Abstract
Background
Economic evaluations are widely used to predict the economic impact of new treatment alternatives. Comprehensive economic reviews in the field of chronic lymphocytic leukemia (CLL) are warranted to supplement the existing analyses focused on specific therapeutic areas.
Methods
A systematic literature review was conducted based on literature searches in Medline and EMBASE to summarize the published health economics models related to all types of CLL therapies. Narrative synthesis of relevant studies was performed focusing on compared treatments, patient populations, modelling approaches and key findings.
Results
We included 29 studies, the majority of which were published between 2016 and 2018, when data from large clinical trials in CLL became available. Treatment regimens were compared in 25 cases, while the remaining four studies considered treatment strategies with more complex patient pathways. Based on the review results, Markov modelling with a simple structure of three health states (progression-free, progressed, death) can be considered as the traditional basis to simulate cost effectiveness. However, more recent studies added further complexity, including additional health states for different therapies (e.g. best supportive care or stem cell transplantation), for progression-free state (e.g. by differentiating between with or without treatment), or for response status (i.e. partial response and complete response).
Conclusions
As personalized medicine is increasingly gaining recognition, we expect that future economic evaluations will also incorporate new solutions, which are necessary to capture a larger number of genetic and molecular markers and more complex patient pathways with individual patient-level allocation of treatment options and thus economic assessments.
Supplementary Information
The online version contains supplementary material available at 10.1007/s40259-023-00583-9.
Key Points
| This study provides a review of economic evaluations in the field of chronic lymphocytic leukemia (CLL). |
| Narrative synthesis of relevant studies was performed focusing on compared treatments, patient populations, modelling approaches and key findings. |
| This review defined the traditional approach of modelling in this field, but also highlighted that model complexity should be increased to address more complex research questions. |
Introduction
Chronic lymphocytic leukemia (CLL) is a lymphoproliferative B-cell malignancy characterized by the proliferation and accumulation of morphologically mature but immunologically dysfunctional B-cell lymphocytes [1]. The most common presentation is an asymptomatic lymphocytosis detected by incidental blood tests. Patients with progressive disease have a rising lymphocytosis, adenopathy, hepatosplenomegaly and bone marrow infiltration resulting in bone marrow failure with anemia and thrombocytopenia [2]. CLL is the most common type of leukemia in Western populations with an incidence of 4.2/100,000, and the incidence is slightly higher in males. Worldwide, 191,000 cases and 61,000 deaths are attributed to CLL every year [3, 4].
Since in most cases CLL remains an incurable disease, the goals of therapy are to improve quality of life (QoL) and to prolong survival; however, several surrogate endpoints are used in clinical trials, such as response rate, minimal residual disease (MRD) status or progression-free-survival (PFS), as improvement in these endpoints are associated with better survival. Ultimately, in most patients, survival and QoL depend on the effect of treatment sequences given along the course of the disease [4]. The decision to start CLL treatment is taken according to IWCLL (International Workshop on Chronic Lymphocytic Leukemia) criteria, when the patient’s symptoms or blood counts indicate that the disease has progressed to a point where it may affect QoL [5]. Only patients with progressive disease require therapy, whereas for patients with early disease, the management is based on a watch-and-wait strategy [4]. However, QoL can be significantly impacted by infections during the watch-and-wait strategy due to immune dysfunction inherent to the CLL itself.
CLL therapy has faced several major developments over the last 2 decades. Initially, the basis of therapy was single-agent chemotherapy, but later chemo-immunotherapy (CIT) became standard of care, especially for younger and fit patients [6]. For patients with comorbidities or unable to tolerate intensive CIT regimens, low intensity chemotherapy combined with antibody-based therapies have been shown to be effective in large clinical trials [7]. Although CIT regimens were the standard first-line treatments for patients with CLL for years, oral targeted therapy has recently emerged as an alternative and often preferred treatment option based on Bruton Tyrosine Kinase (BTK) and protein B-cell lymphoma 2 (BCL-2) inhibitors [8]. Currently, multiple effective therapy options are available. However, these are relatively newly developed molecules with high costs. Time on therapy can become lengthy for monotherapy since it is recommended to continue until disease progression or significant toxicity. In addition, there is a need for continuous monitoring during therapy, which adds an additional cost burden on the public healthcare system [9].
More and more attention is being focused on personalized management of patients with CLL due to recent advances in molecular and genomics profiling that correlates with outcome on different treatment options. This relates to diagnosis (e.g. immunophenotyping), response predictors (e.g. del(17p)/TP53 mutation), treatment (e.g. BCR signaling inhibitors, BCL-2 antagonists, chimeric antigen receptor T cells also known as CAR-T cells) or methods to evaluate MRD [10]. Genetic markers, including immunoglobulin heavy chain variable region gene (IGHV) mutation status, TP53 mutation or a deletion at chromosome 17p, are becoming the basis of personalized treatment in CLL, and such markers can guide health professionals to make treatment decisions. It is expected that targeted treatment can lead to decreased side effects and increased effectiveness by considering genetic tumor phenotype information and other parameters (i.e. comorbidities, concomitant medication, germline genetics, adverse events) [8].
To predict the economic impact of the new treatment alternatives and the personalized treatment approaches in CLL for payers, economic evaluations should be performed using various modelling approaches [11]. The use of health economic models to support healthcare decisions in the field of oncology is widely accepted [12]. The results of the economic analyses provide information on the potential costs and benefits of new cancer treatments and therapy sequences, compared with standard treatment, which are particularly important for decision makers as demand for better treatments increases while the healthcare budgets remain less flexible [13].
There has been a growing amount of literature about the health economic aspects of CLL since the early 2000s [14]. A systematic literature review published in 2014 identified nine studies that reported economic models to estimate the cost effectiveness of a treatment for CLL [15]. It found that the majority of CLL studies adopted a Markov model approach. The economic models were subject to key uncertainties; in particular, a lack of data on the long-term effectiveness of treatment [15]. A more recent study published in 2019 reviewed articles related to cost effectiveness, especially for CLL-targeted therapies. It provided a narrative review and a summary of the available evidence on the different types of targeted therapies [16]. Although the former study is a comprehensive review, it was published before the appearance of the most advanced therapies in the field in 2014, and the latter study focused only on targeted therapies. Therefore, at the time of preparing our study, an up-to-date analysis that reviews the economic evaluations of all potential therapies for CLL patients was warranted.
The present study was conducted to systematically collect and synthesize current evidence on the economic evaluations in the field of CLL management. We performed a systematic literature review to identify published health economics models related to all types of CLL therapies, and provide a comprehensive overview of the key modelling approaches and their conclusions.
Methodology
The aim of the literature review was based on focused review questions, which enabled efficient planning of the literature search. The review questions in Supplementary Material I (see electronic supplementary material [ESM]) provide an explicit statement of questions being addressed in this work. The systematic literature review was conducted and reported in compliance with the PRISMA 2020 Statement [17], a guide for standard reporting of systematic literature reviews.
Literature Search and Screening
The literature search covered Medline (via PubMed) and EMBASE (via SCOPUS) databases. The search strategy was developed with a search string identifying all hits potentially relevant for the disease area in focus (CLL) and for economic evaluations. We used either specific keywords or relevant subject headings of PubMed and EMBASE. The developed search strategy is presented in Supplementary Material II (see ESM) in a tabular format. The search was performed in June 2021.
Search hits were de-duplicated first (as overlap was expected between the two databases), followed by title and abstract screening for exclusion by two researchers independently to ensure internal quality control. To assess eligibility for inclusion in the review, the potentially relevant articles were next evaluated in full text by two researchers independently. Full-text papers that did not fulfil the inclusion criteria were excluded, and reasons for exclusion were documented. In case of disagreement between the two independent researchers on inclusion/exclusion, a third, senior researcher was invited to decide on inclusion. Inclusion/exclusion of papers were documented with EndNote. The process was documented in tabular format and was presented with a PRISMA flowchart.
