Abstract
Background and Aims
The intestinal mucosa undergoes a continual process of proliferation, differentiation, and apoptosis. An imbalance in this highly regimented process within the intestinal crypts is associated with several intestinal pathologies. Although metabolic changes are known to play a pivotal role in cell proliferation and differentiation, how glycolysis contributes to intestinal epithelial homeostasis remains to be defined.
Methods
Small intestines were harvested from mice with specific hexokinase 2 (HK2) deletion in the intestinal epithelium or LGR5+ stem cells. Glycolysis was measured using the Seahorse XFe96 analyzer. Expression of phospho-p38 mitogen-activated protein kinase, the transcription factor atonal homolog 1, and intestinal cell differentiation markers lysozyme, mucin 2, and chromogranin A were determined by Western blot, quantitative real-time reverse transcription polymerase chain reaction, or immunofluorescence, and immunohistochemistry staining.
Results
HK2 is a target gene of Wnt signaling in intestinal epithelium. HK2 knockout or inhibition of glycolysis resulted in increased numbers of Paneth, goblet, and enteroendocrine cells and decreased intestinal stem cell self-renewal. Mechanistically, HK2 knockout resulted in activation of p38 mitogen-activated protein kinase and increased expression of ATOH1; inhibition of p38 mitogen-activated protein kinase signaling attenuated the phenotypes induced by HK2 knockout in intestinal organoids. HK2 knockout significantly decreased glycolysis and lactate production in intestinal organoids; supplementation of lactate or pyruvate reversed the phenotypes induced by HK2 knockout.
Conclusions
Our results show that HK2 regulates intestinal stem cell self-renewal and differentiation through p38 mitogen-activated protein kinase/atonal homolog 1 signaling pathway. Our findings demonstrate an essential role for glycolysis in maintenance of intestinal stem cell function.
Keywords: Intestinal Stem Cells, Glycolysis, HK2, Metabolism
Abbreviations used in this paper: 2-DG, 2-desoxyglucose; 4-OHT, 4-hydroxytamoxifen; ANG4, angiogenin 4; CGA, chromogranin A; DEFA, defensin; GFP, green fluorescent protein; HK2, hexokinase 2; IBD, inflammatory bowel disease; IF, immunofluorescence; ISC, intestinal stem cell; ISH, in situ hybridization; KO, knockout; LYZ, lysozyme; MAPK, mitogen-activated protein kinase; MUC2, mucin 2; OXPHOS, oxidative phosphorylation; PBS, phosphate-buffered saline; qPCR, quantitative real-time reverse transcription polymerase chain reaction; TAM, tamoxifen; WT, wild-type
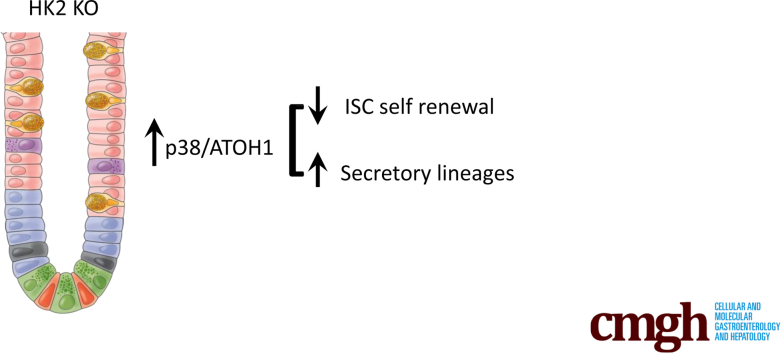
Graphical abstract
Summary.
Our findings demonstrate that glycolytic activity is necessary to maintain intestinal stem cell (ISC) self-renewal and that limiting glycolysis contributes to the generation of secretory lineages from ISCs. The inability to fine-tune glycolysis in ISCs subsequently disturbs the balance between self-renewal and differentiation.
The mammalian intestinal mucosa undergoes a dynamic process of constant and rapid renewal characterized by active proliferation of crypt-based stem cells localized near the base of the crypts.1 As these cells progress up the crypt-villus axis, there is cessation of proliferation and subsequent differentiation into 1 of 4 main primary cell types (enterocytes, goblet cells, Paneth cells, and enteroendocrine cells).1,2 An imbalance in this highly regimented and orderly process is associated with common intestinal pathologies, including colorectal cancer and inflammatory bowel disease (IBD), which cause significant morbidity and mortality.3,4
Metabolic changes play a pivotal role in regulating cell proliferation and differentiation.5 Normal tissue- and cell-specific metabolic pathways are tightly regulated during differentiation and perform unique functions in specific cell contexts.5 Compared with their differentiated progeny, stem cells are characterized by distinct metabolic activities with a higher glycolysis and a lower fraction of oxidative phosphorylation (OXPHOS).6 Moreover, Paneth cells provide lactate to support the high OXPHOS and mitochondrial activity in LGR5+ intestinal stem cells (ISCs) of small intestinal organoids.7 Previously, we showed that intestinal cell differentiation is associated with decreased glycolysis.8 However, it is not entirely known if and how the altered glycolysis affects the fate of discrete subtypes of intestinal epithelial cells and their molecular characteristics during differentiation, and this represents a major gap in the understanding of intestinal homeostasis. Elucidation of the effects of altered metabolism that governs development, survival, and function of discrete intestinal cell types may provide more suitable and selective therapeutic approaches for improving intestinal regeneration and function in patients.
Hexokinases (HKs) are critical enzymes regulating the irreversible bioconversion of glucose to glucose-6-phosphate in glucose metabolism. Four major HK isoforms, denoted as HK1, HK2, HK3, and HK4 (also known as glucokinase or GCK), are expressed in mammalian tissues.9 Clinical and animal studies indicate that HK2 likely plays a critical role in the pathogenesis of diseases, such as ischemic injury and cancers.10, 11, 12 In the intestine, HK2, a major contributor to increased glycolysis,13 is predominantly expressed by intestinal epithelial cells.14 Loss of epithelial HK2 protects against acute intestinal inflammation14; however, its overall function in ISC proliferation and differentiation remains poorly understood. Considering the importance of glycolysis in intestinal disorders, it is critical to better understand the exact roles that glycolysis and HK2 play in ISC function. Here, we provide evidence that HK2 plays an important role in regulation of ISC self-renewal and differentiation. Complete ablation of HK2 is embryonically lethal.15 Therefore, we used HK2f/f; Villin-creERT2 and HK2f/f; LGR5-EGFP-IRES-creERT2 conditional intestinal knockout (KO) models and found that HK2 contributes to the maintenance of ISC function.
Results
HK2 Is Regulated by Wnt Signaling in Intestinal Cells
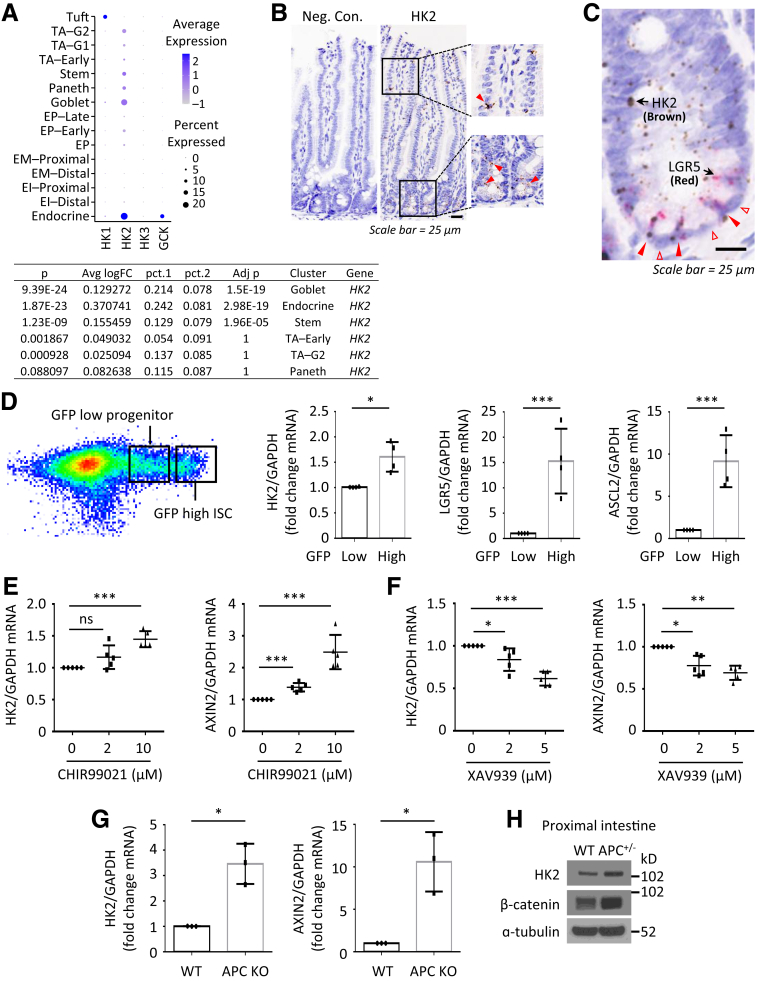
To determine the role of glycolysis in the intestine, we first determined the differential expression of HKs in intestinal cells. Analysis of single-cell transcriptome data of small intestine epithelial cells from Haber et al.16 demonstrated that HK2 was mainly expressed in goblet, enteroendocrine, and stem cells (Figure 1A). In contrast, HK1 and HK4 (GCK) are mainly expressed in Tuft and endocrine cells, respectively, whereas HK3 is less expressed in intestinal epithelium. These results suggest that HK2 is the main isoform expressed in the intestinal epithelium. Ubiquitously expressed HK2 was noted in crypts as shown by in situ hybridization (ISH) (Figure 1B). Moreover, dual ISH confirmed the concordance between LGR5 and HK2 expression in the intestine (Figure 1C). These data suggested a possible role for HK2 in the maintenance of goblet, enteroendocrine, and LGR5+ ISCs in the small bowel. To further analyze the concordance of LGR5 and HK2 expression, we isolated intestinal crypts from LGR5-EGFP-IRES-creERT2 knock-in mice by flow cytometry of intestinal cells with high green fluorescent protein (GFP) expression (LGR5-GFPhigh ISCs) and the more differentiated GFPlow progenitors.17 Similar to the expression pattern of ISC markers LGR5 and ASCL2, HK2 was mainly expressed in LGR5-GFPhigh ISCs (Figure 1D).
