Abstract
Acinetobacter baumannii is a leading cause of antibiotic-resistant nosocomial infections with high mortality rates, yet there is currently no clinically approved vaccine formulation. During the onset of A. baumannii infection, neutrophils are the primary responders and play a major role in resisting the pathogen. Here, we design a biomimetic nanotoxoid for antivirulence vaccination by using neutrophil membrane-coated nanoparticles to safely capture secreted A. baumannii factors. Vaccination with the nanotoxoid formulation rapidly mobilizes innate immune cells and promotes pathogen-specific adaptive immunity. In murine models of pneumonia, septicemia, and superficial wound infection, immunization with the nanovaccine offers significant protection, improving survival and reducing signs of acute inflammation. Lower bacterial burdens are observed in vaccinated animals regardless of the infection route. Altogether, neutrophil nanotoxoids represent an effective platform for eliciting multivalent immunity to protect against multidrug-resistant A. baumannii in a wide range of disease conditions.
Keywords: nanovaccine, Acinetobacter baumannii, pneumonia, sepsis, wound infection
Graphical Abstract
The discovery of penicillin and other antibiotics revolutionized the management of bacterial infections that were once life-threatening; however, in the past several decades, the overuse of antibiotics has engendered dangerous multidrug-resistant pathogens that are difficult to eradicate.1 A recent study revealed that antimicrobial resistance is responsible, either directly or indirectly, for millions of deaths annually across the world.2 Among the six leading pathogens responsible for more than 70% of cases is Acinetobacter baumannii, a nosocomial pathogen that causes ventilated-associated pneumonia, meningitis, and sepsis, as well as infections of the urinary tract, skin, and other soft tissues.3-4 Despite the rising incidence of A. baumannii infection, treatment options are largely limited to antibiotic cocktails, with novel therapeutics such as phage therapy being explored in the clinic.5-6 While therapeutic strategies are highly valuable, prophylactic vaccines provide a powerful approach for the management of pathogenic diseases. Many experimental vaccines for A. baumannii have been reported, but unfortunately none of them have moved past clinical trials.7 One notable obstacle involves selecting the appropriate antigenic targets, as the plasticity of A. baumannii can quickly render monovalent vaccines ineffective.8
Cellular nanoparticles, consisting of a nanoparticulate core camouflaged with a cell membrane coating, have recently been gaining significant traction for use in biomedical applications.9-11 In particular, antivirulence vaccination with nanotoxoids serve as a promising strategy to help overcome antibiotic-resistant superbugs by targeting the virulence factors that bacteria use for their proliferation and survival.12 This reduces the direct selective pressure on individual pathogens, thus minimizing the opportunities for resistance to develop.13-14 Nanotoxoid vaccines are constructed by utilizing cellular nanoparticles to detain and neutralize harmful bacterial virulence factors, thus enabling them to be safely introduced into a host for immune priming.15-17 As cellular nanoparticles are capable of capturing a wide range of bacterial virulence factors, this approach can be leveraged to facilely generate multivalent nanovaccines.18 Multiantigenic nanotoxoids have been successfully engineered against methicillin-resistant Staphylococcus aureus using red blood cell nanoparticles and a clinical isolate of Pseudomonas aeruginosa using macrophage nanoparticles, and each formulation was able to effectively protect against live bacterial infection in murine models.19-20
To invade its host, A. baumannii secretes a plethora of virulence factors such as phospholipases, hemolytic factors, endotoxins, porins, elastase, and capsular polysaccharides.3-4 Mechanistically, many virulence factors exert their cytotoxic activity by interacting with the cell membrane, making them prime candidates for inclusion into nanotoxoid formulations.4, 21-22 Neutrophils are oftentimes the first to be recruited to sites of A. baumannii infection in a cytokine- and chemokine-dependent manner.23-24 It has been shown that administration of the chemokine CXCL2 prior to A. baumannii challenge accelerates pathogen clearance in mice.25 On the other hand, neutrophil depletion sensitizes animals to infection and significantly enhances the lethality of A. baumannii.23, 25
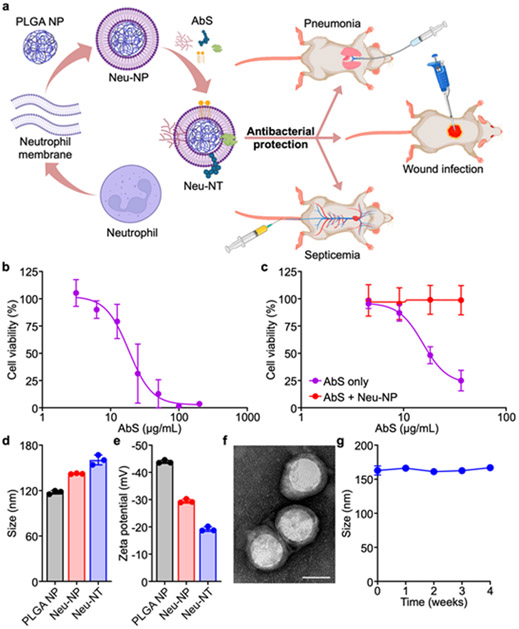
Given the critical immunological role of neutrophils in resisting A. baumannii infections, we engineered biomimetic neutrophil membrane-coated nanoparticles (denoted ‘Neu-NP’) for capturing secreted A. baumannii virulence factors to form nanotoxoids, termed Neu-NT (Figure 1a). Neu-NP were found to effectively attenuate the cytotoxicity of the bacterial proteins, and the corresponding Neu-NT were shown to potently elicit the anti-A. baumannii immunity in a dose-dependent manner. Further studies revealed that Neu-NT vaccination had a major impact on dendritic cell (DC) and B cell activation. Immunization with Neu-NT either subcutaneously or intranasally elicited high antibody titers against A. baumannii, induced the formation of germinal centers in both the spleen and the draining lymph node (dLN), and increased the number of memory B cells. Most importantly, vaccination with Neu-NT successfully protected mice in pneumonia, septicemia, and wound infection models, and this enhanced efficacy was also associated with significantly reduced inflammation.
