Abstract
We conducted a systematic review and meta-analysis of randomized control trials to formally assess the safety and efficacy of autologous whole cell vaccines as immunotherapies for solid tumors. Our primary safety outcome was number, and grade of adverse events. Our primary efficacy outcome was clinical responses. Secondary outcomes included survival metrics and correlative immune assays. We searched MEDLINE, Embase, and the Cochrane Central Register of Controlled Trials for studies published between 1946 and August 2020 using any autologous whole cell product in the treatment of any solid tumor. The Cochrane Randomized Controlled Trial risk of bias tool was used to assess risk of bias. Eighteen manuscripts were identified with a total of 714 patients enrolled in control and 808 in vaccine arms. In 698 patients receiving at least one dose of vaccine, treatment was well tolerated with a total of 5 grade III or higher adverse events. Clinical response was reported in a minority (n = 2, 14%) of studies. Autologous cell vaccines were associated with improved overall (HR 1.28, 95% CI 1.01–1.63) and disease-free survival (HR 1.33, 95% CI 1.05–1.67) over thirteen and ten trials respectively. Where reported, immune assays correlated well with clinical outcomes. Our results suggest that autologous whole cell vaccination is safe and efficacious in increasing survival in patients undergoing treatment for solid tumors.
Registration: PROSPERO CRD42019140187.
Subject terms: Cancer, Surgical oncology, Cancer immunotherapy
Introduction
Surgical excision represents a cornerstone in the treatment of solid tumors. Despite significant advances in adjuvant therapies, disseminated disease remains a chief cause of mortality following operative management1. Immunotherapies have shown promising results in the treatment of certain solid malignancies and early studies suggest that they may provide benefit in the adjuvant setting following surgical treatment of solid tumors2–4.
Autologous whole cell vaccination represents one approach to immunotherapy in which a patient’s own tumor cells serve as a source of antigen. Following ex vivo isolation and manipulation, these cells are rendered replication defective and re-administered to the patient along with an immunogen, to produce an anti-tumor response in vivo. This approach has several theoretical advantages over other forms of immunotherapy. These include the breadth of antigenic coverage included in the vaccine, and the diversity of immune cell populations that can be recruited by such a product. Furthermore, unlike other approaches to cancer vaccination, the autologous product is highly specific to a patient’s tumor but does not require sequencing level knowledge of the cancer’s antigenic landscape5,6.
Numerous early-stage clinical trials have employed autologous whole cell vaccines as adjuvants to the surgical treatment of cancer. Currently, two autologous whole cell products are being investigated in this context in phase III clinical trials7,8.
While the results of these studies are eagerly anticipated, several other RCTs have been previously published showing positive results. Despite this, there is little formal consensus on the overall safety and efficacy of these products or in which disease they are most likely to be of benefit. In order to guide further development in this field, we have conducted a systematic review of randomized control trials investigating the use of autologous whole cell vaccines in the treatment of solid tumors. Our primary safety outcome was number and grade of adverse events while our primary efficacy outcome was clinical response. Secondary outcomes included survival metrics and correlative immune assays while tertiary outcomes focussed on health utility and economic factors. The results of this study serve to guide further investigations into the use of autologous whole cell vaccines in treating solid tumors and facilitate the design of future clinical trials.
Methods
The results in this manuscript are presented in accordance with Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) guideline (Appendix 1)9. The protocol has been documented on PROSPERO (CRD42019140187) and previously published6. Our protocol was designed for use both in the current systematic review, as well as in a systematic review investigating the use of autologous cell vaccination in the treatment of hematologic maligancies6.
Search strategy
The search strategy employed in this manuscript was developed in collaboration with an information specialist and clinical expert in the field and is available in Appendix 2. The search was applied to MEDLINE (OVID interface, including In-Process and Epub Ahead of Print), Embase (OVID interface), and the Cochrane Central Register of Controlled Trials (Wiley Interface) for articles published between 1946 and August 6th, 2020. Forward and reverse citation searching were also performed on any studies which were deemed to meet eligibility criteria10. No language restrictions were imposed on the search. Clinicaltrias.gov was not included in the search as a decision was made to focus on results of published RCTs.
Eligibility criteria
Eligible studies were clinical trials which employed an autologous whole cell vaccine product in a solid malignancy. Any study employing an autologous whole cell product as part of the intervention was considered, however studies using only allogeneic or lysate vaccines were excluded. No restrictions were placed in terms of patient demographics, publication date, disease site, or prior/concurrent treatments. Only randomized controlled trials were considered for this review and single arm studies, non-randomized trials, and randomized trials without a control group were excluded. Similarly, only complete manuscripts detailing clinical trials were included. Case reports, conference abstracts, letters, reviews, and editorials were not considered.
Study selection and data collection processes
Titles and abstracts of all studies captured by our search strategy were screened by two out of four possible reviewers independently (DJB, MAK, STK, ABM) for eligibility using Distiller Systematic Review Software (DistillerSR, Evidence Partners, Ottawa, Canada). Any conflicts were flagged by the software and reviewers met to resolve the conflict. In the event of two reviewers not reaching a consensus, a third reviewer was included to facilitate resolution. Studies that were deemed eligible at this point then proceeded to full text screening which was similarly carried out in duplicate using DistillerSR. Studies that were deemed eligible at both the levels of title/abstract and whole text screening were included in the review and proceeded to data extraction. Data extraction was performed in duplicate (DJB, MAK) using standardized forms in DistillerSR. Extracted data included study details (country and year of publication, recruitment period, follow up period, patients enrolled in treatment and control groups), patient characteristics (malignancy and stage), intervention details (fresh vs. frozen and fixed vs. irradiated vaccine, dosage and administration details, adjuvants used, and prior/concurrent interventions), adverse events reported, clinical response data, overall and disease-free survival, and results of immune assays. Prior to data extraction, forms were pilot tested by two independent reviewers (DJB, JM). As with study selection, conflicts were resolved first through discussion between reviewers and then by a third reviewer if a consensus was not reached.
Risk of bias
Risk of bias was assessed by two independent reviewers (DJB, MAK) using the Cochrane Randomized Controlled Trial risk of bias tool11. Discrepancies were flagged by the Distiller software and reviewers met for conflict resolution. If a consensus was not obtained by two reviewers, a third party (JM) was used as a tie breaker.
Outcomes
The primary outcomes assessed by this systematic review were safety and efficacy of the autologous cell products based on reported adverse events (AEs) and clinical response respectively. Clinical responses included complete response (CR) and overall response (OR). In our published protocol, clinical response was primarily intended to be applied to our companion study of autologous cell vaccination hematologic malignancies where CR and OR could be reported in the context of minimal residual disease6,12. We nevertheless recorded and reported on this outcome where it was reported in studies investigating solid tumors.
