Abstract
Psoriasis is a common chronic inflammatory skin disease characterized by inflammatory cell infiltration and epidermal hyperplasia. However, the regulatory complexity of cytokine and cellular networks still needs to be investigated. Here, we show that the expression of FXYD3, a member of the FXYD domain-containing regulators of Na+/K+ ATPases family, is significantly increased in the lesional skin of psoriasis patients and mice with imiquimod (IMQ)-induced psoriasis. IL-17A, a cytokine important for the development of psoriatic lesions, contributes to FXYD3 expression in human primary keratinocytes. FXYD3 deletion in keratinocytes attenuated the psoriasis-like phenotype and inflammation in an IMQ-induced psoriasis model. Importantly, FXYD3 promotes the formation of the IL-17R-ACT1 complex by competing with IL-17R for binding to TRAF3 and then enhances IL-17A signaling in keratinocytes. This promotes the activation of the NF-κB and MAPK signaling pathways and leads to the expression of proinflammatory factors. Our results clarify the mechanism by which FXYD3 serves as a mediator of IL-17A signaling in keratinocytes to form a positive regulatory loop to promote psoriasis exacerbation. Targeting FXYD3 may serve as a potential therapeutic approach in the treatment of psoriasis.
Keywords: FXYD3, TRAF3, IL-17 signaling, Keratinocytes, Psoriasis
Subject terms: Inflammation, Immunological disorders
Introduction
Psoriasis is a common chronic skin disease characterized by keratinocyte hyperplasia and the infiltration of inflammatory cells and affects 2–3% of the global population [1]. Although the exact pathogenesis mechanism of psoriasis remains elusive, the complex communication between keratinocytes and immune cells has been highlighted in the contexts of disease initiation and maintenance. Recently, the IL-23-IL-17 axis has been shown to play a key role in psoriasis. Antagonists of IL-17, or the blocking of IL-17RA, showed promising therapeutic effects in patients with psoriasis, suggesting the importance of the IL-17 cytokine in psoriasis pathogenesis [2]. The skin epidermis, mainly composed of keratinocytes, plays an integral physiological role in the establishment and maintenance of the skin barrier. Keratinocytes are key participants in both the initiation and exacerbation of psoriasis [1, 3]. Keratinocytes are also the main target cells of proinflammatory cytokines such as IL-17A within the skin. These are able to induce the production of chemokines (e.g., CXCL1, CXCL2, CCL20, CXCL8) or antimicrobial proteins such as β-defensins, S100A8 and S100A9, leading to the recruitment of increased numbers of IL-17-producing T cells and neutrophils into the skin [4–6]. Recent multivariate studies have revealed the important role of IL-17-mediated NF-κB activation in keratinocytes in the initiation and maintenance of psoriasis [7–9]. Although the role of IL-17 is fairly well understood, it remains unclear whether specific epithelial signaling pathways or tissue-specific mediators of IL-17-mediated immune responses occur in psoriasis.
FXYD3, also known as Mat-8, belongs to the FXYD protein family, and all members of this family have an FXYD domain [10]. The FXYD family contains seven members (FXYD1-7) that are expressed in various tissues and exert diverse functions [11]. FXYD3 is mainly expressed in the skin as well as in the colon, stomach, and uterus. It has been reported to contribute to the progression of breast cancer and pancreatic ductal adenocarcinomas as well as have more general roles in cell proliferation and survival [12, 13]. Our previous study revealed a critical role for the SOX9/FXYD3/Src axis in boosting nongenomic estrogen signaling and SOX9 nuclear entry to maintain ER+ breast cancer stem cells and endocrine resistance [14]. However, the pathological roles and mechanisms of FXYD3 in skin diseases such as psoriasis remain largely unknown.
In this study, we observed that FXYD3 is mainly expressed in epidermal keratinocytes and that it is expressed at significantly higher levels in the skin lesions of psoriasis patients than in normal skin. This suggests that FXYD3 may have an important function in regulating psoriasis exacerbation. Correspondingly, FXYD3 deficiency in keratinocytes reduced inflammation and disease severity in an IMQ-induced psoriasis-like skin model. In response to IL-17 stimulation, FXYD3 competes with IL-17R to interact with TRAF3 and promotes the formation of an IL-17R-ACT1 complex. FXYD3 deficiency thereby inhibits the IL-17-mediated expression of chemokines and antimicrobial peptides. Furthermore, IL-17A increases the expression of FXYD3 in keratinocytes, forming a psoriasis-related inflammatory circuit. Given the importance of IL-17 signaling in psoriasis, these findings provide novel insights into FXYD3 function as a potential diagnostic and therapeutic target.
Materials and methods
Mice
FXYD3fl/fl mice on a C57BL/6J background were generated by Nanjing Biomedical Research Institute of Nanjing University (Nanjing, China). K14-Cre mice on a C57BL/6J background were obtained from the Model Animal Research Center of Nanjing University. All mice were bred and maintained at the Zhejiang University Laboratory Animal Center under specific pathogen-free conditions. C57BL/6 mice were purchased from SLACCAS (Shanghai, China). Age-matched and sex-matched mice were used for all experiments. All animal experiments were conducted according to the protocol approved by the Institutional Animal Care and Use Committee of Zhejiang University School of Medicine.
Human subjects
Psoriatic skin samples were obtained from the Department of Dermatology and Venereology, the Second Affiliated Hospital, Zhejiang University School of Medicine. Normal control skin sections consisted of healthy tissue from the resection edges of cutaneous biopsies that appeared to be normal at the histological level. The expression of FXYD3 and TRAF3 was detected by IHC staining and scored from 0 to 3 according to the percentage of positive cells and staining intensity. The staining score was calculated by multiplying the intensity score by the staining extent score. The use of pathological specimens and the review of all pertinent patient records were approved by the Ethics Review Board of the Second Affiliated Hospital, Zhejiang University School of Medicine.
Immunohistochemistry and immunofluorescence staining assay
Skin samples were fixed in 4% paraformaldehyde and embedded in paraffin at the Histology Core Facility of Zhejiang University School of Medicine, following the manufacturer’s standard protocol (reagents purchased from Beijing Zhongshan Jinqiao Biotechnology Company). All H&E- and immunohistochemistry-stained sections were imaged using an Olympus BX61 microscope. The epidermal thickness of the skin was calculated as the area of epidermis/the length of epidermis [15]. Fluorescence images were taken using an LSM880 microscope and analyzed using ImageJ software. The following primary antibodies were used: FXYD3 (ab205534, clone EPR17160), Ki67 (ab279653, clone B56), K14 (ab7800, clone LL002), K10 (ab76318, EP1607IHCY) and CD31 (ab182981, clone EPR17259) from Abcam; TRAF3 (Santa Cruz, sc-947) from Santa Cruz Biotechnology; and phospho-p38 (CST, 4511S) and phospho-ERK (CST, 4370S) from Cell Signaling Technology.
Cell cultures
Mouse primary keratinocytes were prepared as described previously [16]. To isolate primary mouse epidermal keratinocytes, whole skin from neonatal mice was suspended in Dispase II (Sigma‒Aldrich cat. D4693, 2 mg/mL) overnight at 4 °C. The next day, after 12–18 h in dispase, the epidermis was placed into TrypLE solution (GIBCO cat. 12604021) for 10 min at 37 °C with gentle shaking. The cells were cultured in Medium 154CF (GIBCO cat. M154CF500) supplemented with 0.05 mM calcium chloride and human keratinocyte growth supplement (GIBCO cat. S0015). The NHEK cell line (normal human epidermal keratinocytes, NHEK) was a gift from Prof. Yuping Lai (Life Science Institute, East China Normal University, Shanghai, China) and cultured in EpiLife medium (GIBCO cat. MEPI500CA) supplemented with EDGS (GIBCO cat. S0125), 0.06 mM CaCl2 and Pen-Strep (100 units/ml penicillin and 100 µg/ml streptomycin). The HaCaT cell line was kindly provided by Prof. Honglin Wang (Shanghai Jiao Tong University School of Medicine, Shanghai, China). HaCaT, HeLa and 293 T cells were cultured in DMEM/high glucose containing 10% fetal bovine serum.
