], [35], [36], [37], [38], [39], [40], [41], [42], [43], [44], [45], [46], [47], [48], [49], [50], [51], [52], [53], [54], [55], [56], [57], [58], [59], [60], [61], [62], [63], [64], [65], [66], [67], [68]] | |
| Hip | [[69], [70], [71], [72], [73], [74], [75]] | |
| Vertebra | [17,20,76] | |
| Knee | [[77], [78], [79], [80], [81]] | |
| Not described | [[82], [83], [84], [85], [86], [87], [88], [89], [90], [91], [92], [93], [94], [95], [96], [97], [98], [99], [100], [101], [102], [103], [104], [105], [106], [107], [108], [109]] | |
| CD44 | Femur | [13,15,17,22,26,110] |
| Hip | [75,111] | |
| Iliac crest | [32,36,37,40,44,47,53,55,59,112] | |
| Vertebra | [76] | |
| Knee | [[78], [79], [80], [81]] | |
| Not described | [[83], [84], [85],91,95,97,99,107,113] | |
| CD166 | Femur | [15,17,22,114,115] |
| Hip | [71,111,116] | |
| Iliac crest | [37,46,47,52,117] | |
| Vertebra | [17] | |
| Knee | [79,80] | |
| Not described | [33,84,85,104,107] | |
| CD29 | Femur | [18,22,115] |
| Hip | [111] | |
| Iliac crest | [35,36,39,47,48,57,68,78,80,118] | |
| Knee | [67,69,70] | |
| Not described | [83,84,87,100,105,107,113] | |
| STRO-1 | Femur | [22,27,119,120] |
| Hip | [73,116] | |
| Iliac crest | [62,118,121,123] | |
| Not indicated | [90,91,97,103,116,124,125] | |
| CD146 | Femur | [20,110] |
| Hip | [73] | |
| Iliac crest | [20,41,45,48,49,62,122] | |
| Not described | [83,84,87,90,94,97,103] | |
| CD271 | Femur | [110,126] |
| Hip | [73] | |
| Iliac crest | [122] | |
| Not described | [90] |
3.2. Cartilage cell surface markers
11.3% of the articles evaluated cell surface markers from cells cultured in vitro from cartilage tissue. As previously described for bone marrow the most frequent markers were CD105+, CD90+ and CD73+, followed by CD44+, CD166+, CD29+, STRO-1+ and CD146+ (Table 3).
Table 3.
Cell surface markers assessed from in vitro studies in cells isolated from cartilage in femur, hip, iliac crest, vertebra and knee. Negative cell surface markers were not included, such as CD45, CD34, CD14, CD11b, CD79a, CD19 and HLA-DR.
| Cell Surface markers | Anatomical site | Reference |
|---|---|---|
| CD105, CD90 and CD73 | Femur | [127] |
| Hip | [71] | |
| Knee | [38,81,117,[127], [128], [129], [130], [131], [132], [133]] | |
| Not described | [71,[134], [135], [136]] | |
| CD44 | Knee | [81,[128], [129], [130],132] |
| Not described | [135,136] | |
| CD166 | Femur | [127] |
| Hip | [71] | |
| Knee | [[127], [128], [129], [130],132,133,137] | |
| Not described | [28,135,138] | |
| CD29 | Knee | [81,128,129,131,136,137] |
| STRO-1 | Knee | [129] |
| Not described | [134,136] | |
| CD146 | Knee | [128,132] |
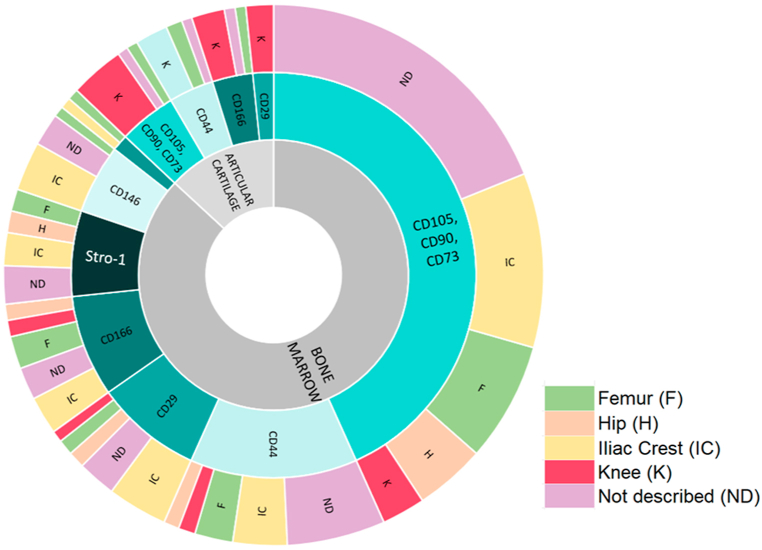
Fig. 3 summarizes cell surface markers identified in the literature in bone marrow and cartilage.
Fig. 3.
Summary of cell surface markers in bone marrow and cartilage. Percentage of articles evaluating cell surface markers in vitro. Inner circle: bone marrow (BM) and articular cartilage (AC). Middle circle describes cell surface markers for BM and AC. Outer circle describes anatomical sites where markers were identified to characterize possible MSCs. Abbreviations: BM: Bone marrow, AC: Articular cartilage, ND: Not described, IC: Iliac crest, F: Femur, H: Hip, K: Knee.
3.3. In situ evaluated cell surface markers
From our search, only 4% of the publications evaluated cell surface markers in situ from the following anatomical sites: vertebrae, femur and iliac crest. The most frequent ones identified by IHC were CD105, CD90 and CD73 [136,139,140]. Additionally, CD271 expression was observed in histological MSCs studies in femoral heads of osteoarthritic patients [55,139,140]. Last, perivascular endothelial cells surrounding endothelial capillaries were identified by immunofluorescence and IHC and were positive for CD146 and CD271 [45].
4. Discussion
This is an original work set out to validate which cell surface markers have been identified in the literature for hMSCs related to the skeletal system, as there is a need to determine the true surface markers of these stem cells; thus a scoping review was performed. Based on our search, it was evidenced the most common cell surface markers for MSCs characterization were those proposed by the ISCT in 2006: CD105 (82.9%), CD90 (75.0%), CD73 (52.0%); yet other markers were identified: CD44 (42.1), CD166 (30.9%), CD29 (27.7%), STRO-1 (17.8%), CD146 (15.1%) and CD271 (7.9%) in cells cultured in vitro derived from different locations of the skeletal system, such as bone marrow and cartilage. Our search included articles from 1994 to 2021 in PubMed, Scopus and Web of Science, where most reports were performed in studies with cells isolated from human adult bone marrow, followed by cartilage obtained from surgical procedures and last non-reported site of isolation. 96% of the studies identified cell surface markers from cells cultured in vitro, and only six articles (4%) carried out in situ evaluations using IHC. To date it remains to be determined if these proteins correspond to true stemness markers, as contradictory results have been reported in the literature. Therefore, it is important to establish the function of each surface marker according to the niche that characterizes skeletal tissues, where MSCs have been identified.
In this study it was evidenced BM was the preferred site of isolation, obtained from pelvic gridle comprised of femur, iliac crest, and hip. However, many studies did not define site of isolation (Fig. 3, ND), which might be important. Moreover, to a lesser extent knee was used as a structure to attain MSCs. Last, vertebra was also employed to obtain bone marrow MSCs, yet very few studies used this bone (one to three publications), thus they do not appear in Fig. 3. It remains to be determined if anatomical location influences surface marker expression. The pelvic gridle originates during embryological development for the somatopleure, and the vertebra from somites. On the other hand, knee cartilage was the other important element to obtain MSCs. This tissue also develops from the somatopleure, yet the process is different compared with endochondral ossification [141]. Therefore, we hypothesize anatomical site, and embryological origin could be key in characterizing surface marker expression, which remains to be established. Another issue to consider is overall, many studies use remnants of bone or cartilage tissue from surgeries in patients with skeletal pathologies, and these cells might be affected by inflammatory cytokines [142].
Multipotent mesenchymal stromal cells were first identified from BM; however, the acronym MSC has been widely used to describe cells from different tissues, as a case in point, cells from non-skeletal sources such as placenta, umbilical cord and adipose tissue [7]. In those studies, multipotent stromal cell surface marker from BM were employed to characterize their phenotype (mainly CD90, CD105, CD73), highlighting their low specificity to determine stemness in skeletal tissues [8,143,144]. Hence, researchers have opted to use other epitopes. Among surface markers proposed to identify MSCs are those suggested by Robey in 2014 [10], including CD105+, CD90+, CD146+ and CD271+. In their work, they highlight the importance of verifying the characteristics of a stem cell, first by demonstrating clonicity and also by transplanting cells into a mouse to verify their differentiation capacity to form bone, stroma and adipocytes in vivo [10].
Regarding these proteins CD105 (endoglin) corresponds to a glycoprotein of the cell membrane, which is a receptor for transforming growth factor beta-1 and beta-3. It is mainly expressed in vascular endothelial cells, the syncytiumtrophoblast, and to a lesser extent in monocytes, fibroblasts and chondrocytes [145]. Additionally, CD90 (Thy-1 cell surface antigen THY1) is a protein that is bound to glycophosphatidylinositol, present in intercellular interactions [146]. It has been identified in various cell types, such as hematopoietic stem cells HSC [147] and fibroblasts [148]. Furthermore, CD146 (melanoma cell adhesion molecule MCAM) is present in vascular endothelial cells, where MCAM is a cell adhesion protein key in angiogenesis processes. Cells characterized as possible MSCs that express CD146 have been described to be multipotent, a feature required by a stem cell [149]. In addition, they have a greater capacity to support hematopoiesis, as demonstrated by Sorrentino et al. in hBM, where CD146+ MSCs secreted growth factors capable of controlling HSC function [150]. Hence, CD146 becomes an important candidate to identify possible MSCs [151]. Last, CD271 (nerve growth factor receptor, NGFR) is not expressed as a universal marker in MSCs, as the name describes it has been observed ubiquitously in brain and more specific in meninges, but has been recognized in human adult bone marrow [152].