Secondary outcomes included survival metrics (overall and disease-free survival) and immune assays. Information on immune assays included whether specific assays including ELISpot, delayed type hypersensitivity (DTH), and intracellular cytokine staining (ICS) were performed and whether positive outcomes as defined by the studies were observed and corelated with clinical outcomes.
Tertiary outcomes were metrics of reported health-related quality of life, health utility, and biological assays that correlate with efficacy.
Data analysis
Studies were pooled using Comprehensive Meta-Analyst (version 3; Biostat Inc., USA). For dichotomous outcomes (e.g. CR and OR), risk ratios were calculated using a random-effects analysis based on the Der-Simonian Laird model and presented with 95% confidence intervals. For time-to-event outcomes (i.e. disease-free and overall survival), hazard ratios were calculated using a random-effects generic inverse-variance model, and presented with 95% confidence intervals. Statistical heterogeneity was assessed using the I2 statistic. Thresholds for determining heterogeneity were as recommended by the Cochrane Handbook (with 30–60% representing moderate, 50–90% representing substantial, and 75–100% representing considerable heterogeneity)13. Publication bias was evaluated using funnel plots and Egger’s regression, where sufficient data were available (Cochrane recommends at least 10 studies)13. A priori subgroup analyses included cancer type, or by type of adjuvant administered.
Deviations from published protocol
In our published protocol we initially considered all clinical trials using autologous whole cell vaccines in the treatment of solid tumors without specifying that the study be an RCT. Our team decided to focus only on randomized control trials as they would provide the most robust evidence. Due to the range in dates over which studies were published and difficulties in obtaining updated contact information we did not contact authors for missing information.
Ethics approval and consent to participate
Ethics approval is not required for this systematic review as the review uses solely published literature.
Results
Study selection and characteristics
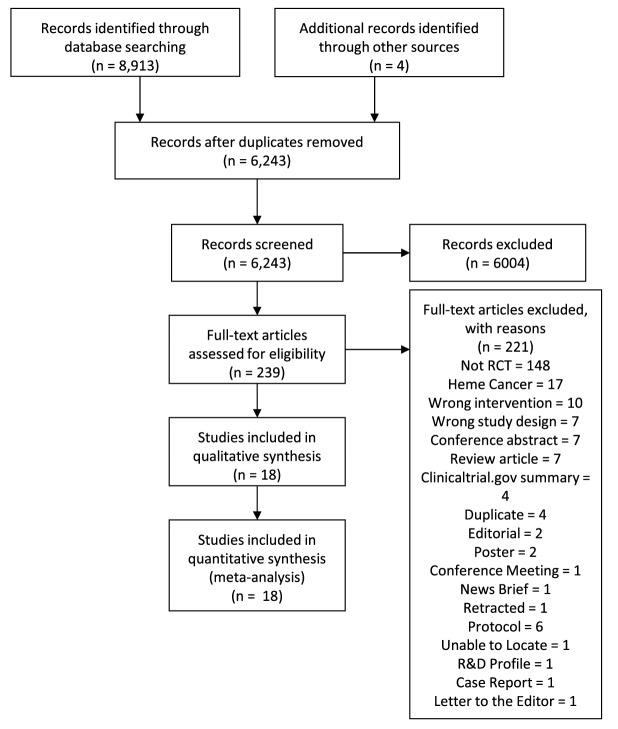
Our search strategy yielded 8913 citations representing 6243 unique manuscripts. After screening, a total of 14 randomized clinical trials met eligibility and an additional four reports were identified through forward and reverse citation searching and were deemed to meet eligibility criteria. Four of these studies were updates on trials reported by an earlier study captured in our systematic review. Thus, a total of 14 unique randomized clinical trials were included in our systematic review and meta-analysis (Fig. 1). In total there were 714 patients in the control groups, 808 patients enrolled to receive vaccination, and 698 being vaccinated with at least one dose. The characteristics of the trials are described in Table 1. In brief, studies spanned from 1977 to 2020 with half of the reports being published prior to 2000 and the other half in 2000 or after. The most common countries out of which trials were based were the United States (n = 4) and China (n = 3), with two trials occurring in the United Kingdom and one study in Italy, Germany, the Netherlands, Australia, and Israel respectively. The most frequent disease site was the colon/rectum (n = 5), followed by hepatocellular carcinoma (HCC) (n = 3), and renal cell carcinoma (RCC) (n = 2). One trial was performed in each of ovarian cancer, glioblastoma, lung cancer, and melanoma. Reported follow up times ranged from 12 months to a mean +/− SD of 116.1 +/− 23.8 months. In most studies, the patients were given the vaccine as an adjuvant following resection of the primary tumor. However, in the studies by Bota et al., and Embleton et al./McIllmurray et al., patients enrolled in the trial were undergoing surgery for a recurrence after previous therapy14–16. Similarly, Schulze et al. used an autologous cell product as an adjuvant following surgical resection of liver metastasis from a previously treated synchronous or metachronous colorectal tumor17. In the study by Hoover et al. some patients had had prior therapies while others had not18.
Figure 1.
Study selection flow chart.
Table 1.
Study characteristics.