The following exclusion criteria were applied during literature screening (the same criteria was used for the initial title and abstract screening and for the full-text screening): duplicates; no English abstract; article not reporting original data (e.g., letter, editorial, comment, or non-systematic review); the primary focus of the study is not CLL patients; studies not reporting an economic model or not using modelling techniques; former systematic literature reviews and meta-analyses with/without relevant topic.
Former systematic literature reviews with relevant topic were excluded but collected separately and the references of the papers were screened for further inclusion. To further increase the sensitivity of the systematic review, a so-called snowball approach was adopted, in which the reference lists of the included studies were screened for additional relevant papers.
Data Collection and Data Synthesis
Data from included studies were extracted to an MS Excel file. A pilot, annotated data extraction file was circulated to all reviewers (three researchers working independently), together with three example studies. Following the pilot extraction, the data extraction grid was finalized according to the comments. Data extraction was limited to findings relevant to the research topic. The data extraction sheet included fields on (1) the general characteristics of the included studies; (2) the modelled study population; (3) model characteristics and methods; (4) quantification of costs and (5) quantification of benefits. All relevant data were extracted from the included papers in the final data extraction spreadsheet. Extracted data were double-checked by an independent reviewer for quality assurance of the systematic literature review.
A narrative synthesis of the extracted data was performed according to the review questions, without meta-analysis of study findings. This report includes a summary of the investigated technologies, compared treatments, modelled patient population and the type of modelling approach. It is important to note that this brief summary only includes the information collected from scientific papers. Conference materials, such as poster presentations or abstracts of an oral presentation, were not excluded from the review, but they were not included in the narrative synthesis report and the below results, as they generally include a less comprehensive and detailed description about the modelling approach and the characteristics of the analysis.
Risk of bias assessment of the included studies was performed with the ECOBIAS checklist [18]. This tool was developed to conduct a risk-of-bias assessment in model-based economic evaluations. It includes an overall checklist and more specific aspects focusing on bias related to structure, data and consistency, with a total of 22 items.
Results
In total, we identified 1138 records from EMBASE and 433 records from PubMed before June 2021. After the initial title and abstract screening, we considered 111 records in the full-text review phase. There were 77 records that were conference materials, and did not fall under the subject of this review. The remaining 34 studies were carefully reviewed in full text, and five of them were excluded because no economic modelling was performed. The remaining 29 studies were subject to data extraction. A flow chart of the literature review is presented in Supplementary Material III (see ESM).
General Characteristics and Investigated Therapies
The objectives of these studies are listed in Supplementary Material IV (see ESM). The study location, the type of investigated technologies, the treatment line and specific characteristics of the patient population are shown in Table 1. The first study was published in 1991 (the only study before 2000), whereas the majority of the studies were published between 2016 and 2018 (17/29), when the large clinical trials about CIT (e.g., CLL8, CLL10, CLL11 or COMPLEMENT 1) and about targeted therapies (e.g. RESONATE trials) were available as primary data sources. There were ten studies from the UK, seven from the US, two from Canada and Spain, and a single study from eight countries (Australia, Finland, France, Germany, Netherlands, New Zealand, Portugal and Ukraine).
Table 1.
General information about the included studies
| References | Study country | Specific patient population characteristics | No. compared regimens | Abbreviation of the compared regimens | Treatment line of the compared regimens |
|---|---|---|---|---|---|
| Studies comparing treatment regimens (n = 25) | |||||
| Weeks et al. [25] | USA | Not defined | 2 | Ig vs No Ig | Not defined |
| Dervaux et al. [39] | France | Not defined | 18 combinations | Mini-CHOP vs F vs Clb (in different combinations) | First line; relapsed/refractory |
| Scott and Scott [23] | New Zealand | Patients who were able to tolerate third-line treatment | 2 | A vs FC-R | Third line |
| Main et al. [29] | UK | Not defined | 3 | FC-R vs FR; FC-R vs Clb | First line |
| Hornberger et al. [27] | USA | Not defined | 2 | FC-R vs FC | First line |
| Woods et al. [42] | UK | Patients not eligible for fludarabine-based therapy | 2 | B vs Clb | First line |
| Adena et al. [26] | Australia | Not defined | 2 | FC-R vs FC | First line; relapsed/refractory |
| Kongnakorn et al. [22] | USA | Not defined | 3 | B vs A vs Clb | First line |
| Mandrik et al. [30] | Ukraine | Not defined | 2 | FC-R vs FC | First line; relapsed/refractory |
| Becker et al. [32] | UK | Patients unsuitable for full-dose fludarabine-based therapy | 6 | G-Clb vs R-Clb vs Clb vs B vs R-B vs O-Clb | First line |
| Blommestein et al. [33] | Netherlands | Patients unsuitable for full-dose fludarabine-based therapy | 4 | G-Clb vs R-Clb vs O-Clb vs Clb | First line |
| Casado et al. [34] | Spain | Patients with comorbidities that make them unsuitable for full-dose fludarabine-based therapy | 2 | G-Clb vs R-Clb | First-line |
| Danese et al. [24] | USA | Not defined | 2 | R-Chemo vs Chemo | First line; relapsed/refractory |
| Herring et al. [40] | Canada | Patients for whom fludarabine-based therapies are considered inappropriate | 2 | O-Clb vs Clb | First line |
| Müller et al. [31] | Germany | Not defined | 2 | FC-R vs FC | First line |
| Soini et al. [37] | Finland | Patients unsuitable for fludarabine-based therapy | 5 | G-Clb vs O-Clb vs R-B vs R-Clb vs Clb | First line |
| Hatswell et al. [44] | UK | Double refractory (refractory to fludarabine and alemtuzumab) | 2 | O vs BSC | Relapsed/refractory |
| Howard et al. [28] | UK | Not defined | 2 | FC-R vs FCM-miniR | First line |
| Paquete et al. [35] | Portugal | Patients who are unsuitable for full dose fludarabine-based therapy | 3 | G-Clb vs R-Clb vs Clb | First line |
| Williams et al. [46] | UK | Not defined | 2 | FC-R vs FC | First line |
| Barnes et al. [38] | USA | Patients without a 17p deletion | 2 | Ibr vs Clb/G-Clb (theoretical alternative) | First line |
| Casado et al. [43] | Spain | Not defined | 2 | Ide-R vs R | Relapsed/refractory |
| Mistry et al. [45] | UK |
Indication 1: relapsed/refractory patients who had the del(17p)/TP53 aberration Indication 2: relapsed/refractory patients who lacked the del(17p)/TP53 aberration |
2 | Ven vs BSC | Relapsed/refractory |
| Sinha and Redekop [36] | UK | Patients with comorbidities that make them unsuitable for full-dose fludarabine-based therapy | 2 | Ibr vs G-Clb | First line |
| Vreman et al. [41] | UK | Not defined | 2 | Acp vs Ibr | Relapsed/refractory |
| Study | Study country | Specific patient population characteristics | No. compared scenarios | Description of compared treatment scenarios |
|---|---|---|---|---|
| Studies comparing treatment strategies / patient pathways (n = 4) | ||||
| Buchanan et al. [19] | UK | Not defined | 5 |
Scenario 1: Genetic testing + no Ibr Scenario 2: Genetic testing + Ibr Scenario 3: No genetic or genomic testing Scenario 4: Genomic testing + Ibr Scenario 5: Genomic testing + Ibr + O |
| Chen et al. [21] | USA | Not defined | 2 |
Scenario 1: CIT regimens will be replaced by oral targeted therapies by 2025 Scenario 2: CIT would remain the standard of care for the period |
| Patel et al. [20] | USA | Not defined | 2 |
Scenario 1: The patient pathway is simulated with Ibr in the 1st line Scenario 2: The patient pathway is simulated with Ibr in the 3rd line |
| Lachaine et al. [9] | Canada | Not defined | 2 |
Scenario 1: CIT regimens remain the standard of care until 2025 Scenario 2: The use of oral targeted therapy increases gradually over time until 2025 |
A alemtuzumab, Acp acalabrutinib, B bendamustine, BSC best supportive care, CHOP cyclophosphamide-vincristine-prednisone-doxorubicin, CIT chemo-immunotherapy, Clb chlorambucil, F fludarabine, FC fludarabine-cyclophosphamide, FCM-miniR fludarabine-cyclophosphamide-mitoxantrone-low-dose rituximab, FC-R fludarabine-cyclophosphamide-rituximab, G-Clb obinutuzumab-chlorambucil, Ibr ibrutinib, Ide-R idelalisib-rituximab, Ig immunoglobulin, O-Clb ofatumumab-chlorambucil, R rituximab, R-B rituximab-bendamustine, R-Chemo rituximab + chemotherapy, R-Clb rituximab-chlorambucil, Ven venetoclax
In 25 studies, specific treatment regimens (at least two or more) were compared and evaluated in terms of the cost effectiveness based on economic modelling (Table 1). Among these, one study performed a trial-based economic evaluation also (for short-term outcomes), besides the economic modelling. In addition, an early economic modelling was performed in another study based on premature clinical data. Details on the specific patient population of the investigated studies were collected in 12 studies. In eight of these studies, only patients unsuitable for fludarabine-based therapy (and/or with comorbidities) were considered. In two cases, patient selection was linked to del(17p)/TP53 mutation status, in one study only double refractory (refractory to fludarabine and alemtuzumab) patients were included, while in one study only patients unable to tolerate third-line treatment were considered.