On the other hand, in 2018 Chan et al. reported the identification of MSCs related to the skeletal system in humans with the capacity of self-renewal and multipotential, characterized by the following cell surface markers: PDPN+, CD146-, CD73+, CD164+ [11]. These cells isolated from the growth plate generated progenitors for bone, cartilage and stroma, but not adipose tissue. However, this report has not echoed in the community, as it was not found in our scoping review. Additionally, according to Chan et al. CD146- characterizes a stem cell, whereas CD146+ identifies a progenitor cell. In contrast, Robey et al. point out CD146+ defines one of the cell surface markers associated with possible skeletal stem cells [10]. Collectively, Robey et al. and Chan et al. make clear a unique cell surface marker to determine self-renewal capacities and differentiation does not exist for skeletal tissue.
The present scoping review identified other markers used to determine MSCs: CD44+, CD166+, CD29+ and STRO-1+ in BM, and cartilage. CD44 corresponds to hyaluronic acid receptor, which mediates cell-cell and cell-matrix interactions, with affinity for ligands such as osteopontin, collagens and metalloproteases (MMPs). Additionally, it participates in hematopoietic processes and is associated with receptor tyrosine-protein kinase (ERBB2) and Hyaluronan mediated motility receptor (HMMR). CD166 (Activated Leukocyte Cell Adhesion Molecule, ALCAM) is expressed on osteoblasts and HSC residing in the hematopoietic niche and may be key in regulatory processes in bone formation [153]. On the other hand, CD29 (integrin beta 1) are heterodimers that bind ligands which are components of the extracellular matrix proteins, such as fibronectin, laminin, collagen and thrombospondin. In addition, they regulate processes, such as cell proliferation, differentiation, cell adhesion, and cell migration in the context of mineralization, bone development and angiogenesis [154]. Last, STRO-1 first identified in 1991 was described to bind to BM stromal elements [155], yet its identity has not been totally described [156]. These markers are not exclusive of the skeletal system, they are found in other tissues, such as: CD44 in cervix [157], CD166 in epithelium of the Fallopian tubes, CD29 in liver, particularly in bile ducts (preliminary results).
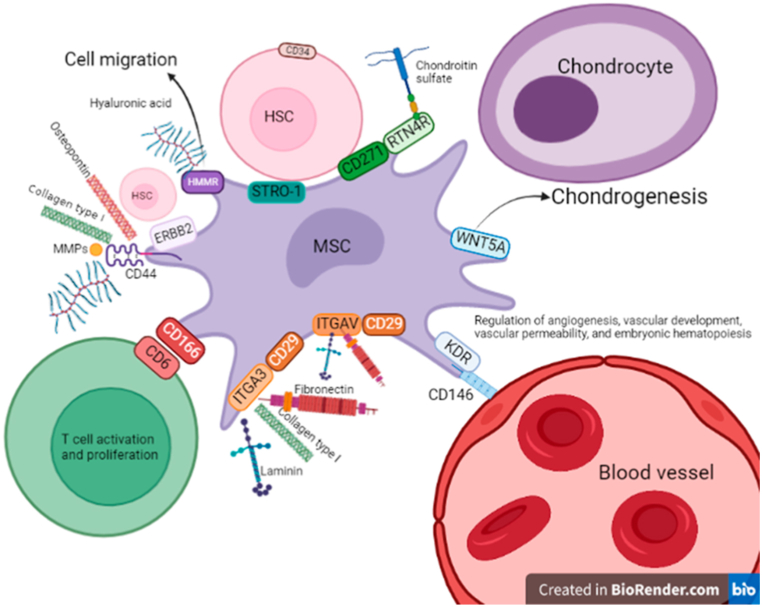
Collectively, our results established MSCs surface markers reported in the literature related to the skeletal system remain to be determined. Therefore, we hypothesize MSCs surface antigen can change according to anatomical location based on their niche. For example, the periosteum contains and outer fibrous layer and an inner layer where skeletal stem cells must reside to differentiate into osteoblasts and promote cortical bone maintenance [158]. The growth plate is the main site of longitudinal bone growth and stem cells are found in this tissue [159]. Stromal cells in the BM make part of the niche that supports hematopoiesis, regulating proliferation, self-renewal, differentiation and HSC migration [160]. On the other hand, articular cartilage antagonizes vascular invasion and prevents friction between two skeletal elements. During development, it derives from the interzone, an anatomical location where mesenchymal stem cells migrated [161]. However, in adult tissues it does not house stem cells [162]. Based on surface antigen markers not including those by the ISCT, we propose the following niche and possible cell-cell and cell-extracellular matrix interaction. In adult BM CD146 associated with Vascular endothelial growth factor receptor 2 (KDR) has been suggested as a stem cell marker candidate related with vessel endothelium (pericyte phenotype); and CD271 stromal cell epitope related to CD34 HSCs, hence hematopoiesis [163] (Fig. 4). In conclusion, through surface markers we can suggest a niche and thus cell function, as aforementioned. Moreover, it is likely more than one epitope can to be expressed by the same cell.
Fig. 4.
Possible MSCs-cell/extracellular matrix interactions based on cell surface markers. Using STRING database interactions among cell surface markers and cells expressing them were established. CD44 is a receptor that associates with bone extracellular matrix proteins (osteopontin and collagen Type I, hyaluronic acid). In association with ERB-B2 it plays a function in hematopoiesis. CD166: expressed by stromal cells it interacts with CD6 in T cells to sustain their proliferation and activation. CD29: Receptor in association with ITG-alpha-3 binds to fibronectin, laminin and collagen type I. In association with ITGAV binds to fibronectin and laminin. CD146: Plays a role in cell adhesion to vascular endothelium when co-expressed with KDR. WNT5A is co-expressed with CD146 that could be associated with chondrogenesis. CD271: Stromal cells expressing CD271 are in direct contact with CD34 positive HSCs. CD271 can be associated with RTN4R, whose function is to bind to chondroitin sulfate. STRO-1: Is a protein that binds to CD34 HSCs cells. Abbreviations: ERBB2: Receptor tyrosine-protein kinase erbB-2; HMMR: Hyaluronan mediated motility receptor; MMPs: matrix metalloproteinases, ITG-alpha-3: Integrin alpha-3; ITGAV: Integrin alpha-V; KDR: Vascular endothelial growth factor receptor 2; WNT-5A: Wingless Protein Wnt-5a, RTN4R: (Reticulon-4 receptor) chondroitin sulfate receptor; HSC: Hematopoietic stem cell.
Even though, all studies used cell epitopes to characterize possible MSCs, an identified weakness of our study was the lack of possibility to evaluate the quality of the publications data because there is no standardized procedure for cell isolation and culture, therefore, there is high heterogeneity among studies. In addition, the searched databases included basic research and clinical investigations, with a variety of techniques for cell isolation and different research questions, hence no standardized protocol exists for MSCs isolation. Furthermore, most reports did not demonstrate the two criteria a stem cell must comply: self-renewal and differentiation. It has been described isolated cells could be progenitors and not stem cells that under non physiological conditions are submitted to differentiation processes that cannot be confirmed by staining methodologies, since the gold standard to evaluate differentiation capacity is in vivo transplantation [10]. Additionally, it was recognized almost all publications were carried out in cultured cells. It has been evidenced that cell surface markers can change according to cell niche (in situ) and upon cell manipulation, such as trypsinization procedures for cell sorting [5]. This disparity among cell epitopes for skeletal stem cells in the literature could be generating contradictory results.
To expand on the aforementioned, expression of some epitopes could be forced in an artificial manner when cells are cultured in vitro, such as stage-specific embryonic antigen-4 (SSEA-4) [164,165]. Furthermore, it has been evidenced cell culture confluence can diminish the expression of cell surface markers, such as CD49f (integrin α6), as well as induce the expression of CD106, CD49d and CD200 [165]. Moreover, certain illnesses, mainly inflammatory diseases, such as osteoarthritis (OA) can produce growth factors and cytokines, which can modify MSCs phenotype. This is also true for growth factors used in culture [142,166]. It is critical to evaluate expressed markers in vivo, as well as to identify their possible anatomical location in situ, because their phenotype must be well characterized before they are employed in a clinical setting. Furthermore, pathologists can further characterize skeletal tissue malignancies using these markers.
Although MSCs have been employed for clinical applications, future developments aim at using MSCs in different fields. For example, in cancer treatments to inhibit proliferative cells in myeloid chronic leukemia [167], as demonstrated in an in vitro study with MSCs from murine origin characterized by presenting CD 44 and CD90; or cardiomyocyte development [168], by presenting again the same markers CD44 and CD90. However, before thinking of clinical applications the biology of stem cells must be further determined. The field has now advanced with norms for their use in research, such as ISO 24651–2022, which specifies requirements for biobanking in human mesenchymal stromal cells derived from bone marrow (hBM-MSCs) [169]. In this norm, section “hBM-MSCs characterization immunophenotyping by flow cytometry”, it is detailed these cells must express CD105, CD90, CD73, CD44, CD146 and CD271, which is in agreement with the CD markers identified in this scoping review. Hence, we propose these proteins must be identified in situ in different tissues from the skeletal system with specific origins (somatopleure and somites). Our research group is currently assessing the presence of these markers in bone and cartilage during embryological and fetal development. We hope this future study will shed light on the niche that houses MSCs and their cell surface markers.