| Studies | Country | Multi-center? | Centralized manufacture protocol? | Malignancy | Stage | Patients (n) | Recruitment Period | Follow up period | Previous interventions | Vaccine name | Trial sponsor | ||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Control | Vaccine Group | ||||||||||||
| enrolled | vaccinated | ||||||||||||
|
Oh (2020) Oh (2016) |
USA | Yes | Yes | Ovarian | III, IV+ | 11 | 20 (31)° | 20 (31)° | 2011–2015 | Median 43.4mo (10.1–77.6mo) | No | Vigil® | Gradalis, Inc |
| Bota (2018) | USA | No | NA | Glioblastoma | IV+ | 4† | 5† | 5† | NR | NR | Surgery, chemotherapy, radiotherapy | ERC1671 (Gliova™) | Eiptopoietic Research Corporation |
| Schulze (2009) | Germany | Yes | NR | Colon | I–IV+ | 25 | 25 | 25 | 1991–1998 | Mean (+/− SD): 116.1 ± 23.8mo vaccine, 112.4 ± 18.5 control | Surgery, chemotherapy, radiotherapy for synchronous / metachronous colon or rectal cancer | N/A | Unclear |
| Peng (2006) | China | No | NA | HCC | I, II | 35 | 40 | 32 | 1999–2003 | Median +/− SD: 33.6 ± 8.7 | NR | N/A | Science Developing Plan Fund of Guandgong Province |
| Uyl-de Groot (2005), Vermorken (1999) | Netherlands | Yes | Yes | Colon | II, III | 126 | 128 | 128 | 1987–1996 | Median 5.8 years | No | OncoVAX | Intracel Corporation |
| Peng (2005) | China | No | NA | HCC | I–III | 26 | 30 | 24 | 2000–2003 | Median > 42 months | NR | N/A | Natural Science Foundation of Guangdong Province |
| Kuang (2004) | China | No | NA | HCC | I–III | 21 | 19 | 18 | 2001–2003 | Median 15 months (8–28) | NR | N/A | Ministry of Education, Culture, Sports, Science and Technology of Japan |
| Harris (2000) | United States | Yes | No | Colon | II, III | 153 | 205 | 205* | 1986–1993 | Median 7.6 years | No | N/A |
Public Health Service Grants (National Cancer Institute, National Institute of Health, Department of Health and Human Services) Intracel |
| Galligioni (1996) | Italy | NR | NR | RCC | I–III | 54 | 60 | 60 | 1987–991 | 61 months | NR | N/A | Unclear |
| Hoover (1993) | United States | Yes | No | Colon / Rectum | I–III | 39 | 50 | 41 | 1981–1990 | Median 93 months (colon), 57 months (rectal) | None within 5 years of enrolment | N/A |
National Institutes of Health Organon Teknika/Biotechnology Research Institute |
| Gray (1989, 1988) | Australia | Yes | Yes | Colon / Rectum | II, III | 145 | 148 | 129 | 1978–1981 | 5 years | NR | N/A | Unclear |
| Adler (1987) | Israel | NR | NR | RCC | I–IV+ | 19 | 24 | 24 | 1980–1985 | Median 30 months | NR | N/A | Frieda and Shmuel Sharfhartz Memorial Cancer Research Fund |
| Souter (1981) | United Kingdom | NR | NR | Lung | I, II | 49 | 46 | 34 | NR | NR | No | N/A | Cancer Research Campaign Grant |
| Embleton (1978), McIllmurray (1977) | United Kingdom | No | NA | Melanoma | II | 7 | 8 | 8 | NR | 12 months | No | N/A | Cancer Research Campaign Grant |
NA not applicable, NR not reported, HCC Hepatocellular Carcinoma, RCC Renal Cell Carcinoma.
°In the study by Oh (2016), 20 patients were randomized to the treatment group and 11 to the control; subsequently 10 additional patients were de-randomized and placed into treatment group. Our survival analysis focusses only on those who were randomized.
†Numbers from interim analysis.
*Although 205 patients were vaccinated, only 150 patients were deemed “analyzable” based on adequacy of vaccines and sufficient follow up information.
Intervention details
In all trials the primary intervention used was surgery (Table 2). In twelve out of fourteen trials, the vaccine was given exclusively as an adjuvant following surgery with curative intent. In the study by Adler et al. an autologous vaccine product was given as an adjuvant to patients with stage III or lower renal cancer while the vaccine represented a palliative measure in surgically treated patients with stage IV disease19. In the trial described by Bota et al., patients had glioblastoma that was treated with surgery in which an R2 resection (gross residual tumor) was performed14. Twelve of fourteen studies specified whether a fresh or frozen product was given, and in all 12, the vaccine had been frozen prior to administration. Eleven trials specifically reported irradiating cells prior to administration whereas the remaining three trials employed a fixed cell product. Only three studies reported experiencing issues with vaccine quality, however 10 studies did not report on this metric. In all but one trial (which also employed endolymphatic installation), vaccines were administered exclusively through a subcutaneous route. Reported doses ranged from 0.1 to 100 × 106 cells per dose, with numbers of doses administered ranging from 1 to 24. No study reported on a correlation with number of doses and outcomes. The bulk of the studies employed BCG (six trials) or GM-CSF (five trials) with alternative or additional adjuvants being miRNA engineered into the vaccine, Newcastle Disease Virus (NDV), Interleukin-2 (IL-2), Vibrio cholerae neuraminidase (VCN), and C. parvum. In three trials the vaccine was given alongside another therapy including chemotherapy, radiation, or hormone therapy.
Table 2.
Intervention Details.
| Studies | vaccine manufacturing considerations | Vaccine administration | Vaccine adjuvant | Other interventions | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Fresh vs frozen | Irradiated cells? | Fixed cells? | Quality control issues? | Lowest dose (E6 cells) | Highest dose (E6 cells) | # Doses | Administration route | Identity | Incorporation strategy | Primary intervention | Concurrent therapies | |||
| Intended | Provided | |||||||||||||
|
Oh (2020) Oh (2016) |
Frozen | Yes | No | NR | 4 | 10 | 4–12 | mean 7.8 | Subcutaneous | GM-CSF; TGFβ targeting miRNA | Transfection | Surgery + Chemotherapy | None | |
| Bota (2018) | Frozen | Yes | No | NR | 0.1 | 1 | 2 autologous cells, 3 allogeneic cells | 2 autologous cells, 3 allogeneic cells* | Subcutaneous | GM-CSF, CPA, allogenic lysate | Mixed with vaccine | Surgery | Chemotherapy | |
| Schulze (2009) | Frozen | Yes | No | NR | 10 | 6 | 6 (18 patients), 7 (1 patient), 11 (1 patient), 12 (1 patient), 3 (2 patients), 4 (1 patient), 5 (3 patients) | Subcutaneous | NDV | Infection (non-replicating) | Surgery | None | ||
| Peng (2006) | Frozen | NR | Yes | NR | NR; dose consists of 40uL of cells | 3 | 3 | Subcutaneous | GM-CSF, Il-2 microspheres, tuberculin | Mixed with vaccine | Surgery | None | ||
| Uyl-de Groot (2005), Vermorken (1999) | Frozen | Yes | No | NR | 10 | 4 | 4 (101 patients), 3 (1 patient), 1 (1 patient), unknown (25 patients) | Subcutaneous | BCG | Mixed with vaccine | Surgery | None | ||
| Peng (2005) | Frozen | NR | Yes | NR | NR; consists of 10uL of cells | 3 | 3 (24 patients), unspecified (6 patients) | Subcutaneous | GM-CSF, Il-2, tuberculin | Mixed with vaccine | Surgery | None | ||
| Kuang (2004) | NR | NR | Yes | NR | NR; dose consists of 40uL of cells | 3 | Unclear | Subcutaneous | Il-2, GM-CSF | Mixed with vaccine | Surgery | None | ||
| Harris (2000) | Frozen | Yes | No | Yes | 10 | 3 | Unclear | Subcutaneous | BCG | Mixed with vaccine | Surgery | None | ||
| Galligioni (1996) | Frozen | Yes | No | NR | 10 | 3 | Unclear | Subcutaneous | BCG | Mixed with Vaccine | Surgery | None | ||
| Hoover (1993) | Frozen | Yes | No | Yes | 10 | 3 | 4 (1 patient), 3 (40 patients), 1 (1 patient) | Subcutaneous | BCG | Mixed with Vaccine | Surgery | Radiation | ||
| Gray (1989, 1988) | Frozen | Yes | No | No | 0.5 | 18 | NR | Subcutaneous | BCG; VCN treatment of cancer cells | Scarification | Surgery | None | ||
| Adler (1987) | Frozen | Yes | No | NR | 3 | 100 | 5–6 | ≤ 24 / Unclear | Subcutaneous & Endolymphatic Installation | BCG | Mixed with Vaccine | Surgery | Hormone Therapy | |
| Souter (1981) | Frozen | Yes | No | Yes | 20 | 1 | 1 | Subcutaneous | C. parvum | Mixed | Surgery | None | ||
| Embleton (1978), McIllmurray (1977) | NR | Yes | No | NR | 50 | 1 | 1 | Subcutaneous | BCG | Mixed with Vaccine | Surgery | None | ||
GM-CSF granulocyte macrophage colony stimulating factor, TGFβ tumor growth factor beta, miRNA microRNA, NR none reported, CPA cyclophosphamide, NDV Newcastle disease virus, Il-2 interleukin 2, VCN vibrio-cholera neuraminidase.
