Summary
Background
Knowing the prevalence of multimorbidity among adults across continents is a crucial piece of information for achieving Sustainable Development Goal 3.4, which calls for reducing premature death due to non-communicable diseases. A high prevalence of multimorbidity indicates high mortality and increased healthcare utilization. We aimed to understand the prevalence of multimorbidity across WHO geographic regions among adults.
Methods
We performed a systematic review and meta-analysis of surveys designed to estimate the prevalence of multimorbidity among adults in community settings. We searched PubMed, ScienceDirect, Embase and Google Scholar databases for studies published between January 1, 2000, and December 31, 2021. The random-effects model estimated the pooled proportion of multimorbidity in adults. Heterogeneity was quantified using I2 statistics. We performed subgroup analyses and sensitivity analyses based on continents, age, gender, multimorbidity definition, study periods and sample size. The study protocol was registered with PROSPERO (CRD42020150945).
Findings
We analyzed data from 126 peer-reviewed studies that included nearly 15.4 million people (32.1% were male) with a weighted mean age of 56.94 years (standard deviation of 10.84 years) from 54 countries around the world. The overall global prevalence of multimorbidity was 37.2% (95% CI = 34.9–39.4%). South America (45.7%, 95% CI = 39.0–52.5) had the highest prevalence of multimorbidity, followed by North America (43.1%, 95% CI = 32.3–53.8%), Europe (39.2%, 95% CI = 33.2–45.2%), and Asia (35%, 95% CI = 31.4–38.5%). The subgroup study highlights that multimorbidity is more prevalent in females (39.4%, 95% CI = 36.4–42.4%) than males (32.8%, 95% CI = 30.0–35.6%). More than half of the adult population worldwide above 60 years of age had multimorbid conditions (51.0%, 95% CI = 44.1–58.0%). Multimorbidity has become increasingly prevalent in the last two decades, while the prevalence appears to have stayed stable in the recent decade among adults globally.
Interpretation
The multimorbidity patterns by geographic regions, time, age, and gender suggest noticeable demographic and regional differences in the burden of multimorbidity. According to insights about prevalence among adults, priority is required for effective and integrative interventions for older adults from South America, Europe, and North America. A high prevalence of multimorbidity among adults from South America suggests immediate interventions are needed to reduce the burden of morbidity. Furthermore, the high prevalence trend in the last two decades indicates that the global burden of multimorbidity continues at the same pace. The low prevalence in Africa suggests that there may be many undiagnosed chronic illness patients in Africa.
Funding
None.
Keywords: Multimorbidity, Systematic review, Meta-analysis, Global prevalence, Chronic disease
Research in context.
Evidence before this study
We searched PubMed, ScienceDirect, and Google Scholar for peer-reviewed papers and research reports on the prevalence of multimorbidity, using the search words 'prevalence' and 'multimorbidity' and similar terms published between January 1, 2000 and December 31, 2021. One meta-analysis combined 68 studies from 1992 to 2017 and showed that the global pooled prevalence of multimorbidity in community settings was 33.1%. In 2021, another meta-study focused on articles that investigated people in community settings from Latin America and the Caribbean.
Added value of this study
This research used studies until 2021 to analyze multimorbidity prevalence in community settings worldwide. South America has the highest prevalence of multimorbidity when comparing prevalence estimates across geographic regions. The prevalence difference was obtained across age groups, gender, country and income level, and study periods. For the first time in a subgroup study, we stratified the number of conditions to estimate the prevalence of multimorbidity. Studies that included mental health in the definition of multimorbidity resulted in a high pooled prevalence. Our research also uses statistical techniques to estimate the pooled prevalence of multimorbidity in adults while capturing heterogeneity in the estimates. This study summarizes the available evidence and encourages policymakers to use more standardized methods to reduce the burden of multimorbidity, which is a critical step toward meeting the sustainable development goal (SDG) goal of reducing premature mortality from non-communicable diseases by one-third through prevention and treatment by 2030.
Implications of all the available evidence
Our findings show that the landscape of multimorbidity prevalence has increased in the last two decades though it has remained relatively unchanged since 2010, implying a slow reduction in the burden of multimorbidity. About half of the South American adult population had multimorbidity, and thus these countries should take it as a priority agenda to develop more sustainable and integrated models of care. Research like this is crucial as the world tries to balance lowering the expense of multimorbidity on society and improving healthcare outcomes.
Introduction
Multimorbidity has emerged as a significant public health issue in the world. It is typically defined as the presence of two or more chronic conditions at the same time in one individual.1 Multimorbidity has increased in various population groups due to population aging, lifestyle changes, improved socioeconomic conditions, and improved diagnostic capabilities by health services.2, 3, 4 Due to a lack of data from low-income countries and the use of different definitions of multimorbidity, a recent systematic review highlighted the need to estimate the prevalence of multimorbidity and patterns of multimorbidity.5
The high prevalence of multimorbidity has several negative consequences, including a high mortality rate, increased healthcare utilization, and increased healthcare expenses, influencing overall functioning and quality of life.6, 7, 8, 9, 10 According to a recent review and meta-analysis, those with at least two morbidities have a 1.73 times higher risk of death than people without multimorbidity.8 Moreover, healthcare demands and costs of multimorbidity continue to rise as populations age.11
Although few systematic reviews and meta-analyses on multimorbidity in community settings have been published in recent years, these included fewer studies or are restricted to a specific geographic region.12, 13, 14, 15 According to a systematic review and meta-analysis of studies with data collected between 1992 and 2017, the global pooled prevalence of multimorbidity in community settings was 33.1% (95% confidence interval: 30.0–36.3%).12 This prior study, however, did not look at how multimorbidity patterns changed over time or gave insight into multimorbidity definitions based on the number of conditions.
In recent years, many studies have been conducted to identify the clinical patterns of chronic conditions.14,16, 17, 18, 19 Two systematic reviews on multimorbidity identified depression, hypertension, and diabetes as the most prevalent co-occurring chronic diseases.5,20 Another study of multimorbidity identified cardiovascular and metabolic diseases as the most common diseases, followed by mental health disorders and musculoskeletal conditions.21 In a multi-national cross-sectional study of non-institutionalized adults aged 50 and over in Finland, Poland, Spain, China, Ghana, India, Mexico, Russia, and South Africa, hypertension, cataract, and arthritis were the most prevalent comorbid conditions.22 A study conducted in Germany among health-insured individuals aged 65 and older identified three broad multimorbidity patterns–cardiovascular/metabolic disorders, anxiety/depression disorders, and pain/neuropsychiatric disorders.23 It indicates that mental health disorders were prevalent in the studies, so we examined the prevalence of multimorbidity with and without mental health disorders.
These findings provide an explanation for the clinical patterns as well as the burden of multimorbidity that was observed among the studied people. An accurate and up-to-date prevalence estimation is critical to assess the impact of multimorbidity on public health and project effective and integrative interventions to reduce premature death due to multimorbidity. It is challenging to conduct a meta-analysis to estimate a global prevalence as the different studies used a different number of diseases and disease combinations. There is no gold standard for quantifying multimorbidity; definitions of multimorbidity and statistical approaches for evaluating prevalence differ greatly.24, 25, 26, 27, 28 But the trade-off of generating pooled estimate of multimorbidity exceed the drawbacks of the variability in the data. However, the prevalence of multimorbidity was not thoroughly assessed based on geographic regions, country's economic level, age, study periods, and the number of diseases considered for defining multimorbidity.
Given the growing concern about the rising burden of chronic diseases, understanding the prevalence of multimorbidity in the adult population is critical for developing preventive strategies. As a result, we conducted a systematic review and meta-analysis to examine the global and regional prevalence of multimorbidity and changes in multimorbidity prevalence over time among the adult population in community settings.
Methods
Search strategy
We searched PubMed, Google Scholar, Embase and ScienceDirect online databases to select peer-reviewed papers for our systematic review and meta-analysis. We screened observational studies (cross-sectional and baseline in a cohort) to determine the global prevalence of multimorbidity in the adult population in community settings. Our search included articles published in any language between January 2000 and December 2021, which would help minimize data heterogeneity and provide a more precise estimate of global multimorbidity prevalence. The screening was conducted primarily in English, but we also utilized the Google translation tool for article selection. A description of search terms is given in Appendix A. The search results were compiled using Mendeley citation management software. In addition to the database search, we explored references of selected studies and previously published systematic reviews on similar topics to incorporate all potential pertinent articles to construct our summary estimates. The Preferred Reporting Items for Systematic Review and Meta-analysis (PRISMA) checklist was followed in this study.29 The protocol was registered in the PROSPERO database (CRD42020150945).
Selection criteria
Our systematic review included studies that (1) defined multimorbidity as having more than one underlying chronic conditions; (2) documented multimorbidity as the outcome of interest; (3) provided the number of participants in the study, with at least 200; (4) defined multimorbidity in the article, with at least five chronic conditions; (5) were observational studies, either cross-sectional or cohort, including adults 18 years and above; (6) published in years 2000–2021; and (7) were conducted in a community setting. Furthermore, only the recent study was considered if more than one study studied the same population. Only prevalence at baseline was included when the design was a cohort. Studies were excluded if they (1) focused only on comorbidity, (2) defined multimorbidity as more than two diseases (3) studied only inpatients or outpatients in hospital and primary care settings, (4) studied institutional population, i.e., people in nursing home, old home etc., (5) included acute conditions in the list of conditions, (6) used less than 5 conditions to define multimorbidity, or (7) were qualitative, interventional studies, opinion articles, conference presentations, books, letters, editorials, reviews, dissertations/theses, or abstracts.
Data extraction and quality assessment
Using Covidence, two independent reviewers (S.R.C. and D.C.D.) screened the articles. The reviewers examined successively the titles, abstracts, and full texts of all possibly relevant articles identified by our searches. The differences in article selection and data extraction were handled by consensus and, if necessary, discussion with another reviewer (A.H.). Two independent reviewers (S.R.C. and T.C.S.) created a data-extraction form to establish the type of information to be extracted. The reviewers (S.R.C. and T.C.S.) recorded pertinent data on the name of the first author, study settings (e.g., country, year of publication, study period (start-end year), region), and study conduct (e.g., study design, population age and male percentage, number of study participants, data sources, method of ascertainment of morbidity, and minimum number of conditions included in multimorbidity), prevalence of multimorbidity, and number of participants with multimorbidity from the published article only. We further stratified the articles based on the country's income level (World Bank classification by income, GNI per capita).30 Moreover, the study participants were cross tabulated by age group and gender, and multimorbidity was documented whenever possible. If the prevalence of multimorbidity was not directly given, it was manually computed from the data supplied in the articles. In studies providing longitudinal prevalence estimates over a period, we utilized baseline prevalence. After settling any differences, the two reviewers (S.R.C. and T.C.S.) independently extracted the data, discussed the inputs, and revised the extracted data. Unresolved issues were resolved by involving a third reviewer (J.B.).
The Newcastle-Ottawa Scale (NOS), the tool for assessing the quality of non-randomized research, was used to determine the risk of bias for individual studies.31 The eight items of NOS are categorized into three domains of potential bias, namely “selection (representativeness of the sample, sample size, non-respondents, ascertainment of the exposure),” “comparability (the subjects in different outcome groups are comparable, based on the study design or analysis; and confounding factors are controlled),” and “outcome (assessment of the outcome and statistical test)”.31, 32, 33 A few points on the NOS were modified to be relevant to our research question (Supplementary File 1). The articles' methodological stringency, lucidity, and clarity are reflected in the subjective scores. However, we did not eliminate any articles based on their quality scoring. A study can be given one star for each item within the selection and outcome categories. For comparability, a maximum of two stars can be awarded. Thus, a cross-sectional study can be awarded a maximum of 10 stars (10 points), and a cohort study can be awarded a maximum of 9 stars (9 points). Overall, the studies were categorized as “low risk of bias (8–10 stars)”, “moderate risk of bias (6–7 stars)”, and “high risk of bias (0–5 stars)”. Two independent reviewers (S.R.C. and D.C.D.) assessed the quality of the included studies, and the discrepancies were resolved with discussion with the third reviewer (A.H.). The PRISMA statement consists of a 27-item checklist given in Supplementary File 2.
Statistical analysis
The statistical analysis was performed using meta and metafor packages in the R statistical software (version 4.1.1). Multimorbidity prevalence was estimated as the ratio of the number of people with multimorbidity (numerator) and sample size (denominator). The numerator was derived from the percentage of people with multimorbidity when the numerator was not available. We obtained the pooled prevalence (with 95% CIs) of multimorbidity among the overall population from all studies and subgroups. The pooled prevalence was estimated using a random-effects model that allows the actual effect size to vary from study to study. The calculated proportion from each study and the combined effect estimate with 95% CI were represented graphically using forest plots. We assessed potential publication bias by visually observing the symmetry of funnel plots and using Egger's test. The I2 statistic was used to quantify heterogeneity across the selected studies. The I2 statistic indicates the proportion of overall variation across studies due to heterogeneity rather than chance. Subgroup analysis was carried out to determine the pooled prevalence for each group and look for potential explanations for the heterogeneity. Geographical region (Africa, Asia, Europe, North America, Oceania, and South America); WB/WHO income region (High, Upper-middle, Low- and Lower-middle); Study design (Cross-sectional, Cohort); Multimorbidity (5–9 conditions, 10–19 conditions, ≥20 conditions); Mental health included in the multimorbidity definition (Yes or No); Age groups of study participants (≥30 years, ≥40 years, ≥50 years, ≥60 years) and Gender (male and female) were considered for sub-group analysis. We conducted a trend analysis to see the global multimorbidity prevalence over time (2000–2021). We also conducted sensitivity analyses to assess the findings' robustness in consideration of sample size, multimorbidity prevalence, multimorbidity definitions based on the number of conditions studied, and NOS overall quality of the studies. Two-sided P < .05 was considered statistically significant.
Role of the funding source
There was no funding available for this study. All of the study's data was accessible to all of the authors, and the corresponding author had responsibility for publication.
