Abstract
The NLRP3 inflammasome plays an essential role in resistance to bacterial infection. The nervous system secretes multiple neuropeptides affecting the nervous system as well as immune cells. The precise impact of the neuropeptide CGRP on NLRP3 inflammasome activation is still unclear. Here, we show that CGRP negatively regulates the antibacterial process of host cells. CGRP prevents NLRP3 inflammasome activation and reduces mature IL-1β secretion. Following NLRP3 inflammasome stimulation that triggers endosome leakage, CGRP internalized to endosomal compartments is released into the cell cytosol. Cytosolic CGRP binds directly to NLRP3 and dismantles the NLRP3-NEK7 complex, which is crucial for NLRP3 inflammasome activation. CGRP administration exacerbates bacterial infection, while the treatment with a CGRP antagonist has the opposite effect. Our study uncovers a unique role of CGRP in inhibiting inflammasome activation during infections, which might shed new light on antibacterial therapies in the future.
Keywords: Neuropeptide, CGRP, NLRP3, Inflammasome, Suppressor
Subject terms: Infection, Antimicrobial responses
Introduction
Innate immunity is important in controlling pathogenic infection [1]. Pattern recognition receptors such as Toll-like receptors (TLRs) and NOD-like receptors (NLRs) are utilized by the host to detect invading microbes or danger signals [2]. Toll-like receptor activation leads to cytokine production in immune cells. Among the secreted cytokines, interleukin-1β (IL-1β) is essential for leukocyte recruitment to the site of infection [3]. IL-1β is produced as a prototype protein that needs further processing to become its mature form by inflammasomes [4–8]. Unlike other inflammasomes that either function in restricted tissues or detect limited pathogens, NLRP3 senses multiple species of pathogens, including bacteria and viruses, as well as some sterile infections in a wide range of cell types [9, 10]. The NLRP3 inflammasome is also involved in antitumor immunity and chronic diseases [11, 12].
Typical NLRP3 inflammasome activation requires two consequential steps [13, 14]. One is a priming step to send Toll-like receptor signals into cells to upregulate the expression of vital genes required for NLRP3 inflammasome activation. Lipopolysaccharide (LPS) is the most common priming reagent for this purpose. Another is stimuli to nucleate NLRP3, apoptosis-associated speck-like protein containing a CARD (ASC) and caspase-1 to a final functional machinery, in which caspase-1 becomes activated and cleaves substrates such as pro-IL-1β and the pore-forming protein gasdermin D (GSDMD) [15, 16]. These stimuli include bacterial toxins, ATP and crystalline particles [17]. Moreover, ATP binding to NLRP3 and the association between NLRP3 and mitotic Ser/Thr kinase NEK7 are prerequisites for NLRP3 oligomerization and inflammasome activation [18, 19]. The NLRP3 inflammasome can be modulated by a number of factors either from the microbe or from the host itself [20–22]. Recently, neuroimmune interactions have attracted widespread attention due to the vital role of the nervous system in infections [23]. Whether neuropeptides have an effect on the NLRP3 inflammasome remains unclear.
Calcitonin gene-related peptide (CGRP) is a small neuropeptide that is secreted by brain parabrachial nucleus neurons [24] or by nociceptor neurons in the lung post-infection [25]. CGRP binds to its receptor CALCRL on recipient cells to transduce signals inside cells [26]. CGRP is also internalized into early endosomes and continues to signal there [27]. The disulfide bond in the CGRP N-terminus is crucial for its function, and CGRP lacking the first 7 amino acids becomes an antagonist for CGRP signaling [28]. CGRP is considered to be an inducer of migraine and acts as a negative regulator of the type 2 innate lymphoid cells (ILC2s) [29–33]. In addition, CGRP has a similar composition to antimicrobial peptides. This molecule can block virus replication and promote microbiota homeostasis [34]. However, its direct role in antimicrobial activities is mysterious. Whether CGRP affects the NLRP3 inflammasome also needs to be determined.
Here, we show that CGRP negatively regulates host anti-microbe immunity in the lung. Cgrp knockout mice have an enhanced survival rate and diminished bacterial burden in infected mouse organs. CGRP inhibits activation of the NLRP3 inflammasome and decreases the production of mature IL-1β. After NLRP3 inflammasome stimulation, CGRP internalized into endosomal compartments is released into the cytosol. CGRP binds directly to NLRP3 and hinders complex formation between NLRP3 and NEK7. Administration of CGRP lacking the first 7 amino acids alleviated bacterial infections. Our study uncovers a cellular role of CGRP in the process of inhibiting inflammasome activation during bacterial infections that might shed new light on antibacterial therapies with neuropeptides or their analogs in the future.
Results
Neuropeptide CGRP plays an essential role in controlling bacterial infection
The gene Calca (Calcitonin Related Polypeptide Alpha) encodes two proteins, namely, CGRP and Calcitonin, in different tissues (here, the CGRP-encoding gene is Cgrp and Calcitonin-encoding gene is Calcitonin). CGRP is upregulated in the lung during infection, while its precise role in antibacterial immune responses is still unclear [25]. Therefore, to test the role of CGRP in lung infections, we generated knockout mice specifically deficient for Cgrp but not Calcitonin (Fig. S1a). Cgrp was absent in the lungs of the Cgrp knockout mice, and the expression of Calcitonin was not affected by Cgrp deletion in the thyroid (Fig. S1b, c). After infections with bacteria such as Klebsiella pneumoniae and Staphylococcus aureus, Cgrp but not Calcitonin expression levels were enhanced (Fig. S1d, e). Nociceptor neurons were the main source of secreted CGRP in the lung post-K. pneumoniae infection (Fig. S1f). When lethal doses of K. pneumoniae were administered, the Cgrp-deficient mice showed an enhanced survival rate compared with their wild-type counterparts (Fig. 1a). Bacterial loads in the Cgrp knockout mice were also lower than those in the wild-type mice 2 days post-infection (Fig. 1b–d). Similar effects of Cgrp deletion on S. aureus infection were also observed (Fig. S1g, h). When we investigated the immune cells in the lungs 24 h post-infection, we found that more leukocytes were present in the lungs of the Cgrp knockout mice than in those of the control mice (Fig. S1i). Among the leukocytes existing in infected lungs, the cell number of macrophages but not neutrophils was significantly elevated (Fig. S1j, k), suggesting a substantial role of macrophages in the early phase of infection. When we assessed the lung pathologies in the mice infected with K. pneumoniae for 2 days, we found that the wild-type mice showed extensive leukocyte infiltration, while the Cgrp-deficient mice had almost normal lung morphologies (Fig. 1e), indicating that the Cgrp knockout mice had suppressed bacterial invasion two days post-infection. To assess the secreted cytokine profile in infected lungs, we performed an enzyme-linked immunosorbent assay (ELISA) array comprising various important cytokines involved in bacterial infections (Fig. 1f). We found that IL-1β protein levels were abnormally increased in Cgrp-deficient lung homogenates. The elevated IL-1β protein was further confirmed in the bronchoalveolar lavage fluid (BALF) of infected lungs (Fig. 1g). However, the mRNA level of Il1b in BALF macrophages was unchanged (Fig. 1h). Administration of anti-CGRP antibody to mouse lungs protected mice from K. pneumoniae infection (Fig. S1l). CGRP did not influence bacterial proliferation (Fig. S1m), suggesting that CGRP might affect bacterial infection by regulating the immune responses of immune cells in the lung.
Fig. 1.
CGRP suppresses host immunity against bacterial infection. a Cgrp+/+ and Cgrp–/– mice were inoculated intratracheally with 3 × 104 CFU of K. pneumoniae, followed by survival rate examinations within a period of 8 days. n = 10 for each group. b–d Cgrp+/+ and Cgrp–/– mice were inoculated intratracheally with 3 × 104 CFU of K. pneumoniae for 2 days, followed by bacterial load determinations using homogenates from the lung (b), liver (c) or spleen (d). n = 5 for each group. e Lung pathologies were examined in lungs from the Cgrp+/+ and Cgrp–/– mice inoculated intratracheally with 3 × 104 CFU of K. pneumoniae for 2 days. Scale bar, 50 μm. f Cgrp+/+ and Cgrp–/– mice were inoculated intratracheally with 3 × 104 CFU of K. pneumoniae for 24 h, followed by ELISAs of the indicated cytokines using supernatants of lung homogenates. g, h Cgrp+/+ and Cgrp–/– mice were inoculated intratracheally with 3 × 104 CFU of K. pneumoniae for 24 h. Bronchoalveolar lavage fluids were collected and subjected to an ELISA for IL-1β protein levels in supernatants (g) or an RT‒PCR analysis for Il1b mRNA levels in the pelleted macrophages (h). i Calcrlflox/flox (Calcrl+/+) and Calcrlflox/flox;Lyz2-Cre (Calcrl–/–) mice were inoculated intratracheally with 3 × 104 CFU of K. pneumoniae, followed by survival rate examinations within a period of 8 days. n = 10 for each group. j–l Calcrlflox/flox (Calcrl+/+) and Calcrlflox/flox;Lyz2-Cre (Calcrl–/–) mice were inoculated intratracheally with 3 × 104 CFU of K. pneumoniae for 2 days, followed by bacterial load determinations using homogenates from lung (j), liver (k) or spleen (l). n = 5 for each group. m Calcrlflox/flox (Calcrl+/+) and Calcrlflox/flox;Lyz2-Cre (Calcrl–/–) mice were inoculated intratracheally with 3 × 104 CFU of K. pneumoniae for 24 h. Bronchoalveolar lavage fluids were collected and subjected to an ELISA for IL-1β protein levels in supernatants. p.i. post infection, NS nonsignificant. Data are shown as the means ± SDs. *P < 0.05; **P < 0.01; ***P < 0.001. Experiments were repeated three times with similar results
The above observation about CGRP and macrophages suggested that CGRP might negatively affect macrophages’ anti-infection activity. We first assessed whether macrophages expressed CGRP post-infection. We found that CGRP was not expressed by macrophages post-K. pneumoniae infection (Fig. S1n), suggesting that macrophages are not sources for CGRP secretion in the lung during infections. However, the expression level of the CGRP receptor Calcrl was upregulated by K. pneumoniae stimulation (Fig. S1o). To test whether CGRP affects macrophages through CALCRL, we generated mice with macrophage Calcrl depletion by crossing Calcrlflox/flox mice with Lyz2-Cre mice that induce deletion of floxed flanking sequences in myeloid cells. We found that the Calcrl-deficient mice had an elevated survival rate compared with their WT counterparts, similar to the Cgrp knockout mice post-K. pneumoniae infection (Fig. 1i). Consistently, the bacterial loads in the Calcrl knockout mice were decreased (Fig. 1j–l). Moreover, the IL-1β protein levels in the BALF of the Calcrl-deficient mice infected with K. pneumoniae were elevated (Fig. 1m), indicating that CALCRL is critical for CGRP’s function in immune cells. Taken together, these results suggest that the neuropeptide CGRP plays an essential role in controlling bacterial infection by acting on macrophages.