Author contribution statement
Luisa Nathalia Fonseca, Maria Lucia Gutierrez: Conceived and designed the experiments; Performed the experiments; Analyzed and interpreted the data; Wrote the paper. Santiago Bolivar: Conceived and designed the experiments; Performed the experiments; Analyzed and interpreted the data. Tatiana Agudelo, Liz Daniela Beltrán, Daniel Camargo, Nestor Correa, Maria Alexandra Del Castillo, Sebastián Fernández de Castro, Valeria Fula, Gabriela García, Natalia Guarnizo, Valentina Lugo, Liz Mariana Martínez, Verónica Melgar, María Clara Peña, Wilfran Arbey Pérez, Nicolás Rodríguez: Performed the experiments; Analyzed and interpreted the data. Andrés Pinzón, Mercedes Olaya: Analyzed and interpreted the data. Sonia Luz Albarracín: Analyzed and interpreted the data; Contributed reagents, materials, analysis tools or data.
Funding statement
Dr. Maria Lucia Gutierrez and Luisa Nathalia Fonseca were supported by Pontificia Universidad Javeriana, Bogotá-Colombia [20036, Proposal ID 10340 & Master Student scholarship].
Data availability statement
Data included in article/supp. material/referenced in article.
Declaration of interest's statement
The authors declare no competing interests.
References
- 1.Bianco P., Robey P.G. Skeletal stem cells. Development (Camb.) 2015;142(6):1023–1027. doi: 10.1242/dev.102210. Mar 15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Friedenstein A.J., Piatetzky-Shapiro, Petrakova K.V. Osteogenesis in transplants of bone marrow cells. Development. 1966;16(3):381–390. Dec 1. [PubMed] [Google Scholar]
- 3.Owen M., Friedenstein A.J. Ciba Foundation Symposium. John Wiley & Sons, Ltd; 1988. Stromal stem cells: marrow-derived osteogenic precursors; pp. 42–60. [DOI] [PubMed] [Google Scholar]
- 4.Caplan A.I. Mesenchymal stem cells. J. Orthop. Res. 1991;9(5):641–650. doi: 10.1002/jor.1100090504. Sep. 1. [DOI] [PubMed] [Google Scholar]
- 5.Robey P. Mesenchymal stem cells”: fact or fiction, and implications in their therapeutic use. F1000Res. 2017;6 doi: 10.12688/f1000research.10955.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Li H., Wen F., Qi Z.C., Zhou J.J., Zhu Y.J., Cheng P., et al. Human umbilical cord-derived mesenchymal stem cells co-cultured with hepatocytes can differentiate into hepatocyte-like cells. Chin. J. Tissue Eng. Res. 2013;17(32):5772–5777. [Google Scholar]
- 7.Fathi E., Vandghanooni S., Montazersaheb S., Farahzadi R. Mesenchymal stem cells promote caspase-3 expression of SH-SY5Y neuroblastoma cells via reducing telomerase activity and telomere length. Iran J Basic Med Sci. 2021;24(11):1583. doi: 10.22038/IJBMS.2021.59400.13187. Nov 1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Fukuchi Y., Nakajima H., Sugiyama D., Hirose I., Kitamura T., Tsuji K. Human placenta-derived cells have mesenchymal stem/progenitor cell potential. Stem Cell. 2004;22(5):649–658. doi: 10.1634/stemcells.22-5-649. Sep. [DOI] [PubMed] [Google Scholar]
- 9.Dominici M., Le Blanc K., Mueller I., Slaper-Cortenbach I., Marini F.C., Krause D.S., et al. Minimal criteria for defining multipotent mesenchymal stromal cells. The International Society for Cellular Therapy position statement. Cytotherapy. 2006;8(4):315–317. doi: 10.1080/14653240600855905. [DOI] [PubMed] [Google Scholar]
- 10.Robey P.G., Kuznetsov S.A., Riminucci M., Bianco P. Bone marrow stromal cell assays – in vitro and in vivo. Methods Mol. Biol. 2014;1130:279. doi: 10.1007/978-1-62703-989-5_21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Chan C.K.F., Gulati G.S., Sinha R., Weissman I.L., Chang H.Y., Longaker M.T. Identification of the human skeletal stem cell. Cell. 2018;175:43–56.e21. doi: 10.1016/j.cell.2018.07.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Spitzhorn L.S., Megges M., Wruck W., Rahman M.S., Otte J., Ö Degistirici, et al. Human iPSC-derived MSCs (iMSCs) from aged individuals acquire a rejuvenation signature. Stem Cell Res. Ther. 2019;10(1) doi: 10.1186/s13287-019-1209-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Ng C.P., Sharif A.R., Heath D.E., Chow J.W., Zhang C.B.Y., Chan-Park M.B., et al. Enhanced exvivo expansion of adult mesenchymal stem cells by fetal mesenchymal stem cell ECM. Biomaterials. 2014;35(13):4046–4057. doi: 10.1016/j.biomaterials.2014.01.081. [DOI] [PubMed] [Google Scholar]
- 14.Salerno A., Brady K., Rikkers M., Li C., Caamaño-Gutierrez E., Falciani F., et al. MMP13 and TIMP1 are functional markers for two different potential modes of action by mesenchymal stem/stromal cells when treating osteoarthritis. Stem Cell. 2020;38(11):1438–1453. doi: 10.1002/stem.3255. [DOI] [PubMed] [Google Scholar]
- 15.Granchi D., Ochoa G., Leonardi E., Devescovi V., Baglìo S.R., Osaba L., et al. Gene expression patterns related to osteogenic differentiation of bone marrow-derived mesenchymal stem cells during Ex vivo expansion. Tissue Eng. C Methods. 2010;16(3):511–524. doi: 10.1089/ten.TEC.2009.0405. [DOI] [PubMed] [Google Scholar]
- 16.Piccinato C.A., Sertie A.L., Torres N., Ferretti M., Antonioli E. High OCT4 and low p16INK4A expressions determine in vitro lifespan of mesenchymal stem cells. Stem Cell. Int. 2015;2015 doi: 10.1155/2015/369828. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Quintin A., Schizas C., Scaletta C., Jaccoud S., Applegate L.A., Pioletti D.P. Plasticity of fetal cartilaginous cells. Cell Transplant. 2010;19(10):1349–1357. doi: 10.3727/096368910X506854. Oct 1. [DOI] [PubMed] [Google Scholar]
- 18.Etheridge S.L., Spencer G.J., Heath D.J., Genever P.G. Expression profiling and functional analysis of Wnt signaling mechanisms in mesenchymal stem cells. Stem Cell. 2004;22(5):849–860. doi: 10.1634/stemcells.22-5-849. [DOI] [PubMed] [Google Scholar]
- 19.Henze K., Herten M., Haversath M., Busch A., Brandau S., Hackel A., et al. Surgical vacuum filter-derived stromal cells are superior in proliferation to human bone marrow aspirate. Stem Cell Res. Ther. 2019;10(1) doi: 10.1186/s13287-019-1461-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Herrmann M., Hildebrand M., Menzel U., Fahy N., Alini M., Lang S., et al. Phenotypic characterization of bone marrow mononuclear cells and derived stromal cell populations from human iliac crest, vertebral body and femoral head. Int. J. Mol. Sci. 2019;20(14) doi: 10.3390/ijms20143454. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Boiret N., Rapatel C., Veyrat-Masson R., Guillouard L., Guérin J.J., Pigeon P., et al. Characterization of nonexpanded mesenchymal progenitor cells from normal adult human bone marrow. Exp. Hematol. 2005;33(2):219–225. doi: 10.1016/j.exphem.2004.11.001. Feb. [DOI] [PubMed] [Google Scholar]
- 22.Nasef A., Zhang Y.Z., Mazurier C., Bouchet S., Bensidhoum M., Francois S., et al. Selected Stro-1-enriched bone marrow stromal cells display a major suppressive effect on lymphocyte proliferation. Int J Lab Hematol. 2009;31(1):9–19. doi: 10.1111/j.1751-553X.2007.00997.x. Feb 1. [DOI] [PubMed] [Google Scholar]
- 23.Rocha B., Calamia V., Casas V., Carrascal M., Blanco F.J.J., Ruiz-Romero C. Secretome analysis of human mesenchymal stem cells undergoing chondrogenic differentiation. J. Proteome Res. 2014;13(2):1045–1054. doi: 10.1021/pr401030n. [DOI] [PubMed] [Google Scholar]
- 24.Harichandan A., Sivasubramaniyan K., Bühring H.J. Prospective isolation and characterization of human bone marrow-derived MSCs. Adv. Biochem. Eng. Biotechnol. 2013;129:1–17. doi: 10.1007/10_2012_147. [DOI] [PubMed] [Google Scholar]
- 25.Pacini S., Barachini S., Montali M., Carnicelli V., Fazzi R., Parchi P., et al. Mesangiogenic progenitor cells derived from one novel CD64brightCD31brightCD14neg population in human adult bone marrow. Stem Cell. Dev. 2016;25(9):661. doi: 10.1089/scd.2015.0344. May 1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Porter R.M., Liu F., Pilapil C., Betz O.B., Vrahas M.S., Harris M.B., et al. Osteogenic potential of reamer irrigator aspirator (RIA) aspirate collected from patients undergoing hip arthroplasty. J. Orthop. Res. 2009;27(1):42–49. doi: 10.1002/jor.20715. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Kafienah W., Mistry S., Perry M.J., Politopoulou G., Hollander A.P. Pharmacological regulation of adult stem cells: chondrogenesis can Be induced using a synthetic inhibitor of the retinoic acid receptor. Stem Cell. 2007;25(10):2460–2468. doi: 10.1634/stemcells.2007-0059. Oct 1. [DOI] [PubMed] [Google Scholar]