The specific therapies from the 25 studies that investigated treatment regimens are shown in Table 1. A simple two-arm comparison was performed in 18 studies, three studies compared three treatment arms, while four-arm, five-arm and six-arm comparisons were done in one study each. There was one study where 18 different combinations of three treatment regimens were defined (both first-line and refractory treatment lines were considered in defining the combinations). There were 15 studies where first-line treatment was investigated, four where only relapsed/refractory patients were included and four where both of them were considered. In one study, the treatment line was not clear, while in one study only the third line was considered. In the majority of studies (n = 17/25), CIT regimens were investigated at least in one arm. Targeted therapies were investigated only in five studies (three on ibrutinib, including one where acalabrutinib was used on the comparator arm, one on idelalisib and one on venetoclax).
We identified four studies that not only investigated specific treatment regimens, but had a broader perspective and compared treatment strategies. Treatment strategies were defined based on the anticipated use of targeted therapies and genetic testing among CLL patients in the future. These studies tried to cover a longer treatment pathway from first line to relapse-refractory treatment lines based on certain features of the available therapies (specific examples are discussed in more detail below). Those four studies that compared treatment strategies are summarized below:
One study evaluated a total of five treatment strategies; two strategies that reflected varying genetic testing practices (defined as assays solely targeting specific genes of interest); two related to genomic testing (defined as genome-wide testing, simultaneously scrutinizing multiple genes); and one where no genetic or genomic testing is used. Selection of specific therapies in the first line and subsequent lines were linked with the use of genetic or genomic testing and the utilization of the information from these tests [19].
Another study simulated multiple lines of therapies during the course of CLL management and assumed two scenarios on the use of ibrutinib. In one scenario, the targeted therapy was considered as the first line, while in the other scenario it was considered as the third line [20].
A third study simulated the expected evolving management of CLL patients until 2025 with two scenarios. One scenario assumed that CIT regimens will be replaced by oral targeted therapies first in the relapse setting, and then as the first-line treatment. The other scenario assumed that CIT would remain the standard of care for the period [21].
A similar study aimed to quantify the economic burden of oral targeted therapies when compared with CIT regimens. It also simulated the CLL patient population under varying treatment options until 2025. A scenario where CIT remains the standard of care was compared with another scenario where the use of oral targeted therapy increases gradually over time [9].
Modelling Approaches of the Included Studies
A great majority of the studies (25/29) used Markov or partitioned survival modelling method with mutually exclusive health states. Among the four studies that did not follow the logic of mutually exclusive health states, there was only one study where a more complex patient-level discrete event simulation methodology was used [22]. In case of the remaining three studies, one used a decision tree modelling method [23], one used an epidemiological simulation model [24], and in one study the modelling method was not described in detail, therefore, no clear method was identified [25]. The modelling approaches of the studies are presented in Table 2.
Table 2.
Summary of modelling approach in the included studies
| References | Modelling methodology (additional complexity to the simple 3 health state structure) | Number of health states and their names |
|---|---|---|
| Weeks et al. [25] | No clear description | N/A |
| Dervaux et al. [39] | Markov modelling (health states by therapy type) | 4 in 1st line: Response to 1st line, Progression after 1st line without 2nd line, Progression after 1st line with 2nd line, Death; 3 in 2nd line: Response to 2nd line, Progression after 2nd line, Death |
| Scott and Scott [23] | Decision tree | N/A |
| Main et al. [29] | Markov modelling | 3: Progression-free, Progressed, Death |
| Hornberger et al. [27] | Markov modelling | 3: Progression-free, Progressed, Death |
| Woods et al. [42] | Markov modelling (health states by therapy type and by response) | 6: Stable disease, Partial response, Complete response, Progressive disease, Best supportive care, Death |
| Adena et al. [26] | Markov modelling | 3: Progression-free, Progressed, Death |
| Kongnakorn et al. [22] | Discrete event simulation | N/A |
| Mandrik et al. [30] | Markov modelling | 3: Progression-free, Progressed, Death |
| Becker et al. [32] | Markov modelling (health state for no treatment) | 4: Progression-free with therapy, Progression-free without therapy, Progressed, Death |
| Blommestein et al. [33] | Markov modelling (health state for no treatment) | 4: Progression-free with therapy, Progression-free without therapy, Progressed, Death |
| Casado et al. [34] | Markov modelling (health state for no treatment) | 4: Progression-free with therapy, Progression-free without therapy, Progressed, Death |
| Danese et al. [24] | Epidemiological simulation model | N/A |
| Herring et al. [40] | Markov modelling (health states by therapy type and by response) | 9: Stable disease, Partial response, Complete response, Progressive multifocal leukoencephalopathy, Progression to 2nd line, Progression to 3rd line, Progression to 4th line, Best supportive care, Death |
| Müller et al. [31] | Markov modelling | 3: Progression-free, Progressed, Death |
| Soini et al. [37] | Markov modelling (health state for no treatment) | 4: Progression-free with therapy, Progression-free without therapy, Progressed, Death |
| Buchanan et al. [19] | Markov modelling (health states by therapy type) | 5: Progression-free, Progressed, Best supportive care, Bone marrow transplant, Death |
| Chen et al. [21] | Partitioned survival modelling (health state for no treatment) | 4: Progression-free with therapy (Watchful waiting), Progression-free without therapy, Progressed, Death |
| Hatswell et al. [44] | Markov modelling (health states by therapy type) | 5: Progression-free, Progressed, Best supportive care, Bone marrow transplant, Death |
| Howard et al. [28] | Partitioned survival modelling (health states for no treatment) | 4: Progression-free with therapy (Watchful waiting), Progression-free without therapy, Progresseda, Death |
| Paquete et al. [35] | Partitioned survival modelling | 3: Progression-free, Progresseda, Death |
| Williams et al. [46] | Markov modelling | 3: Progression-free, Progressed, Death |
| Barnes et al. [38] | Markov modelling (Health state for no treatment) | 4: Progression-free with therapy, Progression-free without therapy, Progressed, Death |
| Casado et al. [43] | Partitioned survival modelling | 3: Progression-free, Progresseda, Death |
| Mistry et al. [45] | Markov modelling (Health states by therapy type) | 7: Progression-free, Progression to 2nd line, Progression to 3rd line, Progression to 4th line, Progression to 5th line, Best supportive care, Death |
| Sinha and Redekop [36] | Partitioned survival modelling | 3: Pre-progression, Post-progressiona, Death |
| Vreman et al. [41] | Partitioned survival modelling | 3: Pre-progression, Post-progressiona, Death |
| Patel et al. [20] | Markov modelling (Health states by therapy type) | 6: 1st line therapy, Progression to 2nd line, Progression to 3rd line, Progression to 4th line, Best supportive care, Death |
| Lachaine et al. [9] | Markov modelling (Health state for no treatment) | 4: Progression-free with therapy (Watchful waiting), Progression-free without therapy, Progressed, Death |
aPartitioned survival modelling was used, in which progression is not modelled directly as a state, but the time in progression state is derived from the difference in the area between the overall survival and progression-free survival curves
The simplest model structure for studies with mutually exclusive health states for CLL was a three-state Markov model structure (progression-free state, progression state and death). This was used in six models to evaluate the cost effectiveness of specific treatment regimens for CLL patients [26–31]. However, on top of this simple structure, we identified different approaches in the other studies that add more complexity to the modelling. These approaches are discussed below.
