Abstract
The balance between inflammatory T helper type 17 (Th17) and immunosuppressive regulatory T (Treg) cells is critical for maintaining immune homeostasis in the human body and is tightly regulated under healthy conditions. An increasing number of studies have reported that deubiquitinases (DUBs) play a vital role in regulating Th17- and Treg-cell differentiation. However, the biological functions of only a small fraction of DUBs in Th17- and Treg-cell differentiation are well defined. In this study, we identified ubiquitin-specific peptidase 1 (USP1) as a vital regulator of CD4+ T-cell differentiation. USP1 promoted Th17-cell differentiation but attenuated Treg-cell differentiation, thereby promoting the development of inflammatory diseases. Mechanistically, USP1 in CD4+ T cells enhanced the activity of RORγt but promoted the proteasomal degradation of Foxp3 through deubiquitination and stabilization of TAZ in vitro and in vivo. Notably, ML323, a specific inhibitor of the USP1/UAF1 deubiquitinase complex, inhibited Th17-cell differentiation and promoted Treg-cell differentiation in vitro and in vivo, indicating that ML323 might be a promising candidate for the treatment of diseases associated with an imbalance between Th17 and Treg cells. Our study highlights the critical role of USP1 in regulating adaptive immune responses and suggests that USP1 might be a drug target for the treatment of diseases associated with an imbalance between Th17 and Treg cells.
Keywords: USP1, TAZ, Th17, Treg
Subject terms: Lymphocyte differentiation, Autoimmunity
Introduction
CD4 helper T cells (CD4+ T cells) are among the main cells involved in the acquired immune response and play important roles in body development and homeostasis. Following T-cell antigen receptor (TCR) stimulation and synergistic effects mediated by cytokines, naïve CD4+ T cells differentiate into distinct subsets, including T helper type 1 (Th1), Th2, Th17, and regulatory T (Treg) cells [1, 2]. Th17 cells represent a proinflammatory subset that provides defense against pathogenic infections and tumors but can also cause autoimmunity and tissue damage [3]. In contrast, Treg cells represent a subset of specialized T cells that are essential for the regulation of immune responses and maintenance of peripheral tolerance [4]. Th17-driven inflammation and the occurrence of autoimmunity are normally controlled by Treg cells and anti-inflammatory cytokines, such as IL-10 [5]. The balance between Th17 and Treg cells is tightly regulated under normal conditions. Quantitative and functional changes in Th17 and Treg cells result in abnormal immune responses, leading to inflammation, cancer, or autoimmune diseases [6, 7]. Thus, understanding the mechanisms regulating the balance between Th17 and Treg cells would help in the development of therapies for inflammatory diseases or resolution of infections by pathogens.
It is widely acknowledged that the functions and differentiation of Th cells are tightly regulated by different lineage-specific transcription factors. Fxop3 is a specific transcription factor that drives Treg-cell lineage differentiation and is induced by the cytokine TGF-β. Furthermore, TGF-β combined with IL-6 induces the expression of the transcription factor RORγt and drives Th17-cell production [8]. Thus, the balance between Fxop3 and RORγt is crucial in the balance between Th17 and Treg cells. Transcriptional co-activator with PDZ-binding motif (TAZ) is a Hippo signaling effector protein that is associated with mesenchymal stem cell differentiation, human embryonic stem cell self-renewal, and tumor progression across multiple cancer types [9–11]. A previous study showed that TAZ is a critical co-activator of RORγt and can target Foxp3 for proteasomal degradation by decreasing its acetylation, thereby promoting Th17-cell differentiation while reducing Treg-cell differentiation [12].
Ubiquitination is an important protein modification implicated in the regulation of diverse biological processes, including the immune response [13]. The ubiquitination process is catalyzed by the sequential activity of ubiquitin-activating (E1), ubiquitin-conjugating (E2), and ubiquitin-ligating (E3) enzymes, while its reversal, deubiquitination, is mainly performed by deubiquitinases (DUBs) [14]. There is increasing evidence that both ubiquitination and deubiquitination play crucial roles in the orchestration of the immune response by ensuring the proper functioning of different cell types, including Th17 and Treg cells [15]. A large number of E3 ubiquitin ligases and DUBs exist in mammalian cells and recognize distinct substrates [16]. However, the biological functions of only a limited number of E3 ubiquitin ligases and DUBs have been described, especially in the regulation of the adaptive immune response.
Ubiquitin-specific peptidase 1 (USP1) is one of the best-characterized DUBs and is considered to play vital regulatory roles in DNA repair processes [17], tumor pathogenesis [18], antiviral innate immunity [19], and NOD-like receptor protein 3 (NLRP3) inflammasome activation [20]. The deubiquitinase activity of USP1 can be promoted by its binding partner USP1-associated factor 1 (UAF1, also called WD40 repeat containing protein 48 [WDR48]) [21]. In the regulation of acquired immunity, USP1 is upregulated in activated T cells and functions as a modulator in memory responses. USP1 deficiency results in a gradual loss of memory CD8+ T cells [22]. However, the specific role of USP1 and its underlying mechanisms in CD4+ T-cell activation and differentiation remain unclear.
In this study, we found that USP1 promoted Th17-cell differentiation but attenuated Treg-cell differentiation, thereby promoting the development of inflammatory diseases. Mechanistically, USP1 in CD4+ T cells enhanced the activity of RORγt and promoted proteasomal degradation of Foxp3 by deubiquitinating and stabilizing TAZ. These results suggest that USP1 is an important target for the regulation of adaptive immune responses.
Results
USP1-regulated CD4+ T-cell differentiation in vivo
The ability of USP1 to modulate CD4+ T-cell responses and the underlying mechanisms remain unclear. To study the function of USP1 in CD4+ T cells, we generated mice with USP1 conditionally knocked out in CD4+ T cells (Usp1CKO) by crossing Usp1flox/flox (Usp1fl/fl) and CD4-Cre mice. USP1 immunoblotting results for splenocytes from Usp1fl/fl and Usp1CKO mice showed a lack of the USP1 protein in Usp1CKO mice (Supplementary Fig. 1A). Usp1CKO mice showed expected Mendelian ratios and survival rates comparable to those of control Usp1fl/fl mice (Supplementary Fig. 1B). Usp1CKO mice did not show any distinct abnormalities in thymocyte development or peripheral T- and B-cell homeostasis, including intracellular IL-17A expression and the proportion of Treg cells among splenic CD4+ T cells (Supplementary Fig. 1C–I).
The role of USP1 in shaping CD4+ T-cell responses was elucidated by immunizing Usp1fl/fl and Usp1CKO mice with the MOG(35–55) peptide in complete Freund’s adjuvant (CFA). Notably, CD4+ T cells from Usp1CKO mice showed decreased intracellular IFN-γ and IL-17A expression after MOG(35–55) peptide immunization compared to CD4+ T cells from Usp1fl/fl mice (Fig. 1A). Usp1CKO splenocytes exhibited a higher proportion of Treg cells (CD25+ Foxp3+) among CD4+ T cells (Fig. 1B). After immunization with the MOG(35–55) peptide, Usp1CKO splenocytes exhibited less secretion of IFN-γ and IL-17A but increased secretion of IL-10 (Fig. 1C). Furthermore, Usp1CKO mice exhibited significantly fewer effector-memory T cells (Te/Tem cells) in the spleen than their Usp1fl/fl counterparts (Fig. 1D). Activation analysis showed that CD4+ T cells from Usp1CKO mice exhibited reduced CD69 expression relative to Usp1fl/fl CD4+ T cells (Fig. 1E). Consistently, USP1-deficient CD4+ T cells exhibited a reduced activation ability; thus, USP1 deficiency in CD4+ T cells restricted experimental autoimmune encephalomyelitis (EAE) progression (Fig. 1F). Usp1CKO mouse sera contained lower levels of IFN-γ, IL-17A, and GM-CSF and higher levels of IL-10 than Usp1fl/fl mouse sera (Fig. 1G). Intracellular IL-17A expression after MOG(35–55) peptide immunization was decreased in CD4+ T cells from the central nervous system (the spinal cord and brain) of Usp1CKO mice compared to those from the central nervous system of Usp1fl/fl mice (Fig. 1G). However, the proportion of Treg cells was increased in Usp1CKO CD4+ T cells (Fig. 1I).