CGRP suppresses NLRP3 inflammasome activation
CGRP deficiency leads to abnormal IL-1β secretion, prompting us to assess the activation status of inflammasomes, which are required for IL-1β maturation and secretion in macrophages post-K. pneumoniae infection. We stimulated LPS-primed bone-marrow-derived macrophages (BMDMs) with canonical stimuli together with CGRP. CGRP stimulation significantly inhibited the NLRP3 inflammasome but not the NLRC4 or AIM2 inflammasome (Fig. 2a). Costimulation of various inflammasome initiators with CGRP restored cell viability and reduced cell death caused by the activation of the NLRP3 inflammasome but not the NLRC4 or AIM2 inflammasome (Fig. 2b, c). Moreover, similar inhibitory effects on NLRP3 inflammasome activation by CGRP were observed when CGRP was coincubated with lysosome-destroying reagents that activate the NLRP3 inflammasome (Fig. 2d–f). However, when CGRP was added alongside LPS to prime macrophages, the inhibitory effects disappeared (Fig. S2a–c), suggesting that the inhibitory effect of CGRP was functional only when CGRP was accompanied by NLRP3 stimulation. Consistently, the expression levels of Nlrp3, Il1b or Calcrl were not influenced by the addition of CGRP during the LPS priming step (Fig. S2d–f). To determine how CGRP prohibits NLRP3 inflammasome activation, we tested whether CGRP blocked potassium efflux, which leads to NLRP3 inflammasome activation. However, CGRP addition had little effect on potassium efflux (Fig. S2g). Extracellular ATP is an NLRP3 stimulus that causes potassium efflux after binding and activating the ion channel P2X7R [35]. Increased ATP stimulation led to decreased cellular potassium, while CGRP addition did not change this trend (Fig. S2h), suggesting that CGRP does not block potassium efflux to inhibit NLRP3 inflammasome activation.
Fig. 2.
CGRP specifically inhibits the NLRP3 inflammasome. a–c WT BMDMs primed with 1 μg/ml LPS for 3 h were stimulated with 10 μM nigericin for 30 min, 2 mM ATP for 30 min, 0.5 μM gramicidin for 1 h, 2 μg/ml poly(dA:dT) for 4 h or Salmonella at an MOI of 10 for 4 h with or without the presence of 25 ng/ml CGRP. Cell culture supernatants and cell pellets were collected for immunoblotting with antibodies against the indicated proteins (a). Percentages of viable cells were determined by examining cellular ATP levels in cell pellets (b), and percentages of cells undergoing pyroptosis were calculated by assessing released LDH in cell culture supernatants (c). d–f WT BMDMs primed with 1 μg/ml LPS for 3 h were stimulated with 2 μM LLOMe, 500 μg/ml silica, 250 μg/ml Alum, 200 μg/ml MSU, 200 μg/ml CPPD, and 200 μg/ml Nano-SiO2 for 6 h with or without the presence of 25 ng/ml CGRP. Cell culture supernatants and cell pellets were collected for immunoblotting with antibodies against the indicated proteins (d). Percentages of viable cells were determined by examining cellular ATP levels in cell pellets (e), and percentages of cells undergoing pyroptosis were calculated by assessing released LDH in cell culture supernatants (f). g Calcrl+/+ and Calcrl–/– BMDMs primed with 1 μg/ml LPS for 3 h were stimulated with 10 μM nigericin for 30 min, 2 mM ATP for 30 min or 500 μg/ml silica for 6 h in the presence of 25 ng/ml CGRP. Cell culture supernatants and cell pellets were collected for immunoblotting with antibodies against the indicated proteins. h WT BMDMs primed with 1 μg/ml LPS for 3 h were stimulated with 10 μM nigericin for 30 min, 2 mM ATP for 30 min or 500 μg/ml silica for 6 h in the presence of 25 ng/ml CGRP or CGRP (8–37). Cell culture supernatants and cell pellets were collected for immunoblotting with antibodies against the indicated proteins. i Macrophages isolated from mouse lungs were primed with 1 μg/ml LPS for 3 h, followed by incubation with 10 μM nigericin in the presence of 25 ng/ml CGRP or CGRP (8–37) for 30 min. Cell culture supernatants were subjected to ELISA to determine IL-1β protein levels. Sup., supernatants. Data are shown as the means ± SDs. Similar results were observed for at least three repeats
Since CALCRL is involved in CGRP-mediated suppression of antibacterial activities, we then examined the inhibitory role of CGRP in NLRP3 inflammasome activation in Calcrl-deficient cells. CGRP blocked NLRP3 inflammasome activation in wild-type BMDMs but not in Calcrl knockout BMDMs (Fig. 2g). Calcrl deficiency abolished the CGRP-mediated increase in cell viability and decrease in cell death in macrophages (Fig. S2i, j), further suggesting that CGRP acts directly on macrophages to inhibit NLRP3 inflammasome activation. CGRP lacking the first 7 amino acids is a CGRP antagonist that binds CALCRL normally but fails to induce internalization [27]. When CGRP (8–37) was added with inflammasome stimulators to macrophages, the NLRP3 inflammasome formed normally (Fig. 2h). Cell viability in the cells treated with CGRP (8–37) was not increased compared with that in the cells treated with CGRP (Fig. S2k). Moreover, there were more dead cells among the CGRP (8–37)-treated cells than the CGRP-treated cells (Fig. S2l). We also isolated mouse lung macrophages and stimulated NLRP3 inflammasome activation in the presence of CGRP or CGRP (8–37). Similar to the results observed in BMDMs, CGRP but not CGRP (8–37) abolished IL-1β secretion in lung macrophages (Fig. 2i). Overall, these results indicate that CGRP suppresses NLRP3 inflammasome activation in macrophages.
CGRP enters the macrophage cytosol after NLRP3 inflammasome stimulation
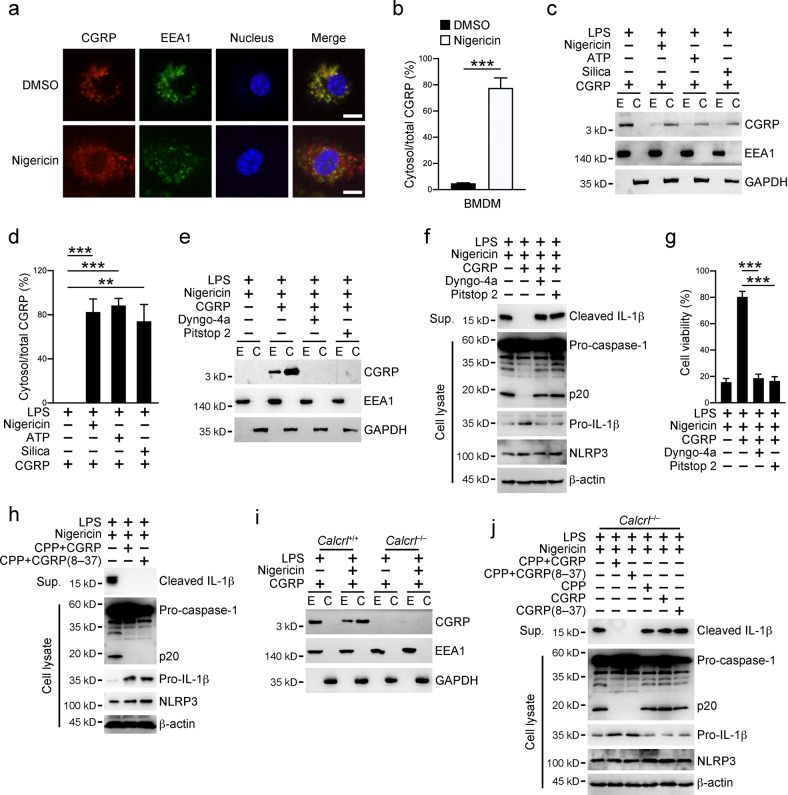
To determine how CGRP inhibits the NLRP3 inflammasome, we stained macrophages incubated with CGRP alone or together with the inflammasome stimulus nigericin (Fig. 3a, b). We found that CGRP localized with the early endosome marker EEA1 without coincubation with nigericin, indicating that CGRP is internalized into endosomal compartments in macrophages. However, CGRP escaped from the endosomal compartments post-nigericin stimulation. This phenomenon prompted us to examine the cellular localization of CGRP after NLRP3 inflammasome activation. Through endosome isolation and cell fractionation, we found that CGRP localized mainly in the cytosolic fractions after stimulation with various NLRP3 stimuli (Fig. 3c, d). To validate the fidelity of our endosomal fractionation procedure, we treated cells with internalization inhibitors to block CGRP endocytosis. CGRP was not present in either the endosomal or cytosolic fractions after these inhibitor treatments (Fig. 3e and Fig. S3a). Moreover, internalization inhibitors abolished CGRP-mediated NLRP3 inflammasome inhibition with respect to IL-1β secretion, cell viability decrease and cell death augmentation (Fig. 3f, g and Fig. S3b). As noted, CGRP was only present in the endosomal compartments in the resting state (Fig. S3c). We also tested the internalization ability of the CGRP antagonist CGRP (8–37). As expected, CGRP (8–37) failed to enter the endosomal compartments, let alone enter the cytosol (Fig. S3d, e). However, when peptide CGRP (8–37) was transfected into the cell cytosol through cell-penetrating peptides, CGRP (8–37) was able to inhibit the NLRP3 inflammasome similar to full-length CGRP (Fig. 3h and Fig. S3f, g), indicating that CGRP functions in the cytosol to inhibit NLRP3 inflammasome activation. Finally, we examined the role of the CGRP receptor CALCRL in CGRP internalization. In Calcrl-deficient cells, CGRP failed to enter the endosomal compartments or be released into the cytosol after NLRP3 inflammasome activation (Fig. 3i and Fig. S3h). Although CGRP incubation failed to inhibit the NLRP3 inflammasome in Calcrl-deficient cells, peptide CGRP or CGRP (8–37) transfection through cell-penetrating peptides led to significant inhibition of the NLRP3 inflammasome (Fig. 3j), accompanied by increased cell viability and decreased cell death (Fig. S3i, j). In summary, these results suggest that CGRP enters the macrophage cytosol to inhibit the NLRP3 inflammasome.