Figure 1.
HK2 is a target gene of Wnt signaling in intestinal cells. (A) Expression of HKs was analyzed by scRNA sequencing. “pct.1” is the percentage of HK2 expressing cells in the selected cell type (eg, Goblet). The “pct.2” is the percentage of HK2 expressing cells in the rest of cells (eg, all non-Goblet cells). EI, enterocyte immature; EM, enterocyte mature; EP, enterocyte progenitor. TA, transit amplifying; G1, G1/S cell-cycle phase; G2, G2/M cell-cycle phase. ( B) RNAscope brown staining of HK2 in intestinal crypts from WT mice. (C) HK2 is mainly expressed in LGR5-positive cells but not in Paneth cells. RNA scope duplex staining of HK2 (brown) with LGR5 (red) in WT jejunum crypts. (D) HK2 is more highly expressed in GFPhigh cells. Small intestine crypts from the LGR5-EGFP-IRES-creERT2 mice were harvested and digested with Typsin LE for 30 minutes to obtain single cells. GFPhigh and GFPlow cells were isolated by fluorescence-activated cell sorting. Expression of ASCl2, LGR5, and HK2 was determined by qPCR. Data represent mean ± SD (n = 4 mice). (E and F) Small intestinal crypts from jejunum of WT mice were harvested and seeded in 24-well plates. Organoids were treated with either vehicle control (DMSO) or Wnt agonist CHIR99021 (D) or Wnt antagonist XAV939 (E) for 3 days. Total RNA was extracted and expression of HK2 and AXIN2 was analyzed by qPCR (n = 5 mice). (G) Small intestinal crypts from APCf/f and APCf/f; Villin-creERT2 mice were harvested and seeded into 24-well plates and cultured for 24 hours followed by treatment with 4-OHT (1 mM) for 24 hours to induce knockout of APC expression. Four days after removing 4-OHT, total RNA was extracted and expression of HK2 and AXIN2 was determined by qPCR (n = 3 mice). (H) Western blotting analysis of protein expression in WT and APCf/+; Villin-cre mice. Intestinal mucosa from 3-month-old mice was collected for analysis. ∗P < .05; ∗∗P < .01; ∗∗∗P < .005.
Because Wnt signaling plays a critical role in ISC self-renewal and differentiation, we next used the organoid culture system to determine whether HK2 expression was regulated by Wnt signaling. Treatment with CHIR99021, a Wnt agonist,18 increased mRNA expression of HK2 and AXIN2, a well-known Wnt target gene (Figure 1E). In contrast, treatment with the tankyrase inhibitor, XAV939, which antagonizes Wnt,18 dose-dependently decreased HK2 and AXIN2 mRNA expression (Figure 1F). We next determined whether loss of APC could increase HK2 expression because APC mutation leads to hyperactivation of Wnt signaling.19 We isolated the crypts from APCf/f (wild-type [WT]) or APCf/f; Villin-creERT2 mice and cultured for growing organoids. The organoids were treated with 4-hydroxytamoxifen (4-OHT) to induce the deletion of APC. As shown in Figure 1G, APC deletion (APC KO) resulted in increased HK2 and AXIN2 mRNA expression. Similarly, the increased HK2 expression was further demonstrated in the intestinal epithelium isolated from APC f/+; Villin-cre mice (Figure 1H). These results demonstrate that HK2, which is mainly expressed in ISCs, is regulated by Wnt signaling, suggesting that HK2 may play a critical role in ISC self-renewal and differentiation.
HK2 Loss Increases Intestinal Cell Differentiation
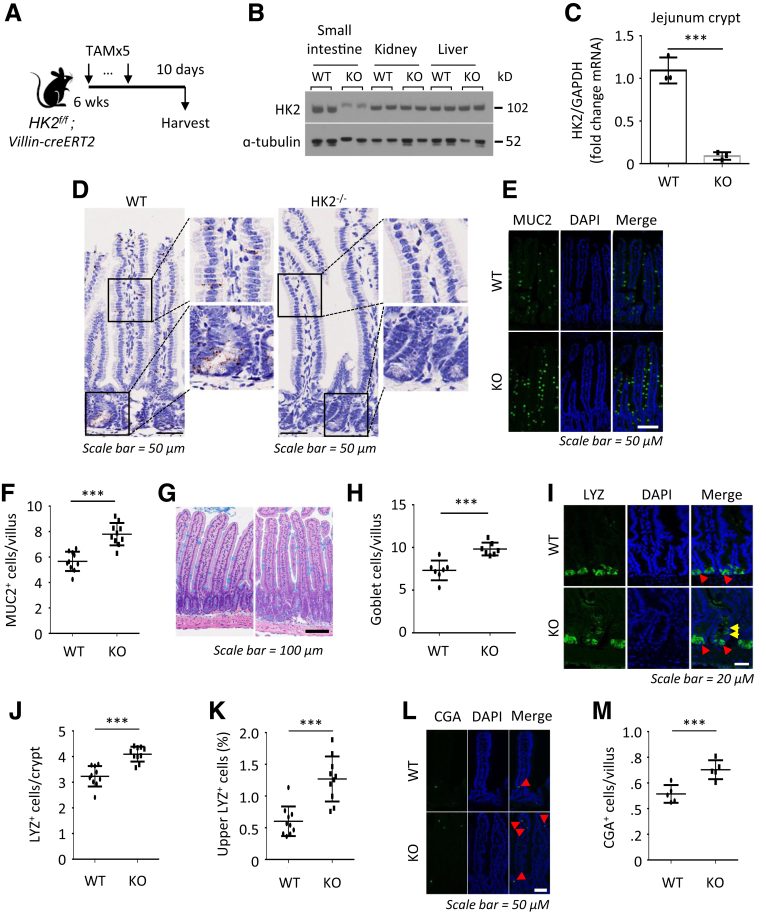
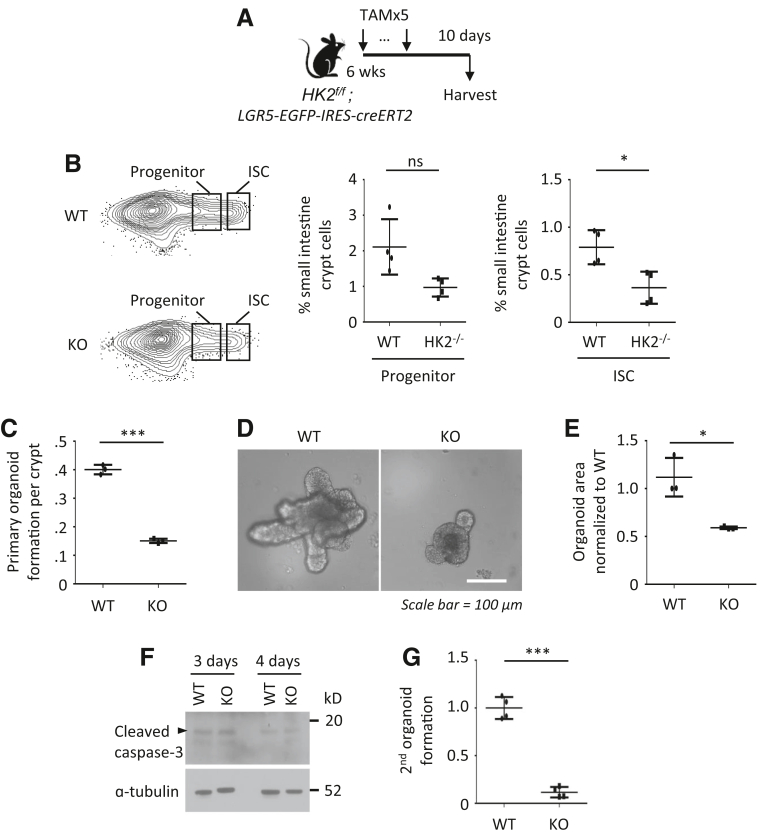
We have shown that intestinal cell differentiation is associated with decreased glycolysis.8 To determine the role of HK2 in the intestine, we crossed HK2f/f mice to Villi-creERT2 mice and engineered HK2f/f; Villin-creERT2, a conditional intestinal KO model that disrupts HK2 in all intestinal epithelial cell types on tamoxifen (TAM) administration. We administered 5 doses of TAM starting at postnatal day 45 to HK2 KO (HK2f/f; Villin-creERT2) and WT control mice (HK2f/f); 5 days post-TAM, intestinal tissues were harvested for analysis (Figure 2A).
Figure 2.