*in the study by Bota et al.(2018) it was unclear how many cycles of the 2 autologous cell and 3 allogeneic cell vaccines were provided.
Primary outcomes
Safety
Adverse event reporting for control arm patients was inconsistently reported and therefore no pooled analysis could be performed. The data for adverse events is therefore reported descriptively and in tabular format (Table 3). Overall, the vaccine products were well tolerated with a total of five adverse events grade 3 or greater. In the trial by Bota et al., four grade 3 adverse events were reported in patients in the treatment arm while eight were reported in the control arm14. These events were mostly neurological in nature including headaches and gait disturbances. In the study by Gray et al., one patient in the vaccine arm developed end stage renal failure20,21. Notably, there were no deaths associated with administration of the autologous cell products in any study. The most frequently reported single adverse event was local complications such as erythema or pruritis. Fever was also a commonly reported AE, while other AEs that were not local complications or fever included headaches, dizziness, nausea or lymphadenopathy. Some studies reported adverse events as number of events while others reported the total number of patients experiencing the event on any injection and others provided both metrics.
Table 3.
Adverse events.
| Study | Vaccinated patients (n) | Doses provided per patient | Serious (Grade 3+) adverse events (n of events) |
All adverse events (n of events) | Number of patients experiencing any AE | ||
|---|---|---|---|---|---|---|---|
| Total (n) |
Local complication (n) | Fever (n) |
|||||
|
Oh (2020) Oh (2016) |
31° | mean 7.8 (max 12) | None | 93 | 93 | 0 | NR |
| Bota (2018)† | 5† | 2 autologous cells, 3 allogeneic cells* | 4 | 162 | 67 | NR | NR |
| Schulze (2009) | 25 | 6 (18 patients), 7 (1 patient), 11 (1 patient), 12 (1 patient), 3 (2 patients), 4 (1 patient), 5 (3 patients) | None | 5 | 4 | 0 | 4 |
| Peng (2006) | 32 | 3 | None | 62 | 7 | 30 | 20 |
| Uyl-de Groot (2005), Vermorken (1999) | 128 | 4 (101 patients), 3 (1 patient), 1 (1 patient), unknown (25 patients) | None | NR | NR | NR | 128 |
| Peng (2005) | 24 | 3 (24 patients), unspecified (6 patients) | None | 72 | 72 | 0 | 24 |
| Kuang (2004) | 18 | Unclear | None | NR | NR | 0 | 18 |
| Harris (2000)± | 205 | Unclear | None | NR | NR | NR | 162 |
| Galligioni (1996) | 60 | Unclear | None | NR | NR | NR | NR |
| Hoover (1993) | 41 | 4 (1 patient), 3 (40 patients), 1 (1 patient) | None | NR | 82 | NR | 41 |
| Gray (1989, 1988) | 129 | NR | 1 | NR | NR | NR | NR |
| Adler (1987) | 24 | ≤ 24 / Unclear | This study did not report on adverse events | ||||
| Souter (1981) | 34 | 1 | This study did not report on adverse events | ||||
| Embleton (1978), McIllmurray (1977) | 8 | 1 | None | NR | 4 | NR | NR |
NR not reported.
°31 patients received vaccination; 20 of these patients were randomized to the vaccine group and 11 additional patients were placed in the vaccine group in a de-randomized fashion.
†Bota (2018) was the only study to provide information on AEs for control; 8 serious AEs, 58 total AEs in control group.
*in the study by Bota et al. (2018) it was unclear how many cycles of the 2 autologous cell and 3 allogeneic cell vaccines were provided.
±although only 150 patients were considered “analyzable” in the study by Harris (2000), 205 were vaccinated.
Efficacy
Overall response was reported in two studies in which vaccinated patients had measurable disease at the time of vaccination (Supplementary Fig. 1)14,19. There was no significant difference in overall response between the vaccine and control groups (RR 1.86; 95% CI 0.48–8.16), however data remains limited. There were no studies which reported complete response.
Secondary outcomes: overall and disease-free survival
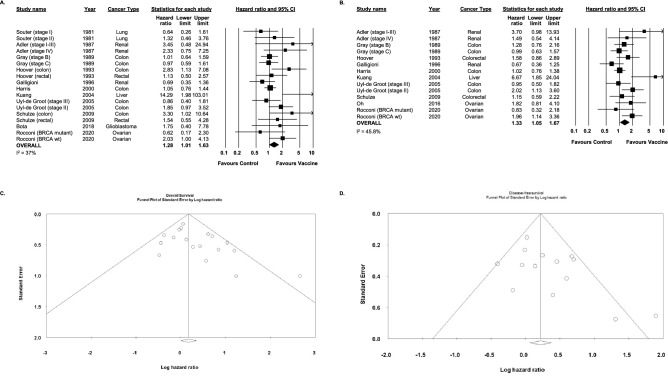
Thirteen studies reported on OS (Fig. 2a). Cumulatively, vaccination was associated with a statistically significant improvement in OS (HR 1.28, 95% CI 1.01–1.63 I2 = 37%). Similarly, in the ten studies in which DFS was reported, survival was improved in patients receiving vaccination (HR 1.33, 95% CI 1.05–1.57 I2 = 45.8%, Fig. 2b). Meta-bias assessment suggested publication bias for overall survival (Fig. 2c, Egger’s regression intercept 1.36, 95% CI 0.13–2.59, p < 0.05) but not disease-free survival (Fig. 2d, Egger’s regression intercept 1.76, 95% CI 0.12–3.64, p = 0.06).