Imiquimod model of psoriasis
Seven- to ten-week-old mice received a daily topical dose of 62.5 mg IMQ cream (5%, Aldara, 3 M Pharmaceuticals) on the shaved dorsal back or 25 mg on the ears for 3–6 days. An objective scoring system was used to score skin inflammation based on the clinical Psoriasis Area and Severity Index (PASI) according to a previous report [17]. Erythema, scaling, and thickening were scored independently from 0 to 4 (0, none; 1, slight; 2, moderate; 3, marked; 4, very marked). The cumulative score (erythema score plus scaling score plus thickening score) served as the severity of inflammation index (scale 0–12).
Skin cell isolation and flow cytometry
Single cells from the ear were generated according to a previously reported study [18]. The antibodies for flow cytometry included APC-CD45 (Biolegend, 109814), PE-CF594-CD3 (Biolegend, 100245), FITC-βTCR (eBioscience, 11-5961-81), PE-γδTCR (Biolegend, 107507), BV605-CD11b (Biolegend, 101237), BV650-LY6G (Biolegend, 127641), PE-Cy7-CD11c (Biolegend, 117317), AF700-MHCII (Biolegend, 107621), BV421-F4/80 (Biolegend, 123131), and APC-Cy7-LY6C (Biolegend, 128025); the Zombie Aqua™ Fixable Viability Kit (Biolegend, 423101) was also used. The cells were analyzed using a BD Fortessa Cell Analyzer. The data were analyzed using FlowJo software.
RNA isolation and quantitative RT‒PCR
Total RNA from skin tissue or cells was isolated using TRIzol reagent (Takara) according to the manufacturer’s directions. Reverse transcription was performed using ReverTra Ace® qPCR RT Master Mix (TOYOBO) according to the manufacturer’s instructions. Real-time PCR was performed using SYBR Green Master Mix (Vazyme, Nanjing, China). The mRNA expression levels of the examined genes were normalized and then determined using the 2−ΔΔCt method. Primer sequences are shown in Table S1.
IL-17A neutralization experiment
For the IL-17A neutralization experiment, anti-mouse IL-17A (BioxCell cat. BE0173, 100 μg) or isotype control (BioxCell cat. BE0083) was intraperitoneally injected into the mice before IMQ application, and injections were repeated every other day.
Immunoprecipitation and western blotting
Mouse primary epidermal keratinocytes were stimulated with IL-17A (R&D, 421-ML-025) at the indicated time point. Cells were then harvested and lysed in lysis buffer. SDS‒PAGE and immunoblot analysis were performed using the indicated antibodies. For exogenous co-IP, epitope-tagged plasmids were transfected into HEK293T cells, and the cells were lysed in lysis buffer (5% glycerol, 1% Nonidet P-40, 1 mM EDTA, 100 mM NaCl, 20 mM Tris-HCl pH 8.0, and protease inhibitors). Each sample supernatant was incubated with anti-Flag M2 beads (Sigma, M8823) or anti-HA beads (MCE, HY-K0201) at 4 °C overnight. For endogenous co-IP, primary epidermal keratinocytes or HaCaT cells were stimulated with IL-17A, lysed in lysis buffer and incubated with anti-FXYD3, anti-ACT1, or anti-TRAF3 and protein G magnetic beads (MCE, HY-K0202). The proteins were then eluted by loading buffer for immunoblot assay with the indicated antibodies. The antibodies used in this study included phospho-p65 (CST, 3033S), phospho-p38 (CST, 4511S), phospho-ERK (CST, 4370S), phospho-JNK (CST, 4668 S), p38 (CST, 8690S), JNK (CST, 9252S), p65 (Proteintech, 10745-1-AP), ERK (CST, 4695S), β-tubulin (Proteintech, 10068-1-AP), ACT1 (Santa Cruz, sc-11444, sc-100647), TRAF3 (Santa Cruz, sc-6933, sc-947), IL17R (Santa Cruz, sc-376600), Flag (Abmart, M20008S), HA (Proteintech, 51064-2-AP), β-tubulin (Proteintech, 10068-1-AP), and FXYD3 (Abcam, ab205534).
Plasmid construction and transfection
Flag-tagged and HA-tagged FXYD3 were generated by PCR and subcloned into a pcDNA3.1 eukaryotic expression vector (Invitrogen). Truncated FXYD3 was amplified from vectors expressing HA-full-length FXYD3 and cloned into pcDNA3.1-HA expression vectors. Expression plasmids for Flag-tagged TRAF2, TRAF3, TRAF5, TRAF6, and ACT1 and plasmids for HA-IL-17R, HA-tagged TRAF3, or its variants, including TRAF3-ΔTRAF, TRAF3-ΔZ, TRAF3-ΔRing, TRAF3-ΔCT, and TRAF3-TRAF, were kindly provided by Prof. XiaoJian Wang (Zhejiang University, Hangzhou, China). FXYD3 was cloned into the pLenti-puro lentivirus vector for overexpression. The plasmids were transfected into HEK293T cells or HeLa cells with jetPRIME® reagent (Polyplus) according to the standard protocol.
RNA interference
Small interfering RNA (siRNA) was transfected into NHEKs and primary mouse keratinocytes using Lipofectamine™ 3000 Transfection Reagent (Invitrogen, L3000015) according to the manufacturer’s protocol. The following siRNA oligonucleotide sequences were used: negative control siRNA (5′-UUCUCCGAACGUGUCACGU-3′), FXYD3 siRNA (5′-GCCAAUGACCUAGAAGAUAAA-3′), TRAF3 siRNA1 (5′-CCACCGGAGAGAUGAAUAU-3′), TRAF 3 siRNA2 (5′-CAAGGUGUUUAAGGAUAAU-3′)
Statistical analysis
All statistical analyses were performed using GraphPad Prism 8 software and are presented as the mean ± SEM. The results were analyzed using Student’s t test (two groups) or ANOVA (more than two groups). A P value of <0.05 was considered to indicate statistical significance.
Results
FXYD3 is upregulated in the epidermis of patients with psoriasis and in the IMQ mouse model
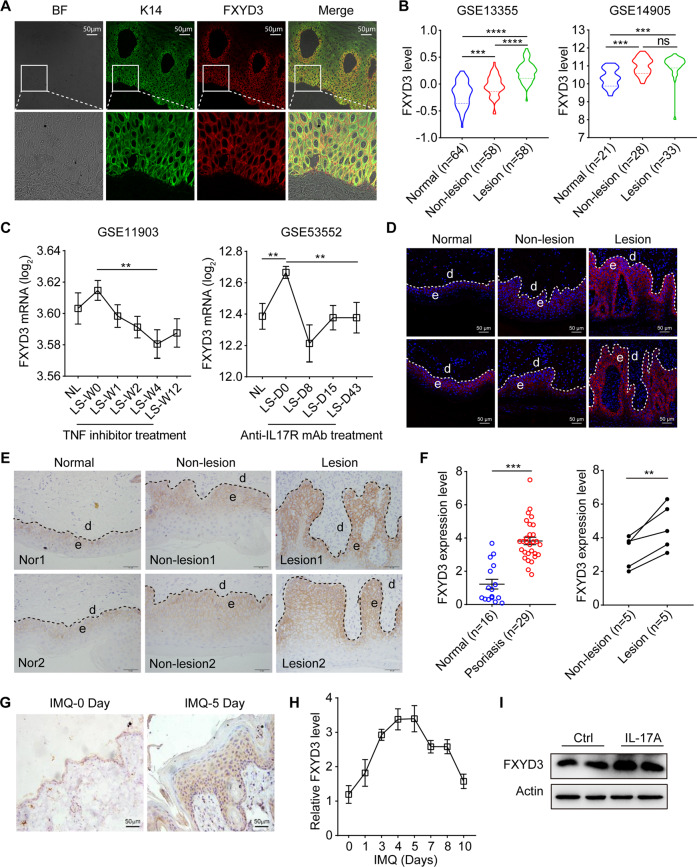
To study the relationship between psoriasis and FXYD3, we first examined the expression pattern of FXYD3 in skin biopsies. As shown in Fig. 1A, the FXYD3-positive signal (red) mainly colocalized with the K14-positive signal (green) in the epidermis, indicating that FXYD3 was predominantly expressed in keratinocytes, especially in the basal and spinous cell layers (Fig. 1A). GEO analysis (GSE13355 and GSE14905) showed that FXYD3 mRNA expression was significantly increased in lesional skin compared with normal skin from healthy individuals. Moreover, FXYD3 mRNA levels in lesional skin were also higher than those in the adjacent nonlesional skin of psoriasis patients in GSE13355, although the levels were comparable in GSE14905 (Fig. 1B). Analysis of pretherapy and posttherapy clinical data of patients with psoriasis from public GEO databases showed that FXYD3 mRNA expression was significantly decreased after the application of a TNF inhibitor for 4 weeks or after treatment with anti-IL-17 receptor antibody for 43 days (Fig. 1C).