HK2 loss increases differentiation of secretory cells. (A) Schematic of HK2f/f; Villin-creERT2 mouse model, including the timeline of TAM injection and tissue collection. (B) Western blotting analysis of HK2 expression in small intestine mucosa, kidney, and liver tissues from WT and HK2 KO mice. (C) Expression of HK2 in WT and HK2 KO jejunum crypts was determined by qPCR (n = 3 mice). (D) The expression of HK2 in WT and HK2 KO mice was analyzed by ISH. (E) IF staining of jejunum from WT and HK2 KO mice with DAPI and MUC2. (F) Quantification of MUC2+ goblet cells in villus (n = 10 mice; at least 20 villi were counted from each mouse). (G) AB staining of jejunum of WT and HK2 KO mice. (H) Quantification of AB+ goblet cell in villi (at least 20 villi counted per mouse; n = 7 mice). (I) IF staining of jejunum from WT and HK2 KO mice with DAPI and LYZ. (J) Quantification of LYZ+ Paneth cells in crypt (n = 10 mice; at least 20 crypts were counted from each mouse). (K) Quantification of number of LYZ+ cells in the upper crypts based on LYZ IF staining (n = 10 mice; at least 20 crypts were counted from each mouse). (L) IF staining of jejunum from WT and HK2 KO mice with DAPI and CGA. (M) Quantification of CGA+ EE cell in crypts (n = 5 mice; at least 20 villi were counted from each mouse). ∗∗∗P < .005.
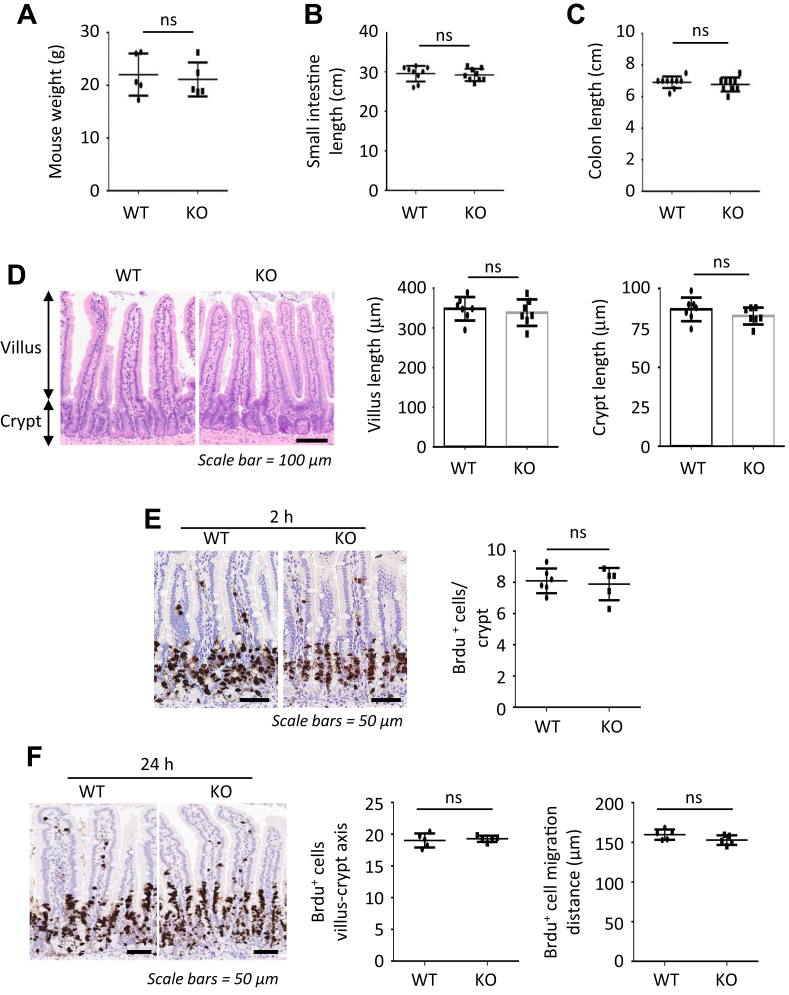
Administration of TAM specifically reduced HK2 expression in the intestinal epithelium as noted by Western blot (Figure 2B), quantitative real-time reverse transcription polymerase chain reaction (qPCR) (Figure 2C), and ISH (Figure 2D). Intestinal HK2 loss resulted in an increase in the number of goblet cells in the small bowel as noted by mucin 2 (MUC2) (Figure 2E and F) and Alcian blue staining (Figure 2G and H). Staining the intestinal sections from HK2 KO mice for lysozyme (LYZ) revealed an obvious increase in Paneth cells (Figure 2I–K). Moreover, the number of LYZ+ cells in the upper crypts of HK2 KO mice was also significantly increased (Figure 2I and K). Furthermore, increased chromogranin A (CGA)+ enteroendocrine cells were found in the small intestine of HK2 KO mice (Figure 2L and M). Thus, our studies indicate that HK2 KO results in ISC differentiation toward the secretory lineage. HK2 KO did not alter body weight (Figure 3A), colon and small intestinal length (Figure 3B and C), morphology of the small intestine and villus length or crypt depth (Figure 3D), and the proliferation as determined by Brdu incorporation (Figure 3E and F).
Figure 3.
Effects of HK2 loss on the intestinal architecture. (A) Body weight from WT and HK2 KO mice (n = 5 mice). (B) The length of small intestine was determined in WT and HK2 KO mice (n = 9 mice). (C) The length of colon was determined in WT and HK2 KO mice (n = 9 mice). (D) Hematoxylin-eosin staining of jejunum slices from WT and HK2 KO mice; the length of the villi and crypts was analyzed (at least 20 villi or crypts measured per mouse; n = 7 mice). (E and F) BrdU staining of jejunum of WT and HK2 KO mice and quantification of BrdU+ cell in villi and villius-crypt axis (at least 20 crypts or villius-crypt axis counted per mouse; n = 56 mice), 2 (E) or 24 (F) hours after BrdU administration.
Loss of HK2 Represses Intestinal Stemness
To further investigate the role of HK2 in regulating ISC maintenance, we generated a stem cell–specific HK2 KO mouse model HK2f/f; LGR5-EGFP-IRES-creERT2 mouse, where the LGR5 knock-in allele permits the isolation of LGR5-GFPhigh containing ISCs by flow cytometry and the deletion of HK2 in the GFPhigh subset of ISCs on TAM administration (Figure 4A). This model is often used to quantify GFPhigh containing ISCs and GFPlow progenitors.20, 21 We ablated HK2 in 6-week-old LGR5-GFP mice (Figure 4A) and intestinal cells with high GFP expression (Lgr5-GFPhigh ISCs) and lower GFP expression (Lgr5-GFPlow progenitors) were gated. HK2 loss decreased the frequency of LGR5-GFPhigh containing ISCs by 50% (Figure 4B).
Figure 4.
Loss of HK2 represses intestinal stemness. (A) Schematic of the mouse model, including the timeline of TAM injection and tissue collection. (B) Frequency of EpCAM+/PI-/Lgr5-GFPhigh ISCs and Lgr5-GFPlow progenitors in crypt cells from WT and HK2 KO mice by flow cytometry (n = 4 mice). (C–E) WT and HK2f/f; Villin-creERT2 mice were injected with 5 dosages of TAM, and small intestine crypts were collected. Organoid-forming assay is shown in C (n = 3 mice). The picture of organoids from WT and HK2 KO mice is shown in D. Quantification of the organoid area from WT and HK2 KO mice is shown in E (at least 30 organoids were measured per mouse; n = 3 mice per group). (F) The crypts from HK2f/f and HK2f/f; Villin-creERT2 mice were treated with 4-OHT for 24 hours to induce KO of HK2. Organoids were harvested 3 or 4 days after removal of 4-OHT, and cleaved caspase-3 was determined by Western blot. (G) HK2 KO decreases the secondary colony formation. The number of organoids per well (n = 4) were counted and expressed as a fold change compared with WT. ∗P < .05; ∗∗∗P < .005.
Intestinal organoids, generated from whole crypts, represent an established model system for studying intestinal development and maintenance. We next determined the effects of HK2 loss on growth of intestinal organoids. The activity of ISCs was assessed based on their ability to drive the formation of organoids.22,23 Crypts were isolated from WT control or HK2f/f; Villin-creERT2 mice at Day 5 after the last TAM injection and cultured to grow organoids. Organoids cultured from WT control mice continued to grow and underwent extensive budding. By contrast, HK2 deletion led to a decrease in crypt organoid-forming capacity (Figure 4C) and the formation of small organoids (Figure 4D and E) with no effect on cell death (Figure 4F). As an assessment of the renewal capacity of organoid-forming cells,24 we next tested whether HK2 was required for the formation of secondary organoids. Interestingly, HK2-deficient organoids generated secondary organoids at much lower rates than HK2 WT control organoids (Figure 4G). Our results suggest that HK2 loss represses ISC self-renewal (ie, fewer ISC numbers with less organoid-forming potential).