Fig. 1.
USP1 deficiency attenuated the generation of hyperinflammatory CD4+ T-cell responses in vivo. A–F Usp1fl/fl and Usp1CKO mice were immunized with the MOG(35–55) peptide in CFA, and the mice were harvested on Day 20. A Splenocytes were restimulated directly ex vivo, and the intracellular production of IFN-γ and IL-17 by CD4+ T cells was determined. Pooled data are presented in the right panel. B The expression of CD25 and Foxp3 on splenic CD4+ T cells was detected. Pooled data are presented in the right panel. C Splenocytes were stimulated for 48 h with the MOG(35–55) peptide, and cytokine production was measured by ELISA. D Flow cytometric analysis of the frequencies of naïve (Tn, CD44loCD62Lhi), effector-memory (Te/Tem, CD44hiCD62Llo) and central memory (Tcm, CD44hiCD62Lhi) T-cell populations in splenic CD4+ T cells from Usp1fl/fl and Usp1CKO mice. E Flow cytometric analysis of CD69 in splenic CD4+ T cells. F–I Usp1fl/fl and Usp1CKO mice were immunized with the MOG(35–55) peptide in CFA and pertussis toxin to induce EAE. F The graph shows clinical scores for EAE (n = 5 for Usp1fl/fl and Usp1CKO mice). G Mice were harvested on Day 28, and the concentrations of IFN-γ, IL-17, GM-CSF and IL-10 in the serum were measured by ELISA. H Cells from the central nervous system (the spinal cord and brain) were restimulated directly ex vivo, and the intracellular production of IL-17A by CD4+ T cells was determined. Pooled data are presented in the right panel. I The expression of CD25 and Foxp3 on CD4+ T cells from the central nervous system was detected. Pooled data are presented in the right panel. Data shown are the mean ± SD. **P < 0.01 and ***P < 0.001 by an unpaired t test. Data are representative of three independent experiments with similar results
Next, we investigated whether ML323, a specific allosteric inhibitor of the USP1/UAF1 deubiquitinase complex, regulates CD4+ T-cell differentiation in vivo. Notably, ML323 treatment resulted in substantial decreases in the frequencies of Th1 and Th17 cells (Supplementary Fig. 2A) and a significant increase in the frequency of Treg cells (Supplementary Fig. 2B). ML323 treatment reduced MOG(35–55) peptide-induced IFN-γ and IL-17A production while increasing IL-10 production (Supplementary Fig. 2C). The percentage of Te/Tem cells and expression of CD69 were reduced in CD4+ T cells from ML323-treated mice (Supplementary Fig. 2D, E). Moreover, ML323 treatment restricted EAE progression (Supplementary Fig. 2F). Sera from WT ML323-treated mice contained lower levels of IFN-γ, IL-17, and GM-CSF but a higher level of IL-10 than those from DMSO-treated control mice (Supplementary Fig. 2G). Taken together, these results show that USP1 is a critical regulator of CD4+ T-cell differentiation in vivo.
USP1 was dispensable for the TCR signaling pathway in CD4+ T cells
To further determine the function of USP1 in CD4+ T cells, the properties of CD4+ T cells were assessed after stimulation of the TCR with an antibody specific for the invariant signaling protein CD3 (anti-CD3) plus an antibody specific for the costimulatory receptor CD28 (anti-CD28). Real-time PCR and immunoblot analyses (Supplementary Fig. 3A, B) confirmed increased expression of USP1 in CD4+ T cells stimulated with the anti-CD3 plus anti-CD28 antibodies (Th0 cells). However, there was no difference in the expression of USP1 among Th0, Th1, Th2, and Th17 cells (Supplementary Fig. 3A, B). The expression of the activation markers CD69, CD44, and CD62L on CD4+ T cells, as well as the production of the cytokines IFN-γ and IL-2, was unaffected by deletion of USP1 following treatment with the anti-CD3 and anti-CD28 antibodies (Supplementary Fig. 3C, D). No differences in proliferation were observed between Usp1fl/fl and Usp1CKO CD4+ T cells stimulated with the anti-CD3 or anti-CD28 antibodies (Supplementary Fig. 3E). To further study the role of UPS1 in regulating the TCR signaling pathway, the effect of ML323 treatment on CD4+ T cells was examined. Consistently, the activation, IFN-γ and IL-2 production, and proliferation of CD4+ T cells were unaffected by ML323 treatment following stimulation with the anti-CD3 plus anti-CD28 antibodies (Supplementary Fig. 3F–H). Together, these results indicate that USP1 is dispensable for the TCR signaling pathway in CD4+ T cells.
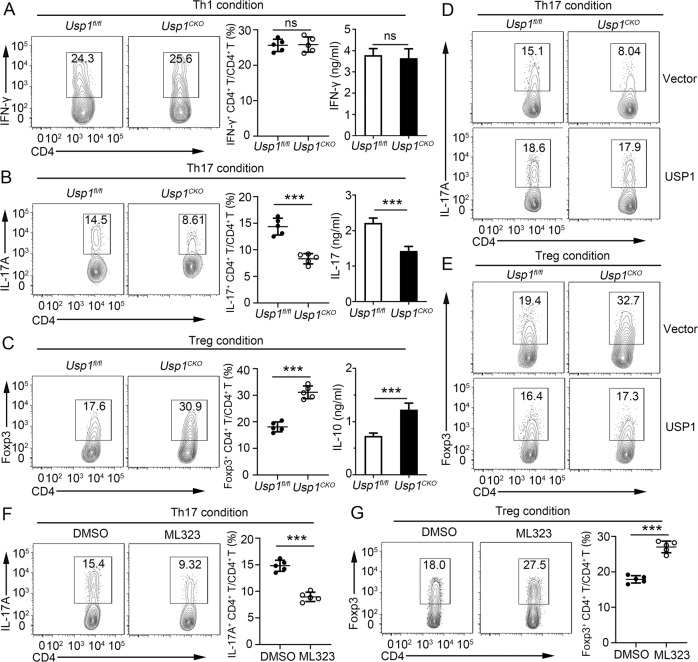
USP1 enhanced Th17-cell differentiation but attenuated Treg-cell differentiation
To further assess the role of USP1 in the regulation of CD4+ T-cell differentiation, we differentiated Usp1fl/fl and Usp1CKO naïve CD4+ T cells under different polarizing conditions. We found that the frequency of IFN-γ+ CD4+ T cells and the level of IFN-γ were comparable between Usp1fl/fl and Usp1CKO naïve CD4+ T cells under Th1-cell differentiation conditions (Fig. 2A). However, USP1 deficiency resulted in a significantly lower frequency of IL-17A+ CD4+ T cells and decreased IL-17A production after Th17 skewing (Fig. 2B). In contrast, USP1 deficiency resulted in a much greater frequency of Foxp3+ CD4+ cells and higher IL-10 production after Treg skewing (Fig. 2C). Furthermore, reintroduction of USP1 into Usp1CKO CD4+ T cells rescued Th17-cell differentiation but attenuated Treg-cell differentiation (Fig. 2D, E, Supplementary Fig. 3I, J). To further confirm the role of UPS1 in the regulation of CD4+ T-cell differentiation, the effect of ML323 treatment was examined. Consistently, ML323 treatment impaired the generation of Th17 cells and facilitated the differentiation of Treg cells (Fig. 2F, G). These results suggest that USP1 plays a critical role in balancing the development of Th17 and Treg cells.
Fig. 2.
