Abstract
The majority of breast cancer patients is treated with breast-conserving surgery (BCS) combined with adjuvant radiation therapy. Up to 40% of patients has a tumor-positive resection margin after BCS, which necessitates re-resection or additional boost radiation. Cathepsin-targeted near-infrared fluorescence imaging during BCS could be used to detect residual cancer in the surgical cavity and guide additional resection, thereby preventing tumor-positive resection margins and associated mutilating treatments. The cysteine cathepsins are a family of proteases that play a major role in normal cellular physiology and neoplastic transformation. In breast cancer, the increased enzymatic activity and aberrant localization of many of the cysteine cathepsins drive tumor progression, proliferation, invasion, and metastasis. The upregulation of cysteine cathepsins in breast cancer cells indicates their potential as a target for intraoperative fluorescence imaging. This review provides a summary of the current knowledge on the role and expression of the most important cysteine cathepsins in breast cancer to better understand their potential as a target for fluorescence-guided surgery (FGS). In addition, it gives an overview of the cathepsin-targeted fluorescent probes that have been investigated preclinically and in breast cancer patients. The current review underscores that cysteine cathepsins are highly suitable molecular targets for FGS because of favorable expression and activity patterns in virtually all breast cancer subtypes. This is confirmed by cathepsin-targeted fluorescent probes that have been shown to facilitate in vivo breast cancer visualization and tumor resection in mouse models and breast cancer patients. These findings indicate that cathepsin-targeted FGS has potential to improve treatment outcomes in breast cancer patients.
Key words: Cysteine cathepsins, Breast cancer, Targeted molecular imaging, Fluorescence-guided surgery, Near-infrared fluorescence imaging
Introduction
To date, breast cancer remains the most frequently diagnosed cancer and the leading cause of cancer-related mortality in women worldwide, representing about 25% of all cancer cases and 15% of all cancer deaths [1]. Most newly diagnosed breast cancer patients can be treated with breast-conserving surgery (BCS) combined with adjuvant radiation therapy [2–5]. Although such treatment offers better cosmetic results and equivalent survival outcomes compared with total mastectomy, BCS is associated with an increased risk of tumor-positive resection margins [5–9]. Margin status in turn is a critical determinant of local recurrence and in some cases disease-specific mortality [7–11].
BCS based on visual and tactile feedback assisted by current localization techniques, such as implanted radioactive iodine seeds, still results in tumor-positive resection margin rates up to 40% [12–15]. A tumor-positive resection margin after BCS necessitates re-resection or boost radiation therapy, resulting in worse cosmetic outcomes and increased morbidity, complication risks, and healthcare costs [16–18]. Evidently, there is an unmet need for a method to detect tumor-positive margins at the time of surgery to guide immediate resection of residual tumor tissue and prevent additional mutilating treatments.
Numerous pathology and imaging methods for intraoperative guidance and margin assessment have been evaluated to decrease tumor-positive resection margin rates after BCS, but most have significant clinical and technical limitations that have precluded widespread adoption [15, 19]. An ideal method for margin assessment during BCS would be able to detect tumor-positive margins rapidly, non-invasively, in real-time with high spatial accuracy. A technique that could meet these requirements is intraoperative tumor-targeted near-infrared (NIR) fluorescence imaging (FI).
Tumor-targeted NIR fluorescence-guided surgery combines the administration of a contrast agent, consisting of a fluorophore and a targeting moiety, with the use of a fluorescence-sensitive camera, matched to operate in the range of NIR fluorescence light (700–900 nm) [20]. It allows for rapid, real-time optical imaging of large surface areas by selectively highlighting cells that overexpress certain molecular targets.
In BCS, tumor-targeted NIR FI for tumor-positive resection margin detection could be used both in vivo on the resection cavity surfaces and ex vivo on the resected specimen [21–23]. However, due to the soft, pliable nature of a resected breast cancer specimen, its geometry does no longer accurately correspond to that of the resection cavity [24]. Consequently, correlating an ex vivo detected tumor-positive margin to the in vivo location of the residual tumor that needs to be excised is extremely difficult. Therefore, detection of residual tumor tissue on the surgical cavity walls seems to be the most promising approach.
Multiple contrast agents directed at different molecular targets are under extensive investigation for NIR fluorescence-guided BCS [25]. However, the cathepsin-targeted contrast agents are the most developed subgroup that has been shown to enable in vivo margin assessment in breast cancer patients [21, 23, 26–29].
Cathepsins are an important group of proteolytic enzymes that play a major role in both normal cellular physiology and disease [30, 31]. They are categorized according to the catalytic amino acids in their active site as either serine (cathepsin A and G), aspartic (cathepsin D and E), or cysteine (cathepsin B, C, F, H, K, L, O S, V, W, and X) proteases. This review focuses on the largest and best-studied group in breast cancer, the cysteine cathepsins. The cysteine cathepsin family in humans comprises 11 members that are involved in numerous intra- and extracellular processes [32]. These proteases are synthesized as pro-enzymes that are activated under mildly acidic conditions. However, not all members are equally dependent on pH and they differ in cellular location, tissue expression, and substrate specificity [30, 33, 34].
Cysteine cathepsins are mainly, but not exclusively, located within the endo-lysosomal system, where they are crucial for lipid and protein metabolism, autophagy, and antigen presentation [35, 36]. In addition, extra-lysosomal cathepsins located in the nucleus and mitochondrial matrix contribute to cell-cycle control and apoptosis initiation [33, 37]. Secreted cysteine cathepsins have been shown to participate in extracellular matrix (ECM) remodeling by degrading abundant components such as collagen and fibrin [34]. Most cysteine cathepsins like B, C, F, H, and L are expressed ubiquitously and share a broad spectrum of substrates [33, 34]. In contrast, cathepsin K and S are rather substrate specific and are expressed by certain cell types only [38–40]. The key role cysteine cathepsins play in this broad range of biochemical processes indicates that these proteases are essential for normal tissue homeostasis.
Dysregulated activity of cysteine cathepsins is associated with various pathological conditions, including atherosclerosis, neurodegenerative disease, osteoporosis, arthritis, and cancer [41–46]. In numerous cancer types, increased cysteine cathepsin enzymatic activity drives tumor progression, proliferation, invasion, and metastasis through a variety of different mechanisms [47, 48]. Given their crucial contribution to protein catabolism, it is plausible that cancer cells utilize cathepsins to meet their increased metabolic need [30, 49–52]. Additionally, secreted cathepsins mediate ECM degradation to facilitate cancer invasion and dissemination [47, 49]. There is also increasing evidence that the proteolytic products of extracellular molecules targeted by cathepsins, such as receptors and cell adhesion molecules, can induce cancer promoting signaling cascades [49].
During neoplastic transformation, the normally tightly regulated activity of cysteine cathepsins is altered by gene amplifications and the formation of transcript variants [53]. Another interesting phenomenon frequently reported is a shift in ratio between cysteine cathepsins and their endogenous inhibitors, such as cystatins and stefins, resulting in cathepsin upregulation [54]. This aberrant cathepsin activity is not restricted to one particular cancer type and occurs in both tumor cells as well as tumor-associated cells such as fibroblasts, myoepithelial cells, endothelial cells, and various immune cells, particularly tumor-associated macrophages (TAMs) [53]. Their expression pattern in tumor tissue and the extent to which they are upregulated varies between different types of cancer, stressing the importance of research specific to cancer type [55].
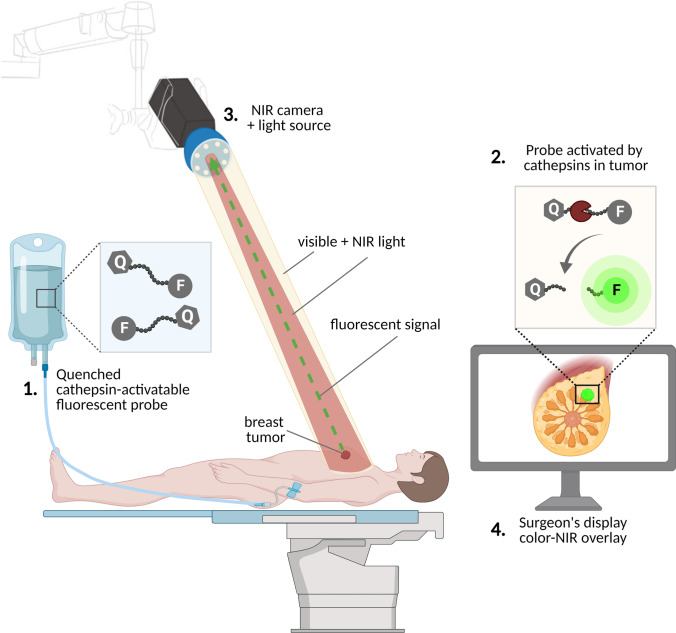
Over recent decades, it has been shown that various members of the cysteine cathepsin family have a more than tenfold overexpression and a more than 50-fold increase in enzymatic activity in breast cancer compared with healthy breast tissue [56–58]. Until recently, the expression of cysteine cathepsins in breast cancer has been assessed mainly for its prognostic value. However, because cysteine cathepsins are upregulated in many breast cancer subtypes, they are also extensively researched as molecular targets [54, 59]. The use of cathepsin-targeted NIR FI during breast cancer surgery could help to identify residual tumor on the surgical cavity surfaces and guide additional excision, thereby minimizing tumor-positive margins, the need for re-resection, and local recurrence (Fig. 1).
Fig. 1.
Cathepsin-targeted fluorescence-guided surgery. 1 A quenched, cathepsin-activatable fluorescent probe is administered intravenously prior to surgery or topically during surgery. 2 The probe is activated by cysteine cathepsins overexpressed by tumor and/or stromal cells. 3 The fluorescence signal generated by the activated probe is detected using a NIR sensitive camera system and 4 displayed on a screen in the operating theater. Q, quencher; F, fluorophore; pacman shape, cysteine cathepsin activating the probe; green dotted arrow, fluorescent signal generated by the activated probe in the tumor after illumination with NIR light from the camera system. Abbreviations: NIR, near infrared.
In this review, we first discuss the expression of the individual cysteine cathepsins in breast cancer to substantiate their potential use as a target for fluorescence-guided breast cancer surgery. The scope of this review is restricted to the most extensively investigated cysteine cathepsins B, C, K, L, O, S, and V. We summarize their specific role in cancer progression, cellular sources, and correlation to clinicopathological characteristics such as molecular subtype (Table 1). In addition, we will provide an overview of the cathepsin-targeted fluorescent probes that have been investigated for fluorescence-guided breast cancer surgery both preclinically and in patients.
Table 1.