Results
Identification and selection of studies
A flowchart of the literature search to select the relevant articles is summarized in the PRISMA format and is presented in Fig. 1. The initial search retrieved 8003 studies from the three pre-specified databases. After excluding the duplicates, the titles and abstracts were screened for a further selection of probable articles. Subsequently, the investigators selected 376 articles based on eligibility criteria for full-text review. By manual searching through the included papers’ reference lists and reference lists of previous systematic reviews on similar topics, 12 studies were considered for scrutiny, resulting in the total number of potential articles being 388. After excluding 262 studies in full-text review, finally, 126 studies with a total of 15,400,421 (approximately 15.4 million) people were included in the systematic review and meta-analysis. Sample sizes in the studies range from 264 to 3,759,836.3,27,34, 35, 36, 37, 38, 39, 40, 41, 42, 43, 44, 45, 46, 47, 48, 49, 50, 51, 52, 53, 54, 55, 56, 57, 58, 59, 60, 61, 62, 63, 64, 65, 66, 67, 68, 69, 70, 71, 72, 73, 74, 75, 76, 77, 78, 79, 80, 81, 82, 83, 84, 85, 86, 87, 88, 89, 90, 91, 92, 93, 94, 95, 96, 97, 98, 99, 100, 101, 102, 103, 104, 105, 106, 107, 108, 109, 110, 111, 112, 113, 114, 115, 116, 117, 118, 119, 120, 121, 122, 123, 124, 125, 126, 127, 128, 129, 130, 131, 132, 133, 134, 135, 136, 137, 138, 139, 140, 141, 142, 143, 144, 145, 146, 147, 148, 149, 150, 151, 152, 153, 154, 155
Fig. 1.
PRISMA flow diagram for study selection.
Characteristics of the studies
Table 1 shows the characteristics of the included studies. The 126 population-based studies were conducted across 54 countries. Six of the 126 research included were carried out in multiple countries. The majority of the studies (n = 47) were conducted in Asia, followed by Europe (n = 27), South America (n = 19), Africa (n = 10), North America (n = 14), Oceania (n = 6), and various continents (n = 3). Between 2000 and 2021, 53 studies were carried out in high-income countries (HICs), 48 in upper middle-income countries (UMICs), and 24 in low- and lower-middle-income countries (Low- and LMICs). Most of the studies (121 studies) were cross-sectional in design, and the remaining five had a cohort design, from which we used data from the baseline assessment. When defining multimorbidity, 37 studies looked at 5–9 diseases, 64 studies at 10–19 diseases, and 24 studies at more than 20 diseases.
Table 1.
Characteristics of the included studies in the meta-analysis (according to the order of year).
| Author [Ref] | Country | WB income country | Year of publication | Study period | Study design | Source of data | Ascertainment of morbiditiesa | Sample size | Age, y | Mean/median age, y | Gender (male %) | Number of conditions included | Prevalence, % |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Dhungana et al.,34 | Nepal | Low- or LMIC | 2021 | 2016–2018 | Cross-sectional | NCD (non-communicable diseases) survey 2018 in Nepal | Objective | 8931 | ≥20 | 46.7 | 42.2 | 7 | 14.0 |
| Zhang et al.,35 | China | UMIC | 2021 | 2017 | Cross-sectional | Beijing Longitudinal Study of Aging (BLSA) | Self-reported | 1837 | ≥60 | NA | 44.3 | 12 | 53.2 |
| Keetile et al.,36 | Botswana | UMIC | 2020 | 2016 | Cross-sectional | Survey on Chronic Non-Communicable Diseases in Botswana (NCDs survey) | Self-reported | 1178 | ≥15 | NA | 30.9 | 10 | 5.4 |
| Zou et al.,37 | China | UMIC | 2020 | 2004–2008 | Cross-sectional | A baseline dataset from China Kadoorie Biobank (CKB) study, a Chinese population-based cohort study | Self-reported and Objective | 512,888 | 30–79 | NA | 41.0 | 16 | 15.9 |
| Ma et al.38 | China | UMIC | 2020 | 2015–2106 | Cross-sectional | China Health and Retirement Longitudinal Study (CHARLS) | Self-reported | 19,656 | ≥45 | 60.2 | 48.3 | 14 | 54.3 |
| Kim et al.,39 | Korea | HIC | 2020 | 2016 | Cross-sectional | Korea National Health and Nutrition Examination Survey (KNHANES) | Self-reported | 68,590 | ≥19 | NA | NA | 39 | 23.7 |
| Kshatri et al.40 | India | Low- or LMIC | 2020 | 2019–2020 | Cross-sectional | A cross-sectional study | Self-reported | 725 | 60–106 | 70.2 | 52.1 | 18 | 48.8 |
| Kyprianidou et al.41 | Cyprus | HIC | 2020 | 2018–2019 | Cross-sectional | A cross-sectional study | Self-reported | 1140 | ≥18 | 40 | 43.7 | 47 | 28.6 |
| de Melo et al.42 | Brazil | UMIC | 2020 | 2013–2014 | Cross-sectional | National Health Survey database | Self-reported | 11,697 | ≥60 | 70.1 | 40.1 | 13 | 53.1 |
| Zhang et al.43 | USA | HIC | 2020 | 2012–2017 | Cross-sectional | National Health Interview Survey (2012–2017) of Asian Indians, Chinese, and NHWs (non-Hispanic whites) | Self-reported | 132,666 | ≥18 | NA | 48.5 | 10 | 38.2 |
| Li et al.44 | China | UMIC | 2019 | 2017 | Cross-sectional | A community-based cross-sectional health interview and examination survey | Self-reported and Objective | 4833 | ≥60 | NA | 45.5 | 5 | 16.1 |
| Aminisani et al.45 | Iran | UMIC | 2020 | 2017–2018 | Cross-sectional | Prospective Epidemiological Research Studies in Iran (PERSIAN) | Self-reported | 1493 | ≥50 | 61.6 | 38 | 36 | 36.6 |
| Craig et al.46 | Jamaica | Low- or LMIC | 2020 | 2007–2008 | Cross-sectional | Jamaica Health and Lifestyle Survey 2007/2008 (JHLS-II) | Self-reported | 2551 | 15–74 | NA | NA | 11 | 24.1 |
| Vargese et al.47 | India | Low- or LMIC | 2020 | 2017 | Cross-sectional | A register based cross sectional study | Self-reported | 525 | ≥18 | 47.4 | 46.9 | 12 | 16.2 |
| Lee et al.48 | Korea | HIC | 2020 | 2014 | Cross-sectional | 2014 Korean Health Panel Survey | Self-reported | 11,232 | ≥18 | 57.5 | 49.6 | ≥20 | 34.8 |
| Zhao et al.49 | China | UMIC | 2020 | 2011–2015 | Cross-sectional | China Health and Retirement Longitudinal Study (CHARLS) for 2011, 2013, and 2015 | Self-reported | 11,817 | ≥50 | 62 (median) | 48.8 | 11 | 61.9 |
| Wister et al.50 | Canada | HIC | 2020 | 2010 | Cross-sectional | Canadian Longitudinal Study on Aging (CLSA) dataset | Self-reported | 15,711 | 45–85 | 62 | 49 | 27 | 64 |
| Yao et al.51 | China | UMIC | 2019 | 2011–2015 | Cross-sectional | China Health and Retirement Longitudinal Study (CHARLS) | Self-reported | 19,841 | ≥50 | NA | 48.6 | 14 | 42.4 |
| Zhang et al.52 | China | UMIC | 2019 | 2015 | Cross-sectional | China Health and Retirement Longitudinal Survey (CHARLS) 2015 | Self-reported | 11,707 | ≥60 | 70.5 | 48.7 | 14 | 43.6 |
| Laires et al.53 | Portugal | HIC | 2019 | 2014 | Cross-sectional | Fifth Portuguese National Health Interview Survey, conducted in 2014 | Self-reported | 15,196 | 25–79 | NA | 44 | 15 | 43.9 |
| Ba et al.54 | Vietnam | Low- or LMIC | 2019 | 2018 | Cross-sectional | A cross-sectional study | Self-reported | 1680 | ≥15 | 38 | 50.1 | 9 | 16.4 |
| Khan et al.55 | Bangladesh | Low- or LMIC | 2019 | 2015–2016 | Cross-sectional | A large-scale cross-sectional study | Self-reported | 12,338 | ≥35 | 58.5 | 48.6 | 6 | 8.4 |
| Singh et al.56 | South Asia | Low- or LMIC | 2018 | 2010–2011 | Cross-sectional | Cardiometabolic Risk Reduction in South Asia Surveillance Study | Self-reported and Objective | 16,287 | ≥20 | 41 | 47.3 | 5 | 9.4 |
| Lai et al.57 | Hong Kong | HIC | 2019 | 2008 | Cross-sectional | The Thematic Household Survey (THS) on health-related topics | Self-reported | 17,396 | ≥35 | NA | 48.5 | 14 | 8.8 |
| Bao et al.58 | China | UMIC | 2019 | NA | Cross-sectional | Cross-sectional community health survey | Self-reported | 18,137 | ≥45 | 61.4 | 47.6 | 19 | 20.8 |
| Hu et al.59 | Taiwan | HIC | 2019 | 2003–2013 | Cross-sectional | The National Health Insurance Research Database | Self-reported | 1,429,527 | ≥20 | NA | NA | 20 | 30.4 |
| Park et al.60 | Korea | HIC | 2019 | 2013–2015 | Cross-sectional | Sixth Korean National Health and Nutrition Examination Survey (KNHANES) conducted in 2013–2015 | Self-reported | 8370 | ≥50 | 62.5 | 46.3 | 10 | 39 |
| Hernandez et al.61 | Ireland | HIC | 2019 | NA | Cross-sectional | Irish population study | Self-reported | 6101 | ≥50 | NA | 46.3 | 31 | 73.3 |
| Frolich et al.,62 | Denmark | HIC | 2019 | 2012 | Cross-sectional | Danish national administrative and health registries | Objective | 1,397,173 | ≥16 | NA | 48.4 | 16 | 21.6 |
| Chang et al.,63 | South Africa | UMIC | 2019 | 2014–2015 | Cross-sectional | Population-based survey conducted in The Health and Ageing in Africa: a longitudinal study of an INDEPTH Community in South Africa (HAALSI) Programme | Self-reported and Objective | 3889 | ≥40 | 61.7 | 45.2 | 10 | 69.4 |
| Nguyen et al.,64 | England | HIC | 2019 | 2004–2005 | Cross-sectional | English Longitudinal Study of Aging (ELSA) wave 2 | Self-reported | 9171 | ≥50 | 66.4 | 44.5 | 26 | 80.8 |
| dos Santos Costa et al.,65 | Brazil | UMIC | 2018 | 2014 | Cross-sectional | Cross-sectional population-based study | Self-reported | 1451 | ≥60 | NA | 37 | 29 | 92.8 |
| Cheung et al.,66 | Hong Kong | HIC | 2018 | 2016–2017 | Cross-sectional | Baseline well-being assessment of the Jockey Club Community eHealth Care Project | Self-reported | 2618 | ≥60 | NA | 47.5 | 7 | 41.8 |
| Zemedikun et al.,67 | UK | HIC | 2018 | 2006–2010 | Cross-sectional | UK Bio-bank, a major collaborative research project | Self-reported and Objective | 502,643 | 40–69 | 58 | 45.6 | 36 | 19 |
| El Lawindi et al.,68 | Egypt | Low- or LMIC | 2018 | 2016–2017 | Cross-sectional | A community-based cross-sectional study | Self-reported | 2317 | ≥18 | 36.2 | 54.9 | 16 | 19.6 |
| Stanley et al.,70 | New Zealand | HIC | 2018 | 2014 | Cross-sectional | National-level routine health data on hospital discharges and pharmaceutical dispensing | Objective | 3,489,747 | ≥18 | NA | 48.2 | 30 | 27.9 |
| Araujo et al.,71 | Brazil | UMIC | 2018 | 2015 | Cross-sectional | Cross-sectional population-based study | Self-reported | 4001 | ≥18 | NA | 47.2 | 12 | 29 |
| Jankovic et al.,72 | Serbia | UMIC | 2018 | 2013 | Cross-sectional | 2013 National Health Survey (NHS 2013) of the Serbian population | Self-reported | 13,765 | ≥20 | 51.8 | 46 | 13 | 30.2 |
| Chen et al.,73 | China | UMIC | 2018 | 2011–2012 | Cross-sectional | China Health and Retirement Longitudinal Study 2011 | Self-reported | 3737 | ≥45 | NA | 51.9 | 16 | 45.5 |
| Nunes et al.,74 | Brazil | UMIC | 2018 | 2015–2016 | Cross-sectional | The Brazilian Longitudinal Study of Aging (ELSI-Brazil) | Self-reported | 9412 | ≥50 | 62.9 | 46 | 19 | 67.8 |
| Mondor et al.,75 | Canada | HIC | 2018 | 2005–2012 | Cross-sectional | The Canadian Community Health Survey (CCHS) (2005–2011/12) | Objective | 27,195 | ≥18 | NA | 48.6 | 17 | 33.5 |
| Mounce et al.,76 | England | HIC | 2018 | 2002–2003 | Cohort | The English Longitudinal Study of Aging (ELSA) cohort | Self-reported | 4564 | ≥50 | NA | 43.7 | 15 | 34 |
| Ge et al.,77 | Singapore | HIC | 2018 | 2015–2016 | Cross-sectional | Population Health Index (PHI) survey | Objective | 1940 | ≥21 | 51.4 | 43.9 | 17 | 35 |
| Camargo-Casas et al.,78 | Colombia | UMIC | 2018 | 2012 | Cross-sectional | Salud, Bienestery, Envejecimiento Bogota (SABE-B), (Health, Well-being and Ageing Study) | Self-reported | 2000 | ≥60 | 71.1 | 36.6 | 12 | 40.4 |
| Amaral et al.,79 | Brazil | UMIC | 2018 | 2010 | Cross-sectional | A project entitled “Conditions of health, quality of life and depression in elderly persons assisted under the Family Health Strategy in Senador Guiomard, Acre” | Self-reported | 264 | 60–102 | NA | 39 | 14 | 66.3 |
| Puth et al.,80 | Germany | HIC | 2017 | 2012–2013 | Cross-sectional | National telephone health interview survey “German Health Update” (GEDA2012) | Self-reported | 19,294 | ≥18 | NA | 48.3 | 17 | 39.6 |
| Waterhouse et al.,81 | South Africa | UMIC | 2017 | 2007–2008 | Cross-sectional | Wave 1 (2007–08) of the South African Study on Global Ageing and Adult Health | Self-reported and Objective | 3055 | ≥50 | NA | 39.6 | 8 | 12.9 |
| Alimohammadian et al.,69 | Iran | UMIC | 2017 | 2004–2008 | Cross-sectional | Golestan cohort data | Self-reported | 49,946 | 40–75 | NA | 42.4 | 8 | 19.4 |
| Wang et al.,82 | Australia | HIC | 2017 | 2007 | Cross-sectional | 2007 National Survey of Mental Health and Wellbeing (NSMHWB) | Self-reported | 8820 | 16–85 | 44 | 49.7 | 8 | 28.8 |
| Kunna et al.,83 | China | UMIC | 2017 | 2008–2010 | Cross-sectional | World Health Organization Study on Global AGEing and Adult Health (SAGE) Wave 1 (2007–2010) | Self-reported and Objective | 11,814 | ≥50 | NA | 46.4 | 8 | 29.7 |
| Lujic et al.,84 | Australia | HIC | 2017 | 2005–2009 | Cohort | The 45 and Up Study, The PBS (Pharmaceutical Benefits Scheme) database, The NSW (New South Wales) Admitted Patient Data Collection (APDC) | Self-reported | 90,352 | ≥45 | 70.2 | 44.3 | 8 | 37.4 |
| Nunes et al.,85 | Brazil | UMIC | 2017 | 2013 | Cross-sectional | Population-based data from the Brazilian National Health Survey | Self-reported | 60,202 | ≥18 | 43.7 | 44.9 | 22 | 22.2 |
| Mini et al.,86 | India | Low- or LMIC | 2017 | 2011 | Cross-sectional | United Nations Population Fund (UNFPA) in the year 2011 on ‘Building Knowledge Base on Population Ageing in India’ | Self-reported | 9852 | ≥60 | 68 | 47 | 12 | 30.7 |
| Larsen et al.,87 | Denmark | HIC | 2017 | 2013 | Cross-sectional | Danish national health survey conducted in 2013 | Self-reported | 162,283 | ≥16 | 47.8 | 49 | 15 | 37 |