Fig. 1.
FXYD3 is upregulated in the epidermis of patients with psoriasis and IMQ-treated mice. A Immunofluorescent staining of FXYD3 (red) and K14 (green) in human skin. Bar = 50 µm. B The mRNA expression level of FXYD3 in the skin from normal, nonlesional skin, and lesional skin of psoriasis patients was analyzed using the Gene Expression Omnibus (GEO) database. C GEO data analysis of FXYD3 mRNA levels before and after TNF inhibitor (left) or anti-IL-17R antibody (right) treatment in patients with psoriasis. D Immunofluorescent labeling of FXYD3 in the skin from healthy controls and psoriatic patients. Bar = 50 µm. E Immunohistochemical staining for FXYD3 in two healthy and two psoriatic human skin sections. Bar = 50 µm. F Semiquantitative scoring of FXYD3 in the epidermis detected by IHC. G Representative images of the expression of FXYD3 in the skin from IMQ-treated mice. Bar = 50 µm. H The mRNA expression of FXYD3 in the epidermis on Days 1, 3, 4, 5, 7, 8 and 10 during the application of IMQ in mice (n = 6/group). I Immunoblotting analysis of FXYD3 expression in NHEKs stimulated with IL-17A for 24 h. Error bars show the mean ± SEM. *, P < 0.05; **, P < 0.01; ***, P < 0.001. The P value was determined using two-tailed unpaired Student’s t test (F) or one-way ANOVA (B and C). Data are representative of three independent experiments
To gain further insight into the correlation between FXYD3 and the progression of psoriasis, we also collected skin tissue samples from psoriasis patients for comparison with samples from healthy donors and performed immunofluorescence and immunohistochemical analysis of FXYD3 on the skin sections. The level of FXYD3 was markedly increased in epidermal keratinocytes of lesional skin but only slightly increased in nonlesional skin of psoriasis patients compared to normal skin from healthy donors (Fig. 1D–F). In the IMQ-induced psoriasis model, the level of FXYD3 was significantly increased in the epidermis and was highly correlated with the pathological progression of psoriasis (Fig. 1G, H; Fig. S1A). To further investigate which factor induced FXYD3 upregulation in keratinocytes, we analyzed multiple cytokines, including IL-17A, IL-22, IL-6, TNF-α and IFN-γ. Among these cytokines, IL-17A, TNF-α and IL-22, especially IL-17A, promoted the expression of FXYD3 (Fig. 1I and Fig. S1B). Taken together, these data indicate that the expression of FXYD3 is upregulated in the lesions and correlated with psoriasis exacerbation.
FXYD3 ablation in keratinocytes attenuates psoriasis-like phenotypes in an IMQ mouse model
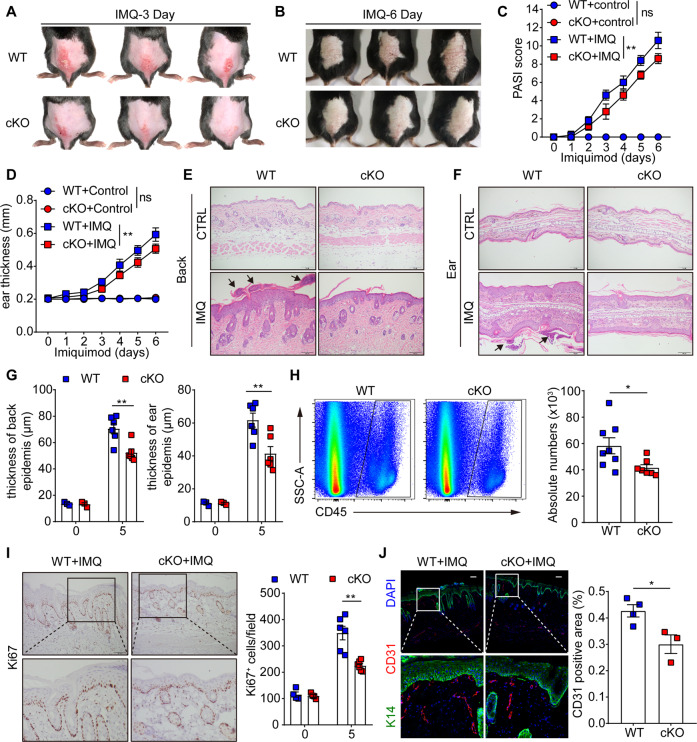
To investigate the function of FXYD3 in the epidermis in vivo, we generated K14creFXYD3f/f (hereafter referred to as cKO) mice by crossing FXYD3 floxed (WT) mice with K14-Cre mice, in which FXYD3 was selectively deleted in keratinocytes (Fig. S2A). Compared to those in WT mice, FXYD3 mRNA levels in cKO mice were significantly lower in the epidermis (Fig. S2B). We next treated WT and cKO mice with IMQ cream on the shaved back or ear daily for 6 days. The knockout of FXYD3 in keratinocytes significantly reduced the scores for psoriasis disease phenotypes, including erythema, scaling, and thickening (Fig. 2A, B). The PASI score and ear thickness measurement also decreased in the cKO mice (Fig. 2C, D). Microscopic examination of hematoxylin and eosin-stained skin sections from IMQ-treated cKO mice showed decreased epidermal thickening and the presence of Munro’s microabscesses (black arrow) in the back and ear skin (Fig. 2E–G). After IMQ treatment, the infiltration level of CD45+ immune cells into the skin of mice was greater in WT mice than in cKO mice (Fig. 2H).
Fig. 2.
FXYD3 ablation in KCs ameliorates the IMQ-induced psoriasis-like pathological phenotype in mice. A, B Representative images of dorsal back from WT and FXYD3 cKO mice (n = 3/group) treated with imiquimod (IMQ) for 3 days and 6 days. C Daily scoring of the back skin in WT and cKO model mice (n = 6/group). D Ear thickness of WT and cKO mice was measured on the days indicated (n = 6/group). E–G Representative images of back and ear stained by hematoxylin and eosin and statistical analysis of measurements of epidermal thickness. Scale bars, 100 μm. H Flow cytometry analysis of CD45+ cells in the ears of mice (n = 7–8/group). I Immunohistochemistry labeling of Ki67 in mouse skin samples (n = 6/group). Scale bars, 100 μm. J Immunofluorescence images of back sections stained with DAPI (blue), CD31 (red) and K14 (green) (n = 3–4/group). Scale bars, 100 μm. Error bars show the mean ± SEM. *, P < 0.05; **, P < 0.01; ***, P < 0.001. The P value was determined using two-tailed unpaired Student’s t test (H and J) or two-way ANOVA (C, D, G and I). Data are representative of three independent experiments
To investigate the histological features of WT and cKO mice in the IMQ model, we performed an immunohistochemical analysis of Ki67 on the back skin where IMQ-treated cKO mice showed reduced Ki67+ cells in the epidermis (Fig. 2I). Similar results were found in dorsal skin; in cKO mice, higher expression levels of keratin 10 (K10) reflected increased differentiation, whereas decreased expression of K14 in the epidermal layer reflected reduced keratinocyte proliferation (Fig. S1C, D). In addition, skin sections were stained with CD31 to assess angiogenesis, another classic hallmark of psoriasis. IMQ induced a significantly increase in the number of cutaneous blood vessels in the dermis of the dorsal skin in WT mice. This phenomenon was still observed in cKO mice, but the change was significantly less pronounced (Fig. 2J). Taken together, these results indicate that FXYD3 plays an important role in promoting psoriasis development.