- 28.Tornero-Esteban P., Peralta-Sastre A., Herranz E., Rodríguez-Rodríguez L., Mucientes A., Abásolo L., et al. Altered expression of Wnt signaling pathway components in osteogenesis of mesenchymal stem cells in osteoarthritis patients. PLoS One. 2015;10(9) doi: 10.1371/journal.pone.0137170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Zhou Y., Liu C., He J., Dong L., Zhu H., Zhang B., et al. KLF2+ stemness maintains human mesenchymal stem cells in bone regeneration. Stem Cell. 2020;38(3):395–409. doi: 10.1002/stem.3120. Mar 1. [DOI] [PubMed] [Google Scholar]
- 30.Baghaei K., Hashemi S.M., Tokhanbigli S., Rad A.A., Assadzadeh-Aghdaei H., Sharifian A., et al. Isolation, differentiation, and characterization of mesenchymal stem cells from human bone marrow. Gastroenterol Hepatol Bed Bench. 2017;10(3):208–213. [PMC free article] [PubMed] [Google Scholar]
- 31.Ren J., Wang H., Tran K., Civini S., Jin P., Castiello L., et al. Human bone marrow stromal cell confluence: effects on cell characteristics and methods of assessment. Cytotherapy. 2015;17(7):897–911. doi: 10.1016/j.jcyt.2015.03.607. Jul. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Manfrini M., di Bona C., Canella A., Lucarelli E., Pellati A., D'Agostino A., et al. Mesenchymal stem cells from patients to assay bone graft substitutes. J. Cell. Physiol. 2013;228(6):1229–1237. doi: 10.1002/jcp.24276. Jun 1. [DOI] [PubMed] [Google Scholar]
- 33.Carrancio S., López-Holgado N., Sánchez-Guijo F.M., Villarón E., Barbado V., Tabera S., et al. Optimization of mesenchymal stem cell expansion procedures by cell separation and culture conditions modification. Exp. Hematol. 2008;36(8):1014–1021. doi: 10.1016/j.exphem.2008.03.012. [DOI] [PubMed] [Google Scholar]
- 34.Lubis A.M., Sandhow L., Lubis V.K., Noor A., Gumay F., Merlina M., et al. Isolation and cultivation of mesenchymal stem cells from iliac crest bone marrow for further cartilage defect management. Acta Med. Indones. 2011;43(3):178–184. [PubMed] [Google Scholar]
- 35.Xin J., Ding W., Hao S., Jiang L., Zhou Q., Wu T., et al. Human bone marrow mesenchymal stem cell-derived hepatocytes express tissue inhibitor of metalloproteinases 4 and follistatin. Liver Int. 2015;35(10):2301–2310. doi: 10.1111/liv.12797. Oct. [DOI] [PubMed] [Google Scholar]
- 36.Hung S.C., Chen N.J., Hsieh S.L., Li H., Ma H.L., Lo W.H. Isolation and characterization of size-sieved stem cells from human bone marrow. Stem Cell. 2002;20(3):249–258. doi: 10.1634/stemcells.20-3-249. May 1. [DOI] [PubMed] [Google Scholar]
- 37.Fickert S., Schroter-Bobsin U., Groß A.F., Hempel U., Wojciechowski C., Rentsch C., et al. Human mesenchymal stem cell proliferation and osteogenic differentiation during long-term ex vivo cultivation is not age dependent. J. Bone Miner. Metabol. 2011;29(2):224–235. doi: 10.1007/s00774-010-0215-y. [DOI] [PubMed] [Google Scholar]
- 38.Ayatollahi M., Soleimani M., Tabei S.Z., Kabir Salmani M. Hepatogenic differentiation of mesenchymal stem cells induced by insulin like growth factor-I. World J. Stem Cell. 2011;3(12):113–121. doi: 10.4252/wjsc.v3.i12.113. Dec 26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Tomuleasa C.I., Foris V., Soriţǎu O., Páll E., Fischer-Fodor E., Lung-Illes V., et al. Effects of 60Co γ-rays on human osteoprogenitor cells. Rom. J. Morphol. Embryol. 2008;50(3):349–355. [PubMed] [Google Scholar]
- 40.Yu B.Y., Chen P.Y., Sun Y.M., Lee Y.T., Young T.H. Response of human mesenchymal stem cells (hMSCs) to the topographic variation of poly(3-hydroxybutyrate-co-3-hydroxyhexanoate) (PHBHHx) films. J. Biomater. Sci. Polym. Ed. 2012;23(1–4):1–26. doi: 10.1163/092050610X541386. [DOI] [PubMed] [Google Scholar]
- 41.Rozemuller H., Prins H.J., Naaijkens B., Staal J., Bühring H.J., Martens A.C. Prospective isolation of mesenchymal stem cells from multiple mammalian species using cross-reacting anti-human monoclonal antibodies. Stem Cell. Dev. 2010;19(12):1911–1921. doi: 10.1089/scd.2009.0510. [DOI] [PubMed] [Google Scholar]
- 42.Ayatollahi M., Geramizadeh B., Zakerinia M., Ramzi M., Yaghobi R., Hadadi P., et al. Human bone marrow-derived mesenchymal stem cell: a source for cell-based therapy. Int J Organ Transplant Med. 2012;3(1):32–39. [PMC free article] [PubMed] [Google Scholar]
- 43.Xiao K., Fang Z., Gao X., Zhao J., Huang R., Xie M. Membrane complement regulatory protein reduces the damage of transplanting autologous bone marrow mesenchymal stem cells by suppressing the activation of complement. Injury. 2017;48(10):2089–2094. doi: 10.1016/j.injury.2017.08.008. [DOI] [PubMed] [Google Scholar]
- 44.Adesida A.B., Mulet-Sierra A., Jomha N.M. Hypoxia mediated isolation and expansion enhances the chondrogenic capacity of bone marrow mesenchymal stromal cells. Stem Cell Res. Ther. 2012;3(2) doi: 10.1186/scrt100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Tormin A., Li O., Brune J.C., Walsh S., Schütz B., Ehinger M., et al. CD146 expression on primary nonhematopoietic bone marrow stem cells is correlated with in situ localization. Blood. 2011;117(19):5067–5077. doi: 10.1182/blood-2010-08-304287. May 12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Alsalameh S., Amin R., Gemba T., Lotz M. Identification of mesenchymal progenitor cells in normal and osteoarthritic human articular cartilage. Arthritis Rheum. 2004;50(5):1522–1532. doi: 10.1002/art.20269. [DOI] [PubMed] [Google Scholar]
- 47.Mareschi K., Rustichelli D., Calabrese R., Gunetti M., Sanavio F., Castiglia S., et al. Multipotent mesenchymal stromal stem cell expansion by plating whole bone marrow at a low cellular density: a more advantageous method for clinical use. Stem Cell. Int. 2012;2012 doi: 10.1155/2012/920581. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Jones E.A., English A., Kinsey S.E., Straszynski L., Emery P., Ponchel F., et al. Optimization of a flow cytometry-based protocol for detection and phenotypic characterization of multipotent mesenchymal stromal cells from human bone marrow. Cytometry B Clin Cytom. 2006;70(6):391–399. doi: 10.1002/cyto.b.20118. [DOI] [PubMed] [Google Scholar]
- 49.Houshmand B., Behnia H., Khoshzaban A., Morad G., Behrouzi G., Dashti S.G., et al. Osteoblastic differentiation of human stem cells derived from bone marrow and periodontal ligament under the effect of enamel matrix derivative and transforming growth factor-beta. Int. J. Oral Maxillofac. Implants. 2013;28(6):e440–e450. doi: 10.11607/jomi.te24. [DOI] [PubMed] [Google Scholar]
- 50.Turner S., Balain B., Caterson B., Morgan C., Roberts S. Viability, growth kinetics and stem cell markers of single and clustered cells in human intervertebral discs: implications for regenerative therapies. Eur. Spine J. 2014;23(11):2462–2472. doi: 10.1007/s00586-014-3500-y. [DOI] [PubMed] [Google Scholar]
- 51.Chen X., Wang L., Hou J., Li J., Chen L., Xia J., et al. Study on the dynamic biological characteristics of human bone marrow mesenchymal stem cell senescence. Stem Cell. Int. 2019;2019 doi: 10.1155/2019/9271595. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Bayes-Genis A., Roura S., Soler-Botija C., Farré J., Hove-Madsen L., Llach A., et al. Identification of cardiomyogenic lineage markers in untreated human bone marrow-derived mesenchymal stem cells. Transplant. Proc. 2005;37(9):4077–4079. doi: 10.1016/j.transproceed.2005.09.103. [DOI] [PubMed] [Google Scholar]
- 53.Scanu M., Mancuso L., Cao G. Evaluation of the use of human Mesenchymal Stem Cells for acute toxicity tests. Toxicol. Vitro. 2011;25(8):1989–1995. doi: 10.1016/j.tiv.2011.07.006. Dec 1. [DOI] [PubMed] [Google Scholar]
- 54.Crapnell K., Blaesius R., Hastings A., Lennon D.P., Caplan A.I., Bruder S.P. Growth, differentiation capacity, and function of mesenchymal stem cells expanded in serum-free medium developed via combinatorial screening. Exp. Cell Res. 2013;319(10):1409–1418. doi: 10.1016/j.yexcr.2013.04.004. Jun. [DOI] [PubMed] [Google Scholar]
- 55.Prins H.J., Braat A.K., Gawlitta D., Dhert W.J.A., Egan D.A., Tijssen-Slump E., et al. In vitro induction of alkaline phosphatase levels predicts in vivo bone forming capacity of human bone marrow stromal cells. Stem Cell Res. 2014;12(2):428–440. doi: 10.1016/j.scr.2013.12.001. Mar 1. [DOI] [PubMed] [Google Scholar]
- 56.Ruben R.R., Beatriz R.N., Patricia M., Salvador A.C., Patricia R.T., Piedad N.A., et al. Impact of a porous Si-Ca-P monophasic ceramic on variation of osteogenesis-related gene expression of adult human mesenchymal stem cells. Appl. Sci. 2018;8(1) [Google Scholar]