Fig. 3.
CGRP enters macrophage cytosol. a, b WT BMDMs primed with 1 μg/ml LPS for 3 h were further incubated with 25 ng/ml CGRP with or without 10 μM nigericin for 30 min. Cells were fixed and stained with antibodies against CGRP and EEA1 (a). Percentages of cytosolic CGRP versus total CGRP inside one cell were calculated, and at least 100 cells were counted (b). Scale bar, 10 μm. c, d WT BMDMs primed with 1 μg/ml LPS for 3 h were stimulated with 10 μM nigericin for 30 min, 2 mM ATP for 30 min or 500 μg/ml silica for 6 h in the presence of 25 ng/ml CGRP. Cell pellets were then subjected to endosome isolation and cell fractionation, followed by immunoblotting with antibodies against the indicated proteins (c). Percentages of cytosolic CGRP versus total CGRP inside cells were calculated (d). e WT BMDMs primed with 1 μg/ml LPS for 3 h were stimulated with 10 μM nigericin together with 25 ng/ml CGRP for 30 min in the presence of 20 μM Pitstop 2 or 10 nM Dyngo-4a as indicated. Cell pellets were then subjected to endosome isolation and cell fractionation, followed by immunoblotting with antibodies against the indicated proteins. f, g WT BMDMs primed with 1 μg/ml LPS for 3 h were stimulated with 10 μM nigericin together with 25 ng/ml CGRP for 30 min in the presence of 20 μM Pitstop 2 or 10 nM Dyngo-4a. Cell culture supernatants and cell pellets were collected for immunoblotting with antibodies against the indicated proteins (f). The percentages of viable cells were determined by examining cellular ATP levels in cell pellets (g). h WT BMDMs were transfected with peptides CGRP or CGRP (8–37) through cell-penetrating peptides (CPP) for 3 h. Cells were then primed with 1 μg/ml LPS for 3 h and stimulated with 10 μM nigericin for 30 min. Cell culture supernatants and cell pellets were collected for immunoblotting with antibodies against the indicated proteins. i Calcrl+/+ and Calcrl–/– BMDMs primed with 1 μg/ml LPS for 3 h were incubated with 25 ng/ml CGRP with or without 10 μM nigericin for 30 min. Cell pellets were then subjected to endosome isolation and cell fractionation, followed by immunoblotting with antibodies against the indicated proteins. j Calcrl–/– BMDM cells were transfected with the peptides CGRP or CGRP (8–37) through cell-penetrating peptides (CPP) for 3 h. Cells were then primed with 1 μg/ml LPS for 3 h and stimulated with 10 μM nigericin for 30 min. Otherwise, Calcrl–/– BMDM cells primed with 1 μg/ml LPS for 3 h were stimulated with 10 μM nigericin together with 25 ng/ml CGRP or CGRP (8–37) for 30 min. Cell culture supernatants and cell pellets were collected for immunoblotting with antibodies against the indicated proteins. E endosome, C cytosol, Sup. supernatants. Data are shown as the means ± SDs. ***P < 0.001. Experiments were repeated three times with similar results
CGRP associates with NLRP3
To determine how CGRP participates in NLRP3 inflammasome inhibition, we screened a mouse bone marrow cDNA library with CGRP as bait using the yeast two-hybrid system. Seven out of 16 positive colonies were identified as containing the NLRP3 transcript. We then cloned full-length NLRP3 and validated the interaction between CGRP and NLRP3 in yeast cells (Fig. 4a). We also tested the association between these two proteins in mammalian cells. GFP-tagged CGRP but not GFP alone interacted with NLRP3 in HEK293T cells (Fig. 4b). Moreover, recombinant GST-tagged CGRP but not GST itself pulled NLRP3 from NLRP3-expressing cell lysates (Fig. 4c). When CGRP was added to BMDMs along with the NLRP3 inflammasome stimulator nigericin, endogenous NLRP3 was found to interact with CGRP (Fig. 4d), indicating a physiological association between the two. Moreover, CGRP (8–37) was shown to interact with NLRP3 either in yeast cells by a yeast two-hybrid assay or in mammalian cells by coimmunoprecipitation (Fig. S4a, b). When we truncated NLRP3 into two parts in the middle, we found that both parts were associated with CGRP (Fig. S4c), suggesting that there might be multiple CGRP binding sites on NLRP3. To pinpoint the exact regions on NLRP3 that bind CGRP, we performed detailed truncation experiments (Fig. 4e). NLRP3 (378–427) and NLRP3 (678–727) were inferred to be responsible for CGRP association through tiling truncations (Fig. 4f, g). To confirm these speculations, we generated NLRP3 mutants lacking these two regions alone or together. We found that NLRP3 lacking amino acids 378–427 and 678–727 failed to associate with CGRP, while NLRP3 lacking either of these two regions bound CGRP normally (Fig. S4d), suggesting that these two regions work together to provide a binding cavity for CGRP. Moreover, truncated NLRP3 with amino acids 378–427 or 678–727 interacted with CGRP (Fig. S4e, f). We then restored wild-type and truncated NLRP3 in Nlrp3 knockout iBMDM cells (Fig. S4g). We found that CGRP failed to associate with the truncated NLRP3 lacking both of these regions in these cells (Fig. 4h). To our surprise, NLRP3 inflammasome activation was abolished in cells containing any of these three NLRP3 truncated isoforms (Fig. 4i, j and Fig. S4h). NLRP3 truncations lacking either of these two regions were still able to associate with CGRP but failed to induce inflammasome activation. The discrepancy between NLRP3 regions responsible for CGRP binding and inflammasome initiation might be caused by these NLRP3 truncations losing their interactions with some vital regulators inside cells. Together, these results suggest that CGRP associates with NLRP3 to exert its inhibitory role during inflammasome activation.
Fig. 4.
CGRP interacts with NLRP3. a Plasmids encoding Gal4 DNA binding domain (BD)-tagged CGRP and Gal4 activating domain (AD)-tagged NLRP3 were cotransfected into the yeast strain AH109. Transformants were grown in the indicated selection media. b Plasmids encoding FLAG-tagged NLRP3, Myc-tagged GFP or GFP-CGRP were cotransfected into HEK293T cells for 24 h, followed by immunoprecipitation with antibody against Myc or with control IgG. Immunoprecipitates and cell lysates were immunoblotted with antibodies against the indicated proteins. c Recombinant GST or GST-tagged CGRP was incubated with cell lysates of HEK293T cells overexpressing FLAG-tagged NLRP3, followed by a GST pulldown assay. Precipitates and cell lysates were immunoblotted with antibodies against the indicated proteins. d WT BMDMs primed with 1 μg/ml LPS for 3 h were stimulated with 10 μM nigericin with or without 25 ng/ml CGRP for 30 min. Cells were then lysed and subjected to immunoprecipitation with an antibody against NLRP3 or control IgG. Immunoprecipitates and cell lysates were immunoblotted with antibodies against the indicated proteins. e Schemes for mouse NLRP3 truncations. f Plasmids encoding FLAG-tagged NLRP3 N-terminal truncations, Myc-tagged GFP or GFP-CGRP were cotransfected into HEK293T cells for 24 h, followed by immunoprecipitation with antibody against FLAG or with control IgG. Immunoprecipitates and cell lysates were immunoblotted with antibodies against the indicated proteins. g Plasmids encoding FLAG-tagged NLRP3 C-terminal truncations, Myc-tagged GFP or GFP-CGRP were cotransfected into HEK293T cells for 24 h, followed by immunoprecipitation with antibody against FLAG or with control IgG. Immunoprecipitates and cell lysates were immunoblotted with antibodies against the indicated proteins. h Nlrp3–/– iBMDMs restored with FLAG-tagged full-length or truncated isoforms of NLRP3 were primed with 1 μg/ml LPS for 3 h and then stimulated with 10 μM nigericin together with 25 ng/ml CGRP for 30 min. Cells were then lysed and subjected to immunoprecipitation with an antibody against FLAG or with control IgG. Immunoprecipitates and cell lysates were immunoblotted with antibodies against the indicated proteins. i, j Nlrp3–/– iBMDMs restored with full-length or truncated isoforms of NLRP3 were primed with 1 μg/ml LPS for 3 h and then stimulated with 10 μM nigericin for 30 min. Cell culture supernatants were collected for ELISAs to determine the secreted IL-1β protein levels (i). The percentages of viable cells were determined by examining cellular ATP levels in cell pellets (j). Data are shown as the means ± SDs. Similar results were observed for at least three repeats
CGRP disrupts the interaction between NLRP3 and NEK7
We next sought to determine how CGRP inhibited NLRP3 inflammasome activation in the cytosol. NLRP3 contains an ATP hydrolase motif that is essential for NLRP3 activation [36, 37]. To assess whether CGRP affected NLRP3 hydrolytic ability, we incubated CGRP with purified NLRP3 and radiolabeled ATPs (Fig. 5a). CGRP had little effect on the hydrolytic activity of NLRP3. Moreover, CGRP did not hinder the ATP binding capability of NLRP3 (Fig. S5a). In addition to ATP catalysis, NLRP3 requires NEK7 to form oligomers during inflammasome activation [17, 38]. To examine whether CGRP could affect the association between NLRP3 and NEK7, we conducted competition assays to examine the association between NLRP3 and NEK7 in the presence of increasing amounts of CGRP in mammalian cells (Fig. 5b). CGRP increase led to undetectable NEK7 associated with NLRP3, suggesting that CGRP has a higher binding affinity for NLRP3 than NEK7 does. Moreover, CGRP (8–37) expelled NEK7 from NLRP3 (Fig. S5b). Consistently, NEK7 failed to compete with CGRP or CGRP (8–37) for NLRP3 binding (Fig. 5c and Fig. S5c). To further test whether NLRP3 could bind CGRP and NEK7 at the same time, we transfected these three plasmids into HEK293T cells (Fig. 5d). When we immunoprecipitated CGRP from cell lysates, only NLRP3 was detected in the immunoprecipitants, suggesting exclusive binding of CGRP and NEK7 for NLRP3. The same situation was observed for CGRP (8–37) when cotransfected with NLRP3 and NEK7 (Fig. S5d). To exclude the possibility that CGRP might bind NEK7 to hinder NEK7’s association with NLRP3, we assessed the binding ability of CGRP with NEK7 in HEK293T cells (Fig. 5e). We found that CGRP did not interact with NEK7. Moreover, when we immunoprecipitated NEK7 from cells cotransfected with CGRP and NLRP3, the association of NEK7 with NLRP3 was observed only in the absence of CGRP (Fig. 5f). The presence of CGRP or CGRP (8–37) strongly dissociated the NLRP3-NKE7 complex (Fig. 5f and Fig. S5e).