FXYD3 ablation in keratinocytes led to decreased IMQ-induced psoriasis-like inflammation
We further studied whether FXYD3 affected IMQ-induced skin inflammation. Single-cell suspensions isolated from ear skin were stained with LY6G, CD11b, CD11c, MHCII, F4/80, LY6C, CD3, TCRβ, or TCRγδ and analyzed by flow cytometry. We performed unbiased clustering analysis by t-distributed stochastic neighbor embedding (t-SNE) using CD45+ live+ single cells. A single t-SNE map was created from the signal strength of the immune marker to define specific cellular lineages expressed using a blue‒green-yellow‒red continuous color scale (Fig. 3A). We identified several cell populations, including neutrophils (CD11b+ Ly6G+), dendritic cells (CD11c+ MHCII+), macrophages (CD11b+ CD11c− Ly6G− F4/80+), monocytes (CD11b+ Ly6G− CD11c− F4/80− Ly6C+) and T-cell subsets, via conventional and t-SNE guided gating (Fig. 3B; Fig. S3A). The distributions and relative frequencies of CD45+ immune cell subsets were compared between IMQ-treated WT and cKO mice (Fig. 3C). Notably, FXYD3 deficiency in keratinocytes markedly decreased both the absolute number and percentage of neutrophils, monocytes and macrophages. DCs and γδ T-cell counts were also decreased in psoriatic skin in cKO mice, although the frequencies of γδ Thi cells were higher than those in WT mice (Fig. 3D, E). Next, we analyzed whether FXYD3 influences the expression of inflammatory mediators and chemokines in the skin. FXYD3 knockout in keratinocytes decreased the expression of IL-6, CXCL1, CCL20, S100A8 and S100A9 compared to that in wild-type mice, while the models showed similar expression levels of CXCL2 (Fig. 3F). Furthermore, we also found decreased gene expression of IL-23, IL-17A, IL-17F, IL-1β and IL-22, except for a similar level of TNF-α compared to wild-type mice (Fig. 3G). To further assess whether FXYD3 could also regulate psoriasis development by regulating keratinocyte proliferation, we treated primary keratinocytes with IL-17A in vitro for cell cycle experiments. The results showed that FXYD3 knockout in keratinocytes did not alter the percentage of cells in the active phases of the cell cycle (S and G2/M phases) (Fig. S3B). These results were also confirmed by CCK-8 assays with transient knockdown of FXYD3 using siRNA in NHEKs or overexpression of FXYD3 in HaCaT cells (Fig. S3C, D), which further indicated that FXYD3 does not directly regulate the proliferation of keratinocytes. These data showed that FXYD3 is involved in IMQ-induced skin inflammation and is important in mediating the recruitment of inflammatory immune cells in psoriasis.
Fig. 3.
FXYD3 ablation in keratinocytes decreased IMQ-induced psoriasis-like inflammation. A t-SNE analysis of CD45+ cells with the CD11b, CD11c, F4/80, CD3, LY6G, LY6C, αβTCR, MHCII and γδ TCR markers. The colors in the expression-level heatmaps (right panel) represent the median intensity values for each marker. B Distribution of the different immune cell subsets in the t-SNE analysis. C Relative clustering of skin immune cells from WT and cKO mice. D Absolute numbers of immune cell subsets in the skin of WT and cKO mice (n = 5–6/group). E Percentages of various immune cells among CD45+ skin cells from WT and cKO mice (n = 5–6/group). F, G Quantitative PCR analysis of cytokines, chemokines, and antimicrobial peptides from the back skin of WT and cKO mice (n = 5–8/group). Error bars show the mean ± SEM. *, P < 0.05; **, P < 0.01; ***, P < 0.001. The P value was determined using two-tailed unpaired Student’s t test (F) or two-way ANOVA (D and E). Data are representative of three independent experiments
FXYD3 contributes to psoriasis-like inflammation by promoting IL-17-mediated signaling
Given that IL-17 is a signature cytokine that responsively stimulates keratinocytes to express various cytokines, chemokines, and antimicrobial peptides, such as CXCL1, CCL20, S100A8 and S100A9 [6, 19], we hypothesized that FXYD3 is involved in IL-17A signaling in keratinocytes. To test this, we isolated primary keratinocytes and stimulated them with IL-17A in vitro. We found that the IL-17-induced phosphorylation of p65, p38, and ERK1/2 was decreased in FXYD3-knockout keratinocytes (Fig. 4A). Consistent with this, the IL-17-induced phosphorylation of signaling molecules was reduced in FXYD3-silenced NHEK compared to the negative control siRNA (siNC)-transfected NHEKs (Fig. S4A). Immunohistochemistry staining of p-p38 and p-ERK in the epidermis was significantly weaker in the IMQ-treated FXYD3-deficient mice than in the WT mice (Fig. 4B). Furthermore, after IL-17A treatment, the levels of CXCL1, CXCL2, CCL20, S100A8 and S100A9 in FXYD3-deficient keratinocytes were lower than those in wild-type cells (Fig. 4C).
Fig. 4.
FXYD3 contributes to psoriasis-like inflammation via its promotion of IL-17-mediated signaling. A Western blot of p65, phospho-p65, p38, phospho-p38, JNK, phospho-JNK, ERK, and phospho-ERK in primary keratinocytes treated with IL-17A (100 ng/ml). B Representative images of p-p38 and p-ERK IHC staining in the backs of WT and cKO mice treated with IMQ for 5 days. The expression levels were calculated on the right. Scale bar, 100 μm or 50 μm. C Relative expression of IL-17A target genes in primary keratinocytes from WT and cKO mice treated with IL-17A for 3 and 6 h. D Representative images of back stained by hematoxylin and eosin and epidermal thickness measurements. The mice were treated with intraperitoneal injections of an anti-IL-17A antibody or isotype control (100 µg) on Days 0, 2, and 4 during IMQ application (n = 3–6/group). E Daily scoring of the back skin in WT-isotype, WT-anti-IL-17A, cKO-isotype and cKO-anti-IL-17A model mice (n = 3–6/group). F CXCL1, CCL20, S100A8 and S100A9 mRNA levels were measured in the back skin of WT and cKO mice treated with anti-IL-17A or isotype antibodies. Error bars show the mean ± SEM. *, P < 0.05; **, P < 0.01; ***, P < 0.001. The P value was determined using two-way ANOVA (C and E) or one-way ANOVA (D and F). Data are representative of three independent experiments
To further determine whether FXYD3-mediated promotion of IL-17-mediated signaling was responsible for the psoriasis-like phenotype in the IMQ model, we used a blocking antibody against IL-17A during the induction of psoriasis. Consistent with the results presented in Fig. 2, the psoriasis phenotype was attenuated in cKO mice compared with WT mice. More importantly, injection of an anti-IL-17 antibody greatly inhibited the psoriasis-like phenotype in WT mice but not in cKO mice (Fig. 4D, E). These results suggested that IL-17-stimulated psoriasis pathogenesis might already be defective in FXYD3-cKO mice and therefore is not further reduced by an anti-IL-17 antibody. The levels of the target genes of IL-17A, such as CXCL1, CCL20, S100A8 and S100A9, were substantially decreased in cKO mice compared with WT mice. However, the levels of these genes in WT mice were comparable to those in cKO mice after blocking IL-17A (Fig. 4F). These results suggest that FXYD3 deficiency in keratinocytes restricts psoriasis development, likely by suppressing IL-17-mediated signaling.
FXYD3 interacts with TRAF3
IL-17 signaling begins with the binding of IL-17A/F cytokines to their receptors IL-17RA and IL-17RC. Upon ligand binding, the heterodimeric receptor complex recruits the adaptor protein ACT1, which further recruits tumor necrosis factor receptor-associated factor 6 (TRAF6) to trigger the activation of NF-κB and mitogen-activated protein kinases (MAPKs) [20, 21]. Phosphorylated ACT1 also recruits TRAF2 and TRAF5 to mediate the mRNA stability of IL-17 target genes [22, 23]. The IL-17R-ACT1 complex is also associated with MEKK3 and MEKK5 via TRAF4 to activate ERK5, resulting in keratinocyte proliferation and tumor formation [24]. Intriguingly, recent progress has also shown that TRAF3 binds to the CBAD domain in IL-17RA, interferes with the IL-17R-Act1 interaction, and negatively regulates IL-17 signaling [25].