Separate Health State for no Treatment
We identified eight studies where the progression-free state was separated into two different health states [9, 21, 32–37]. In one state, patients were under treatment without progression, while in the other state, patients were not treated and had no progression. This allows the impact of having treatment in terms of both costs and benefits to be captured within the no-progression state.
Separate Health States According to Therapy Type
We identified eight studies where certain therapy types had separate health states, such as for best supportive care or stem cell transplantation [19, 20, 36, 38–42]. Similarly, in some of these studies, the different lines of therapies were modelled as separate health states, which reflects the assumption that the number therapy lines in CLL has an impact on patient outcomes. This approach allows the impact of each specific therapy to be captured in terms of both costs and benefits among those who require treatment.
Separate Health States According to Response
We identified two studies where the level of patients’ response to the therapy was considered in different health states [40, 42]. For instance, partial response and complete response were added as health states of separate treatment outcomes. This allows one to capture the impact of achieving different levels of response in terms of both costs and benefits.
Partitioned Survival Modelling
We identified six studies where partitioned survival modelling was used while assuming that patients were transitioning between mutually exclusive health states [21, 41, 43–46]. In this approach, progression is not modelled directly as a state, but the time in progression state is derived from the difference in the area between the overall survival and progression-free survival curves. One of these studies even compared the effect of using Markov modelling or using the partitioned survival method [46]. It validated the applicability of both methods, but also concluded that due to the different assumptions, the selection of the modelling method impacts on the cost-effectiveness results.
It is important to note that these additional approaches to the traditional three-state model are not mutually exclusive, and the various approaches could be combined to capture more complex simulation problems appropriately. We found examples for this:
A study used a model structure, where the progression-free state was divided as patients with and without treatment. In addition, the best supportive care and ibrutinib therapy had separate health states [36].
Another study defined partial and complete response health states after first-line therapy. In addition, the second, third and fourth line of therapies also had separate states [40].
Some of those studies that performed partitioned survival modelling also had more a complex structure than the simple-three state approach. One of them included a watchful waiting state before first-line therapy, which allows that some patients enter the model without having treatment [21]. Another study with partitioned survival modelling had a model structure with a separate health state for the best supportive care, which was considered separate from the other CLL treatment regimens [41].
Summary of Study Findings
The great majority of studies calculated the total cost per quality-adjusted life-year (QALY) gain in the cost-effectiveness analyses (n = 26). In these studies, the costs and the QALYs were modelled for each compared alternative and then the incremental cost-effectiveness ratio (ICER) was calculated expressing the net cost per a QALY gain. Table 3 includes the cost per QALY gain in the base-case scenario of each study and the study conclusion. The main results of the studies are also briefly discussed by highlighting the main patterns below.
Table 3.
Summary of results and conclusions of the included studies
| References | Country | Abbreviation of the compared regimens | Cost per QALY gain in base case scenario + Study conclusion | |
|---|---|---|---|---|
| Studies comparing treatment regimens and calculating cost per QALY gain (n = 26) | ||||
| Main et al.[29] | UK | FC-R vs FR; FC-R vs Clb | 13,189 GBP (FC-R vs FR); 6422 GBP (FC-R vs Clb) | Addition of R to FC is cost effective |
| Hornberger et al. [27] | USA | FC-R vs FC | 23,530 USD | |
| Adena et al. [26] | Australia | FC-R vs FC | 42,906 AUD | |
| Mandrik et al. [30] | Ukraine | FC-R vs FC | 8704 USD (1st line); 11,056 USD (R/R) | |
| Müller et al. [31] | Germany | FC-R vs FC | 17,979 EUR | |
| Williams et al. [46] | UK | FC-R vs FC | 16,308 GBP (Partitioned Survival); 13,189 GBP (Markov) | |
| Woods et al. [42] | UK | B vs Clb | 11,960 GBP | Bendamustine is cost effective in 1st line |
| Kongnakorn et al. [22] | USA | B vs A vs Clb | 50,619 USD (B vs Clb); dominant (B vs A) | |
| Becker et al. [32] | UK | G-Clb vs R-Clb vs Clb vs B vs R-B vs O-Clb | <30,000 GBP for each comparator | Obinutuzumab + chlorambucil is cost effective in 1st line |
| Blommestein et al. [33] | Netherlands | G-Clb vs R-Clb vs O-Clb vs Clb | <30,000 EUR for each comparator | |
| Casado et al. [34] | Spain | G-Clb vs R-Clb | 24,838 EUR | |
| Soini et al. [37] | Finland | G-Clb vs O-Clb vs R-B vs R-Clb vs Clb | 29,334 EUR (G-Clb vs Clb); 43,958 EUR (R-Clb vs Clb); 59,316 EUR (RB vs Clb); 82,159 EUR (O-Clb vs Clb) | |
| Paquete et al. [35] | Portugal | G-Clb vs R-Clb vs Clb | 20,397 EUR (G-Clb vs Clb); dominated (G-Clb vs R-Clb) | |
| Barnes et al. [38] | USA | Ibr vs Clb / G-Clb (theoretical alternative) | 189,000 USD | Ibrutinib is not cost effective in 1st line, price reduction is needed |
| Sinha and Redekop [36] | UK | Ibr vs G-Clb | 75,648 GBP | |
| Patel et al. [20] | USA | Ibr in the 1st line vs Ibr in the 3rd line | 2,350,041 USD | |
| Scott and Scott [23] | New Zealand | A vs FC-R |
46,016 NZD (Alemtuzumab); 60,012 (FCR) Alemtuzumab is cost effective in 3rd-line treatment |
|
| Howard et al. [28] | UK | FC-R vs FCM-miniR |
10,651 EUR (Cost saving with QALY loss) FCM-miniR produces a cost saving and QALY loss in 1st line |
|
| Herring et al. [40] | Canada | O-Clb vs Clb |
68,647 CAD Ofatumumab + chlorambucil is cost effective in first line |
|
| Hatswell et al. [44] | UK | O vs BSC |
130,563 GBP Ofatumumab is not cost effective for double-refractory patients |
|
| Mistry et al. [45] | UK | Ven vs BSC |
39,940 GBP (del(17p)/TP53); 47,370 GBP (non-del(17p)/TP53) Venetoclax is not cost effective for relapsed or refractory patients |
|
| Casado et al. [43] | Spain | Ide-R vs R |
29,990 EUR Idelalisib + rituximab is cost effective for relapsed or refractory patients |
|
| Vreman et al. [41] | UK | Acp vs Ibr |
61,941 GBP Acalabrutinib is not cost effective in relapsed patients |
|
| Weeks et al. [25] | USA | Ig vs No Ig |
6,000,000 USD Immune globulin is not cost effective in patients with high risk for infection |
|
| Buchanan et al. [19] | UK | Genetic testing + no Ibr vs Genetic testing + Ibr vs No genetic or genomic testing vs Genomic testing + Ibr vs Genomic testing + Ibr + O |
8565 GBP (Scenario 1 vs 3); 31,153 GBP (Scenario 2 vs 3); 50,559 GBP (Scenario 4 vs 3); 177,198 GBP (Scenario 5 vs 3) Use of genomic testing was not cost effective |
|
| Chen et al. [21] | USA | CIT regimens will be replaced by oral targeted therapies by 2025 vs CIT would remain the standard of care for the period |
189,000 USD More sustainable pricing strategies for targeted therapies are needed |
|
A alemtuzumab, Acp acalabrutinib, AUD Australian dollars, B bendamustine, BSC best supportive care, CAD Canadian dollars, CHOP cyclophosphamide-vincristine-prednisone-doxorubicin, CIT chemo-immunotherapy, Clb chlorambucil, EUR Euros, F fludarabine, FC fludarabine-cyclophosphamide, FCM-miniR fludarabine-cyclophosphamide-mitoxantrone-low-dose rituximab, FC-R fludarabine-cyclophosphamide-rituximab, GBP British pounds, G-Clb obinutuzumab-chlorambucil, Ibr ibrutinib, Ide-R idelalisib-rituximab, Ig immunoglobulin, NZD New Zealand dollars, O-Clb ofatumumab-chlorambucil, R rituximab, R-B rituximab-bendamustine, R-Chemo rituximab + chemotherapy, R-Clb rituximab-chlorambucil, R/R relapsed or refractory, USD United States dollars, Ven venetoclax
The addition of rituximab to the fludarabine–cyclophosphamide combination was investigated in six studies, four from Europe and a single study from Australia and the US [26, 27, 29–31, 46]. All studies used CIT in first line based on the CLL8 clinical trial, but a study from Ukraine also considered relapsed or refractory patients. All the studies found that the addition of rituximab is a cost-effective alternative, when taking into account the national cost-effectiveness thresholds.