Figure 2.
(A,B) Hazard ratio and accompanying 95% confidence interval for overall survival (A) and disease-free survival (B) in randomized control trials of patients treated with autologous whole cell vaccines for solid tumors. (C) Funnel plot for studies reporting on overall survival. (D) Funnel plot for studies reporting on disease-free survival.
Secondary outcomes: correlative immune assays
Eleven trials reported some form of immune assay (Table 4). The most frequently employed assay was a delayed type hypersensitivity (DTH) response, which was carried out in eight of 14 total trials. Of these eight trials, seven reported positive DTH responses in one or more patients and one study did not observe any DTH responses. In five of the seven trials patients who had a DTH response were more likely to have a better clinical outcome. The two remaining trials did not correlate responses with clinical outcomes. Other types of assays performed included an ELISPOT assay as well as various phenotypic analyses, stimulation tests, and counts of cell populations.
Table 4.
Immune Assays.
| Study | DTH/ELISPOT assays | ICS | Other immune assays? | Phenotypic analysis? | |||
|---|---|---|---|---|---|---|---|
| Assay | Stimulus | Positive responses? | Correlation with clinical outcomes? | ||||
|
Oh (2020) Oh (2016) |
ELISPOT | Whole Cell | Yes | NR | No | No | No |
| Bota (2018) | Neither | NA | NA | NA | No | CD3/CD4 + count; maximal and end of treatment | Yes |
| Schulze (2009) | Neither | NA | NA | NA | No | No | No |
| Peng (2006) | DTH | Lysate | Yes | Yes | No | No | No |
| Uyl-de Groot (2005), Vermorken (1999) | DTH | Whole Cell | No | NA | No | No | No |
| Peng (2005) | DTH | Lysate | Yes | Yes | No | Flow cytometry; proportion of CD3, CD4, CD8, CD16, and CD56 positive cells | Yes |
| Kuang (2004) | DTH | Lysate | Yes | Yes | No | No | No |
| Harris (2000) | DTH | Whole Cell | Yes | Yes | No | No | No |
| Galligioni (1996) | DTH | Whole Cell | Yes | NR | No | No | No |
| Hoover (1993) | DTH | Whole Cell | Yes | NR | No | No | No |
| Gray (1989, 1988) | Neither | NA | NA | NA | No | No | No |
| Adler (1987) | DTH | Whole Cell | Yes | Yes | No | In vitro lymphocyte stimulation, immune response to tuberculin | No |
| Souter (1981) | Neither | NA | NA | NA | No | No | No |
| Embleton (1978), McIllmurray (1977) | Neither | NA | NA | NA | No | In vitro cytotoxicity and leukocyte responses to stimulants PHA, PWM, ConA | No |
DTH delayed type hypersensitivity, ICS intracellular cytokine staining, ELISPOT enzyme linked immunospot, NR not reported, NA not applicable, PHA phytohaemagglutinin, PMW pokeweed mitogen, ConA concanavalin A.
Tertiary outcomes: health utility and economic assessment
Measures of quality of life and economic value of the autologous whole cell vaccine treatment were reported in one study (7%). Uyl-de Groot (2005) reported 5.51 quality adjusted life years (QALYs) in patients receiving AWCV compared to 4.65 in patients in the control group (confidence intervals and whether study was reporting mean or median was unclear). With these estimates, the cost per QALY of the AWCV was US $22,56122.
Subgroup analyses
Statistically significant differences were not observed between different disease sites with respect to improvement in overall or disease-free survival (Supplemental Fig. 2). Similarly, there was no statistically significant differences in overall or disease free survival based on the adjuvant employed (Supplemental Fig. 3). Due to insufficient data other planned subgroup analyses were not performed, including fresh vs frozen vaccine (all studies used fresh vaccine); single vs multidose (only two studies clearly reported delivering only a single dose); and centralized vs non-centralized manufacturing (only two studies reported non-centralized manufacturing).
Risk of bias
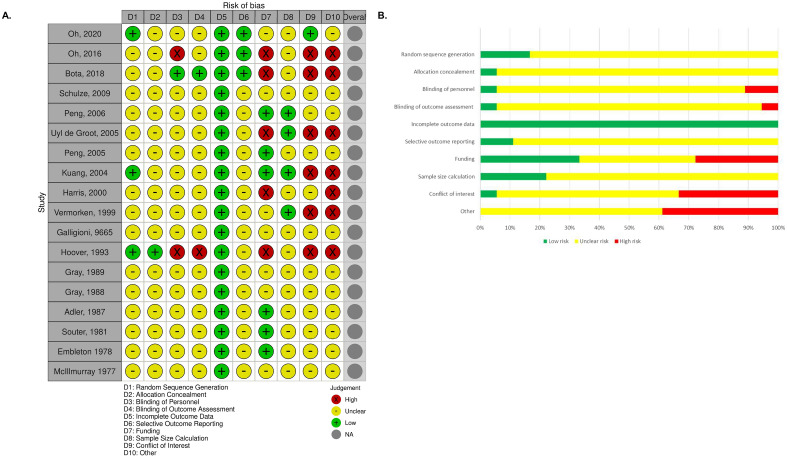
The majority of the studies included in the review were at an unclear risk of bias (Fig. 3). Fifteen out of eighteen manuscripts did not provide details on their random sequence generation, while the methods for allocation and blinding of personnel were not specific in seventeen and fifteen of the eighteen papers respectively. Pre-specified protocols were referenced in two studies, making it difficult to ascertain whether selective outcome reporting occurred. Furthermore, details of sample size calculations were provided in a minority (n = 4) of studies. The area with the greatest clear risk of bias was conflict of interest with six studies having at least one author with a significant conflict. Industry sponsorship was acknowledged in five manuscripts while seven additional manuscripts did not clearly report sources of funding.
Figure 3.
(A,B) Risk of Bias within individual studies (A) and across studies (B).
Discussion
Disseminated disease remains the major cause of cancer-specific mortality following the operative management of solid tumors, despite advances in adjuvant therapies1,23–25. Here we show that autologous whole cell vaccination represents a strategy that is safe and can extend survival in patients who have undergone tumor resection. The results of this systematic review support further investigation into these therapies.