| Gu et al.,88 | China | UMIC | 2017 | 2013 | Cross-sectional | A cross-sectional study | Self-reported | 2452 | ≥60 | 69.2 | 51.5 | 13 | 49.4 |
| Dhalwani et al.,89 | England | HIC | 2017 | 2008–2013 | Cohort | The English Longitudinal Study of Ageing (ELSA) 4, 5, 6 | Self-reported | 5476 | ≥50 | 61 (median) | 47 | 18 | 21.1 |
| Nunes et al.,90 | Brazil | UMIC | 2016 | 2012 | Cross-sectional | A population-based cross-sectional study | Self-reported | 2927 | ≥20 | 45.7 | 41.1 | 11 | 29.1 |
| Picco et al.,91 | Singapore | HIC | 2016 | 2012–2013 | Cross-sectional | The Well-being of the Singapore Elderly (WiSE) study | Self-reported | 2565 | ≥60 | NA | 43.5 | 10 | 51.5 |
| Palladino et al.,92 | 16 countries | HIC | 2016 | 2011–2012 | Cross-sectional | Survey of Health, Ageing and Retirement in Europe (SHARE) in 2011–12 | Self-reported | 56,427 | ≥50 | 66 | 44.1 | 13 | 37.3 |
| Cossec et al.,93 | France | HIC | 2016 | 2012 | Cross-sectional | Health, Health Care and Insurance Survey from 2012 (Enquête Santé et Protection Sociale) called ESPS | Self-reported | 4236 | 56–105 | 69.6 | 43 | 7 | 14.9 |
| Vadrevu et al.,104 | India | Low- or LMIC | 2016 | 2009 | Cross-sectional | A cross-sectional survey | Self-reported | 815 | ≥40 | 54.9 | 51.3 | 6 | 44.1 |
| Marengoni et al.,95 | Sweden | HIC | 2016 | 2001–2004 | Cross-sectional | Swedish National study on Aging and Care in Kungsholmen (SNAC-K) | Objective | 3155 | ≥60 | 74.4 | 35.7 | ≥5 | 52.4 |
| Jovic et al.,96 | Serbia | UMIC | 2016 | 2013 | Cross-sectional | 2013 National Health Survey (NHS 2013) of the Serbian population | Self-reported | 13,103 | ≥20 | 49.4 | 48.1 | 12 | 26.9 |
| Su et al.,97 | China | UMIC | 2016 | 2013 | Cross-sectional | A large-scale survey initiated by Shanghai Health and Family Planning Commission | Self-reported | 2058 | ≥80 | NA | 42.1 | 10 | 49.2 |
| Ramond-Roquin et al.,98 | Canada | HIC | 2016 | 2010 | Cross-sectional | The Program of Research on the Evolution of a Cohort Investigating Health System Effects (PRECISE) | Self-reported | 1710 | 25–75 | 51.3 | 40.5 | 21 | 63.8 |
| Lenzi et al.,99 | Italy | HIC | 2016 | 2012 | Cross-sectional | The hospital discharge record (HDR) database, the mental health information system, residential mental healthcare discharge records, the outpatient pharmaceutical database, the regional mortality register database | Objective | 3,759,836 | ≥18 | NA | 48 | 26 | 15.3 |
| Dung et al.,100 | Vietnam | Low- or LMIC | 2016 | 2011 | Cross-sectional | Vietnam Ageing Survey (VNAS) | Self-reported | 2789 | ≥60 | 71.9 | 39.7 | 12 | 43.9 |
| Valadares et al.,101 | Brazil | UMIC | 2016 | 2012–2013 | Cross-sectional | Cross-sectional population-based study | Self-reported | 749 | 45–60 | 52.5 | 0 | 11 | 53 |
| Pache et al.,102 | Switzerland | HIC | 2015 | 2003–2006 | Cross-sectional | Population-based study | Objective | 3714 | 35–75 | 49.6 | 47 | 27 | 56.3 |
| Afshar et al.,103 | 28 countries | NA | 2015 | 2003 | Cross-sectional | World Health Survey (2003) | Self-reported | 125,404 | ≥18 | NA | 48.5 | 6 | 7.8 |
| Roberts et al.,104 | Canada | HIC | 2015 | 2011–2012 | Cross-sectional | Canadian Community Health Survey 2011/12 | Self-reported | 105,406 | ≥20 | NA | 44.1 | 9 | 12.9 |
| Arokiasamy et al.,105 | 6 Countries | Low- or LMIC | 2015 | 2007–2010 | Cross-sectional | World Health Organization Study on Global AGEing and Adult Health (SAGE) Wave 1 (2007–2010) | Self-reported and Objective | 42,236 | ≥18 | NA | 50.7 | 8 | 21.9 |
| Ha et al.,106 | Vietnam | Low- or LMIC | 2015 | 2010 | Cross-sectional | Population-based study | Objective | 2400 | ≥60 | 72.6 | 34.8 | 6 | 39.2 |
| Wang et al.,107 | China | UMIC | 2015 | 2012 | Cross-sectional | Jilin Provincial Chronic Disease Survey | Self-reported | 21,435 | 18–79 | NA | NA | 18 | 24.7 |
| Wang et al.,108 | China | UMIC | 2015 | 2010–2011 | Cross-sectional | Confucius Hometown Aging Project in Shandong, China (June 2010–July 2011) | Self-reported and Objective | 1480 | ≥60 | 68.5 | 40.6 | 16 | 90.5 |
| Nunes et al.,109 | Brazil | UMIC | 2015 | 2008 | Cross-sectional | A population-based cross-sectional study | Self-reported | 1593 | ≥60 | NA | 37.2 | 17 | 81.3 |
| Chung et al.,110 | Hong Kong | HIC | 2015 | 2011–2012 | Cross-sectional | Thematic Household Survey (THS) conducted by the Census and Statistics Department (C&SD) of the Hong Kong SAR Government | Self-reported | 25,780 | ≥15 | NA | 47.8 | 46 | 12.5 |
| Hussain et al.,3 | Indonesia | UMIC | 2015 | 2007–2008 | Cross-sectional | Fourth wave of Indonesian Family Life Survey (IFLS-4) | Self-reported and Objective | 9438 | ≥40 | NA | 48.4 | 11 | 35.7 |
| Ruel et al.,111 | Australia | HIC | 2014 | 2000–2002 | Case-sectional | North West Adelaide longitudinal Health Study (NWAHS) | Self-reported and Objective | 1854 | ≥18 | 50 | 48 | 8 | 32 |
| Mahwati et al.,112 | Indonesia | UMIC | 2014 | 2007–2008 | Cross-sectional | The fourth survey of the Indonesian Family Life Survey (IFLS) which held in 2007 | Self-reported | 2960 | ≥60 | NA | 46 | 9 | 15.8 |
| Islam et al.,27 | Australia | HIC | 2014 | 2009 | Cross-sectional | A cross-sectional survey | Self-reported | 4574 | ≥50 | 69.3 | NA | 11 | 52 |
| Banjare et al.,113 | India | Low- or LMIC | 2014 | 2011–2012 | Cross-sectional | A cross-sectional survey | Self-reported | 310 | ≥60 | NA | 49.4 | 21 | 56.8 |
| Hien et al.,114 | Burkina Faso | Low- or LMIC | 2014 | 2012 | Cross-sectional | Cross-sectional study among community-dwelling elderly | Objective | 389 | ≥60 | 69 | 55.3 | 15 | 65 |
| Orueta et al.,115 | Spain | HIC | 2013 | 2007–2011 | Cross-sectional | Primary care electronic medical records, hospital admissions, and outpatient care databases | Objective | 452,698 | ≥65 | NA | 42.5 | 47 | 61.1 |
| Aguiar et al.,116 | Brazil | UMIC | 2013 | 2011 | Cross-sectional | A cross-sectional, population-based study | Self-reported | 622 | ≥50 | 64.1 | 0 | 12 | 58.2 |
| Alaba et al.117 | South Africa | UMIC | 2013 | 2008 | Cross-sectional | South African National Income Dynamics Survey (SA-NIDS) of 2008 | Self-reported | 11,638 | ≥18 | 40 | 39 | 6 | 4 |
| Wu et al.,118 | China | UMIC | 2013 | 2010 | Cross-sectional | SAGE-China Wave 1 | Self-reported and Objective | 13,157 | ≥50 | 62.6 | 48.1 | 8 | 18.9 |
| Phaswana-Mafuya et al.,119 | South Africa | UMIC | 2013 | 2008 | Cross-sectional | National population-based cross-sectional survey | Self-reported | 3638 | ≥50 | NA | 42.5 | 8 | 22.5 |
| Jerliu et al.,120 | Kosovo | UMIC | 2013 | 2011 | Cross-sectional | A nationwide cross-sectional study | Self-reported | 1890 | ≥65 | 73.4 | 50.2 | 6 | 45.2 |
| Kiliari et al.,121 | Cyprus | HIC | 2013 | 2008 | Cross-sectional | A nationally based survey | Self-reported | 465 | 18–88 | 53 | 43.2 | 27 | 28.5 |
| Fuchs et al.,122 | Germany | HIC | 2012 | 2008–2009 | Cross-sectional | Telephone health interview surveys in representative samples of the German adult population (German Health Update, GEDA) | Self-reported | 21,262 | 18–100 | 48.8 | 48.5 | 22 | 40.1 |
| MacHado et al.,123 | Brazil | UMIC | 2012 | 2005 | Cross-sectional | A secondary analysis of a cross-sectional population-based study | Self-reported | 377 | 40–65 | NA | 0 | 5 | 39.3 |
| Kirchberger et al.,124 | Germany | HIC | 2012 | 2008–2009 | Cross-sectional | The population-based KORA-Age project | Self-reported | 4067 | 65–94 | 73.4 | 48.8 | 13 | 58.6 |
| Agborsangaya et al.,125 | Canada | HIC | 2012 | 2010 | Cross-sectional | Health Quality Council of Alberta (HQCA) 2010 Patient Experience Survey | Self-reported | 5010 | ≥18 | 46.7 | 47.7 | 16 | 19 |
| Tucker-Seeley et al.,126 | USA | HIC | 2011 | 2004 | Cross-sectional | The Health and Retirement Study (HRS) | Self-reported | 7305 | ≥50 | 65 | 46.4 | 6 | 35.4 |
| Khanam et al.,127 | Bangladesh | Low- or LMIC | 2011 | 2004 | Cross-sectional | A descriptive cross-sectional study | Objective | 452 | 60–92 | 69.5 | 45.1 | 9 | 53.8 |
| Taylor et al.,128 | Australia | HIC | 2010 | 2004–2006 | Cross-sectional | North West Adelaide Health Study (NWAHS Stage 2) | Self-reported and Objective | 3206 | ≥20 | NA | NA | 7 | 17.1 |
| Loza et al.,129 | Spain | HIC | 2009 | 1999–2000 | Cross-sectional | A national health survey | Self-reported and Objective | 2192 | ≥20 | NA | 46.3 | 9 | 29.7 |
| Minh et al.,130 | 5 countries | Low- or LMIC | 2008 | 2005 | Cross-sectional | 2005 cross-site study of 8 sites in 5 Asian countries | Self-reported | 18,494 | 25–64 | NA | 50 | 7 | 7.2 |
| Camargo-Casas,78 | Columbia | UMIC | 2018 | 2012 | Cross-sectional | NA | Self-reported | 2000 | ≥60 | 71.1 | 36.6 | NA | 40.4 |
| Wilk et al.131 | Canada | HIC | 2021 | 2015–2018 | Cross-sectional | Canadian Community Health Survey (CCHS), 2015–2018 | Self-reported | 100,803 | ≥20 | 47.9 | 48.9 | 5 | 8.1 |
| Tomita et al.132 | Tanzania | Low- or LMIC | 2021 | 2017–2018 | Cross-sectional | The Dar es Salaam Health and Demographic Surveillance System (HDSS) | Self-reported | 2299 | ≥40 | 53.0 | 32.4 | 8 | 24.8 |
| Smith et al.133 | Ireland | HIC | 2021 | 2009–2013 | Cross-sectional | Irish Longitudinal Study on Ageing (TILDA) Survey | Self-reported | 5946 | ≥50 | 62.7 | 51.7 | 14 | 50.3 |
| Delpino et al.,134 | Brazil | UMIC | 2021 | 2019 | Cross-sectional | The Brazilian National Health Survey 2019 | Self-reported | 65,803 | 18–59 | NA | 47.8 | 14 | 22.3 |
| Marthias et al.,135 | Indonesia | UMIC | 2021 | 2014 | Cross-sectional | The Indonesian Family Life Survey 2014 (Wave – 5) | Self-reported and Objective | 3678 | ≥50 | 65 (median) | 46.1 | 10 | 22.0 |
| Zhang et al.136 | China | UMIC | 2021 | 2019 | Cross-sectional | A cross-sectional study | Self-reported and Objective | 3250 | ≥60 | NA | 46.6 | 26 | 30.3 |
| Lin et al.,137 | Taiwan | HIC | 2021 | 2017–2019 | Cross-sectional | A community-based survey | Self-reported | 3739 | 65–85 | 72.9 | 42.8 | 7 | 27.8 |
| Nicholson et al.,138 | Canada | HIC | 2021 | 2015 | Cross-sectional | The Canadian Longitudinal Study on Aging (CLSA) | Self-reported | 11,161 | 65–85 | NA | 47.5 | 15 | 75.3 |
| Bezerra et al.,139 | 17 countries | HIC | 2021 | 2015 | Cross-sectional | Survey of Health, Aging and Retirement in Europe (SHARE) 2015 (Wave – 6) | Self-reported | 63,844 | ≥50 | NA | 44.3 | 13 | 33.6 |
| Koyanagi et al.,140 | 48 countries | Low- or LMIC | 2021 | 2002–2004 | Cross-sectional | The World Health Survey 2002–2004 | Self-reported | 224,842 | ≥18 | 38.3 | 49.3 | 10 | 3.8 |
| Shi et al.,141 | Brazil | UMIC | 2021 | 1998–2013 | Cross-sectional | The National Sample Household and Brazilian National Health Survey | Self-reported | 795,271 | ≥18 | NA | 47.2 | 9 | 18.3 |
| Wang et al.,142 | China | UMIC | 2021 | 2018 | Cross-sectional | A cross-sectional survey | Self-reported | 1871 | ≥60 | 83.6 | 39.0 | 33 | 74.3 |
| He et al.,143 | China | UMIC | 2021 | 2014–2019 | Cohort | Annual health examination data set in the Xinzheng electronic health Management | Self-reported and Objective | 50,100 | ≥65 | 69.2 (median) | 46.1 | 7 | 31.4 |
| Ballesteros et al.,144 | Colombia | UMIC | 2021 | 2015 | Cross-sectional | Colombian population-based survey Health, Wellbeing and Aging (Salud, Bienestar y Envejecimiento—SABE) | Self-reported | 17,571 | ≥60 | 69.2 | 44.3 | 10 | 62.3 |
| Mohamed et al.,145 | Kenya | LMIC | 2021 | 2003–2015 | Cross-sectional | Nairobi Urban Health & Demographic Surveillance System (NUHDSS) | Self-reported and Objective | 2003 | ≥40 | 48.8 | 46.0 | 16 | 28.7 |
| Kanungo et al.,146 | India | Low- or LMIC | 2021 | 2017–2019 | Cross-sectional | Longitudinal Ageing Study in India (LASI), Wave-1 | Self-reported | 59,764 | 45–116 | 60.2 | 45.9 | 12 | 50.4 |
| Oh et al.,147 | USA | HIC | 2020 | 2001–2003 | Cross-sectional | The National Survey of American Life | Self-reported | 5191 | ≥18 | 42.2 | 63.1 | 22 | 54.1 |
| King et al.,148 | USA | HIC | 2019 | 2013–2014 | Cross-sectional | The National Health and Nutrition Examination Survey (NHANES) | Self-reported and Objective | 5541 | ≥20 | NA | 48.2 | 11 | 59.6 |
| Bowling et al.149 | USA | HIC | 2019 | 2011–2016 | Cross-sectional | The National Health and Nutrition Examination Survey (NHANES), 2011–2016 | Self-reported and Objective | 4217 | ≥50 | 56.7 | 48.7 | 12 | 72.4 |
| Keats et al.150 | Canada | HIC | 2017 | 2009–2015 | Cohort | Atlantic Partnership for Tomorrow's Health (PATH) study | Self-reported | 18,709 | ≥35 | NA | 30.0 | 18 | 38.2 |
| Quinaz Romana et al.151 | Portugal | HIC | 2019 | 2013–2016 | Cross-sectional | The National Health Examination Survey (INSEF) | Objective | 4911 | ≥25 | NA | 47.5 | 20 | 38.3 |
| de Souza et al.152 | Brazil | UMIC | 2019 | 2001–2002 | Cohort | A longitudinal study of municipal technical and administrative employees in Rio de Janeiro | Self-reported and Objective | 733 | ≥24 | 41.6 | 33.8 | 15 | 45.6 |
| Costa et al.153 | Brazil | UMIC | 2020 | 2013–2014 | Cross-sectional | Brazilian National Survey | Self-reported and Objective | 23,329 | ≥20 | 37.9 | 47.2 | 14 | 10.9 |
| Keomma et al.154 | Brazil | UMIC | 2020 | 2015 | Cross-sectional | The ISA-Capital health survey | Self-reported and Objective | 1019 | ≥60 | 67.7 | 40.3 | 10 | 40 |
| Jürisson et al.155 | Estonia | HIC | 2021 | 2015–2017 | Cross-sectional | Estonian Health Insurance Fund | Objective | 909,477 | ≥25 | 53.4 | 45.9 | 55 | 39.8 |
Ascertainment of morbidities- Objective: medical records/clinical examinations.