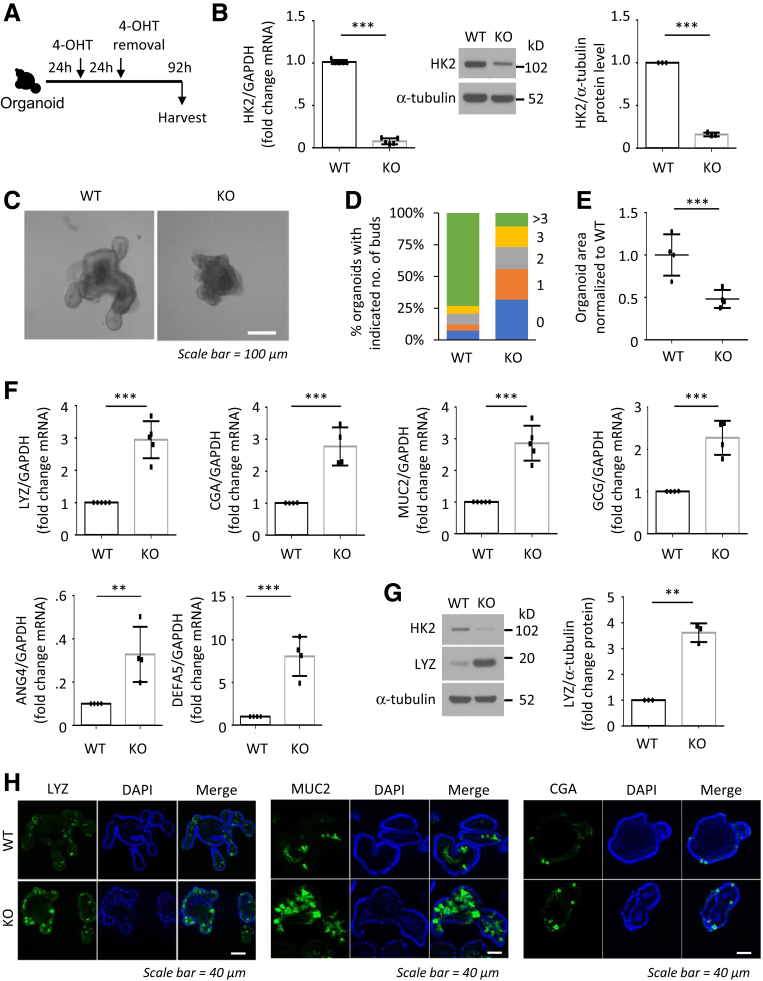
To further determine the role of HK2 in intestinal cell differentiation, crypts isolated from WT control or HK2f/f; Villin-creERT2 mice were cultured, and the organoids were treated with 4-OHT to induce HK2 deletion (Figure 5A). Treatment with 4-OHT results in the decreased expression of HK2 (Figure 5B), formation of small organoids, and decreased number of lobes per organoid (Figure 5C–E), another indicator of stem cell function.25 As observed in mouse intestine, HK2 deletion in organoids increased levels of many secretory cell markers, such as MUC2, LYZ, and CGA and GCG (Figure 5F-G). Paneth cells contain distinct cytoplasmic granules for exocytosis of antimicrobial peptides, such as LYZ, defensins (DEFA), and angiogenin 4 (ANG4). We next determined whether HK2 KO affects expression of ANG4 and DEFA alpha 5 (DEFA5). Consistent with the increased number of Paneth cells, increased expression of ANG4 and DEFA5 was noted in HK2 KO mice compared with WT mice (Figure 5F), further demonstrating that HK2 deficiency promotes Paneth cell differentiation. HK2 deletion in organoids increased number of secretory cell lineages was also demonstrated by IF staining (Figure 5H). Together, these data demonstrate that loss of HK2 in the small bowel not only represses ISC self-renewal but also shifts early differentiation within the crypt toward the secretory lineage (ie, greater numbers of Paneth, goblet, and enteroendocrine cells).
Figure 5.
HK2 deficiency promotes secretory cell differentiation in organoids. (A) Schematic of the organoid model, including the timeline of 4-OHT treatment and organoid collection. (B) HK2 expression was determined by qPCR (n = 5 mice) and Western blot (n = 3 mice). (C) Representative image of WT and HK2 KO organoids. (D) Quantification of the budding number of WT and HK2 KO organoids (at least 30 organoids were measured per mouse). One representative result from 3 biologic independent experiments is shown. (E) Organoid size derived from indicated genotypes was quantified (at least 30 organoids were measured per mouse; n = 4 mice). (F) qPCR analysis for MUC2, LYZ, ANG4, and DEFA5, CGA and GCG in organoids from indicated genotypes (n = 4–5 mice). (G) LYZ protein expression in WT and HK2 KO organoids determined by Western blot. LYZ signals from 3 mice were quantitated densitometrically and expressed as fold change with respect to α-tubulin. (H) IF staining of LYZ (left), MUC2 (middle), and CGA (right) in WT and HK2 KO organoids. ∗∗P < .01; ∗∗∗P < .005.
HK2 regulates ISC self-renewal and differentiation through the regulation of p38 mitogen-activated protein kinase (MAPK)/ATOH1 pathway
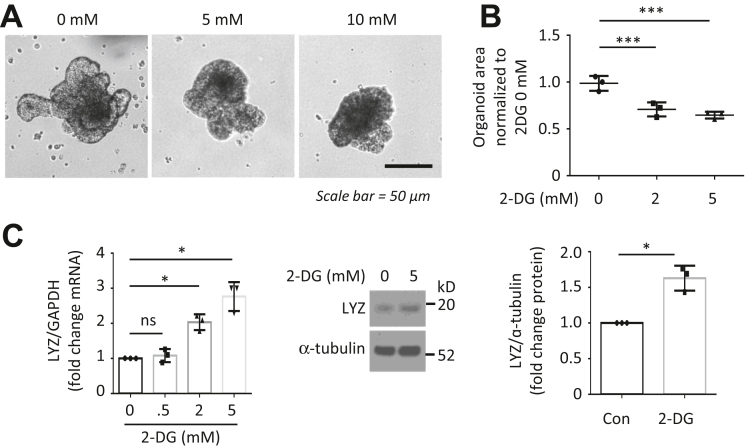
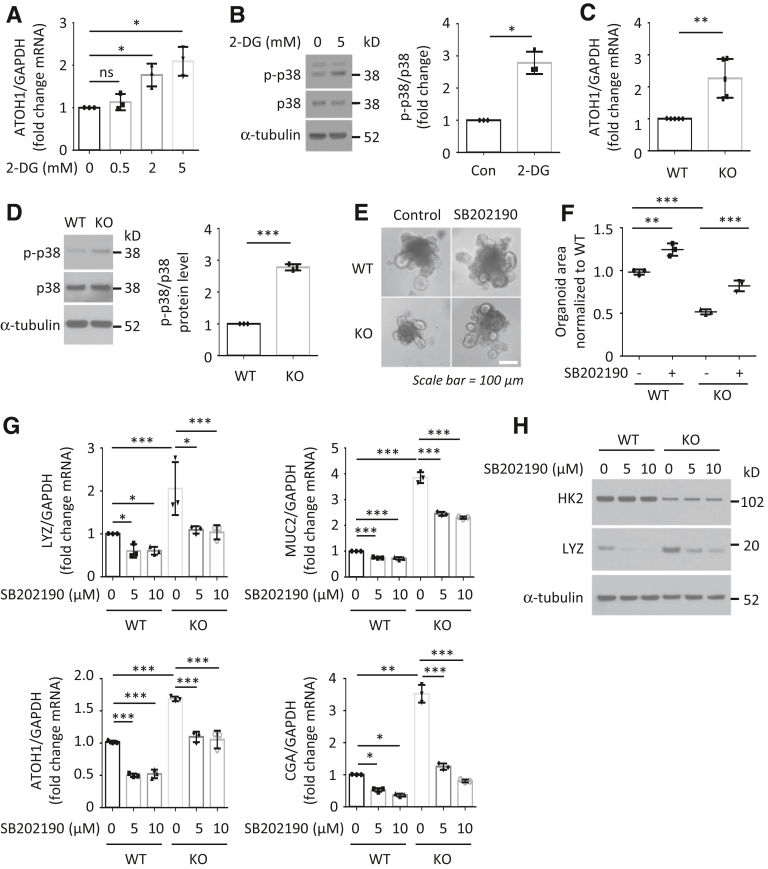
To determine the mechanisms involved in the regulation of ISC function by HK2/glycolysis, we used glycolysis inhibitor 2-desoxyglucose (2-DG). Similar to HK2 KO, treatment of organoids with 2-DG repressed organoid growth and increased differentiation (Figure 6A–C). Interestingly, we found that 2-DG treatment resulted in an increase in ATOH1 mRNA expression (Figure 7A). Because ATOH1 is required for differentiation of secretory cell lineages in the small bowel,26 our results suggest that glycolysis regulates secretory lineage generation through ATOH1. p38 MAPK alpha is required for secretory differentiation.27 Moreover, 2-DG has been shown to induce energy stress through the inhibition of glycolysis and subsequent activation of the p38 MAPK pathway in mouse embryonic fibroblast cells.28 We next determined whether inhibition of glycolysis using 2-DG affects the activation of p38 MAPK in intestinal cells. As shown in Figure 7B, treatment of intestinal cells with 2-DG resulted in the increased activation of p38 MAPK as noted by the increased phosphorylation of p38 MAPK. Consistent with results using 2-DG, we found that HK2 KO increased ATOH1 mRNA expression (Figure 7C) and p38 MAPK phosphorylation in HK2 KO organoids (Figure 7D). Therefore, these results demonstrate that HK2 regulates secretory cell differentiation through increased ATOH1 expression and activation of p38 MAPK.
Figure 6.
Treatment with glycolysis inhibitor 2-DG repressed organoid growth. Small intestinal organoids from WT mice were treated with 2-DG for 3 days. (A) Morphology of organoids. (B) Quantification of the organoid size from control and 2-DG treatment (at least 30 organoids were measured per mouse; n = 3 mice). (C) The expression of LYZ mRNA was detected by qPCR and Western blot (n = 3 mice). ∗P < .05; ∗∗∗P < .005.
Figure 7.
HK2 regulates ISC self-renewal and differentiation through the regulation of p38 MAPK/ATOH1 pathway. (A and B) Small intestine organoids from WT mice were treated with 2-DG for 3 days. Treatment with 2-DG increased ATOH1 mRNA expression (A) and p38 phosphorylation (B). Phosphorylated p38 MAPK expression from 3 separate experiments was quantitated densitometrically and expressed as fold change with respect to total p38 MAPK. (C and D) Crypts from HK2f/f and HK2f/f; Villin-creERT2 mice were treated with 4-OHT for 24 hours to induce KO of HK2. HK2 KO increased the expression of ATOH1 in organoids as noted in C (n = 5 mice). HK2 KO increased p38 phosphorylation in intestinal organoids as shown in D. Phosphorylated p38 MAPK signals from HK2 WT (n = 3 mice) and HK2 KO (n = 3 mice) were quantitated densitometrically and expressed as fold change with respect to total p38 MAPK. (E–H) Crypts from HK2f/f and HK2f/f; Villin-creERT2 mice were treated with 4-OHT for 24 hours to induce KO of HK2; the p38 inhibitor SB202190 was added together with 4-OHT for 24 hours and alone for an additional 4 days. Inhibition of p38 MAPK attenuated the decrease in organoid size on HK2 KO as noted in E. Quantification of the size of organoids derived from indicated genotypes is shown in F (at least 30 organoids per mouse were measured; n = 3 mice). Inhibition of the p38 pathway attenuated the increased expression of secretory markers in HK2 KO organoids as shown in G (n = 3 mice). Inhibition of the p38 pathway attenuated the increased LYZ protein expression in HK2 KO organoids as shown in H. ∗P < .05; ∗∗P < .01; ∗∗∗P < .005.