- 57.Kalamegam G., Abbas M., Gari M., Alsehli H., Kadam R., Alkaff M., et al. Pelleted bone marrow derived mesenchymal stem cells are better protected from the deleterious effects of arthroscopic heat shock. Front. Physiol. 2016;7(MAY) doi: 10.3389/fphys.2016.00180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Walter M.N.M., Kohli N., Khan N., Major T., Fuller H., Wright K.T., et al. Human mesenchymal stem cells stimulate EaHy926 endothelial cell migration: combined proteomic and in vitro analysis of the influence of donor-donor variability. J. Stem Cells Regen. Med. 2015;11(1):P18–P24. doi: 10.46582/jsrm.1101004. May 30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Herencia C., Martínez-Moreno J.M., Herrera C., Corrales F., Santiago-Mora R., Espejo I., et al. Nuclear translocation of β-catenin during mesenchymal stem cells differentiation into hepatocytes is associated with a tumoral phenotype. PLoS One. 2012;7(4) doi: 10.1371/journal.pone.0034656. Apr 10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Guan Q., Li Y., Shpiruk T., Bhagwat S., Wall D.A. Inducible indoleamine 2,3-dioxygenase 1 and programmed death ligand 1 expression as the potency marker for mesenchymal stromal cells. Cytotherapy. 2018;20(5):639–649. doi: 10.1016/j.jcyt.2018.02.003. [DOI] [PubMed] [Google Scholar]
- 61.Gudleviciene Z., Kundrotas G., Liudkeviciene R., Rascon J., Jurga M. Quick and effective method of bone marrow mesenchymal stem cell extraction. Open Med. 2015 Jan 1;10(1):44–49. doi: 10.1515/med-2015-0008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Proksch S., Steinberg T., Schulz S., Sauerbier S., Hellwig E., Tomakidi P. Environmental biomechanics substantiated by defined pillar micropatterns govern behavior of human mesenchymal stem cells. Cell Transplant. 2012 Nov 1;21(11):2455–2469. doi: 10.3727/096368912X637037. [DOI] [PubMed] [Google Scholar]
- 63.Churchman S.M., Boxall S.A., McGonagle D., Jones E.A. Predicting the remaining lifespan and cultivation-related loss of osteogenic capacity of bone marrow multipotential stromal cells applicable across a broad donor age range. Stem Cell. Int. 2017;2017 doi: 10.1155/2017/6129596. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Sundin M., D'Arcy P., Johansson C.C., Barrett A.J., Lönnies H., Sundberg B., et al. Multipotent mesenchymal stromal cells express FoxP3: a marker for the immunosuppressive capacity? J. Immunother. 2011 May;34(4):336–342. doi: 10.1097/CJI.0b013e318217007c. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Friis T., Haack-Sørensen M., Mathiasen A.B., Ripa R.S., Kristoffersen U.S., Jørgensen E., et al. Mesenchymal stromal cell derived endothelial progenitor treatment in patients with refractory angina. Scand. Cardiovasc. J. 2011 Jun;45(3):161–168. doi: 10.3109/14017431.2011.569571. [DOI] [PubMed] [Google Scholar]
- 66.Ng M.H., Doustjalali S.R., Pathak R., Sabet N.S., Khor S.F., Lokanathan Y., et al. Two dimensional protein map standardization of human bone marrow stromal cells. J. Proteonomics Bioinf. 2013;6:4. [Google Scholar]
- 67.Diaz-Romero J., Romeo S., Bovée J.V.M.G., Hogendoorn P.C.W., Heini P.F., Mainil-Varlet P. Hierarchical clustering of flow cytometry data for the study of conventional central chondrosarcoma. J. Cell. Physiol. 2010 Nov 1;225(2):601–611. doi: 10.1002/jcp.22245. [DOI] [PubMed] [Google Scholar]
- 68.Wei X.F., Chen Q.L., Fu Y., Zhang Q.K. Wnt and BMP signaling pathways co-operatively induce the differentiation of multiple myeloma mesenchymal stem cells into osteoblasts by upregulating EMX2. J. Cell. Biochem. 2019 Apr 1;120(4):6515–6527. doi: 10.1002/jcb.27942. [DOI] [PubMed] [Google Scholar]
- 69.Stiehler M., Rauh J., Bünger C., Jacobi A., Vater C., Schildberg T., et al. In vitro characterization of bone marrow stromal cells from osteoarthritic donors. Stem Cell Res. 2016 May 1;16(3):782–789. doi: 10.1016/j.scr.2016.03.013. [DOI] [PubMed] [Google Scholar]
- 70.Dusfour G., Maumus M., Cañadas P., Ambard D., Jorgensen C., Noël D., et al. Mesenchymal stem cells-derived cartilage micropellets: a relevant in vitro model for biomechanical and mechanobiological studies of cartilage growth. Mater. Sci. Eng. C. 2020 Jul 1;112 doi: 10.1016/j.msec.2020.110808. [DOI] [PubMed] [Google Scholar]
- 71.Cournil-Henrionnet C., Huselstein C., Wang Y., Galois L., Mainard D., Decot V., et al. Phenotypic analysis of cell surface markers and gene expression of human mesenchymal stem cells and chondrocytes during monolayer expansion. Biorheology. 2008;45(3–4):513–526. [PubMed] [Google Scholar]
- 72.Le Pape F., Richard G., Porchet E., Sourice S., Dubrana F., Férec C., et al. Adhesion, proliferation and osteogenic differentiation of human MSCs cultured under perfusion with a marine oxygen carrier on an allogenic bone substitute. Artif. Cell Nanomed. Biotechnol. 2018 Feb;46(1):95–107. doi: 10.1080/21691401.2017.1365724. [DOI] [PubMed] [Google Scholar]
- 73.Brady K., Dickinson S.C., Guillot P.V., Polak J., Blom A.W., Kafienah W., et al. Human fetal and adult bone marrow-derived mesenchymal stem cells use different signaling pathways for the initiation of chondrogenesis. Stem Cell. Dev. 2014;23(5):541–554. doi: 10.1089/scd.2013.0301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Nasef A., Chapel A., Mazurier C., Bouchet S., Lopez M., Mathieu N., et al. Identification of IL-10 and TGF-β transcripts involved in the inhibition of T-lymphocyte proliferation during cell contact with human mesenchymal stem cells. Gene Expr. 2007;13(4–5):217–226. doi: 10.3727/000000006780666957. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Choi Y.A., Lim J., Kim K.M., Acharya B., Cho J.Y., Bae Y.C., et al. Secretome analysis of human BMSCs and identification of SMOC1 as an important ECM protein in osteoblast differentiation. J. Proteome Res. 2010 Jun 4;9(6):2946–2956. doi: 10.1021/pr901110q. [DOI] [PubMed] [Google Scholar]
- 76.Salamanna F., Cepollaro S., Contartese D., Giavaresi G., Brodano G.B., Griffoni C., et al. Biological rationale for the use of vertebral whole bone marrow in spinal surgery. Spine. 2018;43(20):1401–1410. doi: 10.1097/BRS.0000000000002626. [DOI] [PubMed] [Google Scholar]
- 77.Block T.J., Marinkovic M., Tran O.N., Gonzalez A.O., Marshall A., Dean D.D., et al. Restoring the quantity and quality of elderly human mesenchymal stem cells for autologous cell-based therapies. Stem Cell Res. Ther. 2017;8(1) doi: 10.1186/s13287-017-0688-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Mareddy S., Crawford R., Brooke G., Xiao Y. Clonal isolation and characterization of bone marrow stromal cells from patients with osteoarthritis. Tissue Eng. 2007;13(4):819–829. doi: 10.1089/ten.2006.0180. [DOI] [PubMed] [Google Scholar]
- 79.Sakaguchi Y., Sekiya I., Yagishita K., Ichinose S., Shinomiya K., Muneta T. Suspended cells from trabecular bone by collagenase digestion become virtually identical to mesenchymal stem cells obtained from marrow aspirates. Blood. 2004 Nov 1;104(9):2728–2735. doi: 10.1182/blood-2003-12-4452. [DOI] [PubMed] [Google Scholar]
- 80.Mareddy S., Broadbent J., Crawford R., Xiao Y. Proteomic profiling of distinct clonal populations of bone marrow Mesenchymal stem cells. J. Cell. Biochem. 2009;106(5):776–786. doi: 10.1002/jcb.22088. [DOI] [PubMed] [Google Scholar]
- 81.Oda T., Sakai T., Hiraiwa H., Hamada T., Ono Y., Nakashima M., et al. Osteoarthritis-derived chondrocytes are a potential source of multipotent progenitor cells for cartilage tissue engineering. Biochem. Biophys. Res. Commun. 2016 Oct 21;479(3):469–475. doi: 10.1016/j.bbrc.2016.09.085. [DOI] [PubMed] [Google Scholar]
- 82.Sobreiro-Almeida R., Tamaño-Machiavello M.N., Carvalho E.O., Cordón L., Doria S., Senent L., et al. Human mesenchymal stem cells growth and osteogenic differentiation on piezoelectric poly(vinylidene fluoride) microsphere substrates. Int. J. Mol. Sci. 2017 Nov;18(11) doi: 10.3390/ijms18112391. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Munshi A., Mehic J., Creskey M., Gobin J., Gao J., Rigg E., et al. A comprehensive proteomics profiling identifies NRP1 as a novel identity marker of human bone marrow mesenchymal stromal cell-derived small extracellular vesicles. Stem Cell Res. Ther. 2019;10(1) doi: 10.1186/s13287-019-1516-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Wagner W., Horn P., Castoldi M., Diehlmann A., Bork S., Saffrich R., et al. Replicative senescence of mesenchymal stem cells: a continuous and organized process. PLoS One. 2008 May;3(5) doi: 10.1371/journal.pone.0002213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Tsai C.C., Huang T.F., Ma H.L., Chiang E.R., Hung S.C. Isolation of mesenchymal stem cells from shoulder rotator cuff: a potential source for muscle and tendon repair. Cell Transplant. 2013;22(3):413–422. doi: 10.3727/096368912X656090. [DOI] [PubMed] [Google Scholar]