USP1 enhanced Th17-cell differentiation but attenuated Treg-cell differentiation in vitro. A–C Purified naïve CD4+ T cells from Usp1fl/fl or Usp1CKO mice were isolated; stimulated under standard Th1, Th17 or Treg conditions; and harvested on Day 5. Flow cytometric analysis of intracellular IFN-γ (A), IL-17A (B) or Foxp3 (C) in CD4+ T cells and the associated pooled data (near right). ELISA results for IFN-γ, IL-17A, and IL-10 in the culture medium are presented in the far right panels. D, E Flow cytometric analysis of intracellular IL-17A (D) or Foxp3 (E) in Usp1fl/fl and Usp1CKO naïve CD4+ T cells infected with a control retrovirus (Vector) or retrovirus expressing USP1 and differentiated under standard Th17 or Treg conditions. F, G Purified naïve CD4+ T cells from Usp1fl/fl or Usp1CKO mice were isolated, treated with the USP1 inhibitor ML323 (30 μM), stimulated under standard Th17 or Treg conditions and harvested on Day 5. Flow cytometric analysis of intracellular IL-17A (F) or Foxp3 (G) in CD4+ T cells and the associated pooled data (near right). Data shown are the mean ± SD. **P < 0.01 and ***P < 0.001 by an unpaired t test. Data are representative of three independent experiments with similar results
USP1 balances Th17/Treg-cell development in a T-cell-intrinsic manner in vivo
Next, we conducted in vivo competitive adoptive CD4+ T-cell transfer assays to confirm the intrinsic role of USP1 in regulating Th17/Treg-cell development. Rag1−/− recipient mice received CD45.1+ Usp1fl/fl and CD45.2+ Usp1CKO naïve CD4+ T cells at a ratio of 1:1 and were subsequently immunized with MOG(35–55) in CFA (Fig. 3A). Although there was no significant difference in the CD4+ T-cell percentage between CD45.1+ Usp1fl/fl and CD45.2+ Usp1CKO cells (Fig. 3B), CD45.2+ Usp1CKO CD4+ T cells exhibited a considerably decreased percentage of Th17 cells, a higher proportion of Treg cells, and a comparable frequency of Th1 cells compared to CD45.1+ Usp1fl/fl CD4+ T cells (Fig. 3C, D, Supplementary Fig. 4A). Activation analysis showed similar expression of CD69 in CD45.1+ Usp1fl/fl and CD45.2+ Usp1CKO cells (Supplementary Fig. 4B).
Fig. 3.
USP1 enhanced Th17-cell differentiation but attenuated Treg-cell differentiation in a T-cell-intrinsic manner in vivo. A Schematic of the experimental design of competitive adoptive CD4+ T-cell transfer assays. B Spleens were harvested, and the percentages of Usp1fl/fl (CD45.1+) and Usp1CKO (CD45.2+) cells in CD4+ T-cell populations were determined. C Splenocytes were restimulated directly ex vivo, and the intracellular production of IL-17 by CD45.1+ or CD45.2+ CD4+ T cells was determined. Pooled data are presented in the right panel. D The expression of CD25 and Foxp3 on CD45.1+ or CD45.2+ CD4+ T cells was determined. Pooled data are presented in the right panel. E–H Purified Usp1fl/fl or Usp1CKO naïve CD4+ T cells were adoptively transferred into Rag1−/− mice. The recipient mice were immunized with the MOG(35–55) peptide in CFA and pertussis toxin to induce EAE. E The graph shows the clinical scores for EAE (n = 5). F Splenocytes were restimulated directly ex vivo, and the intracellular production of IL-17 by CD4+ T cells was determined. Pooled data are presented in the right panel. G The expression of CD25 and Foxp3 on CD4+ T cells from spleens was detected. Pooled data are presented in the right panel. H The concentrations of IFN-γ, IL-17, TNF-α and IL-2 in serum were measured by ELISA. Data shown are the mean ± SD. **P < 0.01 and ***P < 0.001 by an unpaired t test. Data are representative of three independent experiments with similar results
To further understand the impact of Usp1CKO CD4+ T cells on the in vivo immune response, Rag1−/− recipient mice were administered Usp1fl/fl and Usp1CKO naïve CD4+ T cells and subsequently immunized with the MOG(35–55) peptide in CFA. Rag1−/− mice receiving Usp1CKO CD4+ T cells developed significantly attenuated EAE compared with those receiving Usp1fl/fl CD4+ T cells (Fig. 3E). In addition, after stimulation with the MOG(35–55) peptide, Usp1CKO CD4+ T cells included significantly lower percentages of Th17 and Th1 cells and a higher percentage of Treg cells than Usp1fl/fl CD4+ T cells (Fig. 3F, G, Supplementary Fig. 4C). Activation analysis showed decreased expression of CD69 in Usp1CKO CD4+ T cells (Supplementary Fig. 4D). After stimulation with the MOG(35–55) peptide, Usp1CKO CD4+ T cells exhibited less secretion of IFN-γ and IL-17A but higher secretion of IL-10 (Fig. 3H). Therefore, these results further demonstrate that USP1 deficiency in CD4+ T cells attenuates Th17-cell differentiation and promotes Treg-cell differentiation.
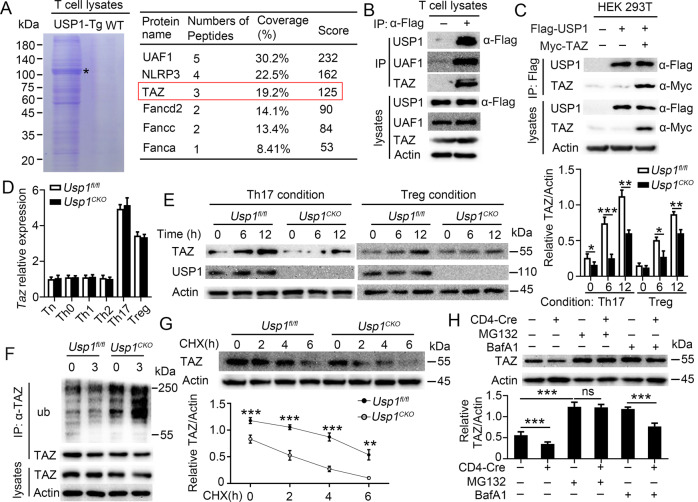
USP1 deubiquitinated and stabilized TAZ in CD4+ T cells
Next, we sought to explore the molecular mechanism underlying CD4+ T-cell differentiation modulated by USP1. For this, we first used anti-FLAG beads to immunoprecipitated proteins from lysates of CD4+ T cells from wild-type (WT) or USP1-Tg mice, in which Flag-tagged USP1 was overexpressed in CD4+ T cells, and then further analyzed the resultant immunoprecipitants by mass spectrometry. Notably, the immunoprecipitants included UAF1, NLRP3, TAZ, and other proteins (Fig. 4A). A previous study showed that TAZ drives Th17-cell differentiation but blocks Treg-cell differentiation [12]. Interestingly, our above results show that USP1 plays the same functions in CD4+ T-cell differentiation. Hence, we investigated whether USP1 acts by regulating TAZ in CD4+ T cells. We found that endogenous USP1 and TAZ formed a stable complex in CD4+ T cells from WT or USP1-Tg mice (Fig. 4B). The results also showed that USP1 interacted with TAZ in HEK293 cells cotransfected with the USP1 and TAZ proteins (Fig. 4C). TAZ expression did not differ at the mRNA level between Usp1fl/fl and Usp1CKO CD4+ T cells (Fig. 4D) but showed a significant difference at the protein level (Fig. 4E). As USP1 is a DUB, we investigated whether USP1 regulates TAZ protein levels through its deubiquitinase activity. We found that USP1 deficiency potentiated TAZ ubiquitination (Fig. 4F) and promoted TAZ degradation (Fig. 4G). TAZ degradation in Usp1CKO CD4+ T cells was completely blocked by the proteasome inhibitor MG132 (Fig. 4H).
Fig. 4.