Cysteine cathepsins in breast cancer
| Cathepsin | Main cellular source | Cellular localization | Specific role in metastasis to | Breast cancer subtype | Expression in increasing grade and stage | References |
|---|---|---|---|---|---|---|
| Cathepsin B |
Tumor cells Macrophages Fibroblasts Endothelial cells Myoepithelial cells |
Lysosomal Extracellular |
Lung Bone |
IBC HER2 + ER + PR + |
Increases | [56, 58, 60–83] |
| Cathepsin C |
Tumor cells (that metastasized to the lung) Leukocytes Fibroblasts |
Lysosomal | Lung |
ER + PR + HER2 + TNBC |
Unknown | [84–87] |
| Cathepsin K |
Tumor cells (that metastasized to the bone) Myofibroblasts |
Lysosomal Extracellular |
Bone |
ER + PR + HER2 + TNBC |
Increases | [38, 91–99] |
| Cathepsin L |
Tumor cells Macrophages Myofibroblasts |
Lysosomal Extracellular |
Lung |
ER + PR + HER2 + TNBC |
Increases | [56–58, 61, 66, 67, 95, 100–106, 108] |
| Cathepsin O | Tumor cells | Lysosomal | Unknown | ER + | Unknown | [109–112] |
| Cathepsin S |
Tumor cells Macrophages |
Lysosomal Extracellular |
Brain |
TNBC ER + PR + |
Unknown | [30, 33, 69, 95, 105, 113–117] |
| Cathepsin V |
Tumor cells Stromal fibroblasts |
Lysosomal | Unknown |
ER + HER2 + |
Increases | [33, 118–121] |
Shown are the cellular sources, cellular localization, specific roles, associated breast cancer subtypes, and the influence of grade and stage on expression for the different cysteine cathepsins discussed in this review
Abbreviations: IBC, inflammatory breast cancer; HER2, human epidermal growth factor receptor 2, ER, estrogen receptor; PR, progesterone receptor; TNBC, triple negative breast cancer
Cysteine Cathepsin Expression in Breast Cancer
Cathepsin B
Cathepsin B is one of the more ubiquitously expressed members of the cysteine cathepsin family that has been a main focus of research in breast cancer. It has both carboxypeptidase and endopeptidase activity and its substrates include ECM components such as laminin, fibronectin, collagen types I and IV, and proteoglycans [60]. Lah et al. were the first to report significantly higher cathepsin B levels in breast cancer tumors compared with matched normal breast tissue samples [58]. This was confirmed in additional studies, where reports detailed its increased activity across different histological and molecular subtypes of breast cancer [56, 58, 61–64]. Upregulation of this protease is a prognostic factor for (disease free) survival of breast cancer patients, and its enzymatic activity and level of expression have been shown to increase with advancing tumor grade [57, 65–67].
The cellular sources of cathepsin B in breast cancer are predominantly tumor cells and macrophages and, to a lesser extent, stromal components such as myoepithelial cells, fibroblasts, myofibroblasts, and endothelial cells of the neo-vasculature [61, 62]. Both in vitro and in vivo studies have demonstrated that the cellular origin of cathepsin B overexpression can change during tumor progression [68]. In the primary tumor, cathepsin B derived from tumor cells rather than macrophages promotes cancer progression, while at the metastatic site stromal-derived cathepsin B seems to be the main driver of tumor progression [68–70]. It is well established that expression is not confined to the lysosomal compartment, as translocation of cathepsin B to the cell membrane or secretion into the extracellular space during neoplastic transformation have been reported [71–74]. This redistribution is associated with malignant progression that could be related to exposure of the enzyme to a distinct set of substrates. Additionally, translocation to the cell membrane makes the protease easily accessible to cathepsin B-targeted probes.
The important contribution of cathepsin B to breast cancer progression becomes clear in cell line and murine model studies. Knockdown or selective inhibition of cathepsin B suppresses cancer invasion and metastasis to the lung and bone, whereas an increased cathepsin B expression and activity promotes metastatic spread [75–79].
Several underlying molecular mechanisms of cathepsin B overexpression and its contribution to cancer progression have been elucidated, some of which seem to be breast cancer subtype-specific. In human epidermal growth factor receptor 2 (HER2)-positive breast cancer, for instance, overexpression is the result of an increased transcription of the cathepsin B gene due to an HER2-activated kinase signaling network [80]. In hormonal receptor-positive breast cancer cells, interleukin 6, known for its stimulation of aromatase expression, has been shown to induce cathepsin B expression [81]. In vitro studies revealed a functional role of upregulated cathepsin B in pericellular proteolysis of the ECM and basement membrane components, driving tumor invasion [71, 82]. Interestingly, inflammatory breast cancer cells have been identified as particularly benefitting from cathepsin B abundance [56, 58, 61, 62]. Both inflammatory breast cancer cell lines and patient samples show elevated levels of cathepsin B, with the latter displaying a positive correlation with the number of lymph node metastases [83].
In conclusion, cathepsin B is upregulated in various breast cancer subtypes and has a broad spectrum of cellular sources at the primary and metastatic site. It plays a prominent role in many tumor-promoting processes and seems to be specifically implicated in breast cancer metastasis to the lung and bone. Jointly, the data suggest that the upregulation and partly extracellular localization of cathepsin B may be a prime target for imaging purposes.
Cathepsin C
Cathepsin C is a ubiquitously expressed lysosomal aminopeptidase required for the activation of pro-inflammatory neutrophil serine proteases such as elastase and proteinase 3, signifying its role as a mediator of inflammation [84]. Cathepsin C is necessary for normal mammary gland development [85]. During mammary carcinogenesis, cathepsin C expression and enzymatic activity is elevated, mainly in stromal cells like leukocytes and fibroblasts, but in tumor cells as well [86].
A wealth of information on the specific role of cathepsin C in breast cancer was derived from a series of experiments by Xiao et al., ascribing this enzyme a key role in lung metastasis [87]. In human cell lines derived from primary and metastatic tumors and in tissue samples of different molecular breast cancer subtypes, significantly higher cathepsin C levels in lung metastases than in primary breast tumors were observed. Additionally, human-transgenic mouse orthotopic breast cancer models revealed a higher lung metastasis burden in cathepsin C overexpressing mice and a significantly reduced lung metastasis capacity in the respective knockdown counterparts, indicating a potential therapeutic role for cathepsin C inhibition [87].
The functional role on a molecular level of cathepsin C is related, at least in vitro, to induction of signaling pathways that result in neutrophil recruitment and the formation of neutrophil extracellular traps (NETs)—web-like structures composed of granule proteins and decondensed chromatin that promote tumor progression and metastasis [87]. This is in line with the frequently observed exploitation of NETs by cancer cells, promoting their dissemination [88, 89]. The clinical relevance of these findings was highlighted by elevated neutrophil infiltration and NET formation in human lung metastasis as compared with the primary breast tumor and a positive correlation of these factors with cathepsin C expression [87]. Interestingly, the authors reported cathepsin C enzymatic activity and expression and the correlating NET formation to be higher in patients with triple-negative breast cancer (TNBC) compared to hormonal receptor-positive breast cancer.
To summarize, in breast cancer, cathepsin C holds a specialized role during the early stages of pulmonary colonization. By stimulating a signaling cascade leading to the activation and exploitation of neutrophils, this enzyme promotes cancer cell proliferation in the lung. The mainly lysosomal location of cathepsin C could hinder its accessibility to probes, making it less ideal as an imaging target [90]. On the other hand, increased cathepsin C activity and expression in tumor (and associated) cells of all breast cancer subtypes does make this protease a promising target for imaging of lung metastases.
Cathepsin K
Increasing evidence provides insight into how breast cancer cells take advantage of yet another member of the cathepsin family, cathepsin K. Cathepsin K is an endopeptidase that functions in the lysosomal and extracellular environment [91]. As a potent collagenase, this enzyme has a very specialized function during bone remodeling; thus, under physiological conditions, its expression and secretion are mainly limited to osteoclasts [38, 92]. However, an early study demonstrated that this protease is also expressed in primary breast cancer cells and bone metastases [93]. Subsequent studies confirmed its expression and increased enzymatic activity in bone-residing breast cancer cells and primary tumor cells, while demonstrating its absence in non-cancerous breast tissue and soft tissue metastases [91, 93–96]. Importantly, cathepsin K expression is consistently higher within bone-residing breast cancer cells than in the primary tumor, suggesting that this enzyme has a central role in creating the necessary microenvironmental conditions for breast cancer cells to metastasize to bone.
One of the molecular mechanisms through which cathepsin K contributes to breast cancer progression is by activation of ECM degrading matrix metalloproteinases, which enhances the invasiveness and metastatic capacity of breast cancer cells [97]. Additionally, secreted cathepsin K itself can degrade ECM components [98].
As for associations with other clinicopathological characteristics, assessment of both hormonal receptor-positive and -negative cell lines and patient samples at different stages imply a stage rather than receptor-dependent expression [91, 93, 95, 99].
To conclude, cathepsin K plays a major role in the metastatic spread of breast cancer cells to the bone. Its overexpression and increased enzymatic activity in bone-residing breast cancer cells and its extracellular localization indicates the potential associated with this protease for the imaging of bone metastases.
Cathepsin L
A considerable number of experiments have been performed to investigate the expression and role of cathepsin L in breast cancer. Cathepsin L is a lysosomal and extracellular endopeptidase whose expression is upregulated in breast cancer cells and TAMs, and which increases with advancing tumor grade [58, 61, 67]. Its overexpression and increased enzymatic activity have been observed across various breast cancer subtypes and is a prognostic factor for (disease free) survival of breast cancer patients [56, 57, 61, 66, 67, 95, 100–102]. Cathepsin L has been shown to play a major role in the process of lung metastasis [101, 103, 104]. In vitro ribonucleic acid (RNA) interference and pharmacological inhibition studies demonstrated that breast cancer cells use cathepsin L to enhance their proliferative, invasive, and migratory capacity by degrading ECM components[101–106]. This effect was also observed in vivo, as mice injected with cathepsin L knockdown breast cancer cells or treated with a selective cathepsin L inhibitor exhibited significantly smaller tumors compared to the controls with functional cathepsin L [101, 103, 105].
One of the mechanisms by which cathepsin L is upregulated is the loss of stress-induced shutdown of selective messenger RNA (mRNA) translation [103]. Under physiological conditions, cellular stress conditions, such as hypoxia, induce general shutdown of protein biosynthesis [107]. Due to tumor-associated resistance to stress conditions, breast cancer cells can maintain high levels of cathepsin L. This aberrant cathepsin L activity in turn results in upregulation of the mammalian target of rapamycin (mTOR), a key regulator of tumor cell proliferation, tumor growth, survival, and angiogenesis [102]. In addition, it has been shown that the p53 gene is activated by tumor-associated stress conditions, resulting in the downregulation of cystatins and, as a consequence, increased cathepsin L activity [108].
In conclusion, across a variety of breast cancer subtypes significant overexpression and amplified enzymatic activity of cathepsin L is observed, which increases with advancing grade and stage and has an important role during lung metastasis. It has also been established that breast cancer cells can maintain high cathepsin L levels, prioritizing its expression during stress conditions. Both cathepsin L upregulation in breast cancer cells of different molecular subtypes and its partly extracellular localization make cathepsin L a suitable target for intraoperative fluorescence imaging of breast cancer.
Cathepsin O
Currently, only a limited number of studies have been conducted to examine the expression and role of cathepsin O in breast cancer. Nevertheless, these few studies show that cathepsin O is highly expressed by breast cancer cells [109]. The overexpression is associated with estrogen receptor (ER) status and a decreased response tamoxifen. Genome-wide association studies found overexpression of cathepsin O is caused by variants of small nucleotide polymorphisms (SNP) near the CSTO gene [109–112]. Upregulation of cathepsin O has been shown to facilitate downregulation of the breast cancer 1 gene (BRCA1), by activating protein degradation pathways and through modulation of transcription regulators. Since BRCA1 is important for deoxyribonucleic acid (DNA) double-strand break repair, the upregulation of cathepsin O contributes to the neoplastic transformation of breast cancer cells [109–112].
Its expression in ER-positive breast cancer cells suggests cathepsin O could be a suitable target for tumor imaging. However, cathepsin O expression and activity in tumor cells versus normal breast tissue, its cellular sources and localization should first be more extensively investigated for all breast cancer subtypes.
Cathepsin S
Cathepsin S is a lysosomal and extracellular endopeptidase [30, 33]. Its overexpression and increased enzymatic activity have been reported in hormone responsive cell lines, yet it is expressed to a much higher extent in the more aggressive TNBC subtype [95, 113–115]. Tumor cells and tumor-stromal macrophages have been identified as the main cellular sources, whereas increased stromal expression is associated with higher tumor grade [115, 116].