We identified five studies that investigated first-line CLL patients who were not eligible for fludarabine-based therapy and received chlorambucil-based CIT regimens or chlorambucil alone [32–35, 37]. All the studies were conducted in Europe and used data from the CLL11 trial. All the studies concluded that when considering the national cost-effectiveness thresholds, obinutuzumab + chlorambucil is a cost-effective treatment regimen for fludarabine-ineligible first-line CLL patients.
Ibrutinib was investigated in three studies for first-line CLL patients, two of which were conducted in the US and one in the UK [20, 36, 38]. Different comparator alternatives were used in these studies, but all of them found that ibrutinib is not cost effective in first line. Interestingly, each study considered a base-case scenario with a given list price of ibrutinib, and then calculated the discount that would be required for being under the cost-effectiveness threshold.
Besides ibrutinib, alternative targeted therapies were also investigated in three studies [41, 43, 45]. One study compared venetoclax with best supportive care for relapsed or refractory patients in the UK and found that it was not cost effective in the base-case model. Another study compared the combination of idelalisib + rituximab with rituximab for relapsed or refractory patients in Spain and found that it was cost effective. The third study compared acalabrutinib with ibrutinib and found that it was not cost effective in relapsed patients in the UK.
Regarding the studies comparing treatment strategies, results of two studies are important to highlight [19, 21]. A study that compared alternatives based on using genetic and genomic testing in the UK found that the use genomic testing was unlikely to be cost effective using the standard ICER threshold. It highlighted that either a societal costing perspective or a lower price for targeted therapies could make the use of genomic testing cost effective in the UK. The other study compared the scenario where CIT regimens are replaced by oral targeted therapies by 2025 to the scenario where CIT remains the standard of care for the period. It concluded that the ICER would be above the cost-effectiveness threshold in the US, and therefore more sustainable pricing strategies for targeted therapies are needed.
Results of the remaining studies are not detailed here as they either investigated a therapy which has been mostly outdated since the publication of the paper or because they were similar to the abovementioned patterns with some minor changes. However, all results are presented in Table 3.
Results of the Risk of Bias Assessment
The completed ECOBIAS checklist of the 29 included studies can be found in Supplementary Material V (see ESM). Most studies had low risk of bias, however, two important issues were revealed. Internal consistency checking or validation procedures were only reported for the minority of the studies (7/29). This might be only a reporting bias, as validation is usually a standard element of model development, so it can be anticipated that it was done for more studies but not reported. The other issue was related to costing. While the currency was clearly reported for all studies, the year of costing was not clear for eight of the 29 studies. This is an important bias in the analyses as all studies used multiple data sources for costing, and in many cases the cost data was reflecting on different years. Other aspects of the quality assessment were generally reported with sufficient clarity. The study perspective was clearly defined for all studies, the costing approach was detailed in almost all of the cases (28/29), discounting was applied in the majority of the studies (24/29) and the model structure was also clearly presented for 28 evaluations. Sensitivity analysis to quantify uncertainties were incorporated into most studies (27/29). All other aspects of the risk of bias assessment are included in Supplementary Material V (see ESM).
Discussion
This study provides a review of economic evaluations in the field of CLL. Compared with previous studies [15, 16], this review is more up to date and did not limit the type of therapies that were considered. These are important aspects due to the recent advancements in the therapeutic area of CLL. We identified 29 scientific publications that included economic modelling to estimate the cost effectiveness of different treatment regimens and treatment strategies. It was important to differentiate between regimens and strategies, because in case of the former, the direct effects of specific therapies (single agent or combination) are assessed compared to other(s), which can be considered the traditional method of economic modelling. On the other hand, the simulation of treatment strategies includes multiple specific therapies during the course of the disease and adds more complexity to the simulated pathway, which resonates with the whole disease modelling approach in the scientific literature [47]. This approach usually requires more data and/or more assumptions to provide valid estimations, but on the other hand, it also provides better opportunities to capture complex issues such as the impact of new advancements on the whole patient pathway. The modelling results in these cases can be used in wider areas of decision support, such as defining actual clinical or financial guidelines for a disease.
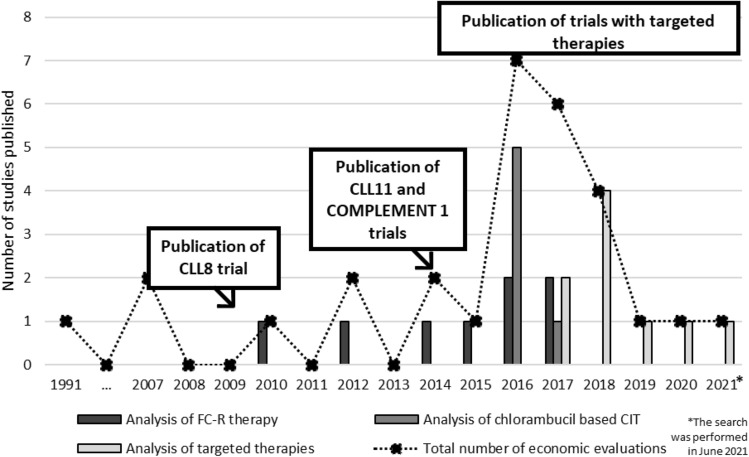
We were able to clearly distinguish three important trends in the literature on cost-effectiveness models in CLL; these are illustrated in Fig. 1. First, CIT regimens, mainly FC-R, were compared with chemotherapies during the 2010s, mostly based on the CLL8 trial results [48]. These studies provided justification for changing the therapeutic landscape and accelerated the use of CIT. Then, large clinical trials were published that compared CIT regimens with each other, such as the CLL10 or CLL11 trials [7, 49]. These well designed and highly publicized trials were used to build new cost-effectiveness models and support the reimbursement of different regimens, mostly favouring the obinutuzumab + chlorambucil combination based on the CLL11 trial results. The third trend includes the current ongoing tendency that attempts the simulation of treatment strategies, mainly predicting how targeted therapies will change the whole landscape of CLL treatment. This also includes simulating the impact of specific therapies such as ibrutinib, idelalisib or venetoclax. This trend is clearly ongoing as, after our literature search, further studies were published on venetoclax [50, 51]. The number of economic evaluations peaked in 2016 and 2017 when the three trends were overlapping (Fig. 1).
Fig. 1.
Publishing economic evaluations in chronic lymphocytic leukemia (CLL)—timeline and trend. CIT chemo-immunotherapy, FC-R fludarabine-cyclophosphamide-rituximab
Importantly, we were also able to identify a key modelling study that aimed to take into account the effect of genomic/genetic testing within a cost-effectiveness simulation [19]. The molecular features of the disease such as TP53 mutation, del(17p) and IGHV mutational status are known predictors of the efficacy of treatment strategies, thus impacting the health economics for different subgroups of patients [52, 53]. The identified study [19] could provide an important basis for upcoming works on analyzing personalized solutions. This will be very important as it can be anticipated that new therapeutic options will take advantage of improvements in molecular diagnostics and will focus on specific patient groups (e.g. patients at high risk for early relapse) [54].