Across 14 randomized clinical trials, 698 patients received at least one dose of autologous whole cell vaccine with only five reported high grade (grade III+) adverse events. Four of the high-grade AEs were reported in a single study of glioblastoma patients that also reported a total of 8 in the control group. This outcome suggests that concurrent therapies or the disease site (brain) of this patient cohort, rather than the vaccine itself, were contributing factors14. In the majority of the remaining studies, concurrent therapies were not provided (see Table 2) and AEs were only listed for the vaccination group. Thus, most of the AEs that were directly attributed to vaccination were mild, taking the form of fevers or injection site reactions, and not requiring medical intervention (see Table 3). In one study, however, a high grade AE was observed in the form of renal failure secondary to deposition of immune complexes in the glomerulus20. It should be noted that the remaining patients in this trial were not reported to develop similar AEs and the adjuvant, BCG, has been used extensively since without documented serious AEs18,22,26,27. Overall the incidence of high grade adverse events noted in our study is significantly less than what has been described for patients receiving checkpoint blockade or chemotherapy as a component of the treatment of solid tumors28–30. Integrated analysis of AEs between studies was complicated by inconsistent reporting metrics with some articles reporting the total number of AEs occurring and others reporting the number of patients experiencing an AE. While some of the studies captured in our review predate the publication of current standardized reporting practices, adherence to these conventions will enable better cross-study comparison in future works31,32.
In addition to safety, the other primary outcome in our systematic review was efficacy based on clinical response. This was included as a primary outcome because the protocol published for this systematic review was also designed for use in a study of autologous cell vaccines in patients with hematologic malignancies6. This metric is of limited value in the current manuscript given that the majority of studies employed autologous vaccines in the adjuvant setting in the context of patients who had previously undergone curative tumor resection. Thus, patients only had measurable disease at the time of vaccination in two studies limiting useful assessment of clinical response in the current manuscript. The cumulative risk ratio for overall response across all studies was 1.86 (95% CI 0.48 8.16, Supplemental Fig. 1). The two studies which reported on overall response were conducted in drastically different time periods in disparate malignancies14,19. Patients with measurable disease that is not removed by surgery typically represent advanced disease. Therefore, clinical response in the context of solid tumors is more likely to be reported in the context of advanced disease which may be inherently more resistant to immunotherapy33–35.
Although analyzed as a secondary outcome, the results obtained for survival outcomes may be more useful in terms of describing the efficacy of these therapeutics as there is more data available for this outcome. Overall, autologous whole cell vaccines were shown to increase the overall and disease-free survival relative to the respective randomized control populations (Fig. 2). The beneficial effects of vaccination on survival were significantly greater in patients with earlier stage disease in three studies where this comparison was made19,21,22. Larger tumors have also been found to be more immunosuppressive and it is possible that more locally advanced disease has further dampened the immune response33–35. Although the opposite trend was observed by Souter et al., the overall survival was not significantly different between treatment and control groups for either stage I or II lung cancer36. An interesting observation made by both Hoover et al. and Schulze et al. is the superior performance of autologous whole cell vaccines in cancers of the colon relative to those of the rectum17,18. The immunobiology of this phenomenon remains unclear. There is an emerging consensus that right sided colonic tumors typically exhibit higher antigenic loads and better responses to immunotherapies than left and the highly immunogenic DNA mismatch repair deficient (MMRD) tumors are more prevalent on the right, providing one possible explanation37,38. There were no statistically significant differences in overall survival based on the adjuvants employed, although experience with adjuvants such as NDV were quite limited and there was significant variability in these subgroups (Supplemental Fig. 3). No study captured in this review reported on differences in outcomes associated with number of doses given, although evidence for benefit from more doses has been observed in the use of autologous whole cell vaccines for hematologic malignancies39–42.
Recently, the results of a phase IIb RCT investigating the use of an autologous cell vaccine as an adjuvant to standard of care surgery and chemotherapy in stage III/IV high grade serous, endometroid, and clear cell ovarian cancers were published. This trial demonstrated a modest improvement in recurrence free survival in the group receiving the vaccine although this did not reach statistical significance (p = 0.078). Nevertheless, no grade 3 or 4 adverse events were attributed to the vaccine which is in agreement with out findings43.
In most instances where an immune assay was carried out positive results were obtained, at least in some patients (see Table 4). The most frequently employed investigation was the DTH assay. This assay is relatively simple and amenable to use in clinical trials with large volumes of patients. DTH responses are commonly used in other approaches to cancer vaccination and thus allow for a comparison to other therapies as well44,45. Although the DTH results appeared to correlate well with clinical outcomes in this study, the assay is limited in that it does not provide information on the immune subpopulations mediating the response and is less useful in quantifying the magnitude of response beyond a binary answer.
A significant limitation to this study is the variability in terms of protocols and time periods during which trials were conducted. Half of the 18 reports captured in this systematic review were published prior to the year 2000 with the remaining being published subsequently. Technical knowledge, reporting standards, and our ability to accurately assess residual disease have advanced considerably. Knowledge of tumor immunology and cancer immunotherapy has also expanded significantly over the timeframe in which studies are published. It is therefore less informative to rely on quantitative outcomes reported in earlier studies than it is to consider the overall trends that were established. The variability in disease sites investigated, while reducing the power of our report to make conclusions regarding any one tumor type, provides a broader overview of the efficacy of autologous whole cell vaccination. While comparison between outcomes in different disease sites can be performed based on our analysis (Supplemental Fig. 2), it is most reliable in studies which carried out comparisons in the setting of a single trial.
Of the 808 enrolled patients, 698 received at least one dose of vaccine representing a successful vaccination rate of 86%. This rate is significantly better than what was observed in our systematic review of autologous whole cell vaccination in hematologic malignancies (58%) but still suggests that the ability to manufacture vaccines represents a barrier in the context of solid tumors12. In the current study, the main reason for patients not receiving vaccination was withdrawal or being lost to follow-up followed by post-operative complications / death and patients becoming ineligible18,20,36,46–48. Unlike our systematic review of autologous whole cell vaccination in hematologic malignancies manufacturing challenges represented a reason for which only a minority of patients were not vaccinated12.
Overall, our systematic review and meta analysis indicate that autologous whole cell vaccination represents a safe strategy that can potentially extend overall survival when used as an adjuvant to surgical management of solid tumors. Our review was limited by the broad publication range, limited power to make conclusions in any one disease site, and inconsistencies in reporting standards across studies. Given the significant advances that have been made in basic tumor immunobiology and immunotherapy as a field in the last decade, there is strong rationale to continue exploring autologous whole cell vaccines as an adjuvant strategy to the surgical management of solid tumors.