Fig. 5.
CGRP expels NEK7 from NLRP3. a FLAG-tagged NLRP3 was immunoprecipitated from HEK293T cells overexpressing FLAG-NLRP3 through anti-FLAG antibody-conjugated beads. Beads containing NLRP3 were incubated with 100 μCi [γ-32P]ATP in the presence of 100 ng/ml CGRP or 10 mM ATP, followed by washing with PBS every 10 min. The signals of radioactive ATP were detected through liquid scintillation. b Plasmids encoding FLAG-tagged NLRP3, HA-tagged NEK7 and increasing amounts of Myc-tagged GFP or GFP-CGRP were cotransfected into HEK293T cells for 24 h, followed by immunoprecipitation with antibody against FLAG or with control IgG. Immunoprecipitates and cell lysates were immunoblotted with antibodies against the indicated proteins. c Plasmids encoding FLAG-tagged NLRP3, Myc-tagged GFP or GFP-CGRP and increasing amounts of HA-tagged NEK7 were cotransfected into HEK293T cells for 24 h, followed by immunoprecipitation with antibody against FLAG or with control IgG. Immunoprecipitates and cell lysates were immunoblotted with antibodies against the indicated proteins. d Plasmids encoding FLAG-tagged NLRP3, Myc-tagged GFP or GFP-CGRP and HA-tagged NEK7 were cotransfected into HEK293T cells for 24 h, followed by immunoprecipitation with an antibody against Myc or with control IgG. Immunoprecipitates and cell lysates were immunoblotted with antibodies against the indicated proteins. e Plasmids encoding Myc-tagged GFP or GFP-CGRP and HA-tagged NEK7 were cotransfected into HEK293T cells for 24 h, followed by immunoprecipitation with antibody against Myc or with control IgG. Immunoprecipitates and cell lysates were immunoblotted with antibodies against the indicated proteins. f Plasmids encoding Myc-tagged GFP or GFP-CGRP, FLAG-tagged NLRP3 and HA-tagged NEK7 were cotransfected into HEK293T cells for 24 h, followed by immunoprecipitation with antibody against HA or with control IgG. Immunoprecipitates and cell lysates were immunoblotted with antibodies against the indicated proteins. g WT BMDMs primed with 1 μg/ml LPS for 3 h were stimulated with 10 μM nigericin with or without 25 ng/ml CGRP for 30 min. Cells were lysed and subjected to immunoprecipitation with an antibody against NLRP3 or with control IgG. Immunoprecipitates and cell lysates were immunoblotted with antibodies against the indicated proteins. h, i WT BMDMs (h) or Calcrl knockout cells (i) primed with 1 μg/ml LPS for 3 h were stimulated with 10 μM nigericin with or without 25 ng/ml CGRP for 30 min. Cells were lysed and subjected to immunoprecipitation with an antibody against NLRP3 or with control IgG. Immunoprecipitates and cell lysates were immunoblotted with antibodies against the indicated proteins. j Nlrp3–/– iBMDMs restored with FLAG-tagged full-length or truncated isoforms of NLRP3 were primed with 1 μg/ml LPS for 3 h and then stimulated with 10 μM nigericin for 30 min. Cells were then lysed and subjected to immunoprecipitation with an antibody against FLAG or with control IgG. Immunoprecipitates and cell lysates were immunoblotted with antibodies against the indicated proteins. k Nlrp3–/– iBMDMs restored with full-length or truncated isoforms of NLRP3 were primed with 1 μg/ml LPS for 3 h and then stimulated with 10 μM nigericin for 30 min. Cells were then fixed and stained with an antibody against ASC, followed by the examination of ASC specks inside cells. One hundred cells were assessed for each group. Data are shown as the means ± SDs. Experiments were repeated three times with similar results
When BMDMs were costimulated with NLRP3 inflammasome stimulator and CGRP, endogenous NLRP3 was found to associate with CGRP but not with NEK7 (Fig. 5g). CGRP inhibited the association between NLRP3 and NEK7 in WT but not Calcrl knockout cells (Fig. 5h, i). In addition, transfection of peptide CGRP (8–37) into BMDMs led to dissociation of the NLRP3-NEK7 complex (Fig. S5f), further supporting the notion that CGRP expels NEK7 from NLRP3 inside cells. Due to the importance of NEK7 in NLRP3 inflammasome activation and the finding that NLRP3 lacking any of the CGRP binding regions failed to be functional, we examined the interactions between NEK7 and NLRP3 truncations lacking amino acids 378–427, 678–727 or both. NLRP3 lacking any of the CGRP binding regions no longer bound NEK7 (Fig. 5j and Fig. S5g), indicating that these CGRP binding sites are essential for the NLRP3-NEK7 interaction. Correspondingly, NLRP3 inflammasome activation was abolished in Nlrp3 knockout iBMDMs restored with any of the NLRP3 truncations (Fig. 5k). Overall, these results suggest that CGRP disrupts the interaction between NLRP3 and NEK7 in vivo.
CGRP-mediated NLRP3 inflammasome suppression facilitates bacterial invasion
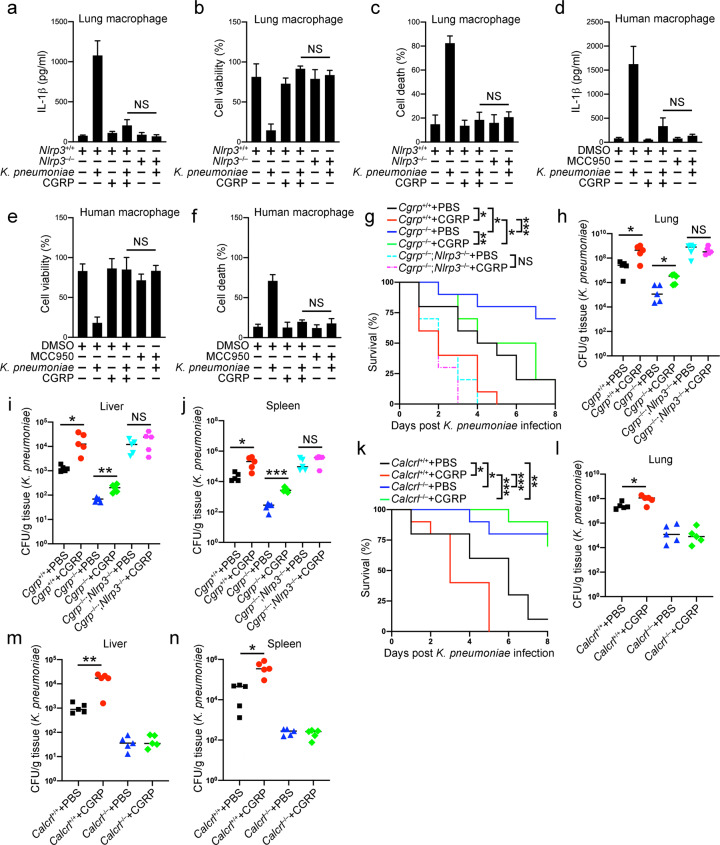
To test the physiological role of CGRP in bacterial infection, we incubated mouse lung macrophages with K. pneumoniae and CGRP (Fig. 6a–c). K. pneumoniae infection led to mature IL-1β secretion, decreased cell viability and increased cell death. CGRP addition strongly inhibited K. pneumoniae-induced inflammasome activation and the corresponding consequences. Moreover, CGRP administration had almost the same effect as Nlrp3 deficiency, further suggesting the involvement of CGRP-mediated NLRP3 inflammasome inhibition during bacterial infection. Similar results were observed in BMDMs (Fig. S6a–c). We also generated human macrophages from donor peripheral blood cells and infected these cells with K. pneumoniae. CGRP inhibits IL-1β secretion in a similar way as the NLRP3 inhibitor MCC950 in human macrophages. CGRP treatment also enhanced cell viability and reduced cell death in these cells, similar to MCC950 (Fig. 6d–f).
Fig. 6.