Global and regional prevalence of multimorbidity
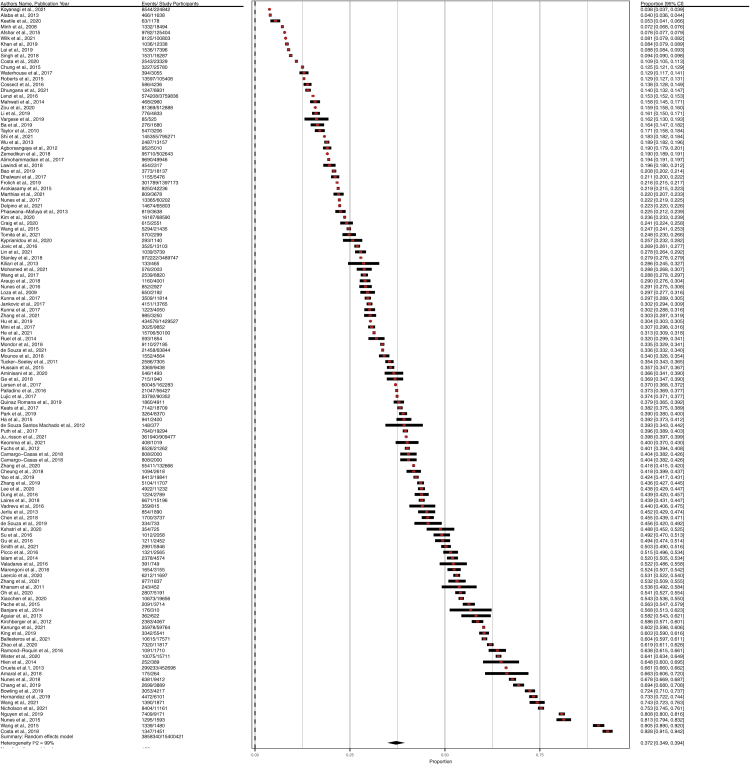
The prevalence of multimorbidity among the adult population ranged from 4.0% to 92.8% in the studies. Prevalence estimates along with confidence intervals for multimorbidity are shown in Fig. 2 by using a forest plot. The random-effects overall pooled estimated (126 studies) prevalence of multimorbidity was 37.2% (95% CI = 34.9%–39.4%, I2 = 99.7%). The pooled proportion of multimorbidity was the highest in South America with 45.7% (95% CI = 39.0%–52.5%, I2 = 99.0%). On the other hand, the pooled prevalence of multimorbidity was the lowest in Africa with 28.2% (95% CI = 15.6%–40.8%, I2 = 99.0%). However, studies from Asia, Europe, North America, and Oceania were calculated to have the pooled prevalence of multimorbidity 35% (95% CI = 31.4%–38.5%, I2 = 99.3%), 39.2% (95% CI = 33.2%–45.2%), 43.1% (95% CI = 32.3%–53.8%), and 32.5% (95% CI = 26.8%–38.2%, I2 = 98.9%), respectively.
Fig. 2.
Forest Plot of the Overall Prevalence of multimorbidity in community settings.
Subgroup analysis
The subgroup analysis of the prevalence of multimorbidity by continents, study design, number of diseases included in multimorbidity, age, and gender is shown in Table 2. The forest plots are given in the Supplementary File 3. Of note, 85 studies reported the prevalence of multimorbidity in males and females. According to the table, the pooled prevalence of multimorbidity was higher among female participants (39.4%, 95% CI = 36.4–42.4%, I2 = 99.6%) than male participants (32.8%, 95% CI = 30.0–35.6%, I2 = 99.6%). The Fig. 3 shows the gender segregation of pooled prevalence of multimorbidity by geographic regions. Female participants from South America (prevalence 50.1% and 95% CI = 39.7–60.4%) appeared to have the most multimorbid conditions in the world. Multimorbid illnesses were notably more prevalent in European and North American women than in male participants.
Table 2.
Summary results of subgroup analysis.
| Subgroup | No of studies | Weighted Mean agea (SE) | Pooled prevalence of Multimorbidity | 95% CI | I2 (%) | |
|---|---|---|---|---|---|---|
| WHO geographic Region | Africa | 10 | 49.71 (10.9) | 0.282 | 0.156–0.408 | 99.9 |
| Asia | 47 | 57.76 (11.6) | 0.350 | 0.314–0.385 | 99.9 | |
| Europe | 27 | 58.16 (9.6) | 0.392 | 0.332–0.452 | 99.6 | |
| North America | 14 | 54.61 (6.1) | 0.431 | 0.323–0.538 | 99.9 | |
| Oceania | 6 | 58.38 (13.3) | 0.325 | 0.268–0.382 | 98.3 | |
| South America | 19 | 56.38 (13.4) | 0.457 | 0.390–0.525 | 99.9 | |
| WB/WHO income region | High | 53 | 56.61 (9.7) | 0.386 | 0.353–0.419 | 99.9 |
| Upper-middle | 48 | 60.43 (12.5) | 0.387 | 0.355–0.419 | 99.9 | |
| Low and Low-middle | 24 | 53.19 (11.93) | 0.321 | 0.243–0.40 | 99.8 | |
| Study design | Cross-sectional | 121 | 56.46 (11.06) | 0.374 | 0.351–0.396 | 99.3 |
| Cohort | 5 | 62.7 (6.71) | 0.324 | 0.279–0.369 | 96.7 | |
| Number of conditions included for defining multimorbidity | 5–9 conditions | 37 | 57.54 (12.64) | 0.250 | 0.223–0.278 | 97.9 |
| 10–19 conditions | 64 | 60.15 (9.96) | 0.413 | 0.376–0.450 | 99.9 | |
| ≥20 conditions | 24 | 53.44 (8.47) | 0.457 | 0.393–0.500 | 99.9 | |
| Gender | Female | 85 | – | 0.394 | 0.364–0.424 | 99.9 |
| Male | 85 | – | 0.328 | 0.300–0.356 | 99.2 | |
| Mental health included in Multimorbidity definition | Yes | 91 | 57.62 (11.02) | 0.384 | 0.359–0.410 | 99.3 |
| Nob | 28 | 61.12 (11.56) | 0.332 | 0.271–0.392 | 98.9 | |
| Age of the study participants | ≥30 years | 76 | 65.2 (6.26) | 0.444 | 0.393–0.494 | 99.9 |
| ≥40 years | 71 | 65.86 (5.69) | 0.457 | 0.402–0.512 | 99.9 | |
| ≥50 years | 58 | 67.42 (4.63) | 0.472 | 0.420–0.525 | 99.9 | |
| ≥60 years | 33 | 70.91 (2.01) | 0.510 | 0.441–0.580 | 98.3 | |
| Overall | 126 | 56.95 (10.85) | 0.373 | 0.349–0.394 | 99.0 | |
The weighted mean age and standard error (SE) were calculated based on the available study sample size and the study participant's mean/median age.
Because the disease list was not mentioned in a few of the articles, we assumed these articles may not contain mental health.
Fig. 3.
Regional differences of pooled prevalence of multimorbidity by gender.
Based on the continents of the studies, the estimated pooled prevalence of multimorbidity was found 38.6% (95% CI = 35.3%–41.9%, I2 = 99.2%) in high-income countries, 38.7% (95% CI = 35.5–41.9%, I2 = 99.2%) in upper middle-income countries (UMICs), and 32.1% (95% CI = 24.3–40.0%, I2 = 99.5%) in Low- and LMICs. In the case of the number of diseases included in the multimorbidity, the prevalence was found 44.7% (95% CI = 39.5%–50.0%, I2 = 99.3%) among the studies that considered ≥20 diseases. The prevalence of multimorbidity was 25.0% (95% CI = 22.3–27.8%, I2 = 99.0%) for studies with 5–9 diseases, and 41.3% (95% CI = 37.6%–45.0%, I2 = 99.0%) for studies with 10–19 diseases. When mental health is included in the multimorbidity definition, the prevalence (38.4%, 95% CI = 35.9–41.0%, I2 = 99.0%) was higher than without inclusion of mental health (33.2%, 95% CI = 27.1–39.2%, I2 = 99.1%).
Among the different age groups of the study participants, the highest prevalence was found in the studies that included the respondents more than 60 years with 51.0% (95% CI = 44.1%–58.0%). The pooled prevalence was 44.4% (95% CI = 39.3%–49.4%, I2 = 99.1%) among the participants with 30 years and above. When the study participants were ≥40 years and ≥50 years, the pooled proportion of multimorbidity was 45.7% (95% CI = 40.2%–51.2%, I2 = 99.0%) and 47.2% (95% CI = 42.0%–52.5%, I2 = 99.1%), respectively.
There was a difference in the prevalence of multimorbidity by study design among the studies. The pooled prevalence of multimorbidity was 37.4% (95% CI = 35.1%–39.6%, I2 = 99.0%) for cross-sectional studies, and 32.4% (95% CI = 27.9%–36.9%, I2 = 96.7%) for cohort studies.
Trends of global multimorbidity prevalence over time
The global prevalence of multimorbidity by 5-year interval is displayed in Fig. 4, considering studies that contains 10 or more diseases. The five-year span was categorized based on the year in which investigations were done. If a study was completed between 2013 and 2016, we assumed it was conducted between 2011 and 2015 because the majority of years fell within the interval. The study was removed from the analysis if it did not belong to any of the groups. We excluded papers that reported a multimorbidity prevalence of less than 10% or greater than 80% in order to minimize variability in trend analysis. The prevalence of multimorbidity has been on the rise globally since 2000, but it has remained rather stable since 2011. The trend analysis with the studies that considered ten or more illnesses in multimorbidity classifications, showed that the global prevalence of multimorbidity remained high, exceeding 40%.
Fig. 4.
Pooled prevalence of multimorbidity by year.
Sensitivity analysis for global prevalence
We conducted sensitivity analyses including studies with more than 1000 participants, removing studies from Africa, and removing studies that showed prevalence of less than 20% and more than 80%. The reasons for removing studies with less than 1000 participants are to increase estimate reliability and precision of the estimate with the studies with a larger sample size. Furthermore, we excluded papers with extreme prevalence estimates of less than 20% and more than 80% because these values could lead to heterogeneity in predicting worldwide prevalence. Forest plots are reported in Supplementary File 4. When considering studies of more than 1000 participants, the global prevalence among participants tends to be 36.1% (95% CI = 33.7–38.4%, I2 = 98.8%), which is in line with the findings of the meta-analysis with 126 studies. After excluding African studies, the prevalence was 37.9% (95% CI = 35.4%–40.2%), which is comparable to the meta-analysis with 126 studies. We also found the global prevalence was higher than the overall pooled prevalence after removing studies with extreme prevalence. The results showed the prevalence 42.3% (95% CI = 39.8–44.7%, I2 = 98.8%) after excluding studies with extreme prevalence. The findings excluding studies with extreme prevalence are, therefore, higher than the meta-analysis of 126 studies. With high-quality papers (minimal bias according to NOS), we found the prevalence to be 36.6% (95% CI = 33.6–39.5%, I2 = 99.8), which imply a similar result that we analyzed in the meta-analysis of 126 studies. Moreover, the studies using self-reported multimorbid data indicate a prevalence of 38.3% (95% CI = 35.1–41.5%), but the studies with data from medical records indicate a prevalence of 34.3% [95% CI = 30.3–38.2%].