Because inhibition of p38 MAPK has been shown to repress ATOH1 expression in intestinal epithelial cells,27 we next tested whether activation of p38 MAPK mediates the phenotypes induced by HK2 KO. Organoids derived from HK2 KO mice showed decreased growth, which was rescued by addition of SB202190, a selective p38 MAPK inhibitor (Figure 7E and F). Moreover, HK2 KO increased expression of LYZ, MUC2, ATOH1, and CGA; this increase was blocked by inhibition of p38 MAPK (Figure 7G and H). Importantly, these results suggest that activation of p38 MAPK is required for HK2 KO repression of ISC self-renewal and promotion of ISC differentiation.
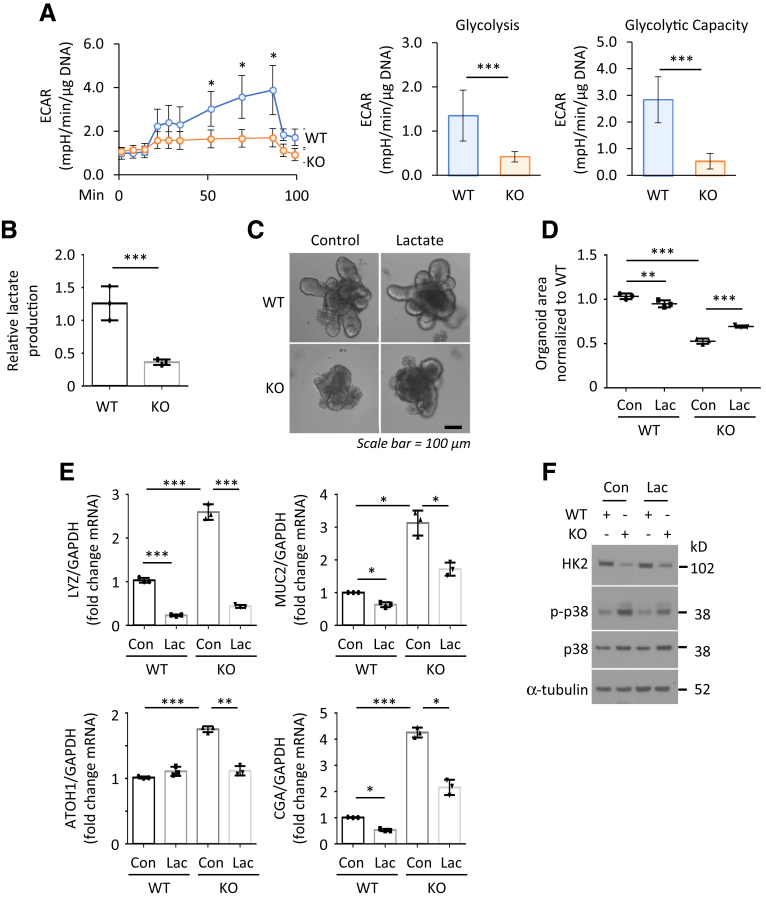
Lactate Reverses the Phenotypes Induced by HK2 Knockout
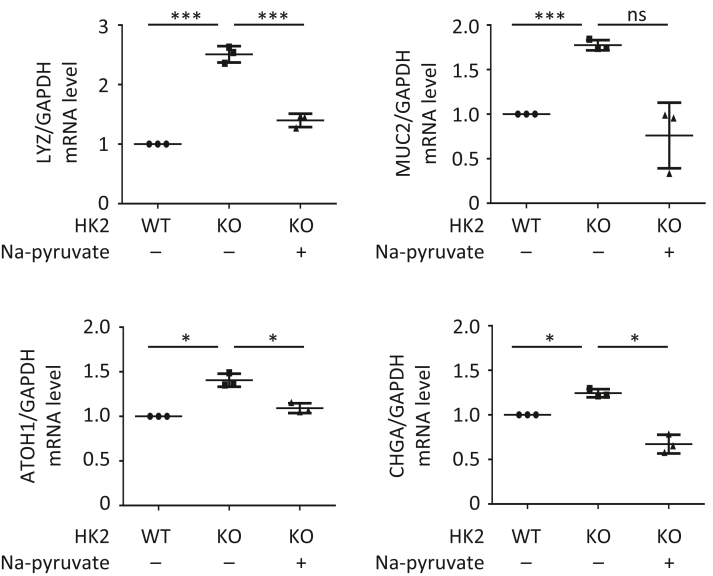
To further study the HK2/glycolytic activity involved in HK2-dependent regulation of ISC function, we next assessed glycolysis by measuring extracellular acidification rate in crypt organoids as described.29 As shown in Figure 8A, HK2 KO results in decreased extracellular acidification rate, which is associated with decreased glycolytic capacity. Consistent with these results, decreased lactate production was noted in HK2 KO organoids compared with HK2 WT organoids (Figure 8B), indicating impairment of glycolytic activity in organoids with HK2 deletion. We next tested whether supplementation of lactate could rescue the repressed organoid size and increased secretory lineages in the HK2 KO organoids. As shown in Figure 8C and D, supplementation of lactate partially restored the organoid size in the 4-OHT-induced HK2 KO organoids. We further observed that lactate attenuates or blocks the increased secretory lineage markers and ATOH1 expression in the HK2-deficient organoids (Figure 8E). Moreover, addition of lactate attenuates the increased p38 MAPK phosphorylation induced by HK2 KO in organoids (Figure 8F). Similar results were noted in organoids treated with pyruvate, another glycolytic product. Pyruvate attenuates or blocks the increased secretory lineage markers and ATOH1 expression in the HK2-deficient organoids (Figure 9). These results show that lactate/pyruvate treatment rescues the phenotypic consequences of HK2 deficiency in intestinal cells and further implicates a crucial role for HK2/glycolysis in promoting ISC self-renewal and repressing ISC differentiation.
Figure 8.
Lactate reverses the phenotypes induced by HK2 KO. (A) Seahorse analysis of intestinal WT and HK2 KO organoids (n = 4 wells). One representative result from 3 biologic independent experiments is shown. (B) The crypts from HK2f/f and HK2f/f; Villin-creERT2 mice were incubated, and organoids were treated with 4-OHT for 24 hours to induce KO of HK2. Three days after 4-OHT treatment, culture media was collected, and lactate in the media was measured and results normalized by total DNA amount (n = 3 mice). (C–F) The crypts from HK2f/f and HK2f/f; Villin-creERT2 mice were cultured and crypt organoids treated with 4-OHT for 24 hours to induce KO of HK2. Lactate (10 mM) was added to the medium together with 4-OHT for 24 hours and continuous treatment for an additional 4 days. Supplementation of lactate attenuated the decrease in organoid size induced by HK2 KO as shown in C. Quantification of the size of organoids derived from indicated genotypes is shown in D (at least 30 organoids were measured per mouse; n = 3 mice). Supplementation of lactate attenuated the increased expression of secretory markers in HK2 KO organoids as shown in E (n = 3 mice). Supplementation of lactate attenuated activation of p38 MAPK associated with HK2 KO as noted in the Western blot shown in F. ∗P < .05; ∗∗P < .01; ∗∗∗P < .001.
Figure 9.
Pyruvate reverses the increase of secretory cell differentiation induced by HK2 KO. The crypts from HK2f/f and HK2f/f; Villin-creERT2 mice were cultured and crypt organoids treated with 4-OHT for 24 hours to induce KO of HK2. Sodium pyruvate (10 mM) was added to the medium together with 4-OHT for 24 hours and continuous treatment for an additional 4 days. qPCR analysis for MUC2, LYZ, CGA, and ATOH1 mRNA expression in organoids from indicated genotypes (n = 3 mice). ∗P< .05; ∗∗∗P < .005.
Discussion
Metabolic changes are known to play a pivotal role in cell proliferation and differentiation.5 However, it is not entirely known if and how the altered glycolysis affects the fate of discrete subtypes of intestinal epithelial cells and their molecular characteristics during differentiation. The present study identifies HK2 as a critical enzyme for the maintenance of ISC homeostasis. Deleting HK2 in LGR5+ISCs repressed ISC stemness, and HK2 KO or inhibition of glycolysis increased the number of Paneth, goblet, and enteroendocrine cells and repressed ISC self-renewal. Moreover, HK2 regulates ISC self-renewal and differentiation through the regulation of the p38 MAPK/ATOH1 signaling pathway. Importantly, these findings demonstrate that fine-tuned glycolysis is critical for the maintenance of ISC function.
Our findings identify HK2 as a target gene of Wnt signaling in the intestinal epithelium. Activation of Wnt/β-catenin has been shown to promote glycolysis.30,31 Our results suggest that Wnt regulates glycolysis, at least in part, through regulation of HK2 expression. Moreover, Wnt signaling plays a critical role in ISC self-renewal and differentiation. The balance between self-renewal and differentiation in ISCs is regulated by Wnt signaling. We found that HK2 is mainly expressed in LGR5-GFPhigh ISCs compared with the more differentiated GFPlow progenitors. In addition, our findings show that HK2 contributes to ISC self-renewal and HK2 KO promotes ISC differentiation into Paneth cells, goblet cells, and enteroendocrine cells. Therefore, we show that HK2, as a target gene of Wnt signaling, mediates the function of Wnt signaling in the regulation of ISC self-renewal and differentiation.