Figure 1.
Formulation and characterization of Neu-NT. (a) Neutrophil membrane-coated nanoparticles (Neu-NP) are complexed with A. baumannii secreted proteins (AbS), and vaccination with the resulting nanotoxoid (Neu-NT) formulation protects animals in models of pneumonia, septicemia, and superficial wound infection. Created with BioRender. (b) Cell viability of neutrophils after incubation with different concentrations of AbS for 3 days (n = 3, mean ± SD). (c) Cell viability of neutrophils following incubation with different concentrations of AbS with or without Neu-NP for 3 days (n = 4, mean ± SD). (d,e) Size (d) and surface zeta potential (e) of PLGA NP, Neu-NP, and Neu-NT (n = 3, mean ± SD). (f) Representative TEM image of Neu-NT (scale bar: 50 nm). (g) Stability of Neu-NT in 10% sucrose over time at 4 °C (n = 3, mean ± SD).
Results and Discussion
The fabrication of Neu-NP was first optimized by mixing different amounts of neutrophil membrane with preformed poly(lactic-co-glycolic acid) (PLGA) nanoparticle cores.26 To achieve membrane coating, the mixture was sonicated, and the stability of the resulting Neu-NP was evaluated in phosphate buffered saline (PBS) (Figure S1). At low cell membrane to PLGA ratios, the nanoparticles rapidly aggregated in PBS due to incomplete coating. Increasing the amount of membrane led to progressively more stable Neu-NP, and an optimal coating ratio of 1:1 was chosen for use in further studies in order to maximize coverage while minimizing waste. The A. baumannii LAC-4 strain, a multidrug-resistant and hypervirulent clinical isolate that reliably colonizes mice,27 was utilized as the source of antigenic material. Toxic virulence factor secretions were obtained by saturated ammonium sulfate precipitation from the supernatant of A. baumannii cultured to the stationary phase.4, 28 The precipitated A. baumannii secreted proteins (denoted ‘AbS’), which likely contained a mixture of outer membrane proteins, phospholipases, lipases, hemolysins, and serine proteases,28-29 were then desalted by size exclusion chromatography and concentrated with centrifugal filters for further use. Mass spectrometry analysis was performed to annotate the proteins in the AbS preparation (Table S1). The cytotoxicity of AbS was evaluated on neutrophils, which were differentiated from the MPRO clone 2.1 cell line after treatment with all-trans retinoic acid for 3 days,30 and an IC50 value of 18.5 μg/mL was observed (Figure 1b). To evaluate the neutralization capacity of Neu-NP, the nanoparticles were preincubated with AbS, and the cytotoxicity of the resulting mixture on neutrophils was measured (Figure 1c). Under the experimental conditions, Neu-NP were able to fully attenuate the toxicity of AbS due to the presence of the neutrophil membrane. Notably, harsh heat treatment of AbS at 100 °C for 4 hours failed to eliminate its cytotoxic effects (Figure S2). This highlighted the unique advantage of the nanotoxoid approach, which enables the safe delivery of toxic antigens that would otherwise be too dangerous to administer in their native form.19 For further study, the final Neu-NT formulation was synthesized by complexing 4 μg of AbS with 100 μg of Neu-NP, a ratio at which cytotoxicity was completely abrogated to enable safe in vivo administration. Compared with bare PLGA cores, the size of the nanoparticles increased after both membrane coating and AbS complexation, with the final Neu-NT formulation exhibiting an average hydrodynamic diameter of approximately 160 nm (Figure 1d). The surface zeta potential also increased progressively from −44 mV to −19 mV during the fabrication progress (Figure 1e). Transmission electron microscopy (TEM) revealed that Neu-NT possessed a core–shell structure characteristic of cell membrane-coated nanoformulations (Figure 1f).31-33 When stored at 4 °C, Neu-NT remained stable in 10% sucrose for a minimum of 4 weeks (Figure 1g).
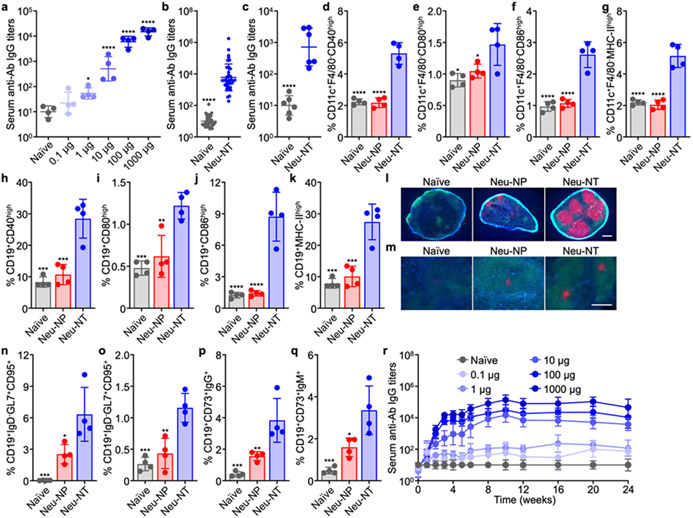
The immunological activity of Neu-NT was assessed by administering the nanovaccine to CD-1 mice. The dose-dependent antibody response against A. baumannii was first evaluated by subcutaneously vaccinating the mice on days 0, 7, and 14, followed by sample collection on day 21 (Figure 2a). At a 10-μg dosage of Neu-NT, anti-A. baumannii immunoglobulin G (IgG) titers in the serum were significantly elevated. The antibody response began to saturate at approximately 100 μg of Neu-NT, and this dosage was selected for further study (Figure 2b). When the serum from Neu-NT vaccinated mice was incubated with A. baumannii, dose-dependent bactericidal activity was observed (Figure S3). In contrast, such activity was not observed when using naïve serum or heat-inactivated serum, indicating that the effect could likely attributed to complement activation.34-35 Immunity against A. baumannii could also be established by intranasally administering Neu-NT (Figure 2c); the lower serum antibody titers compared with subcutaneous administration could likely be attributed to the various mucosal barriers hindering delivery.36 After confirming successful antibody titer generation, we sought to characterize the innate immune response elicited by Neu-NT in greater detail. The nanovaccine was subcutaneously injected and allowed to transport via the lymphatic system to the dLNs, which were collected after 1 day for immunophenotyping. Three major antigen-presenting cell (APC) populations were analyzed for signs of maturation and activation (Figure S4a). When looking at CD40, CD80, CD86, and MHC-II, it was revealed that Neu-NT significantly increased the expression of each marker in both DCs (Figure 2d-g) and B cells (Figure 2h-k), but not in macrophages (Figure S4b).37 In comparison, Neu-NP alone had minimal effect on APC activation, indicating that immune activation by Neu-NT was largely mediated by the immunogenicity of AbS, which contained immunostimulatory molecular patterns (Figure S5).