Publication bias
The Egger test found that there was no statistically significant publication bias (P > .05) among the 83 population-based studies evaluating the relationship between gender and multimorbidity status. However, the Egger test revealed a statistically significant publication bias among the 126 population-based studies for proportion (Supplementary File 5). We also have applied trim-and-fill method to adjust for this publication bias in the analysis. We see that the procedure identified and trimmed 42 added studies. The overall effect estimated by the trim-and-fill is 26.71% (95% CI = 0.2350–0.2799). Our initial estimate with 126 studies was 37.1%, which is substantially larger than the bias-corrected effect. If we assume that publication bias affected our findings, the trim-and-fill method allows us to hypothesize that our initial results were overstated because of publication bias, and the global estimate when controlling for selective publication might be 26.71%. Moreover, considering the odds ratio in a funnel plot we found a high existence of publication bias in our study. Consequently, publication bias may be a cause of heterogeneity in investigating overall proportion.
Discussion
This study analyzed data from 126 studies that involved nearly 15.4 million people from 54 countries, providing an up-to-date global multimorbidity prevalence of 37.2% (95% CI = 34.9–39.4%). A previous meta-analysis with studies until 2017 found that 33.1% had multimorbidity in the adult population aged 18 and older living in the community.12 In comparison to that meta-analysis including studies in community settings, we found a higher prevalence of multimorbidity. Another meta-analysis that included studies from both community and healthcare settings estimated the overall prevalence of multimorbidity was 42.4% (95% CI = 38.9–46.0%) among adults.156 The inclusion of studies from primary care and health care settings in the meta-analysis resulted in a higher pooled prevalence than ours.
The sub-group analysis by region showed significant differences in the pooled prevalence of multimorbidity. Our analysis showed that the prevalence of multimorbidity was highest in South America. The result is consistent with a meta-analysis that found that the pooled proportion of multimorbidity in Latin America and the Caribbean was as high as 43% (95% CI: 35–51%).157 Africa had the lowest prevalence of multimorbidity, according to our analysis. The result could be attributable to the low age group of participants in the African studies compared to other geographic regions. The lowest rate of multimorbidity in Africa should be interpreted with caution because it raises the possibility that many people living with chronic illnesses in Africa are going undiagnosed.
In subgroup analysis, the prevalence of multimorbidity was lower in Low- and LMICs than in UMICs and HICs. The prevalence of multimorbidity was highest in UMICs. This difference is consistent with another study's findings, where a meta-analysis in community settings found that the pooled multimorbidity prevalence was higher in HICs than LMICs.12,156 The majority of the survey included in the meta-analysis were from HICs and UMICs, with a few studies conducted in Low-income countries. It may reflect the differences in diagnostic and data management systems among HICs, UMICs, and Low- and LMICs. According to a study, the disparity in prevalence estimates between HICs and LMICs could be due to the fewer publications on multimorbidity prevalence in LMICs because of limited understanding and importance of multimorbidity in LMICs compared to HICs.158 People in low-income countries may be less likely to seek treatment for diseases than those in high-income countries. Therefore, the prevalence in low-income countries may be underestimated if diseases are defined using medical records.
The pooled prevalence of multimorbidity was higher for the cross-sectional study design than for the cohort study type in this meta-analysis. This disparity in multimorbidity prevalence could be due to study designs with varying levels of methodological differences, such as various study populations, sampling procedures, sample coverage, sample sizes, data collection, and so on. Besides, we considered the baseline sample for a cohort study design that might contribute to the lower prevalence.
For included studies, the more the number of diseases evaluated for multimorbidity, the higher the prevalence. When examining 20 or more conditions for multimorbidity, the prevalence was 44.7%, but it was lowered to 41.3% for 10–19 diseases and 25.0% for 5–9 diseases to define multimorbidity. According to a study, the different combinations of illnesses may cause the prevalence of multimorbidity to differ significantly.156,159 A range of different combinations of multimorbidity definitions has been proposed in the literature, ranging from a list of 16 chronic diseases to 291 diseases.156,158, 159, 160, 161 Furthermore, the pooled estimate of multimorbidity prevalence with the studies those included mental health in the definition of multimorbidity was greater. Previous studies identified a correlation between multimorbidity and mental health.20,162,163 Our findings, the higher prevalence of multimorbidity with the studies that included mental health, reveal consistency with the findings of previous research.
Our study showed that prevalence estimates varied substantially according to age and gender. Our research showed that females had a higher pooled prevalence of multimorbidity than males. It indicates an association between gender and multimorbidity (evidence of which was provided in multiple studies).69,162,163 According to our findings, multimorbidity increases with age. While the prevalence estimates varied between and within age groups, our meta-analysis indicated that a large proportion of individuals over 60 had multimorbidity. It is well established that the prevalence of multimorbidity increases in very old persons.164, 165
The calculation of the global prevalence of multimorbidity based on the study's publication interval of 5-year is one of the most important findings of our research. According to our findings, the prevalence of multimorbidity has changed considerably over the previous two decades but has remained relatively consistent since 2011. This suggests a gradual decline in the global burden of multimorbidity. The plateau observed in multimorbidity prevalence since 2011 may be attributable to a handful of the 19 studies that showed low prevalence in 2016–2021. Therefore, this conclusion should be studied further. Over the years, the global prevalence of multimorbidity among adults has exceeded 40 percent, indicating a high burden of multimorbidity exists over years.
One of the study's strengths was its strong study selection and screening protocols. Because of our rigorous search approach and inclusion criteria, we were able to conduct the largest systematic review of multimorbidity prevalence in community settings to date. The majority of the papers included in the review were of high quality. The comprehensive subgroup analyses demonstrate that our findings are applicable to a wide range of contexts. One important finding of our study is the estimation of the global prevalence of multimorbidity by year of publication. This review did, however, have several limitations. To report multimorbidity prevalence, the majority of the studies in our sample used self-reported data. As a result, such research findings were prone to response bias. High heterogeneity between studies in our meta-analysis implies that the prevalence of multimorbidity varies between studies. To overcome this constraint, we used a random-effects model and performed subgroup analyses. Furthermore, considerable heterogeneity may indicate that the prevalence of multimorbidity varies significantly by geographical region, country income classification, gender, age group, number of diseases considered for multimorbidity, or study methodology.
The high prevalence of multimorbidity highlights the need for healthcare reforms and improvements in several continents. Policymakers should commit to increasing multimorbidity awareness, particularly in relation to mental health management, supporting innovation, maximizing the use of existing resources, and coordinating the efforts of multiple countries to reduce the burden and fatal effects of multimorbidity. More than half of the global adult population over the age of 60 has multimorbid illnesses, and female adults are more prone to develop multimorbidity than male adults. Therefore, management should incorporate these findings into healthcare policies, and countries, particularly in South America, should aim to increase their preventative efforts and build more integrated care models to reduce the burden.
Contributors
A.H., S.R.C., D.C.D., and T.C.S. contributed to the study concept, literature search, and design. A.H., S.R.C., D.C.D., T.C.S. and J.B. contributed to the data acquisition. A.H. and S.R.C. accessed the data and contributed to the data analysis. A.H., S.R.C., and J.B. contributed to the data interpretation. A.H., S.R.C. and D.C.D. drafted the manuscript. All authors contributed to the critical revision of the manuscript.
Data sharing statement
Because this meta-analysis was based on data extracted from previously published research, most of the data and study materials are available in the public domain. For further discussions, we invite interested parties to contact the corresponding author.
Declaration of interests
All other authors declare no competing interests.
Acknowledgement
We appreciate the statistical advice provided by the University of Sharjah's College of Health Sciences. We appreciate the five anonymous reviewers' insightful comments.
Footnotes
Supplementary data related to this article can be found at https://doi.org/10.1016/j.eclinm.2023.101860.
Contributor Information
Saifur Rahman Chowdhury, Email: saifur@mcmaster.ca.
Dipak Chandra Das, Email: dipak.das@northsouth.edu.
Tachlima Chowdhury Sunna, Email: tachlima.sunna@northsouth.edu.
Joseph Beyene, Email: beyene@mcmaster.ca.
Ahmed Hossain, Email: ahmed.hossain@northsouth.edu.
Appendix
.
Appendix A.
Search strategy.
| A. PubMed | ||
| #1 | (“Prevalence” OR “Surveillance” OR “Surveys” OR “Epidemiology”) AND (“Multimorbidity” OR “Multi-morbidity” OR “Multimorbidities” OR “Multi-morbidities” OR “Multi morbidity” OR “Multi morbidities”) | 3734 |
| #2 | (“Risk factors” OR “Determinants” OR “Predictors”) AND (“Multimorbidity” OR “Multi-morbidity” OR “Multimorbidities” OR “Multi-morbidities” OR “Multi morbidity” OR “Multi morbidities”) | 1500 |
| #3 | (“Aging” OR “Gender”) AND (“Multimorbidity” OR “Multi-morbidity” OR “Multimorbidities” OR “Multi-morbidities” OR “Multi morbidity” OR “Multi morbidities”) | 1708 |
| Searching date starting from 2000/01/01 to 2021/12/31All the entries were under ‘All fields’ categories | ||
| B. Google Scholar | ||
| #1 | (“Prevalence” OR “Surveillance” OR “Surveys” OR “Epidemiology”) AND (“Multimorbidity” OR “Multi-morbidity” OR “Multimorbidities” OR “Multi-morbidities” OR “Multi morbidity” OR “Multi morbidities”) | 18,913 |
| #2 | (“Risk factors” OR “Determinants” OR “Predictors”) AND (“Multimorbidity” OR “Multi-morbidity” OR “Multimorbidities” OR “Multi-morbidities” OR “Multi morbidity” OR “Multi morbidities”) | 16,500 |
| #3 | (“Aging” OR “Gender”) AND (“Multimorbidity” OR “Multi-morbidity” OR “Multimorbidities” OR “Multi-morbidities” OR “Multi morbidity” OR “Multi morbidities”) | 17,103 |
| Searching date starting from 2000/01/01 to 2021/12/31 | ||
| C. ScienceDirect | ||
| #1 | (“Prevalence” OR “Surveys” OR “Epidemiology”) AND (“Multimorbidity” OR “Multi-morbidity” OR “Multimorbidities” OR “Multi-morbidities” OR “Multi morbidity” OR “Multi morbidities”) | 4104 |
| #2 | (“Risk factors” OR “Determinants” OR “Predictors”) AND (“Multimorbidity” OR “Multi-morbidity” OR “Multimorbidities” OR “Multi-morbidities” OR “Multi morbidity” OR “Multi morbidities”) | 4133 |
| #3 | (“Aging” OR “Gender”) AND (“Multimorbidity” OR “Multi-morbidity” OR “Multimorbidities” OR “Multi-morbidities” OR “Multi morbidity” OR “Multi morbidities”) | 4391 |
| Searching date starting from 2000/01/01 to 2021/12/31 | ||
| D. Embase | ||
| #1 | (“Prevalence” OR “Surveillance” OR “Surveys” OR “Epidemiology”) AND (“Multimorbidity” OR “Multi-morbidity” OR “Multimorbidities” OR “Multi-morbidities” OR “Multi morbidity” OR “Multi morbidities”) | 8713 |
| #2 | (“Risk factors” OR “Determinants” OR “Predictors”) AND (“Multimorbidity” OR “Multi-morbidity” OR “Multimorbidities” OR “Multi-morbidities” OR “Multi morbidity” OR “Multi morbidities”) | 3616 |
| #3 | (“Aging” OR “Gender”) AND (“Multimorbidity” OR “Multi-morbidity” OR “Multimorbidities” OR “Multi-morbidities” OR “Multi morbidity” OR “Multi morbidities”) | 7138 |
| Searching date starting from 2000/01/01 to 2021/12/31 | ||
Appendix A. Supplementary data
S1. Study quality assessment details for cohort, and cross-sectional studies by New-Castle Ottawa Scale.
S2. Preferred Reporting Items for Systematic Reviews and Meta-analysis (PRISMA) checklist.
Forest plots of subgroup analysis.
Sensitivity analysis results.
Funnel plot and publication bias results.