Immature intestinal epithelial cells, a product of the ISCs, differentiate into absorptive or secretory cells. We found that loss of HK2 increased the expression of ATOH1, which is required for secretory lineage generation in the intestine,26 implying that HK2 regulates secretory cell differentiation through the regulation of ATOH1 expression. We also found that HK2 regulated ATOH1 through p38 MAPK signaling. Activation of p38 MAPK contributes to aging associated ISC exhaustion.32 p38 MAPK inhibition is required for long-term maintenance of human intestinal organoids.33 In addition, intestine-specific p38a KO mice showed a decreased secretory differentiation in intestine.27 Our findings identify p38 MAPK and ATOH1 as downstream signaling molecules of HK2 and reveal a critical role for HK2/p38 MAPK/ATOH1 pathway in the regulation of secretory lineage generation in the intestine.
Importantly, we demonstrate that HK2 deficiency promotes ISC differentiation resulting in increased secretory lineage cells. Recently, it has been shown that deletion of HK2, which is highly expressed in intestinal epithelium from patients with IBD, protects against dextran sulfate sodium–induced acute intestinal colitis in mice.14 However, the mechanisms involved in this protection are not clear. Alterations in intestinal epithelial cells subtypes, including reduced numbers of goblet and Paneth cells, are frequently observed in inflammatory conditions.34 Dysfunction of goblet and Paneth cells is often observed in patients with IBD and can contribute to disease pathogenesis.35, 36, 37, 38 In addition, Paneth cell defects contribute to the origin of gut inflammation in models of IBD,39 and Paneth cell dysfunction contributes to mucosal dysbiosis in patients with IBD and in mouse models.36,40,41 Mucus secreted from goblet cells serves as an important barrier to prevent pathogens from invading the mucosa.42 The abnormal differentiation of goblet cells results in the deficient synthesis and secretion of mucins and thus intestinal mucosal barrier dysfunction.42 Our findings suggest that deletion of HK2 may contribute to attenuate the dextran sulfate sodium–induced acute intestinal inflammation by promoting ISC differentiation into Paneth and goblet cell lineages. Although targeted HK2 inhibition can attenuate dextran sulfate sodium–induced acute intestinal inflammation, our results show that HK2 deletion can also repress ISC self-renewal, suggesting a limitation in the consideration of targeting HK2 as a therapeutic option for chronic inflammation.
Compared with the differentiated progeny cells, stem cells are often characterized by distinct metabolic activities with higher glycolysis and a lower fraction of mitochondrial OXPHOS.6 We showed that HK2 KO in ISCs decreased ISC self-renewal. Moreover, HK2 KO decreased glycolytic activity and lactate production in intestinal cells, whereas supplementation of lactate or pyruvate reversed these phenotypes induced by HK2 KO. Our results demonstrate that glycolysis in ISCs is critical for the maintenance of ISC function. Our study establishes a paradigm wherein glycolysis plays a direct role in controlling ISC fate. Previously, we have shown that intestinal cell differentiation is associated with decreased glycolysis.8 In agreement with our findings, other investigators have shown that proliferative cells in intestinal crypt are characterized by a glycolytic metabolic phenotype, whereas differentiated intestinal epithelium maintain an OXPHOS phenotype.43 Activating mitochondrial OXPHOS by substitution of glucose with galactose has shown to promote ISC differentiation.7 Together, these findings suggest that ISCs show a preference for glycolysis for self-renewal but shift to an OXPHOS-dependent metabolism driving differentiation.
Previously, Rodriguez-Colman et al.7 showed that OXPHOS in ISCs and glycolysis in Paneth cells are required to support ISC function; however, this study was limited by using only in vitro epithelial cell cultures. In addition, whether and how altered glycolysis in ISCs affects ISC self-renewal and differentiation is not known. In contrast, our study provides several lines of evidence demonstrating a critical role of glycolysis to directly promote ISC function: (1) HK2, a major contributor to increased glycolysis,13 is mainly expressed in ISC but not in Paneth cells; (2) specific deletion of HK2 in ISCs repressed ISC stemness; and (3) inhibition of glycolysis or specific deletion of HK2 in ISCs promotes ISC differentiation. Our results demonstrate that glycolysis in ISCs is required to support ISC function.
In conclusion, our findings demonstrate that glycolysis is necessary and sufficient to maintain ISC self-renewal and that limiting glycolysis contributes to the generation of secretory lineages from ISCs. The inability to fine-tune glycolysis in ISCs subsequently disturbs the balance between self-renewal and differentiation. Elucidation of the effects of altered glycolysis that govern the function of discrete intestinal cell types may provide more suitable and selective therapeutic approaches for improving intestinal regeneration and function in patients.
Materials and Methods
Mouse Strains
To investigate the necessity of HK2 in maintaining ISC functions, we obtained HK2f/f mice from Dr Nissim Hay44 (College of Medicine, University of Illinois at Chicago) and crossed them with LGR5-EGFP-IRES-creERT2 mice (B6.129P2-Lgr5tm1(cre/ERT2)Cle/J, Strain #008875) to generate HK2f/f; LGR5-EGFP-IRES-creERT2 mice. HK2f/f; Villin-creERT2 mice were generated by crossing HK2f/f mice to Villi-creERT2 mice (B6.Cg-Tg[Vil1-cre/ERT2]23Syr/J, Strain #020282). As control animals, we used littermate HK2f/f mice, referred to as WT mice. Apc15lox mice (B6.129P2-Apctm1Rsmi/RfoJ, Strain #029275) were crossed with Villin-creERT2 mice to generate APCf/f; Villin-creERT2 mice. APC/Villin-cre mouse colonies were established by mating Villin-cre mice (B6.Cg-Tg[Vil1-cre]1000Gum/J, Strain # 021504) with APC mice (C57BL/6-Apctm1Tyj/J, Strain #009045).
Mice were maintained on a 12 hours light/dark schedule in filter top isolators with autoclaved water under specific pathogen-free conditions and fed autoclaved standard laboratory chow ad libitum. Age- and gender-matched mice were used for all experiments. All animal procedures were conducted in accordance with National Institutes of Health guidelines and were approved by the University of Kentucky Institutional Animal Care and Use Committee.
Mouse Intestinal Crypt Isolation and Organoid Culture
Intestinal crypts and villi were isolated as previously reported.45 Briefly, mouse small intestine was opened longitudinally, washed with ice-cold phosphate-buffered saline (PBS) to remove the luminal contents, and cut into 2- to 4-mm pieces. The intestinal fragments were incubated in ice-cold PBS containing 10 mM EDTA for 60 minutes at 4°C. Crypts were released by shaking with ice-cold PBS. Washing in ice-cold PBS was repeated until most of the crypts were released, as determined by microscopic analysis. Crypt suspensions were passed through a 70-μm cell strainer and centrifuged at 300 × g for 5 minutes. Isolated crypts were mixed with Matrigel and cultured in IntestiCult Organoid Growth Medium (Mouse, stem cell tech Catalog # 06005) as described previously.7 Colony-forming efficiency was calculated by plating 200 crypts and assessing organoid formation 3 days after initiation of cultures as described.21 The organoid area was measured as described.46 Briefly, the surface area of organoids was measured by taking several random nonoverlapping photographs of organoids using a microscope. Photographs were analyzed using ImageJ software. Organoid perimeters for area measurements were defined manually. Measurements from HK2 WT organoids were compared with HK2 KO organoids to determine the relative area change. Secondary colony formation was determined as described.24 Briefly, the same number of crypts from HK2f/f and HK2f/f; Villin-creERT2 mice was cultured and treated with 4-OHT for 24 hours to induce KO of HK2. After removal of 4-OHT, crypt organoids were continually cultured for 4 days. Then, 1 well of organoids from WT and HK2 KO was incubated for 2 minutes in TrypLE Express (Invitrogen) to form fragments of ∼10–20 cells and then plated into 4 wells. The number of organoids recovered from each well was quantified.
Seahorse Analysis
Agilent Seahorse Analyzer was used to measure extracellular acidification rate in organoids as described.47 Briefly, intestinal crypts from HK2f/f and HK2f/f; Villin-creET2 mice were seeded in 24-well plates and treated with 4-OHT for 24 hours to induce HK2 deletion. Two days after 4-OHT treatment, the organoids were transferred to XFe96 cell culture microplates and cultured for an additional 24 hours before measurement. Extracellular acidification rate was measured on addition of glucose to induce glycolytic flux, oligomycin to induce the glycolytic reserve, and 2-DG to inhibit glycolysis. Extracellular acidification rate values per group were normalized to the total amount of DNA present in all wells of the according group.
In Situ Hybridization
ISH was carried out on the Ventana Discovery Ultra platform, using RNAScope VS Universal HRP (for single-plex) (Advanced Cell Diagnostics) or RNAScope VS Duplex Reagent (dual stain) (Advanced Cell Diagnostics) with RNAScope probes directed against Ms-HK2 and Ms-LGR5 (Advanced Cell Diagnostics). Staining conditions were optimized on the test tissue using RNAScope’s Ms-PPIB probe as a positive control, and DapB probe as negative control before staining with probes of interest.
Histology
Sections from WT and HK2 KO mice were stained with hematoxylin-eosin as described.48 Morphology was assessed in high-quality sections containing nonfragmented villi. Villus height and crypt depth were measured with Aperio ImageScope – Pathology Slide Viewing Software (Leica Biosystems Imaging, Inc, Vista, CA) as described.45 Briefly, crypt depth was measured as the length between the bottom of the crypts and the crypt-villus junction; villus height was determined by the length between the crypt-villus junction and the top of the villus. At least 20 well-oriented crypts and villi were counted per mouse by a single blinded observer.