Figure 2.
Immune response to Neu-NT vaccination. (a) Serum anti-A. baumannii (anti-Ab) IgG titers on day 21 after subcutaneous immunization with increasing amounts of Neu-NT on days 0, 7, and 14 (n = 4, geometric mean ± SD). (b) Serum anti-Ab IgG titers on day 21 after subcutaneous immunization with 100 μg of Neu-NT on days 0, 7, and 14 (N = 36 pooled from 6 independent experiments, geometric mean ± SD). (c) Serum anti-Ab IgG titers on day 21 after intranasal immunization with 100 μg of Neu-NT on days 0, 7, and 14 (n = 6, geometric mean ± SD). (d-k) Percentage of CD11c+F4/80− DCs (d-g) and CD19+ B cells (h-k) with high expression of maturation markers CD40 (d,h), CD80 (e,i), CD86 (f,j), and MHC-II (g,k) in the dLN 1 day after subcutaneous immunization with 100 μg of Neu-NP or Neu-NT (n = 4, mean ± SD). In (l-q), mice were subcutaneously vaccinated with 100 μg of Neu-NP or Neu-NT on days 0, 7, and 14, followed by analysis on day 21. (l,m) Representative histological sections of the dLN (l) and spleen (m) (blue: CD19, green: IgD, red: GL7; scale bars: 200 μm). (n,o) Percentage of CD19+ B cells with the IgD−GL7+CD95+ germinal center phenotype in the dLN (n) and spleen (o) (n = 4, mean ± SD). (p,q) Percentage of CD19+CD73+ memory B cells with the IgG+ (p) and IgM+ (q) phenotypes in the dLN (n = 4, mean ± SD). (r) Serum anti-Ab IgG titers over time from mice vaccinated as in (a) (n = 4, geometric mean ± SD). *p < 0.05, **p < 0.01, ***p < 0.001, and ****p < 0.0001 (compared to naïve in (a) and Neu-NT in all other studies). Statistical analysis was performed using one-way ANOVA with Dunnett’s multiple comparison tests in (a) or Tukey’s post-hoc analysis in all other studies.
To study the adaptive immune response against Neu-NT, mice were subcutaneously vaccinated on day 0, followed by booster doses on days 7 and 14. On day 21, immune cell populations in the dLN and spleen were analyzed. Immunofluorescence staining of dLN cryosections revealed the strong presence of multiple germinal centers following Neu-NT vaccination (Figure 2l and Figure S6). Germinal centers are vital for the activation, proliferation, and differentiation of antigen-specific B cells, and their successful formation is indicative of an activated adaptive immune response.38-40 In the spleen, germinal center formation was similarly more abundant in the samples from mice receiving Neu-NT (Figure 2m and Figure S7). Quantitatively, it was determined by flow cytometry that Neu-NT vaccination resulted in a significantly higher number of CD19+IgD− B cells with the GL7+CD95+ germinal center phenotype in the dLN (Figure 2n and Figure S8a). In contrast, the number of germinal center B cells was much lower for mice vaccinated with Neu-NP, and they were nearly undetectable in naïve mice. Similar trends were observed when quantifying the germinal center B cells in the spleen (Figure 2o and Figure S8b). With respect to long-term immunity, the number of CD19+CD73+ memory B cells positive for the IgG (Figure 2p) and IgM (Figure 2q) isotypes in the dLN was significantly higher in mice vaccinated with Neu-NT.41 Serum IgG antibodies also remained elevated for at least 6 months (Figure 2r and Figure S9).