CGRP administration exacerbates bacterial infection. a–c Macrophages from Nlrp3+/+ and Nlrp3–/– lungs primed with 1 μg/ml LPS for 3 h were incubated with K. pneumoniae at an MOI of 10 for 1 h with or without the presence of 25 ng/ml CGRP. Cell culture supernatants were collected for ELISAs to determine the secreted IL-1β protein levels (a). Percentages of viable cells were determined by examining cellular ATP levels in cell pellets (b), and percentages of cells undergoing pyroptosis were calculated by assessing released LDH in cell culture supernatants (c). d–f Macrophages derived from human peripheral blood mononuclear cells were primed with 1 μg/ml LPS for 3 h and then incubated with K. pneumoniae at an MOI of 10 for 1 h with or without the presence of 25 ng/ml CGRP. Cell culture supernatants were collected for ELISAs to determine the secreted IL-1β protein levels (d). Percentages of viable cells were determined by examining cellular ATP levels in cell pellets (e), and percentages of cells undergoing pyroptosis were calculated by assessing released LDH in cell culture supernatants (f). g Cgrp+/+, Cgrp–/– and Cgrp–/–;Nlrp3–/–mice were inoculated intratracheally with 3 × 104 CFU of K. pneumoniae. Moreover, 2 μg CGRP was administered intranasally to each mouse once a day. Survival rates were examined within a period of 8 days. n = 10 for each group. h–j Cgrp+/+, Cgrp–/– and Cgrp–/–;Nlrp3–/– mice were inoculated intratracheally with 3 × 104 CFU of K. pneumoniae. Moreover, 2 μg CGRP was administered intranasally to each mouse once a day. Bacterial load determinations using homogenates from lung (h), liver (i) or spleen (j) were performed 2 days later. n = 5 for each group. k Calcrlflox/flox (Calcrl+/+) and Calcrlflox/flox;Lyz2-Cre (Calcrl–/–) mice were inoculated intratracheally with 3 × 104 CFU of K. pneumoniae. Moreover, 2 μg CGRP was administered intranasally to each mouse once a day. Survival rates were examined within a period of 8 days. n = 10 for each group. l–n Calcrlflox/flox (Calcrl+/+) and Calcrlflox/flox;Lyz2-Cre (Calcrl–/–) mice were inoculated intratracheally with 3 × 104 CFU of K. pneumoniae. Moreover, 2 μg CGRP was administered intranasally to each mouse once a day. Bacterial load determinations using homogenates from lung (l), liver (m) or spleen (n) were performed 2 days later. n = 5 for each group. Data are shown as the means ± SDs. *P < 0.05; **P < 0.01; ***P < 0.001. Similar results were observed for at least three repeats
We then assessed the therapeutic role of CGRP in mouse infection models. CGRP administration decreased the survival of both wild-type and Cgrp-deficient mice infected with K. pneumoniae (Fig. 6g). Bacterial loads in wild-type and Cgrp-deficient mouse organs were also both increased by CGRP treatments (Fig. 6h–j). NLRP3 deficiency abrogated the inhibitory effect of CGRP during bacterial infection (Fig. 6h–j). In contrast, administration of the CGRP antagonist CGRP (8–37) alleviated the infection in the wild-type but not Cgrp knockout mice (Fig. S6d–g), probably by antagonizing endogenous CGRP that was induced during infection in the wild-type mice. Furthermore, CGRP failed to exacerbate survival in Calcrl-deficient mice infected with K. pneumoniae (Fig. 6k). Bacterial loads were also comparable between the PBS-treated and CGRP-treated Calcrl knockout mice infected with K. pneumoniae (Fig. 6l–n), suggesting that the inhibitory role of CGRP in NLRP3 inflammasome activation depends on the receptor CALCRL in vivo. Moreover, administration of the CGRP antagonist CGRP (8–37) alleviated the infection in the wild-type but not Calcrl knockout mice (Fig. S6h–k). Altogether, our results indicate that CGRP-mediated NLRP3 inflammasome suppression is conducive to bacterial invasion, while a CGRP antagonist alleviates infection.
Discussion
In the present study, we showed that Cgrp deficiency alleviates bacterial infection. The protein level of IL-1β was increased in Cgrp knockout mouse lung tissues post-infection. CGRP acts on macrophages to suppress NLRP3 inflammasome activation. Upon NLRP3 inflammasome stimulation, internalized CGRP enters the macrophage cytosol and associates with NLRP3. CGRP expels NEK7 from NLRP3, leading to suppressed NLRP3 inflammasome activation. Administration of a CGRP antagonist ameliorated mouse survival post-bacterial infection. This distinct feature of the neuropeptide CGRP in immune regulation will shed new light on antibacterial therapies in the future.
Recently, a number of neuropeptides have been reported to affect immune cells. The neuropeptide NMU is expressed in lung-resident ILC2s and maintains ILC2 cell numbers and effector function [39]. ILC2s also express the neuropeptide CGRP post-stimulation [33]. The inhibitory CGRP signaling in ILC2s tunes the type 2 innate immune responses [40]. The neuropeptide FF binds its receptor on adipose tissue macrophages and increases M2 activation [41]. Neuropeptide Y is expressed in adipose tissue macrophages, and deletion of this peptide leads to attenuated adipose tissue inflammation [42]. These studies imply close connections between the neuro and immune systems. However, the existence of CGRP signaling in macrophages lacks direct evidence. In our study, we found that CGRP expression levels were enhanced post-bacterial infection in the lung. Macrophages do not express CGRP but have upregulated expression of the CGRP receptor CALCRL. CGRP binds CALCRL on macrophages and is internalized into early endosomal compartments, where CGRP enters the cell cytosol when cells are challenged with NLRP3 inflammasome stimuli. Whether these aforementioned neuropeptides enter the cytosol of target cells needs to be further investigated.
The NLRP3 inflammasome can be activated by a series of stimuli, including membrane pore-forming toxins, crystalline particles and other sterile triggers [17, 38]. These stimuli influence target cells in a wide range. Crystalline particles cause lysosomal rupture to activate the NLRP3 inflammasome [43]. Moreover, the endosomal compartments can be destroyed by these stimulations [44]. CGRP is internalized into the early endosome and continues to transduce signals to cells [27]. When endosomal compartments are damaged, CGRP leaks into the cytosol and binds NLRP3 in macrophages. Moreover, during inflammasome activation, NLRP3 is close to the endosomal apparatus [45], indicating the involvement of endosomal compartments in NLRP3 inflammasome regulation. CGRP leaking from endosomes binds NLRP3 with an affinity much stronger than that of NEK7. Without NEK7’s help, NLRP3 fails to form oligomerized structures to initiate inflammasome activation. There might be more CGRP binding targets in macrophages and other immune cells, such as innate lymphoid cells, which are worth exploring in the future.
Overall, we found an inhibitory role of CGRP in macrophage inflammasome activation and deciphered the molecular mechanism by which CGRP binds and prohibits NLRP3 activation, which might initiate new lines of thinking on neuroimmune networks.
Materials and methods
Antibodies and reagents
Antibodies against NLRP3 (15101), IL-1β (12703, 12426), cleaved IL-1β (83186, 63124), caspase-1 (3866, 24232, 2225), ASC (13833, 67824) and CGRP (14959) were purchased from Cell Signaling Technology (Massachusetts). Antibodies against caspase-1 (ab207802) and NEK7 (ab133514) were purchased from Abcam (Cambridge). Antibodies against CGRP (AF6495) were purchased from Beyotime (Shanghai). The antibody against EEA1 (3704-1) was purchased from Epitomics (California). Anti-c-Myc magnetic beads (88843) were purchased from Pierce (Massachusetts). Antibodies against the 6xHis tag (MA121315) were purchased from Invitrogen (Massachusetts). Antibodies against FLAG tag (F3165) and β-actin (A1978) and anti-FLAG M2 magnetic beads (M8823) were purchased from Sigma-Aldrich (Missouri). Antibodies against c-Myc (sc-40), GST (sc-138), GAPDH (sc-32233) and EEA1 (sc-137130) were purchased from Santa Cruz Biotechnology (Texas). Anti-HA tag antibody (HX1820) was purchased from Huaxingbio (Beijing). Fc block antibody (C247) was purchased from MBL Beijing Biotech (Beijing). Mouse anti-CD45 (11-0451-81), anti-CD11b (48-0112-82), anti-CD11c (17-0128-42), anti-Siglec-F (12-1702-82), anti-Ly6G (12-9668-82), anti-CD64 (12-0641-82) and anti-Ly6C (12-5932-82) antibodies were purchased from Thermo Fisher Scientific (Massachusetts). HRP-conjugated goat anti-mouse IgG (SA00001-1) and HRP-conjugated goat anti-rabbit IgG (SA00001-2) were purchased from Proteintech Group (Pennsylvania). Alexa Fluor Plus 488-conjugated goat anti-mouse IgG (A32723) and Alexa Fluor 594-conjugated goat anti-rabbit IgG (A32740) were purchased from Invitrogen. Antibodies against EEA1 (bs-11250R), Alexa Fluor Plus 488-conjugated donkey anti-goat IgG (bs-0294D-AF488) and Alexa Fluor 594-conjugated donkey anti-rabbit IgG (bs-0295D-AF594) were purchased from Bioss (Beijing).
Glutathione Sepharose 4B resin (17075601) was purchased from Cytiva (Massachusetts). Protein A/G PLUS-Agarose beads (sc-2003) were purchased from Santa Cruz Biotechnology. DAPI (2879038) was purchased from PeproTech (BioGems, California). The Human Monocyte Nucleofector Kit was purchased from Lonza (Basel). Dropout (DO)/–Leu/–Trp and DO/–Ade/–His/–Leu/–Trp supplement, SMART MMLV reverse transcriptase (639524), X-α-Gal (630462) and carrier DNA (630440) were purchased from Clontech, TaKaRa Bio (California). A random primer (hexadeoxyribonucleotide mixture, pd(N)6) (3801) was purchased from TaKaRa Bio. Protease inhibitor cocktail (11697498001) was purchased from Roche (Basel). A total RNA extraction kit (8034111) was purchased from Dakewe (Beijing). Ni-NTA agarose beads (R90115) were purchased from Invitrogen. LPS (B46894) was purchased from Innochem (Beijing). Nigericin (481990) was purchased from Merck Millipore (Massachusetts). Human M-CSF (300-25) and murine M-CSF (315-02) were purchased from PeproTech. Silica (381276) was purchased from Sigma-Aldrich. LLOMe (GC44079) was purchased from GlpBio (California). CPPD (tlrl-cppd), Alum (tlrl-aloh), MSU (tlrl-msu), Nano-SiO2 (tlrl-sio-2) and poly(dA:dT) (tlrl-patn) were purchased from InvivoGen (California). Rat CGRP (1161) and CGRP (8–37) (1169) were purchased from R&D Systems (Minnesota). Human CGRP (CGRP15-P-5) was purchased from Alpha Diagnostic International (Texas). Disuccinimidyl suberate (ab141274), ATP-Agarose (ab270535), Pitstop 2 (ab144650), Dyngo-4a (ab120689) and cell-penetrating peptides (ab142343) were purchased from Abcam. A minute endosome isolation and cell fractionation kit (ED-028) was purchased from Invent Biotechnologies (Minnesota). The CellTiter-Glo luminescent cell viability assay kit (G7570) and CytoTox 96 nonradioactive cytotoxicity assay kit (G1780) were purchased from Promega (Wisconsin).