Supplementary Information
Author contributions
Concept and design: R.C.A., N.K., M.L., D.A.F. Acquisition of data: D.J.B., M.A.K., A.B.M., D.B., B.W., L.G., R.S. Analysis or interpretation of data: J.M., D.J.B., M.G., R.C.A., D.A.F., M.L. Drafting of the manuscript: D.J.B., M.A.K., J.M., R.C.A. Critical revision of the manuscript: D.J.B., J.M., M.A.K., D.A.F., M.L., R.C.A. Statistical analysis: J.M. Obtained funding: N.K., R.C.A., M.L., D.F, J.S.D. Administrative, technical, or material support: All authors. Supervision: N.K., R.C.A., M.L., D.F., J.S.D., M.A.K. The authors consent to the publication of this manuscript.
Funding
We are grateful for the generous support from the Terry Fox Research Institute, Ontario Institute for Cancer Research, BioCanRx, as well as from the Ottawa Hospital Foundation.
Data availability
All data generated or analysed during this study are included in this published article [and its supplementary information files].
Competing interests
The authors declare no competing interests.
Footnotes
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
The online version contains supplementary material available at 10.1038/s41598-023-29630-9.
References
- 1.Chaffer CL, Weinberg RA. A perspective on cancer cell metastasis. Science. 2011 doi: 10.1126/science.1203543. [DOI] [PubMed] [Google Scholar]
- 2.Brahmer J, et al. Nivolumab versus docetaxel in advanced squamous-cell non–small-cell lung cancer. N. Engl. J. Med. 2015 doi: 10.1056/nejmoa1504627. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Khammari A, et al. Adoptive TIL transfer in the adjuvant setting for melanoma: Long-term patient survival. J. Immunol. Res. 2014 doi: 10.1155/2014/186212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Apostolopoulos V, et al. Pilot phase III immunotherapy study in early-stage breast cancer patients using oxidized mannan-MUC1 [ISRCTN71711835] Breast Cancer Res. 2006 doi: 10.1186/bcr1505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Keenan BP, Jaffee EM. Whole cell vaccines—Past progress and future strategies. Semin. Oncol. 2012;39:276–286. doi: 10.1053/j.seminoncol.2012.02.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Khan ST, et al. Safety and efficacy of autologous tumour cell vaccines as a cancer therapeutic to treat solid tumours and haematological malignancies: A meta-analysis protocol for two systematic reviews. BMJ Open. 2020;10:e034714. doi: 10.1136/bmjopen-2019-034714. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.NCT03495921: Vigil + Irinotecan and Temozolomide in Ewing’s Sarcoma (VITA). ClinicalTrials.gov.
- 8.NCT02448173: A Multicenter Study of Active Specific Immunotherapy With OncoVax® in Patients With Stage II Colon Cancer. Clinical Trials.gov.
- 9.Page MJ, et al. The PRISMA 2020 statement: An updated guideline for reporting systematic reviews. BMJ. 2021 doi: 10.1136/bmj.n71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Lefebvre, C., Manheimer, E. & Glanville, J. Searching for studies. In: Higgins JPT, Green S, editor. Cochrane Handbook for Systematic Reviews of Interventions Version 5.1.0 (Updated March 2011. Cochrane Collab. (2011).
- 11.Higgins JPT, et al. The Cochrane collaboration's tool for assessing risk of bias in randomized control trials. BMJ. 2011 doi: 10.1136/bmj.d5928. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Bastin DJ, et al. Safety and efficacy of autologous whole cell vaccines in hematologic malignancies: A systematic review and meta-analysis. Hematol. Oncol. 2021 doi: 10.1002/hon.2875. [DOI] [PubMed] [Google Scholar]
- 13.Higgins, J. P. T. et al. Cochrane handbook for systematic reviews of interventions version 6.2 [updated February 2021]. Cochrane Handbook for Systematic Reviews of Interventions (2021).
- 14.Bota DA, et al. Phase II study of ERC1671 plus bevacizumab versus bevacizumab plus placebo in recurrent glioblastoma: Interim results and correlations with CD4+ T-lymphocyte counts. CNS Oncol. 2018 doi: 10.2217/cns-2018-0009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Embleton MJ, Ransom JH, McIllmurray MB, Reeves WG. Immunological monitoring in a controlled trial of immunotherapy in stage IIB malignant melanoma. Br. J. Cancer. 1978 doi: 10.1038/bjc.1978.77. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.McIllmurray MB, Embleton MJ, Reeves WG, Langman MJS, Deane M. Controlled trial of active immunotherapy in management of stage IIB malignant melanoma. Br. Med. J. 1977;1:540–542. doi: 10.1136/bmj.1.6060.540. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Schulze T, et al. Efficiency of adjuvant active specific immunization with Newcastle disease virus modified tumor cells in colorectal cancer patients following resection of liver metastases: Results of a prospective randomized trial. Cancer Immunol. Immunother. 2009 doi: 10.1007/s00262-008-0526-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Hoover HC, et al. Adjuvant active specific immunotherapy for human colorectal cancer: 6.5-year median follow-up of a phase III prospectively randomized trial. J. Clin. Oncol. 1993 doi: 10.1200/JCO.1993.11.3.390. [DOI] [PubMed] [Google Scholar]
- 19.Adler, A. et al. Active specific immunotherapy of renal cell carcinoma patients: A prospective randomized study of hormono-immuno-versus hormonotherapy. Preliminary report of immunological and clinical aspects. J. Biol. Response Mod.https://pubmed.ncbi.nlm.nih.gov/3330126/ (1987). [PubMed]
- 20.Gray BN, Walker C, Andrewartha L, Freeman S, Bennett RC. Melbourne trial of adjuvant immunotherapy in operable large bowel cancer. Aust. N. Z. J. Surg. 1988 doi: 10.1111/j.1445-2197.1988.tb00966.x. [DOI] [PubMed] [Google Scholar]
- 21.Gray BN, Walker C, Andrewartha L, Freeman S, Bennett RC. Controlled clinical trial of adjuvant immunotherapy with BCG and neuraminidase-treated autologous tumour cells in large bowel cancer. J. Surg. Oncol. 1989 doi: 10.1002/jso.2930400109. [DOI] [PubMed] [Google Scholar]
- 22.Uyl-de Groot CA, et al. Immunotherapy with autologous tumor cell-BCG vaccine in patients with colon cancer: A prospective study of medical and economic benefits. Vaccine. 2005 doi: 10.1016/j.vaccine.2005.01.015. [DOI] [PubMed] [Google Scholar]
- 23.Sohal DPS, Walsh RM, Ramanathan RK, Khorana AA. Pancreatic adenocarcinoma: Treating a systemic disease with systemic therapy. J. Natl Cancer Inst. 2014 doi: 10.1093/jnci/dju011. [DOI] [PubMed] [Google Scholar]