- 86.Teti G., Cavallo C., Grigolo B., Giannini S., Facchini A., Mazzotti A., et al. Ultrastructural analysis of human bone marrow mesenchymal stem cells during in vitro osteogenesis and chondrogenesis. Microsc. Res. Tech. 2012 May;75(5):596–604. doi: 10.1002/jemt.21096. [DOI] [PubMed] [Google Scholar]
- 87.Zieker D., Schäfer R., Glatzle J., Nieselt K., Coerper S., Kluba T., et al. Lactate modulates gene expression in human mesenchymal stem cells. Langenbeck's Arch. Surg. 2008 May;393(3):297–301. doi: 10.1007/s00423-008-0286-6. [DOI] [PubMed] [Google Scholar]
- 88.Yang Z., Dong P., Fu X., Li Q., Ma S., Wu D., et al. CD49f acts as an inflammation sensor to regulate differentiation, adhesion, and migration of human mesenchymal stem cells. Stem Cell. 2015 Sep;33(9):2798–2810. doi: 10.1002/stem.2063. [DOI] [PubMed] [Google Scholar]
- 89.Hafizi M., Hajarizadeh A., Atashi A., Kalanaky S., Fakharzadeh S., Masoumi Z., et al. Nanochelating based nanocomplex, GFc7, improves quality and quantity of human mesenchymal stem cells during in vitro expansion. Stem Cell Res. Ther. 2015 Nov;6:226. doi: 10.1186/s13287-015-0216-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Muñiz C., Teodosio C., Mayado A., Amaral A.T., Matarraz S., Bárcena P., et al. Ex vivo identification and characterization of a population of CD13high CD105+ CD45- mesenchymal stem cells in human bone marrow. Stem Cell Res. Ther. 2015;6(1) doi: 10.1186/s13287-015-0152-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Tondreau T., Lagneaux L., Dejeneffe M., Delforge A., Massy M., Mortier C., et al. Isolation of BM mesenchymal stem cells by plastic adhesion or negative selection: phenotype, proliferation kinetics and differentiation potential. Cytotherapy. 2004;6(4):372–379. doi: 10.1080/14653240410004943. [DOI] [PubMed] [Google Scholar]
- 92.Rallapalli S., Bishi D.K., Verma R.S., Cherian K.M., Guhathakurta S. A multiplex PCR technique to characterize human bone marrow derived mesenchymal stem cells. Biotechnol. Lett. 2009;31(12):1843–1850. doi: 10.1007/s10529-009-0106-2. [DOI] [PubMed] [Google Scholar]
- 93.Qu C., Kaitainen S., Kröger H., Lappalainen R., Lammi M.J. Behavior of human bone marrow-derived mesenchymal stem cells on various titanium-based coatings. Materials. 2016;9(10) doi: 10.3390/ma9100827. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Kaltz N., Ringe J., Holzwarth C., Charbord P., Niemeyer M., Jacobs V.R., et al. Novel markers of mesenchymal stem cells defined by genome-wide gene expression analysis of stromal cells from different sources. Exp. Cell Res. 2010;316(16):2609–2617. doi: 10.1016/j.yexcr.2010.06.002. [DOI] [PubMed] [Google Scholar]
- 95.Meinel L., Karageorgiou V., Fajardo R., Snyder B., Shinde-Patil V., Zichner L., et al. Bone tissue engineering using human mesenchymal stem cells: effects of scaffold material and medium flow. Ann. Biomed. Eng. 2004;32(1):112–122. doi: 10.1023/b:abme.0000007796.48329.b4. [DOI] [PubMed] [Google Scholar]
- 96.Lee W.C., Shi H., Poon Z., Nyan L.M., Kaushik T., Shivashankar G v, et al. Multivariate biophysical markers predictive of mesenchymal stromal cell multipotency. Proc. Natl. Acad. Sci. U. S. A. 2014 Oct;111(42):E4409–E4418. doi: 10.1073/pnas.1402306111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Fouriki A., Dobson J. Oscillating magnet array-based nanomagnetic gene transfection of human mesenchymal stem cells. Nanomedicine (Lond). 2014 May;9(7):989–997. doi: 10.2217/nnm.13.74. [DOI] [PubMed] [Google Scholar]
- 98.Wu Y., Ren M., Yang R., Liang X., Ma Y., Tang Y., et al. Reduced immunomodulation potential of bone marrow-derived mesenchymal stem cells induced CCR4+CCR6+ Th/Treg cell subset imbalance in ankylosing spondylitis. Arthritis Res. Ther. 2011 Feb;13(1):R29. doi: 10.1186/ar3257. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 99.Tsai A.C., Liu Y., Ma T. Expansion of human mesenchymal stem cells in fibrous bed bioreactor. Biochem. Eng. J. 2016;108:51–57. [Google Scholar]
- 100.Vanella L., Raciti G., Barbagallo I., Bonfanti R., Abraham N., Campisi A. Tissue transglutaminase expression during neural differentiation of human mesenchymal stem cells. CNS Neurol. Disord.: Drug Targets. 2015;14(1):24–32. doi: 10.2174/1871527314666150116111339. [DOI] [PubMed] [Google Scholar]
- 101.Liu Q., Zhu H., Dong J., Li H., Zhang H. Defective proliferative potential of MSCs from pediatric myelodysplastic syndrome patients is associated with cell senescence. Int. J. Clin. Exp. Pathol. 2015;8(10):13059–13066. [PMC free article] [PubMed] [Google Scholar]
- 102.H’Ng C.H., Camp E., Anderson P.J., Zannettino A.C.W., Gronthos S. CMTM8 is a suppressor of human mesenchymal stem cell osteogenic differentiation and promoter of proliferation via EGFR signaling. Stem Cell. Dev. 2020 Jul 1;29(13):823–834. doi: 10.1089/scd.2020.0007. [DOI] [PubMed] [Google Scholar]
- 103.Detela G., Bain O.W., Kim H.W., Williams D.J., Mason C., Mathur A., et al. Donor variability in growth kinetics of healthy hMSCs using manual processing: considerations for manufacture of cell therapies. Biotechnol. J. 2018 Feb 1;13(2) doi: 10.1002/biot.201700085. [DOI] [PubMed] [Google Scholar]
- 104.Lopez-Villar O., Garcia J.L., Sanchez-Guijo F.M., Robledo C., Villaron E.M., Hernández-Campo P., et al. Both expanded and uncultured mesenchymal stem cells from MDS patients are genomically abnormal, showing a specific genetic profile for the 5q- syndrome. Leukemia. 2009 Jan 8;23(4):664–672. doi: 10.1038/leu.2008.361. [DOI] [PubMed] [Google Scholar]
- 105.Fernandez-Rebollo E., Franzen J., Goetzke R., Hollmann J., Ostrowska A., Oliverio M., et al. Senescence-associated metabolomic phenotype in primary and iPSC-derived mesenchymal stromal cells. Stem Cell Rep. 2020 Feb 11;14(2):201–209. doi: 10.1016/j.stemcr.2019.12.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Ede B.C., Asmaro R.R., Moppett J.P., Diamanti P., Blair A. Investigating chemoresistance to improve sensitivity of childhood T-cell acute lymphoblastic leukemia to parthenolide. Haematologica. 2018 Aug 31;103(9):1493–1501. doi: 10.3324/haematol.2017.186700. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Estrada-González P.K., Gómez-Ceja L., Montesinos J.J., Mayani H., Chávez-González A., Meillón L., et al. Decreased frequency, but normal functional integrity of mesenchymal stromal cells derived from untreated and Imatinib-treated chronic myeloid leukemia patients. Leuk. Res. 2014 May 1;38(5):594–600. doi: 10.1016/j.leukres.2014.02.011. [DOI] [PubMed] [Google Scholar]
- 108.Kim Y., Jekarl D.W., Kim J., Kwon A., Choi H., Lee S., et al. Genetic and epigenetic alterations of bone marrow stromal cells in myelodysplastic syndrome and acute myeloid leukemia patients. Stem Cell Res. 2015 Mar 1;14(2):177–184. doi: 10.1016/j.scr.2015.01.004. [DOI] [PubMed] [Google Scholar]
- 109.Wang Z., Jiang R., Wang L., Chen X., Xiang Y., Chen L., et al. Ginsenoside Rg1 improves differentiation by inhibiting senescence of human bone marrow mesenchymal stem cell via GSK-3 β and β-catenin. Stem Cell. Int. 2020;2020 doi: 10.1155/2020/2365814. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Kowal J.M., Schmal H., Halekoh U., Hjelmborg J.B., Kassem M. Single-cell high-content imaging parameters predict functional phenotype of cultured human bone marrow stromal stem cells. Stem Cells Transl Med. 2020 Feb 1;9(2):189–202. doi: 10.1002/sctm.19-0171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Nasef A., Chapel A., Mazurier C., Bouchet S., Lopez M., Mathieu N., et al. Identification of IL-10 and TGF-beta transcripts involved in the inhibition of T-lymphocyte proliferation during cell contact with human mesenchymal stem cells. Gene Expr. 2007;13(4–5):217–226. doi: 10.3727/000000006780666957. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Li H.Z., Chen Z., Hou C.L., Tang Y.X., Wang F., Fu Q.G. Uric acid promotes osteogenic differentiation and inhibits adipogenic differentiation of human bone mesenchymal stem cells. J. Biochem. Mol. Toxicol. 2015;29(8):382–387. doi: 10.1002/jbt.21707. [DOI] [PubMed] [Google Scholar]