References
- 1.Moffat K., Mercer S.W. Challenges of managing people with multimorbidity in today's healthcare systems. BMC Fam Pract. 2015;16:129. doi: 10.1186/s12875-015-0344-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.GBD 2015 Mortality and Causes of Death Collaborators Global, regional, and national life expectancy, all-cause mortality, and cause-specific mortality for 249 causes of death, 1980-2015: a systematic analysis for the Global Burden of Disease Study 2015. Lancet. 2016;388(10053):1459–1544. doi: 10.1016/S0140-6736(16)31012-1. Erratum in: Lancet. 2017 Jan 7;389(10064):e1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Hussain M.A., Huxley R.R., Al Mamun A. Multimorbidity prevalence and pattern in Indonesian adults: an exploratory study using national survey data. BMJ Open. 2015;5(12) doi: 10.1136/bmjopen-2015-009810. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Ryan A., Wallace E., O'Hara P., Smith S.M. Multimorbidity and functional decline in community-dwelling adults: a systematic review. Health Qual Life Outcomes. 2015;13:168. doi: 10.1186/s12955-015-0355-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Zhang X., Padhi A., Wei T., et al. Community prevalence and dyad disease pattern of multimorbidity in China and India: a systematic review. BMJ Glob Health. 2022 Sep;7(9) doi: 10.1136/bmjgh-2022-008880. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.McPhail S.M. Multimorbidity in chronic disease: impact on health care resources and costs. Risk Manag Healthc Policy. 2016;9:143–156. doi: 10.2147/RMHP.S97248. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Makovski T.T., Schmitz S., Zeegers M.P., Stranges S., van den Akker M. Multimorbidity and quality of life: systematic literature review and meta-analysis. Ageing Res Rev. 2019;53:100903. doi: 10.1016/j.arr.2019.04.005. [DOI] [PubMed] [Google Scholar]
- 8.Nunes B.P., Flores T.R., Mielke G.I., Thumé E., Facchini L.A. Multimorbidity and mortality in older adults: a systematic review and meta-analysis. Arch Gerontol Geriatr. 2016;67:130–138. doi: 10.1016/j.archger.2016.07.008. [DOI] [PubMed] [Google Scholar]
- 9.Soley-Bori M., Ashworth M., Bisquera A., et al. Impact of multimorbidity on healthcare costs and utilisation: a systematic review of the UK literature. Br J Gen Pract. 2021;71(702):e39–e46. doi: 10.3399/bjgp20x713897. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.WHO MiNDbank - multiple chronic conditions: a strategic framework - optimum health and quality of life for individuals with multiple chronic conditions. https://www.mindbank.info/item/2098
- 11.Barnett K., Mercer S.W., Norbury M., Watt G., Wyke S., Guthrie B. Epidemiology of multimorbidity and implications for health care, research, and medical education: a cross-sectional study. Lancet. 2012;380(9836):37–43. doi: 10.1016/S0140-6736(12)60240-2. [DOI] [PubMed] [Google Scholar]
- 12.Nguyen H., Manolova G., Daskalopoulou C., Vitoratou S., Prince M., Prina A.M. Prevalence of multimorbidity in community settings: a systematic review and meta-analysis of observational studies. J Comorb. 2019;9 doi: 10.1177/2235042x19870934. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Fortin M., Stewart M., Poitras M.E., Almirall J., Maddocks H. A systematic review of prevalence studies on multimorbidity: toward a more uniform methodology. Ann Fam Med. 2012;10(2):142–151. doi: 10.1370/afm.1337. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Violan C., Foguet-Boreu Q., Flores-Mateo G., et al. Prevalence, determinants and patterns of multimorbidity in primary care: a systematic review of observational studies. PLoS One. 2014;9(7) doi: 10.1371/journal.pone.0102149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Pati S., Swain S., Hussain M.A., et al. Prevalence and outcomes of multimorbidity in South Asia: a systematic review. BMJ Open. 2015;5(10) doi: 10.1136/bmjopen-2014-007235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Gruneir A., Bronskill S.E., Maxwell C.J., et al. The association between multimorbidity and hospitalization is modified by individual demographics and physician continuity of care: a retrospective cohort study. BMC Health Serv Res. 2016;16:154. doi: 10.1186/s12913-016-1415-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Schäfer I., von Leitner E.C., Schön G., et al. Multimorbidity patterns in the elderly: a new approach of disease clustering identifies complex interrelations between chronic conditions. PLoS One. 2010;5(12) doi: 10.1371/journal.pone.0015941. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Pati S., Swain S., Knottnerus J.A., Metsemakers J.F.M., Van Den Akker M. Magnitude and determinants of multimorbidity and health care utilization among patients attending public versus private primary care: a cross-sectional study from Odisha, India. Int J Equity Health. 2020;19(1):57. doi: 10.1186/s12939-020-01170-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Sinnige J., Braspenning J., Schellevis F., Stirbu-Wagner I., Westert G., Korevaar J. The prevalence of disease clusters in older adults with multiple chronic diseases--a systematic literature review. PLoS One. 2013;8(11) doi: 10.1371/journal.pone.0079641. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Prados-Torres A., Calderón-Larrañaga A., Hancco-Saavedra J., Poblador-Plou B., van den Akker M. Multimorbidity patterns: a systematic review. J Clin Epidemiol. 2014;67(3):254–266. doi: 10.1016/j.jclinepi.2013.09.021. [DOI] [PubMed] [Google Scholar]
- 21.Garin N., Koyanagi A., Chatterji S., et al. Global multimorbidity patterns: a cross-sectional, population-based, multi-country study. J Gerontol A Biol Sci Med Sci. 2016;71(2):205–214. doi: 10.1093/gerona/glv128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.van Oostrom S.H., Picavet H.S., de Bruin S.R., et al. Multimorbidity of chronic diseases and health care utilization in general practice. BMC Fam Pract. 2014;15:61. doi: 10.1186/1471-2296-15-61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Wallace E., Salisbury C., Guthrie B., Lewis C., Fahey T., Smith S.M. Managing patients with multimorbidity in primary care. BMJ. 2015;350:h176. doi: 10.1136/BMJ.H176. [DOI] [PubMed] [Google Scholar]
- 24.Laux G., Kuehlein T., Rosemann T., Szecsenyi J. Co- and multimorbidity patterns in primary care based on episodes of care: results from the German CONTENT project. BMC Health Serv Res. 2008;8:14. doi: 10.1186/1472-6963-8-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Larsen F.B., Pedersen M.H., Friis K., Glümer C., Lasgaard M. A latent class analysis of multimorbidity and the relationship to socio-demographic factors and health-related quality of life. A national population-based study of 162,283 Danish adults. PLoS One. 2017;12(1) doi: 10.1371/JOURNAL.PONE.0169426. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Diederichs C., Berger K., Bartels D.B. The measurement of multiple chronic diseases--a systematic review on existing multimorbidity indices. J Gerontol A Biol Sci Med Sci. 2011;66(3):301–311. doi: 10.1093/gerona/glq208. [DOI] [PubMed] [Google Scholar]
- 27.Islam M.M., Valderas J.M., Yen L., Dawda P., Jowsey T., McRae I.S. Multimorbidity and comorbidity of chronic diseases among the senior Australians: prevalence and patterns. PLoS One. 2014;9(1) doi: 10.1371/journal.pone.0083783. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Ofori-Asenso R., Chin K.L., Curtis A.J., Zomer E., Zoungas S., Liew D. Recent patterns of multimorbidity among older adults in high-income countries. Popul Health Manag. 2019;22(2):127–137. doi: 10.1089/pop.2018.0069. [DOI] [PubMed] [Google Scholar]
- 29.Moher D., Liberati A., Tetzlaff J., Altman D.G. Preferred reporting items for systematic reviews and meta-analyses: the PRISMA statement. PLoS Med. 2009;6(7) doi: 10.1371/journal.pmed.1000097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.World Bank Country and Lending Groups – World Bank data help desk. https://datahelpdesk.worldbank.org/knowledgebase/articles/906519-world-bank-country-and-lending-groups
- 31.Wells G., Shea B., O'Connell D., et al. The Newcastle-Ottawa Scale (NOS) for assessing the quality of nonrandomised studies in meta-analyses. 2013. http://www.ohri.ca/programs/clinical_epidemiology/oxford.asp
- 32.Lo C.K., Mertz D., Loeb M. Newcastle-Ottawa Scale: comparing reviewers' to authors' assessments. BMC Med Res Methodol. 2014;14:45. doi: 10.1186/1471-2288-14-45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Hossain A., Nasrullah S.M., Tasnim Z., Hasan M.K., Hasan M.M. Seroprevalence of SARS-CoV-2 IgG antibodies among health care workers prior to vaccine administration in Europe, the USA and East Asia: a systematic review and meta-analysis. eClinicalMedicine. 2021;33:100770. doi: 10.1016/j.eclinm.2021.100770. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Dhungana R.R., Karki K.B., Bista B., Pandey A.R., Dhimal M., Maskey M.K. Prevalence, pattern and determinants of chronic disease multimorbidity in Nepal: secondary analysis of a national survey. BMJ Open. 2021;11(7):1–7. doi: 10.1136/bmjopen-2020-047665. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Zhang L., Sun F., Li Y., Tang Z., Ma L. Multimorbidity in community-dwelling older adults in Beijing: prevalence and trends, 2004–2017. J Nutr Health Aging. 2021;25(1):116–119. doi: 10.1007/s12603-020-1467-4. [DOI] [PubMed] [Google Scholar]
- 36.Keetile M., Navaneetham K., Letamo G. Prevalence and correlates of multimorbidity among adults in Botswana: a cross-sectional study. PLoS One. 2020;15(9) doi: 10.1371/journal.pone.0239334. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Zou S., Wang Z., Bhura M., Zhang G., Tang K. Prevalence and associated socioeconomic factors of multimorbidity in 10 regions of China: an analysis of 0.5 million adults. J Public Health (Oxf) 2022;44(1):36–50. doi: 10.1093/pubmed/fdaa204. [DOI] [PubMed] [Google Scholar]
- 38.Ma X., He Y., Xu J. Urban-rural disparity in prevalence of multimorbidity in China: a cross-sectional nationally representative study. BMJ Open. 2020;10(11):e038404. doi: 10.1136/bmjopen-2020-038404. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Kim J., Keshavjee S., Atun R. Trends, patterns and health consequences of multimorbidity among South Korea adults: analysis of nationally representative survey data 2007-2016. J Glob Health. 2020;10(2):20426. doi: 10.7189/JOGH.10.020426. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Kshatri J.S., Palo S.K., Bhoi T., Barik S.R., Pati S. Prevalence and patterns of multimorbidity among rural elderly: findings of the AHSETS study. Front Public Health. 2020;8:582663. doi: 10.3389/fpubh.2020.582663. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Kyprianidou M., Panagiotakos D., Faka A., Kambanaros M., Makris K.C., Christophi C.A. Prevalence of multimorbidity in the Cypriot population; a cross-sectional study (2018-2019) PLoS One. 2020;15(10):e0239835. doi: 10.1371/journal.pone.0239835. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Melo L.A., Lima K.C. Prevalence and factors associated with multimorbidities in Brazilian older adults. Cien Saude Colet. 2020;25(10):3869–3877. doi: 10.1590/1413-812320202510.34492018. Portuguese, English. [DOI] [PubMed] [Google Scholar]
- 43.Zhang Y., Misra R., Sambamoorthi U. Prevalence of multimorbidity among Asian Indian, Chinese, and non-Hispanic White adults in the United States. Int J Environ Res Public Health. 2020;17(9):3336. doi: 10.3390/ijerph17093336. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Li X., Cai L., Cui W.L., et al. Association of socioeconomic and lifestyle factors with chronic non-communicable diseases and multimorbidity among the elderly in rural southwest China. J Public Health (Oxf) 2020;42(2):239–246. doi: 10.1093/pubmed/fdz020. [DOI] [PubMed] [Google Scholar]
- 45.Aminisani N., Rastgou L., Shamshirgaran S.M., Sarbakhsh P., Ghaderi S., Hyde M. Predictors of multimorbidity among the Kurdish population living in the Northwest of Iran. BMC Public Health. 2020;20(1):1094. doi: 10.1186/s12889-020-09214-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Craig L.S., Hotchkis D.R., Theal K.P., Cunningham-Myrie C., Hernande J.H., Gustat J. Prevalence and patterns of multimorbidity in the Jamaican population: a comparative analysis of latent variable models. PLoS One. 2020;15(7):e0236034. doi: 10.1371/journal.pone.0236034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Vargese S.S., Mathew E., Johny V., Kurian N., Gayathri A.V., Raju A.S. Prevalence and pattern of multimorbidity among adults in a primary care rural setting. Clin Epidemiol Glob Health. 2020;8(2):482–485. doi: 10.1016/j.cegh.2019.10.014. [DOI] [Google Scholar]
- 48.Lee Y., Kim H., Jeong H., Noh Y. Patterns of multimorbidity in adults: an association rules analysis using the Korea health panel. Int J Environ Res Public Health. 2020;17(8):2618. doi: 10.3390/ijerph17082618. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Zhao Y., Atun R., Oldenburg B., et al. Physical multimorbidity, health service use, and catastrophic health expenditure by socioeconomic groups in China: an analysis of population-based panel data. Lancet Glob Health. 2020;8(6):e840–e849. doi: 10.1016/S2214-109X(20)30127-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Wister A., Rosenkrantz L., Shashank A., Walker B.B., Schuurman N. Multimorbidity and socioeconomic deprivation among older adults: a cross-sectional analysis in five Canadian cities using the CLSA. J Aging Environ. 2020;34(4):435–454. doi: 10.1080/26892618.2020.1734138. [DOI] [Google Scholar]
- 51.Yao S.S., Cao G.Y., Han L., et al. Prevalence and patterns of multimorbidity in a nationally representative sample of older Chinese: results from the China health and retirement longitudinal study. J Gerontol A Biol Sci Med Sci. 2020;75(10):1974–1980. doi: 10.1093/gerona/glz185. [DOI] [PubMed] [Google Scholar]