Bacteria and CFU determination
K. pneumoniae serotype 2 strain (43816) was purchased from ATCC. Salmonella enterica serovar typhimurium (CGMCC 1.1194) was obtained from the Institute of Microbiology, Chinese Academy of Sciences. S. aureus (NCTC8325) was obtained from the University of Science and Technology of China. Bacteria were preserved in LB plates and inoculated into liquid LB medium, followed by shaking at 37 °C for 16 h. Bacteria either from culture or tissue homogenates were diluted and spread into LB plates to determine the CFU numbers.
Mice and infection
Cgrp knockout mice were generated using CRISPR/Cas9 technology. Briefly, guide RNAs targeting introns flanking exon 5 of the mouse Calca gene together with Cas9 mRNA were introduced into fertilized mouse, e.g., through microinjection to generate Cgrp knockout offspring. F0 founder mice were identified through sequencing and crossed with wild-type mice to generate the F1 generation. Guide RNA sequences were as follows: gRNA1, 5ʹ-TTGGGTTTTTGAATACCGCT-3ʹ; gRNA2, 5ʹ-TTGTGACCATTCTACCATAT-3ʹ; gRNA3, 5ʹ-GACAATCACAAGGCCATTAG-3ʹ; gRNA4, 5ʹ-ACATTGTAGTGTATACTACT-3ʹ. Genotyping primers were as follows: forward primer, 5ʹ-TAAGACTGGGGTGCTTTCACTTTA-3ʹ; reverse primer: 5ʹ-TATTCTGCACTTAGCTTTAGCTCCT-3ʹ. The product length of the targeted allele was 601 bp and 1324 bp for the wild-type allele. Lyz2-Cre (004781) mice were purchased from the Jackson Laboratory (Maine). Calcrlflox/flox (CKOCMP-54598-Calcrl) and Nlrp3–/– (KOCMP-216799-Nlrp3) mice were purchased from Cyagen Biosciences (Jiangsu, China).
Mice were inoculated intratracheally with 3 × 104 CFU of K. pneumoniae, followed by survival rate examinations within a period of 8 days or bacterial load determination 2 days later. Otherwise, mice were inoculated intratracheally with 2 × 108 CFU of S. aureus, followed by survival rate examinations within a period of 8 days or bacterial load determination 2 days later. For CGRP or CGRP (8–37) administration, 2 μg CGRP was administered intranasally to each mouse once a day.
BALF collection and lung single-cell preparation
For bronchoalveolar lavage fluid (BALF) collection, mice were anesthetized, followed by thoracic cavity opening and a flush of circulating blood cells by injecting approximately 10 ml of ice-cold PBS into the right ventricle. The mouse neck was then dissected to expose the trachea. A puncture needle was inserted into the upper end of the trachea, followed by repeated rinsing with 0.8 ml PBS five times. BALF collections were centrifuged at 300 × g at 4 °C for 5 min, followed by ELISAs of the supernatants or macrophage isolation in the cell pellets. For generation of lung single cells, mouse lungs were dissected to remove airways following circulating blood cell removal. Lung tissues were then minced into pieces smaller than 1 mm in diameter, followed by incubation with 5 U/ml DNase I and 300 μg/ml Liberase TL at 37 °C for 30 min. Dissociated lung tissues were passed through a 100 μm cell strainer, followed by washing with ice-cold PBS. Cells incubated with mouse Fc block antibody for 30 min on ice were subjected to flow cytometry-based analysis or sorting. CD45+ was the marker for leukocytes. CD45+CD11c+CD64+ were markers for macrophages, and CD45+CD11b+CD11c–Ly6G+ were markers for neutrophils.
Primary cell separation and culture
For generation of BMDMs, bone marrow cells were flushed from mouse femurs and tibias, followed by red blood cell removal using red blood cell lysis buffer. Cells were resuspended in alpha-MEM containing 10% (v/v) fetal bovine serum and 10 ng/ml M-CSF and grown at 37 °C with a 5% CO2 humidified atmosphere for 7 days. For mouse lung macrophages, bronchoalveolar lavage fluids were collected, and cell pellets were resuspended and stained with fluorescence-conjugated antibodies, followed by cell sorting in a flow cytometric sorter. Alveolar macrophages were CD45+CD11chiSiglec-Fhi cells. For production of human macrophages, human blood samples collected from healthy donors were mixed with an equal volume of saline and then added to lymphocyte separation medium, followed by centrifugation and PBMC collection. Human PBMCs were incubated with APC-conjugated anti-CD14 antibody on ice for 30 min, followed by incubation with anti-APC-conjugated magnetic beads. Monocytes were enriched on a magnetic separator. For analysis of the purity of sorted monocytes, cells were stained with APC-conjugated anti-CD14 antibody, followed by FACS examination. Cells with a purity above 95% were used for subsequent experiments. Cells were resuspended in RPMI-1640 medium containing 10% (v/v) fetal bovine serum and 50 ng/ml human M-CSF, followed by growth at 37 °C with a 5% CO2 humidified atmosphere for 7 days. Healthy blood samples were provided by volunteer researchers. Informed consent was obtained from all subjects, and the experiments conformed to related principles. The study was licensed by the Ethics Committee of the Institute of Microbiology, Chinese Academy of Sciences.
Inflammasome induction
For inflammasome activation, cells were incubated with 1 μg/ml LPS for 3 h, followed by stimulation with 10 μM nigericin for 30 min, 2 mM ATP for 30 min, 0.5 μM gramicidin for 1 h, 2 μg/ml poly(dA:dT) for 4 h, Salmonella at an MOI of 10 for 4 h, 2 μM LLOMe for 6 h, 500 μg/ml silica for 6 h, 250 μg/ml Alum for 6 h, 200 μg/ml MSU for 6 h, 200 μg/ml CPPD for 6 h and 200 μg/ml SiO2 for 6 h. Cells were grown at 37 °C with a 5% CO2 humidified atmosphere.
Plasmid transfection and lentiviral production
Transfection reagent JetPRIME (Polyplus Transfection) was used to conduct transfection in HEK293T cells. HEK293T cells were plated 14 h earlier to achieve a density of approximately 70% when transfection was performed. Lentiviral plasmids containing exogenous genes of interest and packaging plasmids pSPAX2 and pMD2.G were transfected into HEK293T cells at a ratio of 4:3:1. Forty-eight hours later, the supernatants were collected, centrifuged at 1000 rpm for 5 min and filtered using 0.45 μm sterile syringe filters (Merck Millipore). The filtrate was then concentrated with a 100 kD Amicon ultra centrifugal filter unit (Merck Millipore). iBMDM cells were incubated with lentiviruses for 6 h, followed by culture medium replacement with fresh medium. Puromycin (2 μg/ml) was added to the medium 48 h post-infection for 3 days.
Protein transfection using cell-penetrating peptides
For a single well of 6-well plate macrophages, 10 μg of CGRP or CGRP (8–37) was incubated with 10 μg cell-penetrating peptides in 100 μl of PBS at room temperature for 30 min, followed by addition of the peptide-CPP mixture to the cell medium. The culture medium was replaced with fresh medium 3 h later.
Cell viability and cytotoxicity assay
Cell pellets from cells stimulated with inflammasome activators were used to measure cell viability by assessing cellular ATP levels with a CellTiter-Glo luminescent cell viability assay kit (Promega). The supernatants from cells stimulated with inflammasome activators were collected and subjected to a lactate dehydrogenase (LDH) assay to assess cell death using a CytoTox 96 nonradioactive cytotoxicity assay kit (Promega). Both the supernatants and cell pellets were collected for immunoblotting analyses.
Knockout cell line generation and rescue
LentiCRISPRv2 vectors expressing CRISPR‒Cas9 targeting guide RNA (gRNA) designed for target genes and a GFP selection marker were used to generate knockout cell lines. The gRNA targeting NLRP3 was 5ʹ-gttctttatccactgccgag-3ʹ. Guide RNAs were designed using the GPP sgRNA Designer on the GPP Web Portal of Broad Institute. The gRNA-expressing plasmids and packaging plasmids were transfected into HEK293T cells for 48 h, followed by supernatant collection and lentivirus concentration. iBMDM cells were incubated with lentiviruses at an MOI of 1, followed by culture medium replacement with fresh medium 6 h later. GFP-positive cells were isolated in a flow cytometric sorter and sequenced. Nlrp3-deficient iBMDM cells were infected with lentiviruses expressing various NLRP3 mutants at an MOI of 1 for 6 h, followed by culture medium replacement with fresh medium 6 h later. The medium was replaced with fresh medium containing 2 μg/ml puromycin 48 h post infection. Cells stably expressing NLRP3 mutants were obtained one week later.
ELISA
ELISA kits detecting mouse IL-1α (BMS611), CXCL1 (EMCXCL1), CXCL10 (BMS6018) and CXCL12 (EMCXCL12) were purchased from Thermo Fisher Scientific. ELISA kits detecting human IL-1β (D711068), mouse IL-1β (D721017), IL-2 (D721020), IL-3 (D721107), IL-4 (D721021), IL-6 (D721022), IL-10 (D721023), IL-12 p40 (D721174), IL-17 (D721024), TNF-α (D721026) and M-CSF (D721172) were purchased from BBI Life Sciences (Shanghai). Mouse lung homogenates, BALF and cell culture supernatants were collected and subjected to ELISAs following the manufacturer’s instructions.