- 113.Curran J.M., Chen R., Hunt J.A. Controlling the phenotype and function of mesenchymal stem cells in vitro by adhesion to silane-modified clean glass surfaces. Biomaterials. 2005 Dec;26(34):7057–7067. doi: 10.1016/j.biomaterials.2005.05.008. [DOI] [PubMed] [Google Scholar]
- 114.Ng C.P., Sharif A.R.M., Heath D.E., Chow J.W., Zhang C.B.Y., Chan-Park M.B., et al. Enhanced ex vivo expansion of adult mesenchymal stem cells by fetal mesenchymal stem cell ECM. Biomaterials. 2014 Apr;35(13):4046–4057. doi: 10.1016/j.biomaterials.2014.01.081. [DOI] [PubMed] [Google Scholar]
- 115.Sivasubramaniyan K., Harichandan A., Schumann S., Sobiesiak M., Lengerke C., Maurer A., et al. Stem Cells Dev; 2013. Prospective Isolation of Mesenchymal Stem Cells from Human Bone Marrow Using Novel Antibodies Directed against Sushi Domain Containing 2. Vol. 22, Stem Cells and Development; pp. 1944–1954. [DOI] [PubMed] [Google Scholar]
- 116.Stiehler M., Rauh J., Bünger C., Jacobi A., Vater C., Schildberg T., et al. In vitro characterization of bone marrow stromal cells from osteoarthritic donors. Stem Cell Res. 2016;16(3):782–789. doi: 10.1016/j.scr.2016.03.013. [DOI] [PubMed] [Google Scholar]
- 117.Diaz-Romero J., Nesic D., Grogan S.P., Heini P., Mainil-Varlet P. Immunophenotypic changes of human articular chondrocytes during monolayer culture reflect bona fide dedifferentiation rather than amplification of progenitor cells. J. Cell. Physiol. 2008 Jan;214(1):75–83. doi: 10.1002/jcp.21161. [DOI] [PubMed] [Google Scholar]
- 118.Manfrini M., Di Bona C., Canella A., Lucarelli E., Pellati A., D'Agostino A., et al. Mesenchymal stem cells from patients to assay bone graft substitutes. J. Cell. Physiol. 2013;228(6):1229–1237. doi: 10.1002/jcp.24276. [DOI] [PubMed] [Google Scholar]
- 119.Letchford J., Cardwell A.M., Stewart K., Coogans K.K.S., Cox J.P.L., Lee M., et al. Isolation of C15: a novel antibody generated by phage display against mesenchymal stem cell-enriched fractions of adult human marrow. J. Immunol. Methods. 2006 Jan 20;308(1–2):124–137. doi: 10.1016/j.jim.2005.10.015. [DOI] [PubMed] [Google Scholar]
- 120.Janeczek A.A., Tare R.S., Scarpa E., Moreno-Jimenez I., Rowland C.A., Jenner D., et al. Transient canonical Wnt stimulation enriches human bone marrow mononuclear cell isolates for osteoprogenitors. Stem Cell. 2016 Feb 1;34(2):418–430. doi: 10.1002/stem.2241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Naung N.Y., Suttapreyasri S., Kamolmatyakul S., Nuntanaranont T. Comparative study of different centrifugation protocols for a density gradient separation media in isolation of osteoprogenitors from bone marrow aspirate. J Oral Biol Craniofac Res. 2014 Sep 1;4(3):160–168. doi: 10.1016/j.jobcr.2014.11.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Tormin A., Li O., Brune J.C., Walsh S., Schütz B., Ehinger M., et al. CD146 expression on primary nonhematopoietic bone marrow stem cells is correlated with in situ localization. Blood. 2011 May 12;117(19):5067–5077. doi: 10.1182/blood-2010-08-304287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Gothard D., Cheung K., Kanczler J.M., Wilson D.I., Oreffo R.O.C. Regionally-derived cell populations and skeletal stem cells from human foetal femora exhibit specific osteochondral and multi-lineage differentiation capacity in vitro and ex vivo. Stem Cell Res. Ther. 2015 Dec 18;6(1):1–17. doi: 10.1186/s13287-015-0247-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Tsimbouri P., Gadegaard N., Burgess K., White K., Reynolds P., Herzyk P., et al. Nanotopographical effects on mesenchymal stem cell morphology and phenotype. J. Cell. Biochem. 2014 Feb 1;115(2):380–390. doi: 10.1002/jcb.24673. [DOI] [PubMed] [Google Scholar]
- 125.González-García C., Moratal D., Oreffo R.O.C., Dalby M.J., Salmerón-Sánchez M. Surface mobility regulates skeletal stem cell differentiation. Integrative Biology. The Royal Society of Chemistry. 2012;4:531–539. doi: 10.1039/c2ib00139j. [DOI] [PubMed] [Google Scholar]
- 126.Attar-Schneider O., Zismanov V., Drucker L., Gottfried M. Secretome of human bone marrow mesenchymal stem cells: an emerging player in lung cancer progression and mechanisms of translation initiation. Tumor Biol. 2016 Apr 1;37(4):4755–4765. doi: 10.1007/s13277-015-4304-3. [DOI] [PubMed] [Google Scholar]
- 127.Pretzel D., Linss S., Rochler S., Endres M., Kaps C., Alsalameh S., et al. Relative percentage and zonal distribution of mesenchymal progenitor cells in human osteoarthritic and normal cartilage. Arthritis Res. Ther. 2011;13(2) doi: 10.1186/ar3320. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Vinod E., Kachroo U., Ozbey O., Sathishkumar S., Boopalan P.R.J.V.C. Comparison of human articular chondrocyte and chondroprogenitor cocultures and monocultures: to assess chondrogenic potential and markers of hypertrophy. Tissue Cell. 2019 Apr;57:42–48. doi: 10.1016/j.tice.2019.01.007. [DOI] [PubMed] [Google Scholar]
- 129.Williams R., Khan I.M., Richardson K., Nelson L., McCarthy H.E., Analbelsi T., et al. Identification and clonal characterisation of a progenitor cell sub-population in normal human articular cartilage. PLoS One. 2010;5(10) doi: 10.1371/journal.pone.0013246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Bernstein P., Sperling I., Corbeil D., Hempel U., Fickert S. Progenitor cells from cartilage-No osteoarthritis-grade-specific differences in stem cell marker expression. Biotechnol. Prog. 2013 Jan 1;29(1):206–212. doi: 10.1002/btpr.1668. [DOI] [PubMed] [Google Scholar]
- 131.Vinod E., Kachroo U., Amirtham S.M., Ramasamy B., Sathishkumar S. Comparative analysis of fresh chondrocytes, cultured chondrocytes and chondroprogenitors derived from human articular cartilage. Acta Histochem. 2020;122(1) doi: 10.1016/j.acthis.2019.151462. [DOI] [PubMed] [Google Scholar]
- 132.English A., Jones E.A., Corscadden D., Henshaw K., Chapman T., Emery P., et al. A comparative assessment of cartilage and joint fat pad as a potential source of cells for autologous therapy development in knee osteoarthritis. Rheumatology. 2007 Nov 1;46(11):1676–1683. doi: 10.1093/rheumatology/kem217. [DOI] [PubMed] [Google Scholar]
- 133.Diaz-Romero J., Gaillard J.P., Grogan S.P., Nesic D., Trub T., Mainil-Varlet P. Immunophenotypic analysis of human articular chondrocytes: changes in surface markers associated with cell expansion in monolayer culture. J. Cell. Physiol. 2005 Mar;202(3):731–742. doi: 10.1002/jcp.20164. [DOI] [PubMed] [Google Scholar]
- 134.Katoh S., Yoshioka H., Iwasaki M., Senthilkumar R., Rajmohan M., Karthick R., et al. A three-dimensional in vitro culture environment of a novel polymer scaffold, yielding chondroprogenitors and mesenchymal stem cells in human chondrocytes derived from osteoarthritis-affected cartilage tissue. J. Orthop. 2021 Jan 1;23:138–141. doi: 10.1016/j.jor.2021.01.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.Fickert S., Fiedler J., Brenner R.E. Identification of subpopulations with characteristics of mesenchymal progenitor cells from human osteoarthritic cartilage using triple staining for cell surface markers. Arthritis Res. Ther. 2004;6(5):R422–R432. doi: 10.1186/ar1210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.Joos H., Wildner A., Hogrefe C., Reichel H., Brenner R.E. Interleukin-1 beta and tumor necrosis factor alpha inhibit migration activity of chondrogenic progenitor cells from non-fibrillated osteoarthritic cartilage. Arthritis Res. Ther. 2013 Sep 13;15(5):1–13. doi: 10.1186/ar4299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137.Jayasuriya C.T., Hu N., Li J., Lemme N., Terek R., Ehrlich M.G., et al. Molecular characterization of mesenchymal stem cells in human osteoarthritis cartilage reveals contribution to the OA phenotype. Sci. Rep. 2018 Dec 1;8(1):7044. doi: 10.1038/s41598-018-25395-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138.Ma X., Guo Z., Gao W., Wang J., Liu Y., Gao F., et al. LncRNA-NEF is downregulated in postmenopausal osteoporosis and is related to course of treatment and recurrence. J. Int. Med. Res. 2019 Jul 1;47(7):3299–3306. doi: 10.1177/0300060519847854. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139.Campbell T.M., Churchman S.M., Gomez A., McGonagle D., Conaghan P.G., Ponchel F., et al. Mesenchymal stem cell alterations in bone marrow lesions in patients with hip osteoarthritis. Arthritis Rheumatol. 2016 Jul 1;68(7):1648–1659. doi: 10.1002/art.39622. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140.Sanjurjo-Rodriguez C., Baboolal T.G., Burska A.N., Ponchel F., El-Jawhari J.J., Pandit H., et al. Gene expression and functional comparison between multipotential stromal cells from lateral and medial condyles of knee osteoarthritis patients. Sci. Rep. 2019 Dec 1;9(1):1–12. doi: 10.1038/s41598-019-45820-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141.Young N.M., Capellini T.D. Out on a limb. Developmental Approaches to Human Evolution. 2015 Nov 20:101–137. [Google Scholar]