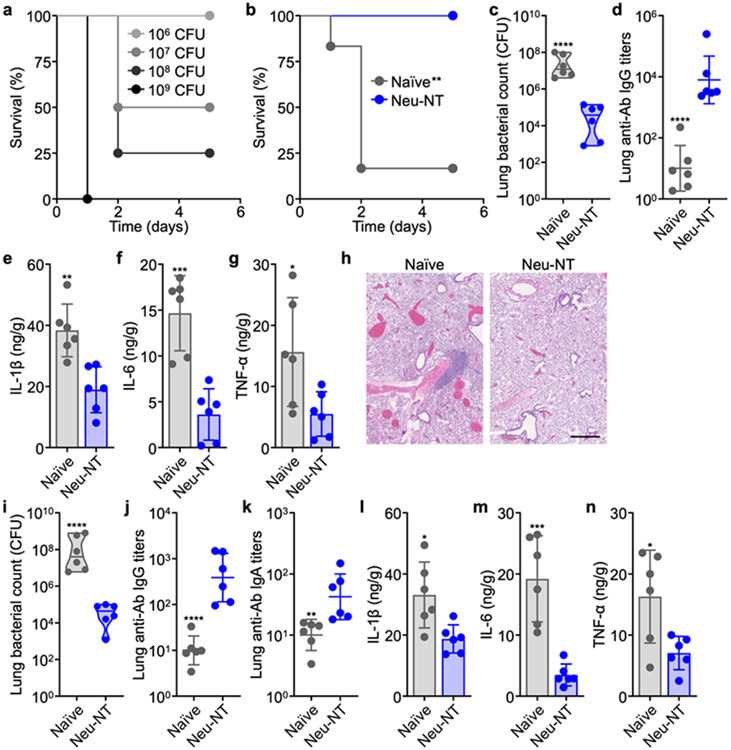
Having validated that Neu-NT could effectively elicit immune responses against A. baumannii, its prophylactic efficacy was evaluated using multiple models of live bacterial infection. To establish a pneumonia model, A. baumannii was inoculated intratracheally (Figure 3a). Whereas the majority of unvaccinated mice succumbed to a bacterial challenge dose of 108 CFU, those subcutaneously vaccinated with Neu-NT were completely protected and remained healthy (Figure 3b). In a separate study in which mice were challenged with a nonlethal dose of bacteria, quantification of the lung bacterial burden revealed a roughly 1000-fold improvement for the mice vaccinated with Neu-NT (Figure 3c). This protection was associated with higher levels of A. baumannii-specific IgG titers in the lungs (Figure 3d). As bacterial infection can provoke an excessive immune system that may lead to undesirable toxicity in the form of a cytokine storm,42-44 the levels of important proinflammatory cytokines such as interleukin (IL)-1β, IL-6, and tumor necrosis factor α (TNF-α) in the lungs of A. baumannii-infected mice were examined (Figure 3e-g). While all three cytokines were elevated in naïve mice, the concentrations in the lungs of mice immunized with Neu-NT were considerably lower. This effect can likely be attributed to the successful activation of adaptive immunity to facilitate the clearance of A. baumannii, thus reducing the need for innate immune responses to be propagated.27, 45-46 In the animals vaccinated with Neu-NT, histopathological analysis of the lungs following infection revealed noticeably less immune cell infiltrates in the lung parenchyma (Figure 3h and Figure S10). There were significantly more distention of blood vessels and filling of the alveoli with blood in naïve mice, which are signs of pulmonary congestion due to infection.47-48 A thickening of the alveolar septum was also observed. Using an intranasal route of vaccination, comparable antivirulence efficacy results were observed, although the IgG titers were lower in magnitude (Figure 3i,j). Importantly, lung IgA antibody levels were elevated in the immunized mice, which demonstrated the ability of Neu-NT to promote mucosal immunity when administered via a mucosal route (Figure 3k).49-50 When analyzing the lung proinflammatory cytokine profile after infection, the concentrations of IL-1β, IL-6, and TNF-α were all lower in animals intranasally vaccinated with Neu-NT (Figure 3l-n).
Figure 3.
Protective efficacy of Neu-NT in a pneumonia model. (a) Survival of mice intratracheally challenged with increasing numbers of A. baumannii (n = 4). (b) Survival of mice over time after subcutaneous vaccination with 100 μg of Neu-NT on days 0, 7, and 14, followed by intratracheal challenge with 108 CFU of A. baumannii on day 21 (n = 6). In (c-n), mice were either subcutaneously (c-h) or intranasally (i-n) vaccinated with 100 μg of Neu-NT on days 0, 7, and 14, intratracheally challenged with 107 CFU of A. baumannii on day 21, and then euthanized for analysis on day 22. (c) Bacterial burden in the lungs of mice (n = 6, geometric median). (d) Anti-A. baumannii (anti-Ab) IgG titers in the lungs (n = 6, geometric mean ± SD). (e-g) Concentrations of IL-1β (e), IL-6 (f), and TNF-α (g) in the lungs (n = 6, mean ± SD). (h) Representative H&E-stained sections of the lungs (scale bar: 500 μm). (i) Bacterial burden in the lungs of mice (n = 6, geometric median). (j,k) Anti-Ab IgG (j) and IgA (k) titers in the lungs (n = 6, geometric mean ± SD). (l-n) Concentrations of IL-1β (l), IL-6 (m), and TNF-α (n) in the lungs (n = 6, mean ± SD). *p < 0.05, **p < 0.01, ***p < 0.001, and ****p < 0.0001 (compared to Neu-NT). Statistical analysis was performed using Mantel–Cox test in (b) and Student’s unpaired t-test in all other studies.
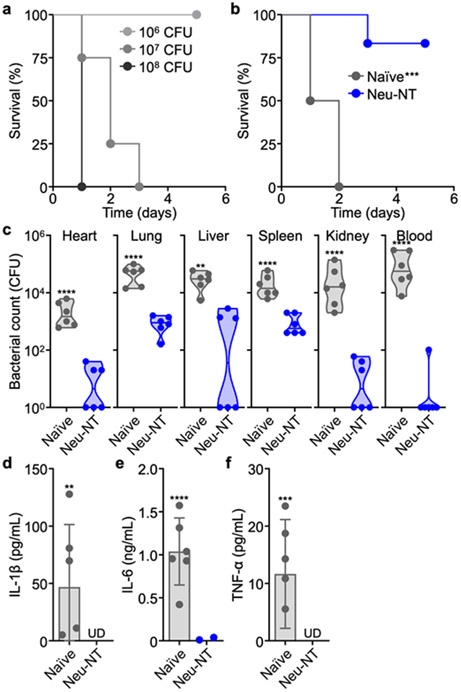
The efficacy of Neu-NT was next tested in a systemic model of infection. To establish a septicemia model, A. baumannii was intravenously injected through the lateral tail vein (Figure 4a). A bacterial challenge of 107 CFU resulted in complete lethality, whereas only 1 out of the 6 mice subcutaneously vaccinated with Neu-NT succumbed to infection (Figure 4b). In a separate study, mice were challenged with 106 CFU of A. baumannii, and bacterial counts in the heart, lungs, liver, spleen, kidneys, and blood were enumerated (Figure 4c). In all organs, a significantly lower number of bacteria was detected for mice that were immunized with Neu-NT. Furthermore, the concentration of the proinflammatory cytokines IL-1β, IL-6, and TNF-α in the serum following infection was nearly undetectable in all vaccinated mice (Figure 4d-f).