- 52.Zhang R., Lu Y., Shi L., Zhang S., Chang F. Prevalence and patterns of multimorbidity among the elderly in China: a cross-sectional study using national survey data. BMJ Open. 2019;9(8):1–9. doi: 10.1136/bmjopen-2018-024268. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Laires P.A., Perelman J. The current and projected burden of multimorbidity: a cross-sectional study in a Southern Europe population. Eur J Ageing. 2019;16(2):181–192. doi: 10.1007/s10433-018-0485-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Ba N.V., Minh H.V., Quang L.B., et al. Prevalence and correlates of multimorbidity among adults in border areas of the Central Highland Region of Vietnam, 2017. J Comorb. 2019;9 doi: 10.1177/2235042X19853382. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Khan N., Rahman M., Mitra D., Afsana K. Prevalence of multimorbidity among Bangladeshi adult population: a nationwide cross-sectional study. BMJ Open. 2019;9(11) doi: 10.1136/bmjopen-2019-030886. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Singh K., Patel S.A., Biswas S., et al. Multimorbidity in South Asian adults: prevalence, risk factors and mortality. J Public Health. 2019;41(1):80–89. doi: 10.1093/pubmed/fdy017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Lai F.T.T., Guthrie B., Wong S.Y.S., et al. Sex-specific intergenerational trends in morbidity burden and multimorbidity status in Hong Kong community: an age-period-cohort analysis of repeated population surveys. BMJ Open. 2019;9(1) doi: 10.1136/bmjopen-2018-023927. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Bao X.Y., Xie Y.X., Zhang X.X., et al. The association between multimorbidity and health-related quality of life: a cross-sectional survey among community middle-aged and elderly residents in southern China. Health Qual Life Outcomes. 2019;17(1):107. doi: 10.1186/s12955-019-1175-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Hu R.H., Hsiao F.Y., Chen L.J., Huang P.T., Hsu W.W.Y. Increasing age- and gender-specific burden and complexity of multimorbidity in Taiwan, 2003-2013: a cross-sectional study based on nationwide claims data. BMJ Open. 2019;9(6):1–10. doi: 10.1136/bmjopen-2018-028333. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Park B., Lee H.A., Park H. Use of latent class analysis to identify multimorbidity patterns and associated factors in Korean adults aged 50 years and older. PLoS One. 2019;14(11) doi: 10.1371/journal.pone.0216259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Hernández B., Reilly R.B., Kenny R.A. Investigation of multimorbidity and prevalent disease combinations in older Irish adults using network analysis and association rules. Sci Rep. 2019;9(1):1–12. doi: 10.1038/s41598-019-51135-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Frølich A., Ghith N., Schiøtz M., Jacobsen R., Stockmarr A. Multimorbidity, healthcare utilization and socioeconomic status: a register-based study in Denmark. PLoS One. 2019;14(8) doi: 10.1371/journal.pone.0214183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Chang A.Y., Gómez-Olivé F.X., Payne C., et al. Chronic multimorbidity among older adults in rural South Africa. BMJ Glob Health. 2019;4(4) doi: 10.1136/bmjgh-2018-001386. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Nguyen H., Chua K.C., Dregan A., et al. Factors associated with multimorbidity patterns in older adults in England: findings from the English Longitudinal Study of Aging (ELSA) J Aging Health. 2020;32(9):1120–1132. doi: 10.1177/0898264319891026. [DOI] [PubMed] [Google Scholar]
- 65.Costa C.D.S., Flores T.R., Wendt A., et al. Inequalities in multimorbidity among elderly: a population-based study in a city in Southern Brazil. Cad Saúde Pública. 2018;34(11) doi: 10.1590/0102-311X00040718. [DOI] [PubMed] [Google Scholar]
- 66.Cheung J.T.K., Yu R., Wu Z., Wong S.Y.S., Woo J. Geriatric syndromes, multimorbidity, and disability overlap and increase healthcare use among older Chinese. BMC Geriatr. 2018;18(1):1–8. doi: 10.1186/s12877-018-0840-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Zemedikun D.T., Gray L.J., Khunti K., Davies M.J., Dhalwani N.N. Patterns of multimorbidity in middle-aged and older adults: an analysis of the UK Biobank data. Mayo Clin Proc. 2018;93(7):857–866. doi: 10.1016/j.mayocp.2018.02.012. [DOI] [PubMed] [Google Scholar]
- 68.El Lawindi M.I., Salem M.R., Razik M.M.A., Balas E.A. Socioeconomic predictors of morbidities in a rural setting: a community based study. Internet J Epidemiol. 2018;15(1):1–7. doi: 10.5580/IJE.53712. [DOI] [Google Scholar]
- 69.Alimohammadian M., Majidi A., Yaseri M., et al. Multimorbidity as an important issue among women: results of a gender difference investigation in a large population-based cross-sectional study in West Asia. BMJ Open. 2017;7(5) doi: 10.1136/bmjopen-2016-013548. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Stanley J., Semper K., Millar E., Sarfati D. Epidemiology of multimorbidity in New Zealand: a cross-sectional study using national-level hospital and pharmaceutical data. BMJ Open. 2018;8(5):1–12. doi: 10.1136/bmjopen-2018-021689. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Araujo M.E.A., Silva M.T., Galvao T.F., Nunes B.P., Pereira M.G. Prevalence and patterns of multimorbidity in Amazon Region of Brazil and associated determinants: a cross-sectional study. BMJ Open. 2018;8(11) doi: 10.1136/bmjopen-2018-023398. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Jankovic J., Mirkovic M., Jovic-Vranes A., Santric-Milicevic M., Terzic-Supic Z. Association between non-communicable disease multimorbidity and health care utilization in a middle-income country: population-based study. Public Health. 2018;155:35–42. doi: 10.1016/j.puhe.2017.11.014. [DOI] [PubMed] [Google Scholar]
- 73.Chen H., Cheng M., Zhuang Y., Broad J.B. Multimorbidity among middle-aged and older persons in urban China: prevalence, characteristics and health service utilization. Geriatr Gerontol Int. 2018;18(10):1447–1452. doi: 10.1111/ggi.13510. [DOI] [PubMed] [Google Scholar]
- 74.Nunes B.P., Batista S.R.R., Andrade F.B., Souza Junior P.R.B., Lima-Costa M.F., Facchini L.A. Multimorbidity: the Brazilian Longitudinal Study of Aging (ELSI-Brazil) Rev Saude Publica. 2018;52Suppl 2(Suppl 2):10s. doi: 10.11606/S1518-8787.2018052000637. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Mondor L., Cohen D., Khan A.I., Wodchis W.P. Income inequalities in multimorbidity prevalence in Ontario, Canada: a decomposition analysis of linked survey and health administrative data. Int J Equity Health. 2018;17(1):90. doi: 10.1186/s12939-018-0800-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Mounce L.T.A., Campbell J.L., Henley W.E., Tejerina Arreal M.C., Porter I., Valderas J.M. Predicting incident multimorbidity. Ann Fam Med. 2018;16(4):322–329. doi: 10.1370/afm.2271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Ge L., Yap C.W., Heng B.H. Sex differences in associations between multimorbidity and physical function domains among community-dwelling adults in Singapore. PLoS One. 2018;13(5) doi: 10.1371/journal.pone.0197443. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Camargo-Casas S., Suarez-Monsalve S., Zepeda M.U.P., García-Peña C., Cano-Gutierrez C.A. Multimorbidity, depressive symptoms, and self-reported health in older adults: a secondary analysis of the Sabe Bogota Study. Rev Invest Clin. 2018;70(4):192–197. doi: 10.24875/RIC.18002478. [DOI] [PubMed] [Google Scholar]
- 79.Amaral T.L.M., Amaral C.D.A., de Lima N.S., Herculano P.V., Do Prado P.R., Monteiro G.T.R. Multimorbidity, depression and quality of life among elderly people assisted in the family health strategy in Senador Guiomard, Acre, Brazil. Ciên Saúde Colet. 2018;23(9):3077–3084. doi: 10.1590/1413-81232018239.22532016. [DOI] [PubMed] [Google Scholar]
- 80.Puth M.T., Weckbecker K., Schmid M., Münster E. Prevalence of multimorbidity in Germany: impact of age and educational level in a cross-sectional study on 19,294 adults. BMC Public Health. 2017;17(1):1–7. doi: 10.1186/s12889-017-4833-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Waterhouse P., Van Der Wielen N., Banda P.C., Channon A.A. The impact of multi-morbidity on disability among older adults in South Africa: do hypertension and socio-demographic characteristics matter? Int J Equity Health. 2017;16(1):1–10. doi: 10.1186/s12939-017-0537-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Wang L., Palmer A.J., Cocker F., Sanderson K. Multimorbidity and health-related quality of life (HRQoL) in a nationally representative population sample: implications of count versus cluster method for defining multimorbidity on HRQoL. Health Qual Life Outcomes. 2017;15(1):1–12. doi: 10.1186/s12955-016-0580-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Kunna R., San Sebastian M., Stewart Williams J. Measurement and decomposition of socioeconomic inequality in single and multimorbidity in older adults in China and Ghana: results from the WHO study on global AGEing and adult health (SAGE) Int J Equity Health. 2017;16(1):1–17. doi: 10.1186/s12939-017-0578-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Lujic S., Simpson J.M., Zwar N., Hosseinzadeh H., Jorm L. Multimorbidity in Australia: comparing estimates derived using administrative data sources and survey data. PLoS One. 2017;12(8):e0183817. doi: 10.1371/journal.pone.0183817. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Nunes B.P., Chiavegatto Filho A.D.P., Pati S., et al. Contextual and individual inequalities of multimorbidity in Brazilian adults: a cross-sectional national-based study. BMJ Open. 2017;7(6):e015885. doi: 10.1136/bmjopen-2017-015885. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Mini G.K., Thankappan K.R. Pattern, correlates and implications of non-communicable disease multimorbidity among older adults in selected Indian states: a cross-sectional study. BMJ Open. 2017;7(3):1–6. doi: 10.1136/bmjopen-2016-013529. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Larsen F.B., Pedersen M.H., Friis K., Gluèmer C., Lasgaard M. A latent class analysis of multimorbidity and the relationship to socio-demographic factors and health-related quality of life. A national population-based study of 162,283 Danish adults. PLoS One. 2017;12(1):e0169426. doi: 10.1371/journal.pone.0169426. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Gu J., Chao J., Chen W., et al. Multimorbidity in the community-dwelling elderly in urban China. Arch Gerontol Geriatr. 2017;68:62–67. doi: 10.1016/j.archger.2016.09.001. [DOI] [PubMed] [Google Scholar]
- 89.Dhalwani N.N., Zaccardi F., O'Donovan G., et al. Association between lifestyle factors and the incidence of multimorbidity in an older English population. J Gerontol A Biol Sci Med Sci. 2017;72(4):528–534. doi: 10.1093/gerona/glw146. [DOI] [PubMed] [Google Scholar]
- 90.Nunes B.P., Camargo-Figuera F.A., Guttier M., et al. Multimorbidity in adults from a southern Brazilian city: occurrence and patterns. Int J Public Health. 2016;61(9):1013–1020. doi: 10.1007/s00038-016-0819-7. [DOI] [PubMed] [Google Scholar]
- 91.Picco L., Achilla E., Abdin E., et al. Economic burden of multimorbidity among older adults: impact on healthcare and societal costs. BMC Health Serv Res. 2016;16:173. doi: 10.1186/s12913-016-1421-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Palladino R., Tayu Lee J., Ashworth M., Triassi M., Millett C. Associations between multimorbidity, healthcare utilisation and health status: evidence from 16 European countries. Age Ageing. 2016;45(3):431–435. doi: 10.1093/ageing/afw044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Le Cossec C., Perrine A.L., Beltzer N., Fuhrman C., Carcaillon-Bentata L. Pre-frailty, frailty, and multimorbidity: prevalences and associated characteristics from two French national surveys. J Nutr Health Aging. 2016;20(8):860–869. doi: 10.1007/s12603-016-0802-2. [DOI] [PubMed] [Google Scholar]
- 94.Vadrevu L., Kumar V., Kanjilal B. Rising challenge of multiple morbidities among the rural poor in India: a case of the Sundarbans in West Bengal. Int J Med Sci Public Health. 2016;5(2):343. doi: 10.5455/ijmsph.2016.25082015129. [DOI] [Google Scholar]
- 95.Marengoni A., Angleman S., Meinow B., et al. Coexisting chronic conditions in the older population: variation by health indicators. Eur J Intern Med. 2016;31:29–34. doi: 10.1016/j.ejim.2016.02.014. [DOI] [PubMed] [Google Scholar]
- 96.Jovic D., Vukovic D., Marinkovic J. Prevalence and patterns of multi-morbidity in Serbian adults: a cross-sectional study. PLoS One. 2016;11(2) doi: 10.1371/journal.pone.0148646. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Su P., Ding H., Zhang W., et al. The association of multimorbidity and disability in a community-based sample of elderly aged 80 or older in Shanghai, China. BMC Geriatr. 2016;16(1):178. doi: 10.1186/s12877-016-0352-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Ramond-Roquin A., Haggerty J., Lambert M., Almirall J., Fortin M. Different multimorbidity measures result in varying estimated levels of physical quality of life in individuals with multimorbidity: a cross-sectional study in the general population. Biomed Res Int. 2016;2016 doi: 10.1155/2016/7845438. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Lenzi J., Avaldi V.M., Rucci P., Pieri G., Fantini M.P. Burden of multimorbidity in relation to age, gender and immigrant status: a cross-sectional study based on administrative data. BMJ Open. 2016;6(12) doi: 10.1136/bmjopen-2016-012812. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Dung L.D., Long G.T. Gender differences in prevalence and associated factors of multi-morbidity among older persons in Vietnam. Int J Ageing Dev Ctries. 2016;1(2):113–132. [Google Scholar]
- 101.Valadares A.L., Lui-Filho J.F., Costa-Paiva L., Pinto-Neto A.M. Middle-aged female sexual dysfunction and multimorbidity: a population-based study. Menopause. 2016;23(3):304–310. doi: 10.1097/GME.0000000000000533. [DOI] [PubMed] [Google Scholar]
- 102.Pache B., Vollenweider P., Waeber G., Marques-Vidal P. Prevalence of measured and reported multimorbidity in a representative sample of the Swiss population disease epidemiology - chronic. BMC Public Health. 2015;15(1):1–8. doi: 10.1186/s12889-015-1515-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Afshar S., Roderick P.J., Kowal P., Dimitrov B.D., Hill A.G. Multimorbidity and the inequalities of global ageing: a cross-sectional study of 28 countries using the World Health Surveys. BMC Public Health. 2015;15(1):1–10. doi: 10.1186/s12889-015-2008-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Roberts K.C., Rao D.P., Bennett T.L., Loukine L., Jayaraman G.C. Prevalence and patterns of chronic disease multimorbidity and associated determinants in Canada. Health Promot Chronic Dis Prev Can. 2015;35(6):87–94. doi: 10.24095/hpcdp.35.6.01. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Arokiasamy P., Uttamacharya U., Jain K., et al. The impact of multimorbidity on adult physical and mental health in low- and middle-income countries: what does the study on global ageing and adult health (SAGE) reveal? BMC Med. 2015;13:178. doi: 10.1186/s12916-015-0402-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Ha N.T., Le N.H., Khanal V., Moorin R. Multimorbidity and its social determinants among older people in southern provinces, Vietnam. Int J Equity Health. 2015;14:50. doi: 10.1186/s12939-015-0177-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Wang S.B., D'Arcy C., Yu Y.Q., et al. Prevalence and patterns of multimorbidity in northeastern China: a cross-sectional study. Public Health. 2015;129(11):1539–1546. doi: 10.1016/j.puhe.2015.06.013. [DOI] [PubMed] [Google Scholar]
- 108.Wang R., Yan Z., Liang Y., et al. Prevalence and patterns of chronic disease pairs and multimorbidity among older Chinese adults living in a rural area. PLoS One. 2015;10(9) doi: 10.1371/journal.pone.0138521. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Nunes B.P., Thumé E., Facchini L.A. Multimorbidity in older adults: magnitude and challenges for the Brazilian Health System. BMC Public Health. 2015;15:1172. doi: 10.1186/s12889-015-2505-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Chung R.Y., Mercer S., Lai F.T.T., Yip B.H.K., Wong M.C.S., Wong S.Y.S. Socioeconomic determinants of multimorbidity: a population-based household survey of Hong Kong Chinese. PLoS One. 2015;10(10):e0140040. doi: 10.1371/journal.pone.0140040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Ruel G., Lévesque J.F., Stocks N., et al. Understanding the evolution of multimorbidity: evidences from the North West Adelaide Health Longitudinal Study (NWAHS) PLoS One. 2014;9(5) doi: 10.1371/journal.pone.0096291. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Mahwati Y. Determinants of multimorbidity among the elderly determinants of multimorbidity among the elderly population in Indonesia. Natl Public Health J. 2014;9(2):187–193. doi: 10.21109/kesmas.v9i2.516. [DOI] [Google Scholar]