USP1 deubiquitinated and stabilized TAZ in CD4+ T cells. A Identification and the list (the right panel) of UAF1, NLRP3, TAZ, Fancd2, Fancc and Fanca identified by mass spectrometry in a Flag-tagged USP1 precipitation assay performed with CD4+ T-cell lysates from WT or USP1-Flag Tg mice. *, migration of USP1. B Immunoprecipitation (IP) and immunoblot (IB) analysis of CD4+ T cells isolated from WT or USP1-Flag Tg mice. C IP and IB analysis of HEK293 cells transfected with the indicated plasmids for 24 h. D, E Purified naïve CD4+ T cells from Usp1fl/fl or Usp1CKO mice were stimulated under standard Th0, Th1, Th2, Th17 or Treg conditions and harvested on Day 5. TAZ expression levels were detected by qPCR (D) or western blotting (E). The densitometric quantification of band intensity is presented in the right panel. F–H TAZ IB (F–H) and ubiquitination (F) analysis using whole-cell extracts of Usp1fl/fl or Usp1CKO CD4+ T cells stimulated with anti-CD3 and anti-CD28 antibodies for the indicated times (F, G) or 3 h (H). MG132 was added (in F or +) or not (−). Data shown are the mean ± SD. *P < 0.05; **P < 0.01; ***P < 0.001 by two-way ANOVA (in D, E) or an unpaired t test (in G, H). Data are representative of three independent experiments with similar results
Furthermore, an enzymatically inactive USP1 mutant (USP1C90A) was constructed. We found that USP1 but not USP1C90A reduced the TAZ degradation rate and catalyzed TAZ ubiquitination in HEK293T cells, indicating that USP1 could reverse TAZ ubiquitination and stabilize TAZ through its deubiquitinase activity (Fig. 5A, B). After transfecting USP1 or USP1C90A into Usp1fl/fl and Usp1CKO naïve CD4+ T cells, we found that the expression of TAZ was markedly attenuated in the absence of USP1, whereas overexpression of USP1 but not USP1C90A restored TAZ expression (Fig. 5C, D and Supplementary Fig. 5A, B). In addition, USP1 overexpression but not USP1C90A overexpression in naïve CD4+ T cells recovered the expression of IL-17A and diminished the expression of Foxp3 (Fig. 5E, F and Supplementary Fig. 5C, D). Taken together, these results demonstrate that USP1 deubiquitinates and stabilizes TAZ in CD4+ T cells and regulates CD4+ T-cell differentiation through its deubiquitinase activity.
Fig. 5.
USP1-regulated CD4+ T-cell differentiation through deubiquitinating enzyme activity. A Western blot analysis of extracts of HEK293T cells transfected with the indicated plasmids and increasing doses of an expression vector for USP1-Flag or USP1C90A-Flag (wedge). The densitometric quantification of band intensity is presented in the lower panel. B Immunoblot (IB) and ubiquitination analysis of extracts of HEK293T cells transfected with the indicated plasmids. C, D TAZ and USP1 IB analysis using whole-cell extracts of Usp1fl/fl and Usp1CKO naïve CD4+ T cells infected with a control retrovirus (Vector) or retrovirus expressing USP1 or USP1C90A and differentiated under standard Th17 (C) or Treg conditions (D). E, F Flow cytometric analysis of intracellular IL-17A (E) or Foxp3 (F) in Usp1fl/fl and Usp1CKO naïve CD4+ T cells infected with a control retrovirus (Vector) or retrovirus expressing USP1 or USP1C90A and differentiated under standard Th17 or Treg conditions. Data shown are the mean ± SD. ***P < 0.001 by an unpaired t test. Data are representative of three independent experiments with similar results
The USP1/UAF1 deubiquitinase complex regulated CD4+ T-cell differentiation
The deubiquitinase activity of USP1 can be promoted by its binding partner, UAF1. Since the mass spectrometry results showed that USP1 interacted with UAF1 in CD4+ T cells (Fig. 4A), we explored whether USP1 regulates CD4+ T-cell differentiation by interacting with UAF1. We found that endogenous USP1 and UAF1 formed a stable complex in CD4+ T cells from WT or USP1-Tg mice (Supplementary Fig. 6A). Similar to the results for USP1, UAF1 deficiency in CD4+ T cells did not affect TAZ expression at the mRNA level but did affect TAZ expression at the protein level (Supplementary Fig. 6B, C). Notably, CD4+ T cells from Uaf1CKO mice showed decreased Th17-cell differentiation but increased Treg-cell differentiation compared to those from Uaf1fl/fl mice (Supplementary Fig. 6D, E). UAF1 deficiency in CD4+ T cells restricted EAE progression (Supplementary Fig. 6F) and reduced the production of IFN-γ, IL-17, and GM-CSF while enhancing the production of IL-10 in the serum (Supplementary Fig. 6G). Taken together, these results indicate that UAF1 regulates CD4+ T-cell differentiation by interacting with USP1 to form the USP1/UAF1 deubiquitinase complex.
USP1-regulated CD4+ T-cell differentiation depends on TAZ
To assess whether TAZ is the primary target of USP1 in the regulation of CD4+ T-cell differentiation, mice with double conditional knockout of USP1 and TAZ in CD4+ T cells (Usp1CKOTazCKO) were generated. Then, Usp1fl/fl, Usp1CKO, Tazfl/fl, TazCKO, and Usp1CKOTazCKO mice were immunized with the MOG(35–55) peptide to evaluate the in vivo role of TAZ. The results showed no differences in intracellular IFN-γ and IL-17 levels or the expression of Foxp3 and CD25 between the TAZ-deficient groups, regardless of the USP1 deficiency status (Supplementary Fig. 7A and Fig. 6A, B). The EAE score and serum levels of IFN-γ, IL-17, and IL-10 were significantly different between Usp1fl/fl and Usp1CKO mice. However, this difference was not observed when mice were also deficient in TAZ (Supplementary Fig. 7B, C). Upregulated mRNA expression of IL-17A under Th17 conditions and decreased mRNA expression of IL-10 under Treg conditions were observed in Usp1CKO CD4+ T cells compared to Usp1fl/fl CD4+ T cells. Similarly, these differences disappeared when TAZ was also deficient (Supplementary Fig. 7D). Notably, USP1 had no effect on RORγt or Foxp3 mRNA expression (Supplementary Fig. 7D). Luciferase results showed that USP1 did not promote the activity of the IL-17A promoter in the absence of TAZ, whereas after TAZ was cotransfected, USP1 enhanced the activity of the IL-17A promoter in a dose-dependent manner (Supplementary Fig. 7E). A previous study showed that TAZ regulated the acetylation (Ac) and ubiquitination (Ub) of Foxp3 [12]. Our results showed that USP1-deficient Treg cells exhibited greater acetylation but less ubiquitination of endogenous Foxp3 than did Usp1fl/fl cells (Supplementary Fig. 7E). In addition, overexpression of USP1 in TazCKO naïve T cells had no impact on the differentiation of Th17 or Treg cells (Fig. 6C, D). Conversely, TAZ overexpression in Usp1CKO naïve T cells facilitated Th17-cell differentiation and impaired the generation of Treg cells (Fig. 6E, F). These results demonstrated that USP1 promoted Th17-cell differentiation and impaired Treg-cell differentiation in a TAZ-dependent manner.
Fig. 6.
USP1-regulated CD4+ T-cell differentiation in a TAZ-dependent manner. A, B Usp1fl/fl, Usp1CKO, Tazfl/fl, TazCKO and Usp1CKOTazCKO mice were immunized with the MOG(35–55) peptide in CFA, and the mice were harvested on Day 20. A Splenocytes were restimulated directly ex vivo, and the intracellular production of IL-17 by CD4+ T cells was determined. Pooled data are presented in the right B. The expression of CD25 and Foxp3 on CD4+ T cells from spleens was detected. Pooled data are presented in the right panel. C, D Flow cytometric analysis of intracellular IL-17A (C) or Foxp3 (D) in TazCKO naïve CD4+ T cells infected with a control retrovirus (Vector) or retrovirus expressing USP1 and differentiated under standard Th17 or Treg conditions. E, F Flow cytometric analysis of intracellular IL-17A (E) or Foxp3 (F) in Usp1CKO naïve CD4+ T cells infected with a control retrovirus (Vector) or retrovirus expressing TAZ and differentiated under standard Th17 or Treg conditions. Data shown are the mean ± SD. ***P < 0.001 by an unpaired t test. Data are representative of three independent experiments with similar results
USP1 balanced Th17/Treg-cell differentiation in human CD4+ T cells
The results demonstrated that USP1 enhanced Th17-cell differentiation but attenuated Treg-cell differentiation through deubiquitination and stabilization of TAZ in mouse CD4+ T cells. Next, we sought to clarify whether USP1 regulates human CD4+ T-cell functions through the same molecular mechanism. Similarly, no difference was found in USP1 expression among different T helper cell subsets, both at the mRNA and protein levels, TAZ was expressed at higher levels in Th17 and Treg cells (Fig. 7A, B). USP1 overexpression significantly promoted Th17-cell differentiation but attenuated Treg-cell differentiation (Fig. 7C, D). Correspondingly, the generation of Th17 cells was impaired, but the differentiation of Treg cells was facilitated when USP1 function was inhibited by ML323 treatment (Fig. 7E, F). Neither USP1 overexpression nor USP1 inhibition by ML323 affected the expression of the activation markers CD69, CD62L, and CD44; production of the cytokines IL-2 and IFN-γ; or proliferation of human CD4+ T cells following stimulation with anti-CD3 and anti-CD28 antibodies (Supplementary Fig. 8A–F). Taken together, these results indicate that USP1 regulates Th17/Treg-cell differentiation in human CD4+ T cells.