Figure 4.
Protective efficacy of Neu-NT in septicemia model. (a) Survival of mice intravenously challenged with increasing numbers of A. baumannii (n = 4). In (b-f), mice were subcutaneously vaccinated with 100 μg of Neu-NT on days 0, 7, and 14 and intravenously challenged with A. baumannii on day 21. (b) Survival of mice over time after intravenous challenge with 107 CFU of A. baumannii (n = 6). (c) Bacterial load in the individual organs of mice 1 day after intravenous challenge with 106 CFU of A. baumannii (n = 6, geometric median). (d-f) Concentrations of IL-1β (d), IL-6 (e), and TNF-α (f) in the serum 1 day after intravenous bacterial challenge with 106 CFU of A. baumannii (n = 6, mean ± SD; UD: undetectable). **p < 0.01, ***p < 0.001, and ****p < 0.0001 (compared to Neu-NT). Statistical analysis was performed using Mantel–Cox test in (b) and Student’s unpaired t-test in all other studies.
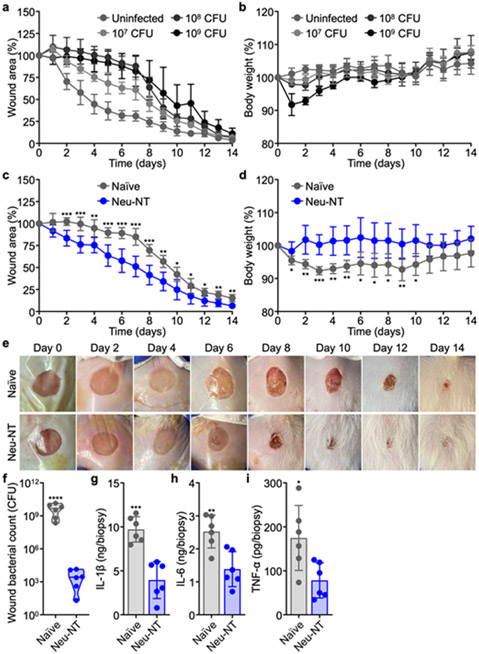
A. baumannii commonly infects open wounds in hospital and battlefield settings.51-53 A corresponding murine model of infection was established by using an 8-mm skin biopsy punch to remove a circular area of dorsal skin,54 followed by the topical application of A. baumannii. Challenge with high doses of bacteria resulted in delayed wound healing and a significant loss of body weight (Figure 5a,b).54 Following infection with 108 CFU of A. baumannii, the wound closure rate for mice subcutaneously vaccinated with Neu-NT was significantly faster compared with unvaccinated mice; the vaccinated mice also maintained their body weight better post-infection (Figure 5c-e and Figure S11). Following the same experimental setup (Figure S12), bacterial burden on day 5 was quantified by taking an 8-mm biopsy of the wound site (Figure 5f). Compared to unvaccinated mice, the bacterial counts in mice vaccinated with Neu-NT were reduced by greater than 6 orders of magnitude. There was also marked reduction of IL-1β, IL-6, and TNF-α levels in the wound bed of the immunized mice (Figure 5g-i).
Figure 5.
Protective efficacy of Neu-NT in a wound infection model. (a,b) Change in wound area (a) and body weight (b) over time of mice infected with increasing numbers of A. baumannii in the wound bed (n = 4, mean ± SD). In (c-i), mice were subcutaneously vaccinated with 100 μg of Neu-NT on days 0, 7, and 14 and infected with 108 CFU of A. baumannii in the wound bed on day 21. (c,d) Change in wound area (c) and body weight (d) over time of mice after infection with A. baumannii (n = 6, mean ± SD). (e) Representative photographs of the wound bed on different days after infection. The Tegaderm film dressing was removed on day 7. (f) Bacterial count in an 8-mm biopsy of the wound 5 days after infection (n = 6, geometric median). (g-i) Concentrations of IL-1β (g), IL-6 (h), and TNF-α (i) in an 8-mm biopsy of the wound 5 days after infection (n = 6, mean ± SD). *p < 0.05, **p < 0.01, ***p < 0.001, and ****p < 0.0001 (compared to Neu-NT). Statistical analysis was performed using Student’s unpaired t-test.
Conclusions
In summary, a neutrophil-mimicking nanoparticle construct was used to capture and deactivate the cytotoxicity of virulence factors secreted by A. baumannii. This neutralization strategy was more effective than harsh heat denaturation, faithfully preserving the bacterial antigens in their native state.55 By vaccinating mice with Neu-NT, potent immune responses against A. baumannii were successfully elicited. Vaccination promoted the rapid maturation of DCs and B cells, which subsequently led to germinal center formation and a robust humoral immune response. In mice immunized with Neu-NT, anti-A. baumannii IgG titers remained elevated for several months. Prophylactic efficacy was evaluated in a pneumonia model of infection, where it was demonstrated that subcutaneous or intranasal Neu-NT vaccination fully protected mice from an intratracheal A. baumannii challenge. Similar results were shown in a septicemia infection model, where subcutaneously vaccinated mice survived lethal intravenous bacterial challenge, which led to reduced bacterial burdens in all major organs. Lastly, in a wound infection model, Neu-NT immunization considerably accelerated the wound healing rate. In all infection models, there was marked reduction in the production of inflammatory cytokines, suggesting that the enhanced protection offered by Neu-NT could also be accompanied by a reduced risk of septic shock.56 Altogether, the data demonstrate the broad clinical applicability of the nanotoxoids, which enable the facile and safe delivery of toxic bacterial virulence factors for vaccination against bacterial infections.