Evidence for the contribution of this protease to tumor progression is reflected by the profound impact of knockdowns and pharmacological inhibition in TNBC cell lines as well as in animal models [105, 114, 116]. These studies show that interference with cathepsin S activity using small-interfering RNA or targeted inhibition prevents invasion and metastasis of breast cancer cells. This is consistent with impeded invasive and migratory capacity in highly metastatic cell lines upon cathepsin S inhibition [105].
Cathepsin S has been shown to have a prominent role in orchestrating breast to brain metastasis [114, 116]. During the early stages of brain metastasis, the main cellular source for cathepsin S are tumor cells, with limited stromal cell contribution. This pattern shifts during late stages, where stromal cells become the main cellular source and tumor-derived cathepsin S expression decreases. However, the experimental downregulation of both sources is required to limit metastasis. The underlying molecular mechanism of cathepsin S-driven brain metastasis is the proteolysis of the junctional adhesion molecule B, expressed on the blood–brain barrier. Breast tumor cells equipped with cathepsin S are capable of migrating across this strict and highly selective barrier by enabling the cleavage of these restricting junction proteins [116].
Another mechanism by which cathepsin S contributes to breast cancer progression is the proteolytic degradation of the aforementioned BRCA1, resulting in suppressed DNA double-strand break repair activity [117]. Contrary to expectation, this does not make breast cancer cells more susceptible to chemotherapy. In fact, cathepsin S overexpression in the tumor seems to inhibit the effects of chemotherapy on breast cancer cells, possibly by enabling TAMs to provide survival signals [69].
Taken together, these studies indicate the relevance of cathepsin S in the progression of breast cancer cells, especially TNBC, and show its contribution to brain metastasis by facilitating the crossing of the blood–brain barrier. Increased cathepsin S expression and activity in breast cancer and its part extracellular localization would make this enzyme a perfect target for the tumor imaging of TNBC and its brain metastases.
Cathepsin V
Cathepsin V is a mainly lysosomal endopeptidase that shares structural similarities with cathepsin L; however, it exerts distinct functions and has a confined tissue distribution [33]. Under physiological conditions, this protease is primarily expressed within the thymus, testis, and corneal epithelium [118]. Data on its involvement in breast cancer is scarce; however, an early study has demonstrated elevated expression in breast cancer cell lines and tissues compared with non-cancerous breast tissue [118].
More recently, cathepsin V expression has been associated with advancing tumor grade, distant metastasis, and breast cancer recurrence [119, 120]. Expression of cathepsin V has been demonstrated in different molecular subtypes of breast cancer and seems to increase during the transition of ductal carcinoma in situ (DCIS) to invasive breast carcinoma, suggesting this protease contributes to the tumor’s invasiveness [121]. Because of its role in tissue invasion, cathepsin V has been included in the genetic signature list for the oncotype DX, an array that comprises the expression of 21 genes and is used to quantify the likelihood of distant recurrence in the ER-positive sub-population [120].
On the molecular level, cathepsin V promotes the degradation of GATA binding protein 3 (GATA3) in ER-positive breast cancer cells [119]. GATA3 is a crucial transcription factor for the normal development of mammary glands and its depletion has been strongly associated with breast cancer progression due to loss of normal cellular differentiation, adhesion, and proliferation [122, 123]. This is in line with the finding that high GATA3 levels are associated with better outcomes in ER-positive patients [124].
In summary, cathepsin V seems to play an important role in the progression from DCIS towards invasive breast cancer. The available data indicates that elevated cathepsin V levels promote the progression of ER-positive cancer cells by degradation of GATA3. Cathepsin V upregulation in breast cancer cells compared to normal breast tissue indicates that this protease could be useful for targeted FI. A possible disadvantage is its mainly lysosomal localization, which could make it less accessible to an imaging probe.
Cathepsin-Targeted Probes for Fluorescence-Guided Breast Cancer Surgery
In recent decades, numerous cathepsin-targeted FI agents have been developed. Initially, cathepsin-targeted probes were used to visualize cathepsin activity in vitro to study their role in cellular (patho)physiology. However, since the introduction of clinical NIR FI systems, cathepsin-targeted probes are under extensive investigation for their possible use in intraoperative tumor visualization. Because of the increased expression and proteolytic activity of cysteine cathepsins in breast tumors of virtually all molecular subtypes, these proteases show great potential as targets for fluorescence-guided breast cancer surgery in a large patient population. Moreover, since cysteine cathepsins derive from both tumor cells and tumor-stromal cells, such as TAMs, cathepsin-targeted probes will potentially result in a more homogeneous tumor signal compared to probes targeting tumor cell-specific proteins, such as epidermal growth factor receptor (EGFR), that often display high intratumor and interpatient heterogeneity [125, 126].
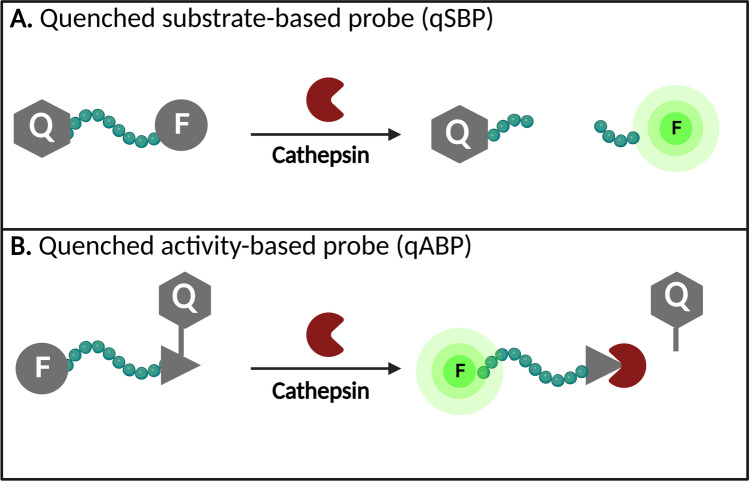
Most cysteine cathepsin-targeted contrast agents are so-called turn-ON (or quenched) probes that only fluoresce after activation. They can either be activated by one specific cathepsin or be pan-reactive, targeting multiple cysteine cathepsins at once. The advantage of activatable agents over traditional fluorophores is the substantial reduction in off-target (or background) fluorescence and an increased speed of detection since no time is required to clear from non-target tissues [127]. The activatable cathepsin-targeted fluorescence agents can be subdivided into substrate-based probes (SBP) and activity-based probes (ABPs). SBPs require enzymatic cleavage of the probe’s substrate to become activated, while ABPs form covalent bonds at the catalytic site of cathepsins and therefore do not diffuse from the target enzyme after activation, resulting in prolonged signal retention at the target location (Fig. 2) [59].
Fig. 2.
Different types of quenched cathepsin-activatable fluorescent probes. A Quenched substrate-based probe. The probe is activated by enzymatic cleavage of the peptide linker by a target cathepsin. Upon cleavage, two fragments—one containing the quencher and the other the now unquenched fluorophore—are released. B Quenched activity-based probe. The probe covalently binds in the active site of the target cathepsin forming a permanent bond. Upon binding the active site, the quencher is released and the probe is activated. Abbreviations: qSPB, quenched substrate-based probe; qABP, quenched activity-based probe; Q, quencher; F, fluorophore.
Completed and onging clinical trials have shown that the process from NIR fluorescence probe development to first-in-human trials and subsequent clinical translation is expensive and time-consuming, stressing the imporance of selecting the probes with the most potential in preclinical research (NCT03659448) [128]. The following sections will therefore review both cathepsin-targeted probes that have only been investigated in preclinical research as well as cathepsin-targeted contrast agents that have already been used for fluorescence-guided breast cancer surgery in patients. The details of these probes are summarized in Table 2.
Table 2.
Cysteine cathepsin-targeted fluorescence probes in breast cancer
| Probe name | Probe type | Target | Fluorophore | Phase | References |
|---|---|---|---|---|---|
| BMV083 | qABP | Cathepsin S | Cy5 | Preclinical | [129] |
| BMV109 | qABP | Cathepsin B, L, and S | Cy5 | Preclinical | [130, 131] |
| VGT-309 | qABP | Cathepsin B, L, and S | ICG | Preclinical | [132] |
| BMV157 | qABP | Cathepsin S | Cy5 | Preclinical | [133] |
| 6QC-NIR | qSBP | Cathepsin B, L, and S | DyLight780-B1 | Preclinical | [134] |
| 6QC-ICG | qSBP | Cathepsin B, L, and S | ICG | Preclinical | [135, 136] |
| DEATH-CAT-FNIR | AND-Gate | Cathepsin L (and caspase 3) | Heptamethine cyanine | Preclinical | [136] |
| YBN14 | Photosensitized qABP | Cathepsin B, L, and S | Bacteriochlorin | Preclinical | [137] |
| 8h6 | DARPin | Cathepsin B | Cy5.5 | Preclinical | [138] |
| Not specified | qABP | Cathepsin S | Cy5.5 | Preclinical | [139] |
| MP-cL3 | ABP | Cathepsin L | Cy5 | Preclinical | [140] |
| LUM015 | qSBP | Cathepsin B, K, L and S | Cy5 | Preclinical/clinical |
Shown are the cathepsin-targeted fluorescent probes for intraoperative breast cancer imaging that have been investigated preclinically and in human patients
Abbreviations: ABP, activity-based probe; qABP, quenched activity-based probe; qSBP, quenched substrate-based probe; AND-Gate, probe that requires activation by two different targets; DARPin, designed ankyrin repeat protein; Cy5, cyanine 5; ICG, indocyanine green; Cy5.5, cyanine 5.5.
Preclinical
Various quenched substrate-based (qSBP) and activity-based probes (qABPs) have been investigated in breast cancer cell lines and mouse models. The cathepsin S-directed qABP BMV083, developed by Verdoes et al., allowed for in vivo breast cancer visualization in a syngeneic orthotopic mouse breast cancer model [129]. Withana et al. showed that an altered version of this probe, BMV109, targeting both cathepsin B, L, and S, had enhanced imaging properites in vivo. In addition, it enabled rapid imaging of enzyme activity in fresh frozen human breast cancer tissue sections [130, 131]. Suurs et al. developed the cathepsin B-, L-, and, S-targeted probe VGT-309, which contains the same cathepsin recognition sequence as BMV109 but a different fluorophore and quencher. In a syngeneic orthotopic breast cancer mouse model, tumors were well delineated in vivo by the fluorescent signal and VGT-309 could be used for fluorescence-guided resection [132]. Bender et al. designed a potent cathepsin S-targeted qABP, BMV157, that could demarcate breast cancer margins with substantial contrast to surrounding healthy tissue in vivo in a syngeneic orthotopic mouse model. However, due to the selectivity of BMV157 for cathepsin S, its fluorescence signal in breast tumors was weaker than that of BMV109 [133].
Further improvement of BMV109 by Ofori et al. resulted in the quenched substrate-based probe 6QC-NIR, designed to exploit the latent lysosomotropic effect [134]. This causes accumulation of the fluorescent fragments of the probe in lysosomes following proteolytic cleavage by cathepsin B, L, or S, preventing diffusion from the target (tumor) location. In a syngeneic orthotopic mouse model of breast cancer, the use of 6QC-NIR provided evidence of its ability to visualize tumors and guide resection using real-time intraoperative FIwith the FDA-approved da Vinci Surgical System and its integrated NIR fluorescence camera system (Firefly mode; Intuitive Surgical, Sunnyville California, USA). Moreover, the use of cathepsin-targeted FI with 6QC-NIR allowed detection of additional breast cancer lesions that were invisible under white light [134]. Yim et al. further optimized the 6QC-NIR probe by replacing the fluorophore Dylight 780-B1 with the FDA-approved fluorophore indocyanine green (ICG). Compared to 6QC-NIR, 6QC-ICG had an enhanced fluorescence signal and an improved sensitivity during fluorescence-guided breast cancer surgery in a syngeneic orthotopic mouse model using commercially available FI cameras that were optimized to detect ICG [135].