Fig. 7.
USP1-regulated human CD4+ T-cell differentiation. A, B Purified human naïve CD4+ T cells were isolated; stimulated under standard Th0, Th1, Th2, Th17 or Treg conditions; and harvested on Day 5. USP1 and TAZ expression levels were detected by qPCR (A) or western blotting (B). C, D Purified human naïve CD4+ T cells were isolated, stimulated under standard Th17 or Treg conditions and harvested on Day 5. Flow cytometric analysis of intracellular IL-17A (C) or Foxp3 (D) in CD4+ T cells and the associated pooled data (right). E, F Purified human naïve CD4+ T cells were isolated, treated with the USP1 inhibitor ML323 (30 μM), stimulated under standard Th17 or Treg conditions and harvested on Day 5. Flow cytometric analysis of intracellular IL-17A (E) or Foxp3 (F) in CD4+ T cells and the associated pooled data (right). Data shown are the mean ± SD. **P < 0.01 and ***P < 0.001 by an unpaired t test. Data are representative of three independent experiments with similar results
Discussion
The Th17/Treg-cell balance is critical in maintaining immune homeostasis in the human body and is tightly regulated under normal conditions. An increasing number of studies have reported that DUBs play a vital role in regulating Th17- and Treg-cell differentiation. USP7, USP21, and USP44 stabilize Foxp3 by mediating its deubiquitination, subsequently promoting the development and differentiation of Treg cells and their suppressive functions [23–27]. USP4-, USP15- and USP17-mediated deubiquitination increases the protein stability of RORγt and IL-17 expression, which promotes Th17-cell differentiation [28–30]. However, the biological functions of only a limited number of DUBs have been characterized, limiting the development of DUB-based therapies. In this study, we identified USP1 as a regulator of Th17- and Treg-cell differentiation. In CD4+ T cells, USP1 stabilized TAZ through deubiquitination, subsequently activating RORγt and promoting the proteasomal degradation of Foxp3 (Supplementary Fig. 9). Targeting these USP family members might regulate the Treg/Th17 balance and limit the occurrence and development of related diseases.
USP1, one of the best-characterized DUBs, deubiquitinates various substrates and plays vital roles in various cell types. In macrophages, the USP1-UAF1 complex enhances IFN regulatory factor 3 (IRF3) activation and subsequent IFN-β secretion by stabilizing TBK1 and enhancing the antiviral response [19]. In addition, the USP1-UAF1 deubiquitinase complex reverses the polyubiquitination of NLRP3 and suppresses its ubiquitination-mediated degradation [20]. It has been shown that USP1 regulates the monoubiquitination of the Fanconi anemia protein FANCD2 and deubiquitinates proliferating cell nuclear antigen [31]. Knocking out USP1 in mice results in genomic instability and a Fanconi anemia phenotype [32]. Notably, NLRP3 has been reported to play a vital role in CD4+ T-cell differentiation, including that of Th1, Th2, and Th17 cells [33–36]. Deletion of FANCD2 and FANCA dysregulates the suppressive activity of Tregs, which functionally exacerbates graft-versus-host disease (GVHD) in mice [37]. Our previous results showed that in CD4+ T-cell lysates, USP1 interacted with TAZ as well as NLRP3, FANCD2, and FANCA (Fig. 4A). Since these molecules play important roles in the regulation of CD4+ T-cell differentiation, whether USP1 also regulates CD4+ T-cell differentiation by regulating these molecules is worthy of further study.
A previous study showed that USP1 deficiency did not affect effector CD8+ T-cell proliferation or survival following primary infection; however, a gradual loss of memory CD8+ T cells occurred over time [22]. We observed changes in memory CD4+ T cells in Usp1CKO mice, but USP1 deficiency did not directly affect memory CD4+ T-cell production, as demonstrated by adoptive transfer assays. In addition, we found that USP1 deficiency did not affect the expression of the activation markers CD69, CD62L, and CD44; production of the cytokines IL-2 and IFN-γ; or proliferation of CD4+ T cells following stimulation with anti-CD3 and anti-CD28 antibodies. These results demonstrated that USP1 was dispensable for the TCR signaling pathway in CD4+ T cells. The decreased expression of intracellular IFN-γ and activation markers and levels of Te/Tem cells in Usp1CKO mice compared with those in Usp1fl/fl mice might be caused by an increase in Treg cells.
TAZ plays important roles in various biological processes, and ubiquitination is a key regulatory mechanism of TAZ activation. Phosphorylated TAZ binds to SCF (β-TrCP) E3 ubiquitin ligase, leading to TAZ ubiquitination and degradation [38, 39]. OTUB2, a member of the DUB family, plays important roles in development and tumorigenesis by deubiquitinating and activating TAZ [40]. The deubiquitinase USP7 promotes head neck squamous cell carcinoma progression by deubiquitinating and stabilizing TAZ [41]. A self-amplifying USP14-TAZ loop drives progression and liver metastasis in pancreatic ductal adenocarcinoma [42]. In our study, we found that USP1-regulated the reciprocal differentiation of Th17 and Treg cells by deubiquitinating and stabilizing TAZ. Previous studies have reported that USP1 and TAZ form a complex that alters TAZ ubiquitination and is related to the metastatic properties of breast cancer and progression of osteosarcoma [43, 44], indicating that the USP1-TAZ complex plays important roles in various biological functions.
In summary, we identified USP1 as a vital regulator of CD4+ T-cell differentiation; it promoted Th17-cell differentiation but attenuated Treg-cell differentiation in a T-cell-intrinsic manner through deubiquitination and stabilization of TAZ. Notably, ML323, a specific inhibitor of the USP1/UAF1 deubiquitinase complex, inhibited Th17-cell differentiation and promoted Treg-cell differentiation in vitro and in vivo, indicating that ML323 might be a promising candidate for the treatment of diseases associated with an imbalance in Th17 and Treg cells. Together, our study results highlight the critical role of USP1 in regulating adaptive immune responses and suggest that USP1 might be a drug target for the treatment of diseases associated with an imbalance in Th17 and Treg cells.
Materials and methods
Mice
C57BL/6 mice (wild-type, WT) were obtained from the Lab Animal Center of Southern Medicine University (Guangzhou, China). Usp1flox/ flox (Usp1fl/fl), Uaf1fl/fl and Tazfl/fl mice on the C57BL/6J background were generated by Cyagen Biosciences Inc. (Guangzhou, China) using CRISPR-Pro technology. CD4-Cre mice were obtained from the Shanghai Research Center for Model Organisms (Shanghai, China). Usp1fl/fl, Uaf1fl/fl or Tazfl/fl mice were crossed with CD4-Cre mice to generate Usp1fl/fl; CD4-Cre (Usp1CKO) mice, Uaf1CKO mice, and TazCKO mice, respectively. Usp1CKO mice were crossed with TazCKO mice to generate Usp1CKOTazCKO mice. CD45.1+ and Rag1−/− mice were purchased from Nanjing Biomedical Research Institute (Nanjing, China). USP1-transgenic (USP1-Tg) mice were generated by Biocytogen (Shanghai, China). To generate mice with CD4+ T-cell-specific overexpression of USP1-Flag (USP1-Tg mice), USP1-transgenic mice were crossed with CD4-Cre mice. All mice were on the C57BL/6 background and maintained in the Lab Animal Center of Southern Medicine University under specific pathogen-free conditions. All animal experiments were conducted in accordance with protocols approved by the Medical Ethics Board and the Biosafety Management Committee of Southern Medical University (approval number SMU-L2017213). All mice were used between 6 and 12 weeks and were randomly divided into different groups.