Supplementary Material
Acknowledgements
This work is supported by the Defense Threat Reduction Agency Joint Science and Technology Office for Chemical and Biological Defense under Grant Numbers HDTRA1-18-1-0014 and HDTRA1-21-1-0010 and the National Institutes of Health under Award Number R21AI159492.
Footnotes
Supporting Information
The Supporting Information is available free of charge on the ACS Publications website at DOI: 10.1021/acs.nano-lett.XXXXXXX.
Materials and methods; Neu-NP coating optimization; heat treatment of AbS; bactericidal activity of immune sera; innate immune response in the dLN after vaccination with Neu-NT; endotoxin quantification; germinal center formation in the dLN after Neu-NT vaccination; germinal center formation in the spleen after Neu-NT vaccination; gating strategy for immunophenotyping dLN cells and splenocytes to detect germinal center formation and memory B cells; dose-dependent antibody titers after vaccination with Neu-NT; histological analysis of lungs after A. baumannii infection; wound size monitoring following A. baumannii infection; monitoring of mice with wound infections for enumeration and cytokine studies; antibodies for flow cytometry, immunofluorescence, and ELISA experiments (PDF)
Mass spectrometry analysis of AbS (XLSX)
The authors declare no competing financial interest.
References
- (1).Davies J; Davies D, Origins and Evolution of Antibiotic Resistance. Microbiol. Mol. Biol. Rev 2010, 74, 417–433. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (2).Murray CJL; Ikuta KS; Sharara F; Swetschinski L; Robles Aguilar G; Gray A; Han C; Bisignano C; Rao P; Wool E; Johnson SC; Browne AJ; Chipeta MG; Fell F; Hackett S; Haines-Woodhouse G; Kashef Hamadani BH; Kumaran EAP; McManigal B; Agarwal R , et al. , Global Burden of Bacterial Antimicrobial Resistance in 2019: A Systematic Analysis. Lancet 2022, 399, 629–655. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (3).Lee CR; Lee JH; Park M; Park KS; Bae IK; Kim YB; Cha CJ; Jeong BC; Lee SH, Biology of Acinetobacter baumannii: Pathogenesis, Antibiotic Resistance Mechanisms, and Prospective Treatment Options. Front. Cell. Infect. Microbiol 2017, 7, 55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (4).Aliramezani A; Soleimani M; Fard RMN; Nojoomi F, Virulence Determinants and Biofilm Formation of Acinetobacter baumannii Isolated from Hospitalized Patients. Germs 2019, 9, 148–153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (5).Schooley RT; Biswas B; Gill JJ; Hernandez-Morales A; Lancaster J; Lessor L; Barr JJ; Reed SL; Rohwer F; Benler S; Segall AM; Taplitz R; Smith DM; Kerr K; Kumaraswamy M; Nizet V; Lin L; McCauley MD; Strathdee SA; Benson CA , et al. , Development and Use of Personalized Bacteriophage-Based Therapeutic Cocktails To Treat a Patient with a Disseminated Resistant Acinetobacter baumannii Infection. Antimicrob. Agents Chemother . 2017, 61, e00954–17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (6).Hesse S; Adhya S, Phage Therapy in the Twenty-First Century: Facing the Decline of the Antibiotic Era; Is It Finally Time for the Age of the Phage? Annu. Rev. Microbiol 2019, 73, 155–174. [DOI] [PubMed] [Google Scholar]
- (7).Mat Rahim N; Lee H; Strych U; AbuBakar S, Facing the Challenges of Multidrug-Resistant Acinetobacter baumannii: Progress and Prospects in the Vaccine Development. Hum. Vaccines Immunother 2021, 17, 3784–3794. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (8).Imperi F; Antunes LCS; Blom J; Villa L; Iacono M; Visca P; Carattoli A, The Genomics of Acinetobacter baumannii: Insights into Genome Plasticity, Antimicrobial Resistance and Pathogenicity. IUBMB Life 2011, 63, 1068–1074. [DOI] [PubMed] [Google Scholar]
- (9).Fang RH; Kroll AV; Gao W; Zhang L, Cell Membrane Coating Nanotechnology. Adv. Mater 2018, 30, 1706759. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (10).Zeng ZL; Pu KY, Improving Cancer Immunotherapy by Cell Membrane-Camouflaged Nanoparticles. Adv. Funct. Mater 2020, 30, 2004397. [Google Scholar]
- (11).Zhang C; Pu K, Molecular and Nanoengineering Approaches Towards Activatable Cancer Immunotherapy. Chem. Soc. Rev 2020, 49, 4234–4253. [DOI] [PubMed] [Google Scholar]
- (12).Guo Z; Kubiatowicz LJ; Fang RH; Zhang L, Nanotoxoids: Biomimetic Nanoparticle Vaccines against Infections. Adv. Ther 2021, 4, 2100072. [Google Scholar]
- (13).Zhou J; Kroll AV; Holay M; Fang RH; Zhang L, Biomimetic Nanotechnology toward Personalized Vaccines. Adv. Mater 2020, 32, 1901255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (14).Totsika M, Disarming Pathogens: Benefits and Challenges of Antimicrobials That Target Bacterial Virulence Instead of Growth and Viability. Future Med. Chem 2017, 9, 267–269. [DOI] [PubMed] [Google Scholar]