RNA extraction and RT‒PCR
A total RNA extraction kit (Dakewe, Beijing) was used to extract RNA from cells according to the manufacturer’s instructions. For reverse transcription, 1 μg RNA was mixed with 2 μl of N6 primers and 7 μl of DEPC water, followed by incubation at 72 °C for 2 min. The mixture was then chilled on ice for 2 min and mixed with 4 μl of 5x first-strand buffer (TaKaRa Bio), 2 μl of 100 mM DTT, 2 μl of 10 mM dNTP mix (Solarbio, Beijing) and 2 μl of SMART MMLV reverse transcriptase (Clontech, TaKaRa Bio). The mixture was incubated under the following conditions: at 25 °C for 10 min, at 42 °C for 1 h and at 75 °C for 10 min. cDNA samples were diluted for further PCR analysis. Primers for PCR identification were as follows: 5ʹ-tggccactctcagtgaagaag-3ʹ (forward) and 5ʹ-ttgaggtcttgtgtgtacgt-3ʹ (reverse) for mouse Calcitonin; 5ʹ-cagatcaggaggtgtggtg-3ʹ (forward) and 5ʹ-gtctttcatcagcctttctt-3ʹ (reverse) for mouse Cgrp; 5ʹ-agaaggttacaaagatctg-3ʹ (forward) and 5ʹ-atgatgagagagatgatcag-3ʹ (reverse) for mouse Calcrl; 5ʹ-tggctgtgtggatctttgc-3ʹ (forward) and 5ʹ-ccagcaaacccatccactct-3ʹ (reverse) for mouse Nlrp3; 5ʹ-tgccaccttttgacagtgatg-3ʹ (forward) and 5ʹ-tgatgtgctgctgcgagatt-3ʹ (reverse) for mouse Il1b; 5ʹ-gaagatctggcaccacacc-3ʹ (forward) and 5ʹ-accagaggcatacagggaca-3ʹ (reverse) for mouse Actb.
Recombinant protein expression and purification
For recombinant expression of GST-tagged CGRP, CGRP cDNA was cloned into the pGEX-6P-1 vector. Plasmids were transformed into E. coli strain BL21, and the bacteria grew in LB medium with 100 μg/ml ampicillin at 37 °C for 8 h, followed by the addition of IPTG to a final concentration of 0.5 mM and shaking at 150 rpm at 37 °C for 3 h. Cells were harvested and resuspended in PBS containing 1% Triton-X-100 and lysed by sonication. Cell lysates were centrifuged at 13,000 rpm at 4 °C for 30 min and filtered through sterile syringe filters. The filtered lysate was loaded on a column containing glutathione sepharose 4B resin, followed by washing with PBS containing 1% Triton-X-100. The protein was eventually eluted with 1 ml of 10 mM L-glutathione solution.
Resin-based pulldown assay
Ni-NTA resins or glutathione sepharose 4B resins were washed twice with PBS and blocked with 1% BSA in PBS at 4 °C for 1 h. Targeting protein-expressing cell lysates were mixed with recombinant GST-tagged proteins and BSA-blocked glutathione sepharose 4B resins at 4 °C for 2 h. Resins were washed three times with PBS containing 1% Triton X-100, followed by sample preparation for SDS‒PAGE and immunoblotting.
Endosome isolation and cell fractionation
A minute endosome isolation and cell fractionation kit was used. Briefly, cells were washed with ice-cold PBS and centrifuged to remove supernatant, followed by addition of buffer A. After a 30 s mixing on a vortex, cell suspensions were transferred to a centrifuge tube column and centrifuged at 16,000 × g for 30 s. Liquids in the tube bottom were collected and mixed on a vortex, followed by centrifugation at 700 × g for 3 min. Supernatants were transferred to a 1.5 ml tube and centrifuged at 16,000 × g at 4 °C for 1 h. Supernatants were transferred again to a new 1.5 ml tube, followed by the addition of a half volume of buffer B. After incubation at 4 °C for 2 h, samples were centrifuged at 10,000 × g at 4 °C for 30 min. Supernatants containing cytosols and pellets containing endosomes were collected separately and examined by immunoblotting.
Immunoprecipitation
HEK293T cells were seeded on 6-well culture plates and transfected with plasmids 16 h later. At 24 h post-transfection, cells were washed twice with PBS and lysed in a prechilled buffer containing 150 mM NaCl, 50 mM Tris (pH 7.5), 1 mM EDTA, 1% Triton-X-100, 1% protease inhibitor cocktail and 10% glycerol on ice for 30 min. For immunoprecipitation using primary cells, cells post-stimulation or treatments were lysed in a prechilled PBS buffer containing 1% Triton-X-100 and 1% protease inhibitor cocktail on ice for 30 min. Cell lysates were centrifuged at 13,000 rpm at 4 °C for 10 min. Primary antibodies and their isotype control IgG were immobilized to AminoLink Coupling Resin (Thermo Fisher Scientific) following the manufacturer’s instructions. Supernatants were incubated with immobilized antibodies at 4 °C for 2 h. Resins were washed three times with PBS containing 1% Triton-X-100, followed by immunoprecipitant detachment in 0.1 M glycine-HCl, pH 2.7. Supernatants were neutralized by adding 1/10 the volume of 2 M Tris-HCl, pH 8.0, followed by immunoblotting with the indicated antibodies.
Immunofluorescence
Cells adhered to covers precoated with poly-L-lysine were stimulated with inflammasome activators, followed by fixation with 4% paraformaldehyde for 10 min at room temperature. Cells were then washed twice with PBS and permeabilized with PBS containing 1% Triton-X-100 for 10 min, followed by washing with PBS twice and blocking in 10% normal goat serum at 37 °C for 30 min. Cells were incubated with primary antibody for 2 h, washed with PBS three times and further incubated with fluorescence-conjugated secondary antibody for 1 h. Nuclei were stained with DAPI. For coimmunostaining using antibodies with the same origin, Lightning-Link kits (ab236553 and ab269900, Novus Biologicals) were used to directly link fluorescence to primary antibodies. Cells were visualized through an UltraVIEW VoX imaging system (PerkinElmer).
Yeast two-hybrid screening
Yeast strain AH109 was inoculated into 5 ml of YPDA medium and shaken at 250 rpm at 30 °C for 16 h. The overnight culture was transferred to a flask containing 50 ml of YPDA and incubated at 30 °C for 3 h. Yeast cells were collected by centrifugation at 4000 rpm for 10 min and resuspended in 1XTE/LiAc buffer (pH 7.5) containing 0.1 M lithium acetate, 0.1 M Tris-HCl and 10 mM EDTA. A mixture of plasmid DNA and carrier DNA was added to the yeast cell suspension and vortexed to mix well. PEG/LiAc buffer containing 40% polyethylene glycol 3350, 0.1 M lithium acetate, 0.1 M Tris-HCl and 10 mM EDTA was then added to the suspension and vortexed at a high speed. The suspension was shaken at 200 rpm at 30 °C for 30 min, followed by the addition of DMSO and heat shock in a 42 °C water bath for 15 min. Cells were chilled on ice for 2 min and resuspended to plated on appropriate SD agar plates. A mouse bone marrow cDNA library was used for Y2H screening with Gal4 DNA binding domain (BD)-tagged CGRP as bait. BD-tagged full-length CGRP or CGRP truncation and Gal4 DNA activating domain (AD)-tagged NLRP3 were cotransfected into yeast strain AH109, and double-positive clones were selected on SD medium lacking adenine, histidine, leucine and tryptophan.
Statistical analysis
No statistical methods were used to predetermine the sample size. Experiments were independently repeated at least three times to achieve statistical significance. No randomization or blinding procedures were used in this study. No samples were excluded from the analysis. Data are shown as the means ± SDs of three technical replicates. Data with a normal distribution determined by the Shapiro–Wilk normality test were statistically analyzed by two-tailed Student’s t tests if not specified. The Gehan-Breslow-Wilcoxon test was used for the analysis of survival data. Data were analyzed by GraphPad Prism 9.0. P values ≤ 0.05 were considered significant (*P < 0.05; **P < 0.01; ***P < 0.001); NS means nonsignificant when P > 0.05.
Supplementary information
Acknowledgements
We thank Ting Li (Peking University) for technical help. This work was supported by the National Natural Science Foundation of China (81922031, 82271790, 92169113), Beijing Natural Science Foundation (7212067), National Key R&D Program of China (2019YFA0111800, 2022YFC2302900), Strategic Priority Research Programs of the Chinese Academy of Sciences (XDB29020000), Key Research Program of Frontier Sciences of Chinese Academy of Sciences (ZDBS-LY-SM025), CAS Project for Young Scientists in Basic Research (YSBR-010), Fok Ying Tung Education Foundation to PX, and Youth Innovation Promotion Association of CAS to SW.
Author contributions
FZ, DY, and XQ designed and performed experiments and analyzed data; JM, WL, QL, CW, YL, DJ, YZ, YQ, and SW performed the experiments and analyzed the data; SW and PX initiated the study, designed and performed experiments, analyzed data, and wrote the paper.
Competing interests
The authors declare no competing interests.
Footnotes
These authors contributed equally: Fangrui Zhu, Dou Yu, Xiwen Qin, Yan Qian.
Contributor Information
Shuo Wang, Email: wangshuo@im.ac.cn.
Pengyan Xia, Email: xiap@pku.edu.cn.
Supplementary information
The online version contains supplementary material available at 10.1038/s41423-022-00968-w.