To increase tumor selectivity, Widen et al. synthesized a so-called AND-Gate probe, DEATH-CAT-FNIR, that required activation by cathepsin L and caspase 3, a different type of protease [136]. This AND-Gate design diminishes the off-target signal by requiring both processing events to be present in the same location. During fluorescence-guided breast cancer surgery using the da Vinci Firefly in a syngeneic orthotopic mouse model, the DEATH-CAT-FNIR had comparable fluorescence signal intensity to the 6QC-ICG probe and a much improved signal in comparison to 6QC-NIR. In addition, DEATH-CAT-FNIR demonstrated a lower background signal in healthy organs and facilitated detection of residual breast cancer cells after resection [136].
Ben-Nun et al. developed a cathepsin B, L, and S targeted, photosensitized qABP. This probe, YBN14, was able to visualize breast tumors in vivo in a syngeneic subcutaneous mouse model and was succesfully used for photodynamic therapy (PDT), a method for cancer treatment that involves the activation of a photosensitive molecule by a light source to cause selective cytotoxic damage to cancer cells [137]. Kramer et al. constructed DARPin 8h6, a highly selective cathepsin B-directed, fluorescently labeled designed ankyrin repeat protein (DARPin), which is a small antibody mimetic. In both a congenic and syngeneic orthotopic breast cancer mouse model, DARPin 8h6 highlighted mammary tumors both in and ex vivo with a considerable contrast to healthy tissue [138]. A cathepsin S-directed, lipidated qABP was designed by Hu et al. and has been demonstrated to visualize breast tumors in vivo with a high tumor to background ratio [139]. Porreba et al. developed the cathepsin L selective ABP MP-cL3, which has been shown to label breast cancer cells in vitro [140]. The PEGylated cathepsin K-, L-, and S-targeted qSBP probe LUM015, designed by Whitley et al., exhibited an increased fluorescence signal ex vivo in breast cancer compared with normal muscle tissue in an orthotopic mouse model [141].
Clinical
To the best of our knowledge, LUM015 is the only cathepsin-targeted contrast agent for fluorescence-guided breast cancer surgery that has been introduced in the clinic.
A first-in-human phase I clinical trial showed that preoperative intravenous LUM015 administration did not raise safety signals and resulted in tumor-specific fluorescence that could be detected upon ex vivo imaging of resected breast cancer tissues [141]. A subsequent dose-escalation pilot study demonstrated that LUM015 allows direct identification of residual tumor in the breast cancer patient’s surgical cavity with a high tumor-to-background ratio of the fluorescent signal [23]. In a phase II clinical trial of 45 breast cancer patients who underwent BCS, the LUM015 fluorescence signal facilitated detection of 84% of all tumor-positive cavity surfaces in the eight patients with tumor-positive margins. In addition, two patients with tumor-positive margins after standard of care surgery were spared second surgeries because additional tissue was excised at sites of high LUM015 signal [142]. In an additional phase II clinical trial of 55 breast cancer patients undergoing BCS, the tumor-to-background ratio of the fluorescence signal ranged between 3.8 and 5.7 [143]. An ongoing multicenter phase II clinical trial (NCT03321929) and a phase III randomized controlled trial (NCT03686215) will further investigate the feasibility and added clinical value of LUM015 for the intraoperative detection of residual tumor during breast cancer surgery.
The previously described cathepsin-targeted probe VGT-309 is currently under investigation in a phase II clinical trial in 40 patients undergoing surgery for primary lung cancer or lung metastases (NCT05400226). The preliminary results in two patients have already been published and illustrate the successful clinical translation of VGT-309 and its potential to improve surgical management of patients undergoing cancer resection [144]. However, VGT0309 has not yet been tested in breast cancer patients.
Future Perspectives: Topical Application of Cathepsin-Targeted Imaging Probes
To date, cathepsin-targeted activatable imaging probes for fluorescence-guided breast cancer surgery have been administered intravenously, necessarily days or hours prior to surgery. Alternative to systemic delivery, cathepsin-targeted turn-ON probes could be topically applied onto the breast cancer patient’s surgical cavity to rapidly differentiate between tumor and healthy tissue, as has been demonstrated in a recent preclinical study [145]. The main advantages of topical application compared with intravenous administration are (1) faster probe activation (minutes instead of days/hours), hence more compatible with normal surgical workflow, (2) a better identification of tumor cells at the resection margin that have not necessarily generated a vascular system yet, (3) a decreased chance of side effects due to a diminished systemic load, (4) a potentially more cost-effective probe development process due to the possibility of using a micro-dose, and (5) it offers a more patient-friendly approach, as patients do not have to visit the hospital prior to surgery for IV injection [146].
Conclusions
In this review, we have discussed the expression and role of cysteine cathepsins in breast cancer and their applicability to fluorescence-guided breast cancer surgery. The cysteine cathepsins B, C, K, L, O, S and V are all highly overexpressed by and have an increased proteolytic activity in virtually all breast cancer subtypes and play major roles in tumor progression. Most of these proteases have been shown to be suitable targets for fluorescence-guided breast cancer surgery, a technique that can identify tumors, facilitate residual tumor identification and guide additional resection. The first and only cathepsin-targeted probe introduced in the clinic thus far shows promising results, possibly preventing the need for re-excision and—potentially—tumor recurrence. Various other cathepsin-targeted probes demonstrate great potential for intraoperative breast cancer imaging in preclinical studies. Due to their particular design, some of these probes offer certain advantages, such as retention of the fluorescence signal in the target cells or a lower background signal, probably making them the most preferable agents for fluorescence-guided breast cancer surgery. Clinical translation of these promising cathepsin-targeted fluorescent probes in the near future could greatly improve treatment outcomes for breast cancer patients, especially if used in a topical application protocol that is more patient friendly and easily integrated into the surgical workflow.
Funding
This research was funded by The National Institutes of Health (NIH), grant number 5R01CA246678.
Declarations
Conflict of Interest
The authors declare no competing interests.
Footnotes
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Contributor Information
Daan G. J. Linders, Email: d.g.j.linders@lumc.nl
Okker D. Bijlstra, Email: o.d.bijlstra@lumc.nl
Laura C. Fallert, Email: l.c.fallert@lumc.nl
Denise E. Hilling, Email: d.e.hilling@lumc.nl
Ethan Walker, Email: yvv@case.edu.
Brian Straight, Email: brian_straight@akrotomeimaging.com.
Taryn L. March, Email: t.l.march@lumc.nl
A. Rob P. M. Valentijn, Email: a.r.p.m.valentijn@lumc.nl
Martin Pool, Email: m.pool@lumc.nl.
Jacobus Burggraaf, Email: kb@chdr.nl.
James P. Basilion, Email: jxb206@case.edu
Alexander L. Vahrmeijer, Email: a.l.vahrmeijer@lumc.nl
Peter J. K. Kuppen, Email: p.j.k.kuppen@lumc.nl
References
- 1.Bray F, Ferlay J, Soerjomataram I, Siegel RL, Torre LA, Jemal A. Global cancer statistics 2018: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin. 2018;68(6):394–424. doi: 10.3322/caac.21492. [DOI] [PubMed] [Google Scholar]
- 2.Waks AG, Winer EP. Breast cancer treatment: a review. JAMA. 2019;321(3):288–300. doi: 10.1001/jama.2018.19323. [DOI] [PubMed] [Google Scholar]
- 3.Kramer S, Darsow M, Kummel S, Kimmig R, Rezai M. Breast-conserving treatment of breast cancer–oncological and reconstructive aspects. Gynakol Geburtshilfliche Rundsch. 2008;48(2):56–62. doi: 10.1159/000118932. [DOI] [PubMed] [Google Scholar]
- 4.Schwartz GF, Veronesi U, Clough KB, Dixon JM, Fentiman IS, Heywang-Kobrunner SH, Holland R, Hughes KS, Mansel RE. Margolese R and others Consensus conference on breast conservation. J Am Coll Surg. 2006;203(2):198–207. doi: 10.1016/j.jamcollsurg.2006.04.009. [DOI] [PubMed] [Google Scholar]
- 5.Fisher B, Anderson S, Bryant J, Margolese RG, Deutsch M, Fisher ER, Jeong JH, Wolmark N. Twenty-year follow-up of a randomized trial comparing total mastectomy, lumpectomy, and lumpectomy plus irradiation for the treatment of invasive breast cancer. N Engl J Med. 2002;347(16):1233–1241. doi: 10.1056/NEJMoa022152. [DOI] [PubMed] [Google Scholar]
- 6.Kreike B, Hart AA, van de Velde T, Borger J, Peterse H, Rutgers E, Bartelink H, van de Vijver MJ. Continuing risk of ipsilateral breast relapse after breast-conserving therapy at long-term follow-up. Int J Radiat Oncol Biol Phys. 2008;71(4):1014–1021. doi: 10.1016/j.ijrobp.2007.11.029. [DOI] [PubMed] [Google Scholar]
- 7.Komoike Y, Akiyama F, Iino Y, Ikeda T, Akashi-Tanaka S, Ohsumi S, Kusama M, Sano M, Shin E, Suemasu K, et al. Ipsilateral breast tumor recurrence IBTR after breast-conserving treatment for early breast cancer: risk factors and impact on distant metastases. Cancer. 2006;106(1):35–41. doi: 10.1002/cncr.21551. [DOI] [PubMed] [Google Scholar]
- 8.Nottage MK, Kopciuk KA, Tzontcheva A, Andrulis IL, Bull SB, Blackstein ME. Analysis of incidence and prognostic factors for ipsilateral breast tumour recurrence and its impact on disease-specific survival of women with node-negative breast cancer: a prospective cohort study. Breast Cancer Res. 2006;8(4):R44. doi: 10.1186/bcr1531. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Bijker N, Peterse JL, Duchateau L, Julien JP, Fentiman IS, Duval C, Di Palma S, Simony-Lafontaine J, de Mascarel I, van de Vijver MJ. Risk factors for recurrence and metastasis after breast-conserving therapy for ductal carcinoma-in-situ: analysis of European Organization for Research and Treatment of Cancer Trial 10853. J Clin Oncol. 2001;19(8):2263–2271. doi: 10.1200/JCO.2001.19.8.2263. [DOI] [PubMed] [Google Scholar]