The modelling approaches of the identified studies have been comprehensively investigated. We found that Markov modelling with a model structure of three health states (’progression-free’, ’progressed’, ’death’) can be considered the traditional basis to simulate the cost effectiveness of CLL therapies. However, many studies have added further complexity to this simple structure. These choices between methodological solutions can be justified by the research questions of the modelling exercise, though they may also be reflective of the availability of resources and opportunities to increase model complexity. Most notably, we listed those cases where additional health states were defined for different therapies, for response status and for no treatment in stable disease. The consideration of therapy type is important, for instance, because the QoL of CLL patients is compromised not only by the disease but also by the type of treatment regimen [55]. The response types (e.g. complete or partial responses, achieving undetectable MRD) should be considered to impact the health economic modelling including different interactions with different types of treatment. Therefore, economic evaluations that consider the effect of biomarkers should reflect this when establishing the model structure. Also, the option of having a ‘no treatment’ health status is also relevant to appropriately reflect the length of the wait and watch approach, which might be different based on the patients’ risk factors and earlier treatment regimens [56].
From the modelling methodology perspective, another important observation was that only one study used discrete event simulation (DES) [22]. A patient-level simulation method is mostly used when the individual patient history is important for the analysis and must be taken into account during the simulation. On the other hand, in Markov cohort models, the probability of a given transition is independent of the timing of earlier transitions [57]. A DES model was used in the case where bendamustine, alemtuzumab and chlorambucil therapies were compared. However, no clear justification was provided for the selection of this modelling methodology. Although the authors stated that the application of the DES approach is a strength of the study, because the model emulates health care processes more realistically, no further clarification was provided [22]. Another study found that the selection of the methodology to simulate patient survival does have an important effect on the evaluation results (partitioned survival modelling vs Markov modelling) [46].
Our study has important limitations. First, in this review, we did not include conference materials. Although they were identified through the literature search, we decided to not include them because we wanted to capture the modelling methodology in detail, and most conference materials have severe limitations in this respect. Second, the results and the interpretation of the cost-effectiveness analyses were only briefly described, since these were already captured in the literature on hematologic malignancies [58], and more importantly, the results are highly context-dependent with limited generalizability across the whole disease area. Thirdly, our literature search was conducted in June 2021 and since then new studies have been published investigating the combination of targeted therapies with monoclonal antibodies. Venetoclax was combined with rituximab for relapsed or refractory patients in Switzerland, and venetoclax was combined with obinutuzumab for first-line treatment of patients in the US [50, 51]. These studies were not investigated with our careful approach in this review due to time limitations. However, they support some of our findings such as the modelling approach, as both used the traditional three-state study design with partitioned survival modelling. Fourthly, we considered just two major literature databases (Medline, EMBASE) and did not apply a forward citation search. Finally, some important methodological aspects were not included in the work, because we wanted to keep the focus on the general characteristics and on the modelling approaches. Therefore, aspects of quantifying the costs and benefits, reliability of data sources or management of uncertainty should be further investigated in future studies.
Conclusion
The traditional approach of three-state Markov models was the cornerstone among the 29 health economic reports identified in CLL. However, this review highlights that model complexity should be further increased to address more complex research questions. These include (1) modelling subgroups of patients based on genetics and comorbidities, (2) considering different qualities of responses (complete response, partial response, MRD response) that interact differently with different treatment approaches, (3) including the risk of adverse events on different therapies and (4) considering different types and durations of therapeutic options. As personalized medicine approaches are gaining more recognition in hematology in general and in CLL in particular, future health economic models should also incorporate new strategies to capture the individualized patient pathways appropriately.
Supplementary Information
Below is the link to the electronic supplementary material.
Declarations
Funding
This work was conducted within the CLL-CLUE project, which received funding from the European Commission’s European Partnership for Personalised Medicine (ERAPerMed) programme. LL, SFR, AMP and AGN were supported by a grant of the Romanian National Authority for Scientific Research and Innovation, CCCDI—UEFISCDI, project number ERANET-PERMED-CLL-CLUE, within PNCDI III. TA was supported by the Academy of Finland (grant 344698), European Union’s Horizon 2020 Research and Innovation Programme (ERA PerMed CLL-CLUE project), the Cancer Society of Finland, and the Norwegian Cancer Society (Grant 216104). CN was additionally supported by the Danish Cancer Society.
Conflict of interest
The authors declare that that they have no conflict of interest.
Ethics approval
This study did not require ethical approval, because there was no involvement of human participants, their data or biological material.
Patient consent to participate/publish
Informed consent to participate or publish was not required for this study.
Data availability
The datasets generated and analyzed during the current study are available from the corresponding author on reasonable request.
Code availability
Not applicable for this research. No specific codes were developed for this study.
Authors contributions
LL, ZK and MC designed the study. LS, SFR, AIG, AMP, AGN conducted the literature review under the supervision of MC and LL. LL, ZK, CUN, TA and CM reviewed the collected data and prepared the draft manuscript. All authors reviewed and accepted the final version of the manuscript.
References
- 1.Moia R, Patriarca A, Deambrogi C, Rasi S, Favini C, Kodipad AA, et al. An update on: molecular genetics of high-risk chronic lymphocytic leukemia. Expert Rev Hematol. 2020;13(2):109–116. doi: 10.1080/17474086.2020.1697225. [DOI] [PubMed] [Google Scholar]
- 2.Mulligan SP, Tam CS. Chronic lymphocytic leukemia: diagnosis and clinical staging. Advances in the treatment of B-cell chronic lymphocytic leukemia; 2012. p. 6–15.
- 3.Mukkamalla SKR, Taneja A, Malipeddi D, Master SR. Chronic Lymphocytic Leukemia. StatPearls. Treasure Island (FL): StatPearls Publishing Copyright © 2021, StatPearls Publishing LLC.; 2021.
- 4.Eichhorst B, Robak T, Montserrat E, Ghia P, Niemann CU, Kater AP, et al. Chronic lymphocytic leukaemia: ESMO clinical practice guidelines for diagnosis, treatment and follow-up. Ann Oncol. 2021;32(1):23–33. doi: 10.1016/j.annonc.2020.09.019. [DOI] [PubMed] [Google Scholar]
- 5.Hallek M, Cheson BD, Catovsky D, Caligaris-Cappio F, Dighiero G, Döhner H, et al. iwCLL guidelines for diagnosis, indications for treatment, response assessment, and supportive management of CLL. Blood. 2018;131(25):2745–2760. doi: 10.1182/blood-2017-09-806398. [DOI] [PubMed] [Google Scholar]
- 6.WHO. Chronic lymphocytic leukemia. 2014 Review of Cancer Medicines on the WHO List of Essential Medicines. 2014.