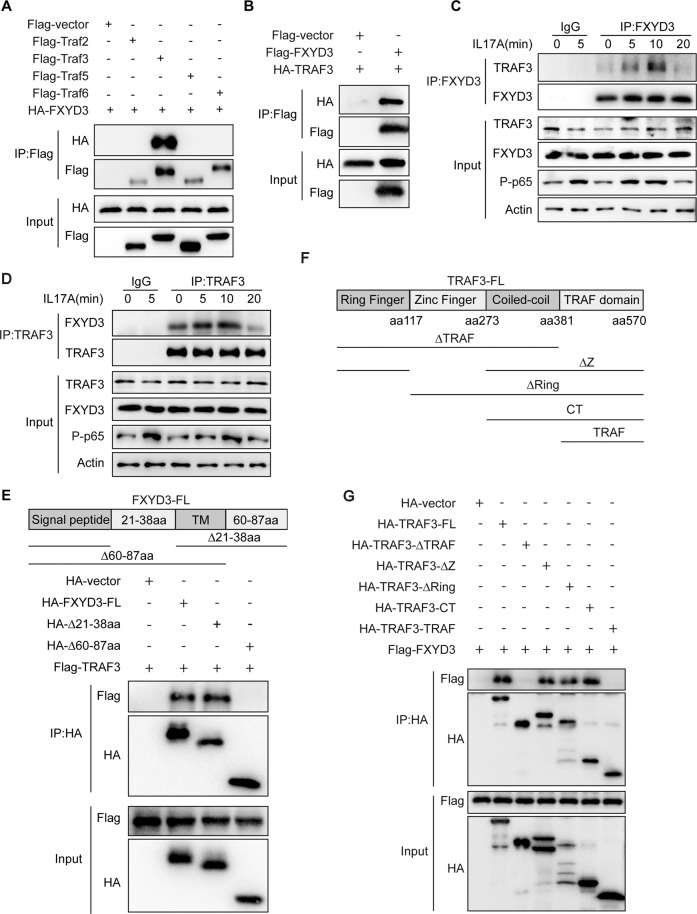
As our results showed that FXYD3 promotes IL-17-mediated signaling pathways, including those involving NF-κB and MAPKs, we next examined how FXYD3 influences IL-17 signaling. FXYD3 was immunoprecipitated from the lysates of HEK293T cells stably overexpressing IL-17 signaling-associated molecules to determine which proteins might interact with FXYD3. The results showed that FXYD3 physically associates with TRAF3 but not IL-17R, ACT1, TRAF2, TRAF4 or TRAF5 (Fig. 5A, B, Fig. S5A). Whole-cell lysates of HaCaT were immunoprecipitated with anti-FXYD3 or control IgG and western blotted with anti-TRAF3. Consistent with the results from the overexpression system, IL-17 stimulation also led to the association of FXYD3 with TRAF3 (Fig. 5C). We next immunoprecipitated TRAF3 with anti-TRAF3 or control IgG and checked the binding protein using FXYD3 antibodies. Similar results were noted in that TRAF3 interacted with FXYD3 in an IL-17 signaling-dependent manner (Fig. 5D). To examine which domain is required for their interactions with TRAF3, we made different deletion mutants of FXYD3 and performed coimmunoprecipitation. We found that the constructs containing the C-terminal domain (HA-∆21-38aa) associated with TRAF3, whereas constructs without the C-terminal domain (HA-∆60-87aa) did not, indicating that the C-terminal domain of FXYD3 is responsible for its interaction with TRAF3 (Fig. 5E). As TRAF3 contains ring finger, zinc finger, coil-coil and TRAF domains (Fig. 5F), we constructed different deletion mutants and examined the potential domain for TRAF3 to interact with FXYD3. We found that the coil-coil and TRAF domains were necessary for the interaction of TRAF3 with FXYD3 (Fig. 5G).
Fig. 5.
FXYD3 interacts with TRAF3. A Coimmunoprecipitation and immunoblotting of HEK293T cells transfected with HA-FXYD3 plasmid and Flag-empty vector or HA-FXYD3 together with Flag-TRAF2/3/5/6. B Flag-vector or Flag-FXYD3 was coexpressed with HA-TRAF3 in 293T cells and immunoprecipitated with anti-Flag beads. C, D Immunoblot analysis of protein immunoprecipitation (IP) with antibodies against FXYD3 or TRAF3 in HaCaT cells treated with IL-17A (100 ng/ml). E FXYD3 deletion mutants are shown in the top panel. HA-vector, HA-tagged FXYD3, HA-∆21-38aa or HA-∆60-87aa mutants were coexpressed with Flag-tagged TRAF3 in HEK293T cells. Whole-cell lysates were immunoprecipitated with anti-HA and then immunoblotted with anti-HA or anti-Flag. F, G HA-TRAF3 or its deletion mutants and Flag-FXYD3 were cotransfected into 293T cells. Whole-cell lysates were immunoblotted with anti-HA or anti-Flag antibodies. Data are representative of three independent experiments
FXYD3 enhances IL-17-mediated signaling by targeting TRAF3
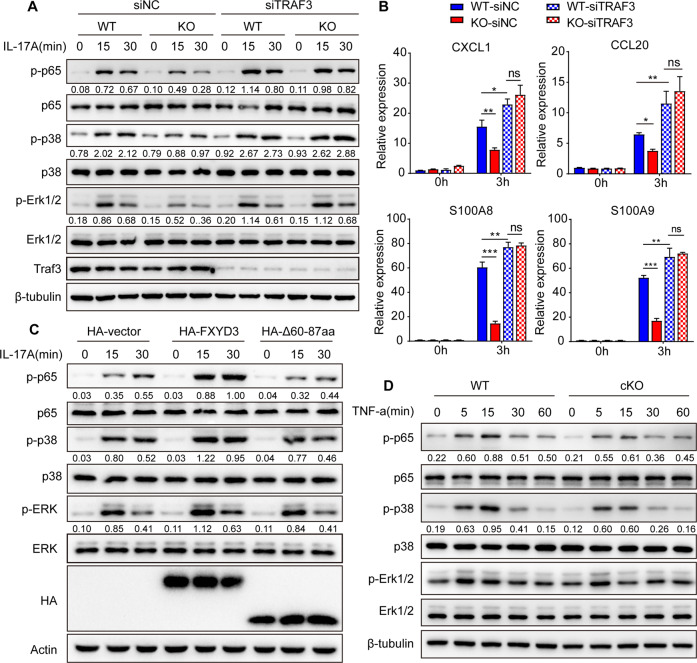
To further confirm whether FXYD3 positively regulates IL-17-mediated signaling by targeting TRAF3, we transfected control siNC or siTRAF3 into WT and cKO primary keratinocytes and stimulated them with IL-17A in vitro. Consistent with previous results [25], IL-17A-induced phosphorylation of p65, p38 and ERK was significantly increased in siTRAF3 cells compared with siNC cells (Fig. 6A). Under IL-17A stimulation, FXYD3 deficiency impaired the phosphorylation of p65, p38 and ERK induced by IL-17A in keratinocytes transfected with control siRNA but did not impair the phosphorylation of p65, p38 and ERK in keratinocytes transfected with TRAF3-specific siRNA (Fig. 6A). In addition, the knockout of FXYD3 diminished IL-17-induced CXCL1, CCL20, S100A8 and S100A9 expression in siNC primary keratinocytes but not in siTRAF3 primary keratinocytes (Fig. 6B). Given that the C-terminal domain of FXYD3 is responsible for its interaction with TRAF3, we reasoned that deletion of this domain in FXYD3 may fail to promote IL-17A signaling. To test this hypothesis, we transfected HeLa cells with HA-vector, HA-FXYD3 or HA-∆60-87aa and stimulated them with IL-17A (Fig. 6C). Indeed, we found that overexpression of FXYD3 increased IL-17-mediated signaling, whereas deletion of the C-terminal domain of FXYD3 failed to promote IL-17A signaling (Fig. 6C). TRAF3 has been shown to interact with TRAF2 or TNF receptor 2 (TNFR2) to inhibit TNF signaling [26, 27]. Therefore, we also examined whether FXYD3 regulates TNF-α signaling. WT and cKO primary keratinocytes were treated with TNF-α for 5, 15, 30, or 60 min. The activation of p65, p38 and ERK was reduced in cKO keratinocytes over all time periods (Fig. 6D). Taken together, these results showed that FXYD3 promotes IL-17 signaling by targeting TRAF3.