EAE model
C57BL/6, Usp1fl/fl, Usp1CKO, Tazfl/fl, TazCKO, and Usp1CKOTazCKO mice and recipient Rag1−/− mice reconstituted with Usp1fl/fl or Usp1CKO CD4+ T cells were immunized subcutaneously with 200 μg of MOG(35–55) peptide emulsified in CFA (Difco Laboratories, USA) with 400 μg of Mycobacterium tuberculosis H37Ra on Day 0. To induce EAE development and assess the severity of EAE, mice also received 200 ng of pertussis toxin (Sigma, USA) by intraperitoneal injection on Days 0 and 2. Symptoms of EAE were monitored daily using a classic previously described clinical scoring system ranging from 0 to 5 as follows: 0, no disease; 1, tail paralysis; 2, weakness in hind limbs; 3, paralysis of hind limbs; 4, paralysis of hind limbs and severe hunched posture; and 5, moribund or death [45].
MOG(35–55) recall assay
Splenocytes were isolated from mice with EAE and restimulated with 100 μg/ml MOG(35–55) in complete RPMI 1640 medium for 48 h. The cell culture supernatants were collected for enzyme-linked immunosorbent assay (ELISA).
Mouse naïve T-cell isolation and an in vitro T-cell activation assay
Spleen and lymph node cells were isolated from mice. CD4+ T cells were negatively selected using The EasySepTM Mouse Naive CD4+ T-Cell Isolation Kit (Miltenyi, Germany). Purified naïve T cells were stimulated with plate-bound anti-CD3 (1 μg/ml or the indicated concentration) and soluble anti-CD28 antibodies (1 μg/ml) in replicate wells of 96-well plates (1 × 105 cells per well) for flow cytometry analysis and ELISA, 12-well plates (1 × 106 cells per well) for qPCR, and 6-well plates (5 × 106 per well) for western blot assays.
Human naïve T-cell isolation and an in vitro T-cell activation assay
Human peripheral blood mononuclear cells were isolated from the peripheral blood of healthy donors by Ficoll centrifugation. Human naïve CD4+ T cells were negatively selected using the EasySepTM Human Naive CD4+ T-Cell Isolation Kit (Miltenyi, Germany). Purified human naïve T cells were stimulated with plate-bound anti-CD3 (1 μg/ml or the indicated concentration) and soluble anti-CD28 antibodies (1 μg/ml) in replicate wells of 96-well plates (1 × 105 cells per well) for flow cytometry analysis and ELISA, 12-well plates (1 × 106 cells per well) for qPCR, and 6-well plates (5 × 106 per well) for western blot assays. Informed consent was obtained in accordance with the Declaration of Helsinki and the guidelines of the Institutional Review Board of Southern Medical University. Written informed consent was obtained from all participants for the use of PBMC samples.
Flow cytometry analysis
For intracellular cytokine staining assays, T cells isolated from the spleen or nervous system of mice or from in vitro cultures were stimulated for 1.5 h with 100 mg/ml MOG(35–55) or PMA (50 ng/ml, Thermo Fisher Scientific, USA) and ionomycin (500 ng/ml, Thermo Fisher Scientific) before Brefeldin A (10 μg/ml, eBioscience, USA) was added to the culture for 3.5 h. As previously described [46], for surface staining, cells were harvested, washed, and stained for 30 min on ice with mixtures of fluorophore-conjugated mAbs or isotype-matched controls. For intracellular cytokine staining (ICS), cells were stained for surface molecules, fixed for 20 min in IC Fixation buffer (Thermo Fisher Scientific), and incubated for 1 h in permeabilization buffer (Thermo Fisher Scientific) with appropriate anti-mouse mAbs. The antibodies used in this study are listed in Supplementary Table 1. The cell phenotype was analyzed on a flow cytometer (BD LSR II) (BD Biosciences, USA) or an Attune NxT (Thermo Fisher Scientific). Data were acquired as the fraction of labeled cells within a live-cell gate and analyzed using FlowJo software (TreeStar). All gates were set on the basis of the isotype-matched control antibodies.
CFSE T-cell proliferation assay
Purified naïve T cells were labeled with 2.5 μM CFSE, and then 5 × 104 T cells/well were stimulated with anti-CD3 and anti-CD28 antibodies. The T cells were cultured for 72 h, and proliferation was determined by flow cytometry analysis of CFSE dilution.
CD4+ T-cell differentiation
Purified naïve CD4+ T cells were stimulated with plate-bound anti-CD3 (2 μg/ml) and anti-CD28 (2 μg/ml) alone (Th0) or under Th1 (10 ng/ml IL-12 and 10 μg/ml anti-IL4; PeproTech, USA), Th2 (20 ng/ml IL-4 and 10 μg/ml anti-IFN-γ, PeproTech), Th17 (2.5 ng/ml TGF-β, 15 ng/ml IL-6, 10 μg/ml anti-IFN-γ and 10 μg/ml anti-IL4; PeproTech) or Treg (1.5 ng/ml TGF-β, 10 μg/ml anti-IFN-γ and 10 μg/ml anti-IL4; PeproTech) conditions. After 5 days of stimulation, the cells were subjected to flow cytometry, ELISA, or qPCR analyses.
Enzyme-linked immunosorbent assay (ELISA)
Cytokine production in the supernatants of in vitro cell cultures or serum samples from mice was measured by ELISA to assess mouse IFN-γ, IL-17, TNF-α and IL-2 (ExCell Bio, China) according to the manufacturer’s protocol.
Quantitative PCR (qPCR) analysis
Total RNA was isolated with TRIzol (Thermo Fisher Scientific) according to the manufacturer’s instructions. One milligram of RNA was reverse transcribed into cDNA with random RNA-specific primers using a high-capacity cDNA reverse transcription kit (Applied Biosystems, USA). An Eppendorf Master Cycle Realplex2 and SYBR Green PCR Master Mix (Applied Biosystems) were used for real-time PCR (40 cycles). The primer sequences used for PCR are listed in Supplementary Table 2.
Plasmid constructs and transfection
Recombinant vectors encoding Flag-USP1, Flag-USP1C90A, Myc-TAZ and HA-ub were cloned into pcDNA3.1 (Sangon Biotech, China). The same plasmids or indicated quality plasmids were transfected into HEK293T cells with Lipofectamine 2000 (Invitrogen) according to the manufacturer’s instructions.
Immunoblot, coimmunoprecipitation and ubiquitination assays
Experiments were performed as previously described [47]. Spinal cords or cells were washed three times with ice-cold PBS and then lysed in Nonidet P-40 lysis buffer containing 150 mM NaCl, 1 mM EDTA, 1% Nonidet P-40, and 1% protease and phosphatase inhibitor cocktail (Biotool). Equal amounts (20 mg) of cell lysates were resolved on 8 ± 15% polyacrylamide gels and transferred to PVDF membranes. The membranes were blocked in 5% nonfat dry milk in PBST and incubated overnight with appropriate primary antibodies at 4 °C. The membranes were incubated at room temperature for 1 h with appropriate HRP-conjugated secondary antibodies and visualized with Plus-ECL (PerkinElmer, CA) according to the manufacturer’s protocol. For immunoprecipitation assays, lysates were immunoprecipitated with IgG or appropriate antibodies and protein G Sepharose beads. The precipitates were washed three times with lysis buffer containing 500 mM NaCl, followed by immunoblot analysis. For deubiquitination assays, cells were lysed with lysis buffer, and the supernatants were denatured at 95 °C for 5 min in the presence of 1% SDS. The denatured lysates were diluted with lysis buffer to reduce the concentration of SDS below 0.1%, followed by immunoprecipitation with the indicated antibodies. The immunoprecipitants were subjected to immunoblot analysis with anti-ubiquitin chains. The antibodies used in this study are listed in Supplementary Table 1.
Retroviral packaging and transduction
Genes encoding wild-type USP1, USP1C90A, or TAZ were separately cloned into the retroviral vector pMXs containing IRES-regulated GFP (Youbio, China). Each of the resulting plasmids was transfected into a packaging cell line, PLAT-T, using FuGENE6 (Roche, Switzerland). After incubation for 24 h, the culture supernatant was harvested and condensed as a viral stock. CD4+ T cells were stimulated with anti-CD3 and anti-CD28 antibodies for 24 h. The cells were then infected with retrovirus in the presence of 0.5 μg/ml polybrene for 24 h and cultured further in the presence of 30 U/ml IL-2 for 3 days. The cells were washed with fresh medium and stimulated with plate-bound anti-CD3 and anti-CD28 antibodies under Th17 or Treg conditions. After 5 days of stimulation, the cells were subjected to flow cytometry, ELISA, or qPCR analyses.