- 7.Goede V, Fischer K, Busch R, Engelke A, Eichhorst B, Wendtner CM, et al. Obinutuzumab plus chlorambucil in patients with CLL and coexisting conditions. N Engl J Med. 2014;370(12):1101–1110. doi: 10.1056/NEJMoa1313984. [DOI] [PubMed] [Google Scholar]
- 8.Hallek M, Al-Sawaf O. Chronic lymphocytic leukemia: 2022 update on diagnostic and therapeutic procedures. Am J Hematol. 2021;96(12):1679–1705. doi: 10.1002/ajh.26367. [DOI] [PubMed] [Google Scholar]
- 9.Lachaine J, Beauchemin C, Guinan K, Thebault P, Aw A, Banerji V, et al. Impact of oral targeted therapy on the economic burden of chronic lymphocytic leukemia in Canada. Curr Oncol. 2021;28(1):332–345. doi: 10.3390/curroncol28010037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Montserrat E, Bauman T, Delgado J. Present and future of personalized medicine in CLL. Best Pract Res Clin Haematol. 2016;29(1):100–110. doi: 10.1016/j.beha.2016.08.009. [DOI] [PubMed] [Google Scholar]
- 11.Vellekoop H, Huygens S, Versteegh M, Szilberhorn L, Zelei T, Nagy B, et al. Guidance for the harmonisation and improvement of economic evaluations of personalised medicine. Pharmacoeconomics. 2021;39(7):771–788. doi: 10.1007/s40273-021-01010-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Miller JD, Foley KA, Russell MW. Current challenges in health economic modeling of cancer therapies: a research inquiry. Am Health Drug Benefits. 2014;7(3):153–162. [PMC free article] [PubMed] [Google Scholar]
- 13.Bullement A, Cranmer HL, Shields GE. A review of recent decision-analytic models used to evaluate the economic value of cancer treatments. Appl Health Econ Health Policy. 2019;17(6):771–780. doi: 10.1007/s40258-019-00513-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Kasteng F, Sobocki P, Svedman C, Lundkvist J. Economic evaluations of leukemia: a review of the literature. Int J Technol Assess Health Care. 2007;23(1):43–53. doi: 10.1017/S0266462307051562. [DOI] [PubMed] [Google Scholar]
- 15.Marsh K, Xu P, Orfanos P, Gordon J, Griebsch I. Model-based cost-effectiveness analyses for the treatment of chronic lymphocytic leukaemia: a review of methods to model disease outcomes and estimate utility. Pharmacoeconomics. 2014;32(10):981–993. doi: 10.1007/s40273-014-0187-1. [DOI] [PubMed] [Google Scholar]
- 16.Harkins RA, Patel SP, Flowers CR. Cost-effectiveness of new targeted agents in the treatment of chronic lymphocytic leukemia. Cancer J (United States). 2019;25(6):418–427. doi: 10.1097/PPO.0000000000000411. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Page MJ, McKenzie JE, Bossuyt PM, Boutron I, Hoffmann TC, Mulrow CD, et al. The PRISMA 2020 statement: an updated guideline for reporting systematic reviews. BMJ. 2021;372:n71. doi: 10.1136/bmj.n71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Adarkwah CC, van Gils PF, Hiligsmann M, Evers SM. Risk of bias in model-based economic evaluations: the ECOBIAS checklist. Expert Rev Pharmacoecon Outcomes Res. 2016;16(4):513–523. doi: 10.1586/14737167.2015.1103185. [DOI] [PubMed] [Google Scholar]
- 19.Buchanan J, Wordsworth S, Clifford R, Robbe P, Taylor JC, Schuh A, et al. Using genomic information to guide ibrutinib treatment decisions in chronic lymphocytic leukaemia: a cost-effectiveness analysis. Pharmacoeconomics. 2017;35(8):845–858. doi: 10.1007/s40273-017-0519-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Patel KK, Isufi I, Kothari S, Davidoff AJ, Gross CP, Huntington SF. Cost-effectiveness of first-line vs third-line ibrutinib in patients with untreated chronic lymphocytic leukemia. Blood. 2020;136(17):1946–1955. doi: 10.1182/blood.2020004922. [DOI] [PubMed] [Google Scholar]
- 21.Chen Q, Jain N, Ayer T, Wierda WG, Flowers CR, O'Brien SM, et al. Economic burden of chronic lymphocytic leukemia in the era of oral targeted therapies in the United States. J Clin Oncol. 2017;35(2):166–174. doi: 10.1200/JCO.2016.68.2856. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Kongnakorn T, Sterchele JA, Salvador CG, Getsios D, Mwamburi M. Economic implications of using bendamustine, alemtuzumab, or chlorambucil as a first-line therapy for chronic lymphocytic leukemia in the US: a cost-effectiveness analysis. Clinicoecon Outcomes Res. 2014;6:141–149. doi: 10.2147/CEOR.S55095. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Scott WG, Scott HM. Economic evaluation of third-line treatment with alemtuzumab for chronic lymphocytic leukaemia. Clin Drug Investig. 2007;27(11):755–764. doi: 10.2165/00044011-200727110-00002. [DOI] [PubMed] [Google Scholar]
- 24.Danese MD, Reyes CM, Gleeson ML, Halperin M, Skettino SL, Mikhael J. Estimating the population benefits and costs of rituximab therapy in the United States from 1998 to 2013 using real-world data. Med Care. 2016;54(4):343–349. doi: 10.1097/MLR.0000000000000486. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Weeks JC, Tierney MR, Weinstein MC. Cost effectiveness of prophylactic intravenous immune globulin in chronic lymphocytic leukemia. N Engl J Med. 1991;325(2):81–86. doi: 10.1056/NEJM199107113250202. [DOI] [PubMed] [Google Scholar]
- 26.Adena M, Houltram J, Mulligan SP, Todd C, Malanos G. Modelling the cost effectiveness of rituximab in chronic lymphocytic leukaemia in first-line therapy and following relapse. Pharmacoeconomics. 2014;32(2):193–207. doi: 10.1007/s40273-013-0125-7. [DOI] [PubMed] [Google Scholar]
- 27.Hornberger J, Reyes C, Shewade A, Lerner S, Friedmann M, Han L, et al. Cost-effectiveness of adding rituximab to fludarabine and cyclophosphamide for the treatment of previously untreated chronic lymphocytic leukemia. Leuk Lymphoma. 2012;53(2):225–234. doi: 10.3109/10428194.2011.605918. [DOI] [PubMed] [Google Scholar]
- 28.Howard DR, Munir T, McParland L, Rawstron AC, Chalmers A, Gregory WM, et al. Clinical effectiveness and cost-effectiveness results from the randomised, phase IIB trial in previously untreated patients with chronic lymphocytic leukaemia to compare fludarabine, cyclophosphamide and rituximab with fludarabine, cyclophosphamide, mitoxantrone and low-dose rituximab: the attenuated dose rituximab with chemotherapy in chronic lymphocytic leukaemia (ARCTIC) trial. Health Technol Assess. 2017;21(28):vii–373. doi: 10.3310/hta21280. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Main C, Pitt M, Moxham T, Stein K. The clinical effectiveness and cost-effectiveness of rituximab for the first-line treatment of chronic lymphocytic leukaemia: an evidence review of the submission from Roche. Health Technol Assess. 2010;14(Suppl. 2):27–32. doi: 10.3310/hta14suppl2-04. [DOI] [PubMed] [Google Scholar]
- 30.Mandrik O, Corro Ramos I, Knies S, Al M, Severens JL. Cost-effectiveness of adding rituximab to fludarabine and cyclophosphamide for treatment of chronic lymphocytic leukemia in Ukraine. Cancer Manag Res. 2015;7:279–289. doi: 10.2147/CMAR.S79258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Müller D, Fischer K, Kaiser P, Eichhorst B, Walshe R, Reiser M, et al. Cost-effectiveness of rituximab in addition to fludarabine and cyclophosphamide (R-FC) for the first-line treatment of chronic lymphocytic leukemia. Leuk Lymphoma. 2016;57(5):1130–1139. doi: 10.3109/10428194.2015.1070151. [DOI] [PubMed] [Google Scholar]
- 32.Becker U, Briggs AH, Moreno SG, Ray JA, Ngo P, Samanta K. Cost-effectiveness model for chemoimmunotherapy options in patients with previously untreated chronic lymphocytic leukemia unsuitable for full-dose fludarabine-based therapy. Value Health. 2016;19(4):374–382. doi: 10.1016/j.jval.2015.12.018. [DOI] [PubMed] [Google Scholar]
- 33.Blommestein HM, de Groot S, Aarts MJ, Vemer P, de Vries R, van Abeelen AF, et al. Cost-effectiveness of obinutuzumab for chronic lymphocytic leukaemia in The Netherlands. Leuk Res. 2016;50:37–45. doi: 10.1016/j.leukres.2016.09.005. [DOI] [PubMed] [Google Scholar]