- 10.Park CC, Mitsumori M, Nixon A, Recht A, Connolly J, Gelman R, Silver B, Hetelekidis S, Abner A, Harris JR et al (2000) Outcome at 8 years after breast-conserving surgery and radiation therapy for invasive breast cancer: influence of margin status and systemic therapy on local recurrence. J Clin Oncol 18(8):1668–75 [DOI] [PubMed]
- 11.Clarke M, Collins R, Darby S, Davies C, Elphinstone P, Evans V, Godwin J, Gray R, Hicks C, James S, et al. Effects of radiotherapy and of differences in the extent of surgery for early breast cancer on local recurrence and 15-year survival: an overview of the randomised trials. Lancet. 2005;366(9503):2087–106. doi: 10.1016/S0140-6736(05)67887-7. [DOI] [PubMed] [Google Scholar]
- 12.Brouwer de Koning SG, VranckenPeeters M, Jozwiak K, Bhairosing PA, Ruers TJM. Tumor resection margin definitions in breast-conserving surgery: systematic review and meta-analysis of the current literature. Clin Breast Cancer. 2018;18(4):e595–e600. doi: 10.1016/j.clbc.2018.04.004. [DOI] [PubMed] [Google Scholar]
- 13.Chagpar AB, Killelea BK, Tsangaris TN, Butler M, Stavris K, Li F, Yao X, Bossuyt V, Harigopal M, Lannin DR, et al. A randomized, controlled trial of cavity shave margins in breast cancer. N Engl J Med. 2015;373(6):503–10. doi: 10.1056/NEJMoa1504473. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.McCahill LE, Single RM, Aiello Bowles EJ, Feigelson HS, James TA, Barney T, Engel JM, Onitilo AA. Variability in reexcision following breast conservation surgery. JAMA. 2012;307(5):467–475. doi: 10.1001/jama.2012.43. [DOI] [PubMed] [Google Scholar]
- 15.Pleijhuis RG, Graafland M, de Vries J, Bart J, de Jong JS, van Dam GM. Obtaining adequate surgical margins in breast-conserving therapy for patients with early-stage breast cancer: current modalities and future directions. Ann Surg Oncol. 2009;16(10):2717–2730. doi: 10.1245/s10434-009-0609-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Bartelink H, Maingon P, Poortmans P, Weltens C, Fourquet A, Jager J, Schinagl D, Oei B, Rodenhuis C, Horiot JC, et al. Whole-breast irradiation with or without a boost for patients treated with breast-conserving surgery for early breast cancer: 20-year follow-up of a randomised phase 3 trial. Lancet Oncol. 2015;16(1):47–56. doi: 10.1016/S1470-2045(14)71156-8. [DOI] [PubMed] [Google Scholar]
- 17.Grant Y, Al-Khudairi R, St John E, Barschkett M, Cunningham D, Al-Mufti R, Hogben K, Thiruchelvam P, Hadjiminas DJ, Darzi A, et al. Patient-level costs in margin re-excision for breast-conserving surgery. Br J Surg. 2019;106(4):384–394. doi: 10.1002/bjs.11050. [DOI] [PubMed] [Google Scholar]
- 18.Vos EL, Siesling S, Baaijens MHA, Verhoef C, Jager A, Voogd AC, Koppert LB. Omitting re-excision for focally positive margins after breast-conserving surgery does not impair disease-free and overall survival. Breast Cancer Res Treat. 2017;164(1):157–167. doi: 10.1007/s10549-017-4232-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Maloney BW, McClatchy DM, Pogue BW, Paulsen KD, Wells WA, Barth RJ. Review of methods for intraoperative margin detection for breast conserving surgery. J Biomed Opt. 2018;23(10):1–19. doi: 10.1117/1.JBO.23.10.100901. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Vahrmeijer AL, Hutteman M, van der Vorst JR, van de Velde CJ, Frangioni JV. Image-guided cancer surgery using near-infrared fluorescence. Nat Rev Clin Oncol. 2013;10(9):507–518. doi: 10.1038/nrclinonc.2013.123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Lamberts LE, Koch M, de Jong JS, Adams ALL, Glatz J, Kranendonk MEG, Terwisscha van Scheltinga AGT, Jansen L, de Vries J, Lub-de Hooge MN, et al. Tumor-specific uptake of fluorescent bevacizumab-IRDye800CW microdosing in patients with primary breast cancer: a phase I feasibility study. Clin Cancer Res. 2017;23(11):2730–2741. doi: 10.1158/1078-0432.CCR-16-0437. [DOI] [PubMed] [Google Scholar]
- 22.Voskuil FJ, Vonk J, van der Vegt B, Kruijff S, Ntziachristos V, van der Zaag PJ, Witjes MJH, van Dam GM. Intraoperative imaging in pathology-assisted surgery. Nat Biomed Eng. 2022;6(5):503–514. doi: 10.1038/s41551-021-00808-8. [DOI] [PubMed] [Google Scholar]
- 23.Smith BL, Gadd MA, Lanahan CR, Rai U, Tang R, Rice-Stitt T, Merrill AL, Strasfeld DB, Ferrer JM, Brachtel EF, et al. Real-time, intraoperative detection of residual breast cancer in lumpectomy cavity walls using a novel cathepsin-activated fluorescent imaging system. Breast Cancer Res Treat. 2018;171(2):413–420. doi: 10.1007/s10549-018-4845-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Tang R, Coopey SB, Specht MC, Lei L, Gadd MA, Hughes KS, Brachtel EF, Smith BL. Lumpectomy specimen margins are not reliable in predicting residual disease in breast conserving surgery. Am J Surg. 2015;210(1):93–98. doi: 10.1016/j.amjsurg.2014.09.029. [DOI] [PubMed] [Google Scholar]
- 25.Hernot S, van Manen L, Debie P, Mieog JSD, Vahrmeijer AL. Latest developments in molecular tracers for fluorescence image-guided cancer surgery. Lancet Oncol. 2019;20(7):e354–e367. doi: 10.1016/S1470-2045(19)30317-1. [DOI] [PubMed] [Google Scholar]
- 26.Tummers QR, Hoogstins CE, Gaarenstroom KN, de Kroon CD, van Poelgeest MI, Vuyk J, Bosse T, Smit VT, van de Velde CJ, Cohen AF, et al. Intraoperative imaging of folate receptor alpha positive ovarian and breast cancer using the tumor specific agent EC17. Oncotarget. 2016;7(22):32144–55. doi: 10.18632/oncotarget.8282. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Dintzis SM, Hansen S, Harrington KM, Tan LC, Miller DM, Ishak L, Parrish-Novak J, Kittle D, Perry J, Gombotz C, et al. Real-time visualization of breast carcinoma in pathology specimens from patients receiving fluorescent tumor-Marking agent tozuleristide. Arch Pathol Lab Med. 2019;143(9):1076–1083. doi: 10.5858/arpa.2018-0197-OA. [DOI] [PubMed] [Google Scholar]
- 28.Unkart JT, Chen SL, Wapnir IL, Gonzalez JE, Harootunian A, Wallace AM. Intraoperative tumor detection using a ratiometric activatable fluorescent peptide: a first-in-human phase 1 study. Ann Surg Oncol. 2017;24(11):3167–3173. doi: 10.1245/s10434-017-5991-3. [DOI] [PubMed] [Google Scholar]
- 29.Voskuil FJ, Steinkamp PJ, Zhao T, van der Vegt B, Koller M, Doff JJ, Jayalakshmi Y, Hartung JP, Gao J, Sumer BD, et al. Exploiting metabolic acidosis in solid cancers using a tumor-agnostic pH-activatable nanoprobe for fluorescence-guided surgery. Nature Communications. 2020;11(1):3257. doi: 10.1038/s41467-020-16814-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Olson OC, Joyce JA. Cysteine cathepsin proteases: regulators of cancer progression and therapeutic response. Nat Rev Cancer. 2015;15(12):712–729. doi: 10.1038/nrc4027. [DOI] [PubMed] [Google Scholar]
- 31.Reiser J, Adair B, Reinheckel T. Specialized roles for cysteine cathepsins in health and disease. J Clin Invest. 2010;120(10):3421–3431. doi: 10.1172/JCI42918. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Fonovic M, Turk B. Cysteine cathepsins and extracellular matrix degradation. Biochim Biophys Acta. 2014;1840(8):2560–2570. doi: 10.1016/j.bbagen.2014.03.017. [DOI] [PubMed] [Google Scholar]
- 33.Brix K, Dunkhorst A, Mayer K, Jordans S. Cysteine cathepsins: cellular roadmap to different functions. Biochimie. 2008;90(2):194–207. doi: 10.1016/j.biochi.2007.07.024. [DOI] [PubMed] [Google Scholar]
- 34.Vidak E, Javorsek U, Vizovisek M, Turk B (2019) Cysteine cathepsins and their extracellular roles: shaping the microenvironment. Cells 8(3) [DOI] [PMC free article] [PubMed]
- 35.Rudensky A, Beers C. Lysosomal cysteine proteases and antigen presentation. Ernst Schering Res Found Workshop. 2006;56:81–95. doi: 10.1007/3-540-37673-9_5. [DOI] [PubMed] [Google Scholar]
- 36.Turk B, Turk D, Turk V. Lysosomal cysteine proteases: more than scavengers. Biochim Biophys Acta. 2000;1477(1–2):98–111. doi: 10.1016/S0167-4838(99)00263-0. [DOI] [PubMed] [Google Scholar]
- 37.Goulet B, Baruch A, Moon NS, Poirier M, Sansregret LL, Erickson A, Bogyo M, Nepveu A. A cathepsin L isoform that is devoid of a signal peptide localizes to the nucleus in S phase and processes the CDP/Cux transcription factor. Mol Cell. 2004;14(2):207–219. doi: 10.1016/S1097-2765(04)00209-6. [DOI] [PubMed] [Google Scholar]
- 38.Drake FH, Dodds RA, James IE, Connor JR, Debouck C, Richardson S, Lee-Rykaczewski E, Coleman L, Rieman D, Barthlow R, et al. Cathepsin K, but not cathepsins B, L, or S, is abundantly expressed in human osteoclasts. J Biol Chem. 1996;271(21):12511–6. doi: 10.1074/jbc.271.21.12511. [DOI] [PubMed] [Google Scholar]
- 39.Nakagawa TY, Brissette WH, Lira PD, Griffiths RJ, Petrushova N, Stock J, McNeish JD, Eastman SE, Howard ED, Clarke SR, et al. Impaired invariant chain degradation and antigen presentation and diminished collagen-induced arthritis in cathepsin S null mice. Immunity. 1999;10(2):207–17. doi: 10.1016/S1074-7613(00)80021-7. [DOI] [PubMed] [Google Scholar]
- 40.Riese RJ, Mitchell RN, Villadangos JA, Shi GP, Palmer JT, Karp ER, De Sanctis GT, Ploegh HL, Chapman HA. Cathepsin S activity regulates antigen presentation and immunity. J Clin Invest. 1998;101(11):2351–2363. doi: 10.1172/JCI1158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Atley LM, Mort JS, Lalumiere M, Eyre DR. Proteolysis of human bone collagen by cathepsin K: characterization of the cleavage sites generating by cross-linked N-telopeptide neoepitope. Bone. 2000;26(3):241–247. doi: 10.1016/S8756-3282(99)00270-7. [DOI] [PubMed] [Google Scholar]
- 42.Hou WS, Li Z, Buttner FH, Bartnik E, Bromme D. Cleavage site specificity of cathepsin K toward cartilage proteoglycans and protease complex formation. Biol Chem. 2003;384(6):891–897. doi: 10.1515/BC.2003.100. [DOI] [PubMed] [Google Scholar]