Mass spectrometry
Proteins from CD4+ T cells were coimmunoprecipitated with anti-Flag antibodies. The immunoprecipitants were fractionated using SDS‒PAGE and visualized by Coomassie staining, followed by mass spectrometry.
Transfection and a luciferase reporter assay
Experiments were performed as previously described [48]. Cells cultured in 12-well plates were transfected with the indicated plasmids using Lipofectamine 2000 (Thermo Fisher Scientific). At 48 h after transfection, the cells were washed with PBS and lysed in Reporter Lysis Buffer (Thermo Fisher Scientific). Luciferase reporter activity was measured in triplicate using a dual-luciferase reporter assay system (Promega, Madison, WI, USA) according to the manufacturer’s protocol and quantified using a 96-well plate luminometer (Promega). The firefly luciferase-to-Renilla luciferase ratios were determined and defined as the relative luciferase activity.
Statistics
All experiments were performed at least three times. When shown, multiple samples represent biological (not technical) replicates of mice randomly sorted into each experimental group. No blinding was performed during animal experiments. Determination of significant differences was performed with Prism 8 (GraphPad Software, Inc.) using unpaired two-tailed t tests (to compare two groups with similar variances) or two-way ANOVA with Bonferroni’s multiple comparison test (to compare more than two groups).
Supplementary information
Acknowledgements
We thank all members of the Department of Rheumatology and Clinical Immunology, Zhujiang Hospital, for helpful discussions and input.
Author contributions
SFH, GLJ, LSL and PW designed the research; XTZ, PW, XXZ, YPZ, JLS, STH, YTC, DNN, XLY, and HYM conducted the research; XTZ, QHY, LGJ and SFH analyzed data; XTZ, XXZ and SFH wrote the paper; GLJ, LSL, PW and SFH provided essential reagents or materials; and SFH, GLJ and LSL as the corresponding authors conducted the experiments. All authors read and approved the final paper.
Funding
This work was funded by grants from the National Natural Science Foundation of China (81971805, 81901614, 81901659, 32070906, 82072384, 82074160, and 82272239).
Competing interests
The authors declare no competing interests.
Ethics approval
All procedures followed were in accordance with protocols approved by the Medical Ethics Board and the Biosafety Management Committee of Southern Medical University.
Footnotes
These authors contributed equally: Xiaotong Zhu, Peng Wang, Xiaoxia Zhan.
Contributor Information
Laisheng Li, Email: lilaish@mail.sysu.edu.cn.
Ligang Jie, Email: Jieligang@hotmail.com.
Shengfeng Hu, Email: hushengfeng@smu.edu.cn.
Supplementary information
The online version contains supplementary material available at 10.1038/s41423-022-00969-9.
References
- 1.Kurup S, Butler N, Harty J. T cell-mediated immunity to malaria. Nat Rev Immunol. 2019;19:457–71. doi: 10.1038/s41577-019-0158-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Borst J, Ahrends T, Bąbała N, Melief CJM, Kastenmüller W. CD4 T cell help in cancer immunology and immunotherapy. Nat Rev Immunol. 2018;18:635–47. doi: 10.1038/s41577-018-0044-0. [DOI] [PubMed] [Google Scholar]
- 3.Fan NW, Dohlman TH, Foulsham W, McSoley M, Singh RB, Chen Y, et al. The role of Th17 immunity in chronic ocular surface disorders. Ocul Surf. 2021;19:157–68. doi: 10.1016/j.jtos.2020.05.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Schlöder J, Shahneh F, Schneider F, Wieschendorf B. Boosting regulatory T cell function for the treatment of autoimmune diseases—That’s only half the battle! Front Immunol. 2022;13:973813. doi: 10.3389/fimmu.2022.973813. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Jin W, Zheng Y, Zhu P. T cell abnormalities in systemic sclerosis. Autoimmun Rev. 2022;21:103185. [DOI] [PubMed]
- 6.Lee GR. The Balance of Th17 versus Treg Cells in Autoimmunity. Int J Mol Sci. 2018;19:730. doi: 10.3390/ijms19030730. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Zhang W, Liu X, Zhu Y, Liu X, Gu Y, Dai X, et al. Transcriptional and posttranslational regulation of Th17/Treg balance in health and disease. Eur J Immunol. 2021;51:2137–50. doi: 10.1002/eji.202048794. [DOI] [PubMed] [Google Scholar]
- 8.Littman D, Rudensky A. Th17 and regulatory T cells in mediating and restraining inflammation. Cell. 2010;140:845–58. doi: 10.1016/j.cell.2010.02.021. [DOI] [PubMed] [Google Scholar]
- 9.Hong JH, Hwang ES, McManus MT, Amsterdam A, Tian Y, Kalmukova R, et al. TAZ, a transcriptional modulator of mesenchymal stem cell differentiation. Science. 2005;309:1074–8. doi: 10.1126/science.1110955. [DOI] [PubMed] [Google Scholar]
- 10.Varelas X, Sakuma R, Samavarchi-Tehrani P, Peerani R, Rao BM, Dembowy J, et al. TAZ controls Smad nucleocytoplasmic shuttling and regulates human embryonic stem-cell self-renewal. Nat Cell Biol. 2008;10:837–48. doi: 10.1038/ncb1748. [DOI] [PubMed] [Google Scholar]
- 11.Zanconato F, Cordenonsi M, Piccolo S. YAP/TAZ at the Roots of Cancer. Cancer Cell. 2016;29:783–803. doi: 10.1016/j.ccell.2016.05.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Geng J, Yu S, Zhao H, Sun X, Li X, Wang P, et al. The transcriptional coactivator TAZ regulates reciprocal differentiation of TH17 cells and Treg cells. Nat Immunol. 2017;18:800–12. doi: 10.1038/ni.3748. [DOI] [PubMed] [Google Scholar]
- 13.Zinngrebe J, Montinaro A, Peltzer N, Walczak H. Ubiquitin in the immune system. EMBO Rep. 2014;15:28–45. doi: 10.1002/embr.201338025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Sun SC. Deubiquitylation and regulation of the immune response. Nat Rev Immunol. 2008;8:501–11. doi: 10.1038/nri2337. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Yang XD, Sun SC. Deubiquitinases as pivotal regulators of T cell functions. Front Med. 2018;12:451–62. doi: 10.1007/s11684-018-0651-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Beck DB, Werner A, Kastner DL, Aksentijevich I. Disorders of ubiquitylation: unchained inflammation. Nat Rev Rheumatol. 2022;18:435–47. doi: 10.1038/s41584-022-00778-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Liang F, Miller AS, Tang C, Maranon D, Williamson EA, Hromas R, et al. The DNA-binding activity of USP1-associated factor 1 is required for efficient RAD51-mediated homologous DNA pairing and homology-directed DNA repair. J Biol Chem. 2020;295:8186–94. doi: 10.1074/jbc.RA120.013714. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Lim KS, Li H, Roberts EA, Gaudiano EF, Clairmont C, Sambel LA, et al. USP1 Is Required for Replication Fork Protection in BRCA1-Deficient Tumors. Mol Cell. 2018;72:925–41.e4. doi: 10.1016/j.molcel.2018.10.045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Yu Z, Song H, Jia M, Zhang J, Wang W, Li Q, et al. USP1-UAF1 deubiquitinase complex stabilizes TBK1 and enhances antiviral responses. J Exp Med. 2017;214:3553–63. doi: 10.1084/jem.20170180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Song H, Zhao C, Yu Z, Li Q, Yan R, Qin Y, et al. UAF1 deubiquitinase complexes facilitate NLRP3 inflammasome activation by promoting NLRP3 expression. Nat Commun. 