Immunofluorescence, Immunohistochemistry, and Alcian Blue Staining
Immunofluorescence (IF), immunohistochemistry, and Alcian blue staining were performed as described previously.48 Tissues were processed for routine IF or immunohistochemistry staining using the following antibodies: rabbit anti-LYZ (Diagnostic BioSystems, Pleasanton, CA), anti-MUC2 (Thermofisher), and anti-CGA (Santa Cruz, Dallas, TX). Negative control animals (including no primary antibody or isotype-matched mouse IgG) were used in each assessment. Alcian blue staining was performed according to standard protocol using Alcian blue pH 2.5 Stain Kit (Dako, Carpinteria, CA).
BrdU Assay
Five days after the final TAM administration, mice were injected with 100 μL of BrdU (10 mg/mL, intraperitoneally) (BD Pharmingen) at 2 or 24 hours before sacrifice. A small section of jejunum tissues was processed for routine immunostaining. For proliferation, the percentage of BrdU-positive cells relative to total cell number per crypt or per villus-crypt axis was quantified. Migration distance was measured as the distance from the bottom of crypts to foremost BrdU positive enterocytes as described.49
Fluorescence-Activated Cell Sorting
Crypts were harvested from the proximal jejunum (∼10 cm) of LGR5-eGFP-IRES-creERT2 mice by 60-minute incubation with ice-cold EDTA/PBS (10 mM) and filtered through a 70-μm strainer. Crypts were dissociated by incubating with TypLE for 20 minutes (Gibco, Waltham, MA; 12604013) at 37o C. TrypLE was stopped by adding Advanced DMEM (Gibco; 12491015) containing 10% fetal bovine serum (Gibco; 10270106) and dissociated cells were passed through a 40-μm strainer. For EpCAM+ isolation, cells were stained with PI (Life Technologies) and anti-EpCAM antibody (eBioscience) and gated on GFPhigh (stem cells) and GFPlow (progenitors). Cell sorting was performed on a BD fluorescence-activated cell sorting Aria II System as described.50
Quantitative Real-Time Reverse Transcription Polymerase Chain Reaction Analysis
Total RNA was extracted and treated with DNase (Promega, Madison, WI). Synthesis of cDNA was performed using reagents in the TaqMan Reverse Transcription Reagents Kit (Applied Biosystems, Foster City, CA). TaqMan probe and primers were purchased from Applied Biosystems. qPCR was performed as described previously.8
Lactate Production Analysis
The crypts from HK2f/f and HK2f/f; Villin-creERT2 mice were collected and cultured. Crypt organoids were treated with 4-OHT for 24 hours to induce KO of HK2. After removal of 4-OHT, organoids were continually cultured for 2 days. Culture medium was replaced with the fresh medium 24 hours before organoid harvesting. The lactate concentration in the medium was measured using an EnzyChrom L-Lactate Assay Kit (BioAssay Systems ECLC-100). The DNA isolated from the organoids was used for normalization of lactate concentration.
Western Blot Analysis
Western blotting was performed as described.8 Antibodies to HK2 (Genetex), phospho-p38, p38, and α-tubulin (all from Cell Signaling) and LYZ (Diagnostic BioSystems) were used.
Reanalysis of Bulk RNA-Seq
We reanalyzed the single-cell transcriptome data of small intestine epithelial cells (EpCAM+, CD45-, TER-119-, and CD31-)16 containing 7216 cells. The Unique Molecular Identifier count matrix and cell cluster labels were retrieved from GSE92332. Unique Molecular Identifier counts were normalized by sequencing depth for each cell, multiplied by a scale factor 10,000, and then natural log transformed.51 Dot plots were used to visualize the expression of selected genes.51 Dot size represents the fraction of cells in the cluster that express the gene; color indicates the mean expression in expressing cells, relative to other clusters. Differential expression tests were carried out using FindAllMarkers function based on Wilcoxon rank sum test.51
Statistical Analysis
For in vitro experiments and in vivo studies, comparisons of HK2, LYZ+, MUC2+, and CGA+, extracellular acidification rate, morphology, IF, and immunohistochemistry between 2 groups were performed using 2-sample Student t test or analysis of variance for multiple groups with contrast statements. Adjustment in P values because of multiple pairwise testing between groups was performed using the Holm step-down procedure. Linear mixed models were used for in vivo studies comparing WT and HK2 KO to account for multiple observations from multiple crypts and villi per mouse. Normality and equality of variance assumptions for study end points were assessed to determine validity of statistical tests and models. Bar graphs represent mean ± standard deviation levels in each group. For in vitro studies, 3 replicates were used for each cell culture condition and each experiment was repeated at least 3 times. Representative data from the repeat experiments are presented. All data from animal samples with measurement of study end points were included in the analysis. All authors had access to the study data and have reviewed and approved the final manuscript.
Acknowledgements
The authors thank Dr Nissim Hay for providing HK2f/f mice; Donna Gilbreath for manuscript preparation; and the Biospecimen Procurement and Translational Pathology Shared Resource Facility, the Flow Cytometry & Immune Monitoring Shared Resource Facility, Redox Metabolism Shared Resource Facility, and the Biostatistics and Bioinformatics Shared Resource Facility of the University of Kentucky Markey Cancer Center (supported by National Cancer Institute grant P30 CA177558, to BME).
CRediT Authorship Contributions
Chang Li, PhD (Formal analysis: Equal; Investigation: Equal)
Yuning Zhou, MD (Formal analysis: Equal; Investigation: Equal)
Ruozheng Wei, PhD (Investigation: Equal)
Dana L. Napier, PhD (Investigation: Equal)
Tomoko Sengoku, PhD (Investigation: Equal)
Michael C. Alstott, PhD (Investigation: Equal)
Jinpeng Liu, PhD (Formal analysis: Equal)
Chi Wang, PhD (Formal analysis: Equal; Methodology: Equal)
Yekaterina Y. Zaytseva, PhD (Investigation: Equal)
Heidi L. Weiss, PhD (Formal analysis: Equal; Methodology: Equal)
Qingding Wang, PhD (Conceptualization: Equal; Formal analysis: Equal; Methodology: Equal; Visualization: Equal; Writing – original draft: Equal)
B. Mark Evers, MD (Conceptualization: Equal; Formal analysis: Equal; Funding acquisition: Lead; Methodology: Equal; Visualization: Equal; Writing – original draft: Equal)
Footnotes
Conflicts of interest The authors disclose no conflicts.
Funding This work was supported by National Institutes of Health grants R01 DK48498 (to BME) and P30 CA177558 (to BME). The funding agencies had no role in the study design, collection, analysis, or interpretation of data.
Contributor Information
Qingding Wang, Email: qingding.wang@uky.edu.
B. Mark Evers, Email: mark.evers@uky.edu.
References
- 1.Santos A.J.M., Lo Y.H., Mah A.T., et al. The intestinal stem cell niche: homeostasis and adaptations. Trends Cell Biol. 2018;28:1062–1078. doi: 10.1016/j.tcb.2018.08.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Yeung T.M., Chia L.A., Kosinski C.M., et al. Regulation of self-renewal and differentiation by the intestinal stem cell niche. Cell Mol Life Sci. 2011;68:2513–2523. doi: 10.1007/s00018-011-0687-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Gersemann M., Wehkamp J., Stange E.F. Innate immune dysfunction in inflammatory bowel disease. J Intern Med. 2012;271:421–428. doi: 10.1111/j.1365-2796.2012.02515.x. [DOI] [PubMed] [Google Scholar]
- 4.Hammoud S.S., Cairns B.R., Jones D.A. Epigenetic regulation of colon cancer and intestinal stem cells. Curr Opin Cell Biol. 2013;25:177–183. doi: 10.1016/j.ceb.2013.01.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Shyh-Chang N., Daley G.Q., Cantley L.C. Stem cell metabolism in tissue development and aging. Development. 2013;140:2535–2547. doi: 10.1242/dev.091777. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Wei P., Dove K.K., Bensard C., et al. The force is strong with this one: metabolism (over)powers stem cell fate. Trends Cell Biol. 2018;28:551–559. doi: 10.1016/j.tcb.2018.02.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Rodriguez-Colman M.J., Schewe M., Meerlo M., et al. Interplay between metabolic identities in the intestinal crypt supports stem cell function. Nature. 2017;543:424–427. doi: 10.1038/nature21673. [DOI] [PubMed] [Google Scholar]
- 8.Wang Q., Zhou Y., Rychahou P., et al. Ketogenesis contributes to intestinal cell differentiation. Cell Death Differ. 2017;24:458–468. doi: 10.1038/cdd.2016.142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Robey R.B., Hay N. Mitochondrial hexokinases, novel mediators of the antiapoptotic effects of growth factors and Akt. Oncogene. 2006;25:4683–4696. doi: 10.1038/sj.onc.1209595. [DOI] [PubMed] [Google Scholar]
- 10.Zhu W., Huang Y., Pan Q., et al. MicroRNA-98 suppress Warburg effect by targeting HK2 in colon cancer cells. Dig Dis Sci. 2017;62:660–668. doi: 10.1007/s10620-016-4418-5. [DOI] [PubMed] [Google Scholar]
- 11.Bustamante M.F., Oliveira P.G., Garcia-Carbonell R., et al. Hexokinase 2 as a novel selective metabolic target for rheumatoid arthritis. Ann Rheum Dis. 2018;77:1636–1643. doi: 10.1136/annrheumdis-2018-213103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Garcia S.N., Guedes R.C., Marques M.M. Unlocking the potential of HK2 in cancer metabolism and therapeutics. Curr Med Chem. 2019;26:7285–7322. doi: 10.2174/0929867326666181213092652. [DOI] [PubMed] [Google Scholar]