References
- 1.Fitzgerald KA, Kagan JC. Toll-like receptors and the control of immunity. Cell. 2020;180:1044–66. doi: 10.1016/j.cell.2020.02.041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Liu G, Gack MU. Distinct and orchestrated functions of RNA sensors in innate immunity. Immunity. 2020;53:26–42. doi: 10.1016/j.immuni.2020.03.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Charmoy M, Hurrell BP, Romano A, Lee SH, Ribeiro-Gomes F, Riteau N, et al. The Nlrp3 inflammasome, IL-1β, and neutrophil recruitment are required for susceptibility to a nonhealing strain of Leishmania major in C57BL/6 mice. Eur J Immunol. 2016;46:897–911. doi: 10.1002/eji.201546015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Mitchell PS, Sandstrom A, Vance RE. The NLRP1 inflammasome: new mechanistic insights and unresolved mysteries. Curr Opin Immunol. 2019;60:37–45. doi: 10.1016/j.coi.2019.04.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Sharma BR, Kanneganti TD. NLRP3 inflammasome in cancer and metabolic diseases. Nat Immunol. 2021;22:550–9. doi: 10.1038/s41590-021-00886-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Wlodarska M, Thaiss CA, Nowarski R, Henao-Mejia J, Zhang JP, Brown EM, et al. NLRP6 inflammasome orchestrates the colonic host-microbial interface by regulating goblet cell mucus secretion. Cell. 2014;156:1045–59. doi: 10.1016/j.cell.2014.01.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Zhao Y, Yang J, Shi J, Gong YN, Lu Q, Xu H, et al. The NLRC4 inflammasome receptors for bacterial flagellin and type III secretion apparatus. Nature. 2011;477:596–600. doi: 10.1038/nature10510. [DOI] [PubMed] [Google Scholar]
- 8.Zhu S, Ding S, Wang P, Wei Z, Pan W, Palm NW, et al. Nlrp9b inflammasome restricts rotavirus infection in intestinal epithelial cells. Nature. 2017;546:667–70. doi: 10.1038/nature22967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Ma J, Zhu F, Zhao M, Shao F, Yu D, Ma J, et al. SARS-CoV-2 nucleocapsid suppresses host pyroptosis by blocking Gasdermin D cleavage. Embo j. 2021;40:e108249. doi: 10.15252/embj.2021108249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Newton K, Dixit VM, Kayagaki N. Dying cells fan the flames of inflammation. Science. 2021;374:1076–80. doi: 10.1126/science.abi5934. [DOI] [PubMed] [Google Scholar]
- 11.Liang JJ, Fraser IDC, Bryant CE. Lipid regulation of NLRP3 inflammasome activity through organelle stress. Trends Immunol. 2021;42:807–23. doi: 10.1016/j.it.2021.07.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Zhivaki D, Kagan JC. NLRP3 inflammasomes that induce antitumor immunity. Trends Immunol. 2021;42:575–89. doi: 10.1016/j.it.2021.05.001. [DOI] [PubMed] [Google Scholar]
- 13.Andreeva L, David L, Rawson S, Shen C, Pasricha T, Pelegrin P, et al. NLRP3 cages revealed by full-length mouse NLRP3 structure control pathway activation. Cell. 2021;184:6299–6312. doi: 10.1016/j.cell.2021.11.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Gaidt MM, Ebert TS, Chauhan D, Schmidt T, Schmid-Burgk JL, Rapino F, et al. Human monocytes engage an alternative inflammasome pathway. Immunity. 2016;44:833–46. doi: 10.1016/j.immuni.2016.01.012. [DOI] [PubMed] [Google Scholar]
- 15.Liu X, Zhang Z, Ruan J, Pan Y, Magupalli VG, Wu H, et al. Inflammasome-activated gasdermin D causes pyroptosis by forming membrane pores. Nature. 2016;535:153–8. doi: 10.1038/nature18629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Shi J, Zhao Y, Wang K, Shi X, Wang Y, Huang H, et al. Cleavage of GSDMD by inflammatory caspases determines pyroptotic cell death. Nature. 2015;526:660–5. doi: 10.1038/nature15514. [DOI] [PubMed] [Google Scholar]
- 17.He Y, Zeng MY, Yang D, Motro B, Núñez G. NEK7 is an essential mediator of NLRP3 activation downstream of potassium efflux. Nature. 2016;530:354–7. doi: 10.1038/nature16959. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Duncan JA, Bergstralh DT, Wang Y, Willingham SB, Ye Z, Zimmermann AG, et al. Cryopyrin/NALP3 binds ATP/dATP, is an ATPase, and requires ATP binding to mediate inflammatory signaling. Proc Natl Acad Sci USA. 2007;104:8041–6. doi: 10.1073/pnas.0611496104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Sharif H, Wang L, Wang WL, Magupalli VG, Andreeva L, Qiao Q, et al. Structural mechanism for NEK7-licensed activation of NLRP3 inflammasome. Nature. 2019;570:338–43. doi: 10.1038/s41586-019-1295-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Garib FY, Rizopulu AP, Kuchmiy AA, Garib VF. Inactivation of Inflammasomes by Pathogens Regulates Inflammation. Biochem (Mosc) 2016;81:1326–39. doi: 10.1134/S0006297916110109. [DOI] [PubMed] [Google Scholar]
- 21.Lamkanfi M, Dixit VM. Modulation of inflammasome pathways by bacterial and viral pathogens. J Immunol. 2011;187:597–602. doi: 10.4049/jimmunol.1100229. [DOI] [PubMed] [Google Scholar]
- 22.Man SM, Kanneganti TD. Regulation of inflammasome activation. Immunol Rev. 2015;265:6–21. doi: 10.1111/imr.12296. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Douglas B, Oyesola O, Cooper MM, Posey A, Tait Wojno E, Giacomin PR, et al. Immune system investigation using parasitic helminths. Annu Rev Immunol. 2021;39:639–65. doi: 10.1146/annurev-immunol-093019-122827. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Palmiter RD. The parabrachial nucleus: CGRP neurons function as a general alarm. Trends Neurosci. 2018;41:280–93. doi: 10.1016/j.tins.2018.03.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Blake KJ, Jiang XR, Chiu IM. Neuronal regulation of immunity in the skin and lungs. Trends Neurosci. 2019;42:537–51. doi: 10.1016/j.tins.2019.05.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Edvinsson L, Haanes KA, Warfvinge K, Krause DN. CGRP as the target of new migraine therapies—successful translation from bench to clinic. Nat Rev Neurol. 2018;14:338–50. doi: 10.1038/s41582-018-0003-1. [DOI] [PubMed] [Google Scholar]
- 27.De Logu F, Nassini R, Hegron A, Landini L, Jensen DD, Latorre R, et al. Schwann cell endosome CGRP signals elicit periorbital mechanical allodynia in mice. Nat Commun. 2022;13:646. doi: 10.1038/s41467-022-28204-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Russo AF. Calcitonin gene-related peptide (CGRP): a new target for migraine. Annu Rev Pharm Toxicol. 2015;55:533–52. doi: 10.1146/annurev-pharmtox-010814-124701. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Durham PL. Calcitonin gene-related peptide (CGRP) and migraine. Headache. 2006;46:S3–8. doi: 10.1111/j.1526-4610.2006.00483.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Nagashima H, Mahlakõiv T, Shih HY, Davis FP, Meylan F, Huang Y, et al. Neuropeptide CGRP Limits Group 2 Innate Lymphoid Cell Responses and Constrains Type 2 Inflammation. Immunity. 2019;51:682–695. doi: 10.1016/j.immuni.2019.06.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Sui, P, Wiesner DL, Xu J, Zhang Y, Lee J, Van Dyken S, et al. Pulmonary neuroendocrine cells amplify allergic asthma responses. Science. 2018;360(6393):eaan8546. [DOI] [PMC free article] [PubMed]
- 32.Wallrapp A, Burkett PR, Riesenfeld SJ, Kim SJ, Christian E, Abdulnour RE, et al. Calcitonin gene-related peptide negatively regulates alarmin-driven type 2 innate lymphoid cell responses. Immunity. 2019;51:709–723. doi: 10.1016/j.immuni.2019.09.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Xu H, Ding J, Porter C, Wallrapp A, Tabaka M, Ma S, et al. Transcriptional atlas of intestinal immune cells reveals that neuropeptide α-CGRP modulates group 2 innate lymphoid cell responses. Immunity. 2019;51:696–708. doi: 10.1016/j.immuni.2019.09.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Assas MB. Anti-migraine agents from an immunological point of view. J Transl Med. 2021;19:23. doi: 10.1186/s12967-020-02681-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Di Virgilio F, Dal Ben D, Sarti AC, Giuliani AL, Falzoni S. The P2X7 receptor in infection and inflammation. Immunity. 2017;47:15–31. doi: 10.1016/j.immuni.2017.06.020. [DOI] [PubMed] [Google Scholar]
- 36.Coll RC, Hill JR, Day CJ, Zamoshnikova A, Boucher D, Massey NL, et al. MCC950 directly targets the NLRP3 ATP-hydrolysis motif for inflammasome inhibition. Nat Chem Biol. 2019;15:556–9. doi: 10.1038/s41589-019-0277-7. [DOI] [PubMed] [Google Scholar]
- 37.Tapia-Abellan A, Angosto-Bazarra D, Martinez-Banaclocha H, de Torre-Minguela C, Ceron-Carrasco JP, Perez-Sanchez H, et al. MCC950 closes the active conformation of NLRP3 to an inactive state. Nat Chem Biol. 2019;15:560–4. doi: 10.1038/s41589-019-0278-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Shi H, Wang Y, Li X, Zhan X, Tang M, Fina M, et al. NLRP3 activation and mitosis are mutually exclusive events coordinated by NEK7, a new inflammasome component. Nat Immunol. 2016;17:250–8. doi: 10.1038/ni.3333. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Wallrapp A, Riesenfeld SJ, Burkett PR, Abdulnour REE, Nyman J, Dionne D, et al. The neuropeptide NMU amplifies ILC2-driven allergic lung inflammation. Nature. 2017;549:351–6. doi: 10.1038/nature24029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Motomura Y, Kobayashi T, Moro K. The neuropeptide CGRP induces bipolar syndrome in group 2 innate lymphoid cells. Immunity. 2019;51:598–600. doi: 10.1016/j.immuni.2019.09.015. [DOI] [PubMed] [Google Scholar]
- 41.Waqas SFH, Hoang AC, Lin YT, Ampem G, Azegrouz H, Balogh L, et al. Neuropeptide FF increases M2 activation and self-renewal of adipose tissue macrophages. J Clin Invest. 2017;127:2842–54. doi: 10.1172/JCI90152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Park S, Komatsu T, Hayashi H, Mori R, Shimokawa I. The role of neuropeptide Y in adipocyte-macrophage crosstalk during high fat diet-induced adipose inflammation and liver steatosis. Biomedicines. 2021;9:1739. doi: 10.3390/biomedicines9111739. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Hornung V, Bauernfeind F, Halle A, Samstad EO, Kono H, Rock KL, et al. Silica crystals and aluminum salts activate the NALP3 inflammasome through phagosomal destabilization. Nat Immunol. 2008;9:847–56. doi: 10.1038/ni.1631. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Lee B. NLRP3 activation in response to disrupted endocytic traffic. bioRxiv. 2021.09.15.460426. 10.1101/2021.09.15.460426.
- 45.Chen J, Chen ZJ. PtdIns4P on dispersed trans-Golgi network mediates NLRP3 inflammasome activation. Nature. 2018;564:71–76. doi: 10.1038/s41586-018-0761-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.