- 43.Nixon RA, Cataldo AM, Mathews PM. The endosomal-lysosomal system of neurons in Alzheimer’s disease pathogenesis: a review. Neurochem Res. 2000;25(9–10):1161–1172. doi: 10.1023/A:1007675508413. [DOI] [PubMed] [Google Scholar]
- 44.Wang B, Sun J, Kitamoto S, Yang M, Grubb A, Chapman HA, Kalluri R, Shi GP. Cathepsin S controls angiogenesis and tumor growth via matrix-derived angiogenic factors. J Biol Chem. 2006;281(9):6020–6029. doi: 10.1074/jbc.M509134200. [DOI] [PubMed] [Google Scholar]
- 45.Yasuda Y, Kaleta J, Bromme D. The role of cathepsins in osteoporosis and arthritis: rationale for the design of new therapeutics. Adv Drug Deliv Rev. 2005;57(7):973–993. doi: 10.1016/j.addr.2004.12.013. [DOI] [PubMed] [Google Scholar]
- 46.Yasuda Y, Li Z, Greenbaum D, Bogyo M, Weber E, Bromme D, Cathepsin V. a novel and potent elastolytic activity expressed in activated macrophages. J Biol Chem. 2004;279(35):36761–36770. doi: 10.1074/jbc.M403986200. [DOI] [PubMed] [Google Scholar]
- 47.Joyce JA, Baruch A, Chehade K, Meyer-Morse N, Giraudo E, Tsai FY, Greenbaum DC, Hager JH, Bogyo M, Hanahan D. Cathepsin cysteine proteases are effectors of invasive growth and angiogenesis during multistage tumorigenesis. Cancer Cell. 2004;5(5):443–453. doi: 10.1016/S1535-6108(04)00111-4. [DOI] [PubMed] [Google Scholar]
- 48.Tan GJ, Peng ZK, Lu JP, Tang FQ. Cathepsins mediate tumor metastasis. World J Biol Chem. 2013;4(4):91–101. doi: 10.4331/wjbc.v4.i4.91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Rudzinska M, Parodi A, Soond SM, Vinarov AZ, Korolev DO, Morozov AO, Daglioglu C, Tutar Y, Zamyatnin AA, Jr (2019) The role of cysteine cathepsins in cancer progression and drug resistance. Int J Mol Sci 20(14) [DOI] [PMC free article] [PubMed]
- 50.Mason SD, Joyce JA. Proteolytic networks in cancer. Trends Cell Biol. 2011;21(4):228–237. doi: 10.1016/j.tcb.2010.12.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Kamphorst JJ, Nofal M, Commisso C, Hackett SR, Lu W, Grabocka E, Vander Heiden MG, Miller G, Drebin JA, Bar-Sagi D, et al. Human pancreatic cancer tumors are nutrient poor and tumor cells actively scavenge extracellular protein. Cancer Res. 2015;75(3):544–53. doi: 10.1158/0008-5472.CAN-14-2211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Perera RM, Stoykova S, Nicolay BN, Ross KN, Fitamant J, Boukhali M, Lengrand J, Deshpande V, Selig MK, Ferrone CR, et al. Transcriptional control of autophagy-lysosome function drives pancreatic cancer metabolism. Nature. 2015;524(7565):361–5. doi: 10.1038/nature14587. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Mohamed MM, Sloane BF. Cysteine cathepsins: multifunctional enzymes in cancer. Nat Rev Cancer. 2006;6(10):764–775. doi: 10.1038/nrc1949. [DOI] [PubMed] [Google Scholar]
- 54.Soond SM, Kozhevnikova MV, Townsend PA, Zamyatnin AA Jr (2019) Cysteine cathepsin protease inhibition: an update on its diagnostic, prognostic and therapeutic potential in cancer. Pharmaceuticals (Basel) 12(2):87 [DOI] [PMC free article] [PubMed]
- 55.Jedeszko C, Sloane BF. Cysteine cathepsins in human cancer. Biol Chem. 2004;385(11):1017–1027. doi: 10.1515/BC.2004.132. [DOI] [PubMed] [Google Scholar]
- 56.Sun T, Jiang D, Zhang L, Su Q, Mao W, Jiang C. Expression profile of cathepsins indicates the potential of cathepsins B and D as prognostic factors in breast cancer patients. Oncol Lett. 2016;11(1):575–583. doi: 10.3892/ol.2015.3960. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Thomssen C, Schmitt M, Goretzki L, Oppelt P, Pache L, Dettmar P, Janicke F, Graeff H. Prognostic value of the cysteine proteases cathepsins B and cathepsin L in human breast cancer. Clin Cancer Res. 1995;1(7):741–746. [PubMed] [Google Scholar]
- 58.Lah TT, Kokalj-Kunovar M, Strukelj B, Pungercar J, Barlic-Maganja D, Drobnic-Kosorok M, Kastelic L, Babnik J, Golouh R, Turk V. Stefins and lysosomal cathepsins B, L and D in human breast carcinoma. Int J Cancer. 1992;50(1):36–44. doi: 10.1002/ijc.2910500109. [DOI] [PubMed] [Google Scholar]
- 59.Schleyer KA, Cui L. Molecular probes for selective detection of cysteine cathepsins. Org Biomol Chem. 2021;19(28):6182–6205. doi: 10.1039/D1OB00225B. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Schmitz J, Gilberg E, Loser R, Bajorath J, Bartz U, Gutschow M. Cathepsin B: Active site mapping with peptidic substrates and inhibitors. Bioorg Med Chem. 2019;27(1):1–15. doi: 10.1016/j.bmc.2018.10.017. [DOI] [PubMed] [Google Scholar]
- 61.Lah TT, Kalman E, Najjar D, Gorodetsky E, Brennan P, Somers R, Daskal I. Cells producing cathepsins D, B, and L in human breast carcinoma and their association with prognosis. Hum Pathol. 2000;31(2):149–160. doi: 10.1016/S0046-8177(00)80214-2. [DOI] [PubMed] [Google Scholar]
- 62.Yano M, Hirai K, Naito Z, Yokoyama M, Ishiwata T, Shiraki Y, Inokuchi M, Asano G. Expression of cathepsin B and cystatin C in human breast cancer. Surg Today. 2001;31(5):385–389. doi: 10.1007/s005950170126. [DOI] [PubMed] [Google Scholar]
- 63.Premzl A, Puizdar V, Zavasnik-Bergant V, Kopitar-Jerala N, Lah TT, Katunuma N, Sloane BF, Turk V, Kos J. Invasion of ras-transformed breast epithelial cells depends on the proteolytic activity of cysteine and aspartic proteinases. Biol Chem. 2001;382(5):853–857. doi: 10.1515/bchm.2001.382.5.853. [DOI] [PubMed] [Google Scholar]
- 64.Saleh Y, Siewinski M, Sebzda T, Jelen M, Ziolkowski P, Gutowicz J, Grybos M, Pawelec M. Inhibition of cathepsin B activity in human breast cancer tissue by cysteine peptidase inhibitor isolated from human placenta: immunohistochemical and biochemical studies. Folia Histochem Cytobiol. 2003;41(3):161–167. [PubMed] [Google Scholar]
- 65.Levicar N, Kos J, Blejec A, Golouh R, Vrhovec I, Frkovic-Grazio S, Lah TT. Comparison of potential biological markers cathepsin B, cathepsin L, stefin A and stefin B with urokinase and plasminogen activator inhibitor-1 and clinicopathological data of breast carcinoma patients. Cancer Detect Prev. 2002;26(1):42–49. doi: 10.1016/S0361-090X(02)00015-6. [DOI] [PubMed] [Google Scholar]
- 66.Foekens JA, Kos J, Peters HA, Krasovec M, Look MP, Cimerman N, Meijer-van Gelder ME, Henzen-Logmans SC, van Putten WL, Klijn JG. Prognostic significance of cathepsins B and L in primary human breast cancer. J Clin Oncol. 1998;16(3):1013–1021. doi: 10.1200/JCO.1998.16.3.1013. [DOI] [PubMed] [Google Scholar]
- 67.Lah TT, Cercek M, Blejec A, Kos J, Gorodetsky E, Somers R, Daskal I. Cathepsin B, a prognostic indicator in lymph node-negative breast carcinoma patients: comparison with cathepsin D, cathepsin L, and other clinical indicators. Clin Cancer Res. 2000;6(2):578–584. [PubMed] [Google Scholar]
- 68.Bengsch F, Buck A, Gunther SC, Seiz JR, Tacke M, Pfeifer D, von Elverfeldt D, Sevenich L, Hillebrand LE, Kern U, et al. Cell type-dependent pathogenic functions of overexpressed human cathepsin B in murine breast cancer progression. Oncogene. 2014;33(36):4474–84. doi: 10.1038/onc.2013.395. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Shree T, Olson OC, Elie BT, Kester JC, Garfall AL, Simpson K, Bell-McGuinn KM, Zabor EC, Brogi E, Joyce JA. Macrophages and cathepsin proteases blunt chemotherapeutic response in breast cancer. Genes Dev. 2011;25(23):2465–2479. doi: 10.1101/gad.180331.111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Gocheva V, Wang HW, Gadea BB, Shree T, Hunter KE, Garfall AL, Berman T, Joyce JA. IL-4 induces cathepsin protease activity in tumor-associated macrophages to promote cancer growth and invasion. Genes Dev. 2010;24(3):241–255. doi: 10.1101/gad.1874010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Victor BC, Anbalagan A, Mohamed MM, Sloane BF, Cavallo-Medved D. Inhibition of cathepsin B activity attenuates extracellular matrix degradation and inflammatory breast cancer invasion. Breast Cancer Res. 2011;13(6):R115. doi: 10.1186/bcr3058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Rozhin J, Sameni M, Ziegler G, Sloane BF. Pericellular pH affects distribution and secretion of cathepsin B in malignant cells. Cancer Res. 1994;54(24):6517–6525. [PubMed] [Google Scholar]
- 73.Sloane BF, Moin K, Sameni M, Tait LR, Rozhin J, Ziegler G. Membrane association of cathepsin B can be induced by transfection of human breast epithelial cells with c-Ha-ras oncogene. J Cell Sci. 1994;107(Pt 2):373–384. doi: 10.1242/jcs.107.2.373. [DOI] [PubMed] [Google Scholar]
- 74.Gabrijelcic D, Svetic B, Spaic D, Skrk J, Budihna M, Dolenc I, Popovic T, Cotic V, Turk V, Cathepsins B. H and L in human breast carcinoma. Eur J Clin Chem Clin Biochem. 1992;30(2):69–74. [PubMed] [Google Scholar]
- 75.Withana NP, Blum G, Sameni M, Slaney C, Anbalagan A, Olive MB, Bidwell BN, Edgington L, Wang L, Moin K, et al. Cathepsin B inhibition limits bone metastasis in breast cancer. Cancer Res. 2012;72(5):1199–209. doi: 10.1158/0008-5472.CAN-11-2759. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Edgington-Mitchell LE, Rautela J, Duivenvoorden HM, Jayatilleke KM, van der Linden WA, Verdoes M, Bogyo M, Parker BS. Cysteine cathepsin activity suppresses osteoclastogenesis of myeloid-derived suppressor cells in breast cancer. Oncotarget. 2015;6(29):27008–27022. doi: 10.18632/oncotarget.4714. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Parker BS, Ciocca DR, Bidwell BN, Gago FE, Fanelli MA, George J, Slavin JL, Moller A, Steel R, Pouliot N, et al. Primary tumour expression of the cysteine cathepsin inhibitor Stefin A inhibits distant metastasis in breast cancer. J Pathol. 2008;214(3):337–46. doi: 10.1002/path.2265. [DOI] [PubMed] [Google Scholar]
- 78.Vasiljeva O, Papazoglou A, Kruger A, Brodoefel H, Korovin M, Deussing J, Augustin N, Nielsen BS, Almholt K, Bogyo M, et al. Tumor cell-derived and macrophage-derived cathepsin B promotes progression and lung metastasis of mammary cancer. Cancer Res. 2006;66(10):5242–50. doi: 10.1158/0008-5472.CAN-05-4463. [DOI] [PubMed] [Google Scholar]
- 79.Duivenvoorden HM, Rautela J, Edgington-Mitchell LE, Spurling A, Greening DW, Nowell CJ, Molloy TJ, Robbins E, Brockwell NK, Lee CS, et al. Myoepithelial cell-specific expression of stefin A as a suppressor of early breast cancer invasion. J Pathol. 2017;243(4):496–509. doi: 10.1002/path.4990. [DOI] [PubMed] [Google Scholar]