Fig. 6.
FXYD3 enhances IL-17-mediated signaling by targeting TRAF3. A WT and KO keratinocytes were transfected with si-negative or si-TRAF3 and stimulated with IL-17A (100 ng/ml). Western blot was preformed for p65, phospho-p65, p38, phospho-p38, ERK, and phospho-ERK. B WT and KO keratinocytes were transfected with si-negative or si-TRAF3 and stimulated with IL-17A (100 ng/ml) for 0 or 3 h. The expression of CXCL1, CXCL2, CCL20, S100A8 and S100A9 was analyzed by real-time PCR. C HeLa cells were transfected with HA-vector, HA-FXYD3 or its deletion mutant HA-∆60-87aa and then stimulated with IL-17A (100 ng/ml) for 15 or 30 min. Whole-cell lysates were immunoblotted with anti-p65, anti-phospho-p65, anti-p38, anti-phospho-p38, anti-ERK, anti-phospho-ERK, anti-HA and anti-actin antibodies. D WT and KO keratinocytes were stimulated with TNF-α (50 ng/ml), and immunoblot analysis was performed for p65, phospho-p65, p38, phospho-p38, ERK, and phospho-ERK. Error bars show the mean ± SEM. *, P < 0.05; **, P < 0.01; ***, P < 0.001. The P value was determined using two-way ANOVA (B). Data are representative of three independent experiments
FXYD3 competes with IL-17R to interact with TRAF3
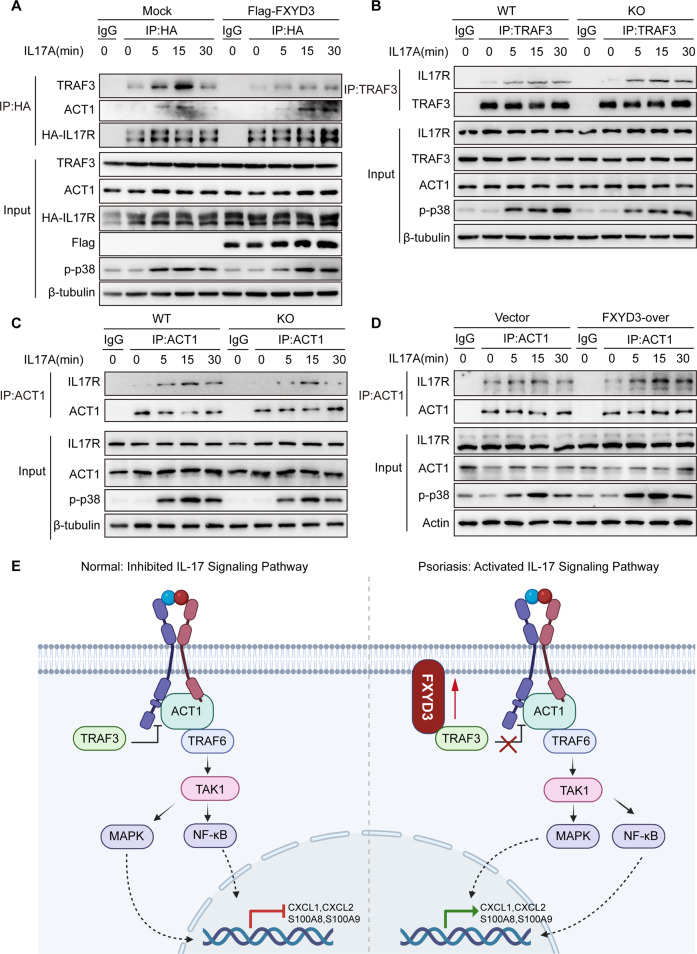
TRAF3 has been shown to act as an inhibitor of IL-17 signaling by competing with ACT1 to bind IL-17R and interfere with the IL-17R-ACT1 interaction [25]. We first checked whether FXYD3 interferes with the interaction between IL-17R and TRAF3 in IL-17 signaling. HeLa cell lines stably expressing HA-tagged IL-17R were transfected with Flag-vector or Flag-tagged-FXYD3-expressing plasmids, and whole-cell lysates were immunoprecipitated with anti-HA and control IgG. The overexpression of FXYD3 inhibited IL-17-induced interactions between IL-17R and TRAF3 but promoted the interaction between IL-17R and ACT1 (Fig. 7A). To confirm our results, we next used immunoprecipitation with either TRAF3 or ACT1 antibodies or IgG control in mouse primary keratinocytes to check the effects upon binding with IL-17R. Similarly, we found that TRAF3 could interact with IL-17R in an IL-17 signal-dependent manner, and the interaction was notably increased in FXYD3-KO primary keratinocytes (Fig. 7B). In addition, the interaction of IL-17R and ACT1 was inhibited in FXYD3-KO primary keratinocytes compared to WT primary keratinocytes (Fig. 7C). Consistent with this, the overexpression of FXYD3 in HaCaT cells increased the IL-17-induced interactions of ACT1 and IL-17R (Fig. 7D). To evaluate the distribution and expression level of TRAF3 in psoriasis, we collected skin tissues from psoriasis patients and healthy donors for immunohistochemistry staining. Immunohistochemistry staining showed (Fig. S6A) that TRAF3 was widely expressed in the epidermis and that the amount of TRAF3 was comparable in the epidermis of psoriasis patients and the healthy controls (Fig. S6B). Consistently, the expression level of TRAF3 did not show any significant difference in epidermis samples from WT-CTRL, cKO-CTRL, WT-IMQ and cKO-IMQ mice (Fig. S6C, D), indicating that FXYD3 has no effect on the expression of TRAF3. Taken together, our results suggest that FXYD3 interferes with the interaction of IL-17R and TRAF3 and promotes the formation of the IL-17R-ACT1 complex to play a positive role in IL-17A signaling.
Fig. 7.
FXYD3 competes with IL-17R to interact with TRAF3 and promote the formation of the IL-17R-ACT1 complex. A Flag-vector or Flag-FXYD3 was transfected into HeLa cells expressing HA-IL-17RA and stimulated with IL-17A (100 ng/ml). Whole-cell lysates were immunoprecipitated (IP) with anti-HA or control IgG and then subjected to immunoblot analysis with the indicated antibodies. B WT and KO keratinocytes were stimulated with IL-17A (100 ng/ml) for 0, 5, 15 or 30 min, and then whole-cell lysates were immunoprecipitated (IP) with anti-TRAF3 or control IgG. Whole-cell lysates were also immunoblotted with the indicated antibodies. C WT and KO keratinocytes were stimulated with IL-17A (100 ng/ml) for 0, 5, 15 or 30 min, and then whole-cell lysates were immunoprecipitated (IP) with anti-ACT1 or control IgG. Whole-cell lysates were also subjected to immunoblotting with the indicated antibodies. D HaCaT cells expressing Flag-vector or Flag-FXYD3 were stimulated with IL-17A (100 ng/ml) for 0, 5, 15 or 30 min. Whole-cell lysates were immunoprecipitated (IP) with anti-ACT1 or control IgG, followed by immunoblotting with the indicated antibodies. E A working model for the FXYD3 regulatory mechanism that promotes psoriasis. Data are representative of three independent experiments
Discussion
FXYD3, a family member of FXYD-domain-containing regulators of Na+/K+ ATPases, has been shown to be overexpressed in human and mouse breast tumors, as initiated by the neu and ras oncogenes [28], and to promote cellular proliferation in breast cancer, prostate cancer and pancreatic cancer [13, 29]. FXYD3 is also highly expressed in the epithelial cells of the skin. However, the role of FXYD3 in this context remains largely unknown. In this study, we demonstrated that FXYD3 exacerbates the development of psoriasis: FXYD3 competes with IL-17R to interact with TRAF3 and promotes the formation of the IL-17R-ACT1 complex, which then enhances IL-17 signaling in keratinocytes.