- (15).Wang F; Fang RH; Luk BT; Hu CJ; Thamphiwatana S; Dehaini D; Angsantikul P; Kroll AV; Pang Z; Gao W; Lu W; Zhang L, Nanoparticle-Based Antivirulence Vaccine for the Management of Methicillin-Resistant Staphylococcus aureus Skin Infection. Adv. Funct. Mater 2016, 26, 1628–1635. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (16).Hu CM; Zhang L, Nanotoxoid Vaccines. Nano Today 2014, 9, 401–404. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (17).Hu CM; Fang RH; Luk BT; Zhang L, Nanoparticle-Detained Toxins for Safe and Effective Vaccination. Nat. Nanotechnol 2013, 8, 933–938. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (18).Lapek JD Jr.; Fang RH; Wei X; Li P; Wang B; Zhang L; Gonzalez DJ, Biomimetic Virulomics for Capture and Identification of Cell-Type Specific Effector Proteins. ACS Nano 2017, 11, 11831–11838. [DOI] [PubMed] [Google Scholar]
- (19).Wei X; Gao J; Wang F; Ying M; Angsantikul P; Kroll AV; Zhou J; Gao W; Lu W; Fang RH; Zhang L, In Situ Capture of Bacterial Toxins for Antivirulence Vaccination. Adv. Mater 2017, 29, 1701644. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (20).Wei X; Ran D; Campeau A; Xiao C; Zhou J; Dehaini D; Jiang Y; Kroll AV; Zhang Q; Gao W; Gonzalez DJ; Fang RH; Zhang L, Multiantigenic Nanotoxoids for Antivirulence Vaccination against Antibiotic-Resistant Gram-Negative Bacteria. Nano Lett . 2019, 19, 4760–4769. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (21).Choi CH; Lee EY; Lee YC; Park TI; Kim HJ; Hyun SH; Kim SA; Lee SK; Lee JC, Outer Membrane Protein 38 of Acinetobacter baumannii Localizes to the Mitochondria and Induces Apoptosis of Epithelial Cells. Cell. Microbiol 2005, 7, 1127–1138. [DOI] [PubMed] [Google Scholar]
- (22).Gaddy JA; Arivett BA; McConnell MJ; Lopez-Rojas R; Pachon J; Actis LA, Role of Acinetobactin-Mediated Iron Acquisition Functions in the Interaction of Acinetobacter baumannii Strain ATCC 19606T with Human Lung Epithelial Cells, Galleria mellonella Caterpillars, and Mice. Infect. Immun 2012, 80, 1015–1024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (23).Breslow JM; Meissler JJ Jr.; Hartzell RR; Spence PB; Truant A; Gaughan J; Eisenstein TK, Innate Immune Responses to Systemic Acinetobacter baumannii Infection in Mice: Neutrophils, but Not Interleukin-17, Mediate Host Resistance. Infect. Immun 2011, 79, 3317–3327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (24).Renckens R; Roelofs JJ; Knapp S; de Vos AF; Florquin S; van der Poll T, The Acute-Phase Response and Serum Amyloid A Inhibit the Inflammatory Response to Acinetobacter baumannii Pneumonia. J. Infect. Dis 2006, 193, 187–195. [DOI] [PubMed] [Google Scholar]
- (25).van Faassen H; KuoLee R; Harris G; Zhao X; Conlan JW; Chen W, Neutrophils Play an Important Role in Host Resistance to Respiratory Infection with Acinetobacter baumannii in Mice. Infect. Immun 2007, 75, 5597–5608. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (26).Wei X; Gao J; Fang RH; Luk BT; Kroll AV; Dehaini D; Zhou J; Kim HW; Gao W; Lu W; Zhang L, Nanoparticles Camouflaged in Platelet Membrane Coating as an Antibody Decoy for the Treatment of Immune Thrombocytopenia. Biomaterials 2016, 111, 116–123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (27).Harris G; Kuo Lee R; Lam CK; Kanzaki G; Patel GB; Xu HH; Chen W, A Mouse Model of Acinetobacter baumannii-Associated Pneumonia using a Clinically Isolated Hypervirulent Strain. Antimicrob. Agents Chemother 2013, 57, 3601–3613. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (28).Weber BS; Kinsella RL; Harding CM; Feldman MF, The Secrets of Acinetobacter Secretion. Trends Microbiol . 2017, 25, 532–545. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (29).Chakravarty B, Genetic Mechanisms of Antibiotic Resistance and Virulence in Acinetobacter baumannii: Background, Challenges and Future Prospects. Mol. Biol. Rep 2020, 47, 4037–4046. [DOI] [PubMed] [Google Scholar]
- (30).Gupta D; Shah HP; Malu K; Berliner N; Gaines P, Differentiation and Characterization of Myeloid Cells. Curr. Protoc. Immunol 2014, 104, 22F.5.1–22F.5.28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (31).Bahmani B; Gong H; Luk BT; Haushalter KJ; DeTeresa E; Previti M; Zhou J; Gao W; Bui JD; Zhang L; Fang RH; Zhang J, Intratumoral Immunotherapy using Platelet-Cloaked Nanoparticles Enhances Antitumor Immunity in Solid Tumors. Nat. Commun 2021, 12, 1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (32).Zhou J; Miyamoto Y; Ihara S; Kroll AV; Nieskens N; Tran VN; Hanson EM; Fang RH; Zhang L; Eckmann L, Co-Delivery of Antigens and Adjuvant in Polymeric Nanoparticles Coated with Native Parasite Membranes Induces Protective Mucosal Immunity against Giardia lamblia. J. Infect. Dis 2022, DOI: 10.1093/infdis/jiac074. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (33).Wei X; Ying M; Dehaini D; Su Y; Kroll AV; Zhou J; Gao W; Fang RH; Chien S; Zhang L, Nanoparticle Functionalization with Platelet Membrane Enables Multifactored Biological Targeting and Detection of Atherosclerosis. ACS Nano 2018, 12, 109–116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (34).Nielsen TB; Yan J; Luna BM; Talyansky Y; Slarve M; Bonomo RA; Spellberg B, Monoclonal Antibody Requires Immunomodulation for Efficacy Against Acinetobacter baumannii Infection. J. Infect. Dis 2021, 224, 2133–2147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (35).McIntosh ED; Broker M; Wassil J; Welsch JA; Borrow R, Serum Bactericidal Antibody Assays – The Role of Complement in Infection and Immunity. Vaccine 2015, 33, 4414–4421. [DOI] [PubMed] [Google Scholar]