- 80.Rafn B, Nielsen CF, Andersen SH, Szyniarowski P, Corcelle-Termeau E, Valo E, Fehrenbacher N, Olsen CJ, Daugaard M, Egebjerg C, et al. ErbB2-driven breast cancer cell invasion depends on a complex signaling network activating myeloid zinc finger-1-dependent cathepsin B expression. Mol Cell. 2012;45(6):764–76. doi: 10.1016/j.molcel.2012.01.029. [DOI] [PubMed] [Google Scholar]
- 81.Ibrahim SA, El-Ghonaimy EA, Hassan H, Mahana N, Mahmoud MA, El-Mamlouk T, El-Shinawi M, Mohamed MM. Hormonal-receptor positive breast cancer: IL-6 augments invasion and lymph node metastasis via stimulating cathepsin B expression. J Adv Res. 2016;7(5):661–670. doi: 10.1016/j.jare.2016.06.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Rothberg JM, Bailey KM, Wojtkowiak JW, Ben-Nun Y, Bogyo M, Weber E, Moin K, Blum G, Mattingly RR, Gillies RJ, et al. Acid-mediated tumor proteolysis: contribution of cysteine cathepsins. Neoplasia. 2013;15(10):1125–37. doi: 10.1593/neo.13946. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Nouh MA, Mohamed MM, El-Shinawi M, Shaalan MA, Cavallo-Medved D, Khaled HM, Sloane BF. Cathepsin B: a potential prognostic marker for inflammatory breast cancer. J Transl Med. 2011;9:1. doi: 10.1186/1479-5876-9-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Korkmaz B, Lamort AS, Domain R, Beauvillain C, Gieldon A, Yildirim AO, Stathopoulos GT, Rhimi M, Jenne DE, Kettritz R. Cathepsin C inhibition as a potential treatment strategy in cancer. Biochem Pharmacol. 2021;194:114803. doi: 10.1016/j.bcp.2021.114803. [DOI] [PubMed] [Google Scholar]
- 85.Lilla JN, Werb Z. Mast cells contribute to the stromal microenvironment in mammary gland branching morphogenesis. Dev Biol. 2010;337(1):124–133. doi: 10.1016/j.ydbio.2009.10.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Ruffell B, Affara NI, Cottone L, Junankar S, Johansson M, DeNardo DG, Korets L, Reinheckel T, Sloane BF, Bogyo M, et al. Cathepsin C is a tissue-specific regulator of squamous carcinogenesis. Genes Dev. 2013;27(19):2086–98. doi: 10.1101/gad.224899.113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Xiao Y, Cong M, Li J, He D, Wu Q, Tian P, Wang Y, Yang S, Liang C, Liang Y, et al. Cathepsin C promotes breast cancer lung metastasis by modulating neutrophil infiltration and neutrophil extracellular trap formation. Cancer Cell. 2021;39(3):423–437. doi: 10.1016/j.ccell.2020.12.012. [DOI] [PubMed] [Google Scholar]
- 88.Flemming A. Tumours use NETs as physical shields. Nat Rev Immunol. 2020;20(6):352–353. doi: 10.1038/s41577-020-0327-0. [DOI] [PubMed] [Google Scholar]
- 89.Masucci MT, Minopoli M, Del Vecchio S, Carriero MV. The emerging role of neutrophil extracellular traps (NETs) in tumor progression and metastasis. Front Immunol. 2020;11:1749. doi: 10.3389/fimmu.2020.01749. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.van Oosten M, Crane LM, Bart J, van Leeuwen FW, van Dam GM. Selecting potential targetable biomarkers for imaging purposes in colorectal cancer using TArget Selection Criteria (TASC): a novel target identification tool. Transl Oncol. 2011;4(2):71–82. doi: 10.1593/tlo.10220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Le Gall C, Bellahcene A, Bonnelye E, Gasser JA, Castronovo V, Green J, Zimmermann J, Clezardin P. A cathepsin K inhibitor reduces breast cancer induced osteolysis and skeletal tumor burden. Cancer Res. 2007;67(20):9894–9902. doi: 10.1158/0008-5472.CAN-06-3940. [DOI] [PubMed] [Google Scholar]
- 92.Dai R, Wu Z, Chu HY, Lu J, Lyu A, Liu J, Zhang G. Cathepsin K: The action in and beyond bone. Front Cell Dev Biol. 2020;8:433. doi: 10.3389/fcell.2020.00433. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Littlewood-Evans AJ, Bilbe G, Bowler WB, Farley D, Wlodarski B, Kokubo T, Inaoka T, Sloane J, Evans DB, Gallagher JA. The osteoclast-associated protease cathepsin K is expressed in human breast carcinoma. Cancer Res. 1997;57(23):5386–5390. [PubMed] [Google Scholar]
- 94.Duong LT, Wesolowski GA, Leung P, Oballa R, Pickarski M. Efficacy of a cathepsin K inhibitor in a preclinical model for prevention and treatment of breast cancer bone metastasis. Mol Cancer Ther. 2014;13(12):2898–2909. doi: 10.1158/1535-7163.MCT-14-0253. [DOI] [PubMed] [Google Scholar]
- 95.Chen B, Platt MO. Multiplex zymography captures stage-specific activity profiles of cathepsins K, L, and S in human breast, lung, and cervical cancer. J Transl Med. 2011;9:109. doi: 10.1186/1479-5876-9-109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Allinen M, Beroukhim R, Cai L, Brennan C, Lahti-Domenici J, Huang H, Porter D, Hu M, Chin L, Richardson A, et al. Molecular characterization of the tumor microenvironment in breast cancer. Cancer Cell. 2004;6(1):17–32. doi: 10.1016/j.ccr.2004.06.010. [DOI] [PubMed] [Google Scholar]
- 97.Andrade SS, Gouvea IE, Silva MC, Castro ED, de Paula CA, Okamoto D, Oliveira L, Peres GB, Ottaiano T, Facina G, et al. Cathepsin K induces platelet dysfunction and affects cell signaling in breast cancer - molecularly distinct behavior of cathepsin K in breast cancer. BMC Cancer. 2016;16:173. doi: 10.1186/s12885-016-2203-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Teitelbaum SL. Bone resorption by osteoclasts. Science. 2000;289(5484):1504–1508. doi: 10.1126/science.289.5484.1504. [DOI] [PubMed] [Google Scholar]
- 99.Kleer CG, Bloushtain-Qimron N, Chen YH, Carrasco D, Hu M, Yao J, Kraeft SK, Collins LC, Sabel MS, Argani P, et al. Epithelial and stromal cathepsin K and CXCL14 expression in breast tumor progression. Clin Cancer Res. 2008;14(17):5357–67. doi: 10.1158/1078-0432.CCR-08-0732. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Harbeck N, Alt U, Berger U, Kruger A, Thomssen C, Janicke F, Hofler H, Kates RE, Schmitt M. Prognostic impact of proteolytic factors (urokinase-type plasminogen activator, plasminogen activator inhibitor 1, and cathepsins B, D, and L) in primary breast cancer reflects effects of adjuvant systemic therapy. Clin Cancer Res. 2001;7(9):2757–2764. [PubMed] [Google Scholar]
- 101.Li Y, Ai X, Zou C, Liu Y, Ma L, Men J, Liu D, Sheng L, Ruan X, Liu H, et al. Discovery of a novel and selective cathepsin L inhibitor with anti-metastatic ability in vitro and in vivo against breast cancer cells. Bioorg Chem. 2021;115:105256. doi: 10.1016/j.bioorg.2021.105256. [DOI] [PubMed] [Google Scholar]
- 102.Qin G, Cai Y, Long J, Zeng H, Xu W, Li Y, Liu M, Zhang H, He ZL, Chen WG. Cathepsin L is involved in proliferation and invasion of breast cancer cells. Neoplasma. 2016;63(1):30–36. doi: 10.4149/neo_2016_004. [DOI] [PubMed] [Google Scholar]
- 103.Tholen M, Wolanski J, Stolze B, Chiabudini M, Gajda M, Bronsert P, Stickeler E, Rospert S, Reinheckel T. Stress-resistant translation of cathepsin L mRNA in breast cancer progression. J Biol Chem. 2015;290(25):15758–15769. doi: 10.1074/jbc.M114.624353. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Yuan L, Liu J, He W, Bao Y, Sheng L, Zou C, Hu B, Ge W, Liu Y, Wang J, et al. Discovery of a novel cathepsin inhibitor with dual autophagy-inducing and metastasis-inhibiting effects on breast cancer cells. Bioorg Chem. 2019;84:239–253. doi: 10.1016/j.bioorg.2018.11.025. [DOI] [PubMed] [Google Scholar]
- 105.Yuan L, Sheng L, He W, Zou C, Hu B, Liu J, Ge W, Liu Y, Wang J, Ma E. Discovery of novel cathepsin inhibitors with potent anti-metastatic effects in breast cancer cells. Bioorg Chem. 2018;81:672–680. doi: 10.1016/j.bioorg.2018.09.029. [DOI] [PubMed] [Google Scholar]
- 106.Sudhan DR, Siemann DW. Cathepsin L inhibition by the small molecule KGP94 suppresses tumor microenvironment enhanced metastasis associated cell functions of prostate and breast cancer cells. Clin Exp Metastasis. 2013;30(7):891–902. doi: 10.1007/s10585-013-9590-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Galluzzi L, Yamazaki T, Kroemer G. Linking cellular stress responses to systemic homeostasis. Nat Rev Mol Cell Biol. 2018;19(11):731–745. doi: 10.1038/s41580-018-0068-0. [DOI] [PubMed] [Google Scholar]
- 108.Mori J, Tanikawa C, Funauchi Y, Lo PH, Nakamura Y, Matsuda K. Cystatin C as a p53-inducible apoptotic mediator that regulates cathepsin L activity. Cancer Sci. 2016;107(3):298–306. doi: 10.1111/cas.12881. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Cairns J, Ingle JN, Wickerham LD, Weinshilboum R, Liu M, Wang L. SNPs near the cysteine proteinase cathepsin O gene (CTSO) determine tamoxifen sensitivity in ERalpha-positive breast cancer through regulation of BRCA1. PLoS Genet. 2017;13(10):e1007031. doi: 10.1371/journal.pgen.1007031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Bond HM, Scicchitano S, Chiarella E, Amodio N, Lucchino V, Aloisio A, Montalcini Y, Mesuraca M, Morrone G. ZNF423: A new player in estrogen receptor-positive breast cancer. Front Endocrinol (Lausanne) 2018;9:255. doi: 10.3389/fendo.2018.00255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Hato Y, Kondo N, Yoshimoto N, Endo Y, Asano T, Dong Y, Nishimoto M, Takahashi S, Fujii Y, Nakanishi R, et al. Prognostic impact of a single-nucleotide polymorphism near the CTSO gene in hormone receptor-positive breast cancer patients. Int J Clin Oncol. 2016;21(3):539–47. doi: 10.1007/s10147-015-0913-5. [DOI] [PubMed] [Google Scholar]
- 112.Ingle JN, Liu M, Wickerham DL, Schaid DJ, Wang L, Mushiroda T, Kubo M, Costantino JP, Vogel VG, Paik S, et al. Selective estrogen receptor modulators and pharmacogenomic variation in ZNF423 regulation of BRCA1 expression: individualized breast cancer prevention. Cancer Discov. 2013;3(7):812–25. doi: 10.1158/2159-8290.CD-13-0038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Gautam J, Bae YK, Kim JA. Up-regulation of cathepsin S expression by HSP90 and 5-HT7 receptor-dependent serotonin signaling correlates with triple negativity of human breast cancer. Breast Cancer Res Treat. 2017;161(1):29–40. doi: 10.1007/s10549-016-4027-1. [DOI] [PubMed] [Google Scholar]