- 24.Bugge AS, et al. Cause-specific death after surgical resection for early-stage non-small-cell lung cancer. Eur. J. Cardio-thoracic Surg. 2018 doi: 10.1093/ejcts/ezx274. [DOI] [PubMed] [Google Scholar]
- 25.Chapuis PH, et al. Recurrence and cancer-specific death after adjuvant chemotherapy for Stage III colon cancer. Color. Dis. 2019 doi: 10.1111/codi.14434. [DOI] [PubMed] [Google Scholar]
- 26.Galligioni E, et al. Adjuvant immunotherapy treatment of renal carcinoma patients with autologous tumor cells and bacillus Calmette-Guèrin: Five-year results of a prospective randomized study. Cancer. 1996 doi: 10.1002/(SICI)1097-0142(19960615)77:12<2560::AID-CNCR20>3.0.CO;2-P. [DOI] [PubMed] [Google Scholar]
- 27.Harris JE, et al. Adjuvant active specific immunotherapy for stage II and III colon cancer with an autologous tumor cell vaccine: Eastern Cooperative Oncology Group study E5283. J. Clin. Oncol. 2000 doi: 10.1200/jco.2000.18.1.148. [DOI] [PubMed] [Google Scholar]
- 28.Xing P, et al. Incidence rates of immune-related adverse events and their correlation with response in advanced solid tumours treated with NIVO or NIVO+IPI: A systematic review and meta-analysis. J. Immunother. Cancer. 2019 doi: 10.1186/s40425-019-0779-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Pearce A, et al. Incidence and severity of self-reported chemotherapy side effects in routine care: A prospective cohort study. PLoS ONE. 2017 doi: 10.1371/journal.pone.0184360. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Huang YF, Xie WJ, Fan HY, Du J. Comparative risks of high-grade adverse events among FDA-approved systemic therapies in advanced melanoma: systematic review and network meta-analysis. Front. Oncol. 2020 doi: 10.3389/fonc.2020.571135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Ioannidis JPA, et al. Better reporting of harms in randomized trials: An extension of the CONSORT statement. Ann. Intern. Med. 2004 doi: 10.7326/0003-4819-141-10-200411160-00009. [DOI] [PubMed] [Google Scholar]
- 32.Begg C, et al. Improving the quality of reporting of randomized controlled trials: The CONSORT statement. J. Am. Med. Assoc. 1996 doi: 10.1001/jama.276.8.637. [DOI] [PubMed] [Google Scholar]
- 33.Diaz-Montero CM, et al. Increased circulating myeloid-derived suppressor cells correlate with clinical cancer stage, metastatic tumor burden, and doxorubicin-cyclophosphamide chemotherapy. Cancer Immunol. Immunother. 2009 doi: 10.1007/s00262-008-0523-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Kono K, et al. CD4(+)CD25high regulatory T cells increase with tumor stage in patients with gastric and esophageal cancers. Cancer Immunol. Immunother. 2006 doi: 10.1007/s00262-005-0092-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Kim SI, Cassella CR, Byrne KT. Tumor burden and immunotherapy: impact on immune infiltration and therapeutic outcomes. Front. Immunol. 2021 doi: 10.3389/fimmu.2020.629722. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Souter RG, Gill PG, Gunning AJ, Morris PJ. Failure of specific active immunotherapy in lung cancer. Br. J. Cancer. 1981 doi: 10.1038/bjc.1981.217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Baran B, et al. Difference between left-sided and right-sided colorectal cancer: A focused review of literature. Gastroenterol. Res. 2018 doi: 10.14740/gr1062w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Li P, et al. A relationship to survival is seen by combining the factors of mismatch repair status, tumor location and age of onset in colorectal cancer patients. PLoS ONE. 2017 doi: 10.1371/journal.pone.0172799. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Ho VT, et al. Biologic activity of irradiated, autologous, GM-CSF-secreting leukemia cell vaccines early after allogeneic stem cell transplantation. Proc. Natl. Acad. Sci. USA. 2009 doi: 10.1073/pnas.0908358106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Biagi E, et al. Responses to human CD40 ligand/human interleukin-2 autologous cell vaccine in patients with B-cell chronic lymphocytic leukemia. Clin. Cancer Res. 2005 doi: 10.1158/1078-0432.CCR-05-0484. [DOI] [PubMed] [Google Scholar]
- 41.Spaner DE, Hammond C, Mena J, Foden C, Deabreu A. A phase I/II trial of oxidized autologous tumor vaccines during the ‘watch and wait’ phase of chronic lymphocytic leukemia. Cancer Immunol. Immunother. 2005 doi: 10.1007/s00262-004-0626-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Burkhardt UE, et al. Autologous CLL cell vaccination early after transplant induces leukemia-specific T cells. J. Clin. Invest. 2013 doi: 10.1172/JCI69098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Rocconi RP, et al. Gemogenovatucel-T (Vigil) immunotherapy as maintenance in frontline stage III/IV ovarian cancer (VITAL): A randomised, double-blind, placebo-controlled, phase 2b trial. Lancet Oncol. 2020 doi: 10.1016/S1470-2045(20)30533-7. [DOI] [PubMed] [Google Scholar]
- 44.Disis, M. L. et al. Delayed-type hypersensitivity response is a predictor of peripheral blood T-cell immunity after HER-2/neu peptide immunization. Clin. Cancer Res.https://pubmed.ncbi.nlm.nih.gov/10778962/ (2000). [PubMed]
- 45.Niu J, et al. Retrospective comparative study of the effects of dendritic cell vaccine and cytokine-induced killer cell immunotherapy with that of chemotherapy alone and in combination for colorectal cancer. Biomed. Res. Int. 2014 doi: 10.1155/2014/214727. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Peng, B. et al. Autologous tumor vaccine lowering postsurgical recurrent rate of hepatocellular carcinoma. Hepatogastroenterology.https://pubmed.ncbi.nlm.nih.gov/16795983/ (2006). [PubMed]
- 47.Peng BG, et al. Tumor vaccine against recurrence of hepatocellular carcinoma. World J. Gastroenterol. 2005 doi: 10.3748/wjg.v11.i5.700. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Sapienza LG, Gomes MJL, Maliska C, Norberg AN. Hemoptysis due to fungus ball after tuberculosis: A series of 21 cases treated with hemostatic radiotherapy. BMC Infect. Dis. 2015 doi: 10.1186/s12879-015-1288-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
All data generated or analysed during this study are included in this published article [and its supplementary information files].