- 113.Banjare P., Pradhan J. Socio-economic inequalities in the prevalence of multi-morbidity among the rural elderly in Bargarh district of Odisha (India) PLoS One. 2014;9(6) doi: 10.1371/journal.pone.0097832. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Hien H., Berthé A., Drabo M.K., et al. Prevalence and patterns of multimorbidity among the elderly in Burkina Faso: cross-sectional study. Trop Med Int Health. 2014;19(11):1328–1333. doi: 10.1111/tmi.12377. [DOI] [PubMed] [Google Scholar]
- 115.Orueta J.F., Nuño-Solinís R., García-Alvarez A., Alonso-Morán E. Prevalence of multimorbidity according to the deprivation level among the elderly in the Basque Country. BMC Public Health. 2013;13:918. doi: 10.1186/1471-2458-13-918. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Aguiar L.B., Baccaro L.F., de Souza Santos Machado V., Pinto-Neto A.M., Costa-Paiva L. Disability and multimorbidity in women older than 50 years: a population-based household survey. Menopause. 2015;22(6):660–666. doi: 10.1097/GME.0000000000000355. [DOI] [PubMed] [Google Scholar]
- 117.Alaba O., Chola L. The social determinants of multimorbidity in South Africa. Int J Equity Health. 2013;12(1):1. doi: 10.1186/1475-9276-12-63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Wu F., Guo Y., Kowal P., et al. Prevalence of major chronic conditions among older Chinese adults: the Study on Global AGEing and adult health (SAGE) wave 1. PLoS One. 2013;8(9) doi: 10.1371/journal.pone.0074176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Phaswana-Mafuya N., Peltzer K., Chirinda W., et al. Self-reported prevalence of chronic non-communicable diseases and associated factors among older adults in South Africa. Glob Health Action. 2013;6:20936. doi: 10.3402/gha.v6i0.20936. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Jerliu N., Toçi E., Burazeri G., Ramadani N., Brand H. Prevalence and socioeconomic correlates of chronic morbidity among elderly people in Kosovo: a population-based survey. BMC Geriatr. 2013;13:22. doi: 10.1186/1471-2318-13-22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Kiliari N., Theodosopoulou E., Papanastasiou E. Multimorbidity and unmet citizens' needs and expectations urge for reforms in the health system of Cyprus: a questionnaire survey. JRSM Open. 2014;5(1) doi: 10.1177/2042533313515860. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Fuchs J., Busch M., Lange C., Scheidt-Nave C. Prevalence and patterns of morbidity among adults in Germany. Results of the German telephone health interview survey German Health Update (GEDA) 2009. Bundesgesundheitsblatt Gesundheitsforschung Gesundheitsschutz. 2012;55(4):576–586. doi: 10.1007/s00103-012-1464-9. [DOI] [PubMed] [Google Scholar]
- 123.De Souza Santos MacHado V., Valadares A.L.R., Da Costa-Paiva L.S., Moraes S.S., Pinto-Neto A.M. Multimorbidity and associated factors in Brazilian women aged 40 to 65 years: a population-based study. Menopause. 2012;19(5):569–575. doi: 10.1097/gme.0b013e3182455963. [DOI] [PubMed] [Google Scholar]
- 124.Kirchberger I., Meisinger C., Heier M., et al. Patterns of multimorbidity in the aged population. Results from the KORA-Age study. PLoS One. 2012;7(1) doi: 10.1371/journal.pone.0030556. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Agborsangaya C.B., Lau D., Lahtinen M., Cooke T., Johnson J.A. Multimorbidity prevalence and patterns across socioeconomic determinants: a cross-sectional survey. BMC Public Health. 2012;12(1):201. doi: 10.1186/1471-2458-12-201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Tucker-Seeley R.D., Li Y., Sorensen G., Subramanian S. Lifecourse socioeconomic circumstances and multimorbidity among older adults. BMC Public Health. 2011;11:313. doi: 10.1186/1471-2458-11-313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Khanam M.A., Streatfield P.K., Kabir Z.N., Qiu C., Cornelius C., Wahlin Å. Prevalence and patterns of multimorbidity among elderly people in rural Bangladesh: a cross-sectional study. J Health Popul Nutr. 2011;29(4):406–414. doi: 10.3329/jhpn.v29i4.8458. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Taylor A.W., Price K., Gill T.K., et al. Multimorbidity: not just an older person's issue. BMC Public Health. 2010;10(718):1–10. doi: 10.1186/1471-2458-10-718. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Loza E., Jover J.A., Rodriguez L., Carmona L. Multimorbidity: prevalence, effect on quality of life and daily functioning, and variation of this effect when one condition is a rheumatic disease. Semin Arthritis Rheum. 2009;38(4):312–319. doi: 10.1016/j.semarthrit.2008.01.004. [DOI] [PubMed] [Google Scholar]
- 130.Van Minh H., Ng N., Juvekar S., et al. Self-reported prevalence of chronic diseases and their relation to selected sociodemographic variables: a study in INDEPTH Asian sites, 2005. Prev Chronic Dis. 2008;5(3):A86. [PMC free article] [PubMed] [Google Scholar]
- 131.Wilk P., Stranges S., Bellocco R., et al. Multimorbidity in large Canadian urban centres: a multilevel analysis of pooled 2015-2018 cross-sectional cycles of the Canadian Community Health Survey. J Comorb. 2021;11 doi: 10.1177/26335565211058037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Tomita A., Leyna G.H., Kim H.Y., et al. Patterns of multimorbidity and their association with hospitalisation: a population-based study of older adults in urban Tanzania. Age Ageing. 2021;50(4):1349–1360. doi: 10.1093/ageing/afab046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Smith L., Shin J.I., Ghayda R.A., et al. Physical multimorbidity and incident urinary incontinence among community-dwelling adults aged ≥50 years: findings from a prospective analysis of the Irish Longitudinal Study on Ageing. Age Ageing. 2021;50(6):2038–2046. doi: 10.1093/ageing/afab151. [DOI] [PubMed] [Google Scholar]
- 134.Delpino F.M., Wendt A., Crespo P.A., et al. Occurrence and inequalities by education in multimorbidity in Brazilian adults between 2013 and 2019: evidence from the National Health Survey. Rev Bras Epidemiol. 2021;24(suppl 2) doi: 10.1590/1980-549720210016.supl.2. English, Portuguese. [DOI] [PubMed] [Google Scholar]
- 135.Marthias T., Anindya K., Ng N., et al. Impact of non-communicable disease multimorbidity on health service use, catastrophic health expenditure and productivity loss in Indonesia: a population-based panel data analysis study. BMJ Open. 2021;11(2) doi: 10.1136/bmjopen-2020-041870. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.Zhang C., Xiao S., Shi L., et al. Urban-rural differences in patterns and associated factors of multimorbidity among older adults in China: a cross-sectional study based on Apriori algorithm and multinomial logistic regression. Front Public Health. 2021;9:707062. doi: 10.3389/fpubh.2021.707062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137.Lin M.H., Chang C.Y., Wu D.M., Lu C.H., Kuo C.C., Chu N.F. Relationship of multimorbidity, obesity status, and grip strength among older adults in Taiwan. Int J Environ Res Public Health. 2021;18(14):7540. doi: 10.3390/ijerph18147540. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138.Nicholson K., Griffith L.E., Sohel N., Raina P. Examining early and late onset of multimorbidity in the Canadian Longitudinal Study on Aging. J Am Geriatr Soc. 2021;69(6):1579–1591. doi: 10.1111/jgs.17096. [DOI] [PubMed] [Google Scholar]
- 139.Bezerra de Souza D.L., Oliveras-Fabregas A., Espelt A., et al. Multimorbidity and its associated factors among adults aged 50 and over: a cross-sectional study in 17 European countries. PLoS One. 2021;16(2) doi: 10.1371/journal.pone.0246623. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140.Koyanagi A., Smith L., Shin J.I., et al. Multimorbidity and subjective cognitive complaints: findings from 48 low- and middle-income countries of the World Health Survey 2002-2004. J Alzheimers Dis. 2021;81(4):1737–1747. doi: 10.3233/JAD-201592. [DOI] [PubMed] [Google Scholar]
- 141.Shi X., Lima S.M.D.S., Mota C.M.M., Lu Y., Stafford R.S., Pereira C.V. Prevalence of multimorbidity of chronic noncommunicable diseases in Brazil: population-based study. JMIR Public Health Surveill. 2021;7(11) doi: 10.2196/29693. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142.Wang X., Yao S., Wang M., et al. Multimorbidity among two million adults in China. Int J Environ Res Public Health. 2020;17(10):3395. doi: 10.3390/ijerph17103395. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143.He K., Zhang W., Hu X., et al. Relationship between multimorbidity, disease cluster and all-cause mortality among older adults: a retrospective cohort analysis. BMC Public Health. 2021;21(1):1080. doi: 10.1186/s12889-021-11108-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144.Ballesteros S.M., Moreno-Montoya J., Grooten W.J.A., Barrera-López P., de la Hoz-Valle J.A. Socioeconomic variation of multimorbidity in Colombian older adults. Sci Rep. 2021;11(1):22738. doi: 10.1038/s41598-021-02219-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 145.Mohamed S.F., Haregu T.N., Uthman O.A., et al. Multimorbidity from chronic conditions among adults in urban slums: the AWI-Gen Nairobi Site Study findings. Glob Heart. 2021;16(1):6. doi: 10.5334/gh.771. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 146.Kanungo S., Ghosal S., Kerketta S., et al. Association of oral health with multimorbidity among older adults: findings from the longitudinal ageing study in India, wave-1, 2017-2019. Int J Environ Res Public Health. 2021;18(23):12853. doi: 10.3390/ijerph182312853. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 147.Oh H., Glass J., Narita Z., Koyanagi A., Sinha S., Jacob L. Discrimination and multimorbidity among Black Americans: findings from the National Survey of American Life. J Racial Ethn Health Disparities. 2021;8(1):210–219. doi: 10.1007/s40615-020-00773-z. [DOI] [PubMed] [Google Scholar]
- 148.King D.E., Xiang J., Pilkerton C.S. Multimorbidity trends in United States adults, 1988–2014. J Am Board Fam Med. 2018;31(4):503–513. doi: 10.3122/jabfm.2018.04.180008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 149.Bowling C.B., Deng L., Sakhuja S., Morey M.C., Jaeger B.C., Muntner P. Prevalence of activity limitations and association with multimorbidity among US adults 50 to 64 years old. J Gen Intern Med. 2019;34(11):2390–2396. doi: 10.1007/s11606-019-05244-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 150.Keats M.R., Cui Y., DeClercq V., et al. Multimorbidity in Atlantic Canada and association with low levels of physical activity. Prev Med. 2017;105:326–331. doi: 10.1016/j.ypmed.2017.10.013. [DOI] [PubMed] [Google Scholar]
- 151.Quinaz Romana G., Kislaya I., Salvador M.R., Cunha Gonçalves S., Nunes B., Dias C. Multimorbilidade em Portugal: Dados do Primeiro Inquérito Nacional de Saúde com Exame Físico [Multimorbidity in Portugal: results from the first National Health Examination Survey] Acta Med Port. 2019;32(1):30–37. doi: 10.20344/amp.11227. Portuguese. [DOI] [PubMed] [Google Scholar]
- 152.Souza A.S.S., Faerstein E., Werneck G.L. Multimorbidade e uso de serviços de saúde em indivíduos com restrição de atividades habituais: Estudo Pró-Saúde [Multimorbidity and use of health services by individuals with restrictions on habitual activities: the Pró-Saúde Study] Cad Saúde Pública. 2019;35(11) doi: 10.1590/0102-311X00155118. Portuguese. [DOI] [PubMed] [Google Scholar]
- 153.Costa Â.K., Bertoldi A.D., Fontanella A.T., et al. Does socioeconomic inequality occur in the multimorbidity among Brazilian adults? Rev Saude Publica. 2020;54:138. doi: 10.11606/s1518-8787.2020054002569. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 154.Keomma K., Bousquat A., César C.L.G. Prevalence of multimorbidity in older adults in São Paulo, Brazil: a study with ISA-Capital. Rev Saude Publica. 2022;56:69. doi: 10.11606/s1518-8787.2022056004252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 155.Jürisson M., Pisarev H., Uusküla A., Lang K., Oona M., Kalda R. Prevalence of chronic conditions and multimorbidity in Estonia: a population-based cross-sectional study. BMJ Open. 2021;11(10) doi: 10.1136/bmjopen-2021-049045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 156.Ho I.S., Azcoaga-Lorenzo A., Akbari A., et al. Variation in the estimated prevalence of multimorbidity: systematic review and meta-analysis of 193 international studies. BMJ Open. 2022;12(4) doi: 10.1136/bmjopen-2021-057017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 157.Huaquía-Díaz A.M., Chalán-Dávila T.S., Carrillo-Larco R.M., Bernabe-Ortiz A. Multimorbidity in Latin America and the Caribbean: a systematic review and meta-analysis. BMJ Open. 2021;11(7) doi: 10.1136/BMJOPEN-2021-050409. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 158.Xu X., Mishra G.D., Jones M. Mapping the global research landscape and knowledge gaps on multimorbidity: a bibliometric study. J Glob Health. 2017;7(1):010414. doi: 10.7189/jogh.07.010414. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 159.Harrison C., Britt H., Miller G., Henderson J. Examining different measures of multimorbidity, using a large prospective cross-sectional study in Australian general practice. BMJ Open. 2014;4(7) doi: 10.1136/BMJOPEN-2013-004694. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 160.Ferrer A., Formiga F., Sanz H., Almeda J., Padrós G. Multimorbidity as specific disease combinations, an important predictor factor for mortality in octogenarians: the Octabaix study. Clin Interv Aging. 2017;12:223–231. doi: 10.2147/CIA.S123173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 161.Quiñones A.R., Markwardt S., Botoseneanu A. Multimorbidity combinations and disability in older adults. J Gerontol A Biol Sci Med Sci. 2016;71(6):823–830. doi: 10.1093/gerona/glw035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 162.van den Bussche H., Koller D., Kolonko T., et al. Which chronic diseases and disease combinations are specific to multimorbidity in the elderly? Results of a claims data based cross-sectional study in Germany. BMC Public Health. 2011;11:101. doi: 10.1186/1471-2458-11-101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 163.Schulze T., Maercker A., Horn A.B. Mental health and multimorbidity: psychosocial adjustment as an important process for quality of life. Gerontology. 2014;60(3):249–254. doi: 10.1159/000358559. [DOI] [PubMed] [Google Scholar]
- 164.Marengoni A., Angleman S., Melis R., et al. Aging with multimorbidity: a systematic review of the literature. Ageing Res Rev. 2011;10(4):430–439. doi: 10.1016/j.arr.2011.03.003. [DOI] [PubMed] [Google Scholar]
- 165.Wang Z., Peng W., Li M., et al. Association between multimorbidity patterns and disability among older people covered by long-term care insurance in Shanghai, China. BMC Public Health. 2021;21(1) doi: 10.1186/s12889-021-10463-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
S1. Study quality assessment details for cohort, and cross-sectional studies by New-Castle Ottawa Scale.
S2. Preferred Reporting Items for Systematic Reviews and Meta-analysis (PRISMA) checklist.
Forest plots of subgroup analysis.
Sensitivity analysis results.
Funnel plot and publication bias results.