- 114.Gautam J, Banskota S, Lee H, Lee YJ, Jeon YH, Kim JA, Jeong BS. Down-regulation of cathepsin S and matrix metalloproteinase-9 via Src, a non-receptor tyrosine kinase, suppresses triple-negative breast cancer growth and metastasis. Exp Mol Med. 2018;50(9):1–14. doi: 10.1038/s12276-018-0135-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Wilkinson RDA, Burden RE, McDowell SH, McArt DG, McQuaid S, Bingham V, Williams R, Cox OT, O’Connor R, McCabe N, et al. A novel role for cathepsin S as a potential biomarker in triple negative breast cancer. J Oncol. 2019;2019:3980273. doi: 10.1155/2019/3980273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Sevenich L, Bowman RL, Mason SD, Quail DF, Rapaport F, Elie BT, Brogi E, Brastianos PK, Hahn WC, Holsinger LJ, et al. Analysis of tumour- and stroma-supplied proteolytic networks reveals a brain-metastasis-promoting role for cathepsin S. Nat Cell Biol. 2014;16(9):876–88. doi: 10.1038/ncb3011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Kim S, Jin H, Seo HR, Lee HJ, Lee YS. Regulating BRCA1 protein stability by cathepsin S-mediated ubiquitin degradation. Cell Death Differ. 2019;26(5):812–825. doi: 10.1038/s41418-018-0153-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Santamaria I, Velasco G, Cazorla M, Fueyo A, Campo E, Lopez-Otin C. Cathepsin L2, a novel human cysteine proteinase produced by breast and colorectal carcinomas. Cancer Res. 1998;58(8):1624–1630. [PubMed] [Google Scholar]
- 119.Sereesongsaeng N, McDowell SH, Burrows JF, Scott CJ, Burden RE. Cathepsin V suppresses GATA3 protein expression in luminal A breast cancer. Breast Cancer Res. 2020;22(1):139. doi: 10.1186/s13058-020-01376-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Paik S, Shak S, Tang G, Kim C, Baker J, Cronin M, Baehner FL, Walker MG, Watson D, Park T, et al. A multigene assay to predict recurrence of tamoxifen-treated, node-negative breast cancer. N Engl J Med. 2004;351(27):2817–26. doi: 10.1056/NEJMoa041588. [DOI] [PubMed] [Google Scholar]
- 121.Toss M, Miligy I, Gorringe K, Mittal K, Aneja R, Ellis I, Green A, Rakha E. Prognostic significance of cathepsin V (CTSV/CTSL2) in breast ductal carcinoma in situ. J Clin Pathol. 2020;73(2):76–82. doi: 10.1136/jclinpath-2019-205939. [DOI] [PubMed] [Google Scholar]
- 122.Asselin-Labat ML, Sutherland KD, Barker H, Thomas R, Shackleton M, Forrest NC, Hartley L, Robb L, Grosveld FG, van der Wees J, et al. Gata-3 is an essential regulator of mammary-gland morphogenesis and luminal-cell differentiation. Nat Cell Biol. 2007;9(2):201–9. doi: 10.1038/ncb1530. [DOI] [PubMed] [Google Scholar]
- 123.Kouros-Mehr H, Bechis SK, Slorach EM, Littlepage LE, Egeblad M, Ewald AJ, Pai SY, Ho IC, Werb Z. GATA-3 links tumor differentiation and dissemination in a luminal breast cancer model. Cancer Cell. 2008;13(2):141–152. doi: 10.1016/j.ccr.2008.01.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Yoon NK, Maresh EL, Shen D, Elshimali Y, Apple S, Horvath S, Mah V, Bose S, Chia D, Chang HR, et al. Higher levels of GATA3 predict better survival in women with breast cancer. Hum Pathol. 2010;41(12):1794–801. doi: 10.1016/j.humpath.2010.06.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Gonzalez-Conchas GA, Rodriguez-Romo L, Hernandez-Barajas D, Gonzalez-Guerrero JF, Rodriguez-Fernandez IA, Verdines-Perez A, Templeton AJ, Ocana A, Seruga B, Tannock IF et al (2018) Epidermal growth factor receptor overexpression and outcomes in early breast cancer: a systematic review and a meta-analysis. Cancer Treat Rev 62:1–8 [DOI] [PubMed]
- 126.Martelotto LG, Ng CKY, Piscuoglio S, Weigelt B, Reis-Filho JS. Breast cancer intra-tumor heterogeneity. Breast Cancer Res. 2014;16(3):210. doi: 10.1186/bcr3658. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Mieog JSD, Achterberg FB, Zlitni A, Hutteman M, Burggraaf J, Swijnenburg RJ, Gioux S, Vahrmeijer AL (2021) Fundamentals and developments in fluorescence-guided cancer surgery. Nat Rev Clin Oncol 19(1):9–22 [DOI] [PubMed]
- 128.Tanyi JL, Chon HS, Morgan MA, Chambers SK, Han ES, Butler KA, Langstraat CL, Powell MA, Randall LM, Vahrmeijer AL et al (2021) Phase 3, randomized, single-dose, open-label study to investigate the safety and efficacy of pafolacianine sodium injection (OTL38) for intraoperative imaging of folate receptor positive ovarian cancer 39(15_suppl):5503–5503
- 129.Verdoes M, Edgington LE, Scheeren FA, Leyva M, Blum G, Weiskopf K, Bachmann MH, Ellman JA, Bogyo M. A nonpeptidic cathepsin S activity-based probe for noninvasive optical imaging of tumor-associated macrophages. Chem Biol. 2012;19(5):619–628. doi: 10.1016/j.chembiol.2012.03.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Verdoes M, Oresic Bender K, Segal E, van der Linden WA, Syed S, Withana NP, Sanman LE, Bogyo M. Improved quenched fluorescent probe for imaging of cysteine cathepsin activity. J Am Chem Soc. 2013;135(39):14726–14730. doi: 10.1021/ja4056068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Withana NP, Garland M, Verdoes M, Ofori LO, Segal E, Bogyo M. Labeling of active proteases in fresh-frozen tissues by topical application of quenched activity-based probes. Nat Protoc. 2016;11(1):184–191. doi: 10.1038/nprot.2016.004. [DOI] [PubMed] [Google Scholar]
- 132.Suurs FV, Qiu SQ, Yim JJ, Schroder CP, Timmer-Bosscha H, Bensen ES, Santini JT, Jr, de Vries EGE, Bogyo M, van Dam GM. Fluorescent image-guided surgery in breast cancer by intravenous application of a quenched fluorescence activity-based probe for cysteine cathepsins in a syngeneic mouse model. EJNMMI Res. 2020;10(1):111. doi: 10.1186/s13550-020-00688-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Oresic Bender K, Ofori L, van der Linden WA, Mock ED, Datta GK, Chowdhury S, Li H, Segal E, Sanchez Lopez M, Ellman JA, et al. Design of a highly selective quenched activity-based probe and its application in dual color imaging studies of cathepsin S activity localization. J Am Chem Soc. 2015;137(14):4771–7. doi: 10.1021/jacs.5b00315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.Ofori LO, Withana NP, Prestwood TR, Verdoes M, Brady JJ, Winslow MM, Sorger J, Bogyo M. Design of protease activated optical contrast agents that exploit a latent lysosomotropic effect for use in fluorescence-guided surgery. ACS Chem Biol. 2015;10(9):1977–1988. doi: 10.1021/acschembio.5b00205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.Yim JJ, Tholen M, Klaassen A, Sorger J, Bogyo M. Optimization of a protease activated probe for optical surgical navigation. Mol Pharm. 2018;15(3):750–758. doi: 10.1021/acs.molpharmaceut.7b00822. [DOI] [PubMed] [Google Scholar]
- 136.Widen JC, Tholen M, Yim JJ, Antaris A, Casey KM, Rogalla S, Klaassen A, Sorger J, Bogyo M. AND-gate contrast agents for enhanced fluorescence-guided surgery. Nat Biomed Eng. 2021;5(3):264–277. doi: 10.1038/s41551-020-00616-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137.Ben-Nun Y, Merquiol E, Brandis A, Turk B, Scherz A, Blum G. Photodynamic quenched cathepsin activity based probes for cancer detection and macrophage targeted therapy. Theranostics. 2015;5(8):847–862. doi: 10.7150/thno.10854. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138.Kramer L, Renko M, Zavrsnik J, Turk D, Seeger MA, Vasiljeva O, Grutter MG, Turk V, Turk B. Non-invasive in vivo imaging of tumour-associated cathepsin B by a highly selective inhibitory DARPin. Theranostics. 2017;7(11):2806–2821. doi: 10.7150/thno.19081. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139.Hu HY, Vats D, Vizovisek M, Kramer L, Germanier C, Wendt KU, Rudin M, Turk B, Plettenburg O, Schultz C. In vivo imaging of mouse tumors by a lipidated cathepsin S substrate. Angew Chem Int Ed Engl. 2014;53(29):7669–7673. doi: 10.1002/anie.201310979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140.Poreba M, Rut W, Vizovisek M, Groborz K, Kasperkiewicz P, Finlay D, Vuori K, Turk D, Turk B, Salvesen GS, et al. Selective imaging of cathepsin L in breast cancer by fluorescent activity-based probes. Chem Sci. 2018;9(8):2113–2129. doi: 10.1039/C7SC04303A. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141.Whitley MJ, Cardona DM, Lazarides AL, Spasojevic I, Ferrer JM, Cahill J, Lee CL, Snuderl M, Blazer DG, 3rd, Hwang ES, et al. A mouse-human phase 1 co-clinical trial of a protease-activated fluorescent probe for imaging cancer. Sci Transl Med. 2016;8(320):3204ra4. doi: 10.1126/scitranslmed.aad0293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142.Smith BL, Lanahan CR, Specht MC, Kelly BN, Brown C, Strasfeld DB, Ferrer JM, Rai U, Tang R, Rice-Stitt T, et al. Feasibility study of a novel protease-activated fluorescent imaging system for real-time, intraoperative detection of residual breast cancer in breast conserving surgery. Ann Surg Oncol. 2020;27(6):1854–1861. doi: 10.1245/s10434-019-08158-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143.Lanahan CR, Kelly BN, Gadd MA, Specht MC, Brown CL, Hughes KS, Tang R, Rai U, Brachtel EF, Rice-Stitt T, et al. Performance of a novel protease-activated fluorescent imaging system for intraoperative detection of residual breast cancer during breast conserving surgery. Breast Cancer Res Treat. 2021;187(1):145–153. doi: 10.1007/s10549-021-06106-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144.Kennedy GT, Holt DE, Azari FS, Bernstein E, Nadeem B, Chang A, Sullivan NT, Segil A, Deshpande C, Bensen E et al (2022) A cathepsin targeted quenched activity-based probe facilitates enhanced detection of human tumors during resection. Clin Cancer Res [DOI] [PMC free article] [PubMed]
- 145.Walker E, Linders DGJ, Abenojar E, Wang X, Hazelbag HM, Straver ME, Bijlstra OD, March TL, Vahrmeijer AL, Exner A et al (2022) Formulation of a thermosensitive imaging hydrogel for topical application and rapid visualization of tumor margins in the surgical cavity. Cancers (Basel) 14(14) [DOI] [PMC free article] [PubMed]
- 146.Santamaría G, Velasco M, Farré X, Vanrell JA, Cardesa A, Fernández PL (2005) Power Doppler sonography of invasive breast carcinoma: does tumor vascularization contribute to prediction of axillary status? 234(2):374–380 [DOI] [PubMed]