- 34.Casado LF, Burgos A, González-Haba E, Loscertales J, Krivasi T, Orofino J, et al. Economic evaluation of obinutuzumab in combination with chlorambucil in first-line treatment of patients with chronic lymphocytic leukemia in Spain. Clinicoecon Outcomes Res. 2016;8:475–484. doi: 10.2147/CEOR.S114524. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Paquete AT, Miguel LS, Becker U, Pereira C, Pinto CG. Cost-effectiveness analysis of obinutuzumab for previously untreated chronic lymphocytic leukaemia in portuguese patients who are unsuitable for full-dose fludarabine-based therapy. Appl Health Econ Health Policy. 2017;15(4):501–512. doi: 10.1007/s40258-017-0321-2. [DOI] [PubMed] [Google Scholar]
- 36.Sinha R, Redekop WK. Cost-effectiveness of ibrutinib compared with obinutuzumab with chlorambucil in untreated chronic lymphocytic leukemia patients with comorbidities in the United Kingdom. Clin Lymphoma Myeloma Leuk. 2018;18(2):e131–e142. doi: 10.1016/j.clml.2017.12.005. [DOI] [PubMed] [Google Scholar]
- 37.Soini E, Hautala A, Poikonen E, Becker U, Kyttälä M, Martikainen J. Cost-effectiveness of first-line chronic lymphocytic leukemia treatments when full-dose fludarabine is unsuitable. Clin Ther. 2016;38(4):889–904.e14. doi: 10.1016/j.clinthera.2016.02.005. [DOI] [PubMed] [Google Scholar]
- 38.Barnes JI, Divi V, Begaye A, Wong R, Coutre S, Owens DK, et al. Cost-effectiveness of ibrutinib as first-line therapy for chronic lymphocytic leukemia in older adults without deletion 17p. Blood Adv. 2018;2(15):1946–1956. doi: 10.1182/bloodadvances.2017015461. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Dervaux B, Lenne X, Theis D, D'Alche-Gautier M-J, Rufat P, Cazin B, et al. Cost effectiveness of oral fludarabine in chronic lymphocytic leukaemia: the French case. J Med Econ. 2007;10(4):339–354. doi: 10.3111/13696990701571585. [DOI] [Google Scholar]
- 40.Herring W, Pearson I, Purser M, Nakhaipour HR, Haiderali A, Wolowacz S, et al. Cost effectiveness of ofatumumab plus chlorambucil in first-line chronic lymphocytic leukaemia in Canada. Pharmacoeconomics. 2016;34(1):77–90. doi: 10.1007/s40273-015-0332-5. [DOI] [PubMed] [Google Scholar]
- 41.Vreman RA, Geenen JW, Hövels AM, Goettsch WG, Leufkens HGM, Al MJ. Phase I/II clinical trial-based early economic evaluation of acalabrutinib for relapsed chronic lymphocytic leukaemia. Appl Health Econ Health Policy. 2019;17(6):883–893. doi: 10.1007/s40258-019-00496-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Woods B, Hawkins N, Dunlop W, O'Toole A, Bramham-Jones S. Bendamustine versus chlorambucil for the first-line treatment of chronic lymphocytic leukemia in England and Wales: a cost-utility analysis. Value Health. 2012;15(5):759–770. doi: 10.1016/j.jval.2012.03.1389. [DOI] [PubMed] [Google Scholar]
- 43.Casado LF, Hernández JÁ, Jarque I, Echave M, Casado MA, Castro A. Cost-utility analysis of idelalisib in combination with rituximab in relapsed or refractory chronic lymphocytic leukaemia. Eur J Haematol. 2018;100(3):264–272. doi: 10.1111/ejh.13007. [DOI] [PubMed] [Google Scholar]
- 44.Hatswell AJ, Thompson GJ, Maroudas PA, Sofrygin O, Delea TE. Estimating outcomes and cost effectiveness using a single-arm clinical trial: ofatumumab for double-refractory chronic lymphocytic leukemia. Cost Eff Resour Alloc. 2017;15(1):1–8. doi: 10.1186/s12962-017-0071-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Mistry H, Nduka C, Connock M, Colquitt J, Mantopoulos T, Loveman E, et al. Venetoclax for treating chronic lymphocytic leukaemia: an evidence review group perspective of a NICE single technology appraisal. Pharmacoeconomics. 2018;36(4):399–406. doi: 10.1007/s40273-017-0599-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Williams C, Lewsey JD, Mackay DF, Briggs AH. Estimation of survival probabilities for use in cost-effectiveness analyses: a comparison of a multi-state modeling survival analysis approach with partitioned survival and Markov decision-analytic modeling. Med Decis Mak Int J Soc Med Decis Mak. 2017;37(4):427–439. doi: 10.1177/0272989X16670617. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Tappenden P, Chilcott J, Brennan A, Squires H, Glynne-Jones R, Tappenden J. Using whole disease modeling to inform resource allocation decisions: economic evaluation of a clinical guideline for colorectal cancer using a single model. Value Health. 2013;16(4):542–553. doi: 10.1016/j.jval.2013.02.012. [DOI] [PubMed] [Google Scholar]
- 48.Hallek M, Fischer K, Fingerle-Rowson G, Fink AM, Busch R, Mayer J, et al. Addition of rituximab to fludarabine and cyclophosphamide in patients with chronic lymphocytic leukaemia: a randomised, open-label, phase 3 trial. Lancet. 2010;376(9747):1164–1174. doi: 10.1016/S0140-6736(10)61381-5. [DOI] [PubMed] [Google Scholar]
- 49.Eichhorst B, Fink AM, Bahlo J, Busch R, Kovacs G, Maurer C, et al. First-line chemoimmunotherapy with bendamustine and rituximab versus fludarabine, cyclophosphamide, and rituximab in patients with advanced chronic lymphocytic leukaemia (CLL10): an international, open-label, randomised, phase 3, non-inferiority trial. Lancet Oncol. 2016;17(7):928–942. doi: 10.1016/S1470-2045(16)30051-1. [DOI] [PubMed] [Google Scholar]
- 50.Barbier M, Durno N, Bennison C, Örtli M, Knapp C, Schwenkglenks M. Cost-effectiveness and budget impact of venetoclax in combination with rituximab in relapsed/refractory chronic lymphocytic leukemia in Switzerland. Eur J Health Econ. 2022;23(5):837–846. doi: 10.1007/s10198-021-01398-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Chatterjee A, Shapouri S, Manzoor BS, Ravelo A, Sail K, Qendri V, et al. Cost-effectiveness of a 12-month fixed-duration venetoclax treatment in combination with obinutuzumab in first-line, unfit chronic lymphocytic leukemia in the United States. J Manag Care Spec Pharm. 2021;27(11):1532–1544. doi: 10.18553/jmcp.2021.27.11.1532. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Moia R, Patriarca A, Schipani M, Ferri V, Favini C, Sagiraju S, et al. Precision medicine management of chronic lymphocytic leukemia. Cancers (Basel) 2020;12(3):642. doi: 10.3390/cancers12030642. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Niemann CU. Cost-effectiveness targeting CLL. Blood. 2020;136(17):1896–1898. doi: 10.1182/blood.2020006949. [DOI] [PubMed] [Google Scholar]
- 54.Guarente V, Sportoletti P. Lessons, challenges and future therapeutic opportunities for PI3K inhibition in CLL. Cancers (Basel). 2021;13(6):1280. doi: 10.3390/cancers13061280. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Holtzer-Goor KM, Schaafsma MR, Joosten P, Posthuma EF, Wittebol S, Huijgens PC, et al. Quality of life of patients with chronic lymphocytic leukaemia in the Netherlands: results of a longitudinal multicentre study. Qual Life Res. 2015;24(12):2895–2906. doi: 10.1007/s11136-015-1039-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Muchtar E, Kay NE, Parikh SA. Early intervention in asymptomatic chronic lymphocytic leukemia. Clin Adv Hematol Oncol. 2021;19(2):92–103. [PubMed] [Google Scholar]
- 57.Standfield L, Comans T, Scuffham P. Markov modeling and discrete event simulation in health care: a systematic comparison. Int J Technol Assess Health Care. 2014;30(2):165–172. doi: 10.1017/S0266462314000117. [DOI] [PubMed] [Google Scholar]
- 58.Saret CJ, Winn AN, Shah G, Parsons SK, Lin PJ, Cohen JT, et al. Value of innovation in hematologic malignancies: a systematic review of published cost-effectiveness analyses. Blood. 2015;125(12):1866–1869. doi: 10.1182/blood-2014-07-592832. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The datasets generated and analyzed during the current study are available from the corresponding author on reasonable request.