- 142.Hermida-Gómez T., Fuentes-Boquete I., Gimeno-Longas M.J., Muiños-López E., Díaz-Prado S., de Toro F.J., et al. Quantification of cells expressing mesenchymal stem cell markers in healthy and osteoarthritic synovial membranes. J. Rheumatol. 2011 Feb 1;38(2):339–349. doi: 10.3899/jrheum.100614. [DOI] [PubMed] [Google Scholar]
- 143.Eirin A., Zhu X.Y., Krier J.D., Tang H., Jordan K.L., Grande J.P., et al. Adipose tissue-derived mesenchymal stem cells improve revascularization outcomes to restore renal function in swine atherosclerotic renal artery stenosis. Stem Cell. 2012 May;30(5):1030–1041. doi: 10.1002/stem.1047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144.Huang G.T.J., Gronthos S., Shi S. Mesenchymal stem cells derived from dental tissues vs. those from other sources: their biology and role in Regenerative Medicine. Journal of Dental Research. J Dent Res. 2009;88:792–806. doi: 10.1177/0022034509340867. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 145.Zanone M.M., Favaro E., Camussi G. Endoglin (CD105) is not a specific selection marker for endothelial cells in human islets of Langerhans. Reply to Wheeler-Jones CPD, Clarkin CE, Farrar CE et al. [letter] Diabetologia. 2013;56(1):225–226. doi: 10.1007/s00125-012-2765-0. [DOI] [PubMed] [Google Scholar]
- 146.Rege T.A., Hagood J.S. Thy‐1 as a regulator of cell‐cell and cell‐matrix interactions in axon regeneration, apoptosis, adhesion, migration, cancer, and fibrosis. Faseb. J. 2006 Jun;20(8):1045–1054. doi: 10.1096/fj.05-5460rev. [DOI] [PubMed] [Google Scholar]
- 147.Craig W., Kay R., Cutler R.L., Lansdorp P.M. Expression of Thy-1 on human hematopoietic progenitor cells. J. Exp. Med. 1993 May 1;177(5):1331–1342. doi: 10.1084/jem.177.5.1331. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 148.Saalbach A., Wetzig T., Haustein U.F., Anderegg U. Detection of human soluble Thy-1 in serum by ELISA. Fibroblasts and activated endothelial cells are a possible source of soluble Thy-1 in serum. Cell Tissue Res. 1999;298(2):307–315. doi: 10.1007/s004419900079. [DOI] [PubMed] [Google Scholar]
- 149.Russell K.C., Phinney D.G., Lacey M.R., Barrilleaux B.L., Meyertholen K.E., O'Connor K.C. In vitro high-capacity assay to quantify the clonal heterogeneity in trilineage potential of mesenchymal stem cells reveals a complex hierarchy of lineage commitment. Stem Cell. 2010 Apr;28(4):788–798. doi: 10.1002/stem.312. [DOI] [PubMed] [Google Scholar]
- 150.Sorrentino A., Ferracin M., Castelli G., Biffoni M., Tomaselli G., Baiocchi M., et al. Isolation and characterization of CD146+ multipotent mesenchymal stromal cells. Exp. Hematol. 2008 Aug;36(8):1035–1046. doi: 10.1016/j.exphem.2008.03.004. [DOI] [PubMed] [Google Scholar]
- 151.Schwab K.E.E., Gargett C.E.E. Co-expression of two perivascular cell markers isolates mesenchymal stem-like cells from human endometrium. Hum. Reprod. 2007;22(11):2903–2911. doi: 10.1093/humrep/dem265. [DOI] [PubMed] [Google Scholar]
- 152.Cattoretti G., Schiro R., Orazi A., Soligo D., Colombo M.P. Bone marrow stroma in humans: anti-nerve growth factor receptor antibodies selectively stain reticular cells in vivo and in vitro. Blood. 1993 Apr 1;81(7):1726–1738. [PubMed] [Google Scholar]
- 153.Hooker R.A., Chitteti B.R., Egan P.H., Cheng Y.H., Himes E.R., Meijome T., et al. Activated leukocyte cell adhesion molecule (ALCAM or CD166) modulates bone phenotype and hematopoiesis. J. Musculoskelet. Neuronal Interact. 2015;15(1):83. [PMC free article] [PubMed] [Google Scholar]
- 154.Clemetson K.J. Blood glycoproteins. N. Compr. Biochem. 1997 Jan 1;29(PART B):173–201. [Google Scholar]
- 155.Simmons P.J., Gronthos S., Ohta S., Graves S.E. Human bone marrow stromal cell precursors: identification and developmental potential. Bone Marrow Transplant. 1995;15(SUPPL. 1) [Google Scholar]
- 156.Fitter S., Gronthos S., Ooi S.S., Zannettino A.C.W. The mesenchymal precursor cell marker antibody STRO-1 binds to cell surface heat shock cognate 70. Stem Cell. 2017 Apr 1;35(4):940–951. doi: 10.1002/stem.2560. [DOI] [PubMed] [Google Scholar]
- 157.Davidson B., Goldberg I., Gotlieb W.H., Ben-Baruch G., Kopolovic J. CD44 expression in uterine cervical intraepithelial neoplasia and squamous cell carcinoma: an immunohistochemical study. Eur. J. Gynaecol. Oncol. 1998;19(1):46–49. PMID: 9476059. [PubMed] [Google Scholar]
- 158.Dwek J.R. The periosteum: what is it, where is it, and what mimics it in its absence? Skeletal Radiol. 2010 Apr;39(4):319. doi: 10.1007/s00256-009-0849-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 159.Kronenberg H.M. Developmental regulation of the growth plate. Nature. 2003 May 15;423(6937):332–336. doi: 10.1038/nature01657. [DOI] [PubMed] [Google Scholar]
- 160.Pinho S., Frenette P.S. Haematopoietic stem cell activity and interactions with the niche. Nat. Rev. Mol. Cell Biol. 2019 May 1;20(5):303. doi: 10.1038/s41580-019-0103-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 161.Chen S., Fu P., Wu H., Pei M. Meniscus, articular cartilage and nucleus pulposus: a comparative review of cartilage-like tissues in anatomy, development and function. Cell Tissue Res. 2017 Oct 1;370(1):53–70. doi: 10.1007/s00441-017-2613-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 162.Hollander A.P., Dickinson S.C., Kafienah W. Stem cells and cartilage development: complexities of a simple tissue. Stem Cell. 2010 Nov;28(11):1992–1996. doi: 10.1002/stem.534. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 163.Kenswil K.J.G., Pisterzi P., Sánchez-Duffhues G., van Dijk C., Lolli A., Knuth C., et al. Endothelium-derived stromal cells contribute to hematopoietic bone marrow niche formation. Cell Stem Cell. 2021 Apr 1;28(4):653–670.e11. doi: 10.1016/j.stem.2021.01.006. [DOI] [PubMed] [Google Scholar]
- 164.Suila H., Pitkänen V., Hirvonen T., Heiskanen A., Anderson H., Laitinen A., et al. Are globoseries glycosphingolipids SSEA-3 and -4 markers for stem cells derived from human umbilical cord blood? J. Mol. Cell Biol. 2011 Apr;3(2):99–107. doi: 10.1093/jmcb/mjq041. [DOI] [PubMed] [Google Scholar]
- 165.Lee R.H., Seo M.J., Pulin A.A., Gregory C.A., Ylostalo J., Prockop D.J. The CD34-like protein PODXL and ά6-integrin (CD49f) identify early progenitor MSCs with increased clonogenicity and migration to infarcted heart in mice. Blood. 2009 Jan 22;113(4):816–826. doi: 10.1182/blood-2007-12-128702. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 166.Gharibi B., Hughes F.J. Effects of medium supplements on proliferation, differentiation potential, and in vitro expansion of mesenchymal stem cells. Stem Cells Transl Med. 2012 Nov;1(11):771–782. doi: 10.5966/sctm.2010-0031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 167.Fathi E., Valipour B., Farahzadi R. Targeting the proliferation inhibition of chronic myeloid leukemia cells by bone marrow derived-mesenchymal stem cells via ERK pathway as a therapeutic strategy. Acta Med. Iran. 2020 Aug 10;58(5):199–206. [Google Scholar]
- 168.Adibkia K., Ehsani A., Jodaei A., Fathi E., Farahzadi R., Barzegar-Jalali M. Silver nanoparticles induce the cardiomyogenic differentiation of bone marrow derived mesenchymal stem cells via telomere length extension. Beilstein J. Nanotechnol. 2021;12:786–797. doi: 10.3762/bjnano.12.62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 169.ISO 24651:2022 Biotechnology — Biobanking — Requirements for human mesenchymal stromal cells derived from bone marrow. https://www.iso.org/obp/ui/#iso:std:iso:24651 Available from: ed-1:v1:en.
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Data included in article/supp. material/referenced in article.