- 13.Roberts D.J., Miyamoto S. Hexokinase II integrates energy metabolism and cellular protection: Akting on mitochondria and TORCing to autophagy. Cell Death Differ. 2015;22:248–257. doi: 10.1038/cdd.2014.173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Hinrichsen F., Hamm J., Westermann M., et al. Microbial regulation of hexokinase 2 links mitochondrial metabolism and cell death in colitis. Cell Metab. 2021;33:2355–2366. doi: 10.1016/j.cmet.2021.11.004. [DOI] [PubMed] [Google Scholar]
- 15.Heikkinen S., Pietilä M., Halmekytö M., et al. Hexokinase II-deficient mice. Prenatal death of homozygotes without disturbances in glucose tolerance in heterozygotes. J Biol Chem. 1999;274:22517–22523. doi: 10.1074/jbc.274.32.22517. [DOI] [PubMed] [Google Scholar]
- 16.Haber A.L., Biton M., Rogel N., et al. A single-cell survey of the small intestinal epithelium. Nature. 2017;551:333–339. doi: 10.1038/nature24489. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Yilmaz O.H., Katajisto P., Lamming D.W., et al. mTORC1 in the Paneth cell niche couples intestinal stem-cell function to calorie intake. Nature. 2012;486:490–495. doi: 10.1038/nature11163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Telias M., Ben-Yosef D. Pharmacological manipulation of Wnt/β-catenin signaling pathway in human neural precursor cells alters their differentiation potential and neuronal yield. Front Mol Neurosci. 2021;14 doi: 10.3389/fnmol.2021.680018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.van Lidth de Jeude J.F., Meijer B.J., Wielenga M.C.B., et al. Induction of endoplasmic reticulum stress by deletion of Grp78 depletes Apc mutant intestinal epithelial stem cells. Oncogene. 2017;36:3397–3405. doi: 10.1038/onc.2016.326. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Cheng C.W., Biton M., Haber A.L., et al. Ketone body signaling mediates intestinal stem cell homeostasis and adaptation to diet. Cell. 2019;178:1115–1131. doi: 10.1016/j.cell.2019.07.048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Mihaylova M.M., Cheng C.W., Cao A.Q., et al. Fasting activates fatty acid oxidation to enhance intestinal stem cell function during homeostasis and aging. Cell Stem Cell. 2018;22:769–778. doi: 10.1016/j.stem.2018.04.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Li X.X., Zhang H.S., Xu Y.M., et al. Knockdown of IRE1alpha inhibits colonic tumorigenesis through decreasing beta-catenin and IRE1alpha targeting suppresses colon cancer cells. Oncogene. 2017;36:6738–6746. doi: 10.1038/onc.2017.284. [DOI] [PubMed] [Google Scholar]
- 23.Llado V., Nakanishi Y., Duran A., et al. Repression of intestinal stem cell function and tumorigenesis through direct phosphorylation of beta-catenin and Yap by PKCzeta. Cell Rep. 2015;10:740–754. doi: 10.1016/j.celrep.2015.01.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Stine R.R., Sakers A.P., TeSlaa T., et al. PRDM16 maintains homeostasis of the intestinal epithelium by controlling region-specific metabolism. Cell Stem Cell. 2019;25:830–845. doi: 10.1016/j.stem.2019.08.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Nalapareddy K., Nattamai K.J., Kumar R.S., et al. Canonical Wnt signaling ameliorates aging of intestinal stem cells. Cell Rep. 2017;18:2608–2621. doi: 10.1016/j.celrep.2017.02.056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Yang Q., Bermingham N.A., Finegold M.J., et al. Requirement of Math1 for secretory cell lineage commitment in the mouse intestine. Science. 2001;294:2155–2158. doi: 10.1126/science.1065718. [DOI] [PubMed] [Google Scholar]
- 27.Otsuka M., Kang Y.J., Ren J., et al. Distinct effects of p38alpha deletion in myeloid lineage and gut epithelia in mouse models of inflammatory bowel disease. Gastroenterology. 2010;138:1255–1265. doi: 10.1053/j.gastro.2010.01.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Zheng M., Wang Y.H., Wu X.N., et al. Inactivation of Rheb by PRAK-mediated phosphorylation is essential for energy-depletion-induced suppression of mTORC1. Nat Cell Biol. 2011;13:263–272. doi: 10.1038/ncb2168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Bas T., Augenlicht L.H. Real time analysis of metabolic profile in ex vivo mouse intestinal crypt organoid cultures. J Vis Exp. 2014;93 doi: 10.3791/52026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Pate K.T., Stringari C., Sprowl-Tanio S., et al. Wnt signaling directs a metabolic program of glycolysis and angiogenesis in colon cancer. EMBO J. 2014;33:1454–1473. doi: 10.15252/embj.201488598. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Kawada K., Toda K., Sakai Y. Targeting metabolic reprogramming in KRAS-driven cancers. Int J Clin Oncol. 2017;22:651–659. doi: 10.1007/s10147-017-1156-4. [DOI] [PubMed] [Google Scholar]
- 32.He D., Wu H., Xiang J., et al. Gut stem cell aging is driven by mTORC1 via a p38 MAPK-p53 pathway. Nat Commun. 2020;11:37. doi: 10.1038/s41467-019-13911-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Sato T., Stange D.E., Ferrante M., et al. Long-term expansion of epithelial organoids from human colon, adenoma, adenocarcinoma, and Barrett's epithelium. Gastroenterology. 2011;141:1762–1772. doi: 10.1053/j.gastro.2011.07.050. [DOI] [PubMed] [Google Scholar]
- 34.Jäger S., Stange E.F., Wehkamp J. Inflammatory bowel disease: an impaired barrier disease. Langenbecks Arch Surg. 2013;398:1–12. doi: 10.1007/s00423-012-1030-9. [DOI] [PubMed] [Google Scholar]
- 35.Adolph T.E., Tomczak M.F., Niederreiter L., et al. Paneth cells as a site of origin for intestinal inflammation. Nature. 2013;503:272–276. doi: 10.1038/nature12599. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Liu T.C., Gurram B., Baldridge M.T., et al. Paneth cell defects in Crohn's disease patients promote dysbiosis. JCI Insight. 2016;1 doi: 10.1172/jci.insight.86907. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Velcich A., Yang W., Heyer J., et al. Colorectal cancer in mice genetically deficient in the mucin Muc2. Science. 2002;295:1726–1729. doi: 10.1126/science.1069094. [DOI] [PubMed] [Google Scholar]
- 38.McGuckin M.A., Eri R.D., Das I., et al. Intestinal secretory cell ER stress and inflammation. Biochem Soc Trans. 2011;39:1081–1085. doi: 10.1042/BST0391081. [DOI] [PubMed] [Google Scholar]
- 39.Liu T.C., Kern J.T., Jain U., et al. Western diet induces Paneth cell defects through microbiome alterations and farnesoid X receptor and type I interferon activation. Cell Host Microbe. 2021;29:988–1001. doi: 10.1016/j.chom.2021.04.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Gulati A.S., Shanahan M.T., Arthur J.C., et al. Mouse background strain profoundly influences Paneth cell function and intestinal microbial composition. PLoS One. 2012;7 doi: 10.1371/journal.pone.0032403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Salzman N.H., Hung K., Haribhai D., et al. Enteric defensins are essential regulators of intestinal microbial ecology. Nat Immunol. 2010;11:76–83. doi: 10.1038/ni.1825. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Yang S., Yu M. Role of Goblet cells in intestinal barrier and mucosal immunity. J Inflamm Res. 2021;14:3171–3183. doi: 10.2147/JIR.S318327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Stringari C., Edwards R.A., Pate K.T., et al. Metabolic trajectory of cellular differentiation in small intestine by Phasor Fluorescence Lifetime Microscopy of NADH. Sci Rep. 2012;2:568. doi: 10.1038/srep00568. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Patra K.C., Wang Q., Bhaskar P.T., et al. Hexokinase 2 is required for tumor initiation and maintenance and its systemic deletion is therapeutic in mouse models of cancer. Cancer Cell. 2013;24:213–228. doi: 10.1016/j.ccr.2013.06.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Li C., Zhou Y., Rychahou P., et al. SIRT2 contributes to the regulation of intestinal cell proliferation and differentiation. Cell Mol Gastroenterol Hepatol. 2020;10:43–57. doi: 10.1016/j.jcmgh.2020.01.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Sorrentino G., Perino A., Yildiz E., et al. Bile acids signal via TGR5 to activate intestinal stem cells and epithelial regeneration. Gastroenterology. 2020;159:956–968. doi: 10.1053/j.gastro.2020.05.067. [DOI] [PubMed] [Google Scholar]
- 47.Ludikhuize M.C., Meerlo M., Gallego M.P., et al. Mitochondria define intestinal stem cell differentiation downstream of a FOXO/Notch axis. Cell Metab. 2020;32:889–900. doi: 10.1016/j.cmet.2020.10.005. [DOI] [PubMed] [Google Scholar]
- 48.Zhou Y., Rychahou P., Wang Q., et al. TSC2/mTORC1 signaling controls Paneth and goblet cell differentiation in the intestinal epithelium. Cell Death Dis. 2015;6:e1631. doi: 10.1038/cddis.2014.588. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Sun X., Yang Q., Rogers C.J., et al. AMPK improves gut epithelial differentiation and barrier function via regulating Cdx2 expression. Cell Death Differ. 2017;24:819–831. doi: 10.1038/cdd.2017.14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Zwiggelaar R.T., Lindholm H.T., Fosslie M., et al. LSD1 represses a neonatal/reparative gene program in adult intestinal epithelium. Sci Adv. 2020;6 doi: 10.1126/sciadv.abc0367. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Stuart T., Butler A., Hoffman P., et al. Comprehensive integration of single-cell data. Cell. 2019;177:1888–1902. doi: 10.1016/j.cell.2019.05.031. [DOI] [PMC free article] [PubMed] [Google Scholar]