Psoriasis is a common, chronic skin disease mediated by both the innate and adaptive immune response in which keratinocytes, dendritic cells and T cells play important roles. Immunological and genetic studies have identified the IL-23/IL-17 axis as a key driver of psoriasis pathogenesis [4, 30]. As the main target cells for proinflammatory factors, keratinocytes responsively secrete a large amount of proinflammatory molecules and chemokines, which then play important roles in amplifying the immune response in lesional skin [31–33]. Our studies found that FXYD3 expression was significantly increased in the epidermis of patients with psoriasis compared to that of healthy controls. For patients with psoriasis, the level of FXYD3 was significantly decreased posttherapy after the application of a TNF inhibitor or an anti-IL-17 receptor antibody. Interestingly, increased FXYD3 expression levels were observed in NHEKs stimulated with cytokines such as IL-17A and TNF-α, which are known to promote psoriatic inflammation, suggesting the existence of a positive feedback loop between immune cells and keratinocytes. FXYD3 knockout in keratinocytes alleviated psoriasis-like skin inflammation in vivo, reduced the phosphorylation of p65, p38, ERK and JNK and decreased CXCL1, CCL20, S100A8, and S100A9 expression in vitro, clearly linking FXYD3 to IL-17A signaling. Furthermore, the IL-17 blocking antibody inhibited the psoriasis-like phenotype in WT mice but not in KO mice. Mechanistically, FXYD3 could interact with TRAF3, a negative mediator of IL-17A signaling, thus promoting the formation of the IL-17R-ACT1 complex; therefore, FXYD3 plays a necessary role in mediating a positive feedback loop to amplify inflammation.
Although the involvement of the IL-17 family in various autoimmune and inflammatory diseases has been studied, the role of the IL-17 cytokine family has also been a hot topic related to psoriasis [31, 34, 35]. IL-17R signaling mainly recruits the adaptor molecule ACT1 by a cytoplasmic motif known as the SEFIR domain to stimulate downstream pathways. IL-17RA additionally contains a C-EBP-β activation domain (CBAD), the function of which has been associated with the negative regulation of signaling. TRAF3 and A20, two signaling inhibitors, have similarly been reported to associate with the CBAD domain [25, 36]. Here, we found that FXYD3 specifically interacts with TRAF3 and positively regulates IL-17 signaling by targeting TRAF3. The use of deletion mutants confirmed that TRAF3 interacts with FXYD3 through the coil-coil and TRAF domains, while TRAF3 also binds to IL-17R via the TRAF domain. Our data suggest that FXYD3 and IL-17R compete with each other to associate with TRAF3.
TRAF3 plays a variety of context-dependent regulatory roles in many types of immune cells. TRAF3 has also been reported to interact with TRAF2 or TNFR2 to inhibit TNF signaling [27]. Correspondingly, the knockdown of TRAF3 increased the synergy of IL-17- and TNF-mediated induction of proinflammatory genes [25]. Here, we also showed that FXYD3 promotes TNF-α-mediated signaling in keratinocytes. TRAF3, as a poorly understood member of the TRAF family, plays essential roles in different signaling pathways, including the control of inflammation, antiviral immunity and cell survival [37]. However, the possibility that TRAF3 plays other roles in keratinocytes requires further exploration.
In summary, our study identified a novel role of FXYD3: FXYD3 regulates IL-17 signaling to promote the development of psoriasis. FXYD3 competes with IL-17R to interact with TRAF3, promoting the formation of the IL-17R-ACT1 complex. This further contributes to the activation of downstream signaling and the production of proinflammatory factors. On the other hand, IL-17A can upregulate FXYD3 in keratinocytes in psoriatic lesions, which contributes to an inflammatory circuit. Antagonists of IL-17 or blockade of IL-17RA has shown clinical efficacy in the treatment of psoriasis. However, targeting IL-17 has been associated with worsening or outbreaks of inflammatory bowel disease, and IL-17A blockers are also associated with an increased risk for mucocutaneous candidiasis [38]. More accurate drug targets still need to be developed, and our present study suggests that FXYD3 may have potential as a target for psoriasis treatment.
Supplementary information
Acknowledgements
We thank Prof Xiaojian Wang for sharing the plasmid. We are grateful to Yanwei Li, Jingyao Chen, Qiong Huang and Guifeng Xiao from the Core Facility of Zhejiang University School of Medicine for their technical support. We also thank Jian Wu from Zhejiang University Laboratory Animal Center for daily mouse breeding. We thank the Key Laboratory for Immunity and Inflammatory Diseases of Zhejiang Province, China for support. This work was supported by grants from the National Natural Science Foundation of China (91842103, 31870907, 81930041) and the Natural Science Foundation of Zhejiang Province (Z19H100001).
Author contributions
QW, LL, MX, and WY designed the experiments. WY, RH, and HQ conducted the experiments and prepared the figures. WL, YX, WL, TW, PZ, and MX contributed to all experiments; WY, LL, and QW wrote the paper. All authors read and approved the final paper.
Competing interests
The authors declare no competing interests.
Footnotes
These authors contributed equally: Wenjuan Yang, Rukun He, Hao Qu.
Contributor Information
Meng Xia, Email: xiameng.dream@zju.edu.cn.
Lihua Lai, Email: lailihua@zju.edu.cn.
Qingqing Wang, Email: wqq@zju.edu.cn.
Supplementary information
The online version contains supplementary material available at 10.1038/s41423-023-00973-7.
References
- 1.Greb JE, Goldminz AM, Elder JT, Lebwohl MG, Gladman DD, Wu JJ, et al. Psoriasis. Nat Rev Dis Prim. 2016;2:16082. doi: 10.1038/nrdp.2016.82. [DOI] [PubMed] [Google Scholar]
- 2.Kim J, Krueger JG. Highly Effective New Treatments for Psoriasis Target the IL-23/Type 17 T Cell Autoimmune Axis. Annu Rev Med. 2017;68:255–69.. doi: 10.1146/annurev-med-042915-103905. [DOI] [PubMed] [Google Scholar]
- 3.Lowes MA, Suarez-Farinas M, Krueger JG. Immunology of psoriasis. Annu Rev Immunol. 2014;32:227–55. doi: 10.1146/annurev-immunol-032713-120225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Ghoreschi K, Balato A, Enerbäck C, Sabat R. Therapeutics targeting the IL-23 and IL-17 pathway in psoriasis. Lancet. 2021;397:754–66.. doi: 10.1016/S0140-6736(21)00184-7. [DOI] [PubMed] [Google Scholar]
- 5.Lowes MA, Bowcock AM, Krueger JG. Pathogenesis and therapy of psoriasis. Nature. 2007;445:866–73. doi: 10.1038/nature05663. [DOI] [PubMed] [Google Scholar]
- 6.Lynde CW, Poulin Y, Vender R, Bourcier M, Khalil S. Interleukin 17A: toward a new understanding of psoriasis pathogenesis. J Am Acad Dermatol. 2014;71:141–50. doi: 10.1016/j.jaad.2013.12.036. [DOI] [PubMed] [Google Scholar]
- 7.Wang M, Zhang S, Zheng G, Huang J, Songyang Z, Zhao X, et al. Gain-of-Function Mutation of Card14 Leads to Spontaneous Psoriasis-like Skin Inflammation through Enhanced Keratinocyte Response to IL-17A. Immunity. 2018;49:66–79.e5. doi: 10.1016/j.immuni.2018.05.012. [DOI] [PubMed] [Google Scholar]