- (36).Neutra MR; Kozlowski PA, Mucosal Vaccines: The Promise and the Challenge. Nat. Rev. Immunol 2006, 6, 148–158. [DOI] [PubMed] [Google Scholar]
- (37).Zhou J; Karshalev E; Mundaca-Uribe R; Esteban-Fernandez de Avila B; Krishnan N; Xiao C; Ventura CJ; Gong H; Zhang Q; Gao W; Fang RH; Wang J; Zhang L, Physical Disruption of Solid Tumors by Immunostimulatory Microrobots Enhances Antitumor Immunity. Adv. Mater 2021, 33, 2103505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (38).Allen CD; Okada T; Cyster JG, Germinal-Center Organization and Cellular Dynamics. Immunity 2007, 27, 190–202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (39).Klein U; Dalla-Favera R, Germinal Centres: Role in B-Cell Physiology and Malignancy. Nat. Rev. Immunol 2008, 8, 22–33. [DOI] [PubMed] [Google Scholar]
- (40).De Silva NS; Klein U, Dynamics of B Cells in Germinal Centres. Nat. Rev. Immunol 2015, 15, 137–148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (41).Weisel NM; Joachim SM; Smita S; Callahan D; Elsner RA; Conter LJ; Chikina M; Farber DL; Weisel FJ; Shlomchik MJ, Surface Phenotypes of Naive and Memory B Cells in Mouse and Human Tissues. Nat. Immunol 2022, 23, 135–145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (42).Tisoncik JR; Korth MJ; Simmons CP; Farrar J; Martin TR; Katze MG, Into the Eye of the Cytokine Storm. Microbiol. Mol. Biol. Rev 2012, 76, 16–32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (43).Fajgenbaum DC; June CH, Cytokine Storm. N. Engl. J. Med 2020, 383, 2255–2273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (44).Teijaro JR, Cytokine Storms in Infectious Diseases. Semin. Immunopathol 2017, 39, 501–503. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (45).Dinarello CA, Overview of the IL-1 Family in Innate Inflammation and Acquired Immunity. Immunol. Rev 2018, 281, 8–27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (46).Krebs VL; Okay TS; Okay Y; Vaz FA, Tumor Necrosis Factor-Alpha, Interleukin-1beta and Interleukin-6 in the Cerebrospinal Fluid of Newborn with Meningitis. Arq. Neuropsiquiatr 2005, 63, 7–13. [DOI] [PubMed] [Google Scholar]
- (47).Pritt BS; Aubry MC, Histopathology of Viral Infections of the Lung. Semin. Diagn. Pathol 2017, 34, 510–517. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (48).Kradin RL; Digumarthy S, The Pathology of Pulmonary Bacterial Infection. Semin. Diagn. Pathol 2017, 34, 498–509. [DOI] [PubMed] [Google Scholar]
- (49).Fujkuyama Y; Tokuhara D; Kataoka K; Gilbert RS; McGhee JR; Yuki Y; Kiyono H; Fujihashi K, Novel Vaccine Development Strategies for Inducing Mucosal Immunity. Expert Rev. Vaccines 2012, 11, 367–379. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (50).Holmgren J; Czerkinsky C, Mucosal Immunity and Vaccines. Nat. Med 2005, 11, S45–S53. [DOI] [PubMed] [Google Scholar]
- (51).Sebeny PJ; Riddle MS; Petersen K, Acinetobacter baumannii Skin and Soft-Tissue Infection Associated with War Trauma. Clin. Infect. Dis 2008, 47, 444–449. [DOI] [PubMed] [Google Scholar]
- (52).Tekin R; Dal T; Bozkurt F; Deveci O; Palanc Y; Arslan E; Selcuk CT; Hosoglu S, Risk Factors for Nosocomial Burn Wound Infection Caused by Multidrug Resistant Acinetobacter baumannii. J. Burn Care Res 2014, 35, e73–e80. [DOI] [PubMed] [Google Scholar]
- (53).Michalopoulos A; Falagas ME, Treatment of Acinetobacter Infections. Expert Opin. Pharmacother 2010, 11, 779–788. [DOI] [PubMed] [Google Scholar]
- (54).Thompson MG; Black CC; Pavlicek RL; Honnold CL; Wise MC; Alamneh YA; Moon JK; Kessler JL; Si Y; Williams R; Yildirim S; Kirkup BC Jr.; Green RK; Hall ER; Palys TJ; Zurawski DV, Validation of a Novel Murine Wound Model of Acinetobacter baumannii Infection. Antimicrob. Agents Chemother 2014, 58, 1332–1342. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (55).Angsantikul P; Fang RH; Zhang L, Toxoid Vaccination against Bacterial Infection using Cell Membrane-Coated Nanoparticles. Bioconjug. Chem 2018, 29, 604–612. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (56).Parrillo JE, Pathogenetic Mechanisms of Septic Shock. N. Engl. J. Med 1993, 328, 1471–1477. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.