2020;11:6042. doi: 10.1038/s41467-020-19939-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Cohn MA, Kee Y, Haas W, Gygi SP, D’Andrea AD. UAF1 is a subunit of multiple deubiquitinating enzyme complexes. J Biol Chem. 2009;284:5343–51. doi: 10.1074/jbc.M808430200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Omilusik KD, Nadjsombati MS, Yoshida TM, Shaw LA, Goulding J, Goldrath AW. Ubiquitin Specific Protease 1 Expression and Function in T Cell Immunity. J Immunol. 2021;207:1377–87. doi: 10.4049/jimmunol.2100303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.van Loosdregt J, Fleskens V, Fu J, Brenkman AB, Bekker CP, Pals CE, et al. Stabilization of the transcription factor Foxp3 by the deubiquitinase USP7 increases Treg-cell-suppressive capacity. Immunity. 2013;39:259–71. doi: 10.1016/j.immuni.2013.05.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Li Y, Lu Y, Wang S, Han Z, Zhu F, Ni Y, et al. USP21 prevents the generation of T-helper-1-like Treg cells. Nat Commun. 2016;7:13559. doi: 10.1038/ncomms13559. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Zhang J, Chen C, Hou X, Gao Y, Lin F, Yang J, et al. Identification of the E3 deubiquitinase ubiquitin-specific peptidase 21 (USP21) as a positive regulator of the transcription factor GATA3. J Biol Chem. 2013;288:9373–82. doi: 10.1074/jbc.M112.374744. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Yang J, Wei P, Barbi J, Huang Q, Yang E, Bai Y, et al. The deubiquitinase USP44 promotes Treg function during inflammation by preventing FOXP3 degradation. EMBO Rep. 2020;21:e50308. doi: 10.15252/embr.202050308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Wang L, Kumar S, Dahiya S, Wang F, Wu J, Newick K, et al. Ubiquitin-specific Protease-7 Inhibition Impairs Tip60-dependent Foxp3+ T-regulatory Cell Function and Promotes Antitumor Immunity. EBioMedicine. 2016;13:99–112. doi: 10.1016/j.ebiom.2016.10.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Yang J, Xu P, Han L, Guo Z, Wang X, Chen Z, et al. Cutting edge: Ubiquitin-specific protease 4 promotes Th17 cell function under inflammation by deubiquitinating and stabilizing RORγt. J Immunol. 2015;194:4094–7. doi: 10.4049/jimmunol.1401451. [DOI] [PubMed] [Google Scholar]
- 29.He Z, Wang F, Ma J, Sen S, Zhang J, Gwack Y, et al. Ubiquitination of RORγt at Lysine 446 Limits Th17 Differentiation by Controlling Coactivator Recruitment. J Immunol. 2016;197:1148–58. doi: 10.4049/jimmunol.1600548. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Han L, Yang J, Wang X, Wu Q, Yin S, Li Z, et al. The E3 deubiquitinase USP17 is a positive regulator of retinoic acid-related orphan nuclear receptor γt (RORγt) in Th17 cells. J Biol Chem. 2014;289:25546–55. doi: 10.1074/jbc.M114.565291. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Rennie ML, Lemonidis K, Arkinson C, Chaugule VK, Clarke M, Streetley J, et al. Differential functions of FANCI and FANCD2 ubiquitination stabilize ID2 complex on DNA. EMBO Rep. 2020;21:e50133. doi: 10.15252/embr.202050133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.van Twest S, Murphy VJ, Hodson C, Tan W, Swuec P, O'Rourke JJ, et al. Mechanism of Ubiquitination and Deubiquitination in the Fanconi Anemia Pathway. Mol Cell. 2017;65:247–59. doi: 10.1016/j.molcel.2016.11.005. [DOI] [PubMed] [Google Scholar]
- 33.Zhang Q, Liu W, Wang H, Zhou H, Bulek K, Chen X, et al. TH17 cells promote CNS inflammation by sensing danger signals via Mincle. Nat Commun. 2022;13:2406. doi: 10.1038/s41467-022-30174-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.D'addio F, Vergani A, Potena L, Maestroni A, Usuelli V, Ben Nasr M, et al. P2X7R mutation disrupts the NLRP3-mediated Th program and predicts poor cardiac allograft outcomes. J Clin Investig. 2018;128:3490–503. doi: 10.1172/JCI94524. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Arbore G, West EE, Spolski R, Robertson A, Klos A, Rheinheimer C, et al. T helper 1 immunity requires complement-driven NLRP3 inflammasome activity in CD4+ T cells. Science. 2016;352:aad1210. doi: 10.1126/science.aad1210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Bruchard M, Rebé C, Derangère V, Togbé D, Ryffel B, Boidot R, et al. The receptor NLRP3 is a transcriptional regulator of TH2 differentiation. Nat Immunol. 2015;16:859–70. doi: 10.1038/ni.3202. [DOI] [PubMed] [Google Scholar]
- 37.Du W, Erden O, Wilson A, Sipple JM, Schick J, Mehta P, et al. Deletion of Fanca or Fancd2 dysregulates Treg in mice. Blood. 2014;123:1938–47. doi: 10.1182/blood-2013-09-528018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Shanzer M, Adler J, Ricardo-Lax I, Reuven N, Shaul Y. The nonreceptor tyrosine kinase c-Src attenuates SCF(β-TrCP) E3-ligase activity abrogating Taz proteasomal degradation. Proc Natl Acad Sci USA. 2017;114:1678–83. doi: 10.1073/pnas.1610223114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Huang W, Lv X, Liu C, Zha Z, Zhang H, Jiang Y, et al. The N-terminal phosphodegron targets TAZ/WWTR1 protein for SCFβ-TrCP-dependent degradation in response to phosphatidylinositol 3-kinase inhibition. J Biol Chem. 2012;287:26245–53. doi: 10.1074/jbc.M112.382036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Zhang Z, Du J, Wang S, Shao L, Jin K, Li F, et al. OTUB2 Promotes Cancer Metastasis via Hippo-Independent Activation of YAP and TAZ. Mol Cell. 2019;73:7–21.e27. doi: 10.1016/j.molcel.2018.10.030. [DOI] [PubMed] [Google Scholar]
- 41.Li J, Dai Y, Ge H, Guo S, Zhang W, Wang Y, et al. The deubiquitinase USP7 promotes HNSCC progression via deubiquitinating and stabilizing TAZ. Cell Death Dis. 2022;13:677. doi: 10.1038/s41419-022-05113-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Zhao C, Gong J, Bai Y, Yin T, Zhou M, Pan S, et al. A self-amplifying USP14-TAZ loop drives the progression and liver metastasis of pancreatic ductal adenocarcinoma. Cell Death Differ. 2022. 10.1038/s41418-022-01040-w. Online ahead of print. [DOI] [PMC free article] [PubMed]
- 43.Mussell A, Shen H, Chen Y, Mastri M, Eng KH, Bshara W, et al. USP1 Regulates TAZ Protein Stability Through Ubiquitin Modifications in Breast Cancer. Cancers. 2020;12:3090. [DOI] [PMC free article] [PubMed]
- 44.Yuan P, Feng Z, Huang H, Wang G, Chen Z, Xu G, et al. USP1 inhibition suppresses the progression of osteosarcoma via destabilizing TAZ. Int J Biol Sci. 2022;18:3122–36. doi: 10.7150/ijbs.65428. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Fu Y, Wang P, Zhao J, Tan Y, Sheng J, He S, et al. USP12 promotes CD4(+) T cell responses through deubiquitinating and stabilizing BCL10. Cell Death Differ. 2021;28:2857–70. doi: 10.1038/s41418-021-00787-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Fu Y, Zhan X, Wang Y, Jiang X, Liu M, Yang Y, et al. NLRC3 expression in dendritic cells attenuates CD4(+) T cell response and autoimmunity. EMBO J. 2019;38:e101397. doi: 10.15252/embj.2018101397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Huang Y, He S, Chen Y, Sheng J, Fu Y, Du X, et al. UCHL1 Promoted Polarization of M1 Macrophages by Regulating the PI3K/AKT Signaling Pathway. J Inflamm Res. 2022;15:735–46. doi: 10.2147/JIR.S343487. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Geng J, Yu S, Zhao H, Sun X, Li X, Wang P, et al. The transcriptional coactivator TAZ regulates reciprocal differentiation of T(H)17 cells and T(reg) cells. Nat Immunol. 2017;18:800–12. doi: 10.1038/ni.3748. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.