- 8.Matsumoto R, Dainichi T, Tsuchiya S, Nomura T, Kitoh A, Hayden MS, et al. Epithelial TRAF6 drives IL-17-mediated psoriatic inflammation. JCI Insight. 2018;3:e121175. [DOI] [PMC free article] [PubMed]
- 9.Ha HL, Wang H, Pisitkun P, Kim JC, Tassi I, Tang W, et al. IL-17 drives psoriatic inflammation via distinct, target cell-specific mechanisms. Proc Natl Acad Sci USA. 2014;111:E3422–31. doi: 10.1073/pnas.1400513111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Crambert G, Li C, Claeys D, Geering K. FXYD3 (Mat-8), a new regulator of Na,K-ATPase. Mol Biol Cell. 2005;16:2363–71. doi: 10.1091/mbc.e04-10-0878. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Geering K. FXYD proteins: new regulators of Na-K-ATPase. Am J Physiol Ren Physiol. 2006;290:F241–50. doi: 10.1152/ajprenal.00126.2005. [DOI] [PubMed] [Google Scholar]
- 12.Liu CC, Teh R, Mozar CA, Baxter RC, Rasmussen HH. Silencing overexpression of FXYD3 protein in breast cancer cells amplifies effects of doxorubicin and gamma-radiation on Na(+)/K(+)-ATPase and cell survival. Breast Cancer Res Treat. 2016;155:203–13. doi: 10.1007/s10549-015-3667-x. [DOI] [PubMed] [Google Scholar]
- 13.Kayed H, Kleeff J, Kolb A, Ketterer K, Keleg S, Felix K, et al. FXYD3 is overexpressed in pancreatic ductal adenocarcinoma and influences pancreatic cancer cell growth. Int J Cancer. 2006;118:43–54. doi: 10.1002/ijc.21257. [DOI] [PubMed] [Google Scholar]
- 14.Xue Y, Lai L, Lian W, Tu X, Zhou J, Dong P, et al. SOX9/FXYD3/Src Axis Is Critical for ER(+) Breast Cancer Stem Cell Function. Mol Cancer Res. 2019;17:238–49. doi: 10.1158/1541-7786.MCR-18-0610. [DOI] [PubMed] [Google Scholar]
- 15.Zheng Y, Danilenko DM, Valdez P, Kasman I, Eastham-Anderson J, Wu J, et al. Interleukin-22, a T(H)17 cytokine, mediates IL-23-induced dermal inflammation and acanthosis. Nature. 2007;445:648–51. doi: 10.1038/nature05505. [DOI] [PubMed] [Google Scholar]
- 16.Li F, Adase CA, Zhang LJ. Isolation and Culture of Primary Mouse Keratinocytes from Neonatal and Adult Mouse Skin. J Vis Exp. 2017;125:56027. [DOI] [PMC free article] [PubMed]
- 17.van der Fits L, Mourits S, Voerman JS, Kant M, Boon L, Laman JD, et al. Imiquimod-induced psoriasis-like skin inflammation in mice is mediated via the IL-23/IL-17 axis. J Immunol. 2009;182:5836–45. doi: 10.4049/jimmunol.0802999. [DOI] [PubMed] [Google Scholar]
- 18.Broggi A, Cigni C, Zanoni I, Granucci F. Preparation of Single-cell Suspensions for Cytofluorimetric Analysis from Different Mouse Skin Regions. J Vis Exp. 2016;110:e52589. [DOI] [PMC free article] [PubMed]
- 19.Prinz I, Sandrock I, Mrowietz U. Interleukin-17 cytokines: Effectors and targets in psoriasis-A breakthrough in understanding and treatment. J Exp Med. 2020;217:e20191397. [DOI] [PMC free article] [PubMed]
- 20.Amatya N, Garg AV, Gaffen SL. IL-17 Signaling: The Yin and the Yang. Trends Immunol. 2017;38:310–22. doi: 10.1016/j.it.2017.01.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Li X, Bechara R, Zhao J, McGeachy MJ, Gaffen SL. IL-17 receptor-based signaling and implications for disease. Nat Immunol. 2019;20:1594–602. doi: 10.1038/s41590-019-0514-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Sun D, Novotny M, Bulek K, Liu C, Li X, Hamilton T. Treatment with IL-17 prolongs the half-life of chemokine CXCL1 mRNA via the adaptor TRAF5 and the splicing-regulatory factor SF2 (ASF) Nat Immunol. 2011;12:853–60. doi: 10.1038/ni.2081. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Bulek K, Liu C, Swaidani S, Wang L, Page RC, Gulen MF, et al. The inducible kinase IKKi is required for IL-17-dependent signaling associated with neutrophilia and pulmonary inflammation. Nat Immunol. 2011;12:844–52. doi: 10.1038/ni.2080. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Wu L, Chen X, Zhao J, Martin B, Zepp JA, Ko JS, et al. A novel IL-17 signaling pathway controlling keratinocyte proliferation and tumorigenesis via the TRAF4-ERK5 axis. J Exp Med. 2015;212:1571–87. doi: 10.1084/jem.20150204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Zhu S, Pan W, Shi P, Gao H, Zhao F, Song X, et al. Modulation of experimental autoimmune encephalomyelitis through TRAF3-mediated suppression of interleukin 17 receptor signaling. J Exp Med. 2010;207:2647–62. doi: 10.1084/jem.20100703. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Cabal-Hierro L, Rodriguez M, Artime N, Iglesias J, Ugarte L, Prado MA, et al. TRAF-mediated modulation of NF-kB AND JNK activation by TNFR2. Cell Signal. 2014;26:2658–66. doi: 10.1016/j.cellsig.2014.08.011. [DOI] [PubMed] [Google Scholar]
- 27.He L, Grammer AC, Wu X, Lipsky PE. TRAF3 forms heterotrimers with TRAF2 and modulates its ability to mediate NF-{kappa}B activation. J Biol Chem. 2004;279:55855–65. doi: 10.1074/jbc.M407284200. [DOI] [PubMed] [Google Scholar]
- 28.Morrison BW, Leder P. neu and ras initiate murine mammary tumors that share genetic markers generally absent in c-myc and int-2-initiated tumors. Oncogene. 1994;9:3417–26. [PubMed] [Google Scholar]
- 29.Grzmil M, Voigt S, Thelen P, Hemmerlein B, Helmke K, Burfeind P. Up-regulated expression of the MAT-8 gene in prostate cancer and its siRNA-mediated inhibition of expression induces a decrease in proliferation of human prostate carcinoma cells. Int J Oncol. 2004;24:97–105. [PubMed]
- 30.Gaffen SL, Jain R, Garg AV, Cua DJ. The IL-23-IL-17 immune axis: from mechanisms to therapeutic testing. Nat Rev Immunol. 2014;14:585–600. doi: 10.1038/nri3707. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Xu M, Lu H, Lee YH, Wu Y, Liu K, Shi Y, et al. An Interleukin-25-Mediated Autoregulatory Circuit in Keratinocytes Plays a Pivotal Role in Psoriatic Skin Inflammation. Immunity. 2018;48:787–98.e4. doi: 10.1016/j.immuni.2018.03.019. [DOI] [PubMed] [Google Scholar]
- 32.Tonel G, Conrad C. Interplay between keratinocytes and immune cells-recent insights into psoriasis pathogenesis. Int J Biochem Cell Biol. 2009;41:963–8. doi: 10.1016/j.biocel.2008.10.022. [DOI] [PubMed] [Google Scholar]
- 33.Moos S, Mohebiany AN, Waisman A, Kurschus FC. Imiquimod-Induced Psoriasis in Mice Depends on the IL-17 Signaling of Keratinocytes. J Invest Dermatol. 2019;139:1110–7. doi: 10.1016/j.jid.2019.01.006. [DOI] [PubMed] [Google Scholar]
- 34.Vandeghinste N, Klattig J, Jagerschmidt C, Lavazais S, Marsais F, Haas JD, et al. Neutralization of IL-17C Reduces Skin Inflammation in Mouse Models of Psoriasis and Atopic Dermatitis. J Invest Dermatol. 2018;138:1555–63. doi: 10.1016/j.jid.2018.01.036. [DOI] [PubMed] [Google Scholar]
- 35.Lauffer F, Jargosch M, Baghin V, Krause L, Kempf W, Absmaier-Kijak M, et al. IL-17C amplifies epithelial inflammation in human psoriasis and atopic eczema. J Eur Acad Dermatol Venereol. 2020;34:800–9. doi: 10.1111/jdv.16126. [DOI] [PubMed] [Google Scholar]
- 36.Garg AV, Ahmed M, Vallejo AN, Ma A, Gaffen SL. The Deubiquitinase A20 Mediates Feedback Inhibition of Interleukin-17 Receptor Signaling. Sci Signaling. 2013;6:ra44. [DOI] [PMC free article] [PubMed]
- 37.Hacker H, Tseng PH, Karin M. Expanding TRAF function: TRAF3 as a tri-faced immune regulator. Nat Rev Immunol. 2011;11:457–68. doi: 10.1038/nri2998. [DOI] [PubMed] [Google Scholar]
- 38.Griffiths CEM, Armstrong AW, Gudjonsson JE, Barker JNWN. Psoriasis. Lancet. 2021;397:1301–15. doi: 10.1016/S0140-6736(20)32549-6. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.